4
The Process
As discussed in Chapter 3, integrated coastal management (ICM) is a dynamic and continuing process for managing coastal systems in a manner that is responsive to scientific information and human expectations. With a focus on wastewater considerations, this chapter describes the application of the various steps in the process and the tools and methods needed to implement the process for managing coastal environments. While the Committee on Wastewater Management for Coastal Urban Areas is not aware of any particular situation in which integrated coastal management is being implemented at the fullest possible extent, it has identified several examples where elements of ICM are being developed and used. These examples are described throughout this chapter.
DYNAMIC PLANNING
The bulk of problem analysis and assessment takes place within the dynamic planning process (see items 1-4 in Figure 3.1). The power of dynamic planning lies in the bringing together of all relevant data and points of view to identify issues, and the use of a comparative risk assessment approach. Dynamic planning maximizes the use of information in the decision-making process. Most important, it ensures that the major risk management decisions are informed by a complete risk assessment.
Set Goals
In a large coastal area with multiple problems and inputs, the setting of goals is a complex and iterative process involving the balancing of expecta-
tions from different sectors of the community. It is seemingly simple but sometimes difficult to identify the important issues relative to wastewater in our coastal environment. This difficulty is due in part to our ignorance of all the goods, services, and other values the coastal environment provides and in part to our individual goals, biases, and perceptions. Coastal resources are, for the most part, a public commons. It is therefore very important that the dynamic planning process be an open and public one that involves all sectors of the communities that may be affected.
Identify Resources
The first step in setting goals for coastal resources in a region is to identify and inventory those resources. This inventory should take a broad interpretation of what may be considered resources in order to arrive at a truly comprehensive starting point for integrated coastal management. It should encompass both the natural and the built environment.
The most obvious resources of a region may be recreational areas (e.g., areas for boating, swimming, scuba diving, surfing) and fisheries. Also of importance would be ecological habitat, birds, wildlife, areas for aesthetic enjoyment, and other environmental attributes. Ports, shipping channels, and other features of the built environment should be included in the inventory as well.
Review Existing Scientific Knowledge
It is important that the goal-setting process be informed by the best available scientific information for a region. The point of this step of the process is to understand what is known about a region as well as to identify what is not known. This review should also serve to bring all participants in the goal-setting process to some common understanding of what is known about a region's resources and environmental characteristics and processes. However, incomplete and imperfect scientific knowledge is not an excuse for delaying action until more research is done. The ICM process should be used to determine if reasonable management decisions can be made, based on existing knowledge.
Assess Human Expectations
A key to the success of dynamic planning is the development of an adequate understanding of human expectations for coastal resources. Expectations may differ considerably from person to person. Often these different perspectives will identify issues that are quite different. Although there may be conflicting objectives or goals behind the issues, frequently
the underlying desires will be similar. For example, long-term viability of commercial fisheries and protection of rare species often appear to be in conflict in the short-term, but in the long-term both rely on protection of the ecosystem. Increasingly there is a variety of sophisticated social science techniques for assessing public expectations and values. Use of these techniques can be valuable and informative in addition to the traditional techniques of public hearings and comment which may often elicit only a relatively narrow, albeit important, perspective.
Public Expectations. The public communicates its expectations in the form of societal values (e.g., ecosystem preservation, protection of endangered species, and pristine beach fronts) and human needs (including recreational uses, fisheries, coastal development, transportation, manufacturing, agriculture, and waste management). Often values and needs will conflict with each other so it is important to understand them well. Out of such understanding those interests that may not have been immediately obvious can become more apparent. Additionally, principles for accommodating apparently conflicting uses and values can be developed.
Public expectations also will change over time. Identification of new health hazards, results from risk assessments, data from monitoring programs, and results of research into ecosystem impacts lead to changes in how issues are defined over time and the identification of new problems. Issues formerly of concern are usually dropped from consideration when they no longer need as much attention. New scientific information, depending on how it is communicated to the public, can change public expectations and drastically shift public attitudes toward single issues in exclusion of others. Public expectations also differ over time and among various subgroups within the population. Recently, there has been concern about environmental inequity expressed by primarily poor and minority populations who have become increasingly alarmed that their adverse environmental exposures may be greater than for more affluent populations (EPA 1992a, b).
To identify public expectations, it is necessary to involve the public in the planning process from the outset and continuously. To ensure that all issues are on the table at the outset, efforts need to be made to reach diverse groups and individuals who are concerned (NRC 1989a).
While public expectations are quite diverse, a common theme often can be identified. That common interest is appreciation or use of resources. Various parts of the public tend to identify issues relative to wastewater management in terms of whether the coastal resources with which they are concerned are protected. Consumers want to be assured that seafood is plentiful and safe to consume. Surfers, divers, and swimmers want to be certain that it is safe and pleasant to be in the water and walk on the beach. Commercial and recreational fishermen expect that the productive quality of the coastal waters is protected from pollution. Residents in the region
may be concerned about the effect of water quality on property values and the local economy. Some want the coastal environment protected for wildlife such as marine mammals and shore birds or simply want to know that the environment is viable, healthy, and sustainable for future generations.
Professional Perspectives. Just as public objectives may vary depending on the particular resource use that a segment of the population values in the marine environment, so will professional objectives vary depending on the particular expertise and interests of the professional in question. As illustrated by several examples, the range of views is vast. A public health practitioner will want to maximize the degree to which human health is protected. Traditionally this philosophy has been articulated through practices that erect the maximum number of barriers between humans and those stressors that could adversely affect human health. An environmentalist may expect maximum protection of the environment and that it remain unaltered. At the other extreme, one might find private entrepreneurs who will strive to minimize the cost of resource utilization in favor of its exploitation. In the middle might be the scientist who favors management objectives that are clearly related to well-understood scientific cause and effect relationships or an economist intent on developing marine-related resources and finding a balance between economic benefits and protection of the environment. One might also find the consulting engineer or government official who must define a wastewater control strategy that is practicably achievable, economically acceptable, and approaches the environmental objectives of the most interests.
Political Decision Making. The objectives of political decisionmakers often will be unstated because the political environment is one in which the process of decision making tends to dominate the need to articulate the goals of the outcome of the process. Political leaders are often freed from the need to articulate their ultimate objectives for wastewater management. There are, however, at least two circumstances in which their objectives become clear. One happens when there is a public outcry to protect a particular resource, such as ''Save the Bay!" The second is when there is a dramatic need to exploit the marine environment for the sake of human welfare, resulting in a cry to "Save Our Jobs!" Although in the short-term they may appear to be in conflict in the political process, in the long-term (usually longer than the term of office of the relevant political leaders) these two objectives usually complement each other.
Defining Issues and Setting Goals
The last step in the goal-setting process is the synthesis of the information and expectations assessed in the preceding three steps into a set of
issues. General goals then should be set around each of these issues. While these goals define a starting point for addressing a region's problems, they can and probably should be revised as new information is discovered and public expectations shift. As the dynamic planning process proceeds, some perspectives may change and the established goals may need to be revisited. Multiway dialogues must be established to bring together the various points of view.
As the foregoing discussion suggests, there is not a simply stated set of goals for wastewater management. Therefore, it follows that the selection of issues will depend somewhat on the viewpoint of the particular participants involved. These viewpoints will generally fall into one of the two general objectives for coastal protection stated in Chapter 2: 1) to restore and maintain the ecological integrity of coastal areas and 2) to maintain important human uses associated with those areas. Both views are valid when analyzed from the stance of the societal values each seeks to protect. The range of viewpoints held will determine how tradeoffs among competing interests will be established.
The development of a rational set of goals, and thereby selection of issues, depends on the skilled blending and balancing of several quite different values, including:
-
economic interests, such as those of coastal developers or commercial fishers;
-
personalized expectations, such as those of scuba divers, swimmers, or sport fishers;
-
rigorous scientific demands, such as those of the basic scientists;
-
conservative analyses, such as those of the ecological and public health sciences;
-
preservation interests, as posed by environmentalists; and
-
fiscal considerations, as posed by public agencies, ratepayers, and taxpayers.
At this stage, if a large number of issues has been identified, it may be necessary to do a risk screening in order to reduce the universe of concerns to the most major ones. For example, for a Pacific coastal area such as Santa Monica Bay, there has been no concern about dissolved oxygen in the water column, but there are significant public concerns about maintaining safety of bathing beaches, particularly near storm drain outlets. In Long Island Sound, the reopening of the extensive contaminated shellfish beds is not a high priority for most people, although it may become important in the future. On the other hand, eutrophication and associated hypoxia present a clear and growing danger to the fish and shellfish stocks of the sound, a danger about which the public is far less aware.
Define the Geographic Extent of Concerns
Once issues have been identified and goals have been set, it is time to define the geographic extent of the associated problems. The importance of this step in the dynamic planning process is that coastal problems occur on different scales. No problem can be addressed adequately and effectively if it is not tackled on the scale at which it occurs. Wastewater and stormwater associated effects occur across the spectrum of scales from very localized changes in benthic populations around the end of an outfall to large-scale nutrient enrichment due to point and nonpoint source inputs occurring over hundreds of square kilometers. Problem domains should encompass the resources affected by the issue of concern and the probable contributory sources. With the environmentally-based identification of the geographic extent of an issue, there also needs to be an involvement of the administrative authorities responsible for the relevant activities in these regions. If these authorities were not a part of the original goal-setting process, goals should be revisited with their involvement.
Resources
For each issue identified in the goal-setting process, there will be a relevant geographic extent of concern. These domains may relate to marine phenomena, such as current transport and upwelling; geographic boundaries, such as drainage area or ridge line; hydrologic phenomena, such as river transport; atmospheric fallout; animal behaviors, such as migration and breeding patterns; and regions for human expectations, such as the demand for products, housing, or other goods from the coastal area.
Sources
Known or presumed sources of contaminants must also be taken into account in defining environmental domains. Where are the outfalls and CSOs? From which portions of the watershed are nutrients being discharged? What are the significant diffuse or nonpoint sources of contaminants and nutrients? Are there septic tanks or other sources of pathogens? Are there aerial inputs of nutrients or contaminants of concern and, if so, from where? Changes in human activities may alter the contributions of various sources over time. Research and monitoring can improve understanding of the relative importance and regions of impact for various sources.
Administrative Authorities
While the inclusion of all important environmental processes and sources
of stress to the coastal resources of concern inevitably will lead to the definition of large areas of geographic extent for certain problems, the need to define areas over which management strategies can be effectively coordinated and implemented may require that areas be narrowed somewhat. In the initial analysis, however, these areas should be defined as large as necessary to include the important processes and sources of concern. Later, based on an understanding of these functions, areas of geographic extent can be narrowed in a well-informed manner.
Assess and Compare Risks
A central principle of ICM is that the setting of priorities for action and allocation of effort toward addressing problems should be guided by an understanding of the relative magnitudes of risks to ecologic and human health. Thus, the third major step in the dynamic planning process is to assess and compare risks.
Assessing Risk
Risk assessment is a tool to distill large amounts of scientific and technical information into a form that indicates where the greatest threats to human and ecosystem health are likely to occur. It is an analytic tool that can be used to estimate potential adverse impacts of urban wastewater and stormwater on the various organisms, populations, communities, and ecosystems inhabiting coastal waters, as well as on the various uses we make of the coastal environment. Risk assessments have been used extensively to determine human cancer risk (NRC 1983). More recently, risk assessments have been used to address other human health outcomes such as reproductive toxicity and developmental impairment. Of late, the risk assessment paradigm has been extended beyond human health to broader environmental and ecosystem impacts (EPA 1990, NRC 1993). The results of such assessments can inform risk managers of the probability and extent of environmental impacts resulting from exposure to different levels of stress. This process allows the maximum amount of available scientific information to be used in the decision-making process.
The risk assessment process consists of four steps: hazard identification, exposure assessment, dose-response assessment, and risk characterization. Hazard identification involves defining the inherent ability of some stress to cause harm. Exposure assessment involves quantifying the likely dose of the agent that may be expected to reach the target organs or the magnitude of the stress on the system (e.g., a sediment or water column concentration). The dose-response assessment involves estimating the adverse effect or response due to an exposure. The next step, risk character-
ization, involves the calculation or estimation of potential impacts based on hazard and exposure, i.e., risk is a function of exposure times hazard,
Risk = f [(exposure)(hazard)]
The process of determining risk to the environment from anthropogenic stresses involves a greater multiplicity of effects or endpoints, more complexity, and often more uncertainty than assessing human health risk. Also, ecological risk assessments involve various levels of biological organization and there is great regional variability among populations, communities, and ecosystems. For these and other reasons, a universally accepted methodology for ecological risk assessments has not been constructed yet.
Identify Hazards to Ecosystems and Human Health. The identification of hazards to ecosystems and human health should, in effect, take place within the goal-setting and domain definition processes. It is the identification of issues of concern and affected resources that point to the hazards of concern in the region.
Screen for Priority Issues. At this point in the process, the number of hazards identified may be too large to manage effectively. If so, two techniques may be used to narrow down the list of identified issues to one that contains the most significant hazards. It may be possible to screen the issues based on what is already known about their relative importance in the region. Some issues may be agreed upon as being less important than others. Initial efforts could then be focused on the ones of greatest concern with the understanding that those of less concern will be addressed at a later date.
A review of the issues may reveal that many of them have a common root cause. For example, regional-scale eutrophication, seagrass dieback, and nuisance algal blooms all result from excess nutrient enrichment. Thus, it may be appropriate to group these issues together in conducting a risk analysis on nutrient loadings.
Determine Dose-Response Relationships. The dose-response relationship is the one relation between the dose of an agent administered or received and the incidence of an adverse effect in the exposed population (NRC 1983). This step is perhaps one of the most important in the dynamic planning process because the results produced are useful in many ways. For example, once the dose-response relationship is determined, it is possible to establish exposure levels which will produce a particular level of response. This approach was taken in the setting of a goal of 40 percent reduction of nutrient loadings to the Chesapeake Bay (see Box 4.1). A general approach for assessing the dose-response relationship for nutrients and eutrophication is presented in Appendix A.
|
Box 4.1 SETTING GOALS AND DEFINING DOMAINS FOR NUTRIENT CONTROL IN THE CHESAPEAKE BAY The Chesapeake Bay Program provides an example of dynamic planning at the regional level that addresses problems occurring across multiple jurisdictions. With specific regard to nutrients, the program has now gone through three iterations of the goal-setting process. The Chesapeake Bay Program is the cooperative effort of the District of Columbia, Virginia, Pennsylvania, Maryland, the Chesapeake Bay Commission, the U.S. Environmental Protection Agency (EPA), and other federal agencies to restore the Chesapeake Bay. The original Chesapeake Bay Program, begun in 1978, targeted three specific issues of concern: nutrient enrichment, toxic substances, and the decline in submerged aquatic vegetation. These issues were identified as the major concerns facing the region based on existing scientific information. In 1983, with the signing of the Chesapeake Bay Agreement, participants agreed to a major action program addressing a wide range of issues, including nutrient reduction. While many specific actions were undertaken, no overall goal for nutrient reduction was established at that time. From 1983 to 1987, program participants developed a state-of-the-art three-dimensional hydrodynamic water quality model of the watershed and conducted research to develop a better understanding of nutrient sources and their impact on the bay. As discussed further in the Assessing Risks section of this chapter and in Appendix A, nutrient enrichment can cause anoxia and hypoxia, dieback of seagrasses, and nuisance algal blooms. While the bay program was not following a formalized framework for integrated coastal management, the approach taken in regard to nutrients clearly illustrates the application of the ICM concepts presented in this report. From the mid-1980s on, the program has evolved to embody important elements of ICM, including reevaluation and feedback. The Chesapeake Bay is the largest estuary in the contiguous United States. Nutrients enter the bay from both point and nonpoint sources throughout the watershed. Point sources include municipal and industrial wastewater discharges. Nonpoint sources include runoff from cropland and farm wastes, urban and suburban runoff, ground water discharges, and atmospheric deposition. Because the sources of nutrients to the bay occur throughout the watershed, the Chesapeake Bay Program defined its domain of analysis as the watershed that is shown in Figure 4.1. This domain includes the entire drainage area of the bay, which extends beyond the jurisdictional domains of the program participants into the states of West Virginia, New York, and Delaware. Thus, although those states chose not to be involved in the program, the analysis was designed to develop an understanding of nutrient inputs that derive from those states as well. The Chesapeake Bay Model is a computer simulation of processes in the watershed and the bay itself. This model was developed and then used to determine the level of nutrient loadings at which deleterious oxygen depletion in the mainstem of the bay would be stopped. Using loading estimates for 1985 as the base year, it was predicted that a 40 percent reduction in nutrient loadings would mitigate the hypoxia and anoxia in the mainstem sufficiently to encourage recovery of the bay's living resources. It is important to note, how- |
Using existing levels of exposure, such as concentrations of a constituent of concern in the water column, sediments, or shellfish, one can determine the likelihood that an adverse effect will occur.
Characterize Exposure. Exposure characterization is the step in which the degree to which the critical elements of the ecosystem or humans are exposed to various sources of concern is determined. Exposure characterization can be very complex in the context of the coastal zone. The key factor to take into account when characterizing sources and exposure in the coastal zone is that environmental concentrations of a constituent of concern will vary considerably depending on where the source enters the system and how many different sources a particular constituent is associated with. For example, seepage from septic systems adjacent to a shallow and enclosed bay is likely to result in locally increased concentration of nutrients and, if sited inappropriately, pathogens. If the bay also receives stormwater runoff that contains significant concentrations of these contaminants, the problem would be compounded. It may also be difficult to determine the relative contributions of the two sources.
Characterizing exposures to humans can also be confounding because of the multitude of behavioral factors associated with human exposures. These are discussed further in the section below on human health risks.
Assessing Human Health Risks. The World Health Organization states that "health is a state of complete physical, mental and social well-being and is not merely the absence of disease or infirmity" (WHO 1948). Rene Dubos defined health as "expressions of the success or failure experienced by the organism in its efforts to respond adaptively to environmental challenges" (Dubos 1965). In the coastal urban environment, human health issues of concern include not only acute and chronic toxicity but also other contributors to human well-being, such as nutritional value of fish and shellfish stocks, recreational opportunities, and contributions of the coastal ecosystem to mental well-being. As an example of the latter type of effect, algal blooms or fish kills that diminish the recreational opportunities in the coastal area would create stress as well as economic consequences for those whose livelihood depends on recreation. While recognizing the full breadth of human health affected by damage to the coastal environment, the approach used here will focus on assessing risks for acute and chronic illnesses caused by exposure to hazardous chemicals and microbiological stressors. Within integrated coastal management, other stressors will be considered as part of other human expectations (such as economic value of a recreational resource) even though there may be direct or indirect health consequences.
Adverse human health effects can range from minor to severe to fatal, and are usually classified as either acute or chronic. Acute effects or illnesses occur with short-term exposures, are of short latency, and usually
recovery occurs. Examples are acute toxicity from exposure to a toxicant and acute gastroenteritis. Chronic effects or diseases usually result from long-term or repeated exposures, may have longer latency periods, and have longer duration. Examples include cancer, neurotoxicity, and infections associated with chronic diseases such as hepatitis A and liver disease, and coxsackie viruses and diabetes. Developmental effects and reproductive toxicity, while conditions of a long-term nature, may result from short-term exposure to harmful agents.
Two principles guide the evaluation of human stressors: 1) the dose makes the poison and 2) there is specificity between agent and effects. Other issues that must be considered are latency (time between exposure and effect); possibility of secondary spread (i.e., from person to person); and the possibility of additivity, synergism, or antagonism between multiple exposures. All of these factors are used in developing risk assessment models to extrapolate from high to low doses in humans and to extrapolate between animal species.
In coastal urban wastewater and urban runoff, the two major classes of contaminants that are of potential concern to human health are hazardous chemicals and infectious agents. These include metals and organic chemicals that may pose varying risks depending on the method of disposal and ultimate environmental fate (i.e., disposal in the ocean, land disposal, or incineration). The toxicity of metals may vary by route of exposure, and by physical and chemical form such as valence state, whether in organic or inorganic state, whether sorbed or dissolved, and whether hydrated or complexed. For example, inorganic, but not organic, arsenic is a carcinogen (Gibb and Chen 1989). Cadmium is considered to be carcinogenic by the inhalation but not the oral route (Life Systems, Inc. 1989; IRIS 1993). Hazardous organic chemicals have entered coastal waters from a number of sources, most of which are due to industrial and agricultural activities. Many of these, like DDT and PCBs, have since been banned but continue to be present in sediments and the tissues of aquatic organisms and water fowl.
Infectious agents of concern include bacteria (e.g., campylobacter, salmonellae, v. cholerae), viruses (e.g., poliovirus, coxsackie, echovirus, adenovirus, and hepatitis A), and parasites (e.g., cryptosporidium, giardia, and entamoeba). Further information on infectious agents is contained in Appendix B. Exposure takes place while swimming in contaminated waters or eating contaminated shellfish. Several diseases can result. These range from subclinical infection to acute, self-limited respiratory, gastrointestinal, skin, or ear infections to extreme gastrointestinal and liver disease (e.g., cholera and viral hepatitis) and other potentially terminal diseases.
Most of these pathogens are derived from human feces; their presence in the environment is often associated with a source of domestic wastewater (e.g., septic tanks, combined sewer overflows, or sewage treatment plant
discharges with an inadequate reduction of pathogen levels before disposal). For illness to occur, there must be ingestion, aspiration, or inhalation of a sufficient number of viable organisms. There are, as well, some zoonotic (animal derived) pathogens that may be present in urban runoff water including the protozoa giardia and cryptosporidium and the bacteria salmonellae and campylobacter. It is unknown to what extent these zoonotic organisms pose a threat in coastal areas.
There are also poisonings from shellfish toxins elaborated by microorganisms. Such poisonings include neurotoxic shellfish poisoning, paralytic shellfish poisoning, ciguatera poisoning, scromboid poisoning, and domoic acid poisoning. It is unknown to what extent wastewater disposal impacts shellfish poisoning problems.
Risk assessors must not only look at risks to the general population ("average" exposures) but also at special populations that may be at greatest risk. For exposures in the coastal ecosystem, there are important developmental, immunological, and behavioral differences between individuals that can affect exposure and risk. For example, infants and toddlers who play in the ocean are more likely to swallow or aspirate seawater contaminated with pathogens when their heads are submerged than are adults. Methyl mercury in fish is most toxic to humans during gestation because of greater vulnerability of the developing brain. Newborn infants and the elderly are more susceptible to infectious diseases because of being relatively immunodeficient.
Human behavior is tied to the expectations people hold for the coastal environment. Behaviors that may lead to greater risk of exposure include swimming, scuba diving, consuming fish caught from local piers, eating raw fish and shellfish, and eating organ meats of fish and shellfish (such as the crab hepatopancreas, which is an ingredient in some Chinese recipes). These factors should be considered in the construction of exposure scenarios for human health risk assessments.
Recently, methods have been developed to assess risks from exposure to infectious agents in seawater and in shellfish (Cabelli et al. 1983, Fleisher 1991, Rose and Gerba 1991). These studies use human epidemiological data and water quality or shellfish monitoring for either indicators of infectious agents or for the agents themselves in order to establish the relationship between disease and water contamination. However, the limitations of epidemiological studies and monitoring for indicators are a source of some uncertainty in these risk assessments. Extrapolation from animal studies to humans has not been used for infectious agents.
Excess lifetime risks of disease incidence can be identified via clinical observation alone for very large risks (between 1/10 and 1/100). Most epidemiological studies can detect risks down to 1/1,000 and very large studies can examine risks in the 1/10,000 range. Risks lower than 1/10,000
cannot be studied with much certainty using epidemiological tools. For many diseases such as cancer, risk assessors often must extrapolate from experimental animal studies to humans because of inherent limitations in studying rare endpoints in human epidemiological studies. For example, Figure 4.2 shows the relationship between event levels of risk and the ability to identify cancer risk in the human population. Since regulatory policy generally strives to limit risks below 1/100,000 for life-threatening diseases like cancer, these lower risks are estimated by making inferences about the shape of the dose-response curve and extrapolations from effects to humans at higher doses or from animal testing. Imperfect though this system is, it has the advantage of incorporating all of the available information and creating usable estimates of risk that can be helpful for decision making.
Assessing Ecological Risks. For problems related to wastewater inputs to the coastal environment, ecological risk assessment can best be broken into two parts: effects due to excessive nutrient inputs and effects from toxic substances. The effects of nutrient inputs to coastal ecosystems have received a tremendous amount of scientific study over the past two decades. The role of nutrients in coastal waters and the risks associated with excess
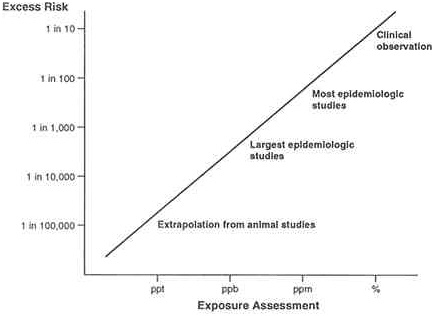
FIGURE 4.2 Sensitivity of epidemiology in detecting risks of regulatory concern.
inputs are well known and described in Appendix A. In moderation, nutrient inputs to estuaries and coastal seas can be considered beneficial. They result in increased production of phytoplankton (the microscopic algae floating in water), which in turn can lead to increased production of fish and shellfish. However, excess nutrients can be highly damaging, leading to effects such as anoxia and hypoxia from eutrophication, nuisance algal blooms, dieback of seagrasses and corals, and reduced populations of fish and shellfish. Eutrophication also may change the plankton-based food web from one based on diatoms toward one based on flagellates or other phytoplankton which are less desirable as food to organisms at higher trophic levels (Doering et al. 1989). Coastal waters receive large inputs of nutrients from both point and nonpoint sources. This is particularly true for estuaries, many of which receive nutrient inputs at rates up to 10,000 times higher per unit area than heavily fertilized agricultural fields (Nixon et al. 1986). As a result of these inputs, many estuaries and coastal seas throughout the world are increasingly experiencing such problems as anoxia and nuisance algal blooms (see Appendix A).
The degree of risk posed by nutrients varies among regions and among different types of ecosystems. In general, the more enclosed the water body and the less water available for dilution, the greater the threat. However, even large areas on the continental shelf can sometimes become anoxic from excess algal production as was demonstrated in the New York Bight in 1976 (Mearns and Word 1982). The degree of density stratification and mixing in the water body are also critical in assessing its sensitivity to low-oxygen events. The degree of risk from nutrients is further affected by the dominant organisms present; for instance, coral reefs and sea grass beds are particularly sensitive to nutrient inputs.
In most estuaries and coastal seas of the temperate zone, nitrogen is the primary element of concern which controls eutrophication. This phenomenon is in sharp contrast to eutrophication in lakes where phosphorus is often the limiting element. Phosphorus is also limiting in tropical lagoons and may be limiting in some temperate estuaries during at least some times during the year. Exactly which element is more critical is a result of differences in the ratio of nitrogen to phosphorus in external inputs, to differences in rates and controls of nitrogen fixation, and to differences in recycling of elements from bottom sediments (Howarth 1988). The relative abundances of other elements can also be important in controlling eutrophication. For instance, some evidence suggests that toxic algal blooms frequently become prevalent only after the ratio of silicon to nitrogen or phosphorus becomes low, that is when silicon is in relatively short supply (Smayda 1989, Officer and Ryther 1980). Silicon is required by diatoms but not by other phytoplankton species; thus as long as silicon is available, diatoms can outcompete other species are suppress blooms of toxic algae. High
levels of iron may also be involved in the formation of toxic algal blooms (Graneli et al. 1986; Cosper et al. 1990). These issues are discussed in detail in Appendix A.
The effects of toxic substances on ecological systems have proven more difficult to study than the effects of nutrients or than human health effects. In general, the science of ecological risk assessment for toxic substances is not as well developed as that for human health risk assessment. The two techniques, however, involve the same fundamental principles. In the case of ecological risk assessment, the causative agent is generally referred to as a stressor, and adverse effects are identified as stresses on an ecosystem. Sometimes, ecological risk assessment can be easier than human health risk assessment. For example, controlled experiments can be performed directly on the systems of concern eliminating the need for extrapolation from high doses to low doses or sensitive subpopulations. These advantages are, for the most part, however, outweighed by the greater complexity in organization and response of ecosystems to stresses.
Several indicators of the health of an ecosystem can be used to assess the hazard of a particular stress. These indicators include rates of primary production or other processes; trophic structure; survival of sensitive species; species diversity; and population of fisheries and shellfish stocks, as well as endangered species of birds and mammals. Specific measures include population counts, growth, survival, reproduction, and recruitment.
Responses to toxic chemical stresses can take place at four levels of biological organization: 1) biochemical and cellular, 2) organismal, 3) population, and 4) community and ecosystem. Within each of these levels, there are multiple potential endpoints that could be considered. Not all responses are disruptive in nature, and they do not necessarily result in degeneration at the next level of organization. Only when the compensatory or adaptive mechanisms at one level begin to fail do deleterious effects become apparent at the next level (Capuzzo 1981). However, failures at various levels are often exceedingly difficult to discern, and so changes in populations or in ecosystems may occur without any change at the organismal level ever being detected. In general, ecological risk assessments should be performed using the most sensitive measure of stress. For aquatic ecosystems, change in community structure is an important ecological concern and appears to be sensitive to toxic chemical response (Schindler 1987, Howarth 1991). A variety of toxic agents predictably cause changes, with loss of sensitive species (e.g., amphipods) which will result in domination by weedy or opportunistic species (e.g., capitellid worms). Species diversity usually decreases although this is generally a very insensitive measure of change compared to loss of sensitive species (Howarth 1991). Table 4.1 shows the response levels of marine organisms to chemical contaminants at the four levels of ecological organization.
TABLE 4.1 Responses of Marine Organisms to Chemical Contaminants at the Four Levels of Biological Organization (Adapted from Capuzzo 1981. Reprinted, by permission, from Oceanus, 1981, Volume 24:1.)
|
Level |
Types of Responses |
Effects at Next Level |
|
Biochemical-Cellular |
Toxication Metabolic impairment Cellular damage |
Toxic metabolites Disruption in energetics and cellular processes |
|
|
Detoxication |
Adaptation |
|
Organismal |
Physiological changes Behavior changes Susceptibility to disease Reproductive effort Larval viability |
Reduction in population performance |
|
|
Adjustment in rate functions Immune responses |
Regulation and adaptation of populations |
|
Population |
Age/Size structure Recruitment Mortality Biomass Adjustment of reproductive output and other demographic characteristics |
Effects on species productivity and coexisting species and community |
|
|
|
Adaptation of population |
|
Community and Ecosystem |
Species abundance Species distribution Biomass Trophic interactions |
Replacement by more adaptive competitors Reduction of secondary production |
|
|
Ecosystem adaptation |
No change in structure and function |
Ecosystems vary in their sensitivity to stress by type and region, and therefore ecological health risk assessments should be specific to the setting of concern. Sensitivity is determined by both physical and ecological parameters. Due to dilution, coastal systems that are more open to hydrologic flows (e.g., most of the Pacific coast with the exception of enclosed bays) may be less sensitive to effects of toxic substances although, of course, many toxic substances can be bioaccumulated. Regional differences in
community or ecosystem structure will also make a difference. For example, marine ecosystems already subject to natural stresses such as large river plumes may be more resistant to the effects of toxic chemical stress. Areas receiving only small nutrient and sediment inputs and having few major storms that affect the bottom are the most sensitive to the effects of toxic chemical stress (Howarth 1991). Some types of ecosystems, such as coral reefs, are also notoriously sensitive to the effects of toxic substances (Jackson et al. 1989).
The types of stress exhibited in ecosystems are not easily recognizable as resulting from one specific stressor or another. Ecological effects such as population shifts or declines can result from a variety of stressors acting independently or synergistically. It can be difficult to tease apart the effects of a variety of co-occurring stressors. Often concentrations of toxic substances and nutrients tend to covary. Areas subjected to overharvesting of resources and habitat alteration also tend to be those receiving excess nutrients and toxic substances.
The assessment of ecological systems must take into account the spatial and temporal scales at which the effect of concern occurs. The spatial scale of a given effect should correlate with the geographic extent defined in the second step of the dynamic planning process. The temporal scale includes the expected timing and duration of a particular stress, such as pulse loadings from a stormwater discharge, as well as the time required for an ecosystem to recover once the stress has been removed.
The ecological risk assessment process is guided by many questions: What level of biological organization and which potential endpoints should be considered? One of the difficulties in determining risk at the population, community, or ecosystem level is that the myriad of physical, chemical, and biological interactions among individuals and populations is not known or well understood. Since the number of interactions increases with the complexity of the biological system, the uncertainties in risk characterizations may increase accordingly. The basic elements for consideration in ecological health risk assessment are shown in Table 4.2.
The fact that ecological risk characterizations are difficult and the results relatively uncertain at higher levels of biological organization does not imply that they cannot be conducted. For instance, both commercial fisheries quotas and migratory waterfowl hunting bag limits are the results of ecological risk assessments that determined the numbers of individuals that could be lost (caught or killed) without having an unacceptable effect at the population or higher level. For many such assessments, a long history of trial and error and professional judgements are important factors in limiting uncertainty. Applying pesticides that are awaiting registration to microcosms, mesocosms, and field plots helps determine risks at the community level and higher. In these experiments, effects of the chemical on various
TABLE 4.2 Basic Elements for Consideration in Ecological Health Risk Assessment (Source: EPA 1991)
|
1. STRESS - The type, properties, temporal and spatial patterns, and interactions of the stresses are of fundamental importance in defining the temporal and spatial dimensions and the potential types of ecological effects. 2. ECOLOGICAL ORGANIZATION - Ecological organization represents the level of biological complexity (for both ecological endpoints and indicators) at which the ecological risk assessment is conducted. In theory, the scale of ecological organization chosen for the ecological risk assessment is dependent upon both the spatial and temporal scales of the stress and the co-occurring ecosystem component affected by the stress. 3. ECOSYSTEM TYPE - Ecological assessments are currently ecosystem specific, that is, assessments describe the risk of ecological effects for aquatic, terrestrial, or wetlands categories of ecosystems and/or their respective sub categories. 4. SPATIAL SCALE - Spatial scale delineates the area over which the stress is operative and within which the ecological effects may occur. Indirect ecological effects may greatly expand the spatial scale required for the assessment. 5. TEMPORAL SCALE - Temporal scale defines the expected duration for the stress, the time scale for expression of direct and indirect ecological effects, and the time for the ecosystem to recover once the stress is removed. |
interactions between and among individuals and populations may be observed without having a complete knowledge of the interactions beforehand.
The EPA uses a relatively simple method for assessing risk to aquatic organisms from single chemicals. It is called the Quotient Method, whereby the concentration of a chemical in a water body is compared to a previously determined safe or acceptable concentration for that substance, e.g., a water quality criterion or a water quality standard. If the quotient, Q,
for a particular situation approaches or is greater than one, that body of water is considered to be at risk from that chemical; the smaller the quotient the lower the risk. Even with the inherent problems of extrapolating toxicities from one species to another and using chronic to acute ratios to determine the acceptable concentrations, the Quotient Method has some merit because it is simple and easy to understand (Table 4.3). It is not, however, capable of determining risk from nonchemical stresses. Also, it is not often easy to decide what is an ''acceptable concentration." The Water Quality Criteria are based on a variety of different types of studies (Table 4.3), but virtually all of these are laboratory based and generally of short duration. Studies of effects of toxic substances in natural ecosystems over longer periods of time
have frequently found major effects that were not predicted by such short-term laboratory studies (Schindler 1987, Jackson et al. 1989, Howarth 1991).
Many of the chemicals in urban wastewater that have the potential to adversely affect aquatic organisms are hydrophobic and sorb, or attach themselves, to sediments. The resulting contaminated sediments can affect organisms living in direct contact with the solids as well as those residing in the overlying waters since the sediments themselves can act as a source of the toxic substances. The Quotient Method, as now used, does not assess hazardous substances in sediments directly. This limitation and the fact that there are numerous coastal areas that have contaminated sediments (NRC 1989b) have led to the development of methods to establish acceptable chemical levels in bottom materials. These methods, sometimes called Sediment Quality Criteria or Sediment Quality Values, can use models to predict whether a given mass loading of chemicals from an effluent will likely result in toxic sediments. These models also can be used to gauge the existing or potential adverse biological impacts of existing contaminated sediments.
TABLE 4.3 Water Quality Assessment Methods (Adapted from Rand and Petrocelli 1985)
|
Method |
Concept |
|
Median Lethal Concentration (LC50) |
The concentration of a substance in water that results in death of 50 percent of the test organisms when exposed for a specified time, e.g., 48 hours. |
|
Median Effective Concentration (EC50) |
The concentration of a substance in water that results in some sublethal effect on 50 percent of the test organisms when exposed for a specified time. |
|
No Observed Effect Level (NOEL) |
The highest concentration of a substance which results in no adverse effect on the exposed test organisms relative to controls. |
|
Water Quality Criteria |
The concentrations of a substance in water that correspond to various effects levels. |
|
Water Quality Standard |
The concentration of a substance or the degree or intensity of some potentially adverse condition that is permitted in a water body. An effluent standard refers to a concentration or intensity of impact permitted in an effluent. |
The EPA has compiled ten methods that have the potential to assess sediment quality relative to chemical contaminants (EPA 1989). Some of the methods involve chemical analyses that allow for the establishment of chemical specific criteria (e.g., an acceptable level of phenanthrene in sediments). These methods should allow risk to be assessed using the Quotient Method with sediment concentrations rather than water concentrations. Other methods involve only biological observations that limit the results to assessment of whether a sediment is toxic. Still others combine chemical and biological measurements. Brief descriptions of the ten methods are given in Table 4.4.
Aesthetics. Adverse aesthetic impacts include unpleasant sights, noxious odors, and unpleasant tactile sensations (such as from contact with the algae Pillayella litoralis). Adverse aesthetic impacts discourage recreational uses and thus can have significant economic impacts. Multiple sources of materials, including combined sewer overflows (CSOs) and urban runoff, as well as commercial ships, recreational boaters, and beachgoers can cause aesthetic offense. Aesthetic impacts can be quantified, albeit through indirect methods. For example, many jurisdictions survey beaches for plastics and other floatable solids and report numbers of objectionable items per unit length per time period.
The range and volume of plastic wastes that end up in the world's oceans are enormous. Typical are a variety of bottles, ropes, and fishnets. There are no reliable estimates of the total volume of such wastes nor the contribution from urban areas to the marine environment. Beach surveys finding condoms and plastic tampon inserters do not identify reliably the source of debris as wastewater, stormwater, recreational boaters, or beachgoers. Plastic debris is not only of aesthetic concern but also can carry pathogens, be mistaken for food and harm marine animals that ingest it, or entangle organisms and strangle them. The Center for Marine Conservation's Coastal Cleanup program cleared 4,347 miles of beaches and waterways of almost 3 million pounds of trash in 1991. Approximately 60 percent of the debris was plastic (Younger and Hodge 1992).
Some of the materials that cause the most aesthetic offense in the coastal marine environment are those that both mobilize public concern and cause significant environmental threats. Floatables, oil and grease, and materials that wash up on shorelines are visible signs of patterns of waste disposal and general human conduct that may also have other impacts on coastal water. As such, they are powerful symbols of more widespread problems. Garbage and syringes washing up on the New Jersey beach in the summer of 1987 did more to alarm the public than did numerous scientific studies. Similarly, when a lawyer jogging along Quincy Bay realized he was treading in human feces, the lawsuit that led to the beginning of the effort to
TABLE 4.4 Sediment Quality Assessment Methods (Source: EPA 1989)
|
Method |
Concept |
|
Bulk Sediment Toxicity |
Test organisms are exposed to sediment which may contain unknown quantities of potentially toxic chemicals. At the end of a specified time period, the response of the test organism is examined in relation to a specified biological endpoint. |
|
Spiked-Sediment Toxicity |
Dose-response relationship are established by exposing test organisms to sediments that have been spiked with known amounts of chemicals or mixtures of chemicals. |
|
Interstitial Water Toxicity |
Toxicity of interstitial water is quantified and identification evaluation procedures are applied to identify and quantify chemical component responsible for sediment toxicity. The procedures are implemented in three phases to characterize interstitial water toxicity, identify the suspected toxicant, and confirm toxicant identification. |
|
Equilibrium Partitioning |
A sediment quality value for a given contaminant is determined by calculating the sediment concentration of the contaminant that would correspond to an interstitial water concentration equivalent to the U.S. EPA water quality criterion for the contaminant. |
|
Tissue Residue |
Safe sediment concentrations of specific chemicals are established by determining the sediment chemical concentration that will result in acceptable tissue residues. Methods to derive unacceptable tissue residues are based on chronic water quality criteria and bioconcentration factors, chronic dose-response experiments or field correlations, and human health risk level from the consumption of freshwater fish or seafood. |
|
Freshwater Benthic Community |
Environmental degradation is measured by evaluating alterations in freshwater benthic community structure. |
|
Marine Benthic Community Structure |
Environmental degradation is measured by evaluating alterations in marine benthic community structure. |
|
Sediment Quality Triad |
Sediment chemical contamination, sediment toxicity, and benthic infauna community structure are measured on the same sediment. Correspondence between sediment chemistry, toxicity, and biological effects is used to determine sediment concentrations that discriminate conditions of minimal, uncertain, and major biological effects. |
|
Method |
Concept |
|
Apparent Effects Threshold |
An AET is the sediment concentration of a contaminant above which statistically significant biological effect (e.g., amphipod mortality in bioassays, depressions in the abundance of benthic infauna) would always be expected. AET values are empirically derived from paired field data for sediment chemistry and a range of biological effects indicators. |
|
International Joint Commission |
Contaminated sediments are assessed in two stages 1) an initial assessment that is based on macrozoobenthic community structure and concentration of contaminants in sediments and biological tissues, and 2) a detailed assessment that is based on a phased sampling of the physical, chemical, and biological aspects of the sediment, including laboratory toxicity bioassays. |
clean up Boston Harbor was filed. In many places, the aesthetic quality of the shoreline has deteriorated, and most of the causes represent serious threats to the ecological integrity of the marine coastal environment.
Compare Risks
A comparison of risks can be helpful in guiding the setting of priorities. Those risks determined to be more important or most unacceptable can then be the focus of risk management options. A clearly articulated comparison or ranking can help to focus efforts on the more important issues.
A determination of highly accurate and precise risk estimates may not be possible but the available data may be adequate to allow the various risks to be compared and ranked on a relative basis as highest or lowest or high, medium, or low. Risk comparisons could be applied to answer questions such as, does biochemical oxygen demand (BOD) from urban stormwater pose as much risk to a receiving water body as nutrients from publicly owned treatment works (POTWs) and agricultural runoff? In this case, a knowledge of the relationship between nutrients and algal blooms can lead to an estimate of the oxygen consumed by respiration and by decomposition of the excess algae, which can then be compared with the relatively more certain estimate of the oxygen consumed by BOD. Another comparison might be, does coastal habitat alteration (e.g., increased sedimentation due to urbanization, wetlands filling, and shoreline alteration) pose as much risk as hazardous chemicals from wastewater?
The risk comparison process should be carried out in the context of the
goals established for the region and with the participation of the interested parties in the region. Since the comparison of risks is not a precise quantitative ranking exercise, it might be helpful to adopt a set of guidelines for comparing risks. These guidelines would help insure that each risk receives adequate scrutiny and that judgements about whether one is more important than another are consistent within a region and based in science. While comparison guidelines will likely vary from region to region due to differences in expectations among the residents of a region and due to differences in ecosystems and the types of problems faced, there are at least four generic criteria applicable to both human health and ecological risks that should be used in comparing risks (EPA 1990):
-
the area or numbers of individuals affected,
-
the level of biological organization affected and the importance within the area,
-
the temporal dimension of the effects and potential for recovery, and
-
the risk estimate itself.
As an example of how these criteria could be used for human health risks, data from Santa Monica Bay were used to compare the risk to humans from swimming with the risk from eating contaminated fish. Using the data, Table 4.5 was constructed. Both of these risks would merit public health concerns. The risk to swimmers is for a generally milder disease but
TABLE 4.5 Comparing Human Health Risks from Swimming Versus Eating Contaminated Fish from Santa Monica Bay
|
Activity |
Exposure |
Hazard |
Range of Risk Estimate |
Number Exposed |
|
Swimming |
Swallow 100 ml water |
Enteric virus infection |
1 x 10-2 to 2 x 10-4 |
44 x 106 person-days/yr |
|
Eating Fish: |
|
|
|
|
|
caught from boats |
23 g fish/day for 70 years |
Cancer1 |
3 x 10-4 to 3 x 10-6 |
320,000 persons/year2 |
|
caught from piers |
23 g fish/day for 70 years |
Cancer |
4 x 10-4 to 7 x 10-4 |
unknown |
|
1 Based on consumption of five different species of fish in a hypothetical "average creel" (Pollock et al. 1991). 2 Based on 80,000 anglers surveyed in 1987 by the California Department of Fish and Game. Fish caught were shared by an unknown number of persons, here assumed to be four per angler (MBC-AES 1988). |
||||
TABLE 4.6 Comparative Risk Criteria (Source: MBC-AES 1988)
|
Activity |
Area or Number Affected |
Level of Biological Organization |
Temporal Dimension |
Risk Estimate |
|
Swimming |
44 x 106 persondays/year1 |
Human |
Short (infection) |
1 x 10-2 to 2 x 10-4 |
|
Eating Fish |
320,000 persons/ year |
Human |
Long (cancer) |
10-4 to 10-5 |
|
1 For 1987. |
||||
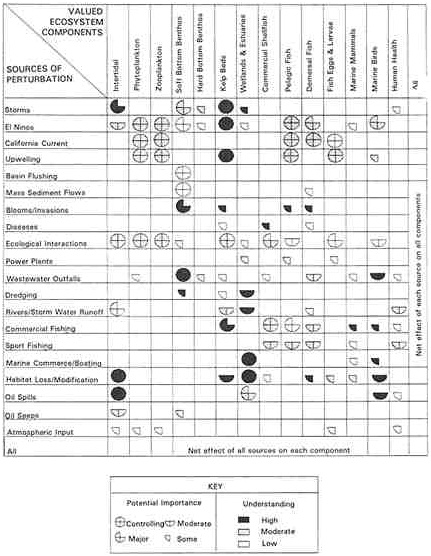
the probability of contracting the illness is high. Among the large number of persons using the beaches, it can be expected that many infants and elderly individuals would be likely to develop severe manifestations or sequelae. The cancer risk, on the other hand, is below the level of detection for the population at risk (approximately 96 excess cases are expected even assuming all 320,000 exposed persons in the Santa Monica region consume 4.6 grams per day for 70 years of Santa Monica Bay fish—an unlikely scenario). A comparison of the risks relative to the comparative risk criteria is given in Table 4.6. Although there are several important information gaps, it appears that in this analysis the swimming-associated risks would be of higher priority. The analysis not only provides relative risk information but also points to weaknesses in the database—the paucity of information about numbers of anglers and lack of data on microbial and chemical shellfish contamination. This information can be fed back to the planning of research and monitoring plans, with risks to be assessed as new data are collected. In fact, for Santa Monica Bay, a survey of anglers is under way to develop estimates on numbers who catch fish and the quantities caught to gain information about vulnerable populations at risk (e.g., infants, the elderly, the immunosuppressed). This information should help refine the comparative assessment. An ideal comparative risk assessment would include health, ecologic, and aesthetic impacts. Table 4.7 shows an array that was developed as part of the Santa Monica Bay Restoration Project. A description of the project and some of the issues to be addressed appears in the Santa Monica Bay case example beginning on page 114. Table 4.7 provides a semiquantitative assessment of the relative importance of a number of stressors for a range of ecosystem components. This model illustrates the numbers of stressors that may need to be considered for a comparative risk assessment and assists in screening for the stressors of concern, including urban wastewater. A more detailed analysis of individual risks would be needed for the next level of priority setting.
Risks, once assessed, must be communicated to all those who are con-
cerned about the process, especially members of the public and stakeholders. Risk communication in clear terms can help give a sense of the relative ranking of various risks.
Santa Monica Bay: A Case Example
Santa Monica Bay in Southern California is currently being evaluated for management options under authority of the Santa Monica Bay Restoration Project, an activity supported by EPA under the National Estuary Program. Santa Monica Bay is a 690 square kilometer indentation along the Southern California Bight. The project is an ambitious and difficult undertaking. It is bounded on the northwest by Point Dume and on the southeast by Point Fermin as shown in Figure 4.3. The bay extends from the shoreline of Los Angeles and the adjacent cities of Santa Monica, El Segundo, and Redondo Beach westward to depths greater than 500 meters. Offshore, the bay drops off into the 750-meter-deep Santa Monica Basin. Two princi-
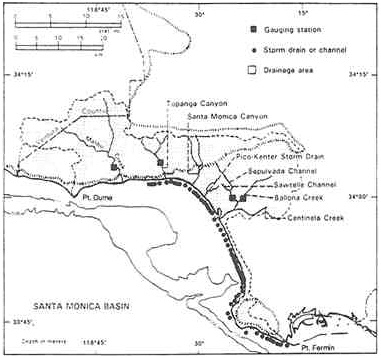
FIGURE 4.3 Natural drainage into Santa Monica Bay. Each dot represents one storm drain or channel (modified from Los Angeles County Department of Public Works maps). (Source: SMBRP 1993. Reprinted, by permission, from Santa Monica Bay Restoration Project, 1993.)
pal inlets of Santa Monica Bay are Marina del Rey, the largest marina in the nation, and the adjoining wetlands of Ballona Creek and King Harbor in Redondo Beach. The shoreline is entirely within the county of Los Angeles.
The Santa Monica Bay Restoration Project defines "the Bay" to include part of the San Pedro Bay shelf, namely the western end along the Palos Verdes Peninsula. Unlike the bay itself, the shelf along the Palos Verdes Peninsula is steep and narrow; within 5 kilometers of shore it plunges into the 850-meter-deep San Pedro Basin.
The present land drainage area of the bay is extremely narrow, extending no more than 24 kilometers inland. The actual drainage area is 850 square kilometers (328 square miles) and includes communities such as Malibu, Santa Monica, west Los Angeles, Beverly Hills, and part of Hollywood in the northeast corner of the drainage triangle; to Westchester, El Segundo, Manhattan Beach, Hermosa Beach, Redondo Beach, and part of Torrance along the central shoreline; and Palos Verdes Estates, Rancho Palos Verdes, and San Pedro on the southern shoreline.
The Bay's Ecosystem
Santa Monica Bay encompasses all the major habitats and ecosystems of the midlatitude Pacific coastline of the United States. The pelagic ecosystem is the largest and supports epipelagic and mesopelagic populations of fish, invertebrates, and algae. Inshore waters of the bay support one of the major areas for eggs and juveniles of keystone species such as northern anchovy and Pacific sardine. Inshore, shallow-water rocky outcrops support related species that compete for space with a large variety of green, red, and brown algae; seaweeds; and kelp. Sandy beaches support dense colonies of mole crabs and other small crustaceans, polychaetes, and clams while rocky intertidal areas are rich in additional seaweeds and attached and free-living invertebrates. Kelp forests are critical habitat for many species of fish, invertebrates, birds, and mammals and have been an economic (harvested) resource as well. The Ballona wetlands have been reduced by the construction of Marina del Rey from 8.5 square kilometers to 0.65 square kilometers. A smaller wetland occurs at the mouth of Malibu creek.
Major Uses of the Bay
The principal uses of Santa Monica Bay include recreation (swimming, boating, diving, fishing), sport and commercial fishing, aesthetic enjoyment, coastal developments, shipping, industrial cooling water, and waste disposal (domestic and industrial). The full market value of residential, commercial, and industrial development along the bay exceeds $30 billion.
The 22 beaches of Santa Monica Bay attract 44 million person days of
visitors each year, principally during holidays, weekends, hot weather periods, and the summer. Since 1985, however, attendance has dropped due, possibly, to increasing news coverage of environmental conditions (MBC-AES 1988). Surfing occurs primarily along the Malibu coast, where waves usually impact the coastline at a better angle than in the center of the bay. Diving is a popular year-round sport principally along the Palos Verdes and Malibu coasts. Boating is also a year-round activity. In 1986, tourism was estimated to contribute $232 million, more than 3,000 jobs, and $4.2 million in tax revenues to Santa Monica's economy (MBC-AES 1988).
A major bait purse seine fishery operates within the bay. Sport anglers contribute nearly $4 million to the local economy. In 1987, nearly 80,000 sport anglers took almost half a million fish, mostly Pacific bonito, chub mackerel, and barred sandbass.
The three major industrial and municipal disposal uses of the bay area are power generation, oil refining, and wastewater disposal. The Hyperion Treatment Plant of the City of Los Angeles discharges 370 million gallons per day (MGD) of treated wastewater 8.5 kilometers (5 miles) offshore and 60 meters deep, below the seasonal pycnocline. The Whites Point outfalls of the County Sanitation Districts of Los Angeles County discharge 360 MGD of treated wastewater 60 meters deep, 3 kilometers offshore, near the east end of the Palos Verdes Shelf. Other sources of contaminants to Santa Monica Bay include return cooling water from three power generating stations, one oil refinery, 68 storm drainage channels, over 7,200 private vessels at two marinas, 92 tanker off loadings per year, oil spills, 2,200 metric tons per year of litter, oil seeps, stormwater runoff, and aerial fallout from smog, brushfires, and other sources.
A special situation of considerable concern exists off Whites Point. During the 1950s and 1960s, the pesticide DDT was discharged to the ocean through the Los Angeles County outfalls. The emissions were discovered in 1969 and promptly controlled (Carry and Redner 1970). However, by that time, marine biota in the Los Angeles area and beyond was contaminated and hundreds of metric tons of the pesticide had accumulated in surface sediments. Since then, deposition from natural and sewage solids have buried this field under 20 to 25 centimeters of sediment. These contaminated sediments, however, apparently continue to be a chronic source of DDT to fish and wildlife in the bay (Mearns et al. 1991).
Identification of Issues of Concern
Issues of concern regarding the environmental quality of Santa Monica Bay deal with human health and marine resources as well as the scope and costs of additional pollution control, clean-up, and abatement activities.
During 1990 and 1991, the Santa Monica Bay Restoration Project con-
ducted workshops with the stakeholders and the public and identified five classes and numerous subclasses of issues or resource use conflicts. The five basic concerns identified were:
-
Swimming and water contact: Is it safe to swim in the bay?
-
Seafood contamination: Is it safe to eat seafood from the bay?
-
Wetlands: How can wetlands be restored and protected?
-
Marine ecosystems and habitats: Are marine ecosystems protected?
-
Fish and shellfish stocks: Are fisheries protected?
Swimming and Water Contact Issues
The area of concern for water contact issues is defined by where people come in contact with potentially contaminated water. This domain includes the entire bay shoreline out to where people can conceivably swim or dive. The State of California defines the offshore swimmable boundary as the 30-foot depth line and 1,000 feet from shore. The California Ocean Plan (CSWRCB 1990) requires that water quality within kelp beds, which are popular diving spots, must meet bathing water standards. However, boaters may come in contact with water not only within marinas, boat harbors, and near shore but also offshore, including over the deep ocean outfalls. Thus all offshore surface waters could be considered as possible routes of exposure and as issue boundaries.
The risk of swimming-associated acute gastroenteritis infection was estimated by the Southern California Association of Governments based on fiftieth and ninetieth percentile enterococcus levels applied to a model developed by the EPA, which assumes head immersion and 100 milliliters of water intake (MBC-AES 1988). For 17 stations between Topanga (near Malibu) and Malaga Cove (Palos Verdes), fiftieth percentile dry-weather acute gastroenteritis infection risk ranged from 0.2 per 1,000 persons (2 x 104) at Malaga Cove to 11.3 per 1,000 persons (1.1 x 10-2) at Pulga Canyon (north of Santa Monica) and Venice Beach; the highest risks (10-2) were along the Santa Monica coast between these two points. During wet weather, risk increased sharply at the southern sites but only slightly at the Santa Monica area sites. Ninetieth percentile risks were one to ten times higher than the fiftieth percentile risks for dry weather and about twice those for wet weather. No estimates were made for respiratory illness risks or for risks associated with stormwater flows during storm events.
Viruses have been detected in stormwater in other areas at levels between 2.6 and 106 plaque-forming units per liter (PFU/L) (O'Shea and Field 1992). Taking the average level (12 PFU/L) and assuming it is similar to that of Santa Monica stormwater, estimates of infection risks could range from 2.7 x 10-1 to 2 x 10-2 when exposure occurs shortly after a storm event.
All these estimates are in general agreement that there is a seasonally-variable 1/100 to 1/5,000 chance of experiencing illness while bathing along Santa Monica Bay beaches. The enterococcus based model suggests that the risk, at least during dry weather, is ten times lower at beaches along the southern and northernmost shores than in the vicinity of Santa Monica and Venice.
Seafood Contamination
Because they are immobile, contaminated harvestable molluscan shellfish, such as mussels, clams, and rock scallops, are limited in areal extent, on the order of several meters to several kilometers from wastewater inputs. Mussels at several locations in Santa Monica Bay have in the past contained concentrations of DDT, PCBs, chlordane, and several metals approaching levels of concern (Phillips 1988). These data, however, have not yet been used in risk assessments. There are no data to support an assessment of risks from pathogens and toxicants in shellfish.
In contrast, finfish and mobile crustaceans, such as crab, lobster, and shrimp, have been the focus of intensive seafood consumption and risk studies. Several fish species of concern have potentially large distribution ranges that encompass tens of square kilometers. The highest levels of DDT and PCB contamination have been found in white croaker, which is among the most frequently caught and consumed fish. The white croaker effectively defines a boundary of concern that extends out to about 100 meters deep and from San Pedro to the central-northern shore of Santa Monica Bay.
The California Environmental Protection Agency Office of Environmental Health Hazard Assessment conducted a comprehensive study of chemical contamination of fish that was weighted by frequency of catch for various species (Pollock et al. 1991). They identified several chemical contaminants of concern: DDT and related compounds, PCBs, chlordane, mercury, and tributyltin. In contaminated areas, the white croaker, a bottom-feeder, was the most contaminated fish but corbina, queen fish, surfperch species, and sculpin (a.k.a. scorpion fish) were also relatively contaminated. Bonita, mackerel, halibut, sand dab, barracuda, opaleye, and halfmoon had the lowest level of contaminants. Cancer risks from PCBs and DDT and related compounds were the most significant health risks and ranged from 10-3 to 10-6 for a lifetime exposure to a particular species, depending on the location and species. Several general guidelines were issued as a result of the study including: eat a variety of fish species, consume fish caught from several locations, and trim fat (which concentrates DDTs and PCBs) from fillets. A number of site-specific advisories were issued recommending that
anglers limit consumption or not eat certain fish species caught from specific locations.
Wetlands
Boundaries of wetlands are not limited to those in current existence but may expand to the natural historical boundaries, such as the wetland areas where Marina del Rey is now situated. The Santa Monica Bay Restoration Project also considers the potential for restoration or development of freshwater wetlands at historical sites, including within areas now occupied by storm drains (SMBRP 1992). Since wetlands contribute to the nourishment and reproduction of migratory birds, the boundary may reach as far as the extent of these migrations.
Ecological Health: Wetlands versus Marine Habitats versus Fisheries
How do we set priorities for marine ecosystems? One way is to evaluate current injury to each and then compare them on the basis of the fraction of habitat in need of restoration. Comparison of risks to various marine ecosystems might be made on several bases including total productivity of each, number of threatened or endangered species in each, or fraction of total habitat injured.
An attempt is made here to compare two habitats, the subtidal benthos and wetlands. About 11.4 square kilometers of the Santa Monica Bay sea floor are projected to incur changed benthic communities as a result of current inputs of suspended solids from sewage inputs. This area includes about 1.6 square kilometers, 8 kilometers offshore in the center of Santa Monica Bay shelf and 9.8 square kilometers located 3 kilometers offshore of the Whites Point area of the Palos Verdes shelf. Combined, this area affects about 1.7 percent of the 690-square-kilometer shelf of Santa Monica Bay. By contrast, there were historically about 9.2 square kilometers of wetland habitat at Ballona Creek and this has been highly modified by development (e.g., Marina del Rey) to less than 0.69 square kilometers, a reduction of over 92 percent. Therefore, on an areal basis, there is the possibility of comparing a 1.7 percent injury to the seafloor of the bay with a 92 percent injury to the major wetland of the bay (MBC-AES 1988).
In addition, there has been no documentation of a threatened or endangered marine invertebrate or fish in the bay, whereas the existing wetland is one of the few remaining habitats for the endangered Belding's Savannah Sparrow and possibly other terrestrial and shore species.
Develop and Compare Alternatives for Risk Management
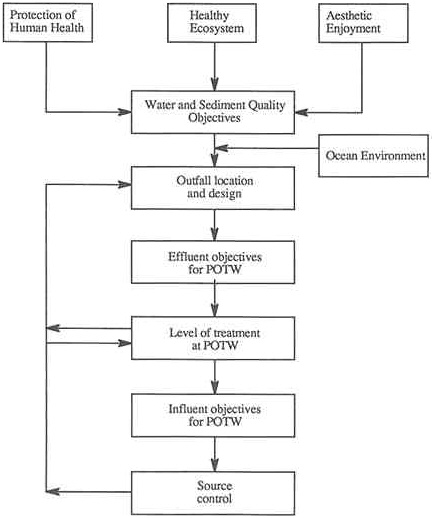
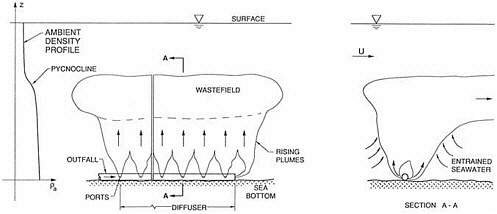
The next step in integrated coastal management is to develop management alternatives for reducing the priority risks to coastal water quality, i.e., solving the problems. Most often the best solutions will involve a combination of actions, including engineering works (e.g., treatment plants and outfalls), source control for pollutants entering POTWs, or reduction of inputs from diffuse sources. This step involves engineering design of systems using the environmental-quality driven approach for both structural and nonstructural measures. To illustrate the engineering process this section focuses at first on a single major discharger of wastewater effluent from a POTW; then later in the section the discussion extends to other situations involving multiple point sources and diffuse sources. However, the same general concepts apply—i.e., working back from water and sediment quality objectives to find the optimum set of control measures needed.
The Need for Problem-Focused Management
Problems must be defined carefully and risks assessed as described in the preceding section. It is not enough to say ''Clean up Boston Harbor." Rather, the dynamic planning process must be specific about exactly what pollution problems exist, what their severity and distribution are, and what the associated risks posed to ecosystems and human populations are. Close examination of all sources of a problem for coastal water bodies is required because one source may represent only a minor fraction of the problem and its tight control would be useless without a holistic approach that would address the bulk of the problem.
The purpose of this section is to explain the process of managing risks and developing alternative management strategies. Often, but not always, these risks can be managed by meeting numerical standards, such as water or sediment quality standards, concentration limits of pathogens in shellfish, or some other environmentally based standard.
After the precise nature of the problems and the sources that contribute to them are understood, the range of technical management options for their solution can be defined. Table 4.8 on pages 122-125 provides a list of possible problems that could be associated with a single major wastewater discharge and indicates what remedial actions may be taken for each. It illustrates in a descriptive way that different problems require different responses in order for pollution control efforts to be effective. There is no single approach to solve all problems even when they all are due to a single wastewater discharge. A similar table should be developed showing possible control measures and their effectiveness for all sources that the risk assessment process has shown to be of concern for a specific coastal man-
agement region. It is important to understand that it is easier to develop explicit control strategies as implied by Table 4.8 for sources such as outfalls from sewage treatment plants than for diffuse sources such as rivers, storm runoff channels, or atmospheric inputs.
Lessons from Existing Situations
In developing pollution control systems, much can be learned by studying the existing coastal pollution situation through detailed field observations and computer modeling. This effort will reveal the relative importance of different sources and information about the processes that are currently important in the coastal environment. For example, continuing with the hypothetical example discussed in Table 4.8, if the discharge point of an outfall for a POTW is moved to a new site much further offshore or the treatment is upgraded, then predictions can be made of the new ambient water quality to be achieved by modifying this one source while leaving unchanged all the other sources, such as CSOs, urban runoff, river discharges, atmospheric deposition, and other diffuse sources. Furthermore, when making water quality predictions, engineers usually have some past experience with discharges in the same region to provide data for model calibration and general guidance.
Ambient Water-Quality Objectives
The risk to human health and ecosystems and to our aesthetic appreciation of coastal water is linked directly to ambient water quality, which is often defined by measurable parameters such as concentrations of suspended solids, toxics, nutrients, bacteria and viruses, oil and grease, and dissolved oxygen. For instance, the risk management of pathogens has been systematized by setting standards for surrogates such as coliform bacteria or enterococci bacteria which can be monitored easily. Each individual discharger does not do a risk assessment for pathogens but uses a surrogate of fecal contamination. The deficiency in this approach is the inadequacy of the surrogate for predicting the presence (or absence) of various pathogens and not in the approach itself. (A more detailed discussion of this practice appears in Chapter 2 and Appendix B). Thus, bathing water standards can be set at various levels of bacteria or other appropriate organisms. Such limits constitute an explicit water quality standard used as an objective for engineers who design systems. Similarly, standards (or objectives) may be established to manage many risks. Setting objectives and standards is an important intermediate step for the design of management strategies and engineering systems.
Setting acceptable water-quality or sediment-quality standards for some
TABLE 4.8 Options for Improving Coastal Waters Affected by a Wastewater Treatment Plant Discharge (Brooks 1988)
|
Assumed present system: primary treatment, short outfall. |
||
|
Assumed ocean environment density stratification due to temperature and/or salinity variation with depth during most seasons of the year. |
||
|
Water Quality Problem |
Effective Remedial Action |
Comments |
|
1. Pathogens/coliform counts too high at target areas (swimming areas, shellfish beds) |
• Longer diffuser • Longer outfall(discharge farther offshore) • Disinfection |
• Higher initial dilution, possible submerged plume • Increases travel time back to shore and die-off •Some methods (e.g., chlorine) are injurious to marine organisms |
|
2. Undesirable biostimulation due to excessive nutrients (e.g., algal blooms) |
• Longer outfall • Keep nutrients below photic zone • Nutrient removal |
•Improved dispersal to open ocean (nutrients less or not harmful) •Submerged effluent plume in naturally turbid waters • With some methods, increased sludge volume, requires larger disposal site |
|
|
x Secondary treatment (not a remedy) |
• Nutrients not significantly removed but may be more available • Increased light penetration due to particle removal |
|
3. Excessive turbidity or light extinction |
• None, if turbidity is natural • Increased removal of particles by chemical coagulation and/or secondary biological treatment |
• Increased light transmission may increase biological growth •Increased sludge production |
|
4. Excessive oil and grease (slicks, grease balls, shoreline deposits) |
• Better diffuser • Source control for sewer system • Better removal of oil and grease in primary tanks • Secondary treatment· |
• Increased dilution • Check other marine and land sources |
|
5. Trace contaminants in water column; bioconcentration in fish and birds |
• Source control • Better diffuser • Move discharge farther away from biologically sensitive areas • Improved treatment (e.g., secondary or advanced primary) |
• Higher dilution (more spread around, no decrease in total mass) • Many toxicants transferred to sludge |
|
6. Benthic accumulation of organic matter in sensitive areas |
• Advanced primary • Physical-chemical treatment • Secondary treatment (part or all of wastewater flow) • Move discharge away from sensitive areas |
• Removes more particles by polymer (or other chemical) addition; makes more sludge • Further particle removal (makes even more sludge) • Increased energy requirements; increased sludge production with increased handling, transportation, and disposal costs • Only really effective approach |
|
7. Trace contaminants in bottom sediments (effects on benthic organisms) |
• Industrial pretreatment • Pollution prevention • Greater particle removal (advanced primary or secondary) • Move discharge further offshore |
• May increase sludge and toxics disposal by industry elsewhere • Away from sensitive areas to places with better natural dis[ersion of water and sediments |
|
Assumed present system: primary treatment, short outfall. |
||
|
Assumed ocean environment density stratification due to temperature and/or salinity variation with depth during most seasons of the year. |
||
|
Water Quality Problem |
Effective Remedial Action |
Comments |
|
8. Benthic release of contaminants deposited in earlier years · |
• Cover up with additional natural sediments • Continued wastewater particle discharge with control of toxics at source • Excavation and removal |
• May require disruption in the area of the new sediment source • Delay implementing secondary treatment (if at all). Source control of toxics is essential • Resuspension may cause rerelease • Need new disposal site • Temporary increase in release of toxics into water column |
|
9. Depression of dissolved oxygen: |
• Better diffuser |
• Higher initial dilution to a lower concentration of BOD |
|
A. Due directly to wastewater discharge |
• Relocate discharge to area of better fluhing • Secondary treatment • Secondary treatment with nitrification |
• More dissolved oxygen resource tapped • Lowers oxygen demand • Lowers oxygen demand |
|
B. Due to excessive plankton growth and then decay in deeper waters |
• Reduce biostimulation |
• See #2 above |
|
10. Other aesthetic problems |
|
|
|
A. Floating solids of wastewater origin |
• Better removal in sedimentation tanks • Effluent screening • Combined sewer overflow control |
• Need to control floatables in wet weather overflows |
|
B. Refuse |
• Better cleaning of streets and paved areas • Public education |
• Need to control other sources of refuse(e.g., solid waste from shipping traffic) |
|
11. Occasional breakdown of treatment plants; toxic spills |
• Long outfall • Good diffuser • Improved maintenance • In-line sewer and treatment plant flow equalization |
|
water uses (such as protection of ecosystems) is not easy and is subject to continual improvement as we learn more about various risks. For example, with respect to total nitrogen, we may have only some generalized brackets rather than specific numbers, but these too can be very useful as targets. When the water and sediment quality objectives are uncertain, an engineer normally would devise a system which will limit ambient pollutant concentrations at the lower end of the band of uncertainty to provide a safety margin. At later times, when more is understood and standards are changed or made more certain, then wastewater disposal systems and other activities can be adjusted to be most effective in achieving the goals.
Finally, the expression of water quality or environmental objectives may be stated in a way that is totally devoid of numerical description. A classic example is the one contained in the language of Section 301(h) of the Clean Water Act which requires that a "balanced indigenous population (of flora and fauna) be maintained." The California Ocean Plan also requires that "Marine communities, including vertebrate, invertebrate, and plant species, shall not be degraded" (CSWRCB 1990). A description of the California Ocean Plan is provided in Box 4.2. These qualitative standards require that the dischargers and regulators develop an agreement as to what these objectives mean on a case-by-case basis and how compliance will be evaluated on a local or regional basis.
Management of uncertainty is part of the design of environmental solutions. It is incorrect to say that if everything is not known, nothing can be done in terms of a wastewater disposal project. With sufficient empirical experience, which has been developed in many cases, successful projects can be built. The challenge of the future is to keep responding to new information so that as time goes on those things that are really necessary and worthwhile are continued and those things that have little or no benefit according to our developing knowledge base can be terminated.
The Environmental-Quality Driven Approach
The next step in risk management is to design engineering control systems that may be used to achieve compliance with the various water-quality and sediment-quality objectives that are established to manage risks to human health and ecosystems. In using the term environmental-quality driven approach, the implication is that other media effects will be included even though water and sediment are central. Sediment quality objectives are recent developments, as it is now well understood that contaminated sediments are significant contributors to exposure to the ecosystem and humans (through shellfish and benthic fish).
The design of engineering systems based on water and sediment quality objectives is shown schematically in Figure 4.4. The figure and the follow-
|
Box 4.2 THE CALIFORNIA OCEAN PLAN The California Ocean Plan is the state of California's Water Quality Control Plan for Ocean Waters established and administered by the State Water Resources Control Board under the California Water Code, and approved by the U.S. Environmental Protection Agency under Section 303(c)(1) of the Federal Clean Water Act. It provides the basis for regulation of point and nonpoint discharges to California's ocean waters. Periodic Review First promulgated on July 6, 1972, the plan has a built-in triennial review process to accommodate advances in scientific understanding and to respond to public inputs on the criteria for protection of various beneficial uses. The plan was updated in 1978, 1983, 1988, and 1990; and is currently undergoing another review. Use of an Environmental Quality Driven Approach The Ocean Plan establishes water quality objectives for California's ocean waters. These objectives are designed to ensure that more than 66 physical, biological, and chemical parameters are within acceptable ranges, that designated beneficial uses are protected, and that human health is protected from potential risks associated with water contact and fish and shellfish consumption. Toxics The Ocean Plan was one of the earliest water quality plans to address toxic pollutant inputs by establishing water quality requirements based on protection of marine biota, preceding federal efforts to promulgate applicable marine water-quality standards. For toxic pollutants, the plan was unique in that it did not mandate technology per se, but established ambient water-quality standards that could be met through a variety of management or technologic controls. Effluent limitations for toxics are established for each discharger so that ambient water-quality requirements will be met after initial dilution. Dischargers can then set the most effective combination of controls (e.g., initial dilution, treatment, or source control) to meet requirements. Pathogens The plan establishes an ambient total and fecal coliform standard for receiving waters and a requirement to monitor enterococcus levels. Disinfection for control of pathogens is not required and is in fact discouraged because of the effluent toxicity associated with most disinfectants. One of the plan's general requirements for locating an outfall discharge point is that "Waste that contains pathogenic organisms or viruses should be discharged a sufficient distance |
|
from shellfishing and water-contact sports areas to maintain applicable bacterial standards without disinfection." If it is not possible to locate the discharge a sufficient distance away, disinfection is required. Dissolved Oxygen The plan establishes an ambient water-quality standard that limits reduction in dissolved oxygen levels to 10 percent of the natural value. It does not establish a standard for BOD in effluent nor does it establish treatment requirements for BOD. Effluent Limitations The plan establishes some standards without regard to initial dilution or receiving water conditions. Specific effluent limitations are established for suspended solids (60 mg/l or 75 percent removal, whichever is less restrictive), settleable solids, turbidity, oil and grease, pH, acute toxicity, and radioactivity. Additional Highlights of the Ocean Plan
The Ocean Plan is Characterized by Its
|
ing discussion refer to a municipal wastewater disposal system in order to illustrate the concepts; however, the same concepts and procedures can be extended to water bodies with multiple inputs from point and diffuse sources.
The first step (top of Figure 4.4) is the expression of the environmental objectives, in terms of water quality objectives or standards, from which the engineering design may proceed. The chart then displays what appears to be a backwards calculation: from these standards is derived the combination of engineering measures that is best suited to reach the prescribed water quality. There are three basic types of engineering components for a municipal wastewater system:
-
the outfall(s), including location and characteristics of multiport diffusers used for high dilution (see Appendix C);
-
the treatment works, with various possible types and levels of treatment (see Appendix D); and
-
source control (or source reduction) to limit the amount of toxic substances or other pollutants entering the sewer system and the treatment plant (see Appendix D).
These three parts constitute a system in which changes in one part will change the need for the others. For instance, a long outfall with high initial dilution generally reduces the need for secondary treatment; or better source control reduces the need for toxics removal at the POTW and simplifies the sludge disposal problem. Only by considering all three components and their associated environmental and financial costs and benefits at once can the optimum combination be found.
The conceptual design then proceeds with a trial choice of outfall and related treatment levels and source control programs necessary to meet the ambient water quality standards. The process involves complex modeling of the transport and fates of contaminants in the ocean after initial dilution. Given a certain outfall configuration, effluent limits needed at the POTW can be determined. To meet these limits, appropriate treatment components and upstream source control measures are then selected.
As a practical matter, such modeling must be done in the forward sense (sources? treatment? outfall? transport and fate? effects), but with iterations it is conceptually the same as Figure 4.4 with the reversed order of steps. However, with experience it is not difficult to work backwards from water-quality and sediment-quality standards to get approximate solutions for the three system components, which are then used as the initial iteration for the detailed forward water quality modeling. The use of modeling as a means for implementing a water-quality driven approach is discussed in further detail in the following section and Appendix C.
System Components
Source Control. Source control is the collective term used to describe all the ways that POTWs regulate the inputs of toxic materials into their sewer systems. These ways include prescribed effluent limits for industrial dischargers, effluent charges, and outright prohibitions. Source control programs implemented by many POTWs have been very effective in reducing the toxicity of both effluents and sludge. In the 1970s, regulators stated that one of the reasons for mandatory secondary treatment was the reduction in toxics in effluent, even though the toxics were then captured in the digested sludge. But in the 1980s, concern over the toxicity of sludge provided the impetus for implementation of stringent source control programs.
Treatment Plants. The various types of treatment plants are summarized briefly in Chapter 2 and in more detail in Appendix D. A study of performance and costs for various treatment components and combinations has been undertaken by the Committee on Wastewater Management for Coastal Urban Areas, and the results are presented in Appendix D.
For marine discharge, the usual configuration is full secondary treatment except for POTWs that have 301(h) waivers and operate at primary, or chemically-enhanced primary, or partial secondary treatment levels. In chemically enhanced primary treatment, sedimentation is enhanced by the addition of polymers or other coagulants that can increase the removal rate for suspended solids from about 55 to 65 percent for the traditional primary sedimentation up to 75 to 85 percent, or even higher, for the enhanced process. It is interesting to note that 85 percent removal of suspended solids, even though in a primary tank, is equivalent to the federal requirement for suspended solids removal for secondary treatment (for influent suspended solids less than 200 mg/l).
The environmental-quality driven approach will encourage further innovation in wastewater treatment focused on controlling water and sediment quality as needed.
Outfalls. An ocean outfall is a pipeline that conveys liquid effluent from a treatment plant to the receiving water. For the discharge end of the outfall, engineering practice has progressed in the last four decades from a simple open-ended pipe near the shore to the use of large multiple-port diffusers discharging the effluent in deep water far offshore. An example of such an outfall is the 27,400-foot one of the Orange County Sanitation Districts of California. As shown in the plan and profile in Figure 4.5, the outfall includes a 6,000-foot-long diffuser with 503 discharge ports and terminates at a water depth of 200 feet. Figure 4.6 is a schematic drawing of the discharge of buoyant effluent into a density-stratified receiving water
FIGURE 4.5 Schematic plan and profile of the 120-inch outfall, County Sanitation Districts of Orange County, California. (In metric units, the overall length is 8.35 km, the diffuser length is 1.83 km, the diffuser depth is 53-60 m, and pipe diameter is 3.05 m.) (Source: Koh and Brooks 1975. Reproduced, with permission, from the Annual Review of Fluid Mechanics, Vol. 7, © 1975 by Annual Reviews Inc.)
from a typical multiport diffuser at the end of an ocean outfall. Wastewater effluent, being effectively fresh water, rises in the ocean and mixes rapidly with the receiving water. Since the ocean is usually density-stratified due to surface heating or freshwater river discharges, the effluent mixes first with denser bottom water. The resulting mixture can become neutrally buoyant before the rising plume reaches the ocean surface. Neutral buoyancy leads to the formation of a submerged waste field or plume, which is then advected horizontally by the prevailing currents.
The mixing occurring in the rising plume is affected by the buoyancy and momentum of the discharge and is referred to as initial dilution. It is typically completed within a matter of minutes. This phase of the mixing process is under some control by the design engineer since it depends on the diffuser details such as length, jet diameter, jet spacings, and discharge depth. The initial dilution is also controlled partially by nature since it depends on the density stratification and currents in the receiving water.
A typical, large discharge diffuser (for a flow of 5 m3/s) might be a kilometer in length and located in 60 m water depth at a distance of 10 km offshore. There might be several hundred discharge jets (typical diameter 10 cm) spaced along the diffuser. The initial dilution obtainable for such a diffuser would be expected to be in the hundreds to a thousand depending on details (mainly flow rate and density stratification). The initial dilution and waste field submergence can be estimated with a fair degree of confidence, thanks to three decades of engineering research on the mixing processes in buoyant jets and plumes. A number of computer models exist in the literature, each of which can provide predictions of sufficient reliability to make rational decisions on design choices.
In more complicated situations the water-quality driven approach can also include systems for CSOs controls and measures for abating diffuse sources.
Transport and Fates Modeling: Predicting Ambient Water and Sediment Quality
Mathematical and conceptual models are extremely useful in the development of risk management alternatives. Models are used to explain observed processes which disperse and modify pollutants in the ocean and to predict how various management options can be expected to perform. Various submodels may be combined to produce an overall model that relates pollutant inputs to water and sediment quality for single and multiple sources. These models are fundamental to the water-quality driven approach for system design because the limits on emission for any discharge or nonpoint source may be back-calculated using the models. Because of the various length and time scales associated with different problems (Table 4.9), a
variety of models is needed-one mathematical model cannot provide answers to all questions. These models are analogous to the emissions-to-air-quality models used in developing air pollution control programs.
To be successful, there must be good predictive capability for the dominant factors that determine the engineering choices for satisfying the standards. These factors are determined by sensitivity analyses as well as the experience of the modeler. Thus for engineering purposes it is not necessary to understand every process if more knowledge would have no effect on the choice of control strategy. For example, it is not necessary to understand the behavior of a certain pollutant at a location where the input is far below any possible threshold value of concern. Or another example, if the range of uncertainty of a biological effect is a factor of 10, the effect may be of no importance if the upper end of the range is fully acceptable.
The preceding discussion relates to the wastewater disposal system for a municipality including source control programs, a treatment plant, and outfall. The three kinds of information required apply equally well to all other types of pollutant sources and the approach to devising an engineering system. In the case of CSOs, for example, it is necessary to have a set of water quality objectives; some knowledge of the environment; and information on the amount, quality, frequency, and distribution of existing CSOs.
TABLE 4.9 Typical Length and Time Scales of Effects Associated with Typical Pollutant Problems for Coastal Wastewater Discharges
|
Pollutant |
General Length Scale |
General Time Scale |
|
Ammonia toxicity (if any) |
One or two kilometers |
Few hours |
|
Other acute toxicity (if any) |
Few kilometers |
Less than 1 day |
|
Coliforms |
Up to 10 kilometers |
Up to a week |
|
Bacterial pathogens |
Up to 10 kilometers |
Up to a week |
|
Viral pathogens |
Up to 10 kilometers |
Up to 4 months1 |
|
Protozoan pathogens |
Up to 10 kilometers |
Up to 3 months2 |
|
Deposition of organic matter |
10 kilometers |
Few days |
|
Oil and grease (wastewater origin) |
Few kilometers |
Few days |
|
BOD-caused DO decrease |
Few kilometers |
Few days |
|
Nutrients (nitrogen) |
Up to 100 kilometers |
Months to a few years |
|
Regional hypoxia |
Up to 100 kilometers |
Months to a few years |
|
Heavy metals (sediments) |
Few kilometers |
Years to decades |
|
Synthetic organics (sediments) |
10s of kilometers |
Decades |
|
1 Melnick and Gerba 1980, Goyal et al. 1984. 2 G. Vessey, MacQuarie University, Sydney, Australia, personal communication, 1992. |
||
If there are multiple sources contributing to the water quality impairment, then environmental modeling must integrate the effect of all sources and develop scenarios for different degrees of control and handling of different sources. Sources of the same kinds can be combined into classes to simplify the modeling, such as one for a large number of small POTWs all affecting the water quality of a large body of water such as Long Island Sound.
Predictive models have a number of uncertainties and need improvement, but nonetheless appropriate engineering systems for wastewater disposal and diffuse source control can be designed to meet prescribed water-and sediment-quality objectives. Since modeling for design of a management plan for pollution control always has some uncertainty covered by safety factors, it is cost-effective to implement a system (such as a waste treatment plants and an outfall) in a stepwise, flexible manner to allow for continuous feedback of operating experience and observed impacts on the coastal waters. In fact, there are few situations where there is not already an existing discharge that serves as a prototype to study before and during upgrading the system. For example, the full effect of upgrading primary treatment on coastal water quality might well be observed before proceeding to more advanced treatment levels. Or source control efforts for specific chemicals can be focused on dischargers whose concentrations are observed to be too high. This approach is always self-correcting, as the discharger is committed to take as many steps as necessary to solve any problem.
Diffuse Sources—Modeling and Control
An integrated management approach must also address the impacts of diffuse sources. The term diffuse sources describes the types of inputs to coastal water bodies other than municipal and industrial wastewater. These sources are diffuse in origin, such as urban storm runoff, and are usually intermittent. Current laws and regulations have redefined some of these discharges as point sources for which permits are now required. Herein the functional term diffuse sources is used for describing the nature of the sources, without making the legal distinctions.
Combined Sewer Overflows and Stormwater Outlets. Nearly all outlets for stormwater flows and CSOs discharge almost at the shoreline through either open channels or pipes. Because of the intermittent nature of the flows, often at discharge rates far greater than dry-weather sewage flows, and scattered locations, it is not feasible to use long outfalls or multipleport diffusers, as is the practice for wastewater effluents from POTWs. In addition, because of the low use factor, such pipes tend to fill up with sand
and marine detritus to such an extent that the hydraulic capacity may be lost. Tide gates may be used to reduce saltwater and sand intrusion, but they must be readily accessible for maintenance rather than deep below the water surface.
Due to the general lack of feasible options to increase the initial dilution significantly at the discharge points, it is necessary to impose corrective measures on land, such as the banning of toxics for dispersed urban use (e.g., the elimination of lead from gasoline), better street cleaning, detention basins, skimming devices for floatables, and storage and diversions to treatment plants during dry weather. These measures may apply to either stormwater outlets or CSOs.
At one time, sewer separation was a common technique for abating CSOs as well as localized flooding. This practice was soon realized to be very expensive and unacceptably disruptive. Furthermore, in many areas it is impractical to separate all stormwater connections from sanitary wastestreams because a sizeable number of these take place on private property. Thus abatement efforts shifted toward maximizing the amount of wet weather flow treated at the wastewater treatment plant. Such efforts include enhancing in-line storage coupled with near surface or deep tunnel storage capacity. High-rate satellite treatment in a stand alone mode (e.g., vortex solids separators, and mechanical screening) has been used in a number of applications across the country over the last 20 years to decrease abatement costs; however, performance has often been disappointing. The most recent example is the large swirl concentrator installation in Washington, D.C. that is achieving suspended solids removal rates of only 25 percent and for which no measurable water-quality improvements have been found (Nemura and Pontikakis-Coyne 1991). In the last five years, high rate treatment has been packaged with satellite treatment concepts creating compact efficient solutions, but performance of these systems is not yet known. Also, during this time period, the cost of deep rock tunneling has decreased which makes the more efficient and dependable deep tunnel storage option increasingly attractive (see Appendix D for more details).
Natural Streams and Rivers. Coastal cities are often located where major streams discharge to the ocean directly or through estuaries. In these cases, the urban coastal waters may receive a large dose of pollutants washed from the entire watershed down the drainage system. These pollutants, which must be controlled at their sources, include nutrients and pesticides from farms, forests, and urban areas; dry and wet weather acid deposition on the watershed; products of chemical weathering; sediments; refuse; animal excretions; and other natural organic matter.
Various stream modification techniques, sometimes with adverse environmental consequences, can help to make the water flow easily into the
ocean, and jetties may guide the flow slightly offshore, although they are usually built for sand control and stabilization of the outlet position. After discharge into an estuary or the ocean, the initial dilution is determined by the dynamics of the density-stratified flow in which a freshwater layer spreads laterally over the denser brackish water or seawater. Dilutions achieved in the first few minutes or fraction of an hour will remain low (i.e., less than 10). Subsequent mixing occurs at the density interface until it disappears. If the river water quality is managed to meet ambient water requirements, then initial dilution should not be an issue. Only long-term problems, such as eutrophication or toxics in sediments, will drive the planning of control measures, such as reduction of nutrients flowing from farmlands or mines into rivers. Source reduction measures have to be applied to the whole watershed with the tightness of control depending on what is needed to avoid problems of bioaccumulation of toxics in the food chain and eutrophication of semi-enclosed water bodies.
Fine sediments (i.e., silt and clay) in river discharges may be beneficial (if uncontaminated) in diluting or covering up previously contaminated deposits from other sources. Sediments carried by rivers replace beach material eroded by wave action. Also, increased turbidity due to finer (colloidal) suspended sediments reduces light penetration, a factor which may help to control excess growth in water bodies high in nutrients. However, the turbidity may be aesthetically unacceptable to the populace.
Atmospheric Deposition. Atmospheric deposition of pollutants directly onto a coastal water body in the form of precipitation or dry deposition is by its nature widely dispersed. The initial dilution is thus not relevant, and only the regional circulation and sedimentation processes determine how much becomes trapped in sediments versus the net transport to the deep ocean. If the residence time of a body of coastal water is short (days to months), the capture of airborne pollutants in the sediments will tend to be less than for lakes where the residence time is of the order of years, as is the case for the Great Lakes, where at present the principal new source of PCBs is atmospheric deposition.
The modeling of the transport and fate of airborne pollutants is similar to that for other input pathways, except that the first step will be vertical diffusion into the upper mixed layer of the ocean, typically to a depth of 10 meters. Whether such inputs are important must be evaluated on a case-by-case basis in comparison to the magnitude of the other sources. It may be found in some cases, as for nitrate in Chesapeake Bay, that as the strengths of other sources are reduced, the remaining depositional source may become relatively significant and must be included in the development of overall control strategies. Of course, control of atmospheric deposition can only be done back at the sources of emission of the pollutant in question to
the atmosphere; the motivation for such control will usually come from other environmental problems first (such as acid deposition on forests and fresh waters), except in cases where the marine problem may be more acute than any land-based ecological or health risks. For example, there may be more bioconcentration of pesticides and PCB's in marine food chains than on land; on the other hand, acid deposition per se is of no consequence in coastal waters because of the huge buffering capacity of the sea.
In summary, for most pollutants direct deposition on coastal waters is a small term in their overall environmental mass balance; there are cases, however, in which direct deposition is a significant factor. Deposition on land areas and subsequent delivery to the shore by storm channels and rivers is often more significant. For example, before unleaded gasoline, the main pathway for lead transport to the ocean in southern California was urban storm runoff (Huntzicker et al. 1975).
Ocean Sludge Disposal
Disposal of digested sludge to the ocean, prohibited by federal law in the United States, is still practiced in other parts of the world. Digested sludge is a suspension of very small organic particles (averaging about 10 to 20 microns) that are similar to small particles still remaining in wastewater effluents. In recent years, because of source control, the concentration of contaminants in digested sludge has been steadily decreasing. Reduction in toxicants is required for safe land disposal and incineration as well. For discussion of other methods of sludge disposal see Appendix D.
The modeling of a possible sludge discharge (typically 100 times less in volume, but much more concentrated in particulate matter than effluent), whether by pipeline or by ship, proceeds by the same kind of transport and fate modeling as described above. The water-and sediment-quality driven approach applies for the development of whether, and, if so, how, sludge can be safely disposed of in the ocean. The principal uncertainty in the transport and fates modeling is the rate at which sludge particles will sink to the bottom. In other words, the size of the deposition footprint is in question. Much can be learned from study of existing sites such as The 106 Mile Dump Site off the New York Bight, where solids tended to reach the bottom somewhat faster than predicted, which resulted in a higher deposition rate near the source than expected.
The primary environmental impact associated with ocean disposal of digested sludge, if it is done far offshore, appears to be possible changes in sediments (Van Dover et al. 1992). On one hand, contaminants may be deposited on the ocean floor and enter the food web. On the other, in some circumstances, sludge disposal may provide beneficial nutrients to the ocean. It is reasonable that the question of sludge disposal to the sea be re-exam-
ined taking into account improved understanding of sludge particles' behavior and sediment quality criteria now being developed.
Digested sludge may be a beneficial resource; when it can be recycled feasibly in a beneficial way, it certainly should be. Sometimes, however, the extra costs of energy and facilities and the cross-media impacts of dewatering the sludge and transporting it to places where it can be used beneficially may not outweigh the environmental costs of ocean disposal.
Cross-Media Considerations
All of the foregoing discussion focused primarily on water and sediment quality and the development of appropriate engineering works and management strategies to produce the desired water and sediment quality while still disposing of wastewater and storm water. The integrated approach obviously requires that full consideration be given to the cross-media impacts of various actions for disposal of wastewater with respect to the land and the atmosphere. Some examples of these cross-media consequences are presented in Table 4.10. There are, of course, other examples as well, but the points are illustrative.
One general point is worth more attention. The building and operation of treatment plants, including the facilities for the processing of sludge, consume materials in their construction and use energy to operate. Materials extraction and production (such as steel for reinforcing bars or copper for windings in motors) have significant environmental costs and consume an important resource base. Energy to run plants requires the extraction of fuels and contributes CO2 to the atmosphere, which may affect global warming. Digested sludge produces methane but this energy is not free. If the treatment plant, through energy conservation, were to have a surplus of electric power generated from the methane, then it could be put into the power grid and substituted for other sources. Secondary treatment is an energy-intensive process, the full environmental impacts of which should be considered along with the benefits and costs.
As a final example, the city of Los Angeles was recently required to stop discharging digested sludge to the ocean via a seven-mile sludge outfall and instead now has an EPA-approved disposal system consisting of dewatering and incineration even though the Los Angeles basin is one of the most severely impacted air pollution regions in the country. The option of moving the ocean discharge point much further offshore to water over 1,000-feet-deep has been predicted to have low cost and few offshore impacts. It was rejected by the EPA because the Clean Water Act prohibited such a project regardless of the environmental consequences of alternative options (LA/OMA 1980, Brooks 1983).
TABLE 4.10 Examples of Possible Cross-Media Consequences of System Choices for Ocean Disposal of Wastewater
|
Item |
Potential Impacts or Hazards |
|
Chlorination |
• Hazardous manufacturing, transport, and storage and handling at the treatment plant |
|
|
• Impact on marine organisms |
|
Sludge, prohibited for ocean disposal |
• More energy used for dewatering for disposal elsewhere to reduce transportation costs |
|
|
• Centrate treatment and disposal |
|
|
• Ground water, land, and food contamination from land disposal, unless pathogens, metals, and toxics are controlled |
|
|
• Air pollutant emissions from incineration, including toxics, metals, and greenhouse warming gases |
|
Increasing levels of treatment |
• More sludge for disposal |
|
|
• More energy required for treatment and sludge processing |
|
|
• Associated impacts of extracting, refining, and burning fuels |
|
Watershed controls on nitrogen release |
• Reduces need for advanced treatment nitrogen removal |
|
Source control of metals and toxics for reduction in effluent |
• Reduces toxics in sludge |
Costs and Feasibility
It is important to describe the costs associated not only with each of the alternatives for the solution of particular problems but also for the whole range of problems. A good understanding of the financial cost of a particular management option results from an analysis of the capital and operating costs of the project over time. The analysis also must take into account the cost of financing capital expenditures
Consideration must be given to determining which sector of society will bear what portion of the costs. The simplest example of the impact of this consideration is seen in the construction of municipal treatment plants. In these cases, the end of federal grant support means that customers now
bear a high proportion of, or all of, both construction and operating costs in their sewer rates. Where costs can be borne initially by the private sector, there may be opportunities to consider possible financial incentives or disincentives to induce the private sector to assume the burden. If examination of a particular risk suggests that suburban development requires effective management of stormwater, then a choice is posed about whether the necessary infrastructure should be provided by government or by developers.
These financial considerations need to be displayed in as great detail as are the technical solutions to the range of problems being considered. The comparative analysis of the cost factors associated with particular solutions will further define the practical achievability of any particular technical management option. It is noted that sometimes a more expensive solution will be favored because it is characteristically more reliable than less costly alternatives.
Finally, managers must consider the feasibility of each option within the range of solutions which have been developed. Feasibility is a function of a wide range of factors, including:
-
public acceptability,
-
legal authority, and
-
institutional capacity.
One illustration will demonstrate the impact of these factors. In some situations, the engineering and cost analysis may suggest that the most technically practical solution to a wastewater management problem is discharge of advanced primary effluent through a very long outfall. However, this solution might be unacceptable to the public for nontechnical reasons, or may be illegal under existing statutory authorities.
Expectations and Benefits in Relation to Costs
After the various risks are analyzed with respect to possible technical solutions, costs, and feasibility, it is necessary to consider the public's evaluation of benefits in relation to costs for various possible solutions. For example, in a particular urban setting (e.g., Point Loma, San Diego), the public may desire to have swimmable water in adjacent kelp beds even though the cost may be much higher than if these benefits were forsaken. If maintaining a certain use is considered to be too expensive, then expectations may be scaled back. The question then is a matter of degree and choice: how much preservation of natural ecosystems and other beneficial uses is the public willing to pay for? In different places, the balance can and will be struck in different ways.
For environmental managers, the challenge is often to find ways to provide the most environmental protection for the money; i.e., engineering
and other solutions must be cost effective and be perceived by the public to have produced significant long-term benefits in relation to the cost. At the same time, public expectations at a particular moment when management decisions must be made may lead to a choice that is either inadequate to protect the environment or that is quite costly with only marginal environmental benefits.
In summary, residuals must be managed and returned to the natural ecosystem by some procedure; the ocean disposal option cannot be considered in an absolute sense, and its risks, costs, and benefits must be evaluated in comparison to the other management and disposal options.
Summary
The main points in risk management for wastewater impacts on urban coastal waters are:
-
Integrated coastal management requires the use of the environmental-quality driven approach to model and manage the effects of single or multiple discharges and diffuse pollution sources and to make effective regional control strategies. The primary environmental objectives to be met are water-quality and sediment-quality standards for the receiving waters.
-
Predictive models have a number of uncertainties and need improvement, but, nonetheless, appropriate engineering systems for wastewater disposal and diffuse source control can be designed to meet prescribed water and sediment quality objectives.
-
There is much to be learned from existing discharge situations that is useful to support modeling and engineering efforts for designing new or upgraded facilities.
-
The ability to develop and use mathematical and conceptual models is ahead of the field of confirming the accuracy of models. More effort is needed to study prototype systems after construction to evaluate the preconstruction modeling and analysis.
-
A continuous, responsive approach is needed for future management of major discharge areas. This approach includes ongoing ocean studies and flexibility of management to modify the discharge system as needed in response to new research findings, new problems, or new environmental objectives. The corollary of this step is the need to proceed in a stepwise fashion, implementing the most effective steps first.
-
Coastal water quality management must be site or region specific because of widely varying conditions along the coastline of the United States.
-
Because of the wide range of spatial and time scales of various ocean processes and the various time scales of different water quality problems, different modeling approaches are required for different pollutants.
SELECTION, POLICY, AND INSTITUTIONS
Institutional Arrangements
The institutional setting for regulating wastewater discharges in the coastal environment is complex, fragmented, and compartmentalized. While the federal Clean Water Act provides a basic regulatory framework, the interplay of natural resource management, regional and local planning, programs for nonpoint and point source discharges, preventive and remedial actions, and multiple levels of government leads to an institutional setting far more diverse and extensive than the act alone would imply. Having a clear picture of this institutional setting is essential to any attempt to improve wastewater management.
Institutions are fragmented in at least three different ways: hierarchically, geographically, and functionally. Hierarchical fragmentation occurs when responsibilities are divided among two or more levels of government. Multiple political jurisdictions lead to geographic fragmentation. The further division of programs according to function adds still another layer of fragmentation. Within any particular watershed or region, the presence of multiple agencies, each with separate but relevant programs, requirements, and responsibilities, creates enormous complexity.
The Clean Water Act itself involves multiple levels of government and considerable complexity because it incorporates sewage treatment requirements, stormwater management, toxicant regulation, estuary management, and funding mechanisms, among other things. While the basic scheme of uniform federal requirements implemented through delegated state programs is straightforward, it leaves coastal states to struggle with some of the most difficult issues. Each state and local jurisdiction must decide how to mesh growth and land-use planning with water quality objectives; it must regulate toxicants in discharges in the absence of comprehensive federal regulations; and funding must be found for upgrading wastewater treatment in the absence of federal construction grants.
As the need for effective pretreatment and pollution prevention programs is recognized, municipal wastewater treatment requires a significantly increased regulatory role for local governments. Despite fragmentation and jurisdictional complexity throughout the nation, it is possible to discern an emerging trend toward better integration of planning, resource management, cross-media issues, and interjurisdictional concerns. Many coastal states provide promising examples of attempts to manage growth, to link land use and water resource management, and to control sources of water pollution in an overall context. Considerable integration and alignment of institutional arrangements has been achieved in some places. Signs of progress include the development of estuary protection strategies (e.g., for the Chesapeake
Bay), regional water resource agencies, and integrated growth management and land-use strategies.
There is no one right approach to better integration of environmental decision making. What is doable in one setting may be out of the question in another. Flexibility in approach and implementation is as necessary as leadership and long-term commitment of funding and political will.
Improving the institutional framework for water quality protection is a significant challenge. Increased integration of planning and implementation is desirable both within water resource functions and between water and other related resource decisions. Efforts at integrated environmental decision making are hopeful signs, but only a beginning. Most water pollution control decisions of this decade will in all likelihood continue to take place in a complex and fragmented institutional setting. Yet greater awareness of the broader context should assist both ad hoc and structural efforts to achieve better integration.
Development, Selection, and Implementation
Other sections of this report discuss goals and objectives for coastal wastewater management, sources of pollutants, and various strategies for the control of these pollutants. Application of the tools of risk management within a dynamic planning process, as described above, leads to identification of one or more strategies that meet the stated goals and requirements. Each strategy may consist of a variety of pollution reduction and pollution control initiatives, as well as other actions or modified behavior. The final steps in the planning process are to turn each strategy into a management plan, then to choose among alternative plans.
A management plan 1) describes a desired outcome as defined by the risk management strategy, 2) specifies the policies and actions necessary to achieve that outcome, and 3) assigns responsibility for implementing specific actions. Just as the process of risk management takes account of a wide range of possible actions and interventions in seeking the most appropriate strategy, it is important to consider all feasible management tools in devising management plans. But coastal wastewater management takes place in a fragmented, multi-institutional setting, where no one entity has the means or the mandate to set overall objectives or compel specific actions by others. A management plan in this situation takes the form of a set of tools that—directly or indirectly, through compulsion, incentive, motivation, or other means—cause necessary actions to be taken. The actions include various kinds of pollution prevention, pretreatment, wastewater reuse, treatment, and disposal practices.
Effective management plans do not rely on a single tool to achieve all required actions. Each kind of tool has unique advantages and disadvan-
tages and may be more suited for one type of application than another. A comprehensive plan should incorporate a range of regulatory mechanisms and choose individual techniques to fit individual problems. The application of these tools can be overlapping—more than one policy can apply to a given activity. Command-and-control regulations, for example, might provide a regulatory floor that applies to all waste dischargers, while a combination of economic incentives, education initiatives, and other instruments of voluntary compliance are used to achieve progress beyond the command and control floor. The following sections outline the command and control approach as it is now practiced for wastewater management. Other kinds of regulatory tools are described and evaluated, using command and control as the basis of comparison.
No wastewater management strategy should be considered in isolation. An intricate network of laws, regulations, and policies is already in place and must be recognized in the development of any new policy. Improvements in the flexibility in these requirements would do much to facilitate effective management action. The same caution holds for existing institutions. Attempts should always be made to improve and rationalize institutions, but the end result of such efforts may fall short of the optimum. A management plan should, therefore, build on existing elements when it is reasonable to do so, superseding and rejecting only when such changes are imperative.
Tools for Management
Environmental quality in coastal areas is the result of numerous individual decisions by residential and business organizations, and governments regarding the use of resources and the discharge of pollutants. If environmental quality is to be protected, those individual decisions must reflect consideration of environmental consequences. One way to accomplish this is to set standards and compel individuals and organizations to observe them on threat of sanction. This command-and-control approach, the most common management strategy in the United States, is embodied in the Clean Water Act and other environmental legislation.
Many other management tools are available. They include economic incentives structured to induce voluntary behavior consistent with environmental quality goals; growth management, used to restrain human activity in sensitive coastal zones; education, which seeks to provide the basis for informed individual action; and financing mechanisms designed to facilitate the implementation of desired actions.
Economic incentives offer important advantages over the more familiar command-and-control instruments (see Appendix E). Properly designed and implemented, they tend to minimize the total cost of meeting environ-
mental objectives. They promote technological innovation and improvement, both in pollution control and pollution prevention. Even though today's command-and-control instruments are more effective and flexible than their counterparts of twenty years ago, economic incentives may be designed to address sources beyond the reach of conventional regulation. However, experience to date suggests that economic instruments are often used most effectively in conjunction with command-and-control measures (Boland 1989).
Growth management is a comprehensive and integrative approach to planning for balancing resource protection and economic development, focused on implementing strategies. Growth management strategies offer some control over the total quantity of pollutants generated in sensitive environments. They also present opportunities for integration across environmental media. Education is another means of reducing pollution at the source. Carefully designed, interactive education programs can produce significant long-range reductions in the quantity and toxicity of materials discharged to the environment. A more detailed discussion of education initiatives is contained in Appendix E.
Wastewater management programs can be financed in numerous ways. These ways include the use of general tax revenue, dedicated tax revenue, user charges, intergovernmental transfers, and debt (long term or short term). Some of the characteristics of particular methods can be regarded as purely financial, such as the ability to provide sufficient revenue, the stability of the revenue stream, or the associated administrative details. Other properties of financing methods have important implications for wastewater management (see Appendix E for more details).
Differences in financing methods result in differences in the total cost burden imposed by wastewater management. More importantly, different financing methods produce very different incidences of cost—across sectors of the economy, across political jurisdictions, and across periods of time. The choice of financing method affects incentives for efficient management, as well. A more detailed presentation of these issues is contained in Appendix E.
The ultimate importance of these considerations derives from the willingness of the public, and various sectors of the public, to pay for improved wastewater management. If the cost of proposed programs is too high, or perceived as unfairly allocated, public and political support will be eroded or lost.
Selection
The planning process described in this report is intended to produce a number of competing management plans. These arise in part from the existence of alternative strategies for wastewater management (different as-
sessments of relative risk, different allocations of abatement effort, etc.). Alternatives may also reflect consideration of different kinds and types of management tools. Each alternative plan has a unique set of likely consequences. While all alternatives are designed to protect the fundamental functions of the ecosystem, they may do so in somewhat different ways. All alternatives are intended to maintain important human uses of the resource, yet they may not all result in exactly the same set of protected uses. Economic costs will differ, as will such critically important characteristics as impacts on the distribution of income, political and social acceptability, etc. Some plans may be flexible and easily modified, while others are slow to adapt to changing circumstances. Some may promote innovation and individual initiative, while others lock in existing methods and technology. For these reasons and more, selection of the appropriate plan is only partly the responsibility of experts.
It is the task of planners and analysts to insure that all plans meet the following tests:
Adequacy—Each plan must satisfy the primary goals of wastewater management: to protect the fundamental functions and biological richness of the ecosystem and to maintain important human uses.
Integration—Each alternative must consider the full range of human activities linked to wastewater generation, as well as the full range of environmental effects resulting from wastewater management actions. All of this should be undertaken for a geographic area large enough to minimize important inter-area impacts.
Comprehensiveness—In developing the final plan, it is essential that all significant alternatives be considered, with respect to both control strategy, management tools, and costs. This consideration should encompass, among other things, alternatives that illustrate selected tradeoffs among the objectives (i.e., improved ecosystem protection at the expense of human activities, and increased human use at the expense of ecosystem protection).
Non-inferiority—No alternative should be put forward for selection that is, in all important respects, inferior to some other alternative. It follows, then, that each final candidate is preferred to some other candidates in one or more respects. The final set of alternatives are, in this sense, the best of all possible alternatives.
Properly applied, the planning process should give rise to novel and innovative solutions, as well as conventional and familiar approaches. Each will have various advantages and disadvantages. As wastewater management requirements become more severe, it can be expected that unconventional regulatory techniques will come to seem more conventional. Whether conventional or unconventional, each plan that meets the criteria must be
fully described together with its expected consequences so that political leadership, regulatory agencies, and the public can make responsible choices.
MONITORING, INFORMATION MANAGEMENT, AND RESEARCH
The information developed through research, monitoring, and data management activities drives the planning process and keeps it dynamic. Information on the status of the coastal environment is obtained through monitoring. Data management allows for effective use of monitoring and research information. Research provides knowledge about the significance of various environmental changes, importance of different impacts, and potential for various technologies and management controls to be effective in mitigating impacts and protecting the environment. Research activities can also contribute new insights to the understanding of human expectations for the coastal environment and the economic impacts of alternative management strategies.
One important strength of the ICM process is that it is responsive to new information inputs and can respond to trends in coastal problems or new scientific findings with less delay than required by federal statutory changes and regulations. The linkage between the planning process and research, monitoring, and data management activities is critical to the success of a continuing, iterative ICM program.
Monitoring
The National Research Council report Managing Troubled Waters: The Role of Marine Environmental Monitoring defines monitoring in the marine environment as ''a range of activities needed to provide management information about environmental conditions or contaminants." Monitoring is generally conducted to gather information about compliance with regulations and permit requirements, model verification, and trends (NRC 1990). The report concluded that monitoring can strengthen environmental management in several ways: 1) defining the extent and severity of problems, evaluating actions, and detecting emerging problems; 2) when coupled with research and predictive modeling, supporting integrated decision making; and 3) guiding the setting of priorities for management programs. It also concluded that comprehensive monitoring of regional and national trends was needed to better assess the extent of pollution problems and address broader public concerns (NRC 1990).
Since the release of that report, the U.S. Environmental Protection Agency has begun to implement a national and regional monitoring program called
the Environmental Monitoring and Assessment Program (EMAP). EMAP will tend to provide a framework and a set of uniform data and quality objectives for regional programs. However, there is region-specific monitoring needed in many areas to examine identified issues, close data gaps found in the planning process, and monitor the performance of risk management strategies chosen.
In addition to the recent work of the EPA, the National Oceanic and Atmospheric Administration (NOAA) has had a significant marine monitoring program in place since 1984. NOAA's National Status and Trends (NS&T) Program monitors for selected metals and organic compounds in sediments and benthic organisms at nearly 300 coastal locations in the United States. The fundamental objective of the NS&T Program is to assess long-term trends in the concentrations of these toxic materials.
An effective ICM system requires a monitoring approach significantly different from approaches used in the past. As the National Research Council found in 1990,
. . . monitoring designed principally to meet regulatory compliance needs generally does not adequately answer questions about the regional and national risks of pollutant inputs to public health, coastal environmental quality, or living resources. The reason is that compliance monitoring typically does not address potential effects removed from specific discharge points, including overall responses of the ecosystem to anthropogenic and natural stresses (NRC 1990).
Data analyses should be available in forms that not only address the current concerns but also allow for identification of new trends. In this manner, monitoring becomes the vehicle through which the process is accountable to the public and useful to practitioners.
Reliance on technology-based standards has led to universal monitoring of effluent parameters such as total suspended solids, BOD, and chemical oxygen demand, while far field and specific effects remain unexamined in many cases. For ecosystem effects, most useful information has been generated by special research projects in specific areas, with hardly any contribution from routine compliance-driven monitoring programs. For health effects, there is monitoring for pathogens and for toxic chemicals. These monitoring programs are designed to enhance risk management strategies that may include posting warnings, fishing limits, and shellfish bed and beach closure strategies, as well as to examine long-term trends.
Information Management
The ICM process is designed to make the fullest use possible of information relating to coastal systems and their management. It is an informa-
tion intensive process that requires effective data and information management. Information and data should be collected and maintained in forms that are accessible to users and compatible with other data in the system. All too often monitoring data are collected and stored, but in forms that are of relatively little use for purposes of analysis (NRC 1990).
The results of scientific analyses should be presented in user friendly formats to all parties involved in the process ranging from scientists and engineers to politicians and the general public. Advances in computing technologies now allow for the display of information in a wide variety of graphical and pictorial formats. Monitoring information and modeling results can be manipulated in a variety of innovative ways to display information about coastal systems including three dimensional presentations of modeling scenarios.
Research
Areas where additional information in needed should be identified in the course of the dynamic planning process. For example, in the course of assessing and comparing risks, it should become clear where there are important uncertainties and data gaps. Research aimed at reducing these uncertainties would improve understanding and provide insight on how to manage the risks. The survey of anglers now under way to develop better estimates of fish consumption in Santa Monica Bay is a good example of a research effort aimed at refining information on risks. In developing management options, research to determine the potential efficacy of various management and control measures will be needed. The need for information should drive the areas of research targeted in an ICM program.
In addition to the specific kinds of research identified in the course of the ICM process, there is an array of more basic research needs in areas where greater understanding is required for fundamental understanding of environmental processes, ecological systems, human health, technological and engineering issues, and economic and policy effects. More specific research needs are identified in the discussion of relevant topics throughout this report and its appendices.
SUMMARY
This chapter has provided a description of how the ICM process should be applied to coastal areas. Several examples describe cases where some ICM concepts are being applied already.
The complexity of the ICM process reflects that of coastal systems including the physical and ecological interactions in the coastal zone and the human expectations and actions that affect them. ICM involves an
iterative and dynamic planning process in which scientific information, engineering expertise, economic analyses, and public participation are combined to identify management alternatives that meet coastal objectives. The selection and implementation step of the ICM process is structured to select the most appropriate management plan and develop adequate institutional arrangements to ensure effective implementation. Research, monitoring, and data management are the activities that provide feedback on how well management controls are working, bring new information into the planning process, and further knowledge about coastal systems.
The following chapter, Benefits, Barriers, Solutions, and Implementation, examines issues relating to the application of ICM to urban coastal areas in the United States and provides recommendations for implementing ICM.
REFERENCES
Boland, J.J. 1989. Environmental Control Through Economic Incentive: A Survey of Recent Experience. Presented at the Prince Bertil Symposium on Economic Instruments in Environmental Control, Stockholm School of Economics, Stockholm, Sweden, June 12-14.
Brooks, N.H. 1983. Evaluation of key issues and alternative strategies. Pp. 709-759 in Ocean Disposal of Municipal Wastewater: Impacts on the Coastal Environment, E.P. Myers and E.T. Haring, eds. MITSG 83-33. Massachusetts: MIT Sea Grant College Program.
Brooks, N.H. 1988. The Experience with Ocean Outfalls to Dispose of Primary and Secondary Treated Sewage. Freeman Lecture, Boston Society of Civil Engineers, April 1988. Presented at Massachusetts Institute of Technology.
Cabelli, V.J., A.P. Dufour, L.J. McCabe, and M.A. Levin. 1983. A marine recreational water quality criterion consistent with indicator concepts and risk analysis. Journal of the Water Pollution Control Federation 55:1306-1314.
Capuzzo, J.M. 1981. Predicting Pollution Effects in the Marine Environment. Oceanus 24(1):25-33.
Carry, C.W., and J.R. Redner. 1970. Pesticides and Heavy Metals. Whittier, California: County Sanitation Districts of Los Angeles County.
CBP (Chesapeake Bay Program). 1992. Progress Report of the Baywide Nutrient Reduction Reevaluation. Washington, D.C.: U.S. Environmental Protection Agency.
Cosper, E.M., C. Lee, and E.J. Carpenter. 1990. Novel "brown tide" bloom in Long Island embayments: A search for the causes. In Toxic Marine Phytoplankton, E. Graneli, B. Sundsttrom, L. Edler, and D.M. Anderson, eds. New York: Elsevier.
CSWRCB (California State Water Resources Control Board). 1990. California Ocean Plan, Water Quality Control Plan for Ocean Waters of California. Sacramento, California: CSWRCB.
Doering, P.H., C.A. Oviatt, L.L. Beatty, V.F. Banzon, R. Rice, S.P. Kelly, B.K. Sulivan, and J.B. Frithsen. 1989. Structure and Function in a Model Coastal Ecosystem: Silicon, the Benthos and Eutrophication. Mar. Ecol. Prog. Ser. 52:287-299.
Dubos, R. 1965. Man Adapting. New Haven, Connecticut: Yale University Press.
EPA (U.S. Environmental Protection Agency). 1986. Ambient Water Quality Criteria Document for Bacteria. EPA A440/5-84-002. Washington, D.C.: U.S. Environmental Protection Agency.
EPA (U.S. Environmental Protection Agency). 1989. Sediment Classification Methods Compendium, Draft Final Report. U.S. Environmental Protection Agency, Watershed Protection Division.
EPA (U.S. Environmental Protection Agency). 1990. Reducing Risk: Setting Priorities and Strategies for Environmental Protection. Washington, D.C.: Science Advisory Board.
EPA (U.S. Environmental Protection Agency). 1991. Ecological Risk Assessment Guidelines, Strategic Planning Workshop. Miami, Florida. April 30 - May 2, 1991.
EPA (U.S. Environmental Protection Agency). 1992a. Environmental Equity: Reducing Risk for all Communities, Vol. 1: Workgroup Report to the Administrator. EPA230-R-92008. Washington, D.C.: U.S. EPA, Policy, Planning, and Evaluation.
EPA (U.S. Environmental Protection Agency). 1992b. Environmental Equity: Reducing Risk for All Communities, Vol. 2: Supporting Document. EPA230-R-92-008A. Washington, D.C.: U.S. EPA, Policy, Planning, and Evaluation.
Fleisher, J.M. 1991. A reanalysis of data supporting U.S. federal bacteriological water quality criteria governing marine recreational waters. Research Journal of the Water Pollution Control Federation 63:259-265.
Gibb, H., and C. Chen. 1989. Is Inhaled Arsenic Carcinogenic for Sites Other than the Lung? PB 90-130675. Washington, D.C.: U.S. EPA, Office of Health and Environmental Assessment.
Goyal, S.M., W.N. Adams, M.L. O'Malley, and D.W. Lear. 1984. Human pathogenic viruses at sewage sludge disposal sites in the middle Atlantic region. Appl. Environ. Microbiol. 48:758-763.
Graneli, E., H. Persson, and L. Edler. 1986. Connection between trace metals, chelators, and red tide blooms in the Laholm Bay, SE Kattagat—An experimental approach. Mar. Env. Res. 18:61-78.
Howarth, R.W. 1988. Nutrient limitation of net primary production in marine ecosystems. Annual Review of Ecology & Systematics 19:89-110.
Howarth, R.W. 1991. Comparative response of aquatic ecosystems to toxic chemical stress. Pp. 169-195 in Comparative Analysis of Ecosystems: Patterns, Mechanisms, and Theories, J.J. Cole, G. Lovett, and S. Findlay, eds. New York: Springer-Verlag.
Huntzicker, J.J., S.K. Friedlander, and C.I. Davidson. 1975. A material balance for automobile emitted lead in the Los Angeles basin. Pp. 33-77 in Air-Water-Land Relationships for Selected Pollutants in Southern California, final report to the Rockefeller Foundation, J.J. Huntzicker, S.K. Friedlander, and C.I. Davidson, eds. Pasadena, California: W.M. Keck Laboratories, California Institute of Technology.
IRIS (Integrated Risk Information System). 1993. Cadmium. CASRN 7440-43-9. U.S. Environmental Protection Agency.
Jackson, J.B.C., J.D. Cubit, B.D. Keller, V. Batista, K. Burns, H.M. Caffey, R.L. Caldwell, S.D. Garrily, C.D. Getter, C. Gonzalelz, H.M. Guzman, K.W. Kaufmann, A.H. Knap, S.C. Levings, M.J. Marholl, R. Steger, R.C. Thompson, and E. Weil. 1989. Ecological effects of a major spill on Panamanian coastal marine communities. Science 243:37-44.
Koh, R.C.Y., and N.H. Brooks. 1975. Fluid mechanics of waste-water disposal in the ocean. Ann. Rev. Fluid Mechanics 7:187-211.
LA/OMA. 1980. Los Angeles/Orange County Metropolitan Area Regional Wastewater Solids Management Program. Final Facilities Plan/Program and Summary of Final EIS/EIR. Whittier, California.
Life Systems, Inc. 1989. Toxicological Profile for Cadmium. Oak Ridge, Tennessee: Agency for Toxic Substances and Disease Registry, Oak Ridge National Laboratory.
MBC-AES (MBC Applied Environmental Sciences). 1988. The State of Santa Monica Bay: Part One: Assessment of Conditions and Pollution Impacts. Los Angeles, California: Southern California Association of Governments.
Mearns, A.J., and J.Q. Word. 1982. Forecasting the effects of sewage solids on marine benthic communities. Pp. 495-512 in Ecological Stress in the New York Bight; Science and Management, G.F. Mayer, ed. Columbia, South Carolina: Estuarine Research Federation.
Mearns, A.J., M. Matta, G. Shigenaka, D. MacDonald, M. Buchman, H. Harris, J. Golas, and G. Lavenstein. 1991. Contaminant Trends in the Southern California Bight: Inventory and Assessment. NOAA Technical Memo NOS ORCA 62. Seattle, Washington: National Oceanic and Atmospheric Administration.
Melnick, J.L., and C.P. Gerba. 1980. The ecology of enteroviruses in natural waters. CRC Crit. Rev. Environ. Control 10:65-93.
Nemura, A., and E. Pontikakis-Coyne. 1991. Water Quality Benefits of CSO Abatement in the Tidal Anacostia River. Washington, D.C.: Metropolitan Washington Council of Governments.
Nixon, S.W., C. Oviatt, J. Frithsen, and B. Sullivan. 1986. Nutrients and the productivity of coastal marine ecosystems. J. Limnol. Soc. S. Africa 12:43-71.
NRC (National Research Council). 1983. Risk Assessment in the Federal Government: Managing the Process. Washington, D.C.: National Academy Press.
NRC (National Research Council). 1989a. Improving Risk Communication. Washington, D.C.: National Academy Press.
NRC (National Research Council). 1989b. Contaminated Sediments. Washington, D.C.: National Academy Press.
NRC (National Research Council). 1990. Managing Troubled Waters: The Role of Marine Environmental Monitoring. Washington, D.C.: National Academy Press.
NRC (National Research Council). 1993. Issues in Risk Assessment. Washington, D.C.: National Academy Press.
Officer, C.B., and J.H. Ryther. 1980. The possible importance of silicon in marine eutrophication. Mar. Ecol. Prog. Ser. 3:83-91.
O'Shea, M.L., and R. Field. 1992. Detection and disinfection of pathogens in storm-generated flows. Can. J. Microbiol. 38:267-276.
Phillips, P.T. 1988. California State Mussel Watch Ten Year Data Summary 1977-1987. Water Quality Monitoring Report No. 87-3. Sacramento, California : State Water Resources Control Board.
Pollock, G.A., I.J. Uhaa, A.M. Fan, J.A. Wisniewslei, and I. Withenell. 1991. A Study of Chemical Contamination of Marine Fish of Southern California. II. Comprehensive Study. Sacramento, California: Office of Environmental Health Hazard Assessment, California Environmental Protection Agency.
Rand, G.M., and S.R. Petrocelli (eds). 1985. Fundamentals of Aquatic Toxicology Methods and Applications. Washington, D.C.: Hemisphere Publishing Corporation. Pp. 651-657.
Rose, J.B., and C.P. Gerba. 1991. Use of risk assessment for development of microbial standards. Water Science Technology 24:29-34.
Schindler, D.W. 1987. Determining ecosystem responses to anthropogenic stress. Can. J. Fish. Aquat. Sci. 44:6-25.
Smayda, T.J. 1989. Primary production and the global epidemic of phytoplankton blooms in the sea: A linkage? Pp. 449-483 in Novel Phytoplankton Blooms: Causes and Impacts of Recurrent Brown Tides and Other Unusual Blooms. Lecture Notes on Coastal and Estuarine Studies, E.M. Cosper, E.J. Carpenter, and V.M. Bricelj, eds. Berlin: Springer-Verlag.
SMBRP (Santa Monica Bay Restoration Project). 1992. Progress Update: Santa Monica Bay Restoration Project 1991-92. Monterey Park, California: Santa Monica Bay Restoration Project.
SMBRP (Santa Monica Bay Restoration Project). 1993. Santa Monica Bay Characterization Report. Monterey Park, California: Santa Monica Bay Restoration Project.
Van Dover, C.L., J.F. Grassle, B. Fry, R.H. Garritt, and V.R. Starczak. 1992. Stable isotope evidence for entry of sewage-derived organic material into a deep-sea food web. Nature 360:153-155.
WHO (World Health Organization). 1948. Preamble, Constitution. Geneva: World Health Organization.
Younger, L.K., and K. Hodge. 1992. 1991 International Coastal Cleanup Results. R. Bierce and K. O'Hara, eds. Washington, D.C.: Center for Marine Conservation.