13
Biotechnology Case Study
GEORGE B. RATHMANN
I want to describe a bit of the history of the biotechnology field to give you a strong sense of the importance of this field, not just in itself but as a prelude to a new technology as it develops over the next century. I then relate that history to some questions that have been raised and finally relate my conclusions with respect to biotechnology to the objectives of the conference.
As rocky as the road for biotechnology was in the United States, what we see coming up on the world scene is much more difficult, much more serious. We desperately need a legal system to solve the problems, and it is our hope that there are ways of dealing with these issues.
The biotech era really dawned when Watson and Crick defined the structure of deoxyribonucleic acid (DNA). As with many world-shattering discoveries, this was simple and concise—a publication of one page outlining the structure of DNA (Nature, April 25, 1953, p. 737). They also had the vision to say it would affect not only how we looked at deoxyribonucleic acid, but how we looked at life itself and our ability to understand living systems. There would be products, there would be opportunities, and there would be new insights that would be most important. All that was recognized in a one-page article.
As important and earth shaking as that was, from the standpoint of the commercialization of biotechnology, something nearly as important occurred on June 17, 1980, when the Supreme Court ruled that live organisms could be patented. It was well recognized as important at the time, but I think few
people realized how important it was for launching the commercialization of biotechnology.
In that patent, Dr. Ananda Chakrabarty, who was at G.E. at the time, claimed an organism that would digest oil. The invention was never commercialized, but it told the world that this field was going to be important and there were going to be commercial opportunities. An investment in trying to understand the biochemistry of life would pay off in the sense that the intellectual property could be protected. Within four months (October 14, 1980), the biotechnology company Genentech went public and jolted Wall Street with a rise in its stock price from $35 to $71 1/4. So it is clear that as of that date, biotechnology assumed increasing commercial importance.
At that time, in October 1980, I was looking at the opportunity to start a biotech company called Amgen and we were putting out a document that we hoped would raise $15 million. Partly because of Genentech's success, we were able to raise $19 million—with only a scientific advisory board, one employee, and promises for two future hires. So it certainly had a profound effect on whether Amgen would ever be. As a matter of fact, within a year, Amgen, Genetics Institute, Immunex, Genetics Systems, Chiron, and many others companies were formed. Within two years, more than 100 companies were formed as this era was launched.
Now, the Chakrabarty decision made it look simple: life forms were patentable. Genentech, Cetus and many others afterwards launched public offerings, recognizing the commercial potential that biotechnology would lead to new discoveries of valuable intellectual property, which could be protected by patents. In reality, it was not quite that simple and the launchings were not that consistent.
Venture capital funds vacillated quite a bit, although after 1980 there was a very substantial influx of venture capital (Figure 13-1). There were periods when it went down, and periods when it went up. Although these look like gigantic numbers, remember it takes about a quarter of a billion dollars to bring a pharmaceutical product to market. It probably takes more than that to commercialize something important in agriculture, food, or other areas. So this flow of venture capital was actually inadequate to keep it going. Of course, the public made the difference, but it can be seen that this was not exactly a consistent, reliable source of funds, either.
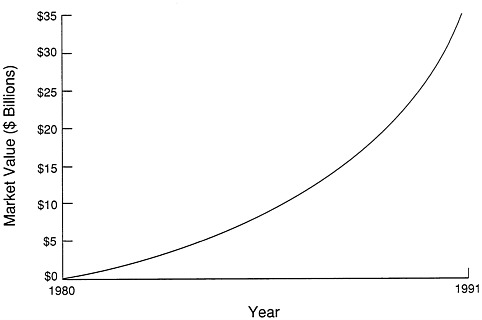
If we smooth everything out, the market value of biotechnology stocks moved dramatically from 1980, when it was literally zero, to 1991, when it was more than $35 billion (Figure 13-2). Those of us in the industry saw some very serious bumps in that curve. In 1987 some biotech companies lost 30-40 percent of the value of the company in a matter of a few days. When you finally smooth everything out, it looks a lot simpler and surer than it felt.
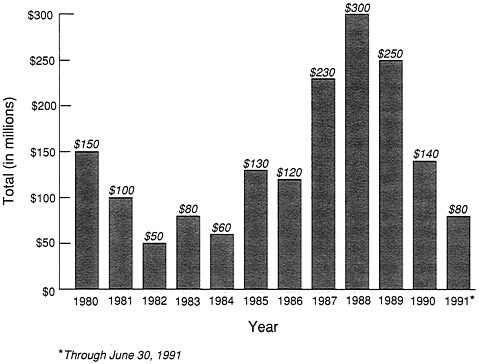
FIGURE 13-1 Venture Capital Disbursements in Biotechnology
Source: Venture Economics and Ernst and Young
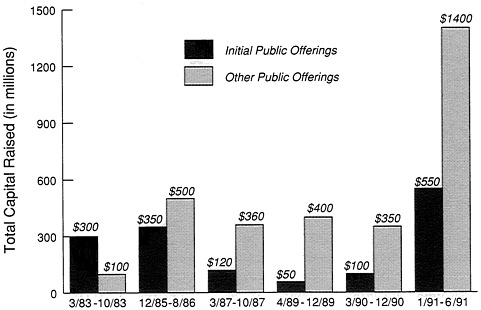
Figure 13-3 shows the amount of capital raised through public stock offerings. In 1991, more money was raised in six months than for many years, and as a matter of fact, when the total figures came in for the year they exceeded $4 billion—equal to all the money that had been raised in the previous years since the launching of commercial biotechnology. Of course, the big news is $550 million in initial public offerings. Those are new companies whose survival may mean wonderful improvements to our lives around the world. At the same time they will be facing some of the rocky roads that the earlier companies faced. So we can see that it is not a steady, easy trip.
Product sales in the industry today have reached about $8 billion and are expected to reach $20 billion by the year 2000. That may be a very conservative figure. The drug industry worldwide by that time will be well over $200 billion, and biotechnology is contributing roughly half of the most important products today. By the time the year 2000 comes around, biotechnology-derived products could be even more important. Of course there should be many other parts of the biotech industry that are commercially interesting by that time.
So we are looking at something of great importance to the economy of the country and to international trade, which is discussed below.
I was asked by the National Research Council to address several questions. The first was, What adjustments in intellectual property rights have been made? Well, of course, the first is the allowance of claims to living organisms. The United States certainly led the way there. It was a very important opportunity that organisms that produced a pharmaceutical material could be claimed in patents. We had something tangible to claim even if the product being produced was already known or already had been defined.
One of the things that has been evolving over the last few years, and certainly in 1987 had a pretty dismal outlook, is referred to as In re Durden. This case implied that just because you have a novel starting material on which you carry out a process to produce another material, the process is not automatically patentable. That case was often interpreted much more severely to mean that unless the process is highly inventive, mere novelty because of novel starting materials does not make it patentable. So it was not possible in 1987 to get claims to the process that was going to produce, for example, in Amgen's case, erythropoietin by using a novel organism.
Because inventors could not claim the process, they had a very serious problem. They could not invoke any rights at all against companies who used their organism overseas, produced the product, and brought it in. They did not have a final product claim; they did not have a process claim; and there was no mechanism for protecting against the direct theft of the organism overseas—copying it, or following the teachings of the patent, and then just shipping the product to the United States.
However, an evolution has occurred since then. Certainly, a lot of process claims have now been granted. There is a bill authored by Congressman Boucher that would give guidance to the Patent Office to make sure it issues those claims. Without those claims, the organism patent is meaningless with respect to overseas competition. What if the overseas country does not issue the organism patent? The organism has only one purpose—to produce the protein, so the inventor is left with no protection against importation. Amazingly enough, the inventor is protected from infringement in the United States by U.S. companies but is unable to stop foreign infringement and U.S. importation. The trade implications are clear.
This has been a very serious problem that is now being addressed. Yet there are still concerns from people who wonder if it is really "fair" to keep foreign companies from bringing their products into the United States. They ask, "Isn't that protectionism?" This a very strange interpretation of fairness. I think these inventions are clearly being copied and misappropriated by foreign companies. Changes may or may not move smoothly, but these issues should be resolved in the next few years, and more and more compa-
nies are availing themselves of the process protection, though some opportunities have been abandoned after In re Durden objections.
There have been great differences in the interpretation of the scope of claims. My initial discussion is limited to the United States because global issues have really only come into play in the least five years. Even in the United States, the scope of claims has been quite a difficult issue with which to deal. The questions stated are, If the claims are too broad, doesn't it mean we are inhibiting the diffusion of technology? If the claims are too narrow, doesn't it mean that the inventor really is disadvantaged? I could say a lot about that, but in actual fact I will cite the record. A Boston court in the United States leaned toward a pretty narrow interpretation of the claims. In a Delaware court, a jury decided that the Genentech case should be very broadly interpreted and cover structures quite different from the ones that were defined in the patent simply because all the rest were straightforward once the patent teachings were available. So these are still issues, but I think we will move toward a pretty clear understanding over the next few years.
The effect on biotechnology advancement has not been smooth even in this country. Patent uncertainty has encouraged second entrants, who then plead that since they made such a significant investment, believing they were not going to be prevented from manufacturing the product, the terms of the claims of the patents should be relaxed. This has certainly been an expensive mistake in many cases.
Major delays in issuance of patents have prevented some innovators from pushing their products as rapidly as they could, because they feared that they might never have coverage and once they proved the success of the product, it could be duplicated relatively readily. I think many of us in the business got a lot of encouragement from the Orphan Drug Act, because that act suggested that we at least could get six years of protection if we were the first to have a product approved for an orphan indication. If we never received adequate patent protection, we still might be able to recoup our investments, which was very comforting. There has been a lot of controversy about the Orphan Drug Act and whether it should serve as a kind of substitute for the Patent Act. Nevertheless, it helped an embryonic biotechnology industry raise money and sustain its early critical momentum.
Finally, patents played a key role in attracting pharmaceutical companies' investments. These were very important for some companies in the early days. Even though the pharmaceutical companies were not the innovators, they certainly helped support many new biotechnology companies and they clearly needed the confidence of patent exclusivity.
As stated in congressional testimony by Dr. P. Roy Vagelos, Chairman of Merck & Co., "To sustain their ability to discover and develop products which form the basis of American competitiveness, U.S. pharmaceutical
companies count on renewed government support ... in strengthening international protection of intellectual property rights." We can illustrate that perhaps even more significantly in the biotech industry.
For example, in 1986 a pharmaceutical product would cost about $94 million and take somewhere between 10 and 20 years before entering the market. Some kind of protection is certainly required before that kind of investment is made. The figure today is $240 million. That number has been challenged by Congress and looked at many ways by the Office of Technology Assessment (OTA); the latest OTA study says that costs may often be that high, although sometimes they may be lower. However, it does not require a lot of arithmetic to figure this out. The pharmaceutical industry in this country alone spends about $10 billion on R&D per year, and about 30 new products—30 new molecular entities—are approved each year. That comes out to be more than $300 million invested for each success.
In fact, there are at most only four or five new therapeutic products approved each year that are important and if you divide by that, you arrive at astronomical figures for important new therapeutics. Also, all this investment is required years before you can enter the market and start to get a return. So this certainly fits the pattern of something that requires protection, and patents look like the way to do it.
In 1986 the average development time of a new pharmaceutical product was 10 years. The interesting thing is that biotechnology has compressed that time. Because of the rational design of these products, their remarkable efficiency and safety profile, and the understanding and cooperation of the U.S. Food and Drug Administration, the average development time is about four to seven years today for biotechnology products, which is a big help. However, it is still a long time and a large investment.
So let us review how biotechnology was commercialized. What happened is not particularly logical, not what anyone would have deduced sitting around a table trying to decide what was going to happen. When a biotech company decided it wanted to launch a product, it had to build a company to launch the product. All the different stages and structures had to be built—the vectors and expression systems, purifications, scale-up, manufacturing, clinical testing, regulatory submissions, and marketing. Surprisingly enough, almost all of these things were in place in major pharmaceutical companies, yet almost every single important invention was done by independent biotechnology companies. That is the fact; that is what we have to deal with. How were they able to do all this, why would they be the first to do it, and was it effective? Is it not terribly inefficient to have to create a company for each new product? That is what was done.
Small, start-up biotechnology companies were responsible for many miracle drugs. For example, Amgen developed erythropoietin, and we now know that 10 milligrams per year, one-fiftieth of an aspirin tablet, will
prevent 20 or more transfusions for people that are deficient in erythropoietin—and there are many more. Chiron produced the answer to hepatitis C, which is something that has plagued society and challenged scientists for more than 30 years—a well-defined disease about which nothing could be done. Cetus discovered ways of amplifying genes. Individual inventors, individual small companies, are pioneering and finding important new molecules and insights that are changing the way medicine is practiced today. This was done in a way that perhaps was hardly predictable—small, independent companies got started and did this all on their own—but this is exactly what happened. Sometimes it occurred with the support of large companies, but none of the key innovations and developments throughout the field were made by the large companies.
As I said, it was a fairly rocky road. I think that is important. The fragility of a new technology and the need for immediate action are more critical than making long-range plans to do wonderful things over long periods of time. These companies are fragile and their viability is always in question. Their survival is in jeopardy at all times. Take 1989 as an example. Headlines blared, "Clouds gather over the biotech field." Interestingly enough, firms were stumbling on regulation and patent problems. The patent situation looked very confused at that time. It was very difficult again to get financing, and the feeling was that many companies would go out of business and some did.
If we look at the number of financings, we see what has faced this emerging technology—and will probably apply to every new technology—big financing surges, dry spells, big surges. The dry spell in 1984 and 1985 seemed to last forever. We learned it can take eight quarters before you see another chance to raise money. When 1987 came along, the stock market wilted, and 1988, 1989, and 1990—one after another—were all very bad years. Of course, 1991 salvaged a lot of companies, but those were dangerous times for fragile, embryonic businesses.
So some protection is required. There is no question that patent protection fits the need in terms of the large investment required over a long period of time. The question is always asked, however, whether keeping the inventions secret would work. Well, it doesn't. Once the gene has been described, it is trivial to produce the product. Even if the gene is not described anywhere, once the structure is out, once the product is available even in clinical trials, the structure can be determined and often easily duplicated at a much lower cost. The cost is even lower because the copier only has to copy winners. He does not have to duplicate the losers. The copier avoids the major investments that the innovator had to make.
So international protection becomes the issue today. The problems in obtaining worldwide protection are difficult. There are many countries that do not honor the patent system. Surprisingly, countries that do not have
strong patent systems (e.g., China, India, Argentina, Brazil) are not troublesome to the biotech field, although the pharmaceutical industry has expressed concern. However, international trade competition with countries that purport to have a patent system is a very serious issue.
For example, Japan is a strong competitor. In Japan, patent flooding surrounds innovator's patents. The Japanese patent office grants narrow patents instead of broad ones. I think it is pretty obvious to those in this industry that small companies need broad patents. If you are going to try to compete in the marketplace with giants, you had better know that you have some reasonable protection against obvious duplication or partial duplication. The Japanese system has not produced many biotechnology innovations and has not produced biotechnology companies. Our problems with the Japanese system are narrow patents, sometimes taking 10 or more years to issue, and patent flooding, which surrounds the inventor's contribution and forces him to join up with a large, entrenched Japanese company to survive.
To summarize, developing countries have concerned some industries, but they have not been competitive in biotechnology. Europe has awarded strong patents that afford U.S. innovators reasonable protection. Japan has been a very serious issue. Today we see two companies in Japan enjoying the products of Amgen—two products approaching a billion dollars in sales, at prices two to four times that of the products in this country, guaranteeing high profits. It is very easy to see what is going to happen over the long term. Those companies are going to be able to invade other countries in the field of biotechnology and be very active participants in trade.
The question then is, Can the United States dictate or influence international patent practices? Well, somehow it has to. This sounds unfair to some, but it is equally unfair to have misappropriation of intellectual property.
We know the history of what happened: Japan behind, Japan even, Japan ahead. The outlook is very serious. If we think back about that 20year period around the 1960s when U.S. patents were not being upheld, that may have been why it was easy for the Japanese to move in and take over the territory.
Now, for future challenges: The federal government's patenting of the genome was a hypothetical question until a short time ago. Would this be serious? It has now become a very practical question. The U.S. Patent Office is currently examining the NIH's application for patents on certain gene sequences. In the meantime, the Industrial Biotechnology Association has held discussions with Reid Adler of the National Institutes of Health (NIH), biotech executives and administration officials who are examining this issue. What should the NIH do with respect to all of these gene patents? A good start is to provide a forum between industry, NIH and other inter-
ested parties to see if we can understand whether these patents should be applied for, whether they should be issued, and if issued, how they should be handled.
Finally, can patents be issued faster? The U.S. Patent and Trademark Office's numbers on the average time of application pendency are very strange and not helpful. The Patent Office has always figured out ways to say it is doing things in two years when, in fact, there has not been a useful biotech patent that has taken less than four years, and usually five. If we cannot get meaningful numbers, I don't think the problem can be solved. I think the Patent Office is misleading all of us.
In terms of the conference objectives, I would like to close with these thoughts concerning a few final issues: First, with respect to international perception of the importance of intellectual property rights, the world acknowledges that the United States was the pioneer in biotechnology, and that it was done by risk capital, as well as federal support of R&D, originally. The positive contribution to human welfare is acknowledged worldwide. That does not mean that all the countries in the world want to give strong patent protection for biotechnology, which is a very difficult issue.
Second, with respect to biotechnology patents, in the United States, the road has been rocky but reasonably satisfactory. Worldwide protection will ultimately be critical. It is sad that this did not occur long ago. Because of this lack, we are seeing companies in foreign countries appropriating U.S. technology to get started.
Finally, with respect to conflict resolution, the most precious resource of a budding new industry or budding new technology is time. The solutions have to be time sensitive. Grandiose solutions that involve 60 or 70 countries, and take years and years, will mean that a lot of the companies will fail before the solutions are in place. I think people should be aware of that.
I would remind you of one last thing. This is an industry of small companies. If you look at the profile of public biotechnology companies, only 13 percent have more than 300 employees, and none have more than 2,000 employees. If we look at all biotech companies (publicly and privately held), there are only 3 percent with more than 300 employees. We are dealing with a very, very broad-based, small-company business and my remarks apply as well to my firm, ICOS, which we started within the last year, as well as to the largest biotech companies, which are still relatively small. These are the companies seeking patent protection. Strong protection can hardly ''disadvantage small companies" as some critics suggest.