6
Assessing Exposure and Risk
This chapter has two major parts: exposure assessment and risk assessment. The exposure assessment section addresses issues that include environmental monitoring for indoor allergens, exposure measures, and internal dose. The risk assessment section describes the general nature of the process, including exposure assessment, and presents an example that uses dust mite exposure data in assessing the risk of sensitization.
EXPOSURE ASSESSMENT
Assessing exposure involves numerous techniques to identify contaminants, contaminant sources, environmental exposure media, transport through each medium, chemical and physical transformations of the contaminant, routes of entry to the body, intensity and frequency of contact, and spatial and temporal concentration patterns of the contaminant (NRC, 1991). Once an estimate of personal exposure is constructed for the time period of interest, it may be adjusted to estimate the internal dose; it may also be further refined to estimate the biologically effective dose for a critical target tissue. In practice, personal exposure measurements may be qualitative or quantitative. The use of quantitative estimates to reflect biologically active concentrations requires a detailed understanding of deposition and the kinetics of transport, metabolism, and clearance.
Assessment of exposure is a multistep process (Figure 6-1). The specific elements in each step of the process are usually tailored to the types of information that are available or that can be collected, and to the agent of interest.
Aeroallergens are common both outdoors and indoors. For example, exposure to pollens and spores from plant material may occur during time spent outdoors; the pollens and spores may be transported inside offices or other buildings in which additional exposure may occur. Indoors, dust mites, mammals, and fungal growth are examples of sources for aeroallergens. For both indoor and outdoor environments, sources must be recognized,
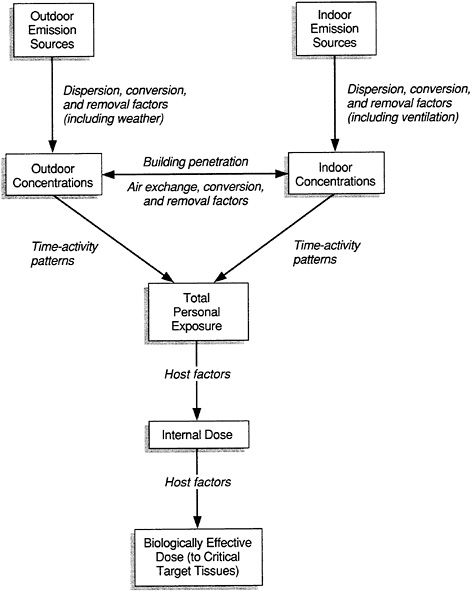
FIGURE 6-1 Framework for exposure assessment. Source: NRC, 1985.
methods of dispersion within the space determined, and factors that may aid in removal evaluated. Once sources have been identified, sampling strategies that allow some estimation of exposure can be devised.
Monitoring for Indoor Allergens
GENERAL PRINCIPLES
Monitoring for allergens can help determine the cause of one or more cases of allergic disease (in which instance, sampling and analysis modes that yield information on the range of possible organisms must be chosen) or characterize environments with respect to specific allergens. Measurements can be semiquantitative (e.g., ''presence or absence" or "low, medium, or high"); or, alternatively, modes can be chosen for sampling and analysis that will give accurate, precise documentation for a specific population regarding a more or less well-defined allergen.
Although monoclonal antibody immunoassays measure specific allergens, most allergen monitoring includes sampling for indicators rather than the actual aerosolized allergen. Table 6-1 is a list of aeroallergens and commonly used indicators. In developing a sampling strategy to collect the data for testing a particular hypothesis, important decisions must be made on where, when, and how to sample.
Where to Sample
The majority of allergen samples are collected either from ambient air in one or more environments or from potential allergen reservoirs. Ambient
TABLE 6-1 Aeroallergens and Sampling/Analysis Modes
|
Common Name |
Aeroallergen |
Indicators |
Sample |
Analysis |
|
Thermophilic actinomycetes |
Glycoproteins |
Living cells |
Air, bulk |
Culture |
|
Molds |
Glycoproteins |
Living cells Spores Antigens |
Air, bulk |
Culture Microscopy Immunoassay |
|
Arthropods |
Glycoproteins |
Antigens Whole animals Guanine |
Bulk Bulk Bulk |
Immunoassay Microscopy Chemistry |
|
Mammals |
Glycoproteins |
Antigens |
Air, bulk |
Immunoassay |
|
Pollen |
Glycoproteins |
Pollen grains Antigens |
Air Bulk |
Microscopy Immunoassay |
air sampling sites should be chosen to represent the range of conditions that might occur. Often, practical considerations require that a single site in each community or in each interior be chosen for all air sampling. Outdoor monitoring is usually done at single sites on rooftops to avoid undue contributions of local sources. Indoors, the site at which a group of people spend a majority of their time (e.g., the living room of residences, open office spaces in large buildings) or at which one or more individuals experience symptoms is usually chosen for air sampling. Material is collected from reservoirs from which exposure can be assumed or extrapolated using mathematical models (Swanson et al., 1990).
When to Sample
Allergen levels in both air and reservoirs change over time as well as in space. Ideally, it should be possible to evaluate air samples continuously over time. As a substitute, multiple discrete samples can be taken over relatively short periods of time, or long-term samples can be taken and analyzed as a time-weighted average or in discrete units.
Sampling for allergens always occurs in a complex environment, and consideration must be given to factors that might modify source strengths, emission rates, accuracy and precision of sample collection, or sample analysis methods and health effects (O'Rourke et al., 1990). Controls are necessary for each step of the investigation.
How to Sample
Sample collection methods include observation, bulk or reservoir sampling, and air sampling. Observational sampling can include sensory perceptions of an indicator (e.g., odors or visible fungus growth) and observation of factors known to be related to specific kinds of sources. (For example, mites are likely found in carpeting on grade-level concrete floors.) Observations can be formalized or casual, the type used by most environmental investigators.
Source or reservoir samples have been used as indicators of exposure to most indoor aeroallergens. Dust samples have been collected for analysis of microorganisms (Gravesen, 1978; Saad and el-Gindy, 1990), arthropod allergens (Ishii et al., 1979; Swanson et al., 1989), and mammalian allergens (Ohman and Lorusso, 1987). Dust collection can be standardized by using a dust collector that maintains a constant flow, vacuuming a given surface for a standard length of time or vacuuming carefully measured sections of a surface, or by using a combination of these methods. A number of measured areas of each substrate can be sampled to estimate variability in space. Vacuum-collected dust samples are weighed before or after sieving to remove hair and other large irrelevant fibers and particles. The levels of allergen per gram of dust can then be used to calculate source strengths
at the time of sampling. Similar sampling at different times allows an estimation of patterns and rates of change in source strengths. Similar principles apply to the collection of liquid samples. If liquid reservoirs are well-stirred, a single sample can be representative of the entire reservoir.
Surface samples are prepared by swabbing or pressing a plate of culture medium or a sticky tape against a surface; such samples are useful for identifying obvious microbial contamination. Many samples must be taken to allow characterization of the surfaces in a space, and such data may or may not be relevant to allergen exposures.
Ideally, human respiratory exposure is measured using air samples taken near the breathing zone of individuals (personal sampling; Macher and First, 1984). Most allergen sampling, however, is done to characterize ambient aerosols. Air sample collection involves drawing a representative sample of the aerosol into a collection device and removing particles in an unbiased way and in a form that allows appropriate analysis.
Volumetric air samples for indoor allergen analysis are usually collected by suction devices. To accurately represent the aerosol, suction samples should be collected under isokinetic conditions in which ambient air flows into the sampler parallel to and at the same rate and direction of suction. In still air and in cases in which the sampling orifice is at an angle to ambient air movement, suction samplers tend to oversample small particles, which are easy to divert from their original path. When air is moving into the sampler orifice faster than the suction rate, small spores will tend to follow streamlines around the orifice and be undercollected. In most sampling protocols, isokinetic conditions are not present. For the small filter cassette samplers and the low flow rate suction impactors (e.g., the Andersen type) used in indoor environments, the error introduced is small. However, high-volume filter samplers and, possibly, the portable suction impactors (SAS, RCS) pull air into the sampler at a rate much higher than ambient air speeds; consequently, small particles are preferentially collected. It is also important to collect a small enough sample that the aerosol in the space is not changed during sampling. High-volume filtration devices process large amounts of air and can actually act as air cleaners.
Once particles have entered the sampler, they must be removed from the airstream. The two most commonly used methods are inertial impaction and filtration. Inertial impaction allows the collection of particles that are able to cross the airstream lines inside a sampler and thus stop at the collection surface. In general, large particles are more efficiently collected by inertial impaction than are small particles. The impaction samplers are often rated by the 50 percent cutpoint (the particle diameter at which 50 percent of particles entering the sampler will be retrieved; ACGIH, 1989). Ratings are set for the commonly used aeroallergen samplers listed in Table 6-1.
Because particle capture is related to both particle diameter and to the speed at which the particle is moving, one can control the size of the particles collected by changing the speed with which particles approach the collection surface. The cascade impactors (e. g., Andersen, 1958) fractionate aerosols by accelerating air through smaller and smaller orifices.
The nature of the impaction surface will also influence the efficiency of particle collection. Unless a particle is traveling at exactly the right speed to stop at the collection surface, it may hit the surface and bounce back into the airstream. This bounce is a function both of particle inertia and of the stickiness of the collection surface. For culture plate impactors, bounce is probably minimal; particles tend to penetrate the agar surface rather than bounce off. For spore traps, however, the collection surface must be coated with adhesive, and it gradually becomes less sticky as more and more particles are trapped. Sampling times must therefore be short enough so that overloading does not occur. Spore traps not only collect spores but all kinds of other particles that might be in the air. In aerosols in which levels of nonbiological particulates far exceed spore concentrations, surfaces often overload before a significant number of biological particles have been collected.
The nature of the impaction surface often determines the kind of analysis that can be used. Adhesive (greased) surfaces are usually analyzed by light microscopy. Agar surfaces are most commonly used for cultural analysis, but they can also be homogenized and assayed using immunochemical or biochemical techniques (Tovey et al., 1981a; Yoshizawa et al., 1991).
Modifications of the Hirst spore trap (the Burkard, Kramer-Collins, and Lanzoni spore traps [Solomon et al., 1980b]) are increasingly used for evaluating outdoor allergen aerosols. These suction devices collect 10 liters of air per minute, impacting particles on a moving, greased tape (over seven days) or on to a microscope slide (over 24 hours). The orifice of the sampler is designed to collect particles as small as about 3 µm efficiently. The trap commonly used outdoors has a wind vane so that the orifice faces into the wind. Similar indoor traps have no wind vane, and the orifices face upward. The recording Burkard version collects particles continuously over 7 days or 24 hours. The 7-day and 24-hour slide samples can be analyzed as 24-hour averages or in time increments of as little as 1 hour. Other devices collect samples in discrete bands over 24 hours (Samplair) or on one spot over a few minutes (Burkard personal).
The rotorod has been used for many years to evaluate outdoor allergens, in spite of the fact that it grossly underestimates fungus spore levels. The particle collection efficiency of these rotating arm impactors depends on the rotational speed and the width of the collecting surfaces (the rods). The wide collection surfaces commonly used for allergen monitoring (1.59 mm) efficiently collect particles as large as 10 µm; however, small particles
tend to follow the streamlines around the surfaces and are missed. The rotorod efficiently collects most pollen types but is less useful for allergen-carrying particles common in indoor air.
Impingers are suction devices that impact particles onto a surface submersed in a liquid. Impingers will not collect hydrophobic particles (like most fungus spores) efficiently unless surfactants are added to the impinger fluid. These devices are especially useful for collecting bacterial aerosols in highly contaminated environments because the resulting suspension of cells in a liquid can be diluted for analysis (Macher and First, 1984; Morey, 1990b).
Suction samplers that collect particles by filtration efficiently trap all particles above the rated pore size of the filter. In addition, particles smaller than the rated pore size are collected by diffusion or impaction. For example, at low flow rates, a 0.8-µm filter probably collects 50 percent or more of the particles in an aerosol larger than 0.1 µm (see Sakaguchi et al., 1989). As the flow rate increases, the filter will capture a higher and higher percentage of smaller particles. The filter's stickiness and kind of pore structure, as well as whether it can be adequately pulverized, all contribute to the ease with which particles can be removed from it for assay.
Extraneous chemicals that remain in some filters after manufacturing can directly change the nature of some aerosols or interfere in assays used to analyze the collected material. Endotoxin assays are particularly susceptible to this kind of interference (Milton et al., 1989), and carefully designed controls should be used each time a new filter medium is employed. Unless spore concentrations are very high, the low flow rate of standard personal cassette samplers (as are commonly used for asbestos) collects too few spores to allow precise estimates of concentration. However, in agricultural or some industrial environments, filter collections can produce valuable information on fungal spore and bacterial counts.
Gravity or settle sampling is the simplest method of collecting an air sample, but these samples are never representative of the aerosol. Large particles tend to be overrepresented, and even small changes in air speed and direction drastically change the fraction of the aerosol collected. Other methods can be used to collect particles from aerosols, including electrostatic precipitation, which is commonly used to clean the air. These devices are not commonly used for collection of aeroallergens.
TYPES OF SAMPLE ANALYSIS
Once an aerosol or a reservoir sample has been collected, it must be analyzed before meaningful data are produced. Assay types used for analyzing allergens include culture, microscopy, immunoassays, biochemical assays, and bioassays. Of these, the most commonly used are culture, microscopy, and immunoassay.
Cultural assays evaluate viable units as indicators of the presence of allergen-carrying particles. The content of the allergen itself may or may not be accurately represented. Cultural assays always quantitatively underestimate levels of allergen-carrying microbes (because only culturable cells are counted) and are biased with respect to the kinds of microbial allergen sources recovered. No single culture medium or set of environmental conditions will allow capture of all the different organisms in a mixed aerosol; however, investigators usually choose culture media that allow recovery of the broadest range of different organisms. Special-purpose media are likely to underestimate even the organisms they are designed to recover. Moreover, some organisms in air or in relatively hostile reservoirs have adapted to minimal conditions and will not grow if rich culture media are provided. Although minimal media are often used to recover bacteria from water samples, they have not been routinely used for fungi. Many of the species of Penicillium and Aspergillus that are common in indoor air grow well in media containing large amounts of salt, sugar, or glycerol (Verhoeff et al., 1990a,b). There are also media that inhibit fast-growing fungi such as Rhizopus or Trichoderma, which can quickly multiply and mask other organisms (Burge et al., 1977; Verhoeff et al., 1990a,b).
Because researchers have yet to discover a generic biomarker for fungal growth and because it appears that fungi differ allergenically at the species level, each fungus that releases allergens into the environment must be identified accurately and precisely so that specific diagnostic and treatment materials can be produced. For some kinds of fungi, generic identification can be completed on the isolation medium. For others (including most fungal species) and for all bacteria and yeasts, subculturing on diagnostic media is required. In addition, for bacteria and some fungi, physiological tests are necessary for species identification.
For particles that are morphologically distinct, direct microscopy is a straightforward approach to studying aerosols. Although very few fungi or pollens are identifiable to the species level microscopically, broader categories can be identified and useful information obtained. Categories of fungus spores that can be identified and counted range from spores of Epicoccum nigrum (a monospecific genus with very distinctive spores) to categories such as "colorless basidiospores," which include hundreds of different kinds of fungi. If a cultural sampler is used in conjunction with a spore trap, spore morphology comparisons can be made and the kinds of identifiable spores expanded. Although pollen is usually stained for microscopic examination, fungus spores often do not take up stains well, and important color characteristics can be masked.
Sample methods that allow microscopic evaluation include suction impaction (as used in the Burkard and Lanzoni spore traps) and rotating impactors (the rotorod). Filter samples can also be analyzed microscopically,
although the clearing necessary to produce optically good samples requires the use of organic oils and solvents, which tend to cause fungus spores to collapse. Relatively smooth surfaced filters (e.g., polycarbonate, teflon) can be mounted in 1% acid fuchsin in lactic acid for counting. Filter samples can also be analyzed using fluorescent stains and epifluorescence microscopy. Water samples are commonly analyzed for bacterial content using the fluorescent dye acridine orange, which stains nucleic acids inside the bacterial cells (Palmgren et al., 1986). The melanin pigments in many fungus spores mask this fluorescence, and some fungal spore walls apparently prevent entry of the dye. However, the method is promising for indoor aerosols of bacteria (including actinomycetes) and colorless fungus spores.
Scanning electron microscopy has also been used to analyze filter samples, and bacteria and fungus spores can be readily seen and counted on smooth-surfaced filters (Pasanen et al., 1989). The level of identification is not as high as for light microscopy, however, and preparation methods are cumbersome for routine counts.
Immunoassays measure the actual allergen rather than an indicator (e.g., viable spores) and are essential for most amorphous allergens including those from mites, cats, and cockroaches. The most sensitive and specific environmental immunoassays for allergens are based on immobilized monoclonal antibodies that bind specific allergen in unknown samples.
Immunodiffusion is a relatively crude immunoassay that is used as a diagnostic test to detect allergen-specific immunoglobulin G (IgG) antibodies in patient sera. It has also been used to demonstrate exposure under the theory that specific IgG may represent exposure to allergens rather than actual disease. Extracts of air or reservoir samples have been used in place of defined allergens to indicate such exposures (Reed and Swanson, 1987).
Finally, measuring the amount of guanine in a sample (a biochemical assay) has been used to estimate the amount of mite allergen in dust samples. Other types of sample analyses have not been applied to allergens.
THE IDEAL AIR MONITOR
No single method is sufficient to detect and monitor all of the different allergens that can be present in indoor environments (Samson, 1985). Currently, the most adaptable collection devices are probably the filter collectors because filter samples can be examined directly by using either visible light, epifluorescence, or electron microscopy. In addition, they can be washed to provide allergens for immunoassay or viable cells for culture. For some allergens, methods of analyzing filter samples have been well defined. For others, especially viable cells, much work remains to be done to define the conditions under which recoveries are optimal. The advantages
of filters (especially personal samplers) are many. They fulfill most of the characteristics of the ideal sampler:
-
They are efficient for collection of small particles.
-
They can be efficiently analyzed using several modes.
-
They can be used for both personal and ambient sampling.
-
They are inexpensive and portable.
Designing a sampling strategy requires consideration of the nature of the allergen source, the nature (including the size and expected concentrations) of the allergen-containing particles, and parameters that influence respiratory exposure to the allergen-containing particles. Each of these parameters influences the choice of sampling method (i.e., observation or reservoir or air sampling) and analytical approach, the sampling plan (amounts of sample to be collected, times and locations to be sampled), and approaches for analysis and interpretation of the data.
Exposure
As mentioned earlier in this chapter, many of the methods used for estimating environmental concentrations of aeroallergens are not truly representative of an individual's exposure to actual allergens. For example, ambient air monitoring is often used to represent personal exposure, and indicators rather than specific inhalable allergens are usually monitored.
AMBIENT DATA AND TIME-ACTIVITY LOGS
One of the ways in which ambient data can be used to estimate personal exposure is to use questionnaires or time-activity logs to catalogue the amount of time people spend in the various areas for which concentration data are available. Examples of the use of area monitoring combined with activity logs have been reported in several studies (Lebowitz et al., 1989; O'Rourke et al., 1989, 1990; Quackenboss et al., 1989a,b, 1991a,b). In these studies, the contribution of indoor concentrations to total personal exposure exceeded that of outdoor concentrations because of the difference in time spent in the two environments. For example, indoor exposure to total pollen, Gramineae, total mold, Cladosporium, and Aspergillus/Penicillium-type spores was two to four times greater than outdoor exposure.
Questionnaires may also be used to determine occupancy rates or activity in the indoor environment under study (Lebowitz et al., 1989; Quinlan et al., 1989). Results from the questionnaires or logs can then be linked with the results of observations to develop categorical estimates of exposure potential in areas for which no concentration information is available.
INDICATOR DATA TO REPRESENT EXPOSURE
An example of the use of indicator data to represent exposure to an allergen is the use of counts of whole house dust mites in dust samples. The presence and quantity of mites in a sample viewed through the microscope indicates only the potential for allergen exposure, and is not a direct measure of exposure itself. House dust mite allergen is associated with fecal particles rather than with the mite itself. Even the counting of fecal particles must be considered a surrogate measure since the concentration of allergen on any one particle cannot be predicted. An accurate measure of exposure to dust mite allergen, as well as other allergens, requires the use of specific immunoassays.
NATURE OF ALLERGEN-CARRYING PARTICLES
Little or no Der p I has been detected in the air in undisturbed rooms (de Blay et al., 1991b; Platts-Mills et al., 1986; Swanson et al., 1989; Yasueda et al., 1989), and after a disturbance in a room, concentrations of airborne mite allergen fall rapidly. It is likely, therefore, that the majority of allergen is carried on particles with large aerodynamic diameter (e.g., greater than or equal to 10 μm). The levels that become airborne depend critically on the form of the disturbance and vary from 5 to 200 ng of Der p I/m3. Because the mean allergen content of mite fecal material and the size of the particles are known, it is possible to estimate the actual amounts of allergen that could enter the lung at a given ambient concentration. For particles of 10-μm aerodynamic diameter, only 5–10 percent would be expected to enter the lung during gentle mouth breathing (Svartengren et al., 1987; Task Group on Lung Dynamics, 1966). Given that each particle contains 0.2 ng of Der p I (Tovey et al., 1981b), an airborne level of 20 ng/m3 means that about 100 fecal particles could be expected to enter the mouth per hour and that from 5 to 10 of these would reach the lung. Use of this technique requires the collection of sufficient mass to measure concentration reliably, a difficult task for aerosols as transient as those of mite fecal particles.
Inhaled allergens from other sources can also differ from generally measured arthropods, particles, grains or spores. For instance, far more allergen is present on amorphous pollen (grain fragments) than assumed from whole grain measurements (Schumacher et al., 1988).
Internal/Actual Dose
Exposure measurements, within the constraints of the methods used, characterize the ambient environment. For example, the number of pollen grains counted may include some that are outside the respirable range and
that cannot be inhaled. Similarly, reported concentrations of allergen are not "adjusted" to reflect the percentage that may be exhaled. Measured concentrations are thus estimates of exposure but not doses to the body. To estimate dose, the following must be known for particulates that enter the respiratory tract:
-
Relationship between the exposure measure and allergen concentration
-
Variability of allergen content in the respirable fraction
-
Percentage of deposition
-
Breathing rate(s)
-
Duration of exposure(s).
No examples have been published in which all of these variables are known, thus allowing a calculation of the internal dose of an aeroallergen.
BIOLOGICALLY EFFECTIVE DOSE
Once contact has occurred, the kinetics of uptake for exposure measures, distribution to the target tissue, and clearance/detoxification must be calculated to estimate a biologically effective dose. These data are not currently available for aeroallergens.
RISK ASSESSMENT
In general, risk assessment is a process designed to evaluate the potential relationship that may exist between exposure to a particular agent, e.g., aeroallergen, and a particular effect, e.g., sensitization or allergic disease. This section describes a number of issues that may be considered in conducting a risk assessment for aeroallergens and an example using data on environmental levels of dust mite allergen and the occurrence of sensitization. The discussion is not exhaustive; rather, it shows the range of issues that can be included for study when data are available or that can be included in a research design to provide data for performing a risk assessment.
Steps in a Risk Assessment
Risk assessment is a process that is composed of four basic components, or steps: (1) hazard identification, (2) exposure assessment, (3) dose-response assessment, and (4) risk characterization (NRC, 1983b). Risk assessment for noncoarcinogens is currently in the process of being formalized in the literature (Pierson et al., 1991; Shoaf, 1991). Figure 6-2 shows the elements of research and exposure assessment and their relationship to the processes of risk assessment and risk management. The four basic steps of risk assessment are described briefly below, followed by an example.
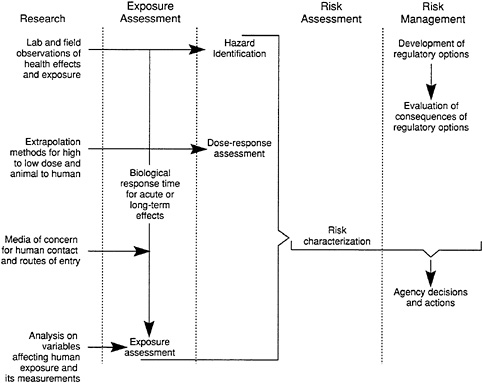
FIGURE 6-2 Elements of research and exposure assessment and their relationship to the process of risk assessment and risk management. Source: NRC, 1991.
STEP 1: HAZARD IDENTIFICATION
Important aspects of hazard identification include a complete description of the aeroallergen and its potential to affect human health adversely at some level of exposure or dose, an understanding of the number of people who are potentially exposed, the background levels of exposure in the environment, the nature of the health effect of interest, and the level of exposure thought to be associated with an increased risk of the health effect. Other exposures that potentially contribute to the development of the health outcome should also be identified.
STEP 2: EXPOSURE ASSESSMENT
The measurement of exposure to allergens is a rapidly advancing field. Instrumentation and approaches were described earlier in this chapter; this discussion summarizes some of the issues that arise in using aeroallergen exposure data for a risk assessment.
Generally, hazard identification (step 1 above) comprises the review of a number of studies from the literature. Often in those reports, more than one sampling protocol will be described, and different instrumentation or interview methods are likely to have been used. It may be difficult to synthesize data presented in different formats (e.g., range only, graphically only, mean values only). In addition, the material collected for environmental samples may be analyzed for different components. For example in the literature on dust mites, several different exposure metrics are reported, including mite counts, immunochemical assays of allergen, and guanine determinations (Platts-Mills and de Weck, 1989). Within this framework, individual assays may differ among laboratories (Lau et al., 1989; Sporik et al., 1990).
An understanding of how the exposure and thus the health effect of interest may be influenced by other factors is essential in interpreting the data. For example, seasonal variation strongly influences exposure to some allergens and resultant reporting of symptoms (O'Rourke, 1992; O'Rourke et al., 1989; Platts-Mills et al., 1987). Characteristics of the exposure site, including type of flooring, kind of ventilation system, and other such features, may affect the nature of exposures as well. The possible role of altitude in allergen exposure has also been investigated (D. Charpin et al., 1991).
Available exposure data must be summarized in spite of all of these issues and problems. In cases in which differences in technique or analysis are thought to provide incomparable results, appropriate conversion factors are often estimated.
STEP 3: DOSE-RESPONSE ASSESSMENT
The information developed in steps 1 and 2 above is used to estimate the dose from the exposure, and to model the dose-response relationship. The exposure-response relationship may be estimated when too little information is available to allow extrapolation from exposure to dose. For noncarcinogens, this effort has traditionally focused on identification of a ''threshold exposure" below which no health effects are observed (Pierson et al., 1991); this threshold is now known as the No Observed Adverse Effect Level (NOAEL; Shoaf, 1991).
The value of the NOAEL, as determined from modeling, depends on the quality of the data and the model selected. Examples of various models include linear, probability (Rose and Gerba, 1991), and exponential and beta-Poisson forms (Regli et al., 1991). Shoaf (1991) reviews the statistical considerations in their use.
Once the NOAEL has been determined, it is divided by an uncertainty factor (UF). Traditionally, a factor of 10 has been used to account for
variability in responses among a human population. When a Lowest Observed Adverse Effect Level (LOAEL) is determined, a UF of 10 is applied. Additional uncertainty in the data is accounted for by use of a modifying factor, varying from 1 to 10, to further reduce the NOAEL (Shoaf, 1991).
STEP 4: RISK CHARACTERIZATION
The relationship between dose (or exposure) and response is used to characterize the risk within a population. For aeroallergens, this relationship might be expressed as the number of new cases expected at a given exposure level or the distribution of expected change in the severity of symptoms with changing exposure levels.
Example of a Risk Assessment: Exposure to Dust Mite Allergen and Sensitization for Asthma
STEP 1: HAZARD IDENTIFICATION
When dust mites were first reported in the literature in the 1970s, it was suggested that levels of more than 500 mites/g of dust in a house were likely to produce symptoms of asthma in allergic individuals. During the 1980s, data accumulated demonstrating a dose-response relationship between reservoir concentrations of dust mite allergens at home and both sensitization and asthma (Table 6-2). Results of this kind led to the proposal of threshold levels for reservoir concentrations. Two separate thresholds have been considered: more than 2 µg of Der p I/g of dust has been associated with increased prevalence of sensitization, whereas 10 µg increases the risk of symptomatic or acute asthma. In an 11-year prospective study of 68 children, Sporik and colleagues (1990) found that exposure1 to high levels of allergen at age 1 increased the risk of asthma and was inversely related to the age of onset of asthma in atopic children. In subsequent studies of children hospitalized in the south of England, it was found that about 80 percent were both sensitized and exposed to high levels of relevant allergen at home (Sporik et al., in press). These results suggest that in areas in which all houses contain high levels of mite allergen, sensitivity to mites is a major risk factor, not only for wheezing but for hospitalization of children with asthma.
Evidence to support a quantitative relationship between exposure to mites and asthma has also been found in studies from Denmark, Australia, Germany, and France (Table 6-2). Two studies in particular strongly imply that high levels of domestic exposure can increase the prevalence of asthma.
TABLE 6-2 Evidence for Regarding Specific Levels of Dust Mite Exposure as Risk Factors for Asthma
|
Study Topics |
Location of Study |
Levels Associated with Increased Risk of Disease |
|
Mites in houses of patients with asthma |
||
|
Voorhorst et al., 1967 |
The Netherlands |
500 mites/g |
|
Korsgaard, 1983b |
Denmark |
100 mites/g |
|
Peat et al., 1987 |
Australia |
100 mites/g |
|
Dowse et al., 1985 |
Papau New Guinea |
1,000 mites/g |
|
Mite allergen levels in houses of patients with asthma |
||
|
Platts-Mills et al., 1987 |
United States |
2-10 µg of group I/g |
|
Lau et al., 1989 |
Berlin |
2 µg of Der p I/g |
|
D. Charpin et al., 1991 |
Marseilles |
2 µg of group I/g |
|
Sporik et al., 1990 |
England |
10 µg of Der p I/g |
|
Arruda et al., 1991* |
São Paulo, Brazil |
10 µg of Der p I/g |
|
Level of exposure associated with change in bronchial reactivity |
||
|
Platts-Mills et al., 1992 |
London, U.K. |
13.5 µg/g reduced to 0.2 µg/g |
|
Provisional standards for dust mite exposure levels |
||
|
Platts-Mills et al., 1992 |
|
2 µg of group I allergen/g of dust (equivalent to 10 mites/g) |
|
|
|
10 µg of group I allergen/g of dust (equivalent to 500 mites/g) |
|
* In São Paulo, all houses have high levels of mite allergen. |
||
In Papua New Guinea, the disease appeared in a group of highland villages and increased to a prevalence of about 7 percent; the previous rate had been less than or equal to 0.5 percent. This increase coincided with the introduction of blankets that the men wrapped around their heads at night. The men with asthma all tested positive on skin tests for dust mites, and dust from the blankets contained an average of 1,140 mites/g of dust (standard deviation = 868; Dowse et al., 1985).
D. Charpin and others (1991) compared children growing up in a suburb of Marseilles, which is a seaport, with those growing up in Briançon at an elevation of 6,000 feet. The average concentration of mite allergen in mattress dust from Marseilles was 15.8 µg/g, compared with 0.36 µg/g in Briançon. In parallel, the prevalence of skin sensitivity to dust mites in Briançon was 4.1 percent compared with 16.7 percent in Marseilles. Finally, the prevalence of asthma in the mountains was lower (4 percent) than that at sea level (6.7 percent).
Because there is a general misconception that levels of allergen will (or should) be higher in the houses of patients with allergic disease, it is important to understand the actual situation. Within a defined area, most houses have similar levels of allergen. For example, on the Gulf Coast, in São Paulo, Brazil, or in southern England, "all" houses have high levels of dust mite allergen; similarly, in inner-city Atlanta, Georgia, or Wilmington, Delaware, most houses have high levels of cockroach allergen. In suburban areas, about 50 percent of all houses have a cat and consequent high levels of cat allergen. In each of these areas, it is the individuals who develop IgE antibodies and have continued exposure to the relevant allergen who are at risk of developing allergy and asthma.
Two hazards exist: sensitization and allergic disease. From the data, it appears that the exposure initiating each outcome may be different. For the purposes of this example, sensitization was chosen as the endpoint.
STEP 2: EXPOSURE ASSESSMENT
Because the common allergens are thought to cause or exacerbate asthma by the inhalation route, measuring inhaled allergen might seem to be the best method for determining exposure (Price et al., 1990; Swanson et al., 1985; Tovey et al., 1981b). However, there are two reasons for using values from reservoir samples instead in this example. First, the majority of exposure data available from the literature are based on measurements of allergen in dust collected from carpets, mattresses, sofas, and other such items. Second, the quantities that become airborne apparently are quite small, commonly 5–50 ng/m3, and appear to depend on the level of physical activity in the home during the time of sampling.
This example uses data from four studies that contain both exposure information and data on the prevalence or incidence of sensitization (D. Charpin et al., 1991; Lau et al., 1989; Price et al., 1990; Sporik et al., 1990). The underlying assumption of this risk assessment is that the cumulative exposure (i.e., duration multiplied by intensity) is proportional to the sensitization concentration; this approach differs from that of previous reports where the exposure value at the time of the survey was related to the health outcome. In using the literature data presented in Table 6-3, three operational guidelines were developed a priori:
-
The exposure concentration is expressed as micrograms of Der p I per gram of collected dust. In instances in which the range is reported, the midpoint is used as the average; if the range is not given, the maximum value is estimated from the graphical display of the data and the midpoint is estimated.
-
Exposure that is reported as the total of allergens Der p I and Der f I is assumed to consist of 50 percent Der p I.
TABLE 6-3 Data for Dust Mite Risk Assessment: Example
|
Exposure concentration (µg Der p I/g dust) |
|
|
|
|
|
|
Reported |
(Estimated) |
Years of Exposure |
Cumulative Exposure (µg of Der p I/g dust/year) |
Percent Sensitized |
Reference |
|
<2 |
(1.0) |
6a |
6 |
0b |
Sporik et al., 1990 |
|
2–10 |
(6.0) |
6 |
36 |
38 |
|
|
11–50 |
(31.0) |
6 |
186 |
50 |
|
|
>50 |
(75.0)c |
6 |
450 |
78 |
|
|
<0.2d |
(0.1) |
10e |
1 |
17f |
Lau et al., 1989 |
|
>5 |
(100.0) |
10 |
1,000 |
86 |
|
|
0.2–0.5 |
(0.35)f |
10 |
3.5 |
36 |
|
|
0.5–5 |
(2.75)f |
10 |
27.5 |
67 |
|
|
8d |
8.0 |
10e |
80 |
17g |
D. Charpin et al., 1991 |
|
0.2 |
0.2 |
10 |
2 |
4 |
|
|
<0.5 |
(0.35) |
8e |
2 |
19h |
Price et al., 1990 |
|
<1 |
(0.5) |
8 |
4 |
32 |
|
|
<2 |
(1.0) |
8 |
8 |
43 |
|
|
a Taken as average age at sensitization; 40 percent are sensitized by age 5, and 60 percent are sensitized by ages 5–11 on average, 10 percent per year. Therefore, 50 percent are sensitized by age 6. b See Table 2 in Sporik et al., 1990. c See Figure 1 of Sporik et al., 1990. d Concentration of antigen is estimated. e Years taken as average age. f See Figure 3 of Lau et al., 1989. g See Table 4 of D. Charpin et al., 1991. h See Table II of Price et al., 1990. |
|||||
-
Time is expressed in years. For a population that is surveyed cross-sectionally, exposure is taken to be equal to age; the average years of exposure thus are equal to the average age. For populations that are surveyed prospectively, the age at sensitization is equal to the years of exposure; if an age is not given, years of exposure are assumed to equal the average follow-up period.
STEP 3: DOSE-RESPONSE ASSESSMENT
Exposure is estimated in this example from several different studies that use slightly different protocols for reporting the results from reservoir sampling. For example, Sporik and colleagues (1990) report the highest concentrations from samples in different parts of the house. Price and
coworkers (1990) report the value from a single, composite sample collected from several locations in the house.
Appropriate models to relate exposure information to dose are not known for these data sets, and two assumptions were made:
-
log normal distribution of the exposure variable, and
-
a linear relationship in exposure-response.
The base-10 logarithm of the estimated cumulative exposure was used in the analysis.
Figure 6-3 shows the results of the committee's analysis. The data fit an equation given by:
This can be simplified (by rounding off) to:
Using the linear assumption, neither a NOAEL nor a LOAEL is identified, because the line intercepts the y-axis at a positive value (approximately 10%). This indicates the lack of a threshold concentration below which sensitization would not occur, i.e., it is not possible to achieve zero risk of sensitation. The R2 value is 0.6, indicating that 60 percent of the variability in linear trend is explained by the model.
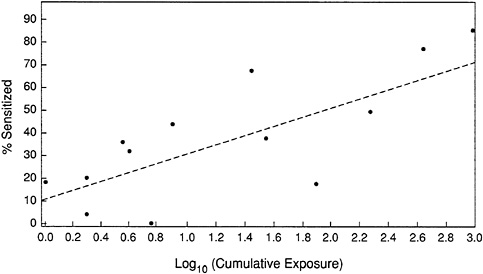
FIGURE 6-3 An exposure-response analysis of dust mite allergens.
Step 4: Risk Characterization
The equation developed from the exposure-response model can be rewritten as
(3)
or
(4)
Once the risk (percent sensitized) is set, the relationship can be solved for concentration in the reservoir, since the value of years will be known for a given population. Alternatively, if the duration and concentration are known, the risk (percent sensitized) can be estimated. For example, assuming 5 years of exposure at a concentration of 2 µg/g, the risk of sensitization would be 32 percent.
These calculations are based on data that were abstracted from reports by researchers using various methods, and must therefore be considered "soft"; the results are presented only as an example of how a risk assessment for sensitization could be performed.
CONCLUSIONS AND RECOMMENDATIONS
Some of the issues to be considered when undertaking a risk assessment for aeroallergens have been discussed and an example has been given by using data from the literature on dust mite exposure and the development of sensitization. The data can be described by a linear model and indicates that there is a positive relationship between cumulative exposure to dust mite allergen and the risk of sensitization.
Some residual sensitization (i.e., approximately 10 percent in this example) will occur irrespective of exposure to dust mite allergen according to these estimates. This finding is consistent with the knowledge that other factors may also result in sensitization. For this reason, information on cross-reactivity of allergenic agents in study subjects is desirable, and important to the analysis of potential mechanisms of sensitization.
Research Agenda Item: Determine whether a practical method could be developed to measure concentrations of dust mite allergens that are capable of sensitizing humans.
Variability in the methods used in the multiple study protocols reported to date is high. More uniformity in the collection of exposure data would be useful in the risk assessment process. Although reservoir sampling has yielded meaningful results that assist in remediating exposure, further development
of standardized air sampling collection and analytical methods is needed.
Research Agenda Item: Standardize methods of collecting and analyzing indoor allergen samples to facilitate comparative and collaborative studies.
Improved, standardized methods of collecting and analyzing indoor allergen samples would be particularly valuable in establishing the relationship between reservoir samples, personal exposure measures, levels of activity, and the potential for airborne exposure of sufficient magnitude to induce negative health outcomes.
Research Agenda Item: Quantitate the relationship of allergens in reservoirs (and on surfaces) to aerosols and develop monitoring methods for quantitating airborne-allergen concentrations in personal breathing zones.
Assessment of exposure is a rapidly advancing, complex, and multistep process that entails numerous variables and estimations. Most monitoring, for example, is often based on sampling for indicators rather than the actual allergen. There is a need for developing improved methods for estimating environmental concentrations of aeroallergens and the resultant individual exposures.
Research Agenda Item: Develop appropriate exposure metrics for specific indoor allergens that are analogous to time-weighted averages and permissible-exposure limits for industrial chemicals.
Methods for determining the effects of indoor allergens can be divided into two general categories: patient testing and environmental testing. Data from both kinds of testing can be useful to the physician in directing the treatment, control, and prevention of allergic disease. There are, however, no effective means currently available to physicians or other medical professionals for obtaining quantitative information on environmental exposures.
Recommendation: Establish effective mechanisms for medical professionals to acquire assessments of potential exposure to indoor allergens in residential environments.





















