9
Dermatological Effects of Mustard Agents and Lewisite
Probably more research has been done on the dermatological effects of sulfur mustard than any of its other effects. This chapter reflects this extensive research in its length and in the level of detail presented in certain sections. Exposure to both sulfur mustard and Lewisite causes acute injury to the skin, including redness, swelling, blisters, ulceration, and necrosis. Sulfur mustard is more effective under conditions of heat and moisture; it appears to damage the skin by disrupting cell proliferation. The arsenic in Lewisite disrupts enzyme activity.
A considerable body of evidence links acute and chronic exposure to sulfur mustard with the long-term development of pigmentary disorders and skin ulcers in humans. Evidence also links sulfur mustard with the development of cutaneous cancers and precancers in both animals and humans. There is insufficient information, however, regarding the long-term effects of Lewisite on the skin.
ANATOMY AND PHYSIOLOGY OF SKIN
The skin is the largest organ in the body, making up approximately 18 percent of the total body mass. Anatomically, the skin is divided into three layers: the epidermis, dermis, and subcutaneous fat (Figure 9-1 ). The severity of burns to the skin is classified according to how many of these layers are damaged (Figure 9-1). The skin serves to protect all of the vital organs of the body from external trauma, invasion by infectious agents, and invasion by noxious substances. It also serves to prevent outward movement of body fluids and other vital substances. Through special anatomical arrangement of the cutaneous circulation and the
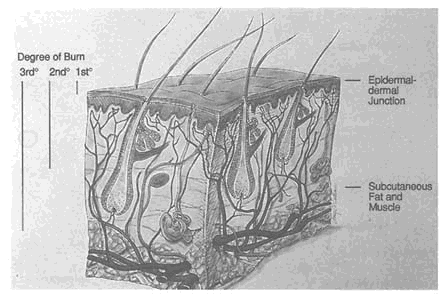
FIGURE 9-1 Anatomy of human skin showing skin layers, hair follicles, sebaceous and sweat glands. The degree of the burn is determined by the depth of damage. First degree burns involve only the epidermis, and cause edema and erythema without vesiculation. Second degree burns involve the epidermis and dermis, and usually result in blisters or dermal necrosis. Third degree burns extend into the subcutaneous fat, muscle, or bone and often cause substantial scarring. SOURCE: Reprinted from Sams and Lynch, 1990, with permission from Churchill Livingstone.
biochemical and physiological activity of the adnexal structures (eccrine sweat glands, sebaceous glands, and apocrine glands), the skin assists in the regulation of body temperature and the excretion, manufacture, and absorption of electrolytes, vitamins, nitrogenous matter, and other organic substances.
Epidermis
The epidermis occupies the outermost layer of skin and is paramount to maintenance of mammalian homeostasis. It, among the three layers of skin, offers the human body considerable protection from entry of noxious chemicals and microorganisms; it prevents uncontrolled outward movement of fluids, electrolytes, and many organic substances. Large burns due to thermal, chemical, or ultraviolet injury, if they destroy large amounts of epidermis, can lead to an enormous loss of fluid, electrolytes, proteins, and other organic materials through an
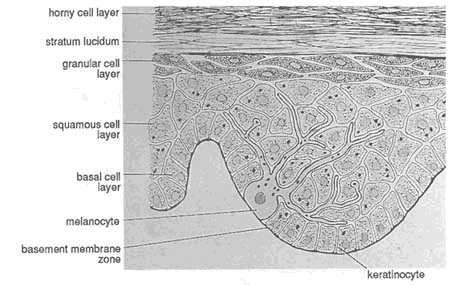
FIGURE 9-2 Layers of the epidermis. As specific cells in the basal layer replicate and differentiate, they move toward the skin surface. The major change in the cells is called ''keratinization" in which the cells become filled with a fibrous protein called keratin. Fully keratinized cells are constantly sloughed off at the skin surface. Other types of epidermal cells, such as melanocytes, have other functions. SOURCE: Reprinted from Sams and Lynch, 1990, with permission from Churchill Livingstone.
unprotected dermis. Such burns are quite painful because loss of an intact epidermis exposes cutaneous nerve endings to air, heat, cold, and other direct stimuli.
The epidermis contains several resident cell populations whose responses to certain stimuli can be protective or destructive. The Langerhans cell participates actively in recognition of and presentation of antigens. Lymphocytes respond to signals from Langerhans cells and other macrophages/monocytes and act in an appropriate fashion to antigens. Melanocytes, pigment-forming cells, protect the skin from harmful ultraviolet (UV) radiation.
The epidermis is composed of four biologically distinct layers of stratified squamous epithelium (Figure 9-2). The deepest layer, usually one cell thick, contains cells that continuously replicate and produce new cells at a rate sufficient to maintain an appropriate number of cells in the upper three layers of the epidermis. Based on location and function, this layer has been called the basal cell layer or germinative layer of the epidermis. Basal cells produce large quantities of the nucleic acids and nucleoproteins required in the process of cell division. Much
like the rapidly dividing cells of bone marrow, the intestinal tract, and hair matrix, basal cells are very sensitive to chemicals that affect nucleic acid synthesis. Sulfur mustard is one such agent.
Thickness of the epidermis varies greatly depending principally on the body site and the number of cornified cell layers within the stratum. The stratum corneum is thinnest on the scrotum, on the flexor surfaces of the forearms, within the axillae, and around the eyes. These are body sites through which sulfur mustard penetrates best and exerts its most profound effects after acute exposure. It has been estimated that the entire epidermis renews itself every 45 to 75 days. Sulfur mustard inhibits cell replication within the basal layer of the epidermis and thus disrupts this pattern, resulting in blister formation.
Basal cells of the epidermis are attached to the dermis through the basal lamina, which is often referred to as the basement membrane zone, or epidermal-dermal junction (Figure 9-3). A variety of collagen-like fibrils within basal cells, traverse and attach to the basal lamina within the dermis. Other collagen-like filaments are thought to serve as "anchoring rods" between dermis and epidermis, and dermis and basal lamina. Injury to or destruction of one or more types of anchoring structures causes separation of cells, giving rise to the formation of vesicles and blisters. It has been postulated that proteases released by sulfur mustard, acting on attachment structures between basal cells and basal lamina, give rise to blister formation (Papirmeister et al., 1991). Destruction of the epidermis followed by "shedding" exposes underlying tissue that is devoid of pigmentation and color. When exposed, the underlying tissue, or dermis, imparts a glistening "whitish" appearance to skin even in the darkest of races.
Dermis
The dermis makes up the greatest mass of human skin. It contains cells and fibers that contribute to the skin's elasticity and resiliency (elastic fibers and collagen fibers) and serves as a major force in protecting the internal organs from injury due to external mechanical forces. It is a true supporting structure for cutaneous blood vessels, nerves, and epidermal adnexal structures. Blister fluid is made up principally from fluid released from the dermis. The depth of injury to the dermis and underlying subcutaneous tissues will determine the depth and extent of skin ulceration. Injury to the upper levels of the dermis results in superficial, rapidly healing ulcers. Injury to the entire dermis results in deep, slow-healing skin ulcers.
The predominant cell type found within the dermis, the fibrocyte, is limited in distribution in the normal active dermis; so is the metaboli-
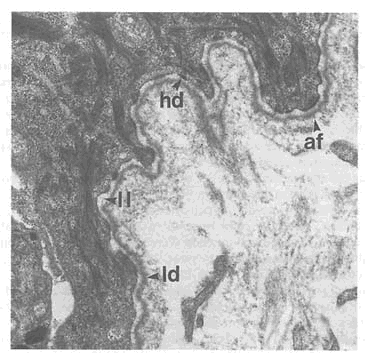
FIGURE 9-3 Epidermal-dermal junction. This electron micrograph of human skin shows the specialized attachments anchoring the epidermis to the underlying dermis. Structures include anchoring fibrils (af), hemidesmosomes (hd), lamina leucida (ll), and lamina densa (Id). Micrograph provided by Henry Bogaars, Brown University.
cally active cell, the fibroblast. Only after injury and during the process of wound healing do fibroblasts proliferate. Large numbers of lymphocytes and monocytes also accumulate in the dermis after injury. Through the production of lymphokines/monokines and other soluble proteins, lymphocytes and monocytes stimulate fibroblast and endothelial cell proliferation and migration, the first step in wound healing and ultimately scar formation. Proliferation of fibroblasts is accompanied by an accelerated production of collagen and mucoproteins and by scar formation. In the normal healing of a cutaneous wound, the accelerated production and degradation of collagen are regulated, through a process of "remodeling," and the degree of scar formation is limited. Unlimited or unrestrained wound healing results in the formation of hypertrophic scars and keloids. Wound infection, which may follow skin injury from sulfur mustard exposure, can cause continued and uncontrolled stimulation of collagen production and ultimately hypertrophic scar and keloid formation.
Eccrine Sweat Glands
Eccrine sweat glands are tube-like invaginations of the epidermis that lie within the normal dermis and are distributed over the entire human body surface. Although exact numbers appear to be related to individual and adaptive factors, there are from 2 million to 4 million glands in the skin of each human being. In an average human, the palms and soles contain the largest number of sweat glands per unit surface area; the back and buttocks contain the least. Excretion of sweat is under emotional and thermoregulatory control, except under resting conditions. Under resting conditions sweating is periodic and involves alternating groups of sweat glands. This form of sweating is invisible or inapparent, and is described as "insensible sweating."
Environmental temperatures above 31°C to 32°C provoke thermoregulatory sweating—a generalized outbreak of sweating and an increase in the number of functioning glands. Areas supplied with few sweat glands may, at this time, be more physiologically active. Thus, at high temperatures, glands of the trunk, thighs, and extremities that respond to thermal stimuli excrete large amounts of sweat. Eccrine sweat glands of the palms, soles, axillae, groin, and forehead respond maximally to emotional stimuli. Under conditions that are stressful these glands are stimulated to produce large volumes of sweat. Sulfur mustard-induced injury to the skin, under wartime conditions, is seen most often in areas that contain thermally and emotionally stimulated sweat glands.
Sweat that is excreted intermittently contains large quantities of chlorides, urea, uric acid, and ammonia. Profuse sweat contains considerably less of these substances, including sodium chloride, and is often pure water. Sulfur mustard is activated by water, yet in the presence of 5 percent sodium chloride it has a markedly reduced effect on human skin (Renshaw, 1946). The decrease in sodium chloride in profuse sweat may account for sulfur mustard's profound cutaneous effects under conditions of high temperature and high humidity. In contrast, the most profound effects of Lewisite, which is deactivated by water, occur under conditions of low temperature, low humidity, and dry skin.
Apocrine Glands
Apocrine sweat glands develop from the follicular epithelium of the pilosebaceous unit, as do the sebaceous glands. The viscous secretions of this gland differ markedly from those of the eccrine sweat gland and are emptied into the canal of hair follicles, rather than directly onto the surface of the skin. In human beings, the apocrine glands are limited in their distribution to the armpit, groin, and pubic regions, around the anus and umbilicus, in a linear band above the umbilicus, and in the
external auditory canal. Physiologically, apocrine glands perform little or no useful function. Their secretions serve as rich culture media for gram-negative bacteria. The action of bacteria on apocrine secretions is in part the cause of "offensive" body odors. Subsequent to sulfur mustard skin injury, the large numbers of gram-negative bacteria residing in apocrine areas are often responsible for secondary bacterial infections. Infection, as stated earlier, is frequently associated with hypertrophic scar formation, a common occurrence in the scrotal area of men after sulfur mustard exposure.
Melanocyte System
Skin color in humans is determined by a number of factors, the most important of which is the overall epidermal cell content of melanin. Hemoglobin, the tissue content of carotenoids, keratin, collagen, and the thickness of the keratinizing layers of the epidermis also contribute to the coloring of the skin. Yet, the total color contribution of all other factors combined does not equal that made by melanin. Ultraviolet light, heat, trauma, and a variety of topically applied chemicals can stimulate melanin production and increase skin pigmentation, usually at the site of exposure. Some systemically administered agents can cause increased generalized skin pigmentation.
Melanin is a dense, insoluble polymer derived, in part, from conversion of the amino acid tyrosine by the copper-containing enzyme tyrosinase into an alkyl-insoluble brown chromoprotein. Melanogenesis, the formation of melanin, occurs within specialized cells, called melanocytes (Figure 9-4). Each melanocyte synthesizes a specialized cytoplasmic organelle, called a melanosome, on which the hydroxylation and polymerization of tyrosine to dopa and then to melanin occur. Darkly pigmented races produce large quantities of melanin; Northern European races produce very little melanin and incompletely melanized melanosomes. Darkly pigmented races respond to minimal external and internal stimuli with sharply increased skin pigmentation.
Melanin pigmentation is of substantial benefit in skin. Intense UV light exposure can cause varying degrees of burn damage to unprotected epidermal keratinocytes, adversely affecting cell nuclei, DNA, RNA, structural and enzymatic proteins, and cell membranes. Metabolic alterations caused by UV light injury stimulate epidermal cells either to attempt self-repair or to die, depending on the degree of injury. Severe injury to the epidermal basal cells can lead to faulty cell repair, mutation, and ultimately the development of cancer. Death of epidermal basal cells causes loss of cell-to-basement membrane and cell-to-cell adhesions. Separation of cells from the basement membrane results in subepidermal blister formation. Sulfur mustard-induced blisters are subepidermal in location.
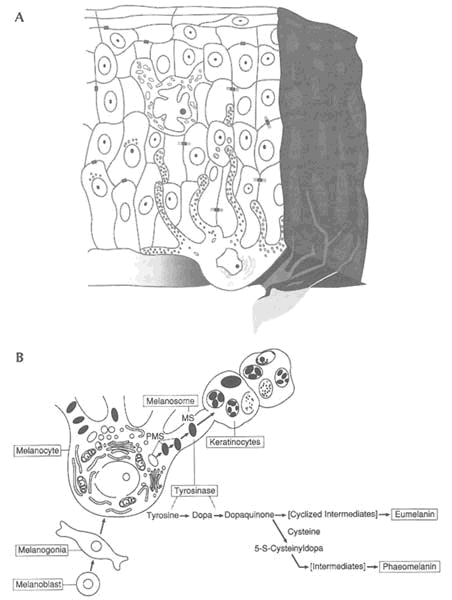
FIGURE 9-4 (A) Epidermal melanin unit. Melanocytes, located in the epidermal layer of the skin, have numerous cellular extensions. These extensions are filled with granules containing the pigment melanin. Melanin granules are "injected" into surrounding epithelial cells from the extensions. (B) Summary of major events in melanocyte development. A cascade of biochemical reactions produces melanin, which protects epidermal cells from damage from the sun. SOURCE: (A) Reprinted from Quevedo, 1969, with permission from American Zoologist. (B) Reprinted from Jimbow et al., 1976, with permission from Williams &Wilkins.
Radiomagnetic emissions from the sun, principally UV light of the A and B spectra, exert a significant influence on skin pigmentation. Polypeptide hormones from the anterior pituitary gland, especially melanocyte-stimulating hormone (MSH), also enhance melanin pigmentation. In women, estrogens stimulate an increase in melanocyte and pigmentary responses in facial, genital, and areolar skin. Chemicals such as theophylline, caffeine, cholera toxin, and prostaglandin E increase the effect of MSH on skin pigmentation. Finally, heat, inflammation, and mechanical injury also stimulate increased pigment formation, especially in the skin of darkly pigmented persons. Based on clinical descriptions of individuals exposed to toxic doses of sulfur mustard, sulfur mustard can also potentiate skin pigmentation. Topical nitrogen mustard, when applied to the skin in the treatment of psoriasis and cutaneous T-cell lymphoma, causes increased skin pigmentation through mechanisms that are as yet unknown.
Injury to the skin of sufficient intensity to cause destruction of melanocytes will result in skin that is devoid of pigmentation. White patches of skin will be noted in even the darkest of pigmented races (leukoderma). Thus, skin that has been subjected to injuries with locally varying intensities, such as after sulfur mustard and Lewisite exposure, will characteristically show areas of depigmentation alternating with areas of hyperpigmentation. In fact, over time the process of healing reveals significant changes in the patterns of pigmentation.
Following skin injury, epithelial cells surrounding the external orifices of the hair follicle and other adnexa proliferate and migrate outward from their source to repopulate skin devoid of epithelium. Epithelial cells from surrounding normal skin also contribute to the process of repair in skin devoid of epithelium. Melanocytes surrounding uninjured hair follicles are stimulated to replicate and increase the production of melanin. Regenerated epithelial cells surrounding the orifices of the hair follicles, then, are the first cells to receive new pigment. The clinical picture of melanocytes repopulating skin is referred to as having a "salt and pepper" appearance. The salt and pepper appearance of skin after sulfur mustard exposure is often written about with some degree of bewilderment. Yet, this is a process that occurs commonly after mechanical and chemical injury to skin.
As melanocytes grow and repopulate normal skin, there is a tendency for overmelanization of any given area. Overmelanized skin at the edges of a healing wound is characteristically darker than skin distal to the healing site. As healing progresses, such skin will eventually return to its normal color and appearance. Normalization of this process often takes 6 to 12 months. The inherent skin color of the affected individual usually determines the amount of time required to return to a normal state.
In many ways, acute and chronic sulfur mustard skin injury mimics
injury caused by a variety of toxic chemicals, and mechanical devices. Unsophisticated and untrained observations of sulfur mustard skin injury have often led to distorted accounts of such injuries. Any interpretations of published and unpublished data should be made based on a knowledge of normal and abnormal morphologic, biochemical, and physiological responses of normal and injured human skin.
ACUTE EFFECTS AND BIOLOGICAL MECHANISMS
Sulfur Mustard
Sulfur mustard is an oily substance that is freely soluble in animal oils, fats, and organic solvents (lipophilic). It is only slightly soluble in water, yet water is required for activation. When delivered as a liquid or vapor, the skin plays a very important role as a portal of entry for sulfur mustard. The lipophilic nature of sulfur mustard and the affinity of skin for lipophilic substances make the skin a fairly good transport system for this agent. After cutaneous exposure to sulfur mustard, high levels appear immediately, but transiently, within the skin. A portion of a given dose passes rapidly from the skin into the bloodstream to elicit toxicity at distant sites. However, even under the most ideal circumstances, only a very small portion, probably only 20 percent, of a single dose of sulfur mustard penetrates human skin (Cullumbine, 1947; Renshaw, 1946). Of this amount, about 12 percent reacts with components in the skin, principally within the epidermis. The remainder (about 8 percent) is absorbed systemically. At a temperature of 21°C, sulfur mustard rapidly penetrates human skin. Renshaw (1946) noted that sulfur mustard liquid or saturated vapor penetrates human skin at a rate of 1 to 4 mg/cm2/min at 21°C. Any increase in ambient temperature causes increased penetration.
There is substantial individual variation in the cutaneous response to sulfur mustard. In general, however, the effects of sulfur mustard on the skin depend on a number of factors including the dose of drug delivered, delivery medium (vapor or liquid), length of exposure of skin cells, degree of hydration of the skin, temperature of the atmosphere, thickness and surface area of the exposed skin, presence or absence of infection, and the intactness of exposed skin. Large dosages of sulfur mustard vapor delivered at 1,000-10,000 mg·min/m 3 (Ct), or liquid at 40-100 mg/cm2 over a long exposure time, will yield significant systemic toxicity, including death. Small vapor dosages at 50 Ct, or liquid at 10-20 mg/cm² and a short exposure time, yield limited local toxicity. Local toxicity is manifest not only in the skin, but also in the eye and mucous membrane of the respiratory tract.
The time of onset of visible cutaneous effects is related to dose and
method of delivery. Microscopically, cutaneous effects begin to appear almost immediately after sulfur mustard contact with skin. Large dosages yield an immediate and profound effect in 1 to 2 hours. Necrosis of the skin following the delivery of large vapor dosages does not appear instantaneously, usually occurring after variable periods of latency. Total necrosis of the skin may occur. Small vapor dosages yield delayed skin effects that may occur 7 to 14 days after exposure. Interestingly, the timing of the onset of sulfur mustard cutaneous reactions is not unlike that observed in cutaneous reactions associated with common Rhus (poison ivy oleoresin) dermatitis.
The precise mechanism whereby increased humidity or increased moisture on the skin potentiates sulfur mustard effect is unknown. However, it is possible to assume that wetting the skin alters the permeability of skin cells, thereby increasing the ability of sulfur mustard to penetrate to metabolically active layers; 5 percent, but not 4 percent, sodium chloride-containing water reduces sulfur mustard effects on the skin. It is postulated that 5 percent sodium chloride decreases the solubility of sulfur mustard in water, decreases the overall rate at which sulfur mustard molecules become activated, or alters sulfur mustard penetrability through skin (Renshaw, 1947).
The mechanism whereby an increase in environmental temperature increases the adverse effect of sulfur mustard is also unknown. Increased environmental temperature may simply increase body temperature, stimulating eccrine sweat gland activity and a concomitant increase in hydration of the skin. It is also possible that profuse sweating and a concomitant increase in the amount of pure water on the skin cause activation of greater quantities of sulfur mustard at the site. Blistering of the skin by exposure to UV light is also enhanced by increased temperature and humidity.
The site of skin exposure and the thickness of skin may often determine the type of cutaneous responses experienced upon exposure to sulfur mustard. Thick skin is purported to be less affected by the irritant effects of sulfur mustard. Likewise, young skin, thought to be morphologically thinner than old skin, and female skin, thought to be thinner and more delicate than male skin, are suspected of reacting more severely at all sites upon a given exposure to sulfur mustard (Mathias, 1987; Renshaw, 1946). Racial factors and skin color appear to have even less well defined relationships. It has been generally accepted that black skin reacts in a different manner than white skin to contact allergens and irritants. Yet there are no good experimental data to support the concept that there are substantial differences in the cutaneous response of black or white skin to antigen and injury. Weigand and colleagues (1974) described a difference between black and white skin in the number of cell layers in the stratum corneum, but these differences
are no greater than those variations seen in the different body regions of a single individual. According to Nagy and colleagues (1946), there is no difference in the rate of sulfur mustard penetration between the skin of whites and blacks. It is suggested that well-designed contemporary studies are needed to better define the resistance or susceptibility of specific skin thicknesses and types to injury.
Clinical and Microscopic Observations
The most useful clinical descriptions of acute sulfur mustard effects on human skin have been reported by Momeni and colleagues (1992), Smith and Dunn (1991), and a group of clinicians at the Hospital of the University of Ghent Medical School, Belgium (Willems, 1989). These reports were based principally on the examination of patients and patient records of individuals exposed to sulfur mustard during the Iran-Iraq conflict of 1980-1988.
Gross morphologic changes in the skin induced by sulfur mustard are characterized by the appearance of an intense period of itching followed by erythema and edema (signs of inflammation), as well as blister (vesicle) formation, denudation of skin, ulceration, and necrosis. Immediate color changes are often described in affected skin and occur in response to stimulation of melanogenesis, probably an effect akin to the "immediate darkening" effect seen after a specific type of acute UV exposure, and from the darkening effect of "cooked" protein within epidermal cells. Increased darkening of the skin from increased melanogenesis at the periphery of mustard-induced blisters is also characteristically observed. Peripheral darkening is often associated with larger areas of hyperpigmented skin that occur at sites where erythema and edema without previous blister formation had existed. Exfoliation and deep ulceration may occur at these sites. Tissue dosages of sulfur mustard vapor required to induce erythema vary between 0.1 and 1 mg/cm2. Vesication can be expected in 50 percent of a population at tissue dosages of 1-2 mg/cm2.
It should be remembered that the site of exposure may be associated with variations in the skin's response to the same amount and extent of sulfur mustard exposure. At the same dosage and time of exposure, loose tissue (less compact dermis) as seen on the face, especially around the eye, and on the genitalia may respond with edema without blistering. However, tissue sites having a very dense dermis, as on the back, may respond with erythema and blister formation without edema.
Healing of the skin is variable and, in the absence of secondary bacterial infection, may proceed without residual defects. The minimally injured hair follicles and other adnexal structures contribute greatly to the healing wound. Tissue reepithelialization often begins and spreads
from these sites, as well as from epithelium at the periphery of the injured site. Time for healing varies depending on the degree of tissue injury, residual skin necrosis, and presence or absence of infection.
Scar formation following sulfur mustard injury in specific anatomical areas may be profound and disabling. As has been stated, the genital regions are especially susceptible to sulfur mustard injury. Severe scarring of scrotal and penile tissue can cause deformity and impair sexual performance.
Microscopically, the epidermis and dermis respond adversely to the irritant effects of sulfur mustard. The National Defense Research Committee group at Harvard made an extensive histological study of a large series of experimental sulfur mustard burns of varying severity in human subjects (Renshaw, 1946). Within the epidermis, and within three hours after sulfur mustard dosages that produce erythema, only a few scattered basal cells show nuclear changes consisting of swelling, loss of chromatin, and dispersion of chromatin to the nuclear periphery, clear vesiculation, vacuolization of the cytoplasm around the nucleus, and in some cells vacuolar or hydropic degeneration of the cytoplasm and pyknosis of nuclei. Later, disintegration of the cytoplasmic membrane of basal cells becomes prominent. In some areas, but more often in more severely damaged skin, these degenerative changes may be seen throughout the basal layer of the epidermis.
Ultrastructural studies of human skin have been supplemented with studies of mustard-exposed human skin grafts on athymic nude mice. Ultrastructurally, the type of cellular injury seen in human skin does not appear to differ from that observed subsequent to a wide variety of toxic insults that lead ultimately to epithelial cell degeneration. The sequence of events begins within basal keratinocytes and always within the cell nucleus. Extensive condensation and margination of heterochromatins and loss of euchromatins are followed by blebbing of the nuclear membrane (blebbing of the nuclear membrane is also evident on light microscopy at this stage). Cell lysis begins with the formation of paranuclear vacuoles, swelling of rough and smooth surface endoplasmic reticulum, dissociation of free rosettes of polyribosomes, loss of mitochondrial structure, cytoplasmic vacuolization, and eventual disruption of the plasma membrane.
These changes are probably confined principally to basal cells of the epidermis, because basal cells are the most active metabolic cells, actively and continuously synthesizing nucleic acids and nucleoproteins that are vital for cell growth and division. Antineoplastic agents such as sulfur mustard and nitrogen mustard derivatives exert their most prominent cytotoxic effects on cells that are actively producing large quantities of nucleic acids and nucleoproteins in preparation for cell division. Cross-linking of DNA is one of the most important cytopathic
effects caused by sulfur mustard and other alkylating agents (Wheeler, 1967). Cells whose repair systems are overwhelmed by large concentrations of alkylating agents and are unable to sustain effective nucleic acid repair ultimately succumb to cell injury and then death.
Vesiculating and necrotizing dosages of sulfur mustard produce microscopic effects similar to erythemogenic dosages, differing only in the extent of injury to the epithelium. Basal cell degeneration is more widespread, and liquefaction necrosis involves multiple neighboring cells rather than isolated foci. Initially limited dermal-epidermal separation followed by extensive separation causes formation of microscopic then macroscopic vesicles. Gross blistering then becomes obvious.
Although residing within the dermis, adnexal structures such as hair follicles, sebaceous glands, and eccrine glands are morphologic derivatives of epidermis. Therefore, tissue injury within these structures occurs in a manner similar to that seen in normal epidermis. Tissue injury is considerably less within the epidermis of hair follicles; sweat glands show only minor defects, as do sebaceous glands.
In some situations, necrotic degeneration of the entire epidermis has been described following blister formation and after the delivery of large dosages of sulfur mustard. Such an occurrence is to be expected after complete degeneration of the basal cell layer has taken place. An intact basal cell layer is important to survival of the entire epidermis. Cells above the level of the basal cell layer are engaged in the process of differentiation and are not programmed for survival, as are normal basal cells. The end product of the process of differentiation is a nonviable, completely cornified cell. Unlike Lewisite, sulfur mustard has been shown to be relatively inactive in interfering with protein and enzyme synthesis. The action of sulfur mustard on protein and enzymes required in the formation of keratin protein does not appear to be effective enough to cause injury to differentiating epithelial cells. This assumption, however, does not rule out an overwhelming dosage of sulfur mustard acting as individual cell poisons in replicating and differentiating epithelial cells.
Factors responsible for damage to the underlying dermis after injury induced by sulfur mustard are not totally understood. Unlike lower mammalian species, dermal injury in human skin is not often as extensive as epidermal (Renshaw, 1946). During the early erythema and edema stages of skin injury, dilatation of papillary dermal capillaries, thickening of the capillary wall, and endothelial cell swelling are noted. Dermal cellular infiltrates are sparse, accumulating principally in a perivascular location. Lymphocytes predominate early, followed by an invasion of polymorphonuclear leukocytes. Perivascular edema is fairly prominent.
As injury progresses and vesiculation occurs, capillaries beneath
vesicles show signs of necrosis. Capillary thrombosis and disruption of capillary wall integrity do not occur until there is evidence of dermal necrosis. Inflammation is increased in the presence of vesiculation and, in fact, may become quite extensive throughout the papillary and reticular dermis. Mononuclear and polymorphonuclear leukocytes appear equally prominent in the presence of epidermal and dermal necrosis. Langerhans cells appear to migrate from the epidermis and collect in clusters at the dermal-epidermal junction.
Fibroblasts, cells that are principally engaged in collagen formation and are basically resting cells in the absence of tissue injury, appear to experience some injury even during the erythematous stage. Pyknosis of the nuclei of fibroblasts becomes prominent and increases as local tissues enter the stage of epidermal and dermal necrosis. Collagen bundles appear not to be affected by injury, retaining a fairly normal morphologic appearance through, and up to, the appearance of blisters.
Mechanisms of Skin Injury
Our ability to explain or understand the mechanisms that lead to sulfur mustard injury in human skin has been encumbered by the lack of an appropriate animal model system. There are no animals in which it has been possible to reproduce, in its entirety, the effects of sulfur mustard on human skin (Mitcheltree et al., 1989; Renshaw, 1946). Numerous investigators using rabbits, pigs, cows, rats, mice, and guinea pigs, both normal and hairless varieties, have made attempts to replicate sulfur mustard human skin injury. Some gross and microscopic similarities to human skin responses have been noted in a few animal species, but for as-yet-undiscovered anatomical and physiological reasons human and animal injury differ to a significant degree. Sulfur mustard penetration of mammalian skin, other than human, occurs rapidly and to fairly deep levels. For a given dosage, higher dermal concentrations are achieved in nonhuman mammalian skin, and hence more profound tissue damage is noted in the dermis rather than epidermis. Injuries to animal skin develop and heal more rapidly than injuries to human skin, despite the same degree and severity of injury. Microblisters rather than macroblisters characteristically appear in the skin of all laboratory species tested. This response seems to occur at all effective dosage levels. The lack of a suitable animal model has also severely limited research directed toward the development of potential prophylactic or treatment agents.
To date, in vitro studies using primary cultures of newborn rat epidermal keratinocytes, or human skin grafts placed on athymic nude mice, have provided the largest body of useful information relative to sulfur mustard toxicity in skin (Papirmeister et al., 1991). These studies
have uncovered a number of clues to the possible mechanism of sulfur mustard skin toxicity. Based on data reported from these systems two postulates have emerged to explain the pathogenesis of sulfur mustard skin toxicity.
DNA Alkylation and Protein Activation. Using cultures of primary epidermal human keratinocytes, Papirmeister and coworkers (1991) have uncovered evidence that alkylation of DNA, reduced tissue levels of oxidized nicotinamide adenine dinucleotide (NAD+), and activation of cellular proteases may account for sulfur mustard-induced blister formation in human skin. According to the authors, the key reaction begins with the intramolecular cyclization of sulfur mustard to form an electrophilic ethylene episulfonium intermediate, which, in the presence of water, alkylates a number of tissue macromolecules including DNA, RNA, and protein. Alkylation of DNA ultimately results in single-strand breaks within basal cells of the epidermis, which in turn trigger activation of DNA repair enzymes, particularly poly-(adenosine diphosphateribose) polymerase (PADPRP). Excessive PADPRP activity causes inhibition of glycolytic and respiratory enzymes, impaired glucose uptake, and depleted stores of oxidized NAD+ . NAD+ depletion results in inhibition of glycolysis, buildup of glucose-6-phosphate (a substrate in the hexose monophase shunt), and, ultimately, activation of cellular proteases. Proteases released from epidermal cells are thought to cause disruption of dermal-epidermal attachments and resultant blister formation.
In studies using cultured human epidermal cells, Martens (1991) has accumulated some preliminary data to show that after exposure to 0.3 mM sulfur mustard, glucose utilization was 50 percent inhibited, and NAD+ content within cells decreased to 50-60 percent of control. The onset of sulfur mustard-induced NAD+ depletion preceded the inhibition of glycolysis. Martens states that his data support the Papirmeister group's postulated mechanism of sulfur mustard damage to human skin. Smith and Dunn (1991) have stated also that studies at their institute are consistent with the Papirmeister proposal. They add, however that ''the process would appear to require an active inflammatory response and altered fluid dynamics in the affected tissue to generate . . . blisters."
DNA Cross-Linking. A simpler postulate is advanced by Bernstein and colleagues (1985, 1987). Using rat keratinocytes cultivated on nylon membranes, their experiments—in which low-dose sulfur mustard (0.01-0.5 nmol/cm²), caused significant inhibition of 3H
thymidine uptake—show that DNA is the most important target of sulfur mustard. Uptake of 3H uridine (RNA synthesis) and 14C leucine (protein synthesis) was not affected by low-dose sulfur mustard in this system. Concentrations on the order of 10-500 nM/cm² were required to inhibit 3H uridine and 14C leucine incorporation. Bernstein proposes that alkylation of nucleophilic residues of macromolecules creates interstrand and intrastrand cross-linking. Synthesis of DNA in particular but also RNA and protein is inhibited, thereby blocking cells at the interface of G2/M phases of the cell cycle. Disruption of cell proliferation in the germinative cell population of the skin occurs, eventually death of the affected cells takes place, and separation of the epidermis from the dermis causes blister formation. Yet to be explained in this postulate are the ultimate fate of alkylated DNA and how alkylation is related to cell death and vesication.
Other investigators (Crathorn and Roberts, 1966; Lawley and Brookes, 1965) have found that cytotoxicity of sulfur mustard was not associated with inhibition of RNA or protein synthesis in a certain type of tumor cell (Hela) and in the bacteria Escherichia coli, respectively, and that disturbance of growth was specifically associated with inhibition of DNA synthesis. Their data may be advanced in support of Bernstein's theory of blister formation.
That sulfur mustard does indeed induce cross-links in DNA has been shown by Sorscher and Conolly (1989), using primary cultures of newborn rat epidermal cells. DNA cross-linking in sulfur mustard-exposed cells was seen at 5 mm sulfur mustard, but not at a lower dose of 2.5 mm sulfur mustard. Increased cross-linking was not observed in cultures treated with 10 and 20 mm sulfur mustard.
Lewisite
Lewisite is a lipophilic substance; therefore, absorption through the skin is a primary route of entry into the body. Percutaneous absorption may be associated with systemic toxicity, manifested by pulmonary edema, diarrhea, agitation, weakness, hypothermia, and hypotension. Systemic toxicity secondary to Lewisite exposure occurs more rapidly, and toxicity is more severe, than after exposure to sulfur mustard.
Cutaneous reactions after exposure to Lewisite vary depending on the atmospheric temperature, relative humidity, presence or absence of water, physical state of the agent (vapor or liquid), and concentration and duration of agent delivered to the skin. The maximum effect of Lewisite on skin takes place under conditions of low temperature, low humidity, and dry nonalkaline terrain (Gates et al., 1946).
Under standard conditions of temperature and humidity, Lewisite
vapor (12 mg/m3 or less) produces erythema of the skin. Higher concentrations (1,000 mg·min/m3) under conditions that yield hot, dry skin may induce small, shallow, turbid vesicles that coalesce to form larger blisters (Goldman and Dacre, 1989). Liquid Lewisite (0.2 mg/cm2) on the skin produces an immediate (10-12 seconds) stinging and burning sensation at the site of application (Davis, 1944). This is followed within 5-15 minutes by a cooked-skin appearance, characteristically dull dead-white or grayish skin similar to that seen after an acid burn. Shortly thereafter, erythema develops around the contaminated site. The central region of the burn becomes urticaria-like and lemon in color. Later, puckering of the skin occurs around adnexal orifices to give the appearance of "tanned pigskin." Six to eight hours later, pinpoint vesiculation appears, shortly replaced by large bullae that cover the entire erythematous area. Characteristically there is a very sharp line of demarcation between Lewisite-burned skin and normal skin, making it fairly simple to distinguish between the burn of Lewisite and sulfur mustard. Delivery of a large quantity of Lewisite to intact skin over a prolonged period can result in deep penetration of agent through subcutaneous tissue into muscle, with attendant edema and necrosis.
Although Lewisite injuries are often described as healing at a more rapid rate than those due to sulfur mustard, Davis (1944) states that "it has been thought by some observers to heal more rapidly than mustard gas burns, but I cannot assent to this opinion." Davis also notes that residual pigmentation in small Lewisite burns is not as characteristic as in sulfur mustard burns. Rather, Lewisite is more prone to leave residual atrophic scarring.
Lewisite lethality in man when delivered via skin is also related to physical state on delivery. According to data presented to this committee by Colonel Richard Solana of the U.S. Army Medical Research Institute of Chemical Defense (Appendix A), the LD50 for humans is estimated to be 40 mg/kg for liquid Lewisite, and 100,000 mg·min/m 3 for Lewisite vapor. The LD50 often quoted for liquid Lewisite is considered low by many investigators (Goldman and Dacre, 1989).
Less is known about Lewisite than sulfur mustard penetration through human skin. Axelrod and Hamilton (1947), in studies using 10 microcuries of radioactive arsenic (74As) per milligram of Lewisite, applied 475 µg of Lewisite to the skin of humans for 10 and 15 minutes. During each experiment 0.43 cm2 of skin was exposed, and biopsy specimens were taken 24 hours after exposure. Radioautographs of exposed tissue showed Lewisite to be confined principally to the epidermis; very little was found in the dermis, mostly around blood vessels and in some but not all hair follicles. Radioactivity within the epidermis was confined to dead epithelial cells. There appeared to exist massive necrosis of most of the involved epidermis. In similar experi-
ments using pigskin, labeled Lewisite was deposited primarily in the hair and hair follicle, with a small amount within the epidermis. Ferguson and Silver (1947) described similar experiments using the skin of guinea pigs: Lewisite could be found within the epidermis in 2 minutes and the dermis within 10; it remained concentrated within the dermis for about 30 minutes and then began to disappear; only traces were detectable in the skin after 24 hours.
The histopathologic changes in skin after Lewisite exposure have been described by Davis (1944), Cameron and colleagues (1946), and McGown and colleagues (1985, 1987). Unlike sulfur mustard exposure, Lewisite causes early and complete necrosis of the epidermis in humans. The necrotic process also involves the dermis where it is principally vascular in location. Capillary degeneration and perivascular leukocyte infiltration accompany Lewisite vesiculation. Feister and colleagues (1989) state that there is evidence to show that, like vesication after sulfur mustard exposure, vesication subsequent to Lewisite injury occurs within the lamina lucida. However, it is not clear which anatomical structures are disrupted to cause epidermal-dermal separation. Studies in the human skin-grafted nude mouse system suggest that epidermal-dermal necrosis precedes epidermal-dermal separation.
It is assumed that upon entry of Lewisite into the aqueous medium of the intact skin it is rapidly hydrolyzed to a stable, water-soluble, but highly toxic derivative 2-chlorovinylarsine oxide (Lewisite oxide) and hydrochloric acid. Feister and colleagues (1989) postulate that Lewisite oxide may be the principal metabolite and major cytotoxic form of Lewisite within tissues. It is also believed that the trivalent form of arsenic, which is highly reactive in biological systems, is responsible for the overt toxicity of all arsenicals, including Lewisite, to living systems. Trivalent arsenicals exert their toxic effects through interactions with active tissue sulfhydryl groups (Peters, 1955; Peters et al., 1946; Squibb and Fowler, 1983). Trivalent arsenicals interact directly with protein sulfhydryl or thiol (sulfhydryl attached to a carbon) groups.
Because a vast array of critical enzymes contain thiol groups that interact with arsenicals, the end result of this interaction is enzyme inactivity. This ability of arsenicals to inhibit tissue enzyme activity in a variety of animal systems has made them valuable tools in the study of the biochemistry of specific enzymes, their mechanisms of action, and sites of action. A body of work, beginning with the separate investigations of R.A. Peters and C. Voegtlin in the 1920s, supports the concept that cell death from Lewisite results from the inhibition of the pyruvate-dehydrogenase complex, causing energy depletion within the cell (Peters, 1955; also see Feister et al., 1989). Numerous studies have shown that addition of arsenic to isolated mitochondria produces an inhibition of cellular respiration, the oxidation of tricar-
boxylic acid cycle substrates, and oxidative phosphorylation (Squibb and Fowler, 1983). An alternative and as yet unproven theory of Lewisite toxicity postulates inhibition of glycolysis secondary to arsenical inhibition of the hexokinase enzyme. The resultant inhibition of each of the subsequent biochemical steps in energy metabolism leads to energy depletion and cell death.
EVIDENCE OF LONG-TERM HEALTH EFFECTS OF MUSTARD AGENTS
Animal Studies and Cellular Bioassays
Animal studies of sulfur mustard effect on skin have been directed principally at defining a role for this agent in the process of carcinogenesis (Fox and Scott, 1980; Heston, 1953; McNamara et al., 1975). Such studies have been performed in a variety of animals including dogs, guinea pigs, rabbits, rats, and mice, as described in Chapter 6. Although several routes of administration have been used, subcutaneous injection of fairly large dosages of sulfur mustard was the most successful route producing cutaneous papillomas and sarcomas. Long-term exposure to sulfur mustard vapor also produced squamous cell and basal cell cancers in rats. These experiments, although crude, suggest that similar acute and chronic exposure in humans may be carcinogenic.
Human Studies
The persistence of lesions following sulfur mustard exposure is directly related to the duration and severity of the exposure. Secondary infection will often influence the process of healing and, in turn, may influence the eventual outcome of skin injury. Injury that results in erythema and edema without vesicle formation is almost always followed by complete healing and no residual cutaneous defects. Sites characterized by early and diffuse hyperpigmentation are exceptions. In such sites, erythema may be followed by exfoliation and skin necrosis. Blistering wounds and necrotic wounds, which characteristically leave large areas of skin devoid of protective epithelium, melanocyte, and intact adnexal structures, are often followed by permanent residual skin defects. Skin damage is intensified in the presence of secondary bacterial infection of unepithelialized skin. The effect of infection is intensified by the action of systemically absorbed sulfur mustard on bone marrow. Intense skin exposure sufficient to cause severe vesiculation and skin necrosis is almost always associated with systemic toxicity. Destroyed or diminished bone marrow activity denotes reduced numbers of or destruction of replicating marrow stem cells (also see Chapter 10).
Reduced granulocyte and other marrow-derived cells in the peripheral blood cause a diminished protective effect from polymorphonuclear leukocytes, macrophages, monocytes, and other cell types that are active in the destruction and scavenging of organisms that invade and impede healing of wounds.
Residual cutaneous lesions most often take the form of scars that result from uncontrolled fibroblastic activity and overgrowth of connective tissue during the process of wound repair. Even well cared for wounds over body sites and parts that are not easily immobilized, such as shoulders, knees, elbows, and male genitalia, often heal with severe residual scar formation. Pigmentation is often altered (either increased or decreased) at these sites, although the degree of alteration does not differ from that observed in injuries caused by burns and other forms of physical and chemical insult. In the absence of melanocyte destruction, hyperpigmentation will predominate. If melanocytes are locally destroyed, and inward migration from destroyed adnexal structures does not occur, depigmentation will predominate. Some previously injured sites have been described as "sensitive" to subsequent mechanical injury. These sites may show recurrent blisters after mild injury.
Skin tumors (basal cell, squamous cell, and Bowen's intraepidermal squamous cell cancer) and rapidly spreading skin ulcers that are resistant to therapy have been reported (Inada et al., 1978; Jackson and Adams, 1973; Klehr, 1984; Wada et al., 1963). To date, the number of cutaneous cancers reported subsequent to acute and chronic sulfur mustard exposure is low, and it is unclear whether some of these cutaneous cancers are related to the carcinogenic effects of sulfur mustard or are related to the presence of chronic skin ulcers (Jackson and Adams, 1973). The occurrence of skin cancers at the site of old scar formation is an acknowledged biological phenomenon (Novick et al., 1977; Treves and Pack, 1930). It appears that cutaneous cancers following acute sulfur mustard exposure usually localize in cutaneous scars, whereas those following chronic exposure can occur on any exposed site (Inada et al., 1978). Many questions remain unanswered in sulfur mustard human carcinogenicity. Some subjects who develop cutaneous cancers after chronic sulfur mustard exposure, particularly Bowen's disease, have had exposure to multiple potential cancer-causing agents, including Lewisite (Inada et al., 1978; Wada et al., 1963). Yet, Kravitz and McDonald (1978) have reported cutaneous cancer induction following chronic topical application of nitrogen mustard in the treatment of cutaneous T-cell lymphoma.
Occupational Exposure
Long-term effects of sulfur mustard exposure have been most frequently described in people previously employed in the manufacture of
mustard gas (Büscher, 1932; Easton et al., 1988; Inada et al., 1978; Klehr, 1984). Pigmentary disorders, skin ulcers, and cutaneous cancers and precancers have been the most common entities described. Klehr described a group of World War II (WWII) German mustard gas workers as having multiple skin tumors, even in unexposed skin, and numerous painful ulcerations that tend to spread. Klehr's report is principally descriptive and is without valid comparative data or control populations, as are most other reports of occupational diseases associated with sulfur mustard exposure. Of 53 workers remaining alive and examined, 34 percent experienced multiple skin tumors, and 45 percent experienced arterial vascular ulcers of the lower extremities. Klehr's clinical description of the kinds of ulcers seen gives the impression that they are not unlike leg ulcers commonly found in individuals with chronic arterial and venous disease. The lack of a control population and the very general descriptive and retrospective nature of this report make it difficult to place value on the content.
Wada and colleagues (1962, 1963) and Inada and colleagues (1978) describe findings in a group of former workers exposed for variable periods to a variety of "war gases" manufactured on Okuno-jima island in Japan (see Chapter 6). Of 488 workers, 115 showed pigmentary skin changes consisting of hyperpigmented and depigmented raindrop spots, mostly on covered skin of the trunk and extremities. Another 22 cases with Bowen's disease, basal cell carcinoma, and other hyperkeratotic skin lesions were described. Of 5 cases extensively described, the average time between initial exposure to sulfur mustard and the development of tumors varied between 31 and 46 years. The number of years worked in the facility varied from 3 to just over 15 years. Most workers in this facility wore protective clothing, which was described as "defective and ill-fitting" most of the time: numerous instance of skin burns, blisters, and other cutaneous injuries were reported. Although an adequate control population was not simultaneously studied, 77 workers who were engaged in clerical and guard duty at the same facility, did not develop evidence of long- or short-term defects.
The principal drawback to assigning a cause and effect relationship solely to sulfur mustard exposure at this facility may be found in the background comments: before 1937, workers whose tasks were limited to the production of "war gases" worked in the manufacture of gases of all types, including mustard gas and Lewisite (Wada et al., 1962). Also missing from these studies is a statistical comparison of the number of cases of Bowen's disease one could expect to find in a comparable population of nonexposed Japanese. In a nationwide 5-year study of skin cancer, the overall incidence of skin cancer among 851,685 new patient visits to dermatologic clinics at major Japanese universities was
0.10 percent (Miyaji, 1963). Comparable figures have been reported by other Japanese authors (Kitamura, 1954), although a study in Hiroshima, the site of a Japanese pre-WWII war gas factory, reported an incidence of 0.16 percent (Hosokawa, 1961). When the geographical distribution of skin cancers in Japan was examined, there was a greater incidence in southern and western Japan (including the prefecturate of Hiroshima), areas where the largest amount of annual sunlight is seen (Miyaji, 1963).
In a report on the British occupational experience, Easton and colleagues (1988) looked at mortality data from a World War II mustard gas manufacturing site in Cheshire, England. The observed number of deaths from skin cancer was zero, versus the expected number of two. The implication from this study is that there is a low or nonexistent death rate from skin cancer in this cohort of individuals, and certainly the death rate is lower than generally expected in a group of exposed workers. Indeed, the incidence of all expected diseases and deaths has been less in the British workers than in workers from other nations, a difference attributed to better worker protection measures in British war gas factories.
Battlefield Exposure
To date, there has been only a single report describing delayed toxic effects of sulfur mustard exposure during battlefield operations. Balali (1986), in a prospective study of delayed toxic effects, has followed a cohort of Iranian solders exposed to mustard gas during the Iran-Iraq war. After two years of observation, 41 percent of the exposed victims are experiencing pigmentary disorders. No other abnormalities have as yet surfaced.
Medical Therapeutic Exposure
For a number of years, Russian and Eastern European physicians have studied the effects of a topical preparation containing sulfur mustard 0.005 percent in petrolatum (psoriasin) on a hyperproliferative disease of the skin, psoriasis. The delivery of therapeutic dosages requires about 0.01 µg psoriasin/cm2 of skin. This amount results in inhibition of DNA synthesis sufficient to reduce basal cell replication, causing a return of the bulk of cells back to a state comparable to normal, yet the cells' ability to repair DNA cross-linking is not impaired. This dosage level is 10-100 times lower than that required to cause erythema in normal skin (Renshaw, 1946). Short-term (15 days) observation of patients treated with psoriasin reveals cutaneous hyperpigmentation like that seen after the application of nitrogen mustard to the skin,
stimulation of hair growth, and cutaneous sensitization (Turanow et al., 1977). Long-term effects are yet to be reported.
Experimental Exposure
Renshaw (1946) has reported on the development of contact sensitivity in man following localized exposure to liquid sulfur mustard. Cutaneous sensitivity may be seen within 8 days following the first application, and a more pronounced effect is seen after four weeks. The incidence of hypersensitivity varies between 30 and 65 percent of exposed individuals. Sensitivity may be immediate (urticaria) or delayed (dermatitis) and appears to last for a lifetime. Sensitivity also includes flares of old, healed sulfur mustard injured sites after a fresh application of sulfur mustard to normal unaffected skin.
EVIDENCE OF LONG-TERM HEALTH EFFECTS OF LEWISITE
The long-term health effects of Lewisite on skin are unknown. There is an extensive bibliography on the long-term effects of arsenicals on specific organ systems, but with few exceptions the skin is omitted from these studies. There is considerable controversy over which arsenicals are most toxic to human and animal tissues (e.g., inorganic arsenicals versus organic arsenicals, versus trivalent, versus pentavalent). The more recent literature leans toward the conclusion that most long-term effects attributable to arsenicals are due to exposure to inorganic trivalent arsenic (Goldman and Dacre, 1989; Squibb and Fowler, 1983).
Epidemiologic studies have clearly demonstrated a real association between chronic adverse reactions and occupational exposure to inorganic arsenic in pesticides, herbicides, fungicides, and animal disinfectants, and in smelter workers. In medicine, preparations such as Fowler's solution, asiatic pills, and Donovan's solution that contain trivalent elemental arsenic have been associated with long-term effects including dermatitis, hyperpigmentation, loss of hair, disseminated cutaneous keratoses, palmar hyperkeratosis, and cutaneous cancer, including basal cell, squamous cell, and Bowen's intraepidermal squamous cell cancer. Yet the long-term administration of organic arsenicals in the treatment of syphilis, trypanosomal diseases, parasitic infestations, relapsing fever, and yaws has not been associated with any of the adverse reactions outlined above.
Animal Studies and Cellular Bioassays
Most animal studies of the long-term effects of Lewisite and other arsenicals on skin and other organs have been directed toward the
elucidation of carcinogenesis (Fraumeni, 1975; IARC, 1980; Kennaway, 1942; National Research Council, 1977; Pershagen, 1981). Squibb and Fowler (1983) state emphatically, "The question as to whether arsenic is a direct carcinogen ... remains unanswered at this time. Epidemiological data clearly indicate that exposure to arsenic increases the incidence of skin, lung, liver and lymphoid cancer in humans, however, animal studies designed to confirm the carcinogenic potential of arsenic and its compounds have been, for the most part, negative." Goldman and Dacre (1989) state, "There is still reservation about accepting arsenic as a carcinogen because of the failure to demonstrate that arsenic in any form has resulted in an increased incidence in the production of tumors in experimental animals."
Human Studies
Occupational Exposure
The most often quoted evidence of Lewisite-induced cutaneous cancer is a case of Bowen's disease that developed 8 years after Lewisite-produced injury (Krause and Grussendorf, 1978) and the multiple keratoses and skin cancers in the group of Japanese "war-gas" factory workers described above (Inada et al., 1978). The questions regarding the Japanese study in terms of sulfur mustard effects also apply here.
Arsenic has been linked to the production of human cancer by many investigators (Allen, 1967; Graham and Helwig, 1959; Graham et al., 1961; Montgomery and Waisman, 1941). Roth (1956) described arsenic-induced cancers among vineyard workers, as well as a striking multiplicity of arsenic-induced cancers. Sommers and McManus (1953) also called attention to the multiplicity of lesions and the involvement of internal organs as well as skin. Arsenic may produce keratoses (keratinized protuberances of skin, particularly on the palms and soles), squamous cell cancer, basal cell cancer, multicentric intraepithelial basal cell carcinoma, and Bowen's intraepidermal squamous cell cancer.
Convincing proof of the etiologic linkage of arsenic to neoplasms is the demonstration of arsenic in tumors. Two fairly simple tests have been used: (1) the Osborne test, which demonstrates the presence of yellowish-green crystals in specially stained histologic sections of skin; and (2) the direct differential chemical analysis of fragments of tissue for arsenic. Montgomery and Waisman (1941) have shown that normal skin will contain 0.00008 mg of arsenic per gram of tissue; whereas, cancerous skin will contain 0.00024 to 4.3 mg of arsenic per gram of tissue. Arsenic has been recovered from human skin up to 30 or more years after administration of the compound. Graham and colleagues (1961) in
an analysis of normal and involved skin found arsenic in increased amounts in a significantly greater proportion of Bowen's disease patients than in lesions of control patients with other dermatoses. These findings were based on a review of material from 15 patients with keratoses and a definite history of ingestion or contact with arsenic, versus patients without such history. In a review of data from another series of studies, Graham and Helwig (1959) conclude that ''our observations strongly suggest that arsenic could be one of the causes of Bowen's disease and that the systemic and cutaneous cancers in these patients may well represent the systemic manifestations of this strong chemical carcinogen."
Battlefield Exposure
It has been stated that the value of Lewisite as a military agent depends in large degree on whether the necessary dosages can be "set up in the field." Field experiences indicate that dosages sufficiently large enough to impact on military operations "are probably not attainable with any reasonable expenditure of munitions" (Gates et al., 1946). Neither saturation of fields, nor delivery of thickened and unthickened Lewisite vapor through bombs and airplane spray, has proven of value. The casualty-producing properties of sulfur mustard far outweigh those of Lewisite, and for this reason there has been no known battlefield use of Lewisite.
Medical Therapeutic Exposure
As stated above, inorganic as well as organic arsenicals have been used for medicinal purposes. Inorganic arsenicals have been used since the time of Hippocrates (460-377 B.C.). Due to their low comparative toxicities, organic arsenicals supplanted the general use of inorganic arsenicals in medicine during the early 1900s. However, the use of inorganic arsenicals was not totally eliminated, and many products were sold as "over-the-counter" home remedies and tonics through the latter half of this century. In individuals exposed to inorganic arsenicals through this route, all of the adverse reactions described earlier have been seen. Cutaneous cancers, basal cell, squamous cell, and Bowen's disease have been well described in these populations. In numerous instances, systemic metastatic cancers of the internal organs have been associated with a large number and variety of cutaneous cancers (Sommers and McManus, 1953).
Experimental Exposure
Other than sensitization subsequent to Lewisite application to skin, described above, there is a paucity of information regarding long-term
effects of acute or chronic exposure to Lewisite from experimental situations.
SUMMARY
Gaps in the Literature
There is a wealth of information on the acute and short-term effects of sulfur mustard on human and animal skin. However, there is a paucity of literature describing delayed or long-term effects. Based on a small body of information derived from fairly crude data, observation periods as long as 35-45 years may be required to produce meaningful human data. To our knowledge, the only prospective study of long-term cutaneous effects of acute sulfur mustard exposure on human skin is that of Balali (1986). This study is now in its fourth or fifth year and should provide very valuable information in 15-20 years.
Human data derived from patients previously treated in Russian and Eastern European studies of the agent psoriasin may also be useful in determining the delayed effect of short-term administration of suberythema dosages of sulfur mustard. We are now approaching 20 to 25 years from the beginning of these studies. Follow-up of those participants, if properly done, now would be of invaluable help in determining delayed effects of acute sulfur mustard exposure. It is possible that some studies were designed using chronic dosing and adequate control populations. If so, patients in this category may aid in determining if chronic sulfur mustard administration in subinjury dosages, like nitrogen mustard, may lead to the development of cutaneous cancer.
Ideally, if one were able to determine successfully who participated, and when, in the variety of experiments carried out by the U.S. Armed Forces and its Allies in World War II, an examination of this group, potentially numbering in the thousands, would serve as an excellent source of data on the long-term effects of sulfur mustard on the skin.
There are also numerous gaps in the literature relative to the acute and long-term effects of Lewisite skin exposure. Lewisite has been subjected to much less intense investigation than sulfur mustard. Very little is known regarding its specific effect on skin; data on such basic areas as absorption, disposition, and excretion after skin exposure are minimal. Although much is known of Lewisite's biochemical interactions, little is known of the morphologic sites of these interactions. Microscopic examination of affected skin has yet to be pursued in depth. Most studies have been impaired, as has been work on sulfur mustard exposure, by the lack of good animal model systems.
Studies on the carcinogenicity or noncarcinogenicity of Lewisite
need to be broadened and pursued with greater intensity. The information obtained from these studies, unlike studies of sulfur mustard exposure, will have broad application in industry, farming, and medicine.
Conclusions
Despite the many years that the problem of acute sulfur mustard toxicity to human skin has been known and observed, its long-term effects after acute and chronic exposure remain obscure. Unfortunately, large volumes of pertinent literature on experimental studies of human exposure remain obscure or destroyed. Despite the flaws in the literature explored to date, it is possible to conclude that (1) the evidence indicates a causal relation between acute, severe exposure to mustard agents and increased pigmentation and depigmentation in human skin; (2) acute and severe exposure can lead to chronic skin ulceration, scar formation, and the development of cutaneous cancer; and (3) chronic exposure to minimally toxic and even subtoxic doses can lead to skin pigmentation abnormalities and cutaneous cancer. The evidence would nevertheless be strengthened by (a) intensive data review; (b) physical examination of identifiable victims of experimentation during and preceding former wars, and the comparison of these individuals with matched cohorts of nonexposed persons; and (c) continued prospective evaluation of individuals with recent battlefield and experimental exposure. It should also be emphasized that scarring of scrotal and penile tissue, quite likely in mustard agent exposure, can impair sexual performance.
There is insufficient information, however, to establish a causal relationship between Lewisite exposure and long-term adverse effects on skin.
REFERENCES
Allen AC. 1967. The Skin: A Clinicopathological Treatise. 2nd ed. New York: Grune and Stratton.
Axelrod DJ, Hamilton JG. 1947. Radio-autographic studies of the distribution of Lewisite and mustard gas in skin and eye tissues. American Journal of Pathology 23:389-411.
Balali M. 1986. First report of delayed toxic effects of yperite poisoning in Iranian fighters. In: Heyndricks B, ed. Terrorism: Analysis and Detection of Explosives. Proceedings of the Second World Congress on New Compounds in Biological and Chemical Warfare. Gent, Belgium: Rijksuniversiteit. 489-495.
Bernstein IA, Brabec MJ, Conolly RC, Gray RH, Kulkarni A, Mitra R, Vaughan FL. 1985. Chemical Blistering: Cellular and Macromolecular Components. AD-A190 313. Ann Arbor, MI: University of Michigan.
Bernstein IA, Bernstam L, Brown R, Fan L, Feng HW, Ku W, Locey B, Ribeiro P, Scavarelli R, Vaughan FL, Zaman S. 1987. Macromolecular and cellular effects of sulfur mustard
on keratinocyte cultures. In: Proceedings of the 6th Chemical Defense Bioscience Review. Frederick, MD. 243-250.
Büscher H. 1932. Green and Yellow Cross. Hamburg, Germany: Himmelheber. Translated from the German in 1944 by N Conway, Kettering Laboratory of Applied Physiology, Cincinnati, OH.
Cameron GR, Carleton HM, Short RHD. 1946. Pathological changes induced by Lewisite and allied compounds. Journal of Pathology and Bacteriology 58:411-422.
Crathorn AR, Roberts JJ. 1966. Mechanism of the cytotoxic action of alkylating agents in mammalian cells and evidence for the removal of alkylated groups from deoxyribonucleic acid. Nature 211:150-153.
Cullumbine H. 1947. Medical aspects of mustard gas poisoning. Nature 159:151-153.
Davis J. 1944. Dermatologic aspects of vesicant war gases (dichloroethyl sulfide and dicholorovinylarsine). Journal of the American Medical Association 126:209.
Easton D, Peto J, Doll R. 1988. Cancers of the respiratory tract in mustard gas workers. British Journal of Industrial Medicine 45:652-659.
Feister A, Papirmeister B, Robinson S, Kiebzak G, McNally R, Ford R, Baggett J, Gottlieb J, Bareis D. 1989. Sulfur Mustard and Lewisite: Current Perspectives and Future Directions. Prepared for the U.S. Army Medical Research Institute of Chemical Defense. Unpublished.
Ferguson RL, Silver SD. 1947. A method for the visual demonstration of Lewisite in skin. American Journal of Clinical Pathology 17:37-38.
Fitzpatrick TB, Eisen AZ, Wolff K, Freedberg IM, Austeru KF. 1979. Dermatology in General Medicine: Textbook and Atlas. 2nd ed. New York: McGraw-Hill.
Fox M, Scott D. 1980. The genetic toxicology of nitrogen and sulphur mustard. Mutation Research 75:131-168.
Fraumeni JF Jr. 1975. Respiratory carcinogenesis: an epidemiologic appraisal. Journal of the National Cancer Institute 55:1039-1046.
Gates M, Williams JW, Zapp JA. 1946. Arsenicals. In: Division 9, National Defense Research Committee, comp. Chemical Warfare Agents, and Related Chemical Problems. Summary Technical Report of Division 9, NDRC. Washington, DC: Office of Scientific Research and Development.
Goldman M, Dacre J. 1989. Lewisite: its chemistry, toxicology, and biological effects. Reviews of Environmental Contamination and Toxicology 110:75-115.
Graham JH, Helwig EB. 1959. Bowen's disease and its relationship to systemic cancer. AMA Archives of Dermatology 80:133-159.
Graham JH, Mazzanti GR, Helwig EB. 1961. Chemistry of Bowen's disease: relationship to arsenic. Journal of Investigative Dermatology 37:317-332.
Heston WE. 1953. Occurrence of tumors in mice injected subcutaneously with sulfur mustard and nitrogen mustard. Journal of the National Cancer Institute 14:131-140.
Hosokawa T. 1961. Studies on skin tumors: statistical observation on skin tumors. Hiroshima Medical Journal.
Inada S, Hiragun K, Seo K, Yamura T. 1978. Multiple Bowen's disease observed in former workers of a poison gas factory in Japan with special reference to mustard gas exposure. Journal of Dermatology 5:49-60.
International Agency for Research on Cancer (IARC). 1980. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans. Vol. 23, Some Metals and Metallic Compounds. Lyon: IARC.
Jackson R, Adams RH. 1973. Horrifying basal cell carcinoma: a study of 33 cases and a comparison with 435 non-horror cases and a report on four metastatic cases. Journal of Surgical Oncology 5:431-463.
Jimbow K, et al. 1976. Some aspects of melanin biology: 1950-1975. Journal of Investigative Dermatology 67:72-89.
Kennaway E. 1942. A contribution of the mythology of cancer research. Lancet 2:769-772.
Kitamura K. 1954. Tumors of skin. J Dermatol and Venereal Dis 64:303-312.
Klehr NW. 1984. Late manifestations in former mustard gas workers, with special consideration of the cutaneous findings. Zeitschrift fur Hautkrankheiten 59:1161-1170. [In German]
Krause H, Grussendorf El. 1978. Syntopy of Bowen's disease and "Lost"-induced scar. Hautarzt 29:490-493. [In German]
Kravitz P, McDonald CJ. 1978. Topical nitrogen mustard-induced carcinogenesis. Acta Dermato-Venereologica 58:421-425.
Lawley P, Brookes P. 1965. Molecular mechanism of the cytotoxic action of difunctional alkylating agents and of resistance to this action. Nature 206:480-483.
Martens ME. 1991. Glucose metabolism and NAD+ content in cultured human epidermal keratinocytes exposed to sulfur mustard (abstract). FASEB Journal 5:A823.
Mathias CGT. 1987. Clinical and experimental aspects of cutaneous irritation. In: Marzulli FN, Maibach HI, eds. Dermatotoxicology. 3rd ed. Washington, DC: Hemisphere Publishing.
McGown EL, Van Ravenswaay T, Damlao CR. 1985. Histologic changes caused by application of Lewisite analogs to mouse skin and human skin xenografts. San Francisco: Letterman Army Institute of Research. AD-A159-554.
McGown EL, Van Ravenswaay T, Dumlao CR. 1987. Histologic changes in nude mouse skin and human skin xenografts following exposure to sulfhydryl reagents: arsenicals. Toxicologic Pathology 15:149-156.
McNamara BP, Owens EJ, Christensen MK, Vocci FJ, Ford DF, Rozimarek H. 1975. Toxicological Basis for Controlling Levels of Mustard in the Environment. Edgewood Arsenal Special Publication EB-SP-74030. Aberdeen Proving Ground, Maryland: U.S. Army Armament Command. Edgewood Arsenal Biomedical Laboratory.
Mitcheltree LW, Mershon MM, Wall HG, Pulliam JD, Manthei JH. 1989. Microblister formation in vesicant-exposed pig skin. Journal of Toxicology—Cutaneous and Ocular Toxicology 8:309-319.
Miyaji T. 1963. Skin cancer in Japan: a nationwide 5 year survey. In: Urbach F, ed. Conference on Biology of Cutaneous Cancer. National Cancer Institute Monograph No. 10. 55-70.
Momeni AZ, Enshaeih S, Meghadi M, Amindjavaheri M. 1992. Skin manifestations of mustard gas. A clinical study of 535 patients exposed to mustard gas. Archives of Dermatology 128:775-580.
Montgomery H, Waisman M. 1941. Epithelioma attributable to arsenic. Journal of Investigative Dermatology 4:365-383.
Nagy SM, Golumbic C, Stein WH, Fruton JS, Bergmann M. 1946. The penetration of vesicant vapors into human skin. Journal of General Physiology 29:441-469.
National Research Council. Division of Medical Sciences. 1977. Arsenic. Medical and Biologic Effects of Environmental Pollutants. Washington, DC: National Academy of Sciences.
Novick M, Gard DH, Hardy SB, Spira M. 1977. Burn scar carcinoma: a review and analysis of 46 cases. Journal of Trauma 17:809-817.
Papirmeister B, Feister AJ, Robinson SI, Ford RD. 1991. Medical Defense Against Mustard Gas: Toxic Mechanisms and Pharmacological Implications. Boca Raton, FL: CRC Press.
Pershagen G. 1981. The carcinogenicity of arsenic. Environmental Health Perspectives 40:93-100.
Peters RA. 1955. Biochemistry of toxic agents: I. Present status of knowledge of biochemical lesions induced by trivalent arsenical poisoning. Bulletin of Johns Hopkins Hospital 97:1-20.
Peters RA, Sinclair HM, Thompson RHS. 1946. An analysis of the inhibition of pyruvate
oxidation by arsenicals in relation to the enzyme theory of vesication. Biochemical Journal 40:516-524.
Quevedo WC, Jr. 1969. The control of color in mammals. American Zoologist 9:531-540.
Renshaw B. 1946. Mechanisms in production of cutaneous injuries by sulfur and nitrogen mustards. In: Division 9, National Defense Research Committee, comp. Chemical Warfare Agents, and Related Chemical Problems. Summary Technical Report of Division 9, NDRC. Washington, DC: Office of Scientific Research and Development.
Renshaw B. 1947. Observations on the role of water in the susceptibility of human skin to vesicant injury. Journal of Investigative Dermatology 9:75-85.
Roth F. 1956. Chronic arsenicism and cancers among vineyard workers in the Moselle Valley. Zeitschrift fur Krebsforschung und Klinische Onkologie 61:287-319.
Sams WM, Lynch PJ. 1990. Principles and Practice of Dermatology. New York: Churchill Livingstone.
Smith WJ, Dunn MA. 1991. Medical defense against blistering chemical warfare agents. Archives of Dermatology 127:1207-1213.
Sommers S, McManus RG. 1953. Multiple arsenical cancers of skin and internal organs. Cancer 6:347-359.
Sorscher D, Conolly R. 1989. Pretreatment of primary rat cutaneous epidermal keratinocyte culture with a low concentration of MNNG: effect on DNA cross-linking measured in situ after challenge with bis-2-chloroethyl sulfide. Journal of Toxicology and Environmental Health 27:367-379.
Squibb KS, Fowler BA. 1983. The toxicity of arsenic and its compounds. In: Fowler BA, ed. Biological and Environmental Effects of Arsenic. Vol. 6. Amsterdam: Elsevier Science. 233-269.
Stedman's Medical Dictionary. 1976. 23rd ed. Baltimore: Williams & Wilkins.
Treves N, Pack GT. 1930. Development of cancer in burn scars: analysis and report of 34 cases. Surgery, Gynecology, and Obstetrics 51:749-782.
Turanow NM, Trofimonwa LJ, Bolschakowa GM, Chapilowa WI. 1977. Experience report from the USSR on the management of psoriasis patients using "Psoriasin." Zeitschrift fur Hautkrankheiten 52:1045-1049. [In German]
Wada S, Nishimoto Y, Miyanishi M, Katsuta S, Nishiki M. 1962. Review of Okuno-Jima poison gas factory regarding occupational environment. Hiroshima Journal of Medical Sciences 11:75-80.
Wada S, Yamada A, Nishimoto Y, Tokuoka S, Miyanishi M, Katsuta S, Umisa H. 1963. Neoplasms of the respiratory tract among poison gas workers. Journal of the Hiroshima Medical Association 16:728-745.
Weigand DA, Haygood C, Gaylor G. 1974. Cell layers and density of Negro and Caucasian stratum corneum . Journal of Investigative Dermatology 62:563-568.
Wheeler GP. 1967. Some biochemical effects of alkylating agents. Proceedings of the Society for Experimental Biology and Medicine 26:885-892.
Willems JL. 1989. Clinical management of mustard gas casualties. Annales Medicinae Militaris Belgicae 3:S1-61.































