10
Other Physiological Effects of Mustard Agents and Lewisite
Exposure to mustard agents and Lewisite may have long-term effects on a number of other physiological systems. This chapter reviews the animal experimental data and human epidemiological studies that have focused on the following systems:
-
immune system, including blood lymphocytes, lymphatic tissues, and bone marrow;
-
gastrointestinal system;
-
blood system;
-
nervous system, including autonomic and higher-order functions; and
-
reproductive risks.
EFFECTS ON THE IMMUNE SYSTEM
Anatomy and Physiology
The human body has the ability to resist many types of organisms or toxic agents to which it may be exposed. This capacity is called immunity. The immune response includes specific actions of blood lymphocytes (one type of white blood cells) and is facilitated by other white blood cells including neutrophils, monocytes, macrophages, eosinophils, and basophils.
The leukocytes are the mobile units of the body's protective system. They circulate throughout the body, moving in and out of tissues via the circulatory system and lymphatic system. The leukocytes are formed
partially in the bone marrow and partially in the lymph tissue, but after formation they are transported in the blood to different parts of the body where they are to be used.
The development of the human immune system begins late in the fetal period, is functioning at birth, and reaches maximum capacity near the time of puberty. In human adults the majority of circulating lymphocytes are T cells and the remainder are B cells and NK (natural killer) cells (a lymphocyte that can nonspecifically destroy certain virally infected or tumor cells). The production of B cells and T cells continues, albeit at a reduced rate, throughout life (Twomey, 1982).
The normal function of the immune system involves a complex sequence of cellular and biochemical events. After exposure to an antigen (a molecule that stimulates a specific immune response), there is phagocytosis (ingestion) of the antigen by macrophages during which the antigen undergoes intracellular breakdown by enzymatic hydrolysis. After hydrolysis, the fragments of the antigen move to the surface of the macrophage for reaction with specific T lymphocytes called helper-inducer T cells. Activation of these T lymphocytes occurs only if the interacting lymphocytes have specific receptors that bind to a complex of antigen fragments and a special protein derived from the major histocompatibility complex (Twomey, 1982).
The generation of antibody-producing plasma cells (B cells) and cytotoxic cells (T cells) requires the presence of biochemical factors (lymphokines and cytokines) secreted by T cells and macrophages. Clonal expansion increases the number of these specifically reactive T and B cells, so that subsequent exposure to the same antigen leads to a rapid specific immune response. As an immune response occurs, a decrease of the T cells is likely, and negative feedback into earlier phases prevents excessive reaction. Thus, the specific antibody reacts with the offending antigen to cause neutralization or inactivation while effecter T cells inactivate or destroy cellular targets. When these mechanisms are functioning properly, the immune system recognizes and eliminates foreign agents quickly and efficiently. Opportunities for dysfunction can occur at any point along the pathway of cellular and biochemical processes, resulting in a variety of immunological effects from hypersensitivity to immunodeficiency, as illustrated in Figure 10-1.
For example, exposure to immunotoxicants can cause immunosuppression, resulting in altered host resistance. The outcome of immune suppression is influenced by the dose and mechanism of action of the immunotoxicants, along with concomitant exposure to other agents such as bacteria, viruses, parasites, or chemicals at levels that might normally be innocuous. In its suppressed condition, the immune system does not respond adequately to hazardous agents. Adverse consequences are those of severe disseminated infectious diseases caused by
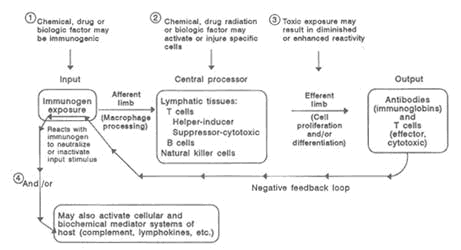
FIGURE 10-1Model of the competent immune system depicting sites of potential effects of the major components by toxic factors. SOURCE: National Research Council, 1992.
a variety of agents that are usually not pathogenic. Age, poor nutrition, and stress (physiologic and psychologic) can exacerbate the development of such immunologic disease (Golub, 1987).
Xenobiotics can also act as sensitizers to stimulate the immune system as antigens by provoking a substantial immune response that leads to hypersensitivity. Immunologic tissue damage can result from activation of the cellular and biochemical systems of the host. The interactions of an antigen with a specific antibody or with effecter lymphocytes trigger the sequence of humoral and cellular events to produce the pathophysiologic effects that lead to tissue injury or disease (Vos, 1977).
Chemicals that suppress bone marrow function can affect reserves of stem cells that are needed for cell replacement. Blood line cells are derived from stem cells, which can develop into many cell types (pluripotent) and which, in adult humans, are primarily in bone marrow. Within the marrow microenvironment, these self-renewing cells mature into committed progenitors, which are in peripheral cells. Stem cells often appear to be sensitive targets for therapeutic and environmental toxicants, most likely because of their rapid proliferation. Xenobiotics or various drugs that are toxic to the myelocytes of the bone marrow can cause profound immunosuppression due to loss of stem cells.
Animal Studies
In a review of the literature, a distinct impression arises that sulfur mustard has been consistently observed to cause pathogenic states of
decreased immunoresponsiveness in animals. One of the earliest clues of this effect emerged from a series of animal experiments by Hektoen and Corper (1920). They observed that dogs and rabbits experienced depressed antibody formation after intravenous and intraperitoneal (IP) exposure to sulfur mustard. The sulfur mustard had a restraining effect on precipitation and on lysis—two of the principal ways antibodies can inactivate invading foreign agents—and profoundly modified the leukocyte count of the blood in experimental animals.
This series of studies placed sulfur mustard in a class with other leukocytic toxins such as benzene, which has frequently been associated with myelotoxicity expressed as leukopenia, pancytopenia, anemia, and aplastic or hypoplastic bone marrow (Dean and Murray, 1991).
Later studies with rabbits demonstrated that the number of leukocytes increased immediately after inhalation exposure but later diminished morphologically. The polymorphonuclear basophil (a type of white blood cell) showed abnormal developments of the nucleus and dissolution of the granules. The lymphocytes, which produce acquired immunity, also showed degenerative changes (Hektoen and Corper, 1920).
Similar results were achieved in experiments in which the route of absorption was intravenous: polymorphonuclear basophils increased following injection and then diminished rapidly, apparently disappearing from the peripheral blood. Zimmerman (1942) reported that lymphocyte disintegration began within 5 hours of intravenous injection of sulfur mustard; within 24 hours most of the lymphocytes had disappeared.
Quantitative histologic investigation of the effects of intravenously injected sulfur and nitrogen mustard on albino rats suggested a decreased immunoresponsiveness, expressed as leukopenia, lymphopenia, and neutropenia (the disappearance of the respective blood cells), as well as hypoplasia and hyperemia of bone marrow. In the lymphoid organs, tissue decreased in volume because of the destruction of lymphocytes (Kindred, 1947). The author further noted that the bone marrow reacted more slowly to the mustards than did the lymphoid organs, but it became hyperplastic. There was some destruction of cells, particularly of the mature granulocytes that protect the body against invading agents by ingesting them (Kindred, 1949).
These data on the albino rat have been supplemented by studies showing that dogs exposed to sulfur and nitrogen mustard experience toxic effects on lymphoid organs and bone marrow. The results of this action were observed in the quantitative decrease of cells in the peripheral blood of poisoned animals. The extent of cellular intoxication was directly related to the amount of the mustard injected (Kindred, 1949). Spurr (1947) further elucidated the influence of mustard com-
pounds on immune function by simultaneous intramuscular injection of typhoid vaccine and nitrogen mustard into rabbits. The data suggest that the toxicity of nitrogen interferes with or suppresses antibody-forming mechanisms of the lymphocyte.
More recent studies have generally confirmed earlier evidence that laboratory animals exposed to mustards experience changes in cells of the immune system that result in undesirable effects (i.e., immunosuppression, alteration of host defense mechanisms against pathogens and neoplasia). Coutelier and colleagues (1991) noted a marked decrease in the number of spleen cells in mice one week after receiving a relatively high dose of sulfur mustard. B lymphocytes were relatively more affected than T lymphocytes by sulfur mustard; similar results were seen in humans, where toxicity for B lymphocytes led to a decrease in B-cell number following exposure to nitrogen mustard compounds.
Blank and colleagues (1991) compared the immunotoxicity of sulfur mustard and nitrogen mustard on humoral and cell-mediated immunity of mice. The effects on thymic and splenic weight, spleen cell number, and the formation of antibody were similar to earlier laboratory results. Both compounds induced splenic and thymic weight loss. When splenic cellularity was depressed, the total number of cells producing antibody response was decreased. Only when sulfur mustard reached lethal levels were the total spleen cells producing antibody response at a level equivalent to that observed following nitrogen mustard administration. Hence, the immunotoxic effect of nitrogen mustard could be distinguished from general toxicity and was tolerated at a higher dose than was sulfur mustard. Nitrogen mustard had an additional immunotoxic effect of decreasing host resistance to tumor cells that was not observed with sulfur mustard. The reason for these differences is unclear.
Human Studies
Evidence that sulfur mustard causes immunosuppression in humans has emerged from several lines of investigation. The earliest evidence came from clinical observations of humans directly exposed to sulfur mustard during World War I (WWI), who showed significant quantitative and qualitative changes in the circulating elements of the immune system. Stewart (1918) studied 10 fatal cases of mustard poisoning and observed striking depression of bone marrow production of white blood cells. For example, in one case the patient showed a total leukocyte count of 7,630/mm3 on the second day after exposure (gassing), 6,650 on the third day, and 270 on the sixth day, 24 hours before death. In another case a total leukocyte count of 35,000 was measured on the second day after gassing, which dropped to 16,000 on the third day, and to 172 on the seventh day, six hours before death.
Krumbhaar (1919a,b) observed that among the first changes in the circulating blood of patients exposed to sulfur mustard was an exhaustion of leukocyte-forming centers. This downward trend in leukocyte count ultimately leads to severe leukopenia. Thus the leukocyte counts in four fatal cases were observed to fall steadily from (1) 10,200 to 2,900; (2) 17,800 to 3,200; (3) 20,400 to 7,600; and (4) 36,000 to 14,000/mm3. Postmortem examination of the femoral bone marrow revealed only a slight mottling, shown histologically to be due to primordial cells and megablasts with a greater or lesser disappearance of normoblast, myelocytes, and adult forms. This was interpreted as an inadequate attempt at blood regeneration, and the resulting lack of leukocytes in the bloodstream suggested a weakening of the immune system. Even in bronchopneumonia that frequently supervenes in heavily gassed patients, little or no reactive rise in leukocyte count was observed.
In another study, the femoral bone marrow in 55 of 75 autopsies of mustard gas-exposed patients was examined (Krumbhaar and Krumbhaar, 1919). The results: 14 marrows were classified as showing almost no regenerative potential; 8 showed only slight reaction; and only 13 showed moderate reaction. In no case was the marrow as hyperplastic as in ordinary lobar pneumonia or acute infections accompanied by leukopenia. The authors concluded that
the blood and bone marrow changes are due to direct action of the poison and not the secondary infections (1) because they have been found well marked in cases where infection was slight or absent; (2) because influenza, typhoid, malaria, and such leucopenia infections played no part in these cases; and (3) because the kind of infection found to be present (pyogenic) does not lead to leucopenia or impaired bone marrow function.
In 1946, Anslow and Houck reviewed classified literature concerning the pharmacological action of sulfur and nitrogen mustard. They reported that evidence from soldiers gassed or burned in World War I was essentially the same as seen in experimental animals. Marked leukopenia and loss of reactivity of the bone marrow were observed in severe cases of mustard intoxication. In mask volunteers, sublethally exposed to sulfur mustard under temperate or tropical climate conditions, there was a moderate to marked leukocytosis appearing as early as four hours after exposure. This was followed by a moderate reduction in the number of leukocytes in the blood.
Another set of clinical observations comes from reports on over 600 sulfur mustard casualties following the release of sulfur mustard in Bari harbor, Italy, in December 1943 (Alexander, 1947). The effects upon the leukocytes in the circulating blood were most severe: white
blood cell counts of 100 cells/mm3 or less were recorded; lymphocytes were the first to disappear; and granulocytes were also severely affected but lagged behind the lymphocytes in their rate of decrease. Not all cases demonstrated a sharp decline in white blood cell count, but all casualties with an extremely low leukocyte count died. Infection was a dominant feature, as it was among sulfur mustard casualties during the Iran-Iraq conflict in 1984 and 1986. These patients experienced leukopenia accompanied by total bone marrow aplasia, which included extensive losses of myeloid stem cells (Balali, 1986; Eisenmenger et al., 1991). These findings are further evidence of an association between suppression of immunologic functions and an increased incidence of infectious disease.
In one of the few studies of long-term effects, Zandieh and colleagues (1990) measured the cell-mediated immunity in three groups of Iranians exposed to sulfur mustard: (1) three months to two years after exposure; (2) one to two years after exposure; and (3) two years after exposure. In comparison to normal controls, T lymphocytes showed a significant decrease of 50 percent in all three groups; helper-inducer T cells were significantly decreased in 52 percent of the first and second groups; and T suppressor cells were increased in 53 percent of the first group and 22 percent of the second and third groups. These measurements indicate that depression of the cell-mediated immunity was observed one, two, and three years after exposure to sulfur mustard. Several recent case reports from the Bahar Medical Laboratory in Tehran, Iran, describe similar long-term effects: about 100 patients exposed to sulfur mustard were observed for a year, and the investigator found alterations in B and T lymphocytes. In addition, the phagocytic activity of these patients was reduced to 20 percent of that of a normally functioning immune system (Balali, 1986).
The search for chemical agents with antitumor activities has provided another clue that sulfur and nitrogen mustards are immunotoxic. As alkylating agents, they form covalent linkages with biologically important molecules, resulting in disruption of cell function, especially cell division. As a result, these agents are particularly toxic to rapidly proliferating cells including neoplastic, lymphoid, and bone marrow cells. Because of these immunosuppressive effects, sulfur and nitrogen mustards have particular clinical importance. Nitrogen mustards were the first nonhormonal agents to show significant antitumor activities in humans, producing dramatic tumor regression in lymphoma patients (Colvin and Chabner, 1990). The significance of the immunosuppression produced by alkylating agents in the setting of cancer therapy is uncertain. The major concerns are (1) the danger of increased susceptibility to infection in the immunosuppressed host and (2) the potential interference with a host immune response to the
tumor. Spitz (1948) conducted a histological analysis of postmortem tissue of 57 cases consisting of a variety of lymphomas, leukemias, and other malignant tumors treated with nitrogen mustards. Attributable to mustard therapy in these cases was a consistent, apparently accumulative hypoplasia of bone marrow. This hypoplasia was correlated with the degree of leukopenia and thrombocytopenia that had existed prior to death.
The immunosuppressive effects of alkylating agents have also been demonstrated in treating autoimmune disease and as adjunctive therapy in organ transplantation procedures, to prevent rejection by the recipient. Immunosuppressive treatments, like exposure to sulfur mustard, result in increased incidence of infectious disease. There is a well-established association between the therapeutic use of chemical immunosuppressants, such as those used in organ transplant therapy, and an increased incidence of infectious disease in humans (Ehrke and Mihich, 1985).
Conclusions
Evidence from animal experiments consistently confirms that mustard agents affect immune system functions. Animal models are most valuable in studying the physiological and molecular mechanisms involved in mustard agent effects. In general, this review accorded greater weight to data derived from studies in more than one animal species or test system, on results that have been reproduced in different laboratories and on data that indicated a dose-response relationship. However, results from animal studies cannot be used alone either to affirm or to negate relationships between exposure to mustard agents and chronic disorders, nor can they be used to estimate accurately the size of the effects in humans.
Clinical observations in humans provide the most direct evidence of the immunologic effects of mustard agents. In this review, greater significance was accorded to observations in humans that provide clear evidence that mustard agent exposure is associated with bone marrow toxicity expressed as leukopenia, pancytopenia, or a plastic or hypoplastic bone marrow. Underrepresented in these works is information on chronic or delayed effects. This may be attributable to the fact that patients, if they survive the acute effects, experience a number of secondary infections and may in fact die from them. Finally, the data presented here indicate that clinical studies as a whole support a close parallelism between animal experiments and observations in humans regarding the immunosuppressive properties of mustard agents.
EFFECTS ON SYSTEMS OTHER THAN THE IMMUNE SYSTEM
Gastrointestinal Effects
Although not the major focus of most studies of mustard agents, gastrointestinal effects have been documented in some animal studies. The most common findings have been intestinal histopathological changes, including destruction of the mucosa and shedding of epithelial elements (Graef et al., 1948; Papirmeister et al., 1991). Gavage studies have also shown epithelial hyperplasia of the forestomach (Sasser et al., 1989b).
In humans, gastrointestinal effects of mustard agents are commonly seen in people with acute high exposures. Common symptoms include nausea and vomiting, both immediately after exposure and as a delayed effect (Papirmeister et al., 1991; Schonwald, 1992). Several mechanisms may account for these effects including (1) a direct and immediate cholinergic effect, (2) an inflammatory reaction of the upper gastrointestinal mucosa, (3) a delayed radiomimetic effect on the small intestine, and (4) physical stress secondary to skin and other effects from mustard agent exposure (Papirmeister et al., 1991). In some individuals there may be chronic gastrointestinal symptoms, but these appear to occur only secondarily to other chronic health problems due to acute high exposures to mustard agents.
Exposure of dogs to high doses of Lewisite produces some injury to the intestinal mucosa and focal necrosis of the liver with peribiliary hemorrhage (Cameron et al., 1946, 1947; Gates et al., 1946). Exposure of rats by lavage produced lesions of the forestomach including necrosis of the stratified epithelium (Sasser et al., 1989a). No information could be found on the effects of Lewisite on the human gastrointestinal systems.
Hematological Effects
In animals exposed to very high doses (i.e., LD50) of mustard agents, aplastic changes occur in the bone marrow. Lymphatic damage also occurs, including cellular depletion of the splenic sinuses and resultant disappearance of lymphocytes from the blood (Graef et al., 1948; Papirmeister et al., 1991). Similar hematological changes occur in humans after very high exposures to mustard agents. Marrow suppression including anemia, thrombocytopenia, and leukopenia has been seen (Papirmeister et al., 1991; Willems, 1989). Severe leukopenia appears to occur only after very high exposures, and secondary infections can be a significant contributor to the mortality in this group.
Laboratory studies have also demonstrated that exposure to Lewisite
may cause a hemolytic anemia similar to that seen with arsine exposure (Goldman and Dacre, 1989). As before, there are no data regarding hematological effects on humans.
Neurological Effects
Extremely high exposures to mustard agents can cause central nervous system excitation leading to convulsions in animals (Anslow and Houck, 1946). Cardiac rhythm irregularities including atrioventricular block may also occur at high levels of exposure (Anslow and Houck, 1946). Renal changes have also been reported, but usually as a late complication in fatal exposures (Papirmeister et al., 1991).
In humans, acute neurological symptoms are common with high exposures to mustard agents, including severe depression and changes in mentation. These symptoms are produced both directly by the chemical and secondarily to other physiological changes (see also Chapter 11). Follow-up of workers in German chemical warfare plants showed a high prevalence of various neurological disorders including impaired concentration, diminished libido, and sensory hypersensitivity (Lohs, 1975). To what extent these can be attributed to mustard agents rather than other chemical warfare agents is unclear, but other exposures at this facility included known nerve agents. Renal and cardiac effects do not appear to occur after human exposures to mustard gas other than as secondary effects in severely affected individuals.
Neurological effects have not been documented in animals after acute exposures to Lewisite, but this apparently has not been well researched. Acute exposure to high levels of Lewisite leads to a shock syndrome due to increased capillary permeability (Goldman and Dacre, 1989). Some direct cardiotoxic effects can be seen with intravenous administration. No direct evidence exists that Lewisite may cause neurological problems in humans. However, arsenic is a well-known neurotoxin, and peripheral neuropathy has been described in humans after a single exposure to arsenic (LeQuesne and McLeod, 1977).
Conclusions
Gastrointestinal, hematological, and neurological effects are common after acute high exposures to mustard agents. These effects can be attributed primarily to the known toxicological effects of these agents and secondarily to effects on other organ systems (i.e., shock, burns). Although effects on these other organ systems have not been a focus of follow-up studies of people exposed to mustard agent, the available evidence provides no indication of persistent or delayed effects other
than those secondary to other conditions related to exposure to mustard agents.
There is insufficient information to link Lewisite with long-term health effects on the hematological, gastrointestinal, and neurological systems.
REPRODUCTIVE RISKS
Sulfur Mustards
Sulfur mustard causes cross-linking of DNA and is known to alkylate DNA at the 0-6 position of guanine. This observation is consistent with the known mutagenic potential of sulfur mustard (see Chapter 6). It is a bacterial and mammalian mutagen. Sulfur mustard causes chromosome breakage and induces sister chromatid exchanges in a wide variety of cells. Epidemiologic studies have also led the International Agency for Research on Cancer to classify sulfur mustard as a human carcinogen. These observations underscore the potential of this compound to induce genetic damage. They also suggest that sulfur mustards could be a reproductive toxin.
Animal Studies
Sulfur mustards induce dominant lethal mutations in Drosophila. Luening (1952) observed gross deletions in chromosomes in Drosophila offspring. Sonbati and Auerbach (1960) also showed that sulfur mustard can cause heritable mutations in germ cells in the offspring of treated Drosophila. Sasser and colleagues (1989c) administered sulfur mustard orally to rats in sesame oil for 5 days per week for 10 weeks at doses of 0, 0.08, 0.2, and 0.5 mg/kg. Treated and untreated males were mated with treated females and untreated females and their fetuses evaluated after 14 days. The observed effects included early fetal resorptions and preimplantation losses, as well as decreases in live embryo implants. A significant increase in abnormal sperm was detected in males exposed to the highest dose. The authors concluded that the timing of these effects was consistent with an effect during the post-meiotic stages of spermatogenesis, possibly involving the generally sensitive spermatids. In an earlier experiment, Rozmiarek and colleagues (1973) exposed pregnant female rats to 0.1 mg/m3 of sulfur mustard by inhalation; exposure during the interval of gestation failed to produce any fetal malformations.
In an extensive study, Hackett and colleagues (1987) studied maternal toxicity, intrauterine mortality, and developmental toxicology in rats and rabbits. Distilled sulfur mustard, diluted in sesame oil, was delivered by intragastric intubation in a volume of 1 ml/300 g rat and 1 ml/4
kg rabbit. Rats were dosed daily from 6 to 15 days with 0, 0.5, 1.0, and 2.0 mg/kg; rabbits received 0, 0.4, 0.6, and 0.8 mg/kg on 6-19 days. In rats, indicators of maternal toxicity were observed at all dose levels, but significant fetal effects (decreased weights, reduced ossification, and skeletal anomalies) occurred only at the highest dose (2.0 mg/kg). In rabbits, maternal toxicity occurred at the highest dose level, body weights of fetuses were reduced, but no other fetal effects were observed.
Finally, Sasser and colleagues (1989c) studied rats to evaluate the effects of sulfur mustards on reproduction. Groups of rats (27 females and 20 males per group per generation) were force-fed with 0, 0.03, 0.1, or 0.4 mg/kg sulfur mustard for 13 weeks prior to mating and throughout gestation, parturition, and lactation in a 42-week two-generation study. There were no significant effects on reproductive function or pregnancy outcome in either generation. Growth of adult F1 rats of both sexes was reduced by the highest exposure, as was the growth of their F1 and F2 offspring during lactation. A dose-related lesion of the squamous epithelial mucosa of the forestomach was observed in both sexes, and benign neoplasms of the forestomach were found in about 10 percent of the intermediate (8/94) and high-dose (10/94) groups. The NOEL in this study was <0.03 mg/kg for toxicity and > 0.4 mg/kg for reproductive effects.
Human Studies
In humans, two studies have attempted to evaluate the potential of sulfur mustard to induce adverse reproductive outcomes. Yamakido and colleagues (1985) used electrophoresis to study blood protein variants in 456 children of 325 workers exposed occupationally to sulfur mustard and Lewisite at the Okuno-jima poison gas factory; children were divided into three exposure groups based upon parental job category within the plant. The blood protein analysis revealed 6 types of protein variants in 11 children, and 11 variant erythrocyte proteins in 25 children. Examination of 32 of the 36 families of interest showed all of the detected variants to be familial and not exposure induced. One protein variant was found in one child that did not differ electrophoretically, but did differ in its enzymatic activity. This variant was not inherited. The female child in question was mentally retarded and had a normal g-banded karyotype but was born with a cleft palate and hypomyotonia (significant decrement in normal muscle tone). The significance of these findings is unclear, as this variant might have been the result of either (1) a germ cell mutation in an intron sequence (a DNA sequence outside of the region coding for the RNA and subsequent protein) that controls expression of the gene of interest, or (2) aberrant
translation or another mechanism unrelated to exposure-induced reproductive anomalies.
In consultation with Dr. Sanford S. Leffingwell (Special Programs Group, Center for Environmental Health, Centers for Disease Control, U.S. PHS) the committee attempted to estimate the power of the Yamakido and colleagues (1985) study to detect induced mutations in the offspring of exposed workers. Of the 456 subjects, 87 were children of workers with the highest likelihood for exposure, 75 were children of workers with intermediate likelihood for exposure, and 294 were children of workers with relatively low likelihood of exposure. The results suggest that an effect could be detected with reasonable probability only if the mutation risk were increased at least 100-fold in the least-exposed group (i.e., from 10-6 to 10-4) or 1,000-fold in the smaller, more-exposedgroups (i.e., from 10-6 to 10-3). Thus, the Yamakido study did not evaluate a sufficiently large sample to detect induced mutations in the offspring of exposed workers of the Okuno-jima war gas factory.
One additional report suggests that sulfur mustard may be responsible for the induction of cleft lip and palate in the offspring of exposed parents. Taher (1992) studied 21,138 live births at Najmeia Hospital in Tehran between 1983 and 1988, following the use of sulfur mustards in the Iraq-Iran conflict. He asserts that parental sulfur mustard exposures were associated with 30 of the 79 cases of cleft lip and palate that were recorded among newborns in this hospital during this period. No actual exposure data exist, although parents were questioned about their exposure to mustard gas, as well as any family history of clefts, rubella during pregnancy, dietary deficiency, and drug use during pregnancy. No information is presented regarding whether mustard exposure was maternal or paternal or both. It is also unclear if the population captured by this hospital represents the geographic area of heaviest mustard exposure during the Iran-Iraq conflict. Further, it is unclear if this incidence of clefts is truly elevated relative to other regions in this country or other parts of the world. Therefore, this study is hardly definitive. It appears to contain, however, the only other human data that attempt to address the reproductive toxicity of sulfur mustards.
It should also be noted that other animal studies have shown that the structurally similar nitrogen mustards are potent teratogens (Danforth and Center, 1954; Haskin, 1948; Murphy et al., 1958; Sanyal et al., 1981).
Summary and Conclusions
Data exist on the reproductive toxicity of sulfur mustards in more than one animal species, but it would be useful to have additional studies to examine the extent of the variability between species. More inhalation and cutaneous exposure studies would also be very helpful,
as these exposure results would more accurately mimic human exposure. Certainly studying larger numbers of animals would provide a more sensitive measure of the possible magnitude of any reproductive risk associated with exposure to sulfur mustards. Short-term, high-dose exposures would also be helpful in attempting to examine any dose-rate effects. It should also be noted that the quality of the human data on the reproductive toxicity of sulfur mustards is quite poor. There has been insufficient follow-up of the occupational or battlefield cohorts to determine the nature of any reproductive toxicity or teratogenic effects attributable to these exposures.
Evidence suggests a causal relationship between sulfur mustard exposure and reproductive toxicity in laboratory animals, but the database is far too small and uncertain to allow a clear understanding of human reproductive risk from exposure to sulfur mustards. Sulfur mustards can cause genetic alterations in the sperm of male rats after inhalation or gastric exposure, but rodent and rabbit studies showed that sulfur mustards are not detectable teratogens in animals. The human data are difficult to interpret: it is unclear if it is significant that the Japanese child with one nonheritable variant protein had the same congenital malformation (cleft palate) that was reported in association with parental mustard exposure in Iran.
Lewisite
Introduction
The literature addressing the reproductive toxicity of Lewisite is small; in fact, few data exist that advance the precise biologic fate of Lewisite in humans. Arsenic itself is known to be embryotoxic and teratogenic, and while Lewisite is an organic arsenical, in highly alkaline environments inorganic arsenic could be formed. These facts led the committee to examine the reproductive effects of inorganic and organic arsenicals, as well as Lewisite itself.
Inorganic Arsenicals. Ancel (1946) reported that sodium arsenate induced a significant reduction in size of offspring when chickens were given this compound orally. James and colleagues (1966) also reported a similar decreased size in offspring of ewes fed sodium arsenite during gestation. Ferm and Carpenter (1968) studied intravenous administration of sodium arsenate (20 mg/kg) in pregnant golden hamsters; the treatment resulted in exencephaly when injected on the eighth day of gestation. Genitourinary abnormalities, cleft lip, cleft palate, microanophthalmia, and ear deformities also were observed when exposure occurred at varying times during
gestation (Ferm, 1974; Ferm et al., 1971; Holmberg and Ferm, 1969). Ferm and Saxon (1971) also reported renal agenesis in hamsters treated with arsenic. Hood and Bishop (1972), studying IP injection of pregnant mice using 45 mg/kg of sodium arsenate, found a variety of malformations in the surviving fetuses.
Finally, Luego and colleagues (1969) report the case of a 17-year-old girl who ingested ''a dose" of inorganic arsenic during the third trimester of pregnancy. She gave birth to a baby weighing 1.1 kg who died 11 hours later. The child had very high levels of arsenic in the liver, brain, and kidney. This clearly shows that anionic arsenic can pass through the mammalian placental barrier. This is consistent with work by Morris and colleagues (1938).
Organic Arsenicals. Organic arsenicals appear to be stored in the placenta at high concentrations, but they appear not to cross the placental membrane with any great ease in humans, cats, or rabbits (Eastman, 1931; Underhill and Amatruda, 1923). Several studies of the teratogenicity of a variety of organic arsenicals have been completed. Sodium dimethylarsonate was given by gavage to CD rats (7.5-60 mg/kg per day) and CD-1 mice (200-600 mg/kg per day) on day 7-16 of gestation. Sacrifice of the animals on day 18 (mice) and day 21 (rats) showed that there was both maternal and fetal toxicity in both species (Rogers et al., 1981). Cleft palate was seen at the middle and highest doses in the mouse. In the rat, genesis of irregular rugae (stomach folds) was observed to be dose related. Both species had maternal toxicity at doses below the maximum.
Frost and colleagues (1964) found no teratogenic effects in feeding studies (0.01, 0.02, and 0.05 percent arsanilic acid) following seven generations of rats. Harrison and colleagues (1980) studied intravenous administration of sodium dimethylarsonate in pregnant CD-1 mice. On day 9 and 10 they observed increased fetal resorption and mortality rates, with increased incidence of skeletal malformations and exencephaly. Hamsters given 900-1,000 mg/kg of sodium dimethylarsonate on day 9, 11, and 12 of pregnancy had a significant dose-related incidence of fetal resorptions (Hood et al., 1982a,b). The same work showed that IP injection of methanearsonic acid into hamsters (500 mg/kg) also induced both fetotoxicity and teratogenicity.
The sodium salts of the methanearsonates administered by IP injection in rodents (500-1,500 mg/g) also caused maternal mortality (Harrison et al., 1980; Hood, 1985). Inorganic arsenic injected at doses of 5-15 mg/kg induced developmental defects in mice (Hood, 1972; Hood and Bishop, 1972). Finally, Willhite (1981) injected 20-100 mg/kg of methylarsonic acid or 20-100 mg of dimethylarsinic acid intravenously into golden hamsters on the eighth day of gestation. In this work there was no teratogenic response.
Animal Studies
Two animal studies of the teratology of Lewisite appear to exist in the literature. Hackett and colleagues (1987) studied the reproductive effects of Lewisite administered daily by intragastric intubation at 0.5, 1.0, and 1.5 mg/kg in rats and 0.07, 0.2, and 0.6 mg/kg in rabbits. These doses were chosen after careful study of the toxic effects of larger dose ranges in these animals. The highest dose used in the study of rats (1.5 mg/kg) did not cause any toxicity or teratogenicity in the maternal animals or their fetuses. In the rabbits, maternal mortality occurred in all but one of the Lewisite treatment groups. This mortality ranged from 14 percent (0.07 mg/kg) to 100 percent (1.5 mg/kg). At the 0.6-mg/kg dose a decrease in maternal body weight gains and an increase in fetal stunting occurred, with decreased fetal weights.
The second study (Sasser et al., 1989a) reported similar negative results. Rats were administered 0, 0.10, 0.25, or 0.60 mg/kg of Lewisite per day in sesame oil by gavage prior to mating, during mating, and after mating until the birth of offspring. The dams received Lewisite during lactation. At weaning, a selected group of both genders continued to receive Lewisite during adolescence, mating, and throughout gestation. Lewisite did not affect reproductive organ weights, performance, or fertility in the two generations. No adverse effects on any offspring were observed. Histologic study showed no changes in male or female reproductive organs.
The committee also received information from Colonel Richard Solana of the U.S. Army Research Institute of Chemical Defense concerning unpublished data on the reproductive toxicity of Lewisite (Appendix A). The litters of approximately 140 rats were tested. Preconception maternal exposures were to either 0.045 or 0.002 mg/cm3 by inhalation for 4 hours per day, 5 days per week for 4 months. Subsequently, the exposed females were mated to unexposed males. After 21 days the pregnant rats were sacrificed and reproductive outcomes assessed. The numbers of corpus lutea and implantations, intrauterine mortality, number and physical dimensions of fetuses, and the degree of ossification of the long bones were measured. The interpretation of the data concluded that no significant exposure-related differences were observed.
Human Studies
The work of Yamakido and colleagues (1985) on the offspring of workers at the Okuno-jima poison gas factory includes individuals exposed to Lewisite. As is noted above, this study is difficult to interpret as clearly negative, since one child was found to have an activity-variant enzymatic protein that was not familial. If this represents a significant finding, it cannot be attributed with any certainty to mustard exposure alone, Lewisite exposure alone, or the combination of the two.
Summary and Conclusions
Serious gaps clearly exist in our knowledge concerning the potential of Lewisite to cause reproductive problems. There appears to be no measurable reproductive toxicity of Lewisite in rodents when exposure was by gavage or inhalation. Our ability to extrapolate from these data to humans is limited, however. The conversion of Lewisite to other toxic forms of arsenic in vivo in animals and man is poorly understood. The comparability of the biochemical conversion of the Lewisite to potentially teratogenic arsenicals between animals and man is unknown.
More studies of multiple species are needed to define more accurately any differences between species. Also clearly needed are studies of the biologic fate of Lewisite in man. The precise nature of the products of bioconversion of Lewisite should be more thoroughly studied. The precise form of arsenic that is a potent teratogen in animals is not entirely clear. This should be combined with studies of the mechanism of action of teratogenic arsenicals. It then would be more clear that the differences in reproductive toxicity between some arsenicals and Lewisite are not related to the bioconversion of Lewisite or to select species-specific action of these compounds.
The studies of the reproductive toxicity of Lewisite in laboratory animals are negative and are therefore insufficient to indicate a causal relationship between exposure and adverse reproductive outcomes.
REFERENCES
Alexander SF. 1947. Medical report of the Bari harbor mustard casualties. Military Surgeon 101:1-17.
Ancel P. 1946. Experimental research on spina bifida. Archives d'Anatomie Microscopique et de Morphologie Experimentale 36:45-68. [In French]
Anslow WP, Houck CR. 1946. Systemic pharmacology and pathology of sulfur and nitrogen mustards. In: Division 9, National Defense Research Committee. Chemical Warfare Agents, and Related Chemical Problems. Summary Technical Report of Division 9, NDRC. Washington, DC: Office of Scientific Research and Development.
Balali M. 1986. First report of delayed toxic effects of yperite poisoning in Iranian fighters. In: Heyndricks B, ed. Terrorism: Analysis and Detection of Explosives. Proceedings of the Second World Congress on New Compounds in Biological and Chemical Warfare. Gent: Rijksuniversiteit. 489-495.
Blank JA, Joiner RL, Houchens DP, Dill GS, Hobson DW. 1991. Comparative immunotoxicity of 2,2'-dichlorodiethyl sulfide and cyclophosphamide: evaluation of L1210 tumor cell resistance, cell-mediated immunity, and humoral immunity. International Journal of Immunopharmacology 13:251-257.
Cameron GR, Carleton HM, Short RHD. 1946. Pathological changes induced by Lewisite and allied compounds. Journal of Pathology and Bacteriology 58:411-422.
Cameron GR, Courtice FC, Short RHD. 1947. Disturbances of function induced by Lewisite (2-chlorvinyldichlorarsine). Quarterly Journal of Experimental Physiology 34:1-28.
Colvin M, Chabner BA. 1990. Alkylating agents. In: Chabner BA, Collins JM, eds. Cancer
Chemotherapy: Principles and Practice. Philadelphia: J.B. Lippincott.
Coutelier JP, Lison D, Simon O, Willems J. 1991. Effect of sulfur mustard on murine lymphocytes. Toxicology Letters 58:143-148.
Danforth CH, Center E. 1954. Nitrogen mustard as a teratogenic agent in the mouse. Proceedings of the Society for Experimental Biology and Medicine 86:705-707.
Dean JH, Murray MJ. 1991. Toxic responses of the immune system. In: Amdur MO, Doull J, Klaassen CD, eds. Casarett and Doull's Toxicology. New York: Pergamon.
Eastman NJ. 1931. The arsenic content of the human placenta following arsphenamine therapy. American Journal of Obstetrics and Gynecology 21:60-64.
Ehrke MJ, Mihich E. 1985. Effects of anticancer agents on immune response. Trends in Pharmacological Sciences 6:412-417.
Eisenmenger W, Drasch G, von Clarmann M, Kretschmer E, Rolder G. 1991. Clinical and morphological findings on mustard gas [bis(2-chloroethyl)sulfide] poisoning. Journal of Forensic Science 36:1688-1698.
Ferm VH. 1974. Effects of metal pollutants upon embryonic development. Reviews on Environmental Health 1:237-259.
Ferm VH, Carpenter S. 1968. Malformations induced by sodium arsenate. Journal of Reproduction and Fertility 17:199-201.
Ferm VH, Saxon A. 1971. Amniotic fluid volume in experimentally induced renal agenesis and anencephaly. Experientia 27:1066-1068.
Ferm VH, Saxon A, Smith BW. 1971. The teratogenic profile of sodium arsenate in the golden hamster. Archives of Environmental Health 22:557-560.
Frost DV, Main BT, Cole J, Sanders PG, Perdue HS. 1964. Reproduction studies in rats with arsanilic acid. Federation Proceedings 3:291.
Gates M, Williams JW, Zapp JA. 1946. Arsenicals. In: Division 9, National Defense Research Committee, comp. Chemical Warfare Agents, and Related Chemical Problems. Summary Technical Report of Division 9, NDRC. Washington, DC: Office of Scientific Research and Development.
Goldman M, Dacre J. 1989. Lewisite: its chemistry, toxicology, and biological effects. Reviews of Environmental Contamination and Toxicology 110:75-115.
Golub ES. 1987. Immunology, A Synthesis. Sunderland, MA: Sinauer Associates. 55 p.
Graef I, Karnofsky DA, Jager VB, Krichesky B, Smith HW. 1948. The clinical and pathologic effects of the nitrogen and sulfur mustards in laboratory animals. American Journal of Pathology 24:1-47.
Hackett PL, Sasser LB, Rommereim RL, Cushing JA, Buschbom RL, Kalkwarf DR. 1987. Teratology studies of Lewisite and sulfur mustard agents: effects of Lewisite in rats and rabbits. PNL-6408, Pt. 1. Richland, WA: Pacific Northwest Laboratory. AD-A198-423.
Harrison WP, Frazier WP, Maxxanti EM, Hood RD. 1980. Teratogenicity of disodium methanarsonate and sodium dimethylarsonate (sodium cacodylate) in mice. Teratology 21:43A.
Haskin D. 1948. Some effects of nitrogen mustard on the development of external body form in the fetal rat. Anatomical Record 102:493-511.
Hektoen L, Corper HC. 1920. The effect of mustard gas (dichloroethylsulphid[e]) on antibody formation. Journal of Infectious Diseases 28:279-285.
Holmberg RE, Ferm VH. 1969. Interrelationship of selenium, cadmium, and arsenic in mammalian teratogenesis. Archives of Environmental Health 18:873-877.
Hood RD. 1972. Effects of sodium arsenite on fetal development. Bulletin of Environmental Contamination and Toxicology 7:216-220.
Hood RD. 1985. Cacodylic Acid: Agricultural Uses, Biological Effects, and Environmental Fate. VA Monograph Series. Washington, DC: U.S. Veterans Administration.
Hood RD, Bishop SL. 1972. Teratogenic effects of sodium arsenate in mice . Archives of Environmental Health 24:62-65.
Hood RD, Harrison WP, Vedel GC. 1982a. Evaluation of arsenic metabolites for prenatal effects in the hamster. Bulletin of Environmental Contamination and Toxicology 29:679-687.
Hood RD, Vedel GC, Zaworotko MJ, Tatum FM. 1982b. Distribution of arsenite and methylated metabolics in pregnant mice (abstract). Teratology 25:50A.
James LF, Lazor VA, Binns W. 1966. Effects of sublethal doses of certain minerals on pregnant ewes and fetal development. American Journal of Veterinary Research 27:132-135.
Kindred JE. 1947. Histologic changes occurring in the hemopoietic organs of albino rats after single injections of 2-chloroethyl vesicants: a quantitative study. Archives of Pathology 43:253-295.
Kindred JE. 1949. The blood cells and the hemopoietic and other organs of dogs given intravenous injections of 2-chloroethyl vesicants. Archives of Pathology 47:378-398.
Krumbhaar EB. 1919a. Role of the blood and the bone marrow in certain forms of gas poisoning. Journal of the American Medical Association 72:39-41.
Krumbhaar EB. 1919b. Bone marrow changes in mustard gas poisoning. Journal of the American Medical Association 73:715.
Krumbhaar EB, Krumbhaar HD. 1919. The blood and bone marrow in yellow cross gas (mustard gas) poisoning. Journal of Medical Research 40:497-506.
LeQuesne PM, McLeod JG. 1977. Peripheral neuropathy following a single exposure to arsenic. Journal of Neurological Sciences 32:437-451.
Lohs K. 1975. Delayed Toxic Effects of Chemical Warfare Agents. Stockholm International Peace Research Institute Monograph. Stockholm: Almqvist & Wilksell International.
Luego G, Cassady G, Palmisano P. 1969. Acute maternal arsenic intoxication with neonatal death. American Journal of Diseases of Children 117:328-330.
Luening KG. 1952. Studies on the origin of apparent gene mutations in Drosophila melanogaster. Acta Zoologica 33:193.
Morris HP, Laug EP, Morris HJ, Grant RL. 1938. The growth and reproduction of rats fed diets containing lead acetate and arsenic trioxide. Journal of Pharmacology and Experimental Therapeutics 64:420-445.
Murphy ML, Del Moro A, Lacon C. 1958. The comparative effects of five polyfunctional alkylating agents on the rat fetus, with additional notes on the chick embryo. Annals of the New York Academy of Sciences 68:762-782.
National Research Council. Committee on Biologic Markers. Subcommittee on Immunotoxicology. 1992. Biologic Markers in Immunotoxicology. Washington, DC: National Academy Press.
Papirmeister B, Feister AJ, Robinson SI, Ford RD. 1991. Medical Defense Against Mustard Gas: Toxic Mechanisms and Pharmacological Implications. Boca Raton, FL: CRC Press.
Rogers EH, Chernoff N, Kavlock RJ. 1981. The teratogenic potential of cacodylic acid in the rat and mouse. Drug and Chemical Toxicology 4:49-61.
Rozmiarek H, Capizzi RL, Papirmeister B, Fuhrman WH, Smith WJ. 1973. Mutagenic activity in somatic and germ cells following chronic inhalation of sulfur mustard (abstract). Mutation Research 21:13-14.
Sanyal MK, Kitchin KT, Dixon RL. 1981. Rat conceptus development in vitro: comparative effects of alkylating agents. Toxicology and Applied Pharmacology 57:14-19.
Sasser LB, Cushing JA, Kalkwarf DR, Mellick PW, Buschbom RL. 1989a. Toxicology studies on Lewisite and sulfur mustard agents: subchronic toxicity study of Lewisite in rats. AD-A217 886. Richland, WA: Pacific Northwest Laboratory.
Sasser LB, Miller RA, Kalkwarf DR, Buschbom RL, Cushing JA. 1989b. Toxicology studies on Lewisite and sulfur mustard agents: subchronic toxicity of sulfur mustard (HD) in rats. AD-A214-555. Richland, WA: Pacific Northwest Laboratory.
Sasser LB, Miller RA, Kalkwarf DR, Buschbom RL, Cushing JA. 1989c. Toxicology studies
on Lewisite and sulfur mustard agents: two-generation reproduction study of sulfur mustard (HD) in rats. Richland, WA: Pacific Northwest Laboratory. AD-A216-423.
Schonwald S. 1992. Mustard gas. PSR Quarterly 2:92-97.
Sonbati EM, Auerbach C. 1960. The brood pattern for intragenic and intergenic changes after mustard gas treatment of Drosophila males. Zeitschrift fur Vererbungslehre 91:253-258.
Spitz S. 1948. The histological effects of nitrogen mustards on human tumors and tissues. Cancer 1:383-398.
Spurr CL. 1947. Influence of nitrogen mustards on the antibody response. Proceedings of the Society for Experimental Biology and Medicine 64:259.
Stewart MJ. 1918. Report on cases of poisoning by mustard gas (dichlorethylsulphide) with special reference to the histological changes and to alterations in the leukocyte count. Chemical Warfare Medical Committee (London). Report 17. As cited in: Smith H. 1943. Review of the literature on the systemic action of mustard gas to August 1, 1943. OSRD Report No. 1717. New York University. Prepared for the Office of Scientific Research and Development.
Taher AA. 1992. Cleft lip and palate in Tehran. Cleft Palate Craniofacial Journal 29:15-16.
Twomey JJ, ed. 1982. The Pathophysiology of Human Immunologic Disorders. Baltimore: Urban & Schwarzenberg.
Underhill FP, Amatruda FG. 1923. The transmission of arsenic from mother to fetus. Journal of the American Medical Association 81:2009-2015.
Vos JG. 1977. Immune suppression as related to toxicology. CRC Critical Reviews in Toxicology 5:67-101.
Willems JL. 1989. Clinical management of mustard gas casualties. Annales Medicinae Militaris Belgicae 3:S1-61.
Willhite CC. 1981. Arsenic-induced axial skeletal (dysraphic) disorders. Experimental and Molecular Pathology 34:145-158.
Yamakido M, Nishimoto Y, Shigenobu T, Onari K, Satoh C, Goriki K, Fujita M. 1985. Study of genetic effects of sulphur mustard gas on former workers of Okuno-jima poison gas factory and their offspring. Hiroshima Journal of Medical Sciences 34:311322.
Zandieh T, Marzban S, Tarabadi F, Ansari H. 1990. Defects of cell-mediated immunity in mustard gas injury after years (abstract). Scandinavian Journal of Immunology 32:423.
Zimmerman T. 1942. As cited in: Smith HW. 1943. Review of the literature on the systemic action of mustard gas to August 1, 1943. OSRD Report No. 1717. New York University. Prepared for the Office of Scientific Research and Development.




















