3
History and Analysis of Mustard Agentand Lewisite Research Programsin the United States
This chapter begins with an introduction that briefly describes sulfur mustard and Lewisite and their effects, accompanied by an overview of their development. This is followed by a description of the organization of chemical warfare research during World War I (WWI) and the postwar period of 1919 to 1940, including the development of Lewisite and nitrogen mustard. The major focus of this chapter, however, is to describe the research programs and protocols relating to mustard agents and Lewisite, initiated just prior to World War II (WWII) and continued throughout the war.
This committee also investigated as many protocols and supporting military documents as it could obtain for use in estimating the possible exposure levels experienced by the men who participated in the mustard and Lewisite tests. These estimates were intended to put into context the concentrations of vesicant used in animal and other types of experiments, which the committee was also charged to survey. As these protocols were investigated, it became apparent to the committee that the full body of knowledge available to the wartime scientists, especially information relevant to the long-term health outcomes of exposure to these agents, was not applied in the conduct of the human experimentation. Thus, this chapter begins to address compelling questions that emerged through the course of this study regarding the appropriate use of the existing scientific and medical literature in WWII testing programs, the lack of medical follow-up of human research subjects, and the probable exposure levels experienced by these subjects.
Finally, the chapter overviews the research programs since the end of WWII, including the continuing investigations concerning the mechanisms of toxicity of these agents. Description of the chemical stockpile
disposal program is also included. The chapter concludes with an outline of some of the conclusions drawn by the committee from analysis of the historical records and calculations of exposure levels.
INTRODUCTION
Sulfur Mustard
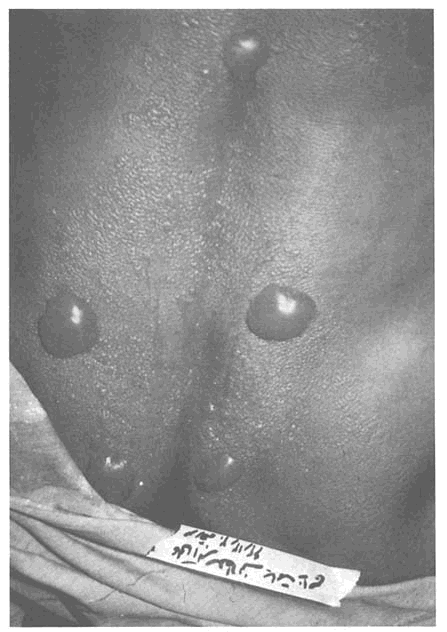
Sulfur mustard (C4H8Cl2S) is one of a class of chemical warfare agents known as vesicants because of their ability to form vesicles, or blisters, on exposed skin (see Figure 3-1). During WWI, exposed troops described the odor of this agent as a stench like mustard or garlic, hence its common name. Table 3-1 summarizes some characteristics of mustard agents and Lewisite. First noted for its toxic properties by dye chemists in the late 1880s, sulfur mustard has been referred to by a number of synonyms: S-mustard, to distinguish it from nitrogen mustard; ''Lost" or "S-Lost," from the names of two chemists who suggested it be used as a war gas (Lommel and Steinkopf); "yellow cross," for the identifying mark on WWI shells containing sulfur mustard; or Yperite, after the site of its first use in 1917. Although commonly and inaccurately referred to as mustard gas, the agent is a liquid at room temperature.
Sulfur mustard produces skin blisters and damage to the eyes and respiratory tract, and it can be lethal at sufficiently high doses. It is a cellular poison and mutagen and a recognized human carcinogen. Battlefield use of sulfur mustard decreases the opponent's ability to fight by producing chemical burns on tissues that come into contact with either vapors or liquid droplets and aerosols. Exposed skin surfaces, eyes, and the linings of both the respiratory and the gastrointestinal tracts are all at risk, and the risks increase dramatically under hot, humid conditions.
From a military standpoint, one of sulfur mustard's most useful properties is its persistence. Droplets of this agent released in an explosion can deposit on numerous surfaces, evaporating slowly and posing a risk from inhalation as well as contact with the skin. Indeed, this very set of conditions was observed in WWI after mustard shelling (Haber, 1986). One reason for this persistence is the characteristic freezing temperature of sulfur mustard (13°C to 15°C). Droplets or bulk quantities would thus be expected to remain where initially deposited during cool or winter weather, under forest canopies, or under overgrown vegetation. Under certain conditions, bulk quantities of mustard agent spilled or splashed onto the soil would not degrade for months.
The exact date of the first sulfur mustard synthesis is somewhat unclear, but the first report may have been by Despretz in 1822. An 1860 report by Neimann describes a delayed-effect vesicant oil as a reaction product of ethylene on a mixture of sulfur chlorides. At that time, this
TABLE 3-1 Chemical and Physical Data
|
|
Sulfur Mustard |
Lewisite |
Nitrogen Mustard |
|
Chemical Abstracts Registry Number |
505-60-2 |
541-25-3 |
51-75-2 |
|
Chemical formula |
C4H8Cl2S |
C2H2AsCl3 |
C5H11Cl2N |
|
Chemical structure |
CH2-CH2Cl |
H AsCl2 |
CH2-CH2Cl |
|
|
S |
|
H3C-N |
|
|
CH2-CH2Cl |
Cl H |
CH2-CH2Cl |
|
Synonyms |
1,1'-Thiobis(2-chloroethane); 2,2'-dichloroethyl sulfide; bis(2-chloroethyl) sulfide; ,'-dichloroethyl sulfide; mustard gas; Schwefel-Lost; S-Lost; Yperite; yellow cross; Senfgas; Kampstoff "Lost"; dichlorodiethyl sulfide |
Chlorovinyldichloroarsine; 2-chlorovinyldichloroarsine; chlorovinylarsine dichloride; dichloro(2-chlorovinyl)arsine |
Mechlorethamine; chlormethine; 2-chloro-N-(2-chloroethyl)-Nmethylethanamine; Stickstofflost; di(chloroethyl)methylamine |
|
Abbreviations |
H (Levinstein mustard), HD (distilled mustard), HT (impure mixture) |
L |
HN2; related compounds include HN1, ethylbis(chloroethyl)amine; and HN3, tris(-chloroethyl)amine |
|
Melting point |
13C-14C |
0.1C |
-60C |
|
Boiling point |
215C-217C at 760 mm Hg |
190C at 760 mm Hg (decomposes) |
87C at 18 Hg |
|
Molecular weight |
159.08 |
207.32 |
156.1 |
|
Solubility |
Very sparingly soluble in water; soluble in oily solvents; high lipid solubility |
Insoluble in water, soluble in ordinary organic solvents |
Very slightly soluble in water |
|
Appearance and odor |
Colorless when pure, normally yellow to brown oily liquid, slight garlic-type odor |
Liquid, faint odor of geranium |
Liquid, faint fishy odor |
|
SOURCES: Budavari, 1989; Hazardous Substances Databank, 1991; IARC, 1975; Somani, 1992; U.S. Army CRDEC, 1988, 1990. |
|||
product was identified as a compound [(C2H4)2S2Cl2] different from sulfur mustard; however, the observed severe skin blistering, latent period of several hours, and subsequent slow healing are all typical of skin exposure to sulfur mustard. At about the same time, Guthrie (1859, 1860) published investigations describing yet another variant compound (thought to be C4H4S2Cl2), also produced from sulfur chloride in reaction with ethylene. The odor was "pungent," resembling that of "oil of mustard." Guthrie noted destruction of the epidermis when the thin skin between the fingers and around the eyes was exposed to the ''vapour" of this compound. When the liquid was allowed to remain on the skin, blister formation was observed. Finally, in 1886, a process to produce significant quantities of pure sulfur mustard was described by Meyer using sodium sulfide, ethylene chlorohydrin, and hydrochloric gas (Jackson, 1936; Meyer, 1886; Prentiss, 1937; West, 1919). This process was the one eventually used by the German war factories to fill the shells fired at Ypres (Haber, 1986).
Lewisite
Lewisite (C2H2AsCl3) is a vesicant that contains organic arsenic. During WWI, a U.S. chemical warfare research laboratory investigating arsenic compounds as potential war gases developed the potent vesicant, subsequently named "Lewisite" after the research group director. Purified Lewisite is a colorless, oily liquid at room temperature with a faint "geranium-like" odor. More volatile than sulfur mustard, this agent can be used as a vapor over large distances and has been mixed with sulfur mustard to achieve greater effectiveness in combat. With a freezing point between -18°C and 0°C, Lewisite is effective over a wider temperature range than sulfur mustard.
Lewisite is also a cellular poison, but works via a different mechanism than sulfur mustard. It is readily absorbed through the skin and respiratory tract, but moist tissues are particularly vulnerable and eyes exhibit the greatest sensitivity (Trammell, 1992; Watson and Griffin, 1992). In contrast to sulfur mustard, Lewisite exposure is characterized by immediate onset of pain. The agent is lethal at sufficient doses, produces chromosomal aberrations in some mammalian cellular assays, and is a systemic poison when absorbed into the bloodstream. Finally, some evidence suggests that Lewisite might be a carcinogen (Centers for Disease Control, 1988).
The development of Lewisite as a war gas was made by W. Lee Lewis in 1918, while working at the Chemical Laboratory of the Catholic University of America in Washington, D.C. (Lewis and Perkins, 1923). The thrust of the work in this laboratory during WWI was the evaluation of substituted arsines (arsenic-containing chemicals) as potential chem-
ical warfare agents. Lewis had noticed a paragraph in a 1904 student dissertation by J.A. Nieuwland that documented the formation of an "extremely poisonous" substance after a reaction of arsenic chloride with dry acetylene in the presence of aluminum chloride (cited in Lewis and Perkins, 1923). The toxicity that had caused Nieuwland to stop further work on the reaction spurred Lewis to investigate the substance more fully. In addition, Lewis and his group worked out safer and more efficient production methods and elaborated plans for large-scale production (Lewis and Perkins, 1923; Lewis and Steigler, 1925). A production plant was eventually constructed in Willoughby, Ohio, and approximately 150 tons of Lewisite were in transit to Europe when the Armistice was signed in November 1918. The vessel was sunk at sea (Spiers, 1986; Tarbell and Tarbell, 1981; Trammell, 1992), and all experimental work with Lewisite in the U.S. Chemical Warfare Service abruptly ceased until WWII (Gates et al., 1946).
RESEARCH PROGRAMS OF WORLD WAR I AND THE POSTWAR PERIOD
As outlined above, prior to the actual use of sulfur mustard as a war gas in 1917, the substance was little more than an interesting compound produced, along with hundreds of other compounds, by the emerging science of industrial chemistry in the last half of the nineteenth century. Thus, tragically, the combat casualties of WWI became the first large group of experimental subjects in studies of the medical effects of sulfur mustard. Organized research into chemical warfare agents began in earnest in Britain and France after the German chlorine gas attack in 1915. In the United States, it was 1917 before a formally organized chemical warfare research program was established. The history of the program has been documented by various authors and summarized by Cochrane (1946) in a classified report released to the public in 1991. The program began with an offer from the Bureau of Mines to the National Research Council (NRC) to mobilize the bureau's unique and specialized laboratories toward the investigation of poison gases.1 With the U.S. declaration of war against Germany in 1917, the NRC Committee on Noxious Gases was formed to administer the research programs concerning poison gases, including sulfur mustard and later Lewisite.
In the United States and Europe, much of the research was focused on methods of mass production of sulfur mustard, development of other vesicants and war gases, and development of better gas masks and other
|
1 |
The National Research Council was in 1917 and is today part of the National Academy of Sciences. The NRC was directly involved in defense research programs during both World War I and World War II. A description of this involvement is included in Appendix C. |
equipment to protect troops from chemical attack. The overall research program was divided into sections, each of which was responsible for specific types of research, ranging from gas production processes to treatment of gas casualties. As the program matured, the various organizational structures were modified. The details of these modifications are not presented here because they are largely irrelevant to consideration of the health effects of mustard agents and Lewisite. One modification, however, may have set the stage for how research into these substances' medical effects has been conducted and directed ever since.
In 1918, a presidential order moved the research program from essentially civilian control under NRC to military control under the War Department. This move gave birth to the Chemical Warfare Service (CWS), which, to the present day, is responsible for the majority of research concerning chemical and biological warfare agents. As time went on this administrative change altered the direction of almost all investigations into the toxicology of vesicants and other chemical agents. Mainstream biomedical science is "hypothesis driven": when interesting results are obtained by an investigator, either that investigator or other groups begin further research to better understand what has been discovered, even if the interesting results are not directly relevant to the original questions being asked. In addition, the results of most biomedical research are published in "open literature," critically reviewed by outside experts and available to all.
In contrast, most military research is "applications driven": priorities are determined on the basis of military needs (e.g., treatment of acute injuries, development of protective clothing), and results not directly relevant to the original questions are seldom pursued. Such research is commonly classified and is published only for other military groups. The tight controls and restrictions on military research can result in a "stunted" body of literature that presents major limitations to later assessments in areas that were never pursued—in this case, the long-term health effects caused by exposure to chemical agents in general, and mustard agents and Lewisite in particular.
Researchers in the medical aspects of chemical warfare began their work in 1917 with few of the guideposts that are normally available from previous studies. The only literature available on the medical effects of sulfur mustard and Lewisite was that produced by the English and French, who had only a small head start with their research programs. Nevertheless, perusal of the significant papers published after the war from these groups reveals that multiple lines of investigation were quickly initiated and pursued. Some of the work done by the medical research groups examined the mechanisms of absorption of mustard agents into human skin, the effects of various ointments and antidotes
on the severity of blisters and skin damage, and the pathological changes in the respiratory tract following inhalation of sulfur mustard vapor. Cochrane's history lists 22 papers and books published by 1920, which stood as the distillation of all significant investigations completed in the United States during WWI (Cochrane, 1946).
With the end of the war, the research program came to an end and CWS decreased drastically in size. The many research and production locations were also completely consolidated at the Edgewood Arsenal in Maryland. After a short period of relative uncertainty about its continued existence, CWS became part of the U.S. Army by an amendment to the National Defense Act on July 1, 1920.
Two later books included comments on the long-term effects of warfare gases. In 1925, Vedder published Medical Aspects of Chemical Warfare, in which he discounted any significant long-term sequelae of gassing and introduced the concept that "neurasthenic" conditions (an archaic term describing lassitude, decreased energy, and impaired functioning that, in Vedder's work, seemed to be used as a synonym for "psychosomatic") were the underlying factors in most veterans' claims of disability. Colonel Harry L. Gilchrist, in contrast, published an extensive comparative study on WWI casualties in 1928 that included detailed clinical descriptions of men who had been gassed in combat and a carefully researched chapter on the probable residual health effects of various gases (Gilchrist, 1928a,b). Gilchrist's work was a major contribution to knowledge about vesicant toxicity. Based on clinical examination of human gas victims and some animal experiments, as well as later follow-up studies of WWI veterans, Gilchrist found that the long-term effects of sulfur mustard were mainly respiratory, including emphysema, chronic asthma, and chronic bronchitis. Chronic conjunctivitis and corneal opacities were also described later by Gilchrist and Philip B. Matz (1933a,b; also see Appendix B for excerpt from Gilchrist and Matz, 1933b).
The period from 1920 until 1936 also saw the establishment of a Medical Research Division within the CWS. This group continued toxicological studies of chemical warfare agents, including sulfur and nitrogen mustards and Lewisite; investigated the lethal and sublethal concentrations of the agents; and renewed investigations into protective ointments. In addition, the group formalized what was known regarding treatment of gas casualties and attempted to examine the residual effects of exposure to various chemical warfare agents. Its work involved both animal and human experimentation. The experiments with human subjects, however, used only a few subjects mostly drawn from personnel, including the scientists themselves, working at Edgewood Arsenal.
For mustard agents and Lewisite, no major breakthroughs were made by the research efforts between 1920 and 1936. According to Cochrane,
the lack of progress was traceable to a variety of factors. One of these factors was funding, often in short supply in a peacetime environment. Also, to appease public concern about poison gas production, significant efforts were spent trying to find peacetime uses for these agents. Another factor was the constant struggles and competition between different branches of the military, different departments within the CWS, and different scientific disciplines. Cochrane reports that the medical researchers, in direct competition with apparently more productive chemists, were especially vulnerable to funding shifts.
Thus, by the dawn of WWII, what was known about mustard agents and Lewisite (or many other agents, for that matter) was not organized into a cohesive body of literature. The clinical picture of the acute effects of exposure and some of the mechanisms of toxicity were well known (Gilchrist, 1928a,b; Vedder, 1925). There were clear guidelines for treatment of casualties, but the treatments were solely palliative. No effective ointments had been developed and nothing was available to prevent skin and lung damage. Even less was known about the long-term effects. So unorganized was the scientific base concerning vesicants that, when the 1941 version of the training manual for treatment of gas casualties (TM 8-285) was prepared for use in treating expected casualties in WWII, it did not include the carefully documented long-term effects of exposure reported by Gilchrist in 1928 and by Gilchrist and Matz in 1933.
This omission, not explained in Cochrane's history or elsewhere, was surprising to this committee. It is improbable that CWS did not know of Gilchrist's work, because it had been published in "open, nonclassified" literature, including one journal. In retrospect, we know that such an omission may well have unfavorably influenced the treatment and long-term follow-up of gassed soldiers in WWII, had such casualties occurred. In terms of the WWII testing programs with human subjects, this omission—coupled with an apparent disregard for the long-term effects of gas exposure—may have contributed to the absence of follow-up of these human subjects, despite the fact that the end points of many of the experiments were skin injury and burns (also see Chapter 4).
TESTING PROGRAMS AND CHEMICAL WARFARE PRODUCTION IN WORLD WAR II
As the war in Europe eroded U.S. neutrality, preparations began to revitalize and expand the activities of the Chemical Warfare Service. In order to obtain a greater base of scientific expertise, the War Department again came to the National Research Council for help (see Appendix C). In 1941, the research effort was reorganized and subsumed under a newly established Office of Scientific Research and Development
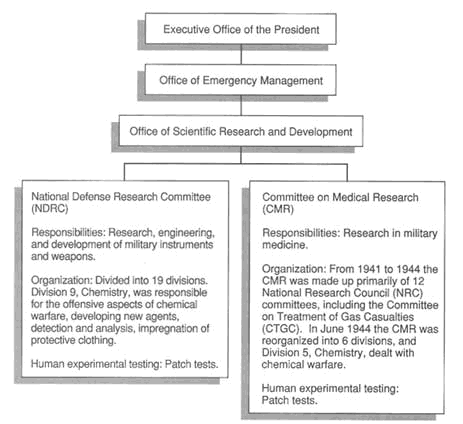
FIGURE 3-2 Organization of World War II civilian scientific research and testing programs. Chamber and field tests were conducted by the Chemical Warfare Service and the Navy Department, Office of Research and Inventions. Civilian researchers from the NDRC and CMR worked in close communication with the military. SOURCE: OSRD, 1946; Stewart, 1948.
(OSRD), eventually comprised of two working branches, the Committee on Medical Research (CMR) and the National Defense Research Committee (NDRC), and an Advisory Council. NRC's Committee on the Treatment of Gas Casualties (CTGC), experts in the fields of medicine and biological sciences, began working closely with the CMR to aid the effort by administering and supervising government grants to researchers and universities for a wide range of research regarding chemical warfare agents (see Figure 3-2 for OSRD organizational chart).
One of the first assignments given to the CTGC was to review the scientific literature on the physiological effects of sulfur mustard and Lewisite, and on the methods tested for protection against injury and treatment of gas burns. The focus of the literature review was on acute
toxicity data, but included longer-term effects. The first reviews were distributed among the various NDRC and CMR groups in August 1943 (Smith, 1943). There is no evidence that these reviews resulted in any changes or modifications of the protocols for the treatment or follow-up of human subjects in the experimental programs. On the subject of long-term effects of exposure, only Vedder's work, and not Gilchrist's, is mentioned in this review.
The focus of the following section is on the experiments conducted in the United States using human subjects; consideration of the animal experiments is included in later chapters, which survey the scientific literature. Similar experiments were also conducted in Great Britain, Canada, Australia, and the Soviet Union, as well as in Germany and Japan. The details of these experiments and these countries' chemical weapons programs are not included here. However, numerous references that provide information regarding these programs were examined by the committee, are included in the bibliography, and are specifically cited in this report where appropriate. One of the richest sources for information on the gas chamber experiments was reports released from the Naval Research Laboratory (NRL). Other primary source information regarding gas chamber experiments in locations other than the NRL was obtained by the committee from Edgewood Arsenal in Aberdeen, Maryland.
CWS carried out three basic types of experiments with human subjects. According to Cochrane, these testing programs involved the use of approximately 60,000 human subjects. Patch, or drop, tests were the most common and were used to assess the efficacy of a multitude of protective or decontamination ointments, treatments for mustard agent and Lewisite burns, effects of multiple exposures on sensitivity, and the effects of physical exercise on the severity of chemical burns. In addition, drop application of liquid mustard agents was commonly used in basic training to raise single blisters to impress upon the trainees the toxicity of these agents and the need for immediate responses to any orders to don gas masks. Chamber tests of various types were conducted to test the effectiveness of protective clothing, all of which had been impregnated with chemicals to retard vapor penetration. Finally, field tests involved the contamination of large or small areas of land with sulfur mustard or Lewisite. Human subjects were used in field tests to test protective clothing, to monitor the effects of the agents on animals in the test sites, and to take measurements of agent concentrations in soil and water samples. Table 3-2 summarizes the known major locations of these tests and the types of experiments done in each location.
Many veterans who were subjects in the chamber tests have obtained detailed records of their exposures from the Naval Research Laboratory. These reports often employ an outdated scientific notation. Table 3-3
TABLE 3-2 Known Gas Testing Facilities and Test Typesa
TABLE 3-3 Concentration Versus Cumulative Exposure Level: Explanation of Notations in NRL Reports and Modern Literature
|
Concentration |
|
|
γ/La |
Used in NRL reports to signify micrograms (µg, also called gamma) per liter (1, according to modern notation) or µg/l |
|
mg/m3 |
milligrams per cubic meter, equivalent to µg/l |
|
ppm |
parts per million, a volume to volume measurement that, at 25°C at sea level, is equal to 6.5 mg/m3 of sulfur mustard |
|
Cumulative Exposure |
|
|
CT |
Used in NRL reports to signify concentration (C) multiplied by time (T); equal to mg·min/m3, modern notation uses t to signify time (Ct) |
|
a The use of L to signify liters is confusing because L is also the abbreviation for Lewisite. However, used with micrograms in the NRL reports, L signified liters. SOURCE: Taylor et al., 1943. |
|
summarizes the various notations used in NRL reports, along with other sources, to express concentrations, and compares these with modern notation. In addition, this table illustrates the difference between atmospheric concentration and the concept of cumulative exposure, in which
both concentration and time of exposure are noted. For purposes of this report, the more modern notation is used and cumulative exposures are expressed in Ct (equivalent to mg min/m3) to avoid repetition, because the vast majority of chamber tests were conducted in 60-minute trials. To place the concentrations that follow into additional context, Table 3-4 summarizes the concentrations required to produce specific physiological effects.
For further comparison, it may be useful to consider two additional calculations. First, according to the International Agency for Research on Cancer (IARC, 1975), the average and maximum atmospheric concentrations of sulfur mustard in combat zones were estimated to be approximately 20 and 33 mg/m3. To determine exposure levels, however, one must consider the duration of exposure to a given atmospheric concentration. The exposure threshold for death from respiratory damage has been estimated to be between 1,000 and 1,500 mg·min/m3 (Ct, see Table 3-4). Thus, fatal exposures on the battlefield in WWI must have lasted between 50 and 75 minutes (the product of 50 minutes and 20 mg/m3 would equal a Ct of 1,000), if the estimated atmospheric concentrations were sustained, or longer if the concentrations dropped substantially.
Recent Centers for Disease Control (CDC) recommendations for safe levels of exposure to mustard agents and Lewisite provide a second useful frame of reference. Responding to the mandated destruction of all unitary lethal and chemical munitions, CDC published chemical agent control limits for atmospheric exposures to chemical munitions in the Federal Register in 1988. For general population exposures, the limits are 0.0001 and 0.003 mg/m3 for sulfur mustard and Lewisite, respectively. For workers directly involved in munitions removal and destruction, the limits (averaged over 8 hours) are 0.003 mg/m 3 for both agents (CDC, 1988).
It is important to remember, when considering these comparisons, that the battlefield and estimated safe occupational levels of sulfur mustard and Lewisite refer to atmospheric concentrations, rather than "dose" level. However, it is the concentration and cumulative exposure to an unprotected target tissue (e.g., the eye, skin, or breathing passages) that determines the dose received and thus the damage to tissues from these agents. The presence of protective clothing and/or a gas mask reduces considerably the amount of agent reaching such a target tissue. Sections of this chapter to follow contain estimates and analysis of the probable cumulative exposures achieved in the chamber and field tests, as well as occupational situations.
Patch or Drop Tests
Information on the specific protocols used in patch tests was obtained from a variety of sources, including archived materials from the NRC
TABLE 3-4 Summary of Major Biological End Points Characterizing Sulfur Mustard Exposure to Humans (at 16°C-27°C unless otherwise noted)
|
|
Effects End Points |
||||
|
Exposure Route |
Thresholda |
Injured (nondisabling) |
Incapacitationb |
Death or Permanent Injury |
References |
|
Eye/vapor (mg·min/m3) |
2 (at = 32°C) 12 |
50-100 |
200 (ICt50)c |
>800 |
Gates and Moore, 1946; McNamara et al; 1975; Papirmeister et al., 1991; Project Coordination Staff, 1946; Stepanov and Popov, 1962 Urbanetti, 1988; U.S. Army, 1974; U.S. Army CRDEC, 1990; U.S. Army and Air Force, 1975 |
|
Respiratory tract/vapor (mg·min/m3) |
12-70 |
<100 |
200 (ICt50) |
1,000 1,500 (LCt50)d |
Ganas, 1969; McNamara et al., 1975; Project Coordination Staff, 1946 Robinson, 1967; Sidell, 1990; Stepanov and Popov, 1962; Stroykov, 1970; U.S. Army, 1974; U.S. Army CRDEC, 1990 |
|
Skin/vapor (mg·min/m3) |
5 (maximum safe Ct) 50 (at 32°C) 100-200 |
100-300 |
1,000 (ICt50 at 32°C, low humidity) 2,000 (ICt50 at 21°C-27°C, humid) |
10,000 (LCt50) |
Gates and Moore, 1946; McNamara et al., 1975; Papirmeister et al., 1991; Project Coordination Staff, 1946; Sidell and Hurst, 1992; U.S. Army, 1974; U.S. Army CRDEC, 1990 |
|
Skin/liquid |
10 µg 10-20 µg/cm2 32 mg/man (HD) 3.5-4 mg/man (HT) |
No reported estimates |
770 mg/man 3,000-7,000 mg/man |
100 mg/kg (LD50) 64 mg/kg (reported human lethal dose) |
Papirmeister et al., 1991; Robinson, 1967; Sidell, 1990; U.S. Army, 1974; U.S. Army CRDEC, 1990; WHO, 1970 |
|
a Threshold is considered first indication of nondisabling signs and symptoms (eye reddening, erythema, and blisters). b Incapacitation is considered inability to perform designated duties. c The Ct expected to incapacitate 50 percent of those exposed. d The Ct expected to cause death in 50 percent of those exposed. SOURCE: Adapted from Papirmeister et al., 1991, Watson and Griffin, 1992. |
|||||
Committee on Treatment of Gas Casualties, the Summary Technical Report of the National Defense Research Committee (OSRD, 1946, declassified in 1960), and from the Fasciculus on Chemical Warfare Medicine, Volume III: Skin and Systemic Poisons (NRC, 1945, declassification date unknown). It should also be noted that some subjects participated in tests in which only the protective ointments were applied to test skin sensitivity to the ointments themselves (many of the ointments were found to be highly irritating and corrosive to the skin). Analysis of the amounts of vesicant used was difficult, however, due to great variability in reporting of concentrations and cumulative exposures in individual experiments.
There were three types of delivery systems for patch testing. One type was called ''Edgewood Rods," which were stainless steel rods with tips of varying diameters that were dipped into liquid sulfur or nitrogen mustard, or Lewisite, and then touched to the skin of a subject, usually on the forearm. A second type, "drod," was constructed from a small syringe that could deliver a measured amount of liquid to the skin. Various types of "vapor cups" were also used. The most common was the Edgewood Vapor Cup, a small glass cup similar to a beaker in which a section of filter paper saturated with liquid vesicant was placed. The cup was placed on the skin, allowing the vapor to rise from the filter paper and contact the skin. The cumulative exposures achieved in the vapor cups have been estimated to be 40,000 to 78,000 Ct. In some experiments, vapor cups were left on the skin for 15 minutes; in others, the cups were applied every 5 minutes for up to 3 hours and 40 minutes; in yet others, the cups were left on for more than an hour. Liquid patch tests, employing rods or drods, were more common than vapor cup tests and exhibited a wide variability in cumulative exposures.
Most of these experiments involved the application of liquid vesicant either before or after some test ointment. Most often, there were two or more sites on the forearm to which the vesicants were applied, thus providing for control sites at which no ointments were applied, and the liquids were allowed to remain on the skin for up to 2 minutes. The amounts used in these types of patch tests ranged from 0.15 to 7 mg for mustard agents and 1.4 to 7 mg for Lewisite. In some experiments, concentrations were expressed in micrograms. In still other experiments of this type, concentrations were also expressed as dilutions, ranging from 1:100 to 1:50,000 sulfur mustard, or Lewisite, to solvent. To further complicate analysis, a number of different solvents were used, including benzene, alcohol, paraffin oil, and chloroform.
Chamber Tests
Because the chamber tests were largely designed for the technical development of protective clothing, these tests were conducted by CWS
and NRL in communication with the NDRC, rather than the CMR and CTGC. Thus, the sources used for information on concentrations and protocols for chamber tests included the Summary Technical Report of the NDRC and NRL technical reports. However, these sources are not exhaustive, and the details of chamber tests in locations such as Edgewood Arsenal and Great Lakes Naval Training Center were not made available to the committee for evaluation. Further, only the NRL has maintained accurate records of the individuals who participated in the tests (close to 2,500 men). Lacking similar information from the other locations, the total number of individuals involved in chamber tests is unknown. The vast majority of those participating in chamber tests were Caucasian men. A small number of African American and Japanese American soldiers were recruited for tests to determine possible differential skin effects of sulfur mustard on members of these races.
Similar to patch tests, there were a variety of types of chamber tests. For some chamber tests, the major questions were how long, under what conditions of temperature, and under what concentrations of gas would chloramide- or activated carbon-impregnated clothing afford protection of personnel against chemical attack with vesicants. The vesicant used most often was sulfur mustard, but nitrogen mustard and, probably, Lewisite were also used. These tests were called "man-break" tests. The common procedure was to equip men with gas masks2 and clothe them in the impregnated suits (see Figure 3-3). The men would then enter the gas chamber (Figure 3-4) and remain there for periods from 60 minutes to 4 hours. The interiors of the chambers were most often maintained at 90°F and 65 percent relative humidity, because investigators were specifically interested in the durability of protective clothing under tropical conditions. Following the period in the chamber, the men wore their gas masks for an additional 5 minutes and remained in the suits for additional periods of time, ranging from 4 to 24 hours3 (Taylor et al., 1943; see Appendix D for excerpts and the Military Reports section, U.S. Navy, of the Bibliography for a complete listing of NRL reports examined). Twenty-four hours after each chamber trial, the men were examined for reddening of the skin (erythema), evidence that the vapor had penetrated the
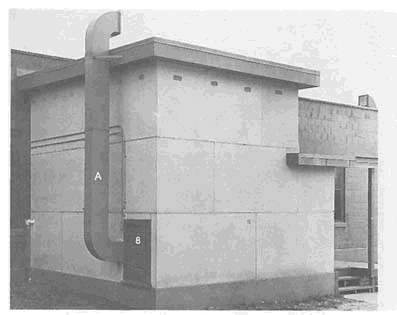

FIGURE 3-4 (A) Naval Research Laboratory (NRL) gas chamber. Interior dimensions of this chamber were 10 ft. by 15 ft., with a 12 ft. high ceiling. The chamber was designed to fit ten men and allow room for moderate exercise. (B) Photograph of the inside of a similar gas chamber used at Edgewood Arsenal for World War II chamber tests (the numbers refer to specific equipment). SOURCE: Taylor et al., 1943. Photographs provided by Naval Research Laboratory (A) and U.S. Army Chemical and Biological Defense Agency (B).
suits and burned the skin. The men were required to repeat the procedure and enter the chambers either every day or every other day until they developed moderate to intense erythema. The number of trials tolerated depended largely on the vesicant concentration in the chambers. For example, a technical report by Taylor and his colleagues documents a specific set of 60-minute trials in which, at a cumulative sulfur mustard exposure level of 600 Ct per trial, the average number of trials tolerated was 5.3, representing a low of 4 trials and a high of 14 trials (Taylor et al., 1945). In trials with cumulative exposures of 9,600 Ct per trial, the average number of trials tolerated was estimated at two, because all the men developed some erythema after the first trial.
The anatomical locations and intensity of erythema are reported in Taylor's paper for each individual. The majority of men experienced intense erythema that was widespread over their bodies, especially in moist areas of skin folds, such as behind the knee and under the arms, in large areas of the chest and shoulders, and on their arms and legs. Little involvement of areas such as the scrotum and buttocks was reported for this particular set of trials, possibly because the men wore an extra layer of impregnated undergarments.
In another set of trials reported by Heinen and his colleagues (1945), multiple cumulative exposure levels ranged from 50 to 700 Ct. In these experiments, the subjects were engaged in different levels of physical activity before, during, and after the chamber trials. No data, however, are included regarding the tests in which activity was performed inside the chambers. Significantly, subjects in this set of trials were not completely dressed in protective clothing. Most were dressed in standard issue attire but wore carbon-impregnated suspenders. It was thought that the suspenders would protect a strip of skin that could then be compared with skin areas that were unprotected. In addition, only a few men were given impregnated underwear. Results from this set of trials are documented with photographs that show burns to the genital areas of many of the men. In one series, of 24 men participating, 13 experienced crusted lesions to the scrotum that were characterized as severe, and 8 experienced severe lesions to the penis. These lesions took up to one month to heal, according to the report.
In general, these two reports are representative of many of the chamber tests conducted at NRL (Washington, D.C.), Edgewood Arsenal (Maryland), Great Lakes Naval Training Center (Illinois), and, for a short time, at Camp Sibert, Alabama. The concentrations, times of exposures, and types of chemical agents used in other locations may not be similar, however, and full reports of other chamber tests were not made available to this committee. There is evidence that some chamber tests may have been done with higher cumulative exposures, because
Taylor and colleagues (1945) refer to ranges of 3,000 to 11,000 Ct of sulfur mustard used at Edgewood Arsenal.
Chamber tests were also conducted in Panama, North Carolina, and Maryland. These tests were called "wear tests," designed to see how the impregnated clothing stood up to use under possible combat conditions. Thus, the men wore the protective suits during drills and combat simulations, ranging from 1 day of amphibious training to 6 weeks of simulated combat. Following this, the men entered gas chambers in these suits, for 60-minute trials (as in the man-break tests), until erythema developed. The data reported in the Summary Technical Report of the NRDC (OSRD, 1946) show a range of 1.0 to 2.2 hours chamber time before erythema developed in this group of subjects. These data are difficult to compare with other chamber tests, however, because only micrograms of sulfur mustard were reported without the usual accompanying notation regarding volume. If one assumes that the micrograms listed were per liter, then the exposures ranged from 300 to 2,400 Ct in this series of chamber trials. Finally, some men participated in arm chamber tests of protective ointments or clothing materials. In these, the arms of the men were placed in a wind tunnel with cumulative exposures reported to be 1,200 Ct.
Some physiological measurements, including temperature and blood counts, were done on men participating in some of the tests, but these physiological measurements were not generally reported in the technical summaries. For those men who participated in Naval Research Laboratory tests, records of the experimental conditions, as well as any physiological measurements, were kept for each test subject and are available from NRL to individuals through the Freedom of Information Act (also see Chapter 4).
Field Tests
Many field tests with mustard gas were conducted with human subjects, but relatively little information is available. Known field tests were conducted by the United States and Australia in various locations (Freeman, 1991; Gillis, 1985; OSRD, 1946) (Table 3-2). Apparently, 1,000 U.S. servicemen participated in these field tests, a number that is supported by the discovery of a list of 1,000 servicemen recommended for special citation for participation in CWS testing programs (Cochrane, 1946; see also Appendix E). There is also evidence that some U.S. field tests involved human subjects who were not protected by clothing or even gas masks. The Summary Technical Report of the NDRC (OSRD, 1946, Table 8, p. 58) presents data about the exposure levels of mustard gas required to produce injuries in man, based on field tests in varied temperatures and climates in which none of the men wore protective
clothing and only some of the men wore gas masks. The cumulative exposures reported for these tests ranged from 50 to 10,000 Ct.
The appendixes to the Report of the Chemical Warfare Service Conference of October 10-13, 1944, obtained from the National Archives, describe various field tests (CWS, 1944). Some of these tests may have contributed data to the summary mentioned above. Appendix VIII of the conference report outlines field tests in which bombing runs dropped from 125 to 550 tons of sulfur mustard over a specified area. Subjects wearing varying levels of protective clothing traversed the area in simulated patrols from 1 to 72 hours following the bombing. Such a protocol required the men to drop to the ground intermittently, thus coming into direct contact with contaminated surfaces. The resultant injuries were classified on the basis of the men's probable fitness for combat. Evidence of accidents during such trials can also be found in CWS documents. For example, one note describes how a group of men involved in a field test removed their gas masks after a rain storm and within two hours experienced ocular pain; three were hospitalized with acute conjunctivitis (Adler, 1944).
Gas Production, Gas Handling, and Chemical Warfare Production
Preparations for chemical warfare before and during WWII involved many additional people in the production, handling, shipping, and training to use and defend against chemical warfare agents. By the end of the war, the four CWS production facilities had produced close to 175 million pounds of ordinary sulfur mustard (H) and over 9 million pounds of distilled, purified sulfur mustard (HD) (Brophy et al., 1959). These production sites were at Edgewood Arsenal in Maryland, Huntsville Arsenal in Alabama, Pine Bluff Arsenal in Arkansas, and Rocky Mountain Arsenal in Colorado. An additional 40 million pounds of Lewisite and 200,000 pounds of nitrogen mustard were produced. Once produced, the agents were shipped to various storage facilities, depots, and proving grounds around the United States and were shipped overseas through ports such as Seattle, New York, New Orleans, and others.
This elaborate network of supply, coupled with the needs for training and chemical weapons testing, required many people from both the military and the civilian sectors. In 1939, CWS listed 803 enlisted men on its personnel rolls; this number grew to over 5,500 by December 1941 and over 61,000 by June 1943. Some 17 percent of military personnel assigned to CWS units were African Americans, a very high percentage when compared to all other units of the War Department. Women from the Women's Army Auxiliary Corps (WAAC) were also assigned to
CWS in jobs ranging from clerks and housekeepers to chemists and toxicologists. Civilian workers numbered 7,000 at the beginning of the war and 28,000 by 1943, of whom 40 percent were female and 45 percent were African Americans. The latter percentage was lower at the Rocky Mountain Arsenal, where there were fewer African Americans available from the surrounding community (Brophy and Fisher, 1959).
Although many of the specific jobs performed by these military and civilian personnel did not involve handling of, or even proximity to, warfare gases, the number of documented injuries was quite high. CWS, in fact, had the worst safety record of any branch of the War Department in both 1942 and 1943, the peak years of production (Brophy and Fisher, 1959). According to these authors, the safety record improved considerably after that, becoming among the best in the War Department by the end of the war. Nevertheless, the dismal safety record meant that many injuries were themselves studied by those involved in the CWS research branches or in studies contracted under NRC's Committee on Treatment of Gas Casualties. For example, many of the eye injuries at Edgewood were referred to and studied at the Johns Hopkins University Medical School under CTGC contracts (Andrus et al., 1948).
One study of these accidental injuries, reported that over 1,000 cases of mustard poisoning, resulting in eye, ear, nose, and throat symptoms, occurred at Edgewood Arsenal over a two-year period (Uhde, 1946). Of these, 790 were eye injuries; these injuries occurred to both males and females. Slow leaks of mustard vapor accounted for close to 80 percent of the problems. An additional 7 percent were from short-term exposures and accidents, such as explosions and mistaken use of real mustard in training exercises designed for simulated gas exposure. While the study did not present adequate information with which to judge the overall severity of injuries, it does report one death from sulfur mustard poisoning during this period. Little information is available from other locations, but Cochrane (1946) noted that during the first two weeks of December 1941, 577 patients were treated for eye and respiratory tract injuries from exposure to chemical warfare agents, especially sulfur mustard. The CWS locations where these injuries occurred were not reported. Finally, there is anecdotal evidence that the atmospheric concentrations of sulfur mustard around manufacturing areas at Edgewood Arsenal exceeded the odor threshold concentrations and thus may have been high enough to cause physiological effects (Howard Skipper, personal communication; see also Appendix A).
It is important to note that CWS personnel were exposed to a variety of toxic materials. For example, in addition to mustard agents, gases such as phosgene (a choking agent), hydrogen cyanide and cyanogen chloride (blood poisoning agents), and chloroacetophenone (tear gas)
were also produced at the arsenals. Other personnel were involved in biological warfare research and production, in locations such as Fort Detrick, Maryland, and a civilian plant in Terre Haute, Indiana (Brophy and Fisher, 1959). Chemicals including napalm and white phosphorus were also stored and packed into bombs by CWS personnel. Even the production and testing of gas masks and filter canisters involved the use of toxic chemicals such as asbestos.4 Finally, many people, including women, were assigned duties in the preparation of impregnated clothing, the most common method of which involved the use of two extremely toxic chemicals, chloroamide and acetylene tetrachloride.
The Bari Harbor Disaster
The only combat casualties from sulfur mustard in WWII were those injured or killed following a German air raid on the harbor of Bari, Italy, on December 2, 1943 (Alexander, 1947; Cochrane, 1946; Gage, 1946; Harris and Paxman, 1982; Infield, 1976; Perera and Thomas, 1986). Under conditions of secrecy, 2,000 bombs, each of which held 60 to 70 pounds of sulfur mustard, had been loaded on the merchant marine ship S.S. John Harvey before it had sailed from Baltimore to Bari. During the raid on Bari harbor, the John Harvey was sunk and some of its load of mustard bombs was damaged, causing liquid mustard to spill out into water already heavily contaminated with an oily slick from other damaged ships. Men who abandoned their ships for the safety of the water became covered with this oily mixture that provided an ideal solvent for sulfur mustard. The casualties were pulled from the water and sent to medical facilities unaware of what they carried with them on their clothes and skin. Equally unaware were the medical personnel who treated these casualties. Before a day passed, symptoms of mustard poisoning appeared in both the casualties and the medics. This disturbing and puzzling development was further compounded by the arrival of hundreds of civilians for treatment; they had been poisoned by a cloud of sulfur mustard vapor that blew over the city from some of the bombs that had exploded when the ship sank.
As the medical crisis worsened, little information was available about what was causing these symptoms. U.S. military command did not want to reveal to the enemy its preparations to position sulfur mustard in Europe for possible use against German forces. Eventually, however, the secret could not be kept (Harris and Paxman, 1982). The destroyer
U.S.S. Bistera, well outside the harbor and undamaged by the raid, had pulled 30 men from the water in a rescue effort. By the next day, the officers and crew of the Bistera were blinded from the effects of the sulfur mustard carried onto the ship by those rescued. Bari was overloaded with casualties by then, and the Bistera and its crew struggled to nearby Taranto for treatment. Soon the U.S. command had no choice but to confirm the cause of these injuries. With the assistance of Colonel Stewart Alexander, a military physician with extensive knowledge of mustard poisoning, better precautions and treatment were begun. By the end of the disaster, over 600 victims of mustard poisoning were treated from the harbor area alone; of these, 83 died (Alexander, 1947). Close to 1,000 civilians from the town also died (Harris and Paxman, 1982). Unfortunately, no long-term medical follow-up of survivors of the Bari harbor disaster has been reported.
Medical Applications of Chemical Warfare Research
As history has repeatedly shown, the experience of medical personnel and researchers in wartime can lead to major innovations in medical treatment practices. Such was the case with chemical warfare research in WWII. Numerous advances were made in the treatment of metal poisoning, development of antibiotics, treatment of burn injuries, and other areas. Many of these advances were reviewed soon after the war in a two-volume summary Advances in Military medicine (Andrus et al., 1948). The story of the use of nitrogen mustard as a cancer chemotherapy agent is especially relevant to the present report.
Nitrogen mustards were first synthesized in the 1930s. These compounds were modifications of sulfur mustard and were found to have greater systemic toxicity than sulfur mustard (Gilman and Philips, 1946; OSRD, 1946). Particularly potent was the effect of nitrogen mustard on cells that are actively proliferating, including the lymphoid tissue, bone marrow, and certain cells lining the gastrointestinal tract. During WWII, the Committee on the Treatment of Gas Casualties authorized a contract between the Office of Scientific Research and Development and Yale University (Andrus et al., 1948). Under this contract, Louis C. Goodman headed a group that was responsible for the study of the pharmacologic effects of nitrogen mustards. The group, including Alfred Gilman, Frederick Philips, and Roberta Allen, focused its efforts on the study of the cytotoxic properties of nitrogen mustard. Enlisting the help of anatomist Thomas Dougherty, the group expanded its work to examine the effect of nitrogen mustard on experimental tumor cells in mice. It was found, but not published until later, that systemic administration of nitrogen mustard caused dramatic regression of these mouse tumors. These data formed the experimental basis of the first clinical trials of
nitrogen mustard as a cancer chemotherapy agent (Gilman, 1946, 1963; Gilman and Philips, 1946; Rhoads, 1947).
Although the concept of chemotherapy does not seem radical today, in 1942 the idea of injecting poisons into cancer patients, especially poisons marked ''compound X" due to their classified status, would have been viewed by most physicians as "the act of a charlatan" (Gilman, 1963). With the help of Gustav Lindskog however, clinical trials were begun in December 1942 with a patient dying of lymphosar-coma for whom all other treatments had failed. The patient's tumors regressed, the outlook brightened, and another patient was begun on the nitrogen mustard therapy. In all, six patients made up this first trial. However, as had happened in the animal studies, the tumors reappeared as the bone marrow recovered, and no long-lasting cure was attained. The challenge remained to establish the regimen of therapy that would kill the cancer cells completely, yet preserve enough of the bone marrow to regenerate needed healthy cells. In addition, there was no reason to assume that all types of cancer cells would be equally affected by nitrogen mustard therapy.
The Yale group dispersed in June 1943, but clinical trials with nitrogen mustard continued in several other locations. By 1948, close to 150 patients in the terminal stages of Hodgkin's disease, lymphosarcoma, or certain leukemias had been treated with this agent (Gilman and Cattell, 1948). The best results were obtained in cases of Hodgkin's disease. Derivatives of nitrogen mustard (hydrochloride forms) are still used today, particularly for treatment of lymphoma, in a regimen that includes an array of other drugs and chemicals administered with the nitrogen mustard (see also Chapter 6).
RESEARCH, USE, AND DISPOSAL OF CHEMICAL WEAPONS AFTER WORLD WAR II
Postwar Research Programs
The vast majority of the post-WWII research concerning mustard agents and Lewisite has been done in animal studies or in model systems, such as skin tissue culture. This research has been aimed toward the development of pretreatments to prevent mustard toxicity or toward improved treatments against acute poisoning. Emphasizing these issues, Papirmeister and colleagues reviewed the literature on sulfur mustard in 1991, including consideration of all such work published up to 1990. For the purposes of the present report, discussion is confined to only those research programs that used human subjects.
Once WWII was over, all of the research programs of the Chemical Warfare Service were scaled down. Very little research was done during
the period between 1946 and 1950, and by the time research in chemical and biological weapons was revitalized in the 1950s, military priorities had shifted to agents perceived to pose greater threats than sulfur mustard or Lewisite. For example, improvements to early nerve gases developed in WWII gave new importance to the development of antidotes to nerve agents. Chemicals with intense psychoactive properties, such as lysergic acid diethylamide (the hallucinogen LSD) and phencyclidine (PCP, known on the street as "angel dust") were also of special interest. Most of this research was done at or supervised by personnel from Edgewood Arsenal; it involved approximately 6,700 human subjects between 1950 and 1975. Only a few projects tested sulfur mustard or Lewisite.
Other groups that participated in this research included the Central Intelligence Agency and the Special Operations Division of the Department of the Army (Taylor and Johnson, 1975). As has been documented in numerous government and popular press publications, abuses of human subjects in these research programs began to emerge almost as soon as the projects were begun but were largely covered up until the early 1970s (Harris and Paxman, 1982; Taylor and Johnson, 1975; see also Appendix F). Finally, congressional hearings into these abuses in 1974 and 1975 resulted in fuller disclosures, eventual notification of all subjects as to the nature of their chemical exposures, and compensation of a few families of those who had died while serving as human subjects in these projects (Harris and Paxman, 1982; Taylor and Johnson, 1975).
As part of its effort to rectify the abuses discovered, the Department of the Army asked the National Research Council to assess the likelihood of long-term health consequences of exposure to the chemicals tested and to report on the current health status of the soldiers who participated in the 1950-1975 testing programs. The resulting study was published in three volumes in 1982, 1984, and 1985. The vast majority of these test subjects, however, had been exposed to nerve agents or hallucinogenic drugs. In the 1984 volume, the NRC committee reported that only 150 individuals had been exposed to vesicants. In a section on vesicants, no conclusions were drawn for Lewisite on the basis of scanty information; for sulfur mustard, however, the group concluded:
Mustard gas is mutagenic in various organisms and test systems. One cannot readily predict the degree of genetic risk that it poses for man, however, because data on its mutagenicity in mammalian germ cells are very limited, and the mutagenic potency of mustards varies considerably among assay systems. Nevertheless, the available evidence suggests that the possibility of mutagenic effects of mustard gas in human germ cells should not be disregarded. The clear muta-
genicity of mustard gas in various assays is consistent with its carcinogenic potential.
Mustard gas is not only a vesicant, but also a systemic poison. Its acute effects have been demonstrated in bone marrow, intestinal tract, and respiratory tract. It can cause blindness and permanent skin scarring with a potential for skin tumors. It probably can also cause acute and chronic bronchitis. Other nonmalignant chronic effects have not been adequately documented.
Single exposures, even if severe, as in military service, are not associated with statistically verifiable increases in mortality from tuberculosis and cancer; but repeated small exposures, such as occur in industrial operations, do increase cancer deaths significantly.
The NRC committee's 1985 report summarized the investigations of the current health status of test subjects and concluded that the number of subjects exposed to mustard gas was too small to detect any long-term health effects. Also cited were the only long-term follow-up studies of WWI sulfur mustard casualties. Overall mortality and morbidity data for a sample of men treated for sulfur mustard injuries in American Expeditionary Forces hospitals from August through November 1918 revealed a slightly increased incidence of lung cancer among gassed veterans, but this increase was not sufficiently high for statistical significance (Beebe, 1960). A further study of this cohort 10 years later did not alter these results (Norman, 1975). 5 To the present committee's knowledge, no human subjects have been used in tests of mustard agents or Lewisite in the United States since the 1960s.
Continuing Use of Sulfur Mustard and Other Chemical Weapons in International Conflicts
Military use of sulfur mustard was a topic at the Paris Conference on the Prohibition of Chemical Weapons in January 1939. Due to continued use of these weapons around the world, however, chemical weapons bans remain an ongoing issue of negotiation at the current chemical convention talks in Geneva, Switzerland. The Stockholm International Peace Research Institute (SIPRI) published a book analyzing the historical, technical, military, legal, and political aspects of chemical and biological warfare in 1971 (SIPRI, 1971). This document reports use of sulfur mustard by the Egyptians in Yemen in 1965 and the Iraqis against the Kurds in 1965.
|
5 |
Review and analysis of the Beebe and Norman papers are included in Chapter 6. |
Numerous reports of use of other agents, including tear gas, smokes, and herbicides, are also reported. Additional reports have surfaced of use of sulfur mustard by the Vietnamese in Cambodia and Laos between 1976 and 1980 (Medema, 1986). There are more recent reports of use of sulfur mustard and cyanide by Armenians against the Azerbaijanis in the Nakhichevan Autonomous Republic (CBW News, 1992).
Probably the greatest use of sulfur mustard, however, has been in the ongoing conflicts between Iran and Iraq, and many of these incidents have been confirmed (D'Halluin and Roels, 1984; Dunn, 1986a,b; Heyndrickx and Heyndrickx, 1984; Mandl and Freilinger, 1984; Medema, 1986; Physician's for Human Rights, 1989; Requena et al., 1988; United Nations Security Council Reports, 1986, 1987, 1988a,b,c,d). Some of the Iranian casualties were treated in European hospitals and thus could be documented medically. These patients suffered from pulmonary, eye, and skin lesions at similar incidence levels as battlefield casualties from WWI. In WWI, 80 to 90 percent of sulfur mustard casualties suffered skin lesions, 86 percent suffered eye involvement, and 75 percent had pulmonary damage (Sidell and Hurst, 1992). Among the Iranian casualties, 83 percent suffered skin lesions, 92 percent had eye problems, and 95 percent had pulmonary damage (Balali-Mood and Navaeian, 1986).
There are also sketchy data that indicate that some Iranian soldiers may have been exposed to Lewisite. London physicians who examined and treated the lesions of these soldiers reported that the signs exhibited were similar to those associated with Lewisite, rather than sulfur mustard (Perera, 1985). For example, pain occurred very quickly following vapor exposure, and skin lesions showed none of the pigmentation changes characteristic of sulfur mustard exposure. In addition, the victims reported that the agent did not smell like garlic, as does sulfur mustard.
U.S. Chemical Stockpile Disposal Program
The U.S. stockpile of sulfur mustard, currently stored at seven military installations on the continental United States (Aberdeen Proving Ground, Maryland; Anniston Army Depot, Alabama; LexingtonBlue Grass Army Depot, Kentucky; Pine Bluff Arsenal, Arkansas; Pueblo Depot Activity, Colorado; Tooele Army Depot, Utah; Umatilla Depot Activity, Oregon) and one location in the South Pacific (Johnston Island, U.S. Pacific Territory), is under congressional mandate for destruction (Carnes, 1989; Carnes and Watson, 1989). Lewisite is stored in only one location, Tooele Army Depot, in ton containers. Although the locations listed here are the official storage facilities, it is not known on how many former military bases small amounts of agents such as sulfur mustard were left or buried when the bases were deactivated. For
example, it was recently discovered that a dumping site, used to dispose of 55-gallon drums of sulfur mustard in the mid-1940s, now lies near a large business complex in Edison, New Jersey (Gallotto, 1992). This site may be one of many located on what was once the Raritan Arsenal, where reports of former soldiers claim that toxic chemicals were poured into pits, along with the emptied drums and shells, treated with lime, and covered over with soil. Such reports are not only relevant to the issue of toxic waste from chemical weapons production in this century; they also point out locations, not apparent in official CWS histories, where military and possibly civilian personnel were exposed to chemical agents during WWII.
The Department of Defense (DoD) Authorization Act of 1986 (P.L. 99-145) directed and authorized the Secretary of Defense to destroy the United States' aging and obsolete stockpile of lethal unitary chemical munitions and bulk agent by September 1994. In response, DoD established the Chemical Stockpile Disposal Program in 1986, but the target completion date has been postponed to 2004. Unitary munitions contain a lethal chemical agent at the time the munition is loaded; in contrast, binary munitions contain agent precursors that mix and react to form lethal agent after the munition is fired. The unitary stockpile includes the vesicant agents sulfur mustard and Lewisite, as well as organophosphate nerve agents. All but approximately 6 percent of the U.S. stockpile of unitary munitions and bulk agent is currently stored in the continental United States as bombs, cartridges, mines, projectiles, spray tanks, and ton containers. Approximately 60 percent of the unitary stockpile tonnage is stored in bulk as ton containers, spray tanks, or similar large containers. The remainder is stored on Johnston Island, including the North Atlantic Treaty Organization's stockpile that was moved in 1990 from a military site near Clausen, Germany.
DoD has tested, considered, and discarded a number of proposed disposal methods in favor of high-temperature incineration (Carnes, 1989; Carnes and Watson, 1989; U.S. Department of the Army, 1988). The first step in this approach involves "reverse assembly" of the munition inside an explosive-containment room, resulting in the separation of agent from any explosive materials and munition hardware or containers. These different fractions are sent to separate incinerators, and materials are incinerated by a specially designed system using four two-staged furnaces (a furnace and an afterburner) for each component (e.g, liquid agent, contaminated metal parts). Temperatures reach between 540°C and 1370°C for the furnaces and approximately 1090°C for the afterburners. Stack gases and incinerator ash are treated in advanced pollution-abatement systems intended to ensure safe handling and eventual disposal in a hazardous waste facility (see Carnes, 1989, for details of emission control systems).
Incineration remains a matter of continuing controversy among environmental groups, citizens who live near some of the proposed incineration sites, and the involved government agencies. The Programmatic Environmental Impact Statement, released by the Department of the Army in 1988, concludes that timely disposal of the stockpile of chemical weapons entails less of a hazard than continued storage.
Technical support and oversight for the Chemical Stockpile Disposal Program (and the companion Chemical Stockpile Emergency Preparedness Program that assists nearby communities in developing emergency preparedness programs) is provided by numerous Army commands and a host of civilian institutions. These include the National Center for Environmental Health of the Centers for Disease Control (U.S. Department of Health and Human Services), Federal Emergency Management Agency, U.S. Environmental Protection Agency, U.S. Department of Agriculture, as well as state and local planning agencies in the 10 affected states, and two national laboratories (Oak Ridge National Laboratory, Oak Ridge, Tennessee; Argonne National Laboratory, Argonne, Illinois). Analyses of vesicant toxicity and long-term health risks from these groups have been considered, along with other information, in generating the present report.
CONCLUSIONS AND FURTHER ANALYSIS
The committee reached two principal conclusions based on its analysis of the chemical warfare testing programs from WWI through 1975. These conclusions relate directly to the estimated level of exposure to mustard agents and Lewisite experienced by the WWII chamber and field test subjects and to the exposures of workers in the Chemical Warfare Service during WWII. In addition, the committee's conclusions are pertinent to the health care concerns of those who have been injured by use of these agents in recent wars and conflicts, or who may be exposed in the future from belligerent use of these agents or through accidental exposure during their disposal.
-
The lack of follow-up health assessments of the human subjects in WWII gas chamber tests and field tests severely diminished the amount and quality of information that could be applied in the assessment of long-term health consequences of exposure to mustard agents and Lewisite. Although the reasons underlying the lack of follow-up health assessments are not explicit from the numerous documents and materials considered by this committee, a number of factors may have played a role:
-
There was no unified body of information, based on WWI research and the research done in the period from 1918 to 1939, when
-
-
research was intensified in the early pre-WWII period. This lack of information seems to have contributed directly to a lack of appreciation for the serious long-term health risks associated with exposure to mustard agents and Lewisite, specifically chronic bronchitis, emphysema, chronic laryngitis, corneal opacities, chronic conjunctivitis, and keratitis.
-
Scientific inquiry was controlled by the military establishment, whose primary concern was with acute rather than long-term injury. This control also probably contributed to the paucity of animal or other types of studies, following WWII, aimed toward elucidation of long-term consequences of damage to specific physiological systems. For example, no long-term follow-up was done on workers involved in chemical warfare materials production, despite the high level of injuries that occurred.
-
The atmosphere of immediacy caused by the outbreak of war, and the resulting prioritization of expected combat injuries, at least strengthened the focus on acute damage from chemical warfare agents, and at worst dampened any sensitivities that were present regarding the future health of human subjects or chemical warfare production workers.
-
Once the war was over, there may also have been ambiguities about which federal department or agency should have had responsibility for follow-up of veterans. Although the former Veterans Administration (VA) had that role traditionally, the VA could not have been expected to know about the testing programs and their possible effects on the health of human subjects without communication from the military.
-
Finally, and related to the issue of responsibility for follow-up, the continued secrecy maintained by the military regarding the WWII testing programs also created a barrier to follow-up assessments of exposed individuals. Even during the present study, which follows a five-year period of intensifying public scrutiny of these WWII programs, obtaining certain types of information was not easy and often involved piecing together bits of data from numerous sources. In fact, this committee was commonly required by many DoD and Department of the Army offices to file all requests under the Freedom of Information Act. These requirements were often imposed even on the present Department of Veterans Affairs (VA), when it attempted to aid this committee by making certain requests for information regarding the possible existence of records of individuals who participated in the testing programs (see Appendix E). The most valuable primary source data were received from the Naval Research Laboratory and NRC's Office of Archives and Information Services. This committee is especially grateful to the NRL for its commitment to open its files. The NRL
-
stands alone among sections of the Department of Defense in the maintenance of files and reports, and the sharing of those files with this committee and with the affected veterans.
-
The levels of exposure to mustard agents or Lewisite experienced by the test human subjects may have been much higher than inferred in the summaries of the experiments and field tests. As in all chemical exposures, such exposure levels directly relate to the types and severities of exposure-induced injuries and diseases. One can infer the cumulative exposures to the skin of chamber subjects strictly on the basis that skin damage was the end point of these experiments (see Table 3-4). Therefore, if all other types of exposures were held to zero, these subjects received between 100 and 300 Ct. As has been documented, some of the subjects were hospitalized for as long as "a month or so" (Taylor et al., 1943). Thus, exposures to the skin may have been as high as 1,000-2,000 Ct. Under the hot, humid conditions in the chambers, however, lower exposure levels would have resulted in similar injuries (Papirmeister et al., 1991). The dose to the skin from such exposures would have been as high as those observed under battlefield and occupational conditions. Further, some sulfur mustard would also have been absorbed from the skin into the systemic circulation.
In the chamber experiments, unmasked subjects were required to remain in their protective clothing from 4 to 24 hours following chamber trials, allowing ample opportunity for additional contact and inhalation exposures from contaminated surfaces and clothing. Another factor that probably resulted in some inhalation exposure of subjects in the chamber tests was vomiting during the period subjects were in the chamber. This was reported by at least one of the subjects who spoke at the public hearing; this person reported conjunctivitis and laryngitis following such a vomiting incident on his seventh day of testing (Elmer Hood, public hearing statement; see also Appendix G). Vomiting presumably would result in removal of the mask while in the chamber, with a resulting inhalation exposure of unknown duration at the chamber concentration being tested.
The most important route of additional exposure in the chamber and field tests was probably gas mask leakage. From the information available to the committee, it appears that the vast majority of the human subjects in the chamber and field tests wore full-face gas masks during their exposures. In fact, the documented exposures at the Naval Research Laboratory were delivered at concentrations and for durations that would have caused lethal respiratory effects if the subjects had not been equipped with respiratory protection. Thus, exposure of the respiratory tract and eye to the agent would have depended on the
|
BOX 3-1 ODOR THRESHOLD FOR SULFUR MUSTARD AND LEWISITE: COMPARISON WITH TISSUE DAMAGE THRESHOLDS Even when enough agent had broken through their gas mask canisters to produce symptoms, chamber test subjects may not have noticed it, at least by odor. The odor threshold for sulfur mustard is reported to be about 0.6 mg/m3, and the median concentration of Lewisite detectable by odor is reported to be 14 to 23 mg/m3 (OSRD, 1946). However, both agents have effects on the eye and respiratory tract at lower concentrations (Papirmeister et al., 1991; Urbanetti, 1987). For example, sulfur mustard exposure at a concentration of 0.5 mg/m3 for 30 minutes (15 Ct) would result in both respiratory and eye symptoms (see Table 3-4). For Lewisite, such irritating effects are reported to be noticeable at concentrations estimated to be as low as 6 to 8 mg/m3 (Papirmeister et al., 1991; Urbanetti, 1987). Thus, for sulfur mustard exposures at 0.5 mg/m3, an exposure of only about 25 minutes (12.5 Ct) could be expected to cause eye and respiratory tract symptoms without the subject being aware of the exposure, at least by odor. |
protection factor (PF) afforded by the gas masks. The PF of a full-face respirator (e.g., a gas mask) is calculated as the ratio of the ambient concentration of the contaminant to the concentration inside the mask, which in turn depends on both leakage around the respirator and contaminant penetration of the gas mask canister. A PF of 100 equals a penetration of 1 percent of the contaminant into the mask (Adley and Uhle, 1969). A PF of 50 to 100, based primarily on leakage around facemasks, has been reported for relatively modern (post-WWII) full-face respirators (Hyatt, 1976). Estimates from industrial hygiene research, however, indicate that the level of protection achieved in actual use of a respirator is usually below the stated PF for that respirator (National Institute of Occupational Safety and Health, 1974). Thus, modern respirators are likely to function closer to the lower PF estimate of 50. In practical terms, even if the respirator actually achieved a PF of 100, subjects exposed to a concentration of 100 mg/m3 of sulfur mustard would be breathing a concentration as high as 1 mg/m3 inside the mask, corresponding to a cumulative exposure of 60 Ct over a single 60-minute trial. At even lower concentrations—under the odor threshold (0.6 mg/m3)—the subjects may well have been unaware of any leakage through their masks (see Box 3-1). Information on the breakthrough capacity of the gas mask cartridges used in the WWII chamber tests was not available to the committee, but it is known that prolonged use of cartridges can result in breakthrough of the agent by exceeding the capacity of the absorbent filter material (Stampfer, 1982).
In the NRL chamber test reports examined by the present committee, when gas mask types were listed, the masks used were Mark III or Mark
IV Navy diaphragm gas masks. These masks were probably equipped with M9A1 (prior to July 1943) or M9A2 canisters that contained Whetlerite, a copper-, chromium-, and silver-impregnated activated charcoal as the sorbent (Brophy et al., 1959). The PF afforded by these masks for sulfur mustard or Lewisite was not available to the committee. However, an individual involved in this testing reported that the WWII British and U.S. masks were very effective in removing sulfur mustard (Howard Skipper, personal communication; see Appendix A). Yet some chamber tests were conducted at high concentrations. For example, a test conducted at chamber concentration of 100 mg/m3 for 60 minutes would have resulted in a cumulative, unprotected, exposure of 30,000 Ct over five trials. Even an assumed PF of 1,000 for the gas mask (10 times greater than that estimated for modern full-face respirators) would have resulted in concentrations as high as 0.1 mg/m3 in each trial, corresponding to a cumulative exposure of 6 Ct just from the inspired air in each trial. This would have been below the odor threshold for sulfur mustard and, over five trials, would have resulted in a cumulative inhalation exposure of 30 Ct, enough to cause signs and symptoms in the eyes and respiratory tract (see Table 3-4). If a more realistic estimate is used, such as a mask with a PF of 100, the per trial exposure would have been 60 Ct. Over five trials then, a subject could have had an inhalation exposure of 300 Ct, more than sufficient to cause an incapacitating injury (see Table 3-4). It is important to remember that any such inhalation exposure would have been in addition to any skin exposure through breakdown of the protective clothing.
It is important to note also that the gas masks and clothing used in the NRL tests were worn repeatedly by the subjects. In at least one series of studies, it was reported that the rubber of the gas mask facepieces and connecting tubes absorbed enough sulfur mustard after 12 to 15 exposures to cause conjunctivitis, laryngitis, and erythema of the face (Taylor et al., 1943). Therefore it is clear that some exposure to the respiratory tract occurred from absorption of sulfur mustard on masks. Finally, as mentioned previously, the special diaphragm element in the types of gas masks used in the NRL chamber tests was eventually shown to provide an additional route of mask leakage, independent of the filter capacity (Brophy et al., 1959).
The presence of erythema of the face, conjunctivitis, laryngitis, or bronchitis within 24 to 72 hours following an exposure to sulfur mustard or Lewisite would be clear evidence that a significant inhalation and eye exposure had occurred, even if the subject was wearing a mask during the exposure. Conversely, it would appear that a lack of such symptoms following even a low-level exposure of 5 to 6 days to sulfur mustard would indicate a cumulative exposure (Ct) of less than about 12 Ct (see Table 3-4). However, in terms of the Centers for Disease Control's
estimates of permissible exposure levels (CDC, 1988), the exposures actually reaching the breathing zone of chamber subjects (from the above example, 0.1 mg/m3 sulfur mustard breakthrough with a gas mask rated at 1,000 PF) may have been more than 1,000 times the general population agent control limits (0.0001 mg/m3 for sulfur mustard), and 33 times the control limits for occupational exposure (0.003 mg/m3 for sulfur mustard). In reality, some of the subjects in the chamber tests and field trials almost certainly breathed concentrations 10 or more times the 0.1 mg/m3 level for at least a part of their exposures.
The focus here on chamber and field test subjects is not meant to discount the probable exposure levels experienced by those who were involved in the production or handling of mustard agents and Lewisite. Indeed, as outlined above, the poor safety record of the Chemical Warfare Service during the peak years of production, the high rate of agent-induced injuries, and the anecdotal reports of perceptible odors of sulfur mustard in the manufacturing areas argue that workers and gas handlers were often exposed to levels of mustard agents and Lewisite sufficient to cause short- and long-term health effects. Thus, these individuals should also be considered at risk for any of the adverse health effects this report identifies.
In conclusion, the dose of sulfur mustard to the skin, eye, and respiratory tracts of the human subjects was substantial, especially in the case of the subjects involved in the chamber tests. Doses to the skin were probably equivalent to those received under combat conditions. Consideration of the probable gas mask leakage, additional exposures from contact or vapors from the clothing, accidents, and the documented signs and symptoms in the chamber test records indicate that the doses received by the human subjects were equivalent to those received in occupational exposures and, perhaps, even battlefield exposures.
REFERENCES
Adler FH. 1944. Report of consultant in ophthalmology on 6 cases of H vapor burns occurring at Bushnell Field Installation on April 20, 1944. Memo to Colonel C.P. Rhoads, dated May 1, 1944. Available at the National Archives, Suitland Reference Branch, Suitland, MD. Record Group 175, Group 4B, Folder 319.1.
Adley FE, Uhle RJ. 1969. Protection factors and self-contained compressed-air breathing apparatus. American International Hygiene Association Journal 30:355-359.
Alexander SF. 1947. Medical report of the Bari Harbor mustard casualties. Military Surgeon 101:1-17.
Andrus EC, Bronk DW, Carden GA Jr, Keefer CS, Lockwood JS, Wearn JT, Winternitz MC, eds. 1948. Advances in Military Medicine. Science in World War II: Office of Scientific Research and Development. Boston: Little, Brown and Company.
Balali-Mood M, Navaeian A. 1986. Clinical and paraclinical findings in 233 patients with sulfur mustard poisoning. In: Heyndricks B, ed. Terrorism: Analysis and Detection of
Explosives. Proceedings of the Second World Congress on New Compounds in Biological and Chemical Warfare. Gent: Rijksuniversiteit. 464-473.
Beebe G. 1960. Lung cancer in World War I veterans: possible relation to mustard gas injury and 1918 influenza epidemic. Journal of the National Cancer Institute 25:12311252.
Brophy LP ,Fisher G. 1959. The Chemical Warfare Service: Organizing for War. United States Army in World War II: The Technical Services. Washington, DC: Office of the Chief of Military History, Department of the Army.
Brophy LP ,Miles WD, Cochrane RC. 1959. The Chemical Warfare Service: From Laboratory to Field. United States Army in World War II: The Technical Services. Washington, DC: Office of the Chief of Military History, Department of the Army.
Budavari S, ed. 1989. The Merck Index. 11th ed. Rahway, NJ: Merck & Co.
Carnes SA. 1989. Disposing of chemical weapons: a desired end in search of an acceptable means. Environmental Professional 11:279-290.
Carnes SA, Watson AP. 1989. Disposing of the U.S. chemical weapons stockpile: an approaching reality. Journal of the American Medical Association 262:653-659.
CBW News, Quarterly Bulletin of Chemical and Biological Weapons Issues. 1992. 9(July): 8.
Centers for Disease Control (CDC). 1988. Final recommendations for protecting the health and safety against potential adverse effects of long-term exposure to low doses of agents: GA, GB, VX, Mustard Agent (H, HD,T) and Lewisite (L) . Federal Register 53:8504-8507.
Chemical Warfare Service (CWS). 1944. Report of Chemical Warfare Service Conference. October 10-13, 1944. Available at the National Archives, Suitland Reference Branch, Suitland, MD. Record Group 175, Group 4B, Folder 337.
Cochrane RC. [1946]. Medical research in chemical warfare. Available through the U.S. Army Chemical Defense Research, Development and Engineering Center, Aberdeen Proving Ground, MD.
Despretz M. 1822. Chlorine compounds. Ann Chim Phys 21:428. [In French]
D'Halluin F, Roels H. 1984. Autopsy observations in an Iranian soldier exposed to war gases. Archives Belges (Supplement):284-290.
Dunn P. 1986a. The chemical war: journey to Iran. Nuclear, Biological and Chemical Defense and Technology International 1(2):28-37.
Dunn P. 1986b. The chemical war: Iran revisited-1986. Nuclear, Biological and Chemical Defense and Technology International 1(3):32-39.
Freeman K. 1991. The unfought chemical war. Bulletin of the Atomic Scientists 47:30-39.
Gage EL. 1946. Mustard gas (dichloroethyl sulfide) burns: in clinical experiences. West Virginia Medical Journal 42:180-185.
Gallotto AA. 1992. Corps tries to ease Raritan Arsenal cleanup fear. Sunday Star-Ledger, Newark, New Jersey. February 2, 1992.
Ganas P. 1969. New developments in chemical and biological warfare. Forces Aeriennes Francaises 24:449-475.
Gates M, Moore S. 1946. Mustard gas and other sulfur mustards. In: Division 9, National Defense Research Committee. Chemical Warfare Agents, and Related Chemical Problems. Summary Technical Report of Division 9, NDRC. Washington, DC: Office of Scientific Research and Development. 30-58.
Gates M, Williams JW, Zapp JA. 1946. Arsenicals. In:Division 9, National Defense Research Committee, comp. Chemical Warfare Agents, and Related Chemical Problems. Summary Technical Report of Division 9, NDRC. Washington, DC: Office of Scientific Research and Development.
Gilchrist HL. 1928a. A Comparative Study of World War Casualties from Gas and Other Weapons. Washington, DC: U.S. Government Printing Office.
Gilchrist HL. 1928b. Chemical warfare and its medical significance. Military Surgeon 63:477-492.
Gilchrist HL, Matz PB. 1933a. The residual effects of warfare gases. Medical Bulletin (US Veterans Administration) 9:339-390.
Gilchrist HL, Matz PB. 1933b. The Residual Effects of Warfare Gases. Washington, DC: U.S. Government Printing Office.
Gillis RG. 1985. Australian Field Tests with Mustard Gas 1942-1945. Department of Defence, Australia.
Gilman A. 1946. Symposium on advances in pharmacology resulting from war research: therapeutic applications of chemical warfare agents. Federation Proceedings 5:285-292.
Gilman A. 1963. The initial clinical trial of nitrogen mustard. American Journal of Surgery 165:574-578.
Gilman A, Cattell M. 1948. Systemic agents: action and treatment. In: Andrus EC, Bronk DW, Carden GA Jr, Keefer CS, Lockwood JS, Wearn JT, Winternitz MC, eds. Advances in Military Medicine. Science in World War II: Office of Scientific Research and Development. Boston: Little, Brown. 546-564.
Gilman A, Philips FS. 1946. The biological actions and therapeutic applications of the b-chloroethyl amines and sulfides. Science 103:409-415.
Guthrie F. 1859. On some derivatives of the olefines. Quarterly Journal of the Chemical Society 12:109-126.
Guthrie F. 1860. On some derivatives of the olefines. Quarterly Journal of the Chemical Society 13:129-135.
Haber L. 1986. The Poisonous Cloud. Oxford:Clarendon Press. 250-257.
Harris R, Paxman J. 1982. A Higher Form of Killing: The Secret Story of Chemical and Biological Warfare. New York: Hill and Wang.
Heinen JH, Carhart HW, Taylor WH, Stolp BN, Conner JC, Clausen NM. 1945. Chamber Tests with Human Subjects. IX. Basic Tests with H Vapor. NRL P-2579. Washington, DC: Naval Research Laboratory.
Heyndrickx A, Heyndrickx B. 1984. Treatment of Iranian soldiers attacked by chemical and microbiological war gases. Archives Belges (Supplement):157-159.
Hyatt. 1976. Respirator Protection Factors. Los Alamos:Los Alamos Scientific Laboratory of the University of California.
Infield GB. 1976. Disaster at Bari. London: New English Library.
International Agency for Research on Cancer (IARC). 1975. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. Vol. 9, Some Aziridines, N,S- & O-Mustards and Selenium. Lyon: IARC.
Jackson KE. 1936. The history of mustard gas. Journal of the Tennessee Academy of Science 11:98-106.
Lewis WL, Perkins GA. 1923. The ß-chlorovinylchloroarsines. Industrial and Engineering Chemistry 15:290.
Lewis WL, Stiegler HW. 1925. The b-chlorovinyl chloroarsines and their derivatives. Journal of the American Chemical Society 47:2546-2556.
Mandl H, Freilinger G. 1984. First report on victims of chemical warfare in the Gulf war treated in Vienna. Archives Belges (Supplement):330-340.
McNamara BP, Owens EJ, Christensen MK, Vocci FJ, Ford DF, Rozimarek H. 1975. Toxicological Basis for Controlling Levels of Mustard in the Environment. Edgewood Arsenal Special Publication EB-SP-74030. Aberdeen Proving Ground, Maryland: U.S. Army Armament Command. Edgewood Arsenal Biomedical Laboratory.
Medema J. 1986. Mustard gas: the science of H. Nuclear, Biological, and Chemical Defense and Technology International 1:66-71.
Meyer V. 1886. Compounds of thiodiglycol. Chemischte Berichte 19:3259-3266. [In German]
National Institute of Occupational Safety and Health. 1974. Research Report. Publication No. 74-104.
National Research Council (NRC). Committee on Toxicology. 1985. Possible Long-Term Health Effects of Short-Term Exposure to Chemical Agents. 3 vols. Washington, DC: National Academy Press.
National Research Council. Division of Medical Sciences. Committee on Treatment of Gas Casualties. 1945. Fasciculus on Chemical Warfare Medicine. Volume III, Skin and Systemic Poisons. Washington, DC: Prepared for the Committee on Medical Research of the Office of Scientific Research and Development.
Niemann A. 1860. The action of brown sulfur chloride on ethylene gas. Annalen der Chemie und Pharmazie 113:288. [In German]
Norman JE Jr. 1975. Lung cancer mortality in World War I veterans with mustard gas injury, 1919-1965. Journal of the National Cancer Institute 54:311-318.
Office of Scientific Research and Development (OSRD). National Defense Research Committee. 1946. Summary Technical Report of Division 9, NDRC. Washington, DC: NDRC. AD-234 249.
Papirmeister B, Feister AJ, Robinson SI, Ford RD. 1991. Medical Defense Against Mustard Gas: Toxic Mechanisms and Pharmacological Implications. Boca Raton, FL: CRC Press.
Perera J. 1985. Lewisite: new gas weapon in the Gulf war. New Scientist 105(1450):8.
Perera J, Thomas A. 1986. Britain's victims of mustard gas disaster. New Scientist 109:26-27.
Physicians for Human Rights. 1989. Winds of Death: Iraq's Use of Poison Gas Against Its Kurdish Population. Somerville, MA: Physicians for Human Rights.
Prentiss AM. 1937. Vesicant agents. In: Chemicals in Warfare: A Treatise on Chemical Warfare. 1st ed. New York: McGraw-Hill. 177-300.
Project Coordination Staff, Chemical Warfare Service. 1946. Technical Aspects of Chemical Warfare in the Field. 2 vols. Washington, DC: Chemical Warfare Service.
Requena L, Requena C, Sanchez M, Jaqueti G, Aguilar A, Sanchez-Yus E, Hernandez Moro B. 1988. Chemical warfare: cutaneous lesions from mustard gas. Journal of the American Academy of Dermatology 19:529-536.
Rhoads CP. 1947. Edward Gamaliel Janeway lecture: the sword and the ploughshare (dichloroethyl sulfide poisoning at Bari, 1943 and work of Chemical Warfare Service, especially on nitrogen mustards or chloroethylamines). Journal of Mt. Sinai Hospital 13:299-309.
Robinson JP. 1967. Chemical warfare. Science Journal 4:33-40.
Sidell FR. 1990. Clinical Notes on Chemical Casualty Care. USAMRICD Technical Memorandum 90-1. Aberdeen Proving Ground, MD: U.S. Army Medical Research Institute of Chemical Defense.
Sidell FR, Hurst CG. 1992. Clinical considerations in mustard gas poisoning . In: Somani SM, ed. 1992. Chemical Warfare Agents. San Diego: Academic Press.
Smith HW. 1943. Progress Report on Review of the Literature on the Systemic Action of Mustard Gas. OSRD Report 1717. Washington, DC: National Defense Research Committee.
Somani SM. 1992. Chemical Warfare Agents. New York: Academic Press.
Spiers EM. 1986. Chemical Warfare. Urbana, IL: University of Illinois Press.
Stampfer JF. 1982. Respirator canister evaluation for nine selected organic vapors. American International Hygiene Association Journal 43:319-328.
Stepanov AA, Popov VN. 1962. [Chemical Weapons and Principles of Antichemical Defense]. Translated by Joint Publications Research Service. JPRS 15107. Washington, DC: JPRS.
Stewart I. 1948. Organizing Scientific Research for War. Science in World War II: Office of Scientific Research and Development. Boston: Little, Brown.
Stockholm International Peace Research Institute (SIPRI). 1971. The Problem of Chemical and Biological Warfare: A Study of the Historical, Technical, Military, Legal, and Political Aspects of Chemical and Biological Warfare and Possible Disarmament Measures . Vol. 1, The rise of chemical and biological weapons. Stockholm: Almqvist & Wiksell.
Stroykov, KN. 1970. Medical Aid for Toxic Agent Victims. Moscow: Meditsina. [In Russian]. As cited in Papirmeister B, Feister AJ, Robinson SI, Ford RD. 1991. Medical Defense Against Mustard Gas: Toxic Mechanisms and Pharmacological Implications. Boca Raton, FL: CRC Press.
Tarbell DS, Tarbell AT. 1981. Roger Adams: Scientist and Statesman. Washington, DC: American Chemical Society.
Taylor JR, Johnson WN. 1975. Research Report Concerning the Use of Volunteers in Chemical Agent Research. DAIG-IN 21-75. Washington, D.C.: Department of the Army, Office of the Inspector General and Auditor General.
Taylor WH, Carhart HW, Daily LE. 1943. Chamber Tests with Human Subjects. I. Design and Operation of Chamber. II. Initial Tests of Navy Issue Protective Clothing Against Vapor. NRL P-2208. Washington, DC: Naval Research Laboratory.
Taylor WH, Carhart HW, Heinen JH. 1945. Chamber Tests with Human Subjects. VII. The Effect of Concentration of H Vapor and Time of Exposure on the Protection Afforded by CC-2 Impregnated Clothing. NRL P-2528. Washington, DC: Naval Research Laboratory.
Trammell GL. 1992. Toxicodynamics of organoarsenic chemical warfare agents. In: Somani SM, ed. Chemical Warfare Agents. San Diego: Academic Press.
Uhde GJ. 1946. Mustard gas (dichloroethyl sulfide) burns of human eyes in World War II. American Journal of Ophthalmology 29:929.
United Nations, Security Council. 1986. Report of the mission dispatched by the Secretary-General to investigate allegations of the use of chemical weapons in the conflict between the Islamic Republic of Iran and Iraq. March 12, 1986. S/17911, S/17911/ Addendum 1, and S/17911/Addendum 2. New York: United Nations.
United Nations, Security Council. 1987. Report of the mission dispatched by the SecretaryGeneral to investigate allegations of the use of chemical weapons in the conflict between the Islamic Republic of Iran and Iraq. May 8, 1987. S/18852 and S/18852/ Addendum 1. New York: United Nations.
United Nations, Security Council. 1988a. Report of the mission dispatched by the Secretary-General to investigate allegations of the use of chemical weapons in the conflict between the Islamic Republic of Iran and Iraq. April 25, 1988. S/19823 and S/19823/Addendum 1. New York: United Nations.
United Nations, Security Council. 1988b. Report of the mission dispatched by the Secretary-General to investigate allegations of the use of chemical weapons in the conflict between the Islamic Republic of Iran and Iraq. July 20, 1988. S/20060 and S/20060/Addendum 1. New York: United Nations.
United Nations, Security Council. 1988c. Report of the mission dispatched by the Secretary-General to investigate allegations of the use of chemical weapons in the conflict between the Islamic Republic of Iran and Iraq. July 25, 1988. S/20063 and S/20063/Addendum 1. New York: United Nations.
United Nations, Security Council. 1988d. Report of the mission dispatched by the Secretary-General to investigate allegations of the use of chemical weapons in the conflict between the Islamic Republic of Iran and Iraq. August 19, 1988. S/20134. New York: United Nations.
Urbanetti JS. 1987. In: Chan P, ed. Proceedings of the Vesicant Workshop, February 1987. Aberdeen Proving Ground, MD: U.S. Army Medical Research Institute. AD-A188 222.
Urbanetti JS. 1988. Battlefield chemical injury. In: Loke J, ed. Pathophysiology and Treatment of Inhalation Injuries. New York: Marcel Dekker.
U.S. Army. 1974. Chemical agent data sheets, Vol. 1. Technical Report EO-SR-74001. Edgewood Arsenal, MD.
U.S. Army. 1988. Final Programmatic Environmental Impact Statement for the Chemical Stockpile Disposal Program. Aberdeen Proving Ground, MD .
U.S. Army and U.S. Air Force. 1975. Military chemistry and chemical compounds. Field Manual No. FM3-9, Regulation No. AFR 355-7.
U.S. Army Chemical Research, Development, and Engineering Center (CRDEC). 1988. Lewisite. Material safety data sheet. Aberdeen Proving Ground, MD: U.S. Army Chemical Research, Development and Engineering Center.
U.S. Army Chemical Research, Development, and Engineering Center (CRDEC). 1990. HD and THD. Material safety data sheet. HCSDS No. 20058A. Aberdeen Proving Ground, MD: U.S. Army Chemical Research, Development and Engineering Center.
Vedder EB. 1925. The Medical Aspects of Chemical Warfare. Baltimore: Williams & Wilkins.
Watson AP, Griffin GD. 1992. Toxicity of vesicant agents scheduled for destruction by the Chemical Stockpile Disposal Program. Environmental Health Perspectives 98:in press.
West CJ. 1919. History of mustard gas. Science 49:412-417.
Willems JL. 1989. Clinical management of mustard gas casualties. Annales Medicinae Militaris Belgicae 3:S1-61.
World Health Organization. 1970. Health Aspects of Chemical and Biological Weapons: Report of a WHO Group of Consultants. Geneva: WHO.