5
Chemistry of Sulfur Mustard and Lewisite
SULFUR MUSTARD
This chapter reviews the important chemical reactions of sulfur mustard and Lewisite. It is included for those readers interested in these chemical reactions and their concomitant biological effects. Thus, the chapter may be of interest only to those readers with a background in chemistry. To ensure brevity, all sources used in the preparation of this chapter are listed at the end of the chapter and are not specifically cited in the text.
The synthesis and chemistry of sulfur mustard, or mustard gas, have been studied and reviewed extensively. Chemical and physical data regarding sulfur mustard were presented in Table 3-1. Meyer first prepared pure sulfur mustard by the reaction of thiodiglycol with phosphorus trichloride (5-2). Thiodiglycol was prepared by the reaction of 2-chloroethanol with potassium sulfide (5-1):
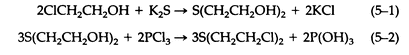
Concentrated hydrochloride, thionyl chloride, and phosgene have all been used in place of phosphorus trichloride.
Sulfur mustard was produced for use in warfare by what is known as the Levinstein process, the reaction of ethylene with sulfur dichloride. The fundamental reactions are the addition of sulfur dichloride to ethylene to form 2-chloroethylsulfenyl chloride (5-3) and the addition of that compound to a second molecule of ethylene (5-4):
So-called Levinstein mustard gas as manufactured on a large-scale contains 69.3 percent sulfur mustard new and 71.5 percent after aging. To this day, no one knows exactly what is in this material, but physiological tests have disclosed no appreciable difference between it and the highly purified material used in chemical studies. Sulfur mustard is a heavy, somewhat oily liquid that is clear or straw colored when pure but dark when crude. Its molecular weight is 159.08, boiling point 215°C-217°C, freezing point 14.45°C, specific gravity 1.27. It is sparingly soluble in water but very soluble in organic solvents, animal oils, and fats. It is stable for weeks at room temperature, slowly hydrolyzed by water, and destroyed by strong oxidizing agents.
Studies on the mechanism and kinetics of the hydrolysis of sulfur mustard have shown that the first step in this reaction is the formation of a transient cyclic sulfonium cation, which then reacts quickly with water to form 2-chloroethyl-2-hydroxysulfide and a hydrogen ion. The reaction sequence is repeated to give dithioglycol (5-5):
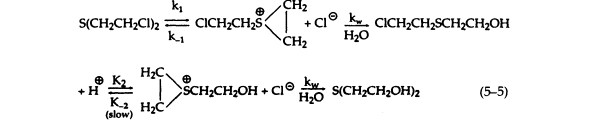
To ensure pure first-order kinetics, sulfur mustard is predissolved in a polar organic solvent, and its concentration is kept low in solution so that the rate of the reverse reactions become negligible compared to kw. The overall reaction—the formation of dithioglycol and 2 HCl—can be described as a quasi-monomolecular process with first-order kinetics. The rate constant for the hydrolysis of sulfur mustard, as determined by acid production, is markedly dependent on temperature and the presence of chloride ion, which retards the hydrolysis rate without altering the reaction products. The retardation of hydrolysis by added chloride is consistent with the reversibility of the activation step to cyclic sulfonium ion. The rate of hydrolysis is not pH dependent and is not altered by metal ions.
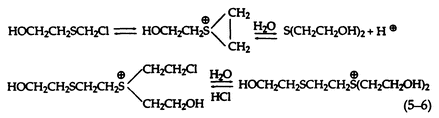
At greater substrate concentrations in the absence of an organic solvent, however, the reaction is more complex, since both dissolution and reaction take place simultaneously and the initial product from the reaction with water accumulates in the aqueous phase and reacts with the sulfonium cation to form a dimeric sulfonium cation (5-6):
This secondary reaction may occur via a transient dithiane disulfonium ion intermediate (5-7):
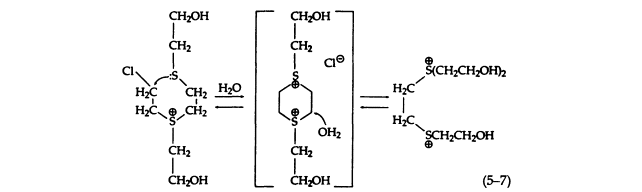
It should be emphasized that all the sulfonium salts (5-6), especially the 2-chloroethyl compound, possess noteworthy toxicity. This toxicity may be due to the decomposition of the sulfonium salts under physiological conditions to form alkylating moieties. The conversion of these sulfonium salts to reactive species is considerably slower than for sulfur mustard. The chemical reactions of the sulfonium salts have been studied in detail, but it is not known whether they are actually formed in vivo . It is certainly possible that such toxic products might be formed on moist areas of the skin, which is consistent with the high susceptibility of these regions to the vesicant action of sulfur mustard. The physiological effects and toxicities of the sulfonium salts need to be investigated, since the proposed mechanism of the cytotoxicity of sulfur mustard is based on the simplified SN1 hydrolysis and is not fully understood.
The relative affinities of nucleophiles are quantitatively described by their competition factors, which compare the rate of constants for bimolecular reactions of cyclic ethylene sulfonium ion with a given nucleophile (Ka) and water (Ko), respectively (5-8):
The dimensions of Fa are 1/concentration, so the reciprocal of Fa is the concentration of nucleophile that must be present in water so that it reacts with 50 percent of the sulfur mustard. An extensive list of
competition factors for sulfur mustard was compiled during World War II. It should be emphasized, however, that despite the large differences in affinities of some nucleophiles, the overall rates of reaction of sulfur mustard are approximately equal. This is consistent with the proposed reaction mechanism, in which the rate-limiting step in the reaction of sulfur mustard in aqueous media is the formation of the cyclic sulfonium intermediate.
In addition to the potential contribution of sulfonium salts to the biologic activity of sulfur mustard, the oxidized forms of sulfur mustard may also be of importance. The reactions of the sulfoxide [OS(CH2CH2Cl)2] are much slower than those of the sulfone [O2S(CH2 CH2Cl)2], leading to a detoxification mechanism (oxidation of sulfur mustard to its sulfoxide). The sulfone, on the other hand, is quite reactive via the elimination of HCl to form the divinylsulfone to which nucleophiles add (5-9):
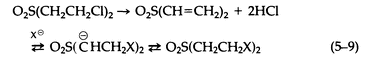
The sulfone is particularly important, since conjugates of it have been identified in the urine of rats dosed intravenously with sulfur mustard.
Reaction of Sulfur Mustard with Various Nucleophiles
Sulfur mustard reacts with sodium salts of alcohols (R; ethanol, methanol, etc.) to give ethers, but the yields are only fair (5-10):
With the corresponding sulfur compounds, almost quantitative yields are obtained (5-11):
The formation of the dimethyl derivative, which is harmless and can be distilled, has been used to characterize sulfur mustard.
With salts of organic acids, esters of thiodiglycol are produced (5-12):
Sulfur mustard reacts readily with secondary amines, but one amine group of the product may be eliminated (5-13):
With ammonia and primary amines, a thiomorpholine is formed (5-14):
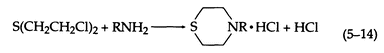
Two molecules of amine may react with one of sulfur mustard (5-15):
Tertiary amines form quaternary ammonium salts (5-16):
When heated with a concentrated aqueous solution of thiourea, sulfur mustard gives the isothiouronium salt, which is decomposed by aqueous NaOH. Acidification produces the mercaptan in high yield
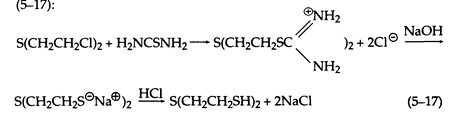
Reactions of Biologic Importance
As is obvious from the chemistry described above, sulfur mustard can react with a number of important functional groups of the large variety of compounds present in cells and tissues. The reactive groups that are of greatest interest are the sulfhydryl group; the phosphate and pyrophosphate ions; organic phosphates such as nucleotides and phospholipids; aromatic nitrogen atoms such as in nicotinamide, adenine, cytosine, and histidine; the carboxyl groups of amino acids and of intermediates of glucose metabolism; the sulfides such as methionine and thiodiglycol; and the amino groups of amino acids, peptides, purines, and pyrimidines. It should be noted, however, that at physiologic pH, most amines are present predominantly in the protonated form rather than as the free base, diminishing the probability of extensive reaction with sulfur mustard.
Evidence that the cytotoxicity of sulfur mustard is due to the alkylation of DNA was first obtained in the late 1940s from studies with bacteria, DNA-containing bacterial viruses, and transforming DNA. The later discovery that the sensitivity of bacterial and mammalian cells is critically dependent on the cell's capacity for repairing sulfur mustard-induced DNA damage strongly supports the DNA target hypothesis.
The relevance of DNA damage and repair to the vesicant action of sulfur mustard is supported by the observation that inhibitors of DNA repair significantly exacerbate skin injury.
Sulfur mustard at neutral pH alkylates purines, pyrimidines, nucleosides, and nucleotides, preferentially at N-7 of guanine and N-1 of adenine. Reactions with 0-6 and N-2 of guanine and N-6 of adenine have also been reported. The following products have been isolated from the reaction of sulfur mustard with DNA (5-18):
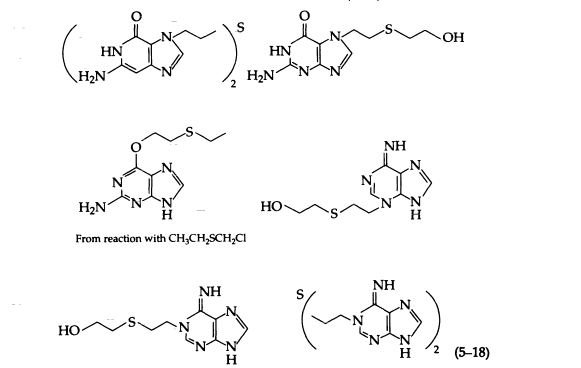
Sulfur mustard, because of its bifunctional nature, is more cytotoxic than is its monofunctional analogue. The molecular basis for this greater toxicity is the ability of sulfur mustard to form interstrand cross-links between guanines of the double helix, which prevents strand separation during replication. In addition, 7-alkylguanines and 3-alkyladenines of DNA are unstable and are released spontaneously from sulfur mustard-treated DNA at physiologic pH and temperature by cleavage of the N-9 glycosyl bond to give an apurinic site. Opening of the imidazole ring of this alkylated purine may also occur under physiologic conditions.
Although sulfur mustard also reacts with RNA, proteins, and phospholipids, the consensus of opinion has been for some time that it is the alkylation of DNA that is by far the most important of its actions. The interstrand DNA cross-link produced by bifunctional mustard com-
pounds is probably the lesion that produces lethality at the lowest frequency of occurrence and at the lowest concentration of the agent. However, cell death from this lesion is delayed for a number of hours, until the cell replicates its DNA or undergoes division. At higher cellular exposures, mechanisms other than DNA cross-linking become important and produce more rapid cell death. The acute damage to the cornea, mucous membranes, and skin seen with sulfur mustard is probably generated by one or more of these other mechanisms.
One mechanism that may be involved in acute damage is nicotinamide adenine dinucleotide (NAD) depletion. The nuclear enzyme poly-(adenosine diphosphoribose) polymerase is activated by DNA strand breaks, such as those produced by sulfur and nitrogen mustards. The enzyme cleaves NAD between nicotinamide and adenine diphosphoribose (ADP) and joins the ADP molecules into polymers of ADP-ribose, which are then linked to nuclear proteins, including the enzyme itself. This process can rapidly deplete cellular pools of NAD, which is required for ATP synthesis. The subsequent depletion of ATP rapidly produces loss of energy-dependent functions in the cell and results in cell death.
Other potential mechanisms of rapid cell death are related to the rapid inactivation of sulfhydryl peptides, especially glutathione, and proteins. These sulfhydryl compounds are critical to maintaining the appropriate oxidation-reduction state of cellular components. In particular, enzymes that maintain calcium homeostasis are sulfhydryl dependent, and sulfhydryl depletion may lead to elevated cellular calcium levels and cell death. Glutathione is also thought to be critical in reducing reactive oxygen species in the cell and preventing lipid peroxidation and loss of membrane integrity.
The toxicities of sulfur mustard to specific organs and tissues are described in detail in subsequent sections of this report. Essentially all of the data on the effects of sulfur mustard on humans are derived from either gas exposure or topical application to the skin. Because of the extensive use of nitrogen mustards in cancer chemotherapy, there is an extensive body of literature on these compounds in man after systemic administration, with doses and clinical follow-up. Since the fundamental mechanisms of interaction of sulfur mustard and nitrogen mustards with biological molecules are very similar, it should be useful to consider the major effects of nitrogen mustards, especially the long-term effects, in trying to ascertain the long-term clinical effects of sulfur mustard. The acute effects of nitrogen mustard are initially nausea and vomiting, followed in a few days by hematopoietic depression. At higher doses, neurotoxicity and damage to the gastrointestinal epithelium are seen. The major delayed effect of nitrogen mustards has been carcinogenesis, especially the development of myelocytic leukemia, although an in-
crease in other types of tumors now seems certain. Another long-term effect of nitrogen mustard treatment is pulmonary fibrosis, produced by damage to the pneumocytes.
Certainly, hematopoietic depression is seen with sulfur mustard exposure in man, although (except in massive exposures) the degree and frequency do not seem to be as intense or frequent as with the nitrogen mustards. This difference is likely due to the more direct exposure to the bone marrow of the nitrogen mustards when given by systemic exposure. This same rationale probably explains why acute leukemia has not been recognized as a consequence of sulfur mustard exposure. However, the increased incidence of solid tumors seen with nitrogen mustard would support the conclusion that exposure of the lungs and skin to sulfur mustard produces a carcinogenic effect on these tissues. Similarly, the delayed pulmonary toxicity seen in a small percentage of patients treated with nitrogen mustards would suggest that long-term damage to the lungs would be expected with intense exposure of the lungs to sulfur mustard.
LEWISITE
The preparation of Lewisite (L-1) by the original procedure is complicated and dangerous. It involves the reaction of acetylene with arsenic trichloride, by using aluminum chloride as a catalyst. The reaction yields three principal products (5-19):
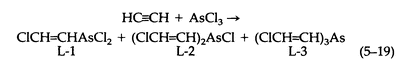
The optimum yield of Lewisite is about 20 percent, obtained along with L-2, L-3, tar, and an explosive material. Acetylene reacts with AsCl3 in hydrochloric acid solution, with mercuric chloride as a catalyst, to give Lewisite in 80 to 85 percent yield (based on AsCl 3). Cuprous chloride and ethanolamine hydrochloride used together, however, constitute the best catalyst for the reaction.
The hydrolysis of Lewisite by water involves the following equilibria (5-20):
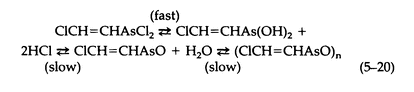
The only substance isolated is polymeric 2-chlorovinylarsinoxide, a white insoluble powder.
The cold aqueous media of pH = 0.5 Lewisite decomposes as follows (5-21):
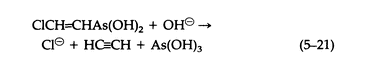
The vesicant character of arsenicals such as Lewisite is not a property of the AsCl2 group exclusively, since carefully prepared solutions of corresponding oxide or dihydroxide are equally vesicant. Lewisite reacts with sodium alkoxides to give derivatives that are volatile, vesicant liquids (5-22) that hydrolyze irreversibly on contact with water:
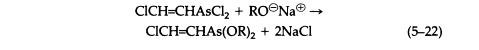
Reaction with sodium mercaptides gives the analogous thioethers (5-23), which are only slightly soluble in water and in general are hydrolyzed reversibly, giving toxic and sometimes vesicant solutions, although the equilibrium generally favors thioether formation (5-24):
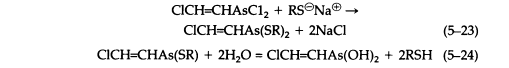
Aqueous and alcoholic solutions of sodium dialkyldithiocarbamates react readily with Lewisite to give crystalline, sharp-melting solids that are useful for its characterization (5-25). These dithiocarbamates are much more stable than the simple thioethers. However, hydrolysis of cyclic thioethers, such as the reaction product of Lewisite and BAL (British Anti-Lewisite) (5-26) is negligible.
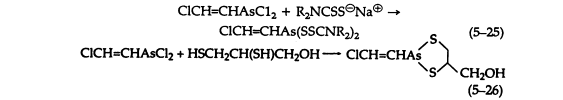
Alkali hydrolyzes all of these compounds with the evolution of acetylene (5-21). Hydrogen peroxide causes decomposition of the ethers and thioethers in neutral or acid solution, giving free arsenic acids.
Little information is available in the literature concerning the reactions of Lewisite with biologically important molecules, although it is reasonable to assume that, as with sulfur mustard, DNA is a major target.
REFERENCES
Annals of the New York Academy of Sciences. 1958. Comparative clinical and biological effects of alkylating agents. 68:657-1266.
Annals of the New York Academy of Sciences. 1969. Biological effects of alkylating agents. 163:589-1029.
Berger NA. 1985. Poly(ADP-ribose) in the cellular response to DNA damage. Radiation Research 101:4-15.
Colvin M, Chabner BA. 1990. Alkylating agents. In: Chabner BA, Collins JM, eds. Cancer Chemotherapy: Principles and Practice. Philadelphia: J.B. Lippincott.
Gates M, Williams JW, Zapp JA. 1946. Arsenicals. In: Division 9, National Defense Research Committee, comp. Chemical Warfare Agents, and Related Chemical Problems. Summary Technical Report of Division 9, NDRC. Washington, DC: Office of Scientific Research and Development.
Grunicke H, Putzer H, Scheidl F, Wolff-Schreiner E, Grunewald K. 1982. Inhibition of tumor growth by alkylation of the plasma membrane. Biosci Rep 2:601-604.
Jarman GN. 1959. Chemical Corps experience in the manufacture of Lewisite in metal-organic compounds. In: Advances in Chemistry. Vol. 23. Washington, DC: American Chemical Society.
Papirmeister B, Feister AJ, Robinson SI, Ford RD. 1991. Medical Defense Against Mustard Gas: Toxic Mechanisms and Pharmacological Implications. Boca Raton, FL: CRC Press.
Reid EE. 1958. Mustard gas. In: Organic Chemistry of Bivalent Sulphur. Vol. 2. New York: Chemical Publishing. 237-451.
Ross WCJ. 1962. Biological Alkylating Agents: Fundamental Chemistry and Design of Compounds for Selective Toxicity. Butterworths: London.
Waters WA, Williams JH. 1950. Hydrolyses and derivatives of some vesicant arsencials. Journal of the Chemical Society (London) 18-22.










