BOUNCING BALLS OF CARBON
The Discovery and Promise of Fullerenes
by Elizabeth J. Maggio

By early 1993, less than 3 years after the existence of a new form of carbon called buckminsterfullerene was confirmed, the scientific community's infatuation with these so-called buckyballs had turned into a love affair. Some might call it an obsession. Neatly packaged by Mother Nature into never-before-seen hollow molecules that resemble infinitesimally small soccer-balls or geodesic domes, buckyballs now have celebrity status. Science magazine declared the buckyball molecule the 1991 "Molecule of the Year" because, said the editors, "rarely has one molecule so swiftly opened the door to a new field of science."
BUCKYBALLS AND THE OTHER FULLERENES KEEP ON ROLLING
The rotund carbon with the cutesy nickname has turned up on t-shirts and coffee mugs; it is the object of several patent proceedings; and it has spurred a commercial venture to produce buckyballs in bulk for the hundreds, if not thousands,
of researchers around the world eager to get their hands on a precious gram or two. In 1992 the 10 most frequently cited papers in chemistry, according to Science Watch, were all on buckyball-related studies aimed at uncovering the molecule's secrets and potential commercial applications.
A buckyball molecule contains 60 carbon atoms, chemically designated as C60, that bond into 12 pentagons and 20 hexagons with the same arrangement as the faces of a soccer-ball (see Figures 9.1 and 9.2). Its shape also follows the same geometric principles that underlie the geodesic dome invented by American architect R. Buckminster Fuller, after whom it was named. Actually, buckminsterfullerene (a.k.a. buckyball) is only one—although the roundest, most abundant, and most popular—of a whole family of similar molecules generally referred to as "fullerenes." The second most common fullerene is C70, whose 70 carbon atoms bond into what some say looks like a rugby ball (see Figure 9.1). "There are a lot of cousins in the carbon family that all share the same molecular form," said University of California, Los Angeles, chemist Robert L. Whetten. He and his colleagues at UCLA and UC-Santa Barbara have unlocked a number of fullerene secrets. They detected molecules with 76, 84, 90, and 94 carbon atoms, and other scientists have shown that giant versions with hundreds of carbon atoms exist.
Buckyballs and the other fullerenes constitute a third form of pure crystalline carbon, something entirely different from diamond and graphite, which were the only two crystalline forms of pure carbon thought to exist until scientists literally stumbled onto the fullerenes. The excitement they are generating is fueled not only by their aesthetic quality but also by their remarkable philosophical and commercial appeal.
For some researchers it has been a humbling experience to discover that modern science did not know everything about the very element that is critical for all earthly life. Apparently, nature has been making fullerenes all along wherever combustion is taking place. In fact, some scientists think they may be one of the most abundant molecules in the universe and that space may even be sprinkled with fullerenes, churned out by certain carbon-rich stars. There is even speculation that the incredibly stable buckyballs may have played a role in the development of organic life on earth.
On the practical end, the fullerenes are supremely tinkerable; being
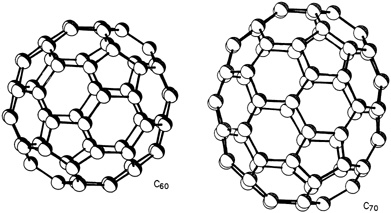
FIGURE 9.1 Schematic drawing showing soccer-ball structure of a buckyball, C60(left), and the rugby-ball structure of the next most common fullerene, C70, (right). Clusters are drawn to scale. (Courtesy of IBM Almaden Research Center.)
empty, round, microscopic cages, they offer the possibility of being stuffed, covered, and packed together with other atoms in experiments limited only by the scientist's imagination. "New branches of materials science and chemistry are opened up by these carbon molecules and their solids. Nobody has shown yet that there's a billion dollar industry in this, but some promising properties have already been discovered," said physicist Donald S. Bethune, of the IBM Almaden Research Center in San Jose. UCLA's Whetten has been on the trail of a particularly enticing fullerene property: superconductivity, perhaps the hottest subject in materials science today. And researchers such as Bethune and his colleagues at IBM have already succeeded in performing one of the neatest fullerene tricks so far—locking up one or more metal atoms inside individual carbon cages, creating what are called metallofullerenes whose properties are just beginning to be explored. Fullerenes may also have a role in the emerging field of nanotechnology in which electronic components, such as semiconductors, are miniaturized down to the atomic or molecular level.
"This is the best science story I've ever come across," commented Robert D. Johnson, a member of the IBM team. "If you had dreamed up fullerenes, it wouldn't be as fantastic as it is turning out to be in real life." Added Bethune, "Beyond the immediate challenge of making, characterizing, and finding applications for these new molecular structures, the
most important thing that has happened in this field is the enormous expansion of people's sense of possibilities. Carbon atoms can be joined to each other and to other molecules, atoms, and clusters in ways that had not previously been imagined. Perhaps this shouldn't have come as such a surprise to beings whose own existence depends so heavily on the marvelous chemical versatility of carbon."
A CARBON PRIMER
Just how versatile is carbon? Of all the elements, not one forms more compounds than carbon; they number in the hundreds of thousands and are the basis for an entire subdivision of chemistry—organic chemistry.
Carbon is exceptional for a number of reasons but most of all for its versatility in bonding to other atoms (especially to other carbon atoms) by sharing its four available electrons in single, double, or triple bonds. Add to this the ease with which carbon can shift its bonding to make a more favorable arrangement. Other elements are not this flexible. For example, the formula for acetylene, a welding gas, is C2H2. The two carbon atoms are linked by a strong triple bond, and each carbon's remaining single bond attaches to a hydrogen atom. However, if tempted with two more hydrogen atoms, the carbons will gladly lessen their grip on each other and change their triple bond to a double one, so that each atom can take on another hydrogen, creating ethylene, C2H4, which is used in the manufacture of plastics. The carbons can accommodate yet another pair of hydrogen atoms by changing their double bond to a single one—the weakest of all—to form ethane, C2H6, a fuel and refrigerant.
Before the fullerene discovery, it was gospel among chemists that pure carbon had only two distinct crystalline forms: diamond and graphite. (Other carbon material, such as charcoal and coke, has an amorphous quality but appears to consist primarily of tiny graphite crystals.) Diamond is the hardest substance known, while graphite is so soft and slippery that it is the basis for pencil lead. Such different characteristics result from the very different ways in which the carbon atoms are arranged. Diamond is a lattice whose basic

FIGURE 9.2 Computer simulation of a soccer-ball-shaped buckyball (C60). (Courtesy of University of Arizona/Dennis Lichtenberger.)
|
Hollow Spherical Molecules "Predicted" in 1966 With tongue-in-cheek musing in the November 3, 1966, issue of the British magazine New Scientist, columnist D. E. H. Jones (writing under the pseudonym Daedalus) made some strikingly accurate predictions about hollow spherical molecules, predating the discovery of the fullerenes by nearly two decades. Some of that column is excerpted here: There is a curious discontinuity between the densities of gases around 0.001 relative to water, and liquid and solids from 0.5 to 25 or so; this week Daedalus has been contemplating ways of bridging this gap and has conceived the hollow molecule, a closed spherical shell of a sheet-polymer like graphite, whose molecules are flat sheets of benzene-hexagons. He proposes to modify the high-temperature graphite process by introducing suitable impurities into the sheets to warp them (rather like "doping" semiconductor crystals to cause discontinuities), reasoning that the curvature thus produced will be transmitted throughout the sheet to its growing edges so that it will ultimately close on itself. … Such fascinating new materials would have a host of uses, in novel shock-absorbers, thermometers, barometers and so on, and possibly in gas-bearings where the rolling contact of the spherical molecules would lower friction even further. Daedalus was worried that they might deform under pressure until he realized that if synthesized in a normal atmosphere they would be full of gas and resilient like little footballs: he is now seeking ways of incorporating "windows" into their structure so that they can absorb or exchange internal molecules, so as to produce a range of super molecular-sieves capable of entrapping hundreds of times their own weight of such small molecules as can enter the windows. SOURCE: Reprinted, with permission, from New Scientist (Nov. 3, 1966). |
framework is a carbon tetrahedron. This is a three-dimensional pyramid structure of five carbon atoms, one at each vertex of the tetrahedron and one in the center. The atomic arrangement of graphite is completely different. The carbon bonds into a two-dimensional hexagonal ring with an atom at each of its six points. Hexagons bond to more hexagons and eventually form flat sheets that stack one over the other. The sheets themselves are only weakly held together, giving rise to graphite's slipperiness and flakiness.
One hexagonal graphite fragment or one tetrahedral piece of a
diamond lattice is not a stable chemical entity. Their carbon atoms still have unused "dangling" bonds that readily latch on to more carbon, leading to the growth of macroscopic crystals. In dramatic contrast, a fullerene is a closed carbon molecule. There are no edges to a fullerene sphere; no atoms left with "dangling" bonds. Once formed, it cannot add additional atoms and grow. It was this property that made buckyballs stick out like sore thumbs in mass spectra of carbon vapor and opened a new chapter on carbon chemistry.
AN UNEXPECTED DISCOVERY
The fullerene story is a relatively short one—less than a decade old—that, perhaps, could have unfolded years earlier if researchers doing the first theoretical studies on a new carbon structure had not written of their work in their native tongue—Japanese. Japanese scientists had made calculations showing that 60 carbon atoms theoretically could be bonded into a stable round molecule that looked like a soccer-ball, a shape technically called a truncated icosahedron. (Strangely, the concept of hollow round molecules that very nearly match the description of a buckyball was put forth in a tongue-in-cheek column that appeared in the New Scientist in 1966. See Box on p. 262.) But the Japanese papers did not make a big hit in the international scientific community. "Language was one barrier," said Bethune, "plus nobody had any notion that this was really possible. It was a purely theoretical exercise."
Still, the synthesis of highly symmetrical molecules, especially of carbon, has been a classic quest in organic chemistry, and in the early 1980s researchers attempted, unsuccessfully, to synthesize the equivalent of buckyballs. They failed, said Bethune, because they relied on the classical approach used by chemists when trying to synthesize something new: start with an existing molecule and modify it through a logical sequence of steps, each accompanied by detailed analysis of intermediate products on which the next modification could be made. "It was time consuming," said Bethune, "akin to mountain climbing. You have to find a particular route up the mountain and along the way there are difficulties to overcome. If you're successful, however, you reach the top." But as those researchers would later discover, to their surprise, the path to fullerenes is amazingly easy: vaporize some graphite and the liberated carbon atoms spontaneously assemble themselves into hollow round balls of varying sizes. "The fact that the fullerenes were not synthesized by chemical intentional design," commented Whetten, "is a painful one for us chemists!"
First Hints of Something Strange
It was pure serendipity that fullerenes were stumbled upon at all. There were hints of their existence in the early 1980s from pioneering basic research being done at Exxon to study how vaporized atoms of different elements cluster back together. The Exxon scientists were running through the periodic table, said Bethune, and had noted strange behavior when they came to carbon. But the fullerenes remained theoretical oddities until 1985 when a landmark paper hit the British journal, Nature, written by British chemist Harold W. Kroto of the University of Sussex and Rice University chemists Robert F. Curl and Richard E. Smalley, along with Rice graduate students at the time, James R. Heath and Sean C. O'Brien. This U.S.-British team reported finding convincing evidence for the existence of a new form of pure carbon—a hollow round molecule, which they named "buckminsterfullerene." At the Houston campus of Rice University, Smalley had been doing experiments, similar to those at Exxon, using a supersonic cluster-beam device he had developed. Across the Atlantic in England, Kroto was heavily involved in his studies of how certain carbon-rich stars may be littering space with carbon molecules in the form of long chains. Eventually the two scientists got together to use Smalley's experimental setup to simulate carbon star chemistry and see what might be happening in outer space. The device vaporized the surface of a rotating disk of graphite using a pulsed laser. The liberated carbon atoms were carried away in a stream of helium gas that expanded as it exited a nozzle, allowing the floating carbon atoms to cool, react, and form clusters. The scientists then ionized the carbon clusters, accelerated them and measured their masses and relative abundances as they whizzed through a time-of-flight mass spectrometer.
The resulting mass spectra showed signals from a wide range of carbon clusters containing anywhere from 38 to 120 atoms, with a curious peak from a 60-atom cluster. The researchers theorized that the laser had torn off fragments of the graphite, liberating pieces of its flat network of hexagonal carbon rings, which then collided and linked up into variously sized clusters. (Bethune's work at IBM would later turn up evidence that the graphite is completely broken down into individual atoms before forming fullerenes.) Then things got exciting and confusing. By allowing more time for the carbon vapor to condense, the researchers discovered that the mass spectrum signals from most of the carbon clusters very nearly disappeared with the exception of that curious 60-atom cluster. Its peak shot straight up like the Washington
Monument, overpowering the readout. The only other noticeable peak, although much smaller, corresponded to a 70-atom cluster. ''As the experiments progressed it became impossible to ignore the antics of the C60 peak, which varied from relative insignificance to total dominance depending on the clustering conditions," Kroto wrote in an article describing these experiments for the journal Science.
A Soccer-Ball Solution
There was no way to account for the extraordinary stability of the big C60 peak. Carbon is notoriously reactive. If this cluster were a flat sheet of carbon hexagons, it would have numerous free edges with which to bond with additional carbon atoms and continue to grow in size. What was stopping it at 60 atoms? Somehow the loose ends of the flat cluster would have to be tied up. The only plausible answer, concluded the researchers, would be if the cluster had closed into a sphere, and that was unheard of in chemistry.
Undaunted by the proposal, they consulted the work of R. Buckminster Fuller, the twentieth-century American architect/inventor who developed the geodesic dome, which is basically a closed network of five-sided (pentagonal) and six-sided (hexagonal) faces. The eighteenth-century Swiss mathematician Leonhard Euler elaborated the geometry behind such a sphere and showed that it had to have 12—and only 12—pentagons in order to close; the number of hexagons could vary, down to two. The soccer-ball is a common example of such a sphere. It has 32 flat faces—20 hexagons and 12 pentagons—which was the exact structure proposed for C60. The researchers theorized that as the carbon clusters were growing in the condensing vapor they rearranged their bonding to produce pentagonal as well as hexagonal rings of atoms that closed the clusters into soccer-ball-like spheres.
Suddenly, the C60 peak made sense. It was "… a most elegant and, at the time, overwhelming solution—the truncated icosahedron cage," recounted Kroto. It was too good not to be true. Not only would a geodesic structure for this cluster have 60 vertices or points marking the location of each carbon atom, but such an arrangement satisfied all the chemical requirements of the carbon atoms, such as bonding. With no loose ends to attract more atoms, this C60 molecule would be superstable. "This structure necessitated the throwing of all caution to the wind … and it was proposed immediately," wrote Kroto. With no independent confirmation that they were correct, the team went ahead anyway and named the new carbon molecule. After discarding such names as
"ballene," "spherene," "soccerene,'' and "carbosoccer," they settled on "buckminsterfullerene," apologizing for the 20-letter, six-syllable moniker. Eventually, buckminsterfullerene became known as simply "buckyball."
All fullerenes have an even number of carbon atoms, which follows from their geodesic geometry, as well as the required 12 pentagons. The varying number of hexagons control their shape. Buckyballs, with 20 hexagons, are the roundest fullerene. The others become progressively out of round with a different number of hexagons in their structure. For example, the atoms in the next most common fullerene, C70, are arranged into 12 pentagons and 25 hexagons, which make it slightly elongated, more like a rugby ball than a soccer-ball (see Figure 9.1). In the report of their discovery, the researchers explain that C60 is uniquely stable and thus the most abundant form because its arrangement of pentagons and hexagons distributes strain perfectly. The second most stable arrangement is that of C70, whose signal always appears along with C60 in mass spectra of vaporized carbon, although to a lesser degree. The researchers calculated that the other fullerenes are not as stable because the pentagons in their structures are located in strained positions that make them vulnerable to chemical attack. That's why they virtually disappeared from mass spectra taken after the carbon vapor was given more time to condense into clusters.
The 1985 Nature paper, titled simply "C60: Buckminsterfullerene," sent a buzz through the scientific community. But even though other researchers were getting the same results as the Kroto-Smalley team, and theorists were coming up with ways in which such hollow spherical molecules should behave, doubts about the existence of buckyballs persisted. The problem was simply that scientists couldn't make enough of it to poke and prod to confirm the proposed structure. All they had were a relatively few transient molecules that could only be studied briefly as they were whisked along in the helium gas that carried them. They didn't hang around long enough or in great enough numbers to be analyzed with conventional methods.
Capturing Buckyballs
Bethune was one of many scientists intrigued by the reports coming out about fullerenes, and in late 1989 he began his own cluster research program at the IBM Almaden Research Center. "Here was this beautiful picture of fullerenes, an amazing new class of carbon compounds," recalled Bethune, "without any proof that it was right." At that point,
only one spectroscopic result had been reported, and it gave very little useful information.
Bethune and his colleagues—Mattanjah de Vries and Heinrich Hunziker of IBM along with Gerard Meijer of the University of Nijmegen, The Netherlands—began working on a way to produce enough fullerenes to characterize. "We had this notion that if fullerenes were so stable and chemically inert, as scientists were claiming, you could collect them," said Bethune. "Why not?" The IBM group set up a system to vaporize graphite. However, instead of trying to analyze the resulting carbon vapor for the brief time it stayed around, they let it condense as soot on a substrate and then probed it for the presence of fullerenes. They placed a piece of copper about a half inch above the graphite, turned on the laser, and caught the escaping soot, which left a distinct dark gray haze on the copper substrate. A second laser was then used to gently knock the collected carbon clusters into an inert carrier gas so they could be analyzed with a sensitive mass spectrometer designed and built by Hunziker, de Vries, and H. Russell Wendt.
When the results were read out by the laboratory's computer, both C60 and C70 made a remarkable showing and were accompanied by signals from other fullerenes up to around 200 atoms in size. The scientists had succeeded in trapping, storing, and accumulating fullerenes on a surface. "We felt like we had suddenly stumbled into a long lost treasure cave," said Bethune. "It was apparently unbelievably simple to produce and capture a whole family of fullerenes. Furthermore, they survived exposure to the atmosphere and room temperature." The IBM researchers estimated that they were making and—more importantly—collecting a nanogram (one billionth of a gram) of buckyballs with each laser pulse. Soon it would be possible to accumulate enough buckyballs for conventional chemical and physical analytical studies that would confirm their structure.
It was the summer of 1990, and the dam was about to crack wide open on fullerenes.
While the IBM group was working on collecting more fullerene-containing soot with its laser deposition method, a U.S.-German team of physicists reported evidence that they had produced soot containing a relatively large amount, roughly 1 percent, of C60 using an even simpler technique. Their fullerene generator, said Bethune, was little more than "a pencil lead and battery." A graphite rod was attached to the terminals of a battery or other power supply, and 100 amps of current was sent through the sample until it smoked and deposited soot on the inside of the helium-filled chamber. The researchers had taken an infrared
spectrum of the collected soot, and it matched the theoretical calculations already made for a soccer-ball-shaped fullerene.
This work was a collaboration between Wolfgang Krätschmer and his graduate student Konstantinos Fostiropoulos at Germany's Max Planck Institute for Nuclear Physics in Heidelberg and Donald Huffman at the University of Arizona in Tucson. They hadn't set out to join the fullerene foray. In fact, one signature of the C60 and C70 fullerenes had appeared in optical analyses of a sample of soot they made back in 1983–2 years before the existence of fullerenes was proposed—only they didn't know it! Like the Kroto-Smalley team, the Krätschmer-Huffman group had been carrying out laboratory simulations of carbon chemistry in stars. They were vaporizing graphite, scraping off the resulting soot from inside the evaporation chamber, and analyzing it optically. They noticed that when the helium atmosphere in their device was at one particular pressure the resulting soot strongly absorbed light at deep ultraviolet wavelengths. A plot of the optical data showed a strange pair of "humps," something the scientists had never seen in all the years they had been analyzing the spectra of carbon dust. They dubbed this particular batch of soot their "camel" sample and put it away after not being able to figure out what the humps meant.
Then 2 years later, in 1985, they read about the proposed buckminsterfullerene carbon molecule in Nature and wondered if those curious humps in the absorption spectrum of the soot they made in 1983 had any connection. "The seeming unlikeliness of this hypothesis, together with some difficulty in reproducing the experiment, led the researchers to put the project on the back burner," recalled Curl and Smalley in a history of the fullerene discovery they wrote for Scientific American . By 1989, however, the Krätschmer-Huffman team became intrigued enough by the possible connection between the proposed fullerenes and their "camel" sample that they decided to give it a second look. They repeated the conditions of their 1983 experiment and this time successfully produced more soot with the double hump spectrum. Analysis of how the material absorbed infrared light not only agreed with what the theorists had already calculated for C60 but also indicated that the "camel" method produced soot with a relatively large quantity of the curious molecules—more than anyone had been able to generate before.
"When we learned of this result," said Bethune, "we immediately tried their recipe for making soot." The IBM team set up a similar "pencil-lead and battery" device and let the current run until the graphite
glowed too bright to look at. The bar broke in about a minute. The researchers ran a soot sample through their mass spectrometer, and the unmistakable signature of the fullerene family appeared on their screen. They vaporized more graphite bars and collected about 20 milligrams of soot, which they put into a small cylinder and heated to nearly 850°F (500° C) to see if anything would sublime out. "We got this beautiful yellow thin film to form on a quartz substrate," Bethune recalled, "and when we looked at it with the mass spectrometer, we saw that the film was almost pure C60." Heating the soot to an even higher temperature yielded another thin film; this time the mass spectrometer told them they had both C60 and C70. "This seemed pretty convincing that the Krätschmer-Huffman team was correct. They had made C60, and not just a little bit."
Now that the IBM group had large enough quantities of purified fullerenes captured as thin films on substrates, Bethune hurried to get samples to various team members who had been waiting to begin a series of sophisticated analyses that would prove—or disprove—the molecule's novel structure. They immediately tried obtaining a Raman spectrum, and began work on photographing the molecules using a scanning tunneling microscope (STM). The big push at IBM and elsewhere, however, was to do nuclear magnetic resonance (NMR) testing that would provide the final, critical piece of evidence for the proposed structure.
Bethune already had in hand the Raman spectrum, which agreed with a soccer-ball structure for C60, when he left in early September 1990 to address the Fifth International Symposium on Small Particles and Inorganic Clusters in Konstanz, Germany. Excited and optimistic, he concluded his talk by saying: "While very soon many more measurements will be done to completely confirm these results, it already appears quite certain that the idea of molecular soccer-balls is realized in nature, and that very soon you will be able to buy them from your favorite chemical company." Bethune's announcement was the first at any major scientific meeting of the production, partial purification and characterization of macroscopic quantities of both C60 and C70 . Upon returning home from the meeting, Bethune was met at the airport by an excited team member who took him directly to the IBM labs. New test results were in. Jet lag couldn't dampen the thrill when Bethune saw the first buckyball photographs and, more importantly, the NMR result everyone had been waiting for, the one that cleared up any lingering doubts that the C60 molecule looks like a miniature soccer-ball.
FINGERPRINTING AND PHOTOGRAPHING BUCKYBALLS
Theorists had already calculated what the test results should be if the atoms in buckyballs really were arranged in soccer-ball fashion. The U.S.-German team had already obtained the first diagnostic result, an infrared absorption spectrum that matched the theoretical calculations and which the IBM team confirmed shortly afterwards. The remaining tests were increasingly more sophisticated, but they would inch researchers closer and closer to the final answer.
The Raman Spectrum
The IBM team was the first to get a key molecular fingerprint, called a Raman spectrum, from the thin films of C60 they had sublimed from soot prepared with the "camel" recipe. "Raman spectroscopy tells us some of the vibrational frequencies of a molecule," Bethune explained. "Just as a musical instrument can be identified by its characteristic
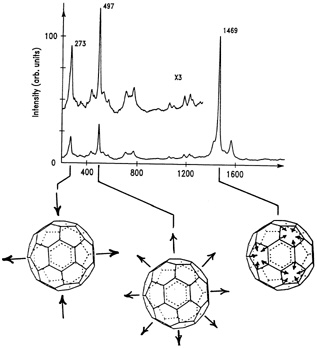
FIGURE 9.3 Raman spectrum of C60showing the three vibrational modes predicted for a soccer-ball structure: "squashing" mode (left); "breathing" mode (middle); "pentagon pinch" mode (right). (Courtesy of IBM Almaden Research Center.)
[vibrational] sound, a molecule's vibrational frequencies tell us about its structure." The molecule's proposed high symmetry would drastically simplify this test. Team members Hal J. Rosen and Wade Tang made the measurements with their lab's Raman spectrometer. This device illuminated the thin film of C60 and then measured how much energy the light lost after scattering off the buckyballs. The energy loss is directly related to vibrations excited in the carbon molecules by the light scattering. The resulting Raman spectrum had three distinct peaks, each corresponding to molecular vibrational frequencies that the theorists had predicted for a soccer-ball structure. The first peak corresponded to a buckyball vibrating in a "squashing" mode, as if the molecule were being pressed between two hands and then released. The second peak corresponded to a "breathing mode," meaning that the C60 molecule was simply expanding and contracting. The last peak, corresponding to the "pentagon pinch" mode, told the researchers that the buckyballs' pentagons and hexagons were expanding and contracting out of phase with each other (see Figure 9.3).
The Raman spectrum, which also complemented the infrared absorption spectrum obtained earlier, was strong support—but not conclusive evidence—for a soccer-ball structure. That would come from the results of NMR studies of buckyballs.
NMR—The Chemist's Proof And a Surprise
Both the IBM group and Kroto's team at the University of Sussex succeeded in obtaining the decisive "single-line" readout from NMR analysis of C60. To chemists, this was the ultimate proof of the molecule's structure. The test worked because a small amount of the carbon atoms in buckyballs are the isotope 13C, which naturally occurs (at about 1.1 percent) along with ordinary carbon atoms, 12C. The isotope has an extra neutron in its nucleus, making it detectable by NMR. Ordinary carbon, with an equal number of protons and neutrons, is not detectable by NMR.
IBM team member Bob Johnson was in charge of the NMR analysis. Sublimed films of C60 were first dissolved in solvent. The fullerene solution was then placed in the NMR device and exposed to a magnetic field. The C60 molecules with the 13C isotope in their structure responded to the magnetic field by generating a tiny magnetic field of their own. This was measured to reveal the geometry and chemical environment surrounding the magnetically active isotopes and thus the entire molecule's structure. It didn't matter where, among buckyball's 60
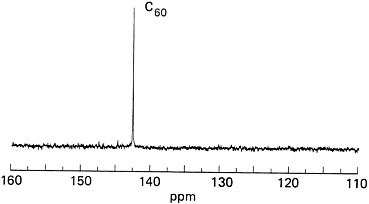
FIGURE 9.4 The "chemist's proof" for C60's soccer-ball structure: a single line on the 13C NMR spectrum. (Courtesy of IBM Almaden Research Center.)
atoms, the magnetically active 13C isotopes occurred, said Bethune. Their NMR reading would be the same for a 13C in any location if buckyballs were truly as symmetrical as suspected. "Every carbon atom in the molecule is just like every other carbon as far as geometry and chemistry go." If C60 weren't symmetrical, the NMR spectrum would consist of multiple peaks. But the single beautiful line told the researchers they were looking at soccer-ball-shaped buckyballs (see Figure 9.4).
The British group also did NMR fingerprinting of the next most common fullerene, C70. Because it has 10 extra atoms, giving it a slightly elongated, rugby-ball shape, this fullerene is not as symmetrical as a round buckyball. The molecule has five nonequivalent groups of atoms, and this produced an NMR spectrum with five distinct lines, a dead giveaway to the slightly out-of-round shape. The IBM group took an additional analytical step and subjected C70 to a more sophisticated examination called 2D "INADEQUATE" NMR. ("INADEQUATE" stands for "Incredible Natural Abundance Double Quantum Transfer Experiment.) This identified which carbon atoms are bonded to each other and, when combined with the NMR spectrum obtained by the British team, completely confirmed the rugby-ball structure for C70. To do this more exacting test, however, Bethune and Meijer first had to enrich the natural 13C isotope content of their fullerene sample. They did this by packing off-the-shelf 13C into a hole bored into a graphite rod before burning it into soot and subliming out C70.
Unexpectedly, this little chemistry trick performed for the 2D NMR analysis also turned up new insight into how fullerenes form in a condensing carbon vapor. When buckminsterfullerene was first proposed, the scientists theorized that carbon came off the vaporized graphite in clumps—essentially graphite fragments—that grew in size through collisions with other fragments and eventually closed into
fullerene spheres. However, when the IBM researchers prepared their isotope-enriched samples of C70' they found that the 13C was randomly mixed in with the regular carbon atoms. "This implies that the graphite must have been completely atomized before forming fullerenes," said Bethune, "and argues against formation mechanisms that involve tearing multiatom fragments from the graphite."
Surprise: Buckyballs Can't Sit Still
While NMR analysis of C60 in solution allowed researchers to study the molecule's symmetry and bonding, the same kind of analysis done on solid fullerene (referred to as "fullerite") turned out to be a powerful tool for probing the detailed geometry of buckyballs and their properties. And, in fact, solid-state NMR analysis carried out independently by Costantino Yannoni of the IBM group and Robert Tycko at AT&T Bell Labs in Murray Hill, New Jersey, revealed a surprising fact: even when solidified into a crystal (when atoms and molecules normally are locked into place by their neighbors), buckyballs are about as sedate as a five year old in a dentist's chair; they're jittery molecules that don't like to sit still. "At room temperature, they nearly ignore each other," Bethune said, "and spin wildly with no correlation between the orientations of adjacent molecules." Such solids are referred to as "plastic crystals,'' and a few others exist in nature, such as a hydrocarbon called adamantane (C10H16). Its carbon atoms form a tetrahedron-like structure that is studded on the outside with the hydrogen. The sides of the molecule bulge out, giving it a somewhat roundish shape. Adamantane used to be the "classic solid rotator" that held the spin record for plastic crystals until buckyballs came along, said IBM team member Johnson, but the new carbon has clearly broken the record for slipperiness. Even though a buckyball is six times larger, it spins three times faster than adamantane, which is slowed by the hydrogen "bumps" on its surface, Johnson explained.
How fast can buckyballs spin in a solid? The IBM group clocked them at around 20 billion revolutions per second at room temperature and found that this is almost as fast as if the molecules were in gas phase, when there is no hindrance at all to rotation. "That's an incredible rate, pretty wild," said Johnson who added that perhaps buckyballs should be named the "new" classic rotator. Further research at IBM showed that the only way to slow buckyballs is to put them in deep freeze. At 8° F (-13° C), their rotation rate drops abruptly. "That's associated with suddenly the molecules beginning to care about the
orientation of their neighbors, and the whole (crystal) lattice undergoes a phase transition where the relative orientation of all these things is specified," explained Bethune. Even then, the molecules aren't completely stationary. They continue to twitch, with a ratchet-like motion, between favorable orientations. Only when the temperature plummets to 77 degrees above absolute zero (around -200° C) will buckyballs stop fidgeting. Later, IBM's Yannoni used this deep-freeze method to keep buckyballs still while making the first NMR measurements of bond lengths between carbon atoms. Because of C60's symmetry, only two bond lengths had to be measured, said Bethune, to specify the position of every atom in the molecule and to calculate the diameter of the sphere: 7.1 angstroms.
Buckyball's propensity to spin about wildly even in solid form made it difficult for other researchers to do x-ray crystallography of their structure. Then scientists at the University of California, Berkeley, led by Joel Hawkins, hit on an ingenious idea to pin down the jumpy molecules. They used "molecular pliers," said Bethune. The researchers had added osmium tetroxide to a C60 solution and made crystals out of the chemical hybrid. Molecules of the metal oxide gripped individual buckyballs like clamps, breaking their slippery symmetry, and making it possible to crystallize them and do x-ray analysis of their atomic positions.
First Buckyball Photos
As 1990 came to a close, with test results undeniably supporting a soccer-ball structure, the world got to see buckyballs for the first time. Formal portraits were made by both the IBM team and a group led by the University of Arizona's Huffman using a scanning tunneling microscope (STM). The photos (see Figure 9.5) were released in back-to-back reports that appeared in the December 13 issue of Nature. Unlike a common light microscope, the STM visualizes individual molecules by recording changes in electric current as a pen-like instrument, whose tip is only a few atoms wide, gently moves over molecules that have been deposited on a specially prepared surface. The IBM researchers put their molecules on the surface of a single crystal of gold. Repeated scanning, done under ultra-high-vacuum conditions, revealed a picture of hexagonally arrayed, round molecules spaced about 11 angstroms apart (1 angstrom = 10-10m) and which fit the predicted diameter for buckyballs, said Bethune. The STM images, made by team member Robert Wilson, also revealed some taller features that the scientists suspect are

FIGURE 9.5 Smaller, dark-colored mounds are buckyballs (C60) photographed with the scanning tunneling microscope. Larger, lighter-colored balls are believed to be C70. (Courtesy of IBM Almaden Research Center.)
the slightly larger C70 molecules mixed in with the C60. While they could clearly identify buckyballs in the STM scans, they couldn't see the individual atoms that make up the hollow cages. They speculated that the molecules' propensity to spin may have blurred the view. Other researchers later found ways to bind the buckyballs more tightly to the surface, revealing in some cases internal structure, according to Bethune.
Buckyballs by the Bushel
Both the IBM team and Harry Kroto's Sussex group continued to concentrate their research efforts on analyzing the fullerene structure. During this time, the U.S.-German collaboration of Krätschmer and Huffman, now joined by University of Arizona physicist Lowell D.
|
Buckyballs: Over 84 Billion Sold In less than a decade, buckyballs have been transformed from bizarre signals detected in mass spectra of vaporized graphite to a hot commercial product. In early 1993, pure buckyballs were selling for $1000 a gram, with a discount for orders of five grams or more. Commercialization of buckyballs began on September 27, 1990, when a team of scientists led by Donald R. Huffman of the University of Arizona in Tucson, and Wolfgang Krätschmer of the Max Planck Institute for Nuclear Physics in Heidelberg, Germany, reported in the journal Nature a process they developed to make high-yield, fullerene-containing soot. That same day, Tucson-based Research Corporation Technologies (RCT) announced filing for worldwide patent protection for the process on behalf of Huffman and Krätschmer. It also filed patents for the various fullerene products produced from the soot. RCT is a nonprofit technology transfer company that evaluates, patents, and commercializes inventions from research institutions. It subsequently licensed the Materials and Electrochemical Research Corp. (MER), also of Tucson, to produce useful research quantities of buckyballs and other fullerenes and to encourage the commercialization and development of new applications for this new form of carbon, according to MER Vice President for Business Development Chuck Hassen. MER markets a range of fullerene products, in addition to pure buckyballs, at much less cost to scientists than if they had to set up their own fullerene generators, and then separate and purify the products they needed for their research, said Hassen. A popular seller is the solvent-extractable part of raw soot made with the Huffman-Krätschmer method. It contains 85 to 90 percent C60; 10 to 13 percent C70; and tiny amounts of C76, C78, and C84. In early 1993, a gram of this extract was going for $200—and less than half that price for volume orders. That represents a 93 percent reduction from MER's first sale in January 1991, said Hassen, who expects even further reductions as improvements are made. In other commercial developments, RCT has licensed Regis Chemical Company of Morton Grove, Illinois, to manufacture the Buckyclutcher I, the trademark for a chromatography column developed at the University of Illinois at Urbana-Champaign for fullerene separation. RCT has also filed international patents on a process developed at Northwestern University that uses C70 to greatly enhance the growth of diamond films used in a variety of commercial applications. |
Lamb, reported another breakthrough in the fullerene saga in the September 27, 1990 issue of Nature: They had succeeded in mass producing large quantities of nearly pure C60 crystals. The mixture also contained a smaller amount of C70 crystals plus traces of larger fullerenes. They had mixed their "camel recipe" soot with the solvent benzene and then evaporated the liquid, leaving behind dark brown to black microscopic crystals, which the scientists subjected to x-ray diffraction studies and additional infrared and mass spectrum analyses. All tests turned up additional convincing evidence for the soccer-ball structure of the C60.
When the soot was mixed with benzene, the clear liquid turned wine red due to the presence of both C60 and C70, quite a startling color change for pure carbon soot! "When they saw the color red develop, they realized they were looking at the first concentrated solution of fullerenes ever seen," wrote Curl and Smalley. "They were the first to observe this roundest of all round molecules, and they knew that chemistry books and encyclopedias would never be quite the same. Now there were three known forms of pure carbon: the network solids, diamond and graphite, and a new class of discrete molecules—the fullerenes."
Currently prepared extracts of soot made with an optimized version of the Krätschmer-Huffman "camel" recipe are nearly 90 percent C60 (buckyballs) and around 10 percent C70. The U.S.-British team that first proposed the existence of buckyballs later realized that they weren't able to produce more than a few fullerene molecules initially because their method didn't keep the vaporized carbon hot enough, long enough, for a larger quantity of the two ultrastable fullerenes to form. The accidentally discovered Krätschmer-Huffman method did. It is so successful at cranking out the carbon balls that it is being patented and has been licensed to a company that is now selling buckyballs in bulk to scientists around the world. (See Box on p. 276.)
Nonfullerene Fullerenes
Soon after scientists got used to the idea that nature, indeed, was packing carbon atoms into miniature soccer-balls and geodesic domes, bizarre forms that Bethune calls "nonfullerene fullerenes" started popping up. In late 1991 Japanese researchers reported the discovery of what have been dubbed "buckytubes," hollow microscopic tubules that appear to be layers of graphite rolled into cylinders. The Japanese scientists, with the NEC Corporation, had been vaporizing carbon by attaching one graphite rod to the positive electrode of a power supply and a second rod to the negative electrode. The positive rod vaporized,
liberating positively charged carbon ions that were pulled to the negative rod where they built up into a deposit. "If you break that off and put it in an electron microscope, what you see are tubular structures layered with different numbers of walls," said Bethune. "In some cases, you can see that the ends of some of them actually close to make closed-end tubes." The Japanese researchers speculate that the buckytubes are extremely strong and could be the basis for a new carbon fiber technology, for example, to reinforce composite materials.
Researchers in Switzerland made a surprise finding about buckytubes while studying them with an electron microscope running at a much higher power setting than normally used. The electron microscope works by bombarding a sample with electrons, and the high-intensity, high-voltage beam the researchers were using caused the buckytubes to transform into something else. "They would see these tubes begin to shrink," said Bethune, "and eventually anneal into something like 'buckyonions.'" These are spherical particles, structured like an onion, whose nested carbon atom layers are spaced apart like bulk graphite. Buckyonions have been seen with up to 10 million carbon atoms. The Swiss researchers suspect that buckyonions are more stable than buckytubes or even finite pieces of graphite. Their report appeared in Nature, and a picture of their ''buckyonions" appeared on the cover with the title: "The Ultimate Fullerene?"
Perhaps the most unusual nonfullerene fullerene described by Bethune is a material called "schwarzite," computer modeled by a team from cornell University. It consists of a network of graphite-like sheets that are both convex and concave, giving the material an undulating structure. "Locally, they look like two-dimensional sheets, but globally they're all linked together by various tubes and connections … leading to a porous 3D form of graphite," Bethune explained. The researchers calculated that each unit cell of crystalline schwarzite would consist of 80 six-carbon rings and 24 seven-carbon rings. Even more bizarre is random schwarzite, whose irregular form consists of hundreds of carbon rings containing not only five and six atoms each but seven, eight, and even nine atoms.
Schwarzite, speculate the researchers, could be a new form of strong yet lightweight materials, if it were real. Right now it exists in theory only, given form by computer modeling. No one has made schwarzite yet … or have they? Bethune wonders if Isaac Silvera, Nancy Chen, and Fred Moshary of Harvard, working in collaboration with the IBM team, may have made similar structures when they compressed tiny samples of C60 under the extraordinarily high pressures created by a diamond
anvil cell. "When you squeeze it hard enough, the material undergoes a phase transition from a fairly dark material to a relatively transparent one," said Bethune. Before and after Raman spectra taken of the material also changed. "At first the lines in the spectrum looked like C60, but the spectrum of the compressed material showed that it was not C60, not diamond, not graphite, and apparently not ordinary amorphous carbon. So it's something different, but whether it's random schwarzite or not isn't yet known."
1001 USES FOR BUCKYBALLS-MAYBE
Even before researchers confirmed the existence of buckyballs and the other fullerenes in 1990, they were already speculating about what they could do with such neat carbon spheres. The last time chemists got this excited was over benzene, a hexagonally shaped ring of six carbon atoms, each attached to a hydrogen atom. Although benzene was isolated in 1825, it took 40 years to determine its structure. Once scientists learned how to manipulate the molecule, benzene became the chemical backbone for a phenomenal number of products, from drugs to plastics, that define modern industrialized society.
Fullerene scientists are hoping for at least as much, if not more, practical impact from their newly found carbon. "The physical stability and relative inertness of the fullerenes allow them to be assembled into very unusual solids," notes Bethune, "thus opening up a new branch of materials science." A particularly exciting aspect of fullerenes is that, since they are hollow spheres, chemistry can be done both on the molecule's exterior surface (''exohedral chemistry") and inside the sphere ("endohedral chemistry").
When talking about practical applications for the fullerenes, Bethune and other scientists enamored of the carbon balls are quick to caution that it is still a big unknown; there's lots of promise but nothing immediately practical. Bethune pulled out a piece of paper divided into two columns. The one on the left was marked Forms of Carbon with Practical Uses or Significance, and the one on the right was Forms of Carbon with NO Practical Uses or Significance. The left column was filled with such things as diamond, graphite, organic chemicals, and polymers. The right column was empty. "The question is," he asked, "where to put all the fullerenes. Obviously, statistics argue that they should go in the left column, based on the simple preponderance of evidence. But if they end up over in the right column with no practical use, it will still be interesting because they will be the only form of carbon there!"
From Insulator to Conductor to Superconductor
The electronics industry would be a big beneficiary of any practical applications to come out of fullerene studies. Scientists already know that, depending on its form, a buckyball solid can have different electrical properties. Unadulterated solid buckminsterfullerene is an insulator at low temperatures; an electric current can't flow through it like it does in a metal. Yet in 1991 researchers at Bell Labs used doping techniques to turn a buckyball solid into not only an electrical conductor but a superconductor as well. Superconductivity is one of the most sought after properties in the world of high technology. It means that a material offers no electrical resistance and lets a current flow through it unhindered. Superconducting materials have already been developed, but their big drawback to widespread commercial application, especially for such futuristic projects as magnetically levitated trains, is that they have to be cooled to temperatures not that much higher than absolute zero before they will work. The cooling requirements alone may be prohibitively expensive. Materials that become superconducting at higher and higher temperatures have long been sought.
What the Bell Labs scientists did was turn C60 into an artificial metal by slipping potassium atoms into the spaces between buckyballs that had been crystallized into a solid thin film (see Figure 9.6). In a report of their work in Physics Today, they described how the transformation from insulator to conductor took place right before their eyes: the pristine yellow color of the pure C60 thin film gradually turned a metallic gray with increased exposure to a potassium vapor. As the color changed, the thin film became more and more electrically conductive. The surprise came when the researchers chilled this artificial metal to about 18 degrees Kelvin (18 degrees above absolute zero) and discovered that it became superconducting. This transition temperature was surprisingly high by superconducting standards. An interesting aside is that in the mid-1960s scientists were able to make a superconductor by sandwiching potassium atoms between flat sheets of graphite, but its transition temperature was only a few tenths of a degree above absolute zero. Somehow, curling up graphite sheets into closed hollow spheres confers special superconducting properties on buckyballs when they are doped with an alkali metal.
A strange thing occurred, however, during the Bell Labs experiments. If the researchers exposed the carbon film too long to the potassium vapor, it lost its superconducting powers and reverted back to being an insulator. The researchers theorized that there is a limit to how
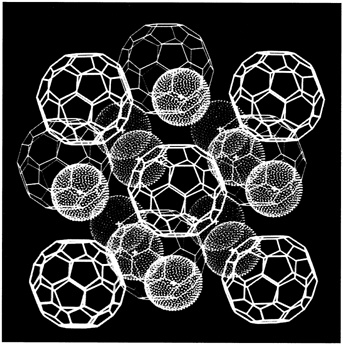
FIGURE 9.6 Computer graphic showing how potassium atoms (smaller balls) fit in between larger buckyballs to make an artificial metal that becomes superconducting when chilled to 18 degrees Kelvin. (Courtesy of University of California, Los Angeles/State University of New York.)
much potassium should be allowed to slip into the carbon network to make it superconducting, and they speculated that a three-to-one ratio of potassium to buckyballs was required. Whetten and his co-workers at UCLA began painstaking experiments to quantify the superconducting formula for this particular artificial metal and confirmed the Bell Labs group's suspicions that a three-to-one ratio is correct. Through this work the UCLA team also found a method to produce this artificial metal so that a large fraction of it ends up with the correct potassium-to-C60 ratio for superconductivity. The original material prepared by the Bell Labs team was only about 1 percent superconducting. Within months—attesting to the pace of fullerene advances—the UCLA team improved the material first to 60 percent superconducting and then all the way to 100 percent.
In the meantime, the Bell Labs and UCLA researchers were also trying to slip other atoms into the buckyball crystals to see the effect on
the superconductivity. With rubidium the artificial metal became superconducting at 29 degrees Kelvin, described as "sizzling" by one of the scientists. Soon after, researchers got the transition temperature up to a stifling 33 degrees Kelvin by using a mixture of rubidium and cesium. There are other superconducting materials with much higher transition temperatures, for example, the so-called high Tc ceramic oxides, but they are planar materials that conduct electricity only in two dimensions. On the other hand, doped buckyballs (as of early 1993) are the high-temperature record holder for three-dimensional superconductors, according to the Bell Labs scientists, and are particularly good candidates for making such things as superconducting wires.
It is still too early to tell if fullerene superconductors will ever be practical. Already scientists have encountered a big obstacle: The material readily reacts with oxygen, and researchers have to be scrupulously careful to keep samples out of contact with air during experiments. "As the Bell Labs people are always careful to point out," said Bethune, "it's not clear whether a spontaneously combustible superconductor is going to be of much use!" Research is already under way at other laboratories to overcome this drawback as well as to improve the transition temperature. On a more positive note, Whetten's group at UCLA showed that once the doped fullerene is made into a superconductor it is stable; it won't loose its powers even after extended heating and mixing. This means it could be tough enough to withstand processing into useful components.
Prisoners in a Carbon Cage
To make superconducting fullerene materials, scientists packed "guest" atoms in between the C60 molecules. But what if they could somehow stuff "guest" atoms, especially metal atoms, into the carbon cages? What would such an exotic material do? Fascinated by the possibility, scientists had already put it on the top of their wishlist as soon as the hollow geodesic structure for the fullerenes was proposed. "Such species would be an interesting new class of molecules," said Bethune, "with tunable electronic, optical, vibrational, and chemical properties depending on what atoms you put inside.''
What seems like a wild idea has turned out to be as easy to do as making plain vanilla fullerenes. Right after the U.S.-British team first detected the peak for C60 in the mass spectrum of carbon vapor and realized what they had, they tried an experiment to see if they could get a guest atom to take up residence inside some of the carbon cages by
vaporizing a graphite disc impregnated with the metal lanthanum. Sure enough the mass spectrum of the carbon vapor had a strong peak where a substance containing both C60 and lanthanum would be. "They took that as evidence that they had produced a metal-containing fullerene," said Bethune. Such carbon-wrapped metals are called metallofullerenes, and since those initial experiments, researchers have succeeded in stuffing one or more different metal atoms into fullerene cages. To date, lanthanum, yttrium, scandium, calcium, uranium, hafnium, titanium, and zirconium have been successfully incorporated, and many other guest atoms are being tried. Researchers have proposed a kind of shorthand to describe how many atoms are trapped in which kind of fullerene molecule. For example, one lanthanum atom inside a buckyball would be designated "La@C60," while "Sc3@C82" would mean three scandium atoms inside C82, a metallofullerene recently made by the IBM group and by Shinohara and his colleagues in Japan.
Initial experiments on stuffing atoms into carbon cages produced a variety of metallofullerenes but in such minute quantities that the only indication of their presence were signals in mass spectra taken of the samples. That changed in early 1992, when Smalley's group at Rice reported a breakthrough that made it possible to produce macroscopic quantities of metallofullerenes. The IBM team then went on to make a milligram or so of C82 carbon cages containing single lanthanum atoms, enough to allow them to do spectroscopic studies and determine its electronic structure. They made this metallofullerene by pressing powdered graphite and lanthanum oxide into a rod, backing it at about 2500° F for 2 hours, and then vaporizing it. The researchers collected the soot and extracted the fullerenes and metallofullerenes from it with solvent. After evaporating the liquid, they were left with a black powder that contained about 2 percent La@C82, the rest being empty C60 and C70 cages. They ran the sample through a series of tests that revealed this metallofullerene to be a truly exotic molecule: there was evidence that the lanthanum atom had transferred its three valence electrons to the fullerene cage. Electrically speaking, said Bethune, this metal-containing carbon cage looked like the C 60 cages in the artificial metal that had been created by packing alkali atoms between crystallized buckyballs in a three-to-one ratio: both have three extra electrons. "So this might give a different route to making a fullerene superconductor." Calculations by researchers at IBM-Zurich suggest that the trapped atom isn't suspended in the center of the cage but is off to the side, attached fairly strongly to the wall of its carbon prison. But there have been other proposals, said Bethune, and the question of exact structure isn't settled yet.
Next, the IBM group tried making a metallofullerene with the metal scandium and got a surprise. Since this atom is smaller than lanthanum, more of them slipped into the carbon molecules—up to three. The case of Sc3@C82 may turn out to be particularly interesting. While some researchers think that the three scandium atoms are in the form of individual ions stuck to the inside of the carbon cage, Bethune speculates that they may have bonded into a molecule. If so, this fullerene confinement system may make it possible to spectroscopically examine molecular ions of this rare-earth metal at room temperature, instead of under the deep freeze conditions usually required because of scandium's high reactivity.
"Beyond characterizing these exotic molecules, we would really like to build materials with them," said Bethune. "This would require production in greater quantities and purification techniques. Purification has proved to be a difficult challenge. Nevertheless, the possibilities that these novel species offer, both for scientific study and for practical applications, are very exciting and still largely unexplored." For example, isolating clusters or molecules of different atoms inside fullerene cages or even tubes may make it possible to fabricate exotic new structures at the nanometer scale, said Bethune. A nanometer is one-billionth of a meter, a size so small that "nanotechnologists" work with building blocks that are only a few atoms in size to make the tiniest components physically possible. Shrinking components down to nano sizes, in theory, could lead to the development of speedier and more efficient electronic and optical devices, especially for computing and communications. The impact of nano-size transistors on computers, for example, could rival the impact that the first tiny transistor devices had when they replaced bulky vacuum tubes.
"The ability to manipulate matter on an atomic scale and create unique materials and devices with custom-designed properties has universal appeal. It marks a triumph of human ingenuity and imagination over the natural rules by which materials are formed," commented Yale solid-state physicist Mark A. Reed in an article about nanotechnology in Scientific American. The big problem, though, with such ultra-small structures is that any "defects" in them would be disastrous. Since fullerenes are unblemished spheres of carbon atoms, noted UCLA's Whetten, they could be perfect nanocrystal building blocks.
A Fullerene Ready for Market
One fullerene technology may soon make it out of the laboratory and into the marketplace, according to Bethune. It's a C70-based treatment
that has proved itself to be practical for enhancing the growth of diamond films. Because of their superior properties, including smoothness, hardness, high-temperature resistance, and chemical stability, diamond films are used to coat such things as cutting tools and to make electronic devices such as semiconductors as well as optical thin films for lenses and infrared windows. Worldwide demand for diamond films and coatings is projected to reach $260 million in 1995 and nearly $2 billion by the year 2000, according to Research Corporation Technologies. This Tucson-based technology transfer company has filed international patents on the C70 treatment method, developed at Northwestern University, and is leading efforts to commercialize it.
The fullerene is used to pretreat a surface so that the fine-grain polycrystalline diamond film can take hold and grow. Conventional pretreatments, such as diamond grit abrasion, are time consuming and expensive and not particularly suitable for anything other than relatively small, flat surfaces. The fullerene pretreatment overcomes this. An ultra-thin layer of C70 is first deposited on a surface and then bombarded with hydrogen and carbon ions. This opens up the fullerene cages, exposing free ends to which the diamond film can attach and grow.
Other Promising Uses and a Real Long Shot
Making nonlinear optical materials is another area in which fullerenes have a good potential for practical application, said Bethune. It has been shown that fullerenes, especially C60, have the ability to react to light by changing their behavior, a very important property to the optics industry. A common example of a product made with nonlinear optical material are eyeglasses that automatically darken with increased exposure to sunlight. The metallofullerenes that the IBM group is working on may also have promising nonlinear optical properties.
Scientists have manipulated the fullerences in a variety of ways and have come up with materials ranging from buckyball polymers to buckyball magnets. But there remains one buckyball property—incredible strength—that is aching for an application, and UCLA's Whetten has a long-shot suggestion: rocket propellant.
It turns out that the hexagon/pentagon geometry that makes geodesic domes such strong building structures also endows buckyballs with superior strength. The seams where the hexagonal and pentagonal faces of the molecule meet are highly resistant to compression, according to Whetten, who tested this by getting these carbon cages moving along at about 15,000 miles per hour and then crashing them into a stainless steel
wall. The molecules simply rebounded, like bullets off Superman's chest, without so much as a dent. "They are resilient beyond any particle known," said Whetten. So what can you do with these superballs? It may seem like science fiction now, but Whetten fancies that they could be shot out of a spacecraft engine as some kind of exotic propellant!
BUCKYBALL POSTSCRIPT: HOW IT ALL STARTED
The entire incredible story of the discovery of fullerenes started as an effort to understand carbon chemistry in outer space. It is, says buckyball's British codiscoverer Harold W. Kroto of the University of Sussex, "a prime example of the way in which an interest in fundamental problems for their own intrinsic sake, irrespective of their predicted use, can yield results of applied significance."
Before getting caught up in fullerene fame, Kroto had been conducting a long-term research project to understand the origins of linear carbon chains detected in space. Signals from these molecules, which are mixed in with the gaseous matter between the stars, had been picked up by ground-based radiotelescopes and identified by comparison with similar signals obtained from laboratory-made molecules. This work was part of a long and fruitful collaboration between astronomy and chemistry that has turned up a rich menu of a variety of cosmic molecules, from ammonia to formaldehyde, by comparing laboratory data with astronomical observations. Kroto, in fact, had participated in the discovery of several different carbon chains, including the detection of one of the most complex molecules ever found in space, HC11N.
At first, scientists suspected that the carbon chains were being formed by reactions between molecules and ions in interstellar gas. Kroto was interested in another possibility, that the carbon chains were forming in the outer atmosphere of certain carbon-rich stars, called red giants, that are constantly throwing out large amounts of dust. "In such stars the possibility that some symbiotic chain/dust chemistry [may be taking place], perhaps related to soot formation, seemed worth considering …," he wrote in a summary of his work that appeared in Science.
To better understand the process, however, Kroto needed to carry out laboratory simulations of the chemistry going on in these carbon stars. Rice University chemist Richard Smalley had just the device: a machine that could vaporize graphite and then analyze the carbon clusters that formed. Kroto and Smalley got together, and the rest is history.
During their experiments, the U.S.-British team found that vaporized
graphite not only formed fullerenes but also precursors of linear carbon chains. The two go hand in hand, according to Kroto, and the possibility that space is also full of fullerenes is being actively pursued. He speculated that the spontaneous formation of fullerenes out of a chaotic carbon vapor may be related to the mechanism that formed the first particles in space. "Indeed, that vital link in planet formation, the primordial solid particle, may well have been carbonaceous. …" In addition to linear carbon chains and related molecules, said Kroto, "a new character, C60, has emerged, whose shadowy role, like that of the Third Man, has only now come to light."
In their now-famous Nature report announcing the discovery of buckminsterfullerene, Kroto and his colleagues wrote: "Because of its stability when formed under the most violent conditions, it [C60] may be widely distributed in the Universe. For example, it may be a major constituent of circumstellar shells with high carbon content. It is a feasible constituent of interstellar dust and a possible major site for surface-catalysed chemical processes which lead to the formation of interstellar molecules. Even more speculatively, C60 or a derivative might be the carrier of the diffuse interstellar lines." Those lines have befuddled scientists for decades. They are signals from molecular material thinly spread out among the stars that scientists have been unable to identify. Kroto suggests that electrically charged buckyballs (ionized by starlight) may be a good candidate for at least some of the mystery molecules and adds that metallofullerenes—metal atoms trapped in the carbon cages—may also be worth looking for in space because of their ease of formation and resiliency.
In their report confirming the existence of buckyballs, the U.S.-German research team notes that both the visible and infrared absorption spectra they obtained from their C60 samples do not match up with any of the interstellar signals. They pointed out, however, that the data are from solid C60' not the ionized buckyballs suggested by Kroto. "Nevertheless, these data should now provide guidance for possible infrared detection of the C60 molecule, if it is indeed as ubiquitous in the cosmos as some have supposed," said the researchers.
While no one has reported finding cosmic buckyballs or confirmed their possible role in planet formation, the rotund carbon has turned up in somewhat related places: 600-million-year-old earth rock and a tiny meteorite. A Russian geochemist reported finding both C60 and C70 in a rock called shungite, found only in a remote part of Russia near Finland. Shungite is a hard black rock made up of mostly coal-like carbon. How the fullerenes got inside is a mystery. Lastly, scientists found fullerenes
while examining a dent in the formerly orbiting Long-Duration Exposure Facility (LDEF), which was returned to earth by the Space Shuttle. Apparently, a tiny carbon-rich meteorite had hit the satellite and left behind a fragment of itself in the resulting dent. The scientists were able to demonstrate that the fullerenes were not formed by the collision with LDEF but were truly cosmic—left behind by the meteorite.
BIBLIOGRAPHY
Baum, R. M. 1991. Systematic chemistry of C60 beginning to emerge. Chemical & Engineering News 69(50):17–20.
Bethune, D. S., G. Meijer, W. C. Tang, and H. J. Rosen. 1990. The vibrational Raman spectra of purified solid films of C60 and C70. Chemistry and Physics Letters 174:219–222.
Dagani, R. 1992. Nanostructured materials promise to advance range of technologies. Chemical & Engineering News 70(47):18–24.
Hawkins, J. M., A. Meyer, T. A. Lewis, S. Loren, and F. J. Hollander. 1991. Crystal structure of osmylated C60: Confirmation of the soccer-ball framework. Science 252(April 12):312–313.
Hebard, A. F. 1992. Superconductivity in doped fullerenes. Physics Today 45(11):26–32.
Holczer, K., O. Klein, S.-M. Huang, R. B. Kaner, K.-J. Fu, R. L. Whetten, and F. Diederich. 1991. Alkali-fulleride superconductors: synthesis, composition, and diamagnetic shielding. Science 252(May 24):1154–1157.
Johnson, R. D., G. Meijer, and D. S. Bethune. 1990. C60 has icosahredral symmetry. Journal of the American Chemical Society 112:8983-8984.
Johnson, R. D., G. Meijer, J. R. Salem, and D. S. Bethune. 1991. 2D nuclear magnetic resonance study of the structure of fullerence C70. Journal of the American Chemical Society 113:3619.
Johnson, R. D., M. S. de Vries, C. S. Yannoni, D. S. Bethune, and J. R. Salem. 1992. Electron paramagnetic resonance studies of lanthanum-containing C82. Nature 355:239–240.
Johnson, R. D., C. S. Yannoni, H. C. Dorn, J. R. Salem, and D. S. Bethune. 1992. C60 rotation in the solid state: Dynamics of a faceted spherical top. Science 255:1235–1238.
Jones, D. E. H. 1966. Ariadne. New Scientist 245(Nov. 3):245.
Krätschmer, W., L. D. Lamb, K. Fostiropoulos, and D. R. Huffman. 1990. Solid C60: A new form of carbon. Nature 347(Sept. 27):354–358.
Kroto, H. W., J. R. Heath, S. C. O'Brien, R. F. Curl, and R. E. Smalley. 1985. C60: Buckminsterfullerene. Nature 318(6042):162–163.
Meijer, G., and D. S. Bethune. 1990. Laser deposition of carbon clusters on surfaces: a new approach to the study of fullerenes. Journal of Chemical Physics 93:7800–7802.
Meijer, G., and D. S. Bethune. 1990. Mass spectroscopic confirmation of the presence of C60 in laboratory-produced carbon dust. Chemistry and Physics Letters 175:1–2.
Moshary, F., N. H. Chen, I. F. Silvera, C. A. Brown, H. C. Dorn, M. S. de Vries, and D. S. Bethune. 1992. Gap reduction and the collapse of solid C60 to a new phase of carbon under pressure. Physical Review Letters 69:466–469.
Pennisi, E. 1992. Scaling chemistry's peaks. Science News 141(16):250–251.
Pennisi, E. 1992. Buckyballs combine to make giant fullerenes. Science News 142(10):149.
Reed, M. A. 1993. Quantum dots. Scientific American 268(1):118–123.
Stucky, G. D., and J. E. MacDougall. 1990. Quantum confinement and host/guest chemistry: Probing a new dimension. Science 247(Feb. 9):669–678.
Teresko, J. 1991. Buckminsterfullerenes. Industry Week 240(Nov. 4):38–44.
Wilson, M. A., L. S. K. Pang, G. D. Willett, K. J. Fisher, and I. G. Dance. 1992. Fullerenes—preparation, properties, and carbon chemistry. Carbon 30(4):675–689.
Wilson, R. J., G. Meijer, D. S. Bethune, R. D. Johnson, D. D. Chambliss, M. S. de Vries, H. E. Hunziker, and H. R. Wendt. 1990. Imaging C60 clusters on a surface using a scanning tunneling microscope. Nature 348:621–622.
Yannoni, C. S., P. P. Bernier, D. S. Bethune, G. Meijer, and J. R. Salem. 1991. An NMR determination of the bond lengths in C60. Journal of the American Chemical Society 113:3190.
Yannoni, C. S., R. D. Johnson, G. Meijer, D. S. Bethune, and J. R. Salem. 1991. Carbon-13 NMR study of C60 in the solid state: Molecular motion and carbon chemical shift anisotropy. Journal of Physical Chemistry 95:9–10.
Yannoni, C. S., M. Hoinkis, M. S. de Vries, D. S. Bethune, J. R. Salem, M. M. Crowder, and R. D. Johnson. 1992. Scandium clusters in fullerene cages . Science 256:1191–1192.
RECOMMENDED READING
Amato, I. 1991. Doing chemistry in the round. Science 254(Oct. 4):30–31.
Crabb, C. 1993. More fun with buckyballs. Discover 14(Jan.):72–73.
Curl, R. F. and R. E. Smalley. 1991. Fullerenes. Scientific American 265(Oct.):54–63.
Kroto, H. 1988. Space, stars, C60, and soot. Science 242(Nov. 25):1139–1145.
Pennisi, E. 1991. Buckyballs still charm. Science News 140(Aug. 24): 120–123.
Taubes, G. 1990. Great balls of carbon. Discover 11(Sept. ):52–59.
































