3
Perinatal and Pediatric Toxicity
BOTH ACUTE AND CHRONIC toxic reactions in the young are often considered together under the title of developmental toxicity. Such toxicity can be further subdivided by the organ system involved or by whether the toxic effect occurred before or after birth. The developmental purview of the committee extends from the beginning of the third trimester through 18 years of age; however, no single theoretical framework or unifying set of principles readily applies to so broad a developmental span. Teratology, the study of congenital malformations, has traditionally focused on the process of organogenesis, the sensitive period in prenatal development when birth defects can be induced by exposure to either endogenous (e.g., endocrine) or exogenous (e.g., xenobiotic) agents. One view of teratogenesis is that this type of abnormal development represents a special form of embryotoxicity.
Developmental toxicology includes the study of chemically induced alterations of the normal sequence of developmental processes. It both encompasses and expands the domain of abnormal development beyond that implied by teratology. Although the term denotes adverse chemical effects on development, its end points are not restricted to gross anatomical defects but encompass multiple expressions of abnormal outcome. This research specialty combines basic principles, concepts, and working assumptions from several disciplines, including developmental and cellular biology, pharmacology, and toxicology. A major objective is to understand how exogenous agents interfere with the normal progression of developmental events to produce phenotypically abnormal cells, tissues, organs, and function. Since this report's focus begins with the third trimester, the committee does not directly consider the teratogenicity of pesticides, i.e.,
their potential to produce gross structural malformations. Rather, the focus is on processes that occur after the completion of organogenesis and continue well into the postnatal period. However, the origins of this broader concern with peri- and postnatal toxicology are inextricably rooted in experimental teratology.
Studies of the toxicity of xenobiotic compounds in children have demonstrated the potential for either acute or chronic exposure to result in serious malfunctions at a later age. This potential exists because of the developmental character of the physiologic/biochemical/molecular function of the young individual. While a biologic system is developing, a toxic event can alter one aspect of that development so that all subsequent reactions are altered or modified. For example, transient elevations of serum bilirubin during the newborn period may produce changes in the basal ganglia of the brain that may not become apparent until several years later but are then permanent in nature.
ACUTE TOXICITY
In this section, the committee discusses and summarizes the relative sensitivity of infants, children, and adults to the acute toxicity of chemicals. Acute toxicity here is defined as toxicity resulting from a single exposure to a chemical. The injury may be immediate or delayed in onset. Both lethality and target organ injury will be considered as toxic end points. A limited number of findings from studies of laboratory animals are summarized where data on humans are inadequate. Because of the meager data base on age-dependent acute toxicity of pesticides, some examples of pharmacologic effects and adverse effects of therapeutic agents in pediatric and adult populations are described. Attention is focused, in turn, on age-related differences in the lethality of pesticides and other chemicals, differential effects of cholinesterase inhibitors in immature and mature subjects, and age-related effects of toxic and pharmacologic actions of selected therapeutic agents.
Data on age-related susceptibility to the lethal effects of chemicals are largely limited to acute LD50 studies in laboratory animals. Done (1964) was one of the first investigators to compile the results of LD50s and other measures of lethality of a variety of chemicals in immature and mature animals. Immature animals were more sensitive to 34 chemicals, whereas mature animals were more sensitive to 24 compounds. Thiourea was 50 to 400 times more toxic (i.e., lethal) in adult than in infant rats. Conversely, chloramphenicol was 5 to 16 times more toxic in 1- to 3-day-old rats. Thus, Done (1964) concluded that immaturity does not necessarily entail greater sensitivity and that age-dependent toxicity is chemical dependent. Goldenthal (1971) tabulated LD50 values for newborn and neonatal animals
as compared to adult animals primarily from data submitted by pharmaceutical firms in drug applications. Approximately 225 of these compounds were more acutely toxic (lethal) to neonates, whereas about 45 were more toxic to adult animals. Almost all the age-related differences in LD50s in the reports of Goldenthal (1971) and Done (1964) were less than 1 order of magnitude; indeed, most varied no more than two- to threefold.
As discussed in Chapter 2, there are important differences between immature laboratory animals and humans. Nonprimate species are generally less mature at birth than are humans. Newborn mice and rats are among the most immature of commonly used test species, so it is not surprising that they often differ markedly from adult animals in sensitivity to chemicals. This phenomenon is particularly evident in the paper by Goldenthal (1971), who reported five times as many chemicals to be more acutely toxic to newborn than to adult animals. Since full-term human newborns are more mature, such pronounced age-dependent differences in toxicity would not be anticipated. Maturation in rodents is very rapid, so that even a few days of age can result in a marked disparity in test results (Done, 1964). Furthermore, organs and their associated functions mature at different rates in different species. Uncertainty in extrapolating findings among different species of mature animals is appreciable. When the additional variable of interspecies maturation patterns is introduced, the choice of an appropriate animal model for pesticide toxicity of neonates, infants, and children becomes even more complex.
The relative acute lethality of pesticides to immature and mature animals has been the subject of a number of studies. Goldenthal (1971), in his extensive compilation of LD50 values for newborn and adult animals, included several fungicides, herbicides, and the insecticide heptachlor. Each of these compounds was more toxic to newborn than to adult rats. Gaines and Linder (1986) more recently contrasted the acute toxicity of 36 pesticides given orally to weanling (4 to 6 weeks old) and to young adult Sherman rats. Age-related differences, where they existed, were usually no more than two- to threefold. Weanlings were more sensitive than adults to only 4 of the 36 compounds. Lu et al. (1965) observed that 14- to 16-day-old rats were intermediate between newborns (most sensitive) and adults (least sensitive) in their susceptibility to malathion poisoning. Such findings are in agreement with the observation that physiological and biochemical processes, which govern the pharmacodynamics of pesticides, mature quite rapidly in rodents. Indeed, metabolism and renal clearance of xenobiotic compounds and their metabolites soon approach and may exceed adult capacities in rodents within 2 to 3 weeks. This same phenomenon occurs in humans, albeit at a somewhat slower pace (i.e., within the first weeks to months of life). Higher metabolism
may confer protection against pesticides or increased susceptibility to injury, depending on the relative toxicity (and rate of elimination) of the parent compound compared to its metabolites. The findings of Lu et al. (1965) are a good case in point. These investigators contrasted acute oral LD50 values for newborn, 14- to 16-day-old, and young adult Wistar rats. The adult animals were the most resistant to malathion, as would be anticipated, since adult rats most efficiently metabolize organophosphates and organophosphates are metabolically inactivated (Benke and Murphy, 1975). Conversely, the older rats of Lu et al. (1965) were the most sensitive to the acute toxicity of dieldrin. Thus, susceptibility to acute pesticide toxicity appears to be a function of age, species, and chemical.
Limitations of acute lethality data should be recognized. Acute doses of chemicals high enough to cause death may damage organ systems by mechanisms that are quite different from those that produce biological effects from chronic exposures to lower levels. MacPhail et al. (1987) examined age-related effects of a number of pesticides on lethality, serum chemistry, and motor activity in weanling and adult male rats. Although age was generally not an important determinant of toxicity for most of the pesticides, there were age-related differences in the effects of carbaryl and diazinon on motor activity. These results could not have been predicted on the basis of LD50 values for the two groups, leading MacPhail et al. (1987) to conclude that mortality may be a poor predictor of morbidity and that nonlethal end points should be used to assess the age-dependency of the neurobehavioral toxicity of pesticides. More sensitive indices should also be used to monitor other potentially vulnerable systems in infants and children, including the hormonal and reproductive systems, the immune system, the nervous system, developmental effects, and carcinogenesis/mutagenesis. Unfortunately, relatively few well-controlled studies have been conducted, particularly in humans, in which sensitive end points are used to assess the relative toxicity of comparable doses of pesticides or other chemicals in pediatric and adult populations.
Cholinesterase inhibition, a mechanism by which organophosphate and carbamate insecticides produce excessive cholinergic effects, is a sensitive end point that can be monitored in humans and other mammals. Brodeur and DuBois (1963) reported that weanling (23-day-old) rats were more susceptible than adults to the acute toxicity of 14 of 15 organophosphates tested. The greater toxicity of parathion in weanling rats was tentatively attributed to deficient hepatic detoxification of parathion and its bioactive oxygen analogue, paraoxon (Gagne and Brodeur, 1972). A comprehensive investigation was reported by Benke and Murphy (1975) in five age groups of male and female Holtzman rats: 1, 12 to 13, 23 to 24, 35 to 40, and 56 to 63 days old. There was a progressive decrease in susceptibility to poisoning by parathion and parathion-methyl with increasing age up to
35 to 40 days for both sexes. Detailed experiments were conducted to determine the influence of aging on metabolic activation of the two compounds, as well as on detoxification systems (e.g., aryl esterase-catalyzed hydrolysis, glutathione-dependent dearylation and dealkylation, and binding in the liver and plasma). Benke and Murphy (1975) concluded that increased detoxification of the active oxygen analogues of parathion and parathion-methyl was largely responsible for the lower acute toxicity of the two insecticides in adult animals. Murphy (1982) subsequently pointed to two other factors that contributed to the lower sensitivity of adult rats to organophosphates: greater binding to noncritical tissue constituents and more rapid catabolism of the parent compounds.
The limited information available suggests that immature humans also experience greater susceptibility to organophosphate- and carbamate-induced cholinesterase inhibition and related effects. In 1976 in Jamaica, 79 people were acutely poisoned as a result of eating parathion-contaminated flour (Diggory et al., 1977). Seventeen of the patients died. Case-fatality ratios were highest (i.e., 40%) among children ranging from newborns to 4 years of age. Zwiener and Ginsburg (1988) presented the clinical histories of 37 infants and children exhibiting moderate to severe organophosphate and carbamate toxicity. Although most of these patients ingested the pesticides, six became intoxicated after playing on sprayed surfaces. Zwiener and Ginsburg (1988) noted that 76% of their subjects were younger than 3 years old. The investigators found there was a paucity of information in the literature on the toxicity of cholinesterase inhibitors in infants and children.
Parathion contamination of stored foodstuffs (Diggory et al., 1977) and aldicarb contamination of crops (Goldman et al., 1990) have resulted in the most widespread outbreaks of foodborne pesticide toxicity in North America. Goldman and co-workers investigated more than 1,000 cases of illness caused by consumption of aldicarb-contaminated watermelons and cucumbers. Unfortunately, infants and children were not studied as a subpopulation at risk. The investigators did calculate doses of aldicarb sulfoxide that produced illness in the general population and estimated that a 10-kg child could readily consume enough of the pesticide on watermelons to experience toxicity. The U.S. Environmental Protection Agency (EPA, 1988) concluded that infants and children are at the greatest risk of acute aldicarb toxicity. This conclusion was based on dietary consumption and contamination patterns, however, rather than on the greater sensitivity of infants and children to this potent cholinesterase inhibitor.
Although immature humans appear to be more susceptible than adults to the acute effects of cholinesterase inhibitors, the age-dependency of this phenomenon is not entirely clear. Some of the most applicable information has been provided by a study of the perinatal development of
human blood esterases (Ecobichon and Stephens, 1973). Erythrocyte acetylcholinesterase and plasma pseudocholinesterase and arylesterase activities were measured in premature newborns of varying gestational age as well as in full-term newborns, children of different ages, and adults. Apparent Km values for the three enzymes did not vary significantly with age for a variety of substrates, indicating that the enzyme properties were similar in all age groups. Enzymatic activity, however, did vary significantly with age. Levels of all three enzymes progressively increased during gestation, then rose markedly during the first year of life. Thereafter, erythrocyte cholinesterase and pseudocholinesterase activities increased gradually to adult levels. If one were to assume that one of these peripheral enzymes (e.g., erythrocyte cholinesterase) reflects brain acetylcholinesterase levels, then the most pronounced effects of cholinesterase inhibitors may be expected to occur in newborns, neonates, and infants, since a chemically induced depression of enzymatic activity may be more apparent when baseline cholinesterase levels are relatively low. Ecobichon and Stephens (1973) provided evidence of another mechanism of increased susceptibility of newborns—namely, diminished detoxification capacity (i.e., significantly lower plasma arylesterase and paraoxon hydrolysis activities). Children 2 to 8 years old had slightly lower activities than adults, suggesting that younger children may be somewhat more susceptible to cholinesterase inhibitors. The consequences of brain acetylcholinesterase inhibition on nervous system development and postnatal function remain largely unexplored.
Because of the paucity of data on the age-dependency of acute toxicity of pesticides in humans, the remainder of this section focuses on relative effects of therapeutic agents in pediatric and adult populations. Substantially more information should be available on drugs, due to their common use in all age groups and stringent requirements by the Food and Drug Administration (FDA) for demonstration of safety and efficacy. Data from well-controlled, parallel studies in infants, children, and adults, however, are quite limited for most drugs.
Done et al. (1977) reported what was termed a therapeutic orphan problem—namely, that safety and efficacy for children had not been proved for 78% of new drugs then marketed in the United States. A 1990 survey by the American Academy of Pediatrics revealed that the labeling of 80% of new drugs approved by the FDA between 1984 and 1989 did not include information on pediatric use. The FDA's policy has allowed the marketing of drugs that have been approved for adults but not studied in children, as long as labeling included disclaimers and no instructions about pediatric use. Without adequate information, physicians commonly prescribe such medications for children, possibly placing pediatric populations at increased risk of uncertain efficacy or adverse reactions.
The FDA (1992) proposed to amend labeling requirements for prescription drugs to promote their safe and effective use in children. Misunderstandings and concern about legal and ethical implications have limited clinical research in pediatric populations. The newly proposed guidelines provide alternative ways to assess effectiveness and safety in children without necessarily having to conduct comprehensive studies. Results from well-controlled studies in adults can be extrapolated to children under some circumstances, although separate pharmacokinetic studies are needed to establish appropriate pediatric dosage regimens. The intent of the proposed amendment is to provide more complete information on labeling of prescription drugs concerning use and possible hazards for children.
Several instances of severe adverse effect from pharmaceutical agents in pediatric populations have attracted widespread attention. During the 1950s, chloramphenicol produced a pallid cyanosis, which progressed to circulatory collapse and death in some newborns (Sutherland, 1959). This so-called gray baby syndrome has been attributed to the diminished hepatic glucuronide conjugation and renal secretory capacities of newborns. Weiss et al. (1960) reported blood half-lives of 26, 10, and 4 hours for chloramphenicol at birth, at 10 to 16 days of age, and in children 4 to 5 years old, respectively. Thus, there is a substantial increase in chloramphenicol metabolism and excretion capacity during the first days and weeks of life. Decreased metabolic and excretory capacities of newborns and neonates have been associated with exaggerated toxicity of a number of other chemicals, including benzyl alcohol (Gershanik et al., 1982), hexachlorophene (Tyrala et al., 1977), and diazepam (Nau et al., 1984). The hexachlorophene poisonings appeared to be associated with increased percutaneous absorption as well as deficient metabolism in newborns. Floppy infant syndrome in babies born to mothers given diazepam is apparently the result of a number of age-dependent factors, including a smaller volume of distribution and thus greater target organ concentrations of the lipophilic drug due to a smaller adipose tissue volume in newborns, increased amounts of free diazepam due to displacement of the drug from plasma protein binding sites by elevated free fatty acid levels, and a prolonged half-life as a result of diminished oxidative and conjugative metabolism (Warner, 1986). As discussed in Chapter 2, most physiological processes that govern the kinetics of drugs and other chemicals mature during the first year after birth. Indeed, profound changes in some processes (e.g., phase I and II metabolism) occur during the first days and weeks of life (Morselli, 1989). Thus, the most pronounced differences from adults in susceptibility to drug toxicity would be expected in newborns, neonates, and infants; the youngest are most likely to experience the most aberrant responses.
The net effect of immature physiological and biochemical processes on drug efficacy and toxicity is difficult to predict. The various processes mature of different rates and may enhance or offset one another. Local anesthetics provide a good illustration. These drugs are commonly administered to the mother during labor and delivery and may readily enter the maternal circulation and cross the placenta (Tucker and Mather, 1979). Cardiovascular depression and respiratory depression in newborns have occasionally been reported, although subtle neurophysiological impairment and behavioral changes are probably more common consequences (Dodson, 1976; Ostheimer, 1979). Premature and full-term newborns exhibit lower plasma protein binding of local anesthetics. This should result in increased amounts of free drug and a more pronounced pharmacologic response, but the greater volume of distribution in newborns reduces the concentration of drug at sites of action. Rates of hepatic microsomal metabolism and plasma pseudocholinesterase-catalyzed hydrolysis of anesthetics such as procaine are quite low in newborns. This deficit in metabolism, coupled with the larger distribution volume that must be cleared of drug, accounts for the prolonged half-life and long duration of action of lidocaine and its analogues in neonates (Morselli, et al., 1980).
Hepatic metabolism and renal clearance of xenobiotic compounds change dramatically during the first year of life. Phase I metabolic reactions (e.g., oxidation) may rise from one-fifth to one-third of the adult rate during the first 2 to 3 postnatal weeks to two to six times the adult rate (Neims et al., 1976; Morselli, 1989). Different isozymes and enzymes mature at different ages. Certain phase II (e.g., glucuronidation) reactions do not reach adult levels for months, while maturation of alcohol dehydrogenase activity may take as long as 5 years (Kearns and Reed, 1989). The majority of xenobiotics, however, are metabolized most rapidly by individuals between 2 to 4 months and about 3 years of age. Thereafter, drug metabolism gradually declines to adult levels (Warner, 1986). Development of renal function displays a similar age-dependency. Glomerular filtration increases dramatically during the first week of life, approaching and exceeding adult values within 3 to 5 months. Renal tubular secretory and absorptive processes mature more slowly (Kearns and Reed, 1989). Older infants and children, therefore, may be less susceptible than adults to drugs that are metabolized to less toxic, more readily excretable metabolites. Spielberg (1992) noted that clearance of nearly all anticonvulsant drugs is quite limited in newborns, especially premature newborns. Conversely, clearance of such drugs (e.g., phenytoin, phenobarbital, carbamazepine, and diazepam) in infants and children, when calculated on a milligram-per-kilogram-of-body-weight basis, was well above that in adults until around puberty. Thus, children are less likely than adults to exhibit toxicity and require higher doses (on a milligram-per-kilogram-
of-body-weight basis) of anticonvulsants to achieve therapeutic levels. In contrast, infants and children may be at greater risk from other drugs and chemicals that undergo metabolic activation (i.e., conversion to bioactive or cytotoxic metabolites). Unfortunately, there is lack of information on such agents in humans in the published literature.
There was concern that acetaminophen (Tylenol), a drug that undergoes metabolic activation to hepatocytotoxic metabolite(s) via a P-450-mediated mixed-function oxidase (MFO) pathway, would cause increased morbidity and mortality in young children. This concern was never realized, however, since hepatotoxicity in young children was found to be less severe than in adults, and has rarely (Rumack, 1984). Acetaminophen is metabolized by several parallel pathways. The two major detoxification pathways involve conjugation of the parent compound with sulfate or glucuronide. Thus only a small fraction of the drug remains to be oxidized by the P-450-mediated pathway to a reactive intermediate (N-acetyl-p-benzoquinonimine). This metabolite is conjugated with glutathione to produce nontoxic products or can bind covalently to cell proteins and nucleic acids, causing cellular injury (Hinson et al., 1990). Although prepubescent children have relatively high hepatic MFO activity, they also exhibit a greater capacity than adults to detoxify acetaminophen by phase II metabolic reactions, primarily sulfate conjugation (Miller et al., 1977). Also, higher glutathione levels in the young may contribute to protection from hepatotoxicity. Thus, the lower susceptibility of children to acetaminophen poisoning is due to their greater capacity to eliminate the drug by nontoxic pathways (Kauffman, 1992).
Clinical trials in infants and children are relatively infrequent for most classes of drugs, but this is not the case for many antineoplastic agents. Although some types of childhood cancer are refractory to chemotheraphy, others have excellent cure rates (Petros and Evans, 1992). Therefore, phase I clinical trials are frequently conducted in both adult and pediatric populations to define the maximum tolerated dose (MTD) for appropriate dosage schedules in phase II trials. Antineoplastic agents include a wide variety of different types of chemicals that act by diverse mechanisms. Thus, results of phase I studies of anticancer drugs afford scientists some of the most comprehensive data sets for contrasting toxic effects of chemicals in children and adults. The investigations typically involve repetitive dosage regimens lasting days or weeks, however, rather than single, acute exposures.
Comparable clinical trials of antineoplastic agents in pediatric and adult patient populations have revealed toxic effects that are often similar qualitatively but different quantitatively (Glaubiger et al., 1982; Marsoni et al., 1985; Evans et al., 1989). In compilation of data on 16 compounds for which there had been comparable phase I trials in adults and children,
TABLE 3-1 Maximum Tolerated Dose (MTD) of Some Anticancer Drugs in Children and Adults
|
|
MTD (mg/m2) |
||
|
Drug |
Children |
Adults |
Ratio, MTD for Children/ MTD for Adults |
|
Dianhydrogalactitiol |
25 |
30 |
0.83 |
|
5-Azacytidine |
200 |
225 |
0.89 |
|
TIC mustard |
900 |
1,000 |
0.90 |
|
Piperazinedione |
3 |
3 |
1.0 |
|
VP16-213 |
150 |
125 |
1.20 |
|
Diglycoaldehyde |
=7,500 |
6,000 |
1.25 |
|
m-AMSA |
50 |
40 |
1.25 |
|
Daunomycin (mg/kg) |
1.0 |
0.8 |
1.25 |
|
Adriamycin (mg/kg) |
0.8 |
0.6 |
1.33 |
|
VM-26 (mg/kg) |
4.0 |
3.0 |
1.33 |
|
3-Deazauridine (leukemia patients) |
8.2 |
6.0 |
1.40 |
|
Azaserine (mg/kg) (total dose) |
156 |
108 |
1.44 |
|
Anhydro-5-flouro-cyclocytidine |
=300 |
=200 |
1.50 |
|
Dihydroxyanthracenedione |
18 |
12 |
1.5 |
|
3-Deazauridine (solid tumors) |
2.8 |
1.5 |
1.85 |
|
Cyclocytidine |
600 |
300 |
2.00 |
|
ICRF-187 |
>2,750 |
1,250 |
>2.20 |
|
SOURCE: Glaubiger et al., 1982. |
|||
the types of toxic effects that limited further dosage escalation were generally the same (Glaubiger et al., 1982). As shown in Table 3-1, the MTD for children was higher than that for adults for 13 of the compounds. Similar findings were reported by Marsoni et al. (1985). These investigators compared the MTDs and recommended phase II doses in children and adults for 14 drugs in patients with solid tumors and 8 drugs in patients with acute leukemia. Children with solid tumors exhibited a greater dose tolerance for 12 of the 14 drugs. Children with leukemia appeared to have tolerances similar to those of adults.
Data on daunomycin in relation to the incidence of congestive heart failure in children and adults have been compiled. Children seem to be more sensitive than adults to this complication at comparable doses, even through the MTD is approximately 20% higher in children than in adults.
The greater tolerance of children to many anticancer drugs may be attributable to higher rates of metabolic or renal clearance. Both Glaubiger et al. (1982) and Marsoni et al. (1985) expressed MTDs on a milligram-per-square-meter rather than a milligram-per-kilogram-of body-weight basis. Had the relative doses been calculated as milligram per kilogram, the interage differences should have been even more pronounced. Pinkel (1958) observed that pediatric patients tolerated more methotrexate on a
milligram-per-kilogram basis than did adults, but the MTDs were similar when calculated on the basis of body surface area. Methotrexate is eliminated primarily by glomerular filtration and active renal tubular secretion of the parent compound. It is not surprising, therefore, that children with relatively high renal function exhibit greater rates of plasma elimination than do adults (Wang et al., 1979). In a study of 47 patients (3 to 39 years old) receiving methotrexate, Bleyer (1977) found a significantly higher incidence of neurotoxicity in the adults. Conversely, young infants have diminished renal function and exhibit lower systemic clearance and a greater potential for injury than do children (McLeod et al., 1992).
As maturation of xenobiotic metabolism and renal function generally parallel one another during the first year of life, it is not surprising that neonates and young infants may be at increased risk of injury from anticancer drugs that undergo metabolic inactivation. Vincristine is one such drug. It is detoxified in the liver and eliminated primarily via biliary excretion. Woods et al. (1981) reported a significantly higher incidence of neurotoxicity and hepatotoxicity in small infants than in children receiving vincristine. On the other hand, compounds that undergo metabolic activation may place children at greater risk than neonates or adults, since children have a higher metabolic capacity. Marsoni et al. (1985) observed that indicine N-oxide was one of the few anticancer drugs tested to have a lower MTD in children than in adults. Indicine N-oxide is believed to be converted to the toxic metabolite dehydroindicine by the liver. Cyclophosphamide is another drug that undergoes metabolic activation to cytotoxic metabolites. Certain of its metabolic pathways, however, also involve inactivation/detoxification. The half-life of cyclophosphamide is shorter in children (1 to 6.5 hours) than in adults (4 to 10 hours) (Crom et al., 1987). Although metabolic activation of drugs such as cyclophosphamide may be highest in children, the operability of concurrent detoxification pathways and inactivation of the reactive metabolites, coupled with rapid urinary excretion of the metabolites, apparently combine to hasten the elimination and thereby to negate expression of greater toxicity in children.
Because of the rapid increase in human immunodeficiency virus (HIV) positive children and the significant morbidity and mortality of the resultant disease, drugs for HIV treatment are being tested in both pediatric and adult populations. One of the most widely tested anti-HIV drugs is azidothymidine (AZT, Restrovir). McKinney et al. (1991) studied the effects of AZT in 88 children (mean age, 3.9 years; range, 4 months to 11 years). Maha (1992) reported that the efficacy and incidence of side effects (e.g., hematological abnormalities, primarily neutropenia) were similar in both adults and children but noted that the mean duration of therapy was
much longer in the cohort of children, suggesting that they tolerated AZT somewhat better than did adults.
NEUROTOXICITY
Postnatal Effects of Neurotoxicants
Studies in animals suggest that the nature of an injury is determined by the stage of brain development at the time of exposure rather than by the relationship of the insult to the time of the birth event. Measures of brain development (e.g., gross brain weight and measures of biochemical change, physiologic function, and microanatomic structure) indicate that the processes and timing of brain development relative to birth differ among species (Himwich, 1973). These considerations are important in evaluating and comparing neurodevelopmental toxicology data from laboratory animals and human epidemiologic studies, especially when exposures occurred during the prenatal and weanling stages, reflecting different stages of brain development in different species. In humans, significant brain development and structural alteration occur until at least 4 to 6 years of age. It is plausible, therefore, that effects could result from exposures occurring several years after birth.
Studies evaluating microanatomic development of the brain indicate that the numerous brain structures have differing peak periods of growth. Therefore, toxic exposures at a particular time would differentially affect the structures undergoing peak development. Studies in animals indicate that exposures at different stages of brain development have differing effects on brain and behavioral function (Rodier, 1980). These critical periods or windows of vulnerability must be seriously considered when evaluating neurotoxic effects.
Because human brain development continues for years after birth, it can be hypothesized that postnatal exposure to xenobiotic compounds would alter the structure or function of the human nervous system. If this hypothesis is correct, there should be evidence of children suffering measurable effects from neurotoxic exposures at levels that do not affect adults. An alternative hypothesis suggests that children are less vulnerable because of the increased plasticity of the developing brain. In this case, children could be less vulnerable to insult. Unfortunately, the epidemiologic literature on childhood effects of neurotoxins is extremely difficult to evaluate because of the complex nature brain function and because of the multiple factors that affect brain development and confound evaluation.
The data on prenatal and early childhood exposure to lead indicate that effects occur at levels well below those that are toxic to adults (Bellinger
et al. 1987). Irradiation studies also suggest vulnerability of the developing brain. Studies on fetal alcohol syndrome (FAS) and on neonatal drug addiction are based on less accurate dose data than are the lead studies, but the occurrence of permanent changes in brain capacity from fetal exposure is strongly suggestive of special vulnerability of the fetus. Damage from a given level of oxygen deprivation (anoxia) is generally more severe for the developing brain than for the mature brain (Menkes, 1981). In certain cases, vulnerability of the infant to neurotoxins may be related not only to the stage of neurologic development but also to the immaturity or failure of various other protective barriers. For example, the vulnerability of the neonatal brain to bilirubin exposure resulting in kernicterus may be related to the immaturity of the so-called blood-brain barrier. Bilirubin concentrations in the 40s (mg/dl) appear to cause no adverse effects in adults, but are not tolerated in children.
The data strongly suggest that exposure to neurotoxic compounds at levels believed to be safe for adults could result in permanent loss of brain function if it occurred during the prenatal and early childhood period of brain development. This information is of particular relevance to dietary exposure to pesticides, since policies that established safe levels of exposures to neurotoxic pesticides for adults could not be assumed to adequately protect a child less than 4 years of age. Knowledge of the degree of variations in neurotoxic dose levels between children and adults is necessary for establishing risk of exposure to the developing brain. Unfortunately, only minimal data are available on the effects of exposure at levels likely to occur in the food supply. The expansion of the knowledge base, particularly the refinement of animal models, is an important first step.
Measuring Neurotoxic Effects in Humans
Techniques for measuring neurotoxic effects attempt to match the various types of neurologic functions (Bondy, 1986; Triebig et al., 1987; Weiss, 1988). Acute severe clinical effects such as seizure, coma, or death are clear, measurable and points, whereas more subtle effects that occur at low exposures must be measured with more sensitive techniques.
Effects involving the peripheral nervous system can be assessed with the use of nerve conduction tests. Stimulus-response times are measured in animals to evaluate more complete reflex arcs. Specific sensory function may be quantified by using vibratory sensitivity measures (Singer et al., 1982; Wu et al., 1985). Specific sensory pathways may be measured by using evoked brain responses for auditory or visual signals (Otto, 1986; Weiss, 1988). Neurotoxic effects can be measured with electroencephalogram (EEG) technology (Dyer and Boyes, 1983; Dyer, 1985) and with
biochemical measurements of neurotransmitter and neuroendocrine levels (Healy et al., 1984; Rosecrans et al., 1982; Finkelstein et al., 1988).
Cognitive and behavioral processes can be measured by testing a multiplicity of pathways and functions with methods that evaluate altered behavior in animals or psychological testing in humans. Unfortunately, testing is complicated by the fact that cognitive and behavioral outcomes can be influenced by many factors other than exposure to neurotoxins. Rigorous experimental or statistical designs are necessary to control for such confounding variables (see, for example, Weiss, 1983; Tilson and Mitchell, 1984; Weiss, 1988; Annau, 1990) Behavioral and developmental assessments have been conducted in children and in adults to identify age-related vulnerabilities to neurotoxins (Pearson and Dietrich, 1985). Animal models for behavioral and developmental studies are being evaluated (Buelke-Sam and Mactutus, 1990; Stanton and Spear, 1990; Tyl and Sette, 1990).
Vorhees (1986) attempted to define the areas of behavioral dysfunction that could be affected by prenatal brain damage, stating that behavioral teratogenesis could be expressed as impairment of several categories of neurobehavioral functions (e.g., sensory, cognitive, motor), delayed behavioral maturation of these functions, or other indices of compromised behavioral competence. He further noted that ''the behavioral effects of some teratogens, even if concomitant with physical defects, may be the most significant devastating and noncorrectable of all the effects observed within the syndrome (associated with the teratogen)" (Vorhees, 1986, p.43).
The Lead Model
The most extensive body of data describing the effects of a neurotoxin on the postnatal developing brain pertains to childhood exposure to lead. Progress in this area is reviewed below to illustrate the issues involved in evaluating the developmental effects of neurotoxins.
In the 1970s, researchers found that lead had measurable effects on the behavioral and cognitive function of children (Perino and Erinhart, 1974; Needleman et al., 1979; Graef, 1980) at blood-lead levels (20 to 40 µg/dl) considerably lower than the threshold previously considered to cause clinical lead disease or biochemical effects in adults.
During the 1980s, neurobehavioral and neurotoxic effects of lead exposure were found in children and in the human fetus at progressively lower levels of exposure (Moore et al., 1982; Needleman, 1983; Winneke et al., 1985; Bellinger et al., 1986; Mayer-Popken et al., 1986; Dietrich et al., 1987; Ruff and Bijur, 1989). Exposure levels resulting in blood-lead levels than 20 µg/dl were implicated. Measures of neurophysiologic
and neurochemical disturbances (Otto and Reiter, 1984; Alfano and Petit, 1985; Otto et al., 1985; Moore et al., 1986) have supported the findings of toxic effects from exposure to low lead levels in humans (<30 µg/dl) and in animals. By testing for subtle neurologic, cognitive, and behavioral effects in children, these investigators elucidated neurodevelopmental toxic effects of lead.
The research that produced these findings was characterized by a variety of methodological approaches:
-
Multifaceted approaches included a range of methods for biochemical and neurophysiologic measurements in animals and (where ethically possible) humans.
-
Studies to evaluate subtle neurologic and developmental effects of lead included innovative methods that combined extensive batteries of different psychologic tests and rigorous statistical design focusing on various constructs to control confounding variables.
-
Investigators looking for lead toxicity did not assume safe levels of exposure or protective effects. When biochemical alteration in function was found at what was considered to be subclinical levels of exposure, researchers looked for methods that would measure subtle functional changes.
Pesticides as Neurotoxicants
Many classes of compounds are used as pesticides. Some of them are known neurotoxicants. Important subclasses of the substances in use are known to have neurotoxic effects. Organophosphates and carbamates are used for demonstration purposes in this section of the extensive data—not because they present greater potential risk than other compounds.
Data suggest that in addition to short-term effects, there are other neurologic effects of a long-term nature in adult humans. For example, symptoms of organophosphate-induced delayed neurotoxicity have been found several weeks after acute exposure and have continued for many months (Whorton and Obrinsky, 1983; Vasilesque et al., 1984; Cherniak, 1988). An intermediate syndrome starts several days after acute exposure and involves paralytic symptoms for many days (Senanayake and Karalliedde, 1987). Abnormal nerve conduction velocities have also been observed in some settings involving low-level, long-term exposure (Misra et al., 1988). Neurobehavioral and psychiatric effects have been reported in some epidemiologic studies of adult populations (Maizlish et al., 1987) and in studies of adult animals (Overstreet, 1984).
The evidence on chronic effects, particularly neurobehavioral effects of
organophosphate and carbamate exposure, is less well established, but is strongly suggestive. Similar to the data on lead, there is strong evidence that acute, high-level exposure results in severe systemic disease caused by biochemical mechanisms that affect the nervous system directly. In addition, the data suggest that more long-term effects may result from exposure and that low-level exposure may have subtle, but measurable, effects on neurologic function.
The emerging data suggest that neurotoxic and behavioral effects may result from low-level chronic exposure to some organophosphate and carbamate pesticides. Sophisticated methods will be required to pursue this line of research. For many other pesticides, the data are far less complete. However, when animal studies have shown that a pesticide functions by disrupting neurologic cellular function and when systemic toxic effects are known to occur after high-level acute exposures, the possibility of low-level chronic neurotoxic and behavioral effects must be considered.
Effects of Pesticides in Children
In reviewing the data on the effects of pesticides, two questions must be addressed: Is there evidence that pesticides cause neurotoxic effects in children after acute exposure to high doses? Is there reason to suspect low-level, long-term developmental effects different from effects in adults?
Acute exposure of children to pesticides and resultant disease similar to neurotoxic effects in adults has been described for a range of pesticides, including organophosphate, carbamates, and organochlorines (Hayes 1970; Mortenson, 1986). Pediatric cases involving neurotoxic effects due to acute exposure continue to be reported for other pesticides (e.g., Roland et al., 1985, who reported on exposure to insect repellents and encephalopathy). Data on children as segments of larger exposed populations have also been reported (e.g., CDC, 1986).
Very few pesticides have been well studied for effects on neurologic development in humans and animals. Studies on polychlorinated biphenyls (PBBs) and polychlorinated biphenyls (PCBs) strongly suggest developmental effects from low-level exposures similar to the effects found for lead.
Data on exposure of humans were generated following a 1973–1974 exposure to PBBs in Michigan. Neuropsychological and developmental data were collected on children who were exposed in utero and during infancy. Physiological testing showed significant differences that were related to measures of body dose (Weil et al., 1981; Seagull, 1983).
In Taiwan, children exposed in utero to PCBs in contaminated cooking
oil experienced deficits in developmental testing and abnormalities in behavioral assessment (Rogan et al., 1988). This study did not include good body burden measures, but sample sizes were large, permitting elucidation of more subtle effects.
Data on most compounds are not as extensive as those on PBBs, PCBs, and lead. Nevertheless, the pattern shown in the data on those compounds generate concern about the vulnerability of the developing human brain to any neurotoxic pesticides.
Levels of Pesticides Affecting Children
Although the vulnerability of the developing brain to neurotoxic exposure is of serious concern, it is entirely unclear from the data available whether exposures at levels consistent with usual dietary exposures would pose a substantial risk to the long-term neurologic development of children in general or to particular subgroups of children that are neurologically vulnerable.
It is theoretically possible that certain children with preexisting neurologic conditions such as hyperactivity might be more vulnerable to certain low-level neurotoxic exposures. There has been a scientific controversy surrounding the effects of "food additives" (i.e., dyes, flavors, and sugar) on children diagnosed as hyperactive. Responses vary with the study methodology, but even studies that do show effects do not show that all children in the hyperactive subpopulation are affected. These studies do not quantify effects of trace pesticide exposures, but they do raise the question, What would the dose curve for neurodevelopmental toxicants look like, and would all children be similarly vulnerable?
Comparability of Neurotoxicity Effects in Laboratory Animals
An evaluation of the accuracy with which adverse effects are detected across species (Stanton and Spear, 1990) was included in the proceedings of a workshop on "Similarities and Differences Between Children and Adults: Implications for Risk Assessment," sponsored by the International Life Sciences Institute (Kimmel et al., 1990). Species were subdivided into rodents, nonhuman primates, and humans and compared across several categories of neurobehavioral function (sensory, motivational/arousal, cognitive, motor, social). Such an analysis is extremely complex, and required a meticulously detailed comparison of hundreds of research reports for the seven toxicants considered. Overall, the investigators concluded that despite wide species differences in neurobehavioral functional categories, there was close agreement across species for the neurotoxic agents reviewed. Agents that produced cognitive, motor, and sensory
deficits in humans generally resulted in corresponding deficits in laboratory animals. Although this relationship held up well at higher doses, comparability across species at lower doses was more difficult to assess. When the outcome measures were operationally similar, however, effects across species were observed with a high degree of reliability. This observation provides an essential basis for adequately predicting and formulating risk assessment guidelines for agents with potential developmental neurotoxicity.
IMMUNOTOXICITY
The primary function of the immune system is to provide resistance to pathogenic agents and surveillance against neoplastic cells. These functions are accomplished by both specific antibodies and cellular components of the immune system. Environmental agents may exert an influence on the immune system by altering cellular function or communication or by serving as a foreign structure and inducing a specific immune response. Altered immune function can result in impaired health by predisposing individuals to infectious disease, malignancy, or autoimmune disease. Because the immune system is not fully developed until adolescence, immunotoxic effects of environmental exposure in children and adults may differ.
Effects of Environmental Agents on the Immune System
Environmental agents may affect the immune system in a variety of ways. The potential outcomes can be summarized as follows:
-
immunosuppression, or depressed function of the immune system;
-
altered host resistance against infections or neoplastic agents;
-
hypersensitivity, or autoimmune reactivity; and
-
uncontrolled proliferation of immune components, such as lymphoma or leukemia (see section, "Carcinogenesis and Mutagenesis," below).
Animal Studies
Most of the studies investigating the effects of pesticides on the immune system have been conducted in animals and have focused on immunosuppression or impaired host resistance following subchronic exposure. For example, host resistance was evaluated in adult Swiss-Webster and B6C3F1 mice following exposure to aldicarb (0.1 to 1,000 ppb) in drinking water (Thomas and Ratajczak, 1988; Thomas et al., 1990). After daily consumption
for 34 days, no effect was noted on host resistance to infectious viral challenge, the functional ability of interferon-induced splenic NK cells to lyse YAC-1 lymphoma target cells, or cytotoxic T-cell function. In addition, there was no change in production of splenic antibody resulting from immunization with sheep erythrocytes, no effect on spleen lymphocyte blastogenesis to B- and T-cell mitogens, and no effect on the mixed lymphocyte culture response, blood counts, differential leukocyte counts, body weight, or relative lymphoid organ weights. The studies concluded that no exposure-related immunologic effects resulted from environmentally relevant concentrations of aldicarb.
The immunotoxic effect of sublethal exposure to dieldrin and aminocarb has also been examined (Fournier et al., 1988). Mice were exposed to the pesticides by gavage or intraperitoneal injection of sublethal (<LD50) doses in corn oil or dimethyl sulfoxide on two occasions, then subsequently infected with mouse hepatitis virus (MHV3). Resistance to the viral infection indicated the status of cell-mediated immunity. Dieldrin increased the cumulative mortality of animals, whereas aminocarb did not. In addition, splenic lymphocytes from the dieldrin-treated mice were found to be functionally suppressed, as evidenced by their reduced ability to respond in a mixed lymphocyte culture. Aminocarb-exposed lymphocytes were not affected. These data indicate that cell-mediated immunity may be affected by pesticide exposure.
The immunotoxicity of captan was evaluated in rats and mice following oral administration (LaFarge-Frayssinet and Declöitre, 1982). Animals were fed a diet with or without 0.3% (wt/wt) captan [cis-N-(trichloromethylthio)-4-cyclohexene-1,2-dicarboximide] for 7, 14, 21, and 42 days. After 14 days of treatment, antibody formation was found to be depressed by about 70% in both species. The effect waned by day 42. Other effects noted on day 14 were reduced splenic T- and B-cell proliferation to mitogens. These responses also improved by day 42.
The effects of lindane, malathion, and dichlorophos on the immunocompetence of rabbits were assessed (Dési et al., 1978). Doses of 1/2.5 to 1/40 of the LD50 were given orally, in capsules, five times per week for 5 to 6 weeks. Animals were intravenously immunized weekly with Salmonella typhi, and antibody titers were assessed. Each of the pesticides caused a decreased antibody titer. Depression of red blood cell cholinesterase activity correlated with the immune suppression to show dose response.
Oral ingestion of lindane- and carbaryl-containing food increased antibody production in response to the antigenic stimulus, sheep red blood cells, in mice. However, decreased resistance to infection was noted following feeding of lindane. Duration of giardiasis was increased in mice, although nonreaginic antibody levels to the parasite were elevated (André et al., 1983).
Studies in mice with the organophosphorus pesticide O,O,S-trimethyl phosphorothioate (an impurity in malathion) demonstrated the ability of this chemical to block both generation of cytotoxic T lymphocytes and antibody responses at doses that did not affect body weight or splenic lymphocyte number (Rodgers et al., 1986). The macrophage appeared to be the affected splenic cell type. The suppression was reversible. Recovery time was dependent on the dose administered. A dose of 1 mg/kg was immunotoxic.
By contrast, another malathion impurity, O,S,S-trimethyl phosphorodithioate, was immunostimulatory (Rodgers et al., 1987). At nontoxic doses, mice demonstrated elevated cytotoxic T lymphocyte responses and heightened humoral immune responses.
The immunotoxic effects of the herbicide 2,3,7,8-tetrachlorodibenzop-dioxin (TCDD) have been studied extensively. In laboratory animals, the immune system appears to be a sensitive target organ. Immunosuppression is characterized by depressed cell-mediated immunity, which is most evident after perinatal exposure during the period of thymic organogenesis. The mechanism of immunosuppression in mice appears to be a defect in T-cell regulation, because nude mice (which lack T-cell populations) were more resistant than their normal littermates (Kerkvliet and Brauner, 1987). Exposure of adult animals to a TCDD concentration of 2.7 µg/kg resulted in depressed humoral immunity (Exon, 1984). In animals, the response is dependent on Ah locus, suggesting a genetic basis for susceptibility.
In the rat, the developing immune system has been shown to be more susceptible than the immune system of the adult to the immunotoxic effects of TCDD (Vos and Moore, 1974; Faith and Moore, 1977). Fetal and neonatal rats were exposed to TCDD through maternal dosing (5 µg/kg). The doses were administered by gavage on day 18 of gestation and on days 0, 7, and 14 of postnatal life. At this concentration, TCDD suppressed the developing immune system but not the immune system of the adult (Faith and Moore, 1977). In mice treated only at 1 month of age (not during the fetal or neonatal periods), there was reduced spleen cell response to phytohemagglutinin (PHA), which was not observed in mice treated at 4 months (Kerkvliet and Brauner, 1987). However, this effect was noted only at a toxic level of TCDD.
Few studies have examined the development of hypersensitivity following exposure to pesticides in laboratory animals. Localized dermal sensitivity has been reported for some pesticides such as naled, malathion, captan, Difulatan, DDT, and Omite (Ercegovich, 1973).
Studies in Humans
No studies have been conducted to examine the immunotoxic effects of pesticides on infants or children. Immunologic effects of chronic exposure to aldicarb in adults were investigated as a result of groundwater contamination by this carbamate pesticide in Wisconsin from 1981 to 1985 (Thomas et al., 1990). Levels of >1 to <61 ppb had been measured (enforcement standard for groundwater is 10 ppb). The average aldicarb level in the groundwater was 16.1 ppb. Adult women from 18 to 70 years of age were examined for immune status in 1985. The 23 women who consumed the contaminated groundwater were compared for health status, immune function, and fluid intake with 27 who consumed water with no known contamination. Aldicarb levels in the groundwater samples averaged 16.1 ppb. Results suggested an association between consumption of aldicarb and T-cell subset abnormalities, elevated response to Candida stimulation, increased number of T8 cells, and increased percentage of T8 to T4 cells. The T-cell analyses were repeated on three more occasions and gave reproducible results. Dose-response data indicated a statistically significant association between aldicarb levels (using well-water values from individual households) and T4:T8 abnormalities as well as Candida stimulation results. However, although the stimulation results differed between groups, values for both groups were within normal limits. In addition, there was no self-reported clinical evidence of adverse health effects in the study groups (Thomas et al., 1990).
Health effects in humans from TCDD exposure were examined. In 1971 TCDD-contaminated sludge waste was mixed waste oil and sprayed for dust control on residential, recreational, and commercial areas in eastern Missouri (Hoffman et al., 1986). Some reduction in activities in these areas was recommended in 1982. As a consequence, the longest period of exposure was 11 years. Individuals were exposed at nine residential sites. At least 1 ppb TCDD was found in all soil samples. Levels as high as 2,200 ppb were found in some samples.
The study involved 155 unexposed persons and 154 people exposed for 6 or more months. The exposed group had increased frequencies of abnormal T-cell subsets (10.4% compared with 6.8%). The T4:T8 ratio was less than 1 (8.1% compared with 6.4%). The exposed group had an increased frequency of anergy (11.8% compared with 1.1%) and relative anergy (35.3% compared with 11.8%). Anergy was correlated with the length of time the individual lived in the area. Chloracne was not observed. These results suggest an effect of TCDD exposure on the T-cell component of the immune system; however, the effect did not produce any clinical illness (Hoffman et al., 1986).
Hypersensitivity to pesticides has been examined. Few problems of dermatitis were noted after exposure to DDT and lindane, which were applied to the skin and clothing of individuals to control disease vectors (Ercegovich, 1973). Furthermore, there are no documented reports of sensitization to pesticides as a result of food or environmental exposure, nor are there reports of antibodies in sera from individuals exposed to pesticides, as would be expected if pesticides functioned as haptens and induced allergic responses.
CARCINOGENESIS AND MUTAGENESIS
Carcinogenesis is a multistage or multistep process by which a normal cell loses its ability to control its rate of proliferation and differentiation and becomes a cell from which a tumor may arise. These alterations may occur as a result of mutagenesis, which involves direct alteration of the structure of DNA, or as a result of nongenotoxic mechanisms that alter the expression of DNA or indirectly lead to mutagenesis. An increased rate of cell proliferation is an example of an indirect mechanism that can lead to carcinogenesis by increasing the likelihood that spontaneous mutation will occur or by decreasing the time available to repair DNA damage. Children may be more susceptible than adults to carcinogenesis or mutagenesis because as developing organisms, their rates of growth and thus of cell proferation are much greater. Experimental and epidemiologic observations do not always support this, however.
Carcinogenesis in the Developing Organism
Animal Studies
Comparisons of tumor incidence observe in rodents at the same age and at the same dose rate but after different exposure durations indicate that tumor incidence is not solely a function of total accumulated lifetime dose but may depend on age at first exposure as well (Gaylor, 1988). This conclusion is supported by the observations of Toth (1968) and Rice (1979), who reported that in comparison to older animals, newborn and young animals are generally more susceptible to chemically induced tumor induction at some sites (including lung and liver) but are often more resistant to tumors at other sites (such as skin and breast). For example, intraperitoneal injections of the solvent urethane in mice produced a sixfold higher rate of leukemia when treatment was begun shortly after birth than when it was begun at about 45 days of age (Berenblum et al., 1966). Sensitivity to the induction of preneoplastic cells in the pancreas by the antibiotic azaserine is maximal in postnatal rats when the level of pancreatic DNA
synthesis is high, whereas treatment is less effective in weanlings and ineffective in adults (Longnecker et al., 1977). When perinatal administration of ethylenethiourea was combined with 2 years of dietary administration to rats and mice, the incidence of thyroid tumors was slightly enhanced as compared to that obtained in the absence of perinatal exposure (NTP, 1992). By contrast, a number of studies do not support the conclusion that younger animals are more susceptible carcinogenesis or mutagenesis than older animals. For example, Greenman (1987) failed to demonstrate an effect of age on 2-acetylaminofluorene-induced bladder cancer in mice and found that younger animals were more resistant to histopathologic changes in both the bladder and the liver. Singh et al. (1986) treated both young and old mice with ethylnitrosourea and observed that genetic alterations in bone marrow cells occurred with a greater frequency among older animals. Methylcholanthrene did not produce skin tumors when applied to new born mice but did produce tumors in 42% of the mice treated as adults (Toth, 1968).
Anisimov (1983) surveyed the literature to determine the effects of aging on tumor latency, incidence, and size at different sites in different species for a variety of chemicals. Although these are not pesticides, the studies provide further evidence of end organ changes with age that may be applicable in the study of pesticide toxicity (Table 3-2). It is apparent from the table that results are contradictory and generelizations are impossible. Skin painting experiments with the dermal carcinogen dimethylbenz-[a]anthracene, for example, showed that younger mice are both more susceptible (Lee and Peto, 1970) and less susceptible (Stenbäck et al., 1981) than older mice to skin tumors. Increasing the age at which the carcinogen diethylnitrosamine was administered to rodents both increased (mice; Clapp et al., 1977) and decreased (rats; Reuber, 1976) the number of esophageal and forestomach tumors observed. The experiments that have been performed in animals to evaluate the effects of aging on susceptibility to chemical carcinogenesis clearly demonstrate that age may be an important factor but do not support the conclusion that younger animals are always more susceptible than older animals.
Cancer risk can thus be a function of age at first exposure, although increasing the age at first exposure does not necessarily decrease susceptibility. One explanation for this inconsistency is that as the number of cells in a target tissue increases with age, the total number of cell divisions may also increase, even if the mitotic rate decreases. There are likely to be a multitude of factors in addition to age and rates of cell proliferation that modulate carcinogenesis.
Increased susceptibility to carcinogenesis at younger ages, when it occurs, may be attributable to two factors: increased rates of cell proliferation and differing metabolic capabilities. The many roles that cell proliferation
TABLE 3-2 Effect of Aging on Latency, Incidence, and Size of Tumors at Different Sites
|
Site |
Animal Species |
Carcinogenic Agent |
Age Group (months) |
Effect of Aging |
Reference |
|
Skin |
Mouse |
MC, BP, TC |
2–4 and 12–13 |
No effect |
Peto et al. (1975); Cowdry and Suntzeff (1944) |
|
MC, DMBA |
1.5–4 and 12–13 |
Decrease |
Lee and Peto (1970); Cowdry and Suntzeff (1944) |
||
|
DMBA |
2 and 11 |
Increase |
Stenbäck et al. (1981) |
||
|
DMBA |
14–20 and 22–24 |
Increase |
Ebbesen (1977) |
||
|
UV-light |
2–3 and 10 |
Decrease |
Blum et al. (1942) |
||
|
Fast neutrons |
1–3 and 21 |
Decrease |
Castanera et al. (1971) |
||
|
Electrons |
1 and 13 |
Decrease |
Burns et al. (1981) |
||
|
Soft tissues |
Mouse |
BP, DBA |
1–3 and 6 |
Increase |
Dunning et al. (1936) |
|
MC |
6 and 20 |
Increase |
Franks and Carbonell (1974) |
||
|
MC |
3–4 and 12 |
Decrease |
Saxen (1954); Stutman (1979) |
||
|
DMBA |
2–6 and 13 |
Increase |
Stenbäck et al. (1981) |
||
|
Plastic films |
1 and 15.5 |
Increase |
Paulini et al. (1975) |
||
|
Moloney sarcoma virus |
3 and 30 |
Increase |
Pazmino and Yuhas (1973) |
||
|
Rat |
BP,MNU |
3–4 and 9–14 |
Increase |
Maiski et al. (1978); Anisimov (1982);Ovsyannikov and Anisimov (1983) |
|
|
Bone |
Rat |
Radionuclides |
2–3 and 8–10 |
No effect |
Sundaram (1963); Streltsova and Moskalev (1964) |
|
Mammary gland |
Rat |
DMBA, MC |
Maximal sensitivity at 50 to 75 days |
|
Huggins et al. (1961); Russo and Russo (1978) |
|
DMBA, MNU |
3–4 and 14–16 |
Decrease |
Syn-mao (1962); Anisimov (1981) |
||
|
FBAA |
1–6 and 12 |
Decrease |
Stromberg and Reuber (1975) |
||
|
Estrogens |
1 and 20 |
Increase |
Geschickter (1939) |
||
|
75Se-selenomethionine |
3 and 24–26 |
Increase |
Dedov (1982) |
||
|
Liver |
Mouse |
DMH |
2–3 and 12–13 |
No effect |
Turusov et al. (1979) |
|
Rat |
CCI4 |
1–6 and 12 |
Increase |
Reuber and Glover (1967) |
|
|
|
FBAA, DENA, AFB1 |
1–6 and 12 |
Decrease |
Reuber and Lee (1968); Stromberg and Reuber (1975); Kroes et al. (1975) |
|
|
|
DMNA |
1.5 and 18 |
Decrease |
Savchenkov et al. (1980) |
|
|
Frog |
DMNA, DMN |
2 and 12–18 |
Increase |
Khudoley (1981) |
|
Esophagus and forestomach |
Mouse |
DENA |
2.5 and 17 |
Increase |
Clapp et al. (1977) |
|
Rat |
DENA |
1-6 and 12 |
Decrease |
Reuber (1976) |
|
|
Stomach |
Rat |
MNNG |
1.5–4.5 and 9 |
Decrease |
Kimura et al. (1979) |
|
Colon |
Mouse |
DMH |
3 and 12 |
Increase |
Turusov et al. (1979, 1981); Zimmerman et al. (1982) |
|
Rat |
|
8–10 and 18 |
Decrease |
Pozharisski et al. (1980) |
|
|
DMH |
|
2 and 7 |
Moon and Fricks (1977) |
||
|
Pancreas |
Mouse |
MNU |
3, 12, and 24 |
Increase |
Zimmerman et al. (1982) |
|
Kidney |
Rat |
FBAA, MNU, DMNA |
1–6 and 12–18 |
Decrease |
Reuber (1975); Savchenkov et al. (1980); Anisimov (1981) |
|
Bladder |
Mouse |
DMBA (in vitro) |
1.5–2 and 28–30 |
Increase |
Summerhayes and Franks (1979) |
|
Lung |
Mouse |
DENA |
2.5 and 12 |
Increase |
Clapp et al. (1977) |
|
MNU |
3 and 24 |
Increase |
Zimmerman et al. (1982) |
||
|
DBA, urethane |
2.4 and 11–12 |
Decrease |
Dourson and Baxter (1981) |
||
|
Rat |
Fast neutrons |
3 and 21 |
Increase |
Castanera et al.(1971) |
|
|
Pleura |
Rat |
Asbestos |
2 and 10 |
Increase |
Berry and Wagner (1976) |
|
Uterus |
Mouse |
DMH |
2 and 12 |
Increase |
Turusov et al. (1979, 1981) |
|
Rat |
MNU |
3 and 14 |
Increase |
Anisimov (1981) |
|
|
Vagina |
Mouse |
DMBA |
3 and 18 |
Increase |
Anisimov (1982) |
|
Ovary |
Mouse |
X-rays |
2 and 12 |
Decrease |
Cosgrove et al. (1965) |
|
Testis |
Rat |
Fast neutrons |
3 and 21 |
Increase |
Castenera et al. (1971) |
may play in carcinogenesis are described above; overall, increased rates of cell proliferation can contribute to an increased likelihood of carcinogenesis. For example, polycyclic aromatic hydrocarbons and aflatoxin B1, produce liver tumors when administered to newborn rodents but not when administered to older animals, presumably because the liver proliferates rapidly in the developing organism but slowly in older animals. Differing metabolic capabilities may contribute to greater susceptibility if the developing organism has less competent detoxifying or conjugating abilities than the adult. Conversely, less competent activating enzymes may protect the developing animal from chemicals that require metabolic activation to their reactive forms to elicit effects. Ethylnitrosourea, which does not require metabolic activation, is very effective as a carcinogen in neonatal rodents as compared to adults, whereas diethylnitrosamine, which requires activation, is not (Vesselinovitch et al., 1979). In addition, there may be age-related differences in DNA repair abilities and in the fidelity of DNA replication.
Human Studies
Epidemiologic studies of the effects of age on susceptibility to carcinogenesis are conflicting. The risk of bladder cancer associated with employment in a ''hazardous occupation" (e.g., an industry believed to be associated with an increased risk of bladder cancer, such as the rubber or leather industries, or work with dyestuffs, paint, and other organic chemicals) was greater in younger people (Hoover and Cole, 1973), whereas the risk of nasal cancer among nickel workers increased in proportion to age at beginning of exposure (Doll et al., 1970). Tucker et al. (1987) demonstrated that chemotherapeutic treatment of children with cancer using alkylating agents, which can form adducts with DNA and induce mutations, resulted in a significantly elevated risk of secondary leukemia. No study has been performed to determine whether similar treatment of adults has the same outcome, however, so it is not possible to conclude that children are more susceptible to chemically induced carcinogenesis on the basis of these limited data. Evidence from epidemiologic studies is thus inadequate to demonstrate a consistent increased susceptibility to carcinogenesis among children, nor would one assume that children would regularly be more susceptible to toxic end points in pesticide toxicity. These data emphasize the need to evaluate each pesticide specifically for age-related toxicity. The incidence of most cancers in humans increases with age, with the exception of certain tumor types that are associated with childhood and that are suspected to result from inborn genetic alterations or prenatal genetic damage. An example of a childhood tumor is retinoblastoma, in which a mutation occurs in the retinoblast population resulting from
genetic damage either before or after conception, creating a population of altered retinal cells that is very susceptible to malignant transformation.
From 1973 through 1989, the incidence of cancer among children of all races from 0 to 14 years old increased 7.6%. The greatest increases were observed for acute lympocytic leukemia (23.7%), brain and nervous system cancers (28.6%), and cancers of the kidney and renal pelvis (26.9%). The incidence of several other childhood cancers decreased (bones and joints, -15.1%; Hodgkins disease, -1.5%; non-Hodgkins lymphomas, -0.9%). During the same period, total cancer incidence for the entire U.S. population increased approximately 16.1% (Miller et al., 1992).
METABOLISM AND PHARMACOKINETICS
Data on pharmacokinetics are basic to considerations of the relative risks of toxic injury from pesticides in both children and adults. The fundamental goal of pharmacokinetic studies is to delineate the uptake and disposition of pesticides, drugs, and other chemicals in the body. A basic tenet of toxicology is that toxic responses are a function of the concentration of the active chemical in target tissues. Thus the degree and duration of a toxic effect depend on the quantity of the reactive form of a chemical that reaches its target site and the length of time the agent remains there. These factors in turn depend on the magnitude of systemic absorption, binding, distribution, metabolism, interaction with cellular components, and elimination of the chemical from the tissue and body. The important structural and functional differences between infants and adults can have an impact on one or more of these pharmacokinetic processes, which in turn may result in different effects of chemicals on the two age groups.
This section focuses on age-related factors that influence the pharmacokinetics of pesticides, drugs, and other chemicals in humans. The study subjects are grouped as follows: premature newborns, full-term newborns, neonates (birth to 4 weeks), infants (4 weeks to 1 year), young children (1 to 5 years), older children (6 to 12 years), and adolescents (13 to 18 years). Consideration in this section is largely limited to information from studies in humans, since there are major difficulties in extrapolating from immature animals to immature humans. Nonprimate species are less mature in many respects than humans at birth. Maturation in most lower animals, however, is quite rapid; some adult-like characteristics and functions are attained in as little as 14 to 21 days in rodents. A difference of only a few days in exposure age can thus have a marked effect on the handling of a chemical and its ensuing effects in such species (Done, 1964; Neims et al., 1976). Various body structures and associated functions mature at different rates in different species. Utmost care must be exercised
in selecting an appropriate animal model for developmental pharmacokinetic and toxicology studies, in interpreting the data, and in extrapolating the data to humans. Animal studies are presented here when data on humans are inadequate or when findings in animals elucidate ontogenetic mechanisms.
For infants and children, exposure to pesticides occurs primarily through ingestion, inhalation, and through the skin. The newborn may have previously encountered chemical agents in utero, but an in-depth examination of in-utero exposure is beyond the prescribed scope of this report. The major emphasis in this section is the absorption and disposition of ingested chemicals. Because children put all kinds of things into their mouths, they are at risk of ingesting pesticides from nonfood sources, including contaminated household objects, ornamental plants, sod, and paint. In certain situations, significant exposure may result from inhalation of pesticides or skin contact with contaminated surfaces (see Chapter 7). Dermal and inhalation exposures are also addressed because they may contribute to the total systemic dose and need to be considered when establishing prudent levels of dietary intake for infants and children.
Dermal and Pulmonary Exposure
The skin area of the infant per unit of body weight is double that of the adult, whereas the permeability of the infant's skin, except for those born prematurely, appears to be similar to that of the adult. These are important factors to remember when considering dermal absorption or penetration of xenobiotic compounds. The stratum corneum (the outer layer of the skin, which serves as the barrier to penetration by chemicals) is fully developed in the human newborn. Studies of the bacteria-inhibiting agent hexachlorophene in premature and full-term infants, the hormone testosterone in infant and adult monkeys (Wester et al., 1977), and alcohols in premature and full-term infants and human adults have shown no differences in penetration, but differences in absorption have been shown for fatty acids (Wester and Maibach, 1982).
There is little evidence to suggest that percutaneous absorption of chemicals varies greatly with age during the preadolescent period, since the overall thickness of the stratum corneum remains relatively constant throughout postnatal development (Rasmussen, 1979). There is a paucity of information, however, from well-controlled studies on percutaneous absorption of chemicals in this age group. McCormack et al. (1982) observed no difference in the rate of penetration of a series of alcohols through premature, full-term newborn, and adult skin specimens in vitro. They did find differences in penetration of a series of fatty acids, which the investigators attributed to differences in solubilization of the fatty
acids in epidermal lipids. Wester et al. (1977) reported that the percutaneous absorption of testosterone was similar in the newborn and adult rhesus monkey.
Some studies have been conducted to assess the age-dependency of dermal absorption of pesticides in rodents. Solomon et al. (1977) found no significant differences between newborn and adult guinea pigs in blood or brain concentrations of the insecticide γ-benzene hexachloride following its topical application. Knaak et al. (1984), however, reported that the fungicide triadimefon was more rapidly absorbed through the skin of young rats than through the skin of adult rats. Shah et al. (1987) contrasted the percutaneous absorption of 14 pesticides in young (33-day-old) versus adult (82-day-old) female rats. The pesticides studied were structurally diverse, in that they included organophosphates, carbamates, organometallics, chlorinated hydrocarbons, biological insecticides, and a triazine compound. No clear age-related pattern of absorption was found. At least four of the compounds (atrazine, carbaryl, chlordecone, and chlorpyrifos) were absorbed more extensively by the younger animals. Six compounds, however, were better absorbed by the adults, and the others seemed to be equally well absorbed by both age groups. No one class of compounds was better absorbed in one particular age group, other than the organometallics, which were more extensively absorbed by the adult rats. Skin penetration was not well correlated with the octanol-water partition coefficients of this diverse group of chemicals (Shah et al., 1987).
The premature human newborn may be a special case. Studies have shown that formation of the stratum corneum is incomplete until just before birth at full term (Singer et al., 1971). Greaves et al. (1975) reported that premature newborns bathed in an antibacterial hexachlorophene solution had considerably higher blood levels of hexachlorophene than did full-term newborns monitored by Curley et al. (1971). Tyrala et al. (1977) similarly observed an inverse relationship between body weight and post-conceptional age versus blood hexachlorophene concentrations in a group of 54 premature and full-term newborns. Subjects with large areas of abraded skin exhibited particularly high blood levels. Thus diminished effectiveness of the epidermal barrier to absorption was apparently a major determinant of the elevated blood levels of hexachlorophene. Another important factor was the reduced capacity of premature and full-term newborns to metabolize and eliminate hexachlorophene. Dermal hexachlorophene exposures have resulted in a number of cases of brain damage in newborns (Powell et al., 1973; Shuman et al., 1974), suggesting that this very early age group could be at increased risk of toxicity from direct skin contact with pesticides.
Several factors may contribute to increased percutaneous absorption
and toxicity of pesticides in neonates and infants. Dermal absorption of a variety of chemicals is markedly increased under diapers and rubber pants. These materials retard the evaporation of volatile chemicals and enhance the hydration and temperature of the skin, thereby increasing penetration by water-soluble chemicals. Damage to the stratum corneum, as in diaper rash, circumvents this barrier layer. In addition, some skin surfaces such as the male scrotum and the face are more absorbent than skin in other areas of the body.
The ratio of surface area to body weight in newborns and infants is approximately 2.5-fold greater than that of adults. Thus, if the exposed area of skin and percutaneous absorption rate in a neonate and adult were equivalent, the neonate would receive almost three times the systemic dose, on a kilogram-of-body-weight basis (Wester and Maibach, 1982). Nevertheless, there are few data to indicate which types of chemicals may be more extensively absorbed through the skin of neonates and infants.
The alveolar epithelium is another potential portal of entry into the body for pesticides. Most xenobiotics are absorbed through the alveolar epithelium into the pulmonary (blood) circulation by simple passive diffusion, rather than specific active transport processes (Schanker, 1978). Therefore, changes in several parameters with age may be of consequence in pulmonary absorption of pesticides, including alveolar surface area, thickness of alveolar membranes, porosity and other properties of the membranes, pulmonary blood flow, and respiratory volume. The normal respiratory volume of the resting infant is approximately twice that of the resting adult, when expressed per unit of body weight. The structural development of the human lung is known to continue postnatally (Hislop and Reid, 1981; Langston, 1983). There is a marked increase in alveolar surface area for the first 18 to 24 months of life. Thereafter, pulmonary structures continue to increase in size, and alveolar surface area increases gradually throughout childhood (Thurlbeck, 1982). There is a progressive increase up to 18 years of age in collagenous elastic fiber bundles in the alveolar walls. Some of these factors may counteract others in terms of their influence on the pulmonary absorption of chemicals. For example, the effect of increased respiratory volume may be offset by the infant's smaller surface area for absorption. Unfortunately, little information is available on the pulmonary absorption and bioavailability of xenobiotic compounds in infants and children.
One group of investigators has studied the pulmonary absorption of nonvolatile drugs in neonatal and adult animals. Hemberger and Schanker (1978) injected measured doses of a series of drugs into the tracheas of neonatal (3 to 27 days of age) and adult rats. Systemic absorption was determined by assay of the quantity of drug remaining in the lungs
and trachea after 2 hours. Lipid-soluble compounds (procainamide and sulfisoxazole) were absorbed at similar rates by neonates and adults, indicating that the properties of the alveolar epithelium do not change substantially with age. Lipid-insoluble compounds (p-aminohippuric acid, mannitol, and tetraethylammonium bromide), however, were absorbed about twice as readily by the 3- to 12-day-old rats as by rats 18 days old or older. The lipid-soluble drugs were absorbed much more rapidly than the lipid-insoluble drugs, but the amount of all drugs absorbed per unit of time was directly proportional to the administered concentration, leading Hemberger and Schanker (1978) to conclude that there was no evidence for an absorption mechanism other than simple diffusion in either the neonate or the adult rats. Lipid-insoluble substances are believed to be absorbed in the lung primarily by diffusion through aqueous channels, or pores (Schanker, 1978). Because the alveolar membrane is reported to become thinner in neonatal rats as they age (Burri et al., 1974), Hemberger and Schanker (1978) concluded that greater porosity must account for the greater absorption of lipid-insoluble drugs in neonatal rats.
Oral Exposure
The gastrointestinal tract is the major portal of entry of pesticides into the body. Absorption depends on the physical and chemical properties of the pesticide, as well as on conditions within the gastrointestinal tract itself. Some of the more important conditions, or factors, include gastric emptying and intestinal motility, gut flora, acid and enzyme secretory activity, mucosal structure and surface area, cellular transport systems, and gastrointestinal blood supply. All these change with postnatal development. A change in one may tend to enhance chemical absorption, whereas a change in another may have the opposite effect. The complexity of the system, with its multiple, interrelating factors, makes it difficult to predict the net effect of maturation on absorption of pesticides and other chemicals.
Absorption
The majority of information on gastrointestinal absorption of chemicals in infants and children has come from nutrition and drug studies. The musculature of the gastrointestinal tract is relatively thin and quiescent before birth but then must rapidly make the transition from placental to intestinal nutrition (Balistreri, 1988). Ingested nutrients entering the gut and regulatory hormones secreted in response to food are important in the adaptation to extrauterine life. For example, plasma levels of the
peptide YY increase substantially after consumption of milk during the first 2 weeks of life (Adrian et al., 1986). Peptide YY reduces the rate of gastric emptying and slows intestinal transit, thereby increasing the efficiency of absorption of nutrients. The rate and extent of absorption of orally administered drugs are often quite variable in newborns, largely because of irregular, unpredictable gastric emptying and peristalsis (Warner, 1986). Some drugs (such as digoxin and diazepam) appear to be absorbed as efficiently by full-term newborns and neonates as by adults. Premature and full-term newborns, however, exhibit slow, incomplete oral absorption of other drugs, including phenobarbital, chloramphenicol, rifampin, valproate, and phenytoin (Morselli et al., 1980; Morselli, 1989). Although poor bioavailability of phenytoin is generally seen during the first month of life, infants absorb the drug well. Similarly, infants absorb valproate at a rate comparable to that of adults.
Absorption of Proteins and Related Large Molecules. The microvillus surface of the adult small intestine serves as a barrier to penetration by many substances, particularly large, charged molecules such as proteins. There is evidence from human and animal studies, however, that the immature intestine can allow passage of intact macromolecules. Antibodies, including immunoglobulin, bovine serum albumin, α-lactalbumin, and other antigens, are reported to be absorbed intact by newborns (Grand et al., 1976). Acquisition of both allergies and passive immunity has been attributed to gastrointestinal absorption of intact proteins by neonates (see section on "Immunotoxicity," above). The period of macromolecular uptake varies in laboratory animals, from as short as 1 to 2 days after birth in guinea pigs to as long as 23 to 24 days in rabbits (Hoffmann, 1982). Selective absorption of intact γ-globulins from colostrum occurs in ruminant species for only a few days, but absorption is retained by suckling rats for up to 20 days. Absorption of α-globulins and other proteins such as cholera toxin has been shown to involve high-affinity binding to specific receptors on the microvillus surface of intestinal mucosal cells in rodents. The binding is followed by invagination of the membrane to form protein-filled vesicles, which migrate through the cytoplasm to the basolateral surface of the cell, where the vesicle contents are extruded (Walker and Isselbacher, 1974). Because this energy-dependent, endocytotic process involves receptor binding of specific proteins, it is unlikely that pesticides would be absorbed in significant quantities in human neonates by this mechanism. And, indeed, most data indicate that uptake of proteins in humans is limited and nonselective, rather than a specific, receptor-dependent transport process as is found in the rat (Walker and Isselbacher, 1974). Because macromolecular uptake in human newborns appears to be nonselective, there could be a transitory period during
which compounds that are normally excluded by the gastrointestinal tract of children and adults are absorbed.
Age-Related Changes in Absorption. Although there is a paucity of data on absorption of drugs as related to age in human neonates and infants, some definitive investigations have been conducted using laboratory animals. Hoffmann (1982) summarized the results of a number of animal studies. The rate of penetration of four model compounds (antipyrine, sodium salicylate, tetraethylammonium bromide, and phenosulfonphthaline) from everted intestinal sacs of rats was evaluated in an in vitro experiment. Penetration of all compounds was two to three times greater for 10- than for 30-day-oldrats, which in turn exhibited somewhat greater penetration rates than did adult animals. In an in vivo study, disappearance of the compounds from the duodenum of anesthetized rats was examined. Antipyrine, sodium salicylate, and tetraethylammonium bromide were each absorbed more rapidly by 10-day-old rats than by adult rats. These differences in the rate of absorption were not reflected by differences in blood levels, apparently because of a greater volume of distribution in the neonatal rats. Large synthetic molecules such as polyvinylpyrrolidinone have also been found to be well absorbed in neonatal and suckling mammals, as have heavy metals (Hoffmann, 1982). Closure, or decrease to the low-level uptake characteristic of adult animals, generally has been found to occur at the time of weaning.
Closure has been associated with structural and functional maturation of intestinal epithelial cells. It has been suggested that a marked reduction in pinocytotic activity is largely responsible for this phenomenon. Pinocytosis is believed to be the primary mechanism for nonselective absorption of macromolecules by the neonatal intestinal epithelium in mammals (Lecce, 1972). Bierring et al. (1964) observed large numbers of pinocytotic vacuoles associated with phagosomes at the base of microvilli in the intestinal epithelial cells of human fetuses. Udall and Walker (1982) saw such vacuoles associated with an extensive apical tubular network system, as well as pseudopodlike cytoplasmic extensions projecting through the lamina propria in the intestine of 1-week-old rabbits. The intestinal epithelium of the adult rabbit showed a marked decrease in the tubular network and pseudopods, which accompanied cessation of systemic uptake of bovine serum albumin. Pang et al. (1983) found that the membrane of newborn rabbits had a significantly higher lipid-to-protein ratio than did that of adult animals. Electron spin resonance spectra revealed that the membranes from the newborn rabbits were more disorganized and fluid, which could account for the more efficient penetration and diffusion of macromolecules during the perinatal period.
Absorption and Retention of Lead and Other Heavy Metals Although
lead and other heavy metals are not commonly used now as pesticides by themselves, they have been used in the past. Lead, in particular, has been widely used and studied. Lead is still of major concern as a health hazard for a number of reasons, including its prevalence in the environment and the increased sensitivity of children to its adverse effects. Barltrop (1965) reported one of the earliest investigations indicating that blood lead concentrations were age related. In a study group of 470 London children, lead levels in blood increased to a maximum in 3 year olds and then progressively decreased during the next 4 to 5 years of life. Subsequent studies demonstrated that young children had higher lead blood levels than adults living with the children (Barltrop et al., 1974; McNeil et al., 1975).
Research findings for both laboratory animals and humans indicate that increased absorption and retention of lead are important factors in the relatively high incidence of lead toxicity in the young. Suckling rats were shown to absorb a greater percentage of ingested lead than did older rats (Kostial et al., 1971). The increased absorption of lead and other divalent cations, including iron, calcium, cadmium, mercury, and manganese, typically lasted until the animals were weaned (Kostial et al., 1978). Ziegler et al. (1978) conducted metabolic balance studies in 14- to 746-day-old human subjects consuming cow's milk, infant formula, fruit juice, and strained fruits or vegetables that contained known small amounts of lead. Absorption and retention of lead increased with increasing dietary lead intake. Net absorption and retention averaged approximately 42% and 32%, respectively, when intake exceeded 5 µg/kg bw/day in 61 balance studies conducted by Ziegler et al. (1978). Data of other investigators indicate that human adults absorb only about 10% of ingested lead (Hursh and Suomela, 1968; Rabinowitz et al., 1976). Thus, greater gastrointestinal absorption, in concert with the increased retention and target organ deposition, appears to contribute significantly to the higher risk of lead poisoning in infants and young children.
Increased lead absorption in the very young is commonly attributed to pronounced pinocytotic activity in the gastrointestinal epithelium, but there appear to be other determinants as well. Because absorption decreases substantially when animals are weaned, Kostial et al. (1978) studied the effect of cow's milk on lead uptake. Inclusion of milk in the diet resulted in a substantially greater absorption not only of lead but also of cadmium, mercury, and manganese in 6- or 18-week-old rats. Still, absorption of the metals by the milk-fed animals was not as great as in 1-week-old suckling rats. The mechanism of milk's effect is unknown, although it is possible that the metals may bind to some milk constituent, and this binding would facilitate their penetration of the gastrointestinal mucosal barrier. Barltrop (1982) reported that raising dietary fat content from 5% to 10% enhanced lead absorption by 80% in rats and proposed
that certain protein deficiencies could result in enhanced absorption because protein diets with a high sulfhydryl content impaired gastrointestinal mucosal binding and uptake of lead. Trace element deficiencies may also play a role. Six and Goyer (1972) demonstrated that iron deficiency resulted in increased lead deposition and toxicity in young rats, possibly because of increased lead absorption. Iron deficiency has been commonly associated with lead poisoning in children. Low dietary intake of calcium has also been shown to lead to greater lead uptake in immature animals and humans (Ziegler et al., 1978).
Other Factors Affecting Oral Absorption
There are additional physiological and morphological processes undergoing continuous maturational changes after birth that can affect gastrointestinal absorption of metals and other chemicals. The microflora of the gut changes considerably during the neonatal and infancy period (Long and Swenson, 1977). The fecal flora in milk-fed infants exhibit negligible demethylating ability, in contrast to that of weaned children and adults. This difference could be important for a chemical such as methylmercury, which is absorbed much more readily than inorganic mercury. Infants would be expected to absorb more of an ingested dose of methylmercury because much of it would not be demethylated in the gut (Rowland et al., 1983). The same would be true for the methylmercury that reenters the gastrointestinal tract via the bile.
Gastric pH varies considerably, falling during the initial hours after birth but returning to neutrality for 10 to 15 days; thereafter, it declines gradually, not reaching adult levels until about 2 years of age (Morselli et al., 1980). Agunod et al. (1969) observed that chemically stimulated hydrochloric acid secretion was low in the gastric juice of neonates but increased until it approached the lower limit of the normal range for adults after 3 months.
Achlorhydria may result in diminished absorption and bioavailability of acidic compounds. The converse should be true for basic compounds. The relatively small surface area of the neonatal intestine proportionally reduces absorption of all chemicals. Although villi and microvilli are present in the intestinal epithelium of newborns, cell proliferation is apparently quite slow (Grand et al., 1976). Autoradiographic experiments in rats demonstrated clearly that the villi of the small intestine of sucklings were shorter and that epithelial cell migration proceeded at a rate of 20%, or less than that, of weaned animals (Koldovsky et al., 1966). Varga and Csaky (1976) found that the blood supply to the gastrointestinal tract of rats changed with age. Fractional blood flow to the total gastrointestinal tract decreased from 20% in 20-day-old rats to 8% to 12% in adult animals.
The net effect of the aforementioned factors on pesticide absorption in the immature individual is hard to predict because they sometimes oppose one another, change at different rates in the maturing organism, and often are ill-defined in humans.
Distribution and Uptake of Chemicals
Distribution of a chemical to sites of action in different tissues, following systemic absorption, is governed by a number of factors. These include plasma protein binding, extracellular fluid volume, adipose tissue mass, organ blood flow, tissue uptake, and tissue binding. The factors exert their influence concurrently and may compete with one another. They may change to varying degrees at different rates during postnatal development. Thus, the net effect of maturation on the quantity of a particular chemical reaching a target tissue is difficult to ascertain. There are few data on binding and distribution of pesticides in infants and children, but a variety of drugs have been relatively well studied (Kearns and Reed, 1989). These are used to illustrate how chemical distribution and uptake can vary with age in the developing individual.
Protein Binding Numerous chemicals, including a variety of pesticides, bind reversibly to plasma proteins. As long as the compounds are bound, they are not able to leave the bloodstream and reach sites of action (i.e., produce biological effects) in extravascular tissues. Although increased plasma protein binding thereby generally reduces the maximum bioactivity of chemicals, binding can prolong their effects by slowly releasing them to sites of action as well as to sites of inactivation or elimination. Much of our current knowledge of how altered plasma protein binding affects the health of infants and children has been gained from clinical studies of therapeutic agents.
Many drugs exhibit significantly lower plasma protein binding in premature and full-term newborns than in adults (Table 3-3). Investigators typically take blood samples from the umbilical cord at the time of delivery and determine the percentage of free, or unbound, drug in the sample. A diverse group of therapeutic agents, including both acidic and basic compounds, exhibit considerably reduced plasma protein binding in perinatal subjects (Morselli et al., 1980; Morselli, 1989). This group includes such common drugs as phenobarbital, digoxin, theophylline, phenytoin, lidocaine, imipramine, and diazepam. Though data for certain age groups are lacking for a number of these drugs, it appears that the age at which binding reaches adult levels is compound-specific. Rane et al. (1971), in a comprehensive study of the age-dependency of the plasma protein binding of phenytoin (a weak acid), found that adult values were approached in infants and young children 3 months to 2 years old. The
TABLE 3-3 Protein Binding of Some Drugs in Cord Plasma in Relation to Adult Plasma
|
Lower Binding |
Higher Binding |
|
Acid Drugs |
|
|
Ampicillin |
Valporic acida (indirect evidence) |
|
Benzylpenicillin |
|
|
Nafcillin |
Salicyclic acida |
|
Naproxen |
Sulfisoxazolea |
|
Salicylates |
Cloxacillina |
|
Phenytoin (same or lower binding) |
Flucloxacillina |
|
Phenylbutazone |
|
|
Phenobarbitone |
|
|
Pentobarbitone |
|
|
Cloxacillin |
|
|
Flucloxacillin |
|
|
Sulfamethoxypyrazine |
|
|
Sulfaphenazole |
|
|
Sulfadimethoxine |
|
|
Sulfamethoxydiazine |
|
|
Neutral drugs |
|
|
Digoxin |
|
|
Dexamethasone |
|
|
Basic drugs |
|
|
Diazepam |
Diazepama |
|
Imipramine |
|
|
Desmethylimipramine |
|
|
Bupivacaine |
|
|
Lidocaine |
|
|
Propranolol |
|
|
Metocurinea |
|
|
D-Tubocurarinea |
|
|
a As compared with maternal plasma. SOURCE: Adapted from Rane, 1992. |
|
percent of unbound phenytoin diminished little thereafter in succeeding age groups. Values comparable to those of adults for binding of acidic drugs are often reached during the second to third year of life, whereas γ-globulins, which are believed to be important in binding of nonacidic compounds, may not attain adult levels until ages 7 to 12 years (Morselli et al.,1980). Reduced binding of imipramine has been reported in children younger than 10 years (Windorfer et al., 1974).
Quantitative and qualitative differences in circulating plasma proteins have been well documented (Morselli, 1976), and it is known that during the perinatal period there is a decrease in plasma protein binding. There appear to be four primary reasons for this decrease. First, the concentration
of albumin, the most important binding protein for most compounds, is reduced. Second, there is a persistence of fetal albumin, which has a lower affinity for many drugs. Third, levels, of γ-globulins and lipoproteins are also low postnatally. Fourth, a transient hyperbilirubinemia is universally present during the first few days after birth (Done, 1964; Nau et al., 1984). Bilirubin competes with drugs, particularly acidic ones, for albumin-binding sites. Rane et al. (1971), for example, demonstrated a correlation between the unbound fraction of phenytoin in umbilical cord blood and the total concentration of bilirubin in plasma of neonates. Conversely, large doses of highly bound drugs can displace bilirubin from plasma proteins, resulting in jaundice. Shortly after birth, lipolysis occurs, resulting in an elevation in free fatty acids in the blood. Free fatty acids compete with and can displace some drugs from plasma protein-binding sites. Nau et al. (1984) conducted a comprehensive study in which they measured binding of diazepam and N-desmethyldiazepam, its major active metabolite, in the serum of adults, fetuses, and neonates 1 to 11 days old. The free fatty acid concentration and free fraction of diazepam and its metabolite were highest on day 1. During the next 10 days of life, there were progressive, parallel decreases in serum free fatty acids, albumin, and free fraction of drug and metabolite. Bilirubin seemed to play a less important role in diazepam binding because it was relatively low on day 1 (the day the free fraction was highest) and peaked on day 3 during the time the free fraction was diminishing. Nau et al. (1984) concluded that increased plasma free fatty acids and albumin and decreased bilirubin levels, coupled with deficient metabolism and elimination of diazepam, predisposed the newborn to excessive, potentially adverse effects of the drug.
The extent of plasma protein binding can have a major impact on the magnitude and duration of chemical action and toxicity. Diminished binding, as mentioned previously, results in higher concentrations of free drug available for diffusion from the blood to sites of action in target tissues and sites of metabolism and/or excretion. Thus, diminished binding typically results in an increased intensity of pharmacological effects and potential for toxicity but a shorter duration of action. Clearance is directly proportional to the free fraction of chemical for compounds for which elimination is dependent on diffusion across cell membranes (e.g., into metabolizing liver cells) or glomerular filtration (and urinary excretion). Decreased binding will therefore normally result in increased elimination of a chemical. The neonate, however, often exhibits compromised hepatic metabolic and renal clearance capacities, so the duration of biological effects may be longer than anticipated.
Distribution Volumes The distribution of xenobiotics and many natural
compounds in the body is known to change with age. As mentioned previously, infants have a higher percentage of water in lean body tissues than do adults. The additional water is primarily extracellular, so that the volume of extracellular fluid per unit of body weight in infants is about twice that of adults (Widdowson and Dickerson, 1964). As a result, water-soluble chemicals have a greater volume (per unit of body weight) in which to distribute. The newborn is exceptionally resistant to the skeletal muscle relaxants succinylcholine and decamethonium. This resistance can be attributed to the distribution of these small, highly ionized molecules in the relatively large extracellular fluid volume, which in effect reduces their concentration and their resulting pharmacologic action. Penicillins have a higher volume of distribution* in neonates because of lower plasma protein binding and higher extracellular water content. Although decreased plasma protein binding of a drug such as lidocaine would be expected to result in an increased amount of free drug and thus an exaggerated pharmacologic response in newborns, its greater volume of distribution reduces its concentration at the site of action. One age-related difference negates the other. Morselli et al. (1980) pointed out that newborns need twice as long as adults to eliminate lidocaine because of the large distribution volume that must be cleared of the drug. In the newborn, the decreased metabolism of lidocaine is apparently offset by increased renal clearance; a low glomerular filtration rate is offset by diminished tubular reabsorption of the drug. Therefore, despite a number of age-dependent differences that affect pharmacokinetics, in this instance the differences nullify one another such that lidocaine's total body clearance and pharmacologic potency are comparable in newborns and adults.
Most drugs have a larger volume of distribution during infancy and early childhood, although the reverse is true for some other compounds (Done et al., 1977). The decreased binding and increased extracellular fluid volume typically seen postnatally result in a greater volume of distribution for relatively polar chemicals. Many drugs, however, have volume of distribution values in excess of the extracellular fluid volume or total body water as a result of their solubility in body fat. Adipose tissue usually makes up a smaller percentage of body weight in newborns and infants than in adults. Thus diazepam, a lipophilic drug, has a somewhat smaller volume of distribution in neonates and infants than in adults (Morselli et al.,
1980). Distribution volume differences, which may be observed for the first 10 years of life, appear to disappear more slowly than most other age-dependent differences that alter pharmacokinetics.
Barriers to Distribution Barriers to tissue uptake, as well as distribution within organs, may vary with age, in conjunction with morphological and functional maturation. In most regions of the body only the vascular endothelium serves as a barrier to diffusion of chemicals from the bloodstream into surrounding tissue. Generally, diffusion is limited to the un-ionized, more lipid-soluble form of chemicals. In some organs (such as the liver), there are pores in the endothelium and gaps between adjacent endothelial cells, which facilitate passage of large, charged molecules. The vascular endothelium of certain organs (such as the brain and testes), however, is devoid of pores and pinocytotic vesicles, has tight cell junctions, and is encased in specialized pericapillary cells. The blood-brain barrier limits entry into the central nervous system to un-ionized, lipophilic compounds (Benet et al., 1990).
There is speculation that neonates and infants may be more susceptible to chemically induced neurotoxicity, in part because of the immaturity of their blood-brain barrier. Watanabe et al. (1990) point out that the central nervous system in developing individuals is potentially vulnerable to chemicals for a protracted period because the central nervous system requires longer than most other organ systems for cellular differentiation, growth, and functional organization. Therefore, any increase in accessibility to cytotoxic agents because of delayed maturation of the blood-brain barrier could have serious consequences.
One of the most commonly cited examples of this phenomenon is lead poisoning in infants and children. It is argued that children exhibit neurological disturbances at lower blood lead concentrations than adults, suggesting that lead enters the central nervous system of children in larger amounts (Barltrop, 1982). Although data on humans are lacking, laboratory studies show that heavy metals accumulate in the brain of immature animals in much greater amounts than in adults (Jugo, 1977; Kostial et al., 1978). These investigators found substantially higher levels of lead, mercury, and manganese in the brains of 1- and 2-week-old rats than in older animals given the agents by intravenous or intraperitoneal injection. The greater toxicity of morphine in immature rats was associated with higher brain-to-blood ratios (Kupferberg and Way, 1963). Pylkko and Woodbury (1961) attributed changes in the convulsant effects of strychnine and brucine in rats to maturation of the blood-brain barrier. The time of maturation of the blood-brain barrier in the rat appears to vary, ranging from about 1 week for 5,7-dihydroxytryptamine (Sachs and Jonsson, 1975) to as long as 3 weeks for cadmium (Wong and Klaassen,
1980). It is not known when this barrier becomes fully functional in humans.
Retention
Immature humans and laboratory animals typically exhibit greater systemic retention of heavy metals than do adults. Ziegler et al. (1978) found that retention of lead in human infants (14 to 746 days old) was dose dependent. Although urinary and fecal excretion of lead increased with dose, they apparently could not compensate for lead intake at higher doses. Investigations have shown greater whole body retention, elevated blood levels, and higher target organ concentrations in young animals than in older animals given heavy metals by injection (Kostial et al., 1978; Wong and Klaassen, 1980). Possible explanations for these age-related differences include diminished excretory capacity, high growth rates and high rate of protein synthesis, altered binding to proteins and other ligands in tissues, higher extracellular fluid volume, and greater permeability of tissue barriers in the immature organism. The brain and testes, two organs believed to have effective barriers to ionized molecules in the adult, exhibited substantially higher levels of lead, mercury (Kostial et al., 1978), and cadmium (Wong and Klaassen, 1980) in young rats. Conversely, the kidneys, another target organ, contained smaller amounts of these compounds in the young animals. Such differences may have significant toxicological implications. Data on tissue distribution as it relates to age are generally lacking for most other classes of chemicals.
Metabolism of Xenobiotic Compounds
The human newborn exhibits decreased capacity to metabolize a variety of drugs and other xenobiotic compounds (Warner, 1986; Reed and Besunder, 1989). The premature newborn is usually more deficient than the full-term newborn, although metabolic functions increase rapidly in both during the initial days after birth. A deficiency in MFO activity does not necessarily entail greater susceptibility to toxicity; indeed, it may have the opposite effect (Done, 1964). Inefficient metabolism would make the newborn more susceptible to the action of compounds that are converted to less active, more readily excreted metabolites. Conversely, inefficient metabolism should confer protection against the compounds that are metabolically activated (i.e., converted to reactive, cytotoxic metabolites). Human fetuses and newborns have higher MFO activity, in relation to adult values, than do nonprimates (Neims et al., 1976). Thus, human newborns should be more sensitive to chemicals requiring metabolic activation, whereas most laboratory animals should be more sensitive to chemicals that are detoxified and eliminated via the MFO system.
TABLE 3-4 Risk Assessment for Infants and Children: Pharmacokinetic Factors
|
Drug |
Newborn t1/2 (hrs) |
Adult t1/2 (hrs) |
|
Drugs with Low Hepatic Clearance |
||
|
Aminophylline |
24–36 |
3–9 |
|
Amylobarbitone |
17–60 |
12–27 |
|
Caffeine |
103 |
6 |
|
Carbamazepine |
8–28 |
21–36 |
|
Diazepam |
25–100 |
15–25 |
|
Mepivacaine |
8.7 |
3.2 |
|
Phenobarbitone |
21–100 |
52–120 |
|
Phenytoin |
21 |
11–29 |
|
Tolbutamide |
10–40 |
4.4–9 |
|
Drugs with Intermediate or High Hepatic Clearance |
||
|
Meperidine |
22 |
3–4 |
|
Nortriptyline |
56 |
18–22 |
|
Morphine |
2.7 |
0.9–4.3 |
|
Lidocaine |
2.9–3.3 |
1.0–2.2 |
|
Propoxyphene |
1.7–7.7 |
1.9–4.3 |
|
SOURCE: Adapted from Rane, 1992. |
||
It is difficult to generalize about age-dependent deficiencies in the metabolism of xenobiotic compounds because different enzymatic pathways seem to exhibit dissimilar maturational patterns (Neims, 1982). There are a number of forms of cytochrome P-450 in the human liver that appear to have distinctive substrate specificity and unique developmental patterns. Pelkonen et al. (1973) found that fetal liver was much more deficient in aryl hydroxylase activity than in other hepatic microsomal monooxygenases. Metabolism of caffeine and theophylline, which initially undergo N-demethylation, is particularly slow in the human newborn. Neonates exhibit a plasma half-life for caffeine of approximately 4 days, as compared to 4 hours for adults (Aldridge et al., 1979). These investigators observed that adult levels and patterns of caffeine metabolism were reached at 7 to 9 months of age. Glucuronidation is one of the most inefficient pathways for metabolism during early development and may take the longest to mature (Done et al., 1977). In contrast, mixed-function-catalyzed oxidation of a number of other drugs increases rapidly during the first days of life, soon approaching and exceeding adult values (Neims, 1982). Thus, the ontogeny of metabolism of the xenobiotic compounds, and its implications in toxicology, is quite compound-specific.
The time course of postnatal development of MFOs has been delineated for several drugs (see Table 3-4). Loughnan et al. (1977) measured the plasma half-life of phenytoin in 2-day- to 96-week-old subjects given the drug intravenously. Although the half-life was prolonged and variable during the first week of life for full-term newborns, the premature newborn
exhibited even longer and more inconsistent values. Adult values seemed to be reached by 7 weeks of age, but the number of study subjects more than 1 week old was too small to be definitive. Neims et al. (1976) used the data of several investigators to estimate phenytoin half-life values as a function of age. A marked increase in phenytoin metabolism (i.e., a decrease in half-life) was observed during the first few days postnatally, followed by a progressive increase to levels two- to threefold greater than those of adults in neonates and infants more than 2 weeks old. The large interindividual variability seen in newborns diminished with age. A similar, rapid increase in metabolism and a decrease in variability with age were seen when data for phenobarbital were evaluated (Neims et al., 1976). Both drugs are metabolized by aromatic hydroxylation. Clearance of many drugs, when normalized to unit body weight, is two- to fourfold greater in infants and young children than in older children and adults. As illustrated in Figure 3-1, plasma levels and the dose of theophylline required to maintain therapeutic levels vary substantially with age. Such increases in metabolic clearance, evident for many drugs from the age of 2 to 3 months to the age of 2 to 3 years, tend to decline gradually during childhood until adult values are reached (Morselli et al., 1980).
Metabolism of xenobiotic compounds in the newborn and neonate may be both qualitatively and quantitatively different from that in the adult. Although chloramphenicol is primarily metabolized by hydrolysis and by glucuronidation, a glycolic acid derivative not found in adults has been identified in newborns (Morselli et al., 1980). Premature newborns, unlike infants and adults, exhibit substantial N-methylase activity. This enzyme, acting in the presence of N-demethylase deficiency, can convert theophylline to caffeine. This process is the opposite of what occurs in adults. Aldridge et al. (1979) evaluated the pattern of caffeine metabolites as affected by age in neonates and infants. The researchers found that the proportion of individual metabolites varied until an adultlike metabolite pattern was reached at 7 to 9 months. The toxicological implications of age-dependent qualitative differences in the metabolism of xenobiotic compounds are for the most part unknown.
Enzyme Development. The ontogeny of metabolism of xenobiotic compounds in humans remains largely unexplored. The number of investigations undertaken during fetal and neonatal periods is largely dictated by the availability of tissue. Accordingly, there have been a considerable number of studies of fetal hepatic enzymic differentiation during the first and second trimesters, but relatively few studies during the third trimester and postnatally (Rane and Sjoqvist, 1972; Neims et al., 1976). The major components of the monooxygenase systems are present in fetal liver during midgestation. Smooth endoplasmic reticulum, the principal site of localization
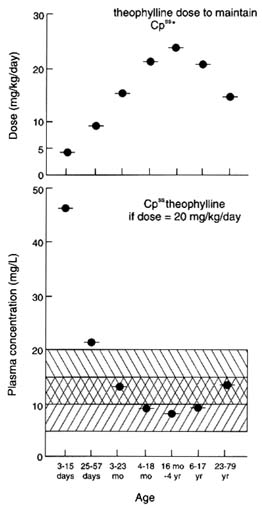
FIGURE 3-1 Theophylline dose requirements and plasma concentrations. (Top) Estimated dose requirements of theophylline (mg/kg/day) to maintain a steady-state plasma concentration Cpss of 10 mg/liter. (Lower) Estimated plasma concentrations of theophylline at steady state if dose is kept at 20 mg/kg/day. Shaded areas indicate tentative therapeutic level for bronchodilation and antiapneic activity.
SOURCE: Aranda, 1984.
of the MFO system in the hepatocyte, is also present at this time (Gillette and Stripp, 1975). Levels of cytochrome P-450 and other monooxygenase components appear to remain relatively constant through parturition. Measurements at term have shown that P-450 levels and NADPH-cytochrome c reductase activity are each about 50% of adult values (Aranda et al., 1974). Postnatal increases in enzyme activity could conceivably result from any one or a combination of the following: increased synthesis of enzyme, conversion of inactive to active enzyme, decreased catabolism of enzyme, disappearance of an endogenous inhibitor, or appearance of an activating substance. Studies of the differentiation of a variety of liver
enzymes during the late fetal and postnatal periods indicate that de novo synthesis is largely responsible for increased enzymatic activity (Greengard, 1977). This synthesis is attributed to gene expression as a result of natural (hormones and other endogenous substrates) and unnatural (drugs and other chemicals) stimuli encountered in the extrauterine environment. It is possible that relative proportions of different forms, or isozymes, of P-450 change with development of the individual. Warner (1986) pointed out that the period of most rapid MFO metabolism (from 2 or 3 months to 3 years) coincides with the period when concentrations of endogenous substrates (such as steroid hormones) are low. The gradual decline in metabolism to adult levels at puberty parallels the increase in sex steroids accompanying maturation.
Liver Development The liver undergoes a series of integrated morphological and functional changes perinatally, including, differentiation of hepatocytes and emergence of constitutive enzymes. One important function, which requires coordinated maturation of a series of processes, is enterohepatic circulation. Bile flow depends on the adequate synthesis, conjugation, secretion, and recirculation of bile acids (Balistreri et al., 1983). Suchy et al. (1981) found that serum levels of cholylglycine and chenodeoxycholate, the two major bile acids, became markedly elevated in normal neonates during the first 4 days of life. The levels then gradually declined over the next 4 to 6 months to values typical of children and adults. The initial increase, which the investigators termed physiologic cholestasis, was attributed to impared intestinal reabsorption and hepatic transport processes. They speculated that the transport deficit could be caused by functional immaturity in hepatocellular uptake, binding, conjugation, or secretion of bile acids.
Two important possible consequences of the physiologic cholestasis during the first months of life are inefficient intestinal fat digestion and inhibition of biliary excretion. Impaired fat digestion could be of toxicologic importance when lipophilic chemicals are ingested with oils. Studies in rats have demonstrated that halogenated hydrocarbons such as carbon tetrachloride are more poorly absorbed and less acutely toxic when given in oils than when given undiluted or in aqueous vehicles (Kim et al., 1990). The oils serve as a reservoir in the gut to retard systemic absorption of lipophilic chemicals. Biliary excretion is one of the two major pathways for elimination of chemicals from the body. Bilirubin, a product of hemoglobin catabolism, can accumulate in the body if its excretion in the bile is unduly hindered. A transient physiological hyperbilirubinemia is commonly seen in newborns, but it usually lasts only a few days, rather than months as does physiological cholestasis (Suchy et al., 1981). The physiologic hyperbilirubinemia is generally attributed primarily to the
newborn's diminished glucuronidation capacity, which in turn is believed to result from low hepatic microsomal uridine diphosphoglucuronyltransferase activity (Kawade and Onishi, 1981). Glucuronide conjugation is depressed to a greater extent and for a longer time during perinatal development than sulfate or glycine conjugation (Dutton, 1978). There have been a number of reports of toxicities associated with neonates' decreased ability to conjugate and eliminate chemicals in the bile and urine. Chemicals implicated in such cases include chloramphenicol (Sutherland, 1959; Weiss et al., 1960), hexachlorophene (Tyrala et al., 1977), benzyl alcohol (Gershanik et al., 1982), and diazepam (Nau et al., 1984).
Excretion
Renal excretion is the principal pathway for elimination of most chemicals from the body. Although volatile parent compounds and metabolites can be exhaled, this route of elimination is of little quantitative significance for most pesticides in current use. As described above, biliary excretion may play a role in elimination of some parent compounds and metabolites, notably conjugates formed in phase II-type reactions of liver metabolism. The conjugates may be eliminated concurrently in both bile and urine.
The kidneys are anatomically and functionally immature at birth. Although nephrogenesis is complete in the full-term human newborn, anatomic immaturity is still manifest for several weeks (Lorenz and Kleinman, 1988). The final phase of postnatal anatomical kidney development is that of increase in nephron size. Renal blood flow is relatively low in the neonate as a result of receiving a smaller percentage of total cardiac output and high intrarenal vascular resistance. Developmental changes in glomerular filtration rate parallel changes in renal blood flow (Lorenz and Kleinman, 1988). Glomerular filtration rate in premature newborns may be as low as 5.0% of that in adults, whereas in full-term newborns it is typically 30% to 40% of adult values. The glomerular filtration rate increases rapidly in the infant, becoming equivalent to that of the adult per unit of surface area within 10 to 20 weeks (West et al., 1948). Glomerular function appears to be more advanced at birth than renal tubular function (Weil, 1955). Tubular functions include both active and passive reabsorptive and secretory processes for specific agents. The deficient transport processes and reduced glomerular filtration rate produce smaller medullary solute gradients than are produced in adults, which in turn result in a diminished capacity of the neonate to concentrate urine. Maturation of tubular transport systems is relatively slow, in that maximum capacity may not be reached until about 8 months of age (West et al., 1948; Calcagno and Rubin, 1963).
Excretion of chemicals by the kidneys depends primarily on glomerular
filtration and tubular secretion and reabsorption. A decrease in one or the other in neonates can result in delayed clearance of a chemical from the bloodstream and the body. Under such circumstances, the chemical may have a prolonged duration of action and there may be an increased risk of toxicity. Aminoglycoside antibiotics, such as kanamycin and gentamicin, are examples of compounds that are not metabolically degraded or bound to plasma proteins to a significant extent. They are primarily eliminated by glomerular filtration. Total body clearance values for premature newborns are only about 5% of adult values, whereas full-term newborn values are about 10% to 30% of the values of adults. Increase in aminoglycoside clearance during the neonatal period is closely related to maturation of glomerular function and correlates well with increased creatinine clearance (McCracken et al., 1971). Penicillins, on the other hand, form a class of compounds eliminated primarily by the kidneys via tubular secretion. Newborns and neonates given penicillins typically exhibit prolonged blood half-lives. Morselli et al. (1980) note that glomerular filtration and tubular secretion mature more rapidly than does tubular reabsorption, so clearance values for some compounds may be quite high during the first 2 to 24 months of life.
SCALING AND REGRESSION ANALYSIS
Scaling is the mathematical process used to adjust the dosage of therapeutic drugs or toxic substances to achieve comparable effects between animals of different size in one species or between different species of animals of markedly different sizes. In this section, body weight, surface area, metabolic rate, and regression analysis will be used as variables to relate dosages of compounds and body size. The choice of an appropriate scaling variable is important because of the need to compare toxicities in newborn animals weighing hundreds of grams and then extrapolating these findings to human infants weighing several kilograms and to human adults weighing 50 to 100 kg.
Although there are sound theoretical bases for choosing any one of the variables that can bridge great differences in size, there is no current consensus on which is most appropriate. Part of the difficulty in using a single approach is that the rate of development of the processes involved in absorption, distribution, metabolism, and excretion of xenobiotic compounds vary with age and across species. Thus, although some agreement is necessary to establish one system of scaling so that one set of studies can be compared with another, no scaling method will resolve all the differences between animals of different ages and of different species.
Body Weight
Traditionally, the therapeutic effectiveness or the toxicity of xenobiotic compounds in animals of different sizes has been evaluated by comparing dosages based on weight and expressing these as, for example, milligrams of compound per kilogram of body Weight or micrograms of compound per 100 g of body weight. In comparing animals that differ in size as much as mice and rats or rats and human adults do, or immature and mature animals, the values per unit of weight have often been found to be unpredictable for equivalent levels of therapeutic efficacy or toxicity. For example, the therapeutic dose of the anticancer drug methotrexate in the mouse is 1.5 mg/kg; in the rat, 0.5 mg/kg; and in humans, 0.07 mg/kg (Pinkel, 1958). Within one species, the vitamin K analog synkavite at a dosage of 0.16 mg/kg produces a bilirubin level of more than 30 mg/dl in the newborn rat, but only 3 mg/dl in the adult rat. In other words, a dose of 0.64 mg/kg in the adult rat is required to increase bilirubin levels by the same amount that is produced by a dose of slightly less than 0.04 mg/kg in the newborn rat (Wynn, 1963). In pesticide studies, the ratio of the LD50 of adult to newborn rats was 2.4 for parathion (3.6 mg/kg for adults and 1.5 mg/kg for 23-day-old rats), whereas for octamethyl pyrophosphate the LD50 ratio was reversed (0.2; the adult LD50 was 10 mg/kg and for the 23-day-old rat LD50 was 49 mg/kg) (Brodeur and DuBois, 1963).
Other Effects of Body Size
Another way to scale dosages of xenobiotic compounds between large and small animals is on the basis of some function of body size other than weight. The growth rate varies from organ to organ or component to component in relation to increases in body size. Examination of this phenomenon led to the use of the allometric expression in the form
y = aBx,
in which y is the weight of an organ or component of the body, B is total body weight, a is a constant, and x is an exponent of body weight. Transforming this equation yields.
log y = x log B + log a,
and then a plot of log y versus log B yields a simple linear graph with x as the slope and log a as the intercept. This type of representation indicates that the growth of a particular organ bears some constant relationship to the growth of the animal, but that relationship may vary from structure to
structure. In physiology, it became apparent that a variety of physiological functions also increased allometrically with body size. These relationships were extensively reviewed by Boxenbaum (1982) and Boxenbaum and D'Souza (1990).
When these allometric concepts were applied to metabolic rate, the equation would be
metabolic rate = a·wtx,
where a is a constant that depends on the units used for metabolic rate and weight (wt), and x would be a fraction that was less than 1.0. Brody (1945) recognized that the expression for metabolic rate was similar to the expression for the surface area of the body. He further made the assumption that since the rate of cooling of a solid body was proportioned to its surface area, by analogy the metabolic rate should be directly proportional to surface area. Thus if surface are = a·wt2/3, then metabolic rate = a·wt2/3. (For an extensive discussion of these relationships, see Calabrese [1986].)
When the potency or toxicity of a substance is related to the persistence of some concentration of the compound in the body, the effect will be related to the rate at which the original substance is metabolized to less potent or to more potent compounds. In many cases, the rate at which a xenobiotic compound is metabolized will be related to the overall metabolic rate of the animal. Because smaller animals, in general, have greater metabolic rates per unit of body mass than do larger animals, the rate of metabolism of many substances per unit of body weight will be more rapid in smaller animals than in larger ones.
Except for the energy required for growth (a small fraction of energy consumption), energy intake and expenditure are essentially equivalent. Total energy expenditure involves the energy required for resting metabolism, for diet-induced thermogenesis, and for physical activity. The total expenditure of energy is the overall metabolic rate, and this can be closely approximated by the total energy intake when body weight is relatively stable. The advantage of this is that energy intake is generally more easily measured than is metabolic rate.
Surface Area
A variety of methods related to metabolic rate have been developed to equalize the values for energy expenditure (and consumption) between individuals of different sizes. The two principal approaches have been to use a power function of weight, such as weight2/3 or weight3/4, or to use calculated surface area. Although surface area is reasonably approximated by weight2/3, it is more precisely a power function of weight with an
additional factor based on a power function of height or length. The surface area methodology has been the more widely used of these two approaches, although weight2/3 and surface area have often been used interchangeably despite the fact that they are not actually equivalent (Brody, 1945; Lindstedt, 1987). Values for usual energy intakes by human individuals of different sizes and the relationship between kilocalories per kilogram of body weight and kilocalories per square meter of body surface area are shown in Table 3-5. Because of the comparability of energy requirements throughout infancy and childhood when calculated on a surface area basis (˜2,000 kcal/m2), it has became common to assume that the metabolism of therapeutic drugs would also be comparable on this basis. Therefore, many medication dosages for children are calculated in terms of milligrams per square meter.
Although these assumptions are reasonably valid for many drugs in individuals of a comparable age group, they are not necessarily valid across all age groups or for all drugs (Lamanna and Hart, 1968). Almost all the comparisons between animals of different sizes (within and across species) in which surface area has been used to compare physiological functions, metabolism of drugs, or other body parameters have used comparisons between mature animals. Because of differences in the rate of maturation of individual organs and their functions, within and between species, as the animal proceeds from birth to maturity, it is not likely that the simple surface area relationship will be true for the comparison of immature and mature animals. Infants, children, and adults, just as immature and mature animals of other species, differ from each other in stages of functional development of individual organ systems, the processes of growth, and maturation of enzyme systems involved in the metabolism of xenobiotic compounds, as well as in their metabolic rates expressed on a weight or surface-area basis (see Table 3-5). Compounds such as chloramphenicol, which is poorly detoxified in the newborn (Calabrese, 1978); acetaminophen, which is excreted primarily as a sulfate conjugate in the young and as a glucuronide in the adult (Sonawane, 1982); isoniazid, which is acetylated at a reduced rate in the newborn (Nyhan, 1961); and caffeine, which is poorly biotransformed in the infant (Neims, 1982), are examples of drugs that differ in metabolism and toxicity in relation to age, whether doses are compared in terms of weight or surface area.
Metabolic Rate
Another method for comparing children of all sizes with adults in order to establish equivalent dosages of drugs is to relate the dose directly to metabolic rate (Calabrese, 1986). Dosage is represented as the amount per unit of metabolic rate, e.g., milligrams per 1,000 kcal per 24 hours. This
TABLE 3-5 Representative Values of Weight, Length (Height), Surface Area, and Caloric Intake for Individuals at Various Ages
|
Age (years) |
Weight (kg) |
Length (Height) (cm) |
Surface Area (m2) |
Caloric Intake |
Kilocalorie/Kilograma |
Kilocalorie/Square Meterb |
|
Premature birth |
1.0 |
36 |
0.09 |
140 |
140 |
1,555 |
|
Full-term birth |
3.5 |
50 |
0.21 |
400 |
115 |
1,905 |
|
2 |
13.0 |
88 |
0.56 |
1,200 |
92 |
2,140 |
|
8 |
25.0 |
127 |
0.93 |
2,000 |
80 |
2,150 |
|
13, female |
46.0 |
157 |
1.43 |
2,200 |
48 |
1,540 |
|
13, male |
45.0 |
156 |
1.40 |
2,800 |
62 |
2,000 |
|
Adult, female |
57.0 |
163 |
1.60 |
2,300 |
40 |
1,440 |
|
Adult, male |
70.0 |
178 |
1.85 |
3,000 |
43 |
1,620 |
|
a Kilocalories per kilogram are given to indicate the three-to fourfold differences in metabolic rate when calculated on a weight basis. b Kilocalories per square meter are shown to demonstrate the relative stability of this measure throughout childhood and also to emphasize the differences between children and adults, even on this basis. SOURCE: Based on data from Hamill et al., 1979; NRC, 1989. |
||||||
TABLE 3-6 Comparison of Doses Across Age Groups, Based on Weight, Surface Area, and Metabolic Rate
|
|
Dose Basis |
||
|
Age (years) |
100 mg/kg |
3,784 mg/Square Metera |
2,333 mg/1,000 kcala |
|
Premature birth |
100 |
340 |
327 |
|
Full-term birth |
350 |
795 |
933 |
|
2 |
1,300 |
2,120 |
2,800 |
|
8 |
2,500 |
3,520 |
4,666 |
|
13, female |
4,600 |
5,410 |
5,130 |
|
13, male |
4,500 |
5,300 |
6,530 |
|
Adult, female |
5,700 |
6,050 |
5,365 |
|
Adult, male |
7,000 |
7,000 |
7,000 |
|
NOTE: To illustrate the relative differences in dosage on these three bases, the dosage for the adult male is held constant in absolute figures (7,000). By dividing that dose by the adult male's weight, surface area, and metabolic rate, one obtains the figures of 100 mg/kg (column 2), 3,784 mg/m2 (column 3), and 2,333 mg/1,000 kcal (column 4). These figures are then used to calculate the absolute dosages for each individual on each basis. These data demonstrate that the three bases for equating drug dosage between individuals of different ages and weights will result in absolute dosages that may vary several fold for the same age and size person. a These values per square meter (m2) and per 1,000 kcal were used to provide equivalent adult dosages. |
|||
method has the theoretical advantage of directly relating dosage to the variable that is commonly responsible for differences in drug metabolism between individuals of different sizes and ages. An example of dosages based on weight, surface area, and metabolic rate is given in Table 3-6, in which the adult dose is made constant. With the exception of the very-low-birth-weight infant, dosages based on kilocalories consumed (or metabolized) yield higher values for children than the other approaches for equivalent adult dosage. Even this approach can yield erroneous results, as illustrated by Schmidt-Nielsen (1972), whose calculations of the dosage of lysergic acid needed to produce the condition called musth in the male elephant were as follows:
|
Based on body weight, elephant versus cat |
= 297 mg |
|
Based on metabolic rate, elephant versus cat |
= 80 mg |
|
Based on body weight, elephant versus human |
= 8 mg |
|
Based on metabolic rate, elephant versus human |
= 3 mg |
|
Based on brain weight, elephant versus human |
= 0.4 mg |
Another problem arises when one considers whether compounds are metabolized to less toxic or to more toxic substances in the liver. This difference is of particular importance in extrapolation from one animal
species to another, especially to the human species. For example, the extent of the postnatal development of cytochrome P-450-dependent oxidations is contingent on both species and substrate as well as on the sex of the animal. In the hydroxylation of aromatic compounds, maximum activity is reached at about 30 days in male and female rats but decreases after that in the female and is constant or slowly increasing in males for another 30 days (Klinger, 1982). In humans, there appears to be a relatively consistent difference; humans have a significantly lower rate of hepatic metabolism than found in other mammalian species. Boxenbaum and D'Souza (1990) discussed this difference between human and other animal species in detail and used the term neoteny to explain these differences. Neoteny is the ''retention of formerly juvenile characteristics by adult descendants … produced by retardation of somatic development" (Gould, 1977).
The significance of this concept for pesticide toxicology is that the hepatic metabolism of many xenobiotics in the mature human subject will occur at a reduced rate when compared to the rates in other mature animals even when corrected for scaling difference. It remains uncertain at this time how the problem of neoteny applies to immature animals. This is an area that deserves investigation although the studies will be complex because of the difficulty in identifying comparable stages of maturity in young animals. Further description of the maturation of enzyme systems is provided in the monograph by Calabrese (1986) entitled Age and Susceptibility to Toxic Substances and in the detailed review by Klinger (1982).
Regression Analysis
An alternative approach to scaling that has overcome some of the problems mentioned is that of regression equations that use the allometric concept without a predetermined power value. In this approach, the log of dose for a specific outcome is plotted against the log of body weight. For many substances and many animal species a linear relationship exists, but the slopes and intercepts are specific for each compound and each species. Krasovskii (1976) explained this process in detail, and after examining the action of several hundred compounds stated, "[I]t was shown that the regularities of the comparative sensitivity of the animals to 80–85% of the substances can be characterized by a straight line equation." When this approach was used to evaluate human toxicity of 107 substances based on data from four to six animal species, the results indicated there was a transfer error that did not exceed a value of 300% to 400% between calculated and observed values.
For risk assessment purposes, the problem becomes simpler as long as
both exposure and toxicity are expressed on the same basis, e.g., micrograms per kilogram, micrograms per square meter, or micrograms per 1,000 kilocalories. To clarify this concept, assume that pesticide X is used on food A. The residue at the point of consumption is found to be 100 µg/100 g (1 µg/g) of food A. If the average adult consumption of food A is found to be 210 g with a total energy intake of 3,000 kcal/day (Table 3-5) and the average 2-year-old male infant consumes the same proportion of his total calories—1,200—as food A (Table 3-5), the following data apply:
Adult: 210 g of food A in a 3,000 kcal total intake
2 year old: (1,200 kcal ÷ 3,000 kcal) × 210 g food A = 84 g food A
Adult exposure = 210 g × 1 µg/g = 210 µg of pesticide X
2-year-old exposure = 84 µg × 1 µg/g = 84 µg of pesticide X
If these intakes are then scaled by weight, surface area, or calories for both adult and child, the value of intake of pesticide X becomes 70 µg/1,000 kcal for both (see Table 3-7).
If toxicity is evaluated on the same basis as exposure is assessed (i.e., on the basis of weight, surface area, or calories metabolized) using the same ratio of dosages, the results will be identical whatever basis is used. For example, if the no-observed-effect level (NOEL) were 6 µg/kg for the adult and 13 µg/kg for the child, then the NOEL expressed on the basis of surface area would be 228 µg/m2 in the adult and 300 µg/m2 in the child. On a metabolic rate basis, the NOEL for adult and child would both be 140 µg/1,000 kcal. Thus, on whatever basis one calculated toxicity, both the infant and adult would be consuming X at half the NOELs for their age group. Similar calculations would be needed to translate these ratios from the human species to the species to be used for toxicity testing.
Because the diets of small children are limited in diversity, it would be reasonable for the 2 year old to consume twice as much of food A as the adult as a proportion of his total energy intake. In this situation, the amount of residue ingested by the infant would be 13 µg/kg, 300 µg/m2, and 140 µg/1,000 kcal. Because of the relative increase in intake by the child compared to the adult, the child would be at the child's NOEL whatever the basis of the calculation.
If the efficacy or toxicity of a compound is not related to its rate of metabolism, using energy consumption as a basis for relating dosages probably would not provide equivalent levels of toxicity. In acute toxicity, for example, if the toxicity depended on peak concentration, comparable dosage would be on a simple weight basis because volumes of distribution based on body water compartments are more closely related directly to weight than to surface area or metabolic rate. Even under such circumstances, differences in rates of absorption, extent of protein binding,
TABLE 3-7 Expression of Exposure Values for the Adult and Child
|
|
Consumption and Physiological Parameters |
Pesticide X Exposure Values |
|||||
|
Age |
Pesticide X |
Weight |
Surface Area |
Food A |
Weight |
Surface Area |
Food A |
|
Adult |
210 µg |
70 kg |
1.85 m2 |
3,000 kcal |
3.0 µg/kg |
114 µg/m2 |
70 µg/1,000 kcal |
|
Child |
84 µg |
13 kg |
0.56 m2 |
1,200 kcal |
6.5 µg/kg |
150 µg/m2 |
70 µg/1,000 kcal |
plasma half-life, or concentration at the receptor site could modify the dosage generated on a weight basis.
In chronic exposures, the differential rate of development of metabolizing enzymes such as glucuronyltransferase and the P-450-dependent mixed-function oxidases can have an impact on toxicity that is independent of overall metabolic rate. If the parent compound were the toxic substance, delayed enzymatic degradation would enhance toxicity. However, if a metabolite were the source of toxicity, the slower metabolism would result in reduced toxicity.
If the metabolism of a xenobiotic material is fully elucidated, it would be appropriate to use a reference base or denominator (weight, surface area, or metabolic rate) that best reflects the pharmacokinetics of that compound. When the developmental pharmacokinetics of a substance are not well delineated, as is often the case for pesticides, it has been demonstrated empirically that on a weight basis, the toxicity difference between immature and mature animals, based on comparative LD50s, is usually less than a factor of 5 and has only occasionally been reported to exceed a factor of 10 for any pesticide studied to date (Calabrese, 1986).
CONCLUSIONS AND RECOMMENDATIONS
Conclusions
In this chapter, the committee summarized the mechanisms of toxicity and explored the potentially vulnerable organ systems or functional systems in the developing animal. In addition, it reviewed data on toxicants that provide the basis for these conclusions. Although the committee was interested in drawing conclusions from toxicity testing of pesticides, in many cases there were no data on developing animals. To illustrate the principles of toxicity as they pertain to developing animals, the committee therefore utilized information from testing of other toxicants, including drugs.
As described in Chapter 2, the nervous system, the immune system, and reproductive systems continue to develop after birth. This observation heightens concern that toxicity during these postnatal developmental stages or periods may have lasting consequences throughout adult life.
-
Differences in toxicity between young and mature animals may be in either direction but are generally modest. The younger animal may be more sensitive or may be less sensitive than the older animal to comparable levels of exposure of toxic agents. The direction of these differences appears to be compound specific as well as age specific because toxicity may not vary linearly with age. In those instances where such measures
-
as LD50s are significantly different, the differences are usually less than 1 order of magnitude and often substantially less.
-
Data on age-dependent pharmacokinetics of pesticides are lacking for most animals, and the data base on pharmacokinetics and metabolism of drugs in immature humans is also limited. Available information reveals that some functional immaturities offset or cancel one another, whereas others tend to be additive. For example, inefficient gastrointestinal absorption of the anti-inflammatory drug indomethacin is offset by decreased biliary and renal clearance. Maturation of most biochemical and physiological processes occurs within the first 2 years; indeed, substantial changes occur within the first days and weeks of life. Compared to adults, therefore, neonates and infants can be anticipated to have the greatest differences in pharmacokinetics and susceptibility to pesticide toxicity—the youngest being the most likely to exhibit aberrant responses. Metabolic and renal clearance of many xenobiotics reaches and exceeds adult levels (when expressed on a body weight basis) during the first year. Therefore, older infants and young children may metabolize pesticides more extensively and eliminate them more rapidly than adults. This rapid elimination may confer increased resistance or susceptibility to toxicity, depending on the nature of the compound to which the individual is exposed.
-
On the basis of our understanding of mechanisms of action of toxicants in mature animals, including the human adult, it is generally possible to predict that similar mechanisms of action will occur in immature animals, including the human infant, child, or adolescent (i.e., biochemical mechanisms of toxicity are similar across age and developmental stages). For example, if a toxicant is cytotoxic (causes cell death) in the adult, it should cause cell death in the immature animal by the same mechanism. This principle suggests that mechanisms of action should also be comparable across species.
-
Studies of the toxicity of xenobiotic compounds in developing mammals—both laboratory animals and humans—demonstrate the potential for acute and chronic toxicity. Toxicity in the perinatal and pediatric periods is of special concern, since systems and structures under development at those times are important for survival over the lifetime of the mammal.
-
Studies in laboratory animals have demonstrated an age-related difference in acute toxicity; however, the direction of the difference is dependent on the chemical, and the magnitude of the effect is usually no more than 1 order of magnitude and often is considerably less. A developing
-
animal may be more, less, or equally sensitive to a given chemical than is an adult.
-
Studies of the influence of age on the toxicity of xenobiotic compounds in laboratory animals may provide an incomplete or inaccurate picture of similar effects in humans. Rodents are less mature at birth than humans. Thus, more pronounced age-related differences would be anticipated in rodents than in humans during the neonatal period. Rodents and most other commonly used test animals mature very rapidly during this time, so that differences of even a few days in age can profoundly affect susceptibility to the toxicity of xenobiotic compounds.
-
Very few data were found on the relative toxicity of pesticides in immature and mature humans. There is, however, a limited data base on pharmaceutical agents. As in animals, age dependency of humans to therapeutic and side effects is drug and age dependent. Since premature and full-term newborns are the most different anatomically and physiologically from adults, it follows that they typically exhibit the most pronounced differences from adults in sensitivity to drugs.
-
Pharmacokinetic processes control the amount of bioactive chemicals in target organs and, in turn, the magnitude and duration of toxic effects. The net effect of immaturity on the various processes that affect chemical disposition is difficult to predict. The situation is complex in a number of respects. Most laboratory animals are less mature than humans at birth, although maturation in animals is more rapid. In addition, various body structures and associated functions mature at different rates in different species.
-
Comparison of exposures between immature and mature animals and across species is complex, and no single mathematical expression is universally applicable. The toxicity of a pesticide varies with its rate-limiting pharmacokinetic processes.
-
Data from studies in humans (e.g., in children and adults treated with cytotoxic chemotheraupetic agents) show that toxic effects are similar qualitatively but may differ quantitatively. That is, the types of toxic effects that limit treatment were similar in children and adults; however, the dose at which treatment limitations were reached was different. Indeed, for some drugs, the maximum tolerated dose is greater in developing than in human adults (i.e., such drugs are less toxic to infants and children).
-
Studies of the ontogeny of plasma esterases in humans suggest that immature individuals may be at greater risk for acute toxic effects of pesticides that are cholinesterase inhibitors. The increase in sensitivity appears to be greatest during the first months of life, apparently because of relatively low levels of esterases and a diminished capacity to detoxify such pesticides.
-
Although the principles of developmental toxicity following in utero exposure have received considerable attention, there has been little attention to the principles of developmental toxicity following exposure to pesticides in the postnatal and perinatal periods.
-
The central nervous system (CNS) continues to develop during the second and third trimester and during postnatal life. These developmental processes include many—if not all—the developmental processes that occur during prenatal development. As expected, alteration of the development of the nervous system can be blatant or silent—until the function is needed.
-
The immune system is responsible for modulating host defenses against a range of human diseases. The successful development and functioning of the immune system require recognition and response to a range of cellular and circulating signals acting by endocrine, autocrine, and paracrine mechanisms. These complex control systems offer multiple opportunities for disruption by environmental chemicals—such as agricultural pesticides.
-
Assessment of the effects of pesticides on the developing human nervous system is difficult because the methodology for such assessment is complex and poorly delineated. Development of the CNS is characterized by exacting architectural complexity and localization of function occurring over a prolonged period postnatally. The effects of altered neurologic development may be measured either as anatomic or behavioral and cognitive outcomes.
-
The scientific consensus on appropriate neurodevelopmental outcome measures for evaluation of exposures (in animal models and human epidemiologic studies) is still evolving (NRC, 1992). Measurement of these end points is complicated, not only because of the elaborate nature of the end points that are measured but also because the timing of the insult may change the outcome and the functional end points may not be manifested until long after the exposure.
-
Despite the difficulties in measuring effects, exposure to xenobiotic compounds has been found to alter CNS development at the anatomic and functional level. The alteration in development can be irreversible, thus resulting in permanent loss of function. These damaging effects of
-
xenobiotic compounds on the CNS of the developing organism can occur at exposure levels that are safe for the adult.
-
Certain classes of pesticides, including organophosphates, carbamates, and organochlorines, are known to have neurotoxic effects, especially as the result of high-dose acute exposures. Generally, data are insufficient for evaluation and determination of the neurodevelopmental effects of low-dose exposure to these broad classes of agents, and risk assessment for low-level exposures is not possible using current data. Nevertheless, the biochemical (neurotoxic) mode of action of these classes of compounds and the distinctive qualitative vulnerability of the child's developing nervous system makes the evaluation of low-dose neurodevelopmental effects a concern.
Recommendations
-
The establishment of a standard developmental assessment model or protocol would allow one to interpret toxicity studies in immature animals in a systematic manner. The committee recommends that a standard protocol for evaluation of immature animals be established and required as part of the basic assessment of pesticides for toxicity to immature animals.
-
Given the potential for lifelong effects following perinatal or pediatric toxicity, it is essential to develop toxicity testing procedures that specifically evaluate in appropriate animal models vulnerability during the developmental period and the adverse effects, if any, over the life of the animal.
-
Given that toxicity is generally age related, consideration of this phenomenon must be included in regulatory action, testing methodologies, and public health policies.
-
In general, it is possible to extrapolate data on the end points of toxic concern from adults to developing humans, although the dose that produces the toxicity is likely to be different. The developing human frequently seems to be more resistant than the adult to cytotoxicity from the anticancer and anti-AIDS drugs tested.
-
Extrapolation of toxicity data from laboratory animals to humans—especially those for developing organisms—may be inaccurate. Careful attention to species differences in disposition and metabolism as well as in stages of maturation of organ systems is essential for accurate policy development and public health protection.
-
Greater attention is needed to develop a broader understanding of the principles guiding developmental toxicity of organisms, especially
-
humans, following birth and during critical periods of postnatal development, including infancy and puberty.
-
Neurodevelopmental effects must be part of the battery of end points evaluated for toxicants, including pesticides and agricultural chemicals.
-
Analysis of the impact or toxicity of agricultural chemicals on the immune system is essential. Regulatory development of a battery of consensus tests is critical to protect the developing immune system. At present, there is a paucity of information on the effects of many chemicals on the developing and indeed on the mature immune system.
-
Although it is extremely difficult to assess neurodevelopmental effects, the CNS may be peculiarly vulnerable during a prolonged period of development, even if the exposure is at a level known to be safe for adults. Thus, a feasible, streamlined, and publicly credible method of assessment must be developed. Effectiveness of animal model and epidemiologic evaluation must be considered. Regulatory development of a battery of consensus tests will be difficult but necessary to ensure public confidence. Agencies involved should actively support research and innovation in this area of assessment.
-
Since the kinetics of a variety of chemicals can be profoundly different in immature and mature subjects, the influence of immaturity on pesticide kinetics and toxicity is complex and must therefore be assessed on a case-by-case and chemical-by-chemical basis.
-
When the pharmacokinetics of a specific compound are understood well enough to indicate that the metabolism (and elimination) of the substance is, in fact, proportional to the animal's rate of metabolism, as is often the case, then comparisons on a metabolic rate basis, on a surface area basis, or weight2/3 basis would be reasonable. If surface area is used for adjusting from animals to humans, it should also be used for adjusting from infants to adults. In the many situations when such data are not available, the use of a simple body weight relationship for toxicity testing may be used as long as potential exposure is calculated on the same basis. Since most dietary pesticide exposure data are based on body weight, this is an added reason to use body weight as the basis for examining toxicity. In any case, scaling methods should be consistent.
REFERENCES
Adrian, T.E., H.A. Smith, S.A. Calvert, A. Aynsley-Green, and S.R. Bloom. 1986. Elevated plasma peptide YY in human neonates and infants. Pediatr. Res. 20:1225–1227.
Agunod, M., N. Yamaguchi, R. Lopez, A.L. Luhby, and G.B. Glass. 1969. Correlative
study of hydrochloric acid, pepsin and intrinsic factor secretion in newborns and infants. Am. J. Dig. Dis. 14:400–414.
Aldridge, A., J.V. Aranda, and A.H. Neims. 1979. Caffeine metabolism in the newborn. Clin. Pharmacol. Ther. 25:447–453.
Alfano, D.P., and T.L. Petit. 1985. Postnatal lead exposure and the cholinergic system. Physiol. Behav. 34:449–455.
André, F., J. Gillon, C. André, S. LaFont, and G. Jourdan. 1983. Pesticide-containing diets augment anti-sheep red blood cell nonreaginic antibody responses in mice but may prolong murine infection with Giardia muris. Environ. Res. 32:145–150.
Anisimov, V.N. 1981. Carcinogenesis and ageing. I. Modifying effects of aging on N-methyl-N-nitrosourea-induced carcinogenesis in female rats. Exp. Pathol. 19:81–90.
Anisimov, V.N. 1982. Carcinogenesis and ageing. III. The role of age in initiation and promotion of carcinogenesis. Exp. Pathol. 22:131–147.
Anisimov, V.N. 1983. Role of age in host sensitivity to carcinogens. Pp. 99–112 in Modulators of Experimental Carcinogenesis: Proceedings of a Symposium Organized by IARC and the All-Union Cancer Research Center of the USSR Academy of Medical Sciences, V. Turusov and R. Montesano, eds. IARC Scientific Publ. No. 51. Lyon, France: International Agency for Research on Cancer.
Annau, Z. 1990. Behavioral toxicology and risk assessment. Neurotoxicol. Teratol. 12:547–551.
Aranda, J.V. 1984. Maturational changes in theophylline and caffeine metabolism and disposition: Clinical implications. Pp. 868–877 in Proceedings of the Second World Conference on Clinical Pharmacology and Therapeutics, L. Lemberger, and M.M. Reidenberg, eds. July 31–August 5, 1983. Washington, D.C.: American Society for Pharmacology and Experimental Therapeutics.
Aranda, J.V., S.M. MacLeod, K.W. Renton, and N.R. Eade. 1974. Hepatic microsomal drug oxidation and electron transport in newborn infants. J. Pediatr. 85:534–542
Balistreri, W.F. 1988. Anatomical and biochemical ontogeny of the gastrointestinal tract and liver. Pp. 33–57 in Nutrition During Infancy, R. C. Tsang and B. L. Nichols, eds. Philadelphia: Hanley and Belfus.
Balistreri, W.F., J.E. Heubi, and F.J. Suchy. 1983. Immaturity of the enterohepatic circulation in early life: Factors predisposing to "physiologic" maldigestion and cholestasis. J. Pediatr. Gastroenterol. Nutr. 2:346–354.
Barltrop, D. 1965. The relationship between some parameters employed in the diagnosis of lead poisoning in childhood with a special reference to the excretion of delta-aminolaevulinic acid. Thesis. University of London.
Barltrop, D. 1982. Nutritional and maturational factors modifying the absorption of inorganic lead from the gastrointestinal tract. Pp. 35–41 in Banbury Report, No. 11: Environmental Factors in Human Growth and Development, V.R. Hunt, M.K. Smith, and D. Worth, eds. New York: Cold Spring Harbor Laboratory.
Barltrop, D., C.D. Strehlow, I. Thorton, and J.S. Webb. 1974. Significance of high soil lead concentrations for childhood lead burdens. Environ. Health Perspect. 7:75–82
Bellinger, D., A. Leviton, H.L. Needleman, C. Waternaux, and M.B. Rabinowitz. 1986. Low-level lead exposure and infant development in the first year. Neurobehav. Toxicol. Teratol. 8:151–161.
Bellinger, D., A. Leviton, C. Waternaux, H. Needleman, and M. Rabinowitz. 1987. Longitudinal analyses of prenatal and postnatal lead exposure and early cognitive development. New Engl. J. Med. 316:1037–1043.
Benet, L.Z., J.R. Mitchell, and L.B. Sheiner. 1990. Pharmacokinetics: The dynamics of drug absorption, distribution, and elimination. Pp. 3–32 in The Pharmacological Basis of Therapeutics, 8th ed., A.G. Gilman, T.W. Rall, A.S. Nies, and P. Taylor, eds. New York: Pergamon Press.
Benke, G.M., and S.D. Murphy. 1975. The influence of age on the toxicity and metabolism of methyl parathion and parathion in male and female rats. Toxicol. Appl. Pharmacol. 31:254–269.
Berenblum, I., L. Boiato, and N. Trainin. 1966. On the mechanisms of urethane leukemogenesis in newborn C57BL mice. II. Influence of thymectomy and of subsequent thymus reimplantation. Cancer Res. 26:361–363.
Berry, G., and J.C. Wagner. 1976. Effect of age at inoculation of asbestos on occurrence of mesotheliomas in rats. Int. J. Cancer. 17:477–483.
Bierring, F., H. Andersen, J. Egberg, F. Bro-Rasmussen, and M. Matthiessen. 1964. On the nature of the meconium corpuscles in human fetal intestinal epithelium. I. Electron microscopic studies. Acta Path. Microbiol. Scand. 61:365–376.
Bleyer, A.W. 1977. Clinical pharmacology of intrathecal methotrexate. II. An improved dosage regimen derived from age-related pharmacokinetics. Cancer Treat. Rep. 61:1419–1425.
Blum, H.F., H.G. Grady, and J.S. Kirby-Smith. 1942. Relationships between dosage and rate of tumor induction by ultraviolet radiation. J. Natl. Cancer Inst. 3:91–97.
Bondy, S.C. 1986. The biochemical evaluation of neurotoxic damage. Fundam. Appl. Toxicol. 6:208–216.
Boxenbaum, H. 1982. Interspecies scaling, allometry, physiological time, and the ground plan of pharmacokinetics. J. Pharmacokinet. Biopharm. 10:201–227.
Boxenbaum, H., and R.W. D'Souza. 1990. Interspecies pharmacokinetic scaling, biological design and neoteny. Adv. Drug Res. 19:139–196.
Brodeur, J., and K.P. DuBois. 1963. Comparison of acute toxicity of anticholinesterase insecticides to weanling and adult male rats. Proc. Soc. Exp. Biol. Med. 114:509–511.
Brody, S. 1945. Bioenergetics and Growth. New York: Reinhold.
Buelke-Sam, J., and C. Mactutus. 1990. Testing methods in developmental neurotoxicity for use in human risk assessment. Conference on the Qualitative and Quantitative Comparability of Human and Animal Developmental Neurotoxicity. Work Group II Report. Neurotoxicol. Teratol. 12:269–274.
Burns, F.J., E.V. Sargent, and R.E. Albert. 1981. Carcinogenicity and DNA break repair as function of age in irradiated rat skin. Proc. Am. Assoc. Cancer Res. 22:91. (Abstract)
Burri, P.H., J. Dbaly, and E.R. Weibel. 1974. The postnatal growth of the rat lung. I. Morphometry. Anat. Rec. 178:711–730.
Calabrese, E.J. 1978. Pollutants and High Risk Groups. New York: Wiley and Sons. 266 pp.
Calabrese, E.J. 1986. Age and Susceptibility to Toxic Substances. New York, Chichester, Brisbane, Toronto, Singapore: Wiley-Interscience Publication, John Wiley and Sons, Inc.
Calcagno, P.L., and M.I. Rubin. 1963. Renal extraction of PAH in infants and children . J. Clin. Invest. 43:1632.
Castanera, T.J., D.C. Jones, D.J. Kimeldorf, and V.J. Rosen. 1971. The effect of age at exposure to a sublethal dose of fast neutrons on tumorigenesis in the male rat. Cancer Res. 31:1543–1549.
CDC (Centers for Disease Control). 1986. Aldicarb food poisoning from contaminated melons. MMWR 35(16):254–258.
Cherniak, M.C. 1988. Toxicological screening for organophosphorus-induced delayed neurotoxicity: Complications in toxicity testing. Neurotoxicology 9:249–272.
Clapp N.K., W.C. Klima, and L.H. Cacheiro. 1977. Temporal advancement of diethylnitrosamine carcinogenesis in aging mice. Proc. Am. Assoc. Cancer Res. 18:62. (Abstract)
Cosgrove, G.E., A.C. Upton, L.H. Smith, and J.N. Dent. 1965. Radiation glomerulo-sclerosis and other late effects: Influence of radiological factors and AET. Radiat. Res. 25:725–735.
Cowdry, E.V., and V. Suntzeff. 1944. Influence of age on epidermal carcinogenesis induced by methylcholanthrene in mice. Yale J. Biol. Med. 17:47–58.
Crom, W.R., A.M. Glynn-Barnhart, J.H. Rodman, M.E. Teresi, R.E. Kavanagh, M.L. Christensen, M.V. Relling, and W.E. Evans. 1987. Pharmacokinetics of anticancer drugs in children. Clin. Pharmacokin. 12:168–213.
Curley, A., R.E. Hawk, R.D. Kimbrough, G. Nathenson, and L. Finberg. 1971. Dermal absorption of hexachlorophene in infants. Lancet 2:296–297.
Dedov, V.I. 1982. Late sequelae of the internal irradiation of the endocrine system of female rats. Izv. Akad. Nauk SSSR. Ser. Biol. 3:454–458.
Dési, I., L. Varga, and D. Farkas. 1978. Studies on the immunosuppressive effect of organochlorine and organophosphoric pesticides in subacute experiments. J. Hyg. Epidemiol. Microbiol. Immunol. 22:115–122.
Dietrich, K., K. Krafft, R. Bornschein, P. Hammond, O. Berger, P. Succop, and M. Bier. 1987. Low-level fetal lead exposure effect on neurobehavioral development in early infancy. Pediatrics 80:721–730.
Diggory, H.J.P., P.J. Landrigan, K.P. Latimer, A.C. Ellington, R.D. Kimbrough, J.A. Liddle, R.E. Cline, and A.L. Smrek. 1977. Fatal parathion poisoning caused by contamination of flour for international commerce. Am. J. Epidemiol. 106:145–153.
Dodson, W.E. 1976. Neonatal drug intoxication: Local anesthetics. Pediatr. Clin. N. Am. 23:399–411.
Doll, R., L.G. Morgan, and F.L. Speiger. 1970. Cancer of the lung and nasal sinuses in nickel workers. Br. J. Cancer 24:623–632.
Done, A.K. 1964. Developmental pharmacology. Clin. Pharmacol. Therap. 5:432–479.
Done, A.K., S.N. Cohen, and L. Strebel. 1977. Pediatric clinical pharmacology and the ''therapeutic orphan." Annu. Rev. Pharmacol. Toxicol. 17:561–573.
Dourson, M.L., and C.S. Baxter. 1981. Reduced prevalence and growth rate of urethane-induced lung adenomas in ageing adult strain A mice. Toxicology 20:165–172.
Dunning, W.F., M.R. Curtis, and F.D. Bullock. 1936. The respective roles of heredity and somatic mutation in the origin of malignancy. Am. J. Cancer 28:681–712.
Dutton, G.J. 1978. Developmental aspects of drug conjugation, with special reference to glucuronidation. Annu. Rev. Pharmacol. Toxicol. 18:17–35.
Dyer, R.S. 1985. The use of sensory evoked potentials in toxicology. Fundam. Appl. Toxicol. 5:24–40.
Dyer, R.S., and W.K. Boyes. 1983. Use of neurophysiological challenges for the detection of toxicity. Fed. Proc. 42(15):3201–3206.
Ebbesen, P. 1977. Effect of age on non-skin tissues on susceptibility of skin grafts to 7,12-dimethylbenz(a)anthracene(DMBA) carcinogenesis in BALB/c mice, and effect of age of skin graft on susceptibility of surrounding recipient skin to DMBA. J. Natl. Cancer Inst. 58:1057–1060.
Ecobichon, D.J., and D.S. Stephens. 1973. Perinatal development of human blood esterases. Clin. Pharmacol. Therap. 14:41–47.
EPA (U.S. Environmental Protection Agency). 1988. Aldicarb. Special Review Technical Support Document. Office of Pesticides and Toxic Substances. Washington, D.C.: U.S. Environmental Protection Agency.
Ercegovich, C.D. 1973. Relationship of pesticides to immune responses. Fed. Proc. 32:2010–2016.
Evans, W.E., W.P. Petros, M.V. Relling, W.R. Crom, T. Madden, J.H. Rodman, and M. Sunderland. 1989. Clinical pharmacology of cancer chemotherapy in children. Clin. Pharmacol. 36:1199–1230.
Exon, J.H. 1984. The immunotoxicity of selected environmental chemicals, pesticides and heavy metals. Pp. 355–368 in Chemical Regulation of Immunity in Veterinary Medicine. New York: Liss.
Faith, R.E., and J.A. Moore. 1977. Impairment of thymus-dependent immune functions by exposure of the developing immune system to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). J. Toxicol. Environ. Health 3:451–464.
FDA (Food and Drug Administration). 1992. Specific requirements on content and format for labeling for human prescription drugs: Proposed revision of pediatric use subsection in the labeling. Fed. Regist. 57:47423–47427.
Finkelstein, Y., M. Wolf, and A. Biegon. 1988. Brain acetylcholinesterase after acute parathion poisoning: A comparative, quantitative, histochemical analysis - post mortem. Ann. Neurol. 24(2):552–557.
Fournier, M., G. Chevalier, D. Nadeau, B. Trottier, and K. Krzystyniak. 1988. Virus-pesticide interactions with murine cellular immunity after sublethal exposure to dieldrin and aminocarb. J. Toxicol. Environ. Health 25:103–118.
Franks, L.M., and A.W. Carbonell. 1974. Effect of age on tumor induction in C57BL mice. J. Natl. Cancer Inst. 52:565–566.
Gagne, J., and J. Brodeur. 1972. Metabolic studies on the increased susceptibility of weanling rats to parathion. Can. J. Physiol. Pharmacol. 50:902–915.
Gaines, T.B., and R.E. Linder. 1986. Acute toxicity of pesticides in adult and weanling rats. Fundam. Appl. Toxicol. 7:299–308.
Gaylor, D.W. 1988. Risk assessment: Short-term exposure at various ages. In Phenotypic Variation in Populations: Relevance to Risk Assessment, A.D. Woodhead, M.A. Bender, and R.C. Leonard, eds. New York: Plenum.
Gershanik, J., B. Boecler, H. Ensley, S. McCloskey, and W. George. 1982. The gasping syndrome and benzyl alcohol poisoning. New Engl. J. Med. 307:1384–1388.
Geschickter, C.F. 1939. Mammary carcinoma in the rat with metastasis induced by estrogen. Science 89:35–37.
Gillette, J.R., and B. Stripp. 1975. Pre- and postnatal enzyme capacity for drug metabolite production. Fed. Proc. 34:172–178.
Glaubiger, D.L., D.D. von Hoff, J.S. Holcenberg, B. Kamen, C. Pratt, and R.S. Ungerleider. 1982. The relative tolerance of children and adults to anticancer drugs. Front. Radiat. Therap. Oncol. 16:42–49.
Goldenthal, E.L. 1971. A compilation of LD50 values in newborn and adult animals. Toxicol. Appl. Pharmacol. 18:185–207.
Goldman, L.R., M. Beller, and R.J. Jackson. 1990. Aldicarb food poisonings in California, 1985–1988: Toxicity estimates for humans. Arch. Environ. Health 45:141–147.
Gould, S.J. 1977. Ontogeny and Phylogeny. Cambridge, Mass.: Belknap Press of Harvard Press. 520 pp.
Graef, J.W. 1980. Management of low level lead exposure. In Low Level Lead Exposure: The Chemical Duplication of Current Research, M.L. Needleman, ed. New York: Raven Press.
Grand, R.J., J.B. Watkins, and F.M Torti. 1976. Development of the human gastrointestinal tract: A review. Gastroenterology 70:790–810.
Greaves, S.J., D.G. Ferry, E.G. McQueen, D.S. Malcom, and P.M. Buckfield. 1975. Serial hexachlorophene blood levels in the premature infant. New Zealand Med. J. 81:334–336.
Greengard, O. 1977. Enzymic differentiation of human liver: Comparison with the rat model. Pediatr. Res. 11:669–676.
Greenman, D.L., A. Boothe, and R. Kodell. 1987. Age-dependent responses to 2-acetylaminofluorene in BALB/c female mice. J. Toxicol. Environ. Health 22:113–129.
Groth, D.H., W.B. Coate, B.M. Ulland and R.W. Hornung. 1981. Effects of aging on the induction of angiosarcoma. Environ. Health Perspect. 41:53–57.
Hamill, P.V.V., T.A. Drizd, C.L. Johnson, R.B. Reed, A.F Roche, and W.M. Moore. 1979. Physical growth: National Center for Health Statistics Percentiles. Am. J. Clin. Nutr. 32:607–629.
Hayes, W.J. 1970. Epidemiology and general management of poisoning by pesticides. Pediatr. Clin. N. Am. 17:629.
Healy, M.P., M. Aslam, P.G. Harrison, and N.P. Fernando. 1984. Lead induced convulsion in young infants. J. Clin. Hosp. Pharm. 9(3):199–207.
Hemberger, J.A., and L.S. Schanker. 1978. Pulmonary absorption of drugs in the neonatal rat. Am. J. Physiol. 234:C191–C197.
Himwich, W. 1973. Problems in interpreting clinical changes in developing and aging animals. Prog. Brain Res. 40:13–23.
Hinson, J.A., D.W. Roberts, R.W. Benson, K. Dalhoff, S. Loft, and H.E. Poulsen. 1990. Mechanism of paracetamol toxicity (Letter). Lancet 335:732.
Hislop, A., and L. Reid. 1981. Growth and development of the respiratory system. Pp. 390–432 in Pediatrics, 2nd ed., J.A Davis and J. Dobbing, eds. London: Hinemann.
Hoffman, R.E., P.A. Stehr-Green, K.B. Webb, R.G. Evans, A.P. Knutsen, W.F. Schramm, J.L. Staake, B.B. Gibson, and K.K. Steinber. 1986. Health effects of long-term exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J. Am. Med. Assoc. 255:2031–2038.
Hoffmann, H. 1982 Absorption of drugs and other xenobiotics during development in experimental animals. Pharmacol. Ther. 16:247–260.
Hoover, R., and P. Cole. 1973. Temporal aspects of occupational bladder carcinogenesis. New Engl. J. Med. 288:1041–1043.
Huggins, C., L.C. Grand, and F.P. Brillantes. 1961. Mammary cancer induced by a single feeding of polynuclear hydrocarbons, and its suppression. Nature 189:204–207.
Hursh, J.B., and J. Suomela. 1968. Absorption of 212Pb from the gastrointestinal tract of man. Acta Radiol. Ther. Phys. Biol. 7:108–120.
Jugo, S. 1977. Metabolism of toxic heavy metals in growing organisms: A review. Environ. 13:36–46.
Kaplan, H.S. 1947. Observation on the radiation-induced lymphoid tumors of mice. Cancer Res. 7:141–147.
Kauffman, R.E. 1992. Acute acetaminophen overdose: An example of reduced toxicity related to developmental differences in drug metabolism. Pp.79–103 in Similarities and Differences Between Children and Adults: Implications in Risk Assessment, P.S. Guzelian, C.J. Henry, and S.S. Olin, eds. Washington, D.C.: ILSI Press.
Kawade, N., and S. Onishi. 1981. The prenatal and postnatal development of UDP glucuronyltransferase activity toward bilirubin and the effect of premature birth on this activity in the liver. Biochem. J. 196:257–260.
Kearns, G.L., and M.D. Reed. 1989. Clinical pharmacokinetics in infants and children: A reappraisal. Clin. Pharmacokinet. 17:29–67.
Kerkvliet, N.I., and J.A. Brauner. 1987. Mechanisms of 1,2,3,4,6,7,8-heptachlorodibenzo-p-dioxin (HpCDD)-induced humoral immune suppression: Evidence of primary defect in T-cell regulation. Toxicol. App. Pharmacol. 87:18–31.
Khudoley, V.V. 1981. The role of age in carcinogenesis induced by N-nitrosodimethylamine and N-dimenthylnitramine in frog Rana temporaria L. Vopr. Onkol. 10:67–71.
Kim, H.J., J.V. Bruckner, C.E. Dallas, and J.M. Gallo. 1990 Effect of oral dosing vehicles on the pharmacokinetics of orally administered carbon tetrachloride in rats. Toxicol. Appl. Pharmacol. 102:50–60.
Kimmel, C.A., D.C. Rees, and E.Z. Francis, eds. 1990 Special issue on the qualitative and quantitative comparability of human and animal developmental neurotoxicity. Neurotoxicol. Teratol. 12:173–292.
Kimura, M., T. Fukuda, and K. Sato. 1979. Effect of aging on the development of gastric cancer in rats induced by N-methyl-N-nitroN-nitrosoguanidine. Gann. 70:521–525.
Klinger, W. 1992. Biotransformation of drugs and other xenobiotics during postnatal development . Pharmacol. Ther. 16:377–429.
Knaak, J.B., K. Yee, C.R. Ackreman G. Zweig, and B.W. Wilson. 1984. Percutaneous absorption of triadimefon in the adult and the young male and female rat. Toxicol. Appl. Pharmacol. 72:406–416.
Koldovsky, O., P. Sunshine, and N. Kretchmer. 1966. Cellular migration of intestinal epithelia in suckling and weaned rats. Nature 212:1389–1390.
Kostial, K., I. Simonovic, and M. Pisonic. 1971. Lead absorption from the intestine in newborn rats. Nature 233:564.
Kostial, K., D. Kello, S. Jugo, I. Rabar, and T. Maljkovic. 1978. Influence of age on metal metabolism and toxicity. Environ. Health Perspect. 25:81–86.
Krasovskii, G.N. 1976. Extrapolation of experimental data from animals to man. Environ. Health Perspect. 13:51–58.
Kroes, R., J.W. Weiss, and J.H. Weisburger. 1975. Immune suppression and chemical carcinogenesis. Recent Results Cancer Res. 52:65–76.
Kupferberg, H.J., and E.L. Way. 1963. Pharmacological basis for increased sensitivity of the newborn rat to morphine. J. Pharmacol. Exper. Ther. 141:105–112.
LaFarge-Frayssinet, C., and F. Declöitre. 1982. Modulatory effect of the pesticide captan on the immune response in rats and mice. J. Immunopharmacol. 4:43–52.
Lamanna, C., and E.R. Hart. 1968. Relationship of lethal toxic dose to body weight of the mouse. Toxicol. Appl. Pharmacol. 13:307–315.
Langston, C. 1983. Normal and abnormal structural development of the human lung. Pp. 75–91. in Abnormal Functional Development of the Heart, Lungs, and Kidneys: Approaches to Functional Teratology, R.J. Kavlock and C.T. Grabowski, eds. New York: A.R. Liss.
Lecce, J.G. 1972. Selective absorption of macromolecules into intestinal ephithelium and blood by neonatal mice. J. Nutr. 102:69–75.
Lee, P.N., and R. Peto. 1970. The effect of the age of mice on the incidence of skin cancer. Br. J. Cancer 24:849–852.
Lindop, P.J. and J. Rotblat. 1962. The age factor in the susceptibility of man and animals to radiation. I. The age factor in radiation sensitivity in mice. Br. J. Radiol. 35:23–31.
Lindstedt, S.L. 1987. Allometry: Body size constraints in animal design. Pp. 65–79 in Drinking Water and Health: Pharmacokinetics in risk Assessment, Vol. 8. Washington, D.C.: National Academy Press.
Long, S.S., and R.M. Swenson. 1979. Development of anaerobic fecal flora in healthy newborn infants. J. Pediatr. 91:283–301.
Longnecker, D.S., J. Frech, E. Hyde, H.S. Lilja, and J.D. Yager, Jr. 1977. Effect of age on nodule induction by azaserine and DNA synthesis in rat pancreas. J. Natl. Cancer Inst. 58:1769–1775.
Lorenz, J.M., and L.I. Kleinman. 1988. Ontogeny of the kidney. Pp. 58–85 in Nutrition During Infancy, R.C. Tsang and B.L. Nichols, eds. St. Louis, Mo.: C.V. Mosby.
Loughnan, P.M., A. Greenwald, W.W. Purton, J.V. Aranda, G. Watters, and A.H. Neims. 1977. Pharamacokinetic observations of phenytoin disposition in the newborn and young infant. Arch. Dis. Child. 52:302–309.
Lu, F.C., D.C. Jessup, and A. Lavallée. 1965. Toxicity of pesticides in young versus adult rats. Food Cosmet. Toxicol. 3:591–596.
MacPhail, R.C., S. Padilla, and L.W. Reiter. 1987. Age-related effects of pesticides. Second International Symposium on the Performance of Protective Clothing. Tampa, Fla. (Abstract).
Maha, M. 1992. Retrovir® (AZT): A comparison of clinical trial results in the adults and children. Pp. 107–112 in Similarities and Differences Between Children and Adults: Implications in Risk Assessment, P.S. Guzelian, C.J. Henry, and S.S Olin, eds. Washington, D.C.: ILSI Press.
Maiski, I.N., B. Botev, G.P. Airapetian, T.A. Pokrovskaya, T. Vatseva, L. Markholev, I. Vatev, and I. Mladenov. 1978. Development of induced tumors in rats of different ages. Onkologiia Sofia. 15:83–87.
Maizlish, N.,M. Schnenker, J. Seiber, S. Samuels. 1987. A. behavioral evaluation of pest control workers in short-term low-level exposure to the organophosphate diazinon. Am. J. Indust. Med. 12(2):153–172.
Marsoni, S., R.S. Ungerleider, S.B. Hurson, R.M. Simon, and L.D. Hammershaimb.
1985 Tolerance to antineoplastic agents in children and adults. Cancer Treat. Rep. 69:1263–1269.
Mayer-Popken, O., W. Denkhaus, and H. Konietzko. 1986 Lead content of fetal tissues after maternal intoxication. Arch. Toxicol. 58(3):203–204.
McCormack, J.J., E.K. Boisits, and L.B. Fisher. 1982. An in vitro comparison of the permeability of adult versus neonatal skin. Pp. 149–164 in Neonatal Skin, Structure and Function, H.I. Maibach and E.K. Boisits, eds. New York: Dekker.
McCracken, G.H., N.R. West, and L.J. Horton. 1971. Urinary excretion of gentamicin in the neonatal period. J. Infect. Dis. 123:257–262.
McKinney, R.E., Jr., M.A. Maha, E.M. Connor, J. Feinberg, G.B. Scott, M. Wulfsohn, K. McIntosh, W. Borkowski, J.F. Modlin, P. Weintrub, K. O'Donnell, R.D. Gelber, G.K. Rogers, S.N. Lehrhman, and C.M. Wilfert 1991. A multicenter trial of oral zidovudine in children with advanced human immunodeficiency virus disease. New Engl. J. Med. 324:1018–1025.
McLeod, H.L., M.V. Relling, W.R. Crom, K. Silverstein, S. Groom, J.H. Rhodman, G.K. Rivera, W.M. Crist, and W.E. Evans. 1992. Distribution of antineoplastic agents in the very young child Br. J. Cancer 66(Supp.):S23–S29.
McNiel, J.L., J.A. Ptasnik, and D.B. Croft. 1975. Evaluation of long-term effects of elevated blood lead concentrations in asymptomatic children. Arh. Hig. Toksikol.(Yugoslavia) 26(Supp.):97–118.
Menczer, J., H. Komarov, M. Shenboum, V. Insler, and B. Czernobilsky. 1979. Attempted induction of granulosa cell tumor in BALB-c mice by gonadotropin administration . Gynecol. Invest. 8:314–322.
Menkes, J.H., ed. 1981. Metabolic disease of the nervous system. In Textbook of Child Neurology. Philadelphia: Lea & Febiger.
Miller, B.A., L.A.G. Reis, B.F. Hankey, C.L. Kosary, and B.K. Edwards, eds. 1992. Cancer Statistics Review: 1973–1989. NIH Pub. No. 92-2789. Bethesda, MD.: National Cancer Institute.
Miller, R.P., R.J. Roberts, and L.J. Fischer. 1977. Acetaminophen elimination kinetics in neonates, children and adults. Clin. Pharmacol. Therap. 19:284–294.
Mistra, U.K., D. Nag, W.A. Khan, and P.K. Ray. 1988. A study of nerve conduction velocity, late responses and neuromuscular synapse functions in organophosphate workers in India. Arch. Toxicol. 61:496–500.
Moon, R.C., and C.M. Fricks. 1977. Influence of gonadal hormones and on 1,2-dimethylhydrazine-induced colon carcinogenesis. Cancer 40:2502–2508.
Moore, M.R., A. Goldburg, I.W. Bushnell, R. Day, and W.M. Fyro. 1982. Prospective study of the neorological effects of leads in children. Neurobehav. Toxicol. Teratol. 4(6):739–743.
Moore, M.R., M.J. McIntosh, and I.W. Bushnell. 1986. The neurotoxicology of lead. Neurotoxicology 7(2):591–556.
Morselli, P.L. 1976. Clinical pharmacokinetics in neonates. Clin. Pharmacokin. 1:81–98.
Morselli, P.L. 1989. Clinical pharmacology of the perinatal period and early infancy. Clin. Pharmacokinet. 17:13–28.
Morselli, P.L., Franco-Morselli, and L. Bossi. 1980. Clinical pharmacokinetics in newborns and infants: Age-related differences and therapeutic implications. Clin. Pharmacokin. 5:485–527.
Mortenson, M.L. 1986. Management of acute childhood poisoning caused by selected insecticides and pesticides. Pediatr. Clin. N. Am. 33(2):421–445.
Murphy, S.D. 1982. Toxicity and hepatic metabolism of organophosphate insecticides in developing rats. Pp.125–134 in Report No.11: Environmental Factors in Human Growth and Development, V.R. Hunt, M.K. Smith, and D. Worth, eds. New York: Cold Spring Harbor Laboratory.
Nau, H., W. Luck, and W. Kuhnz. 1984. Decreased serum protein binding of diazepam and its major metabolite in the neonate during the first postnatal week relate to increased free fatty acid levels Br. J. Clin. Pharmacol. 17:92–98.
Needleman, H.L. 1983 Lead at low dose and the behavior of children. Neurotoxicology 4(3):121–133.
Needleman, H.L., C. Gunnoe, A. Leviton, R. Reed, H. Peresie, C. Maher, and P. Barrett. 1979. Deficits psychologic and classroom performance of children with elevated dentine lead levels. New Engl. J. Med. 300:689–695.
Neims, A.H. 1982. The effect of age on drug disposition and action. Pp. 529–537 in Banbury Report No. 11: Environmental Factors in Human Growth and Development, V.R. Hunt, M.K. Smith, and D. Worth, eds. New York: Cold Spring Harbor Laboratory.
Neims, A.H., Warner, P.M. Loughnan, and J.V. Aranda. 1976. Developmental aspect of the hepatic cytochrome P450 monooxygenase system. Annu. Rev. Pharmacol. Toxicol. 16:427–445.
NRC (National Research Council). 1989. Recommended Dietary Allowances: 10th Edition Washington, D.C.: National Academy Press. 302 pp.
NRC (National Research Council). 1992. Environmental Neurotoxicology. Washington, D.C.: National Academy Press.
NTP (National Toxicology Program). 1992. NTP Technical Report on the Perinatal Toxicity and Carcinogenicity Studies of Ethylene Thiourea in F/344 rats and B6C3F1 mice (feed studies). NTP Technical Report 388. Research Triangle Park, N.C.: National Toxicology Program.
Nyhan, W.L. 1961. Toxicity of drugs in the neonatal period. J. Pediatr. 59:1–20.
Ostheimer, G.W. 1979. Neurobehavioral effects of obstretric analgesia. Br. J. Anaesth. 51:355–405.
Otto, D.A. 1986. Electrophysical Assessment of Neutoxicity in Children. Health Effects Research Laboratory. Research Triangle Park, N.C.: U.S. Environmental Protection Agency.
Otto, D., and L. Reiter. 1984. Developmental changes in slow cortical potentials of young children with elevated lead body burden. Neurophysiological considerations. Ann. N.Y. Acad. Sci. 425:377–383.
Otto, D., G. Robinson, G.S. Bauman, S. Schroeder, P. Mushak, D. Klienman, and L. Boon. 1985. 5-year follow-up study of children with low-to-moderate lead absorption: Electrophysiological evaluation. Environ. Res. 38(1):168–186.
Overstreet, D.M. 1984. Behavioral plasticity and the cholinergic system program. Neuropsychopharmacol. Biol. Psychiatry 8(1):135–151.
Ovsyannikov, A.I. and V.N. Anisimov. 1983. Effect of age on carcinogenesis induced by single subcutaneous administration of N-nitroso-N-methylurea in the rat. Vopr. Onkol.
Pang, K.-Y., J.L. Bresson, and W.A. Walker. 1983. Development of the gastrointestinal mucosal barrier: Evidence for structural differences in the microvillus membranes from newborn and adult rabbits. Biochim. Biophys. Acta 727:201–208.
Paulini, K., G. Beneke, B. Körner, and R. Enders. 1975. The relationship between
the latent period and animal age in the development of foreign body sarcomas. Beitr. Pathol. 154:161–169.
Pazmino, N.H. and L.M. Yuhas. 1973. Senescent loss of resistance to murine sarcoma virus (Moloney) in the mouse. Cancer Res. 33:2668–2672.
Pearson, D.T., and K.N. Dietrich. 1985. The behavioral toxicology and teratology of childhood: Models, methods, and implications for intervention. Neurotoxicology 6(3):165–182.
Pelkonen, O., E.H. Kaltiala, T.K.I. Larmi, and N.T. Karki. 1973. Comparisons of activities of drug-metabolizing enzymes in human fetal and adult livers. Clin. Pharmacol. Ther. 14:840–846.
Perino, J., and C.B. Erinhart. 1974. The relation of subclinical lead level to cognitive and sensorimotor impairment in black preschoolers. J. Learn. Dis. 7:26–30.
Peto, R., F.J. Roe, P.N. Lee, L. Levy, and J. Clack. 1975. Cancer and ageing in mice and men. Br. J. Cancer. 32:411–426.
Petros, W.P., and W.E. Evans. 1992. Antineoplastic agents. Pp 390–405 in Pediatric Pharmacology: Therapeutic Principles in Practice, 2nd ed., S.J. Yaffe and J.V. Aranda, eds. Philadelphia: Saunders.
Pinkel, D. 1958. The use of body surface area as a criterion of drug dosage in cancer chemotherapy. Cancer Res. 18:853–856.
Powell, H., O. Swarner, L. Gluck, and P. Lampert. 1973. Hexachlorophene myelinopathy in premature infants. J. Pediatr. 82:976–981.
Pozharisski, K.M., L.G. Prvanova, V.N. Anisimov, and V.F. Klimashevski. 1980. Effect of age on carcinogenesis in intestines. Exp. Onkol. 2:20–21.
Pylkko, O.O., and P.M. Woodbury. 1961. The effect of maturation of chemically induced seizures in rats. J. Pharmacol. Exp. Ther. 131:185–190.
Rabinowitz, M.B., G.W. Wetherill, and J.D. Kopple. 1976. Kinetic analysis of lead metabolism in healthy humans. J. Clin. Invest. 58:260–270.
Rane, A. 1992. Drug disposition and action in infants and children. Pp. 10–21 in Pediatric Pharmacology: Therapeutic Principles in Practice, S.J. Yaffe, and J.V. Aranda, eds. Philadelphia: Saunders.
Rane, A., and F. Sjoqvist. 1972. Drug metabolism in the human fetus and newborn infant. Pediatr. Clin. North Am. 19:37–49.
Rane, A., P.K.M. Lunde, B. Jalling, S.J. Yaffe, and F. Sjoqvist. 1971. Plasma protein binding of diphenylhydantion in normal and hyperbilirubinemic infants. Peidatr. Pharmacol. Ther. 78:877–882.
Rasmussen, J.E. 1979. Percutaneous absorption in children. Pp. 25–38 in Year Book of Dermatology, R.L. Dobson, ed. Chicago: Year Book Medical.
Reed, M.D., and J.B. Besunder. 1989. Developmental pharmacology: Ontogenetic basis of drug disposition. Pediatr. Clin. North Am. 36:1053–1074.
Reuber, M.D. 1975. Hyperplastic and neoplastic lesions of the kidney in Buffalo rats of varying ages ingesting N-4-(4-flourobiphenyl)acetamide. J. Natl. Cancer Inst. 54:427–429.
Reuber, M.D. 1976 Effects of age and sex on lesions of the oesophagus in Buffalo strain rats ingesting diethylnitrosamine. Exp. Cell Biol. 44:65–72.
Reuber, M.D., and E.L. Glover. 1967. Hyperplastic and early neoplastic lesions of the liver in Buffalo strain rats of various ages given subcutaneous carbon tetrachloride. J. Natl. Cancer Inst. 38:891–899.
Reuber, M.D., and C.W. Lee. 1968. Effect of age and sex on hepatic lesions in
Buffalo strain rats ingesting diethylnitrosamine. J. Natl. Cancer Inst. 41:1133–1140.
Rice, J.M. 1979. Perinatal Carcinogenesis. NCI Monogragh No. 51. DHEW Publ. No. (NIH) 79–1622. Washington, D.C.: Government Printing Office.
Richman, D.D., M.A. Fischl, M.H. Grieco, M.S. Gottlieb, P.A. Volberding, O.L. Laskin, J.M. Leedom, J.E. Groopman, D. Mildvan, M.S. Hirsch, G.G. Jackson, D.T. Durack, S. Nusinoff-Lehrman, and the AZT Collaborative Working Group. 1987. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex: A double-blind, placebo-controlled trial. New Engl. J.M. ed. 317:192–197.
Rodgers, K.E., T. Imamura, and B. H. Devens. 1986. Organophosphorus pesticide immunotoxicity: Effects of O,O,S-trimethyl phosphorothiaote on cellular and humoral immune response systems. Immunopharmacology 12:193–202.
Rodgers, K.E., N. Leung, C.F. Ware, and T. Imamura. 1987. Effects of O,S,S,-trimethyl phosphorodithioate on immune function. Toxicology 43:201–216.
Rodier, P.M. 1980. Chronology of neuron development: Animal studies and their clinical implications. Med. Child Neurol. 22:525–545.
Rogan, W.J., B.C. Gladan, K.-L. Hung, S.-L. Koong, L.-Y. Shih, J.S. Taylor, Y.-C. Wu, D. Yang, N.B. Ragan, and C.-C. Hsu. 1988. Congenital poisoning by polychlorinated biphenyls and their contaminants in Taiwan. Science 241:334–336.
Roland, E.H., J.E. Jan, and J.M. Riss. 1985. Toxic encephalopathy in a child after brief exposure to insect repellants. J. Can. Med. Assoc. 132(2):155–156.
Rosecrans, J.A., J.-S. Hong, R.E. Squibb, J.H. Johnson, W.E. Wilson, and H.A. Tilson. 1982. Effects of perinatal exposure to chlordecone (kepone) on neuroendocrine and neurochemical responsiveness of rats to environmental challenges. Neurotoxicology 3(2):131–142.
Rowland, I.R., R.D. Robinson, R.A. Doherty, and T.D. Landry. 1983. Are developmental changes in methylmercury metabolism and excretion mediated by the intestinal microflora? Pp. 745–758 in Reproductive and Developmental Toxicity of Metals, T.W. Clarkson, G.F. Nordberg, and P.R. Sager, eds. New York: Plenum.
Ruff, H.A., and P.E. Bijur. 1989. The effects of low to moderate lead levels on neurobehavioral functions of children J. Dev. Behav. Pediatr. 10(2):103–109.
Rumack, B.H. 1984. Acetaminophen overdose in young children. Am. J. Dis. Child.138:428–433.
Russo, J., and I.H. Russo. 1978. DNA labeling index and structure of the rat mammary gland as determinants of its susceptibility to carcinogenesis. J. Natl. Cancer Inst. 61:1451–1459.
Sachs, C., and G. Jonsson, 1975. 5,7-Dihydroxytryptamine induced changes in the postnatal development of central 5-hydroxytryptamine. Med. Biol. 53:156–168.
Savchenkov, M.F., V.V. Benemansky, and V.Y. Levina. 1980. Blastomogenic activity of dimethylnitrosamine in animals of different age. Hyg. Sanit. 4:17–20.
Saxen, E.A. 1954. On the factor of age in the production of subcutaneous sarcomas in mice by 20-methylcholantrene. J. Natl. Cancer Inst. 14:1547–569.
Schanker, L.S. 1978. Drug absorption from the lung. Biochem. Pharmacol. 27:381–385.
Schmidt-Nielsen, K. 1972. How Animals Work. London: Cambridge University Press.
Seagull, E.A.W. 1983. Developmental abilities of children exposed to polybrominated biphenyls (PBB). Am. J. Public Health 3:281–285.
Senanayake, N., and L. Karalliedde. 1987. Neurotoxic effects of organophosphorus insecticides: An intermediate syndrome. New Engl. J. Med. 316:761–763.
Shah, P.V., H.L. Fisher, M.R. Sumler, R.J. Monroe, N. Chernoff, and L.L. Hall. 1987. Comparison of the penetration of 14 pesticides through the skin of young and adult rats. J. Toxicol. Environ. Health 21:353–366.
Shuman, R.M., R.W. Leech, and E.C. Alvord, Jr. 1974. Neurotoxicity of hexachlorophene in the human: I. A clinicopathologic study of 248 children . Pediatrics 54:689–695.
Singer, E.J., P.C. Wegmann, M.D. Lehman, M.S. Christensen, and L.J. Vinson, 1971. Barrier development, ultrastructure, and sulfhydryl content of the fetal epidermis. J. Soc. Cosmet. Chem. 22:119–137.
Singer, R., M. Moses, J. Valciukas, R. Lilis, and I.J. Salikoff. 1982. Nerve conduction velocity studies of workers employed in the manufacture of phenoxy herbicides. Environ. Res. 29:297–311.
Singh, S.M., J.F. Toles, and J. Reaume, 1986. Genotype- and age-associated in vivo cytogenetic alterations following mutagenic exposures in mice. Can. J. Genet. Cytol. 28:286–293.
Six, K.M., and R.A. Goyer. 1972. The influence of iron deficiency on tissue content and toxicity of ingested lead in the rat. J. Lab. Clin. Med. 79:128–136.
Solomon, L.M., D.P. West, J.F. Fitzcoff, and A.M. Becker. 1977. Gamma benzene hexachloride in guinea pig brain after topical application. J. Invest. Dermatol. 68:310–312.
Sonawane, B.R. 1982. Developmental aspects of acetaminophen hepatotoxicity: Influence of age and acute starvation. Pp. 507–520 in Banbury Report No. 11: Environmental Factors in Human Growth and Development, V.R. Hung, M.K. Smith, and D. Worth, eds. New York: Cold Spring Harbor Laboratory.
Spielberg, S.P. 1992. Anticonvulsant adverse drug reactions: Age dependent and age independent. Pp. 104–106 in Similarities and Differences Between Children and Adults: Implications in Risk Assessment, P.S. Guzelian, C.J. Henry, and S.S. Olin, eds. Washington, D.C.: ILSI Press.
Stanton, M.E. and L.P. Spear. 1990. Conference on the qualitative and quantitative comparability of human and animal developmental neurotoxicity. Work group I report: Comparability of measures of developmental neurotoxicity in humans and laboratory animals. Neurotoxicol. Teratol. 12:261–267.
Stenbäck, F., R. Peto, and P. Shubik. 1981. Initiation and promotion at different ages and doses in 2200 mice: II Decrease in promotion by TPA with ageing. Br. J. Cancer 44:15–23.
Streltsova, V.N., and Y.I. Moskalev. 1964. Blastomogenic Effect of Ionizing Radiation. Moscow: Meditsina Press.
Stromberg, K., and M.D. Reuber. 1975. Histopathology of breast lesions induced in BUF rats of varying ages by ingestion of N-4-(4'-fluorobiphenyl) acetamide. J. Natl. Cancer Inst. 54:1223–1230.
Stutman, O. 1979. Chemical carcinogenesis in nude mice: Comparison between nude mice from homozygous matings and hetozygous matings and effect of age and carcinogen dose. J. Natl. Cancer Inst. 62:353–358.
Suchy, F.J., W.F. Balistreri, J.E. Heubi, J.E. Searcy, and R.S. Levin. 1981. Physiologic
cholestasis: Elevation of the primary serum bile acid concentrations in normal infants. Gastroenterology 80:1037–1041.
Summerhayes, I.C., and L.M. Franks. 1979. Effects of donor age on neoplastic transformation of adult mouse bladder epithelium in vitro . J. Natl. Cancer Inst. 62:1017–1023.
Sundaram, K. 1963. Longterm consequences of 90Sr in rats and the problem of carcinogenesis. Pp. 139–144 in Cellular Basis and Aetiology of Late Somatic Effects of Ionizing Radiation, R.J.C. Harris, ed. London: Academic Press.
Sutherland, J.M. 1959. Fatal cardiovascular collapse of infants receiving large amounts of chloramphenicol. J. Dis. Child. 97:761–767.
Syn-mao, L. 1962. Role of Hormonal and Nervous Factors in Development of Induced Mammary Tumors in Rats. Thesis. University of Kiev.
Thomas, P.T., and H.V. Ratajczak. 1988. Assessment of carbamate pesticide immunotoxicity. Toxicol. Ind. Health 4:381–390.
Thomas, P., H. Ratajczak, D. Demetral, K. Hagen, and R. Baron. 1990. Aldicarb immunotoxicity: Functional analysis of cell-mediated immunity and quantitation of lymphocyte subpopulations. Fundam. Appl. Toxicol. 15:221–230.
Thurlbeck, W.M. 1982. Postnatal human lung growth. Thorax 37:564–571.
Tilson, H.A., and C.L. Mitchell. 1984. Neurobehavioral techniques to assess the effects of chemicals on the nervous system. Annu. Rev. Pharmacol. Toxicol. 24:425–450.
Toth, B. 1968. A critical review of experiments in chemical carcinogenesis using newborn animals. Cancer Res. 28:727–738.
Triebig, G., I. Cxuzda, H.J. Krekeler, and K.H. Schaller. 1987. Pentachlorophenol and the peripheral nervous system: A longitudinal study in exposed workers. Br. J. Ind. Med. 44:638–641.
Tucker, G.T., and L.E. Mather. 1979. Clinical pharmacokinetics of local anesthetics. Clin. Pharmacokinet. 4:241–278.
Tucker, M.A., A.T. Meadows, J.D. Boice, M. Stovall, O. Oberlin, B.J. Stone, J. Birch, P.A. Vo'te, R.N. Hoover, J.F. Fraumeni, and the Late Effects Study Group. 1987. Leukemia after therapy with alkylating agents for childhood cancer. J. Natl. Cancer Inst. 78:459–464.
Turosov, V.S., L.S. Baslova, and V.A. Krutovskikh. 1979. Effect of age, castration and pregnancy on 1,2-dimethylhydrazine-induced carcinogenesis in CBA mice. Bull. Exp. Biol. Med. 87:458–460.
Turusov, V.S., N.S. Lanko, and Y.D. Parfenov. 1981. Effect of age, on induction by 1,2-dimethylhydrazine tumors of intestines in mice. Bull. Exp. Biol. Med. 91:705–707.
Tyl, R., and W.F. Sette. 1990. Weight of evidence and quantitative evaluation of developmental neurotoxicity data. Conference on the Qualitative and Quantitative Comparability of Human and Animal Developmental Neurotoxicity. Work Group III Report. Neurotoxicol. Teratol. 12:237–280.
Tyrala, E.E., L.S. Hillman, R.E. Hillman, and W.E. Dodson. 1977. Clinical pharmacology of hexachlorophene in newborn infants. J. Pediatr. 91:481–486.
Udall, J.N. and W.A. Walker. 1982. Macromolecular transport across the developing intestine. Pp. 187–198 in Banbury Report No 11: Environmental Factors in Human Growth and Development, V.R. Hunt, M.K. Smith, and D. Worth, eds. New York: Cold Spring Harbor Laboratory.
Varga, F., and T.Z. Csaky. 1976. Changes in the blood supply of the gastrointestinal tract in rats with age. Pflugers Arch. 364:129–133.
Vasilesque. C., M. Alexander, and A. Dan. 1984. Delayed neuropathy after organophosphorus insecticide poisoning: A clinical, electrophysiological, and nervebiopsy study. J. Neurol. Neurosurg. Psych. 47:543–548.
Vesselinovitch, S.D., K.V.N. Rao, and N. Mihailovich. 1979. Neoplastic response of mouse tissues during perinatal age periods and its significance in chemical carcinogenesis. In Perinatal Carcinogenesis, NCI Monogragh No. 51, DHEW Publ. No. (NIH) 79-1622, J.M. Rice, ed. Washington, D.C.: U.S. Government Printing Offi.
Vorhees, C.V. 1986. Principles of behavioral teratology. Pp. 23–48 in Handbook of Behavioral Teratology, E.P. Riley and C.V. Vorhees, eds. New York: Plenum.
Vos, J.G., and J.A. Moore. 1974. Suppression of cellular immunity in rats and mice by maternal treatment with 2,3,7,8, tetrachlorodibenzo-p-dioxin. Int. Arch. Allergy 47:777–794.
Walker, W.A., and K.J. Isselbacher. 1974. Uptake and transport of macromolecules by the intestine: Possible role in clinical disorders. Gastroenterology 67:531–550.
Wang, Y.-M., W.W. Sutow, M.M. Romsdahl, and C. Perez. 1979. Age-related pharmacokinetics of high-dose methotrexate in patients with osteosarcoma. Cancer Treat. Rep. 63:405–410.
Warner, A. 1986. Drug use in the neonate: Interrelationship of pharmacokinetics, toxicity and biochemical immaturity. Clin. Chem. 32:721–727.
Watanabe, T., K. Matsuhashi, and S. Takayama. 1990. Placental and blood-brain barrier transfer following prenatal and postnatal exposures to neuroactive drugs:Relationship with partition coefficients and behavioral teratogenesis. Toxicol. Appl. Pharmacol. 105:66–77.
Weil, W.B., Jr. 1955. The evaluation of renal function in infancy and childhood. Am. J. Med. Sci. 229: 678–694.
Weil, W., M. Spencer, D. Benjamen, and E. Seagull. 1981. The effect of polybrominated biphenyl of infants and young children. J. Pediatr. 98(1):47–51.
Weiss, B. 1983. Behavioral toxicology of heavy metals. Pp. 1–50 in Neurobiology of the Trace Elements: Neurotoxicology and Neuropharmacology, Vol. 2, I.E. Dreosti and R.M. Smith, eds. Clifton, N.J.: Humana. 320 pp.
Weiss, B. 1988. Behavior as an early indicator of pesticide toxicity. Toxicol. Ind. Health 4:351–360.
Weiss, C.F., A.J. Glazko, and J.K. Weston. 1960. Chloramphenicol in the newborn infant: A physiological explanation of its toxicity when given in excessive doses. New Engl. J. Med. 262:787–794.
West, J.R., H.W. Smith, and H. Chasis. 1948. Glomerular filtration rate, effective renal blood flow, and maximal tubular excretory capacity in infancy. J. Pediatr. 32a:10–18.
Wester, R.C., and H.I. Maibach. 1982. Percutaneous absorption: Neonate compared to the adult. Pp. 3-15 in Banbury Report No. 11: Environmental Factors in Human Growth and Development, V.R. Hunt, M.K. Smith, and D. Worth, eds. New York: Cold Spring Harbor Laboratory.
Wester, R.C., P.K. Noonan, M.P. Cole, and H.I. Maibach. 1977. Percutaneous absorption of testosterone in the newborn rhesus monkey: Comparison to the adult. Pediatr. Res. 11:737–739.
Whorton, M.D., and D.L. Obrinsky. 1983. Persistence of symptoms after mild to moderate acute organophosphate poisoning among 19 farm field workers. J. Toxicol. Environ. Health 11:347–354.
Widdowson, E.M., and J.W.T. Dickerson. 1964. Chemical composition of the body. Pp. 1–247 in Mineral Metabolism: An Advanced Treatise, C.L. Comar and F. Bronner, eds. New York: Academic Press.
Windorfer, A., W. Kuenzer, and R. Urbanek. 1974. The influence of age on the activity of acetylsalicylic acid-esterase and protein salicylate binding. Eur. J. Clin. Pharmacol. 7:227–231.
Winneke, G., U. Beginn, T. Ewert, C. Harvestadt, U. Kraemer, C. Krause, H.L. Thron, and H.M. Wagner. 1985. Comparing the effects of perinatal and later childhood lead exposure on neuropsychological outcome. Environ. Res. 38(1):155–167.
Wong, K.-L., and C.D. Klaassen. 1980. Tissue distribution and retention of cadmium in rats during postnatal development: Minimal role of hepatic metallothionein. Toxicol. Appl. Pharmacol. 53:343–353.
Woods, W.G., M. O'Leary, and M.E. Nesbit. 1981. Life-threatening neuropathy and hepatotoxicity in infants during induction therapy for acute lymphoblastic leukemia. J. Pediatr. 98:642–645.
Wu, M.-F., J.R. Ison, J.R. Wecker, and L.W. Lapham. 1985 Cutaneous and auditory function in rats following methyl mercury poisoning. Toxicol. Appl. Pharmacol. 79:377–388.
Wynn, R.M. 1963. Relationship of menadiol tetrasodium diphosphate (synkavite) to bilirubinemia and hemolysis in the adult and newborn rat. Am. J. Obstet. Gynecol. 86:495–503.
Ziegler, E.E., B.B. Edwards, R.L. Jensen, K.R. Mahaffey, and S.J. Forman. 1978. Absorption and retention of lead by infants. Pediatr. Res. 12:29–34.
Zimmerman, J.A., L.D. Trombetta, T.H. Carter, and S.H. Weisbroth. 1982. Pancreatic carcinoma induced by N-methyl-N'-nitrosourea in aged mice. Gerontology 28:114–120.
Zwiener R.J., and C.M. Ginsburg. 1988. Organophosphate and carbamate poisoning in infants and children. Pediatrics 81:121–126.














































































