2
Principles of Bioremediation
The key players in bioremediation are bacteria—microscopic organisms that live virtually everywhere. Microorganisms are ideally suited to the task of contaminant destruction because they possess enzymes that allow them to use environmental contaminants as food and because they are so small that they are able to contact contaminants easily. In situ bioremediation can be regarded as an extension of the purpose that microorganisms have served in nature for billions of years: the breakdown of complex human, animal, and plant wastes so that life can continue from one generation to the next. Without the activity of microorganisms, the earth would literally be buried in wastes, and the nutrients necessary for the continuation of life would be locked up in detritus.
Whether microorganisms will be successful in destroying man-made contaminants in the subsurface depends on three factors: the type of organisms, the type of contaminant, and the geological and chemical conditions at the contaminated site. This chapter explains how these three factors influence the outcome of a subsurface bioremediation project. It reviews how microorganisms destroy contaminants and what types of organisms play a role in in situ bioremediation. Then, it evaluates which contaminants are most susceptible to bioremediation in the subsurface and describes the types of sites where bioremediation is most likely to succeed.
THE ROLE OF MICROBES IN BIOREMEDIATION
The goal in bioremediation is to stimulate microorganisms with nutrients and other chemicals that will enable them to destroy the contaminants. The bioremediation systems in operation today rely on microorganisms native to the contaminated sites, encouraging them to work by supplying them with the optimum levels of nutrients and other chemicals essential for their metabolism. Thus, today's bioremediation systems are limited by the capabilities of the native microbes. However, researchers are currently investigating ways to augment contaminated sites with nonnative microbes—including genetically engineered microorganisms—specially suited to degrading the contaminants of concern at particular sites. It is possible that this process, known as bioaugmentation, could expand the range of possibilities for future bioremediation systems.
Regardless of whether the microbes are native or newly introduced to the site, an understanding of how they destroy contaminants is critical to understanding bioremediation. The types of microbial processes that will be employed in the cleanup dictate what nutritional supplements the bioremediation system must supply. Furthermore, the byproducts of microbial processes can provide indicators that the bioremediation is successful.
How Microbes Destroy Contaminants
Although bioremediation currently is used commercially to cleanup a limited range of contaminants—mostly hydrocarbons found in gasoline—microorganisms have the capability to biodegrade almost all organic contaminants and many inorganic contaminants. A tremendous variety of microbial processes potentially can be exploited, extending bioremediation's utility far beyond its use today. Whether the application is conventional or novel by today's standards, the same principles must be applied to stimulate the right type and amount of microbial activity.
Basics of Microbial Metabolism
Microbial transformation of organic contaminants normally occurs because the organisms can use the contaminants for their own growth and reproduction. Organic contaminants serve two purposes for the organisms: they provide a source of carbon, which is one of the basic building blocks of new cell constituents, and they provide electrons, which the organisms can extract to obtain energy.
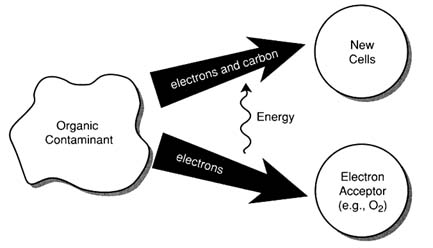
FIGURE 2-1
Microbes degrade contaminants because in the process they gain energy that allows them to grow and reproduce. Microbes get energy from the contaminants by breaking chemical bonds and transferring electrons from the contaminants to an electron acceptor, such as oxygen. They "invest" the energy, along with some electrons and carbon from the contaminant, to produce more cells.
Microorganisms gain energy by catalyzing energy-producing chemical reactions that involve breaking chemical bonds and transferring electrons away from the contaminant. The type of chemical reaction is called an oxidation-reduction reaction: the organic contaminant is oxidized, the technical term for losing electrons; correspondingly, the chemical that gains the electrons is reduced. The contaminant is called the electron donor, while the electron recipient is called the electron acceptor. The energy gained from these electron transfers is then "invested," along with some electrons and carbon from the contaminant, to produce more cells (see Figure 2-1). These two materials—the electron donor and acceptor—are essential for cell growth and are commonly called the primary substrates. (See Box 2-1 and the glossary for definitions of these and other key terms.)
Many microorganisms, like humans, use molecular oxygen (O2) as the electron acceptor. The process of destroying organic compounds with the aid of O2 is called aerobic respiration. In aerobic respiration, microbes use O2 to oxidize part of the carbon in the contaminant to carbon dioxide (CO2), with the rest of the carbon used to produce new cell mass. In the process the O2 gets reduced, produc-
|
BOX 2-1 KEY TERMS FOR UNDERSTANDING BIOREMEDIATION Microorganism: An organism of microscopic size that is capable of growth and reproduction through biodegradation of "food sources," which can include hazardous contaminants. Microbe: The shortened term for microorganism. Oxidize: The transfer of electrons away from a compound, such as an organic contaminant. The coupling of oxidation to reduction (see below) usually supplies energy that microorganisms use for growth and reproduction. Often (but not always), oxidation results in the addition of an oxygen atom and/or the loss of a hydrogen atom. Reduce: The transfer of electrons to a compound, such as oxygen, that occurs when another compound is oxidized. Electron acceptor: The compound that receives electrons (and therefore is reduced) in the energy-producing oxidation-reduction reactions that are essential for the growth of microorganisms and bioremediation. Common electron acceptors in bioremediation are oxygen, nitrate, sulfate, and iron. Electron donor: The compound that donates electrons (and therefore is oxidized). In bioremediation the organic contaminant often serves as an electron donor. Primary substrates: The electron donor and electron acceptor that are essential to ensure the growth of microorganisms. These compounds can be viewed as analogous to the food and oxygen that are required for human growth and reproduction. Aerobic respiration: The process whereby microorganisms use oxygen as an electron acceptor. Anaerobic respiration: The process whereby microorganisms use a chemical other than oxygen as an electron acceptor. Common "substitutes" for oxygen are nitrate, sulfate, and iron. Fermentation: The process whereby microorganisms use an organic compound as both electron donor and electron acceptor, converting the compound to fermentation products such as organic acids, alcohols, hydrogen, and carbon dioxide. |
|
Cometabolism: A variation on biodegradation in which microbes transform a contaminant even though the contaminant cannot serve as the primary energy source for the organisms. To degrade the contaminant, the microbes require the presence of other compounds (primary substrates) that can support their growth. Reductive dehalogenation: A variation on biodegradation in which microbially catalyzed reactions cause the replacement of a halogen atom on an organic compound with a hydrogen atom. The reactions result in the net addition of two electrons to the organic compound. Intrinsic bioremediation: A type of bioremediation that manages the innate capabilities of naturally occurring microbes to degrade contaminants without taking any engineering steps to enhance the process. Engineered bioremediation: A type of remediation that increases the growth and degradative activity of microorganisms by using engineered systems that supply nutrients, electron acceptors, and/or other growth-stimulating materials. |
ing water. Thus, the major byproducts of aerobic respiration are carbon dioxide, water, and an increased population of microorganisms.
Variations on Basic Metabolism
In addition to microbes that transform contaminants through aerobic respiration, organisms that use variations on this basic process have evolved over time. These variations allow the organisms to thrive in unusual environments, such as the underground, and to degrade compounds that are toxic or not beneficial to other organisms.
Anaerobic Respiration. Many microorganisms can exist without oxygen, using a process called anaerobic respiration. In anaerobic respiration, nitrate (NO3-), sulfate (SO42-), metals such as iron (Fe3+) and manganese (Mn4+), or even CO2 can play the role of oxygen, accepting electrons from the degraded contaminant. Thus, anaerobic respiration uses inorganic chemicals as electron acceptors. In addition to new cell matter, the byproducts of anaerobic respiration may include nitrogen gas (N2), hydrogen sulfide (H2S), reduced forms of metals, and methane (CH4), depending on the electron acceptor.
Some of the metals that anaerobic organisms use as electron acceptors are considered contaminants. For example, recent research has demonstrated that some microorganisms can use soluble uranium (U6+) as an electron acceptor, reducing it to insoluble uranium (U4+). Under this circumstance the organisms cause the uranium to precipitate, decreasing its concentration and mobility in the ground water.
Inorganic Compounds as Electron Donors. In addition to organisms that use inorganic chemicals as electron acceptors for anaerobic respiration, other organisms can use inorganic molecules as electron donors. Examples of inorganic electron donors are ammonium (NH4+), nitrite (NO2-), reduced iron (Fe2+), reduced manganese (Mn2+), and H2S. When these inorganic molecules are oxidized (for example, to NO2-, NO3-, Fe3+, Mn4+, and SO42-, respectively), the electrons are transferred to an electron acceptor (usually O2) to generate energy for cell synthesis. In most cases, microorganisms whose primary electron donor is an inorganic molecule must obtain their carbon from atmospheric CO2 (a process called CO2 fixation).
Fermentation. A type of metabolism that can play an important role in oxygen-free environments is fermentation. Fermentation requires no external electron acceptors because the organic contaminant serves as both electron donor and electron acceptor. Through a series of internal electron transfers catalyzed by the microorganisms, the organic contaminant is converted to innocuous compounds known as fermentation products. Examples of fermentation products are acetate, propionate, ethanol, hydrogen, and carbon dioxide. Fermentation products can be biodegraded by other species of bacteria, ultimately converting them to carbon dioxide, methane, and water.
Secondary Utilization and Co-metabolism. In some cases, microorganisms can transform contaminants, even though the transformation reaction yields little or no benefit to the cell. The general term for such nonbeneficial biotransformations is secondary utilization, and an important special case is called co-metabolism. In co-metabolism the transformation of the contaminant is an incidental reaction catalyzed by enzymes involved in normal cell metabolism or special detoxification reactions. For example, in the process of oxidizing methane, some bacteria can fortuitously degrade chlorinated solvents that they would otherwise be unable to attack. When the microbes oxidize methane, they produce certain enzymes that incidentally destroy the chlorinated solvent, even though the solvent itself cannot support
microbial growth. The methane is the primary electron donor because it is the organisms' primary food source, while the chlorinated solvent is a secondary substrate because it does not support the bacteria's growth. In addition to methane, toluene and phenol have been used as primary substrates to stimulate co-metabolism of chlorinated solvents.
Reductive Dehalogenation. Another variation on microbial metabolism is reductive dehalogenation. Reductive dehalogenation is potentially important in the detoxification of halogenated organic contaminants, such as chlorinated solvents. In reductive dehalogenation, microbes catalyze a reaction in which a halogen atom (such as chlorine) on the contaminant molecule gets replaced with a hydrogen atom. The reaction adds two electrons to the contaminant molecule, thus reducing the contaminant.
For reductive dehalogenation to proceed, a substance other than the halogenated contaminant must be present to serve as the electron donor. Possible electron donors are hydrogen and low-molecular-weight organic compounds (lactate, acetate, methanol, or glucose). In most cases, reductive dehalogenation generates no energy but is an incidental reaction that may benefit the cell by eliminating a toxic material. However, researchers are beginning to find examples in which cells can obtain energy from this metabolic process.
Microbial Nutritional Requirements for Contaminant Destruction
Regardless of the mechanism microbes use to degrade contaminants, the microbes' cellular components have relatively fixed elemental compositions. A typical bacterial cell is 50 percent carbon; 14 percent nitrogen; 3 percent phosphorus; 2 percent potassium; 1 percent sulfur; 0.2 percent iron; and 0.5 percent each of calcium, magnesium, and chloride. If any of these or other elements essential to cell building is in short supply relative to the carbon present as organic contaminants, competition for nutrients within the microbial communities may limit overall microbial growth and slow contaminant removal. Thus, the bioremediation system must be designed to supply the proper concentrations and ratios of these nutrients if the natural habitat does not provide them.
How Microbes Demobilize Contaminants
In addition to converting contaminants to less harmful products, microbes can cause mobile contaminants to be demobilized, a strat-
egy useful for containing hazardous materials. There are three basic ways microbes can be used to demobilize contaminants:
-
Microbial biomes can sorb hydrophobic organic molecules. Sufficient biomass grown in the path of contaminant migration could stop or slow contaminant movement. This concept is sometimes called a biocurtain.
-
Microorganisms can produce reduced or oxidized species that cause metals to precipitate. Examples are oxidation of Fe2+ to Fe3+, which precipitates as ferric hydroxide (FeOH3(s)); reduction of SO42- to sulfide (S2-), which precipitates with Fe2+ as pyrite (FeS(s)) or with mercury (Hg2+) as mercuric sulfide (HgS(s)); reduction of hexavalent chromium (Cr6+) to trivalent chromium (Cr3+), which can precipitate as chromium oxides, sulfides, or phosphates; and, as mentioned previously, reduction of soluble uranium to insoluble U4+, which precipitates as uraninite (UO2).
-
Microorganisms can biodegrade organic compounds that bind with metals and keep the metals in solution. Unbound metals often precipitate and are immobilized.
Indicators of Microbial Activity
In the process of degrading or demobilizing contaminants, microbes cause changes in the surrounding environment that are important to understand when evaluating bioremediation.
Chemical Changes
Bioremediation alters the ground water chemistry. These chemical changes follow directly from the physiological principles of microorganisms outlined above. Microbial metabolism catalyzes reactions that consume well-defined reactants—contaminants and O2 or other electron acceptors—converting them to well-defined products.
The specific chemical reactants and products can be determined from the chemical equations for the reactions the microbes catalyze. These equations are familiar to anyone with a basic understanding of microbiology. For example, the chemical equation for the degradation of toluene (C7H8) is:
Thus, when bioremediation is occurring, the concentration of inorganic carbon (represented by CO2) should increase as the concentrations of toluene and oxygen decrease. Another example is the dechlo-
rination of trichloroethane (C2H3Cl3, or TCA) to dichloroethane (C 2H4Cl2, or DCA) by hydrogen-oxidizing anaerobic bacteria:
Here, TCA and hydrogen (H2) decrease as DCA, hydrogen ion (H+), and chloride ion (Cl-) increase. The formation of hydrogen ion may cause the pH to decrease, depending on the ground water chemistry.
In general, under aerobic conditions, one should expect to observe a drop in the O2 concentration when microbes are active. Similarly, under anaerobic conditions, concentrations of other electron acceptors— NO3-, SO42-, Fe3+, Mn4+—will decrease, with a corresponding increase in the reduced species of these compounds (N2, H2S, Fe2+, and Mn2+, respectively). Under both types of conditions the inorganic carbon concentration should increase, because organic carbon is oxidized. The inorganic carbon may take the form of gaseous CO2, dissolved CO2, or bicarbonate ion (HCO3-).
Adaptation by Native Organisms
In addition to producing chemical changes in the ground water, bioremediation can alter the metabolic capabilities of native microorganisms. Often, microorganisms do not degrade contaminants upon initial exposure, but they may develop the capability to degrade the contaminant after prolonged exposure. Several mechanisms have been proposed to explain metabolic adaptation, including enzyme induction, growth of biodegrading populations, and genetic change. However, these proposals remain largely speculative because methodological limitations usually preclude rigorous understanding of how microbial communities develop, both in laboratory tests and at field sites. Regardless of the mechanisms, adaptation is important because it is a critical principle in ensuring the existence of microorganisms that can destroy the myriad new chemicals that humans have created and introduced into the environment.
Adaptation occurs not only within single microbial communities but also among distinct microbial communities that may evolve a co-operative relationship in the destruction of compounds. One community may partially degrade the contaminant, and a second community farther along the ground water flow path may complete the reaction. This type of coupling occurs naturally in anaerobic food chains that convert plant-derived organic compounds to methane. Such coupling has obvious applications for bioremediation of sites bearing contaminant compounds whose complete metabolism may require alternation between anaerobic and aerobic processes.
Growth of Predators
Although bacteria are the agents for biodegradation during bioremediation, other organisms that prey on bacteria also may grow as a result of bioremediation. Protozoa are the most common bacterial predators. Just as mammalian predators, such as wolves, can only be supported by certain densities of their prey, microbial protozoan predators flourish only when their bacterial prey are in large, rapidly replenished supplies. Thus, the presence of protozoa normally signifies that enough bacteria have grown to degrade a significant quantity of contaminants.
Complicating Factors
The basic principles of how microbes degrade contaminants are relatively straightforward. Yet many details of microbial metabolism are not yet understood, and the successful use of microbes in bioremediation is not a simple matter. A range of factors may complicate bioremediation. Some of the key complicating factors are the unavailability of contaminants to the organisms, toxicity of contaminants to the organisms, microbial preference for some contaminants or naturally occurring chemicals over other contaminants, partial degradation of contaminants to produce hazardous byproducts, inability to remove contaminants to very low concentrations, and aquifer clogging from excessive biomass growth.
Unavailability of Contaminants to the Organisms
Readily biodegradable contaminants may remain undegraded or be biodegraded very slowly if their concentrations in the ground water are too low. The problem of too low concentrations usually is caused by unavailability, in which the contaminant is sequestered from the microorganisms. Sequestering of organic contaminants can occur when the contaminant is dissolved in a nonaqueous-phase liquid—a solution that does not mix easily with water and therefore travels through the ground separately from the ground water. Sequestering of organic contaminants can also occur if the contaminant is strongly adsorbed to soil surfaces or is trapped in pores too small for circulating ground water to penetrate easily. In these cases, almost all of the contaminant is associated with the solid, the nonaqueous-phase liquid, or the pores, and the very small concentrations that dissolve in the water support very small or zero biodegradation rates.
Sequestering of metals and other inorganic contaminants occurs most frequently when they precipitate.
One possible strategy for overcoming the unavailability problem is to add chemical agents that mobilize the contaminants, causing them to move with the ground water. Such chemical agents are already used at some sites to increase the efficiency of conventional pump-and-treat ground water cleanup systems. However, their use to facilitate bioremediation is more complex than their use for pump-and-treat systems because the mobilizing agents not only affect the physical properties of the contaminants but may also affect the activity of the microorganisms.
Organic contaminants can be mobilized by adding surfactants. When only small surfactant concentrations are applied, the surfactant molecules accumulate at solid surfaces, reduce the surface tension, and, in principle, increase the spreading of organic contaminants. This spreading might improve contaminant transfer to the water and thereby accelerate bioremediation, but evidence is not clear for actual subsurface conditions. When large concentrations of surfactant are added, the surfactant molecules join together in colloids, called micelles. Organic contaminants dissolve into the micelles and are transported with the water inside them. However, biodegradation usually is not enhanced by contaminant transfer into the micelles because the true aqueous-phase concentration is not increased.
Metals can be mobilized by adding chemicals called complexing agents, or ligands, to which the metals bond. The formation of metalligand bonds dissolves precipitated metals, increasing their mobility. However, the effectiveness of strong ligands, such as ethylene-diaminetetra-acetic acid (EDTA), in enhancing biodegradation is not yet proven. One potential limitation of using ligands to mobilize metals is that microbes may degrade the ligands, releasing the metals and causing them to precipitate again.
In some cases, bacteria produce their own surfactants and ligands that are useful in mobilizing trapped contaminants. In these cases the main purpose of the microorganisms is to produce mobilizing agents, not to biodegrade the contaminants. Bacterially mediated mobilization makes trapped contaminants more accessible for cleanup with pump-and-treat technology; it is potentially less costly than injecting commercial surfactants.
Toxicity of Contaminants to the Organisms
Just as contaminant concentrations that are too low can complicate bioremediation, high aqueous-phase concentrations of some con-
taminants can create problems. At high concentration, some chemicals are toxic to microbes, even if the same chemicals are readily biodegraded at lower concentrations. Toxicity prevents or slows metabolic reactions and often prevents the growth of the new biomass needed to stimulate rapid contaminant removal. The degree and mechanisms of toxicity vary with specific toxicants, their concentration, and the exposed microorganisms. Microbial cells cease to function when at least one of the essential steps in their myriad physiological processes is blocked. The blockage may result from gross physical disruption of cell structure or competitive binding of a single enzyme essential for metabolizing the toxicant.
Presence of Multiple Contaminants and Natural Organic Chemicals
Frequently, contaminated sites contain a combination of several man-made organic contaminants and naturally occurring organic chemicals from decayed plant and animal matter. When such mixtures of organics are present, microbes may selectively degrade the compound that is easiest to digest or that provides the most energy. Microbiologists have long been aware that complex mechanisms regulating microbial metabolism may cause some carbon compounds to be ignored while others are selectively used. This phenomenon, known as diauxy, could have serious implications for bioremediation efforts if the targeted contaminant is accompanied by substantial quantities of preferred growth substrates.
Mixtures do not always cause problems and sometimes can promote bioremediation. For example, biomass that grows primarily to degrade one type of organic compound may also degrade a second compound present at a concentration too low to support bacterial growth by itself.
Incomplete Degradation of Contaminants
In some cases, contaminants may not be fully degraded by the organisms. Partial degradation may diminish the concentration of the original pollutant but create metabolic intermediates that in some cases are more toxic than the parent compound. There are two main reasons why intermediates build up. In one case a so-called dead-end product is produced. Dead-end products may form during cometabolism, because the incidental metabolism of the contaminant may create a product that the bacterial enzymes cannot transform any further. For example, in the cometabolism of chlorinated phenols, dead-end products such as chlorocatechols, which are toxic, some-
times build up. In the second case the intermediate builds up even though the compound can be fully degraded, because some of the key bacterially mediated reactions are slow. For example, vinyl chloride, a cancer-causing agent, may build up during trichloroethylene (TCE) biodegradation. The bacteria can convert TCE to vinyl chloride relatively quickly, but the subsequent degradation of vinyl chloride usually occurs slowly.
Inability to Remove Contaminants to Low Concentrations
Microorganisms may sometimes be physiologically incapable, even when environmental conditions are optimal, of reducing pollutant concentrations to very low, health-based levels, because the uptake and metabolism of organic compounds sometimes stops at low concentrations. This may be caused by the cells' internal mechanisms for regulating what reactions they perform or by an inability of the capable microbial populations to survive given inadequate sustenance. Regardless of the mechanism, if the final contaminant concentration fails to meet the cleanup goal, other cleanup strategies (microbiological or other) may have to be implemented to effectively reduce the concentration to acceptable levels. Research on augmenting sites with nonnative microbes and controlling cells' genetic capabilities and internal regulation may lead to means for overcoming this limitation.
Aquifer Clogging
Stimulating the growth of enough microorganisms to ensure contaminant degradation is essential to in situ bioremediation. However, if all the organisms accumulate in one place, such as near the wells that supply growth-stimulating nutrients and electron acceptors, microbial growth can clog the aquifer. Clogging can interfere with effective circulation of the nutrient solution, limiting bioremediation in places that the solution does not reach. Protozoan predators may help mitigate clogging. In addition, two engineering strategies can help prevent clogging: (1) feeding nutrients and substrates in alternating pulses and (2) adding hydrogen peroxide as the oxygen source. Pulse feeding prevents excessive biomass growth by ensuring that high concentrations of all the growth-stimulating materials do not accumulate near the injection point. Hydrogen peroxide prevents excessive growth because it is a strong disinfectant, until it decomposes to oxygen and water.
CONTAMINANTS SUSCEPTIBLE TO BIOREMEDIATION
A critical factor in deciding whether bioremediation is the appropriate cleanup remedy for a site is whether the contaminants are susceptible to biodegradation by the organisms at the site (or by organisms that could be successfully grown at the site). Some compounds are more easily degraded by a wide range of organisms than others, and systems for encouraging biodegradation are better established for some compounds than for others. Table 2-1 provides an overview of classes of compounds and their inherent suitability for bioremediation. The table is intended to deliver a broad perspective on how chemical and microbiological properties jointly affect prospects for bioremediation, and the judgments it presents are generalities that, of course, have exceptions. The table shows that bioremediation treatment technology is well established for certain classes of petroleum hydrocarbons but that the technologies for treating all other classes are still emerging. Commercial development of bioremediation technologies for these other compounds is possible, but it will require further research and the scaling up of lab discoveries for application in the field.
The table's first column shows the contaminant's frequency of occurrence at hazardous waste sites. It indicates the magnitude of the problem the contaminant poses. The second column indicates the state of development of bioremediation technologies for cleaning up the contaminant. In this column, ''established" means that bioremediation of the contaminant has been tried successfully many times at the commercial scale. "Emerging" means that the concepts underlying bioremediation of the contaminant have been tested in the laboratory and, in some cases, tested successfully at a limited number of field sites under controlled conditions. "Possible" means that evidence from lab tests indicates future potential for bioremediation to successfully cleanup the compound. The third column presents the evidence leading the committee to believe that the contaminant can be cleaned up successfully with bioremediation in the future, even though established bioremediation technology does not yet exist. It indicates what types of organisms can degrade the contaminant and how easily they can act. The fourth column describes contaminant properties that may limit bioremediation. The key limiting properties are the contaminant's tendency to sorb to subsurface solids and to partition into a nonaqueous-phase that travels separately from the ground water. As discussed previously in this chapter, both of these properties—sorption and nonaqueous-phase formation—decrease the amount of contaminant available to the microorganisms, slowing
TABLE 2-1 Contaminant Susceptibility to Bioremediation
|
Chemical Class |
Frequency of Occurrence |
Status of Bioremediation |
Evidence of Future Success |
Limitations |
|
Hydrocarbons and Derivatives |
|
|
|
|
|
Gasoline, fuel oil |
Very frequent |
Established |
|
Forms nonaqueous-phase liquid |
|
Polycyclic aromatic hydrocarbons |
Common |
Emerging |
Aerobically biodegradable under a narrow range of conditions |
Sorbs strongly to subsurface solids |
|
Creosote |
Infrequent |
Emerging |
Readily biodegradable under aerobic conditions |
Sorbs strongly to subsurface solids; forms nonaqueous-phase liquid |
|
Alcohols, ketones, esters |
Common |
Established |
|
|
|
Ethers |
Common |
Emerging |
Biodegradable under a narrow range of conditions using aerobic or nitrate-reducing microbes |
|
|
Halogenated Aliphatics |
|
|
|
|
|
Highly chlorinated |
Very frequent |
Emerging |
Cometabolized by anaerobic microbes; cometabolized by aerobes in special cases |
Forms nonaqueous-phase liquid |
|
Less chlorinated |
Very frequent |
Emerging |
Aerobically biodegradable under a narrow range of conditions; cometabolized by anaerobic microbes |
Forms nonaqueous-phase liquid |
|
Halogenated Aromatics |
|
|
|
|
|
Highly chlorinated |
Common |
Emerging |
Aerobically biodegradable under a narrow range of conditions; cometabolized by anaerobic microbes |
Sorbs strongly to subsurface solids; forms nonaqueous phase—solid or liquid |
|
Less chlorinated |
Common |
Emerging |
Readily biodegradable under aerobic conditions |
Forms nonaqueous phase—solid or liquid |
|
Polychlorinated Biphenyls |
|
|
|
|
|
Highly chlorinated |
Infrequent |
Emerging |
Cometabolized by anaerobic microbes |
Sorbs strongly to subsurface solids |
|
Less chlorinated |
Infrequent |
Emerging |
Aerobically biodegradable under a narrow range of conditions |
Sorbs strongly to subsurface solids |
|
Nitroaromatics |
Common |
Emerging |
Aerobically biodegradable; converted to innocuous volatile organic acids under anaerobic conditions |
|
|
Metals (Cr, Cu, Ni, Pb, Hg, Cd, Zn, etc.) |
Common |
Possible |
Solubility and reactivity can be changed by a variety of microbial processes |
Availability highly variable—controlled by solution and solid chemistry |
bioremediation. In general, the least biodegradable contaminants are those with the strongest tendency to sorb.
The table groups contaminants into five classes: petroleum hydrocarbons and derivatives, halogenated aliphatics, halogenated aromatics, nitroaromatics, and metals. Each class is discussed in more detail below.
Petroleum Hydrocarbons and Derivatives
Petroleum hydrocarbons and their derivatives are naturally occurring chemicals that humans have exploited for a wide range of purposes, from fueling engines to manufacturing chemicals. The representative types of petroleum hydrocarbons and derivatives listed in Table 2-1 are gasoline, fuel oil, polycyclic aromatic hydrocarbons (PAHs), creosote, ethers, alcohols, ketones, and esters. Each of these chemicals has a broad range of industrial applications. For example, PAHs are released when crude oil is refined and from the manufacture of petroleum products such as plastics. Creosote is used in wood preservatives. Ethers, esters, and ketones are components of chemicals ranging from perfumes, to anesthetics, to paints and lacquers, to insecticides.
Gasoline, fuel oil, alcohols, ketones, and esters have been successfully bioremediated at contaminated sites via established bioremediation procedures. Gasoline, in particular, has been the focus of substantial biodegradation and bioremediation research. The gasoline components benzene, toluene, ethylbenzene, and xylene (together known as BTEX) are relatively easy to bioremediate for several reasons:
-
They are relatively soluble compared to other common contaminants and other gasoline components.
-
They can serve as the primary electron donor for many bacteria widely distributed in nature.
-
They are rapidly degraded relative to other contaminants shown in Table 2-1.
-
The bacteria that degrade BTEX grow readily if oxygen is available.
Under many circumstances, ether bonds show considerable chemical stability and therefore resist microbial attack. High-molecular-weight compounds such as creosotes and some PAHs are also only slowly metabolized—partly as a result of their structural complexity, low solubility, and strong sorptive characteristics. Thus, bioremediation techniques for these latter classes of petroleum derivatives are still emerging.
Halogenated Compounds
Halogenated compounds are compounds with halogen atoms (usually chlorine, bromine, or fluorine) added to them in place of hydrogen atoms. Although halogenated organic compounds have been found in nature, these are not significant compared to the synthetic chemicals listed in the middle portion of Table 2-1. When halogen atoms are introduced into organic molecules, many properties, such as solubility, volatility, density, and toxicity, change markedly. These changes confer improvements that are valuable for commercial products, such as solvents used for degreasing, but they also have serious implications for microbial metabolism. The susceptibility of the chemicals to enzymatic attack is sometimes drastically decreased by halogenation, and persistent compounds often result. Consequently, bioremediation technologies for these chemicals are still emerging.
There are two broad classes of halogenated chemicals: halogenated aliphatics and halogenated aromatics.
Halogenated Aliphatics
Halogenated aliphatic compounds are compounds built from straight chains of carbon and hydrogen with varying numbers of hydrogen atoms replaced by halogen atoms. Halogenated aliphatics are effective solvents and degreasers and have been widely used in manufacturing and service industries, ranging from automobile manufacturing to dry cleaning. Some highly chlorinated representatives of this class, such as tetrachloroethene, are completely resistant to attack by aerobic microbes but are susceptible to degradation by special classes of anaerobic organisms. In fact, recent evidence shows that certain anaerobes can completely dechlorinate tetrachloroethene to the relatively nontoxic compound ethene, which is readily decomposed by aerobic microbes.
As the degree of halogenation in aliphatics diminishes, susceptibility to aerobic metabolism increases. The less halogenated ethenes may be destroyed by cometabolism when certain aerobic microbes are supplied with methane, toluene, or phenol, as described earlier in this chapter. Thus, a common treatment rationale for the highly chlorinated aliphatics is to remove the chlorine atoms anaerobically, with methanogens, and then complete the biodegradation process using aerobic cometabolism. However, routine procedures for implementing anaerobic/aerobic sequencing to bioremediate sites contaminated with chlorinated aliphatic materials are not yet established at the commercial scale.
Halogenated Aromatics
Halogenated aromatics are compounds built from one or more halogen-bearing benzene rings. Examples include chlorinated benzenes, used as solvents and pesticides; pentachlorophenol, used in fungicides and herbicides; and polychlorinated biphenyls (PCBs), once widely used in electrical transformers and capacitors. The aromatic benzene nucleus is susceptible to aerobic and anaerobic metabolism, although the latter occurs relatively slowly. Overall, however, the presence of halogen atoms on the aromatic ring governs biodegradability. A high degree of halogenation may prevent aromatic compounds from being aerobically metabolized, as is the case for highly chlorinated PCBs. However, as discussed above for the aliphatic compounds, anaerobic microbes can remove chlorine atoms from the highly halogenated aromatics. As the halogen atoms are replaced by hydrogen atoms, the molecules become susceptible to aerobic attack. Thus, a possible bioremediation scenario for treating soils, sediments, or water contaminated with halogenated aromatic chemicals is anaerobic dehalogenation followed by aerobic destruction of the residual compounds. It should be noted, however, that when certain substituent groups accompany the halogens on the aromatic ring, aerobic metabolism may proceed rapidly, as is the case for pentachlorophenol.
Nitroaromatics
Nitroaromatics are organic chemicals in which the nitro group (–NO2) is bonded to one or more carbons in a benzene ring. A familiar example is trinitrotoluene (TNT), which is used in explosives. Laboratory research has shown that both aerobic and anaerobic microbes can convert many of these compounds to carbon dioxide, water, and mineral components. Recent field tests have confirmed that anaerobic microbes can transform nitroaromatics to innocuous volatile organic acids, like acetate, which then may be mineralized.
Metals
The metals listed in Table 2-1 are common pollutants inadvertently released during the manufacture of various industrial products, from steel to pharmaceuticals. Microorganisms cannot destroy metals, but they can alter their reactivity and mobility. Schemes for using microorganisms to mobilize metals from one location and scavenge the metal from another location have been applied to mining operations. Microbes produce acids that can leach metals, like cop-
per, from low-grade ores. This same approach should be feasible for bioremediation purposes, but it has not been proven. Microorganisms can also demobilize metals by transforming them to a form that precipitates (see "How Microbes Demobilize Contaminants," earlier in this chapter).
ENVIRONMENTS AMENABLE TO BIOREMEDIATION
The suitability of a site for bioremediation depends not only on the contaminant's biodegradability but also on the site's geological and chemical characteristics. The ideal site for in situ bioremediation is one that is as controllable and easy to interpret as the small, laboratory-incubated flask experiments used to test pollutant biodegradation. The site most amenable to bioremediation, like the lab flask, has favorable chemical characteristics and relatively uniform geology. Site characteristics are rarely ideal, however. Each site is a unique section of landscape that presents an unpredictable variety of environmental conditions. Properties such as soil type, geological strata, and water chemistry vary not only from site to site but also within an individual site. Furthermore, site complexity and lack of site data commonly obscure the true type and severity of the contamination. It is normal in implementing bioremediation—or any other cleanup technology—to revise cleanup plans continually as more information becomes available during the remediation.
It is important to realize that no single set of site characteristics will favor bioremediation of all chemical contaminants. For example, certain compounds can only be metabolized under anaerobic conditions, while metabolism of others requires oxygen. When the degradation mechanisms for two co-occurring contaminants are mutually exclusive, difficult choices need to be made or sequential treatment schemes need to be devised.
Two Types of Bioremediation: Intrinsic and Engineered
A principal concern in determining whether the site environment is appropriate for in situ bioremediation is the type of bioremediation to be implemented. Bioremediation can be grouped into two broad types: intrinsic and engineered. Figure 2-2 illustrates the differences between the two.
Intrinsic bioremediation manages the innate capabilities of naturally occurring microbial communities to degrade environmental pollutants without taking any engineering steps to enhance the process. It differs from no-action alternatives in that it requires thorough docu-
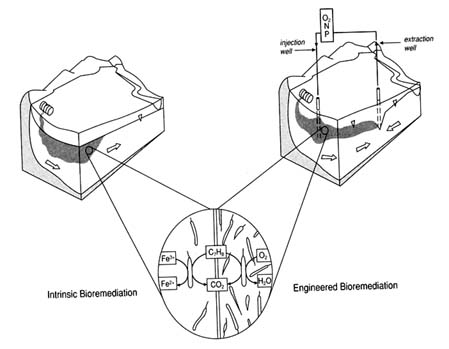
FIGURE 2-2 The differences between intrinsic and engineered bioremediation. In intrinsic bioremediation, left, native subsurface microbes degrade the contaminants without direct human intervention. In the close-up view, the native microbes use iron (Fe3+) as an electron acceptor to degrade toluene (C7H8), a representative contaminant, and convert it to carbon dioxide (CO2). In engineered bioremediation, right, oxygen (O2), nitrogen (N), and phosphorus (P) are circulated through the subsurface via an injection and extraction well to promote microbial growth. In this case the microbes use oxygen as the electron acceptor, converting it to water (H2O) as they degrade the toluene. Note that, as pictured in the close-up view, considerably more microbes are present in the engineered bioremediation system than in the intrinsic system. Consequently, contaminant degradation occurs more quickly in the engineered system. Intrinsic bioremediation requires extensive monitoring to ensure that the contaminant does not advance more quickly than the native microbes can degrade it.
mentation of the role of native microorganisms in eliminating contaminants via tests performed at field sites or on site-derived samples of soil, sediment, or water. Furthermore, the effectiveness of intrinsic bioremediation must be proven with a site-monitoring regime that routinely analyzes contaminant concentrations. The terms "natural," "passive," and ''spontaneous" bioremediation and "bioattenuation"
|
BOX 2-2 INTRINSIC BIOREMEDIATION OF A CRUDE OIL SPILL— BEMIDJI, MINNESOTA In August 1979 an oil pipeline burst near Bemidji, Minnesota, spilling approximately 100,000 gallons of crude oil into the surrounding ground water and soil. In 1983 researchers from the U.S. Geological Survey (USGS) began monitoring the site carefully to determine the crude oil's fate. They discovered that, although components of the crude oil initially migrated a short distance, native microorganisms capable of degrading the oil have prevented widespread contamination of the ground water. The microbes went to work with no human intervention, showing that intrinsic bioremediation can be effective for containing spills of petroleum products. In the years following the spill, portions of the crude oil dissolved in the flowing ground water and moved 200 m from the original spill site. The undissolved crude oil itself migrated 30 m in the direction of ground water flow, and crude oil vapors moved 100 m in the overlying soil. However, the USGS researchers' detailed monitoring shows that the contaminant plume has not advanced since 1987, and the researchers have attributed this halt to intrinsic bioremediation. Three types of evidence convinced the researchers that intrinsic bioremediation was largely responsible for containing the crude oil. First, modeling studies showed that if the oil were not biodegradable, the plume would have spread 500 to 1200 m since the spill (see Figure 2-3). Second, the concentrations of Fe2+ and CH4 increased dramatically in the portion of the contaminant plume where oxygen was not present—evidence of an increase in activity by anaerobic organisms capable of degrading certain crude oil components, such as toluene. Third, concentrations of the crude oil components benzene and ethylbenzene, which are susceptible to aerobic degradation but less susceptible to anaerobic degradation, remained relatively stable in the anaerobic portion of the plume but decreased dramatically at the outer edges of the plume, where mixing with oxygenated water allowed aerobic degradation to occur. The evidence from this site shows that, in hydrologic settings where intrinsic bioremediation rates are fast relative to hydrologic transport rates, native microbes can effectively confine contaminants to near the spill source without further human intervention. However, it is essential for such sites to have detailed, long-term monitoring plans to ensure that the contamination is, indeed, contained. At some sites, the rates of hydrologic transport outpace the rates of intrinsic bioremediation, and additional engineering steps to contain or remove the contamination will be necessary. |
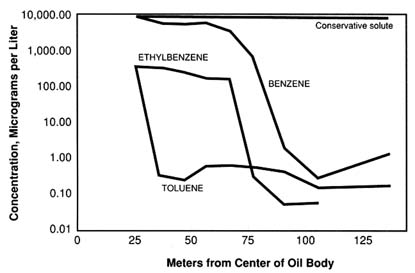
FIGURE 2-3 Concentrations of the crude oil components toluene, ethylbenzene, and benzene at various distances from the center of the Bemidji, Minnesota, oil spill. These concentrations have remained relatively stable at the levels shown here since 1987. Note that the contaminant concentrations are very high near the center of the plume but that they drop dramatically within 100 m of the spill. If the contaminants were not biodegradable, this concentration drop would not occur, and the contamination would have spread much farther, as shown by the hypothetical concentration of a nondegradable solute (called a "conservative solute") pictured here. Thus, at this site, intrinsic bioremediation has effectively confined the contamination to a small region.
SOURCE: Baedecker et al. (in press), reprinted with permission.
have also been used to describe intrinsic bioremediation. Box 2-2 describes a Minnesota site where researchers documented that intrinsic bioremediation prevented the further spreading of crude oil contamination.
Engineered bioremediation is the acceleration of microbial activities using engineered site-modification procedures, such as installation of wells to circulate fluids and nutrients to stimulate microbial growth. The principal strategy of engineered bioremediation is to isolate and control contaminated field sites so that they become in situ bioreactors. Other terms used to describe engineered bioremediation include "biorestoration" and "enhanced bioremediation."
As summarized in Box 2-3 and described below, the site conditions that influence a bioremediation project's success differ for intrinsic and engineered bioremediation.
Site Conditions for Engineered Bioremediation
Because engineered bioremediation uses technology to manipulate environmental conditions, the natural conditions are less important for engineered than for intrinsic bioremediation. For engineered bioremediation, the critical property influencing success is how well the subsurface materials at the site transmit fluids. For systems that circulate ground water, the hydraulic conductivity (the amount of ground water that moves through a unit section of the subsurface in a given time) in the area containing the contaminant should be on the order of 10-4 cm/s or greater (the precise value is site specific). For systems that circulate air, the intrinsic permeability (a measure of how easily fluids flow through the subsurface) should be greater than 10-9 cm2. For both types of systems, the contaminated area will be much more difficult to treat if it has crevices, fractures, or other irregularities that allow channeling of fluids around contaminated material. Land near river deltas, floodplains of large rivers, and areas where sand and gravel aquifers were formed from the melting of glaciers can be uniform over large areas. On the other hand, braided stream channels can contain a substantial number of irregularities that complicate bioremediation system design.
At high concentrations, contaminants (including petroleum products and chlorinated solvents) that form a nonaqueous-phase liquid can exclude water or air from pores in the subsurface. Nonaqueous-phase liquids restrict access of the remedial fluids and gases and complicate engineered bioremediation. In most cases such contaminants at residual concentrations of less than 8000 to 10,000 mg/kg of soil do not significantly affect water or air flow, because at this level
|
BOX 2-3 SITE CHARACTERISTICS THAT FAVOR IN SITU BIOREMEDIATION Engineered bioremediation requires installing wells and other engineering systems to circulate electron acceptors and nutrients that stimulate microbial growth. Key site characteristics for engineered bioremediation are:
Intrinsic bioremediation destroys contaminants without human intervention, as the population of native microbes capable of degrading the contaminant increases naturally. The process requires thorough site monitoring to demonstrate that contaminant removal is occurring. Key characteristics of sites amenable to intrinsic bioremediation are:
|
the contaminants are essentially nonmobile and occupy much less pore space than the water. The specific concentration value at which nonaqueous-phase contaminants begin interfering with fluid circulation varies depending on the contaminant (the value is higher for denser contaminants) and the soil.
Site Conditions for Intrinsic Bioremediation
If intrinsic bioremediation is the only option, ambient site conditions must be accepted as constraints for meeting cleanup goals, because intrinsic bioremediation by definition occurs without adding anything to the site. Only a fraction of the contaminated sites offer naturally occurring hydrogeochemical conditions in which microorganisms can degrade contaminants quickly enough to prevent them from spreading without human intervention.
The critical site characteristic for intrinsic bioremediation is predictability of ground water flow in time and space. Predictable water flow is essential for determining whether the native microbes will be able to act in all the places where the contaminant might travel in all seasons and for determining whether the microbes can act quickly enough to prevent the contamination from spreading with the flowing ground water. The hydraulic gradient and trajectory of ground water flow should be consistent through the seasons and from year to year. To ensure predictability of flow, the fluctuation in the water table should not vary more than about 1 m, although the precise number is site specific. In addition, the trajectory of regional flow should not change by more than about 25 degrees from the primary flow direction. These circumstances are more likely in upland landscapes with humid, temperate climates. In contrast, contaminant plumes in estuaries or the flood plains of large rivers often behave unpredictably.
Another valuable characteristic is the presence in the aquifer of minerals such as carbonates to buffer pH changes that would otherwise result from biological production of carbon dioxide or other acids or bases. Carbonates in the aquifer matrix can be expected when limestone or dolomite are the parent material or when limestone dust or sand occurs in glacial outwash. Carbonates can also occur as shell material in beach deposits.
Intrinsic bioremediation is more extensive when the ambient ground water surrounding the spill has high concentrations of oxygen or other electron acceptors. The importance of ambient concentrations of nitrate, sulfate, and ferric iron as potential electron acceptors that can stimulate microbial growth in the absence of oxygen is too often
ignored. Most ground waters have more nitrate and sulfate than oxygen. This is particularly true in agricultural areas that have been overfertilized and in arid regions where gypsum is dissolved in the ground water.
The concentration of electron acceptors required to ensure bioremediation varies with the contaminant's chemical characteristics and the amount of contamination. More soluble contaminants and large contaminant sources require larger electron acceptor concentrations. Natural ground water circulation conditions at the site also influence the required amount of electron acceptor. The circulation patterns should provide enough mixing between contaminated water and surrounding water that the organisms never consume all of the electron acceptors within the bioremediation region. If the electron acceptor supply becomes depleted, bioremediation will slow or cease.
Also necessary for intrinsic bioremediation is the presence of the elemental nutrients that microbes require for cell building, especially nitrogen and phosphorus. Although nutrients must be present naturally for intrinsic bioremediation to proceed, the quantity of nutrients required is much less than the quantity of electron acceptors. Therefore, a nutrient shortage is less likely to limit intrinsic bioremediation than an inadequate electron acceptor supply.
Impact of Site Heterogeneity on Bioremediation
Observation of the geological cross section at a typical excavation site reveals a complex patchwork of layers, lenses, and fingers of different materials. Indeed, two overriding characteristics of the subsurface are that it is intricately heterogeneous and difficult to observe. The patterns of variability of the properties that govern the flow of water and the transport of chemicals are so complex that it is not possible to predict these properties quantitatively or even to interpolate them with accuracy from sparse observations. In practice, estimation of subsurface hydrogeochemical properties depends on site-specific measurements from water or soil samples and well tests. However, the inherent unobservability of the system means that there is usually insufficient information to characterize the site with certainty.
A consequence of this complexity and heterogeneity, in combination with the poor observability of the subsurface, is that completely reliable prediction of chemical transport and fate is out of reach in most real-world cases. In evaluating a proposed intrinsic or engineered bioremediation scheme, one must consider how it may per-
form under variable and not perfectly known conditions. A scheme that works optimally under specific conditions but poorly otherwise may be inappropriate for in situ bioremediation.
FURTHER READING
While this chapter has briefly reviewed the principles underlying successful bioremediation, the references listed in Table 2-2 provide more thorough coverage of the key disciplines related to bioremediation. The list is not exhaustive. The references it provides were selected to represent the diversity of attitudes, perspectives, and paradigms that are pertinent to understanding bioremediation.
TABLE 2-2 Recommended Sources for Obtaining In-Depth Information About the Disciplines Pertinent to Bioremediation
|
Discipline |
Reference |
Synopsis |
|
Environmental microbiology |
Chapelle, F. H. 1993. Ground-Water Microbiology and Geochemistry. New York: John Wiley & Sons. |
Reviews how the growth, metabolism, and ecology of microorganisms affect ground water chemistry in both pristine and chemically contaminated aquifer systems. |
|
|
Gibson, D. T. 1984. Microbial Degradation of Organic Compounds. New York: Marcel Dekker. |
Provides a detailed survey of how microorganisms metabolize organic compounds. Each chapter, written by a different expert, focuses on a different class of compounds. |
|
|
Madsen, E. L. 1991. Determining in situ biodegradation: facts and challenges. Environmental Science and Technology 25:1661-1673. |
Reviews principles and limitations of environmental microbiology as they apply to determining in situ biodegradation. Proposes useful approaches, especially as applicable to academic research. |
|
|
Madsen, E. L., and W. C. Ghiorse. 1993. Ground water microbiology: subsurface ecosystems processes. Pp. 167-213 in Aquatic Microbiology: An Ecological Approach, T. Ford, ed. Cambridge, Mass.: Blackwell Scientific Publishers. |
Reviews major concepts and methodological developments that determine our understanding of microorganisms and the processes they carry out in subsurface ecosystems. |
|
|
VanLoosdrecht, M. C. M., J. Lyklema, W. Norse, and A. J. B. Zehnder. 1990. Influences of interfaces on microbial activity. Microbiologic Reviews 54:75-87. |
Provides a critical and cross-disciplinary review of how surfaces affect microbial activity and substrate availability. |
|
Hydrobiology |
Dominic, P. A., and F. W. Schwartz. 1990. Physical and Chemical Hydrogeology. New York: John Wily & Sons. |
Reviews principles and practice of ground water hydrology, with emphasis on environmental applications. |
|
|
Freeze, R. A., and J. A. Cherry. 1979. Groundwater. Englewood Cliffs, NJ.: Prentice-Hall. |
Provides a comprehensive presentation of the theory, principles, and practice of hydrogeology. |
|
Environmental engineering |
McCarty, P. L. 1988. Bioengineering issues related to in situ remediation of contaminated soils and groundwater. Pp. 143-162 in Environmental Biotechnology: Reducing Risks from Environmental Chemicals Through Biotechnology, G. S. Omenn, ed. New York: Plenum Press. |
Discusses engineering issues relevant to in situ bioremediation. |
|
|
Rittmann, B. E., A. J. Valocchi, E. Seagren, C. Ray, and B. Wrenn. 1992. A Critical Review of In Situ Bioremediation. Chicago: Gas Research Institute. |
Provides a comprehensive critical review of the microbiological, engineering, and institutional possibilities and restrictions for in situ bioremediation. |
|
Statistics |
ASCE Task Committee. 1990. Review of geostatistics in geohydrology, parts I and II. ASCE Journal of Hydraulic Engineering 116(5):612-658. |
Discusses geostatistical techniques and how they can assist in the solution of estimation problems, including interpolation, averaging, and network design. |
|
Discipline |
Reference |
Synopsis |
|
Contaminant fate and transport |
Fetter, C. W. 1993. Contaminant Hydrogeology. New York: Macmillan Publishing Co. |
Gives a comprehensive treatment of ground water contaminants and their transport, retardation, and transformation in the subsurface. Particularly strong on the chemistry of organic contaminants. |
|
|
National Research Council. 1990. Ground Water Models. Washington, D.C.: National Academy Press. |
Provides a thorough review of the theory, use, limitations, and applications of computer modeling applied to the subsurface. |
|
|
Sahwney, B. L., and K. Brown, eds. 1989. Reactions and Movement of Organic Chemicals in Soils. Madison, Wisc.: Soil Science Society of America. |
Provides a thorough review of sorption-desorption behavior of contaminants in soil. Includes chapters on contaminant movement and transformation. |
|
Commercial application |
Hinchee, R. E., and R. F. Olfenbuttel, eds. 1992. In Situ Bioreclamation: Applications and Investigations for Hydrocarbon and Contaminated Site Remediation. Boston: Butterworth-Heinemann. |
Papers in this compendium discuss field and research studies of in situ and on-site bioremediation. |































