A Regulator's Perspective on In Situ Bioremediation
John M. Shauver
Michigan Department of Natural Resources
Lansing, Michigan
SUMMARY
Bioremediation, like any technology applied to clean up a contaminated site, must first be approved by government regulators who ultimately must agree that the technology has a reasonable chance to reduce the contaminant(s) at the site to acceptable levels. This paper describes the information that regulators need to make their decision. Basically, this information comprises descriptions of the site, the specific cleanup process, and the overall approach to site cleanup. The paper also answers the questions of who, what, when, where, and how in the context of bioremediation on the basis of my 24 years of experience as a regulator.
INTRODUCTION
During the past 20 years, various companies and individuals have developed or claim to have developed biological treatment processes that could cleanup various wastes generated by human activities. These wastes include polychlorinated biphenyls (PCBs), crude oil, refined crude oil products, crude oil wastes, and DDT, to name a few. One problem that the proponents of such treatment technology face is state and federal regulations. It is often hard for the regulated community to understand what is required to ensure that the regulator will approve a proposed treatment process.
This paper describes what a regulator looks for in a proposal to cleanup (remediate) a site to legal standards. The guidance provided here is a condensation of the requirements of the many statutes and regulations used by the Michigan Department of Natural Resources. The paper reflects my view, after 24 years as a regulator, of what information needs to be routinely provided to evaluate a cleanup technology before it is applied to a particular site. Complex sites with unique or unusual features may have to be characterized in greater detail before a cleanup technology can be chosen. Also, the regulated community (potentially responsible parties) must realize that the cleanup process itself is but one facet of the overall site cleanup. To gain approval for implementation of a cleanup process, the responsible party should supply information that includes:
-
a description in three dimensions of the site and of the type and extent of contamination,
-
a detailed description of the cleanup process(es) to be applied to the site, and
-
a detailed description of the approach to overall site cleanup.
SITE DESCRIPTION
The site description should specifically identify the types and amounts of chemical(s) released to the soil and ground water and other phases of the site environment. The description should also include estimates of the rate of movement of the contaminants through the various phases of the environment and of where they are likely to end up. The regulator's response to a given situation depends strongly on the rate of transport and the likely fate of the contaminants.
The site is the three-dimensional area contaminated by the chemicals that have been released. The site is not limited to legal property boundaries. In fact, it usually involves more than one property owner, and the owners may not all be responsible for the contamination. The site description should also include the vertical, horizontal, and lateral extent of contamination, which includes:
-
soil type(s), permeability, porosity of the soils and/or aquifer, and concentrations of contaminants in soil;
-
if appropriate, depth to ground water, rate and direction of flow, concentrations of contaminants in ground water, and concentrations of naturally occurring or other compounds (inorganic or organic) that may interfere with the treatment process;
-
if appropriate, concentrations of contaminants in the air, prevailing wind direction, and nearest human receptors; and
-
concentrations of contaminants in surface waters and sediments.
In any site description the regulator will place great emphasis on identifying the location of the source(s) of contamination. Removal of these sources, or hot spots (identified by an adequate site investigation), is the most effective way to limit migration of chemicals off site. In addition, elimination of the source of the contamination as early as possible is one of the most cost-effective ways to limit future cleanup costs.
A site description should also describe the process that caused the release. This is important because the regulator is required to determine the full extent of the type of contamination at the site. If the material released is gasoline, for example, it is very important to know whether it is leaded or unleaded and whether it came from a hole in a tank; an overfilled tank; or faulty pipes, valves, or other fittings. If the release is described as crude oil, it is important to know if brine, condensate, or other materials are present as well. The description of the cause of the release allows the regulator to identify its source and thus the most highly contaminated areas of the site.
PROCESS DESCRIPTION
The responsible party should provide a detailed description of the treatment process to be used. The engineer who is accustomed to describing an activated carbon process should provide the same level of detail for a biological process. The description should show how the process chosen will contain, destroy, or remove the contaminants to meet legal standards. If biological treatment is chosen, the regulator must be given data that show the ability of the organisms present in or added to the contaminated area to safely and effectively treat the chemical(s) on the site.
When living organisms are proposed to cleanup a site, the regulator expects to see a detailed description of the organisms' requirements for oxygen, nutrients, temperature, moisture, and pH. We must be sure the organism will thrive long enough to treat the chemicals to legal cleanup standards. In addition, if an anaerobic treatment scenario (such as one using iron or sulfur) is proposed, the regulator needs to know that native microbes are capable of the proposed metabolism and that ambient or added nutrients will be available in amounts likely to allow effective treatment but not likely to cause rapid plugging of the delivery wells and/or the soils.
We must be able to determine that the use of bacteria in the soils and ground water (if unsuccessful) will not prevent other treatment technologies from being applied. Use of organisms without adequate information or controls in the past has resulted in severe plugging problems in ground water monitoring wells and/or the aquifer itself. Such loss of permeability not only prevents delivery of the nutrients and oxygen necessary to sustain biological activity to cleanup the soils or aquifer but may seriously hamper use of other technologies.
OVERALL SITE CLEANUP DESCRIPTION
A very important part of the description of the overall approach to site cleanup is the method(s) to be used to prevent movement of the contaminants farther off site through the soil or to or through the ground water or other medium. Containment to prevent further spread of the chemicals is as important in the regulator's mind as any other part of the cleanup. The regulator needs a complete description of the steps to be taken to prevent further movement of the chemicals through the soil, air, ground water, or surface water.
For example, contaminated ground water may be moving down-gradient at 15 cm per day. Purge and capture wells would have to be installed to pump this contaminated ground water back upgradient to the treatment system to prevent further movement of the contaminants off site. If the water is discharged to the ground surface via an infiltration bed, and if it contains volatile organic chemicals (VOCs) that would be released, the responsible party needs to demonstrate adequate control of VOC discharge to the air.
The description also should cover equipment necessary to achieve the cleanup. With biological treatment systems, equipment may be needed for adjustment of the pH of the ground water, removal of iron or other interfering substances before treatment, oxygen/air delivery or oxygen reduction, and identification and monitoring of tracers and nutrients added. For example, if the proposal is to use aerobic bacterial decomposition of the contaminant(s) and the contamination exists to a depth of 15 m below ground water surface in soils with a permeability of 10-7 cm/s, the regulator will be interested in how the responsible party intends to deliver oxygen or air and related nutrients to the organisms.
Also necessary is a description of the monitoring procedures to be used to show that the cleanup system is operating properly. When using biological systems, the responsible party must show that the organisms are, in fact, doing the job. For example, if an aerobic process is used, the level of oxygen in and around the plume of
contamination in the ground water will have to be monitored to ensure that the organisms have sufficient oxygen to decompose the chemicals in the ground water. This type of monitoring may be in addition to or in place of simply monitoring for the contaminant itself. In addition, if nutrients are added, they may also be contaminants and require monitoring. Nitrate, for example, is a chemical of concern that may have to be added to a biological treatment system as a nutrient or may be proposed as an electron acceptor in an anaerobic treatment process. In Michigan the drinking water supplies may not contain more than 5 mg/1 of nitrate. Therefore, if nitrate is used, the regulatory agency will require that it be monitored in addition to other monitoring requirements.
CONCLUSION
A regulator looks for the data necessary to determine that a proposed treatment technology, if properly installed and operated, will reduce the contaminant concentrations in the soil and water to legally mandated limits. In this sense the use of biological treatment systems calls for the same level of investigation, demonstration of effectiveness, and monitoring as any conventional system.
An Industry's Perspective on Intrinsic Bioremediation
Joseph P. Salanitro
Shell Development Company
Houston, Texas
SUMMARY
Laboratory and field evidence is now sufficient to demonstrate that soil microorganisms in aquifers are responsible for a significant portion of the attenuation of aromatic compounds—benzene, toluene, ethylbenzene, and xylenes (BTEX)—from fuel spills to the subsurface environment. Most subsoils contain indigenous microbes that can biodegrade low levels of BTEX (ppb to low ppm), given enough dissolved oxygen in the ground water. With adequate site characterization, analysis, and monitoring, this type of intrinsic bioremediation can shrink plumes and control the migration of hydrocarbons. In situ biodegradation processes, properly monitored, should be considered practical, cost-effective alternatives for managing low-risk, hydrocarbon-contaminated ground waters that are unlikely to affect drinking water wells.
PROBLEM IDENTIFICATION
Accidental releases of fuels from underground storage tanks over the past 10 to 20 years have been responsible for the presence of hydrocarbons, mainly water-soluble aromatic compounds (benzene, toluene, ethylbenzene, and xylenes, or BTEX), in aquifers. In most states, government agencies have required the regulated industry to
restore ground water at such sites to drinking water (health) standards—for example, 1 to 5 parts per billion (ppb) benzene (Marencik, 1991). Corrective actions taken include removal of free product and contaminated soil, site assessments (soil borings and monitoring wells), and determination of the extent of contamination in subsoils and ground water. For a majority of the sites, the ground water hydrocarbon (BTEX) levels are low, on the order of 100 to 1000 ppb. Higher levels are often associated with soil and ground water samples taken near the spill area.
Technologies that have been used to control migration of hydrocarbon plumes or to remediate subsurface soils include soil venting (vadose zone) and sparging (saturated zone) and ground water extraction and treatment (pump and treat) (Mackay and Cherry, 1989; Newman and Martinson, 1992). In addition to these operations, extensive soil and ground water surveying must be done to assess the extent of contamination and the effectiveness of the cleanup method. Current estimates for site assessment and in situ or ex situ restoration of subsoils and ground water to health standard criteria indicate that these operations may be costly ($500,000 to $2 million per site) and not cost-effective and that they may not achieve restoration within time periods of years or decades (Travis and Doty, 1990).
Many contaminated ground waters (e.g., at fuel service station sites) are in shallow aquifer zones, are not used directly for human consumption, and do not even affect downstream drinking water wells. Furthermore, good field evidence indicates that plumes in these ground waters reach a stable condition in which contaminants of concern (BTEX) are biodegraded at some rate by indigenous hydrocarbon-utilizing soil bacteria. This type of unassisted in situ biodegradation has been termed natural attenuation or intrinsic bioremediation.
Industry has been confronted with very large operating and cleanup costs for subsoils and ground water in the restoration of underground fuel storage tank sites to drinking water standards. Where thorough site characterization warrants its use, intrinsic bioremediation offers a way to manage non-migrating or shrinking BTEX plumes in low-risk aquifers that do not affect drinking water wells. Evidence that this natural process is occurring has been obtained from laboratory and field observations.
EVIDENCE FOR INTRINSIC BIOREMEDIATION OF AROMATIC HYDROCARBONS IN
It is now widely recognized that the most significant factor in the time-dependent decrease of BTEX compounds in aquifers is degrada-
tion by soil microbes. Studies reported for laboratory slurry microcosms of subsoil and ground water show that microbes in many soils inherently biodegrade aromatic hydrocarbons at varying rates (5 to 50 percent per day) (Barker et al., 1987; Chiang et al., 1989; Gilham et al., 1990; Hutchins et al., 1991; Kemblowski et al., 1987; Major et al., 1988; and Thomas et al., 1990). These biodecay rates are usually first order; they occur with low levels of hydrocarbon (50 to 10,000 ppb); and they are rapid with adequate dissolved oxygen (e.g., 2 to 3 mg oxygen per milligram of hydrocarbon). Field estimates of hydrocarbon biodegradation rates calculated from fate and transport models using data from upstream and downstream monitoring wells have shown that plume BTEX compounds usually decrease at rates of 0.5 to 1.5 percent per day (Barker et al., 1987; Chiang et al., 1989; Kemblowski et al., 1987; Rifai et al., 1988; and Wilson et al., 1991). Laboratory and field data suggest that in a well-studied sandy aquifer a minimum, or threshold, level (≥1 to 2 ppm) of dissolved oxygen may be required to sustain hydrocarbon degradation (Chiang et al., 1989).
It should be emphasized that laboratory and field data have confirmed that all BTEX compounds can be biodegraded under aerobic conditions (dissolved oxygen in ground water) in aquifer subsoils in which oxygen is the terminal electron acceptor. Soil microcosm experiments or enrichments of aquifer material have shown that toluene and xylenes can be degraded by microbes under iron-reducing, denitrifying, and sulfate-reducing (anaerobic or very low dissolved oxygen) conditions when ferric ion (Fe3+), nitrate ion (NO3-), and sulfate ion (SO42), respectively, serve as electron acceptors (Beller et al., 1992; Edwards et al., 1992; Hutchins, 1991; Lovley et al., 1989; and Zeyer et al., 1986). Field evidence is insufficient, however, to demonstrate that BTEX is biodegraded under anaerobic conditions in an aquifer.
LEVELS OF INTRINSIC ATTENUATION IN GROUND WATER
Evidence from site characterization, ground water monitoring, and modeling at field sites suggests that there may be two levels of intrinsic bioremediation. Figure 1 shows these aspects of a plume in which one is stabilized (A) and the other is reducing (B) in size and extent of contamination. In Figure 1 A the hydrogeological features indicate that ground water velocity (also BTEX and dissolved oxygen) and recharge are slow because of low permeability of the aquifer subsoil. Dissolved oxygen is low within the plume (e.g., <1 ppm). Oxygen is detected in monitoring wells at the edges and is respon-
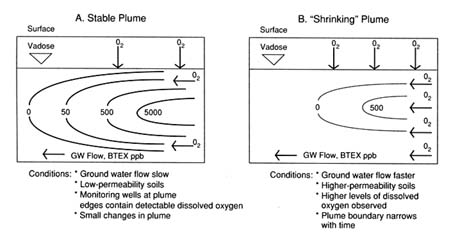
FIGURE 1 Levels of intrinsic bioremediation in aquifers.
sible for the biodegradation of low levels (ppb) of BTEX. Another indirect indicator of soil microbial degradation in aquifers low in dissolved oxygen may be the presence of dissolved ferrous ion (Fe2+) above background well levels. It is known that various ferric oxides in soil can be used (as electron acceptors) by anaerobic iron-reducing bacteria to completely metabolize some aromatic compounds, such as toluene and phenol (Lovley et al., 1989). Therefore, when dissolved oxygen is low, ferric iron may substitute for oxygen, and this biodegradation process may result in elevated concentrations of ferrous ion in ground water.
At the next level of intrinsic bioremediation, plumes noticeably shrink over time, with significant decreases in shape and extent (Figure 1B). This type of plume behavior is observed in aquifers that usually are more permeable (e.g., sandy subsoils), that exhibit higher ground water velocities, and that are higher in dissolved oxygen (higher aquifer reaeration rate) in many monitoring wells. Published examples of plumes undergoing significant intrinsic attenuation of BTEX are those at the Borden (Barker et al., 1987), Traverse City (Rifai et al., 1988; Wilson et al., 1991), and Michigan gas plant (Chiang et al., 1989) sites. Monitoring wells at the periphery show significantly higher dissolved oxygen (e.g., ≥1 ppm) and lower BTEX concentrations, which are consistent with a predominantly biodegradation-driven mass reduction in the aquifer. Examination of monitoring well BTEX levels within the flow path of upstream and downstream segments may also match the biodecay rates (about 1 percent per day) calculated from fate and transport models for BTEX and dissolved oxygen
(e.g., BIOPLUME II, Rifai et al., 1988). These plumes may initially shrink (narrow) in the longitudinal direction because the high infiltration rate of oxygen continues to enhance degradation of hydrocarbons to low concentrations at the edges. Continued monitoring also indicates that because of the higher dissolved oxygen, more BTEX is degraded and the plume may recede closer to the hydrocarbon source. It should be emphasized that the degree to which these reductions in plume BTEX occur depends on the removal of the free-phase and sorbed hydrocarbons from the contaminated zones. For example, a fluctuating water table could continue to flush more BTEX into the plume from the source area. Removal and management of the contaminant source, therefore, are important prerequisites for successfully implementing intrinsic bioremediation at field sites.
FUTURE DIRECTIONS
Laboratory research and field research have contributed to our understanding of intrinsic bioremediation of BTEX in aquifers as a viable option for managing and controlling hydrocarbon plumes. Research in several areas, however, could enhance the validity and overall regulatory acceptability of the plume containment process. For example, important factors for understanding contaminant behavior and predicting the time for remediation may include (1) a better understanding of aquifer parameters (e.g., recharge and water table fluctuations); (2) tools for quantifying subsoil sources of hydrocarbons and their potential for transport into ground water; and (3) user-friendly ground water models that use monitoring well, hydrogeological, and soil microbiology data to predict the transport and fate of contaminants. Geochemical and biological indicators of in situ biodegradation in addition to BTEX and dissolved oxygen, such as the formation of carbon dioxide and other microbial metabolites as well as ferrous ion, may also help verify intrinsic biodegradation processes in aquifers. Information on the limits of degradation of soil contaminants (e.g., optimum BTEX and dissolved oxygen concentrations and supplemental nutrient effects) and on the widespread occurrence of BTEX degraders in aquifers would also improve our understanding of plume management. Finally, it is important that demonstrated in situ biodegradation gain acceptance by the regulatory authorities and that intrinsic bioremediation be considered a valid and cost-effective means of controlling pollutant migration in low-risk aquifers. Biodegradation in aquifers will continue to play a major role in the management of low levels of soluble hydrocarbons from fuel spills to the subsurface.
REFERENCES
Barker, J. G., G. C. Patrick, and D. Major. 1987. Natural attenuation of aromatic hydrocarbons in a shallow sand aquifer. Ground Water Monitoring Review 7:64-71.
Beller, H. R., D. Grbic-Galic, and M. Reinhard. 1992. Microbial degradation of toluene under sulfate-reducing conditions and the influence of iron on the process. Applied and Environmental Microbiology 58:786-793.
Chiang, C. Y., J. P. Salanitro, E. Y. Chai, J. D. Colthart, and C. L. Klein. 1989. Aerobic biodegradation of benzene, toluene and xylene in a sandy aquifer—data analysis and computer modeling. Groundwater 27:823-834.
Edwards, E. A., L. E. Wills, M. Reinhard, and D. Grbic-Galic. 1992. Anaerobic degradation of toluene and xylene by aquifer microorganisms under sulfate-reducing conditions. Applied and Environmental Microbiology 58:794-800.
Gilham, R. W., R. C. Starr, and D. J. Miller. 1990. A device for in situ determination of geochemical transport parameters: 2. Biochemical reactions. Ground Water 28:858-862.
Hutchins, S. R. 1991. Biodegradation of monoaromatic hydrocarbons by aquifer microorganisms using oxygen, nitrate or nitrous oxide as the terminal electron acceptor. Applied and Environmental Microbiology 57:2403-2407.
Hutchins, S. R., G. W. Sewall, D. A. Kovac, and G. A. Smith. 1991. Biodegradation of aromatic hydrocarbons by aquifer microorganisms under denitrifying conditions. Environmental Science and Technology 25:68-76.
Kemblowski, M. W., J. P. Salanitro, G. M. Deeley, and C. C. Stanley. 1987. Fate and transport of residual hydrocarbons in groundwater—a case study. Pp. 207-231 in Proceedings of the Petroleum Hydrocarbons and Organic Chemicals in Groundwater Conference. Houston: National Water Well Association and American Petroleum Institute.
Loveley, D. R., M. J. Baedecker, D. J. Lonergan, I. M. Cozzarelli, E. J. P. Phillips, and D. I. Siegal. 1989. Oxidation of aromatic contaminants coupled to microbial iron reduction. Nature 339:297-300.
Mackay, D. M., and J. A. Cherry. 1989. Groundwater contamination: pump and treat remediation. Environmental Science and Technology 23:630-636.
Major, D. W., C. I. Mayfield, and J. F. Barker. 1988. Biotransformation of benzene by denitrification in aquifer sand. Ground Water 26:8-14.
Marencik, J. 1991. State-by-state summary of cleanup standards. Soils 23:14-51.
Newman, W. A., and M. A. Martinson. 1992. Let biodegradation promote in situ soil venting. Remediation 2:277-291.
Rifai, H. S., P. B. Bedient, J. T. Wilson, K. M. Miller, and J. M. Armstrong. 1988. Biodegradation modeling at an aviation fuel spill site. American Society of Civil Engineers Journal of Environmental Engineering 114:1007-1029.
Thomas, J. M., V. R. Gordy, S. Fiorenza, and C. H. Ward. 1990. Biodegradation of BTEX in subsurface materials contaminated with gasoline. Water Science Technology 22:53-62.
Travis, C. C., and C. B. Doty. 1990. Can contaminated aquifers at Superfund sites be remediated? Environmental Science and Technology 24:1464-1466.
Wilson, B. H., J. T. Wilson, D. H. Kampbell, B. E. Bledsoe, and J. M. Armstrong. 1991. Biotransformation of monoaromatic and chlorinated hydrocarbons at an aviation gasoline spill site. Geomicrobiology Journal 8:225-240.
Zeyer, J., E. P. Kuhn, and R. P. Schwarzenbach. 1986. Rapid mineralization of toluene and 1,3-dimethylbenzene in the absence of molecular oxygen. Applied and Environmental Microbiology 52:944-947.
Bioremediation from an Ecological Perspective
James M. Tiedje
Center for Microbial Ecology
Michigan State University
East Lansing, Michigan
SUMMARY
The ecological approach to bioremediation is distinctly different from the traditional engineering approach: it focuses on such principles as microbial natural selection rather than on mass balances of pollutants. Questions derived from certain basic ecological principles, including specificity and diversity, can serve as key guides in determining the feasibility of bioremediation at a particular site. Similarly, certain kinds of evidence in the biological record, such as numbers of organisms, are strongly indicative of successful bioremediation. A shift in paradigm—emphasizing the ecological principles governing biodegradation instead of contaminant mass balances—would greatly advance the understanding of bioremediation.
INTRODUCTION
I suggest that there are at least two conceptual approaches to hazardous waste bioremediation. In the dominant approach, derived from engineering, mass balance and stirred tank reactor philosophy dominate. An alternative, or ecological, approach focuses on such principles as microbial natural selection and niche fitness characterization. Reliance on the engineering approach has brought us to an impasse—namely, that nature is not a stirred tank reactor, and thus
the mass balance and predictive models of such systems are often inadequate or too expensive. In ecology, however, one recognizes from the beginning that nature is heterogeneous; to understand nature, one focuses on key principles governing the behavior of populations and does not attempt to achieve mass balances. Thus, I suggest that we consider a shift in paradigm—to consider the important ecological principles governing biodegradation and reduce the emphasis on achieving a mass balance for the pollutant.
This paper emphasizes the ecological approach and key questions related to it. The differences in the philosophies underlying the ecological and engineering approaches are substantial. As details of both approaches are developed, some of the underlying factors may merge into the same issues. Nonetheless, the emphasis in the ecological approach is not on quantification of pollutants but on whether principles are met, since it is known that biological communities respond according to these principles.
The first part of this paper reviews basic ecological principles important to the evaluation and success of in situ bioremediation. The second part converts these principles into key questions about the feasibility of bioremediation for a particular site. Finally, the paper outlines ways to determine whether the ecological principles, especially the principle of natural selection, are met.
BASIC PRINCIPLES
Specificity
An ecological approach recognizes a key principle in biology—specificity. Specificity provides the fitness advantage in a niche. In terms of pollutant degradation, this means that organisms are relatively specific for particular substrates (chemicals) and for particular environmental conditions (the niche). Oxidation by biological organisms is the extreme opposite of oxidation by combustion. The former is specific for particular chemicals, while the latter is entirely nonspecific. The specificity of biological organisms is conferred by such features as membrane selectivity, permeases, regulatory proteins controlling enzyme synthesis, and the structure of the enzyme-active site. There is too great a tendency to generalize about bioremediation as a class of technology, like combustion, which obscures the fact that biodegradability should always be discussed together with the particular chemical.
Although specificity may seem to be a disadvantage for bioremediation, in fact it provides one of the cost advantages of the
technique because resources are focused only on the target chemical. In combustion, for example, external resources are needed to oxidize all organic compounds, while in biodegradation resources go only to the compounds that can reach the enzyme's catalytic site. In cometabolism, where external resources are often needed, this feature is extremely important.
Microbial Diversity
Diversity, nature's counter to specificity, results from evolution, in which organisms diversify from their progenitors to occupy new niches. Because of the heterogeneity in nature, there are many niches and thus a naturally high degree of biodiversity. For bacteria, diversity seems to be exceedingly high; there are likely more than 10,000 species per gram of soil (Torsvik et al., 1990). Fungi also seem to be very diverse, with an estimated 1.6 million species on earth (Hawksworth, 1991). Most of these organisms have never been isolated, let alone studied. For example, Bergey's Manual, which describes all known bacteria, includes only 3000 to 4000 species, and most of these are not from soil or water (Holt, 1989).
This great diversity is important to bioremediation in two ways. First, it means some diversity in the mechanisms that confer specificity. For example, a small number of the organisms that degrade benzoate will also be able to degrade chlorobenzoate or perhaps dichlorobenzoate, because the active site pocket is slightly modified in these variants to allow access to the bulkier chlorine group. This principle seems to be important in the metabolism of polychlorinated biphenyls (PCBs), since the oxygenase of some toluene- and naphthalene-degrading organisms can attack PCBs (Kuhm et al., 1991). Generally, the principle applies to structurally similar chemicals or chemicals subject to the same mechanism of attack. Thus, specificity is not absolute but usually limits the range of substrates attacked to very few.
The second reason that diversity is important is that it is thought to lead to a more robust and stable process because diverse species are likely to include specialists for assimilating low and high pollutant concentrations; for tolerances for different pHs, metals, and solvents; for different growth rates; and for different resistances to phage infection or protozoan grazing. For example, among benzene degraders in a gram of soil, there may be hundreds or even thousands of indigenous strains that may vary in these other important ecological traits. Original ecological dogma was that more diversity leads to stability, but current evidence from macroecology suggests
that less complex systems are more stable (e.g., Begon et al., 1990). However, no evidence exists on the relationship between stability and diversity for a microbial process. In any case, higher diversity among pollutant degraders should lead to emergence of the most fit organisms for the degradation and hence enhance degradation performance.
If high diversity and large populations of pollutant degraders already exist in the habitat, it becomes virtually impossible to successfully introduce an inoculum. The native organisms both preemptively colonize the niche and are likely more fit for the niche. Thus, super biodegraders, whether natural or genetically engineered, stand little chance against a significant indigenous population that can degrade the target chemical.
Biogeography of Biodegraders
Bacteria have been on earth for 3 billion years, an extremely long period of time. Indeed, 85 percent of bacterial existence to date occurred before the continental plates began to drift apart. Thus, the organisms have had a very long time to evolve, adapt, and disperse. This long period likely also led to excellent survival strategies, so that organisms can persist outside their optimum niches for many years. A century ago, Beijerinck, a famous Dutch microbiologist, stated that ''everything [bacterial types] is everywhere, the environment selects." This remains the accepted dogma. Extended to biodegrading organisms, this dogma suggests that biodegradative traits found in one soil or water would be found in most other soils or waters around the world. The global distribution of such traits has not yet been fully evaluated (and is the subject of research), but general experience suggests that the dogma is true, at least at the level of the particular activity, if not the identical strain. Hence, there may be some local variation, but it likely occurs at the variety or strain level and is probably not apparent at the process level. In other words, biodegradation proceeds on similar substrates and at similar rates even though some of the strains are slightly different.
The importance of this biogeographical analysis to bioremediation is that it suggests that biodegrading populations are similar at many sites. The portion of biodegrading organisms in the total community at a given site may be somewhat similar to that at other sites if selection has not already occurred. Thus, if the total population is high, as in a fertile surface soil, the biodegrading population will be high. In contrast, in the vadose zone and aquifer soil, which are impoverished in organic matter, the total populations will be lower and hence
so will be the biodegrading populations. At sites where biodegrading populations are likely to be large based on similarity to other sites, it would be difficult to successfully inoculate a biodegrading organism.
Pollutants as Analogs of Natural Products
Biodegradation occurs when organisms have enzymes that can attack the substrate. Natural selection throughout evolutionary history has maintained those enzymes because they enhance fitness. Thus, pollutant degradation occurs because this enzyme probably also metabolizes an analogous natural product in order for selection to have preserved the gene sequence. It is often very difficult to identify the natural substrate for the biodegrading enzyme without obvious structural analogs. For example, halogenated chemicals are rare in nature, and the natural substrates for enzymes involved in reductive dehalogenation are completely unknown (Mohn and Tiedje, 1992).
The corollary of this situation is that bond types (or structures) not known in nature are often not metabolized. Since these new substrates are a potential energy resource, they exert selective pressure for organism variants to use them. To acquire basically new enzymatic traits through natural evolution is thought to take a very long time, probably hundreds or thousands of years. If one wants to biodegrade these nonnatural chemicals in our lifetime to cleanup hazardous waste, the task will likely involve protein and gene engineering, a process not financially feasible in the foreseeable future.
Natural Selection
Ecological systems are driven by the resources available and the competition for them among the community members. For pollutant degradation, the major question is whether the pollutant is an energy resource—will an organism grow on the chemical as a substrate? If so, there is strong selective pressure for the degrading population to outgrow others, thereby amplifying the rate of degradation. It is useful to group chemicals into two classes of biodegradability: (1) those that support the growth of microbial populations and (2) those that are cometabolized (in other words, they do not support growth but are partly metabolized, usually through only a step or two of the complete metabolic pathway). Organisms that carry out cometabolism are not naturally selected and, therefore, are much more difficult to manage in nature. For this reason the distinction of these two classes is important.
When pollutants are growth substrates, major advantages accrue: (1) the catalyst grows logarithmically with no external input of resources; (2) the proper growth, activity, and distribution of the microbial population (which is very difficult to manage under other circumstances) is an inherent outcome of natural selection for the primary energy substrate; and (3) growth substrates are almost always completely oxidized to carbon dioxide, leaving no toxic intermediates. Less than complete pollutant destruction by natural selection is usually due to limitation by some other resource, most commonly the electron acceptor. Because of these advantages, chemicals that are growth substrates have not and should not become widespread pollution problems. This is because the limitations on natural selection disappear as the chemical becomes more widely distributed. Examples of chemicals that are growth substrates are benzene, toluene, xylenes, naphthalene, chlorophenols, acetone, nitrilotriacetic acid, and 2,4-D. Whenever a pollutant is a growth substrate, bioremediation should be seriously considered. Even if the waste contains mixtures of chemicals, some of which are growth substrates and others not, bioremediation may still be advantageous because it can reduce other remediation costs, such as the amount of activated carbon needed.
Cometabolism usually results from relaxed specificity of an enzyme. No sequential metabolic pathway or energy coupling to adenosine triphosphate production typically occurs. Therefore, natural selection cannot be achieved through this secondary (pollutant) substrate. If cometabolism is to be used, it must be done by managing a primary substrate that selects for growth of active organisms, induces the activity, and/or provides a necessary oxidant or reductant to drive the reaction. Sometimes the primary and secondary substrates are competitive inhibitors, which may require more sophisticated management, such as pulse feeding or precise concentration control. Cometabolic processes typically accumulate intermediates, some of which may be toxic.
Cometabolic reactions seem to be the only ones that show activity on many of the recalcitrant chlorinated solvents, such as perchloroethylene (PCE), trichloroethylene (TCE), carbon tetrachloride, and chloroform. Laboratory testing and field testing are beginning to show that it may be possible to successfully manage a cometabolic process in situ. Nonetheless, the experimentation, field testing, and monitoring will all need to be more extensive than for pollutants that are growth substrates.
THREE KEY QUESTIONS
I suggest that the following key questions, in the indicated order of priority, are a basic guide to successful bioremediation:
-
Is the chemical degradable?
-
Is the environment habitable?
-
What is the rate-limiting factor and can it be modified?
Is the Chemical Biodegradable?
The first question is whether the chemical is biodegradable, because bioremediation cannot be accomplished if no organism exists that can degrade the chemical. Biodegradability must be established if it is not already well-documented in the literature. Subquestions are whether the chemical is a growth substrate, for the reasons discussed above, and whether the biodegrading organism exists at the site.
A focus on the biodegradability of the pollutant is also important because it suggests the time until application and the research needed for application, as shown in Figure 1. In the figure, biodegradability is indicated by the frequency of the biodegrading populations within the total soil community. The higher frequency implies several benefits to bioremediation, including greater diversity among the populations of degraders, less chance of encountering patches devoid of organisms, and a rather global distribution of this biodegradative property at most sites, which allows extrapolation of information among sites. If organisms are widespread, they cannot be limiting to biodegradation. Hence, environmental factors are then the focus for ensuring or enhancing bioremediation.
The time until field application of a bioremediation technology can also be predicted by the biodegradability scale of Figure 1. When natural degrading organisms are widespread, application is more immediate because conditions may be met naturally or, if not, technology exists for removing some of the environmental limitations. However, when organisms do not exist or are rare, the time until application is more distant because successful addition or distribution of organisms is difficult to achieve, especially in the subsurface (Harvey et al., 1989). It is even more difficult to genetically engineer a new catalytic property; this approach is far from any practical application to bioremediation.
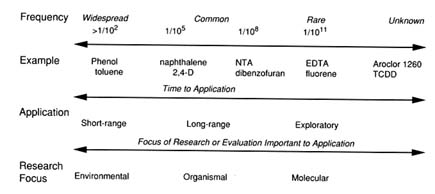
FIGURE 1 Relationship of frequency of biodegraders in the community to application of bioremediation.
Is the Environment Habitable?
The second question—is the environment habitable?—comprises two issues. First, does the environment contain toxic chemicals that make it difficult or impossible for microbes to live? Many polluted sites contain mixtures of chemicals and metals, some at high concentrations, that may pickle the environment so that bioremediation is not feasible. The second issue is the availability of sufficient life-sustaining growth factors, such as nutrients, particularly nitrogen and phosphorus; appropriate electron acceptors; and perhaps other growth factors that might be contained in soil organic matter. Nutrient supply can be evaluated by considering whether the proper carbon-nitrogen-phosphorus (C:N:P) ratio is likely to be met by the soil environment. A C:N:P ratio of 30:5:1 is needed for unrestricted growth of soil bacteria (Paul and Clark, 1989). Microbial growth in most subsoils is not limited by nitrogen and phosphorus as long as the new carbon being provided is not in amounts greater than tens of parts per million. This is often the case with pollutant chemicals. Since nitrogen and phosphorus are inexpensive, however, they are often added as insurance.
What Is the Rate-Limiting Factor and Can It Be Modified?
Too often in bioremediation there is a solution in need of a problem. Thus, effort or money is spent to modify something that is not ratelimiting. To avoid this waste, the rate-limiting parameter must first be defined. In doing so, it is worthwhile to consider the ecosystem in its entirety and to recognize the three key components: sub-
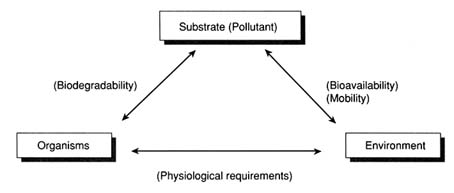
FIGURE 2 Interrelationships of essential components that determine successful bioremediation.
strate (pollutant), biodegrading organism, and environment, as depicted in Figure 2. Factors that reflect this interrelationship and that can limit biodegradation are shown in parentheses in Figure 2.
If biodegradability and habitability have been established, the most common limiting parameter is oxygen, since it has relatively low solubility in water and is in high demand as an oxidant for all biological respiration. Thus, schemes for injection of oxygen or hydrogen peroxide into soil or aquifers are common. Such treatments overcome a rate limitation if the site is anaerobic. Alternative electron acceptors are possible, and nitrate is particularly attractive because of its high electron-accepting capacity in water, its leachability in soils, its low toxicity, and its low cost. Research on denitrification-driven bioremediation is in its infancy, however. The frequency of this type of biodegrading population in soil is not known, but it almost certainly is lower than for oxygen-respiring organisms.
Other treatments to meet physiological requirements include addition of nutrients, adjustment of pH, and removal of toxicants by leaching, precipitation, or some form of inactivation. As stated above, nutrient addition is common, probably because it is easy and cheap and may occasionally provide some benefit, not because it has been a well-documented requirement for many sites.
A second important limitation on biodegradation is the availability of the chemical to the organisms, or bioavailability. Bioavailability is limited when the pollutant is dissolved in organic matter or trapped in micropores in the soil matrix. Substantial work is under way to attempt to understand and enhance the bioavailability of water-in-soluble chemicals. The ecological approach to this problem, however, would be to focus on ensuring that the local environment con-
tains zones that would support natural selection if and when the chemical became available, and not on the immediate (and impossible) recall of that chemical from all microsites.
A related issue, but on a slightly larger scale, is the movement of the chemical or organism so that the two come into contact. Mobility is not a limitation for water-soluble chemicals, which move through soil easily, but it is a severe problem for very insoluble chemicals. In this case, movement of organisms is all that is feasible if physical mixing is not possible.
CONCLUSION
Returning to the ecological approach, the key point in determining whether bioremediation is successful is to establish whether the conditions of natural selection can be expected to be met within the site vicinity. The point is not to determine pollutant mass balances; it is not to ensure that all heterogeneity can be understood and accounted for; and it is not even to worry about local concentrations above regulatory targets if conditions of the surrounding environment ensure that natural selection will occur. This approach recognizes that energy from organic matter is the key limitation for microbial growth and that if the appropriate enzymes and required environmental conditions exist, there is no way to prevent complete biodegradation. Thus, the first criterion for successful bioremediation is documentation of the conditions for natural selection, namely: (1) is the chemical a growth substrate for microbes? (2) is the site habitable (nontoxic) for microbial life? (3) is there sufficient electron acceptor? The ecological approach suggests that more emphasis should be placed on documenting adequate electron acceptor supply and less on measuring the actual pollutant.
A second line of evidence for a successful bioremediation is whether the biological record suggests that natural selection has occurred. This evidence was well illustrated by Madsen et al. (1990) for a plume from a coal tar site. Types of evidence in the biological record include (1) increased rate of pollutant mineralization; (2) increased populations of microorganisms (e.g., total microbial populations, the biodegrading population, and grazers of those populations); and (3) chemical gradients that show a discontinuity caused by respiratory consumption of electron donors (pollutant) and electron acceptors. At contaminated sites, this kind of evidence in the biological record would be strongly indicative of successful intrinsic bioremediation and its persistence as long as the conditions for natural selection can be ensured.
ACKNOWLEDGMENTS
The author's research on biodegradation has been funded by the U.S. Environmental Protection Agency and the National Institute of Environmental Health Sciences Superfund Program.
REFERENCES
Begon, M., J. L. Harper, and C. R. Townsend. 1990. Ecology: Individuals, Populations and Communities. Cambridge, Mass.: Blackwell Scientific Publications.
Harvey, R. W., L. H. George, R. L. Smith, and D. R. LeBlanc. 1989. Transport of microspheres and indigenous bacteria through a sandy aquifer: results of natural- and forced-gradient tracer experiments. Environmental Science and Technology 23:51-56.
Hawksworth, D. L. 1991. The fungal dimension of biodiversity: magnitude, significance, and conservation. Mycological Research 95:641-655.
Holt, J. G. 1989. Bergey's Manual of Systematic Bacteriology. Baltimore: Williams & Wilkins.
Kuhm, A. E., A. Stolz, and H. J. Knackmuss. 1991. Metabolism of naphthalene by the biphenyl-degrading bacterium Pseudomonas paucimobilis Q1. Biodegradation 2:115-120.
Madsen, E. L., J. L. Sinclair, and W. C. Ghiorse. 1990. In situ biodegradation: microbiological patterns in a contaminated aquifer. Science 252:830-833.
Mohn, W. W., and J. M. Tiedje. 1992. Microbial reductive dehalogenation. Microbiological Reviews 56:482-507.
Paul, E. A., and F. G. Clark. 1989. Soil microbiology and biochemistry. San Diego: Academic Press.
Torsvik, V., J. Goksoyr, and F. L. Daae. 1990. High diversity in DNA of soil bacteria. Applied and Environmental Microbiology 56:782-787.
In Situ Bioremediation: The State of the Practice
Richard A. Brown
Groundwater Technology, Inc.
Trenton, New Jersey
William Mahaffey
ECOVA Corporation
Redmond, Washington
Robert D. Norris
Eckenfelder, Inc.
Nashville, Tennessee
SUMMARY
Since the pioneering work by Dick Raymond during the 1970s and early 1980s, in situ bioremediation has been widely used to cleanup aquifers contaminated with petroleum hydrocarbons. A need for better performance led to development of the use of hydrogen peroxide and direct injection of air into the aquifer as sources of oxygen, which was a critical problem in bioremediation. Bioremediation has developed in two branches. The first has been engineering techniques and mathematical models for applying bioremediation to readily degradable contaminants. The second branch has focused on ways to address more recalcitrant contaminants such as chlorinated solvents, polychorinated biphenyls, and pesticides. Work on these more challenging problems has met with some success in the laboratory, but the techniques have yet to be commercialized, largely because of failure to establish and maintain critical control parameters in the subsurface. Continued improvements in the technology will result from efforts in site delineation, engineering controls, use of nonindigenous microorganisms, and field methods for evaluating the microbiological processes.
INTRODUCTION
Bioremediation was first used commercially in 1972 to treat a Sun Oil gasoline pipeline spill in Ambler, Pennsylvania (Raymond et al., 1977), and has been used almost as long as simple pump-and-treat technology. In situ bioremediation was one of the first technologies that was able to bring a site to closure by significantly and permanently reducing soil and ground water contamination, predating in situ processes such as soil vapor extraction and air sparging.
The evolution of in situ bioremediation has had three important aspects: microbiology, engineering, and applications. The microbiological aspects have been concerned with basic metabolic processes and how to manipulate them. Much of this work has been and continues to be laboratory scale and is currently directed at recalcitrant substrates such as polychlorinated biphenyls (PCBs), chlorinated solvents, and pesticides. The second aspect, the engineering of in situ bioremediation, has been concerned with field-scale systems needed to provide the substances required for the metabolic processes, such as oxygen, moisture, and nutrients (Brown and Crosbie, 1989). The most difficult aspect of development has been the translation of laboratory results to field applications. Finally, specific types of bioremediation have been developed to treat specific types of contaminants or matrixes. For example, a significant outgrowth of in situ bioremediation has been the development of ex situ soil biotreatment (Brown and Cartwright, 1990), which has become a cost-effective and widely applied on-site technology. The engineering aspects of bioremediation have produced the greatest successes in the commercial use of the method, leading to the development of specific applications.
Bioremediation has been a successful technology when properly used. It is also an oversold technology, having more promise than results. Understanding the practice of in situ bioremediation—its legitimate uses and potential results—requires an examination of historical developments in microbiology, the current status of the practice of bioremediation, and new developments in bioremediation. This examination illustrates the successes, limitations, and continued needs of bioremediation technology.
HISTORICAL DEVELOPMENTS
The development of bioremediation has been predicated on an evolving use of indigenous microorganisms to biodegrade a variety of organic compounds in soils and wastewater. A large body of information about biooxidation mechanisms and products and the
effects of reaction conditions was available before the technology was commercialized. The microorganisms that could degrade various classes of compounds under both aerobic and anaerobic conditions and the effects of and requirements for pH, nutrients, oxygen, temperature, redox potential, and moisture were all reasonably well established before in situ bioremediation was practiced commercially.
Early studies in hydrocarbon metabolism were reported by Tausson (1927), who isolated bacterial strains capable of oxidizing naphthalene, anthracene, and phenanthrene. Subsequently, Sisler and Zobell (1947) demonstrated that marine bacteria could rapidly oxidize benzo[a]anthracene to carbon dioxide. Senez and co-workers (1956) were the first to suggest that normal alkanes were enzymatically attacked at the first carbon atom (C1 position). Finally, Leadbetter and Foster (1959) were the first to observe, define, and report on the co-oxidation of hydrocarbons previously considered resistant to oxidation and assimilation.
Early in the development of bioremediation, oxygen availability was seen as a critical factor (Floodgate, 1973; Zobell, 1973). The concept of introducing water amended with nutrients and oxygen (using in well aeration) to promote biodegradation was first tried by Dick Raymond in 1972 at the Ambler pipeline spill mentioned earlier. This technology was patented by Raymond in 1974.
From 1975 to 1983, Raymond and co-workers (Jamison et al., 1975) conducted several demonstration projects with the support of the American Petroleum Institute (API). These studies demonstrated the feasibility of in situ bioremediation; the observed reductions in soil and ground water contamination were sufficiently encouraging to stimulate widespread interest in the technology. This early work identified oxygen supply as crucial if the technology was to be generally applicable. This finding led to the innovative use of hydrogen peroxide as an oxygen carrier (Brown et al., 1984).
Laboratory tests at the Texas Research Institute (1982) demonstrated that hydrogen peroxide could be a source of oxygen for bacteria and could be tolerated at concentrations up to 1000 mg/l. API and FMC Corporation supported a field test in Granger, Indiana, that demonstrated that hydrogen peroxide could be used on a field scale (American Petroleum Institute, 1987). The use of hydrogen peroxide as an oxygen source and as an agent for maintaining well performance was subsequently patented (Brown et al., 1986).
During 1983-1986, several commercial in situ bioremediation projects using hydrogen peroxide as the oxygen source were implemented and in some cases reduced hydrocarbons (Frankenberger et al., 1989) and BTEX (benzene, toluene, ethylbenzene, xylenes) (Norris and Dowd,
1993) to below detection limits. Because of the potential for more efficient oxygen supply, the use of hydrogen peroxide expanded interest in bioremediation. However, even though hydrogen peroxide did significantly improve oxygen supply, it, too, had severe limitations: in the treatment of vadose zone (unsaturated) soils and the instability of hydrogen peroxide in certain types of soils (Britton, 1985), which can cause problems such as too rapid decomposition and formation plugging.
The first change in the use of hydrogen peroxide came with the development of soil vapor extraction (SVE), which is now recognized as a more efficient supplier of oxygen for unsaturated soils and which has replaced the use of hydrogen peroxide (Brown and Crosbie, 1989). While the focus of soil vapor extraction has always been removal of volatiles, it was observed that the process of vapor recovery could also result in substantially increased biodegradation rates (Thornton and Wooten, 1982; Wilson and Ward, 1986). Several recent tests, such as those conducted by the U.S. Air Force, have demonstrated a high degree of biooxidation versus physical removal (Miller et al., 1990).
The development of SVE led to a broadening of remedial technology. Because soil vapor extraction could physically remove volatile organics, bioremediation became less of a stand-alone technology. Site remediation became an integrated approach using SVE and bioremediation.
Concerns with hydrogen peroxide stability led to a search for other soluble electron acceptors. Several tests were conducted to evaluate nitrate as an alternate electron acceptor for degradation of monoaromatic (except benzene) and polyaromatic compounds. Nitrate is inexpensive, is easily transported through the formation, and appears to cause fewer problems than oxygen. However, nitrate does not result in degradation of aliphatic compounds, and its use may be limited by state and local regulations and concerns for nitrite formation and potential for eutrophication.
The most recent innovation in bioremediation technology has been the use of air sparging to oxygenate ground water (Brown and Jasiulewicz, 1992). Air sparging involves injecting air below the water table to saturate the ground water with air (and thus provide oxygen), as shown in Figure 1. The process can also transfer volatile components to the unsaturated zone for capture by a vapor recovery system. Currently, air sparging is receiving great attention because it is relatively inexpensive and can distribute oxygen across the entire site at one time rather than relying on an oxygen front moving across the site. In formations where air sparging is applicable, it has supplanted hydro-
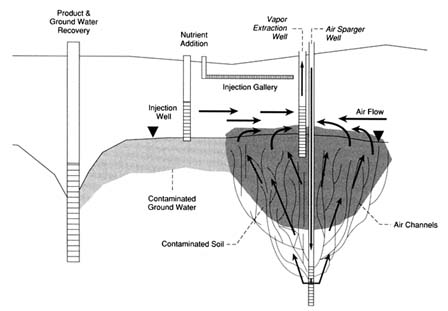
FIGURE 1 Diagram of integrated remedial system.
gen peroxide. Air sparging provides the same benefits to saturated zone treatment that soil vapor extraction has to vadose zone treatment.
CURRENT USES
The application of bioremediation is continually changing. Initially, the technique was viewed as a primary treatment process—able, potentially, to treat a wide range of organic compounds in soil and ground water. The advent of soil vapor extraction and air sparging, however, has diminished the importance of bioremediation as a stand-alone system for contaminants that are relatively volatile and thus readily removed physically by sparging and venting. As a result, bioremediation has evolved in two directions: as part of an integrated system for treating highly mobile (volatile and/or soluble) and/or degradable substrates, such as gasoline or diesel fuel, and as a primary system for treating nonmobile or recalcitrant substrates such as heavier petroleum products and, potentially, PCBs and pesticides.
Treatment of Degradable Mobile Contaminants
In the integrated treatment of hydrocarbon fuels or other mobile and degradable substances, bioremediation, or biodegradation, has become an effective incremental technology in conjunction with SVE and air sparging. Biodegradation occurs readily during the aeration of petroleum hydrocarbons (Miller et al., 1990). The degree to which biodegradation occurs relative to other removal processes, such as volatilization, depends on the properties of the contaminant and the rate of air flow and other environmental factors. Biodegradation can be enhanced by adjusting air flow and moisture and by adding nutrients. Physical removal is enhanced by increasing air flow.
The design of bioremediation strategies is highly site specific. It depends on contaminant properties and distribution, lithology, infrastructure (buildings, pavement, utilities, etc.), regulatory requirements, and client-specific issues such as site usage and time requirements. For instance, soil permeability and layering of highly permeable or very tight soils may preclude one or more technologies or restrict design options. Generally, most in situ processes have had little success in clay-based soils.
Many sites are now being remediated using multiple technologies. Where free-phase hydrocarbons are present, it is almost always advisable to remove the recoverable free-phase liquids. This typically leaves small pockets of free-phase liquids as well as soils contaminated with several thousand parts per million of adsorbed-phase organics. Pump-and-treat methods will satisfactorily remove only those contaminants with water solubilities in excess of 10,000 mg/1. Thus, remediation of most sites requires the incorporation of technologies that can remove or destroy substantial quantities of contaminants.
For volatile biodegradable contaminants, a combination of in situ bioremediation, air sparging, and/or vapor extraction may be the best strategy, provided the soil properties and site infrastructure permit. Designs that emphasize air sparging and vapor recovery are likely to lead to faster remediation than systems that emphasize bioremediation. The latter, accomplished by using intermittent or low air flow rates, offers the advantage of minimizing off-gas treatment as a trade-off for speed of remediation.
Integration of technologies will typically provide the most cost-effective remedial design. Thus, where unsaturated soils are contaminated by biodegradable substances with vapor pressures exceeding approximately 1.0 mm Hg, a combination of vapor recovery and bioremediation is likely to be used. Where saturated zones are con
taminated largely by compounds with vapor pressures exceeding 1.0 mm Hg and with Henry's Law constants exceeding 10-5 atm m3/mole, air sparging can be used to provide oxygen and physically transfer contaminants to the unsaturated zone for capture with a vapor extraction system.
For biodegradable contaminants with minimal volatility, bioremediation may be a stand-alone technology. Polyaromatic hydrocarbons (PAHs), heavy fuels, and plasticizers, for example, respond primarily to bioremediation alone. The oxygen, however, may be provided by air sparging and/or vapor extraction techniques. In fractured bedrock, highly stratified aquifers, or where the saturated interval is no more than about 1 m, oxygen is more aptly provided through recirculated ground water using hydrogen peroxide.
Resistant Organics
Recent years have seen continued progress with microbial degradation of chlorinated solvents, pesticides, PCBs, and nitroaromatic compounds. In general, however, the current state of technology does not permit these classes of compounds to be treated on a commercial scale. Similarly, there is little evidence that nonindigenous microorganisms have been used successfully on a commercial scale for in situ bioremediation.
With highly degradable substances, intrinsic bioremediation can be used as the final treatment when the contaminant load has been reduced to the point that the ambient nutrient levels and oxygen diffusion are sufficient to support biodegradation. With this unassisted bioremediation, treatment costs can be very low.
FUTURE OF THE TECHNOLOGY
The engineering aspects of bioremediation have produced the greatest successes in commercial use of the methods. This is due primarily to a substantial body of information that existed on microbial use of petroleum hydrocarbons as sources of carbon and energy for growth. Raymond's pioneering efforts in the commercialization of bioremediation for petroleum hydrocarbons were based on 45 years of research in biodegradation. In considering the future of bioremediation it is wise to maintain perspective on the historical elements of microbiology and biotechnology that support the engineering breakthroughs. One must also acknowledge the current technical limitations of in situ bioremediation, which fall into three major and highly interactive areas: physical/chemical, microbiological, and site assessment.
Physical/Chemical Limitations
Major engineering advances have already been made in overcoming physical/chemical constraints on in situ bioremediation systems, particularly in the area of oxygenation. However, certain physical/chemical elements still significantly affect the microbiological component of in situ bioremediation. Of these, the molecular architecture of organic pollutant molecules has the greatest implications.
Size and the extent and type of functional group substitution dictate the bioavailability and biodegradability of a molecule. Bioavailability through desorption is greatly reduced by solubility limitations as well as degree of hydrophobicity, both of which depend on molecular size and functional group substituents. Surfactants may improve bioavailability, but they are of no avail where the microbial populations lack the catabolic capacity to biodegrade the molecule(s) of concern.
Another important factor is that single-substance contamination is rare in most polluted environments. Microbial biodegradation of multicomponent mixtures is not as well understood as many would believe. Biodegradation of complex mixtures is often assumed to occur if the contaminants are known to be biodegradable and substrate interactions are known to be not important. However, at least two studies involving gasoline (Barker et al., 1987; Wilson et al., 1990) reported that some BTX (benzene, toluene, xylenes) constituents persisted above regulatory action levels, even after stimulation of bioremediation by addition of inorganic nutrients and various electron acceptors. A number of investigators (Alvarez and Vogel, 1991; Arvin et al., 1989; Bouwer and Capone, 1988) have recognized and begun to investigate the importance of substrate interactions.
Microbiological Limitations
The unpredictability of biodegradation adds to the importance of continued research on metabolic processes such as adaptation, co-oxidation, diauxy, catabolite repression, and competitive inhibition. Central requirements of in situ bioremediation are that the contaminants are biodegradable, that the appropriate microbial populations are present, and that the microbes are able to thrive. The understanding of metabolic pathways in biodegradation and of the factors that control microbial populations continues to grow, thus increasing the potential for bioremediation.
Research into the biodegradation of chlorinated organics illustrates the importance of continued microbiological research. Chlorinated solvents and many other halogenated compounds (e.g., PCBs)
were previously thought to be recalcitrant both aerobically and anaerobically. However, the early 1980s witnessed major advances in our fundamental knowledge of the biodegradation of chlorinated organics. Bouwer et al. (1981) demonstrated the anaerobic degradation of halogenated 1- and 2-carbon compounds. Subsequent research on trichloroethylene (TCE) (Vogel and McCarty, 1985) and perchloroethylene (Fathepure et al., 1987) demonstrated that these compounds were cometabolized through a reductive dehalogenation mechanism by a consortium of anaerobic organisms. Researchers at General Electric Corporation (Bedard et al., 1987; Quensen et al., 1988) identified a reductive dehalogenation mechanism for PCBs. Bedard and her co-workers further demonstrated novel aerobic processes that degraded the more refractory orthosubstituted PCB congeners and have isolated a number of bacterial strains that are highly efficient in degrading the more highly chlorinated congeners. TCE was shown to be co-oxidized by methanotrophic bacteria supplied with methane (Wilson and Wilson, 1985) and by a strain of Pseudomonas cepacia (G4) supplied with phenol or toluene (Nelson et al., 1987).
There has been a plethora of laboratory investigations to identify beneficial microbial processes but relatively few field pilot studies demonstrating the efficacy of in situ bioremediation for recalcitrant compounds and little commercialization of novel microbial processes. Extensive field studies by researchers at Stanford University used stimulation of methanotrophs to co-oxidize TCE under nearly ideal field conditions. To date, the technology has not been commercialized. Another in situ field study was performed by General Electric Corporation in the summer of 1991. While limited in scope, this study provided field-scale data for evaluating aerobic biodegradation of PCBs by naturally occurring microorganisms. Despite these partially successful field studies, there has been little progress toward commercialization of new bioremediation processes for in situ application.
The disparity between research success and commercialization reflects the difficulty of maintaining critical control parameters (e.g., the requirement of methane for co-oxidation of TCE and the coincident competitive inhibition of TCE degradation in the presence of excess methane). Further, many research studies use highly adapted cultures that are not readily dispersed throughout the formation or maintained in the presence of predators. To date, there has been only a preliminary report suggesting that the injection of a specific degrader population, P. cepacia strain G4, for co-oxidizing TCE may be effective under highly ideal site conditions (Nelson et al., 1990). These results are currently being reevaluated by further field pilot testing.
Site Assessment Limitations
An important element of any field pilot program is that the site be well characterized and that statistically valid sampling plans be used during the site investigation and remediation. Several critical elements of an environmental sampling plan are:
-
a definition of the time-space population(s) of interest;
-
development of field-sampling designs and sample measurement procedures that will yield representative data from the defined populations; and
-
assessment of the uncertainty of estimated quantities through means, trends, and average values.
Evaluation of the applicability of bioremediation requires answers to some basic questions, such as: What is the validity of assuming that the enumeration of specific degrader populations can be used to assess the degradative potential at a site or that these populations can be adequately stimulated to degrade the pollutants? How many site samples must be analyzed by treatability methods to demonstrate a biodegradative rate enhancement sufficient to achieve a regulatory level? What are the acceptable standardized methods? How well do the data from these test methods predict actual field results, and to these results justify the costs of obtaining these data? The answers to these questions are likely to vary from site to site and will be greatly influenced by experimental design. Field performance can be predicted from laboratory experiments only through development of appropriate mathematical models that are verified over time by demonstrating good correlation of laboratory and field data.
Future Needs
The future of bioremediation lies in overcoming the limitations of the technologies. Clearly, the enormous costs of site remediations and the goal of eliminating future liability constrain the development of new technologies. The most significant advances will be those that result in the development of predictable, efficient, lower-cost methods of remediation. Some of the limitations are physical/chemical and will be overcome by purely engineering methods; other solutions will be uniquely biological. In addition to the identification of new microbial capabilities for degrading chemical pollutants, other biotechnical offshoots will evolve. These can be viewed as bioaugmentation, analytical methods, and process innovations.
Bioaugmentation
Genetic engineering to improve catabolic capacity has enormous potential for obviating cellular regulatory control over the expression of biodegradative pathways. This technology offers the distinct advantage of constructing new biodegradative pathways by eliminating misrouting of metabolites to end products that inhibit further biodegradation of a pollutant (Reineke and Knackmuss, 1990). The use of specially constructed strains to biodegrade a heretofore recalcitrant pollutant would expand the range of compounds and therefore the number of sites amenable to bioremedial technologies. However, until the release of genetically engineered organisms is more acceptable from a social and regulatory perspective, this technology will be of use only from an academic perspective.
An alternative to classical genetic engineering is laboratory breeding of organisms under appropriate selective pressures to enrich for strains with the desired phenotypic characteristics. This process was effective in isolating a single strain of bacteria capable of degrading chlorobenzenes from the coculture and in the selective breeding of a bacterium that degrades toluene and one that degrades chlorobenzoate. In addition to developing improved strains, a great deal must be done in developing inoculation systems that assure that the desired strain(s) compete effectively and establish residence long enough to achieve the remedial objective.
Analytical Methods
Field analytical techniques for monitoring for the presence of specific degrader populations or levels of contaminants that are as easy to use as home pregnancy tests would revolutionize the environmental industry. Such methods as nucleic acid probes and monoclonal antibody tests have been developed but are not widely used because of their relatively high cost and low reliability. Are these deficiencies inherent in the technology or is further development required?
It would seem that monitoring methods that could provide direct evidence of the performance of in situ bioremediation processes would go a long way toward validating treatment effects early in the remediation process and even provide the mechanism for stimulus-response control of the process. Methods for on-line analysis of general metabolic end results, such as carbon dioxide production and oxygen consumption, are used fairly routinely. However, as the Stanford field pilot program demonstrated, additional benefit can be gained by tracking the levels of specific transient metabolic products of the biological
process. These observations beg the question of whether our fundamental knowledge of biodegradative pathways can be used to suggest and/or develop similar methods for classes of contaminants in addition to petroleum hydrocarbons.
Process Innovations
A number of new technologies, biological and chemical, could be used to enhance bioremediation. With increasing knowledge of anaerobic biodegradation, it should not be long before we witness the use of this microbial process to encourage in situ biorestoration of sites contaminated with chlorinated solvents, PCBs, chlorinated pesticides, or other halogenated organics that otherwise resist microbial degradation. On purely thermodynamic grounds, it is not unreasonable to suppose that a treatment-train approach using both anaerobic and aerobic biodegradation would be the most efficient way to handle such compounds as PCE and PCBs.
A second possibility involves in situ soil flushing, a technology derived from tertiary recovery of petroleum from oil fields. Surfactant/polymer floods are used to essentially wash product or pollutants from the subsurface for above-ground recovery. Typically, this process will leave behind residual contaminants and polymer/surfactant. The potential of using in situ bioremediation to treat these residuals (biopolishing) has received minimal investigation.
CONCLUSION
Bioremediation technology has evolved over 20 years of commercial life. It started as one of the first primary treatment processes, able to address both soil and ground water contamination. It has since become an incremental technology, directed at accelerating the remediation of sites contaminated by petroleum hydrocarbons and other degradable substrates.
The evolution of bioremediation has resulted primarily from engineering work. Most advances in commercial application have been tied to improving oxygen availability. The technology has evolved from simple in well aeration to chemical carriers such as hydrogen peroxide or nitrate and, finally, to aeration technology—soil vapor extraction and air sparging. In the course of this evolution the importance of the biological pathway has declined as physical removal processes have evolved.
The future of bioremediation lies in addressing those contaminants that are not easily extracted physically, such as PAHs, PCBs,
and pesticides. This, approach, however, requires advances in the fundamental knowledge of microbial ecology and biodegradation pathways. Application of new microbial processes requires better monitoring and mathematical modeling as well as improved subsurface engineering. Such advances will lead to better understanding and use of natural or enhanced in situ bioremediation.
REFERENCES
Alvarez, P. J., and T. M. Vogel. 1991. Substrate interactions of benzene, toluene, and para-xylene during microbial degradation by pure cultures and mixed-culture aquifer slurries. Applied and Environmental Microbiology 57(10):2981-2985.
American Petroleum Institute (API). 1987. Field Study of Enhanced Subsurface Biodegradation of Hydrocarbons Using Hydrogen Peroxide as an Oxygen Source. API Pub. 4448. Washington, D.C.: API.
Arvin, E., B. K. Jensen, and A. T. Gunderson. 1989. Substrate interactions during aerobic biodegradation on benzene. Applied and Environmental Microbiology 55(12):3221-3225.
Barker, J. F., G. C. Patrick, and D. Major. 1987. Natural attenuation of aromatic hydrocarbons in a shallow sand aquifer. Ground Water Monitoring Review 7(1):64-71.
Bedard, D. L., M. L. Haberl, R. J. May, and M. J. Brennan. 1987. Evidence for novel mechanisms of polychlorinated biphenyl metabolism in Alcaligenes eutrophus H850. Applied and Environmental Microbiology 53(5):1103-1112.
Bouwer, E. J., B. E. Rittmann, and P. L. McCarty. 1981. Anaerobic degradation of halogenated 1 carbon and 2 carbon organic compounds. Environmental Science and Technology 15(5):596-599.
Bouwer, E. J., and D. G. Capone. 1988. Effects of co-occurring aromatic hydrocarbons on degradation of individual polycyclic aromatic hydrocarbons in marine sediment slurries. Applied and Environmental Microbiology 54(7):1649-1655.
Britton, L. N. 1985. Feasibility Studies on the Use of Hydrogen Peroxide to Enhance Microbial Degradation of Gasoline. API Pub. 4389. Washington, D.C.: API.
Brown, R. A., R. D. Norris, and R. L. Raymond. 1984. Oxygen transport in contaminated aquifers. Proceedings of Petroleum Hydrocarbons and Organic Chemicals in Groundwater: Prevention, Detection, and Restoration, November 5-7, Houston. Worthington, Ohio: National Well Water Association.
Brown, R. A., R. D. Norris, and R. L. Raymond. 1986. U.S. Patent 4,588,506, Stimulation of Biooxidation Process in Subterranean Formations. May 13.
Brown, R. A., and J. Crosbie. 1989. Oxygen Sources for In Situ Bioremediation. Greenbelt, Md.: Hazardous Materials Control Research Institute.
Brown, R. A., and R. T. Cartwright. 1990. Biotreat sludges and soils. Hydrocarbon Processing (Oct.):93-96.
Brown, R. A., and F. Jasiulewicz. 1992. Air sparging: a new model for remediation. Pollution Engineering (July):52-57.
Fathepure, B. Z., J. P. Nengu, and S. A. Boyd. 1987. Anaerobic bacteria that dechlorinate perchloroethene. Applied and Environmental Microbiology 53(11):2671-2674.
Floodgate, G. D. 1973. Theory concerning the biodegradation of oil in natural waters. In Microbial Degradation of Oil Pollutants, D. G. Ahearn and S.P. Meyers, eds., Publ. No. LSU-SG-73-01. Baton Rouge: Louisiana State University, Center for Wetland Resources.
Frankenberger, W. T., Jr., K. D. Emerson, and D. W. Turner. 1989. In situ bioremediation of an underground diesel fuel spill: a case history. Environmental Management 13(3):325-332.
Jamison, V. M., R. L. Raymond, and J. O. Hudson, Jr. 1975. Biodegradation of high-octane gasoline in groundwater. Developments in Industrial Microbiology 16:305-312.
Leadbetter, E. R., and J. W. Foster. 1959. Oxidation products formed from gaseous alkanes by the bacterium Pseudomonas methanica. Archives of Biochemistry and Biophysics 82:491-492.
Miller, R. N., R. E. Hinchee, C. M. Vogel, R. R. DuPont, and D. C. Downey. 1990. A field scale investigation of enhanced petroleum hydrocarbon biodegradation in the vadose zone at Tyndall AFB, Florida. In Proceedings of Petroleum Hydrocarbons and Organic Chemicals in Ground Water: Prevention, Detection, and Restoration, Oct. 31-Nov. 2, Houston. Worthington, Ohio: National Water Well Association.
Nelson, M. J. K., S. O. Montgomery, W. R. Mahaffey, and P. H. Pritchard. 1987. Biodegradation of trichloroethylene and involvement of an aromatic biodegradative pathway. Applied and Environmental Microbiology 53(5):949-954.
Nelson, M. J., J. V. Kinsella, and T. Montoya. 1990. In situ biodegradation of TCE contaminated groundwater. Environmental Progress 9(3):190-196.
Norris, R. D., and K. Dowd. 1993. Successful in situ bioremediation in a low permeability aquifer. In Bioremediation: Field Experience, P. E. Flathman, J. Exner, and D. Jerger, eds. Chelsea, Mich.: Lewis Publishers.
Quensen, J. F., J. M. Tiedje, and S. A. Boyd. 1988. Reductive dechlorination of polychlorinated biphenyls by anaerobic microorganisms from sediments. Science 242:752-754.
Raymond, R. L., V. W. Jamison, and J. O. Hudson. 1977. Beneficial stimulation of bacterial activity in groundwater containing petroleum hydrocarbons. American Institute of Chemical Engineers Symposium Series 73(166):390-404.
Reineke, W., and H. J. Knackmuss. 1990. Hybrid pathway for chlorobenzoate metabolism in pseudomonas sp. B13 derivatives. Journal of Bacteriology 142(2):467-473.
Senez, J. C., and M. Konovaltschikoff-Mazoyer. 1956. Formation d'acides gras dans les cultures de Pseudomonas ruginosa sur n-heptane. Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences 242:2873-2875.
Sisler, F. D., and C. E. Zobell. 1947. Microbial utilization of carcinogenic hydrocarbons. Science 106:521-522.
Tausson, W. C. 1927. Naphthalin als Kohlenstoffquelle für Bakterien. Planta 4:214-256.
Texas Research Institute, Inc. 1982. Enhancing the Microbial Degradation of Underground Gasoline by Increasing Available Oxygen, Final Report. Washington, D.C.: American Petroleum Institute.
Thornton, J. C., and W. L. Wooten. 1982. Venting for the removal of hydrocarbon vapors from gasoline contaminated soil. Journal of Environmental Science and Health A17(1):31-44.
VanLocke, R., A. M. Verlinde, W. Verstraeta, and R. DeBorger. 1979. Microbial release of oil from soil columns. Environmental Science and Technology 13(March):346-348.
Vogel, T. M., and P. L. McCarty. 1985. Biotransformation of tetrachloroethylene to trichloroethylene, dichloroethylene, vinyl chloride, and carbon dioxide under methanogenic conditions. Applied and Environmental Microbiology 49(5):1080-1083.
Wilson, J. T., and B. H. Wilson. 1985. Biotransformation of trichloroethylene in soil. Applied and Environmental Microbiology 49(1):242-243.
Wilson, J. T., and C. H. Ward. 1986. Opportunities for bioremediation of aquifers contaminated with petroleum hydrocarbons. Journal of Industrial Microbiology 27:109-116.
Wilson, J., L. Leach, J. Michalowski, S. Vandergrift, and R. Calloway. 1990. In situ reclamation of spills from underground storage tanks: new approaches for site characterization, project design, and evaluation of performance. In Proceedings: Environmental Research Conference on Groundwater Quality and Waste Disposal, May 2-4, 1989, Washington, D.C., I. P. Muraka and S. Cordle, eds. Palo Alto, Calif.: Electric Power Research Institute.
Zobell, C. E. 1973. Microbial degradation of oil: present status, problems, and perspectives, P. 15 in The Microbial Degradation of Oil Pollutants, D. G. Ahearn and S. P. Meyers, eds. Publ. No. LSU-SG-73-01. Baton Rouge: Louisiana State University, Center for Wetland Resources.
Engineering Challenges of Implementing In Situ Bioremediation
Lisa Alvarez-Cohen
University of California
Berkeley, California
SUMMARY
The use of in situ bioremediation to destroy ground water contaminants essentially requires the creation and management of a subsurface bioreactor. Physical and chemical conditions within the subsurface environment can be manipulated to optimize microbial growth by using hydrodynamic or gas-phase controls. Requisite factors for successful application of in situ bioremediation include adequate aquifer permeability; a suitable microbial population; sufficient hydrodynamic control for plume containment and delivery of required electron donors, electron acceptors, and/or nutrients; and a complete monitoring system.
Evaluating the progress of in situ bioremediation and proving that the microbes are responsible for contaminant degradation can be challenging because of the inaccessibility of the subsurface bioreactor, aquifer heterogeneities, and the wide range of potential contaminant fates. However, overlapping lines of evidence from a range of field-monitoring techniques may provide suitable indication of successful in situ bioremediation.
INTRODUCTION
In situ bioremediation involves the stimulation of microorganisms within a subsurface aquifer to degrade ground water contami-
nants—that is, management of a subsurface bioreactor to carry out specific biological degradations. Management of a subsurface bioreactor can be passive, involving only the monitoring of naturally occurring microbial degradations, or it can be active, involving engineered systems for the manipulation of physical and chemical conditions within the subsurface environment. Subsurface conditions can be effectively altered with hydrodynamic controls, using water as the delivery and transport system, or with gas-phase control within the vadose zone (unsaturated subsurface). Hydrodynamic controls involve manipulation of ground water flow and may include injection wells or infiltration galleries for the introduction of water to the subsurface along with production wells for ground water withdrawal. Gas-phase controls may take the form of vacuum extraction or venting systems, which may be accompanied by direct injection of gas to the vadose zone or sparging of gas into the ground water. The subsurface bioreactor or zone of biostimulation would then occur between the injection and withdrawal systems. However, the inherent heterogeneity and inaccessibility of the subsurface make in situ bioprocesses much more difficult to monitor and control than above-ground engineered systems. The following discussion addresses some of the unique engineering challenges associated with the use of in situ bioremediation to treat contaminated aquifers.
SUBSURFACE BIOREACTOR REQUIREMENTS
Before applying in situ bioremediation to a contaminated aquifer, it is necessary to evaluate the feasibility of engineering a subsurface bioreactor at the specific site to carry out the biological degradations of interest. Engineering feasibility depends on a number of factors; principal among them are aquifer permeability, heterogeneity, and geochemical characteristics, as well as the nature and distribution of the contaminants. As with above-ground bioreactors, providing appropriate environmental conditions, residence times, and substrate availability are fundamental requirements for promotion of efficient biodegradation reactions.
Subsurface Investigation
The practicality of a subsurface bioreactor depends on aquifer characteristics that can best be evaluated by a thorough site investigation, including tracer studies. A combination of existing site data and both direct and indirect measurements can be used to evaluate these characteristics and define the nature and extent of subsurface
contamination. Boreholes and monitoring wells can permit direct sampling of aquifer material and ground water, which is useful for aquifer characterization and development of a three-dimensional assessment of contaminant distribution.
In determining well placement for site assessment, the cost associated with each boring must be weighed against the information lost in the spaces between borings, a balance that is highly dependent on the heterogeneity of the aquifer. It is also necessary to place wells upgradient of the contamination source to provide information on background water quality as well as downgradient for information on the location and size of the pollutant plume. Preliminary information on aquifer composition and plume location derived from remote sensing by geophysical techniques can be useful for optimizing well placement and, consequently, for decreasing the number of wells necessary for adequate monitoring (Benson et al., 1988). Additionally, gas surveys (analyses of gas samples from within the unsaturated zone) are useful for detecting volatiles that diffuse up from the water table. Such surveys may be advantageous for decreasing the number of monitoring wells since they offer additional information on location and migration of volatile plumes (LaGrega et al., 1992).
Tracer tests are additional direct measurement tools that are useful for estimating the direction and velocity of ground water flow and the hydraulic conductivity, porosity, and dispersivity of the aquifer. Tracers also facilitate estimation of contaminant residence times within the biostimulation zone, which is useful for predicting degradation efficiency.
Permeability
Adequate permeability for the transport of solutions delivering nutrients or other compounds required for stimulation of the desired microbial population within the subsurface bioreactor is essential to in situ bioremediation. Additionally, the aquifer must be sufficiently permeable that the increased microbial mass and volume will not cause extensive plugging of the aquifer pores, thus restricting further ground water movement. The proposed rule of thumb (Thomas and Ward, 1989) is that aquifers with overall hydraulic conductivities of 10-4 cm/s or greater would be most amenable to in situ bioremediation (10-4 cm/s hydraulic conductivity corresponds roughly to an intrinsic permeability of 10-9 cm2 for clean water at typical subsurface temperatures). However, it has been shown that microbial growth in aquifer material can cause permeabilities to decrease by a factor of 1000 (Taylor et al., 1990). Additionally, modeling that considered
microbial growth, transport, and biofilm shearing has shown that high-porosity media with widely distributed pore sizes in the small-diameter range are much more susceptible to biofouling than high-porosity media with a narrow pore size range. These results suggest that both permeability and pore size distribution must be considered in determining the feasibility of in situ bioremediation (Taylor and Jaffe, 1991).
Environmental Conditions
It is important to analyze the environmental parameters inside the intended zone of biostimulation that could exert significant impact on microbial growth and degradation potential. Microbial metabolism is substantially affected by temperature: the metabolism of subsurface populations tends to accelerate with increased subsurface temperatures within typical (nongeothermal) ranges. Although temperatures within the top 10 m of the subsurface may fluctuate seasonally, subsurface temperatures down to 100 m typically remain within 1° to 2°C of the mean annual surface temperature (Freeze and Cherry, 1979), suggesting that bioremediation within the subsurface would occur more quickly in temperate climates (Lee et al., 1988).
Additional factors that may limit microbial activity have been summarized elsewhere (Ghiorse and Balkwill, 1985; Ghiorse and Wilson, 1988). They include pH values outside the range of neutral (pH<6, pH>8), desiccating moisture conditions, and extreme redox (reduction-oxidation) potentials. Each of these factors may be mitigated or controlled within a desired range with varying levels of success using hydrodynamic controls.
Monitoring
Monitoring of ground water and aquifer conditions over time is necessary for assessing activity within the subsurface bioreactor and evaluating the progress of the bioremediation. Monitoring wells typically are installed between the injection and production wells so as to detect microbial growth and contaminant degradation within the biostimulation zone. Additional wells installed upgradient from the contamination provide background characterization data, while wells installed beyond the downgradient contaminant boundaries are useful for detection of plume expansion or migration. Factors that should be analyzed in the monitoring samples include contaminant concentration, microbial numbers, electron donor and acceptor concentrations, oxygen demand, degradation products, pH, and major ion con
centrations. Sample collection and handling procedures have been summarized elsewhere (Barcelona et al., 1990).
AQUIFER PREPARATION
Before applying in situ bioremediation, the source of contamination must be detected and mitigated, major accumulations of free product must be removed, and mechanisms for plume containment must be installed. Contaminants entering the subsurface partition into different phases due to sorption, volatilization, and dissolution processes. Contaminant partitioning impedes pump-and-treat removal methods and may decrease the contaminant's availability to microbial degradation.
Source and Free Product Removal
The first step in most aquifer remediation efforts is removal or mitigation of the contaminant source: excavation of leaking underground storage tanks, plugging or repairing leaking surface impoundments or landfill liners, restricting intentional or unintentional land application, and similar measures. Removal typically is achieved by excavating the most contaminated surface soils and drilling wells to pump out the most concentrated source material. Liquid contaminants, which often exist as nonaqueous-phase liquids, or free product, are drawn into the subsurface by gravity and capillary action within the porous media. Free product that is lighter than water, such as petroleum hydrocarbons, tends to migrate downward through the unsaturated zone until hitting an impermeable layer or the water table, where it spreads laterally. Free product that is denser than water, such as many of the chlorinated solvents, would continue to migrate downward through the water table and saturated zone until reaching an impermeable barrier. Free product can be removed from an aquifer by direct pumping using production wells alone or combinations of injection and production wells to cause directional migration of the floating or sinking product. However, most pumping strategies will be capable of removing only part of the free product from the subsurface, leaving the remainder of the organic material trapped in pores as residual free product, dissolved in the surrounding ground water as a contaminant plume, sorbed onto the solid subsurface material, or volatilized into the gas-filled pores of the unsaturated zone. Additionally, the location and removal of sinking free product typically represent much more of an engineering challenge than that of floating product, often resulting in low recovery efficiency.
Plume Containment
In situ bioremediation typically requires times on the order of months to years to reduce contaminants to acceptable levels. During that time the contaminants must not be allowed to spread outside the bioremediation zone and thereby escape treatment.
A contaminant plume can be contained by physical or hydrodynamic controls or a combination of both. Physical controls include low-permeability vertical walls installed to physically block the transport of the plume and/or to inhibit the flow of clean ground water into the contaminated zone. The most commonly used physical containment barrier is the slurry trench wall, which typically is composed of a mixture of bentonite and soil or bentonite and cement. A slurry wall keyed into a confining impermeable layer can significantly decrease localized ground water flow and lengthen the ground water flow path. Grout curtains, vibrating beam walls, and synthetic sheet curtains are also used on a limited basis for physical containment. Physical barriers are most effective with shallow aquifers underlayed by a solid confining layer of bedrock or clay (LaGrega et al., 1992).
Hydrodynamic controls are used alone or in conjunction with physical controls. They are especially suited for use with in situ bioremediation since biostimulation amendments could be added with the control water. Hydrodynamic controls typically consist of combinations of injection and extraction wells and/or infiltration galleries that manipulate ground water flow in order to prevent undesirable plume movement. Wells are situated so that their radii of influence (area of water drawdown or mounding) overlap, allowing control of water within the entire treatment zone as well as effective manipulation of the level of the water table. Radii of influence are computed by iterative application of steady pumping rates with drawdown equations appropriate to the specific aquifer conditions. Plume direction, shape, and migration speed can each be effectively manipulated by hydrodynamic controls, which regulate the detention time and amendment delivery within the biostimulation zone (Barcelona et al., 1990; Knox et al., 1986).
IN SITU BIOSTIMULATION
Stimulation of microbial populations within a subsurface bioreactor requires an appropriate carbon source; electron donors/acceptors for energy production; and inorganic nutrients such as nitrogen, phosphorus, and some trace metals. Also required are proper conditions within the aquifer, such as appropriate pH, temperature, moisture content, and redox potential.
Indigenous microbial populations from many aquifers have been shown to be capable of degrading a wide range of organic contaminants, obviating the need for introduction of exogenous cultures in most bioremediation applications (Lee et al., 1988). However, in the absence of appropriate indigenous strains, introduction of laboratory-enriched populations or even genetically engineered microorganisms may be possible.
To detect the presence of microbial populations capable of degrading the contaminant of interest, laboratory feasibility studies should be conducted. In these studies an aseptically collected sample of subsurface material is exposed to the contaminant of interest under simulated aquifer conditions and is analyzed for contaminant degradation and the concurrent appearance of degradation products. Aseptic aquifer samples are collected by withdrawing an uncontaminated section of a drilling core using a sterile paring device (Lee et al., 1988). Radiotracers may also be used as an analytical tool to confirm contaminant degradation and to trace the degradation sequence. Additional information that may be collected from feasibility studies includes the range of nontoxic contaminant concentrations, nutrient and electron donor/acceptor requirements for optimizing cellular growth and contaminant degradation, and estimates of microbial acclimation periods and growth rates. Adequate detention time for the contaminants within the biostimulation zone must be maintained to allow the degradation reaction to reduce the contaminant concentrations to the desired levels, and acclimation periods may be necessary before the indigenous population becomes capable of carrying out the degradation reaction (Wilson et al., 1985).
Role of Nutrients, Electron Donors, Acceptors
Microorganisms produce energy by moving electrons between an electron donor and an electron acceptor. These reactions can be carried out aerobically, using oxygen as the electron acceptor, or anaerobically, using nitrate, sulfate, carbon dioxide, or other oxidized species as the electron acceptor. Many organic contaminants can be used as a primary substrate for microbial metabolism, in which case the contaminant serves as an electron donor and sometimes also as the major carbon source for the microbial cells. Therefore, some degradation reactions produce energy and usable carbon, resulting in microbial growth. Hence, bioremediation of a primary substrate has a built-in termination mechanism: as the contaminant/substrate is consumed, resulting in depleted concentrations, microbial growth slows and ceases. The hydrocarbons in gasoline and other petroleum de
rivatives can be aerobically degraded and used as a primary substrate for growth by a wide range of naturally occurring microorganisms (Armstrong et al., 1991; Ridgway et al., 1990). Bioremediation of gasoline-contaminated aquifers by indigenous microflora has been successfully implemented a number of times (Jamison et al., 1976; Lee et al., 1988; Thomas and Ward, 1989), although researchers have sometimes reported that the indigenous microbial population required a period of adaptation before degradation commenced (Armstrong et al., 1991).
Alternate Substrates
Because of the nature of contaminant partitioning within the subsurface, contaminants may be transported through the aquifer in dilute ground water plumes at concentrations that provide insufficient energy and/or carbon to support microbial growth. Additionally, since aquifers may be used as drinking water sources, the allowable levels of many ground water contaminants are set in the range of micrograms per liter, requiring reduction of contaminants to concentrations lower than those required for microbial reproduction. Under these circumstances, it may be necessary to supply an alternate substrate for the microorganisms in order to promote degradation by secondary metabolism. Similarly, contaminants that do not benefit microorganisms by providing energy or carbon can sometimes be degraded in the presence of an alternate microbial substrate by cometabolism. Some examples of cometabolic degradations are those catalyzed by the mono- or dioxygenase enzymes of methane-, propane-, or toluene-oxidizing bacteria, which use the contaminant as electron donor and oxygen as electron acceptor, or those carried out by anaerobic bacteria capable of using the contaminant as the electron acceptor. A wide range of chlorinated solvents, including trichloroethylene and vinyl chloride, have been shown to be cometabolically degraded by methane- and toluene-oxidizing bacteria. For the application of either secondary metabolism or cometabolism, use of an alternate substrate presents an additional engineering challenge and potential limit on the reaction rate. However, the use of an alternate substrate also enables the degradation reaction to be maintained at contaminant concentrations below those required to support microbial growth and much lower than those possible for degradations in which the contaminant is used by the microorganisms as a primary substrate. Therefore, alternate substrates can increase the potential for attaining contaminant removal to regulatory levels.
Nutrient Delivery
Nutrients typically are delivered by controlling ground water flow using injection wells or infiltration galleries coupled with downstream production wells. In the most common configuration, ground water withdrawn from production wells downgradient from the biostimulation zone is amended with the nutrients required for biostimulation, treated if necessary to remove contaminants, and reintroduced to the aquifer upgradient of the biostimulation zone using the injection wells or infiltration galleries. Water from an external source is required if the flow of withdrawn water is insufficient to control the subsurface flow or if it is infeasible to reinject the withdrawn ground water. The rate of nutrient delivery to the biostimulation zone, therefore, is often limited by the solubility of the nutrients in water and the reinjection flow rate.
Alternately, gaseous nutrients or substrates such as oxygen or methane may be delivered to the biostimulation zone by sparging, the direct injection of gas into the saturated aquifer to effect in situ dissolution of the gas into solution. However, mobilization of volatile contaminants into the gas phase may necessitate additional gasphase controls.
When the limiting nutrients for microbial growth are added to the subsurface, excessive microbial growth may occur around the injection zone, causing significant plugging of the permeable media and limiting the reinjection flow. Innovative methods for discouraging well plugging while promoting dispersed microbial growth throughout the zone of contamination are required. One such method, which has been shown in field studies to reduce localized plugging associated with cometabolic bioremediation, is alternating pulses of electron donor and electron acceptor in the reinjection water. Since both electron donor and acceptor are required for microbial metabolism, advective and dispersive processes within the aquifer must mix the nutrients before conditions promote microbial growth, causing cells to grow dispersed throughout the aquifer and producing a large biostimulation zone (Semprini et al., 1990).
Oxygen, Air, Hydrogen Peroxide
Because of the low solubility of oxygen in water, the major kinetic limitation on aerobic bioremediation reactions is often the availability of oxygen (Lee et al., 1988; Thomas and Ward, 1989). This is especially the case with high BOD (biological oxygen demand) compounds such as petroleum hydrocarbons. Air sparging of water can supply 8
mg/l dissolved oxygen, while sparging with pure oxygen can deliver 40 mg/l and with hydrogen peroxide more than 100 mg/l oxygen. Therefore, while air sparging is the simplest and most common oxygen delivery technique, the use of oxygen or hydrogen peroxide may speed the bioremediation process and decrease the pumping required. However, in some cases the increased cost and potential explosion hazard associated with pure oxygen may more than offset its increased delivery efficiency.
Application of hydrogen peroxide to in situ bioremediation is limited by its toxicity to microbes and its potential for causing aquifer plugging. Two molecules of peroxide are required to produce one molecule of oxygen:
Although this reaction can be catalyzed by microorganisms, it is also catalyzed by naturally occurring compounds in aquifer material. The highly reactive nature of hydrogen peroxide results in chemical oxidations of organic and inorganic compounds, producing precipitants that may contribute to aquifer plugging and may decrease the oxygen-carrying capacity of the water (Spain et al., 1989). Additionally, both metal-catalyzed and microbially induced decomposition of hydrogen peroxide may produce oxygen at concentrations above water saturation, causing bubbles to form and further decreasing aquifer permeability (Morgan and Watkinson, 1992; Pardieck et al., 1992). To mitigate undesirable peroxide reactions, phosphate is sometimes added before the peroxide to precipitate iron and thereby diminish the metal-catalyzed decomposition. Additional chelating agents have been shown to decrease metal-catalyzed decomposition; however, in a biologically active zone the majority of peroxide decomposition would be expected to be biologically induced (Morgan and Watkinson, 1992). Therefore, the dual actions of precipitation of oxidation products and bubble formation typically limit the practical concentration for addition of hydrogen peroxide in ground waters to 100 mg/l or less (corresponding to 47 mg/l oxygen or less).
The reactivity of hydrogen peroxide in aquifers can be expected to vary considerably from site to site and may not result in significant plugging problems in very highly permeable soils and gravels. However, peroxide is also capable of causing mobilization of undesirable metals such as lead and antimony, producing additional ground water contamination. Therefore, it is important to do laboratory feasibility studies before using hydrogen peroxide in an aquifer since the range of potential adverse reactions is so great.
Finally, hydrogen peroxide can be significantly toxic to microbial populations at relatively low concentrations. The toxicity of the compound reportedly is species specific and depends on cell density (Lee et al., 1988; Pardieck et al., 1992). Significant toxicity has been reported for peroxide concentrations as low as 3 mg/l, whereas other studies have shown addition of 270 mg/l to exert no adverse effects. Additionally, acclimation of microorganisms to slowly increasing concentrations of peroxide has been reported with successful additions greater than 2000 mg/l (Pardieck et al., 1992). Again, laboratory feasibility studies will be necessary to determine the tolerance range of the indigenous microbial population.
The toxic effects of hydrogen peroxide on microbes have a side benefit that can be exploited through careful control of the injection stream. A relatively high concentration of hydrogen peroxide in the injection water may be useful for controlling the growth of biofilms within the immediate vicinity of the injection wells or infiltration galleries, providing the peroxide concentration decreases sufficiently with migration through the aquifer to preclude toxic microbial effects within the biostimulation zone, bubble formation, and precipitation of oxides.
Inorganic Nutrients
Studies of the effects of inorganic nutrient addition on bioremediation rates have yielded varying results. Experiments using nitrogen and phosphorous amendments have shown that they enhanced metabolic activities in some aquifer samples while having no significant effect in other samples from the same aquifer (Swindoll et al., 1988). Others reported that addition of nitrogen and phosphorus enhanced in situ gasoline degradation (Jamison et al., 1976). A series of microcosm and field studies suggest that enhancement of biodegradation by addition of inorganic nutrients is extremely case specific (Baker and Herson, 1990). It has been further suggested that not only are inorganic nutrients not always effective but in some cases they inhibit microbial degradation (Morgan and Watkinson, 1992). Morgan and Watkinson have also shown that phosphate addition in combination with hydrogen peroxide may cause precipitation of insoluble salts during migration through the aquifer, decreasing the permeability within the biostimulation zone.
Hence, the evidence indicates that chemical analysis is not adequate for predicting necessary nutrient amendments. Laboratory or field studies with aquifer material are needed to determine nutrient amendments required to promote maximum cell growth and con
taminant degradation and to predict potential reactivity between the aquifer material and the amendments.
Microbial Introduction
Although in situ bioremediation is a developing technology and is currently the subject of many research studies, little is known about the movement of microbial cells through the subsurface matrix or the feasibility of introducing a stable mixed population of organisms into a contaminated site for the purpose of remediation (Thomas and Ward, 1989). Microbial populations suitable for introduction to a contaminated aquifer may have been selectively enriched in the laboratory or genetically engineered to carry out specific degradation reactions, to resist certain toxic effects, or to grow preferentially under specific environmental conditions. However, while laboratory-enriched populations may be added to the subsurface with little regulatory concern, the introduction of genetically engineered cultures currently is not allowed in the United States.
Successful microbial introduction requires a range of factors: (1) the population must be capable of surviving and growing in the new environment; (2) the microorganisms must retain their degradative abilities under the new conditions; (3) the organisms must come in contact with the contaminants; and (4) the electron donors/acceptors and nutrients necessary for microbial growth and contaminant degradation must be made available to the population (Thomas and Ward, 1989). Once the microorganisms are injected into the aquifer, there must be some mechanism for dispersing them throughout the biostimulation zone before they attach to the solid matrix and carry out the degradation reaction of interest. Cell transport within porous media is highly dependent on the characteristics of both the solid media and the microbial cells. Experiments have shown that the conditions that best promote microbial transport in porous media include (in order of their importance) highly permeable media, ground water of low ionic strength, and small-diameter cells (Fontes et al., 1991). To date, there has been little convincing evidence for successful in situ remediation of aquifers resulting from introduced microbial populations.
DETERMINING THE SUCCESS OF IN SITU BIOREMEDIATION
Perhaps the biggest challenges associated with managing a subsurface bioreactor for in situ bioremediation are evaluating its progress
in the field and determining when sufficient contaminant destruction has occurred to warrant discontinuation of the biostimulation. For in situ bioremediation to be deemed successful, it must be shown that the mass of contaminant in the aquifer has been decreased to desired levels and that the microbial population caused the decrease. Factors such as the heterogeneous nature and inaccessibility of subsurface aquifers, together with competing concurrent processes that affect the form and location of the contaminant (such as volatilization, sorption, dissolution, migration, and dilution), all conspire to confound mass balance analyses. Therefore, specific documentation of the successful application of in situ bioremediation for the destruction of aquifer contaminants is extremely rare. It has been asserted that true proof of in situ bioremediation requires convergent lines of independent evidence of microbial degradation in the field (Madsen, 1991). These include diminished contaminant concentrations within both the horizontal and the vertical dimensions of the plume; increased microbial growth on the contaminant of interest in samples taken from the biostimulation zone; and detection of metabolic products coupled with diminished substrate concentrations (Madsen, 1991). These types of evidence may not be readily obtainable because of the complexity of the concurrent physical, chemical, and biological processes involved, aquifer heterogeneities, and site-monitoring limitations. However, a range of innovative sampling techniques may be incorporated into the field-monitoring methods in order to measure and quantify in situ bioremediation and provide a preponderance of supporting evidence.
Field-Monitoring Methods
The following is a sampling of the diverse field methods that have been applied with varying degrees of success to determine and quantify the success of in situ bioremediation:
-
Two field studies in which in situ bioremediation was based on cometabolism were performed within an initially uncontaminated confined aquifer (Semprini et al., 1990, 1991). A series of tracer studies were conducted using bromide to determine flow characteristics and capture efficiency and chlorinated organics to determine sorption and retardation factors and to evaluate the initial degradation potential within the aquifer. Afterward the aquifer was enriched for methane-oxidizing microorganisms that aerobically degrade chlorinated organics in one field study and for denitrifying organisms that anaerobically degrade chlorinated organics in the other. Compari-
-
sons of chlorinated organic concentrations recovered before and after biostimulation, and experiments in which the required electron donors and/or acceptors were eliminated, indicated that in situ bioremediation was successful in both field studies. The transport and degradation of the organics were quantified and the reaction kinetics were calculated by fitting models to the experimental data. The extensive instrumentation and monitoring facilities used in these studies provided much more accurate mass balances than could be expected for typical field applications.
-
Natural gradient tracer tests were used to compare methane oxidation activities in pristine and sewage-contaminated aquifers (Smith et al., 1991). Two inert ion tracers, chloride and bromide, and an inert dissolved gas tracer, hexafluoroethane, were used to determine advective and diffusive ground water characteristics. Methane break-through curves were measured at both sites, and methane oxidation was estimated from differences between tracer and methane recoveries. Methane oxidation was confirmed by injecting carbon-13-labeled methane and by recovering carbon-13-labeled carbon dioxide. Quantification of the methane degradation was possible, and a one-dimensional transport-and-decay model was used with the field data to determine kinetic degradation parameters.
-
Push/pull tests were used in a field study to determine whether oxygen addition was enhancing the subsurface degradation of polynuclear aromatics (Borden et al., 1989). Ground water was extracted from the aquifer, mixed with aromatics and chloride tracer, oxygenated or deoxygenated, and then rapidly reinjected into the bioactive zone. Samples from the reinjection well were analyzed periodically to determine oxygen, aromatics, chloride, and conductivity. The tracer was used to determine the recapture efficiency and to help compensate for dilution factors. While a comparison of results from the oxygenated and deoxygenated tests suggested that oxygen enhanced aromatic degradation, thus proving successful bioremediation, data from the push/pull test alone were not sufficient to quantify the degradation.
-
Indirect evidence for remediation of coal tar constituents was collected by conducting laboratory comparisons of degradation activities and microbial distributions in contaminated and pristine core samples (Madsen et al., 1991). Increased numbers of contaminant-degrading microorganisms in the contaminated cores, coupled with increased populations of protozoans within the contaminant plume, provided evidence for in situ bioremediation but did not permit quantification of the degradation.
-
In situ bioremediation of jet fuel was qualitatively demon-
-
strated in an actively vented region of the unsaturated zone by comparing ratios of stable isotopes (carbon-13/carbon-12) associated with carbon dioxide from atmospheric and vented gas samples (Hinchee et al., 1991). Active jet fuel degradation was confirmed by assuming that higher isotope ratios indicated atmospheric or plant respiratory origin of the carbon dioxide, while lower ratios indicated petroleum hydrocarbon degradation. However, while this approach may offer qualitative evidence for in situ bioremediation, the results are nonquantifiable.
CONCLUSIONS
In situ bioremediation is the management of a subsurface bioreactor to carry out specific biological degradations of ground water contaminants. Successful implementation should include a thorough aquifer characterization, removal of contaminant source and free product, plume containment, laboratory feasibility studies, installation and operation of biostimulation controls, and continuous monitoring.
Although proving the success of in situ bioremediation is challenging, a variety of field methods can be used to provide adequately convincing evidence of success. Quantification of in situ bioremediation, however, is much more difficult, requiring mass balances that may be achievable only under the most controlled circumstances.
ACKNOWLEDGMENTS
This work was sponsored in part by the National Institute of Environmental Health Sciences under grants P42-ES047905 and P42-ES04705 and by the U.S. Department of Energy Junior Faculty Research Award Program administered by Oak Ridge Associated Universities.
REFERENCES
Armstrong, A. Q., R. E. Hodsen, H. M. Hwang, and D. L. Lewis. 1991. Environmental factors affecting toluene degradation in ground water at a hazardous waste site. Environmental Toxicology and Chemistry 10:147-158.
Baker, K. H., and D. S. Herson. 1990. In situ bioremediation of contaminated aquifers and subsurface soils. Geomicrobiology Journal 8:133-145.
Barcelona, M., W. Wehrmann, J. F. Keely, and W. A. Pettyjohn. 1990. Contamination of Groundwater: Prevention, Assessment, Restoration. Park Ridge, N.J.: Noyes Data Corp.
Benson, R. C., M. Turner, P. Turner, and W. Vogelsong. 1988. In situ, time-series measurements for long-term groundwater monitoring. Pp. 58-72 in Ground-Water Contamination: Field Methods, A. G. Collins and A. I. Johnson, eds. Philadelphia: ASTM Publications.
Borden, R. C., M. D. Lee, J. M. Thomas, P. B. Bedient, and C. H. Ward. 1989. In situ measurement and numerical simulation of oxygen limited biotransformation. Groundwater Monitoring Review 9:83-91.
Fontes, D. E., A. L. Mills, G. M. Hornberger, and J. S. Herman. 1991. Physical and chemical factors influencing transport of microorganisms through porous media. Applied and Environmental Microbiology 57:2473-2481.
Freeze, R. A., and J. A. Cherry. 1979. Groundwater. Englewood Cliffs, N.J.: Prentice-Hall.
Ghiorse, W. C., and D. L. Balkwill. 1985. Microbial characterization of subsurface environments. In Groundwater Quality, C. H. Ward, W. Giger, and P. L. McCarty, eds. New York: John Wiley & Sons.
Ghiorse, W. C., and J. T. Wilson. 1988. Microbial ecology of terrestrial subsurface. Advances in Applied Microbiology 33:107-172.
Hinchee, R. E., D. C. Downey, R. R. Dupont, P. K. Aggarwal, and R. N. Miller . 1991. Enhancing biodegradation of petroleum hydrocarbons through soil venting. Journal of Hazardous Materials 27:315-325.
Jamison, V. W., R. L. Raymond, and J. O. Hudson. 1976. Biodegradation of high-octane gasoline in groundwater. Developments in Industrial Microbiology 16:305-312.
Knox, R. C., L. W. Canter, D. G. Kincannon, E. L. Stover, and C. H. Ward. 1986. Aquifer Restoration: State of the Art. Park Ridge, N.J.: Noyes Publications.
LaGrega, M. D., P. L. Buckingham, and J. C. Evans. 1992. The ERM Group's Hazardous Waste Management. New York: McGraw-Hill.
Lee, M. D., J. M. Thomas, R. C. Borden, P. B. Bedient, C. H. Ward, and J. T. Wilson. 1988. Biorestoration of aquifers contaminated with organic compounds. Chemical Rubber Company Critical Reviews in Environmental Control 18:29-89.
Madsen, E. L. 1991. Determining in situ biodegradation. Environmental Science and Technology 25(10):1663-1673.
Madsen, E. L., J. L. Sinclair, and W. C. Ghiorse. 1991. In situ biodegradation: microbiological patterns in a contaminated aquifer. Science 252:830-833.
Morgan, P., and R. J. Watkinson. 1992. Factors limiting the supply and efficiency of nutrient and oxygen supplements for the in situ biotreatment of contaminated soil and groundwater. Water Research 26(1):73-78.
Pardieck, D. L., E. J. Bouwer, and A. T. Stone. 1992. Hydrogen peroxide use to increase oxidant capacity for in situ bioremediation of contaminated soils and aquifers: a review. Journal of Contaminant Hydrology 9:221-242.
Ridgway, H. F., J. Safarik, D. Phipps, and D. Clark. 1990. Identification and catabolic activity of well-derived gasoline degrading bacteria from a contaminated aquifer. Applied and Environmental Microbiology 56:3565-3575.
Semprini, L., P. V. Roberts, G. D. Hopkins, and P. L. McCarty. 1990. A field evaluation of in situ biodegradation of chlorinated ethenes. Groundwater 28:715-727.
Semprini, L., G. D. Hopkins, P. V. Roberts, and P. L. McCarty. 1991. In situ biotransformation of carbon tetrachloride, freon-113, freon-11, and 1,1,1,-TCA under anoxic conditions. Pp. 41-58 in On-Site Bioreclamation, R. E. Hinchee and R. F. Olfenbuttel, eds. Boston: Butterworth.
Smith, R. L., B. L. Howles, and S. P. Garabedian. 1991. In situ measurement of methane oxidation in groundwater by using natural-gradient tracer tests. Applied and Environmental Microbiology 57(7):1997-2004.
Spain, J. C., J. D. Milligan, D. C. Downey, and J. K. Slaughter. 1989. Excessive bacterial decomposition of H2O2 during enhanced biodegradation. Groundwater 27:163-167.
Swindoll, C. M., C. M. Aelion, and F. K. Pfaender. 1988. Influence of inorganic and organic nutrients on aerobic biodegradation and on the adaptation response of subsurface microbial communities. Applied and Environmental Microbiology 54(1):212-217.
Taylor, S. W., and P. R. Jaffe. 1991. Enhanced in situ biodegradation and aquifer permeability reduction. Journal of Environmental Engineering 117(1):25-46.
Taylor, S. W., P. C. D. Milly, and P. R. Jaffe. 1990. Biofilm growth and the related changes in the physical properties of a porous medium; permeability . Water Resources Research 26(9):2161-2169.
Thomas, J. M., and C. H. Ward. 1989. In situ biorestoration of organic contaminants in the subsurface. Environmental Science and Technology 23(7):760-766.
Wilson, J. T., J. F. McNabb, J. W. Cochran, T. H. Wang, M. B. Tomson, and P. B. Bedient. 1985. Influence of microbial adaptation on the fate of organic pollutants in groundwater. Environmental Toxicology and Chemistry 4:721.
Modeling In Situ Bioremediation
Philip B. Bedient and Handadi S. Rifai
Rice University
Houston, Texas
SUMMARY
The problem of quantifying biodegradation of subsurface pollutants can be addressed by using models that combine physical, chemical, and biological processes. Developing such models is difficult, however, for reasons that include the lack of field data on biodegradation and the lack of numerical schemes that accurately simulate the relevant processes. This paper reviews modeling efforts, including BIOPLUME II.
INTRODUCTION
One of the aquifer remediation methods that has been gaining more widespread attention recently is bioremediation, the treatment of subsurface pollutants by stimulating the growth of native microbial populations. The purpose is to biodegrade complex hydrocarbon pollutants into simple carbon dioxide and water. The technology is not novel; biodegradation of organic contaminants has been recognized and utilized in the wastewater treatment process for years.
Bioremediation is not without its problems, however. The most important are the lack of well-documented field demonstrations, preferably quantitative, of the effectiveness of the technology and its long-term effects, if any, on ground water systems. Other problems in-
clude the possibility that the biodegradation process will generate undesirable intermediate compounds that are more persistent in the environment than the parent compounds.
MODELING BIODEGRADATION PROCESSES
The problem of quantifying biodegradation in the subsurface can be addressed by using models that combine physical, chemical, and biological processes. Developing such models is not simple, however, because of the complex nature of microbial kinetics, the limitations of computer resources, the lack of field data on biodegradation, and the lack of robust numerical schemes that can simulate the physical, chemical, and biological processes accurately. Several researchers have developed ground water biodegradation models. The main approaches used for modeling biodegradation kinetics are:
-
first-order degradation models,
-
biofilm models (including kinetic expressions),
-
instantaneous reaction models, and
-
dual-substrate Monod models.
These are described in more detail in the next section. A more thorough discussion of models can be found in an earlier National Research Council report (National Research Council, 1990).
Previous Modeling Efforts
McCarty et al. (1981) modeled the biodegradation process using biofilm kinetics. They assumed that substrate concentration within the biofilm changes only in the direction normal to the surface of the biofilm and that all the required nutrients except the rate-limiting substrate are in excess. The model employs three basic processes: mass transport from the bulk liquid, biodecomposition within the biofilm, and biofilm growth and decay. The authors evaluated the applicability of the biofilm model to aerobic subsurface biodegradation using a laboratory column filled with glass beads. The experimental data and the model predictions were relatively consistent.
Kissel et al. (1984) developed differential equations describing mass balances on solutes and mass fractions in a mixed-culture biological film within a completely mixed reactor. The model incorporates external mass transport effects, Monod kinetics with internal determination of limiting electron donor or acceptor, competitive and sequential reactions, and multiple active and inert biological fractions that vary spatially. Results of hypothetical simulations involving competition between heterotrophs that derive energy from an
organic solute and autotrophs that derive energy from ammonia and nitrite were presented.
Molz et al. (1986) and Widdowson et al. (1987) presented one-and two-dimensional models for aerobic biodegradation of organic contaminants in ground water coupled with advective and dispersive transport. A microcolony approach was used in the modeling effort: microcolonies of bacteria are represented as disks of uniform radius and thickness attached to aquifer sediments. Associated with each colony was a boundary layer of a given thickness across which substrate and oxygen are transported by diffusion to the colonies. The authors' results indicated that biodegradation would be expected to have a major effect on contaminant transport when proper conditions for growth exist. Simulations of two-dimensional transport suggested that under aerobic conditions microbial degradation reduces the substrate concentration profile along longitudinal sections of the plume and retards the lateral spread of the plume. Anaerobic conditions developed in the center of the plume because of microbial consumption and limited oxygen diffusion into the plume's interior.
Widdowson et al. (1988) extended their previous work to simulate oxygen- and/or nitrate-based respiration. Basic assumptions incorporated into the model include a simulated particle-bound microbial population comprised of heterotrophic facultative bacteria in which metabolism is controlled by lack of an organic carbon electron donor source (substrate), an electron acceptor (oxygen and/or nitrate), a mineral nutrient (ammonium), or all three simultaneously.
Srinivasan and Mercer (1988) presented a one-dimensional, finite difference model for simulating biodegradation and sorption processes in saturated porous media. The model is formulated to accommodate a variety of boundary conditions and process theories. Aerobic biodegradation was modeled using a modified Monod function; anaerobic biodegradation was modeled using Michaelis-Menten kinetics. In addition, first-order degradation was allowed for both substances. Sorption was incorporated using linear, Freundlich, or Langmuir equilibrium isotherms for either substance.
MacQuarrie and Sudicky (1990) used the model developed by MacQuarrie et al. (1990) to examine plume behavior in uniform and random flow fields. In uniform ground water flow, a plume originating from a high-concentration source will experience more spreading and slower normalized mass loss than a plume from a source of lower initial concentration because dissolved oxygen is more quickly depleted. Large ground water velocities produced increases in the rate of organic solute mass loss because of increased mechanical mixing of the organic plume with oxygenated ground water.
Development and Application of BIOPLUME
Borden and Bedient (1986) developed the first version of the BIOPLUME model. They developed a system of equations to simulate the simultaneous growth, decay, and transport of microorganisms combined with the transport and removal of hydrocarbons and oxygen. Simulation results indicated that any available oxygen in the region near the hydrocarbon source will be rapidly consumed. In the body of the hydrocarbon plume, oxygen transport will be rate-limiting and the consumption of oxygen and hydrocarbon can be approximated as an instantaneous reaction. The major sources of oxygen, this research concluded, are transverse mixing, advective fluxes, and vertical exchange with the unsaturated zone.
Rifai et al. (1987, 1988) expanded and extended the original BIOPLUME and developed a numerical version of the biodegradation model (BIOPLUME II) by modifying the U.S. Geological Survey (USGS) two-dimensional method of characteristics model (Konikow and Bredehoeft, 1978). The basic concept used in developing BIOPLUME II includes the use of a dual-particle mover procedure to simulate the transport of oxygen and contaminants in the subsurface.
Biodegradation of the contaminants is approximated by the instantaneous reaction model. The ratio of oxygen to dissolved contaminants consumed by the reaction is determined from an appropriate stoichiometric model (assuming complete mineralization). In general, the transport equation is solved twice at every time step to calculate the oxygen and contaminant distribution:
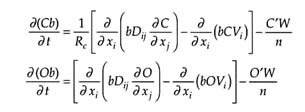
where C and O are the concentration of contaminant and oxygen, respectively; C' and O' are the concentration of contaminant and oxygen in a source or sink fluid; n is the effective porosity; b is the saturated thickness; t is time; xi and xj are Cartesian co-ordinates; W is the volume flux per unit area; Vi is the seepage velocity in the direction of xi; Rc is the retardation factor for the contaminant; and Dij is the coefficient of hydrodynamic dispersion.
The BIOPLUME II model simulates dissolved contaminant concentrations vertically averaged over the thickness of the aquifer. The
two plumes are combined using the principle of superposition to simulate the instantaneous reaction between oxygen and the contaminants, and the decrease in contaminant and oxygen concentrations is calculated from:
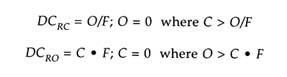
where DCRC and DCRO are the calculated changes in the concentrations of contaminant and oxygen, respectively, caused by biodegradation, and F is the ratio of oxygen to contaminant consumed.
The only input parameters to BIOPLUME II that are required to simulate biodegradation are the amount of dissolved oxygen in the aquifer prior to contamination and the oxygen demand of the contaminant determined from a stoichiometric relationship. Other parameters are the same as would be required to run the standard USGS model in two dimensions (Konikow and Bredehoeft, 1978).
Borden et al. (1986) used the first version of the BIOPLUME model to simulate biodegradation of polycyclic aromatic hydrocarbons at the Conroe Superfund site in Texas. Oxygen exchange with the unsaturated zone was simulated as a first-order decay in hydrocarbon concentration. The loss of hydrocarbon because of horizontal mixing with oxygenated ground water and resulting biodegradation was simulated by generating oxygen and hydrocarbon distributions independently and then combining them by superposition. Simulated oxygen and hydrocarbon concentrations closely matched the observed values at the Conroe site.
Rifai et al. (1988) used BIOPLUME II to model biodegradation of aviation fuel at the U.S. Coast Guard Station, Traverse City, Michigan (Figure 1). Vertically averaged plume data came from 25 wells. The modeling results along the centerline of the contaminant plume were good, and the BIOPLUME II results matched the field observations except in an area between monitoring well M30 and the pumping wells.
Chiang et al. (1989) used BIOPLUME II to characterize hydrocarbon biodegradation in a shallow aquifer. They measured the soluble hydrocarbon concentrations and dissolved oxygen levels in monitoring wells. Results from 10 sampling periods over 3 years showed a significant reduction in total benzene mass with time. The natural attenuation rate was calculated to be 0.95 percent per day. Spatial relationships between dissolved oxygen and total benzene, toluene,
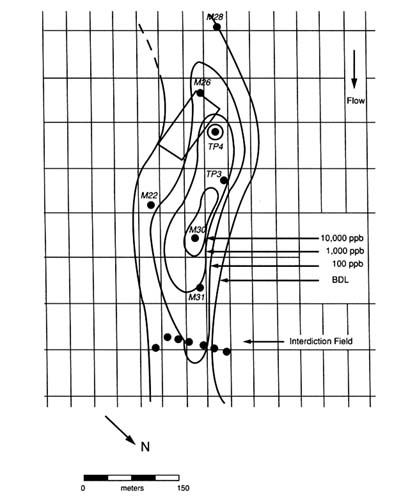
FIGURE 1 Aviation fuel plume at the Traverse City field site (quarter 2,1986).
SOURCE: Rifai et al. (1988). Journal of Environmental Engineering, Vol. 114:1021. Copyright © by the American Society of Civil Engineers (ASCE).
Reprinted with permission of ASCE.
and xylene (BTX) were shown to be strongly correlated by statistical analyses and solute transport modeling using BIOPLUME II. The results were remarkably consistent with field data on the presence of high or low levels of BTX and dissolved oxygen in several monitoring well samples.
REFERENCES
Borden, R. C., and P. B. Bedient. 1986. Transport of dissolved hydrocarbons influenced by reaeration and oxygen limited biodegradation: 1. Theoretical development. Water Resources Research 22:1973-1982.
Borden, R. C., P. B. Bedient, M. D. Lee, C. H. Ward, and J. T. Wilson. 1986. Transport of dissolved hydrocarbons influenced by oxygen limited biodegradation: 2. Field application. Water Resources Research 22:1983-1990.
Chiang, C. Y., J. P. Salanitro, E. Y. Chai, J. D. Colthart, and C. L. Klein. 1989. Aerobic biodegradation of benzene, toluene, and xylene in a sandy aquifer—data analysis and computer modeling. Ground Water 27(6):823-834.
Kissel, J. C., P. L. McCarty, and R. L. Street. 1984. Numerical simulation of mixed-culture biofilm. American Society of Civil Engineers Journal of Environmental Engineering Division 110:393.
Konikow, L. F., and J. D. Bredehoeft. 1978. Computer Model of Two-Dimensional Solute Transport and Dispersion in Ground Water: Automated Data Processing and Computations. Techniques of Water Resources Investigations of the U.S. Geological Survey. Washington, D.C.: U.S. Geological Survey.
MacQuarrie, K. T. B., and E. A. Sudicky. 1990. Simulation of biodegradable organic contaminants in groundwater: 2. Plume behavior in uniform and random flow fields. Water Resources Research 26(2):223-239.
MacQuarrie, K. T. B., E. A. Sudicky, and E. O. Frind. 1990. Simulation of biodegradable organic contaminants in groundwater: 1. Numerical formulation in principal directions. Water Resources Research 26(2):207-222.
McCarty, P. L., M. Reinhard, and B. E. Rittmann. 1981. Trace organics in groundwater. Environmental Science and Technology 15(1):40-51.
Molz, F. J., M. A. Widdowson, and L. D. Benefield. 1986. Simulation of microbial growth dynamics coupled to nutrient and oxygen transport in porous media. Water Resources Research 22:107.
National Research Council. 1990. Ground Water Models: Scientific and Regulatory Applications. Washington, D.C.: National Academy Press.
Rifai, H. S., P. B. Bedient, R. C. Borden, and J. F. Haasbeek. 1987. BIOPLUME II Computer Model of Two-Dimensional Contaminant Transport Under the Influence of Oxygen Limited Biodegradation in Ground Water User's Manual Version 1.0. Houston: Rice University, National Center for Ground Water Research.
Rifai, H. S., P. B. Bedient, J. T. Wilson, K. M. Miller, and J. M. Armstrong. 1988. Biodegradation modeling at a jet fuel spill site. American Society of Civil Engineers Journal of Environmental Engineering Division 114:1007-1019.
Srinivasan, P., and J. W. Mercer. 1988. Simulation of biodegradation and sorption processes in ground water. Ground Water 26(4):475-487.
Widdowson, M. A., F. J. Molz, and L. D. Benefield. 1987. Development and application of a model for simulating microbial growth dynamics coupled to nutrient and oxygen transport in porous media. Pp. 28-51 in Proceedings of the Association of Ground Water Scientists and Engineers/International Ground Water Model Center, Holcomb Research Center Institute Conference on Solving Ground Water Problems with Models. Dublin, Ohio: National Ground Water Association.
Widdowson, M. A., F. J. Molz, and L. D. Benefield. 1988. A numerical transport model for oxygen- and nitrate-based respiration linked to substrate and nutrient availability in porous media. Water Resources Research 24(9):1553-1565.
Testing Bioremediation in the Field
John T. Wilson
U.S. Environmental Protection Agency
Robert S. Kerr Environmental Research Laboratory
Ada, Oklahoma
SUMMARY
An operational definition for success of in situ bioremediation at field scale includes meeting regulatory goals for ground water quality in a timely fashion at a predictable cost. Current practice for site characterization does not adequately define the amount of contamination subject to bioremediation. As a result, laboratory estimates of the requirements for electron acceptors and mineral nutrients and of the time required for remediation have much uncertainty. Another aspect of success is the capacity to continue to meet regulatory goals for ground water quality after the active phase of remediation is complete. In contrast to laboratory studies, the extent of remediation achieved at field scale is influenced by dilution of compounds of regulatory concern in circulated water and by partitioning of the regulated compounds between water and residual nonaqueous-phase oily material. The extent of weathering of residual oily-phase material and the hydrologic environment of the residual have a strong influence on the potential for ground water contamination after active remediation ceases.
INTRODUCTION
Transfer of bioremediation laboratory research to the field is often a frustrating and unsatisfying activity. Part of the problem has to do with the levels of inquiry in the laboratory and in the field. Laboratory studies deal with biochemical or physiological processes. Appropriate controls ensure that only one mechanism is responsible for the phenomena under study. During field-scale implementation of bioremediation technology, several processes operate concurrently. They may involve several distinct mechanisms for biological destruction of the contaminant, as well as partitioning to immobile phases, dilution in ground water, and volatilization.
Experimental controls are usually unavailable during full-scale implementation of in situ bioremediation because the technology is applied uniformly to the contaminated area. As a result, performance monitoring that is limited to the concentration of contaminants in ground water over time, and perhaps the concentrations of nutrients and electron acceptors, cannot ensure that the biological process developed in the laboratory was responsible for contaminant removal at full scale.
The appropriate equivalent of experimental controls is a detailed characterization of the site, the flow of remedial fluids, and the flux of amendments. This characterization allows an assessment of the influence of partitioning, dilution, or volatilization and provides a basis for evaluating the relative contribution of bioremediation.
APPROPRIATE SITE CHARACTERIZATION
Most plumes of organic contamination in ground water originate from spills of refined petroleum hydrocarbons, such as gasoline, or chlorinated solvents, such as trichloroethylene. These substances enter the subsurface as nonaqueous-phase oily liquids, traveling separately from the ground water. As long as the oily-phase liquid is present in the subsurface, it can act as a continuing source of contamination because contaminants contained in the nonaqueous phase will dissolve in the ground water.
Traditionally, monitoring wells have been used to define the extent of contamination in the subsurface environment. However, wells cannot determine the extent of contamination by oily-phase materials. If monitoring well data are the only data available, it is difficult to estimate the total contaminant mass subject to remediation within an order of magnitude.
As an example, Kennedy and Hutchins (1992) estimated the mass
of alkylbenzenes released to a shallow water table aquifer from a pipeline spill of refined petroleum products. The contaminated area was roughly circular with a diameter of 150 m. Thirteen monitoring stations were located uniformly across the spill. At each station a series of continuous cores was taken extending from clean material above the spill, through the spill, to clean material below it. Then monitoring wells were installed in the boreholes used to acquire the cores.
The cores were extracted and analyzed for the content of BTEX (benzene, toluene, ethylbenzene, xylenes) and total petroleum hydrocarbons. Ground water was collected and analyzed for the same parameters. Data from the 13 stations were subjected to geostatistical analysis to estimate the total contaminant mass in the aquifer and the total mass dissolved in the ground water (see Clark, 1979, for a description of geostatistics). Kennedy and Hutchins (1992) used proprietary computer software to estimate the total mass of contaminants. SURFER, available from Golden Software, Denver, Colorado, supports linear kriging of plan two-dimensional data following the trapezoidal rule, Simpson's rule, and Simpson's 3/8 rule. Lacking information on the structure of the data, Kennedy and Hutchins ran all three simulations and took the numerical average.
Of 320 kg of benzene in the aquifer, only 22 kg was dissolved in the ground water; of 8800 kg of BTEX compounds, only 82 kg was dissolved; and of 390,000 kg of total petroleum hydrocarbons, only 115 kg was dissolved. Monitoring well data grossly underestimated the extent of contamination. This research shows the need for site characterization techniques that can accurately estimate the total mass of contaminants subject to bioremediation.
Estimates of Total Contaminant Mass
Estimates of total contaminant mass in the subsurface are required to predict the demand for nutrients and electron acceptor that must be met to complete the remediation. If the total demand for nutrients and electron acceptor has been estimated, the rate of supply of the limiting requirement can be used to estimate the time required for remediation.
The most rigorous approach involves the collection of cores from the contaminated regions of the subsurface environment, followed by extraction and analysis of the cores for the contaminants of concern. A wireline piston sampler (Zapico et al., 1987) makes it possible to collect representative continuous cores, even in noncohesive material.
To preclude losses to volatilization and biodegradation, current good practice recommends against shipping core samples to a laboratory for extraction. When cores are subsampled and extracted in the field, the variability between replicate subsamples is much smaller (Siegrist and Jenssen, 1990). Standard operating procedure at the Robert S. Kerr Environmental Research Laboratory uses a paste sampler to take replicate subcores of each core sample. Each subcore weighs 10 to 15 g and represents 10 to 15 cm of vertical core. The subcore is delivered to a 40-ml vial, containing 5 ml of methylene chloride, which is sealed with a Teflon-faced septum. The sample is dispersed in the solvent to begin extraction, preclude volatilization, and prevent biodegradation. The vials are shipped to the laboratory for subsequent extraction and analysis.
Unless the cores are screened to identify those that deserve analysis, this approach may be too expensive to be practical for general use. The data set reported by Kennedy and Hutchins (1992) contained more than 400 analyses. However, there are several techniques that can reduce the analytical burden. Headspace analysis can be used to screen cores in the field to determine whether the depth interval represented by a core is contaminated with oily-phase hydrocarbons, so that field extraction and analysis of the core are justified.
Aquifer samples can be equilibrated with the headspace of a plastic bag before analysis by a field gas chromatograph, organic vapor analyzer, or explosimeter (Robbins et al., 1989). Alternately, a plug can be removed from a core with a paste sampler and the inlet to an explosimeter or organic vapor analyzer inserted directly into the cavity (Kampbell and Cook, 1992). These techniques are inexpensive and generate data in real time, which allows the screening information to be used to guide decisions about depth and location of subsequent cores.
Often the meter's response in the field headspace analyses has a strong correlation to the content of total petroleum hydrocarbons. In these cases, results from a limited number of expensive core analyses can be extrapolated to a large number of inexpensive field headspace analyses. Kampbell and Cook (1992) compared the hydrocarbon vapor concentrations in the cavities left when plugs were removed from cores for extraction to the content of total petroleum hydrocarbons in the cores. The correlation coefficient between meter response and hydrocarbon content was 0.957 on a set of 24 cores and 0.801 on a set of 64 cores.
Estimates of Contaminant Dilution in Ground Water
In the process of remediation, prodigious quantities of water may be circulated through an oily-phase spill. The compounds of regulatory concern, such as the BTEX compounds, are often more water-soluble than the other components of refined petroleum hydrocarbons. As the circulated water sweeps through the spill, the more water-soluble components partition into the water and are diluted out. The concentration of regulated compounds will drop because of simple dilution.
Downs et al. (1989, in press) quantitatively described this effect in a pilot-scale demonstration of in situ bioremediation of a jet fuel spill using nitrate as the electron acceptor. They used a tracer to estimate the volume of recirculated water, and they cored the area perfused by ground water to estimate the quantity of total hydrocarbons and the quantity of hydrocarbons of regulatory concern. Simple partitioning theory was used to calculate the distribution of hydrocarbons of concern between recirculating ground water and the residual jet fuel.
Estimate of Recirculated Volume of Water
An infiltration gallery sited above the spill effectively perfused a plan surface area of 130 m2 with nitrate-amended ground water. The infiltrated water was recovered in five purge wells. In Figure 1 a computer model predicts flow paths from the infiltration gallery to the recovery wells. In Figure 2 a cross section shows the relationship of the infiltration gallery, the contaminated interval, and the recovery wells. A pulse of chloride was used to trace the flow of water to the recovery wells. The volume of circulated water was considered to be the pumping rate multiplied by the travel time of the chloride between infiltration and the recovery wells. The arrival time at each well was weighted by its pumping rate to calculate the overall residence time, and circulated volume, between infiltration and recovery. Figure 3 shows breakthrough curves of the chloride tracer.
In the demonstration the average residence time was 10 days and the circulated volume was 10,900 m3.
Estimate of Partitioning Between Oil and Water
The area of the spill that was perfused by the infiltration gallery was cored to determine the total quantity of jet fuel and the quantities of benzene, toluene, ethylbenzene, and the xylenes. Smith et al.

FIGURE 1 Hydraulic model of in situ bioremediation of a JP-4 spill at the Traverse City demonstration site. Ground water amended with mineral nutrients and nitrate as an electron acceptor was recharged through an infiltration gallery. The model predicted flow lines from the infiltration gallery to pumping wells (PP5 to PP9) that capture and recirculate ground water. To capture the infiltrated water containing nitrate, more water was pumped than was delivered to the gallery. As a result, some of the flow lines to the wells originate in uncontaminated ground water upgradient of the spill.
(1981) reported empirical partition coefficients for the compounds between JP-4 jet fuel and water. To estimate the distribution of an individual BTEX compound between fuel and water, the published partition coefficients were multiplied by the ratio of the volume of JP-4 under the infiltration gallery to the volume of water in circulation. The distribution between oil and fuel was used to calculate the fraction of total material in oil or water. To predict the equilibrium solution concentration of a BTEX compound in circulation, the quantity of the compound originally in the fuel was multiplied by the fraction that should partition to water and was divided by the circulated volume of ground water.
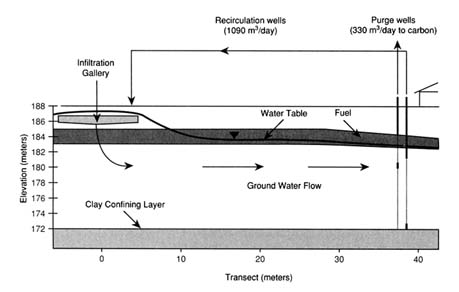
FIGURE 2 Cross section of a JP-4 spill at the Traverse City demonstration site. Ground water was amended with mineral nutrients and nitrate as an electron acceptor. Water was recirculated to an infiltration gallery installed above the JP-4 spill. It moved vertically across the spill, then laterally through the aquifer to the recovery wells. Part of the recovered water was recirculated; part was purged.
Table 1 compares the predicted dilution of benzene, toluene, and o-xylene to the actual concentration in monitoring wells before recirculation of ground water. Dilution alone produced at least a fivefold reduction in concentration.
To maintain hydraulic control over the spill, a fraction of the recovered water was discharged to waste. This flow was replaced with clean water from the aquifer. If the circulation system behaved as a completely mixed reactor, solutes in the circulated water would be diluted at a first-order rate of 0.03 per day. Dilution of a BTEX compound was estimated by multiplying the rate of dilution of circulated water by the portion of the total mass that partitioned to the circulated water.
Estimate of Bioremediation
The actual behavior of benzene is depicted in Figure 4. The dashed line is the calculated equilibrium concentration of benzene in the recirculated water based on partitioning and dilution. The solid line
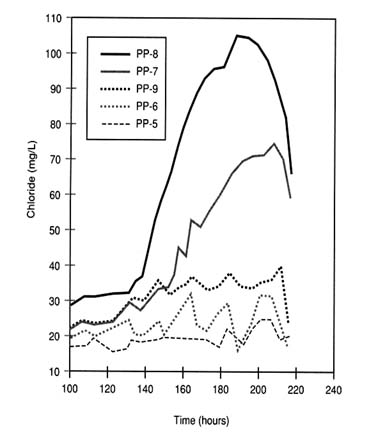
FIGURE 3 Breakthrough of chloride from a tracer test conducted to evaluate the hydraulic model of water flow. Chloride was added to water supplied to the infiltration gallery. The concentration of chloride that breaks through is proportional to the number of flow lines originating in the infiltration gallery, compared to flow lines originating upgradient in the aquifer.
shows concentrations in the recirculation well that captured the greatest portion of infiltrated water. Concentrations rose slowly over time, overshot the prediction at about two recirculation volumes, then showed good agreement with the prediction for another recirculation volume. Then biological acclimation occurred, and benzene was removed from the circulated water. Concentrations dropped below the analytical detection limit over a 2-day period. Concentrations of other BTEX compounds were not reduced (compare data for o-xylene in Figure 5), which established that the removal was a biological process. If a physical or chemical process had been responsible for the
TABLE 1 Comparison of Concentrations (µg/1) of BTEX Compounds in Ground Water After Bioremediation to the Concentrations Expected from the BTEX Content of the Residual Petroleum Hydrocarbons
|
Compound |
Concentration Prior to Bioremediation |
Concentration After Bioremediation |
Concentration Predicted from Residual |
|
Benzene |
760 |
<1 |
2 |
|
Toluene |
4500 |
<1 |
15 |
|
Ethylbenzene |
840 |
6 |
6 |
|
m p-Xylene |
2600 |
23 |
27 |
|
o-Xylene |
1380 |
37 |
18 |
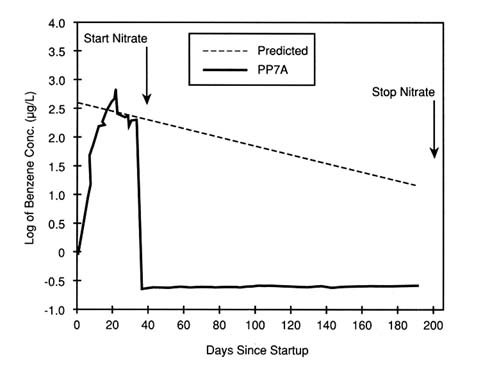
FIGURE 4 Bioremediation of a JP-4 jet fuel spill using nitrate. Comparison of the depletion of benzene in circulated ground water to the depletion predicted from dilution and partitioning.
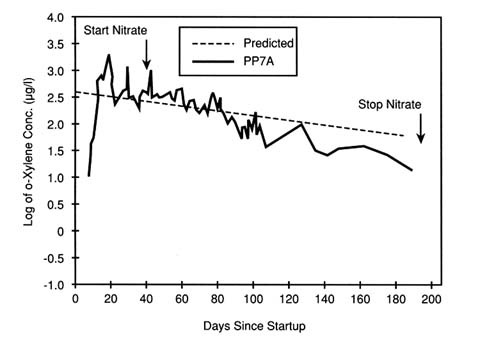
FIGURE 5 Comparison of the depletion of o-xylene in circulated groundwater to the depletion predicted from dilution and partitioning in a JP-4 jet fuel spill.
benzene removal, other BTEX compounds (such as o-xylene) would also have been removed because these other compounds have physical and chemical properties similar to those of benzene but are less readily biodegradable.
Note from Figure 4 that removal of benzene occurred before nitrate was added to the system. This effect had not been predicted in the laboratory treatability study conducted as part of the design of the pilot-scale demonstration (Hutchins et al., 1991a) and has not been conclusively reproduced at laboratory scale since then.
CRITERIA FOR SUCCESS AT FIELD SCALE
The criteria for success at each remediation site are unique, depending on the particular requirements of state and federal regulators and the particular concerns of the site owner. However, requirements at many sites can be generalized to the following:
-
Concentrations of substances of regulatory concern in ground water at the end of active bioremediation will be less than the cleanup goals established by the regulatory authorities.
-
Concentrations of substances of regulatory concern in ground water will not rise above the cleanup goals, within a prescribed period of monitoring, after active bioremediation is concluded.
-
The site owner will enjoy beneficial use of the property during remediation and will be allowed to sell or transfer it when remediation has been complete.
The following considerations have a direct bearing on the first two requirements.
Can Any Oily-Phase Residual Support a Plume?
Monitoring wells can provide a misleading picture of the course of bioremediation. Pumping, or seasonal changes in regional water tables, can drop ground water elevations below the depth interval occupied by oily-phase contaminants. Water produced by monitoring wells may be clean, but contamination will return when pumping stops or recharge raises the regional water table elevation. Changes in the stage of nearby rivers or lakes, combined with seasonal variations in recharge, may alter the slope of the water table (hydraulic gradient), which will change the trajectory of the plume of contamination. Plumes may actually move away from monitoring wells under these conditions, then return to them later.
To supplement data from monitoring wells, many regulatory authorities require a measure of residual oily-phase material left after bioremediation. Cleanup goals are usually set with the conservative assumption that the relative composition of oily-phase material does not change during remediation. As a result, concentrations of oily-phase material that are determined to be protective of ground water quality are low, on the order of 10 to 100 mg total petroleum hydrocarbon per kilogram of aquifer material (Bell, 1990).
Bioremediation, particularly innovative bioremediation that uses an electron acceptor other than oxygen, can remove the compounds of regulatory concern from the subsurface while leaving significant amounts of oily-phase hydrocarbons. The issue is whether any residual oily-phase hydrocarbon is capable of producing a plume of contamination at concentrations that exceed the cleanup goal.
The JP-4 bioremediation demonstration at Traverse City, Michigan, was used to evaluate the importance of partitioning of contaminants between ground water and residual oily material. The concen-
trations of BTEX compounds in recirculated ground water were compared to concentrations in the weathered oily-phase residual.
When infiltration with nitrate brought the concentrations of BTEX compounds in the JP-4 spill below action levels, infiltration was stopped and concentrations of BTEX compounds in the aquifer were measured under natural conditions. The JP-4 contaminated interval was cored and analyzed for residual total hydrocarbons and concentrations of BTEX compounds. The reduction in concentration of total petroleum hydrocarbons was minimal, from 2000 mg/kg to 1400 mg/kg. The concentrations of BTEX compounds in the residual oil were divided by the fuel-to-water partition coefficients of Smith et al. (1981) to predict the capacity of the residual to contaminate ground water. The concentrations measured in ground water under natural conditions were near or below the predicted concentrations (Hutchins et al., 1991b; see Table 1).
Apparently ground water quality is controlled by the relative concentration of organic contaminants in the weathered oily-phase residual and not by the absolute amount of weathered total petroleum hydrocarbons. The relative concentrations of organic contaminants can be used to predict the concentrations in ground water in contact with the oily-phase residual.
Accounting for Spatial Heterogeneity
Bioremediation is difficult to assess in heterogeneous geological material. Often, oily-phase material is associated with fine-textured material with low hydraulic conductivity. Remedial fluids tend to pass around the fine-textured material. Because the flux of nutrients and electron acceptor through the fine-textured material is small, there is little opportunity for bioremediation, and significant concentrations of contaminants can remain in subsurface material.
These relationships will be illustrated in a case history from an industrial site in Denver, Colorado (Nelson et al., in press). At this site, a temporary holding tank under a garage leaked used crankcase oil, diesel fuel, gasoline, and other materials into a shallow water table aquifer. Figure 6 shows the relationship between the garage, the work pit containing the leaking holding tank, and the approximate area of the spill.
Remediation involved removal of separate oily phases, in situ bioremediation with hydrogen peroxide and mineral nutrients, and bioventing. Ground water flow under ambient conditions was to the north or northeast. The flow of water during the remediation paralleled the natural gradient. Water was produced from a recovery well
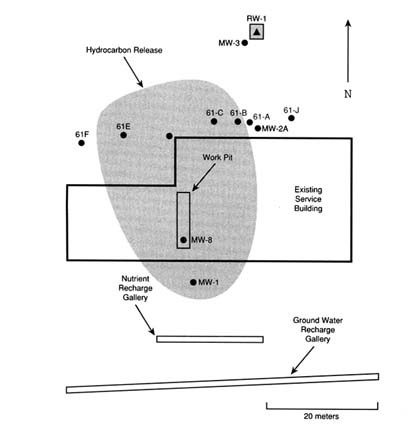
FIGURE 6 Infrastructure at an in situ bioremediation project in Denver, Colorado. A holding tank in a work pit under a garage leaked petroleum hydrocarbons to the water table aquifer. Ground water was pumped from a recovery well (RW-1) and filtered through activated carbon. The flow was split. Part was amended with hydrogen peroxide and mineral nutrients and recharged in a nutrient recharge gallery. The remainder was recharged in a ground water recharge gallery. The system was designed to sweep hydrogen peroxide and nutrients under the service building. MW-1, 2A, and 3 are monitoring wells, and 61A through 61J are boreholes for cores.
on the northeast side of the spill. The flow from the well was split; part of the flow was amended with hydrogen peroxide and nutrients and recharged to the aquifer in a nutrient recharge gallery on the south side of the spill (Figure 6). The remainder of the flow was delivered to a ground water recharge gallery to the south of the nutrient recharge gallery. From 3 to 6 gpm (11 to 22 l/min) was deliv-
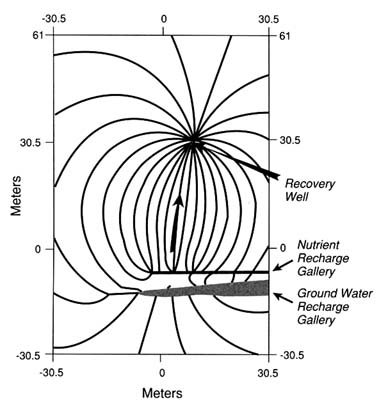
FIGURE 7 Hydraulic model of flow from the nutrient recharge gallery to the recovery well. Note that the spill is contained within the flow path from the gallery to the well and that the well captures all the flow lines from the nutrient recharge gallery.
ered to the nutrient recharge gallery, and 4 to 8 gpm (15 to 30 l/min) was delivered to the ground water recharge gallery, for a total flow of 9 to 11 gpm (34 to 42 l/min). Figure 7 presents a mathematical model of the flow paths from the galleries to the recovery well. The system was designed to sweep the ground water containing hydrogen peroxide and mineral nutrients through the spill to the recovery well.
The system was operated from October 1989 to March 1992. At a flow of 10 gpm (38 l/min), 10 to 15 pore volumes would have been exchanged in the area between the nutrient recharge gallery and the recovery well.
Remediation of Ground Water at the Denver Site
Table 2 compares the reduction in concentrations of benzene and total BTEX compounds in ground water that was achieved by in situ bioremediation at the site in Denver. Monitoring wells MW-1 and MW-8 (shown in Figure 6) are in areas with oily-phase hydrocarbons. Well MW-2A is just outside the region with oily-phase hydrocarbon. Well MW-3 is a significant distance from the region with oily-phase hydrocarbon; it sampled the plume of contaminated ground water that moved away from the spill.
Before remediation, concentrations in wells MW-1 and MW-8 were equivalent. Well MW-1 was closest to the nutrient recharge gallery, and the aquifer surrounding MW-1 was completely remediated; BTEX compounds were undetectable in ground water. In well MW-8, immediately adjacent to the point of release, the concentration of benzene was reduced at least one order of magnitude, and the concentrations of benzene and BTEX compounds in well MW-3 were also reduced an order of magnitude.
It is of particular interest that significant concentrations of benzene or total BTEX never developed in the pumped recovery well (RW-1). The BTEX compounds were monitored twice a month from July 1989 to March 1992. Benzene was detected only twice, at a concentration of 2 µg/1. The other BTEX compounds were never detected. Water from contaminated flow paths sampled by MW-3 was probably diluted by uncontaminated water from other flow paths to RW-1 (compare Figure 7). This behavior illustrates the contrast in contaminant concentrations between passive monitoring wells and pumped wells.
TABLE 2 Reduction in Concentration (µg/1) of Hydrocarbon Contaminants in Ground Water Achieved by In Situ Bioremediation
|
|
Benzene |
Total BTEX |
||||
|
Well |
Before |
During |
After |
Before |
During |
After |
|
MW-1 |
220 |
<1 |
<1 |
2030 |
164 |
<6 |
|
MW-8 |
180 |
130 |
16 |
1800 |
331 |
34 |
|
MW-2A |
? |
11 |
0.8 |
? |
1200 |
13 |
|
MW-3 |
11 |
5 |
2 |
1200 |
820 |
46 |
|
RW-1 |
<1 |
2 |
<1 |
<1 |
2 |
<1 |
Remediation of Subsurface Material at the Denver Site
Water in the monitoring wells and the recirculation well contained low concentrations of contaminants by March 1992. Active remediation was terminated, and the site entered a period of postremediation monitoring.
In June 1992 core samples were taken from the aquifer to determine the extent of hydrocarbon contamination remaining and whether a plume of contamination could return once active remediation ceased. The site was cored along a transect downgradient of the release. The transect extended laterally from clean material, through part of the spill, into clean material on the other side. In each borehole, continuous cores extended vertically from clean material above the spill, through the spill, to clean material below. The cores were extracted and analyzed for total petroleum hydrocarbons and for the concentrations of individual BTEX compounds.
The relationships between the land surface, the water table, the region containing hydrocarbons, and the bedrock are presented in Figure 8. Significant amounts of hydrocarbons remain within a narrow interval, approximately 0.6 m thick, near the water table. The total saturated thickness of the aquifer was approximately 6 m. At the time of sampling the elevation of the water table was 1610 m (5280.5 ft) above mean sea level, and all the hydrocarbons were below the water table.
The highest concentrations of hydrocarbons at the Denver site were obtained in samples from the borehole (D) closest to the work pit. Table 3 presents the vertical distribution of BTEX compounds and total petroleum hydrocarbons (TPH) in borehole D. The material in the interior of the spill had higher proportions of BTEX compounds. Table 4 makes the same comparison at the most contaminated depth interval along the transect. Material closer to the spill had higher concentrations of TPH and greater relative proportions of BTEX compounds.
Figure 9 plots the percentage of BTEX in the residual oil after bioremediation against the total content of hydrocarbon. Obviously, the materials with lower residual concentrations of hydrocarbons are more extensively weathered.
Infiltration of hydrogen peroxide and mineral nutrients at an aviation gasoline spill in Michigan preferentially removed BTEX compounds from the oily-phase gasoline, leaving a total petroleum hydrocarbon residual low in aromatic hydrocarbons (Wilson et al., in press). At the Denver site, apparently, a cortex of material that has been physi-
FIGURE 8 Cross section showing the vertical relationship of the land surface, water table, residual hydrocarbon after bioremediation, and the lower confining layer of the aquifer. The cross section runs through core boreholes depicted in Figure 6.
TABLE 3 Vertical Extent of Total BTEX Compounds and Total Petroleum Hydrocarbons (mg/kg) at Borehole D, the Most Contaminated Borehole in the Transect (Figure 8)
|
Elevationa |
TPH |
BTEX |
Benzene |
Color and Texture |
|
1609.711 to 1609.458 |
<44 |
<1 |
<0.2 |
Brown sand |
|
1609.458 to 1609.354 |
227 |
5.1 |
<0.2 |
Brown sand |
|
1609.354 to 1609.230 |
860 |
101 |
<0.2 |
Black sand |
|
1609.230 to 1609.101 |
1176 |
206 |
4.3 |
Black sand |
|
1609.101 to 1609.050 |
294 |
27 |
0.68 |
Black sand |
|
1609.050 to 1608.949 |
273 |
7.4 |
0.26 |
Black sand |
|
1608.949 to 1608.821 |
<34 |
<1 |
<0.2 |
Black sand |
|
1608.821 to 1608.492 |
<24 |
<1 |
<0.2 |
Brown to yellow sand |
|
a Meters above sea level. |
||||
TABLE 4 Lateral Distribution of Total BTEX Compounds and Total Petroleum Hydrocarbons (mg/kg) Along the Transect (Figure 8) at the Most Contaminated Depth Interval
|
Borehole |
TPH |
BTEX |
Benzene |
|
B |
167 |
0.8 |
<0.2 |
|
C |
156 |
3.5 |
<0.2 |
|
D |
1176 |
260 |
4.3 |
|
E |
156 |
3.5 |
0.06 |
FIGURE 9 Relationship between extent of hydrocarbon contamination (concentration of total petroleum hydrocarbons) and the extent of biological and chemical weathering (reduction in percentage of BTEX compounds in total petroleum hydrocarbons) in core material after bioremediation at the Denver site.
cally and biologically weathered surrounds a central core of material that has not been depleted of BTEX compounds.
The concentration of an individual petroleum hydrocarbon in solution in ground water in contact with oily-phase hydrocarbon can be predicted by Raoult's law. The solution concentration in water should be proportional to the mole fraction of the hydrocarbon in the oily phase. Assume that the weathered material is weathered because it is in effective contact with moving ground water that supplied nutrients and electron acceptors, and the residual is not weathered because it was not in effective contact and the supply of nutrients and electron acceptors was inadequate. If partitioning between moving ground water and the weathered oily residual controls the concentration of hydrocarbons in the water, the 10-fold reduction in concentrations of benzene and BTEX compounds seen in the weathered core material (Table 3) would produce the 10-fold reduction in concentrations of benzene and BTEX compounds seen in the monitoring wells (Table 2).
Do Mass Transfer Effects Limit Development of a Plume?
The usual expectation for in situ bioremediation is total removal of the contaminant from the subsurface environment. The extent of remediation more commonly achieved is removal of the contaminant from the circulated ground water.
In situ bioremediation merely accelerates the natural physical and biological weathering processes that occur in the subsurface. The oily material in most intimate contact with the circulated ground water is weathered to the greatest extent. After extensive remediation of the more transmissive regions, the release of contaminants to the circulated ground water is controlled by diffusion and slow advection from the subsurface material that still contains significant quantities of contaminants. The relationships are presented in Figure 10.
In such circumstances the disposition of contamination in the ground water can only be understood as a dynamic system. This release may best be described through the chemical engineering concept of a mass transfer coefficient. As the circulated water passes through the weathered spill, a certain quantity of hydrocarbon is transferred to the water; the amount transferred is directly proportional to the exposure time of the water in the contaminated area. If the circulated water contains enough nutrients and electron acceptor to meet the demand of the contaminants transferred from the fine-textured material, the plume will be destroyed by biological activity as rapidly as it is produced.
When active remediation is stopped, the concentration of elec-
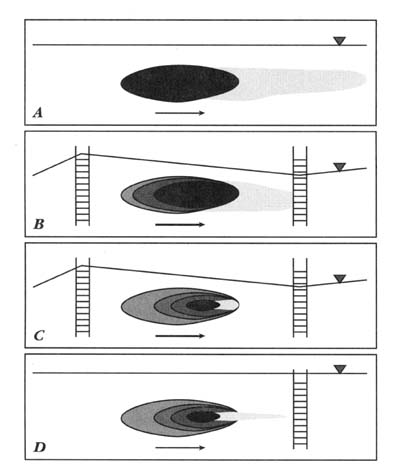
FIGURE 10 Schematic representation of in situ bioremediation in heterogeneous geological material. A fresh release (panel A) is rapidly weathered (panel B) due to increased flow of water and increased concentration of electron acceptor. As weathering progresses, aromatic hydrocarbons such as the BTEX compounds are restricted to regions with low hydraulic conductivity (panel C). After bioremediation the flux of aromatic hydrocarbons from the residual core to the moving ground water is controlled by mass transport limitations. The extent of the plume produced is controlled by the supply of electron acceptor (panel D). Although greatly attenuated, the plume may be regenerated under ambient conditions.
TABLE 5 Contrast in Conditions During Active In Situ Bioremediation and Conditions at the Termination of Remediation at a Spill from an Underground Storage Tank in Denver Colorado
|
Parameter |
During Active Remediation |
Ambient Conditions After Remediation |
|
Introduced concentration of oxygen |
470 mg/l |
5.5 mg/l |
|
Hydraulic gradient |
0.097 m/m |
0.0012 m/m |
|
Interstitial flow velocity |
2.4 m/day |
0.03 m/day |
|
Travel time of water across the spill |
20 days to recovery well |
1500 days to monitoring well |
|
Maximum oxygen demand supported |
20 mg/l per day |
0.004 mg/l per day |
tron acceptor returns to ambient conditions in the aquifer, and the hydraulic gradient returns to the normal condition. As a result, the residence time of water in the spill area is longer, and the total amount of hydrocarbon transferred to the water is greater, although the supply of electron acceptor for biological destruction of the hydrocarbon is less (compare panels C and D in Figure 10).
These relationships are well illustrated by the performance of bioremediation at the Denver site (Table 5). The hydraulic conductivity in the depth interval containing the hydrocarbons is approximately 8.5 m per day (David Szlag, University of Colorado, Boulder, personal communication). The distance from the nutrient recharge gallery to the recovery well is 45 m. Assume that the distance from the upgradient edge of the spill, through the spill, to monitoring wells downgradient at the property boundary is also 45 m. Assuming an effective porosity of 0.35, Darcy's law can be used to estimate the interstitial flow velocity of the ground water and its residence time along the flow path. When active remediation was terminated, the residence time of water was 75 times longer, and the supply of oxygen was 85 times less than conditions during active remediation (compare Table 5).
In June 1992 core material from the spill was assayed for the potential rate of oxygen consumption. Core material was dewatered by placing it in a Buchner funnel and applying a partial vacuum. Then the core material was sealed in a glass mason jar. After 24 hours, a sample of air in contact with the core material was passed through an oxygen-indicating tube to estimate oxygen consumption potential. Acclimated material exerted oxygen demands of 6 to more than 36 mg/kg core material per day (Table 6), equivalent to 40 to more than 240 mg/l ground water per day. At these rates, the oxy-
TABLE 6 Relationship Between the Potential Oxygen Uptake Rate (mg oxygen per kg per day) of Freshly Collected Core Material and the Location of the Core Material with Respect to the Interval Contaminated with Hydrocarbons
|
|
Position of Borehole in the Transect |
|||||
|
Location in Borehole |
A |
B |
C |
D |
E |
F |
|
Just Above |
|
|
<4 |
|
7.4 |
|
|
Within |
15.5 |
>30 |
>36 |
|
>34 |
23.5 |
|
Just Below |
6.0 |
<3 |
5.7 |
7.3 |
|
21 |
gen supplied as hydrogen peroxide during active remediation would be consumed in 2 to 12 days.
In the assay the microbial consumption of oxygen was faster than the rate of supply during active remediation. If the microbes in the aquifer expressed the potential rate of oxygen consumption, oxygen would have been depleted before the recharge water moved across the spill. In the absence of oxygen, BTEX compounds would have partitioned to the ground water and should have been detected in the monitoring wells. In fact, oxygen concentrations between 2 and 5 mg/l were always present in water produced by the recovery well, and BTEX compounds were virtually absent. Oxygen consumption must have been limited by mass transfer of hydrocarbon to the ground water circulated through the spill.
Relationship to Siting and Sampling Monitoring Wells
No established procedures exist for determining under ambient conditions whether the mass transfer of hydrocarbons from oily residual material will exceed the supply of oxygen or other natural electron acceptors. As a result, it is impossible to predict if natural bioremediation will prevent the regeneration of a plume, or if a plume of contaminated ground water will regenerate and at what concentrations. Ground water moving under the natural gradient must be allowed to travel all the way through the spill and then to the monitoring wells before it is possible to determine whether mass transfer effects will reestablish a plume.
An assessment of natural hydrologic conditions at a site will be necessary to intelligently locate compliance monitoring wells and determine an appropriate schedule of monitoring. Required are an un-
derstanding of the average natural hydraulic gradient and the hydraulic conductivity in the depth interval containing residual hydrocarbon. This information can be used to predict the velocity and trajectory of potential plumes of contaminated water. The frequency of monitoring can be adjusted to reflect the expected time required for ground water to travel through the area containing residual hydrocarbon to the point of compliance.
CONCLUSIONS
-
When oily-phase materials are to be remediated, core analyses are required to estimate the total mass of contaminant subject to remediation.
-
Headspace analyses in the filed can be used to screen core samples to identify those that deserve further analysis in the laboratory. If properly benchmarked by a limited number of laboratory analyses, field headspace techniques can provide a rapid and affordable estimate of total contaminant concentrations.
-
Simple ground water flow models can estimate the volume of water circulated through a spill during in situ bioremediation. This information can be coupled with simple partitioning theory to estimate the apparent attenuation due to dilution.
-
In situ bioremediation frequently leaves a residual of weathered oily-phase material.
-
Partitioning theory can be used to predict the concentrations of BTEX compounds in ground water in contact with the weathered oily residual.
-
After extensive in situ bioremediation, pockets of fine-textured material may still contain high concentrations of contaminants.
-
Mass transfer effects control the access of residual organic contaminants to moving ground water.
-
Under proper conditions, natural biodegradation supported by ambient concentrations of electron acceptors and mineral nutrients may destroy organic contaminants as fast as they escape from the oily-phase residual.
-
At the present state of science, only long-term monitoring can determine if natural biodegradation will prevent the regeneration of a plume of contaminated ground water.
ACKNOWLEDGMENTS
This work was supported by the United States Air Force through Interagency Agreement RW 57935114 between the Armstrong Labo-
ratory Environics Directorate (U.S. Air Force) and the R. S. Kerr Laboratory (U.S. Environmental Protection Agency). This work was also supported by the U.S. Environmental Protection Agency through the Bioremediation Field Initiative. It has not been subjected to agency review and therefore does not necessarily reflect the views of the agency, and no official endorsement should be inferred.
REFERENCES
Bell, C. E. 1990. State-by-state summary of cleanup standards. Soils: Analysis, Monitoring, Remediation (Nov.-Dec.):10-16.
Clark, I. 1979. Practical Geostatistics. London: Applied Science Publishers.
Downs, W. C., S. R. Hutchins, J. T. Wilson, R. H. Douglas, and D. J. Hendrix. 1989. Pilot project on biorestoration of fuel-contaminated aquifer using nitrate: part I—field design and ground water modeling. Pp. 219-233 in Proceedings, Petroleum Hydrocarbons and Organic Chemicals in Ground Water: Prevention, Detection, and Restoration, Nov. 15-17, 1989, Houston. Dublin, Ohio: National Water Well Association.
Downs, W. C., S. R. Hutchins, J. T. Wilson, R. H. Douglas, and D. J. Hendrix. In press. Nitrate-mediated biodegradation of BTEX in JP-4 contaminated soil and ground water. In Bioremediation: Field Experiences, P. E. Flathman, D. E. Jerger, and J. H. Exner, eds. Chelsea, Mich.: Lewis Publishers.
Hutchins, S. R., G. W. Sewell, D. A. Kovacs, and G. A. Smith. 1991a. Biodegradation of aromatic hydrocarbons by aquifer microorganisms under denitrifying conditions. Environmental Science and Technology 25(1):68-76.
Hutchins, S. R., W. C. Downs, J. T. Wilson, G. B. Smith, D. A. Kovacs, D. D. Fine, R. H. Douglass, and D. J. Hendrix. 1991b. Effect of nitrate addition on biorestoration of fuel-contaminated aquifer: field demonstration. Ground Water 29(4):571-580.
Kampbell, D. H., and M. L. Cook. 1992. Core assay method for fuel contamination during drilling operations. Pp. 139-140 in Subsurface Restoration Conference Proceedings. Houston: Rice University, National Center for Ground Water Research.
Kennedy, L. G., and S. R. Hutchins. 1992. Applied geologic, microbiological, and engineering constraints of in situ BTEX bioremediation. Remediation 3(1):83-108.
Nelson, C., R. J. Hicks, and S. D. Andrews. In press. In situ bioremediation: an integrated system approach. In Bioremediation: Field Experiences, P. E. Flathman, D. E. Jerger, and J. H. Exner, eds. Chelsea, Mich.: Lewis Publishers.
Robbins, G. A., R. D. Bristol, and V. D. Roe. 1989. A field screening method for gasoline contamination using a polyethylene bag sampling system. Ground Water Monitoring Review (Fall):87-97.
Siegrist, R. L., and P. D. Jenssen. 1990. Evaluation of Sampling Method Effects on Volatile Organic Compound Measurements in Contaminated Soils. Environmental Science and Technology 24(9):1387-1392.
Smith, J. H., J. C. Harper, and H. Jaber. 1981. Analysis and Environmental Fate of Air Force Distillate and High Density Fuels. Report ESL-TR-81-54. Tyndall Air Force Base, Fla.: Engineering and Services Laboratory, Air Force Engineering and Services Center.
Wilson, J. T., J. M. Armstrong, and H. Rifai. In press. A full scale field demonstration on the use of hydrogen peroxide for in situ bioremediation of an aviation gaso-
line-contaminated aquifer. In Bioremediation: Field Experiences, P. E. Flathman, D. E. Jerger, and J. H. Exner, eds. Chelsea, Mich.: Lewis Publishers.
Zapico, M. M., S. Vales, and J. A. Cherry. 1987. A wireline piston core barrel for sampling cohesionless sand and gravel below the water table. Ground Water Monitoring Review 7(3):74-82.
























































































