6
Measles and Mumps Vaccines
BACKGROUND AND HISTORY
Measles
Measles formerly afflicted virtually all children before they reached adolescence. It is a viral infection caused by a member of the paramyxovirus group. Conventionally, the diagnosis of measles is made clinically on the basis of its signs and symptoms, which include a characteristic rash. The diagnosis can be confirmed by a laboratory test that detects antibodies to the measles virus. It is also possible to isolate the measles virus, but this effort often fails. Therefore, failure to isolate the virus is not an argument against the diagnosis. A diagnosis of measles based solely on clinical appearance could be erroneous, because a number of other exanthematous diseases can resemble measles.
The disease can be quite debilitating, and its complications are among the most serious consequences of childhood exanthematous infections (Robbins, 1962). These include otitis media, croup, diarrhea, hemorrhagic rash, pneumonia, parainfectious encephalitis, and subacute sclerosing panencephalitis. Whatever its toll in industrialized countries, where the measles fatality rate is 1 per 10,000 cases (Babbott and Gordon, 1954), measles has been a far greater scourge in developing countries, with case fatality rates as high as 1,000 per 10,000 cases (Morley, 1974).
For these reasons, efforts to prevent measles have been extraordinary.
The initial method of prevention depended on postexposure prophylaxis with immune gamma globulin (Stokes et al., 1944). This method, although quite effective, was marred by several difficulties. It required vigilance with respect to exposure and almost immediate action, because if gamma globulin was given more than 4 days after the exposure, it was no longer effective at preventing disease, although it did attenuate it. Moreover, the prevention it afforded was short-lived, because the injected antibodies tended to disappear within about 2 months. An effort to allow the infection to take place, but in an attenuated form, by injecting less immune globulin was usually successful. The consequence of this was a milder case of clinical measles and a resulting lifelong immunity. However, the titration was not always perfect, and in some children the disease was inadvertently prevented, and therefore, they were soon susceptible again, whereas other children developed nearly full-blown measles, with all the risks of serious morbidity and complications.
The next step in prevention efforts was the development of a killed vaccine. The killed vaccine was derived from the Edmonston strain, which was originally isolated in 1954 (Enders and Peebles, 1954). The component antigen was the virus inactivated by formalin and precipitated by alum. Although this vaccine was in use for nearly 4 years (1963 to 1967), it was abandoned when analysis indicated that it provided only short-lived immunity and it was found that formerly vaccinated children developed severe reactions called ''atypical measles'' after their immunity waned and they became infected with the wild-type measles virus (Centers for Disease Control, 1967).
Development of a live attenuated measles vaccine began a new era in the prevention of this disease. The initial vaccine was derived from the Edmonston strain, which was attenuated by serial passage in various tissue cultures and ultimately grown in chicken embryo cells. The resulting variant was named the Edmonston B strain. It was quite immunogenic, but it was not free of side effects. One-third of the recipients developed high fever, and half of the recipients had a rash. Nevertheless, none of the recipients acted ill. Administration of the vaccine with immune globulin of the proper titer attenuated the reaction without interfering with the induction of permanent immunity.
In the meantime, two vaccines derived from the Edmonston B strain were developed by additional serial passage in chicken embryo cells that were maintained at a lower than optimal temperature. The resulting more-attenuated Enders strain (Hilleman et al., 1968a,b) was the product of an additional 40 passages of the Edmonston B strain; the Schwarz strain was the product of an additional 85 passages of the Edmonston B strain (Schwarz, 1964). Each vaccine induced immunity, and the side effects from these further attenuated vaccines were substantially reduced. The more-attenu-
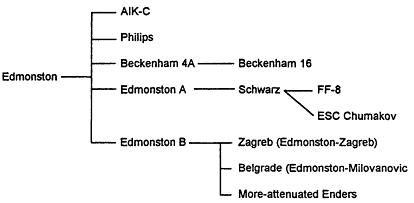
FIGURE 6-1 Derivation of measles vaccine strains. Sources: Adapted from Plotkin and Mortimer (1988, p. 189) and Hirayama M. (1983).
ated Enders strain vaccine is currently in use in the United States; the Schwarz strain is used elsewhere in the world. Other strains have been developed and used in various smaller population groups. 'Figure 6-1 illustrates the derivation of many measles vaccines from the Edmonston strain. Table 6-1 lists the measles vaccines used in the United States.
TABLE 6-1
Measles Vaccines Used in the United States
|
Attenuation |
Strain |
Trade Name |
Manufacturer |
Years in Use |
|
Live attenuated |
Edmonston B |
Rubeovax |
Merck Sharpe & Dohme |
1963-1975 |
|
|
|
M-Vac |
Lederle |
1963-1975 |
|
|
|
Pfizer-vax; |
Pfizer |
1963-1975 |
|
|
|
Measles-L |
|
|
|
|
|
Generic |
Lilly, Parke Davis, Philips Roxane |
1963-1975 |
|
Live, more attenuated |
Schwarz (derived from Edmonston A) |
Lirugen |
Pitman Moore-Dow |
1965-1976 |
|
|
More-attenuated Enders (derived from Edmonston B) |
Attenuvax |
Merck Sharp & Dohme |
1968-presen |
|
Source: Adapted from Plotkin and Mortimer (1988, p. 189). |
||||
Mumps
Unlike measles, mumps is not considered a globally devastating disease. Nevertheless, because of its complications, it was targeted for prevention by use of a vaccine. The complications that prompted this were epididymoorchitis, aseptic meningitis, meningoencephalitis, and deafness (usually, but not exclusively, unilateral) (Coll, 1974).
Before a vaccine was developed, there was no effective means of preventing this disease. Mumps is rare in the first year of life, and its rarity has been attributed to the passive protection rendered by maternal antibodies (Meyer, 1962). Nevertheless, immune globulin injections administered after exposure do not prevent mumps (Reed et al., 1967).
Development of mumps vaccine had two stages. Initially, there was an inactivated vaccine (Enders, 1946). It was not sufficiently effective, in that it offered protection only to some 80 percent of the recipients and the protection lasted for less than 1 year. Therefore, investigators undertook efforts to develop an attenuated strain of mumps virus that could be used as a live vaccine.
The Jeryl Lynn strain, the mumps virus strain used in mumps vaccines in the United States, came about by numerous passages in vitro, first in embryohated ben's eggs and then in chicken embryo cells (Buynak and Hilleman, 1966). The seroconversion rate was nearly 97 percent. Subsequently, two other strains were developed by similar attenuation of a wild-type isolate. They are Leningrad-3-Parkow and Urabe AM9, which were generated in the former Soviet Union and Japan, respectively.
The American Academy of Pediatrics recommends that measles-mumps-rubella vaccine (MMR) be given at age 15 months and at entry into middle or junior high school. The Advisory Committee on Immunization Practices recommends that MMR be administered at 15 months and then again at school entry at age 4 to 6 years. ("MMR" is used in this report to indicate any multivalent vaccine preparation directed against measles, mumps, and rubella. No association with a specific manufacturer is intended or should be inferred.)
BIOLOGIC EVENTS FOLLOWING IMMUNIZATION
Measles
Although the measles vaccine is administered by injection rather than by the natural, respiratory route of infection, the host response is similar to that evoked by the wild-type virus in all but two respects. The immunized subject develops humoral and cellular immune responses some 48 hours earlier than the naturally infected host, and the recipient of the vaccine does
not develop clinical measles. Three classes of immune globulin (IgA, IgG, and IgM) are produced and are detectable in the serum and nasal mucus of vaccinated subjects (Bellanti et al., 1969).
The standard test of immunity to measles is based on the detection of serum antibodies by the enzyme-linked immunosorbent assay method. Although the titers of these antibodies induced by the vaccine tend to be somewhat lower than those resulting from natural infection (Schwarz and Anderson, 1965), immunity acquired by vaccination is long-lasting (Krugman, 1983).
Mumps
Following the administration of mumps vaccine, seroconversion is slower and the antibody titers achieved are lower than those following natural infection. A neutralizing antibody response can be detected in some recipients 2 weeks after vaccine administration; in others, it can be delayed for up to 6 weeks (Hilleman et al., 1968a,b). It is assumed that this immunity is long-lasting, but this has not yet been established.
ENCEPHALOPATHY AND ENCEPHALITIS
Clinical Description
Encephalopathy refers to any acute or chronic acquired abnormality of, injury to, or impairment of the function of the brain. Symptoms can include alterations in state of consciousness or behavior, convulsions, headache, and focal neurologic deficits. Encephalitis refers to an encephalopathy caused by an inflammatory response in the brain. This is usually manifested with systemic constitutional symptoms, particularly pleocytosis of the cerebrospinal fluid (CSF). However, the terms encephalopathy and encephalitis have been used imprecisely and even interchangeably in the literature. The discussion that follows uses the terminologies of the authors of the reports. However, if the authors used the term encephalitis, but there was no documentation of pleocytosis in the CSF, "encephalitis" is used in quotation marks. The annual incidence of encephalitis for the years 1950 to 1981 in Olmsted County, Minnesota, was 7.4 per 100,000 people (Beghi et al., 1984; Nicolosi et al., 1986). The incidence in children less than age 1 year was 22.5, in children between ages 1 and 4 years it was 15.2, and in children between ages 5 and 9 years it was 30.2 per 100,000. Other estimates of encephalopathy for children less than age 2 years were somewhat lower than those reported by Beghi et al. and Nicolosi et al. cited above. Other estimates for annual incidence range from 5 per 100,000 people (Walker et al., 1988) to 10 per 100,000 people (Gale et al., 1990). Chapter 3 contains a discussion of encephalopathy.
History of Suspected Association
The occurrence of encephalitis following a natural measles virus infection is well described. The condition is quite severe, often leading to permanent brain damage or even death. There may be no detectable pathologic lesion, but in most cases some edema and demyelination are noted. Early studies of the adverse events associated with measles vaccine concentrated on "encephalitis." These are described below (Landrigan and Witte, 1973; Nader and Warren, 1968).
The first report of encephalopathy following vaccination with the live attenuated Edmonston B (Rubeovax) measles vaccine appeared in 1967 (Trump and White, 1967). A 2-year-old girl developed unsteadiness 7 days following vaccination. This was followed by pronounced generalized ataxia (diagnosed as cerebellar ataxia), fever, vomiting, and an exanthem. There was pleocytosis in the CSF 1 month after vaccination. The ataxia persisted for at least 8 months. Because of the child's history and physical and laboratory findings, the investigators attributed the condition to measles vaccination. Two early case series investigations of neuralgic disorders following measles vaccination included reports of "encephalitis." These are discussed below.
Mumps affects the central nervous system as well, but it is more likely to cause meningitis than encephalitis (Azimi et al., 1969). This condition tends to be self-limited and has a good prognosis. Cases of pure encephalitis following mumps are rare, but they can be quite severe.
Evidence for Association
Biologic Plausibility
Chapter 3 contains a discussion of the biologic plausibility for certain types of encephalopathies and vaccination. As described above, natural (wild-type) measles virus infection is associated with a well-described, frequently very severe encephalitis.
Case Reports, Case Series, and Uncontrolled Observational Studies
Many uncontrolled observational studies in the literature describe the occurrence of encephalopathy after administration of measles vaccine. These are reviewed first. Data from similar studies regarding multivalent preparations are described next. Individual case reports and unpublished case reports from U.S. Public Health Service passive surveillance systems are discussed last. There are no data regarding monovalent mumps vaccine and encephalopathy.
Measles Vaccine The first published case of encephalopathy (acute cerebellar ataxia) attributed to measles vaccine was discussed above (Trump and White, 1967). Retrospective analyses of populations who have received measles vaccine have been reported from many countries, including the United States. These uncontrolled observational studies provide no information on the concurrent background rates of encephalopathy. Table 6-2 summarizes case series and uncontrolled observational studies in which the incidence rates of encephalopathy or encephalitis following administration of measles vaccine were calculated by the authors.
Two case series addressed early concerns in the United States that measles vaccine might cause encephalitis. The first was a report of 23 cases of neurologic disease following measles vaccination in the United States from January 1965 to February 1967 (Nader and Warren, 1968). The authors characterized 18 of the 23 cases as "encephalitis" (described as including disturbances of sensorium, seizure, major loss of motor function, and cerebral edema; no data are provided regarding pleocytosis in the CSF). The interval from vaccination to the onset of symptoms ranged between 3 and 24 days. Postmortem findings in one case revealed herpes simplex virus in brain tissue. There were two cases of aseptic meningitis, two cases of cerebellar ataxia, and one case of extraocular muscle paralysis. The authors estimated a rate of 1.5 reported cases of "encephalitis" within a 4-week period of vaccination per 1 million doses of vaccine distributed. They compared this with a background rate of 2.8 cases of encephalitis (unrelated to vaccination or known parainfectious causes) per 1 million children for any 4-week period. The authors concluded, ''No single clinical or epidemiologic characteristic appears consistently in the reports of cases of possible neurologic sequelae of measles vaccination" (p. 998).
A review of 84 patients with neurologic disorders occurring within 30 days of vaccination against measles virus reported to the Centers for Disease Control from 1963 to 1971 revealed 59 patients with extensive neurologic disorders, which included encephalomyelitis (Landrigan and Witte, 1973). The cases reported by Nader and Warren (1968) and discussed above are a subset of the data of Landrigan and Witte (1973). Although in all 59 patients the onset of symptoms occurred between 1 and 25 days after vaccination, in 45 it coincided with the period of maximal viral replication (6 to 15 days after vaccination). Of 50 patients for whom follow-up information was available (follow-up presumably from 1963 to sometime before 1973), 26 recovered fully, 5 died (2 of the 5 had pathologic features of Reye syndrome), and 19 were left with permanent neurologic damage. Thirteen of the 59 patients were classified as having encephalomyelitis. Long-term follow-up of 12 of the patients showed residual neurologic signs in 3 patients. Long-term follow-up was available for 31 of 36 patients considered to have encephalopathy. Ten of those 31 patients recovered fully, 5 died,
TABLE 6-2 Rates of Encephalitis/Encephalopathy After Measles Vaccination from Uncontrolled Studies
|
Reference |
Years Covered |
Measles Vaccine Used |
Condition |
No. of Cases |
Calculated Rate per Million Doses |
|
Nader and Warren, 1968 |
January 1965 to February 1967 |
Live attenuated |
Encephalitis |
18 |
1.5 |
|
Landrigan and Witte, 1973 (overlaps with Nader and Warren, 1968) |
1963 to 1971 |
Live attenuated |
Encephalomyelitis (including transverse myelitis) |
13 |
1.2 |
|
|
|
|
Encephalopathy |
36 |
|
|
Hirayama, 1983 |
1978 to 1982 (?) |
Schwarz |
Encephalitis/ encephalopathy |
8 |
3.7 |
|
|
|
Biken-CAM |
|
4 |
2.9 |
|
White, 1983 |
1965 to 1976 |
Unspecified |
Encephalitis |
7 |
1.8 |
|
Koch et al., 1989 |
1987 |
MMR (unspecified) |
Meningitis/ encephalitis |
5 |
11 |
|
Fescharek et al., 1990 |
1976 to 1989 |
More attenuated Enders |
Meningitis/ encephalitis |
16 (total) |
3 |
|
|
|
(measles, measles-mumps, MMR) |
|
5* |
1 |
|
* The authors thought that 5 of the 16 cases were possibly causally related to the vaccine. |
|||||
and 16 were left with neurologic residua. The authors calculated rates of "encephalitis" of 1.16 cases per 1 million doses of vaccine distributed.
Among the recipients of more than 3 million doses of measles vaccine (various strains, but mostly the Schwarz strain) in the United Kingdom between 1968 and 1974, there were 47 cases of "encephalitis" (Beale, 1974). The report does not discuss the criteria used for the diagnosis. Data on the occurrence of encephalitis in temporal relation to administration of measles vaccine for the years 1965-1976 in Canada showed a rate of 1.79 cases of encephalitis per 1 million doses of vaccine distributed (White, 1983). These data are based on hospital admissions associated with International Classification of Diseases codes for "viral encephalitis unspecified" and "acute viral encephalitis.''
In a report from the former East Germany (Dietzsch and Kiehl, 1976), there were 7 central nervous system (CNS) complications out of 174,725 immunizations with an unstated vaccine, but it was probably one of the strains from the former Soviet Union. Two febrile seizures, four cases of encephalopathy, and one case of encephalitis (there was pleocytosis in the CSF) were reported. Few clinical details were reported. Two of the patients with encephalopathy and the patient with encephalitis recovered completely, one patient with encephalopathy was left with a residual paralysis, and another died of leukemia.
A report from the former Soviet Union (Ozeretskovskii and Gurvich, 1991) referred to cases of encephalitis and encephalitic reaction caused by a measles vaccine (probably the Smorodintsev strain), but offered no primary data. The authors quote three rates per 100,000 vaccinees: 0.1, 0.02, and 190 cases. The rate of 0.02 is far below the acknowledged background rate of encephalitis and the rate of 190 is far above any rates quoted anywhere for encephalitis/encephalopathy after receipt of measles vaccine. Considering the imprecision of the definition of "encephalitis" and "encephalitic reaction" and the discrepancy of the rates, it is impossible to interpret that report.
A report of adverse events associated with measles vaccine in Japan from 1978 to 1983 cited 12 cases of "encephalitis" or "encephalopathy," without describing them, and derived a rate of 3.7 cases of "encephalitis'' per 1 million vaccinees administered the Schwarz vaccine and 2.9 cases per 1 million vaccinees administered the Biken-CAM vaccine (Hirayama, 1983). A follow-up to that report published 5 years later (Isomura, 1988) mentioned 16 cases of "encephalitis" (4 more cases than the earlier report), but provided no details. The incidence rate for "encephalopathy" and "encephalitis" following measles vaccination appeared to be lower than the observed incidence of encephalitis from all causes among age-matched controls (Hirayama, 1983; Isomura, 1988). This comparison was not derived from a controlled cohort study, however.
Measles Vaccine-Containing Preparations In an analysis of 433 spontaneous reports to a vaccine manufacturer in the Federal Republic of Germany (former West Germany) between 1976 and 1989 (Fescharek et al., 1990), 6 of 16 reports of "meningitis" or "encephalitis" were thought by the authors to be possibly related to measles, measles-mumps, or measles-mumps-rubella vaccine, leading to a rate of 1 case per 1 million doses distributed, as calculated by the authors. The vaccine strains are those currently licensed in the United States, that is, the more attenuated measles vaccine and Jeryl Lynn mumps vaccine. Assuming that all 16 reports of cases of "meningitis'' and "encephalitis" were causally related to the vaccine, the rate would increase to about 3 cases per 1 million doses distributed, which is within the range reported in other countries, as described above.
A study based on a new passive surveillance system in Canada reported a rate of 1.1 cases of meningitis or encephalitis, without distinguishing between the two, per 100,000 doses of MMR (Koch et al., 1989). (This high rate was probably due to the inclusion of meningitis in the survey.) It was estimated that more than 8 million doses of MMR were distributed in Canada during the reporting period.
A description of 212 adverse events associated with MMR reported to Swedish health authorities from 1982 to 1984 (when an estimated 700,000 doses of MMR were sold) includes 17 reports of transient, serious cases of neurologic symptoms: 3 patients with "encephalitic symptoms" who were treated at the hospital, 7 patients with "encephalitic symptoms" who were not hospitalized, 5 patients with acute symptoms with motor difficulties, 1 patient with seizures and fever, and 1 patient with hemiparesis (Taranger and Wiholm, 1987). "Encephalitic symptoms" included tiredness, whining, irritability, and mood changes with or without fever. No mention of CSF pleocytosis was made. Follow-up of at least 1 year showed that one 18-month-old boy who had developed symptoms of mild encephalitis with balance problems had residua of foot dragging and stumbling when he was tired.
Case Reports Many case reports describe encephalitis or encephalopathy following administration of measles vaccine. Because isolation of measles virus is problematic and exposure to wild-type measles virus is common, it is difficult to assess a possible role of measles or measles vaccine in the occurrence of encephalopathy or encephalitis in an individual case. Typical case reports follow.
A 5-year-old received a live measles vaccine and developed fever two weeks later (Alves et al., 1992). Three days after the onset of fever, the boy presented with hemiparesis, dysarthria, and a generalized rigid-akinetic syndrome. A spinal tap obtained four days later showed pleocytosis. One month later he was diagnosed with postencephalitic parkinsonism. He responded to levodopa therapy. The parkinsonism persisted for the 2 years
between the time of vaccination and publication of the report (Alves et al., 1992). A 14-month-old girl received the wellcome measles vaccine and developed convulsions 12 days later (Barbor and Grant, 1969). She became confused, restless, and then unconscious. Although the authors called this an encephalitis, there was no CSF pleocytosis on days 13 or 21 postvaccination. She made little progress in the 4 months between hospitalization and publication of the report. An electroencephalographic record of slow waves, which are not characteristic of measles encephalitis, and possible slight head trauma 9 days after vaccination suggested a temporal, not a causal, relation between the convulsions and the measles vaccination. A 13-month-old girl was admitted to the hospital with involuntary jerking movements of her limbs 10 days after receiving a further attenuated Enders live measles vaccine (Jagdis et al., 1975). She was afebrile, although she had fever for 2 days prior to admission. The CSF was turbid and showed pleocytosis. She had a convulsion followed by apnea. She died 13 days after vaccination. Postmortem examination suggested viral encephalitis; Cowdry type A inclusion bodies suggested measles virus as the etiologic agent, but no measles virus was isolated. She had no known exposure to wild-type measles virus, but an epidemic in the community was ending. Haun and Ehrhardt (1973) described a boy age 11 months who developed drowsiness, convulsions, and coma 12 days following vaccination with the L-16 SSW measles vaccine (a variant derived from the Soviet strain Leningrad-16). There was pleocytosis in the CSF. He died the same day as onset of symptoms. Autopsy findings were suggestive of disseminated intravascular coagulation as the cause of death. A boy age 2 years was administered live measles vaccine 10 days before the development of persistent convulsions (Starke et al., 1970). The child suffered convulsions accompanied by unconsciousness until his death a month later. He had experienced convulsions in the first year of life during a bout of pneumonia. The autopsy stated there was CNS death, "encephalitis" following measles inoculation, and septic pulmonary infarction. No further details are given.
Several reports of encephalopathy following measles vaccination can be found in the Vaccine Adverse Event Reporting System (VAERS) (submitted between November 1990 and July 1992). As with many VAERS reports, the information that is supplied is frequently inadequate to support or reject a diagnosis or to exclude the possibility that other factors are responsible for the disorder, if the case was encephalopathy or encephalitis. The committee found that 17 VAERS reports were suggestive of encephalopathy or encephalitis in vaccinees (mostly MMR) from ages 5 months to 16 years. Reported latencies ranged from 1 to 14 days after immunization. The patients presented with symptoms such as fever, ataxia, somnolence, convulsions, and flaccid paralysis. Several reports contained too little information to suggest a diagnosis or to shed light on causality.
A specific type of measles encephalopathy, immunosuppressive measles encephalopathy (IME), has been documented in two immunosuppressed children following vaccination against measles. IME is distinct from acute measles encephalitis and subacute sclerosing panencephalitis. It has an incubation period of 5 weeks to 6 months. In one case of a 7-year-old girl with acute lymphoblastic leukemia (Valmari et al., 1987), measles virus was isolated from her CSF approximately 10 weeks after she received MMR (which contains the more attenuated measles vaccine used in the United States). The authors believed the isolated virus was vaccine strain rather than the wild-type strain because the child had no contact with natural measles during the 5 weeks to 6 months prior to the onset of symptoms. A previously described case of IME in a leukemic child involved the Schwarz strain vaccine virus (Mitus et al., 1962). Measles virus was cultured from throat and conjunctiva, but not from postmortem brain tissue.
Controlled Observational Studies
The National Childhood Encephalopathy Study, a case-control study described in detail in Chapter 5, reported a significant association between measles vaccination and onset of either convulsions or encephalopathy within 7 to 14 days of receiving the vaccine (Alderslade et al., 1981). However, a separate analysis of those diagnosed with encephalitis or encephalopathy was not performed
Controlled Clinical Trials
A report from India (Kumar et al., 1982) described 206 children injected with the Schwarz strain of measles vaccine and 206 children who were not immunized. A 14-month-old girl was diagnosed with encephalitis (fever, vomiting, semi-consciousness, weakness, occasional white blood cells in the CSF) on postvaccination day 10. At the time the report was published, she was reported to be recovering "gradually." There were no cases of encephalitis in the controls, but the numbers are far too small to detect an association.
Causality Argument
There is demonstrated biologic plausibility that measles vaccine might cause encephalopathy. Although there are a number of reports of encephalitis or encephalopathy following immunization with measles vaccines of various strains, the rates quoted are impossible to distinguish from background rates. Good case-control or controlled cohort studies of these conditions in similar unvaccinated populations, which are necessary for deter-
mining the causal relation between measles and mumps and encephalopathy and encephalitis, are lacking. No conclusive evidence of the occurrence of encephalopathy or encephalitis resulting from the administration of measles vaccine was identified. There are no data regarding the occurrence of encephalopathy following administration of monovalent mumps vaccine. It is therefore not possible to implicate specifically either the measles or mumps component of MMR.
Conclusion
The evidence is inadequate to accept or reject a causal relation between measles or mumps vaccine and encephalitis or encephalopathy.
ASEPTIC MENINGITIS
Clinical Description
Aseptic meningitis is defined as an inflammation of the meninges associated with pleocytosis of the CSF. In the early stage of aseptic meningitis polymorphonuclear leukocytes predominate, but within 8 to 16 hours this changes to a predominance of mononuclear cells. There may be some elevation of protein, but in general, the glucose level is normal. In patients with aseptic meningitis associated with mumps, there may be hypoglycorrhachia. Bacterial cultures are negative. The description of aseptic meningitis in the wake of mumps vaccine administration follows this pattern, except that hypoglycorrhachia was not mentioned in the reports.
The yearly incidence of aseptic meningitis for the years 1950 to 1981 in Olmsted County, Minnesota, was 10.9 per 100,000 people (Nicolosi et al., 1986). The annual incidence was markedly higher in children less than age 1 year (82.4 per 100,000) and slightly higher in children between ages 1 and 4 years (16.2 per 100,000) and in children between ages 5 and 9 years (18.8 per 100,000).
History of Suspected Association
Mumps disease is clearly associated with aseptic meningitis. The committee was charged with investigating a possible causal relation between only mumps vaccine and aseptic meningitis.
Evidence for Association
Biologic Plausibility
Mumps disease has been found to be clearly associated with aseptic meningitis. Mumps virus (both wild-type and vaccine strains) has been isolated from the CSF of patients with aseptic meningitis.
Case Reports, Case Series, and Uncontrolled Observational Studies
The ability to isolate mumps virus from the CSF of patients presenting with symptoms of meningitis and to determine the type of the isolate as a wild-type or a vaccine strain indicates that mumps vaccine can cause aseptic meningitis. Many case series and observational studies have documented cases of meningitis after vaccination with mumps virus-containing vaccine. Of particular interest are the cases in which the vaccine strain was identified. This has been done extensively with the Urabe strain. Data concerning the Urabe strain mumps vaccine will be presented first. Data related to the Jeryl Lynn strain (that used in the United States) are presented last.
In 1989, Gray and Bums published two letters (Gray and Bums, 1989a,b) in The Lancet concerning a 3-year-old girl presenting with aseptic meningitis 21 days after vaccination with MMR. Fluorescent-antibody tests identified the isolated virus as mumps virus (Gray and Bums, 1989a), and soon thereafter, this virus was identified by nucleotide sequencing analysis as the Urabe strain (Gray and Burns, 1989b).
Identification of the mumps virus as the Urabe vaccine strain by nucleotide sequence analysis of the isolates from eight patients with meningitis in Canada led to suspension of the sale of that vaccine in Canada in May 1990 (Brown et al., 1991). Using the polymerase chain reaction to amplify the genetic signal, investigators from Japan also typed mumps virus isolated from patients with meningitis as a vaccine strain, most probably Urabe (Mori et al., 1991; Yamada et al., 1990).
Most recently, the Nottingham (United Kingdom) Public Health Laboratory isolated mumps virus from the CSF of eight children following administration of Urabe-containing MMR (Colville and Pugh, 1992). Seven of the isolates resembled the vaccine strain (the sample from the eighth patient could not be typed). Vaccination occurred 17 to 24 days prior to the lumbar puncture. The rate of virologically confirmed and suspected MMR-associated meningitis was calculated to be 1 case per 3,800 doses. None of the children had severe illness, and no sequelae were seen. Colville and Pugh (1992) reviewed laboratory records from an approximately 3-year period and determined that there were excess cases of lymphocytic meningitis in the group that recently received MMR compared with the incidence in
those who had not recently been vaccinated with MMR. More cases of Urabe strain-related meningitis have been identified in the United Kingdom, and use of the Urabe vaccine strain has been suspended in that country.
A Urabe strain-containing MMR was released in Canada in 1986. Soon after that, cases of mumps meningitis began to appear. In an investigation at Montreal Children's Hospital of four patients with meningitis that appeared within 19 to 26 days after receipt of the Urabe-containing vaccine, mumps virus was isolated from the patients' CSF, as detected by hemadsorption inhibition with mumps antisera (McDonald et al., 1989). This did not distinguish the vaccine strain from the wild-type strain; however, none of the four patients were known to have had contact with an individual with natural mumps virus infection. The illnesses were not severe, and all patients recovered without sequelae.
Retrospective studies of mumps-associated meningitis and reports from surveillance systems provide more data regarding a relation between mumps vaccine and meningitis. Cizman et al. (1989) retrospectively reviewed the medical records of 2,418 children hospitalized and treated for aseptic meningitis at University Medical Center in Ljubljana, Yugoslavia, between 1979 and 1986. The etiology of the aseptic meningitis was assessed by serologic tests and isolation of the virus from CSF, urine, feces, or throat swabs. They confirmed the presence of mumps virus strains by the complement fixation test with a specific antiserum. They also tested for poliovirus, Central European tick-borne encephalitis virus, and herpes simplex virus. In 115 children, the onset of aseptic meningitis occurred within 30 days of vaccination against measles and mumps (Leningrad 3 strain), leading to an attack rate of approximately 1 per 1,000 immunized children, as calculated by the authors. Most of the cases occurred between 11 and 25 days after vaccination. The attack rate in immunized 6- to 8-year-old children was 3.5 times greater than that in immunized 1- to 3-year-old children. None of the children had sequelae. Signs of parotitis and virologic findings suggestive of mumps infection were found in 65 of the children, although only 1 child had a history of exposure to mumps. Much more enterovirus was isolated from children with nonvaccine-associated aseptic meningitis than from the 115 children with vaccine-associated aseptic meningitis. Although the authors did not calculate a rate of aseptic meningitis and they did not report how many cases of aseptic meningitis they finally attributed specifically to mumps vaccination, they were clearly concerned about the high incidence and, on the basis of in vitro tests, believed that their vaccine was inadequately attenuated compared with the Jeryl Lynn strain.
Introduction of vaccination for measles, mumps, and rubella (using the Urabe strain mumps vaccine) in Japan in 1989 coincided with early reports of mumps vaccine-associated meningitis. This prompted surveillance efforts in Japan to study the problem. Pediatricians at 24 hospitals in the
Gunma Prefecture were asked to fill out a questionnaire regarding clinical details and laboratory findings for patients with aseptic meningitis without a history of vaccination with MMR and for patients with parotitis and convulsive disorders within 2 months of vaccination with MMR during an 8-month period in 1989 (Fujinaga et al., 1991). There were 35 cases of aseptic meningitis within 2 months of vaccination with MMR. These patients had no history of contact with individuals with natural mumps virus infection. Mumps meningitis was seen in 38 patients with no history of vaccination, and meningitis resulting from other causes was seen in 46 patients. Mumps virus, but no other viral isolates, was detected by indirect immunofluorescence in 13 patients with aseptic meningitis who had been vaccinated within the 2 previous months, but who were negative for contact with wild-type mumps virus. Characterization of virus in samples from 13 patients by the polymerase chain reaction and nucleotide sequence determination or by restriction enzyme analysis determined that all 13 viruses were of the Urabe strain. They referred to these as the virus-positive group. They divided the remaining 22 patients into two groups: 11 patients who seroconverted (the serum-positive group) and 11 patients who had clinical signs of meningitis but from whom virus was not isolated and who had not seroconverted. They calculated incidence rates for the virus-positive group, the serum-positive group, and the clinical meningitis group for the 2-month period of 3, 2.5, and 1.5 cases per 1,000 children vaccinated with MMR, respectively. The estimated background incidence of acute neurologic diseases in the Gunma Prefecture for the years 1987 and 1988, by comparison, was 0.37 per 1,000 children.
A nationwide surveillance of neurologic complications after mumps vaccine administration in Japan during 1989 (which presumably included the data from the report described above [Fujinaga et al., 1991]) revealed 311 suspected cases of vaccine-related meningitis among 630,157 vaccinations with MMR (Sugiura and Yamada, 1991). Of 222 CSF samples examined, 99 samples contained mumps virus, and 96 of these were shown by molecular biology techniques to be the Urabe strain. The incidence rates of suspected or laboratory-confirmed aseptic meningitis were 1 in 2,026 and 1 in 6,564 people administered MMR, respectively. The authors noted that these incidence rates were higher than the estimated incidence rate among those who received monovalent Urabe strain mumps vaccine before or during the survey period. They also noted that all patients with aseptic meningitis recovered without sequelae.
Data concerning aseptic meningitis in association with the Jeryl Lynn strain mumps virus are more scarce than those related to the Urabe strain. Virus was isolated from a patient with symptoms of meningitis beginning 20 days after vaccination with the Jeryl Lynn strain of mumps vaccine (that used in the United States) (Ehrengut and Zastrow, 1989). The isolated
virus, obtained from a swab of the orifice of Stenson's duct and from the CSF, was identified as the vaccine strain on the basis of the morphology of the cytopathic effect but not by molecular analysis. Fescharek and colleagues (1990) described the isolation of mumps virus from two patients with meningitis reported to the pharmaceutical firm Behringwerke AG in the former West Germany. The mumps vaccine administered was Jeryl Lynn (that used in the United States), but identification of the virus as wild-type or vaccine strain was not attempted.
Eleven cases of meningitis following receipt of MMR in the United States (the Jeryl Lynn strain of mumps vaccine) reported in VAERS (submitted between November 1990 and July 1992) were examined by the committee. In no case was the strain identified or the virus isolated. The latencies from vaccination to symptoms ranged from 3 days to 2 weeks. In some patients the clinical symptoms seemed supportive of a diagnosis of meningitis, but intercurrent infections were seen in two of the patients, insufficient information was available for three patients, and encephalopathy was possible for another patient.
Controlled Observational Studies
None.
Controlled Clinical Trials
None.
Causality Argument
There is strong biologic plausibility that mumps virus could cause aseptic meningitis. Wild-type mumps virus clearly does so. Isolation of the virus and typing by molecular biologic techniques as the vaccine strain of mumps virus from patients who developed aseptic meningitis following immunization with mumps vaccine provide evidence of a causal relation. This relation is firmly established for the Urabe strain. The incidence appears to be approximately 1 case per few thousand vaccine recipients. The matter is unclear with regard to the Jeryl Lynn strain (that used in the United States), because in the sole reported case in which the virus was identified as the "vaccine strain," the isolated virus was typed by the older morphologic technique and not by molecular analysis. In the two other published cases of Jeryl Lynn-associated mumps meningitis, the virus was not typed as the vaccine or wild-type strain. VAERS contains several reports of what probably is meningitis after administration of Jeryl Lynn mumps vaccine-containing preparations, but the reports do not describe virus isolation or typ-
ing. A recent study of various commercial mumps vaccine preparations demonstrates the existence of two populations of Jeryl Lynn strain virus in commercial vaccine preparations, with sequence variation of up to 4.4 percent for some genes (Afzal et al., 1992). Only one population of the Urabe strain was detected. The authors hypothesized that one of the populations could interfere with the growth of the other, thus influencing rates of adverse reactions. There are no data to substantiate this hypothesis directly.
Conclusion
The evidence is inadequate to accept or reject a causal relation between the Jeryl Lynn strain mumps vaccine and aseptic meningitis.
SUBACUTE SCLEROSING PANENCEPHALITIS
Clinical Description
Subacute sclerosing panencephalitis (SSPE) is a rare subacute encephalitis accompanied by demyelination. The entire course of SSPE may be one of slow progressive deterioration, but variable periods of remission can occur. The usual duration is about 12 to 24 months to a vegetative state or death. A more complete discussion of SSPE can be found in Chapter 3.
History of Suspected Association
Laboratory findings implicate a measles-like virus as the cause of SSPE. Epidemiologic data have also linked SSPE to prior measles infection.
The first report of SSPE in a patient with a negative history for measles but a positive history of vaccination with live attenuated measles vaccine was reported in 1968 (Schneck, 1968). The child had received measles vaccine with immune globulin 3 weeks prior to the onset of symptoms. The clinical course accelerated 10 weeks after vaccination, and the child died 18 months after vaccination. Serologic studies were not performed, but postmortem histologic examination of the brain supported a diagnosis of SSPE. Several more case reports of SSPE in children negative by history for measles but positive for receipt of the measles vaccine followed and are described in more detail below.
The dramatic decline in the number of measles cases in the United States from 1964 to 1968 paralleled a decline in the number of cases of SSPE starting in the early 1970's. Only 4.2 new cases of SSPE per year, on average, were reported from 1982 to 1986 (Dyken et al., 1989). This is in contrast to the 48.6 new cases of SSPE per year, on average, reported from 1967 to 1971. This decline is attributed to the increased use of measles
vaccine, introduced in the United States in 1963. However, a report of data from the National Registry for Subacute Sclerosing Panencephalitis showed that the proportion of newly diagnosed cases of SSPE occurring in children identified by history as vaccinated against measles increased approximately threefold from 1967 to 1974 (Modlin et al., 1977). These data are discussed in more detail below.
The first publication in 1972 of data in the newly established National Registry for Subacute Sclerosing Panencephalitis in the United States reported 14 patients (of a total of 219 records in the registry) who had received a measles vaccine prior to the onset between 1960 and 1970 of SSPE (Jabbout et al., 1972). Six of the 14 patients were reported to have had measles prior to the onset of SSPE as well. The interval between vaccination and the onset of SSPE was 1 year or more in all 14 cases. The specific type of measles vaccine administered is not known.
The committee was charged with investigating a possible causal relation between measles vaccine only and SSPE.
Evidence for Association
Biologic Plausibility
SSPE is a recognized sequela of measles infection, and it is biologically plausible that it could occur after administration of the live attenuated viral vaccine. Identification of the cause of SSPE as wild-type or vaccine-strain measles virus has not been possible. The viruses isolated from patients with SSPE differ from the known measles viruses. The viruses may have become altered by the prolonged residence in the brains of the patients, or they may have been different at the time of the original infection.
Case Reports, Case Series, and Uncontrolled Observational Studies
The first published case report of SSPE in a child with a history of vaccination with live attenuated measles vaccine appeared in 1968 and was described above (Schneck, 1968). In the following 5 years, several more reports of SSPE in individuals vaccinated against measles appeared (Cho et al., 1973; Gerson and Haslam, 1971; Jabbour et al., 1972; Klajman et al., 1973; Landrigan and Witte, 1973; Parker et al., 1970; Payne et al., 1969). These reports represented a total of 22 patients with SSPE, 7 of whom had a history of both measles and measles vaccination (Gerson and Haslam, 1971; Jabbour et al., 1972). The other 15 patients had a negative history for measles and a positive history for receipt of live attenuated measles vaccine (Cho et al., 1973; Jabbour et al., 1972; Klajman et al., 1973; Landrigan and Witte, 1973; Parker et al., 1970; Payne et al., 1969; Schneck, 1968). For
two of those 15 patients, exposure to measles virus was probable, but clinical measles was not recorded (Landrigan and Witte, 1973; Parker et al., 1970). The latency between vaccination against measles and the onset of SSPE symptoms ranged from 3 weeks (Landrigan and Witte, 1973; Schneck, 1968) to 5 years (Cho et al., 1973).
The absence of prevaccination serology and the inability to characterize the cause of SSPE as wild-type or vaccine-strain measles virus in all cases preclude, as discussed below, a determination that the SSPE was caused by administration of the live attenuated measles vaccine. A negative history of natural measles disease in unimmunized persons is always suspect because measles infection can occur subclinically without rash. No case reports of SSPE definitively show that the cause of SSPE in a specific patient was the vaccine-strain virus and not the wild-type virus.
In 1978 the question about SSPE and measles vaccine surfaced again in response to a report concerning a boy who at age 7 years showed signs of SSPE, including deterioration in school performance, incontinence, and forgetfulness (Dodson et al., 1978). Within a few weeks of receiving live attenuated measles vaccine at age 8 years, the patient's symptoms progressed. At 2.5 to 3 months after vaccination, the patient died. SSPE was diagnosed by high measles virus titers in serum and CSF and a high ratio of immunoglobulin G/albumin in serum and CSF. At age 13 months he had suffered a mild illness considered by history to be measles. The authors hypothesized that the measles vaccine accelerated an already evolving SSPE.
The National Registry for Subacute Sclerosing Panencephalitis was founded in 1969, in response to an interest in the effects of measles vaccine on the incidence of SSPE (Schacher, 1968). Originally housed at the University of Tennessee Center for Health Sciences, it now resides at the University of South Alabama. The registry now includes data on more than 575 patients (Paul R. Dyken, University of South Alabama, Mobile, personal communication, 1993). The number of new cases of SSPE documented in the registry decreased from 46 in 1967 to 33 in 1972 to 13 in 1974 (Modlin et al., 1977). The average number of new reports of SSPE per year from 1982 to 1986 was 4.2 (Dyken et al., 1989) and is now about 1, although underreporting is suspected (Paul R. Dyken, University of South Alabama, Mobile, personal communication, 1993).
Analysis of 375 confirmed cases of SSPE that occurred in the United States from 1960 to 1974 (Modlin et al., 1977) demonstrated a decreasing incidence of SSPE beginning in the early 1970's. From 1967 to 1970 the proportion of new cases of SSPE associated with measles vaccine was less than 13 percent, but it increased to 20.6 percent in 1973 and 38.5 percent in 1974. This prompted the authors to note:
Although far from conclusive, the data presented here suggest that live,
attenuated measles vaccine virus may be capable of contributing to the pathogenesis of SSPE. However, the risk of SSPE following vaccination, if any, appears less than the risk following natural measles (Modlin et al., 1977, p. 511).
A review of the data in the registry of patients with SSPE whose onset occurred up to 1986 (Dyken et al., 1989), which included the 375 patients described by Modlin et al. (1977) in the study described above and 200 additional patients, confirmed the continuing decline in the incidence of SSPE and the increase in the proportion of patients with SSPE who had a history of measles vaccination.
Reports of patients with SSPE from other countries after the institution of measles immunization campaigns have supported a role for measles disease in the pathogenesis of SSPE. The very high levels of hemagglutination inhibition (HAI) antibody in the serum and CSF of 100 patients with SSPE observed in Tehran, Iran, between 1977 and 1982 (Mirchamsy, 1983) compared with the HAI antibody levels in patients known to have been vaccinated against measles suggest that these patients had naturally acquired measles. Similarly, all 70 patients with SSPE reported to the Virusdiagnostic Laboratory in Stuttgart, Germany, between 1967 and 1978 were negative for measles vaccination by history (Enders-Ruckle, 1978). Of 26 patients with documented SSPE in Northern Ireland between 1965 and 1985, none had a history of measles vaccination (Morrow et al., 1986). Beersma and colleagues (1988) described 77 patients with SSPE in The Netherlands whose onset of symptoms occurred between 1976 and 1986. Only two of the patients had received a measles vaccine. One of the two patients developed clinical measles 1 week after vaccination and SSPE 9 years later. The other child developed SSPE 1.5 years after vaccination against measles. Prior measles virus infection could not be ruled out. Eleven of 215 patients with SSPE identified in Japan between 1966 and 1985 had received measles vaccine but had not had measles virus infection by history. A total of 184 patients had a history of measles virus infection but not vaccination against measles (Okuno et al., 1989).
There are no reports of SSPE in VAERS (submitted between November 1990 and July 1992), nor is there a discussion of SSPE in the surveillance reports from the data base of the Monitoring System for Adverse Events Following Immunization (MSAEFI), which preceded VAERS.
Controlled Observational Studies
Because SSPE is such a rare condition, study of its etiology is best done by using a case-control design. Patients known to have SSPE are compared with individuals without SSPE to determine whether the proportions of certain characteristics or factors thought to be disease related are
similar in the two groups. In this way, a number of possible etiologic factors can be investigated in a single study.
In the years between the two reviews of the data in the SSPE registry discussed above, a case-control study of patients in the SSPE registry was reported (Halsey et al., 1980). Fifty-two patients with SSPE were compared with controls (49 playmates and 49 hospitalized children) matched for age, sex, and race. Children with SSPE were more likely than their age-matched controls to have had measles (odds ratio [OR], 7; 95% confidence interval [95% CI], 2.5 to 19.6), but they were less likely than controls to have received measles vaccine (OR, 0.28; 95% CI, 0.11 to 0.70). The age of infection with measles virus for children with SSPE was significantly less than that for controls who had measles. There was no difference in age at the time of vaccination between those subjects and controls who did not have a prior measles infection. The same proportion of cases as controls had more than one measles vaccination.
If the etiology of SSPE has changed over the years such that a proportion of all cases were due to the vaccine, then the demographic and epidemiologic characteristics of the SSPE cases would be expected to change as well. Two such ecologic studies have been reported. When U.S. patients whose SSPE was diagnosed between 1956 and 1975 were compared with those whose SSPE was diagnosed between 1976 and 1986, there was no difference in the ratio of males to females or in the proportion of African Americans with SSPE (Dyken et al., 1989). The question of latency was assessed by dividing the patients into three groups: those with a history of measles only, those with a history of both measles and measles vaccination, and those with a history of measles vaccination only. The latency to the onset of SSPE for each of the three groups increased between the periods of 1956-1966 and 1980-1986. The latency to the onset of SSPE for the group with a history of measles vaccine only was shorter than the latencies for the groups with a history of measles, but this difference was not statistically significant.
Similar analyses were done for cases of SSPE in Romania. Cernescu et al. (1990) compared 50 patients whose SSPE onset was in 1978-1979 with 62 patients whose SSPE onset was in 1988-1989. The patients in the 1978-1979 cohort were diagnosed before the national measles immunization program in Romania was implemented in 1979. For the 1988-1989 cohort, they found an increased mean age at the time of onset (6.1 versus 12.1 years) and a difference in the ratio of males to females (2.7:1 versus 0.76:1). They also reported that 76 percent of the cases of SSPE from the 1978-1979 cohort reported a primary measles infection at less than 2 years of age, compared with only 47 percent of the 1988-1989 cohort. The mean interval from the time of measles to the onset of SSPE also increased, from 54 to
106 months, as had the proportion of cases with extreme levels of measles antibody (36 versus 88.7 percent).
Controlled Clinical Trials
No controlled clinical trials of measles vaccination have provided data on the incidence of SSPE. The Medical Research Council of the United Kingdom reported follow-up data on the incidence and complications of wild-type measles infection from a randomized trial of 36,000 patients who received either live measles vaccine or killed vaccine followed later by live measles vaccine or no vaccine. Follow-up was for up to 4 years and 9 months (Medical Research Council, 1971). No mention was made of SSPE, indicating either that there were no cases or that it was not an outcome that was examined. Because other neurologic events were noted and because SSPE is such a striking and serious disease, it is likely that any cases of SSPE would have been reported, if they had occurred.
Causality Argument
There is no question that measles virus is causally related to SSPE. Therefore, it is biologically plausible that there is a link between receipt of live attenuated measles vaccine and SSPE. There is strong evidence that if such an association does exist, it would be very weak compared with the association between a naturally acquired measles infection and SSPE. This evidence is mainly temporal; that is, the incidence of SSPE has decreased dramatically in parallel with widespread measles immunization. There have been only two new cases of SSPE in U.S. citizens reported to the National Registry of Subacute Sclerosing Panencephalitis since 1989 (Paul R. Dyken, University of South Alabama, Mobile, personal communication, 1993). Neither patient had a history of natural measles infection. One patient was immunized at 15 months of age.
It is likely that at least some patients with SSPE have had unrecognized measles infection prior to immunization, and that the SSPE is directly related to this measles infection. Evidence for this comes from Krugman et al. (1962), who reported that before the use of measles vaccine, 15 percent of children whose parents reported no history of measles were found to be immune to the infection. In addition, data on 375 children in the National Registry for Subacute Sclerosing Panencephalitis obtained from Modlin et al. (1977) indicated that four children who had SSPE but no history of measles or measles vaccination in fact had elevated measles virus antibody titers.
The data of Cernescu et al. (1990) showing that the characteristics of patients with SSPE onset in 1978-1979 (prior to national measles immuni-
zation) differ from those of patients with SSPE onset in 1988-1989 indicate a possible change in the nature of the disease since the introduction of measles vaccine and a concurrent decrease in the incidence of measles. If such a change is confirmed by other studies (and this will be difficult, because there are so few new cases of SSPE), it could indicate a different etiology for current SSPE cases compared with those in the past. It could also merely indicate a change in the time of life at which a child is infected with measles and subsequently develops SSPE (e.g., since the beginning of widespread immunization, perhaps only infants who are too young for immunization are infected with measles virus and only a proportion of these develop SSPE).
It will be difficult to obtain other evidence for a causal relation between measles vaccine and SSPE. First, the number of cases of SSPE in the United States is now so low that detection of even moderately strong associations may be difficult. Second, the period of time between infection with the measles virus and development of SSPE is quite long, and if an association between measles vaccine and SSPE exists, a similarly long latency (perhaps 10 years or more) would be expected. Even if the latencies for the two conditions were different and the difference were moderately large, the difference would be difficult to detect because the range of time from measles infection to SSPE is fairly long and the number of new cases of SSPE is low.
Although application of new scientific methods, such as RNA sequencing, could be used to describe more completely the virus that causes SSPE, the well-known genetic alterations of the virus from wild-type measles virus will confound interpretation of the data and make it unlikely that investigators will be able to determine whether there is an independent association between measles vaccine and the development of SSPE.
There has been some concern as to whether measles vaccine could exacerbate preexisting SSPE (Dodson et al., 1978) and whether a second dose of measles vaccine could more often result in SSPE (Halsey, 1990). After publication of the case report of Dodson et al. (1978) of an 8-year-old boy with SSPE whose condition appeared to have been exacerbated by administration of the measles vaccine, Halsey et al. (1978) reported data suggesting that such a concern was not warranted. The National Registry for Subacute Sclerosing Panencephalitis contained records of nine patients who received attenuated or killed measles vaccine after the onset of SSPE symptoms. Four of the nine patients died an average of 3.6 years after the onset of SSPE symptoms and 2.4 years after vaccination. The remaining five patients on record at that time were still alive an average of 10.5 years after the onset of SSPE symptoms and 9.3 years after vaccination. Halsey and colleagues argued that the variability in the course of SSPE rendered the assertions of Dodson et al. (1978) questionable. The same data set
contained evidence that the proportion of SSPE patients who received more than one dose of vaccine was the same as for the control population.
Conclusion
The evidence is inadequate to accept or reject a causal relation between measles vaccine and SSPE.
RESIDUAL SEIZURE DISORDER
Clinical Description
A residual seizure disorder (RSD) caused by vaccination can be defined as a seizure that occurs within 72 hours of vaccination and that is followed by two or more afebrile seizures during the next 12 months (U.S. Department of Health and Human Services, 1992). Subsequent seizures would be anticipated in succeeding years. Approximately 0.5 to 2 percent of the population experience epilepsy. It can occur at any age. Chapter 3 contains a more lengthy discussion of RSD. The cases of RSD reported in this section would not necessarily fit the criteria for RSD presented above. The committee accepted an author's statement that a case was RSD. In addition, the committee considered all cases in which a person experienced repeated (more than one) afebrile seizures to be RSD to not exclude incorrectly any true cases of RSD.
Evidence for Association
Biologic Plausibility
Naturally acquired measles infection is associated with encephalitis, and patients with encephalitis can present with seizures. There are no specific data bearing on the biologic plausibility of an association between measles or mumps vaccine and RSD.
Case Reports, Case Series, and Uncontrolled Observational Studies
The National Collaborative Perinatal Project followed about 54,000 pregnant women, living in 13 cities in the United States, between 1959 and 1966 (Hirtz et al., 1983). Among the children born to those women, 2,766 children experienced at least one seizure within the first 7 years of life. Thirty-nine of those children experienced a convulsion within 2 weeks following an immunization. One child had convulsions following two separate immunizations (against measles and smallpox), so there were a total of 40 seizures. Ten seizures occurred following measles vaccination, generally with
a latency of 7 to 10 days. All but one of the seizures were associated with fever; however, the vaccine administered to the child with the febrile seizures was not specified. The children were followed for up to 7 years, and all 10 children who had received measles vaccination ''did well'' with no long-term neuralgic sequel.
Nader and Warren (1968) described 23 cases of neuralgic disease that followed administration of measles vaccine and that were reported to the U.S. National Communicable Disease Center between 1965 and 1967. During that time, 15 million doses of measles vaccine were distributed throughout the United States. Eleven of the 23 patients were reported to have seizures or convulsions (three of which were noted to be accompanied by fever), and there was one case of persistent spastic quadriplegia. One of the cases of seizures persisted after the acute phase of the illness.
Beale (1974) reported on the measles vaccine experience in the United Kingdom. From 1968 to 1974, more than 3 million children were immunized with Schwarz or Beckenham 31 measles vaccines. Adverse reactions were reported to the governmental Committee on Safety of Medicines. There were 57 febrile convulsions associated with the Schwarz vaccine and 65 associated with the Beckenham 31 vaccine. No other data describing the nature of the seizures or long-term follow-up of the patients were available.
In a study of voluntary reporting of reactions to vaccination in the North West Thames region of England between 1975 and 1981, when approximately 170,000 children received live measles vaccine (as well as other childhood vaccines), there were 26 reports of convulsions without evidence of neuralgic damage following measles vaccination (Pollock and Morris, 1983). No further details were provided, except that at follow-up the children were normal.
Maspero and colleagues (1991) reported a case series of 1,148 children immunized in 1990 in Lombardy, Italy, with the Edmonston-Zagreb vaccine strain and compared them with a case series of children in a nearby district immunized from 1980 to 1987 with the Schwarz vaccine strain. The authors reported that they saw no neuralgic events following administration of the Edmonston-Zagreb vaccine. There was no comparable statement regarding the incidence of neuralgic outcomes in the population immunized with the Schwarz strain.
A 19-month-old Japanese boy was immunized with measles vaccine (Schwarz strain) and 11 days later developed a fever and prolonged (30 minutes) convulsions with loss of consciousness (Abe, 1985). He had four more brief convulsions over the next 6 months, all with fever, and his electroencephalogram exhibited transient abnormalities 14 months later. The report indicated that 2.5 years following the first seizure, the boy's development appeared to be normal.
Haun and Ehrhardt (1973) described an 11-month-old child who devel-
oped clonic seizures and CSF pleocytosis within 12 days of receiving the Leningrad-16 SSW measles vaccine strain and died soon thereafter. (This case is discussed again in Chapter 10.)
Griffin and colleagues (1991) examined the records of a cohort of children in Tennessee enrolled in the Medicaid program who had received MMR or measles-rubella vaccine (MR) in their first 3 years of life to estimate the incidence of neuralgic outcomes. As determined from computerized records, children who were enrolled in the Medicaid program within 90 days of birth in one of four counties, who had a Tennessee birth certificate indicating a birth date within the study period (approximately 1974 to 1984), and who received during those years at least one diphtheria and tetanus toxoid and pertussis vaccine (DPT) immunization at ages 29-365 days and at least one MMR or MR immunization between 12 and 36 months of age were included in the study. Follow-up began at the time of the first MMR or MR immunization and was restricted to the first 36 months of life. Of the population of 18,364 children enrolled in the Medicaid program who received immunizations, 100 were confirmed to have had a seizure. Of these, 77 had febrile seizures (4 children had seizures between days 7 and 14 postimmunization and none were recurrent), 15 had febrile seizures (1 child had two seizures at 1 and 3 days postimmunization, and another child had a seizure at 29 days postimmunization), and 8 had seizures associated with other acute neuralgic illnesses. Most seizures occurred more than 30 days following the immunization. It is possible that there was underascertainment of seizure cases in this cohort, because only those patients for whom a medical claim was filed were counted. Thus, children who moved, went off the Medicaid program, or whose parents did not file a claim were not counted as seizure cases. The authors made no attempts to follow seizure cases for long-term problems.
Fescharek et al. (1990) described convulsions that occurred in 41 patients following administration of vaccine containing measles antigen, mumps antigen, or both. Seven of the 41 patients had convulsions that were not accompanied by fever. More detailed information was not supplied. It is not clear whether any of the convulsions represented the early signs of an RSD.
A report from the passive surveillance system used to detect adverse events following immunization in Canada provided the rates of occurrence of adverse events but not long-term outcomes (Koch et al., 1989). Included in that report were all adverse events reported prior to the end of 1988 for individuals who had received immunizations at any time in 1987. For the purposes of classification, convulsions/seizures were defined as those involving muscle contractions and a decreased level of consciousness, with or without a fever, and had to have been diagnosed by a physician. Forty-four cases were classified as convulsions/seizures following the administration of MMR; the associated rate was 9.3 cases per 100,000 doses. Although
some follow-up beyond 1 year was done to identify residual disorders, the authors did not provide data regarding seizures.
Controlled Observational Studies
The committee was not able to identify any controlled observational studies that reported on the possible association between measles or mumps vaccine and RSD.
Controlled Clinical Trials
As noted above, the Medical Research Council of the United Kingdom reported follow-up data (up to 4 years and 9 months) from a randomized trial of 36,000 patients who received either live attenuated measles vaccine or killed vaccine followed by live attenuated vaccine or no vaccine (Medical Research Council, 1971). Although follow-up was designed to examine the incidence and complications of wild-type measles infection, had an RSD occurred, it might have been noted in such a long-term study. There was no mention of RSD.
Causality Argument
There is evidence that acute seizures are possible sequel of immunization with measles and mumps vaccines. Therefore, it is biologically plausible that there is a connection between immunization and RSD. However, it would be essential to rule out the possibility that the acute cases described in the literature are not febrile seizures, which are common in children and which would not be expected to lead to an RSD. The available data are from case reports and case series; there are no data from observational studies that would allow the calculation of a risk of RSD for vaccinated as opposed to unvaccinated individuals. Perhaps most important, none of the available cases can be confirmed as RSD on the basis of the report alone.
Conclusion
The evidence is inadequate to accept or reject a causal relation between measles vaccine and residual seizure disorder.
There is no evidence bearing on a causal relation between mumps vaccine and residual seizure disorder.
The evidence is inadequate to accept or reject a causal relation between multivalent measles or mumps vaccines and residual seizure disorder.
SENSORINEURAL DEAFNESS
Clinical Description
Sensorineural deafness refers to hearing impairment resulting from disturbances of the cochlea or auditory nerve. The ability to hear high frequencies is often selectively lost. No population-based incidence rates were identified.
History of Suspected Association
This condition, which can be unilateral or bilateral, is characteristic of natural mumps infection and is reported in about 4 percent of cases of mumps. Partial or complete recovery is common.
Evidence for Association
Biologic Plausibility
Viral infections of the cochlea are known to occur. Sensorineural deafness can be a complication of natural mumps virus infection.
Case Reports, Case Series, and Uncontrolled Observational Studies
A 7-year-old girl who had audiometry 2 years earlier for an unstated reason developed total deafness in the left ear 11 days after an injection of MMR. This was not preceded by any symptoms such as dizziness or earache. There was no recovery of hearing (Nabe-Nielsen and Walter, 1988a,b). A 3-year-old girl was evaluated because of bilateral deafness. At the age of 15 months she received MMR. Ten days later, she developed high fever, headache, ataxia, and irritability, which lasted several days. Nystagmus was noted. She recovered spontaneously, but soon after she was noted to have hearing impairment. On evaluation at the age of 3 years, she had moderate to severe bilateral, unremitting sensorineural deafness (Brodsky and Stanievich, 1985).
Controlled Observational Studies
None.
Controlled Clinical Trials
None.
Causality Argument
There is demonstrated biologic plausibility that mumps vaccine could cause sensorineural deafness, in that wild-type mumps virus is associated with the condition. The biologic plausibility for a causal relation between measles vaccine and sensorineural deafness is less firm. Although cases of sensorineural deafness following administration of mumps and measles vaccines have been reported, the timing of onset and other nonspecific features make it impossible to distinguish vaccine from nonvaccine causation. Virus isolation would be helpful in assessing causality when the data are as scarce as described for the causal relation between measles and mumps vaccines and sensorineural deafness; however, such data are lacking.
Conclusion
The evidence is inadequate to accept or reject a causal relation between measles or mumps vaccines and sensorineural deafness.
OPTIC NEURITIS
Clinical Description
Patients with optic neuritis present with unilateral or bilateral impairment of vision. This process can be transient, with full recovery following, or the loss of vision can be permanent. In most instances the underlying pathogenesis is demyelination involving the optic nerve. Chapter 3 contains a more detailed discussion of optic neuritis. No population-based incidence rates were identified.
History of Suspected Association
Measles virus and measles vaccine have long been studied for their ability to cause demyelinating disorders. The committee was charged with investigating a possible causal relation between only measles vaccine and optic neuritis.
Evidence for Association
Biologic Plausibility
Chapter 3 contains a description of the general biologic plausibility for a role for vaccines, particularly live viral vaccines, in causing demyelinat-
ing disorders. There are no data bearing on the biologic plausibility that measles vaccine specifically can cause optic neuritis.
Case Reports, Case Series, and Uncontrolled Observational Studies
There are several reports of optic neuritis following measles vaccination. A 6-year-old boy developed bilateral optic neuritis 18 days after an injection of MMR. He was treated with corticosteroids and experienced a complete resolution after several weeks (Kazarian and Gager, 1978). Marshall et al. (1985) described a 16-month-old girl who experienced an acute loss of vision 16 days after an injection of MMR. Two days earlier she felt warm to the touch and developed a cough, conjunctivitis, and a generalized maculopapular rash. Examination revealed diffuse chorioretinitis and papilledema, which ultimately evolved into a "salt and pepper" pattern. Seven months later she improved, but she had macular scarring. Riikonen (1989) described 18 children with optic neuritis following infection, vaccination, or both. Of those 18, 10 went on to develop multiple sclerosis. Six of these children had been vaccinated between 3 days and 1 month before the onset of optic neuritis, but none had received measles vaccine during that time period. All 18 of the children were reported to have received measles vaccine (unspecified) between 12 and 18 months of age; the age of onset of optic neuritis ranged from 5 years 2 months to 14 years 10 months.
Controlled Observational Studies
None.
Controlled Clinical Trials
None.
Causality Argument
There is demonstrated biologic plausibility of a causal relation between optic neuritis and measles vaccine, in that measles virus is associated with demyelinating disorders. The number of reported cases is too small and the data contained within the reports are too equivocal to support a positive association between measles vaccine and optic neuritis. As discussed in Chapter 3, optic neuritis can result from many causes and is frequently associated with multiple sclerosis.
Conclusion
The evidence is inadequate to accept or reject a causal relation between measles vaccine and optic neuritis.
TRANSVERSE MYELITIS
Clinical Description
Transverse myelitis is a focal, demyelinating lesion that can occur in isolation or as a component of diffuse demyelinating diseases such as acute disseminated encephalomyelitis and multiple sclerosis. Transverse myelitis is characterized by an acute onset of signs of spinal cord disease, usually involving the descending motor tracts and the ascending sensory fibers, suggesting a lesion at one level of the spinal cord. Chapter 3 contains a general discussion of transverse myelitis. The annual incidence of transverse myelitis in Rochester, Minnesota, from 1970 to 1980 was estimated to be 0.83 per 100,000 people (Beghi et al., 1982). The authors noted that this incidence is approximately sixfold higher than the rate calculated for Israel. They attribute this to differences in case ascertainment.
History of Suspected Association
Measles virus is known to be associated with demyelinating disorders. The committee was charged with investigating a possible causal relation between only measles vaccine and transverse myelitis.
Evidence for Association
Biologic Plausibility
Chapter 3 contains an in-depth discussion of the biologic plausibility of a relation between vaccines and demyelinating disorders. Measles virus is associated with central demyelinating diseases.
Case Reports, Case Studies, and Uncontrolled Observational Studies
A case report (in abstract form) linking transverse myelitis with live attenuated measles vaccine was identified (Clark et al., 1977). Thirteen days after vaccination with Schwarz strain measles vaccine, a 16-year-old girl developed symptoms of transverse myelitis. Measles virus was recovered from her throat and stool. The authors hypothesized a relation between
vaccine-associated demyelination and cell-mediated responses to measles virus antigens and myelin basic protein.
As mentioned earlier, Landrigan and Witte (1973) used data voluntarily submitted to the Centers for Disease Control regarding neuralgic disorders following administration of measles vaccine. From 1963 to 1971, 84 cases of neuralgic disorders with onset of less than 30 days after administration of live measles vaccine were reported in the United States. One of the case patients was diagnosed as having transverse myelitis. No further information was provided.
VAERS contains one report (submitted between November 1990 and July 1992) of transverse myelitis developing shortly after MMR vaccination alone and one after MMR given in conjunction with DPT, oral polio vaccine (OPV), and Haemophilus influenzae type b (Hib) vaccine. The temporal and clinical details in those reports are insufficient for proper evaluation.
Controlled Observational Studies
None.
Controlled Clinical Trials
None.
Causality Argument
There is demonstrated biologic plausibility for a causal relation between measles vaccine and transverse myelitis, in that measles virus is well associated with demyelinating disorders. Two cases of transverse myelitis following administration of measles vaccine and two cases following administration of MMR were identified. These cases were temporally associated with administration of the vaccine; there was no other evidence that associated the vaccine and the adverse event. No data from observational or experimental studies lend support to the hypothesized association. The incidence of transverse myelitis unrelated to vaccine is estimated to be about 1 case per 100,000 population (Beghi et al., 1982). Thus, the number of cases identified do not appear to be above background rates, and any study designed to detect an excess number of cases over the background would have to be very large.
Conclusion
The evidence is inadequate to accept or reject a causal relation between measles vaccine and transverse myelitis.
GUILLAIN-BARRÉ SYNDROME
Clinical Description
Guillain-Barré syndrome (GBS) is characterized by the rapid onset of flaccid motor weakness with depression of tendon reflexes and inflammatory demyelination of peripheral nerves (Asbury and Gibbs, 1990). The annual incidence of GBS appears to be approximately 1 per 100,000 people for adults. The data are not definitive, but the annual incidence of GBS in children under age 5 years appears to be approximately the same. The annual incidence of GBS in children over age 5 years and teenagers appears to be lower. Chapter 3 contains a detailed discussion of GBS.
History of Suspected Association
A possible relation between live attenuated viral vaccines and demyelinating disease has been investigated for many years, as described in Chapter 3. There is no specific information suggesting an association between measles vaccine and GBS. The committee was charged with investigating a possible causal relation between only measles vaccine and GBS.
Evidence for Association
Biologic Plausibility
Chapter 3 contains a detailed discussion of the arguments that vaccine can cause demyelination, including GBS. GBS has been described in a few patients following natural (wild-type) measles infection (Lidin-Janson and Straanegard, 1972). Thus, GBS appears to be a rare but possible sequela of measles.
Case Reports, Case Series, and Uncontrolled Observational Studies
Grose and Spigland (1976) reported two cases of GBS that developed in patients within 1 week after immunization with measles vaccine. One of these patients, a 19-month-old girl, was part of a study of 24 patients with GBS for whom serologic studies were performed as part of an effort by the authors to identify possible causal viral agents. She received a combined
measles (Moraten strain) and rubella vaccine 5 days before the development of symptoms (unable to stand and support her own weight). The authors eliminated the possibility that the neuralgic reaction was unlikely to be related to rubella vaccine, because the rubella virus titers indicated that the child was already immune to rubella virus when she was given the vaccine. Four years later the authors saw a second patient with characteristics similar to those of their first one. A 10-month-old girl was given measles vaccine (Moraten strain), as well as her second doses of DPT and OPV, and 4 days later she developed early symptoms of GBS. Both children had a primary immune response to measles antigen, as demonstrated by the seroconversion following immunization.
Norrby (1984) described a 12-year-old girl who became ill with a disorder diagnosed as GBS soon after being vaccinated with MMR, but her CSF protein levels were normal, which casts doubt on the diagnosis. The other findings were supportive of a diagnosis of GBS. The authors presented summary data from Merck Sharp & Dohme indicating that 1 in 60 million doses of MMR has been associated with GBS. Landrigan and Witte (1973) used data voluntarily submitted to the Center for Disease Control regarding neuralgic disorders following administration of measles vaccine. From 1963 to 1971, 84 cases of neuralgic disorders with onset less than 30 days after live attenuated measles virus vaccination were reported in the United States, but these did not include GBS. In a review of adverse event reports submitted between 1976 and 1989 to the Behringwerke AG pharmaceutical firm in the former West Germany, Fescharek and colleagues (1990) described three cases of GBS following vaccination with measles or mumps vaccines (the specific vaccines used in the three patients were not identified). Two of the cases were thought to be related to something other than the vaccines; however, this was not elaborated. Assuming that all three cases were causally related, the authors calculated an incidence of 1 in 1.8 million doses of vaccine distributed.
Summary data from MSAEFI record eight cases of GBS following measles immunization reported between 1979 and 1990. One patient received measles-rubella vaccine and seven received MMR. Nine VAERS reports (submitted between November 1990 and July 1992) reviewed by the committee describe the occurrence of GBS after measles immunization. Three of the five VAERS reports indicating the occurrence of GBS after vaccination with MMR alone met the diagnostic criteria for GBS as outlined in Chapter 3. The patients reported in the other four reports received other vaccines in addition to MMR.
Controlled Observational Studies
None.
Controlled Clinical Trials
None.
Causality Argument
There is biologic plausibility for a causal relation between measles vaccine and GBS. GBS has been shown to follow natural measles virus infection. As described in Chapter 3, several vaccines and viruses are suspected of playing a role in GBS. Reports in the literature describing a possible relation between GBS and measles vaccine are case reports, case series, and uncontrolled observational studies. These include at most a total of six cases of GBS reported in the published literature and seven cases from VAERS. These cases were temporally related to vaccination; however, lack of clinical details and other antecedent events preclude a determination of a causal relation.
Conclusion
The evidence is inadequate to accept or reject a causal relation between measles vaccine and GBS.
INSULIN-DEPENDENT DIABETES MELLITUS
Clinical Description
Diabetes mellitus is a genetically determined disease manifested by abnormal metabolism of carbohydrate, protein, and fat (Fajans, 1989; Kaplan, 1990). Type I or insulin-dependent diabetes mellitus (IDDM) is associated with an insufficiency of insulin secretion by pancreatic beta cells and is characterized by an absolute need for injected insulin to sustain life. In most cases the onset of IDDM is in childhood, but it may occur at any age. Almost all diabetes in children is insulin-dependent. Approximately 10 to 15 percent of diabetics in industrialized countries have IDDM. The annual incidence of IDDM in the United States is about 12 to 14 new cases per 100,000 children ages 0 to 16 years. By age 20, approximately 0.3 percent of individuals will have developed IDDM.
History of Suspected Association
Although the pathogenesis of IDDM is not completely understood, most investigators feel that both environmental and genetic factors are involved, and there are compelling data suggesting that viruses may be one of the
most important environmental triggers of pancreatic beta cell destruction in individuals with a genetic predisposition for IDDM (Banatvala et al., 1987; Maclaren, 1992). Genetic susceptibility has been associated with certain histocompatibility locus antigens (HLAs) on chromosome 6 (Gutierrez-Lopez et al., 1992; Maclaren, 1992).
Evidence favoring a role for environmental factors such as viral infections in the development of IDDM includes the finding that only about one of every two or three pairs of identical twins who develop IDDM are concordant for the disease, and individuals at highest genetic risk for IDDM, as well as rodents genetically homogeneous for spontaneously developing IDDM, do not always acquire the disease (Gutierrez-Lopez et al., 1992; Lipton et al., 1992; Maclaren, 1992).
Several different mechanisms appear to be involved in the pathogenesis of virus-induced IDDM. These have been summarized by Yoon and colleagues and consist of four main categories: (1) direct destruction of pancreatic beta cells by cytolytic viruses without the stimulation of an autoimmune reaction, (2) viral triggering of an autoimmune response, either by molecular mimicry or by altering the immunologic appearance of beta cell antigens, (3) cumulative insults to beta cells by environmental factors such as viral infections and toxins (Dahlquist, 1991; Tishon and Oldstone, 1987; Yoon et al., 1987b), and (4) persistent vital infection resulting in an altered ability to produce insulin with or without progressive beta cell destruction over a period of time (Oldstone, 1989; Tishon and Oldstone, 1987; Yoon et al., 1987a; Yoon and Ray, 1985).
There is no notable history of a suspected association between monovalent measles vaccine and IDDM. Suspicion of an association between mumps vaccine and IDDM is based on the ability of the wild-type mumps virus to cause pancreatitis (Association for the Study of Infectious Disease, 1974; Craighead, 1975; Prince et al., 1978), individual cases of IDDM with onset shortly following acute clinical mumps infections (Gamble et al., 1980; Harris, 1899; Hinden, 1962; Kremer, 1947; McCrae, 1963; Messaritakis, 1971; Otten et al., 1984; Patrick, 1924; Peig et al., 1981), clusters of IDDM after mumps epidemics (Dacou-Voutetakis et al., 1974), and large epidemiologic studies demonstrating parallel curves between outbreaks of mumps disease and new cases of IDDM (Gunderson, 1927; Sultz et al., 1975). Some cases of IDDM with clinical onset temporally related to immunization with mumps vaccine have been reported in the literature and VAERS (submitted between November 1990 and July 1992) (Blom et al., 1991; Helmke et al., 1986; Otten et al., 1984; Pawlowski and Gries, 1991; Quast et al., 1979; Sinaniotis et al., 1975; Taranger and Wiholm, 1987).
Evidence for Association
Plausibility
Pancreatitis is a well-recognized clinical feature of epidemic parotitis, with an incidence ranging from less than 1 to as high as 25 percent (Association for the Study of Infectious Disease, 1974; Craighead, 1975). Since 1899, there have been many reports of abrupt-onset IDDM in individuals of all ages within a few days to weeks following mumps infection or exposure to mumps infection in household members or close contacts (Harris, 1899; Hinden, 1962; Kremer, 1947; McCrae, 1963; Messaritakis, 1971; Otten et al., 1984; Patrick, 1924; Peig et al., 1981). One study found a significant excess of consultations for mumps in the 6 months before the onset of IDDM, particularly in the month prior to the onset of symptoms, in 1,663 children with recently diagnosed IDDM in Great Britain and Wales (P < 0.001) (Gamble et al., 1980).
There have been reports of clusters of IDDM following epidemics of mumps disease (Dacou-Voutetakis et al., 1974) and cyclic variations in incidence curves for IDDM resembling those seen for epidemics of infectious diseases (Gundersen, 1927; Sultz et al., 1975). Some data demonstrate that the curves of the incidence rates of IDDM in children parallel those for epidemics of parotitis and mumps encephalitis, with a lag of from 2 to 4 years (Gundersen, 1927; Sultz et al., 1975). Some investigators attribute the sharp rise in the incidence of IDDM in boys in 1950 to 1960 to the common practice of purposefully exposing boys to mumps in the 1950s, since mumps orchitis occurs less commonly as a complication of mumps disease in children than adults (Sultz et al., 1975).
There have been numerous case reports of IDDM following infection with viruses other than the mumps virus, the most common being coxsackievirus and rubella virus. One of the most convincing reports of the ability of viruses to induce acute-onset IDDM was published by Yoon and colleagues in 1979. They isolated a variant of coxsackievirus B4 from autopsy specimens of a 10-year-old boy's pancreas. The child had developed diabetic ketoacidosis within 3 days of onset of symptoms of a flu-like illness and died 7 days later. He had lymphocytic infiltration of the islets of Langerhans, necrosis of beta cells, and a rise in the neutralizing antibody titer to this virus. One of several inbred strains of mice inoculated with the human viral isolate developed diabetes, and fluorescein-labeled antiviral antibody staining revealed antigens of the same virus in the mouse beta cells.
Since then other cases of a temporal association between the onset of IDDM and well-documented coxsackievirus B4 and B5 infections have been reported (Champsaur et al., 1982; Gladisch et al., 1976). Additional evidence suggesting that coxsackieviruses may cause pancreatic damage and
subsequent IDDM has been provided by a report by Jenson and colleagues who found evidence of insulitis and beta cell damage in pancreatic sections from four of seven neonates who died of coxsackievirus B infection, although this finding does not prove that the infants would have developed IDDM if they had lived (Jenson et al., 1980).
Evidence that persistent viral infection may cause IDDM comes from studies of patients with the congenital rubella syndrome and experimental evidence that rubella infection in rabbit and hamster models causes pancreatic beta cell damage (Menser, 1978; Rayfield et al., 1986). Rubella virus has been isolated from the pancreases of several patients with congenital infections (De Prins et al., 1978; Monif, 1974), and inflammation of the pancreas has been reported in other children with congenital rubella (Bunnell and Monif, 1972; Patterson et al., 1981). Epidemiologic studies have shown that the prevalence of IDDM among children with congenital rubella infection is high in some countries, but not in others, suggesting that only patients with congenital rubella plus a genetic predisposition for developing IDDM are affected (Menset et al., 1978; Rubinstein et al., 1982). Indeed, it has been demonstrated that the frequencies of the HLAs DR2 and DR3 are significantly lower and higher, respectively, in patients with IDDM and congenital rubella than in those without IDDM (Rubinstein et al, 1982).
Experiments in animals have demonstrated that viruses such as coxsackievirus, encephalomyocarditis virus, mengovirus, reovirus, and lymphocytic choriomeningitis virus are capable of inducing IDDM, but most studies have shown that both the strain of the virus and the genetic susceptibility of the animal are important in the development of IDDM (Menser et al., 1978; Rayfield et al., 1986; Tishon and Oldstone, 1987; Yoon and Ray, 1985; Yoon et al., 1978, 1979, 1987a,b). This is illustrated particularly well by the ability of certain strains of lymphocytic choriomeningitis virus to stimulate the onset of IDDM and of others to prevent it (Dyrberg et al., 1988; Oldstone, 1988; Oldstone et al., 1990a,b; Tishon and Oldstone, 1987).
Infection with live (but not inactivated) mumps and rubella viruses, and coxsackievirus B4 has been found to lead to increased expression of HLA class I molecules and minor decreases in insulin secretion in cultured human beta cells (Parkkonen et al., 1992). Several common viruses including mumps virus, coxsackievirus B, and reovirus type 3 can infect human pancreatic beta cells in vitro and destroy them (Parkkonen et al., 1992; Prince et al., 1978; Yoon and Ray, 1985).
Case Reports, Case Series, and Uncontrolled Observational Studies
The case reports implicating measles vaccine as a potential cause of IDDM involve administration of the mumps vaccine at the same time.
There have been several cases of IDDM reported following MMR, measles-
mumps, or mumps vaccination. The committee heard presentations at its May 1992 public meeting from two parents whose daughters developed IDDM after receiving MMR (see Appendix B). There have been four cases of IDDM reported to VAERS (submitted between November 1990 and July 1992) following receipt of MMR and one or two following receipt of mumps vaccine (both reports might represent the same child, since both were 6-year-old males who developed IDDM following mumps immunization). The ages have ranged from 1 to 27 years, with one case each being reported at ages 1, 7, 18, and 27 years. There was either one or two cases in a child age 6 years, as explained above. The onset of symptoms of IDDM in these patients ranged from 2 days to 2.3 months after immunization, with one case occurring at 2 days, two at 6 weeks, and one at 2.3 months. The intervals in the others were not specified.
In 1975, Sinaniotis and colleagues reported the onset of IDDM 1 month after receipt of mumps vaccine in a 6.5-year-old boy. In 1991, Pawlowski and Gries described an 11-year-old boy who had mumps disease at age 16 months and then received measles-mumps vaccine 5 months before the onset of IDDM. He had severe abdominal pain and fever 1 week after immunization.
In 1984, Otten and colleagues reported three cases of IDDM, with onset in one case 10 days and in two cases 3 weeks after mumps vaccine in children 3, 2, and 16 years of age, respectively. They noted that the two younger children were positive for HLAs DR3 and DR4 and that the older boy was positive for DR4. In 1986, Helmke and colleagues reported seven children who developed IDDM in the second to fourth week following mumps or measles-mumps vaccination. All seven children were positive for DR4, and three were also positive for DR3.
In 1979, Quast and colleagues noted that in the first 2 years after mumps and measles-mumps vaccines were introduced in the former West Germany, two cases of IDDM with onset 7 and 10 days after immunization with measles-mumps and mumps vaccines, respectively, were reported to the manufacturer, Behringwerke AG.
In 1990, Fescharek and colleagues noted that 20 cases of IDDM were reported to the manufacturer, Behringwerke AG, from 1976 through 1989, a period during which about 5 million doses of mumps vaccine were distributed in the former West Germany, giving a rate of 1 for every 250,000 doses distributed. The two cases of IDDM identified by Quast et al. (1979) are probably part of the more extensive study from the records of Behringwerke AG (Fescharek et al., 1990). For 19 of 20 patients, the interval between immunization and the onset of symptoms was reported, and this ranged from 3 days to 7 months. Twelve cases began within 30 days of immunization. The annual number of new cases of IDDM was assumed to be about
12 per 100,000 on the basis of a mean value of the incidences of IDDM in comparable countries, since no data were available for the former West Germany. It was estimated that for every 5 million children vaccinated against mumps, 50 spontaneous cases of IDDM would have been expected by random coincidence within 30 days after immunization.
In 1987, Taranger and Wiholm noted that three cases of IDDM diagnosed within 1 month of MMR immunization were reported to the pharmaceutical department of the Swedish Health Authorities during the 3-year period (1982 to 1984), when 700,000 doses of the vaccine were sold. All were 12-year-old girls. They noted that one had developed symptoms of IDDM 2 to 3 weeks before being immunized. Prospective data on the incidence of IDDM in children in Sweden since 1977 revealed that one to two girls at that age were expected to develop IDDM during each 1-month period. Thus, they concluded that the number of cases reported after receipt of MMR did not exceed the expected background frequency.
Sultz and colleagues (1975) conducted interviews with 112 parents of diabetic children in Erie County, New York (approximately one-third of all cases identified for the 25 years from 1946 through 1971), and noted that IDDM was preceded by mumps disease or exposure to mumps virus in almost 50 percent of the children and by mumps vaccination in an additional 11 percent. The median lag time was 3 years (mean, 3.8 years).
Controlled Observational Studies
In 1991, Blom and colleagues reported the results of a nationwide controlled study in Sweden evaluating vaccinations, infections, and the use of medicines during the year preceding the diagnosis of IDDM as possible risk determinants for IDDM in children 0 to 14 years of age. The study included 339 children with recent-onset IDDM and 528 control children matched for age, sex, and county. The data were obtained from mailed questionnaires that indicated that the purpose of the study was to reveal possible relations between different childhood diseases and environmental factors. It did not mention that the study was focusing on IDDM. They found no evidence that vaccinations increased the risk of developing IDDM in childhood. Mumps and MMR vaccinations had no significant effect on the relative risk of developing IDDM (for mumps: OR, 1.75; 95 percent CI, 0.54 to 5.70; for MMR: OR, 0.95; 95 percent CI, 0.71 to 1.28). However, measles vaccination was associated with a significantly decreased relative risk of developing IDDM (OR, 0.74; 95 percent CI, 0.55 to 1.00). Other data from that study demonstrated a lack of association between any specific infectious agent and IDDM, although children with IDDM had more infections during the year prior to diagnosis.
Controlled Clinical Trials
None.
Causality Argument
There is no demonstrated biologic plausibility to suggest a causal relation between monovalent measles vaccine and IDDM. Indeed, the available data demonstrate a decreased relative risk for IDDM in individuals who have received measles vaccination (Blom et al., 1991).
There is evidence suggesting that mumps virus infection can trigger the onset of IDDM in some individuals. Biologic plausibility data implicating the mumps virus in the pathogenesis of IDDM include (1) the association between viral infections, including mumps, and IDDM in humans, (2) the detection of circulating autoantibodies against pancreatic antigens, particularly islet cells, during convalescence from mumps infection as well as early in the course of IDDM, and (3) in vitro studies demonstrating that the wild-type mumps virus can infect human pancreatic beta cells.
Data regarding a possible association between mumps vaccine and IDDM are limited to the cases noted above of a temporal relation between mumps immunization and the onset of symptoms of IDDM (Fescharek et al., 1990; Helmke et al., 1986; Otten et al., 1984; Pawlowski and Gries, 1991; Quast et al., 1979; Sinaniotis et al., 1975; Taranger and Wiholm, 1987). It should be noted that because the etiology of IDDM may be multifactorial, any temporal relation with mumps vaccine may be because other factors (such as toxins, nutrients, or other infections) have already destroyed enough pancreatic beta cells that even minor damage by the mumps vaccine virus may trigger the onset of diabetic symptoms.
The incidence of IDDM shortly following mumps immunization of children in Sweden and Germany was at or below expected background levels (Blom et al., 1991; Fescharek et al., 1990). It should be noted, however, that most of the postulated mechanisms of the pathogenesis of IDDM (autoimmune, cumulative environmental effects, or persistent infection) suggest that there may be a prolonged interval between vaccination and the onset of symptoms of IDDM. Even if the vaccine virus were to cause IDDM by direct cytolysis of pancreatic beta cells, it will be difficult to document this by isolating the virus from pancreatic tissue since, currently, there is a very low mortality early in the course of IDDM, when the virus would most likely be present.
Conclusion
The evidence is inadequate to accept or reject a causal relation between measles or mumps vaccine and IDDM.
STERILITY DUE TO ORCHITIS
Clinical Description
Sterility is the inability to produce offspring. Orchitis is inflammation of the testis, which is manifested by swelling and tenderness and is usually of infectious origin, such as tuberculosis, mumps, enterovirus, syphilis, or certain fungal diseases. Orchitis is also referred to as testitis, didymitis, and orchiditis. No population-based incidence rates were identified.
History of Suspected Association
There is no suspected association between measles vaccine alone and orchitis or sterility. The possibility of an association between mumps vaccine and sterility secondary to orchitis has been suspected on the basis of reports of orchitis following infection with wild-type mumps virus.
Evidence for Association
Biologic Plausibility
There are no data bearing directly on the biologic plausibility of an association of orchitis or sterility with measles vaccine. The most compelling argument for biologic plausibility regarding orchitis following mumps vaccine are the reports of orchitis following infection with the wild-type mumps virus. In 1950, Werner (1950a) reported that mumps is complicated by orchitis in one-fifth of all cases of mumps occurring in males after puberty. He also found that mumps orchitis had been the cause of testicular atrophy in 43 percent of 44 cases of obvious testicular atrophy found in an examination of 2,000 random males 14 to 34 years of age. In another study, he analyzed seminal fluid from 49 males with a past history of mumps orchitis (Werner, 1950b). The age of onset of orchitis ranged from 10 to 27 years, with a median age of 16 years. The interval between the onset of orchitis and the study ranged from a few months to 9 years, with a median of 4 years. Some degree of testicular atrophy was apparent in 39 of 49 patients. Some 51 percent of 49 patients from 18 to 27 years of age with a past history of mumps orchitis had semen with mean sperm counts and motilities that were lower than those for semen from control subjects. Only one man had azoospermia. Testicular atrophy was no more common among patients with abnormal semen specimens than it was in the group with orchitis as a whole. Mumps orchitis was felt, on the basis of seminal fluid examination, to have impaired fertility in 13 percent of the individuals with a past history of the disease. Werner (1950b) concluded that since only 1.7
percent of all males with mumps contract orchitis (but 20 percent or more of males who develop mumps after puberty have orchitis) and only 13 percent of those with orchitis had impaired fertility attributable to mumps orchitis, mumps orchitis is not an important cause of sterility in males.
Penttinen and colleagues (1968) found that 15 to 25 percent of men who contracted mumps developed orchitis, and in about 10 to 20 percent of these men it was bilateral and, thus, had the potential to cause sterility. Two other studies have found that 30 to 38 percent of postpubertal males with mumps develop orchitis (Beard et al., 1977; Philip et al., 1959). They reported bilateral involvement in 17 and 37 percent of cases, respectively. Mumps orchitis was found to be most common in the second through fourth decades of life (Beard et al., 1977).
A review of the records of 2,482 patients with mumps (about half of whom were under 15 years of age) admitted to 16 infectious disease units in England and Wales revealed that 333 of 1,513 males developed orchitis and 71 males had orchitis and meningitis or encephalitis (Association for the Study of Infectious Disease, 1974). Interestingly, 5 of 969 females had oophoritis. The authors noted that orchitis was second only to CNS involvement as a complication of mumps. There were no recorded sequel, but the investigators did not attempt to follow up these patients for sterility because ''the practical difficulties associated with such a study are formidable'' (p. 555).
In 1954, Sandler reported azoospermia in a 34-year-old male who developed bilateral orchitis following mumps disease. Although his azoospermia persisted for over 1 year, he subsequently fathered a child.
In a retrospective study, McKendrick and Nishtar (1966) found that several men had fathered children following either unilateral or bilateral mumps orchitis.
Several authors concluded that since orchitis is usually unilateral, it is rarely a cause of permanent sterility (Association for the Study of Infectious Disease, 1974; Bendersky-Malbec, 1982; Penttinen et al., 1968; Werner, 1950b).
Case Reports, Case Series, and Uncontrolled Observational Studies
The data regarding mumps vaccine-associated orchitis in the literature are in two reports of surveillance for adverse reactions to immunization in other countries. Three cases of orchitis were reported following administration of MMR in Canada in 1987, for an incidence of 0.5 cases per 100,000 doses of MMR distributed (Koch et al., 1989). Six cases of suspected orchitis were reported following administration of measles or mumps (Jeryl Lynn strain) vaccine in the former West Germany from 1976 through 1989 (Fescharek et al., 1990). During that period of time an estimated 5.5 mil-
lion doses of measles, mumps, and measles-mumps vaccines and MMR were sold. Two of these patients had hydroceles. The other four patients recovered after 2 to 3 days "with only slight inconvenience to the vaccinee" (p. 447).
There have been 11 reports in VAERS (submitted between November 1990 and July 1992) of orchitis, or possible orchitis, following vaccination with MMR (10 reports) or mumps vaccine (1 report). The ages of the vaccinees ranged from 19 months to 26 years, with a median age of 12 years. The interval from immunization to the onset of symptoms was noted for nine patients and ranged from 1 to 34 days, with a median of 16 days. Six patients recovered, and no outcome was reported for five patients. Five cases were bilateral, and four cases were unilateral (all on the left side); for two cases the laterality was not specified. Seven patients were stated to have orchitis. The diagnosis was questionable in four patients. In one case the patient had been seen in the past for problems with his testicles. Following immunization he had three episodes of testicular swelling and pain, with each episode lasting about 2 hours. In another case, a urologist diagnosed torsion versus mild epididymitis and could not rule out the possibility that the condition was related to MMR. In another case, the patient did not have swollen testicles when examined on two occasions by a physician, although he was reported by his mother to have swollen testicles and swollen legs. In another, the patient had a swollen left testicle that was not red or hot to the touch and was diagnosed as having "testis disease."
In 1976, Borsche reported that after about 1,000 vaccinations with monovalent mumps vaccine, no side effects were noted.
A study of live attenuated mumps vaccine (Rubini strain) in monkeys, 13 adult males, and 60 children aged 15 to 24 months revealed no inflammation, swelling, or pain of the testes (Gluck et al., 1986).
Controlled Observational Studies
Penttinen and colleagues (1968) found that the frequency of orchitis as a complication of mumps was two to three times lower among recipients of mumps vaccine than among nonvaccinated servicemen. Furthermore, they found that the rate of orchitis was 25 times lower among the vaccinees than among nonvaccinated men, suggesting that the vaccine afforded protection from mumps orchitis.
Controlled Clinical Trials
Schwarz and colleagues (1975) looked for, but did not find, orchitis in 1,232 children who received MMR (Jeryl Lynn strain or a placebo).
Causality Argument
There is no evidence bearing on a causal relation between measles vaccine and orchitis or sterility.
Biologic plausibility for a causal relation between mumps vaccine and orchitis stems from the relation between wild-type mumps virus and orchitis (Association for the Study of Infectious Disease, 1974; Bendersky-Malbec, 1982; British Medical Journal, 1980; Sandler, 1954; Werner, 1950a,b) and from the temporal correlation between mumps vaccine and orchitis in several individual cases. Because orchitis is uncommon, it seems possible that cases temporally related to mumps vaccination are caused by the vaccine virus. However, it is also possible that at least some of the cases of orchitis following administration of mumps vaccine were caused by infection with wild-type virus, because the impetus to immunize males often is exposure to mumps disease. It should be noted that even though millions of doses of mumps vaccine have been administered, very few cases of mumps vaccine-associated orchitis have been reported.
There are no case reports of sterility in the literature or in VAERS (submitted between November 1990 and July 1992). However, these data are difficult to obtain because of the number of years required for follow-up. Because there are case reports of bilateral orchitis following administration of mumps vaccine, there is a possibility of sterility, and thus, a causal relation between mumps vaccine and sterility has not been fully studied.
Conclusion
There is no evidence bearing on a causal relation between measles vaccine and orchitis or sterility.
The available evidence is inadequate to accept or reject a causal relation between mumps vaccine and orchitis.
The available evidence is inadequate to accept or reject a causal relation between mumps vaccine and sterility.
THROMBOCYTOPENIA
Clinical Description
Thrombocytopenia is a decrease in the number of platelets, the cells involved in blood clotting. Thrombocytopenia may stem from the failure of platelet production, a shortened platelet life span, or an abnormal distribution of platelets within the body (Lee et al., 1993). Normal platelet counts are on the order of 150,000 to 450,000/mm3. Thrombocytopenia occurs in
children of all ages, with an incidence of 31.9 cases (defined as a platelet count less than 150,000/mm3) per 1 million children under age 15 years per year (Cohn, 1976). Approximately 70 percent of cases occur following viral illnesses (Lightsey, 1980). In most cases, thrombocytopenia in children is mild and transient, and it is often discovered only incidentally when a complete blood count is performed. Severe thrombocytopenia associated with spontaneous bleeding, including bleeding into the skin, is called thrombocytopenic purpura.
History of Suspected Association
In 1966, Oski and Naiman reported a decrease of greater than 25,000/ mm3 in the platelet counts of 38 of 44 (86 percent) subjects immunized with live, attenuated Edmonston B measles vaccine. The lowest platelet counts were observed 1 week following immunization, and the platelet counts returned to prevaccination levels after 3 weeks in all but two patients. There were no petechiae and no purpura or bleeding problems in any of the patients. Nieminen and colleagues (1993) found acute thrombocytopenic purpura in 23 of approximately 700,000 children after they were immunized with MMR. The mean interval between immunization and purpura was 19 days. There also have been several individual case reports of thrombocytopenia following measles vaccination in the literature and VAERS. These studies are described in detail in a later section.
Evidence for Association
Biologic Plausibility
There is demonstrated biologic plausibility that measles or mumps vaccines could be associated with thrombocytopenia on the basis of experience with wild-type virus infections. Early case reports of purpura and bleeding associated with measles did not provide sufficient data to indicate the cause of bleeding. Specifically, they did not differentiate isolated thrombocytopenia from the thrombocytopenia found in disseminated intravascular coagulation. Severe hemorrhage is a well-documented, but rare, complication of infection with measles virus. It is known as the "black measles" because of hemorrhage into the skin (Hudson et al., 1956) and most likely results from disseminated intravascular coagulation. The first case of fatal purpura associated with measles was reported by Jackson in 1890. Hudson et al. (1956) reported 2 cases of thrombocytopenic purpura in patients with measles and reviewed 20 other cases reported in the literature. They found that the hemorrhagic manifestations began an average of 6 days (range, 2 to 14 days) after the onset of the measles rash. The number of circulating plate-
lets ranged from 5,000 to 90,000 (average, 30,900) in the 12 patients for whom counts were available. In 1934, Perlman reported that there was "a rather constant tendency for the platelet count to drop below normal" (p. 602) in 50 random cases of measles. No data on the degree of thrombocytopenia were provided. Thrombocytopenia has been described as a rare complication of mumps disease (Graham et al., 1974).
Case Reports, Case Series, and Uncontrolled Observational Studies
Even though large numbers of doses of live attenuated measles vaccine have been administered, very few cases of thrombocytopenia have been reported. In 1965, Katz noted that he was aware of two cases of idiopathic thrombocytopenic purpura and one case of hemolytic-uremic syndrome after approximately 5 million doses of live attenuated measles vaccine had been administered over 6 years. The hemolytic-uremic syndrome is characterized by the triad of hemolytic anemia, thrombocytopenia, and acute renal insufficiency (Kaplan et al., 1987; Levin et al., 1989; Srivastava and Bagga, 1992). Rare cases of this syndrome have been documented following immunization (Srivastava and Bagga, 1992). In addition to the case noted above following administration of live attenuated measles vaccine (Katz, 1965), there have been other cases following administration of mumps vaccine (Dosik and Tricarico, 1970) and MMR (Taranger and Wiholm, 1987).
There are several individual case reports in the literature of thrombocytopenia following administration of live attenuated measles vaccine alone (Alter et al., 1968; Bach and Allard, 1974; DeRitis and Pecorari, 1990; Giroud et al., 1983; Kiefaber, 1981; Medical Journal of Australia, 1980) or concomitantly with immune globulin (Bachand et al., 1967; Saxton, 1967; Wilhelm and Paegle, 1967). Cases of thrombocytopenia also have been reported following administration of measles-mumps vaccine (von Muhlendahl, 1989) and MMR (Azeemuddin, 1987; Neiderud, 1983), but not after administration of mumps vaccine alone (other than that associated with hemolytic-uremic syndrome discussed above). The vast majority of cases of thrombocytopenia occur following the first dose of measles vaccine, but thrombocytopenia has been documented following administration of a second dose as well (Wiersbitzky et al., 1992). It should be noted that comparatively few individuals have received more than one dose of measles vaccine to date.
Case series and uncontrolled observational studies provide the bulk of the information regarding measles and measles-containing vaccines and thrombocytopenia. Taranger and Wiholm (1987) and Bottiger and colleagues (1987) found that 16 cases of thrombocytopenia following administration of MMR were reported to the Swedish health authorities over a 3-year period from 1982 through 1984, when an estimated 700,000 doses of MMR (using
the same strains that are used in the United States) were sold and approximately 590,000 children were immunized. One other child had hemolytic-uremic syndrome (as noted above), but the child's platelet count was not reported. Fourteen cases of thrombocytopenia occurred in 18-month-old children. Eight of 13 patients with thrombocytopenia considered to have been vaccine related recovered without therapy. One patient had a second episode, and two patients responded to prednisone therapy within 2 months. Only one patient remained thrombocytopenic at 2 years of follow-up, but that patient had no clinical symptoms. Three other patients had a transient petechial rash, but platelet counts were not determined at the time of the rash.
In 1987, a new passive surveillance system for adverse reactions to vaccines was implemented in Canada (Koch et al., 1989). Five cases of thrombocytopenia were reported to this new system, for a cumulative incidence of 1 case per 100,000 doses of MMR distributed.
Fescharek and colleagues (1990) reported 11 cases of thrombocytopenia following administration of measles vaccine between 1976 and December 1989, over which period an estimated 5.5 million doses of measles and measles-mumps vaccines and MMR were sold in the former West Germany. All cases of thrombocytopenia occurred following immunization with vaccines containing the measles virus antigen (the same strain that is used in the United States). The lowest platelet count was 9,000. All 11 patients recovered either spontaneously or after steroid therapy.
Data suggesting that MMR can cause thrombocytopenic purpura have recently been reported by Nieminen and colleagues (1993). They found that 23 of about 700,000 children immunized with MMR in Finland (which uses the same vaccine strains as those used in the United States) developed thrombocytopenic purpura a mean of 19 days (median, 17 days; range, 7 to 59 days) following vaccination. The patients' ages ranged from 1.2 to 7.3 years. The median platelet nadir was 4,000/mm3, with a range of 1,000 to 45,000/mm3. Fifteen of the patients' platelet counts returned to greater than 100,000 within 1 month, 20 within 2 months, and 22 within 6 months. Thirty months later, one patient had a second episode associated with an infection.
Bone marrow aspirates were performed in 13 patients, and all revealed "at least normal" (p. 268) numbers of megakaryocytes. Platelet survival was decreased in both of the two patients studied, and platelet-associated immunoglobulin was detected in 10 of 15 patients tested. Five of six lost antibodies on follow-up 62 to 206 days after immunization, but one remained weakly positive at 132 days. Five of 15 patients had glycoproteinspecific platelet antibody.
There have been 12 reports in VAERS (submitted between November 1990 and July 1992) of thrombocytopenia following administration of measles
vaccine (1 report) or MMR (11 reports). One case occurred after receipt of measles vaccine alone, six after receipt of MMR alone, and five cases after receipt of MMR plus one or more other vaccines (DPT, OPV, or Hib vaccine). The ages of the patients ranged from 12 months to 18 years. Seven of the 10 patients whose ages were given were 2 years old or younger. The interval between immunization and the onset of symptoms for the nine patients for whom these data were provided ranged from 6 to 27 days, and for six patients it was less than 14 days. Platelet counts were provided for 10 patients, and the lowest count for each was 4,000, 9,000, <10,000, 11,800, 17,000, 38,000, 43,000, 43,000, 55,000, and 65,000/mm3. Five patients were reported to have recovered. The outcomes in the other seven patients were not specified.
Controlled Observational Studies
Data suggesting that the live measles vaccine can cause thrombocytopenia were provided by Oski and Naiman (1966), who found decreases in platelet counts in most subjects following administration of live attenuated measles vaccine (this vaccine is no longer used in the United States). They determined platelet counts of 59 individuals on a weekly basis until 21 days after administration of either live measles vaccine, live measles vaccine plus gamma globulin, gamma globulin alone, or killed measles vaccine. Twenty-five of 28 patients who received immune globulin along with measles vaccine and 13 of 16 patients who received measles vaccine alone had transient decreases in their platelet counts (mean maximum decrease of 92,000/mm3 [representing a 37 percent decrease from the original platelet count] for those who received immune globulin versus 108,000/mm3 [36 percent decrease from the original platelet count] for those who received measles vaccine alone). The median decrease in the 38 patients whose platelets fell by more than 25,000/mm3 was in the range of 76,000 to 100,000/mm3. In contrast, only 1 of the 10 patients who received gamma globulin alone and none of the 5 patients who received killed measles vaccine experienced a decline in platelet count.
In over 50 percent of patients vaccinated with live attenuated measles vaccine, the platelet count fell more than 75,000/mm3 below its original level. The most dramatic decline was 317,000/mm3 in an iron-deficient infant, whose original platelet count had been 570,000/mm3. The lowest documented platelet count was 64,000/mm3. The maximum depression was noted at 1 week, and postimmunization platelet counts returned to preimmunization levels in all but two vaccinees by 3 weeks postimmunization. In those patients whose platelet counts were determined more frequently, decreases were seen by 3 days postimmunization. A decrease in the platelet
count of one infant was documented after each of three challenges with live attenuated measles vaccine.
The decrease in platelet counts was greater in children less than 2 years of age than in those over 12 years of age (126,000 versus 51,000/mm3). It is not known whether maternal antibody plays a role in the accentuated platelet count decrease in infants less than 1 year of age. However, it seems unlikely since there was little difference between the degree of thrombocytopenia seen in patients who received measles vaccine alone and that seen in patients who received immune globulin along with the measles vaccine. It should be noted that measles vaccine is rarely given to infants who have the highest levels of maternal antibody (those less than 6 months of age).
Serial bone marrow aspirates from three patients were examined, and a decrease in the number of megakaryocytes was demonstrated by the third day postimmunization. The megakaryocytes also demonstrated morphologic alterations characterized by vacuolization of the cytoplasm and nucleus. In addition to the bone marrow findings, stable or decreasing plasma acid phosphatase determinations also suggested that the decrease in platelet count was more likely the result of decreased platelet production rather than increased destruction. This is in contrast to data of Hudson and colleagues (1956) suggesting that the thrombocytopenia following wild-type measles virus infection is caused by increased destruction of platelets.
Although Oski and colleagues (1966) speculated that the vaccine virus may replicate in the bone marrow since the onset of the vaccine-induced thrombocytopenia occurred during the incubation period of the live attenuated measles virus infection following immunization, they were unable to isolate the virus from the bone marrow on either of two occasions. It has been noted that the thrombocytopenia following wild-type virus infection occurs later, usually about 1 week following the onset of rash, not during the incubation period. Data suggesting that the measles vaccine virus can suppress the bone marrow are provided in a study by Olivares et al. (1989), who found a significant decrease in hemoglobin concentration by days 9 and 14 following administration of live attenuated measles vaccine (Schwarz strain) in infants studied prospectively at 0, 4, 9, 14, 21, and 30 days postimmunization. Platelet counts were not determined in that study.
Controlled Clinical Trials
None.
Causality Argument
Evidence that wild-type measles virus is associated with thrombocytopenia provides biologic plausibility that measles vaccine could also be associated
with thrombocytopenia. Evidence from the study by Oski et al. (1966), individual reports in the literature, and VAERS suggests a causal relation between measles vaccine and thrombocytopenia. However, the measles vaccine strain (the live attenuated Edmonston B strain) studied by Oski is no longer used in the United States. The evidence concerning the live, more attenuated measles vaccine strain currently used in the United States is scarce. Although transient decreases in platelet counts following measles vaccination or from other nonvaccine causes may be common, clinically significant thrombocytopenia is extremely rare, especially considering the very large number of doses of measles vaccine that have been administered.
The reports of thrombocytopenia following mumps diseases provide biologic plausibility that mumps vaccine could be associated with thrombocytopenia. The evidence bearing on a causal relation between mumps vaccine and thrombocytopenia consists of one report of a child who had thrombocytopenia as part of hemolytic-uremic syndrome following receipt of mumps vaccine (Dosik and Tricarico, 1970).
Published reports of passive surveillance systems from several countries provide evidence that MMR is associated with clinically significant thrombocytopenia within two months of vaccination. On the basis of data from Finland and Sweden, the incidence appears to be on the order of 1 per 30,000 to 40,000 vaccinated children. This is a sixfold higher incidence than that reported in the only study of background incidence of thrombocytopenia identified by the committee (Cohn, 1976). The committee could not identify the component of MMR responsible for the thrombocytopenia, but the data from Oski and from the experience with wild-type measles virus suggest that the measles vaccine component of MMR might be responsible for the thrombocytopenia that occurs after MMR.
Conclusion
The evidence establishes a causal relation between MMR and thrombocytopenia. On the basis of data from Finland and Sweden, the incidence appears to be on the order of 1 per 30,000 to 40,000 vaccinated children.
The evidence is inadequate to accept or reject a causal relation between monovalent measles and mumps vaccines and thrombocytopenia.
Risk-Modifying Factors
Because so little information is available, the committee does not have the means to recommend any precautions to prevent clinically significant thrombocytopenia from occurring after administration of live attenuated measles vaccine or MMR. One child in the series by Nieminen and colleagues (1993) had had acute idiopathic thrombocytopenic purpura 9 months prior to the episode following MMR administration. Children with a prior his-
tory of thrombocytopenia may be at increased risk for developing thrombocytopenia following MMR.
ANAPHYLAXIS
Clinical Description
Anaphylaxis and anaphylactic shock refer to an acute, severe, and potentially lethal systemic allergic reaction. Most cases resolve without sequel. Signs and symptoms begin within minutes to a few hours after exposure. Death, if it occurs, usually results from airway obstruction caused by laryngeal edema or bronchospasm and may be associated with cardiovascular collapse. Chapter 4 contains a detailed discussion of anaphylaxis.
History of Suspected Association
The suspected relation between measles and mumps vaccines and anaphylaxis is based on several reports of anaphylactic reactions following administration of measles or measles-mumps vaccines or MMR in the literature and VAERS (Aukrust et al., 1980; Fescharek et al., 1990; Herman et al., 1983; McEwen, 1983; Pollock and Morris, 1983; Taranger and Wiholm, 1987; Thurston, 1987; Van Asperen et al., 1981). No reports of anaphylaxis following administration of monovalent mumps vaccine have been published, but the 1991 Red Book states that since 1967 there have been rare, isolated reports of allergic reactions (American Academy of Pediatrics, Committee on Infectious Diseases, 1991).
Anaphylaxis to egg proteins has been considered to be a relative contraindication to immunization with live attenuated virus vaccines grown in eggs or in tissue culture cells of chicken embryo origin (American Academy of Pediatrics, Committee on Infectious Diseases, 1991). In 1983, Herman et al. reported generalized urticaria, angioedema, and respiratory difficulty after immunization with MMR in two children who had allergy to egg white protein (ovalbumin). Both had serum immunoglobulin E (IgE) reactive with the ovalbumin-related antigens in measles vaccine and MMR.
Evidence for Association
Biologic Plausibility
Concern has been raised regarding the safety of egg-derived vaccines in individuals who are sensitive to egg protein, since some individuals are exquisitely sensitive. Measles and mumps viruses in currently available monovalent and combination (measles-mumps and MMR) vaccines are grown
in cell cultures of chicken embryo fibroblasts (rubella virus is grown in human diploid cell culture). Egg-related antigens can be detected in measles and mumps vaccines, but in extremely small quantities (much less than in the egg-derived vaccines).
Herman and colleagues (1983) reported that two children with systemic allergic reactions to egg white protein (ovalbumin) had anaphylactic reactions to MMR. Both children had serum IgE reactive with ovalbumin-related antigens in the vaccine. They had no detectable IgE directed against the measles vaccine, although IgE directed at ovalbumin was present.
Vaccine components other than egg protein have been implicated in triggering severe allergic reactions to live attenuated virus vaccines. Previously available live attenuated virus vaccines contained small amounts of antibiotics such as penicillin and streptomycin, but currently they have only trace amounts (25 µg/ml) of neomycin sulfate. They also have trace amounts of proteins such as chick embryo tissue culture protein and human serum albumin.
Case Reports, Case Series, and Uncontrolled Observational Studies
In 1981, Van Asperen et al. reported immediate reactions following administration of live attenuated measles vaccine (Rimevax) in three children in Australia. The reactions began within 30 minutes of immunization and consisted of vomiting, fever, and a rash; two of the patients also had cyanosis.
Pollock and Morris (1983) reported that nine reactions in the 170,000 children who received measles vaccine fell into their category of ''anaphylaxis and collapse'' during a 7-year period (January 1975 through December 1981) of intensified voluntary reporting of vaccine reactions in the North West Thames region of England. Some were felt to be vasovagal reactions, and some included slight facial swelling or pallor. It was unclear whether any of these reactions were anaphylactic.
Fifteen reports of reactions occurring within 30 minutes of vaccination with live attenuated measles virus (Rimevax) were received by the Adverse Drug Reactions Advisory Committee in Australia for the period February 1980 to March 1982 (McEwen, 1983). The most common findings were vomiting, changes in skin coloring, and disturbances of breathing. The mechanism of the reactions was unknown. Insufficient detail was provided to determine whether any of these reactions represented anaphylaxis. All individuals survived, and in 10 individuals the symptoms resolved without active treatment. The authors noted that the role that therapy played in the recovery of the other five children could not be assessed.
In a report from India (Sokhey, 1991), measles vaccine was involved in five incidents of adverse reactions in children. In three incidents, symp-
toms were felt to be typical of toxic shock. In one incident, 6 of the 12 children who received measles vaccine died. The six children who survived were said to have been saved by timely hospitalization and appropriate (but unspecified) treatment. Most of the incidents were felt to have been caused by contamination of the vaccines with pathogens, because the quality of the sterilization procedures was unsatisfactory. These incidents, however, were not described in sufficient detail to eliminate the possibility of anaphylactic shock.
In 1980, Aukrust et al. reported severe hypersensitivity or intolerance reactions to measles vaccine in six children. No description of the reactions was provided, although the authors felt that the children had immediate hypersensitivity reactions "most probably due to allergy." This measles vaccine was grown in monkey kidney cells (Aukrust et al., 1980). Trace amounts of calf serum proteins, but not egg antigens, were demonstrated in measles vaccine by crossed immunoelectrophoresis, but the exact cause of the untoward reactions was not identified. Four of the cases received the same lot of vaccine, suggesting the presence of an allergenic contaminant. The possibility of lot contamination also was suggested by the higher incidence of severe hypersensitivity reactions in Norway than in other countries (1 case per 15,000 to 20,000 doses versus 1 to 2 cases per 1 million doses, respectively) provided with the same vaccine by the same manufacturer.
Thurston (1987) reported two cases of anaphylaxis in his private practice. The first was an 18-month-old boy who developed bradycardia, cyanosis, periorbital edema, widespread erythema, and hypotonia 5 minutes after receiving MMR. He responded immediately to epinephrine. The second was a 16-month-old girl who started crying and who developed widespread erythema, cyanosis, decreased respiration, and wheezing 5 minutes after receiving MMR. She also responded to epinephrine.
The Swedish health authorities reported that no case with a clear picture of anaphylactic shock following administration of MMR was reported from 1982 to 1984, during which time 700,000 doses of MMR were sold in Sweden (Taranger and Wiholm, 1987). Five children had reactions that were described as anaphylactic or hypersensitivity reactions. These included a 12-year-old boy who developed urticaria with dyspnea and suspected laryngeal edema a couple of hours after immunization. He was treated with epinephrine and cortisone. Two 12-year-olds received epinephrine because of local redness and general symptoms of paleness and itching. An 18-month-old child was reported to have a diagnosis of mucocutaneous syndrome, but no further information was provided. A 5.5-year-old boy received cortisone and antihistamine orally for small urticaria.
In Germany, five reports of so-called immediate reactions were received after distribution of approximately 5.5 million doses of measles, mumps, and measles-mumps vaccines and MMR (Fescharek et al., 1990). Three
reports were of siblings who all collapsed after having been vaccinated shortly after one another. These were considered to be probable psychosomatic reactions. Another was the possible aspiration of a piece of candy, and the last case was called an "anaphylactic reaction" but was not described further.
In the new passive surveillance system for adverse reactions to vaccines in Canada, 30 of the 511 reports of adverse events following administration of MMR in 1987 were of allergic reaction, for an estimated incidence of 6.6 reports per 100,000 doses distributed (Koch et al., 1989). No details of these reactions were given.
Nine cases of possible anaphylactic reactions following administration of measles or mumps vaccines have been reported to VAERS (submitted between November 1990 and July 1992). All of these reactions followed receipt of MMR. The ages of the patients ranged from 4 to 31 years, with a median age of 11 years. None of the reactions were described in sufficient detail to verify the diagnosis of anaphylaxis. Also, prompt treatment may have prevented some of the individuals from developing full-blown anaphylaxis. Reactions in six of the patients had at least one component of an anaphylactic reaction. Three cases probably did not represent anaphylaxis. One child began to cry 2 to 4 hours after vaccination, and she complained that she could not hear; then she became pale and sweaty. Her symptoms resolved after she was given a drink of 7-Up. Another had a probable vasovagal reaction. Another case listed complaints of weakness, dizziness, nausea, blurred vision, and decreased hearing in the right ear immediately after the vaccine was administered, but the patient went home with no residual effects.
Egg allergy refers to an IgE-mediated immediate reaction to ovalbumin, the most severe manifestation being anaphylactic shock. Most patients who react to the ovalbumin skin test can ingest ovalbumin without any difficulties. Genuine anaphylactic reactions are very rare. Most physicians feel that patients with severe systemic reactions to egg proteins should be considered to be at some increased risk for a severe systemic reaction to measles and mumps vaccines. Most also agree that patients who are skin test positive to egg protein but who do not have clinical symptoms are not at any higher risk of an adverse reaction than the general population. There is disagreement, however, on the usefulness of vaccine skin testing in predicting those children who should receive the vaccines in graded (desensitizing) doses in order to avert life-threatening reactions.
Several reports in the literature describe safe measles and MMR immunization of patients with varying degrees of allergic symptoms to egg protein, including severe immediate reactions (Brown and Wolfe, 1967; Bruno et al., 1990; Di Cristofano, 1989; Greenberg and Birx, 1988; Kamin et al., 1963, 1965; Kemp et al., 1990).
Herman and colleagues (1983) advocate intracutaneous testing with diluted vaccine prior to immunization with measles vaccine in children with systemic reactions to egg protein. They employed graded doses of measles vaccine to safely immunize six patients who had severe allergic hypersensitivity reactions to ovalbumin and IgE anti-measles vaccine antibody and positive reactions after intracutaneous or intradermal testing with the vaccine.
Lavi and colleagues (1990) gave MMR to 90 egg-allergic children after performing skin tests with diluted MMR. They confirmed the reliability of negative reactions to skin tests with diluted MMR in predicting that the vaccine can safely be administered to such children. However, they stressed the importance of skin testing with diluted MMR because three patients who had positive reactions to MMR skin tests developed generalized urticaria, despite graded challenges, suggesting that they may have developed more pronounced reactions if MMR had been given in a routine fashion. Indeed, Puvvada and colleagues (1993) reported systemic reactions after just intradermal skin testing with MMR in two patients with severe systemic reactions to egg.
Fasano and colleagues (1992) reported a case series of 140 children with egg hypersensitivity in whom the safety of MMR was evaluated. Sixty-nine of the 140 children already had received MMR and were not tested. Two of the remaining 71 children had positive skin prick reactions to MMR. Three of six egg-allergic children, as well as three of six normal adult control subjects, had positive responses to MMR intradermal testing. All 71 children were given MMR in the standard dose under close observation, and the only child with an immediate reaction had four small hives 15 to 25 minutes after immunization. However, systemic reactions to MMR were documented in two nonallergic children. Both were skin test negative to egg and neomycin. One had a positive skin prick test to MMR and its individual components (antigens). The other was skin prick test negative, but intradermal test positive to the same components (antigens). The authors concluded that MMR skin testing was not helpful in predicting an adverse reaction.
Skin prick testing with MMR was performed on subjects with documented or suspected systemic allergies to antigens other than egg before they were immunized with MMR (Juntunen-Backman et al., 1987). Of 135 individuals, 126 had negative skin prick tests to MMR. Mild generalized urticaria or fever was noted in 2 of the 122 vaccinees who eventually were vaccinated with MMR. The authors concluded that children with common forms of systemic allergy can safely be vaccinated with MMR.
MMR contains neomycin sulfate, which may be responsible for some immediate reactions to the vaccine (Kwitten et al., 1993). Elliman and Dhanraj (1991) reported safe MMR vaccination in one child, despite neomycin
allergy documented by a positive reaction to skin patch testing with 20 percent neomycin sulfate.
Controlled Observational Studies
None.
Controlled Clinical Trials
None.
Causality Argument
Evidence from individual reports in VAERS and the literature is consistent with a causal relation between measles vaccine and MMR and anaphylaxis. The most compelling evidence consists of the cases reported by Herman and colleagues (1983) and by Thurston (1987). All four patients received MMR. In most cases of MMR-associated anaphylaxis, the precise component of the vaccine responsible for the severe reaction was not identified. In addition to the measles and mumps antigens, egg proteins, antibiotics, and other contaminants have been implicated. Reported cases of anaphylaxis following administration of these vaccines are extremely rare, and several reports suggest that anaphylaxis to measles vaccine is overreported and that not all of the cases are substantiated (Fescharek et al., 1990; McEwen, 1983; Pollock and Morris, 1983; Sokhey, 1991; Taranger and Wiholm, 1987; Thurston, 1987; Van Asperen et al., 1981). In 1983, Pollock and Morris estimated that anaphylaxis or collapse within 24 hours of vaccination occurred with a frequency of about 9 cases per 170,000 doses of measles vaccine administered in a large region of England over 7 years. In children with a history of anaphylactic reactions to egg, only five cases of immediate allergic reaction had been reported after distribution of more than 174 million doses of measles vaccine in the United States (American Academy of Pediatrics, Committee on Infectious Diseases, 1991).
Conclusion
The evidence establishes a causal relation between MMR and anaphylaxis.
The evidence favors acceptance of a causal relation between measles vaccine and anaphylaxis.
Because these conclusions are not based on controlled studies, the criteria for the diagnosis of anaphylaxis are variable, and pharmacologic intervention complicates the diagnosis, no reliable estimate of incidence or rela-
tive risk is available. Estimates from the studies described above range from 1 per 20,000 to 1 per 1 million doses distributed.
The evidence is inadequate to accept or reject a causal relation between mumps vaccine and anaphylaxis.
Risk-Modifying Factors
Most anaphylactic reactions occur in individuals who have no known risk factors for severe reactions to these vaccines; thus, no special precautions can be taken. Patients who have demonstrated severe systemic reactions to egg protein or neomycin may be at increased risk of anaphylaxis following receipt of measles or mumps vaccines, and guidelines for immunizing such patients have been provided by the Committee on Infectious Diseases of the American Academy of Pediatrics (1991). Patients with allergies to other antigens, including chickens and feathers, are not at increased risk of severe allergic reactions to these vaccines.
DEATH
A detailed discussion of the evidence regarding death following immunization can be found in Chapter 10. Only the causality argument and conclusions follow. See Chapter 10 for details.
Causality Argument
The data relating death and measles or mumps vaccine are from case reports and case series. The largest series comes from India, but toxic shock syndrome caused by the unhygienic conditions involved in the immunization program was the apparent cause of death reported for eight of nine patients. Evidence based on RNA sequencing techniques has linked measles vaccine and measles infection to subsequent death in some severely immunocompromised children. In contrast, studies of the immunogenic response to measles vaccine in children infected with human immunodeficiency virus, which causes acquired immune deficiency syndrome, have not recorded any deaths from measles infection.
The evidence favors the acceptance of a causal relation between measles vaccine and anaphylaxis. The evidence establishes a causal relation between MMR and thrombocytopenia and anaphylaxis. Anaphylaxis and thrombocytopenia can be fatal. Although there is no direct evidence of death as a consequence of measles vaccine-related anaphylaxis or of MMR-related thrombocytopenia or anaphylaxis, in the committee's judgment measles vaccine could cause fatal anaphylaxis and MMR could cause fatal
thrombocytopenia or fatal anaphylaxis. There is no evidence or reason to believe that the case fatality rate of vaccine-related thrombocytopenia or anaphylaxis would differ from the case fatality rates for these adverse events associated with any other cause.
Conclusion
The evidence establishes a causal relation between vaccine-strain measles virus infection and death. The conclusion is based on case reports in immunocompromised individuals and not on controlled studies. No relative risk can be calculated. However, the risk of death from measles vaccine-strain infection would seem to be extraordinarily low.
The evidence establishes a causal relation between MMR and death from anaphylaxis or complications of severe thrombocytopenia. There is no direct evidence for this; the conclusion is based on the potential of thrombocytopenia and anaphylaxis to be fatal. The risk would seem to be extraordinarily low.
The evidence favors acceptance of a causal relation between measles vaccine and death from anaphylaxis. There is no direct evidence for this; the conclusion is based on the potential of anaphylaxis to be fatal. The risk would seem to be extraordinarily low.
The evidence is inadequate to accept or reject a causal relation between measles and mumps vaccines and death from causes other than those listed above.
REFERENCES
Abe T, Nonaka C, Hiraiwa M, Ushijima H, Fujii R. Acute and delayed neuralgic reaction to inoculation with attenuated live measles virus. Brain and Development 1985;7:421-423.
Afzal MA, Pickford AR, Forsey T, Minor PD. Heterogeneous mumps vaccine. Lancet 1992;340:980-981.
Alderslade R, Bellman MH, Rawson NS, Ross EM, Miller DL. The National Childhood Encephalopathy Study: a report on 1,000 cases of serious neurological disorders in infants and young children from the NCES research team. In: Department of Health and Social Security. Whooping Cough: Reports from the Committee on the Safety of Medicines and the Joint Committee on Vaccination and Immunization. Lond: Her Majesty's Stationery Office; 1981.
Alter HJ, Scanlon RT, Schechter GP. Thrombocytopenic purpura following vaccination with attenuated measles virus. American Journal of Diseases of Children 1968; 115:111-113.
Alves RS, Barbosa ER, Scarf M. Postvaccinal parkinsonism. Movement Disorders 1992;7:178-180.
American Academy of Pediatrics, Committee on Infectious Diseases. The Red Book. Report of the Committee on Infectious Diseases, 22nd edition. Elk Grove, IL: American Academy of Pediatrics; 1991.
Asbury AK, Gibbs CJ Jr, eds. Autoimmune neuropathies: Guillain-Barré syndrome. Annals of Neurology 1990;27(Suppl.):S1-S79.
Association for the Study of Infectious Disease. A retrospective survey of the complications of mumps. Journal of the Royal College of General Practitioners 1974;24:552-556.
Aukrust L, Almeland TL, Refsum D, Aas K. Severe hypersensitivity or intolerance reactions to measles vaccine in six children: clinical and immunological studies. Allergy 1980;35:581-587.
Azeemuddin S. Thrombocytopenia purpura after combined vaccine against measles, mumps, and rubella (letter). Clinical Pediatrics 1987;26:318.
Azimi PH, Cramblett HG, Haynes RE. Mumps meningoencephalitis in children. Journal of the American Medical Association 1969;207:509-512.
Babbott FL Jr., Gordon JE. Modem measles. American Journal of Medical Science 1954;228:334-361.
Bach C, Allard M. Purpura thrombopenique aigu apres vaccination antirougeoleuse. A propos d'une observation. [Acute thrombocytopenic purpura after measles vaccination. Report of a case.] Semaine des Hopitaux de Paris 1974;50:589-593.
Bachand AJ, Rubenstein J, Morrison AN. Thrombocytopenic purpura following live measles vaccine. American Journal of Diseases of Children 1967;113:283-285.
Banatvala JE et al. Insulin-dependent (juvenile-onset, type I) diabetes mellitus: Coxsackie B viruses revisited. Progress in Medical Virology 1987;34:33-54.
Barbor PR, Grant DB. Encephalitis after measles vaccination. Lancet 1969;2:55-56.
Beale AJ. Measles vaccines. Proceedings of the Royal Society of Medicine 1974;67:1116-1119.
Beard CM, Benson RC, Kelalis PP, Elveback LR, Kurland LT. The incidence and outcome of mumps orchitis in Rochester, Minnesota, 1935-1974. Mayo Clinic Proceedings 1977;52:3-7.
Beersma MFC, Kapsenberg, JG, Renier WO, Galama JMD, Van Druten EN, Lucas, CJ. Subacute sclerosing panencephalitis in the Netherlands (1976-1986). Nederlands Tijdschrift voor Geneeskunde 1988;132:1194-1199.
Beghi E, Kurland LT, Mulder DW. Incidence of acute transverse myelitis in Rochester, Minnesota, 1970-1980, and implications with respect to influenza vaccine. Neuroepidemiology 1982;1:176-188.
Beghi E, Nicolosi A, Kurland LT, Mulder DW, Hauser WA, Shuster L. Encephalitis and aseptic meningitis, Olmsted County, Minnesota, 1950-1981. I. Epidemiology. Annals of Neurology 1984;16:283-294.
Bellanti JA, Sanga RL, Klutinis B et al. Antibody responses in serum and nasal secretions of children immunized with inactivated and attenuated measles-virus vaccines. New England Journal of Medicine 1969;260:628-633.
Bendersky-Malbec N. Les oreillons en 1982: mise au point sur le vaccin anti-ourlien. [Mumps in 1982: current situation concerning anti-mumps vaccine.] Concours Medecin 1982;104:167-177.
Blom L, Nystrom L, Dahlquist G. The Swedish childhood diabetes study: vaccinations and infections as risk determinants for diabetes in childhood. Diabetologia 1991;34:176-181.
Borsche A. Wie gefahrlich sind Impfungen im Kindesalter? [What are the hazards of vaccinations in childhood?] Zietschrift für Allemeinmedizin 1976;52:666-674.
Bottiger M, Christenson B, Romanus V, Taranger J, Strandell A. Swedish experience of two dose vaccination programme aiming at eliminating measles, mumps, and rubella. British Medical Journal 1987;295:1264-1267.
British Medical Journal. Prevention of mumps (editorial). British Medical Journal 1980;281:1231-1232.
Brodsky L, Stanievich J. Sensorineural hearing loss following live measles virus vaccination. International Journal of Pediatric Otorhinolaryngology 1985 :10:159-163.
Brown EG, Furesz J, Dimock K, Yarosh W, Contreras G. Nucleotide sequence analysis of
Urabe mumps vaccine strain that caused meningitis in vaccine recipients. Vaccine 1991;9:840-842.
Brown FR, Wolfe HI. Chick embryo grown measles vaccine in an egg-sensitive child. Journal of Pediatrics 1967;71:868-869.
Bruno G, Giampietro PG, Grandolfo ME, Milita O, Businco L. Safety of measles immunization in children with IgE-mediated egg allergy (letter). Lancet 1990;335:739.
Bunnell CE, Monif GR. Interstitial pancreatitis in the congenital rubella syndrome. Journal of Pediatrics 1972;80:465-466.
Buynak EB, Hilleman MR. Live attenuated mumps virus vaccine. I. Vaccine development. Proceedings of the Society for Experimental Biology and Medicine 1966;123:768-775.
Centers for Disease Control. Recommendations of the Public Health Service Advisory Committee on Immunization Practices: measles vaccines. Morbidity and Mortality Weekly Report 1967;16:169-271.
Cernescu C, Popescu-Tismana G, Alaicescu M, Popescu L, Cajal N. The continuous decrease in the number of SSPE annual cases ten years after compulsory anti-measles immunization. Revue Roumaine de Virologie 1990;41:13-18.
Champsaur HF, Bottazzo GF, Bertrams J, Assan R, Bach C. Virologic, immunologic, and genetic factors in insulin-dependent diabetes mellitus. Journal of Pediatrics 1982;100:15-20.
Cho CT, Lansky LJ, D'Souza BJ. Panencephalitis following measles vaccination. Journal of the American Medical Association 1973;224:1299.
Cizman M, Mozetic M, Radescek-Rakar R, Pleterski-Rigler D, Susec-Michieli M. Aseptic meningitis after vaccination against measles and mumps. Pediatric Infectious Disease Journal 1989;8:302-308.
Clark C, Hashim G, Rosenberg R. Transverse myelitis following rubeola vaccination. Neurology 1977;27:360.
Cohn J. Thrombocytopenia in childhood: an evaluation of 433 patients. Scandinavian Journal of Hematology 1976;16:226-240.
Coll JR. The incidence and complications of mumps. General Practitioner 1974;24:545-551.
Colville A, Pugh S. Mumps meningitis and measles, mumps, and rubella vaccine. Lancet 1992;340:786.
Craighead JE. The role of viruses in the pathogenesis of pancreatic disease and diabetes mellitus. Progress in Medical Virology 1975;19:161-214.
Dacou-Voutetakis C, Constantinidis M, Moschos S, Vlachou C, Matsaniotis MD. Diabetes mellitus following mumps: insulin reserve. American Journal of Diseases of Children 1974;127:890-891.
Dahlquist G, Blom L, Lonnberg G. The Swedish childhood diabetes study: a multivariate analysis of risk determinants for diabetes in different age groups. Diabetologia 1991;34:757-762.
De Prins F, Van Assche FA, Desmyter J, DeGroote J, Gepts W. Congenital rubella and diabetes mellitus. Lancet 1978;1:439-440.
De Ritis L, Pecorari R. Porpora trombocitopenica in seguito a vaccinazione antimorbillosa. [Thrombocytopenic purpura following measles vaccination.] Pediatria Medica e Chirurgica 1990;12:161-163.
Di Cristofano A. La vaccinazione contro il morbillo in bambini allergici all'uovo. [Measles vaccination in children allergic to eggs.] Professioni Infermieristiche (Roma) 1989;42:24-30.
Dietzsch HJ, Kiehl W. Zentralnervose Komplikationen nach Mmasern-Schutzimpfung. [Central nervous complications after measles vaccination.] Deutsche Gesundheit-Wesen 1976:31:2489-2491.
Dodson WE, Pasternak J, Trotter JL. Rapid deterioration in subacute sclerosing panencephalitis after measles immunization (letter). Lancet 1978;1:767-768.
Dosik H, Tricarico F. Haemolytic-uraemic syndrome following mumps vaccination. Lancet 1970;1:247.
Dyken PR, Cunningham SC, Ward LC. Changing character of subacute sclerosing panencephalitis in the United States. Pediatric Neurology 1989;5:339-341.
Dyrberg T. Schwimmbeck PL, Oldstone MBA. Inhibition of diabetes in BB rats by virus infection. Journal of Clinical Investigation 1988;81:928-931.
Ehrengut W, Zastrow K. Komplikationen "nach" Mumpsschutzimpfungen in der Bundesrepublik Deutschland (einschliesslich Mehrfachschutzimpfungen). [Complications "following" mumps vaccinations in the Federal Republic of Germany (including combined vaccine immunizations).] Monatsschrift Kinderheilkunde 1989;137:398-402.
Elliman D, Dhanraj B. Safe MMR vaccination despite neomycin allergy (letter). Lancet 1991;337:365.
Enders JF. Techniques of laboratory diagnosis, tests for susceptibility, and experiments on specific prophylaxis. Journal of Pediatrics 1946;29:129-142.
Enders JF, Peebles TC. Propagation in tissue culture of cytopathic agents from patients with measles. Proceedings of the Society for Experimental Biology and Medicine 1954;86:277-286.
Enders-Ruckle G. Frequency, serodiagnosis and epidemiological features of subacute sclerosing panencephalitis (SSPE) and epidemiology and vaccination policy for measles in the Federal Republic of Germany (FRG). Developments in Biological Standardization 1978;41:195-207.
Fajans SS. Diabetes Mellitus. In: DeGroot LJ et al., ed. Endocrinology. Philadelphia: W.B. Saunders; 1989.
Fasano MB, Wood RA, Cooke SK, Sampson HA. Egg hypersensitivity and adverse reactions to measles, mumps, and rubella vaccine. Journal of Pediatrics 1992;120:878-881.
Fescharek R, Quast U, Maass G, Merkle W, Schwarz S. Measles-mumps vaccination in the FRG: an empirical analysis after 14 years of use. II. Tolerability and analysis of spontaneously reported side effects . Vaccine 1990;8:446-456.
Fujinaga T, Motegi Y, Tamura H, Kuroume T. A prefecture-wide survey of mumps meningitis associated with measles, mumps and rubella vaccine. Pediatric Infectious Disease Journal 1991;10:204-209.
Gale JL, Thapa PB, Bobo JK, Wassilak SGF, Mendelman PM, Foy HM. Acute neurological illness and DTP: report of a case-control study in Washington and Oregon. Sixth International Symposium on Pertussis Abstracts. DHHS Publication No. (FDA) 90-1162. Bethesda, MD: U.S. Public Health Service, U.S. Department of Health and Human Services; 1990.
Gamble DR. Relation of antecedent illness to development of diabetes in children. British Medical Journal 1980;281:99-101.
Gerson K, Haslam RH. Subtle immunologic abnormalities in four boys with subacute sclerosing panencephalitis. New England Journal of Medicine 1971;285:78-82.
Giroud M, Page G, Genelle B, Lacroix X. Les thrombopenies postvaccinales avec purpura [Postvaccinal thrombopenia with purpura]. Journal de Medecine de Lyon 1983;64:97-100.
Gladisch R, Hofmann W, Waldherr R. Myokarditis und Insulitis nach Coxsackie virus Infektion. Zeitsch rift fur Kardiologie 1976;65:837-849.
Gluck R, Hoskins JM, Wegmann A, Just M, Germanier R. Rubini, a new live attenuated mumps vaccine virus strain for human diploid cells. Developments in Biological Standardization 1986;65:29-35.
Graham DY, Brown CH, Benrey J, Butel JS. Thrombocytopenia. Journal of the American Medical Association 1974;227:1161-1164.
Gray JA, Bums SM. Mumps meningitis following measles, mumps, and rubella immunization (letter). Lancet 1989a;2:98.
Gray JA, Bums SM. Mumps vaccine meningitis (letter). Lancet 1989b;2:927.
Greenberg MA, Birx DL. Safe administration of mumps-measles-rubella vaccine in egg-allergic children. Journal of Pediatrics 1988;113:504-506.
Griffin MR, Ray WA, Mortimer EA, Fenichel GM, Schaffner W. Risk of seizures after measles-mumps-rubella immunization. Pediatrics 1991;88:881-885.
Grose C, Spigland I. Guillain-Barré syndrome following administration of live measles vaccine. American Journal of Medicine 1976;60:441-443.
Gunderson E. Is diabetes of infectious origin? Journal of Infectious Diseases 1927;41:197-202.
Gutierrez-Lopez MD, Bertera S, Chantres MT, Vavassori C, Dotman JS, Trucco M, et al. Susceptibility to Type 1 (insulin-dependent) diabetes mellitus in Spanish patients correlates quantitatively with expression of HLA-DQ(alpha) ARG 52 and HLA-DQ(beta) non-Asp 57 alleles. Diabetologia 1992;35:583-588.
Halsey N. Risk of subacute sclerosing panencephalitis from measles vaccination. Pediatric Infectious Disease Journal 1990;9:857-858.
Halsey NA, Schubert W, Jabbour JT, Preblud SR. Measles vaccine and the course of subacute sclerosing panencephalitis (letter). Lancet 1978;2:783.
Halsey NA, Modlin JF, Jabbour JT, Dubey L, Eddins DL, Ludwig DD. Risk factors in subacute sclerosing panencephalitis: a case-control study. American Journal of Epidemiology 1980;111:415-424.
Harris HF. A case of diabetes mellitus quickly following mumps. Boston Medical and Surgical Journal 1899;140:465-469.
Haun U, Ehrhardt G. Zur problematik postvakzinaler Komplikationen nach Masemschutzimpfung. [Postvaccinal complications following protective vaccination against measles.] Deutsche Gesundheit-Wesen 1973;28:1306-1308.
Helmke K, Otten A, Willems WR, Brockhaus R, Mueller-Eckhardt G, Stief T et al. Islet cell antibodies and the development of diabetes mellitus in relation to mumps infection and mumps vaccination. Diabetologia 1986;29:30-33.
Herman JJ, Radin R, Schneiderman R. Allergic reactions to measles (rubeola) vaccine in patients hypersensitive to egg protein. Journal of Pediatrics 1983;102:196-199.
Hilleman MR, Buynak EB, Weibel RE, Stokes JJ, Whitman JE Jr, Leagus MB. Development and evaluation of the Moraten measles virus vaccine. Journal of the American Medical Association 1968a;206:587-590.
Hilleman MR, Buynak EB, Weibel RE, et al. Live attenuated mumps-virus vaccine. New England Journal of Medicine 1968b;278:227-232.
Hinden E. Mumps followed by diabetes. Lancet 1962;1:1381.
Hirayama M. Measles vaccines used in Japan. Reviews of Infectious Diseases 1983;5:495-503.
Hirtz DG, Nelson KB, Ellenberg JH. Seizures following childhood immunizations. Journal of Pediatrics 1983;102:14-18.
Hudson JB, Weinstein L, Chang T, et al. Thrombocytopenic purpura in measles. Journal of Pediatrics 1956;48:48-56.
Isomura S. Measles and measles vaccine in Japan. Acta Paediatrica Japonica 1988;30:154-162.
Jabbout JT, Duenas DA, Sever JL, Krebs HM, Horta-Barbosa L. Epidemiology of subacute sclerosing panencephalitis (SSPE). Journal of the American Medical Association 1972;220:959-962.
Jackson H. A fatal case of purpura with a few notes on the recent literature of this disease. Archives of Pediatrics 1890;7:951.
Jagdis F, Langston C, Gutwith M. Encephalitis after administration of live measles vaccine . Canadian Medical Association Journal 1975;112:972-975.
Jenson AB, Rosenberg HS, Notkins AL. Pancreatic islet cell damage in children with fatal viral infections. Lancet 1980:2:354-358.
Juntunen-Backman K, Peltola H, Backman A, Salo OP. Safe immunization of allergic children against measles, mumps, and rubella. American Journal of Diseases of Children 1987;141:1103-1105.
Kamin PB, Fein BT, Britton HA. Live, attenuated measles vaccine. Journal of the American Medical Association 1963;185:99-102.
Kamin PB, Fein BT, Britton HA. Use of live, attenuated measles virus vaccine in children allergic to egg protein. Journal of the American Medical Association 1965;193:1125-1126.
Kaplan BS, Proesmans W, et al. The hemolytic uremic syndrome of childhood. Seminars in Hematology 1987:24:148-160.
Kaplan SA. Clinical Pediatric Endocrinology. Philadelphia: W.B. Saunders; 1990.
Katz SL. Immunization with live attenuated measles virus vaccine: five years' experience. Archiv für die Gesamte Virusforschung 1965;16:222-230.
Kazarian EL, Gager WE. Optic neuritis complicating measles, mumps, and rubella vaccination. American Journal of Ophthalmology 1978:86:544-547.
Kemp A, Van Asperen P, Mukhi A. Measles immunization in children with clinical reactions to egg protein. American Journal of Diseases of Children 1990;144:33-35.
Kiefaber RW. Thrombocytopenic purpura after measles vaccination (letter). New England Journal of Medicine 1981;305:225.
Klajman A, Sternbach M, Ranon L, Drucker M, Geminder D, Sadan N. Impaired delayed hypersensitivity in subacute sclerosing panencephalitis. Acta Paediatrica Scandinavica 1973:62:523-526.
Koch J, Leer C, McCarthy R, Carter A, Cuff W. Adverse events temporally associated with immunizing agents—1987 report/Manifestations facheuses associees dans le temps a des agents immunisants—rapport de 1987. Canada Diseases Weekly Report/Rapport Hebdomadaire des Maladies au Canada 1989;15:151-158.
Kremer HU. Juvenile diabetes as a sequel to mumps. American Journal of Medicine 1947:3:257-258.
Krugman S. Further-attenuated measles vaccine: characteristics and use . Reviews of Infectious Diseases 1983;5:477-481.
Krugman S, Giles JP, Jacobs AM, Friedman H. Studies with live attenuated measles-virus vaccine. American Journal of Diseases of Children 1962;103:353-363.
Kumar R, Chandra R, Bhushan V, Srivastava BC. Adverse reaction after measles immunization in a rural population. Indian Pediatrics 1982:19:605-610.
Kwitten PL, Rosen S, Sweinberg SK. MMR vaccine and neomycin allergy (letter). American Journal of Diseases of Children 1993;147:128-129.
Landrigan PJ, Witte JJ. Neuralgic disorders following live measles-virus vaccination. Journal of the American Medical Association 1973;223:1459-1462.
Lavi S. Zimmerman B, Koren G, Gold R. Administration of measles, mumps, and rubella virus vaccine (live) to egg-allergic children. Journal of the American Medical Association 1990;263:269-271.
Lee GR, Foerster J, Athens JW, Lukens JN, eds. Wintrobe's Clinical Hematology, 9th edition. London: Lea & Febiger: 1993.
Levin M, Walter MD S, Barratt TM. Hemolytic uremic syndrome. Advances in Pediatric Infectious Disease 1989;4:51-81.
Lidin-Janson G, Strannegard O. Two cases of Guillain-Barré syndrome and encephalitis after measles. British Medical Journal 1972;2:572.
Lightsey AL. Thrombocytopenia in children. Pediatric Clinics of North America 1980:27:293-308.
Lipton RB, Kocova M, LaPorte RE, Dorman JS, Orchard TJ, Riley WJ, Drash Al, Becker DI,
Trucco M. Autoimmunity and genetics contribute to the risk of insulin-dependent diabetes mellitus in families: islet cell antibodies and HLA DQ heterodimers. American Journal of Epidemiology 1992;136:503-512.
Maclaren N, Atkinson M. Is insulin-dependent diabetes mellitus environmentally induced? New England Journal of Medicine 1992:327:348-349.
Marshall GS, Wright PF, Fenichel GM, Karzon DT. Diffuse retinopathy following measles, mumps, and rubella vaccination. Pediatrics 1985;76:989-991.
Maspero A, Cancellieri V, Della Rosa C. Reazioni secondarie alla vaccinazione contro il morbillo. [Adverse reactions to measles vaccine.] Giornale di Malattie Infettive e Parassitarie 1991;43:499-503.
McCrae WM. Diabetes mellitus following mumps. Lancet 1963:1:1300-1301.
McDonald JC, Moore DL, Quennec P. Clinical and epidemiologic features of mumps meningoencephalitis and possible vaccine-related disease. Pediatric Infectious Disease Journal 1989;8:751-755.
McEwen J. Early-onset reaction after measles vaccination: further Australian reports. Medical Journal of Australia 1983:2:503-505.
McKendrick GDW, Nishtar T. Mumps orchitis and sterility. Public Health 1966:80:277-278.
Medical Journal of Australia. Measles vaccination: thrombocytopenia. Medical Journal of Australia 1980:1:561.
Medical Research Council. Vaccination against measles: clinical trial of live measles vaccine given alone and live vaccine preceded by killed virus. The Practitioner 1971:206:458-466.
Menser MA, Forrest JM, Bransby RD. Rubella infection and diabetes mellitus. Lancet 1978;1:57-60.
Messaritakis J. Diabetes following mumps in sibs. Archives of Disease in Childhood 1971;46:561.
Meyer MB. An epidemiologic study of mumps: its spread in school and families. American Journal of Hygiene 1962:75:259.
Mirchamsy H. Measles immunization in Iran. Reviews of Infectious Diseases 1983;5:491-494.
Mitus A, Holloway A, Evans AE, Enders JF. Attenuated measles vaccine in children with acute leukemia. American Journal of Diseases of Children 1962;103:243-248.
Modlin JF, Jabbour JT. Witte J J, Halsey NA. Epidemiologic studies of measles, measles vaccine, and subacute sclerosing panencephalitis. Pediatrics 1977;59:505-512.
Monif GR. Rubella virus and the pancreas. Medecine et Chirurgie Digestives 1974;3:195-197.
Mori I, Torii S, Hamamoto Y, Kanda A, Tabata Y, Nagafuji H. [Virological evaluation of mumps meningitis following vaccination against mumps.] Kansenshogaku Zasshi [Journal of the Japanese Association for Infectious Diseases] 1991;65:226-283.
Morley DC. Measles in the developing world. Proceedings of the Royal Society of Medicine 1974;67:1112-1115.
Morrow JI, Dowey KE, Swallow MW. Subacute sclerosing panencephalitis in Northern Ireland: twenty years' experience. Ulster Medical Journal 1986:55:124-130.
Nabe-Nielsen J, Walter B. Unilateral total deafness as a complication of the measles-mumps-rubella vaccination. Scandinavian Audiology, Supplementum 1988a:30:69-70.
Nabe-Nielsen J, Walter B. Unilateral deafness as a complication of the mumps, measles, and rubella vaccination. British Medical Journal 1988b;297:489.
Nader PR, Warren RJ. Reported neurologic disorders following live measles vaccine. Pediatrics 1968:41:997-1001.
Neiderud J. Thrombocytopenic purpura after a combined vaccine against morbilli, parotitis and rubella. Acta Paediatrica Scandinavica 1983;72:613-614.
Nicolosi A, Hauser WA, Beghi E, Kurland LT. Epidemiology of central nervous system infections in Olmsted County, Minnesota, 1950-1981. Journal of Infectious Diseases 1986;154:399-408.
Nieminen U, Peltola H, Syrjala MT, Makipernaa A, Kekomaki R. Acute thrombocytopenic purpura following measles, mumps and rubella vaccination: a report on 23 patients. Acta Paediatrica 1993;82:267-270.
Norrby R. Polyradiculitis in connection with vaccination against morbilli, parotitis and rubella. Lakartidningen 1984;81:1636-1637.
Okuno Y, Nakao T, Ishida N, Konno T, Mizutani H, Fukuyama Y, et al. Incidence of subacute sclerosing panencephalitis following measles and measles vaccination in Japan. International Journal of Epidemiology 1989;18:684-689.
Oldstone MBA. Prevention of Type I diabetes in nonobese diabetic mice by virus infection. Science 1988;239:500-502.
Oldstone MBA. Viruses can cause disease in the absence of morphological evidence of cell injury: implication for uncovering new diseases in the future. The Journal of Infectious Diseases 1989;159:384-389.
Oldstone MBA, Ahmed R, Salvato M. Viruses as therapeutic agents II. Viral reassortants map prevention of insulin-dependent diabetes mellitus to the small RNA of lymphocytic choriomeningitis virus. Journal of Experimental Medicine 1990a;171:2091-2100.
Oldstone MBA, Tishon A, Schwimmbeck PL, Shyp S, Lewicki H, Dyrberg T. Cytotoxic T lymphocytes do not control lymphocytic choriomeningitis virus infection of BB diabetes-prone rats. Journal of General Virology 1990b;71(Pt. 4):785-791.
Olivares M, Walter T, Osorio M, Chadud P, Schlesinger L. Anemia of a mild viral infection: the measles vaccine as a model. Pediatrics 1989;84:851-855.
Oski FA, Naiman JL. Effect of live measles vaccine on the platelet count. New England Journal of Medicine 1966;275:352-356.
Otten A, Helmke K, Stief T, Mueller-Eckhard G, Willems WR. Federlin K. Mumps, mumps vaccination, islet cell antibodies and the first manifestation of diabetes mellitus type I. Behring Institute Mitteilungen 1984;75:83-88.
Ozeretskovskii NA, Gurvich EB. Pobochnoe deistvie vaktsin kalendaria profilakticheskikh privivok. [The side effects of vaccines used in prophylactic inoculation schedules.] Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1991;5:59-63.
Parker JC, Klintworth GK, Graham DG, Griffith JF. Uncommon morphologic features in sub-acute sclerosing panencephalities (SSPE) . American Journal of Pathology 1970;61:275-291.
Parkkonen P, Hyoty H, Koskinen L, Leinikki P. Mumps virus infects beta cells in human fetal islet cell cultures upregulating the expression of HLA class I molecules. Diabetologia 1992:35:63-69.
Patrick A. Acute diabetes following mumps. British Medical Journal 1924;2:802.
Patterson K, Chandra RS, Jenson AB. Congenital rubella, insulitis, and diabetes mellitus in an infant. Lancet 1981:1:1048-1049.
Pawlowski B, Gries FA. Mumpsimpfung und Typ-I-Diabetes. [Mumps vaccination and type-I-diabetes.] Deutsche Medizinische Wochenschrift 1991;116:635.
Payne FE, Baublis JV, Itabashi HH. Isolation of measles virus from cell cultures of brain from a patient with subacute sclerosing panencephalitis. New England Journal of Medicine 1969;281:585-589.
Peig M, Ercilla G, Millan M, Gomis R. Postmumps diabetes mellitus (letter). Lancet 1981:1:1007.
Penttinen K, Cantell K, Somer P, Poikolainen A. Mumps vaccination in the Finnish defense forces. American Journal of Epidemiology 1968;88:234-244.
Perlman EC. Purpuric and cerebral manifestations following measles. Archives of Pediatrics 1934;51:596-604.
Philip RN, Reinhard KR, Lackman DB. Observations on a mumps epidemic in a ''virgin'' population. American Journal of Hygiene 1959;69:91-111.
Plotkin SA, Mortimer EA. Vaccines. Philadelphia: W.B. Saunders Co., 1988.
Pollock TM, Morris J. A 7-year survey of disorders attributed to vaccination in North West Thames region. Lancet 1983;1:753-757.
Prince GA, Jenson AB, Billups LC, Notkins AL. Infection of human pancreatic beta cell cultures with mumps virus. Nature 1978;271:158-161.
Puvvada L, Silverman B, Bassett C, Chiaramonte LT. Systemic reactions to measles-mumps-rubella vaccine skin testing. Pediatrics 1993;91:835-836.
Quast U, Hennessen W, Widmark RM. Vaccine induced mumps-like diseases. Developments in Biological Standardization 1979;43:269-272.
Rayfield EJ, Kelly K J, Yoon JW. Rubella virus-induced diabetes in the hamster. Diabetes 1986;35:1278-1281.
Reed D, Brown G, Merrick R, et al. A mumps epidemic on St. George Island, Alaska. Journal of the American Medical Association 1967;199:113-117.
Riikonen R. The role of infection and vaccination in the genesis of optic neuritis and multiple sclerosis in children. Acta Neurologica Scandinavica 1989;80:425-431.
Robbins FC. Measles: clinical features, pathogenesis, pathology, and complications. American Journal of Diseases of Children 1962;103:266-273.
Rubinstein P, Walker ME, Fedun B, Witt ME, Cooper LZ, Ginsberg-Fellner F. The HLA system in congenital rubella patients with and without diabetes. Diabetes 1982;31:1088-1091.
Sandler B. Recovery from sterility after mumps orchitis. British Medical Journal 1954;11:795.
Saxton NL. Thrombocytopenic purpura following the administration of attenuated live measles vaccine. Journal of the Iowa Medical Society 1967;57:1017-1018.
Schacher SA. An epidemiological approach to subacute sclerosing panencephalitis. Neurology 1968;18(1 Pt 2):76-77.
Schneck SA. Vaccination with measles and central nervous system disease. Neurology 1968;18(Pt 2):79-82.
Schwarz AJ. Immunization against measles: development and evaluation of a highly attenuated live measles vaccine. Annales de Paediatrie 1964;202:241-252.
Schwarz AJ, Anderson JT. Immunization with a further attenuated live measles virus vaccine. Archiv fur Gesamte Virusforschung 1965;16:273-278.
Schwarz A J, Jackson JE, Ehrenkranz NJ, Ventura A, Schiff GM, Walters VW. Clinical evaluation of a new measles-mumps-rubella trivalent vaccine. American Journal of Diseases of Children 1975;129:1408-1412.
Sinaniotis CA, Daskalopoulou E, Lapatsanis P, Doxiadis S. Diabetes mellitus after mumps vaccination (letter). Archives of Disease in Childhood 1975;50:749-750.
Sokhey J. Adverse events following immunization: 1990. Indian Pediatrics 1991;28:593-607.
Srivastava RN, Bagga A. Hemolytic uremic syndrome: recent developments. Indian Pediatrics 1992;29:11-24.
Starke G, Hlinak P, Nobel B, Winkler C, Kaesler G. Maserneradikation—eine Moglichkeit? Ergebnisse und Erfahrungen mit der Masernschutzimpfung 1967 bis 1969 in der DDR [Measles control—a possibility? Results and experiences with measles vaccination from 1967 to 1969 in the German Democratic Republic.] Deutsche Gesundheit-Wesen 1970;25:2384-2390.
Stokes JJ, Maris EP, Gelles SS. Chemical, clinical, and immunologic studies on the products of human plasma fractionation. XI. The use of concentrated normal human serum gamma globulin (human immune serum globulin) in the prevention and attenuation of measles. Journal of Clinical Investigations 1944;23:531-540.
Sugiura A, Yamada A. Aseptic meningitis as a complication of mumps vaccination. Pediatric Infectious Disease Journal 1991;10:209-213.
Sultz HA, Hart BA, Zielezny M, Schlesinger ER. Is mumps virus an etiologic factor in juvenile diabetes mellitus? Journal of Pediatrics 1975;86:654-656.
Taranger J, Wiholm BE. Litet antal biverkningar rapporterade efter vaccination mot massling-passjuka-roda hund. [The low number of reported adverse effects after vaccination against measles, mumps, rubella.] Lakartidningen 1987;84:948-950.
Thurston A. Anaphylactic shock reaction to measles vaccine (letter). Journal of the Royal College of General Practice 1987;37:41.
Tishon A, Oldstone MBA. Persistent virus infection associated with chemical manifestations of diabetes. American Journal of Pathology 1987:126:61-72.
Trump RC, White TR. Cerebellar ataxia presumed due to live, attenuated measles virus vaccine. Journal of the American Medical Association 1967:199:129-130.
U.S. Department of Health and Human Services. U.S. Public Health Service, National Vaccine Injury Compensation Program; Revision of the Vaccine Injury Table; Proposed Rule. Federal Register, 42 CFR Part 100, August 14, 1992;57(158):36877-36885.
Valmari P, Lanning M, Tuokko H, Kouvalainen K. Measles virus in the cerebrospinal fluid in postvaccination immunosuppressive measles encephalopathy. Pediatric Infectious Disease Journal 1987:6:59-63.
Van Asperen PP, McEniery J, Kemp AS. Immediate reactions following live attenuated measles vaccine. Medical Journal of Australia 1981;2:330-331.
von Muhlendahl KE. Nebenwirkungen und Komplikationen der Masern-Mumps-Impfung. [Side effects and complications of measles-mumps vaccination.] Monatsschrift Kinderheilkunde 1989:137:440-446.
Walker AM, Jick H, Perera DR, Knauss TA, Thompson RS. Neurologic events following diphtheria-tetanus-pertussis immunization. Pediatrics 1988;81:345-349.
Werner CA. Mumps orchitis and testicular atrophy. I. Occurrence. Annals of Internal Medicine 1950a;32:1066-1074.
Werner CA. Mumps orchitis and testicular atrophy. II. A factor in male sterility. Annals of Internal Medicine 1950b:32:1075-1086.
White F. Measles vaccine associated encephalitis in Canada (letter). Lancet 1983;2:683-684.
Wiersbitzky S, Bruns R, Schroder C, Warmuth M. Thrombozytopenische Purpura nach Impfung mit Lebendvakzine (Mumps-Masern-Roteln-Schutzimpfung)? [Thrombocytopenic purpura following immunization with a live vaccine (mumps-measles-rubella vaccination)?] Kinderarztliche Praxis 1992:60:28-29.
Wilhelm DJ, Paegle RD. Thrombocytopenic purpura and pneumonia following measles vaccination. American Journal of Diseases of Children 1967;113:534-537.
Yamada A, Takeuchi K, Tanabayashi K, Hishiyama M, Takahashi Y, Sugiura A . Differentiation of the mumps vaccine strains from the wild viruses by the nucleotide sequences of the P gene. Vaccine 1990:8:553-557.
Yoon JW, Ray UR. Perspectives on the role of viruses in insulin-dependent diabetes. Diabetes Care 1985;8(Suppl. 1):39-44.
Yoon JW, Onodera T, Notkins AL. Virus-induced diabetes mellitus: XV. Beta cell damage and insulin-dependent hyperglycemia in mice infected with Coxsackie virus B-4. Journal of Experimental Medicine 1978:148:1068-1080.
Yoon JW, Austin M, Onodera T, Notkins AL. Virus-induced diabetes mellitus. New England Journal of Medicine 1979:300:1173-1179.
Yoon JW, Eun HM, Essani K, Roncari DA, Bryan LE. Possible mechanisms in the pathogenesis of virus-induced diabetes mellitus. Medecine Clinique et Experimentale [Clinical and Investigative Medicine] 1987a:10:450-456.
Yoon JW, Kim CJ, Pak CY, McArthur RG, et al. Effects of environmental factors on the development of insulin-dependent diabetes mellitus. Medecine Clinique et Experimentale [Clinical and Investigative Medicine] 1987b;10:457-469.





































































