4
Training Pathways
A generation ago medical research was conducted largely by physicians, most of whom had little formal training in science (Smith, 1989). Clinical investigation was focused on disease and disease processes and was conducted largely at the patient level. Advances in cell biology and molecular genetics are bringing investigators closer to discovering how genes direct and influence normal human development as well as disease. Developments in areas such as neurobiology, immunology, and developmental biology present new challenges for designing and testing innovative treatments and preventions. Furthermore, new methodologies for assessing the outcomes of current and new medical technologies are evolving rapidly. Rigorous clinical research training is required to ensure valid results, inferences, and conclusions to improve health care practices. Yet, there is a growing concern that too few people are being trained to conduct sophisticated studies on the advances presented by these new developments in science and technology (Kelley, 1988; Martin, 1991).
Numerous criticisms have been leveled at the U.S. system of undergraduate and graduate medical education, including a growing divergence between patient needs and physician training; excessive emphasis on research and service in research-intensive universities at the expense of teaching; poor integration between the preclinical and clinical components of medical education; changes in hospital-based clinical training and the move to more ambulatory care, as a result of which trainees are unable to observe the entire course of disease; and a teaching style that fails to engender the development of faculty role models or imbue students with problem-solving skills and positive attitudes for lifelong learning (Cantor et al., 1991; Goodman et al., 1991). Moreover, along with the growing
complexities of the U.S. health care system and its burgeoning problems, medical students are expected to become increasingly compassionate and caring as well as more aware and knowledgeable about patients' insurance coverage, case law, and ethics.
Dentistry, nursing, and other health professional groups also encounter barriers to clinical research careers that may or may not be similar to the barriers found in medicine. For example, unlike medicine, where there is extensive graduate medical education, the dental school curriculum is designed to prepare dentists who can practice dentistry upon graduation—after four years of graduate education. The dentistry curriculum thus combines didactic course work and clinical skills development during those four years, which brings into question the amount of time that dental students can commit to developing research skills (Appendix A). Although nurses, pharmacists, and allied health professionals generally acquire their clinical practice skills at the undergraduate level, most acquire their research skills in doctoral programs. In the past, many of these doctoral programs have been in other fields, such as education or psychology. New doctoral programs in nursing and allied health disciplines are being created, however (Appendix B; Selker, 1994).
The committee did not have the expertise to judge the effectiveness or the quality of programs in dentistry, nursing, and the allied health professions. The committee therefore sought input from the appropriate professional groups through task forces, commissioned papers, or written comments. Most groups felt that there were obstacles in the training pathways leading to careers in patient-oriented clinical research. Some of these were seen as peculiar to a given profession, whereas others were viewed as generic to all health care groups. The complete task force reports on dentistry and on nursing and clinical psychology can be found in Appendixes A and B, respectively, and the background paper by Dr. Selker elaborates on clinical research in the allied health professions (1994). Where appropriate, however, the concerns of those groups will be noted in the text.
The committee believes that health care professionals in all fields should be well-versed in the sciences underpinning the practice of health care. Sophisticated scientific and quantitative preparation empowers health care practitioners to pose insightful questions about human biology and behavior, to retrieve and critically analyze information for use in solving clinical problems, and to remain open to unexpected new possibilities. The diverse responsibilities in the various professional groups engaged in clinical research require that they have different kinds and levels of educational and scientific backgrounds. Unlike doctoral programs, in which the goal is to train highly skilled research scientists, the primary goal of health professional schools is to blend the scientific knowledge base with clinical skills to prepare highly qualified and competent practitioners of health care. In a health care environment in which health care knowledge and technology are accelerating rapidly and new discoveries are reported almost daily,
preparing health practitioners who are well-grounded in the biological, social, behavioral, information, and quantitative sciences becomes ever more challenging. Clearly, all health care professionals should have a firm grasp of the traditional biomedical sciences as well as the social and behavioral sciences (Association of American Medical Schools, 1992b; Greenlick, 1992). Newer interdisciplinary biological sciences such as molecular biology, molecular genetics, and neuroscience, as well as increasingly sophisticated quantitative methods in areas such as medical effectiveness research, are also expanding the boundaries of knowledge for health care.
To begin to analyze the many perceived obstacles in the pathways leading to clinical research careers at the professional school level, the committee posed several generic questions:
-
Is the present system for clinical research training inadequate?
-
What does society want and expect students to know?
-
Are professional schools organized to meet these goals?
-
Are the faculty and administration committed to change?
-
Are resources available for effecting change where changes are needed?
To approach these questions, the committee developed a list of issues that were addressed by the subcommittees examining issues affecting clinical research careers in the precollege and undergraduate periods, during graduate education, and during postdoctoral training. The committee examined the recruitment into scientific careers and the retention of those interested in pursuing research careers. Clearly, issues that affect students early are the quality and quantity of hands-on research experiences that are directly related to resources and quality of teaching. If students are unprepared or ''turned off" to science and mathematics early in the educational process (that is, during their education from kindergarten through grade 12 [K–12]), should mechanisms be developed to change the environment and inspire interest in these fields? The influence of role models and mentors throughout the education and training pathway also have an effect on decisions to pursue scientific careers (Cameron, 1991). As students move into college, some of the same factors concerning quality of scientific curricula apply, but other factors can also affect their career choices, including income potential, job availability and security, and economic factors. Extensive length of training, accumulating educational debt, absence of quality research experiences and funding for research training, lack of time for engaging in research activities, lack of effective mentoring, and other lifestyle factors are some of the factors confronting health professionals who are interested in graduate education and postgraduate training (Applegate 1990; Smith, 1989). Furthermore, the demographics of the United States are changing, and the committee recognizes that changes in the education and training environment must be sensitive to gender and cultural differences and encourage increasing numbers of these groups to pursue research
careers. Thus, this chapter examines the barriers and obstacles to research careers throughout the education and training pathway. Many of the issues confronting individuals are generic to all scientific careers, while some are specific to clinical research careers. The distinctions will be noted where applicable. It should be noted, however, that the committee has been hindered in its analyses by the extreme lack of outcomes data for research training programs and for factors affecting career choice.
Although the audience for this report might question the relevance of K–12 science experiences and their relationship to clinical research careers, the committee felt that it was important to reemphasize obstacles throughout the entire education and training pathways for clinical investigators. All too often, reports of this nature focus too narrowly on the late stages of training and neglect the earlier stages of education that influence the pool from which scientific talent will be drawn. Because each successive level of the training pathway relies on the preparation of the talent pool of the previous level, the committee felt that it would be productive to examine obstacles to scientific careers, particularly clinical investigative careers, from kindergarten to the achievement of a career as an established scientist.
The first portion of this chapter presents an overview of existing efforts to stimulate interest in careers in the sciences and health professions among students of all ages. Particular attention is paid to activities that involve or encourage students to become interested in scientific investigation. Because the committee membership did not have professional educators at the K–12 levels or at the undergraduate level, they chose to draw upon the work of others who have considered this issue. Among the sources relied on were Educating Scientists and Engineers: Grade School to Grad School (U.S. Congress, Office of Technology Assessment, 1988a); Nurturing Scientific Talent: A Discussion Paper (National Academy of Sciences, Government-University-Industry Research Roundtable, 1987); Fulfilling the Promise: Biology Education in the Nation's Schools (National Research Council, 1990); and By the Year 2000; First in the World (Federal Coordinating Committee for Science, Engineering and Technology, Committee on Education and Human Resources, 1991). To supplement these sources, the committee commissioned a paper by Marcia Matyas formerly of the American Association for the Advancement of Science, "Early Exposure to Research: Opportunities and Effects" (Matyas, 1994) from which this section of the report draws heavily.
The following sections of the chapter closely examine what is known, or not known, about professional education and training for careers in clinical investigation. These sections are supplemented by excerpts from the workshop "Clinical Research and Research Training: Spotlight on Funding" (Appendix D) the task force reports (Appendixes A, B, and C), and commissioned papers on training programs of the National Institutes of Health (NIH), models for
postdoctoral clinical research training, the influence of resident review committees and certification boards on research training, and mentoring.
DEMOGRAPHICS
The committee recognizes that the recruitment and retention of scientists and health professionals into careers as clinical investigators must reflect the changing demographics of the United States (U.S. Congress, Office of Technology Assessment, 1985). Unlike nursing, which has been dominated by women, scientists and academic physicians in the past have characteristically been white males. Women now constitute nearly half of all medical students in U.S. medical schools and earn slightly more than a third of all life sciences doctorates (National Research Council, 1987b, 1991). The picture is not as hopeful for African-Americans, Hispanics, and native Americans, who remain underrepresented in research and medicine (National Research Council, 1987a). This is of considerable concern because by the turn of the century, one third of the children living in the United States will be members of minority groups. These demographic data indicate that special efforts are needed to recruit members of these groups to pursue careers in patient-oriented clinical research (Robert Wood Johnson Foundation, 1987).
KINDERGARTEN TO COLLEGE
The decision to pursue a career in the sciences or health professions is the result of the interaction of many educational, psychosocial, and environmental factors. Exposure to science and mathematics instruction beginning in elementary school profoundly influences career choice (Federal Coordinating Council on Science, Engineering and Technology, 1991). Most commonly, school-age children get their first exposure to science by conducting hands-on experiments in the classroom. Other factors not directly related to the formal educational process are important as well. For example, many decisions to pursue a career in the sciences are the result of personal characteristics, such as positive motivation and good study habits. The expectations of parents, teachers, and peers; adequate mentoring; the presence of career opportunities; good occupational status; and job security also clearly play a role. Students can also be influenced by their participation in informal science experiences offered through museums or youth clubs (Matyas and Malcom, 1991). The committee believes that life experiences and the quality of science education during the formative years have a profound effect on the future talent pool from which highly capable clinical investigators will be drawn at later stages of the education pathway.
Classroom Experience
There are some 45 million students and 2.5 million teachers in the nation's 60,000 public and 40,000 private elementary and secondary schools. Because of the diversity of schools, school districts, and local control over education, the quality and effectiveness of science and mathematics education can be equally diverse. With the exception of a few magnet science high schools with the stated goal of fostering greater interest in scientific careers, most schools and school districts cannot or do not emphasize one subject area over another.
Although hands-on science activities are an ideal way to stimulate student interest in science, for a variety of reasons, many students are not introduced to these kinds of science experiences. For one thing, most students have only minimal exposure to science-related instruction. According to one national survey of teachers, an average of only 18 minutes a day is devoted to science in grades kindergarten–3; in grades 4–6, the average exposure is 29 minutes (Weiss et al., 1989). Far more time is spent teaching mathematics and reading. When hands-on or laboratory activities are used in the classroom, they are seldom truly experimental. More typically they are "cookbook" activities, with prescribed outcomes designed to illustrate specific phenomena. Students rarely have the chance to develop their own hypotheses, design and execute experiments, and draw conclusions.
Teachers are probably the most critical ingredient in a young person's education. Good teaching can inspire students and foster intellectual pursuits by promoting interest in the subject matter, comprehension, and perseverance. Poor teaching can stifle learning, leading to student disinterest and complacency. According to the Federal Coordinating Council on Science, Engineering and Technology (FCCSET) Committee on Education and Human Resources (1991), less than one third of the nation's elementary, middle school, and high school math and science teachers meet coursework standards established by their own professional organizations. Elementary school teachers often are expected to teach science and mathematics, yet they have taken little or no course work in these subjects. High school math and science teachers are less likely, on average, than teachers in other fields to have concentrated in their primary teaching field during college (Federal Coordinating Council on Science, Engineering and Technology, 1991). As a group, teachers at each grade level are more likely to rely on didactic methods than hands-on experimentation, small-group problem-solving, or demonstrations.
Not only is it difficult to recruit highly talented teachers with science backgrounds but it is also difficult to retain the highly skilled teachers already in the system. Although teacher salaries grew nearly 25 percent in real terms from 1983 to 1988, budget cutbacks at the federal, state, and local levels over the past few years have forced many public school teachers to forgo salary raises or even to take reductions in compensation and benefits. It has been estimated that for
every science or math teacher entering teaching for the first time, 13 leave the profession (Federal Coordinating Council on Science, Engineering and Technology, 1991).
Educational quality also is heavily dependent on the availability of resources—including not only money but also up-to-date texts and instructional materials. Teacher morale declines as these professionals are asked to do more with increasingly inadequate resources and outdated instructional materials. Furthermore, most schools do not have adequate equipment or facilities to allow routine laboratory experimentation. This is especially true in elementary and middle schools. For K–12 teachers, inadequate facilities, lack of materials for individualized instruction, and insufficient funds for purchasing equipment and supplies were among the problems most often cited as "serious" impediments to teaching science.
Science Fairs and Competitions
In contrast to the classroom experience, science fairs and competitions often provide valuable exposure to research. Although many science fairs accept nonexperimental projects, it is becoming increasingly common to require students to conduct background research, develop a hypothesis, and conduct a series of experiments to prove or disprove the hypothesis. The International Science and Engineering Fair and the Westinghouse Talent Search are among the largest such initiatives in the United States.1
Another forum for student involvement in research is the American Junior Academy of Science, which allows high school students to present their research at the annual meeting of the American Association for the Advancement of Science. Publications such as the Journal of High School Science Research and the Journal of Student Research provide high school students with the opportunity to publish their studies. Although these programs and activities involve thousands of students each year, their focus is almost exclusively on high school students. Despite this progress, the majority of U.S. students finish their precollege years without having had a significant research experience (Matyas and Malcom, 1991).
For many precollege students, the primary opportunity to engage in hands-on science activities comes through informal experiences, such as visits to science museums, or participation in youth organizations, such as Boy Scouts of the USA, Girl Scouts of the USA, Girls, Inc. (formerly Girls Clubs of America, Inc.), and church groups (Matyas and Malcom, 1991). Parents can also facilitate
TABLE 4-1 Science Classroom Activities Used by Teachers During Their Most Recent Science Lesson by Grade Level, 1985–1986
|
|
Percentage of Classes |
|
|
|
Science Classroom Activity |
K–6 |
7–9 |
10–12 |
|
Lecture Discussion Demonstrations Hands-on or laboratory materials Use of computers Working in small groups Doing seat work from textbook Completing supplemental work sheets Assigning homework |
74 87 52 51 2 33 31 38 28 |
83 82 42 43 5 35 45 44 54 |
84 80 44 39 5 36 35 37 52 |
|
Source: Weiss, 1987. |
|||
these activities at home by providing toys and materials that encourage exploration and experimentation.
Specific Initiatives
A number of programs have been designed to give precollege students experience with hands-on, inquiry-based science. A few engage students in actual research projects (Table 4-1). For the most part, programs that involve students in research are targeted at the high school level and reach limited numbers of students.
Student research experiences also can be indirectly affected by programs aimed at improving the science literacy of teachers and parents. In-service programs, for example, can help teachers acquire knowledge of content and teaching methods to incorporate laboratory components into the science curriculum. Workshops can inform teachers and parents about research opportunities that allow children to become involved, either directly with an individual researcher or through a formal program.
Effecting Change
On the positive side, there is evidence that science and mathematics education is receiving increasing attention by policymakers at many levels. Among the goals established in 1989 by the nation's governors for improving the U.S. educational system, for example, was that U.S. students become first in the world in science and mathematics achievement by the year 2000 (Federal Coordinating Committee for Science, Engineering, and Technology, 1991). Subsequently, the FCCSET established strategic objectives for improving students' preparation in the sciences and mathematics.
Concern about a future shortage of scientists and engineers has spurred expanded federal investment in an effort to increase student interest in science, mathematics, and engineering. In fiscal year 1992, federal agencies participating in the FCCSET Committee on Education and Human Resources2 requested that nearly $180 million be spent on student opportunities and incentives. This reflects a 56 percent increase over 1990 budget levels. An additional $100.5 million was requested by the Department of Defense for Reserve Officers' Training Corps scholarships, many of which go to students majoring in science or engineering.
It is difficult to estimate the level of financial commitment to science education by colleges, universities, industry, and professional societies. It is the committee's sense, however, that there has been an overall increase in both funding for and activities related to enhancing precollege science education.
Federal Programs
Certain federal agencies offer students the chance to gain research experience through summer apprenticeship programs. These programs usually enroll students in grades 10 through 12. A number of agencies conduct Saturday academy programs, which run during the academic year. The NIH's Biomedical Research Assistant Saturday Scholars program, for example, involves 90 junior and senior high school students in hands-on laboratory activities on Saturday mornings. NIH has also initiated a new program called the Science Education Partnership program to encourage careers in the biomedical sciences. The National Oceanic and Atmospheric Administration also sponsors a Saturday academy for junior and senior high school students (Matyas, 1994).
A new NIH program, the Biomedical Preparatory School, gives high school students course credits for time spent in agency laboratories. Under the U.S. Department of Defense's Junior Science and Humanities Program, some 10,000 high school students annually participate in regional meetings where they present their research findings. The National Science Foundation's (NSF's) Young Scholars Program, which targets minority students, lets students work side by side with researchers (National Science Foundation, 1990). In 1992, approximately 8,000 students participated in the program. NSF also encourages minority student involvement in research through its Summer Science Camps and Comprehensive Regional Centers for Minorities.
Nonfederal Programs
There is also a significant nonfederal attempt to provide research experiences to precollege students. The 1992 Directory of Student Science Training Programs for Precollege Students lists 428 such programs, almost all of which are implemented at or by colleges and universities (Science Service, Inc., 1991). A small number of programs are hosted by science museums; industrial and professional societies participate only rarely in such efforts.
Summary
Although some attempts are being made to increase students' interest in science and mathematics, current initiatives fall short in a number of respects. Most science education efforts function more to retain students already in the science career pipeline than to recruit new entrants. In general, the younger the student, the less intensive the research experience is likely to be. The number of students who participate in such activities is relatively small compared with the number of students at the early high school level who are interested in a science or engineering career. In 1977, among 7 million high school sophomores, roughly 730,000 expressed an interest in a future career in science or engineering. The kinds of programs described here, however, have the capacity to serve less than one third of these students. To tap into the larger pool of interested students, additional ways of involving students in research activities are needed, as is greater involvement of the public and private scientific communities.
RESEARCH EXPERIENCES FOR UNDERGRADUATE STUDENTS
In many respects, undergraduate education and training in the United States rival or surpass those of comparable educational systems in most other countries around the globe. The U.S. research enterprise, which depends heavily on the flow of talented undergraduates into academic and industrial laboratories, is also one of the strongest in the world. For all of its strengths, however, U.S. higher education, particularly in the sciences, is facing numerous challenges. Rising tuition costs, for example, present significant barriers for many high school students hoping to enroll in college. Of particular concern, however, is that students who do gain entry into the higher education system appear to be showing less and less interest in studying science and mathematics (U.S. Department of Education, Office of Educational Research and Improvement, 1991; Lapoint et al. 1989). The proportion of college freshmen planning to major in the two subjects dropped by half between 1966 and 1988, from 11.5 to 5.8 percent (Green, 1989).
There is also evidence of considerable attrition into other fields among undergraduates who initially show an interest in the sciences (Hewitt and Seymour, 1991). Although 70 percent of business majors and more than 60 percent of education and social science majors earned their baccalaureate degrees in four years (Cooperative Institutional Research Program, 1982), fewer than 40 percent of students initially majoring in biology received their degrees; the remainder either obtained non-science degrees or dropped out of college. The committee believes that few, if any, students who are turned off to science at the time they enter college will pursue research careers.
At the undergraduate level, it is government and academia that are most involved in encouraging student involvement in science. To a lesser extent, professional societies encourage student interest in science-related studies through scholarship and research internships. Industry supports student research activities through scholarships and cooperative and summer internship programs. Most industry-supported programs, however, target students interested in engineering and the physical sciences rather than the life sciences (Matyas and Malcom, 1991).
Institutional Programs
Academic institutions are strong sponsors of student involvement in research. Often these efforts are part of the regular curriculum. For example, many liberal arts colleges require students to conduct a research project as part of their graduation requirements. Some institutions have programs specifically intended to encourage student participation in ongoing faculty research projects.
Many such efforts were catalyzed by federal initiatives, such as the National Science Foundation's now-defunct Undergraduate Research Program.
Like precollege programs, research opportunities for undergraduate students are often available during the summer months. One example of a successful program is the Summer Undergraduate Research Fellowships (SURFs) at the California Institute of Technology (1991). More than 1,300 students have participated in SURFs since its inception in 1979. Students work on a research project throughout the 10-week fellowship and then present their findings at a scientific meeting. More than 20 percent of SURF recipients have been coauthors of papers published in peer-reviewed scientific journals.
Among other similar academic initiatives is Carnegie Mellon University's Undergraduate Research Associates Program, which places strong emphasis on research participation among women and minorities, and the University of Kentucky College of Medicine Employment Opportunities Program, which provides research and work activities in medicine and a variety of other health fields including nursing, dentistry, and hospital administration (Matyas, 1994).
Federal Programs
Most federal programs that support student research activities do so through either summer research experiences or cooperative ventures in which the student alternates work at a federal research facility with formal course work at a college or university. Table 4-2 provides a partial list of the programs currently operated or funded by the federal government (Matyas, 1994). Many are focused on the needs of underrepresented minorities, women, and people with disabilities.
The National Institute of General Medical Sciences (NIGMS) sponsors the Minority Access to Research Careers (MARC) program, a major component of which is the Honors Undergraduate Research Program. Since 1977, the MARC Honors Undergraduate Research Program has provided tuition and stipend support to over 2,700 junior and senior honors students at predominantly minority institutions. Among its other goals, the MARC Honors Undergraduate Research Program strives to prepare minority students to compete for entry into graduate programs in the biomedical sciences. To date, the majority of students participating in the program have majored in the biological sciences (Garrison and Brown, 1985). A 1985 Institute of Medicine (IOM) evaluation of NIH's MARC Honors Undergraduate Research Program found that over three quarters of former MARC students went on to enroll in or complete graduate or professional studies. Thus, there is a strong indication that the MARC Honors Undergraduate Research Program promotes minority student enrollment in graduate or professional schools.
It is worth noting, however, that NIGMS's MARC Honors Undergraduate Research Program has had some unintended, albeit positive, results. Although
TABLE 4-2 Selected Federal Agencies Sponsoring Undergraduate Research Programs
|
Federal Agency |
Program |
|
U.S. Department of Commerce |
National Institute of Standards and Technology (NIST) Student Cooperative Program (work/study) and Student ''Q" program (summer co-op) |
|
U.S. Department of Defense |
Science and Engineering Co-op Program |
|
U.S. Department of Energy |
Minority Undergraduate Training for Engineering Careers (MUTEC) Galludet University Program (summer) Research Partnership Program (year-round) Minority Access to Engineering-Related Careers Science and Engineering Research Semester |
|
U.S. Department of Health and Human Services |
Minority Access to Research Careers (MARC) Honors Undergraduate Research Training Program Minority Biomedical Research Support Program (MBRS) |
|
U.S. Department of Interior |
Minority Participation in Earth Sciences |
|
|
|
|
U.S. Department of Justice |
Forensic Science Research and Training (FSRTC) Summer Intern Program |
|
|
|
|
Environmental Protection Agency |
Minority Research Apprentice Program Cooperative Education Program Federal Junior Fellowship Program |
|
National Aeronautics and Space Administration |
Baccalaureate Cooperative Education Program Advanced Design Program |
|
National Science Foundation |
Research Experiences for Undergraduates (REU) Research Careers for Minority Scholars Engineering Senior Design Projects to Aid the Disabled |
|
Source: Matyas, 1994. |
|
the program was initially designed to encourage minority students to pursue Ph.D.s in the biomedical sciences, it has proven to be an excellent recruitment tool for bringing minority students into the medical profession. Only about seven percent of the undergraduate students who participate in MARC ultimately receive a Ph.D. MARC students often receive bachelors and even masters' degrees in the sciences or, more often, M.D. degrees, instead of pursuing a Ph.D. In a 1985 IOM evaluation of the program, over 40 percent of MARC Honors Undergraduate Research Program participants who went on to graduate or professional schools were training to be physicians (Institute of Medicine, 1985). Preliminary findings from a 1992 review of the MARC program are similar (Matyas, 1994). It is unclear, however, how many of these minority physicians have joined the faculty ranks or have become clinical investigators in other employment sectors such as government or industry.
The NSF's Research Experiences for Undergraduates (REU) program, begun in 1987, is designed to provide undergraduate students with hands-on research experience. It has many of the same objectives as NIGMS's MARC Undergraduate Research Honors Program, including encouraging undergraduates to attend graduate school in the sciences or engineering. During its first three years, REU supported 11,000 students, over half of whom attended predominantly undergraduate institutions. A 1990 evaluation of NSF's REU program revealed similar findings (National Science Foundation, 1990). Among one group of students, for example, participation in REU increased the proportion of students planning to acquire a master's or doctorate degree from 75 to 92 percent. Nearly 70 percent of participants enrolled in graduate school immediately following graduation.
In 1989, NIH initiated a similar program, Research Supplements for Underrepresented Minorities, to allow scientists with active NIH grants to add a minority high school student, undergraduate student, graduate student, or postdoctoral fellow to their research teams. Since its inception, the program has supported over 650 minority researchers.
There are also a number of federal initiatives that, through their support of academic institutions and faculty, indirectly buttress the undergraduate research experience. Within the Public Health Service, the NIH Minority Biomedical Research Support program has provided resources to over 90 minority colleges and universities to allow state-of-the-art research by faculty and students. The former Alcohol, Drug Abuse, and Mental Health Administration (ADAMHA) supported a program, Minority Institutions Research and Development Programs, that provided support for the "enhancement of existing research infrastructure" (Federal Coordinating Committee for Science, Engineering and Technology, Committee on Education and Human Resources, 1991).
Similarly, the NSF has a series of initiatives—the Faculty Enhancement Program, the Research in Undergraduate Institutions Program, and the Instrumentation and Laboratory Improvement Program—intended to increase the
number and quality of research experiences for undergraduate students. NSF also sponsors efforts to improve the research infrastructures at predominantly minority institutions: Comprehensive Regional Centers for Minorities, Alliances for Minority Participation, Research Improvement in Minority Institutions, and Minority Research Centers of Excellence.
Program Shortcomings
Programs intended to stimulate interest in research among undergraduate students suffer from a number of shortcomings. Efforts to recruit and retain underrepresented groups more often than not are focused on engineering, not science (Matyas and Malcom, 1991). In addition, the majority of such initiatives target minority students rather than women, people with disabilities, or the general student population. According to one study, less than 10 percent of efforts by colleges and universities to recruit students interested in science specifically target women (Matyas and Malcom, 1991). More significant perhaps is that the kinds of initiatives geared to attract women undergraduates are less likely to involve opportunities for scientific research.
Special efforts to encourage students with disabilities to participate in science and engineering activities are extremely rare. More often than not, funds are provided to support individual students' laboratory or research activities. With funding from NSF, the American Association for the Advancement of Science is developing a six-school model program for recruiting the disabled, the Access to Engineering program. The committee is unaware of any similar effort to draw the disabled into medical research careers.
When majority and minority groups are taken as a whole, academic institutions and federal agencies are most likely to facilitate the involvement of students in nonengineering research activities. These programs, however, tend to involve highly motivated and high-achieving students in their sophomore and junior years who already have made a commitment to a science or engineering career. In many instances, the programs act more as vehicles of retention or affirmation than of recruitment.
Finally, although many programs involve students in biomedical research, rarely do precollege or undergraduate students participate in patient-oriented clinical research. NSF sponsors a program, Bioengineering and Aiding the Disabled, in which senior undergraduate engineering students design a piece of equipment to assist a person with a disability. NIH's Research Supplements for Underrepresented Minorities supports minority students or postdoctoral fellows involved in clinical research. Through its Explorer Post program, the Centers for Disease Control and Prevention recruits students ages 14 to 21 to attend lectures, go on field trips, and participate in basic and clinical research activities (Matyas, 1994). In addition to these federal initiatives, there are a few programs scattered
in various academic institutions that expose students to clinical research, but a full inventory of these programs has not been made.
Assessing Program Effectiveness
To determine whether programs that expose students to the world of research encourage them to pursue research careers, one needs to know what the goals of the effort were and whether those goals were matched to appropriate activities. Goals may be specific or general, long-range or short-term. In all but the most exemplary programs, well-defined, measurable goals are lacking (Malcom, 1983). Many programs appear ineffective because their goals are set either too high or too low.
Even if a program appears successful in meeting its objectives, without a means to measure that success it is difficult for sponsors to decide whether continued investments are worthwhile. Studies of precollege and undergraduate programs designed to recruit and retain women or minorities in the sciences and engineering have found that less than half the programs did any formal studies of effectiveness (George et al., 1987, Lockheed et al., 1985, Malcom, 1983; Matyas and Malcom, 1991). Part of the reason for this poor record is that sponsors traditionally have budgeted only a small fraction of program monies for program evaluation. More recently, however, sponsors have begun to encourage and even require more extensive program evaluation and outcomes assessments.
Results obtained by those programs that have conducted formal evaluations indicate that the effects of early research experiences appear to have been positive. For example, in a number of studies examining precollege intervention programs, the integration of content knowledge with hands-on, inquiry-oriented laboratory activities, especially over a period of several years, was one of the critical characteristics of an exemplary program (George et al., 1987; Lockheed et al., 1985; Malcom, 1983; Matyas and Malcom, 1991).
In summary, although evaluations of research experience programs are not regularly completed, the evaluations that do exist suggest that these strategies are effective in encouraging high-achieving students who are already interested in science or engineering to continue their studies. There are strong classroom data and isolated programmatic data indicating that early research experiences may also have a positive effect on students who have average or poor academic skills and moderate or low interest in science or engineering careers (Kyle, 1984; Massachusetts Institute of Technology, 1990; Office of Technology Assessment, 1988a).
Designing Effective Programs
By establishing clear and measurable goals, selecting program activities that are proven effective for the target group, and designing and implementing an evaluation plan, effective new programs can be established with relative ease. It is important for program directors to approach program design and evaluation as a research problem whose results are used to assess what is and what is not working, to refine strategies, and to continue testing as the program is implemented in future years. These are the hallmarks of an effective program.
Establishing Specific Goals
The goals of a program intended to interest students in the sciences should be clear and measurable. Is the goal of the program to facilitate students' pursuit of research careers in science? Will it distinguish between students who are interested in pursuing an M.D. as opposed to a Ph.D.? Will the program focus only on specific science fields? These are some of the questions that must be considered as a program's goals are established.
Goals should also identify the program's target group, taking into consideration such features as student age, race, and academic achievement level. For example, if the goal of the program is to confirm the research career goals of students who are already high achievers and highly motivated, research experiences that occur late in the undergraduate period will be beneficial. If one of the goals is to entice students who may have little natural interest in research, then earlier research experiences—starting in the precollege and early undergraduate years—will be more effective.
Selecting Appropriate Strategies
There should be a clear match between the goals of the program and the activities of the participants. To identify the best activities to include and strategies to use in a program, a number of factors should be considered, including the age group of the participants, the timing of the program (summer, academic year, or year-round), and the available funding.
Activities and goals also should be matched to available funds. For its Summer Science Camps, for example, NSF budgets $100,000 for residential programs and $60,000 for commuter programs. Approximately 60 students participate in the average four-week session. If students are required to pay a fee for participation, financial aid should be offered. A common mistake made by new programs is to scale back activities to match the available funds without modifying the program's goals accordingly. In cases such as this, the goals are
not met—not because the program itself was ineffective but because the goals did not reflect the actual scope or scale of the program effort.
Designing an Evaluation Plan
The design of an evaluation plan should begin when the program goals are being set and the activities are being selected. Program directors often make the mistake of waiting until the program is well under way before considering evaluation, only to realize that they have missed the opportunity to assess changes in attitudes, perceptions, motivation, and interest.
Most careful evaluations include both formative and summative components. Formative evaluation provides feedback to the program staff about how well individual program components are working. For example, students may complete an evaluation form addressing the application process, a particular seminar series, or program social functions. Summative evaluations attempt to assess the overall impact of the program. This information may be provided by exit interviews or surveys of participants and, more importantly, by later surveys to identify the long-term impact of the program on the studies and careers of its participants.
Characteristics of Successful Programs
Programs designed to encourage precollege and undergraduate students to pursue careers in the sciences—particularly in clinical research—will be successful only if their component activities and the strategies for carrying them out are effective. A number of studies have attempted to define the characteristics of successful programs (George et al., 1987; Lockheed et al., 1985; Malcom, 1983; Matyas and Malcom, 1991) (see box Characteristics of Successful Programs). When designing such an initiative, it is important to discover as much as possible about other similar efforts. Much can be learned by contacting those in charge of ongoing programs.
A number of institutions have moved from sponsoring isolated programs to implementing a set of articulated activities designed to "pump" students through the science and engineering "pipeline." One example of this coordinated approach is the Comprehensive Regional Center for Minorities (CRCM) at the University of Puerto Rico. Under CRCM, more than a dozen regional college and university campuses provide exposure to science and engineering for precollege and undergraduate students, K-12 teachers, and college and university faculty (George, 1991).
A similarly integrated strategy has been adopted by the University of Kentucky College of Medicine Education Outreach Center, which sponsors
|
CHARACTERISTICS OF SUCCESSFUL PROGRAMS
|
programs for K-12, undergraduate, and graduate students; partnerships and research programs for teachers; and community outreach efforts, such as a science telephone hotline for student questions and a computer bulletin board, Science Spoken Here.
The progression from single, one-time programs to coordinated, longer-term efforts is an important step toward structural reform, institutional commitment, and line item budgets, which are among the goals of most intervention efforts.
Conclusions
Although crucial data are lacking, the committee believes that research experiences during the precollege and undergraduate years can have a strong and positive impact on students' interest in and commitment to future studies and careers in the sciences. Feedback from more than 30 years of involving students in laboratory research has provided important information about what does and what does not work in such programs. Much of this information is being put to use in the hundreds of research experience programs currently being implemented by federal agencies, colleges and universities, industry, and others.
At the same time, the efforts made to date serve only a small segment of the students who are interested in science as a possible future career. Not all of these students are currently achieving high grades, but many have the potential to do so. There is a much larger population of students who also need to feel the excitement and satisfaction of participating in research activities. Reaching these students will require work on a number of levels.
First, programs currently proven to be effective should be used as models for expanding existing efforts. Second, new program models, which serve the needs of the "second tier" of students, should be developed (Tobias, 1990). NIH's Biomedical Preparatory School, which is geared to a diverse group of students, including those with less-than-perfect academic records, is a good example of this strategy. This should not be perceived as a lowering of standards to reach the second tier; rather, programs should be developed to encourage academic achievement and inspire these students to pursue health professional and clinical investigative careers.
Finally, there need to be systemic changes in science education at both the precollege and undergraduate levels so that research is not a special activity for only a few select students during a few weeks in the summer. Research should be embedded in the science curriculum so that the skills that every young toddler knows—generating hypotheses, designing and conducting experiments, and drawing conclusions—are not lost from the repertoire of learning skills but are formalized and reinforced throughout the precollege and undergraduate years. Programs should also be developed to foster clinical research training. Such exposure could include participation in data collection or other activities in clinical research.
HEALTH PROFESSIONAL SCHOOLS
Although much of the preceding discussion might be considered generic to all scientific and preprofessional careers, this section examines factors that affect students in the health professions. Because the task force reports on dentistry and on nursing and clinical psychology are appended to the report (see Appendixes A
and B, respectively), readers will be referred to those appendixes for specific information pertaining to those professions.
Physician-Scientists
To examine the human resource pool for clinical research in medicine, it will be useful to review the numbers and demographics of applicants and matriculants to medical schools since World War II. Following the war there was an immediate jump in the number of applicants, from about 12,300 in 1943 to more than 21,500 in the late 1940s, probably resulting in part from the Servicemens Readjustment Act of 1944 (Ahrens, 1992). Although the number of applicants surged, the number of those accepted into medical school during the same period grew only slightly, from approximately 6,500 to 7,400. By the mid-1950s the number of applicants dropped to about 15,000 a year with about 8,000 accepted.
In 1958, the Bayne-Jones report was released (U.S. Department of Health and Welfare, 1958). That report called for more physicians and more medical schools to train them. Two years earlier, the Health Research Facilities Act authorized a Public Health Service (PHS) program to expand the capacity and improve the quality of the nation's medical research facilities (Institute of Medicine, 1990). Thus, between the mid-1950s and the mid-1970s the number of medical schools grew from 83 to 114. Over the same period, the number of available slots in medical schools nearly doubled from about 8,000 to 15,000. The number of applicants grew as well, from 15,000 to a peak of about 42,600 in 1974 (Jonas et al., 1992). From 1974, the annual number of applicants declined steadily until the 1988–1989 academic year, when 27,671 students applied for about 17,000 slots in the nation's 126 medical schools. Since 1980 the number of students accepted has hovered around 17,200. The decline in applicants changed the applicant/acceptance ratio over this period. Whereas the ratio was 2.83 applicants for each slot in 1974, the ratio had dropped to 2.10 by 1980 and reached a low of 1.56 in 1988 (Ahrens, 1992; Tudor, 1988). Actual first-year enrollments over the same period have been slightly lower, hovering between 16,800 and 17,200, and annual graduating classes have fluctuated between 15,300 and 16,300 nationwide (Association of American Medical Schools, 1992a).
The fairly level number of enrollments throughout the 1980s combined with a decline in the numbers of applicants raised concern in many sectors about the quality and preparedness of medical school applicants. The proportion of students with 3.5 to 4.0 grade point averages declined slightly, from 46.6 to 43.7 percent between 1987 and 1989, but grew to 46.2 percent by 1991. This drop in the percentage of first-year enrollees was accompanied by a concomitant rise in the percentage of students entering with B and C averages. These concerns, however, have been neither confirmed nor denied. The number of applicants rose again over
the ensuing two years, to about 33,300, and the applicant/acceptance ratio rebounded to nearly 1.94. Of the entering 1991 class, 7.3 percent had master's or doctoral degrees.
Women did not begin entering medical schools in large numbers until the late 1970s. For example, women constituted only 12.8 percent of applicants in 1971 but grew to 31.8 percent 10 years later. In 1991, 41.1 percent (13,700 of 33,301) of the applicant pool were women and 58.9 percent (19,601) were men. Although the percentage of women in the various medical schools covers a wide range—from a low of 23 percent to a high of 71 percent—about 39.8 percent of the 1991 first-year class were women. Data on the grades and class standings of the women entering medical school show that the overall quality of the applicants has been maintained (Jonas et al., 1992).
The race and ethnicity of medical students have changed remarkably over the past decade as well (Jonas et al., 1992). Ten years ago, only about 16 percent of first-year students were members of minority groups. The class entering medical school in 1991 was made up of almost 30 percent racial and ethnic minorities. Although this demonstrates a dramatic change on the surface, progress by the subgroups shows startling differences. For example, the proportion of Asians and Pacific Islanders has grown from 5.1 percent of the entering class in 1982 to nearly 16 percent of the entering class in 1991, thus exceeding their representation in the general population. At the same time, the proportion of students from all other minority groups has increased only slightly. Because enrollments have remained level, the growing numbers of women and minority students have been realized with a concomitant decrease in the number of white, non-Hispanic men. The decline in the number of white males applying to medical school may suggest that other more favorable career options are competing with medicine.
What's Wrong with Medical Education?
Although the previous discussion examined the quantitative aspect of the physician talent pool, the committee was concerned about the qualitative issues for encouraging medical students to pursue research careers, particularly clinical investigative careers. A recent survey of medical students, house staff, and junior faculty at the University of California, San Francisco, revealed three commonly perceived disadvantages to an academic career involving research: (1) reduced research funding; (2) the culture and politics of research, including bureaucracy and sexism; and (3) decreased emphasis on clinical care and relevant health issues. Personal barriers included decreased funding and competition for scarce resources, too much competition for positions, and the clash of family commitments with a career that provides insufficient leisure time (Martin, 1991). Furthermore, there is general consensus that the difficulty of simultaneously maintaining competency in both science and medicine requires that time be set aside for
training in both (Smith, 1989). Students perceive the conflicting demands of research and clinical care and have a growing sense that it is impossible to do both well (Martin, 1991). In addition, career decisions involving two professionals married to each other often work against the decision to enter research training or a research career.
Thus, the committee posed several questions about the effectiveness of medical education in promoting clinical research careers.
-
What is wrong with medical education as it pertains to inspiring clinical research careers?
-
Are the expectations of medical students clearly delineated by the faculty?
-
What are the barriers to research participation during medical school?
-
Can change be effected during medical school to encourage participation in clinical research?
It was clearly stated in the introduction to this report that research is a social and political process that requires communication, interpersonal relationships, and scientific exchange to uncover new knowledge about natural phenomena. To approach the answers to these issues affecting medical education as it pertains to clinical research training, the committee examined factors affecting medical students and residents such as curriculum, student indebtedness, role models and mentors, available time for conducting research, and enculturation into clinical research environments. For many, these issues overlap with those of medical students choosing to engage in preclinical research activities as well.
UNDERGRADUATE MEDICAL EDUCATION
As early as 1910, the Flexner Report highlighted the importance of basic science for medical education (Flexner, 1910). Twenty years ago, the charge was made that medical students were becoming scientific illiterates, and observers continue to bemoan the lack of analytical skills being taught to ensuing classes (Ahrens, 1992). Consistent with reports that medical students are less scientifically skilled is the impression that they are also less scientifically inclined. Thus, two critical questions must be asked. First, is the medical school science curriculum and science culture adequate for preparing physicians to be scientifically literate and enthusiastic about science? Second, is there something about science as a career that is a far more powerful influence on career choice than any exposure to science? The answers to these questions require a variety of approaches if there is to be an increase in the supply of physician-scientists.
In 1988, the IOM's study Resources for Clinical Investigation concluded that there are a number of reasons why clinical research has lost a great deal of its appeal for physicians in training. These include the large debt borne by recent
M.D. graduates, the discrepancy between the incomes of clinical investigators and those of their colleagues who have chosen to enter the more lucrative pathway of private practice, the increasing difficulty clinical investigators experience in getting funds for their research from NIH and other sources, and uncertainties about advancement in the academic community, where accomplishments in laboratory research come sooner and, consequently, are often held in higher regard than those in clinical investigation (Institute of Medicine, 1988a). Six years later, few of these reasons have disappeared, although the validities of some, such as the debt burden, have been called into question.
Medical School Science Curriculum and Culture
Today, medical education centers on the accumulation of an ever-increasing number of facts. Medical students are measured by their ability to recount these facts, often at the expense of enhancing their analytical skills. According to some analysts, even though current students know many more facts, they have little appreciation of the scientific method that was employed to develop this knowledge base and have minimal skills in analyzing clinical science questions (Bishop, 1984; Bryan, 1992; McManus, 1991). Possibly because the thrust of the medical school curriculum is directed toward the accumulation of facts to prepare practicing physicians, many believe that it offers few opportunities for developing analytical skills. At the very least, schools should provide each student with an opportunity to have a first-hand experience with the variability of biological and clinical data, to learn how to formulate a testable hypothesis, to endure the tedium of data collection, and to organize and interpret results (Segal et al., 1990). This should be required not only of those choosing research pathways but of all medical students to ensure that they become informed and analytical consumers of published reports in peer-reviewed journals (Reigelman et al., 1983).
Thirty years ago teachers in the preclinical sciences were expected to give lectures and monitor student learning activities during laboratory exercises. Laboratory exercises have been vastly reduced in modern medical curricula and lectures are now distributed more widely among specialists. In one medical school, for example, first-year medical students were lectured by 136 different faculty members, and second year students were lectured by 183 different teachers (Abrahamson, 1991). A decade ago, NIH Director James Wyngaarden maintained that ''one of the casualties of [the] new medical curriculum has been the simulated research laboratory experiences common to many basic-science courses" (Wyngaarden, 1979, p. 1258). Medical students are not receiving the laboratory experiences necessary to understand the scientific method, and they are rarely exposed to scientists as role models who can provide consistency in both the learning and the practice of science. The result is that not only are there fewer
physicians trained and capable of conducting research but there are also a smaller number of physicians capable of critically evaluating the medical research literature. The number of physicians training in research has not kept pace with the growth in the physician population (Institute of Medicine, 1989d).
Numerous studies have called for reform. Fewer didactic lectures, more small-group teaching, increased supervision of students learning clinical skills, and more interdisciplinary efforts that emphasize making basic science relevant to the clinical practice of medicine are among the efforts under way in the nation's medical schools (Association of American Medical Schools, 1984, 1992b; Jonas et al., 1991). Few schools, however, have a specific curriculum requirement for research.
A number of schools are experimenting with an alternative curriculum. Rush Medical College in Chicago instituted a problem-based curriculum in 1984 in response to a set of perceived problems in medical education, including the following:
-
an emphasis on fact memorization over problem-solving and reasoning skills,
-
limited instruction in assessing the medical literature in the preclinical curriculum,
-
an overcrowded schedule of lectures and laboratory sessions, frequently coupled with poor attendance by students,
-
limited direct orientation of basic science education to a clinical career,
-
a need to instruct students more clearly on habits of lifelong learning, and
-
a need to more fully develop appropriate professional attitudes and practices (Goodman et al., 1991).
A similar statement was made in the Association of American Medical Colleges report, General Professional Education of the Physician and College Preparation for Medicine, which again stressed the pitfalls of lecturing (Association of American Medical Colleges, 1984). Yet, there remain strong perceptions that even with reform in the medical school curriculum, the barriers to a satisfying research career remain significant enough to be a disincentive for many. Moreover, although many efforts are under way to improve the medical school curriculum, it is not clear whether research skills have been included as part of the overall goals of these changes. If they are included, it is not clear what the measures of effectiveness for research preparedness are in these new curricula.
TABLE 4-3 Career Choice Preference by Medical School Graduates from 1989 to 1991
|
M.D. Graduates' Preferences (First Choices) for Career Activities |
|
1989 Graduates |
1990 Graduates |
1991 Graduates |
|||
|
|
|
No. |
Percent |
No. |
Percent |
No. |
Percent |
|
Full-time academic faculty Appointment: |
|||||||
|
|
Basic science teaching and research |
146 |
1.30 |
152 |
1.30 |
132 |
1.20 |
|
|
Clinical sciences |
3,223 |
28.80 |
3,341 |
28.80 |
3,104 |
27.10 |
|
Salaried basic scientist: |
|||||||
|
|
Basic medical sciences |
16 |
0.10 |
21 |
0.02 |
22 |
0.02 |
|
|
Clinical sciences |
36 |
0.03 |
30 |
0.03 |
29 |
0.03 |
|
Clinical Practice: |
|||||||
|
|
Private clinical practice |
6,254 |
56.00 |
6,560 |
56.50 |
6,365 |
55.70 |
|
|
Salaried clinical practice |
1,251 |
11.20 |
1,247 |
10.70 |
1,442 |
12.60 |
|
Source: Beran, 1994; Graduation Questionnaire, Association of American Medical Colleges. Washington, D.C. |
|||||||
Research Interests of Medical Students
Some evidence points to a decrease in interest in postgraduate research activities among medical school graduates. For example, a graduation questionnaire administered by the Association of American Medical Colleges queries senior medical students on their preferences for career activities, including their desire to engage in research. Consistently, less than 1 percent indicate that becoming a salaried research scientist is their first choice (Table 4-3) (Beran, 1994). Just slightly over 1 percent indicate a preference for a full-time academic faculty appointment in basic science teaching and research. These results are not surprising; this probably represents a fraction of students who have, for some reason, chosen to pursue research careers rather than patient care.
Far more fourth-year medical students—27 to 28 percent—indicate a preference for a full-time academic appointment in clinical science rather than basic science. It should be noted, however, that an appointment in clinical science or a clinical department does not directly translate into a preference for a clinical research career. When asked to estimate the degree of involvement in research anticipated during their medical careers, between 13 and 15 percent indicate significant involvement (several years set aside for full-time research or 25 percent or more of a continuous career devoted to research pursuits) (Table 4-4) (Beran, 1994). Approximately 40 percent note that they anticipate involvement
TABLE 4-4 Degree of Involvement in Research Activity During Career as Indicated by Graduating Medical Students, 1989–1991
|
Expected Extent of Research Involvement |
1989 Graduates |
1990 Graduates |
1991 Graduates |
||||
|
|
No. |
Percent |
No. |
Percent |
No. |
Percent |
|
|
Exclusively |
28 |
0.3 |
34 |
0.3 |
43 |
0.4 |
|
|
Significantly involved (several years set aside for full-time research or more than 25 percent of continuous career devoted to research pursuits) |
1,708 |
15.3 |
1,669 |
14.4 |
1,480 |
12.9 |
|
|
Somewhat involved (one year or less than 25 percent of continuous career) |
4,529 |
40.5 |
4,655 |
40.1 |
4,646 |
40.6 |
|
|
Limited involvement (e.g., occasional participation in clinical trials) |
4,084 |
36.5 |
4,405 |
37.9 |
4,291 |
37.5 |
|
|
Not involved |
649 |
5.8 |
81 |
0.7 |
672 |
5.9 |
|
|
Source: Beran, 1994; Graduation Questionnaire, Association of American Medical Colleges. Washington, D.C. |
|||||||
in research (one-year or less than 25 percent of a continuous career), and about 37 percent anticipate limited involvement (occasional participation in clinical trials).
On the positive side, a separate survey reported that physician-scientists most enjoyed the intellectual environment of research and the freedom that came with it, as well as the opportunities to teach. What they least liked were the pressures of time and the need to succeed, lack of support from superiors, and financial concerns (Martin, 1991). Thus, the perceptions of those who might pursue research accurately reflect the perceptions of those who presently conduct research.
Research Participation by Medical Students
When to undertake research training remains a point of controversy if one chooses to become a clinical investigator. Although some studies have questioned whether medical school research experiences are a factor in generating
more physician-scientists (Woods, 1979), most would agree that research experiences during medical school are influential in encouraging some, if not total, research involvement during the medical career (Davis and Kelley, 1982; Segal et al., 1990). Another issue is whether the overall good of training more scientifically literate physicians is sufficient (with the increased likelihood that this will result in more physician-hours in research) or whether new and innovative efforts should be made to encourage more physicians to dedicate their careers to research (Bishop, 1984).
There is concern that most medical residents are hesitant to begin a research activity because of their lack of knowledge about the career possibilities in research and also because of a deficiency in basic research skills (Martin, 1991). Thus, some individuals are already "lost" to research if they have not been exposed before they begin their residencies. If research experience during medical school is a reasonable predictor of postgraduate research activity, the opportunity to take time off for research during medical school or during the first full year following receipt of the M.D. degree has the potential to encourage more physicians to pursue research pathways than programs providing brief research experiences during the residency and fellowship years.
To remedy this situation, some have suggested that there be a period of research prior to or during medical school in order for the student to decide whether he or she enjoys the activity and is good at it (Smith, 1989). Several studies have indicated that medical students who have been exposed to a research experience during their medical education are more likely to engage in research during their postgraduate years (Davis and Kelley, 1982; Jennett, 1988; Paiva et al., 1975; Segal et al., 1990). Some medical schools (for example, Duke, Yale, Case Western Reserve, and the University of Pennsylvania among others) have implemented programs in which medical students are encouraged or required to take one-year off from medical studies to participate in research. To the committee's knowledge, the students in these programs have not been tracked in any systematic fashion to determine whether they have continued to pursue research activities.
In addition to funding training programs, which will be discussed below, another serious constraint confronting medical students who choose to engage in research is time. It has been estimated that M.D.s spend less than 50 percent of their time in the laboratory during research training, compared with nearly 75 percent for Ph.D.s (Martin, 1991). This can jeopardize the quality of the research experience. The first two years of the standard medical curriculum are crammed with course work and the learning of facts. The third year is generally filled with clinical rotations to introduce students to the various specialties that often influence their career choices. Time permitting, some students choose to do a research elective. The summers between the second and third or the third and fourth years are often the only significant blocks of time available for a serious commitment to research. The length of time available, however, is often two to
three months or less. The focus on obtaining a residency position during the fourth-year preoccupies students, although this period also is used for research electives.
Even when students choose to engage in research, the committee believes that most choose to perform studies in the laboratory rather than patient studies. Laboratory experiments that are frequently predesigned or already under way with the possibility of publication at the end of the research period are particularly attractive to students. Some research experiences allow students to develop their own hypotheses and to test them. Nonetheless, these opportunities are valuable from the standpoint that the students are surrounded by the culture and socialization of research. Furthermore, the student has a reasonable expectation of presenting the findings at a regional or national meeting and possibly publishing in a peer-reviewed journal.
At the same time, the committee believes that few opportunities exist to expose medical students to research involving patients. Unlike discrete laboratory projects, human studies are frequently multiyear studies in which a student might not be able to develop an independent portion of the project. Thus, a growing consensus of opinion postulates that the traditional medical school curriculum is not equipped to provide the necessary scientific training for clinical investigators, even for the most motivated of students. With the exception of the M.D.-Ph.D. track and a few other special programs, research experiences frequently occur during residency or following residency in a fellowship.
Training Programs for Medical Students
A few programs allow medical students to gain research experiences. These programs are funded by the federal government, the private sector, and institutions themselves. For example, NIH sponsors a short-term training grant program (referred to as a T35 training grant) to medical schools to support brief training experiences for medical students (predoctoral professional students are not generally appointed on institutional National Research Service Award training grants [T32]). The T35 program generally pays a small stipend (for example, $1,000) for 8 to 10 weeks of research experience, generally during the summer. The 1989 NIH review of the training programs indicated that between 1,000 and 1,400 short-term appointments were supported annually by NIH throughout the 1980s (National Institutes of Health, 1989a). The review panel examined the research interests of medical school graduates who were supported on T35 training grants and concluded that program participants were twice as likely to indicate an interest in a research career as were their fellow graduates.
Because of a lack of programs or deficiencies in existing programs, Duke University, Johns Hopkins University, University of Pennsylvania, and Washington University initiated The Four Schools Physician-Scientist Program
in Internal Medicine in 1989 (Four Schools Physician-Scientist Program in Internal Medicine, 1991). In this program, two third year medical students are selected from each institution to participate in a six year, fully funded program of research and clinical training. The obvious advantages of this program are the total immersion into a scientific culture, exposure to other institutions, and relief of debt burden. This program is in its infancy but may provide a useful prototype for future investment in physician-scientist training. Whether these students will later participate in clinical research activities has not been determined.
In the private sector, the Howard Hughes Medical Institute (HHMI) sponsors Medical Student and Postdoctoral Training Fellowships. HHMI sponsors a national competition to encourage an interlude of basic research at NIH or elsewhere during medical school to encourage an interest in research. As of 1992, the program had placed 230 students from 73 medical schools in various NIH laboratories. Students spend 40 to 80 hours a week in the laboratory and must give a presentation of their work to their fellow students. They are also provided housing during their time at NIH. As with the previous programs and the Medical Scientist Training program discussed below, it is unclear whether these experiences enhance an individual's view of patient-oriented research.
Dual-Degree Programs
One approach to increasing the supply of physicians trained to conduct research is the development of dual-degree programs. Many medical schools offer students the opportunity to earn graduate and professional degrees in addition to the doctor of medicine degree. A combined M.D. and Ph.D. is offered at 109 schools, a combined M.D. and master's degree is available at 42 schools, a combined M.D. and doctor of jurisprudence (J.D.) degree is available in 10 schools, and a combined M.D. and master of public health degree (M.P.H.) is available in 29 schools (Jonas et al., 1991). These programs provide the student with the opportunity to undertake a unique approach to medical education. The program most touted in its record of producing physician-scientists has been the NIH-sponsored Medical Scientist Training (MST) program, which was initiated in 1962 and which is administered through the National Institute of General Medical Sciences (NIGMS) (Bickel et al., 1981).
In the MST program, students selected by admissions committees at each school pursue M.D. and Ph.D. degrees simultaneously. After spending two years in the standard medical school curriculum, students engage in a research project under the supervision of a scientist-mentor for a minimum of three years. This research project forms the basis of a thesis that is defended by the student in order to obtain the Ph.D. degree. Finally, the student completes one-year of clinical rotations, after which both degrees are awarded.
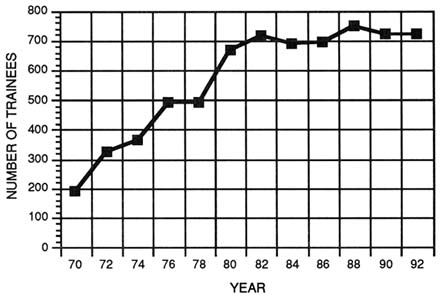
FIGURE 4-1 Total number of participants in the Medical Scientist Training program from 1970 to 1992. (Source: National Institutes of Health, National Institute of General Medical Sciences.)
The obvious advantages of the M.D.-Ph.D. program are that it requires an early commitment to science and provides continuity with the standard basic science components of the medical school curriculum, and students do not accumulate a large debt burden because the NIGMS program pays tuition costs, stipends, and some laboratory expenses for six years. Other advantages are that the student is exposed to the culture of the scientific environment and is expected to achieve scientific competency upon graduation from medical school. Nevertheless, it is not known whether these programs are effective for preparing students to undertake research involving human subjects. The disadvantage is that there often is at least a three-year hiatus between the completion of thesis work and the opportunity to return to scientific work following residency (Smith, 1989).
Data collected in 1990 on the MST program by the NIGMS revealed that of the 126 medical schools, 109 listed M.D.-Ph.D. training opportunities. Approximately 1,500 students were enrolled in these programs (Martin, 1991). NIGMS funds MST programs at only 29 of the medical schools, accounting for about 700 students annually (Figure 4-1). The 80 MST programs at the remaining medical schools are funded through institutional resources or the private sector. The NIH has invested more than $400 million through its MST
program in some 2,000 double-degree graduates since its inception in the 1960s (Ahrens, 1992).
MST Program Outcomes
To date, information on the extent to which NIH's MST program has actually achieved its goal of producing independent physician-investigators, regardless of the area of research, has been sparse. One study conducted in 1981 suggested that MST program graduates outperformed their counterparts in securing faculty positions and academic promotions, obtaining NIH research grants, and publication activity (Bickel et al., 1981; Sherman et al., 1981). The extent to which these graduates were involved in basic biomedical, clinical, or patient-oriented research, however, was not examined.
Assessments of more recent graduates, although confined to individual programs and involving no comparison groups, give some sense of the research orientations of these physician-investigators. One survey of 148 MST program graduates from Washington University found 86 percent of those who completed their postgraduate training were employed in academic positions. Of this group, nearly two thirds were employed in clinical departments, 69 percent had received NIH grants, 11 percent were Howard Hughes Medical Institute investigators, and 6 percent were recipients of clinician-scientist awards from Pfizer or Squibb (Freiden and Fox, 1991).
Examinations of the outcomes for MST program graduates from other such programs reveal similar employment and research activity patterns (Bradford et al., 1986; Freiden and Fox, 1991; Martin, 1991; McClellan and Talalay, 1992). For example, a survey of M.D.-Ph.D. programs at eight medical schools revealed the following:
-
Of those students who had completed their postgraduate or residency training, more than 90 percent had gone on to academic or institute research positions.
-
Approximately six percent went into private practice.
-
Four percent took research positions at NIH, in research institutes, and in industry.
-
Of those who took faculty appointments, most were in departments of medicine.
-
On average, it takes about seven years to complete the dual-degree program.
Such "snapshots" of individual programs suggest that the MST program may be instrumental in the production of patient-oriented researchers. At the same time, several important questions remain. Current data do not allow a
determination of the effectiveness of the MST program in comparison with the effectiveness of other NIH mechanisms for training physician-scientists and patient-oriented researchers (for example, the Clinician-Investigator and Physician-Investigator Awards). In addition, the relative effectiveness of MST program support for training M.D.-Ph.D. researchers in comparison with the effectiveness of similar programs that do not receive such support has not been established.
This latter question is particularly important, because about half of the medical students enrolled in dual-degree programs in the 1990-1991 school year received no MST program support, and of those who received an M.D. degree in 1990, equal numbers had either graduated from dual-degree programs or had already earned their Ph.D. (Howard Hughes Medical Institute, 1992). There is some evidence suggesting that this distribution may differ across clinical specialties (Prystowsky, 1992). For example, few M.D.-Ph.D. recipients are in surgical departments, which suggests that these programs are not often designed to encompass research in the surgical disciplines. Determination of the performance of this training support, in relation to the performance of dual-degree programs or Ph.D. training prior to the receipt of the M.D. degree, would thus yield valuable data for guiding future initiatives to augment the pool of physician-investigators involved in patient-oriented research.
Whether characteristics of the training program or the preselection of the trainees is responsible for the apparent success as measured by the above indicators is not clear. There has been no study in which a control group matched for prior performance has been used to assess the outcomes of the MST program. At the very least one can assume that if intelligent, motivated people are supported financially for several years and protected from taking on responsibilities other than their research, they are likely to do research, publish papers, receive grants, and be promoted (Bland and Schmitz, 1986; Brancati, 1992; Ahrens, 1992). The success of the MST program of NIH supports this contention for a significant proportion (more than 60 percent) of MST program participants who have taken academic or institute research positions. Nevertheless, there have been no systematic analyses of the significance of research exposure in medical school in deciding on a research career, that is, how many students who were not previously so inclined turn to science as a result of such exposure. Furthermore, although the MST program is believed to be effective at training physicians to perform basic research, its effectiveness in providing training for patient-oriented research is unproven. Because many of these investigators have entered the research workforce in recent years and may not have shown their full potential as independent investigators, continued tracking and program evaluation will be useful for determining program outcomes.
FIGURE 4-2 Average first-year, in-state medical school tuition for academic years 1981–1982 to 1990–1991. (Source: Association of American Medical Colleges, Section for Operational Studies.)
Training in Research Ethics
Insufficient laboratory experiences and deficiencies in those experiences are not the only dilemmas experienced by physicians who choose research pathways. Scientific ethics should be introduced at some point in training for all individuals wishing to become scientists. The need for reliable instruction in scientific ethics and proper standards of behavior was evident in a survey of biomedical trainees at the University of California, San Diego. Fifty-one percent reported a first-hand observation of some kind of unethical research conduct, a personal history of unethical behavior, or a willingness to modify, perhaps even to fabricate, experimental data to get a paper accepted or, more likely, to win a research grant (Kalichman and Friedman, 1992).
Courses that formally address scientific ethics are rarely offered, much less required, in either basic science or clinical programs. Trainees from clinical departments, however, are more likely than those from basic science departments to have had a course in which scientific ethics were discussed (Kalichman and Friedman, 1992). NIH is now requiring that all institutions that receive training
funds have a formal program in research ethics. Many research institutions have formulated policies for the conduct of research and scientific recordkeeping. Furthermore, all institutions that perform research involving human subjects must provide assurance to the sponsor of the ethical treatment of human subjects in their research. It is unclear, however, whether there are mechanisms to convey this information in any systematic fashion to research trainees, or whether they merely learn by trial and error in the human studies approval process.
Financing and Debt
The rapidly growing indebtedness of medical students is raising serious concerns throughout the medical education community. The committee felt strongly that the rising debt levels of medical students are deterring individuals from pursuing research careers. To further elucidate this issue, the committee commissioned a paper by Robert Beran on student indebtedness, and from which this section draws heavily (Beran, 1994).
During the 1980s, medical school tuition increased more rapidly than it had in earlier decades. Average tuition for all types of medical schools, private and public, more than doubled between 1981 and 1991 (Figure 4-2) (Association of American Medical Colleges, 1991). In the 1989–1990 academic year, tuition ranged from $8,650 to $24,300, with a median of $17,116. Student living expenses can top $10,000, and fees add several hundred dollars to the bill.
As the cost of attending medical school increases, the proportion of these costs supported by scholarships has dropped and students have been forced to make up the difference through loans (Beran, 1994; Hughes et al., 1991) (Figure 4-3). In the 1980-81 academic year, 34 percent ($137 million) of the $401.9 million in total financial assistance provided to medical students was in the form of scholarships. By the 1990–1991 academic year, the scholarship proportion had dropped to about 23 percent of the $826.5 million in student aid. This decline in the amount of aid provided through scholarship programs is largely the result of a reduction in funds available from federally sponsored scholarship programs. In 1980–1981, of the total scholarship funds ($180 million), more than 64 percent was provided through the National Health Service Corps (NHSC) ($50 million, or 36 percent) and the Armed Services Health Professional Scholarship (HPSP) program ($38 million, or 28 percent); about 17 percent was contributed from institutional funds. In the 1990–1991 academic year, however, the proportion of funds available from the NHSC program was less than 1 percent of the $186.5 million total scholarship aid, and that from the HPSP program was about 30 percent. By contrast, the proportion of scholarship funds available from the institutions rose to 41 percent.
It has long been suggested that high levels of educational debt may be a strong deterrent to interest in a career in academic research. Although the effects
FIGURE 4-3 Loans, scholarships with practice obligations (*), and scholarships without practice obligations awarded to medical students for academic years 1974–1975 through 1988–1989. (Source: Reprinted, with permission, from Hughes et al. [1991], p. 405. Copyright 1991 by The New England Journal of Medicine.)
of debt on a career decision during residency training are largely unknown, an examination of inquiries regarding career choice and interest in research at the time of medical school graduation have not lent support to this concern. The committee reviewed the responses provided through the Association of American Medical Colleges Graduation Questionnaire. It should be noted, however, that the responses at this point of one's educational pathway may not truly reflect career choices and that querying trainees during residency or fellowship may be better indicators of career selections.
From 1980 to 1990, the average indebtedness of medical school graduates almost tripled, from about $16,500 to about $46,200 in nominal dollars (Figure 4-4) (Table 4-5). When corrected for inflation, the growth of indebtedness over the past decade was 81 percent for all schools, 65 percent for public schools, and
FIGURE 4-4 Average educational debt for medical students upon graduation (top panel) and percent of medical school graduates with educational debt (bottom panel), 1980–1990. (Source: Association of American Medical Colleges, Section for Operational Studies.)
105 percent for private schools (Table 4-6) (Beran, 1994). The proportion of medical students graduating with debt has hovered between 75 and 82 percent over the past decade (Figure 4-4).
TABLE 4-5 Trends in Mean Education Debt of Medical School Graduates for Selected Years from 1980 to 1990 in Current Dollars
|
School Type |
1980 |
1985 |
1989 |
1990 |
Decade Increase (percent) |
|
All types |
$16,493 |
$29,943 |
$43,374 |
$46,354 |
181 |
|
Public school |
$14,907 |
$25,718 |
$34,568 |
$38,189 |
156 |
|
Private school |
$18,493 |
$36,417 |
$53,226 |
$58,898 |
218 |
|
Source: Beran, 1994; Graduation Questionnaire, Association of American Medical Colleges. Washington, D.C. |
|||||
TABLE 4-6 Trends in Mean Education Debt of Medical School Graduates for Selected Years from 1980 to 1990 in Constant 1980 Dollars
|
School Type |
1980 |
1985 |
1989 |
1990 |
Decade Increase (percent) |
|
All types |
$16,493 |
$22,936 |
$28,135 |
$29,907 |
81 |
|
Public school |
$14,907 |
$19,700 |
$22,952 |
$24,639 |
65 |
|
Private school |
$18,493 |
$27,895 |
$35,341 |
$38,000 |
105 |
|
Source: Beran, 1994; Graduation Questionnaire, Association of American Medical Colleges. Washington, D.C. |
|||||
The mean educational debt levels for 1988, 1989, and 1990 medical school graduates who expect significant involvement in research during their medical careers is below the mean debt for all indebted medical graduates. The mean debt for the 1988 graduates anticipating research involvement was $37,821, whereas the mean debt for all indebted 1988 graduates was $38,489. Graduates expecting to pursue an exclusive career in research graduated with a mean debt of $30,015. Students graduating in 1989 who expected significant research involvement had a mean educational debt of $40,885. The mean debt for all 1989 graduates with debt was $42,374. The mean debt for the 1990 groups of graduates who expected significant involvement in research was $45,150, whereas it was $46,224 for all 1990 graduates. From these data, one could conclude that excessive debt does not appear to deter graduates' interest in research (Beran, 1994). These data, however, deal with mean debt. To gain a clearer understanding of the influence that debt has on career choice, the committee would need data on the range of debt for those
choosing to pursue research careers. Furthermore, these data do not account for consumer debt, which can add additional burdens for trainees.
Although the empirical data seem to indicate that debt may not influence career choice, anecdotal reports argue otherwise. Intuitively, the pressures of an academic career and a growing likelihood that a young physician cannot service these huge debts on academic salaries leads the committee to be concerned that debt does play a key role in career choice—turning young physicians away from academic research careers. An analysis by the Association of American Medical Colleges shows that an annual income of $60,000, the starting academic salary for many internal medicine specialties and subspecialties, is insufficient for servicing debt loads of $75,000 or more (Association of American Medical Colleges, 1991).
GRADUATE MEDICAL EDUCATION
Many forces are responsible for shaping the content and structure of graduate medical education (GME). The primary goal of GME is to prepare novice medical school graduates to provide the highest quality of medical care on the basis of the vast knowledge of medical science. Confluent with that objective, the committee believes that a certain cohort must also be well prepared to pose and answer relevant scientific questions, both at the fundamental level and at the level of patients and populations. Furthermore, the scientific preparation of physicians not only should adequately prepare them for academic careers but also should be responsive to the needs of other employment sectors where clinical research talent is needed, such as government and industry. Thus, the committee explored the forces, personal as well as professional, shaping GME in an effort to find ways to overcome the barriers to investigative careers.
To probe these factors in more depth, the committee commissioned a number of papers on issues that it felt were particularly influential in developing career pathways for physician-scientists at the postdoctoral level. One paper, by Georgine Pion of Vanderbilt University, examined the training programs offered through NIH (Pion, 1994). Two other papers, one by David Atkins and colleagues at the University of Washington (Atkins et al., 1994) and one by Thomas Lee and Lee Goldman at the Brigham and Women's Hospital in Boston Lee and Goldman, 1994), dealt with models for postdoctoral training. Linda Blank of the American Board of Internal Medicine drafted a paper on the roles of the resident review committees and the certification boards on research career pathways (Blank 1994), and Judith Swazey prepared a paper on mentors and role models (Swazey, 1994). All of these papers provided valuable information to the committee in preparing this portion of the report.
How Long Should Training Be and What Should the Training Involve?
The duration of research training has long been considered a key factor in preparing for a successful research career (Levey et al., 1988; Oates, 1982). Most would agree that less than 12 months of research training is inadequate to prepare independent investigators in either the basic sciences or clinical research (National Institutes of Health, 1989a). Levey et al. (1988) have shown that at least two years of postdoctoral research training is required. Some have suggested that at least three years of training in modern biological science is necessary to prepare most individuals to perform as independent investigators (Goldstein, 1986). Similar suggestions have been made for preparing clinicians to conduct clinical effectiveness research (Goldman et al., 1990). The advantage to scientific training after the receipt of the M.D. degree is that there is temporal continuity between training and a research career. It was the model employed by many who are clinical investigators today. The drawback of this model is that by the time a student has completed training, he or she might have already decided on a clinical career without being exposed to research, and some of the flexibility in the system is thus lost.
For medical school faculty in departments of medicine, the length of postdoctoral research training was a significant predictor for subsequent involvement as an active researcher and principal investigator for a peer-reviewed research grant (Levey et al., 1988). The more that the physician is deeply immersed in the primary literature surrounding basic biology, the more likely it is research will to lead to fundamental discoveries that will further the understanding of disease processes (Martin, 1991). Along these same lines, Safran et al.(1992) has shown improved performances on various clinical knowledge measures following the implementation of a scientific curriculum in a surgical residency program. The committee believes that trainees who are exposed to clinical investigation will gain an appreciation for the results of clinical research and be prepared to pose pertinent research questions regarding humans.
Several recommendations have emerged to encourage postdoctoral research training. A 1986 survey of full-time faculty in departments of medicine advocated incorporating formal course work, particularly in the basic sciences and statistics, with less time allocated to patient care (Levey et al., 1988). In 1989, a National Research Council committee made the following recommendations (National Academy of Sciences, 1989) regarding changes that should be made in the postdoctoral institutional training programs for physician-scientists:
-
a true consortium between the clinical and preclinical departments of the institution, with shared responsibilities for the design and administration of the program;
-
selection of trainees on the basis of evidence of some previous experience in research and overall promise;
-
formal course work in the physical and biochemical sciences sufficient to give graduates a theoretical background comparable to those of people with graduate degrees in the biological sciences;
-
not less than three years of research training, primarily in direct research experience under the supervision of a mentor; and
-
modules of instruction specifically tailored to the needs of the physician trainee in such areas as basic laboratory techniques, chromatography, radioimmunoassay, protein purification, advanced instrumental techniques, fundamental principles of enzymology and molecular biology, subcellular fractionation techniques, computer technology, evaluation of experimental data, epidemiology, statistics and database management, as well as grant and manuscript writing.
Although training should always be individualized, generic skills are needs. They include experimental design, biostatistics, data analysis, ethics of human experimentation and research ethics, scientific writing and presentation, general laboratory skills, including computing, and critical evaluation of scientific information (Institute of Medicine, 1988a). The investigator, for example, must understand the differences between randomized controlled trials and other experimental and nonexperimental designs. Trainees also need to be attentive to sampling methods, sample size, and analytical methods (Institute of Medicine, 1988a).
Some argue that these skills are less appreciated early in medical school and might best be taught to the beginning investigator, trainee, or fellow (Institute of Medicine, 1988a). Yet, there are dangers in waiting to introduce these concepts. Most residents elect to do research training when they are in their late twenties or thirties. Their Ph.D. counterparts might already have invested 10 years in the research laboratory. Furthermore, many clinical fellows have no coursework requirements and have no contact with basic scientists or clinical investigators. These problems point to the need for more training of physician-scientists where the research is conducted, as well as the need for formal, rigorous course work.
A few programs have attempted to provide experiences for students to conduct research during or immediately after their medical school training. The NIH Physician-Scientist Training Awards (K11 and K12) have been available since 1985. Both institutional (K12) and individual (K11) awards are made for training non-Ph.D. physicians for five years following residency. The primary intent of the awards, however, is to ensure that a period of time is spent in basic science laboratories.
Although much postgraduate training occurred at NIH in the past, most postdoctoral research training has now shifted to universities and medical centers. Furthermore, many trainees are remaining at the institutions where they completed their residencies to conduct postdoctoral research. Critics charge that this encourages institutions to select their own graduates rather than to select the best candidates in a nationally competitive manner (Martin, 1991).
The usual course for research training after receipt of the M.D. is two years of training in one of the clinical subspecialties while on a training grant. After the traineeship, individuals can apply for a two-year fellowship that allows them to pursue a research problem under the guidance of a faculty adviser or mentor. Training grants and fellowships are especially critical in enabling M.D.s to ''buy out" of other administrative and clinical responsibilities that are not usually faced by Ph.D. scientists. The appropriate length of training and payback provisions have been debated frequently over the past few years (National Institutes of Health, 1989a).
Edwin Cadman, chairman of the Department of Medicine at Yale, has suggested that the best way to improve prospects for physician-scientists is to envision a future in which they spend a longer time in training, have higher salaries during training, are less dependent on the federal government for support during training, are more concentrated at research-intense medical schools, and are more concerned about population health (Cadman, 1990). Thus, postgraduate training must be streamlined to permit both adequate clinical training and the ability to continue in research.
External Factors Affecting Research Training During GME
Residency Review Committees and Certification Boards
The committee was interested to know what effect, if any, residency review committees (RRCs) and certifying boards have in promoting or hindering research careers among physicians. Both organizations have the ability to establish requirements for research training in medical subspecialties, although neither provides funding or an organizational framework to accomplish this.
Role of RRCs
The nation's 24 RRCs accredit roughly 6,900 residency training programs, which are collectively responsible for establishing the clinical training requirements of some 85,000 medical residents. (See box for list of specialties represented.) Certification boards for these 24 specialties evaluate M.D.
|
AMERICAN BOARD OF MEDICAL SPECIALTIES American Board of Allergy and Immunology American Board of Anesthesiology American Board of Colon and Rectal Surgery American Board of Dermatology American Board of Emergency Medicine American Board of Family Practice American Board of Internal Medicine American Board of Medical Genetics American Board of Neurological Surgery American Board of Nuclear Medicine American Board of Obstetrics and Gynecology American Board of Opthamology American Board of Orthopaedic Surgery American Board of Otolaryngology American Board of Pathology American Board of Pediatrics American Board of Physical Medicine and Rehabilitation American Board of Plastic Surgery American Board of Preventive Medicine American Board of Psychiatry/Neurology American Board of Radiology American Board of Surgery American Board of Thoracic Surgery American Board of Urology |
candidates to verify that they have received adequate preparation to practice as specialists in their respective fields.
The commissioned paper by Linda Blank details the analysis she performed to assess the research requirements of the RRCs, some of which is encapsulated here. The executive secretaries of all 24 RRCs were surveyed to determine which program standards for formal training (so-called special requirements) include research experiences to obtain a description of the research criteria, to confirm the status of the research experience and documentation, and to describe any planned changes in the research experience.
Although 22 of 24 RRCs include research components in their accreditation requirements, only seven RRCs require their residents to have a research experience. Ten RRCs insist that residents should have research experience during training, and four other RRCs encourage such opportunities. The rationale
for requiring or emphasizing research experiences or research training is to enhance one's clinical training to become a competent clinician.
Determination of the presence (or absence) of research in a residency program is left to RRC field surveyors, who visit residency programs as a part of the accreditation process. With the exception of information obtained from these reviews, no data are available on any programs to determine the actual levels of participation of residents in research training activities. In 1991 the RRC for internal medicine introduced a computerized system to collect and analyze accreditation data, but it is the only RRC so far to do so.
The nature and length of available research experiences are not specified in the special requirements of RRCs and are specific to the individual training program. With the exception of allergy and immunology, which requires that one quarter of the two-year residency be spent conducting research, no time commitments are specified by any of the other RRCs in their special requirements. Overall, the special requirements for research training are universally vague and difficult to interpret and measure. The committee is concerned that the RRCs do not place enough emphasis on the importance of an academic, discovery-oriented milieu for effective clinical training. The committee believes that experiences for some should go beyond exposure or superficial introduction in research methods and should have rigorous training to prepare residents in research training to undertake independent investigations with human subjects.
Role of Certification Boards
Requirements for specialty board certification generally include specified accredited training, practice experience (for some specialties), and licensure and examination. Research experience is not required for certification, although two boards—for preventive medicine and pediatrics—recognize research as an important element of clinical training. All 24 member boards of the American Board of Medical Specialties were surveyed to assess the availability of certification pathways for physicians who seek careers in clinical investigation, the number of candidates who use these pathways, examination performances, outcomes, and any expected changes in the pathways and impacts of certification and recertification for specialist who choose a career in clinical investigation (Blank, 1994).
Three of the boards—anesthesiology, dermatology, and internal medicine—offer special pathways for clinical investigators. In anesthesiology, clinical investigators are required to spend five years (rather than four, as is required for clinical-track residents) to complete the training requirement, including one and a half years conducting research.
In dermatology, research training takes place during the second or third year of residency. Residents who follow a career path in investigative dermatology usually spend more time on research during these years, although all residents are encouraged to participate in basic or clinical research at some point in their training. During an average year, between 5 and 8 percent of dermatology residents focus their training on research, and 3 of the 101 accredited training programs have 20 percent or more of their residents request additional time for basic or clinical research, according to the board.
In 1983 the American Board of Internal Medicine (ABIM) established the four-year clinical investigator pathway (CIP), which includes two years of research. From 1985 through 1990, 125 candidates completed the CIP, and 103 (82 percent) of them are now certified. Of the 80 in this group for whom career status is known, 45 (44 percent) are in private practice (including three who indicate that they remain involved in research) and 35 (34 percent) are in academic medicine (including 23 in research and 3 who combine research with teaching or consultation). From the listing in the 1991–1992 Directory of Medical Specialties (1991), 6,612 internists are listed as recertified by the ABIM, and 65 (or 1 percent) indicate that their major career activity is medical research. Only one of the other certifying boards—nuclear medicine—is discussing a clinical investigator pathway similar to that operated by the ABIM.
Many boards recently have initiated time-limited certification. That is, certificates have a built-in time limitation (for example, 10 years). At the end of the established period, practicing physicians will be required to take a recertification examination to continue to practice as a specialist. These programs are still too new to measure their effects on the careers of clinical researchers. The committee is very concerned that if clinical investigators, many of whom have very narrow academic interests, are required to maintain a broad-based practice to meet recertification requirements in 10-year increments, clinical research may suffer. There are no firm data to support this contention, but the committee raises it as a matter that should be watched closely.
Liaison Committee for Medical Education
Although the Liaison Committee for Medical Education (LCME) relates to medical education rather than GME, it is appropriate to consider its role here, along with the RRCs and certification boards, as an influential organization that affects the preparation of physicians. The LCME is the national authority that accredits medical education programs leading to the M.D. degree. It was formed in 1942 under the sponsorship of the Association of American Medical Colleges and the Council on Education of the American Medical Association. LCME is recognized by the Secretary of the U.S. Department of Education, the Council on Postsecondary Education, the U.S. Congress in various health-related laws, and
state licensure boards (Liaison Committee for Medical Education, 1991). Thus, if changes are to be made in medical education to encourage clinical research, the committee recognizes that the LCME, its sponsors, and other parties must also be participants in these changes.
BARRIERS TO CLINICAL RESEARCH TRAINING
Physicians interested in undertaking research—particularly patient-oriented research, where the opportunities and needs seem to be greatest—during the postgraduate training period face a series of obstacles. Several of the most important are discussed below.
Inadequate Training in Research Methods
Although the conclusions of clinical studies are discussed regularly during clinical training, the methods involved in developing such studies rarely receive systematic scrutiny. Today's medical students and residents receive only desultory instruction in the basics of biostatistics, epidemiology, and health services research (Neinstein and Mackenzie, 1989). Patient-oriented researchers need expertise in study designs (such as case-control and cohort studies), and they must be familiar with the strengths and limitations of statistical techniques (such as logistic regression and Cox proportion hazards analyses). Ideally, researchers should also understand and be able to measure the costs of therapy, treatment outcomes, quality of life, and cost-effectiveness, among other variables (Atkins et al., 1994; Lee and Goldman, 1994, Roper, 1988; U.S. Government Accounting Office 1994). No single investigator is likely to master all of these skills. To be successful in a clinical research field, each investigator must be prepared to interact not just with other physicians but also with researchers in related disciplines, such as the social and quantitative sciences.
In some institutions much of the clinical research occurs in clinical research centers (CRCs), which represent a longstanding program of patient-oriented research. Funding for CRCs has declined, however, as research on the cellular and molecular bases of disease has increased. Furthermore, the model of research conducted in CRCs, which relies on detailed measurements in a small number of patients, may be too narrow for training some physicians in other important areas of clinical research, particularly studies involving the diagnosis, prognosis, and treatment of disease. Schools of public health and divisions of epidemiology and biostatistics at large medical centers offer these subjects and other relevant courses. With only 24 schools of public health and 126 medical schools in the United States, however, a minority of postdoctoral training programs have access to these resources.
Although large multicenter trials account for a substantial proportion of the clinical research conducted at many major academic centers, these sorts of studies may not provide the best training opportunities for new investigators. A junior investigator in a large randomized trial may contribute to data collection but may not be involved in the design, data analysis, or manuscript preparation. Therefore, participation in a multicenter randomized trial is unlikely to adequately prepare an independent clinical investigator unless this experience is supplemented by other training.
Inadequate Mentoring
In addition to being inadequately trained to conduct independent research through such experiences as peripheral participation in a clinical trial, research fellows pursuing clinical projects may encounter a limited supply of experienced mentors to guide them through their research endeavors. Faculty engaged in clinical research may not have been adequately trained in research methods and are even less likely to have received adequate training in clinical research methods. Many have numerous competing commitments, and they may not have the time or the resources to assist and guide the trainees' in their projects.
To further elucidate the attributes of effective mentoring, the committee commissioned Judith Swazey of the Acadia Institute to draft a paper, "Advisors, Mentors, and Role Models in Graduate Professional Education: Implications for the Recruitment, Training, and Retention of Physician-Investigators," (Swazey, 1994). In conclusion, there are few empirical data on what constitutes effective mentoring and the outcomes of mentoring. However, the committee believes that mentors who commit themselves to advising and guiding trainees through the maze of research are critical players in the research careers of young investigators. The committee also believes that some form of midcareer program to aid established investigators in becoming more effective mentors could help alleviate the shortage of clinical investigator mentors that now exists.
Timing of Training
For some specialties, the optimal timing of research training is not clear. For instance, if residents in surgery go into the laboratory for one-year following the second year of clinical training, they will have to complete three clinical years after they complete their time in the laboratory. By the time they finish their residency, the data they accumulate may be too old for use as preliminary results for a grant application (see Appendix C). If instead, they go into the laboratory after the third or fourth year, many surgeons feel uncomfortable with the level of their clinical skills when they return to the senior year of residency. Furthermore,
brief and sporadic intervals in a laboratory are probably inadequate to prepare physicians for a research career, not to mention a clinical investigative career.
Waiting to begin research training until after the completion of clinical training has two primary drawbacks. First, many of the brightest residents will have been lost to an academic career by the passage of time. Second, if they train in research (outside the clinical setting) for two or more years after they complete their clinical training, they will feel clinically inadequate when they begin their career.
The time and expense involved in conducting clinical studies may significantly hamper research fellows who are trying to complete advanced medical training concurrently. Many patient-oriented projects take too long to complete or are too costly to attract trainees for relatively brief fellowships. In contrast, laboratory projects with established investigators can often take advantage of the existing resources of a productive laboratory, generate data more quickly, and incur more modest marginal costs. Thus, a different type of reward system may be warranted since publication may not be the currency of achievement for those clinical research trainees involved in long-term research projects.
Consistent and continuous involvement in clinical research activity throughout the training period might be one option for maintaining an interest and gaining an aptitude for clinical research—an objective that runs counter to the training requirements of residency review committees and certification boards noted earlier (Blank, 1994). This may be combined with a one-, two-, or three-year fellowship to specialize in designing and conducting clinical studies.
Competing Commitments
The average resident spends as many as 100 hours in the hospital each week delivering patient care and on clinically related issues while, at the same time, pursuing a meaningful clinical education (Safran et al., 1992). Because clinical research trainees frequently are in clinical care environments as well, the competing demands of patient care and research commitments are difficult to balance (Littlefield, 1984 and 1986). Ironically, if clinical research trainee spends time seeing patients who are not involved in a research protocol, the likelihood that the trainee will receive research support for patient-oriented studies in the future is reduced. A survey by the American Federation for Clinical Research found that every 10 hours of clinical work a week was associated with a 23 percent decrease in the odds of having federal or nonprofit foundation grant support (Lee et al., 1991). The data demonstrate an association between increased nonresearch responsibilities and decreased probability of funding, but they do not prove a cause-and-effect relationship. This does not imply that clinical research
trainees should not provide patient care but, rather, that clinical research time may need to be protected from clinical training demands.
AVENUES FOR POSTDOCTORAL RESEARCH TRAINING
The vast majority of support for research training comes from the federal government—primarily NIH. Over the years, however, federal support research training has been politically charged. In 1974, the Nixon administration impounded the research training funds in an effort to phase out all research training. Congress reacted immediately by passing the National Research Service Award (NRSA) Act, which authorized training through the Public Health Service agencies, primarily NIH, ADAMHA, and the Health Resources Service Administration. Training was thus restored and funded as a separate line item in annual appropriations. Recently, however, funding has fluctuated as positions have been cut and restored and stipends have been readjusted (Institute of Medicine, 1990).
NIH currently funds training grants to support a number of trainees within an institution (T32 awards), individual research fellowships (F32 awards), and several types of career development awards (K awards). In fiscal year 1992, NIH obligated about $314 million, or about 3.5 percent of its total budget, to support research training grants (T32 awards) and fellowships (F32 awards). An additional $101.6 million was committed to career development awards (National Institutes of Health, 1993b).
A handful of private foundations and philanthropies also fund research training. In addition, a small number of programs around the country—supported by foundations and academic institutions—are taking innovative steps to improve the competence of clinical investigators. (See the section Model Programs for Research Training later in this chapter and the background papers by Atkins et al., 1994, and Lee and Goldman, 1994.) Although it is a substantial force in the area of research funding, industry is a relatively minor player when it comes to support for clinical research training.
Federal Support from NIH
Although NIH offers a variety of research training and career development opportunities, T32 training awards and F32 fellowship awards are the most common mechanisms for funding postdoctoral training of young investigators. Training grants and fellowships differ in a number of ways. For example, training grant applications are reviewed by special review panels convened by the individual institutes, and awards are made to program directors at universities or research institutes for training a certain number of individuals. The T32 awards,
which may be renewed by the awardee institution every five years, provide one-year or more of postdoctoral research training in a specific research area or clinical subspecialty. The actual selection of the trainees is left to the grant directors in the recipient institutions. Fellowship applications are most commonly reviewed by the initial review groups (IRGs) in the Division of Research Grants of NIH, and they are awarded to individual fellows. Fellowships are usually awarded for two years under the preceptorship of a mentor, but they may be extended for an additional year. Because of their focus, institutes are, in general, most interested in developing investigators in a particular field of research through training grants; IRG reviewers are more concerned with the substance of the research proposal presented in the fellowship application.
Financing course work in clinical research training presents a substantial problem in some training programs. Although tuition for courses is covered in predoctoral programs that support Ph.D. candidates, tuition support is not necessarily provided under the grants that support postdoctoral students—the stage when many physician-scientists require it. Although NIH institutional training grants may permit the inclusion of some tuition expenses, these must be anticipated in advance and may not be available from grants already in force. Individual NRSA fellowships (F32 awards) provide salary stipends but not tuition expenses.
Funding for training grants and fellowships grew from $180 million in 1980 to $314 million in 1992 (National Institutes of Health, 1993b). After correcting for inflation, funding actually declined from the late 1970s (Institute of Medicine, 1990). Furthermore, stipend readjustments in 1989 trimmed about 1,000 positions, which were reinstated in 1990.
Although NIH has supported about 11,000-12,000 training positions annually over the last decade, only about 5,400-5,600 of these have been postdoctoral positions (Figure 4-5). In 1992 the number of postdoctoral positions reached an estimated 5,814, surpassing the previous high of 5,690 in 1987. Of the 5,814 positions available in 1992, 2,651 (45.6 percent) were awarded to M.D.s and 3,163 (54.4 percent) were awarded to Ph.D.s. This ratio of awards to M.D.s and Ph.D.s has changed over the decade as well. Although less than 40 percent of postdoctoral awards were made to M.D.s in the early 1980s, the proportion of postdoctoral awards to M.D.s is approaching 50 percent. Of the 2,651 postdoctoral awards to M.D.s in 1992, 2,336, or 88.1 percent, were awarded through institutional training grants, and only 11.9 percent (315 awards) were individual fellowships. By contrast, 40.9 percent, or 1,293, of the 3,163 awards to Ph.D.s were individual awards (Figure 4-6).
A number of other observations can be made about fellowship and training applications and awards for M.D.s and Ph.D.s. From 1977 through 1989, the annual number of T32 training grant applications from physicians was roughly equal to the number of applications from those with a Ph.D. (Tables 4-7 and 4-8). The success rates for physicians have ranged from 44 percent in 1979 to 78
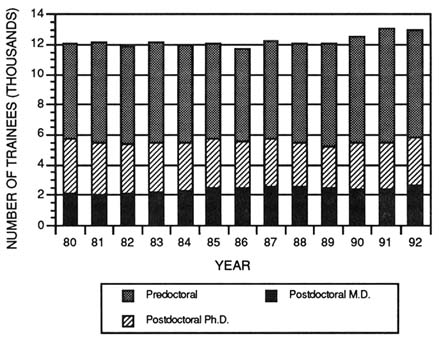
FIGURE 4-5 Number and distribution of predoctoral and postdoctoral training positions support by the NIH. (Source: National Institutes of Health, 1993b.)
FIGURE 4-6 Distribution of NIH postdoctoral training positions between individual and institutional training grants for M.D.s and Ph.D.s in 1992. (Source: National Institutes of Health, 1993b.)
TABLE 4-7 Applications and Awards for Training Grants (T32 awards) and Individual Fellowship Awards to Principal Investigators with a Ph.D.
|
|
Number of Applications |
Rates |
||||
|
Award Type and Year |
Reviewed |
Approved |
Awarded |
Approval |
Award |
Success |
|
Training grant awards |
||||||
|
1977 1978 1979 1980 1981 1982 1983 1984 1985 1986 1987 1988 1989 |
306 277 175 271 179 197 192 161 288 211 222 272 183 |
250 235 133 248 162 190 184 146 276 191 219 257 178 |
145 173 77 200 103 131 152 91 200 96 144 139 99 |
82 85 76 92 91 96 96 91 96 91 99 95 97 |
58 74 58 81 63 69 83 62 72 50 66 54 56 |
48 60 44 74 56 63 78 57 69 45 63 51 54 |
|
Mean |
226 |
|
135 |
94 |
65 |
59 |
|
Fellowship awards |
||||||
|
1977 1978 1979 1980 1981 1982 1983 1984 1985 1986 1987 1988 1989 |
252 334 311 310 324 290 297 362 428 436 364 396 489 |
214 279 264 269 267 253 270 326 384 401 345 376 472 |
132 168 164 137 122 137 147 151 198 147 163 166 144 |
85 84 85 87 82 87 91 90 90 92 95 95 97 |
62 60 62 51 46 54 54 46 52 37 47 44 30 |
52 50 53 44 38 47 50 42 46 34 44 42 29 |
|
Mean |
353 |
|
152 |
90 |
51 |
45 |
|
Source: Reprinted, with permission, from Ahrens (1992). Copyright 1992 by the Oxford University Press, Inc. |
||||||
percent in 1983, and is currently hovering around 50 percent. Over this same period the success rate for Ph.D.s has been slightly lower (Ahrens, 1992).
Over the same period, the number of F32 fellowship applications by Ph.D.s (range 1,332 to 1,648) greatly exceeded those by M.D.s (range, 252 to 489).
TABLE 4-8 Applications and Awards for Training Grants (T32 awards) and Individual Fellowship Awards (F32 awards) to Principal Investigators with an M.D.
|
|
Number of Applications |
|
Rates |
|
|
|
|
Award Type and Year |
Reviewed |
Approved |
Awarded |
Approval |
Award |
Success |
|
Training grant awards |
||||||
|
1977 1978 1979 1980 1981 1982 1983 1984 1985 1986 1987 1988 1989 |
352 289 255 298 135 181 210 171 261 160 180 235 244 |
246 232 186 273 122 168 192 158 253 149 176 225 242 |
130 150 96 176 62 94 131 87 164 61 114 133 112 |
70 80 73 92 90 93 91 92 97 93 98 96 99 |
53 65 52 65 51 56 68 55 65 41 65 59 46 |
37 49 37 57 43 51 60 50 62 38 60 56 45 |
|
Mean |
229 |
|
116 |
94 |
57 |
50 |
|
Fellowship awards |
||||||
|
1977 1978 1979 1980 1981 1982 1983 1984 1985 1986 1987 1988 1989 |
1,332 1,463 1,667 1,421 1,474 1,500 1,439 1,452 1,602 1,575 1,468 1,430 1,648 |
1,165 1,290 1,502 1,277 1,292 1,339 1,296 1,324 1,512 1,478 1,444 1,384 1,588 |
747 876 945 649 485 619 628 543 709 415 700 600 468 |
87 88 90 90 88 89 90 91 94 94 98 97 96 |
64 68 63 51 38 46 49 41 47 28 49 43 30 |
56 60 56 45 33 41 44 37 44 26 47 42 28 |
|
Mean |
1,498 |
|
645 |
92 |
49 |
44 |
|
Source: Reprinted, with permission, from Ahrens (1992), p. 161. Copyright 1992 by the Oxford University Press, Inc. |
||||||
Over those 13 years, however, the number of fellowship applications by M.D.s nearly doubled, while those by Ph.D.s increased by less than 20 percent. The success rates for F32 applications between the two groups were nearly identical during the 1980s, although like other NIH awards, the rates fell over the period. More important, the actual number of awards for Ph.D.s has declined from a high
FIGURE 4-7 Number of NIH individual fellowship (F32 award) applications for studies involving the use of human subjects or human materials and those not involving human materials by all applicants. (Source: National Institutes of Health, Division of Research Grants.)
of 945 in 1979 to 468 in 1989. The number of awards to M.D.s fluctuated somewhat during the period but, for the most part, hovered between 135 and 165. There were more than twice as many F32 fellowship applications as awards for both M.D.s and Ph.D.s, which suggests that the scientific merits of the two groups' applications were judged to be nearly identical (Ahrens, 1992).
The emphasis on clinical research through these training mechanisms is difficult to discern. Because the training grants are awarded to institutions and managed locally, it is not possible to determine the nature of the training. The fellowship applications, however, must pass through the same institutional review board process as regular grant applications, and they are so identified on the cover sheet of the application. Of the 1,600 to 2,000 F32 fellowship applications submitted annually by both M.D.s and Ph.D.s, between 18 and 20 percent indicate that they intend to use human subjects or materials (Figures 4-7 and 4-8). Of the 300 to 400 applications from M.D.s, about 100 to 150 (30-40 percent) indicate the intent to use of human subjects or human materials (Figures 4-9 and 4-10). The success rates of applications not indicating the use of human subjects or human materials parallels the success rate of those applications that so indicate (Figure 4-11). The number of studies not involving humans or human
FIGURE 4-8 Proportion of NIH individual fellowship (F32 award) applications by all applicants for studies involving the use of human subjects or human materials and those not using human subjects or materials. (Source: National Institutes of Health, Office of Extramural Research.)
materials far exceeds the number of studies involving humans. If the fellowship awards for studies indicating the use of humans or human materials show the same pattern as the committee's analysis of R01 grants, which found that only about one third of the awards indicating the use of human subjects or human materials would be for patient-oriented research, then less than 50 of the more than 300 F32 awards made annually to M.D.s are likely to be for research involving patient contact.
Although NIH has tracked the number of M.D. and Ph.D. recipients of fellowship awards and appointments on training grants, very little is known about the demographics of the trainees. For example, no data have been collected on the gender and race compositions of the trainees in the T32 training program and the F32 fellowship program. Although some data are available for those programs that encourage minority participation in research, the involvement of these awardees in clinical research is not easily determined.
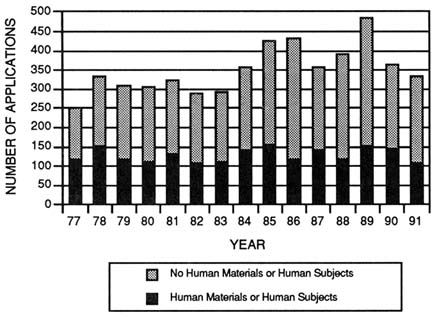
FIGURE 4-9 Number of NIH individual fellowship (F32 award) applications by all applicants for studies involving the use of human subjects or human materials and those not using human subjects or materials. (Source: National Institutes of Health, Office of Extramural Research.)
ASSESSING THE OUTCOMES OF TRAINING PROGRAMS
Since the inception of the NRSA program in the early 1970s, considerable time, money, and intellectual capital have been invested in reviewing, monitoring, and modifying the mechanisms for NIH research training. In the process, many data describing various trainee characteristics and possible relationships to subsequent outcomes have accumulated. The most recent internal evaluation was completed in 1989 and was reported in Review of the National Institutes of Health Biomedical Research Training Programs (National Institutes of Health, 1989a). That report devoted much attention to the recruitment and research training of professional doctorates. Recommendations focused on several issues, including early recruitment of talented individuals into biomedical research careers; the optimal structure of research training, such as length of time for training, modification of the payback provision for NRSA training, combining M.D.s and Ph.D.s in the same programs, and performance reviews; integrating research training with clinical certification requirements; trainee stipends and
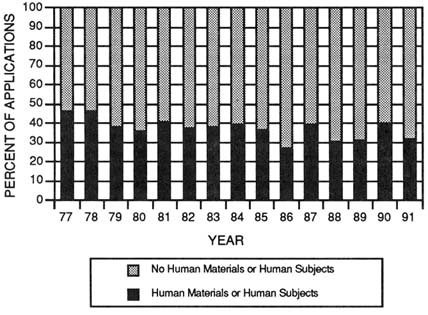
FIGURE 4-10 Proportion of NIH individual fellowship (F32 award) applications by M.D. applicants for studies involving the use of human subjects or human materials and those not using human subjects or materials. (Source: National Institutes of Health, Office of Extramural Research.)
education costs; K-series awards; and data collection and monitoring. Although the report addresses research training, the implication is that the recommendations should enhance research training for performing laboratory-based research. The panel stresses at the outset of the report's executive summary that the report should address ''areas of research training not currently addressed adequately or systematically, e.g., clinical trial design and methodology, biostatistics, epidemiology, and population demography" (National Institutes of Health, 1989a, p. 1). To redress these deficiencies, the panel recommended programs at the master's degree level in epidemiology, biostatistics, or related topics and nondegree, certificate programs with emphases on epidemiology and biostatistics (National Institutes of Health, 1989a).
Despite repeated analyses over two decades, the causal linkages between research training and outcomes have yet to be identified. It appears, however, that the training grant mechanism is less successful in inducing such trainees to apply for NIH grants than is the fellowship program (Figure 4-12) (Institute of Medicine, 1989d; Quantum Research Corporation, 1991). Less than 20 percent physicians trained on T32 training grants eventually succeed in obtaining funding
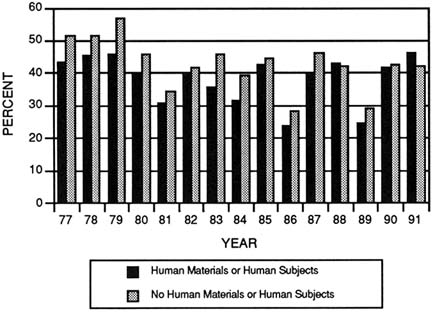
FIGURE 4-11 Success rates for NIH individual fellowship (F32 award) applications by M.D. applicants for studies involving the use of human subjects or human materials and those not using human subjects or materials. (Source: National Institutes of Health, Office of Extramural Research.)
as principal investigators on NIH grants. By contrast, those who have received F32 fellowship awards have a higher success rate than the trainees receiving support from T32 awards (Quantum Research Corporation, 1991). Moreover, success in competing for grants seems to correlate with more than two years of training (Institute of Medicine, 1989d). Since it has been shown that fellowship awardees are more successful than those trained on training grants in obtaining subsequent NIH funding and the ratio of traineeships to fellowships is higher for M.D.s (Figure 4-6), it could be inferred that Ph.D.'s might have a competitive advantage over M.D.s in obtaining NIH research grant awards.
This is not to suggest that these training mechanisms are ineffective. The available information suggests that individuals supported by NIH generally outperform other groups in research-related outcomes. The measures of success, however, are generally limited to participation rates in the NIH grant system. Whether these outcomes can be confidently attributed to the receipt of funds from NIH for training remains unclear, and any effect is likely to be small. As funds for NIH become increasingly constrained, the use of data showing the ability of
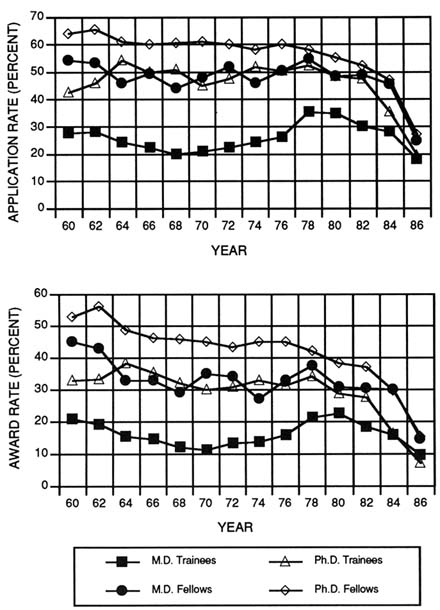
FIGURE 4-12 Application (top) and award (bottom) rates by NIH-supported M.D. and Ph.D. trainees and fellows for NIH research grants. Data for years 1982–1986 may not reflect all applicants since many may not yet have applied when the tabulations were made in 1991. (Source: National Institutes of Health, Office of Extramural Research.)
trainees to garner NIH research grants as a measurable outcome of effective training may become less and less reliable.
The time, duration, and quality of early research experiences appear to have a positive influence on the outcome of postdoctoral research training. For example, successful investigators have had longer research experiences at each stage of their careers than those who have received training but have not chosen investigative careers (Lee et al., 1991; Levey et al., 1988). Similarly, medical school research experience was strongly associated with postgraduate research involvement (Segal et al., 1990).
What is not known is how the proportion of NIH-trained individuals who go on to pursue careers in research might be increased, whether there are differences among various subgroups of trainees, and how to make fair comparisons of outcomes across the variety of training mechanisms. Furthermore, there are few data on how many training programs and fellowships might include some aspect of patient-oriented research.
Postdoctoral Ph.D. Training
The outcomes for individuals who received NIH postdoctoral traineeships and fellowships, primarily in the biomedical sciences, have been examined in relation to an assortment of post hoc-constructed comparison groups. In general, these studies have found that postdoctoral recipients, regardless of the sponsor, perform better on all research-related measures than those who did not choose to pursue postdoctoral study.
For example, NIH postdoctoral recipients were three times as likely as Ph.D. recipients with no postdoctoral plans to have applied for research support from the Public Health Service (56.9 percent of NIH-supported postdoctoral researchers did so compared with 19.6 percent of postdoctoral investigators without such training). Of those who applied, NIH-supported Ph.D. applicants were almost twice as successful in obtaining later grant support (Garrison and Brown, 1985). If the duration of training is viewed as a critical dimension of intensity, these findings suggest that a more intense "dose" of training may contribute to the production of more active researchers.
NIH-trained postdoctoral researchers were also more likely to apply for Public Health Service grants than were Ph.D.s whose training was supported by other sources (56.9 percent with training supported by NIH applied for grants compared with 34.5 percent of postdoctoral recipients trained through other means) (Garrison and Brown, 1985). More recent tabulations on the rate of application for grant awards from NIH from NIH-supported trainees by Quantum Research Corporation (1991) verify the same pattern correlating NIH-supported training with higher application and success rates in garnering research funding from NIH (Table 4-9).
TABLE 4-9 Percentage of First-Time Ph.D. Grant Applicants and Recipients with Prior NIH-Supported Training, 1964-1989
|
Fiscal Year of First Application for Award |
All Applicants |
All Recipients |
Applicants with Training |
Recipients with Training |
Trainee Application Rate |
Trainee Award Rate |
|
1964 1965 1966 1967 1968 1969 1970 1971 1972 1973 1974 1975 1976 1977 1978 1979 1980 1981 1982 1983 1984 1985 1986 1987 1988 1989 |
1,267 1,221 1,254 1,247 1,176 1,204 1,369 1,339 1,458 1,559 1,692 1,782 1,975 2,219 2,222 2,245 2,370 2,247 2,396 2,376 2,321 2,597 2,651 2,488 2,763 2,863 |
867 688 660 728 527 625 483 581 803 601 1,087 1,181 917 918 1,247 1,516 1,169 1,121 1,109 1,283 1,264 1,385 1,467 1,388 1,453 1,251 |
434 480 547 572 617 657 832 804 824 889 980 1,110 1,193 1,331 1,377 1,360 1,427 1,361 1,500 1,351 1,327 1,467 1,377 1,286 1,322 1,302 |
345 321 328 379 321 394 337 406 552 406 725 835 629 647 852 1,037 801 765 804 891 854 922 892 842 862 689 |
34.3 39.3 43.6 45.9 52.5 54.6 60.8 60.0 56.5 57.0 57.9 62.3 60.4 60.0 62.0 56.1 60.2 60.6 62.6 56.9 57.2 56.5 51.9 51.7 47.8 45.6 |
39.8 46.7 49.7 52.1 60.9 63.0 69.8 69.9 68.7 67.6 66.7 70.7 68.6 70.5 68.3 68.4 68.5 68.2 72.5 69.4 67.6 66.6 60.8 60.7 59.3 55.1 |
|
Source: Quantum Research Corporation, 1991. |
||||||
Multivariate analyses of outcomes data (such as success in obtaining grants and academic employment) that controlled for such things as the effect of selectivity of the baccalaureate institution and the prestige of the doctoral institution on training outcomes of produced small multiple R2 values, ranging from 0.06 to 0.14 (Garrison and Brown, 1985). This indicates that several other factors foster successful career paths—factors that have not been tapped by existing databases.
Postdoctoral M.D. Training
Three studies have attempted to tease out the role of postdoctoral training for M.D.s (Garrison and Brown, 1985; National Institutes of Health, 1986; National Research Council, 1976). The findings have been fairly inconclusive because of the difficulties associated with retrospectively devising appropriate comparison groups with existing data. Physicians who have been recipients of National Research Service Award traineeships and fellowships have been contrasted with M.D.s without postdoctoral training and M.D.s who reported their primary activities to be research or teaching, but who had not pursued formal postdoctoral study. Among other findings, previously reported differences in research-related outcomes between M.D.s with postdoctoral NIH-supported appointments and M.D.s without them were significantly reduced in more recent analyses, which included those in research and teaching positions as the group of comparison. Recent tabulations by Quantum Research Corporation (1991) indicate that NIH-supported M.D. trainees were more successful in obtaining NIH research grants than were those who were not supported by NIH during training (Table 4-10).
Several studies have examined the performance of physician-investigators, relating outcomes to gross measures of length and type of training (Levey et al., 1988; Sherman, 1983, 1989). A strong relationship between the existence and length of formal research training and outcomes has emerged. For instance, in terms of academic employment, grant application and award rates, and average time spent in research, the performance of M.D.-Ph.D.s, a group that has undergone a formal sequence of research training, regardless of whether they had pursued postdoctoral study, outstripped that of M.D.s who did not have a Ph.D. Furthermore, if one accepts the notion that recipients of NIH postdoctoral fellowships also possess appropriate research training credentials (because their selection is based on the decisions of NIH peer review study sections), it is not surprising that NIH fellows consistently outperformed their postdoctoral trainee counterparts (National Institutes of Health, 1989a).
Although most of these retrospective analyses indicate that previous NIH support is correlated somewhat with obtaining later grant funding, the changes in the support of research may affect this measure as an outcome. As competition for NIH research funds increases, trainees may have less chance of acquiring NIH funds. Even good training and preparation may not be sufficient to garner funding, and the use of NIH funding as the yardstick of success may skew the outcomes of these programs. Thus, the committee acknowledges that outcomes must take into account other measures of research involvement.
TABLE 4-10 Percentage of First-Time M.D. Grant Applicants and Recipients with Prior NIH-Supported Training, 1964–1989
|
Fiscal Year of First Application for Award |
All Applicants |
All Recipients |
Applicants with Training |
Recipients with Training |
Trainee Application Rate |
Trainee Award Rate |
|
1964 1965 1966 1967 1968 1969 1970 1971 1972 1973 1974 1975 1976 1977 1978 1979 1980 1981 1982 1983 1984 1985 1986 1987 1988 1989 |
1,108 999 1,026 930 765 663 590 597 722 697 722 804 927 1,019 1,036 973 1,074 983 935 1,000 1,028 1,150 1,159 1,029 1,070 1,145 |
652 582 616 542 363 376 266 310 432 314 460 444 406 419 481 556 469 464 409 494 546 592 521 577 561 512 |
394 395 480 444 394 389 363 369 447 417 416 496 533 616 632 581 661 584 544 550 560 586 548 485 480 516 |
265 279 328 296 224 258 188 215 303 186 294 294 251 279 332 401 336 317 287 303 343 363 289 317 322 269 |
35.6 39.5 46.8 47.7 51.5 58.7 61.5 61.8 61.9 59.8 57.6 61.7 57.5 60.5 61.0 59.7 61.5 59.4 58.2 55.0 54.5 51.0 47.3 47.1 44.9 45.1 |
40.6 47.9 53.2 54.6 61.7 68.6 70.7 69.4 70.1 59.2 63.9 66.2 61.8 66.6 69.0 72.1 71.6 68.3 70.2 61.3 62.8 61.3 55.5 54.9 57.4 52.5 |
|
Source: Quantum Research Corporation, 1991. |
||||||
Research Career Development Awards
NIH sponsors a series of career development awards including the Physician-Scientist Award (K11 award), for M.D.s without prior research experience; a modified form of the Research Career Development Award (K04 award), which requires a minimum of three years of previous research experience; and the Clinical Investigator Award (K08 award), which requires five years of prior research training and is intended primarily for physician-investigators. In addition
to the individual K11 awards, NIH supports an Institutional Physician-Scientist Award, the K12 award.
The K awards are heterogeneous in many respects, including the amount of research training expected. To date there has been very little evaluation of these transitional training-research mechanisms, particularly with regard to patient-oriented research (Biddle et al., 1988; Carter et al., 1987). NIH has plans to collect systematic information about program similarities and differences as a precursor to developing more comprehensive evaluation efforts.
In 1991, $99 million was allocated for all individual career development awards (CDAs). Of this, about $4.3 million supported 66 M.D. recipients of K04 awards, $38.7 million supported 499 M.D. recipients of K08 awards, and $24.4 million supported 306 Physician-Scientist Awards (K11 awards). An additional $4.9 million was awarded for Institutional Physician-Scientist Awards (K12 awards). Some trends for the K awards are noteworthy. On the one hand, the K04 awards, which required previous research experiences, have declined by more than half over the past 10 years, from 787 in 1982 to 313 in 1992. On the other hand, the number of Clinical Investigator Awards (K08 awards) grew from 160 to 527 over the same period, and the number of K11 awards, initiated in 1984, grew to 321.
As with all of the preceding grant and training program data, accurate data on the amount of patient-oriented studies supported through K awards are difficult to uncover. About 40 to 45 percent of K award applications indicate the intent to use human materials or human subjects; this percentage is consistently a few percentage points higher than that for the application pool for regular research grants (R01 awards). Ahrens has performed analyses on a sample of 243 abstracts from Physician-Scientist Awards to determine the fraction that are patient-oriented. He concluded that about 30 percent included some research involving humans (Ahrens, 1992).
Clinical Associate Physician Program
Although any of above training programs could be used by trainees pursuing a career in patient-oriented research, the only awards specifically designed to foster this type of investigation are those supported through the Clinical Associate Physician (CAP) program. CAP awards are funded by the General Clinical Research Center branch of the National Center for Research Resources (see Chapter 3 for a description of the General Clinical Research Center program). Each center is allowed a maximum of two CAPs. Recently, the training period has been extended from two to three years. Since the program's inception in 1974, more than 260 clinical investigators have been trained through the CAP program. About 40 new CAP awards are made each year (Figure 4-13).
FIGURE 4-13 Number of clinical associate physician (CAP) fellows supported annually by the General Clinical Research Center program and the amount of funding, 1983–1992. (Source: National Center for Research Resources.)
Preliminary results from an ongoing analysis of the CAP program demonstrate that the CAP alumni are successful in obtaining subsequent funding from NIH. More than 40 percent of the physicians in the CAP program have received NIH funding as principal investigators. Similar numbers of K08 award recipients are successful in obtaining funding. An unknown number are probably involved in research as coinvestigators, but that number has not been determined. A survey of the clinical associate physicians is under way and should reveal how many are actually involved in NIH-sponsored research and receive funding from other sources. Given the nature of the program, it is likely that a high percentage of those funded are actively involved in patient-related clinical research. These results indicate that the CAP program and the K08 award program are effective in training competitive clinical investigators.
Non-NIH Federal Support
Although NIH is the major supporter of health-related training, including that targeted at training patient-oriented researchers, there are several other federal sponsors of health-related training. For example, prior to its reorganization and incorporation into NIH, the National Institute of Mental Health, the National Institute of Drug Abuse, and the National Institute of Alcoholism and Alcohol Abuse supported about 1,500 NRSA fellowships and traineeships (pre- and postdoctoral) totaling $32.9 million in fiscal year 1990 (Alcohol, Drug Abuse, and Mental Health Administration, 1991).
Specific research training opportunities in health services research are supported by the Agency for Health Care Policy and Research (AHCPR), primarily in the form of dissertation awards and individual and institutional postdoctoral training awards. In fiscal 1992, AHCPR invested about $3 million in training through NRSA fellowships and traineeships—equal to only about 1 percent of NIH allocations for training. The U.S. Department of Veteran's Affairs also has a small program of research training efforts in this area. Postdoctoral training for physicians who are pursuing a master's degree in public health is available for individuals who are interested in health care delivery research questions relevant to the services provided by the U.S. Department of Veteran's Affairs.
Since 1951, the Centers for Disease Control and Prevention has sponsored a combined training and service epidemiology training program for postdoctoral training, the Epidemic Intelligence Service. Working under the supervision of practicing epidemiologists at the various Centers for Disease Control and Prevention sites, trainees develop their epidemiologic skills during a two-year fellowship. Although the program focuses on preparing trainees with epidemiologic skills to work in public health, it also encourages active participation in population research. More than 1,700 professionals have served in the Epidemic Intelligence Service. About 80 percent of the participants are physicians; other health professionals such as nurses and dentists with master's degrees in public health are also accepted into the program. One interesting aspect of the program is that the American Board of Preventive Medicine recognizes the training program fulfills the certification requirements of one-year of supervised training and field experience. Many of the alumni are employed in public health agencies, including the Centers for Disease Control and Prevention; about 12 percent are on university faculty (Thacker et al., 1990). Whereas the program may be effective in training public health epidemiologists, it might serve as a model for patient-oriented clinical research.
The Food and Drug Administration also has developed an extensive intramural program for training Food and Drug Administration staff. For example, the Center for Drug Evaluation and Research operates a staff college that helps to train its medical reviewers. Enrollees can take courses in drug law and
regulatory procedures, basic and applied statistical methods, chemistry and biotechnology, immunology, pharmacology, and clinical trials (U.S. Department of Health and Human Services, Food and Drug Administration, 1992; Peck 1988). Effectiveness of these types of programs in preparing clinical investigators, rather than train individuals to assess regulatory requirements for new drugs and devices, is not known.
Private Support
There is no current, comprehensive source of information about private sources of funding for clinical research training. A 1983 report by the Rand Corporation listed 75 foundations that provided some support for training physicians and as well as those with Ph.D.s (Carter, 1983). In 1981, according to the report, foundations funded some 400 individual junior faculty and postdoctoral awards for M.D.s. These numbers are certainly out of date, although it is not known whether they under- or overestimate the present level of funding. Non-federally supported M.D.-Ph.D. programs may be supporting as many as 700 double-degree candidates, although this number also cannot be verified (Ahrens, 1992).
Informal contacts with several voluntary health agencies and foundations by Institute of Medicine staff revealed that many support training, particularly of M.D.s. When queried whether their training programs specifically support patient-oriented clinical research trainees, most responded that they did not. Exceptions to this are the American Cancer Society, which recently started a junior faculty program for human investigation training, and the American Heart Association, which has an equally broad set of training programs. More frequently, these organizations and foundations support fundamental research training pertaining to their specific missions.
Some medical specialty groups have taken research training into their own hands. The Orthopaedic Research and Education Foundation (supported by individual contributions of members of the American Academy of Orthopaedic Surgeons and the Orthopaedic Research Society), for example, raised $3.8 million in 1991, nearly all of which went to fund peer-reviewed research and research training activities (Orthopaedic Research and Education Foundation, 1991). Again, it is not clear how much of these training funds is used to support patient-oriented clinical research training.
Health policy and health services research training are promoted by several private foundations. For example, the Robert Wood Johnson Foundation funds a Clinical Scholars Program, which has trained postdoctoral physicians in health services research since 1969 (Piccirillo, 1992; Shuster et al., 1983). Predoctoral and postdoctoral training are also supported by the Pew Charitable Trusts and Harvard Medical School's Clinical Effectiveness Program, the latter funded by the
Kellogg Foundation, the Klingenstein Fund, NIH, and the Health Resources and Services Administration (Goldman et al., 1990).
Payback of Debt
As mentioned previously, many medical residents accrue a large amount of education-related debt from undergraduate and medical schools. Under the current rules, payback must begin in the third year of postgraduate training. To accommodate this financial burden, research training often is either omitted to facilitate earlier entry into practice or is used as a time to moonlight to earn money for debt repayment. Neither scenario is likely to permit adequate or high-quality training for research on human subjects.
Other than established training programs that pay stipends during research training, some novel programs are focusing on mechanisms to repay educational debt and retain trainees. One notable example is the NIH program for AIDS researchers begun in 1989. In this program, physician research trainees are recruited to NIH to engage in AIDS research. Trainees are encouraged by the opportunity to relieve their educational debt load. NIH allows $20,000 in debt relief for each year served to a maximum of $40,000. In addition, the trainees are paid a stipend for living costs. In the first three years this program was under way, 19 trainees were accepted into the program each year. Although this is a promising avenue for encouraging ongoing participation in research, it is not evident how many of these trainees are actually engaging in patient-oriented research. Moreover, this is small program in an area of great need. It is too early for the program to have any measurable outcomes for continued participation rates in research.
Federal programs such as those described above require authorization through public law. The NIH Revitalization Act of 1993 expanded this opportunity to other areas at the discretion of the NIH director (U.S. Congress, 1993).
MODEL PROGRAMS FOR RESEARCH TRAINING
Although many of the methodologic advances in patient-oriented research have been developed in graduate schools of public health and divisions of general medicine, investigators in subspecialties of medicine and other departments are increasingly recognizing the need for training in these techniques (Goldman, 1991; Goldman et al., 1986). This trend is the reflection of a paradigm shift in which new ''horizontal" relationships are formed within a medical center, crossing the "vertical" divisions defined by preclinical sciences and clinical specialties and subspecialties (Kelley, 1992). These horizontal relationships may be defined by diseases, such as cancer, or by research methodologies. At many universities
molecular biologists have developed informal or formal research interactions that play more active day-to-day roles in their lives than interactions with their subspecialties do. The same kind of cross-disciplinary associations are developing among investigators interested in advanced patient-oriented research methodologies.
A number of programs have been initiated around the country to provide investigators with the skills needed to perform patient-oriented research. A selection of these is described below; this is followed by a discussion of some of the characteristics common to most such initiatives.
Overview of Selected Programs
Robert Wood Johnson Clinical Scholars Program
One of the oldest, largest, and most successful of the existing research training programs is the Clinical Scholars Program (CSP) (Shuster et al., 1983), which was started in 1969 by the Commonwealth Fund and Carnegie Corporation. Since 1973, CSP has been funded by the Robert Wood Johnson Foundation. Each year some 25 new fellows are enrolled in six programs at seven universities and their affiliated Veterans Affairs Hospitals. The foundation does not encourage the pursuit of advanced degrees.
From 1971 through 1992 there were 600 graduates of CSP, of whom 363 (61 percent) are currently in academic medicine and another 31 (5 percent) are in government. Slightly more than half of the graduates were from internal medicine. Many have assumed leadership roles at their institutions and at various federal agencies, including AHCPR.
University of Michigan School of Public Health
The University of Michigan School of Public Health supports a program in clinical research design and statistical analyses that can lead to a master's of science degree (Penchanksy et al., 1988). The program's required core courses are taught during 18 sessions, each of which is held about once a month and lasts for four days. Student participants include physicians at various levels of training and other health care personnel.
Harvard Clinical Effectiveness Program
The Harvard Clinical Effectiveness Program provides methodologic training to postdoctoral trainees during an intensive two-month summer session
administered by the Harvard School of Public Health (Goldman et al., 1990). The program was initiated in 1986 in response to interest generated among medical subspecialty fellows supported by NIH training grants.
The curriculum provides 15 credits (of the 40 needed for a master's of science or master's of public health degree) at the Harvard School of Public Health. During this period, fellows are required to be completely free of clinical responsibilities. A prerequisite for all applicants for the program is a commitment to an academic career that will utilize the methodologic skills taught in the program. All applicants must be sponsored by the chief of their clinical subspecialty division or department, who must pay the trainee's tuition (currently about $6,000) with individual or institutional training grants or other institutional funds.
Of the 80 physicians who have enrolled in the summer curriculum and who have finished their clinical training, 68 (85 percent) hold full-time academic positions and another 4 (5 percent) are in government or nonprofit research positions.
Other Programs
Among other academic centers that sponsor patient-oriented research training programs are those at Johns Hopkins University, the Mayo Clinic, Stanford University, the University of California at San Francisco, and McMaster University in Canada (Neufield, 1989).
Common Characteristics
In most instances, the programs are coadministered by schools of public health and departments of medicine. Several programs are affiliated with degree-granting schools of public health; others actively involve divisions of epidemiology and biostatistics within the medical school.
Strong emphasis is placed on issues of study design such as formulation of the research question, types of study design, subject selection, randomization, measurement, sample size, bias, pretests, quality control, compliance, discontinuing criteria, closing a trial, alternative designs, including observational studies, cohort studies, cross-sectional studies, case-control studies, and hybrid designs and multicenter trials.
All curricula stress in-depth training in statistics and epidemiology. Among the topics often covered are discrete and continuous probability theories, linear and logistic regression techniques, analysis of variance and covariance, nonparametric testing, graphical displays, data transformation, contingency-table analysis, life-table and survival-analysis techniques, mathematical modeling, meta-analysis,
cost-effectiveness and cost-utility analysis, measurement error, global and specific health and functional status instruments, and questionnaire and interview design.
All programs offer training in the use of computer software and data management, as well as in the ethics of clinical research (for example, conflicts of interest, authorship, misconduct, subject selection, informed consent, institutional review boards, confidentiality, financial issues, and replication). Specific training in research management (such as resource estimation and personnel management) is included in a few of the programs. All programs include some information on how to pursue funding, and most instruct participants on how to prepare a grant proposal. Communications skills, however, are infrequently addressed.
All programs combine a basic instructional curriculum with research activities under a faculty mentor. The total duration of training ranges from 18 months to three years, with most lasting two years. The first-year in most programs consists of an introductory curriculum in research methods, with the remainder of the time devoted to elective course work and a mentored research project.
The least uniform aspect of the programs reviewed by the committee is the funding mechanism. Although a large number of options were mentioned, only the few programs with department of medicine or hospital support seemed to have resources dedicated to administering their respective programs. At least one program was assisted by substantial foundation support, and others were pursuing similar funding from outside organizations. Tuition costs varied substantially, from $23,000 per year for a two-year program to $5,000 for an eight-week summer course. Finally, these programs tend to be oriented more toward population-based research or clinical trials rather than toward human pathophysiology or biology.
Remaining Obstacles
The difficulty of obtaining stable sources of funding has been the major obstacle for newly created fellowships in clinical research and may prevent other institutions from developing similar programs. Salary support for fellows can be provided through customary subspecialty training grants, but support of faculty time is often problematic. Developing and sustaining these programs require a substantial commitment of faculty and administrative time. Established programs offer from 130 to 250 classroom hours over periods of 4 to 24 months for classes of 10 to 50 fellows each. Although some of this time is accounted for by existing courses offered through other schools or departments, much of it involves new courses and seminars designed specifically to meet the needs and abilities of clinically trained physicians.
Foundation and departmental funding has been obtained to support faculty in individual programs, but this is often directed to program development, not the continuing obligations of faculty involved in teaching courses or acting as mentors to fellow-initiated projects. The ability and willingness of departments of medicine to support these activities through clinically generated revenues vary from center to center.
Ensuring program support is complicated by the variety of medical specialties and subspecialties served in such programs. No umbrella organization exists at NIH to fund comprehensive training for a variety of fellows, whose stipends are supported by separate NIH institutes. Reliance on tuition support from a collection of training grants and individual sponsors with varying budget regulations makes program planning more precarious and less efficient than if centralized support was available from a single entity at NIH or some other major sponsor.
Finally, tuition alone may not address the need to support faculty involvement as mentors. Faculty whose research activities are well-funded may not need additional support to supervise fellows who participate in their research activities. Because funding for patient-oriented research is modest, however, prospective mentors are likely to have limited extramural support to help fellows' projects. Furthermore, a substantial time commitment is required from faculty to be effective mentors, especially when fellow-initiated projects involve topics and methods outside their current research activities. Unless specific support for this time is available, mentorship is likely to be unsatisfactory for fellows and faculty alike.
CONCLUSIONS
In conclusion, the committee found that data do not exist to make an accurate assessment of the number of patient-oriented clinical investigators or the number who are being trained. Whereas career pathways for those choosing to pursue basic science investigation are clearly delineated, with established rewards and measures of productivity, comparable training pathways for patient-oriented clinical research careers are not. Given the current economic and social climate, identification of the best and most efficient ways to produce patient-oriented researchers has assumed additional importance. The escalation of health care costs, the increasing failure of the "safety net" to guarantee adequate health care for all citizens, and the emergence of AIDS and other still incurable diseases have strongly accentuated the critical need for research on the prevention, diagnosis, management, and treatment of disease. Current advances in molecular biology hold significant promise, but those advances can be fully exploited only by well-trained and committed investigators.
The responsibilities and expectations of faculty who engage in basic research are straightforward, with agreed upon standards for judging success and rewarding achievement. The same is not true for individuals choosing to become clinical investigators or faculty members who participate in clinical research. Few programs rigorously train clinical scientists to provide them with a substantive foundation in clinical research methods. The responsibilities and expectations of the clinical research faculty are ambiguous and there are no agreed upon standards for measuring success. Furthermore, there appears to be few rewards even when consensus agrees that success has been achieved. Given this scenario, it is clear that medical students and other health professionals do not perceive clinical research pathways as viable options for academically based careers or careers in other employment sectors as well.
The prospect of a significant infusion of funds for postdoctoral research training may be low, given such problems as the federal budget deficit and other economic woes. What is more likely is a scenario in which resources for research training remain constant or increase only minimally. Thus, future policy and program decisions will most likely involve such issues as identifying which training mechanisms work best, what is needed for their implementation in other settings, and how such programs could be fine-tuned to increase their efficiency. In addition, situations of constant or reduced funding will require policymakers to decide which mechanisms should be eliminated or scaled back to permit the expansion of other programs or experiments with promising new strategies.
The committee concluded that some means must be developed for determining what programs are, in fact, training patient-oriented clinical investigators. Only then can the scientific community be confident that an appropriate number are being trained. Once those programs are identified and suitable outcomes measures are determined, the programs that are effective in training patient-oriented clinical investigators should be expanded. Some programs, such as the Clinical Associate Physician program, train this type of investigator by design. Since the General Clinical Research Center infrastructure is already in place around the country, these centers seem to be an appropriate place to begin developing programs that involve medical students and residents in human research.
Finally, the desire for change will have to come from all sectors with an interest in clinical research and professional education. The federal government will have to assume the leadership role in effecting change, but it will need the full cooperation of the academic medical centers, the pharmaceutical biotechnology and medical device industries, medical and life insurance companies, professional societies, and organizations with a stake in professional education and certification for all groups of clinical investigators. All of those listed above as well as other groups need to work together progressively to improve the training of patient-oriented clinical investigators and create rewarding career paths to encourage clinicians to pursue careers in clinical research. The










































































