3
Clinical Research Funding and Infrastructure
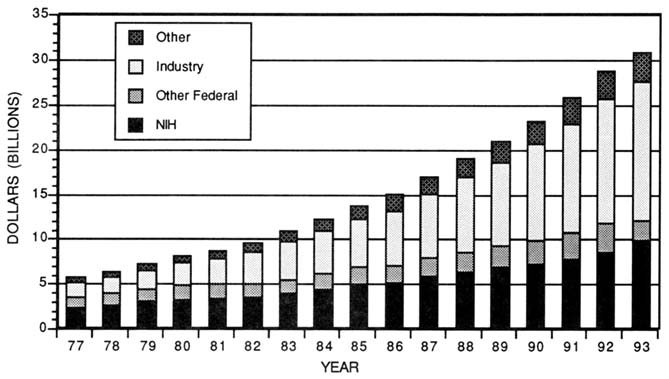
About $22 billion (16.7 percent) of the estimated $132 billion currently invested in research and development (R&D) by all sectors in the United States is health-related (National Institutes of Health, 1993b; National Science Foundation, 1992). The federal government is generally regarded as the primary sponsor of biomedical research, but, in fact, funding comes from a myriad of public and private sponsors, each with their own objectives or missions. During the 1980s, however, the portion supported by the federal government plummeted, decreasing from 59 percent in 1980 to an estimated 41 percent in 1992 (Figure 3-1) (National Institutes of Health, 1993b). The most notable change in this ratio over the past decade has been the growing contribution by industry, which has grown from 31 to 48 percent over the same period. Of the remainder, private nonprofit organizations supported about 4 to 5 percent, and state and local governments supported a small amount of health research.
Although less well appreciated, the contributions by the academic health centers themselves cover many of the costs of performing research (Commonwealth Fund, 1985). For example, many centers sponsor a variety of research activities with their own funds, such as covering the costs of starting up newly independent investigators, providing bridging funds for ongoing, high-quality research activities that fell just short of the funding level because of limited federal funds, and funding other projects that for various reasons may not or cannot be funded by other sponsors. Private philanthropy is not easily quantified, because it can be derived from a variety of private sources and large gifts or commitments to build research infrastructure may not be included in the accounting for research.
Also intertwined in this labyrinth of clinical research funding is the role of third-party payers. Although third-party payers, particularly Medicare, have underwritten some of the costs of medical education, the costs of experimental or investigational therapies have not generally been allowed as reimbursable, even though the results of clinical studies will define future standards for medical care. The growing concerns about cost-containment and a shift toward managed care are having an effect on what insurers will cover, even in the use of standard therapy (Antman et al., 1988 and 1989; Wittes 1987b). The committee is concerned that these coverage decisions might not be based on the best and most up-to-date information. Furthermore, cost-containment decisions might encourage the use of outmoded therapies rather than foster the timely introduction of truly novel or innovative therapies that could lead to long-term savings. Some feel that insurers and other third-party payers have a fundamental interest in and responsibility for supporting evaluative, patient-oriented clinical research to engage in coverage decisions and to facilitate the adoption of more cost-effective care (leaf, 1989; Newcomer, 1990). The total costs of clinical research cannot be shifted to insurers, but they are participants in providing care and should support and promote definitive studies that will define standards of care, assess the effectiveness of current therapies, and provide new effective therapies. Thus, the committee includes here a section on the roles and responsibilities of third-party payers.
Realizing how critical funding is to successful research careers, particularly the perception by clinical scientists of their inability to garner funds for patient-oriented studies, the committee devoted time to develop a clearer understanding of the research funding base. Many of the commissioned papers included some reference to the tenuous nature of research funding, and the committee sponsored an invitational workshop, "Clinical Research and Training: Spotlight on Funding," in June 1992. This chapter explores trends in research funding by the various sectors. Because of inadequate data collection methods by research sponsors, it was frequently impossible to disaggregate research funds devoted to patient-oriented clinical research from other research funds. When possible, however, the trends in funding for patient-related research are elaborated. Since academic research careers are closely intertwined with the investigator-initiated, peer-reviewed grant system in the Public Health Service, including the National Institutes of Health (NIH) (and previously the Alcohol, Drug Abuse and Mental Health Administration [ADAMHA]), the committee focused considerable attention on this process.
THE BIG PICTURE
Prior to World War II, health research was sponsored primarily by industry, academic institutions, and private individuals (Ginzberg and Dutka, 1989).
Following the war, policy changes initiated by Vannevar Bush, then head of the Office of Scientific Research and Development, began the surge of federal investment in university-based fundamental research. Bush and his colleagues formulated a set of proposals intended to sustain the nation's wartime research momentum and direct it toward civilian goals. These policies, outlined in his report to the President, "Science—the Endless Frontier" (Bush, 1945), proposed a coordinated federal policy of investing in research and the training of new researchers that would be driven by scientific merit rather than by political or geographical interests. This approach became the cornerstone of the peer-reviewed, academically based system now in place for federally sponsored, competitive extramural research grant programs. From the end of the war to the mid-1960s, the federal government invested heavily in health research and allocated resources to build health research facilities and to create programs to train health researchers (Institute of Medicine, 1990). Moreover, the synergism between federally sponsored research and research sponsored by industry and the private nonprofit sectors thrust the United States into the forefront of biomedical research.
In the 1960s, increasing allocations for the war in Southeast Asia and the Cold War buildup began to constrain the federal resources available for domestic programs, including health research. In the 1970s, the health research budget plateaued, and high inflationary pressures further reduced the purchasing power of research funds. Over the past decade, the nation's expenditures for health research have tripled when measured in current dollars. After adjusting for inflation, which was relatively low throughout the 1980s, this investment grew by 65 percent (Figure 3-1).
The health research enterprise has been highly successful, but the system has become increasingly stressed in recent years. The most significant reason is the concern over growing federal debt and recent legislation attempting to reduce the huge annual federal budget outlays. The 1980's policy of increased spending but decreased taxes has put the U.S. government in a precarious financial position and has mortgaged the country for many years to come. There are anticipated decreases in defense spending, but expectations for increased funding in other categories of the federal budget remain low. Recent attempts to reduce federal deficits have increased the competition for scarce funds for all federally financed programs. State funds and those from private sector sources have been unable to compensate for the slower growth of available federal funds. The increasing competition among worthy projects has often resulted in concessions to short-term needs rather than longer-term investments. The combination of increasing research costs and increasingly constrained funding has sent shock waves throughout the academic research community (Lederman, 1991; Movsesian, 1990). The broad array of research sponsors and the decentralized nature of the research of thousands of individual investigators are responsible for the success of health research over the past half-century.
FEDERAL SUPPORT FOR HEALTH SCIENCES R&D
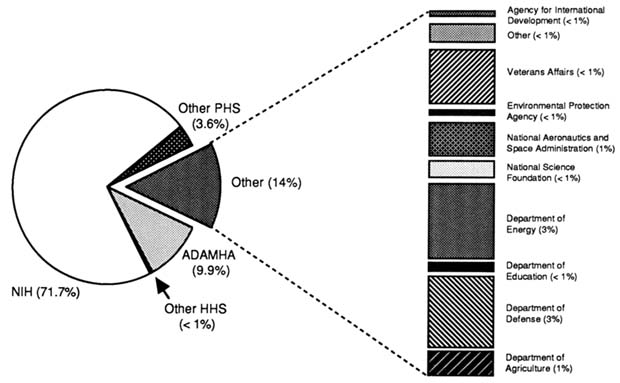
As a result of the postwar policy changes, the federal government became the largest single sponsor of health research, and programs that support health research can be found in numerous federal agencies (U.S. Congress, Office of Technology Assessment, 1991). About three fifths of these funds now come from programs in the U.S. Department of Health and Human Services (DHHS), including those in the Public Health Service (PHS) (Figure 3-2) (National Institutes of Health, 1993b). Within the PHS, NIH—which now includes the National Institute of Alcohol and Alcohol Abuse, the National Institute of Drug Abuse, and the National Institute of Mental Health—allocate the largest percentage of federal funds for health-related research (Figure 3-2). Research funds are also appropriated for the Centers for Disease Control and Prevention (CDC); the Health Care Financing Administration; the Health Resources Administration; the Food and Drug Administration (FDA); the Health Services Administration; the Office of Health Research, Statistics, and Technology; the Agency for Health Care Policy and Research; and the Office of the Assistant Secretary for Health in PHS. Other federal departments and agencies have budgets for health sciences research as well, most notably the Departments of Agriculture, Defense, Education, Energy, and Veterans Affairs and the National Science Foundation (see Figure 3-2). Even though some agencies have only a minimal role in sponsoring clinical research, they may require highly talented clinical investigators to carry out their mission, such as investigators at FDA and CDC. Thus, the committee sought to determine the fraction of federally sponsored research that involved human subjects.
National Institutes of Health
Of the nearly half of all financial support for health research that comes from federal sources, about three quarters is disbursed through NIH. The postwar policy decision to support fundamental research in academic institutions stimulated steady increases in NIH's budget (Figure 3-3). The most rapid growth in the NIH budget occurred between 1955 and 1965. From the late 1960s to 1980, budget growth for NIH leveled off. During the 1980s, however, congressional appropriations to NIH increased an average of 10 percent a year, resulting in a 2 percent per annum real growth in the NIH budget (National Institutes of Health, 1991). Many of the increases over the past few years can be attributed to the growth in funding for AIDS research. The new initiative for research into women's health issues has not yet stimulated growth in the NIH budget, but the Clinton administration and U.S. Congress have the opportunity to make these changes.
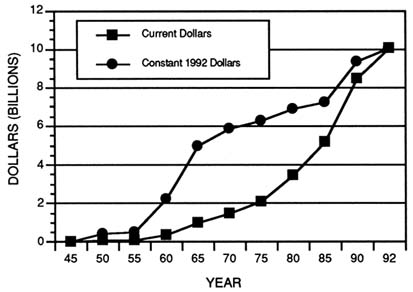
FIGURE 3-3 NIH appropriations from 1945 to 1992 in current and constant 1992 dollars. (Source: U.S. Department of Health and Human Services, Public Health Service, 1991e.)
Allocations among extramural and intramural NIH programs and program management have not changed significantly since the late 1970s (Figure 3-4). Only about 10 to 12 percent of NIH budget is allocated for research conducted intramurally. Nearly 80 percent of the NIH budget is allocated to extramural programs for research and training at universities and other research institutions both in the United States and abroad (National Institutes of Health, 1993b). Most of these extramural research funds are allocated through peer review processes for research grants and cooperative agreements. A small fraction of these funds are allocated for research contracts as well. Nevertheless, since the expansion of NIH extramural programs began in the mid-1940s, R&D grants have been, and continue to be, the cornerstone of NIH and ADAMHA extramural support for health research.
Intramural Research
Although the intramural research program at NIH includes a broad portfolio of activities such as basic research, training, communication of scientific findings, development of policies on biomedical research priorities, and
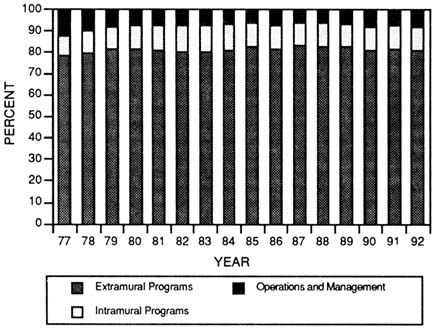
FIGURE 3-4 Allocation of the NIH budget from 1977 to 1992.
(Source: National Institutes of Health, 1993b.)
translation of research findings into more effective medical care, the committee was most concerned about the research activities and training at the Warren Grant Magnuson Clinical Center. The clinical center was established in 1953 on the Bethesda, Maryland, campus of NIH to facilitate research using human subjects that could not be conducted at academic medical centers for various reasons (Ahrens, 1992). The clinical center currently has about 500 patient beds, or about 50 percent of all the research beds in the country. The remaining 50 percent are located throughout the country in academic health centers that are largely supported by NIH institutes and centers. Since its inception, the clinical center has served as a training ground for clinical investigators, may of whom are now on the faculty at academic health centers around the country. Resource limitations precluded the committee from undertaking a comprehensive assessment of the clinical center, but the committee drew upon several studies done in the 1980s that examined the structure of NIH (Institute of Medicine, 1985), the intramural program (Institute of Medicine, 1988b), and research at the clinical center (Ahrens, 1992; Institute of Medicine, 1987; National Institutes of Health, 1986).
There are two primary advantages for scientists who conduct research at the clinical center: (1) they do not have to compete for resources through the extramural peer review system and (2) they are not distracted from their research by obligations to teach or provide clinical services to the general public like their counterparts at academic medical centers are (Ahrens, 1992). Many criticisms of the intramural program have surfaced over the past decade suggesting that the quality of intramural research has declined. Some have suggested that peer review of the intramural research community is not as rigorous as that of the extramural research community. Further, it has recently been suggested that the organization of research groups has stifled cutting-edge investigations. It has been argued, on the other hand, that scientific oversight within NIH is as rigorous for intramural research scientists as peer review is for the extramural research community. The intramural research budget has not grown in real terms over the past decade, and intramural research scientists are therefore competing internally for scarce resources. The clinical center has accredited training programs in some fields, increasing the teaching requirements of the staff physician-scientists.
Although the NIH campus served as a primary training ground for health scientists in the 1950s and 1960s, there were signs in the 1980s that NIH was beginning to have difficulty attracting and retaining scientists, including clinical investigators. During the 1980s, there was speculation that the intramural research program was not performing at the same level of quality demonstrated in the past. The relatively low government salary scales, noncompetitive fringe benefits, and the other bureaucratic constraints of working in a federal agency are thought to be contributory (Institute of Medicine, 1988b). It also has been postulated that the military draft may have been a driving force encouraging research-oriented scientists to pursue research training there during the mid to late 1960s.
In response to these concerns and to the suggestion that the intramural program could benefit by shifting to the private sector, the Institute of Medicine (IOM) conducted an in-depth review of the program in 1988 (Institute of Medicine, 1988b). The IOM study committee concluded that the intramural program has made, and continues to make, valuable contributions to understanding basic biological and disease processes. For example, an analysis by Ahrens of 36 physician-scientists at the clinical center revealed that intramural scientists publish more papers on both clinical and nonclinical research than their counterparts in medical schools do (Ahrens, 1992). Moreover, the first gene therapy protocols have been carried out at the clinical center.
Despite difficulties in effectively coordinating activities across institutes and responding efficiently to new challenges or crises, the IOM study committee concluded that the federated organizational structure of NIH has helped meet the nation's biomedical research goals. To maintain the intramural program's excellence and credibility and to improve in areas which it is deficient, the study committee recommended some changes in NIH administration as well as in the
scope of responsibilities of scientific administrators directing the intramural programs (Institute of Medicine, 1988b).
Beyond the general problems associated with the intramural program, the clinical center presents specific problems because of its role and position in the federation of institutes. According to the an NIH report, the center has struggled with an identity problem (National Institutes of Health, 1986). In one sense, the center might be viewed as a hospital with all the requisite responsibilities associated with patient care. In another sense, the center appears to be a collection of the clinical research fiefdoms of the activities of each separate institute, where ''The Clinical Center per se has very little power in determining its practical management, its clinical research program, or its fiscal decisions." (National Institutes of Health, 1986). Indeed, the clinical center budget is determined by the individual institutes on the basis of their research involvement and previous bed allocation. Although a medical board composed of the clinical directors from each institute help guide policy at the clinical center, some believe that an external advisory board should be constituted along the lines of the institute advisory councils to review protocols to ensure appropriate allocation of clinical center resources and an appropriate level of research activity for each institute.
Although the committee understands that there are problems with the physical infrastructure of the clinical center and intramural clinical investigators share the same career obstacles, the committee did not feel that it had enough information or insight to make recommendations concerning the intramural program. Furthermore, a recent report on the NIH intramural program by an ad hoc panel has proposed a new, yet smaller (about 200 bed) clinical center (Marshall, 1994).
Extramural Programs at NIH
R&D grants, particularly investigator-initiated research project grants (R01), are the cornerstone of the extramural research program at NIH. To more clearly understand the support base for clinical research, the committee felt that it would be useful to recap some of the problems and policy changes that have affected the entire extramural research community over the past decade. When possible, the committee's analysis focused on patient-oriented clinical research.
As growth in the NIH budget slowed during the mid-1970s, competition for grants intensified, and the number of new and competing renewal grants awarded by NIH fluctuated annually (Institute of Medicine, 1979; Seggel, 1985). Through the 1970s the number of funded new and competing proposals ranged from as few as 3,500 in 1976 to as many as 5,900 in 1979. The number funded annually did not follow any particular pattern, but depended on the cumulative grant portfolio and funds appropriated for a particular institute. These erratic patterns meant that
even outstanding grant proposals often were not funded. Scientists began to feel that obtaining funding from NIH was unpredictable and had no regard for an investigator's previous research accomplishments or the significance of one's research. Moreover, the decreasing proportion of research grants being awarded to physicians raised concerns that the number of physician-investigators was declining and that measures must be taken to turn this situation around (Kelley, 1980; Thier et al., 1980; Wyngaarden, 1979).
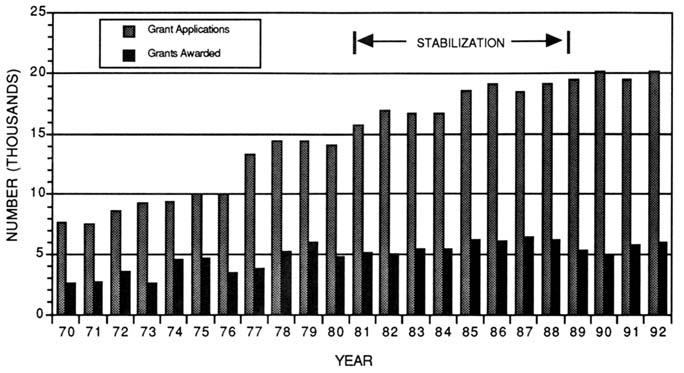
The 1979 and 1980 reports by Institute of Medicine for the DHHS Steering Committee for the Development of a Health Research Strategy reexamined these concerns about the future of federal support for new and ongoing health research in light of impending federal budget constraints. The Steering Committee called for five-year plans and evaluative procedures to be established for all of the health-related agencies in DHHS and emphasized the need to stabilize the science base by making investigator-initiated research projects the first priority in NIH and ADAMHA research budgets (Institute of Medicine, 1979 and 1980). The 1979 Steering Committee report suggested that the minimum number of competitive research grant awards for fiscal year 1981 be 5,000 for NIH and 569 for ADAMHA (Seggel, 1985). Although NIH was able to fund the recommended number of new and competing awards, appropriations for 1981 allowed ADAMHA to fund only 284 new and competing awards that year—only half the recommended level. As a result, U.S. Congress and the executive branch agreed on a policy that specified the minimum number of new and competing grants NIH and ADAMHA would be required to fund each year—a "stabilization policy." Thus, establishing the number of new and competing proposals to be funded became an integral part of the federal budget policy that remained in place through fiscal year 1988 (Institute of Medicine, 1990).
The stabilization policy prevented erosion of the nation's scientific base by maintaining minimum annual numbers of investigator-initiated research grants. The total number of research project grants sponsored by NIH grew from 15,500 to 20,867 between 1977 and 1988. Research project grants increased from 51 percent of the total NIH extramural budget in 1978 to 67 percent in 1989 (Figure 3-5). Funding for research project grants grew from $ 2.5 billion in 1977 to $ 3.9 billion by 1989, when measured in constant 1988 dollars. Along with this growth, the expectation of funding may have encouraged scientists to submit more grant applications, which increased from 14,142 in 1980 to 20,154 by 1990 (Figure 3-6) (National Institutes of Health, 1993b). It should also be noted, however, that the number of amended applications grew significantly over the same period and now makes up nearly 30 percent of the application pool. By 1987, the number of new and competing awards made annually reached a peak of 6,400 for NIH and 600 for ADAMHA.
Other forces were affecting the available pool of funds for scientists competing for funding in 1990. Despite added appropriations from Congress throughout the 1980s, the funds available were never adequate to fund fully the
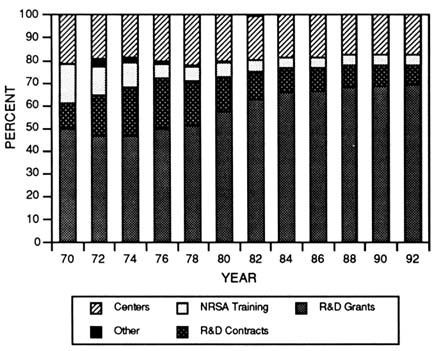
FIGURE 3-5 NIH extramural awards as a percentage of the extramural budget from 1970 to 1992. (Source: National Institutes of Health, 1993a.)
agreed upon number of awards. In order to comply, NIH and ADAMHA were forced into a policy of reducing ongoing research commitments (continuing awards for already approved and funded grants) as well as the amounts paid to new and competing awards in what is commonly referred to as "downward negotiation"—a recent practice for reconciling NIH and ADAMHA research grant commitments with annual appropriations by making across-the-board reductions in all grant awards. Although no negotiations between the scientist and NIH or ADAMHA actually occur, these budget "cuts" placed additional burdens on scientists; they were expected to perform the research outlined in their proposals with less than the recommended amount of funding.
Although NIH and ADAMHA were increasing the numbers of new and competing awards through the stabilization policy, another policy affected the pool of available funds. The research community felt that the average three-year award period for traditional research project grants (R01) was too short and did not allow sufficient time to achieve research goals or for long-term research program planning. Frequent renewals placed too much emphasis on the writing of grants
and attending to administrative details, distracted scientists from their research, and overburdened the review groups with competing renewals. Responding to these calls, NIH and ADAMHA instituted a policy to increase gradually the length of grant awards beginning in 1986. The intended result of increasing award periods was to provide more stability in research activities and scientists' careers and, perhaps, to discourage the number of multiple grant applications by individual investigators. In addition, longer award periods were viewed as a way to reduce the administrative workload for NIH and ADAMHA study sections by reducing the number of competitive renewal applications processed each year. As a result of this policy change, the average length of R01 awards increased from 3.3 years in 1980 to 4.1 years in 1990 (U.S. Department of Health and Human Services, Public Health Service, 1992c). Lengthening the award periods, however, also obligated NIH and ADAMHA appropriations further into the future.
The policy for lengthening award periods was linked to increasing the average award size (Kennedy, 1990, U.S. General Accounting Office, 1988). According to the U.S. Department of Commerce, the costs of performing health research outpaces the average annual rise in consumer prices and has developed a deflator index known as the Biomedical Research and Development Price Index to account for this difference. Even factoring in this accelerated cost increase, the average size of an R01 grant award grew from $114,6000 to $134,400 in inflation-adjusted dollars (1982 = 100) between 1980 and 1991. These accumulating obligations for future years reduced the funds available to meet annual targets of new and competing grant awards. Obligations for noncompeting continuations grew from 67 to 68 percent of the NIH extramural research budget in the mid-1980s to more than 76 percent in 1990 (Figure 3-7). Although this appears to be a small percentage shift, these growing obligations for noncompeting awards represented about $350 million that was not available for funding new and competing renewal grant applications. As a result, NIH awarded only about 5,400 new and competing awards in 1989, and dropped even further in 1990 to a devastating low of 4,600 (National Institutes of Health, 1993b). Not only were new and competing awards declining, but in 1989 the total number of grants dropped to 20,681, and this number dropped yet even further in 1990 to 20,316.
The House report accompanying the appropriations for 1991 cited congressional concern about the conundrum of increased appropriations for NIH but the declining numbers of new and competing awards (U.S. House of Representatives, 1991). Specific instructions were relayed to NIH to roll back the average length of awards to no more than four years. In an attempt to buttress the system, Congress also appropriated funds with the expectation of reaching 6,000 new and competing awards; nevertheless, only about 5,800 were funded in 1991. Appropriations for 1992 also kept the number of new and competing awards at about 5,800.
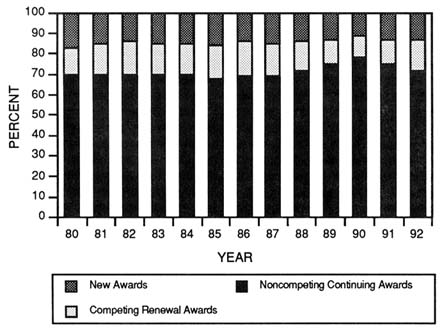
FIGURE 3-7 Percentage of amount awarded by NIH for competing and noncompeting grant awards from 1980 to 1992. (Source: National Institutes of Health, Division of Research Grants.)
The annual budget process for fiscal year 1993 was peculiar in its own right. For the first-time in more than a decade, the tables were turned between the President's budget request and congressional appropriations. Standard protocol throughout the 1980s was a presidential budget request for NIH that just slightly exceeded the previous year's appropriations. Subsequently, Congress would add to the request, giving NIH an increase that would cover inflation plus a small amount more. Unlike previous years, however, the President requested a large increase in the NIH budget for 1993. Congressional appropriations were far below the President's request and barely kept the NIH budget ahead of inflation. As a result, new and competing awards were about 5,800 in 1991 and 6,000 in 1992. With extreme budget pressures on the federal government, it is not clear how the annual budget ritual will be played out over the next few years.
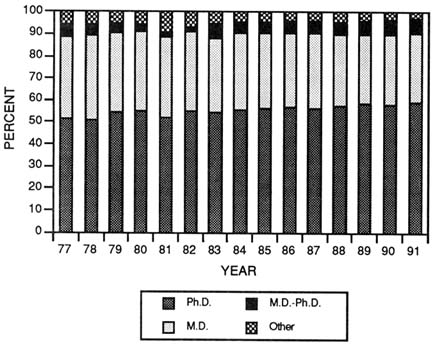
FIGURE 3-8 Degrees of principal investigators on traditional research project grant (R01) applications indicating the use of human materialsor human subjects. (Source: National Institutes of Health, Division of Research Grants.)
Setting Program Priorities Through Peer Review
Over the past two decades, much attention has been focused on the success rates of physician-scientists in peer review competition for NIH and ADAMHA research grants. The clinical research effort was measured by the number of clinicians (physician- or dentist-scientists) obtaining grant support in the 1950s and 1960s. More than 50 percent of all current research project grant applications for studies using human subjects or materials are led by a Ph.D. as the principal investigator (Figure 3-8), and thus, the proportion of applications from M.D. principal investigators is decreasing (Vaitukaitis, 1991). This imbalance is slightly offset by a growing proportion of principal investigators with M.D. and Ph.D. degrees.
Applications, Awards, and Success Rates Although annual awards for new and competing grants from NIH have hovered between 5,000 and 6,000 throughout the 1980s, the number of applications grew from 14,142 to 20,154
TABLE 3-1 Distribution of All NIH Grant Awards by Degree of the Principal Investigator for Selected Years from 1970 to 1987
between 1980 and 1990, far exceeding the ability of NIH to fund even a reasonable fraction (Figure 3-6).
In 1970 the fraction of grant awards to M.D. principal investigators was 36.7 percent, with 51.3 percent going to Ph.D.s and 5.9 percent going to M.D.-Ph.D.s. Although the number of awards to M.D.s increased (along with the overall number of NIH grants), the fraction to M.D.s declined to 26.2 percent and the fraction to M.D.-Ph.D.s declined to 3.7 percent by 1987. There has been a concomitant rise in the number of awards to Ph.D.s who garner about two thirds of grant awards (Table 3-1).
The committee examined the success rates among the three groups for all research grants and for those involving human subjects or materials. In neither comparison was there an appreciable difference in success rates among the groups. Thus, perceived differences in the quality of grants among the various groups are not substantiated, even when the proposal involves research on humans or human materials (Figure 3-9).
Costs of Human Studies The costs of performing clinical research are higher than those for performing preclinical research (Kimes et al., 1991). Whatever the reasons, grant awards that indicate the use of human materials or human subjects are consistently larger than other grants (Figure 3-10).
Peer Review More than 2,000 scientists are involved in the NIH peer review system (National Institutes of Health, 1992b). This unique system, in which nongovernment scientists are entrusted with public monies for distribution
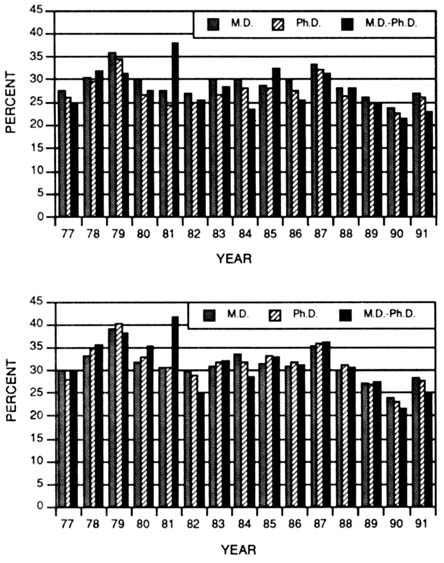
FIGURE 3-9 Success rates of M.D.s, PH.D.s and M.D.-Ph.D.s for competing traditional research project (R01) grant applications indicatingthe use of human materials (top panel) or human subjects compared to those not usinghuman materials or subjects (bottom panel) from 1977 to 1991. (Source:National Institutes of Health, Division of Research Grants.)
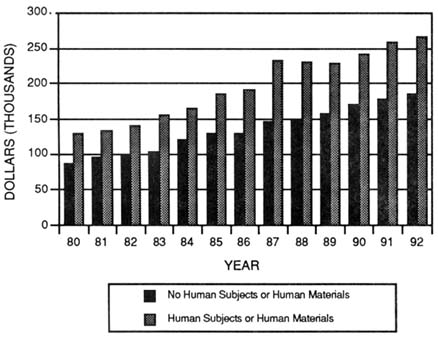
FIGURE 3-10 Average award size of NIH research project grants comparing awards for studies using human materials or human subjects with thoseawards for studies not using human materials or subjects. (Source: NationalInstitutes of Health, Division of Research Grants.)
to their colleagues for the pursuit of scientific knowledge, is considered the best in the world. The committee believes that these scientists should be commended for giving their time to support a system built on public trust. Nevertheless, many concerns have been aired about peer review of grant applications for both preclinical and clinical research.
Studies involving patients pose special methodologic challenges that are not encountered in laboratory bench research. In bench research, the subjects of the experiment are selected to ensure that they are virtually identical. In clinical research, by contrast, the populations under study, even in studies involving identical twins, are never truly identical. Whereas in bench research experiments are conducted in such a manner that everything other than the experimental maneuver is applied to the control group, many aspects of clinical research studies cannot be controlled. Moreover, there are fewer constraints on how the results from bench studies can be assessed (i.e., isolation of cellular material, euthanasia of animals, ex vivo studies). In clinical studies, the outcomes must be assessed
in whole patients who agree to participate, and most importantly, the process of measurement must do no harm. Therefore, human research appears to be less scientific to those accustomed to bench research.
The potential consequence of a bias against clinical research is a less favorable review for studies involving patients when compared with bench studies. Since only 20 percent of all initial review group members are physicians familiar with the care of patients, this is of particular concern (National Institutes of Health, 1992b). These concerns have arisen, in part, because of a lack of specific guidelines for grant application referral or assignment to study sections, lack of guidelines for study section administrators (i.e., different study sections employ widely differing strategies), and the lack of oversight of any of the review process and constitution of study sections.
Several reviews of the peer review system have been performed (the most recent in 1991), but the problems mentioned have not been resolved to everyone's satisfaction (National Institutes of Health, 1992c,d). Although the committee could not undertake an analysis of the competency of reviewers for assessing patient-oriented research, it is clear that the number of M.D.s on study sections is very low (U.S. Department of Health and Human Services, Public Health Service, 1986, 1992b). It is also perceived that the M.D.s who are on study sections are oriented more toward basic science than clinical research. Whether this is a cause of inadequate review cannot be substantiated. Furthermore, the number of women and minorities on review panels is not representative of their participation rates in the scientific community, and very few from these groups are M.D.s (National Institutes of Health, 1992b).
The study sections were originally constituted along the lines of scientific disciplines. With minor exceptions, the study sections are suitably constituted to review basic science grant applications. Few, however, focus specifically on human biology or studies involving human subjects. Concern has been expressed that this put clinical research at a disadvantage in the review system. The only data available on the distribution of priority scores for grant applications reveal only minor differences between nonhuman research and research indicating the use of humans or human materials (Figure 3-11). However, NIH has no means of separating applications proposing the use of human materials from those directly involving human subjects. Furthermore, since the content of grant applications is confidential, the committee was unable to perform its own analysis on grant applications. Nonetheless, it is believed that the perception of a bias may have influenced investigators to withhold applications.
Funding Clinical Research
The preceding discussion addressed concerns about all extramural funding by NIH (and previously ADAMHA). The committee is expressly concerned about
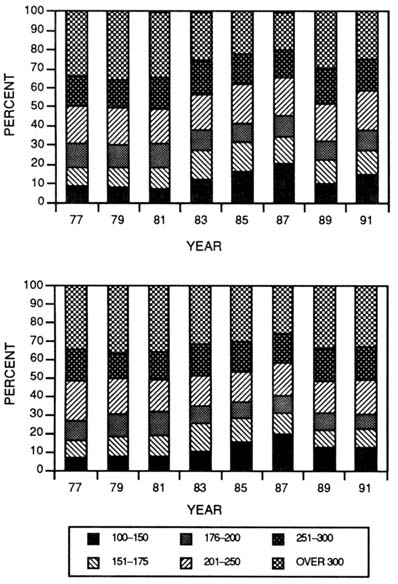
FIGURE 3-11 Distribution of priority scores for traditional research project grant (R01) applications comparing those indicating the use of human materials or subjects (top panel) with those applications not indicating use of humans or human materials (bottom panel). (Source: National Institutes of Health, Division of Research Grants.)
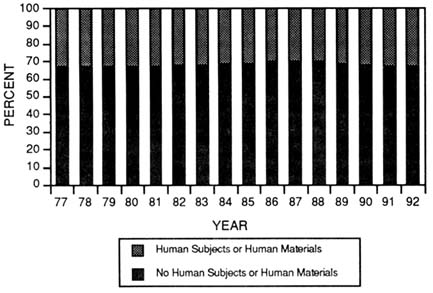
FIGURE 3-12 Distribution of NIH competing traditional research project grant (R01) applications comparing those indicating the use of human materials or subjects with those applications not indicating use of humans or human materials. (Source: National Institutes of Health, Division of Research Grants.)
the fraction of extramural research funds allocated for clinical research, more specifically, funds for patient-oriented clinical research.
Almost every inventory of clinical research supported by NIH in the past has been troubled by ambiguity about what clinical research is, who is performing it, and how much funding is provided (Ahrens, 1992; Institute of Medicine, 1988a; Wyngaarden, 1986). Earlier studies have relied on either the fraction of grants requiring institutional review board (IRB) approval or the number of principal investigators with clinical degrees (M.D., D.D.S., D.O., and the like) who win grant awards. Some analyses cross-link these two measures to arrive at an estimate of clinical research. Although these estimates can be used as surrogate measures of clinical research activities, the committee was concerned that such measures do not accurately portray the amount of clinical research activity that directly involves interactions with human subjects. As indicated in Chapter 1, clinical research can have various meanings to different audiences. The committee agrees with a broad definition encompassing a wide spectrum of research activities, but elected to focus on career pathways leading to patient-oriented clinical research. The next section explores these measures of clinical
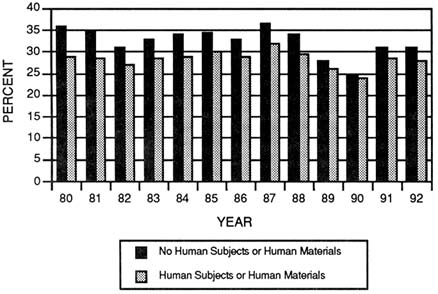
FIGURE 3-13 Success rates of traditional research project grant (R01) applications comparing those indicating the use of human materials or subjects with those applications not indicating use of humans or human materials. (Source: National Institutes of Health, Division of Research Grants.)
research and presents the committee's analysis of a sample of R01 grants indicating use of human subjects or materials.
IMPAC Data
As indicated previously, the number of new and competing research project grant applications submitted to NIH grew from 14,142 in 1980 to 20,154 in 1990. Throughout this period, the fraction of grant applications indicating that the studies intended to use human subjects or materials has remained remarkably constant, at about one third (Figure 3-12) (Vaitukaitis, 1991). However, this is based on the human subject box on PHS grant application form number 398, which includes both proposals for studies that actually involve human subjects and proposals that are exempt under IRB rules for research on human materials such as body fluids, pathological specimens, or certain observational human studies. Nonetheless, the trends in the applications are useful.
Over the past decade, the trend in award rates for grant applications involving humans have been paralleled those grant applications not involving
humans, but have been a couple of percentage points lower (Figure 3-13). When the applications are divided by degree of the principal investigator, M.D.s have a slightly higher success rate than Ph.D.s for studies involving both human and nonhuman subjects (Figures 3-14 and 3-15). However, grant applications from M.D.s for studies not involving humans have a slightly better success rate than grant applications for studies involving humans (Figure 3-16). Again, these data include all IRB reviewed clinical research.
OMAR Data
After a hiatus of several years, the NIH Office of Medical Applications of Research (OMAR) reestablished a centralized inventory of NIH-supported clinical studies in 1985 (National Institutes of Health, 1992a). This provided a single source of information on clinical studies, partially in response to the reporting requirements of the Stevenson-Wydler Technology Transfer Act of 1980. Thus, OMAR collects data from the individual institutes through the representatives of the Coordinating Committee on Assessment and Transfer of Technology at the end of each fiscal year. The working definition for their data collection is the following:
A clinical study is a research study undertaken with nine or more human subjects to evaluate prospectively the diagnostic/prophylactic/therapeutic effect of an intervention (drug, device, regimen, or procedure) used or intended ultimately for use in the practice of medicine or the prevention of disease. The term ''clinical study" does not include registries, epidemiological surveys, or epidemiological studies conducted retrospectively (National Institutes of Health, 1992a).
More details about the data collection and analysis are provided in OMAR's annual reports. The committee felt that it was instructive to show the tabulation of OMAR data and recap the significant findings (Table 3-2). From 1986 and 1987 the number of clinical studies supported by all institutes (except the National Cancer Institute [NCI] and the Division of Research Resources [DRR]) grew by nearly 11 percent, from 1,133 to 1,272. At the same time, OMAR reported that funding for clinical studies grew by more than 27 percent, from $381 million to $501 million. Unfortunately, the OMAR data are not current, and longitudinal comparisons are difficult to unravel.
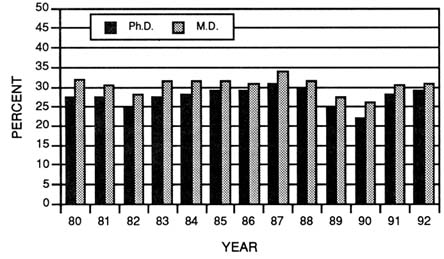
FIGURE 3-14 Success rates of traditional research project grant (R01) applications for those studies indicating the use of human materials or subjects for M.D.s and Ph.D.s. (Source: National Institutes of Health, Division of Research Grants.)
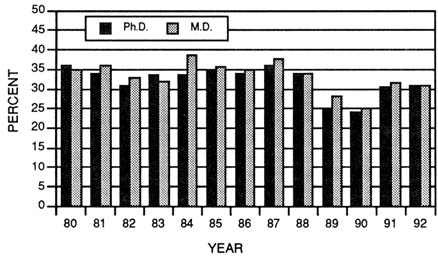
FIGURE 3-15 Success rates of traditional research project grant (R01) applications for those studies not indicating the use of human materials or subjects for M.D.s and Ph.D.s. (Source: National Institutes of Health, Division of Research Grants.)
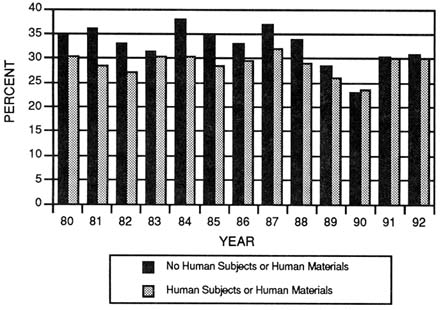
FIGURE 3-16 Success rates of traditional research project grant (R01) applications by M.D.s comparing studies not indicating the use of human materials or subjects with those that use human materials or human subjects. (Source: National Institutes of Health, Division of Research Grants.)
Ahrens' Analysis
Ahrens reported on his longitudinal analysis of abstracts from a random sample of 557 R01 grant awards selected from the years 1977, 1982, and 1987 (Ahrens, 1992). His classification scheme included six separate categories of clinical research in addition to nonclinical research. He concluded that nonclinical research declined from 49 percent of the 1977 sample to about 43 percent of the 1987 sample. The sample sizes, however, were small (less than 2 percent of all R01s for each year). Moreover, abstracts of grant applications are often not representative of the entire application, nor reflective of the work actually performed.
CRISP Data
NIH maintains an information system on Computerized Retrieval of Information on Research Projects (CRISP), which is supported by PHS. As can
TABLE 3-2 NIH Support for Human Studies for Fiscal Years 1985–1987 as Reported by the NIH Office of Medical Applications for Research.
|
Item |
FY 1985 |
FY 1986 |
FY 1987 |
|
Number of studies with supporta |
1,112 |
1,133 |
1,272 |
|
Total obligations for fiscal year |
|
|
|
|
• NIH subtotala |
$243,627,000 |
$251,661,000 |
$345,790,000 |
|
• NCI |
$117,470,428 |
$107,430,356 |
$128,391,447 |
|
• NCRRb |
$15,969,811 |
$21,560,887 |
$26,735,913 |
|
• Total |
$377,067,239 |
$380,652,243 |
$500,917,360 |
|
Average cost per study each yeara |
$219,089 |
$222,119 |
$271,847 |
|
Total number of patients, all studies (projected)a |
445,397 |
493,279 |
562,165 |
|
417 |
477 |
431 |
|
|
$615 |
$555 |
$744 |
|
|
a Excluding the National Cancer Institute (NCI) and the National Center for Research Resources (NCRR). b The National Center for Research Resources was formerly the Division of Research Resources. c Excludes a single study with a sample size in excess of 100,000. Source: National Institutes of Health, 1990. |
|||
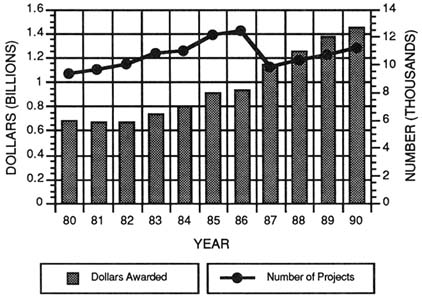
FIGURE 3-17 Results of a search of the Computerized Retrieval of Information on Research Projects (CRISP) system linking the term "clinical" with "human" showing the number of projects and subprojects (on P01s) and the dollars awarded, 1980-1990. (Source: National Institutes of Health, Division of Research Grants.)
be expected, most of the research projects are from NIH (and formerly ADAMHA) including some information on intramural research. Abstracts for each research project are entered into the database with data elements on funding, awarding institute, awardee, awardee's institution, and so forth. Author abstracts are used when possible; otherwise, abstracts are prepared by writers. CRISP files are indexed and search headings are established to be similar to those used for MEDLINE.
Thus, in an attempt to find another measure to determine the level of support for patient-oriented clinical research, the committee asked NIH staff to perform a search linking the terms human with clinical on research supported by NIH from 1980 to 1990. The results of that analysis are shown in Figure 3-17. These data show that support for human or clinical research grew from $682 million in 1980 to $1,458 million in 1990. When adjusted for inflation, this represents 19 percent real growth. Over the same period, the number of projects and subprojects (from P01s) increased from 9,370 to 11,127.
The CRISP database uses research project abstracts to codify the research. Primary, secondary, and tertiary key words for each abstract are entered by NIH staff—not by the grant author. Thus, the search strategy is based on subjective
coding of research projects. When abstracts are missing, they are prepared by writers. Moreover, abstracts frequently provide too little information to determine the actual scope of a research project, and there is no apparent avenue for recording a change in scope by the investigator in the database. Finally, the award amounts entered into the CRISP system are the initial awards by the institute and are not corrected for administrative adjustments including downward negotiation.
Committee's Analysis
The committee recognized the extreme variability and ambiguity in the aforementioned analyses or measures of clinical research. Since the committee focused on patient-oriented research, it wanted to ascertain what fraction of NIH grants that indicated the use of human subjects or materials were actually for patient-oriented clinical research. Because this data element is not captured in any present database, the committee developed a strategy to categorize a random sample of R01 grant awards. Because the committee felt that grant abstracts were unreliable, it chose to perform the analysis using the actual grant files from each of 11 institutes. Data from the National Institute of Dental Research and the National Center for Nursing Research were considered by their respective task forces and were not included in the analysis. Six grants from the National Institute of Environmental Health Sciences were excluded because their files are retained in Research Triangle, North Carolina. Also, since the study was coordinated through the Office of the Deputy Director for Extramural Research at NIH and began in early 1992, the former ADAMHA institutes were not included. Nonetheless, the committee believes that the sample was representative of investigator-initiated grants.
Of the 16,313 R01s that were active in fiscal year 1991, 14,535 were reviewed by IRGs in the Division of Research Grants. Of the latter, in 4,284 or about 30 percent, the human studies box was checked (referred to from now on as IRB positive). The committee estimated that a sample size of approximately 10 percent, or 430 grants, for studies involving human subjects or materials would be sufficient to estimate the fraction of grant awards for studies that actually involve interaction with human subjects.
There were 114 regular and ad hoc IRGs that reviewed IRB-positive grants that year. Many of these IRGs infrequently review grant applications for studies involving humans, that is, less than 25 percent of grant awards resulting from a respective study section review are IRB positive. At the same time, some IRGs frequently review grant applications for studies involving humans. Since a straight random sample across all IRGs would raise the possibility that the sample could be drawn from study sections that only occasionally review IRB-positive grants, or, more likely, from IRGs that review mostly IRB-positive grants, the committee developed a sampling strategy to ensure that IRGs with
TABLE 3-3 Grouping of 1991 R01 Grant Awards Reviewed by NIH Division of Research Grants Initial Review Groups (IRGs) by Prevalence of Institutional Review Board (IRB) Indicator
|
Quartile |
Prevalence of IRB Positivity (%) |
Number of Study Sections |
Number of R01 Awards |
Number of IRB+ R01s |
Average Proportion IRB+ (%) |
|
First Second Third Fourth |
0-25 26-50 51-75 76-100 |
51 40 11 12 |
7,836 4,596 1,227 876 |
875 1,825 760 824 |
11 39 62 94 |
|
Total |
|
114 |
14,535 |
4,284 |
29 |
|
Source: National Institutes of Health, Division of Research Grants. |
|||||
both high and low numbers of IRB-positive grants were evaluated. Thus, the IRGs were placed into four quartiles as shown in Table 3-3. The denominator of interest for this analysis was the 4,284 grants that indicated the use of human subjects or materials. To ensure that the sample represented IRGs that reviewed many IRB-positive grants as well as those that reviewed few IRB-positive grants, a random sample of 100 grants was selected from the first and fourth quartiles. The second and third quartiles were combined, and a sample of 250 grants was selected. Thus, the overall sample was 450, or slightly more than 10 percent of all IRB-positive R01 awards. Because of missing files or unavailable data, 446 grants (10.4 percent of the 4,284 human grants) were actually reviewed. The sample also included representative grants from each of 11 institutes. Table 3-4 shows the target number for review in each group and the actual number read.
Rather than develop an elaborate scheme for classifying the grants, the committee sought to simplify the strategy by using the following categories of research:
-
Fundamental research seeks to answer fundamental questions about the nature of biology through a broad range of basic and clinical research. Most of these studies involve nonhuman materials, although some may involve human materials. Any of these studies may eventually lead to major improvements in the prevention or cure of disease, but for the purpose of this analysis the committee sought to distinguish fundamental research from the two categories described below.
-
Human research is the portion of clinical research in which patients serve directly as the research subjects, often referred to
-
as patient-oriented or patient-related research. For example, this category of clinical research includes research activities such as the characterization of normal and diseased human function; evaluation of new diagnostic, therapeutic, or prognostic techniques, approaches, and devices; evaluation of existent practices or technology in standard practice; and phase I-IV drug trials. Thus, this category of research activity has direct application to the prevention, diagnosis, treatment, or cure of disease in the individual or group of individuals under study; rehabilitation (including quality of life issues) of the patient; or study of human pathophysiology. Furthermore, it involves direct, "hands-on" evaluation of the human subject.
-
Epidemiologic research investigates the circumstances under which disease occurs in populations. It seeks factors that cause disease such as environmental exposures, personal habits, genes, viruses, and the like. Epidemiologic studies both describe the distribution of disease in populations (rates over time and between places) and analyze disease risk determinants. Such research is the source of many ideas about the causes of disease, factors that determine high risk for development of disease, and methods to promote the prevention or control of disease.
Using these categories, the committee sought to unravel the ambiguity of the grant categorization process that lumps all human research together. Thus, this scheme allowed the committee to focus on that portion of clinical research—true human research—that was the central theme of this study.
With the cooperation of the grants managers in each institute, the complete grant files were obtained and available for the analysis. The data were collected in a manner that kept all personal identifiers confidential. The grants were classified according to the three categories of research listed above. Because many grants may have components of human research combined with other experiments, it was necessary to estimate the proportion of effort and funding committed to each category in increments of 10 percent. The committee recognized the potential pitfalls of subjectively estimating percent effort when two or more categories of research were involved.
The results of the analysis are shown in Tables 3-4 and 3-5. Interestingly, 186, or 41.6 percent, of these grants were for fundamental research, as described above in the first category—fundamental research. Of these, 46 did not involve human subjects or materials at all, and another 85 had more or equal amounts of nonhuman research than human materials research. Of the 227, or about 50.8 percent, that involved some human research, 161 were classified as category 2 (human subject research) and 66 were combined fundamental and human research. The remaining 33 grants were in epidemiology. If these data are representative of the entire 4,284 grants for studies involving human research, then 2,180 grants or
TABLE 3-4 Results of Classification of IRB-positive R01 Awards Reviewed by NIH Division of Research Grants Initial Review Groups (IRGs)
|
|
Number of Awards |
|
|
|
|
|
|
Quartile |
Fundamental Research |
Human Research |
Fundamental and Human Research |
Epidemiology |
Target Number |
Total Number |
|
First |
65 |
18 |
12 |
0 |
100 |
95 |
|
Second and third |
119 |
78 |
51 |
4 |
250 |
252 |
|
Fourth |
2 |
65 |
3 |
29 |
100 |
99 |
|
Total |
186 |
161 |
66 |
33 |
450 |
446 |
|
Source: National Institutes of Health, Division of Research Grants. |
||||||
TABLE 3-5 Proportion of Each Quartile Composed of IRB-positive Grant Awards
|
Quartile |
Fundamental Research |
Human Research |
Fundamental and Human Research |
Epidemiology |
Total Number |
|
First |
0.684 |
0.189 |
0.126 |
0 |
0.999 |
|
Second and third |
0.472 |
0.309 |
0.202 |
0.015 |
0.998 |
|
Fourth |
0.020 |
0.656 |
0.030 |
0.292 |
0.998 |
|
Source: National Institutes of Health, Division of Research Grants. |
|||||
about 51 percent of the IRB-positive awards would involve interactions with human subjects.
Next, the proportions of each category of research were determined for each group to derive the stratum-specific proportions that are presented in Table 3-6. Multiplication of these proportions by the number of awards that are IRB positive in each stratum gave an estimate of overall human research in relation to the denominator of 14,535. Thus, the committee concluded that 1,504, or 10.4 percent, of the 14,535 R01 grants active in 1991 were purely for studies involving human subjects; an additional 657, or 4.5 percent, had combined fundamental (human and nonhuman) and human subject research; and less than 2 percent involved human epidemiology. To extrapolate these findings, roughly 84 percent of the R01 grant awards support nonhuman research.
TABLE 3-6 Estimation of the Total Number of Patient-Oriented R01 Research Grant Awards from IRB-Positive Data
|
Quartile |
Fundamental Research |
Human Research |
Fundamental and Human Research |
Epidemiology |
Total Number |
|
First |
599 |
165 |
110 |
0 |
875 |
|
Second and third |
1,220 |
799 |
522 |
38 |
2,579 |
|
Fourth |
16 |
540 |
25 |
241 |
822 |
|
Total |
1,836 |
1,504 |
657 |
279 |
4,275 |
|
Source: National Institutes of Health, Division of Research Grants. |
|||||
The committee is fully aware of the potential pitfalls in this type of analysis. For example, it could be argued that most of the R01 grants for studies involving human subject are reviewed by panels convened by the respective institute rather than IRGs in the Division of Research Grants. Indeed, about 1,800 R01 grants active in 1991 were reviewed by IRGs convened by the institutes; nearly 800 R01 grant awardees indicated the use of human subjects or materials. Because institute review panels are often convened to review grant applications submitted in response to requests for applications, the committee felt that they were not a representative sample of unsolicited, investigator-initiated grant proposals. Another potential gap is human research that is supported by program project awards (P01). It is believed that these large, multifaceted projects frequently include a human research component. Many of these proposals are reviewed by institute review groups as well. The committee did not have the time or the resources to analyze these awards. Program projects, however, are only a small portion of the extramural research budget compared with the R01 portion. Although the committee cannot draw conclusions from these data on the total amount of human research funded by NIH, this exercise demonstrated that the present classification is not useful for accurately determining the fraction that is truly human research. Lastly, this analysis was performed only on grant awards. As mentioned above, analysis of grant applications is not possible, but Cuca has reported on the bias of getting clinical research grants funded through the peer review system (Cuca, 1983; Cuca and McLoughlin, 1987), and Friereich (1990) and Friedman et al. (1991) have examined similar problems specific to cancer research.
R&D Centers
NIH supports nearly 600 centers designed to consolidate related research efforts and resources into a single administrative and programmatic structure. About 100 of these are special resource centers for animals or biotechnology resources. The remaining 500 are specialized centers (P50), center core grants (P30), comprehensive centers (P60), and general clinical research centers (GCRCs) (M01).
Centers, whether they are funded by NIH or an institution's own funds, can serve as vital institutional resources for multidisciplinary research. The funds provided through grants to centers from NIH are to be used for salaries of key staff, operation of shared resources and services, and center administration. These funds also may be used to recruit new talent to the center, to fund investigators who previously have not obtained competitive peer-reviewed federal funding, to provide interim research support for center investigators, and to obtain new shared resources. Although the committee did not perform a detailed analysis of the NIH program for centers, they commissioned Charles Pak of the University of Texas Health Sciences Center to write a paper on the value of the GCRC program, particularly its potential role in training patient-oriented investigators (Pak, 1994), and drew from the 1989 IOM report on the NCI cancer centers program (Institute of Medicine, 1989a).
General Clinical Research Centers The GCRC program, begun in 1959, was designed to support a clinical research infrastructure located within academic medical institutions around the country. Thus, the program was perceived as an extension of the Warren Grant Magnuson Clinical Center located on the NIH campus in Bethesda, Maryland. To this end, a typical GCRC is rather like a miniclinical center that occupies an area in a hospital through a contractual agreement (Ross, 1985). Unlike the Magnuson Clinical Center, which is organized as a collection of the individual institutes' clinical research arms to reflect their own disease orientation, the GCRCs were intended to have a general research orientation that cuts across disciplinary lines and serves all departments.
The goals of the program are the following: (1) to make available to medical scientists the resources that are necessary for the conduct of clinical research; (2) to provide an environment for studies of normal and abnormal body functions and for investigations of the cause, progression, prevention, control, and cure of human disease; (3) to provide an optimum setting for controlled clinical investigations; (4) to encourage collaboration among basic and clinical scientists, to encourage, develop, and maintain a national corps of expert clinical investigators; (5) to serve as an environment for training other health professionals in clinical research; and (6) to provide resources in which advances in basic knowledge can be translated into new or improved methods for patient
care (U.S. Department of Health and Human Services, Public Health Service, 1991a).
Although commonly a designated part of a hospital, each GCRC is designed to support areas within academic medical centers dedicated to patient-related research. These centers can be composed of specialized inpatient and outpatient facilities, laboratories and equipment, and mainframe computers, and the facilities are staffed by specialized personnel, such as biostatisticians, computer systems managers, research nurses and dieticians, and research laboratory technicians. For example, an inpatient clinical research center is a self-contained unit with its own research beds, administration, nursing staff, laboratory, metabolic kitchen, and computerized data analysis facility. Outpatient units are commonly contiguous to the inpatient facility and are becoming an important complement to the center, just as large segments of the medical profession are moving toward ambulatory care.
Center funding is provided through a competitive grant program by the National Center for Research Resources. The principal investigator named on the grant is usually a dean, thus cutting across departmental affiliations. The program director, however, is responsible for administering the grant (even writing the grant proposal) and the day-to-day management of the center, including supervision of the center-supported staff and facilities. The program director is supported by an advisory committee that reviews proposed research protocols for use of the center. Although the GCRC grant supports the research infrastructure for center studies such as room and board for subjects, nursing, and some laboratory support, individual investigators are responsible for securing funding for specialized procedures or their own research.
One estimate suggests that nearly 5,000 research projects involving as many as 7,000 investigators are currently under way in GCRCs (Pak, 1994). The range of topics, in decreasing order of number of projects, include endocrinology, maternal and child health, immunology, cardiovascular disease, diabetes, gastroenterology, cancer, kidney disease, genetics, aging, hypertension, and arthritis.
The program was initiated in 1959, and the first eight centers with 133 patient beds were established in 1960. The number of GCRCs and patient beds grew rapidly, reaching a peak of 1,137 beds in 1967 and 93 centers in 1969 and 1970 (Ahrens, 1992). Through the 1970s the number of centers dropped to 75, and the number of beds declined to 600. The GCRC no longer funds centers in terms of patient beds; rather, funding is based on inpatient bed-days and outpatient visits. There are currently 74 centers nationwide supporting about 130,000 inpatient bed-days and 200,000 outpatient visits.
Although funding for the GCRCs increased from $103 million in 1986 to $127 million in 1992, the program did not realize an increase in 1993. Moreover, much of the growth over the past few years can be attributed to increases in funding for human immunodeficiency virus (HIV) research, which
accounted for $13 million in 1986 and grew to $24 million in 1992. The non-AIDS portion of the GCRCs has thus not kept up with inflation.
The committee is concerned about the future of the GCRCs because they are logical sites for bridging what many believe is a widening gap between laboratory research and human studies. The GCRCs represent a nationwide resource that could be used to increase the number of scientific advances that are translated to the bedside, as well as to continue to advance the understanding of human pathophysiology. Furthermore, GCRCs have supported a Clinical Associates Program (CAP) for several years. To expand this training function, the centers might serve as unique sites for mounting a training program for medical students and residents who choose to perform patient-oriented research (the CAP program will be covered thoroughly in Chapter 4, on clinical research training). GCRCs might also serve as an important interface between industry and academia, and these attributes will be discussed in Chapter 5.
Other Centers Many of the individual institutes support specialized or comprehensive centers such as NCI's cancer centers and the multiarthritis centers of the National Institute of Arthitis and Musculoskeletal and Skin Diseases. This committee did not assess each institute's portfolio of centers, nor did it make a judgment of their value. Much controversy has surrounded the support of centers over the past several years, in part because of the difficulties in obtaining individual investigator-initiated grants. Although this committee also places the highest value on investigator-initiated grants, it also believes that the conduct of human research requires infrastructure and resources that can be efficiently provided through centers. Understandably, funding for centers that have become obsolete or unproductive should be terminated, but new ones can be devised to meet new research challenges.
Centers for Disease Control and Prevention
The mission of the Centers for Disease Control and Prevention (CDC) is to assist state and local health authorities and other health-related organizations decrease the spread of communicable diseases, protect the public from other diseases or conditions amenable to reductions, provide protection from certain environmental hazards, improve occupational safety and health, and disease prevention. CDC is also responsible for licensing clinical laboratories engaged in interstate commerce, conducting foreign quarantine activities aimed at preventing the introduction of disease into the United States, and developing scientific criteria for occupational health hazards. About nine tenths of CDC's budget is allocated to the nonresearch portion of its mission, predominantly through block grants to states.
Of the $982 million appropriated to CDC in fiscal year 1989, only about 10 percent ($100.6 million) was obligated for health research. In constant 1988 dollars, research funds at CDC grew from $56.6 million to $95.5 million between 1984 and 1989. Increases were greatest in fiscal years 1987 and 1988, when research funds grew by 18.8 and 26.8 percent, respectively, in constant dollars. These increases coincided directly with the increasing national emphasis on research into HIV infection.
The National Institute of Occupational Safety and Health (NIOSH) is a research arm of CDC. NIOSH conducts research; develops criteria for occupational safety and health standards; and provides technical services to government, labor, and industry, including training in the recognition, avoidance, and prevention of unsafe or unhealthful working conditions and the proper use of adequate safety and health equipment. Through these activities, NIOSH tries to reduce the high economic and social costs associated with occupational illness and injury. Obligations for research funded by NIOSH grew only slightly between 1984 and 1987, and they declined in the following two years. Of the $70.4 million appropriated to NIOSH for fiscal year 1989, $24.7 million was committed for research, and about $10.1 million was obligated for training.
CDC has been a leader in the nation's efforts to prevent and control the spread of HIV infection, managing a comprehensive HIV prevention program that includes surveillance; epidemiologic and laboratory studies; and prevention through information, education, and risk reduction. Appropriations for AIDS activities for fiscal year 1989 were $382.3 million—39 percent of the CDC budget. The research portion of this allocation was $44.6 million, for epidemiologic and laboratory studies to determine the natural history of the disease and to gain more knowledge about the transmission of HIV. Research funds allocated to other parts of CDC have grown much faster than those to NIOSH
Another part of the CDC, the National Center for Health Statistics (NCHS), is responsible for collecting, maintaining, analyzing, and disseminating statistics on the health, illness, and disability of the U.S. population and the effects of these factors on the U.S. economy. Although this function is not classified as research, there is a large component of epidemiologic studies for the development of databases. NCHS also is responsible for collecting nonhealth data on the numbers of births, deaths, marriages, and divorces in the United States. For fiscal year 1989, $49 million was appropriated to NCHS.
Agency for Health Care Policy and Research
The Agency for Health Care Policy and Research (AHCPR) was established in 1989 as a focal point for health services research in the PHS. Whereas its predecessor, the National Center for Health Services Research, focused on general
health services research, which is the study of the organization, structure, and financing of health care, AHCPR also had a mandate to develop and support research on the quality, appropriateness, and relative effectiveness of clinical intervention. The need, as expressed by Congress, was to reduce inappropriate variations in practice; to reduce, where possible, the uncertainty and lack of information often faced by clinicians and physicians; and, most important, to empower the patient to be a more informed participant in the decision making process (Agency for Health Care Policy and Research, 1992). AHCPR is also in the forefront of developing the field of primary care research (U.S. Department of Health and Human Services, Public Health Service, 1991c, 1991d). As this country attempts to shift the emphasis of medicine to primary care and produce primary care physicians, a sound scientific primary care research base will be necessary. Although the research portfolio of AHCPR spans a broad spectrum of health services research, the committee focused on the portion that involves patient interactions that lead to improved medical practice.
AHCPR's Medical Treatment Effectiveness Program (MEDTEP) seeks to improve the effectiveness and appropriateness of health care through improved understanding of outcomes and alternative interventions (U.S. Department of Health and Human Services, Public Health Service, 1991b). Clinical management of a given condition can be quite variable throughout the country. The outcomes of the available strategies are often equally variable. Thus, MEDTEP is a multifaceted program composed of the following four interrelated components designed to assess the relative effectiveness of alternative strategies for treating common clinical conditions:
-
the development of databases;
-
the conduct and support of research on outcomes, effectiveness, and appropriateness of health care services and procedures;
-
the development of clinical practice guidelines; and
-
the dissemination, assimilation, and evaluation of research findings and clinical practice guidelines.
Although all these areas are vital to improving health care, the committee focused on the second area—the conduct and support of research on outcomes, effectiveness, and appropriateness of health care services and procedures. One of MEDTEP's unique contributions is the focus on common conditions; its relevance to all patients with a given condition (including those with comorbidities) and all providers caring for these patients; and its broad definition of clinical success, which includes symptom relief, quality of life, functional status, patient satisfaction, and costs (Agency for Health Care Policy and Research, 1992).
Although earlier studies analyzed large claims databases to understand differences in the clinical management of given conditions, MEDTEP supports a
small number of clinical effectiveness trials on selected conditions identified by Patient Outcomes Research Teams (PORTs). Unlike the efficacy trials commonly supported by NIH and the pharmaceutical industry, these effectiveness trials will focus on clinical outcomes that occur under ordinary conditions. Each of the PORT projects includes an elaborate five-year program encompassing synthesis of the research on a condition (using meta-analysis), acquisition and analysis of primary and secondary data, development of clinical recommendations, dissemination of findings, and evaluation of the effects of the findings on clinical practice. A sample of PORTs already under way include prostate disease, low back pain, acute myocardial infarction, cataracts, total knee replacement, ischemic heart disease, biliary tract disease, pneumonia, type II diabetes, hip fracture and replacement, prevention of stroke, and delivery by cesarean section and other obstetrical procedures. Clinicians trained in outcomes or effectiveness methodologies are critical to the success of the PORTs. Of the 12 PORTs, 10 are run by clinicians, 1 is run by an economist, and 1 is run by a mathematical statistician. Eventually, AHCPR will support Medical Treatment Effectiveness Research Centers on Minority Populations and a pharmaceutical outcomes program. There are also opportunities for cross-agency collaboration. For example, AHCPR supplemented a clinical trial on otitis media funded primarily by the National Institute of Child and Human Development to collect extra data on the quality-of-life dimensions to the research as well as to track patients not accepted into the trial.
AHCPR had a budget of $120 million in fiscal year 1992. The MEDTEP line accounted for approximately $67 million of the total. Of this, $44 million was allocated for grant and contract research, including interagency agreements with other Public Health Service components. The remainder of the $67 million supports the development of clinical practice guidelines, training through the National Research Service Award (NRSA), dissertation awards, and other programmatic functions of the agency (Raskin, 1992).
With respect to NRSA training, AHCPR's annual budget is relatively small, amounting to approximately $3 million when compared with NIH's $300 million annual training budget. These funds support about 95 individuals through either institutional or individual awards. Of those, about 42 are M.D.s in training; a significant percentage of this number are primary care physicians. AHCPR also supports approximately 20 to 25 predoctoral dissertation awards.
U.S. Department of Veterans Affairs
Historically, the U.S. Department of Veterans Affairs (VA), previously known as the Veterans Administration, has provided health care to veterans through a network of 172 hospitals and centers nationwide. Approximately 130 of these units have medical trainees and about 100 have formal agreements with
medical schools. The VA provides financial support for 8,350 residents and interns—nearly 13 percent of the trainees in the United States. In addition, Congress appropriates R&D funds to the VA to conduct studies pertaining to veterans' health or using veteran patient populations. The VA research program sponsors investigations across a broad spectrum of health research, including basic and clinical sciences, health services (outcomes, cost-effectiveness, and technology assessment), applied research, and rehabilitation and prosthetics (Smith, 1992a).
VA has several attributes that make it a good resource base for clinical research. First, patient recruitment for clinical investigations is easier for VA than for NIH. Second, the costs for the standard medical care portion of clinical investigations are charged to health care delivery funds rather than research dollars, so that only the marginal costs of the research consume research appropriations—a potential model for non-VA research as well. The clinical trials conducted by VA may have a far-reaching impact on research performed by other federal agencies. VA also is exploring ways to enhance its position as a resource base for clinical investigations by more open cooperation with private industry (Institute of Medicine, 1989b).
Recognizing this unique niche for conducting health research, the research management in the VA has developed a new mission statement that emphasizes clinical research, particularly clinically derived research as well as clinically relevant research:
To develop and conduct research representing a continuum of programs—biomedical research, health services research, and prosthetics and rehabilitation—which integrates the clinical needs and research inquiries to enhance the quality of health care delivery to veterans (Smith, 1992b).
The VA R&D budget is a separate line item in the federal budget and is divided into the following three major categories: (1) medical research, (2) rehabilitation research, and (3) health services R&D. In fiscal year 1993, VA was appropriated $232 million to support about 1,500 programs (Smith, 1992a). About 75 percent of the budget supports investigator-initiated biomedical research. The VA research budget, however, has not increased over the past decade when measured in constant dollars (Figure 3-18) (U.S. Department of Veterans Affairs, 1991). Flat budgets and growing research costs have negatively affected the numbers of projects that can be supported, particularly in the medical portion of the research budget (Figure 3-19) (Smith, 1992b; U.S. Department of Veterans Affairs, 1991). Nevertheless, over the past few years the rehabilitation research component has shown a gradual increase, and health services research has shown a tremendous surge in funding. Eight percent of the research budget is directed toward VA cooperative studies (multihospital clinical trials).
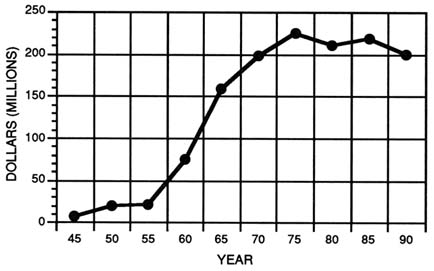
FIGURE 3-18 Appropriations for research in the U.S. Department of Veterans Affairs, 1945–1990. (Source: U.S. Department of Veterans Affairs, 1992)
Although the VA research budget is not very large when compared with that of NIH, it should be noted that salaries for clinical investigators, facility support, and so forth are derived from other portions of the VA budget. If those costs are added in, the budget might approach the equivalent of $500 million (Smith, 1992b). Moreover, if the NIH-funded research being performed in VA facilities is included, the VA research budget approaches the equivalent of $900 million. Although VA is able to leverage a significant amount of research with its modest budget, it is under the same budget pressures as other science agencies competing for scarce resources. For example, the average cost of an investigator-initiated grant in 1987 was $60,000; by 1992, the average cost had increased to $90,000. This growth reflects solely the increasing costs of performing research because salaries and overhead are not included.
All VA-sponsored research is conducted intramurally. About 80 percent of the investigators are clinicians who administer care to veterans at VA medical centers. Eligibility criteria in the VA research system require that applicants for an investigator-initiated grant must have a five-eighths appointment within the VA (Smith, 1992b). This requirement is an attempt to ensure that the investigators are clinicians who are involved in patient care.
Approximately 10 percent of the VA research budget is allocated for career development at all levels (Smith, 1992b). This includes limited salary support for some levels of training for young physician-investigators. Generally, salary
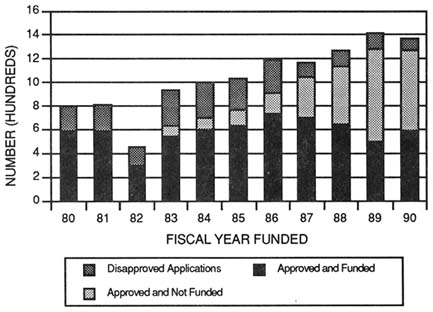
FIGURE 3-19 Disposition of competing research grant applications undergoing merit review for the U.S. Department of Veterans Affairs, 1980-1990. (Source: U.S. Department of Veterans Affairs, 1992)
support for established VA investigators is covered with nonresearch funds. To encourage careers in clinical research, VA is developing a career development program that will cover salaries and provide a small amount of research support. The salaries and positions are then transferred to individual VA hospitals. This program is available for the entire spectrum of clinician-investigators—from those directly out of their residency programs to very senior investigators.
Another new program under development emphasizes the collaboration between the VA research program and academic research through research fellowships. These fellowships would be available to postresidency physicians who would be eligible for fellowships supported by their academic institution, with a contribution from the VA research program of $3,000 to $10,000 a year.
The VA is very concerned that its research program is in jeopardy, however (Smith, 1992a, b). As a percentage of total VA appropriations, the research budget declined from 3.5 percent in 1970 to 1.5 percent in 1993. In the past two years, only budget transfers from the U.S. Department of Defense have kept the VA research budget ahead of inflation. VA believes its programs, particularly the career development program, are in a fragile funding situation. It appears that VA will not be able to fund any new programs at all—no new career development and
no new investigator-initiated awards—and only about a third of continuing competitive renewals will receive funding.
U.S. Department of Education
The National Institute on Disability and Rehabilitation Research (NIDRR) is part of the Office of Special Education and Rehabilitative Services in the U.S. Department of Education (U.S. Department of Education, Office of Special Education and Rehabilitative Services, 1991b). The Rehabilitation Research and Training Centers (RRTCs) are the largest program. Although much of the focus of the RRTCs is on vocational strategies, some support is provided to physicians and allied health professionals for research in rehabilitative medicine. The budget of the NIDRR was $65 million for fiscal year 1993.
U.S. Department of Defense
The U.S. Department of Defense (DOD) conducts health research vital to national security. Three branches conduct intramural and extramural health research: (1) the U.S. Army Medical Research and Development Command (USAMRDC), (2) the Directorate of Life Sciences in the Air Force Office of Scientific Research, and (3) the Life Sciences Programs Directorate of the Office of Naval Research (Institute of Medicine, 1990). While the three branches conduct a significant amount of health sciences research, there is no reliable estimate of how much is performed on human subjects.
Of the three branches, USAMRDC receives the largest allocation of DOD funds for military health sciences research—about 80 percent of the total DOD health sciences research budget. In fiscal year 1989, $252 million was appropriated. The USAMRDC conducts mission-oriented medical R&D designed to support the soldier in the field. This program supports research on increasing efficiency of soldiers by improving instrumentation and new medical knowledge in the following areas: (1) military disease hazards, including infectious diseases, biological warfare defense, and AIDS; (2) combat casualty care, including shock, wound healing, and craniofacial injuries; (3) medical chemical defense; and (4) army systems hazards.
The Directorate of Life Sciences in the Air Force Office of Scientific Research has a health-related research budget much smaller than that of USAMRDC. In 1989 allocations for health research were only $17.1 million. These funds support research in several areas of neuroscience, experimental psychology, toxicology, visual and auditory psychophysics, radiation biology, and cardiovascular physiology.
The Office of Naval Research funds health research through the Life Sciences Programs Directorate. In fiscal year 1989, $24.4 million was allocated to biological and medical sciences and $11.5 million was allocated to cognitive and neural sciences. The 1990 budget had only slight increases for the biological and medical sciences—to $25.3 million—and an increase $13.7 million for the cognitive and neural sciences.
Although the committee is aware that the armed services may not be heavily involved in medical research on human subjects, some research areas may fall into the purview of the armed services. For example, the armed services has supported human studies on vaccines and therapies for tropical diseases. New opportunities are becoming available for the armed services to expand their clinical studies in several areas; for example, funding was recently made available for breast cancer. The Surgical Task Force of this committee has also recommended that the military examine potential involvement in treatment strategies for trauma at established civilian trauma centers, which might expand the knowledge base for treating trauma received on the battlefield (see Appendix C).
NONPROFIT ORGANIZATIONS
Throughout the twentieth century, private nonprofit organizations have played a critical role in funding health sciences research. Many early private foundations were established to benefit particular institutions or to address specific social or health problems with assets generally derived from an individual's or a family's gifts. During the twentieth century, voluntary health agencies, which are also referred to as operating foundations, have proliferated. In addition, a special type of nonprofit organization—the medical research organization—has developed. Each type of organization differs in its mission, governance, and mechanisms of support. Although these organizations make up a limited portion of all health sciences research support, they are vital to the nation's medical research enterprise because of their flexibility and their dedication to curing human disease and suffering.
NIH estimated that private nonprofit organizations contributed about $1,196 million (or about 4.3 percent of the total), to health R&D in 1992 (U. S. Department of Health and Human Services, Public Health Service, 1992c). This figure, however, probably underestimates the role of philanthropy in health sciences research by excluding endowed professorships and donations for facilities and equipment. Another estimate has placed philanthropy at nearly one-quarter of a typical institution's budget for biomedical R&D (Boniface and Rimel, 1987).
Foundations
Although federal investment in health sciences research has eclipsed that of foundations since World War II, foundations still play a vital role in the research enterprise. Few foundations conduct in-house research, because most believe that support for extramural research and research infrastructure provides the most efficient use of funds. Private foundations currently provide a great variety of support mechanisms for health sciences research and use their resources to support new areas of investigation or to augment federal funding. Common categories of foundation support include individual research project grants, predoctoral and postdoctoral fellowships, equipment grants, payment of publication expenses, special library collections grants, and sponsorship of conferences or workshops. Although some foundations support research and research training for physicians and other health professionals, it is unclear how much is being invested in patient-oriented research or clinical research training. Nonetheless, foundations, have provided crucial support in filling gaps in the research agenda that have not been addressed appropriately or profitably by government or industry.
The mechanisms for setting priorities and making funding decisions vary among foundations (Institute of Medicine, 1990). In some instances, funding decisions are made through personal contacts or because of a foundation's interest in a specific disorder. Large, independent foundations may form advisory committees to determine areas of emphasis; proposals may also be subjected to a peer review process similar to that used by NIH. Smaller foundations may not plan program initiatives; instead, they may fund the best unsolicited proposals received in a given time period. The extent of foundation support for health sciences research varies from year to year, depending on the relative timing of costly initiatives.
Voluntary Health Agencies
Voluntary health agencies (VHAs), such as the American Cancer Society and the American Heart Association, play critically important roles in advancing research in their areas of interest. VHAs (often referred to as operating foundations) are private charities supported primarily by public donations. In addition to grants for research and training, these organizations also support activities that include public awareness and education, patient referrals, continuing education for health professionals, and lobbying to increase federal funding for disease-specific research. Now, perhaps over 200 national and regional VHAs actively support health research, and many of VHAs were founded by the families and friends of individuals suffering from a given disease.
The six largest VHAs (in revenues) are, in descending order, the American Cancer Society, the American Heart Association, the March of Dimes-Birth
Defects Foundation, the Muscular Dystrophy Association, the National Easter Seal Society, and the American Lung Association. These six organizations reported combined expenditures for disease-related research of more than $250 million in 1988 (Institute of Medicine, 1990). Because these organizations rely on voluntary contributions, they often are unable to make long-term commitments to research efforts such as multiyear clinical studies. They are effective, however, in responding rapidly to new research initiatives and providing resources to scientists to develop new lines of investigation.
VHAs also can play a critical role in the early stages of scientific career development. Through funding mechanisms such as fellowships and career development awards, these organizations attract young researchers to a specific field and provide them with research funding before they are able to compete successfully for federal support. Grant awards from these organizations commonly range between $20,000 and $50,000.
The effects of the lobbying activities of VHAs cannot be overstated. These organizations have been instrumental in increasing public awareness of the need to fight particular diseases and in soliciting grass-roots support for more federal research funds. They also have been very influential in establishing new institutes at NIH focusing on specific diseases and sets of diseases.
Medical Research Organizations
Medical research organizations (MROs) are unique in the portfolio of nonprofit support for research and research training. MROs are required by law to spend 3.5 percent of their endowments annually on medical research in conjunction with a hospital. The largest MRO, with assets estimated to be more than $6 billion, is the Howard Hughes Medical Institute (HHMI). In recent years HHMI has become the largest single private nonprofit contributor to biomedical research, with 1992 expenditures estimated at $281 million—a total greater than the expenditures of many NIH institutes. The J. David Gladstone Foundation Laboratories for Cardiovascular Disease, which is affiliated with the University of California at San Francisco, is another example and has assets estimated at $118 million.
HHMI traditionally has established large laboratories, with core groups of investigators in universities and hospitals around the United States to facilitate interaction with the larger research community. Investigators are appointed for fixed terms of three to seven years, with full funding provided for faculty and technician salaries as well as research expenses. Investigator productivity is evaluated through research conferences, annual progress reports, and site visits. Recently, HHMI has initiated a program to support individual investigators at institutions that do not have a core HHMI laboratory. This program will expand
HHMI support from approximately 180 investigators in its 30 core laboratories to approximately 250 at more than 40 institutions over the next few years.
HHMI's program is restricted to a few selected areas of research: cell biology and cell regulation, genetics, immunology, neuroscience, and structural biology. A 10-member medical advisory board has ultimate responsibility for the quality of the research program, and scientific review boards comprising scientists in each of the five areas oversee work in their respective fields. Although the HHMI program is highly regarded for its contributions to biomedicine and support for physician-scientists, its role in supporting patient-oriented clinical investigations is less clear. Physicians supported by HHMI may have clinical interests and may conduct clinical investigations, but the research portfolio of the institute is directed at preclinical research. Furthermore, although HHMI is sufficiently large to make major contributions in its selected areas of research, it does not seek to replace the central role of NIH in any field.
In addition to its research portfolio, HHMI has undertaken a broad program to strengthen science education from the precollege to the postdoctoral stages. The graduate science education program funds several levels of graduate training. For instance, doctoral fellowships in the biological sciences (60 yearly) provide predoctoral students with a stipend and cost-of-education allowance for three to five years; medical student research training fellowships (up to 60 a year) are modeled after HHMI's Research Scholars Program, supporting students for a year of research training at any U.S. academic or research institution. HHMI began the Undergraduate Science Education Program in 1988 to award grants to strengthen science education and research in private undergraduate colleges and increase the number of students, especially minorities and women, pursuing careers in the biomedical sciences. The program recently has been expanded to selected public universities.
INDUSTRY
Before World War II, industry funded more than half of all health sciences research in the United States (Boniface and Rimel, 1987; Ginzberg and Dutka, 1989). After the war, industry's support, although still increasing, was outpaced by the investment of the federal government. Since the early 1980s, however, industry has been playing an increasingly important role in health sciences research, focusing primarily on product development. The kinds of industries engaged in health sciences R&D include biotechnology firms and manufacturers of pharmaceuticals, medical devices, and instruments. These industries tend to be very research-intensive, and R&D investment is measured as a percentage of sales. For example, DiMasi et al. (1991) have estimated that it costs more than $230 million for a pharmaceutical firm to bring a new drug to market.
Understandably, corporate research focuses mainly on applied research and product development that moves products to market rather than on the undirected disease-oriented research or fundamental basic biology familiar to NIH. Pharmaceuticals, biologicals, and medical implants all require human studies to reach the market; therefore, clinical investigators and manufacturers need to work together closely to ensure that testing proceeds efficiently and under the strictest scientific methodology. Development and testing requirements for investigative new drugs or devices probably account for these large R&D expenditures (Battelle, 1991, Fletcher, 1989). Also, high levels of research investment have been attributed in part to the commercial potential for biological products such as genetically engineered insulin (Institute of Medicine, 1990). In 1991 industry contributed an estimated $13.5 billion to health research, accounting for about 48 percent of the total national investment in health research (Figure 3-1) (National Institutes of Health, 1993b).
In the past, a great deal of research was performed ''in-house" for proprietary reasons, and industry relied on university research programs to develop basic knowledge and scientific talent. In addition, pharmaceutical firms, and now biotechnology firms, generally contracted with clinicians in academic centers to test compounds in all phases of clinical trials. During the 1980s these established paradigms of academic-industry and industry-government relationships began to shift. Rapid advances in science and changes in the tax code encouraging R&D investment and technology transfer have prompted many special linkages among industry, government, and academic scientists (Witt, 1991). Shared interests in specific problems have helped to create some industry-sponsored cooperative basic research programs located in universities (National Academy of Sciences, Government-University-Industry Research Roundtable, 1986). Industry has also been working closely with NIH to develop numerous therapies, particularly new drugs to fight HIV infection. Although most regard these new linkages favorably, they can create problems of conflict of interest from the level of individual investigators to that of the research institutions themselves. Some of these unique relationships between industry and academic scientists are elucidated in Chapter 5.
The shifting policies of the health insurance industry and Medicare is another problem confronting manufacturing industries that require human testing to bring their products to market. The move toward cost-containment has driven many employers to shop for health plans that provide a certain level of care at the lowest cost to themselves and their employees. More and more employers are choosing to provide managed health care as the only option. Although managed health care can potentially reduce the costs of health care in the short-term, its effects on innovation through drugs, devices, and procedures have not been fully realized (Holmes, 1992; Laetz, 1991; Leaf, 1989; Moody, 1992; Telling, 1992). As the debate over ways to contain the rate of growth of health care intensifies in
TABLE 3-7 Distribution of U.S. R&D Expenditures for Ethical Pharmaceuticals by Function, 1991
|
Function |
Amount ($ million) |
Percent |
|
Clinical evaluation phases, I, II, III |
1,555.0 |
26.7 |
|
Biological screening pharmacologic testing |
984.2 |
16.9 |
|
Synthesis and extraction |
570.8 |
9.8 |
|
Pharmaceutical dosage formulation and stability testing |
447.4 |
9.4 |
|
Toxicology and safety testing |
407.7 |
7.0 |
|
Process development for manufacturing and quality control |
425.2 |
7.3 |
|
Clinical evaluation: phase IV |
233.0 |
4.0 |
|
Regulatory, IND and NDA preparation, submission and processing |
192.2 |
3.3 |
|
Bioavailability studies |
151.4 |
2.6 |
|
Other |
757.1 |
13.0 |
|
Total |
5,824.0 |
100.0 |
|
Source: Reprinted, with permission, from Pharmaceutical Manufacturers Association (1993). Copyright 1993 by the Pharmaceutical Manufacturers Association. |
||
the next few months and years, clinical research must be perceived as a vital part of the U.S. health care system and crucial for improving health.
The costs of pharmaceutical innovation are high. DiMasi et al. (1991) have estimated that it took 12 years and, on average, $231 million (1987 dollars) to bring a new drug to market for drugs tested in humans between 1970 and 1982. Throughout the 1980s, the pharmaceutical industry increased expenditures for R&D above that of the annual increases for R&D allocated to NIH. While the average industrial investment in R&D by industry is about 5 to 6 percent of gross income, pharmaceutical firms invest a high level in R&D expenditures in relation to sales—for example, 13 percent in 1987.
The distribution of R&D expenditures varies by company and type of research. The National Science Foundation reports that nearly 80 percent of industrial R&D is development, about 15 percent is applied R&D, and basic
research accounts for only 5 percent (National Science Foundation, 1988b). There is another way to view investment; that is, approximately one third of a pharmaceutical firm's R&D investment is devoted to discovery and new product development, one third is spent on existing product improvement and expansion of current business, and one third is directed toward process improvement for defending current market shares of products (Institute of Medicine, 1990). Whatever the measure or matrix of investment, a large portion of pharmaceutical R&D is spent on clinical evaluation of drugs in phases I through IV of clinical evaluation (Table 3-7).
Biotechnology is one subcategory of industrial biomedical R&D of particular importance to this committee (Blumenthal et al., 1986a, 1986b). The ability to synthesize proteins and peptides, new biological approaches to drug delivery such as the use of liposomes to encapsulate drugs, and other biological advances are rapidly expanding opportunities for finding and testing new biological therapies (Telling, 1992). The private sector, however, is not the exclusive investor in biotechnology. Whereas the federal government, primarily NIH, has been the primary source of R&D funds for biotechnology, the commercial markets for new biologicals is encouraging increased investment in biotechnology by industry. NIH reported that nearly 22 percent, or $1.02 billion, of its 1988 R&D budget was allocated to research on developing biotechnology techniques or employing the biotechnology (Institute of Medicine, 1990). The size of NIH investment in biotechnology reflects the importance of molecular and cellular biology in biomedicine.
CLINICAL RESEARCH AND THIRD-PARTY PAYERS
A chapter on the resources of funding for clinical research would not be complete without a discussion of the contributions to clinical research by third-party payers. In the United States, the relationship among insurers, subscribers, employers, and providers is unique. For many with employer-based health coverage, the employer establishes the contract of coverage, the employee pays part of the premium for the health coverage that the employer has established, and the third-party payer is the steward of the funds. The policy for those with federally supported coverage, such as Medicare or Medicaid, has been not to cover investigational or experimental therapies. The sad irony is that the 35 million or more people who have no health care coverage have more freedom to enroll in clinical studies or receive experimental therapy because they have no stake in who pays, and no third-party questions their decision.
Unfortunately, very little is known about the amount of support actually contributed by third-party payers. There are no databases that track this investment, and in many instances, it is believed that the less known, the better. Another variable in this equation is the amount of unreimbursed care provided by
hospitals and medical centers for individuals enrolled in clinical studies or trials; for example, those covered under Medicare. The committee was clearly aware of the problems of assessing the level of involvement of third-party payers in clinical research, but it felt strongly that the relevant policy issues should be explored. Thus, while this section is short on data, it draws upon presentations by executives from the insurance sector given at the workshop "Clinical Research and Training: Spotlight on Funding" (see Appendix D).
An unwritten understanding previously promoted, or at least did not discourage, the participation of patients in clinical studies or trials in which third parties contributed patient care costs, whether or not they were cognizant of it. For example, an extra computed tomography scan or other test might be performed to collect longitudinal data, and the claim would be covered without question. Changes in health care coverage in the past 15 years—with the emphasis on reigning in costs by cutting hospital stays and other care not proven to be cost-effective or efficacious—have altered this fragile relationship. The move to prospective payment based on diagnostic-related groups may also have affected the enrollment of patients in clinical studies. With computerized technology, third-party payers are able to scrutinize how patients are being treated and question why they should be supporting experimental studies. Government programs, both Medicare and state Medicaid programs are having the same difficulties that the science agencies are experiencing—scrambling for scarce resources in the federal budget. The for-profit private insurers must be equally concerned with covering the soaring costs for standard therapies and paying dividends to shareholders. Even the nonprofit insurers like Blue Cross and Blue Shield must be concerned with balancing their cash flow. Thus, most third-party payers probably believe that clinical research falls far outside of their boundaries of responsibility.
Old therapies that may or may not be effective are not being adequately assessed, and new medical technologies and therapies are evolving very rapidly. At the same time, biomedical scientists are becoming increasingly sophisticated in understanding the biological bases of disease processes and the heterogeneity of patient populations. This synergism stokes the engine for even more scientific inquiry to define more precise fits between treatments and specific patient populations, not only for the good of science, but certainly for the benefit of the patients. Coupled to this is the public's expectation for increasingly sophisticated health care with cascades of tests and procedures and without concern for how the costs will be covered. Patients with incurable diseases also expect to have access to the best possible care or optimal therapy—even if that involves enrolling in a clinical study for testing an unproven therapy. Viewed purely on a cost basis, standard therapy may be the least expensive in the short run. A well-designed clinical trial, however, may uncover an improved therapy to reduce morbidity or mortality and, it is hoped, improve quality of life, despite the high initial costs. The conundrum that has arisen is that an adversarial situation has
developed between the patients and their doctors, on the one hand, and the patients and the third-party payers, on the other, because the affected parties cannot agree on what portion of clinical investigation is reimbursable (Newcomer, 1990).
The bottom line is that reimbursement of costs is essential. Someone must ultimately pay for the costs associated with experimental therapy as well as the costs of standard medical care, whether it is the federal government (Health Care Financing Administration, NIH, and the like), the third-party payers, the institutions, or other sponsors of research. Following the paradigm of the past, clinical investigators expected that insurers would reimburse the costs of legitimate care associated with sponsored research of the highest quality. At the same time, it was believed that the sponsor of the research should bear the cost associated with the research. Nevertheless, it is frequently difficult to separate the care costs from the research costs, and most of the disagreement focuses on this gray area. This is particularly evident in the treatment of cancer, where almost all treatment is some form of experiment, often using class C (cancer) drugs or combining two or more types of therapies (Antman et al., 1988; Wittes, 1987b).
Another point of contention is the refusal by a payer to allow reimbursement for any care if a covered patient is enrolled in a study Wittes 1987b and 1988). Patients, however, are suing their insurers to allow them to enroll in clinical studies and receive coverage for both the standard care and the experimental therapy, especially when standard therapies are not much improved over no treatment. Although this puts pressure on third-party payers to cover the costs associated with the standard care and those associated with the experimental therapy, moving these controversies into the courts may not be the best way to encourage participation in clinical research. After prolonged legal procedures, as well as high legal costs for the patient and the patient's family, the decision is usually left in the hands of a jury, which is unlikely to have the requisite expertise in experimental medicine. The result fails to serve good medicine, appropriate patient care, or sound reimbursement policy. Furthermore, it ties up everyone's time and resources and prolongs the potential benefit a patient might receive from the investigational therapy. A better route would be to have clinicians, in collaboration with payers, make sound decisions based on clear clinical research data about whether to provide care under an experimental protocol.
One example of growing cooperation among the affected parties is the use of autologous bone marrow transplantation for treating metastatic breast cancer. This is a developing technology that is costly, effort-intensive, and somewhat toxic, but it has shown some promise over standard therapy. Briefly, it consists of harvesting autologous bone marrow from a patient, administering very high doses of chemotherapy or radiation therapy to inactivate the metastatic cells, and then reconstituting the normal hematopoietic elements from the harvested bone marrow. Hospitalization is necessary for anywhere from a few days to several weeks, and the cost for such treatment has been in the range of $75,000 to
$150,000 per patient. That this therapy is available is a tribute to the sponsors of fundamental research, including the National Cancer Institute, the American Cancer Society, and the American Leukemia Society. More interesting, however, is the paradigm for supporting a large, multicenter clinical trial on the therapy. After losing several suits forcing various payers to cover the costs of this therapy, a coalition of third-party payers, including some of the individual Blue Cross and Blue Shield plans, have agreed to fund a demonstration project in which they will accept some responsibility for paying the clinical care costs associated with these particular national studies. This may signal a new paradigm for sharing the costs of clinical research in which payers, clinical investigators, and patients all cooperate to further understanding of novel and innovative therapies.
Third-Party Payers' Perspective
It has become increasingly necessary for plans to serve their subscribers while striving for access to quality health care at an affordable price. Inextricably bound to those objectives are the processes of technology assessment and coverage determination. Medical technology committees, frequently including physicians and subscribers, have become increasingly sophisticated and have established sets of criteria to guide coverage, including cost-effectiveness, legal, ethical, and cultural differences, and distributive justice (Whether the technology is available to all subscribers.). These criteria have been delineated after some years of study to help determine when a technology has reached a stage at which it is no longer considered investigational and can be accepted as eligible for coverage. Once the criteria have been satisfied and the committee has determined that a critical mass of evidence is available to show that a procedure or technology is established as standard care, most will agree that it is eligible for coverage determination (Leaf, 1989). The bigger concern, however, appears to be over who should pay for the initial studies to collect the requisite primary data and how the data should be collected, shared, and analyzed (Antman et al., 1988; Wittes 1987b). For example, FDA approval of a drug or device for a given condition often, although not always, meets the criteria for coverage (Wittes, 1987a).
Another problem arises, however, when an approved drug is used to treat a disease for which it is not approved (off-label use) (Moertel, 1991; U.S. Government Accounting Office, 1991). In some instances, there may be compelling evidence that a particular drug is effective, but the pharmaceutical manufacturer does not choose to add this information to the label because of the costs associated with additional FDA approval or the short time remaining on a sole-source patent. Patients and the provider are often left with few options. At the same time, third-party payers see their role as that of gatekeepers preventing overutilization and expansion of technology beyond its intended use or the continued use of outmoded technology. This scenario presents a serious gap in
the U.S. system of who should pay for what. For example, pharmaceutical patents are time-limited. When an already approved therapy shows promise for another condition and a manufacturer is unable or unwilling to cover the costs associated with gaining approval for the use of the drug in the treatment of that other condition, who should pay for the investigations? Should the government allocate funds for the trials? Should the third-party payers be obligated to participate? Would a coalition of all concerned parties resolve this conundrum?
During the early 1980s, a modification of coverage determination was designed to cover certain therapies that were not yet established, but had demonstrated promising success rates at particular institutions—selective coverage. For example, a payer might determine that a procedure in the hands of skilled physicians looks promising and would cover the associated costs at a particular medical center (for example, the early days of heart transplantation). Thus, a body of evidence might have accumulated demonstrating a procedure had positive outcomes and that it might no longer be considered investigational when performed at that institution. Although this late-stage coverage is appreciated, many resources that were not reimbursed were consumed to reach this stage.
Third-party payers are also concerned about the proliferation of clinical trials for any one condition. Because many of these trials are investigator-initiated, one might find five or six different research groups, each treating patients on a different protocol for the same disease. Depending on the available patient populations, many of these trials might not be able to accrue enough patients to achieve statistical validity. Moreover, the third-party payers may not be able to determine which ones should be covered and which ones should not. Another problem cited is the varied perspectives of the investigators, even those collaborating on one project. By the time one gets through compromising with 5 or 10 very aggressive investigators, each one altering the study design in his or her own way, a study may be misguided and not answer the original question, or perhaps answer questions that were not very germane about the disease in question to society as a whole. These difficulties are not very appealing to insurers and make them disinclined to sponsor clinical studies.
Involvement in Study Design and Data Analyses
If third-party payers are to become significant collaborators in clinical studies and trials, their roles in experimental design and data analysis should be fully elucidated. As a stakeholder, the question arises as to how much of a role the insurer should have in creating the study design. Would it constitute a conflict of interest if an insurer supports a study with a design that could be potentially biased at the outset because of company participation? How can participation be assured without compromising the scientific validity of the investigation?
Data Sharing and Analysis
Once a third-party payer decides to support a study, another question arises; that is, how much access should they have to the data? On the one hand, the insurer will have access to the claims data, from which it could draw certain conclusions. On the other hand, how much access to the scientific data should the insurer be allowed? Again, as a stakeholder, the third-party payer probably believes that it should have complete access to medical information on patients covered under its policies. In the appropriate conduct of randomized clinical trials, however, the study is blinded and the codes cannot be broken until the statistical considerations are met. Other types of clinical studies may warrant other arrangements of data sharing.
In addition to the issues surrounding access of data by the third-party payers, access to third-party payer databases by investigators also raises several issues. The insurers maintain the massive databases required for accounting purposes in the U.S. health care system. Such data bases could be utilized for continuous evaluation of medical practice. Studies of such data might not only provide a sounder basis for reimbursing new and experimental interventions but also could allow reevaluation of older, potentially obsolete or ineffective technologies that should no longer be employed. Numerous deficiencies in these databases, however, preclude this use. For example, each insurer has a claim form that collects and codes information differently. Without a standard type of data collection, comparison of data sets from different sources becomes impossible. Moreover, many of the claims data are probably not complete enough to draw conclusions about the effectiveness or outcomes of different therapies.
Possible Solutions
From the perspective of third-party payers, direct funding of clinical research is not possible. Viewing themselves as custodians of subscribers' funds, they do not have the reserves or the authority to devote resources to underwriting all clinical research, particularly that in which a third-party payer has a commercial interest. However, as custodians of those funds, third-party payers make coverage decisions that affect the health and well-being of their subscribers. Thus, payers have an obligation to find out what works and what does not. Nevertheless, possible solutions that will serve the interests of all parties can be developed without allowing costs to continue to soar (Antman, 1989; Wittes, 1988). The committee believes that high-quality care at a reasonable cost can be retained, while at the same time advancing new and unproven therapies that could potentially improve quality of life.
Much can and should be done to ensure, encourage, and enhance the cooperative investment of time and interest that all parties have in clinical
research, clinical research training, and the responsible utilization of new approaches to the diagnosis and treatment of disease. New approaches must encourage, not discourage, the responsible use of emerging technologies and therapies and the development of new uses for accepted interventions. To increase the quality of life and the effectiveness of health care, all parties need to foster continued innovation, not only in academic and industrial research laboratories but also at the bedside.
There must be a true partnership that meets the goals and expectations of all participating parties and ensures the timely and cost-effective application of the findings of clinical research or the application of new technologies. Meaningful partnerships and coalitions need to be created. These coalitions could include not only university academic health centers but also private foundations, federal and state agencies, the pharmaceutical and biomedical technological industry, and those in the health insurance industry. For example, an interdisciplinary, interorganizational group could be created to help establish guidelines and provide recommendations for the use of, and payment for, therapies that have gone beyond the early investigational phase or for therapies over which there is a degree of controversy. Membership for such a group could be drawn, for example, from the Institute of Medicine, the Agency for Health Care Policy and Research, NIH, the health insurance sector, health-related private foundations, and representative consumers. This group would develop and maintain guidelines and criteria and decide what new procedures, therapies, or devices should or should not be reimbursable. One form of cooperation might be to adopt a uniform policy of paying for experimental therapies for all patients on approved protocols by NIH. Another might be to establish a diagnosis-related group-style prospective payment system that would ensure that adequate numbers of patients were enrolled in a trial to answer a question with adequate statistical power.
The potential advantages of increased cooperation are obvious. By working together rather than through the courts or other judgmental bodies, the committee believes that the appropriateness and effectiveness of care for patients can be improved. Improved outcomes for patients may result in economic savings for the patients and the payers. Thus, knowledge of how best to provide care will be expanded and transmitted to a broad base of qualified providers and, of great importance, to the next generation of practitioners. Of course, any changes need to occur in a climate that recognizes that the resources for health care are under greater stress and pressure than in any other time in U.S. history, whether it be in the academic health centers, pharmaceutical and biotechnology industry, or the insurance industry.
MODELS OF COOPERATION
Undeniably, the U.S. system of health research has been highly successful largely because of the unhindered ability of investigators to pursue intriguing and pertinent questions ranging from very fundamental basic biology to clinical studies requiring the participation of human subjects. Previously, the separation of who should pay for what was of little concern because of plentiful resources and fairly well-established areas of responsibility. Simplistically, NIH and other federal agencies funded investigator-initiated human studies and other clinical studies deemed necessary by advisory bodies or Congress to fill gaps in certain areas, industry has been motivated by the potential for-profits and clinical studies are driven by regulatory concerns, the insurance industry may or may not cover certain aspects of investigational care, and academic health centers and hospitals have underwritten significant portions of unrecovered clinical research expenses out of their own reserves. With the growing attention to health care cost-containment and increasing constraints on the federal research budget, however, the committee fears that fundamental human research may be inadvertently squeezed out of the research portfolio. Clearly, the potential for return on investment continues to be a strong incentive for industry to sponsor clinical trials of test substances, chemical or biological. A large amount of the knowledge base upon which new therapeutic agents may be founded, however, is derived from investigator-initiated preclinical research and studies of human biology and disease that are likely to have no immediate or long-term commercial interest. Thus, the committee believes that new models of cooperation among all parties with a vested interest in health care are necessary for continued progress in human research to further improve modern health care.
To explore these opportunities for increased cooperation among industry, government, academic health centers, and third-party payers, the committee sought examples in other scientific areas to serve as models or prototypes. One notable example is SEMATECH, which was formed during the 1980s in response to the intense international competition in the semiconductor industry. SEMATECH is a consortium of U.S. semiconductor manufacturers working with government and academia; it sponsors and conducts precompetitive cutting-edge research in semiconductor manufacturing technology for U.S. manufacturers. It was originally created out of a concern that the U.S. manufacturers were losing market share and may be forced out of the global market altogether, therefore risking the national security if the U.S. military were reliant on foreign manufacturers for vital computer chips.
The annual budget for SEMATECH is $200 million—far above the ability of many firms to shoulder independently. Half of the budget is raised through corporate memberships, with a ceiling of $15 million to prevent single-company domination and a floor of $1 million. The remaining $100 million is provided through the U.S. Department of Defense through the Advanced Research Projects
Agency. The funds are used to conduct research at SEMATECH by staff and scientists from the member companies. SEMATECH also awards research contracts to small semiconductor research firms and universities.
For member companies to conduct cooperative research through such consortia, they must file for antitrust exemptions with the U.S. Department of Justice. Whereas SEMATECH may appear to be an anomaly in U.S. R&D sector, more than 380 such filings have been recorded. In sum, SEMATECH serves as one prototype that could be duplicated in the health research arena to support fundamental human research (precompetitive) and clinical research training.
In the policy arena, the Government-University-Industry Research Roundtable (GUIRR) of the National Academy of Sciences is a forum of senior-level government science managers, leaders in industrial research, and leaders in university leadership who meet to discuss broad science policy issues affecting all groups. The formation of GUIRR is unique in its own sense because it required approval by the White House Office of Management and Budget to allow senior government managers to meet in closed sessions with industry leaders. Although by its charter GUIRR is prohibited from making recommendations, it serves as a unique forum for science policy discussions and a means to bridge gaps among the federal government, industry, and the academic community.
Some health fields are already taking the initiative to assemble funds through consortia of industry and private philanthropy to promote research and training in specific areas. For example, the Alliance for Aging Research has proposed a National Geriatrics Development Fund to create Leadership Centers in Geriatrics at various academic medical centers with geriatric medicine programs. The Alliance, with the support of the Commonwealth Fund, hopes to raise matching funds from industry and nonprofit foundations to carry out this mission. Awards will be made through a peer-reviewed competition.
Although business as usual has brought the United States to the pinnacle of health research, the committee feels that new paradigms for research cooperation and support are warranted. The committee believes that models of cooperation that could be applied in the area of human research already exist. All parties—industry, academia, government, and third-party payers—need to recognize that clinical research is not someone else's responsibility, but is the collective responsibility of all. One proposal would be to form an alliance or consortia of all parties, similar to SEMATECH, so that critical, fundamental human research can be supported to the benefit of all parties and, most importantly, improve the health of the U.S. public. Thus, funds from government, industry, third-party payers, nonprofit organizations, special interest groups, and academia could be pooled and available for peer-reviewed competition to close gaps of knowledge in particular areas or provide special emphasis in others deemed appropriate or urgent.
CONCLUSIONS
The committee concluded that patient-oriented clinical research is supported by a diverse, yet interlocking network of federal agencies, industry, and private nonprofit organizations that share many common goals. Of these, the federal government is the single largest sponsor of health research in the United States. Of the more than $75 billion the federal government invested in R&D during fiscal year 1993, nearly $11 billion was health-related. Contributions by health-oriented corporations are roughly equal in magnitude, but they are devoted largely to product application developments rather than fundamental discovery research. Contributions by private nonprofit sponsors favor fundamental discovery research, generally in somewhat restricted fields of interest, but represent only about four to five percent of the total U.S. investment in health research.
In light of this investment and the continuing budget limitations, the scientific community must reexamine its resource base to improve its effectiveness and efficiency. Federally sponsored health research by the various agencies is generally mission oriented. Whereas NIH is the primary agency that disburses federal health research funds for investigation into fundamental biological discovery, other agencies such as VA and AHCPR are key players in health research, particularly patient-oriented research. Thus, the committee emphasizes that all types of health research expand the boundaries of knowledge for improving health care and should be considered crucial parts of the realm of health research.
Although industry has been playing an increasingly important role in health research, focusing primarily on product development, it relies heavily on university research programs for fundamental knowledge (both basic and clinical) and talent. Cooperative ventures between universities (or government) and industry provide a unique mechanism for sharing knowledge and for technology transfer, a central policy of the federal government for increasing U.S. economic competitiveness.
Foundations, voluntary health agencies, and other nonprofit organizations have played a very important role in sponsoring health research. The committee believes that these organizations have been particularly helpful by providing crucial support in filling gaps in the nation's research agenda and sponsoring new initiatives. Although the federal government rapidly eclipsed the investment by these organizations following World War II, these organizations have continued to supply a steady stream of research dollars. These funds are used for individual research project, supporting career development awards in specific research fields, equipment, facilities, and various programs of knowledge dissemination. The committee anticipates that these organizations will continue to provide support for the health sciences.
The health insurance industry also is a stakeholder in the realm of clinical research. Third-party payers need to recognize their responsibility to subscribers
and society as a whole for improving health care. The committee does not imply that insurers should foot the entire costs for experimental or investigational therapy, but that they should work with the medical scientific community to determine what works and what does not.
To facilitate cooperation to uncover new knowledge about human disease and improve health care, the committee recommends the formation of an alliance that will bring all parties with a vested interest to the table in support of patient-oriented clinical research. New paradigms of cooperation are warranted to continue to improve the health of the U.S. public.