Technologies for Chemical Measurements
As part of its deliberations, the committee estimated the ease of implementing the chemical technologies described below for in situ applications (Table 3).
MASS SPECTROMETRY (FENN ET AL., 1990)
Mass spectrometry (MS) is useful for a variety of purposes in environmental monitoring and research, including characterization of proteins, distinguishing among sources of compounds by the ratios of various isotopes (e.g., 13C/12C), and measurement of heavy metals. MS is critical for reconstructing the pathways of carbon flow in marine systems. Construction of carbon budgets, recognition of controls on the carbon cycle, and calibration of a CO2 paleobarometer are also important in studies of Earth history. Success in all these studies depends critically on the development and wide availability of adequate MS instrumentation. Due to natural fractionations of stable isotopes, compounds produced in various groups of organisms have slightly different isotopic compositions. Individual compounds are "isotopically labeled" at their source. These natural labels can be followed through the marine carbon cycle, and effects of secondary processes can be dissected in detail. In the past, all those intercompound differences were invisible because the available methods required samples so large that preparative isolation of individual compounds for isotopic analysis was impractical. Recently, however, isotope ratio monitoring techniques have been developed. For analysis of individual organic compounds, the effluent of a
TABLE 3 Analytical Technology Applied to In Situ Measurements
|
Chemical Sensing |
Sample Processing |
Data Analysis, Interpretation |
|||
|
D |
Mass spectrometry |
S |
Chromatography/electrophoresis separations |
E |
Chemometrics |
|
E |
Electrochemistry |
|
|
E |
Communications |
|
E-S |
Fluorometry |
E |
Flow injection analysis/continuous flow analysis |
E |
Data storage/handling |
|
E |
Absorption spectroscopy |
|
|
|
|
|
D |
Raman spectroscopy |
D |
Robotics |
|
|
|
D |
Fourier transform infrared spectroscopy |
|
|
|
|
|
S |
Refractive index |
|
|
|
|
|
D |
Satellite |
|
|
|
|
|
D |
Piezoelectric mass sensing |
|
|
|
|
|
E-S |
Immunochemistry/biochemistry |
|
|
|
|
|
S |
Polymers/new materials |
|
|
|
|
|
D |
Recognition chemistry |
|
|
|
|
|
D = Difficult—Unlikely to see widespread application to in situ measurements in the next 15 years. S = Straightforward—With some investment, could see application in 5 to 15 years. E = Easy—No major technical barriers to application between now and 5 years hence. |
|||||
high-resolution gas chromatograph is routed to a microscale combustion furnace. In turn, the products of combustion (principally CO2, but also N2) are led to the ion source of an isotope mass spectrometer. As the bursts of gas from the combustion of individual chromatographic peaks pass through the ion source, the relative abundance of 13C or 15N can be measured with a precision of about 2 parts in 10,000, good enough to observe natural variations. The entire system operates under conditions of continuous flow, so hundreds of measurements can be made within a single chromatographic run. Determining the sources and fates of individual organic compounds will be a major theme in marine organic geochemical research in the future, and its dependence on MS instrumentation is extreme. Marine laboratories in other countries are quickly entering this area, but laboratories in the United States are experiencing difficulty in finding funding to purchase the necessary instrumentation.
A mass spectrometer is a device in which a sample is ionized and the ions are sorted and counted on the basis of their mass-to-charge ratios. Ions can be formed by several methods. A recently developed method, in which an ion beam sputters a nebulized liquid vapor, leaves molecules with multiple negative or positive charges. This permits the identification of very large molecules. Also, solid samples of molecules with masses of up to 300,000 daltons can be vaporized from surfaces using lasers with wave
lengths matched to the absorption wavelengths of the molecules. Mass analysis can be performed on the resulting ions using a variety of techniques, including: electrostatic, magnetic, double focusing, quadrupole lens, ion trap, time of flight, and Fourier transform ion cyclotron resonance. These methods have potential relevance for marine chemistry, but must circumvent the extreme difficulties of measuring materials dissolved in seawater.
MS can be used for characterizing dissolved organic material (DOM) in seawater and in surface films and particulate organic material (POM) and dissolved material associated with sediments. As organic matter can serve as binding sites for trace metals, DOM identity and concentrations must be known to understand trace metal kinetics in seawater. Characterization of oceanic DOM is also important for a better understanding of biological processes. At present, most of the dissolved organic compounds found in seawater are unidentified. Carbohydrates and proteins, measured as total (free plus combined) hydrolyzable amino acids and sugars, constitute 5 to 20% of the DOM. Other components, such as lipids and low molecular weight organic compounds (e.g., formaldehyde and pyruvate), occur at much lower concentrations. Another identifiable organic constituent of seawater is DMSP [dimethylsulfonio-propionate, a precursor of dimethyl sulfide (DMS)]. DMSP is an important source of DMS to the atmosphere, having a role in formation of cloud condensation nuclei. With the MS systems available now, several thousand different molecules could be identified in seawater in land-based laboratories. A remaining problem is to isolate these compounds, which are present at concentrations many orders of magnitude more dilute than the major dissolved constituents of seawater. Emphasis should be placed on solution of basic problems of collection and purification of substances dissolved in seawater.
Many investigators are using MS to characterize the structures of organic compounds obtained from marine particulate organic matter from sediment traps and from suspended particulates. The origins and alteration of this material are of great interest, and the molecules within it are generally susceptible to structural analysis. There is a great need for advanced instrumentation in this area, particularly for MS-MS systems. American laboratories are generally falling behind those in other countries in equipping for these techniques.
Combinations of chromatography and MS are useful for oceanography. Gas chromatography combined with MS is a mature technique for analyses of complex mixtures and isotopic analysis. Liquid chromatography can be combined with MS to examine polar, labile substances without chemical derivatization, and is already applied in a variety of marine laboratories. This technique is sensitive enough to detect analytes in the picomolar range. Another application of MS is in combination with capillary zone electro-
phoresis. Efficient chromatographic separation can be achieved with this combination.
These methods are ready to use now for land-based applications, but are expensive. Oceanographers are aware of many MS methods, but because of the cost, such methods are not readily available. This problem could be solved by a time-sharing arrangement or the development of national facilities. Mass spectrometers are in general too bulky and require too much stability and power to be used on board ocean-going vessels. Quadrupole mass spectrometers are sometimes taken to sea, however, for various biological experiments, such as studies of respiratory gas exchange by marine organisms. With further advances in ion traps, more MS devices may be used on board ships. Unfortunately, the only way to determine analytes quantitatively is to calibrate the equipment with identical, isotopically labeled molecules. New developments, particularly using internal standards in chromatographic combinations, may alleviate this problem.
Accelerator mass spectrometry (AMS) is now being applied to measure the age of carbon in seawater. AMS is revolutionizing the measurement of light isotopes such as 14C in dissolved CO2 as well as DOC in seawater, requiring as little as a few hundred milliliters of sample. The older beta decay counting methods required 150-liter samples that were difficult and expensive to obtain. An AMS facility began operating at the Woods Hole Oceanographic Institution in 1991 at a cost of several millions of dollars. Seawater samples collected by the WOCE program will be analyzed in this facility. This AMS facility may also eventually be used for measuring 10Be for marine geology research.
Inductively coupled plasma-mass spectrometry (ICP-MS) is revolutionizing the measurements of refractory metals, such as titanium, and can provide a wealth of isotopic information that could only be obtained previously with great difficulty. ICP-MS has been used as a fast and sensitive technique for measuring 230Th in marine sediments (Shaw and Francis, 1991) and barium in seawater (Klinkhammer and Chan, 1990). For the future, advances in the capabilities of mass spectrometers can be expected (Table 4), developed by interdisciplinary groups of academic, government, and industry scientists. It is unlikely, though not impossible, that MS techniques will be appropriate for buoy development.
ELECTROCHEMICAL TECHNIQUES
This section focuses on techniques designed to probe the interaction of an electrode with a seawater sample, especially those that derive a signal from oxidation-reduction reactions. From the point of view of measurements important to chemical oceanography and ocean science, the following electrochemical techniques have the most likelihood of application in the
Table 4 Expected Advances in the Field of Mass Spectrometry
|
|
1991 |
1996 |
2006 |
|
Overall sensitivity of detection |
1–100 picomoles |
1–100 femtomoles |
Attomole |
|
Molecular Weight |
|||
|
Laser matrix time of flight |
300,000 daltons |
106 daltons |
|
|
Electrostatic quadrupole |
130,000 daltons |
106 daltons |
|
|
Time of flight |
<500 daltons |
10,000 daltons |
|
|
Electrostatic-sector |
>3000 daltons |
>10,000 daltons |
|
|
LC-MS |
|||
|
Flow probe |
5–50 picomoles |
|
|
|
Sector |
<1200 daltons |
|
|
|
|
~5000 daltons |
|
|
|
Flow electrostatic quadrupole |
<1 picomole |
Femtomole |
|
|
Electrostatic-ion trap |
~3000 daltons |
|
|
near future: potentiometry, constant-potential techniques at steady state, pulse voltammetry, stripping voltammetry, and coulometric titrations (see Whitfield and Jagner, 1981, for review).
Of these, potentiometry employs the simplest instrumentation, in that it requires only an electrometer for measurement of the potential difference between the sensing electrode and a reference electrode. The other techniques additionally require a current source and control circuitry, usually packaged together as a potentiostat. Coulometric techniques also require a means to determine charge, which is often done by incorporating an electronic integrator into a potentiostat. The technology of modern electronics provides for these purposes robust and inexpensive instruments, many of which include microprocessors suitable for controlling the experiment and manipulating the resulting data.
Potentiometry (Buck, 1984)
The potential of an electrode is related directly to the activities, and thus indirectly to the concentrations, of the chemical species involved in the equilibria that establish the potential. The main virtues of potentiometry are simplicity, very low power requirements, and the possibly small size of the sensors. The main drawback is that an unwanted reaction may enter into determination of the potential and sensitivity may be poor. Potentiometric sensing, as a transducing technique, can be coupled with an infinite variety of novel chemical reactions to solve specific analytical problems, as described below in the section on new chemistry. A wide variety of potentiometric ion-selective electrodes (ISEs) already have been developed (see
TABLE 5 Potentiometric Ion-Selective Electrodes
|
Glass |
|
H+, Na+ |
|
Solid State |
|
Silver sulfide-based pressed pellet type—Ag+, S2-, Cd2+, Cu2+, Pb2+, I-, Br-, Cl- |
|
Single crystal-F-(LaF3) |
|
Organic Membrane (Liquid or Polymer) |
|
Ion exchanger or charged carrier—NO3-, Cl-, Ca2+ |
|
Neutral carrier—K+, Ca2+, Li+, Na+, Mg2+, H+, CO32- |
Table 5), and several are now being used, or are potentially useful, for measuring key ocean elements. The most common use of direct potentiometry (as compared with potentiometric titrations) is for measurement of pH (Culberson, 1981). Most other cation electrodes are subject to some degree of interference from other major ions. Electrodes for sodium, potassium, calcium, and magnesium have been used successfully. Copper, cadmium, and lead electrodes in seawater have been tested, with variable success. Anion-selective electrodes for chloride, bromide, fluoride, sulfate, sulfide, and silver ions have also been tested but have not yet found wide application.
New polymer membrane-based ISEs for nitrate and carbonate exhibit detection limits and selectivities that may be applicable for ocean measurements. In addition, a number of these ISEs can be used as internal transducers for the design of useful potentiometric gas sensors. For example, dissolved CO2 can be detected potentiometrically by using either a glass membrane electrode or a polymer-based carbonate ISE, in conjunction with an appropriate reference electrode, behind an outer gas permeable membrane. Novel differential pCO2 sensors based on two polymer membrane-type pH sensors have also been developed recently.
Constant Potential Techniques at Steady State
Electrochemical detection under convective conditions has been applied widely in freshwater measurements. In addition, seawater measurements have been combined with flow injection analysis (FIA) and high pressure liquid chromatography (HPLC) (D.C. Johnson et al., 1986). Well-developed commercial product lines exist, and detection limits are typically in the range of femtomoles. For in situ, shipboard, and land-based measurements employing HPLC or FIA, electrochemical detection could provide increased capabilities with respect to compound-specific detection and improved detection limits. The limitations of this approach are determined completely by the requirements of FIA or HPLC.
The use of microelectrodes for steady-state measurements is less well developed but has special promise for sensors and monitors in cases where power and size are important constraints (Montenegro et al., 1991). The size and shape of microelectrodes can be tailored specifically to the analytical problem. The nature of the electrode response depends on the time scale of the experiment. Electrode arrays can be fabricated with thousands of small elements. Lithographic procedures raise the possibility of very low per-item cost of manufacturing identical arrays.
Pulse Voltammetry (Osteryoung, 1988; Osteryoung and O'Dea, 1986)
Modern pulse voltammetry employs stepwise changes in potential, the sequence of which is controlled by software. Thus, the exact choice and timing of potential steps can be tailored to the specific analytical problem. Standard pulse sequences routinely employed in electroanalytical investigations include those of square wave, normal pulse, and other voltammetries.
The current resulting from a chemical reduction is directly proportional to concentration and typically can be measured with a relative precision of 0.2% and an absolute accuracy of better than 1%. For typical conditions and electrodes of conventional size, the change in current per change in concentration is on the order of 70 nanoamperes per micromolar. Detection limits in the worst case are about 1 micromolar and are typically 0.1 micromolar; in favorable cases, nanomolar concentration detection limits can be achieved. The linear range, over which signal is proportional to concentration, is typically a factor of 104 to 106, for example 10 nanomolar to 1 micromolar.
The response, though of low resolution, is specific to the reacting species. It is possible, therefore, to determine metals in a specific oxidation state [e.g., As(III)] or as a specific complexed ion. Particulate material does not affect the response of the electrodes to dissolved material. The electrochemical behavior of organic compounds tends to be similar for different compounds in which the same functional group is reacting. Thus, voltammetric measurements can be used to give semiempirical quantification of classes of compounds without going through the expense and difficulty of determining the identity of individual compounds present. Measurements can be carried out at characteristic times of 10 microseconds to 10 seconds, thus providing considerable scope for optimization.
Stripping Voltammetry (Zirino, 1981; Shuman and Martin-Goldberg, 1984; Van der Berg, 1989)
Voltammetry can also be employed in the stripping mode; that is, the material of interest is accumulated in or on the electrode, and once concen-
trated is voltammetrically ''stripped'' from the electrode. Concentration factors of 105 can be achieved routinely, and thus detection limits in the range 10-11 to 10-12 molar can be achieved. At these levels of concentration, fidelity of the sample becomes the factor that controls the quality of the result. This technique has been used widely in oceanographic science, especially for the determination of metals such as lead, copper, cadmium, and zinc. Stripping voltammetry at mercury electrodes yields detection limits in aqueous solution of 30 picomolar for copper and 0.3 nanomolar for zinc (Stoeppler, 1991). Problems of interferences by other elements in either aqueous or amalgam solution have been studied exhaustively, and well-documented procedures exist for eliminating these interferences.
Stripping voltammetry has been used to study the distribution of Zn(II) between labile and inert complexes in seawater with total zinc concentration of 50 nM (Muller and Kester, 1990). Formation constants of Zn(II) with various inorganic complexing agents (e.g., Cl- and NO3-) have been measured by anodic stripping voltammetry at total zinc concentrations of 10 nM (Komorsky-Lovríc and Branica, 1987).
Distributions between labile and inert complexes have been determined in samples with 15 nM Cu(II) and Zn(II) by anodic stripping at a micromercury electrode (Daniele et al., 1989). The technique of absorptive stripping voltammetry holds great promise for the determination of electroactive substances that can be adsorbed at an electrode. New developments in the use of microelectrodes for stripping voltammetry provide the possibility of extending the extensive body of laboratory procedures now employed to in situ measurements. Ligand competition voltammetry is also being carried out on seawater samples.
Coulometry (Bard and Faulkner, 1980)
Coulometry comprises a set of techniques in which the total charge required (not the current, as in potentiometry) to oxidize or reduce the chemical species of interest is measured. The prime virtue of coulometric techniques is that they link the quantity of substance determined directly to the quantity of electrical charge, and thus expensive and often difficult procedures for standardization or calibration can be minimized or eliminated.
Coulometric procedures are robust theoretically in that Q = nFN, where Q is quantity of charge, n is the number of electrons in the reaction, F is the Faraday constant, and N is the number of moles of reactant. This simple relationship is little affected by environmental variations (e.g., temperature or ionic strength). Coulometric procedures are also robust technically, in that the charge is just the integral with respect to time of the current. Coulometry is also easy to automate and operate under remote control. The accuracy of coulometric determinations is typically as good as 0.1%. Preci-
sion of 0.01% can be achieved, and it may be possible to achieve accuracies this good in special cases.
The coulometric principle can be applied in many different ways to determine specific substances. The most obvious and direct way is to electrolyze a sample of known volume at constant potential. However, coulometry can also be applied in titrations at constant current, employing a reagent that is oxidized or reduced in the process. Coulometry may also be employed in titrations by coulometric generation of reagent. Examples include the determination of CO2 by reaction with ethanolamine, with subsequent titration with coulometrically generated base. Another coulometric reagent generation is for determination of SO2 by coulometrically generated iodine. Coulometric titration is now the method of choice for determination of TCO2 in seawater (Johnson et al., 1987). Finally, it can be used for the generation of standards, particularly for substances that are difficult to prepare and store, and for in situ calibration. Coulometry may also be used for detection in liquid chromatography and flow injection analysis.
New electrochemical techniques can be applied directly to presently employed amperometric methods to optimize the potential-time waveform and current sampling scheme. For example, steady-state amperometric measurements at constant potential may be converted to pulsed operation with synchronous sampling of current in a way that improves performance but is transparent to the user. Optimal sampling schemes for individual methods can be developed through laboratory research. This type of development is exemplified by the Endeco® pulsed oxygen sensor. For remote operation, as from buoys, improvement in performance of computers should make it possible to convert amperometric techniques to voltammetric techniques. Determining the voltammetric response (which might consist of 150 data points per concentration measurement) provides better statistical definition of the current-concentration relationship. Knowledge of the voltammetric response could also provide the ability to compensate directly for changes in the properties of the reaction being used to determine concentration. This approach is presently practical in a laboratory research setting and, assuming further improvements in technology of computers, should be possible in oceanographic applications in the near future. Again, this type of development could be made transparent to the user.
The size, shape, and material of the electrode can be tailored to the application. Problems of response time, for example, often can be solved by using a smaller electrode. The use of new electrode materials, including possibilities for modification of the electrode surfaces, could lead to new measurement capabilities. This emphasizes the importance of the development of polymers, new materials, and recognition chemistry, as discussed in the section on new chemistry. Size, shape, and choice of material all present new technical opportunities that can be applied now, but also present many
possibilities for longer-term development. Routine practice in laboratories lags significantly behind present technical capabilities. Modern instrumentation is qualitatively superior to that of even the mid-1980s.
Major commercial efforts have gone into the development of sensors based on microelectrodes fabricated by means of lithography. Commercial products have not resulted because markets have not been identified that would support the volume required for economical production. Deterioration of response with time for in situ measurements might be decreased by employing multiple electrodes and using each only once. For example, present lithographic technology can allow production of line electrodes 20 micrometers wide, individually addressable, with 80-micrometer spacing. A 10-centimeter-long strip would contain 1000 electrodes.
The electrode material also may be manipulated to achieve more specific or more reliably controlled performance. Specific catalysts for desired reactions may be incorporated into the electrode material or bound to the surface of the electrode. A present example is the coating of a carbon or other inert electrode with a polymer film impregnated with a mercuric salt. The resulting electrode is catalytic for reduction of metals, such as Pb2+, that are soluble in mercury. This is an area of research that could pay off through qualitative improvements in accuracy, precision, and response time.
Developments in electroanalytical chemistry are driven by technical advances in electronics, computers, and materials. Present scientific capabilities available in a research laboratory will be applicable for field measurements with the advent of smaller, less expensive, more powerful computers. Miniaturization of electrochemical cells, which can improve performance, especially response time, can be implemented most effectively in the context of miniaturization of control circuitry. Concomitant low cost could make disposable systems a practical reality. Sophisticated data analysis and data handling techniques can, with better facilities for computation, be handled in real time.
SPECTROPHOTOMETRY
Absorbance
Spectrophotometry encompasses a broad family of techniques, but fundamentally is very simple: the analyte in a sample (perhaps with a reagent added) absorbs some fraction of light at some selected wavelength, which may be correlated with the analyte's concentration by Beer's Law. The essence of the method is to compare the amount of light absorbed by a sample in the presence and absence of a specific analyte. The instrumentation necessary for spectrophotometry has been available for nearly half a century; however, diode array detectors have changed the performance of
single-wavelength instruments radically. The various forms of spectrophotometer differ primarily in details of light sources, dispersive element, and detector. The classical Beckman and Cary spectrophotometers can measure absorbance in the ultraviolet (UV) to near-infrared (near-IR) (250 to 1500 nanometers). Classical IR spectrophotometers use different sources and bolometric detectors to cover the range from about 2500 to more than 10,000 nanometers. Fourier transform IR (FT-IR) spectrophotometers use a more complex technique to cover the same wavelength range, with significant advantages (see section on infrared below). Atomic absorption spectrophotometers make use of lamps producing line spectra of particular elements to detect volatilized atoms of those elements by their characteristic absorbance lines. Photoacoustic and thermal lensing spectrophotometries make use of the conversion of absorbed light into heat to detect absorbance indirectly by measuring sound evolved and deflection by changes in refractive index, respectively.
For the purposes of chemical oceanography, spectrophotometry is limited to wavelengths from the UV to near-IR (250 to 1500 nanometers). The various forms of spectrophotometer differ primarily in combinations of light sources, dispersive elements, and detectors.
The principle of spectrophotometric measurement involves the comparison of two similar light levels, leading to an inherent weakness in the technique. In particular, as the concentration of absorbing analyte decreases, the two light levels become more and more similar until they cannot be distinguished. Thus, the sensitivity and accuracy of the method are very dependent upon the stability of the light source, the precision of the detectors, and the reproducibility of the sensor matrix. As a rule of thumb, with non-solid state sources and detectors, absorbance differences of 0.0001 unit are difficult to measure. The maximum extinction coefficient for a molecule in the visible or near-IR region (determined by the oscillator strength) is about 2 × 105 liter mole-1 cm-1. Thus, the detection limit for classical spectrophotometry is perhaps a few nanomoles per liter of "solvent." More commonly, detection limits are set by the presence of interfering contaminants in the sample; this is especially true for complex media like seawater.
Some spectrophotometric techniques work to enhance sensitivity or utility in other ways. The advent of semiconductor diode array detectors permits entire spectra to be acquired simultaneously instead of one wavelength band at a time. Also, automated spectrophotometric analyzers originally developed for clinical use have been adapted for use at sea when many samples must be analyzed over a period of time. Computational techniques for signal averaging, smoothing, integration, and data analysis have been widely implemented in modern instruments. From the standpoint of chemical oceanography, spectrophotometry is a mature technology that is widely used and accepted.
Spectrophotometry can also be employed after reaction of the analyte with a reagent or indicator to enhance or enable an analysis to be made. Many analytes of interest, such as metal ions, are determined indirectly as complexes with organic molecules. Thus, cupric ion [Cu(I)] has a modest absorbance by itself, but has a 1000-fold greater absorbance when complexed with bathocuproine. Moreover, some such complexes are insoluble in water and may be extracted from aqueous solutions as a preconcentration step before spectrophotometry. Preconcentration has been widely used in trace analysis by spectrophotometry.
Ideally, the indicating chemistry is reversible, allowing continuous long-term measurement. If nonreversible chemistry is used to form a color, provision must be made for continuous delivery of the reagent indicator, especially if it consumed in the analysis. In general, this is more difficult than the spectrophotometric measurement itself. Various clever methods have been suggested for continuous reagent delivery, while true long-term, unattended operation is not yet demonstrated. On board ships, however, "field" spectrophotometric instruments have proven rugged and reliable, and are very widely used, particularly for enzyme-based analyses. The sampling rate can be rather high, with continuous operation possible when the spectrophotometer is used with an immersible pump and connecting pipe to the depth of interest.
Developments in traditional forms of spectrophotometry, as well as new methods, could find greater use in ocean measurements. Spectroscopy based on absorption of visible light may have reached a limit in its traditional form, however. Spectrophotometry will remain in wide use due to its ease, low cost, and great versatility. In many ways it remains the first choice for analysis, but its low sensitivity makes it useful for a rather limited spectrum of analytes.
Many analytes listed in Table 1 have been measured spectrophotometrically in seawater for some time, including many metal ions and some gases, although spectrophotometry is the preferred method for only a minority. Some analytes, like alkanes, are spectrophotometrically "silent," or do not form colored complexes with other reagents. Similarly, individual nuclides cannot be distinguished by classical spectrophotometry, and many of the other analytes, such as halogenated pesticides and metal alkyls, are more easily determined by other methods, such as gas chromatography with electron capture detection, or emission spectroscopy. Indeed, many of the analytes, such as zinc or copper, are present at trace levels and are not measurable by spectrophotometry.
Infrared
Infrared spectroscopy is a well-established technique for determining molecular structure. It is essentially an absorption spectrophotometric tech-
nique using a different wavelength. Because spectral bands are much sharper than those in the UV-visible region, highly selective determinations can be made. Information is particularly rich in the mid-IR region of 3 to 15 micrometers. More recently, some very useful results have been obtained using wavelengths of 0.7 to 3 micrometers. IR spectroscopy is not a highly sensitive technique, since absorption coefficients are low, particularly for dissolved samples.
Very promising is the use of FT-IR spectroscopy as a detector for gas or liquid chromatography. With proper extraction and preconcentration steps, FT-IR can be a useful technique for studying organic compounds in the ocean. This approach will be limited to land-based instruments for now, but shipboard instruments are conceivable. As for remote mapping, FT-IR combined with chemometrics shows promise for classifying materials in the surface layer of the ocean (Bronstein and McGrath, 1989; Kolber et al., 1990).
FT-IR is a relatively new method of performing IR adsorption measurements. In classical IR spectrophotometry, the absorbance of a sample is measured as the wavelength (or frequency) is varied. In FT-IR, an entire spectrum is acquired simultaneously as an "interferogram," in which the absorbance data are encoded; the spectral data are obtained by calculating the Fourier transform of the interferogram. The advantages of this approach are its efficiency, in that it effectively scans the entire spectrum at once rather than serially in small increments; its generally higher resolution; and the capability for digital signal averaging and other manipulations that are otherwise difficult. The main disadvantage is the relative cost and complexity of the instruments; instrument costs are decreasing, however.
Instrumentation for IR spectroscopy has gone through a major change since the availability of FT-IR. Improved noise levels and spectral resolution have resulted. Complete systems can be purchased for under $30,000, with little need for user intervention. These systems are not quite rugged enough for oceangoing applications, but future developments in making them more rugged are forthcoming. It is useful to note that entire FT-IR instruments have been sent to explore the atmosphere of Mars, so size, weight, and power requirements are readily met.
Luminescence
Luminescence (emission) spectroscopy encompasses photoluminescence-based techniques such as fluorescence and phosphorescence, plasma-excited luminescence, and chemi-and bioluminescence. Although these techniques differ in their means for raising the sample to an excited electronic state, all are based on measuring the light subsequently emitted when the excited atom or molecule returns to its ground state. These techniques are more
sensitive than the absorption spectrophotometric techniques described above. In absorption spectrophotometry, the instrument must distinguish the difference between two similar light levels, whereas in emission spectrometry (ideally) the blank or background produces no signal at all. The fact that the exciting light in photoluminescence methods is a different color than the emitted light permits the former to be filtered out. In the case of chemi-and bioluminescence there is no exciting light per se, no light filtering is necessary, and detection limits below attomolar (10-18 mole) quantities are achieved with simple instruments.
Fluorescence and phosphorescence techniques are powerful, versatile, and widely used in chemical analysis. While few of the analytes listed in Table 1 are intrinsically fluorescent as solutes in seawater, some can be determined directly or indirectly using added indicator molecules. For example, aluminum ion can be determined by the fluorescence of the 8-hydroxyquinoline complex ion. Hundreds of organic chelators exhibiting this behavior are known. Specificity can be a problem, however, because some compounds will bind up to 25 different ions. Some indicating schemes rely on fluorescence upon binding a chelator to the metal ion, a less useful method because of the higher baseline signals. Luminescence methods are less applicable with nonmetals and anions, because fewer photoluminescent complexes are known. The most widely used emission spectroscopic technique in ocean science is fluorescence, primarily because it permits sensitive determination of chlorophyll. In situ fluorometers designed to measure chlorophyll are now commercially available, an index of the broad interest in this analyte. The sophisticated "double pulse" fluorometer provides a measure of the fraction of chlorophyll actually engaged in photosynthesis, and thus the fraction of photosynthesizing biomass that is functional (Kolber et al., 1990). Chemical reactions designed to attach fluorescent markers to amino acids allow measurement at natural concentrations by high-performance liquid chromatography. This is a model for techniques that might be developed for other types of organic compounds. Fluorescence has also been used to provide semiquantitative measurements of oil (NRC, 1985).
Phosphorescence is growing in importance for condensed phase determinations as techniques become available for reducing the quenching effects of solutes such as oxygen; at present it is practical mainly for solid samples. Today's instruments, with powerful dye lasers for excitation, offer detection limits in the micromolar range; sample preconcentration further increases sensitivity while at the same time minimizing the deleterious effects of high salt concentrations on background levels.
An important adjunct to photoluminescence methods is the use of time resolution. Following excitation, photoluminescent species spend a brief time in the excited state before subsequent re-emission of the photon. For classical fluorescence, this lifetime is in the nanosecond range, whereas for
phosphorescence, the emission commonly takes micro-or milliseconds to appear. Different molecules exhibit lifetimes ranging from 10-3 to 10-11 seconds due to many different phenomena. Thus, by mechanically, electronically, or optically "gating" the detector, photoluminescence emission from interfering species may be temporally filtered out, enhancing the signal-to-noise ratio. The success of this approach may be inferred from published determinations of lanthanides at the 10-13 molar level (Diamandis and Christopoulos, 1990).
Chemiluminescence and bioluminescence offer the most sensitive of all the luminescence-based measurements (equaling radioisotope methods), while requiring only modest instrumentation. These methods are based on light emission from chemically generated excited state molecules. Thus, there is no need for excitation light. For instance, adenosine triphosphate may be detected at attomolar concentrations by hand-held instrumentation in seconds using firefly luciferase. Relatively few analytes of interest participate directly in chemiluminescent reactions, however, so methods must be designed to relate the chemi/bioluminescent intensity to the analyte level using some coupled reaction, which limits this method to a small number of analytes. It might be possible to alter microorganisms genetically to yield the desired analyte-chemiluminescent reaction. Using integrating detectors such as charge-coupled devices or fast photographic film, as little as a few enzyme molecules become detectable (Bronstein and McGrath, 1989). Some recent research has shown that chemiluminescent methods may be adapted for in situ determinations of some metal ions (see the section on flow injection analysis). Nitrate, iron, manganese, copper, and cobalt are all detected with chemiluminescent techniques.
While chemiluminescence is easy, inexpensive, sensitive, and often selective, it remains little used because of the difficulty in coupling recognition chemistry to chemiluminescent reagents. The instrumentation is simpler than for fluorescence and thus can be constructed for in situ use; luminometers for monitoring bioluminescent organisms are commercially available. However, the technique requires consumable reagents, which are somewhat more difficult to incorporate into an immersible sensor for continuous monitoring, although Johnson and his colleagues (see the section on flow injection analysis) have reported progress in this area. Luminol chemiluminescence has been used from time to time for some analytes such as transition metals, but the method is nonspecific and requires use of strongly basic compounds for the reactions. Peroxyoxalate chemiluminescence has been reported as useful for determining certain fluorescent molecules in liquid chromatography, but remains seldom used. A recent advance of substantial interest is the development of bis-adamantanyldioxetane derivatives as chemiluminogenic enzyme substrates (Schaap, 1988). These molecules are extremely stable until hydrolyzed by enzymes such as alkaline phosphatase, whereupon they emit
light. At present, chemiluminescence is not the method of choice for any of the analytes listed in Table 1.
Atomic emission spectroscopy can be employed, generally with an inductively coupled plasma for thermal excitation. The sample is introduced into the plasma as a mist of ultrafine droplets, and the monochromator and detector are set to measure the intensity of an atomic emission line characteristic of the element. This technique is powerful, general, sensitive, linear, and able to measure over 70 elements, and, as a result, is widely used. Response is typically linear over four orders of magnitude in concentration with relative standard deviations of 1 to 3%. In low-salt aqueous solutions, detection limits range from 10 to 1000 nanomolar without preconcentration. Significant problems with saline samples remain, but use of Babington nebulizers alleviates these problems somewhat.
All of these emission techniques have been used to analyze seawater, but their suitability for use at sea or in situ varies. Inductively coupled plasma emission spectroscopy permits determination of 60 elements sequentially or simultaneously with good sensitivity and precision; however, it is not sensitive enough for some of the rare earth elements and trace metals without pre-concentration, and is obviously unsuited for molecules. While inductively coupled plasma techniques are unsuited for in situ use, they have been integrated into flow injection and chromatographic systems. Incorporation of extraction and preconcentration into a continuously operating system appears difficult. Probable future developments in this technology include improvements in sample introduction to the plasma, particularly with techniques such as capillary electrophoresis and supercritical fluid chromatography; optical improvements such as multichannel high-sensitivity detectors and acousto-optic tunable filters; Fourier transform-based instrumentation; and improved chemical methods for extraction and pre-concentration.
Optical and laser technologies also offer the prospect for improvements in fluorescence and phosphorescence determinations. An important development is the use of fluorescence and phosphorescence with optical fiber sensors.
Raman
Raman spectroscopy is based upon the Raman effect, in which a fraction of light incident on a sample will scatter at wavelengths differing from that of the original wavelength. The change in frequency is determined by the composition of molecules in the sample. Thus, a sample of water excited with light having a wavelength of 400 nanometers will emit scattered light not only at 400 nanometers but also at a trio of longer wavelengths (near 463 nanometers), whose frequency difference from the excitation cor-
responds to the stretching frequency of-OH bonds in water. The Raman spectrum thus detects the same phenomena as does infrared spectroscopy, but the relative intensities of bands measured by the two techniques differ.
Raman scattering is a weak phenomenon; before lasers offered powerful, monochromatic light, Raman was an effect observed rather than exploited. Until very recently, spectra often required hours to acquire, even using lasers with several watts of power. A substantial limitation arises from interference by photoluminescent impurities in the sample, generally constraining Raman spectroscopy to longer wavelength lasers, even though the strength of the scattering declines as the fourth power of the frequency of the incident light.
At present, Raman spectroscopy for ocean measurements is limited by the weakness of the phenomenon and the type of equipment required. Seawater exhibits background fluorescence due to dissolved organic solutes, and thus sensitivity is further compromised. An especially important interferant is chlorophyll, which fluoresces upon excitation with argon lasers.
Two variations of Raman spectroscopy offer substantial signal enhancement. The first is resonance Raman spectroscopy, in which the intensity of scattering is enhanced up to 106-fold, increasing the speed and selectivity of this method. In some cases, the wavelength of excitation may be chosen to maximize the enhancement of a particular component in a solution. A second important Raman spectroscopic technique is surface-enhanced Raman spectroscopy (Garrell, 1989). The strength of the signal is greatly enhanced when the solute of interest becomes adsorbed to a metal surface such as etched platinum. This technique has been combined with electrochemical methods so that the adsorption of a solute, such as amino acids, proteins, and other organic molecules, to the surface of an electrode is modulated by electrical potential or redox reactions. This method can be exploited with optical fibers and metal colloids and may ultimately offer the potential for in situ applications. Other important new developments in Raman methods include ultraviolet excitation and Fourier transform Raman spectroscopy. For examination of particles, molecular orbital laser excitation, coupling laser-Raman with scanning electron microscopy, was developed.
Finally, the introduction of new detectors, such as diode arrays and charge-coupled devices (CCDs), has been a boon for Raman spectroscopy. CCDs permit the accumulation of light in the manner of photographic film; additionally, their noise level is lower than that of the photomultiplier tube. In addition, by combining CCDs or diode arrays with optical dispersive elements, entire spectra may be collected in fractions of a second.
Recent work by Angel and Myrick (1990) suggests that certain contaminants might be monitored by Raman spectroscopy in situ through optical fibers, but the above caveats still remain. The depths accessible would be limited mainly by optical loss in the fiber. The use of the fiber permits
the expensive, fragile components to remain aboard ship; even so, it would not be trivial to construct a seagoing Raman spectrometer for shipboard use. A relatively high sampling rate (one per millisecond, for instance) might be achieved. Adequate power and cooling water, and a stable platform must be available. Raman signals can emanate from the fiber itself. Power consumption by the lasers typically used (a few kilowatts) is too great to deploy a Raman spectrometer on an autonomous buoy.
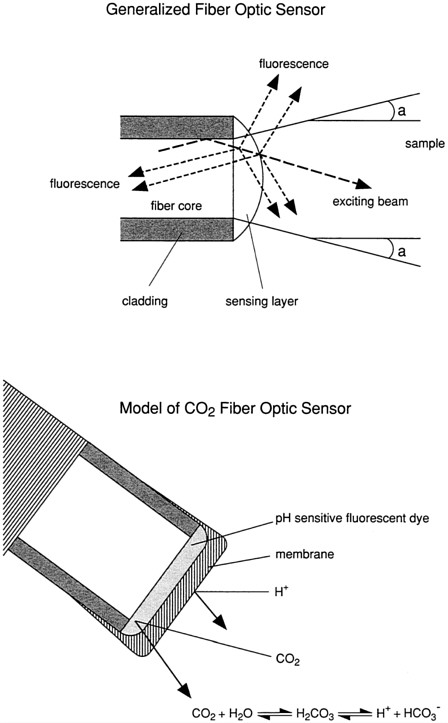
Fiber Optics (Wolfbeis, 1991; Angel, 1987; Seitz, 1988)
Fiber optics can be used to deliver light over long distances. Fiber optic sensors provide a means to measure properties of seawater continuously using changes in the characteristics of a transmitted light beam. To make measurements, light is sent down an optical fiber to excite the sample (Figure 2). The sample in its natural state (for example, chlorophyll) or as reacted with a dye modulates the light by absorbance or it emits fluorescent light, which is carried back to a detector via the same, or a second, fiber. The concentration of the material to be measured can be determined by the degree of absorbance, by the amount of emitted light, or by the excited state lifetime. Optical fibers consist of a ''cladding'' of one type of glass or plastic surrounding a core of another type, so that light is trapped in the core and can be transmitted over long distances. The attenuation of the signal depends on the color of the light and the fiber material. If a larger fiber is used, the signal increases, but so does the noise.
Many analytes do not absorb or emit light. In these cases, indicators must be employed. Optical sensors are made by attaching a selective indicator layer to the distal tip of an optical fiber. Optical sensors employ well-known spectroscopic principles; the innovation is in making sensors for measuring specific substances dissolved in seawater using indicators, biological molecules, or various reagent systems. Some of the advantages of optical sensors are that they are small (50 to 250 micrometers), are free from electromagnetic interference, and allow continuous monitoring. The research challenge is to develop suitable indicators that provide stable measurement. Another advantage is that no direct electrical connections are required. In addition, fiber sensors can be multiplexed; that is, a single instrument can support multiple sensors, for samples at different depths or for different analytes. Optical fibers are useful for applications where it is difficult to transfer power to a remote device because of their low attenuation. Calibration and stability can be improved, in some cases, by employing ratio measurements using indicators having multiple absorption or emission peaks. By ratioing the peaks, calibration curves can be derived that are independent of the absolute dye concentration (at various analyte concentrations), light intensity, and other effects.
One application for fiber optic sensors is for measuring CO2 in seawater. Sensors are being developed with solution-filled tips behind a membrane permeable to CO2. Recent collaborative work has resulted in a CO2 sensor with resolution equivalent to 7 µatm pCO2 (Goyet et al., 1992). For CO2 sensors, reversible indicators are used. In systems where irreversible chemistry may be useful, reagent delivery systems may be employed to replenish reagents continuously (Luo and Walt, 1989).
Sampling with fiber optic sensors can be continuous if needed; otherwise they can be operated discontinuously, with a lower duty cycle. These sensors could be used for laboratory-based or in situ applications. The cost of instrumentation for fiber optic systems should be $25,000 to $50,000. Sensors would need to be replaced periodically (several weeks to many months), depending upon their design. Sensors using fiber optic probes will be available within 5 years for some applications and within 10 years for some others. Sensors for pH, CO2, and O2 are in development now; new sensors should be capable of measuring from high concentrations down to 1 part per million for ions and organic materials. Basic research is still required for specific applications.
Refractive Index (Eisenberg, 1965; Yeung, 1986)
Salinity measurements are most often used in oceanography to determine seawater density. The conventional measure used by oceanographers for determining salinity is conductivity. This is feasible because the salt content of seawater is well defined, as is the temperature-related compressibility. As an alternative, the refractive index of water is a good descriptor of density when temperature is known or can be measured. Refractive index provides a high-precision method for determining the density of pure water. As various salts are added, the refractive index is a less exact predictor of density, although relative measurements can still be useful.
Commercial refractive index instruments are available, with a light beam and two photodiodes. If there is no difference in refractive index, and thus density, between sample and reference cells (such as a quartz crystal), light hits both photodiodes equally. If the reference cell and sample cell differ in density, diffraction pushes the beam to one side. This device does not depend on the absolute intensity of the light beam. The response time is on the order of 0.1 second. The precision of the bench top model is to the sixth or seventh decimal place. The position sensor has a high dynamic range, and the device has no moving parts. This technology is ready for evaluation for oceanographic measurements with very little additional developmental work. A coil of optical fiber with a refractive index close to that of seawater, combining clad and unclad fibers, could possibly provide a more sensitive device, although intensity depends on fiber coil geometry. Calibration will be difficult for these sensors. The effect of biofouling could be
reduced by using relative measurements (since the intensity is not crucial) and by various chemical, optical, and mechanical means. The cost of these devices is $5,000.
PIEZOELECTRIC MASS SENSORS (ALDER AND MCCALLUM, 1983; CAREY AND KOWALSKI, 1986; THOMPSON ET AL., 1986; WARD AND BUTTRY, 1990)
A piezoelectric mass sensor is a device that measures the amount of material adsorbed on its surface by the effect of the adsorbed material on the propagation of acoustic waves. Piezoelectric devices work by converting electrical energy to mechanical energy. There are a number of different piezoelectric mass sensors. Thickness shear mode sensors measure the resonant frequency of a quartz crystal. Surface acoustic wave mode sensors measure the amplitude or time delay. Flexure mode devices measure the resonant frequency of a thin Si3N4 membrane. In shear horizontal acoustic plate mode sensors, the resonant frequency of a quartz crystal is measured.
These piezoelectric crystal oscillators are very accurate mass sensors because their resonant frequencies can be measured precisely with relatively simple electronic circuitry. For certain quartz crystals, the resonant frequency is inversely related to the crystal thickness. A crystal resonating at 5 megahertz is typically 300 micrometers thick. If material is coated or adsorbed on the crystal surface, the resonant frequency will change (decrease) in proportion to the amount of material added. The effect of adsorbed mass on the oscillator frequency varies according to the operational mode of the device. In any case, interpretation of mass via changes in frequency or amplitude assumes that the coated films are rigidly elastic and infinitesimally thin (that is, an extension of the crystal).
Piezoelectric mass sensors have been used for gas phase sensing (for example, SO2, H2S, and HCl) using crystals coated with chemisorbent films (including polymers) that interact specifically with these gases. Even immobilized enzymes have been employed as selective coating materials (for example, formaldehyde sensing using immobilized formaldehyde dehydrogenase; Guilbault, 1984). Indeed, the nature and selectivity of the coating material used to achieve chemical recognition of the target species is the key to fabricating useful analytical sensors based on piezoelectric devices.
The use of piezoelectric mass sensors for solution phase measurements is still under development. Whereas considerable success has been achieved with these devices for detecting the mass of electrodeposited analytes in solution (on thin films of conducting metal electrodes deposited on the quartz crystals), detecting mass changes due to selective chemical interactions at the surface (for example, antibody-antigen reactions) has been less successful. Changes in frequencies do occur, but the direction and magnitude of change are not always predictable. The main reason for this is that
piezoelectric mass sensors respond to the viscosity of the sample in the microenvironment at the crystal-solution interface. This surface viscosity can change (even when bulk phase viscosity does not) as a result of such surface chemical interactions, and also in response to temperature. In fact, piezoelectric devices are very good viscometers. This can be corrected for to some extent by using a reference crystal (in a differential measurement mode) that does not possess the chemically selective reagent on its surface, or a reference crystal with a nonactive form of the same chemically selective coating. Similar differential designs are also required to minimize effects of nonspecific adsorption. In practice, it may be difficult to match the nonspecific binding properties of the reference and analytical crystals. This problem can limit the accuracy of analytical data obtained with these mass-sensitive devices in the presence of multiple analytes. Adsorption onto the surface of the selective chemical coating is generally reversible when the free energy of adsorption is less negative than about-16 kilo-joules per mole.
The rate of sampling with piezoelectric sensors is limited by their physical characteristics and present technology to the millisecond range for applications in the liquid phase. The technique is versatile in that it can be used in a variety of locations. The solid state electronics necessary to operate the piezoelectric sensor are easily miniaturized, and data can be recorded continuously or periodically. A small computer with a reasonable memory could easily record data over long times. There may be some problems in deep-sea locations, simply because of the complications in packaging the sensor for high-pressure environments. although this problem may be surmountable.
The main advantage of piezoelectric devices is that, in principle, any process that results in a mass change at an interface can be measured. However, this very nonselective transduction process is also a major disadvantage in that it mandates the use of even more selective surface chemistries than are required for other types of chemical transducer systems. This will make the implementation of piezoelectric chemical sensing devices for ocean measurements rather difficult, but by no means impossible. Indeed, the coupling of pattern recognition techniques with an array of marginally selective piezoelectric transducers may, in the future, make these devices more useful for quantitative ocean measurements.
NEW CHEMISTRY
Immunochemistry
Immunoassays can be carried out by creating antibodies that bind with molecules of interest (antigens). Antibodies are created by injecting the
antigen into rabbits (or other animals, such as chickens) and then extracting the resulting antibodies. Assays can be made sensitive by labeling a given amount of antigen with fluorescent or radioactive compounds, or enzymes, thus creating a labeled analyte reagent. The difficulty in making antibodies to low-molecular-weight analytes depends on the molecules; it can take 6 months and several animals to produce a useful polyclonal antibody reagent. Monoclonal antibodies are more selective for particular compounds, but are more costly to develop. Antibodies have a shelf life of more than I year, with refrigeration.
An example of an immunoassay is the enzyme-linked immunosorbent assay (ELISA), which uses 96-well plates with antibodies bound on the sides of the wells. The characteristic of interest is the binding constant for the immunoreaction, which is defined as the association constant between antibody and antigen. It is important to generate a good antibody with strong binding.
Of the two classes of immunoassays, heterogeneous and homogeneous, homogeneous assays are generally much faster and easier to automate, although the detection limits are not as low as for heterogeneous assays. Homogeneous assays for small analyte molecules are based on competitive binding, and can be direct or indirect.
A practical factor limiting the routine use of immunochemistry for environmental monitoring is that many U.S. Environmental Protection Agency-approved methods require analyses by gas chromatography and mass spectrometry (GC-MS) for measuring pollutants. This is a case in which state-of-the-art techniques cannot be applied because government regulations mandate use of techniques that were well developed when the regulations were formed. Immunochemistry techniques could be faster and cheaper than GC-MS, although they may not be as sensitive in some cases. Analysis of certain samples may require some extraction steps. For example, many organic compounds bind to particulates and must be extracted before analysis. Whether immunoassays can be implemented with these organic extracts remains to be seen.
It requires about 2 hours to carry out an immunoassay, in either land-based or shipboard laboratories, using a single 96-well microtiter plate and associated plate reader. Simultaneous use of multiple plates can further increase sample throughput. New reagents must be developed for each analyte. It is expensive to make appropriate reagents and develop the chemical procedures, but the subsequent cost per sample is very low. These techniques are available now; what remains is to develop methods, that is, to make antibodies toward the desired analytes.
Making antibodies to some small molecules and ions, particularly if they are insoluble, reactive, volatile, or toxic, can be difficult. Few antibodies to the analytes listed in Table 1 have been developed. A variety of
dissolved organic carbon molecules could be measured by immunoassay. Various chloro-and fluorocarbons may be among those few that can be detected, although the issues of solubility and compound immunogenicity could be important factors, depending on the specific compounds to be sensed. One class of analytes of great importance for sensor development are the toxins released by toxic algae under bloom conditions. Present techniques of detection are to monitor toxin concentrations accumulated by suspension-feeding organisms. In addition, it should be possible to develop reliable and selective immunoassays to detect free metal ion levels (e.g., manganese and iron) using antibodies raised toward specific organic ligand chelates of these metal species. For example, a heterogeneous immunoassay method was recently reported for the detection of Hg(II) with high selectivity over all other heavy metals (Wylie et al., 1991).
Polymers and New Materials
An area that offers tremendous potential for advancing the state of the art of measurement technologies is that of polymer and materials science. This field has been identified as one of the critical technologies essential to maintaining U.S. industrial competitiveness. In recent years, high-temperature superconductors, thin diamond films, and lightweight ultrastrong polymeric materials have been developed. These materials and others should have a major impact on the ultimate successful implementation of ocean instrumentation. These developments will contribute to a variety of disparate areas of sensor and instrument design.
Materials with selective binding or transport properties will have a major impact on sensor design and fabrication. Selectivity in either binding or transport can be exploited for a variety of measurement needs. This selectivity can be either intrinsic, that is, built into the chemical properties of the material, or coupled with selective carriers that allow a non-selective material to be converted into a selective one (see the section on recognition chemistry). An example of the latter is the use of valinomycin as a selective carrier in a polyvinyl chloride membrane to form a potentiometric potassium ion sensor. Advances in the fields of gas separation materials for air purification and membrane development for desalinization are contemporary examples illustrating the importance of selective materials. As these materials are identified, they can be exploited for the design of selective measurement schemes.
A second area in which new materials will have an impact on oceanographic measurements is that of corrosion resistance. Many materials, particularly metals, are incompatible with the ocean environment. Consequently, alternative materials or suitable protection measures must be developed. New corrosion-resistant materials are essential. For example, organic con-
ducting polymers could presumably replace certain metallic conductors. Films of diamond or other materials may be employed to encapsulate materials sensitive to corrosion.
Biofouling is another problem with deployable ocean instrumentation that could be reduced by using new materials. Presently, toxic antifoulants must be released continuously to prevent adhesion and growth of organisms on virtually all submerged materials. This poses a particular problem for analytical instrumentation, as both the antifoulant and biological layers can perturb or interfere with the measurements being made. Ideally, more passive methods to prevent biofouling should be utilized. Advances in understanding microbial adhesion will assist in the design and preparation of suitable new materials. Similar advances in developing new materials for tissue and organ transplants compatible with blood and the responses of the human defense system may be transferable directly to reducing the biofouling on ocean instruments.
Finally, many new materials will affect the development of ocean instrumentation and sensors. These include new optical components, such as lenses, filters, light-emitting diodes and laser diodes, conducting ceramics or organic polymers that can be used in the preparation of new electrodes, piezoelectric devices, and new materials for energy storage. These areas undoubtedly will significantly affect the ability of analytical chemists to design new sensors, develop new transduction schemes, and construct new instruments.
Recognition Chemistry (Dobler, 1981; Lehn, 1985; Izatt and Christensen, 1987)
Successful development and implementation of various chemical sensors for ocean measurements (based on optical, electrochemical, or mass transducers) requires concomitant advances in the design or discovery of organic or inorganic molecules that interact selectively with the important ocean analytes. These developments are particularly important for in situ sensors where no separation of ocean components or addition of external reagents occurs before or during the measurement step.
The importance of recognition chemistry has been highlighted in several of the previous sections. Developments in recognition chemistry are important for the design of in situ sensors and are a high-priority research area. In practice, the surfaces or membranes of any in situ sensing device will contain chemical species that interact selectively and reversibly with the analyte to yield a detectable signal. For example, in the glass membrane pH electrode, already used widely in the ocean for in situ pH measurements, negatively charged silicon oxide (Si-O-) sites of the glass interact with protons. Increased proton activity reduces the amount of negative
charge on the surface of the glass, thereby altering the phase boundary potential between the surface of the glass membrane and the solution. The activities of other cations (for example, Na+ and K+) required to yield the same phase boundary potential are much greater (sometimes as high as 1011 times, depending on the exact composition of the glass material), because these species exhibit much weaker interactions with the Si-O- sites. This is perhaps the simplest, yet most impressive, example of analytically useful chemical recognition and selectivity. The challenge is to achieve this level of selectivity (while maintaining reversibility) within other electrochemical devices, as well as newer optical and piezoelectric devices.
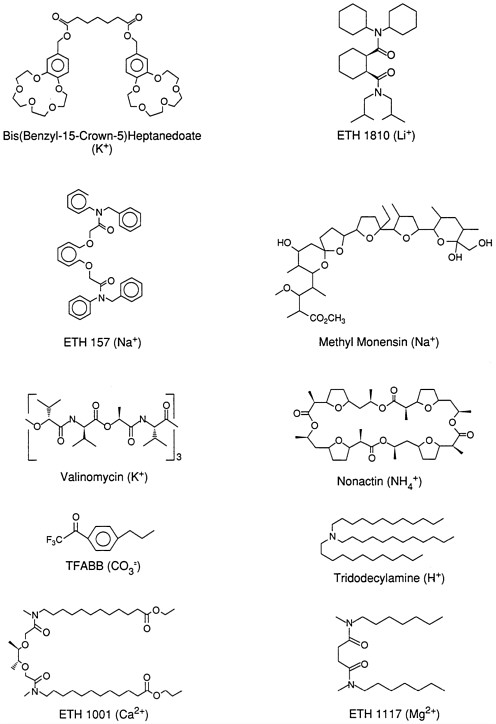
Synthetic organic chemistry is critical to progress in this area. Indeed, the design of metal ion binding ligands for use in sensors, as well as for preparative separation processes in metallurgy and related areas, is currently an active area of research. A wide range of crown ethers, cryptands, hemispherands, and acyclic molecules containing electronegative oxygen atoms or mixed oxygen and nitrogen electron donor atoms in appropriate geometric positions within these ionophore structures have been synthesized, and some of these compounds have been used for the development of relatively selective optical and electrochemical sensors for Na+, Ca2+, Li+, K+, and most recently Mg2+ (Figure 3). Naturally occurring antibiotics with macrocyclic structures, such as valinomycin (for K+) and monensin (for Na+), may also be used for the fabrication of ion sensors. Other molecules possessing various combinations of oxygen and sulfur atoms (as ether links) or all-sulfur-based thiacrown structures exhibit selective recognition of certain transition metals (Ag+, Cu2+, Zn2+, and others). In all cases, cation selectivity is dictated by the size and shape of the binding site formed by the three-dimensional configuration of electronegative oxygen, nitrogen, and sulfur atoms.
The design and synthesis of molecules that interact with specific anionic species has received far less attention. In contrast with cationic recognition, there is no known generic arrangement of electrophilic atoms that provides the basis for recognizing and distinguishing anions. While the synthesis of the macrocyclic structures with multiple positively charged nitrogen sites, in the form of guanidinium or polyamines, has been successful (in terms of binding various anionic species), the resulting structures do not exhibit analytically relevant anionic selectivities. Moreover, the presence of positive charges on these structures, required for anion interaction, is highly pH dependent, and this limits the potential in situ application of such chemical recognition reagents. Indeed, such sensors can only function effectively when the sample solution is buffered or a buffered region exists at the surface of the sensor. An alternative strategy involves the synthesis of organic molecules possessing electrophilic carbon atoms that can react reversibly with certain nucleophilic anions (particularly CO32-) to form an
ionic adducts. Generally, trifluoroacetophenone-type derivatives have proven useful for such purposes, and optical as well as electrochemical carbonate sensors based on this novel recognition chemistry have been developed.
The use of metal ion-ligand complexes as well as organometallic species to achieve guest-host-type recognition has also received considerable attention for the selective binding of both anions and neutral species, including gases. For example, there is evidence suggesting that dibasic phosphate interacts selectively with certain dibenzyltin dichloride structures, and nitrite can bind specifically as an axial ligand to the central Co(III) ion of vitamin B12 structures. These interactions have been used to devise sensors for these anions, although the exact chemistry involved is not yet fully understood. In preliminary studies, metalloporphyrins and metallophthallocyanines with varying metal ion centers [e.g., Mn(III), In(III), Co(II/III), and Sn(IV)] and peripheral structures have also proven useful for achieving some chemical recognition of anions and gases. Here, the nature of the metal center and the exact three-dimensional structure of the surrounding porphine or phthallocyanine ligand can control the degree of binding of anions and gases (for example, O2) as axial ligand to the metal. So-called picket fence-type porphyrins are structures in which the nearly planar metalporphine ring is at the bottom of a pocket formed by large organic molecules attached in one direction from this planar base structure. While certain Co(II) and Fe(II) picket fence porphyrins can bind oxygen gas reversibly, possibilities also exist for incorporating hydrogen binding sites within the appended picket fence chains so that the binding of larger neutral and anionic species can be stabilized within the pockets of these structures.
The use of large organic structures containing three-dimensional chemical recognition pockets is not limited to porphyrin-type molecules. Indeed, various cyclodextrins exist in which the orientation of the repeating carbohydrate structure forms a hydrophobic inner core that can interact selectively with hydrophobic chemical analytes of appropriate size (benzene, pyrene, phenols, and others). Similar principles of recognition by size exclusion or inclusion can be achieved with certain polymeric structures, particularly when target chemical species are present during the polymerization process (for example, polypyrrole). In such cases, called molecular imprinting, the target ionic or neutral species can act as a template to dictate the final three-dimensional structure of the resultant polymeric film, thereby creating a polymeric material with a preferential interaction or permeability toward the target species.
The committee recognizes that the design and synthesis of molecules or structures that exhibit useful chemical recognition properties is extremely difficult, with a high risk of failure. Advanced molecular modeling methods are beginning to be applied to this area, but preliminary results are
mixed. In many cases minimum-energy structures that are predicted to have ideal conformations for selective interactions with target species do not bind the target species with adequate selectivity. Mimicking the inherently high selectivity of biological molecules, such as enzymes and antibodies, may be the most rational approach to achieving selective chemical recognition through classical organic and inorganic chemistry. Indeed, with powerful advances being made in elucidating protein structures (via two-dimensional nuclear magnetic resonance spectroscopy, x-ray crystallography, and other means), it may now be possible to synthesize much smaller molecules with binding sites (for anions, cations, gases, and neutral organics) that are similar to those of proteins that are known to exhibit selective interactions with these target species. Regardless of the approaches taken, it is clear that a considerable investment of time and resources will be required to develop the arsenal of chemically selective and stable host compounds that will be required to measure all the key oceanic species via in situ chemical sensor technology. The committee believes that such an investment would be worthwhile, given the great potential of selective chemical reactions for in situ use in the ocean.
Chromatography and Electrophoresis (Ewing et al., 1989; Pimental and Coonrod, 1987)
Chromatography and electrophoresis are used to separate dissolved constituents in seawater. Chromatography is based on partition of the individual components between gas or liquid passed through a column and the liquid or solid stationary phase. This partition is based on solubilities of dissolved material in the different phases and specific chemical interactions with column components. Electrophoresis separates materials on the basis of electrical charge and size as solvents flow through the plate.
Gas chromatography and liquid chromatography are mature separation techniques that are used for many ocean measurements. They have proven to be invaluable in the analysis of samples for organic compounds, by separating components that have different chemical or physical properties. Instruments are attached to the end of the columns to detect, quantify, and identify the components. Many different types of detectors have been used in conjunction with chromatography. Most notable is the combination of chromatography with mass spectrometry, whereby much information can be obtained from a single sample. Chromatographic techniques can also be used simply as an extraction or preconcentration step before subsequent chemical measurements. Chromatography takes advantage of a number of the properties of molecules and ions in solution and at surfaces. Their behavior in seawater is often vastly different than in fresh water, given the higher ionic strength of seawater, so that greater study of the behavior of
ions and molecules in seawater will be needed to advance the field of analysis of materials dissolved in seawater.
Advances in chromatography in the past 20 years have been achieved both in the chromatographic columns and in detectors that measure concentrations of chemicals in the outflow from the column. Improvements in column chemistry and in flow and solvent characteristics have increased speed, selectivity, and resolution of chromatographic sewparations. Many of the spectroscopic techniques described in this report, including mass spectroscopy, are used at the effluent end of gas chromatographic or high-performance liquid chromatography columns to measure concentrations by a range of so-called ''hyphenated'' techniques such as GC-MS, GC-FTIR, HPLC-UV-fluorescence diode array detectors, and others. Recent developments in computer control of flow regimens and mixtures of carrier phase solvents show promise for improving chromatographic techniques. Capillary chromatography with supercritical liquids and field-flow fractionation also show promise (Pimental and Coonrod, 1987). The latter technique allows separation of larger macromolecules and particles by application of temperature differences or electric fields across a flow.
Chromatographic systems are inexpensive, and are generally rugged enough to be operated on a research vessel or could be modified relatively easily for this purpose. It is possible to pass large volumes of water through an adsorbent in a device that is the size of an double-A battery, for preconcentration and separation.
Ion chromatography is very promising for ocean measurements. Anions and cations with concentrations below parts per billion can be separated and detected. The chromatographic retention time provides information on the chemical form of the ion, its oxidation state, and whether it is complexed with another material.
Future developments that may facilitate ocean measurements from vessels or buoys include miniaturization of chromatographic equipment (so less solvent is needed per analysis), new solvent transport systems, such as electrokinetic transport, to reduce power requirements on the pumps, and more sensitive detectors for liquid chromatography. Certain combinations of very short columns and flow injection analysis are also promising for real-time studies.
Electrophoresis has been used for decades to separate organic molecules on the basis of molecular charge. Capillary zone electrophoresis combines high voltage with electrophoretic mobility to measure ionic compounds. By adjusting the pH, many organic compounds can be made ionic. If one uses fused silica capillaries, there is bulk (electro-osmotic) flow of solution. The combination of electrophoretic and electro-osmotic flows brings improved separation. Detectors are placed at the end of the flow, as in chromatography. This method can be used to determine the major ions in
seawater. Detection with on-column preconcentration is possible for concentrations down to 1 part per billion. There are hybrid methods that allow separation of neutral solutes by interactions similar to liquid chromatography. The power requirements for capillary zone electrophoresis are low, so it could be used to drive flow injection analysis. The minimum cost of these devices is $15,000 for commercial systems.
Flow Injection Analysis and Continuous Flow Analysis
Flow injection analysis (FIA) is a robust method for automating complex chemical analyses (Ruzicka and Hansen, 1988). It is relatively simple and can be adapted for use with a variety of detectors, including spectrophotometers, fluorometers, mass spectrometers, and electrochemical analyzers. It has been used on board ships to determine dissolved nutrients (Johnson et al., 1985) and trace metals (Sakamoto-Arnold and Johnson, 1987; Elrod et al., 1991). Unsegmented continuous flow analysis (CFA) systems based on the principles of FIA can operate in situ over the entire range of depths found in the ocean (Johnson et al., 1986a, 1989).
An FIA system consists of a pump, injection valve, manifold of capillary tubing and a flow-through detector (Figure 4). The injection valve is omitted from CFA systems. Chemical reagents are mixed continuously by pumping them together with a carrier solution. The seawater sample is periodically introduced into the carrier stream by the injection valve in FIA or introduced continuously in CFA. The mixture then flows through the detector. Typical analysis times range from 1 to 8 minutes for chemical determinations in seawater. A variety of manipulations can be performed with FIA systems. These include concentration with resin columns, solvent extraction, dialysis, separation with membranes, and derivatization. FIA systems are commercially available and are also easily constructed.
Flow injection analysis with chemiluminescence detection (FIA-CL) can be used to determine a variety of metals in seawater, including cobalt, copper, manganese, and iron, with detection limits in the picomolar concentration range. These reactions typically involve the formation of a metal complex with an organic ligand and oxidation of the complex. Reaction products in the excited state decay by emitting a photon of light which can be used to quantify the metal concentration. FIA-CL systems are capable of achieving precision and accuracy similar to those obtained by graphite furnace atomic absorption spectrophotometry systems.
A second advantage of FIA and CFA is their ability to operate underwater for extended periods of time (Johnson et al., 1986b). It is often desirable to obtain high resolution profiles of dissolved chemicals to examine their spatial and temporal variability. CFA systems have been used to measure spatial variability in nutrient distributions in the upper ocean (Johnson
FIGURE 4 Schematic of the FIA system used to determine cobalt in seawater. This system incorporates two valves, which allows cobalt to be concentrated on a column of immobilized 8-hydroxyquinoline (8-HQ) and magnesium ions in the seawater matrix to be removed by washing the column with water at pH 5.6. Reprinted with permission from Sakamoto-Arnold and Johnson (1987). Copyright 1987 American Chemical Society.
et al., 1989) and in redox reactive chemical concentrations around hydrothermal vents (Johnson et al., 1986a). These systems have used conventional peristaltic pumps to propel the sample and reagents through the system.
Good spatial and temporal resolution can be obtained with systems based on peristaltic pumps because they produce high flow rates. However, systems based on peristaltic pumps are limited to deployment times of no longer than a few days because of the lifetime of the tubing used in the pump. The feasibility of using osmotic pumps to propel the sample and reagents for longer deployments is now being explored. Osmotic pumps are widely used for in vivo drug delivery systems. They can run for extended periods of time and do not require an external power source, as they operate on the difference in the osmotic pressure between the external seawater and an internal filling solution (Theeuwes and Yum, 1976). Typical flow rates with osmotic pumps are 1 to 10 microliters per hour. A reagent pump would consume a reagent volume of about 10 milliliters per year, and a sample pump would accumulate a sample volume of about 100 milliliters per year. The response time of an osmotically pumped system would be limited by the time required to flush the flow cell of the detector. At these low flow rates, at least 12 minutes would be required to flush a 1-centimeter-path-length photometer cell. However, for long-term deployments of up to 1 year, sampling rates of once per hour would be sufficient. Osmotically pumped CFA systems are now operating in the laboratory, and ocean deployments are expected to begin in the near future.
A CFA system based on osmotic pumps would be extremely simple, and quite inexpensive to build. Instruments based on this design begin to fall within the realm of chemical sensors. A sensor system is usually considered to be much less complex than the typical FIA system. However, the mechanical simplicity of an osmotically pumped CFA system would certainly fall within the framework of a chemical sensor (Ruzicka and Marshall, 1990).
The primary constraints on development of FIA for oceanographic research do not now appear to be the mechanical and electrical systems needed to operate them on board ship or in situ. Long-term stability of reagents will be a problem for deployments with osmotically pumped systems. These reagents must be stable for year-long time periods. The stability problem might be solved by using the time release strategy employed in drug delivery.
Robotics (Hurst and Mortimer, 1987)
Two hundred and fifty million chemical analyses are performed each day in the United States for quality assurance, environmental monitoring, medical diagnostics, toxicology, forensics, commerce, research and development, and other purposes. This large number of analyses is due in part to
a rapid increase in the number of laws mandating chemical testing. The increase in testing has surpassed the increase in the training of analytical chemists, which creates the need for faster, high-quality automated analyses. The need for analyzing an increasing number of samples with a diminishing supply of trained analysts may be addressed by the development of automated chemical instrumentation. To promote the development and use of laboratory automation, the National Institute of Standards and Technology formed the Consortium on Automated Analytical Laboratory Systems in 1990, with members from industry and the federal government.
Different analysis regimens, defined by operational complexity and the number of samples that must be run each day, require different types of automation (Figure 5). Dedicated automation includes processes that are highly automated for repetition of a single task. Although Technicon Autoanalyzers® have been taken to sea for 20 years, these fall into the dedicated automation category and are not good examples of the potential of flexible automation, which allows automation of numerous sequential steps. Flexible automation includes more sophisticated laboratory robots, with a spectrum of operational complexities and a moderate number of samples. These robots are called flexible because they can be reprogrammed for different tasks and for a series of tasks that comprise a laboratory procedure. In actual practice, however, once a robot system is built for a specific analysis, it is rarely reconfigured for another method. The use of robots does not always decrease expense. Software development often accounts for a large percentage of development costs. Setting up automated systems requires expertise in computers and mechanical engineering. Usually it takes so much time to remove system errors that robots are not very flexible. Automated processes should not necessarily be designed with the same steps as those used by human technicians, because of the different sensor sets.
Robots have been used in laboratories for a number of applications, including ELISA, transfer of bacterial plaques from agar plates to liquid media, analysis of cranberry juice, and microwave dissolution of environmental samples. How does the performance of laboratory robots compare with that of technicians? Robots are usually slower than technicians because their sensor sets are inferior to those of humans and thus more testing is required to ascertain completion of actions. Robots do not get interrupted, and their performance does not degrade over long periods of time. Sampling rates by robots depend on the procedure being automated. Technicians usually do better than robots on accuracy and precision in the short run, but robots perform better over the long run. Robotic precision has been improved by switching from volumetric to gravimetric measurements. For example, in making chromatographic calibration standards, better than 1% precision can be achieved, using mixtures robotically prepared by gravimetry.
FIGURE 5 Decision matrix for choosing whether or not to automate a laboratory method, and if so, what kind of automation to use. Courtesy of Zymark Corporation, Hopkinton, Massachusetts, USA.
Robotic errors tend to be major, so that errors can be spotted more easily. In fact, robot-based laboratory procedures can provide a record of all steps for quality control purposes.
Because of vibrations, power stability, and particularly corrosion, commercial laboratory robotic systems available today would have problems on ships. This problem provides an opportunity for research engineers to develop means to modify some sections of the ship to improve power and platform stability. Environmental constraints of a sea-based system were never factored into the design of today's laboratory robots, but the systems could be modified somewhat to reduce these problems. For now, robots would be most feasible for land-based measurements. In the future, however, robots could be important for at-sea measurements, because continuous or repeated measurements are often made over the course of many days.
The base cost for a robot is about $25,000. The average complete cost for an analysis system that actually does a routine analytical task is two to five times this amount. This includes the costs of set-up, creating custom devices, custom software, and training. Land-based systems are available now, and sea-based systems are a distinct future possibility. Time, talent, and money will be needed to develop them. Robots can be set up to carry
out virtually any kind of analysis. Sea-based robotic systems must be designed to handle the power problems, the vibration and stability problems, and the corrosion problems presented by a shipboard environment.
Chemometrics
Analytical chemistry deals with quantitative, numerical concepts and with data. Chemists use statistics routinely to analyze their data and to present it, but usually at an elementary level. A relatively new subfield within chemistry is chemometrics. Chemometrics involves the application of multivariate statistics, mathematics, and computational methods to chemical measurements. It exists at the interfaces among the larger fields of chemistry, mathematics and statistics, and computer science. The goals pursued are varied and include designing and selecting optimal measurement procedures and experiments, gathering the best-quality analytical data, and gleaning the maximum amount of useful chemical information from the data. Specific areas of focus include statistics, sampling, experimental design, optimization, signal processing, factor analysis, resolution, calibration, modeling and parameter estimation, structure-property relations, pattern recognition, library searching, and artificial intelligence. Statistical thinking and methodology pervade this list, although the emphasis varies among items. Martin et al. (1988) suggested that application of chemometric methods to data obtained from marine samples could help classify organic components in samples, detect patterns in the data that merit further study, and serve as a noise filter.
The progress of the field of chemometrics can be traced with the aid of a series of six review articles published at 2-year intervals in Analytical Chemistry (for example, Brown, 1990) which cover noteworthy advances. A series of recent textbooks also cover the field (for example, Massart et al., 1988). Recent topical reviews on subjects related to multivariate analysis of analytical data have appeared (for example, Jurs, 1990). Relatively few formal courses in chemometrics exist at either the undergraduate or graduate level. Chemometrics education is largely ad hoc, via short courses, symposia, textbooks, and tutorials. A compilation of tutorials taken from Chemometrics and Intelligent Laboratory Systems is available (Massart et al., 1990). Topics included are experimental design and optimization, signal processing, multivariate calibrations, and model building. This brief discussion of chemometrics is meant to provide sources of details about chemometrics methods rather than giving the basic information itself. The books and review articles cited provide a thorough introduction to the major topics covered here that could be important for ocean measurements.
Experimental design, response surfaces, and optimization of experiments are all important components of chemometrics. Many trade-offs exist among
the number of experiments performed, the number of variables that can be tested for effect, and the efficiency of approach to the optimum experiment with the fewest number of replications. The simplex method (Walters et al., 1991) has been employed widely for optimization, especially in chromatography. For simplex optimization, the number of experiments needed to reach an optimum set of conditions is less than all possible combinations. This method is evolutionary in that it directs the experiment toward the optimum, the computations are simple, and it is amenable to automation. Self-optimizing analytical systems based on simplex optimization have been described in the literature. The use of self-optimizing analytical instruments would be of great value for unattended instrumentation in ocean measurements. The application of standard experimental design methods to oceanic measurements should be a routine part of designing all experiments, particularly those that are expensive to perform and difficult to repeat.
Calibration and mixture analysis addresses the methods for performing standard experiments with known samples and then using that information optimally to measure unknowns later. Classical least squares, iterative least squares, principal components analysis, and partial least squares have been compared for these tasks, and the trade-offs have been discussed (Haaland, 1992). These methods allow a superior spectroscopic experiment to be performed because they allow the calibration of the method to depend in an optimal way on the experimentation done. These calibration methods ensure that the best measurements are done. They are especially useful for noisy data with substantial interferences, such as those found in many types of ocean science measurements.
Multivariate model building allows the construction of models to connect independent variables and observations. Multiple linear regression analysis, principal components regression, partial least squares, and many other advanced statistical techniques have been used. The goal of such investigations may be estimation of the parameters in a mathematical model to assess the relative importance of the independent variables on the dependent variable, or it may be to generate the ability to predict unknown observations. Validation of the models constructed using either internal validation (such as jackknifing or duplexing) or external prediction (true prediction of unknowns) is an important component of such studies. Another important component of such studies is the determination of how to choose the best set of independent variables from among those available. Principal components analysis, factor analysis, and partial least squares have been used successfully in this area, especially for spectral data.
Classification and clustering methods allow the analysis of data when the observations to be explained are not quantitatively known. Discriminants can classify patterns of data into categories. Clustering methods can
seek common characteristics among subsets of data taken from a larger data set. These classes of analysis can be used fruitfully with very large data sets to search for patterns among the data. Certain chemometric methods have been applied to ocean science for some time, primarily for data analysis. Optimization and some other newer chemometric techniques are just beginning to find their way into marine chemistry. Often, it is desirable to put the data into categories or classes, and these pattern recognition methods are well suited to such tasks.
Some of the software to support chemometric methods is available in commercial form. Other software can be located by searching the literature or compilations such as the Scientific Computing and Automation Resource Directory. However, many methods of chemometrics are available only from the developers or through reports in the scientific literature rather than as software. It is likely that software well suited to ocean science would have to be produced by combining commercially available software with custom-written software to gain the specificity needed.
Communications
One of the primary benefits of chemical sensors is the ability to obtain chemical data in real time. Real-time data can be used to adjust and optimize experimental protocols and sampling design as data are obtained. Communication of these data is not now a problem for chemical sensors that are operated on board ship, or which are tethered to the ship by a hydrographic cable. Electromechanical cables with two or four conductors are almost universally available on research ships. These cables have a bandwidth great enough to transmit data at 4800 baud over 10,000 m. This should be sufficient for most ship-based chemical sensor applications. Far greater bandwidths can be obtained with fiber optic cables. These are not now in general use for oceanography, as there is limited demand for them, but submarine fiber optic cable and repeaters are available.
Data communications is a much greater problem for remotely operated systems, such as those deployed on moorings in mid-ocean. A limited commercial capability for transmitting data via satellite is provided by Service Argos, Inc. The Argos system is flown aboard the NOAA Advanced TIROS-N satellites in a low earth orbit. As a result, Argos transmitters require little power, and complete units weigh as little as 165 grams. An Argos transmitter aboard an oceanographic buoy can send only 32 bytes of data to a satellite on each orbit, however. The mean number of satellite passes per day ranges from 7 at the equator to 28 at the North and South Poles. There is no capability for two-way communication, which would allow instructions to be transmitted to the sensor.
Several other alternatives to the Argos system exist, but all have prob-
lems. The NASA GOES satellites, in geostationary orbits, now have the capability for continuous data transmission at 100 baud. This data rate will become 1200 baud in the near future. The transmitters require 30 watts of power, which is difficult to provide on a continuous basis for year-long time periods, but is feasible for intermittent use. This system can accommodate a limited number of users. Commercial communications satellites can also be used, but the transmitters again require large amounts of power and a large antenna.
Communications will be a problem for retrieving data from remotely operated sensors as they become available. The benefits of near-real-time transmission of data from remote sites are clear. The data can be incorporated into oceanographic models to improve model predictions. Transmission of the data also eliminates the risk of losing the complete data set if the buoy is lost. For telemetered data, processing close to the sensor should be emphasized, to reduce the size of the data stream. For example, computed concentrations, rather than raw data, should be telemetered. Chemometric procedures programmed into the instruments can be used to help in this data reduction process. A new generation of satellites that provide the equivalent of a global cellular communications network will be necessary to take full advantage of chemical sensor systems.