B2
Overview and Background: Mechanism of Action of Antiprogestins
NANCY L. WEIGEL, Ph.D.
Department of Cell Biology, Baylor College of Medicine, Houston
INTRODUCTION
Potent and specific progesterone antagonists have been sought for many years. Although some progress had been made in developing such compounds (Raynaud and Ojasoo, 1986), it has been only a little more than a decade since the first progesterone antagonist, RU 486, was developed by researchers at Roussel-Uclaf (Philibert et al., 1982). Other progesterone antagonists have also been described (Philibert et al., 1981; Neef et al., 1984; Raynaud and Ojasoo, 1986). To date, all of the described progesterone antagonists also show at least some degree of glucocorticoid antagonism (Raynaud and Ojasoo, 1986; Mao et al., 1992). Thus the search for the "ideal" progesterone antagonist continues.
Although the mechanism of action of the various progesterone antagonists may differ, studies suggest that most, if not all of the antagonism, is mediated by the progesterone receptor. The progesterone receptor is a member of a superfamily of nuclear receptors that includes the other steroid receptors as well as the thyroid hormone, retinoic acid, and vitamin D receptors (Evans, 1988).
In order to understand the mechanism of action of antagonists, it is first necessary to understand the structure and function of the receptors themselves. The members of this family of receptors share three regions of homology. The locations of these three regions within the human progesterone receptor are shown in Figure B2.1. The first and most highly conserved is termed C1. It encodes the DNA binding domain and contains two zinc finger structures (Evans, 1988). The other two regions of homology, C2 and C3, are small segments of the carboxyl terminus,

FIGURE B2.1 Location of the conserved regions in the steroid-receptor superfamily. Shown here, as an example, is the structure of the human progesterone receptor. C1 is the conserved region containing the DNA binding domain. C2 and C3 are conserved regions important for ligand binding and receptor dimerization. The numbers represent the number of the first amino acid in each of the conserved regions and the last amino acid in the receptor. SOURCE: Reproduced from Weigel et al. (1993).
which are contained within a larger region important for ligand binding and for receptor dimerization. Upon activation, the receptors dimerize, bind to specific DNA sequences termed steroid response elements, and activate the transcription of target genes. Typically, the activation of receptors is studied by using artificial target genes on plasmids that contain one or more of the specific response elements placed 5' of a promoter and a cDNA for an enzyme such as chloramphenicol acetyl-transferase which can be easily assayed (Denner et al., 1990b). The activity of the receptor can then be studied by transfecting the reporter gene (and a plasmid that expresses receptor if necessary) into a target cell, treating with the desired agonist or antagonist, and measuring the resulting enzyme activity. Although there are many similarities in the mechanisms of action of the various steroid receptors, there are also significant differences. The structure and function of the human progesterone receptor are described below.
STRUCTURE AND FUNCTION OF THE PROGESTERONE RECEPTOR
The human progesterone receptor is expressed as two forms, hPR-B and hPR-A. Both are derived from the same gene, but are produced from different mRNAs (Kastner et al., 1990). The PR-A is essentially a truncated version of the PR-B lacking the 164 amino terminal amino acids (Figure B2.2). These receptor forms share common hormone binding and DNA binding domains. Either form can activate transcription of a target gene in cells co-transfected with the corresponding expression vector for PR-A or PR-B, as well as a suitable reporter plasmid (Bocquel et al., 1989). However, the two forms differ in their relative activities, depending upon the target gene studied (Bocquel et al., 1989). In the cases examined thus far, both forms are expressed in cells and in tissues that contain progesterone receptor (Horwitz and Alexander, 1983; Lessey et al., 1983; Feil et al., 1988). Although it is presumed that both forms exist in the same cells, this has not been unequivocally demonstrated.
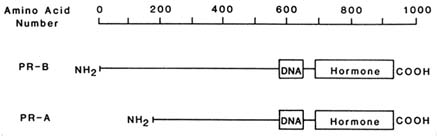
FIGURE B2.2 The structures of hPR-B, hPR-A, and the regions containing the DNA and hormone binding activities.
The Hormone Binding Domain
The ligand binding domain resides in the carboxyl terminal 30 kDa of the progesterone receptor (Figure B2.2). Despite the large size of this domain, analysis of this region using both deletions and point mutations suggests that most of this region is required to maintain the high-affinity hormone binding activity (Carson-Jurica et al., 1990; Danielson, 1991). Photoaffinity labeling studies of the glucocorticoid receptor using R 5020 show that two amino acids, which are about 150 amino acids apart in the linear sequence, are close enough in the three-dimensional structure of the receptor that they both react with the same portion of the R 5020 molecule (Carlstedt-Duke et al., 1988). This suggests that the region is extensively folded back upon itself to produce the binding pocket for the ligand. Consistent with these data is the observation that the entire hormone binding domain is relatively resistant to digestion by a number of proteases when bound with R 5020 (Allan et al., 1992).
Within this domain reside two additional functions of the receptor. The active form of the receptor is a dimer, and the region most important for receptor dimerization is found in the ligand binding domain (Fawell et al., 1990). In addition, this domain is important for transcriptional activation of steroid receptors.
The DNA Binding Domain
The DNA binding domain is a highly conserved region that is responsible for the DNA binding specificity of the receptor. The region contains two zinc finger structures (Umesono and Evans, 1989). The first zinc finger is important for the interaction with DNA, and the second is involved in dimerization with the other receptor molecule (Hard et al., 1990). Although some members of the steroid-receptor family are capable of heterodimerizing with other members of the family (Berrodin et al., 1992; Yen et al., 1992), to date only homodimers of the progesterone receptor have been observed. However, there are two forms of
the progesterone receptor, PR-A and PR-B, and these are capable of forming both homodimers and heterodimers as measured by gel retardation assays (El-Ashry-Stowers et al., 1989) and by immunoprecipitation (DeMarzo et al., 1991). Whether such heterodimerization occurs in vivo and plays a role in preferential activation of specific target genes is not yet known.
Transactivation Function
The amino terminal portion of the progesterone receptor is not required either for DNA binding or for hormone binding. However, it is important for transcriptional activation (Tasset et al., 1990). Studies have shown that there are two regions in the progesterone receptor that are important for transcriptional activation (Bocquel et al., 1989; Meyer et al., 1990). The first is the amino terminus of the protein, and the second resides in the hormone binding domain (Bocquel et al., 1989; Meyer et al., 1990). The relative importance of these two domains depends on both the target gene and the cell type in which they are examined.
Association with Other Proteins
In the absence of hormone, the progesterone receptor is found associated with several non-steroid binding proteins including two heat shock proteins, hsp90 and hsp70, as well as some less well characterized proteins (Dougherty et al., 1984; Smith et al., 1990). The role or roles of these proteins in vivo have not been elucidated. These large receptor complexes each contain a single steroid binding molecule. The receptors are not in the dimer form and do not bind to DNA, which suggests that one role of the non-steroid binding proteins is to inhibit DNA binding of the receptors in the absence of ligand. There is evidence, in the case of the glucocorticoid receptor, that the heat shock protein complex is important for maintaining the integrity of the hormone binding site in the absence of ligand (Pratt et al., 1990), but the progesterone receptor does not appear to require the heat shock proteins to maintain ligand binding activity.
Phosphorylation of the Progesterone Receptor
The steroid receptors, including the human progesterone receptor, are phosphoproteins (Dougherty et al., 1982; Housley and Pratt, 1983; Grandics et al., 1984; Denner et al., 1987). The human progesterone receptor is phosphorylated at numerous sites (Sheridan, et al., 1989), and its phosphorylation is increased in response to hormone treatment. Beck et al. (1992) have shown that the receptor in T47D breast cancer
cells undergoes a rapid (less than 10 minutes) twofold increase in phosphorylation in response to R 5020 administration, followed by a slower additional phosophorylation that results in a receptor form with decreased mobility on SDS (sodium dodecyl sulfate) polyacrylamide gels. Takimoto and coworkers (1992) have shown that the final phosphorylation that alters receptor mobility occurs only if the receptor can bind to DNA. Weigel and coworkers (1992) have shown that the chicken progesterone receptor is phosphorylated during in vitro transcription assays by a DNA-dependent kinase in HeLa nuclear extracts. This enzyme was identified as the double-stranded DNA-dependent kinase purified by Carter et al. (1990). Subsequent studies by Bagchi and coworkers (1992) have shown that the human progesterone receptor undergoes a similar DNA-dependent phosphorylation in vitro, suggesting that this enzyme may be responsible for the DNA-dependent phosphorylation observed in T47D breast cancer cells. In addition to the phosphorylations that are common to both PR-A and PR-B, PR-B has at least two additional phosphorylation sites as judged by mobility on SDS gels and by peptide mapping (Sheridan et al., 1989; Beck et al., 1992). These sites may play a role in the differential activities displayed by the PR-B and PR-A forms.
The role of phosphorylation in progesterone-receptor function has not been determined directly. However, receptor isolated from cells treated briefly with R 5020 so that the phosphorylation is enhanced shows enhanced specific DNA binding (Beck et al., 1992). Moreover, treatment of cells with activators of kinases enhances the receptor-dependent transcriptional activity (Beck et al., 1992), indicating that phosphorylation may also enhance the transcriptional activity of the receptors.
Progesterone-Receptor Function
In the absence of hormone, the progesterone receptors is found in a complex with heat shock proteins in the cytosol fraction of cell homogenates. Based on these types of assays, the unliganded receptor was originally believed to be cytoplasmic. However, immunocytochemical studies suggest that in vivo the unliganded receptor is found in the nucleus (Perrot-Applanat et al., 1992a, b). This binding is less tight than in the presence of hormone since receptor isolated from hormone-treated cells is tightly bound to the nuclear fraction and requires high-salt treatment for extraction. There is evidence that the progesterone receptor cycles continuously between the cytoplasm and the nucleus in the absence of hormone and that this recycling and nuclear retention require energy (Perrot-Applanat et al., 1992a, b). Taken together, the data suggest that in the absence of hormone, the bulk of
the receptor molecules are loosely bound in the nucleus but that they cycle through the cytoplasm with perhaps a relatively short residence time in the cytoplasm. In contrast, the distribution of the glucocorticoid receptor, which also cycles (DeFranco, 1991), appears to favor cytoplasmic localization in the absence of hormone.
A proposed model for the mechanism of progesterone-receptor activation and the possible steps at which antagonists might act to inhibit receptor function are shown in Figure B2.3. Treatment with hormone results in dissociation of the receptor-heat shock protein complex, dimerization of the receptor, and binding to specific DNA response elements termed PREs, or progesterone response elements. Since the glucocorticoid receptor recognizes the same DNA sequence, these elements are also termed GREs or PRE/GREs. Although one or two of these elements placed in the 5' flanking region of a target gene are sufficient to produce a gene whose transcription is activated in response to progesterone (Tsai et al., 1989), natural target genes have much more complicated flanking regions consisting of sequences that bind many transcription factors. In some cases, genes that are steroid inducible do not appear to contain consensus steroid response elements in their 5' flanking regions. Thus, the receptors may also act at nonconsensus sequences through interaction with other factors to induce or repress genes.
Studies in vitro demonstrate that the receptor is important for formation of stable preinitiation complexes that then allow transcription of the target gene (Klein-Hitpass et al., 1990). Characterizing the proteins with which the steroid receptors interact to activate transcription is currently an active area of research. It is likely that cell-specific and target gene-specific transcription factors play important roles in the extent and nature of the response to activation of steroid receptors.
MECHANISMS OF ACTION OF PROGESTERONE ANTAGONISTS
Shown in Figure B2.4 are the structures of progesterone, the commonly used progesterone agonist R 5020, and examples of two antagonists, RU 486 developed by Roussel-Uclaf and ZK 98 299 developed by Schering. Although the two antagonists appear to have very similar structures in this two-dimensional depiction, the C and D rings of RU 486 are fused in the trans position, whereas the C and D rings of ZK 98 299 are fused in a cis position. Thus in three dimensions the structures are quite different, and as described below, the antagonists appear to inhibit progesterone-receptor function by different mechanisms.
Evidence that the progesterone antagonists act through the progesterone receptor comes from many studies. First, antagonists compete
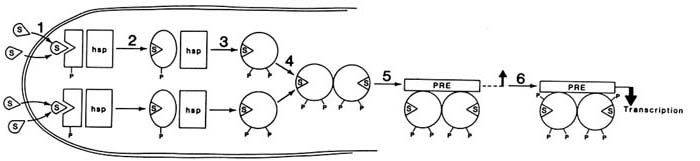
FIGURE B2.3 A model for the mechanism of action of the human progesterone receptor. In the absence of steroid, the receptor is associated with heat shock proteins and is basally phosphorylated. In this model, each P represents a class of phosphorylation sites rather than a single site. Binding of hormone (step 1) results in a conformational change in the receptor (step 2), which allows additional phosphorylation and dissociation of the receptor-heat shock complex (step 3), dimerization between two receptor molecules (step 4), binding to a steroid response element (step 5), and additional phosphorylation and interaction with the appropriate transcription factors to produce a transcriptionally active complex (step 6). NOTE: hsp = heat shock protein complex including hsp90 and hsp70; P = a class of phosphorylation sites; PRE = progesterone response element; S = steroid.
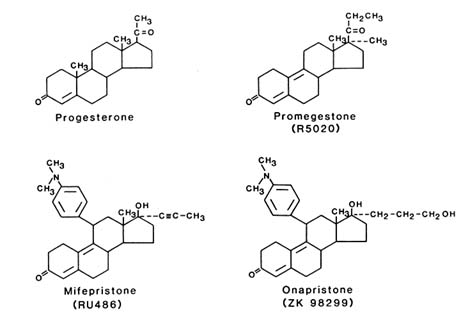
FIGURE B2.4 Structures of progesterone agonists and antagonists. Shown in the upper portion of the figure are the structures of progesterone and the progesterone agonist R 5020 (promegestone) and in the lower portion, the two antagonists RU 486 (mifepristone) and ZK 98 299 (onapristone), which are representative of the classes of antagonists that promote DNA binding and prevent DNA binding, respectively.
with progesterone itself and with progesterone agonists for binding to the progesterone receptor, and with dexamethasone and triamcinolone acetonide for binding to the glucocorticoid receptor (Philibert et al., 1981; Elger et al., 1986; Raynaud and Ojasoo, 1986; Kalimi, 1987). These studies have been done both in extracts containing endogenous receptors and in expression systems, such as the yeast expression system, where binding can be shown to be dependent upon the presence of the expressed receptor (Vegeto, 1992). Similarly, the progesterone-dependent transcriptional activation either of endogenous progesterone receptors (Beck et al., 1993) or of transiently transfected receptors (Meyer et al., 1990) can be inhibited by progesterone antagonists. The primary question is by what mechanism antagonists act to block receptor function. Transcriptional activation of the progesterone receptor is a multistep process and antagonists potentially can act at several places in the process as shown in Figure B2.3. Antagonists may (1) block the binding of progesterone to the receptor, (2) alter or block the conformational changes associated with the binding, (3) block dissociation of the heat shock protein complex, (4) alter or block receptor dimerization, (5) alter or block DNA binding, or (6) alter interaction with
other factors to produce transcriptionally active receptors. Different antagonists may act at different steps in receptor function, and a single antagonist may affect more than one stage of receptor activation.
Binding of Antagonists to Progesterone Receptors
The initial studies of RU 486 showed that the compound competed with progesterone and glucocorticoid agonists for binding to the progesterone and glucocorticoid receptors. Additional information specifically on the mechanism of action of RU 486, and on RU 486 and breast cancer, can be found in the reviews of Mao et al. (1992) and of Horwitz (1992), respectively. Other progesterone antagonists also compete for steroid binding. Surprisingly, RU 486 does not bind to either the chicken or the hamster progesterone receptor (Baulieu, 1989), and none of the other reported antagonists bind to the chicken progesterone receptor. Although there are many amino acid differences in the ligand binding domains of the progesterone receptors, a single amino acid substitution is sufficient to convert the chicken progesterone receptor from a protein that does not bind RU 486 to a protein that does bind it (Benhamou et al., 1992). This exquisite sensitivity suggests that studies of antagonists for potential clinical applications will have to be done with human steroid receptors, since receptors from other species may respond somewhat differently.
Although competition studies suggest that the antagonists are competing for the agonist binding site, detailed studies also showed that the binding of agonists and antagonists was not identical. In particular, the binding of agonists and RU 486 showed different sensitivities to sulfhydryl reagents, suggesting either a different availability of sulfhydryl groups in the receptors or differences in the interaction of the steroids with sulfhydryl groups (Moudgil et al., 1989). That the antagonists do in fact bind differently has been shown in several studies. Allan et al. (1992), using partial proteolysis of in vitro translated 35S-methionine-labeled receptor as an assay for receptor conformation, have shown that all of the receptor is susceptible to proteolysis in the absence of ligand. In the presence of agonist, limited proteolysis produces a resistant fragment of just under 30 kDa. Essentially the same fragment was produced whether the receptor was in the heat shock complex or was dissociated from the heat shock proteins. Fragments of similar sizes were produced by a variety of proteolytic enzymes, suggesting that there is a protease-resistant core in the presence of agonist. When the antagonist RU 486 was used, a resistant fragment, which was approximately 3 kDa smaller than that detected with agonist, was produced, indicating that the conformation induced by RU 486 was different from that produced by the agonist. Immunoprecipitation with C262, a mono-
clonal antibody that is specific for the 14 carboxyl terminal amino acids of the receptor, showed that the receptor fragment from the agonist-containing digest was immunoprecipitated, whereas the smaller fragment from the antagonist-containing digest was not recognized (Allan et al., 1992). This study demonstrated that the carboxyl terminal portion of the receptor is not protected by the antagonist although it is protected by the agonist. Vegeto et al. (1992) described a carboxyl terminal deletion mutant of the progesterone receptor that was incapable of binding the agonist R 5020, but still bound RU 486 with essentially normal affinity. These data imply that the primary determinants of RU 486 binding are different from those of R 5020 binding. It is possible that the bulky hydrophobic substitution on the RU 486 is interacting with another portion of the ligand binding domain, which allows it to bind in the absence of the carboxyl terminal amino acids. Presumably the binding sites for the agonist and antagonist are overlapping; nonetheless, it is clear that the extreme carboxyl terminal portion of the receptor is unnecessary for RU 486 binding, whereas it is critical for binding of R 5020.
Formation of Dimers and DNA Binding Complexes
Initial studies with the glucocorticoid receptor (Moguilewsky and Philibert, 1984; Formstecher et al., 1988) had suggested that RU 486 inhibited dissociation of the receptor-heat shock protein complexes and that the mechanism for antagonism might be due to failure to produce DNA binding complexes. Studies have shown that RU 486 does not block dissociation of the progesterone receptor-heat shock protein complex and that RU 486 does permit receptor dimerization and DNA binding (El-Ashry-Stowers et al., 1989). The receptor is found in the nucleus as a result of RU 486 treatment (Sheridan et al., 1988), and additional studies have shown that the RU 486-bound receptor can compete for the same DNA binding sites in vivo (Meyer et al., 1990). However, the conformations of the dimers and of the DNA complexes are different from those produced with agonist alone. The DNA complexes have typically been examined using a gel retardation assay in which the receptor is mixed with 32P-labeled PRE and unlabeled nonspecific DNA, and the resulting protein-DNA complexes are detected by gel electrophoresis and autoradiography. The free DNA migrates very rapidly, and the bound receptor complexes, which are much larger, migrate more slowly. The receptor produces three dimer forms, A-A, A-B, and B-B. Because of the difference in the sizes and possibly the shapes of these complexes, the three forms are detected as three closely spaced bands in gel retardation assays (El-Ashry-Stowers et al., 1989). In the absence of hormone, the receptor does not bind to DNA and no receptor complexes are detectable. RU 486 also produces
three receptor forms, but these forms have somewhat higher mobility than the agonist-containing forms. These studies show that the conformations of these complexes are different in the presence of agonist compared to antagonist, which is consistent with the proteolytic data described above.
Early studies had suggested that PR dimers could bind either agonist or antagonist but that mixed agonist-antagonist dimers were not produced (Meyer et al., 1990). Other studies (DeMarzo et al., 1992) showed that such heterodimers can be produced. Additional data supporting the fact that heterodimers can be formed comes from the studies of Skafar (1991), who examined the cooperativity of hormone binding in receptor dimers versus monomers. Both RU 486 and R 5020 alone showed cooperative binding to dimers, suggesting that a conformational change is induced in each case that favors binding of a second similar molecule. In dilute solution containing monomers, RU 486 had no effect on the affinity of R 5020 for the receptor, whereas at high concentrations of receptor, which presumably contains predominantly dimers, the cooperativity of R 5020 binding to the receptor was abolished by RU 486. Thus the progesterone antagonists may be able to inhibit progesterone action by binding to a single site of a dimer as well as by binding to both sites.
Although RU 486 and several other progesterone antagonists do cause receptor binding to DNA (El-Ashry-Stowers et al., 1989), another antagonist, ZK 98 299, from Schering apparently does not. Initial studies by Klein-Hitpass and coworkers (1991), as well as subsequent studies (Takimoto et al., 1992; Beck et al., 1993), have shown that under the same conditions in which R 5020 and RU 486 promote DNA binding of the receptor in gel retardation assays, ZK 98 299 does not produce a DNA binding form of the receptor. Thus, this antagonist appears to act by a very different mechanism. Since DNA binding is required for activation of transcription, any antagonist that blocks DNA binding will block the activity of the progesterone receptor. Interestingly, whereas treatment of T47D breast cancer cells with RU 486 produces the receptor form with reduced mobility on SDS gels characteristic of the DNA-dependent phosphorylation, treatment with ZK 98 299 does not (Takimoto et al., 1992). Whether the ZK 98 299 prevents the dissociation of the receptor-heat shock protein complexes, blocks dimerization, or simply alters the conformation of the dimer to reduce the affinity for DNA has not been reported.
Effects of Antagonists on Transcriptional Activation
The effects of antagonists on transcriptional activation of progesterone receptors have been examined by using endogenous receptors in
T47D breast cancer cells, transfected reporters and receptors in a variety of cell lines, and in vitro transcription studies. The effects of the antagonists vary depending upon the system used for analysis of activity.
Meyer and coworkers (1990) have shown that RU 486 acts as an antagonist in HeLa cells transfected with either hPR-A or hPR-B and a reporter gene MMTV-CAT, and that RU 486 exhibits no detectable agonist activity under these conditions. However, if a simpler promoter, PRE/GRE-tk -CAT, is used, the PR-B form shows some agonist activity with RU 486, whereas the PR-A is still inactive. They have shown that the progesterone receptor contains two regions important for transcriptional activation by the receptor termed TAF-1 (the amino terminus of the protein) and TAF-2 (the hormone binding domain). The strength of these domains as transcriptional activators depends both on the reporter gene used and on the cell type (Bocquel et al., 1989). Studies with deletion mutants of the receptor show that RU 486 does not activate TAF-2 (Meyer et al., 1990). However, for genes that predominantly require TAF-1 for transcriptional activation, RU 486, through PR-B, will partially activate the transcription of the gene. It is interesting that PR-A is unable to stimulate transcription in the presence of RU 486. This suggests that the region unique to PR-B may contain an additional activation function not present in PR-A.
Vegeto et al. (1992) found that a mutant of hPR-B, which lacked a portion of the extreme carboxyl terminus, no longer bound R 5020 and was thus not transcriptionally active. However, this mutant did bind RU 486 with normal affinity. The receptor was transcriptionally active in response to RU 486 both when the receptor was expressed in yeast and when it was expressed in mammalian cells. These data, combined with the conformational data and the antibody data described above, suggest that the carboxyl terminal portion of the receptor may act as a repressor (Allan et al., 1992; Vegeto et al., 1992) and that one of the roles of the agonist is to change the conformation of this region and eliminate the repressor activity. In this mutant, the repressor region has been removed, which allows the RU 486 to act as an effective agonist. These studies do not address the issue of whether the deletion produces an active TAF-2 in the presence of RU 486 or whether repression has simply been relieved, allowing TAF-1 to function.
Recent studies by Denner et al. (1990b) and Power et al. (1991a,b) have shown that some of the steroid receptors can be transcriptionally activated in the absence of hormone. This ligand-independent activation occurred in response to activation of kinases or inhibition of phosphatases and was strictly dependent upon the presence of chicken progesterone receptor (Denner et al., 1990b), the orphan receptor COUP-TF (Power et al., 1991a), or other steroid receptors such as the
human estrogen receptor (Power et al., 1991b). Studies with the human progesterone receptor have failed to demonstrate ligand-independent activation of the receptor, although the same compounds, 8-Br cAMP (8-bromoadenosine cyclic 3',5'-phosphate, which stimulates protein kinase A) and okadaic acid (an inhibitor of phosphatases 1 and 2A), which were used for chicken progesterone-receptor studies, do stimulate the progesterone-dependent activity of the human progesterone receptor (Beck et al., 1992).
One major difference between the human progesterone receptor and the chicken progesterone receptor is that the human progesterone receptor absolutely requires ligand to bind to DNA (Beck et al., 1992), to be phosphorylated by the DNA-dependent kinase (Bagchi et al., 1992), and to be active in the in vitro transcription assay (Bagchi et al., 1991), whereas the chicken progesterone receptor does not (Klein-Hitpass et al., 1990; Weigel et al., 1992). However, antagonists such as RU 486 do cause binding of receptor to DNA. Beck and coworkers (1993) have shown that whereas RU 486 is incapable of activating transcription from a reporter gene stably transfected into T47D cells and can block the activity of R 5020, when RU 486 and 8-Br cAMP are given in combination, the RU 486 exhibits a substantial amount of agonist activity. Since T47D cells contain both PR-B and PR-A, it is not clear whether one or both of these receptors are involved in the mediation of transcriptional activation by RU 486. A possible concern with these results is the question of whether the RU 486 was metabolized to produce an agonist as has been observed in some cases with the estrogen antagonist tamoxifen. However, all previous studies with RU 486 suggest that it is not metabolized, and analysis of RU 486 recovered from cells treated with 8-Br cAMP showed that it had not been metabolized to new compounds (Beck et al., 1993). It is of interest to note that whereas okadaic acid and TPA (a phorbol ester that stimulates the activity of protein kinase C) both stimulated R 5020-mediated activation, neither was capable of activating transcription in the presence of RU 486. In contrast, the antagonist ZK 98 299, which blocks receptor binding to DNA, functions as an antagonist in the presence of R 5020, R 5020 + 8-Br cAMP, or RU 486 + 8-Br cAMP. These data support the conclusion that the effects of RU 486 and 8-Br cAMP occur through the classical progesterone receptor, rather than through another molecule. Thus, the same reporter gene that is completely unresponsive to RU 486 alone can be activated under some sets of conditions, provided that an appropriate signal transduction pathway is also activated.
In vitro transcription studies also show that the antagonists that stimulate DNA binding can act as agonists. In vitro transcription mediated by the human progesterone receptor requires the presence of a ligand that produces the DNA binding form of the receptor (Bagchi et
al., 1991). However, Klein-Hitpass and coworkers (1991), using a G-free cassette assay with a PRE as the response element in the 5' flanking region, have found that the antagonists that allow receptor binding to DNA act as agonists. In this assay, ZK 98 299, which does not promote DNA binding, is inactive and can antagonize the activity of both R 5020 and RU 486. Whether the antagonists will function as agonists in all in vitro transcription assays or whether this is again a function of the target gene has not been determined.
Other Effects of Antagonists on Receptors
Several studies have shown that activation of progesterone and glucocorticoid receptors is accompanied by enhanced phosphorylation (Hoeck et al., 1989; Orti et al., 1989; Sheridan et al., 1989; Denner et al., 1990a), and other reports have shown that activation of kinases will enhance activation of the receptors (Denner et al., 1990b; Beck et al., 1992). Besides inhibition of binding, another point at which the antagonists may alter receptor action is by altering receptor phosphorylation. Treatment of T47D breast cancer cells with RU 486 stimulates phosphorylation of the receptor, and the magnitude of the increase in phosphorylation is severalfold more than in the presence of R 5020 (Sheridan et al., 1989; Takimoto et al., 1992). RU 486-treated receptors also exhibit the altered mobility on SDS gels characteristic of receptors isolated from R 5020-treated cells. However, it is possible that the molecule is aberrantly phosphorylated and that this phosphorylation, perhaps in combination with an altered conformation, produces the antagonist activity of the receptors that bind to DNA. In contrast, analysis of the phosphorylation of the RU 486-treated glucocorticoid receptor suggests that RU 486 blocks the normal hormone-dependent phosphorylation of the glucocorticoid receptor (Hoeck et al., 1989; Orti et al., 1989; Mao et al., 1992). Thus, this same antagonist may be acting through different mechanisms in inhibiting the progesterone and the glucocorticoid receptors. Treatment of T47D breast cancer cells with ZK 98 299 results in some enhancement of receptor phosphorylation, but the phosphorylation that alters the mobility on SDS gels is blocked (Takimoto et al., 1992).
Although most of the interest in hormone action has been focused on the activation of receptors, receptor processing subsequent to nuclear binding and receptor recycling are integral parts of receptor function. Very little is known about these steps. Typically, activation of the progesterone receptor results in a decrease in the level of progesterone receptors in T47D cells (Sheridan et al., 1988). RU 486 does not cause this same loss in receptors, which suggests that some of the effects of RU 486 may be to interfere at this step.
SUMMARY AND FUTURE AREAS OF STUDY
Although much has been learned about the mechanism of action of progesterone receptors, there are still many unanswered questions related to receptor function and the role of antagonists in inhibition of progesterone action. This review has focused on the classical nuclear progesterone receptor that appears to mediate most of the progesterone-dependent effects for which an antagonist would be clinically useful. However, in addition to receptor-mediated effects of progesterone, there is evidence of an unrelated membrane-bound progesterone receptor, whose function has not been well characterized, that presumably acts through completely different mechanisms (Ke and Ramirez, 1990). The steroid binding specificities of this receptor may be different from those of the classical receptor. If this is, indeed, a physiologically important molecule, then care may need to be taken to design antagonists that inhibit the nuclear receptor but not the membrane-bound receptor, or vice versa. Moreover, studies with the currently available antagonists suggest that although compounds such as RU 486 bind to both the progesterone and the glucocorticoid receptors, the effect on function of these receptors differs. This characteristic, in addition to the relative binding affinities, might be used to formulate more specific antiprogestins.
Several of the studies described here suggest that those antagonists that promote DNA binding will act as agonists under some conditions. This observation raises the question of whether, for some applications, it would be better to focus on antagonists that block DNA binding and therefore completely block receptor function. On the other hand, there may be instances in which specific partial agonist activity could be beneficial.
The studies utilizing antagonists that promote DNA binding suggest that in some fashion the conformation of the receptor is inappropriate to interact productively with other transcription factors to initiate transcription. However, almost nothing is known about the detailed mechanism of transcriptional activation by steroid receptors. For example, must the receptor dissociate from the gene and then reassociate to reinitiate transcription, or once bound, does it stay bound throughout many cycles of transcription? The RU 486 data suggest that the RU 486-bound receptor may stay associated with the DNA and cause aberrant processing (Sheridan et al., 1988). If dissociation is necessary, then the RU 486 would block transcription after a single round. This might explain the apparent agonist activity in the transcription assays in vitro. Usually, the calculated efficiency of transcription in these assays is not more than one transcript per template, so that the difference between R 5020 and RU 486 might not be detected in this assay. A
detailed understanding of the mechanism of transcriptional activation and the role of antagonists in blocking this activity should be very helpful in designing more effective antagonists.
REFERENCES
Allan, G.F., Leng, X., Tsai, S.Y., et al. Hormone and antihormone induce distinct conformational changes which are central to steroid receptor activation. Journal of Biological Chemistry 267:19513–19520, 1992.
Bagchi, M.K., Tsai, S.Y., Tsai, M-J., et al. Progesterone enhances target gene transcription by receptor free of heat shock proteins hsp 90, hsp 56 and hsp 70. Molecular and Cellular Biology 11:4998–5004, 1991.
Bagchi, M.K., Tsai, S.Y., Tsai, M-J., et al. Ligand and DNA-dependent phosphorylation of human progesterone receptor in vitro. Proceedings of the National Academy of Sciences, USA 89:2664–2668, 1992.
Baulieu, E-E. Contragestion and other clinical applications of RU 486, an antiprogesterone at the receptor. Science 245:1351–1357, 1989.
Beck, C.A., Weigel, N.L., and Edwards, D.P. Effects of hormone and cellular modulators of protein phosphorylation on transcriptional activity, DNA binding, and phosphorylation of human progesterone receptors. Molecular Endocrinology 6:607–620, 1992.
Beck, C.A., Weigel, N.L., Moyer, M.L., et al. The progesterone antagonist RU 486 acquires agonist activity upon stimulation of cAMP signaling pathways. Proceedings of the National Academy of Sciences, USA 1993, in press.
Benhamou, B., Garcia, T., Lerouge, T., et al. A single amino acid that determines the sensitivity of progesterone receptors to RU 486. Science 255:206–209, 1992.
Berrodin, T.J., Marks, M.S., Ozato, K., et al. Heterodimerization among thyroid hormone receptor, retinoic acid receptor, retinoid X receptor, chicken ovalbumin upstream promoter transcription factor, and an endogenous liver protein . Molecular Endocrinology 6:1468–1478, 1992.
Bocquel, M.T., Kumar, V., Stricker, C., et al. The contribution of the N- and C-terminal regions of steroid receptors to activation of transcription is both receptor and cell specific. Nucleic Acids Research 17:2581–2594, 1989.
Carlstedt-Duke, J., Stromstedt, P.E., Persson, B., et al. Identification of hormone-interacting amino acid residues within the steroid binding domain of the glucocorticoid receptor in relation to other steroid hormone receptors. Journal of Biological Chemistry 263:6842–6846, 1988.
Carson-Jurica, M.A., Schrader, W.T., and O'Malley, B.W. Steroid receptor family: Structure and functions. Endocrine Review 11:201–220, 1990.
Carter, T., Vancurova, I., Sun, I., et al. A DNA-activated protein kinase from HeLa cell nuclei. Molecular and Cellular Biology 10:6460–6471, 1990.
Danielson, M. Structure and function of the glucocorticoid receptor. Pp. 39–78 in Nuclear Hormone Receptors. Parker, M.G., (ed). London: Academic Press, 1991.
DeFranco, D.B., Qi, M., Borror, K.C., et al. Protein phosphatases types 1 and/or 2A regulate nucleocytoplasmic shuttling of glucocorticoid receptors. Molecular Endocrinology 5:1215–1228, 1991.
DeMarzo, A.M., Beck, C.A., Onate, S.A., et al. Dimerization of mammalian progesterone receptors occurs in the absence of DNA and is related to the release of the 90-kDa heat shock protein. Proceedings of the National Academy of Sciences, USA 88:72–76, 1991.
DeMarzo, A.M., Onate, S.A., Nordeen, S.K., et al. Effects of the steroid antagonist RU 486 on dimerization of the human progesterone receptor. Biochemistry 31:10491–10501, 1992.
Denner, L.A., Bingman III, W.E., Greene, G.L., et al. Phosphorylation of the chicken progesterone receptor. Journal of Steroid Biochemistry 27:235–243, 1987.
Denner, L.A., Schrader, W.T., O'Malley, B.W., et al. Hormonal regulation and identification of chicken progesterone receptor phosphorylation sites. Journal of Biological Chemistry 265:16548–16555, 1990a.
Denner, L.A., Weigel, N.L., Maxwell, B.L., et al. Regulation of progesterone receptor-mediated transcription by phosphorylation. Science 250:1740–1743, 1990b.
Dougherty, J.J., Puri, R.K., and Toft, D.O. Phosphorylation in vivo of chicken oviduct progesterone receptor. Journal of Biological Chemistry 257:14226–14230, 1982.
Dougherty, J.J., Puri, R.K., and Toft, D.O. Polypeptide components of two 8s forms of chicken oviduct progesterone receptor. Journal of Biological Chemistry 259:8004–8009, 1984.
El-Ashry-Stowers, D., Onate, S.A., Nordeen, S.K., et al. Human progesterone receptor complexed with the antagonist RU 486 binds to hormone response elements in a structurally altered form. Molecular Endocrinology 3:1545–1558, 1989.
Elger, W., Beier, S., Chwalisz, K., et al. Studies on the mechanisms of action of progesterone antagonists. Journal of Steroid Biochemistry 25:835–845, 1986.
Evans, R.M. The steroid and thyroid hormone receptor superfamily. Science 240:889–895, 1988.
Fawell, S.E., Lees, J.A., White, R., et al. Characterization and colocalization of steroid binding and dimerization activities in the mouse estrogen receptor . Cell 60:953–962, 1990.
Feil, P.D., Clarke, C.L., and Satyaswaroop, P.G. Progesterone receptor structure and protease activity in primary human endometrial carcinoma. Cancer Research 48:1143–1147, 1988.
Formstecher, P., Lefebvre, P., and Dautrevaux, M. RU 486 stabilizes the glucocorticoid receptor in a non-transformed high molecular weight form in intact thymus cells under physiological conditions. Journal of Steroid Biochemistry 31:607–612, 1988.
Grandics, P., Miller, A., Schmidt, T.J., et al. Phosphorylation in vivo of rat hepatic glucocorticoid receptor. Biochemical Biophysical Research Communications 120:59–65, 1984.
Hard, T., Kellenbach, E., Boelens, R., et al. Solution structure of the glucocorticoid receptor DNA-binding domain. Science 249:157–160, 1990.
Hoeck, W., Rusconi, S., and Groner, B. Down-regulation and phosphorylation of glucocorticoid receptors in cultured cells. Investigations with a monospecific antiserum against a bacterially expressed receptor fragment. Journal of Biological Chemistry 264:14396–14400, 1989.
Horwitz, K.B. The molecular biology of RU 486. Is there a role for antiprogestins in the treatment of breast cancer? Endocrine Reviews 13:146–163, 1992.
Horwitz, K.B., and Alexander, P.S. In situ photolinked nuclear progesterone receptors of human breast cancer cells: Subunit molecular weights after transformation and translocation. Endocrinology 113:2195–2201, 1983.
Housley, P.R., and Pratt, W.B. Direct demonstration of glucocorticoid receptor phosphorylation by intact L-cells. Journal of Biological Chemistry 258:4630–4635, 1983.
Kalimi, M. Receptor-mediated antiprogestin action of RU 486. Pp. 121–137 in Receptor Mediated Antisteroid Action. Agarwal, M.K., ed. New York: Walter de Gruyter, 1987.
Kastner, P., Krust, A., Turcotte, B., et al. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO Journal 9:1603–1614, 1990.
Ke, F.C., and Ramirez, V.D. Binding of progesterone to nerve cell membranes of rat brain using progesterone conjugated to 125I bovine serum albumin as a ligand. Journal of Neurochemistry 54:467–472, 1990.
Klein-Hitpass, L., Tsai, S.Y., Weigel, N.L., et al. The progesterone receptor stimulates
cell-free transcription by enhancing the formation of a stable preinitiation complex. Cell 60:247–257, 1990.
Klein-Hitpass, L., Cato, A.C., Henderson, D., et al. Two types of antiprogestins identified by their differential action in transcriptionally active extracts from T47D cells. Nucleic Acids Research 19:1227–1234, 1991.
Lessey, B.A., Alexander, P.S., and Horwitz, K.B. The subunit structure of human breast cancer progesterone receptors: Characterization by chromatography and photoaffinity labeling. Endocrinology 112:1267–1274, 1983.
Mao, J., Regelson, W., and Kalimi, M. Molecular mechanism of RU 486 action: A review. Molecular Cellular Biochemistry 109:1–8, 1992.
Meyer, M.E., Pornon, A., Ji, J., et al. Agonistic and antagonistic activities of RU 486 on the functions of the human progesterone receptor. EMBO Journal 9:3923–3932, 1990.
Moguilewsky, M., and Philibert, D. RU 38486: Potent antiglucocorticoid activity correlated with strong binding to the cytosolic glucocorticoid receptor followed by an impaired activation. Journal of Steroid Biochemistry 20:271–276, 1984.
Moudgil, V.K., Anter, M.J., and Hurd, C. Mammalian progesterone receptor shows differential sensitivity to sulfhydryl group modifying agents when bound to agonist and antagonist ligands. Journal of Biological Chemistry 264:2203–2211, 1989.
Neef, G., Beier, S., Elger, W., et al. New steroids with antiprogestational and antiglucocorticoid activities. Steroids 44:349–373, 1984.
Orti, E., Mendel, D.B., Smith, L.I., et al. Agonist-dependent phosphorylation and nuclear dephosphorylation of glucocorticoid receptors in intact cells. Journal of Biological Chemistry 264:9728–9731, 1989.
Perrot-Applanat, M., Guiochon-Mantel, A., and Milgrom, E. Immunolocalization of steroid hormone receptors in normal and tumour cells: Mechanisms of their cellular traffic. Cancer Surveys 14:5–30, 1992a.
Perrot-Applanat, M., Lescop, P., and Milgrom, E. The cytoskeleton and the cellular traffic of the progesterone receptor. Journal of Cell Biology 119:337–348, 1992b.
Philibert, D., Deraedt, R., and Teutsch, G. A potent anti-glucocorticoid in vitro. 8th International Congress of Pharmacology 14631, 1981 (abstract).
Philibert, D., Deraedt, R., Teutsch, G., et al. RU 38486—A new lead for steroidal anti-hormones. 64th Annual Meeting of the Endocrine Society 668, 1982 (abstract).
Power, R.F., Lydon, J.P., Conneely, O.M., et al. Dopamine activation of an orphan member of the steroid receptor superfamily. Science 252:1546–1548, 1991a.
Power, R.F., Mani, S.K., Codina, J., et al. Dopaminergic and ligand-independent activation of steroid hormone receptors. Science 254:1636–1639, 1991b.
Pratt, W.B., Dalman, F.C., Meshinchi, S., et al. The relationship between glucocorticoid receptor binding to Hsp90 and receptor function. Nippon Naibunpi Gakkai Zasshi 66(12):1185–1197, 1990.
Raynaud, J-P., and Ojasoo, T. The design and use of sex-steroid antagonists. Biochemistry 25:811–833, 1986.
Sheridan, P.L., Krett, N.L., Gordon, J.A., et al. Human progesterone receptor transformation and nuclear down regulation are independent of phosphorylation . Molecular Endocrinology 2:1329–1342, 1988.
Sheridan, P.L., Evans, R.M., and Horwitz, K.B. Phosphotryptic peptide analysis of human progesterone receptor: New phosphorylated sites formed in nuclei after hormone treatment. Journal of Biological Chemistry 264:6520–6528, 1989.
Skafar, D.F. Differences in the binding mechanism of RU 486 and progesterone to the progesterone receptor. Biochemistry 30:10829–10832, 1991.
Smith, D.F., Faber, L.E., and Toft, D.O. Purification of unactivated progesterone receptor and identification of novel receptor-associated proteins. Journal of Biological Chemistry 265:3996–4003, 1990.
Takimoto, G.S., Tasset, D.M., Eppert, A.C., et al. Hormone-induced progesterone receptor phosphorylation consists of sequential DNA-independent and DNA-dependent stages: Analysis with zinc finger mutants and the progesterone antagonist ZK98299. Proceedings of the National Academy of Sciences, USA 89:3050–3054, 1992.
Tasset, D., Tora, L., Fromental, C., et al. Distinct classes of transcriptional activating domains function by different mechanisms. Cell 62:1177–1187, 1990.
Tsai, S.Y., Tsai, M-J., and O'Malley, B.W. Cooperative binding of steroid hormone receptors contributes to transcriptional synergism at target enhancer elements. Cell 57:443–448, 1989.
Umesono, K., and Evans, R.M. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell 57:1139–1146, 1989.
Vegeto, E., Allan, G.F., Schrader, W.T., et al. The mechanism of RU 486 antagonism is dependent on the conformation of the carboxy-terminal tail of the human progesterone receptor. Cell 69:703–713, 1992.
Weigel, N.L., Carter, T.H., Schrader, W.T., et al. Chicken progesterone receptor is phosphorylated by a DNA-dependent protein kinase during in vitro transcription assays. Molecular Endocrinology 6:8–14, 1992.
Weigel, N.L., Denner, L.A., Poletti, A., et al. Phosphorylation/dephosphorylation regulates the activity of progesterone receptors. Advances in Protein Phosphatases 7:237–269, 1993.
Yen, P.M., Sugawara, A., and Chin, W.W. Triiodothyronine (T3) differentially affects T3-receptor/retinoic acid receptor and T3-receptor/retinoid X receptor heterodimer binding to DNA. Journal of Biological Chemistry 267:23248–23252, 1992.



















