B8
Use of Antiprogestins in the Management of Endometriosis and Leiomyoma
SAMUEL S.C. YEN, M.D., D.Sc.
Professor and W.R. Person Chair
Department of Reproductive Medicine, University of California
ABSTRACT
Pelvic endometriosis and uterine leiomyomas (fibroids) are the two most common disorders in women during reproductive age. Infertility, pelvic pain, and uterine bleeding are major clinical manifestations. Although their pathogeneses are unclear, both conditions are ovarian steroid dependent, and tumors are endowed with receptors for estrogen (ER) and progesterone (PR). The rationale for inducing regression of these tumor growths with an antiprogestin was formulated soon after the demonstration of the ability of RU 486 to interrupt early pregnancy in women. It was followed by a series of short-term studies showing the effectiveness of RU 486 in inhibiting ovulation, inducing luteolysis, and disrupting endometrial integrity in normally ovulatory women. Concurrently, clinical trials were conducted to determine the beneficial effect and safety of long-term daily administration of RU 486 in patients with endometriosis and fibroids.
For symptomatic endometriosis, in a pilot study, six patients were treated with a daily dose of 100 mg of RU 486 for three months. The treatment resulted in marked improvement of pain scores without discernible change in the extent of endometrial implants. In subsequent studies with a reduced dose (50 mg/day) and extended duration (six months) of RU 486 administration, both pain and endometriosis scores improved significantly.
In patients with leiomyomas, administration of RU 486 at a daily dose of 50 mg for three months induced a decrease in leiomyoma volume by 22 percent at one month, 40 percent at two months, and 50 percent at three months. When the dose of RU 486 was reduced
to 25 mg/day, the decrease of leiomyoma volume was virtually identical to that observed with the 50-mg dose. Further decreasing the dose to 5 mg/day was less effective, with an overall reduction of tumor volume by 25 percent. There was a decrease of immunostaining for PR but not for ER in leiomyomas treated with RU 486.
In all studies, amenorrhea with early to midfollicular range of estradiol levels was induced, and no change in bone mineral density was noted. Ovulatory cycles resumed four to six weeks after completion of treatment. Side effects were observed, which included atypical hot flushes, mild elevation of serum transaminase, and antiglucocorticoid effects at higher doses. Thus, an antiprogesterone may provide a novel mode of long-term (years) medical management for pelvic endometriosis and uterine leiomyomas. Future studies of lower doses and correlation of individual responses with the status of steroid hormone receptors, growth factors, anatomical sites, and vascularity may be helpful in predicting maximal responses of individual patients.
INTRODUCTION
Pelvic endometriosis and uterine leiomyomas (fibroids) are the two most common disorders in women during reproductive age. Infertility, pelvic pain, and uterine bleeding are major clinical manifestations, and hysterectomies are frequently resorted to. Although their pathogeneses are unclear, both conditions are ovarian steroid dependent, and tumors are endowed with receptors for estrogen (ER) and progesterone (PR). The rationale for inducing regression of these tumor growths with an antiprogestin was formulated soon after the demonstration of the ability of RU 486 to interrupt early pregnancy in women (Baulieu, 1989). It was followed by a series of short-term studies showing the effectiveness of RU 486 in inhibiting ovulation, inducing luteolysis, and disrupting endometrial integrity in normally ovulatory women (Schaison et al., 1985; Liu et al., 1987; Garzo et al., 1988; Luukkainen et al., 1988; Roseff et al., 1990). Concurrently, clinical trials were conducted to determine the beneficial effect and safety of long-term daily administration of RU 486 in patients with endometriosis and fibroids.
ENDOMETRIOSIS
Endometriosis is a common disease, affecting as many as 1 in 15 women of reproductive age (Barbieri, 1990). The incidence is much higher among women with infertility (25 percent; Jones and Rock, 1976). In this condition, functioning endometrial gland and stroma have migrated outside the uterine cavity. These ectopic endometriotic
implants are infiltrating lesions involving reproductive organs, peritoneum, bladder, and rectosigmoid colon. On rare occasions, implants may be found outside the abdominal cavity (e.g., in the lung). The depth of infiltration into the fibromuscular tissue of the pelvis is strongly correlated with the severity of pelvic pain. Superficial implants, on the other hand, are found more frequently in patients with infertility (83 percent; Cornillie et al., 1990). Dysmenorrhea, dyspareunia, and pelvic, back, and rectal pain are common symptoms. Definitive diagnosis is based on the visualization of endometriotic implants by laparoscopy.
The histologic features and the density of immunostaining of PR and ER are heterogeneous as contrasted to the uterine endometrium (Metzger et al., 1993). Approximately half of these implants are in phase with normal endometrium, indicating nonuniformity in hormone responsiveness among implants (Lessey et al., 1989). Nonetheless, the growth of endometriosis is stimulated by the cyclic ovarian steroids estrogen (E2) and progesterone (P4), and in the absence of these steroids (e.g., after ovariectomy), endometriosis undergoes regression (Shaw, 1992).
Current medical treatments of endometriosis depend on suppression of ovarian function and induction of endometrial atrophy. Danazol, an isoxazole derivative of 17a-ethynyltestosterone, has been used for the treatment of endometriosis for the past 15 years. The drug has multiple and diverse effects on reproductive tissues. The main biological properties of Danazol are binding to androgen receptors and suppression of the hypothalamic-pituitary axis, resulting in acyclic ovarian function (Dmowski et al., 1983). Although Danazol is effective in the subjective improvement of symptoms and decreasing the growth of implants, the androgenic, anabolic, and hepatic side effects, such as hirsutism, voice deepening, weight gain, edema, headache, and increase in serum transaminases, are major drawbacks to its clinical use. The second effective treatment is the use of a gonadotropin-releasing hormone (GnRH) agonist to down-regulate the pituitary-ovarian axis, thereby inducing hypoestrogenism. The beneficial effect of a GnRH agonist on endometriosis is similar to that of Danazol, but it is devoid of androgenic and anabolic effects. However, the profound hypoestrogenic state and the associated consequence of severe hot flushes and a reduction of bone mass are major limitations for its long-term use (Matta et al., 1988a; Rummon et al., 1988). Both Danazol and a GnRH agonist are approved by the Food and Drug Administration for treatment of endometriosis of six-month duration. It is apparent that the development of novel, effective, and long-term medical treatments with little or no side effects would be a significant advance in the management of symptomatic endometriosis.
Antiprogestin in the Treatment of Endometriosis
Pilot Study
Rationale. Since ectopic endometrial tissue contains both ER and PR, and is sensitive to the hormonal agents that affect these receptors (Lessey et al., 1989), it prompted us to evaluate whether the antiprogestin RU 486 could have some beneficial effects in women with symptomatic pelvic endometriosis (Kettel et al., 1991).
Protocol. Six normal-cycling women with pelvic pain due to endometriosis participated in the pilot study. Their mean age was 29 years (range: 17–40 years). Four of them had failed other hormonal treatments, and none had taken any hormonal medication for at least two months prior to the study. All patients used barrier contraceptives throughout the study. Informed consent was obtained prior to enrollment.
RU 486 was given as two 50-mg tablets per day for three months, starting on cycle day 1. Staging of the disease was assessed at pre- and posttreatment laparoscopy by the American Fertility Society (AFS) Revised Classification (AFS, 1985). Pelvic pain was graded as minimal (+), mild (++), moderate (+++), or severe (++++).
Baseline blood samples were obtained in the early follicular phase of the pretreatment cycle and again during the last month of treatment. Ovarian function was monitored by daily determinations of estrone 3a-glucuronide (E1G), an estrogen metabolite, and pregnanediol 3a-glucuronide (PdG), a progesterone metabolite, on the first void urine samples throughout the treatment period. Values were compared with data collected from 13 regularly cycling women during the menstrual cycle. In addition, 24-hour frequent sampling (10–20 minutes) for luteinizing hormone (LH), adrenocorticotropic hormone (ACTH), and cortisol measurements was performed in the early follicular phase of the pretreatment cycle and in the third month of treatment. Analytical methods are well established in our laboratory.
Outcome. All women became amenorrheic during treatment. Concentrations of PdG were acyclic and remained relatively constant throughout the treatment period. After an initial transient rise in E1G levels during the first month of treatment, values comparable to the early to midfollicular phase range of normal-cycling women (Figure B8.1) were maintained. Serum estradiol (E2) and estrone (E1), testosterone (T), and androstenedione (A) as well as serum follicle-stimulating hormone (FSH), thyroid-stimulating hormone (TSH), and prolactin were unchanged. LH pulse amplitude (but not frequency) was increased
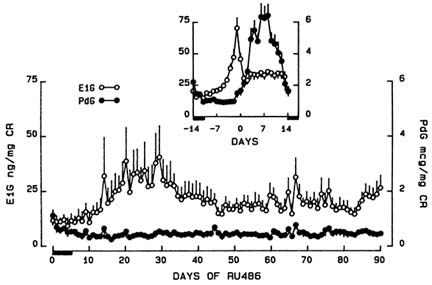
FIGURE B8.1 Mean (±SE) urinary estrone 3a-glucuronide (E1G) and pregnanediol 3a-glucuronide (PdG) normalized to creatinine (CR) in six women during treatment with RU 486, 100 mg/day for three months. Insert: mean (±SE) daily urinary E1G and PdG levels in 13 normally cycling women. Data are centered around the day after the E1G peak (day 0).
(P < .02), suggesting an antiprogesterone effect on the hypothalamic-pituitary. After cessation of RU 486, regular menstrual cycles ensued and the first episode of vaginal bleeding occurred within three to six weeks in all subjects.
Twenty-four-hour mean cortisol (P < .01) and ACTH (P < .05) concentrations were increased after RU 486 treatment. This rise in cortisol and ACTH was most apparent during the early morning hours (2:00 a.m. to 4:00 p.m.) and became indistinguishable during times of secretory quiescence (4:00 p.m. to 2:00 a.m.) (Figure B8.2). With cosinor analysis, the normal circadian rhythm of cortisol and ACTH secretion was maintained, with no change in acrophase (7:36 a.m. versus 7:35 a.m.) after treatment. These findings indicate that RU 486, at 100-mg daily dose, induced an antiglucocorticoid effect.
Treatment resulted in an improvement of pelvic pain in all subjects (Table B8.1). This pain relief persisted over a one- to two-year follow-up interval in three of six patients; pain recurred to a lesser extent in one subject; and two subjects successfully conceived after completing therapy. Follow-up laparoscopy was accomplished in five patients. Endometriotic implants had resolved in only one subject and persisted in all others (Table B8.1). Three subjects reported atypical flushes, and one
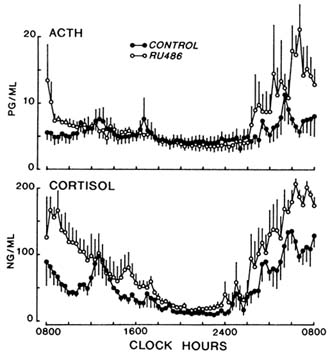
FIGURE B8.2 Mean (±SE) 24-hour secretory patterns of ACTH and cortisol before and after treatment with RU 486 at 100 mg/day for three months.
TABLE B8.1 Six Women with Symptomatic Endometriosis Before and After Treatment with RU 486 (100 mg/day for three months)
|
|
|
AFS Stagea |
|
Pelvic Pain |
|
|
Subject |
Age (years) |
Before |
After |
Before |
After |
|
1b |
26 |
III |
III |
++++ |
+ |
|
2 |
17 |
I |
I |
+++ |
|
|
3 |
34 |
I |
I |
+++ |
+ |
|
4b |
40 |
I |
I |
++ |
|
|
5 |
33 |
I |
O |
+++ |
++ |
|
6 |
24 |
II |
|
+++ |
+ |
|
a The American Fertility Society revised classification of endometriosis. b Conceived. |
|||||
subject complained of anorexia and fatigue during the first four to eight weeks of treatment. No other significant side effects were reported. These preliminary findings offered promise for future investigations using lower doses and longer-term therapy with RU 486, with the aim of avoiding the antiglucocorticoid effect and allowing sufficient time for the
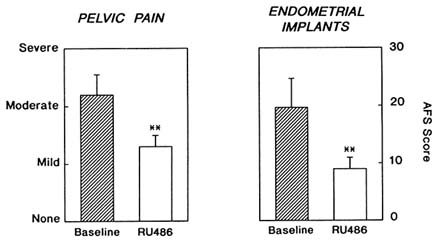
FIGURE B8.3 Clinical scores for pelvic pain and AFS scores for endometriotic implants before and after six months of RU 486 treatment at 50-mg daily dose (**P < .05).
resolution of endometriotic implants, which was not seen in this three-month trial.
Long-Term, Low-Dose Studies
The purpose of this study was to test the efficacy of a reduced dose of RU 486 (50 mg/day), and extended duration of therapy (six months), on symptomatic endometriosis, and to dissociate the antiglucocorticoid effect of RU 486. Nine women with symptomatic endometriosis and previous failure in medical treatment (either no improvement or dropout due to side effects) participated in this study. Daily symptom inventories and urine collections were obtained during baseline, treatment, and recovery cycles. Each subject underwent pre- and posttreatment laparoscopy, and bone mineral density (BMD) determinations. Blood samples for hormone analysis were obtained weekly for four weeks and monthly thereafter. Seven subjects underwent 24-hour frequent sampling (every 10 minutes), before (early follicular phase) and during the last month of therapy.
Outcome. All patients became amenorrheic during therapy and exhibited anovulatory urinary E1G and PdG profiles. All subjects reported a significant decrease in pelvic pain (P < .02) and dysmenorrhea (P < .03), which lasted throughout therapy (Figure B8.3). Laparoscopic assessments of endometriotic implants by AFS scores (two observers)
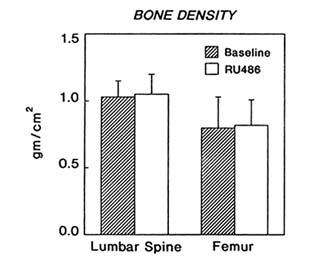
FIGURE B8.4 Bone mineral density of the lumbar spine and femur determined by dual photon x-ray absorptiometry before and after six months of RU 486 treatment at 50-mg daily dose.
showed decreases in eight of nine subjects (19.8 ± 4.9 to 9.4 ± 1.7, P < .05) (Figure B8.3). BMD of the lumbar spine and femur remained constant throughout therapy (Figure B8.4). Serum levels of LH increased during the first month of treatment [11.4 ± 2.6 to 25.3 ± 7.3 IU (international units) per liter, P < .05], with a concomitant increase in testosterone (1.07 ± 0.3 to 1.90 ± 0.4 nmol/liter, P < .05).
Frequent sampling studies demonstrated no change in LH pulse frequency or amplitude. Twenty-four hour mean serum cortisol was unchanged (164.4 ± 13.1 versus 166.2 ± 12.3 nmol/liter) with preservation of the normal circadian rhythm (Figure B8.5). No other hormonal changes occurred.
Side Effects. Two patients developed transient, mild increases in liver transaminase, which normalized at one month. Atypical, mild hot flushes were noted in these patients. No other significant adverse effects were noted. This study demonstrates the effectiveness of long-term, low-dose RU 486 in achieving a condition of ovarian acyclicity and improving both pain and extent of disease without inducing an antiglucocorticoid effect. Thus, RU 486 may provide a safe and well-tolerated alternative for the medical management of endometriosis.
A follow-up study, using a 5-mg daily dose for six months, was conducted in patients with symptomatic endometriosis. In this ongoing study, four patients have completed treatment (three are ongoing). All four patients have had improvement of pelvic pain, amenorrhea, and no
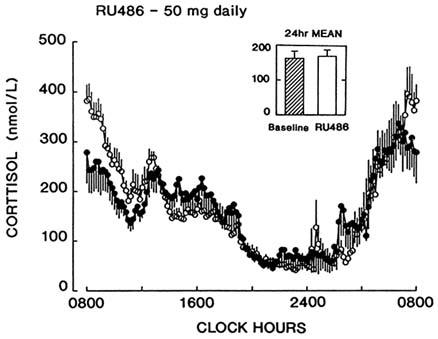
FIGURE B8.5 Twenty-four-hour serum cortisol levels measured at 20-minute intervals before (•) and after (○) six months of RU 486 treatment. Insert: 24-hour mean cortisol values before and at the end of RU 486 treatment.
side effects observed. These preliminary observations have prompted us to extend this study to a larger number of patients with the hope of developing a long-term (years) therapy using antiprogestins in symptomatic endometriosis when fertility is not an issue.
In summary, RU 486 at a 50-mg dose, and possibly at a 5-mg dose for a period of six months has resulted in impressive therapeutic effectiveness. Side effects were limited to slight and reversible elevation of liver transaminase in about 20 percent of the patients, which is similar to the side effects of danazol. The major advantage of this treatment modality may be the potential long-term use (years) in the ''moderation" of symptomatic endometriosis, thereby circumventing a hysterectomy and bilateral ovariectomy, particularly in women of late reproductive years.
LEIOMYOMA (FIBROID TUMOR)
Uterine leiomyomas are common pelvic tumors occurring in up to 20 percent of women over 30 years of age (Buttram and Reiter, 1981). Leiomyomas represent one of the most frequent indications for opera-
tive procedures in women of reproductive age: 600,000 women had hysterectomies in 1991, with an annual cost exceeding $5 billion (Carlson et al., 1993). Although the mechanisms of tumorigenesis are unknown, evidence suggests that leiomyomas are ovarian steroid dependent (Buttram and Reiter, 1981; Lumsden et al., 1989; Rein et al., 1990). Receptors for both estrogen and progesterone have been identified in leiomyomas, and their content is significantly greater than those found in the myometrium (Lumsden et al., 1989; Rein et al., 1990). Pituitary-ovarian down-regulation by GnRH agonists, the only medical treatment currently available, results in 50 percent regression of leiomyomas in women of reproductive age (Friedman et al., 1987; Perl et al., 1987; West et al., 1987; Andreyko et al., 1988; Kessel et al., 1988; Lumsden et al., 1989; Schlaff et al., 1989; Rein et al., 1990; Friedman et al., 1991). The major limitation of the treatment modality is severe hypoestrogenism, with consequent severe hot flushes and bone loss. Treatment is approved by the U.S. Food and Drug Administration for a duration of six months only.
Since leiomyomas and endometriosis share ovarian steroid dependency, we reasoned that RU 486 may have an inhibitory effect on the growth of leiomyomas. That progesterone may play a role in leiomyoma growth is suggested by the finding of a higher mitotic count in leiomyomas obtained during the secretory phase than in the proliferative phase of the menstrual cycle (Kawaguchi et al., 1988). Additionally, when the GnRH agonist and a progestin are coadministered, the expected regression of leiomyoma size observed with the GnRH agonist alone is not achieved (Friedman et al., 1988). Here, we report studies showing an inhibitory effect of RU 486 on the growth of uterine leiomyomas.
Antiprogestin in the Management of Leiomyoma— Dose-Response Studies
Subjects. Thirty-four normally cycling women between the ages of 18 and 45 years with symptomatic uterine leiomyomas were recruited for this study. None of the subjects had taken any hormonal medications for at least three months prior to the study. All subjects used barrier contraception. These subjects participated in the following experiments:
RU 486, 50-mg daily dose for three months (N = 10)
RU 486, 25-mg daily dose for three months (N = 17) (7 underwent uterine blood flow studies); and
RU 486, 5-mg daily dose for three months (N = 7).
Clinical and Laboratory Assessments. Each subject had a pelvic sono-
gram performed prior to initiation of drug therapy and monthly thereafter. This was performed either vaginally or abdominally, with each tumor measured in three dimensions. Subjects were given RU 486 beginning on days 1-3 of the menstrual cycle. Baseline and monthly blood samples for complete blood count and chemistry panel were obtained. Serum samples for hormonal evaluation were obtained before and after initiation of therapy, daily for one week, weekly for four weeks, and monthly thereafter. LH, FSH, E2, E1, A, T, TSH, progesterone (P), dehydroepiandrosterone (DHEA), DHEA-sulfate, cortisol, and prolactin were determined by in-house radioimmunoassays.
Bone mineral density, determined by dual photon x-ray absorptiometry of the spine and hips was assessed (50-mg dose only) before and at the end of therapy. Uterine arterial flow was analyzed by a duplex sonography combining real-time imaging and pulsed Doppler velocimetry by transvaginal scanning. Doppler waveforms were computed and expressed as vascular resistance index (RI) (De Ziegler et al., 1991).
Histology and Receptor Protein Studies. Leiomyomas and myometrial tissue were obtained at surgery from six RU 486-treated patients and six untreated patients in the follicular phase of the cycle. Tissues were fixed in neutral-buffered formalin for 24 hours and embedded in paraffin. Mounted sections (8 µm thick) were used for immunohistochemical analysis (Garcia et al., 1988; Lessey et al., 1989). Briefly, after treatment with pronase-phosphate buffered saline (PBS) solution, sections of tissue were incubated with primary antibody for 30 hours. Secondary antibody, biotinylated goat antirat immunoglobulin G, was applied for 30 minutes, followed by tertiary antibody, streptavidin-alkaline phosphatase for 30 minutes. Samples were developed in McGrady's reagent. Pronase treatment was not required with the PR primary antibody. PR antibody was used at 1:5 dilution. The ER antibody was not diluted. For control, a duplicate section of each slide was stained in a similar manner, except that the primary antibody was eliminated.
Levels of ER and PR were evaluated using an image analysis software program (Image, program provided by Wayne Rasband, National Institutes of Health). This computer program captures an image from the microscope and provides a quantitative analysis of staining intensity. An arbitrary numerical unit is assigned to each slide and its nonimmune control. For each immunostained section, the adjacent nonimmune control was subtracted and the difference reported.
50-mg Dose
Outcome. At the 50-mg dose, a decrease in uterine leiomyoma volume in response to RU 486 is demonstrated. Reductions of 22 percent
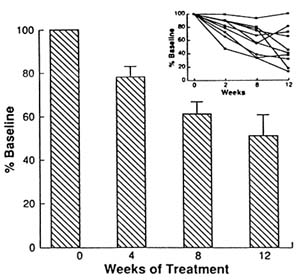
FIGURE B8.6 Percentage change in uterine leiomyoma volume in response to three months of RU 486 treatment. Insert: individual patient response.
at 4 weeks, 39 percent at 8 weeks, and 49 percent at 12 weeks were observed (Figure B8.6). This effect is comparable to that achieved with the GnRH agonist after a six-month treatment (35–50 percent decrease) (Friedman et al., 1987, 1991; Perl et al., 1987; West et al., 1987; Andreyko et al., 1988; Kessel et al., 1988; Lumsden et al., 1989; Schlaff et al., 1989; Rein et al., 1990). Schlaff et al. (1989) have noted that GnRH agonist treatment is accompanied by a significant decrease of both myometrial volume and leiomyoma volume, as assessed by magnetic resonance imaging. In fact, the nonleiomyoma volume, presumably representing normal myometrium, was more responsive than leiomyomas to hypoestrogenism. In our study, response was defined as a decrease in leiomyoma volume and excludes the myometrial component. When both factors are considered, the effectiveness of RU 486 in the regression of leiomyomas may be greater.
Eight of ten (80 percent) patients had a significant response to RU 486 (25 percent or greater reduction in leiomyoma volume) after three months of treatment. This is comparable to a clinical response of 77 percent (25 percent or greater decrease in uterine volume) in patients treated with leuprolide (long-lasting GnRH agonist) for six months (Friedman et al., 1991). Although all but one patient displayed a decrease in leiomyoma volume, individual variations ranging from 15 percent to 90 percent in response to RU 486 were apparent (Figure B8.6
insert). Such variations in response may represent differences in vascularity, cellular composition, and biologic endowment of tumors.
With GnRH agonist treatment, 95 percent of patients in the study by Friedman et al. (1991) experienced some side effects related to the hypoestrogenism. Side effects noted with the use of RU 486 included mild, infrequent, atypical hot flashes (distinct from menopausal hot flushes) in four patients. A transient elevation in serum transaminases accompanied by joint pain was seen at the end of treatment in one patient, with rapid resolution after discontinuation of RU 486. There was no significant change in bone mineral density of the spine and hips after three months of therapy (0.8 ± 0.319 versus 0.835 ± 0.713 g/cm2). Serum E2 and E1 levels remain in the early to midfollicular phase range during therapy.
A significant decrease in both leiomyoma and myometrium observed in PR staining (Figure B8.7a), with unaltered ER staining (Figure B8.7b), after prolonged exposure to RU 486 suggests that RU 486 in vivo may have a direct effect on PR number in these target tissues. The possibility that receptor occupancy by RU 486 may mask immunohistochemical localization of PR protein is unlikely but cannot be excluded (Weigel et al., 1992).
In the ovariectomized rhesus monkey model, Wolf et al. (1989) and Neulen et al. (1990) have demonstrated the ability of RU 486 to antagonize the mitogenic effects of exogenous estrogen on the endometrium. Under estradiol replacement, RU 486 treatment induced a rise in cytosolic and nuclear ER concentrations. Although the mechanisms remain unclear, these observations suggest that RU 486 may alter the functionality of the ER by acting as a noncompetitive antiestrogen (Wolf et al., 1989; Neulen et al., 1990). In our study, there was no clear effect of RU 486 on ER immunoreactivity, suggesting that if an antiestrogenic effect exists, it is likely functional in nature.
The mechanism by which continuous RU 486 acts to induce chronic anovulation is unclear. There is a transient increase in LH, A, and T levels, which may be due to the withdrawal of feedback action of progesterone on the hypothalamic-pituitary axis (see below). However, in the absence of endogenous progesterone, RU 486 may act as an agonist and thereby induce an acyclic hormonal pattern and anovulation (Dierschke et al., 1973; Liu and Yen, 1983; Gravanis et al., 1985; Collins and Hodgen, 1986; Liu et al., 1987). Alternatively, RU 486 may disrupt the hypothalamic-pituitary-ovarian axis by acting as a noncompetitive antiestrogen (Wolf et al., 1989; Neulen et al., 1990).
In summary, we have shown that an antiprogesterone that induces ovarian acyclicity also decreases leiomyoma volume. This decrease in volume is seen in the face of follicular phase levels of estrogen. Thus, an antiprogesterone may provide a novel mode of management for leiomy-
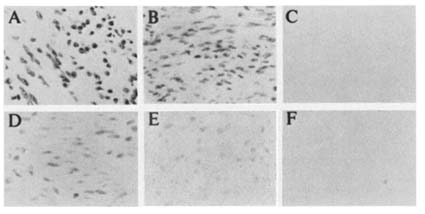
FIGURE B8.7a (A) Immunohistochemical staining of PR in leiomyomas of a control patient. (B) Immunoreactivity of PR seen in myometrium of control patient. (C) Nonimmune control of leiomyomas as in A. (D) Immunohistochemical staining of PR in leiomyomas of RU 486-treated patient. (E) Immunoreactivity of PR seen in myometrium of RU 486-treated patient. (F) Nonimmune control slide of myometrium as in D.
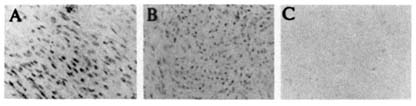
FIGURE B8.7b (A) Immunohistochemical staining of ER in leiomyomas of a control patient. (B) Immunohistochemical staining of ER in leiomyomas of RU 486-treated patient. (C) Nonimmune control slide of leiomyomas seen in A.
omas without undesirable hypoestrogenism. This observation prompted us to conduct two subsequent studies to ascertain the minimal effective dose of RU 486.
25- and 5-mg Doses
The effect of reduced doses of RU 486 on leiomyoma volume was evaluated in three-month treatment with a 25-mg daily dose in 17 patients, and 5 mg in 7 patients. All patients were monitored as described above, with the exception that 24-hour urinary free cortisol was used as an index of antiglucocorticoid effects instead of serum cortisol.
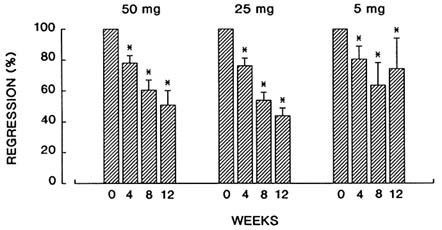
FIGURE B8.8 Comparison of percent change in uterine leiomyoma volume in response to three-month treatment of RU 486 at daily doses of 50, 25, and 5 mg.
Outcome
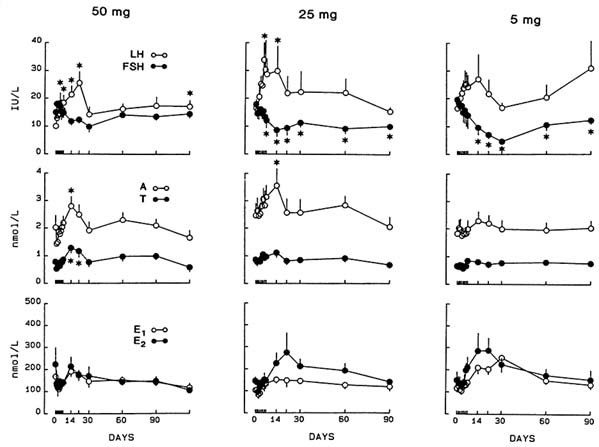
-
Response of Leiomyoma Volume: The regression of the mean leiomyoma volume in patients treated with a 25-mg daily dose of RU 486 was 21.7 percent at one month, 56.6 percent at two months, and 68.4 percent after three months (P < .001). These responses were similar or greater than those observed at a 50-mg daily dose (Figure B8.8). At a 5-mg daily dose, there was a reduction of 36.5 percent after the first month, 27.2 percent after the second month, and 29.2 percent after the third month of therapy (P < .05) (Figure B8.8). Thus, at the lowest dose tested (5 mg), the time-dependent progressive regression of leiomyoma observed with 50-mg and 25-mg doses was no longer apparent.
As with the 50-mg daily dose, all patients became amenorrheic during their treatment. Five patients experienced atypical hot flushes during the first month of treatment, and two patients who used the 25-mg daily dose had mild elevations of liver transaminases, which resolved within one month after they discontinued the medication.
-
Hormonal Changes: Based on our earlier observations that RU 486 exerted multiple sites of action at the hypothalamic-pituitary-ovarian-endometrial axis (Garzo et al., 1988), we anticipated that long-term administration of RU 486 might disclose sequential antagonist and agonist effect on the reproductive axis. As with the 50-mg daily dose, levels of LH, A, and T increased during the first two weeks of treatment with the 25-mg daily dose (Figure B8.9). In contrast, a significant decline in FSH levels was evident in both 25- and 5-mg doses, which lasted for the entire course of treatment. This inhibitory effect of RU 486 on FSH
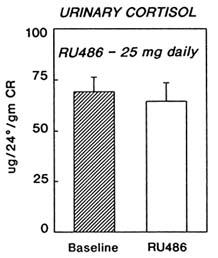
FIGURE B8.10 Twenty-four-hour urinary free cortisol levels (mean ± SE) at baseline and after three months of treatment with RU 486 at 25-mg daily dose.
-
levels, however, was not observed with the 50-mg dose (Figure B8.9). In the face of decreased FSH, serum E2 and E1 levels were maintained in the early to midfollicular phase range. These findings are entirely consistent with our previous studies, which indicated that RU 486 exerted impact at multiple sites with diverse actions (Garzo et al., 1988). Our present finding also suggests that long-term exposure to RU 486 induces a biphasic effect on different target cells in a dose-dependent manner. Further studies are essential to evaluate the specific responses of different target tissues.
At both the 25- and the 5-mg daily doses, urinary free cortisol levels were unchanged (Figure B8.10). Thus, the antiglucocorticoid action can be disassociated from the antiprogesterone action of RU 486 at these doses. A finding is critical for long-term use of RU 486 in the treatment of sex-steroid dependent tumors.
-
Uterine Blood Flow: At the 25-mg daily dose, the change of uterine blood was evaluated. As shown in Figure B8.11, there was an increase in vascular resistance (reflecting a decrease in blood flow) recorded from the uterine arteries after three months of treatment as compared to pretreatment values recorded during the early follicular phase. This finding resembled that reported after GnRH agonist therapy for leiomyoma (Matta et al., 1988b). The mechanism(s) and significance of the increased vascular resistance in response to RU 486 are unclear at the present time.
Conclusion. RU 486 at a 25-mg dose causes a greater than 50 percent reduction, and at a 5-mg dose a small but significant decline, in myoma
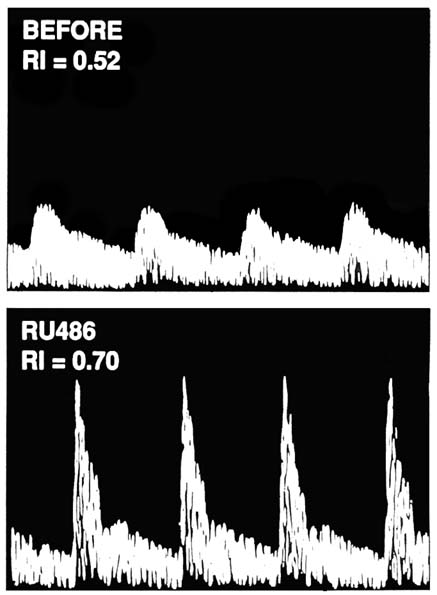
FIGURE B8.11 Doppler ultrasonography of uterine arterial flow velocity waveforms before (top) and after RU 486 treatment (bottom) in a patient with uterine leiomyoma (RI = resistance index).
volume unaccompanied by antiglucocorticoid effects. These findings provide strong support that long-term use of RU 486 may serve as an effective and safe alternative for the management of myoma and
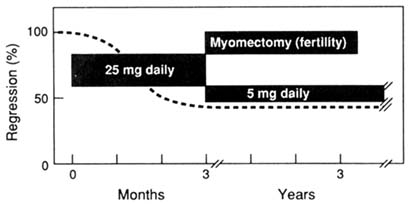
FIGURE B8.12 The therapeutic strategy of a step-down regimen for the use of antiprogestins for long-term (years) suppression in the treatment of endometriosis and leiomyoma. Myomectomy may be performed in cases of infertility associated with leiomyoma following the initial downregulation during the first three months of treatment.
endometriosis. Future experiments to test the effectiveness and safety of long-duration (years) suppression of endometriosis and myoma growth are both necessary and feasible. The therapeutic strategies for the use of antiprogestins are depicted in Figure B8.12. A step-down dose of RU 486 is proposed. At a 25-mg daily dose or lower, a 50 percent down-regulation of these lesions (and pain) is anticipated. This is followed by a reduced daily dose of 5 mg to serve in a long-term (years) maintenance of regression. If proved successful, the advantages include cost-effectiveness, circumvention of the need for a hysterectomy, and a reduction of surgical morbidity.
ACKNOWLEDGMENTS
This report is based on data generated by Drs. L.M. Kettel, A. Murphy, A. Morales, and R. Reinsch, and supported in part by Roussel-Uclaf and San Diego Reproductive Medicine and Education and Research Foundation. The assistance and data analyses provided by Gail Laughlin and the preparation of this manuscript by Laurie Epifano are deeply appreciated.
REFERENCES
American Fertility Society (AFS). Revised American Fertility Society classification of endometriosis. Fertility and Sterility 43:351, 1985.
Andreyko, J.L., Blumenfeld, A., Marshall, L.A., et al. Use of an agonistic analog of GnRH (nafarelin) to treat leiomyomas: Assessment by magnetic resonance imaging. American
Journal of Obstetrics and Gynecology 158:903–910, 1988.
Barbieri, R.L. Etiology and epidemiology of endometriosis. American Journal of Obstetrics and Gynecology 162:565–567, 1990.
Baulieu, E.E. Contragestion and other clinical applications of RU 486, and antiprogesterone at the receptor. Science 245:1351, 1989.
Buttram, V.C., Jr., and Reiter, R.C. Uterine leiomyomata: Etiology, symptomatology, and management. Fertility and Sterility 36:433, 1981.
Carlson, K.J., Nichols, D.H., and Schiff, I. Indication for hysterectomy. New England Journal of Medicine 328:856, 1993.
Collins, R.L., and Hodgen, G.D. Blockade of the spontaneous midcycle gonadotropin surge in monkeys by RU 486: A progesterone antagonist or agonist? Journal of Clinical Endocrinology and Metabolism 63:1270–1276, 1986.
Cornillie, F.J., Osterlynck, D., Lauweryns, J.M., et al. Deeply infiltrating pelvic endometriosis: Histology and clinical significance. Fertility and Sterility 53:978, 1990.
De Ziegler, D., Bessis, R., and Frydman, R. Vascular resistance of uterine arteries: Physiological effects of estradiol and progesterone. Fertility and Sterility 55:775, 1991.
Dierschke, D.J., Yamaji, T., Karsch, F.J., et al. Blockade by progesterone of estrogen-induced LH and FSH release in the rhesus monkey. Endocrinology 92:1496–1501, 1973.
Dmowski, W.P., Headley, S., and Radwanska, E. Effects of danazol on pulsatile gonadotropin patterns and on serum estradiol levels in normally cycling women. Fertility and Sterility 39:49–55, 1983.
Friedman, A.J., Barbieri, R.L., Benacerraf, B.R., et al. Treatment of leiomyomata with intranasal or subcutaneous leuprolide, a GnRH agonist. Fertility and Sterility 48:560–567, 1987.
Friedman, A.J., Barbieri, R.L., Doubilet, P.M., et al. A randomized, double blind trial of a gonadotropin releasing-hormone agonist (leuprolide) with or without medroxyprogesterone acetate in the treatment of leiomyomata uteri. Fertility and Sterility 49:404, 1988.
Friedman, A.J., Hoffman, D.I., Comite, F., et al. Treatment of leiomyomata uteri with leuprolide acetate depot: A double-blind, placebo-controlled, multicenter study. Obstetrics and Gynecology 77:720–725, 1991.
Garcia, E., Bouchard, P., De Brux, J., et al. Use of immunocytochemistry of progesterone and estrogen receptors for endometrial dating. Journal of Clinical Endocrinology and Metabolism 67:80, 1988.
Garzo, V.G., Liu, J., Ulmann A., et al. Effects of an antiprogesterone (RU 486) on the hypothalamic-hypophyseal-ovarian-endometrial axis during the luteal phase of the menstrual cycle. Journal of Clinical Endocrinology and Metabolism 66:508–517, 1988.
Gravanis, A., Schaison, G., George, M., et al. Endometrial and pituitary responses to the steroidal antiprogestin RU 486 in postmenopausal women. Journal of Clinical Endocrinology and Metabolism 60:156–163, 1985.
Jones, H.W., and Rock, J.A. Regulation of female infertility. Pp. 181–210 in Regulation of Human Fertility. Diczfalusy, E., ed. Copenhagen: Scripton, 1976.
Kawaguchi, K., Fuji, S., Konishi, I., et al. Mitotic activity in uterine leiomyomas during the menstrual cycle. American Journal of Obstetrics and Gynecology 160:637–641, 1988.
Kessel, B., Liu, J., Mortola, J., et al. Treatment of uterine fibroids with agonist analogs of gonadotropin-releasing hormone. Fertility and Sterility 49:538, 1988.
Kettel, L.M., Murphy, A.A., Mortola, J.F., et al. Endocrine responses to long-term administration of the antiprogesterone RU 486 in patients with pelvic endometriosis. Fertility and Sterility 56:402–407, 1991.
Lessey, B.A., Metzger, D.A., Haney, A.F., et al. Immunohistochemical analysis of estrogen and progesterone receptors in endometriosis: Comparison with normal
endometrium during the menstrual cycle and the effect of medical therapy. Fertility and Sterility 51:409–414, 1989.
Liu, J.H., and Yen, S.S.C. Induction of midcycle gonadotropin surge by ovarian steroid in women: A critical evaluation. Journal of Clinical Endocrinology and Metabolism 57:797–802, 1983.
Liu, J.H., Garzo, G., Morris, S., et al. Disruption of follicular maturation and delay of ovulation after administration of the antiprogesterone RU 486. Journal of Clinical Endocrinology and Metabolism 65:1135–1140, 1987.
Lumsden, M.A., West, C.P., Hawkins, R.A., et al. The binding of steroids to myometrium and leiomyomata (fibroids) in women treated with the gonadotrophin-releasing hormone agonist Zoladex (ICI 118630). Journal of Endocrinology 121:389–396, 1989.
Luukkainen, T., Heikinheimo, O., Haukkamaa, M., et al. Inhibition of folliculogenesis and ovulation by the antiprogesterone RU 486. Fertility and Sterility 49:961–963, 1988.
Matta, W.H., Shaw, R.W., Hesp, R., et al. Reversible trabecular bone density loss following induced hypo-oestrogenism with the GnRH analogue buserelin in premenopausal women. Clinical Endocrinology 29:45–51, 1988a.
Matta, W.H., Stabile, I., Shaw, R.W., et al. Doppler assessment of uterine blood flow changes in patients with fibroids receiving the gonadotropin-releasing hormone agonist buserelin. Fertility and Sterility 49:1083–1085, 1988b.
Metzger, D.A., Szpak, C.A., and Haney, A.F. Histologic features associated with hormonal responsiveness of ectopic endometrium. Fertility and Sterility 59:83, 1993.
Neulen, J., Williams, R.F., Hodgen, G.D., et al. RU 486 (mifepristone): Induction of dose dependent elevations of estradiol receptor in endometrium from ovariectomized monkeys. Journal of Clinical Endocrinology and Metabolism 71:1074–1075, 1990.
Perl, V., Marquez, J., Schally, A.V., et al. Treatment of leiomyomata uteri with D-Trp6 luteinizing hormone-releasing hormone. Fertility and Sterility 48:383–389, 1987.
Rein, M.S., Friedman, A.J., Stuart, J.M., et al. Fibroid and myometrial steroid receptors in women treated with gonadotropin-releasing hormone agonist leuprolide acetate. Fertility and Sterility 53:1018, 1990.
Roseff, S.R., Kettel, L.M., Rivier, J., et al. Accelerated dissolution of luteal-endometrial integrity by the administration of antagonists of gonadotropin-releasing hormone and progesterone to late-luteal phase women. Fertility and Sterility 54:805, 1990.
Rummon, I.S., Radwanska, E., Ali, A., et al. Bone mineral density in women with endometriosis and during ovarian suppression with gonadotropin-releasing hormone agonists or danazol. Fertility and Sterility 49:792–796, 1988.
Schaison, G., George M., Lestrat, N., et al. Effects of the antiprogesterone steroid RU 486 during midluteal phase in normal women. Journal of Clinical Endocrinology and Metabolism 61:484–489, 1985.
Schlaff, W.D., Zerhouni, E.A., Huth, J.A., et al. A placebo-controlled trial of a depot GnRH analogue (leuprolide) in the treatment of uterine leiomyomata. Obstetrics and Gynecology 74:857–862, 1989.
Shaw, R.W. Treatment of Endometriosis. Lancet 340:1267, 1992.
Weigel, N.L., Beck, C.A., Estes, P.A., et al. Ligands induce conformational changes in the carboxyl-terminus of progesterone receptors which are detected by a site-directed antipeptide monoclonal antibody. Molecular Endocrinology 6:1585–1597, 1992.
West, C.P., Lunsden, M.A., Lawson, S., et al. Shrinkage of uterine fibroids during therapy with goserelin (Zoladex): A LHRH agonist administered as a monthly subcutaneous depot. Fertility and Sterility 48:45–51, 1987.
Wolf, J.P., Hsiu, J.F., Anderson, T.L., et al. Noncompetitive antiestrogenic effect of RU 486 in blocking the estrogen-stimulated luteinizing hormone surge and proliferative action of estradiol on endometrium in castrate monkeys. Fertility and Sterility 52:1055–1060, 1989.