4
Vaccine Demand and Supply
The state of vaccine demand, supply, and innovation on the global level is quite different from that in the United States. Consequently, this chapter examines these trends on a global basis and then explores the domestic conditions of vaccine supply and demand.
GLOBAL DEMAND AND SUPPLY
Demand
The potential size of the worldwide pediatric vaccine market is determined by two factors: the annual worldwide birth cohort (approximately 143 million live births per year) (World Bank, 1993) and the number of vaccines a child receives through adolescence. Eight of the vaccines recommended by the World Health Organization's (WHO's) Expanded Program on Immunization (EPI) should be administered during or shortly after the first year of life (see Appendix G for immunization schedule). According to one estimate, almost 1.5 billion doses of vaccine were used around the world in 1990 (Baudrihaye, 1992) (Table 4-1). Of this amount, North America, Europe, and Japan used just 14 percent of the total, while purchases by the United Nations Children's Fund (UNICEF), the Pan American Health Organization (PAHO), and WHO accounted for approximately 63 percent of the total vaccine used (Baudrihaye, 1992).
Although the number of potential vaccinees in developing countries is
TABLE 4-1 Estimated Worldwide Usage of Vaccines, 1990 (in millions of doses)
|
Vaccine |
North America Europe, and Japan |
UNICEF PAHO, and WHO |
Other |
Total |
|
BCG |
5 |
160 |
20 |
185 |
|
DTP |
40 |
219 |
50 |
260 |
|
Hepatitis B |
15 |
|
35 |
50 |
|
Influenza |
75 |
|
10 |
85 |
|
Measles and combined |
15 |
131 |
30 |
165 |
|
Meningococcal |
10 |
20 |
30 |
60 |
|
Polio (OPV, IPV) |
60 |
450 |
190 |
700 |
|
Rabies |
1 |
3 |
4 |
8 |
|
Total |
211 |
983 |
358 |
1,552 |
|
Percentage of total |
14 |
63 |
23 |
100 |
|
SOURCE: Adapted from N. Baudrihaye, European Federation of Pharmaceutical Industries Association, Brussels, 1992; with additional information provided by Akira Homma, PAHO, 1993; John Gilmartin, UNICEF, 1993; Terrel Hill, UNICEF, 1993. |
||||
much larger than that in the industrialized world (almost 80 percent of the 143 million live births occur in the developing world), the amount spent on vaccines in the industrialized world greatly exceeds that spent by UNICEF, PAHO, and WHO. The total worldwide value of human vaccines sold in 1992 has been estimated to be as high as $3 billion (Technology Management Group, 1993), of which only $65 million represented UNICEF purchases (John Gilmartin, UNICEF, personal communication, 1993).
Regional Demand
Assessments of country-level demand for vaccines must take into account the size of the target population, estimated extent of immunization coverage, anticipated vaccine wastage, number of scheduled doses, and any special immunization campaigns or strategies that would lead to a surge in demand. Determination of demand for vaccines is more problematic when special, intensive immunization strategies are considered (World Health Organization/Children's Vaccine Initiative, 1992c). For example, the ongoing global campaign to eradicate polio in the Americas has led to increased demand for and, at brief intervals, temporary shortages of polio vaccine (Pan American Health Organization, 1992). In 1992, UNICEF
purchased 351 million doses of oral polio vaccine (OPV), which cost $25.6 million including air freight delivery (John Gilmartin, UNICEF, personal communication, 1993; World Health Organization/Children's Vaccine Initiative, 1992b). It is estimated that to duplicate polio eradication efforts elsewhere in the world, the annual purchase of OPV must increase to $87 million (Agency for Cooperation in International Health, 1992).
Vaccine wastage has been identified as another major problem, not only in terms of cost but also in terms of forecasting the demand for vaccine (World Health Organization/Children's Vaccine Initiative, 1992c). Between 1982 and 1992 the demand for EPI vaccines rose 10-fold (Agency for Cooperation in International Health, 1992; UNICEF, 1991b). This kind of growth in demand has forced UNICEF to try to predict the number of doses needed, so that manufacturers will have enough time to increase their production. This has not been an easy task, primarily because the month-to-month variation in demand for a vaccine can vary as much as sevenfold (World Health Organization/Children's Vaccine Initiative, 1992c). Up until 1990, UNICEF's annual forecast of worldwide vaccine demand was fairly accurate. However, in both 1991 and 1992, countries requested substantially less vaccine from UNICEF than estimated (Terrel Hill, UNICEF, personal communication, 1993). The precise reasons for the decreased country demand for UNICEF-supplied vaccine are not fully understood at this time. It is likely, however, that increased local production of vaccines in some countries has led to decreased country-level demand. In addition, improved national census data in many countries may have resulted in a more realistic assessment of vaccine need. Of great concern, however, is that the decreased demand for vaccine may be a result of a slippage in immunization coverage in many countries (Terrel Hill, UNICEF, personal communication, 1993).
Supply
Vaccines are manufactured by both industrialized and developing countries around the world. It is estimated that almost 60 percent of the diphtheria and tetanus toxoids and pertussis vaccine (DTP) currently being used in the world is actually produced in the country that uses it (World Health Organization/Children's Vaccine Initiative, 1992a). The annual production of 500 million doses of EPI vaccines by the People's Republic of China is equivalent to roughly half of all vaccines purchased by UNICEF each year (Agency for Cooperation in International Health, 1992). Currently, OPV is produced or bulk finished in over 25 nations (of which half are considered to be developing countries). (The quality control requirements for the production of OPV differ from those for the finishing
of OPV bulk vaccine. For countries to mount full production of OPV, they must maintain expensive monkey colonies, which are needed for neurovirulence testing and must have considerable staff expertise and training. In contrast, finishing a vaccine that has already been fully tested may reduce the need for such large investments in quality control.) Tetanus toxoid is made in almost 40 countries, DTP is manufactured in approximately 30 countries, and measles and BCG (bacillus Calmette-Guérin) vaccines are produced in approximately 20 countries (Agency for Cooperation in International Health, 1992).
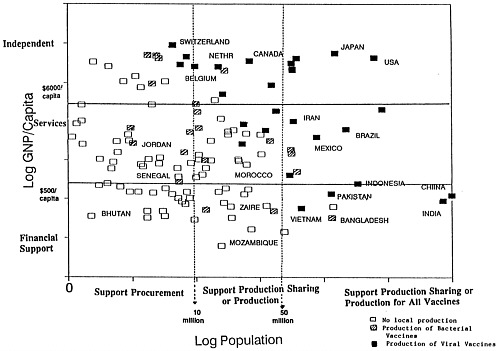
The vaccine supply grid developed by Amie Batson and Peter Evans of the World Health Organization (Figure 4-1) depicts the 130 countries that currently produce vaccines according to per capita gross national product and population size. A number of donor agencies are using the grid to evaluate strategies for helping countries buy vaccines, share vaccine production capabilities, or establish production facilities.
Population expansion, more comprehensive immunization, and greatly increased demands for polio vaccine because of global eradication goals have raised questions about the stability of the global vaccine supply (World Health Organization/Children's Vaccine Initiative, 1992a). According to UNICEF, the demand for OPV peaked in 1990 and declined slightly in 1991 and 1992 (Terrel Hill, UNICEF, personal communication, 1993). The largest single user country, India, is expected to become self-sufficient in OPV production in 1993, thereby reducing demand from UNICEF by at least 50 million doses per year (John Gilmartin, UNICEF, personal communication, 1993). UNICEF's ability to procure adequate levels of vaccine into the future is a concern, given rising vaccine prices and competing priorities for increasingly limited resources (UNICEF, 1991a, 1992a); in addition, some donors, such as the Rotary Foundation, have decreased the amount included in their pledges. Preliminary projections of vaccine requirements through 1995 suggest that there may be significant shortfalls in vaccine supply if UNICEF is unable to secure the procurement of EPI vaccines at very low prices into the future. There is also concern that there are now insufficient funds to buy additional EPI vaccines required for such activities as measles control and neonatal tetanus eradication (Agency for Cooperation in International Health, 1991).
At the 1991 International Meeting on Global Vaccine Supply in Kumamoto, Japan, several issues that may affect the future viability of EPI were discussed. Among them were the need to strengthen monetary, logistical, and supply mechanisms for integrating new vaccines into EPI and the need to improve substandard manufacturing capabilities in some countries (Agency for Cooperation in International Health, 1991).
There is mounting concern about the quality of many of the locally produced vaccines used in EPI programs (Hlady et al., 1992; World Health
Organization, 1992). It has been estimated that more than half of the vaccines produced around the world do not meet accepted WHO standards of quality (Lancet, 1992). Many countries lack functioning national control authorities, and as a result, quality control of locally produced vaccines is emerging as a top-priority concern of EPI and the Children's Vaccine Initiative (CVI).
Procurement
UNICEF is the largest single purchaser of vaccine (in doses) for the developing world. The number of doses of EPI vaccines supplied by UNICEF more than doubled in 5 years (Table 4-2). In 1985, UNICEF bought roughly 366 million doses at a cost of approximately $18 million; by 1992, this had increased to 850 million doses at a total cost of some $65 million, including air freight delivery (UNICEF, 1991a, 1992a,b). Polio vaccine and DTP account for the largest number of doses in the UNICEF procurement; this is followed by tetanus toxoid (TT), and BCG and measles vaccine (Table 4-2).
Every 2 years, UNICEF issues a tender for the purchase of vaccines. UNICEF purchases EPI vaccines from all companies that are prequalified to supply vaccine and that submit bids. Companies whose bids are higher than the winning bid are often asked to resubmit an offer. In theory, the lowest bidder receives two-thirds of the UNICEF market, with each successively higher bidder receiving one-third of the remaining market (Peter Evans, Expanded Program on Immunization, World Health Organization, personal communication, 1993). In general, the lowest bidder is unable to
TABLE 4–2 Vaccines Procured by UNICEF, 1985 and 1990
|
Vaccine |
No. of Doses in 1985 (in thousands) |
Percent of Total |
No. of Doses in 1990 (in thousands) |
Percent |
|
BCG |
66,296 (4)a |
18.11 |
132,004 (5) |
13.65 |
|
DTP |
89,485 (4) |
24.45 |
183,881 (4) |
19.01 |
|
DT |
20,153 (5) |
5.51 |
13,144 (5) |
1.36 |
|
Measles |
36,215 (5) |
9.90 |
86,313 (7) |
8.92 |
|
OPV |
116,772 (5) |
1.90 |
388,510 (6) |
40.17 |
|
TT |
37,049 (4) |
10.12 |
163,400 (6) |
16.89 |
|
Totals |
365,970 (9) |
|
967,254 (11) |
|
|
a Values in parentheses are number of suppliers. SOURCE: Data supplied by UNICEF, 1993. |
||||
provide UNICEF with two-thirds of the total doses required for any vaccine; therefore, several suppliers provide each vaccine, with preference given to the lowest bidders.
UNICEF has used competitive vaccine bids for many years. From 1985 through 1990, the number of suppliers grew from 9 to 11 (Table 4-2). European vaccine manufacturers supply 90 percent of the vaccines used by UNICEF (this includes Connaught Laboratories, Ltd., [Canada], a subsidiary of Pasteur-Mérieux Sérums et Vaccins) (John Gilmartin, UNICEF, personal communication, 1993). Currently, U.S. vaccine manufacturers are invited to participate in the bidding, but have not made offers since at least 1982. That year, a U.S. vaccine manufacturer was criticized in congressional hearings for selling vaccine to PAHO at prices substantially below those quoted to the U.S. government (U.S. Congress, Senate, 1982). This continues to be a sensitive issue in the United States.
To supply vaccine to UNICEF, a company must request and pay for a WHO-organized evaluation of its manufacturing facilities and the country's national biologics control authority. Several lots of the company's vaccine are then tested at one or more of the WHO's collaborating centers, a process also paid for by the company. Only when the vaccine is determined to meet WHO standards, when the facility is approved, and when the national control authority is determined to be reliable is the company licensed to supply UNICEF with vaccines. UNICEF and WHO do not have the capability to monitor the consistency of vaccine lots produced by manufacturers as is currently done in the United States by the U.S. Food and Drug Administration (see Appendix C).
No manufacturers based in developing countries supplied UNICEF with vaccines in 1990 (Table 4-3). Compared with their international competitors, most vaccine manufacturers in developing countries have several disadvantages. They must frequently import raw materials, often paying substantial import duties on these materials. And because vaccine manufacturing is more capital intensive than labor intensive, the low-cost labor pool in developing countries does not offer any advantages. In fact, some have charged that locally produced vaccines are often more expensive than those procured through UNICEF and PAHO (Baudrihaye, 1992; Vandersmissen, 1992).
Through its procurement system, UNICEF has actively sought to expand the base of suppliers both to ensure a stable vaccine supply and to keep the prices charged for EPI vaccines comparatively low (U.S. Congress, Senate, 1982). (In this regard, UNICEF might be reluctant to purchase a ''super" vaccine, such as might result from the CVI, from a single supplier.) UNICEF vaccine prices are a fraction of those commanded elsewhere in the world, including the United States (Table 4-4). Until quite recently, yearly EPI vaccine price increases have barely exceeded inflation. However, in
TABLE 4–3 Companies That Supplied EPI Vaccines to UNICEF, 1990
|
Company |
BCG |
DTP |
TT |
Measles |
Polio |
|
Connaught Laboratories, Ltd. (Canada) |
X |
X |
X |
X |
X |
|
Con Pharma (Canada) |
|
|
X |
X |
|
|
Eisai (Japan) |
|
|
|
X |
|
|
Evans-Medical, Ltd. (United Kingdom) |
X |
|
|
X |
|
|
Behringwerke (Hoechst) (Germany) |
X |
X |
X |
|
|
|
Pasteur Mérieux Sérums et Vaccins (France) |
X |
X |
X |
X |
X |
|
Inter-Export (Yugoslavia) |
|
X |
X |
|
|
|
Japan BCG (Japan) |
X |
|
|
|
|
|
Sclavo (Italy) |
|
|
|
X |
X |
|
SmithKline Beecham (United Kingdom) |
|
|
|
X |
X |
|
Swiss Serum Vaccine Institute (Switzerland) |
|
X |
X |
|
|
|
SOURCE: Data supplied by UNICEF, January 1993. |
|||||
TABLE 4–4 UNICEF prices for EPI Vaccine, 1992
|
Vaccine |
No. of Doses |
Cost($)/Dose |
Cost($)/Series |
|
BCG |
1 |
0.065 |
0.065 |
|
DTP |
3 |
0.0575–0.075 |
0.173–0.225 |
|
Measles |
1 |
0.16 |
0.16 |
|
TT |
3–5 |
0.0325–0.05 |
0.0975–0.25 |
|
OPV |
3 |
0.07–0.085 |
0.210–0.255 |
|
Total |
11–13 |
|
0.705–0.955 |
|
SOURCE: UNICEF Price List, 1992. |
|||
1992, vaccine prices increased 23 percent above the 1990 tender price (Steele, 1992). Vaccine manufacturers have indicated that the low prices quoted to UNICEF for EPI vaccines cannot continue indefinitely because the costs of manufacturing vaccines, research and development, and capital investments are all increasing (Meérieux, 1992; Vandersmissen, 1992).
Although some 90 percent of the vaccine purchased by UNICEF and PAHO are made by several of the largest manufacturers, these purchases amount to less than 10 percent of these company's vaccine revenues (Agency for Cooperation in International Health). Indeed, some companies that supply vaccines to UNICEF do so to utilize their excess capacity, and the prices that they charge generally cover the marginal cost of production (Dupuy and Freidel, 1990; Robbins and Freeman, 1988). Because these vaccine purchases have a minimal impact on the total vaccine revenues of those companies that sell vaccine to UNICEF and PAHO, some have suggested that dependence on these international vaccine suppliers puts the global vaccine supply in a precarious position (Agency for Cooperation in International Health, 1992; Institute of Medicine, 1986). Even though a major UNICEF supplier's exit from the vaccine business might have a minor impact on the firm's bottom line, there is concern that it might have a significant negative impact on the supply of high-quality vaccines to the developing world.
Innovation
There are a number of childhood diseases, including malaria and acute respiratory infections, that claim millions of lives annually and for which effective vaccines are not yet available. Unfortunately, the research and development of new and improved vaccines for exclusively developing-country markets by commercial manufacturers is limited. Most public-sector vaccine institutes in Europe do not have the resources or the mandates required to conduct new vaccine development for developing-country markets. The low prices quoted to UNICEF/PAHO cover the marginal costs of production, but they do not appear to provide sufficient market incentives for international vaccine companies to invest in vaccine research and development.
Furthermore, despite a number of successful programs such as the WHO/UNDP Program for Vaccine Development and the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases, there is no significant international or multinational fund dedicated to the early stages of vaccine development and testing of vaccine for use in the developing world.
New and improved vaccines that are developed and manufactured for
industrialized-country markets do "trickle down" eventually (sometimes after many years) to some developing countries. In some cases, vaccines developed by and for the DOD have been introduced into some developing countries on an ad hoc basis by commercial manufacturers. However, the target groups for these vaccines tend to be adults, not infants and children. This is because the DOD's primary responsibility is to protect young-adult soldiers—not infants and children. Commercial manufacturers have been reluctant to invest in the costly clinical trials required to demonstrate further vaccine efficacy in infants and young children probably because the returns are likely to be small compared with those from other investment opportunities. The prices of new vaccines have been beyond the means of most developing countries and such international buyers as UNICEF and PAHO. As a consequence, no new vaccines have been added to the UNICEF procurement scheme since its inception, despite recommendations that hepatitis B vaccine be included in national immunization programs.
DEMAND AND SUPPLY IN THE UNITED STATES
Demand
The pediatric vaccine market in the United States is predictable, limited, and stable. The size of the market is constrained by two factors: the annual birth cohort—approximately 4 million live births per year (World Almanac and Book of Facts, 1992) and the number of vaccines a child receives through adolescence. Thirteen of the eighteen separate vaccinations recommended by the Advisory Committee on Immunization Practices should be administered during or shortly after the first year of life (see Appendix G for immunization schedule). Three of the remaining four vaccines should be given before age 6 years.
Currently, about 20 million doses of DTP and OPV are distributed each year in the United States (National Vaccine Injury Compensation Trust Fund, 1992). Prior to the measles epidemic of 1989–1991 and the requirement for a second dose of measles vaccine, approximately 10 million doses of measles-mumps-rubella vaccine (MMR) were distributed each year. In 1990, 19 million doses of MMR were distributed; in 1991, this figure dropped to 16 million (National Vaccine Injury Compensation Trust Fund, 1992).
Over the last decade, the public sector has purchased an increasing share of the vaccines sold in the United States (Table 4-5). Currently, almost half of all vaccines purchased in this country are procured with federal or state funds at contract prices. The current trend toward public-sector
TABLE 4–5 Publicly Purchased Doses as a Percentage of Net Doses Distributed in the United States, 1985–1991
procurement of vaccines is of considerable concern to the large commercial manufacturers (Douglas, 1992, 1993; Saldarini, 1992, 1993; Williams, 1993). They argue that sales to the public sector offset those to the private sector, and increasing public sector procurement will lead to further increases in the prices charged to private-sector clients (Garnier, 1993). Those involved in vaccine manufacturing also contend that if the U.S. government emerges as the sole purchaser of vaccines, company investments in vaccine-related research and development would likely decline (Douglas, 1992; Katz, 1993; Saldarini, 1992; Six, 1992). Others (Edelman, 1993; Shalala, 1993) however, suggest that the effects of any large-scale federal procurement policy on the U.S. vaccine industry are uncertain—policies on pricing, funding for product development, and competitive production of vaccines could entice additional manufacturers to enter this industry (Institute of Medicine, 1986; Shalala, 1993).
Supply
Forty vaccines and toxoids and an additional 10 immune globulins and antitoxins are licensed and available for use in the United States (see the box "Vaccines, Toxoids, Immune Globulins, and Antitoxins Available in the United States, 1993). The current supply of most childhood vaccines is plentiful in the United States. This is not to say, however, that all children who should be immunized are or that potential shortages cannot occur. Nevertheless, the problem of less-than-optimal vaccine coverage in the United States is due more to problems of access and to the failure of the
|
Vaccines, Toxoids, Immune Globulins, And Antitoxins Available in the United States, 1993 |
|
Licensed Vaccines and Toxoids |
|
Adenovirus vaccine, live oral, type 4 |
|
Adenovirus vaccine, live oral, type 7 |
|
Anthrax vaccine, adsorbed |
|
BCG (bacillus Calmette Guérin vaccine) |
|
Cholera vaccine |
|
Diphtheria toxoid |
|
Diphtheria toxoid, adsorbed |
|
Diphtheria and tetanus toxoids, adsorbed (TD) |
|
Diphtheria and tetanus toxoids and pertussis vaccine, adsorbed (DTP) |
|
Diphtheria and tetanus toxoids and pertussis vaccine and Haemophilus influenzae type b |
|
Diphtheria and tetanus toxoids and acellular pertussis vaccine adsorbed (DTaP) |
|
Hepatitis B vaccine, plasma derived |
|
Hepatitis B vaccine, recombinant |
|
Haemophilus type b polysaccaride vaccine |
|
Haemophilus b conjugate vaccine (Hib-CV) |
|
Influenza virus vaccine |
|
Japanese encephalitis virus vaccine inactivated |
|
Measles virus vaccine live |
|
Measles, mumps and rubella virus vaccine live (MMR) |
|
Measles and mumps virus vaccine live |
|
Measles and rubella virus vaccine live |
|
Meningococcal polysaccaride vaccine A, C, Y, W135 combined |
|
Mumps virus vaccine live |
|
Pertussis vaccine |
|
Pertussis vaccine adsorbed |
|
Poliovirus vaccine inactivated |
|
Polio vaccine live oral, trivalent |
|
Plague vaccine |
|
Pneumococcal vaccine, polyvalent |
|
Rabies vaccine |
|
Rabies vaccine adsorbed |
|
Rubella vaccine |
|
Rubella and mumps virus vaccine live |
|
Smallpox vaccine |
|
Vaccines, Toxoids, Immune Globulins, And Antitoxins Available in the United States, 1993 |
|
Tetanus toxoid |
|
Tetanus toxoid adsorbed |
|
Tetanus-diptheria (Td) |
|
Typhoid vaccine |
|
Typhoid vaccine, live oral Ty21a |
|
Yellow Fever vaccine |
|
Immune globulins and Antitoxins |
|
Botulism antitoxin |
|
Cytomegalovirus immune globulin intravenous |
|
Diphtheria antitoxin |
|
Hepatitis B immune globulin |
|
Immune globulin |
|
Pertussis immune globulin |
|
Rabies immune globulin |
|
Tetanus antitoxin |
|
Tetanus immune globulin |
|
Vaccinia immune globulin |
public health and medical communities to fully immunize all U.S. children than to deficiencies in supply (Cutts et al., 1992; Peter, 1992).
Between 1966 and 1977, half of all commercial vaccine manufacturers in the United States stopped producing and distributing vaccines (U.S. Congress, House, 1986). During the late 1970s and early 1980s, the exodus from the vaccine business continued. Eli Lilly, Pfizer, Glaxo, Wellcome, Dow Chemical, and Merrell-National Laboratories were among those companies that discontinued their vaccine operations or sold off their vaccine components altogether (see Appendix H). The reasons for the exodus during these years are many, but include U.S. Food and Drug Administration requirements for demonstration of vaccine efficacy1, liability concerns, and poor market returns relative to other product areas. In the United States, the few remaining vaccine manufacturers stayed in the vaccine business as much to meet the public health need (there were no other suppliers for OPV and MMR) as out of corporate commitment to their products.
Although 18 companies and two states are licensed to manufacture selected vaccines for the U.S. market, only a handful of companies supply pediatric vaccines. The supply of two of the vaccines, MMR and OPV, is dependent on sole-source suppliers (U.S. Department of Health and Human Services, 1991). Reliance on such a small number of companies for the production of U.S. pediatric vaccines has not been without problems
(Institute of Medicine, 1985; U.S. Congress, House, 1986). A series of unfortunate events in 1984 and early 1985 led to a shortage of DTP in the United States: two private-sector manufacturers withdrew from the market because of liability concerns (among other reasons), and a third manufacturer experienced some production problems. State manufacturers of DTP could not meet the demand, and the Centers for Disease Control and Prevention (CDC) had to issue a revised immunization schedule that urged physicians to delay giving some DTP booster shots until more vaccine became available (U.S. Congress, House, 1986). The fragility of the nation's vaccine supply had been demonstrated.
In 1983, Congress appropriated funds to the CDC to ensure that a 6-month stockpile of critical vaccines be maintained at all times as a solution to a temporary shortage of vaccine. Although a 6-month stockpile would compensate for short-term interruptions in supply, it is unlikely that U.S. immunization efforts could be sustained if a sole producer of a vaccine were to halt the production and distribution of a needed product. It takes considerably more than 6 months to retrofit an existing production facility to make a new vaccine and longer still to construct a facility from the ground up (George Siber, Massachusetts Department of Public Health, personal communication, 1993).
Pricing
In the United States, commercial manufacturers list two prices for a vaccine: a contract price, which is negotiated on an annual basis with the CDC, and a catalog price, which sets vaccine prices for private-sector clients, such as hospitals, health maintenance organizations, pharmacies, and physicians. As can be seen in Table 4-6, the catalog price for each
TABLE 4-6 Cost and Price (including Excise Tax) of the Basic Series of Childhood Vaccines in the United States, as of March 31, 1993
|
Vaccine |
Price ($) |
No. of Doses |
Cost ($) |
||
|
Contact |
Catalog |
Public Sector |
Private Sector |
||
|
DTaP |
11.01 |
16.33 |
2 |
22.02 |
32.66 |
|
DTP |
5.99 |
10.04 |
3 |
17.97 |
30.12 |
|
Hib-CV |
5.37 |
15.13 |
4 |
21.48 |
60.52 |
|
MMR |
15.33 |
25.29 |
2 |
30.66 |
50.58 |
|
OPV |
2.16 |
10.43 |
4 |
8.64 |
41.72 |
|
Hepatitis B |
6.91 |
10.71 |
3 |
20.73 |
32.12 |
|
Total |
|
|
18 |
121.50 |
247.72 |
|
SOURCE: Division of Immunization, Centers for Disease Control and Prevention, 1993. |
|||||
childhood vaccine is higher than the contract price. The total public-sector cost of the required pediatric vaccines in 1993 is $122, while the cost to private-sector clients is more than double that ($248). Although there are differences in the terms and conditions of vaccine sales to the public and private sectors (companies bear the cost of distributing catalog-priced vaccines and buy back unused doses), sales to the private sector are said to offset those to the public sector. As the percentage of doses procured by the public sector has increased over time, vaccine prices in the private sector have risen substantially.
Excluding the cost of vaccine, the charges associated with administering the complete series of pediatric vaccines may run from as little as $25 at a public health clinic to more than $200 at a private physician's office (Freeman et al., 1993). Thus, the total amount, including vaccine, needed to fully immunize a child in the United States ranges from almost 147 in the public sector to more than $448 in the private sector.
In 1988, in an effort to compensate for adverse events from government-mandated vaccines as well as to offset vaccine manufacturers' liability concerns, an excise tax was added to the price of each of the government-mandated childhood vaccines. Until recently, the taxes—$4.56 per dose of DTP, $4.44 per dose of MMR, and $0.29 per dose of OPV—were paid into a special trust fund that was used to pay the claims of those with vaccine-related injuries. The law establishing the National Vaccine Injury Compensation Program mandated that the excise taxes be collected until 1992, at which point the program was to be reassessed. A provision to extend the National Vaccine Injury Compensation Program was included as part of a larger congressional bill, which was subsequently vetoed for reasons unrelated to the compensation program. Because there was no further congressional action to extend the collection of excise taxes, the Secretary of the Treasury, in accordance with the law, revoked the excise tax in January 1993. This situation has caused some confusion, but is expected to be resolved shortly by Congress. (See Appendix B for a discussion of the National Vaccine Injury Compensation Program.)
The list price for each of the major government-mandated childhood vaccines in both the public and private sectors has increased substantially since 1977 (see Table 4-7). Tables 4-8 and 4-9 show federal contract and private catalog prices, respectively, in constant dollars for OPV, DTP, and MMR for the period 1977-1992. For comparison, the last three columns in Tables 4-8 and 4-9 present the indices used to track changes in the prices of various goods. The first, the Consumer Price Index (CPI), reflects the price rise of a general "basket" of consumer goods; the second, the Pharmaceutical Producer Price Index (PPPI), reflects price changes in ethical pharmaceuticals. Prices in both indices are standardized to the base year of 1983.
TABLE 4-7 Vaccine Prices (in dollars) in the United States from 1977–February 1993
|
Year |
DTP |
OPV |
MMR |
Hib-CV |
||||
|
|
CP |
FC |
CP |
FC |
CP |
FC |
CP |
FC |
|
1977 |
0.19 |
0.15a |
1.00 |
0.30 |
6.01 |
2.42 |
NA |
NA |
|
1978 |
0.22 |
0.15a |
1.15 |
0.31 |
6.16 |
2.35 |
NA |
NA |
|
1979 |
0.25 |
0.15a |
1.27 |
0.33 |
6.81 |
2.62 |
NA |
NA |
|
1980 |
0.30 |
0.15a |
1.60 |
0.35 |
7.24 |
2.71 |
NA |
NA |
|
1981 |
0.33 |
0.15a |
2.10 |
0.40 |
9.32 |
3.12 |
NA |
NA |
|
1982 |
0.37 |
0.15a |
2.75 |
0.48 |
10.44 |
4.02 |
NA |
NA |
|
1983 |
0.45 |
0.42a |
3.56 |
0.58 |
11.30 |
4.70 |
NA |
NA |
|
1984 |
0.99 |
0.65a |
4.60 |
0.73 |
12.08 |
5.40 |
NA |
NA |
|
1985 |
2.80 |
2.21 |
6.15 |
0.80 |
13.53 |
6.85 |
NA |
NA |
|
1986 |
11.40 |
3.01 |
8.67 |
1.56 |
15.15 |
8.47 |
NA |
NA |
|
1987 |
8.92 |
7.69 |
8.07 |
1.36 |
17.88 |
10.67 |
NA |
NA |
|
1988 |
11.03 |
8.46b |
8.07 |
1.36 |
24.11 |
16.18 |
13.75 |
11.00 |
|
1989 |
10.65 |
7.96 |
9.45 |
1.92 |
24.11 |
16.18 |
13.75 |
6.00 |
|
1990 |
10.65 |
6.91 |
9.74 |
1.92 |
24.07 |
14.71 |
14.55 |
5.20 |
|
1991 |
9.97 |
6.25 |
9.91 |
2.09 |
25.29 |
15.33 |
14.55 |
5.16c |
|
1992 |
9.97 |
6.25 |
9.91 |
2.09 |
25.29 |
15.30 |
14.55 |
5.16c |
|
1993 |
10.04 |
5.99 |
10.43 |
2.16 |
25.29 |
15.33 |
15.13 |
5.37 |
|
NOTE: CP, Catalog Price; FC, Federal contract Price; NA, vaccine not licensed. From 1988 to 1992, prices include federal excise tax for the Vaccine Injury Compensation Program. Excise taxes are set at $4.56 per dose of DTP, $4.44 per dose of MMR, and $0.29 per dose of OPV. a No federal contract. The price represents the average price charged to the states. b Federal contract price was $9.62 for a portion of 1988. c Merck federal contract price was $8.25 for use among Native American populations. SOURCE: Division of Immunization, Centers for Disease Control and Prevention, 1993. |
||||||||
It is worth noting that through the early 1980s, the prices of OPV and DTP were quite low, an indication, the committee believes, that manufacturers were treating vaccines much like generic products that cost little to produce, that had high-volume sales, and that had low profit margins. Vaccines appear to have been priced to cover their marginal costs of production. Indeed, companies marketed DTP at roughly $0.15 a dose and OPV at $0.30 a dose to the federal government into the early 1980s.
Beginning in the early 1980s and continuing to the present, vaccine prices have risen substantially. Over the 15-year period from 1977 to 1991, the cumulative increases (in 1993 dollars and excluding the excise tax) in the contract and catalog prices for DTP were $1.55 (1,033 percent increase) and $5.22 (2,847 percent increase) respectively. The cumulative increase in the price of OPV from 1977 through 1992 was $8.62, or 500 percent for the contract price of vaccine, and $1.50, or 862 percent, for the catalog price of
vaccine. From 1977 through 1992, the contract price for MMR increased by $8.47, or 350 percent, wheras the catalog price rose by $14.84, or 247 percent. During this same period, the CPI rose 122 percent and the PPPI jumped 232 percent.
The rate of price increases in the market for DTP, MMR, and OPV has outstripped the rise in prices for the economy as a whole and for ethical pharmaceuticals. Those companies that remained in the vaccine business after the exodus in the 1970s appear now to be treating vaccines much like other pharmaceutical products with a corresponding investment in new facilities and in research and development and with an anticipation of returns.
Vaccine Innovation
The pharmaceutical industry has often been described as a high-risk, high-profit enterprise that is dependent upon the development and marketing of novel products (di Masi et al., 1991; Grabowski and Vernon, 1990; Lasagna, 1992; Office of Technology Assessment, 1991). Most established pharmaceutical firms have viewed the vaccine business as unpromising, characterized by undifferentiated product lines, a high risk of product liability, a few large high-volume, low-price purchasers, and poor patent protection (DeBrock, 1983; Institute of Medicine, 1985, 1986; Nicholas Mellors, Merlin, personal communication, 1993; Vandersmissen, 1992). The exodus of companies from the vaccine business in the 1960s through 1970s (see Appendix H), the relatively low expenditures on research and development into the 1980s (Table 4-10), and the small proportion (less than 5 percent) of vaccine Product License Applications (PLAs) as a total of all PLAs filed at the Center for Biologics and Evaluation Research from 1987 to 1991 would appear at the outset to confirm this assessment.
The pharmaceutical industry devotes a relatively small share of its research and development expenditures to biologics, a category that includes vaccines (Table 4-10). This is hardly surprising since vaccine sales account for less than 5 percent of most diversified companies' total sales (Agency for Cooperation in International Health, 1991; American Cyanamid, 1991; Institute of Medicine, 1992; Merck & Co., Inc., 1991b). Although spending in real terms (as reported to the Pharmaceutical Manufacturers Association) on pharmaceutical and biologics R&D has increased over time, the pattern of investment in biologics R&D has, until very recently, been one of decline. Spending on biologics research fell from 4 percent of the total in 1973 to a little more than 2 percent in 1983. By 1988, spending on biologics R&D had returned to the 1973 level (in relative terms), and it has increased every
TABLE 4-8 Federal Contract Prices for Vaccines in ''Current" Dollars
TABLE 4-9 Private Catalog Prices for Vaccines in "Current" Dollars
year since then. Of more relevance, however, is spending on vaccine R&D as a percentage of total vaccine sales. This percentage decreased substantially from 1976 to 1982 (Table 4-10). Although recent data on vaccine R&D as a percentage of vaccine sales are unreported and unavailable, it is likely that investment in R&D has increased to 12–15 percent of sales, which is similar to that for the overall pharmaceutical industry (Business Week, 1992; Financial Times, 1993).
The total number of Investigational New Drug (IND) applications submitted to the Center for Biologics Evaluation and Research of the U.S. Food and Drug Administration (FDA) has increased dramatically, from 66 in 1980 to just under 558 in 1992 (Zoon and Beatrice, 1993). Almost half of the IND applications filed since the mid-1980s are for biologic products produced by using biotechnology (Figure 4-2). Although almost four times as many IND applications were submitted for therapeutics than for vaccines in 1992 (Figure 4-3), there has been a notable increase in the number of vaccine IND applications filed in the last several years. From 1983 through 1989, an average of 32 vaccine IND applications were filed each year. In 1990, 67 vaccine IND applications were filed; in 1992, 81 were filed (Zoon and Beatrice, 1993). Thus, it appears that the relatively low number of PLAs filed during the late 1980s and early 1990s reflects both the lengthy development timeline of vaccines and the time-consuming FDA licensure process. Many more vaccine-related PLAs can be expected in the future.
There are other signs that vaccines are becoming more important relative to other operations of pharmaceutical companies. For example, Merck & Co., Inc., created the Merck Vaccine Division in 1991, and Lederle-Praxis Biologicals was made a full business unit of American Cyanamid in 1992. Corporate-level reorganization has also translated into major capital investments for some companies. Indeed, Merck & Co., Inc., is investing $150 million in the construction of a biotechnology facility for vaccines in Pennsylvania (Douglas, 1993).
Established pharmaceutical firms with vaccine interests are also actively pursuing promising technologies developed by various biotechnology companies by either licensing the technology or simply buying the company outright (Sugawara, 1992). In 1989, Lederle Laboratories, a unit of the American Cyanamid Corporation, acquired Praxis Biologics, a biotechnology company that had developed a conjugate Haemophilus influenzae type b conjugate vaccine (Hib-CV). Merck & Co., Inc., has entered into a variety of strategic alliances with a variety of companies, including MedImmune, Inc., a biotechnology firm involved in vaccine development (MedImmune, 1991; Merck & Co., Inc., 1991a). By the end of 1992, there were over 75
TABLE 4-10 R&D Expenditures and Sales of All U.S. Pharmaceuticals and of the Biologics Component
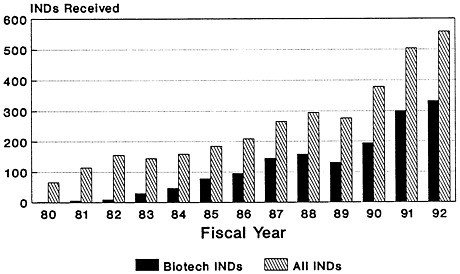
FIGURE 4-2
CBER biotech INDs received, compared to total. SOURCE: Application Review and Policy, Therapeutics Research and Review, Center for Biologics Evaluation and Research, U.S. Food and Drug Administration.
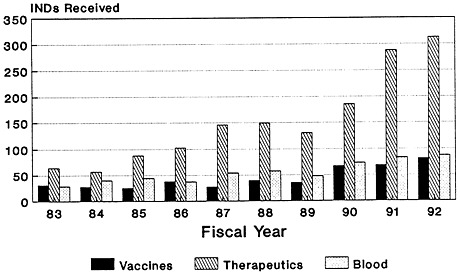
FIGURE 4-3
CBER INDs received, by category. SOURCE: Application Review and Policy, Therapeutics Research and Review, Center for Biologics Evaluation and Research, U.S. Food and Drug Administration.
biotechnology companies worldwide (most of them in the United States) conducting vaccine-related R&D (Oryx Press, 1992).
There is also considerable international activity in the area of vaccine innovation. A number of U.S. companies are entering into cross-licensing arrangements with European and Japanese partners, and European companies are acquiring firms that have access to the U.S. market. In 1989, Institut Mérieux acquired Connaught Laboratories, Inc., and Merck and Company entered into a product development and licensing agreement with Pasteur Mérieux Sérums et Vaccins in 1993. Chiron, a U.S. biotechnology firm, joined with Ciba-Geigy, an established Swiss-based pharmaceutical firm, to purchase the financially troubled Sclavo, an Italian manufacturer of vaccines in 1990 (Chiron Corporation, 1991). A number of biotechnology companies involved in vaccine-related R&D have entered into strategic alliances with Japanese companies (National Research Council, 1992).
A review of the vaccines licensed for use in the United States since 1986 shows that approximately half are new (Table 4-11). This is markedly different from the situation just 10 years ago. A majority of the vaccines licensed in the 1970s were improvements of old vaccines (Institute of Medicine, 1985). A recently licensed vaccine against typhoid resembles a product, in some respects, that might be used in the CVI, but it was developed in part by and for the DOD.
The current vaccine development process in the United States, from basic research through to the production, distribution, and marketing of vaccine products, although poorly integrated, does lead to the development and production of new vaccines for the domestic market, primarily because vaccine manufacturers perceive there to be adequate returns on their investment. An indication of the level of vaccine innovation is the sheer number of vaccines in various stages of development in the United States (Table 4-12). As can be seen, however, few of the vaccines currently being developed by established vaccine manufacturers are for exclusive use in the developing world, simply because such vaccines are perceived to be without sufficient returns on investment. As noted earlier, some vaccines developed by or for the DOD have been introduced into some developing countries on an ad hoc basis by commercial manufacturers. A few small biotechnology firms are working on vaccines of potential benefit to the developing world. As discussed in Chapter 3 and later in this report, few of these companies have the capacity to take a vaccine through to licensure and full-scale manufacture. Most of the development-stage companies working on vaccines of relevance to the developing world do so as part of cooperative research and development agreements (CRADAs) with the U.S. Department of Defense, and to a lesser extent, with the National Institute of Allergy and Infectious Diseases. At this time, the Walter Reed Army Institute of Research has almost 40 CRADAs with private-sector firms, the vast majority
of which are development-stage biotechnology companies based in the United States (LTC Willis. A. Reid, Chief, Office of Research and Technology Applications, Walter Reed Army Institute of Research, personal communication, 1993).
By all accounts, the worldwide vaccine industry appears to be entering a new era of activity and innovation. In the United States, commercial vaccine manufacturers and biotechnology firms are pursuing the development of innovative vaccine products targeted to the industrialized-world market. The development and manufacture of vaccines for exclusively developing-world markets are not attractive investments for either commercial vaccine manufacturers or biotechnology firms because they are unlikely to offer adequate returns on investments under current market arrangements.
TABLE 4-11 Vaccines Licensed for Use in the United States Since 1986
|
Vaccine and Company |
Review Time (months) |
FDA Approval |
Characteristics |
|
BCG live |
18.1 |
05/1990 |
New approval of an old vaccine for limited indication (treatment of carcinoma-in-situ of the urinary bladder |
|
Connaught Laboratories, Inc. |
|
|
|
|
BCG vaccine |
29.5 |
08/1990 |
New approval of old vaccine (also indicated for the treatment of carcinoma-in-situ of the urinary bladder |
|
Organon Teknika Corporation |
|
|
|
|
Diphtheria and tetanus toxoids and acellular pertussis vaccine, adsorbed (DTaP) |
51.5 |
12/1991 |
New acellular pertussis component; shared manufacture with Takeda Chemical Industries, Ltd. |
|
Lederle-Praxis Biologicals |
|
|
|
|
Diphtheria and tetanus toxoids and acellular pertussis vaccine, adsorbed (DTaP) |
27.1 |
08/1992 |
New acellular component; shared manufacture with Biken (Research Foundation of Osaka University) |
|
Connaught Laboratories, Inc. |
|
|
|
|
Diphtheria and tetanus toxoids and pertussis vaccine, adsorbed and Haemophilus influenzae type b conjugate |
14.9 |
03/1993 |
New vaccine |
|
Lederle-Praxis Biologicals |
|
|
|
|
Haemophilus influenzae type b meningococcal outer membrane conjugate vaccine |
24.9 |
12/1989 |
New vaccine (18–60 months) |
|
Merck & Co., Inc. |
|
|
|
|
Haemophilus influenzae type b meningococcal outer membrane conjugate vaccine |
4.1 |
12/1990 |
Infant indication |
|
Merck & Co., Inc. |
|
|
|
|
Haemophilus influenzae type b conjugate vaccine (tetanus toxoid conjugate) |
27.8 |
03/1993 |
New vaccine |
|
Pasteur Mérieux Sérums et Vaccins |
|
|
|
|
Haemophilus influenzae type b and diphtheria CRM 197 protein conjugate vaccine |
22.5 |
12/1988 |
New vaccine (18–60 months) |
|
Praxis Biologics, Inc. |
|
|
|
|
Haemophilus influenzae type b conjugate vaccine |
6.9 |
10/1990 |
Infant indication |
|
Praxis Biologics, Inc. |
|
|
|
|
Vaccine and Company |
Review Time (months) |
FDA Approval |
Characteristics |
|
Haemophilus influenzae type b conjugate (diphtheria toxoid conjugate) |
55.4 |
12/1987 |
New vaccine (18–60 months) |
|
Connaught Laboratories, Inc. |
|
|
|
|
Hepatitis B vaccine, recombinant |
18.0 |
07/1986 |
New vaccine |
|
Merck & Co., Inc. |
|
|
|
|
Hepatitis B vaccine, recombinant |
20.8 |
08/1989 |
Independent introduction |
|
SmithKline Beecham |
|
|
|
|
Influenza virus vaccine |
17.3 |
08/1988 |
New introduction of old vaccine |
|
Evans Medical, Ltd. |
|
|
|
|
Japanese encephalitis virus, inactivated |
30.8 |
12/1992 |
New vaccine |
|
Research Foundation of Osaka University Connaught Laboratories, Inc. |
|
|
|
|
Poliovirus vaccine, inactivated |
16.1 |
11/1987 |
Enhanced poliovirus vaccine |
|
Connaught Laboratories, Inc. |
|
|
|
|
Poliovirus vaccine, inactivated |
93.3 |
12/1990 |
Independent introduction |
|
Pasteur Mérieux Sérums et Vaccins |
|
|
|
|
Rabies vaccine, adsorbed |
93.3 |
03/1988 |
Independent introduction |
|
Michigan Department of Public Health |
|
|
|
|
Rabies vaccine |
42.4 |
12/1991 |
Independent introduction |
|
Connaught Laboratories, Inc. |
|
|
|
|
Typhoid vaccine, live oral (Ty21a) |
92.5 |
12/1989 |
New vaccine |
|
Swiss Serum & Vaccine Institute, Berne |
|
|
|
|
SOURCES: New Drug Approvals in 1991, Pharmaceutical Manufacturers Association, January 1992; New Drug Approvals in 1990, Pharmaceutical Manufacturers Association, January 1991; New Drug Approvals in 1989, Pharmaceutical Manufacturers Association, January 1990; Biotechnology Medicines, Pharmaceutical Manufacturers Association, 1990, Douglas Reynolds, Connaught Laboratories. Swiftwater, Pennsylvania, October 1992; Carolyn Hardegree, Center for Biologics Evaluation and Research, U.S. Food and Drug Administration. |
|||
TABLE 4.12 Selected Vaccines in Development
|
Product and Company U.S. |
Development Status |
|
Adenohepatitis B virus vaccine |
Phase I |
|
Wyeth-Ayerst |
|
|
Acellular pertussis vaccine |
Phase I/II |
|
Massachusetts Department of Public Health |
|
|
Acellular pertussis component |
Phase I |
|
Michigan Department of Public Health |
|
|
Diphtheria and tetanus toxoids and acellular pertussis vaccine, adsorbed |
Phase III (infant efficacy study) |
|
Lederle-Praxis Biologicals |
|
|
Diphtheria and tetanus toxoids and acellular pertussis vaccine, adsorbed |
Phase III |
|
North American Vaccine |
|
|
Diphtheria and tetanus toxoids and accellular pertussis vaccine, adsorbed and inactivated polio vaccine |
Phase III |
|
North American Vaccine |
|
|
TetrammuneTM Diphtheria and tetanus toxoids and pertussis vaccine, adsorbed, and Haemophilus influenzae type b vaccine |
PLA submitted (recommended for approval by FDA advisory committee, ages 2 months up to 7th birthday) |
|
Lederle-Praxis Biologicals |
|
|
Diptheria and tetanus toxoids and acellular pertussis vaccine, adsorbed, and Haemophilus influenzae type b conjugate vaccine |
Phase II (for booster dose at 15–18 months of age or when both vaccines recommended to be given simultaneously) |
|
Lederle-Praxis Biologicals |
|
|
PropediaTM Diphtheria and tetanus toxoids and pertussis vaccine, adsorbed and Haemophilus influenzae type b vaccine (diphtheria toxoid conjugate) |
PLA submitted (for 15–60 months of age as final booster dose in Hib series or as n single dose primary immunization at 15–60 months of age) |
|
Connaught Laboratories, Inc. |
|
|
Act HIBTM + DTP; Diptheria and tetanus toxoids Act HIB and pertussis vaccine, adsorbed, reconstituting Haemophilus influenzae type b conjugate vaccine (tetanus protein conjugate) |
Submitted as part of PLA for alone (for 2–60 months of age) |
|
Connaught Laboratories, Inc./Pasteur Mérieux Sérums et Vaccins |
|
|
Act HIBTM + DTaP; Diptheria and tetanus toxoids and acellular pertussis vaccine, adsorbed, reconstituting Haemophilus influenzae type b conjugate vaccine (tetanus protein conjugate) |
Phase II for 15–60 months of age |
|
Connaught Laboratories, Inc./Pasteur Mérieux Sérums et Vaccins |
|
|
Product and Company |
Development Status |
|
Diphtheria and tetanus toxoids and pertussis vaccine, Haemophilus influenzae type b, hepatitis B vaccine |
Phase I (by summer of 1993) |
|
Merck & Co., Inc./Connaught Laboratories, Inc. |
|
|
Diphtheria and tetanus toxoids and pertussis vaccine, Haemophilus influenzae type b, hepatitis B vaccine and inactivated polio vaccine |
Phase I (by summer of 1993) |
|
Merck & Co., Inc./Connaught Laboratories, Inc. |
|
|
Diphtheria and tetanus toxoids and pertussis vaccine, hepatitis B and Haemophilus influenzae type b conjugate vaccine |
Pre-clinical studies completed; preparing IND submissions |
|
Michigan Department of Public Health/ SmithKline Beecham |
|
|
Diphtheria and tetanus toxoids and acellular pertussis vaccine and hepatitis B vaccine and Haemophilus influenzae type b conjugate vaccine |
Pre-clinical studies completed; preparing IND submissions |
|
Michigan Department of Public Health/ SmithKline Beecham |
|
|
Diptheria and tetanus toxoids and pertussis vaccine, adsorbed, and poliovirus vaccine, inactivated |
PLA submitted |
|
Connaught Laboratory, Inc./Pasteur Mérieux Sérums et Vaccins |
|
|
Act HIBTMHaemophilus influenzae type b conjugate vaccine (tetanus protein conjugate) |
PLA submitted |
|
Connaught Laboratories, Inc./Pasteur Mérieus Sérums et Vaccins |
|
|
Haemophilus influenzae type b conjugate vaccine |
Phase III |
|
Massachusetts Department of Public Health |
|
|
Haemophilus influenzae type b conjugate vaccine, hepatitis B vaccine |
Phase Ill |
|
Merck & Co., Inc. |
|
|
VAQTATM hepatitis A vaccine |
Phase III |
|
Merck & Co., Inc. |
|
|
Hepatitis B vaccine |
Phase II |
|
Connaught Laboratories. Inc. |
|
|
Hepatitis B vaccine |
Phase III |
|
Amgen/Johnson & Johnson |
|
|
Herpes vaccine |
Phase I (adults) |
|
Lederle-Praxis Biologicals |
|
|
PrymeTM lyme disease vaccine (recombinant OspA lipoprotein for Lyme borreliosis) |
Phase I |
|
Connaught Laboratories, Inc. |
|
|
Product and Company |
Development Status |
|
Lyme disease vaccine (recombinant OspA lipoprotein for Lyme borreliosis) |
Phase I (by summer of 1993) |
|
SmithKline Beecham |
|
|
M-M-R®II measles, mumps, rubella, and varicella |
Phase III |
|
Merck & Co., Inc. |
|
|
Measles, mumps, rubella virus vaccine, live |
Project currently inactive |
|
Connaught Laboratories, Inc./Pasteur Mérieux Sérums et Vaccins |
|
|
Meningococcal group B vaccine (outer membrane protein) |
Phase III for 2 years of age and older |
|
Connaught Laboratories, Inc. |
|
|
Pneumococcal conjugate vaccine (streptococcal conjugate vaccine, diphtheria toxoid and tetanus protein conjugates for otitis media and pneumonia) |
Phase I |
|
Connaught Laboratories, Inc./Pasteur Mérieux Sérums el Vaccins |
|
|
Pneumococcal conjugate vaccine, streptococcal pneumonia vaccine, enhanced |
Phase I/II |
|
Lederle-Praxis Biologicals |
|
|
Pneumococcal conjugate vaccine |
Phase II |
|
Merck & Co., Inc. |
|
|
Respiratory syncytial virus vaccine |
Phase I/II |
|
Lederle-Praxis Biologicals |
|
|
Rhesus rotavirus vaccine |
Phase III |
|
Wyeth-Ayerst |
|
|
Rhesus rotavirus vaccine |
Phase II |
|
Merck & Co., Inc. |
|
|
Sabin IPV inactivated Sabin polio vaccine |
Phase III (age 2 months and up) |
|
Lederle-Praxis Biologicals |
|
|
Salmonella, live attenuated |
Phase I |
|
Lederle-Praxis Biologicals |
|
|
Streptococcal group B vaccine |
Phase I |
|
North American Vaccine |
|
|
Varivax® varicella vaccine |
PLA submitted |
|
Merck & Co., Inc. |
|
|
SOURCES: Pharmaceutical Manufacturers Association. 1990. New Medicines in Development for Children. Washington, D.C.; Pharmaceutical Manufacturers Association. 1990. Biotechnology Medicines. Washington, D.C.; Douglas Reynolds, Connaught Laboratories, Inc., October 1992; Glenna Crooks and Ronald B. Ellis, Merck & Co., Inc., May 1993; Jane Scott, Lederle-Praxis Biologicals, February 1993; George Siber, Massachusetts Biologic Laboratories, June 1993; Robert Meyers, Michigan Department of Public Health, May 1993; Dan Soland, SmithKline Beecham, June 1993. |
|
NOTE
REFERENCES
Agency for Cooperation in International Health. 1991. Report of the International Meeting on Global Vaccine Supply, May 23–26, Kumamoto, Japan.
Agency for Cooperation in International Health. 1992. Report of the Second International Meeting on Global Vaccine Supply, August 3–5, Tokyo, Japan.
American Cyanamid. 1991. Form 10-K. Washington, D.C: Securities and Exchange Commission.
Baudrihaye N. 1992. European vaccine manufacturers: Present status and future trench. Vaccine 10:893–5.
Business Week. 1992. R&D Scoreboard: On a clear day you can see progress. June 29. New York: McGraw Hill, Inc.
Chiron Corporation. 1991. Annual Report. Emeryville, California.
Cutts FT, Bernier RH, Orenstein WA. 1992. Causes of low preschool immunization coverage in the United States. Annual Review of Public Health 13:385–398.
DeBrock, LM. 1983. The domestic vaccine industry: The economic framework. Paper presented at the Institute of Medicine Conference on Barriers to Vaccine Innovation, November 28–29, Washington, D.C.
Di Masi J, Hansen RW, Grabowski HG, Lasagna L. 1991. Cost of innovation in the pharmaceutical industry. Journal of Health Economics 10:107–142.
Douglas GR. 1993. Testimony before the Subcommittee on Access to Immunization Services, National Vaccine Advisory Committee. A Public Hearing on the Economic and Commercial Underpinning of Vaccine Supply, February 24, Bethesda, Maryland.
Douglas GR. 1992. Testimony before the Senate Subcommittee on Appropriations. Childhood vaccine research and development Issues. April 8. Washington, D.C.
Dupuy JM Freidel L. 1990. Lag between discovery and producing of new vaccines for the developing world. Lancet 336:733–734.
Edelman, MW. 1993. Testimony of the Children's Defense Fund. Senate Labor and Human Resources Committee and the House Subcommittee on Health and Environment. April 21. Washington, D.C.
Financial Times. 1993. Pharmaceuticals: Research and Development. April 22. Pp. 1–6.
Freeman P, Johnson K, Babcock J. 1993. A health challenge for the states: Achieving full benefit of childhood immunization, Occasional paper. February. The John W. McCormack Institute of Public Affairs, University of Massachusetts at Boston.
Garnier, JP. 1993. Testimony before the Senate Committee on Labor and Human Resources and the House Subcommittee on Health and the Environment. April 21, 1993. Washington, D.C.
Grabowski H Vernon J. 1990. A new look at the returns and risks to pharmaceutical R&D. Management Science 36:804–821.
Hlady GW, Bennett JV, Samadi AR, Begum J, Hafez A, Tarafdar AI, Boring JR. 1992. Neonatal tetanus in rural Bangladesh: Risk factors and toxoid efficacy. American Journal of Public Health 82:1365–1369.
Hoover's Handbook of American Business. 1992. Emeryville, California: The Reference Press, Inc.
Institut Mérieux International. 1990. Annual Report. Lyon, France.
Institute of Medicine. 1985. Vaccine Supply and Innovation. Washington, D.C.: National Academy Press.
Institute of Medicine. 1986. Proceedings of a Workshop on Vaccine Supply and Innovation. Report for the Subcommittee on Oversight and Investigations, Committee on Energy and Commerce. U.S. Congress, House. August. Washington, D.C.
Institute of Medicine. 1992. Proceedings from Working Groups on The Children's Vaccine Initiative: Planning Alternative Strategies Towards Full U.S. Participation. June. Washington, D.C.
Katz S. 1993. Could the Childhood Vaccine Act Be Bad? Letter to the Editor. Pediatrics 91:160.
Lancet. 1992. Noticeboard. Vaccines quality control deficient. Lancet 340:1282.
Lasagna L. 1992. Introductory remarks. Cost containment and pharmaceuticals: Issues for future research. Pharmo Economics 1(Suppl.):1–76.
MedImmune, Inc. 1991. Annual Report. Gaithersburg, Maryland.
Merck & Co., Inc. 1991a. Annual Report. Rahway, New Jersey.
Merck & Co., Inc. 1991b. Form 10-K. Washington, D.C: Securities and Exchange Commission.
Mérieux A. 1992. Industrial Continuity and Response to Global Needs: A Challenging Paradox. Keynote address. Second Meeting of the Consultative Group of the Children's Vaccine Initiative, November 16–17, World Health Organization, Geneva.
National Research Council. 1992. U.S.-Japan Technology Linkages in Biotechnology: Challenges for the 1990s. Washington, D.C.: National Academy Press.
National Vaccine Injury Compensation Trust Fund. 1992. Materials provided at the Meeting of the Advisory Commission on Childhood Vaccines, Health Resources and Services Administration, Rockville. Maryland, December 2–3.
Office of Technology Assessment. 1991. Biotechnology in a Global Economy. Washington, D.C.
Oryx Press. 1992. Bioscan. Vol 6 (Suppl. 4):1583. December. Phoenix, Arizona.
Pan American Health Organization. 1992. Progress in the worldwide polio eradication effort. EPI Newsletter 14(6):1.
Peter G. 1992. Childhood Immunizations. New England Journal of Medicine 327:1794–1800.
Pharmaceutical Manufacturers Association. 1990. Biotechnology Medicines. Washington, D.C.
Pharmaceutical Manufacturers Association. 1990. New Medicines in Development for Children. Washington, D.C.
Robbins A, Freeman P. 1988. Obstacles to developing vaccines for the Third World. Scientific American. November. pp. 126–133.
Saldarini RJ. 1992. Testimony before Senate Subcommittee on Appropriations. Childhood vaccine research and development issues. April 8. Washington, D.C.
Saldarini RJ. 1993. Testimony before the Subcommittee on Access to Immunization Services, National Vaccine Advisory Committee. A Public Hearing on the Economic and Commercial Underpinning of Vaccine Supply, February 24, Bethesda, Maryland.
Six H. 1992. Testimony before Senate Subcommittee on Appropriations. Childhood vaccine research and development issues. April 8. Washington, D.C.
SmithKline Beecham. 1991. Annual Report. Philadelphia.
Steele I. 1992. Vaccine price rise jeopardizes UCI. First Call for Children. A UNICEF Quarterly no.4:1–2.
Sugawara S. 1992. Biotech firms forming more strategic links: Young industry seeks support from mature corporations. October 19. Washington Post, Washington Business: p. 1.
Technology Management Group. 1993. Human Vaccine III. New Haven, Connecticut.
UNICEF. 1991a. Executive Board Action Item: Establishment of a Vaccine Independence Initiative. March 12. New York, New York.
UNICEF. 1991b. Executive Board: Universal Childhood immunization, 1990, Progress Report. March 20. New York, New York.
UNICEF. 1992a. Vaccine Independence Initiative. Project Proposal for Funding by Interested Donors. July 13. New York, New York.
UNICEF. 1992b. Data regarding volume and price of purchased vaccines. Vaccine Supply Division. New York, New York.
U.S. Congress, House. 1986. Childhood Immunizations. A Report prepared by the Subcommittee on Health and the Environment, Committee on Energy and Commerce. September. Washington, D.C.
U.S. Congress, Senate. 1982. Heating to Review Federal and State Expenditures for the Purchase of Children's Vaccines. Subcommittee on Investigations and General Oversight, Committee on Labor and Human Resources, July 22, Washington, D.C.
U.S. Department of Health and Human Services. 1991. Establishments and Products Licensed under Section 351 of the Public Health Service Act. March 1. Rockville, Maryland: U.S. Food and Drug Administration.
Vandersmissen W. 1992. Availability of quality vaccines: The industrial point of view. Vaccine 10:955–957.
Williams D. 1993. Testimony before the Subcommittee on Access to Immunization Services, National Vaccine Advisory Committee. A Public Hearing on the Economic and Commercial Underpinning of Vaccine Supply, February 24, Bethesda, Maryland.
World Almanac and Book of Facts. 1992. New York, New York: Pharos Books.
World Bank. 1993. World Development Report: Investing in Health. Washington, D.C.
World Health Organization. 1992. EPI for the 1990s. Geneva.
World Health Organization/Children's Vaccine Initiative. 1992a. CVI Forum. October. Geneva.
World Health Organization/Children's Vaccine Initiative. 1992b. Report of Second Meeting of the Consultative Group. November 16–17. Geneva.
World Health Organization/Children's Vaccine Initiative. 1992c. Task Force on Situation Analysis. November 16–17. Geneva.
Zoon KC Beatrice MG. 1993. New directions for FDA's Center for Biologics Evaluation and Research (CBER). New Drug Approvals in 1992. February. Washington, D.C.: Pharmaceutical Manufacturers Association.