4
Biologic Markers of Lead Toxicity
In the last few years, considerable interest has developed in discovering and validating new biologic markers for many toxic substances. Identifying new biologic markers has helped scientists to understand much better the mechanisms of toxicity. This has also been the focus for biologic studies of the mechanisms of lead toxicity. Biologic markers are indicators of events in biologic systems or samples. The National Research Council (NRC, 1989a,b) has classified biologic markers into three types—markers of exposure, of effect, and of susceptibility. A biologic marker of exposure is an exogenous substance or its metabolite or the product of an interaction between a xenobiotic agent and some target molecule or cell. A biologic marker of effect is a measurable biochemical, physiologic, or other alteration within an organism that, depending on magnitude, can be recognized as an established or potential health impairment or disease. A biologic marker of susceptibility is an indicator of an inherent or acquired limitation of an organism's ability to respond to the challenge of exposure to a specific xenobiotic substance. This chapter describes biologic markers of exposure, effect, and susceptibility for lead. It also establishes the biologic basis for the assessment of analytic techniques to monitor lead in sensitive populations, which will be described in Chapter 5.
BIOLOGIC MARKERS OF EXPOSURE
Any assessment of the toxicity associated with exposure to lead
begins with measurement of the exposure. In practice, one assesses lead exposure through environmental or biologic monitoring techniques to examine markers of exposure. Lead exposure is the amount of lead (from whatever source) that is presented to an organism; dose is the amount that is absorbed by the organism (NRC, 1990). Various factors—such as blood flow, capillary permeability, transport into an organ or tissue, and number of active binding and receptor sites—determine the path of lead through the body and can influence the biologically effective dose. For example, lead inhaled in dust could be retained in the lungs, removed from the lungs by protective mechanisms and ingested, stored in bone, or eliminated from the body via the kidneys. Toxicity can be observed in the kidneys, blood, nervous system, or other organs and tissues. At any step after exposure, biologic markers of exposure to lead can be detected.
A key component of biologic monitoring of lead exposure is the toxicokinetic and physiologic framework that underlies such monitoring. Screening in the absence of knowledge about lead's in vivo behavior limits the interpretation of monitoring data for public-health risk. For example, if clinical management or regulatory actions are to be effective, the timing of lead exposure that is reflected in a typical blood lead value should be known, as should dose-response relations that link the body lead concentration with adverse health effects.
Lead Absorption
Humans absorb lead predominantly through the gastrointestinal and respiratory tracts. Little uptake occurs through skin, especially in nonoccupational exposures. Lead deposition and absorption rates in the human respiratory tract are complex functions of chemical and physical forms of the element and of anatomic, respiratory, and metabolic characteristics.
Inhaled lead is deposited in the upper and lower reaches of the respiratory tract. Deposition in the upper portion leads to ciliary clearance of lead, swallowing, and absorption from the intestine. Smaller lead particles, especially those less than 1 µm in statistically averaged diameter, penetrate the lower, pulmonary portion of the respiratory tract and undergo absorption from it.
Human studies (Chamberlain, 1983; EPA, 1986a) have shown that about 30–50% of inhaled lead is retained by the lungs (the range reflects mainly particle size and individual breathing rate). These studies have used unlabeled lead aerosol (Kehoe, 1961a,b,c), radiolabeled oxide aerosol (Chamberlain et al., 1978), lead fumes inhaled by volunteers (Nozaki, 1966), ambient air lead around motorways and encountered by the general population (Chamberlain et al., 1978; Chamberlain, 1983), lead salt aerosols inhaled by volunteers (Morrow et al., 1980), and lead in forms encountered in lead operations, fumes, dusts, etc. (Mehani, 1966). Most (over 95%) of whatever lead is deposited in the human pulmonary compartment is absorbed (Rabinowitz et al., 1977; Chamberlain et al., 1978; Morrow et al., 1980). Thus, the overall rate of uptake is governed by lung retention (i.e., 30–50%). Uptake occurs rapidly, generally in a matter of hours.
Evidence of complete and rapid uptake can be gleaned from analysis of autopsy lung tissue (Barry, 1975; Gross et al., 1975). The chemical form of inhaled lead appears to have little effect on uptake rate (Chamberlain et al., 1978; Morrow et al., 1980). Similarly, uptake is little affected by air lead concentration, even when it is greatly in excess of that commonly encountered in nonoccupational settings—up to 450 µg/day (Chamberlain et al., 1978).
The above data apply to adults and are relevant for the sensitive adult population, i.e., pregnant women. In the case of children, no studies have experimentally documented rates of direct uptake of lead from lungs. On anatomic grounds (Hofmann et al., 1979; Hofmann, 1982; Phalen et al., 1985) and metabolic grounds (Barltrop, 1972; James, 1978), however, uptake in adults should be greater than uptake in children.
In nonoccupationally exposed populations, lead uptake from the gastrointestinal tract is its main route of absorption. For adults, the lead content of foods, tap water, and other beverages is of main concern for lead exposure. For infants, toddlers, and older children, ingestion of lead-contaminated nonfood materials—e.g., dust, soil, and leaded-paint chips—is of additional concern. In some cases, such exposure can exceed that occurring through the diet (NRC, 1976, 1980; EPA, 1986a; WHO, 1987).
Various studies of gastrointestinal absorption of lead in adults as derived from measures of metabolic balance (Kehoe, 1961a,b,c) and
isotope distribution (Hursh and Suomela, 1968; Harrison et al., 1969; Rabinowitz et al., 1974, 1980; Chamberlain et al., 1978) have documented that 10–15% of dietary lead is absorbed. The rate rises considerably, to as high as 63%, under fasting conditions (Chamberlain et al., 1978; Rabinowitz et al., 1980; Heard and Chamberlain, 1982). That suggests that lead in tap water and other beverages, which are often imbibed on an empty stomach, undergo higher uptake and pose proportionately greater exposure. Rate of lead uptake over the range of exposures likely to be encountered by the general population, up to at least 400 µg/day, seems similar (Flanagan et al., 1982; Heard et al., 1983).
Studies of lead bioavailability in the human intestine (Chamberlain et al., 1978; Rabinowitz et al., 1980; Heard and Chamberlain, 1982) have indicated that common dietary forms of lead are absorbed to about the same extent. Lead sulfide in one study was absorbed to the same extent as other forms, and in another study was absorbed to the same extent with meals, but during fasting was absorbed less than the chloride. Particle size difference might account for the absorption difference between fasting and meals.
Dietary lead absorption is considerably higher in children than in adults. Results of studies of both Ziegler et al. (1978) with infants and Alexander and colleagues (1973) with children indicate an absorption rate up to 50% from the intestinal tract.
Young children ingest nonfood lead through normal mouthing behavior and particularly through the abnormal, excessive behavior called pica. Substantial uptake of lead and systemic exposure occur because of the high concentrations of lead in such media as dust, soil and leaded paint (Duggan and Inskip, 1985; EPA, 1986a; WHO, 1987); the ingestion of perhaps 100 mg, or even more, of such media (Binder et al., 1986; Clausing et al., 1987; Calabrese et al., 1989; Davis et al., 1990); and a bioavailability of up to 30% (Day et al., 1979; Duggan and Inskip, 1985; EPA, 1986a). The higher intestinal absorption of lead seen in developing versus adult humans is commonly observed in other mammalian systems, including nonhuman primates (Pounds et al., 1978) and rodents (e.g., Kostial et al., 1978).
Percutaneous absorption of inorganic lead in nonoccupational populations is low. Moore et al. (1980) applied 203Pb-labeled lead acetate to intact skin of adult volunteers and obtained an average absorption rate
of only 0.06%. Lilley et al. (1988) applied lead as the metallic powder or nitrate salt solution to one subject's skin; it failed to increase the lead content of either whole blood or urine, but the lead content of sweat far from the area of application increased.
Lead has long been known to cross the placental barrier in humans and other species and become lodged in fetal tissues (Barltrop, 1969; Chaube et al., 1972; Buchet et al., 1978; Alexander and Delves, 1981; Rabinowitz and Needleman, 1982; Borella et al., 1986; Mayer-Popken et al., 1986). The question of when lead begins to enter the fetus during maternal exposure is important, but has not been fully answered. Data of Barltrop (1969) and Mayer-Popken et al. (1986) suggest that lead entry occurs by the third or fourth month; data of Borella et al. (1986) and Chaube et al. (1972) suggest that uptake occurs later.
Lead Distribution
Absorbed lead enters plasma and undergoes rapid removal to various body compartments: erythrocytes, soft tissue, and mineralizing tissue. Removal occurs over a matter of minutes (Chamberlain et al., 1978; DeSilva, 1981). If exposure is constant, a steady state eventually occurs.
Under steady-state conditions (i.e., stable exposure), plasma lead and erythrocyte lead are in equilibrium. The equilibrium fraction of lead in plasma is less than 1% and varies very little (Cavalleri et al., 1978; Everson and Patterson, 1980; DeSilva, 1981; Manton and Cook, 1984), but rises at blood lead concentrations of about 50 µg/dL or higher (DeSilva, 1981; Manton and Cook, 1984).
Lead is removed from whole blood, under steady-state conditions, with a half-life that depends on such factors as total body lead burden, age, magnitude of external exposure, and the method of measuring half-life (according to total circulating lead or absorbed exogenous fraction as measured by isotopic tracer).
Whole-blood lead measured with various protocols of experimental exposure has been found to have a half-life of about 25 days (Griffin et al., 1975; Rabinowitz et al., 1976; Chamberlain et al., 1978). That refers to the first, or short-term, component of blood lead decay. Actual measurements of half-life, commonly obtained through blood
lead changes that occur with reduction in chronic exposure, yield various and generally much higher values than those obtained experimentally; actual measurements reflect a larger contribution of a long-term component, described as many months in half-life.
Early studies by Barry (1975, 1981) and Gross et al. (1975) showed that lead in most soft tissues is usually below 0.5 parts per million (ppm); age-dependent accumulation in kidneys (Indraprasit et al., 1974) and aorta (Barry, 1975; Gross et al., 1975) in nonoccupational populations has been reported.
Available data are not sufficient to show whether soft tissue concentrations have been declining in response to lower air and dietary lead uptake in recent years. Such changes would be registered more readily in the youngest segments of the population, where cumulative body burdens are smaller.
Studies that showed no lead accumulation with age in many soft tissues have been cross-sectional and theoretically would disguise the moderate accumulation that can occur with age but be offset by declining lead exposure in recent years.
The human brain, the principal target organ of lead exposure, has low concentrations of lead—less than 0.2 ppm (wet weight)—on a whole-organ basis when there has been no occupational exposure. Lead content can rise by a factor of several in people with high lead exposure (Barry, 1975). In subjects with lethal poisoning, whole-brain concentrations are above 1 ppm (Okazaki et al., 1963; Klein et al., 1970). Region-specific distribution of lead in the brain has been documented. The highest concentrations are in the hippocampus and frontal cortex (Okazaki et al., 1963; Niklowitz and Mandybur, 1975; Grandjean, 1978).
Barry (1975, 1981) showed that tissue lead concentrations were lower in infants than in older children. Those in older children were not materially different from those in adult women.
A large body of laboratory and clinical evidence shows that lead accumulates with age in human mineralizing tissue, i.e., bones and teeth. Accumulation appears to begin at birth (or even in utero) and continues until the age of 50–60 years, when it starts to decrease through some combination of dietary, metabolic, and hormonal changes (CDC, 1985; EPA, 1986a; Drasch et al., 1987; Drasch and Ott, 1988;
Wittmers et al., 1988). Total lead content in bone can reach 200 mg in nonoccupationally exposed adults and much higher in those occupationally exposed to large concentrations.
Drasch and Ott (1988) have confirmed that bone lead is cumulative at least from birth. Autopsy samples from infants less than 1 year old had a bone lead concentration half that of preschool children (0.33 vs. 0.62 ppm wet weight) and one-fifth that of people 10–20 years old (1.76 ppm). All bone types—cortical bone, such as midfemur, and trabecular bone, such as temporal bone and pelvis—were shown to accumulate lead, but the denser cortical bone had markedly higher concentrations in the two older groups. A sex-based difference in bone lead accumulation was observed in the oldest group for trabecular bone, males having statistically higher concentrations (p < 0.05). Recent measurements of bone lead in adult autopsy samples also documented continued accumulation in adulthood up to at least the age of 50 (Drasch et al., 1987; Wittmers et al., 1988). In the work of Drasch et al. (1987), temporal bone showed age-dependent accumulation throughout adulthood, including the 70s, whereas midfemoral and pelvic samples showed a plateau in middle age and then a decline. The latter decline was pronounced in females and was attributed to osteoporotic changes. Those data support the finding in analysis of NHANES II results that menopausal women have higher blood lead than younger women (Silbergeld et al., 1988). Men were estimated to have a significantly higher total skeletal lead burden than women—mean, 41.4 mg versus 24.1 mg. Comparison of recent analyses with data gathered 10 years earlier in the same laboratory and with identical methods indicated a marked decline of lead in femoral and pelvic samples across adult age groups, amounting to 30–50%.
In similar investigations, Wittmers et al. (1988) examined tibia, skull, rib, ilium, and vertebra from 134 hospital autopsies for lead content as a function of age, lateral and cross-sectional analytic symmetry, and bone composition. Lead content was symmetric in positional location, but not bone type. Lead concentrations rose with age in all sample types, and there was some longitudinal variation within a bone specimen, but not enough to preclude use of single measurements in bone analysis.
Lead Retention and Excretion
EPA (1986a) and ATSDR (1988) analyzed the retention and excretion of lead in humans and animals. Ingested lead that is not absorbed is lost through urinary and fecal excretion. Absorbed lead that is not sequestered in bone or some soft tissues is eventually eliminated through the kidneys or through biliary clearance into the intestine. Deposition in keratinizing tissues (nails and hair) is a minor elimination pathway.
On the basis of various experimental measurements (Kehoe, 1961a,b,c; Rabinowitz et al., 1976; Chamberlain et al., 1978), the following can be said:
-
Urinary loss of lead in adults makes up about two-thirds of total elimination. Fecal lead loss (of lead arising from biliary elimination—i.e., endogenous fecal lead) makes up about one-third. About 8% of the total is eliminated through Hair and nails.
-
Whole-body lead elimination over the short term removes about 50–60% of the newly absorbed lead, with a half-life in adult volunteers of about 20 days (Rabinowitz et al., 1976; Chamberlain et al., 1978). Of the deposited fraction, 50% (i.e., 25% of lead initially absorbed) is eventually eliminated.
-
Infants and children retain 50% of ingested lead (Alexander et al., 1973; Ziegler et al., 1978).
-
Infants (and perhaps preschool children) have slower elimination than adults (Thompson, 1971; Alexander et al., 1973; Chamberlain et al., 1978; Ziegler et al., 1978; EPA, 1986a).
-
Lead elimination through urine might depend on concentration, as estimated by Chamberlain (1983) on the basis of results of studies that reported blood and urine values in adults.
-
Whole-body lead retention in humans subjected to constant exposure is accounted for largely by skeletal accumulation.
Interactions of Lead with Nutrients
The toxicokinetics of lead in humans are affected by the metabolic and nutritional status of the exposed subjects. Nutrition and nutritional deficiencies are of prime concern in very young children, in whom
increased lead exposure is concurrent with deficiencies in many interactive elements, especially calcium and iron (see Markers of Susceptibility). Interactive relations for lead have been reviewed elsewhere (Mahaffey and Michaelson, 1980; EPA, 1986a). Various child and infant nutritional-status surveys have documented iron deficiency in children under 2 years old, especially those in low socioeconomic classifications. They are also the children with the highest prevalences of high body lead (Yip et al., 1981; Mahaffey et al., 1982a; Mahaffey and Annest, 1986).
Similarly, various reports have shown a strong negative correlation between calcium intake and blood lead in children (e.g., Sorrell et al., 1977; Ziegler et al., 1978; Johnson and Tenuta, 1979) and adults (Heard and Chamberlain, 1982). In the analyses of Ziegler et al. (1978), the inverse association of blood lead concentration and calcium intake in infants was seen to extend into the low part of the range of adequate intake.
Other nutrients that interact inversely with lead exposure are zinc (Chisolm, 1981; Markowitz and Rosen, 1981) and phosphorus (Heard and Chamberlain, 1982).
Mathematical Models
Over the years, a number of attempts have been put forth to provide a quantitative, mathematical model of the relation of lead in exposure media to total and toxicologically active lead in the body, the in vivo compartmentalization of lead in the human body, the relation of lead in target tissues and organs to likely biologic markers of exposure and toxicity, and even the relation of direct dose biologic markers to markers of early effect. Modeling approaches to metals in general are described by Clarkson et al. (1988), and specific reviews of lead biokinetic modeling are provided by Mushak (1989) and EPA (1986a).
Models of in vivo toxicokinetics of lead differ greatly, both in their use of empirical data and in the types of lead exposure to which they are applicable. One can broadly group toxicokinetic models of lead into linear and nonlinear forms. We are interested here primarily in models that are applicable to low-concentration lead exposure.
Linear Models
Rabinowitz and co-workers (1976, 1977) used stable isotopic-lead distribution analyses in adult volunteers to develop a three-compartment model of lead disposition. The kinetically discernible compartments were blood (the most mobile, containing 2 mg of lead), soft tissue (of intermediate mobility, containing 0.6 mg of lead), and bone (the largest, most stable, with a half-life of a decade or more and sequestering most total body lead).
The modeling efforts of Kneip and co-workers (Kneip et al., 1983; Harley and Kneip, 1984) expanded earlier approaches to a multiorgan model that can be used to estimate deposition in children of different ages. Figure 4-1 shows the major components of the Harley and Kneip (1984) approach that depicts six tissue compartments.
Table 4-1 presents age-dependent estimates of lead half-lives for bone, kidney, and liver reported by Harley and Kneip (1984) for the age range of 1–20 years. Bone lead in children 1–6 years of age has a half-life that is only one-third that in older children and only about half that in 8-year-olds. In contrast (and as expected), soft-tissue lead half-lives are independent of age. Age dependence (over ages 1–15 years) of tissue burdens of lead was also estimated (see Table 4-2). Blood lead peaks at 2 years and then declines gradually. Bone lead estimates show that lead concentration in 1-year-old infants is about 60% of that in 7-year-olds—not greatly at odds with the laboratory ratio of 1:2 based on autopsy samples for about the same age interval.
The models referred to are for essentially steady-state exposure with associated complete mixing of the linked lead pools, and they use first-order kinetics. They are chronic-exposure modeling approaches and are not to be considered valid for acute lead poisoning.
Nonlinear Models
At low to moderate lead exposure, linear models of lead in humans appear to be as good as any other form of mathematical depiction. However, any model intended to be broadly applicable to higher exposures must account for the known empirical curvilinearity of blood lead as a function of some external lead concentration (e.g., in water or air) and multiple subpools of lead (e.g., in blood and in bone).
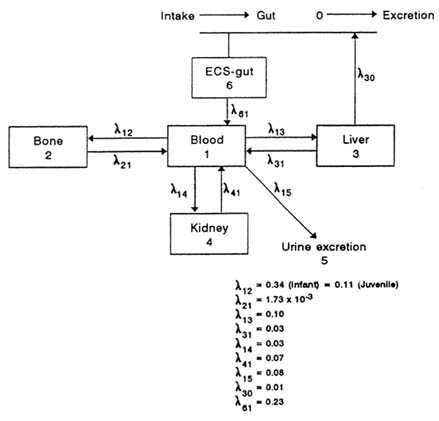
FIGURE 4-1 Linear toxicokinetic model of Harley and Kneip (1984). Model has six components, including initial extracellular space-gut compartments. Coefficients (λ) of compartment entry and exit are as indicated. Source: Kneip et al., 1983. Reprinted with permission from NeuroToxicology; copyright 1983, Intox Press.
The nonlinear model proposed by Marcus (1985) is an attempt to accommodate data that show that plasma lead manifests a concentration-dependent equilibrium with erythrocytes in humans and that blood lead concentration is nonlinear over a broad range of exposure. Workplace exposures represent the high end of the predictive range. The model provides a rather good fit for data from studies by DeSilva (1981) and Manton and Cook (1984) of subjects exposed over a broad range.
TABLE 4-1 Lead Half-Life Estimates by Age in Humans, Based on Linear Modeling
|
Tissue Half-Life, days |
|||
|
Age, yr |
Bone |
Kidney |
Liver |
|
1 |
1,135 |
10 |
23 |
|
3 |
1,135 |
10 |
23 |
|
6 |
1,135 |
10 |
23 |
|
8 |
2,560 |
10 |
23 |
|
15 |
3,421 |
10 |
23 |
|
20 |
3,421 |
10 |
23 |
|
Source: Adapted from Harley and Kneip, 1984, Table 4. |
|||
Chamberlain (1985) used a variant of nonlinear exposure models to rationalize the nonlinear relations of blood lead to media lead for a variable excretory function, dose-dependent urinary excretion, and thus incorporated dose-dependent transfer coefficients.
Biologic Monitoring
In recent years, the amount of lead entering the environment has declined because of regulatory or other risk-reduction actions and the body lead burden considered to pose an unacceptable risk of toxicity has declined. Those simultaneous decreases have shifted attention to small lead exposures and their associated subtle adverse effects. Small exposures add considerably to the complexity of interpreting lead pharmacokinetics. For example, the strong propensity for lead to accumulate in skeletal tissue and then be resorbed into blood with age increases the relative impact of endogenous input to blood at low concentrations. As noted in a recent report to Congress on childhood lead poisoning (ATSDR, 1988), high-dose exposure is not necessary for toxicity.
Despite increasing interest in low-concentration lead exposure, young children still sustain acute and subacute overt poisoning in areas of
TABLE 4-2 Estimates of Age-Dependent Lead Concentrations in Tissue, Based on Linear Modelinga
heavy lead contamination. It is still necessary, therefore, to consider the pharmacokinetics of lead at higher exposures.
Measurement of blood lead right after acute or subacute lead exposure is probably the only means of unambiguously establishing such exposure (Chisolm and Harrison, 1956; Chisolm, 1965; NRC, 1972, 1980; EPA, 1986a). That is because lead is rapidly absorbed into plasma and distributed to erythrocytes and vulnerable tissue sites while there is still a lag in response by most early-effect indicators, such as erythrocyte protoporphyrin.
Carton et al. (1987) showed the relative reliability and suitability of blood lead measures in an outbreak of acute lead poisoning caused by consumption of lead-contaminated flour in a Spanish village. A group of 136 poisoning patients whose blood lead concentrations were known at the outset were examined longitudinally. Of various exposure measures—erythyrocyte protoporphyrin, coproporphyrin, urinary delta-aminolevulinic (ALA), blood delta-ALA-D, urinary lead, and blood lead—blood lead most closely corresponds to the severity of acute or subacute poisoning and to the overall laboratory and clinical pictures in the most severely affected people.
Whole Blood
For epidemiologic and clinical acceptability and utility, lead in the whole blood of chronically exposed populations remains the biologic marker of choice. It has been the traditional view that blood lead generally reflects fairly recent exposure, i.e., exposure 20–30 days before measurement. In cases of relatively stable exposure, however, such a short-term index of lead uptake is still of considerable utility.
The collection of blood lead in children in well-designed studies (either cross-sectional or longitudinal) is subject to problems of interpretation. Child blood lead is highly responsive to changes in exposure in the preceding 1 or 2 weeks. Most studies involve a recruitment phase that precedes blood lead collection (or the first blood collection in a longitudinal study) by a few days to a few months. The recruitment activity itself is an interaction with the child's family or caretakers that might alter their behavior and increase their awareness of potential lead exposure hazards. The primary exposure vector for young children is household dust and surface soil (even if the source is deteriorating lead-based paint), so changes in caretaker behavior that reduce dust exposure might cause a reduction in a child's blood lead concentration between the time of recruitment and the time of blood collection. Such changes include more frequent dusting and handwashing and more effective control of child access to dusty or dirty places. Changes in caretaker behavior are even more likely in longitudinal studies with repeated contacts between investigators and subjects or in cross-sectional studies in communities that have already had long-standing media coverage or community controversy about removal of leaded paint or lead-contaminated soil.
Blood lead concentration as a short-term measure of exposure is less accurate with subjects whose skeletal lead contributes substantially to total blood lead concentration. Estimating total body lead burden is complex and requires that all sources of lead be considered. Consequently, it might be expected that, in people (children and adults) with a high body lead burden lodged in bone, more of the lead in bone would contribute to blood lead concentration and be reflected in the long half-life of removal from blood. With regard to fractional contributions of recent versus cumulative lead exposure to blood lead, various and numerous studies have shown that the major component of a given total blood lead concentration in a young child or adult is recent input, and
the influence of cumulative input increases as a function of age and exposure history (Duggan, 1983; Christoffersson et al., 1984; Harley and Kneip, 1984; Schwartz et al., 1985; Schütz et al., 1987a; Skerfving, 1988). One exception would be retired or reassigned lead workers who received heavy lead exposure in their working careers.
Table 4-3 summarizes data related to the toxicokinetic aspects of blood lead in children. Corresponding data on diverse adult populations are set forth in Table 4-4. The tables should be read for their implications for biologic monitoring, especially for low-dose exposures.
A number of conclusions are to be drawn from Table 4-3, including:
-
Blood lead appears to stabilize in older children, at least enough to preserve rank order, especially when exposure is reduced.
-
Rate of change of blood lead of infants and perhaps older children in response to changes in exposure appears to be a function of current exposure and accumulated body burden.
-
Older children appear to preserve an earlier exposure history (in bone stores), as shown in rank ordering; there might be at least two lead compartments that contribute to blood lead concentration, one of which is large enough to preserve statistical association (consisting of lead in bone), although continuing exposure cannot be ruled out when blood lead concentrations are large.
Data in Table 4-4 can be summarized as follows:
-
Men and women usually exposed to lead in air and diet have a lower rate of change in blood lead in response to exposure changes than those with little or no exposure.
-
Bone lead can be an important source of steady-state blood lead concentration, even under conditions of ordinary exposure. Similarly, occupationally exposed people can accumulate a skeletal burden large enough to become the dominant source of blood lead concentration even after active exposure ceases.
-
Blood lead clearance in substantially exposed people can be described by two components, one of which is rapid (1–2 months) and reflects soft-tissue lead, and one a longer-term bone component that is reflected in reduction of bone lead stores.
TABLE 4-3 Studies of Kinetic Behavior of Lead in Blood of Children
|
Study Group and Exposure |
Half-Life, days |
Comments (References) |
|
Infants, middle class; ambient exposure |
- |
Blood lead very unstable for first 20 mo Rabinowitz et al., 1984) |
|
Infants, middle class; ambient low exposure |
5.6 |
Reanalysis of Ziegler et al. et al. (1978) data; mean-time 8 days (half-life, 5.6 days) (Duggan, 1983) |
|
Infants, low socioeconomic status; heavy ambient exposure |
ca. 300 |
Reflects high body burden plus in utero uptake in urban setting (Succop et al., 1987) |
|
Low-socioeconomic-status children of battery workers; secondary exposure |
- |
Rank order of group preserved over 5 yr; r = 0.74 (Schroeder et al., 1985) |
|
General U.S. child population; varied exposure |
- |
Regression analyses of NHANES blood lead data showed 30-day (best-fit) lag with lead source (Schwartz et al., 1985; Annest and Mahaffey, 1984) |
|
School-age English children; low exposure |
- |
Two blood lead sets, 20 mo apart; rank order preserved (Landsdown et al., 1986) |
|
U.S. children, 4–12 yr old; increased ambient exposure |
- |
Rank order of serial blood lead measures generally preserved (David et al., 1982) |
-
Generally, blood lead half-life is highly variable because of such factors as age, metabolic variability, total body burden, and concentration and duration of exposure.
With careful attention to methodologic details, blood lead concentration analyses by competent laboratories can be used in general population surveys of trends in lead exposure. That has proved especially useful in relating recent declines in blood lead concentration and reductions in such sources as leaded gasoline.
Plasma
Earlier data suggested that plasma lead does not vary across a broad range of total blood lead, but it is now accepted that plasma and erythrocyte lead are in equilibrium and that the plasma fraction of lead is stable up to a blood lead concentration of 50–60 µg/dL, at which the fraction increases (Cavalleri et al., 1978; DeSilva, 1981; Manton and Malloy, 1983; Manton and Cook, 1984).
The existence of an equilibrium of lead between plasma and erythrocytes indicates that some fraction of total erythrocyte lead can be shifted to plasma in responses to downward shifts from steady-state exposure. That accounts for the fast component of blood lead decay, which is commonly faster than that expected from erythrocyte turnover rates.
Plasma lead concentrations at steady state are extremely small, often less than 1% of blood lead concentration, and rarely above 1 µg/dL. Even at blood lead concentrations over 50–60 µg/dL, they go up to only a few micrograms per deciliter. That makes it unlikely that a typical clinical laboratory will routinely analyze plasma lead, owing to such complicating factors as ambient lead contamination and ready hemolysis of high-lead erythrocytes.
Teeth
Human deciduous teeth accumulate lead in substantial quantities over their embryonic and postnatal life, up to the time of shedding. Teeth are anatomically and metabolically diverse, and this affects lead toxicokinetics in the mineralizing matrix.
Like bone, teeth have long been recognized as relatively useful tissues for assessing biologic markers of long-term lead accumulation. Unlike bone, however, teeth irreversibly sequester lead (Cohen, 1970);
TABLE 4-4 Studies of Kinetic Behavior of Lead in Blood of Adults
|
Study Group and Exposure |
Half-Life, days |
Comments (References) |
|
Experimental studies: |
||
|
Volunteers exposed to stable 204Pb in diet |
25 |
Short-term study, nonequilibrium kinetics for bone lead release (Rabinowitz et al., 1976) |
|
Volunteers inhaling 203Pb tracer aerosol |
16 |
Short-term study, nonequilibrium kinetics for bone lead release (Chamberlain et al., 1978) |
|
Volunteers inhaling cold aerosol at two concentrations |
28 (10.9 µg/m3) 26 (3.2 µg/m3) |
Short-term study, lead nonequilibrium kinetics for bone lead release (Griffin et al., 1975) |
|
Epidemiologic studies: |
||
|
Men in England; low ambient exposure |
120–180 |
Serial survey of blood lead after lowered exposure over longer time (Delves et al., 1984) |
|
Women in English town; ambient exposure with tap water lead higher exposure |
180 |
Lead plumbing changed; blood lead followed serially (Thomas et al., 1979) |
|
Study Group and Exposure |
Half-Life, days |
Comments (References) |
|
Retired lead workers |
2,044 (median) |
Mean lead exposure, 23 years; broad range of lives (Schütz et al., 1987a) |
|
Active lead workers, examined after removal to lower exposure |
29 (median) |
Half-life for short component of two-component curve, showing bone lead as do profiles of retired workers (Schütz et al., 1987a) |
|
Lead workers; exposure reduced because of strike at plant |
20–130 |
Broad range of short-term blood lead component (O'Flaherty et al., 1982) |
|
Lead workers; exposure reduced because of medical removal for excessive exposure (≥;60 µg/dL) |
79–130 |
Broad range showing exposure history (Kang et al., 1983) |
|
Lead-poisoned workers removed from active exposure for medical reasons |
619 (median) |
Major input from slower, bone-lead-based component (Hryhorczuk et al., 1985) |
|
Retired lead workers examined with in vivo x-ray fluorescence for tibia lead |
|
Blood lead in ex-workers primarily from bone resorption (Christoffersson et al., 1984) |
and they are more accessible for study because they are shed (see, e.g., EPA, 1986a; Mushak, 1989).
Table 4-5 presents illustrative studies of lead distribution in human teeth and the potential utility of teeth as biologic markers of childhood lead exposure. The data in the table make it clear that lead deposition in teeth is complicated and is a function of age (Steenhout and Pourtois, 1981), region of tooth (e.g., Needleman and Shapiro, 1974; Grandjean et al., 1986), type of tooth (Mackie et al., 1977; Delves et al., 1982), and extent of exposure (EPA, 1986a; Mushak, 1989).
Secondary (circumpulpal) dentin accumulates the largest concentrations of lead and is most sensitive to the extent of lead uptake in other body compartments (through contact with blood lead), so it is probably best suited for examining even subtle exposure. It seems specially attractive to consider low-lead epidemiologic studies in tissues that are major accumulators of lead, as opposed to tissues that only accumulate lower concentrations. Teeth differ in lead content as a function of tooth type, concentrations being higher in incisors than in premolars; this kind of difference is related in part to the fractions of actively accumulating regions in each tooth type (see, e.g., Mackie et al., 1977).
Lead in shed teeth of children, however useful for revealing accumulation, reflects retrospective exposure over a fairly long period and one that encompasses peak sensitivity and peak exposure periods, i.e., at the age of 2–3 years. Hence, this measure remains of less use than some others as a basis for environmental intervention at specific times.
In vivo analysis of lead in teeth seems to have the virtue of providing information on current lead accumulation when used in tandem with serial measurements of blood lead. Shapiro and co-workers (1978) showed a moderate correlation between lead in teeth and blood lead as single measures. However, such an in vivo measure seems to offer little advantage over similar measurements of tibial sites.
Bone
The skeletal system accumulates lead from before birth until at least the sixth decade. As public-health concerns are increasingly shifted to smaller lead exposures, two aspects of bone lead rise in importance. The first is the increasing degree to which bone contributes lead to total blood lead concentration, especially during pregnancy and at later stages
TABLE 4-5 Studies of Kinetic Behavior of Lead in Human Teeth
|
Study Group and Exposure |
Type of Tooth Measure |
Comments (References) |
|
U.S. children; high exposure |
Circumpulpal dentin, shed teeth |
U.S. urban children have higher tooth lead than controls (Needleman and Shapiro, 1974) |
|
U.S. children; range of exposures |
Whole tooth; various tooth types |
Lead varies with tooth type (Mackie et al., 1977) |
|
U.S. urban children; with higher lead exposures |
Incisors in vivo, related to concurrent blood lead |
Correlation of in vivo tooth lead with blood lead (single; r = 0.5) (Shapiro et al., 1978) |
|
Danish children; much lower exposure than U.S. children |
Circumpulpal dentin |
Concentration varies with age and tooth type (Grandjean et al., 1986) |
|
British children stratified by socioeconomic variables |
Tooth crowns (shed tooth minus resorbed pulp) |
Considerable variance with type and position in jaw (Delves et al., 1982) |
|
Belgian children; variable lead exposure |
Whole tooth |
Normalizing for age gives better index of exposure (micrograms per gram per year) (Steenhout and Pourtois, 1981) |
of life—e.g., in osteoporosis in postmenopausal women. The second is toxicokinetic and methodologic: the extent to which real-time monitoring of bone lead can be used to determine unsafe rates of body lead accumulation.
It used to be widely held that the human skeletal system provides a metabolically inert depository for lead and that the huge amounts of lead being sequestered were inconsequential for health-risk assessment. That confidence rested in part on the assumption that bone was kinetically homogeneous as a lead compartment, with a half-life long enough to forestall risk of ready transfer back to blood. Current evidence argues, however, that bone is both a set of compartments for lead deposition and a target for lead toxicity. The dual identity complicates bone lead kinetics when it is applied to long-term modeling. The mobility of bone lead to blood is important. Table 4-6 summarizes studies that helped to characterize bone lead as a potentially toxic fraction of whole-body lead in sensitive populations. (Some of the material overlaps that presented earlier on blood lead.)
Human bone appears to have at least two, and possibly three, kinetically
TABLE 4-6 Studies of the Kinetic Behavior of Lead in Human Bone
|
Study Group and Exposure |
Comments (References) |
|
Swedish retired lead workers; 3–45 years of lead exposure |
Bone lead adds approximately 65% to total blood lead in retirement; accounts for half-life of 5.6 years (Schütz et al., 1987a) |
|
Swedish lead workers; work expo-sure variable; invivo analysis com-pared with chelatable lead |
Chelatable lead well correlated with trabecular, but not cortical bone (Schütz et al., 1987b) |
|
Japanese lead workers at various ages |
Chelatable lead is age-dependent, showing bone contribution (bone lead age-dependent) (Araki and Ushio, 1982) |
|
U.S. urban high-risk children meeting test criteria for in vivo tibial lead vs. chelation test |
Tibial (cortical) lead correlated with, and predictive (with blood lead) of chelatable lead (Rosen et al., 1989) |
distinct lead compartments. Lead in trabecular (spongy) bone appears to be more mobile than lead lodged in cortical (compact) bone, and there appears also to be a fraction of bone lead in equilibrium with the lead in blood (see, e.g., Skerfving, 1988). Trabecular bone seems to be an important source of resorbed lead when high exposure is reduced, e.g., through removal for medical reasons, by retirement of lead workers, or in response to chelation in adults (Schütz et al., 1987a). In young children, in whom only cortical bone has been examined, lead appears to leave cortical bone (tibia) (Rosen et al., 1989).
In the aggregate, the information in Table 4-6 makes it clear that bone lead readily returns to blood in substantial proportions. Although the mobilization is most apparent in people with a history of occupational exposure, bone lead also appears to be a major contributor in older people with ambient exposures to lead. More important, it is clear that bone lead is constantly mobilized in young children as part of physiologic remodeling of bone in the growth process. In addition to having a smaller fraction of total body stores of lead in bone, young children continuously recycle lead from bone to blood and other nonosseous tissues in bone reformation that accompanies the growth process.
Milk
Milk is the primary dietary constituent for young infants. Although the concentration of lead in human milk and infant formulas is relatively low (about 1.7 µg/dL), the volume consumed is large and thereby constitutes the primary source of lead for young infants, amounting to a daily intake of up to 50 µg (Ryu et al., 1985). Lead content of breastfed infants' milk correlates well with their blood lead concentrations until 6 months of age (r = 0.42,p < 0.0003) (Ryu et al., 1985), when infants begin to crawl and walk. At these times of infant and child development, milk lead content accounts for less than 10% of blood lead concentrations (Rabinowitz et al., 1985b).
Placenta
Some studies have found a close correlation between maternal and
newborn blood lead concentrations (Rabinowitz and Needleman, 1982; Korpela et al., 1986), but changes in compartmentalization of lead between blood, soft tissues, and bone of both mother and fetus might well be nonlinear and affected by nutritional status, as well as by marked differences in body composition between the developing fetus and the pregnant woman. In fact, blood lead concentrations during pregnancy have been seen to decline, rise, or evidence no definite trend (Davis and Svendsgaard, 1987).
Birthweight, head circumference, and placental weight were reduced as a function of placental lead content in a group of 100 obstetrically normal infants (Ward et al., 1987). Moreover, in the Port Pirie study in Australia, preterm delivery, defined as birth before the thirty-seventh week of pregnancy, was significantly related to maternal blood lead concentrations at delivery in a dose-response manner (McMichael et al., 1986). The latter study was carried out in 831 pregnant women, and the data were assessed by multivariate techniques. It has also been reported that placental lead content increases with lead exposure (Roels et al., 1978; Khera et al., 1980; Mayer-Popken et al., 1986); and in a limited study of amniotic fluid lead concentrations, it was found that concentrations of lead at term (59.6 ± 8.3 ng/ml) were significantly higher than maternal blood lead (40.4 ± 18.2 ng/ml) and umbilical cord blood lead (37.1 ± 13.5 ng/ml) (Korpela et al., 1986). Moreover, amniotic fluid concentrations failed to correlate with maternal or cord blood concentrations. Measurements of bone lead and blood lead concentrations (in pregnant women throughout the course of pregnancy), assessments of amniotic fluid concentrations, and placental lead concentrations at term collectively hold promise for further characterizing the dynamics of maternal-fetal lead transfer.
Chelatable and Urinary Lead
Spontaneous excretion of lead in nonoccupationally exposed humans is a highly variable process that involves small concentrations of lead (EPA, 1986a). In view of the difficulty of analyzing lead at low concentrations in a complex matrix, urinary lead appears to have little utility for general screening.
In contrast, the plumburesis associated with lead mobilization provides
what is considered the best measure of the potentially toxic fraction of the total body lead burden (see CDC, 1985, 1991; EPA, 1986a). On the basis of various in vitro experimental and epidemiologic studies (Chisolm and Barltrop, 1979; Piomelli et al., 1984; CDC, 1985; EPA, 1986a; Mushak, 1989), chelatable lead is assumed to be a chemical sample of both mobile body compartments—i.e., blood and soft tissues—as well as of subcompartments of bone.
BIOLOGIC MARKERS OF EFFECT
This section examines biologic markers of early subclinical effects of lead that have potential value in the quantitative assessment of human health risk. It serves as a bridge between human lead toxicology and the practice of laboratory screening in analytic toxicology for the evaluation of lead intoxication. Elucidation of biologic markers of effect also sheds light on mechanisms of toxic action. Methodologically, markers of effect are any measurable biochemical, physiologic, or other alteration from normal in humans that shows potential health impairment. The theoretical advantage of markers of effect over markers of exposure is that markers of effect reflect actual biologic responses of the body. A practical advantage in many instances is that markers of effect are relatively independent of the vicissitudes of lead measurement, particularly the contamination of samples with lead.
If a biologic marker is a robust early perturbation in response to low-dose exposure, it might be found in most of the target population. It is important that such an early perturbation not be likely itself to constitute a bona fide adverse effect and that it be useful in avoiding exposure sufficient to produce adverse effects in other organ systems. Given present knowledge, a biologic marker should reliably operate at a blood lead concentration below the 10 µg/dL associated with population IQ decrement, neurobehavioral changes, and deficits in growth indexes in a discernible fraction of young children. Some (e.g., Friberg, 1985) have even argued that markers of effect themselves indicate that it is already too late for monitoring to prevent any toxicologic perturbation at all.
Biologic markers of lead's effects are often confined in usefulness to a range of body burden of lead. As acceptable magnitudes of lead exposure have been reduced, it has been necessary to re-evaluate the
relevance of biologic markers of effect, as is the case with biologic markers based on lead's disturbances of heme synthesis.
This section deals with the epidemiologic utility of established markers of effect, including brief statements on the underlying toxicology and pathology. The markers are discussed in terms of their current usefulness and reliability as the definition of ''safe'' lead exposure continues to change. The section also discusses the use of markers for elucidating mechanisms of toxic action.
Markers Based on Disturbance of Heme Synthesis
Lead affects the biologic synthesis of heme at various steps in a number of important organ systems (see Chapter 2, especially Fig. 2-5). Overall toxicity in the synthetic pathway at moderate exposures is attributable to effects on three enzymes, although others can be affected at larger exposures. The three effects are stimulation of the activity of delta-ALA synthetase (delta-ALA-S), the mitochondrial enzyme that mediates the rate-limiting step in heme formation; inhibition of the activity of the cytosolic enzyme porphobilinogen synthetase (PBG-S or delta-ALA-D); and inhibition of the activity of the mitochondrial enzyme ferrochelatase or inhibition of intramitochondrial movement of iron to the ferrochelatase site.
The steps in heme synthesis are not uniformly sensitive to lead, nor are they all equally useful in development of biologic markers of effect; only some steps are useful this way. Some of the relevant characteristics of biologic markers based on disturbance of heme synthesis are presented in Table 4-7.
Inhibition of the activity of the enzyme delta-ALA-D occurs at a very low body lead burden, indexed as blood lead; the threshold of this effect is 5 µg/dL or even lower (Chisolm et al., 1985; EPA, 1986a). Hernberg and Nikkanen (1970) produced data that allow an estimate of 50% inhibition at a blood lead concentration of 16 µg/dL. Thus, at current exposures in the United States, many people would be expected to have measurable inhibition of the enzyme.
The enzyme is retained in the mature erythrocyte with a function that is vestigial compared with its role in blood-forming and other tissues.
The erythrocyte enzyme's response to lead is similar to its response in other tissues at high lead concentrations (Secchi et al., 1974; Dieter and Finley, 1979; EPA, 1986a), but tissue activity at low concentrations is unknown.
Lead directly affects delta-ALA-D activity by active-site inhibition through thiol-site binding (e.g., Finelli et al., 1975). That behavior produces two problems: one is related to diagnostic utility, in that direct measurement of lead is equivalent to measurement of delta-ALA-D enzyme activity, and vice versa; the second is methodologic, in that measurement of delta-ALA-D activity is affected by lead contamination of the sample just as direct blood lead measurement is. In addition, enzyme activity is affected by zinc contamination: zinc offsets lead inhibition and produces inaccurate results.
The distribution of delta-ALA-D in sensitive populations is genetically polymorphic and occurs as three phenotypes (Doss et al., 1979; Battistuzzi et al., 1981; Astrin et al., 1987). Consequently, delta-ALA-D-activity screening in tandem with phenotype identification (Astrin et al., 1987) distinguishes subjects who are genetically most susceptible at a given blood lead concentration and those who merit maximal protection from exposure.
With increasing inhibition of delta-ALA-D activity, delta-ALA accumulates in the body and eventually in urine. The threshold for urinary ALA accumulation (as blood lead concentration) can be up to 40 µg/dL for workers and children, depending on measurement method (NCR, 1972; Chisolm et al., 1976; Meredith et al., 1978; EPA, 1986a; Okayama et al,. 1989).
Not only does urinary ALA accumulation have a high threshold in terms of magnitude of lead exposure, but its utility in low-exposure screening is generally assumed to be valid for groups, rather than for individuals, such as lead workers (e.g., Roels et al., 1975; Alessio et al., 1979). Statistically, the sensitivity and the specificity of the method are such that high predictability (minimal false negatives or false positives) exists only at high blood lead concentrations (e.g., Okayama et al., 1989); that rules out its use in young children and pregnant women who receive low-dose lead exposures. Reports of screening of high-risk children with colorimetric measurement of urinary ALA indicate poor correlation with blood lead concentrations (Blanksma et al., 1970; Specter et al., 1971; Chisolm et al., 1976).
TABLE 4-7 Heme-Synthesis Disturbances and Effect Markers
|
Lead Effect |
Result |
Marker Threshold, Lead Concentration, µg/dL |
Comments |
|
Inhibition of delta-ALA-D(PBG-S) activity |
Accumulation of ALA in tissues and urine |
5 |
Sensitive for current population blood lead concentrations; problematic relation to tissue effects |
|
Feedback stimulation of delta-ALA-S activity |
Minor contribution to total ALA in urine |
40 |
Not a feasible marker |
|
Accumulation of urinary ALA |
— |
20–40a |
Useful for population screening; limited in individual predictability; not useful for childhood screening |
|
Inhibition of heme formation from protoporphyrin IX |
Accumulation of erythrocyte Protoporphyrin in blood |
15–20 (children) 25–30 (adults) |
Most common screening marker for children and workers; basis of risk scheme, with blood lead, used by CDC in recent advisories, but not a part of the CDC 1991 statement |
|
Lead Effect |
Result |
Marker Threshold, Lead Concentration, µg/dL |
Comments |
|
Impaired use of coproporphyrin |
Accumulation of coproporphyrin in urine |
40 |
Reflects continuing toxicity;supplanted in popularity byerythrocyte protoporphyrin measurement |
|
a Depends of method of measurement (Chapter 5). |
|||
The aspect of heme-synthesis disturbances by lead that has been most widely exploited as a biologic marker or early effect has been the accumulation of the heme precursor erythrocyte protoporphyrin IX or zinc protoporphyrin (EP or ZPP) in blood of children and in some adult populations. EP accumulates in response to lead-related inhibition of the activity of the intramitochondrial enzyme ferrochelatase or lead-related impairment of intramitochondrial iron transport (Chisolm and Barltrop, 1979; CDC, 1985; EPA, 1986a; Moore et al., 1987). EP increase therefore indicates a generalized mitochondrial toxic response. It accumulates only in newly formed erythrocytes during the active lead-exposure period, and it takes weeks after onset of exposure for it to show up. It remains increased after lead exposure ceases, and it decreases in proportion to the turnover rate of the human mature erythrocyte, i.e., about 0.8%/day in the absence of decreased cell survival.
EP accumulation is exponentially and directly correlated with blood lead in children (Chisolm and Barltrop, 1979; Piomelli et al., 1982, 1984; Hammond et al., 1985) and in adults (Grandjean and Lintrup, 1978; Lilis et al., 1978; Valentine et al., 1982; Alessio, 1988). The population threshold of blood lead concentration for a lead-associated EP response is 15–20 µg/dL in children (Piomelli et al., 1982; Hammond et al., 1985) and 25–30 µg/dL in lead workers (Grandjean and Lintrup, 1978).
The utility of EP accumulation in a rapid and cost-effective screening procedure for high-risk children in the United States was recognized early; it was so attractive for screening at the high blood lead concentrations common in the early 1970s that it became part of the screening method advanced by the U.S. Public Health Service in 1975 (CDC, 1975). In its 1975 statement, the Centers for Disease Control and Prevention linked EP of 60 µg/dL of whole blood to later measurement of lead in venous blood. A combination of a blood lead of 30 µg/dL or higher and an EP of 60 µg/dL or higher was taken as evidence of lead toxicity. In 1978, the combination was modified to lead of 30 µg/dL and EP of 50 µg/dL or higher (CDC, 1978). The new CDC statement (CDC, 1991), however, makes it clear that use of EP is not practical or useful for low blood lead concentration in portions of the multitiered approach now being recommended.
Lead-associated EP increase is similar in hematologic result to iron deficiency, so the use of blood EP as a lead-specific marker must take
into account the relative risk of iron deficiency or the actual iron status of the people being screened (CDC, 1985; Mahaffey and Annest, 1986; Marcus and Schwartz, 1987; Piomelli et al., 1987). But it has to be kept in mind that the rare genetic disorder erythropoietic protoporphyria produces high concentrations of EP that are discordant with blood lead concentrations.
Increase in EP concentration, as a measure of lead exposure, differs from increase in urinary coproporphyrin (CP), a heme precursor that was traditionally used as a marker of childhood and worker exposure to lead before the heavy use and popularity of EP measurement. Urinary CP responds only to active lead exposure and intoxication and is a measure that reflects current exposures (Piomelli and Graziano, 1980). The threshold for urinary CP increase appears to parallel that of urinary ALA, being about 40 µg/dL in lead in blood.
The apparent interference of lead in the kidney 1-hydroxylase system, which uses heme, is discussed with respect to vitamin D later in this chapter.
Markers of Other Biologic Systems
Biologic markers that are based on biologic systems other than heme synthesis are presented in Table 4-8.
Lead intoxication produces impairments in blood-forming tissue other than those directly involved in heme synthesis. Lead strongly inhibits an enzyme central in erythropoietic pyrimidine metabolism, pyrimidine-5'-nucleotidase (Py-5'-N). The enzyme catalyzes the hydrolysis of pyrimidine nucleotides from the degradation of ribosomal RNA fragments in maturing erythrocytes. Inhibition leads to accumulation of the nucleotides, and ribosomal catabolism is retarded (Paglia and Valentine, 1975; Angle and McIntire, 1978; Buc and Kaplan, 1978); at high lead exposures, inhibition is severe enough to produce basophilic stippling from undergraded fragments.
The blood-lead threshold for this effect, based on lead-exposed children, appears to be around 10 µg/dL (Angle et al., 1982; Cook et al., 1986, 1987). Sensitivity for predicting a blood lead concentration equal to or greater than 40 µg/dL is 80% (using an enzyme activity mean below 2 standard deviations (SDs)); specificity, as percentage of
TABLE 4-8 Non-Heme-Synthesis Markers of Effect of Lead Exposure
|
Lead Effect |
Result |
Marker Threshold, µg/dL |
Comments |
|
Inhibition of Py-5'-N activity in erythrocytes |
Accumulation of ribosomal fragments in reticulocytes |
5–10 |
Analogous to role of ALA-D activity inhibition; quite sensitive; linkage to verse effects questionable |
|
Inhibition of Na+, K+-ATPase in erythrocyte membrane |
Potassium loss and net sodium gain in cells; altered cell survival |
Not established; in workers, limited correlation with blood lead |
Studies in lead workers; direct measure of lead's presence; subject to contamination |
|
Inhibited hydroxylation of 25-OH-vitamin D |
Reduction in hormonal metabolite 1,25-(OH)2-vitamin D |
10–15 |
Important health effect, but not appropriate for use as marker |
children with an adequate enzyme activity who had blood lead less than 40 µg/dL, was 96 (Cook et al.,1987). The status of this marker for its relevance to lead toxicity is analogous to that of delta-ALA-D, noted above. Like delta-ALA-D, it reflects the presence of biologically active lead at a site not readily probed directly. At some point in increasing lead exposure, lead-related inhibition of the enzyme will produce an accumulation of pyrimidine metabolites. Py-5'-N activity is also quite low in a genetic disorder that produces a hemolytic anemia due to such inhibition and ribosomal fragment buildup (Valentine and Paglia, 1980). Consequently, people with this phenotype, which is rare, are extremely sensitive to lead exposure and merit both identification and added protection from lead exposure.
Lead exposure results in inhibition of erythrocyte membrane Na+,K+- ATPase, which mediates the control of intracellular movement of both these physiologically crucial ions (Raghavan et al., 1981). Inhibition produces loss of cellular K+, but not disturb input of Na+; it produces cell shrinkage, a net increase in sodium concentration, and increased fragility and lysis.
This marker of membrane ATPase has been examined quantitatively only in lead workers (Secchi et al., 1968; Raghavan et al., 1981) in whom it appears that the inhibition correlates well with membrane lead concentrations, but poorly with total blood lead. Its broad applicability to screening of lead workers and other populations is problematic.
In young children, increases in blood lead are associated with disturbances in vitamin D function, notably formation of the hormonal metabolite 1,25-(OH)2-vitamin D, which is crucial for a wide variety of functions (see Chapter 2). The threshold for reduction of concentrations of 1,25-(OH)2-vitamin D in terms of blood lead appears to lie at blood lead concentrations of 10-15 µg/dL (Rosen et al., 1980; Mahaffey et al., 1982b); the effect on vitamin D function is uniformly distributed across the range of blood lead concentrations.
Reductions in plasma concentrations of the hormonal metabolite are not suitable for use as biologic markers of effect. The reductions in this metabolite and some other early effects are now known to occur in some people at quite low concentrations of blood lead (including cognitive and other neurobehavioral end points) and are, in fact, adverse effects, given the crucial function of this hormone in the body, and one
must therefore use other more sensitive effect markers before decrements in circulating concentrations of 1,25-(OH)2-vitamin D would be detected.
Relevance of Current Markers of Effect for Low-Dose Exposures
The utility of commonly used biologic markers of effect is related to the range of body lead burden of concern. Summary comments regarding these are provided in Table 4-9.
Use of increase in EP in lead screening of high-risk populations
TABLE 4-9 Markers of Effect Relative to Low-Dose Lead Exposure
|
Marker |
Threshold, µg/dL |
Effectiveness |
|
Activity of ALA-D (PBG-S) inhibition |
5 |
Sensitivity at low exposure; relevant to tissue effects questionable; useful for phenotype sensitivity to toxicity |
|
EP increase |
15–20 (children) 25–30 (adults) |
Yields too many false-negative results at blood lead around 25 µg/dL; no correlation at blood lead of 15 µg/dL |
|
Urinary ALA increase |
Up to 40 |
Not useful at blood lead of 10–15 µg/dL |
|
Urinary CP increase |
40 |
Not useful at blood lead of 10–15 µ/dL |
|
Py-5'-N activity inhibition |
5–10 |
Quite sensitive at blood lead of 10–15 µg/dL; relevance to toxic effects questionable |
appears to yield an increasingly unacceptable rate of false-negative results as population blood concentrations decline and as the guideline concentrations for what is acceptable exposure also fall (see Chapter 2). Mahaffey and Annest (1986) showed that at the relatively high blood lead concentration of 30 µg/dL, the false-negative rate for blood lead and EP in the NHANES II survey population approached 50%. As seen in Chapter 5, such false-negative rates are lower, but are still high in high-risk child populations like those in Chicago. One would expect the rate to be even higher at lower blood lead concentrations, as seen in analysis of the Chicago screening population cited.
Such measures as urinary ALA and CP apply mainly to relatively high lead exposures, having thresholds of about 40 µg/dL. They would not be of much use at current concentrations of concern, around 10 µg/dL.
Inhibition of activity of both delta-ALA-D and Py-5'-N is significant at blood lead concentrations of 10 µg/dL and less. Consequently, they would technically be useful markers; but they appear to offer little advantage over lead measurement itself, because the presence of erythrocyte lead produces continuing inhibition, and contaminating lead (and zinc in the case of delta-ALA-D) would also affect the measurements of enzyme activities. In the case of delta-ALA-D, and perhaps that of Py-5'-N, use of these markers in tandem with assessment of genetic phenotype would identify subjects at great risk of hematotoxicity even at low doses of exposure. That would be especially important in children homozygous for the ALA-D-2 allele (Astrin et al., 1987), but also important in heterozygous children. A significant number of such phenotypes are seen in traditionally high-risk populations (e.g., Doss et al., 1979; Rogan et al., 1986; Astrin et al., 1987).
Potential Markers of Effect
Despite the limited utility of many of the conventional biologic markers of effect either at high exposures for such organs as the kidney or at increasingly lower general exposures, it is still of interest to consider available information on some candidates for biologic markers. These are classified as enzymes, binding proteins, and metabolites. A summary of the potential markers of effect is given in Table 4-10.
TABLE 4-10 Potentially Useful Markers of Effect of Lead Exposure
|
Marker |
Threshold |
Comments |
|
Enzymes |
|
|
|
Urinary N-acetyl-beta-D-glucosaminidase |
Approximately 60 µg/dL |
Most sensitive measure of tubular injury by lead so far |
|
Erythrocyte nicotinamide adenine dinucleotide (NAD) in exposed subjects and in vitro |
Not measured, but lower than that for Py-5'-N |
Reflects damage to formation and function of NAD; suggests that genetic susceptibility to NAD reduction can be exacerbated by lead exposure |
|
Lead-Binding Proteins |
|
|
|
Erythrocyte low-weight binding proteins in association with lead toxicity |
Protein low in lead workers with overt toxicity |
Serves a protective function analogous to suggested function in animal kidney |
|
Metabolites |
|
|
|
beta-Isobutyric acid (β-IBA) in urine |
Increased threefold at blood lead approximately 60 µg/dL; no threshold calculated |
Suggests that lead is damaging DNA function and formation via increased thymidine degradation to β-IBA |
Enzyme Systems
As noted in Chapter 2, lead is now widely known to induce both glomerular and tubular injury, at least in adults occupationally exposed to lead. In most instances, however, the thresholds for such injury appear to be high and presumably of interest mainly in occupational screening at current OSHA guidelines. Schaller et al. (1980) and Buchet et al. (1980) reported that no discernible kidney dysfunction was observable below 60 µg/dL (Buchet et al., 1980) or over the range 50–85 µg/dL (Schaller et al., 1980). In common with the rest of the field of occupational or environmental metal nephrotoxicity, however, the indicators of what constitutes early kidney injury due to lead have been relatively insensitive until recently.
The lower range of lead-induced nephrotoxicity was examined by Meyer et al. (1984) and Verschoor and co-workers (1987). Their main index for low-concentration lead effect was urinary excretion of the tubule cell lysosomal enzyme N-acetyl-beta-D-glucosaminidase (NAG). This enzyme in urine might well be a promising general marker of early metal-induced kidney damage, in that various workers found it to be more sensitive than beta-2-microglobulin in assessing early cadmium-induced tubular dysfunction in workers (e.g., Chia et al., 1989; Kawada et al., 1989; Mueller et al., 1989).
In their worker group, Verschoor et al. (1987) found that tubular, rather than glomerular, indexes are affected earliest and that urinary NAG concentrations were increased at blood lead below 60 µg/dL and showed a significant correlation with EP.
Nicotinamide adenine dinucleotide (NAD) synthetase (NAD-S) catalyzes the final step in the Preiss-Handler pathway for biologic synthesis of the metabolically common coenzyme NAD (Preiss and Handler, 1958), and it is associated with an end product whose synthesis is impaired in a number of genetic disorders, including thalassemia (Zerez and Tanaka, 1989) and sickle-cell disease (Zerez et al., 1988).
Zerez et al. (1990) found that, in erythrocytes from lead-exposed subjects or treated with lead in vitro, NAD-S activity is obliterated at lead concentrations at which Py-5'-N activity, itself a sensitive measure of lead exposure, still retains 50–70% of activity. The small group of lead-exposed subjects ranged in blood lead from 34 to 72 µg/dL.
Lead-Binding Proteins
The presence of proteins with an avidity for lead in kidney and brain was discussed in Chapter 2. In the erythrocytes of variably exposed lead workers, there is a lead-binding protein that appears to be inversely linked to clinical manifestations of occupational lead intoxication; i.e., the higher the erythrocyte concentration, the more resistant the worker appears to be to overt poisoning (Raghavan et al., 1980, 1981; Lolin and O'Gorman, 1986). The protein's function is reminiscent of kidney cytosol lead-binding protein (e.g., Oskarsson et al., 1982; Goering and Fowler, 1985).
Lolin and O'Gorman (1988) measured the protein in lead workers with various degrees of lead exposure. The protein was quantitated as two peaks, which suggested a heterogenous protein. It was present in erythrocytes from all lead workers, but absent from controls. The threshold for induction of the protein was about 38 µg/dL or less; that indicates some utility for monitoring in occupational exposures. Equally important, concentrations of the protein are significantly lower in people with clinical toxicity. Furthermore, those workers found that the concentration of the erythrocyte lead-binding protein was related to intensity of exposure (past and present), not its duration. That is also typical of inducible proteins.
Lolin and O'Gorman have postulated that the protein is protective in its function and would play a special role in subjects who are particularly susceptible to lead's effect on, e.g., ALA-D activity. They had found earlier (Lolin and O'Gorman, 1986) that there is a type of ALA-D activity inhibition not seen in environmental or nonoccupational exposures. Such protection targeted to preservation of ALA-D activity is consistent with the findings of Oskarsson et al. (1982) and Goering and Fowler (1985) that rat kidney ALA-D activity is notably resistant to lead and that this is due to the presence of a kidney cytosolic lead-binding protein. The analytic data on both worker erythrocyte and rat kidney cytosol protein structure suggest metallothionein, as noted by Goering and Fowler (1985) and Lolin and O'Gorman (1988), but further work is required to establish this fact.
Metabolites
beta-Aminoisobutyric acid (beta-AIB) is a normal degradation product of thymidine, a constituent of DNA. Unlike typical amino acids, it is actively secreted as a catabolic metabolite via the tubule. It is normally excreted at low rates in humans not exposed to lead (6 nmol/µmol of creatinine). Farkas and co-workers (1987) examined the concentrations of this metabolite in urine of workers occupationally exposed to lead and in marmoset monkeys experimentally exposed via tap water. In workers with a mean blood lead concentration of 64 µg/dL and a mean EP of 117 µg/dL, there was a tripling of urinary output of beta-AIB. No threshold was determined for excretion beyond the normal range. In monkeys, there was a dose-dependent increase in urinary excretion. Given the fact of beta-AIB's handling by the kidney, the increase in the metabolite is a marker more of DNA damage through increased degradation of thymidine to beta-AIB.
Identification of Toxicity Mechanisms
Markers of effect not only are useful in the screening of high-risk populations, but also help to establish the various molecular and cellular mechanisms by which lead imparts multiorgan toxicity in those high-risk populations.
Inhibition of 1,25-Dihydroxyvitamin D Formation
As noted elsewhere, lead exposure is associated with reduced blood concentrations of the hormonal metabolite of vitamin D, 1,25-(OH)2-vitamin D. Such reductions with blood lead concentrations of 33–55 µg/dL, furthermore, rival those seen in several disease states (Rosen et al., 1980; Mahaffey et al., 1982b; Rosen and Chesney, 1983). Consequently, reductions in this hormone at lower lead exposures are signaling
early metabolic disturbance. Reduced concentrations of the hormone indicate that lead has two mechanisms of adverse effect that potentially can operate in high-risk populations. The first concerns the toxic consequences of disturbance in the hormone-calcium relationship, and the second concerns the many roles played by 1,25-(OH)2-vitamin D beyond regulatory control of calcium function.
A major mechanisms of cellular lead toxicity appears to be interference in calcium homeostasis and function (Chapter 2). Such interference occurs either directly, via lead-calcium interactions in the cell, or through impaired function of calcium as a second messenger due to disturbed regulation by 1,25-(OH)2-vitamin D (Rasmussen, 1986a,b; Pounds and Rosen, 1988). It implies a risk of impaired handling of vesicular intestinal calcium and intracellular calcium in bone cells. Calcium-based effects are broadly distributed as to tissue and system sites, including the vascular system and developing neural and bone tissue.
As summarized in Table 2-3, other physiologic functions potentially can be altered by reduced concentrations of 1,25-(OH)2-vitamin D. They include parathyroid phospholipid metabolism, cyclic GMP production in skin fibroblasts, rental phosphate reabsorption, and differentiation and proliferation of diverse cell types. In addition, the division, communication, and cytostructural organization of many cell types are affected.
Impairment of Heme Synthesis
Heme is a prosthetic group for many functional proteins involved in cell function and survival, and its formation is obligatory for cellular functions in many tissues, especially blood-forming tissue, muscle, kidney, liver, and brain (EPA, 1986a). Evidence of an effect of lead on heme formation would constitute a far-reaching mechanistic clue to lead toxicity.
Heme formation is an intramitochondrial process. Its inhibition, whether by inhibition of the intramitochondrial enzyme ferrochelatase or by impairment of intramitochondrial delivery of the iron atom to protoporphyrin, can be considered a marker of generalized mitochondrial toxicity of lead in heme formation for a large number of cell and tissue types.
BIOLOGIC MARKERS OF SUSCEPTIBILITY
One factor that can enhance susceptibility to lead toxicity is nutritional status. Various nutritional factors have been shown experimentally to influence absorption and tissue concentrations of lead (Mahaffey, 1985). Although experimental diets can be manipulated to create a wide range of nutritional problems, factors of clinical importance are far more restricted. Those considered of greatest importance to young children, as well as many adults, are total food intake, frequency of food intake, and dietary intake of some trace minerals, notably calcium and iron (Mahaffey, 1985).
Fasting adults have been reported to absorb a substantially greater fraction of dietary lead than nonfasting adults (Rabinowitz et al., 1980; Heard and Chamberlain, 1982). Comparable data on the effects of fasting on lead absorption by children and young nonhuman primates are not available.
To assess the role of dietary calcium in absorption and retention of environmental lead, it is essential to recognize that effects reflect both acute and long-term variation in dietary calcium intake. Numerous studies with experimental animals fed diets low in calcium have established that calcium deficiency increases both tissue retention and toxicity of lead (Mahaffey et al., 1981). Long-term calcium deficiency produces physiologic adaptive mechanisms (Norman, 1990), including increased concentrations of various binding proteins and stimulation of the endocrine and regulatory systems that regulate the concentration of ionized calcium. Parathyroid hormone and 1,25-(OH)2-vitamin D are critical to regulatory control of calcium.
Physiologic controls that react to changes in dietary calcium also affect the biokinetics of lead. Generally, calcium deficiency increases lead toxicity (Mahaffey et al., 1973), but it is not clear that the increase in toxicity occurs predominantly because of physical competition between calcium and lead for absorption. The mechanisms that produce changes in lead absorption as a function of calcium status are not well documented.
Humans can adapt to a wide range of iron requirements and intakes (Cook, 1990). There are two basic principles of adaptation to differences in iron intake: Regulation of iron absorption is achieved by the gastrointestinal mucosa, and fractional absorption depends directly on the metabolic need for iron. How the gastrointestinal tract achieves
adaptation to change in dietary iron intake and change in iron requirement remains an unanswered question in iron metabolism (Cook, 1990).
Iron deficiency increases tissue deposition and toxicity of lead (Mahaffey-Six and Goyer, 1972). Ragan (1977) demonstrated sixfold increases in tissue lead in rats when body iron stores were reduced, but before iron deficiency developed. The influence of iron status on lead absorption has been investigated in adult humans, but with different results (Watson et al., 1980, 1986; Flanagan et al., 1982). It is not clear whether the difference reflects the severity of iron deficiency, differences in analytic approach, or some other undefined factors. A high prevalence of iron deficiency occurs in infants, children, and adolescents because of the need to expand the body's iron pool for growth. Women have higher iron requirements because of menstrual blood losses. The iron requirement of a normal pregnancy is approximately 500 mg, which is distributed to the fetus, placenta, and expanded maternal erythrocyte mass.
It is critical to recognize that the groups of people who have the highest environmental lead exposures are also at greatest risk of iron deficiency (Mahaffey and Annest, 1986). The greatest impact of iron deficiency is in young children, who develop defects in attention span that lead to learning and problem-solving difficulties (Lozoff et al., 1985, 1987; Pollitt et al., 1986, 1989). Data from a longitudinal prospective study in Yugoslavia (Graziano et al., 1990) show the combined effects of lead exposure and iron deficiency among pregnant women, infants, and children. Adverse effects of both conditions on the neurobehavioral and hematopoietic systems were found.
The importance of iron status for low-income families has been recognized for decades. In the early 1970s, a special program for low-income women, infants, and children was begun in the United States. Extensive evaluation of the impact of a program of nutritional supplementation for women, infants, and young children has been reported by Rush et al. (1988a,b).
Children's blood lead concentrations tend to be associated with families' provision of intellectual and sociologic support (Milar et al., 1980; Hunt et al., 1982; Stark et al., 1982; Dietrich et al., 1985). In one study, the scores of mothers on three Home Observation for Measurement of the Environment scales were significantly associated with cumulative blood lead concentrations in infants: maternal involvement
with child, provision of appropriate play materials, and emotional and verbal responsivity of the mother (Dietrich et al., 1985). Scores on such scales are loosely associated with socioeconomic status, although the prevalence of these risk factors varies substantially within all social strata (Pearson and Dietrich, 1985). The association between poor maternal support for the child and blood lead remains after other factors are controlled for (Bornschein et al., 1985).
One intrinsic, biologic factor of growing interest, but that has received little attention, is the distribution in sensitive populations of genetic susceptibilities that potentiate health risk. Few specific biologic markers of susceptibility for lead have been identified.
The nature of lead effects and their genetic potentiation (or attenuation) must be understood both qualitatively and quantitatively. The relative distribution of genetically susceptible segments of the population is of main concern when these segments suffer substantial lead exposure. For example, lead exposure and the hepatoporphyric genetic disorder acute intermittent porphyria both produce accumulation of potentially neurotoxic ALA in plasma and urine (e.g., EPA, 1986a) and show some neuropsychiatric responses. A second genetic disorder, that associated with ALA-D deficiency, might well be a special problem for children at high risk for lead exposure in urban areas of the United States (Astrin et al., 1987). According to available data, any increased, genetically based susceptibility to lead exposure or its adverse effects is based mainly on lead's effects on heme biosynthesis and erythropoiesis.
There is genetic polymorphism for the heme-pathway enzyme ALA-D in human populations. That has been recognized for some time (e.g., Granick et al., 1973), but the molecular genetic basis of the phenomenon has now been described (Battistuzzi et al., 1981). Potluri et al. (1987) identified the gene site at chromosome 9q34. Two common alleles, ALAD-1 and the deficiency ALAD-2, with frequencies of 0.9 and 0.1 in Europe-based populations, give rise to three phenotypes: 1-1, 1-2, and 2-2. Heterozygotic (1-2) people have ALA-D activity of approximately 50% of normal, and severely deficient homozygotic (2-2) people have activity of approximately 2% of normal (Doss et al., 1979). Doss et al. (1982) reported that workers with moderate workplace contact with lead but manifest lead poisoning in the form of high erythrocyte protoporphyrin concentrations were found to be heterozygotic for ALA-D deficiency, and Doss and Muller (1982) described an acute
lead-toxicity response in a person with ALA-D deficiency and moderate lead exposure. Ziemsen et al. (1986) reported that the fraction of lead workers with high blood lead increased as ALAD-2 phenotypy increased. Several recent studies have documented that children apparently heterozygotic or homozygotic for ALAD-2 can be susceptible to lead effects. Rogan et al. (1986) examined a group of children in a large lead-screening program and found that, independently of blood lead, children with ALA-D deficiency also had significantly high EP; results of further testing suggested a problem with the amount of the enzyme, rather than a biochemically defective form. Astrin et al. (1987) have, however, found that ALAD-2 in a large sample of lead-screened children in New York City was correlated with the relative frequency of high blood lead (over 30 µg/dL).
In acute intermittent porphyria, there is significant inhibition of the activity of the enzyme porphobilinogen deaminase, which mediates the conversion of porphobilinogen to uroporphyrinogen I. That leads to accumulation of high concentrations of ALA in urine and other body fluids (Goldberg and Moore, 1980; Moore et al., 1987). In both overt lead poisoning and attacks of acute intermittent porphyria, there are pronounced gastrointestinal, psychomotor, and cardiovascular responses, which have led to suggestions that lead works its adverse neurologic effects through direct action, as well as through the heme pathway (Silbergeld et al., 1982; EPA, 1986a). Conclusive evidence that lead significantly exerts neurotoxicity through the heme pathway, via excessive production of neurotoxic ALA (EPA, 1986a), has not been forthcoming. Studies directed to the hypothesis have entailed large exposures to lead, and it is not clear that low-dose lead exposure would effectively synergize neurologic manifestations of the attack stage of acute intermittent porphyria.
Increased lead exposure affects the liver in various ways (EPA, 1986a). One site of toxicity involves impairment of hepatic biotransformations of endogenous metabolites (e.g., Saenger et al., 1984) and impairment of the detoxification and activation of drugs and other xenobiotic substances via effects on P-450 mixed-function oxidase (Alvares et al., 1976; Meredith et al., 1978) and perhaps the carcinogen-activating P-448 complex. Evidence of genetically differentiated hepatic biotransformation and biodegradation capacity in the P-450 and P-448 systems has been reviewed by Parke (1987). It is already known
that genetic diversity has an impact on biotransformation of various drugs (Parke, 1987). Animal systems show genetic polymorphism in P-450—P-448 systems, but the requisite human studies of the underlying molecular genetics remain to be done.
SUMMARY
The absorption, distribution, retention and excretion of lead in sensitive populations from various sources affect both the biologic monitoring of lead exposure and toxic outcomes. The literature dealing with the quantitative aspects of lead toxicokinetics is extensive and supports a number of conclusions.
Inorganic lead is absorbed principally from the lungs and gastrointestinal tract of humans. About 30–50% of inhaled lead is absorbed from the lower respiratory tract in adults; the proportion is greater in children. Lead in water is absorbed variably: in adults, 10–15% is absorbed after consumption of blood; in children, approximately 50% is absorbed after consumption of food. Under fasting or semifasting conditions, rates of absorption rise considerably for adults and probably for children.
Lead is absorbed into plasma; with steady-state exposure, redistribution of 99% or even more to the erythrocytes occurs. Newly absorbed plasma lead is distributed metabolically to blood, soft tissue, and various compartments of bone, where longer-term storage occurs. It is critical to recognize that skeletal lead stores are continuously remobilized as part of the physiologic remodeling of bone that accompanies the growth process.
Skeletal accumulation of lead occurs through life until the sixth or seventh decade, when lead loss from bone occurs. Among adults, lead is released from bone in response to reductions in exposure or to metabolic stresses and alterations in the skeletal system. In people not occupationally exposed, up to approximately 200 mg of lead can accumulate. In lead workers, much more accumulates. Reversal of accumulation is associated with either dietary changes or, especially in postmenopausal women, bone-mineral homeostatic changes. The latter contribute to the blood lead burden and complicate lead toxicokinetic compartmental analysis.
Lead is excreted by the kidneys and gastrointestinal tract (biliary clearance occurs). Urine is the dominant route in adults. Spontaneous lead excretion is variable and is affected by biologic processes that complicate analysis, but plumburesis associated with chelation probing or chelation therapy is extensive and diagnostically useful in lead poisoning.
Whole blood lead is the most popular and most useful indicator of lead exposure in acute and subacute poisoning. Blood lead measurement is also the most widely used measure of chronic lead exposure.
Blood lead reflects relatively recent exposure in young children who were not excessively and not chronically exposed in their earliest years; but heavily exposed children and adults have a blood lead concentration at any time that integrates recent and older exposures. With respect to quantitative inputs to a given blood lead content, recent contributions account for the major fraction in both children and adults. Recent input is associated with a quick component, and accumulated lead input is reflected in a much slower component, with a longer half-life (e.g., Schütz et al., 1987a).
Older exposures come into play via lead release to blood. One result of this phenomenon is a life-long legacy of earlier exposures. The policy and biomedical consequences are obviously important. For example, one must take account of the extent of such contributions to blood lead concentrations in planning the extent of control actions and concomitant population responses to lead regulatory actions.
At low-dose lead exposures, which induce effects that are of increasing concern, blood lead concentrations around 10 µg/dL or less must be monitored in various populations. About that concentration are distributions of blood lead concentrations—an aggregate variance, which is a complex integral of exposure, interindividual variance, and the scatter associated with laboratory methods. Of those factors, the magnitude of exposure and the quality of method refinements would be the most responsive to attempts at minimization. Individual differences in lead toxicokinetics are intrinsic to populations and not readily amenable to control.
Plasma lead, if it were amenable to clean collection and measurement, would have considerable interpretive value for assessing population lead exposures. Plasma, of course, transports lead to target tissues and major repositories. Contamination and loss problems are formidable
for ready analysis of plasma in the typical clinical or epidemiologic research laboratory. Not only is contamination a problem for plasma analyses, but small rates of erythrocyte hemolysis would increase error. A hemolysis rate of 1% would double the plasma lead content, assuming a 1% equilibrium lead fraction in plasma. Furthermore, we still need to understand the toxicokinetics of plasma lead distribution for various exposure scenarios; that requires that acceptable methods be developed.
Bone lead measurement is the best way to assess body lead accumulation in such populations as high-risk urbanized young children, and it signals to the analyst and the policy-maker how rapidly lead is accumulating in bone. The role of this measure is enhanced considerably when techniques for integrating lifetime exposure are used in tandem with serial measurement of blood lead concentration in variably exposed young children or pregnant women.
Lead is released from bone in response to reductions in exposure or to metabolic stresses and alterations in the skeletal system. Lead appears in several compartments in bone, and these vary in their ability to release lead to the bloodstream. Furthermore, the known distribution characteristics of lead in bone compartments indicate that one can probe both long-term and shorter-term lead storage rates in bone spectrometrically. Such probing can be used both with populations in systematic development (children) and with populations in senescence (post-menopausal women).
Some biologic markers of effects of large exposures to lead have been characterized qualitatively and quantitatively and have been validated for toxicity in human populations. The usefulness of sensitive indicators, such as inhibition of particular enzymes, at very low lead concentrations has the added advantage that genetic susceptibility in lead-exposed subjects can be monitored. This would be the case for at least ALA-D activity distributions in sensitive populations exposed to lead. The sensitivity of some potentially useful biochemical markers has yet to be determined. Effect markers have been extremely helpful in identifying mechanisms of toxic action and in developing a better understanding of the biochemical interactions of lead in humans and other living systems.
Finally, little attention has been given to the identification of biologic markers of susceptibility. Current limited information does show that
















































