2
Adverse Health Effects of Exposure to Lead
Exposure to lead produces a variety of adverse health effects in sensitive populations through its impact on different organs and systems. The nature of the effects is a complex function of such factors as the magnitude of exposure, the physiologic and behavioral characteristics of the exposed person, and the relative importance of the lead-injured organ or system to overall health and well-being. The toxic effects of lead range from recently revealed subtle, subclinical responses to overt serious intoxication. It is the array of chronic effects of low-dose exposure that is of current public-health concern and that is the subject of this chapter. Overt, clinical poisoning still occurs, however, and is also discussed here. We have several reasons for emphasizing low-dose exposure. As recently noted by Landrigan (1989), the subtle effects of lead are bona fide impairments, not just inconsequential physiologic perturbations or slight decreases in reserve capacity. And the effects are associated with magnitudes of lead exposure that are encountered by a sizable fraction of the population in developed countries and thus are potentially found in very large numbers of people.
This chapter summarizes key points about the health effects of exposure of sensitive populations to lead. It deals specifically with various adverse effects of lead in sensitive populations (with emphasis on effects of low-dose exposure), persistence of some important health effects, molecular mechanisms of lead toxicity, and dose-effect relations. As described below, clinical lead poisoning differs between children and adults, in part because their organ systems are affected in different ways and to different extents. In addition, some people are more vulnerable
to lead toxicity and have increased sensitivity, because they suffer from disease, lack proper nutrition, or lack adequate health care. These factors influence exposure patterns and the biokinetics of lead absorption.
CLINICAL INTOXICATION IN CHILDREN
Childhood lead poisoning involves injury in at least three organ systems: the central nervous system (specifically, the brain), the kidney, and the blood-forming organs. Other systems are also affected, but the nature of their toxic injury has not been as well characterized.
Central Nervous System Effects
The nature of lead-associated overt central nervous system injury in children differs with the degree of lead exposure. Blood lead concentrations of about 100–150 µg/dL are associated with a high probability of fulminant lead encephalopathy. Before the widespread clinical use of chelation therapy, lead encephalopathy carried a high rate of mortality, about 65% (Foreman, 1963; NRC, 1972). The use of chelation therapy, pioneered by Chisolm and co-workers (NRC, 1972), has reduced mortality to 1 or 2%, or even less, if the poisoning is recognized and dealt with. The range of blood lead concentrations reported in association with encephalopathy is quite large (NRC, 1972), owing to such factors as individual differences in toxicokinetics and in timing of lead measurement and treatment.
Children are much more sensitive than adults to the neurophatic effects of lead. The central nervous system is principally involved in children and the peripheral nervous system in adults (Chisolm and Harrison, 1956; NRC, 1972; Chisolm and Barltrop, 1979; Piomelli et al., 1984), and thresholds of blood lead concentration for neurofunctional measures are lower in children. The common neuropathologic findings in fatal childhood lead encephalopathy (Pentschew, 1965) are cerebral edema, structural derangement in capillaries, neuronal necrosis, and neuronal loss in isocortex and basal ganglia.
Many children who survive an episode of lead encephalopathy have permanent neurologic sequelae, including retardation and severe behavioral
disorders (Byers and Lord, 1943; Perlstein and Attala, 1966; Rummo et al., 1979).
Renal Effects
Kidney injury in childhood plumbism is most often seen in overt poisoning and involves damage to the proximal tubule. In poisoning with encephalopathy, Chisolm (1962, 1968) has found the presence of the full, albeit transitory, Fanconi syndrome: glycosuria, aminoaciduria, hyperphosphaturia (with hypophosphatemia), and rickets. In overt toxicity after a range of exposures, aminoaciduria is the most consistent finding (Chisolm, 1962).
In chronic high-dose lead exposure, aminoaciduria appears to be the most consistent nephropathic finding. In a group of children with blood lead concentrations of 40–120 µg/dL, Pueschel et al. (1972) found aminoaciduria in those with blood lead of 50 µg/dL or more.
The role of childhood lead poisoning as a known contributor to early adult chronic nephritis found in Australia (e.g., Henderson, 1954; Emmerson, 1963) has not been identified in the United States (Tepper, 1963; Chisolm et al., 1976).
Hematologic Effects
Anemia is common in severe chronic lead poisoning and is reported to be associated with blood lead concentrations of 70 µg/dL and higher (NRC, 1972; CDC, 1978; Chisolm and Barltrop, 1979). However, reanalyses of the hematologic data of Landrigan et al. (1976) in children by Schwartz et al. (1990) indicated that anemia as indexed by hematocrit is present with blood lead concentrations below 70 µg/dL. Typically, the anemia is mildly hypochromic and normocytic and arises from a combination of reduced hemoglobin formation (resulting from either impaired heme synthesis or globin chain formation) and reduction in erythrocyte survival because of hemolysis (Waldron, 1966; Valentine and Paglia, 1980).
INTOXICATION IN ADULTS
The most extensive adult studies are of workers occupationally exposed to lead in battery recycling, lead smelting, alkyl lead manufacturing, plumbing, and pipefitting. These studies are described in other reports (EPA, 1986a; ATSDR, 1988) of lead exposure and are not the focus of this report. This report examines principally the effects of lead exposure in pregnant women as a sensitive population. Other adult populations may be at increased risk of lead intoxication because of large exposures, but they are beyond the scope of this report. For this reason, only a brief summary of effects in adults is presented below.
Central Nervous System and Other Neuropathic Effects
Although lead poisoning after very large exposures in adults can produce central nervous system injury, the exposure threshold is much higher in adults than in children. Blood lead concentrations associated with adult encephalopathy are well above 120–150 µg/dL. The features of adult lead encephalopathy, which can be as abrupt in onset in adults as in children, have been described by Aub et al. (1926), Cantarow and Trumper (1944), and Cumings (1959). They include dullness, irritability, headaches, and hallucination, progressing to convulsions, paralysis, and even death.
The more typical neuropathologic outcome of adult lead poisoning is peripheral polyneuritis involving sensory or motor nerves. There is often pronounced motor dysfunction, such as wrist drop and foot drop in the more advanced cases (Feldman et al., 1977). Changes include segmental demyelination and axonal degeneration (Fullerton, 1966), often with concomitant endoneural edema of Schwann cells (Windebank and Dyck, 1981).
Renal Effects
Occupational chronic lead nephropathy, the most important category of lead-associated kidney injury in adult populations, has been heavily studied for many years in Europe, but not as well in the United States.
In the studies of Wedeen et al.(1975, 1979), renal dysfunction has been established in U.S. lead workers, many of whom had no history of prior lead poisoning.
Generally, the Fanconi syndrome of acute childhood poisoning is not seen in adults with chronic lead poisoning. Proximal tubular injury from lead in adults at early stages of nephropathy is difficult to detect in workers, because of extensive renal reserve (Landrigan et al., 1982). Hyperuricemia is frequent probably because of increased uric acid production (Granick et al., 1978).
Lead has been clearly demonstrated to produce tubular nephrotoxicity in humans and rodents after acute or chronic exposure (Goyer and Rhyne, 1973; Wedeen et al., 1986; Ritz et al., 1988). Tubular proteinuria is a well-known manifestation of metal nephrotoxicity, but inconsistently reported in lead nephrotoxicity (Bonucci and Silvestrini, 1989; Goyer, 1989), perhaps because of the lack of sensitive and specific protein assays (Bernard and Becker, 1988). With the advent of two-dimensional gel electrophoresis (O'Farrell, 1975) and highly sensitive silver staining methods (Merril et al., 1981), it should be possible to separate various nonreabsorbed proteins from the urinary filtrate into lead-specific patterns at an early stage of tubular injury or monitor the low-molecular-weight proteins, such as retinol-binding protein, which is stable at the pH of normal urine (Bernard and Becker, 1988).
Hematologic Effects
Lead workers often show evidence of both marked impairment of heme biosynthesis and increased erythrocyte destruction (EPA, 1986a). Characteristic biochemical and functional indexes of those impairments include increased urinary delta-aminolevulinic acid and erythrocyte zinc protoporphyrin, increased cell fragility, and decreased osmotic resistance, which combine to produce anemia (Baker et al., 1979).
REPRODUCTIVE AND DEVELOPMENTAL EFFECTS
Reproductive and Early Developmental Toxicity
Reproductive toxicity resulting from the high-dose lead exposure is
well established (Rom, 1976). Much of the early literature focused on an increased incidence of spontaneous abortion and stillbirth associated with lead exposure in the workplace (Paul, 1860; Legge, 1901; Oliver, 1911; Lane, 1949). In addition, lead was used as an abortifacient in England (Hall and Cantab, 1905). These outcomes, which are far less common today, presumably involve some combination of gametotoxic, embryotoxic, fetotoxic, and teratogenic effects and define the upper end of the spectrum of reproductive toxicity in humans. Since these earlier reports, industrial exposure of women of childbearing age was restricted by improved industrial hygiene practices, but a recent U.S. Supreme Court decision ruled exclusion illegal. The decision was based on the premise of equal access to the workplace, not on insufficiency of evidence of toxic harm.
Epidemiologic studies of exposed women have reported reproductive effects of lead exposure in both nonoccupational groups (Fahim et al., 1976; Nordstrom et al., 1978a,b) and occupational groups (Panova, 1972). Deficiencies in the design of the studies prevent definitive conclusions, but the studies have helped to direct attention to a potential problem.
Very early preimplantation loss can easily go undetected and might be occurring after moderate-dose and perhaps even low-dose exposure. With the advent of human chorionic gonadotropin assays, it is now possible to detect the onset of pregnancy and early fetal loss during the first 1–2 weeks of pregnancy. Savitz et al. (1989) used data from the National Natality and Fetal Mortality Survey, a probability sample of live births and fetal deaths to married women in 1980, to show that maternal employment in the lead industry was a risk factor for negative pregnancy outcomes, including stillbirth (OR = 1.6) and preterm birth (OR = 2.3). No systematic study has been conducted of the effects of increased lead stores on early fetal loss in women who may have incurred substantial lead exposures during their childhood or during a prior period of employment in a lead-related trade. Such studies are warranted, given the known reproductive toxicity of large exposure to lead.
Several prospective studies have examined the issue of lead's involvement in spontaneous abortion, stillbirth, preterm delivery, and low birthweight. Women in the studies in Boston (Bellinger et al., 1991b), Cleveland (Ernhart et al., 1986), Cincinnati (Bornschein et al., 1989),
and Port Pirie (McMichael et al., 1986; Baghurst et al., 1987a) had average blood lead concentrations during pregnancy of 5–10 µg/dL; almost all had blood lead concentrations less than 25 µg/dL. The Glasgow (Moore et al., 1982) and Titova Mitrovica (Graziano et al., 1989; Murphy et al., 1990) cohorts had average blood lead concentrations of about 20 µg/dL. None of those studies reported an association between maternal blood lead concentrations and spontaneous abortion or stillbirth. However, the Cincinnati and Port Pirie studies found a lead-related decrease in duration of pregnancy, and the Glasgow, Cincinnati, and Boston studies reported a lead-related decrease in birthweight. The Boston study found an increased risk of intrauterine growth retardation, low birthweight, and small-for-gestational-age deliveries at cord blood lead concentrations of 15 µg/dL or more. The Port Pirie study found that the relative risk of preterm delivery increased 2.8-fold for every 10-µg/dL increase in maternal blood lead. In the Cincinnati study, gestational age was reduced about 0.6 weeks for each natural log unit increase in blood lead, or about 1.8 weeks over the entire range of observed blood concentrations. Even after adjustment for the reduced length of pregnancy, the Cincinnati study found reduced infant birthweight (by about 300 g) and birthlength (by about 2.5 cm), and the Port Pirie group reported reduced head circumference (by about 0.3 cm) (Baghurst et al., 1987b). Findings from some of the prospective studies have been extensively reviewed (Davis and Svendsgaard, 1987; Ernhart et al., 1989; Grant and Davis, 1989). However, some striking inconsistencies, yet to be explained, characterize the data on the relationship between prenatal lead exposure and fetal growth and maturation. For instance, in the large cohort (N = 907) of women residing in Kosovo (Factor-Litvak et al., 1991), no associations were seen between midpregnancy blood lead concentrations (ranging up to approximately 55 µg/dL) and either infant birthweight or length of gestation.
Several studies have also looked for evidence of teratogenicity (Needleman et al., 1984; Ernhart et al., 1986; McMichael et al., 1986). Needleman et al. (1984), in a retrospective study of the association between cord blood lead and major or minor malformations in a cohort of 4,354 infants, found a significant increase in the number of minor anomalies observed per child, but no malformation was found to be associated with lead. Unexpectedly, several other factors, such as premature labor and neonatal respiratory distress, were found to be reduced
with increased blood lead. Both Ernhart et al. (1986) and McMichael et al. (1986) tried but failed to replicate these findings; however, these studies lacked the power to detect the small effects reported by Needleman et al. (1984). The Needleman et al. study is important because the minor anomalies in question might reflect general fetal stress and predict developmental disorders (Marden et al., 1964).
Evidence is accumulating that relatively small increases in maternal blood lead during pregnancy can be associated with delayed or retarded growth. Shukla et al. (1987) reported that 260 infants from the Cincinnati prospective lead study experienced retardation in covariate-adjusted growth. More specifically, they found that infants born to women with lead concentrations greater than 8 µg/dL during pregnancy grew at a lower than expected rate if increased lead exposure continued during the first 15 months of life. Conversely, if postnatal lead exposure was small, the infants grew at a higher than expected rate; that suggests a catchup in growth after fetal growth suppression. No lead-related growth effects were observed in infants born to women with blood lead concentrations less than 8 µg/dL. In a later analysis of stature at 33 months of age, Shukla et al. (1991) reported that sustained increases in lead exposure above 20 µg/dL throughout the first 33 months of life are associated with reduced stature. However, prenatal exposure was no longer related to stature at 33 months of age. The reported indication of fetal toxicity is consistent with other previously discussed markers of lead-related fetal toxicity. It is also consistent with cross-sectional studies of lead's relation with physical size.
Several points emerge from a review of those studies, apart from a lead-related retardation of growth itself. First, the specific manifestations of the fetal insult vary among cohorts and might reflect lead's interaction with such cofactors as adequacy of prenatal care, maternal age, ethnicity, and nutritional status. Second, the blood lead concentrations associated with adverse fetal development are low (10–15 µg/dL or even lower) and comparable with those found in a substantial fraction of women of childbearing age (ATSDR, 1988). The validity of the reported association between fetal lead exposure and markers of adverse fetal development is strengthened by the observed negative association between maternal or fetal blood lead concentrations and early physical growth and cognitive development (Bellinger et al., 1987; Dietrich et al., 1987a,b; Vimpani et al., 1989). Thus, the birth-outcome measures,
early physical-growth measures, and early measures of infant development can be viewed as potentially reflecting the fetal toxicity of lead.
Gametotoxicity of lead has been studied primarily in male lead workers. Lancranjan et al. (1975) noted lead-associated disturbances of reproductive competence in lead workers; blood lead concentrations of about 40 µg/dL were associated with asthenospermia and hypospermia, and higher concentrations with teratospermia. Erectile dysfunction was observed in the lead workers, but did not seem dose-dependent. Zielhuis and Wibowo (1976) criticized the design and results of that study, noting potential underestimation of blood lead concentrations.
Wildt et al. (1983) noted that lead-battery workers with blood lead concentrations over 50 µg/dL showed prostatic and seminal vesicular dysfunction compared with controls. However, their study had a number of methodologic problems concerning the measures of dysfunction and exposure monitoring (EPA, 1986a).
More recently, Assennato and co-workers (1986) reported sperm count suppression in lead-battery workers in the absence of endocrine dysfunction. Rodamilans et al. (1988) found that duration of lead exposure of smelter workers was variably associated with endocrine testicular function: workers who had been employed for more than 3 years had decreases in serum testosterone, steroid-binding globulin, and free-testosterone index. In both studies, the mean blood lead concentrations were over 60 µg/dL.
We have already noted longitudinal studies of lead's effects on growth and development in young children. Cross-sectional data are also available from a large population survey. Schwartz et al. (1986) reported that postnatal exposure of U.S. children affects later growth, according to analysis of the large NHANES II data set with respect to height, weight, and chest circumference as a function of blood lead concentration. The three growth milestones in children under 7 years old were significantly and inversely associated with blood lead concentration: height, p < 0.0001; weight, p < 0.001; and chest circumference, p < 0.026. The association was present over the blood lead concentration range of 5–35 µg/dL. These results are consistent with those of Frisancho and Ryan (1991), who found an inverse association between blood lead level and stature in a cohort of 1,454 5–12 year old children in the Hispanic HANES data set, and those of Lauwers and co-workers (1986) in Belgium, who noted statistically significant and inverse associations
among growth indexes and blood lead concentration in children up to the age of 8 years. Nonquantitatively, reduced stature has been seen in children chronically exposed to lead (Johnson and Tenuta, 1979).
Angle and Kuntzelman (1989) in a retrospective pilot study examined 30 children with increased blood lead concentrations (over 30 µg/dL) and erythrocyte protoporphyrin relative to those in a control group. Growth velocity, higher in the high-lead group before 24 months of age, reverted to a net retardation after this age, compared with values in controls. In a longitudinal followup study (Markowitz and Rosen, 1990), lead-poisoned children showed reduced growth velocity, compared with that in age-matched control subjects. Furthermore, impaired growth velocities in the lead-poisoned children did not change substantially from baseline after chelation therapy.
The data on children suggest that endocrinologic disturbances can occur at sensitive points in anthropometric development. Endocrine dysfunction in lead workers with relatively high lead exposure is known (Sandstead et al., 1970; Robins et al., 1983).
Huseman and co-workers (1987) found that height in two lead-poisoned children dropped to below the tenth percentile during intoxication; both subjects demonstrated depressed thyroid-stimulating hormone (TSH) responses to thyrotropin-releasing hormone (TRH), and one showed depression in resting TSH concentrations.
Cognitive and Other Neurobehavioral Effects
Information about the effects of low-level exposure to lead has been obtained principally from two types of epidemiologic studies. One is the cross-sectional or retrospective cohort study, in which children's lead exposure and development are assessed at the same time or in which past lead exposure is estimated. The second type is the prospective longitudinal study, in which children's exposure and development are assessed on multiple occasions. Each type of study has strengths and weaknesses. For clarity, the findings from each type are discussed separately.
Prospective Longitudinal Studies
The findings pertaining to the association between indices of prenatal
lead exposure and early development are mixed. In some cohorts, prenatal exposures corresponding to maternal or cord blood lead concentrations of 10–20 µg/dL were associated with early developmental delays. In the Boston cohort, infants with cord blood lead concentrations between 10 and 25 µg/dL manifested a performance deficit of 4–8 points between 6 and 24 months of age, relative to infants with cord blood lead concentrations below 3 µg/dL (Bellinger et al., 1984a; 1986a,b; 1987). In the Cincinnati cohort, developmental scores at 3 and 6 months of age declined by 6–7 points for each increase of 10 µg/dL in prenatal lead concentrations in the range of 1–27 µg/dL (Dietrich et al., 1987a,b); in addition, 12-month Mental Development Index (MDI) scores were inversely related to infants' blood lead concentrations at 10 days of age. In the Cleveland cohort, increased cord blood lead concentrations were significantly associated with increased numbers of neurologic signs, and increased maternal blood lead concentrations with lower scores on the Bayley Scales and the Kent Infant Development Scale at age 6 months (Ernhart et al., 1986, 1987).
Quite different results were reported from the Australian studies. In the Port Pirie study, developmental assessments were first administered at 2 years of age, at which time MDI scores from the Bayley Scales were not associated with average antenatal, maternal, or cord blood lead concentrations (Baghurst et al., 1987b; Wigg et al., 1988). In the Sydney cohort, neither maternal nor cord blood lead concentration was inversely related to any index of children's development at 6, 12, 24, 36, or 48 months (Cooney et al., 1989a,b). In fact, cord blood lead concentration was positively associated with infants' motor development even after adjustment for covariates. Exposure misclassification is a potential problem in this study. At 12, 18, and 24 months of age, half the children provided capillary (fingerstick) blood samples, and the other half venous blood samples. Given the potential difficulties associated with capillary samples, the mixing of sampling methods at several ages complicates the effort to establish the relative exposures of the children in the cohort. For example, the Sydney group found that at age 3 the average lead concentration in capillary samples was 30% greater than that in venous samples. Mahaffey et al. (1979) noted a similar positive bias of 20% for capillary versus venous samples in the NHANES II data. Although the impact of those differences on exposure assessment in the Sydney cohort is uncertain, the investigators' concern over contamination of the early capillary samples prompted the recruitment of an additional 123 children.
The association between prenatal or perinatal exposure and indexes of overall development was apparent beyond the first year in the Boston cohort (Bellinger et al., 1987) and to a limited extent in the Cincinnati cohort (Dietrich et al., 1989), but not in the Cleveland cohort (Ernhart et al., 1987). In the Boston study, the association between prenatal exposure and children's performance attenuated after 2 years of age. However, children who had high prenatal exposure and high exposure at age 57 months (blood lead concentration greater than 10 µg/dL) ''recovered'' to a smaller extent than did children with high prenatal exposures but lower exposures at age 57 months (Bellinger et al., 1991a). In the Cincinnati cohort, neonatal blood lead concentration (measured at 10 days of age) was inversely associated with performance at age 4 years on all subscales of the Kaufman Assessment Battery for Children (K-ABC), but only among the more disadvantaged children (Dietrich et al., 1991). This association was not evident at 5 years of age on the K-ABC (Dietrich et al., 1992) or at 6.5 years on the Wechsler Intelligence Scale for Children-Revised (Dietrich et al., 1993a). Neonatal blood lead levels were, however, inversely associated with fine motor performance on the Bruininks-Oseretsky Test of Motor Proficiency (Dietrich et al., 1993b).
Several factors could account for the inconsistencies in findings across studies. First, infants might generally be able to compensate for early adversities associated with lead exposure. Second, the adverse impact of the substantial postnatal rise in the exposures of the children in most cohorts might have obscured a persisting effect of prenatal exposure on development. In the Port Pirie study, the absence of an association between antenatal or cord blood lead concentrations and 2-year Bayley scores could reflect the attenuation of an association that would have been detected if assessments had been carried out before age 2. Third, the impact of competing risks for poor development among disadvantaged infants might have overwhelmed a persisting but small effect of prenatal lead exposure. Fourth, the expression of lead insult might be modified over time by the child's social environment. Although an association between prenatal exposure and indexes of overall development might not persist, associations could emerge with respect to more specific aspects of development. For instance, in the Cincinnati cohort, the association between prenatal exposure (maternal blood lead concentration during pregnancy) and performance on the Bayley Scales attenuated by the time the children were 2 years old, but an inverse association
was found between prenatal exposure and children's scores on the Fluharty Speech and Language Screening Test (Dietrich, 1991) at age 30 months. Fifth, the loss in power resulting from cohort attrition might have reduced the probability that a persisting association would be detected.
The latter hypothesis is contradicted, however, by a pattern of increasing consistency in data from the various studies supporting an inverse association between blood lead levels measured in the postnatal period and cognitive function in the late preschool and school-age period. In the Boston cohort, blood lead concentration across a range of 3–20 µg/dL at age 2 years was associated with a decrease of approximately 6 points in children's General Cognitive Index (GCI) scores on the McCarthy Scales at age 57 months (Bellinger et al., 1991a). The coefficients associated with other postnatal blood lead measurements as well as with dentin concentrations were also negative but not statistically significant. The inverse association between cognition and blood lead was still apparent at age 10 years. Children's IQ scores on the Wechsler Intelligence Scale for Children-Revised declined approximately 6 points for each rise of 10 µg/dL in blood lead level at age 2 years, while scores on the Kaufman Test of Educational Achievement declined approximately 9 points (Bellinger et al., 1992). In this cohort, the mean blood lead level at age 2 years was less than 7 µg/dL, with 90% of the values below 14 µg/dL. In the Port Pirie cohort, an increase from 10 to 31 µg/dL in a cumulative index of postnatal blood lead concentrations (particularly concentrations up to age 4 years) was associated with a decrease of approximately 7 points in GCI scores at age 4 years (McMichael et al., 1988) and a decrease of 4–5 points in WISC-R IQ scores at age 7 years (Baghurst et al., 1992). In the data for both studies, no threshold is discernable for the association between increased blood lead level and decreased performance. Moreover, in both studies, children's scores on the Perceptual-Performance subscale of the McCarthy Scales (and the Memory Scale as well in the Port Pirie study) and WISC-R Verbal IQ were most strongly associated with postnatal lead exposure. In the Cincinnati cohort, later postnatal blood lead concentrations, as well as indexes of lifetime blood lead, were weakly associated with children's scores at 4 and 5 years of age on the Simultaneous Processing subscale of the K-ABC, which, like the Perceptual-Performance subscale of the McCarthy Scales, assesses primarily visual-spatial and visual-motor skills
(Dietrich et al., 1991, 1992). Some of these associations were not statistically significant after "full" covariate adjustment, however. Right ear auditory processing skills at age 5 years, assessed by the Filtered Word subtest of the Screening Test for Auditory Processing Disorders, were significantly associated with postnatal blood lead concentrations as well (Dietrich et al., 1992). Scores on the Auditory Figure-Ground subtest were not associated with lead exposure. Assessments of this cohort at age 6 indicated that high postnatal blood lead levels (especially those measured around ages 4 and 5 years) were significantly associated with lower scores on WISC-R IQ (Performance IQ only) (Dietrich et al., 1993a), and various indices of both gross-motor and fine-motor function (Dietrich et al., 1993b).
Preliminary findings from the Yugoslavian prospective study indicate a significant inverse association between blood lead concentration at 2 years of age and concurrent MDI scores, corresponding to a decrease of 2.9 points as blood lead increased from 10 to 30 µg/dL (Wasserman et al., 1991).
The Cleveland study has provided little evidence that postnatal lead exposure is associated with children's development (Ernhart et al., 1989). A significant inverse association between blood lead concentration at age 2 years and IQ scores at age 4 was found, however, if four children identified as influential by regression diagnostics were excluded. The investigators discounted this finding on statistical grounds, however. In the Sydney study, a composite exposure index consisting of blood lead concentrations measured during the first year of life was weakly associated (p = 0.07) with children's adjusted GCI scores on the McCarthy Scales. It appears, however, that the association was positive, rather than negative; i.e., children with greater exposures achieved higher scores (Cooney et al., 1989a,b).
Inconsistencies in the data preclude drawing inferences about modifiers of any association between lead and development. Among children 6 months old and 4 years old in the Cincinnati cohort and children 18 and 24 months old in the Boston cohort, the inverse association between neonatal blood lead concentration and MDI scores was stronger among children below the median social class (Dietrich et al., 1987a,b, 1991; Bellinger et al., 1988). Such interactions have not been observed in all studies, however, or even at other ages within the Boston and Cincinnati cohorts. In addition, the performance of 6-month-old boys in the Cincinnati
cohort was more strongly associated with blood lead concentration than was the performance of 6-month-old girls. To judge by estimates of performance change between 24 and 57 months of age in the Boston cohort, boys recovered more slowly than girls from the adverse effects of higher prenatal exposure. That is consistent with substantial evidence that a wide range of developmental neuropsychiatric disorders are more prevalent among boys than girls (Gualtieri, 1987). In the Port Pirie cohort, however, at ages 2, 4, and 7 years, the performance decrement of girls has consistently been found to be greater than that of boys (McMichael et al., 1992; Baghurst et al., 1992).
It is clear that there are points of both agreement and disagreement in the findings of the prospective studies. A variety of methodologic and substantive explanations can be posited and, at this juncture, it is not yet clear which are correct. In terms of methodologic factors, false positive findings (Type I errors) due to multiple comparisons or to incomplete adjustment for confounding could be responsible for the associations observed in some studies between lead exposure and cognitive development. False negative findings (Type II errors) due to factors such as statistical "over-control" or exposure misclassification may be responsible for the lack of associations reported in some studies.
In terms of substantive explanations, it is possible that the strength of the association, or the likelihood of detecting an association, depends on population characteristics that are not comparable in the various cohorts (e.g., socioeconomic status, medical risk status, lead exposure profiles). One would not necessarily expect all studies to produce the same results, and, indeed, they have not (Mushak, in press). When findings differ, however, the information yield is likely to be the greatest. Each study is likely to contribute only part of the answer to the general question, "Under what exposure conditions do different populations of children manifest a lead-associated impact on growth and development?" It is clear that the complete answer to this question is unlikely to be simply "all" or "none."
In summary, there is relatively little consistency across the set of prospective studies in terms of the association between indices of prenatal lead exposure and later cognitive function. In contrast, as the length of follow-up has increased to include assessments at school-age, striking consistencies are emerging, with all 3 studies (Boston, Cincinnati, Port Pirie) reporting significant inverse associations between blood lead levels
measured in the first few postnatal years and intellectual performance at ages 6 to 10 years.
Table 2-1 summarizes the findings from prospective studies to date with respect to reproductive outcome and early cognitive development. An additional prospective study is being conducted in Mexico City (Rothenberg et al., 1989) but follow-up data at ages older than 30 days have not yet been published.
Cross-sectional and Retrospective Studies
Most of the recent cross-sectional studies of lead and children's cognition have been reviewed by Grant and Davis (1989). Here, an effort is made to identify major themes, including issues on which the data are inconsistent. For reference, basic features of the major studies are listed in Table 2-2.
Because each study has include a global assessment of children's intelligence, this outcome provides the strongest basis for interstudy comparison. To assess the degree to which the results of various studies support a common dose-response relationship between lead exposure and IQ, the mean IQ scores of children in different exposure groups from the various studies are plotted together in Figures 2-1 and 2-2. For studies that report a partial regression coefficient as the measure of association, the best fit line is presented if the authors also provide the intercept or if it can be discerned from a figure in the original report. Information from such studies can contribute to the effort to identify a lowest-observed-effect concentration, if adequate steps are taken to assess the underlying assumption that the lead-IQ association is linear over the range of exposures represented in a cohort. Integrating the findings from separate studies would be facilitated if, in reporting the association between lead and IQ (or any other outcome), investigators provided quantitative measures of effect size, such as regression coefficients and standard errors or adjusted means and standard errors, rather than simply p values, correlations, or percentages of variance (or incremental variance) attributable to, lead. Because of differences in the measurement and interpretation of blood lead and tooth lead, the results of studies relying on these exposure indexes are plotted separately. The nature and extent of adjustment for confounding varies considerably from study to
TABLE 2-1 Prospective Studies
|
Study Site: Key References |
No.a |
Blood Lead Assessment |
Outcome Assessmentb |
Major Findings |
|
Boston: Bellinger et al., |
249 |
Cord low: < 3 µg/dL medium: 6–7 high: 10–25 |
6 mo: BSID 12 mo: BSID 18 mo: BSID |
1. Mental Development Index of BSID inversely related to cord-blood lead group at all ages between 6 and 24 mo of age. |
|
1984a; 1986a,b; |
|
6 mo: |
24 mo: BSID 57 mo: MSCA |
2. The inverse associations strongest for children below median social class. |
|
1987; 1988; 1989b; 1990; 1991; 1991a; |
|
SD = 7.1 12 mo: SD = 6.5 |
10 yr: WISC-R K-TEA |
3. Mental Development Index scores not related to blood lead concentrations measured in first 2 yr of life. |
|
1992 |
|
18 mo: SD = 5.7 24 mo: |
|
4. General Cognitive Index scores at 57 mo inversely related to blood lead concentration measured at 24 mo of age. |
|
|
SD = 6.3 57 mo: SD = 4.1 10 yr: SD = 2.4 |
|
5. General Cognitive Index scores not associated with cord-blood lead group. Among children with high cord-blood lead concurrent blood lead concentration and sociodemographic characteristics associated with extent of recovery or compensation. |
|
|
|
|
|
6. IQ and achievement scores at age 10 yr inversely associated with blood lead measured at 24 mo of age. |
|
Study Site: Key References |
No.a |
Blood Lead Assessment |
Outcome Assessmentb |
Major Findings |
|
Port Pirie: |
723 |
Maternal |
24 mo: BSID |
1. Increased risk of preterm delivery. |
|
|
|
(prenatal): |
48 mo: MSCA |
2. Reduced head circumference at birth. |
|
Baghurst et al, 1987a,b |
|
Cord: 8.3 6 mo: 14.5 |
7 yr: WISC-R |
3. Indexes of prenatal exposure not related to MDI scores at age 2 yr. |
|
Baghurst et al., 1992 |
|
15 mo: 20.9 24 mo: 21.3 |
|
4. Mental Development Index at 24 mo weakly associated with blood lead concentration at 6mo of age. |
|
Wigg et al., 1988 |
|
36 mo: 19.5 48 mo: 16.4 |
|
5. General Cognitive Index scores at age 48 mo inversely related to integrated average of postnatal blood lead concentrations. |
|
McMichael et al., 1988 |
|
Integrated postnatal |
|
|
|
Vimpani et al., 1989 |
|
average to age 4 19.1 |
|
6. IQ at 7 years inversely related to integrated average of postnatal blood lead concentrations. |
|
|
|
Mean lifetime to age 7 19.1 |
|
|
|
Cincinnati: |
305 |
Maternal (prenatal):
|
3 mo: BSID 6 mo: BSID |
1. Low birthweight and reduced duration of gestation. |
|
Dietrich et al., 1987a,b; 1989; 1990; 1991; 1992; 1993a,b |
|
SD = 3.7 Cord: SD = 45 |
12 mo: BSID 24 mo: BSID 39 mo: FSLST 48 mo: K-ABC |
2. Mental Development Index scores at 3 and 6 mo inversely related to prenatal and postnatal blood lead concentrations. |
|
Study Site: Key References |
No.a |
Blood Lead Assessment |
Outcome Assessmentb |
Major Findings |
|
Cincinnati |
|
Neonatal (10 day):
SD = 2.8 |
60 mo: K-ABC SCAN 78 mo: |
3. Mental Development Index at 12 mo inversely associated (indirectly, via birth -weight) with prenatal blood lead concentration. |
|
Dietrich, 1991; 1992 |
|
3 mo: SD = 3.4 |
WISC-R 72 mo: |
4. Mental Development Index at 24 mo positively associated with prenatal blood lead concentration. |
|
Shukla et al., 1989; 1991 |
|
Maximum first yr:
SD = 8.2 |
BOTMP |
5. Mental Development Index scores at 3, 6, 12, and 24 mo not associated with postnatal blood lead concentrations. |
|
|
|
Maximum second yr:
SD = 11.4 |
|
6. Retarded growth in stature. |
|
|
|
24 mo: SD = 9.2 |
|
7. FSLST scores at 39 mo inversely related to prenatal blood lead concentration. |
|
|
|
Mean of 3rd yr: 16.3, SD = 7.8 |
|
8. K-ABC scores at age 4 yr inversely related only to neonatal (10-day) blood lead concentration (poorest children only). |
|
|
|
Mean of 4th yr: 14.1, SD = 7.3 |
|
9. Poorer central auditory processing abilities associated with higher postnatal blood lead concentrations. |
|
|
|
Mean of 5th yr: 11.9, SD = 6.4 |
|
10. K-ABC scores at age 5 yr (simultaneous processing) inversely associated significantly only with mean blood lead concentration in fourth year of life. |
|
|
|
|
|
11. WISC-R performance IQ and BOTMP scores inversely associated with postnatal lead exposure. |
|
Study Site: Key References |
No.a |
Blood Lead Assessment |
Outcome Assessmentb |
Major Findings |
|
Cleveland |
359 |
Maternal (prenatal):
|
Neonatal: NBAS |
1. Neurological soft signs score on GRBE associated with cord-blood lead concentration. |
|
Ernhart et al., 1986; 1987; 1989 |
|
SD = 1.9 Cord: 6 mo: |
Neonatal: GRBE 6 mo: BSID |
2. Mental Development Index, Psychomotor Development Index, and KID scores at 6 mo inversely related to maternal blood lead concentration during pregnancy. |
|
Ernhart and Morrow -Tlucak, 1987 |
|
SD = 3.3 2 yr: SD = 6.5 |
6 mo: KID 12 mo: BSID, SICD |
3. No other associations between either prenatal or postnatal blood lead concentrations and scores or growth indexes. |
|
Morrow-Tlucak and Ernhart, 1987 |
|
3 yr: SD = 5.9 SD = 2.0 |
24 mo: BSID, SICD 3 yr: SB, SICD |
|
|
Ernhart and Greene, 1990 |
|
|
4–10 yr: WPSSI |
|
|
Greene and Ernhart, 1991 |
|
|
|
|
|
Sydney: |
318 |
Maternal delivery:
|
6 mo: BSID 12 mo: BSID |
No association between children's scores and any index of lead exposure. |
|
Cooney et al., 1989a,b |
|
Cord: 6 mo: 12 mo: 18 mo: 24 mo: 30 mo: |
24 mo: BSID 36 mo: MSCA 48 mo: MSCA |
|
|
Study Site: Key References |
No.a |
Blood Lead Assessment |
Outcome Assessmentb |
Major Findings |
|
Sydney |
|
36 mo: 42 mo: 48 mo: Multiplicative factors |
|
|
|
Kosovo, Yugoslavia: |
541 |
Maternal |
|
|
|
Graciano et al., 1990 |
|
|
|
|
|
Wasserman et al., 1992 |
|
|
|
|
|
NOTES: a Numbers of children recruited into cohort. Numbers included in specific analyses vary with cohort attrition and patterns of missing data. b BSID: Bayley Scales of Infant Development MSCA: McCarthy Scales of Children's Abilities NBAS: Neonatal Behavior Assessment Scale GRBE: Gram-Rosenblith Behavioral Examination SICD: Sequenced Inventory of Communication Development KID: Kent Infant Development Scale SB: Stanford-Binet Intelligence Scale WISC-R: Wechsler Intelligence Scale for Children-Revised WPSSI: Wechsler Preschool and Primary Scales of Intelligence FSLST: Fluarty Speech and Language Screening Test K-ABC: Kaufman Assessment Battery for Children SCAN: Screening Test for Auditory Processing Disorders SICD: Sequenced Inventory of Communication Development WISC-R: Wechsler Intelligence Scale for Children-Revised K-TEA: Kaufman Test of Educational Achievement BOTMP: Bruininks-Oseretsky Test of Motor Proficiency |
||||
TABLE 2-2 Major Cross-Sectional Studies of Low-Dose Exposure
|
Study |
No. |
Exposure Index |
IQ Measurement |
Age at Assessment |
Potential Confounders Considered |
|
Needleman et al., 1979 |
158 |
Toot lead |
WISC-R |
7.4 yr |
Mother's age at child's birth, maternal education, father's social class, number of pregnancies, parental IQ |
|
Winneke et al., 1982 |
52 |
Toot lead |
German WISC |
8.5 yr |
Exposure groups matched for age, sex, father's occupational, status |
|
Winneke et al., 1983 |
115 |
Tooth lead |
German WISC |
9.4 yr |
Age, sex,duration of labor, socio-hereditary background (composite of school type and occupational status of parents) |
|
Smith et al., 1983; Pocock et al., 1987 |
402 |
Tooth lead |
WISCR-R |
6 yr |
Maternal IQ, quality of marital relationship, family characteristics, parental interest, family size, social class, birthweight, length of hospital stay after birth, sex |
|
Fergusson et al., 1988 |
724 |
Tooth lead |
WISC-R |
8 yr |
Maternal education, paternal education, birthweight sex, standard of living, maternal emotional responsiveness, maternal avoidance of punishment, number of weatherboard homes resided in |
|
Study |
No. |
Exposure Index |
IQ Measurement |
Age at Assessment |
Potential Confounders Considered |
|
Hansen et al., 1989a,b |
162 |
Tooth lead |
WISC-R |
First grade |
Number of sibship (birth order), maternal education, maternal age, whether child came home from hospital after mother, jaundice, father's socioeconomic status |
|
Bergomi et al., 1989 |
237 |
Tooth lead |
WISC-R |
7.7 yr |
Age, SES, sex |
|
Yule et al., 1981 |
166 |
Blood lead |
WISC-R |
6–12 yr |
Age, social class |
|
Lansdown et al., 1986 |
194 |
Blood lead |
WISC-R |
x = 9.1 yr |
Age, social class |
|
Fulton et al., 1987 |
501 |
Blood lead |
BAS |
6–9 yr |
Parents' vocabulary and matrices scores, child's interest score, age father's education, length of gestation, parental involvement in school, class year, days absent from school, height, car and telephone ownership, whether father is unemployed, sex |
|
Hawk et al., 1986 |
75 |
Blood lead |
SBIT-R |
3–7 yr |
Maternal IQ, H.O.M.E. (measure of home rearing environment), gender |
|
Silva et al., 1988 |
579 |
Blood lead |
WISC-R |
11 yr |
None (no multivariate analysis because blood lead-IQ association not significant in bivariate analyses) |
|
Study |
No. |
Exposure Index |
IQ Measurement |
Age at Assessment |
Potential Confounders Considered |
|
Hatzakis et al., 1989 |
509 |
Blood lead |
WISC-R |
Primary |
Parental IQ, birth order, age, family size, father's age, father's education, occupation, mother's education, alcoholic mother, bilingualism, birthweight, length of hospital stay after birth, walking age, history of CNS disease, history of head trauma, illness affecting sensory function, parental divorce |
study. The legends provide additional information to aid the reader in evaluating this issue.
Figure 2-1 displays the IQ scores of children classified by blood lead concentration, which serves as an index of earlier and current lead exposures. Within each cohort, children with lower mean blood lead concentrations scored higher than children with higher mean concentrations, and the decline with increasing exposure was roughly monotonic. The differences among cohorts in overall performance (i.e., height on the ordinate) are substantial, but not surprising, in view of the many differences among studies, including the IQ test used and its appropriateness to the population sampled, the sociodemographic characteristics of the cohort, and the total body lead burden of the children.
Figure 2-2 displays the IQ scores of children in studies that relied on tooth lead as the exposure index. Within each study, children's scores tended to decline with increasing tooth lead. The consistency in the findings is all the more surprising, in view of interstudy differences in the portion of tooth anatomy sampled for analysis (e.g., whole tooth, crown, circumpulpal dentin, primary dentin) as well as in the type and location of teeth obtained for analysis (Smith et al., 1983; Grandjean et al., 1986; Purchase and Fergusson, 1986; Paterson et al., 1988) and, in many cases, the absence of interlaboratory quality-assurance and quality-control procedures.
To a large extent, evaluation of whether a study provides evidence of an association between lead and IQ has traditionally been based on whether the p value related to the association is less than 0.05. A more efficient use of the information from different studies is to assess the consistency in the magnitude of effect sizes. Studies in which the p value was greater than 0.05 can provide evidence that supports the association, if they all report similar effect sizes. The p value associated with an individual study depends on many factors, including sample size, the dispersion of values for exposure and outcome within a cohort, and the precision achieved in measuring exposure, outcome, and covariables. The p value associated with the result of a single study is somewhat less important when the studies are viewed in aggregate.
A meta-analysis of 24 recent comparable studies of the association between lead and IQ (including most of the studies in Figures 2-1 and 2-2) indicated that, under the null hypothesis of no association between lead concentration and IQ, the overall pattern of reported associations is
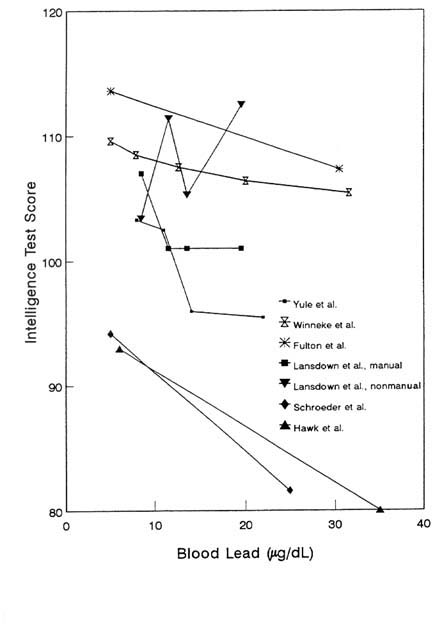
FIGURE 2-1 Blood lead concentrations vs. intelligence test scores. Data from cross-sectional and retrospective cohort studies that relied on blood lead concentration as index of children's exposure. For study to be included, investigators had to present either mean IQ scores for children by blood lead strata or sufficient information about regression of IQ on blood lead to specify regression line (i.e., coefficient for blood lead and intercept of regression line or figure from which they could be determined). Except where noted, scores are adjusted for confounding although control variables vary among studies. Source of information provided for each study depicted is as follows: Yule et al. (1981): mean full-scale WISC-R IQ scores for children in blood lead quartiles. Winneke et al. (1990): WISC scores based on four subscales: Vocabulary, Comprehension, Picture Completion, Block Design; apparently not adjusted for confounding. The group with the highest blood lead levels (mean of 50.1) achieved a mean IQ score of 104.3 (not shown). Fulton et al. (1987): regression of British Ability Scales combined score on blood lead. Based on analysis conducted by Grant and Davis (1989). Lansdown et al. (1986): mean WISC-R IQ scores not presented for complete cohort, only for children stratified by parental occupation (manual vs. nonmanual); apart from stratification on parental occupation, scores are not adjusted for confounding. Hawk et al. (1986): regression of Stanford-Binet IQ scores on blood lead concentration; chunk test evaluating contribution of control and interaction terms was not significant. Schroeder et al. (1985): regression of Stanford-Binet IQ Scores on blood lead concentrations; data represent apparently unadjusted regression of IQ on contemporary blood lead concentration among 6- to 12-year-olds; data selected for inclusion because they are most similar to those from traditional cross-sectional study. Although the model in which blood lead was the only predictor yielded the most precise estimate of the slope of the blood lead-IQ relationship, the slope was reduced from -0.4456 to -0.255 in the model that achieved optimal precision and validity. Source: Adapted from Bellinger and Needleman, 1992.
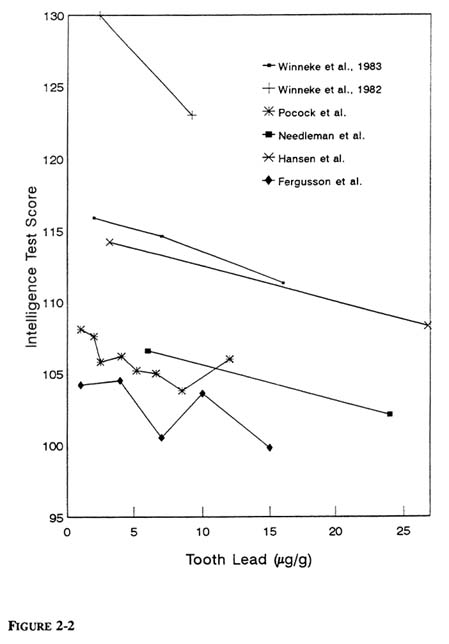
FIGURE 2-2 Tooth lead concentrations vs. intelligence-test scores. Data from retrospective cohort studies that relied on concentration of lead in some portion of tooth as index of children's exposure. For study to be included, investigators had to present either mean IQ scores for children by tooth lead strata or sufficient information about regression of IQ on tooth lead to specify regression line (i.e., the coefficient for tooth lead and intercept of regression line or figure from which they could be determined). Except where noted, scores are adjusted for confounding although control variables vary among studies. Source of information provided for each study depicted is as follows: Winneke et al. (1983): verbal IQ scores from German adaptation of WISC; full-scale IQ scores for children in different tooth lead strata were not provided. Winneke et al. (1982): full-scale IQ scores German adaptation of WISC for matched groups. Pocock et al. (1987): WISC-R scores. Needleman et al. (1979): full-scale WISC-R scores. Hansen et al. (1989): full-scale scores on Danish adaptation of WISC for matched groups. Fergusson et al. (1988): unadjusted full-scale WISC-R scores at age 8 yr; tooth lead values displayed are midpoints of ranges; no range is provided for highest tooth lead strata (12+), so 15 µg/g was chosen; identical pattern was evident in WISC-R scores at 9 yr of age (not shown); adjusted scores were not provided. Source: Adapted from Bellinger and Needleham, 1992.
highly unlikely to have occurred by chance (Needleman and Gatsonis, 1990). Two methods were used to calculate a joint p value: Fisher's method for aggregating p values and Mosteller and Bush's method for calculating a weighted sum of t values. For studies relying on blood lead as the index of children's exposure, both methods yielded a joint p value less than 0.0001. For studies based on tooth lead, the p values were 0.0005 and 0.004. Pooled data from the eight WHO/CEC (World Health Organization and Commission of the European Communities) studies (conducted in Aarhus, Athens, Bucharest, Budapest, Modena, Sofia, Zagreb, and Dusseldorf) resulted in a common regression coefficient of -0.53 (p < 0.1, one-tailed) (Ewers et al., 1989; Winneke et al., 1990).
Despite interstudy differences in the ranges of blood lead represented in a cohort, most studies report a 2- to 4-point IQ deficit for each increase of 10–15 µg/dL in blood lead within the range of 5–35 µg/dL. A threshold for that effect of lead is not evident from the reported studies. It is important to note that the effect sizes estimated on the basis of
prospective longitudinal studies and cross-sectional studies are essentially identical. In a recent meta-analysis of studies reporting on cognitive function at school-age, Schwartz (1992a, in press (a)) calculated the IQ decline over the blood lead range of 10 to 20 µg/dL to be 2.32 points (standard error of 1.27) for longitudinal studies and 2.69 points (standard error of 1.28) for cross-sectional studies. The public-health significance of such an effect size has stimulated spirited debate.
The public health significance of an effect size of this magnitude has stimulated spirited debate. Three issues warrant consideration. First, SEM is a concept that pertains to the performance of an individual, not a group. Specifically, it defines the range, centered around a subject's observed score, within which his or her true score likely to lie. Thus, SEM is not germane in interpreting the importance of group differences in mean score. Second, a property of statistical distributions is that a small difference in mean score between two groups results in substantial differences in frequency of extreme values between the two distributions. The distributional implications of small changes in population mean score have been confirmed by analyses of several lead-study data sets (Needleman et al., 1982; Bellinger et al., 1989a; Davis, 1990). Third, small differences in IQ have been associated with differences in measures of socioeconomic success, such as wages and educational attainment (Griliches, 1977).
Although the search for markers of increased vulnerability has been carried out only with post hoc analyses, results of several studies suggest that children in lower social strata (or whose parents have manual occupations) express an IQ deficit at lower exposures than do children in higher social strata (Harvey et al., 1984; Winneke and Kraemer, 1984; Rabinowitz et al., 1991). Sociodemographic differences are thought to account for the discrepancy between the results of the first (Yule et al., 1981) and second (Lansdown et al., 1986) London studies. Not all studies report socioeconomic differences in vulnerability, however. In one study, the association between increased tooth lead and lower IQ was more prominent among boys than girls (Pocock et al., 1987). The findings should be viewed as preliminary, but they are consistent with patterns reported in two of the prospective studies (Dietrich et al., 1987a; Bellinger et al., 1990). In two recent studies, however, the inverse association between lead level and cognitive performance was stronger among girls than boys (Rabinowitz et al., 1991; Leviton et al., 1993), leaving the issue of sex differences in vulnerability unresolved.
Several obstacles impede efforts to discern whether specific neuropsychologic
deficits are associated with higher lead exposures. First, differences among investigators in interests and experience, as well as national differences in assessment strategies and approaches, have contributed to interstudy differences in the instruments used and the ages at which children were assessed. Second, within an individual study, the instruments used to assess function in different cognitive domains vary in reliability and sensitivity. If children with different exposures perform differently on test A but not on test B, it might be difficult to determine whether the contrast is attributable to lead-associated effects on the skills underlying test A but not those underlying test B or to the superiority of test A in psychometric properties. Third, the specific manifestations of lead's cognitive toxicity might vary with characteristics of a cohort, such as socioeconomic status or other markers of the types of developmental support available to children. For instance, children in lower social strata often begin to manifest language deficits in the second year of life that are attributed to a relative lack of environmental support for the types of linguistic skills assessed in standardized tests. The increased vulnerability of verbal function in such children might make this aspect of cognition most sensitive to toxic exposure. In children from higher social strata, where greater emphasis might be placed on the development of primarily verbal academic skills, this aspect of cognitive function could be more protected. Toxicity might be expressed in other ways, such as visual-spatial or visual-motor integration. Fourth, differences across studies in the cognitive domains found to be associated with lead might reflect differences in the exposure histories of the children in various cohorts and to differences in the exposure index used. Some cognitive functions might be more strongly associated with exposure within the first 2 years of life, and others with later exposure (Shaheen, 1984). For still other functions, the important contrast could be between cumulative and acute exposure (e.g., Winneke et al., 1987, 1988).
As noted in the discussion of the impact of lead on IQ, the importance of p values should not be magnified in assessing the consistency across studies in the association of specific cognitive functions with lead. Numerous studies showing similar effect sizes, some of which might not be statistically significant, are more persuasive than a set of studies showing discrepant effect sizes with similar p values.
There is relatively little consistency across studies in terms of whether verbal IQ or performance IQ is more strongly associated with lead exposure. Some studies report stronger associations for verbal IQ or surrogate scores (Needleman et al., 1979; Ernhart et al., 1981; Yule et
al., 1981; Bergomi et al., 1989; Hansen et al., 1989a,b), and others for performance IQ (Marecek et al., 1983; Shapiro and Marecek, 1984). In some studies, size of exposure was significantly associated with both scales (Hatzakis et al., 1989), and in others, with neither scale (Smith et al., 1983; Winneke et al., 1983; Lansdown et al., 1986 Fergusson et al., 1988; Silva et al., 1988). Similar inconsistency has been reported in the results of IQ testing conducted at school-age in the prospective studies (Baghurst et al., 1992; Bellinger et al., 1992; Dietrich et al., 1993a). Several studies have noted significantly lower reading scores (primarily word-reading) among children with larger exposures (Yule et al., 1981; Fulton et al., 1987; Fergusson et al., 1988; Needleman et al., 1990); other studies have noted similar, but nonsignificant, trends (Ernhart et al., 1981; Smith et al., 1983; Silva et al., 1988). Spelling deficits have also been reported (Yule et al., 1981; Fergusson et al., 1988). Some studies report significant associations between lead exposure and mathematical skills (Fulton et al., 1987; Fergusson et al., 1988); others do not (Yule et al., Smith et al., 1983; Lansdown et al., 1986).
In several studies, children with larger lead exposure did poorly on assessments of visual-spatial or visual-motor skills, with deficits apparent on figure reproduction, visual retention, mazes, eye-hand coordination, and construction tasks (Winneke et al., 1988; McBride et al., 1982; Bellinger et al., 1991a; Hansen et al., 1989a,b). Analysis of the pooled data from the WHO/CEC studies (Ewers et al., 1989) indicated a significant positive association between errors on the Bender-Gestalt Test (German scoring system) and blood lead concentration, particularly on the more difficult trials when perceptual distractions were introduced.
A more consistent finding across studies is an inverse relationship between children's lead concentration and the adequacy of their performance on simple and especially choice reaction-time tasks (Needleman et al., 1979, 1990; Winneke et al., 1983, 1989, 1990; Hunter et al., 1985; Hatzakis et al., 1989; Raab et al., 1990). In the WHO/CEC studies, blood lead concentration was positively associated with errors and negatively associated with hits on a serial-choice reaction-time task (Ewers et al., 1989). Lead exposure was not significantly associated with performance on a delayed-reaction-time task in this set of studies. Larger exposure has also been linked to poorer performance on tests such as the Toulouse-Pieron cancellation test (Bergomi et al., 1989), the
Trail-Making Test, Stroop Test, the Talland Letter Cancellation Test, and the Wisconsin Card Sorting Test (Bellinger et al., in press). If the assumption that such tasks assess children's attention skills is correct, these data are consistent with other findings, based on teachers' ratings, that children with larger exposures are less attentive in the classroom (Needleman et al., 1979); Yule et al., 1984; Hatzakis et al., 1987; Silva et al., 1988; Thomson et al., 1989). Blood lead concentration was not significantly associated with teachers' ratings of classroom behavior in the WHO/CEC studies, however (Ewers et al., 1989). Except for the finding of Hansen et al. (1989a,b) of greater off-task behavior during the continuous-performance task, direct observations of children have not demonstrated behavioral differences between groups of children with varied magnitudes of lead exposure (Bellinger et al., 1984b; Harvey et al., 1984).
Byers and Lord (1943) reported that 19 of 20 children with asymptomatic lead poisoning failed to achieve adequate progress in school, despite IQs in the normal range. Their difficulties were attributed to behavioral dysfunctions, such as distractibility and impulsivity. Although those observations are generally credited with originating studies on so-called subclinical effects of lead exposure, relatively few of the more recent studies have examined performance in school as an outcome variable, apart from collecting teachers' ratings of children's classroom behavior.
The limited data available are generally consistent with the hypothesis that children with greater lead burdens not only perform worse on laboratory and psychometric tests of cognitive function, but also are more frequently classified as learning-disabled and make slower progress through the grades. For instance, in a followup study of a subset of 141 children in the cohort originally identified by Needleman et al. (1979), dentin lead concentrations greater than 20 parts per million (ppm) were associated with increased rates of referral for remedial academic help and with grade retention during the late elementary-school years (Bellinger et al., 1984b). In a cross-sectional study of 200 second-grade Scandinavian children, the risk (adjusted odds ratio) for learning disability among children with circumpulpal-dentin lead concentrations greater than 16 ppm was 4.3 (the reference was the rate among children with concentrations less than 5 ppm) (Lyngbye et al., 1990). Including children with a variety of medical risk factors reduced the odds ratio, but
the risk of learning disability among children with high dentin lead remained double the reference risk.
An assessment of the prevalence of lead-associated learning disabilities over a much longer followup interval was reported by Needleman et al. (1990). At age 18–19 years, children with high dentin lead concentrations had significantly higher rates of reading disability (at least two grades below expected) and failure to graduate from high school; the adjusted odds ratios were 5.8 and 7.4, respectively, when the prevalence among children with dentin lead concentrations below 10 ppm was used as the reference.
In children, early neurobehavioral and other developmental effects have been reported at blood lead concentrations of 10 µg/dL or even lower (and equivalent concentrations in other tissues). Figure 2-3 shows a nonparametric smoothed curve of full-scale IQ versus dentin lead concentration with covariates controlled for. The figure comes from a reanalysis of the data of Needleman et al. (1979) by Schwartz (in press). The analyses of Needleman and co-workers have recently been criticized. It has been suggested that their finding of a significant association between full-scale IQ and dentin lead followed from three critical choices: their exclusion of subjects with characteristics that they felt might be strongly related to the outcome (such as hospitalization for head injuries or residence in non-English-speaking homes), their use of external age adjustment rather than direct control for age in the regression, and their method for assigning subjects to lead-exposure groups. The reanalysis addressed recent criticisms of the original analysis by
-
Including all the subjects, instead of using the exclusion criteria of Needleman and co-workers.
-
Controlling directly for age in the regression model, instead of using indirect standardization.
-
Using the mean dentin lead in each child as the exposure index, instead of a set of categorization rules that discarded discordant values.
The reanalysis also controlled for additional covariates. Dentin lead concentrations were found to be more highly significantly related to full-scale IQ than in the original analysis. Figure 2-3 indicates that the covariate-adjusted association continued to the lowest dentin lead concentration found in the sample, 1 ppm. Although that cannot speak to
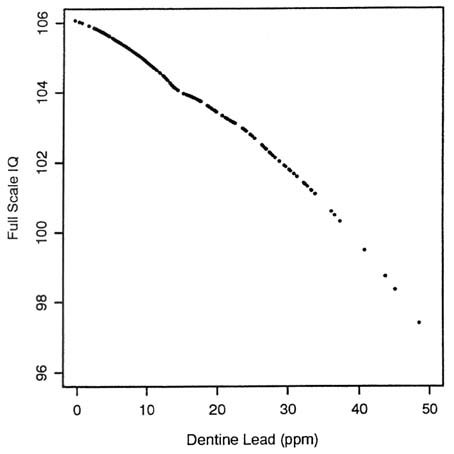
FIGURE 2-3 Nonparametric smoothed plot of full-scale IQ vs. dentin lead from Needleman et al. (1979). After controlling by regression for age, maternal IQ, maternal age, mother's and father's education, mother's and father's SES, number of siblings and hospitalization for lead poisoning. None of subjects excluded in original analysis were excluded from reanalysis. Source: Schwartz, in press. Reprinted with permission from NeuroToxicology; copyright 1992, Intox Press.
effects at lower concentrations, the rarity of concentrations below 1 ppm in industrial societies suggests a lack of an effective threshold. Schwartz
(in press) presents a plot that indicates that smoothing did not distort the relationship. Schwartz also reports a reanalysis of the data of Bellinger et al. (1991a). The Schwartz reanalysis—in addition to addressing the question of the impact of the exclusions, age-control method, and definition of exposure in the original paper of Needleman et al. (1979)—also went to some lengths to examine the sensitivity of the conclusions to those and other factors. The regression coefficients and standard errors for the baseline model (which controlled for age, used mean dentin lead as the exposure index, and used no exclusionary rules) were compared with those for a number of different models. Some additional covariates were included, and some of the original Needleman exclusionary rules were used.
The association between dentin lead and full-scale IQ was insensitive to those changes. To ensure that the association was not driven by a few influential observations, Schwartz used M estimation, a technique that assigns lower weight to points that are far from the predicted regression line to reduce the possible influence of a few anomalous points. Boot-strapping, which calculates mean regression coefficients and confidence intervals by repeatedly resampling observations from the original data set, was also used; it yields inferences that are less sensitive to any assumptions about the distributional properties of the variables and parameters. Both techniques gave results essentially identical with those of the baseline model. Robust variance estimates also yielded the same results. The nonparametric smooth curve mentioned above (Figure 2-3) also indicates that the relationship is not driven by a few selected observations.
Schwartz also examined the association between dentin lead and other outcomes. Those outcomes might be expected to covary with IQ, and an association with them further indicates that the IQ results are not anomalous in these data. In models that control for all the covariates used in the IQ regressions, dentin lead was associated with Teacher Rating Score, with Piaget Mathematics and Reading Scores, with Seshore Rhythm, and with other neurodevelopmental outcomes. The findings suggest that the association between dentin lead and intellectual development in the data of Needleman and co-workers is strong and robust. Bellinger et al. had reported that 20-month blood lead concentrations were associated with 57-month McCarthy General Cognitive Index in their prospective study of lead exposure. Using least-square means, they
showed that children with blood lead of 3–9 µg/dL had significantly lower McCarthy scores than children with blood lead below 3 µg/dL. Figure 2-4 shows a covariate-adjusted nonparametric smoothing of the McCarthy Scores for those children versus blood lead concentrations from Schwartz's reanalysis. A continuous dose-dependent decline is seen to start at 1 µg/dL. Figure 2-5 shows the covariate-adjusted Bayley MDI scores at age 18 months for three categories of cord lead concentration, as reported from the Boston study. More recently, Schwartz (in press), using hockeystick regression, has demonstrated a threshold estimate below 1 µg/dL in the relationship between McCarthy Global Cognitive Index and blood lead in these data. Those studies support the general conclusion that there is growing evidence that there is no effective threshold for some of the adverse effects of lead.
Another neurobehavioral end point is evident in Figure 2-6, which shows the percent of children with hearing levels worse than the reference
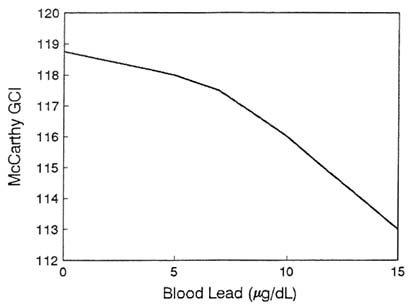
FIGURE 2-4 Nonparametric smoothed plot of McCarthy Global Cognitive Index vs. blood lead at 24 months of age in Boston prospective lead study (Bellinger et al., 1991a). Source: Schwartz, in press. Reprinted with permission from NeuroToxicology; copyright 1992, Intox Press.
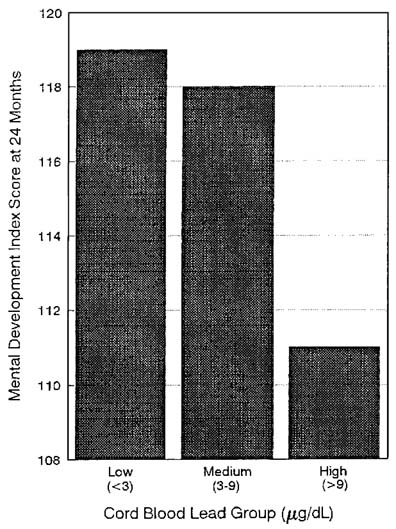
FIGURE 2-5 Mean Bayley Mental Development Index in children aged 24 months, by umbilical cord lead group, after adjustment for covariates, from study of Bellinger and co-workers (1987a,b). Source: Adapted from Schwartz, in press. Reprinted with permission from NeuroToxicology; copyright 1992, Intox Press.
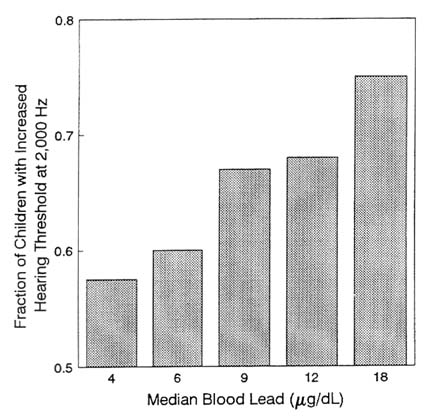
FIGURE 2-6 Fraction of children with hearing worse than reference level of quintiles of blood lead concentration, after adjustment for covariates. Data from Schwartz and Otto (1991). Source: Adapted from Schwartz, in press. Reprinted with permission from NeuroToxicology; copyright 1992, Intox Press.
level, by quintiles of blood lead concentration, after adjustment for covariates. The data are from the Hispanic Health and Nutrition Examination Survey, as reported by Schwartz and Otto (1991). The effects clearly continue to well below 10 µg/dL.
Considerable interest has focused on the persistence of the cognitive deficits seen in lead. The longest followup study, published recently by Needleman and co-workers (1990), showed that some deficits persisted and showed a dose-dependent relationship with lead exposure. Figure 2-7
shows the fraction of children with reading disability, by quartile of dentin lead concentration, after adjustment for covariates.
In the paper of Schwartz and co-workers (1986), children's stature was associated with blood lead concentrations. A hockeystick regression analysis found no evidence of a threshold down to the lowest blood lead concentration in the data (2 µg/dL). At lower ages, Shukla and colleagues (1987, 1989) found an association between integrated postnatal blood lead and child's stature at 33 months. Figure 2-8 shows that relationship, after adjustment for covariates.
Neurotoxic effects of lead in addition to effects on cognition and other neurobehavioral measures in children have been documented in both the central nervous system and the peripheral nervous system (PNS) of both adults and children.
In both lead workers and lead-exposed children, one noninvasive,
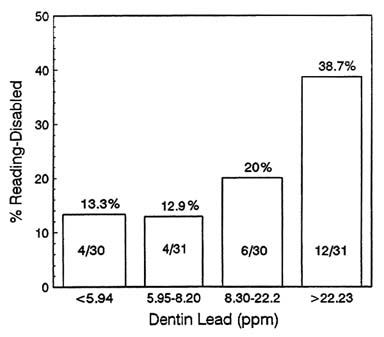
FIGURE 2-7 Percentage of children with reading disability (defined as two or more grades below expected) by quartiles of dentin lead concentration after adjustment for covariates. Data from long-term followup of Needleman and co-workers (1990). Source: Schwartz, in press. Reprinted with permission from NeuroToxicology; copyright 1992, Intox Press.
useful measure of PNS injury is the reduction of conduction velocity in some sensory and motor nerves. By and large, lead workers show impairment of nerve conduction velocity at relatively higher concentrations of blood lead than those associated with either childhood lead neurotoxicity or that related to other toxic end points in adults. Nerve conduction-velocity impairment appears not to be a particularly sensitive measure of neurotoxicity in adults, as it is a measure that reflects advanced manifestation of demyelinating injury involving Schwann cells.
Effects of lead on peripheral nerve function in children are also known, although not as well studied as in adults. Studies of inner-city children (Feldman et al., 1973a,b; 1977) and children residing in smelter communities (Landrigan et al., 1976; Englert, 1978; Winneke et al., 1984; Schwartz et al., 1988) have been reported. Multiple statistical analyses (Schwartz et al., 1988) of nerve conduction-velocity data obtained from a group of asymptomatic smelter-community children described earlier (Landrigan et al., 1976) demonstrated a threshold in children for nerve conduction-velocity reduction of blood lead concentration ranging from 20 to 30 µg/dL, depending on the statistical analysis. One complication with nerve conduction velocity as a toxicity measure is that a dose-dependent biphasic response can be identified, i.e., a U-shaped dose-effect curve across studies and across a broad range of blood lead concentration (e.g., Schwartz et al., 1988; Winneke et al., 1989).
Various assessments of neurophysiologic end points have involved various evoked-potential testing, particularly by Otto (Benignus et al., 1981; Robinson et al., 1985; Otto, 1989). The salient points of the various studies are as follows:
-
Various evoked-potential tests are measures of CNS perturbations in young children, even though some inconsistencies across time and stage of neurologic development suggest that multiple mechanisms are involved.
-
Linear dose-effect data were reported in connection with conditioned slow-wave voltage changes in children, brain-stem evoked-potential latencies, pattern-reversal evoked-potential (PREP) latencies, and PREP amplitude.
-
Such measures seem to be minimally affected by social and cultural factors that complicate psychometric studies.
-
Although much of the evoked-potential information examined by Otto and others might not have clear clinical connections, except for that
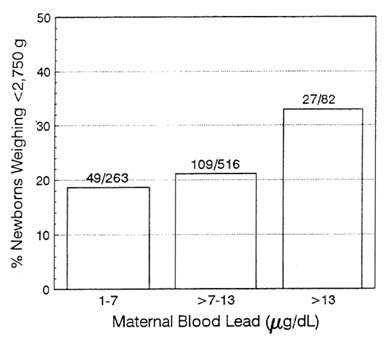
FIGURE 2-8 Percentage of newborns weighing less than 2,750 g by maternal blood lead category, after adjustment for covariates. Data from Dietrich (1991). Source: Schwartz, in press. Reprinted with permission from NeuroToxicology; copyright 1992, Intox Press.
-
linked to hearing impairment (Schwartz and Otto, 1987), any CNS perturbations that occur in developing children should be regarded with the utmost concern.
CARDIOVASCULAR EFFECTS
Hypertension and Pregnancy
Ever since Schedoff and Porockjakoff drew attention to the association of high blood pressure and eclampsia in 1884, there has been increasing interest in this relationship. In normal pregnancy, despite the 30–40% increase in blood volume and cardiac output, arterial pressure falls,
because of a decrease in peripheral vascular resistance. A fall early in gestation lasts until around weeks 18–24 of pregnancy. Between weeks 24 and 26, blood pressure increases to a plateau; it then stays steady until delivery. Women who do not follow that usual pattern and are hypertensive during pregnancy have a higher risk of adverse pregnancy outcome. The reported prevalence of hypertension ranges from 3.5% to 23.6% (Underwood et al., 1967; Russell et al., 1968; Naidoo and Moodley, 1980; Huisman and Aarnoudse, 1986); the lowest of those figures is for a population mostly of Caucasians, and the highest is for a group of nulliparous women at risk of poor pregnancy outcome. The estimates of prevalence have suffered from lack of uniform definition and of standardized measurements of blood pressure.
Hypertension is the disease most often associated with fetal growth retardation (IOM, 1985). Low and Galbraith (1974) attributed 27% of intrauterine growth retardation with an identifiable cause to severe pre-eclampsia, chronic hypertensive vascular disease, or chronic renal disease. Breart et al. (1982) found that intrauterine growth retardation occurred in only 3% of births when the diastolic blood pressure was less than 90 mm Hg, 6% of births at 90–110 mm Hg, and 16% of births when it was higher than 110 mm Hg.
Lead readily crosses the placental barrier during the entire gestational period, and its uptake appears to be cumulative until birth (ATSDR, 1988). McMichael et al. (1986) showed that preterm deliveries and reduction in birthweight were significantly related to maternal blood lead at delivery. Those findings have been documented in other studies in the United States and other countries. The prenatal effects are minimal or disappear and thus do not show compromised neurologic functioning in children.
The consistency of the types of adverse pregnancy outcomes that have been related to hypertension and to blood lead in separate studies is striking, but needs to be better integrated. Rabinowitz et al. (1987) are the only investigators who have reported on the relationship of pregnancy with blood pressure and blood lead during pregnancy. They studied 3,200 live births in Boston by white, middle-class women. They examined umbilical-cord blood lead and reported a significant association with blood pressure at delivery and the presence of hypertension during pregnancy. Schwartz (1991) has reported an association between blood lead and blood pressure in females in a nationally representative sample.
A comprehensive review of both human and animal studies has been published (Boscolo and Carmignani, 1988).
Animal Models of Lead and Blood Pressure
In the last decade, a substantial body of animal data have associated in vivo lead exposure with increased blood pressure in animals. Increases in blood pressure in the rat have been documented by Victery et al. (1982), Iannacone et al. (1981), Perry and Erlanger (1978), Carmignani et al. (1983), Webb et al. (1981), Evis et al. (1987), Boscolo and Carmignani (1988), Bodgen et al. (1991), Nakhoul et al. (1992), and Lal and co-workers (1991). In many of these studies, the blood lead levels involved were quite low. Figure 2-9, for example, shows a plot of the
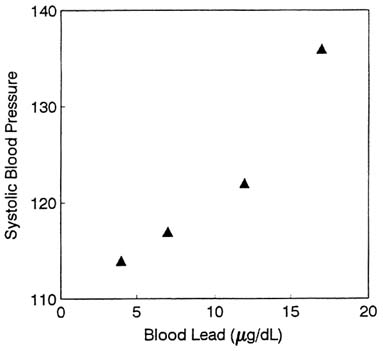
FIGURE 2-9 Response of blood pressure to blood lead concentrations in rats (Boscolo and Carmignani, 1988). Source: Schwartz, 1992b. Reprinted with permission; copyright 1992, CRC Press.
data from Boscolo and Carmignani (1988), taken from the review of Schwartz (1992b). Other studies have documented similar increases in pigeons (Revis et al., 1981).
A common finding in these studies was increased reactivity to alpha-adrenergic stimulation, prolonged response to norepinephrine stimulation, and reduced effectiveness of isoproterenol in lowering blood pressure. These all point to a mechanism involving the modulation of the calcium messenger system that regulates blood pressure. This system is disturbed by lead in other organs as well.
In vitro studies have also supported the role of lead in increasing blood pressure. Isolated tail arteries have shown increased contractile response in studies by Piccini et al. (1977), Chai and Webb (1988), Skoczynska et al. (1986), Carmignani et al. (1983), Iannocone et al. (1981), and Webb et al. (1981). These studies also document increased responsiveness to alpha-adrenergic stimulation. Chai and Webb (1988) report that the contractile response to lead in an isolated rabbit tail artery was increased by a protein kinase C stimulant, and decreased in the presence of a protein kinase C inhibitor. This again suggests the centrality of the calcium messenger system. This system regulates tone in the vascular periphery of humans as well as animals.
Population-Based Epidemiology Studies
Recent epidemiology studies have generally supported the conclusion that the animal results are generalizable to humans. Positive associations between blood lead levels and blood pressure have been reported in essentially all studies, and most of them have reported significant results. The largest studies were the British Regional Heart Study and the NHANES II study. The similarity in the regression coefficients between those two studies has been noted by EPA in the air lead criteria document (EPA, 1986a) as well as by Pocock et al. (1988). Figure 2-10 shows the estimated changes in systolic blood pressure for a change in blood lead from 10 µg/dL to 5 µg/dL from 11 recent studies of the association between blood lead and blood pressure (Schwartz, 1988; Orssaud et al., 1985; Kroumhout, 1988; Pocock et al. 1988; Elwood et al., 1988a,b; Neri et al., 1988; Moreau et al., 1982; de Kort et al., 1987; Sharp et al., 1988). As can be seen, positive and moderate consistent effects are seen in the studies. Other studies have also reported
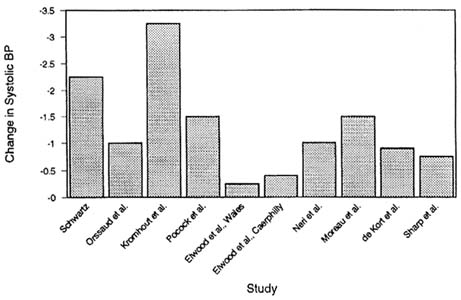
FIGURE 2-10 Reported changes in systolic blood pressure associated with a decrease in blood lead from 10 to 5 µg/dL. Bars: (1) Schwartz, 1988; (2) Orssaud et al., 1985; (3) Kromhout et al., 1985, Kromhout, 1988; (4) Pocock et al., 1985, 1988; (5) Elwood et al., 1988a,b, Welsh Heart Program; (6) Elwood et al., 1988a,b, Caerphilly Collaborative Heart Disease Studies; (7) Neri et al., 1988; (8) Moreau et al., 1982; (9) de Kort et al., 1987; de Kort and Zwinnis, 1988; (10) Sharp et al., 1988. Source: Adapted from Schwartz, 1992b.
significant associations, including Weiss et al., (1986), Moller and Kristensen (1992), Egeland et al. (1992), Apostoli et al. (1992), and Hu (1991). No association was reported by Grandjean et al. (1989), and a mixed result, with positive associations in one model, and negative associations in a model with multiple divalent cations, was reported by Staessen et al. (1991). Overall, a considerable majority reported significant associations. Combined with the strong animal model, mechanistic results, and the moderate concordance of effect size, this suggests overwhelming evidence for the causality of the association. This conclusion was also the consensus of the International Conference on lead in blood pressure (Victery et al., 1988), and of EPA's external Science Advisory Board.
Given the estimated changes in blood pressure from Figure 2-10, a study would need extremely large sample sizes to test whether the expected consequences of increased blood pressure on myocardial infarctions occur. No study done to date has had the power to detect relative risks of 1.05. Two studies have focused on intermediate, and more common, cardiovascular end points. Kirkby and Gyntelberg (1985) reported that lead exposure was associated with electrocardiogram changes associated with ischemic heart disease. This was confirmed in a general population study by Schwartz (1991).
MECHANISMS OF TOXICITY
The search for mechanisms of lead's toxic actions in human and experimental animal populations must depend to some extent on the level of the mechanistic explanations being sought. For example, histopathologic, physiologic, cellular, and subcellular and molecular levels of mechanistic explanation have been invoked in numerous papers on human and experimental lead toxicity. Eventually, all the mechanistic explanations are traced to lead's functions at the molecular level in various cell types and in various subcellular components. Investigators have long sought a global, unifying molecular mechanism of lead's toxic action at all sites in the human body. The diversity of toxic effects has made such an explanation extremely difficult to find, and it is the molecular-level mechanism that is of principal interest in this report.
The consequences of lead action on organ and tissue function are also
highly correlated with reproducible lead-induced dysfunction in cell culture models of neurons and glia, brain capillary epithelium, bone, and several other pertinent cell types (Tiffany-Castiglioni, 1993; Goldstein, in press; Pounds et al., 1991). Finally, the many toxicological effects of lead are well supported in hypothesis and theory by lead-dependent perturbation of critical physiological, biochemical, and molecular events including signal transduction processes, gene expression, mitochondrial function, etc. (Goering, in press; Regan, in press; Shelton, 1993; Simons, in press).
Most attempts to elucidate or define the mechanism of neurotoxic action of lead on cognition and related measures of brain function include altered development and maintenance of the neural network by lead as a vague, but central thesis. The concept that lead might provoke local or global changes in brain development, architecture, organization, and function is reasonable and supported directly and indirectly by the studies from several laboratories. Two broad classifications of mechanisms have been proposed by Silbergeld (1992). First, are the neurodevelopmental mechanisms that result in persistent and irreversible changes in the architecture of the nervous system. The best example of this developmental mechanism is the neural cell adhesion molecule discussed below. Second, lead interferes with signal transduction processes, especially those associated with neurotransmitter function, which may be reversible. Although these two broad mechanisms may overlap if the neuropharmacologic effects of lead contribute to the developmental mechanism, they provide a useful framework to organize information.
Definition of mechanism of action: To assess appropriately the literature developing on mechanisms of action for lead toxicity and neurotoxicity, it is important to note that the cellular and molecular effects of lead are parallel to the effect of lead on nervous system function in humans. That is, at any level of biologic organization, lead toxicity manifests a broad continuum of toxicity from overt at higher levels to multifactorial recondite toxicities at lower exposure levels. A similar broad continuum of toxicities is observed at the cellular level. Thus, it should not be expected that the actions of lead on a single cellular or molecular process will provide an adequate description of the mechanism of action.
In addition, there is not agreement among investigators as to what constitutes a mechanism of action as the definition of mechanism of action, like the definition of beauty, lies in the eye of the beholder. For
example, the underlying process responsible for poor school performance may be best related to another behavioral outcome, such as visual-moter integration. At another, but more remote level, perturbation of signal transduction or gene expression may be responsible for changes in neuronal development and the hard-wiring of the nervous system that may underlie the changes in behavior. At yet another level, interaction of lead with critical sites on specific proteins may explain the effects of lead on signal transduction processes.
Neurotoxicity: The principal neuropathologic feature of acute lead encephalopathy is interstitial edema. Several lines of investigation implicate functional changes in the permeability and barrier properties of the capillary endothelium (Bressler and Goldstein, 1991). These changes in endothelium function may be mediated by the effects of lead on astrocytes possibly through altering calcium homeostasis or by activation of protein kinase C (Gebhart and Goldstein, 1988).
Neurodevelopmental toxicity: The neural cell adhesion molecule (NCAM) is a complex of three polypeptides which regulates many neurodevelopmental processes including neuronal fiber outgrowth and synapse formation (Edelman, 1986). The extracellular domain of the NCAM complex is modified by the addition of sialic acid moieties, with the embryonic form more polysalicylated than the adult form. The sialic acid content determines the strength of interactions between NCAMs on adjacent cells. Chronic lead exposure decreases the rate of NCAM desialylation and the conversion from the embryonic to adult stage in the rat cerebellum (Cookman et al. 1987; Regan, 1989, in press). It is logical that the impaired NCAM desialylation may induce a dys-synchrony in normal cerebellar development, with subsequent altered neuronal structuring contributing reduced fine motor skills and other manifestations of toxicity. However, the biochemical and cellular mechanisms by which lead impairs desialylation remain to be clarified.
Many studies have evaluated the effects of lead on selected parameters of neurotransmitter function and the electrophysiological consequences. Changes in neurotransmitter levels, turnover, and release are well documented in numerous experimental systems, including the neuromuscular junction, synaptosomal preparations, brain tissue slices and cultured neurons. Although there are inconsistencies and contradictions in the findings among these studies, the conclusions are remarkably consistent when the differences in experimental design and the experimental system
are considered (Bressler and Goldstein, 1991; Minnema, 1989; Silbergeld, 1992). Chronic exposure to low levels of lead enhances the basal or spontaneous release of various neurotransmitter from almost all systems investigated. For example, lead concentration of 40 µg/dL increased the frequency of miniature end plate potentials, but did not affect the presynaptic nor the end plate potential after direct stimulation (Atchison and Narahashi, 1984; Cooper et al., 1984; Manalis and Cooper, 1973). In contrast, lead at higher concentrations blocked the evoked release of neurotransmitters in both the peripheral and central nervous system preparations.
Interactions of lead with the calcium, messenger system have received considerable attention during the last twenty years. This attention is the result of the physico-chemical similarity between Pb2+ and Ca2+ and the ubiquitous role of calcium ions as intracellular messengers for transducing electrical and hormonal signals. The interaction of lead with Ca2+ homeostasis and the calcium messenger system has been reviewed in detail (Pounds, 1984; Pounds et al., 1991; Simons, in press; Bressler and Goldstein, 1991).
The concentration of free cytoplasmic calcium ion, [Ca2+]i, is normally maintained between 50 and 150 nM by the calcium homeostasis system. An appropriate hormonal or electrical signal at the plasma membrane is transduced to a cytoplasmic Ca2+ signal by increasing the [Ca2+ ]i in one or more parts of the cell. Lead interferes with the generation of a Ca2+ signal in many cells and nerve terminals. Recent work has extended this understanding by demonstrating that Pb2+ inhibited Ca2+ entry when calcium channels were opened by depolarization (Simons and Pocock, 1987)
Cytoplasmic Ca2+ signals are received by a variety of Ca2+ receptor proteins including calmodulin, protein kinase C, calcimedins, parvalbumins, troponin C and many others. Some of these Ca2+ receptor proteins are specific to certain cell types, while others are ubiquitous. Two of the most versatile and ubiquitous Ca2+ receptor protein are calmodulin and the protein kinase C family. The calmodulin-mediated responses are typically of brief duration. Typically calmodulin-mediated functions include neurotransmitter release, endocrine and exocrine secretion, etc. Protein kinase C is activated by Ca2+ and a lipid metabolite produced by prosphoinositol metabolism, diacylglycerol. Protein kinase C activates protein kinase and prosphatases with both a broad and narrow spectrum
of protein substrates. Protein kinase C-mediated responses are typically of longer duration than calmodulin-mediated responses and include cell division and proliferation, cell-cell communication, organization of the cytoskeleton, etc. Lead can perturb the function of these Ca2+ receptor protein directly by substituting for Ca2+ with more or less activity, or indirectly by interfering with the generation or removal of the Ca2+ signal. For example Pb2+ will effectively and functionally displace or substitute for Ca2+ in calmodulin and other receptor proteins tested to data (Habermann et al., 1983; Fullmer et al., 1985; Richardt et al., 1986). High levels of calmodulin are particularly associated with the nerve terminals where calmodulin-dependent phosphorylation regulates neurotransmitter release. The inappropriate, or prolonged activation of calmodulin by Pb2+ rather than by Ca2+ would logically explain the increased spontaneous neurotransmitter released observed by many investigators.
Protein kinase C (PKC) is not a single protein, but a family of isozymes, most of which are calcium activated. Protein kinase C has a profound effect on cell function, especially the regulation of cell growth and differentiation. Markovac and Goldstein (1988a,b) demonstrated that very low levels of lead substituted for calcium in the activation of protein kinase C enzyme activity. Unfortunately, there is not a clear understanding as to the mechanism by which Ca2+ activates protein kinase C. Thus the exact biochemical mechanism by which lead activates PKC is only speculation. Nevertheless, the activation of PKC by lead has been confirmed in several laboratories using other tissue or cellular preparations, and thus different PKC isozyme patterns (Goldstein, 1993). Very high levels of lead which are not reasonably expected in vivo are required to inhibit PKC activity. Current evidence correlates activation of PKC activity with functional changes in brain microvascular formation in culture after activation by lead. Similar persistent changes in neuronal activity could underlie the more subtle effects of lead on neuronal function. Thus, the Pb2+-protein interactions with Ca2+ receptor proteins and other proteins, such as those of heme biosynthesis, are beginning to be understood (Goering 1993).
Lead has diverse and complex actions on the calcium messenger system, emphasizing the importance of this pathway as a key molecular and cellular target of lead toxicity. Although the effects of lead on these cellular and molecular processes is clearly established, the causal link
between these effects and the subtle effects of chronic, low-level lead exposure is difficult to define with experimental rigor.
Effects on Heme Biosynthesis and Erythropoiesis
EPA (1986a) extensively reviewed the effects of lead on heme biosynthesis and erythropoiesis. This report will summarize the EPA findings and will describe new studies.
The various steps in heme biosynthesis affected by lead are depicted in Figure 2-11. The activity of delta-aminolevulinic acid synthetase, ALA-S (the rate-limiting enzyme), is stimulated in various tissues in the presence of lead intoxication. Stimulation of enzyme activity begins to occur at a blood lead concentration of 40 µg/dL (Meredith et al., 1978). Accumulation of ALA in lead-exposed subjects occurs both by increased delta-ALA-S activity and by inhibition of activity of the extremely lead-sensitive cytosolic enzyme porphobilinogen synthetase (PBG-S, ALA dehydratase [ALA-D]). Of the two sources of ALA accumulation, that associated with ALA-D activity inhibition is predominant. The threshold for ALA-D inhibition is between 5 and 10 µg/dL. ALA accumulation in urine (ALA-U) has generally been assumed to begin in children and adults with blood lead concentration of about 40 µg/dL (e.g., NRC, 1972; EPA, 1986a). Buildup in plasma (ALA-P) can occur at lower blood lead concentrations (Meredith et al., 1978). Recent evidence (Okayama et al., 1989) suggests that the accumulation rate depends on the method of measurement of the metabolite; dose-response calculations of accumulation of ALA in lead workers suggest a threshold of below 20 µg/dL, on the basis of the more specific high-performance liquid chromatography.
Another important step affected by lead is formation of heme from protoporphyrin IX (erythrocyte protoporphyrin; zinc protoporphyrin) in which insertion of iron is inhibited by both inhibition of activity of the enzyme ferrochelatase (Piomelli, 1981; Posnett et al., 1988) and possibly altered transport of iron intramitochondrially (Moore,1988; Marcus and Schwartz, 1987; Piomelli, 1981; Sassa et al., 1973). Lead-associated accumulation of zinc protoporphyrin (ZPP) resembles that due to iron deficiency in young children, and one must adjust for the presence of
iron deficiency when examining lead exposure. Accumulation of ZPP is strongly and logarithmically correlated with blood lead concentrations in both children (e.g., Piomelli et al., 1973, 1982; Lamola et al., 1975; Roels et al., 1976) and adults (e.g., Grandjean and Lintrup, 1978; Lilis et al., 1978). The threshold for response in children is 15–20 µg/dL (Piomelli et al., 1982; Hammond et al., 1985).
Lead-induced impairment of the body heme pool has a broad impact on the entire organism, and this can be readily comprehended as an effect-cascade scheme. In summary:
-
Diminished hemoglobin biosynthesis leads eventually in a dose-dependent manner to anemia (EPA, 1986a; WHO, 1977; Schwartz et al., 1990). The threshold for lead's effect on hematocrit—based on analysis of a group of 579 children's blood data—is approximately 20–25 µg/dL (Schwartz et al., 1990).
-
Heme formation for hemoproteins in neural tissues can be affected, disturbing normal brain-cell energy production (e.g., Whetsell et al., 1984; Holtzman et al., 1981).
-
Lead disturbs heme-mediated formation of 1,25-(OH)2-vitamin D in the kidney, and the disturbance can affect the many functions of vitamin D in calcium balance and metabolism (Edelstein et al., 1984; Mahaffey et al., 1982b; Fowler et al., 1980).
-
Other steps in the heme pathway disturbed by lead include the accumulation of coproporphyrin in urine (CP-U) at an apparent blood-lead threshold of approximately 40 µg/dL, considerably above that for the steps already noted. This disturbance occurs only during lead intoxication (Piomelli and Graziano, 1980), in contrast to the delay observed in ZPP changes.
In general, impairments of erythropoiesis and erythrocyte physiology caused by lead exposure are considered to occur at relatively higher body burdens—i.e., blood lead at or above approximately 40 µg/dL—than is the case for effects on heme biosynthesis.
Iron-deficiency anemia in children is exacerbated by lead (Watson et al., 1980; Yip et al., 1981); lead workers' hemoglobin concentration is inversely and strongly correlated with blood lead concentrations at a threshold of approximately 50 µg/dL. In children, as noted earlier, lead exposure affects hematocrit at a blood-lead threshold of approximately 20–25 µg/dL (Schwartz et al., 1990).
Lead-associated anemia also occurs through direct damage to the
erythrocyte, i.e., hemolytic anemia. This occurs through a combination of increased erythrocyte fragility (Waldron, 1966), increased osmotic resistance (e.g., Horiguchi et al., 1974), and impaired erythropoietic pyrimidine metabolism (Valentine and Paglia, 1980); Angle and McIntire, 1982).
Effects on Vitamin D and Calcium Metabolism
Recent studies document the impairment of vitamin D metabolism and function by lead, and this suggests a risk of a toxic action of lead on the many human bodily functions mediated by vitamin D, particularly during development. Those functions are tabulated in Table 2-3. Two studies of the toxic response in children have appeared in the literature (Rosen et al., 1980; Mahaffey et al., 1982b). In the report of Rosen et al. (1980), a group of lead-intoxicated children with blood lead concentrations between 33 and 120 µg/dL were observed to have had marked decreases in circulating amounts of the vitamin D metabolite 1,25-(OH)2-vitamin D. This association was most pronounced, in a dose-dependent manner, at blood lead concentrations over 62 µg/dL, but reductions were still significant down to 33–55 µg/dL. The fact that chelation therapy in the children returned 1,25-(OH)2-vitamin D to normal without affecting the vitamin D hydroxylation in the kidney indicated that activity of the mitochondrial enzyme P-450 in the kidney might have been impaired.
A second investigation in children also documented a strong negative correlation between blood lead concentrations and serum 1,25-(OH) 2-vitamin D concentrations (Mahaffey et al., 1982b). They found that the slopes of the regression analysis lines for data subsets above and below blood lead of 30 µg/dL were comparable for blood lead and 1,25-(OH)2-vitamin D concentrations.
The decrements in circulating 1,25-(OH)2-vitamin D seen by Rosen et al. (1980) in the blood lead range of 33–55 µg/dL are similar to those seen in children with severe renal insufficiency (Rosen and Chesney, 1983). For the entire blood lead range of 33–120 µg/dL, the metabolite reductions are similar to what is observed in vitamin D-dependent rickets (Type I), oxaloses, hormone-deficient hypoparathyroidism, and aluminum intoxication (Rosen and Chesney, 1983).
Data on experimental animals support an effect of lead on biosynthesis of 1,25-(OH)2-vitamin D. Smith et al. (1981) observed decreases in the
TABLE 2-3 Various Tissues, Cell Types, and Functions Modulated by Vitamin D Hormone
|
Tissue |
Cell Type |
Function |
|
Bone |
Osteoblasts |
Modulates cytosolic Ca2+ |
|
Bone remodeling |
||
|
Gastrointestinal tract |
Enterocyte |
Mineral absorption |
|
Mammary gland |
Mammary explants |
Calcium uptake |
|
Parathyroid gland |
Parathyroid |
Phospholipid metabolism |
|
Pituitary gland |
GH4C1 pituitary cell |
Prolactin synthesis |
|
Modulates cytosolic Ca2+ |
||
|
Heart |
Cardiac muscle |
Calcium uptake |
|
Skin |
Fibroblasts, epidermal |
CGMO production |
|
Kidney |
Tubular cells |
Phosphate reabsorption |
|
Skeletal muscle |
Myoblasts |
Calcium uptake |
|
Pancreas |
β cells |
Insulin secretion |
|
Macrophage |
Phagocytosis |
|
|
|
|
Modulation of protooncogenes |
plasma metabolite concentrations in rats given high doses of lead orally. In chicks fed lead, a dose-dependent reduction in biosynthesis of the dihydroxy hormone in the kidney and reduced concentrations in tissues have been noted (Edelstein et al., 1984).
As can be seen in Table 2-3, 1,25-(OH)2-vitamin D controls bone remodelling and several other metabolic functions. Additional recently
|
Tissue |
Cell Type |
Function |
||
|
|
Lymphocytes |
|
Modulates differentiation and proliferation |
|
|
HL-60 myeloid leukemia cells |
|
|||
|
Diverse |
ROS osteosarcoma cells |
|||
|
Mononuclear cells |
||||
|
Epidermal cells |
||||
|
Cancer cell lines, multiple |
||||
|
|
|
Cell division |
||
|
|
Cell-cell communication |
|||
|
Diverse |
|
Diverse |
Organization of cytoskeleton |
|
|
Endocrine secretion |
||||
|
Neurotransmitter release |
||||
|
|
Platelet release reaction |
|||
|
Exocrine secretion |
||||
characterized functions of this vitamin D were summarized by Reichel et al. (1989). 1,25-(OH)2-vitamin D, for example, controls intestinal vesicular calcium (Nemere and Norman, 1988) and modulates intracellular calcium ion in mouse osteoblasts (Lieberherr, 1987), rat cardiac muscle cells (Walters et al., 1987), and cultured mouse mammary gland (Mezzetti et al., 1988). Other test systems have also been investigated (Chisolm et al., 1988; Sugimoto et al., 1988).
Collectively, a decrease in amounts of the dihydroxy metabolite can potentially produce disturbances in the calcium messenger system and cell functions controlled by calcium ion (Pounds and Rosen, 1988).
Immunoregulatory properties of the dihydroxy metabolite have been noted (e.g., Iho et al., 1986), as has its role in growth and differentiation of cell types other than those of bone (see, e.g., Barsony and Marx, 1988). Other newly revealed functions are shown in Table 2-3.
A number of studies have attempted to measure the functions affected by lead, and these are considered in the section of this chapter dealing with molecular mechanism of lead toxicity.
Carcinogensis
Virtually all the attention to lead as a major public-health problem arises from its noncarcinogenic effects in humans and experimental animals. But questions have been raised about lead carcinogenicity and the topic is briefly summarized here. Available data are from occupational epidemiologic studies, short- and long-term experimental-animal tests, and biochemical and in vitro assessments of lead compounds.
Various studies (Hiasa et al., 1983; Shirai et al., 1984; Tanner and Lipsky, 1984) have shown that dietary exposure to lead acetate at relatively high doses increases the development of renal cancer caused by several known organic renal carcinogens in rats. Hiasa et al. (1983) studied the promoting effects of a diet containing 1% lead acetate on the development of renal tubule-cell tumors after earlier exposure to N-ethyl-N-hydroxyl nitrosamine. They found a 70% incidence of renal tumors in animals given lead acetate and the nitrosamine at 32 weeks and no increase of tumors in animals given either compound alone. Similar results with the nitrosamine were reported by Shirai et al. (1984), who concluded that lead acetate acted as a promoter, and with N-(4'-fluoro-4-biphenyl) acetamide by Tanner and Lipsky (1984), who demonstrated that lead acetate accelerated the onset and development of kidney tumors in rats after chronic exposure.
Kasprzak et al., (1985) used a diet containing 1% lead acetate and supplemented with calcium acetate at 0–6% and showed that addition of calcium acetate to the diet tended to increase the incidence of renal tumors after 58 weeks of lead acetate exposure from 45% to 71%, but decreased the accumulation of lead in the kidneys.
The overall results indicate that lead can act both as a renal carcinogen in rodents and as a promoter of renal carcinogenesis caused by other organic renal carcinogens. The exact minimal doses of lead required to produce the effects are unknown.
About a dozen occupational studies have considered lead exposure versus various types of cancers in such work categories as battery recycling, lead smelting, alkyl lead manufacturing, plumbing, and pipefitting. EPA (1986a) has examined the older studies, and they have been augmented by those of Fanning (1988) for battery operations, Gerhardsson et al. (1986) for alkyl lead production, and Cantor et al. (1986) for plumbing and pipefitting.
Results of some studies suggest a renal-cancer risk (Selevan et al., 1985; Cantor et al., 1986), but results of others do not (e.g., Cooper et al., 1985). In some cases (Selevan et al., 1988), an association with duration of exposure added plausibility to the findings.
In contrast, results of numerous animal studies strongly support a renal-cancer potential for soluble lead salts (see EPA, 1986a; IARC, 1980, 1987), and at least 12 long-term studies (with various rat strains, a mouse strain, and both males and females) documented induction of renal tumors when animals were fed either lead acetate or subacetate (soluble forms of lead). Those animal data meet EPA's criteria for sufficient evidence of carcinogenicity, as published in ''Guidelines for Carcinogen Risk Assessment'' (EPA, 1986b).
The mechanism of lead carcinogenicity in laboratory animals remains unclear. Lead is not mutagenic in most test systems, but it has been shown to be clastogenic, reducing the fidelity of DNA repair polymerases. As described above, lead compounds are also mitogens in rodent kidneys and have been shown to act as tumor promoters and co-carcinogens in various experimental studies. The inconclusive human data and established animal carcinogenicity led to a classification of lead as a probable human carcinogen (IARC, 1980, 1987) and an EPA carcinogen ranking of Group B2 (EP, 1986b). Since the U.S. population has an exposure of approximately 1 µg/kg of body weight per day, the EPA method for extrapolating animal data to humans could be used to estimate a lifetime cancer risk for lead approximately 10-5. Of course, the cancer risks would vary, depending on the extent of exposure if the linearized extrapolation is appropriate for lead based on biology. The noncarcinogenic effects of lead remain of predominant interest in sensitive populations, but the potential carcinogenic effects of lead should be considered as new information becomes available.
Nephropathy
The question arises whether lead has subclinical renal effects, as would be expected, given the array of toxic effects on other organ systems. Several obstacles frustrate efforts to answer the question epidemiologically or experimentally. First, there is a marked reserve capacity of the kidney to function in the face of toxic insult. It might be
some time before the reserve capacity is depleted when people are exposed to large concentrations; this has been shown in workers occupationally exposed to lead. Second, we do not know the mechanisms of nephrotoxic events at the cellular or subcellular level, e.g., in the proximal renal tubules. Finally, there is a dearth of biologic markers specific for lead's nephrotoxic action.
Previous studies (Victery et al., 1984) have shown that lead-ion uptake in proximal renal tubule cells occurs via membrane binding or passive diffusion. Consequently, it is the kidney's intracellular handling of lead that defines the nephrotoxic dose-response relations for lead toxicity.
One can look to several kinds of experimental studies to garner clues as to what is occurring in humans who have subclinical lead exposures. Of particular interest are data on leadbinding proteins in experimental systems.
Oskarsson et al. (1982) showed that the lead-binding patterns in rat kidneys and brain, major target organs for lead toxicity, were consistent with binding to two proteins, which might thus be factors in the intracellular handling and availability of lead. Goering and Fowler (1984, 1985) showed that the inhibition of PBG-S (ALA-D) activity in the rat kidney is mediated by both lead biochelation and zinc availability; the former perhaps helps not only to account for relative resistance to lead in kidney PBG-S (Fowler et al., 1980; Oskarsson and Fowler, 1985a), a cytosolic enzyme otherwise quite sensitive to lead in other tissue (Fowler et al., 1980; Oskarsson and Fowler, 1985a), but such sequestration are linked to the presence of the lead binding proteins. ALA-S and ferrochelatase function were not, however, protected. These observations suggested that either other molecules or the lead-binding proteins were facilitating the mitochondrial uptake of lead, because the mitochondrial inner membrane has previously been shown (Oskarsson and Fowler, 1985a,b) to be highly impermeable to lead in vitro (Oskarsson and Fowler, 1987). Studies of Mistry et al. (1985) show a high affinity of these proteins for lead; other data (Fowler et al., 1985; Mistry et al., 1986; Shelton et al., 1986) indicate that they play a role in the intranuclear transport of lead and in lead-induced changes in renal gene expression and that their biologically active form is a cleavage fragment of alpha-2-microglobulin in the retinol-binding protein family (Fowler and Du Val, 1991) that undergoes aggregation, at least in vitro (Du Val and Fowler, 1989).
With experimental exposures to mercury (Woods and Fowler, 1977; Woods et al., 1984) or inorganic oxyarsenic (Woods and Fowler, 1978; Mahaffey et al., 1981), the resulting porphyrinuria appears to be derived from injury to the kidney itself. Other data are consistent with a lead-associated effect on renal heme formation. Ferrochelatase inhibition (a mechanism of erythrocyte protoporphyrin accumulation) occurs in the kidney (Fowler et al., 1980). The kidney is relatively rich in porphyrins (Zawirska and Medras, 1972; Maines and Kappas, 1977), and lead appears to inhibit the heme-requiring kidney 1-hydroxylase enzyme system (Rosen and Chesney, 1983; Reichel et al., 1989); one function of this system is the formation of 1,25-(OH)2-vitamin D, the hormonal metabolite of vitamin D. Short-term experimental-animal studies with intravenous lead have shown a high correlation between formation and dissolution of lead inclusion bodies in renal tubule cells and changes in total and specific gene regulation at these sites. Such changes in regulation are to be found in various subcellular fractions—mitochondrial, microsomal, cytosolic, and lysosomal fractions with a specific response for each organelle compartment (Mistry et al., 1987).
Chronic exposures in experimental animals have produced similar results with regard to lead induction of specific stress proteins. Comparisons of such data with morphometric analyses of tubule cell populations and effects on heme biosynthesis might permit determination of which biologic markers are of greater utility in delineating specific lead-induced changes in cell functions.
Knowledge of the intracellular handling such as the kidney and brain, is essential to an understanding of the mechanisms of lead toxicity in target cell populations in these tissues. Soluble, high-affinity lead-binding proteins in the kidney and brain of rats were first reported by Oskarsson et al. (1982). Those molecules were not identified in other nontarget tissues, so they might play a role in lead toxicity in these organs at low doses. Later studies (Goering and Fowler, 1984, 1985; Goering et al., 1986) demonstrated that semipurified preparations of the proteins play a major role in mediating lead inhibition of the heme biosynthetic pathway enzyme alpha-aminolevulinic acid dehydratase (porphobilinogen synthetase). Other studies (Mistry et al., 1985, 1986) demonstrated that the kidney lead-binding proteins had a high affinity for lead and were capable of facilitating the cell-free nuclear translocation and chromatin binding of 203Pb. Those molecules thus
appear to act as "receptors" for lead and to regulate its intranuclear uptake, chromatin binding, and changes in proximal tubule cell gene expression (Fowler et al., 1985; Shelton et al., 1986; Mistry et al., 1987; Hitzfeld et al., 1989; Klann and Shelton, 1989).
More recent studies (DuVal et al., 1989; Fowler and DuVal, 1991) have identified the renal lead-binding protein as a cleaved form of the protein alpha-2-microglobulin that locks the first 9-nitrogen-terminal residue and shown that the brain lead-binding protein is a chemically similar protein that is rich in aspartic and glutamic amino acids, but immunologically distinct. It appears that it is the cleared form of the alpha-2-microglobulin that is biologically active. Western blot studies (DuVal et al., 1989) have shown that that protein undergoes aggregation after in vitro exposure to lead. The data suggest that the protein can play an early role in the formation of the pathognomonic cytoplasmic and intranuclear lead inclusion bodies. The inclusion bodies are the main intracellular storage sites for lead in proximal tubule cells after increased or chronic lead exposure (Goyer and Rhyne, 1973; Moore et al., 1973; Fowler et al., 1980; Shelton and Egle 1982; Oskarsson and Fowler 1985a,b; Klann and Shelton 1989).
Previous studies have shown a marked kidney-specific macromolecular binding pattern for lead in renal proximal tubule cells. The binding is followed by several undefined intracellular events that result in the presence of large quantities of lead in renal proximal tubule cell nuclei. The precise sequence of events and relationships to other intracellular lead species have not been completely studied. Such data are central to an understanding of the bioavailability of lead to sensitive cellular processes. The bioactive lead species thus might be centrally involved in the mechanisms of toxicity. Further studies of how lead reacts with them should permit their use as biologic indicators of lead-induced nephropathy, provided that they are excreted in urine. Lead-induced alterations of renal gene expression (Fowler et al., 1985; Mistry et al., 1986; Shelton et al., 1986; Herberson et al., 1987; Hitzfeld et al., 1989) and inhibition of renal heme biosynthetic pathway enzymes with attendant development of metal-specific porphyrinuria patterns (Mahaffey and Fowler, 1977; Mahaffey et al., 1981) are examples of sensitive biochemical systems. Such responses have great potential as biologic indicators of nephropathy, once their relation to the pathophysiology of lead nephropathy is understood.
SUMMARY
Exposure of various sensitive populations to lead induces a wide variety of adverse effects—in the central nervous system of children and fetuses, in various growth indexes of children, in the cardiovascular system of older people, in heme synthesis, and in calcium homeostasis and function. LOELs (lowest-observed-effect levels) for various lead effects are summarized in Table 2-4 for children and Table 2-5 for adults.
The weight of the evidence gathered during the 1980s clearly supports the conclusion that the central and peripheral nervous systems of both children and adults are demonstrably affected by lead at exposures formerly thought to be well within the safe range. In children, blood lead concentrations around 10 µg/dL are associated with disturbances in early physical and mental growth and in later intellectual functioning and academic achievement. Studies of electrophysiologic end points have suggested some of the changes in brain function that might mediate the apparent effects.
Despite impressive advances over the last decade in the methodologic rigor of studies of lead exposure and nervous system function, epidemiology remains limited by opportunity. Therefore, animal studies are critical for interpreting the human data. Factors that an epidemiologist must take account of with statistical analysis (an inevitably imperfect process) can be controlled experimentally with animals. For example, the influence of socioeconomic factors on performance can be eliminated, and the importance of timing, dose, and duration of exposure can be evaluated more precisely.
The extensive evidence gathered in animal studies cannot be reviewed here, but some themes warrant enumeration. First, primates exposed to sufficient lead to produce a blood lead concentration of 25 µg/dL or less manifest a variety of memory, learning, and attentional deficits resembling those observed in humans. Second, the deficits appear to be permanent; they are evident for as long as 10 years in animals whose blood lead is maintained at approximately 15 µg/dL. Third, striking concordance of the human and animal data weighs heavily in favor of the hypothesis that low-dose lead exposure is responsible for some of the developmental and cognitive deficits observed in humans. Many of the
TABLE 2-4 Lowest-Observed-Effect Levels of Blood Lead for Effects in Children
|
LOEL, µg/dL |
Neurologic Effects |
Heme-Synthesis Effects |
Other Effects |
|
<10 to 15 (prenatal and postnatal) |
Deficits in neurobehavioral development (Bayley and McCarthy Scales), electro-physiologic changes,a,b and lower IQc, d |
ALA-D inhibitione |
Reduced gestational age and birthweight; reduced size up to age 7–8 yra,b,e |
|
15–20 |
|
||
|
<25 |
Reduced hematocrit (reduced Hb)f |
|
|
|
30 |
Slower nerve conductione |
|
|
|
40 |
|
Increasing CP-U and ALA-Uc |
|
|
70 |
|
||
|
80–100 |
|
Colic, other gastrointestinal effects, kidney effectse |
|
|
a Data from CDC, 1991. b Data from EPA, 1990a,b. c Data from Bellinger et al., 1992. d Data from Dietrich et al., 1993a. e Data from ATSDR, 1988. f Data from Schwartz et al., 1990. |
|||
TABLE 2-5 Lowest-Observed-Effect Levels of Blood Lead for Effects in Adults
|
LOEL, µg/dL |
Heme Synthesis and Hematologic Effects |
Neurologic Effects |
Renal Effects |
Reproductive Effects |
Cardiovascular Effects |
|
<10 |
ALA-D inhibition |
|
|
|
|
|
10–15 |
|
|
|
|
Increased blood pressure |
|
15–20 |
Erythrocyte protoporphyrin increase in females |
|
|
|
|
|
25–30 |
Erythrocyte protoporphyrin increase in males |
|
|
|
|
|
40 |
Increased ALA-U and CP-U |
Peripheral nerve dysfunction (slower nerve conduction) |
|
|
|
|
50 |
Reduced hemoglobin production |
Overt subencephalopathic neurologic symptoms |
|
Altered testicular function |
|
neurodevelopmental and possibly other toxic effects resulting from lead exposure might not be reversible. It is important to distinguish two aspects of reversibility. The first pertains to biologic plasticity, specifically an organism's ability to repair damage and recover functional capacity. The second pertains to the reality of exposure patterns. Impairment might persist, regardless of an organism's capacity for recovery, as long as exposure is maintained. In a practical sense, the impairment is irreversible. Therefore, the fact that an adverse effect is reversible in the biologic sense does not necessarily mean that it is without potential public-health importance. The key issue is whether exposure is reduced, and expression of an organism's recovery capacities thus permitted.
This chapter documents that lead induces measurable increases in diastolic and systolic blood pressure in human populations and in experimental-animal models of environmentally induced blood-pressure changes.
Lead exposure is not the only risk factor for hypertension, but is more amenable to reduction or prevention than behavioral factors that are refractory to change. Furthermore, the relation of lead to blood pressure persists across a dose-effect continuum, so reducing lead exposure of all magnitudes has public-health and societal ramifications. Lead's impact is noteworthy also because of the importance of associated cardiovascular morbidity and mortality, even for an agent that contributes less than a major risk.
Through various processes, including toxicokinetic and intracellular disturbances, lead impairs calcium homeostasis and functions. The importance of impacts on calcium is that they impair calcium's central role in multiple cellular processes.
Lead produces a cascade of effects on the heme body pool and affects heme synthesis. Some of the effects serve as early measures of body lead burden.
Lead effects on cognition and other neurobehavioral measures need to be evaluated on a population-wide, as well as individual, basis. This evaluation should account for the whole statistical distribution of exposures and associated toxicity.
Unfortunately, the direct identification and linkage of the critical and sensitive biological processes which are targets for these effects remains saltatory. There are many reasons why our ability to define the mechanism(s)
of action for lead toxicity lags behind our ability to detect and quantify the toxicological effects. In addition to the difficulties in defining a mechanism of action as discussed above, these reasons include:
-
. Lead is a catholic toxicant producing adverse effects in most tissues and organs of the body, with a parallel effects on multiple organelles and metabolic processes. This situation makes it extremely difficult to identify and isolate the critical process(s) with sufficient experimental rigor.
-
. There is frequently a long delay between the onset of lead exposure and the development of toxic manifestations, impairing identification of causal relationships between functional and cellular or biochemical events.
-
. Lead causes nonspecific, decremental loss of tissue and organ function, with no important pathognomonic manifestations of toxicity.
-
. The multifactorial nature of the toxicity in the nervous, cardiovascular, skeletal and other organ systems complicate establishing causal relationships between cellular and molecular processes and organ dysfunction.
Nevertheless, these difficulties do not diminish the importance or necessity of these efforts. Continued efforts must be made to bridge experimental animal and human studies, at all level analysis, and to integrate the biochemical and molecular events impacting function at the level of the whole organisms.