3
Lead Exposure of Sensitive Populations
A complete assessment of exposure in sensitive populations requires knowledge of the sources of exposure. That is especially important for lead: it has multiple sources, and knowledge of them helps to define exposure to lead and to identify sensitive populations.
The conventional approach to identifying lead exposure in a population has been to attribute lead intoxication to single sources of lead at high concentrations, such as leaded paint. However, current understanding calls for a more comprehensive view. First, there is a growing consensus that lead induces a continuum of toxic effects in humans, starting with small exposures that cause subtle, but important, early effects. Our understanding of what constitutes a safe exposure has increased; as a result, the upper limit of a safe lead content in blood has declined to one-sixth to one-fourth of what it was in a matter of a few decades. Second, once lead is absorbed from a specific source, it is added to a body burden that contributes to various health effects. Therefore, exposures small enough to have viewed as of little importance now are taken more seriously. In other words, we must consider the aggregate impact of multiple small lead sources in assessing health risk.
This chapter is divided into three sections. The first provides a historical perspective on lead contamination, addressing such topics as natural concentrations of environmental lead and the chronologic record of anthropogenic contamination with lead. The second section discusses the major current sources and pathways of lead exposure in sensitive populations, including paint, air, dust and soil, drinking water and
food. The section includes a brief discussion of occupational lead exposure and ends with sources that can produce large, but not necessarily pervasive, exposure, such as improperly lead-glazed food and beverage containers and lead-based ethnic medicinal preparations. The chapter concludes with a detailed summary.
HISTORICAL OVERVIEW OF ANTHROPOGENIC LEAD CONTAMINATION
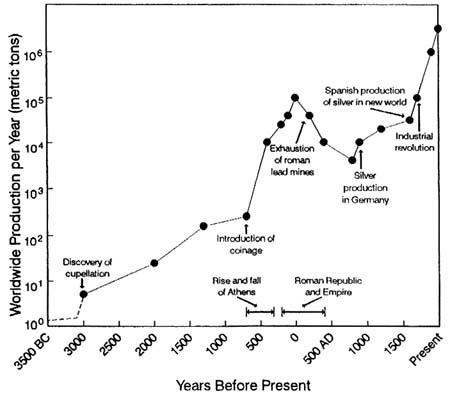
Lead production dates to the discovery of cupellation—a metallurgic process for separating silver from lead ores—some 5,000 years ago (Nriagu, 1985a). However, such anthropologic artifacts as the lead beads in the Hittite ruins of Catal Hüyück from 6500 BC and the lead statuette from the temple of Osiris in Abydos from 3000 BC reveal earlier uses of lead.
The historical record of industrial lead production over the last 5,000 years is illustrated in Figure 3-1. The current production rate is approximately 3.4 million metric tons per year (U.S. Bureau of Mines, 1989). The total amount of lead over the last 5,000 years is estimated to be 300 million metric tons (Flegal and Smith, 1992).
Lead has a long history of wide use. A lead glaze in a Babylonian tablet from 1700 BC has been described; these glazes had become common in China during the Chou Dynasty of 1122–256 BC. In the Roman Empire, lead was used in cooking pots and other utensils, in syrups, in beverage adulterants (e.g., sapa), in medicines, and in the construction of pipes and cisterns to transport water (Nriagu, 1983b). The wide use of lead for the latter explains the word plumbing (from the Latin plumbum, lead). Lead was so pervasive during that period that there is little doubt that lead poisoning was endemic in the Roman population. In fact, it has been speculated (Gilfillan, 1965; Nriagu, 1983b) that chronic lead poisoning contributed substantially to the decline of the Roman Empire.
One of the environmental tragedies of that period is that, despite some Romans' recognition of the problems associated with lead toxicity, awareness did not restrict its use. For example, Vitruvius (Nriagu, 1985b) observed:
Water supply by earthen pipes has advantages. First, if any fault occurs in the work, anybody can repair it. Again, water is much more wholesome from earthenware pipes than from lead pipes, for it seems to be made injurious by lead because some white lead is produced from it; and this is said to be harmful to the human body. Thus if what is produced by anything is injurious, it is not doubtful but that the thing is not wholesome in itself.
We can take example by workers in lead who have complexions affected by pallor. For when, in casting, the lead receives the current of air, the fumes from it occupy the members of the body, and burning then thereupon, robs the limbs of the virtues of the blood. Therefore, it seems that water should not be brought in lead pipes if we desire to have it wholesome.
Current uses of lead are much more extensive. It is still used in some glazes, eating utensils, folk medicines, and plumbing. It is also used in paint pigments, solders, wall and window construction, cosmetics, sheeting of ships, roofs, guttering, containers, sealants, protective coatings, printing type, insecticides, batteries, plastics, lubricants, ceramics, machine alloys, and gasoline additives (NRC, 1980; EPA, 1986a).
The amount of contaminating lead released into the environment closely parallels the record of lead production over the last 5,000 years. Approximately half the lead produced is released into the environment as contamination (NRC, 1980). Current production is about 3.4 million metric tons per year, and current lead release is about 1.6 million metric tons per year. About 150 million metric tons of lead has been released into the environment in the last 5,000 years. The latter value, total release, is probably closer to the total amount of lead put to use, approximately 300 million metric tons, inasmuch as the element is indestructible and cannot be transformed into an innocuous form.
Much of the lead released into the environment is emitted into the atmosphere (about 330,000 metric tons/year) (Nriagu and Pacyna, 1988). Those releases are currently dominated by emissions from leaded gasoline (over 248,000 metric tons/year), but emissions from other sources—including coal and oil combustion, mining, manufacturing, incineration, fertilizers, cement production, and wood combustion—are substantial (Table 3-1). In fact, the latter exceed emissions of the most other contaminants by orders of magnitude.
The magnitude of industrial emissions of lead is illustrated by comparisons with natural emissions of lead and other contaminants. The
TABLE 3-1 Worldwide Emissions of Lead to the Environment, 1983a
sum of industrial lead emissions is approximately 700 times the sum of natural emissions of lead into the atmosphere (Patterson and Settle, 1987; Nriagu, 1989). Emission of industrial lead aerosols to land and aquatic ecosystems is now predominant. It accounts for approximately 15–20% (202,000–263,000 metric tons/year) of the total anthropogenic emission of lead to land (approximately 1,350,000 metric tons/year) and approximately 63–82% (87,000–113,000 metric tons/year) of the total lead that enters aquatic ecosystems (approximately 138,000 metric tons/year) (Nriagu and Pacyna, 1988).
The historical record of atmospheric emissions of industrial lead aerosols has been measured in the environment by various investigators (Figure 3-2). It was initially documented by the 230-fold increase in lead deposition rates in Greenland ice cores over the last 3,000 years, from 0.03 ng/cm2 per year in prehistoric ice cores (800 BC) to about 7 ng/cm2 per year in contemporary ice cores (Murozumi et al., 1969; Ng and Patterson, 1981; Wolff and Peel, 1985). Comparable increases in the Northern Hemisphere have since been documented in pond and lake
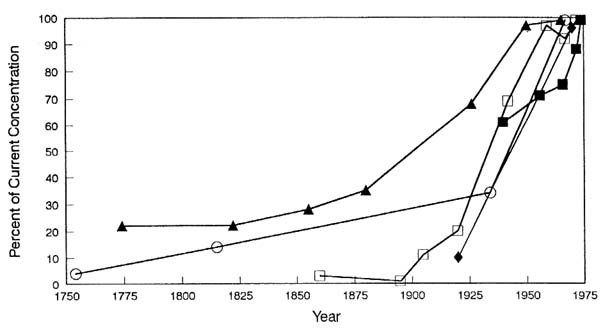
FIGURE 3-2 Lead contamination from industrial aerosols as recorded in chronologic strata. Circles, Greenland snow (Murozumi et al., 1969); squares, dated pond sediment from remote Sierras (Shirahata et al., 1980); open triangles, lake sediments (Edgington and Robbins, 1976); closed triangles, marine sediments (Ng and Patterson, 1982). Source: Adapted from EPA, 1986a, Vol. II.
sediments (Lee and Tallis, 1973; Edgington and Robbins, 1976; Robbins, 1978; Livett et al., 1979; Shirahata et al., 1980; Davis et al., 1982) the oceans (Schaule and Patterson, 1981, 1983; Flegal and Patterson, 1983; Boyle et al., 1986), pelagic sediments (Veron et al., 1987; Hamelin et al., 1988), and marine corals (Shen and Boyle, 1988).
Smaller increases by a factor of 2–5 have been detected in Antarctic ice cores (Boutron and Patterson, 1983, 1986; Patterson et al., 1987) and in the South Pacific (Flegal and Patterson, 1983; Flegal, 1986). The contrast reflects the localization of 90% of lead emissions in the northern hemisphere and the short residence time (10 days) of lead aerosols relative to the interhemispheric mixing rate of 1–2 years (Turekian, 1977; Flegal and Patterson, 1983).
Other releases of lead to the land range from 540,000 to 1,700,000 metric tons/year (Nriagu and Pacyna, 1988). These include industrial lead from commercial wastes, smelter, wastes, and mine tailings (each approximately 300,000 metric tons/year); fly ash (approximately 140,000 metric tons/year); urban refuse (approximately 40,000 metric tons/year); agricultural wastes (approximately 14,000 metric tons/year); animal wastes (approximately 12,000 metric tons/year); solid wastes (approximately 8,000 metric tons/year); wood wastes (approximately 7,000 metric tons/year); municipal sewage sludge (approximately 6,000 metric tons/year); peat (approximately 2,000 metric tons/year); and fertilizers approximately 1,000 metric tons/year). Many of those are projected to increase and become, at least relatively, more important with the reduction in atmospheric emission of gasoline lead.
Nonatmospheric input of industrial lead into aquatic ecosystems is smaller, but still substantial (Nriagu and Pacyna, 1988). It ranges from 25,000 to 50,000 metric tons/year and includes lead from manufacturing (approximately 14,000 metric tons/year), sewage sludge (approximately 9,000 metric tons/year), domestic wastewater (approximately 7,000 metric tons/year), smelting and refining (approximately 6,000 metric tons/year), and mining (1,000 metric tons/year).
Lead contamination in urban areas is often much greater than in remote areas (Table 3-2). That is due to the extensive use of lead in industrial processes and the relatively limited mobility of a sizable fraction of this lead. Long-distance transport of a fraction of the lead to the atmosphere also occurs. Terrestrial, aeolian, and fluvial gradients show that most of the lead emitted in urban areas has remained as a
TABLE 3-2 Environmental Lead Concentrations in Remote and Rural Areas and Urban Areasa
|
|
Remote and Rural Lead Concentration, µg/gb |
Reference |
Urban Lead Concentration, µg/gb |
References |
|
Air |
0.05 |
Lindberg and Harriss, 1981 |
0.3 |
Facchetti and Geiss, 1982; Galloway et al., 1982 |
|
Fresh water |
1.7 x 10-5 |
Elias et al., 1982 |
0.005–0.030 |
EPA, 1986a, Vol. II |
|
Soil |
10–30 |
EPA, 1986a, Vol. II |
150–300 |
EPA, 1986a, Vol. II |
|
Plants |
0.18c |
Elias et al., 1982 |
950d |
Graham and Kalman, 1974 |
|
Herbivores (bone) |
2.0d |
Elias et al., 1982 |
38d |
Chmiel and Harrison 1981 |
|
Omnivores (bone) |
1.3d |
Elias et al., 1982 |
67d |
Chmiel and Harrison 1981 |
|
Carnivores (bone) |
1.4d |
Elias et al., 1982 |
193d |
Chmiel and Harrison, 1981 |
|
a Values can be highly variable, depending on organism and habitat location. b Except µg/m3 in air. c Fresh weight. d Dry weight. |
||||
contaminant in those areas (Huntzicker et al., 1975; Roberts, 1975; Ragaini et al., 1977; Biggins and Harrison, 1979; Palmer and Kucera, 1980; Harrison and Williams, 1982; Ng and Patterson, 1982; Elbaz-Poulichet et al., 1984; Flegal et al., 1989). For example, in the Great Lakes (Flegal et al., 1989), surface-water lead concentrations in the highly industrialized Hamilton harbor (290 pmol/kg) are nearly 50 times higher than those of some offshore waters in Lake Ontario (6.5 pmol/kg). Complementary stable lead-isotope composition measurements show that essentially all (over 99%) of that lead, in even the most remote regions of Lake Ontario and Lake Erie, is derived from releases of industrial lead from Canada and the United States.
Those measurements are consistent with those in numerous other studies that have shown the pandemic scale of lead contamination, which has increased lead concentrations throughout the Northern Hemisphere by a factor of at least 10. Lead concentrations in the atmosphere are now 100 times natural concentrations (Patterson and Settle, 1987). Lead concentrations in remote surface waters of the North Pacific and the North Atlantic are at least 10 times natural concentrations (Flegal and Patterson, 1983; Boyle et al., 1986). Lead concentrations in terrestrial organisms are 100 times natural concentrations (Elias et al., 1982).
Studies incorporating rigorous trace-metal analysis have shown that the natural background lead concentration of North American Indians in pre-Columbian times was 0.3 mg per 70-kg adult (Patterson et al., 1987; Ericson et al., 1991). The body of an average North American urban adult contains 100–1,000 times as much lead.
Some uses of lead are being reduced in the United States and other countries in response to growing concern over pervasive lead toxicity even at low exposures. For example, lead in gasoline has been decreased in recent decades (Figure 3-3), as noted widely (EPA, 1986a; Nriagu, 1990). The United States has also seen a major reduction in the use of lead-soldered cans for foods and beverages (EPA, 1986a; ATSDR, 1988); lead in such containers can increase food lead content by a factor of up to 4,000 over the lead content of fresh food (Settle and Patterson, 1980).
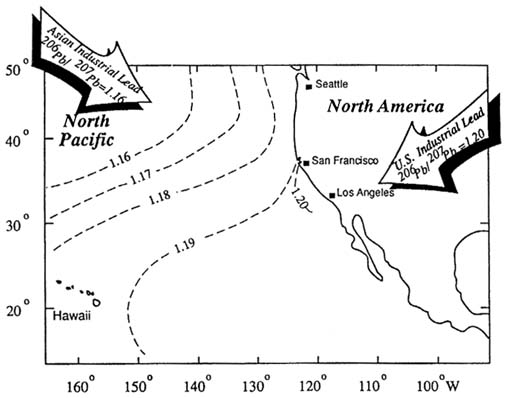
The dispersion of industrial lead is not constrained by national boundaries. For example, stable-isotope composition measurements, which can identify specific sources of industrial lead, have shown that industrial lead from Canada and the United States is transported across the
Great Lakes (Flegal et al., 1989). Similar analyses have documented that over 95% of the lead in the North Pacific represents deposition of Asian and North American industrial lead aerosols (Figure 3-4).
SOURCE-SPECIFIC LEAD EXPOSURE OF SENSITIVE POPULATIONS
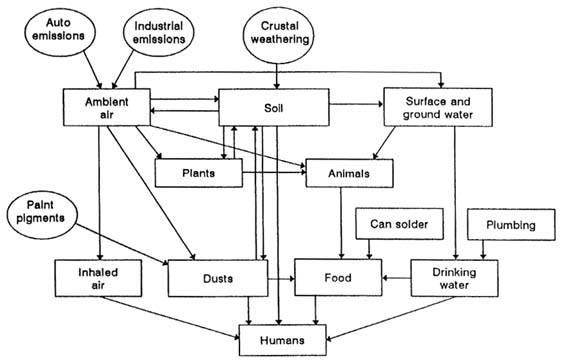
This chapter presents a general picture of the common modes of human exposure to lead—through leaded paint, air (which it enters from leaded gasoline and stationary emission), dust and soil, tap water, the workplace, and miscellaneous sources. Many of the sources and pathways of lead exposure are connected in ways that complicate exposure analysis and frustrate reduction and removal strategies (Figure 3-5); in this regard, lead in the air, lead in paint, and lead in drinking water are of particular concern.
Lead in Paint
Lead-based paint in and around U.S. urban housing has long been recognized as a serious and pervasive source of lead poisoning of young children. It also accounts for exposure to lead through its appearance in dust and soils. This source of lead poisoning has expanded to include workers in housing-lead abatement and homeowners who attempt rehabilitation of old housing. It also affects such workers as salvagers, construction crews, and marine maintenance staff who encounter mobilized lead in burning, cutting, chipping, and grinding.
Physicochemical and Environmental Considerations
Lead compounds have served as pigments for painting media for millennia; for example, the use of white lead pigment—basic lead carbonate—dates to prehistoric times (Friedstein, 1981). Older leaded paints included a linseed-oil vehicle plus a lead-based pigment and in some cases a long-chain fatty acid and a lead-based drying catalyst, or
drier, commonly an organic acid salt of lead. Use as pigment accounted for most of the lead present in older paints. Pigment lead concentrations were high in paints marketed and used before the 1940s; fractions in the final dry film were up to approximately 50% (CDC, 1985).
Physical properties of lead-based paints were such as to lead to their widespread use in homes, in public facilities, and in industrial sites. The most common pigment, basic lead carbonate (2PbCO3Pb(OH) 2), reacted with other paint components to yield a flexible, durable lead soap film. The surface would have excellent durability (weathering) characteristics, but would eventually age, i.e., would either peel or undergo weathering and interior shedding or chalking. Aging yielded paint chips and a constantly renewing surface with concomitant dispersion of the older leaded film as a leaded dust on nearby surfaces.
The period during which leaded paint had the highest amounts of lead and posed the worst toxicity risk in the United States was from around 1875 to the 1940s, when other pigments, such as titanium dioxide, began entering the paint market (e.g., Farfel, 1985). Lead was used in residential paints throughout the 1800s, and it has been estimated (ATSDR, 1988) that 3 million metric tons of lead in various old paints persist in old housing and public facilities in the United States. Lead has been used in other pigments, e.g., as lead oxide and lead chromate.
General Characteristics of Exposure
Given the pervasive nature of leaded paint in homes, elementary schools, day-care centers, etc., and the normal oral exploratory behavior of very young children, it is logical for leaded paint to be a major source of lead for young children. Young children, especially toddlers, can ingest fallen or peelable chips of leaded paint, gnaw intact lead-painted woodwork, and ingest leaded paint dispersed in soils or in dusts adhering to hands. Household-paint dust can also be entrained into the breathing zone of toddlers and inhaled.
Scope of the Problem
In the United States, the distribution of paint lead in housing is a
TABLE 3-3 Estimated Numbers of Children Under 7 Years Old Residing in Lead-Based-Paint U.S. Housing, by Date of Construction
|
Construction Date |
No. Lead-Based-Paint Homes |
No. Children |
|
Pre-1940 |
20,505,000 |
5,885,000 |
|
1940–1959 |
16,141,000 |
4,632,000 |
|
1960–1974 |
5,318,000 |
1,526,000 |
|
Total pre-1975 |
41,964,000 |
12,043,000 |
|
Source: Adapted from ATSDR, 1988. Data from Pope, 1986. |
||
function of housing age, as shown in Table 3-3 (ATSDR, 1988), which also shows numbers of children in housing of different ages (derived from U.S. Bureau of the Census enumerations). In Table 3-3, the national estimate for the number of all housing units having paint with lead at or above the detection minimum of 0.7 mg/cm2 is approximately 42 million, about 52% of the entire U.S. housing inventory. Of the 42 million, approximately 21 million units were built before 1940.
The number of children under 7 years old in lead-based-paint housing is about 12 million, of whom approximately 6 million live in the oldest units, which have the highest concentrations of lead in paint. It has been determined (ATSDR, 1988) that 4.4 million metropolitan children (children in 318 U.S. Standard Metropolitan Statistical Areas) 0.5–5 years old live in the oldest housing.
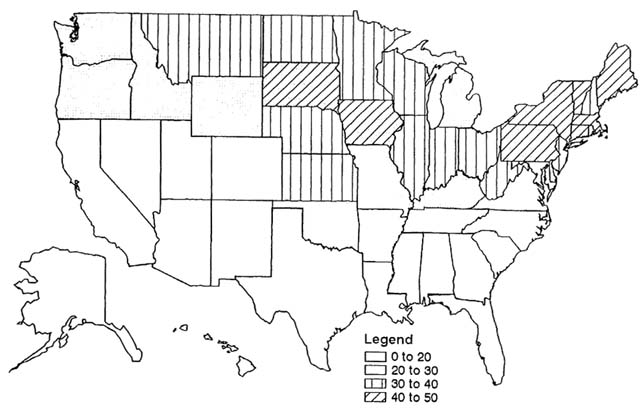
The percentages of housing with leaded paint by date of construction are pre-1940, 99%; 1940–1959, 70%; and 1959–1974, 20% (Pope, 1986). The oldest housing group also had the highest percentages of lead in paint formulations; percentages declined afterward (EPA), 1986a; ATSDR, 1988). The oldest U.S. housing is to be found in the older areas of the nation. Figure 3-6 shows that the northeastern and mid-western areas of the nation have the highest percentages of pre-1940 housing.
In 1989–1990, the U.S. Department of Housing and Urban Development (HUD) conducted a survey to estimate better the extent of the lead-based-paint hazard in the U.S. housing stock (HUD, 1990). Among the 77 million privately owned and occupied homes in the
United States built before 1980, 57 million contained lead-based paint (defined as paint lead concentrations of at least 1.0 mg/cm 2). Families with children under 7 years old occupied an estimated 9.9 million of the 57 million; the 9.9 million included 3.7 million units with deteriorating (e.g., peeling) lead-based paint. The HUD survey provided additional detail on the location of the lead-based paint. Of the 57 million units with lead-based paint, 18 million had the paint only on exterior surfaces, 11 million only on interior surfaces, and 28 million on both.
Pope (1986) also determined (Table 3-4) from U.S. Bureau of the Census (1986) housing-survey data that about 6.2 million U.S. housing units are deteriorating and have leaded paint in unacceptable amounts. (As seen in Table 3-4, almost 1 million of these units were of pre-1940 construction.) Some 1.8 million children under 7 years old live in those units. Of these, 1.2 million are estimated by ATSDR (1988) to have blood lead concentrations over 15 µg/dL.
-
Case reports and case series. As noted in reports from ATSDR (1988) and NRC (1972), the clinical literature of the last 60 years is full of case reports and reviews documenting severe lead poisoning of young children—laboratory evidence of lead in blood, paint chips in the gastrointestinal tract, and no concurrent environmental evidence of other sources of lead exposure.
-
Field epidemiology. A particularly comprehensive data set, quantitatively linking child screening populations to leaded paint, is that from the 1976–1980 screening of children for lead poisoning in Chicago and the accompanying assessment of 80,000 housing units for the presence of leaded paint (Annest and Mahaffey, 1984). Schwartz and Levin (1991) analyzed the data and estimated a relative risk of approximately 15 for lead toxicity in summer months for children who resided in homes with leaded paint.
-
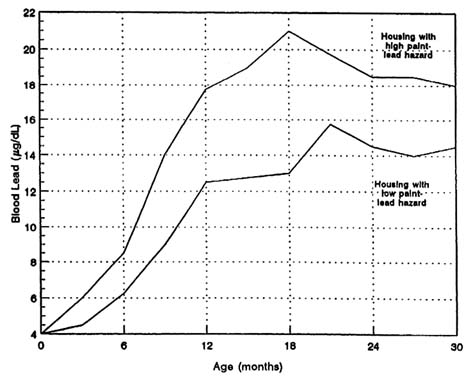
Research epidemiology. Numerous site-specific epidemiologic studies have been critically evaluated (e.g., EPA, 1986a). They have entailed multivariate regression analyses in which the size of the paint lead contributions to blood lead concentrations are calculated. A particularly detailed study is that of children in inner-city housing in Cincinnati, Ohio, with leaded paint and paint-related pathways of exposure (Clark et al., 1985; Bornschein et al., 1987). Figure 3-7 shows data from Clark et al. (1985, 1987) as reanalyzed by this committee;
TABLE 3-4 Estimated Numbers of Children Under 7 Years Old Residing in Unsound and Lead-Based-Paint U.S. Housing, by Age and Criteria of Deterioration
|
Category of Unsoundness |
Construction Date |
No. Unsound Lead-Based-Paint Homes |
No. Children |
|
Peeling paint |
Pre-1940 |
964,000 |
277,000 |
|
1940–1959 |
758,000 |
218,000 |
|
|
1960–1974 |
250,000 |
72,000 |
|
|
Total peeling paint |
Pre-1975 |
1,972,000 |
567,000 |
|
Broken plaster |
Pre-1975 |
1,594,000 |
458,000 |
|
Holes in walls |
Pre-1975 |
2,602,000 |
747,000 |
|
Grand totals |
Pre-1975 |
6,168,000 |
1,772,000 |
|
Source: Adapted from ATSDR, 1988. Data from Pope (1986) and 1983 housing survey data (U.S. Bureau of the Census 1986). |
|||
-
blood lead concentration is seen to change as a function of paint lead in housing.
HUD (1990) cited several published and unpublished studies on the influence of home renovation or abatement of lead-based paint on the blood lead concentration of children living in housing units during these activities. Bellinger et al. (1986b) reported a significant association between blood lead concentrations at age 24 months and recent home-refinishing activities. Rabinowitz et al. (1985a) reported a mean increase in blood lead concentrations (1.4 µg/dL, standard error 0.7) in children in homes recently refinished. Farfel (1987) reported that neither traditional nor modified methods of abating lead-based paint reduced the blood lead concentrations of children living in the residences. Farfel also reported that, at least in the short term, traditional abatement methods resulted in increased blood lead concentrations, presumably because of exposure to lead-laden dust. In contrast, three unpublished studies cited in the 1990 HUD report demonstrated blood lead reductions after traditional abatement methods.
Lead in Air
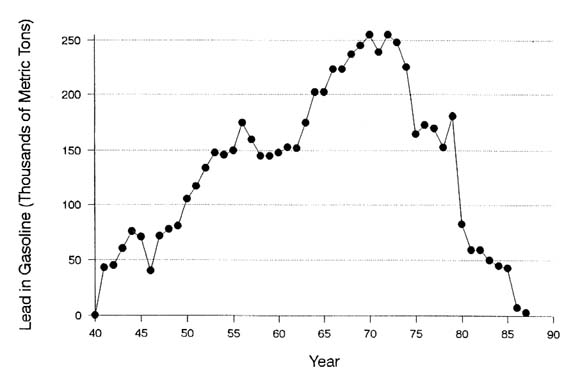
Lead enters air from gasoline and from stationary emissions. Environmental lead contamination from combustion of leaded gasoline has been widely documented in the United States and elsewhere (NRC, 1980; EPA, 1986a), and there is much evidence that it has added substantially to the body lead burdens of affected human populations.
From the 1920s to the late 1980s, lead was added to gasoline in the antiknock additive tetraethyl lead (later, this was mixed with tetramethyl lead). Tetraethyl lead is still used widely as a gasoline additive in many countries.
Physicochemical and Environmental Considerations
Lead in gasoline was emitted typically at approximately 24,000 µg/m3 at the tailpipe in the 1970s (Dzubay et al., 1979), and urban air contained
lead at about 1–10 µg/m3. A combination of air dilution and atmospheric fallout through dry and wet deposition accounts for the difference.
As described in detail elsewhere (EPA, 1986a), air lead from gasoline depends in a complex way on distances from vehicular traffic, lead content of gasoline, and mixing with the atmosphere. In closed spaces, such as garages and tunnels, air lead concentrations are well above those of open areas. Exhaust lead is discharged in such forms as halides and oxides, but these are eventually converted to the sulfate. Once the lead is dispersed, physical and chemical changes occur, including changes in particle size distributions, chemical changes from organic to inorganic lead, and chemical changes in the inorganic species themselves.
Most exhaust lead is deposited near its vehicular source (e.g., Reiter et al., 1977; Harrison and Laxen, 1981), whereas undeposited matter reaches a stable particle form within 100–200 km of its source. Particles approximately 10 µm in diameter are deposited over a broader distance, and there is long-range transport of particles less than 0.1 µm in diameter for up to about a month (e.g., Chamberlain et al., 1979).
General Characteristics of Exposure
The amount of lead consumed in the manufacture of antiknock additives for leaded gasoline has been enormous. In the United States, EPA (1986a) has estimated total consumption for 1975–1984 as 1.1 million metric tons. It has also estimated (EPA, 1986a) that 4–5 million metric tons have been deposited in the environmental in the United States since introduction of alkyl lead additives in the mid-1920s.
Leaded-gasoline use is being phased out in the United States, as a result of a series of regulatory actions beginning in 1973, when EPA promulgated the requirement for unleaded gasoline for use in vehicles with catalytic converters (EPA, 1973), devices that would be damaged by lead. In 1982, EPA promulgated new rules (EPA, 1982) that switched the basis of the standard from an average lead content of all gasoline to an average lead content of leaded gasoline and set a limit of 1.1 g/gal in leaded gasoline. On August 2, 1984, EPA proposed to reduce the permissible amount of lead in gasoline to 0.1 g/gal, effective
January 1, 1986. The final regulation, issued in early 1985 (EPA, 1985), imposed an interim limit of 0.5 g/gal in June 1985. As a result of those actions, lead use in gasoline declined from 175,000 metric tons in 1976 to less than 4,000 metric tons in 1988.
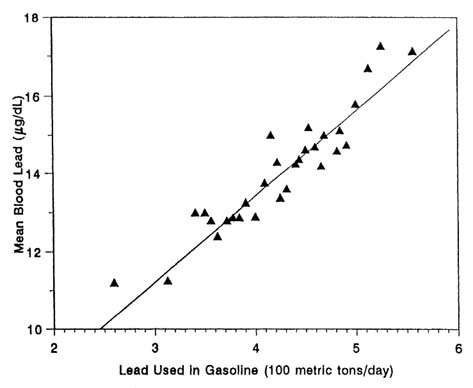
The decline has been accompanied by a remarkable parallel decline in the mean blood lead concentration of the U.S. population (see Figure 1-3). Figure 3-8 shows the adjusted mean blood lead concentrations in the NHANES II survey—controlled for age, race, sex, income, degree of urbanization, region of the country, occupational exposure, dietary intake, and alcohol and tobacco consumption—plotted against national gasoline lead use.
The various analyses of the blood lead-gasoline lead relationship, via the NHANES II data set (Annest et al., 1983; Schwartz and Pitcher, 1989) and data from an isotopic-lead study (Facchetti and Geiss, 1982), show that gasoline lead, via both direct inhalation and exposure to fallout, can account for 50% or more of total blood lead concentration at the earlier air lead contents attributable to gasoline consumption.
Scope of the Problem
Although leaded gasoline is being phased out in the United States, huge quantities of deposited lead remain in environmental compartments from the many decades of use of leaded gasoline starting in the 1920s. Using linear and logistic regression analysis, Schwartz et al. (1985) have estimated the U.S. short- and long-term effect of leaded gasoline in terms of decreases in blood lead concentrations due to the phasing out of leaded gasoline, projected to 1992. The estimates are presented in Table 3-5 for children 0.5–13 years old.
Lead in Dust and Soil
This section deals with a major pathway of exposure to lead: lead in dust and soil. Lead in those media are now recognized to be derived from several sources, including leaded paint and atmospheric lead. The magnitude of the pathway and of the associated health hazards has been documented only recently.
TABLE 3-5 Estimated Reductions in Blood Lead Content Because of Phaseout of Leaded Gasoline, Children 6 Months to 13 Years Olda,b
Physicochemical and Environmental Considerations
Technically, dusts and soils are discrete physicochemical substances. However, both are stable, immobilizable, and relatively permanent depositories of contaminating lead (Yankel et al., 1977; Angle et al., 1984; Brunekreef, 1984; CDC, 1985; EPA, 1986a; ATSDR, 1988). Soils reflect precursor geology; dusts reflect atmospheric fallout and other deposition. Dusts have a wider range of particle sizes, including very small particles. Dusts and soils interact differently in human exposures and numerous recent studies have documented the different types of interactions. Some are discussed below.
Lead is present in dusts and soils at potentially toxic concentrations, primarily because of its use in leaded paint and its fallout from the air (Nriagu, 1978; Brunekreef, 1984; Duggan and Inskip, 1985; EPA, 1986a; ATSDR, 1988). It is often difficult to apportion lead content in soils and dusts accurately to either paint or atmospheric lead, but one or
the other dominates in some circumstances and both contribute importantly in other circumstances.
Dusts and soils in remote communities near to primary lead smelters often are enriched in lead from atmospheric fallout due to these operations, either via direct emission or via re-entrainment of already highly contaminated media (e.g., Yankel et al., 1977; Angle et al., 1984; CDC, 1986a,b; EPA, 1986a; ATSDR, 1988). Soils and surfaces next to high-density roadways have similarly been documented as being heavily contaminated by vehicular exhaust emission of lead particles and other substances (Nriagu, 1978; Brunekreef, 1984; Duggan and Inskip, 1985; EPA, 1986a).
In inner-city areas with tracts of old, deteriorating housing, dusts and soils in adjacent interior and exterior sites are heavily contaminated with leaded paint (Sayre et al., 1974; Charney et al., 1983; Chisolm et al., 1985; Clark et al., 1985; Bornschein et al., 1987; EPA, 1986a; ATSDR, 1988). Studies of leaded-paint weathering in areas low in automobile density have identified contamination of soils and dusts and have shown a contamination pattern consistent with the presence of paint lead. Some inner-city neighborhoods also receive lead fallout from various mobile sources (vehicular exhaust) and stationary sources (secondary smelters, battery plants, and municipal incinerators).
A number of studies have attempted to measure the paint contribution to lead in dusts and soils; some are summarized in Table 3-6. Exterior-paint lead on homes, outbuildings, and such other outside entities as bridges will be transferred to adjacent soils and dusts (Ter Haar and Aronow, 1974; Linton et al., 1980; Landrigan et al., 1982; Fergusson and Schroeder, 1985; Schwar and Alexander, 1988). Movement of soil lead and interior-paint lead to interior dusts has also been documented (e.g., Clark et al., 1985; Bornschein et al., 1987, 1989). The removal of leaded paint from various surfaces warrants extreme caution.
Concentrations of lead in soils in rural areas of the United States are typically less than 30 µg/g of soil. In areas affected by lead mining, industrial emissions or vehicular traffic emitting leaded exhaust, such concentrations can increase by a factor of hundreds or even thousands. Automobile emissions account for most of the lead in soil and dust in suburban and rural areas (Nriagu, 1978; EPA, 1986a), whereas paint, atmospheric fallout from vehicular exhaust, and stationary sources account for most of the lead in urban dust and soil.
TABLE 3-6 Representative Studies of Contribution of Leaded Paint to Lead in Dusts and Soils
|
Study Site |
Study Design |
Results |
References |
|
Lead-painted frame and brick homes, Detroit, Mich., area |
Soil lead vs. distance from test buildings (N = 18 each type) |
Lead in soil 2 ft away 5 times higher than in samples 10 ft away |
Ter Haar and Aronow, 1974 |
|
Lead-painted rural barns and urban homes with leaded paint |
Soil lead vs. distance from two painted building types |
Similar soil content for both building types |
Ter Haar and Aronow, 1974 |
|
Outside areas around homes in small town |
Dust lead samples, curbside vs. at building line; electron and microscopic chemical and surface analysis with element markers |
25–85% of dwelling-line particles were paint flakes |
Linton et al., 1980 |
|
House dust from homes, Christchurch, New Zealand |
House dust lead as function of home age and type: painted surface, brick, etc. |
In homes with leaded paint in interiors, paint lead adds 45% to total dust lead content |
Fergusson and Schroeder, 1985 |
|
Neighboring soils, bridge, Mystic, Conn. |
Distance-stratified soil lead (1cm layer) from bridge during and after lead removal |
Soil lead 8,127 µg/g at bridge; 3,272 µg/g up to 30 m away; 457 µg/g 30–80 m away, and 197 µg/g 100 m away |
Landrigan et al., 1982 |
|
Study Site |
Study Design |
Results |
References |
|
Variable-quality housing, Cincinnati, Ohio |
Dust lead (internal and external) and dust fall rate vs. house age, paint lead, and condition |
All measures much higher in poor housing with paint lead |
Clark et al., 1985 |
|
Variable-quality housing, Cincinnati, Ohio |
Statistical analysis (structural equation modeling) of lead pathway in 18-month-olds |
Paint lead and external-dust lead explain 52% of dust-lead variation; paint lead correlated with external-dust lead |
Bornschein et al., 1987 |
|
Various residential areas and homes undergoing deleading |
Analysis of housedust lead or child blood lead from paint dust generated during and after paint lead removal |
Such dust formation has substantial effect on child exposure and blood lead |
Rey-Alvarez and Menke Hargrave, 1987; Amitai etal., 1987; Farfel and Chisolm, 1987; Rabinowitz et al., 1985a; Charney et al., 1983 |
|
Playground areas at schools undergoing lead-paint removal and repainting |
279 schools in London, England, tested for play-area dust lead before, during, and after removal of old paint |
Substantial increases in play-area dust lead after old-paint removal |
Schwar and Alexander, 1988 |
Atmospheric lead from exhaust and stationary sources is transferred to soils through both dry (Friedlander, 1977; Schack et al., 1985) and wet (Lindberg and Harriss, 1981; Talbot and Andren, 1983; Barrie and Vet, 1984) depositional processes. The proportions of the type of contribution to the total deposition of lead vary markedly; wet depositional processes can account for up to 80% (Talbot and Andren, 1983).
Lead deposition onto soils from vehicular exhaust declines exponentially with distance from the roadway (EPA, 1986a). Much of this lead is immobilized in the top 5 cm of undisturbed soil (Reaves and Berrow, 1984) according to a complex function of geochemistry and pH (Olson and Skogerboe, 1975; Zimdahl and Skogerboe, 1977).
General Characteristics of Exposure
The extent to which lead in dusts and soils is translated into blood lead and later to some adverse effect depends first on the nature of the exposed population. Among sensitive populations, young children are most exposed to lead via dusts and soils, because they commonly put their hands in their mouths and often mouth or ingest contaminated soils.
The relationship of lead in dusts and soils to blood lead in young children has been the subject of various epidemiologic studies in urban areas and in rural areas that have stationary sources, such as smelters. The studies have been examined by EPA (1986a) and ATSDR (1988); the key studies include those of Yankel et al. (1977), Angle et al. (1984), CDC (1986a,b), Bornschein et al. (1987), and Rabinowitz and Bellinger (1988).
The complexities of and relation among sources and pathways have been quantitatively examined by Bornschein and co-workers (1987) on the basis of longitudinal environmental epidemiologic assessments of 18-month-old inner-city children. The children had substantial but not exclusive lead exposure through mobilizable leaded paint in poor-quality housing. The authors found that blood lead concentrations are influenced by the presence of lead in interior and exterior dust through hand pickup of lead in exploratory activity; dust lead is controlled by exterior dust (sampled as surface scrapings) and interior paint; and the lead concentration gradient works from the exterior to the interior, so the
external contamination around a child's residence markedly affects the interior contamination and thus the exposure risk.
Other studies have shown a strong association of soil and interior dust lead with children's blood lead concentration; the various regression-slope estimates for earlier reports have been tabulated by EPA (1986a). The estimates cover a broad range, but suggest that detectable effects of these media on blood lead concentrations would occur at dust and soil concentrations of 500–1,000 µg/g (CDC, 1985). Particle size, chemical species of lead, and type of soil and dust matrices are important modifiers of the soil and dust lead hazard, because they influence lead intake and absorption (Roy, 1977; Barltrop and Meek, 1979; Heyworth et al., 1981; Healey, et al., 1982; Dornan, 1986; Koh and Babidge, 1986; Steele, et al., 1990). For example, particles of different lead-based paints are likely to have different solubilities (e.g., higher for the older lead carbonate paints, lower for the newer lead chromate paints), different particle sizes (large for paint chips ingested directly from walls or window sills, smaller for particles settled on dust or soil, very fine for particles formed by chalking or burned off walls by poorly applied heat guns), and thus different bioavailability for young children.
Scope of the Problem
A total of 20 million or more housing units were built before 1940; they are the units most likely to have old flaking, chalking, and weathering highly leaded paint that is being transferred to soils and becoming dust. The estimated numbers of young children discussed earlier as exposed to leaded paint are simultaneously exposed to lead in dust and soil. Added to the lead in soil and dust from paint in urban areas are the sizable amounts of lead from fallout from heavy vehicular traffic and stationary sources.
Schwartz et al. (1985) have used linear and logistic regression analysis of declines in child blood lead concentration associated with phasing out of leaded gasoline to estimate that 1.35 million children in 1989 and 1.25 million children in 1990 will have blood lead concentrations below 15 µg/dL as a result of the control action. Those numbers, when combined with projections to 1992, reflect a substantial change in dust lead concentrations associated with the decrease in fallout.
Lead in Drinking Water
Lead in tap water—consumed in the home, offices, other worksites, and public buildings—can be a particularly important source of lead exposure of young children, pregnant women, and other people (Moore et al., 1985; Levin, 1986, 1987; Ohanian, 1986; ATSDR, 1988). The potentially major role of tap-water lead in overall human exposure has long been recognized in Europe and older areas of the United States, but only recently has the full scope of the U.S. water-lead problem been examined. Reasons include the complex, heterogeneous nature of water supplies, the absence of detailed current survey data, and the relative exclusion of tap-water lead in environmental exposure assessment of lead-poisoning cases.
Physicochemical and Environmental Considerations
Tap-water lead concentrations are highly variable from house to house and tap to tap, because of differences in soldering, temperature, and water use. Any attempt to measure exposure or compliance with a target concentration must rest on an adequate sample size. For example, Schock et al. (1989) used data from four communities and found that 225–625 samples were required to produce a sample mean within 20% of the population mean (95% confidence limit), depending on the community. If no more than 10% of subjects should be exposed above a given value, the number of samples needed for accurate statistical inference would be even higher.
Lead theoretically can enter tap water at any of several points in the delivery system. A water-treatment plant distributes finished water with very little lead (e.g., Levin, 1986) and little more is added through the distribution lines, but contamination of domestic tap water occurs at five kinds of points in or near residential, public, or office core plumbing: lead connectors (i.e., goosenecks or pigtails), lead service line, lead-soldered joints in copper plumbing, lead-containing drinking fountains and water coolers, and lead-containing brass faucets and other fixtures. A host of chemical and physicochemical variables affect the extent to which any or all of those sites contribute to the water lead content. The
important variables include the relative corrosivity of the water (i.e., acidity, alkalinity, and ion content), the standing time of water in contact with leaded surfaces, the age of lead-soldered joints and other leaded components, the quantity and surface area of lead sites, and the temperature of water in contact with lead surfaces.
In general, the problem with lead connectors and service pipe is associated principally with old housing, built around 1920 or before, in older northeastern American cities, particularly such New England cities as Boston (Worth et al., 1981; Karalekas et al., 1983). Since 1986, federal law has prohibited lead for these uses in new construction.
Solder-lead leaching varies with the age of plumbing and diminishes as the solder sites age, a process assumed to take about 5 years. The extent of lead leaching is strongly affected by the acidity of the water (i.e., pH), as seen in Table 3-7, which summarizes EPA data on pH and age of homes. With corrosive water (pH less than 6.4), it can be seen that soldered joints more than 5 years old still leach sizable amounts of lead in first-draw samples. Copper plumbing with lead-soldered joints came into widespread use in the United States and other developed countries in the 1950s.
Brass faucets and other fixtures containing alloyed lead at various percentages, even below current permissible percentage (8%), can contribute to tap-water contamination (Samuels and Meranger, 1984; Schock and Neff, 1988; Gardels and Sorg, 1989). Gardels and Sorg (1989) reported that newer brass faucets could contaminate standing water closest to the fixtures (less than 250 ml) at over 10 µg/L, an action concentration promulgated by EPA (1991).
In public facilities that serve young children and other sensitive populations, such as kindergartens and elementary schools, additional exposure to lead in tap water can occur (Levin, 1986; ATSDR, 1988; EPA, 1990c). Patterns of water use in schools potentially can allow greater exposures than in homes. For example, lead leaching is at its maximum into standing water, i.e., water generated overnight, during weekends, and during holiday and summer vacation periods. Water contamination in schools and the like can occur in water coolers and fountains, as well as in the expected core plumbing and fixtures (ATSDR, 1988; Gardels, 1989).
TABLE 3-7 Percentage of Variably Collected Water Samples Exceeding Lead at 20 µg/L at Different pH, by Age of House
|
|
|
Samples with Lead over 20 µg/L> |
|
|
Age of House, yr |
pH |
First Flush |
% Fully Flushed (2 min) |
|
0–2 |
<6.4 |
93 |
51 |
|
|
7.0–7.4 |
83 |
5 |
|
|
>8.0 |
72 |
0 |
|
More than 2, less than 6 |
<6.4 |
84 |
19 |
|
|
7.0–7.4 |
28 |
7 |
|
|
>8.0 |
18 |
4 |
|
6 or more |
<6.4 |
51 |
4 |
|
|
7.0–7.4 |
14 |
0 |
|
|
>8.0 |
13 |
3 |
|
Source: ATSDR, 1988. Data from EPA, 1987. |
|||
General Characteristics of Exposure
Tap-water lead affects different groups in different ways. For example, lead-contaminated water can be used in infant formula and in beverages for older children and can be consumed directly. Tap-water lead can be ingested in foods cooked in lead-contaminated water. Furthermore, food surfaces can bind and concentrate water lead (Smart et al., 1981; Moore, 1985).
Lead in tap water is much more bioavailable than lead in food, because it is often consumed during semifasting (between meals) or after fasting (overnight) conditions. According to the data of Heard and Chamberlain (1982) and Rabinowitz et al. (1980), adults' fasting absorption rates can be 60% or higher, compared with rates of 10–15% in association with meals.
The marked reductions in blood lead concentrations of water-consumers
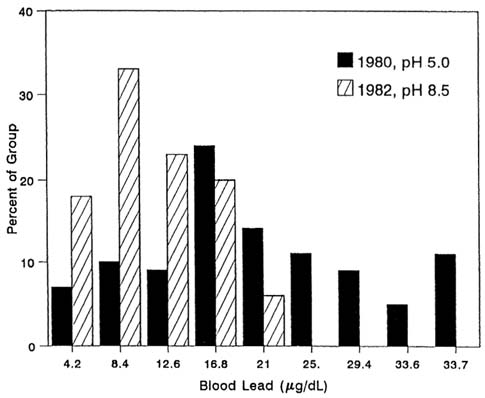
in Glasgow and Ayr, Scotland, after water-treatment steps to reduce corrosivity (see, e.g., Moore et al., 1981; Sherlock et al., 1984; Moore, 1985) constitute convincing evidence of an impact of water lead on blood lead concentration. Figure 3-9 shows blood lead concentration distributions for two periods in a single group of mothers mointored before and after change in tap-water pH in Ayr, Scotland (Richards and Moore, 1984; Sherlock et al., 1984). Significant declines were observed in blood lead values as water was treated between 1980 and 1982.
With respect to case reports of lead intoxication associated with tap water, Cosgrove et al. (1989) reported lead intoxication—blood lead concentrations ranging up to 45 µg/dL—in a toddler found to have been exposed to lead solely from tap water, which entered the home through new copper plumbing with lead-soldered fittings. First-draw water samples averaged 390 µg/L and were as high as 1,080 µg/L.
Environmental epidemiologic studies have attempted to analyze the quantitative relation of tap-water lead to blood lead concentrations in both infants and adults. Some of the studies considered both dietary and water data; others examined only tap-water lead. As can be seen in Table 3-8 and in the very detailed Table 11-51 of EPA (1986a), for mainly first-draw water samples (except Sherlock et al., 1984), the relation of blood lead to tap-water lead is complex and a function of the concentration range of water and blood lead in the cohort. Over a broad range of water concentrations, well above 100 µg/L, the relation is curvilinear (e.g., Worth et al., 1981; U.K. Central Directorate on Environmental Pollution, 1982; Sherlock et al., 1984); it becomes linear at the low end of water content, i.e., less than 100 µg/L (Pocock et al., 1983). At the higher concentrations, the relation is best fitted through logarithmic or cube-root expressions.
Scope of the Problem
Table 3-9, based on data from Levin (1986) and ATSDR (1988), shows the numbers of children up to 13 years old who were at risk of exposure to lead from domestic plumbing. As indicated, 1.8 million children up to 13 years old lived in homes with newly installed lead-soldered plumbing (that is less than 2 years old), of whom 0.7 million
TABLE 3-8 Selected Studies of Relation of Blood Lead to Tap-Water Lead
|
Study Details |
Form of Model |
Reference |
|
524 Boston residents; water lead up to 1,108 µg/L; blood lead up to 71 µg/dL |
ln(blood lead) = ln[0.041 (water lead) 0.000219 (water lead)2] |
EPA (1986a) analysis of Worth et al., 1981 |
|
128 Glasgow mothers; water lead up to 1,060 µg/L; blood lead up to 39 µg/dL |
Blood lead = 13.2 + 1.18 (water lead)1/3 |
U.K. Central Direct., 1982 |
|
126 Glasgow infants of above mothers |
Blood lead = 9.4 + 2.4 (water lead)1/3 |
U.K. Central Direct., 1982 |
|
114 Ayr, Scotland, mothers before and after water treatment |
Blood lead = 5.6 + 2.62 (water lead)1/3 |
Sherlock et al., 1984 |
|
7,735 middle-aged British men; water lead less than 100 µg/L |
Blood lead = 14.48 + 0.062 (water lead) |
Pocock et al., 1983 |
|
Source: Adapted from EPA, 1986a, Vol. III. |
||
TABLE 3-9 Estimated Numbers of Children at Risk of Exposure to Lead in Household Plumbing
|
Housing Type |
Population at Risk |
|
New Housinga |
|
|
8.8 million people in new housing with lead soldered piping: |
|
|
(8.8 million) (7.6% of population less than 5 years old) |
0.7 million |
|
(8.8 million) (12.8% of population 5–13 years old) |
1.1 million |
|
Total number of children at risk in new housing |
1.8 million |
|
Old Housingb |
|
|
If one-third of units built before 1939 contain lead pipes,c then (0.33) (0.29) = 10% of housing has lead pipes: |
|
|
(0.10) (17.8 million children less than 5 years old) |
1.8 million |
|
(0.10) (30.1 million children 5–13 years old) |
3.0 million |
|
Total number of children at risk in old housing |
4.8 million |
|
a Data from Levin, 1986; based on 9.6 million in new homes, of which 92% have metal plumbing. b Data from U.S. Bureau of the Census, 1985. c Data from David Moore, Office of Policy Development and Research, HUD, Submissions to ATSDR, January 1987, and EPA Source: ATSDR, 1988. |
|
were less than 5 years old. The corresponding tally for old leaded plumbing is 4.8 million, of whom 1.8 million were under 5 years old. The two groups yield a total of 6.6 million children.
According to Levin's (1986) analyses, 42 million U.S. residents receive water from public supplies having lead concentrations above 20 µg/L. ATSDR (1988) estimates that 3.8 million of those are chil-
20 µg/L. ATSDR (1988) estimates that 3.8 million of those are children less than 6 years old.
Levin (1987) has used regression-analysis methods described by Schwartz et al. (1985) to estimate that 240,000 children less than 6 years old will have blood lead concentrations over 15 µg/dL, in part because of exposure to lead in tap water.
Lead in the Diet
Physicochemical and Environmental Considerations
Lead contaminates food through various pathways: deposition of airborne lead, binding of soil lead to root crops, use of lead-contaminated water and equipment in processing, use of lead-soldered cans for canned foods, and lead leaching from poorly made lead-glazed food and beverage containers.
Lead is readily deposited on leaf surfaces of edible plants (e.g., Schuck and Locke, 1970) and accumulates over the life of the crop. The deposition rate in areas with high air lead content can measurably increase the lead content of leafy crops, and such surface contamination is difficult to remove by either harvest washing or rainfall (Page et al., 1971; Arvik and Zimdahl, 1974).
Transfer of lead from soil to edible roots is a complex function of physicochemical factors that govern the plant uptake of lead, including those mentioned earlier in this chapter. Camerlynck and Kiekens (1982) reported that normal soils contain exchangeable lead at approximately 1 µg/g or less, and presumably some portion of the mobile lead will bind to plant roots.
Lead in processing water can sometimes be the major contributor to dietary lead (Moore et al., 1979; Smart et al., 1981). However, the more common source of food contamination in processing is the use of lead-soldered cans. When lead is used as seam solder, the material can spatter on the interior of the can or the toxicant can migrate to the canned-food matrix itself. Acidic foods induce more lead release from the soldering material, although the leaching phenomenon also occurs
with relatively low-acidity foods, such as corn and beans, and in all cases the total amounts liberated are a function of the shelf-life of the canned goods. Lead release is accelerated by contact with oxygen once a can is opened. Lead in wine has been shown to be a potentially important source of dietary lead exposure (e.g., Elinder et al., 1988).
Pottery, dinnerware, and other ceramic items are used to store foods. If containers so used have been made with poorly fired leaded glazes, lead can migrate from them into the food (extensively discussed in Lead in Housewares, U.S. House, 1988; see also Wallace et al., 1985). Key factors affecting lead release include characteristics of the glaze, the temperature and duration of food storage, and the acidity of the food. Lead can also be released on extended scrubbing and cleaning of even well-prepared glazes.
Commercial American products have led to fewer problems in this regard than commercial products from other countries—such as countries in southern Europe and Latin America and mainland China—or items made by artisans and hobbyists. Most cases of lead toxicity have been associated with repeated use of vessels with problematic glazes or with prolonged food storage.
Glassware is often decorated with decals or decorative surfaces that contain lead. Those surfaces have a potential for exposure through contact with young children's lips and mouths.
Characteristics of General Exposure
The contribution of foods and beverages to body lead burden, as reflected in blood lead, has been measured in epidemiologic surveys of infants, toddlers, and older people (EPA, 1986a). Various studies have shown that dietary lead can contribute substantially to blood lead in complex ways that reflect the influence of the tap-water lead component, dietary habits, and individual differences in lead toxicokinetics (see, e.g., EPA, 1986a).
Ryu et al. (1983, 1985) found that dietary lead affects blood lead in infants in a simple linear fashion, at least in moderate exposures. Sherlock et al. (1982) and the U.K. Central Directorate on Environmental Pollution (1982) examined the relation in infants and mothers via a duplicate-diet survey. Blood lead in the infants in the U.K. study was
related to dietary lead by both linear and cube-root functions, whereas Sherlock and co-workers found a cube-root relation for mothers and infants. The relation becomes curvilinear when intake exceeds 100 µg/day. The slopes of the curves (µg/dL of blood vs. µg/day), which can be estimated from the above studies for an intake of 100–200 µg/day, are 0.034 for adults (Sherlock et al., 1982), 0.06 for infants (Sherlock et al., 1982), 0.053–0.056 for infants (U.K. Central Directorate, 1982), and 0.16 for infants (Ryu et al., 1985). The relation of Ryu and co-workers has the steepest slope and is based on the lowest average lead intake; the slope might level at much higher lead intakes.
Dietary intakes of lead are being reduced in the United States (EPA, 1986a; ATSDR, 1988). For 2-year-olds, for example, there was a decline of approximately 75%, from 52.9 to 13.1 µg/day, from 1978 to 1985. It should be remembered that there is a distribution of lead content about the average and that the diet-survey numbers are based on relatively small samples, compared with the volume and diversity of the U.S. food supply. Several important factors in the decline include the domestic phaseout of lead-seamed beverage and food cans and the reduction in movement of lead to agronomic crops, as a result of a lowering exposure of growing crops to air lead. The latter is associated with the phaseout of leaded gasoline and tighter stationary-source regulations.
Table 3-10 shows U.S. production of lead-seamed cans in 1980 and 1988; these are production figures supplied by a trade group and do not reflect independent surveys of lead-seamed cans on grocery-store shelves. The latter would include some carryover from past years' production, depending on canned-food shelf life.
Scope of the Problem
As noted by Mushak and Crocetti (1989), virtually all sensitive populations are exposed to some lead in food, owing to the relatively centralized food production and distribution system in the United States and other developed nations. They also estimated on the basis of food-lead concentration distribution profiles, adjustments for lead reduction in foods, intakes from other lead sources—that approximately 5% of the 21 million U.S. children less than 6 years old, or 1 million children,
TABLE 3-10 Changes in Percentage of Lead-Soldered Food and Soft-Drink Cans (millions of cans)a
will have increases in blood lead concentrations because of intake of lead in food.
Although the main theme of this report is ordinary environmental exposure to lead, occupational settings present the highest, continuous lead exposures of all. Furthermore, workers transport lead from work to their homes, where their families, including children, are exposed to it (e.g., Baker et al., 1977; Milar and Mushak, 1982).
Various traditional customs and medications can result in high lead exposures. Reports of such exposures are numerous in the clinical literature from various regions—Arab countries (Aslam et al., 1979), the Indian-Pakistani subcontinent (Pontifex and Garg, 1985), China (CDC, 1983a), and Latin America (Bose et al., 1983; CDC, 1983b; Trotter, 1985; Baer et al., 1987). The preparations often include lead compounds as major or principal ingredients, so the poisoning potential is high.
In the United States, the most familiar type of lead-containing preparation is a Mexican-American folk preparation that contains lead oxides (Bose et al., 1983; Trotter, 1985; Baer et al., 1987). Greta (lead (II) oxide) and azarcon (mixed-valence lead tetroxide, PbO2.2PbO) are used to treat digestive disorders; their use produces diarrhea or vomiting. Use of these medicines is widespread and can result in serious lead poisoning in children.
SUMMARY
It is difficult to rank sources of lead exposure by their importance for health by such simple criteria as numbers of affected persons. Simultaneous exposure to multiple lead sources is inevitable; different sources of lead are often associated with different degrees of lead poisoning, which would make it necessary to rank by effect severity, as well as frequency; and sources differ in distribution among sensitive populations. An alternative is to provide a ranking by relative overall impact, which includes the potential of a source for the most severe poisoning, its relative pervasiveness, estimates of numbers of persons exposed to it, and the relative difficulty of abating it.
The sensitive populations within the general, nonoccupational sector are preschool children, fetuses (via maternal exposure), and pregnant
women (as surrogates for fetuses). On the basis of overall public-health impact on those populations, sources can be combined into two groups. Lead in paint, lead in dusts and soils, and lead in drinking water constitute the more important group today. In that group, leaded paint ranks first in importance for young children, followed closely by lead in dusts and soils, and then by tap-water lead. For adults, tap-water lead is probably the exogenous source of most concern. (Endogenous exposure to lead can occur when subjects mobilize lead and calcium from bone; this typically occurs in adults or in children who break bones.) In the United States, leaded gasoline at present concentrations and dietary lead make up the second group, of somewhat less concern. These statements of importance are relative; they do not imply that any specific source is unimportant as a contributor to lead body burdens or to earlier effects in populations as a whole. The body combines lead absorbed from all sources into one dose.
The phasedown of leaded gasoline is greatly reducing the input of lead to environmental compartments. However, the inventory of 4–5 million metric tons of lead still in the environment because of past leaded-gasoline use will continue to contribute to the risk of exposure of sensitive populations. Outside the United States, various approaches to leaded-gasoline control are being taken, from modest control actions to phaseout and phasedown regulations.
Leaded paint (and its transport to dusts and soils) is a major national source of exposure of children. Dust and soil lead comes from leaded-paint transfer and atmospheric fallout, and many studies have documented its contribution to lead body burdens of young children. Quantitative assessments of the relative contributions of dust and soil lead to total body lead, such as blood lead concentration, have been the subject of diverse studies. In addition, particle size, chemical species of lead, and soil and dust matrices are important modifiers of the soil and dust lead hazard eventually reflected in lead intake and absorption.
Pathways of exposure to tap-water lead are multiple: direct drinking, beverages prepared with contaminated water, and foods cooked in lead-contaminated water. Patterns of leaded-water use can amplify toxicity risk. Ingestion on an empty stomach, a common occurrence, greatly increases the rate of lead absorption. The use of water in elementary schools and other child facilities is intermittent, with extended standing time over weekends and in vacation periods. That allows buildup of lead in fountains and water lines.
Most developed countries, including the United States, have complex food production and food distribution systems that permit lead contamination. Virtually everyone has some exposure to dietary lead, and lead concentrations in food can be quite high. But lead in foods of older children and infants has been reduced through phasing out of lead-soldered cans for milk and fruit juices and reduced input into food crops.
There does appear to be a persisting problem with lead leaching mainly from poorly made and lead-glazed food and beverage pottery. It could also be that even well-made vessels with lead glazes will lose lead through extended surface abrasion, as in scrubbing, washing, and rinsing.