D
Supercritical Water Oxidation and Wet Air Oxidation
Supercritical water oxidation and wet air oxidation—two moderate-temperature, high-pressure processes—have attracted interest because of some potential advantage over components of the baseline system:
-
The processes lend themselves to the use of oxygen rather than air; the volume of off-gases can be reduced as a result.
-
Some potential gas pollutants do not appear to be formed: particulates, NOx, and dioxins, for example (Some nitrogen in feed materials has shown up as N2O).
-
The processes operate with low or moderate concentration of material in water, typically 1 to 10 weight percent; consequently, they are particularly useful when the feed material is already available in dilute form.
-
The processes are like incineration in that they are broadly applicable to any oxidizable organic compound.
Typical operating conditions for these two processes are
-
for wet air oxidation,
-
temperature 200-300ºC,
-
pressure 600-2,000 pounds per square inch; and
-
residence time in reactor, 1-2 hours; and
-
for supercritical water oxidation,
-
temperature 450-600ºC,
-
pressure 3,500+ pounds per square inch, and
-
residence time in reactor, 10 seconds to 2 minutes.
Wet air oxidation has been in commercial use for more than 20 years. There are more than 200 plants in operation worldwide, working on a variety of feedstocks: spent caustics, sludge from municipal and industrial wastewater, pulp and paper waste, metallurgical processing waste streams, etc. Supercritical water oxidation is being actively studied by many research groups; commercial applications are believed to be imminent.
The higher temperature of Supercritical water oxidation (SCWO) compared with wet air oxidation (WAO) has a large effect on reaction rates, which shows up in the different reactor residence times. Additionally, the oxidation process in wet air oxidation is far from complete; 20 to 50 percent of the organic carbon will remain, though in altered form, as small organics such as acetic acid. In contrast, Supercritical water oxidation can oxidize organic material almost completely. The aqueous phase from wet air oxidation will require further treatment; it is usually fed to a biological treatment process. The aqueous phase from Supercritical water oxidation may not need this type of further treatment.
Reaction rates for chemical agents or surrogate compounds have not been measured directly. Test results on wet air oxidation for a large number of related materials have been reported (Copa and Lehmann, 1992). For example, various herbicides and pesticides—phosphorus compounds related to nerve agents—have been destroyed to high destruction removal efficiency (DRE) levels (99.99 percent):
-
round-up—a phosphono compound (i.e., C—P bond);
-
dursban—a thinophosphorus compound; and
-
malathion—a dithiophosphorus compound.
The reaction rates for large molecules appear to be high. Some of the oxidation products, however (e.g., acetic acid), are much more refractory and persist in the wet air oxidation product. They require the higher temperature and pressure of supercritical water oxidation for their oxidation in reasonable time.
Reaction rate data for some small molecules have been reported in the form of first-order reaction rate constants (Testor et al., 1991). Some materials (e.g., methanol), which are intermediates in the oxidation of larger species, show large rate dependence on temperature. The data illustrate why wet air oxidation leaves a large fraction of the organic carbon remaining as small molecules such as methyl alcohol; the higher temperature of supercritical water oxidation ensures a much higher level of destruction.
For example
Half-Life of Methanol 3,500-psi Pressure
|
Temperature (ºC) |
Half-Life (Seconds) |
|
450 |
2,065 |
|
500 |
21 |
|
530 |
1.5 |
Data on surrogates of chemical agents have shown that the C—P bond (as in GB and VX) is the most resistant to cleavage under supercritical water oxidation conditions (E. Gloyna, personal communication with Dr. Walter May, 1993). For example, it was shown that DMMP (dimethoxy methylphosphonate) is hydrolyzed readily to the corresponding methylphosphonic acid.
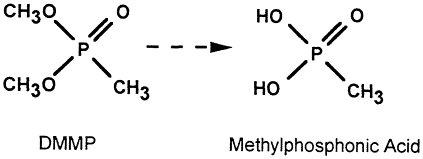
The acid product however was more resistant to attack; 10 percent of theoretical oxygen demand remained in a test at 520ºC, 7 seconds residence time.
Small scale pilot plant tests have recently been carried out on GB and VX, under supercritical water oxidation conditions (ARPA, 1993). Conditions were 4,000 pounds per square inch, 450-525ºC, and residence times of 10 seconds or more. The agents were "completely" destroyed (DRE of more than six 9s). Traces of acetic acid and formic acid were reported in some runs. The data indicate that temperatures and residence times can be chosen to reduce the organic content of the water to negligible levels; no after-treatment for removal of organics should be needed.
No pilot plant tests on mustard have been attempted yet. The reactivity should exceed that of GB and VX, based on some surrogate compound work. (There is no C—P bond, the resistant bond in nerve agents.) Other problems are expected, however.
Corrosion has been a major problem with both supercritical water and wet air oxidation, particularly in the presence of the acidic reaction products that result from chemical agent oxidation. A lot of metals and a few refractories were tested for reactivity or solubility under acidic conditions expected for GB or VX oxidation, at 350 and 550ºC. Some of the results (Gloyna, personal communication with Dr. Walter May, 1993) follow:
|
Material |
Loss rate (mils/year)) |
|
Pt, Pt-Ir, Pt-Rh |
0.1-1 |
|
Ta, Nb |
50-3,000 |
|
Ti |
100 |
|
Ni alloys |
100 |
|
ZrO2 |
10-150 |
|
A12 O3 |
100-800 |
|
SiC |
1,000 |
Platinum or other noble metals stood up well, as shown. They do not stand up to chloride ion, or to chloride-nitrate, at low pH, however. The corrosion rate in HCl or in HCl-HNO3 solutions was increased a thousandfold or more over other reagents such as HF. Other refractory metals (Ta, Nb, Hastelloy C) also dissolved excessively in acidic chloride solution.
A platinum liner was chosen for the pilot reactor used at the Illinois Institute of Technology Research Institute for the GB and VX tests (ARPA, 1993). Mustard however presents a problem because of its high chlorine content (44 weight percent).
Corrosion can be sharply reduced by caustic addition to increase the pH. This raises a serious problem with supercritical water oxidation, however: the salts formed are insoluble in the supercritical fluid under the "usual" supercritical water oxidation conditions. Insoluble salts have led to plugging problems in the reactor. Techniques for handling the problem, or getting around it, are under investigation. The problem will arise with mustard because of the need to control corrosion under supercritical water oxidation conditions. The "plugging" problem is not expected to arise with wet air oxidation; the alkali salts formed at high pH are soluble in the liquid water present.
The control of corrosion by increased pH has another possible disadvantage: the CO2 from the reaction products can react with the excess caustic to form additional salt—sodium carbonate. The total solid product can be increased two- or threefold as a consequence, which is generally considered undesirable.
SUMMARY
The two processes are in different stages of development. Wet air oxidation has been in commercial use for many years. Supercritical water oxidation is under active research and development.
Both processes are generally applicable to organic materials. They have somewhat different advantages and disadvantages:
-
Wet air oxidation does not perform a complete mineralization. The high organic content of the effluent water will require further processing (e.g., biological treatment).
-
Corrosion is expected to be a severe problem with both technologies, requiring control by suitable choice of material and/or control of pH. Corrosion is a particular problem with supercritical water oxidation, where pH control has thus far led to plugging problems.





