2
The Unitary Chemical Agent and Munitions Stockpile
This chapter presents a brief overview of the chemical agents in the U.S. stockpile and their containers and associated munitions. It describes inventories and their locations, and assesses the condition of the stockpile. Comprehensive data on the first two parts can be found in a number of other reports (NRC, 1984; U.S. Army, 1988) including the National Research Council's Alternatives report (NRC, 1993a).
AGENTS
The two principal types of agent in the U.S. stockpile are nerve agents (GB1 and VX2) and blister or mustard agents (H, HD, HT). Each is found in a variety of containers and munitions. The structures of these compounds are shown in Chapter 3.
Nerve agents are organophosphonate compounds that contain phosphorus double-bonded to an oxygen atom and single-bonded to a carbon atom. They are highly toxic or lethal in both liquid and vapor forms. In pure form, the nerve agents are practically colorless and odorless. GB evaporates at about the same rate as water and is relatively nonpersistent in the environment. VX evaporates much more slowly and can persist for a long time under average weather conditions.
Bis(2-chloroethyl)sulfide is the principal active ingredient in blister agents or mustard.3 Mustard has a garlic-like odor. It presents both vapor and
contact hazards. Because it is practically insoluble in water, mustard is very persistent in the environment and can contaminate both soils and surfaces for a long time.
CONTAINERS AND MUNITIONS
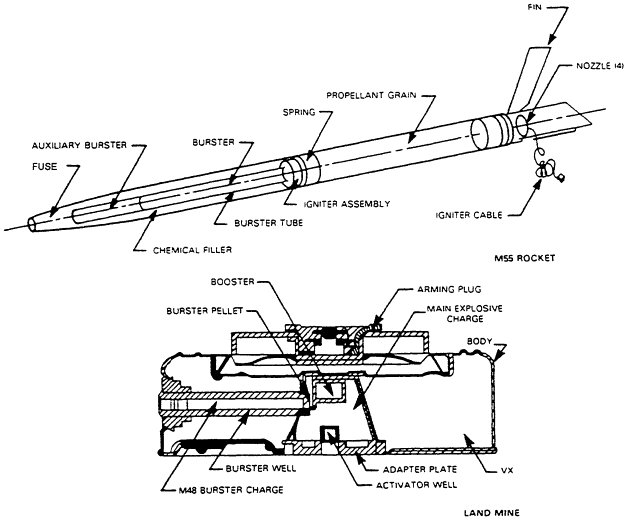
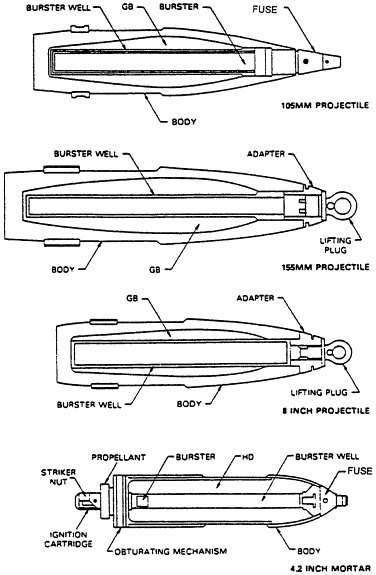
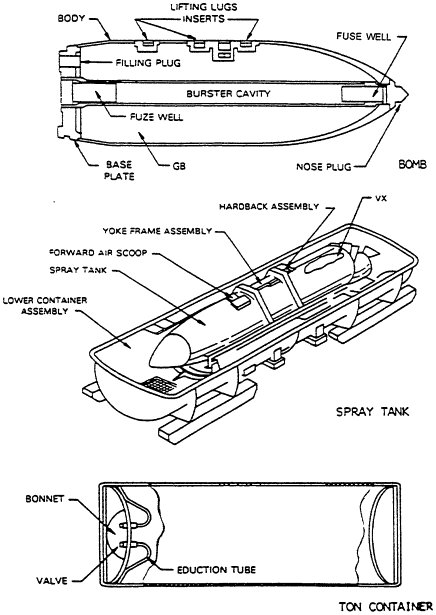
The stockpile of unitary chemical agents can be found in containers (various bombs stored without explosives, aerial spray tanks, and ton containers) and munitions (land mines, M55 rockets, artillery projectiles, and mortar projectiles) (Figures 2-1, 2-2, and 2-3). Some munitions have no explosives or propellant, whereas others contain some combination of fuse, booster, burster, and propellant (Table 2-1). Generally, these components are referred to collectively as energetics. They incorporate a variety of chemical compounds that must be eliminated as part of the chemical stockpile disposal operation.
The fuse, a small, highly sensitive explosive element, initiates an explosive chain by detonating a booster. The booster is an intermediate charge, sensitive enough to be detonated by the fuse and energetic enough to detonate the much larger burster. The burster, the end of the chain, bursts the munition with sufficient energy to disperse the agent held in the munition. The M55 rocket also contains an integral solid rocket propellant that can be removed only by cutting open the rocket.4
GEOGRAPHICAL DISTRIBUTION
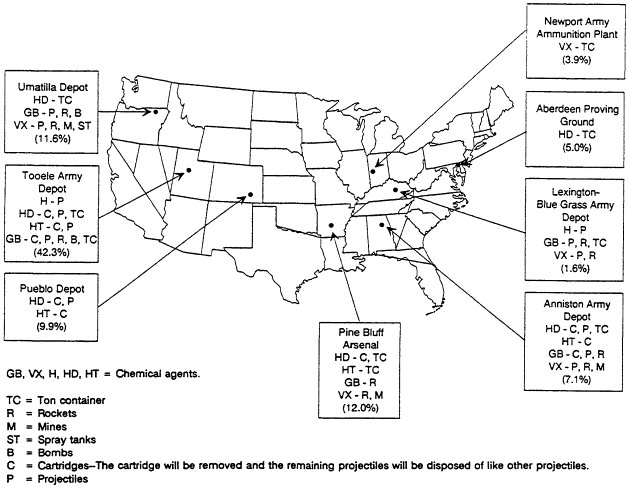
The unitary chemical stockpile is located at eight continental U.S. storage sites (Figure 2-4) and at Johnston Atoll in the Pacific Ocean, about 700 miles southwest of Hawaii. The nature of the stockpile at each continental U.S. site, by type of container or munition and by type of agent, is delineated in Table 2-2. Each site differs in the amount of metals, explosives, propellants, and agent stored (Table 2-3).
TABLE 2-1 Composition of Munitions in the U.S. Chemical Stockpile
|
Munition Type |
Agent |
Fuse |
Burster |
Propellant |
Dunnage |
|
M55 115-mm rocketa |
GB, VX |
Yes |
Yes |
Yes |
Yes |
|
M23 land mines |
VX |
Yesb |
Yes |
No |
Yes |
|
4.2-in. mortars |
Mustard |
Yes |
Yes |
Yes |
Yes |
|
105-mm cartridges |
GB, mustard |
Yes |
Yes |
Yes |
Yes |
|
105-mm projectiles |
GB, mustard |
Yesc |
Yesc |
No |
Yes |
|
155-mm projectiles |
GB, VX, mustard |
No |
Yesc |
No |
Yes |
|
8-in. projectiles |
GB, VX |
No |
Yesc |
No |
Yes |
|
Bombs (500-750 lb) |
GB |
No |
No |
No |
Yes |
|
Weteye bombs |
GB |
No |
No |
No |
No |
|
Spray tanks |
VX |
No |
No |
No |
No |
|
Ton containers |
No |
No |
No |
No |
|
|
a M55 rockets are processed in individual fiberglass shipping containers. b Fuse and land mines are stored together but not assembled. c Some projectiles have not been put into explosive configuration. d GA (Tabun), or ethyl-N, N-dimethylphosphoramidocyanidate, is a nerve agent. e Lewisite, or Dichloro(2-chlorovinyl) arsine, is a volatile arsenic-based blister agent. SOURCE: PEIS, 1988. |
|||||
TABLE 2-2 Chemical Munitions Stored in the Continental United States
|
Chemical munitions (agent) |
APG |
ANAD |
BAD |
NAAP |
PBA |
PUDA |
TEADa |
UMDA |
|
Mustard agent (H, HD, or HT) |
||||||||
|
105-mm projectile (HD) |
|
X |
|
|
|
X |
|
|
|
155-mm projectile (H, HD) |
|
X |
X |
|
|
X |
X |
|
|
4.2-in. mortar (HD, HT) |
|
X |
|
|
|
X |
X |
|
|
Ton container (HD) |
X |
X |
|
|
X |
Xb |
X |
X |
|
Ton container (HT) |
|
|
|
|
X |
|
|
|
|
Agent GB |
|
|
|
|
|
|
|
|
|
105-mm projectile |
|
X |
|
|
|
|
X |
|
|
155-mm projectile |
|
X |
|
|
|
|
X |
X |
|
8-in. projectile |
|
X |
X |
|
|
|
X |
X |
|
M55 rocket |
|
X |
X |
|
X |
|
X |
X |
|
500-lb bomb |
|
|
|
|
|
|
|
X |
|
750-1b bomb |
|
|
|
|
|
|
X |
X |
|
Weteye bomb |
|
|
|
|
|
|
X |
|
|
Ton container |
|
Xb |
Xb |
|
Xb |
|
X |
X |
|
Agent VX |
|
|
|
|
|
|
|
|
|
155-mm projectile |
|
X |
X |
|
|
|
X |
X |
|
8-in. projectile |
|
|
|
|
|
|
X |
X |
|
M55 rocket |
|
X |
X |
|
X |
|
X |
X |
|
M23 land mine |
|
X |
|
|
X |
|
X |
X |
|
Spray tank |
|
|
|
|
|
|
X |
X |
|
Ton container |
|
|
|
X |
|
|
|
|
|
NOTE: APG, Aberdeen Proving Ground, Md.; ANAD, Anniston Army Depot, Ala.; BAD, Blue Grass Army Depot, Ky.; NAAP, Newport Army Ammunition Plant, Ind.; PBA, Pine Bluff Arsenal, Ark.; PUDA, Pueblo Depot Activity, Colo.; TEAD, Tooele Depot, Utah; and UMDA, Umatilla Depot Activity, Ore. a Small quantities of Lewisite and tabun (GA) are stored in ton containers at TEAD. b Small quantities of agent drained as part of the Drill and Transfer System assessment for the M55 rockets. |
||||||||
TABLE 2-3 Approximate Amounts of Metals, Energetics, and Agent Contained in the Chemical Weapons Stockpile (tons), by Site
|
Site |
Ferrous Metal |
Aluminum |
Explosive |
Propellant |
Estimated Agenta |
|
Tooele |
22,000 |
570 |
350 |
175 |
10,500 |
|
Anniston |
13,700 |
1,020 |
451 |
757 |
1,800 |
|
Umatilla |
7,930 |
1,380 |
338 |
1,030 |
2,900 |
|
Pine Bluff |
2,644 |
1,431 |
180 |
1,060 |
3,000 |
|
Lexington |
1,631 |
904 |
115 |
670 |
400 |
|
Pueblo |
10,910 |
0 |
124 |
0 |
2,500 |
|
Newport |
2,455 |
0 |
0 |
0 |
1,000 |
|
Aberdeen |
NAb |
0 |
0 |
0 |
1,300 |
|
JACADS |
NA |
NA |
NA |
NA |
1,700 |
|
TOTAL |
61,270 |
5,305 |
1,558 |
3,692 |
24,800 |
|
a Estimated values, calculated by the Alternatives Committee, based on percentages of the total stockpile at each site, multiplied by 25,000 tons. b NA—not available. SOURCE: Information supplied by the Program Manager for Chemical Demilitarization at a meeting of the Committee on Alternative Chemical Demilitarization Technologies, March 9-10, 1992, National Academy of Sciences. |
|||||
The significance of these tables for selecting disposal technologies is that the storage sites are highly varied, in that
-
they range in size from Tooele with 42.3 percent of the stockpile to Blue Grass with only 1.6 percent;
-
two sites have only liquid agent stored in ton containers—Aberdeen with mustard and Newport with VX;
-
Pueblo has only mustard in artillery projectiles; and
-
all sites except Aberdeen, Newport, and Pueblo have all three agent types and a variety of munitions.
CONDITION OF THE CHEMICAL STOCKPILE
The condition of the unitary chemical agent and munitions stockpile has been a significant factor in the decisions to effect its elimination, as well as in the decisions on how to eliminate it. In the 1984 National Research Council (NRC) report Disposal of Chemical Munitions and Agent, these issues were addressed:
-
all chemical agents maintained in the stockpile by the Army were at least 16 years old (now at least 25 years), some more than 40 years old (now more then 49 years); none was manufactured after 1968;
-
the stockpile was deteriorating, and some munitions had begun to leak;
-
the stockpile was expensive to safeguard and maintain; and
-
the timing of the disposal of the unitary chemical stockpile would likely be affected by a treaty then under consideration.
The Committee on Demilitarizing Chemical Munitions and Agents and its associated Stockpile Assessment Panel recommended the following (NRC, 1984)
The stockpiles of obsolete or unserviceable toxic chemical agents and munitions, including bulk stocks, should be destroyed as soon as possible. For the present time [1984], however, storage is the only option.
According to a 1992 Army report, there have been almost 1,500 "leakers" found since 1982 in the stockpile of unitary chemical agents and munitions, a yearly average of somewhat more than 130 containers and munitions as illustrated by Table 2-4 (Evans, 1993). More than two-thirds of these leakers (907) have been M55 rockets.
In September 1993, the Army informed the committee that an estimated 100-gallon spill from a ton container of mustard was discovered at Tooele Army Depot. Subsequently, the committee received a copy of a "Chemical Event Report" (U.S. Army, 1993c), giving some of the details of the event, and the committee discussed the event with Army personnel. The leak occurred around a corroded plug. This plug was at the "8 o'clock" position in the round end of the container, and the container had drained down to that level. (Normally, containers are positioned with plugs between "10 o'clock" and "2 o'clock" to limit liquid leakage). The leak produced a 10-foot by 12-foot pool on the ground, leading to speculation that leakage had been rapid and that the material may have been under pressure, perhaps due to high temperature (a slow leak would probably have seeped into the ground without
TABLE 2-4 Toxic Chemical Munition Leakers
|
|
|
|
Number of Leakers by Year |
|||||||||||
|
Nomenclature |
Agents |
Leakage Rate (%) |
1982 |
1983 |
1984 |
1985 |
1986 |
1987 |
1988 |
1989 |
1990 |
1991 |
Jan-Jun 1992 |
Total |
|
105-mm cartridge |
H, GB |
0.004 |
|
4 |
1 |
|
10 |
3 |
2 |
11 |
1 |
1 |
|
33 |
|
4.2 in. cartridge |
HD, HT |
0.008 |
|
4 |
|
|
8 |
21 |
2 |
1 |
2 |
3 |
4 |
45 |
|
155-mm projectile |
H, GB, VX |
0.039 |
40 |
36 |
56 |
3 |
11 |
10 |
11 |
15 |
8 |
16 |
67 |
273 |
|
8 in. projectile |
GB, VX |
0.013 |
|
|
6 |
|
|
|
|
|
|
|
|
6 |
|
MC-1 bomb |
GB |
0.67 |
11 |
1 |
|
|
9 |
2 |
8 |
6 |
2 |
7 |
|
46 |
|
MK94 bomb |
GB |
0.384 |
|
|
|
|
3 |
|
|
|
|
2 |
|
5 |
|
MK 116 bomb |
GB |
0.0a |
|
|
|
|
|
|
|
|
|
|
|
0 |
|
M55 rocket |
GB |
0.285 |
20 |
12 |
164 |
212 |
99 |
35 |
67 |
125 |
74 |
68 |
31 |
907 |
|
M55 rocket |
VX |
0.005 |
|
|
2 |
1 |
|
|
|
2 |
4 |
|
|
9 |
|
M23 mine |
VX |
0.087 |
|
4 |
|
|
2 |
|
|
|
|
|
|
6 |
|
TMU-28/B spray |
VX |
0.0 |
|
|
|
|
|
|
|
|
|
|
|
0 |
|
Ton container |
H, GB, VX |
|
11 |
1 |
11 |
19 |
15 |
18 |
35 |
10 |
1 |
16 |
4 |
141 |
|
TOTAL |
|
|
82 |
62 |
240 |
235 |
157 |
89 |
125 |
170 |
92 |
113 |
106 |
1,471 |
|
a None since 1979. SOURCE: Evans, 1993. Information supplied by M. Evans, Office of the Program Manager for Chemical Demilitarization, Aberdeen, MD. |
||||||||||||||
spreading as far). A subsequent check of all containers at that site found another that showed evidence of small seepage. The containers are in an area that is normally inspected every three months.
The significance of the leak is that it was large. Leaks tabulated in Table 2-4 are typically small seepages. The leak was estimated to have had a maximum distance of adverse medical effects of 908 meters (still within chemical storage area boundaries). No agent was detected at the site boundaries, and there were no injuries as a result of the leak. Had the leak been of a volatile nerve agent, the consequences could have been much more serious, and the leak might have triggered an evacuation procedure.
From available information it cannot be said that one large leak portends a significant decrease in stockpile safety. Ton containers have a history of leakages around corroded plugs, with GB the most common offender. There is no direct evidence that the mustard leak was rapid or that the container was pressurized, although this appears to be a plausible explanation. At the time of the writing of this report, there was no further investigation of the incident under way or planned. The Stockpile Committee believes that this incident should be more thoroughly investigated to determine if such leaks are a new phenomenon and if container pressurization is, or will become, an increasing problem. The committee notes that unexpectedly pressurized mustard projectiles were encountered in disposal operation at Johnston Atoll Chemical Agent Disposal System (JACADS).
Although evidence presented in Table 2-4 seems to suggest that the rate of occurrence of leakers does not depend on the duration of storage, age does affect munitions and agents in several ways. Metal corrosion has been observed within burster wells, and resultant metal salts may produce sensitive compounds. Corrosion also inhibits disassembly of artillery projectiles as practiced in the baseline system, and likely to be practiced in any alternative system that processes the liquid agent in a separate stream. GB is known to corrode aluminum, and this has led to the majority of the M55 rocket leakage problems.
Mustard and GB both gel with age, producing a material that can neither be drained from its container nor, if mechanically removed, processed through a facility designed to treat liquids. Disposal operations at JACADS also uncovered some 105-mm mustard projectiles that had developed an increased internal pressure that caused foaming upon extraction of the burster well. The reason for the pressure rise is not understood. Gelled agent and foaming agent, though not direct safety problems, complicate and slow the disposal operations.
M55 PROPELLANT STABILITY
The M55 rocket presents the most serious threat to public safety from an accident initiated by a weapon in storage. The weapon is fused, loaded with agent, has a burster installed to disperse the agent, and is capable of self-propulsion if the rocket motor is ignited. The M55s are stored in igloos with many other rockets, almost 4,000 in each igloo at Blue Grass Army Depot, Kentucky, for example. Each rocket has about 10 pounds of agent. The propellant in the rocket motor is known to deteriorate in such a manner that under certain conditions it can autoignite. Indeed, the threat of autoignition of M55 rocket motors prompted the original U.S. Army Chemical Demilitarization Program, focused in the beginning entirely on that weapon.
The consequences of ignition of a single rocket within an igloo full of rockets would be severe, as confirmed by an Army test in which a small fire was started in such a storage site. The rockets contained agent simulant. After the fire had smoldered for some time, exploding rockets blew out the igloo's door. Some rockets were then propelled from the igloo while violent burning continued within, accompanied by periodic explosions. Clearly, the autoignition of an M55 rocket in a storage igloo would be a disaster of the first order; such an event is totally unacceptable. Double base propellants, the type used in the M55, contain nitrocellulose and nitroglycerin. Nitrocellulose is unstable, decaying in a process that is exothermic, accelerated by increasing temperature, and autocatalyzed by its own acidic nitrate products. Decay self-accelerates, due to both the rising temperature and the accumulating acidic nitrate catalysts. If unchecked, propellant temperature will continue to rise until violent combustion is initiated, particularly in thick propellant sections that cannot effectively dissipate heat.
The minimum propellant grain diameter for autoignition of similar double base unstabilized propellants has been estimated to be 3 inches (SAIC, 1985). The diameter of the M55 grain is 4.5 inches. Thus it presents the potential for autoignition, although the actual critical condition is a function of grain geometry as well as diameter.
A history of double base propellant autoignition has been corrected by the addition of a suitable stabilizer: ''Early double base propellant formulations exhibited a tendency to undergo spontaneous combustion with unfortunate regularity; modern formulations, including the M28 propellant [used in the M55], incorporate a stabilizer to delay spontaneous combustion'' (SAIC, 1985).
Decay of nitrocellulose cannot be inhibited, but a stabilizer, 2-nitrodiphenylamine (2-NDPA), is added to react with the acidic nitrate decay products before they can act as catalysts for further decay. In the process, 2-NDPA is consumed, thus eventually depleting the stabilizer concentration to a dangerous level.
There are differing models for the stabilizer depletion steps. One report (SAIC, 1985) indicates that three "daughter" compounds are formed as acidic nitrates are captured, having strengths of 0.75, 0.50, and 0.25 times that of the parent before becoming totally ineffective. Recent briefings to the committee have described depletion as a two-step process through daughters of 0.50 and 0.25 times the parent strength.
Stabilizer depletion rates are very slow—a small fraction of I percent per year—and essentially constant until levels are low enough to permit increasing autocatalysis, whereupon the propellant degradation and stabilizer depletion rates increase exponentially (SAIC, 1985; U.S. Army, 1993a). While there is agreement about this general model of depletion, substantial disagreements exist on the quantitative aspects of the kinetics of the deterioration process. This is discussed more fully below.
A maximum of about 2 percent 2-NDPA can be added to the propellant without degrading its ballistic properties (U.S. Army, 1993a). Army regulations call for increased surveillance of munitions when the stabilizer concentration diminishes to 0.50 percent and immediate disposal when it reaches 0.20 percent. Samples of propellant that have autoignited have had stabilizer concentration at or less than 0.20 percent (U.S. Army, 1993a).
The M55s were manufactured from 1959 to 1965, with stabilizer levels of about 1.8 percent. Insufficient time has passed for the stabilizer to be depleted, and no instances of M55 autoignition have occurred.
Since the effect of a stabilizer is to delay rather than eliminate autoignition, and in view of the serious consequences that would follow an M55 autoignition, several attempts have been made to estimate the safe storage life of these munitions. The Stockpile Committee has examined reports that address propellant stability (SAIC, 1985; OTA, 1992; MITRE, 1993d; U.S. Army, 1993a), though it has not directly examined original data and analyses.
Table 2-5 summarizes the estimates of safe storage life reported in the above sources. These sources contain significantly differing estimates of the remaining safe storage life. Although the committee is unable to resolve the differences, it can point out that there is considerable uncertainty in all of the results. Because that uncertainty raises the possibility that autoignition might occur prior to completion of the scheduled disposal programs, and because that schedule is in itself uncertain, this subject deserves greater attention.
The cited analyses are all based upon quite limited data and upon differing assumptions of stabilizer behavior. Field samples of M55 rocket propellant were collected in 1980, 1985, and 1989. Samples collected in 1980 were analyzed, but labeling errors prevented correlation with the collection site. Consequently, only analyses based upon the 1985 and 1989 samples have been used in stockpile assessment studies (Baronian, 1994).
TABLE 2-5 Estimated Date at Which 0.5 Percent Stabilizer Levels Would Be Reached in Most Rapidly Deteriorating M55 Lots
|
Source of Estimate |
Year At Which 0.5 % Level Would be Reached |
|
Original estimate of time from date of manufacture |
1986-1992 ± 2a |
|
MITRE, 1993 (based on 1985 samples) |
2008 |
|
MITRE, 1993 (based on accelerated aging data) |
2007 |
|
OTA |
2010 |
|
U.S. Army, 1993a |
1997b |
|
a Data obtained from Science Applications International Corporation (SAIC, 1985). b Mason estimated 2002 as the date to reach 0.2% stabilizer levels; 1997 has been inferred from his paper and analytic method. |
|
-
1985 Science Applications International Corporation (SAIC) Report. The SAIC report provides much of the background on stabilizer behavior and points out that additional testing, using field samples, must be done to refine the estimates of safe storage life. It cites tests performed at the time of manufacture (1959-1965) that projected safe storage life to be, conservatively, 27 years. At the time the SAIC report was prepared, the M55 stockpile was being subjected to a Special Assessment Program (a program of surveillance with a defined level of intensity). In this program, rockets were selected randomly from six locations: Umatilla, Tooele, Blue Grass, Anniston, Pine Bluff, and Johnston Island. The rockets, including their motors, were disassembled. Samples in 3-inch sections were taken from the front, middle, and rear of each propellant grain. A total of 1,302 samples were collected from two motors from each combination of storage site and propellant lot. At the time the SAIC report was prepared, testing on these samples was not complete, but enough data had been collected to show that the overall mean stabilizer content was 1.73 percent and the lowest lot mean was 1.31 percent. The average depletion of 2-NDPA, the virgin stabilizer material included in the propellant, was about 0.2 percent (of the original 1.96 percent). SAIC went on to say that depletion rates would have to be ten times greater than this for the rockets to present a hazard within the next ten years; comparison of the oldest lots of propellant with the newest showed no evidence of such acceleration.
-
The SAIC report notes that of the propellant lot samples showing the lowest concentration (less than 1.5 percent), one was from Johnston Island, while the others were from "small early lots which were manufactured to a lower stabilizer specification." The M55 rockets on Johnston Island have all since been destroyed during Operational Verification Testing (OVT) operations there. The report does not indicate what has happened to the few hundred rockets remaining from these small lots. Obviously, if they remain in the stockpile they justify intensive surveillance and perhaps early destruction.
Finally, the SAIC report presents an analysis of the chemical kinetics of the propellant deterioration process. This analysis shows that three daughter products, themselves stabilizers, are produced by the reaction of 2-NDPA and nitrocellulose. The 2-NDPA diminishes along a declining exponential curve. When the effects of the daughter products are added in, the total "stabilizer capacity," consisting of that of the virgin stabilizer and the daughters combined, declines along a straight line. These observations are important to later aspects of this discussion.
-
1992 Office of Technology Assessment (OTA) Report. The OTA report summarizes a study carried out by the U.S. Army Material Systems Analysis Activity. In this study, conducted in 1985, 393 M55 rockets were randomly selected from the stockpile of 478,000. (Presumably these were the same rockets reported on by SAIC in 1985.) The rockets in the sample were disassembled, and individual components were inspected. Stabilizer levels were reported to be between 1.6 and 2.2 percent. However, because this 1985 assessment was the first since production, it was impossible to quantify stabilizer degradation over time. A worst-case estimate of remaining storage life was made by projecting stabilizer loss for the rocket lot showing greatest loss since manufacture. The conclusion was that the "increased surveillance" stabilizer level (0.50 percent) would be reached in 2010.
-
1993 MITRE Report. The MITRE report contains two estimates. One stems from the same data reported by SAIC (and presumably OTA) and summarized above. After citing numerous uncertainties and error sources, MITRE said, "Given the underlying error problems and the non-comparability of data from before and after 1985, it is difficult to confidently predict remaining safe storage life." MITRE went on, however, to develop a worst case analysis based on the minimum lot mean stabilizer levels noted above. The resulting estimate for the date when stabilizer levels would reach 0.5 percent came out to be 2008.
MITRE also made another bounding prediction based on accelerated aging tests that have been assumed to understate the time to stabilizer depletion. Based on this analysis, MITRE estimated the date at which 0.5 percent 2-NDPA, the virgin stabilizer, would be reached as 2007 at Tooele,
-
the site with the earliest such date, while the 0.2 percent level would be reached in 2019. This projection assumed a decreasing rate of depletion of the stabilizer capacity.
The MITRE report emphasizes an important point not touched on by either SAIC or OTA. MITRE pointed out that "only one rocket needs to ignite or detonate to cause a serious accident. The reliance on lot segment sampling to monitor stockpile safety is based on an assumption of intra-lot homogeneity." MITRE underlined the fact that data show differences as high as 0.4 percent stabilizer content within the same lot segments. Moreover, the example of the low-stabilizer sample in the 1985 assessment showed nearly a 0.3 percent variation between sections of one grain.
-
1993 Mason Report. Mason's analysis (U.S. Army, 1993a) begins with the same 1.31 percent lot mean concentration noted above. From that, he subtracts a 0.14 percent "measured deviation" to arrive at an initial (1985) concentration of 1.17 percent.5 Citing a field-measured depletion rate of 0.00014976 percent per day, and assuming linear depletion of the 2-NDPA at this rate, Mason calculates that the critical 0.20 percent concentration would be reached in 17.7 years. He then concludes, "It is theoretically possible for a single rocket motor to autoignite by the year 2002."
Although Mason did not calculate the date at which 0.5 percent 2-NDPA would be reached, with his stated linear depletion rate this would be 5.5 years before 2002, or about 1996-1997. This makes it evident that Mason's analysis, if accurate, is cause for much greater concern than the others reported above. The difference stems in part from the assumption of linear diminution of stabilizer capacity and in part from the allowance for "measured deviation."
Mason raises another point that remains unresolved. He argues that 2-NDPA may not be as effective as assumed in the analysis cited by OTA and that it might have as little as one-fifth the capacity assumed to absorb oxides of nitrogen, the products of deterioration of the nitrocellulose and themselves catalysts for further deterioration.
Finally, Mason also compared measured concentrations of 2-NDPA with the as-loaded records, finding that some of the measured concentrations were higher than as-loaded values. Several explanations are suggested, but the discrepancy cannot be resolved with certainty. There is also some confusion as to whether measurements indicated 2-NDPA concentration or the combined concentration of 2-NDPA and its daughters.
In summary, deterioration of the rocket propellant in the M55s is potentially a serious hazard to continued safe storage of the chemical agent and munitions stockpile. Most extant estimates of the dates when this danger might arise are at or beyond the end points of the current schedule for destruction of the stockpile. However, at least one analyst puts the date of potential danger much earlier. All of these estimates are very uncertain, based as they are on old and sparse data. Controversy is evident about the reaction kinetics and the effectiveness of the 2-NDPA stabilizer. Moreover, the schedule for the disposal program is also uncertain and it could easily slip into or beyond the dates at which significant depletion of stabilizer is now predicted.
It is the committee's conclusion that the consequences of an M55 autoignition are too great for this level of uncertainty to be tolerated. Thus, the committee recommends that the Army undertake a new and definitive study of M55 rocket propellant, including new sampling if that is appropriate, to develop more reliable assessment of these propellants. This study should address all the issues raised by the studies summarized above and by this discussion, including intralot and intragrain variations; degradation kinetics; the effectiveness of both 2-NDPA and its daughter products as stabilizers; the fate of the few hundred rockets manufactured with low stabilizer levels; and the autoignition characteristics of the M55 grain, given its actual configuration.