Below is the uncorrected machine-read text of this chapter, intended to provide our own search engines and external engines with highly rich, chapter-representative searchable text of each book. Because it is UNCORRECTED material, please consider the following text as a useful but insufficient proxy for the authoritative book pages.
4 Fates INTRODUCTI ON Petroleum introduced to the marine environment goes through a variety of physical, chemical, and biological transformations during its transport by the advective and spreading processes discussed below. This section identifies the major factors controlling each of these processes, reviews the relevant experimental and field evidence for quantitative evaluation of the effect of these various processes on petroleum, and estimates the amount of petroleum hydrocarbons in the marine environment at the present time. Although much of the subsequent discussion deals with the fate of oil spills, this source of oil in the marine environment only accounts for about 15% of the annual input, with chronic discharges being of much greater significance (see Chapter 2, Table 2-22~. The latter are subject to essentially the same kinds of fates but are sometimes more difficult to study owing to the dispersed nature of the inputs and lower concentrations of petroleum compared to oil spills. Advection and spreading begin immediately after introduction of petroleum to the ocean and cause a rapid increase in the exposure area of the oil to subsequent "weathering" processes. These include evaporation, dissolution, vertical dispersion, emulsification, and sedimentation. Involved in all of these processes are chemical factors determined by the specific composition of the petroleum in question. Additionally, photochemical oxidation of some of the components of petroleum can be induced by sunlight. Dark or autooxidation may also occur. The products of these processes include hydrocarbon fractions and reaction products introduced to the atmosphere, slicks and tar lumps on the surface of the ocean, dissolved and particulate hydrocarbon materials in the water column, and similar components in the sediments. While physical and chemical processes are occurring, biological processes also act on the different fractions of the or iginal petroleum in various ways. The biological processes considered include degr adation of petroleum by microorganisms to carbon dioxide or organic components in intermediate oxidation stages, uptake by larger organisms and subsequent metabol ism, storage, or discharge. 270
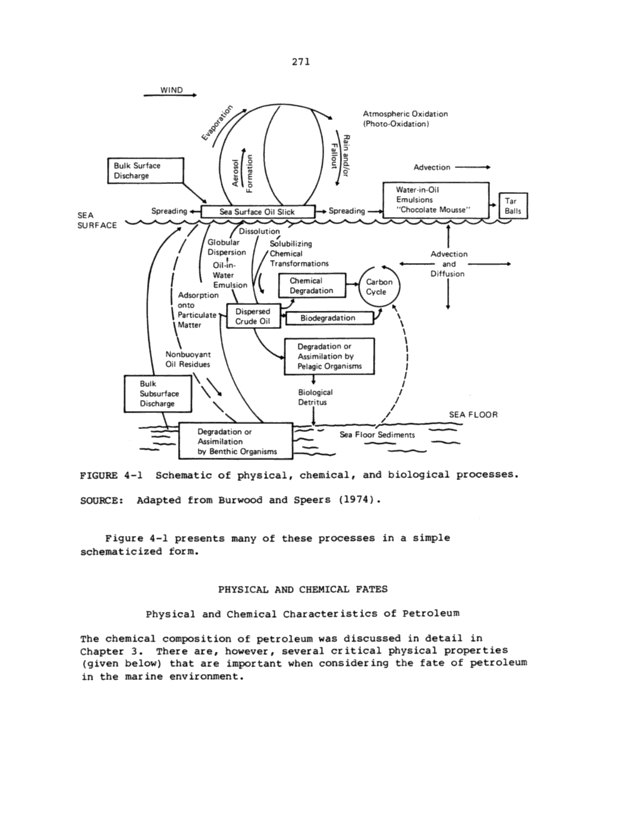
271 Wl ND _ ~ Bulk Surface l Discharge SEA SU RFACE ~/ Atmospheric Oxidation (Photo-Oxidation ) Advection Water-in-Oil Emulsions _ Tar Balls _ Spreading ~Sea Surface Oil Slick l_Spreading-| "Chocolate Mousse" ~ 7 7 In ~ / / Globular / / Dispersion I Water ! Emulsion Solubilizing / Chemical / Transformations /( | Chemical L<Grb: | Adsorption \ L Degradation onto ~ Particulate: Crilde Oi f Biodegradation ~\` \ ~\ Degradation or ~ Nonbuoyant \ Assimilation by l Oil Residues \ Pelagic Organisms , 1r I Biological / Detritus ~ 1 , ~ Bulk \ Subsurface \ Discharge \ _ ~ \ - Degradation or -_ _ Assimilation __ by Benthic Organisms Advection Diff usion SEA F LOO R _ , _ Sea Floor Sediments FIGURE 4-1 Schematic of physical, chemical, and biological processes. SOURCE: Adapted from Burwood and Speers (1974 ~ . Figure 4-1 presents many of these processes in a simple schematicized form. PHYSICAL AND CHEMICAL FATES Physical and Chemical Character istics of Petroleum The chemical composition of petroleum was discussed in detail in Chapter 3 . There are, however, several cr itical physical properties (given below) that are important when consider ing the fate of petroleum in the mar ine environment.
272 Density Density of spilled oil increases as evaporation removes the lighter constituents, but only rarely does the density reach that of seawater. This effect is partially balanced by a density decrease with increasing temperature. The effective density of a slick tends to increase due to weathering, but more significant increases are attributable to (~) the uptake of water by many oils to form emulsions (~moussen), which have higher densities (approaching that of seawater), and (2) association with suspended minerals or organic matter. Oxidation also may cause a density increase, but the products may be quite water soluble and will thus migrate out of the oil. Density plays an obviously important role in the fate of spilled oil, for the density difference between oil and water determines the extent to which the slick is submerged and the residence time of oil droplets which may be propelled downward in the water column by breaking waves. Following the Kurdistan spill there were anecdotal but unsub- stantiated accounts of submerged or neutrally buoyant oil (Vandermeulen, 1981~. It is generally accepted that the density of most weathered oils does not become great enough for neutral buoyancy to occur and result in significant amounts of particles and pancakes in suspended equilibrium in the water column. Viscosity and Pour Point Spill viscosity (resistance to flow) increases with weathering and decreases with increasing temperature. This is important, as it controls the rate of spreading in the gravity-viscous regime. A related property is pour point temperature for oils which is often invoked as an Equivalent to melting point" for organic chemicals. Phenomena associated with the rapid increase in viscosity as the pour point is approached are not well understood. Probably more important is the effect of emulsified water on the bulk viscosity of emulsions. Oils usually have non-Newtonian theologies (flow) characteristics; thus a viscosity measurement is meaningful only in the context of a particular Theological model if the shear conditions are defined. It is appropriate to measure and record low shear rate viscosities using, for example, an Ostwald viscometer. Vapor Pressure Vapor pressure controls evaporation rate and air concentrations of hydrocarbons and, therefore, the fire hazard in the vicinity of spills. Vapor pressures can be estimated using Raoult's law (vapor pressure of a solution equals the product of the vapor pressure of the solvent and the mole fraction of the solvent) if the composition of the mixture is known--which is usually not the case. The use of pseudo-components or analytical expressions for vapor pressure is discussed in the Evaporation section below.
273 TABLE 4-1 Henry 's Law Constants for Selected Hydrocarbonsa Molecular Vapor or Aqueous Henry 's Law Weight Partial Pressure Solvability Constant Compound (at 25°C) (g/mol) (atm) (g/m3) (atm m3/mol ) Methane 16 1.0 24 .1 0 .67 e-Butane 58 1.0 61.4 0.95 n-Hexane 86 0 .2 9.5 1. 81 e-Octane 114 0.019 0.66 3.28 n-Decane 148 0.0017 0.052 4. 83 Cyclohexane 84 0.13 55 0.19 1-Hexane 84 0.24 50 0.41 Benzene 78 0.13 1780 0.0055 Toluene 92 0.038 515 0.0067 o-Xylene 106 0.0087 175 0.0052 Naphthalene 128 0.00011 34 0.00043 Biphenyl 154 0 .000013 7 .5 0 .0002 7 aFor gaseous solutes the solohil ity is at 1.0 atm pressure. SOURCE: Af ter McKay, 1981. Aqueous Solub il ity Henry's law, CP=HC, where p is pressure in atmospheres, C is concentra- tion in solution, and H is Henry' s law constant, can be invoked, although some er ror may be introduced because there is ev idence that mixtures are more soluble than is expected from a direct mole fraction dependence (Leionen et al., 1971), a phenomenon that is at least partially due to the presence of dissolved natural humic-like matter in seawater (Boehm and Quinn, 19731. Henry's law constants for selected hydrocarbons in distilled water are given in Table 4-1. The solubilities of hydrocarbons in seawater are generally some 60-809s of the distilled vrater values owing to the "salting out" effect. ThiS can be character ized by the Setchenow equation (Aquan-Yuen et al ., 1979 ~ . Processes Advection and Spreading Transport of oil spilled onto the sea surface is due to two processes: advection and spreading. Advection is due to the influence of over- lying winds and/or underlying currents. ThiS process may be subdivided further depending on the causes of motion. For example, there may be advection by Stokes drift, Ekman currents, Langmoir circulation, geostrophic currents, or even turbulent flow. Descriptions and mathematical treatments of these various advection processes can be found in texts on general and physical oceanography. The other transport process is spreading, a phenomenon resulting from a dynamic equilibrium between the forces of gravity, inertia, frict ion, v iscosity, and surface tension.
274 1cm 1mm 1 00,um 1 OlJm 1pm J 0.11Jm 0.01pm l DDULL BROWN 6 ~NIFIC ANT 4~ SURFACE CONCENTRATIONS ~ DARKER BROWNS TO BLACK IRIDESCENCE - SILVERY SHEEN /OLORLESS SLICK CAPILLARY WAVES DAMPED / MONOMOLECULAR LAYER I I I ~ I I I 10 102 103 104 10S 106 107 SURFACE CONCENTRATION OF SPILL tLITERS PER SQUARE KILOMETER) FIGURE 4-2 Sur face concentration of spill . SOURCE: Barger et al . ( 1974 ~ . Oil on the sea surface manifests itself as slicks of var table thickness. An approximate classif ication of these slicks is "thin" slicks, less than 10 um thick, to "thick" slicks, often millimeters or even centimeters thick. Generally, the area of thin slick exceeds that of the thick, but most of the oil volume usually resides in the thick slick. Figure 4-2 is a labeled plot of thickness versus surface concentr at ion . Observations of water may show f irst the evidence of oil by damping of capillary waves: the sur face becomes less ~ rough ~ and more "glassy," but no oil is visible. As the slick thickens to 1 Em, light interference effects become apparent, often giving irridescent colors. Further increase in thickness to approximately 10 Em gives darker films. The behavior of thin films is dominated by surface tension (or interracial energy) effects; spreading is promoted when the sum of the oil-water and oil-air infer facial tensions is less than that of the water-air infer facial tension. Behavior in this regime is complicated by the presence of natural organic surface layers on the ocean surface, especial ly in quiescent and biologically productive areas. Although
275 these infer facial tensions can be measured, reliable deduction of behavior is not possible because (1 ) as the oil spreads it evaporates and dissolves, and the infer facial tension changes; (2) oxidation (probably photolytic in or igin) alters the composition of the oil, especially by forming oxygenated compounds with low inter facial tensions; (3 ~ as hydrocarbons dissolve in water they alter the water-air infer facial tension; and (4 ~ spreading induces a change in composition of the o il by select ive d issolut ion and evapor at ion of cer ta in components . Under relatively quiescent conditions, slicks of thickness greater than 10 Am tend to be surrounded by thin slicks; thus, they do not experience a surface tension force to induce spreading. Accordingly, the thick slicks tend to spread more slowly, at a rate controlled by a balance of hydrostatic, viscous, and inertial forces. This fluid flow process can be described mathematically if certain simplifying assump- tions are made. However, the results probably will not have general utility because (1) solutions are very complex; (2) the rheology (flow) of the oil is often complex, i.e., the viscosity is not constant; (3) wave action stretches and compresses the oil slick; (4) water-in-oil emulsions may form; (5) usually the slick is wind driven relative to the water ; (6) the presence of natural surface convergences or diver- gences will cause the oil slick to separate or accumulate; (7) oil composition (as distinct from viscosity or sur face properties) appear s to influence spreading (Fazal and Milgram, 1979~; and (8) the entire spreading process is likely to be profoundly influenced by sea state, especially under rough conditions in which oil may be carr fed by spray. In recent years there have been many attempts to model the fate of oil spills. For example, more than 35 different models are described in a comprehensive repor t by Huang and Monastero (1982~. Because advective processes are the principal controls for the fate of a spill, they are the most frequently modeled. The general consensus of modeling experts is that there is no one universal model that will generally yield predictions that are real istic or undistorted . The modell ing of the many d isper sed smaller sl icks is a ma jor unsolved problem. Wind causes surface water dr if t at a velocity of a few percent of the wind speed . Oil behaves s imilar ly, the consensus be ing that the drift velocity is 3-4% of the wind speed. G.L. Smith (1977) has treated this in some detail, err iving at a dr if t factor of 3 .64 ~ O . 51% . An important observation is that the dr if t factor of the thick slick exceeds that of the thin; thus, the thicker region tends to accumulate at the leading edge of the sl ick, with the th inner reg ion trail ing . Calculation of dr if t is essential in oil spill tra jectory models, but is compl icated by (1 ) the possible influence of Cor iol is forces on the slick (tending to cause diversion to the "right" in the northern hemisphere and "left" in the southern hemisphere), (2 ~ by residual and tidal ocean currents which provide an additional vector, (3) by Stokes surface drift associated with gravity waves (Lange and Hufner fuss, 1978), and (4) the reduction by floating oil of wind stress transmitted to the sea.
276 Comparison of computed and actual trajectories of slicks such as those from the Argo Merchant, Ixtoc blowout, Kurdistan, or Amoco Cadiz suggests that the major sources of uncertainties are (1) lack of reli- able data on wind speed and direction (due to distance from weather stations) and (2) lack of detailed ocean surface current data. Evaporation Evaporation, which may be responsible for the loss of from one- to two-thirds of an oil spill mass in a per lad of a few hour s or a day (Jordan and Payne, 1980), causes considerable changes in chemical com- position and physical properties of the oil. Calculation of evapo- ration rates is cliff icult because the rate depends on a number of factors, all of which may change with time. Observations of evapora- tion rate and attempts to predict that rate have been reported by Kreider (1971), McAuliffe (1977), Mackay et al. (1980b), Butler (1976), and Harrison et al. (1975) and are generally reviewed by Jordan and Payne (1980~. The rate of evaporative loss from a given volume of oil depends on (1) the area exposed, which tends to increase continuously as the slick spreads; (2) the oil phase component vapor pressures, which are a function of oil temperature and composition, and which fall as the lighter components are depleted from the slick; (3) the oil-air mass transfer coeff icient, which depends primarily on the wind speed but also on the hydrocarbon vapor diffusivity; and (4) the possible presence of diffusive barriers such as a water-in-oil emulsion or a "skin" on the oil surface. Thus the "half-lives. for the various hydrocarbon components in the slick cannot be determined, although approximate values can be suggested for defined conditions. The rate of evaporation from a thick, cold slick under calm conditions may be orders of magnitude slower than from a thin, warm slick under stormy conditions. There are two general approaches to calculating evaporation rates. First is a pseudocomponent approach in which the oil is postulated to consist of a number of components or pseudocomponents of defined volatility and with proportions selected to give a mixture with volatil- ity characteristics similar to that of the oil. As evaporation pro- ceeds, the change in oil composition is computed and the falling vapor pressure is calculated from Raoult's law at the desired temperature. This approach has been used by Mackay and Leionen (1975), Yang and Wang (1977), and Mackay and Paterson (19811. The second approach is to postulate an analytical expression for the amount evaporated as a function of time and composition as attempted by Butler (1976) and Mackay et al. (1980b). In the latter case, a method was proposed by which oil distillation data could be used to predict vapor pressures and, hence, evaporation rates. Evaporation rates and composition changes can be measured by simple pan evaporation experiments, either outdoors or in wind tunnels, with an attempt to extrapolate the results to oceanic conditions. There remains a need to improve the prediction of oil evaporation rates and
277 to characterize oil volatility characteristics more accurately by means of information obtained from pan evaporation experiments, distillation temperature data, and evaporation by a controlled air flow bubbled through the oil. Such information probably can be used to estimate the oil fractions evaporated under various defined conditions and to calcu- late the fractional retention of specific hydrocarbons at various times. Such a capability would be invaluable as a means of calculating changes in dens ity or v iscos ity, assess ing changing toxicity, and improving identif ication of slick samples for legal purposes. Although parts of this overall capability are in place, a comprehensive treatment is s til 1 lack ing . Hydrocarbons may evaporate from true solution in sur face water quite rapidly--often with half-lives of an hour or less. This is illustrated by the analytical data reported for samples collected under dispersed oil slicks in which there was evidence of substantial removal of vola- tiles from the water column (McAulif fe et al ., 1980 ~ . In the case of high-molecular-weight hydrocarbons of low Volubility, most may be in colloidal or accommodated form and are not immediately available for evaporation. This topic has been reviewed recently by Mackay et al. (1981a), using calculations based on previous work by Mackay and Leionen (1975) and L'ss and Slater (1974~. Dissolution Dissolved hydrocarbon concentrations in water are particularly important because of their potentiality for exerting a toxic effect on biological systems. They are less important from the viewpoint of the mass lost by the spill, for dissolution of even a few percent of the spill is unlikely. Dissolution is bet ieved to be directly from the slick to the water column and from dispersed oil drops to the water column. In analyzing spill behavior a prediction of dissolution rate is unnecessary because the mass dissolved is negligible compared with that removed by droplet entrainment and can be subsumed in the dispersion rate expression. The extent of dissolution is obviously influenced by the oil's aqueous Volubility which, for a crude oil, is typically 30 mg/L. Most of the dissolved hydrocarbons are the more soluble low molecular weight aromatics such as benzene, toluene, and the xylenes. As the oil evapo- rates, these hydrocarbons are removed; thus the oil Volubility drops and the dissolution rate falls to a negligible value. Some illustrative Volubility data for fresh and weathered crude oils are given by Mackay and Shiu (1975~. Calculations of the rate of dissolution are imprecise, and only Cohen et al. (1980) and Butler (1976) have attempted to make estimates. The most soluble hydrocarbons, which are also the most volatile, are likely to be preferentially removed by evaporation, which is typically orders of magnitude faster. Even when hydrocarbons do dissolve, many are likely to be removed by subsequent evaporation from the water , provided they have sufficient volatility.
278 It should be reemphasized that in the subsequent chapter on Effects the simplest aromatic compounds are shown to be among the most toxic compounds of crude and refined oil, and as they are also the most soluble, their impact on the marine environment is greater than simple mass balance considerations would imply. Dispersion/Vertical Tr anspor t The lifetime of an oil slick on an ocean surface is often controlled by the dispersion or vertical transport of small particles of oil or oil-in-water emulsions into the water column (}lackey et al., 1980a). This 1 if etime usually determines whether a given sl ick is 1 ikely to impact on a particular shoreline that may be, for example, several days drift time from the site of the spill. Dispersion also results in exposure of subsurface marine organisms to particulate and dissolved oil. These organisms, in turn, may mediate the sedimentation of some of the oil through incorporation into fecal pellets. The nature of the fluid mechanics of the event resulting in natural vertical dispersion is not well understood and is undoubtedly complex. Breaking or surface turbulence waves probably cause the oil to be driven into the water column, thus forming a swarm of oil droplets. The larger particles probably rise and coalesce with the slick, while the smaller oil droplets are conveyed with water eddies vertically downward to become permanently incorporated into the water column. These smaller droplets, which do not r ise to the surface, and their associated water medium may be classified as an oil-in-water emulsion. This emulsion formation is only a part of the overall dispersion process . Expressions for natural dispersion rates have been assembled by Mackay et al. ~1980a), Spaulding et al. ~1978), Carver and Williams (1978), and Aravamudan et al. (19811. The simplest approach for includ- ing dispersion in an oil spill model is that used in the SLICKTRAC model by Blaikley et al. (1978), who tabulated estimated vertical dispersion rates expressed as a percentage of the oil per day as a function of sea state and duration of the spill. This tabulation is undoubtedly an oversimplification of a complex phenomenon. A similar approach has been used by Audunson et al. (1980~. Experimental wind-wave tank measurements and a mathematical treatment of this process have been made by Mackay et al. (1981a). Equations were proposed for transport rates as a function of the oil slick thickness, the oil-water infer facial tension, the sea state, and in particular, the fraction of the sea covered by breaking waves. Although there are some data on this fraction, it is only for seas in the absence of oil. As is well known, oil reduces the incidence of breaking waves. Also, the dispersion process is believed to occur even when there are no breaking waves, a possible mechanism being Folding n of the oil when short waves of relatively high amplitude and short wavelength pass through the oil layer. When the surface layer of water is well mixed, vertical eddy diffu- sion presumably causes further transport downward, and hypothetically,
279 Langmair circulation cells may be even more important. Sutcliffe et al. (1963) reported sinking rates of water in the convergences of 2.7-5.7 cm/s with moderate wind speeds . This should be suff icient to overcome the buoyancy of some oil droplets that otherwise would not sink, but direct observations are lacking. Further research on the problem of vertical dispersion is justified. An adequate set of equations cannot be developed until the basic mechanisms are better understood. Emulsification/Mousse Formation (Water-in-Oil) Laboratory studies to evaluate water-in-oil emulsion fornication for different crude oils and petroleum products have demonstrated a depen- dence on the unique chemical compositions of each of the mater ials tested (Payne, 1984, and references therein) . Heavier crudes with high viscosities are, in general, found to form the more stable emulsions (Bocard and Gatellier, 1981), and the presence of asphaltenes and higher-molecular-weight waxes have been found to be positively cor- related with mousse stability (Berridge et al., 1968a,b; Davis and Gibbs, 1975; MacGregor and McLean, 1977; Mackay et al., 1979, 1980a; Twardus, 1980; Bocard and Gatellier, 1981; Bridie et al., 1980~. Slightly differ ing results have been obtained in different investiga- tions, but generally these materials act together in the emulsif ication process, although the asphaltenes do appear to play a more s ignificant role (Bridle et al., 1980; Berridge et al., 1968a,b). The crystallizing properties of the component waxes (near the pour points of the oils tested) are believed to be important in affecting the internal oil- mousse structure and viscosity, and the asphaltenes are believed to act as surfactants, preventing water-water coalescence in the more stable mixtures (Berridge et al., 1968c; Canevari, 1969; Mackay et al., 1973; Bridie et al., 1980; Cairns et al., 1974~. Other indigenous surface- active agents such as metalloporphyrins and nitrogen, sulfur, and oxygen compounds are believed to be equally important. The products of photochemical and microbial oxidation have also been identif fed as having an impor tent role as stabiliz ing agents (Bocard and Gatellier, 1981; Klein and Pilpel, 1974; Burwood and Spears , 1974 ; Zaj ic et al ., 1974 ; Fr iede , 1973 ; Guire et al ., 1973 ~ . In several instances, mousse could only be formed with photochemically or microbially weathered oils which were also subject to evaporation/ d issolution processes . Brega, Niger fan, Zar zatine, and 1 ight Arabian crude oils have all been shown to exhibit this behavior in laboratory studies (Berridge et al., 1968b; Bocard and Gatellier, 1981) . The formation of a stable mousse at the Ixtoc I wellhead was also observed to be delayed until after these processes had been operative for 24-48 hours on the oil released during that blowout (Payne , 1981 ) . No stable mousses could be formed in laboratory studies at any temperature with light petroleum distillates such as gasoline, kerosenes and several diesel fuels (Berridge et al., 1968a,b; Twardus, 1980) and could only be obtained with several 1 ight lube oils when they are fortif fed with wax and asphaltene mixtures obtained from known mousse
280 forming oils such as Kuwait crude (Bridle et al., 1980~. This asphaltene mixture could also contain other surface-active agents of higher molecular weights. Temperature is also a factor in mousse formation, and in several instances at low temperatures approaching the pour point of the heavier oils, stable emulsions have been generated regardless of wax or asphaltene content. Conversely, if stable water-in-oil emulsions are repeatedly exposed to freeze-thaw cycles, some destabilization and separation of water and oil have been noted (Dickens et al., 1981; and Twardus, 1980~. Similar results have been obtained when laboratory generated and real spill water-in-oil emulsions were subjected to prolonged heating or removal from the water column. The absolute amount of water content and the size of water droplets incorporated into various mixtures of mousse also significantly affect their stability and viscosity (8erridge et al., 1968a,b; Mackay et al. , 1980a; Twardus, 1980; Bocard and Gatellier, 1981) . Positive correla- tions of percent water versus mousse stability and viscosity have been noted for several of the crude oils studied (Mackay et al., 1979, 1980a) . In general, with many oils, maximum stability is achieved with a water content in the range of 20-80%; however, at an oil-specific critical point, significant destabilization of the emulsions occurred (Berridge et al., 1968a,b; Twardus, 1980~. Presumably, this reflects enhanced water-water contact and coalescence resulting in ultimate phase separation. In most of the laboratory studies, the presence and/or absence of bacteria and suspended particulate material did not appear to affect emulsion behavior (Berridge et al., 1968a,b; Davis and Gibbs, 1975~. Bacterial growth was generally limited to the surface of the mousse products tested, and is believed to have been inhibited by limited oxygen and nutrient diffusion into the mousse. Toxic materials inher- ent to the oils themselves may also be responsible for these observa- tions, although water content (and in particular the size of the water droplets encapsulated within the mixtures) has also been correlated with the presence of bacteria in the less stable mousses (Berridge et al., 1968a,b). In several laboratory studies significant bacterial utilization of the mousse only occurred after treatment with disper- sants, resulting in the break-up of the material, with concomitant increased surface-to-volume ratios (Bocard and Gatellier, 19811. Physical Properties of Water-in-Oil Emulsions The physical properties of stable emulsions are different from those of the starting crudes, and increases in specific gravity and viscosity have been observed to affect spreading, dispersion, and solution rates (Berridge et al., 19685; Davis and Gibbs, 1975; MacGregor and McLean, 1977; Mackay et al., 1979, 1980a; Twardus, 1980~. Some evidence has also suggested that evaporation of hydrocarbons of lower molecular weight (Cg-C12) is af fected by the emulsion (Twardus, 1980; Nagata and Rondo, 19771. In general, these effects are most significant in the emulsions con- taining greater than 50% water. Water-in-oil emulsions with less water usually have pour points, spreading properties, and viscosities which proportionately resemble those of the starting oils (Twardus , 1980 ; Mackay et al., 1980a).
281 a ~?\407 ° _~3 `~,.0 FIGURE 4-3 Photooxidation of benzo (a) pyrene to the 6 ,12-, 1, 6-, and 3, 6-diones. SOURCE: National Research Council (1972, pp. 67-69) . The flash points and burn points of the water-in-oil emulsions s tudied have been found to vary s ignif icantly with water content, and for medium crudes, in situ combustion was signif icantly inhibited when the water content reached 79% (Twardus, 1980 ~ . For heavier crudes, signif icant combustion inhibition occurred when the water content reached 30% (Twardus, 1980) . Water-dependent increases in viscosity also at feet cleanup procedures, for sk imming, mopping, and pumping of such mixtures becomes more difficult. The sorption capacity of various commercially available sorbant mater ials has also been observed to decrease as the water content in the mousse mixtures increases. This behavior is believed to be inherent to the hydrophobic properties of the sorbant mater ials examined (Twardus, 1980 ~ . Photoox idat ion/Autoox idation Photooxidation About 25% of the average oil spill evaporates and, in the gaseous state, is almost certainly all oxidized photochemically by OH radical and other species in hours or days to CO, CO2, oxygenated organics, "secondary" aerosols, etc. (Altshuler and Bufalini, 1971; Heicklen, 1976~. A proposed reaction for the photooxidation of benzo- [alpyrene to the 6,12-, 1,6-, and 3,6-drones is illustrated in Figure 4-3. These processes prevent oil's reentry into the sea as petroleum. The dissolved fraction of petroleum, such as the aromatics and polar compounds, is also subject to photochemical oxidation (Zafiriou, 1977; Z. ika, 1980 ~ . ~til 1 et al . { 1980 ~ showed that cumene, an alkyl- benzene, could be photooxidized by mechanisms involving the absorption of light by humic substances in natural waters, while PAH absorb sunlight directly and may be oxidized both by direct photolysis and as
282 TABLE 4-2 Petroleum Photooxidation Summary REPORTED PRODUCTS: a Carbon dioxide Organic acids, esters, lactones Diacids Phenols and polyphenols REPORTED EFFECTS: b . Darkening of oil Increased ease of emulsification Changes in oil-on-water spreading Increased formation of soluble organics/carbons Increased toxicity of petroleum or WSF to various organisms Decreased primary production Aldehydes and ketones Hydroperoxides Alcohols Sulfoxides NOTE: References to data in table appear in text. Knot in order of importance. ee section above on emulsification/mousse formation and Chapter 5 section on the toxicity of chemical products. the indirect pathway (Zepp and Cline, 1977; 2epp, 1978; Zepp and Baughman, 1978~. The photooxidation of nondissolved oil, such as slicks, tar balls, films, "sheen, n droplets and microdroplets, and colloidally dispersed oil, has been studied intensively s ~ nce the 1973 NBC workshop. Table 4-2 presents a summary of photooxidation products and ef feats that have been reported in the 1 iterature . Other reviews that have considered oil photooxidation are given by Clark and MacLeod (1977), McAuliffe (1977), Wheeler (1978), Sprague et al. ~1981) , and Jordon and Payne (1980 ~ . Of pnotoox~dation products and Laboratory and Simulated Environmental Studies A series of laboratory and field studies, beginning with Ber ridge et al. (1968a), has elucidated the photolytic conversion under various conditions of petroleum hydrocarbons to a broad range of secondary and tertiary reaction products such as 3- to 4- ring PAH, thiacyclane oxides, methyl esters, salicyclic and phthalic acids, alcohols, ketones, hydroper- oxides, lactones, hydroxyaromatic acids, and polyphenols (Frankenfeld, 1973; Burwood and Speers, 1974; Klein and Pilpel, 1974; Hansen, 1975, 1977; Larson et al., 1977, 1979; Bocard and Gatellier, 1981; Brunnock et al., 1968). Environmental Case Histories . The U.S. group studvino the Amoco Cadiz spill was able to link photooxidation products found in environ- mental samples very closely with those formed in laboratory studies
283 (Carder et al., 1978 ; Patel et al., 1979 ; Overton et al., 1979) . A slick sample less than 10 days old showed a ser ies of C1-C3 benzothiophene sulfoxide homologs, corresponding to the benzothiophenes that were present in the original oil. Usir~g a tungsten lamp with uranium glass f ilter, Overton et al. (1979) photolysized a similar crude suspended in aqueous saline solution with the visible analog of sunlight (without much W) . This exper iment showed again that for some oils, visible and long wave W light can be important in oil photooxida- tion, in agreement with Freegarde et al. (1971) and contrasting with Hansen's (1975) conclusions that wavelengths below 350 nm are needed. Product identification in the Amoco Cadiz studies confirmed in molecular detail the prediction of Berridge et al. (1968a), that the reactivity of oxidized sulfur compounds in a case in which the compounds that are ox~dized--benzothiophenes--are much less reactive than typical thio- organ iCS . There are several reports and papers involving oil photooxidation in the Ixtoc I event. These include the only case in which experienced scientists, observing the occurrence of the event at close range for long periods of time reported seeing apparently light-dependent changes in oil behavior. Atwood and Ferguson (1982) stated that in zone 4 (roughly from lo to 20 nautical miles from the blowout) the light-brown emulsion visibly darkened and formed into black streaks. "This was assumed to result from oxidation of the oil, and the rate seemed to be dependent on sunlight intensity. . . The extent of the zone and rate of mousse formation apparently were dependent on sunlight intensity and wind stress. " Reports by Mackay et al. (1981a,b) and Boehm and Fiest (1980 ~ give varying interpretations of the importance of photooxidation of the oil spilled from Ixtoc I. Generally, they agree that photooxida- tion is minor with regard to material balance considerations but that it produced significant changes in the oil at 50 km and more from the well-read. Over ton et al. (1980) exposed very fresh Ixtoc I oil to natural sunlight, air, and synthetic seawater. The exposures were under a quartz plate, with the oil being added as a 1:10 heptane solution/ dispersion and the heptane being allowed to evaporate. The temperature was controlled, and 4-day Louisiana sung ight exposures were used. Each experiment had a "dark control. Photographs documented the conversion of the oil to a crusted material which broke up into tarry flakes, con- firming experimentally the involvement of photolysis suggested by the anecdotal observations of Atwood and Ferguson (1982) and the report by Patton et al. (1981) that at 750-1,000 km from the well-head on sheen- covered water, the primary mater ial was gram-si zed pancakes and mill~gram-sized flakes--both covered with a 5- to 10-~m thick "skin. " The flakes were enr iched in polar compounds and were denser than pancakes . The polar aromatic fraction of Overton et al. (1980), contained an unresolved mixture as determined by high pressure liquid chromatography (HPLC) . This mixture was absent in unexposed mater ial. The acid fraction, on methylation, yielded numerous peaks, including large amounts of neck to n-Cl1 fatty acid methyl esters (FAMES). Branched FAMES and C1- and C2-alkylated naphthols were also found,
284 as well as substituted 1- to 3-r ing aromatic and heteroaromatic acids. The authors stressed that a separate microbial oxidation simulation exper iment showed many of the same product types, but with different r atlas than those found in the photochemical exper iment. Autooxidation The thermodynamic instability of reduced carbon compounds in the presence of oxygen is not always manifested in a significant reaction rate. When it is, the noncombustion mechanisms involved most commonly are multistep, free radical reactions that are notoriously complex in mechanism and detailed behavior . They are subject to induction periods, and rates are often very sensitive to both inhibitory arid accelerators effects of trace components and to the physicochemical environment. Relevant background mater ial is widely available; basic reviews are given by Fallab (1967) and Nonhebel and Walton (1974), among others. Many constituents of oil are active in these reactions (e.g., branched hydrocarbons, benzylic C-H bonds, aromatic rings). Petroleum also contains potential inhibitors famines, metastable radicals, sulfur compounds) and accelerators (transition metal complexes , selected organics) . The issue is therefore entirely one of rate, and basic theory cannot be of much help in assess ing environmental r ates . However, it can predict the probable products and help to rationalize those actually found. There are only a few relevant observations on dark autooxidations of petroleum in the environment (Kawahara, 1969; Burwood and Speers, 1974; Brunnock et al., 1968; Lysyj and Russell, 1974; Larson et al., 1977; McLean and Betancourt, 1973~. The chemical mechanisms involved in oil autooxidation and photooxidation are probably similar. The formation of virtually all the reported breakdown products (largely oxygenated compounds) can be rationalized by known types of photoreac- tions and autooxidation (Howard and Ingold, 1966; Fallab, 1967; Nonhebel and Walton, 1974; Hendry et al., 1976~. In contrast, we are lacking both good chemical evidence for, and good mechanisms to explain, apparent "polymerization" to asphaltenes, to skins or to Semisolid flakes. n These mechanisms probably involve both free radical pathways and singlet molecular oxygen (Larson and Hunt, 1978~. Whatever the detailed chemical mechanisms involved, in some cases they appear to give products similar to those formed in biological processes (Overtop et al., 1980; Dowty et al., 1974; Patel et al., 19781. Sedimentation Laboratory Studies The various forms of oil in seawater can be sorbed onto settling particles and delivered to the bottom sediments. Meyers and Quinn (1973) suggested that as hydrocarbons become more soluble with increasing temperature and/or interaction with dissolved organic matter, correspondingly smaller amounts are available to associate with suspended mineral particles in seawater. These authors studied the sorption of n-alkanes and polycyclic aromatic hydrocarbons onto bentonite clay in saline solut ions . The heats of sorpt ion indicated
285 physical adsorption of the Van der Waals type. The extent of associa- tion decreased with increasing Volubility of the hydrocarbons from n-alkanes to aromatics. Several other clay minerals were also investigated and showed differing amounts of hydrocarbon association. In more recent studies, Meyers and Gas {1978) investigated the adsorption of hydrocarbons by smectite clay in saline solutions. They found that association increased linearly with increasing n-alkane concentration and with increasing carbon chain length. The degree of association of aromatic hydrocarbons was generally low, and isoalkanes were more effectively adsorbed than n-alkanes containing the same number of carbon atoms. Button (1976) reported that when the Volubility of n-dodecane in saline solution was not exceeded, this n-alkane was readily metabolized by microorganisms but not sorbed by clays. He concluded that findings of the type reported by Meyers and Quinn (1973) may represent the affinity of clays for small, oil-phase particles. Removal of indigenous organic matter from sediment particles by hydrogen peroxide treatment increased the uptake of n-alkanes and No. 2 fuel oil hydrocarbons from saline solutions as compared to that by untreated particles (Meyers and Quinn, 19731. Meyers and Quinn sug- gested that sedimentary organic matter interfered with oil uptake by masking sorption sites in the sediment and/or by binding small particles together, thus reducing the effective surface area. Another possibility is that some of the sedimentary organic matter was released from the particles to the solution, thereby increasing the Volubility of the oil as reported by Boehm and Quinn (1973, 19741. In contrast to the results of this study are the findings of Karickoff et al. (1979), who examined the sorption of the aromatic hydrocarbons (especially pyrene) in saline solutions by pond and river sediments. The partition coefficients were directly related to organic carbon content for a given sediment particle size. The sand fraction was a considerably less effective adsorbent than the fine fractions, and differences in the sorption between the silt and clay fractions were largely due to organic carbon content. The authors concluded that a reasonable estimation of the sorption behavior can be made from a knowledge of the particle size distribution, associated organic matter in the sediment, and the octanol-water distr ibution coefficient of the hydrocarbons . Bassin and Ichiye (1977) studied the flocculation behavior of suspended sediments and crude oil emulsions in both fresh and brackish waters. They demonstrated that oils and clays formed colloids or colloidal electrolytes in the presence of dissolved salts. Oil sedimentation seemed to be caused by adsorption of oil films onto clay particles which were subsequently flocculated by the shrinking of the double-layer charge on the collodial clay particles. Thus, a signifi- cant quantity of oil may be adsorbed to the clays and sedimented with them. They further suggested that the sedimentation of oils by clays in coastal areas is due more to electrolytic flocculation than to the affinity between the oil and the clays. The weathering processes affecting No. 2 fuel oil in saline solu- tions were investigated by Zurcher and Thuer (1978~. They concluded that partial dissolution, adsorption, dispersion, and agglomeration play important roles as initial processes in weathering of oil in
286 natural aquatic systems and result in the fractionation of the original oil mixture. Alkylated benzenes and naphthalenes were enriched in water phase; some high-molecular-weight aliphatic hydrocarbons were adsorbed by clays, and oil droplets were associated with suspended minerals. The latter process depended on the formation of dispersed oil droplets through turbulence and infer facial tension of the oil, and this played a major role in oil sedimentation. Model Ecosystems Gear ing et al . ( 1980 ) studied the partitioning of No. 2 fuel oil among seawater, suspended particulate matter, and sediments at the Marine Ecosystems Research Laboratory (MERL) located at the University of Rhode Island. The fuel oil was added as an oil-water dispersion in semiweekly doses to three systems over a 4-month period, and samples were analyzed for various hydrocarbon fractions. Transport from the water column was examined in relation to their phys ical and chemical properties. The amount of hydrocarbons in the water column associated with suspended particulate matter was inversely proportional to their water solubility. The result was a fractionation of saturated and aromatic hydrocarbons. The eventual settling of the suspended material carried about 50% of the relatively insoluble saturated components and less than 20% of the more soluble aromatic hydrocarbons to the sediments. Once in the sediments, these components were slowly mixed down through the zone of bioturbation (3-4 cm), and 10-20% of the hydrocarbons originally delivered to the sediments persisted for at least 1 year after the end of the oil additions. Hinga et al. (1980) used the MERL facility to study the biogeo- chemistry of 14C-labeled benzo[a]anthracene. They reported that this polycylic aromatic hydrocarbon, having a low water solubility and low rate of metabolism in the water column, became associated with sediments either through direct adsorption on the bottom after turbulent mixing at the sediment-water interface' or by adsorption onto or incorporation into suspended particulate matter, followed by subsequent deposition. Once incorporated into surface sediments, the hydrocarbon and its metabolites were mixed deeper into the sediments by benthic animal activity. R.F. Lee et al. (1978) studied the fate of polycyclic aromatic hydrocarbons added to ecosystem enclosures (CEPEX) suspended In Saanich Inlet, Br itish Columbia, Canada . Their results indicated that aromatic hydrocarbons have a short residence time (on the order of a few days) in mar ine waters and, because of their low solubil ity in water, the higher-molecular-weight components are associated with par- ticles in the water column. After sedimentation, biological degradation became an important factor in their removal. In areas with low concen- trations of suspended material, rates of hydrocarbon sedimentation would be low. F ield Investigations In the case of actual oil spills in the mar ine environment, there are several mechanisms (including those studied under laboratory ana enclosed or controlled mesocosm conditions) by which petroleum can reach the sediments. For the purpose of this discussion, the most important of these are (1) sorption of oil by suspended particles (these particles include terrigenous minerals,
287 plankton and detrital particles, and resuspended bottom sediments), (2) ingestion of oil by zooplankton and incorporation into fecal pellets, (3) weathering of oil by physical/chemical processes, and (4) direct mixing of oil and sediments. The following field investigations illustrate each of the above mechanisms. Sorption of Oil The impact of the Tsesis oil spill in 1977 on the l pelagic ecosystem of the Baltic Sea was the subject of a detailed study by Johansson et al. (1980~. Sediment traps were used to demonstrate the importance of sedimentation as a mechanism for the removal of this No. 5 fuel oil from the water column. Up to 0.7% of the sedimented material collected during the second week after the spill was petroleum hydrocarbons, and the estimated total sedimentation in the impacted areas was 30060 tons. This accounted for 10-15% of the approximately 300 tons of oil spilled. The most probable mechanism for the rapid sedimentation of oil was through adsorption to detritus or clay par- ticles, for particulate levels were high due to wind-induced resuspen- sion of bottom sediments. Another study by McAuliffe et al. (1975) estimates the fates of 35,000-65,000 bbl of crude oil discharged by Chevron Production platform C, Man Pass Block 41 in the Gulf of Mexico. By measuring the concentrations of hydrocarbons in the sediments and subtracting back- ground values, they calculated that less than 1% of the spilled oil was found in the sediments within a 5 mile radius of the platform and suggested that sorption of oil droplets on settling particles was the probable mechanism for sedimenting the hydrocarbons. In addition to sorption reaction in the water column, hydrocarbons can associate with particles before entering estuarine and coastal waters. For example, Van Vleet and Quinn (1977) reported that about 95% of the hydrocarbons discharged from a municipal wastewater secondary treatment plant were associated with suspended solids. This plant is located on the Providence River at the head of Narragansett Bay and is a major source of the suspended petroleum hydrocarbons found in the water column of the bay (Schultz and Quinn, 19771. The material is transported throughout the bay via tidal currents and eventially settles to the bottom, resulting in decreasing sedimentary hydrocarbon concen- trations from the r iver to the mouth of the bay (Hurtt and Quinn, 1979~. DiSalvo and Guard (1975) reported that at least 13.5 tons of pollutant hydrocarbons were present in association with suspended particulate matter in San Francisco Bay at any given time dur ing their sampling period. The source of the particulate hydrocarbons was thought to be suspended material from sewage effluents. Analysis of water samples suggested that hydrocarbons were associated with large particles or with flocculated smaller particles which were able to settle from suspension in less than 16 hours and which accumulated in the shoal areas of the bay. Incorporation of Oil Into Zooplankton Fecal Pellets Forrester (1971) found small (5 um to 2 mm size) droplets of Bunker C oil in the water column following the 1970 wreck of the tanker Arrow in Chedabucto Bay, Nova Scotia. These. droplets were formed by surf action
288 on oiled beaches and by wave action in oil-covered water, and were stirred into the water column to a depth of 80 m in some places. The size range of the oil particles included that of natural foods consumed by zooplankton. Conover ( 1971 ) found that zooplankton ingested small particles of the of] that were dispersed throughout the water column. He reported that up to 109 of the oil was associated with the zooplank- ton and their feces contained up to 7% Bunker C. As much as 20% of the oil was delivered to the bottom sediments as fecal pellets in addition to the particulate oil which was exported from the area by hydrodynamic processes. Weather ing of Oil Patton et al . ( 1981 ) studied the IXtoc I oil spill in the Gulf of Mexico and reported that weathering processes f irst formed a cracked, scaly surface on oil pancakes, which then flaked in turbulent seas. This process exposed fresh oil within the pancakes, wh ich produced a sur face sheen unt it a new sk in formed . After weathering, the skin flaked off on agitation, and the process was repeated. The flaking of these particles seemed to be a significant intermediate in the dispersion of this oil spill, and the increased density of the chemically weathered flakes relative to the pancakes suggested that this process may ultimately result in the sedimentation of oil. Direct Mixing of Oil and Sediments The barge Florida ran aground off West Falmouth, Massachusetts, in September 1969 and spilled about 600 tons of No. 2 fuel oil into Buzzards Bay e Strong winds churned the oil into an oil-water emulsion and drove it into West Falmouth Harbor and Wild Harbor. Oil was incorporated into the sediments under a water depth of at least 10 m due to the intense mixing of oil and water by gale force winds. The effects of this spill have been studied in detail by several workers (e.g., Blumer et al., 1970b; Blumer and Sass, 19727. Most recently, Sanders et al. (1980) reported that 5 years after the spill, partly degraded fuel oil was still present in the sediments of Wild Harbor River and Estuary. ~ spill of Bunker C oil in San Francisco Bay in 1971 was studied by Conomos (1975~. The spilled oil increased in density by evaporative loss of its lighter fractions and/or solution of its low-molecular- weight components, and was mixed through the water column by strong tidal currents and wind. Some of the oil globules carried to the bottom by the turbulence mixed with sandy and gravelly sediments and remained near the bottom. The oil was eventually moved, beaching in eastern San Pablo Bay after being transported landward by the near-bottom water currents. In regard to oil stranded on coastlines, the behavior of spilled oil in different environments is primarily dependent on the porosity of sediments and the energy of the waves acting on the coastline. Rocky shores tend to "self-clean. within a matter of months, whereas soft-sediment lagoons or mangrove swamps act as long term (years to decades) petroleum sinks . On cobble and sandy beaches, oil can sink deeply into the sediments and remain longer than on bare rocks. Pools of oil are likely to collect in hollows among rocks, protected by a
289 skin of weathered oil and may remain essentially unchanged for a long tome. Tidal pumping is the active factor causing penetration into the sediments. Sediment grain size and compaction control the rate of penetration. In muddy sediments, penetration is minimal, and only the upper few centimeters are affected. The breakup of the supertanker Amoco Cadiz (spilling 223,000 tons of crude oil) in March 1978, off the Brittany coast, coincided with the annual rebuilding phase of beaches in which tons of sand are transported onto the shallow winter beach slope (Hess' 19781. This process resulted in the stranding of oil and mousse on the beaches, followed by transport and burial within these beaches. Long et al. (1981) report that beaches having thick sand layers became long term reservoirs in which the oil moved slowly and continuously downward until it reached a level near the water table where it was somewhat stabilized, having a residence time of more than 3 years. For beaches with a thin sand layer overly- ing an impermeable basement, the oil moved laterally along the bedding plane and could be seen Washing out N at outcrops. Summary and Recommendations Advection and spreading are the most important processes affecting the fate of spilled oil. Predictions based on complex mathematical modeling of these processes are unreliable because of the wide spectrum of oil types and the changing environmental conditions occurring during a spill. The best estimate possible at the present time is that the drift velocity is 3-4% of wind speed. Evaporation of oil is the next most important process, accounting for up to one- to two-thirds of the mass lost. Evaporation of various hydrocarbons from aqueous solution is also important. However, the predictability of evaporative behavior is difficult due to the com- plexity of the oil and uncertainties in Volubility and other thermo- dynamic data for individual compounds. Dissolution is considerably less important than evaporation in determining the fate of spilled oil because of the low aqueous Volubility of most components. In order to be able to develop the complex models which will yield the predictive capability required for future spillages of petroleum at sea, better and more comprehensive thermodynamic data are needed on individual hydrocarbons as well as on nitrogen-, sulfur-, and oxygen-con ta i n ing compounds . The movement of oil into the water column is important because it determines the lifetime of a slick. The primary mechanism for the process is believed to be propulsion by surface turbulence of oil into the water column as a "shower" of oil droplets. Modeling awaits an understanding of the exact mechanism for this process. Thus, research leading to a better understanding of the mechanisms for vertical . dispersion of oil is recommended. Our present knowledge of atmospheric photochemistry suggests that almost all of the oil that evaporates is photochemically oxidized in the atmosphere. In surface water, photochemical oxidation may be important, taking place within a time scale of minutes to days. In
290 addition, reaction products may be more or less toxic than their precursors . The signif icance of photooxidation and autooxidation and their products is unknown and needs to be determined . Emulsif ication and mousse formation ar ise from the physical mixing due to wave turbulence when oil is released into the sea and involves surface-active compounds (possibly asphaltenes , porphyr ins, and other nitrogen, sulfur, and oxygen compounds). Products of photochemical and microbial oxidation also can serve as surfactants, but mousse formation may slow bacterial action. In addition, emulsification can be initiated and dispersants added to spills to curb or reduce impacts. Research in this area should be focused on the relationship between chemical . composition and formation and the stability of oil-water emulsions, including the role of photochemical and biochemical reaction products. Sedimentation of spilled oil takes place primarily through sorption on per ticulates or by incorporation into fecal matter. Weathering processes increase the density of floating oil and, when this occurs, incorporation into particles will eventually cause an increase in density above that of seawater so that the oil then sinks below the surface into the water column and, in some cases, eventually to the sediments. A better understanding of interactions of petroleum with particulates in the water column and sediments is needed. - BIOLOGICAL FATES Introduction The biodegradation of petroleum is seen by most workers as one of the principal mechanisms for removal of petroleum from the mar ine environ- ment. This applies particularly to the nonvolatile components of crude oil or refined products. The var ious compounds differ widely in terms of the ir b iodegr adab it ity . Thus al kanes and a lkenes and the s impler monoaromatics are biodegraded quite readily, but the tars and resins are virtually impervious to biological attack. The pathways used for biodegr adation of petroleum tend to fall into two distinct approaches: that used by bacteria, and that of the eukaryotic invertebrate and vertebrate systems. Insufficient data are available from green plants to make broad generalizations on their ability to biodegrade petroleum hydrocarbons. Microorganisms (bacteria, yeasts, fungi) are important in the degradation of petroleum in surface films, slicks, the water column, and sediments. Phytoplankton may degrade hydrocarbons in the water column, but little is known of this. Zooplankton are known to aid in the sedimentation of oil droplets and oil associated with particulate matter through their ingestion of microparticulate oil from the water column, followed by excretion of what is apparently unmodif fed oil in the feces (and rews and Floodgate, 1974; Conover, 1971; among others). Benthic invertebrates such as polychaetes, wh ich normal ly play an impor tent par t in the ox idat ion and recycling of sediment organic matter, also have a significant role in the degradation of sediment-bound oil (e.g., Gardner et al., 1979; Gordon et al ., 1978b ; Lee et al ., 1979 ~ .
291 Fish, marine mammals, and birds can become contaminated through uptake of oil from the water column and through ingestion of oiled food, or in the case of marine mammals and seabirds, through cleaning and preening of oiled fur or plumage. Therefore, these animals can also contribute to the overall biodegradation of petroleum in the marine environment. The understanding of petroleum biodegradation is far from complete because of the complexity of both petroleum and the various metabolic processes. Of the various classes that make up petroleum, most of the attention has been on the hydrocarbons. Some information is available also on the degradation of sulfur-containing compounds, e.g., dibenzo- thiophene. The accumulation and metabolism of various sulfur-, nitrogen-, and oxygen-containing compounds present in petroleum, by marine algae and higher invertebrates and vertebrates, are largely unstudied. Microbial Biodegradation When hydrocarbons become available to a microbial community in a complex mixture such as petroleum, biodegradation of most petroleum compounds occurs simultaneously, but at widely differing rates. Generally the biodegradation of the n-alkanes is most rapid, followed closely by the simple aromatic components. The isoalkanes, cyclo- alkanes, and condensed aromatics are degraded more slowly. Various hydrocarbon components may also influence each other's degradation indirectly through the phenomena of cometabolism or "diauxie. n In the first process, a normally refractory hydrocarbon may be degraded in the presence of a second readily degraded hydrocarbon. In the case of diauxie, the presence of a more easily utilized hydrocarbon represses enzyme induction necessary for metabolism of the second hydrocarbon. The latter is degraded only after the first is exhausted. The following summary of hydrocarbon biodegradation by microbes is based primarily on recent reviews of the current literature (Bertha and Atlas, 1977; Atlas and Bartha, 1981~. Aliphatic Hydrocarbons The biodegradation of normal and branched alkanes was reviewed by McKenna (1971) and Rathledge (19781. Pirnik (1977) reviewed some specific problems related to the biodegradation of methyl-branched alkanes. Alkanes of the C1O-C22 range are the most readily and frequently utilized hydrocarbon substrates. The gaseous alkanes (C1-C4) are degraded by certain groups of microorganisms; because of low Volubility, the Cs-Cg alkanes are attacked by relatively few hydrocarbon degraders. The n-alkanes above C22 are not readily biodegraded because they are only slightly soluble at temperatures within the range normally found in the ocean. Nevertheless, biodegradation of n-alkanes
292 up to C44 in length has been demonstrated (Haines and Alexander, 1974), albeit slowly, particularly at low temperatures. Isoalkanes are less readily utilized in comparison to n-alkanes. Methyl branching in the 2- or 3-position is a hindrance to betaoxida- tion, and relatively few alkane degraders possess mechanisms to bypass such blockage. Further branching, resulting in quaternary carbon atoms, may render an isoalkane completely resistant to microbial biodegradation. Clef ins tend to be more toxic and, at least under aerobic condi- tions, are less readily utilizable than the corresponding alkanes. Theoretically, olefins should be less stable under anaerobic conditions than alkanes, as they can be hydroxylated without a need for oxygenate enzyme systems. The most common type of primary metabolic attack by microorganisms on n-alkanes is mediated by mixed function oxidases (monooxygenases) - that, acting on the terminal carbon, convert the hydrocarbon molecule to a primary alcohol. A cytochrome P450 and a rubredoxin system have been characterized as mediating such oxidations, both resulting in the same primary alcohol product. Although in the great majority of cases the initial attack is directed at the terminal carbon atom of the hydrocarbon molecule (Figure 4-4a), some microorganisms attack hydrocarbons subterminally, converting them to secondary alcohols (Markovetz, 1971) . Oxidation continues to the keto and ester stage. The ester, most commonly a formats or acetate ester, is hydrolyzed, yielding formic or acetic acid and a primary alcohol. The pr imary alcohols, whether der ived from terminal or subterminal oxidations, are further oxidized to aldehydes and fatty acids. The fatty acids are subsequently shortened by C2 units by betaoxidation. In some cases, however, especially when betaoxidation is hindered by branching, the fatty acid is attacked at the other terminal carbon by the process called omegaoxidation. Alternatively, the blockage posed by the methyl branch can be eliminated by the mechanism elucidated by Seubert and Fass (1964~. This pathway essentially elongates the methyl branch by a carboxylation step, and the resulting C2 unit is released . · ~ as acetic acza. Alkanes may be attacked, either as the alkanes at a saturated terminal carbon, or may be oxidized directly at the double bond with formation of an epoxy compound. This is hydrated to a dial, which in turn, is oxidized and cleaved to yield a fatty acid and a primary alcohol. Al icycl ic Hydrocarbons Low-molecular-weight cycloalkanes, such as cyclohexane and decalin, exhibit considerable solvent-type membrane toxicity and serve as growth substrates for microbes only in exceptional cases. At low concentra- tions, in mixed enr ichments, and In the mar ine environment, cyclo- alkanes are degraded at moderate rates. Initial cometabolic attack followed by co~runensal utilization of the products is the main mechanism
293 of biodegradation (Perry, 1977, 1979; Trudgill, 1978; Beam and Perry, 1974~. The metabolic sequence, as illustrated for cyclohexane, is shown in Figure 4-4b. The oxidase responsible for converting the cycloalkane to the cyclic alcohol and the monooxygenase that lactonizes the ring apparently are seldom present in the same microorganism, necessitating the synergistic degradation sequence. Aromatic and Condensed Polyaromatic Hydrocarbons Monoaromatic hydrocarbons have considerable membrane toxicity because of their solvent properties, but in low concentrations they are rapidly utilized by a considerable number of microorganisms. Condensed poly- aromatics having 2-4 r ings are somewhat less toxic and are biodegradable at rates that decrease with the level of condensation. Condensed polyaromatics with 5 and more r ings fail to serve as growth substrates and are eliminated from the environment very slowly. The initial metabolic transformation steps, if any, are cometabolic . The microbial metabolism of aromatic hydrocarbons has been subject to several updated reviews (Gibson, 1968, 1971, 1977; Hopper, 1978~. Using benzene to illustrate the sequence of events (Figure 4-4c), initial bacterial oxidation occurs by dioxygenase attack. The postulated dioxe ten e product is first reduced to cis-1,2-dihydroxy-dihydrobenzene and is oxidized, in turn, to catechol, regenerating NADH (hydrogen form of nicotinamide-adenine dinucleotide) in the process. The catechol ring is opened by either orthocleavage or metacleavage, yielding in the first case, cis, cis-muconic acid, beta-ketoadipic acid, and the succinate plus acetate fragments. In the second case the cleavage yields 2-hydroxy-cis, cis-muconic semialdehyde, and subsequently the pyrovate plus 2-keto-4-pentenoic acid fragments are produced. As reviewed by Cripps and Watkinson (1978), condensed polyaromatic hydrocarbons having 2 or more fused aromatic rings command special interest because some compounds in this group are potential carcino- gens, or may be transformed to carcinogens by microbial metabolism. Two- and three-ring condensed aromatic hydrocarbons such as naphtha- lene, anthracene, and phenanthrene are degraded by successive opening of the aromatic rings, essentially by the mechanism described for benzene. More highly condensed polycyclic aromatic hydrocarbons such as benzota~pyrene and benzota~anthracene (Gibson, 1975, 1976) are cooxidized to dihydrodiols and thus are activated to carcinogens. They apparently are not extensively degraded by pure cultures and are miner al ized to CO2 in the env ironmen t only at extremely slow rates (R.F. Lee and Ryan, 1976; R.F. Lee, 1977a; Her bes and Schwall, 1978~. Recently, Wu and Won g (1981) reported microbial methyl hydroxylation of 7 ,12-dimethylbenzo (a) anthracene, resulting also in carcinogenic activation . Alkylaromatic hydrocarbons with shor t alkyl var. ieties such as toluene may be degraded by the mechanisms described for benzene (Figure 4-4c). Alternatively, the initial attack may occur at the methyl group with a conversion, in several steps, to benzoic acid. Oxidative decar- boxylation leads to catechol that is sub ject to r ing cleavage. Phenyl
294 A CH3 - (CH2 )nCH2 OH 1 CH3 - (CH2 )n-CHs OH ~ 1 CH3 - (CH2 )n - (m + 1 )-CH2 - (CH2 )m-CH3 1 o 11 CH ~-(CH2 )n-CHO CH3 - (CH2 )n - (m ~ 1 )-C-(CH2 )m CH, 1 ' ~ O CH3 - (C H2 )n -COOH CH3 - (CH2 )n - ( m + 1 )-O-C-(CH2 )m-CH3 */ l, ,B-Oxidation HOH2-(CH2 )-COOH CH3 - (CH2 )n - 2-COOH OHC-(CH2 )n-COOH ,B-Oxidation HOOC-(CH2 )n-COOH ,B-Oxidation CH3 - (CH2 )n - ( m + 2 )-CH2 OH + CH3 - (CH2 )m-COOH ~1 CH3 - (CH2 ~n - ( m + 2 )-CHO ,B-Oxidation CH3 - (CH2 )n - (m+ 2)-COOH ~B-Oxidation OHO O 1I1 I' B ~ O2 + ~H ~- 2H ~~o Cyclohexane Cyclohexanone Cyclohexanol COOH CH2 ,B-Oxidation CH2 1 CH2 CH2 COOH COOH CH2 -2H CH2 1 +H2 0 CH2 CH2 CHO Adipic Acid `~-Oxo Hexanoic Acid ,B-Caprolactone + H2O COOH CH2 CH2 1 CH2 CH2 CH2 OH ,B Hydroxy Hexanoic Acid ) FIGURE 4-4 Degradative pathways of petroleum hydrocarbons. (a) n-alkanes: (left) diterminal or omegaoxidation, (center) monoterminal betaoxidation, and (right) subterminal oxidation (Atlas and Bartha, 1973a). (b) An example of metabolism of alicyclic hydrocarbons (Atlas and Bar tha, 1981~. (c) Microbial metabolism of the aromatic ring (simplified) by mete or ortho cleavage, as shown for cyclohexane (Atlas and Bartha, 19811. alkanes with long alkyl chains are regularly metabolized, starting at the terminal carbon of the alkyl moiety (omegaoxidation). Successive betaoxidation steps shorten the alkyl chain to benzoic acid (in the case of odd carhon numbers) or to phenylacetic acid (even carbon numbers). Benzoate is easily degraded as outlined above, but phenyl- acetic acid is more persistent and, in pure culture experiments, often accumulates as an end-product.
295 C FIGURE 4-4 (continued) Benzene Asphaltenes and Res ins ~I)H O2 ~\COOII Catechol ~ COOH Cis Cis-Muconic: Acicl 02- CHO COOH ~OH 2-Hydroxy-Cis Cis Muconic Se~nialdehyde H2 of HCOOH ~ ~ 02~ 0~\ coon ~ ,COOH ,B-Ketoa~iipic Acid ,CoA o CH3 -CH Acetaldehyde COOH Succillic Acid ~0 CH3-C-COOH 11 o - HOOC CH 2 CH, - COO} ~ . . . . . o 11 Cart C SCoA 2-Keto-4 Pyruvic Acid Penten~,ic Acid Acetyl-(~,A Asphaltenes are a heterogeneous and poorly character iced assortment of compounds with high molecular weights and low volatility and Volubility. Analytical techniques are in general inadequate to define the individual chemical structures of asphaltenes and are even less able to follow their fate in the environment (viz., Chapter 3~. However, "tar," high in asphaltenes, is widely distributed throughout the marine environment (Butler et al., 1973; e.g. , physical fates section). As well, both laboratory and practical experience show that these compounds are highly resistant to biodegradation (e.g., Traxler et al., 1965~. Resins comprise the polar and often heterocyclic NSO compounds (compounds containing N. S. O as constituents). When not highly condensed they may be available to limited microbial metabolism. This includes the lower-molecular-weight resin fraction such as phenols, cresols, thiols, thiophenes, pyridines, and pyrroles. The latter have considerable toxicity toward microorganisms, but at least some of them are likely to be biodegraded at low concentrations. Very little work has been published in this area, and available information is restr icted to the condensed dibenzothiophene (Yamada et al. , 1968; Nakatani et al., 1968; Kodama et al ., 1970, 1973; Labor de and Gibson, 1977 ~ . Somewhat paradoxical is the microbially mediated production of long chain alkanes (waxes ~ dur ing biodegradation of petroleum (Walker and Colwell, 1976b). These are produced only as a consequence of bio
296 degradation and not by nonbiological weathering. The mechanism of their formation is as yet unexplored. A head-to-head condensation of reactive biodegradation intermediates (e.g., free radicals) is con- sidered to be a possible explanation for their appearance. Phytoplankton and Marine Algae The evidence suggests that unicellular algae are able to take up and metabolize both aliphatic and aromatic hydrocarbons. However, the extent to which this occurs is only poorly understood. Much less has been done with macroalgae, except to show that such genera as Enteromorpha, and Phylospadix, when exposed to an oil spill, will take up petroleum hydrocarbons (Clark et al., 1973, 1975; Burns and Teal, 1979; Vandermealen and Gordon, 1976~. The uptake and metabolism of aliphatic hydrocarbons to fatty acids by the diatom, Chaetoceros simplex calcitrans, was investigated by Boutry et al. (1977~. A green alga, Scenesdesmus, was reported to metabolize the alkane, heptadecane, in the light but not in the dark (Masters and Zaiic, 1971). Since that time, increasing awareness of work Fucus, the Importance or pnotooxloatlon leaves their result open to question. The ability to metabolize the simple aromatic hydrocarbon, naph- thalene, appears to be fairly widespread. Thus Cerniglia et al. (1980a) found that nine species of blue-green algae, five green algae, one brown alga, and two diatoms were able to oxidize naphthalene under photoautotrophic conditions, with at least six metabolites produced. The alga, Prototheca zopfii, which lacks chlorophyll, was reported to degrade both the aromatic and al iphatic portions of crude oil (Walker et al., 1975~. Blue-green algae have also been reported to metabolize biphenyl and are thought to be capable of metabol iz ing other aromatic hydrocarbons (Cerniglia et al., 1980b). Work in the last 3 years (Cerniglia et al. , 1980b) has led to further understanding of metabolic pathways in phytoplankton (Figure 4-5 ~ . Cernigl~a et al. (1979) reported that a culture of cyanobacteria exposed to 4C-naphthalene metabolized 1.4% of the substrate in hours. The major product was 1-naphthol, with preliminary evidence presented for the formation of both cis- and trans-diols. This would suggest that blue-green algae appear to have attributes of both bacteria {which metabolize naphthalene to cis-diols) and higher organisms twhich produce trans-diols) (Hopper, 19783. A single study has examined, in phytoplankton, the fate of a polycyclic aromatic hydrocarbon. After introduction of 14C-benzo (a) anthracene into a mar ine mesocosm, some of the radioactivity was subsequently found associated with a phytoplankton fraction (Hinge et al., 1980~. Most of the fraction was still in the form of benzofa)- anthracene, but there were signif icant amounts of polar metabolites reported also. However , the possibility cannot be dismissed that associated bacter ia were involved. Axenic cultures are needed for this kind of work. - ~- -
297 - H o AH / Naphthalene 1,2-oxide OH Naphthalene \ H OH A, cis-Naphthalene dihydrodiol o m~ ~N 1-Naphthol H OH 4-Hydroxy-1 -tetralone Further metabolism FIGURE 4-5 Proposed pathways for the metabolism of naphthalene by Oscillatoria sp., strain JCM. SOURCE: Adapted from Cerniglia et al. (1980b). Invertebrates and Vertebrates General Patterns of Hydrocarbon Uptake and Tissue Contamination Unlike microorganisms, animals tend not to utilize petroleum hydro- carbons as a carbon source, but generally oxidize and conjugate the products, rendering the end-products more water soluble, thereby facilitating their elimination via the usual modes of excretion of dissolved substances. All animal groups tested have been capable of taking up petroleum hydrocarbons from either the water column directly or via their food. This uptake can occur directly through the general body integument, across respiratory surfaces (gills, lungs, or other gas-exchange surfaces), and via the gut. Although the precise mechanism of avail- ability of hydrocarbons is a topic about which we still know little, uptake may be simple, nonmediated transport across epithelial layers (Kotyk, 1973~. Bioavailability depends to a considerable degree on whether the hydrocarbons are dissolved in the water column, sorbed by or bound onto particulate sediments or organic material, or bound up in food (see Sedimentation section). The organic content of sediments or particles, for example, can determine the sorption characteristics for hydrocarbons (Means et al., 1979) and therefore the amount of hydrocarbon in solution in natural waters. In bivalves the sorption of specific hydrocarbons and their apparent bioavailability have been found to vary with hydrophobicity (Dunn, 1980~. In fish and some invertebrates, factors related to solubility
298 of hydrocarbons may well be responsible for the greater accumulation or retention of alkylated aromatics as compared to the unsubstituted forms (Roubal et al., 1977; Melancon and Lech, 1979; Neff, 1979~. The degree of correlation between accumulation of some lipophilic foreign compounds by fish and octanol-water partition coefficients (Veith et al., 1979) supports the idea that partitioning into and uptake via the gills (Hunn and Allen, 1974) is a ma jor pathway in these animals. The nature of the compound may also dictate the absorption of hydrocarbons via the gut. Indeed, in mammals the absorption of aliphatic hydrocarbons by the gut was found to be dependent on carbon number (Albro and Fishbein, 1970~. In two species of marine fish, the absorption of hexadecane from contaminated food differed markedly from that of benzofa~pyrene, and the patterns of absorption for the two compounds were quite different in various species (Whittle et al., 19771. The bases for such differences are not yet apparent. The role of bacteria in the guts in metabolizing ingested hydrocarbons in marine vertebrates is also unknown. Macroinvertebrates All invertebrates studied to date readily take up petroleum components, and a majority also metabolize them fairly readily, although little is known of the various metabolic pathways involved when compared to the body of knowledge available on microbial metabolism of hydrocarbons. Experimental studies have demonstrated that the process of elimina- tion of hydrocarbons is initiated within minutes or hours of their uptake, although less is known of metabolite formation and their eventual fate. The route of uptake varies with the organism and its feeding habits. Thus in coDenods {Calanus helaolandicus] Harris et al. (1977) and corner et al. (1976b) demonstrated that dietary uptake of naphthalene was more important than uptake from the water. However, in blue crabs (Callinectes sapidus), hydrocarbon in the food was not accumulated rapidly and was quickly voided in the feces (R.F. Lee and Neahauser, 1976~. While there is no doubt that hydrocarbon contamination may be available from reservoirs within oiled sediments, in many instances the main route appears to be via the water column. Thus Rossi (1977) reported that most of the aromatic hydrocarbons accumulated by the polychaete Neanthes arenaceodentata were derived from water and not from sediments. Soft-shell clams (Mya arenaria) in oiled sediments appear to behave similarly (Vandermeulen et al., 1981, 1982~. Judging from the few studies that have addressed the question of tissue distribution of hydrocarbons, petroleum becomes readily dis- tributed throughout the exposed animals, but storage of hydrocarbon apparently occurs in lipid-rich tissues, and concentrations of hydro- carbons are generally found to be higher in lipid-rich animals. Most analytical work has been done with readily accessible inter- tidal invertebrates such as crabs and bivalves. As a consequence the data base for pelagic and offshore benthic invertebrates is slim, leaving a gap in our understanding of oil distribution in marine organisms generally. In many instances, extrapolation appears to be valid, but comparative corroboration is needed.
299 Bivalves, many of which filter large volumes of water while feeding, can take up and concentrate petroleum hydrocarbons from the water, whether in solution, absorbed to suspended particles, or as finely dispersed oil globules (Anderson, 1975; Boehm and Quinn, 1977; Clement et al., 1980; Disalvo et al., 1975; Dobroski and EPifano, 1980; Farrington and Quinn, Canzonier, 1976; Fucik and Neff, 1977; Hansen et al., 1978; R.F. Lee et al., 1972a, 1978; Neff et al., 1976; Nunes and Benville, 1979; Palmork and Solbakken, 1981; Stainken, 1977; W.C. Wong, 1976~. Reviews of the 1 iterature on the uptake and discharge of petroleum hydrocarbons by bivalves have been presented (R.F. Lee, 1977a; Neff, 1979; National Research Council, 1980~. Numerous studies have shown that bivalves can accumulate hydro- carbons to a level several orders of magnitude above the concentration in the water (Table 4-3~. Table 4-4 is a summary of the accumulation of petroleum hydrocarbons by marine bivalves taken from areas con- taminated by spills or chronic pollution. The maximum concentration of petroleum hydrocarbons in bivalves exposed to oil under laboratory or field conditions was between 300 and 400 ~/g (Tables 4-3 and 4-4~. Clams, oysters, and mussels differed in their rates of hydrocarbon uptake, possibly due to differences in filtering rates and amounts of lipids (Clark and Finley, 1974; Neff et al. , 19761 . Stegeman and Teal (1973) noted that oysters with high lipid content took up more fuel oil (314 ug/g wet weight) from the water than others with less lipid content (161 ~g/g). Burns and Smith (1977) reported that mussel and oyster tissues appeared to be saturated at approximately 30 mg of hydrocarbons per gram of body lipid. In oiled areas, burrowing bivalves such as Mya arenaria or Modiolus demissus have much h igher hydrocarbon concentrations than 1973; Farrington et al., 1982; Fossato and attached epibenthic bivalves such as Mytilus edulis or Crassostrea virginica (R.F. Lee et al., 1981b; Vandermoulen and Gordon, 1976~. Detritus-feedinq bivalves accumulate more hydrocarbons than suspension feeders (Augenfeld et al., 1981; Roesijadi et al., 1978~. Several factors can affect tissue accumulation of hydrocarbons and their subsequent elimination. For example, hydrocarbons accumulated in lipid-rich gametes will be discharged during gamete release. Thus, the seasonal reproductive cycle is an important factor in hydrocarbon accumulation. Maxima for benzota~pyrene and perylene concentrations in Mytilus edulis from Laguna Veneta, Italy, occurred in January with minima in May (Fossato et al., 1979~. Spawning took place from March to April. Temperature and salinity are also parameters affecting uptake. Uptake of polynuclear aromatic hydrocarbons by clams was greater at reduced temperatures, while changes in salinity had little or no effect (FuCik and Neff, 1977~. The discharge rate was not affected by temperature or salinity. Depuration, i.e., elimination, of hydrocarbons by bivalves is not yet completely understood. Depuration does occur, but it depends in part on the manner of contamination and, in most instances, appears to occur only incompletely. Petroleum hydrocarbons accumulated by bivalves maintained under laboratory conditions generally had a half-life of only a few days (Table 4-3~; however! mussels collected from heavily
300 u) c o ·~1 ,{5 o c~ E U) 0o o C) ~: ~4 o ,4 o v o Ll 4) c U) a' - .~, m c . ~ LO c ~o L' o ~3 ~: QJ - o ~4 P* o ~o U) c v 1 a - E" 1 e ~· ~~ C ~~ ~I~ ~_-4 ~u~ ~ iDu ~a - ~10 ~ C.) ~r~ a ~a~ o~ c r ~v ~JJ_1 _1 ~ ~d a'Q. ~- - c --- c ~O c - ~ ^ ~ _ a~ _ 4) ~ cc ~ ~c c ~ 0 ~Om mm ~ ~ ~ ~ O~ ~ O ~O O C I: _1 ~ r- ~ ~- ~ 1- ~ ~- 0_' ~ _ 0 eq 45 4,~ os ~ a~ ~ ~ ~ C ~ C Ll ~ · ~ · ~ · ~ · ~ a,~ - 0 0 ~ c (~ E-4 C~4 - b - ~. - C~ -~G1 b4 U) N ~1 ~. · . . ~O C C 4J U) ~45 d2 Z C~ O~ C - ·~4 U1 ~ · O C O E o ~r ~c~ C · - . ~. . . . . ~ ~ O C O O O O O c ~OD O O O O mt,' O ._, _ 0 0 =- - :>' L~ ~ ~ a ~, _ o ~4 _ _ 0 1 U) #3 ~ 5: - - C 0 ~ C - . - E ~u: · ~ O C ~ ._' ~ t, E X ~ C -4 ~ ~ O C ~ _ 0 o~ 0 4, ~ E X · - ~ ~ E" - c o r. C c~ ·~1 - O C ~, ,,,: C ~ O ~ ~ X ~ 3 - U' o S ~ a' U N O :^ ~ :~: <: :' a, O ~ ~ O CL U) U a ._' ~n o ~U ~U~ r ~r ~4 a ~a ~0 C~ U~ u ~c ~u, ~0 L ~:r ~1 O ~O ~ ~r 0 co 0 0 0 c r~ r~c~ ·~ 0 ~r o o ~DO ~u ~5 ~O O O O O1 - · · O O O ~) C C ~C~ ~ C UO ~C ' U. ~C C U) U) 00 ~C ~U C ~C _ U -01 ~S 1 ~1 ~- U a) 1 16 ^ ~·0 (L~ ~ ~\~- ~- - - S ^ C ~ ~ ~ C ~`= ~ ~ ~5: ~-s - ~ E ~s s s - s 0 ~0 ~ 0 ,. O ~S V ~ a~ 0 ~ ~O N C b; >. ~G5 ~S C, A' N .~4 ~Q. ~C ~s C _ ·e ~-4 CL E C c ~ ~- a) G) ~1 _ ~ ~a, c ~C ~C ~_ ~ .~4 ·_ · - · - C O C U. O O O ~ -4 E _ ~, - 0 _ _ ~_ >, c ~ 0 ~Q' ~ U :, ~_ O O ~_ U ~O . - ~r, t4 · :~ E · ~t ~· C O ~O O - O Z t) Z Z ~Z - U) 0 o Ld Ul 01 ~5 U) O U) ~ o~' --, ~ ._ _ ._ _ a ~n :- :~: ._~ C E ~ - o - v U 0 0 C o ._, v 0 ._, U o U, c ._' o o U1 4) UD .~ - r~ 0` - ~' . E o v a. ~: c ._~ E Q} v o c .. ~a · C) DC · C, O ~1 v:
301 c a) of ~1 ~ . - U ~ _ 0 1 U] :~: - a a: c .,. o o a a) ., m ._. c o u o A: 3 o JJ ad o In o 1 a: E~ ,' CJ~ O c: O ~ ~ n5 ~ ~ ~ 00 O a C) ~ C - - C) ~: ta C o C) N O ~ ~: :r ~: ~: ._ ~n ~ ~ _ C~ r ~co : ~ a a~ a~ u~u~ ~ ~ ~ ~ O ~ ~ ~ ~ - -- C O [~ - ~ cna~. ~a~. ~_1_1 ~_I · · · 3 -I >' ~ --·- ~-~ _ _ a~ ~t ~e q ~~ N _I -I ·· ~_ · 1- C C ~t 1~ 0 ~ ~ ~S:: _ ~a ~_ ~ -- to 10 ~I~ ~ ~ ~ ~ 05 _d -~ ~ ~C a, ~n5 _ ~ ~ ~ u' ~ C C C a) 4 ~- 0 C ~ ^ ~ ~ 4 ra - ~ns ~s 0 0 ~ ·n 0 c c: C C C a, ~0 Y Y ~ ~ ~ ~ · - ~ c~ ~ ES ~c: Y ~ ~ ~ ~ S: ~ UB ~ ~ ~ ~ ~ Ei 4~ ~ ~ ~ ~ ~ - ~ C ~ ~ ~ ~ S · - ~ ~ ~ ~ J: O m ~ c' c' a a ~ h a ~ ~ ~m I I I I I N 1 1 1 ~1 o 1 I ~ ~ N _' N I I 1 _1 1 1 O ~LO ~ ~r 0 r ~0un tD 1 · ~· ·· 1 0 ~0 ~0 0 a' a, 0 O C ~ U) C C: ~ `: a, u, ~ tn u, ln ~ u, ~ ~ eQ C C ~ ~ ~ ~ t) ~ ' - ~ '~' - ~ ' - ~ ~ ~ ~o' - .,. ~. - dJ ~ ~ ~ - - ~ ~ ~. - ^ ~ ~ C ' - ~S ~ lo ~- ~ ~ ~ oS ~- ·~1 JJ ~ C-I ~- , ~ ~ ~ ~ ~ ~ ~ ~ ~ - ~ ~ C ~ ~ S O S O ~ ~ ~ S O ~ ~S O == ~ O a, ~a,, 3~4 ~ ~ ~ ~ ~ ~ ~ N 0 ~ S I -- ~·.4 05 0 ~ QJ-- RS O O ~ ~ -~ ta Q. ~D ·- o5 _I ·- ~ 1 1 _I )~ ~_I ~ )~ ~ ~: ~ ~ ~ n o. ~ ~ N y ~: :' ~._' .,, . - ~ o O O O O S" UO ' ~C) ~ O C~ ._' A5 O ~Z _ ~ ·- c: O C .~4 ~., C ~S n o z t) _I _ ~ ~ O ·_~ ·~4 i.d O4S ~n eq 8 on U} n tn o V 'c . tQ U] . - a' UB u~ £ .`n ~5 V V C) ~ .- ._, .,~ ·- c ~ c: 0 0 0 0 s ~ s v v o z .,, u, 3 ~n 4 ~3 ~- ~o ~z tn .~' aml ac) ~ v ~ ~ ~ £ ,. ~a ~: ~: 3 00 ~n .,' V o tn o o o 00 UO o ~: ,' 3 ~a a) £ c a, 3 C O ~ a, - ~n £ a, ~- O :J o~ o X a, v ~s: a ~: ._' ~.. C) ~5 9 0 ~I cn
302 contaminated areas sometimes required a much longer period for deputation (Table 4-41. There also appear to be inherent species differences; thus, some studies have shown a difference between Mercenaria mercenaria and Mytilus edulis from similarly polluted areas. A weakened physiological state may well affect the rate of deputation. Possibly there are "conservative tissues" or "stable compartments" from which deputation is slow (Stegeman and Teal, 19731. The fate of hydrocarbons in other invertebrates is much the same as in bivalves, dependent on the same environmental and physiological factors for uptake, residence time, tissue distribution, and deputation/ elimination. A major difference that may exist relates to the metabolic fate of hydrocarbons, in particular, those in which metabolism is mediated by cytochrome P450-dependent enzyme systems (mixed function oxygenates). Most invertebrates examined to date appear to have this capability (e.g., crabs, lobster, polychaetes, and bivalves). The metabolism of petroleum hydrocarbons in invertebrates has not received the attention needed, but a range of metabolites has been described including derivatives of simple aromatic hydrocarbons in crabs (e.g., Corner et al., 1976b). Gordon et al. (1978) provided evidence for uptake and removal of hydrocarbons from chronically oiled sediments by the polychaete Arenicola marina. They pointed out, however, that the precise role of the polychaete, as distinct from that of possible microbial interaction, had not been defined. Polychaetes are now known to have mixed function oxidases (R.F. Lee, 1981), but possible effects of their excretion and bioturbation on bacterial activity have received little attention. Fish The nature of the compounds and possible association with blood serum components (flack et al., 1979) may influence deposition in various tissues. Association of hydrocarbons with cell membranes in coho salmon (Oncorhynchus kisutch) was indicated in at least one study (Roubal, 1974), but details of transfer processes through membranes or cells have not been demonstrated for hydrocarbons in aquatic vertebrates. Although in general the levels of hydrocarbons in exposed fish are greatest in the liver, in experiments with a variety of species the levels in neural tissues of fish have equaled or even exceeded those in the liver (Neff et al., 1976; Roubal et al., 1977; Collier et al., 1980~. This would seem to be consistent with the high lipid content and vascularization of these tissues. The observed distribution may be related to the molecular size of the hydrocarbons, for Roubal et al. (1977) observed that aromatics with a high molecular weight were retained more readily in brain tissues of the coho salmon (O. kisutch) than compounds with a smaller molecular weight. Similar observations have also been obtained in other t issues . Eff iciency of uptake of hydrocarbons from the food may be low in f ish in some circumstances (Whittle et al. , 1977) . While the mass of mater ial accumulated via dr inking of water in mar ine f ish under most circumstances would be minimal, the uptake via gills can be very impor- tant (Lee et al., 1972b). Benthic fish, particularly the Pleuronec- tidae, readily take up hydrocarbons from the sediment (Varanasi et al.,
303 1981), possibly via Resorption from sedimentary particles, but the route is uncertain. The contamination of animals in early development deserves close attention, for they may be more susceptible to toxic effects at these stages in their life cycle than as adults. Contamination of fish or invertebrate eggs is either direct, or via exposure of females during oocyte maturation (e.g., Kuhnhold et al., 1979; Hose et al. , 1981; Rossi and Anderson, 1977; Longwell, 1958~. The elimination of hydrocarbons from contaminated animals is affected by numerous factors and is highly variable. Disposition may be accomplished by several routes. One often suggested for fish is direct partitioning through the gills into water. The significance of this pathway has not been established for hydrocarbons in general, but Thomas and Rice (1981) offered evidence that substantial proportions of naphthalene and toluene could be discharged directly via the gills. Whether this is as important as excretion via the kidneys remains to be seen. Birds and Marine Mammals The significance of various pathways in birds . and mammals will differ from that in fish. Entry of hydrocarbons via the respiratory epithelium in lungs of birds and mammals is, barring aspiration of water, restricted mainly to those volatile compounds transported in the atmosphere, whether particulate or in a true gas phase. Thus hydrocarbons in aerosols in the vicinity of an oil spill may be an important source to birds. Sorption to particles apparently is an important factor in the delivery of hydrocarbons to the lungs of terrestrial mammals. Absorption of hydrocarbons by seals immersed in contaminated water has been clearly demonstrated (Engelhardt et al., 1977) but whether by lung or skin was not confirmed. However, the same studies indicated that dietary as well as nondietary routes are quite important in seals and, presumably, in other marine mammals. Absorption via the gut (in part by preening) can occur in seabirds (Grau et al., 1977) as in other waterfowl (Lawler et al., 1978a,b). A blood-brain barrier may exclude saturated aliphatic but not aromatic hydrocarbons in birds (Lawler et al., 1978~. The exposure of avian eggs to hydrocarbons may be direct or maternal. Exposure can occur through direct transfer of oil on the plumage to egg shells in the nest, and small amounts (50-100 pL) have been shown to be toxic to embryos. Contamination of terrestr ial bird (quail) eggs with Bunker C or No. 2 fuel oil via maternal routes resulted in reduced egg production and reduced egg viability (Grau et al., 1977~. Transplacental contamination of developing marine mammals has not been demonstrated. Factors Influencing Petroleum Biodegradation Rates Some of the factors that determine biodegradation rates are inherent to the polluting oil; others are environmental and subject to variation. Organismic factors (abundance of hydrocarbon degraders and their sub- strate range) and certain environmental parameters (pa, salinity) that
304 are nearly uniform and in a favorable range throughout the marine environment (Tait and De Santo, 1972) are not considered here. Composition and Weathering of Petroleum The biodegradable portion of various crude oils ranges from 11 to 90% (Colwell and Walker, 19771. A low percentage of biodegradation may result from a high amount of volatile components, for these ordinarily evaporate before significant biodegradation can take place. Because of this, the biodegradation percentage is often related to the ~topped. (preevaporated) crude rather than the intact one. Low percentages of biodegradation can result also from high proportions of condensed polyaromatic, condensed cycloparaffinic, and asphaltic petroleum components, because these compounds are biodegraded at extremely slow rates if at all. Toxicity of certain petroleum components can delay or prevent the biodegradation of susceptible ones. Atlas and Bartha (1972b) and Atlas (1975) noted such action by volatile components of certain petroleums in the environment. Toxic and lipophilic substances such as pesticides (Seba and Corcor an, 1969; Hartung and Klinger, 1970), polychlorinated biphenyls (Sayler and Colwell, 1976), and mercury (Walker and Colwell, 1976a; Sayler and Colwell, 1976) can be concentrated in the oil slick 102-105 times above their ambient concentration in the water and may inhibit biodegradation of the petroleum. Photooxidation (Burwood and Speers, 1974) may remove methyl branches that block biodegradation, but in high concentrations photooxidation products may become toxic to microorganisms (Van der Linden, 1978~. Formation of mousse (Berr idge et al., 1968c} reduces the surface area and availability of mineral nutrients and O2, thus hindering biodegradation (Atlas et al., 1980; Colwell et al., 1978) Temperature . The nature of the marine environment restricts petroleum biodegradation to the mesophilic and psychrophilic organisms. Hydrocarbon biodegrada- tion has been reported at temperatures below 0°C (ZoBell and Agosti, 1972~. Because of arctic and subarctic oil exploration, this has led to a substantial interest in psychrophilic and psychrotrophic hydro- carbon degraders (Malins, 19771. Generally, the rate and extent of hydrocarbon biodegradation was severely restricted at low water temperatures (Gunkel, 1968; ZoBell, 1969; Mulkins-Phillips and Stewart, 1974~. The temperature dependence of hydrocarbon biodegradation rates can be expressed in terms of Qlo values (Qlo = increases in rate per 10° change in temperature). Gibbs et al. (1975) and GibbS and Davis (1976) determined an average Qlo value of 2.7 over the 6°-26°C range.
305 Hydrostatic Pressure Some crude oils exceed the specific weight of water and others may do so at an advanced stage of weathering. Thus, hydrocarbons can enter the deep-sea environment and, consequently, the effect of hydrostatic pressure on oil biodegradation is of interest. Using an enrichment culture obtained from 4,940-m depth, Schwarz et al. (1974a,b, 1975) found that, at 20° and 25°C, 500-atm pressure delayed biodegradation only moderately. The same pressure at 4°C reduced metabolism by more than 1 order of magnitude as compared to a 1-atm control incubated at the same temperature. The authors concluded that the biodegradation of any petroleum residue that reaches the deep-sea environment will be exceedingly slow (Schwarz et al., 19751. Oxygen The initial attack on hydrocarbons is commonly performed by oxygenates. There have been several reports on anaerobic conversion of hydrocarbons (Senez and Azoulay, 1961; Chateau et al., 1962; Iizaka et al., 1969; Traxler and Bernard, 1969; Parekh et al., 1977~. These were all in vitro studies with isolated cultures. Thus, a pathway appears to exist for the anaerobic utilization of alkanes, with sulfate or nitrate serving as electron acceptors. Nevertheless, anaerobic hydrocarbon biodegradation in the environment is either undetectable, or orders of magnitude lower than aerobic biodegradation (Bailey et al., 1973; Ward and Brock, 1978; Delaune et al., 1980; Ward et al., 19801. Oxygen limitation of petroleum biodegradation is unlikely in the case of sur face sl icks that are in direct contact with atmospher ic oxygen. When oil is dispersed in the water column, oxygen limitation may occur. Complete oxidation of 1 L of oil would exhaust the dissolved oxygen in 320,000-400,000 L of seawater (ZoBell, 19691. Whether or not oxygen actually becomes limiting depends on the oil concentration, the rate of biodegradation, and oxygen replenishment by turbulence (wave and current action). Oxygen limitation of petroleum biodegradation in the water column may occur sometimes, but mineral nutrients are more likely to become limiting before the oxygen is depleted. Marine sediments commonly are anaerobic below a thin surficial layer. The thickness of this layer depends on grain size, organic content, and the degree of physical or biological disturbance of the sediment. Petroleum that becomes incorporated in anaerobic mar ine sediments is essentially immune to biodegradation until some disturbance releases the oil or oxygenates the sediment. The main reason for increased petroleum persistence in fine-grained sediments as compared to coarse-grained ones appears to be a lower rate of oxygen diffusion.
306 Mineral Nutr tents Petroleum-degrading microorganisms need to obtain mineral nutr tents from seawater . Consider ing the composition of seawater (Tait and De Santo, 1972 ~ in relation to mineral nutr lent requirements, actual levels of phosphorus, nitrogen, and iron are likely to approach limit- ing concentrations. Other essential elements appear to be present in suff icient or excess concentration. In vitro exper iments employing relatively high oil-to-seawater ratios have convincingly demonstrated phosphorus and nitrogen 1 imitation of petroleum biodegradation (Atlas and Bar tha, 1972b). Iron limitation was confirmed in clear offshore seawater but not in sediment-r ich coastal seawater (Dibble and Bar the, 19767. The need for phosphorus and nitrogen supplements for optical oil biodegradation activity in seawater was noted also by Bridle and Bos (1971), Reisfeld et al. (1972), Gibbs (1975), and LePetit and N'Guyen (1976) . Based on the data of Atlas and Bar tha (1972b) and Reisfeld et al. (1972), 1.5-2.5% N and 0.2% P (w/w) addition (calculated on the basis of petroleum that was actually degraded) allowed maximal petroleum biodegradation in these in vitro experi- ments. Bridle and Bos (1971) found somewhat higher and Gibbs (1975) somewhat lower requirements, explainable by differing experimental conditions and petroleum-to-seawater ratios. A summary discussion of the relation of mineral nutrient requirements for biodegradation of oil pollutants was provided by Floodgate (1979) with the conclusion that the scarcity of mineral nutrients in seawater is often limiting for petroleum biodegradation, not only in vitro but also under closely s imulated environmental conditions. Rates of Petroleum Biodegradation in the Marine Environment Microbial Degradation Consider ing the multitude of factors that influence petroleum bio- degradation and the var iety of methods employed in its measurement, generalized statements about rates are difficult. Nevertheless, rates are of central interest in terms of the self-pur if ideation capacity of the mar fine environment and need to be def ined at least with the crude accuracy of an order of magnitude. Petroleum biodegradation rates have been estimated in four types of experimental systems in ways that might be applied to spill conditions in temperate waters: (1) fermentation studies, (2) seawater enrichments under optimized conditions, {3) in situ marine enrichments, and (4) in situ petroleum degradation potentials. These are shown in Table 4-S. System 1 provides an estimate of optimal rate and is shown only for compar ison . The rates of system 2 may be approached in situ using nuts ient-stimulated biodegradation as described by Atlas (1977~. Conditions equal to system 3 occur upon prolonged exposure of a marine environment to petroleum pollutants, whereas system 4 measures the potential of ~ marine environment for petroleum biodegradation before the existing microbial population has an opportunity to shift in response to the
307 TABLE 4-5 Estimates Under Temperate Conditions of Hydrocarbon Biodegradation Rates Degradation Rates System (g/m3/day) References 1. High density monocultures in fermenters, optimal conditions 2. Seawater common ity under nutrient-enriched conditions 3. In situ mar ine oil additions (long incubation times) 4. In situ mar ine unoiled condition (short incubation times) 10,000-100,000 6,8 5-2,500 3,5,7,12 0.5-60 1,2,3,4 0.001-0.030 9,10,11 The cited references served as a basis for calculat ions; the above listed figures do not necessarily appear in the papers in this par- ticular form. Key to the references: (1) Atlas and Bronner (19811, (2) Atlas and Bartha (1973b), (3) Atlas et al. (1980) , (4) Atlas et al. (1981) , (5) Caparello and La Rock (1975J, (6) Dibble and Bar tha (1976) , (7) Coty and Leavitt (1971), (8) Kanazawa (1975), (9) Lee (1977a) , (10) Robertson et al. (1973) , (11) Seki (1976), and (12) Walker et al., (19761. petroleum exposure. This potential is greatly influenced not only by the prevailing environmental conditions but also by the previous history of exposure of the site to petroleum pollutants. The relevance of the four systems in Table 4-5 to the biodegrada- tion of oceanic petroleum spills is as follows: The initial values are expected to be in the range observed in system 4. On prolonged contact with the petroleum, population shifts can be expected to occur, and biodegradation rates can approach the values of system 3. Cytochrome P450-Dependent Metabolism One principal route of metabolism of hydrocarbons in metazoans is that initiated by cytochrome P450-dependent polysubstrate monooxygenases (MO) or mixed function oxidases (MFO). The metabolism of hydrocarbons by this route is essentially a means whereby animals can convert these lipophilic compounds to derivatives that are more water soluble and, hence, more readily excreted, thus profoundly affecting the nature, disposition, and effects of hydrocarbon residues in the animal. The scientific literature is replete with numerous studies and several
308 Conjugotion ond Excretion OH ' ~ Broome 1T JO O' I ,, ~ , ~ , _r Epoxide ~ Hydrase OHM oH 1 Con jugalion and Excretion Spontoneous :~ 6INDING TO Conjugation and Excretion FIGURE 4-6 Diagram of cytochrome P450-dependent metabolism of benzo (a) pyrene . reviews of P450 systems and their function in xenobiotic metabolism (for example, Johnson, 1979; Gelboin , 1980 ; Manner ing , 1981) . Br iefly, the P450 complex initially forms epoxide der ivatives of aromatic hydrocarbons, which can then be further metabolized by other enzymes (for example, epoxide hydrolase) and various conjugating enzymes. This subsequent conjugation usually results in detoxification of the epoxide intermediates, which by themselves are reactive and may be mutagenic A scheme describing the biochemical production, cycling, and fate of products of primary and secondary metabolism of an important model aromatic hydrocarbon, benzo (a) pyrene, is depicted in Figure 4-6 . , ~
309 Patterns of P450-Mediated Metabolism The character istics, functions, and induction of P450 systems in aquatic, principally marine, species have been recently reviewed in detail (Bend and James, 1978; Stegeman, 1981; Lech et al., 1981; R.F. Lee, 19811. In fish the liver is the primary site of hydrocarbon metabolism, while the hepatopancreas apparently serves this function in invertebrates. MFO activity has been found in fish species whenever sought. There have been fewer studies to determine the presence or absence of MFO systems in mar ine invertebrates. To date, 18 marine invertebrate spec ies , belong ing to 4 phyla (Annel Ida, Arthropoda, Echinodermata, and Mollusca) are known to contain MEG activity in their hepatopancreas, digestive gland, and other tissues (R.F. Lee, 19811. Tar ine invertebrates belonging to such phyla as Por if era, Platyhel- minthes, Rhynchocoela , Rot if er a , Gastrotr icha , Nematoda, Echiurida, Brachiopoda, Phoronida, and Chaetogratha have not been examined for MFO activity. Numerous studies have estimated the activity of P450- dependent enzyme systems responsible for hydrocarbon metabolism, using benzofa~pyrene as a model substrate (Table 4-67. However, a study comparing activity of the hepatic microsomes of coho salmon (Oncorhynchus kisutch) in vitro indicated that rates of metabolism of different aromatic hydrocarbons are not always equal (Schnell et al., 1980) . A1SO there are multiple forms of cytochrome P450, and the metabolism of different types of compounds may depend on the relative abundance of specific forms of the enzyme. Seabirds and some other waterfowl possess P450 systems (Bend et al., 1977a; Knight et al., 1981; Shackelford and Kahn, 1981; Peakall et al., 1981), but there have been few estimates of the capacity for hydro- carbon metabolism. Similarly, such activity has been little assessed in marine mammals (Engelhardt, 1981~. The only known examples are included in Table 4-6. The rates in some extrahepatic tissues are appreciable (Pohl et al., 1974; Stegeman et al., 1979; Singer and Lee, 1977~. The mass transformed per gram of fish in most tissue is usually only a small fraction of that in liver, yet the significance of metabolism in a given organ to the health of that organ may be great. Aromatic hydro- carbon metabolism has also been demonstrated in embryonic stages of marine fish, even during early development before the appearance of the liver (Binder, 1981~. In at least one species (Fundulus heteroclitus) such activity in embryos ~s very low until hatching, when within 24 hours there is a 10-fold increase in "constitutive" activity. Metabol ism In Vivo As the major site of metabolism of hydrocarbons is the liver, the gall bladder is accordingly a major route of exoretion of metabolites in fish (Lee et al., 1972b; Melancon and Lech, 1978; Collier et al., 1978; Solbakken et al ., 1980; Varanas i and Gmur, 1981~. Thus the analysis of bile might serve to monitor exposure of fish to various foreign chemicals (Statham et al., 1976~. Most of the metabolites appearing in
310 c ~: E o UJ o C) _ ._. u~ 1= ~r _ a' O ~ _ ~ E '. 5: ~ ~ c) ~l a' O O V v E O C~ - U. .,. ~: aJ . - ~: aJ E o U] c E ~n ,i o v ~: ~: - o Q m o U~ o C) ·,- 1 m E~ a, ~ s =: r~U ~ U) ~ · - _ ~C~ ~ _ _~ ~C~ ~; 3 ~ :, r_ ·~ ~- _- ~_'~ _, ~-~ : ~X · _ _ ~ _ · ~_ ~· CO ~ ~{: ~ a ~tO ra 4~ ~ ~ct. ~ , ~ ^ c: · - ' ~ ~ - ~ U] ~C U) U) ~, ~ ~. ~ · n: ~· ~ ~ ~1 EE `: O ~ ~ ~' ~ ~ - 't ~ · ~a) . v ~ ~1 U] U] ~ U] U] tn - U~ ~_ · ~ o o o O V o ~i 4~1 ~ ~D o ~ o · · . - o o o o - ~: .,' a) v o L' QJ E Ul _ ~ ·- ~ ~ E - X O O ~ b; ~ O `: ~ ~ ~ :^ c: m =:- ._, ~: r~ _ ao0~ 0·0 ·O~ O+.0 +1C~+1 o0CO O ··~ 000 _ __~ _ ___ 000 0 000 · ··~ 0 000 +1 +1+1+1 ~D 0 000 1 1· 1 1··- 1 10 1 1000 UJ UlU) U `: VV c ~C: ~a s Q. Q. ~ ~0 0 C) 0 0 V V V :) V ~ V ~U ~U. UJ U] ~ ~. U ~o ~0 o ~V _. ~V ~· - ~ E ~s s u: s ~· - . a 4 ~: UJ :, s" ·_' ·~4 ~: ~ 4 UJ :' C) ~- - V 0 ~ D E O UJ ~:, UB O - :e Ua .,' ~a C. UJ _ V ~V V `: U, · - D ~1 O _ UD C: Qt ·~4 U] O ~ Z UJ :' ~: :' E a O 3 UJ ~a ~ - m ~: ~; -
c As: In x o P4 m - .,. 4~ o - 1 a. m At: 0 Us ,4 A: E C 3 A In .,, E~ .,. U] 311 m ~s E U) a) e) E U] o o ~0 o o CO _~_1 ~4 · ~· ax ~·a~ C ~ ~QJ(IS_ ~m m a, - ~v e ~ r _ n' tn E E U] E U] . E a) U] QJ m n' m c ~_ U) U] a' E E ~ s a' ~: u ~CO r ~r ~cr' ~C ~C ~ 00 0 ~r ~unO ·e · · ·ee ~II OO O _I OOO011 ~1~1 m1 =1 m1m1~1=1 O O COU~Oer oo ~t ~r ~aiOt) I OO o o OoooaD ·e e e e···(D OO O O OOOO +1+1 +1 +1 +1+1+t+10t~ I C ~U ~O~)~) ~+1`0 O ~CD ~O OO O U' ~C~_.O ~O ·e e · ·e·eee OO O O OOOO ~O ~J ~1 a) a :> ._1 ~- - . - ~·_t ~ _~1 _1 _ ~_~_~ ·- ~4 _u)E cn _ tn O·~ ~_ Ug ~ ·-t tt ~JJ S ~C ~O ·,' SL'a) a) c a, ~c' ~ c ~a, ~JJ C O IO C} ~ ~U] ~n ~ Ll ,t u] E cq ~Q ~O te ~t ~ s ~a, ~a ~Q. ~n ~ ~0 ~ 0 c: ~a, O tU ~ rl ~ sq tn D. ~ :' O ~ ~C C U] O U] ·- Ct; 4~ ~ O C) U] ~-- U) C: - ~_ u]- ~ cO ~ ~4 3 ~_ ~E~ U] o . - .,. L~ ~; E U: Q, U) s E~ e e ~e .~' .- ~ aJ ~ - - 4 o o ~ ~ ~ o ~4 cL E E O O E 0 0 0 O O a, O C) - . - E ~ ~ E E E E ~ tn -4 ~ o Qo Ll cn 3 ~ ta S O S ~ C) s U, - `4 ~L4 ~a, ._' . - ~ - 0 JJ 4J t~ C a) a o U] eQ S C) . - - - ~t _' ~n fa ~ _t U] U' :> tq G, Q ~ ~ O tn ~ ~c ~: 3 ~ ~ ~C' - E 12: - ._, ~ m z: Ll Q4 -t E ~ ~ 0 0 a, ~ ~ · - Q4=O a C) _ 3 :, P4 0 mm O 0 1 := Q4O c U) eq o ~ a' ~ u, `: ~3 Q,- - ~ 3 O ~ ~ n- -4 ~ :, ~ ~n tr a ~ E ~ a. `4 3 m m 1 1 a, =~ C) 0 :r 1 - - C) ~n u, cn a O Q Q 3 ~1~1 51 a) ~t 4J n o 3 S 4~
312 TABLE 4-7 Comparison of Metabolites of Naphthalene in Liver, Muscle, and Bile of Starry Flounder (Platichthys stellatus) Metabolites Liver MuscleBile Total conjugates 62.3 + 8.8a 46.1 + 0.391.5 + 0.3 Total nonconjugates 37.7 + 8.8 53.9 ~ 0.38.5 + 0.3 Glucuronides 16.6 + 1.6 23.7 + 1.481.7 + 0.3 Mercapturic acids 10.5 + 6.6 8.0 + 0.98.8 + 0.1 Sulfate/glucosides 34.8 + 14 .8 14.4 + 0.50.9 + 0.1 D ihydrodiol (1,2-isomer) 12.4 + 11.7 23.9 + 0.93.0 + 0.1 Naphthols (l-a 2-) 11.5 + 3.9 21.6 + 0.62.9 + 0.2 Uncharacterized 14.0 + 7.1 8.4 + 0.42.6 + 0.1 NOTE: Data at 12°C and 7 days. data are expressed as percent of total metabolites. SOURCE: Varanasi et al. (1981~. bile are conjugated derivatives of oxygenated forms of hydrocarbon. Excretion of metabolites of aromatic hydrocarbons by other routes, including gills, urine, and skin, has also been indicated for fish and invertebrates. The patterns of hepatic metabolism of hydrocarbons in viva are largely similar to those in vitro, at least as judged from identity of polar derivatives of hydrocarbons isolated from bile. Biliary metabolites of naphthalene, methylnaphthalene, phenanthrene, and benzota~pyrene freed from conjugates have included dihydrodiol, phenolic, and quinone derivatives (Melancon and Lech, 1978; Solbakken et al., 1980; Varanasi and Gmur, 19811. Examples of principal metabolites of some compounds reportedly found in fish bile are listed in Table 4-7. In crabs and shrimp exposed to naphthalene, the metabolites produced included conjugates with glucose and sulfate, dials, and phenolic derivatives (Corner et al., 1973; Lee et al., 1976; Sanborn and Malins, 1980~. Rates of metabolism in viva cannot yet be inferred from in vitro rates. In many cases the rates of biotransforma- tion in viva can be expected to match the intake, and little accumula- tion of parent compound will be evident. When the parent compound is retained at low levels, metabolites may be retained in various tissues for longer periods than the parent (e.g., Varanasi et al., 1979; Melancon and Lech, 19791. The identity of naphthalene metabolites extracted from tissues (muscle and/or liver) of starry flounder (PlatichthyS stellatus) included conjugates as well as phenols and dihydrodiols (Varanasi et al., 1981), but the profile of tissue metabolites differed from that in bile (Table 4-8)0 Metabolites of benzota~pyrene {B(a)P) extracted from liver of English sole
313 TABLE 4-8 Induction by Hepatic Benzo (a) pyrene Hydroxylase in Mar ine Fish by Aromatic Hydrocarbons Spec ies Tr ea tmen t BP Hydroxylase Con tr al Tr ea ted Little skatea dibenzanthracene 0 .009 + 009C 0 .396 + 0 .194 ( intraper itoneal ) Sheepsheada 3-methylcholanthrene 0.070 + 0 .025C 0 .550 ~0 .190 ( i ep - ) Croakerb tsenzo (a)pyrene 0.026 + 0.002d 0.420 + 0 .021 (i-p-) James and Bend [ 19811 . tStegeman 119811. Cnmoles 3-OH-BP equivalent/min/mg microsomal protein. dnmoles [3H]-BP metabolites/min/mg microsomal protein. (Parophrys vetulus ~ included a suite of metabolites like those seen in vitro (Varanasi and Gmur, 1981) . Moreover, a portion of B (a) P in liver in that study was found to be in cellular constituents, as tens ibly the result of formation of active intermediates in viva. The or igin of metabolites in many extrahepatic tissues is not fully known. Given that both oxidative capacity (Stegeman et al., 1979) and con jugating enzymes (James et al ., 1979 ~ occur irk most tissues of f ish , the var. ious types of extrahepatic metabolites possibly are produced in situ. The export of metabolites from the liver to other tissues is also possible, as suggested by studies in mammals. The effects of these metabolites and their further transformation In various tissues are not known. The total flux of metabolites by the var. ious excretory routes is unknown for any mar fine species and needs to be addressed. Factors Influencing Metabolism The rates of hydrocarbon metabolism and elimination can be influenced by a var. iety of environmental and physiological factors . Paramount among these is the induction of cytochrome P450 by hydrocarbons and other environmental pollutants. Polynuclear aromatic hydrocarbons, crude oil, and refined petroleum products, as well as polychlorinated biphenyls and polybrominated biphenyls, are among those compounds that have the capacity to induce increased levels of P450 and increased rates of hydrocarbon metabolism in liver and some extrahepatic tissues of f ish and some inver tebr ates . The phenomenon has been descr ibed in numerous mar ine and freshwater f ish species (e.g ., Payne and Penrose, 1975; Bend et al. , 1977), including embryonic and larval forms (Binder and Stegeman, 1980 ~as well as polychaetes (R. F. Lee, 1981) . Induction
314 is usually described as an increase in the rate of Bta)P metabolism in vitro (e.g., Table 4-8), but metabolism of other hydrocarbons is also elevated, as indicated by the higher rates of methyloaphthalene metabolism in viva in induced trout (Statham et al., 1978~. The increased rates of Bta)P metabolism in fish appear to be attributable to the synthesis of novel forms of cytochrome P450 (Elcombe et al., 1979a,b; James and Bend, 1980; Stegeman et al., 19811. The presence of new cytochrome P450S in crabs has also been suggested after exposure to pollutants (R.F. Lee et al., 1982~. There is growing evidence for induction in fish in the environment. This includes induction of hydrocarbon metabolism in fish by spilled oil in the environment (Payne, 1976; Kurlelec et al., 1977; Stegeman, 1978~. This is not a petroleum-specific phenomenon, however. There is increasing evidence for widespread induction of P450 in fish by chemicals of unknown origin in the environment (e.g., Bend, 1980; Dunn, 1980; Stegeman et al., 19817. The causes of such induction have not been established, although in one case (Dunn, 1980) there was a cor- relation with PAH in the sediment. In the polychaete, Capitella capitata, exposed to crude oil, the third generation had much higher MEO activity than the first or second generation (R.F. Lee, 1981~. Grassle and Grassle (1976, 1977) showed that C. capitata is actually a complex of at least six sibling species based on electrophoretic patterns. Exposure to oil thus may result in selection for species or strains that are resistant to oil because of high MFO activity. Induction of hydrocarbon metabolism in the liver of seabirds has been noted in one study of oiled birds from the Amoco Cadiz. where elevated levels of MFO were observed. Similar induction in mammals has not been demonstrated but is virtually certain to occur, based on comparison with responses to foreign chemicals seen in their terrestrial counterparts. Elevated B(a)P hydroxylase activity was reported in the kidneys of seals fed crude oil (Engelhardt, 1981~. GU11S fed Prudoe Bay crude oil did not show induction 8 days after treatment, but any induction response had probably disappeared by that time. Another type of induction, by DOT Which induces different types of P450 from those of aromatic hydrocarbons), has been demonstrated in puffins (Bend et al., 19771. There are several studies that have disclosed an effect of tempera- ture on disposition of naphthalene in viva. In both coho salmon (Oncorhynchus kisutch) and starry flounder (Platichthys stellatus) there was a pronounced increase in the retention of naphthalene and its metabolites in the tissues with a decrease in temperature (Collier et al., 1978; Varanasi et al., 1981~. Moreover, lower temperature also effected a shift in the pattern of naphthalene metabolism in starry flounder, resulting in a substantially greater proportion of glu- curonides and 1,2-dihydrodiol in liver at the lower temperature a week after exposure (Varanasi et al., 19811. Metabolites in muscle did not follow the same pattern, however. The exact basis for and consequences of such responses are not known and clearly warrant investigation. In addition to effects on disposition and elimination of hydrocar- bons, low temperature also can cause an attenuation of the induction of P450 in fish (Stegeman, 1979~. Further evidence also indicates seasonal
315 and sex-l inked responses to inducers (Stegeman and Chevion, 1980, Forl in, 1980 ~ . Moreover, the many d if ferent types of pollutant compounds present in the environment can influence the metabol ism, disposition, and effects of each other. In a study by Gruger et al. (1981) the pattern of metabolism of 2,6-dimethylnaphthalene in viva in starry flounder was substantially altered by the presence of either naphthalene or cresol in the animal. Such results have implications for the toxicity of mixed chemicals in the environment and also for extrapolations based on studies of biotransformation of single com- pounds . Formation of Mutagenic Metabol ites The metabolism of aromatic hydrocarbons can result in their activation to toxic, and in some cases mutagenic and carcinogenic derivatives. The activity of the metabolites will depend, in part, on which part of the molecule has been metabolized. The patterns of metabolism of some aromatics by f ish liver in vitro have been studied, but the principal effort has been on benzo (a) pyrene (e.g., Ahokas et al ., 1979 ; Bend et al., 1979; Stegeman, 1981; Varanas~ and Gmur , 1981) . The principal phenols, dihydrodiols, and quinone metabolites of benzota~pyrene formed by aquatic species have been reviewed (Stegeman, 1981~. In general, metabolism of Bta)P by teleost fish liver preparations results in formation of high percentages of benz~ring (7,8- and 9,10-0 dihydro- diols) but little K region (4,5-dihydrodiol) . This is also true of extrahepatic and embryonic t issues . The ma jor metabol ite with crabs was shown to be 3-hydroxy benzo {a) pyrene, with minor amounts of diols and other phenols also being produced (Singer et al., 1979~. Metabolism of benzo (a) pyrene in vitro by fish produces derivatives that are mutagenic (Ahokas et al ., 1979 ; Stegeman, 1977 ~ and that bind to DNA {Ahokas et al., 1979 ; Varanasi et al., 1981) . The patterns of in vitro metabolism of some other aromatics by fish have also been established, e.g., the metabolism of 2-methylnaphthalene by trout liver (Breger et al., 1981) . Similar studies on patterns of hydrocarbon metabolism by marine birds and mamnals have yet to be done. In shor t, induct ion can incr ease r ates of metabol ism and d ispos i- tion , but it also increases rates of formation of mutagenic deriva- tives. Thus, hydrocarbon-induced P450s are known to be efficient in forming mutagenic derivatives of carcinogenic hydrocarbons (Wood et al., 1976~. Moreover, a strong induction in fish proceeds with little or no change in the activity of conjugating enzymes that detoxify reactive products (e.g., Statham et al., 1978~. This could shift the steady-state level of toxic derivatives in vivo. The relationsh~ps of metabolism, disposition, and toxic action of many xenobiotics have recently been reviewed (tech and Bend , 1980 ), but for hydrocarbons much r emains to be determined . _
316 Conclusions and Recommendations Microbial degradation is a major mechanism for elimination of petroleum pollutants from the aerobic mar ine environment. The environmental constraints that influence the rate of biodegradation have been defined. Substantial progress has been made toward determining the rate of biodegradation in various marine environments, but further refinement and standardization of methodology are required before reliable rate projections can be made. Evidence to date indicates that uptake of hydrocarbons from food and/or water is a universal phenomenon in animals, with partitioning from water or frown particles or sediments after desorption the key process. The levels of specific compounds in different species or different tissues do not accurately reflect exposure, and this is due to differences in partitioning and in metabolic factors. Metabolic transformation of hydrocarbons occurs in most groups of animals but at widely differing rates. Equilibrium concentrations in tissues are dependent on their ability to metabolize various compounds as well as physical-chemical processes, and in many cases metabolic rates may balance uptake rates with little apparent bioaccumulation. Intermediary metabolites are found in many tissues and may be retained longer than parent compounds. Although considerable progress has been made on the biological fate of petroleum, numerous gaps remain: 1. The fate of hydrocarbons in green plants has received insufficient attention. 2. In order to understand the fate of oil more completely, more work is needed on the metabolism of heterocyclic compounds and non- hydrocarbon compounds in petroleum. Little is known about the slow but possibly significant biodegradation of the hydrocarbons of high molecu- lar weight and the metabolites that might be formed. 3. Some basic cellular processes remain obscure: for example, movement of hydrocarbons across cell membranes and their potential interaction with cellular nucleic acids. Further elucidation of metabolic pathways in animals is needed. 4. Study of the distribution and fate of metabolites is recom- mended, including the question of whether polar compounds that are produced have a significant effect on mousse formation, as described in the phys ical f ates section . AMOUNTS OF HYDROCARBONS IN THE MARINE ENVIRONMENT Introduction A cons iderable amount of data has been gathered on the concentration of hydrocarbons in the oceanic water column, sediments, and biota since the 1975 NRC report. In many studies, however, differentiation between biological, petroleum, pyrogenic, and other sources of these hydrocar- bons was not unequivocal because of the lack of def initive diagnostic
317 parameters and analytical problems associated with handling the trace quantities of hydrocarbons found in most natural samples. The quality of the hydrocarbon data given in the following sections should be viewed in the context of these analytical difficulties, which have been dis- cussed in the chapter on chemical methods. Dissolved Petroleum Hydrocarbons Low-Molecular-Weight Hydrocarbons (C1-C4) Ambient Concentrations Low-molecular-weight hydrocarbon (LMWH) concentrations in most of the ocean are largely influenced by natural processes (e.~., air-sea exchange, seepage or diffusion across the sea-sediment interface, in situ biological production) which produce near-surface concentrations of methane, ethane , propane , and the butanes in the open ocean of 40-150, 0.2, 0.2, and <0.2 nL/L, respectively. Methane in the upper water column of the ocean has been shown by several investigators (Lamontagne et al., 1971, 1973, 1974; MacDonald, 1976; Swinner ton and Lamontagne, 1974; Brooks and Sackett, 1973, 1977; Brooks et al., 1973, 1981a; Scranton and Brewer, 1977; Scranton and Farrington, 1977) to be supersaturated with respect to the partial pressure of methane in the atmosphere. Most of this supersaturation apparently results from in situ biological production (Lamontagne et al ., 1973; Seller and Schmidt, 1974; Scranton and Brewer, 1977; Scranton and Farr ing ton, 1977 ; Brooks et al ., 1981a) and advection from coastal areas (Sackett and Brooks, 1975; Brooks, 1979 ~ . Methane supersaturation appears to be a permanent feature of the mixed layer in the world ocean, except in regions of strong upwelling such as the Yucatan Shelf (Brooks et al., 1973) and in some ice-covered areas of the Antarctic (Lamontagne et al., 1974~. Most of the published profiles of methane in the open ocean have a subsurface maximum of at least twice surface levels. This phenomenon may be due to a combination of factors including outgasing of surface water and higher production rates of biogenic methane at intermediate depths. Methane levels' in the deep ocean are below equilibrium with the atmosphere in areas of dee~water formation, apparently due to methane degradation during advection {Scranton and Brewer, ~ 978) . Less is known about the biological origin and distribution of C2-C4 h~H in the ocean. Sackett and Brooks (1975), Cline and Holmes {1977a,b), and Reitsema e t al . ( 1978 ~ have shown that concentrations of LM~H are s ignif icantly higher in near-bottom waters on the continental shelves and in the vicinity of structural highs and gas seeps. Brooks (1979) reported massive deep methane maxima in the northwest Caribbean Sea which were attr ibuted to submar ine seepage off the Jamaica Ridge system. From another sea floor seep region, Norton Sound, Alaska, Cline and Holmes (1977a,b) observed C2-C4 hydrocarbon concentrations elevated by a factor of 10 or more. Point Source Distr ibutions Because LATH are abundant constituents of crude oils and natural gas, their presence in the water column is a
318 sensitive indicator of petroleum inputs. Through thousands of analyses from hydrocarbon "sniffing and discrete sampling programs, ports and estuar ies--with their associated commercial, petrochemical, and transportation activities and offshore petroleum operations--have been ident if fed as the ma jor man-der ived sources of ~MWH in the Gul f of Mexico. The water column for at least one example of each of these types of inputs has shown [r~WH concentrations to be several orders of magnitude higher than the water column in the open Gulf. The underwater venting of waste gases and br ine discharges, both associated with offshore platforms, was the major source of nonmethane LM~H in coastal surface waters, and was apparently responsible for an increase of 2 orders of magnitude in most Louisiana shelf waters over levels in the open Gulf. Average concentrations of 3,100, 31, and 22 nL/L for methane , ethane, and propane, r espectively, were observed (Brooks et al. ~ 1973, 1977, 1979, 1981b; Brooks, 1976; Brooks and Sackett, 1973, 1977; Sackett and Brooks, 1974, 1975; Sackett, 1977; Wiesenburg et al . 1981b) . Weisenburg et al . (1981b) found that the average 2, 970 pL/L LMWH in brine from the Buccaneer Gas and Oil Field in the northwest Gulf of Mexico caused discernible increases in [MWH levels in surface waters only within 300 m of the platform. Brooks et al. (1978, 1981b) also reported very high ~MMH concentrations in the vicinity of the Ixtoc I well blowout in the Gulf of Mexico. Volatile Liquid Hydrocarbons (C5-C12 ~ Ambient Concentrations Although cat 30-40% of crude oil consists of volatile liquid hydrocarbons (VLH), there is little information available on their distribution in the marine environment. This is primarily a result, until the past few years, of the difficult methodologies involved in VLH measurements. Sauer (1978, 1980), Sauer and Sackett (1980), and Sauer et al. (1978) found that open ocean, nonpetroleum-polluted surface waters contain VLH concentrations of approximately 60 ng/L, while heavily polluted Louisiana shelf and coastal waters reached over 500 ng/L. Aromatics accounted for about 60-85% of the total VL;H in surface waters. Cycloalkane concentrations were a . O ng/L in open ocean water and from 60 to 110 ng/L in polluted waters (about 20% of the total VLH). Total alkanes increased from 15 ng/L in open ocean water to as much as 40 ng/L in polluted shelf waters. These latter elevated levels were shown to be a direct result of the large amounts of brine discharges and underwater venting of waste gases assoczated with offshore production on the Louisiana shelf. An approximately linear relationship existed between anthro- pogenic gaseous and volatile hydrocarbons. Although Schwarzenbach et al. (1979) used a somewhat different technique (i.e., solvent extraction off charcoal versus heat desorption from Tenax-GC), their studies of two coastal stations--one in Vineyard Sound and the second a tidal creek ~n Massachusetts--yielded similar results. They identified approximately 50 compounds with individual abundances of -1-100 ng/L, although concentrations above 20 ng/L were rare. The total VLH recovered from these very near-shore stations
319 ranged from 0.2 to 1.0 ugC/L. Gschwend et al. (1982) and Mantoura et al. (1982) have followed the preliminary report of Schwarzenbach et al. (1979) with further data from sites near Woods Hole, Massachusetts. At one s ite , alkylbenzenes and aldehydes were the ma jor Vms observed. The alkylbenzenes were dominated by anthropogenic inputs and air-sea exchange, with selective biodegradation effecting minor changes in the sununer. Offshore mixing and adsorption on particulates appeared minor. The alkylnaphthalenes showed a contrasting pattern to the alkylbenzenes, which was explained by a dominant winter time source such as space heating oil. Alkanes were frequently petroleum derived, but penta- decane, heptadecane, and pristane showed evidence of strong biogenic sources as well. Gschwend et al. (1980) have reported concentrations of VLHs in the Peru upwelling region of the Pacific. Point Source Distributions Weisenburg et al. (1981a} reported mean VLH concentrations of 1.9 ug/L composed of more than 80% light aromatics (benzene and naphthalenes) around the Buccaneer Gas and Oil Field in the Gulf of Mexico. Sauer (1981b) also reported VLH concentrations in the same field as well as in vents in the Gulf of Mexico. Brooks et al. (1978) found VLH concentrations as high as 19 ug/L around a gas well blowout on the Texas shelf. VLH concentrations of as much as 400 ug/L were reported in the immediate vicinity of the Extol ~ blowout on the Campeche shelf as a result of dispersed oil in the water column (Brooks et al., 1981b). At 6 and 12 miles downplume, VLH concentrations had decreased to 63 and 4 ug/L, respectively. Payne et al. (1980) also reported VLH in the water column around the IXtoc I blowout: benzene and toluene concentrations were 49 and 97 ug/L, respectively, 6 miles from the blowout, with most values further downplume in the 1- to 4-pg/L range. Lysyj et al. (1981) reported aromatic VLH concentra- tions as high as 120 ~g/L in Port Valdez, Alaska, as a result of discharges from a ballast treatment plant. High-Molecular-Weight Hydrocarbons In general, studies on the quantities and sources of high-molecular- weight hydrocarbons (HNWH) in the water column have indicated that the hydrocarbon composition is a function of both biosynthetic and anthro- pogenic sources (Barbier et al., 1973; Brown et al., 1973; Gordon et al., 1974, 1978a, Iliffe and Calder, 1974; Brown and Huffman, 1976; Keizer et al., 1977; Calder, 1977; Boehm et al., 1979; Boehm, 1980~. Reported concentrations of petroleum-derived EIMWH in seawater are difficult to interpret. This is due to the radically different methods of quantification (i.e., gravimetric, GO, GC/MS, IR, fluorescence, etc.) used in various studies and to the problem of contamination during sampling and processing. Another problem encountered is the wide range of compounds grouped together in the hydrocarbon fraction (i.e., alkanes, olefins, isoprenoids, cycloalkanes, and aromatics). Many methods of isolation and quantification will bias the results for or against one or more of these groups of compounds (see Chapter 3, Chemical Methods section). In addition, water processing varies from
320 study to study and can range from no filtration and batch extraction to filtration plus adsorption and reverse phase LC analysis. Concentra- tions of total HMWH mentioned in the literature typically range from 0.2 to 100 ug/L, although the lack of uniform analytical techniques and reporting formats makes comparisons difficult. In most of these studies, certain quantification of the relative proportions of natural and anthropogenic hydrocarbons is impossible. HMWH have been reported for numerous areas of the Atlantic. A preponderance of measurements has given median concentrations of 1-10 ug/L for large areas of the North Atlantic (Levy, 1971; Levy and Walton, 1973; Brown et al., 1973, 1975; Monaghan et al., 1973; Gordon and Keizer, 1974; Barbier et al., 1973; Hardy et al., 1975; Brown and Huffman, 1976; Zsolnay, 1977a; Boehm et al., 1979; Boehm, 19801. Barbier et al. (1973) reported values up to 140 ng/L with an average of 40 ug/L at two stations near Dakar, Africa; Wade and Quinn (1975) observed a range of 13-239 (average 73) ug/L in the Sargasso Sea. Grahl-Neilson (1978) examined the petrogenic hydrocarbons, naphthalene, phenanthrene, and dibenzothiophene in the North Sea, using fluorescence, and concluded petroleum hydrocarbons were below detection limits (~20 ng/L). Mackie et al. (1974) reported n-alkanes from 0.2-4.9 ug/L off Scotland. Mackie et al. (1976) and Hardy et al. (1977) have reported average n-alkane concentrations of 0.166 and 4.5 ug/L, respectively, in the North Sea. Law (1981) using fluorescence in the United Kingdom coastal waters found petroleum hydrocarbons in the 1.1- to 74-pg/L range (mean values were 1.3, 2.5, and 2.6 ug/L in the northern North Sea, southern North Sea, and It ish Sea, respec- tively). Keizer et al . (1977) and Gordon et al. (1974 ~ have reported n-alkanes by GO and petroleum HMWH by fluorescence from <20 to 145 ng/L and 1 ug/L, respectively, in the Sargasso Sea. Boehm (1980) and Boehm et al. (1979) found anthropogenic hydrocar- bons were ubiquitous in the Georges Bank area, except in zooplankton. Petroleum HMWH were apparently from the Argo Merchant oil spill, chronic inputs from ballast washings, and normal ship traffic. Dissolved concentrations were generally in the 0.1- to 2-pg/L range throughout the year, but were above 10 ng/L in the 4 months after the Argo Merchant spill in late 1976. Individual polynuclear aromatics were persistent during the year at levels of 1-10 ng/L' but were elevated in concentration (10-50 ng/L) in early 1977. Variations were patchy and were not related to differences in water mass characteristics (Boehm et al., 19791. Unresolved complex mixtures (UCM) generally accounted for 60-80% of the total determined HMWH. Similar distributions have been observed by Iliffe and Calder (1974), Barbier et al. (1973), and Keizer et al. (1977~. Unusually high concentrations of HMWH (3-12 mg/L) were reported by Harvey et al. (1979) for six samples collected from a depth of about 200 m at five stations on a transect from the eastern Caribbean into the southwest North Atlantic. On the basis of limited chemical char- acterization of this material the authors suggested that it was a "biochemically weathered oil that has not undergone evaporative weathering" and that it probably originated from a natural submarine seep on the Venezuelan shelf. A "conservative estimate of its dimen
321 sign" was used by them to calculate that the layer contained more than 1 megaton of oil, an amount approximating that released in the Ixtoc I spill, and considerably more than the 0.2 mta best estimate given in Table 2-1 of Chapter 2. In several Baltic Sea studies, 57.2 ugC/L of nonolefinic dissolved hydrocarbons and 157 ng/L of aromatic hydrocarbons were reported by Zsolnay (1972, 1973~. A significant correlation between aromatic and saturated hydrocarbons was found, suggesting a common anthropogenic source. A correlation with chlorophyll was noted for waters off north- west Afr ice and Nova Scotia ~ Zsolnay, 1974, 1977b) . Average hydrocarbon concentrations of 4 . 9 ug/L were observed of f Nova Scotia and 4 .6 ng/L off northwest Africa (Zsolnay, 1977b). Zsolnay (1979) reported HMWH averag ing 6.9-25.8 ug/L in the Mediterranean Sea, with the Alboran Sea and the area off Libya having the greatest concentrations. Monaghan et al. (1973) and Brown et al. (1975) also have presented IR data for HMWH from the Mediterranean Sea in the range of <1-195 ug/L. Petroleum hydrocarbons were not typically observed in the water column in the South Texas and Florida outer continental shelf areas of the Gulf of Mexico (Parker et al., 1972; Jeffrey, 1979~; however, Boehm and Feist (1979) and Shokes et al. (1979a,b) have found petrogenic hydrocarbons in both whole and filtered seawater from near-shore Louisiana. Total HASH in seawater at two sites averaged 37 and 5.8 pa/L. Literature values for total hydrocarbons in the open Gulf range from 0.1 ug/L to approximately 76 ug/L (Parker et al., 1972; Koons and Monaghan, 1973; Calder, 1977; Jeffrey, 1979; Iliffe and Calder, 1974; Brown et al., 1973~. Parker et al. (1972) found n- paraffins of ~1 ug/L in the Gulf of Mexico and Caribbean. There is generally a large decrease in HASH between the surface and a depth of about 10 m (Brown et al., 1973; Parker et al., 1972), below which values are generally <1 tlg/L. This may suggest that hydrocarbons are present as particulate matter rather than in true solution. Koons and Monaghan (1973) reported hydrocarbon concentrations from 1 to 8 ug/L in the Gulf of Mexico. Indications of chronic petroleum pollution in the open Gulf of Mexico include aromatic concentrations ranging from 1 to 3 ug/L (Brown et al., 1973) and HMWH concentrations of up to 75 ug/L which were attributed to tanker traffic in the Florida Straits (Iliffe and Calder, 19741. In a further study from the northeast Gulf of Mexico, Calder (1977) estimated dissolved and particulate hydrocarbon concen- trations at 0.4 and 0.3 ug/L, respectively. Aromatic hydrocarbons were estimated by Hiltrabrand {1978) at proposed deep-water port sites in the Gulf of Mexico at 23-24 vg/L in surface water and only O .6-1. 3 ug/L at 16 m. The aromatic hydrocarbons showed a sharp decrease with depth in these prof lies . Kennicutt (1980) found approximately 9-99 ng/L n-alkanes; 1 ng/L aromatics; and 6 ng/L pristine, phytane, olefins, and cycloalkanes for both the dissolved and particulate fractions in the northeast Gulf of Mexico. The major source of these hydrocarbons was inferred to be biological. Less Is known about hydrocarbon distributions in the Pacific. Cretney and Wong (1974) reported some fluorescence in the northeast Pacific off Vancouver Island (0.011-0.027 ug/L as chrysene), and
322 Koons and Brandon (1975 ~ reported values in a hydrocarbon seep region off California (0.4-16 ug/L). In an extensive survey (ca. 350 water samples collected along 17,000 miles of tanker routes in the Pacific), Brown and Searl (1976) reported E~WH had a median concentration of 1.6 1lg/L for the sur face and 0 . 9 ng/L at 10 m. H ASH appeared to be a mixture of both biogenic and petrogenic compounds; approximately 17- 23% of the HMMH were aromatics. In the Indian Ocean, San Gupta et al. (1980) found 0.6 and 26.5 ug/L of petroleum hydrocarbons by W absorbance, with highest values along tanker routes. Petroleum Hydrocarbons in the Surface Microlayer Several studies have reported on the isolation and composition of hydrocarbons in surface films (Garrett, 1967; Duce et al., 1972; Keizer and Gordon, 1973; Ledet and Laseter, 1974; Morris, 1974; Hardy et al., 1975; Wade and Quinn, 1975; Marty and Saliot, 1976; Boehm et al., 1979; Boehm, 1980~. Garrett (1967) first identified hydrocarbons in surface films, and Duce et al. (1972) reported that hydrocarbons (ca. 8.5 ug/L) were concentrated in the surface film by a factor of 1.4 over subsur face concentrations. Morris { 1974 ~ measured alkanes in surface f ilm samples from the Mediterranean and concluded that they contained petroleum hydrocarbons. Ledet and Laseter (1974) found that alkanes at the air-sea interface off Louisiana and Florida were of a mixed biogenic-anthropogenic origin, while Wade and Quinn (1975) observed HASH from 14 to 599 ug/L with an average of 155 ug/L in the micro- layer of the Sargasso Sea and suggested that the major source of hydrocarbons in the surface waters of the Sargasso Sea was small particles of weathered pelagic tars. Concentrations averaged only 73 ug/L at 20-30 cm below the surface. Marty and Saliot (1976) found an enrichment factor averaging 50 in the eastern Atlantic and Mediter- ranean. Dissolved n-alkanes varied from 15-114 ug/L in the micro- layer compared to O . 1-5 .7 ~g/L for underlying water . The particulate n-alkanes varied from 0.3 to 7 . 2 ug/L in the underlying water and from 3 e3 tO 1 ~214 ug/L in the microlayer. Dissolved n-alkanes were generally higher than in particulates, except in polluted waters. Boehm (1980) and Boehm et al. (1979) reported a surface film enrichment of 1.4-90 times that of subsurface water in the Georges Bank area. The surface microlayer contained HMWH (5- to 76-pg/L range) that were mainly petroleum. There have been extensive problems with sampling just the surface microlayer and some values may be low due to entrain- ment of subsurface waters. Particulate Petroleum Hydrocarbons (Tar Balls) Most particulate petroleum residues in the sea are found floating at or near the surface and are called "tar balls.. They are the residues remaining after various physical and chemical processes have acted on floating o'1 for varying periods of time (see Physical and Chemical Fates section) e Butler et al. (1973) , Butler (1975) , and Morris (1971
323 have estimated that 10-30 % of oil discharged to the ocean remains in the form of tar balls and has an estimated residence time on the order of a year. Butler (1975) concluded that small tar lumps are formed by frag- mentation of much older petroleum residues. Tar balls or lumps range in size from less than a millimeter to many centimeters in diameter, although most are quite small (1-10 nun in diameter). Their texture varies from soft to very hard. Some have living organisms on their surfaces [Horn et al . (1970 ~ found barnacles as old as 4 months!, as well as incorporated mineral particles, organisms, shells, and/or organic deter ~ s . Tar balls found near shore or in the littoral zone are often broken into smaller pieces and have incorporated mineral mater ial . The abundance of floating tar has been studied more intensively in the North Atlantic, Mediterranean, Gulf of Mexico, and Caribbean than for the remainder of the oceanic areas. Most of the data collected since 1973 are for the same areas already covered in the 1975 NIP report. The estimate for total tar in the ocean is reduced from 318 to 277 x 103 tons. The average amounts of pelagic tar concentrations and estimated total tar observed in various portions of the world's oceans are summarized in Table 4-9. There are little or no reported data for two-thirds of the world's oceans (e.g., South Atlantic, Antarctic, most of the Arctic, large areas of the Pacific and Indian, and portions of the North Atlantic). The highest reported concentrations continue to be for the Sargasso and Mediterranean seas, with mean values of 10 mg/m . These two seas and the Gulf of Mexico provide the best data for estimating temporal variations in tar concentrations. For example, Morris and Butler (1975) reported a reduction in mean floating tar concentrations in the Ionian Sea, but in other portions of the Mediterranean (e.g., Alboran and Tyrrheian seas) there appeared to be an increase from 1969 to 1975. Knap et al. (1980j, by analyzing beach tars in Bermuda (in the Sargasso Sea), concluded that there had been no decrease in the inputs of petroleum residues to the Atlantic between 1971 and 1979. In the Gulf of Mexico, Jeffrey (1920 ~ determined that the average floating tar concentration was 1.35 mg/m (range 0-10 maim ), based on 220 neuston tows taken on nine cruises between 1972 and 1976. No apparent change in tar ball concentrations was observed dur ing this per iod. In another temporal study in the eastern Gulf of Mexico, Van Vleet et al. (1982a) reported that tar concentrations increased off the southwestern Florida shore from winter to summer due to the increasing influence of the loop current, which has relatively high concentrations of tar--due, apparently, to tanker traff tic through the Car ibbean and Yucatan Strait . This study supports the consensus by most investigators that there is a strong correlation between high levels of tar concentrations and tanker routes . A few investigators have analyzed the composition of pelag ic tars (Butler et al., 1973: JeffreY et al., 1976; Ehrhardt and Derenbach, For the most par t, pelag ic tar s have been classif fed as having the general sources of tanker sludge residues, weathered crude of 1, fuel (Bunker ~ oil residues, and highly weathered residues of indeterminate or igin. The classifications are based on gas small tar lumps are formed by fran ~ . , 1977: van Vleet et al. , 1982b) .
324 TABLE 4-9 Tar Densities on the World Oceans Location and Reference Ar ea Tar (mg/m2 ) (lol2 m2) Max. Mean Total Ta r (103 t) NW Atlantic Marg inal Sea (Morr is, 1971; Morr is and Butler, 1973; McGowan et al., 1974) East Coast Continental Shelf (Attaway et al. 1973; Sherman et al., 1973, 1974; van Dolah et al., 1980 ; Cordes et al., 1980 ) Caribbean (Jeffrey, 1973; Sherman et al., 1973 ; Jeffrey et al., 1974; Geyer and Giammona, 1980; Sleeter et al., 1976) 2 1 2 Gulf of Mexico (Jeffrey, 1973; 2 Sherman et al., 1973; Jeffrey et al., 1974; Light, 1977; Geyer and Giammona, 1980; Koons and Monaghan, 1973; Pequegnat et al., 1979; Van Vleet et al., 1982a,b) Gulf Stream (Morris, 1971; Morris and Butler, 1973; Sherman et al., 1974; Levy, 1977) Sargasso Sea (Polikarpov et al., 7 1971; Attaway et al., 1973; Morris and Butler, 1973; Sherman et al., 1973, 1974; McGowan et al., 1974; Sleeter et al., 1974) Canary and North Equatorial Current (Heyerdahl, 1971a,b; Ehrhardt and Derenbach, 1977; Sleeter et al., 1976) Rest of Northeast Atlantic (McGowan et al., 1974) 8 3 8 North Sea (Oppenheimer et al., 3 1977; Smith, 1976) Baltic South Equatorial Current South Atlantic (Eagle et al., 1979) Mediterranean (Horn et al., 1970; Morris, 1971, 1974; Morris and Culkin, 1974; Morris and Butler, 1975; Morris et al., 1976; Zsolnay et al., 1978) Indian Ocean Southwest Pacific (R.A. Ice, 1973; C.S. Won g et al., 1976) 3 50 2.5 75 45 2.4 1 10 1 1 13.4 1 2 10 1.4 2.8 10 2.2 18 91 10 70 2,270 8 24 10.7 0~5 4 12.1 0.3 0.9 232 0.5 ? ? 25 540 10 25 ? ? ? (large) 0.0003 0.01
325 TABLE 4- 9 (con t inued ) Location and Reference Area Tar (mg/m2) Total Tar . ~ ~ ( 10 ~ me ) Max . Mean ( 10 ~ t ) Kuroshio System (C. S. Wong et al., 1974b, 1976 ) Rest of Northwest Pacific (C.S. Won g et al., 1976) Northeast Pacif ic (C. S. Wong et al., 1974b, 1976) Arctic (C. S. Wong et al., 1974a; Smith, 1976) 10 30 40 13 14 2.1 21 0.4 12 3 0.03 1.2 3 0.1 1.3 Antarctic 10 ? ? Area accounted for 227 (63%) 210 TOTAL AREA 361 277a Assuming density 0 . ~ mg/m2 for areas unaccounted . SOURCE: Modified from National Research Council (1975). chromatographic analyses, sulfur contents, stable isotopes, vanadium and nickel concentrations, and gel permeation . Butler et al. (1973 ~ and Morris and Butler {1973), using gas chromatography, concluded that most of the floating tars collected in the Nor th Atlantic were of a tanker sludge or igin. In samples from the nor theast Atlantic, Ehrhardt and Derenbach (1977) found that 61% were from crude oil sludges, 35 % from crude oil and Bunker oil residues, and 4% of unknown origin. Using molecular character ization by gas chromatography and percentage sulfur of floating tars in the Gulf of Mexico and Caribbean, Jeffrey et al. (1976 ~ found that approximately 30 Be of the tar balls analyzed were tanker sludge residues, based on a bimodal UCM and a high percentage of high-molecular-weight alkanes. Only 2% of the tar balls were identified as fuel oil residues, whereas 65% were crude oils of many origins. Jeffrey (1980 ~ postulated that a significant fraction of the tar collections in the western Gulf or iginates from young seep oil in the southwestern Gulf . goons and Monaghan (1973 ~ also suggested that some of the tar balls they analyzed or iginated from natural seepage. Special mention should be made here of the report Global Oil Pollution by Levy et al. (1981), which gives results of the IGOSS mar ine pollution (petroleum) monitor ing pilot pro ject (MAPMOPP) . The report sonar izes data on "over 85 ,000 visual observations of oil slicks and other floating pollutants, 4000 collections of floating tar balls, 3100 collections of beach tar, and almost 3000 measurements of dissolved/dispersed hydrocarbons " obtained dur ing the per iod 1975-1978 . Some of these data, which are available in other published reports, e.g ., C. S . Won g et al . (1976 ~ are included in Table the data and conclusions of the IGOSS Pilot those presented here. _ . . 4-9. Generally, project are cor~sistent with
326 Petroleum Hydrocarbons in Marine Sediments Petroleum and hydrocarbons of biosynthetic or igin have been investi- gated by many workers in a wide variety of marine sediments. Table 4-10 summarizes some of the results that have appeared in the reviewed literature between 1977 and mid-1981. A similar table covering earlier work has been prepared by Clark and MacLeod (1977a). The studies referenced in Table 4-10 had a variety of objectives and used numerous analytical approaches . Vir tually all of these studies provided detailed information about concentrations of individual constituent molecules of petroleum, in addition to the data on total hydrocarbon concentration. Totals for unpolluted mar ine sediments seldom exceed 50 ug/g. Much more information about the extent of petroleum contamination can be obtained from examination of the k inds and amounts of individual hydrocarbon molecules present In a sediment. This ability to recognize and quantify sedimentary petroleum in the presence of hydrocarbons from other sources is the result of a con- t inu ing evolution in analytical techn iques and increased knowledge of compounds from specif ic sources . Many of the areas class if fed as having little, if any, petroleum pollution reveal, upon detailed examination, small but distinct concentrations of petroleum hydrocar- bons. Recent applications of methods for detailed interpretation of hydrocarbons in marine sediments include Atlas et al. (1981), Barrick et al. (19801, Venkatesan et al. (1980) , and numerous others. A suite of criteria, i.e. , tote' hydrocarbons, UCM, and n-alkanes; pr istane and phytane contents; tr iterpanes; aromatics; and chlor inated hydrocarbons, was used to distinguish the pollutant histor ies of these sediments. Individually, none of these criteria is definitive. Each, as discussed by Venkatesan et al. (1980), and the references they cite, has limitations and requires careful application; yet together, they present a cohesive story (see also Chapter 3, Chemical Methods section for more details). Table 4-10 shows that observed hydrocarbon concentrations vary by more than 6 orders of magnitude on a global basis, with ranges of 3 orders of magnitude common within a given locality. Much of this variation is the result of regional and temporal differences in petroleum input rates combined with variability in sediment type and depositional history. Major inputs take the forms of catastrophic oil spills, of repetitive small spills (typically in harbors), and of petroleum associated with sewage and urban runoff discharged into coastal waters. Because these inputs are all initially added to the water column and are later transferred to sediments, variations in local sedimentation patterns also strongly influence the distribution of hydrocarbons in sediments. The interplay of inputs and sedimenta- tion patterns can be seen in numerous well-studied coastal systems. The coastal system of Narragansett Bay/Rhode Island Sound is an area of complex sedimentation patterns (McMaster, 1960) and substantial petroleum inputs, mostly from the Providence area at the head of the bay. The sedimentary hydrocarbons of this system have been extensively studied (Farrington and Quinn, 1973; Zafiriou, 1973; Van ~leet and Quinn, 1978; Hurtt and Quinn, 1979~. Sewage treatment plants discharge
327 3 ~t_ ,., a, ~css ·· ~ 4.' _ ~_ _ ~ ~C. · · E~ V_=_ ~ o ~o ~ s a~ =_ V_ C C ~ ~ m ~m~ V r ~ ~ C r ~ ~ ~ a' ~ cr ~ a~ ~ c ~. - cn 00 C~ N _I N - ~_1 4 ~O ~ _I _ ~C: -1 ., - _ ~ - ~_ ~d ~ {o ~, (1) ~ ~ `: C ~o ~ ~ ~ ~ ~ S C) ~ ·- ~ · - o- - U. ·- V ~r4 C) · - 4J ·- V ·- V O , ~V I; ~ ~V ~V ~ - - ~ ~ E ~ E ~ E ~ E v O J: O ~ O ~ O s O ~ n ~Q~ ~ ~Q. ~ E Q. ~O ~ ~_4 ~· - ~ O =: E~ ~E~ ·Q _ O V 'V 3 ~ ~4 V C) ~ ~ cr. O ~ C.) - U] `4 E Se :' ~ Z O 0 q'1 . - ~. V ~ . - Ld <: O ~ C~ O _ E _ ·,4 eq ~ ~ ~ l I ~ 3 a .- E _ ~ E U1 V ~ C _ a' .~ ~ ~ ~1 ~nl ~I sl =1 ~ ~ ~ ~CO CD x: u: a I u3 1 1 o ·,. UJ 0c C ~0 r. :~ 3: ,~ _ w a m_ _ o C. C. ~ O _ _ C ~ J: C ~O -0 ~_ ~ O _ E~ o ~U) ~ C) :~; ~s E~ - a ~ · - - :' . ~C V _ ~ r~ E E ~ ~ _ O O a ~3: ~: C) ~ · - - - V O S:L ~ ,~ ~C) _ ~ O O CO C~ C ~ ~: .,._ · - 33 C C C _' ~· - ~ 3 c3 C V~ ~ C: _ ~ _ ~ ~ a, a' ~ a, ~ ~ r~ v ai ~ ai E :> V ~ ~ C ~ - C - ~ C 3 ~C x :>m )c: c~ :c :D v 0 0 ~ 4~ c ~ c _ 4J v 0 0 v ., · - ~· E ~E~ ~o c 4J c r a' ~ ~: O O C aV _ JJ ~ _ e. ~ C: ~C ·-~ a~, -- as ~ C~ \~ _ Ld _ i, _ C4 L ~ C. ~o ~V ~ · - ~ · - ._' ~ ·~4 JJ E ~ E ~ C ~ O ._, ~ · - P4 2 Z 2 Pd Z ._, 4~ C 1 · - 1 ~ V~ C · - a, V ~ ~: ~:^ m u, i,4 ~.4 UJ O ~ Q} U) ~ N l' ~N ~3 U] ~ ·,' V~ o4 P4 ~5 ~ ._' . - V V a V C C ·,4 · - C ~ ·_4 · - V ~ O ·04 _I CO O P4 1 11 1 Z P4 Z ZZ Z O O 1 1 1 ~u-)0 1 1 1 vl vl~ 1 Ql O O ~ 1 :) O U) U] :^ 1 - _I ~ · - O ~ 0 m ~ ~UJ :e Q) ~ CD ~4 ~ ~O .,4 3 (:) S 1 O O O V · - · - V~ U~ ~ a' · - ~ :- U] V . C O V E v ~n C a) ~ CD O L. <: Z U, CC U ~CO a' ms~l CD ~ I U~ 0 o U] c U] o ~ 531 q~ :' 1 u) o l o o~ Q, :3 ~n e `: 3 C: ~ ~ V S C ~ Z J O C _d -04 ~U] O ~ V ~ - - C ~ ~ ~J v1 ~ ~c u~ ra O · - U ~V ~ V C 3 0 O:
328 C o o ~ a' 5: E~ ~ ^ C o ~ 3 c U C O - ~n E a, ~ V) F; Z O 0 ~1 a, . - ~ ~S O ~ ~ O E 3 a _ E C: - p: s .~4 ~5 U' ~ C O ._4 V O J r" ^ a' ~o ~a: co ~a~ ~ ~' - ^ ~ tn a ~. .~ 3,,~,., - ~_ ~a' ~a' ~r~ s~· ~· ~· ~· O C~ - _4 ~- ~. ~,-f ~_ ~ ~,\ ~a ~0 ~·~] ·o ,- a ~C ~ ~ E~· ~· ~-4c c JJ c ~a) c 3 ~5 O~ ~c: ~ ~ ~O ~ C ~ _ - ~ - -C _o~ ^ U] ^ ^ ~U] ~C ~C m~ ~ ~ ~ ~= ~ ~ ~ o~ o Y Y ~ r ~C ~ o cO a) ~ ~ c ~ ~ ~ ~ ~ ~0 ~ ~ ~ a' ~ cn ~ ~ ~ o, ~ ~ro a'~ a' ~ ~ ~a' C5\ ~ ~Q.- ~u, `: ~ ~ a, ~ ~ ~ ~ ~ Y ~Y ~ ~ ~ ~3 Y ~3E 0 L4 - ~ - ~ - ~ - ~ - a) ~ -c - ~ ~ - ~ - ~c - ~O ~ 0 ~ ~ a, ~ ~ ~s a' s5; ~ u ~c~ ~a: ~m a: ~u ~u:E ~a C C C C ~C C C C C C + ~V U ~V - - ~ ~ ~ ~U ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ V . - ~. - ~. - JJ . - V . - C, ~ ~ ~ ~ ~ ~:: ~ ~ V ~ ~ ~ ~ ~V £ ~ £ ~ £ ~ £ ~ ~ £ .m ~ £ ~ £ ~ E: ~ E ~s O s s O s O ~s O s s O s C s s O s O s O s O ~ O - - ~ ~ ~ ~4J ~ ~-~4 ~ ~- - ~ ~. - ~. - ~ ~- 0- ~O E~ ~ra O - (D _ _ _ ~tD tD ~- O c ~0 0 · · _ ~a ~- ,- _ ~a: ~· _ \° ~ a ~_ _ _ ~_ . _ ~ _ ~0 cc 0 c ~cn - o 0 ~ o1 o . · ~ ~1 ~- 1 - 1 - o u ~kD O · o --ut ~a:, ~o · · er a ~0 1 o ~1 O O· O ~1 ~=) ' 1- kD 1 ~1 1 OO tD ~t- ~a ~( ~· ~L.~} a ~,-1 tr) 1 1 1 1 1 1 1 1 1 · · · · 1 ~r ~a ~0 0 ~u ~0 0 0 0 ~o ~0 ~· · · ~ ~ ~0 1 1· ~- ~D ~O a,1 a:' r ~0 0 ~ 0 ~O q. u ~ ~c ~_ _ u~ ~ ~o P4~P4 ~ ro ._. as a ~1 1 1 ~1 ~S: c~ 1~ 1 .- -4 ~ c' C) ~ ~1 Ll L. ~ ~I o9 o ~0 O s .,. m ~ P4 L. 0 ~ £ 3 a) ~ z m U~ n ~4 :, U] o .. ~4 ~ O ~ U] 4~ Q4 UJ J (V ~ L. a' ~ ~ ~a ' ~4 U' . - ~d ~ U U] ~1 S ~ 1 m 1 1 Z Z Z P4 In Z 111 111 J;1~:1 0Lr. ·~ 0 0 _ ~4 1 1 111 0 0 000 o - "c .- ~, ~·- x m ~C .~- ._ ~·_ C C U~ 0 ~ ~ ~0 U] ~0 -- m u, ~ ~ Cd ~0 C ~0 ~ 00 ~ X ~ ~0 ~ ~ ~Z U) c 0 c C C ~ 0 ~ u: ~n 0 X 0~ z ~u,a. l 1 Z Z _, 0 ~D ~4 1 ' 0 C ~t 1 _. ~1 1 1 0 0 y u, C ~: :5 N O .,1 113 ~ ~O U] Q' LJ~ ~:' U]U) ~_ O C ~a ._4 C U] ~_ ~: C' -_1 4) U] m ~n C~ C ~q~ U) m u~ 1 :D u.
329 o _ a' a, _ _.- s · V ._1 .~ ~E 4 ~^~ I_~ _~ c - c - a, ~ 0 ~ ~ -~CO~ 0 I_ a ~_00 c~ ~ ~u~ a ·, ~tt~,-y ~ ~c c 3~ -V ~ - ~ - n ~a m ~s: c o - 1 v C) :n ~ ~ E ~: O v O a, . a' _ 0 ·n 0 0· ~r ~ O- ~N · t~ u: cn ~ c Y Y 1 1 cIct u~ ~D . ~D 1 kD In 1 u' 0 · ~ 0 ~u~ o~ _4 _I _d _I N ~0 - 4 ~0 0 O I C ~ N I V' 1 ~vl' 1 c' ~ 1 ~ 1 i,, ~_. ~-4 L ~: ~1 1 1 1 '.n 0 ° O 0 0 E | ° I E | ° v 0 ~ ~o~ ~ ~ ~O0 . - . - CL . Q. ~ 0 E 0 30 EI . N I N | ~r 4) o cO . - JJ N ._' ~: ~u) r ~C ~o .-. 1 cc ~ · v . ~ - l 0 0 v · - 3 o v Occ 8 Ld U) Co n V o :> .. - c o .~4 - 3 ee V L. c ~ ~ v v o c. · u, ~ - - V 0 - ~n o ~ tO ~JJ -_ c 0 ~cn c a' ~c· - ~ 0 ~· ~ a, 3 ~c V 3 ' ~ C 4J Q. Ql 11 - ~L) t) 3 0 P4 c ~ 0 a 1 · O ~4~ ~ ~ 3 Z 0 E E ~ O V ~ c 3 In 3 c :> ·' - ~ E ~ c v ~0 ~0 · 0 s ~ ~O , ~ C V O L O c CL C2~ 0 ~ 3 3 ~c 11 ~5 ~ V ~ C `0 P4 ~ O ~ 3 ~ a, s ~ 0 t) ct ~ 0 ' N ~ 0 t~ ~ a~ ~ ::) `- - ~ ~ ~c 3 O ~ ~ ~ :> E 3 ~ D 3 c ~ ~ ~ u, c ~ tn _ ~ ~ ~ ~ ~ E Q ^ u, ~ ~ 0 u, ~ c s ~ _ ~ ~ v tn ~ E E O ~ ~ V c C ~ ~ C ~ mo V ~ C 1~ 0 a~ O O ~ oG 00 05 z ~ ~ ~ z m1 ~ a1 =1 o1~1 mIsl.-l. -
330 upward of 103 metric tons of hydrocarbons annually into Narragansett Bay and Rhode Island Sound. Most of this is of fossil origin, and a substantial fraction is surely petroleum derived, although coal-burning (Tripp et al., 1981) and other combustion products (Lake et al., 1979) also contribute. These hydrocarbons are weakly bound to detrital par- ticles and, consequently, sediment throughout the bay. Approximately half of the suspended hydrocarbons are deposited in the vicinity of the discharge and half are dispersed toward the mouth of the bay. In part, this reflects distance from the primary source. It also reflects changes in the sedimentation pattern: sediments tend to become coarser and lower in organic carbon toward the mouth of the bay. Segmentally analyzed cores show that fossil hydrocarbons in Narragansett Bay are essentially a twentieth century phenomenon. Although these sediments are extensively reworked after deposition by burrowing organisms, most show decreasing hydrocarbon concentrations with depth and differences in the molecular composition of the hydro- carbons, which indicate that those in the deeper sediments are biogenic. A few cores show subsurface maxima in petroleum hydrocarbons. These suggest discrete pollution events, possibly oil spills or dumping of polluted dredge spoils. Because it is farther from the city of Providence and has coarser sediments, Rhode Island Sound is lower in sedimentary hydrocarbons than Narragansett Bay. During the period from 1968 through 1970, highly petroleum-polluted dredge spoil from the Providence River, containing 10 metric tons of hydrocarbons, was dumped in the sound (Boehm and Quinn, 1978~. In the vicinity of the dump site, hydrocarbons having the molecular characteristics of degraded petroleum were observed at elevated concentrations. Evidence of dredge spoil hydrocarbons was limited, however, to within 2 km of the dump site, probably due in part to the fact that the finest, most polluted spoil was subsequently covered with coarser, less contaminated material, which tended to prevent dispersal. The trends outlined above for the Narragansett Bay/Rhode Island Sound system appear repeatedly elsewhere (Wakeham and Farrington, 1980~. Detailed comparison, however, shows many differences. Differences in present pollution rates and pollution histories, as well as sedimenta- tion patterns and other environmental variables, make each area dis- tinct. At present, the accurate, quantitative prediction of petroleum accumulation in sediments which will result from a particular pollution event at a particular location is simply not possible. The rate of loss of hydrocarbons from chronically polluted sediments is not easily measured. In areas affected by acute oil spills, hydro- carbon half-lives in sediments range from months in exposed locations to decades in sheltered areas (Hoffman and Quinn, 1979; Gilfillan et al., 1981; and others). While similar processes of physical flushing and biochemical degradation must be at work in chronically polluted sediments, direct experimental evidence that defines the rates of these processes is not available.
331 Petroleum Hydrocarbons in Mar ine Organisms Pelagic B'ota Very little information is available on petroleum hydrocarbons in pelagic mar ine organisms . Organisms themselves produce various isoprenoids, particularly pristane, olefins, and straight-chain saturated paraffins, and these have been studied in some detail (Blumer et al., 1963, 1969, 1970a, 1971; Burns and Teal, 1973) . By contrast, cycloalkanes and aromatic hydrocarbons ar e not normally found in organisms, and therefore the implication of oil pollution as a source of hydrocarbons in mar ine biota is usually based on their presence along with an UCM. In the open ocean, concentrations of hydrocarbons in organisms are low and the or igin of the hydrocarbons is not always easily determined. In areas where large inputs of hydrocarbons have occurred, HMWE] in organisms can be directly related to petroleum pollution . For plankton, Boehm (1980) and Bieri (1979) found that zooplankton appeared free of petroleum hydrocarbons in the Georges Bank and Flor Ida shelf areas, respectively, although at least in the Georges Bank area other components of the system contained signif icant petroleum. Morr is et al. (1976) and Burns and Teal (1973) reported petroleum hydrocarbon concentrations in two phytoplankters from the Sargasso Sea at 10-20 ug/L. In the Gulf of Mexico, petrogenic HM~H have been observed in zooplankton samples, but have been attr ibu ted to the incorporation of tar balls in the samples (Carder, 1976; Parker et al., 1972) or con- tamination by offshore production activities (Middleditch et al., 1979a ,b ) ~ For h igher troph ic levels, metabolic processes determine the internal concentrations of petroleum hydrocarbons. Processes of ingestion, metabolism, excretion, and lipid storage, which are both active and selective, lead to petroleum concentrations which ar e strongly influenced, not only by the organism's external environment, but also by its behavior, physiology, and biochemistry (see Biological Fates section) . Factors such as these increase the var lability of hydrocarbon concentrations in organisms and make elucidation of the causes of that var lability more difficult. Horn et al. (1970) found tar balls in the stomachs of saury collected in the Mediterranean. Teal (1976 ~ found evidence of tar ball ingestion in a galatheid from the Nares Abyssal Pla~n in the Nor th Atlantic . Morr is et al. (1976) found petroleum contamination in most levels of the Sargasso Sea community. Petroleum-der ived HM~I ranged from 16 to 3,230 pg/g dry weight in crabs, fish, and shrimp from the Sargasso area. Middleditch et al. (1977, 1979b) found n-alkanes ranged from 0 to 16 ug/g in muscle and from 0 to 13 ug/g in livers of f ish around a production platform with some evidence of petroleum-derived HMWH. Bieri (1979) found no evidence of petroleum hydrocarbons in fish from the Flor ida shelf, except in one sample which correlated with evidence of petroleum in underlying sediments. Benthic Biota Hydrocarbons are naturally present in most, if not all, l mar ine organisms as a result of endogenous biosynthesis and dietary exposure. In addition, many mar ine organisms have been found to contain
A ~9 ._1 c: lo o c ~ - ~ :: c 3 o o . - ~q S U o :^ := o 1 m EN lo a) a, a' Z: So o :c E~ .,. a' a: ~ C! ~1 0 l .-, 4J _ I: X ~. ~ o t) o 0 ,' `: 0 UC U] C ~ ~:' m m -4 ~ 0 JJ a _ 3 3 _ _ o' O4 - er tD 332 cn ., 3 3 U] o L. ~5 a :, . C.) C U) U! U] ~ ~: e" m ~: a, Q) U' L. m o Q. - o v -- - ~ ~- o · eo ~r~ ax r~ a' ~cs~ ~a' - ~- - ~ a' s ~c - c - UD JJ ~ C C ~ kD ~ ~D - - ~ - - 3 c ~ ~ ~ c ~ ~ u~ u) E~ 0 ~ 3 ~ - ~ ~ ~' 3 ~<1~ ~ ~ - c' ~ ~c E 3 3 -4 - c c c ~ ~ a, c a\ c `: ^ c z ~ ~ ~- 0~ E O E O ~ ~ ~a 1 r ~_ ~ ~ ~5 ~ ~ _ a' ~ ai u ~u~ E 3 ec ~1 c c s ~ ~ ~ O ~ C - ~ a) ~ :> c ~ c ~ ~ ~ 3 3 0 ~c u' ~m m m ~<: ~n ~ C ~ · - Y ~ ~ ,~, 3 E O 1 nJ c I u' E C s 3 0 m m U) c o E E D 3 3 a, O O O 4 ~ ~ V O O O v Ll C 0 3 m ~ ~ a Q c~ a :- ~ m m U) ~q ~5 ~ N N N N m m ~1 C~ o . ~ CJ ~ o z v oe U) ~a m0 0 C ~C) o c . - C~ O a 3 3 ~ U' sO O CdZ Z ta o~ U) U) x: ~ m m ~U] S" N N N N ~3 m m 0 ~ SV I rasl=~ cl ~1 ~ m' ° _ ~ V U, U: ~ V ~ gr, 0 ·5: ra 0 ' C) C ~ 0 ~ C C c s cD Y 3 ~n ~ 0 ~ O O 3 ~) Z a'1 r~ c · > U) O U) ' Z :^ =: ro ~m~ m ~c `= ~ m ~ ~ ~ ~ ~n ~ ~ m ~ 0 0 0 0 ~ 0 ~ u, ~ v ~0 Q. ~ u, c ~ ~ ~ ~ u ~ c' C ~1 ~ ~ ~ 3 . - 3 . - 3 .~4 d V L. ~ ~ ~ ~ ,`: ~ ~ ~ V 0 ~ ~ O ~ O ~ C ~ O ' - 3 N ~ S ~ C) ~ ~ ~ U ~ 0 45: N ~ X Q~ U) 4~ U) Q) ~Q 3 47 3 ~ S S eC; :] 3 m z c: c: ~ x c O z z m :^ m
· Oe ~ _ ~__I U~ _I 0\ ~a) ~C ~r ~ ~ JJ -V a'v :, ~ v ~a' ~a) m ~ ~ ta - a' C ~- ~ ~ ~ 0 ~0 ·0 ~ - 0 CO · -UB ~U] ~ U) - ~3 ~ ~ ~ ,4 _ ~,. ~ ~ ~ ~ ~ ,4 ~ a~ Z ~ ~z a' Q1 Z ~- E~ Z a, ~1 1 ~- G1 1 1- 1 1 - 1 1 - - cr ~ a' Y ~ ~ u, ~: ~ 3 s ~ ) ~s ~ ~ ~ s ~ ~l _ ~t ,~ _ ~,~ _ i,, ~ ,8 _ ,~ s ~:' c ~n c ~c ~c ~m c ~E" E V O L 0 V ) ~v a) v ~v =-- e ~Q. ·-v v~ ~ v ~a `: E ~E ~E ~ 04~ ~ O ~o V o V ~, ~1 ~O u, ~ 1 ~V ~u, U~ C~ vl V :^ 3 ~ _ _ 1 ct. ~D V ~ vl - _ Q) V 3 G' - 3 - a, N 1 1 CO V t)1 _ ~ 3 3 _ _ a ~ c, a a a a ~ y tn U] o c) - 3 ~ ~O O O Z c) v v ~n tn la ~O O C) C O ~a · - C ~_ 3 s U' O ~ Z 3 ,1 ~ ~ a) ~ V m1 4~ ~ ~ v E ~ _. 0 C., C X ~ ~ Ll - - O 3 v ,4 ~ ~ .:1 ~a ~ O C ~ a, 4J U) ·~ O Z o C~ v U. o C) C _t ~ a, ceq 3 o z eq :3 Ll ~4 a, C' c ~ C) C~ U] 0 V cn ~o :~ O ~C) m eq t4 3 N ~O m z X C vl :31 U] 333 -~1 ta 1 o . - v :1 c ~Q · L. C o ~C o o .,. v 0~ 0 ~n ~: 0 ~4 z I0 5 0 .-, ~: ~ .,- Z _ ~ _ c) vO o ~ :e cn tn o ·-, oc s 1 4 ~C ~ ·- ~ ~ · - 3 c E :~- - ,0 1 14 ~ O v U] ra ·~ 0 vs . - 3 · . ·. ' s s 0 ~ ~ V V C a :>~- - oo .Q V a, ~ ~ ~ al ~ a' v t) D. 3 a) ~ ~ 0 3 a :~ ~1 o1 ~1 a'1
334 hydrocarbons from petroleum and other pollution sources. Table 4-11 summer izes some of the information about petroleum in benthic organisms that has been reported in the reviewed literature between 1977 and 1981. A similar table cover ing earlier work has been prepared by Clark and Macleod (1977a). These tables show that petroleum has been detected in numerous taxa, in a variety of locations representing different marine environmental types, and resulting from several k inds of pollution sources . Other work (not tabulated) shows that mar ine organisms in many parts of the world ocean are free of detectable petroleum. Petroleum has been found in association with several genera of marine benthic algae and vascular plants: Enteromorpha, Polysiphonia, Fucus, Salicornia, Spartina, and Zostera. In the Case of FUCUS collected in a small boat harbor (Straw and Wiggs, 1979), petroleum was only an external coating. For Enteromorpha, Polysiphonia, Salicornia, and Spartina from a heavily polluted marsh (Burns and Teal, 1979), however, petroleum was incorporated into the plant tissues. Several workers have investigated the occurrence of petroleum in bivalve mollusks (Table 4-11, mussels, clams, oysters) . An extensive investigation of petroleum hydrocarbons, as well as other organic and inorganic pollutants in marine bivalves, especially mussels (Mytilus sps.) and oysters (Crassostrea and Ostrea sps.), has been carried out in several countries. In the United States, scientists have examined the hydrocarbons in tissues of mussels (Mytilus edulis and M. californianus) and oysters (Crassostrea virginica and Ostrea equestris) . . along the coastline of the 48 contiguous states (Goldberg et al., 1978; Farrington et al., 1980, 1982~. To quote Farrington et al. (1980, p. 17), ~A general consensus from these studies is that elevated concentra- tions as much as two orders of magnitude above background in remote areas are found near known or suspected sources of inputs of fossi~ fuel compounds. . . . Detailed analyses often make possible identifi- cation of probable major sources for the fossil fuel compounds." Both petroleum and pyrogenic inputs are suggested by the data (Farrington et al., 1982~. Hydrocarbons in the oyster Pinctada margaratifera from Arabian Gulf waters of coastal Kuwait were determined by Anderlini et al. (1981~. Animals from all six stations sampled showed evidence of petroleum (UCM, phytane) as well as biogenic hydrocarbons. The highest petroleum concentration (approximately 300 ug/g dry weight) was observed at the station closet to Kuwait's major oil loading and refinery facilities. This concentration is comparable to those observed by the U.S. Mussel Watch for bivalves collected near urban areas. Grahl-Neilson (1978) monitored the concentrations of individual aromatic hydrocarbons in seven species of benthic invertebrates for 1 year following a spill of 2,000 tons of crude oil near the coast of Norway. All species showed substantial reductions in total hydrocarbon concentrations with time. Within this general trend, however, there were marked differences apparently related to species, pollution history of sampling sites, and molecular identity of the various aromatic hydrocarbons.
335 Summary and Recommendations Several U.S. agencies, including the Bureau of Land Management, the National Oceanic and Atmospheric Administration, and the Environmental Protection Agency, as well as those of other countries, have supported studies on the amount of petroleum in the water column, sediments, and organisms in the ocean. Results have been accumulated during the past 10 years so that several conclusions can be drawn. For the water column, PHC concentrations vary by several orders of magnitude and are related to the proximity to petroleum sources, e.g., offshore and shore-based coastal production and refining activities as well as transportation practices and accidents. The amount of particulate- bound petroleum, primarily floating oil residues (viz., tar balls), also varies by orders of magnitude, with the highest concentration associated with tanker shipping lanes. Significant decreases have not been observed in amounts of petroleum recorded in the most intensely studied areas, such as the Mediterranean Sea, north central Atlantic Ocean, and the Gulf of Mexico. In marine sediments, elevated PHC concentrations are related to the proximity of sewage and industrial outfalls, offshore dumping sites, and accidental discharges. The extent and history of pollution sedimentation, and related environmental factors are characteristic for each geographical area. Thus, a primary research recommendation is studies of the rate of loss of PHCS from chronically polluted sediments _ . need to be done so that accurate accounting of these compounds can be obtained. Relatively little information is available on PRC concentrations in pelagic organisms, mainly because of analytical problems, that is, in differentiating between PHCS and hydrocarbons produced by organisms in nature and micro tar balls caught in plankton net tows made in heavily traversed oceanic areas. PHCS are usually detected in samples of benthic organisms collected from polluted areas, but not from areas f ree of spills or accidents . REFERENCES Ahokas, J.T., H. Saarni, D.W. Nebert, and O. Pelkonen. 1979. The in vitro metabolism and covalent binding of benzo (a) pyrene to DNA catalyzed by trout liver microsomes. Chem. Biol. Interact. 25: 103-111. Albro, P.~., and L. Fishbein . 1970 . Absorption of aliphatic hydrocarbons in rats . Biochim. Biophys . Acta 219: 437-446 . Altshuler, A.P. and J.J. Bufalini. 1971. Photochemical aspects of air pollution. Rev. Environ. Sci. Technol. 5 :39-64 . Anderlini, V.C., L. Al-Hormi, B.W. DeLappe, R.W. Risebrough, W. Walker II, B.R.T. Simoneit, and A.S. Newton. 1981. Distribution of hydrocarbons in the oyster Pinctada margaratifera, along the coast of Kuwait. Mar . Pollut. Bull. 12:57-62.
336 Anderson, J.W. 1975 . Laboratory studies on the effects of oil on mar ine organisms: an overview. Publication 4349 . Amer ican Petroleum Institute, Washington, D.C. Andrews, A. R., and G. D. Floodgate . 1974 . Some observations on the interactions of mar ine protozoa and crude of' residues . Mar . Biol . 25:7-12. Aquan-Yuen, M., D. Mackay, and W.Y. Shiu. 1979. Solubility of hexane, phenanthrene, chlorobenze and p-dichlorobenzene in aqueous solutions . J. Chem. Eng. Data 24:30. Aravamudan, K. S., P. K. Ray , and G. Marsh . 1981. Simplif fed models to predict the breakup of oil on rough seas pp. 153-159. In Proceedings, 1981 Oil Spill Conference, Amer ican Petroleum Institute, Washington, D.C. Atlas, R.M. 1975. Effects of temperature and crude oil composition on petroleum biodegradation . Appl . Microbiol . 30: 3 96-40 3 . Atlas, R.M. 1977 . Stimulated petroleum biodegradation . Cr. it. Rev . 5: 371-386 . Atlas, R.M., and R. Bartha. 1972a. Biodegradation of petroleum in seawater at low temperatures. Can. J. Microbial. 18: 1851-1855 . Atlas, R.M., and R. Bartha. 1972b. Degradation and mineralization cuff petroleum in seawater: limitation by nitrogen and phosphorus. Biotechnol. Bioeng. 14: 309-317 . Atlas, R.M., and R. Bartha. 1973a. Fate and effects of oil pollution in the marine environment. Residue Rev. 49 :49-85 . Atlas, R.M., and R. Bartha. 1973b. Stimulated biodegradation of oil slicks using oleophilic fertilizers. Environ. Sci. Technol. 7 :538-541. Atlas, R.M., and R. Bar~cha. 1981. Microbial Ecology--Fundamentals and Applications. Addison-Wesley, Reading, Mass . Atlas, R.M., and A. Bronner . 1981. Microbial hydrocarbon degradation within intertidal zones impacted by the Amoco Cadiz oil spillage, pp. 251-256. In Proceedings of the International Symposium on the Amoco Cadiz: Fate and Effects of the Oil Spill. Centre Oceanologique de Bretagne, Brest, France . UNESCO. Atlas, R.M., G. Roubal, A. Bronner, and J. Haines . 1980 . Microbial degradation of hydrocarbons in mousse from Ixtoc I, pp. 1-24. In Proceedings of Conference on Researcher/P ierce Ixtoc I Cruises . National Oceanographic and Atmospher ic Administration, AOMI, Miami, Fla. Atlas, R.M., P.D. Boehm, and J.A. Calder. 1981. Chemical and biological weathering of oil from the Amoco Cadiz spillage within the littoral zone. Estuarar ine Coastal Mar . Sci . 12: 589-608 . Attaway , D., J.R. Jadamec , and W. McGowan . 1973 . Rust in floating petroleum found in the marine environment. U.S. Coast Guard, Groton, Conn. Atwood, D. K., and R. Ferguson 1982 . Example study of the weather ing of spilled petroleum in a tropical mar ine environment--Ixtoc I . Bull . Mar . Sci. 32 :1-13. Audunson , T ., V. Dalen , J . P. Math ison , J . Haldor sen , and F. Krogh . 1980. SLICKEORCAST--A simulation program for oil spill emergency track~ng and long-term contingency planninge Proc. Petromar 1980, Monaco, Mz~y 26-30 . Continental Shelf Institute.
337 Augenfeld, J.M., R.G. Riley, B.L. Thomas , and J.W. Anderson. 1981. The fate of polyaromatic hydrocarbons in an intertidal sediment exposure system. I. Bioavailability to Macoma inquinata (Mollusca: Pelecypoda) and Abarenicola pacifica (Annelida:Polychaeta). Mar. Environ. Res . ~ in press ~ . Bailey, N.J.L., A.M. Jobson, and M.A. Rogers. 1973. Bacterial degradation of crude oil: compar ison of f ield and exper imental data . Chem. Geol . 11: 203-221. Barbier, M., D. Joly, A. Saliot, and D. Tourres. 1973. Hydrocarbons from sea water . Deep Sea Res. 20: 305-314. Barger, W.R., D.R. Sherard, and W.D. Garrett. 1974. Determination of the Thickness of Petroleum Films on Water . Memorandum Repor t 2883. Naval Research Laboratory, Washington, D.C. Barrick , R.C., J. I . Hedges , and M.L. Peterson . 1980 . Hydrocarbon geochemistry of the Puget Sound region. I. Sedimentary acyclic hydrocarbons . Geochim. Cosmochim. Acta 44: 1349-1362 . Bartha, R., and R.M. Atlas. 1977 . The microbiology of aquatic oilepills . Adv. Appl. Microbiol . 22: 225-226 . Bassin, N.J., and T. Ichiye. 1977. Floculation behavior of suspended sediments and oil emulsions . J. Sed. Petrol . 47: 671-677 . Beam, H.W., and J.J. Perry. 1974. Microbial degradation of cycloparaffinic hydrocarbons via cometabolism and commensalism. J. Gen. Microbiol. 82 :163-169. Bend, J.R. 1980. Induction of drug-metabolizing enzymes by polycyclic aromatic hydrocarbons: mechanism and some implications in environmental health research, p. 93. In Environmental Chemicals, Enzymic Mechanisms and Human Disease. Ciba Foundation Symposium No . 76 . Excerpta medica, Amsterdam. Bend , J. R., and M. O. James . 1978 . Xenobiotic metabol ism in mar ine and freshwater species, p. 128. In D.C. Malins and J.R. Sargent eds. Biochemical and Biophysical Perspectives in Mar ine Biology. Vol . 4. Academic Press, New York. Bend, J.R., D.S. Miller, W.B. Kinter, and D.B. Peakall. 1977a. DDE-induced microsomal mixed-function oxidases in the puf f in (Fratercula arctica) . Biochem. Pharmacol. 26: 1000-1001. Bend, J.R., M.O. James, and P.M. Dansette. 1977b. In vitro metabolism of xenobiotics in some mar ine animals . Ann. N.Y. Acad. Sci. 298: 505-521. Bend, J.R., L.M. Ball, T.H. Elmamlouk, M.O. James, and R.M. Philpot. 1979. Microsomal mixed-function oxidation in untreated and polycyclic aromatic hydrocarbon-treated fish. In M.A.Q. Khan, J.u . Lech, and J.J. Menn, eds. Pesticide and Xenobiotic Metabolism in Aquatic Organisms, p. 297 . Amer ican Chemical Society, Washington, D.C. Berr idge , S.A., R.A. Dean, R.G. Fallows , and A. FiSh. 1968a. The properties of persistent oils at sea. J. Instit. Petr . 54: 300-309 . Berr idge , S.A., R.A. Dean, R.G. Fallows , and A. FiSh. 1968b. The properties of persistent oils at sea, pp. 2-11. In Peter Hepple ed. Proceedings of a Symposium, Scientif ic Aspects of Pollution of the Sea by Oil. Institute of Petroleum, London.
338 Berr idge , S.A., M. T. Thew , and A.G. Lor iston-Clarke . 1968c . The formation and stability of emulsions of water in crude petroleum and similar stocks, pp. 35-59 . In P. Hepple, ed. Scientific Aspects of Pollution of the Sea by Oil. Institute of Petroleum, London . Beslier, A., J.L. Birrien, L. Cabioch, C. Larsonneur, and L. Le Borgne. 1980. La pollution des Bales de Morlaix et de Lannion por les hydrocarbures de 1' Amoco Cadiz: Repartition sur les fonds et evolution. Heloglander Meeresunters 33 :209-224. Bieri, R. 1979. Hydrocarbons in demersal fish, macro-epifauna, and zooplankton, Vol. II, Chapter 9. MAFLA final report prepared by Dames and Moore . Contract AA550-CT7-34 . Bureau of Land Management, Wash ington, D. C. B inder, R. L. 1981. Xenobiotic monooxygenase activity and the r esponse to inducers of cytochrome P-450 dur ing embryonic and larval development in f~sh. Ph.D. thesis, WHOI/MIT Joint Program in Oceanography . B inder, R. L., and J . J. Stegeman . 1980 . Induction of aryl hydrocarbon hydroxylase activity in embryos of an estuar ine f ish . Biochem. Pharmacol . 29: 949-951. Blikely, D.R., G.F.L. Dietzel, A.W. Glass, P.J. van Kleef. 1977. Slicktrak--A computer simulation of offshore oil spills, cleanup, effect, and associated costs, pp. 4S-53. In Proceedings, 1977 Oil Spill Conference. American Petroleum Institute, Washington, D.C. Blumer, M., M. M. Mullin, and D. W. Thomas. 1963. Pristane in zooplankton . Science 140: 974 . Blumer, M., J.C. Robertson, J.E. Gordon, and J. Sass. 1969. Phytol-derived Cl9 di- and tri-olefinic hydrocarbons in marine zooplankton and fishes. Biochemistry 8 :4067-4074. Blumer , M., and J. Sass . ~ 972. Oil pollution : Persistence and degradation of spilled fuel oil. Science 176 :1120-1122. Blumer, M., G. Sooza, and J. Sass. 1970a. Hydrocarbon pollution of edible shellfish by an oil spill. Mar . Biol. 5 :195-202. Blumer, M., J. Sass, G. Souza, H. Sanders, F. Grassle, and G. Hampson . 1970b. The West Falmouth oil spill. Reference 70-44. woods Hole Oceanographic Institution, Woods Hole, Mass . Blumer, M., M.M. Mullin, and D.W. Thomas. 1970c. Pristane in the mar ine environment . Heloglander Wissenschaf tliche Merr esunter suchungen 10: 18 7-201 . Blumer, M., R. R. L. Guillard , and Y. Chase . 1971. Hydrocarbons of marine phytoplankton. Mar. Biol. 8 :183-189. Bocard, C. and C. Gatellier. 1981. Breaking of fresh and weathered emulsions by chemicals, pp. 601-607 . In Proceedings, 1981 Oil Spill Conference. Publication 4334. Amer ican Petroleum Institute, Washington, D. C. Boehm, P.D. 1980. Evidence for the decoupling of dissolved, particul ate, and surface microlayer hydrocarbons in northwestern Atlantic continental shelf water s . Mar . Chem. 9: 255-281. Boehm, P. D., and D. L. Fiest . 1979 . Determine hydrocarbon composition and concentrations of ma jor components of the mar ine ecosystem, Vol. VI . In W.B. Jackson and G.W. Faw, eds . Biological/Chemical
339 Survey of Texamo and Caplin Sector Salt Dome Br ine Disposal Sites off Louisiana, 1978-1979 . Technical Memorandum NMFS-SEFC-32. NOAA, Rockville, Md. Boehm, P. D. and D. L. Feist. 1980 . Surface water column transport and weathering of petroleum hydrocarbon during the Ixtoc I blowout in the Bay of Campeche and their relation to sur face oil and microalga compositions, pp. 169-185. In Proceedings of the Conference on the Preliminary Scientif ic Results from the Researcher/Pierce Cruise to the Ixtoc I Blowout. NOAA, Office of Marine Pollution Assessment, Boehm, P.I). and J.G. Quinn. 1973. the dissolved organic matter Acta 37: 2459-2477 . Rockv ille, Md . Solubilization of hydrocarbons by in sea water . Geoch im. Cosmoch im. Boehm, P.D. and J.G. Quinn. 1974 e The volubility behavior of No. 2 fuel oil in sea water. Mar. Pollut. Bull. 5~7~:101-105. Boehm, P.D., and J.G. Quinn. 1976. The effect of dissolved organic matter in seawater on the uptake of mixed individual hydrocarbons and No. 2 fuel oil by a mar ine f ilter-feeding bivalve (Mercenaria mercenar ia) . Estuarine and Coastal Mar. Sci. 4:93-105. Boehm, P.D., and J.G. Quinn. 1977. The persistence of chronically accumulated hydrocarbons in the hard shell clam Mercenaria mercenaria. Mar. Biol. 44:227-233. Boehm, P.D. and J.G. Quinn. 1978. Benthic hydrocarbons of Rhode Island Sound. Estuar ine Coastal Mar . Sci . 6: 471-494 . Boehm, P . D ., W. G . Ste inhauer , D. L. Fiest , N . Mosesman , J . E . Bar ak, and G.H. Perry. 1979. A chemical assessment of the present levels and sources of hydrocarbon pollutants in the Georges Bank region, pp. 333-341. In Proceedings, 1979 Oil Spill Conference. Amer ican Petroleum Institute, Washington, D. C . Boutry, L. -C., M. Bordes , A. Feur ier , M. Bar bier , and A. Saliot. 1977 . La diatomee mar ine Chaetoceros simplex calcitrans Paulsen et son environment. IV. Relations avec le milieu de culture: etude des hydrocarbures. J. Exp. Mar . Biol. Ecol. 28 :41-51. Breger, R.K., R.B. Franklin, and J.J. Lech. 1981. Metabolism of 2-methylnaphthalene to isomeric dihydrodiols by hepatic microsomes c:>f rat and rainbow trout. Drug Me tab. DiSp. 9 (2) :88-93. Br idle , A. L., and J. Bos . 1971. Biological degradation of mineral oil ~n seawater . J. Inst. Pet. London 57: 270-277 . Bridie, A.L., Th.H. Wanders, W. Zegveld, and H.B. Van der Hei jde. 1980 . Formation, prevention, and break ing of sea water oil emulsions "Chocolate Mousses. n Mar. Pollut. Bull. ~n cr ude 2: 434-348 . Brooks, J.M. 1976. Flux of light hydrocarbons into the Gulf of Mexico v~a runoff, pp. 135-200. In H. Windom and R.A. Duce, eds. Marine Pollutant Transfer . D. C. Heath and Co., Lexington , Mass . Brooks, J. M. 1979 . Deep methane maxima in the northwest Car ibbean Sea: possible seepage along the Jamaica Ridge. Science 206: 1060-1071. Brooks, J.M., and W.M. Sackett. 1973. Sources, sinks and concentrations of light hydrocarbons in the Gulf of Mexico. J. Geophys . Res . 78: 5248-5258 .
340 Brooks, J.M. and W.M. Sackett. 1977. Significance of low-molecular- weight hydrocarbons in marine waters, pp. 445-448. In R. Campos and J. Goni, eds. Proceedings of the 7th International Meeting on Organic Geochemistry. Espanola de Micropaleontologia, Madrid. Brooks, J.M., A.D. Fredericks, W.M. Sackett, and J.W. Swinnerton. 1973. Baseline concentrations of light hydrocarbons in the Gulf of Mexico. Environ. Sci. Technol. 7:639-642. Brooks, J.M., B.B. Bernard, and W.M. Sackett. 1977. Input of low molecular-weight-hydrocarbons from petroleum operations into the Gulf of Mexico, pp. 373-384. In D.A. Wolfe, ed. Fate and Effects of Petroleum Hydrocarbons in Mar ine Ecosystems and Organisms . Pergamon, New Yor k . Brooks , J.M., B.B. Bernard, T.C. Sauer, Jr ., and H. Abdel-Reheim. 1978. Environmental aspects of a well-flowout in she Gulf of Mexico. Environ. Sci . Technol. 12: 695-703 . Brooks, J.M., B.B. Bernard, W.M. Sackett, and J.R. Schwarz . 1979. Natural gas seepage on the south Texas shelf, pp. 371-378. Paper No. OTC 3411 Offshore Technology Conference Houston, Tex . Brooks , J.M., D.F. Reid, and B.B. Bernard. 1981a. Methane in the upper water column of the northwest Gul f of Mexico . J. Geophys . Res. 86:11029-11040. Brooks, J.M., D.A. Wiesenburg, R.A. Burke, Jr., and M.C. Kennicutt. 1981b. Gaseous and volatile hydrocarbons input from a subsurface oil spill in the Gulf of Mexico. Environ. Sci. Technol. 15:91-959. Brown, R.A., and H. L. Huffman . 1976 . Hydrocarbons in open ocean waters. Science 191:847-849. Brown, R.A., and T.D. Searl. 1976. Nonvolatile hydrocarbons along tanker routes of ~che Pacific Ocean, pp. 259-274. Paper No. 2448, Vol. 1. Offshore Technology Conference, Houston, Tex. Brc~wn, R.A., T.D. Searl, J.J. Elliott, B.G. Phillips, D.E. Brandon, and P. H. Monaghan . 1973 . Distr ibution of heavy hydrocarbons in some Atlantic Ocean waters, pp. 505-519. In Proceedings, Joint Conference on Prevention and Control of Oil Spills . Amer ican Petroleum Institute, Washington, D. C . Brown, R.A., J.J. Elliot, and T.D. Searl. 1974. Measurement and c}1aracter ization of nonvolatile hydrocarbons in ocean water, pp . 1 31-133. In Marine Pollution Monitoring (Petroleum) . NBS Spec~al Publication 409 . National Bureau of Standards, Gaithersburg, Md . Brown, R.A., J.J. Elliot, J.M. Kelicher, and T.D. Searl. 1975. Sampling and analysis of nonvolatile hydrocarbons in ocean water, pp. 172-187 . In T. R. P. Gibb , Jr ., ed. Analytical Methods in Oceanography. Advances in Chemistry Ser. ies 147 . Amer ican Chemical Society, Washington, D.C. Brunnock , J.V., D.F. Duckworth , and G.G. Stephens. 1968 . Analysis of beach pollutants. J. Inst. Petrol. 54: 30-325 . Burns, K.A. 1976. Hydrocarbon metabolism in the intertidal fiddler crab Uca pugnax. Mar . Biol. 36: 5-11. Burns, K.A., and J.L. Smith. 1977. Distribution of petroleum hydrocarbons in Westernport Bay (Australia): results of chronic low level inputs, pp. 442-453. In D. Wolfe, ed. Fate and Effects of Petroleum Hydrocarbons in Mar ine Ecosystems and Organisms . Pergamon, New York.
341 Burns, K.A. and J.~. Teal. 1973. Hydrocarbons in the pelagic Sargasso community. Deep Sea Res. 20:207-211. Burns, K.A., and J.M. Teal. 1979. The West Falmouth oil spill: hydrocarbons in the salt marsh ecosystem. Estuarine Coastal Mar. Sci. 8:349-360. Burwood, R., and G.C. Speers. 1974. Photooxidation as a factor in the environmental dispersal of crude oil. Estuarine Coastal Mar. Sci. 2:117-135. Butler, J.N. 1975. Evaporative weathering of petroleum residues: the age of pelagic tar. Mar. Chem. 3: 9-21. Butler, J.N. 1976. Transfer of petroleum residues from sea to air: evaporative weathering. In H.L. Windon and R.A. Duce, eds. Marine Pollutant Transfer Lexington Books, Lexington, Mass. Butler, J.N., B.F. Morris and J. Sass. 1973. Pelagic tar from Bermuda and the Sargasso Sea, Special Publication 10. Bermuda Biological Station for Research, St. George's West. Button, D.K. 1976. The inf luence of clay and bacter ia on the concentration of dissolved hydrocarbons in saline solution. Geochim. Cosmochim. Acta 40:435-440. Cairns, R.J.R., D.M. Grist, and E.L. Neustadter. 1974. The effect of crude oil-water infer facial properties on water-crude oil emulsion stability, pp. 135-151. In A.L. Smith, ed. Theory and Practice of Emulsion Technology. Academic Press, New York. Calder, J.A. 1976. Hydrocarbons from zooplankton of the eastern Gulf of Mexico. In Proceedings of a Symposium on Sources, Effects, and Sinks of Hydrocarbons in Aquatic Environments. American Institute of Biological Sciences, Arlington, Va. Calder, J.A. 1977. Seasonal variations of hydrocarbons in the water column of the MAFLA lease area, pp. 432-441. In D.A. wolfe, ed. Fate and Effects of Petro~eum Hydrocarbons in Marine Ecosystems and Organisms. Pergamon, New York. Calder, J.A., J. Lake, and J. Laseter. 1978. Chemical composition of selected environmental and petroleum samples from the Amoco Cadiz oil spill. In W.H. Hess, ed. The Amoco Cadiz Oil Spill. A Preliminary Sczentific Report. NOAA, EPA, Washington, D.C. Canevari, G.P. 1969. General dispersant theory, pp. 171-177. In Proceedings, Jo~nt Conference on Prevention and Control of Oil Spills. NTIS Report PB 194-395. National Technical Information Service, Springfm ld, Va. Caparello, D.M., and P.A. La Rock. 1975. A radioisotope assay for the quantification of hydrocarbon biodegradation potential in environmental samples. Microb. Ecol. 2:28-42. Cerniglia, C.E., D.T. Gibson, and C. Van Baalen. 1979. Algal oxidation of aromatic hydrocarbons; formation of 1-naphthol from naphthalene by Agmencllum quadruplicatum, strain PR-6. Biochem. Biophys. Res. Commun. 88: 50-58. Cerniglia, C.E., D.T. Gibson, and C. Van Baalen. 1980a. Oxidation of naphthalene by cyanobacteria and microalgae. J. Gen. Microbiol. 116:495-500. Cerniglia, C.E., C. Van Baalen, and D.T. Gibson. 1980b. Oxidation of biphenyl by the cyanobacterium, Oscillator ia sp., strain JCM. Arch. Microbiol. 125:203-207.
342 Chouteau , J ., E. Azoulay, and J . E. Senez . 1962 . Anaerobic formation of n-heptene-1 from n-heptane by resting cells of Pseudomonas aeruginosa. Nature 194:576-578. Clark, R.C., and J.S. Finley. 1973. Parraffin hydrocarbon pattern in petroleum polluted mussels. Mar. Pollut. Bull. 4:172-176. Clark, R.C., and J.S. Finley. 1974. Acute effects of outboard motor effluent on two marine shellfish. Environ. Sci. Technol. 8:1009-1014. Clark, R.C., and J.S. Finley. 1975. Uptake and loss of petroleum hydrocarbons by mussels, Mytilus edulis, in laboratory exper iments. Fishery Bull . 73: 508-515 . Clark, R.C., and W.D. MacLeod. 1977a. Inputs, transport mechanisms, an observed concentrations of petroleum in the mar ine environment, pp. 91-223. In D.C. Malins, ed. Effects of Petroleum on Arctic and Subarctic Environments and Organisms Vol. 1, Nature and Fate of Petroleum Academic Press, New York. Clark, R.C., Jr., and W.D. MacLeod, Jr. 1977b. Photochemical modification, pp. 129-132. In D.C. Malins, ed. Effects of Petroleum on Arctic and Subarctic Marine Environments and Organisms. Vol. 1, The Nature and Fate of Petroleum. Academic Press, New York. Clark, R.C., Jr., J.S. Finley, B.G. Patten, D.F. Stefoni, and E.E. DeNike. 1973. Interagency investigations of a persistent oil spill on the Washington coast, pp. 793-808. In Proceedings, Joint Conference on Prevention and Control of Oil Spills. American Petroleum Institute, Washington, D.C. Clark, R.C., J.S. Finley, B.G. Patten, and E.E. DeNike. 1975. Long-term chemical and biological effects of a persistent oil spill following the grounding of the General M.C. Meigs. In 1975 Conference on Prevention and Control of Oil Pollution American Petroleum Institute, Washington, D.C. Clark, R.C., Jr., B.G. Patten, and E.E. DeNike. 1978. Observations of a cold-water intertidal community after 5 years of a low level, persistent oil spill from the General M.C. Meiggs. J. Fish. Res. Board Can. 35:754-765 ~ Clement, L.E., M.S. Stekol, and D.G. Shawl 1980. Accumulation, fractionation and release of oil by the intertidal clam Macoma balthica. Mar. Biol. 57:41-50. Cline, J.D. and M.L. Holmes. 1977a. Submarine seepage of natural gas in Norton Sound, Alaska. Science 198:1149-1153. Cline, J.D. and M.L. Holmes. 1977b. Anomalous gaseous hydrocarbons in Norton Sound: biogenic or thermogenic? Offshore Technology Conference, Houston, Texas. Paper No . OTC 30S2, 1: 81-86 . Cohen, Y., D. Mackay, and W.Y. Shiu. 1980. Mass transfer rates between oil slicks and water. Can J. Chem. Eng. 58:569. Collier, T.K., L.C. Thomas, and D.C. Malins. 1978. Influence of environmental temperature on disposition of dietary naphthalene in coho salmon (Oncorhynchus kisutch): isolation and identification of individual metabolites. Comp. Biochem. Physiol. 61C:23-28. Collier, T.K., M.K. Krahn, and D.C. Malins. 1980. The disposition of naphthalene and its metabolities in the brain of rainbow trout (Salmo gairdneri). Environ. Res . 23: 35-41.
343 Colwell, R.R., and J.D. Walker. 1977. Ecological aspects of microbial degradation of petroleum in the mar ine environment. Crit. Rev. Microbiol. 5:423-445. Colwell, R.R., A.L. Mills, J.D. Walker, P. Garcia-Tello, and V. Campos-P. 1978. Microbial ecology studies of the Metula spill in the Straits of Magellan. J. Fish. Res. Board Can. 35:573-580. Conomos, T.J. 1975. Movement of spilled oil as predicted by estuarine nontital drift. Limmol. Oceanogr. 20:159-173. Conover, R. J. 1971. Some relations between zooplankton and Bunker C oil on Chedabucto Bay following the wreck of the tanker Arrow. J. Fish. Res. Board Can. 28:1327-1330. Cordes , C ., L . Atk inson , R. Lee , and J . Blanton . 1980 . Pelag ic tar of f Georgia and Florida in relation to physical processes. Mar. Pollut. Bull. 11: 315-317 . Corner , E.D.S., C.C. Kilvington, and S.C.M. O'Hara. 1973. Qualitative studies on the metabolism of naphthalenes in Maia squinado tHerbst) . J. Mar . Biol. ASSOC. U. K. 53: 819-832. Corner, E.D.S., R.P. Harris, K.J. Whittle, and P.R. Mackie. 1976a. Hydrocarbons in mar ine zooplankton and f ish, pp. 71-10 5 . In A. P.M. Lockwood, ed. Effects of Pollutants on Aquatic Organisms. Cambridge University Press, London. Corner, E.D.S., R.P. Harris, C.C. Kilvington, and S.C.M. O'Hara. 1976b. Petroleum compounds in the marine food web: short-term exper iments on the fate of naphthalene in Calanus . J. Mar . Biol . Assoc. U.K. 56:121 - 133. Coty, V.F., and R.I. Leavitt. 1971. Microbial protein from hydrocarbons. Devel. Ind. Microbiol. 12: 61-71. Cretney, W.J. and C.S. Wong. 1974. Fluorescence monitoring study at ocean weather station "p," pp. 175-180. In Marine Pollution Monitoring (Petroleum). NBS Special Publication 409. National Bureau of Standards, Gaithersburg, Md. Cripps, R.E., and R.J. Watkinson. 1978. Polycyclic aromatic hydrocarbons: metabolism and environmental aspects, pp. 113-134. In J.R. Watkinson, ed. Developments in Biodegradation of Hydrocarbons. Applied Science Publishers, London. Crisp, P.T., S. Brenner, M.I. Venaktesan, E. Ruth, and I.R. Kaplan. 1979. Organic chemical characterization of sediment-trap particulates from San Nicholas, Santa Barbara, Santa Monica, and San Pedro Basins, California. Geochim. Cosmachim. Acta 43:1791-1801. navis, S.J. and C.F. Gibbs. 1975. The effect of weathering on a crude oil exposed at sea. Water Res. 9:275-289. Delaune, R.D., G.A. Hambrick III, and W.H. Patrick, Jr. 1980. Degradation of hydrocarbons in oxidized and reduced sediments. Mar. Pollut. Bull. ~ 1:103-106. Dibble, J.T., and R. Bar tha . 1976. The effect of iron on the biodegradation of petroleum in seawater. Appl. Environ. Microbiol. 31:544-550. Dickens, D.F., I.A. Buist, and W.M. Pistruzak. 1981. Dome's petroleum study of oil and gas under sea ice, pp. 183-189. In Proceedings, 1981 Oil Spill Conference. Publication 4334. American Petroleum Institute, Washington, D.C.
344 Dicks, B., and K. Iball . 1981. Ten years of saltmarsh monitor ing--the case history of a Southampton Water saltmarsh and a changing refinery effluent discharge, pp. 361-374. In Proceedings, 1981 Oil Spill Conference . Publication 4334. Amer loan Petroleum Institute, Washington, D. C. DiSalvo, L.H. and J.E. Guard. 1975. Hydrocarbons associated with suspended particulate matter in San Francisco Bay waters, pp. 169-173. In Proceedinas. 1975 Conference On Prmu~ntinn And ~^ntro1 of Oil Pollution. American Petroleum Institute, Washington, D.C. DiSalvo, L.H., H.E. Guard, and L. Hunter. 1975. Tissue hydrocarbon burden of mussels as potential monitor of environmental hydrocarbon insult. Environ. Sci. Technol. 9:247-251. Dobroski, C.J., and C.E. Epifano. 1980. Accumulation of benzota~pyrene in a larval bivalve via trophic transfer. Can. J. Fish. Aquat. Sci. 37:2318-2322. Dowty, B.J., NeE. Brightwell, J.L. Laseter, and G.W. Griffin. 1974. Dye-sensitized photo-oxidation of phenanthrene. Biochem. Biophys. Res. Comms. 57:452-455. Duce, R.A., J.G. Quinn, C.E. Olney, S.R. Piotrowicz, B.J. Ray, and T.L. Wade. 1972. Enrichment of heavy metals and organic compounds in the surface micro-layer of Narragansett Bay, Rhode Island. Science 176:161-163. Dunn, B.P. 1980. Polycyclic aromatic hydrocarbons in mar ine sediments, bivalves, and seaweeds: analysis by high-pressure liquid chromatography, pp. 367-377. In A. Bjorseth and A.J. Dennis, eds. Proceedings of the Fourth International Symposium on Polynuclear Aromatic Hydrocarbons. Batelle Press, Columbus, Ohio. Dunn, B.P., and H.F. Stich. 1976. Release of the carcinogen benzota~pyrene from environmentally contaminated mussels. Bull. Environ. Contam. Toxicol. 15:398-401. Eagle, G.A., A. Green, and J. Williams. 1979. Tar ball concentrations in the ocean around the Cape of Good Hope before and after a major oil spill. Mar. Pollut. Bull. 10:321-325. Ehrhardt, M. 1972. Petroleum hydrocarbons in oysters from Galveston Bay. Environ. Pollut. 3: 257-271. Ehrhardt, M., and J. Derenbach. 1977. Composition and weight per area of pelagic tar collected between Portugal and south of the Canary Islands. Meteor. Forsch-Ergebnisse A(l9~:1-9. Ehrhardt, M., and J. Heinemann. 1975. Hydrocarbons in blue mussels from the Kiel Bight. Environ. Pollut. 9 :263-282. Elcombe, C.R., R.B. Franklin, and J.J. Lech. 1979a. Induction of hepatic microsomal enzymes in rainbow trout, p. 319 . In M.A. Q. Khan, J.J. Lech, and J.J. Menn, eds. Pesticide and Xenobiotic Metabolism in Aquatic Organisms . American Chemical Society, Washington, D.C. Elcombe, C.R., R.B. Franklin, and J . J. Lech. 1979b. Induction of microsomal hemoproteints) P-4S0 in the rat and rainbow trout by polyhalogenated biphenyls. Ann. N.Y. Acad. Sci . 320 : 193 . Elmamlouk, T.H., T. Gessner, and A.C. Brownie. 1974. Occurrence of cytochrome P-450 in hepatopancreas of Homarus Americanus. Comp. Biochem. Physiol. 48B:419.
345 Engelhardt, F.R. 1981. Hydrocarbon metabolism and cortisol balance in oil-exposed r ing seals, Phoca h ispida. Comp. Biochem. Phys iol . ~ in press) . Engelhardt, F. R., J. R. Geraci , and T. G. Smith . 1977 . Uptake and clearance of petroleum hydrocarbons in the ringed seal, Phoca hispida. J. fish. Res. Board Can. 34~8) :1143-1147. Fallab, S. 1967. Reactions with molecular oxygen. Agnew. Chem. Int . Edition 6:496-507. Farrington, J.W., and J.G. Quinn. 1973. Petroleum hydrocarbons in Narragansett Bay. I. Survey of hydrocarbons in sediments and clams (Mercenaria mercenaria). Estuarine Coastal Mar. Sci. 1:71-79. . Farrington, J.W., and B.W. Tripp. 1977. Hydrocarbons in western North Atlantic surface sediments. Geochim. Cosmochim. Acta 41:1627-1641. Farrington, J.W., N.M. Frew, P.M. Gschwend, and B.W. Tripp. 1977. Hydrocarbons in cores of northwestern Atlantic coastal and continental margin sediments. Estuar~ne Coastal Mar . Sci . 5:793-808. Farrington, J.W., J. Albaiges, K.A. Burns, B.P. Dunn, P. Eaton, J.L. Laseter, P. L. Parker, and S. Wise. 1980 . Fossil fuels, pp. 7-77 . In The International Mussel Watch. National Academy of Sciences, Washington, D. C. Farrington, J.W., A.C. Davis, N.W. Frew, and K.S. Rabin. 1981. No. 2 fuel oil compounds in Mytilus edulis: retention and release after an oil spill. Mar . Biol. 66 :15-26. Faza1, R.A., and H.J. Milgram. 1979. The effect of surface phenomena on the spreading of oil on water. Report MITSG 79-31. MIT Sea Grant College Program. Floodgate, G. D. 1979 . Nutr ient limitation, pp. 107-118. In A.W. Bourquin and P. H. Pr itchard, ed. Proceedings of Workshop, Microbial Degradation of Pollutants in Marine Environments. EPA-66019-79-012. Environmental Research Laboratory, Gulf Breeze' Fla. Forlin, L. 1980. Effects of Clophen A50, 3-methylcholanthrene, pregnenolone 16a-carbonitrile and phenobarbital on the hepatic microsomal cytochrome P-450 dependent monooxygenase system in rainbow trout, Salmo gairdneri, of different age and sex. Toxicol. Appl. Pharmacol. 54:420. ~orrester, W.D. 1971. Distribution of suspended oil particulates following the grounding of the tanker Arrow. J. Mar. Res. 29:151-170. Fossato, V. U. 1975 . Elimination of hydrocarbons by mussels. Mar . Pollut. Bull. 6: 7-10 . Fossato, V.U., and W.J. Canzon~r. 1976. Hydrocarbon uptake and loss by the mussel Mytilus edulis. Mar . Biol. 36: 243-250 . Fossato, V. U., C. Nasci , and F. Dolci . 1979 . 3 ,4-benzopyrene and perylenein mussels, Mytilus sp., from the Laguna Veneta, Northeast Italy. Mar . Environ. Res. 2 :47-53. Frankenfeld, J. S . 1973. Factors governing the fate of oil at sea: var. iations in the amounts and types of dissolved or dispersed materials during the weather~ng process. In Proceedings, Joint Conference on Pre~rention and Control of Oil Spills. American Petroleum Institute, Washington, D. C.
346 Freegarde, M., C.G. Hatchard, and C.A. Parker e 1971. Oil spilt at sea: its identification, determination and ultimate fate. Lab. Practice 20~1~:35-40. Friede, J.D. 1973. The isolation and chemical and biological properties of microbial and emulsifying agents for hydrocarbons. Progress Report Ad 770-630. National Technical Information Service, O.S. Department of Commerce, Springfield, Va., 5 pp. Fucik, K.W., and J.M. Neff. ~ 977. Effect of temperature and salinity of naphthalene uptake in the temperate clam, Rangia cuneata, and ache boreal clam, Protothaca staminea, pp. 305-312. In D. Wolfe, ed. Fate and Effects of Petroleum Hydrocarbons in Mar ine Organisms and Ecosystems. Pergamon, New York. Gardner, W.S., R.F. Lee, K.R. Tenore, and L.W. Smith. 1979. Degradation of selected polycyclic aromatic hydrocarbons in coastal sediments: importance of microbes and polychaete worms. Water Air Soil Pollut. 11:339-347. Garrett, W.D. 1967. Stabilization of air bubbles at the air-sea interface by surface active material. Deep Sea Res. 14:666-672. Garver, D.R., and G.N. Williams. 1978. Advances in oil spill trajectory modelling. Proc. Oceans '78. Marine Technology Society and IEEE, Washington, D.C., September 6-8, 1978. Gear ing, P. J. J.N. Gearing, T.F. Lytle, and J . S. . Lytle . 19 76. Hydrocarbons in 60 northeast Gulf of Mexico shelf sediments: a preliminary survey. Geochim. Cosmochim. Acta 40 :1005-1017 . Gear ing , P. J ., J. N. Gear ing , R. J. Pruell , T. L. Wade , and J . G. Quinn . 1980 . Par titioning of No. 2 fuel oil in controlled estuar ine ecosystems: sediments and suspended par ticulate matter . Environ. Sci. Technol. 14~9~:1129-1136. Gelboin, H.V. 1980. Benzota~pyrene metabolism, activation, and carcinogenesis: role and regulation of mixed-function oxidases and related enzymes. Physiol. Rev. 60:1107-1155. Geyer, R.A., and C.P. Giammona. 1980. Naturally occurring hydrocarbons in the Gulf of Mexico and Caribbean Sea, pp. 37-106. In R.A. Geyer, ed. Mar ~ne Environmental Pollution. Vol. . I, Hydrocarbons . Elsevier, New York. Gibbs, C. F. 1975 . Quantitative studies in mar ine biodegradation of oil . I . Nutr ient limitation at 14C. Proc . R. Soc . London, Ser . B 188: 61-82. Gibbs, C.F., and S.J. Davis. 1976. The rate of microbial degradation of oil in a beach gravel column. Microb. Ecol. 3: 55-64 . Gibbs, C.F., K.B. Pugh, and A.R. Andrews. 1975. Quantitative studies in marine biodegradation of oil. II. Effects of temperature. Proc. R. Soc. London, Ser. B 188:83-94. Gibson, D.T. 1968. Microbial degradation of aromatic compounds. Science 161:1093-1097. Gibson, D.T. 1971. The microbial oxidation of aromatic hydrocarbons. Crit. Rev. Microbiol. 1:199-223. Gibson, D.T. 1975. Oxidation of the carcinogens benzofa~pyrene and benzotajanthracene to dihydrodiols by a bacterium. Science 189:295-297.
347 Gibson, D. T. 1976 . Microbial degradation of polycyclic aromatic hydrocarbons, pp. 57-66 . In J.M. Sharpley and A.M. Kaplan, eds. Proceedings of the Third International Biodegradation Symposium. Applied Science Publishers, London. Gibson, D.T. 1977. Biodegradation of aromatic petroleum hydrocarbons, pp. 36-46. In D. Wolfe, ed. Fate and Effects of Petroleum Hydrocarbons in Mar ine Ecosystems and Organisms. Pergamon, New Yor k . Gilfillan, E.S., and J.H. Vandermuelen. 1978. Alternation in growth and physiology of soft-shell clams, Mya arenaria, chronically oil ed with Bunker C from Chedabucto Bay, Nova Scotia, 1970-76. J. Fish. Res . Board Can. 35: 630-636 . Gilfillan, E.S., D.S. Page, R.P. Gerber, S. Hansen, J. Cooley, and J. Hotham. 1981. Fate of the Zoe Colocotroni oil spill and its effects on infaunal communities associated with mangroves, pp. 353-360. In Proceedings, 1981 Oil Spill Conference. American Petroleum Institute, Wash ington, D.C. Goldberg , E . D., V. T . Bowen , J .W. Farrington, G . Harvey , J . H . Martin, P.L. Parker, R.W. Risebrough, W. Robertson, E. Schneider, and E. Gamble. 1978. The Mussel Watch. Environ. Conserv. 5 :101-125 . Gordon, D.C., Jr., and P.D. Keizer. 1974. Estimation of petroleum hydrocarbons in seawater by fluroescence spectroscopy: improved sampling and analytical methods . Technical Report 481. Fisher ies and Mar ine Sciences, Environment Canada, Ottowa, Ontar io. Gordon, D.C., P.D. Keizer, and J. Dale. 1974. Estimates using fluorescence spectoscopy of the present state of petroleum hydrocarbons contamination in the water column of the nor thwest Atlantic Ocean. Mar . Chem. 2: 251-261. Gordon, D.C., P.D. Keizer, and J. Dale. 1978a. Temporal variations and probable or ~ g ins of hydrocarbons in the water column of Bedfor d Basin, Nova Scotia. Estuarine Coastal Mar. Sci. 7:243-256. Gordon, D.C., J. Dade, and P.D. Keizer. 1978b. Importance of sediment working by the deposit-feeding polychaete Arenicola marina on the weathering rate of sediment-bound oil. J. Fish. Res. Board Can. 35: 591-603 . Grahl-Nielson, O. 1978 . The Ekof ish Bravo blowout: petroleum hydrocarbons in the sea, pp. 476-487 . In Proceedings of the Conference on Assessment of Ecological Impacts of Oil Spills. Amer ican Institute of Biological Sciences . Arlington, Va . Grahl-Nielsen, O., K. Westrheim, and S. Wilhelmsen. 1979. Petroleum hydrocarbons in the North Sea, pp. 629-632. In Proceedings, 1979 0~1 Spill Conference . Amer ican Petroleum Institute, Wash ~ngton, D.C. Grassle , J. F., and J . P. Grassle . 1977 . Tempor al adaptations in s ibling species of Capitella, pp. 177-189. In B.C. Coull, ed. Ecology of Mar ine Benthos. University of South Carol~na Press, Columbia. Grassle, J.P. , and J.F. Grassle. 1976. Sibling species in the marine pollution indicator Capitella (polychaete). Science 192:567-659. Grau, C.R., T. Roudybush, J. Dobbs, and J. Wathen. 1977. Altered yolk structure and reduced hatchability of eggs from birds fed single doses of petroleum oils. Science 195:779-781.
348 Gruger, E.H., Jr., J.V. Schnell, P.S. Fraser, D.W. Brown, and D.C. Malins. 1981. Metabolism of 2,6-dimethylnaphthalene in starry flounder (Platichthys stellatus) exposed to naphthalene and p-cresol. Aquat. Toxicol. 1:37-48. Gschwend, P.M., O.C. Zafiriou, and R.B. Gagosian . 1980 . Volatile organic compounds in seawater from the Peru upwelling region. Limnol . Oceanogr . 25 :1044-1053 . Gschwend , P. M., O. C. Zaf ir iou , R. F. Mantoora , R. P. Swar zenbach , R. B . Gagosian. 1982. Volatile organic compounds at the coastal site; I. Seasonal Ear Cations. Environ. Sci . Technol . 16: 31-37 . Guire , P. E., J. D. Fr iede , and R. K. Gholson . 1973 . Production and characteriza~icn of emulsifying factors from hydrocarbonoclastic yeast and bacteria, pp. 229-231. In D.G. Ahern and S.P. Meyers , eds. The Microbial Degradation of Oil Pollutants. Publication LSU-Sg-73-01. Center for Wetland Resources, Louisiana State University, Baton Rouge. Gunkel, W. 1968 . Bacter iological investigations of oil-polluted sediments from the Cornish coast following the Torrey Canyon disaster . Helgolander Wiss. Meeresunters. 17:151-158. Haines, J.K., and M. Alexander. 1974. Microbial degradation of high-molecular weight alkanes. Appl. Microbiol. 28:1084-1085. Hansen, H.P. 1975. Photc~chemical degradation of petroleum hydrocarbon sur face films on seawater. Mar. Chem. 3:183-195. Hansen, H.P. 1977. Photodegradation of hydrocarbon surface films. Rapp. P.-v. Reun. Cons. Int. Explor. Mer 171:101-106. Hansen, N., V.B. Jensen, H. Applequist, and E. Morch. 1978. The uptake and release of petroleum hydrocarbons by the marine mussel Mytilus edulis. Pron . Water Tech . 10: 351-359 . Hardy , R., P . R. Mack ie , K. J. Wh ittle , A. D. Mc Intyre , and R. A. A. Blackman. 1975. Occurrence of hydrocarbons in surface films, subsurface water and sediment around the U.K., p. 71. In A.D. McIntyre and R. Whittle, eds. Petroleum Hydrocarbons in the Marine Environment. Proceedings from the TCES Workshop held in Aberdeen, Sept. 9-12, 1975. International Council for the Exploration of the Sea. 171:71. Hardy, R. , P.R. Mackie, K.J. Whittle, A.D. McIntyre, and R.A.A. Blackman . 1977 . Occurrence of hydrocarbons in surface films, subsurface water, and sediment around the United Kingdom. Proceedings from the ICES Workshop held in Aberdeen, Sept. 9-12, 1975. International Council for the Exploration of the Sea. 171:61-65. Harris, R.P., V. Berdugo, E.D.S. Corner, C.C. Kilvington, and S.C.M. O'Hara. 1977. Factors affecting the retention of a petroleum hydrocarbon by marine planktonic copepods, pp. 286-304. In D.A. Wolfe ed. Fate and Ef fects of Petroleum Hydrocarbons in Mar ine Ecosystems and Organisms. Pergamon, New York. Harrison, W., M.A. Winn~ck, P.T.Y. Kwang, and D. Mackay. 1975. Crude oil spills: disappearance of aromatic and aliphatic components from small sea-surface slicks. Environ. Sci. Technol 9: 231-234. , -
349 Hartung, R., and G.W. Klinger. 1970. Concentration of DOT by sedimented polluting oils. Environ. Sci. Technol. 4: 407-410 . Harvey, G.R., A.G. Regue jo, P.A. MaGillivary, and J.M. Tokar. 1979. Observations of a subsur face oiler ich layer in the open ocean . Science 205: 999-1001. Heicklen, J. 1976 . Atmospher ic Chemistry. Academic Press, New York, 406 pp. Hendry, D.G., C.W. Gould, D. Scheutzle, M.G. Syz, and F.R. Mayo. 1976. Auto~oxidations of cyclohexane and its auto-oxidation products. J. Organ. Chem. 41~1) :1-459. Herbes , S. E., and L. R. Schwall . 1978 . M, crobial transformation of polycyclic aromatic hydrocarbons in pr istine and petroleum contaminated sediments. Appl. Environ. Microbiol. 35: 306-316 . Hess, W.N., ed. 1978. The Amoco Cadiz oil spill: a preliminary scientif ic report . NOAA/EPA Special Report . NTIS No . PB-285-805 . National Technical Information Service, Springf~e ld, Va. Heyerdahl, T. 1971a. Atlantic Ocean pollution and biota observed by the "Ran expedition. Biol. Conserv. 3 :164-167. Heyerdahl, T. 197lb. The Ra Expeditions, chaps. 8-11. Doubleday, New York. Hiltrabrand, R.R. 1978. Estimation of aromatic hydrocarbons in seawater at proposed deepwater port (DWP) sites in the Gulf of Mexico. Mar e Pollut. Bull. 9:19-21. Hinga, K.R., M.E.Q. Pilson, R.F. Lee, J.W. Farrington, K. Tjessem, and A.C. Davis. 1980. Biogeochemistry of benzanthracene in an enclosed marine ecosystem. Environ. Sci. Technol. 14:1136-1143. Hoffman, E.J. and J.G. Quinn. 1979. Gas chromatographic analyses of Argo Merchant oil and sediment hydrocarbons at the wreck site. l Mar . Pollut. Bull. 10: 20-24 . Hopper, D.J. 1978. Microbial degradat~on of aromatic hydrocarbons, pp. 85-112 . In J. R. Watk inson ed. Developments in Biodegradation of Hydrocarbons Vol. 1. Applied Science Publishers, London. Horn, M. H., J.M. Teal , and R. H. Backus . 1970 . Petroleum lumps on the surface of the sea. Sc~ence 168: 245-246 . Hose , J . E ., J . B . Hannah , M. L . Landolt , B.. S . Miller , S.. P . Felton , and W.T. Iwaoka. 1981. Uptake of benzo (a~pyrene by gonadal tissue of flatfish (family Pleuronectidae) and its effect on subsequent egg development. J. Toxicol. Environ. Health 7 :991. Howard, J. A., and K. U. Ingold . 1966 . Absolute rate constants for hydrocarbon auto-oxidat, on. III. Methylstyrene, -methlstyrene and indene. Can. J. Chem. 44 :1113-1118. Huang, J.C., and F.C. Monastero. 1982. Review of the state-of-the-art of oil spill simulation models. Final report. Amer ican Petroleum Institute, Washington, D.C. Hunn, J.B., and J.L. Allen. 1974. Movement of drugs across the gills of fishes. Ann. Rev. Pharmacol. 14 :47-55. Hurtt, A.C., and J.G. Quinn. 1979. Distribution of hydrocarbons in Narragansett Bay sediment cores. Environ. Sci. Technol . 13: 829-836 . Iizaka, H., M. Ilida, and S. Fu jita. 1969. Formation of -n-decene-1 from n-decane by resting cells of C. rugosa. Z. Allg. Mikrobiol. 9: 223-226 .
350 Iliffe, T.M., and J.A. Calder. 1974. Dissolved hydrocarbons in the eastern Gulf of Mexico Loop Current and the Car ibbean Sea. Deep Sea Res. 21:481-488. James, M.O., and J.R. Bend. 1980. Polycyclic aromatic hydrocarbon induction of cytochrome P-450 dependent mixed-function oxidases in marine fish. Toxicol. Appl. Pharmacol. 54:117. James, M.O., E.R. Bowen, P.M. Dansette, and J.R. Bend. 1979. Epoxide hydrase and glutathione S-transferase activities with selected alkene and arene oxides in several marine species. Chem. Biol. Interactions 25:321-344. Jeffrey, L.M. 1973. Preliminary report on floating tar balls in the Gulf of Mexico and Caribbean Sea. Sea Grant Project 53399. Texas A&M University, College Station. Jeffrey, L.M. 1979 . Water column particulates and dissolved hydrocarbons, Vol. II, Chap. 25. In MAFLA final report prepared for Dames and Moore. Contract AA550-CT7-34. Bureau of Land Management, Washington, D.C. Jeffrey, L.M. 1980. Petroleum residues in the marine environment, pp. 163-179 . In R.A. Geyer ed . Mar ine Environmental Pollution. Vol. Hydrocarbons. Elsevier, New York. Jeffrey, L.M., W.E. Pequegnat, E.A. Kennedy, A. Vos , and B.N. Jane . 1974 . Pelagic tar in the Gulf of Mexico and Car ibbean Sea, pp. 233-235 . In Mar ine Pollution Monitor ing (Petroleum) . Special Publication 409 . National Bureau of Standards, Gaithersburg, Md. Jeffrey, L.M., A. Vos, and N. Powell. 1976. Progress report on the chemistry of environmental tars of the Gulf of Mexico and Atlant~c. Report for Naturally Occurring Hydrocarbons in the Gu' f of Mexico. Project S5173. Texas A&M University, College Station. Johansson , S. ., U. Larsson , and P . Boehm. 1980 . The Tses is oil spill . Mar . Pollut. Bull . 11: 284-293. Johnson, E. F. 1979 . Multiple forms of cytochrome P-450: cr. iter ia and signif icance, pp. 1-26 . In E. Hodgson, J. R. Bend, and R.M. Philpot, eds . Reviews in Biochemical Toxicology. Jordan, R.E., and J.R. Payne. 1980. Fate and weathering of petroleum spilled in the mar ine environment: a literature review and synopsis. Ann Arbor Science Publishers, Ann Arbor, Mich. Kamahara, F.K. 1969. identification and differentiation of heavy residual oil and asphalt pollutants in surface waters by comparative ratios of infrared absorbances. Environ. Sci. Technol. 3:150-153. Kanazawa, M. 1975. Productzon of yeast from e-paraffins, pp. 438-453. In S.R. Tannenbaum and D.I.C. Wang, ed. Single Cell Protein, Vol. 2. MIT Press, Cambridge, Mass. Karickhoff, S.W., D.S. Brown, and T.A. Scott. 1979. Sorption of hydrophobic pollutants on natural sediments. Water Res. 13:241-248. Keizer, P.D., and D.C. Gordon. 1973. Detection of trace amounts of oil and seawater by fluorescence spectroscopy. J. Fish. Res. Board Can. 30 :1039-1046 . Keizer, P.D., D.C. Go~don, Jr., and J. Dale. 1977. Hydrocarbons in eastern Canadian marine waters determined by fluorescence spectroscopy and gas-liquid chrome ~ography. J. Fish . Res . Board Can. 34:347-353.
351 Keizer , P. D., T. P. Ahern, J. Dale , and J. H. Vandermuelen . 1978a . Residues of Bunker C oil in Chedabucto Bay, Nova Scotia, 6 years after the Arrow spill. J. Fish. Res. Board Can. 35: 528-535 . Keizer , P. D., J. Dale , and D. C. Gordon, Jr . 1978b. Hydrocarbons in sur f ic ial sediments from the Scotian Shelf . Geoch im. Cosmoch im . Acta 42:165-172. Rennicutt, M.D., II. 1980. Particulate and dissolved lipids in seawater . Ph. D. thesis. Texas A&M University, College Station . Klein, A.E., and N. Pilpel. 1974. The effects of artificial sunlight upon floating oils. Water Res . 8: 79-83. Knap, A.H., T.M. I1iffe, and J.N. Butler. 1980. Has the amount of tar on the open ocean changed in the pas t decade ? Mar . Pollut . Bull. 11: 161-164. Knight, G.C., C.H. Walker, D.C. Cabot, and M.P. Harris. 1981. The activity of two hepatic microsomal enzymes in sea birds. Comp. Biochem. Physiol. 68C:127-132. Kodama , K., S . Nakatani , K. Umehara , K . Shimizu , Y. Minoda , and K . Yamada. 1970. Microbial conversion of petro-sulfur compounds. III. Isolation and identification of products from dibenzothiophene. Agr ic. Biol. Chem. 34 :1320-1324. Rodama, R.K., R. Umehara, S. Shimizu, K. Nakatani, Y. Minoda, and K. Yamada. 1973. Identification of microbial products from dibenzothiophene and its proposed oxidation pathway . Agr ic . Biol . Chem. 37:45-50. Koons, C.B., and D.E. Brandon . 1975. Hydrocarbons in water and sediment samples from Coal Oil Point area, offshore California, pp. 513-552. Paper No. 2387. Offshore Technology Conference, Houston, Tex. Koons, C.B., and P.H. Monaghan. 1973. Petroleum derived hydrocarbons in Gulf of Mexico waters. Trans. Gulf Coast Assoc. Geol. Soc. 16: 170-181. Koons, C.B., and J.P. Thomas. 1979. C15 ~ hydrocarbons in the sediments of the New York Bight, pp. 625-628. In Proceedings, 1979 Oil Spill Conference . Amer ican Petroleum Institute, Washington, D.C. Kotyk, A. 1973 . Mechanisms of non-electrolyte transpor t. Biochem. Biophys. Acta 300 :183-210. Kreider, R.E. 1971. Identif~cation of oil leaks and sp~lls. In Proceedings, Joint Conference on Prevention and Control of Oil Spills. American Petroleum Institute, Washington, D.C. Kuhnhold, W.W, D. Everich, J.J. Stegeman, J. Lake, and R.E. Wolke. 1979. Effects of low levels of hydrocarbons on embryonic, larval and adult winter flounder (Pseudopleuronectes americanus), pp. 677-711. In Proceedings of the Conference on Assessment of Ecological Impacts of Oil Spills. NTIS No. AD-A072 859. National Technical Information Service, Spr ingf ield, Va. Kurelec, B., S. Britvic, M. Rijavec, W.E.G. Muller , and R.K. Zahn . 1977 . Benzo (a~pyrene monooxygenase induction in marine f ish--molecular response to oil pollution. Mar . Biol. 44: 211-216 . Laborde, A.L., and D.T. Gibson. 1977. Metabolism of dibenzothiophene by a Beijerinckia species. Appl. Environ. Microbiol. 34:783-790.
352 Lake, J.L., C. Norwood, C. Dimock, and R. Bower. 1979. Origins of polycyclic aromatic hydrocarbons in estuarine sediments. Geochim. Cosmochim. Acta 43:1847-1854. Lamontagne, R.A., J.W. Swinnerton, and V.J. Linnenbom. 1971. Nonequilibrium of carbon monoxide and methane at the air-sea interface. J. Geophys. Res. 76:5117-5121. Lamontagne, R.A., J.W. Swinnerton, V.J. Linnenbom, and W.D. Smith. 1973. Methane concentrations in various marine environments. J. Geophys. Res. 78:5317-S324. Lamontagne, R.A., J.W. Swinnerton, and V.J. Linnenbom. 1974. C1-C4 hydrocarbons in the North and South Pacific. Tellus 16:71-77. Lange, P., and H. Hufner fuss. 1978. Drift response of monomolecular slicks to wave and wind action. J. Phys. Oceanogr. 8. Larson, R.A. and L. L. Hunt. 1978 . Photo-oxidation of a ref. ined petroleum oil: inhibition by 6-carotene and role of singlet oxygen. Photochem. Photobiol. 28: 553-555 . Larson, R.A., L.A. Hunt, and D.W. Blakenship. 1977. Formation of toxic products from a No. 2 fuel oil by photooxidation. Environ. Sci. Technol. 11~5) :492-496. Larson, R.A., T.L. Bott, L.L. Hunt, and K. Rogenmuser. 1979. Photo-oxidation products of a fuel oil and their anti-microbial activity. Environ . Sci. Technol . 13: 965-969 . Law, R.J. 1978. Petroleum hydrocarbon analysis conducted following the wreck of the supertanker Amoco Cadiz. Mar. Pol' ut. Bull. 9: 293-296 . Law, R.J. 1981. Hydrocarbon concentrations in water and sediments from UK marine waters, determ~ned by fluorescence spectroscopy. Ma~. Pollut. Bull. 12:153-157. Lawler, G.C., W.-A. Loong, and J.L. Laseter. 1978a. Accumulation of saturated hydrocarbons in tissues of petroleum-exposed mallard ducks (Anas platyrhynchos). Environ. Sci. Technol. 12:51-50. Lawler, G. C., W-A. Loong, and J. L. Laseter . 1978b . Accumulation of aromatic hydrocarbons in tissues of petroleum-exposed mallard ducks (Anas platyrhynchos). Environ. Sci. Technol. 12:51-54. Lech, J.J., and J.R. Bend. 1980. The relationship between biotransformation and the toxicity and fate of xenobiotic chemicals in fish. Environ. Health Perspect. 35:115. Lech, J.J., M.J. Vodcnik, and C.R. Elcombe. 1981. Induction of monooxygenase activity in fish. In L. Weber, ed. Aquatic Toxicology. Raven Press, New Yor k . Ledet, E.J., and J.L. Laseter. 1974. Alkanes at the air-sea inter face from offshore Louisiana and Flor ida . Science 186: 261-263 . Lee, R.A. 1973. Uptake of petroleum bydrocarbons by marine copepods. Unpublished manuscript. Lee, R.F. 1977a. Accumulation and turnover of petroleum hydrocarbons in mar ine organisms , pp. 60-70 . In D. Wolfe, ed. Fate and Effects of Petroleum Hydrocarbons in Mar ine Organisms and Ecosystems. Pergamon, New York . Lee, R.F. 1977b. Fate of petroleum components in estuarine waters of the Southeastern United States, pp. 611-616. In Proceedings, 1977 Oil Spill Conference. American Petroleum Institute , Washington , D.C.
353 Lee, R. F. 1981. Mixed function oxygenates (MF) in mar ine invertebrates . Mar . Biol . Letts . 2: 87-105 . Lee , R. F., and M. L. Neuhauser . 1976 . Fate of petroleum hydrocarbons taken up from food and water by the blue crab, Cal' inectes sapidus. Mar. Biol. 37 :363-370. Lee, R.F., and C. Ryan. 1976. Biodegradation of petroleum hydrocarbos by marine microbes, pp. 119-126. In J.M. Sharpley and A.M. Kaplan ed. Proceedings of the Third International Biodegradation Symposium. Applied Science Publishers, London. Lee , R. F., and S. C. Singer . 1980 . Detoxifying enzymes system i n mar ine polychaetes. Increases in activity after exposure to aromatic hydrocarbons. Rapp. R.V. Reun. Cons. Int. Explor . Mer 179:29 . Lee , R. F., G. Sauerheber, and A. A. Benson . 1972a. Petroleum hydrocarbons: uptake and discharge by the marine mussel Mytilus edulis. Science 177: 344-346 . Lee , R. F., R. Sauerheber , and G. H. Dobbs . 1972b. Uptake , metabolism, and discharge of polycyclic aromatic hydrocarbons by marine fish. Mar . Biol. 17 :201. Lee , R. F., C. Ryan , and M. L. Nenhauser . 1976 . Fate of petroleum hydrocarbons taken up from food and water by the blue crab Callinectes sapidus. Mar. Biol. 37:363-370. . Lee , R. F., W. S. Gardner , J.W. Anderson, J.W. Blaylock , and J . Barwell-Clarke. 1978. Fate of polycyclic aromatic hydrocarbons in controlled ecosystem enclosures. Environ. Sci. Technol. 12(7) :832-838. Lee, R.F., S.C. Singer, K.R. Tenor e, W.S. Ga~dner, and R.M. Philpot. 1979. Detoxification system in polychaete worms: importance in the degradation of sediment hydrocarbons, pp. 23-37. In W.B. Vernberg, F.P. Thurberg, A. Calabrese, and F.J. Vernberg, eds. Pollution and Physiology of Marine Organisms. Academic Press, New York. Lee, R.F., J. Stolzenbach, S. Singer, and K.R. Tenore. 1981a. Effects of crude oil on growth and mixed-function oxygenase activity in polychaetes, Nere~s sp., pp. 323-334. In F.J. Vernberg, A. Calabrese, F.P. Thurberg, and W.B. Vernberg, eds. Biological Mon~tor ing of Mar ine Organisms . Academic Press, New York . Lee , R. F ., B . Dornse i£ , F . Gonsoul in , K . Tenor e , and R. Hanson. 1981b. Fate and effects of a heavy fuel oil spill on a Georgias alt marsh. Mar . Environ. Res. 5: 12S-43. Lee, R.F., S.C. Singer, and D.S. Page. 1982. Responses of cytochrome P-450 systems in marine crabs and polychaetes to organic pollutants. Aquat. Toxicol. In press. Leinonen, P.J., D. Mackay, and C.R. Phillips. 1971. A correlation for the solubility of hydrocarbons in water. Can. J. Chem. Eng. 49:747-752. LePetit, J., and M.-H. N'Guyen . 1976 . Besoins en phosphore des bacter ies metabolisant les hydrocarbures en mer . Can. J . Microbiol. 22: 1364-1373 . Levy, E.M. 1971. The presence of petroleum residues off the coast of Nova Scotia, in the Gulf of St. Lawrence and the St. Lawrence River. Water Res. 5:723-733.
354 Levy, E.M. 1972. Evidence for the recovery of waters off the coast of Nova Scotia from the effects of a major oil spill. Water Air Soil Pollut. 1: 144-148 . Levy, E.M. 1977. The geographical distribution of tar in the North Atlantic. Rapp. P.-v. Reun. Cons. Int. Explor. Mer 171:55-60. Levy, E.M., and A. Walton. 1973. Dispersed and particulate petroleum residues in the Gulf of St. Lawrence. J. Fish. Res. Board Can. 30: 261-267. Levy, E.M., M. Ehrhardt, K. Kohnke, E. Sobtchenko, T. Suxuoki, and A. Tokuhiro. 1981. Global oil pollution. Results of MAPMOPO, the IGOSS pilot project on marine pollution (petroleum) monitoring. Intergovermental Oceanographic Commission, UNESCO, Par i s. Light, M. 1977. Report on Gulf of Mexico 1976 fall cruise (DWP Oceano, FY 77-1) CTC Acushnet. U. S. Coast Guard, Groton, Conn . Liss , P. S., and P.G. Slater . 1974. Flux of gases across the air-sea interface. Nature 247 :181-184. Long, B.F.N., J.H. Vandermsulen, and T.P. Ahern. 1981. The evolution of stranded of] within sandy beaches, pp. S19-524. In Proceedings, 1981 Oil Spill Conference . Amer ican Petroleum Institute, Wasington, D.C. Lysyj, I ., and E. C. Russell . 1974 . Dissolutzon of petroleum-der ived products in water . Water Res . 8: 863-868 . Lysyj, I., G. Perkins, J.S. Farlow, and R.W. Morris. 1981. Distribution of aromatic hydrocarbons in Port Valdez, Alaska, pp. 4 7-54 . In Proceedings, 1981 Oil Spill Conference . Amer ican Petroleum Institute, Washington, D. C . Lytle , T. F., and J. S. Lytle . 1979 . Sediment hydrocarbons near an oil r ig . Estuar ine Coastal Mar . Sci . 9: 319-330 . MacDonald, R.W. 1976 . The distr ibution of low-molecular-weight hydrocarbons in the southern Beaufor t Sea. Environ . Sci . Technol . 10: 1241-1246 . MacGregor, C., and A. Y. McLean . 1977 . Fate of crude oil spilled in a simulated arctic environment, pp. 461-463. In Proceedings, 1977 Oil Spill Conference. Publication 4284. Amer ican Petroleum Institute, Washington, D.C. Mackay, D., and P.J. Leionen. 1975. The rate of evaporation of low solubility contaminants from water bodies. Environ. Sci. Technol. 9: 1178-1180 . Mackay, D., and S. Paterson. 1981. A mathematical model of oil spill behavior . Report EE7 . Department of Env' ronment, EPS, Ottawa, On tar io. Mackay, D., and W.Y. Shiu. 197S. The aqueous solubility of weathered crude oils. Bull. Environ. Contamin. and Toxicol. 15:101. Mackay, D., I. Buist, R. Mascarenhas , and S. Paterson. 1979. Experimental studies of dispersion and emulsion formation from oil slicks, pp. 1.17-1.40. In Workshop on the Physical Behavior of Oil in the Mar ine Environment, Pr inceton University. Prepared for the National Weather Service, Silver Spr ing, Md. Mackay , D., I . Buist, R. Mascarenhas , and S. Paterson . 1980a. Oil spill processes and models. Report submitted to Environmental Emergency
355 Branch, Environmental Impact Control Directorate, Environment Protection Service , Environment Canada. Ottawa, Ontario K1A 1C8. Mackay, D., S. Paterson, and S. Nadeau. 1980b. Calculation of the evaporation rate of volatile liquids, p. 361. In Proceedings of the National Conference on Control of Hazardous Mater ial Spills, Louisville, Ky. Mackay, D., I. Buist, R. Mascarenhas and S. Paterson. 1981a. Oil spill processes and models. Report EE8. Department of Environment, Environment Protection Service, Ottawa, Ontar lo K1A 1C8 . Mackay , D ., S . Pater son , P. . D . Boehm , and D. L . Fe ist . 198 1b . Physical-chemical weathering of petroleum hydrocarbons from the Ixtoc 1 blowout--chemical measurements and a weathering model. In Proceedings, 1981 Oil Spill Conference. American Petroleum Institute, Washington, D. C. Mackay, G.C.M., A.Y. McLean, O.J. Betancourt, and B.C. Johnson. 1973. The formation of water-in-oil emulsions subsequent to an oil spill. V. J. Inst. Petroleum 59~5681:164-172. Mackie, P.R., K.J. Whittle, and R. Hardy. 1974. Hydrocarbons in the marine environment. I. n-alkanes in the Firth of Clyde. Estuarine Coastal Mar. Sc'. 2:359-374. Mackie, P.R., R. Hardy, K.J. Whittle, D.V.P. Conway, and A.D. McIntyre. 1976. Relationship between hydrocarbons, chlorophyll, and particulate carbon in the area between Firth and Forth and the Fortes oil field. ICES CM Fish. Improvement Comm. 1976/E:42. International Council for the Exploration of the Seas, Charlottenlund, Denmark. Mackie, P.R., H.M. Platt, and R. Hardy. 1978 . Hydrocarbons in the mar ine environment . II . Distr ibution of n-alkanes in the fauna and environment of the sub-antarctic island of South Georgia. Estuar ine Coastal Mar . Sci . 6: 301-313 . MacLeod, W. D., D.W. Brown, R. G. Jenk ins , and L. S. Ramos . 1977 . Intertidal sediment hydrocarbon levels at two sites on the Strait of Juan de Fuca, pp. 385-396 . In D.A. Wolfe, ed. Fate and Effects of Petroleum Hydrocarbons in Mar ine Ecosystems and Organisms . Pergamon, New York. Flalins, D.C., ed. 1977. Effects of Petroleum on Arctic and Subarctic Mar ine En~rironments and Organisms . Vol . 1, Nature and Fate of Petroleum. Academic Press, New Yor k . Mannering, G.J. 1981. Hepatic cytochrome P-450 linked drug-metabolizing systems, pp . 53-166 . In P . Jenner and B . Testa, eds . Par t B. Concepts in Drug Metabolism. Marcel Dekker, New York. Mantoura, R.F., P.M. Gschwend, OeCe Zafiriou, and K.R. Clark. 1982. Volatile organic compound at a coastal site : ill, short-term var. iations . Environ. Sci . Technol. 16: 38-45 . Markovetz, A. J. 1971. Subterm, nal oxidation of aliphatic hydrocarbons by microorganisms. Crit. Rev. Microbiol. L: 225-237. Marty, J. C., and A. Saliot. 1976. Hydrocarbons (normal alkanes ~ in the surface microlayer of seawater. Deep Sea Res. 23:863-873. Masters, M.J., and J.E. Zajic. 1971. Myxotrophic growth of algae on hydrocarbon substrates. Dev. Ind. Microbiol. 12: 77-86 .
356 Mayo, D.W., D.S. Page, J. Cooley, E. Sorenson, F. Bradley, E.S. Gilfillan, and S.A. Hanson. 1978. Weathering characteristics of petroleum hydrocarbons deposited in fine clay sediments, Searsport, Maine. J. Fish. Res. Board Can. 35:552-562. McAuliffe, C.D. 1977. Dispersal and alteration of oil discharged on a water surface. Chap. 3. In P.D.A. Wolfe, ed. Fate and Effects of Petroleum Hydrocarbons in Marine Organisms and Ecosystems. Pergamon, New York. McAuliffe, C.D., J.C. Johnson, S.H. Greene, G.P. Canevari, and T.D. Searl. 1980. Dispersion and weathering of chemically treated crude oils in the oceans. Environ. Sci. Technol. 14:1509-1518. McGowan, W.E., W.A. Saner, and G.L. Hufford. 1974. Tar ball sampling in the western N. Atlantic, pp. 83-84. In Marine Pollution Monitoring (Petroleum). NBS Special Publication 409. National Bureau of Standards, Gaithersburg, Md. McRenna, E.J. 1971. Microbial degradation of normal and branched alkanes, pp. 73-97. In Degradation of Synthetic Organic Molecules in the Biosphere. National Academy of Sc fences , Wash ington , D. C . McLean, A.Y., and O.J. Betancourt. 1973. Physical and Chemical changes in spilled oil weathering under natural conditions pp. 1249-1256 Paper No. 0TC-1784. Offshore Technology Conference, Houston, Tex. McMaster, R.L. 1960. Sediments of Narragansett Bay system and Rhode Island Sound, Rhode Island . J. Sediment Petrol. 30:249-274. Means, J.C., J.J. Hassett, S.G. Wood, and W.L. Banwart. 1979. Sorption properties of energy-related pollutants and sed~ments, p. 327. In P.W. Jones and P. Leber eds. Polynuclear Aromatic Hydrocarbons. Ann Arbor Science Publishers, Ann Arbor, MiCh. Melancon, M.J., and J.J. Lech. 1978. Distribution and elimination of naphthalene and 2-methylnaphthalene in rainbow trout dur~ng short- and long-term exposures. Arch. Environ. Contam. Toxicol. 7:207-220. Melancon, M.J., Jr., and J.J. Lech. 1979. Uptake, biotransformation, d~sposition, and elimination of 2-methylnaphthalene and naphthalene in several fish specm s, pp. 5-22. In L.L. Marking and R.A. Kimerele eds. Aquatic Toxicology. ASTM STP 667. American Society for Testing and Materials, Philadelphia, Pa. Meyers, P.A., and T.G. Oas. 1978. Comparison of associations of different hydrocarbons with clay particles in simulated seawater. Environ. Sci . Technol . 12: 934-937 . Meyers, P.A., and J.G. Quinn. 1973. Association of hydrocarbons and mineral particles in saline solution. Nature 244:23-24. Middleditch, B.S., B. Basile, and E.S. Chang. 1977. Environmental effects of offshore oil production: alkanes in the region of the Buccaneer oil field. J. Chromatogr. 142:777-785. Middleditch, B.S., E.S. Chang, and B. Basile. 1979a. Alkanes in plankton from the Buccaneer oil field. Bull Environ. Con tam. Toxicol. 21:421-427. Middleditch, B.S., E.S. Chang, B. Basile, and S.R. Missler. 1979b. Alkanes in fish from the Buccaneer oil field. Bull. Environ. Contam. Toxicol. 22:249-257.
357 Mill, T., D.G. Hendry, and H. Richardson. 1980. Free radical oxidants in natural waters. Science 207:886-887. Monaghan, P.H., J.H. Seelinger, and R.A. Brown. 1973. The persistent hydrocarbon content of the sea along certain tanker routes. Preliminary report presented at API Tanker Conference, Hilton Head Island, S.C., May 7-9, 1973. Morris, B.F. 1971. Petroleum: tar quantities floating in the northwestern Atlantic taken with a new quantitative neuston net. Scm nce 173:430-432. Morris, B.F. 1974. Lipid composition of surface films and zooplankton from the eastern Mediterranean. Mar. Pollut. Bull. 5:105-109. Morris, B.F., and J.N. Butler. 1973. Petroleum residues in the Sargasso Sea and on Bermuda beaches, pp. 521-530. In Proceedings, Joint Conference on Prevention and Control of Oil Spills. American Petroleum Institute, Washington, D. C. Morr is , B. F., and J. N. Butler . 1975 . Pelagic tar in the Mediterranean Sea 1974-1975. Environ. Conserv. 2: 275-281. Morris, B.F., and F. Culkin. 1974. Lipid chemistry of eastern Mediterranean surface layers. Nature 250: 640-642. Morris, BeFe ~ J. Cadwallader, J. Geiselman, and J.N. Butler. 1976. Transfer of petroleum and biogenic hydrocarbons in the Sargasso community, pp. 235-259. In H.L. Windom and R.A. Duce, eds. Marine Pollutant Transfer . Lexington Books, Lexington, Mass . Mulkins-Phillips, G.J., andJ.E. Stewart. 1974. Effect of en~rironmental parameters on bacterial degradation of Bunker C oil, crude oils and hydrocarbons. Appl. Microbiol. 28:915-922. Nagata , S., and G. Kondo. 1977 . Photo~oxidation of crude oils . In Proceedings, 1977 Oil Spill Conference. American Petroleum Institute, Washington, D.C. Nakatani, S., T. Akasaki, K. Kodama, Y. Minoda, and K. Yamada. 1968. Microbial conversion of petro-sulfur compounds. II. Culture conditions of dibenzothiophene-utilizing bacter ia. Agr ic. Biol. Chem. 32: 120S-1211. National Research Council. 1972. Particulate Polycyclic Organic Matter. Washington, D. C . National Research Councz1. 1975 . Pollution in the Mar ine Environment. National Academy of Sciences, Washington, D. C . Neff, J.M. 1979. Polycyclic Aro~natic Hydrocarbons in the Aquatic Environment: Sources, Fates and Biologi cal Effects. Applied Science Publishers, London. Neff, J.M., and J.W. Anderson. 1975. Accumulation, release and distribution of benzotalpyrene 14C in the clam Rangia cuneata, pp. 469-471. In Proceedings, 1975 Conference on Prevention and Control of Oil Pollution. American Petroleum Institute, Washington, D.C. Neff , J.M., B.A. Cox, D. Dixit, and J.W. Anderson. 1976 . Accumulation and release of petroleum-der ived aromatic hydrocarbons by four species of mar ine animals. Mar . Biol . 38: 279-289 . Nonhebel, D.C., and J.C. Walton. 1974. Free Radical Chemistry. Cambridge University Press, New York. 572 pp.
358 Nunes, P., and P.E. Benville. 1979. Uptake and deputation of petroleum hydrocarbons in the Manila clam, Tapes semidecussata Reeve. Bull. Environ. Con tam. Toxicol. 21:719-726. Oppenheimer, C.H., W. Gunkel, and G. Gassman. 1977. Microorganisms and hydrocarbons in the North Sea during July-August 1975, pp. 593-610. In Proceedings, 1977 Oil Spill Conference. American Petroleum Institute, Washington, D.C. Overton, E. B., J. R. Patel , and J. L. Laseter . 1979 . Chemical character ization of mousse and selected environmental samples from the Amoco Cadiz oil spill. In Proceedings, 1979 Oil Spill Conference. American Petroleum Institute, Washington, D.C. Overton, E.B., J.L. Laseter, W. Mascarella, C. Rashke, I. Noiry, and J.W. Far r ington. 1980. Photochemical Oxidation of Ixtoc I Oil. Researcher/Pierce Ixtoc I Symposium. Page, D.S., D.W. Mayo, J.F. Cooley, E. Sorenson, E.S. Gilfillan, and S.A. Hanson. 1979. Hydrocarbon distribution and weathering characteristics at a tropical oil spill site, pp. 709-712. In Proceedings, 1979 Oil Spill Conference. American Petroleum Institute, Washington, D.C. Palmork, K.H., and J.E. Solbakken. 1981. Distribution and elimination of (9-14C) phenanthrene in the horse mussel (Modiola modiolus). Bull. Environ. Contam. Toxicol. 26:196-201. Parekh, V.C., R.W. Traxler, and J.M. Sobek. 1977. n-Alkane oxidation enzymes of a Pseudomonad. Appl. Environ. Microbiol. 33:881-884. Parker, P.L., J.K. Winters, and J. Mor tan. 1972. A Base-Line Study of Petroleum in the Gulf of Mexico, pp. 555-581. In Base-Line Studies of Pollutants in the Mar ine Environment (Heavy Metals, Halogenated Hydrocarbons and Petroleum) . National Science Foundation, IDOE, Washington, D.C. Patel, J.R., J.A. McFall, G.W. Griffin, and J.L. Laseter. 1978. Toxic photo-oxygenated products generated under environmental conditions from phenanthrene. Paper presented at the EPA. SyTnposium on Carcinogenic Polynuclear Aromatic Hydrocarbons in the Mar ine Environment. Pensacola Beach, Fla., August ~ 4-18, 1978. Patel, J.R., E.B. Overton, and J.L. Laseter. 1979. Environmenta' photo-oxidation of dibenzothiophenes following the Amoco Cadiz oil spill . Chemosphere 8: 557-561 . Patton, J.S., M.W. Rigler, P.D. Boehm, and D.L. Fiest. 1981. Ixtoc 1 oil spill: flaking of surface mousse in the Gulf of Mexico. Nature 290: 23S-238 . Payne, J . 1984 . Petroleum Spills in the Mar ine Env i ronment. Butterwor th Publishers, Woburn, Mass. (in press). Payne, J.F. 1976. Field evaluatzon of benzota~pyrene hydroxylase induction as a monitor for mar ine pollution. Science 191:945-946. Payne, J.F., and W.R. Penrose. 1975. Induction of aryl hydrocarbon hydroxylase in f ish by petroleum. Bull. Environ. Contam. Toxicol . 14: 112-116 . Payne, J.R., N.W. Flynn, P.J. Mankiewicz, and G.S. Smith. 1980. Surface evaporation/dissolution partitioning of lower-molecular- weight aromatic hydrocarbons in a down-plume transect from the
359 Ixtoc ~ wellhead, pp. 239-266. In Proceedings of the Conference on the Preliminary Scientific Results from the Researcher/Pierce Cruise to the Ixtoc I Blowout. NOAA, Office of Marine Pollution Assessment, Rock~ille, Md. Peakall, D.B., and D.J. Hallett et al. 1981. Toxicity of Prudhoe Bay Crude Oil and its Aromatic Fractions to Nestling Herring Gulls. (in press). Pequegnat. 1979. Pelagic tar concentrations in the Gulf of Mexico over the south Texas continental shelf. Contrib. Mar. Sci. 22:31-39. Perry, J.J. 1977. Microbial metabolism of cyclic hydrocarbons and related compounds. Crit. Rev. Microbiol. 5:387-412. Perry, J.J. 1979. Microbial cooxidations involving hydrocarbons. Microbiol. Rev. 43:59-72. Pirnik, M.P. 1977. Microbial oxidation of methyl branched alkanes. Crit. Rev. Microbiol. 5:413-422. Plack, P.A., E.R. Skinner, A. Rogie, and A.I. Mitchell. 1979. Distr ibution of DDT between lipoproteins of trout serum. Comp . Biochem. Physiol . 62C: 119-126 . Platt, H.M., and P.R. Mackie. 1980 . Distr ibution and fate of aliphatic and aromat~c hydrocarbons in antarctic fauna and environment. Elelogolander Meeresunters. 33: 236-245 . Pohl , R. J., J. R. Bend, A.M. Guar ino , and J. R. Fouts . 1974. Hepatic microsomal mixed-function oxidase activity of several mar ine species from coastal Maine. Drug Metab. Dis . 2: 545-555 . Polikarpov, G.G., V.N. Yegorov, V.N. Ivanov, A.V. Tokareva, and. I.A. Felepov. 1971. Oil areas as an ecological niche. Pr iroda 11 [transl. by N. Precoda], Pollut. Abstr. 3:72. Rathledge, C. 1978. Degradation of aliphatic hydrocarbons, pp. 1-46. In J . R. Watk inson ed . Developments in Biodegr adation of Hydrocarbons Vol. 1. Applied Science Publishers, London. Reed, W.E., I.R. Kaplan, M. Sandstrom, and P. Mankiewicz. 1977. Petroleum and anthropogenic influence on the composition of sediments from the Southern California Bight, pp. 183-188. In Proceedings, 1977 Oil Spill Conference. American Petroleum Institute, Washington, D.C. Reisfeld, A., E. Rosenberg , and D. Gutnick . 1972 . Microbial degradation of o'1: factors affecting oil dispersion in seawater by mixed and pure cultures. Appl. Microbiol. 24: 363-368 . Reitsema, R.J., F.A. Lindberg, and A.J. Kaltenback . 1978. Light hydrocarbons in Gulf of Mexico waters: sources and relation to structural highs. J. Geochem. Explor . 10 :139-151. Robertson, B., S. Arhelger, P.J. Kinney, and D.K. Button. 1973. Hydrocarbon biodegradation in Alaskan waters, pp. 171-184. In D.G. Ahearn and S. P. Meyers, eds . The Microbial Degradation of Oil Pollutants. Publication LSU-SG-73-01. Center for Wetland Resources, Louisiana State University, Baton Rouge. Roesi jadi, G., J.W. Anderson, and J.W. Blaylock. 1978. Uptake of hydrocarbons from marine sediments contaminated with Prudhoe Bay crude oil: influence of feeding type of test species and availability of polycyclic aromatic hydrocarbons. J. FiSh. Res. Board Can. 35:608:614.
360 Rossi, S.S. 1977. Bioavailability of petroleum hydrocarbons from water, sediments and detritus to the marine annelid Neanthes arenaceodentata, pp. 621-625. In Proceedings, 1977 Oil Spill Conference. American Petroleum Institute, Washington, D.C. Rossi, S. S., and J.W. Anderson . 1977 . Accumulation and release of fuel-oil der ived diaromatic hydrocarbons by the polychaete Neanthes arenaceodentata . Mar . Bill. 39: 51-55 . Roubal, W. T. 1974 . Spin-labeling of living tissue--a method for investigating pollutant-host interaction, pp. 367-379. In F. Vernberg and W. Verberg eds. Pollution and Physiology of Marine Organisms. Academic Press, New York. Roubal, W.T., T.K. Collier, and D.C. Malins. 1977. Accumulation and metabolism of cation-14 labeled benzene, naphthalene, and anthracene by young coho salmon (Oncorhynchus kisutch). Arch. Environ. Contam. Toxicol . 5: 513-529 . Sackett, W.M. 1977. Use of hydrocarbon sniffing in offshore exploration. J. Geochem. Explor . 7: 243-254 . Sackett, W.M., and J.M. Brooks. 1974. Use of low-molecular-we~ght hydrocarbon concentrations as indicators of mar ine pollution, pp . 171-173. In Marine Pollution Monitoring (Petroleum). Special Publ' cation 409. National Bureau of Standards, Gaithersburg, Md . Sackett, W.M., and J.M. Brooks. 1975. Origin and distribution of low-molecular-weight hydrocarbons in Gulf of Mexico coastal waters, pp. 211-230 . In T.M. Church, ed. Mar ine Chemistry in the Coastal Environment. ACS Symposium Series 18. American Chemical Society, Washington, D. C. Sanborn, H. R., and D. C. Malins . 1980 . The disposition of aromatic hydrocarbons in adult spot shr imp (Pandalus platyceros ~ and the formation of metabol ites of naphthalene in adult and larval spot shrimp. Xenobiotica 10: 193-200 . Sanders, H.L., J.F. Grassle, G.R. Hampson, L.S. Morse, S. Garner-Price, and C.C. Jones. 1980. Anatomy of an oil spill: long-term effects from the grounding of the barge Florida off West Falmouth, Massachusetts . J. Mar . Res . 38: 265-380 . San Gupta, R., S.Z. Qasim, S.P. Fondekar, and R.S. Topgi. 1980. Dissolved petroleum hydrocarbons in some reg~ons of the northern Indian Ocean. Mar. Pollut. Bull. 11:164-174. Sauer, T.C., Jr. 1978. Volatile liquid hydrocarbons in the mar~ne environment. Ph.D. thesis. Texas A&M University, College Station. Sauer, T.C., Jr. 1980. Volatile liquid hydrocarbons in water of the Gulf of Mexico and Caribbean Sea. Limnol. Oceanogr. 25:338-351. Sauer, T.C., Jr. 1981a. Volatile organic compounds in open ocean and coastal surface waters. Organ. Geochem. 3:91-101. Sauer, T.C. 1981b. Volatile liquid hydrocarbons characterization of underwater hydrocarbon vents and formation waters from offshore production operations. Environ. Scz. Technol . 15 : 917-923 . Sauer, T.C., Jr., and W.M. Sackett. 1980. Gaseous and volatile hydrocarbons in marine environments with emphasis on the Gulf of Mexico, pp. 133-161. In R.A. Geyer, ed. Marine Environment Pollution Vol. I., Hydrocarbons. Elsevier, New York.
361 Sauer , T.C., Jr ., W.M. Sackett, and L.M. Jeffrey. 1978. Volatile liquid hydrocarbons in the surface coastal waters of the Gulf of Mexico. Mar . Chem. 7: 1-16 . Sayler, G.S., and R.R. Colwell. 1976. Partitioning of mercury and chlor inated biphenyl by oil, water and sediment. Environ. Sci . Technc,1 . 10: 1142-1145 . Schnell, J.V., E.H. Gruger, Jr., and D.C. Malins. 1980. Monooxygenase activities of coho salmon (Oncorhynchus kisutch) liver microsomes using three polycyclic aromatic hydrocarbon substrates. Xenobiotica 10: 22 9-23 4 . Schultz, D.M., and J.G. Quinn. 1977. Suspended material in Narragansett Bay: fatty acid and hydrocarbon composition. Organ. Geochem. 1: 27-36 . Schwarz , J. R., J. D. Walker , and R. R. Colwell. 1974a ~ Hydrocarbon degradation at ambient and in situ pressure. Appl. Microbiol. 28: 982-986 . Schwar z , J. R., J. D. Walker , and R. R. Colwell . 1974b. Growth of deep-sea bacter ia on hydrocarbons at ambient and in situ pressure . Dev . Ind. Microbiol. 15: 239-249 . Schwarz, J.R., J.D. Walker , and R.R. Colwell. 1975. Deep-sea bacteria: growth and utilization on n-hexadecane at in si tu temperature and pressure. Can. J. Microbiol. 21: 682-687 . Schwar zenbach , R. P ., R. H. Bromund , P. M. Gschwen , and O. C . Zaf ir iou . 1979. Volatile organic compounds in coastal seawater: preliminary results. J. Organ. Geochem. 1: 45-61. Scranton, M. I., and P.G. Brewer . 1977 . Occurrence of methane in the near-surface waters of the western subtrop~cal North Atlantic. Deep Sea Res . 24 :127-138 . Scranton, M. I., and P.G. Brewer . 1978 . Consumption of dissolved methane in the deep ocean. Limnol. Oceanogr. 23:1207-1213. Scranton , M. I ., and J .W. Farr ington . 1977 . Methane production in the waters of Walvis Bay. J. Geophys. Res . 82 :4947-4953 . Seba, D.B., and E.F. Corcor an. 1969. Surface slicks as concentrators of pesticides in the mar ine environment. Pesticide Monit. J. 3: 190-193 . Seiler, W., and V. Schmidt. 1974. Dissolved non-conservative gases in seawater, pp . 2 19-243 . In E. D. Goldberg, ed . The Sea . Vol. . V. Wiley Intersczence, New York. Seki, H. 1976. Method for estimating the decomposition of hexadecane in the mar ine environment. Appl . Environ . Microbiol . 31: 439-441. Senez, J.C., and E e Azoulay. 1961. Dehydrogenation of paraf f inic bydrocarbons by resting cells and cell free extracts of Pseudomonas aeruginosa. Biochim. Biophys. Acta 47: 307-316 . Seubert, We J and E. Fasse 1964e Untersuchugen uber den bakteriellen Abban von Isoprenoidene Ve Der Mechanismns des Isoprenoidabbanes. Biochem. Z. 341:35-44. Shackelford, M.E., and M.A.Q. Khan. 1981. Hepatic mixed-function oxidase of the mallard duck (Anas platyrhychos). Comp. Biochem. Physiol. 70C.
362 Shaw, D.G., and B.A. Baker. 1978. Hydrocarbons in the marine environment of Port Valdez, Alaska. Environ. Sci. Technol. 12:1200-1205. Shaw, D.G., and J.N. Wiggs. 1979. Hydrocarbons in Alaskan intertidal algae. Phytochem. 18:2025-2027. Shaw, D.G., and J.N. Wiggs. 1980. Hydrocarbons in the intertidal environment of Kachemak Bay, Alaska. Mar. Pollut. Bull. 11:297-300. Sherman, K., J.B. Colton, R.L. Dryfoos, and B.S. Kinnear. 1973. Oil and plastic contamination and fish larvae in surface waters of the northeast Atlantic. MARMAP Operational Test Survey Report: July-August 1972, January-March 1973. Sherman, K., J.B. Colton, R.L. Dryfoos, K.D. Knapp, and B.S. Kinnear. 1974. Distribution of tar balls and nueston sampling in the Gulf Stream system, pp. 83-84. In Marine Pollution Monitoring (Petroleum). Special Publication 409. National Bureau of Standards, Gaithersburg, Md. Shokes, R. , P. Mankind was, R. Sims, M. Guttman, R. Jordan, J. Nemmers, and J . Payne . 1979a . Geochemical basel ine study of the Texoma offshore br ine disposal s ite: Big Hill, fall 1977-Sept . 1978 . Report SAI-012-79-834-LJ. Shokes , R., P . Mank iewicz , R. S ims , M. Guttman , R. Jordan , J. Nemmer s , and J. Payne. 1979b. Geochemical baseline study of the Texoma offshore brine disposal sites: West Hackberry, fall 1977-spring 1978. Report SAI-012-79-835-LJ. Singer, S.C., and R.F. Lee. 1977. Mixed function oxygenase activity in blue crab, Callinectes sapidus: tissue distribution and correlation with changes during molting and development. Biol. Bull. 153:377-386. Singer , S.C., P.E. March, F. Gonsoulin, and R.F. Lee, 1979. Mixed function oxygenase activity in the blue crab, Callinectes sapidus: characterization of enzyme activity from stomach tissue. Comp. Biochem. Physiol. 65C:129. Sleeter, T.D., B.F. Morris, and J.N. Butler. 1974. Quantitative sampling of pelagic tar in the North Atlantic. 1973. Deep Sea Res. 21:773-775. Sleeter, T.D. r B.F. Morris, and J.N. Butler. 1976. Pelagic tar in the Caribbean and Equator ial Atlantic. 1974. Deep Sea Res. 23:467-474. Sleeter, T.D., J.N. Butler, and J.E. Barbash. 1979 . Hydrocarbons in sediments from the edge of the Bermuda Platform, pp. 615-620. In Proceedings, 1979 Oil Sp'l1 Conference. Amer ican Petroleum Institute, Washington, D. C . Smith, G.B. 1976. Pelagic tar ~n the Norwegian coastal current. Mar. Pollut. Bull. 7:70-72. Smith, G.L. 1977. Determination of the leeway of oil slicks, p. 351. In D.A. Wolfe, ed. Fate and Effects of Petroleum Hydrocarbons in Marine Ecosystems and Organisms. Pergamon, New York. Solbakken, J.E., K.H. Palmork, T. Neppelberg, and R.R. Scheline. 1980. Urinary and biliary metabolites of phenanthrene in the coalfish (Pollachius virens). Acta Pharmacol. Toxicol. 46:127.
363 Spaulding , M. L., P. Cornillon , and M. Reed . 1978 . Modelling oil spill fates and i nteractions with f isher ies, pp. 29-34. In D. Mackay and S. Paterson eds. Oil Spill Modelling: Proceedings of a Workshop. Publication EE-12. University of Toronto, Institute for Env ironmental Stud ies . Sprague , J. B ., J. H. Vandermeolen , and P . G. Wells eds . 1981. Oil and Dispersants in Canadian Seas--Research Appraisal and Recommendations. Environment Canada. Ottawa, Ontario. Stainken, D. 1977. The accumulation and deputation of No. 2 fuel oil by the soft shell clam {Mya arenaria) L., pp. 313-322. In D.A. Wolfe, ed. Fate and Effects of Petroleum Hydrocarbons in Marine Organisms and Ecosystems. Pergamon, New York. Statham, C.N., M.J. Melacon, Jr., and J.J tech. 1976. Bioconcentrntion of xenobiotics in trout bile: a proposed monitoring aid for some water-borne chemicals. Science 193:680-681. Statham, C.N., C.R. Elcombe, S.P. Szyjka, and J.J. Lech. 1978. Effect of polycycl~c aromatic hydrocarbons on aepatic microsomal enzymes and disposition of methyloaphthalene in rainbow trout in vivo. Xenobiotica 8:65-71. Stegeman, J.J. 1977. Fate and effects of oil in marine animals. Oceanus 20:59. Stegeman, J.J. 1978. Influence of environmental contamination on cytochrome P-450 mixed-function oxygenates in fish: implications for recovery in the Wild Harbor Marsh. J. Fish. Res. Board Can. 35:668. Stegeman, J.J. 1979. Temperature influence on basal activity and induction of mixed-function oxygenate activity in Fundulus heteroclitus. J. Fish. Res. Board Can. 36:1400-1405. Stegeman, J.J. 1981. Polynuclear aromatic hydrocarbons and their metabolism in the marine environment, pp. 1-60. In H.V. Gelboin and P.O.P. Ts'O eds. Polycyclic Hydrocarbons and Cancer. Vol. 3. Academic Press, New York. Stegeman, J.J., and M. Chevion. 1980. Sex differences in cytochrome P-450 and mixed-funotion o~genase activity in gonadally mature trout. Biochem. Pharmacol . 28: 554-559 . _ , ~ . . . Stegeman, J.J., and H.B. Kaplan. 1981. Mixed-function oxygenate activity and bench (a) Irene metabc~linm in the barnacle Rabanus: eburneus (Crusteacea. Cirrioedia). Come. Biochem. PhYsiol. 68C:55. Stegeman, J.J., and J.H. Teal. 1973. Accumulation, release and retention of petroleum hydrocarbons by the oyster, Crassostrea v~rginica. Mar . Biol . 22: 37-44. Stegeman, J. J., R. L. Binder, and A. Orren . 1979 . Hepatic and extrahepatic microsomal electron transport components and mixed-function oxygenates in the mar ine f ish Stenotomus firer sicolor . Biochem. Pharmacol . 28: 3431-3439 . Stegeman, J.J., A.V. KlOtz, B.R. Wooden, and A.M. Pajor. 1981. Induction of hepatic cytochrome P-450 in fish and the indication of environmental induction in soup (Stenotomus chrysops). Aquat. Toxicol. 1: (in press) .
364 Stegeman, J.J., T.R. Shopek, and W.G. Whilly. 1982. Bioactivation of polynuclear aromatic hydrocarbons to cytotoxic and mutagenic products by marines fish, pp. 201-211. In N. Richards and B.L. Jackson, eds. Carcinogenic Polynuclear Aromatic Hydrocarbons in the Marine Environment. Rep. EPA 600-/9-82-003. U.S. Environmental Protection Agency, Washington, D. C e Sutcliffe, W. H., E. R. Baylor, and D.W. Menzel. 1963. Sea surface chemistry and Langmoir circulation. Deep Sea Res. 10: 233-243. Swinnerton, J.W., and R.A. Lamontagne. 1974. Oceanic distribution of low-molecular-weight hydrocarbons: baseline measurement. Environ. Sci. Technol . 8: 657-663. Tait, R.V., and R.S. De Santo. 1972. Elements of marine ecology. Spr inger, New Yor k . Teal, J.M. 1976. Hydrocarbons uptake by deep-sea benthos, pp 358-371. In Proceedings of Symposium on Sources, Effects and Sinks of Hydrocarbon in the Aquatic Environment. Amer ican University, Washington, D. C. Teal, J.M., K. Burns, and J. Farrington. 1978. Analyses of aromatic hydrocarbons in inter tidal sediments resulting from two spills of No. 2 fuel oil in Buzzards Bay, Massachusetts. J. Fish. Res. Board Can. 35:510-520. Thomas, R.E., and S.D. Rice. 1981. Excretion of aromatic hydrocarbons and their metabolites by freshwater and saltwater Dolly Varden char, pp. 425-448. In F.J. Vernberg, F.P. Thurberg, A. Calabrese, and W.B. Vernberg, eds. Biological Monitoring of Marine Pollutants. Academic Press, New York. Thompson, S., and G. Eglinton. 1978. Composition and sources of pollutant hydrocarbons in the Severn Estuary. Mar. Pollut. Bull. 9:133-136. Traxler, R.W., and J.M. Bernard. 1969. The utilization of n-alkanes by Pseudomonas aeruginosa under conditions of anaerobiosis. Int. Biodeterior. Bull. 5:21-25. Traxler, R.W., P.R. Proteau, and R.N. Traxler. 1965. Action of microorganisms on bituminous materials. I. Effect of bacteria on asphalt viscosit. Appl. Microbiol. 13:838-841. Tripp, B.W., J.W. Farrington, and J.M. Teal. 1981. Unburned coal as a source of hydrocarbons in surface sediments. Mar. Pollut. Bull. 12:122-126. Trudgill, P.W. 1978. Microbial degradation of alicyclic hydrocarbons, pp. 47-84. In J.R. Watkinson ed. Developments in Biodegradation of Hydrocarbons. Vol. 1. Applied Science Publishers, London. Twardus, E.M. 1980. A Study to Evaluate the Combustibility and Other Physical and Chemical Properties of Aged Oils and Emulsions. R & D Division, Environmental Emergency Branch, Environmental Impact Control Directorate, Environmental Protection Service, Environmental Canada, Ottawa, Ontario. Van der Linden, A.C. 1978. Degradation of oil in the marine environment, pp. 165-200. In J.R. Watkinson, ed. Developments in Biodegradation of Hydrocarbons. Vol. 1. Applied Science Publishers, London.
365 Vandermealen, J.H. 1981. Scientific studies during the Kurdistan tanker incident: Proceedings of workshop. Report B1-R-80-3. Bedford Institute of Oceanography, Dartmouth, N. S. Canada . Vandermeulen, JoHo 1982. Some conclusions regarding long-term biological efffects of some ma jor oil spills. Phil. Trans. R. Soc. London, Ser . B 297: 335-351. Vandermeolen , J. H., and D. C. Gordon , Jr . 1976 . Reentry of 5-year-old stranded Bunker C fuel oil from a low-energy beach into the water, sediments and blots of Chedabucto Bay, Nova Scotia. J. Fish. Res. Board Can. 33:2002-2010. Vandermaulen, J.H., B.F.N. Long, and T.P. Ahern. 1981. Bioavailability cuff stranded Amoco Cadiz oil as a function of environmental self-cleaning, pp. 585-598 . In G. Conan, ed. Amoco Cadiz: Fates and Ef feats of the Oil Spill. Centre National pour 1' Exploit . Oceans Brest, France. van Dolah, R.F., V.G. Burrell, Jr., and S.B. West. 1980. The distribution of pelagic tars and plastics in the South Atlantic Bight. Mar . Pollut. Bull . 2: 352-356 . Van Vleet, E.S., and J.G. Quinn. 1977. Input and fate of petroleum hydrocarbons enter ing the Providence River and upper Narragansett Bay from waste water effluents. Environ. Sci. and Technol. 11 :1086-1092. Van Vleet, E.S., and J.G. Quinn. 1978. Contribution of chronic petroleum inputs to Narragansett Bay and Rhode Island Sound sediments . J. Fish . Res . Board. Can . 35: 536-543 . Van Vleet, E.S., W.M. Sackett, F.F. Weber, and S.B. Reinhardt. 1982a. Spatial and temporal Ear iation of crude oil residues in the eastern Gulf of Mexico. In Advances in Organic Geochemistry, 1981. Pergamon, New York (in press). Van Vleet, E.S., W.M. Sackett, Fir. Weber, and S.B. Reinhardt. 1982b. Input of pelagic tar into the northwest Atlantic from the Gulf loop current: chemical character ization and its relationship to Ixtoc I oil Con. Jr . Offshore Aquat. Sci . ~ in press) . Varanasi , U., and D.J. Gmur . 1981. Hydrocarbons and metabolites in English sole (Parophrys vetulus) exposed simultaneously to (3H)benzota~pyrene and ~ '4C) naphthalene in oil-contaminated sediment . Aquat . Toxicol . 1: 4 9-6 8 . Varanasi, U., D.J. Gmur , and P.A. Treseler . 1979. Influence of time and mode of exposure on biotransformation of naphthalene by juvenile s tarry f launder (Platichthys stellatus ~ and rock sole (Lepidopsetta bilineata). Arch. Envoy on. Contam. Toxicol 8: 673-692 . Varanasi , U., D.J. Gmur , and W. L. Reichert. 1981. Effect of environmental temperature on naphthalene metabolism by juvenile starry flounder (Platichthys stellatus). Arch. Environ. Con tam. Toxicol . 10: 203-214 . Veith, D.G., D.L. Defoe, and B.V. Bergstedt. 1979. Measuring and estimating the bioconcentration factor of chemicals in f ish. J. Fish. Res. Board Can. 36:1040. .
366 Venkatesan , M. I ., S.. Brenner , E . Ruth , J.. Bonilla , and I . R. Kaplan . 1980. Hydrocarbons in age-dated sediment cores from two basins in the Southern California Bight. Geochim. Cosmochim. Acta 44:789-802. Wade, T.L., and J.G. Quinn. 1975. Hydrocarbons in the Sargasso Sea surface microlayer . Mar . Pollut. Bull . 6: 54-57. Wakeham, S.G., and J.W. Farrington. Contemporary aquatic sediments, pp. 3-32. In R.A. Baker, ed. Contaminants and Sediments. Vol. 1. Ann Arbor Science Publishers. Ann Arbor, MiCh. Walker J.D., and R. R. Colwell . ~ 976a. Oil , chlor inated biphenyl , mercury and microrganism interactions. Environ. Sci. Technol. 10:1145-1147. Walker, J.D., and R.R. Colwell. 1976b. Long-chain n-alkanes occurring during microbial degradation of petroleum. Can. J. Microbiol. 22:886-891. Walker, J.D., R.R. Colwell, and L. Petrakis. 1975. Degradation of petroleum by an alga, Prototheca zopfii. Appl. Microbiol. 30:79-81. Walker, J.D., R.R. Colwell, and L. Petrakis. 1976. Biodegradation rates of components of petroleum. Can. J. Microbiol. 22:1209-1213. Ward, D.M., and T.D. Brock. 1978. Anaerobic metabolism of hexadecane in mar ine sediments. Geomicrobio1. J. 1:1-9. Ward, D.M., R.M. Atlas, P.D. Boehm, and J.A. Calder. 1980. Microbial biodegradation and the chemical evolution of Amoco Cadiz oil pollutants. Ambio 9:277-283. Wheeler, R. B. 1978 . The fate of petroleum in the mar ine environment. Spec ial Repor t . Exxon Production Research Company, Houston Tex. Whittle , K. J ., J. Murray, P. R. Mack ie , R. Hardy , and J . Farmer . 19 77 . Fate of hydrocarbons in f ish. Rapp. P.-v. Reun. Cons . Int. Explor . Mer 171: 139-142. Wiesenburg , D.A., G. Bodennec, and J.M. Brooks . 1981a. Volatile hydrocarbons around a production platform in the northwest Gulf of Mexico. Bull. Environ. Contam. Toxicol. 27 :167-174. Wiesenberg, D.A., J.M. Brooks, and R.A. Burke, Jr . 1981b. Gaseou~s hydrocarbons around an active offshore gas and oil f~eld. Environ. Sci. Technol. 16:278-282. Wong , C. S ., D. MacDonald, and R. D. Bellegay. 1974a. Distr ibution of tar and other particulate pollutants along the Beaufort Sea coast, Victor ia, B.C. 1974 Inter im Report. Environment Canada, Ocean Chemicals Division, Ocean Aquatic Affairs, Pacific Regulations, Ottawa, Ontar io. Wong, C.S., D.R. Green, and W.J. Cretney. 1974b. Quantitative tar and plastic waste distr ibution in the Pacif ic Ocean. Natur e 247:30-32. Wong, C.S., D.R. Green, and W.J. Cretney. 1976. Distribution and source of tar on the Pacific Qcean. Mar. Pollut. Bull. 7 :102-106 . Wong, W.C. 1976. Uptake and retention of Kuwait crude oil and its effect on oxygen uptake by the soft-shell clam, Mya arenaria. J. Fish. Res. Board Can. 33:2774-2780.
367 Wood, A.W., W. Levin, A.Y.H. Lu, H. Yagi, O. Hernandez, D.M. Jerma, and A.H. Cooney. 1976. Metabolism of benzota~pyrene derivatives to mutagenic products by highly purified hepatic microsomal enzymes. J. Biol . Chem. 251:4882 . Wu, J., and L.K. Wang. 1981. Microbial transformations of 7,12-dimethyl- benzo (a ~ anthracene . Appl . Environ. Microbiol . 41: 843-845 . Yamada, K., Y. Monoda, K. Komada, S. Nakatani, and T. Akasaki. 1968. Microbial conversion of petro-sulfur compounds. I. Isolation and identification of dibenzothiophene-utilizing banter ia . Agr ic. Biol. Chem. 32:840-845. Yang , W.C., and H. Wang. 1977. Modeling of oil evaporation in aqueous environment. Water Res. 11:879-887. Zafiriou, O.C. 1973. Petroleum hydrocarbons in Narragansett Bay. lI. Chemical and isotopic analysis. Estuarine Coastal Mar. Sci. 1:81-87. Zafiriou, O.C. 1977. Marine organic photochemistry previewed. Mar. Chem. 5:497. Zajic, J.E., B. Supplisson, and B. Volesky. 1974. Bacterial degradation and emulsification of No. 6 fuel oil. Environ. Sci. Technol. 8:664-668. Zepp, R.G. 1978. Quantum yields for reaction of pollutants in dilute aqueous solution. Environ. Sci. Technol. 12:327-329. Zepp, R.G., and D.M. Cline. 1977. Rates of direct photolysis in the aquatic environment. Environ. Sci. Technol e 11 359-366. Zepp, R.G., and G.L. Baughman. 1978. Production of photochemical transformation of pollutants in the aquatic environment. In O. Hutzinger, I.H. van Lelyveld, and B.C.J. Zoeteman, eds. Aquatic Pollutants--Transformation of Biological Effects. Pergamon, New York. Zika, R.G. 1980. Marine Organic Photochemistry, Chap. 10. In E.K. Duursma and R. Dawson eds. Marine Organic Chemistry. Elsevier, New York. Zitko, V. 1971. Determination of residual fuel oil contamination of aquatic animals. Bull. Environ. Contam. Toxicol. 5:559-S63 e ZoBell, C.E. 1969. Microbial modification of crude oil in the sea^, pp. 317-326. In Proceedings, Joint Conference on Prevention and Control of Oil Spi~ls. American Petroleum Institute, Washington, D.C. ZoBell, C.E. 1973. Bacterial degradation of mineral oils at low temperatures, pp. 153-161. In D.G. Ahearn and S.P. Meyers, eds . The Microbial Degradation of Oil Pollutants. Publication LSU-SG-73-01. Center for Wetland Resources, Louis iana State Univer sity, Baton Rouge . ZoBell, C.E., and J. Agost~. 1972. Bacterial ox~dation of mineral oils at sub-zero Celsius. Abstracts of the 72nd Annual Meeting. Abstract Ell. American Society of Microbiology, Philadelphia, Pa., April 23-28, 1972. Zsolnay, A. 1972. Preliminary study of the dissolved hydrocarbons and hydrocarbons on par ticulate material in the Gotland Deep of the Baltic. Kieler Meeresforsch. 27:129-134.
368 Zsoloay, A. 1973a. The relative distribution of nonaromatic hydrocarbons in the Baltic in September 1971. Mar. Chem. 1:127-136. Zsolnay, A. 1974. Hydrocarbon content and chlorophyll correlation in the waters between Nova Scotia and the Gulf Stream, pp. 255-256. In Marine Pollution Monitoring {Petroleum) . Special Publication 409. National Bureau of Standards, Gaitherburg, Md. Zsolnay, A. 1977a. Inventory of nonvolatile fatty acids and hydrocarbons in the oceans. Mar. Chem. 5:465-475. Zsolnay, A. 1977b. Hydrocarbon content and chlorophyll correlations in water between Nova Scotia and the Gulf Stream. Deep Sea Res. 24: 199-207 . Z solnay, A. 1979 . Hydrocarbons in the Mediterranean Sea, 1974-1975 . Mar . Chem. 7: 343-352. Z sol nay, A., B.F. Morris, and J.N. Butler . 1978. Relationship between aromatic hydrocarbons and pelagic tar in the Mediterranean Sea, 1974-1975. Environ. Conserve. 5: 295-297. Zurcher , F., and M. Thuer . 1978 . Rapid weather ing processes of fuel oil in natural waters: analyses and interpretations. Environ. Sci. Technol . 12: 838-843.