Medical Devices, Component Materials, and Product Liability
PAUL CITRON
Roughly 40 years ago, the era of implantable medical devices was ushered in. With it, exciting new therapeutic options became available. These devices significantly complemented the medical armamentarium that was at that time limited to pharmaceutical preparations, surgical intervention largely based on excision of diseased tissue and expendable organs, and perhaps most successful of all, "tincture of time."
In the 1950s, medical devices such as large-diameter vascular grafts for the first time permitted surgeons to replace body parts that had become defective. The year 1958 saw the implantation of the first electronic device, the cardiac pacemaker. This revolutionary technology stimulated the heart experiencing bradycardia, a too-slow rate, to a rate approximating resting normal. In this way the heart was once again able to pump sufficient quantities of blood to meet the majority of the patients' hemodynamic requirements.
The 1960s saw the emergence of the mechanical heart valve as a practicable replacement for defective natural valves. Implanted medical devices such as these offered therapy where previously none was available. In many instances they offered the possibility of not only saving patients' lives but also restoring their quality of life. Perhaps the clearest example of the lifesaving capacity of medical devices has been the development of implanted defibrillators. This emerging technology is capable of detecting fibrillation as well as the dangerous heart rhythms that can lead to fibrillation, then automatically delivering a precise shock to the heart to restore normal rhythm. Carefully selected patients who receive such devices would most likely have become victims of what is aptly known as sudden
cardiac death syndrome. Other technologies such as drug delivery systems, orthopedic joint implants, and intraocular lens implants have restored patients to fuller and more productive lives.
Performance Characteristics
It is important to note the hostile nature of the environment in which medical devices must function. Over its evolutionary cycle, the body has created a formidable set of defenses against foreign materials. It recognizes them as being potentially dangerous and vigorously sets out to consume, destroy, or isolate intruders. Obviously these defenses work well. Unfortunately, they do not recognize medical devices as allies and seek to destroy them. Only a limited number of materials such as silicone rubber, certain polyurethanes, a small number of other polymers, and an equally small number of inert metals and exotic alloys have been found to be clinically acceptable for implantation. These materials are used to construct the implant itself or serve as the protective barrier to shield other components that are not clinically acceptable for implantation.
As if the requirement of clinically acceptable tissue response was not enough, implanted materials must also be ''biodurable." That is, they must have the intrinsic physical and mechanical properties to withstand the rigors of the application in which they are used. Consider two examples. A pacemaker lead that connects the stimulator to the heart must be able to maintain its integrity for years while being flexed by the beating heart approximately 38 million times per year (Figure 1). It must also withstand the abrasive action of blood and the wiping action of the heart valve through which most leads pass while still serving its primary role as a stable conduit for electrical signals passing to and from the heart. As another illustration, a prosthetic mechanical heart valve is expected to function flawlessly for the patient's lifetime while being subjected to wear forces and large pressure-induced forces as it opens and closes (Figure 2). It is not unrealistic for a valve to experience in excess of 600 million opening and closing cycles. It must do this without mechanical failure and without appreciably damaging blood cells or causing other serious complications.
These are challenging requirements that are being met with today's technologies. Even though performance is excellent, there is room for improvement. The perfect implant has yet to be developed, and this is primarily related to the effects of the blood coagulation system.
Regulating Medical Devices
The critical nature of medical devices has caused them to come under stringent regulation in many parts of the world. Clearance to market devices
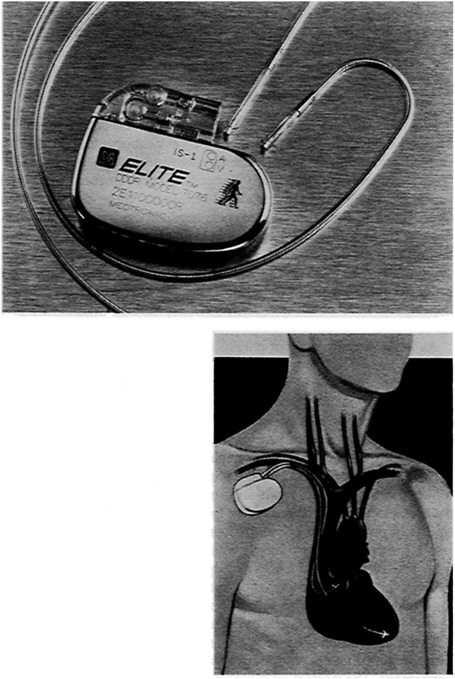
FIGURE 1 Top: A pacemaker system consists of two major parts—the pulse generator, and the lead/electrode which connects the pacemaker to the heart. Bottom: The pulse generator, which contains the circuitry and power source to monitor heart activity and produces stimulation pulses when required, is typically implanted subcutaneously in the pectoral region near the collar bone. Stimulation pulses move along the lead, through the electrode at its far end to cause the heart to contract. In a similar manner, signals produced by the heart travel through the lead/electrode to appropriately alter the pacemaker's operation. SOURCE: Medtronic, Inc.
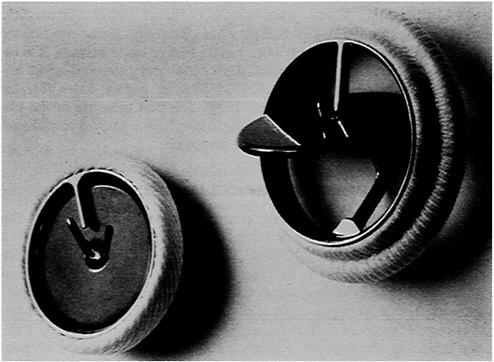
FIGURE 2 A mechanical heart valve must perform perfectly for a lifetime while withstanding wear and pressure-induced forces. A fabric sewing ring allows the valve to be sutured into place. SOURCE: Medtronic, Inc.
in the United States is granted only after the Food and Drug Administration (FDA) has determined through its classification and review procedure that there is reasonable assurance of the safety and effectiveness of the device. Such regulatory requirements are necessary and appropriate. They impart a degree of discipline and thoroughness to the process. They also provide a third-party appraisal of the suitability of a new technology in comparison with other available alternatives. A rigorous but responsive and responsible regulatory process helps to ensure that new medical technologies represent the state of the art, have the real potential to do good as demonstrated in scientifically grounded studies, and reach patients promptly.
IMPORTANCE OF MEDICAL DEVICES
The impact of medical devices has been profound and far reaching. A survey conducted by the National Center for Health Statistics of the Centers for Disease Control and by the FDA's Center for Devices and Radiological Health estimated 11 million Americans in 1988 were alive with one or more implantable products, such as artificial joints, fixation
devices, intraocular lenses, pacemakers, or heart valves (Biomedical Market Newsletter, 1991). This industry has global significance and is one of the few in which the United States has a positive trade balance. It is estimated that in 1992 the U.S. medical device industry produced a $4.0 billion favorable trade balance on $39.7 billion in annual sales (Health Industry Manufacturers Association, 1994). The best measure of the impact of medical device technology, though, is in how it improves and sustains patients' lives. This must never be forgotten.
IMPACT OF PRODUCT LIABILITY
Despite the enormous contribution medical devices have made to the public health, it is a business that frightens many. This fear is largely a consequence of the possibility of liability exposure in the event of device malfunction or failure. As is the case in other businesses, the specter of product liability in medicine is peculiar to the United States. Its influence is growing and is having a chilling effect on innovation. It also damages global competitiveness and increases health care costs directly and indirectly. Ironically, the shadow of product liability may actually be keeping better performing products from the market rather than being a force for improvement.
The Suppliers' Dilemma
Typically, medical device manufacturers must rely on components and raw materials manufactured by other firms in order to produce their final products. While some implants, for instance those used in reconstructive plastic surgery, consist of a single, homogeneous material, the majority of devices integrate a broad range of technologies. In many instances these components are produced for other commercial applications but have also been qualified for use in medical applications.
The dilemma that exists for the raw material or component supplier can be illustrated by the following example. Consider for a moment the mechanical heart valve (Figure 2). To secure such valves to the heart, a fabric sewing ring encircles the valve. The valve is literally sewn into place by the surgeon, who sutures through the fabric into heart tissue. Certain polyester and polytetrafluoroethylene (PTFE) fibers have proven suitable for this use in more than 20 years of clinical experience. To the best of knowledge, there have been no adverse clinical events associated with the fabric or fiber.
Yet, a key manufacturer of the fibers notified the heart valve industry in 1993 that it will discourage future use of its material in permanently implantable products. The company had concluded that selling raw material could not be justified in light of the business risk of litigation from merely having their raw material in permanent implants.
Suppliers Become Liable, Too
Precedent has demonstrated that upstream raw material suppliers can and do get pulled into product liability cases even where they had no direct involvement in the design, specification, or manufacture of the final product. They are viewed as having deep pockets and become defendants. Despite the fact that suppliers of raw materials usually win in court and are found not liable, the cost of proving themselves innocent and the management attention that must be given the matter far exceed the business opportunity. For instance, a supplier can be subjected to hundreds of lawsuits for a medical product in which its material was used as an ingredient. The material can be procured on the open market through commercial supply-house channels without any direct involvement by the supplier in the specification, design, testing, or manufacture of the end product. The out-of-pocket legal costs can, and have, run into the millions of dollars on only dollars worth of raw materials sales.
The medical device industry has seen a growing list of highly reputable material supply companies such as Dow Chemical, Dow-Corning, and DuPont announce their intention to restrict sales to implant manufacturers. These companies have sharply reassessed the extent and manner in which they participate in medical devices. This has shifted the balance in the relationship between suppliers and manufacturers. Formerly, materials were supplied by large, sophisticated chemical companies, with well-established quality procedures, to smaller companies. Litigation has moved the market to a new relationship where small, often undercapitalized start-ups with no manufacturing history provide material to the device companies. Liability concerns have driven mature, technologically well-established firms from this market.
While the impact has been greatest for implanted polymeric and elastomeric materials, it has not been restricted to them. The adverse experience with product liability has caused suppliers of essentially all components used in implants to assess their willingness to supply. For example, certain well-established manufacturers of integrated circuits have refused to supply their chips for implanted devices.
Impacts of Short-Term Solutions
The device industry is engaged in an all-out effort to find and qualify equivalent replacement materials and sources of supply. Although this process is likely to be successful, the resources expended on these initiatives will merely enable the medical device industry to pick up where it was before this occurred. The state of the art will not have been advanced. Some would suggest that the departure of the leading specialty chemical companies from selling materials to implant manufacturers may even
lower the overall quality standard of the portfolio of implantable materials as smaller, undercapitalized, and less sophisticated supply sources step in and attempt to fill the void. What is clear is that the flow of new materials that would permit as yet unmet clinical needs to be addressed will be markedly slowed. The exiting companies have the laboratories from which future breakthroughs would have been likely to come. This is not to say progress has ceased, but rather, it has been slowed—and for the wrong reasons.
In many instances suppliers and medical device companies can contractually shift the risk of product failure to the device manufacturer. This is not a complete answer, however. Suppliers still can be joined in the law-suit and must put up with the expense of discovery procedures and the great inconvenience it entails, as well as adverse publicity. While indemnification can make material available in some cases, it adds significant cost to the final product without adding any value to the physician or patient. If the device company is not financially strong, suppliers will not be comforted by such a shift in risk. This places start-up companies and entrepreneurs at a disadvantage. In this way innovation is negatively affected as are the patients who could have benefited. All of the initiatives targeted at securing continuing supply of components divert R&D dollars from activities that could provide better products to patients.
PRESCRIPTION FOR PROGRESS
The chilling effect of product liability on U.S. medical device innovation and, ultimately, competitiveness, has been outlined above. An obvious remedy is sweeping tort reform. However political reality suggests this will not occur, at least not in the foreseeable future. Perhaps, then, the following steps are a prescription for progress that will remove barriers to medical device innovation and availability.
-
The industry must communicate clearly to patients, physicians, and other sectors of the public the intrinsic limitations of medical technology. Expectations must be in line with the industry's abilities and the state of the art. Yes, medical devices are able to do miraculous things, but the perfect implant has not yet been achieved. Until it is, results will sometimes be imperfect.
-
The medical device industry has an obligation to produce high quality products, track their performance, conduct research to expand understanding of underlying mechanisms of action, and invest in initiatives that build on knowledge gained to produce evolutions of improved products.
-
The FDA, as part of its product approval process, should maintain
-
master files on materials that have been found to be clinically acceptable for implantation and suitable for defined applications. Medical products employing such materials and which secure marketing approval from the FDA should be deemed safe and effective as well as representing an appropriate standard of technology. Product liability actions that may be brought against the manufacturer of the end product would, in consideration of the rigorous FDA qualification process, exclude pain, suffering, and punitive damages as long as the product was produced in compliance with the terms of FDA approval.
-
Component and raw material suppliers should be shielded by law from medical device product liability actions for FDA-approved products if readily available "off-the-shelf" materials meeting specifications are incorporated into implantable products that undergo FDA approval. The burden for demonstrating suitability would fall to the manufacturer of the final product. In instances where modified or custom materials or components are provided, the supplier of raw materials would have similar protection as long as the design specification was met.
-
In those instances when individual patients cannot recover from the manufacturer costs due to device malfunction, a government-administered fund modeled after the one established for children experiencing severe complications from vaccinations would be created. In this way, the good of the many would be preserved while keeping whole those who experience an unexpected problem.
CONCLUSION
Medical device breakthroughs over the last 40 years have had a profound positive impact for millions of patients around the world. These triumphs were made possible by a spirit of discovery and the uniquely American impatience with the status quo. We traditionally want to make things better. But our litigious nature has reached such a level that it is extinguishing the spark of innovation. Methods must be implemented that remove barriers to progress so the process of continual improvement can lead to better products and better outcomes. In this way the root causes behind product liability will be reduced as well.
REFERENCES
Biomedical Market Newsletter. 1991. First medical device implant survey released. September, p. 8.
Health Industry Manufacturers Association. 1994. The Global Medical Device Marketplace Update: Markets for Medical Technology Products. Report #94-1. Washington, D.C.: Health Industry Manufacturers Association.








