Hospital-Acquired Infections: Diseases with Increasingly Limited Therapies
M. N. SWARTZ
The ≈6500 acute-care hospitals in this country represent unique ecological systems and provide the settings for nosocomial (hospital-acquired) infections. The principal components making up these systems are patients, medical-care personnel, equipment and devices employed in the treatment of patients with complicated medical illnesses, and the commensal microbiota of patients and the microbial population in the hospital environment. Modern acute-care hospitals are complex institutions consisting of a variety of specialized components: burn services, oncology wards, coronary care units, intensive care units, and transplantation units. Individual units may have particular nosocomial infection problems related to the type of patient being treated or the nature of their underlying illnesses, procedures employed in individual units, and the selection pressure exerted by antimicrobial usage patterns.
Each year there are ≈37.7 million admissions to acute-care hospitals in the United States; among these, ≈2.1 million patients (5.7%) develop nosocomial infections (1). This is a sizable number and, at first glance, might seem surprising five decades after the beginning of the antibiotic era. A variety of changes account for the current significant role of nosocomial infections. These alterations include changes in the age of hospitalized patients, the nature of their underlying illnesses, the types
M. N. Swartz is chief, James Jackson Firm Medical Services and formerly chief of the Infectious Disease Unit of the Massachusetts General Hospital, Boston.
of surgical and systemic therapy that are now available for treating diseases that were formerly untreatable, and the multitude of antimicrobial drugs currently available and capable of selecting a more resistant microbial flora.
CHANGING FEATURES OF THE POPULATION OF HOSPITALIZED PATIENTS
The increase in mean life expectancy in the population as a whole is reflected in the increasing age of hospitalized patients. In an acute-care community general hospital in Madison, WI, for example, the percentage of patients 65 years of age or older rose from 13% in 1970–1973 to 24% by 1987 (2). Similarly, at the Massachusetts General Hospital, the mean age of admissions increased steadily over a 7-year period (1986–1992) from 54 to 57 years. Increasing age as a risk factor for nosocomial infections is seen in the analysis of nosocomial infections in elderly patients reported by the National Nosocomial Infections Surveillance (NNIS) system between 1986 and 1990 (3). Whereas elderly patients (>65 years of age) represented only 31% of total discharges from hospitals, 54% of nosocomial infections occurred in this group.
CHANGES IN TYPES OF PROCEDURES PERFORMED FOR COMPLEX ILLNESSES
Extensive surgical procedures are now carried out almost routinely, correcting a variety of incapacitating or debilitating illnesses and restoring patients to productive lives. Many of these procedures have been introduced and performed in large numbers only in the past several decades. Innovations in cardiac surgery or orthopedic surgery serve as examples (4, 5). In 1960 the modern era of heart valve replacement began with the first successful placement of a caged-ball valve in the subcoronary aortic valve position. Currently, an estimated 46,000-58,000 prosthetic heart valve replacement procedures are performed annually in the United States (6). The first saphenous vein aortocoronary artery bypass graft for coronary artery disease was carried out in 1967. Over the ensuing 25 years this procedure has become one of the most commonly performed operations in the United States. About 390,000 such procedures are performed each year (6).
Paralleling development of innovative and extensive operative procedures for treatment of cardiac disease have been similar advances in reconstructive orthopedic surgical procedures. The first total hip replacements in this country began in 1966, and current estimates are that ≈125,000 such procedures are performed annually in North America
(William Harris, personal communication). The first total knee replacements were carried out in 1970, and it is estimated that currently ≈125,000 such procedures are performed in North America each year.
The aforementioned surgical procedures have in common the characteristic of prolonged operative time and, in the case of valve and joint replacements, the feature of insertion of a foreign body. Both of these factors are predisposing risks for development of post-operative infections. Such infections include not only operative would infections but also lower respiratory tract infections (secondary to prolonged anesthesia, intubation, etc.), urinary tract infections (secondary to indwelling urinary catheters), and blood stream infection (complicating the use of intravenous catheters while the patient is recovering from extensive surgery).
Consequences of the benefits of modern complex surgical procedures include nosocomial infections, stemming from a variety of factors. These include the extensive nature of the surgical procedures, prolonged operative/anesthesia times, need for medical devices and invasive manipulations during the post-operative period, the often complex nature of the underlying disease or of associated illnesses, and the older ages of hospitalized patients.
Overall nosocomial infection rates vary considerably depending on the type of surgery performed. Among common surgical procedures in acute-care hospitals participating in the NNIS system, the highest rates of overall nosocomial infections occur with gastric surgery (21%), bowel surgery (19%), craniotomies (18%), coronary artery bypass graft procedures (11%), and other cardiac surgery (10%) (7). Orthopedic procedures involving introduction of metallic plates and of prosthetic joints have overall nosocomial infection rates of ≈8%. The fact that more major surgery is now possible than was the case three decades ago carries with it the almost inevitable consequence of a greater burden of nosocomial infections.
Individuals particularly vulnerable to nosocomial infections by virtue of the immunosuppressive treatment of their underlying conditions make up a larger component of hospitalized patients than was the case three or four decades ago. Such patients include those undergoing organ transplantation or intensive chemotherapy of leukemia, lymphoma, and other neoplastic processes. The first successful human renal transplantation was performed in 1954. By 1992, renal transplantation was performed in 10,210 patients annually (8). By 1992 multiple other types of organ transplantation have become commonplace: 3059 liver transplants and 2171 heart transplants annually. Including bone marrow transplants, of which there were ≈4900 performed in 1990 (9), the total number of organ transplants in 1992 numbered >21,400. Nosocomial
infections in transplant patients include those due to the common nosocomial bacterial pathogens but also some due to opportunistic viral and fungal agents. Patients undergoing induction therapy for leukemia or chemotherapy for a variety of cancers are particularly at risk for nosocomial infections by virtue of the neutropenia consequent on cytotoxic drug treatment. In a prospective study over a 42-month period at a cancer research center in New York City, an overall nosocomial infection rate of 48.3 per 100 neutropenic patients (46.3 per 1000 days at risk from neutropenia) was observed (10). The NNIS data, admittedly a different group of patients in different geographic areas, nonetheless provides a frame of reference for comparison: median overall infection rates per 100 discharges for general medical patients (3.5%), for cardiac surgical patients (9.8%), and for patients on the burn/trauma service (14.9%) (11).
CHANGING SPECTRUM OF NOSOCOMIAL PATHOGENS EARLY IN THE ANTIMICROBIAL ERA
Nosocomial infections are not unique to the past few decades but have occurred since care of patients in hospitals began. What has changed over the past half century are the properties and species of infecting microorganisms. Viral agents (herpes group viruses and hepatitis virus) and Mycobacterium tuberculosis, important causes of nosocomial infections, will not be considered here since they have been considered separately earlier in this colloquium.
In the preantibiotic era, the principal microbial causes of nosocomial infections were Gram-positive cocci: Streptococcus pneumoniae, Streptococcus pyogenes and other streptococcal species, and Staphylococcus aureus. Sir William Osler and Henry Christian recognized the role of S. pneumoniae as a cause of terminal pneumonia in patients dying of various other primary illnesses. ''Patients with arteriosclerosis, heart disease, nephritis, etc., not infrequently are carried off by a pneumococcic pneumonia which may give few or no signs" (12).
The introduction of penicillin G into clinical medicine in the mid-1940s was viewed overly optimistically as heralding the elimination of the common bacterial infections, including those acquired within hospitals. Although highly effective initially against S. aureus, a major cause of human infections, subsequent developments belied the initial enthusiasm. Whereas ≈90% of S. aureus isolates at Boston City Hospital prior to 1946 were susceptible to penicillin, by 1952 ≈75% of isolates were resistant (13). In the early 1970s, ≈75% of S. aureus isolates at the Massachusetts General Hospital were resistant to penicillin. Within a few years, 90% were penicillin-resistant. Currently, worldwide >95% of
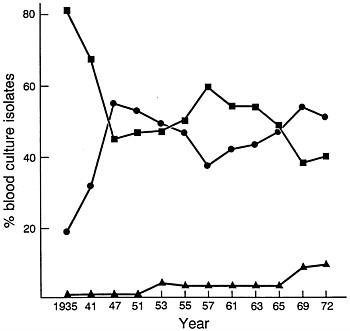
FIGURE 1 Changes in relative percentages of aerobic Gram-positive cocci (■), aerobic Gram-negative bacilli (●), and yeasts (▲) as causes of nosocomial bacteremias/fungemias at Boston City Hospital between 1935 and 1972. Data are plotted from McGowan et al. (15).
S. aureus isolates are resistant to penicillin G as the result of plasmid-mediated penicillinase production (14).
The introduction of penicillin in treatment of human infections was followed in a few years by the introduction of streptomycin, the tetracyclines, erythromycin, and chloramphenicol. As more extensive surgery and cytotoxic drugs were used in treatment and as selection of more antibiotic-resistant microorganisms developed as a result of broad-spectrum antibiotic usage, the frequency of hospital-acquired infections increased. At Boston City Hospital, the frequency of hospital-acquired bacteremias and fungemias steadily increased (with few fluctuations) between 1935 and 1972 from a rate of 3.7 per 1000 hospital admissions to a rate of 14.7 per 1000 admissions (15). Most striking was the change that occurred in the predominant species causing the bloodstream infections. Whereas in 1935 Gram-positive aerobic cocci accounted for 80% of isolates and Gram-negative aerobic bacilli accounted for 20%, by 1972 the relative roles had undergone major changes (Figure 1). By the early 1970s, ≈55% of blood isolates were Gram-negative aerobic bacilli; ≈40%
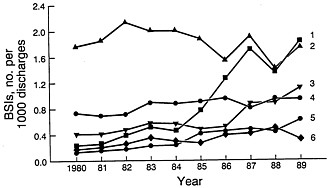
FIGURE 2 Changes in the frequencies of CNS (trace I), Gram-negative bacilli (trace 2), S. aureus (trace 3), others (trace 4), Candida spp. (trace 5), and enterococci (trace 6) as causes of nosocomial bloodstream infections (BSIs) between 1980 and 1989 in NNIS system hospitals. Modified from Banerjee et al. (18) and reproduced with permission (copyright Cahners).
were Gram-positive aerobic cocci, and the remainder were Candida or Torulopsis spp. Between 1941 and 1972, the percentage of S. pneumoniae among bacteremic Gram-positive coccal strains declined from 15 to 4% and that of enterococci increased from 3 to 19%. Among Gram-negative bacilli, the principal changes observed between 1941 and 1972 were a decline in frequency for Escherichia coli from 66 to 27% and an increase in Klebsiella–Enterobacter from 0 to 39% (15, 16).
During the decade of the 1980s, changes in the frequency of nosocomial infections and the spectrum of microorganisms involved continued. Primary bloodstream infections represent ≈8% of all hospital-acquired infections in the United States (3, 17). The frequency of bloodstream infection varies with the category of hospital (18). The rate for small nonteaching hospitals is the lowest (1.3 per 1000 discharges) followed by that for large nonteaching hospitals (2.5 per 1000 discharges). The highest rate (6.5 per 1000 discharges) occurs in large teaching hospitals, followed by the rate (3.8 per 1000 discharges) in small teaching hospitals. The higher rates in the large teaching hospitals presumably reflect the more seriously ill patient population within large tertiary-care hospitals. Increases in the primary bloodstream infection rates occurred between 1980 and 1989 and varied by category from 70 to 279%. The most striking changes were in the rates for individual pathogen groups (Figure 2). Four pathogen groups accounted for most of the increase in nosocomial bloodstream infections in the United States between 1980 and 1989: coagulase-negative staphylococci (CNS), S.
aureus, Enterococcus spp., and Candida spp. (18). The largest percentage increase (75.4%) in bloodstream bacterial infections occurred with CNS in large teaching hospitals. Bloodstream infections with Candida spp. increased in frequency by 487% in large teaching hospitals whereas those with S. aureus increased 176% and those with Enterococcus spp. increased 120%. Aerobic Gram-negative bacilli, which had achieved prominence in the previous two decades, showed no change in frequency in large teaching hospitals.
The spectrum of microbial pathogens involved overall in nosocomial infections differs somewhat from that causing only bacteremia/fungemia (19). Overall, in NNIS hospitals from 1986 to 1989, the five leading pathogens were E. coli (16%), Enterococcus spp. (12%), Pseudomonas aeruginosa (11%), S. aureus (10%), and CNS (9%). The leading role of E. coli reflects the frequency of urinary tract infections in hospitalized patients. Each of the other major categories of nosocomial infections has its own distinct leading pathogen: wound infection, S. aureus; pneumonia, P. aeruginosa and S. aureus; bloodstream infection, CNS. Over the past decade there has been a decline in the frequency of E. coli infections and increases in infections due to CNS (from 4% of total infections to 9%) and Candida albicans (from 2 to 5%). In the past decade the trend has been away from antimicrobial-susceptible species to more resistant pathogens. Even among susceptible species, there has been a shift from readily susceptible to antibiotic-resistant strains.
Overall data for a large number of hospitals are very helpful in identifying major trends. However, they may tend to obscure outbreaks that occur in individual hospitals and important shifts that occur in antimicrobial susceptibilities of pathogens that present problems in individual intensive-care units. For example, among 392 nosocomial infections occurring among 920 neutropenic cancer patients in a cancer unit, Candida spp. (18%) was the most common pathogen followed closely in frequency by CNS (17%) (10). In addition to common microorganisms seen in the multihospital NNIS survey, less frequently observed pathogens such as Clostridium spp., Corynebacterium spp., Aspergillus spp., and herpes viruses may account for 20% of the total pathogen group.
PROBLEMS OF ANTIMICROBIAL RESISTANCE IN SELECTED NOSOCOMIAL PATHOGENS
S. aureus
Methicillin-resistant S. aureus (MRSA) was first described in 1961 in England and over the next decade became an important nosocomial
pathogen in parts of Europe (20). MRSA is resistant to all penicillins, including semisynthetic penicillinase-resistant congeners, penems, and carbapenems. The basis for this resistance is the mecA gene that codes for a new penicillin-binding protein (PBP; PBP2a) with a reduced affinity for all β-lactam antibiotics (21). The mecA gene is chromosomal in location and not subject to dissemination among staphylococcal strains via plasmid spread. However, resistance to other antibiotics such as streptomycin, tetracyclines, trimethoprim, and erythromycin can be spread widely via plasmids and by transposition in many MRSA (21). In the early 1970s, the prevalence of MRSA temporarily receded only to reemerge in the past one and a half decades as a cause of nosocomial outbreaks of infections in Europe, Africa, Asia, Australia, and the United States (22). The prevalence of MRSA among S. aureus isolates reveals marked differences between countries: 0.1% in Denmark in 1988, 4% in Germany in 1989, 26% in France in 1989, and 15% in the United States in 1987–88. However, these represent mean prevalence rates for many hospitals. Although the average prevalence rate of MRSA among 24 hospitals in Italy was 26% in 1986, the survey included hospitals with prevalence rates that varied from 6 to 44% (22). In the NNIS data from U.S. hospitals evaluated over a 17-year period (1975–1991), the percentage of MRSA increased from 2.4% in 1975 to 29% (23). The prevalence of MRSA varied with the size of hospitals. In 1991 it was 14.9% for hospitals with <200 beds, 20.3% for hospitals with 200–499 beds, and 38.3% for hospitals with 500 or more beds.
Nosocomially acquired strains of MRSA do not appear to exhibit enhanced intrinsic clinical virulence in comparison with methicillin-susceptible strains. However, patients who carry MRSA may be at a higher risk of developing infection due to that organism that patients colonized with methicillin-susceptible S. aureus (24). Epidemiologic virulence (efficiency in colonizing patients and in spreading throughout hospitals) has been attributed to some strains (21, 23, 25). A variety of factors may contribute to the prevalence of MRSA in a given hospital: patterns of antibiotic use, biologic properties of circulating MRSA strains, the nature of the patient population, local infection control practices, and frequency of admission of colonized or infected patients from other institutions.
Careful monitoring of S. aureus strains from hospitalized patients in Denmark at a national laboratory has provided data on antibiotic susceptibility and phage type of 523,000 isolates over the interval from 1966 to 1988 (26). The rise in prevalence of MRSA from 3% in 1966 to ≈15% in the period 1967–1970 was followed by a gradual decline to a level of ≈0.2%. The rise in prevalence of MRSA during the late 1960s, and the subsequent decline, was paralleled by changes in phage type.
The increase in prevalence of MRSA appears to have been due to spread in hospitals of one or a few clones of S. aureus belonging to phage-type complex 83A and exhibiting multiple antibiotic resistance (27). In Denmark in the 1950s, the S. aureus strains isolated were frequently of the 52/52A/80/81 complex, but these were replaced increasingly in the early 1960s by tetracycline-resistant strains of the 83A complex; 70% of these were multiresistant (45% methicillin resistant). Subsequently, prevalence of strains of phage complex 83A steadily declined until 1980 and has remained at 6% since 1980. Currently, <2% of strains of S. aureus are multiresistant, and even strains of the 83A complex are no longer multiresistant.
Important factors in the control of the Danish outbreak included the extensive and continual monitoring of nosocomial S. aureus isolates and prompt institution of isolation precautions when MRSA was detected. The basis for the increased prevalence of the epidemic clone(s) of MRSA in the Danish outbreak and their subsequent disappearance is unclear. A role for antimicrobial use patterns in the evolution of resistance has been suggested (27). The use of tetracyclines and streptomycin in the 1960s may have provided some selective pressure for the increase in frequency of multiantibiotic-resistant MRSA. Similarly, the considerable reduction in the quantities of these two drugs used during the early 1970s may have lessened the selective pressure for streptomycin- and tetracycline-resistant subtypes of phage 83A complex MRSA.
In the mid 1980s, the newer fluoroquinolone antimicrobials offered considerable initial promise for dealing with both clinical infections due to MRSA and the carrier state (28). The minimum inhibitory concentration (MIC) for 90% of S. aureus isolates (both methicillin-susceptible and methicillin-resistant isolates) was 0.5 µg/ml (29). In the late 1980s and early 1990s, this promise proved disappointing as resistance rapidly developed, particularly among methicillin-resistant strains. In a large tertiary care hospital in New York City, >80% of MRSA had become resistant to the available fluoroquinolones (14).
CNS
By 1971 ≈65% of CNS had become resistant to penicillin at large urban teaching hospitals such as the Massachusetts General Hospital, and from 1979 on ≈80% of isolates have been resistant. Resistance to penicillinase-resistant penicillins, such as methicillin and oxacillin, has steadily increased. At the Massachusetts General Hospital, the percentage of methicillin-resistant isolates among CNS has risen from 8% in 1971 to 54% in 1992.
The increasing role of CNS as a pathogen in infections involving
prosthetic devices (artificial heart valves, cardiac pacemaker leads, joint replacements, nervous system ventricular shunts, peritoneal dialysis catheters, and polyethylene intravenous catheters) has occurred contemporaneously with development of increasing methicillin-resistance. Staphylococcus epidermidis, a common commensal on human skin, is frequently the species infecting foreign body implants or indwelling venous catheters, but other CNS species may also be involved. Binding of such organisms to foreign-body surfaces and their intercell adherence is enhanced by a biofilm matrix (extracellular "slime layer" consisting of exopolysaccharides) they produce. Colonization within a biofilm appears to protect S. epidermidis cells against opsonization and phagocytosis by polymorphonuclear leukocytes, and it may afford protection against the bactericidal action of antibiotics by acting as a barrier to the latter's penetration (30).
CNS (and S. aureus) have developed resistance mechanisms for evading the inhibitory action of many antimicrobials previously useful in treating infections due to these microorganisms (14). They have acquired plasmid genes encoding β-lactamase production that render them (>90%) resistant to penicillin G. MRSA are resistant to methicillin and other penicillinase-resistant penicillins, cephalosporins, carbapenems, and penems by virtue of a chromosomal gene (mecA) that encodes a new PBP (PB2a) with reduced affinity for all β-lactam antibiotics. Fluoroquinolone resistance in clinical isolates of S. aureus is associated with mutations in the A subunit of the DNA gyrase (31). A second form of resistance to fluoroquinolones in S. aureus is due to a mutation (norA) in the chromosomal gene that codes for a membrane transporter effecting fluoroquinolone efflux driven by the proton gradient across the cell membrane (32). Rifampin resistance in S. aureus is encoded on the chromosomal gene for the DNA-dependent RNA polymerase and involves an alteration in the B subunit to which rifampin binds. Rifampin is not used alone to treat staphylococcal infections because of rapid emergence of resistance.
Staphylococcal infections are not ordinarily treated with aminoglycosides, but a drug in this class is sometimes combined with a penicillinase-resistant penicillin in the treatment of life-threatening infections. Plasmid-mediated aminoglycoside-modifying enzymes (phosphotransferase, adenylylating enzyme, and acetyltransferase) alter the conformation of these antibiotics and interfere with binding of the aminoglycoside to the 30S ribosomal subunit, a necessary step in aminoglycoside action (formation of an unstable initiation complex, thus blocking translation and exerting a bactericidal effect).
Resistance in staphylococci to tetracyclines is encoded on plasmids or transposons that cause synthesis of a new membrane protein that
decreases accumulation of tetracycline by causing active efflux of the drug (33). Resistance to macrolides (erythromycin and clindamycin) is primarily plasmid-mediated and inducible, involving methylation of an adenine in the 23S ribosomal RNA of the 50S ribosomal subunit. This results in decreased binding of the macrolides to their usual targets on the ribosome (34). Trimethoprim resistance is common (≈40%) among CNS and is somewhat more frequent than among S. aureus isolates (35). The same genetic resistance determinant is found on conjugative multiresistant plasmids and on the chromosome in both species. The principal mechanism of resistance has been production of new plasmid-mediated dihydrofolate reductases that are trimethoprim-resistant (36).
The increasing resistance of CNS to methicillin and the ≈38% prevalence of methicillin-resistance among S. aureus isolates in large U.S. hospitals (23), as well as the rapid emergence of fluoroquinolone resistance among MRSA, have required frequent use of alternative antimicrobials. MRSA are resistant as well to cephalosporins, precluding use of the latter for staphylococcal infections. While only 15% of S. aureus isolates are clindamycin-resistant and 25% are erythromycin-resistant, a higher percentage have inducible resistance, and the use of these drugs in severe staphylococcal infections may be limited. A more suitable antimicrobial for life-threatening staphylococcal infections is the bactericidal drug vancomycin. Thus, the continuing problems with nosocomial MRSA infections and infections due to CNS have effected a major increase in the use of vancomycin. They have also led to extensive use of vancomycin in initial treatment of nosocomial fevers and for infections where MRSA and CNS are considered among possible pathogens. In addition, vancomycin usage by the oral route has also increased in the past 15 years in the treatment for Clostridium difficile pseudomembranous colitis.
CHANGES IN ANTIMICROBIAL USAGE OVER THE PAST TWO DECADES
Specific antimicrobial use depends on trends in antimicrobial-resistant pathogens as causes of disease. In the 1970s, ≈50% of antimicrobial usage involved administration to hospitalized patients. Study of parenteral antimicrobial usage at the University of Iowa Hospital between 1978 and 1992 provides an indication of changes that have occurred in major teaching hospitals (37). Whereas only 23% of patients in 1978 received one or more antimicrobials during their hospital stay, this figure rose to 44% by 1991. The principal increases in parenteral drug use were with vancomycin (increasing from use in <1% of patients in 1978 to 10% in 1992), and third-generation cephalosporins (increasing
from use in 1% of patients to 7% of patients by 1992). The quantity of vancomycin used per 1000 patient days increased 161-fold between 1978 and 1992. This experience is typical of that at other general hospitals. Such extensive use has had the potential, and now the reality, of selecting strains of vancomycin-resistant nosocomial pathogens (Enterococcus spp. and Staphylococcus hemolyticus) for which alternative therapies are extremely limited.
The Increasing Problem of Antibiotic Resistance in Enterococcus SPP.
Enterococci are intrinsically more resistant in vitro to penicillin (MIC, 2.5–5 µg/ml) than are other streptococci (MIC, 0.02–0.08 µg/ml). Since the reports of Hunter (38) in 1947 and of Jawetz et al. (39) in 1950, the capacity of penicillin and an aminoglycoside to act synergistically against enterococci has been known. The bactericidal action of this combination is due to limited penicillin inhibition of bacterial cell wall synthesis that permits enhanced uptake of aminoglycoside, the latter then acting lethally on its ribosomal target (40). Treatment of serious enterococcal infections has involved the use of combined penicillin–aminoglycoside therapy since the 1950s. Originally, the aminoglycoside was streptomycin. By the early 1970s, 25–50% of clinical isolates showed high-level (>2000 µg/ml) resistance to streptomycin and kanamycin. Accordingly, gentamicin was substituted for streptomycin. The penicillin and gentamicin combination proved to be synergistic in vitro against all enterococci tested during the 1970s and provided effective therapy. However, in 1979 the first enterococcus isolate with high-level (>2000 µg/ml) resistance to gentamicin was identified in Paris (41). Subsequently, enterococci with high-level gentamicin resistance and resistance to penicillin–gentamicin synergy have been observed worldwide (42). By the early 1990s, in some centers, >50% of enterococcal isolates showed resistance to penicillin–gentamicin synergy. Among Enterococcus faecium isolates, which prior to 1987 had been uniformly susceptible to gentamicin at 200 µg/ml, in some hospitals as many as 70% of isolates showed high-level resistance to gentamicin.
High-level resistance to streptomycin is due either to plasmid-mediated (adenylyltransferase) modification of the drug or, occasionally, can be due to a chromosomal mutation that alters ribosomal affinity for streptomycin (42). High-level gentamicin resistance is based on enzymatic modification of the aminoglycoside by a plasmid-encoded bifunctional enzyme with both 2"-phosphotransferase and 6'-acetyltransferase activities (43). Aminoglycoside modification by this bifunctional enzyme prevents penicillin (or vancomycin) synergy with all available aminoglycosides
except streptomycin. Fortunately, up to 46% of high-level gentamicin-resistant Enterococcus faecalis isolates are still susceptible to streptomycin. While all enterococci exhibit low-level intrinsic resistance to aminoglycosides, the MICs of certain aminoglycosides, (tobramycin, kanamycin, netilmicin, and sisomicin) are higher in E. faecium than E. faecalis. This relative resistance is based on a chromosomally mediated 6'-acetyltransferase and is of sufficient degree to prevent synergy with cell-wall-active antimicrobial agents (42).
To add to the problems produced by high-level gentamicin resistance in treatment of serious enterococcal infections, major resistance to penicillin has emerged. Although a variety of less frequently isolated Enterococcus species (E. gallinarum, E. asseliflavus, E. durans, E. avium, and E. raffinosus) have occasionally been responsible for infections, most clinical isolates have been E. faecalis (85–90%) and E. faecium (5–10%). Penicillin resistance in enterococci has been of two types: (i) higher levels of intrinsic resistance to penicillin and other β-lactams, particularly in E. faecium, and associated with production of greater amounts of a low-affinity PBP (PBP5) (44); (ii) β-lactamase production by enterococci (Bla+) carrying plasmids and exhibiting high-level gentamicin resistance (HLGR) as well. A large outbreak of infections has been caused by a clone identified in the mid-Atlantic area (45). Such a strain (Bla+ HLGR), once ensconced in a hospital, is difficult to eradicate, reminiscent of the problem with MRSA. In one outbreak, the β-lactamase and high-level gentamicin-resistance genes were chromosomal in location, probably on a conjugative transposon (46). Although penicillin resistance due to β-lactamase production is as yet uncommon in enterococci, it presents a major threat, particularly if conjugative spread occurs.
Vancomycin resistance among clinical enterococcal isolates, particularly E. faecium strains highly resistant to penicillin because of their low-affinity PBPs, was first recognized in England and France in the late 1980s, ≈30 years after the glycopeptide had been introduced in clinical use. Subsequent nosocomial outbreaks due to vancomycin-resistant E. faecium have occurred in several hospitals in the United States, including a cardiothoracic intensive care unit in one (47) and a medical-surgical intensive care unit in another (48). In the latter, nondisposable handles of electronic rectal thermometers were the means of spread of enterococci. In outbreaks some patients have developed serious infections such as bacteremia, peritonitis, and pneumonia. In a survey of 10,961 nosocomial enterococcal strains collected between 1989 and 1993 in the NNIS hospital system, 2.5% were found to be vancomycin-resistant (49). The prevalence of vancomycin resistance among nosocomial isolates rose from 0.3% in 1989 to 7.9% in 1993, and hospitals in nine states were sources of resistant isolates. The most striking increase in prevalence of
vancomycin resistance has been found in isolates from patients with nosocomial infections in intensive care units where the percentage rose from 0.4% in 1989 to 13.6% in 1993.
Vancomycin resistance in enterococci consists of several different phenotypes (50). The VanA phenotype consists of inducible high-level resistance to both vancomycin and another glycopeptide, teicoplanin, that is mediated by a transposable element (Tn1546) on a conjugative plasmid in E. faecium, E. faecalis, and E. avium. The expression of the VanB phenotype is inducible in E. faecium and E. faecalis and transferable by conjugation, but the location of the resistance genes is unknown and the resistance is to vancomycin and not to teicoplanin. The VanC phenotype is probably mediated by a chromosomal gene, present in E. gallinarum and E. casseliflavus, constitutive, and confers low-level resistance only to vancomycin.
Normal peptidoglycan synthesis involves the sequential action of D-Ala:D-Ala ligase and D-Ala-D-Ala adding enzyme to ultimately produce D-Ala-D-Ala termini on the pentapeptide side chains of the nascent bacterial cell wall (50, 51). The amide of the terminal D-Ala-D-Ala of vancomycin-susceptible bacteria appears to form a crucial hydrogen bond with a carbonyl group on the vancomycin skeleton (Figure 3) (51). This NH group appears to be essential for vancomycin binding to the cell wall and its inhibition of transglycosylation and transpeptidation of growing peptidoglycan strands.
Vancomycin-resistant bacteria, such as enterococci with the VanA phenotype, have acquired a transposable element, Tn1546, encoding nine genes, on a plasmid. The resistance gene products include a transmembrane sensor protein (VanS) that resembles eukaryotic transmembrane receptors. The latter senses the presence of vancomycin and transduces that information to a response regulator protein (VanR) that is a transcriptional activator of vanH and vanA genes (51). The latter two genes are critical in the subsequent expression of drug resistance (Figure 3). The vanH gene product is a new α-keto acid reductase that produces D-lactate or D-hydroxybutyrate from pyruvate and α-ketobutyrate, respectively. The VanA protein is a homolog of a D-Ala:D-Ala ligase with its specificity altered to preferentially utilize D-α-hydroxy acids such as D-lactate rather than D-Ala as the COOH-terminal component in ester linkage. Thus, a depsipeptide (e.g., D-Ala-D-lactate) is formed by VanA and is incorporated subsequently into the peptidoglycan termini of resistant bacteria (52). The substitution in the depsipeptide of an ester linkage for the amide linkage of the terminal D-Ala-D-Ala dipeptide appears to reduce vancomycin binding to the cell wall by ≈1000-fold, approximating the decreased vancomycin susceptibility of resistant strains. The novel depsipeptide peptidoglycan termini do not interfere
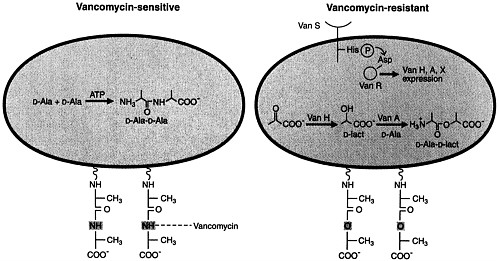
FIGURE 3 Schematic representations of vancomycin-susceptible and -resistant bacteria. Peptidoglycan strands of susceptible bacteria (Left) contain D-Ala-D-Ala termini to which vancomycin binds avidly. Peptidoglycan strands of resistant bacteria (Right) contain D-Ala-D-Lact depsipeptide termini (ester linkage) and have markedly reduced affinity for vancomycin. The plasmidborne transposable element Tn1546 encodes the VanH and VanA proteins that are α-keto acid reductases and depsipeptide ligases (with D-lactate or D-hydroxybutyrate as the COOH-terminal partner), respectively. Reproduced from Walsh (51) with permission (copyright American Association for the Advancement of Science).
with subsequent transpeptidation and strand crosslinkage, allowing formation of structurally intact cell walls of vancomycin-resistant Gram-positive bacteria.
Gram-positive bacteria other than Enterococcus spp. also may be resistant to vancomycin. Some clinical isolates of S. hemolyticus have shown resistance to vancomycin (53). Inherent resistance to vancomycin is a feature of Leuconostoc spp., Pediococcus spp., and Lactobacillus spp., all three rarely causing human infections.
A threat exists of further dissemination of vancomycin resistance to other species via transposition of Tn1546 into broad-host-range plasmids. Barriers do not exist to heterospecific expression of enterococcal resistance genes in Listeria monocytogenes, Bacillus spp., and Streptococcus spp. (50). Transfer of vancomycin resistance from E. faecalis to S. aureus via conjugation in the laboratory raises the frightening possibility of the emergence of glycopeptide resistance in MRSA (54).
RESISTANCE TO THIRD-GENERATION CEPHALOSPORINS AMONG GRAM-NEGATIVE AEROBIC BACILLI
Resistance of Gram-negative pathogens to β-lactam antibiotics is mediated by any one of three mechanisms (production of β-lactamases, reduced permeation of the drug, or alteration of the target site, i.e., PBP) or by several acting in concert. In the mid-1980s, it was noted that resistance was developing among Gram-negative bacilli possessing inducible chromosomal cephalosporinases (e.g., Enterobacter cloacae, Proteus spp., Serratia spp., Citrobacter freundii) when certain of the newer cephalosporins and cephamycins were used in therapy (55). Spontaneous mutation of such organisms to a stably derepressed (induced) state resulted in persistence in these mutants of high levels of β-lactamase even without the presence of an inducer. Such resistance is not transmissible but has been responsible for many instances of therapeutic failure, relapse, or nosocomial spread of such infections (56). Most outbreaks of such nosocomial infections have involved Enterobacter spp., and resistance has been present to virtually all β-lactam antibiotics (including newer cephalosporins and aztreonam) except imipenem.
Further problems in the treatment of infections due to Gram-negative bacilli became evident in the mid 1980s in France and Germany when Klebsiella isolates with plasmid-mediated resistance to broad-spectrum cephalosporins (cefotaxime, ceftriaxone, and ceftazidime) and aztreonam appeared (57). Such resistance soon became evident worldwide (58). The basis of such resistance is the emergence of new extended-spectrum β-lactamases, observed first in Klebsiella pneumoniae and subsequently in E. coli, C. freundii, Serratia marcescens, and Enterobacter
cloacae. These new resistances are mediated by plasmids encoding TEM-1-, TEM-2-, and SHV-1-related (mutated) β-lactamases. These new extended-spectrum β-lactamases differ from the original plasmid-encoded TEM-1 β-lactamase (mediating ampicillin resistance in Gram-negative enteric bacteria) and plasmid-encloded SHV-1 β-lactamase in Klebsiella, by 1–4 amino acids; and, thus, they are designated (TEM-4, SHV-3, etc.) to indicate this relatedness. The prevalence of extended-spectrum β-lactamases in Enterobacteriaceae has risen disturbingly over the past 7 or 8 years. For example, among isolates of K. pneumoniae from patients in 20 French hospitals, the prevalence of such resistance increased from <1% in 1985 to 11% in 1988 (56). In 1988–1989 in one hospital in Athens, Greece, 24% of K. pneumoniae and 4% of E. coli isolates possessed extended-spectrum β-lactamases. Enterobacteriaceae producing extended-spectrum β-lactamases have been responsible for a number of nosocomial outbreaks, usually originating in an intensive care unit and then spreading elsewhere (56). Such dissemination of drug resistance can involve spread of a particular strain of bacteria, horizontal interspecies spread of a resistance plasmid, or spread of resistance genes. Either extensive use of an extended-spectrum cephalosporin in a hospital unit or prior use in a particular patient has usually been associated with such outbreaks. Since K. pneumoniae containing extended-spectrum β-lactamases also often carry genes for amikacin resistance on the same plasmids, high-level use of the aminoglycoside may serve to select for resistant strains.
The potential for selection of increasingly resistant Gram-negative bacillary pathogens in a hospital can be seen in the sequence of events in one U.S. hospital over several years. In 1988–1989, a major outbreak of infections due to Acinetobacter occurred, requiring extensive use of ceftazidime, and to a lesser degree, imipenem, to which the infecting strain was susceptible. Late in 1988 an outbreak of ceftazidime-resistant K. pneumoniae infections began and 155 patients were infected or colonized during a 2-year period (59). Predisposing factors included >2 weeks of hospitalization and >7 days of antibiotic administration. Prior to isolation of the ceftazidime-resistant K. pneumoniae on average almost five antibiotics per patient had been administered. These isolates were resistant to aminoglycosides and to the bactericidal action of all third generation cephalosporins, moxalactam, and cephamycins and were susceptible only to imipenem. The strains examined contained several plasmids and produced β-lactamases with enzymatic characteristics suggesting TEM-10 or TEM-26. The outbreak was finally controlled with a combination of antibiotic (ceftazidime) restriction and barrier precautions for all infected or colonized patients.
The use of imipenem to treat infected patients in the aforementioned
hospital outbreak increased 3- to 4-fold. Subsequently, an outbreak of nosocomial infections (pulmonary and bacteremic) occurred in the surgical intensive care unit of the same hospital due to a strain of multiresistant Acinetobacter calcoaceticus (60). The organism was resistant to all antimicrobials ordinarily employed against Gram-negative aerobic bacilli: aminoglycosides (including amikacin); ampicillin; antipseudomonal penicillins; first-, second-, and third-generation cephalosporins; cephamycins; fluoroquinolones; aztreonam; imipenem; chloramphenicol; etc. The only antimicrobials to which the A. calcoaceticus isolates were susceptible were ampicillin–sulbactam (sulbactam responsible for the bactericidal effect of the combination) and polymyxin.
The above-described sequential outbreaks of nosocomial infections due to increasingly resistant organisms emphasizes the potentially great selective power of extensive antimicrobial use in a given institution, particularly in intensive care units, in favoring emergence of multiresistant pathogens.
CONTROL OF NOSOCOMIAL INFECTIONS DUE TO RESISTANT BACTERIA
In the past the rate of introduction of new antimicrobial drugs has been sufficient to counter those infections caused by organisms resistant to available drugs. Although there are >155 antibiotics (14), the rate of introduction of genuinely new drugs with different modes of action or genuinely different spectra of activity into clinical usage has slackened. Therapeutic options for nosocomial infections are increasingly limited because of antimicrobial resistance.
Control of this group of infections merits consideration of changes in some current practices. (i) Monitoring of nosocomial pathogens and their resistance patterns in acute-care hospitals. Although it is common practice for hospital microbiology laboratories to collect data on the antimicrobial susceptibilities of major pathogens and to provide summary information to physicians, such data commonly is tabulated for the recent year's experience, encompasses results from all parts of the hospital (including ambulatory services), and is provided to the physicians months later. This information is helpful for guidance in antimicrobial selection when a given pathogen has been identified or is suspected but its antimicrobial susceptibilities have not yet been determined. However, such information is less helpful in identifying early phases of nosocomial outbreaks in specialized units, since the data may become available late in the outbreak and may be obscured by collating susceptibilities from all parts of a hospital. In addition, data is not readily available in the form of antimicrobial-resistance patterns. Early detection of outbreaks of nosocomial
infection may be improved by ongoing monitoring of pathogens isolated and their resistance patterns in selected intensive care units on a monthly basis. (ii) Prompt institution of barrier precautions. Early identification of the new multiresistant pathogen serves to alert intensive care unit personnel to the problem and to institute isolation precautions before the strain is widely transmitted within the unit and elsewhere in the hospital. Once the number of infected and colonized patients increases containment becomes much more problematic. (iii) Judicious use of antimicrobial agents. The use of antimicrobials by physicians in hospitals (and elsewhere) requires acute awareness of the increasing problems with resistant organisms. Thus, unnecessary use of an antimicrobial drug has public health implications. Such use may serve to select for resistant organisms that may be carried to other more vulnerable patients and produce serious difficult-to-treat infections. Antibiotic control programs can be an effective means to prevent inappropriate use of antimicrobials in hospitals. Newer antimicrobials should be included in such programs to delay the emergence of resistant strains by limiting unnecessary use of such drugs. Indeed, consideration might be given in some instances to cycling the use of certain antimicrobials in selected intensive care units.
SUMMARY
About 5% of patients admitted to acute-care hospitals acquire nosocomial infections. A variety of factors contribute: increasing age of patients; availability, for treatment of formerly untreatable diseases, of extensive surgical and intensive medical therapies; and frequent use of antimicrobial drugs capable of selecting a resistant microbial flora. Nosocomial infections due to resistant organisms have been a problem ever since infections due to penicillinase-producing Staphylococcus aureus were noted within a few years of the introduction of penicillin. By the 1960s aerobic Gram-negative bacilli had assumed increasing importance as nosocomial pathogens, and many strains were resistant to available antimicrobials. During the 1980s the principal organisms causing nosocomial bloodstream infections were coagulase-negative staphylococci, aerobic Gram-negative bacilli, S. aureus, Candida spp., and Enterococcus spp. Coagulase-negative staphylococci and S. aureus are often methicillin-resistant, requiring parenteral use of vancomycin. Prevalence of vancomycin resistance among enterococcal isolates from patients in intensive care units has increased, likely due to increased use of this drug. Plasmid-mediated gentamicin resistance in up to 50% of enterococcal isolates, along with enhanced penicillin resistance in some strains, leaves few therapeutic options. The emergence of Enterobacteriaceae
with chromosomal or plasmid-encoded extended spectrum β-lactamases presents a world-wide problem of resistance to third generation cephalosporins. Control of these infections rests on (i) monitoring infections with such resistant organisms in an ongoing fashion, (ii) prompt institution of barrier precautions when infected or colonized patients are identified, and (iii) appropriate use of antimicrobials through implementation of antibiotic control programs.
REFERENCES
1. Haley, R. W., Culver, D. H., White, J. W., Morgan, W. M. & Emori, T. G. (1985) Am. J. Epidemiol. 121, 159–167.
2. Scheckler, W. E., Scheibel, W. & Kresge, D. (1991) Am. J. Med. 91, Suppl. 3B, 90S–94S.
3. Emori, T. G., Banerjee, S. N., Culver, D. H., Gaynes, R. P., Horan, T. C., Edwards, J. R., Jarvis, W. R., Tolson, J. S., Henderson, T. S., Martone, W. J., Hughes, J. M. & the National Nosocomial Infections Surveillance System (1991) Am. J. Med. 91, Suppl. 3B, 289S–293S.
4. Moss, A. J., Hamburger, S., Moore, R. M., Jr., Jeng, L. L. & Howie, L. J. (1991) Use of Selected Medical Device Implants in the United States, 1988 (Natl. Cent. Health Statistics, Centers for Disease Control, Atlanta), No. 191.
5. Health Industry Manufacturers Association (1992) Comments to FDA on the Medical Device Tracking Regulation.
6. Survey of Society of Thoracic Surgeons (1992) Ann. Thorac. Surg. Brochure.
7. Horan, T. C., Culver, D. H., Gaynes, R. P., Jarvis, W. R., Edwards, J. R., Reid, C. R. and the National Nosocomial Infections Surveillance System (1993) Infect. Control Hosp. Epidemiol. 14, 73–80.
8. United Network for Organ Sharing (1993) Scientific registry data.
9. Bortin, M. M., Horowitz, M. M. & Rimm, A. A. (1992) Ann. Intern. Med. 116, 505–512.
10. Carlisle, P. S., Gucalp, R. & Wiernik, P. H. (1993) Infect. Control Hosp. Epidemiol. 14, 320–324.
11. Martone, W. J., Gaynes, R. P., Horan, T. C., Emori, T. G. & Jarvis, W. R. (1991) Infect. Control Hosp. Epidemiol. 12, 609–621.
12. Christian, H. A. (1944) The Principles and Practice of Medicine (Appleton–Century, New York), pp. 54–55.
13. Finland, M. (1955) N. Engl. J. Med. 252, 570–580.
14. Neu, H. C. (1992) Science 257, 1064–1073.
15. McGowan, J. E., Jr., Barnes, M. W. & Finland, M. (1975) J. Infect. Dis. 132, 316–335.
16. McGowan, J. E., Jr. (1985) Rev. Infect. Dis. 7, Suppl. 3, S357–S370.
17. Horan, T. C., White, J. W., Jarvis, W. R., Emori, T. G., Culver, D. H., Munn, V. P., Thornsberry, C., Olson, D. R. & Hughes, J. M. (1984) Morbid. Mortal. Wkly. Rep. 35, 1SS, 17SS–29.
18. Banerjee, S. N., Emori, T. G., Culver, D. H., Gaynes, R. P., Jarvis, W. R., Horan, T. C., Edwards, J. R., Tolson, J. S., Henderson, T., Martone, W. J. & the National Nosocomial Infections Surveillance System (1991) Am. J. Med. 91, Suppl. 3B, 86S–89S.
19. Schaberg, D. R., Culver, D. H. & Gaynes, R. P. (1991) Am. J. Med. 91, Suppl. 3B, 72S–75S.
20. Kayser, F. H. (1975) Lancet ii, 650–653.
21. Lyon, B. R. & Skurray, R. (1987) Microbiol. Rev. 51, 88–134.
22. Boyce, J. M. (1991) Infect. Control Hosp. Epidemiol. 12, 79–82.
23. Panlilio, A. L., Culver, D. H., Gaynes, R. P., Banerjee, S., Henderson, T. S., Tolson, J. S., Martone, W. J. & the National Nosocomial Infections Surveillance System (1992) Infect. Control Hosp. Epidemiol. 13, 582–586.
24. Muder, R. R., Brennen, C., Wagener, M. M., Vickers, R. M., Rihs, J. D., Hancock, G. A., Yee, Y. C., Miller, J. M. & Yu, V. I. (1991) Ann. Intern. Med. 114, 107–112.
25. Cookson, B. D. & Phillips, I. (1988) J. Antimicrob. Chemother. 21, Suppl. C, 57–65.
26. Rosdahl, V. T. & Knudsen, A. M. (1991) Infect. Control Hosp. Epidemiol. 12, 83–88.
27. Westh, H., Jarlov, J. O., Kjersem, H. & Rosdahl, V. T. (1992) Clin. Infect. Dis. 14, 1186–1194.
28. Neu, H. C. (1992) Annu. Rev. Med. 43, 465–486.
29. Eliopoulos, G. M. & Eliopoulos, C. T. (1993) in Quinolone Antimicrobial Agents, eds. Hooper, D. C. & Wolfson, J. S. (Am. Soc. Microbiol., Washington, DC), 2nd Ed., pp. 168–169.
30. Peters, G. (1988) J. Antimicrob. Chemother. 21, Suppl. C, 139–148.
31. Hooper, D. C., & Wolfson, J. S. (1993) in Quinolone Antimicrobial Agents, eds. Hooper, D. C. & Wolfson, J. S. (Am. Soc. Microbiol., Washington, DC), 2nd Ed., pp. 98–110.
32. Trucksis, M., Wolfson, J. S. & Hooper, D. C. (1991) J. Bacteriol. 173, 5854–5860.
33. McMurry, L. M., Park, B. H., Burdette, V. & Levy, S. B. (1987) Antimicrob. Agents Chemother. 31, 1648–1650.
34. Weisblum, B., Siddhikol, C., Lai, C. J. & Demohn, V. (1971) J. Bacteriol. 106, 835–847.
35. Galetto, D. W., Johnston, J. L. & Archer, G. L. (1987) Antimicrob. Agents Chemother. 31, 1683–1688.
36. Burchall, J. J., Pelwell, L. P. & Fling, M. E. (1982) Rev. Infect. Dis. 4, 246–254.
37. Pallares, R., Dick, R., Wenzel, R. P., Adams, J. R. & Nettleman, M. D. (1993) Infect. Control Hosp. Epidemiol. 14, 376–382.
38. Hunter, T. H. (1947) Am. J. Med. 2, 436–442.
39. Jawetz, E., Gunnison, J. B. & Colman, V. R. (1950) Science 111, 254–256.
40. Moellering, R. C., Jr., & Weinberg, A. N. (1971) J. Clin. Invest. 50, 2580–2584.
41. Horodniceanu, T., Bougueleret, L., El-Solh, N., Bieth, G. & Delbos, F. (1979) Antimicrob. Agents Chemother. 16, 686–689.
42. Moellering, R. C., Jr. (1991) J. Antimicrob. Chemother. 28, 1–12.
43. Ferretti, J. T., Gilmore, K. S. & Courvalin, P. (1986) J. Bacteriol. 167, 631–638.
44. Fontana, R., Amalfitano, G., Rossi, L. & Satta, G. (1992) Clin. Infect. Dis. 15, 486–489.
45. Wells, V. D., Wong, E. S., Murray, B. E., Coudron, P. E., Williams, D. S. & Markowitz, S. M. (1992) Ann. Intern. Med. 116, 285–292.
46. Rice, L. B., Eliopoulos, G. M., Wennersten, C., Goldmann, D., Jacoby, G. A. & Moellering, R. C., Jr. (1991) Antimicrob. Agents Chemother. 35, 272–276.
47. Karanfil, L. V., Murphy, M., Josephson, A., Gaynes, R., Mandel, L., Hill, B. C. & Swenson, J. M. (1992) Infect. Control Hosp. Epidemiol. 13, 195–200.
48. Livornese, L. L., Dias, S., Samel, C., Romanowski, B., Taylor, S., May, P., Pitsakis, P., Woods, G., Kaye, D., Levison, M. E. & Johnson, C. C. (1992) Ann. Intern. Med. 117, 112–116.
49. Centers for Disease Control (1993) Morbid. Mortal. Wkly. Rep. 42, 597–599.
50. Arthur, M. & Courvalin, P. (1993) Antimicrob. Agents Chemother. 37, 1563–1571.
51. Walsh, C. T. (1993) Science 261, 308–309.
52. Handwerger, S., Pucci, M. J., Vol, K. J., Liu, J. & Lee, M. S. (1992) J. Bacteriol. 174, 5982–5984.
53. Schwalbe, R. S., Stapleton, J. T. & Gilligan, P. H. (1987) N. Engl. J. Med. 316, 927–931.
54. Noble, W. C., Virani, Z. & Cree, R. G. (1992) FEMS Microbiol. Lett. 93, 195–198.
55. Sanders, C. C. (1987) Annu. Rev. Microbiol. 41, 573–593.
56. Sanders, C. C. & Sanders, W. E., Jr. (1992) Clin. Infect. Dis. 15, 824–839.
57. Philippon, A., Labia, R. & Jacoby, G. (1989) Antimicrob. Agents Chemother. 33, 1131–1136.
58. Jacoby, G. A. & Medeiros, A. A. (1991) Antimicrob. Agents Chemother. 35, 1697–1704.
59. Meyer, K. S., Urban, C., Eagan, J. A., Berger, B. J. & Rahal, J. J. (1993) Ann. Intern. Med. 119, 353–358.
60. Urban, C., Go, E., Mariano, N., Berger, B. J., Avraham, I., Rubin, D. & Rahal, J. J. (1993) J. Infect. Dis. 167, 448–451.






















