Herpes Simplex Virus Infections of Women and Their Offspring: Implications for a Developed Society
RICHARD J. WHITLEY
While herpes simplex virus (HSV) infections of humans have been recognized since ancient times (1, 2), it was not until the 18th century that Astruc, physician to the King of France, identified herpes as a cause of genital infection (3). Subsequently, in 1893, Vidal reported human-to-human transmission of HSV infections, identifying the necessity of intimate human contact for spread of infection (2). Studies of the host immune response to HSV during the early 20th century provided insight into a unique property of HSV infection—namely, that neutralizing antibodies were identified in the sera of adults who subsequently developed recurrences, a phenomenon known as reactivation of latent infection (4). Neonatal HSV infection was not described until the 1940s (5, 6); however, the association between newborn disease and genital HSV infection was not made until the late 1960s (1).
With the evolution of our society in developed countries, particularly increasing sexual freedom associated with advances in birth control, and the emergence of sexually transmitted diseases, both horizontal (sexual partners) and vertical (mother to baby) transmission of HSV infection has become prevalent. Today, genital HSV infection exists in over 60 million Americans, most of child-bearing age, and results in the majority of the 1600 cases of neonatal herpes that occur yearly in the United
Richard J. Whitley is professor of pediatrics, microbiology, and medicine at the University of Alabama, Birmingham.
States. This article will review the changing epidemiology of genital and neonatal HSV infections with emphasis on the current status of therapy of the newborn, the cost of disease to society, and the need for the development of appropriate preventive strategies. Since the infected newborn is most likely to develop life-threatening disease and, therefore, incur the greatest costs to society, the baby becomes the starting point for our considerations.
INCIDENCE OF NEWBORN INFECTION
Although centers in the United States caring for infants with neonatal HSV infections have observed fluctuations in disease incidence, the estimated rate of occurrence is approximately 1 in 2500 to 1 in 5000 deliveries yearly (7–9). For unknown reasons, several countries worldwide, such as Africa and the United Kingdom, do not appear to recognize a significant number of cases of neonatal HSV infection in spite of the high prevalence of antibodies to HSV-2 (10, 11). In fact, the incidence of neonatal HSV infections in the United States may reflect the decreasing prevalence of HSV-1 antibodies and, therefore, the absence of transplacental humoral immunity, which might confer protection to the fetus.
PATHOGENESIS OF NEONATAL HSV INFECTIONS
At least four factors influence the incidence of newborn HSV disease. The first is the type of maternal genital infection at the time of delivery. The duration and quantity of viral excretion and the time to total healing vary with primary, initial, and recurrent maternal genital infections, such that primary, initial, and recurrent maternal genital infections, such that primary is most and recurrent is least severe (12, 13). Primary infection is associated with the excretion of 106–108 plaque-forming units of HSV for as long as 14–21 days. In contrast, recurrent infection is associated with a shorter duration of viral excretion (namely, 3–5 days) and at lower quantities (about 102 plaque-forming units of HSV). The incidence of neonatal herpes in babies born to women with primary or initial genital HSV infection is higher (33%) than those with recurrent infection (3%) (14). Second, the mother's HSV antibody status at delivery influences the severity of maternal infection as well as the likelihood of transmission. Transplacental maternal neutralizing and antibody-dependent cell-mediated cytotoxic (ADCC) antibodies have at least an ameliorative effect on acquisition and severity of infection for babies exposed to virus (15–18). Maternal primary infection late in gestation usually does not result in significant passage of maternal transplacental antibodies and, therefore, will increase risk to the fetus
(19). Third, the duration of ruptured membranes is an important indicator of risk for acquisition of neonatal HSV infection. Prolonged rupture of membranes (>6 hr) appears to increase the risk of fetal acquisition of infection, probably as a consequence of ascending infection from the cervix (7). Fourth, the application of fetal scalp monitors in the labor and delivery suite increases the risk of neonatal HSV infection by providing a site of inoculation of virus (20, 21). It should be remembered that between 20% and 60% of women of child-bearing age are HSV-2 seropositive, as discussed below. Thus, the probability of reactivation of latent virus, for the delivery population as a whole, increases and then provides a source of virus for scalp-electrode infection.
HSV infection of the newborn can be acquired at one of three times: in utero, intrapartum, or postpartum. The mother is the usual source of infection. Intrauterine infection occurs in ≈5% of all babies with neonatal HSV infection. These children usually have evidence of skin scarring/lesions at birth, chorioretinitis, and/or hydranencephaly. Intrapartum transmission accounts for about 85% of all cases and results from direct contact of the fetus with infected maternal genital secretions at delivery. Postnatal acquisition accounts for ≈;10% of cases and is the consequence of contact of the baby with an environmental source of HSV, usually a family member or care giver (22–31). Data from the National Institute of Allergy and Infectious Diseases (NIAID) Collaborative Antiviral Study Group (CASG) indicate that the frequency of babies with neonatal HSV-1 infections is nearly 30% (16). Since HSV-1 accounts for only ≈15% of all genital HSV infections in the United States, concern for postnatal acquisition should be high. However, family are as likely, if not more so, to be the source of newborn infection as nurses or hospital aides on the newborn or obstetrical services.
Since the mother is the source of infection in a majority of cases, an understanding of the changing epidemiology of genital HSV infection in women of child-bearing age is essential.
EPIDEMIOLOGY OF MATERNAL HSV INFECTIONS
Type-specific reagents that allow for the unequivocal distinction between HSV-1 and HSV-2 infections have provided the opportunity to define the changing seroprevalence of HSV-2 infections worldwide. The appearance of type-specific HSV-2 antibodies positively correlates with the onset of sexual activity (32–34), although crowded living conditions may contribute to infection (35, 36). The seroprevalence of HSV-2 in healthy women ranges from 10% to 60% in Americans to 77% in Ugandans (37). As many as 50–60% of lower socioeconomic women in the United States and elsewhere develop antibodies to HSV-2 by early
adulthood (38). Antibodies to HSV-2 are virtually nonexistent in nuns (32, 39, 40). Sera collected from pregnant women in the mid-1980s from Padua, Italy, and Orebro and Stockholm, Sweden, defined seroprevalence rates varying between 8% and 28%. Over the ensuing decade, these rates have increased 2-fold. Type-specific antibodies have been found in ≈35% of middle class women and 10–20% of women in higher socioeconomic groups in the United States (41–45).
Overall in the United States, HSV-2 seroprevalence increases from 6.9% at 15–29 years of age to 23.4% by the age of 60. When populations are analyzed according to race, these prevalence rates become 4.6% and 19.7% for Caucasians and 21.8% and 64.7% for Blacks, respectively (46). Factors found to influence acquisition of HSV type 2 include sex (women greater than men), race (people of color more than Caucasians), martial status (divorced versus single or married), and place of residence (city greater than suburban) (47). Clearly, seropositive pregnant women have the capability of reactivating HSV-2 at the time of delivery and, therefore, transmitting infection to their child.
The most important factor that influences acquisition of infection obviously is intimate exposure to an infected individual. Thus, rates of infection are influenced by the number of sexual partners (48–50). For heterosexual women living in the United States, the probability of acquisition of HSV-2 for those having one partner was <10%. The probability increased to 40%, 62%, and >80% if the number of total lifetime sexual partners increased to 2–10, 11–50, or >50, respectively. For heterosexual men, similar data were 0% for one lifetime sexual partner and 20%, 35%, and 70% for each of the subsequent three risk groups, respectively (47). With discordant antibody status between sexual partners, a susceptible female may become infected by an infected partner, creating risk if the woman is pregnant, especially if at term (51).
From the above data, it is apparent that the incidence of HSV-2 infection is a function of exposure to infected individuals; therefore, those with the largest number of sexual partners are most likely to acquire infection within any time frame. In one prospective study of low-risk individuals—namely, college students in Columbia, South Carolina, incidence was ≈2% per year over 4 years (22). The rate of acquisition of HSV-2 infection during pregnancy was 0.2% in Northern California and 2.5% in Birmingham, Alabama.
MATERNAL GENITAL HERPES SIMPLEX INFECTIONS: CLINICAL AND VIROLOGIC PARAMETERS
From the seroprevalence data, genital HSV infection in the woman—pregnant or otherwise—is common. A serious, but uncommon, problem
encountered with HSV infections during pregnancy is that of widely disseminated disease whereby infection can involve multiple visceral sites, in addition to cutaneous dissemination (52–57). Before the availability of acyclovir therapy, the mortality among these pregnant women is reported to be >50%. Fetal deaths occurred in >50% of cases, although mortality did not correlate with the death of the mother.
Maternal primary infection prior to 20 weeks gestation has been associated with spontaneous abortion but not at a high incidence (45). Primary infection during gestation also has been associated with fetal disease in utero (58, 59). Infection that occurs later in gestation has not been associated with the termination of pregnancy (60–62), but fetal morbidity has been documented, as evidenced primarily by intrauterine growth retardation (63).
Localized genital HSV infection is the most common form of infection during pregnancy. Prospective investigations indicate that genital HSV infection occurs at a frequency of about 1% at any time during gestation, as reviewed (7, 64, 65). Most of these infections have been considered recurrent. The frequency of HSV recurrences during gestation should be of concern to women with known histories of infection. Transmission of infection to the fetus is most frequently related to shedding of virus at the time of delivery. Since HSV infection of the fetus is usually the consequence of contact with infected maternal genital secretions at the time of delivery, the determination of viral excretion at this time is of importance. The actual incidence of viral excretion at delivery has been suggested to be 0.01–0.39% for all women, irrespective of past HSV history (7, 44, 66–68). For women with a past history of genital herpes, in a predominantly white, middle-class population, documented recurrent infection occurred in 84% of pregnant women (68). Moreover, asymptomatic viral shedding occurred in at least 12% of the recurrent episodes. Viral shedding from the cervix occurred in 0.56% of symptomatic infections versus 0.66% of asymptomatic infections (44, 66, 69). The incidence of cervical shedding in pregnant women with asymptomatic HSV infection has been reported to average ≈ 3.0% (70). The observed rate of shedding among pregnant women with asymptomatic infection has varied more than that among nonpregnant women (from 0.2% to 7.4%), depending upon the study population and trial design (44, 62, 66, 71, 72). The frequency of recurrences has not been shown to be different from one pregnancy to the next for any given woman (72). Overall, these data indicate that the frequency of cervical shedding is low, rendering the risk of transmission of virus to the infant similarly low when the infection is recurrent in nature (7). The frequency of shedding does not appear to vary by trimester during gestation (68, 71). Given the high seroprevalence of maternal infection, protection for the
fetus must exist or the incidence of neonatal disease would be significantly higher.
Importantly, 70% of infants who develop neonatal disease are born to women who are completely asymptomatic for genital HSV infections at the time of delivery and have neither a past history of genital herpes nor a sexual partner reporting a genital vesicular rash (16). Only 20% of these mothers reported genital HSV infection in their sexual partners.
CLINICAL PRESENTATION
The clinical presentation of babies with neonatal HSV infection is a direct reflection of the site and extent of viral replication. The significance of clinical presentation in the context of developing and developed societies is of utmost relevance in that it teaches the biomedical investigator of useful approaches to decision amelioration. Neonatal HSV infection is almost invariably symptomatic and frequently lethal. Infected babies are divided into three categories—namely, those with (i) disease localized to the skin, eye and/or mouth; (ii ) encephalitis with or without skin, eye and/or mouth involvement; and (iii) disseminated infection, which involves multiple organs, including central nervous system, lung, liver, adrenals, skin, eye, and/or mouth (7, 73). Table 1 summarizes disease classification of 291 babies with neonatal HSV infections studied by the NIAID CASG.
Disseminated Infection
Babies with the worst prognosis for both mortality and morbidity are those with disseminated infection. Disseminated disease involves multiple organs, especially the lung, liver, adrenal glands, and brain. Encephalitis appears to be a common component of this form of infection, occurring in about 60–75% of children with disseminated infection. Mortality in the absence of therapy exceeds 80%; all but a few survivors are impaired. These babies appear not to receive transplacental antibodies.
Encephalitis
Nearly one-third of all babies with neonatal HSV infection have encephalitis only. Babies with disseminated infection probably seed the brain by a blood-borne route, resulting in multiple areas of cortical hemorrhagic necrosis. In contrast, babies who present with only encephalitis likely have axonal transmission of virus to the central nervous system. Death occurs in 50% of babies with localized central nervous
TABLE 1 Demographic and clinical characteristics of infants enrolled in NIAID collaborative antiviral study
|
|
Disease classification |
|
|
|
Characteristic |
Disseminated |
CNS |
Skin, eyes, or mouth |
|
No. of babies |
93 (32) |
96 (33) |
102 (35) |
|
No. of male/no. female |
54/39 |
50/46 |
51/51 |
|
No. Caucasian/no. other |
60/33 |
73/23 |
76/26 |
|
No. premature (<36 wk) |
33 (35) |
20 (21) |
24 (24) |
|
Gestational age, wk |
36.5 ± 0.4 |
37.9 ± 0.4 |
37.8 ± 0.3 |
|
Enrollment age, wk |
11.6 ± 0.7 |
17.4 ± 0.8 |
12.1 ± 1.1 |
|
Maternal age, yr |
21.7 ± 0.5 |
23.1 ± 0.5 |
22.8 ± 0.5 |
|
Clinical findings |
|
||
|
Skin lesions |
72 (77) |
60 (63) |
86 (84) |
|
Brain involvement |
69 (74) |
96 (100) |
0 (0) |
|
Pneumonia |
46 (49) |
4 (4) |
3 (3) |
|
Mortality at 1 yr* |
56 (60) |
13 (14) |
0 (0) |
|
Neurologic impairment of survivors (no. affected/total no.) |
|
||
|
Total |
15/34* (44) |
45/81† (56) |
10/93† (11) |
|
Adenine arabinoside |
13/25† (50) |
25/51† (49) |
3/34† (9) |
|
Acyclovir |
1/6† (17) |
18/27† (67) |
4/51† (8) |
|
Placebo |
1/2† (50) |
2/3† (67) |
3/8† (38) |
|
CNS, central nervous system. Numbers in parentheses are percentages. * Regardless of therapy. † Denominators vary according to number with follow-up available. |
|||
system disease who are not treated and is usually related to brain stem involvement. With rare exceptions, survivors are left with neurologic impairment (74, 75).
Skin, Eye, and/or Mouth Infection
Infection localized to the skin, eyes and/or mouth is associated with no mortality, but it is associated with morbidity. These babies tend to receive large quantities of transplacental neutralizing and ADCC antibodies. Approximately 30% of these children eventually develop evidence of neurologic impairment (73–77). The significant neurologic findings include spastic quadriplegia, microcephaly, and blindness. Important questions regarding the pathogenesis of delayed onset neurologic debility are raised by such clinical observations. Despite normal clinical examinations in early infancy, neurologic impairment has become apparent between six months and 1 year of life. The clinical presentation is similar to that associated with congenital toxoplasmosis or syphilis.
TREATMENT OF NEONATAL HSV INFECTION
While both vidarabine and acyclovir are efficacious therapies for neonatal HSV infection, acyclovir is the treatment of choice in spite of not being licensed for this disease because of established safety for other indications (77). Acyclovir is an acyclic analog of guanosine. Virusspecified thymidine kinase phosphorylates acyclovir to its monophosphate derivative, an event that does not occur to any significant extent in uninfected cells. Acyclovir is then phosphorylated by cellular enzymes to its triphosphate derivative. Acyclovir triphosphate binds viral DNA polymerase, acting as a DNA chain terminator (78, 79). At levels 30 times higher than those used clinically, acyclovir can be teratogenic in the in vitro limb-bud assay, but other animal studies indicate that acyclovir is not a significant teratogen (80). Acyclovir is not a significant mutagen in the Ames test but induces chromosomal mutagenic events in a manner similar to that of caffeine (80). Because of the occasional need for acyclovir therapy during pregnancy, as well as the likelihood of frequent first trimester exposures to drug before pregnancy is recognized, an ''Acyclovir in Pregnancy Registry" is established to gather data on all reported prenatal exposures to oral acyclovir. Though no significant risk to the mother or fetus has been documented, the total number of monitored pregnancies remains too small to detect any epidemiologic risk that is not overwhelming (81). The safety of acyclovir in pregnancy, therefore, has not been unequivocally established. Since acyclovir crosses the placenta and can concentrate in amniotic fluid, there is valid concern about the potential for renal toxicity in the fetus (82). Limited data suggest the safety of acyclovir administration near term for mother and fetus (83).
The efficacy of vidarabine therapy (15 mg per kg per day over 12 hr as a continuous infusion for 10–14 days) rests on the demonstration of a decrease in mortality from 75% to 40% in infants with either disseminated or isolated central nervous system disease, and ≈50% of survivors developed normally (76). Furthermore, therapy decreased progression of disease from localized skin, eye, and mouth involvement to either encephalitis or disseminated disease from 70% in placebo recipients to 32% in vidarabine-treated babies. A subsequent clinical trial at 30 mg per kg per day for 10–14 days decreased progression to 4%. Although there were no deaths among infants with skin, eye, and mouth infection, severe neurologic impairment was decreased from 30% to 10% with vidarabine therapy (74).
Subsequent clinical trials have compared vidarabine to acyclovir for neonatal HSV infections. The NIAID CASG compared outcome for 202 babies with neonatal HSV infection who were randomly treated with
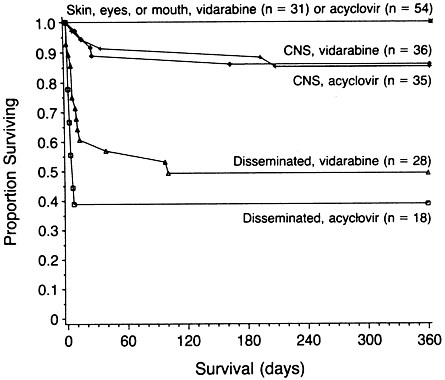
FIGURE 1 Survival of babies with neonatal HSV infection, according to treatment and the extent of disease. CNS, central nervous system. [Reproduced with permission from ref. 84 (copyright New England Journal of Medicine).]
either acyclovir or vidarabine (84). Mortality and morbidity data are summarized in Figure 1 and Table 2, respectively. Notably, there is no difference in mortality between treatment groups. Overall, the mortality was 0%, 18%, and ≈;55% for babies with skin, eye, or mouth disease, encephalitis, or disseminated infection, respectively. For babies with skin, eye, and mouth infection, there were no deaths; 90% and 98% of vidarabine and acyclovir recipients, respectively, were developing normally at 2 years of age. For babies surviving encephalitis, 50% of acyclovir recipients and 43% of vidarabine recipients were developing normally. For survivors of disseminated infection, 62% and 57% of vidarabine and acyclovir recipients, respectively, were normal at a 24-month follow-up. Thus, no significant differences exist between acyclovir and vidarabine therapy for any form of neonatal HSV infection.
Models of relative risk predicting patients at greatest likelihood for death or severe neurologic sequelae have been applied to the data, as
TABLE 2 Assessment of morbidity after 12 months in infants with neonatal HSV infection treated with vidarabine or acyclovir
|
|
Morbidity after 12 mo |
Alive after 12 mo, morbidity unknown |
Dead within 12 mo |
|
||||
|
Extent of disease |
Normal |
Mild |
Moderate |
Severe |
Sub- total |
Total |
||
|
Skin, eye, or mouth infection |
|
|||||||
|
Vidarabine |
22 |
1 |
1 |
1 |
25 |
6 |
0 |
31 |
|
Acyclovir |
45 |
0 |
1 |
0 |
46 |
8 |
0 |
54 |
|
Central nervous system infection |
|
|||||||
|
Vidarabine |
13 |
1 |
5 |
11 |
30 |
1 |
5 |
36 |
|
Acyclovir |
8 |
5 |
6 |
9 |
28 |
2 |
5 |
35 |
|
Disseminated disease |
|
|||||||
|
Vidarabine |
7 |
1 |
0 |
4 |
12 |
2 |
14 |
28 |
|
Acyclovir |
3 |
1 |
0 |
1 |
5 |
2 |
11 |
18 |
|
Total |
98 |
9 |
13 |
26 |
146 |
21 |
35 |
35 |
|
The values given are the numbers of infants. [Modified and reprinted with permission from ref. 84 (copyright New England Journal of Medicine).] |
||||||||
indicated in Table 3. From these data, if disease can be limited to the skin, eye, or mouth, clinical outcome is far superior to any other form of disease. It appears as though the quantity of transplacental maternal antibodies correlates positively to disease that remains limited to the skin, eye, and mouth. A confounding variable, however, is the recognition of ADCC antibodies on the sera of babies who present with encephalitis (18).
To improve outcome, it will be necessary to develop strategies that prevent the development of encephalitis or disseminated disease and institute therapy before coma ensues. Furthermore, neurologic impairment of babies with disease localized to the skin, eyes, and mouth emphasizes the need to further investigate pathogenic mechanisms and treatment options.
COST-BENEFIT ANALYSES OF ANTIVIRAL THERAPY
The deployment of acyclovir in the treatment of neonatal HSV infections and recognition of enhanced survival but with persistent morbidity prompted a cost-benefit analysis of the value of antiviral therapy from a societal perspective. Defining costs as documented for
TABLE 3 Prognostic factors identified by multivariate analyses for neonates with HSV infection
|
|
Relative risk |
|
|
Dominant factors |
Mortality |
Morbidity |
|
Total group (n = 202) |
|
|
|
Extent of disease |
|
|
|
Skin, eyes, or mouth |
1 |
1 |
|
CNS |
5.8* |
4.4* |
|
Disseminated |
33* |
2.1* |
|
Level of consciousness |
|
|
|
Alert or lethargic |
1 |
NS |
|
Semicomatose or comatose |
5.2* |
NS |
|
Disseminated intravascular coagulopathy |
3.8* |
NS |
|
Prematurity |
3.7* |
NS |
|
Virus type |
|
|
|
1 |
2.3† |
1 |
|
2 |
1 |
4.9* |
|
Seizures |
NS |
3.0* |
|
Infants with disseminated disease (n = 46) |
|
|
|
Disseminated intravascular coagulopathy |
3.5* |
NS |
|
Level of consciousness |
|
|
|
Alert or lethargic |
1 |
1 |
|
Semicomatose or comatose |
3.9* |
4.0* |
|
Pneumonia |
3.6* |
NS |
|
Infants with CNS involvement (n = 71) |
|
|
|
Level of consciousness |
|
|
|
Alert or lethargic |
1 |
NS |
|
Semicomatose or comatose |
6.1* |
NS |
|
Prematurity |
5.2* |
NS |
|
Seizures |
NS |
3.4* |
|
|
|
|
|
No. of skin-vesicle recurrences |
|
|
|
<3 |
NA |
1 |
|
≥3 |
NA |
21* |
|
Virus type |
|
|
|
1 |
NA |
1 |
|
2 |
NA |
14†‡ |
|
CNS, central nervous system; NS, not statistically significant (P > 0.05); NA, not applicable (no baby with disease confined to the skin, eyes, or mouth died). [Modified and reprinted with permission from ref. 92 (copyright New England Journal of Medicine).] *P < 0.01. †P < 0.05. †‡ Because of the correlation between virus type and skin-vesicle recurrence, virus type was not significant in the multivariate model; however, it was significant as a single factor. |
||
the care of children at the University of Alabama at Birmingham and assuming an annual incidence of disease of 1600 cases per year, acyclovir treatment had no significant effect on the direct costs of health care for babies with disease, as would be anticipated. However, indirect costs, as associated with the long-term care of the child with neonatal HSV infection, are significantly decreased. The overall reduction of costs to society was from $250,000,000 per year to approximately $215,000,000 (85). In addition to providing an objective assessment of the impact of antiviral therapy on this disease, the model provides an approach to defining the potential value of improvement for subsequent clinical trials, particularly those involving therapeutic interventions.
PREVENTION OF NEONATAL HSV INFECTION
With the increased awareness of serious neonatal HSV infection occurring as a consequence of maternal genital herpes, methods of prevention have attracted attention. An unnecessarily high frequency of Cesarean sections occurs in individuals with a history of recurrent genital herpes. In large part, this increased frequency of Cesarean section is related to maternal request as well as litigious concerns. Several questions remain to be resolved regarding the value of cesarean section in the prevention of neonatal HSV infections. While surgical delivery has been associated with decreased transmission of infection when membranes are ruptured <4 hr, Cesarean section has not been proven efficacious when membranes are ruptured for longer periods of time. Nevertheless, it has been recommended that when membranes are ruptured for up to 24 hr, there is still a time frame within which Cesarean section is of value. While these recommendations seem logical, no data exist from adequately performed clinical investigations to support the recommendation.
Antiviral Prophylaxis
Some investigators have suggested that acyclovir may be useful in preventing the occurrence of neonatal HSV infections in infants who are delivered unknowingly through an infected birth canal. The prophylactic use of acyclovir has also been suggested in pregnant women who have a known history of recurrent genital lesions. No data exist that establish the value of prophylactic antiviral therapy for the newborn. Such recommendations will be of importance in considering the option of vaginal delivery for the women excreting virus at the time of onset of labor.
Suppressive therapy of genital herpes in women with a known history
of recurrent infection may pose significant but undefined risk to the fetus. Suppressive therapy in women with recurrent genital herpes may not prove efficacious. It already has been established from the trials of suppressive acyclovir administered to individuals with frequently recurrent genital herpes that reactivation of virus can occur in spite of the administration of 200 mg of acyclovir three times daily (86). It is not unreasonable to think that virus shedding could occur in women taking acyclovir for suppressive therapy for recurrent genital HSV infection during the last 4 weeks of gestation. Furthermore, the pharmacokinetics and metabolism of acyclovir in the human fetus are totally unknown, although a small study of acyclovir's tolerance and pharmacokinetics at term appears reassuring (83, 87). The possibility of acyclovir fetal nephrotoxicity introduces a potential risk of drug administration, which must be considered. In addition, it must be recognized that the women who are at greatest risk for delivering babies who develop neonatal HSV infection are those least likely to have a history of recurrent genital HSV infection. Thus, those at greatest risk remain to be identified. Perhaps, the detection of type-specific antibodies to glycoprotein (g) G-2 will be of value in identifying those women at greatest risk.
Immunoprophylaxis
Over the past several years, attention has been directed to two arenas of immunoprophylaxis: monoclonal antibody administration and immunization. The technology that has allowed for the humanization of murine monoclonal antibodies as well as the development of human monoclonal antibodies may provide compounds that could be administered to babies delivered through infected birth canals (88, 89). In so doing, the newborn child might be provided a similar level of antibodies as that encountered from transplacental maternal antibody acquisition. Studies performed in newborn mice indicate that administration of either gB- or gD-humanized monoclonal antibodies can significantly decrease acquisition of mice subsequently exposed to HSV (89, 90). It should be noted that these antibodies may have therapeutic value when administered alone or with acyclovir (ref. 91; E. Kern, P. E. Vogt, J. Palmer, M. S. Co, and R.J.W., unpublished results).
Alternatively, the administration of a vaccine—either gB or gD subunit or live-attenuated—to susceptible individuals might prevent primary infection late in gestation and, therefore, shift the risk of the child exposed to HSV at delivery to at the worst 3% for disease acquisition instead of 33%. One promising candidate subunit vaccine from Chiron is undergoing extensive field trials to prevent genital HSV infection and, possibly, to treat individuals with frequently recurrent infection. Likely,
studies with a subunit vaccine will be undertaken in the future in pregnant women or those about to conceive.
CONCLUSION
Significant progress has been made in the diagnosis and management of neonatal HSV infection over the past two decades. Nevertheless, this disease continues to increase among all socioeconomic groups of our society. As the disease has become recognized in the United States, it has become similarly an ever-increasing problem in third world countries. The lack of adequate health care delivery systems in third world countries should cause greater attention to vaccine development. Future efforts must be directed toward the prevention of this disease rather than treatment after its occurrence.
SUMMARY
Herpes simplex virus infections of humans have been known since ancient times. Contemporary society has witnessed a series of devastating manifestations of herpes simplex virus infections—namely, genital herpes simplex virus infection and neonatal herpes simplex virus infection. With the evolution of society, particularly advances in birth control and increasing promiscuity, the seroprevalence of herpes simplex virus type 2 infections has increased worldwide, however, more so in developed societies. As a consequence, individuals of child-bearing age are at risk for either reactivation of herpes simplex virus at termination of gestation or acquisition of a new primary infection at that time. The consequences of vertical transmission of herpes simplex virus from mother to child, resulting in neonatal herpes simplex virus infection, can be devastating. Current efforts, which are directed toward the treatment of neonatal herpes, have established the value of drugs such as vidarabine and acyclovir. However, the real emphasis for future programs is the prevention of herpes simplex virus infections to avoid person-to-person transmission either horizontally or vertically. The development of vaccines directed against herpes simplex virus may be of value toward this end.
REFERENCES
1. Nahmias, A. J. & Dowdle, W. R. (1968) Prog. Med. Virol. 10 110–159.
2. Wildy, P. (1973) in The Herpesviruses, ed. Kaplan, A. S. (Academic, New York), pp. 1–25.
3. Hutfield, D. C. (1966) Br. J. Vener. Dis. 42. 263–268.
4. Andrews, C. H. & Carmichael, E. A. (1930) Lancet i, 857–858.
5. Whitley, R. J. (1990) in Virology, eds. Fields, B. N., Knipe, D. M., Chanock, R., Hirsch, M., Melnick, J., Monath, T. & Roizman, B. (Raven, New York), pp. 1843–1887.
6. Smith, M. G., Lennette, E. H. & Reames, H. R. (1941) Am. J. Pathol. 17, 55–68.
7. Nahmias, A. J., Keyserling, H. L. & Kerrick, G. M. (1983) in Infectious Diseases of the Fetus and Newborn Infant, eds. Remington, J. S. & Klein, J. O. (Saunders, Philadelphia), pp. 636–678.
8. Nahmias, A. J., Keyserling, H. L. & Lee, F. K. (1989) in Viral Infections of Humans: Epidemiology and Control, ed. Evans, A. S. (Plenum, New York), pp. 393–417.
9. Sullivan-Bolyai, J., Hull, H. F., Wilson, C. & Corey, L. (1983) J. Am. Med. Assoc. 250, 3059–3062.
10. Adam, E., Sharma, S. D., Zeigler, O., Iwamoto, K., Melnick, J. L., Levy, A. H. & Rawls, W. E. (1972) J. Natl. Cancer Inst. 48, 65–72.
11. Templeton, A. C. (1970) J. Clin. Pathol. 23, 24–30.
12. Corey, L. (1982) J. Am. Med. Assoc. 248, 1041–1049.
13. Reeves, W. C., Corey, L., Adams, H. G., Vontver, L. A. & Holmes, K. K. (1981) N. Engl. J. Med. 305, 315–319.
14. Brown, Z. A., Benedetti, J., Ashley, R., Burchett, S., Selke, S. , Berry, S., Vontver, L. A. & Corey, L. (1991) N. Engl. J. Med. 324, 1247–1252.
15. Yeager, A. S., Arvin, A. M., Urbani, L. J. & Kemp, J. A., III (1980) Infect. Immun. 29, 532–538.
16. Whitley, R. J., Corey, L., Arvin, A., Lakeman, F. D., Sumaya, C. V., Wright, P. F., Dunkle, L. M., Steele, R. W., Soong, S. J., Nahmias, A. J., Alford, C. A. & National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group (1988) J. Infect. Dis. 158, 109–116.
17. Yeager, A. S. & Arvin, A. M. (1984) Pediatrics 73, 188–193.
18. Kohl, S., West, M. S., Prober, C. G., Sullender, W. M., Loo, L. S. & Arvin, A. M. (1989) J. Infect. Dis. 160, 770–776.
19. Boulter, E. A., Zwartouw, H. T. & Thornton, B. (1982) Br. Med. J. 284, 746.
20. Kaye, E. M. & Dooling, E. C. (1981) Neurology 31, 1045–1055.
21. Parvey, L. S. & Chien, L. T. (1980) Pediatrics 65, 1150–1153.
22. Gibson, J. J., Homung, C. A., Alexander, G. R., Potts, W. A., Lee, F. K. & Nahmias, A. J. (1990) J. Infect. Dis. 162, 306–312.
23. Douglas, J., Schmidt, O. & Corey, L. (1983) J. Pediatr. 103, 908–910.
24. Francis, D. P., Hermann, K. L. & MacMahon, J. R. (1975) Am. J. Dis. Child. 129, 889–893.
25. Hammerberg, O., Watts, J., Chernesky, M., Luchsinger, I. & Rawls, W. (1983) Pediatr. Infect. Dis. 2, 290–294.
26. Kibrick, S. (1979) Pediatrics 64, 390.
27. Light, I. J. (1979) Pediatrics 63, 480–482.
28. Linnemann, C. C., Jr., Buchman, T. G., Light, I. J., Ballard, J. L. & Roizman, B. (1978) Lancet i 964–966.
29. Sullivan-Bolyai, J. Z., Fife, K. H., Jacobs, R. F., Miller, Z. & Corey, L. (1983) Pediatrics 71, 455–457.
30. Sanders, D. Y. & Cramblett, H. G. (1968) Am. J. Dis. Child. 116, 251–256.
31. Yeager, A. S., Ashley, R. L. & Corey, L. (1983) J. Pediatr. 103, 905–907.
32. Nahmias, A. J., Josey, W. E., Naib, Z. M., Luce, C. F. & Fuest, B. (1970) Am. J. Epidemiol. 91, 547–552.
33. Rawls, W. E., Tompkins, W. A. & Melnick, J. L. (1969) Am. J. Epidemiol. 89, 547–554.
34. Adam, E., Kaufman, R. H., Mirkovic, R. R. & Melnick, J. L. (1979) Obstet. Gynecol. 54, 171–173.
35. Wolinska, W. H. & Melamed, M. R. (1970) Acta Cytol. 14, 239–242.
36. Becker, W. B. (1966) S. Afr. Med. J. 40, 109–111.
37. Rawls, W. E., Adam, E. & Melnick, J. L. (1972) in Oncogenesis and Herpesviruses, Scientific Publication II, eds. Biggs, P. M., de The, G. & Payne, L. N. (Int. Agency Res. Cancer, Lyon, France), pp. 424–427.
38. Pagano, J. S. (1975) J. Infect. Dis. 132, 209–223.
39. Evans, A. S. & Dick, E. C. (1964) J. Am. Med. Assoc. 190, 699–708.
40. Rattray, M. C., Corey, L., Reeves, W. C., Vontver, L. A. & Holmes, K. K. (1978) Br. J. Vener. Dis. 54, 262–265.
41. Smith, I. W., Peutherer, J. F. & MacCallum, F. O. (1967) J. Hyg. 65, 395–408.
42. Arvin, A. M. & Prober, C. G. (1990) Ped. Infect. Dis. J. 9, 764–767.
43. Prober, C. G., Corey, L., Brown, Z. A., Hensleigh, P. A., Frenkel, L. M., Bryson, Y. J., Whitley, R. J. & Arvin, A. M. (1992) Clin. Infect. Dis. 15, 1031–1038.
44. Lee, F. K., Coleman, R. M., Pereira, L., Bailey, P. D., Tatsuno, M. & Nahmias, A. J. (1985) J. Clin. Microbiol. 22, 641–644.
45. Nahmias, A. J., Josey, W. E., Naib, Z. M., Freeman, M. G., Fernandez, R. J. & Wheeler, J. H. (1971) Am. J. Obstet. Gynecol. 110, 825–836.
46. Johnson, R. E., Nahmias, A. J., Magder, L. S., Lee, F. K., Brooks, C. & Snowden, C. (1989) N. Engl. J. Med. 321, 7–12.
47. Nahmias, A. J., Lee, F. K. & Bechman-Nahmias, S. (1990) Scand. J. Infect. Dis. 69, 19–36.
48. Rawls, W. E. & Gardner, H. L. (1972) Clin. Obstet. Gynecol. 15, 913–918.
49. Rawls, W. E., Gardner, H. L., Flanders, R. W., Lowry, S. P., Kaufman, R. H. & Melnick, J. L. (1971) Am. J. Obstet. Gynecol. 110, 682–689.
50. Rawls, W. E., Garfield, C. H., Seth, P. & Adam, E. (1976) Cancer Res. 36, 829–835.
51. Kulhanjian, J. A., Soroush, V., Au, D. S., Bronzan, R. N., Yasukawa, L. L., Weylman, L. E., Arvin, A. M. & Prober, C. G. (1992) N. Engl. J. Med. 326, 916–920.
52. Young, E. J., Killam, A. P. & Greene, J. F. (1976) J. Am. Med. Assoc. 235, 2731–2733.
53. Flewett, T. H., Parker, R. G. F. & Philip, W. M. (1969) J. Clin. Pathol. 22, 60–66.
54. Anderson, J. M. & Nicholls, M. W. N. (1972) Br. Med. J. 1, 632.
55. Goyette, R. E., Donowho, E. M., Hieger, L. R. & Plunkett, G. D. (1974) Obstet. Gynecol. 43, 191–196.
56. Hensleigh, P. A., Glover, D. B. & Cannon, M. (1979) J. Reprod. Med. 22, 171–176.
57. Peacock, J. E. & Sarubbi, F. A. (1983) Obstet. Gynecol. 61, 13–18.
58. Baldwin, S. & Whitley, R. J. (1989) J. Teratol. 39, 1–10.
59. Hutto, C., Arvin, A., Jacobs, R., Steele, R., Stagno, S., Lyrene, R., Willett, L., Powell, D., Anderson, R., Wetherman, J., Ratliff, G., Nahmias, A. J. & Whitley, R. J. (1987) J. Pediatr. 110, 97–101.
60. Thong, Y. H., Steele, R. W., Vincent, M. M., Henson, S. A. & Bellanti, J. A. (1973) N. Engl. J. Med. 289, 604–606.
61. Grossman, J. H., Wallen, W. C. & Sever, J. L. (1981) Obstet. Gynecol. 58, 1–4.
62. Harger, J. H., Meyer, M. P. & Amortegui, A. J. (1986) Obstet. Gynecol. 67, 637–642.
63. Brown, Z. A., Vontver, L. A., Benedetti, J., Critchlow, C. W., Sells, C. J., Berry, S. & Corey, L. (1987) N. Engl. J. Med. 317, 1246–1251.
64. Stagno, S. & Whitley, R. J. (1985) N. Engl. J. Med. 313, 1270–1273.
65. Stagno, S. & Whitley, R. J. (1985) N. Engl. J. Med. 313, 1327–1329.
66. Bolognese, R. J., Corson, S. L., Fuccillo, D. A., Traub, R., Moder, F. & Sever, J. L. (1976) Obstet. Gynecol. 48, 507–510.
67. Tejani, N., Klein, S. W. & Kaplan, J. (1979) Am. J. Obstet. Gynecol. 135, 547.
68. Vontver, L. A., Hickok, D. E., Brown, Z., Reid, L. & Corey, L. (1982) Am. J. Obstet. Gynecol. 143, 75–84.
69. Arvin, A. M., Hensleigh, P. A., Prober, C. G., Au, D. S., Yasukawa, L. L., Wittek, A. E., Palumbro, P. E., Paryani, S. G. & Yeager, A. S. (1986) N. Engl. J. Med. 315, 796–800.
70. Hatherley, L. I., Hayes, K. & Jack, I. (1980) Med. J. Aust. 2, 325–329.
71. Guinan, M. E., MacCalman, J., Kern, E. R., Overall, J. C. & Spruance, S. L. (1981) N. Engl. J. Med. 304, 759–763.
72. Harger, J. H., Pazin, G. J., Armstrong, J. A., Breinig, M. C. & Ho, M. (1983) Am. J. Obstet. Gynecol. 145, 784–791.
73. Whitley, R. J. (1990) in Infectious Diseases of the Fetus and Newborn Infants, eds. Remington, J. & Klein, J. (Saunders, Philadelphia), pp. 282–305.
74. Rasmussen, L. & Merigan, T. C. (1978) Proc. Natl. Acad. Sci. USA 75, 3957–3961.
75. Whitley, R. J. & Hutto, C. (1985) Pediatr. Rev. 7, 119–126.
76. Whitley, R. J., Nahmias, A. J., Soong, S.-J., Galasso, G. G., Fleming, C. L., Alford, C. A., Jr., & National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group (1980) Pediatrics 66, 495–501.
77. Whitley, R. & Gnann, J. (1992) N. Engl. J. Med. 327, 782–789.
78. Elion, G. B., Furman, P. A., Fyfe, J. A., de Miranda, P., Beauchamp, L. & Schaffer, H. J. (1977) Proc. Natl. Acad. Sci. USA 74, 5716–5720.
79. Schaeffer, H. J., Beauchamp, L., deMiranda, P., Elion, G. B., Baver, D. J. & Collins, P. (1978) Nature (London) 272, 583–585.
80. Dorsky, D. I. & Crumpacker, C. S. (1987) Ann. Intern. Med. 107, 859–874.
81. Andrews, E. B., Tilson, H. H., Hurin, B. A., Path, F. R. C. & Cordero, J. F. (1988) Am. J. Med. 85, 123–128.
82. Whitley, R. J. & Arvin, A. (1994) in Seminars in Pediatric Infectious Diseases, ed. Baker, C. J. (Saunders, Philadelphia), in press.
83. Frenkel, L. M., Brown, Z. A., Bryson, Y. J., Corey, L., Unadkat, J. D., Hensleigh, P. A., Arvin, A. M., Prober, C. G. & Connor, J. D. (1991) Am. J. Obstet. Gynecol. 164, 569–576.
84. Whitley, R. J., Arvin, A., Prober, C., Burchett, S., Corey, L., Powell, D., Plotkin, S., Starr, S., Alford, C., Connor, J., Jacobs, R. F., Nahmias, A. J., Soong, S.-J. & National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group (1991) N. Engl. J. Med. 324, 444–449.
85. Jue, S., Koanemon, S., Rowe, M., Laughlin, C., Whitley, R. & National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group (1992) Neonatal Herpes Simplex Virus (HSV) Infections: A Cost-Benefit Analysis of Acyclovir Therapy (ICAAC, Anaheim, CA), p. 350 (abstr. 1434).
86. Straus, S. E., Takiff, H. E., Seidlin, M., Bachrach, S., Lininger, L., DiGiovanna, J. J., Western, K. A., Smith, H. A., Lehrman, S. N., Creagl-Kirk, T. & Alling, D. W. (1984) N. Engl. J. Med. 310, 1545–1550.
87. Stray-Pedersen, B. (1990) Lancet 336, 756.
88. Co, M. S., Deschamps, M., Whitley, R. J. & Queen, C. (1991) Proc. Natl. Acad. Sci. USA 88, 2869–2873.
89. Kern, E. R., Vogt, P. E., Co, M. S., Kohl, S. & Whitley, R. J. (1992) Treatment of Herpes Simplex Virus Type 2 Infections in Mice with Murine and Humanized Monoclonal Antibodies (MABS) (Int. Soc. Antiviral Res., Vancouver, Canada) (abstr. 125).
90. Baron, S., Worthington, M. G., Williams, J. & Gaines, J. W. (1976) Nature (London) 261, 505–506.
91. Metcalf, J. F., Koga, J., Chatterjee, S. & Whitley, R. J. (1987) Intervirology 185, 1–11.
92. Whitley, R. J., Arvin, A., Prober, C., Corey, L., Burchett, S., Plotkin, S., Starr, S., Jacobs, R., Powell, D., Nahmias, A. J., Sumaya, C., Edwards, K., Alford, C., Caddell, G., Soong, S.-J. & National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group (1991) N. Engl. J. Med. 324, 450–454.


















