3
Reactor-Produced Radionuclides
HISTORICAL PERSPECTIVE
More than 50 years after the discovery of nuclear fission by Otto Hahn and Fritz Strassmann in 1938 and more than 50 years after the demonstration of a self-sustaining nuclear chain reaction by Fermi and coworkers in 1942, the use of reactor products permeates nearly every field of science. The book by Kamen (1957) documents this progress. A more complete treatment is provided by Stannard (1988). Historically, isotopes were provided to researchers by the Atomic Energy Commission and the Energy Research and Development Agency and are now provided by the U.S. Department of Energy (DOE). Oak Ridge National Laboratory (ORNL) played a pioneering role in the development of the first full-scale operating reactor prototype and the initial production of radioisotopes for applications in medical and biological research (Mirzadeh et al., 1992). Although the tracer principle was established by de Hevesy in the early 1900s (de Hevesy, 1913), the widespread uses of radioactivity began with the production of radionuclides at the reactor at ORNL. Sodium-24 was one of the first radionuclides used to measure the permeability of canine red blood cells in vivo. Although carbon-11-labeled compounds were created shortly after the development of the cyclotron by bombarding boron-10 with deuterons, the boron-10(d,n) reaction, the production of longer-lived carbon-14 by the nitrogen-14(n,p) reaction in the nuclear reactor at ORNL was instrumental in establishing its widespread use throughout the field of biology. The tracer approach was also quickly applied to clinical situations. Among the important ''firsts" were the determination of the speed of peripheral circulation using radium by Blumgart and Yens (1927) and the study of thyroid metabolism using radioactive iodine by Hamilton nd Soley (1939). Uptake, retention, and excretion of radiolabeled phosphorus (phospho-
rus-32) and radiolabeled iodine (iodine-131) provided valuable information about the selectivity of proposed therapeutic regimens. Radioisotopes of iron and chromium were also valuable in applications in hematology. Red blood cell survival, iron physiology, and blood volume were some of the important contributions. In the early 1940s, phosphorus-32 and then sulfur-35 and iodine-131, were used to label antigens and antibodies. In the process of studying the behavior of iodine-131-labeled insulin, Berson and Yalow (1959) developed the sensitive assay system for blood components known as radioimmunoassay, the importance of which was recognized with a Nobel Prize in 1977.
This body of work laid the groundwork for modern biomedical and clinical research, in particular with reference to the tracer principle, which became an invaluable tool (Bizzell, 1966; Mirzadeh et al., 1992). The use of radioisotopes is unique in that it provides a method for measuring biochemical processes in vivo, especially in cases in which the process is easily saturated, since radiation makes it possible to detect and localize quantities as small as only a few thousand radiolabeled molecules. The development of generator technology in the 1960s marked another advance in nuclear medicine research and in clinical nuclear medicine. The Brookhaven National Laboratory (BNL) used fission-product molybdenum-99 to produce the first molybdenum-99/technetium-99m (99Mo/99mTc) generator, revolutionizing the field of nuclear medicine (Tucker et al., 1958). 99mTc, the isotope used in more than 80 percent of the diagnostic nuclear imaging studies performed today, is the short-lived "daughter" resulting from the decay of 99Mo. Simple devices now enable hospitals to extract 99mTc from the 99Mo/99mTc generators as needed, and instant kits provide prepackaged chemicals to simplify its incorporation into organic molecules.
Other reactor-produced radioisotopes continue to play a major role in research, and recent advances in many fields (such as molecular biology, including the Human Genome Project) could not have been accomplished without the use of 32P. In addition, many of the isotopes useful for therapeutic applications, such as strontium-89 for the palliation of metastatic bone pain, are produced in reactors. Two other reactor-produced radioisotopes, samarium-153 and rhenium-186, may also be of use in the treatment of bone cancer and are currently under clinical study. There is therefore a need to maintain a continuous supply of these isotopes both for the benefit of patients and to provide investigators with the tools needed to develop and improve such technologies.
CURRENT APPLICATIONS IN MEDICINE AND PHYSICAL AND LIFE SCIENCES
The peaceful use of radioisotopes has made great progress since 1911, when George de Hevesy demonstrated that various substances could be radiolabeled and subsequently "traced" by spiking his meals with some naturally occurring radioactivity to prove that his landlady was using leftovers instead of fresh food
(Carmain, 1993). In addition to their use in the clinical practice of nuclear medicine and radiology and in the research conducted in those medical fields, radioisotopes have found applications in a wide variety of scientific fields such as nutrition, genetics, molecular biology, pharmacology, drug development, nuclear physics, environmental chemistry, geology, and industrial manufacturing.
Table 3-1 shows the extraordinarily widespread uses and applications of reactor-produced radioisotopes in the biomedical field.
The most important clinical radionuclide currently is 99mTc. Disintegration of this short-lived radionuclide product, which is itself the product of the decay of 99mMo, results in the emission of photons with sufficient energy to be passed through considerable amounts of tissue and to be detected by a sensitive camera. To the extent that the 99mTc can be confined to a specific organ or tissue (a tumor, for example), these photons produce an image of that organ or tissue. Much radiopharmaceutical research focuses on ways of concentrating 99mTc (and other radionuclides) in biological targets of interests, generally by tying it to a substance or molecule that is differentially taken up by that tissue. The 6-hour half-life of 99mTc minimizes the amount of radiation to which the patient is exposed, but puts a premium on scheduling and rapid action once the radiopharmaceutical is produced. The 99mMo/99mTc generator discussed above has vastly simplified this process by allowing hospitals to prepare their own radiopharmaceuticals on-site and when needed. Developed at Brookhaven National Laboratory in the early 1950s as part of the program to separate fission products, the 99/99mTc generator has now become the major source of radionuclides for nuclear medicine (Table 3-2). Other reactor-produced diagnostic radionuclides in common use are iodine-125, iodine-131, and xenon-133.
A potentially very large growth area involves therapeutic radionuclides. Perhaps as many as one-half to three-quarters of all cancer patients receive radiation at some point in their treatment. Cobalt-60 has been a very common source of gamma rays for external-beam treatment, with cesium-137 occasionally substituted for head and neck irradiation. Brachytherapy is often used for very well localized cancers. This originally involved implanting encapsulated "seeds" of radium or radon in or near the tumor, but now iodine-125, iridium-192, or gold-198 used more frequently. A third method has been the object of increasing research; it employs isotopes that naturally accumulate in a specific tissue or that can be attached to a molecule with such specificity. Iodine-131, one of the first isotopes produced with nuclear reactors, has been used for several decades to diagnose and treat thyroid diseases because of its affinity for thyroid tissue. Efforts to expand the therapeutic approach beyond the thyroid have primarily focused on reactor-produced isotopes, although an appreciable number are also produced in accelerators. The major categories of promising therapeutic applications are as follows:
-
Bone agents to reduce bone pain (strontium-89, rhenium-186, rhenium-188, samarium-153, and tin-117).
TABLE 3-1 Selected Reactor-Produced Radionuclides with Biomedical Applications
|
Radionuclides |
Uses |
|
Arsenic-77 |
• In cancer therapy |
|
Bromine-82 |
• In metabolic studies and studies of estrogen receptor content |
|
Calcium-47 |
• In studies of cell function and bone formation of mammals and to produce Scandium-47 |
|
Californium-252 |
• In brachytherapy for treatment of cervical cancer and potentially for treatment of gliomas |
|
Carbon-14 |
• For medical research to trace metabolism of new drugs and other organic carbon-containing molecules |
|
Cerium-141 |
• For research and development on lung densities |
|
Cesium-137 |
• To treat cancer; to measure correct patient dosages of radiopharmaceuticals |
|
Chromium-51 |
• To assess red blood cell survival studies |
|
Cobalt-58 |
• To diagnose pernicious anemia |
|
Cobalt-60 |
• To treat cancer and sterilize surgical instruments |
|
Copper-64 |
• As a clinical diagnostic agent for cancer and metabolic disorders |
|
Copper-67 |
• In cancer therapy and to label antibodies for cancer therapy |
|
Dysprosium-165 |
• To treat rheumatoid arthritis |
|
Dysprosium-166 |
• Decays to holmium-166 which is used in cancer therapy |
|
Einsteinium-253 |
• To radiolabel antibodies for cancer therapy |
|
Erbium-169 |
• To treat rheumatoid arthritis |
|
Fermium-255 |
• To radiolabel antibodies for cancer therapy |
|
Gadolinium-159 |
• In cancer therapy |
|
Gold-199 |
• In cancer therapy and to treat rheumatoid arthritis |
|
Holmium-166 |
• In cancer therapy and to treat rheumatoid arthritis |
|
Iodine-125 |
• As a potential cancer therapeutic agent and for basic biomedical research |
|
Iodine-129 |
• To check radioactivity counters in in vitro diagnostic testing |
|
Iodine-131 |
• To diagnose and treat thyroid disorders including cancer and for basic biomedical research |
|
Iridium-191 |
• To assess cardiac function especially in the pediatric population |
|
Iridium-192 |
• In cancer therapy |
|
Lutetium-177m |
• In cancer therapy and to label antibodies for cancer therapy |
|
Molybdenum-99 |
• To produce technetium-99m, the most commonly used radioisotope in clinical nuclear medicine |
|
Osmium-191 |
• Decays to iridium-191m, which is used for cardiac studies |
|
Osmium-194 |
• Decays to iridium-194, which is used in cancer therapy |
|
Palladium-103 |
• In the treatment of prostate cancer |
|
Phosphorus-32 |
• In cancer treatment, cell metabolism and kinetics, molecular biology, genetics research, biochemistry, microbiology, enzymology, and as a starter to make many basic chemicals and research products |
|
Phosphorus-33 |
• In cancer treatment, molecular biology and genetic research, and biochemical and enzymological studies |
|
Platinum-195m |
• In pharmacokinetic studies of antitumor agents |
|
Radionuclides |
Uses |
|
Rhenium-186 |
• As a bone cancer therapeutic agent and to radiolabel various molecules as cancer therapeutic agents; also used to treat rheumatoid arthritis |
|
Rhenium-188 |
• For treatment of medullary thyroid carcinoma and alleviation of pain in bone metastases |
|
Samarium-145 |
• For treatment of ocular cancer |
|
Samarium-153 |
• To radiolabel various molecules as cancer therapeutic agents and to alleviate bone cancer pain |
|
Scandium-47 |
• In the therapy of cancer |
|
Selenium-75 |
• In protein studies in life science research |
|
Silver-111 |
• In cancer therapy |
|
Strontium-85 |
• To study bone formation and metabolism |
|
Strontium-89 |
• To alleviate metastatic bone pain |
|
Strontium-90 |
• Decays to yttrium-90, which is used in cancer therapy |
|
Sulfur-35 |
• In studies of cell metabolism and kinetics, molecular biology, genetics research, biochemistry, microbiology, enzymology, and as a starter to make many basic chemicals and research products |
|
Technetium-99m |
• The most widely used radiopharmaceutical in nuclear medicine imaging |
|
Tellurium-123m |
• For research and development on lung densities and calibrating; also used in cardiology |
|
Tin-117m |
• For palliative treatment of bone cancer pain |
|
Tritium (hydrogen-3) |
• To make tritiated water, which is used as a starter for thousands of different research products and basics chemicals, and for life science and drug metabolism studies to ensure the safety of potential new drugs |
|
Tungsten-188 |
• Decays to rhenium-188 for treatment of cancer and rheumatoid arthritis |
|
Xenon-133 |
• In nuclear medicine for lung ventilation and perfusion studies |
|
Yttrium-90 |
• To radiolabel various molecules as cancer therapeutic agents |
|
SOURCES: Carmain, 1993; personal communication from T. Rosseel, Oak Ridge National Laboratory, February 11, 1994. |
|
-
Halogen-labeled estrogens for hormone-dependent cancers (bromine-82 and iodine-131).
-
Halogen-labeled nucleosides for cancer treatment (iodine-125, iodine-131).
-
Colloids for synovectomy (dysprosium-165, holmium-166, yttrium-90, and palladium-109).
-
Monoclonal antibodies and tissue-specific peptides for cancer treatment (iodine-125, iodine-131, yttrium-90, copper-64).
An example of drug development from the first of these categories is that of strontium-89 chloride (recently approved by the U.S. Food and Drug Administra-
TABLE 3-2 Technetium-Based Radiopharmaceuticals
|
Generic Name |
Product Name |
Use |
Manufacturer |
|
Technetium generator |
Ultra-TechneKow FM |
Supply of 99mTc |
Mallinckrodt |
|
Technetium generator |
Supply of 99mTc |
Medi-Physics |
|
|
TechneLite |
Supply of 99mTc |
DuPont-Merck |
|
|
Aggregated albumin |
Macrotec |
Lung imaging |
Squibb |
|
TechneScan MAA |
Lung imaging |
Mallinckrodt |
|
|
Pulmolite |
Lung imaging |
DuPont-Merck |
|
|
Aggregated albumin |
Lung imaging |
CIS-US |
|
|
MPI MAA |
Lung imaging |
Merck Sharp & Dohme |
|
|
Albumin colloid |
Microlite |
Imaging of RE system |
Dupont-Merck |
|
Serum albumin |
HSA Kit |
Blood pool imaging |
Medi-Physics |
|
Disofenin |
Hepatolite |
Hepatobiliary imaging |
Dupont-Merck |
|
Exametazime |
Ceretec |
Cerebral perfusion |
Amersham |
|
Lidofenin |
TechneScan HIDA |
Hepatobiliary imaging |
Merck Sharp & Dohme |
|
Mebrofenin |
Choletec |
Hepatobiliary imaging |
Squibb |
|
Medronate |
Osteolite |
Bone imaging |
DuPont-Merck |
|
AN-MDP |
Bone imaging |
CIS-US |
|
|
TecheScan MDP |
Bone imaging |
Merck Sharp & Dohme |
|
|
MDP-Squibb |
Bone imaging |
Squibb |
|
|
Medronate |
Bone imaging |
Medi-Physics |
|
|
TechneScan MDP |
Bone imaging |
CIS-US |
|
|
Mertiatide |
TechneScan MAG3 |
Renal imaging |
Mallinckrodt |
|
Oxidronate |
OsteoSan HDP |
Bone imaging |
Mallinckrodt |
|
Penetate sodium |
DTPA |
Kidney and brain imaging |
Medi-Physics |
|
AN-DTPA |
Kidney and brain imaging |
CIS-US |
|
|
Techneplex |
Kidney and brain imaging |
CIS-US |
|
|
Pyro- and tri- metaphosphates |
TechneScan PYP |
Bone imaging |
Mallinckrodt |
|
Phosphotec |
Bone imaging |
Squibb |
|
|
Pyrolite |
Bone imaging |
DuPont-Merck |
|
|
AN-Pyrotec |
Bone imaging |
CIS-US |
|
|
Red blood cell kit |
Ultratag RBC |
Bloodpool imaging |
Mallinckrodt |
|
RB-SCAN |
Blood pool imaging |
Cadema |
|
|
Sestamibi |
Cardiolite |
Myocardial imaging |
DuPont-Merck |
|
Gluceptate |
Glucoscan |
Kidney and brain imaging |
DuPont-Merck |
|
TechneScan Gluceptate |
Kidney and brain imaging |
Merck Sharp & Dohme |
|
|
Succimer |
DMSA |
Renal studies |
Medi-Physics |
|
Sulfur colloid |
Sulfur Colloid |
Gastrointestinal and organ studies |
Medi-Physics |
|
Tesuloid |
Gastrointestinal and organ studies |
Squibb |
|
|
AN-Sulfur Colloid |
Gastrointestinal and organ studies |
CIS-US |
|
|
Teboroxime |
CardioTec |
Myocardial imaging |
Squibb |
|
SOURCE: R. Brown, Mallinckrodt, Inc., personal communication, April 6, 1994. |
|||
tion) for the palliation of bone pain in patients with metastatic bone cancer. Strontium, rhenium, and tin are all preferentially taken up by bone, most readily at sites of active osteogenesis. Primary bone tumors and areas of metastatic involvement can thus accumulate significantly greater concentrations of radioactive strontium (or rhenium or tin) than surrounding normal bone. In addition, strontium-89 is retained in metastatic bone lesions much longer than it is in normal bone. At present, strontium-89 chloride is approved for use only as an adjunct to external-beam radiotherapy, but in such cases a single injection of strontium-89 has been shown to provide significant pain relief for 6 months (Lewington et al., 1991).
Radiohalogen-labeled estrogens are being tested for their ability to selectively deliver radiation to tumor sites in the breast and female reproductive organs (DeSombre et al., 1992; McLaughlin et al., 1989). Scientists at Mallinckrodt Medical Inc., have attached samarium-153 and holmium-166 to hydroxyapatite particles as potential synovectomy agents for the treatment of rheumatoid arthritis (University of Missouri Research Reactor Center, 1994). Radiohalogen-labeled pyrimidine nucleosides have the potential to deliver short-range (Auger) electrons to restricted sites in tumors (Adelstein,1993).
At a meeting on isotope availability at the Los Alamos National Laboratory, Zalutsky (1992) pointed out that the developments in biotechnology have enhanced the possibility of radioimmunotherapy, that is, raising monoclonal antibodies against specific cell surface antigens on a variety of human cell types, especially tumor cells, and using them to deliver radionuclides to those cells and only those cells. A variation on this strategy involves the use of antibody fragments and even smaller "molecular recognition units" like somatostatin and endothelin. Antibody and peptide carriers that can be used to detect and treat cancer are being developed (Hinkle, 1993; Serafini, 1993, Sugarbaker, 1993). Two are currently available for diagnostic purposes, and commercial companies are examining the use of dozens of new proprietary peptides as antitumor agents (Brice, 1994).
Because nuclides that emit radiation with different relative levels of biological effectiveness and ranges of action are available, an advantage of radioimmunotheraphy is the potential for choosing a radionuclide with physical characteristics that are compatible with a particular tumor type. Reviews discussing the potential utility of a variety of nuclides for antibody-mediated radiotherapy have been provided by Jungermann et al. (1984), Humm (1986), Cobb and Humm (1986), and Zalutsky (1992), but the majority of clinical radioimmunotherapeutic trials to date have used iodine-131 as the radionuclide. Although iodine-131 will probably be an adequate label for some therapeutic applications (particularly if improved labeling methods are used), in many circumstances, other nuclides might offer significant advantages (e.g., yttrium-90). Factors for consideration in selecting a nuclide for therapy include tumor size, proximity of the tumor to radiation-sensitive normal tissue, and the radiosensitivity of the tumor itself. The
ideal nuclide for treating a 0.5- to 1.0-kg advanced-stage liver tumor is likely to be different from the one selected to treat micrometastases of only a few hundred cells. In addition, the degree of heterogeneity in tumor dose deposition that results from regional variations in tumor blood flow, permeability, and antigen expression will dictate the relative efficacy of using radiation. Much work remains to be done in developing radioimmunotherapeutic strategies, and this type of therapy will probably be most useful, at least initially, as an adjunct to less specific forms of treatment such as surgery, chemotherapy, and external-beam radiotherapy. Numerous observers nevertheless see this approach as the door to a whole new era of nuclear medicine (Brice, 1994; Fischman et al., 1993; Schenter, 1993).
SUPPLIES AND SUPPLIERS
Molybdenum-99
Current Sources
In recent years there has been considerable concern about the availability of 99Mo in the United States (e.g., Holmes, 1993; Moody and Peterson, 1992). The sole North American source of this important biomedical isotope is at present a single reactor in Canada. Because 99mTc from the 99Mo/99mTc generator is used in more than 80 percent of nuclear medicine procedures, a guarantee of the continuous availability of 99Mo, which has a 66-hour half-life, is essential. A brief strike at the Canadian processing and shipping plant in 1991 focused attention on the potential problems of a single source of 99Mo, but recently the 99Mo supply situation has stabilized and is slowly becoming broader based.
The dominant worldwide supplier of 99Mo is Nordion International Inc. of Canada, which purchases this radioisotope mainly from a single government reactor (the NRU reactor) at Chalk River, 2 hours away from Nordion's processing facilities in Kanata, Ontario, Canada. A second reactor for 99Mo production by Nordion, the Maple-X reactors, was under construction but is currently on hold pending resolution of a dispute over costs with the Canadian Atomic Energy of Canada Ltd. (AECL), a Crown (Canadian government) Corporation that operates all research reactors in Canada. Nordion International, Inc. was formed more than 20 years ago as the Radiochemical Company, a division of AECL. As the Radiochemical Company, it processed and sold reactor isotopes produced by the Research Company of the AECL. In 1991, MDS Health Group Ltd., a private company and Canada's largest diversified health care company, bought the Radiochemical Company for $165 million and renamed it Nordion International, Inc., upon privatization. According to MDS and Nordion, the key to their purchase was a 23-year guaranteed supply contract with AECL, which was then making isotopes with two reactors, the NRU and NRX reactors, and was about to
begin construction of a third, the Maple-X reactor, whose primary purpose would be isotope production. The NRU reactor was down for repairs for nearly all of 1991, but Nordion was able to meet customer demand from the NRX reactor. The NRX reactor itself was shut down for repairs in January 1992, and in March 1993 the AECL announced that it would not reopen, leaving Nordion without a backup reactor until completion of the Maple-X reactor. In November 1993 AECL announced that despite the $40 million that it had sunk into costs for design, licensing, and a building, projected costs for the Maple-X reactor had grown too high to allow completion. Apparently, neither AECL nor MDS anticipated that the $165 million paid by the latter for Nordion would not go to AECL, but instead would simply disappear into the federal government's consolidated revenue fund.
MDS has filed a lawsuit claiming breach of contract and has asked the court to order AECL to complete construction or pay MDS $300 million in compensation. In a separate action, Nordion and AECL have entered into a prearranged arbitration process as set out in a 1988 agreement. At issue in that process is AECL claim that unforeseen events had forced the closure of the NRX reactor and raised production costs to the extent that AECL is entitled to renegotiate the supply contract.
Arbitration hearings were to begin sometime in the Spring of 1994, but they have been delayed until completion of a report on possible solutions commissioned by the Canadian government's Office of Energy. In any event, Nordion began searching for alternate backup arrangements a year earlier when it became clear that the NRX reactor would not be reactivated. After unsuccessful negotiations with a number of North American reactors, Nordion has struck an agreement with the Institute National des Radioéléments (IRE) in Fleurus, Belgium. IRE can use any of four European reactors for the irradiation of targets: BR-2 in Belgium, SILOE and OSIRIS in France, and HFR in Petten, The Netherlands. All processing takes place at Fleurus, the site of Nordion's European operations. IRE was expected to be capable of producing 50 percent of the world's 99Mo requirements by mid-1994. Table 3-3 provides information about a number of other reactors around the world capable of producing substantial amounts of 99Mo. In most cases a decision to produce 99Mo for sale would require considerable time and money for processing and distribution arrangements. Staff at one of these sites, the University of Missouri Research Reactor (MURR), informed the committee of a much simpler plan that would maintain a supply of 99Mo in case of difficulties with the Chalk River reactor. When Nordion was just beginning its search for backup capabilities, MURR proposed that unprocessed sections of spent reactor fuel be shipped to Nordion for extraction of 99Mo. Such sections would contain several thousand curies of 99Mo, just like the fuel elements normally extracted from the AECL NRU reactor. Details of the cost and feasibility of the plan have not been worked out, but it seems reasonable to investigate whether this can be a cost-effective near-term solution to concerns about reliance on a single reactor for 99Mo.
TABLE 3-3 Reactors with Significant Isotope Production Capability
|
Country |
Location |
Reactor |
Power (MW) |
Isotopes Currently Produced |
|
United States |
Hanford, Washington |
FFTF |
400 |
None; reactor in standby mode |
|
Idaho |
ATR |
250 |
60Co, 63Ni, 192Ir, 153Gd |
|
|
Oak Ridge National Laboratory |
HFIR |
100 |
252Cf and other transuranics, 85Kr, 64Cu, 67Cu, 117mSn, 195mPt, others |
|
|
Brookhaven National Laboratory |
HFBR |
60 |
47Sc, 55Fe, 64/67Cu, 119Sn, 153Sm, 186Re 198/199Au, others |
|
|
University of Missouri |
MURR |
10 |
32P, 192Ir, 35S, 198Au, 186Re, 51Cr, 103Pd, many others |
|
|
Los Alamos National Laboratory |
OWR |
8 |
None; possible production of 99Mo |
|
|
Georgia Tech |
GTTR |
5 |
90Y, 24Na, 18F, 140La |
|
|
Massachusetts Institute of Technology |
MITR-II |
5 |
165Dy, 166Ho, 198Au |
|
|
New Mexico |
Sandia |
2 |
None; possible production of 99Mo |
|
|
Oregon State |
OSTR |
1 |
Variety |
|
|
Belgium |
Mol |
BR-2 |
100 |
99Mo, 133Xe, 131I, 192Ir, others |
|
Sweden |
Nykoping |
Studsvik |
50 |
32P, 60Co, 192Ir, 89Sr, 90Y, others; principal supplier for Amersham |
|
The Netherlands |
Petten |
HFR |
45 |
99Mo, 133Xe, 131I, 192Ir, others |
|
Canada |
Chalk River |
NRU |
40 |
99Mo, 60Co, 14C, 32P, 89Sr, 90Y, 125I, 131I, 137CS, 133Xe, 192Ir |
|
France |
Saclay |
OSIRIS |
70 |
99Mo, 133Xe, 131I, 192Ir, others |
|
Grenoble |
SILOE |
35 |
99Mo, 133Xe, 131I, 192Ir, others |
|
|
Poland |
Warsaw |
Maria |
30 |
Possible fission products |
|
Country |
Location |
Reactor |
Power (MW) |
Isotopes Currently Produced |
|
Australia |
Sydney |
HIFAR |
10 |
99Mo, 64Cu, 67Cu, 153Sm, 166Dy, 186Re, 188Re, 198Au |
|
Russia |
Chelyabinsk |
Mayak |
Unknown |
137Cs, 60Co, 14C, 3H, 88Kr |
|
Obninsk |
IPPE |
Unknown |
131/132/133I, 32/33P, 133Xe, 99Mo, 140Ba, 95Zr, 95Nb, 137Cs, 147Pm |
|
|
Dimitrovgrad |
SM3 |
Unknown |
33P, 60Co, 192Ir, 153Gd, 252Cf |
|
|
Unknown |
SM2 |
100 |
192Ir, 60Co |
|
|
Moscow |
Unknown |
Unknown |
99Mo/99mTc, 98Mo |
|
|
South Africa |
Pelindaba |
Safari |
20 |
99Mo |
Future Sources
One of Nordion's biggest customers, Mallinckrodt Medical Inc., has recently secured its own backup supply of 99Mo by building additional facilities at the reactor site at Petten, The Netherlands, Mallinckrodt's current plan is to produce enough 99Mo to meet its own needs in both Europe and North America but not to go into competition with Nordion for the world market. Their action will substantially reduce that market nevertheless.
DOE, partly in response to the concerns expressed by the U.S. nuclear medicine community, is seriously considering resuming the production of 99Mo using one of two small reactors until recently devoted to defense missions. The Atomic Energy Commission produced 99Mo at the Brookhaven and Oak Ridge National Laboratories until mid-1966, when it stopped production in deference to U.S. commercial production and sales by Union Carbide in Tuxedo, NY, and later, at the General Electric Test Reactor in Pleasanton, Calif. The latter closed in 1977, and Union Carbide's successor, Cintichem, shut down the Tuxedo reactor in 1990. The Isotope Production and Distribution Program (IPDP), with the aid of some quarter of a million dollars from the radiopharmaceutical industry, immediately began a feasibility study that ultimately pointed to the underutilized Omega West reactor at Los Alamos National Laboratory as a potentially viable source of 99Mo and other reactor isotopes (Public Law 101-101, the Energy and Water Development Appropriations Act of 1990, required isotope production and distribution to be self-supporting as of fiscal year 1990). DOE bought the relevant technology from Cintichem and invested $3.5 million dollars for process devel-
opment at Los Alamos National Laboratory, only to have the reactor shut down by a leaking coolant pipe. In addition, the current reactor operator, DOE's Defense Programs division announced plans to halt all use of the reactor, leaving IPDP with all operating costs. In the interim, the major buyers have signed long-term contracts with Nordion or other suppliers, reducing prospects for substantial DOE sales. Additional defects have been discovered since the reactor shut down, the repair of which could cost more than $10 million. Largely because of these recent events, the small (2-megawatt [MW] annular core research reactor at Sandia National Laboratory has been receiving serious consideration as a producer of 99Mo. Start-up costs and perhaps operating costs would be lower, although production capacity would likely be reduced as well.
Although the long-term supply of 99Mo will probably depend in large measure on the outcome of the Maple-X reactor dispute, the committee is convinced that the short-term situation is no longer precarious. Furthermore, the committee is not convinced that DOE can penetrate the market sufficiently at this point to make 99Mo a significant source of net revenue. The committee therefore recommends against government support of a dedicated domestic reactor for 99Mo production.
The Advanced Neutron Source (ANS), a new 330-megawatt (MW) research reactor currently proposed for construction at Oak Ridge National Laboratory, is a potential source for the cost-effective production of 99Mo sometime after 2003. The primary research, education, and training missions of this $2 billion to $3 billion reactor center do not include isotope production at the moment, but focus on condensed-matter research and materials analysis. Its very high rate of neutron production will allow for the high-quantity, high-quality production of any radioisotopes that can be produced by a reactor, and DOE has identified it as a potential future source of isotopes, ''to the extent permitted by its primary mission" (White, 1993). Commercial production of 99Mo or any other short-lived isotope is a major commitment, however, and it is the perception of many of DOE's present isotope customers that IPDP's second-class status at the present facilities is a major hurdle to efficient operation of both IPDP and its customers. If ANS is to be the nation's primary source of reactor isotopes, DOE must ensure the availability of appropriate ports and retrieval vehicles in the design of this reactor and equitable treatment of isotope customers in the plans for its administration. A recent report by Mirzadeh et al. (1944) on the projected capabilities of ANS is very encouraging. It makes it very clear that as the the successor to the High Flux Isotope Reactor, ANS is envisioned as a powerful resource for the production of radioisotopes for a wide variety of scientific and industrial applications.
Research and Development on Alternative Sources
Unlike many reactor-produced radionuclides, which are created by the capture of neutrons from the reactor core by a target nucleus, 99Mo is a fission product, one of the pieces into which highly enriched uranium-235 breaks when it is bom-
barded by the reactor's neutrons. The yield of 99Mo is less than 10 percent, with the remainder largely being long-lived radioactive waste. Public concern over radioactive waste provides significant economic and political incentives for the development of alternate methods for the production of this isotope.
The committee was informed of concepts developed by two different groups in the United States for accelerator-based methods for the production of 99Mo. In one of these, the "Molytron" concept (Schmidt, 1993), the primary beam is high energy protons (60 to 150 million electron volts [MeV], whereas the other, advocated by Morgan (1993) of the North Texas Research Institute, proposes using neutrons generated via accelerator-based methods at low energy.
A different approach is being attempted by researchers at the University of Missouri who have used patented gel technology to develop a 99Mo/ 99mTc generator that produces low-specific-activity 99Mo by neutron activation of 98 instead of the high-specific-activity 99Mo fission product (University of Missouri Research Reactor Center, 1994).
These technologies are far from proven at this point and are competing with a very successful and strongly established commercial operation. The committee recommends that DOE not solicit or evaluate proposals for the development of such methods, but rather leave that work to the private sector.
Other Commercial Radionuclides
Table 3-4 shows the estimated 1992 revenues to reactor operators or their packaging and distributing partners from their best selling products. As mentioned above, there are no real leaders beyond 99Mo and cobalt-60, which is used for external-beam radiation therapy. Unlisted but close behind the other isotopes in table 3-4 would be strontium-89 (which could jump up the list now that it has received approval from the U.S. Food and Drug Administration as an analgesic for bone cancer). Nordion, because its focus is on high -volume, high-revenue isotopes, dominates the market for these radionuclides as well. As in the case of 99Mo, DOE reactors were once prime sources of these isotopes, but DOE with drew from production in deference to domestic commercial suppliers who have since gone out of business. At present DOE controls a large share of the market only for californium-252, iridium-192, and tritium (hydrogen-3). All three of these are long-lived and are or will likely be targets of serious competition from Russia and other former Soviet republics. If short-lived reactor-produced radionuclides become important for cancer therapy, however, and Canada's Maple-X reactor is not completed, the number and condition of North American reactors will be inadequate unless the ANS serves as a major producer.
Research Radionuclides
A comparison of Tables 3-1 and 3-4 makes it obvious that however ably Nordion is serving the needs of commercial radioisotope users, medical and
TABLE 3-4 Leading Commercial Reactor-Produced Radioisotopes
|
Isotope |
Half-Life |
Estimated 1992 Wholesale Demand (thousands of $) |
|
Molybendum-99 |
66 h |
25,000–33,000 |
|
Cobalt-60 |
5.3 yr |
33,000–44,000 |
|
Iridium-192 |
74 days |
4,000–5,000 |
|
Carbon-14 |
5,730 yr |
2,600–3,000 |
|
Xenon-133 |
5.2 days |
2,100—2,600 |
|
Hydrogen 3 (tritium) |
12 yr |
1,600–2,400 |
|
Iodine-131 |
8.0 days |
1,600–2,000 |
|
Californium-252 |
2.6 yr |
1,500–2,400 |
|
Cesium-137 |
29 yr |
1,500—2,000 |
|
Iodine-125 |
60 days |
1,200–1,400 |
|
Phosphorus-32 |
14 days |
600–1,000 |
|
Sulfur-35 |
87 days |
350–500 |
|
Yttrium-90 |
64 h |
250–300 |
|
SOURCES: Arthur Andersen & Co. (1993), except for phosphorus-32 and sulfur-35 (A. Ketring, University of Missouri, personal communication, May 17, 1994) and carbon-14 (C. Marchetti, DuPont-NEN, personal communication, May 18, 1994). |
||
industrial, it is clearly not the driving force in the exploration of new and better uses of radionuclides. In the United States, DOE's national laboratories and DOE-fueled university reactors play a critical role in providing reactor-produced radioisotopes for medical research as well as clinical use, including a number of unique isotopes for medical uses. They produce not only low-demand radioactive and enriched stable isotope products but also a number of associated services to domestic and international customers for medical, research, and industrial applications. However, isotope programs have always depended on the "parasitic" use of reactors, whose primary use was not related to isotope production, and the declining budgets have severely affected the operating times of these reactors, resulting in a curtailed supply of isotopes. Furthermore, the revolving fund provided by the Energy and Water Development Appropriations Act of 1990 (Public Law 101-101) along with the mandate to become financially self-sufficient through sales is already deeply in the red, despite the fact that costs of reactor isotopes have increased beyond the reach of many researchers. This has led to a severe reduction in the development of new radiopharmaceuticals at the very time when research is moving at a rapid pace in complementary areas like molecular biology and cancer diagnosis and treatment (Holmes, 1993).
Although there are a number of large research reactors at universities in the United States, the primary U.S. source of research radionuclides is MURR. In 1993, 56 of 157 MURR publications were on biomedical or life science projects (University of Missouri Research Reactor Center, 1994). Only MURR is cur-
rently producing a substantial number of radionuclides for use in research in radiopharmaceuticals. Isotopes, irradiation, and related services are supplied to approximately 300 clients in 45 industries, 7 state and federal laboratories, and 31 other universities. The shipments of radionuclides over the past year from MURR as well as the medical and nonmedical applications of these isotopes are listed in Table 3-5. Clients are typically charged only the incremental costs for production of the radioisotopes, with the major proportion of the center's $8 million annual operating costs coming from the state of Missouri, research grants, and non-radioisotope-related services (e.g., topaz irradiation to provide its characteristic color). Income from the last of these sources, much like that from DOE sales of enriched stable isotopes, has recently become threatened by the predatory pricing policy of Russian reactor facilities.
Since 1970, DOE's Office of Energy Research has provided the fuel required to operate some 37 (originally 76) university research and training reactors and, since 1987, has been supporting the conversion of designated university reactors from using highly enriched uranium fuel to using low-enrichment uranium fuel (as part of international nuclear weapons nonproliferation activity). However, DOE has not provided support for the operational costs and facility renovation at university reactors in the past (Riggs, 1993), and recent DOE budget constraints do not allow DOE to provide this support.
It is instructive to review the support of reactors in Western Europe. The National Research Council report (1988) on university research reactors indicated that base support in Western Europe for the operation of individual programs is higher than that in the United States. The base support for two major university research reactor-type reactors (Berlin and Munich in Germany) was $2.5 million each in 1985. Federal support for university reactors in the United States, that is, providing fuel, came to only about $2 million for all 40 university research reactors active in 1987. In addition, significant funding for major upgrades and new equipment has been available to the European reactor centers ($50 million for renovations in Berlin and more than $100 million for a new reactor in Munich).
The National Research Council report (1988) went on to recommend that $20 million be made available annually, for at least 3 years, to support university research reactors for facility upgrades (recognizing that as some are upgraded, others might have to be closed) and operational costs (recognizing that simply matching university contributions would generate a federal share of $37 million). Special appropriations of $1 million a year in fiscal years 1990 to 1993 for some specific instrumentation is all that has resulted from that report. The present committee believes that the observations of the 1988 report are still valid and that the $10 million to $20 million being considered for support of the Omega West or the Sandia National Laboratory reactors would be better spent on radionuclide production at university research reactors, especially the larger ones, and specifically the University of Missouri Research Reactor Center.
TABLE 3-5 Isotopes Produced at University of Missouri Research Reactor
|
Radioisotope |
Primary Uses |
Quantity (Ci) Shipped from July 1992 to July 1993 |
|
Sodium-24 |
Hypertension research and tracer for power reactors |
12 |
|
Silicon-31 |
Materials research |
172 |
|
Phosphorus-32 |
7,964 |
|
|
Sulfur-35b |
Radiolabeled biological compounds (DNA) |
5,064 |
|
Calcium-45 |
Biochemistry research tracer |
16 |
|
Scandium-46 |
Biochemistry research tracer and liquid flow tracer |
4.3 |
|
Calcium-47 |
Biochemistry research tracer |
0.002 |
|
Chromium-51 |
Diagnosis of blood cell volume (in use); biochemistry research tracer |
697 |
|
Iron-55 |
Biochemistry research tracer |
115 |
|
Cobalt-58 |
Positron source for materials science research |
5.8 |
|
Iron-59 |
Biochemistry research tracer |
5.7 |
|
Cobalt-60 |
Therapy for cancer (in use); sterilization source and calibration source |
0.2 |
|
Nickel-63 |
Detectors for smoke, explosives, analytical instruments |
76 |
|
Copper-64 |
Diagnosis of and therapy for cancer (clinical trials)c; biochemistry tracer, materials research |
2.0 |
|
Zinc-65 |
Biochemistry research tracer |
0.05 |
|
Copper-67 |
Biochemistry research tracer |
0.01 |
|
Selenium-75 |
Biochemistry research tracer |
16 |
|
Strontium-85 |
Biomedical tracer |
1.3 |
|
Rubidium-86 |
Biochemistry research tracer |
8.2 |
|
Zirconium-95 |
Calibration source |
1.0 |
|
Palladium-103 |
Therapy for prostate cancer (in use) |
1.99 |
|
Ruthenium-103 |
Instrument calibration source |
1.3 |
|
Rhodium-105 |
Therapy for cancer (proposed)d |
0.2 |
|
Palladium-109 |
Therapy for (proposed), cancer |
0.1 |
|
Silver-110m |
Chemical tracer, gamma calibration source |
1.8 |
|
Tin-113 |
Decays to indium-113m |
6.6 |
|
Cadmium-115 |
Therapy for arthritis (proposed) |
0.03 |
|
Tin-119m |
Chemical tracer, gamma calibration source |
0.03 |
|
Tellurium-123m |
Mossbauer source; lung, heart research |
0.04 |
|
Antimony-124 |
Oil well tracer |
5.4 |
|
Tellurium-125m |
Mossbauer source |
0.007 |
|
Tellurium-129m |
Mossbauer source |
0.001 |
|
Barium-133 |
Calibration source for detectors, gamma cameras |
0.2 |
|
Samarium-145 |
Therapy for cancer (proposed) |
0.1 |
|
Samarium-153 |
Therapy for bone cancer (clinical trials) materials research |
51 |
|
Gadolinium-153 |
Diagnosis of osteoporosis (in use) |
4.9 |
|
Europium-154 |
Calibration standard |
0.02 |
|
Terbium-160 |
Gamma source |
0.4 |
|
Dysprosium-165 |
Therapy for arthritis (clinical trials) |
2.3 |
Market Analyses
Proponents of the Fast Flux Test Facility, a 400-MW DOE reactor at the Hanford site in the state of Washington scheduled for decommissioning, have predicted an explosive growth in demand for new approaches to radiotherapy, that is, 1.6 million new cancer cases each year in the United States. The business plan produced for the facility by the Freeman School of Business at Tulane University and the Levy Rosenblum Institute in September 1993 projects a global wholesale market (raw isotopes, not radiopharmaceutical end products) of close to a $1 billion by 2002, a 10-fold increase primarily because of therapeutic radiopharmaceuticals. Other observers have been far less optimistic. Landis et al. (1993), although still projecting substantial growth, caution that the Tulane plan assumes U.S. Food and Drug Administration approval of a large number of radiopharmaceuticals that are now in the research or clinical testing stage. The
timing of these approvals and the cost-effectiveness of the techniques employing the radiopharmaceuticals will determine the extent of actual use of the radioisotopes, making the Tulane assumptions speculative at best.
Arthur Andersen & Co. (1993), in a management study for DOE, is more conservative, pointing out that the isotope market is characterized by high barriers to entry, producers who are government owned or rely on government-owned facilities, and demand driven by less than a handful of major commercial customers. In its market analysis and advice to DOE, it assumed modest annual growth (5 to 10 percent) in the overall radioisotope market, with no large change in the market profile (i.e., cobalt-60 at 40 percent and 99Mo at 34 percent will continue to dominate sales). In fact, their data, substantiated in testimony to the committee by Evans (1993), show that at present no other single radioisotope accounts for more than 5 percent of the market. The rest of the reactor-produced radionuclides, although many in number, are used mainly by researchers, who constitute only 2 percent of the radioisotope market in dollars.
CONCLUSIONS
-
In the short term the supply of reactor-produced radionuclides for commercial use, including 99Mo, is sufficient. Radiopharmaceutical companies state that the present domestic and foreign suppliers are reliable and have or will soon sign long-term supply contracts with existing producers.
-
In the long term if short-lived reactor-produced radionuclides become important for cancer therapy, the present number and condition of production reactors in North America will be inadequate.
-
A federally supported U.S. reactor for the production of research radioisotopes is definitely justified. At present, MURR is playing a major role as the supplier of radionuclides for research facilities and radiopharmaceutical manufacturers. Federal support at present is limited to the provision of reactor fuel and peer-reviewed research grants.
RECOMMENDATIONS
-
In view of the demonstrated reliability of the current sources of commercially valuable isotopes and their steps to secure adequate backup, the committee recommends that the Omega West reactor at Los Alamos National Laboratory or reactors at other facilities NOT be reopened as a dedicated source of molybdenum-99 and other reactor-produced isotopes.
-
To ensure the continued supply of radionuclides (other than molybdenum-99) for medical and research facilities, the committee recommends core support for reactor-based isotope production. The University of Missouri Research Reactor appears to be the best currently available facility that can meet this need.
-
Because reactors have finite lifetimes and because future demands may exceed current capabilities, the committee recommends that DOE ensure that plans for the Advanced Neutron Source reactor reflect the importance of isotope production, and in particular, molybdenum-99, by providing funding at an appropriate amount to ensure availability.
REFERENCES
Adelstein, S. J. 1993. The Auger Process: A Therapeutic Promise? American Journal of Roentgenology 160:707–713.
Arthur Andersen & Co. 1993. U.S. Department of Energy Isotope Production and Distribution Program Management Study. Washington, D.C.
Berson, S. A., and R. S. Yalow. 1959. Quantitative Aspects of the Reaction Between Insulin and Insulin Binding Antibody. Journal of Clinical Investigation 38:1996.
Bizzell, O. M. 1966. Early History of Radioisotopes from Reactors, Isotope and Radiation Technology 4:25–29.
Blumgart, H. L. and Yens, O. C. 1927. Studies on Velocity of Blood Flow: The Method Utilized. Journal of Clinical Investigations 4:1–13.
Brice, J. 1994. Dual-Head SPECT Lifts Nuclear Medicine Market. Diagnostic Imaging 16(1):29–33.
Cobb, L. M., and J. L. Humm. 1986. Radioimmunotherapy of Malignancy Using Antibody Targeted Radionuclides, British Journal of Cancer 54:863–870.
Carmain, E. 1993. The New Alchemy. Nuclear Energy2nd Quarter:2–13.
de Hevesy, G. 1913. Use of Radioelements as Tracers in Chemistry and Physics. Chemistry News 108:166.
DeSombre, E. R., B. Shafii, R. N. Hanson, P. C. Kuivanen, and A. Hughes. 1992. Estrogen Receptor-Directed Radiotoxicity with Auger Electrons: Specificity and Mean Lethal Dose. Cancer Research 62:5752–5758.
Evans, D. J. 1993. Presentation to the Biomedical Isotopes Committee, Institute of Medicine, National Academy of Sciences, Washington, D.C., November 19, 1993.
Fischman, A. J., J. W. Babich, and H. W. Strauss. 1993. A Ticket to Ride: Peptide Radiopharmaceuticals. Journal of Nuclear Medicine 34:2253–2263.
Hamilton, J. G. and M. H. Soley. 1939. Studies in Iodine Metabolism by the Use of New Radioactive Isotope of Iodine. American Journal of Physiology 127:557–572.
Hinkle, G. H. 1993. An Overview of Advances in the Radioimmunodetection of Melanoma. New Perspectives in Cancer Diagnosis and Management 1(3):774–780.
Holmes, R. A. 1993. U.S. Dependence on Foreign Isotopes. Statement to the Subcommittee on Energy, Committee on Science, Space and Technology, U.S. House of Representatives, Washington, D.C., October 14, 1993.
Humm, J. L. 1986. Dosimetric Aspects of Radiolabeled Antibodies for Tumor Therapy . Journal of Nuclear Medicine 27:1490–1497.
Jungermann, J. A., K. H. P. Yu and C. L. Zanelli. 1984. Radiation Absorbed Dose Estimates at the Cellular Level for Some Electron-Emitting Radionuclides for Radioimmunotherapy. International Journal of Applied Radiation and Isotopes. 35:883.
Kamen, M. D., ed. 1957. Applications to Clinical Research. P. 246. In Isotopic Tracers in Biology, 3rd edition. New York: Academic Press.
Landis, J. W., F. P. Baronowski, and E. H. Klevans. 1993. Future Use of the United States Department of Energy's Fast Flux Test Facility. Final Report of the Independent Review Team for Secretary of Energy. Photostatic copy dated September 20, 1993.
Lewington, V. J., A. J. McEwan, D. M. Ackery, R. J. Bayly, D. H. Keeling, P. M. Macleod, A. T. Porter, and M. A. Zivanovic. 1991. A Prospective Randomized Double-Blind Crossover Study
to Examine the Efficacy of Strontium-89 in Pain Palliation in Patients with Advanced Prostate Cancer Metastatic to Bone. European Journal of Cancer27:954–958.
McLaughlin, W. H., R. A. Milius, K. M. Pillai, J. P. Edasery, R. D. Blumenthal, and W. D. Bloomer. 1989. Cytotoxicity of Receptor-Mediated 16a-[125I]Iodo-Estradiol in Cultured MCF-7 Human Breast Cancer Cells. Journal of the National Cancer Institute 81:437–440.
Mirzadeh, S., C. W. Alexander, T. McManamy and F. F. Knapp, Jr. 1994. Projected Medical Isotope Production Capabilities of the Advanced Neutron Source (ANS). Oak Ridge National Laboratory Publication No. 12010/S. Oak Ridge, Tenn.: Oak Ridge National Laboratory.
Mirzadeh, S., R. E. Schenter, A. P. Callahan and F. F. Knapp, Jr. 1992. Production Capabilities in U.S. Nuclear Reactors for Medical Radioisotopes. Oak Ridge National Laboratory Publication No. TM-12010. Oak Ridge, Tenn.: Oak Ridge National Laboratory.
Moody, D. E., and E. J. Peterson, eds. 1992. Proceedings of the DOE Workshop on the Role of a High-Current Accelerator in the Future of Nuclear Medicine. Report No. LA-11579C. Los Alamos, N.M.: Los Alamos National Laboratory.
Morgan, I. L. 1993. Presentation to the Biomedical Isotopes Committee, Institute of Medicine, National Academy of Sciences, Washington, D.C., November 20, 1993.
National Research Council. 1988. University Research Reactors in the United States—Their Role and Value. Washington, D.C.: National Academy Press.
Riggs, J. R. 1993. Statement to the Subcommittee on Energy, Committee on Science, Space and Technology, U.S. House of Representatives, Washington, D.C., October 14, 1993.
Schenter, R. E. 1993. Statement to the Subcommittee on Energy, Committee on Science, Space and Technology, U.S. House of Representatives, Washington, D.C., October 14, 1993.
Schmidt, G. W. 1993. Letter to Hillary R. Clinton, July 20, 1993. Copy provided to Donald Erb at the U.S. Department of Energy and in turn provided to the Committee on Biomedical Isotopes, September 11, 1993.
Serafini, A. N. 1993. Radioimmunoscintigraphy in Melanoma. New Perspectives in Cancer Diagnosis and Management 1(3):83–85.
Stannard, J. N. 1988. Radioactivity and Health: A History. DOE Report No. RL01830-T59. Richland, Wash.: Pacific Northwest Laboratory.
Sugarbaker, E. V. 1993. Recent Clinical Applications of RAID in Malignant Melanoma. New Perspectives in Cancer Diagnosis and Management 1(3):91–93.
Tucker, D., M. W. Greene, A. J. Weiss, and A. Murrenhoff. 1958. Methods of Preparation of Some Carrier-Free Radioisotopes Involving Sorption on Alumina. Report No. BNL-3746. Upton, N.Y.: Brookhaven National Laboratory.
University of Missouri Research Reactor Center. 1994. Annual Report for 1993. Columbia: University of Missouri.
White, W. 1993. Statement to the Subcommittee on Environment, Energy, and Natural Resources, Committee on Government Operations, Washington, D.C., December 6, 1993.
Zalutsky, M. R., and A. S. Narula. 1988. Radiohalogenation of a Monoclonal Antibody Using an N-Succinimidyl 3-(tri-n-butylstannyl) Benzoate Intermediate. Cancer Research 48:1446–1450.
Zalutsky, M. R. 1992. Monoclonal Antibodies: Imaging and Therapy. In D. E. Moody, and E. J. Peterson, eds. Proceedings of the DOE Workshop on the Role of a High-Current Accelerator in the Future of Nuclear Medicine. Report No. LA-11579C. Los Alamos, N.M.: Los Alamos National Laboratory.
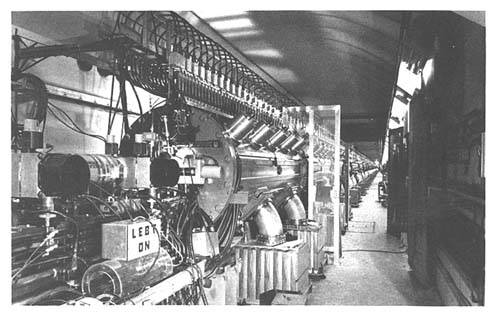
A view looking down into the nine accelerating stations of the proton LINAC at Brookhaven National Laboratory. The LINAC is almost 600-feet long and accelerates the protons to an energy of 200 MeV. The protons are used for isotope production in the Brookhaven Linac Isotope Producer. SOURCE: Brookhaven National Laboratory.






















