2
Recent Trends in Support for Biomedical Research and Development
ENRIQUETA C. BOND AND SIMON GLYNN
The case studies found in the present volume of the series Medical Innovation at the Crossroads explore the role of interdisciplinary and interinstitutional research and development (R&D) in the evolution of modern medical devices and biotechnology drugs. To provide some background for such analysis, this chapter will describe the major sources of financing for biomedical R&D, updating in some respects two useful earlier analyses by Ginzberg and Dutka (1989) and the Institute of Medicine (IOM; 1990). This chapter will also describe recent initiatives to encourage technology transfer by strengthening collaborations between government, industry, and academia. As noted by Weisbrod (1994), a complicated set of financial incentives—such as direct support of R&D in combination with the enormous expansion of health care insurance that has paid for the adoption of technologies—operate to foster technological innovation. As we will see below, both government and industry have substantially increased their investments in R&D but the relative amounts and kinds of investment of the two sectors are changing. Constraints on the federal budget are slowing increases in R&D support by the federal government and leading Congress to demand more evidence of health "payoffs" from these investments.
BIOMEDICAL RESEARCH AND DEVELOPMENT FUNDERS
Total national funding for biomedical R&D exceeded $30 billion in 1993 (U.S. Department of Health and Human Services, 1993). Of this, spending in the
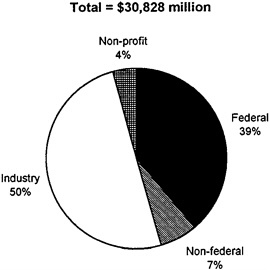
FIGURE 2-1 Health R&D funding by source, 1993. SOURCE: U.S. Department of Health and Human Services, 1993.
health-related components of federal agencies,1 principally the National Institutes of Health (NIH) in the Department of Health and Human Services (DHHS), represented nearly $12 billion, or 39 percent of all spending (see Figure 2-1). Spending by state and local governments contributed about $2 billion, or slightly less than 7 percent of national spending on health R&D.
The difference, $17 billion or 54 percent, is financed by health R&D spending in the private sector—primarily industry, but also private nonprofit organizations such as the Howard Hughes Medical Institute and other foundations. Industry was the largest single sponsor of biomedical R&D in 1993, financing in excess of 50 percent of all biomedical R&D (although, as we will see, the nature of this R&D is different in several respects from research funded by federal spending). Total expenditures by private, nonprofit institutions (including universities) for health R&D were $1.2 billion, or 4 percent of all spending (see Figure 2-1). In addition to this explicit investment of institutional funds, universities also support biomedical R&D through cross-subsidization from patient-care dollars. It has recently been estimated that the amount of such subsidies is around $850 million.
Federal Spending for Biomedical Research and Development
The phenomenon of large-scale public support for biomedical research in the United States is relatively recent. In 1940, for example, the largest funders of biomedical research performed in the United States were corporations and nonprofit organizations, spending $25 million and $17 million, respectively (not adjusted for the effects of inflation). Spending by federal agencies for biomedical research was largely in their own laboratories and equaled only $3 million, or less than 7 percent of all spending (Ginzberg and Dutka, 1989).
From a policy perspective, the expansion of public sector spending for biomedical research can be traced to the dramatic advances achieved by academic research during the 1930s and 1940s, such as the development of radar and the jet engine but also important advances in medicine, including the discovery by Fleming and then development by Flory and Heatley of penicillin to treat infectious disease (see Ginzberg and Dutka, 1989; Rosenberg and Nelson, 1993). Increasingly, basic research was seen as important to social goals, including improved health, and consequently deserving of public sector support. This view was successfully articulated by Vannevar Bush in an influential report, Science—the Endless Frontier, that proposed large-scale public sector funding for basic research, including significant support for biomedical R&D (Bush, 1945). As a consequence of this view, public sector spending for biomedical research, as well as for academic research in all areas, increased enormously as illustrated in Figure 2-2. From 1950 to 1965, appropriations to NIH to expand the funding of
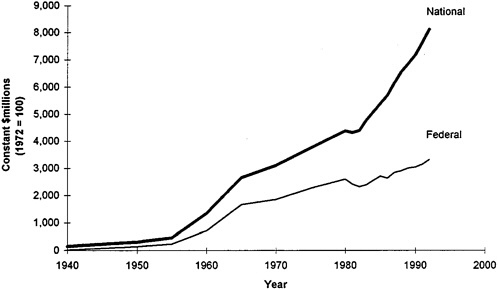
FIGURE 2-2 National and federal funds for health R&D, 1940–1992. SOURCE: Ginzberg and Dutka, 1989; U.S. DHHS, 1993.
biomedical research accelerated in real terms at 18 percent annually, eclipsing spending by corporations and nonprofit organizations (Ginzberg and Dutka, 1989). By 1965, federal spending had become the source of nearly two out of every three dollars spent on biomedical research, largely through programs of NIH.
More recently, federal funding of biomedical research has continued to follow a trend of real, sometimes substantial, increases over inflation. From 1983 to 1993, federal spending for biomedical research increased from $5.4 billion to $12.0 billion in current dollars, or 36 percent adjusting for the effects of inflation2(U.S. DHHS, 1993). Federal spending for basic research in the life sciences increased by more than $3.3 billion3 (National Science Board, 1993). Indeed, the percentage increase in funding for the life sciences over this period was exceeded only by spending for research in mathematics and computer science, although in absolute terms the increase in these areas is comparatively small ($0.35 billion) (Laubach, 1994; National Science Board, 1993).
Despite these increases in federal spending, national spending for biomedical research has increased even faster. From 1983 to 1993, national spending from all sources increased $20 billion, or more than 75 percent (adjusted for the effects of inflation; U.S. DHHS, 1993). During this time period, the significance of federal funding has changed. Figure 2-3 illustrates that relative levels of federal spending are decreasing, from 50 percent of all funding in 1983 to 39 percent in 1993 (even though federal spending for biomedical research, in absolute terms, has increased).
For 1994, $11.4 billion of the $71.6 billion that the federal government was projected to spend on general scientific R&D was to support biomedical research and development (up 6.5 percent from FY 1993;4 American Association for the Advancement of Science, 1993a). NIH appropriations are about 90 percent of this spending5 (see Table 2-1). In addition to NIH, agencies that sponsor academic biomedical research include the National Science Foundation, the Centers for Disease Control and Prevention, the Department of Veterans Affairs, the Department of Defense, and the Department of Energy. Most of this spending by federal agencies is directed to extramural programs as opposed to research in
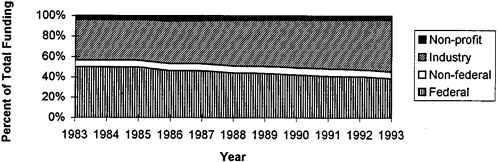
FIGURE 2-3 Trends in sources of funding for biomedical R&D as a percent of total R&D funds, 1983–1993. SOURCE: U.S. Department of Health and Human Services, 1993.
federal laboratories. Universities and academic medical centers are the largest recipients of these extramural funds, receiving nearly 75 percent of all extramural spending (see Figure 2-4; U.S. DHHS, 1993).
Federal Spending for Medical Device Research and Development
Medical devices (a primary focus of this volume) are different from pharmaceuticals and biotechnology in that they constitute a relatively small item within the federal medical research budget. This is because they draw heavily on advances in physics and engineering (see Gelijns and Rosenberg, chapter 1 of this volume). For this reason, the focus on basic advances in disease- and organ-specific research in university research funded by NIH has largely excluded medical device development (Foote, 1992). A recent study estimates that federal spending for biomedical R&D in these areas accounted for only about $422 million in 1992, a decrease of $27 million from 1991 spending (see Table 2-2). Of this, three-quarters was funded by NIH spending (less than 5 percent of NIH appropriations).
There are exceptions to this—appropriations by Congress to NIH are occasionally targeted to specific technologies or systems that require medical device development. Examples include the Artificial Heart Program and the Artificial Kidney Program (Foote, 1992). More recently, the Human Genome Project has specifically solicited interdisciplinary proposals both for developing advanced sequencing and mapping technology and for computational methods to organize and analyze the resulting data (IOM, 1990). In addition to these programs, the National Science Foundation also funds investigator-initiated research in a number of areas that are relevant to innovation in medical devices, including several programs in science and biological engineering (IOM, 1990). As a group, though, these programs represent a relatively small percentage of the university biomedical research enterprise.
TABLE 2-1 Estimated R&D in the National Institutes of Health (in million dollars)
A Change in the Emphasis of Federal Spending
In recent years, concern in Congress over the federal deficit and efforts to control the increase in federal spending have created increased competition for appropriations and focused attention explicitly on the results of public spending for biomedical research; NIH's National Cancer Institute, for example, was recently chastised for failing after 20 years of large-scale public spending to demonstrate
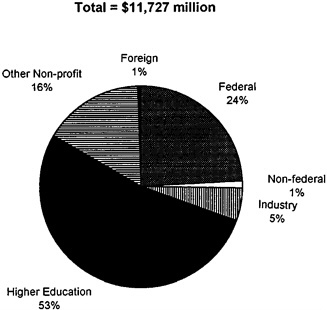
FIGURE 2-4 Distribution of federal health R&D funding by performer, 1992. SOURCE: U.S. Department of Health and Human Services, 1993.
TABLE 2-2 Estimated Federal Spending for Medical Device-related R&D (in million dollars)
|
Agency |
FY 1991 |
FY 1992 |
Percent Change |
|
National Institutes of Health |
378.9 |
354.4 |
-6 |
|
National Science Foundation |
18.0 |
18.0 |
0 |
|
Department of Defense |
22.8 |
15.0 |
-34 |
|
Department of Veterans Affairs |
9.3 |
10.5 |
13 |
|
Food and Drug Administration |
9.4 |
10.2 |
9 |
|
Department of Energy |
2.7 |
5.8 |
115 |
|
National Aeronautics and Space Administration |
4.9 |
4.9 |
0 |
|
Department of Education |
2.3 |
2.3 |
0 |
|
National Institutes of Standards and Technology |
0.5 |
0.5 |
0 |
|
TOTAL |
448.8 |
421.6 |
-6 |
|
SOURCE: Littell, 1994. |
|||
more significant results in the prevention and treatment of cancer (AAAS, 1992).
This trend not only exists within biomedicine, but also in other sectors of the economy (AAAS, 1993a). It reflects the view that in many areas of high technology the globalization of research and the pressure of international competition have introduced a critical time dimension into the stream of product development (NAS, 1992; Office of Science and Technology Policy, 1994). Whereas the United States has enjoyed a competitive advantage in many high technology fields, there is a perception that other countries may have developed more effective means for the direct translation of new knowledge into commercial products. As indicated by a number of forums, reports, and congressional hearings, the consequence of this view is that federal funding for research will become increasingly tied to societal goals. The same emphasis has been echoed by the Carnegie Commission on Science, Technology, and Government (1992).
Several programmatic consequences of this thinking can be identified in the FY 1994 budget. First, new federal research support appears to be concentrated in particular areas of science and technology seen as critical to national goals. These initiatives and their proposed funding levels in the FY 1994 budget are biotechnology research ($4.3 billion, largely through NIH), advanced materials and processing ($2.1 billion), global environmental change research ($1.5 billion), advanced manufacturing technology ($1.4 billion), high-performance computing and communications ($1.0 billion), and science, mathematics, and engineering technology education ($2.3 billion) (National Science Board, 1993).
Second, within NIH this increased focus on the economic and social benefits of publicly-funded biomedical research is expressed in a growing number of focused initiatives required by Congress or the administration (National Science Commission, 1992; Anderson, 1993). For example, the administration explicitly targeted several research initiatives for increased funding in its proposed budget to Congress for fiscal year 1994 (AAAS, 1993b). These initiatives and the proposed funding levels include AIDS research ($1.3 billion), women's health ($61 million, as part of a 15-year project estimated to cost over $650 million), breast cancer research ($216 million), and minority health ($56 million).
Direct Federal Support for Industry
Of the 30 percent of extramural funds not received by universities and medical schools, one-third (11 percent of all extramural funds) was used to fund biomedical R&D in industry in 1992 (see Figure 2-4). These extramural funds represented 6 percent of biomedical R&D performed by industry (U.S. DHHS, 1993).
These funds do not only flow through NIH. The National Institute of Standards and Technology (NIST), for example, is the focus of new spending for applied research and development in several areas, including biomedical R&D.
The NIST component with the largest increase in funding in FY 1994 was the Advanced Technology Program (ATP), which partially funds research and development in individual companies and joint ventures to develop promising, high-risk technologies, where the risks are perceived as too high to pursue without the incentive of federal spending. As examples, projects recently funded by ATP include development of synthetic polymers as bio-absorbable materials for use in orthopedics; development of implantable ''microreactors," containing living cells that are isolated from the body's immune system, to treat diseases requiring bioagents produced by the cells; and the use of multi-photon detector technology to develop radioisotope detection and measurement systems for use in diagnostics (AAAMI, 1994b; AAAS, 1993b). ATP's budget in FY 1994 was $199.5 million (AAAS, 1993a; Robinson, 1994).
At the same time, spending for defense programs is increasingly expected to promote dual-use technology development. In this context, the FY 1994 budget designated the Advanced Research Projects Agency as the lead agency for the Technology Reinvestment Project (TRP) in dual-use technologies, intended to help smaller defense contractors develop technologies for defense that also have commercial applications. Total funding for the TRP initiative is $472 million (AAAS, 1993b; Robinson, 1994). The initiative is divided into 11 technology areas, one of which is health care technology. Medical projects funded under TRP include a prototype ceramic honeycomb system that will separate oxygen from air and deliver the oxygen under pressure without pumps or moving parts; and a consortium in commercializing computer-vision technology originally developed for the National Aeronautics and Space Administration (NASA; AAAMI, 1994e).
In addition to the ATP and TRP initiatives, federal funding also explicitly targets applied research in smaller, technology-intensive companies through the Small Business Innovation Research (SBIR) program started in 1983. Under this program, when a federal agency's extramural research spending exceeds $100 million the agency is required to set aside a fixed percentage of this spending for SBIR projects. SBIR divides research support into three phases. Phase 1 awards are up to $100,000 and are used to evaluate the scientific and technical usefulness of an idea. In Phase 2, projects from Phase 1 with demonstrated potential are funded up to $750,000 to further develop the proposed idea for one or two years. Phase 3 aims for commercialization by private sector investment; no SBIR funds may be used for this purpose. In 1994, NIH set aside 1.5 percent ($125 million) for SBIR awards in the life sciences. For FY 1995, this increases to 2 percent (about $165-170 million), and increases for FY 1997 to 2.5 percent (about $230 million) (AAAMI, 1994c; Robinson, 1994).
The newest program for FY 1994 is the Small Business Technology Transfer (SBTT) program, also intended explicitly to help small business. SBTT is distinct from SBIR in that SBTT also aims to encourage cooperative R&D and technology transfer between small businesses and research institutions. Consequently,
small businesses are required to team with research institutions to receive SBTT funding (no such requirement exists for SBIR). SBTT began a three year pilot program in FY 1994. Funding levels for SBTT were also initially below the levels for SBIR. In 1994, this amounted to $4.1 million for NIH, or approximately 40 awards in FY 1994 (AAAMI, 1994c).
Tax Credits on Incremental Research and Development
As well as direct federal funding to support biomedical research, substantial indirect support for research is provided to industry in the form of tax credits on incremental R&D expenses. According to estimates by the U.S. Treasury Department, this indirect means of federal support for basic R&D has accounted for more than $20 billion from 1981 to 1992 (National Science Board, 1993). No data are available specifically for biomedical R&D. Unfortunately, a tax credit requires positive earnings, not usually enjoyed by the smaller, innovative companies in biotechnology or medical devices.
Industry Spending for Biomedical Research and Development
The largest source of financing for biomedical R&D is spending from private sources. As illustrated in Figure 2-1 above, industry is the main supporter of biomedical R&D in the United States. Industry spending exceeded $15 billion in 1993, or 50 percent of all health R&D, and substantially exceeded all sources of federal financing, including NIH. Industry spending was also the most rapidly increasing source of financing in 1993 (U.S. DHHS, 1993).
Industry performed by far the majority (78 percent) of the $15 billion in biomedical R&D that it funded in 1993 (see Figure 2-5), as well as 40 percent of all spending on health R&D overall (U.S. DHHS, 1993). For-profit sponsorship of research at universities and academic medical centers remains a relatively small proportion of the total investment by industry in R&D. According to the American Association for the Advancement of Science, about 11 percent of funds received by universities in 1991, or $1.35 billion, come from industry; only 5 percent of industrial R&D is allocated for basic or fundamental research (AAAS, 1993b).
Research and Development Investments by Pharmaceutical and Biotechnology Companies
The pharmaceutical sector is the largest industrial funder of biomedical R&D. According to the Pharmaceutical Manufacturers Association (PMA; 1993b), there were 136 pharmaceutical companies operating in the United States in 1993. The member companies of the PMA, representing over 90 percent of U.S. pharmaceutical sales, spent $12.6 billion on biomedical R&D in 1993
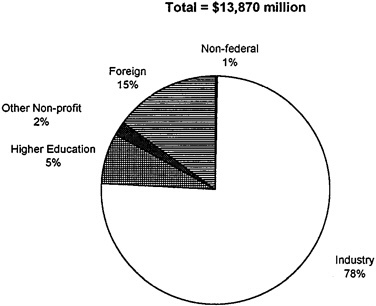
FIGURE 2-5 Distribution of industry health R&D funding by performer, 1992. SOURCE: U.S. DHHS, 1993.
(PMA, 1993b). R&D spending for PMA members has also accelerated faster than federal spending for biomedical R&D, increasing on average 14 percent per year starting in 1970 (see Figure 2-6). This spending for R&D represents about 17 percent of total pharmaceutical sales, or more than double the percentage of R&D spending in other technology-intensive sectors of the U.S. economy (U.S. Department of Commerce, 1994).
Research performed in company laboratories represents over 75 percent of this spending on R&D—about 25 percent is contracted to outside organizations, including universities and academic medical centers (PMA, 1993b). Most R&D spending is in four areas—cardiovasculars, drugs related to the central nervous system, anti-infectives, and neoplasms (PMA, 1993b). Clinical evaluation phases I through IV comprise about 30 percent of R&D spending, and probably represent the largest component of industry funding to universities and academic medical centers (PMA, 1993b).
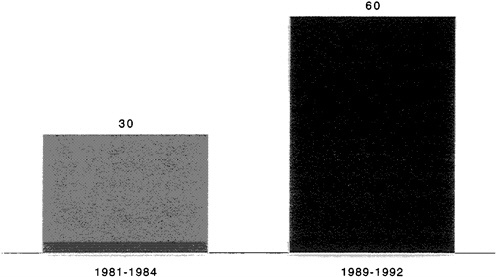
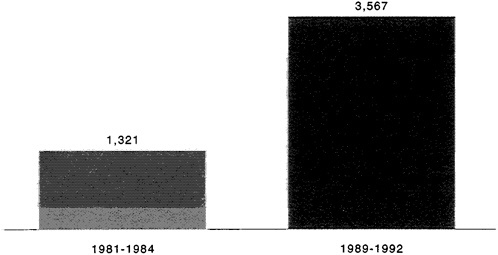
The nature of this research is changing. According to a recent study of the pharmaceutical industry by the Boston Consulting Group (BCG), more than two-thirds of all drugs in development are now aimed at chronic diseases (BCG, 1993). The effects of this changing focus are reflected in the greater complexity and difficulty of pharmaceutical R&D—especially the lack of clear clinical end-points to demonstrate efficacy and longer and larger clinical trials (BCG, 1993). Figures 2-7 and 2-8 show the rate at which clinical trials have increased in duration and size. The rapidly changing health care environment places a premium on

FIGURE 2-6 Total R&D spending, Pharmaceutical Manufacturers Association member companies, 1990–1993. SOURCE: PMA, 1993b.
truly novel drugs. Within this context, the distinction between pharmaceutical companies and biotechnology companies is blurring as pharmaceutical companies are increasingly using biotechnology techniques to develop new drugs. According to the BCG study, 33 percent of research projects in major pharmaceutical companies in 1993 were based on biotechnology, compared with only 2 percent in 1980. In some larger pharmaceutical companies, up to 70 percent of the research projects were based on molecular biology (BCG, 1993).
According to a recent "DataWatch" article in Health Affairs, the biotechnology sector included 1,272 biotechnology companies in 1993, of which 235 are public (Read and Lee, 1994). More than 100 of these companies were started in the last two years. Compared to the larger pharmaceutical sector, biotechnology is relatively small—according to a survey by Ernst and Young (1994), revenues for biotechnology companies were about $7 billion in 1992, compared to revenues of $114 billion for pharmaceutical companies. The biotechnology sector is nonetheless a very large funder of biomedical research. According to the same survey, biotechnology companies spent nearly $5.7 billion on R&D in 1992 (or about half the R&D expenditures for pharmaceuticals), or nearly 80 percent of sales (Read and Lee, 1994). As is obvious from these levels of R&D spending, the overwhelming majority of biotechnology companies are research organizations with essentially no revenues. Moreover, with very few exceptions, development efforts in the majority of these biotechnology companies are several years from approval. Table 2-3 shows the number of drugs currently in development that use biotechnology techniques, including drugs developed by larger pharmaceutical companies. Currently, there are only 19 biotechnology drugs approved for use in the United States (see Table 2-4).
TABLE 2-3 Biotechnology Drugs Currently in Development
Research and Development Investments by the Medical Devices Industry
In contrast to pharmaceuticals and biotechnology, medical devices encompass a more diverse group of products, ranging from disposable needles to sophisticated and expensive modalities such as magnetic resonance imaging (MRI). At present, the Food and Drug Administration (FDA) identifies about 1,700 different types of medical devices, which are developed and manufactured by as many as 11,000 device companies (including foreign companies; Gelijns et al., forthcoming). According to the U.S. Department of Commerce, surgical and medical instruments, surgical appliances and supplies, electromedical equipment, and X-ray equipment were projected to be among the fastest growing sectors of U.S. industry in 1993 (U.S. Department of Commerce, 1993).
In 1993, the medical device industry invested less than 7 percent of sales in R&D, compared to 17 percent in pharmaceuticals (Business Week, 1994). Large variations in R&D spending exist among device categories, however, reflecting the complexity of the type of product involved—developing MRI, of course, is considerably more complicated than developing a new set of surgical instruments. R&D spending also varies depending on company size, mirroring the division of labor between large pharmaceutical companies and biotechnology companies. According to an analysis by the Health Care Technology Institute, device companies with less than $5 million in sales spent 77.5 percent of their sales in 1991 on R&D, or almost the identical figure for biotechnology (although the absolute level of spending is almost certainly less). This spending for R&D compares to 17.2 percent for companies with between $5 and $20 million in
TABLE 2-4 Biotechnology Medicines or Vaccines Approved by the Food and Drug Administration, 1993
|
Product |
Indications |
Company |
Year Approved |
|
Beta interferon |
Multiple sclerosis |
Chiron |
1993 |
|
DNAse |
Cystic fibrosis |
Genentech |
1993 |
|
Factor VIII |
Hemophilia |
Genentech, Genetics Institute |
1993 |
|
IL-2 |
Renal cell cancer |
Chiron |
1992 |
|
Indium-111-labeled antibody |
Cancer imaging |
Cytogen |
1992 |
|
Aglucerase |
Gaucher's disease |
Genzyme |
1991 |
|
G-CSF |
Adjunct to chemotherapy |
Amgen |
1991 |
|
GM-CSF |
Bone marrow transplant |
Immunex |
1991 |
|
Hyaluronic acid |
Ophthalmic surgery |
Genzyme |
1991 |
|
CMV immune globulin |
Prevention of rejection in organ transplants |
MedImmune |
1990 |
|
Gamma interferon |
Chronic granulomatous disease |
Genentech |
1990 |
|
PEG-adenosine deaminase |
Immune deficiency |
Enzon |
1990 |
|
t-PA |
Myocardial infarction, pulmonary embolism |
Genentech |
1990 |
|
Erythropoietin |
Anemia associated with renal failure, AIDS, cancer |
Amgen |
1989 |
|
Hepatitis B antigens |
Diagnosis |
Biogen |
1987 |
|
Alpha interferon |
Cancer, genital warts, hepatitis |
Biogen, Genentech |
1986 |
|
Hepatitis B vaccine |
Prevention |
Biogen, Chiron |
1986 |
|
Human growth hormone |
Deficiency |
Genentech |
1985 |
|
Human insulin |
Type I diabetes |
Genentech |
1982 |
|
SOURCE: "Datawatch: Biotechnology and Innovation Progress Reports," in Health Affairs, Summer, 1994. |
|||
sales, and 4.5 percent for companies with greater than $100 million in sales (Health Care Technology Institute, 1993).
FUNDING RELATIONSHIP BETWEEN INDUSTRY AND UNIVERSITIES
Over the past decade, the number and variety of industry-university relationships has increased dramatically. The two most common forms of collaboration between industry and academia for biomedical R&D are project-specific research support, and consulting arrangements (Blumenthal, 1994). Other frequent types of collaboration—including large-scale investments in academic research centers by industry—have received much more attention, perhaps because of their novelty in the life sciences (Atkinson, 1994). A list compiled by Webster and Etzkowitz of large-scale collaborations between pharmaceutical companies and academia through 1990 is reproduced in Table 2-5.
Despite the dramatic increase in attention to and interest in relationships between universities and industry, there is no centralized source of data to track consulting arrangements or the amount of project-specific biomedical R&D that industry funds in universities (Committee on Small Business, March 11, 1993). Estimates do exist for the subset of university research involving biotechnology techniques, however. Estimates by Blumenthal indicate that industry funding of individual research projects in academia totaled between $85 million and $135 million in 1984, or between 8 and 24 percent of all funds available for biotechnology research in academia (Blumenthal, 1992). These estimates also indicate that spending per project was less than the average size of NIH grants, and that these projects were typically of a shorter duration, suggesting that industry-sponsored research may be more focused and applied in nature than research funded by federal spending (Blumenthal et al., 1986).
Beyond industry funding of individual projects, faculty at major research-intensive universities performing research in biotechnology also receive support through consulting arrangements with industry. According to Blumenthal, 47 percent of biotechnology faculty had consulted for industry at some point over a three-year period ending in 1984 (Blumenthal, 1992). In a separate survey of nearly 700 graduate students and fellows in life sciences departments at six leading universities, 19 percent received some research or educational support from industry (Blumenthal, 1992).
FEDERAL EFFORTS TO ENCOURAGE TECHNOLOGY TRANSFER
Over the past decade, federal legislation has been introduced to encourage collaborations between universities and industry and to improve and extend these interactions. This legislation recognizes that industry must have reasonable expectations of being able to recover its development costs, which may be considerable,
TABLE 2-5 Selected Large-scale Relationships Between Universities/Academic Health Centers and Industry in Biomedical Research (in million dollars)
|
University/Academic Health Center |
Industry Partner |
Funds (million$) |
Duration |
Started |
Focus |
|
Harvard University Medical School |
Takeda |
1.0 per annum |
Open-ended |
1986 |
Angiogenesis factors |
|
Washington University |
Monsanto |
100.0 |
12 |
1982 |
Biomedical research |
|
Massachusetts General Hospital |
Shiseido |
85.0 |
1 |
1989 |
Dermatology |
|
Massachusetts General Hospital |
Hoechst |
70.0 |
12 |
1981 |
Molecular biology |
|
Georgetown University |
Fidia |
62.0 |
Open-ended |
1985 |
Neurosciences |
|
Massachusetts General Hospital |
Bristol-Myers Squibb |
37.0 |
5 |
1990 |
Cardiovascular |
|
Harvard University Medical School |
Monsanto |
23.5 |
12 |
1974 |
Cancer angiogenesis |
|
University of California at San Diego |
Ciba Geigy |
20.0 |
6 |
1990 |
Rheumatoid and osteoarthritis |
|
Harvard University Medical School |
Hoffman-LaRoche |
10.0 |
5 |
1990 |
Medicinal chemistry |
|
Harvard University Medical School |
Dupont |
6.0 |
5 |
1981 |
Genetics |
|
Washington University |
Mallink |
3.8 |
5 |
1981 |
Hybridomas |
|
Yale University |
Bristol-Myers |
3.0 |
5 |
1982 |
Anticancer drugs |
|
Columbia University |
Bristol-Myers |
2.3 |
6 |
1983 |
Gene structure |
|
Johns Hopkins University |
SmithKline Beckman |
2.2 |
5 |
1988 |
Respiratory disease |
|
Yale University |
Celanese |
1.1 |
5 |
1981 |
Enzymes |
|
Johns Hopkins University |
Johnson & Johnson |
1.0 |
Open-ended |
1982 |
Biology |
|
Rochester University |
Kodak (Sterling) |
0.5 |
Open-ended |
1983 |
DNA |
|
SOURCE: Statement of J. L. Wagner and M. E. Gluck, OTA, to the U.S. Congress Subcommittee on Business Opportunities, Regulation, and Energy of the House Committee on Small Business. Adapted from A. J. Webster and H. Etzkowitz, Academic-Industry Relations, The Second Academic Revolution: A Framework Paper for the Proposed Workshop on Academic-Industry Relations (London, England, Science Policy Support Group, 1991). |
|||||
or it will not participate. Patents and licenses on intellectual property developed at universities are, consequently, important for the transfer of technologies, and especially biotechnology, to clinical practice (Committee on Small Business, 1993b; IOM, 1989).
The Stevenson-Wydler and the Bayh-Dole Acts
The first element of this legislative effort is the 1980 Stevenson-Wydler Technology Transfer Act. Broadly, Stevenson-Wydler established an infrastructure for technology transfer from federal laboratories into the universities and industry. To facilitate technology transfer the act requires that each agency with federal laboratories allocate 0.5 percent of the agency research and development budget for this purpose. Federal laboratories having an annual budget exceeding $20 million are also required to provide at least one full-time professional position dedicated to this function (Chen, 1993; IOM, 1989). Despite the failure of Congress to appropriate funds for Stevenson-Wydler, other congressional and executive actions followed which reinforced the policy that federal agencies should actively pursue collaborative relationships with private industry.
The second example of such legislation is the 1980 Bayh-Dole Patent and Trademark Laws Amendment. Essentially, Bayh-Dole enables intellectual property rights to discoveries made using federal funds to be assigned to the university or research institution. It also allows the institution to seek patents and, in turn, to license and collect royalties on these patents, as long as these funds are used for scientific research and are shared with the principal investigator (Chen, 1993).
The apparent effect of Stevenson-Wydler and Bayh-Dole on academic biomedical research is impressive, at least in terms of patents. According to a study performed by the General Accounting Office (GAO), the top 35 research universities (all among the 25 leading recipients of NIH and NSF support during 1989-1990) accounted for 2,043 patent applications and 1,063 patents; of these, 731 were licensed, representing more than $113 million in income from licenses. The majority of these patents and licenses were in the health sciences (Blumenthal, 1992). Indeed, in biotechnology, universities were more efficient in generating patents than private industry—biotechnology companies in the 1980s were realizing more than four times as many patent applications per dollar invested from university research than from their own labs (Blumenthal, 1992).
Federal Technology Transfer Act
The 1986 Federal Technology Transfer Act extended the ideas in Bayh-Dole about university-industry relationships to interactions between federal laboratories and industry. The law authorizes the directors of federal labs to negotiate licensing agreements for inventions made in federal labs, and for the licensing
fees and royalties to be shared with the federal employee/inventor, the remainder to be retained by the laboratory for technology transfer (Chen, 1993; IOM, 1989). Incentives are also offered for companies and federal laboratories to enter into cooperative research and development agreements (Chen, 1993).
It should be stressed that these federal efforts in biomedical research are not restricted to NIH. In addition to NIH, several other agencies sponsor intramural research in technologies that may be relevant to the development, in particular, of medical devices. Several medical technologies developed under contract for NASA, for example, have been successfully transferred to clinical use, including a compliant knee joint for prosthetic and robotic devices that permits rotational movement in three different planes, and a walker for dynamically supporting persons with limited use of their extremities (AAAMI, 1994d).
To encourage the transfer of these technologies and expertise from the federal laboratories (in all areas, not only biomedical research), the National Technology Transfer Center (NTTC) was started in 1993 using a five-year grant from NASA. The intent of NTTC is to transfer technologies from the national laboratories, including NASA, into the commercial sector. NTTC is currently linked to more than 700 federal laboratories at 17 federal agencies, and serves as an electronic ''gateway" to federal research resources, via searches of available technologies (AAAMI, 1994a).
Consequences for University-Industry Relationships
The trends in funding for biomedical R&D identified in this chapter have had remarkable consequences for research universities. First, the scale of the academic research enterprise has increased enormously, in all disciplines. As the universities have expanded their research programs to absorb the increased federal spending for research, the size and complexity of the research enterprise have created an economic imperative for the larger research universities. For example, Columbia University has been a doubling of budget size every 10 years, and expenses increasing at 10 percent compounded annually for the past forty-five years (Cole, 1993). In academic biomedical research, spending by the leading research universities and medical schools has also increased nearly 200 times in response to increases in NIH support for basic and clinical research, from $50 million in 1950 to $9.7 billion in 1993 (nearly 30 times in constant dollars; Ginzberg and Dutka, 1989; U.S. DHHS, 1993).
Federal financing of academic biomedical research is critical for the long-term development of the research base and for immediate program funding. Nonetheless, the emphasis of appropriations for biomedical R&D has shifted, increasing the level of spending required to support the research enterprise but no longer permitting major expansion (AAAS, 1993b). Consequently, universities are seeking new sources of capital to maintain and expand (selectively) the base of academic research.
For major research universities, one of these sources is licensing fees realized under the Stevenson-Wydler and Bayh-Dole acts, especially in the emerging field of biotechnology. The patent on DNA recombinant techniques by Boyer and Cohen is a particularly lucrative example: income from the Cohen-Boyer patents for 1991–1992 represented $14.6 million, or 58 percent of total income from all patents held by Stanford University (total income for 1991–1992 was $25.5 million). The GAO survey of the top 35 universities cited earlier does raise questions as to whether this is a useful source of financing for more than a few universities, however. The universities surveyed reported an average income from licenses of $1.6 million; nine universities reported income in excess of $1.0 million and only six reported income in excess of $2.0 million (Blumenthal, 1992). The relatively few commercially profitable inventions emerging from those institutions and the substantial minimum efficient scale of operation, "… [imply] there is a reasonably high probability that many universities that 'invest' in expanded technology licensing operations in order to produce substantial new sources of income [will fail]" (Feller, 1990, p. 340).
In response to increasing biomedical research opportunities, many larger research universities have also directly or indirectly established ventures to commercialize their academic research. In a recent issue of Health Affairs, Atkinson discussed the university-based venture capital funds that were established at Harvard, Johns Hopkins, and Columbia Universities (Atkinson, 1994). Critics of these arrangements have questioned whether true organizational separation is possible—Harvard University, for example, had rejected a 1980 proposal to invest in a start-up biotechnology firm intended to develop research by a Harvard faculty member because it was considered incompatible with the university's central mission of learning. "The preservation of academic values is a matter of paramount importance to the University," wrote Derek Bok, then president of Harvard University, "and owning shares in a company would create a number of potential conflicts of interest with these values" (Hunnewell, 1994). In 1988, however, the university reversed itself by establishing Medical Science Partners, an enterprise designed to commercialize biomedical research findings. More recently, Harvard has proposed to establish an academic research center, the Harvard Institutes of Medicine, that will also include biotechnology companies (Hunnewell, 1994). In both instances, the university has faced little of the faculty criticism or media attention that accompanied the 1980 proposal. To date, however, no evidence is available on whether these university-based venture capital funds are in fact an effective means of commercializing research findings.
CONCLUDING OBSERVATIONS
Increased investments in R&D have contributed to the development of new knowledge; the strengthening of the pharmaceutical and device industries; the growth of universities, academic health centers, and medical specialties; and
the emergence of the biotechnology industry. The recent science policy statement, Science in the National Interest, reaffirms the federal investment in science, "… both to sustain America's preeminence in science and to facilitate the role of science in the broader national interest" (OSTP, 1994). Technological innovations both to improve and preserve health and to assure economic prosperity are strong and continuing rationales for investments in R&D.
In recent years, the rapid proliferation of collaborations in biological research involving partnerships between universities, industry, and government have greatly extended the frequency, scope, and visibility of such activities. The desire to draw commercial potential out of government-supported science has led to legislative and executive initiatives to promote more frequent and more directed technology transfer collaborations between the sectors. In many instances, as mentioned, it is still too soon to know how effective the different initiatives will be in fostering technology transfer. Continued monitoring and evaluation to assess what works should guide future policy development.
The amounts of dollars contributed for funding of science by the different sectors have changed over the past decade. While at one time government was the major investor in science, today industry supports over 50 percent of health R&D. It seems likely that the federal government will continue to support biomedical R&D handsomely, though perhaps not the continued expansion of the American university system. Consequently, if there is to be future growth of academic research it will depend increasingly on industrial support. Future policies will need to create an environment favorable to such private sector support. As we have noted, federal policies such as those governing intellectual property rights or tax credits for investments in R&D affect the level of support for R&D and the relationships of the different sectors. Furthermore, as U.S. scientists in academia seek more money from industrial sources, such collaborations will raise questions about potential conflicts of interest and about the roles of universities in education and in economic prosperity. Efforts to regulate collaboration between the sectors should seek to eliminate these ambiguities without unnecessarily burdening university-industry agreements.
REFERENCES
American Association for the Advancement of Medical Instrumentation (AAAMI). 1994a. National center facilitates technology transfer. In: Medical Device Research Report 1(1):11. Arlington, Va.: AAAMI.
AAAMI. 1994b. NIST Advanced Technology Program seeks proposals. In: Medical Device Research Report 1(2):6. Arlington, Va.: AAAMI.
AAAMI. 1994c. SBIR, STTR funding opportunities at NIH. In: Medical Device Research Report 1(1):3–5. Arlington, Va.: AAAMI.
AAAMI. 1994d. Technologies available. In: Medical Device Research Report 1(2):13. Arlington, Va.: AAAMI.
AAAMI. 1994e. Technology Reinvestment Project aims to develop 'dual-use' technologies.
In: Medical Device Research Report 1(2):2. Arlington, Va.: AAAMI.
American Association for the Advancement of Science (AAAS). 1992. Research and Development FY 1992. Publication no. 91-17S. Washington, D.C. : AAAS.
AAAS. 1993a. Congressional Action on Research and Development in the FY 1994 Budget. Publication No. 93-35S. Washington, D.C.: AAAS.
AAAS. 1993b. Research and Development FY 1994. Publication No. 93-10S. Washington, D.C.: AAAS.
Anderson, C. 1993. Strategic research wins the day. Science 259:21.
Atkinson, S. H. 1994. University-affiliated venture capital funds. Health Affairs (Summer):159–175.
Blumenthal, D. 1992. Academic-industry relationships in the life sciences. Journal of the American Medical Association 268:3344–3349.
Blumenthal, D. 1994. Growing pains for new academic/industry relationships. Health Affairs (Summer):176–193.
Blumenthal, D., Gluck, M., Louis, K., et al. 1986. University-industry research relationships in biotechnology: Implications for the university. Science 232:1361–1366.
Boston Consulting Group (BCG). 1993. The Changing Environment for U.S. Pharmaceuticals: The Role of Pharmaceutical Companies in a Systems Approach to Health Care. Boston, Mass.: BCG.
Bush, V. 1945. Science—The Endless Frontier. A Report to the President on a Program for Postwar Scientific Research. Washington, D.C.: Office of Scientific Research and Development.
Business Week. 1994. R&D Scoreboard. June 27, pp. 78–103.
Carnegie Commission on Science, Technology, and Government. 1992. Enabling the Future: Linking Science and Technology to Societal Goals . New York, N.Y.: Carnegie Corporation of New York.
Chen, P. 1992. The National Institutes of Health and its interactions with industry. In: Biomedical Research: Collaboration and Conflict of Interest. Porter and Malone, eds. Baltimore, Md.: The Johns Hopkins University Press, pp. 199–221.
Cole, J. R. 1993. Balancing acts: Dilemmas of choice facing research universities. Daedalus (Fall):1–36. Cambridge, Mass.: The American Academy of Arts and Sciences.
Committee on Small Business; Subcommittee on Business Opportunities, Regulation, and Energy; U.S. House of Representatives. 1993a. Statement of J.L. Wagner and M. E. Gluck, Office of Technology Assessment. March 11. Washington, D.C.: U.S. Government Printing Office.
Committee on Small Business; Subcommittee on Business Opportunities, Regulation, and Energy ; U.S. House of Representatives. 1993b. Statement of W. A. Peck, Association of American Medical Colleges. June 17. Washington, D.C.: U.S. Government Printing Office.
Ernst and Young, 1993. Biotech 94: Long-Term Value, Short-Term Hurdles . San Francisco: Ernst and Young.
Feller, I. 1990. Universities as engines of R&D-based economic growth: They think they can. Research Policy 19:335–348.
Foote, S. B. 1992. Managing the Medical Arms Race: Public Policy and Medical Device Innovation. Berkeley, Calif.: University of California Press.
Gelijns, A. C. 1991. Innovation in Clinical Practice. Washington, D.C.: National Academy Press.
Gelijns, A. C. and N. Rosenberg. Forthcoming. Medical device innovation: Opportunities and barriers for small firms. Washington, D.C.: National Academy Press.
Ginzberg, E., and Dutka, A. B.1989. The Financing of Biomedical Research. Baltimore, Md.: The Johns Hopkins University Press.
Health Care Technology Institute. 1994. Insight: Variation in Research and Development Spending Within the Medical Technology Industry. Alexandria, Va.: Health Care Technology Institute.
Hunnewell, S. 1994. The medical-industrial complex. Harvard Magazine (January-February):34–37.
Institute of Medicine (IOM). 1989. Government and Industry Collaboration in Biomedical Research and Education. Washington, D.C.: National Academy Press.
IOM. 1990. Funding for Health Sciences Research: A Strategy to Restore the Balance. Washington, D.C.: National Academy Press.
Laubach, G. 1994. Perspective on Academic Health Centers. Health Affairs (Summer):194–196.
Littell, C. L. 1994. Datawatch. Innovation in medical technology: Reading the indicators. Health Affairs (Summer):226–235.
National Academy of Sciences (NAS). 1986. New Alliances and Partnerships in American Science and Engineering. Washington, D.C.: National Academy Press.
NAS. 1991. Industrial Perspectives on Innovation and Interactions with Universities. Washington, D.C.: National Academy Press.
NAS. 1992. Future National Research Policies within Industrialized Nations. Washington, D.C.: National Academy Press.
National Science Board. 1993. Science and Engineering Indicators—1993 . Pub. no. NSB-93-1. Washington, D.C.: U.S. Government Printing Office.
National Science Commission. 1992. A Foundation for the 21stCentury: A Progressive Framework for the National Science Foundation. Washington, D.C.: National Science Commission.
Office of Science and Technology Policy. 1994. Science in the National Interest. Washington, D.C.
Pharmaceutical Manufacturers Association (PMA). 1993a. Biotechnology Medicines in Development. Washington, D.C.: Pharmaceutical Manufacturers Association.
PMA. 1993b. PMA Annual Survey Report: Trends in U.S. Pharmaceutical Sales and R&D. Washington, D.C.: Pharmaceutical Manufacturers Association.
Read, J. L., and Lee, Jr., K. B. 1994. Datawatch. Health care innovation: Progress report and focus on biotechnology. Health Affairs (Summer):215–225.
Robinson, B. 1994. Promises, promises. Technology Transfer Business (Winter):35–39.
Rosenberg, N., and Nelson, R. R. 1994. American Universities and Technical Advance in Industry. Research Policy 23:223–348.
Technology Transfer Business. 1994. SBIR accolades. (Winter):6.
U.S. Department of Health and Human Services (U.S. DHHS). 1993. NIH Data Book 1993. Pub. no. 93-1261. Washington, D.C.: U.S. Government Printing Office.
U.S. Department of Commerce. 1993. U.S. Industrial Outlook 1993. Washington, D.C.: U.S. Government Printing Office.
U.S. Department of Commerce. 1994. U.S. Industrial Outlook 1994. Washington, D.C.: U.S. Government Printing Office.
Weisbrod, B. A. 1994. The nature of technological change: Incentives matter! In: Institute of Medicine. Medical Innovation at the Crossroads , vol. 4. Adopting New Medical Technology. A. C. Gelijns and H. V. Dawkins, eds. Washington, D.C.: National Academy Press, pp. 8-48.