CHARLES ROY HAUSER
March 8, 1900–January 6, 1970

BY CHARLES K. BRADSHER
CHARLES R. HAUSER characterized himself, and has been characterized by others,1 as a physical organic chemist, but more than his rivals, he devoted his efforts to the study of reactions that have been or could become of interest to the synthetic organic chemist. The index2 to collective volumes I through V of Organic Syntheses shows that Hauser contributed twenty-six articles, almost equal to the combined total for the other ten physical organic chemists whose names appear in Tarbell and Tarbell's1 extended list of prominent American physical organic chemists. Hauser's contributions to synthetic organic chemistry were recognized with the American Chemical Society's Award for Creative Work in Synthetic Organic Chemistry in 1962 and the Synthetic Organic Chemical Manufacturers Association Medal for Creative Research in Organic Chemistry in 1967, leaving no doubt that he qualified also as an outstanding synthetic organic chemist. A quotation from the SOCMA Meeting Call 3 of August 30, 1967, considered his role as being that of a synthetic as well as a physical organic chemist:
. . . The subject of Dr. Hauser's talk will be “Some Applications of Physico-Chemical Principles to Organic Synthesis.” Dr. Hauser will draw on his own experience to illustrate the progress since the 1930's. At that time, the
physico-chemical reactions began to move into the mainstream of development from a base where the work in structures was of limited maturity and reactions were extremely difficult to predict. He will comment on how this pattern has taken shape in terms of the flexibility of the carbon atom, and how it has advanced into the far more sophisticated forms of today, often at the expense of cherished concepts. Incidentally, this latter is a process that leaves him wary of all theories, concepts or generalities that seem too well established.
Interestingly, Tarbell and Tarbell,4 in their brief characterization of Hauser's contribution to organic chemistry, began by discussing his contribution to synthesis: “He developed new and useful synthetic methods and knowledge about reaction mechanisms, for which he had a real instinct.”
In his over 450 publications, Hauser showed wide-ranging interests, but his most memorable work was in the field of bases. By the time of his death, he was certainly the international authority on the role of bases in organic synthesis. It is remarkable that so much was accomplished by someone who had once been a school dropout!
I was asked to write this memoir because I knew Hauser over almost the entire span of his career at Duke University and was the author of an earlier biographical sketch5 on him. Two years after his arrival at Duke I was in a recitation section of his; then after 1939 I was a junior colleague, and at the time of his death I was his department chairman. Despite the long association I cannot claim to have been a close friend, but from records, personal observation, and discussions with former colleagues and students, I know something about Hauser's professional life. Fortunately, Hauser's three children have been kind enough to share with me some of their reminiscences about his life away from the chemistry building. I am indebted to his two daughters, Frances M. Grate and Betty Yourison, and his son,
Charles F. Hauser (also an organic chemist), for their letters. Professor Hauser's widow, Mrs. Madge B. Hauser, survived until 1992.
Hauser's father, also named Charles, but with no middle name, was brought to this country from Germany as a child. As he grew up he had few opportunities for education but learned to support himself as a truck farmer and part-time master carpenter. He married Elizabeth Rogan, and the Hausers were living in San Jose, California, in 1900 when Charles Roy Hauser was born. Shortly before World War I the family moved to Homestead, Florida, a small community about 40 miles southwest of Miami, where the mother set up a boarding house. This is the place that Hauser always thought of as home, and he preferred to be thought of as a Floridian rather than a Californian.
Later he was to amuse his children with accounts of his life in Florida. One concerned his frequent swims in a canal close by his house. On one occasion just before diving in he saw something that looked very large and suspiciously reptilian. A local hunter was called to shoot the alligator, which proved to be 14 feet in length!
Another story concerned the origin of a vision problem that was to trouble him for the rest of his life. The following account is from his son, Charles:
Dad had to drop out of school in the eighth grade because he lost his sight. He thought it was because of poor food, combined with the glare of water and sand of Florida. He worked on the truck farm for a year, during which time his sight returned partially. The local high school principal recognized Dad as a sharp lad and induced him back to school by letting him enter the ninth grade and not miss a year. (Dad said that he wanted to get back to school because he was tired of hoeing tomatoes.)6
With a high school diploma and savings of $200, Hauser entered the University of Florida and acquired a B.S. degree in chemical engineering. His college annual (class of
1923)7 reveals that he was known as “C.R.” and belonged to the American Chemical Society and the Flint Chemical Society plus some engineering associations. It was a custom in those days for yearbook editors to salute each senior with some descriptive phrase, which in Hauser's case was particularly perceptive: “He likes chemistry.”
Hauser stayed on at Florida to acquire the M.S. degree in 1925, working at least part-time as an assistant. For his Ph.D. he moved on to the University of Iowa, where he did research in chloramine chemistry under the direction of G. H. Coleman. At Iowa he also met and married a fellow chemistry graduate student, Madge L. Baltimore, whose technical knowledge was to become important to him later on when his eyesight began to degenerate.
After a year as instructor at Lehigh, he moved to Duke University still with the rank of instructor. When the Hausers arrived in Durham, North Carolina, in 1929 the Neo-Gothic West Campus of Duke University had not been completed, and two years were to elapse before he could move his research to the new chemistry building. Fortunately, his department chairman, Paul M. Gross, appreciated Hauser's zeal for research and provided him with a reasonable share of the very limited resources and facilities available.
Hauser, as we knew him, was a modest gentleman, intense and endlessly worried about details. Physically slight, he had a long-time preoccupation with the need for proper nutrition and exercise. He was one of the Duke faculty's better tennis players but abandoned the game at some time in his forties, when he took up swimming and then walking for physical recreation. His fitness program was evidently a success for it has been reported that as late as in his fifties he could walk on his hands to amuse his grandson.8
A single physical limitation, the sensitivity to light that had made him drop out of school earlier, led to self-im-
posed restrictions that influenced both his personal and professional life. Although light sensitivity did not appear to affect his tennis game before World War II, it was the excuse usually given when he refused invitations to lunch or dinner, even with distinguished visitors, or to attend evening meetings or social functions.
His limited reading was usually done in his office by daylight only, and he was known to walk into a colleague's office, extinguish the lights (with an apology), state his business, and leave turning the lights back on. Despite the willingness of his wife to read to him, he was not as well read as many of his contemporary physical organic chemists. As his daughter Frances (Grate) remembers, “Daddy credited the handicap of his poor eyesight with forcing him to do original thinking—he couldn't read other people's work.”8 His son Charles recalls: “He liked poetry and classical music. He said that classical music (played softly) would relax him so he could think about chemistry.”
Long after Hauser's work had attracted national attention he was not well known on the Duke campus. He kept a low profile, avoiding service on university-wide committees or participation in social events involving the general faculty and appeared content with a modest, almost frugal, life style. Perhaps this inconspicuousness accounts for his being overlooked when in 1953 fourteen distinguished members of the Duke faculty were selected to become the first James B. Duke professors. Only five years later, and still not recognized as a “distinguished professor,” Hauser became the first member of the Duke faculty to be elected to the National Academy of Sciences!
In addition to his numerous research papers and chapters, Hauser had hoped to publish a book on organic reaction mechanisms, embracing the material contained in his graduate course, but he never found a suitable coauthor.
This was unfortunate, for he best communicated his ideas in writing, and worked hard at achieving clarity. The manuscripts that he presented to the typist were usually first typed by him on yellow paper to prevent glare, with all corrections made by overstriking. This format was familiar to his colleagues and graduate students, since we were frequently polled to determine which of alternate word arrangements was clearer. To his students and research associates he emphasized the importance of acquiring writing skills. It was such a shock to him to learn that the University of Florida still had in the university library a copy of his master's thesis written in a more primitive Hauser style that he even made an effort to have it removed.6 He appears to have been less disturbed by the preservation by his family of some verse written by him only a few years later, probably because publication was not contemplated.8 The verses, dedicated to the future Mrs. Hauser, were reasonably competent and in a whimsical rather than sentimental mood. To most of us who knew him only professionally, the possibility of Hauser having an interest in verse or whimsy would have sounded about equally improbable, but his son has assured me that at home his father clearly liked poetry and had an excellent sense of humor.6
Hauser's very limited ability to read by artificial light led not only to his previously cited inability to even scan all of the chemical literature important to his research but also to an even more limited opportunity for general reading. It is quite understandable that this handicap, coupled with his never having been outside the United States, made him seem less sophisticated than many of his university colleagues.8
To each of his graduate students, but especially to those having problems with research or course work, Hauser could be counted on to be friendly and helpful, ready to give advice and inspiration. A student or indeed a postdoc who
might be having difficulties could look forward to having his study or relaxation interrupted by an evening telephone call at home. I have found no support for the Duke legend that less zealous co-workers terminated such conversations by coughing into the telephone, relying on Hauser's well-known fear of catching cold to bring the conversation to an early conclusion. Hauser's interest in his students and co-workers did not end when they left his laboratory; their subsequent careers were followed with interest and, in most cases, justifiable pride.
Hauser once remarked to me that it took him a long time to learn how to do research. Unfortunately, he did not amplify his remark. Records show that six years after completing his Ph.D. degree he had published eight research papers, seven of which were on chloramine chemistry, the subject of his dissertation research. While my first reaction was that he felt that these early papers did not give evidence of the originality characteristic of his later publications, I now feel that what he had learned was how to organize his research, suiting the particular problem or part of it to the level of skill and development that his assistant might have. There is no doubt but that Hauser developed great skill at such organization.
Not long before his death Hauser described his research interests as “fundamental organic chemical studies of mechanisms and syntheses in the fields of condensations, cyclizations, substitutions, eliminations, and molecular rearrangements.”3 There is no doubt that his 450+ papers have provided us with new insights into each of these areas. Perhaps his most important contributions concern an operation central to organic synthesis, the establishment of a new carbon-to-carbon bond. A brief summary follows.
Working with W. B. Renfrow, Jr., in 1937, Hauser showed that the popular belief that the base-catalyzed self-conden-
sation of esters required the presence of two hydrogens on the alpha carbon of the ester was erroneous.9 He found that even ethyl isobutyrate, which has only one alpha hydrogen, undergoes self-condensation in the presence of a sufficiently strong base, in this case the triphenylmethide ion. The authors explained the condensation in terms of a complex equilibrium (Scheme 1) that could also be extended to the self-condensation of ethyl acetate. This will seem familiar because it was to become essentially the modern textbook description of the mechanism.
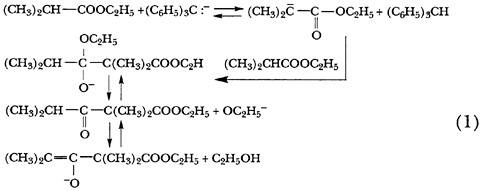
In his second paper of the series in 1938 Hauser enunciated what became Hauser's Rule: In all known condensations of ethyl esters a weaker base is formed than the one used to initiate the reaction. 10
Hauser recognized the kinship between the ester condensation and the Perkin reaction in which, in the classic example, sodium acetate and acetic anhydride are heated together with benzaldehyde to yield, on addition of water, cinnamic acid. Although Perkin himself had assumed that condensation occurred between acetic anhydride and benzaldehyde, the textbook writers of the 1930s usually de-
picted the reaction as occurring between benzaldehyde and sodium acetate, the role of the acetic anhydride being that of a dehydrating agent.11 A plausible defense for such a view had been provided by R. Fittig and F. L. Slocum, who showed that when sodium butyrate is substituted for sodium acetate in the experiment, some alpha-ethylcinnamic acid is formed.12
Hauser concluded that the anion of acetic anhydride was a much more probable intermediate than a doubly charged anion derived from sodium acetate. With David S. Breslow he demonstrated that when sodium butyrate is heated with acetic anhydride, or when sodium acetate is heated with butyric anhydride, essentially the same equilibrium mixture containing both anhydrides is formed, suggesting that the intermediate in Fittig's formation of ethylcinnamic acid most likely was butyric anhydride rather than sodium butyrate.
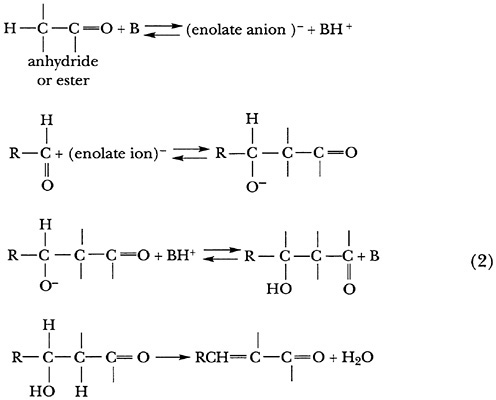
The condensation of benzaldehyde with ethyl acetate in the presence of bases has also been called a Perkin condensation. Although it had long been supposed that reactions of this type occur via an aldol intermediate, no such intermediate had ever been isolated. Hauser and Breslow showed that with the suitable choice of a base and reaction conditions the long-awaited aldol intermediate could be isolated. 13,14
An astute analysis of all results then available led Hauser and Breslow to propose a reaction mechanism (Scheme 2; B = base) that brings out the critical importance of the conjugate acid (derived from the base) in facilitating the dehydration step that serves to permit the reaction to go to completion.
T. S. Stevens had shown that the action of various bases on benzylic quaternary salts (I, Scheme 3) resulted in a 1,2 shift of alkyl groups, quite properly called the Stevens rearrangement.15 In 1951, in collaboration with S. W. Kantor, Hauser found that sodium amide in liquid ammonia caused another type of rearrangement, affording ortho-substituted benzene derivatives (II) in yields of over 90 percent (Scheme 3).16
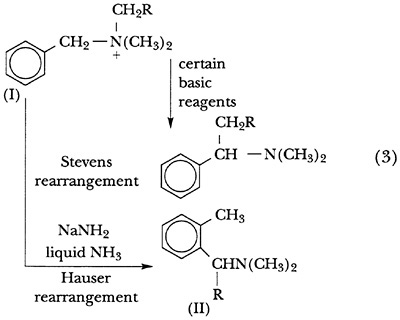
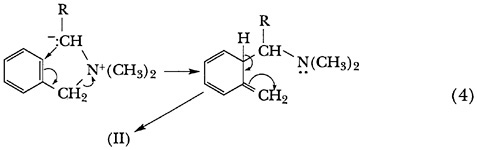
Although the reaction mechanism was obscure, it was correctly proposed in the first paper and confirmed by subsequent work in collaboration with A. J. Weinheimer17 and D. Van Eenam.18 The Hauser rearrangement takes place by what the authors have described as an aromatic nucleophilic mechanism in which the benzene ring serves as an electron acceptor in a five-atom ring displacement (Scheme 4). The ortho-methyl group, which appears in the rearrangement product, did not arrive there by migration from the nitrogen atom, but was instead created from the benzylic methyl group.
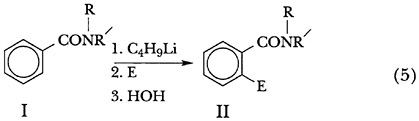
In 1964, in collaboration with W. H. Puterbaugh,19 and as part of a comprehensive study20 of the lithiation of aromatic nuclei and the side chains attached thereto, Hauser carried out the ortho-lithiation of N-methylbenzamide (I,R = H,R' = Me, Scheme 5) and, by reaction of the resulting lithium reagent with ketones, demonstrated the synthetic usefulness of the reaction. While significant as the first example of the ortho-lithiation of a benzamide, it was supplanted in 1977 by a more useful metalation reaction using N,N-diethylbenzamide (I,R = R' = Et) and secondary-butyllithium, reported by P. Beak and R. A. Brown.21
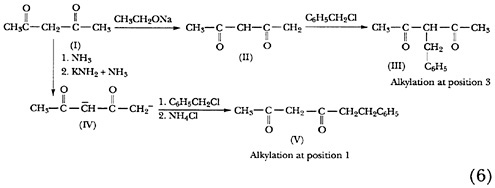
The discovery by Hauser and T. M. Harris that multiple anions may be made to react selectively perhaps will have the greatest impact on organic synthesis.22 This breakthrough has made possible the easy synthesis of compounds that would be difficult to obtain by other means. For example, the monoanion (II, Scheme 6) of acetylacetone (I) has long been known to undergo benzylation at carbon 3. However, if the dianion (IV) was formed by reaction of acetylacetone
with two equivalents of potassium amide in liquid ammonia, alkylation with benzyl chloride afforded the 1-alkylated product (V) in good yield.23 Similar selectivity was shown in the reaction of other dianions24 and, later, higher multiple anions.25
In 1944 Hauser directed a research project on antimalarial drugs for the Office of Scientific Research and Development and was awarded a Certificate of Merit. He served as a consultant for Union Carbide Chemicals Company from 1946 to 1961. He was a visiting lecturer at Ohio State University during the summer of 1956 and in 1961 was awarded a James B. Duke Professorship by Duke University. Hauser was the recipient of three American Chemical Society awards: first, in 1957 the Florida Section Award, given to an outstanding chemist in the Southeast; then in 1962 the Herty Medal, also for an outstanding chemist in the Southeast as well as the ACS Award for Creative Work in Organic Chemistry; and, finally, in 1967 the Medal for Synthetic Organic Chemistry from the Synthetic Organic Chemical Manufacturers Association.
On January 6, 1970, a few months before he was to retire, Charles R. Hauser gave up the fight against a debilitating heart condition that had bothered him for a long time.
Quite characteristically one of his last visitors was a graduate student who talked with him about research.
NOTES
1. D. S. Tarbell and A. T. Tarbell. The History of Organic Chemistry in the United States, 1875-1955. (Nashville: Folio Publishers, 1986):143.
2 Organic Syntheses, Index to Collective Volumes I-V, eds. R. L. Shriner and R. H. Shriner. (New York: John Wiley & Sons):432.
3. I am indebted to the Duke University Archives (William E. King, Archivist) for this and other pertinent information.
4. Tarbell and Tarbell, op. cit., p. 268.
5. McGraw-Hill Modern Scientists and Engineers, vol. 2. (New York: McGraw-Hill, 1980):29-31.
6. Letter. August 17, 1990.
7. The Seminole. (Gainesville: University of Florida, 1923):39. I am indebted to Joyce Dewsbury, coordinator of the University Archives, University of Florida, for a copy of this page.
8. A joint letter from Frances Hauser Grate and Betty Hauser Yourison, September 3, 1990.
9. C. R. Hauser and W. B. Renfrow, Jr. (1937,1).
10. C. R. Hauser and W. B. Renfrow, Jr. (1937,2).
11. For example, J. F. Norris. Principles of Organic Chemistry. (New York: McGraw-Hill, 1931):459. Other examples are cited in note 13.
12. R. Fittig and F. L. Slocum. Ueber die Perkin'sche Reaction: Einwirkung von Benzaldehyde auf einem Gemisch von Essigsaureanhydrid und Buttersaurem Natrium. Justus Liebig's Annalen de Chemie 227(1885):53-55.
13. C. R. Hauser and D. S. Breslow (1939,1).
14. C. R. Hauser and D. S. Breslow (1939,2).
15. T. S. Stevens. Degradation of quaternary ammonium salts. Part II. Journal of the Chemical Society (1930):2107-19.
16. C. R. Hauser and S. W. Kantor (1951).
17. C. R. Hauser and A. J. Weinheimer (1954).
18. C. R. Hauser and D. N. Van Eenam (1956,1; 1957,1,2).
19. C. R. Hauser and W. H. Puterbaugh (1964).
20. C. R. Hauser, F. N. Jones, and M. F. Zinn (1963,1).
21. P. Beak and R. A. Brown. The ortho lithiation of tertiary benzamides. Journal of Organic Chemistry 42(1977):1823-24. See also P. Beak and V. Snieckus. Direct lithiation of aromatic tertiary amides:
An evolving synthetic methodology for polysubstituted aromatics. Accounts of Chemical Research 15(1982):306-12.
22. C. R. Hauser and T. M. Harris (1957,3).
23. C. R. Hauser and T. M. Harris (1958).
24. For example, C. R. Hauser and W. R. Dunnavant (1960).
25. For example, C. R. Hauser, M. L. Miles, and T. M. Harris (1963,2).
SELECTED BIBLIOGRAPHY
1937 With W. B. Renfrow, Jr.Certain condensations brought about by bases. I. The condensation of ethyl isobutyrate to ethyl isobutyrylisobutyrate. J. Am. Chem. Soc. 59:1823-26.
With W. B. Renfrow, Jr.Condensations brought about by bases. II. The condensation of the enolate of ethyl isobutyrate with ethyl benzoate and further observations on the Claisen type of condensation. J. Am. Chem. Soc. 60:463-65.
1939 With D. S. Breslow. Condensations brought about by bases. V. The condensation of the anhydride with the aldehyde in the Perkin synthesis. J. Am. Chem. Soc. 61:786-92.
With D. S. Breslow. Condensations brought about by bases. VI. The mechanism of the Perkin synthesis. J. Am. Chem. Soc. 61:793-98.
1940 With D. S. Breslow. Condensation brought about by bases. IX. The relationship between the Claisen and Perkin type of condensations. J. Am. Chem. Soc. 62:593-97.
With D. S. Breslow. Condensations. XII. A general theory for certain carbon-carbon condensations effected by acidic and basic reagents. J. Am. Chem. Soc. 62:2389-92.
1945 With P. S. Skell. The mechanism of beta-elimination with alkyl halides. J. Am. Chem. Soc. 67:2206-8.
1951 With S. W. Kantor. Rearrangements of benzyltrimethylammonium ion and related quaternary ammonium ions by sodium amide involving migration into the ring. J. Am. Chem. Soc. 73:4122-31.
1953 With W. H. Puterbaugh. Aldol condensation of esters with ketones or aldehydes to form beta-hydroxy esters by lithium amide. Com-
parison with the Reformatsky reaction . J. Am. Chem. Soc. 75:1068-72.
1954 With A. J. Weinheimer. The ortho-substitution rearrangement versus beta-elimination of certain quaternary ammonium ions with sodium amide. Extension of the method of synthesis of vicinal alkyl aromatic derivatives. J. Am. Chem. Soc. 76:1264-67.
1956 With D. N. Van Eenam. Alicyclic amine from rearrangement of 2,4,6-trimethylbenzyltrimethyl ammonium ion and its reconversion to aromatic system. J. Am. Chem. Soc. 78:5698.
With B. O. Linn. Synthesis of certain beta-diketones from acid chloride and ketones by sodium amide. Mono versus diacylation of sodio ketones with acid chlorides. J. Am. Chem. Soc. 78:6066-70.
1957 With D. N. Van Eenam. Rearrangement of 2,4,6-trimethylbenzyltrimethly-ammonium ion by sodium amide to form an exo-methylenecyclo-hexadieneamine and its reactions . J. Am. Chem. Soc. 79:5512-20.
With D. N. Van Eenam. Base-catalyzed elimination and aromatization of a cyclohexadieneamine and its methiodide. J. Am. Chem. Soc. 79:6274-77.
With T. M. Harris. Dicarbanions of dibenzyl ketone, dibenzyl sulfone and alpha, beta, beta-triphenylpropionitrile. J. Am. Chem. Soc. 79:6342.
1958 With T. M. Harris. Condensations at the methyl group rather than the methylene group of benzoyl- and acetylacetone through intermediate dipotasio salts . J. Am. Chem. Soc. 80:6360-63.
1959 With T. M. Harris. Benzylation at the terminal methyl group of certain unsymmetrical beta-diketones through one of two possible intermediate diketones . J. Am. Chem. Soc. 81:1160-64.
1960 With W. R. Dunnavant. Factors in aldol condensations of alkyl acetates with benzophenone and reversals by sodium amide versus lithium amide. Metallic cation effects. J. Org. Chem. 25:1296-1302.
1961 With W. I. O'Sullivan, F. W. Swamer, and W. J. Humphlett. Influence of the metallic cation of certain organometallic compounds on the courses of some organic reactions. J. Org. Chem. 26:2306-10.
1963 With F. N. Jones and M. F. Zinn. Metalations of benzyldimethylamine and related amines with n-butyllithium in ether. Deuteration to form ring and side-chain derivatives. J. Org. Chem. 28:663-65.
With M. L. Miles and T. M. Harris. Aroylation at the terminal methyl group of 1,3,5-triketone to form a 1,3,5,7-tetraketone. J. Am. Chem. Soc. 85:3884.
1964 With W. H. Puterbaugh. Metalation of N-methylbenzamide with excess n-butyllithium. Condensations with electrophilic compounds to form ortho-derivatives. Cyclization . J. Org. Chem. 29:853-56.
1965 With S. Boatman and T. M. Harris. Alkylations at the alpha-prime-methylene or -methinyl group of alpha-formyl cyclic ketones through their dicarbanions. Angular alkylations. J. Am. Chem. Soc. 97:82-86.
With E. M. Kaiser. Kinetic versus thermodynamic control in carbonyl addition reactions of carbanions. Chem. Ind. (London) 1299-1300.
1969 With C. Mao, F. C. Frostick, Jr., E. H. Man, R. M. Manyik, and R. L. Wells. Dual formation of beta diketones from methylene ketones and acetic anhydride by means of boron triflouride. Improved method of synthesis of certain beta diketones. J. Org. Chem. 34:1425-29.




















