
ROWLAND PETTIT
February 6, 1927–December 10, 1981
BY JOHN C. GILBERT
ROWLAND PETTIT, although educated primarily in the area of physical-organic chemistry, focused his scientific research on organo-transition metal chemistry and brought a level of creativity and a “nose” for the issues of significance in the subject that justly earned him international recognition. As one who enjoyed both intellectual and technical challenges, Rolly made contributions that addressed problems ranging from those of largely theoretical and academic interest to those of substantial practical and industrial importance. The broad scope of his research is epitomized by his work on the synthesis and reactions of cyclobutadiene iron tricarbonyl, the role of orbital symmetry in metal-catalyzed isomerizations of strained hydrocarbons, and the mechanisms of the water gas shift reaction and the Fischer-Tropsch process.
Rolly was born in Port Lincoln, Australia, a small town in the southern part of the country, on February 6, 1927. The eldest of four sons, he was the only one to venture into the world of science. Many of the stories that Rolly enjoyed telling about his formative years in Port Lincoln revolved about fishing expeditions in the shark-infested waters near there. Following his precollege education he matriculated
at the University of Adelaide, where he earned his B.Sc. (1949), M.Sc. (1950), and first Ph.D. (1953). Rolly often boasted that he had financed much of his schooling at the pool table; thus, he not only fished for shark but was himself a “pool shark!”1
His graduate research, under the supervision of A. K. MacBeth and G. M. Badger, was in natural products and polynuclear heterocyclic chemistry. Even at this early stage of his career, Rolly had an appreciation for the role of metals in fostering various chemical phenomena, not all of which had to do with his laboratory research. This interest is illustrated by the following story. As a student at Adelaide, Rolly had become enamored of a beautiful young blond, blessed not only with good looks but also with a father who was one of the most prosperous ranchers in the area. The family's affluence included a large swimming pool, a luxury virtually unknown in the region at the time. Because the state of pool technology was primitive by contemporary standards, control of algae was a continuing battle, and this was particularly vexing to “Daddy,” who enjoyed his daily swim. Pettit, aware of the role of copper compounds as effective algicides, saw an opportunity to make a lasting, positive impression on his beloved's father, thereby fortifying his relationship with the daughter. Thus, he dosed the pool liberally with copper sulfate and soon presented a pool filled with crystal-clear, algae-free water for inspection. The father, having expressed his gratitude for this accomplishment, enthusiastically plunged into the pool, only to emerge minutes later with his formerly handsome head of silver-gray hair exhibiting a stunning blue-green cast. Rolly was never again to explore the realm of bioinorganic chemistry!
Upon completion of his Ph.D., Pettit accepted an “Exhibition of 1851 Overseas Fellowship.” These highly competi-
tive awards had been established using profits from the Exhibition (World's Fair) of 1851 and enabled graduates from universities in other parts of the British Empire to go to Britain for two years of study for higher degrees. It was undoubtedly the interest kindled by his experience in the area of polynuclear compounds that led Pettit to take up his fellowship under the tutelage of Michael J. S. Dewar, then the newly appointed professor of chemistry at the Queen Mary College (QMC) of the University of London. In retrospect, this decision was to have enormous influence on the rest of Rolly's scientific career, sparking as it did his life-long fascination with nonbenzenoid aromatic compounds and organometallic chemistry, and furthering his interest in the application of theory to organic chemistry; Rolly had independently learned molecular orbital theory while at Adelaide, although no one in the department at that time either knew this technique or cared about it.
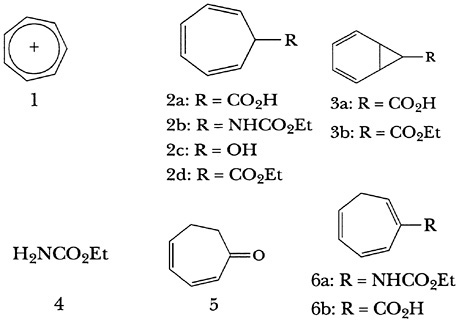
Pettit's best-known accomplishment at QMC was the first intentional synthesis of tropylium ion (1), the first truly stable carbenium ion to be discovered. The reason Pettit's synthesis of this ion was not the first one actually to appear in the scientific literature is unusual and, in retrospect, amusing. The synthesis started with the alleged 2,4,6-cycloheptatriene-1-carboxylic acid (2a). This was one of several isomeric cycloheptatrienecarboxylic acids that Büchner had previously prepared by alkaline isomerization of what he believed to be the norcaradienecarboxylic acid (3a), the ethyl ester (3b) of which was assigned as the product of addition of carbethoxycarbene to benzene. An obvious route from 2a to 1 thus would be by way of a Curtius degradation. However, A. W. Johnson, then at Cambridge University, was known to be trying this route. Consequently, Dewar and Pettit, feeling that it would be improper for them to “poach,” attempted numerous alternative ways of transforming 2a to 1,
all of which failed. At this point the news came from Cambridge that the urethane formed by Curtius degradation of the supposed 2a, and thus assigned structure 2b, hydrolyzed extremely easily to urethane (4) and other unidentified products. Johnson explained the facile hydrolysis in terms of an unprecedented SN1-type reaction, its ease owing to the aromaticity of the tropylium ion (1), derived from 2b by ionization. Because Johnson planned no further work on the problem and since the mechanistic explanation he provided to account for his results seemed dubious, Pettit repeated the preparation and hydrolysis of the alleged urethane 2b, obtaining not only 4, but also 2,4-cycloheptadienone (5), both in nearly quantitative yield. This seemed to refute Johnson's interpretation because hydrolysis of 2b in the manner he suggested should have led to 2,4,6-cycloheptatriene-1-ol (2c), not to a ketone. Rolly's result strongly implied that the structure of the urethane derived from the Curtius degradation had been wrongly formulated by Johnson and was instead an isomer (e.g., 6a, rather than 2b); as an analog of an enamine, such a species would be expected to hydrolyze easily. The structural reassignment in turn meant that the “Büchner acids” were isomers derived from hydrolysis and isomerization of the ester 2d, itself formed by the previously mentioned reaction of carbethoxycarbene and benzene. So, for nearly a year Pettit had been preparing the acid 2a that he wanted but had been converting it to an isomer, 1,4,6-cycloheptatriene-1-carboxylic acid (6b) that could not be transformed into 1! Indeed, all the routes used in attempts to convert “2a,” as assigned by Büchner, to 1 succeeded when applied to the acid originally formulated as “3,” which in fact is properly assigned as 2a. In the meantime, Doering and Knox “scooped” Pettit by realizing that a crystalline material made many
years earlier by Meerwein, and described by him as “chlorocycloheptatriene,” was in fact tropylium chloride.
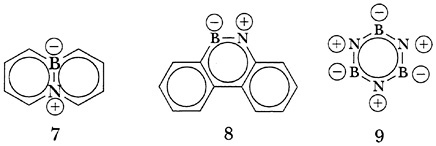
Another notable contribution by Pettit at QMC was the synthesis of what proved to be the first two (7 and 8) of a series of astonishingly stable aromatic “borazaro” compounds, conceptually derived from normal aromatic hydrocarbons by replacing one or more pairs of adjacent carbon atoms by the isoelectronic moiety, B-N+. While borazine (9) is related in this way to benzene, it is highly reactive because the large difference in electronegativity between B- and N+ restricts p-bonding. Compounds 7 and 8, on the other hand, closely resemble the corresponding hydrocarbons, phenanthrene and naphthalene. In fact, 8 fails to undergo the Diels-Alder reaction with maleic anhydride, an unexpected result since naphthalene itself reacts reversibly with this anhydride in a Diels-Alder fashion.
Pettit's completion of the second of his two Ph.D. degrees is an amusing tale in itself, and Michael J. S. Dewar has provided the following account of certain events that led to it.
Pettit's first Ph.D. Degree was from the University of Adelaide. At the time, Examining Committees at universities in Australia always included an outside member, usually from Britain, and I was asked to serve as one of Rolly's examiners. Under the circumstances, no oral examination was normally held because it was impossible for the committee to meet. However, Rolly's professor in Adelaide, G. M. Badger, traveled round the world regularly and was due to visit London just after Rolly had arrived. He suggested that it would be nice to set a precedent by having an oral examination. This was duly arranged, at a convenient period before lunch, in my office, and when we had all gathered, I produced a bottle of sherry to assist the proceedings. There was of course no question about the outcome—Rolly's Ph.D. thesis was superb—so we sat around happily “talking chemistry” until it was time for lunch. The next day Rolly was unwise enough to complain about his oral, saying that he had spent a lot of time reading up on organic chemistry in preparation for a grilling and that all we had in fact done was to spend an hour gossiping about chemists!
This brash statement was destined to haunt him two years later. Rolly had had no intention of submitting a Ph.D. thesis in Britain because he already had his Ph.D. from Adelaide. However, as the end of his two years of fellowship drew to a close, I worked on him, pointing out what a unique distinction it would be to have two Ph.D.'s. I also reminded him that his Fellowship provided funds for typing and binding a Ph.D. thesis and that the money would be wasted if he failed to write one. So he relented and I arranged the oral examination carefully, planning it for our home after dinner, with one of my oldest friends, Christopher Longuet-Higgins, as the
other examiner. After we had lulled Rolly into a mistaken sense of complacency with one of Mary's dinners and a fair amount of good wine, we set to work, having spent the whole afternoon planning it. I forget now what the questions we asked were. Most of them were ones we could not answer ourselves. We reduced Rolly to total incoherence. After about an hour, when we had run out of prepared questions, I said sternly, “Please leave the room, Dr. Pettit, so that we can discuss our verdict.” Mary said he came out, shaking like a jelly, almost in tears, and saying how awful it was that he had let me down by failing so disastrously. After a suitable interval, I summoned him back and said, “Well, Dr. Pettit, I hope you found this oral examination satisfactory?” To his credit, Rolly replied without batting an eyelid, “Yes, Professor Dewar, entirely satisfactory.” It was of course a great piece of good fortune for me to have him as a founding member of my first research group, in which he remained for five years, the last three of which were as an ICI Postdoctoral Fellow.
In the spring of 1957 Rolly accepted a position as an instructor at the University of Texas at Austin (UT-Austin), never having set foot in the States, much less Texas. His recruitment to the Department of Chemistry was done “in the old-fashioned way,” by virtue of personal contacts between eminent scientists. In Rolly's case, Dewar had alerted Doering that Pettit was a superb candidate for an academic post in the United States.2 Doering, in turn, knew of the opening at UT-Austin and put the department in contact with Rolly. It was at this point that he discovered it would be impossible for him to emigrate to the United States with a permanent visa since the quota of these for citizens of Australia was 100 per year and the waiting list was already twenty years long. Indeed, the only option available that would allow him to enter the United States by the fall was as a participant in the “Exchange Visitor Program of the University of Texas,” which was designed to enable foreign students to further their educations in Texas. This type of visa was good for only five years and made its recipients ineligible to apply for a permanent visa until such time that
the holder had spent a minimum of two years back in his or her country of residence; this proviso was soon to challenge Rolly's creativity and power of persuasion. Nevertheless, always the optimist and anxious to embark on his independent scientific career, Rolly elected to come to Texas despite having only a student visa. The outcome of this decision was obviously to be of great mutual benefit.
Rolly found Austin's climate a welcome relief from the damp and cold of England, and he felt right at home in Texas—after all, Aussies are rugged individualists too! Indeed, whenever British visitors concluded their seminars by showing pictures of England under crystal-clear, sunny skies, Rolly used to needle them by asking how long they had to wait for the appropriate climatic conditions needed to obtain such “rare” photographs. Within a year, however, he began to address his visa status, hoping to circumvent the impending requirement for him to spend two years back in Australia. The request for an exception to the regulation was pursued at a number of levels, a process that at various points involved Dr. Alan Waterman, then director of the National Science Foundation; Dr. Wallace Brode, scientific advisor to the Secretary of State; and even the Honorable Lyndon B. Johnson, who was a U.S. senator at the time. Much to Rolly's chagrin, all appeals were to no avail, apparently because President Eisenhower felt strongly that the program under which Pettit had entered the country was designed to give foreign students an education in the United States with the intention that they would return to their homes and use their skills to uplift the economies of their own countries; to make exceptions would be to encourage the “brain drain” phenomenon. One of the effects of the prolonged but unsuccessful effort to evade the long arm of the Immigration and Naturalization Service was to reinforce Rolly's general distaste for paperwork and the necessity to
work through administrative channels. In any case, Rolly was ultimately forced to explore other options and fortunately found a loophole that obviated the need to leave the country for an extended period. The strategy was simple enough, but its risk was great. All he needed to do was to leave the United States, and of course Nuevo Laredo, Mexico, was an easy drive from Austin, and then return immediately; absence from the States, however brief, was sufficient to permit renewal of his visa. The risk was that once having crossed into Mexico, Rolly would not be allowed to reenter the United States because he did not hold the proper type of visa for reentry—a classic Catch-22 situation! Fortunately, the overworked officials at the Immigration and Naturalization Service let the Aussie back in, failing to take proper notice of the type of visa he held. However, by stamping his visa according to standard bureaucratic practice, they provided Rolly with the evidence he needed to prove that he indeed had left the United States.
All of Rolly's visa problems were eventually rendered moot when, in 1959, he married Flora Hunter, a Ph.D. biochemist associated with the Clayton Foundation Institute of Biochemistry at UT-Austin. From this union came two children, George H. and Nancy S. In Flora, Rolly had a wife who succeeded in juggling her own professional career, childrearing, and support of her husband's aspirations in a way that few women could have. Given Rolly's predilection for professional travel and his aforementioned distaste for paperwork, Flora shouldered the lion's share of the tasks of family life and made it possible for him to concentrate on his passion for chemistry. Rolly was extremely proud of Flora's ability to successfully organize his life, provide love and nurturance to the children he too so adored, continue to pursue her own interests in scientific research, and aug-
ment the family's finances with astute actions on the stock market. 3
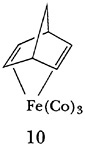
Pettit's early independent scientific career at UT-Austin involved the synthesis and characterization of cationic non-benzenoid aromatic species such as the perinaphthenylium, thiapyrylium, and homotropylium ions. The latter was the first example of a species exhibiting “homoaromaticity,” a theoretical concept first introduced by Saul Winstein. Rolly also initiated his studies on iron tricarbonyl complexes of polyenes and, in 1959, prepared 10 from bicyclo [2.2.1] hepta-1,5-diene, the first such complex of a nonconjugated system. His potential and accomplishments at this point in his career were formally recognized by his being named as an A. P. Sloan fellow. On an informal basis, the fact that UT-Austin had an outstanding young chemist on its hands was noted by the faculty of the Chemistry Department at MIT. Norman Hackerman, then chairman of the department at UT-Austin, learned of this in 1960 when Arthur C. Cope, who was visiting in Austin at the time, mentioned that his faculty had listed the “potentially most able chemists in this country between the ages of 28 and 32 . . . and that Pettit was in that list.”
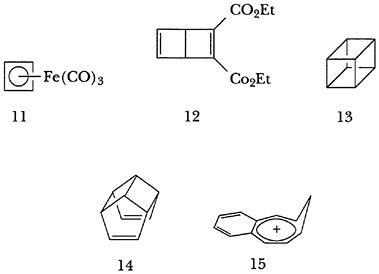
Extension of the investigation of various organoiron complexes eventually led Rolly and his co-workers to the preparation in 1964 of 1,3-cyclobutadiene iron tricarbonyl (11)
and shortly thereafter to the long-sought and theoretically important diene itself.4 Indeed, the facile release of the highly reactive diene from the complex made the hydrocarbon a readily available intermediate from which a number of fascinating molecules were ultimately prepared by the Pettit group and others. Among them were a number of examples of Dewar benzenes, (e.g., [12], as well as cubane [13], hypostrophene [14], and benzohomotropylium ion [15]). Rolly's interest in the properties of highly strained compounds and his unique combination of expertise in organometallic chemistry and theoretical organic chemistry led to seminal studies of metal-catalyzed cycloadditions and rearrangements that stimulated many other scientists to explore the mechanism and application of such processes.
In the area of “pure” organometallic chemistry, compounds prepared in the Pettit group have provided considerable insight into the nature of bonding between organic species and transition metals. Rolly's pioneering work in the synthesis and examination of the properties of the iron tricarbonyl complexes of p-allyl, pentadienyl, and tropylium
cations are noteworthy; his work with the latter species contributed much to the concept of “fluxionality” in molecules, species that Pettit delighted in calling “ring whizzers,” as illustrated by the equilibria in Eq. 1.
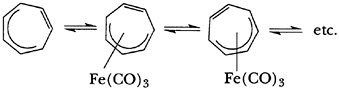
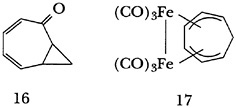
Examination of the chemistry of the iron tricarbonyl complex of the homotropylium ion led to the ingenious preparation of the theoretically interesting 2,3-homotropone (16), and work with the bis(iron tricarbonyl) complex of 1,3,5-cycloheptatriene (17) produced an incredibly stable organometallic cation. The pKR+ value of the corresponding alcohol (8.0), a reflection of cationic stability, is one of the largest such values, if not the largest, recorded.
Pettit recognized the importance of unraveling the mechanistic details of reactions catalyzed by organometallic species. His enviable chemical intuition and ability to design simple yet definitive experiments resulted in important mechanistic understanding of the olefin metathesis (disproportionation) reaction and the Fischer-Tropsch process, both of which are of great industrial importance. In the former case he was able to show that cyclobutane intermediates were not involved and instead suggested the interven-
tion of a metal complex, an insight that has since been confirmed. His use of a binuclear metal carbenoid species as a model compound to elucidate the mechanism of the Fischer-Tropsch reaction, coupled with his definition of the details of the chain-propagating step in the overall transformation, has provided important information in this area. In related work, Pettit and his group demonstrated the synthetic utility of carbon monoxide and water in an iron-catalyzed water gas shift reaction with the intermediacy of an anionic metal hydride. These results have significant implications for addressing the challenge of producing synthetic fuels by metal-catalyzed processes.
Rolly's renewed interest in the mechanism of the Fischer-Tropsch reaction, in particular, on the details of how carbon-carbon bond formation occurs under such catalytic conditions, developed only a year or so before he was diagnosed as having inoperable lung cancer. To be sure, these studies were merely an extension of one of his long-standing interests—that of C-C bond making and breaking as mediated by transition metals. Pettit in fact had just published a seminal communication in the area. He also had a very strong feeling that cracking and depolymerization reactions, of considerable interest to the petrochemical industry, were related to the Fischer-Tropsch reaction (i.e., all these processes involved transition metal-bridging methylene or methylidene reactive intermediates).5
As Rolly began to succumb to the disease and to the heroic yet debilitating attempts to control it by chemotherapy, he was hospitalized and remained so for the last months of his life. He continued to maintain almost daily contact with his research group of about ten individuals through Evan Kyba, who would visit him for anywhere from five minutes to an hour late each afternoon. Rolly would gather his strength for those visits so that he could focus fully on the
discussion of his group's results. He would analyze the data and discuss with Kyba what key experiments were to be performed next. When this was done, Rolly was generally exhausted, and the meeting would be over—he simply did not have the energy for discussion of nonchemical matters. The next morning the results of the previous day's discussions would be communicated to the relevant members of the Pettit research team, and the cycle would continue.
Although there were many projects ongoing at the time, the one that commanded Rolly's interest on an almost daily basis was the effort related to the catalytic hydrogenolytic depolymerization of hydrocarbons. The chemistry required considerable fine tuning of the experimental procedures before meaningful results could be obtained. This took several months, during which Rolly became very frail, but he refused to give in to the inevitable while this crucial piece of work was still unfinished. Evan Kyba still vividly remembers when he presented the last data to Rolly for his inspection. “We've got it!” he exclaimed, “Write it up.” He died less than a week after that meeting. Kyba is convinced that Rolly's interest in that scientific problem kept him alive at least two months longer than any other person would have survived under the circumstances—but such was the force of his intellect that he would not quit until the job was done.
Rolly's research accomplishments earned him election to the National Academy of Sciences in 1973. Earlier he had received the Southwest Regional Award of the American Chemical Society (1968) and membership in the American Academy of Arts and Sciences. He was named to the W. T. Doherty Professorship in Chemistry in 1980.
Although dealing with administrative tasks was not Rolly's forte or of interest to him, he did consent to serve as chairman of the department for a four-year term. This was at the
behest of John Silber, then dean of the College of Arts and Sciences, whose powers of persuasion are legend. I remember well Silber's first and only meeting with the faculty of the department, during which he made it quite clear who was in charge. It was indeed fortunate for us that we had someone with Rolly's strength of character and reputation as our buffer with Dean Silber, as Rolly saw to it that the department continued to grow in quality and reputation of its faculty despite the maelstrom surrounding Silber's tenure as dean. Nevertheless, Rolly's term as chairman probably represented the longest four-year period of his life.
Rolly was an individual with a breadth of interests outside of chemistry. These included the aforementioned love of competitive pool, at which he excelled regardless of how many shots of scotch whiskey he had consumed; the raising of exotic flora, with an emphasis on bromeliads; and, in the last years of his life, ranching in the classic Texas style. The image of him leaning over the pool table, sighting along his personalized cue with those piercingly bright blue eyes, and then successfully making a difficult combination shot is impossible to forget. Similarly, his obvious delight at being able to conduct experiments on the efficacy of calcium carbide in coaxing his beloved bromeliads into bloom typified his excitement about research of all kinds. In an entirely different vein, he was a sight to behold as he tended to his small herd of cattle, handling the ranch equipment, all of which was foreign to this Aussie, with aplomb and ascertaining that his bull, “Taurus,” was behaving himself.6
Rowland Pettit was an exceptional human being, much admired by his colleagues here and abroad, his students, and all those with whom he came in contact. His intelligence, wit, creativity, unrelenting optimism, and general love of life were characteristics that fit this man and brought out the best in all who knew him. He was a person who
lived life to the fullest. As Rolly himself often said, in his usual self-deprecating manner, “All the victorious gladiators have left the arena. Only I lie bleeding in the sand.” Until the very end, he lived to fight another day, and we all miss him.
THE AUTHOR APPRECIATES CONTRIBUTIONS to this memoir from Michael J. S. Dewar, Nathan Bauld, Stephen Martin, Evan P. Kyba, Jeffrey S. McKennis, William Baird, and Michael Edens, all of whom benefited professionally and personally from their relationship with Rowland Pettit.
NOTES
1. Many years later (1970) Rolly finally obtained his own pool table and installed it in the basement of his house. This table invariably became the center of attention at many of the social events he and his wife hosted. It was a rare occasion when he lost at “eight-ball” or straight pool. His talents at the table were also well-known in a pool hall in Columbus, Texas, a small town on the route Rolly traveled on his frequent consulting trips to Exxon Research and Engineering Company in Baytown. The “locals,” most of whom were Hispanic, were soon to learn that the “gringo” from Austin was awesome at the table.
2. It is an interesting coincidence that within five years Rolly was to be instrumental in luring his own mentor, Dewar, to UT-Austin to become the first holder of the Robert A. Welch Chair in Chemistry. Rolly knew that Michael was not entirely happy with his situation at the University of Chicago. One point of contention, according to a letter that Pettit wrote to Al Matsen, a colleague at UT-Austin, in June of 1962, was that the University of Chicago was dragging its feet with regard to providing Dewar with an air conditioner for his office, and Michael was finding the summer heat and humidity of the Windy City unbearable; air conditioning was something that Texans knew about, and it was made clear in the offering letter to Dewar that his research space would all be so equipped.
A somewhat different perspective on the matter has been provided by Dewar himself. He writes, “Curiously enough, five years after going to Austin, Rolly repaid the favour (of Michael's having
helped Pettit to find a position at UT-Austin) by persuading me to follow him there. It was on the surface an idiotic move. I was very happy at the University of Chicago and UTA was then almost unknown. I went because I had heard from Rolly that the Texas Legislature wanted to make UTA a top University and because I had been greatly impressed by the chairman, Norman Hackerman, when I visited Austin in 1957 on Rolly's behalf. With Texas money and Norman in charge, the project seemed feasible and an exciting venture to be in on. Also Rolly was very persuasive.”
3. This is not to say that Rolly was not involved with his family. For example, when the children reached the age when sex education was important, he enthusiastically joined Flora in the purchase of two pure-bred Persian kittens, naming the male, “Joe Kapp,” in honor of the Minnesota Vikings quarterback of the time. Joe eventually performed his fatherly duties but had such a miserable personality that he was banned from the house shortly thereafter. What Nancy and George got out of the experience is not known, but both of them now prefer dogs as pets.
4. The successful synthesis of such iron tricarbonyl complexes evolved from the unsuccessful efforts of Rolly's colleague, Nate Bauld, to prepare the nickel complex of benzocyclobutadiene from the corresponding dibromide. Bauld, knowing that one of Pettit's students, George Emerson, was having great success making dieneiron tricarbonyl complexes, provided Emerson with a sample of the dibromide; the rest is history.
5. There is some belief that Rolly's interest in the Fischer-Tropsch process was triggered by the oil embargo imposed by OPEC in 1973. He took the embargo as an attack on the petrochemical industry, long his favorite, and hoped to teach the cartel a lesson by developing processes that would lessen our dependence on foreign oil.
6. There was a period when Taurus appeared to prefer to “smell the flowers” rather than service the cows, as was intended. Rolly duly consulted with a veterinarian, who told him that Taurus apparently had wandered into a large patch of cockleburrs, which had attached themselves in a manner that made certain activities painful for the bull. The cure recommended was for Pettit to remove the offensive burrs with the cautious use of manicuring scissors. The record is silent on whether this remedy was indeed pursued.
SELECTED BIBLIOGRAPHY
1956 Synthesis of the perinaphthindenylium cation. Chem. Ind. 1306-7.
1962 With J. L. von Rosenberg, Jr. and J. E. Mahler. Bicyclo[5.1.0]octadienyl cation, a new stable carbonium ion. J. Am. Chem. Soc. 84:2842-43.
With G. F. Emerson. p-Allyl iron tricarbonyl cations. J. Am. Chem. Soc. 84:4591-92.
1964 With G. F. Emerson. Diene-iron carbonyl complexes and related species. Adv. Organomet. Chem. 1:1-46.
With J. E. Mahler and D. A. K. Jones. The tropylium-iron carbonyl cation. J. Am. Chem. Soc. 86:3589-90.
1965 With L. Watts and J. D. Fitzpatrick. Cyclobutadiene. J. Am. Chem. Soc. 87:3253-54.
With others. Cyclobutadiene-iron tricarbonyl. A new aromatic system. J. Am. Chem. Soc. 87:3254-55.
1966 With J. E. Barborak and L. Watts. Convenient synthesis of the cubane system. J. Am. Chem. Soc. 88:1328-29.
With R. K. Kochlar. Cyclopentadieneiron tricarbonyl. J. Organomet. Chem. 6:272-78.
With others. Tautomerism in cyclooctatetraene-iron tricarbonyl. J. Am. Chem. Soc. 88:4760-61.
With P. W. Jolly. Evidence for a novel metal-carbene system. J. Am. Chem. Soc. 88:5044-45.
1967 With J. C. Barborak. Stereospecific rearrangements in the homocubyl cation. J. Am. Chem. Soc. 89:3080-81.
1970 With R. E. Davis. Bond localization in aromatic iron carbonyl complexes. J. Am. Chem. Soc. 92:716-17.
With J. Wristers and L. Brener. Mechanism of metal-catalyzed rearrangement of strained cyclobutane and cyclobutene derivatives. J. Am. Chem. Soc. 92:7499-501.
1971 With others. Degenerate Cope rearrangements in hypostrophene, a novel C10H10 hydrocarbon. J. Am. Chem. Soc. 93:4957-58.
With G. S. Lewandos. Mechanism of the metal-catalyzed disproportionation of olefins. J. Am. Chem. Soc. 93:7087-88.
With K. M. Nicholas. Alkyne protecting group. Tetrahedron Lett. 37:3475-78.
1972 With others. Existence of the dianion of cyclobutadiene. J. Chem. Soc., Chem. Commun. 365-66.
1974 With others. (2p + 6p) Cycloaddition reactions between ligands co-ordinated to an iron atom. J. Am. Chem. Soc. 96:7562-64.
1976 With L. W. Haynes. Carbonium ions p-complexed to metal atoms. Carbonium Ions 5:2263-302.
1977 With others. Reductions with carbon monoxide and water in place of hydrogen. I. Hydroformylation reaction and water gas shift reaction. J. Am. Chem. Soc. 99:8323-25.
1980 With others. Homogeneous catalysts for reduction utilizing carbon monoxide and water. Ann. N.Y. Acad. Sci. 333:101-6.
1981 With R. C. Brady III. Mechanism of the Fischer-Tropsch reaction. The chain propagation step. J. Am. Chem. Soc. 103:1287-89.
1982 With W.T.Osterloh and M.E.Cornell. On the mechanism of hydrogenolysis of linear hydrocarbons and its relationship to the Fischer-Tropsch reaction. J.Am.Chem.Soc. 104:3759-61.
With C. E. Sumner, Jr. and J. A. Collier. Chemistry of octacarbonyl (µ-methylene) diiron and its derivatives . Organometallics 1:1350-60.






















