2
Phylogeny from Function: The Origin of tRNA Is in Replication, not Translation
Nancy Maizels
Alan M. Weiner
The diversity evident in contemporary organisms and in the fossil record is remarkable. But perhaps even more remarkable is the fact that all living species, and perhaps all species that have ever lived on earth, share a common biochemistry. In particular, the central pathways of macromolecular synthesis—DNA replication, transcription, and translation—are conserved in all three contemporary kingdoms: Eucarya, Eubacteria, and Archaea (Woese et al., 1990).
The challenge for those interested in biochemical evolution is to deduce, from contemporary molecules and organisms, how these pathways arose. Replication, transcription, and translation all require a multiplicity of interactions among a large cast of macromolecules. It is inconceivable that any of these complex processes arose full blown. A plausible scenario for the evolution of any pathway must therefore provide a persuasive explanation for the selective advantage conferred by each new component as it was added to the pathway.
The discovery of the catalytic properties of RNA (Cech et al., 1981; Guerrier-Takada et al., 1983) showed that a single chemical species could function as both genome and enzyme. This suggested an evolutionary scheme, simple in outline, which is shown in Figure 1. Prebiotic reactions on the primordial earth generated RNA, RNA became the first
Nancy Maizels is associate professor and Alan M. Weiner is professor of molecular biophysics and biochemistry at Yale University School of Medicine, New Haven, Connecticut.
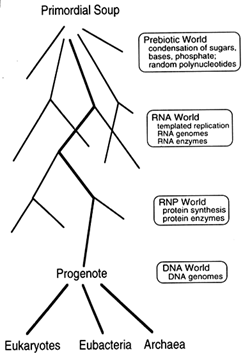
Figure 1 From the primordial soup to the three contemporary kingdoms. Woese (1990) originally used the term "progenote" to describe a common ancestor with an inaccurate translation system, but we make no assumption about the accuracy of translation in the progenote. Accuracy is a matter of degree, and the important point is that translation in this ancestor must have been substantially similar to our own. For clarity, we show DNA as evolving after templated protein synthesis. An interesting alternative, suggested by J. Szostak (Harvard Medical School, personal communication), is that DNA evolved much earlier, perhaps even in the RNA world, as a catalytically inactive storage form of RNA.
self-replicating molecule, early living systems arose with RNA genomes and relatively simple biochemistries, and these evolved into cells with DNA genomes and complex biochemistries. Indeed, the biochemistry of all living organisms is so similar that there is little doubt all life on earth can be traced back to a single common ancestor, which Woese named the "progenote" (Woese, 1990). Moreover, because the translation apparatus is fundamentally similar in all three contemporary kingdoms, this progenote must have been capable of templated protein synthesis substantially like our own.
Here we propose a phylogeny for the origin of tRNA based on the ubiquity and conservation of tRNA-like structures in the replication of contemporary genomes, and we discuss the evidence in contemporary molecules that leads to and supports this phylogeny. The unique aspect of this phylogeny is that it places the origin of tRNA in replication, before the advent of templated protein synthesis. This implies that tRNAs arose before the other components of the translation apparatus, that aminoacyl-tRNA synthetases arose next, and that both tRNA and aminoacyl-tRNA synthetases predated the anticodon and mRNA.
Methods
Molecular Fossils. In the classical terms of paleobiology, there is no fossil record of the most ancient forms of life or the molecules from which they were made. However, just as mineralized bones, shells, or cell walls tell us about the evolution of modern cellular organisms, contemporary biological molecules provide clues regarding the evolution of the earliest forms of life. In order to discuss precellular evolution in terms that are most accessible to a wide variety of disciplines, we use the phrase "molecular fossil" to describe the molecules that are central to this analysis. A molecular fossil is any molecule whose contemporary structure or function provides a clue to its evolutionary history. Molecular fossils are not to be confused with fossil molecules (DNA preserved in amber), or "living fossils" (slowly evolving species such as the coelacanth), or the physical fossils of cells and multicellular organisms that constitute the raw data for tracing more recent evolution. A molecular fossil is, of necessity, an abstraction rather than a tangible object: it records, embodies, and reflects ancient evolution but is not itself ancient.
Many Interesting Macromolecules Are Social and Are Therefore Constrained to Coevolve. There would be no such thing as a molecular fossil if evolution inevitably erased its own footsteps. But many biological macromolecules are social—they interact with other macromolecules. These interactions constrain the evolution of any individual molecule as well as the ensemble of other molecules with which it interacts. As diagrammed in Figure 2, a social macromolecule can change only in ways that preserve its ability to interact with its important partners. A change in any partner that alters one of these interactions will be tolerated only if all other partners change accordingly. Coevolution of this kind is possible for a molecule that interacts with one or a few different partners but becomes more difficult as the size of the ensemble
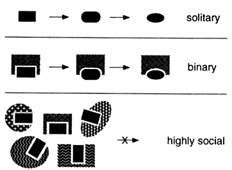
Figure 2 Social macromolecules. Evolution of a solitary macromolecule is almost unconstrained as long as it remains functional. The larger the interacting ensemble of macromolecules, the more difficult it is for any one of them to change.
of partners increases; the larger the ensemble, the deeper the valleys in the adaptive landscape. The most interactive structures and functions are thus the most likely to be preserved in their original forms—they are effectively frozen in time.
Results And Discussion
The Earliest Genomic Tags. We now consider replication in the RNA world (Gilbert, 1986), an era that predates the evolution of either DNA or templated protein synthesis. In these simpler times, enzymes made of RNA replicated genomes made of RNA. Nonetheless, early replicases and genomes had to confront many of the same problems faced by contemporary RNA genomes. Two of these problems are immediately evident:
- Specificity of replication. How did genomes that were destined for replication distinguish themselves from junk RNA or catalytic RNAs that should not be replicated?
- The "telomere problem" (Watson, 1972). How did ancient genomes and replicases prevent loss of 3' terminal sequences during successive rounds of replication?
The replicative strategy of the contemporary bacteriophage Qß suggests a single, powerful solution to both problems. Qß is a (+)-strand RNA phage. As shown in Figure 3, at the very 3' terminus of Qß is a tRNA-like structure, which ends in the sequence CCA. The 3'-terminal tRNA-like structure in the Qß (+)-strand genome serves as a recognition element for the replicase, which initiates synthesis of the (-)-strand at the penultimate C of the CCA terminus. The tRNA-like structure thus ensures specificity of replication. Furthermore, the 3'-terminal CCA of Qß can also function, at least in principle, like a modern telomere: loss of part or all of the CCA sequence could be restored by the CCA-adding enzyme, tRNA nucleotidyltransferase. Regeneration of a CCA terminus
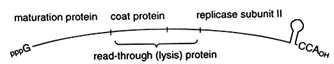
Figure 3 Qß bacteriophage genome. The genome of Qß bacteriophage is a single-stranded RNA with a 3'-terminal tRNA-like genomic tag. The genome also serves as a messenger for four phage proteins, including the catalytic subunit II of Qß RNA replicase.
by this enzyme has in fact been demonstrated for brome mosaic virus (BMV), a plant virus with a 3'-terminal tRNA-like structure very similar to that of Qß (Rao et al., 1989).
These observations about the Qß genome led us to propose that the first tRNA-like structures arose as ''genomic tags" that marked the 3' ends of ancient RNA genomes for replication by RNA enzymes in the RNA world (Weiner and Maizels, 1987; Maizels and Weiner, 1993). The simplest such tags would have been the predecessors of the "top half" of modern tRNA, consisting of a coaxial stack of the TΨC arm on the acceptor stem (see Figure 5). The presence of such 3' terminal tRNA-like structures in two different kingdoms—contemporary bacterial viruses like Qß and plant viruses such as turnip yellow mosaic virus (TYMV) and BMV (reviewed by Hall, 1979; Haenni et al., 1982; Guerrier-Takada et al., 1988)—suggests that these structures date back at least as far as the progenote. The role of tRNA in replication appears to have arisen much earlier, however. As we discuss in greater detail below, molecular fossil evidence suggests that tRNA-like structures were first used for replication of RNA genomes by RNA enzymes in the RNA world and then persisted through the transition from RNA to DNA genomes.
An Early Origin for tRNA Rationalizes the 5' and 3' Processing of tRNAs in Modern Cells. The genomic tag model immediately explains the existence of two enzymes with otherwise puzzling activities in contemporary tRNA processing, RNase P and tRNA nucleotidyltransferase. Transcription of tRNA typically initiates not at the 5' end of the functional molecule, but at a site upstream. The 5' leader sequences are then removed from the "pre-tRNA" by an endonucleolytic cleavage catalyzed by RNase P. RNase P is a ribonucleoprotein, but the RNA component alone is capable of catalysis (Guerrier-Takada et al., 1983). The presence of a catalytic RNA component suggests that RNase P activity arose in the RNA world (Alberts, 1986); use of an RNA component cannot be explained by the need to recognize so many different species of tRNA, since elongation factor Tu accomplishes the same task without the help of RNA. Further attesting to the ancient origin of RNase P, the structure it recognizes is highly conserved: the Escherichia coli enzyme can cleave the 3' tRNA-like structure of TYMV (Green et al., 1988; Guerrier-Takada et al., 1988; Mans et al. , 1990). But why would a tRNA processing enzyme be present in the RNA world? We suggest that the first function of RNase P may have been to free catalytic RNAs from the 3'-terminal genomic tags required for replication; cleavage may have been necessary to activate catalytic function or to prevent replication from interfering with catalysis. If this is the case, it would explain the puzzling fact that tRNAs undergo 5' processing at
all: there is no reason a priori why the mature 5' ends of modern tRNAs could not be generated directly by transcription, yet there is only one instance known in which this is so (Lee et al., 1989).
If early tRNAs functioned as telomeres, it would also explain the surprising fact that all cells contain tRNA nucleotidyltransferase, an activity that can regenerate the 3'-terminal CCA sequence of tRNAs. This activity can be viewed as a telomerase, responsible for maintaining the integrity of the terminal CCA. Interestingly, although eubacteria and archaea encode the 3' terminal CCA of tRNA, eukaryotes do not, and they must rely instead on tRNA nucleotidyltransferase to produce mature tRNAs (Palmer et al., 1992). The primitive state is not yet known, but as tRNA nucleotidyltransferase is present in all cells, it seems most plausible that genomically encoded CCA was devised later to circumvent a slow or inefficient step in tRNA processing. This may also explain why the telomerase function of tRNA nucleotidyltransferase can in certain cases be augmented by the replicase itself. A number of modern RNA and DNA polymerases, including Qß RNA replicase (Blumenthal and Carmichael, 1979) and Taq DNA polymerase will add an untemplated A to the 3' end of a newly synthesized molecule (for example, see Tse and Forget, 1990). Polyadenylation of polymerase II transcripts, which often occurs following a CA dinucleotide (Wigley et al., 1990; Raabe et al., 1993), may be viewed as an exaggerated example of this.
Viruses May Be Clues to Early Evolution. The Qß RNA genome is replicated by an enzyme composed of four subunits. Only subunit II is encoded by the phage genome; the other three subunits—ribosomal protein S1 and elongation factors Tu and Ts (Blumenthal and Carmichael, 1979)—are components of the translation apparatus. If Qß is viewed as no more than a cellular parasite, then this simple bacteriophage appears to have stolen elements of the protein synthesis machinery for its replication. However, if tRNAs arose early to function in replication, it would be natural for factors that recognized tRNA to accompany this molecule as it took on a new role in translation. The presence of translation factors in an RNA replicase may therefore be viewed as evidence that these two processes have long been intimately connected.
In addition, the notion that some modern viruses still reveal their ancient origins allows us to see viruses in a new way. Viruses usually command our attention as vectors of disease, and the extraordinary genomic diversity of modern viruses is rarely appreciated by nonvirologists. There are double-stranded viruses, single-stranded viruses, circular viruses and linear viruses, RNA viruses, DNA viruses, viruses
made of RNA that replicate through DNA intermediates, and viruses made of both RNA and DNA. Why such diversity? We suggest that viruses may have evolved early and that their genomic diversity reflects the variety of replication strategies available before large DNA genomes became the cellular norm. This leads us to predict that the study of modern viruses will provide further insight into early evolution.
Transitional Genomes as Clues to Early Replication Strategies. If the original role of tRNA-like structures was in replication, as suggested by the single-stranded bacteriophage and plant virus genomes, one might expect to find additional examples of contemporary genomes in which tRNA plays that same role. When we first proposed the genomic tag hypothesis (Weiner and Maizels, 1987), only one other example of tRNA involvement in replication was known: In modern retroviruses, tRNAs function as primers for initiation of cDNA synthesis by the retroviral reverse transcriptase. Over the past few years, additional novel replication strategies have been described that employ tRNA-like structures. These appear to link replication of single-stranded RNA viruses with retroviral replication and with the synthesis of modern chromosomal telomeres. In each of these instances, a genomic RNA replicates via a DNA intermediate. We call these "transitional genomes," because they can be viewed as reenacting the transition from an RNA world to the contemporary DNA world.
Three different sorts of transitional genomes appear to link the function of tRNA in replication of RNA genomes with its role in replication of contemporary DNA genomes, as shown in Figure 4. The example most similar to the (+)-strand RNA viruses Qß and TYMV is the Mauriceville plasmid of Neurospora mitochondria (Maizels and Weiner, 1987; Kuiper and Lambowitz, 1988; Akins et al., 1989). This double-stranded DNA plasmid replicates in a most unusual way. First, rolling-circle transcription of the plasmid generates a multimeric RNA (+)-strand. The multimer is cleaved to produce full-length monomeric RNA transcripts, each with a 3'-terminal tRNA-like structure ending in CCACCA, a genomic tag with a reiterated CCA terminus (see Figure 4). The 3'-terminal genomic tag of the monomeric (+)-strand RNA then serves as the initiation site for replication, but in this case the template is copied not into (-)-strand RNA by an RNA replicase, as for Qß and TYMV, but into cDNA by a reverse transcriptase. Moreover, the reverse transcriptase is encoded by the monomeric (+)-strand RNA, which doubles as an mRNA. The similarities between the replicative strategies of bacteriophage Qß and the Mauriceville plasmid are remarkable: a full-length (+)-strand RNA with a 3'-terminal genomic tag encodes the enzyme which copies the genome starting at the penultimate C of the
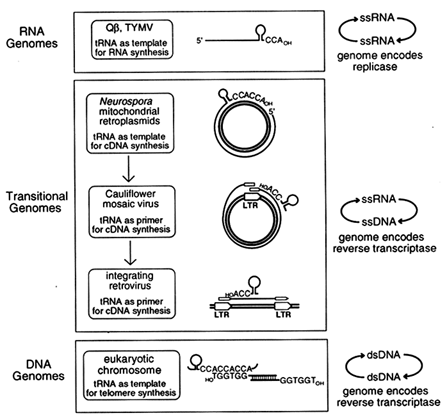
Figure 4 A functional phylogeny for the origin of tRNA in replication. We propose that tRNA began as a 3'-terminal genomic tag on single-stranded RNA genomes where it serves as template for initiation of RNA synthesis. The genomic tag was preserved in transitional replication strategies where it serves as template for initiation of DNA synthesis on the single-stranded RNA genome. Still later, the genomic tag was transformed into the primer for initiation of DNA synthesis in retroviral-like replication strategies. ss, Single-stranded; ds, double-stranded.
genomic tag. Thus the Mauriceville retroplasmid is best described as an RNA genome replicating through a DNA intermediate, with the plasmid DNA serving the same role as the DNA provirus does in the life cycle of a modern retrovirus.
The enzymology of replication of the Mauriceville retroplasmid provides further support for the transitional status of this plasmid genome (Wang and Lambowitz, 1993). The Mauriceville reverse transcriptase
initiates cDNA synthesis without a primer—a feat previously thought to be impossible for a DNA polymerase. This suggests that the transition from RNA to DNA genomes did not require invention of a novel priming activity, but simply the transformation of an RNA polymerase into a DNA polymerase. The ability of Qß replicase to misincorporate DNA bases in the presence of Mn2+ (Blumenthal and Carmichael, 1979) suggests that such a transformation is not implausible.
From Template to Primer. In Qß, TYMV, and the Mauriceville retroplasmid, tRNA is the template for replication. In contrast, tRNA functions not as template but as primer in other transitional genomes which probably arose later. Examples of such genomes are cauliflower mosaic virus (CaMV) and vertebrate retroviruses. The CaMV genome is a circular duplex DNA that replicates as an extrachromosomal element without ever integrating into chromosomal DNA (see Figure 4). Transcription of the viral genome initiates at a unique regulatory region which, like a retroviral long terminal repeat (LTR), consists of an RNA polymerase II promoter just upstream from a polyadenylation signal. The polyadenylation signal is too close to the promoter to function efficiently, so transcription bypasses the signal the first time around and generates a terminally redundant transcript that is slightly longer than full length. This terminally redundant genomic (+)-strand RNA is then converted to a cDNA by the CaMV-encoded reverse transcriptase using tRNA as the primer (Hohn et al., 1985; Covey and Turner, 1986; Sanfacon and Hohn, 1990). The CaMV replication scheme is very similar to that used by retroviruses (also shown in Figure 4), except that subsequent integration of retroviral DNA into the host chromosome generates a DNA provirus with a copy of the regulatory region at either end (hence, LTR). The terminally redundant DNA provirus is topologically equivalent to the circular CaMV genome, and transcription from the upstream promoter of the provirus to the distal polyadenylation signal generates the terminally redundant genomic RNA. Transcription of diverse, but related (Xiong and Eickbush, 1990), retroviral elements is also primed by tRNA (Kikuchi et al., 1986; Chapman et al., 1992).
Specificity and Catalysis Reside in Separate Polymerase Domains. The suggestion that tRNA functioned first as template for the initiation of replication and later as primer is consistent with the domain structure of contemporary polymerases. Many polymerases have two structural domains, one that is catalytic and another that determines template specificity. The classical example of this separation of functions is the σ factor of E. coli RNA polymerase (reviewed by Jaehning, 1991). Similarly, in Qß replicase it is elongation factor Tu that recognizes that
3'-terminal tRNA-like structure, not the bacteriophage-encoded catalytic subunit (Blumenthal and Carmichael, 1979). For tRNA to evolve from template to primer, the replication enzyme would consist—like Qß replicase—of two domains, one catalytic and the other recognizing the 3'-terminal tRNA-like tag. The relative orientation of these two domains would then determine whether the tRNA was used as template or as primer. The remarkable ability of the Mauriceville reverse transcriptase to use RNA either as primer or as template, at least in vitro, suggests that the transition from template to primer may have been straightforward (Wang and Lambowitz, 1993).
A Functional Phylogeny for the Evolution of tRNA in Replication: RNA Genomes to Modern DNA Telomeres. The function of tRNA in the replication of RNA genomes and transitional genomes leads us to propose a phylogeny for the evolution of tRNA in replication. As shown in Figure 4, tRNA-like structures first arose in ancient RNA genomes, where they served as templates for the initiation of replication and also functioned as primitive telomeres. These tRNA-like structures persisted during the evolution of DNA, and they are immortalized today in transitional genomes, RNA genomes that replicate via a DNA intermediate. In the transitional genomes, tRNAs functioned first as template and later as primer for synthesis of a cDNA copy by a reverse transcriptase encoded by the genomic RNA itself.
The role of tRNA in replication is not restricted to viruses and extrachromosomal elements but extends to cellular chromosomes as well. The termini of modern chromosomes are replenished by an enzyme called telomerase, which adds species-specific TnGm repeats, one nucleotide at a time, to an appropriate TnGm primer (reviewed by Blackburn, 1991). Telomerase is a ribonucleoprotein, and its RNA component serves as a built-in template for sequence addition by the reverse transcriptase-like protein subunit. In the Tetrahymena telomerase, for example, a built-in template containing the sequence 5'-CA2C4A2 specifies addition of 5'-T2G4 telomeric repeats. There is an uncanny resemblance between the action of telomerase and the copying of the reiterated CCACCA terminus of a tRNA-like genomic tag by the Mauriceville reverse transcriptase. Moreover, the Mauriceville reverse transcriptase can, like telomerase, initiate at an internal CCA sequence by using a DNA primer (Wang and Lambowitz, 1993). The genomic tag hypothesis suggests that telomere addition can be viewed, from an evolutionary perspective, as abortive replication. Furthermore, if ancient tRNA-like structures were the predecessors of the built-in template in contemporary telomerases, it would explain why modern telomere sequences are variations on a CnAm repeat motif.
How Did tRNA Come to Play a Role in Translation? Covalent linkage of a basic amino acid to a 3'-terminal tRNA-like genomic tag might have improved the efficiency or specificity of replication in an RNA world, perhaps by permitting the negatively charged RNA replicase to bind more tightly to the negatively charged RNA genome. Alternatively, if aminoacylation interfered with replication, charging could have limited the number of genomes in the replicative pool or prevented free genomic tags from competing for the replicase. The aminoacylation activity could have arisen very early, even as a variant of the replicase itself. Aminoacylation chemically resembles RNA polymerization, and a variant replicase could have evolved to catalyze aminoacylation, just as a group I ribozyme, which naturally catalyzes phosphoester bond transfer, can be redesigned to catalyze reactions at a carbon center (Piccirilli et al., 1992). The specificity with which modern group I ribozymes bind L-arginine leaves little doubt that an aminoacyl-tRNA synthetase made of RNA could charge tRNA with considerable specificity (Connell et al., 1993). In any case, as we discuss in greater detail elsewhere (Maizels and Weiner, 1987), replication would have provided the driving force for the first two steps in the evolution of protein synthesis.
The apparent diversity in size and quaternary structure of modern aminoacyl-tRNA synthetases has long been puzzling. All these enzymes perform the same two-step reaction using an enzyme-bound aminoacyl-adenylate intermediate, and one might therefore have expected that all would be descended from a single ancestral protein. This mystery was refined but not clarified by the realization that modern aminoacyl-tRNA synthetases can be divided into two structurally and functionally distinct classes: synthetases with the classical Rossman nucleotide-binding fold charge the 2' hydroxyl of tRNA, and synthetases with a seven-stranded antiparallel b-sheet generally charge the 3' hydroxyl (Cusack et al., 1990; Eriani et al., 1990; Ruff et al., 1991; Cavarelli et al., 1993). However, if aminoacyl-tRNA synthetases first arose in an RNA world, as we suggested (Weiner and Maizels, 1987), and were then transformed by stepwise replacement of RNA with protein as envisioned by White (White, 1982), even a single RNA enzyme could give rise to multiple protein enzymes because there is unlikely to be a unique path for replacement of RNA by protein (Weiner and Maizels, 1987; Benner et al., 1989).
The Top Half of tRNA Is Ancient. The tRNA-like structures in early genomes may have consisted simply of a coaxial stack of the TΦC arm on the CCA acceptor stem. We base this suggestion on two different kinds of evidence (Figure 5 and Table 1). First, the top half of modern
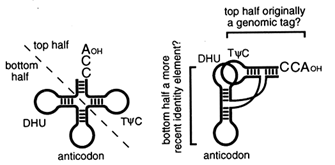
Figure 5 The two halves of contemporary tRNA. The "top half" of tRNA is structurally and functionally independent and may be more ancient than the "bottom half" of the molecule.
tRNA is an independent structural domain that is recognized by RNase P (McClain et al., 1987; Yuan and Altman, 1994), Tu (Rasmussen et al., 1990), tRNA synthetases (McClain, 1993; Schimmel et al., 1993; Saks et al., 1994), and perhaps even ribosomal RNA (Noller et al., 1992). The importance of this domain in almost all macromolecular interactions involving tRNA suggests that it is ancient, as does its structural independence from the bottom half of the molecule. Second, the ability of the cell to distinguish each tRNA from all the others—solving what is usually referred to as the tRNA identity problem—depends to a surprising extent on the identity of specific nucleotides in the top half of the molecule (McClain, 1993; Schimmel et al., 1993; Saks et al., 1994), including the "discriminator base" just inboard from the CCA terminus (Crothers et al., 1972). This suggests that the identity of some tRNAs, and perhaps the specificity of the cognate aminoacyl-tRNA synthetases, was established before the bottom half of tRNA was incorporated into the molecule. Whether the bottom half of tRNA arose as an expansion loop within the top half or as an independent structural and functional domain that was subsequently incorporated into the top half is a question that future work may be able to resolve.
TABLE 1 Enzyme activities for which the top half of tRNA is the primary determinant of recognition
|
RNase P |
McClain et al., 1987; Yuan and Altman, 1994 |
|
elongation factor Tu |
Rasmussen et al., 1990 |
|
tRNA synthetases |
Schimmel et al., 1993; Saks et al., 1994 |
|
CCA-adding enzyme |
Li and Thurlow |
|
ribosomal RNA? |
Noller et al., 1992 |
Conclusions And Future Prospects
The ubiquity and conservation of tRNA in the replication strategies of a variety of contemporary genomes suggest a functional phylogeny for tRNA. This phylogeny is unique in placing the origin of tRNA in replication, prior to the advent of templated protein synthesis. In this scenario, aminoacyl-tRNA synthetase activities would have arisen next, to facilitate or regulate replication, and both tRNA and the aminoacyl-tRNA synthetase activities would have predated the anticodon and mRNA. A corollary is that the top half of modern tRNA may have had a more ancient origin than the bottom half bearing the anticodon.
The genomic tag hypothesis has "explanatory power" (Popper, 1963). It makes sense of—and establishes possible relationships between—otherwise puzzling structures and functions including RNase P, the CCA-adding enzyme, telomerase, contemporary synthetases, and the terminal tRNA-like structures themselves. It is also robust. A number of key experiments alluded to above in support of the genomic tag model were carried out after our original proposal (Weiner and Maizels, 1987), including studies of the Mauriceville retroplasmid (Wang and Lambowitz, 1993), the internal RNA template of telomerase (Blackburn, 1991), cleavage of plant virus tRNA-like structures by RNase P (Green et al., 1988; Guerrier-Takada et al., 1988; Mans et al., 1990), stereospecific binding of an amino acid by RNA (Connell et al., 1993), the ability of a ribozyme to work on a carbon center (Piccirilli et al., 1992), and the division of contemporary synthetases into two classes (Eriani et al., 1990).
What do we expect to learn from this model in the future? One prediction is that there are likely to be other transitional genomes that employ tRNA or tRNA-like structures in their replication. Another is that detailed functional and structural studies of contemporary tRNAs (White, 1982; Pan et al., 1991) will further support the independence of the top and bottom halves of the molecule, explain why contemporary tRNA is a cloverleaf rather than the pseudoknotted structure found in plant viruses (Mans et al., 1990), and unlock the evolutionary history that must lie in the location and function of the (almost) universally modified bases in tRNA. But especially exciting is the possibility that plausible phylogenies will emerge for other key biochemical pathways, grounded in the structure and function of contemporary molecules.
Summary
We propose a phylogeny for the evolution of tRNA that is based on the ubiquity and conservation of tRNA-like structures in the replication of
contemporary genomes. This phylogeny is unique in suggesting that the function of tRNA in replication dates back to the very beginnings of life on earth, before the advent of templated protein synthesis. The origin we propose for tRNA has distinct implications for the order in which other components of the modern translational apparatus evolved. We further suggest that the "top half" of modern tRNA—a coaxial stack of the acceptor stem on the TΨC arm—is the ancient structural and functional domain and that the "bottom half" of tRNA—a coaxial stack of the dihydrouracil arm on the anticodon arm—arose later to provide additional specificity.
References
Akins, R. A., Kelley, R. L. & Lambowitz, A. M. 1989. Characterization of mutant mitochondrial plasmids of Neurospora spp. that have incorporated tRNAs by reverse transcription. Mol. Cell. Biol. 9, 678–691.
Alberts, B. M. 1986. The function of the hereditary materials: Biological catalyses reflect the cell's evolutionary history. Am. Zool. 26, 781–796.
Benner, S. A., Ellington, A. D. & Tauer, A. 1989. Modern metabolism as a palimpsest of the RNA world. Proc. Natl. Acad. Sci. USA 86, 7054–7058.
Blackburn, E. H. 1991. Structure and function of telomeres. Nature (London) 350, 569–573.
Blumenthal, T. & Carmichael, G. C. 1979. RNA replication: Function and structure of Qß replicase. Annu. Rev. Biochem. 48, 525–548.
Cavarelli, J., Rees, B., Ruff, M., Thierry, J. C. & Moras, D. 1993. Yeast tRNA(Asp) recognition by its cognate class II aminoacyl-tRNA synthetase. Nature (London) 362, 181–184.
Cech, T. R., Zaug, A. J. & Grabowski, P. J. 1981. In vitro splicing of the ribosomal RNA precursor of Tetrahymena: Involvement of a guanosine nucleotide in the excision of the intervening sequence. Cell 27, 487–496.
Chapman, K. B., Bystrom, A. S. & Boeke, J. D. 1992. Initiator methionine tRNA is essential for Ty1 transposition in yeast. Proc. Natl. Acad. Sci. USA 89, 3236–3240.
Connell, G. J., Illangesekare, M. & Yarus, M. (1993) Three small ribooligonucleotides with specific arginine sites. Biochemistry 32, 5497–5502.
Covey, S. N. & Turner, D. S. 1986. Hairpin DNAs of cauliflower mosaic virus generated by reverse transcription in vivo. EMBO J. 5, 2763–2768.
Crothers, D. M., Seno, T. & Söll, D. G. 1972. Is there a discriminator base in transfer RNA? Proc. Natl. Acad. Sci. USA 69, 3063–3067.
Cusack, S., Berthet-Colominas, C., Hartlein, M., Nassar, N. & Leberman, R. 1990. A second class of synthetase structure revealed by X-ray analysis of Escherichia coli seryl-tRNA synthetase at 2.5 A. Nature (London) 347, 249–255.
Eriani, G., Delarue, M., Poch, O., Gagloff, J. & Moras, D. 1990. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature (London) 347, 203–206.
Gilbert, W. 1986. The RNA world. Nature (London) 319, 618.
Green, C. J., Vold, B. S., Morch, M. D., Joshi, R. L. & Haenni, A. L. 1988. Ionic conditions for the cleavage of the tRNA-like structure of turnip yellow mosaic virus by the catalytic RNA of RNase P. J. Biol. Chem. 263, 11617–11620.
Guerrier-Takada, C., Gardiner, K., Marsh, T., Pace, N. & Altman, S. 1983. The RNA moiety of RNAase P is the catalytic subunit of the enzyme. Cell 53, 267–272.
Guerrier-Takada, C., van Belkum, A., Pleij, C. W. A. & Altman, S. 1988. Novel reactions of RNAase P with a tRNA-like structure in turnip yellow mosaic virus. Cell 53, 267–272.
Haenni, A.-L., Joshi, S. & Chapeville, F. 1982. tRNA-like structures in the genomes of RNA viruses. Prog. Nucleic Acid Res. Mol. Biol. 27, 85–104.
Hall, T. C. 1979. Transfer RNA-like structures in viral genomes. Int. Rev. Cytol. 60, 1–26.
Hohn, T., Hohn, B. & Pfeiffer, P. 1985. Reverse transcription in CaMV. Trends Biochem. Sci. 10, 205–209.
Jaehning, J. A. 1991. Sigma factor relatives in eukaryotes. Science 253, 859.
Kikuchi, Y., Ando, Y. & Shiba, T. 1986. Unusual priming mechanism of RNA-directed DNA synthesis in copia retrovirus-like particles. Nature (London) 323, 824–826.
Kuiper, M. T. R. & Lambowitz, A. M. 1988. A novel reverse transcriptase activity associated with mitochondrial plasmids of Neurospora. Cell 55, 693–704.
Li, Z. and Thurlow, D. L. Effects of base sequence in the loops of 20 RNA minihelices on recognition by ATP/CTP:tRNA nucleotidyltransferase.
Maizels, N. & Weiner, A. M. 1987. Peptide-specific ribosomes, genomic tags and the origin of the genetic code. Cold Spring Harbor Symp. Quant. Biol. 52, 743–749.
Maizels, N. & Weiner, A. M. 1993. in The RNA World, eds. Gesteland, R. F. & Atkins, J. F. (Cold Spring Harbor Lab. Press, Plainview, NY), pp. 577–602.
Mans, R. M., Guerrier-Takada, C., Altman, S. & Pleij, C. W. 1990. Interaction of RNase P from Escherichia coli with pseudo knotted structures in viral RNAs. Nucleic Acids Res. 18, 3479–3487.
McClain, W. H. 1993. Rules that govern tRNA identity in protein synthesis. J. Mol. Biol. 234, 257–280.
McClain, W. H., Guerrier-Takada, C. & Altman, S. 1987. Model substrates for an RNA enzyme. Science 238, 527–530.
Noller, H. F., Hoffarth, V. & Zimniak, L. 1992. Unusual resistance of peptidyl transferase to protein extraction procedures. Science 256, 1416–1419.
Palmer, J. R., Baltrus, T., Reeve, J. N. & Daniels, C. J. 1992. Transfer RNA genes from the hyperthermophilic Archaeon, Methanopyrus kandleri. Biochem. Biophys. Acta 1132, 315–318.
Pan, T., Gutell, R. R. and Uhlenbeck, O. C. 1991. Folding of circularly permuted transfer RNAs. Science 254, 1361–1364.
Piccirilli, J. A., McConnell, T. S., Zaug, A. J., Noller, H. F. & Cech, T. R. 1992. Aminoacyl esterase activity of the Tetrahymena ribozyme. Science 256, 1420–1424.
Popper, K. R. (1963). Conjectures and Refutations (Routledge and Kegan Paul, London).
Raabe, T., Bollum, F. J. & Manley, J. L. 1993. Primary structure and expression of bovine poly(A) polymerase. Nature (London) 353, 229–234.
Rao, A. L. N., Dreher, T. W., Marsh, L. E. & Hall, T. C. 1989. Telomeric function of the tRNA-like structure of brome mosaic virus RNA. Proc. Natl. Acad. Sci. USA 86, 5335–5339.
Rasmussen, N. J., Wikman, F. P. & Clark, B. F. 1990. Crosslinking of tRNA containing a long extra arm to elongation factor Tu by trans -diamminedichloroplatinum(II). Nucleic Acids Res. 18, 4883–4890.
Ruff, M., Krishanswamy, S., Boeglin, M., Poterszman, A., Mitschler, A., Podjarny, A., Rees, B., Thierry, J. C. & Moras, D. 1991. Class II aminoacyl transfer RNA
synthetases: crystal structure of yeast aspartyl-tRNA synthetase complexed with tRNA(Asp). Science 252, 1682–1689.
Saks, M. E., Sampson, J. R. & Abelson, J. N. 1994. The transfer RNA identity problem: a search for rules. Science 263, 191–197.
Sanfacon, H. & Hohn, T. 1990. Proximity to the promoter inhibits recognition of cauliflower mosaic virus polyadenylation signal. Nature (London) 346, 81–84.
Schimmel, P., Giege, R., Moras, D. & Yokoyama, S. 1993. An operational RNA code for amino acids and possible relationship to the genetic code. Proc. Natl. Acad. Sci. USA 90, 8763–8768.
Tse, W. T. & Forget, B. G. 1990. Reverse transcription and direct amplification of cellular RNA transcripts by Taq polymerase. Gene 88, 293–296.
Wang, H. & Lambowitz, A. M. 1993. The Mauriceville plasmid reverse transcriptase can initiate cDNA synthesis de novo and may be related to reverse transcriptase and DNA polymerase progenitor. Cell 75, 1071–1081.
Watson, J. D. 1972. Origin of concatemeric T7 DNA. Nature New Biol. 239, 197–201.
Weiner, A. M. & Maizels, N. 1987. tRNA-like structures tag the 3' ends of genomic RNA molecules for replication: Implications for the origin of protein synthesis. Proc. Natl. Acad. Sci. USA 84, 7383–7387.
White, H. G., III 1982. Evolution of coenzymes and the origin of pyridine nucleotides. In The Pyridine Nucleotide Coenzymes, eds. Everse, J., Anderson, B. & You, K. (Academic, New York), pp. 1–17.
Wigley, P. L., Sheets, M. D., Zarkower, D. A., Whitmer, M. E. & Wickens, M. 1990. Polyadenylation of mRNA: minimal substrates and a requirement for the 2' hydroxyl of the U in AUAAA. Mol. Cell. Biol. 10, 1705–1713.
Woese, C. (1990). Evolutionary questions: the ''progenote". Science 247, 789.
Woese, C. R., Kandler, O. & Wheelis, M. L. 1990. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc. Natl. Acad. Sci. USA 87, 4576–4579.
Xiong, Y. & Eickbush, T. H. 1990. Origin and evolution of retroelements based upon their reverse transcriptase sequences. EMBO J. 9, 3353–3362.
Yuan, Y. & Altman, S. 1994. Selection of guide sequences that direct efficient cleavage of mRNA by human ribonuclease P. Science 263, 1269–1273.
















