3
Evaluating Hazards and Assessing Risks in the Laboratory
3.A INTRODUCTION
A key element of planning an experiment involves assessing the hazards and potential risks associated with the chemicals and laboratory operations to be employed in a proposed experiment. This chapter provides a practical guide for the laboratory worker engaged in these activities. Section 3.B introduces the sources of information where laboratory workers can find data on toxic, flammable, reactive, and explosive chemical substances as well as physical, biological, and radioactive hazards. Section 3.C discusses the toxic effects of laboratory chemicals. The first part of this section presents the basic principles that form the foundation for evaluating hazards for toxic substances. The remainder of the section describes how the laboratory worker can use this understanding and the sources of information introduced above to assess the risks associated with potential hazards of chemical substances and then to select the appropriate level of laboratory practice as discussed in Chapter 5. Sections 3.D and 3.E present guidelines for evaluating hazards associated with the use of flammable, reactive, and explosive substances and physical hazards, respectively. Finally, there is a brief reference to biohazards and hazards from radioactivity in sections 3.F and 3.G, respectively.
Although the responsibility for carrying out the hazard evaluations and risk assessments described here generally lies primarily with the laboratory worker who will actually be conducting the proposed experiment, this activity often requires consultation with other colleagues and superiors. For example, depending on the level of training and experience of the laboratory worker, the involvement of the worker's immediate laboratory supervisor may be advisable and in some instances essential. In addition, many institutions have environmental health and safety offices, where industrial hygiene specialists are available to advise laboratory workers and their supervisors on issues involved in the assessment of risks of laboratory chemicals. Chemical hygiene officers, required by federal regulation, play similar departmental roles in many institutions.
3.B SOURCES OF INFORMATION
3.B.1 Chemical Hygiene Plan
Beginning in 1991, every laboratory in which hazardous chemicals are in use has been required by federal law to have a written Chemical Hygiene Plan (CHP), which includes provisions capable of protecting personnel from the ''health hazards associated with the chemicals present in that laboratory." All laboratory workers should be familiar with and have ready access to their institution's CHP. In some laboratories, CHPs include standard operating procedures for work with specific chemical substances, and in these cases the CHP may be sufficient as the primary source of information used for risk assessment and experiment planning. However, most CHPs provide only general procedures for handling chemicals, and in these cases prudent experiment planning requires that the laboratory worker consult additional sources for information on the properties of the substances that will be encountered in the proposed experiment.
3.B.2 Material Safety Data Sheets
Federal law requires that manufacturers and distributors of chemicals provide users with Material Safety Data Sheets (MSDSs), which are designed to provide the information needed to protect users from any hazards that may be associated with the product. MSDSs have become the primary vehicle through which the potential hazards of materials obtained from commercial sources are communicated to the laboratory worker. Institutions are required by law to retain and make readily available to workers the MSDSs provided by chemical suppliers.
As the first step in a risk assessment, laboratory workers should examine their plan for a proposed experiment and identify the chemicals whose toxicological properties they are not already familiar with from previous experience. The MSDS for each unfamiliar chemical should then be examined. Procedures for accessing MSDS files vary from institution to institution. In some cases, MSDS files may be present in each laboratory, while in many cases complete files of MSDSs are maintained only in a central location, such as the institution's environmental health and safety office. Some laboratories now have the capability to access MSDSs electronically, either from CD-ROM disks or via computer networks. As a last resort, the laboratory worker can always contact the chemical supplier directly and request that an MSDS be sent by mail.
MSDSs are concise technical documents, generally two to five pages in length. An MSDS typically begins with a compilation of data on the physical, chemical, and toxicological properties of the substance and then provides generally concise suggestions for handling, storage, and disposal. Finally, emergency and first aid procedures are usually outlined. At present there is no required format for an MSDS; however, it is expected that the Occupational Safety and Health Administration (OSHA) will soon adopt a general 16-part format proposed by the American National Standards Insti-
tute (ANSI). The following is a guide to the information typically found in an MSDS:
-
Name of supplier (with address and phone number) and date MSDS was prepared or revised. Toxicity data and exposure limits sometimes undergo revision, and for this reason MSDSs should be reviewed periodically to check that they contain up-to-date information. Phone numbers are provided so that, if necessary, users can contact the supplier to obtain additional information on hazards and emergency procedures.
-
Name of the chemical. For products that are mixtures, this section may include the identity of most but not every ingredient. Common synonyms are usually listed.
-
Physical and chemical properties. Data such as melting point, boiling point, and molecular weight are included here.
-
Physical hazards. This section provides data related to flammability, reactivity, and explosibility hazards.
-
Toxicity data. OSHA and American Conference of Governmental Industrial Hygienists (ACGIH) exposure limits (as discussed below in section 3.C) are listed. Many MSDSs provide lengthy and comprehensive compilations of toxicity data and even references to applicable federal standards and regulations.
-
Health hazards. Acute and chronic health hazards are listed, together with the signs and symptoms of exposure. The primary routes of entry of the substance into the body must also be described. In addition, potential carcinogens are explicitly identified. In some MSDSs, this list of toxic effects is quite lengthy and may include every possible harmful effect the substance can have under the conditions of every conceivable use.
-
Storage and handling procedures. This section usually consists of a list of precautions to be taken in handling and storing the material. Particular attention is devoted to listing appropriate control measures, such as the use of engineering controls and personal protective equipment necessary to prevent harmful exposures. Because an MSDS is written to address the largest scale that the material could conceivably be used on, the procedures recommended may involve more stringent precautions than are necessary in the context of laboratory use.
-
Emergency and first aid procedures. This section usually includes recommendations for firefighting procedures, first aid treatment, and steps to be taken if the material is released or spilled. Again, the measures outlined here are chosen to encompass worst-case scenarios, including accidents on a larger scale than could conceivably occur in a laboratory.
-
Disposal considerations. Many MSDSs provide guidelines for the proper disposal of waste material.
-
Transportation information. It is important to remember that this chapter is concerned only with evaluating the hazards and assessing the risks associated with chemicals in the context of laboratory use. MSDSs, in contrast, must address the hazards associated with chemicals in all possible situations, including industrial manufacturing operations and large-scale transportation accidents. For this reason, some of the information in an MSDS may not be relevant to the handling and use of that chemical in a laboratory. For example, most MSDSs stipulate that self-contained breathing apparatus and heavy rubber gloves and boots be worn in cleaning up spills, even of relatively nontoxic materials such as acetone. Such precautions, however, might be unnecessary in the case of laboratory-scale spills of acetone and other substances of low toxicity.
Originally, the principal audience for MSDSs comprised health and safety professionals (who are responsible for formulating safe workplace practices), medical personnel (who direct medical surveillance programs and treat exposed workers), and emergency responders (e.g., fire department personnel). With the promulgation of federal laws such as the Hazard Communication Standard (29 CFR 1910.1200) and the OSHA Laboratory Standard (29 CFR 1910.1450), the audience for MSDSs has been expanded to include laboratory workers in industrial and academic laboratories. However, not all MSDSs are written to meet the requirements of this new audience effectively.
In summary, among the currently available resources, MSDSs remain the best single source of information for the purpose of evaluating the hazards and assessing the risks of chemical substances. However, laboratory workers should recognize the limitations of MSDSs as applied to laboratory-scale operations:
-
The quality of MSDSs produced by different chemical suppliers varies widely. The utility of some MSDSs is compromised by vague and unqualified generalizations and internal inconsistencies.
-
MSDSs must describe control measures and precautions for work on a variety of scales, ranging from microscale laboratory experiments to large manufacturing operations. Some procedures outlined in an MSDS may therefore be unnecessary or inappropriate for laboratory-scale work. An unfortunate consequence of this problem is that it tends to breed a lack of confidence in the relevance of the MSDS to laboratory-scale work.
-
Many MSDSs comprehensively list all conceivable health hazards associated with a substance without differentiating which are most significant and which are most likely to actually be encountered. This can make it difficult for laboratory workers to distinguish highly hazardous materials from moderately hazardous and relatively harmless ones.
3.B.3 Laboratory Chemical Safety Summaries
As discussed above, although MSDSs are invaluable resources, they suffer some limitations as applied to risk assessment in the specific context of the laboratory. Appendix B introduces the concept of the Laboratory Chemical Safety Summary (LCSS), which is specifically tailored to the needs of the laboratory worker. As indicated in their name, LCSSs provide information on chemicals in the context of laboratory use. These documents are summaries and are not intended to be comprehensive or to fulfill the needs of all conceivable users of a chemical. In conjunction with the guidelines described in this chapter, the LCSS provides essential information required to assess the risks associated with the use of a particular chemical in the laboratory.
The format, organization, and contents of LCSSs are discussed in detail in the introduction to Appendix B. Included in an LCSS are the key physical, chemical, and toxicological data necessary to evaluate the relative degree of hazard posed by a substance. LCSSs also include a concise critical discussion, presented in a style readily understandable to laboratory workers, of the toxicity, flammability, reactivity, and explosibility of the chemical; recommendations for the handling, storage, and disposal of the title substance; and first aid and emergency response procedures.
Appendix B contains LCSSs for 88 chemical substances. Several criteria were used in selecting these chemicals, the most important consideration being whether the substance is commonly used in laboratories. Preference was also given to materials that pose relatively serious hazards. Finally, an effort was also made to select chemicals representing a variety of different classes of substances, so as to provide models for the future development of additional LCSSs.
3.B.4 Labels
Commercial suppliers are required by law to provide their chemicals in containers affixed with precautionary labels. Labels usually present concise and nontechnical summaries of the principal hazards associated with their contents. Note that precautionary labels should not replace MSDSs and LCSSs as the primary source of information for risk assessment in the laboratory. However, labels can serve as valuable reminders of the key hazards associated with the substance.
3.B.5 Additional Sources of Information
The resources described above provide the foundation for risk assessment of chemicals in the laboratory. This section highlights the sources that should be consulted for additional information on specific harmful effects of chemical substances. Although MSDSs and LCSSs include considerable information on toxic effects, in some situations the laboratory worker should seek additional, more detailed information. This step is particularly important when the worker is planning to use chemicals that have a high degree of acute or chronic toxicity or when it is anticipated that work will be conducted with a particular toxic substance frequently or over an extended period of time. Section 3.B of this chapter provides explicit guidelines as to how laboratory workers can use the information in an MSDS or LCSS to recognize when it is necessary to seek such additional information.
The following annotated list provides references on the hazardous properties of chemicals in the approximate order of their utility in assessing risks in the laboratory. The first six references are particularly valuable sources of information, and it is strongly recommended that copies of these be made readily accessible to laboratory workers at all times. A compilation of related materials and recommended resources can be found in the bibliography.
-
Occupational Health Guidelines for Chemical Hazards, U.S. DHHS; F. W. Mackison, R. S. Stricoff, and L. J. Partridge, editors, DHHS (NIOSH) Publication Number 81-123, U.S. Government Printing Office, Washington, D.C., 1981, and a supplement published as DHHS (NIOSH) Publication No. 89-104, U.S. Government Printing Office, Washington, D.C., 1988. The guidelines currently cover almost 400 substances and are based on the information assembled under the Standards Completion Program, which served as the basis for the promulgation of federal occupational health regulations ("substance-specific standards"). Typically five pages in length and written clearly at a level that should be readily understood by laboratory workers, each set of guidelines includes information on physical, chemical, and toxicological properties, signs and symptoms of exposure, and considerable detail on control measures, medical surveillance practices, and emergency first aid procedures. However, some guidelines date back to 1978 and may not be current, particularly with regard to chronic toxic effects.
-
Chemical Safety Data Sheets, Royal Society of Chemistry, five volumes, Cambridge, United Kingdom, 1989-1992. This excellent collection of data sheets summarizes hazard information on more than 500 chemicals. These are more useful for the laboratory worker than most MSDSs and are similar in aim to the LCSSs. Sections include threshold limit values, physical properties, chemical hazards, biological hazards (e.g., vapor inhalation, eye contact, skin contact, swallowing), carcinogenicity, mutagenicity, reproductive hazards, first
-
aid, handling and storage, disposal, and fire precautions. Each summary includes a list of references.
-
A Comprehensive Guide to the Hazardous Properties of Chemical Substances, P. A. Patnaik, Van Nostrand Reinhold, New York, 1992. This particularly valuable guide is written at a level appropriate for the typical laboratory worker. It covers about 1,500 substances; sections in each entry include uses and exposure risk, physical properties, health hazards, exposure limits, fire and explosion hazards, and disposal/destruction. Entries are organized into chapters according to functional group classes, and each chapter begins with a general discussion of the properties and hazards of the class.
-
Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices, 1994-1995, American Conference of Governmental Industrial Hygienists (ACGIH), Cincinnati, Ohio, 1994. A handy booklet listing ACGIH threshold limit values (TLVs) and short-term exposure limits (STELs). These values are under continuous review, and this booklet is updated annually. The ACGIH's multivolume publication Documentation of the Threshold Limit Values and Biological Exposure Indices reviews the data (with reference to literature sources) that were used to establish the threshold limit values.
-
Fire Protection for Laboratories Using Chemicals (NFPA Standard Code No. 45), National Fire Protection Association, Quincy, Massachusetts, 1991. This is the national fire safety code pertaining to laboratory use of chemicals.
-
Bretherick's Handbook of Reactive Chemical Hazards, 4th edition, L. Bretherick, Butterworth, London, 1990. An extremely comprehensive compilation of examples of violent reactions, fires, and explosions due to unstable chemicals, as well as reports on known examples of incompatibility between reactive chemicals.
-
Sax's Dangerous Properties of Industrial Materials, 8th edition, three volumes, Richard J. Lewis, Sr., Van Nostrand Reinhold, New York, 1992. This compilation of data for 20,000 chemical substances contains much of the information found in a typical MSDS, including physical and chemical properties, data on toxicity, flammability, reactivity, and explosibility, and a concise safety profile describing symptoms of exposure. This is a useful reference for checking the accuracy of an MSDS and a valuable resource to assist workers in preparing their own LCSSs.
-
Fire Protection Guide to Hazardous Materials, 10th edition, National Fire Protection Association, Quincy, Massachusetts, 1991. This resource contains hazard data on more than 400 chemicals.
-
Patty's Industrial Hygiene and Toxicology, 4th edition, G. D. Clayton and F. E. Clayton, editors, Wiley-Interscience, New York, 1994, Volume 2, Toxicology (part C). A classic and authoritative reference on the toxicology of different classes of organic and inorganic compounds. The six parts of volume 2 consist of several thousand pages of information organized by functional group class. The focus in Patty's is on health effects; hazards due to flammability, reactivity, and explosibility are not covered.
-
Proctor and Hughes' Chemical Hazards of the Workplace, 3rd edition, G. J. Hathaway, N. H. Proctor, J. P. Hughes, and M. L. Fischman, editors, Van Nostrand Reinhold, New York, 1991. This resource provides an excellent summary of the toxicology of 542 chemicals. Most entries are one to two pages in length and include signs and symptoms of exposure with reference to specific clinical reports.
-
Handbook of Toxic and Hazardous Chemicals and Carcinogens, 3rd edition, two volumes, Marshall Sittig, Noyes Publications, Park Ridge, New Jersey, 1991. This very good reference, which is written with the industrial hygienist in mind, covers 800 substances.
-
Sigma-Aldrich Library of Chemical Safety Data, 2nd edition, Robert E. Lenga, editor, two volumes, Sigma-Aldrich, Milwaukee, Wisconsin, 1988. This compilation of safety data for approximately 14,500 chemicals is in tabular form. It presents considerably less information than is found in a typical MSDS or LCSS, but it is convenient as a single source of information for a very large number of substances.
-
Clinical Toxicology of Commercial Products, 5th edition, Robert E. Gosselin, Roger P. Smith, and Harold C. Hodge, Williams & Wilkins, Baltimore, Maryland, 1984. This reference is designed to assist the physician in dealing with cases of acute chemical poisoning. It contains trade names of products and their ingredients.
-
Casarett and Doull's Toxicology: The Basic Science of Poisons, 4th edition, M. O. Amdur, J. Doull, and C. D. Klaassen, editors, Pergamon Press, New York, 1991. This complete and readable overview of toxicology is a good textbook but is not arranged as a ready reference for handling laboratory emergencies.
-
Catalog of Teratogenic Agents, 7th edition, Thomas H. Shepard, Johns Hopkins University Press, Baltimore, Maryland, 1992. This catalog is one of the best references available on the subject of reproductive and developmental toxins.
-
The Laboratory Environment, R. Purchase, editor, Special Publication Number 136, Royal Society of Chemistry, Cambridge, United Kingdom, 1994.
3.B.6 Computer Services
In addition to computerized MSDSs, a number of computer databases are available that supply data for
creating or supplementing MSDSs. The National Library of Medicine (NLM) and the Chemical Abstracts databases are examples. These and other such databases are accessible through various on-line computer data services; also, most of this information is available as CD-ROM and computer updates. Many of these services can be accessed for up-to-date toxicity information.
3.B.6.1 The National Library of Medicine Databases
The databases supplied by NLM are easy to use and relatively inexpensive. TOXLINE, the best source of information for most people, covers data published from 1981 to the present. For data published in the period from 1965 through 1980, TOXLINE65, a back file of TOXLINE, is also available. The telephone number to call for information and instructions on obtaining an NLM account is 1-800-638-8480.
Other databases supplied by NLM are the Hazardous Substance Data Base (HSDB), the Registry of Toxic Effects of Chemical Substances (RTECS), and the Medical Literature Analysis and Retrieval System (MEDLARS). NLM also supplies other specialized databases called CANCERLIT, DART, GENETOX, IRIS, CCRIS, and CHEMID.
3.B.6.2 Chemical Abstracts Databases
Another source of toxicity data is Chemical Abstracts (CA). In addition to the NLM, several services provide CA, including Knight-Ridder Information (formerly DIALOG), ORBIT, STN, and Ovid Technologies (formerly CD Plus). Searching procedures for CA depend on the various services supplying the database. Searching costs are considerably higher than for NLM databases because CA royalties must be paid. Telephone numbers for the above suppliers are as follows:
|
Knight-Ridder Information |
1-800-334-2564 |
|
ORBIT |
1-800-456-7248 |
|
STN |
1-800-848-6533 |
|
Ovid Technologies |
1-800-289-4277 |
Specialized databases are available from a vendor called Chemical Information Systems (CIS) for aquatic toxicity, dermal toxicity, EPA TSCA FYI, 8(d) and 8(e) studies, and so on. The CIS telephone number is 1800-CIS-USER.
Searching any database is best done using the Chemical Abstracts Service (CAS) Registry Number for the particular chemical. Free text searching is available on most of the databases except MEDLINE, which has a controlled vocabulary. As mentioned above, a menu-driven format is available to aid the inexperienced user. Equipment needed to do a search includes a computer terminal, a modem for accessing the on-line database by telephone, and a printer. Results of the search can also be captured by using an electronic format (e.g., a floppy disk).
3.B.6.3 Informal Forum
The "Letters to the Editor" column of Chemical & Engineering News, published weekly by the American Chemical Society, has become an informal but widely accepted forum for the reporting of anecdotal information on chemical reactivity hazards and other safety-related information. This publication is accessible via full-text searching services provided by STN.
3.C TOXIC EFFECTS OF LABORATORY CHEMICALS
3.C.1 Basic Principles
The chemicals encountered in the laboratory have a broad spectrum of physical, chemical, and toxicological properties and physiological effects. The risks associated with the use of laboratory chemicals must be well understood prior to their use in an experiment. The risk of toxic effects is related to both the extent of exposure and the inherent toxicity of a chemical. As discussed in detail below, extent of exposure is determined by the dose, the duration and frequency of exposure, and the route of exposure. Exposure to even large doses of chemicals with little inherent toxicity, such as phosphate buffer, presents low risk. In contrast, even small quantities of chemicals with high inherent toxicity or corrosivity may cause significant adverse effects. The duration and frequency of exposure are also critical factors in determining whether a chemical will produce harmful effects. In some cases, a single exposure to a chemical is sufficient to produce poisoning. On the other hand, for many chemicals repeated exposure is required to produce toxic effects. For most substances, the route of exposure (through the skin, the eyes, the gastrointestinal tract, or the respiratory tract) is also an important consideration in risk assessment. In the case of chemicals that are systemic toxicants, the internal dose to the target organ is a critical factor.
When considering possible toxicity hazards while planning an experiment, it is important to recognize that the combination of the toxic effects of two substances may be significantly greater than the toxic effect of either substance alone. Because most chemical reactions are likely to produce mixtures of substances whose combined toxicities have never been evaluated, it is pru-
dent to assume that mixtures of different substances (i.e., chemical reaction mixtures) will be more toxic than their most toxic ingredient. Furthermore, chemical reactions involving two or more substances may form reaction products that are significantly more toxic than the starting reactants. This possibility of generating toxic reaction products may not be anticipated by the laboratory worker in cases where the reactants are mixed unintentionally. For example, inadvertent mixing of formaldehyde (a common tissue fixative) and hydrogen chloride could result in the generation of bis(chloromethyl)ether, a potent human carcinogen.
It is essential that all laboratory workers understand certain basic principles of toxicology and learn to recognize the major classes of toxic and corrosive chemicals. The next sections of this chapter summarize the key concepts involved in assessing the risks associated with the use of toxic chemicals in the laboratory.
(Also see Chapter 5, section 5.D.)
3.C.1.1 Dose-Response Relationships
Toxicology, the science of poisons, is the study of the adverse effects of chemicals on living systems. The basic tenet of toxicology is that no substance is entirely safe and that all chemicals result in some toxic effects if a high enough amount (dose) of the substance comes in contact with a living system. Paracelsus (1493-1541) elegantly articulated this simple concept five centuries ago when he noted, "All substances are poisons; there is none which is not a poison. The right dose differentiates a poison...." This is perhaps the most important concept for all laboratory workers to be cognizant of. For example, ingestion of water, a vital substance for life, can result in death if a sufficiently large amount (i.e., gallons) is ingested at one time. On the other hand, sodium cyanide, a highly lethal chemical, will produce no permanent effects if a living system is exposed to a sufficiently low dose. The single most important factor that determines whether a substance will be harmful (or, conversely, safe) to an individual is the relationship between the amount (or concentration) of the chemical and the toxic effect it produces. For all chemicals, there is a range of concentrations that result in a graded effect between the extremes of no effect and death. In toxicology, this is referred to as the dose-response relationship for the chemical. The dose is the amount of the chemical and the response is the effect of the chemical. This relationship is unique for each chemical, although for many similar types of chemicals, the dose-response relationships are very similar. Among the thousands of laboratory chemicals, there is clearly a wide spectrum of doses that are required to produce toxic effects and, in some cases, even death. For most chemicals, a threshold dose has been established (by rule or by consensus) below which a chemical is not considered to be harmful.
Some chemicals (e.g., dioxin) will produce death in laboratory animals upon exposure to microgram doses and therefore are obviously extremely toxic. Other substances, however, may have no harmful effects following doses in excess of several grams. One way to evaluate the acute toxicity (i.e., the toxicity occurring after a single exposure) of laboratory chemicals involves consideration of their lethal dose 50 (LD50) or lethal concentration 50 (LC50) value. The LD50 is defined as the amount of a chemical that when ingested, injected, or applied to the skin of a test animal under controlled laboratory conditions will kill one-half (50%) of the animals. The LD50 is usually expressed in units of milligrams or grams per kilogram of body weight. For volatile chemicals (i.e., chemicals with sufficient vapor pressure that inhalation is an important route of chemical entry into the body), the LC50 is often reported instead of the LD50. The LD50 is the concentration of the chemical in air that will kill 50% of the test animals exposed to it. The LC50 is usually given in units of parts per million, milligrams per liter, or milligrams per cubic meter. Also reported are LC10, and LD10 values, which are defined as the lowest concentration or dose that causes the death of test animals. In general, the larger the value of the LD50 or LC50, the more chemical it takes to kill the test animals and therefore the lower the toxicity of the chemical. Although lethal dose values may vary among animal species and between animals and humans, the relative toxicity of different substances is usually relatively constant, and chemicals that are highly toxic to animals are generally highly toxic to humans.
3.C.1.2 Duration and Frequency of Exposure
Toxic effects of chemicals can occur after single (acute), intermittent (repeated), or long-term, repeated (chronic) exposure. An acutely toxic substance can cause damage as the result of a single, short-duration exposure. Hydrogen cyanide, hydrogen sulfide, and nitrogen dioxide are examples of acute toxins. In contrast, a chronically toxic substance causes damage after repeated or long-duration exposure or causes damage that becomes evident only after a long latency period. Chronic toxins include all carcinogens, reproductive toxins, and certain heavy metals (e.g., mercury, lead) and their compounds. Many chronic toxins are extremely dangerous because of their long latency periods: the cumulative effect of low exposures to such substances may not become apparent for many years.
In a general sense, the longer the duration of exposure, that is, the longer the body (or tissues in the body) is in contact with a chemical, the greater the opportunity for toxic effects to occur. Frequency of exposure also has an important influence on the nature and extent of toxicity. The total amount of a chemical required to produce a toxic effect is generally less for a single exposure than for intermittent or repeated exposures. More total chemical is required to produce toxicity for intermittent or chronic exposure because many chemicals can be eliminated from the body, because tissue injuries can often be repaired, and because adaptation of tissues can occur over time. Some toxic effects occur only after chronic exposure; this is because sufficient amounts of chemical cannot be attained in the tissue by a single exposure. Sometimes a chemical has to be present in a tissue for a considerable time to produce injury. For example, the neurotoxic and carcinogenic effects from exposure to heavy metals usually require long-term repeated exposure.
The time between exposure to a chemical and onset of toxic effects varies depending on the chemical and the exposure. For example, the toxic effects of carbon monoxide, sodium cyanide, and carbon disulfide are evident within minutes. For many chemicals, the toxic effect is most severe between one and a few days after exposure. However, some chemicals produce "delayed" toxicity; in fact, the neurotoxicity produced by some chemicals is not observed until a few weeks after exposure. The most delayed toxic effect produced by chemicals is cancer: in humans, it usually takes 10 to 30 years between exposure to a known human carcinogen and the detection of a tumor.
3.C.1.3 Routes of Exposure
Exposure to chemicals in the laboratory can occur by several different routes: (1) inhalation, (2) contact with skin or eyes, (3) ingestion, and (4) injection. Important features of these different pathways are detailed below.
3.C.1.3.1
Inhalation
Toxic materials that can enter the body via inhalation include gases, the vapors of volatile liquids, mists and sprays of both volatile and nonvolatile liquid substances, and solid chemicals in the form of particles, fibers, and dusts. Inhalation of toxic gases and vapors can produce poisoning by absorption through the mucous membranes of the mouth, throat, and lungs and can also damage these tissues seriously by local action. Inhaled gases and vapors can pass into the capillaries of the lungs and be carried into the circulatory system. This absorption can be extremely rapid. Because of the large surface area of the lungs in humans (about 75 square meters (m2)), this is the main site for absorption of many toxic materials.
The factors governing the absorption of gases and vapors from the respiratory tract differ significantly from those that govern the absorption of particulate substances. Factors controlling the absorption of inhaled gases and vapors include the solubility of the gas in body fluids and the reactivity of the gas with tissues and the fluid lining the respiratory tract. Gases or vapors that are highly water-soluble, such as methanol, acetone, hydrogen chloride, and ammonia, dissolve predominantly in the lining of the nose and windpipe (trachea) and therefore tend to be absorbed from those regions. These sites of absorption are also potential sites of toxicity. Formaldehyde is an example of a reactive, highly water-soluble vapor for which the nose is a major site of deposition. In contrast to water-soluble gases, reactive gases with low water-solubility, such as ozone, phosgene, and nitrogen dioxide, penetrate farther into the respiratory tract and thus come into contact with the smaller tubes of the airways. Gases and vapors that are not water-soluble but are more fat-soluble, such as benzene, methylene chloride, and trichloroethylene, are not completely removed by interaction with the surfaces of the nose, trachea, and small airways. As a result, these gases penetrate the airways down into the deep lung, where they can diffuse across the thin lung tissue into the blood. The more soluble a gas is in the blood, the more of it will be dissolved and transported to other organs.
In the case of inhaled solid chemicals, an important factor in determining if and where a particle will be deposited in the respiratory tract is its size. One generalization is that the largest particles (³5 microns (µm)) are deposited primarily in the nose, smaller particles (1 to 5 µm) in the trachea and small airways, and the smallest particles in the lungs. Thus, depending on the size of an inhaled particle, it will be deposited in different sections of the respiratory tract, and the location can affect the local toxicity and the absorption of the material. In general, particles that are water-soluble will dissolve within minutes or days, and chemicals that are not water-soluble but have a moderate degree of fat-solubility will also clear rapidly into the blood. Those that are not water-soluble or highly fat-soluble will not dissolve and will be retained in the lungs for long periods of time. Metal oxides, asbestos, and silica are examples of water-insoluble inorganic particles that might be retained in the lungs for years.
A number of factors can affect the airborne concentrations of chemicals. Vapor pressure (the tendency of
molecules to escape from the liquid or solid phase into the gaseous phase) is the most important characteristic of a chemical to consider. The higher the vapor pressure, the greater the potential concentration of the chemical in the air. For example, acetone (with a vapor pressure of 180 millimeters of mercury (mmHg) at 20 °C) could reach an equilibrium concentration in air of 240,000 parts per million (ppm), or 24%. (This value is approximated by dividing the vapor pressure of the chemical by the atmospheric pressure—760 mmHg—and multiplying by 1,000,000 to convert to ppm.) Fortunately, the ventilation present in most laboratories prevents an equilibrium concentration from developing in the breathing zone of the laboratory worker.
Even very low vapor pressure chemicals can be dangerous if the material is highly toxic. A classic example is elemental mercury. Although the vapor pressure of mercury at room temperature is only 0.0012 mmHg, the resulting equilibrium concentration of mercury vapor is 1.58 ppm, or about 13 milligrams per cubic meter (mg/m3). The TLV for mercury is 0.05 mg/m3, more than 2 orders of magnitude lower.
The vapor pressure of a chemical increases with temperature; therefore, heating of solvents or reaction mixtures increases the potential for high airborne concentrations. Also, a spilled volatile chemical can evaporate very quickly because of its large surface area, creating a significant exposure potential. It is clear that careful handling of volatile chemicals is very important; keeping containers tightly closed or covered and using volatiles in fume hoods are techniques that should be used to avoid unnecessary exposure to inhaled chemicals.
Certain types of particulate materials can also present the potential for airborne exposures. If a material has a very low density or a very small particle size, it will tend to remain airborne for a considerable time. For example, the very fine dust cloud generated by emptying a low-density particulate (e.g., vermiculite) into a secondary container will take a long time to settle out, and these particles can be inhaled. Such operations should therefore be carried out in a fume hood.
Operations that generate aerosols (suspensions of microscopic droplets in air), such as vigorous boiling, high-speed blending, or bubbling gas through a liquid, increase the potential for exposure via inhalation. Consequently, these and other such operations on toxic chemicals should also be carried out in a hood.
3.C.1.3.2
Contact with Skin or Eyes
Contact with the skin is a frequent mode of chemical injury in the laboratory. Many chemicals can injure the skin directly. Skin irritation and allergic skin reactions are a common result of contact with certain types of chemicals. Corrosive chemicals can cause severe burns when they come in contact with the skin. In addition to causing local toxic effects, many chemicals are absorbed through the skin in sufficient quantity to produce systemic toxicity. The main avenues by which chemicals enter the body through the skin are the hair follicles, sebaceous glands, sweat glands, and cuts or abrasions of the outer layer. Absorption of chemicals through the skin depends on a number of factors, including chemical concentration, chemical reactivity, and the solubility of the chemical in fat and water. Absorption is also dependent on the condition of the skin, the part of the body exposed, and duration of contact. Differences in skin structure affect the degree to which chemicals can be absorbed. In general, toxicants cross thin skin (e.g., scrotum) much more easily than thick skin (e.g., palms). When skin is damaged, penetration of chemicals increases. Acids and alkalis can injure the skin and increase its permeability. Burns and skin diseases are the most common examples of skin damage that can increase penetration. Also, hydrated skin absorbs chemicals better than dehydrated skin. Some chemicals such as dimethyl sulfoxide can actually increase the penetration of chemicals through the skin by increasing its permeability.
Contact of chemicals with the eyes is of particular concern because these organs are so sensitive to irritants. Few substances are innocuous in contact with the eyes; most are painful and irritating, and a considerable number are capable of causing burns and loss of vision. Alkaline materials, phenols, and strong acids are particularly corrosive and can cause permanent loss of vision. Because the eyes contain many blood vessels, they also can be a route for the rapid absorption of many chemicals.
3.C.1.3.3
Ingestion
Many of the chemicals used in the laboratory are extremely hazardous if they enter the mouth and are swallowed. The gastrointestinal tract, which consists of the mouth, esophagus, stomach, and small and large intestines, can be thought of as a tube of variable diameter (about 5 m in length) with a large surface area (about 200 m2) for absorption. Toxicants that enter the gastrointestinal tract must be absorbed into the blood to produce a systemic injury. Sometimes a chemical is caustic or irritating to the gastrointestinal tract tissue itself. Absorption of toxicants can take place along the entire gastrointestinal tract, even in the mouth, and depends on many factors, including the physical properties of the chemical and the speed at which it dissolves. Absorption increases with surface area, permeability, and residence time in various segments of the
tract. Some chemicals increase intestinal permeability and thus increase the rate of absorption. More chemical will be absorbed if the chemical remains in the intestine for a long time. If a chemical is in a relatively insoluble, solid form, it will have limited contact with gastrointestinal tissue, and its rate of absorption will be low. If it is an organic acid or base, it will be absorbed in that part of the gastrointestinal tract where it is most fat-soluble. Fat-soluble chemicals are absorbed more rapidly and extensively than water-soluble chemicals.
3.C.1.3.4
Injection
Exposure to toxic chemicals by injection does not occur frequently in the chemical laboratory. However, it can occur inadvertently through mechanical injury from "sharps" such as glass or metal contaminated with chemicals or when chemicals are handled with syringes. The intravenous route of administration is especially dangerous because it introduces the toxicant directly into the bloodstream, eliminating the process of absorption. Nonlaboratory personnel, such as custodial workers or waste handlers, must be protected from this form of exposure by putting all "sharps" in special trash containers and never in the ordinary scrap baskets. Hypodermic needles with blunt ends are available for laboratory use.
3.C.2 Types of Toxins
Exposure to a harmful chemical can result in local toxic effects, systemic toxic effects, or both. Local effects involve injury at the site of first contact. The eyes, the skin, the nose and lungs, and the digestive tract are typical sites of local reactions. Examples of local effects include (1) ingestion of caustic substances causing burns and ulcers in the mouth, esophagus, stomach, and intestines, (2) inhalation of hazardous materials causing toxic effects in the nose and lungs, and (3) contact with harmful materials on the skin or eyes leading to effects ranging from mild irritation to severe tissue damage. Systemic effects, by contrast, occur after the toxicant has been absorbed from the site of contact into the bloodstream and distributed throughout the body. While some chemicals produce adverse effects on all tissues of the body, other chemicals tend to selectively injure a particular tissue or organ without affecting others. The affected organs (e.g., liver, lungs, kidney, central nervous system) are referred to as the target organs of toxicity. The target organ of toxicity is not necessarily the organ where the highest concentration of the chemical is achieved. Hundreds of different systemic toxic effects of chemicals are known. Systemic effects can result from single (acute) exposures or from repeated or long-duration (chronic) exposures, becoming evident only after a long latency period.
Toxic effects can be further classified as reversible or irreversible. Reversible toxicity is possible because in some cases tissues have the capacity to repair toxic damage, so that the damage disappears following cessation of exposure. Irreversible damage, in contrast, persists even after cessation of exposure. Recovery from a burn is a good example of reversible toxicity; cancer is generally thought to be irreversible.
The chemicals used in the laboratory can be grouped among several different classes of toxic substances. Many chemicals display more than one type of toxicity. The following are the most common classes of toxic substances encountered in laboratories.
3.C.2.1 Irritants
Irritants are noncorrosive chemicals that cause reversible inflammatory effects (swelling and redness) on living tissue by chemical action at the site of contact. A wide variety of organic and inorganic chemicals are irritants, and consequently, skin and eye contact with all chemicals in the laboratory should be avoided.
3.C.2.2 Corrosive Substances
Corrosive substances cause destruction of living tissue by chemical action at the site of contact and can be solids, liquids, or gases. Corrosive effects can occur not only on the skin and eyes, but also in the respiratory tract and, in the case of ingestion, in the gastrointestinal tract as well. Corrosive materials are probably the most common toxic substances encountered in the laboratory. Corrosive liquids are especially dangerous because their effect on tissue generally takes place very rapidly. Bromine, sulfuric acid, aqueous sodium hydroxide solution, and hydrogen peroxide are examples of highly corrosive liquids. Corrosive gases are also frequently encountered. Gases such as chlorine, ammonia, and nitrogen dioxide can damage the lining of the lungs, leading, after a delay of several hours, to the fatal buildup of fluid known as pulmonary edema. Finally, a number of solid chemicals have corrosive effects on living tissue. Examples of common corrosive solids include sodium hydroxide, phosphorus, and phenol. Dust from corrosive solids can be inhaled and cause serious damage to the respiratory tract.
There are several major classes of corrosive substances. Strong acids such as nitric, sulfuric, and hydrochloric acid can cause serious damage to the skin and eyes. Hydrofluoric acid is particularly dangerous and
produces slow-healing, painful burns. Strong bases, such as the metal hydroxides and ammonia, make up another class of corrosive chemicals. Strong dehydrating agents, such as phosphorus pentoxide and calcium oxide, have a powerful affinity for water and can cause serious burns upon contact with the skin. Finally, strong oxidizing agents, such as concentrated solutions of hydrogen peroxide, can also have serious corrosive effects and should never come into contact with the skin or eyes.
3.C.2.3 Allergens
A chemical allergy is an adverse reaction by the immune system to a chemical. Such allergic reactions result from previous sensitization to that chemical or a structurally similar chemical. Once sensitization occurs, allergic reactions can result from exposure to extremely low doses of the chemical. Allergic reactions can be immediate, occurring within a few minutes after exposure. Anaphylactic shock is a severe immediate allergic reaction that can result in death if not treated quickly. If this is likely to be a hazard for a planned experiment, advice on emergency response should be obtained. Allergic reactions can also be delayed, taking hours or even days to develop. The skin is usually the site of such delayed reactions, in which cases it becomes red, swollen, and itchy.
It is important to recognize that delayed chemical allergy can occur even some time after the chemical has been removed. Contact with poison ivy is a familiar example of an exposure that causes a delayed allergic reaction. Also, just as people vary widely in their susceptibility to sensitization by environmental allergens such as dust and pollen, individuals may also exhibit wide differences in their sensitivity to laboratory chemicals. Examples of substances that may cause allergic reactions in some individuals include diazomethane, dicyclohexylcarbodiimide, formaldehyde, various isocyanates, benzylic and allylic halides, and certain phenol derivatives.
3.C.2.4 Asphyxiants
Asphyxiants are substances that interfere with the transport of an adequate supply of oxygen to the vital organs of the body. The brain is the organ most easily affected by oxygen starvation, and exposure to asphyxiants can lead to rapid collapse and death. Simple asphyxiants are substances that displace oxygen from the air being breathed to such an extent that adverse effects result. Acetylene, carbon dioxide, argon, helium, ethane, nitrogen, and methane are common asphyxiants. It is thus important to recognize that even chemically inert and biologically benign substances can be extremely dangerous under certain circumstances. Certain other chemicals have the ability to combine with hemoglobin, thus reducing the capacity of the blood to transport oxygen. Carbon monoxide, hydrogen cyanide, and certain organic and inorganic cyanides are examples of such substances.
3.C.2.5 Carcinogens
A carcinogen is a substance capable of causing cancer. Cancer, in the simplest sense, is the uncontrolled growth of cells, and it can occur in any organ. The mechanism by which cancer develops is not well understood, but the current thinking is that some chemicals interact directly with DNA, the genetic material in all cells, to result in permanent alterations. Other chemical carcinogens can modify DNA indirectly by changing the way the cells grow. Carcinogens are chronically toxic substances; that is, they cause damage after repeated or long-duration exposure, and their effects may become evident only after a long latency period. Carcinogens are particularly insidious toxins because they may have no immediate apparent harmful effects.
3.C.2.6 Reproductive and Developmental Toxins
Reproductive toxins are substances that have adverse effects on various aspects of reproduction, including fertility, gestation, lactation, and general reproductive performance. Developmental toxins are substances that act during pregnancy to cause adverse effects on the embryo or fetus. These effects can include lethality (death of the fertilized egg, the embryo, or the fetus), malformations (this class of substances is also called teratogens), retarded growth, and postnatal functional deficiencies. When a pregnant woman is exposed to a chemical, generally the fetus is exposed as well because the placenta is an extremely poor barrier to chemicals. Reproductive toxins can affect both men and women. Male reproductive toxins can in some cases lead to sterility. Two well-known male reproductive toxins are ethylene dibromide and dibromochloropropane.
3.C.2.7 Neurotoxins
Neurotoxic chemicals can induce an adverse effect on the structure or function of the central and/or peripheral nervous system, which can be permanent or reversible. In some cases the detection of neurotoxic effects may require specialized laboratory techniques, but often they can be inferred from behavior such as slurred speech and staggered gait. Many neurotoxins
are chronically toxic substances whose adverse effects are not immediately apparent. At the present time, because of the limited data available in this area, significant uncertainties attend the assessment of risks associated with work with neurotoxic substances.
3.C.2.8 Toxins Affecting Other Organs
Target organs outside the reproductive and neurological systems can also be affected by toxic substances found in the laboratory. Most of the chlorinated hydrocarbons, benzene, other aromatic hydrocarbons, some metals, carbon monoxide, and cyanides, among others, can produce one or more effects in target organs. Such an effect may be the most probable result of exposure to the particular chemical. Although this chapter does not include specific sections on liver, kidney, lung, or blood toxins, many of the LCSSs mention those effects in the toxicology section.
3.C.3 Assessing Risks Due to the Toxic Effects of Laboratory Chemicals
The first step in assessing the risks associated with a planned laboratory experiment involves identifying which of the chemicals to be used in the proposed experiment are potentially hazardous substances. The OSHA Laboratory Standard (29 CFR 1910.1450) defines a hazardous substance as
a chemical for which there is statistically significant evidence based on at least one study conducted in accordance with established scientific principles that acute or chronic health effects may occur in exposed employees. The term ''health hazard" includes chemicals which are carcinogens, toxic or highly toxic agents, reproductive toxins, irritants, corrosives, sensitizers, hepatotoxins, nephrotoxins, neurotoxins, agents which act on the hematopoietic systems, and agents which damage the lungs, skin, eyes, or mucous membranes.
The OSHA Laboratory Standard further requires that certain chemicals be identified as "particularly hazardous substances" and handled using special additional procedures. Particularly hazardous substances include chemicals that are "select" carcinogens (those strongly implicated as a potential cause of cancer in humans), reproductive toxins, and compounds with a high degree of acute toxicity. Highly flammable and explosive substances make up another category of hazardous compounds, and the assessment of risk for these classes of chemicals is discussed in section 3.D. This section considers the assessment of risks associated with work with specific classes of toxic chemicals, including those that pose hazards due to acute toxicity and chronic toxicity.
3.C.3.1 Acute Toxicants
Acute toxicity is the ability of a chemical to cause a harmful effect after a single exposure. Acutely toxic agents can cause local toxic effects, systemic toxic effects, or both, and this class of toxicants includes corrosive chemicals, irritants, and allergens (sensitizers). Among the most useful parameters for assessing the risk of acute toxicity of a chemical are its LD50 and LC50 values, selected with due regard for the possible routes of exposure. In interpreting these lethal dose and lethal concentration values, the following points should be considered. The LD50 is the mean dose causing death in animals, and it should be recognized that the minimum dose causing death in some proportion of the test population will be much lower, with significant illness or harm short of lethality probably occurring at even lower doses. Finally, it is assumed that the lethal dose for animals (usually rodents) is an appropriate predictor of the lethal dose in humans.
In assessing the risks associated with acute toxicants, it is useful to classify a substance according to the acute toxicity hazard level as shown in Table 3.1. LD50 values can be found in the Laboratory Chemical Safety Summary (LCSS) or MSDS for a given substance, and in references such as Sax's Dangerous Properties of Industrial Materials (Lewis, 1992), Sigma-Aldrich Library of Chemical Safety Data (Lenga, 1988), and A Comprehensive Guide to the Hazardous Properties of Chemical Substances (Patnaik, 1992). Table 3.2 relates test animal LD50 values expressed as milligrams or grams per kilogram of body weight to the probable human lethal dose, expressed in easily understood units, for a 70-kilogram (kg) person.
Special attention must be given to any substance classified according to the above criteria as having a high level of acute toxicity hazard. Chemicals with a high level of acute toxicity make up one of the categories of "particularly hazardous substances" defined by the OSHA Laboratory Standard. Any compound rated as highly toxic in Table 3.1 meets the OSHA criteria for handling as a particularly hazardous substance.
Table 3.3 lists some of the most common chemicals with a high level of acute toxicity that are encountered in the laboratory. These compounds must generally be handled using the additional procedures outlined in Chapter 5, section 5.D. In some circumstances, it may not be necessary to employ all of these special precautions, such as when the total amount of an acutely toxic substance to be handled is a small fraction of the harmful dose. It is an essential part of prudent experiment planning to determine whether a chemical with a high
TABLE 3.1 Acute Toxicity Hazard Level
|
Hazard Level |
Toxicity Rating |
Oral LD50 (Rats, per kg) |
Skin Contact LD50 (Rabbits, per kg) |
Inhalation LC50 (Rats, ppm for 1 h) |
Inhalation LC50 (Rats, mg/m3 for 1 h) |
|
High |
Highly toxic |
<50 mg |
<200 mg |
<200 |
<2,000 |
|
Medium |
Moderately toxic |
50 to 500 mg |
200 mg to 1 g |
200 to 2,000 |
2,000 to 20,000 |
|
Low |
Slightly toxic |
500 mg to 5 g |
1 to 5 g |
2,000 to 20,000 |
20,000 to 200,000 |
TABLE 3.2 Probable Lethal Dose for Humans
|
Toxicity Rating |
Animal LD50 (per kg) |
Lethal Dose When Ingested by 70-kg (150-lb) Human |
|
Extremely toxic |
Less than 5 mg |
A taste (less than 7 drops) |
|
Highly toxic |
5 to 50 mg |
Between 7 drops and 1 teaspoonful |
|
Moderately toxic |
50 to 500 mg |
Between 1 teaspoonful and 1 ounce |
|
Slightly toxic |
500 mg to 5 g |
Between 1 ounce and 1 pint |
|
Practically nontoxic |
Above 5 g |
Above 1 pint |
|
SOURCE: Modified, by permission, from Gosselin et al. (1984). Copyright 1984 by Williams & Wilkins, Baltimore. |
||
degree of acute toxicity should be treated as a "particularly hazardous substance" in the context of a specific planned use. This determination not only will involve consideration of the total amount of the substance to be used, but also will require a review of the physical properties of the substance (e.g., is it volatile? does it tend to form dusts?), its potential routes of exposure (e.g., is it readily absorbed through the skin?), and the circumstances of its use in the proposed experiment (e.g., will the substance be heated? is there likelihood that aerosols may be generated?). Depending on the worker's level of experience and the degree of potential hazard, this determination may require consultation with supervisors and safety professionals.
Because the greatest risk of exposure to many laboratory chemicals is by inhalation, it is essential that laboratory workers understand the use of exposure limits that have been established by agencies such as ACGIH
TABLE 3.3 Examples of Compounds with a High Level of Acute Toxicity
|
Acrolein |
Nickel carbonyl |
|
Arsine |
Nitrogen dioxide |
|
Chlorine |
Osmium tetroxide |
|
Diazomethane |
Ozone |
|
Diborane (gas) |
Phosgene |
|
Hydrogen cyanide |
Sodium azide |
|
Hydrogen fluoride |
Sodium cyanide |
|
Methyl fluorosulfonate |
(and other cyanide salts) |
and OSHA. The threshold limit value (TLV), assigned by the ACGIH, defines the concentration of a chemical in air to which nearly all individuals can be exposed without adverse effects. The TLV-TWA (threshold limit value-time weighted average) refers to the concentration safe for exposure during an entire 8-h workday, while the TLV-STEL (threshold limit value-short term exposure limit) is a higher concentration to which workers may be exposed safely for a 15-min period. OSHA defines the permissible exposure limit (PEL) analogously to the ACGIH values, with corresponding TWA and STEL limits. TLV and PEL values allow the laboratory worker to quickly determine the relative inhalation hazards of chemicals. In general, substances with PELs or TLVs of less than 50 ppm should be handled in a fume hood. Comparison of these values to the odor threshold for a given substance will often indicate whether the odor of the chemical provides sufficient warning of possible hazard. However, individual differences in ability to detect some odors as well as anosmia, or "olfactory fatigue," can limit the usefulness of odors as warning signs of overexposure. LCSSs contain information on odor threshold ranges and whether a substance is known to cause olfactory fatigue. Finally, a variety of devices are available for measuring the concentration of chemicals in laboratory air, so that the degree of hazard associated with the use of a chemical can be assessed directly. The industrial hygiene offices of many institutions can assist labora-
tory workers in measuring the air concentrations of chemicals.
3.C.3.2 Corrosive Substances, Irritants, and Allergens
Lethal dose and other quantitative toxicological parameters generally provide little guidance in assessing the risks associated with corrosives, irritants, and allergens (sensitizers), because these toxic substances exert their harmful effects locally. When planning an experiment that will involve the use of corrosive substances, basic prudent handling practices should be reviewed to ensure that the skin, face, and eyes are protected adequately by the proper choice of corrosion-resistant gloves and protective clothing and eyewear, including, in some cases, face shields. Similarly, LD50 data are not an indicator of the irritant effects of chemicals, and therefore special attention should be paid to the identification of irritant chemicals by consulting LCSSs, MSDSs, and other sources of information. Allergens are another class of acute toxicants whose effects are not included in LD50 data. Individuals may differ widely in their tendency to become sensitized to allergens, so it is prudent to regard compounds with a proven ability to cause sensitization as highly toxic agents. Once a person has become sensitized to an allergen, subsequent contact can lead to immediate or delayed allergic reactions. Furthermore, sensitization to a specific substance can persist for many years. Because an allergic response can be triggered in a sensitized individual by an extremely small quantity of the allergen, it may occur despite personal protection measures that are adequate to protect against the acute effects of chemicals. Laboratory workers should be alert for signs of allergic responses to chemicals.
3.C.3.3 Carcinogens
Because cancer is such a widespread cause of human mortality, and because exposure to chemicals may play a significant role in the onset of cancer, a great deal of attention has been focused on evaluation of the carcinogenic potential of chemicals. However, the vast majority of the substances involved in research, especially in laboratories concerned primarily with the synthesis of novel compounds, have not been tested for carcinogenicity. Compounds that are known to pose the greatest carcinogenic hazard are referred to as "select carcinogens," and they constitute another category of substances that must be handled as "particularly hazardous substances" according to the OSHA Laboratory Standard. A select carcinogen is defined in the OSHA Laboratory Standard as a substance that meets one of the following criteria:
-
It is regulated by OSHA as a carcinogen.
-
It is listed as "known to be a carcinogen" in the latest Annual Report on Carcinogens issued by the National Toxicology Program (NTP) (U.S. DHHS, 1991).
-
It is listed under Group 1 ("carcinogenic to humans") by the International Agency for Research on Cancer (IARC).
-
It is listed under IARC Group 2A ("probably carcinogenic to humans") or 2B ("possibly carcinogenic to humans"), or under the category "reasonably anticipated to be a carcinogen'' by the NTP, and causes statistically significant tumor incidence in experimental animals in accordance with any of the following criteria: (a) after inhalation exposure of 6 to 7 h per day, 5 days per week, for a significant portion of a lifetime to dosages of less than 10 mg/m3; (b) after repeated skin application of less than 300 mg/kg of body weight per week; or (c) after oral dosages of less than 50 mg/kg of body weight per day.
Table 3.4 lists some representative substances that meet the above criteria for classification as OSHA select carcinogens. These chemicals are classified as particularly hazardous substances and should be handled using the basic prudent practices given in Chapter 5, section 5.C, supplemented by the additional special practices outlined in section 5.D. Work with compounds that are possible human carcinogens may or may not require the additional precautions given in section 5.D. For these compounds, the LCSS should indicate whether or not the substance meets the additional criteria listed in category 4 and must therefore be treated as a select carcinogen. If an LCSS is not available, consultation with a safety professional such as a chemical hygiene officer may be necessary in order to determine whether a possible human carcinogen should be classified as a particularly hazardous substance.
Many chemical substances are encountered in the laboratory for which there is no animal test or human epidemiological data on carcinogenicity. In these cases, workers must evaluate the potential risk that the chemical in question is a carcinogenic substance. This determination can sometimes be made on the basis of knowledge of the specific classes of compounds and functional group types that have previously been correlated with carcinogenic activity. For example, chloromethyl methyl ether is a known human carcinogen and therefore is regarded as an OSHA select carcinogen requiring the handling procedures outlined in section 5.D. On the other hand, the carcinogenicity of ethyl chloromethyl ether and certain other alkyl chloromethyl ethers is not established, and these substances do not necessarily have to be treated as select carcinogens. However, because of the chemical similarity of
TABLE 3.4 Examples of Select Carcinogens
these compounds to chloromethyl methyl ether, it is possible that these substances have comparable carcinogenicity, and it is therefore prudent to regard them as select carcinogens requiring the special handling procedures outlined in section 5.D.
Table 3.5 lists important general classes of chemicals for which some members (but not necessarily all) have been identified as being carcinogenic substances. Listed for each general class are representative compounds that are "reasonably anticipated to be carcinogens" based on animal tests, selected from lists of substances identified as carcinogens or potential carcinogens by OSHA, IARC, and the Annual Report on Carcinogens (U.S. DHHS, 1991) published by the National Toxicology Program.
The determination of whether a suspected carcinogenic chemical must be treated as a "particularly hazardous substance" in the context of a particular laboratory use will be affected by the scale and circumstances associated with the intended experiment. The laboratory worker must decide whether the amount and frequency of use, as well as other circumstances, are such that additional precautions beyond the basic prudent practices of section 5.C are required. For example, the large-scale or recurring use of such a chemical might suggest that the special precautions of section 5.D be followed to control exposure, whereas adequate protection from a single use of a small amount of such a substance may be obtained through the use of the basic procedures in section 5.C.
When evaluating the carcinogenic potential of chemicals, it should be noted that exposure to certain combinations of compounds (not necessarily simultaneously) can cause cancer even at exposure levels where neither of the individual compounds would have been carcinogenic. 1,8,9-Trihydroxyanthracene and certain phorbol esters are examples of "tumor promoters." Although not carcinogenic themselves, they can dramatically amplify the carcinogenicity of other compounds. It should also be understood that the response of an organism to a toxicant typically increases with the dose given, but the relationship is not always a linear one. Some carcinogenic alkylating agents exhibit a dose threshold above which the tendency to cause mutations increases markedly. At lower doses, natural protective systems prevent genetic damage, but when the capacity of these systems is overwhelmed, the organism becomes much more sensitive to the toxicant. However, there are differences between individuals in the levels of protection against genetic damage as well as in other defense systems. These differences are determined in part by genetic factors and in part by the aggregate exposure of the individual to all chemicals within and outside of the laboratory.
3.C.3.4 Reproductive and Developmental Toxins
Reproductive toxins are defined by the OSHA Laboratory Standard as substances that cause chromosomal damage (mutagens) and substances with lethal or
TABLE 3.5 Classes of Carcinogenic Substances
|
Alkylating agents |
Hydrazines |
|
a-Halo ethers |
Hydrazine (and hydrazine salts) |
|
Bis(chloromethyl) ether |
1,2-Diethylhydrazine |
|
Methyl chloromethyl ether |
1,1-Dimethylhydrazine |
|
Sulfonates |
1,2-Dimethylhydrazine |
|
1,4-Butanediol dimethanesulfonate (myleran) |
|
|
Diethyl sulfate |
N-Nitroso compounds |
|
Dimethyl sulfate |
N-Nitrosodimethylamine |
|
Ethyl methanesulfonate |
N-Nitroso-N-alkylureas |
|
Methyl methanesulfonate |
|
|
Methyl trifluoromethanesulfonate |
Aromatic amines |
|
1,3-Propanesultone |
4-Aminobiphenyl |
|
Epoxides |
Benzidine (4, 4'-diaminobiphenyl) |
|
Ethylene oxide |
a-Naphthylamine |
|
Diepoxybutane |
ß-Naphthylamine |
|
Epichlorohydrin |
Aniline |
|
Propylene oxide |
o-Anisidine (2-methoxyaniline) |
|
Styrene oxide |
2,4-Diaminotoluene |
|
Aziridines |
o-Toluidine |
|
Ethylenimine |
|
|
2-methylaziridine |
Aromatic hydrocarbons |
|
Diazo, azo, and azoxy compounds |
Benzene |
|
4-Dimethylaminoazobenzene |
Benz[a]anthracene |
|
Electrophilic alkenes and alkynes |
Benzo[a]pyrene |
|
Acrylonitrile |
|
|
Acrolein |
Natural products (including antitumor drugs) |
|
Ethyl acrylate |
Adriamycin |
|
|
Aflatoxins |
|
Acylating agents |
Bleomycin |
|
ß-Propiolactone |
Cisplatin |
|
ß-Butyrolactone |
Progesterone |
|
Dimethylcarbamyl chloride |
Reserpine |
|
|
Safrole |
|
Organohalogen compounds |
|
|
1,2-Dibromo-3-chloropropane |
Miscellaneous organic compounds |
|
Mustard gas (bis(2-chloroethyl)sulfide) |
Formaldehyde (gas) |
|
Vinyl chloride |
Acetaldehyde |
|
Carbon tetrachloride |
1,4-Dioxane |
|
Chloroform |
Ethyl carbamate (urethane) |
|
3-Chloro-2-methylpropene |
Hexamethylphosphoramide |
|
1,2-Dibromoethane |
2-Nitropropane |
|
1,4-Dichlorobenzene |
Styrene |
|
1,2-Dichloroethane |
Thiourea |
|
2,2-Dichloroethane |
Thioacetamide |
|
1,3-Dichloropropene |
|
|
Hexachlorobenzene |
Miscellaneous inorganic compounds |
|
Methyl iodide |
Arsenic and certain arsenic compounds |
|
Tetrachloroethylene |
Chromium and certain chromium compounds |
|
Trichloroethylene |
Thorium dioxide |
|
2,4,6-Trichlorophenol |
Beryllium and certain beryllium compounds |
|
|
Cadmium and certain cadmium compounds |
|
|
Lead and certain lead compounds |
|
|
Nickel and certain nickel compounds |
|
|
Selenium sulfide |
teratogenic (malformation) effects on fetuses. Many reproductive toxins are chronic toxins that cause damage after repeated or long-duration exposures with effects that become evident only after long latency periods. Developmental toxins act during pregnancy and cause adverse effects on the fetus; these effects include embryo lethality (death of the fertilized egg, embryo, or fetus), teratogenic effects, and postnatal functional
TABLE 3.6 Examples of Reproductive Toxins
|
Arsenic and certain arsenic compounds |
Ethylene oxide |
|
Benzene |
Lead compounds |
|
Cadmium and certain cadmium compounds |
Mercury compounds Toluene |
|
Carbon disulfide |
Vinyl chloride |
|
Ethylene glycol monomethyl and ethyl ethers |
Xylene |
defects. Embryotoxins have the greatest impact during the first trimester of pregnancy. Because a woman often does not know that she is pregnant during this period of high susceptibility, women of childbearing potential are advised to be especially cautious when working with chemicals, especially those rapidly absorbed through the skin (e.g., formamide). Pregnant women and women intending to become pregnant should seek advice from knowledgeable sources before working with substances that are suspected to be reproductive toxins. As minimal precautions, the general procedures outlined in Chapter 5, section 5.D, should then be followed for work with such compounds.
Information on reproductive toxins can be obtained from LCSSs, MSDSs, and by consulting safety professionals in the environmental safety department, industrial hygiene office, or medical department of the worker's institution. Literature sources of information on reproductive and developmental toxins include the Catalog of Teratogenic Agents (Shepard, 1992), Reproductively Active Chemicals: A Reference Guide (Lewis, 1991), and "What Every Chemist Should Know About Teratogens" in the Journal of Chemical Education (Beyler and Meyers, 1982). Table 3.6 lists some common materials that are suspected to be reproductive toxins. In some cases it will be appropriate to handle these compounds as particularly hazardous substances using the special additional precautions outlined in section 5.D.
3.D FLAMMABLE, REACTIVE, AND EXPLOSIVE HAZARDS
In addition to the hazards due to the toxic effects of chemicals, hazards due to flammability, explosibility, and reactivity need to be considered in risk assessment. These hazards are described in detail in the following sections. Further information can be found in Bretherick's Handbook of Reactive Chemical Hazards (Bretherick, 1990), an extensive compendium that is the basis for the lists of incompatible chemicals included in various reference works. Bretherick describes computational protocols that consider thermodynamic and kinetic parameters of a system to arrive at quantitative measures such as the Reaction Hazard Index (RHI). So-called "reactive" hazards arise when the release of energy from a chemical reaction occurs in quantities or at rates too great for the energy to be absorbed by the immediate environment of the reacting system, and material damage results. In addition, the "Letters to the Editor" column of Chemical & Engineering News routinely reports incidents with explosive reaction mixtures or conditions.
3.D.1 Flammable Hazards
3.D.1.1 Flammable Substances
Flammable substances, those that readily catch fire and burn in air, may be solid, liquid, or gaseous. The most common fire hazard in the laboratory is a flammable liquid or the vapor produced from such a liquid. An additional hazard is that a compound can enflame so rapidly that it produces an explosion. Proper use of substances that can cause fires requires knowledge of their tendencies to vaporize, ignite, or burn under the variety of conditions of use in the laboratory.
For a fire to occur, three conditions must exist simultaneously: an oxidizing atmosphere, usually air; a concentration of flammable gas or vapor that is within the flammable limits of the substance; and a source of ignition. In most situations, oxygen or air is present. Prevention of the coexistence of flammable vapors and an ignition source is the optimal way to deal with the hazard. When the vapors of a flammable liquid cannot always be controlled, strict control of ignition sources is the principal approach to reduction of the risk of flammability. The rates at which different liquids produce flammable vapors depend on their vapor pressures, which increase with increasing temperature. The degree of fire hazard of a substance depends also on its ability to form combustible or explosive mixtures with air and on the ease of ignition of these mixtures. Also important are the relative density and solubility of a liquid with respect to water and of a gas with respect to air. These characteristics can be evaluated and compared in terms of the following specific properties.
3.D.1.2 Flammability Characteristics
3.D.1.2.1
Flash Point
The flash point is the lowest temperature at which a liquid has a sufficient vapor pressure to form an ignitable mixture with air near the surface of the liquid. Note that many common organic liquids have a flash point below room temperature: for example, acetone (-18 °C), benzene (-11.1 °C), diethyl ether (-45 °C),
|
QUICK GUIDE TO RISK ASSESSMENT FOR HAZARDOUS CHEMICALS The following outline provides a summary of the steps discussed in this chapter that laboratory workers should use to assess the risks of handling toxic chemicals. Note that if a Laboratory Chemical Safety Summary is not already available, then following the protocol outlined here should enable a worker to prepare his or her own LCSS.
|
TABLE 3.7 NFPA Fire Hazard Ratings, Flash Points, Boiling Points, Ignition Temperatures, and Flammable Limits of Some Common Laboratory Chemicals
|
|
NFPA Ratinga |
Flash Point (°C) |
Boiling Point (°C) |
Ignition Point (°C) |
Flammable Limits Temperature (percent by volume) |
|
|
|
|
|
|
|
Lower |
Upper |
|
Acetaldehyde |
4 |
-37.8 |
21.1 |
175 |
4.0 |
60 |
|
Acetic acid (glacial) |
2 |
39 |
118 |
463 |
4.0 |
19.9 |
|
Acetone |
3 |
-18 |
56.7 |
465 |
2.6 |
12.8 |
|
Acetonitrile |
3 |
6 |
82 |
524 |
3 |
16 |
|
Carbon disulfide |
3 |
-30.0 |
46.1 |
90 |
1.3 |
50.0 |
|
Cyclohexane |
3 |
-20.0 |
81.7 |
245 |
1.3 |
8.0 |
|
Diethylamine |
3 |
-23 |
57 |
312 |
1.8 |
10.1 |
|
Diethyl ether |
4 |
-45.0 |
35.0 |
160 |
1.9 |
36.0 |
|
Dimethyl sulfoxide |
1 |
95 |
189 |
215 |
2.6 |
42 |
|
Ethyl alcohol |
3 |
12.8 |
78.3 |
365 |
3.3 |
19.0 |
|
Heptane |
3 |
-3.9 |
98.3 |
204 |
1.05 |
6.7 |
|
Hexane |
3 |
-21.7 |
68.9 |
225 |
1.1 |
7.5 |
|
Hydrogen |
4 |
— |
-252 |
500 |
4 |
75 |
|
Isopropyl alcohol |
3 |
11.7 |
82.8 |
398 |
2.0 |
12.0 |
|
Methyl alcohol |
3 |
11.1 |
64.9 |
385 |
6.7 |
36.0 |
|
Methyl ethyl ketone |
3 |
-6.1 |
80.0 |
515 |
1.8 |
10.0 |
|
Pentane |
4 |
-40.0 |
36.1 |
260 |
1.5 |
7.8 |
|
Styrene |
3 |
32.2 |
146.1 |
490 |
1.1 |
6.1 |
|
Tetrahydrofuran |
3 |
-14 |
66 |
321 |
2 |
11.8 |
|
Toluene |
3 |
4.4 |
110.6 |
480 |
1.2 |
7.1 |
|
p-Xylene |
3 |
27.2 |
138.3 |
530 |
1.1 |
7.0 |
|
a 0, will not burn; 1, must be preheated to burn; 2, ignites when moderately heated; 3, ignites at normal temperature; 4, extremely flammable. SOURCE: Adapted from NFPA (1991b), pp. 325M-11 to 94. |
||||||
and methyl alcohol (11.1 °C). The degree of hazard associated with a flammable liquid also depends on other properties, such as its ignition point and boiling point. Commercially obtained chemicals are now clearly labeled as to flammability and flash point. Consider the example of acetone given in section 3.C.1.3.1. At ambient pressure and temperature, an acetone spill can produce a concentration as high as 23.7% acetone in air. Acetone is not particularly toxic. However, with a flash point of -18 °C and upper and lower flammable limits of 2.6% and 12.8% acetone in air, respectively (see Table 3.7), it is clear that an acetone spill produces an extreme fire hazard. Thus the major hazard given for acetone in the LCSS is flammability.
3.D.1.2.2
Ignition Temperature
The ignition temperature (autoignition temperature) of a substance, whether solid, liquid, or gaseous, is the minimum temperature required to initiate or cause self-sustained combustion independent of the heat source. The lower the ignition temperature, the greater the potential for a fire started by typical laboratory equipment. A spark is not necessary for ignition when the flammable vapor reaches its autoignition temperature. For instance, carbon disulfide has an ignition temperature of 90 °C, and it can be set off by a steam line or a glowing light bulb. Diethyl ether has an ignition temperature of 160 °C and can be ignited by the surface of a hot plate.
3.D.1.2.3 Limits of Flammability
Each flammable gas and liquid (as a vapor) has two fairly definite limits of flammability defining the range of concentrations in mixtures with air that will propagate a flame and cause an explosion. At the low extreme, the mixture is oxygen rich but contains insufficient fuel. The lower flammable limit (lower explosive limit (LEL)) is the minimum concentration (percent by volume) of the fuel (vapor) in air at which a flame is propagated when an ignition source is present. The upper flammable limit (upper explosive limit (UEL)) is the maximum concentration (percent by volume) of the vapor in air above which a flame is not propagated. The flammable range (explosive range) consists of all concentrations between the LEL and the UEL. This range becomes wider with increasing temperature and
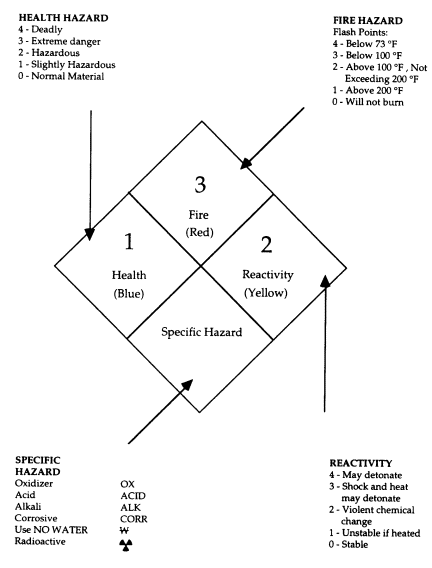
FIGURE 3.1 National Fire Protection Association system for classification of hazards. SOURCE: National Fire Protection Association (1990).
in oxygen-rich atmospheres and also changes depending on the presence of other components. The limitations of the flammability range, however, provide little margin of safety from the practical point of view because, when a solvent is spilled in the presence of an energy source, the LEL is reached very quickly and a fire or explosion will ensue before the UEL can be reached.
3.D.1.3 Classes of Flammability
Several systems are in use for classifying the flammability of materials. Some (e.g., Class I—flammable liquid, etc., see Chapter 4) apply to storage or transportation considerations. Another (Class A, B, C—paper, liquid, electrical fire) concerns the type of fire extinguisher to be used (see Chapter 6, section 6.F.2 on emergency equipment). To assess risk quickly, the most direct indicator is the NFPA (National Fire Protection Association) system, which classifies flammables according to the severity of the fire hazard with numbers 0 to 4 in order of increasing hazard: 0, will not burn; 1, must be preheated to burn; 2, ignites when moderately heated; 3, ignites at normal temperature; 4, extremely flammable (Figure 3.1). Substances rated 3 or 4 under this system require particularly careful handling and storage in the laboratory. Some vendors include the NFPA hazard diamond on the labels of chemicals. The Fire Protection Guide on Hazardous Materials (NFPA, 1991) is a comprehensive listing of flammability data and ratings.
The NFPA fire hazard ratings, flash points, boiling points, ignition temperatures, and flammability limits of a number of common laboratory chemicals are given
in Table 3.7 and in the LCSSs (see Appendix B). The data illustrate the range of flammability found for liquids commonly in use in laboratories. Dimethyl sulfoxide and glacial acetic acid (NFPA fire hazard ratings of 1 and 2, respectively) can be handled in the laboratory without great concern about their fire hazards. By contrast, both acetone (NFPA 3) and diethyl ether (NFPA 4) have flash points well below room temperature.
It should be noted, however, that tabulations of properties of flammable substances are based on standard test methods, which may have very different conditions from those encountered in practical laboratory use. Large safety factors should be applied. For example, the published flammability limits of vapors are for uniform mixtures with air. In a real situation, local concentrations that are much higher than the average may exist. Thus, it is good practice to set the maximum allowable concentration for safe working conditions at some fraction of the tabulated LEL; 20% is a commonly accepted value.
Among the most hazardous liquids are those that have flash points near or below 38 °C (100 °F) because these materials can be hazardous in the common laboratory environment. There is particular risk if their range of flammability is broad. It is important to note, as shown in Table 3.7, that some commonly used substances are potentially very hazardous, even under relatively cool conditions. Some flammable liquids will maintain their flammability even at concentrations of 10% by weight in water. Methanol and isopropyl alcohol have flash points below 38 °C (100 °F) at concentrations as low as 30% by weight in water. HPLC users generate acetonitrile/water mixtures that contain from 15 to 30% acetonitrile in water, a waste that is considered toxic and flammable and thus cannot be added to a sewer.
Because of its extreme flammability and tendency for peroxide formation, diethyl ether should be available for laboratory use only in metal containers. Carbon disulfide is almost as hazardous.
3.D.1.4 Causes of Ignition
3.D.1.4.1 Spontaneous Combustion
Spontaneous ignition (autoignition) or combustion takes place when a substance reaches its ignition temperature without the application of external heat. The possibility of spontaneous combustion should always be considered, especially when storing or disposing of materials. Examples of materials susceptible to spontaneous combustion include oily rags, dust accumulations, organic materials mixed with strong oxidizing agents (e.g., nitric acid, chlorates, permanganates, peroxides, and persulfates), alkali metals (e.g., sodium and potassium), finely divided pyrophoric metals, and phosphorus.
3.D.1.4.2
Ignition Sources
Potential ignition sources in the laboratory include the obvious torch and Bunsen burner, as well as a number of less obvious, electrically powered, sources ranging from refrigerators, stirring motors, and heat guns to microwave ovens (see section 6.C). Whenever possible, open flames should be replaced by electrical heating.
The vapors of most flammable liquids are heavier than air and capable of traveling considerable distances. This possibility should be recognized, and special note should be taken of ignition sources situated at a lower level than that at which the substance is being used. Flammable vapors from massive sources such as spills have been known to descend into stairwells and elevator shafts and ignite on a lower story. If the path of vapor within the flammable range is continuous, as along a floor or benchtop, the flame will propagate itself from the point of ignition back to its source. Metal lines and vessels discharging flammable substances should be bonded and grounded properly to discharge static electricity. There are many sources of static electricity, particularly in cold, dry atmospheres, and caution should be exercised.
3.D.1.4.3
Oxidants Other Than Oxygen
The most familiar fire involves a combustible material burning in air. However, the oxidant driving a fire or explosion need not be oxygen itself, depending on the nature of the reducing agent. All oxidants have the ability to accept electrons, and fuels are reducing agents or electron donors (see Young, 1991).
Examples of nonoxygen oxidants are shown in Table 3.8. When potassium ignites on being added to water, the metal is the reducing agent and water is the oxidant. If the hydrogen produced is ignited, it becomes the fuel for a conventional fire, with oxygen as the oxidant. In ammonium nitrate explosions, the ammonium cation is oxidized by the nitrate anion. These
TABLE 3.8 Examples of Oxidants
|
• Gases: |
fluorine, chlorine, ozone, nitrous oxide, steam, oxygen |
|
• Liquids: |
hydrogen peroxide, nitric acid, perchloric acid, bromine, sulfuric acid, water |
|
• Solids: |
nitrites, nitrates, perchlorates, peroxides, chromates, dichromates, picrates, permanganates, hypochlorites, bromates, iodates, chlorites, chlorates |
hazardous combinations are treated further in section 3.D.2.
(See Chapter 5, section 5.F, for a more detailed discussion on flammable substances.)
3.D.1.5 Special Hazards
Compressed or liquefied gases present hazards in the event of fire because the heat will cause the pressure to increase and the container may rupture (Braker and Mossman, 1980; Braker et al., 1988; Matheson Gas Products, 1983). Leakage or escape of flammable gases can produce an explosive atmosphere in the laboratory. Acetylene, hydrogen, ammonia, hydrogen sulfide, propane, and carbon monoxide are especially hazardous.
Even if not under pressure, a substance in the form of a liquefied gas is more concentrated than in the vapor phase and may evaporate extremely rapidly. Oxygen is an extreme hazard. Liquefied air is almost as dangerous because nitrogen boils away first, leaving an increasing concentration of oxygen. Liquid nitrogen standing for some time may have condensed enough oxygen to require careful handling. When a liquefied gas is used in a closed system, pressure may build up. Hence adequate venting is required. If the liquid is flammable (e.g., hydrogen and methane), explosive concentrations may develop without warning unless an odorant has been added. Flammability, toxicity, and pressure buildup may become more serious on exposure of gases to heat.
(Also see Chapter 5, section 5.G.2.5, for more information.)
3.D.2 Reactive Hazards
3.D.2.1 Water Reactives
Water reactive materials are those that react violently with water. Alkali metals (e.g., lithium, sodium, and potassium), many organometallic compounds, and some hydrides react with water to produce heat and flammable hydrogen gas, which can ignite or combine explosively with atmospheric oxygen. Some anhydrous metal halides (e.g., aluminum bromide), oxides (e.g., calcium oxide), and nonmetal oxides (e.g., sulfur trioxide) and halides (e.g., phosphorus pentachloride) react exothermically with water, and the reaction can be violent if there is insufficient coolant water to dissipate the heat produced.
(See Chapter 5, section 5.G, for further information.)
3.D.2.2 Pyrophorics
For pyrophoric materials, oxidation of the compound by oxygen or moisture in air proceeds so rapidly that ignition occurs. Many finely divided metals are pyrophoric, and their degree of reactivity depends on particle size, as well as factors such as the presence of moisture and the thermodynamics of metal oxide or metal nitride formation. Many other reducing agents, such as metal hydrides, alloys of reactive metals, low-valent metal salts, and iron sulfides, are also pyrophoric.
3.D.2.3 Incompatible Chemicals
Accidental contact of incompatible substances could result in a serious explosion or the formation of substances that are highly toxic or flammable or both. Many laboratory workers question the necessity of following storage compatibility guidelines. The reasons for such guidelines can be made obvious by reading descriptions of the condition of laboratories following California earthquakes in recent decades (see Pine, 1988, 1994). Those who do not live in seismically active zones should take these accounts to heart, as well. Other natural disasters and chemical explosions themselves can set off shock waves that empty chemical shelves and result in inadvertent mixing of chemicals.
Some compounds can pose either a reactive or a toxic hazard, depending on the conditions. Thus, hydrocyanic acid (HCN), when used as a pure liquid/ gas in industrial applications, is incompatible with bases because it is stabilized against (violent) polymerization by the addition of acid inhibitor. HCN can also be formed when cyanide salt is mixed with an acid. In this case, the toxicity of hydrogen cyanide gas, rather than the instability of the liquid, is the characteristic of concern.
Some general guidelines can be applied to lessen the risks involved with these substances. Concentrated oxidizing agents are incompatible with concentrated reducing agents. Indeed, either may pose a reactive hazard even with chemicals that are not strongly oxidizing or reducing. For example, sodium or potassium, strong reducing agents frequently used to dry organic solvents, are extremely reactive toward halocarbon solvents (which are not strong oxidizing agents). Strong oxidizing agents are frequently used to clean glassware. Clearly, it is prudent to use such potent reagents only on the last traces of contaminating material. Tables 3.9 and 3.10 are guides to avoiding accidents involving incompatible substances. Chemicals or classes of chemicals in one column can be hazardous when mixed with those opposite them in the adjacent column. The magnitude of the risk obviously depends on quantities. In ordinary laboratory use, chemical incompatibilities will not usually pose much, if any, risk if the quantity of the substance is small (a solution in an NMR tube or a microscale synthesis). However, storage of commercially obtained chemicals (e.g., in
TABLE 3.9 Partial List of Incompatible Chemicals (Reactive Hazards)
Substances in the left hand column should be stored and handled so that they cannot accidentally contact corresponding substances in the right hand column under uncontrolled conditions.
|
Acetic acid |
Chromic acid, nitric acid, peroxides, permanganates |
|
Acetic anhydride |
Hydroxyl-containing compounds such as ethylene glycol, perchloric acid |
|
Acetone |
Concentrated nitric and sulfuric acid mixtures, hydrogen peroxide |
|
Acetylene |
Chlorine, bromine, copper, silver, fluorine, mercury |
|
Alkali and alkaline earth metals, such as sodium, potassium, lithium, magnesium, calcium, powdered aluminum |
Carbon dioxide, carbon tetrachloride, other chlorinated hydrocarbons (also prohibit the use of water, foam, and dry chemical extinguishers on fires involving these metals—dry sand should be employed) |
|
Ammonia (anhydrous) |
Mercury, chlorine, calcium hypochlorite, iodine, bromine, hydrogen fluoride |
|
Ammonium nitrate |
Acids, metal powders, flammable liquids, chlorates, nitrites, sulfur, finely divided organics, combustibles |
|
Aniline |
Nitric acid, hydrogen peroxide |
|
Bromine |
Ammonia, acetylene, butadiene, butane, other petroleum gases, sodium carbide, turpentine, benzene, finely divided metals |
|
Calcium oxide |
Water |
|
Carbon, activated |
Calcium hypochlorite, other oxidants |
|
Chlorates |
Ammonium salts, acids, metal powders, sulfur, finely divided organics, combustibles |
|
Chromic acid and chromium trioxide |
Acetic acid, naphthalene, camphor, glycerol, turpentine, alcohol, other flammable liquids |
|
Chlorine |
Ammonia, acetylene, butadiene, butane, other petroleum gases, hydrogen, sodium carbide, turpentine, benzene, finely divided metals |
|
Chlorine dioxide |
Ammonia, methane, phosphine, hydrogen sulfide |
|
Copper |
Acetylene, hydrogen peroxide |
|
Fluorine |
Isolate from everything |
|
Hydrazine |
Hydrogen peroxide, nitric acid, any other oxidant |
|
Hydrocarbons (benzene, butane, propane, gasoline, turpentine, etc.) |
Fluorine, chlorine, bromine, chromic acid, peroxides |
|
Hydrocyanic acid |
Nitric acid, alkalis |
|
Hydrofluoric acid (anhydrous) |
Ammonia (aqueous or anhydrous) Hydrogen fluoride |
|
Hydrogen peroxide |
Copper, chromium, iron, most metals or their salts, any flammable liquid, combustible materials, aniline, nitromethane |
|
Hydrogen sulfide |
Fuming nitric acid,a oxidizing gases |
|
Iodine |
Acetylene, ammonia (anhydrous or aqueous) |
|
Mercury |
Acetylene, fulminic acid,a ammonia |
|
Nitric acid (concentrated) |
Acetic acid, acetone, alcohol, aniline, chromic acid, hydrocyanic acid, hydrogen sulfide, flammable liquids, flammable gases, nitratable substances |
|
Nitroparaffins |
Inorganic bases, amines |
|
Oxalic acid |
Silver and mercury and their salts |
|
Oxygen |
Oils, grease, hydrogen, flammable liquids, solids, gases |
|
Perchloric acid |
Acetic anhydride, bismuth and its alloys, alcohol, paper, wood, grease, oils (all organics) |
|
Peroxides, organic |
Acids (organic or mineral), (also avoid friction, store cold) |
|
Phosphorus (white) |
Air, oxygen |
|
Phosphorus pentoxide |
Alcohols, strong bases, water |
|
Potassium chlorate |
Acids (see also chlorates) |
|
Potassium perchlorate |
Acids (see also perchloric acid) |
|
Potassium permanganate |
Glycerol, ethylene glycol, benzaldehyde, sulfuric acid |
|
Silver and silver salts |
Acetylene, oxalic acid, tartaric acid, fulminic acid,a ammonium compounds |
|
Sodium |
See alkali metals (above) |
|
Sodium nitrite |
Ammonium nitrate and other ammonium salts |
|
Sodium peroxide |
Any oxidizable substance, such as ethanol, methanol, glacial acetic acid, acetic anhydride, benzaldehyde, carbon disulfide, glycerol, ethylene glycol, ethyl acetate, methyl acetate, furfural |
|
Sulfuric acid |
Chlorates, perchlorates, permanganates |
|
a Produced in nitric acid-ethanol mixtures. SOURCE: Reproduced, by permission, from Hazards in the Chemical Laboratory, 4th edition, L. Bretherick, Ed. (1986). |
|
500-g jars or 1-L bottles) should be carefully managed from the standpoint of chemical compatibility.
3.D.3 Explosive Hazards
3.D.3.1 Explosives
An explosive is any chemical compound or mechanical mixture that, when subjected to heat, impact, friction, detonation, or other suitable initiation, undergoes rapid chemical change, evolving large volumes of highly heated gases that exert pressure on the surrounding medium. The term applies to materials that either detonate or deflagrate. Heat, light, mechanical shock, and certain catalysts initiate explosive reactions. Hydrogen and chlorine react explosively in the presence of light. Acids, bases, and other substances catalyze the explosive polymerization of acrolein, and many metal ions can catalyze the violent decomposition of hydrogen peroxide. Shock-sensitive materials include acetylides, azides, nitrogen triiodide, organic nitrates, nitro compounds, perchlorate salts (especially those of heavy metals such as ruthenium and osmium), many organic peroxides, and compounds containing diazo, halamine, nitroso, and ozonide functional groups.
Table 3.11 lists a number of explosive compounds. Some are set off by the action of a metal spatula on the solid; some are so sensitive that they are set off by the action of their own crystal formation. Diazomethane (CH2N2) and organic azides, for example, may decompose explosively when exposed to a ground glass joint. The mechanisms of the explosions of nitro-aromatic compounds have been reviewed by Brill and James (1993).
3.D.3.2 Peroxides
Organic peroxides are among the most hazardous substances handled in the chemical laboratory. They are generally low-power explosives that are sensitive to shock, sparks, or other accidental ignition. They are far more shock-sensitive than most primary explosives such as TNT.
Also potentially hazardous are compounds that undergo autooxidation to form organic hydroperoxides and/or peroxides when exposed to the oxygen in air (see Table 3.12). Especially dangerous are ether bottles that have evaporated to dryness. A peroxide present as a contaminant in a reagent or solvent can be very hazardous and change the course of a planned reaction. Autoxidation of organic materials (solvents and other liquids are most frequently of primary concern) proceeds by a free-radical chain mechanism. For the substrate R—H, the chain is initiated by ultraviolet
|
 |
TABLE 3.11 Functional Groups in Some Explosive Compounds
|
Structural Feature |
Compound |
|
—C=C— |
Acetylenic compound |
|
—C=C-M |
Metal acetylide or carbide |
|
—C=C—X |
Haloacetylide |
|
|
Diazo compounds |
|
|
Nitroso compounds |
|
|
Nitroalkanes, C-nitro and polynitroaryl compounds, polynitroalkyl compounds, trinitroethyl compounds |
|
C-O-N=O |
Acyl or alkyl nitrites |
|
C-O-NO2 |
Acyl or alkyl nitrates |
|
C-O-O-C |
Alkyl or acyl peroxides |
|
|
Alkyl hydroperoxides |
|
|
Dialkyl peroxycarbonates |
|
CNO-M |
Metal fulminates or aci-nitro salts, oximates |
|
—N3 |
Organic azides, acyl azides Metal azides, metal azide complexes |
|
M(CO)n |
Transition metal-carbonyl compounds |
|
—C=N |
Metal cyanides, organic nitriles, cyanogen halides |
|
SOURCE: Adapted from Bretherick (1990), pp. S20-S22. |
|
light, by the presence of a radical source, and by the peroxide itself. Oxygen adds to the R radical, producing the peroxy radical R—O—O. The chain is propagated when the peroxy radical abstracts a hydrogen atom from R—H. Excluding oxygen by storing potential peroxide-formers under an inert atmosphere (N2 or argon) or under vacuum greatly increases their safe storage lifetime. In some cases, stabilizers or inhibitors (free-radical scavengers that terminate the chain reaction) have been added to the liquid to extend its storage lifetime. Because distillation of the stabilized liquid will remove the stabilizer, the distillate must be stored with care and monitored for peroxide formation.
Note that alkali metals and their amides may form peroxides on their surfaces. Do not apply standard peroxide tests to such materials because they are both water and oxygen reactive!
For purposes of managing the storage of chemicals that can form peroxides upon aging, the three classes given in Table 3.13 provide useful distinctions. As part of its Chemical Hygiene Plan (CHP), an institution should provide guidelines for handling these three classes. For example, if on-site incineration is available, disposal of chemicals in Class III after 3 months might be recommended. Various time limits for disposal of the different classes have been given.
3.D.3.3 Other Oxidizers
Oxidizing agents may react violently when they come into contact with reducing materials, and sometimes with ordinary combustibles. Such oxidizing agents include the halogens, oxyhalogens and organic peroxyhalogens, chromates, and persulfates as well as peroxides. Inorganic peroxides are generally stable. However, they may generate organic peroxides and hydroperoxides in contact with organic compounds, react violently with water (alkali metal peroxides), and form superoxides and ozonides (alkali metal peroxides). Perchloric acid is a powerful oxidizing agent with organic compounds and other reducing agents. Perchlorate salts can be explosive and should be treated as potentially hazardous compounds.
For many years, sulfuric acid—dichromate mixtures were used to clean glassware (a sulfuric acid—peroxy-disulfate solution is now recommended because disposal of chromate is a problem). Confusion about cleaning baths has led to explosions on mixing potas-
TABLE 3.12 Types of Compounds Known to Autooxidize to Form Peroxides
|
• Aldehydes |
|
• Ethers, especially cyclic ethers and those containing primary and secondary alkyl groups (never distill an ether before it has been shown to be free of peroxide) |
|
• Compounds containing benzylic hydrogens |
|
• Compounds containing allylic hydrogens (C = C—CH), including most alkenes; vinyl and vinylidene compounds |
|
• Compounds containing a tertiary C—H group (e.g., decalin and 2,5-dimethylhexane) |
TABLE 3.13 Classes of Chemicals That Can Form Peroxides Upon Aging
sium permanganate with sulfuric acid and nitric acid with alcohols.
3.D.3.4 Dusts
Suspensions of oxidizable particles (e.g., flour, coal dust, magnesium powder, zinc dust, carbon powder, and flowers of sulfur) in the air can constitute a powerful explosive mixture. These materials should be used with adequate ventilation and should not be exposed to ignition sources. Some solid materials, when finely divided, are spontaneously combustible if allowed to dry while exposed to air. These materials include zirconium, titanium, Raney nickel, finely divided lead (such as prepared by pyrolysis of lead tartrate), and catalysts such as activated carbon containing active metals and hydrogen.
3.D.3.5 Explosive Boiling
Not all explosions result from chemical reactions. A dangerous, physically caused explosion can occur if a hot liquid or a collection of very hot particles comes into sudden contact with a lower-boiling-point material. Sudden boiling eruptions occur when a nucleating agent (e.g., charcoal, ''boiling chips") is added to a liquid heated above its boiling point. Even if the material does not explode directly, the sudden formation of a mass of explosive or flammable vapor can be very dangerous.
3.D.3.6 Other Considerations
The hazards of running a new reaction should be considered especially carefully if the chemical species involved contain functional groups associated with explosions (see Table 3.11) or are unstable near the reaction or work-up temperature, if the reaction is subject to an induction period, or if gases are by-products. Modern analytical techniques (see Chapter 5, section 5.G) can be used to determine reaction exothermicity under suitable conditions.
Even a small sample may be dangerous. Furthermore, the hazard is associated not with the total energy
released, but rather with the remarkably high rate of a detonation reaction. A high-order explosion of even milligram quantities can drive small fragments of glass or other matter deep into the body. It is important to use minimum amounts of these hazardous materials with adequate shielding and personal protection. A compound is apt to be explosive if its heat of formation is more than about 100 calories per gram (cal/g) less than the sum of the heats of formation of its products. In making this calculation, a reasonable reaction should be used in order to yield the most exothermic products.
Scaling up reactions can introduce several hazards. The current use of microscale teaching methods in undergraduate laboratories unfortunately increases the likelihood that graduate students and others may be unprepared for a number of problems that can arise when a reaction is run on a larger scale. These include heat buildup and serious hazard of explosion from the use of incompatible materials. The rate of heat input and production must be weighed against that of heat removal. Bumping of the solution or a runaway reaction can result when heat builds up too rapidly. Exothermic reactions can "run away" if the heat evolved is not dissipated. When scaling up experiments, sufficient cooling and surface for heat exchange should be provided, and mixing and stirring rates should be considered. Detailed guidelines for circumstances that require a systematic hazard evaluation and thermal analysis are given in Chapter 5, section 5.G.
Another situation that can lead to problems is a reaction susceptible to an induction period; particular care must be given to the rate of reagent addition versus its rate of consumption. Finally, the hazards of exothermic reactions or unstable or reactive chemicals are exacerbated under extreme conditions, such as high temperature or high pressure used for hydrogenations, oxygenations, or work with supercritical fluids.
3.D.4 The Dirty Dozen
In laboratories carrying out moderate- to large-scale synthetic chemistry, it is generally recognized that certain substances tend to be responsible for more than their share of accidents (see also Chapter 5, section 5.G.6). In some laboratories these perennial "bad actors" are known as the "Dirty Dozen" (see Table 3.14). Although accident statistics for such laboratories show that most accidents lead to cut hands and back injuries (Kaufmann, 1990), enough workers have had incidents with these elements and compounds to make extreme caution advisable. Inappropriate mixing or handling of certain compounds can also produce hazardous toxic gases. Institutions might find it useful to prepare their own lists as part of their Chemical Hygiene Plans.
3.E PHYSICAL HAZARDS
3.E.1 Compressed Gases
Compressed gases can expose the worker to both mechanical and chemical hazards, depending on the gas. Hazards can result from the flammability, reactivity, or toxicity of the gas, from the possibility of asphyxiation, and from the gas compression itself, which could lead to a rupture of the tank or valve.
3.E.2 Nonflammable Cryogens
Nonflammable cryogens (chiefly liquid nitrogen) can cause tissue damage from extreme cold because of contact with either liquid or boil-off gases. In poorly ventilated areas, inhalation of gas due to boil-off or spills can result in asphyxiation. Another hazard is explosion from liquid oxygen condensation in vacuum traps or from ice plug formation or lack of functioning vent valves in storage Dewars. Because 1 volume of liquid nitrogen at atmospheric pressure vaporizes to 694 volumes of nitrogen gas at 20 °C, the warming of such a cryogenic liquid in a sealed container produces enormous pressure, which can rupture the vessel. (See Chapter 5, section 5.G, for detailed discussion.)
3.E.3 High-Pressure Reactions
Experiments carried out at pressures above one atmosphere can lead to explosion from equipment failure. Hydrogenation reactions are frequently carried out at elevated pressures. A potential hazard is the formation of explosive O2/H2 mixtures and the reactivity/pyrophoricity of the catalyst (see section 3.D). High pressures can also be associated with the growing use of supercritical fluids (see McHugh and Krukonis, 1994; Bright and McNally, 1992).
3.E.4 Vacuum Work
Precautions to be taken when working with vacuum lines and other glassware used at subambient pressure are mainly concerned with the substantial danger of injury in the event of glass breakage. The degree of hazard does not depend significantly on the magnitude of the vacuum because the external pressure leading to implosion is always one atmosphere. Thus, evacuated systems using aspirators merit as much respect as high-
TABLE 3.14 The "Dirty Dozen"
|
1. Organic azides |
Explosion hazards, especially with ground glass joints |
|
2. Perchlorate salts of organic, |
Explosion hazards organometallic, and inorganic complexes |
|
3. Diethyl ether |
Fires (see also entry 10 below) |
|
4. Lithium aluminum hydride |
Fires on quenching |
|
5. Sodium, potassium |
Fires on quenching |
|
6. Potassium metal |
Fires on quenching |
|
7. Sodium-benzophenone ketyl still pots |
Fires on quenching |
|
8. Palladium on carbon |
Fires on removal from the inert atmosphere, especially if wet with organic solvent or when contacting combustible materials such as filter paper |
|
9. Heat |
Exothermic reactions causing violent spills on scale-up due to inadequate provision for heat removal |
|
10. Ethers with a-hydrogen atom |
Dangerous peroxide concentration during distillation; explosion hazards, especially with ground glass joints |
|
11. Carbon monoxide |
Toxicity and role in forming nickel tetracarbonyl from steel gas lines and autoclaves |
|
12. Organic peroxides |
Sensitivity to shock, sparks, and other forms of accidental detonation; sensitivity to heat, friction, impact, and light, as well as to strong oxidizing and reducing agents |
vacuum systems. Injury due to flying glass is not the only hazard in vacuum work. Additional dangers can result from possible toxicity of the chemicals contained in the vacuum system, as well as from fire following breakage of a flask (e.g., of a solvent stored over sodium or potassium).
Because vacuum lines typically require cold traps (generally liquid nitrogen) between the pumps and the vacuum line, precautions regarding the use of cryogens should be observed also. Health hazards associated with vacuum gauges have recently been reviewed (Peacock, 1993). The hazards include the toxicity of mercury used in manometers and McLeod gauges, overpressure and underpressure situations arising with thermal conductivity gauges, electric shock with hot cathode ionization systems, and the radioactivity of the thorium dioxide used in some cathodes.
3.E.5 Ultraviolet, Visible, and Near-Infrared Radiation
Ultraviolet, visible, and near-infrared radiation from lamps and lasers in the laboratory can produce a number of hazards. Medium-pressure Hanovia 450 Hg lamps are commonly used for ultraviolet irradiation in photochemical experiments. Powerful arc lamps can cause eye damage and blindness within seconds. Some compounds, for example, chlorine dioxide, are explosively photosensitive.
When incorrectly used, the ultraviolet, visible, or near-infrared light from lasers poses a hazard to the eyes of the operators and other people present in the room and is also a potential fire hazard. Depending on the type of laser, the associated hazards can include mutagenic, carcinogenic, or otherwise toxic laser dyes
and solvents or flammable solvents, ultraviolet or visible radiation from the pump lamps, and electric shock from power supplies for lamps.
Lasers are classified according to their relative hazards: Class I lasers, including laser printers, compact disc players, and unfocused laser diodes, are either completely enclosed or have such a low output of power that even a direct beam in the eye could not cause damage. Class II lasers, including supermarket scanners and visible laser bar code scanners, are visible light lasers with power of less than 1 milliwatt (mW). These can be a hazard if a person stares into the beam and resists the natural reaction to blink or turn away. Class IIIA lasers have powers between 1 and 5 mW and can present an eye hazard if a person stares into the beam and resists the natural reaction to blink or turn away, or views the beam with focusing optical instruments. Class IIIB lasers are visible, ultraviolet, and infrared lasers with powers in the 5 to 500 mW range and produce eye injuries instantly from both direct and specularly reflected beams. Class IV lasers are visible, ultraviolet, and infrared lasers with continuous powers in excess of 500 mW or pulse energies in excess of a threshold that depends on wavelength and pulse duration. Class IV lasers present all of the hazards of Class III lasers and may also produce eye or skin damage from diffuse scattered light. Anyone who is not the authorized operator of a laser system should never enter a posted laser-controlled laboratory if the laser is in use.
3.E.6 Radiofrequency and Microwave Hazards
Radiofrequency (RF) and microwaves occur within the range 10 kilohertz (kHz) to 300,000 megahertz (MHz) and are used in RF ovens and furnaces, induction heaters, and microwave ovens. Extreme overexposure to microwaves can result in the development of cataracts and/or sterility. Microwave ovens are increasingly being used in laboratories for organic synthesis and digestion of analytical samples. Use of metal in microwave ovens can result in arcing and, if a flammable solvent is present, in fire or explosion. Superheating of liquids can occur. Capping of vials and other containers used in the oven can result in explosion from pressure buildup within the vial. Inappropriately selected plastic containers may melt.
3.E.7 Electrical Hazards
The electrocution hazards of electrically powered instruments, tools, and other equipment can almost be eliminated by taking reasonable precautions, and the presence of electrically powered equipment in the laboratory need not pose a significant risk. Many electrically powered devices are used in homes and workplaces in the United States, often with little awareness of the safety features incorporated in their design and construction. But, in the laboratory, as well as elsewhere, it is critical that these features not be defeated by thoughtless or ignorant modification. The possibility of serious injury or death by electrocution is a very real one if careful attention is not paid to engineering, maintenance, and personal work practices. Equipment malfunctions can lead to electrical fires. Every worker should know the location of electrical shutoff switches and/or circuit breaker switches and should know how to turn off power to burning equipment by using these switches.
Some special concerns arise in laboratory settings. The insulation on wires can be eroded by corrosive chemicals, organic solvent vapors, or ozone (from ultraviolet lights, copying machines, and so forth). Eroded insulation on electrical equipment in wet locations such as cold rooms or cooling baths must be repaired immediately. In addition, sparks from electrical equipment can serve as an ignition source in the presence of flammable vapor. Operation of certain equipment (e.g., lasers, electrophoresis equipment) may involve high voltages and stored electrical energy. The large capacitors used in many flash lamps and other systems are capable of storing lethal amounts of electrical energy and should be regarded as "live" even if the power source has been disconnected.
Loss of electrical power can produce extremely hazardous situations. Flammable or toxic vapors may be released from freezers and refrigerators as chemicals stored there warm up; certain reactive materials may decompose energetically upon warming. Hoods may cease to function and to protect workers. Stirring (motor or magnetic) required for safe reagent mixing may cease. Return of power to an area containing flammable vapors may ignite them.
3.E.8 Magnetic Fields
Increasingly, instruments that generate large static magnetic fields (e.g., frequently, NMR spectrometers) are present in research laboratories. Such magnets typically have fields of 25,000 to 160,000 gauss (2.5 to 16 teslas), far above Earth's magnetic field, which is about 0.5 G. The magnitude of these large static magnetic fields falls off rapidly with distance, which is fortunate, because effects on magnetic media such as credit cards and computer disks are thus limited (see Chapter 6, Table 6.1). Strong attraction occurs when the magnetic field is above 50 to 100 G and increases by the seventh
power as the separation is reduced. However, this highly nonlinear falloff of magnetic field with distance results in an insidious hazard. Objects made of ferromagnetic materials such as ordinary steel may be scarcely affected beyond a certain distance but at a slightly shorter distance may experience a significant attraction to the field. If the object is able to move still closer, the attractive force increases rapidly, and the object can become a projectile aimed at the magnet. Objects ranging from scissors, knives, wrenches, and other tools and keys to oxygen cylinders, buffing machines, and wheelchairs have been pulled from a considerable distance to the magnet itself.
Superconducting magnets use liquid nitrogen and liquid helium coolants. Thus, the hazards associated with cryogenic liquids (see section 3.E.2) are of concern, as well.
There is no epidemiological evidence that exposure to static magnetic fields results in adverse effects on human health (Persson and Stahlberg, 1989; Budinger, 1992). The health effects of electromagnetic fields remain unresolved (Hileman, 1993). The effects of electromagnetic fields on protein biosynthesis, similar to those seen in response to heat shock, and the response of cells to changes in electrical stimulation have been reported (Blank, 1983).
3.E.9 Cuts, Slips, Trips, and Falls
Among the most common injuries in laboratories are back injuries and injuries arising from broken glass and from slipping or tripping. Cuts can be minimized by the use of correct procedures (e.g., the procedure for inserting glass tubing into rubber stoppers and tubing, which is taught in introductory laboratories), through the appropriate use of protective equipment, and by careful attention to manipulation. Spills resulting from dropping chemicals not stored in protective rubber buckets or laboratory carts can be serious because the worker can fall or slip into the spilled chemical, thereby risking injury from both the fall and exposure to the chemical. Chemical spills resulting from tripping over bottles of chemicals stored on laboratory floors are part of a general pattern of bad housekeeping that can also lead to serious accidents. Wet floors around ice, dry ice, or liquid nitrogen dispensers can be slippery if the areas are not carpeted and if drops or small puddles are not wiped up as soon as they form. Attempts to retrieve 5-gallon bottles of distilled water, jars of bulk chemicals, and rarely used equipment stored on high shelves have often led to back injuries in laboratory environments. Careful planning of where to store difficult-to-handle equipment and containers (because of weight, shape, or overall size) can therefore be expected to reduce the incidence of back injuries.
3.F BIOHAZARDS
Biohazards are a concern in laboratories in which microorganisms or material contaminated with them is handled. These hazards are usually present in clinical and infectious disease research laboratories, but may also be present in any laboratory in which bodily fluids or tissues of human or animal origin are handled. Occasionally, biohazards are present in testing and quality control laboratories, particularly those associated with water and sewage treatment plants and facilities involved in the production of biological products and disinfectants. Teaching laboratories may introduce low-risk infectious agents as part of a course of study in microbiology for advanced students.
A consensus code of practice for controlling biohazards, Biosafety in Microbiological and Biomedical Laboratories, was first produced by the Centers for Disease Control and Prevention and the National Institutes of Health in 1984; the third and most recent edition was published in 1993 (U.S. DHHS, 1993).
(Also see Chapter 5, section 5.E.)
3.G HAZARDS FROM RADIOACTIVITY
The discussion in this section provides a brief primer on the hazards arising from radioactivity. A comprehensive treatment of radiation laboratory safety is given in Shapiro (1990).
Unstable atomic nuclei eventually achieve a more stable form by emission of some type of radiation. These nuclei or isotopes are termed radioactive. The energy emitted from a decaying nucleus may be alpha, beta, or gamma particles or electromagnetic radiation gamma rays or x-rays, as discussed below. Radiation that has enough energy to ionize atoms into ions and electrons is denoted ionizing radiation. Ionizing radiation can also be produced by machines such as particle accelerators and x-ray machines.
-
Alpha particles are charged particles containing two protons and two neutrons and are emitted from certain heavy atoms such as uranium and thorium. An alpha particle can be stopped by a sheet of paper but is very damaging inside the body.
-
Beta particles are electrons emitted with very high energy from many radioisotopes. Positively charged counterparts of beta particles are called positrons. Positronic and electron emissions from radioactive atoms can be shielded by thin metal foils or one-quarter inch of plastic. Tritium (3H), phosphorus-32, and carbon-14
-
are beta emitters. Beta particles are usually stopped by the skin but can cause serious damage to skin and eyes.
-
Gamma rays and x-rays, extremely energetic photons, have no mass or charge. Gamma rays are generally emitted from the nucleus during nuclear decay, and x-rays are emitted from the electron shells. Gamma rays are also produced by particle accelerators and nuclear reactors. Extremely dense materials such as lead or depleted uranium are required to shield against these very energetic, penetrating forms of radiation.
-
Neutrons, uncharged particles, are emitted from the nucleus during decay. Shielding materials for neutrons include water, paraffin, boron, and concrete.
Radioactive decay rates are reported in curies (1 curie (Ci) = 3.7 x 1010 disintegrations per second) or in the International System of Units (SI) in becquerels (Bq) (1 becquerel = 1 disintegration per second). The decay rate provides a characterization of a given source, but provides no absolute guide as to the hazard of the material. The hazard depends on the nature of, as well as the rate of production of, the ionizing radiation. In characterizing human exposure to ionizing radiation, it is assumed that the damage is proportional to the energy absorbed. The radiation absorbed dose (rad) is defined in terms of energy absorbed per unit mass: 1 rad = 100 ergs/g (SI: 1 gray (Gy) = 1 joule/kg = 100 rads). For electromagnetic energy, the roentgen (R) produces 1.61 x 1012 ion pairs per gram of air (SI: 1 coulomb/kg = 3.876 R).
For evaluation of the risk of exposure to ionizing radiation in humans, the dose equivalent in rem (roentgen equivalent man) is defined as
where the absorbed dose is given in rads, Q is the quality factor, and N is the tissue factor. Q is 1 for x-rays and gamma radiation of any energy, and for beta radiation. For alpha radiation, Q is 20. For neutrons, Q is 2 to 10, depending on their energy. In the United States, the applicable Standards for Protection Against Radiation from Sealed Gamma Sources (U.S. National Committee on Radiation Protection and Measurements, 1960), defines dose equivalents as follows: for x-ray, gamma ray, and electron radiations, Q x N = 1 and so 1 rad = 1 rem; for neutrons or high-energy protons, Q x N = 10 and 1 rem = 0.1 rad.
Damage may occur directly as a result of the radiation interacting with a part of the cell or indirectly by the formation of toxic substances within the cell. The extent of damage incurred depends on many factors, including the dose rate, the size of the dose, and the site of exposure. Effects may be short-term or long-term. The acute short-term effects associated with large doses and high dose rates, for example, 100,000 mrads (100 rads) in less than 1 week, may include nausea, diarrhea, fatigue, hair loss, sterility, and easy bruising. In appropriately managed workplaces, such exposures are impossible unless various barriers, alarms, and other safety systems are deliberately destroyed or bypassed. Above 600 rads, all exposures are probably fatal. Long-term effects, which develop years after the exposure, are primarily observed as cancer. Exposure of the fetus in utero to radiation is of concern, and the risk of damage to the fetus increases significantly when doses exceed 15,000 mrems. The U.S. Nuclear Regulatory Commission has set limits for whole-body occupational exposure at 500 mrems per quarter and 2,000 mrems/year and recommends that student exposures not exceed 500 mrems/year. Exposure limits are lower in facilities operated by the Department of Energy and other agencies. No completely safe limit of exposure is known.
As with all laboratory work, protection of the worker against the hazard consists of good facility design, operation, and monitoring, as well as good work practices on the part of the worker. The ALARA (as low as reasonably achievable) exposure principle is central to both levels of protection. The amount of radiation or radioactive material used should be minimized. Exposures should be minimized by shielding radiation sources and workers and visitors and by use of emergency alarm and evacuation procedures. Physical distance between personnel and radiation sources should be maximized, and whenever possible, robotic or other remote operations should be used to reduce exposure of personnel.
(Also see Chapter 5, section 5.E.)


































