MYRON LEE BENDER
May 20, 1924–July 29, 1988
BY FRANK H. WESTHEIMER
MYRON LEE BENDER played a major role in bringing enzymology within the compass of chemistry and made outstanding contributions to our understanding of reaction mechanisms in organic chemistry and enzymology. In particular, he and his coworkers unscrambled the kinetics of the action of the serine proteases. They showed how to reconcile the rate data with a two-step mechanism for the hydrolytic process, wherein an enzyme molecule is acylated as it cleaves a peptide bond, and subsequently is regenerated when the acylated enzyme is hydrolyzed. Since the proteases constitute one of the leading systems in the study of enzymes at a molecular level, Bender's research was of great importance to the development of bio-organic chemistry.
While this body of work probably constitutes the most important of Bender's achievements, his earlier contribution to the detailed mechanism of the hydrolysis of esters would alone be sufficient to provide his work with lasting distinction.
Later, he and Koshland working independently invented a chemical procedure to convert the single serine residue in the protease subtilisin to a cysteine and thereby tested the importance of that single change in enzyme structure
on the catalytic activity of that enzyme—a sort of site-directed mutagenesis prior to the time when this objective could be achieved by the methods of molecular biology. Additionally, he and his coworkers prepared interesting models for enzymic processes. He made numerous other contributions to mechanistic organic chemistry and mechanistic enzymology and trained an influential group of bioorganic chemists. He was honored for his elegant science, notably by election to the National Academy of Sciences, and was active and inventive throughout his life.
BIOGRAPHICAL
Bender was born and grew up in St. Louis, Missouri. He took his undergraduate degree at Purdue University and obtained his Ph.D. in chemistry there with H. B. Hass. He bore a birthmark (not unlike that of Mr. Gorbachev) on his face, an angioma that affected his circulatory system and presumably led to glaucoma. It may even have been related to the strokes he suffered late in life. But neither the birthmark, the glaucoma, nor his strokes affected his spirit, his friendships, or his originality, and his strokes interfered only temporarily with the development of his research. He never complained, and his scientific productivity was enormous.
In 1952, while he was on the staff at the Illinois Institute of Technology, Bender married Muriel Schulman. It was a splendid marriage. Muriel was a loving, loyal, and helpful wife, who accompanied him to meetings in the United States and abroad. The Benders had three fine sons who survive them; obviously he and Muriel enjoyed each other's company and that of their family. Their marriage bond was true to the end; Muriel was ill at the time of Myron's death, and survived him by only a few weeks.
ORIGINAL RESEARCH
After Purdue Bender spent a postdoctoral year at Harvard with Paul Bartlett and then won an Atomic Energy Commission postdoctoral fellowship, which he exercised in 1950 in my laboratory at the University of Chicago. He arrived with an original research plan—a method to test for the reality of the tetrahedral intermediate that had long been postulated in the hydrolysis of the esters and amides of carboxylic acids. Prior to Bender's work the experimental evidence for this postulate was rather indirect. Bender offered a firm experimental basis for the tetrahedral intermediate. He carried out the hydrolysis of ethyl benzoate and other esters marked with 18O in the carbonyl oxygen atom and showed that the remaining starting material lost label as the reaction progressed.1 This is what would be predicted if the formation of the tetrahedral intermediate is reversible and if the intermediate is sufficiently long-lived to undergo proton transfer before decomposition. In fact, the demonstration of oxygen exchange into the unreacted ester during hydrolysis would be hard to explain without a tetrahedral intermediate. This work comes as close to a proof of mechanism as can be found in physical-organic chemistry (see Figure 1). Furthermore, the reaction is an essential one in both chemistry and biochemistry.
THE TETRAHEDRAL INTERMEDIATE
In Figure 1 the primed rate constants (e.g., k1′) for species substituted with 18O and are only slightly smaller than the constants (not primed—e.g., k1) for compounds carrying the normal isotope. Exchange of isotopically-labeled oxygen, here designated as O, into the residual, unhydrolyzed ester takes place provided that k−1′ (and, of course, k−1) and k2 (and, of course, k−2) are not small compared to k4 and k4′.
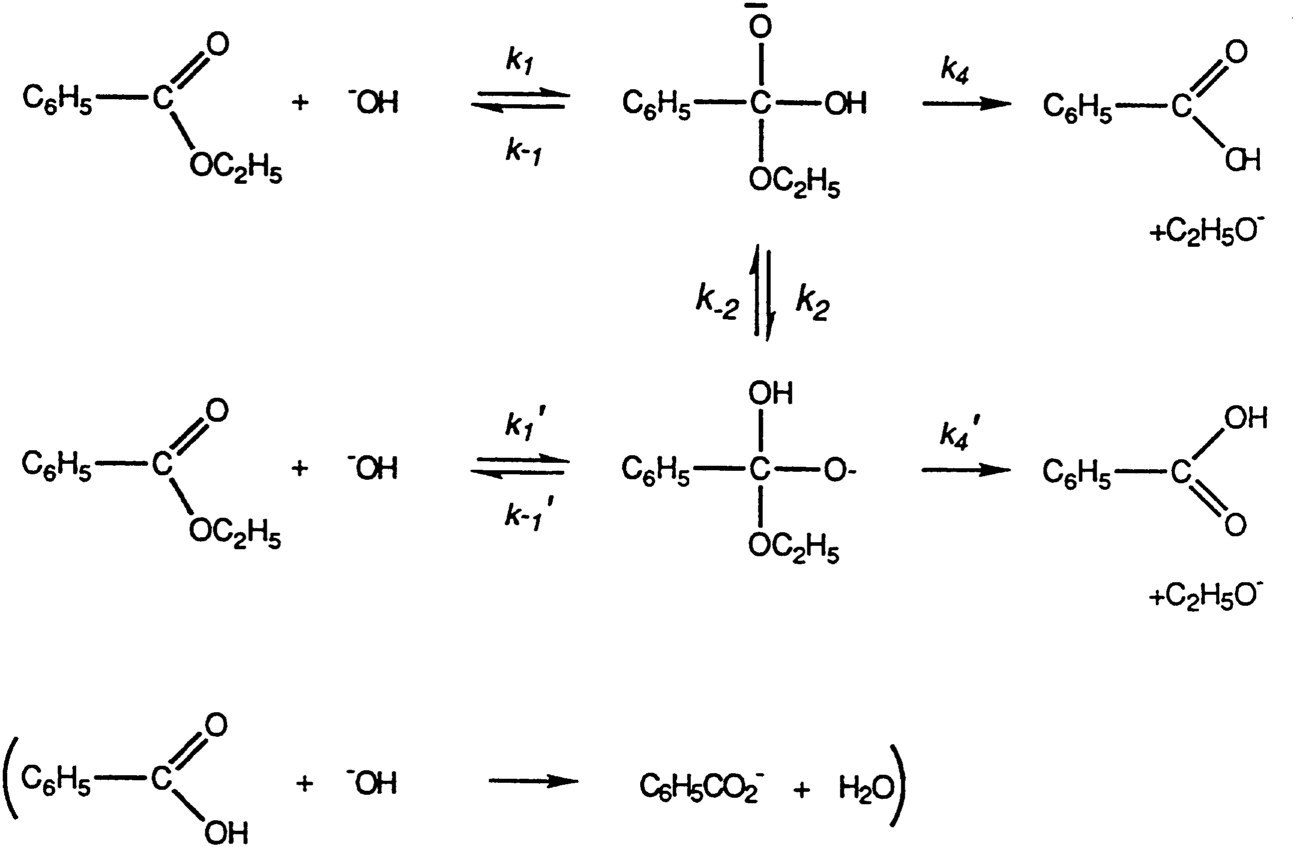
FIGURE 1 The exchange of oxygen between ester and solvent during alkaline hydrolysis.
Bender released the isotopic oxygen by pyrolysis of the ethyl benzoate to give CO2 for mass-spectrometric analysis (see Figure 2).
This research was ideally suited to the time and place. In 1951 mechanistic chemistry was coming into its own with an interested community of physical-organic chemists ready to examine and applaud new initiatives. At the University of Chicago, Harold Urey and his collaborators had constructed an isotope ratio mass spectrometer—this was before accurate mass-spectrometers were commercially available—and Urey was willing to arrange for analyses of Bender's samples. Bender could not readily have developed his idea in many other places in the world. The project solved an important problem and brought Bender instant recognition from the community of physical-organic chemists. He subsequently enlarged the research in this area.2, 3, 4
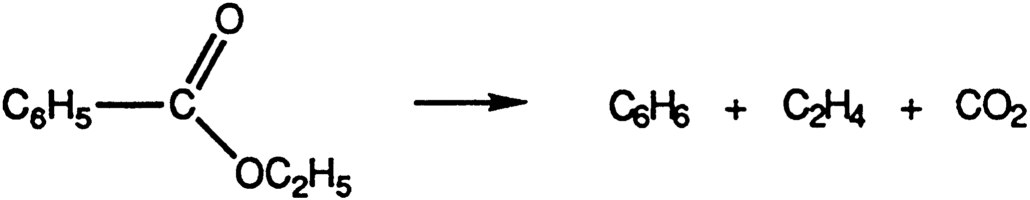
FIGURE 2 Pyrolysis of ethyl benzoate.
The conclusions of this work are correct, have largely been confirmed, and have proved of great importance in the development of physical-organic chemistry, while one must note that some of the later work5 has not gone entirely unchallenged.6 The data, both from Bender's laboratory and from that of his critics, show that the exchange of isotopic oxygen in many cases accompanies the alkaline hydrolysis of esters and it is reasonable that, in some instances, the ratios of rate constants in the scheme above are such as to obscure that exchange. Since the exchange does occur in a number of examples it offers firm evidence supporting a tetrahedral intermediate in ester hydrolysis. This is the point that Bender made, a point that contributed so much to the development of reaction mechanisms in organic chemistry.
ILLINOIS INSTITUTE OF TECHNOLOGY
Subsequent to this brilliant start on research Bender was appointed an instructor at the University of Connecticut. He was there for only one year. Fortunately for the progress of bio-organic chemistry, however, Bender immediately was appointed to the staff of the Illinois Institute of Technology. There he continued his investigations of the hydrolyses of esters and amides7 and in 1954-55 published the first of his papers on the hydrolysis of esters catalyzed by alpha chymotrypsin.4, 7
He investigated intramolecular catalysis in the hydrolysis of esters and amides, demonstrated the imidazole catalysis8 in the hydrolysis of p-nitrophenyl acetate, and investigated the enzyme-catalyzed exchange of 18O between solvent and carboxylic acids.9 Most significantly, he and his coworkers demonstrated spectrophotometrically the existence of an acyl enzyme intermediate in the chymotrypsin-catalyzed hydrolysis of o-nitrophenyl cinnamate.10
A two-step mechanism (or perhaps one should say, a two-step pathway) for the chymotrypsin-catalyzed hydrolysis of esters and amides had previously been developed on the basis of the work of A. K. Balls and Brian Harley and their coworkers. Balls11 noted that chymotrypsin and trypsin were stoichiometrically inactivated by a nerve gas (diisopropyl fluorophosphonate). He also stoichiometrically acylated chymotrypsin by treating the enzyme at low pH with p-nitrophenyl acetate or p-nitrophenyl pivalate.12 His work identified the specific serine residue at the active site of the enzyme.13
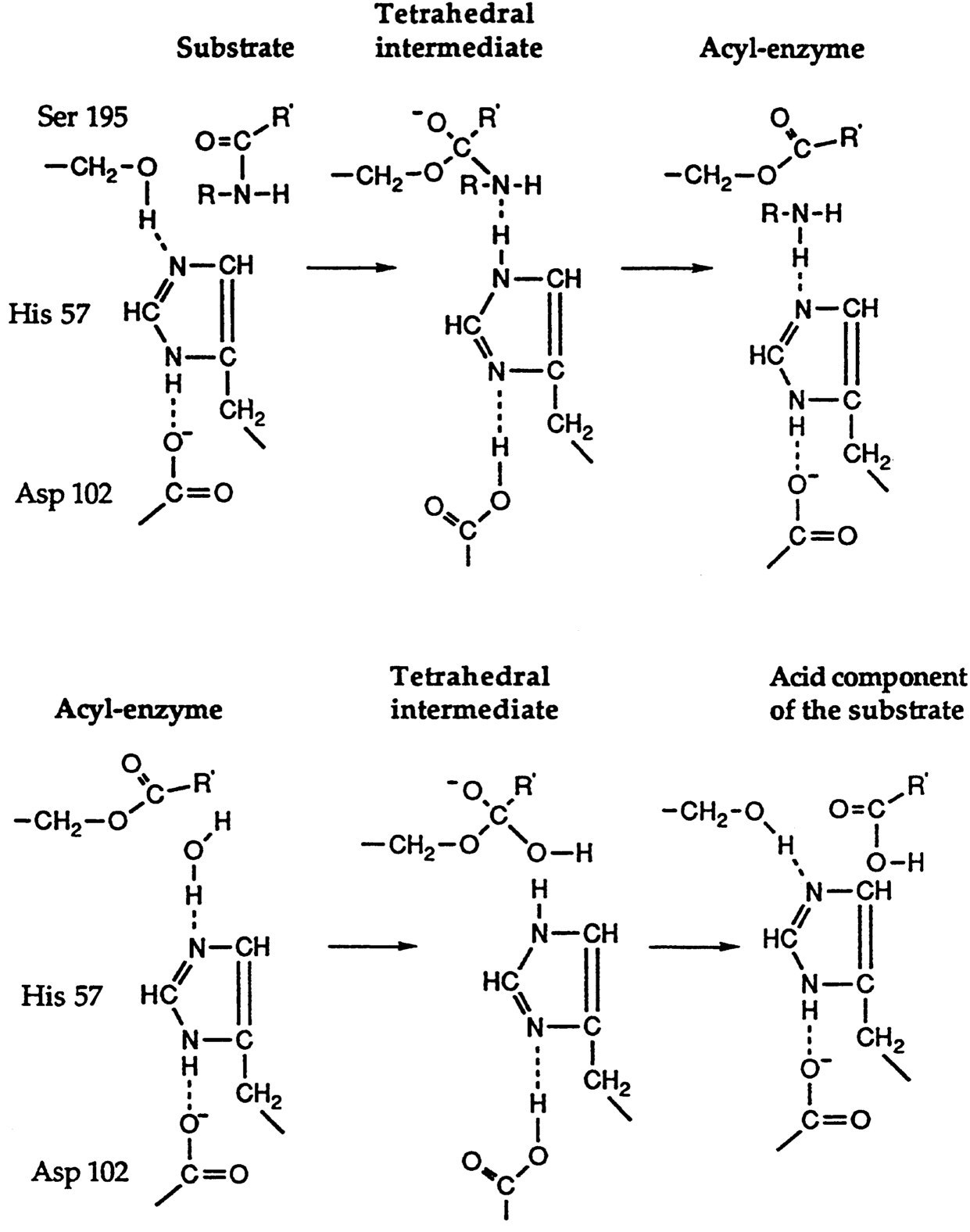
Hartley and Kilby14 demonstrated that, when chymotrypsin acts on p-nitrophenyl acetate, a “burst” of nitrophenol is released that is stoichiometric with the quantity of chymotrypsin that is employed. These experiments, like those of Balls, strongly suggested that a specific hydroxyl group in chymotrypsin is acylated during enzymatic catalysis and that this hydroxyl group is regenerated when the acetyl ester of the enzyme is subsequently hydrolyzed (see Figure 3).
Bender's spectroscopic demonstration10 that a cinnamate ester of chymotrypsin is formed during the enzymic hydrolysis of o-nitrophenyl cinnamate fits with, and strongly reinforces, the earlier work of Balls and Hartley. Of course, as one examines the Balls-Hartley pathway more closely, one realizes that each step in the formation or decomposition of an ester or amide presumably proceeds through a
a tetrahedral intermediate of the type that Bender had demonstrated for the non-enzymic hydrolysis of esters. However, since the nucleophile for the serine proteases is a serine hydroxyl group rather than a water molecule, the type of experiment that Bender invented cannot be applied to the enzymic processes. One must accept the tetrahedral intermediate by analogy, rather than demonstrate it by experiment.
Today many more details of the mechanism are known. In particular, the participation of a histidine residue as a base in the mechanism (as shown in Figure 3) comes from the work of Shaw and his coworkers,14 who showed the N-tosylphenylalanyl chloromethyl ketone reacts stoichiometrically with a histidine residue of the enzyme. The histidine serves to pull a proton from the hydroxyl group of the essential serine residue, and thus makes it much more nucleophilic. Subsequent X-ray crystallographic studies16 confirmed in every detail the mechanism of action of the serine proteases that had been developed through a study of the chemistry and kinetics (see below) of the process, and disclosed an additional feature—the participation in the active site of an aspartate residue along with those of histidine and serine. The function of the aspartate is apparently to form a hydrogen bond to the N-H proton of the histidine and make it more nucleophilic.
NORTHWESTERN UNIVERSITY
In 1960 Bender was appointed an associate professor at Northwestern University and was soon promoted to professor. In the few years after he was appointed he and his coworkers—in particular Burt Zerner and F. J. Kézdy—put the mechanism of action of the serine esterases on a firm footing.
KINETICS
Zerner and Bender17,18 reconciled the reaction kinetics for the action of the serine esterases with the two-step pathway shown in the equations above. A vast body of kinetic data had been published19 on the enzymatic hydrolyses of esters and amides, but attempts to interpret these data had served only to confuse the literature. The two-step mechanism for the enzymatic hydrolysis of esters and amides that had been suggested by Balls' labeling experiments and Hartley 's “burst” experiments could, however, be confirmed by reaction kinetics. Scientific theory is always best established when the same conclusion can be obtained in two or more entirely different ways. The importance of Bender and Zerner's kinetic analysis can scarcely be overestimated. The serine esterases/peptidases occupy a special place in the history of mechanistic enzymology, and these kinetics are essential to the understanding of these processes. Some scientists today write as if all the mechanisms of enzyme action were established by X-ray crystallography. With respect to the serine proteases—perhaps the most important example of mechanistic enzymology—X-ray crystallography largely confirmed what had already been established by protein chemistry and enzyme kinetics.
The kinetic analysis to the two-step mechanism led to the equation shown below:
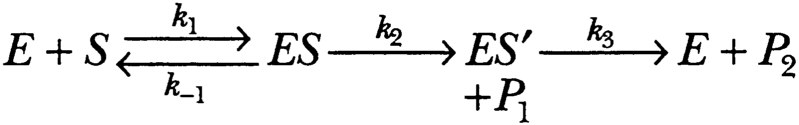
Here P1 is ammonia, an alcohol, or a peptide. P2 is an acylated amino acid or peptide residue. E is the serine esterase or peptidase. ES is a Michaelis complex and ES′ is the acylated enzyme.
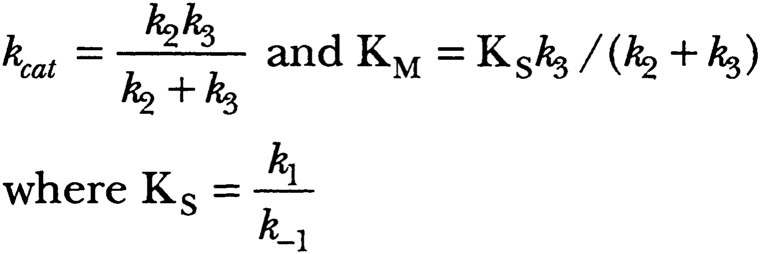
These equations can be simplified when either the first step (acylation of the enzyme to form an acylated enzyme as intermediate) or the second step (hydrolysis of this intermediate) is clearly rate-limiting.
For amides where k3» k2, kcat = k2, and k2 is different for and characteristic of each substrate; further, KM = KS (binding constant).
For esters, however, where k2 » k3, kcat = k3, and kcat is therefore the same for all esters of any particular acid, while KM is smaller than KS, and does not represent the binding constant of substrate to enzyme. The predictions from these equations could be tested from the mass of data that had already been accumulated and from specific experiments that Bender and Zerner designed to test them.
In particular, the formulation predicts that, at saturating substrate concentrations, all esters of N-acetyltryptophan, for example, will react at the same rate (i.e., the rate of the hydrolysis of the chymotryptic ester of acetyltryptophan). In other words, the value of kcat will be the same for all these esters. On the other hand, the Michaelis constant will correctly represent the affinity of the substrate for the enzyme. When, however, the first step is rate limiting (as in the hydrolysis of the tryptophanyl amide) each substrate will react at a different maximal velocity and the Michaelis constant will correctly represent the binding of substrate to enzyme.
Bender and his coworkers demonstrated that the abundant data for the hydrolysis of various substrates by chymo-
TABLE 1 N-Acetyl-L-Tryptophan Derivatives
|
kcat' sec−1 |
KM × 105, M |
|
|
Amide |
0.026 |
730 |
|
Ethyl ester |
26.9 |
9.7 |
|
Methyl ester |
27.7 |
9.5 |
|
p-Nitrophenyl ester |
30.5 |
0.2 |
trypsin accord with these conclusions. Some of the data for derivatives of N-acetyltryptophan are assembled in Table 1. Many more data are in the original papers.
Note that the ethyl ester and the p-nitrophenyl ester of acetyl-tryptophan are hydrolyzed with the same rate constant, but with vastly different Michaelis constants, whereas the amide reacts much more slowly, but with a much larger Michaelis constant. These data are consistent with rapid reaction of esters with the enzyme, followed by rate-limiting hydrolysis of a common intermediate, whereas the amide is hydrolyzed with rate-limiting acylation of the enzyme.
Bender had previously provided spectroscopic evidence for the formation of an acetylated enzyme with unnatural substrates. Now Kezdy and Bender22 answered some of the last objections to this mechanism when they demonstrated spectroscopically the formation of an intermediate with natural substrates and showed that the decomposition of the acyl-enzyme intermediate occurred at a rate consistent with that calculated by the kinetic scheme of Bender and Zerner. It was primarily on the basis of these studies of enzyme mechanism that Bender was elected to the National Academy of Sciences in 1968. Undoubtedly his detailed kinetic analysis of an enzyme-catalyzed reaction constitutes one of his major scientific achievements.
SITE-DIRECTED MUTAGENESIS
Along with these studies of reaction kinetics and the spectroscopic identification of acyl-enzyme intermediates, Bender made several other significant contributions to enzymology. In particular, Polgar and Bender20 invented a kind of site-directed mutagenesis. Their methodology differs from, and is entirely independent of, modern work with nucleic acids and is much less general and convenient than the latter. But they accomplished their work many years before it was possible to manipulate nucleic acids, and Polgar and Bender's research, together with that of Neet and Koshland (see below), demonstrated the importance of making small changes in the structures of enzymes. Until this time enzyme mechanisms had been tested by changing the substrate; now they could also be tested by changing the structure of the catalyst.
Polgar and Bender converted subtilisin into thiosubtilisin by chemical transformation of the essential serine residue to a cysteine. The same chemistry was independently achieved by Neet and Kosland.21 Today this type of transformation is accomplished almost routinely through synthesis and expression of appropriately modified nucleic acids. In 1968 the modification of a single amino acid in an enzyme was an important departure and illustrated the type of information about enzyme action that could be obtained by specific substitution of one amino acid by another. Thiosubtilisin turned out to be a much poorer enzyme than subtilisin—in fact, it hardly qualifies as an enzyme at all—but the research in 1966 demonstrated the importance of the precise nature of enzyme-substrate interactions.
MODEL SYSTEMS
Concurrent with his research on enzymes, Bender initi-
ated a series of studies of model systems. This work included the rapid internal reactions of phthalates and such molecules and internal catalytic reactions that more closely resemble enzymic action. He chose to work with cyclodextrins, whose cavities serve as binding sites for substrates. His research followed and enormously amplified the prior studies of Fritz Cramer23 and his coworkers. Bender (and more or less concurrently, several other researchers) attached various functional groups to the cyclodextrin to catalyze the hydrolysis of esters).24, 25, 26, 27 Similarly, he worked with bicyclic systems, where the molecular geometry placed catalytic sites close to ester linkages, much as they must be in the active site of serine esterases.28 In the structure shown in Figure 4, for example, Bender used an imidazole residue to simulate the histidine in chymotrypsin, and a carboxylate residue, properly placed, to simulate the aspartate in chymotrypsin.
Most models for esterases are active only in the hydrolysis of highly activated esters such as p-nitrophenyl esters; this model hydrolyzed an ordinary ester. It presented a fast internal reaction that effected the hydrolysis of an aliphatic ester.22 Of course, this was not catalysis, since the reaction is stoichiometric, and is an internal process; a large number of fast internal reactions are well known. This study demonstrated that our understanding of the groups needed for catalysis is accurate and sufficient. In particular, Bender and his coworkers showed the importance of the aspartate residue in the catalytic triad of the serine esterases. They synthesized a model28 similar to that shown in Figure 4, but lacking the carboxylic acid residue, and then showed that the rate of hydrolysis of the ester could be increased 2500-fold by the addition of 0.5 M benzoate ion. They thus provided a kinetic verification for the efficacy of the catalysis that had been postulated, on the basis of X-ray structure,
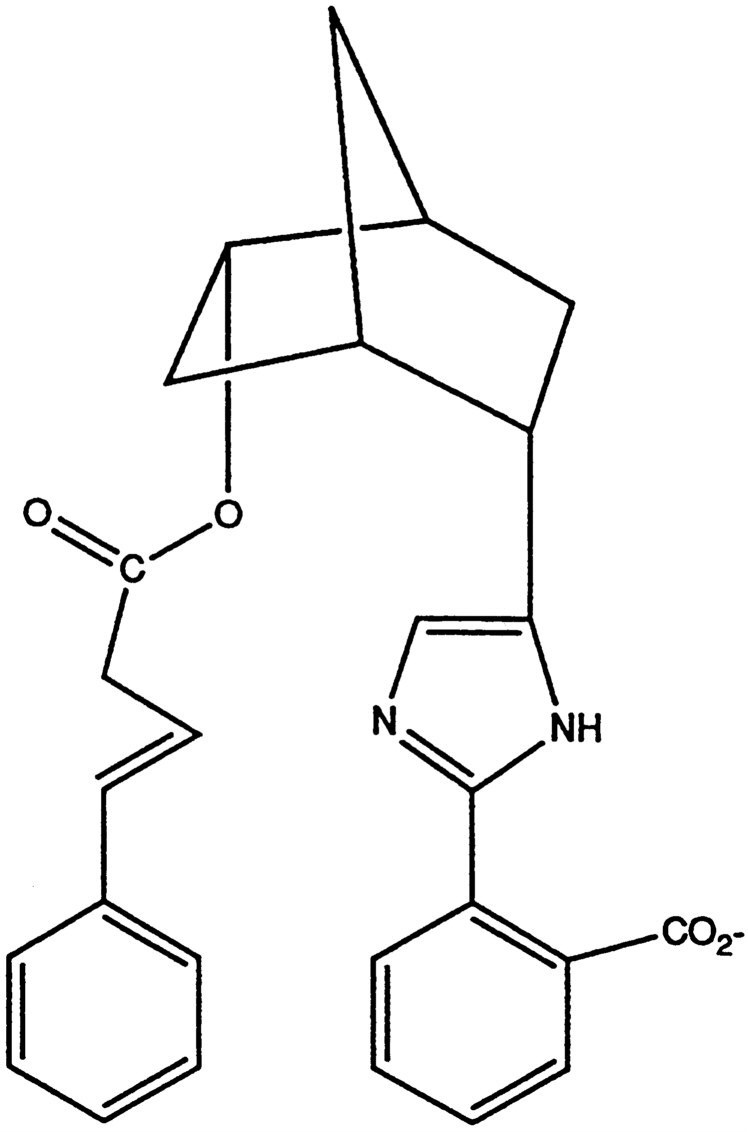
FIGURE 4 A model for a serine esterase.
for the aspartate residue of the catalytic triad. In this case, contrary to most of our understanding of the mechanism of action of the serine esterases, X-ray analysis preceded chemistry. The work on benzoate catalysis was carried out shortly before Bender's death and showed how active he was right up to the end of his life.
In summary, the two-step mechanism for the serine esterases was postulated on the basis of good evidence by Brian Hartley and A. K. Balls and the essential serine had been identified. But the mechanism was strongly reinforced
by Bender's spectroscopic studies, and a proper understanding of the reaction kinetics came directly from his work. Furthermore, the detailed mechanism and understanding of enzymic hydrolysis rest in substantial part on Bender's prior work on the oxygen exchange that accompanies ester hydrolysis. In fact, our understanding of the mechanism of enzymic hydrolysis of esters and amides comes in large part from Bender' s probing experiments and critical examinations of the resulting data. All of these accomplishments distinguish Myron Bender as a major contributor to the development of bio-organic chemistry in our time.
THE WRITER THANKS PROFESSORS Jack Kirsch and Jeremy Knowles for their helpful suggestions concerning this manuscript.
NOTES
1. M. L. Bender. Oxygen exchange as evidence for the existence of an intermediate in ester hydrolysis. J. Am. Chem. Soc. 73:1626 (1951).
2. M. L. Bender and H. d'A. Heck. Carbonyl oxygen exchange in general-base catalyzed ester exchange. J. Am. Chem. Soc. 89:1211 (1967).
3. M. L. Bender, R. D. Ginger, and K. C. Kemp. Oxygen exchange during the hydrolysis of amides and the enzymatic hydrolysis of esters. J. Am. Chem. Soc. 76:3350 (1954).
4. M. L. Bender, R. R. Stone, and R. S. Dewey. Kinetics of isotopic oxygen exchange between substituted benzoic acids and water. J. Am. Chem. Soc. 78:319 (1956).
5. M. L. Bender and R. J. Thomas. The concurrent alkaline hydrolysis and isotopic oxygen exchange of a series of p-substituted methyl benzoates. J. Am. Chem. Soc. 83:4189 (1961).
6. S. A. Shain and J. F. Kirsch. Absence of carbonyl exchange concurrent with the alkaline hydrolysis of substituted methyl benzoates. J. Am. Chem. Soc. 90:5848 (1968).
7. M. L. Bender and B. W. Turnquest. The acidic, basic, and chymotrypsin-catalyzed hydrolysis of some esters. J. Am. Chem. Soc. 77:4271 (1955).
8. M L. Bender and B. W. Turnquest. The imidazol-catalyzed hydroysis of p-nitrophyenyl acetate. J. Am. Chem. Soc. 79:1652 (1957).
9. M. L. Bender and K. C. Kemp. The kinetics of the α-chymotrypsin-catalyzed oxygen exchange of carboxylic acids. J. Am. Chem. Soc. 79:116 (1957).
10. M. L. Bender and B. Zerner. The formation of the acyl-enzyme intermediate, trans-cinnamoyl-α-chymotrypsin. J. Am. Chem. Soc. 83:2391 (1961).
11. E. F. Jansen, M.-D. F. Nutting, and A. K. Balls. J. Biol. Chem. 179:189, 201 (1949). A. K. Balls and E. F. Jansen. Adv. Enzym. 13:321 (1952).
12. A. K. Balls and F. L. Aldrich. Acetyl-chymotrypsin. Proc. Natl. Acad. Sci. USA. 41:190 (1955). L. E. McDonald and A. K. Balls. J. Biol. Chem. 227:727 (1957).
13. N. K. Schaffer, S. C. May, and W. H. Summerson. J. Biol. Chem. 202:67 (1963).
14. B. S. Hartley and B. A. Kilby. Biochem. J. 56:288 (1954).
15. P. M. Blow, J. Birktoft, and B. S. Hartley. Role of a buried acid group in the mechanism of action of chymotrypsin. Nature 221:337 (1969).
16. E. B. Ong, E. Shaw, and G. Schoelmann. J. Am. Chem. Soc. 86:1271 (1964). J. Biol. Chem. 240:694 (1965).
17. B. Zerner and M. L. Bender. The relative rates of hydrolysis of ethyl, methyl, and p-nitrophenyl esters of N-acetyl-L tryptophan. J. Am. Chem. Soc. 85:358 (1963).
18. B. Zerner and M. L. Bender. The kinetic consequences of the acyl-enzyme mechanism for the reactions of specific substrates with chymotrypsin. J. Am. Chem. Soc. 86:3669 (1964). B. Zerner, R. P. M. Bond, and M. L. Bender. Kinetic evidence for the formation of acyl-enzyme intermediates in the α -chymotrypsin-catalyzed hydrolysis of specific substrates. J. Am. Chem. Soc. 86:3674 (1964).
19. C. Niemann. Alpha chymotrypsin and the nature of enzyme catalysis. Science 143:1287 (1964).
20. L. Polgar and M. L. Bender. A new enzyme containing a synthetically-formed active site. J. Am. Chem. Soc. 88:2319 (1966).
21. K. E. Neet and D. E. Koshland, Jr. The conversion of serine at the active site of subtilisin to cysteine: A chemical mutation. Proc. Natl. Acad. Sci. USA 56:1606 (1966).
22. F. J. Kezdy and M. L. Bender. The observation of acyl-enzyme
intermediates in the α-chymotrypsin-catalyzed hydrolysis of specific ester substrates at low pH. J. Am. Chem. Soc. 86:937 (1964). F. J. Kezdy and M. L. Bender. The acylation of α-chymotrypsin by N-acetyl-L-tryptophan. J. Am. Chem. Soc. 86:938 (1964).
23. F. Cramer and H. Hettler. Inclusion compound of cyclodextrins. Naturwiss. 54:625 (1967).
24. R. Breslow and A. W. Czarnik. J. Am. Chem. Soc. 105:1390 (1983). D. Hilbert and R. Breslow. Bioorg. Chem. 12:206 (1984).
25. R. C. VanEtten, J. F. Sebastian, G. A. Clowes, and M. L. Bender. Acceleration of phenyl ester cleavage by cycloamyloses. J. Am. Chem. Soc. 89:3242 (1967). R. L. VanEtten, J. F. Sebastina, G. A. Clowes, and M. L. Bender. J. Am. Chem. Soc. 89:3253 (1967).
26. Y. Kitaura and M. L. Bender. Ester hydrolysis catalyzed by modified cyclodextrins. Bioorg. Chem. 4:237 (1975).
27. V. T. D'Souza and M. L. Bender. Miniature organic models for enzymes. Acc. Chem. Res. 20:146 (1987).
28. M. Kumiyama, M L. Bender, M. Utakea, and A. Takeda. Model for charge-relay. Proc. Natl. Acad. Sci. USA 74:2634 (1977).
SELECTED BIBLIOGRAPHY
1951 Oxygen exchange as evidence for the existence of an intermediate in ester hydrolysis. J. Am. Chem. Soc. 73 : 1626.
1957 With B. W. Turnquest. The imidazole-catalyzed hydrolysis of p-nitrophenyl acetate. J. Am. Chem. Soc. 79:1652.
1958 With Y. L. Chow and F. Chloupek. Intramolecular catalysis of hydrolytic reactions. II. The hydrolysis of phthalamic acid. J. Am. Chem. Soc. 80:5380.
1959 With G. R. Schonbaum and J. Nakamura. Direct spectrophotometric evidence for an acyl-enzyme intermediate in the chymotrypsin-catalyzed hydrolysis of o-nitrophenyl cinnamate. J. Am. Chem. Soc. 81:4746.
1960 Mechanisms of catalysis of nucleophilic reactions of carboxylic acid derivatives. Chem. Rev. 60:53-113.
1963 With F. J. Kézdy and B. Zerner. Intramolecular catalysis in the hydrolysis of p-nitrophenyl salicylates. J. Am. Chem. Soc. 85:3017.
1964 With F. J. Kézdy and C. R. Gunter. The anatomy of an enzymatic catalysis: µ-chymotrypsin. J. Am. Chem. Soc. 86:3714.
1965 With J. A. Reinstein, M. S. Silver, and R. Mikulak. Kinetics and mechanism of the hydroxide ion and morpholine-catalyzed hydrolysis of methyl o-formylbenzoate. Participation by the neighboring aldehyde group. J. Am. Chem. Soc. 87:4545.
With F. J. Kézdy. Mechanism of action of proteolytic enzymes. Annu. Rev. Biochem. 34:49-76.
1967 With L. Polgar. The reactivity of thiol-subtilisin, an enzyme containing a synthetic functional group. Biochemistry 6:610.
With R. C. Van Etten, J. F. Sebastian, and G. A. Clowes. Acceleration of phenyl ester cleavage by cycloamyloses, a model for enzymatic specificity. J. Am. Chem. Soc. 89:3242.
With R. L. Van Etten, G. A. Clowes, and J. B. Sebastian. The mechanism of the cycloamylose-accelerated cleavage of phenyl esters. J. Am. Chem. Soc. 89:3253.
1969 With L. Polgar. Chromatography and activity of thiol-subtilisin. Biochemistry 8:136.
1971 With P. Valenzuela. The difference between α- and δ-chymotrypsins. Preparation and alkaline dependence of α-chymotrypsin-catalyzed hydrolysis of N-acetyl-L-tryptophan methyl ester (ATME). The involvement of alanine-149 in α-chymotrypsin catalysis. J. Am. Chem. Soc. 93:3783.
1974 With K. Tanizawa. The application of insolubilized chymotrypsin to kinetic studies on the effect of aprotic dipolar organic solvents. J. Biol. Chem. 249:2130.
1977 With M. Komiyama, M. Utaka, and A. Takeda. Model for charge relay. Acceleration of carboxylate anion in intramolecular general base-catalyzed ester hydrolysis by the imidazolyl group. Proc. Natl. Acad. Sci. USA 74:2634.
1979 With T. A. Grooms. Modification, purification, and characterization of the enzyme with altered specificity. J. Molecular Catalysis 6:359.
1981 With H.-L . Wu. and D. A. Lace Elimination of cannibalistic denaturation by immobilization or inhibition . Proc. Natl. Acad. Sci. USA78:4118.
1984 With M. Komiyama. Cyclodextrins as enzyme models. In The Chemistry of Enzyme Action. Edited by M. I. Page. Elsevier Science Publishers : 505-27.
With I. M. Mallick, V. T. D'Souza, M. Yamaguchi, J. Lee, P. Chalabi, and R. C. Gadwood. An organic chemical model of the acylchymotrypsin intermediate. J. Am. Chem. Soc. 106:7252.
1985 With V. T. D'Souza, K. Hanabusa, T. O'Leary, and R. C. Gadwood. Synthesis and evaluation of a miniature organic model of chymotrypsin . Biochem. Biophys. Res. Commun. 128:727.
1987 Chapter 4. In Cyclodextrins (cycloamyloses) as Enzyme Models. Edited by M. I. Page and A. W. Williams. The Royal Society of Chemistry.
Kinetic studies of immobilized enzymes in apolar solvents. In Methods in Enzymology. Edited by K. Mosbach. 135:537.
With V. T. D'Souza, X. L. Lu, and R. D. Ginger. Thermal and pH stability of β-benzyme. Proc. Natl. Acad. Sci. USA 84:673.
With V. T. D'Souza. Miniature organic models of enzymes. Accts. Chem. Res. 20:146.
With H.-L. Wu and G.-Y. Shi. Preparation and purification of microplasmin. Proc. Natl. Acad. Sci. USA 84:8292.
With H.-L. Wu, G.-Y. Shi, and R. C. Wohl. Structure and formation of microplasmin. Proc. Natl. Acad. Sci. USA84:8793.
BOOKS
Mechanisms of Catalysis of Carboxylic Acid Derivatives (in Russian). Moscow:Mir Publishing Company. 1064:1-192.
Mechanisms of Homogeneous Catalysis from Protons to Proteins. New York: Wiley-Interscience (1971):xii, 686.
With L. J. Brubacher. Catalysis and Enzyme Action. New York: McGraw-Hill Book Company (1973):xiv, 203. In Japanese: Kyoto: Kagaku
Dojim (1975):vii, 201. In Spanish: Barcelona: Editorial Reverte, S.A. (1977):vi, 199.
With M. Komiyama. Cyclodextrin Chemistry. New York: Springer-Verlag KG (1978):viii, 96. In Japanese: Tokyo: Japan Scientific Societies Press (1979):iv, 166.
With R. J. Bergeron and M. Komiyama. The Bioorganic Chemistry of Enzymatic Catalysis. New York: Wiley-Interscience (1984):xii, 312. In Russian: Moscow: Mir Publishing Company (in press 1986).
With V. T. D'Souza. Chymotrypsins: Real and Artificial. In preparation.