The Chemistry of Poisons in Amphibian Skin
JOHN W. DALY
Poisonous substances occur throughout nature and are particularly well-known from plants, where they presumably serve in chemical defense against herbivores. Poisons can also serve as venoms, which are introduced into victims by coelenterates; molluscs; various arthropods, including insects, spiders, and scorpions; gila monsters; and snakes, by a bite or sting, or as toxins, such as those produced by bacteria, dinoflagellates, and other microorganisms. Examples of poisons of plant origin encompass a wide range of substances, including many alkaloids; a variety of terpenes and steroids, some of which occur as saponins; and unusual secondary metabolites such as the trichothecenes, pyrethroids, and dianthrones (1, 2). Another wide range of presumably defensive substances occur in marine invertebrates, including steroid and terpenoid sapogenins, tetrodotoxins, a variety of polyether toxins, and alkaloids (3, 4). Poisons also occur in terrestrial invertebrates and vertebrates, where they serve as chemical defenses by insects and other arthropods (5, 6), by fish (7), and by amphibians (8). Recently, a toxic alkaloid was characterized from the skin and feathers of a bird (9), where it confers some protection against predation by humans. Chemical defenses can be directed either against predators or against microorganisms. The present paper is concerned with the chemical nature, origin, and function of poisons present in amphibian skin. Many of the substances in amphibians
John Daly is chief, Laboratory of Bioorganic Chemistry, at the National Institutes of Health, Bethesda, Maryland
might better be categorized as ''noxious" rather than "poisonous," although at high enough dosages all of these compounds would be poisons.
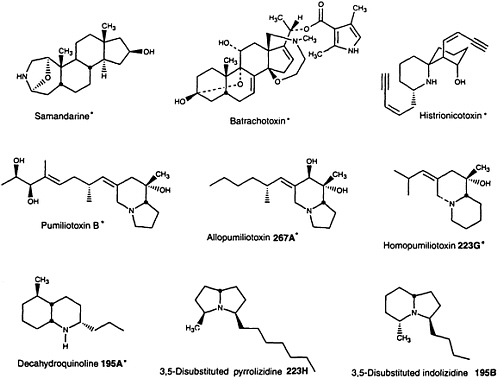
Toads and salamanders have been considered noxious creatures for centuries and indeed the majority of amphibians have now been found to contain noxious and sometimes poisonous substances in their skin secretions (8). The type of biologically active substance found in amphibians appears to have phylogenetic significance. Thus, indole alkylamines are typically present in high levels in bufonid toads of the genus Bufo, phenolic amines in leptodactylid frogs, vasoactive peptides in a great variety in hylid frogs, particularly of genus Phylomedusa (10), and bufadienolides in parotoid glands and skins of bufonid toads of the genus Bufo as well as in skin of related bufonid genera Atelopus and probably Dendrophryniscus and Melanophryniscus (11). The water-soluble alkaloid tetrodotoxin occurs in newts of the family Salamandridae, toads of the brachycephalid genus Brachycephalus and the bufonid genus Atelopus, and now in one frog species of the dendrobatid genus Colostethus (12). Lipophilic alkaloids have been found only in salamanders of the salamandrid genus Salamandra; in frogs of the dendrobatid genera Phyllobates, Dendrobates, Epipedobates, and Minyobates, the mantellid genus Mantella and the myobatrachid genus Pseudophryne; and in toads of the bufonid genus Melanophryniscus. More than 70 other genera from 11 amphibian families do not have skin alkaloids. The distribution of various lipophilic alkaloids in amphibians is given in Table 1 and structures are shown in Figure 1.
The origin and function of poisons and noxious substances found in amphibians are only partially known. The high levels of amines, including such well-known biogenic amines as serotonin, histamine, and tyramine and derivatives thereof, found in skin of various toads and frogs (8), undoubtedly are synthesized by the amphibian itself. They are stored in granular skin glands for secretion upon attack by a predator, whereupon their well-known irritant properties on buccal tissue would serve well in chemical defense. The high levels of vasoactive peptides, such as bradykinin, sauvagine, physaelaemin, caerulein, bombesin, dermorphins, etc., presumably also serve in defense against predators, although many, including the magainins, have high activity as antimicrobials (13) and thus might also serve as a chemical defense against microorganisms. Skin secretions from one hylid frog are used in "hunting magic" folk rituals by Amazonian Indians; such secretions contain many vasoactive peptides (10) and a peptide, adenoregulin, that can affect central adenosine receptors (14). The peptides of frog skin are synthesized by the amphibian and indeed additional peptides are being deduced based on cDNAs for their precursors (15). The various hemolytic proteins of certain amphib-
TABLE 1 Occurrence of lipid-soluble alkaloids in amphibians
|
|
PTX-A class |
|
Izidine alkaloid |
|
|||||||||
|
Family and genus |
SAM |
BTX |
HTX |
PTX |
aPTX |
hPTX |
DHQ |
3,5-P |
3,5-I |
5,8-I |
1,4-Q |
Epi |
Pseudophry |
|
Salamandridae |
|||||||||||||
|
Salamandra |
+ |
— |
— |
— |
— |
— |
— |
— |
— |
— |
— |
— |
— |
|
Dendrobatidae |
|||||||||||||
|
Phyllobates |
— |
+ |
+ |
+ |
— |
— |
+ |
— |
+ |
— |
— |
— |
— |
|
Dendrobates |
— |
— |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
— |
— |
|
Epipedobates |
— |
— |
+ |
+ |
+ |
— |
+ |
— |
— |
+ |
+ |
+ |
— |
|
Minyobates |
— |
— |
— |
+ |
+ |
— |
+ |
— |
— |
+ |
+ |
— |
— |
|
Mantellidae |
|||||||||||||
|
Mantella |
— |
— |
— |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
— |
— |
|
Myobatrachidae |
|||||||||||||
|
Pseudophryne |
— |
— |
— |
+ |
+ |
— |
— |
— |
+ |
— |
— |
— |
+ |
|
Bufonidae |
|||||||||||||
|
Melanophryniscus |
— |
— |
— |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
— |
— |
|
SAM, samandarines; BTX, batrachotoxins; HTX, histrionicotoxins; PTX, pumiliotoxins; aPTX, allopumiliotoxins; hPTX, homopumiliotoxins; DHQ, 2,5-disubstituted decahydroquinolines; 3,5-P, 3,5-disubstituted pyrrolizidines; 3,5-I and 5,8-I, disubstituted indolizidines; 1,4-Q, 1,4-disubstituted quinolizidines; Epi, epibatidine; Pseudophry, pseudophrynamines. With the exception of 3,5-P and 3,5-I, these alkaloids are not known to occur in arthropods (see text). Histrionicotoxins may occur in Minyobates and Mantella, but the evidence is not conclusive. |
|||||||||||||

FIGURE 1 Structures of lipophilic amphibian alkaloids. Alkaloids indicated by asterisks represent structural classes that have not been detected in nature except in and, in the case of batrachotoxins, in one species of bird (9).
ians are certainly of endogenous origin. The steroidal bufadienolides appear to be synthesized from cholesterol by the bufonid toads (16). It has been suggested that structurally similar and toxic lucibufagins of fireflies might also be produced by the insect from dietary cholesterol (17). However, toxic cardenolides in monarch butterflies appear to be sequestered from milkweed plants by the larvae (18). The chemical defensive attributes of the highly toxic bufadienolides are due to effects on membrane Na+/K+-ATPase.
The origin of tetrodotoxins in amphibians and higher organisms remains enigmatic. Thus, puffer fish raised in hatcheries do not contain tetrodotoxin (19), and likely biosynthetic precursors are not incorporated into tetrodotoxin with newts (20). Feeding nontoxic puffer fish with tetrodotoxin does not result in sequestration, but feeding toxic ovaries from wild puffer fish does (19). A bacterial origin for tetrodotoxin has been suggested, but such a source fails to explain the fact that one Central American species of toad of the genus Atelopus contains mainly tetrodotoxin; another Central American species contains mainly chiriquitoxin, which is a unique but structurally similar toxin; and yet another contains mainly zetekitoxin, which is another unique, probably structurally related toxin (see ref. 12). Chiriquitoxin, while related in structure to tetrodotoxin, differs in the carbon skeleton (21). The chemical defensive attributes of tetrodotoxin are due to blockade of voltage-dependent sodium channels and hence cessation of neuronal and muscle activity.
The origin of the lipophilic alkaloids in dendrobatid frogs, engendered by the observation that the frogs, which are used by Colombian Indians to poison blow darts, when raised in captivity contain none of the toxic batrachotoxins present in wild-caught frogs (22), remains to be investigated. In contrast, the toxic samandarines from fire salamanders are present in the skin glands of the salamander through many generations of nurture in captivity (G. Habermehl, personal communication). The various lipophilic alkaloids of amphibians all have marked activity on ion channels and hence through such effects would serve effectively as chemical defenses, even though some have relatively low toxicity.
The batrachotoxins were the first class of unique alkaloids to be characterized from skin extracts of frogs of the family Dendrobatidae (see ref. 23 for a review of amphibian alkaloids). Batrachotoxin was detected in only five species of dendrobatid frogs and these frogs were then classified as the monophyletic genus Phyllobates, based in part on the presence of batrachotoxins (24). However, levels of batrachotoxins differ considerably, with the Colombian Phyllobates terribilis containing nearly 1 mg of batrachotoxins per frog, while the somewhat smaller Phyllobates bicolor and Phyllobates aurotaenia, also from the rain forests of the Pacific versant in Colombia, contain 10-fold lower skin levels (8). The two
Phyllobates species from Panama and Costa Rica contain either only trace amounts of batrachotoxin or for certain populations of Phyllobates lugubris no detectable amounts. Batrachotoxins are unique steroidal alkaloids, which were unknown elsewhere in nature until the recent discovery of homobatrachotoxin at low levels in skin and feathers of a Papua New Guinean bird of the genus Pitohui (9). In the dendrobatid frogs, three major alkaloids are present—namely, batrachotoxin, homobatrachotoxin, and a much less toxic possible precursor, batrachotoxinin A. The latter, when fed to nontoxic captive-raised P. bicolor using dusted fruit flies, is accumulated into skin glands but is not converted to the more toxic esters batrachotoxin and homobatrachotoxin (25). Dendrobatid frogs of another genus would not eat the batrachotoxinin-dusted fruit flies. Batrachotoxins depolarize nerve and muscle by specific opening of sodium channels; the sodium channels of the Phyllobates species are insensitive to the action of batrachotoxin (22).
Further examination of extracts of dendrobatid frogs over nearly 3 decades led to the characterization of nearly 300 alkaloids, representing some 18 structural classes (see ref. 23). Several classes remain unknown in nature except in frog skin (see Table 1), and their origin remains obscure in view of the relatively recent finding that frogs of the dendrobatid genera Dendrobates and Epipedobates, like Phyllobates, do not have skin alkaloids when raised in captivity (26). The distribution of the various alkaloids of amphibians is pertinent to any speculation as to their origin (see Table 1).
The so-called pumiliotoxin A class of "dendrobatid alkaloids" is as yet known only in nature from frog/toad skin. The class consists of alkaloids with either an indolizidine (pumiliotoxins and allopumiliotoxins) or a quinolizidine (homopumiliotoxins) ring, in each case with a variable alkylidene side chain. The pumiliotoxin A class occurs in skin of all of the amphibian genera that contain lipophilic alkaloids with the exception of the fire salamanders, which contain only samandarines. In spite of a wide distribution in the alkaloid-containing frogs, there are species and/or populations of frogs that have no pumiliotoxin A class alkaloids or only trace amounts. Members of pumiliotoxin A class are active toxins with effects on sodium and perhaps calcium channels and, thus, would serve well in defense against predators.
Histrionicotoxins represent another major class of dendrobatid alkaloids. They contain a unique spiropiperidine ring system and side chains with acetylenic, olefinic, and allenic groups. Histrionicotoxins remain known in nature only from dendrobatid frogs of the general Phyllobates, Dendrobates, and Epipedobates. They are probably absent in the tiny dendrobatid frogs of the genus Minyobates. Histrionicotoxins were detected in a single Madagascan frog of the mantellid genus Mantella (27)
obtained through the pet trade but have not been detected in any extracts of several Mantella species collected in Madagascar (28). Histrionicotoxins do not occur in all species of the above dendrobatid frog genera or in all populations of a single species (8). Their occurrence within populations of a species on a single small island can vary from high levels to none.
The decahydroquinolines are the third major class of dendrobatid alkaloids still known only from frog/toad skin. Decahydroquinolines occur in skin of all the frog/toad genera that have lipophilic alkaloids with the sole exception of the Australian myobatrachid frogs of the genus Pseudophryne that contain only (allo)pumiliotoxins and a series of indole alkaloids unique in nature to this genus of frogs—namely, the pseudophrynamines (29).
A series of simple bicyclic alkaloids could be considered to make up a major "izidine" class of alkaloids in the dendrobatid and other frogs. These include the 3,5-disubstituted pyrrolizidines, the 3,5-disubstituted and 5,8-disubstituted indolizidines, and the 1,4-disubstituted quinolizidines. The 3,5-disubstituted pyrrolizidines and the 3,5-disubstituted indolizidines are not unique to frogs, having been reported from ants (see ref. 6). Ants thus represent a potential dietary source for such alkaloids in dendrobatid and other frogs. Indeed, feeding experiments with ants of the genus Monomorium that contain a 3,5-disubstituted indolizidine and a 2,5-disubstituted pyrrolidine resulted in a remarkable selective accumulation, into the skin of the dendrobatid frog Dendrobates auratus, of the indolizidine but not of the pyrrolidine (25). It should be noted that some dendrobatid frogs do contain significant levels in skin of such 2,5-disubstituted pyrrolidines and of 2,6-disubstituted piperidines, neither of which appears to be sequestered into skin, at least by D. auratus. The 5,8-disubstituted indolizidines and 1,4-disubstituted quinolizidines remain as yet unknown in nature except from frog/toad skin (Table 1).
There are also a number of alkaloids characterized from skin extracts of dendrobatid frogs that have a rather limited distribution within the many species that have been examined and are as yet known only from frog skin. The tricyclic gephyrotoxins occur along with the more widely distributed histrionicotoxins in only a few species and populations of dendrobatid frogs (8). The tricyclic cyclopenta[b]quinolizidines occur in only one species, a tiny Colombian frog Minyobates bombetes (30). The potent nicotinic analgesic epibatidine occurs only in four dendrobatid species of the genus Epipedobates found in Ecuador (31).
Two classes of dendrobatid alkaloids have potential dietary sources. The first are the pyrrolizidine oximes (32), whose carbon skeleton is identical to that of nitropolyzonamine, an alkaloid from a small millipede (33). Indeed, raising the dendrobatid frog D. auratus in Panama on leaf-litter arthropods, gathered weekly, resulted in skin levels of the
pyrrolizidine oxime 236 even higher than levels in wild-caught frogs from the leaf-litter site (34). The second are the tricyclic coccinelline alkaloids that have been found in several frogs/toads. The coccinellines occur as defensive substances in a variety of small beetles (see ref. 6). Thus, beetles represent a possible dietary source for coccinelline-class alkaloids in frog/toad skin. Indeed, the beetle alkaloid precoccinelline is a significant alkaloid in the skin of D. auratus raised in Panama on leaf-litter arthropods (34). The other alkaloids that were found in skin of D. auratus raised on leaf-litter arthropods are three other tricyclic alkaloids, perhaps of the coccinelline-class but of unknown structure, two 1,4-disubstituted quinolizidines, a gephyrotoxin, a decahydroquinoline, and several histrionicotoxins. With the exception of the pyrrolizidine oxime 236, skin levels of the various alkaloids in the captive-raised frogs were low compared to levels of alkaloids in wild-caught frogs from the leaf-litter collection site or from the parental stock of D. auratus on a nearby island (34). Individual variation in wild-caught frogs appears significant, which complicates the comparisons. However, the lack of any pumiliotoxins and the relatively low levels or absence of decahydroquinolines and histrionicotoxins in the captive-raised frogs suggests that dietary sources for these alkaloids have been missed in the paradigm using large funnels to collect the arthropods from the leaf litter.
In summary, poisons used in chemical defense are widespread in nature. In amphibians, the defensive substances seem to be elaborated by the amphibian in the case of amines, peptides, proteins, bufadienolides, and the salamander alkaloids of the samandarine class. For the tetrodotoxin class of water-soluble alkaloids, the origin is unclear, but symbiotic bacteria have been suggested for marine organisms (4). For the so-called dendrobatid alkaloids, a dietary source now appears a likely explanation for the lack of skin alkaloids in dendrobatid frogs raised in captivity. Certainly, dendrobatid frogs of the dendrobatid genera Phyllobates, Dendrobates, and Epipedobates, which in the wild contain skin alkaloids, have highly efficient systems for accumulating selectively into skin a variety of dietary alkaloids (25, 34). A biological system for sequestration of alkaloids for chemical defense finds precedence in the transfer of pyrrolizidine alkaloids from plants via aphids to ladybug beetles (35). Accumulation of cantharidins in muscle of ranid frogs after feeding on beetles has been documented (36). Frogs of the dendrobatid genus Colostethus, which in the wild do not contain skin alkaloids, do not accumulate dietary alkaloids (25).
The proposal that all alkaloids found in skin glands of dendrobatid frogs and used in chemical defense against predators have a dietary origin leads to many questions. First, the profile of alkaloids has been found in many instances to be characteristic of a species or a population.
Thus, either the systems responsible for sequestration of alkaloids differ in selectivity among different species and/or populations of dendrobatid frogs or the small arthropod fauna presenting itself and used as a diet by different species and/or populations varies even within a small island. The latter appears more likely. It was noted that the dendrobatid frogs raised on leaf-litter in Panama shared more alkaloids with a population of D. auratus from the leaf-litter site than they did with the parental population from a nearby island (34). The second major question concerns what small insects or other arthropods contain such toxic and/or unpalatable alkaloids as the batrachotoxins, the pumiliotoxins, and the histrionicotoxins, the decahydroquinolines, the 5,8-disubstituted indolizidines, the 1,4-disubstituted quinolizidines, and epibatidine. It is remarkable that such small, presumably distasteful arthropods have escaped the attention of researchers. Whether frogs intent on sequestering defensive alkaloids seek out such prey is unknown. With regard to the frogs/toads from the Madagascan family Mantellidae, the Australian family Myobatrachidae and the South American genus Melanophryniscus of the family Bufonidae, which also contain many of the dendrobatid alkaloids, it is unknown whether sequestering systems are present or even whether captive-raised frogs will lack skin alkaloids. If such systems are present, then it is remarkable from an evolutionary standpoint that such unrelated lineages of toads/frogs have independently developed systems for sequestering alkaloids into skin glands from a diet of small, presumably noxious insects for use by the toad/frog in chemical defense.
SUMMARY
Poisons are common in nature, where they often serve the organism in chemical defense. Such poisons either are produced de novo or are sequestered from dietary sources or symbiotic organisms. Among vertebrates, amphibians are notable for the wide range of noxious agents that are contained in granular skin glands. These compounds include amines, peptides, proteins, steroids, and both water-soluble and lipid-soluble alkaloids. With the exception of the alkaloids, most seem to be produced de novo by the amphibian. The skin of amphibians contains many structural classes of alkaloids previously unknown in nature. These include the batrachotoxins, which have recently been discovered to also occur in skin and feathers of a bird, the histrionicotoxins, the gephyrotoxins, the decahydroquinolines, the pumiliotoxins and homopumiliotoxins, epibatidine, and the samandarines. Some amphibian skin alkaloids are clearly sequestered from the diet, which consists mainly of small arthropods. These include pyrrolizidine and indolizidine alkaloids from ants, tricyclic coccinellines from beetles, and pyrrolizidine oximes, pre-
sumably from millipedes. The sources of other alkaloids in amphibian skin, including the batrachotoxins, the decahydroquinolines, the histrionicotoxins, the pumiliotoxins, and epibatidine, are unknown. While it is possible that these are produced de novo or by symbiotic microorganisms, it appears more likely that they are sequestered by the amphibians from as yet unknown dietary sources.
REFERENCES
1. Whittaker, R. H. & Feeny, P. P. (1971) Science 171, 757-770.
2. Balandrin, M. F., Klocke, J. A., Wurtele, E. S. & Bollinger, W. H. (1985) Science 228, 1154-1160.
3. Scheuer, P. J. (1990) Science 248, 173-177.
4. Yasumoto, T. & Murata, M. (1993) Chem. Rev. 93, 1897-1909.
5. Eisner, T. & Meinwald, J. (1966) Science 153, 1341-1350.
6. Jones, T. H. & Blum, M. S. (1983) Alkaloids: Chemical and Biological Perspectives (Wiley, New York), Vol. 1, pp. 33-84.
7. Tachibana, K. (1988) Bioorg. Mar. Chem. 2, 124-145.
8. Daly, J. W., Myers, C. W. & Whittaker, N. (1987) Toxicon 25, 1023-1095.
9. Dumbacher, J. P., Beehler, B. M., Spande, T. F., Garraffo, H. M. & Daly, J. W. (1992) Science 258, 799-801.
10. Erspamer, V., Melchiorri, P., Erspamer, G. F., Montecucchi, P. C. & De Castiglione, R. (1985) Peptides 6, 7-12.
11. Flier, J., Edwards, M. W., Daly, J. W. & Myers, C. W. (1980) Science 208, 503-505.
12. Daly, J. W., Gusovsky, F., Myers, C. W., Yotsu-Yamashita, M. & Yasumoto, T. (1994) Toxicon 32, 279-285.
13. Bevins, C. L. & Zasloff, M. (1990) Annu. Rev. Biochem. 59, 395-414.
14. Daly, J. W., Caceres, J., Moni, R. W., Gusovsky, F., Moos, M., Jr., Seamon, K. B., Milton, K. & Myers, C. W. (1992) Proc. Natl. Acad. Sci. USA 89, 10960-10963.
15. Richter, K., Egger, R., Negri, L., Corsi, R., Severini, C. & Kreil, G. (1990) Proc. Natl. Acad. Sci. USA 87, 4836-4839.
16. Porto, A. M. & Gros, E. (1971) Experientia 27, 506.
17. Eisner, T., Wiemer, D. F., Haynes, L. W. & Meinwald, J. (1978) Proc. Natl. Acad. Sci. USA 75, 905-908.
18. Brower, L. P., van Zandt Brower, J. & Corvino, J. M. (1967) Proc. Natl. Acad. Sci. USA 57, 893-898.
19. Matsui, T., Hamada, S. & Konosu, S. (1981) Bull. Jpn. Soc. Sci. Fish. 47, 535-537.
20. Shimizu, Y. & Kobayashi, M. (1983) Chem. Pharm. Bull. 31, 3625-3631.
21. Yotsu, M., Yasumoto, T., Kim, Y. H., Naoki, H. & Kao, C. Y. (1990) Tetrahedron Lett. 31, 3187-3190.
22. Daly, J. W., Myers, C. W., Warnick, J. E. & Albuquerque, E. X. (1980) Science 208, 1383-1385.
23. Daly, J. W., Garraffo, H. M. & Spande, T. F. (1993) in The Alkaloids, ed. Cordell, G. A. (Academic, San Diego), Vol. 43, Chap. 3, pp. 185-288.
24. Myers, C. W., Daly, J. W. & Malkin, B. (1978) Bull. Am. Mus. Nat. Hist. 161, 307-365.
25. Daly, J. W., Secunda, S. I., Garraffo, H. M., Spande, T. F., Wisnieski, A. & Cover, J. F., Jr. (1994) Toxicon 32, 657-663.
26. Daly, J. W., Secunda, S. I., Garraffo, H. M., Spande, T. F., Wisnieski, A., Nishihira, C. & Cover, J. F., Jr. (1992) Toxicon 30, 887-898.
27. Daly, J. W., Highet, R. J. & Myers, C. W. (1984) Toxicon 22, 905-919.
28. Garraffo, H. M., Caceres, J., Daly, J. W., Spande, T. F., Andriamaharavo, N. R. & Andriantsiferana, M. (1993) J. Nat. Prod. 56, 1016-1038.
29. Daly, J. W., Garraffo, H. M., Pannell, L. K. & Spande, T. F. (1990) J. Nat. Prod. 53, 407-421.
30. Spande, T. F., Garraffo, H. M., Yeh, H. J. C., Pu, Q.-L., Pannell, L. K. & Daly, J. W. (1992) J. Nat. Prod. 55, 707-722.
31. Spande, T. F., Garraffo, H. M., Edwards, M. W., Yeh, H. J. C., Pannell, L. & Daly, J. W. (1993) J. Am. Chem. Soc. 114, 3475-3478.
32. Tokuyama, T., Daly, J. W., Garraffo, H. M. & Spande, T. F. (1992) Tetrahedron 48, 4247-4258.
33. Meinwald, J., Smolanoff, J., McPhail, A. T., Miller, R. W., Eisner, T. & Hicks, K. (1975) Tetrahedron Lett. 2367-2370.
34. Daly, J. W., Garraffo, H. M., Spande, T. F., Jaramillo, C. & Rand, A. S. (1994) J. Chem. Ecol. 4, 943-955.
35. Witte, L., Ehmke, A. & Hartmann, T. (1990) Naturwissenschaften 77, 540-543.
36. Eisner, T., Conner, J., Carrel, J. E., McCormick, J. P., Slagle, A. J., Gans, C. & O'Reilly, J. C. (1990) Chemoecology 1, 57-62.