The Chemistry of Phyletic Dominance
JERROLD MEINWALD AND THOMAS EISNER
Whether we chose as our criterion Erwin's generous estimate of 30 million species of insects (1) or the somewhat more modest number favored by Wilson (2), it is clear that insects have achieved formidable diversity on Earth. On dry land they literally reign supreme. It has been estimated that there are some 200 million insects for each human alive (3). The eminence of the phylum Arthropoda among animals is very much a reflection of the success of the insects alone.
A number of factors have contributed to the achievement of dominance by insects. They were the first small animals to colonize the land with full success, thereby gaining an advantage over latecomers. Their exoskeleton shielded them from desiccation and set the stage for the evolution of limbs, tracheal tubes, and elaborate mouthparts, adaptations that were to enable insects to become agile, energetically efficient, and extraordinarily diverse in their feeding habits. Metamorphosis opened the option for insects to exploit different niches during their immature and adult stages. The evolution of wings facilitated their dispersal as adults, as well as their search for mates and oviposition sites. And there were subtle factors such as the acquisition of a penis (the aedeagus), which enabled insects to effect direct sperm transfer from male to female, without recourse to an outer aquatic environment for fertilization (3).
Jerrold Meinwald is Goldwin Smith Professor of Chemistry and Thomas Eisner is Schurman Professor of Biology and director of the Cornell Institute for Research in Chemical Ecology at Cornell University, Ithaca, New York.
A factor not often appreciated that also contributed to insect success is the extraordinary chemical versatility of these animals. Insects produce chemicals for the most diverse purposes—venoms to kill prey, repellents and irritants to fend off enemies, and pheromones for sexual and other forms of communication. The glands responsible for production of these substances are most often integumental, having been derived by specialization of localized regions of the epidermis. In insects, as in arthropods generally, the epidermis is fundamentally glandular, being responsible for secretion of the exoskeleton. In their acquisition of special glands, arthropods appear to have capitalized upon the ease with which epidermal cells can be reprogrammed evolutionarily for performance of novel secretory tasks. The glandular capacities of arthropods are known to anyone who has collected these animals in the wild. Arthropods commonly have distinct odors and are often the source of visible effluents that they emit when disturbed (Figure 1). Much has been learned in recent years about the function and chemistry of arthropod secretions. The insights gained have been fundamental to the emergence of the field of chemical ecology. Our purpose here is to focus on some aspects of the secretory chemistry of these animals, with emphasis on a few recent discoveries.
ARTHROPOD CHEMICAL DEFENSES
In our earliest collaborative publication, we described the dramatic chemical defense mechanism of the whip scorpion, Mastigoproctus giganteus (6). This ancient arachnid is able to spray a well-aimed stream of ˜85% acetic acid [CH3CO2H] containing 5% caprylic acid [CH3(CH2)6CO2H] at an assailant. The role of the caprylic acid proved to be especially interesting: it facilitates transport of the acetic acid through the waxed epicuticle of an enemy arthropod. It is likely that this simple strategy of using a lipophilic agent to enable a more potent compound to penetrate a predator's cuticle has helped this species to survive for as long as 400 million years. Many other independently evolved arthropod defensive secretions make use of the same strategy (9). The whip scorpion defense mechanism helped us to appreciate the fact that chemistry need not be complex to be effective. The virtue of simplicity is further illustrated by the defensive use of mandelonitrile and benzoyl cyanide, easily decomposed precursors of hydrogen cyanide (HCN), by certain millipedes and centipedes (10, 11).
While arthropod defensive secretions often rely for their effect on well-known aliphatic acids, aldehydes, phenols, and quinones, there are many cases in which compounds capable of whetting the appetite of any natural products chemist are utilized. For example, steroids play a
dominant role in the defensive chemistry of dytiscid beetles, and of the large water bug, Abedus herberti. This bug discharges a mixture of pregnanes in which desoxycorticosterone is the main component (12). A family of much more highly functionalized steroids, the lucibufagins, serves to render some species of firefly (lampyrid beetles) unpalatable to predatory spiders and birds (13-15). These cardiotonic steroids are closely related to the bufadienolides, whose only known occurrence among animals is in the poison glands of certain toads (16). The discovery
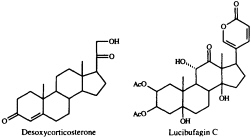
that the lucibufagins also show antiviral properties (17) has prompted us to seek a technique for joining a preformed a-pyrone nucleus to a steroidal framework, since up to now there has been no general, convenient synthetic route to these steroidal pyrones. Our search has recently met with success (18), using the Pd0-promoted coupling of 5-trimethylstannyl-2-pyrone with an enol triflate (Eq. 1).
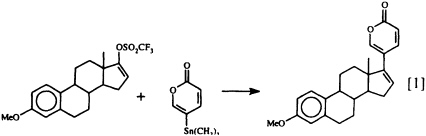
This direct synthesis of steroidal pyrones should make a variety of structures related to the lucibufagins (as well as to the toad-derived bufadienolides) readily available for biological investigation for the first time. How the insects themselves manage to obtain their defensive pregnanes and steroidal pyrones remains a mystery, since insects are generally considered to lack the enzymatic machinery essential for steroid biosynthesis (19). In fact, we do not yet know whether these insect defensive steroids are produced de novo or whether they are derived
directly or indirectly from a dietary source; this is a subject which merits further study.
Perhaps the most interesting arthropodan defensive compounds from the point of view of structural diversity are the alkaloids. While alkaloids had long been believed to arise only as a consequence of plant secondary metabolism, it has become apparent over the last few decades that arthropods are both prolific and innovative alkaloid chemists. The millipede Polyzonium rosalbum, once thought to secrete camphor (20), in fact gives off a camphoraceous/earthy aroma produced by the spirocyclic isoprenoid imine polyzonimine (21).
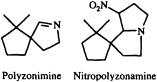
The biosynthesis of this imine and its congener, nitropolyzonamine (22), would be another challenging area for future exploration.
We have recently characterized the heptacyclic alkaloid chilocorine from the ladybird (coccinellid) beetle Chilocorus cacti (23). In spite of its superficial complexity, this structure is easily dissected into two tricyclic moieties, A and B, each of which can be regarded as an acetogenin which has been elaborated from a straight chain of 13 carbon atoms stitched together at three points by a trivalent nitrogen atom.
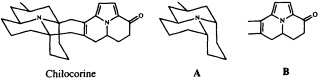
It is intriguing to note that while the azaphenalene skeleton of part structure A is, in fact, commonly found among coccinellid alkaloids (24), the azaacenaphthylene skeleton of B is known, again combined with fragment A, only from the recently described hexacyclic alkaloid exochomine (25). Since pyrroles are not basic, we would expect tricyclic compounds resembling B to have been missed in a conventional alkaloid isolation scheme, and we anticipate that a targeted search for these novel pyrroles may well turn up additional examples of this otherwise unknown ring system.
Aside from yielding the most complex insect alkaloids so far characterized, coccinellid beetles are sources of a wide array of structural types.
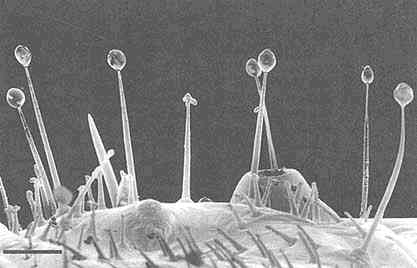
FIGURE 2 Glandular hairs on surface of pupa of the Mexican bean beetle, Epilachna varivestis. The secretory droplets contain such azamacrolides as epilachnene (27). (Bar = 0.1 mm.)
Perhaps the star performer in this arena is the Mexican bean beetle, Epilachna varivestis. The adult produces a defensive alkaloid cocktail containing more than a dozen pyrrolidines, piperidines, an azabicyclo[3.3.1]nonane, and azaphenalenes (26). Interestingly, the pupa of this beetle, which is densely covered with glandular hairs, secretes an entirely different group of defensive alkaloids, the azamacrolides, which function as highly effective ant repellents (Figure 2). We have described these unique macrocyclic compounds, of which the most important example is epilachnene, in a recent ''advertisement" (27). They, too, appear to be constructed from a fatty acid precursor to which a basic nitrogen has been joined. We have carried out a few exploratory biosynthetic experiments with larvae of E. varivestis to determine the possible sources of both the 14-carbon straight chain and the ethanolamine moieties of epilachnene (28). Our results are summarized below:
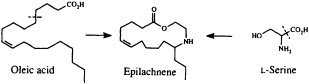
9,10-Dideuteriooleic acid was shown to be converted to dideuterioepilachnene by the larvae (presumably after two chain-shortening ß-oxidations), confirming our expectation that the long carbon chain can be derived from an appropriate fatty acid. Both 2H- and 15N-labeled L-serine are incorporated into the alkaloid as well, accounting for the origin of the ethanolamine unit (28). The unique step in this scheme, as in the most plausible biosynthetic sequences for practically all the coccinellid beetle alkaloids so far characterized, is the joining of a nitrogen substituent to a fatty acid chain. A variety of intriguing mechanistic hypotheses might be put forward to rationalize this process, which does not seem to have any close biochemical precedent. Once more, additional experimental work will be needed to clarify what it is that the beetles actually do, but it seems likely that our understanding of secondary metabolism will make at least a small leap forward as the result of a mechanistic study of this novel carbon-nitrogen bond-forming process.
SPIDER VENOMS
In addition to their widespread exploitation of organic compounds for defensive purposes, arthropod species often use offensive chemical weaponry. Many spiders possess venoms capable of paralyzing their prey, and consequently spider venoms have become a popular hunting ground for neurotoxins of potential neurochemical and neurotherapeutic utility (29). We joined forces with colleagues at Cambridge NeuroScience Research (CNS), Inc., to pursue several problems in this area. In one of these, we studied the venom of Dolomedes okefinokensis, a "fishing spider" capable of immobilizing vertebrate prey. The venom of this spider is a typically complex mixture, as revealed by its high-performance liquid chromatogram, which shows the presence of at least several dozen components. Nevertheless, guided by a microscale neurochemical bioassay (30), Kazumi Kobayashi and her CNS colleagues were able to isolate a reversible L- and R-type voltage sensitive calcium channel blocker that appeared to be of interest. We established that this compound, designated CNS 2103, is the 4-hydroxyindole-3-acetic acid amide of a long-chain polyamine. The structure, based on ultraviolet absorption and 1H-NMR spectroscopic data, along with conventional and tandem mass spectrometry, was determined to be that given below.
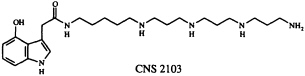
To confirm this structure, we devised a synthetic route to CNS 2103 which has the virtue of being easily modified to give access to a variety of unnatural analogs of the spider neurotoxin as well (31).
It is of particular interest that polyamines closely related to CNS 2103 have been found not only in other spider species (29) but also in the venom of the solitary digger wasp Philanthus triangulum (32). The similarity of these wasp and spider neurotoxins provides a notable example of convergence in the evolution of secondary metabolites aimed at a common target.
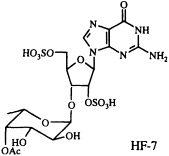
Polyamines are readily and reversibly protonated to give water-soluble polycations, and it seems likely that their mode of action is related to this capability (33). In our collaborative work on another venom constituent, HF-7, isolated from the funnel-web spider Hololena curta, we encountered an entirely differently constituted blocker of non-N-methyl-D-aspartate-glutamate-sensitive calcium channels. This compound turned out to be complementary to the cation-forming polyamines, in the sense that it can exist as a mono- or di-anion. Because of its unusual and entirely unanticipated structure, HF-7 proved more difficult to characterize than CNS 2103. The ultraviolet absorption spectrum of HF-7 pointed to the presence of a guanine chromophore. We had no success in obtaining mass spectroscopic data, however, until we resorted to negative ion fast atom bombardment (FAB) mass spectrometry, which established a molecular weight of 631. Loss of 80 atomic mass units (SO3) from the m/z 630 parent negative ion gave rise to a base peak corresponding to the molecular formula C18H24N5O13S (m/z obs. 550.1036; calc. 550.1091), implying the composition C18H25N5O16S2 for HF-7 itself.
On the basis of a number of two-dimensional NMR experiments, we were finally able to conclude that HF-7 is an acetylated bis(sulfate ester) of a guanosine fucopyranoside, although the exact position of the sulfate groups, the point of attachment of the fucose to the ribose ring, and the absolute configuration of the fucose moiety remained uncertain (34). Because of the very limited supply of this material, and because a family of compounds closely related to the natural product should prove useful in studying structure-activity relationships, we set out to synthesize a set of candidate guanosine fucopyranosides of clearly defined structure and stereochemistry. This synthetic effort has enabled us to characterize HF-7 itself by direct comparison of the natural product with several unambiguously constructed reference compounds (35). As a result of this work, the structure of HF-7 shown below is firmly established.
The occurrence of sulfated nucleoside glycosides in spider venoms, or for that matter in any other natural source, does not seem to have been previously noted. We look forward to the possibility that this novel
arachnid metabolite will prove to be the forerunner of a significant group of anionic neuroactive agents.
CHEMICAL CONTRIBUTIONS TO ARTHROPOD DOMINANCE
In this discussion, we have restricted ourselves to the consideration of only a few examples of arthropod chemistry. From these alone, it is evident that insects synthesize defensive compounds by using all of the major biosynthetic pathways, producing acetogenins, simple aromatics and quinones, isoprenoids, and alkaloids. In addition, some of the millipedes, coccinellid beetles, and spiders we have studied utilize biosynthetic pathways that have yet to be characterized.
While arthropod secretions are often the result of de novo syntheses, there are also many instances in which insects pursue an alternative defensive strategy: the sequestration of ready-made defensive compounds from plant or even animal sources. A simple example is provided by larvae of the Australian sawfly Pseudoperga guerini (Figure 3), which store the defensive terpenes from ingested eucalyptus, segregating them in a specialized sac, and then regurgitating the mixture in response to attack (36). It is interesting to compare the arthropods' ability to acquire useful natural products with our own species' long history of searching for compounds in nature that can be put to use in a variety of contexts, which has resulted in discoveries of compounds as broadly important as the pyrethroids, quinine, digitoxin, penicillin, the avermectins, and taxol. Although our own searching and screening techniques may well be of unparalleled sophistication, our activities as "chemical prospectors" are certainly not entirely without antecedent.
The preceding falls far short of conveying a true impression of the chemical skills of arthropods. Excluded from our discussion are the diverse signaling agents that mediate such vital insectan functions as food location, mate attraction, social bonding, and alarm communication. Other contributors to this colloquium address some of these topics. While

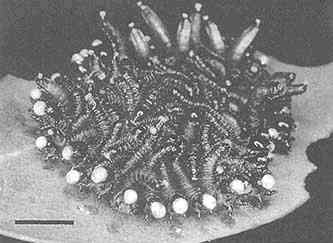
FIGURE 3 Australian sawfly (Pseudoperga guerini). (Top) Female, guarding clutch of larvae recently emerged from her eggs. At this stage, the larvae are unable to fend for themselves when attacked. (Bottom) Older larvae, responding to disturbance by regurgitating droplets of oil from ingested eucalyptus. The droplets are potently deterrent to predators. The newly emerged larvae on left have not as yet accumulated sufficient dietary oil for defense. [Bars = 0.5 cm (Left), 1 cm (Right).]
insects are by no means unique in having evolved such functions, they may well be the group of animals that took chemical performance in the service of the functions to its most sophisticated expression. The adage "better living through chemistry" may indeed be more applicable to insects than to the industrial giant that coined it (37).
What we know already about insect chemistry is tantalizing, but there can be no question that the best is yet to come. Only a tiny percentage of insects have so far been subject to even the most cursory chemical study. There is no telling what, in the line of molecular novelty and chemical ecological ingenuity, the remainder might have to offer.
SUMMARY
Studies of arthropod defensive chemistry continue to bring to light novel structures and unanticipated biosynthetic capabilities. Insect alkaloids, such as the heptacyclic acetogenin chilocorine and the azamacrolides, exemplify both of these aspects of arthropod chemistry. Spider venoms are proving to be rich sources of neuroactive components of potential medical interest. The venom of a fishing spider, Dolomedes okefinokensis, has yielded a polyamine which reversibly blocks L- and R-type voltage-sensitive calcium channels. Most recently, we have characterized, from the funnel-web spider Hololena curta, a sulfated nucleoside glycoside which serves as a reversible blocker of glutamate-sensitive calcium channels. The ability to synthesize or acquire an extremely diverse array of compounds for defense, offense, and communication appears to have contributed significantly to the dominant position that insects and other arthropods have attained.
The support of our research on insect-related chemistry by National Institutes of Health Grants AI12020 and AI2908, National Science Foundation Grant MCB-9221084, and by Hatch Project Grants NY(C)-191424 and NY(C)-191425, as well as by the Schering-Plough Research Institute, the Merck Research Laboratories, and Cambridge NeuroScience, Inc., is gratefully acknowledged.
REFERENCES
1. Erwin, T. L. (1983) in Tropical Rain Forest: Ecology and Management, eds. Sutton, S. L., Whitmore, T. C. & Chadwick, A. C. (Blackwell, Edinburgh), pp. 59-75.
2. Wilson, E.O. (1988) in Biodiversity, ed. Wilson, E.O. (Natl. Acad. Press, Washington, DC), pp. 3-18.
3. Eisner, T. & Wilson, E. O. (1977) in The Insects: Readings from Scientific American , eds. Eisner, T. & Wilson, E. O. (Freeman, San Francisco), pp. 3-15.
4. Carrel, J. E. & Eisner, T. (1974) Science 183, 755-757.
5. Meinwald, J., Jones, T. H., Eisner, T. & Hicks, K. (1977) Proc. Natl. Acad. Sci. USA 74, 2189-2193.
6. Eisner, T., Meinwald, J., Monro, A. & Ghent, R. (1961) J. Insect Physiol. 6, 272-298.
7. Eisner, T., Alsop, D., Hicks, K. & Meinwald, J. (1978) Handb. Exp. Pharmacol. 48, 41-72.
8. Eisner, T., Kluge, A. F., Ikeda, M. I., Meinwald, Y. C. & Meinwald, J. (1971) J. Insect Physiol. 17, 245-250.
9. Attygalle, A. B., Smedley, S. R., Meinwald, J. & Eisner, T. (1993) J. Chem. Ecol. 19, 2089-2104.
10. Eisner, T., Eisner, H. E., Hurst, J. J., Kafatos, F. C. & Meinwald, J. (1963) Science 139, 1218-1220.
11. Jones, T. H., Conner, W. E., Meinwald, J., Eisner, H. E. & Eisner, T. (1976) J. Chem. Ecol. 2, 421-429.
12. Lokensgard, J., Smith, R. L., Eisner, T. & Meinwald, J. (1993) Experientia 49, 175-176.
13. Eisner, T., Wiemer, D. F., Haynes, L. W. & Meinwald, J. (1978) Proc. Natl. Acad. Sci. USA 75, 905-908.
14. Meinwald, J., Wiemer, D. F. & Eisner, T. (1979) J. Am. Chem. Soc. 101, 3055-3060.
15. Goetz, M., Wiemer, D. F., Haynes, L. W., Meinwald, J. & Eisner, T. (1979) Helv. Chim. Acta 62, 1396-1400.
16. Nakanishi, K. (1974) in Natural Products Chemistry, eds. Nakanishi, K., Goto, T., Itô, S., Natori, S. & Nozoe, S. (Academic, New York), Vol. 1, p. 469.
17. Wilson, G. R. & Rinehart, K. L. (1989) U.S. Patent 4,847,246.
18. Liu, Z. (1994) Dissertation (Cornell Univ., Ithaca, NY).
19. Nakanishi, K. (1974) in Natural Products Chemistry, eds. Nakanishi, K., Goto, T., Itô, S., Natori, S. & Nozoe, S. (Academic, New York), Vol. 1, p. 526.
20. Cook, O. F. (1900) Science 12, 516-521.
21. Smolanoff, J., Kluge, A. F., Meinwald, J., McPhail, A. T., Miller, R. W., Hicks, K. & Eisner, T. (1975) Science 188, 734-736.
22. Meinwald, J., Smolanoff, J., McPhail, A. T., Miller, R. W., Eisner, T. & Hicks, K. (1975) Tetrahedron Lett., 2367-2370.
23. McCormick, K. D., Attygalle, A. B., Xu, S.-C., Svatos, A., Meinwald, J., Houck, M. A., Blankespoor, C. L. & Eisner, T. (1994) Tetrahedron 50, 2365-2372.
24. Ayer, W. A. & Browne, L. M. (1977) Heterocycles 7, 685-707.
25. Timmermans, M., Braekman, J.-C., Daloze, D., Pasteels, J. M., Merlin, J. & Declercq, J.-P. (1992) Tetrahedron Lett. 33, 1281-1284.
26. Attygalle, A. B., Xu, S.-C., McCormick, K. D. & Meinwald, J. (1993) Tetrahedron 49, 9333-9342.
27. Attygalle, A. B., McCormick, K. D., Blankespoor, C. L., Eisner, T. & Meinwald, J. (1993) Proc. Natl. Acad. Sci. USA 90, 5204-5208.
28. Attygalle, A. B., Blankespoor, C. L., Eisner, T. & Meinwald, J. (1994) Proc. Natl. Acad. Sci. USA 91, 12790-12793.
29. McCormick, K. D. & Meinwald, J. (1993) J. Chem. Ecol. 19, 2411-2451.
30. Kobayashi, K., Fischer, J. B., Knapp, A. G., Margolin, L., Daly, D., Reddy, N. L., Roach, B., McCormick, K. D., Meinwald, J. & Goldin, S. M. (1992) Soc. Neurosci. Abstr. 18, 10.
31. McCormick, K. D., Kobayashi, K., Goldin, S. M., Reddy, N. L. & Meinwald, J. (1993) Tetrahedron 49, 11155-11168.
32. Nakanishi, K., Goodnow, R., Konno, K., Niwa, M., Bukownik, R., Kallimopoulos, T. A., Usherwood, P., Eldefrawi, A. T. & Eldefrawi, M. E. (1990) Pure Appl. Chem. 62, 1223-1230.
33. Nakanishi, K., Choi, S.-K., Hwang, D., Lerro, K., Oriando, M., Kalivretenos,
A. G., Eldefrawi, A., Eldefrawi, M. & Usherwood, P. N. R. (1994) Pure Appl. Chem. 63, 671-678.
34. McCormick, K. D. (1993) Dissertation (Cornell Univ., Ithaca, NY).
35. McCormick, J. (1994) Dissertation (Cornell Univ., Ithaca, NY).
36. Morrow, P. A., Bellas, T. E. & Eisner, T. (1976) Oecologia 24, 193-206.
37. Hounshell, D. A. & Smith, J. K., Jr. (1988) Science and Corporate Strategy: DuPont R&D 1902-1980 (Cambridge Univ. Press, Cambridge, U.K.), p. 221.












