8
Conclusions and Recommendations
The HIV epidemic has taught scientists, clinicians, public health officials, and the public that new infectious agents can still emerge. The nation must be prepared to deal with a fatal illness whose cause is initially unknown but whose epidemiology suggests it is an infectious disease. The AIDS epidemic has also taught us another powerful and tragic lesson: that the nation's blood supply—because it is derived from humans—is highly vulnerable to contamination with an infectious agent. A nation's blood supply is a unique, essential, life-giving resource. Whole blood and many blood products are lifesaving for many people. As a whole, our nation's system works effectively to supply the nation with necessary blood and blood products and its quality control mechanisms check most human safety threats. The events of the early 1980s, however, revealed an important weakness in the system—in its ability to deal with a new threat that was characterized by substantial uncertainty. The potential for recurring threats to the blood supply led this Committee to reappraise the processes, policies, and resources through which our society attempts to preserve its supply of safe blood and blood products.
GENERAL CONCLUSIONS
The events and decisions that the Committee has analyzed underscore the difficulty of decisionmaking when the stakes are high, when decisionmakers may have personal or institutional biases, and when knowledge is imprecise and incomplete. The Committee attempted to understand the complexities of the decisionmaking process during the period analyzed in this report and develop lessons to protect the blood supply in the future. In retrospect, the system was
not dealing well with contemporaneous blood safety issues such as hepatitis, and was not prepared to deal with the far greater challenge of AIDS.
By January 1983, the Centers for Disease Control (CDC) had accumulated enough epidemiological evidence to conclude that the agent causing AIDS was almost certainly transmitted through blood and blood products and could be sexually transmitted to sexual partners. The conclusion that the AIDS agent was blood-borne rested on two findings. First, AIDS was occurring in transfusion recipients and individuals with hemophilia who had received AHF concentrate; these AIDS patients did not belong to any other known high-risk group for contracting AIDS. Second, the epidemiologic pattern of AIDS was similar to hepatitis B, another blood-borne disease. However, the magnitude and consequences of the risk for transfusion and blood product recipients was not known at this time. Furthermore, the epidemiological pattern of the new disease was difficult to interpret because, unlike most infectious diseases, there seemed to be several years between exposure leading to infection and the development of symptoms. As a result, physicians and public health officials underestimated the large number of infectious people who had no symptoms of AIDS but could transmit the disease to others and therefore substantially understated the risk of infection.
Compared to the pace of many regulatory and public health decision processes, the federal government responded relatively swiftly to the early warnings that AIDS might be transmitted through blood and blood products. Public and private sector officials considered a range of clinical and public health interventions for reducing the risk of AIDS transmission through blood and blood products. This period, however, was characterized by a great deal of scientific uncertainty about the risks of HIV infection through blood and blood products and about the costs and benefits of the available options. The result, the Committee found, was a pattern of responses which, while not in conflict with the available scientific information, was very cautious and exposed the decisionmakers and their organizations to a minimum of criticism. This limited response can be seen in the refusal of blood banks in 1983 and 1984 to screen for and defer homosexuals or use surrogate tests (Chapter 5), in the Food and Drug Administration's (FDA) cautious and inadequate regulatory approach to the recall of potentially contaminated AHF concentrate (Chapter 6), and in the failure of physicians and the National Hemophilia Foundation to disclose completely the risks of using AHF concentrate and the alternatives to its use (Chapter 7).
Blood safety is a shared responsibility of many diverse organizations. They include U.S. Public Health Service agencies such as the CDC, the FDA, and the National Institutes of Health (NIH), and private-sector organizations such as community blood banks and the American Red Cross, blood and plasma collection agencies, blood product manufacturers, groups such as the National Hemophilia Foundation (NHF), and others. The problems the Committee found
were inadequate leadership and inadequate institutional decisionmaking processes in 1983 and 1984. No person or agency was able to coordinate all of the organizations sharing the public health responsibility for achieving a safe blood supply.
Decisionmaking Under Uncertainty
The management of a public health risk requires an evolving process of decisionmaking under uncertainty. It includes interpretive judgment in the presence of scientific uncertainty and disagreement about values. Public health officials must characterize and estimate the magnitude of the risk, which involves considering both the likelihood that infection might occur in various circumstances, and the costs and benefits associated with each of the possible uncertain outcomes. They must also develop and test public health and clinical care strategies, and communicate with the public about the risk and strategies for reducing it. When confronted with a poorly understood and anomalous public health threat, inertia often influences decisions. It is often easier to maintain the status quo than to make a change. In fact, regulatory policymakers, health scientists, and medical experts often require substantial scientific evidence before informing the public and adopting remedial action. Lack of scientific consensus becomes a kind of amplifier for the usual discord and conflict that can be expected whenever an important science-based public policy decision—one profoundly affecting lives and economic interests—must be made. First, uncertainty creates opportunities for advocates of self-interested and ideological viewpoints to advance plausible arguments that favor their desired outcome. Second, uncertainty intensifies bureaucrat cautiousness.
In the course of its investigations, the Committee learned several lessons about decisionmaking under uncertainty. These are set out here both as general lessons and to provide a framework for the recommendations that follow.
Risk Perception
Risk perception is shaped by social tensions, and cultural, political, and economic biases (Douglas 1985). It is important to understand the different contexts in which risk is perceived and the complex system of beliefs, values, and ideals that shape risk perception (Nelkin 1989). There are several other factors that influence risk perception, including locus of control, the type of risk posed by the threat, and the time interval involved in evaluating the risk. For example, people tend to underestimate risks that they perceive to be under their control, risks associated with a familiar situation, and low probability events (Douglas 1985). People have difficulty accepting estimates of a risk that is
involuntary, uncertain, unfamiliar, and potentially catastrophic (Fischoff 1987). The epidemic caused by HIV in the blood supply illustrates these patterns of perception and behavior with respect to risk.
Risk Assessment Versus Risk Management
A central precept of risk management is to separate the assessment of risk from the management of its consequences (NRC 1983). Otherwise, risk managers tend to bias their estimates of the magnitude of the risk in favor of their preconceived notions about appropriate or desirable policy choices. The events that the Committee studied provide examples of what can happen when this precept is not followed. When there is uncertainty, it may be necessary to assess risk by making subjective estimates rather than by obtaining objective measures. Such was the case in 1983 when, as part of implicit risk-benefit calculations about donor screening and deferral, blood banks and blood product manufacturers had to make judgments about the risk that their products could transmit AIDS (see Chapter 5). Anticipating the consequences of taking action, which is in the domain of risk management, may bias risk estimates toward values that support risk-averse action. When blood bank officials estimated the risk of transmitting AIDS as ''one per million" transfusions, they chose a rate that was low enough to justify their reluctance to take further action. Despite mounting evidence that the risk was much higher, they maintained their original estimate throughout 1983. If the CDC had made numeric estimates of the risk, and the blood banks, blood product manufacturers, or the FDA had used these estimates in a formal analysis of the decision problem, they might have reached different conclusions about, for example, surrogate testing for AIDS.
Consider the Full Range of Possibilities
When there is uncertainty about the facts that will determine the consequences of a decision, a systematic approach is usually best (NRC 1994). One important principle is to consider the full range of assumptions and alternative actions, not only worst-case scenarios. In the events studied by the Committee, systematic denial of worst-case scenarios was a recurring theme, as can be seen in the way that the NHF and the FDA discussed the CDC's warnings in 1982 and early 1983. The plasma fractionators introduced a worst case scenario of their own at the July 1983 Blood Products Advisory Committee (BPAC) meeting, when they estimated that three or four suspect donors and an automatic recall policy could lead to recall of all of the nation's supply of AHF concentrate (Chapter 6). A closely related principle is to scrutinize the evidence
to ascertain what is based on fact, what is a "best-guess" estimate, and what is simply untested conventional wisdom.
One approach to such an analysis would be to use a formal group process to systematically sample expert opinion on relevant factors such as the probability of infection and the economic and noneconomic costs and benefits of each of the possible outcomes. Often these officials should use decision analysis, which takes into account the likelihood of events and the magnitude of their outcomes, as a tool to compare the expected value of the outcome of the policy alternatives under consideration. Two somewhat analogous models to consider include those used in Institute of Medicine studies to establish priorities for the development of new vaccines (IOM 1985) and to evaluate the artificial heart program of the National Heart, Lung, and Blood Institute (IOM 1991). The book Acceptable Risk (Fischoff, et al. 1981) also offers sensible approaches to dealing with this kind of situation.
Risk Reduction Versus Zero Risk
Decisionmakers tend to seek zero-risk solutions even when they are unattainable or unrealistically costly (NRC 1994). In doing so, they may run the risk of failing to implement solutions that are less effective but are certain to reduce illness. The failure to adopt risk-reduction strategies can be seen in the resistance of blood banks to screening for homosexual activity or using surrogate tests for AIDS (Chapter 5) and in FDA's limited approach to product recall decisions (Chapter 6). Chapter 7 also points out that many risk-reduction strategies for individuals with hemophilia were available but not fully disclosed or recommended. The perfect should not be the enemy of the good.
Risk Communication
Risk communication is a sensitive area because of its influence on the perceptions and behaviors of health professionals and consumers, regulatory policies, and public decisionmaking (Nelkin 1989). Many public health officials and physicians wish to appear in command and infallible. When uncertain, they remain silent rather than disclose their ambivalence (NRC 1989). In the Committee's view, however, the greater the uncertainty, the greater the need for communication. The Committee's analysis of physician–patient communications at the beginning of the AIDS era illustrates the tragedies that can accompany silence about risks (Chapter 7). Risk-communication skills are equally important when presenting information to the general public. The blood banks' reluctance to acknowledge the risk of transfusion-associated AIDS (Chapter 5) seems to
have been due in part to the difficulties that they foresaw in presenting this information to potential donors and recipients.
Other important principles of risk communication are that the source of the information must be credible, the process should be open and two-way, and the message should be balanced and accurate (NRC 1989). When there was no other sources of information for physicians treating people with hemophilia and for their patients, the NHF and its Medical and Scientific Advisory Council (MASAC) took on an important risk-communication role—providing what would now be called "clinical practice guidelines." The NHF's credibility in this area was eventually seriously compromised by its financial connections to the plasma fractionation industry.
Bureaucratic Management of Potential Crises
Federal agencies had the primary responsibility for dealing with the national emergency posed by the AIDS epidemic. The Committee scrutinized bureaucratic function closely, and came to the following conclusions about the management of potential crises.
Coordination and Leadership
A crisis calls for extraordinary leadership. Legal and competitive concerns may inhibit effective action by agencies of the federal government. Similarly, when policymaking occurs against a backdrop of a great deal of scientific uncertainty, bureaucratic standard operating procedures designed for routine circumstances seem to take over unless there is a clear-cut decisionmaking hierarchy. An effective leader will insist upon coordinated planning and execution. Focusing efforts and responsibilities, setting timetables and agendas, and assuming accountability for expeditious action cannot be left to ordinary standard operating procedures. These actions are the responsibilities of the highest levels of the public health establishment.
The Public Health Service failed to bring these leadership functions to bear when CDC scientists raised concerns about the blood supply at the January 4, 1983 meeting but received no public support from the director of the CDC or the office of the Assistant Secretary for Health. Similarly, the record does not indicate that the highest levels of the FDA or the PHS were involved in responding to advice from the BPAC regarding donor deferral or product recall. Part of this leadership problem may stem from major changes in the PHS leadership that took place during this period: the leadership of the FDA, the
CDC, and the NIH, and the person serving as the Assistant Secretary for Health all changed between 1982 and 1984.
Advisory Mechanisms
In the early 1980s, the FDA and other agencies did not have a systematic approach to conducting advisory committee proceedings. Such an approach requires that agencies tell their advisory committees what is expected of them, keep attention focused on high-priority topics, and independently evaluate the advice offered. No regulatory process should have its information base effectively controlled by an advisory panel. Public agencies must be able to generate and analyze the information that they need to assure that decisions serve the needs of the public. The FDA failed to observe this principle when it allowed statements and recommendations of the BPAC to go unchallenged, apparently because it could not independently analyze the information (Chapter 6).
Because mistakes will always be made and opportunities sometimes missed, regulatory structures must be organized and managed to assure both the reality and the continuous appearance of propriety. The prominence of representatives from blood banks and blood product manufacturers on the BPAC, with no balancing influence from consumers and no process within the FDA to evaluate its recommendations (Chapter 6), is a failure of advisory committee management. Perhaps advisory committees should contain fewer topical experts and more members with expertise in principles of good decisionmaking and the evaluation of evidence. A committee so constituted might run a reduced risk of standing accused of having conflicts of interest.
Analytic Capability and Long-Range Vision
Leadership passes to the organization that has access to information and the ability to analyze it. Federal agencies should avoid exclusive reliance upon the entities which they regulate for analysis of data and modeling of decision problems. The FDA should have had some independent capacity to analyze the information presented at the July 1983 BPAC meeting that suggested that with only three or four suspect donors, an automatic recall policy would completely deplete the nation's supply of AHF concentrate (Chapter 6). In addition, there did not seem to be any focus within the Public Health Service prepared to, or charged to, analyze the options, costs, and benefits of the options for protecting the blood supply that were discussed at the January 4, 1983, meeting convened by CDC.
In addition, agencies need to monitor more systematically the long-term outcomes of blood transfusion and blood product infusion and to think far ahead
to anticipate both new technologies and new threats to the safety of the blood supply. Because new pathogens can enter the blood supply and be propagated very rapidly through it, a low level of suspicion about a threat should trigger high-level consideration of how to manage and monitor the problem.
Through its fact-finding interviews and through written documents, the Committee found little evidence that the PHS agency heads and the Assistant Secretary for Health were involved in making decisions about protecting the blood supply in 1983 and 1984 when HIV was becoming increasing apparent as a threat. Most decisions and interagency communication seems to have occurred several levels below the top.
Presumptive Regulatory and Public Health Triggers
The Committee believes that the Public Health Service should prepare for future threats to the blood supply by specifying in advance the types of actions that should occur once the level of concern passes a threshold. In the face of scientific uncertainty, the PHS needs a series of criteria or triggers for taking regulatory or other public health actions to protect the safety of blood and blood products. The Committee favors a series of triggers in which the response is proportional to the magnitude of the risk and the quality of the information on which the risk estimate is based. Not all triggers should lead to drastic or irrevocable actions; some merely require careful consideration of the options or developing new information. This general principle is detailed by examples in each of the Committee's four areas of inquiry. Table 8.1 summarizes these triggers and corresponding actions.
Product Treatment
Whenever they propose new methods of protecting the safety of the blood supply, blood regulatory agencies must perform cost-utility or cost-benefit analyses to evaluate whether the intervention will advance the public health at reasonable costs. If manufacturers do not have market incentives, resources, or access to data to test promising methods, public agencies should create incentives or provide resources or access to data. In this case, the trigger is a new proposal to increase safety, and the action is for the public sector to assume responsibility for thorough analysis and development, or to create incentives for industry to do so.
Table 8.1. Triggers for Taking Actions in Response to Uncertain Risks
|
Trigger Risks |
Action |
|
Product Treatment |
|
|
Proposal to increase safety |
Public sector to assume responsibility for thorough analysis and development |
|
Initiation of risk-benefit or cost-benefit analysis |
Ensure that the analysis takes into account possible secondary and other benefits |
|
Donor Screening |
|
|
Identification of a high-risk population |
Self-deferral and segregation of lots |
|
Plasma fractionators' action to increase screening |
All companies consider why they should not follow suit and set in motion a consensus development mechanism |
|
Availability of a surrogate test or other partially effective interventions that have minimal risks |
Use the test/intervention unless it is certain to be redundant prior to realizing its benefits |
|
Recall |
|
|
Implementation of a new test or treatment process |
Recall untested or untreated products as expeditiously as possible |
|
Beginning of a recall action |
Provide clear guidance and monitoring |
|
Communication of Risk |
|
|
New information relevant to a public health agency's actions |
Tell affected communities what they need to know to make an informed choice among listed options: the facts, the gaps in knowledge, and the implications thereof |
When performing a cost-effectiveness analysis of new treatments for blood products, the potential to protect against other threats should always be a part of the analysis. Here, the trigger is the initiation of a cost-effectiveness analysis, and the action is to ensure that the analysis takes into account secondary benefits.
Donor Screening
Whenever epidemiologists identify a high-risk donor group, the FDA should immediately tell blood banks to create a way to defer that group and tell collection agencies to segregate and separately treat supplies obtained from those populations. Concerns about stigmatizing subpopulations and maintaining the supply of blood products should influence the means of taking actions, not whether to take action. In this case, the trigger to action is the identification of a high-risk population, and the action is deferral and segregation of lots.
Whenever any segment of the industry institutes a donor screening program, the FDA should require all segments of the industry to follow suit with actions that they believe will be at least as effective in promoting safety. Public regulators have a responsibility to monitor these efforts and to forge consensus or to impose the most effective methods as information concerning efficacy becomes available. Here, the trigger is one company's action to take an additional safety measure, and the response is for all companies to follow suit, or to be held accountable when they do not.
Blood banks should use a partially effective intervention that has little or no risk unless they can show that a better method will rapidly supersede it. In this case the trigger is the availability of an inherently risk-free, partially effective intervention, and the response to use that test/intervention unless it is certain to become redundant prior to realizing its full benefits.
Recall
When a test or treatment makes a product safer, manufacturers should withdraw all stocks of untested or untreated product as quickly as possible. Where immediate complete withdrawal might injure the public health, withdrawals should be partial or staged. Here, the trigger is the implementation of a new test or treatment process, and the action is to recall untested or untreated products as expeditiously as possible, given other considerations of public health.
A limited, staged, or selective recall places responsibility on public regulatory agencies to establish criteria for selecting lots for recall, to provide processes to permit effective implementation of the recall by industry, and to monitor the recall to assure that removal of the products occurs in the prescribed manner. In this case the trigger is the initiation of a recall action, and the response is to provide clear guidance and monitoring.
Communication to Patients and Providers
Whenever new information triggers inquiry into a possible threat to the blood supply, both patients and their physicians should have access to the information. Public officials should presume that candid statements and rigorous actions will enhance rather than erode public confidence and that persons using blood or blood products have the right to understand fully the risks and benefits of using these products. In this case, the trigger is new information relevant to the public health, and the action is to tell affected individuals what they need to make an informed choice: the facts, the gaps in knowledge, and the implications thereof.
RECOMMENDATIONS
The Committee's charge was to learn from the events of the early 1980s the lessons that would help the nation prepare for future threats to the blood supply. The Committee identified potential problems with the system in place at that time (as summarized earlier in this chapter) and proposes changes that, if implemented in the early 1980s, might have moderated some of the effects of the AIDS epidemic on recipients of blood and blood products. This analysis has led the Committee to the following recommendations for Public Health Service agencies, for the blood and plasma fractionation industry, and for health care providers and the public. These recommendations address both public health options and individual clinical options.
The Committee is mindful of several caveats. First, the Committee is acutely aware of the difficulties of retrospective analysis, as described in Chapter 1. Second, the Committee has not considered its recommendations from perspectives other than blood safety. Finally, the Committee tried to identify opportunities for institutional change that would respond to the problems that the Committee diagnosed. The Committee based its recommendations on the institutions as they functioned in the early 1980s, not as they exist now. The organizations responsible for blood safety and public health will have to evaluate their current policies and procedures to see if they fully address the issues raised by our recommendations.
The Public Health Service
Several federal agencies necessarily play important, often different roles in managing a public health crisis such as the contamination of blood and blood products by the AIDS virus. The National Blood Policy of 1973 charged the
Public Health Service (including the CDC, the FDA, and the NIH) with responsibility for protecting the nation's blood supply.
Leadership
The Committee has come to believe that a failure of leadership contributed to delay in taking effective action, at least during the period from 1982 to 1984. This failure led to incomplete donor screening policies, weak regulatory actions, and insufficient communication to patients about the risks of AIDS.
In the event of a threat to the blood supply, the PHS must, as in any public health crisis, insist upon coordinated action. The Secretary of Health and Human Services is responsible for all the agencies of the Public Health Service,1 and therefore the Committee makes
Recommendation 1: The Secretary of Health and Human Services should designate a Blood Safety Director, at the level of a deputy assistant secretary or higher, to be responsible for the federal government's efforts to maintain the safety of the nation's blood supply.
Choosing a "lead person" is important because it is in the nature of federal agencies and their leaders to be at once competitive and protective. This condition is healthy in reasonable measure and in normal times. However, a serious threat to public health requires that agencies communicate, cooperate, and learn to view the world through each other's lenses. Once there is an action plan, the Secretary of Health and Human Services must hold the agency leaders accountable for enforcing cooperation in implementing the plan.
To be effective in coordinating the various agencies of the PHS, the Blood Safety Director should be at the level of a deputy assistant secretary or higher, and should not be a representative of any single PHS agency. When a threat does arise, the Blood Safety Director should create a crisis management team.
One such action was to establish, in July 1982, the Committee on Opportunistic Infections in Hemophiliacs (see Chapter 3). This group seems to have been organized by the CDC, but there is no record of its operations after August of that year.
Blood Safety Council
The AIDS crisis revealed that the institutions in place to ensure blood safety, both public and private, were unable to work cooperatively toward a common goal of a safe blood supply. The institutions were not accountable to anyone but themselves, and they failed to cooperate, to coordinate their activities, and to communicate effectively with physicians and the public. The Committee has become convinced that the nation needs a far more responsive and integrated process to detect, evaluate, and respond to emerging threats to the blood supply. To this end the Committee makes
Recommendation 2: The PHS should establish a Blood Safety Council to assess current and potential future threats to the blood supply, to propose strategies for overcoming these threats, to evaluate the response of the PHS to these proposals, and to monitor the implementation of these strategies. The Council should report to the Blood Safety Director (see Recommendation 1). The Council should also serve to alert scientists about the needs and opportunities for research to maximize the safety of blood and blood products. The Blood Safety Council should take the lead to ensure the education of public health officials, clinicians, and the public about the nature of threats to our nation's blood supply and the public health strategies for dealing with these threats.
Supplying safe blood and blood products to the nation—a public good—requires the cooperation of public and private institutions. The Blood Safety Council would give voice to the public's interest in having these institutions cooperate and would provide opportunities for them to do so.
The lessons of HIV transmission through blood and blood products show the need for an advisory council with a significantly greater level of diversity, responsibility, and authority than the current Blood Products Advisory Committee of the FDA. The BPAC is limited by the regulatory mission of the FDA which it advises, and there is no other body primarily concerned with blood safety as a whole. Representatives from governmental agencies, academia, the blood bank community, industry, and the public all have relevant expertise and perspectives and should be involved in the Blood Safety Council. A broad-based range of expertise in areas of hematology, infectious diseases, epidemiology, blood product manufacturing, blood collection and delivery, risk assessment, consumer advocacy, and cost-benefit analysis is essential.
The proposed Blood Safety Council would facilitate the timely transmission of information, assessment of risk, and initiation of appropriate action both during times of stability and during a crisis. The Council should report to the Blood Safety Director (see Recommendation 1). The Council would not replace
the PHS agencies responsible for blood safety but would complement them by providing a forum for them to work together and with private organizations. The PHS agencies would be represented on the Council (see below and Figure 8.1). The Council would not have its own surveillance capability, but would work with CDC and FDA to interpret the information that those organizations can provide. It would not carry out or fund research itself, but would work with those at NIH and in the private sector to identify priorities for blood safety research. The Council would not have regulatory power, but would inform FDA actions from a blood safety rather than a product-specific perspective.
The organizations and groups that should be included in the Blood Safety Council, and the reasons for including them, are as follows:
-
The FDA can provide a direct link between itself, the essential regulatory agency responsible for the safety of blood and blood products, and important sources of information, scientific support, and disease surveillance findings.
-
The CDC can provide expertise in epidemiology, infectious diseases, and immunology as well as communicate the results of ongoing disease surveillance studies. The CDC's newly established emerging infectious disease program would also provide valuable information.
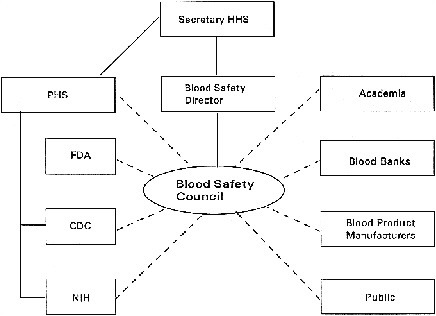
Figure 8.1. Blood Safety Council relationships.
-
The NIH can provide scientific expertise and the means to communicate information about essential research needs to the appropriate institutes for support of research.
-
Representatives from academia can bring independent scientific and medical expertise, especially in hematology, infectious diseases, epidemiology, risk assessment, and cost-effectiveness analysis.
-
Representatives from the volunteer blood collection community can bring experience with blood safety concerns and the knowledge of blood bank operations that is necessary to evaluate proposed change.
-
Representatives from the private-sector blood product manufacturers and biotechnology companies can bring both experience with blood safety concerns and knowledge of plasma fractionation operations.
-
Representatives of the general public (who may in the future require blood transfusions) and individuals who currently require frequent use of blood products, such as hemophilia patients, bring important perspectives on the trade-offs that must be considered in evaluating response options.
The Blood Safety Council should consider the following activities and issues:
Surveillance. Although the FDA and the CDC keep track of events in blood and blood product recipients, their surveillance systems are passive and incomplete. The Blood Safety Council should work with the CDC to design a system of active surveillance for adverse reactions in blood recipients, as described in Recommendation 5 below. If such a system is established, the Council would benefit from its results and should participate in its governance.
Expert Panel on Best Practices. Drawing on its members' knowledge about blood and blood product safety concerns, and about clinical alternatives, the Blood Safety Council could establish a panel of experts to provide the public and providers of care with information about risks and benefits, alternatives to using blood products, and recommended best practices, as described in more detail in Recommendation 13 below.
Investigate Methods to Make Blood Products Safer. The Council should evaluate new methods to make blood and blood products safer. One promising approach is double inactivation in the preparation of blood products, which minimizes the risk of transmission of infectious pathogens in the blood of the donor pool. At present, the FDA requires only a single inactivation process (usually solvent detergent or heat treatment) for most blood products manufactured in the United States. With the goal of maximizing the safety of the
blood supply at minimal added cost, the Blood Safety Council should encourage the FDA to evaluate double inactivation methods and expeditiously relicense products manufactured by the improved technologies, if appropriate. The Blood Safety Council should also consider, at least yearly, in a public forum, opportunities to maximize the safety of the blood supply.
Another promising approach is to reconsider minimum pool size requirements in plasma product manufacturing. The FDA currently requires a large number of donors to be included in plasma pools used in the manufacture of plasma products in order to ensure a wide range of antibodies in preparations of intravenous gamma globulin. Pooling of plasma obtained from numerous donors, although permitting some economy of scale, also increases the risk that a large fraction of manufactured blood products will be contaminated by a single infected donor. The Blood Safety Council should consider this issue and address the safety and efficiency trade-offs in changing the minimum pool size.
The Blood Safety Council would provide information relevant to the decisions that individuals as well as public and private decisionmakers need to make. The forum would not have direct regulatory or other authority, but would function as a forum for holding the organizations with authority responsible for blood safety. In short, the Blood Safety Council could advocate the public's need for a responsible process for decisionmaking about public health policy. The following examples illustrate how regular public discussions of blood safety issues, in the presence of representatives from the relevant organizations' perspectives, could provide an opportunity to hold the organizations with authority accountable for blood safety.
If it had existed in the 1970s, for instance, the Blood Safety Council might have called for the development of heat-treated AHF concentrate to reduce the risk of hepatitis, which would have also reduced the risk of HIV transmission. It would have been able to do so if the NIH and blood products industry representatives on the Council had been called upon to make periodic reports to the Council during the 1970s about their efforts to deal with the hepatitis problem. These representatives would have fed the discussions of the Council back into their own organizations' decisionmaking.
In 1983, the Council could have provided a forum for CDC to present its concerns about HIV in the blood supply and held the FDA, the NHF, and the blood banks and fractionators accountable for responding constructively. CDC created a forum on its own by convening the January 4, 1983, meeting in Atlanta, but as the Committee's analysis indicates, the follow-up on this meeting was insufficient. If a standing Blood Safety Council had existed, the CDC scientists who had concerns about the safety of blood and blood products would have had an opportunity to hold blood collection organizations accountable for their decisions regarding donor deferral and surrogate testing. It would also provide an opportunity to hold plasma fractionators and the FDA accountable for its decisions with regard to heat-treated AHF.
Later that year, the Council could have provided a mechanism to evaluate the claims that automatic recall of AHF would have virtually eliminated the supply of AHF. As the analysis in Chapter 6 indicates, neither the BPAC nor the FDA staff had the capacity to analyze claims that a automatic recall would have such an effect. The Blood Safety Council could have insisted that the FDA commission a formal decision analysis of the options for surrogate testing, or the Council might have performed such an analysis itself. The FDA would retain its regulatory authority, and continue to get advice from the BPAC, but the Council would have provided critical information relevant to the agency's decision.
Finally, if the Council had established an expert panel on best practices as described above and in Recommendation 13, hemophilia patients and their physicians would have had a more credible source of information about the risks of HIV infection and their clinical options than the NHF was able to provide. The operations of such a panel are described below under Recommendation 13.
Compensation Policy
When a product or service provided for the public good has inherent risks, the common law tort system fails to protect the rightful interests of patients who suffer harm resulting from the use of those products or services. Each claim requires extended, costly, and complex adjudicative procedures to establish liability. The results are erratic and unpredictable, and therefore inequitable (IOM 1985).
The doctrine of strict liability holds manufacturers accountable for injuries that are incurred from products that are inherently dangerous because diligence cannot fully eliminate their risks. The public health imperative of assuring enough vaccine for widespread use argues for limits on the strict liability doctrine for vaccine-related injuries. The chief concern is that fear of liability will discourage manufacturers from producing a vital public good. To vitate this concern, a federal compensation system has removed vaccine-related injuries from the scope of strict liability laws (Mariner 1992). The federal government established a mechanism for compensating individuals suffering harm from vaccine-related complications. Its rationale is that consent to undergo vaccination confers benefits to the entire community.
Blood-product-related injuries have also been removed from the scope of strict liability law by blood shield laws, which are in force in most states, and which protect society's interests in having an adequate blood supply. The blood shield laws serve to protect providers and manufacturers of blood and blood products from liability claims in instances where they take all due care to ensure the safety of the product. These laws, however, are unique in the manner in which they limit liability. The shield laws have made it difficult, and often impossible, to obtain compensation for HIV infection acquired from blood or
blood products. To address this asymmetry between the protection that blood shield laws offer for manufacturers and adequate protection of individual rights, the Committee makes
Recommendation 3: The federal government should consider establishing a no-fault compensation system for individuals who suffer adverse consequences from the use of blood or blood products.2
An effective no-fault system requires prospective standards and procedures to guide its operations. In a no-fault system, individual plaintiffs would not have to prove that their adverse outcome was a result of negligence related to manufacture of a blood product. Therefore, there needs to be an objective, science-based process to establish which categories of adverse outcomes are caused by blood-borne pathogens and which individual cases deserve compensation. As with vaccines, a tax or fee paid by all manufacturers or by the recipients of blood products could finance a compensation system. Rather than attempt to allocate blame for HIV infections through blood and blood products, some countries have established such no-fault compensation programs for individuals infected with HIV as a result of their use of blood and blood products. Countries fund these programs in a variety of ways, including direct government support and joint public/private resources.
Making recommendations about compensating affected individuals for damages incurred in the past is outside the Committee's mandate. However, had there been a no-fault compensation system in the early 1980s, it could have relieved much financial hardship suffered by many who became infected with HIV through blood and blood products in the United States. The no-fault principles outlined in this recommendation might serve to guide policymakers as they consider whether to implement a compensation system for those infected in the 1980s.
The Centers for Disease Control and Prevention
The CDC has an indispensable role to play in protecting our nation's health: to detect potential public health risks and sound the alert. Because of its expertise in detecting and evaluating possible infectious disease outbreaks, the Committee believes that the CDC should take responsibility for a surveillance system to detect adverse outcomes from blood and blood products. The following two
recommendations embody an important principle: separating the assessment of risk from the management of the consequences of risk. The FDA, in its role as guarantor of the safety of the blood supply, has the responsibility for managing threats to the blood supply. The CDC should detect potential threats and assess the magnitude of the danger.
Early Warning Systems
A nation needs individuals and organizations that identify problems and raise concerns that may be difficult to confront. The CDC plays this role in the Public Health Service. The CDC appears to have been prescient in raising the possibility that the blood supply was contaminated early in the AIDS epidemic, but it was relatively ineffective in convincing other agencies of the potential gravity of the situation. In order to improve CDC's efficacy in this critical role, the Committee makes
Recommendation 4: Other federal agencies must understand, support, and respond to the CDC's responsibility to serve as the nation's early warning system for threats to the health of the public.
Officials in the government, scientists, and physicians in the private sector seem to have discounted the CDC warnings about the transmissibility of AIDS through blood and blood products because the swine flu episode in the 1970s had cost the agency considerable credibility. If, in 1983, the involved public and private organizations had the attitude called for in this recommendation, CDC's recommendations regarding donor screening and surrogate testing might have led to earlier, more effective screening and donor deferral policies.
Consistent with the precept of separating risk assessment and risk management as described above, CDC's role is to characterize and assess risks, and communicate this to others. The FDA and other organizations have the responsibility to manage the risks through regulation, clinical practice guidelines, and other means. The Committee believes that CDC should be able to play its designated role without fearing loss of credibility if it sometimes proves to be wrong. Implementing this recommendation may be difficult. As a start, the Secretary of Health and Human Services should insist that an agency that wishes to disregard a CDC alert should support its position with evidence that meets the same standard as that used by the CDC in raising the alert.
Surveillance
In order to carry out its early warning responsibility effectively, the CDC needs good surveillance systems. Because blood products are derived from
human beings and may contain harmful biologic agents that were present in the blood of a donor, blood products are inherently risky, a principle long recognized by blood shield laws. The Committee, believing that the degree of surveillance should be proportional to the level of risk, makes
Recommendation 5: The PHS should establish a surveillance system, lodged in the CDC, that will detect, monitor, and warn of adverse effects in the recipients of blood and blood products.
If such a system had existed in 1982, data about the risks of HIV transmission through blood and blood products might have been available sooner and might have been more definitive. In dealing with newly approved pharmaceuticals, the FDA increasingly demands careful post-approval study of potential adverse effects (the so-called ''Phase IV Trial"). Two existing systems for vaccine adverse events—the CDC/FDA Vaccine Adverse Event Reporting System (VAERS) and the CDC's Large-Linked Database (LLDB)—might be useful models (Institute of Medicine 1994).
The Food and Drug Administration
The FDA has legal authority to protect the safety of the nation's blood supply. Accordingly, it is the lead federal agency in regulating blood-banking practice, the handling of source plasma, and the manufacture of blood products from plasma. The Committee found cause for concern when it evaluated the FDA's actions in protecting the public from HIV in the nation's blood supply during the 1980s. The record reveals many opportunities to improve the agency's capacity to deal with crises involving the blood supply, most notably with respect to the safety of AHF concentrate. In responding to these opportunities, the Committee's recommendations focus on decisionmaking and the role of advisory committees in formulating the FDA's response to crises.
Risk Reduction
In a crisis, decisionmakers may become so preoccupied with seeking solutions that will dramatically reduce danger that they will fail to implement solutions that are less effective but are likely to improve public safety to some degree. Partially effective risk-reducing improvements, as described herein, can save lives, pending the development of more efficacious safety measures. In order that the perfect not be the enemy of the good, the Committee makes
Recommendation 6: Where uncertainties or countervailing public health concerns preclude completely eliminating potential risks, the FDA should encourage, and where necessary require, the blood industry to implement partial solutions that have little risk of causing harm.
In the event of a future threat to the blood supply, the FDA should encourage small, low-risk solutions to large, difficult problems. The FDA's actions during the early 1980s are evidence that the agency should change its attitude toward regulation in order to adopt this proactive approach. Some examples from Chapter 6 illustrate how the FDA might have encouraged practices that would have reduced the risk faced by recipients of blood or a blood product.
Example: Destroy Unscreened Blood When Possible. When hospital blood banks first started to screen donors by questioning them for risk factors, there was a period of transition during which its stocks contained two classes of blood or plasma: blood from screened donors, which was relatively safe; and blood from unscreened donors, which had a higher probability of containing HIV. Within a few weeks of starting to screen donors, blood from unscreened donors would have been either used or discarded. In the instructions contained in its letter of March 24, 1983, the FDA could have recommended that blood banks adopt a policy of using blood from screened donors whenever possible during the transition period, a policy that some blood banks may have adopted on their own. Requiring all blood banks to adopt this policy would not have compromised the nation's blood supply, and it would have prevented at least a few instances in which a patient received an infected unit of blood.
Example: Destruction of Potentially Contaminated Cryoprecipitate. Blood banks store cryoprecipitate from a single unit of donated blood in the frozen state for up to one year. The FDA could have issued a directive that required the blood banks to check their inventory of frozen cryoprecipitate and destroy possibly contaminated units whenever they learned of a previous donor who had AIDS or was strongly suspected of having AIDS.
Example: Phased Recall. In July 1983, there was considerable reluctance to recall untreated Factor VIII concentrate at a time when much of the supply was almost certainly contaminated with HIV. The FDA apparently feared that the ensuing shortage of Factor VIII would have caused more harm than the HIV virus. A phased withdrawal would have been a compromise between no withdrawal and immediate total withdrawal. This middle path might have avoided a factor concentrate shortage and still reduced the number of hemophiliacs who became infected.
Example: Lookback. The FDA formally instituted a "lookback" policy in 1991, years after it was clear that AIDS had a long incubation period during which a patient could transmit HIV through sexual contact or contact with blood. Lookback required blood banks to contact recipients of blood from infected donors and notify them that they might be a HIV carrier and should be tested for HIV antibodies. Earlier action on lookback might have reduced secondary transmission of HIV.
Decision Processes
In all fields, decisionmaking under uncertainty requires an iterative process. As the knowledge base for a decision changes, the responsible agency should reexamine the facts and be prepared to change its decision. The agency should also assign specific responsibility for monitoring conditions and identifying opportunities for change. In order to implement these principles at the FDA, the Committee makes
Recommendation 7: The FDA should periodically review important decisions that it made when it was uncertain about the value of key decision variables.
An example illustrates the principle of iterative decisionmaking. During 1983, most blood bank officials opposed asking prospective male donors if they had ever had sex with a man. They were worried that regular donors might take offense and stop donating blood. They were also concerned about some gays would lie about their homosexuality and donate blood in reprisal for being singled out as the target of the questioning. Eventually, some blood collection centers began to ask questions about sexual preference. If the FDA had carefully monitored these experiments, it would have soon learned that the blood bank officials' fears were groundless. The FDA might then have revised its requirements for donor screening to include direct questions about high-risk sexual practices.
Regulatory Efforts
Although the FDA has a great deal of regulatory power over the blood products industry, the agency appears to regulate by expressing its will in subtle, understated directives. This informal approach to regulation is often necessary to permit a timely response and to preserve needed flexibility. The FDA used this approach, for example, in July 1983 when it issued recommendations to withdraw lots of AHF concentrate that plasma fractionators had identified as
containing material from a donor that had AIDS. The language in the July 1983 communication failed to specify, however, whether the agency considered the recommendations to be binding on industry. While most regulated industries might have interpreted these letters as mandatory, that question should not have been left to the judgment of individual entities. Taking this into account, the Committee makes
Recommendation 8: Because regulators must rely heavily on the performance of the industry to accomplish blood safety goals, the FDA must articulate its requests or requirements in forms that are understandable and implementable by regulated entities. In particular, when issuing instructions to regulated entities, the FDA should specify clearly whether it is demanding specific compliance with legal requirements or is merely providing advice for careful consideration.
In 1983, the FDA chose a middle ground when faced with the decision to withdraw all AHF concentrate. The agency recommended that plasma fractionators withdraw individual lots of AHF concentrate when a donor was suspected of having AIDS. This decision was certainly defensible. However, the process for this "case-by-case" withdrawal was seriously compromised by the vagueness of the criteria specified for a recall. The agency failed to specify a process for deciding whether a donor may have had AIDS. The agency should have specified a process for reviewing donors who did not fully satisfy the diagnostic criteria for AIDS but who were suspected of having the disease. When deciding whether to withdraw a lot of AHF concentrate, the FDA asked plasma fractionators to take into account the time of the donation in relation to the diagnosis of AIDS and the effect of the recall on product availability. However, the FDA did not specify parameters for assessing either of these decision criteria. With greater forethought, the FDA could have avoided the potential for a seriously flawed implementation of a policy that otherwise appeared to balance benefits, risks, and harms.
Advisory Committees
The FDA made several decisions in 1983 that appear to have been influenced by the blood-industry-based (profit and nonprofit) members of the BPAC. The BPAC membership did not include individuals with expertise in the social, ethical, political, and economic aspects of the issues that BPAC was deliberating at the time. The FDA apparently did not seek independent analysis of the recommendations made by the members of the BPAC, some of whom were employed by the blood industry. In the early 1980s, the FDA appeared too
reliant upon analyses provided by industry-based members of the BPAC and the BPAC. For example, see the discussion in Chapter 6 of the July 19, 1983, BPAC meeting which resulted in the decision for case-by-case rather than automatic recall of lots of AHF when one donor was suspected of having AIDs. Chapter 6 also contains a discussion of the December 15, 1983, BPAC meeting, which effectively curtailed actions on surrogate testing of blood for months. The Committee's analysis of the FDA's management of its advisory committee leads to the following three recommendations:
Recommendation 9: The FDA should ensure that the composition of the Blood Products Advisory Committee reflects a proper balance between members who are connected with the blood and blood products industry and members who are independent of industry.
The FDA should select some BPAC members because they can provide independent judgment, question the analyses provided by blood-industry-based BPAC members, and hold the FDA accountable for a high standard of public responsiveness. The BPAC should have at least one voting member who is a representative of consumer interests. BPAC members who vote to establish policy should have neither the appearance of a conflict of interest nor a true conflict of interest.
An agency that is practiced in orderly decisionmaking procedures will be able to respond to the much greater requirements of a crisis. The BPAC meetings cited before Recommendation 9 above provide examples to support this recommendation. Applying this principle to the use of advisory committees, the Committee makes
Recommendation 10: The FDA should tell its advisory committees what it expects from them and should independently evaluate their agendas and their performance.
The FDA staff and its advisory committees should structure their relationship so that they invigorate each other. The agency should hold an advisory committee accountable for its performance through periodic independent evaluation. By placing unresolved issues on future agendas, the committee can hold the FDA accountable for taking follow-up action between committee meetings. The IOM Committee to Study the Use of Advisory Committees by the Food and Drug Administration makes further recommendations to strengthen the FDA advisory committee system (IOM 1992).
Advisory committees provide scientific advice to the FDA; they do not make regulatory decisions for the agency (IOM 1992). As Chapter 6 indicates, the
FDA in 1983 did not independently verify the estimates of the risk of blood-product-related HIV infection. The FDA did not analyze the public health implications of the BPAC's recommendation against automatic recall of AHF concentrate that contained plasma from donors suspected of having AIDS. The FDA's lack of independent information and its own analytic capacity meant that it had little choice but to incorporate the advice of the BPAC into its policy recommendations. To ensure the proper degree of independence between the FDA and the blood products industry, the Committee makes
Recommendation 11: The FDA should develop reliable sources of the information that it needs to make decisions about the blood supply. The FDA should have its own capacity to analyze this information and to predict the effects of regulatory decisions.
Communication to Physicians and Patients
One of the crucial elements of the system for collecting blood and distributing blood products to patients is the means by which to convey concern about the risks inherent in blood products. In today's practice of medicine, in contrast to that of the early 1980s, patients and physicians each accept a share of responsibility for making decisions. Patients' informed consent is required for risky procedures. From early 1983, it was clear that AHF concentrate was a risky product. The failure to tell hemophilia recipients of Factor VIII concentrate about the risks of this treatment and about alternative treatments seems especially serious in the light of present-day emphasis on the autonomy of patients in decisions involving their health.
Clinical Practice
One powerful lesson of the AIDS crisis is the importance of telling patients about the potential harms of the treatments that they are about to receive. The NHF dedicated itself to providing information to individuals with hemophilia and their physicians. Their strategy, however, was seriously flawed. As discussed in Chapter 7, the NHF provided treatment advice, not the information on risks and alternatives that would enable physicians and patients to decide for themselves on a course of treatment. Hemophilia patients did not have the basis for informed choice about a difficult treatment decision.
Considerable scientific and medical uncertainties characterized the early years of the AIDS epidemic. For individuals medically dependent on the use of blood and blood products, these uncertainties created complex dilemmas about clinical options for their continued care. In instances of great uncertainty, it is
crucial for patients to be fully apprised of the full range of options available to them and to become active participants in the evaluation of the relative risks and benefits of alternative treatments. As the case studies in Chapter 7 indicate, the failure to communicate adequately about these options prevented many hemophiliacs from making choices in which they accepted responsibility for balancing the risk of AIDS and the risks of bleeding. Ultimately the failure to communicate led to a powerful sense of betrayal that exacerbated the tragedy of the epidemic for many patients and their families. To encourage better communication, the Committee makes
Recommendation 12: When faced with a decision in which the options all carry risk, especially if the amount of risk is uncertain, physicians and patients should take extra care to discuss a wide range of options.
Medicine has many "gray areas" in which the correct course of action is not clear. Guidelines should identify these areas and spotlight the importance of full disclosure of risks, discussion of the broadest range of clinical options, and incorporation of the patient's preferences into an individualized recommendation. Given the inherent risks and uncertainties in all blood products, the public and the providers of care need expert, unbiased information about the blood supply. This information includes risks and benefits, alternatives to using blood products, and recommended best practices. As Chapter 7 indicates, the NHF (the only organization that stepped in to provide information to hemophiliacs and the physicians who were treating them) focused on practice recommendations rather than complete information on risks and options. In order to provide the public and providers of care with the information they need, the Committee makes
Recommendation 13: An expert panel should be created to inform the providers of care and the public about the risks associated with blood and blood products, about alternatives to using them, and about treatments that have the support of the scientific record.
One lesson of the AIDS crisis is that a well-established, orderly decisionmaking process is important for successfully managing a crisis. This applies as much to clinical decisionmaking as to the public health decision process addressed by the earlier recommendations. As the narrative indicates, there are both public health and clinical approaches to reducing the risk of blood-borne diseases. The Blood Safety Council called for in Recommendation 2 would deal primarily with risk assessment and in the public health domain, actions that would reduce the chance that blood products could be vectors of infectious agents. The primary responsibility of the expert panel on best practices called for in Recommendation 13 would be to provide the clinical information that
physicians and their patients need to guide their individual health care choices. To be most effective, this panel should be lodged in the Blood Safety Council (see Recommendation 2) so that both bodies can interact and coordinate their activities in order to share information about emerging risks and clinical options.
Any organization that supplies this information must adhere to accepted norms for documenting evidence. The Committee believes that the public's interest would be best served by creating one publicly accountable source of this information. This function would build on the experience of the Agency for Health Care Policy and Research, which has an established guideline development process and issues guidelines on topics such as the management of chronic pain, screening for AIDS, and management of urinary incontinence (El-Sadr, et al. 1994; Jacox, et al. 1994).
Experience in developing practice guidelines for hemophilia treatment and blood transfusion is an important element of preparedness for future threats to the blood supply. There are now well-established processes such as those recommended by the IOM Committee to Advise the Public Health Service on Practice Guidelines (IOM 1990, 1992) and used by the Agency for Health Care Policy and Research. The U.S. Preventive Services Task Force (1989) uses another system process. Guideline developers should perform a thorough literature search, identify well-designed studies, describe fully the evidence on harms and benefits, and explain the connection between the evidence and the recommendations. They should seek critical evaluation from a wide spectrum of individuals and organizations and should periodically reexamine the recommendations in the light of changing knowledge.
Credibility
During the early 1980s, in its role as the guardian of the interests of the hemophilia patient community, the NHF was the principal source of information about using blood products. The outcome of the NHF efforts was that individuals with hemophilia and their families lost faith in the NHF as the rightful steward of their interests. The reasons discussed in Chapter 7 include the NHF's unwavering recommendation to use AHF concentrate, its dependence on funds contributed by the plasma fractionation industry, and the composition of the NHF expert panel (MASAC) that formulated treatment recommendations (e.g., the panel's lack of infectious disease experts and decision analysts).
Toward the end of providing the highest-quality, most credible information to patients and providers, the Committee makes
Recommendation 14: Voluntary organizations that make recommendations about using commercial products must avoid conflicts
of interest, maintain independent judgment, and otherwise act so as to earn the confidence of the public and patients.
One of the difficulties with using experts to give advice is the interconnections that experts accumulate during their careers. Organizations that regulate an industry may get advice from the same experts who advise the industries. Organizations that give treatment advice may rely on experts whose employer relies upon support from industry. As a result, an expert may have a history of relationships that raise concerns about whether he or she can be truly impartial when advising a course of action in a complex situation. The Committee believes that the best way to avoid these risks is to choose some panelists who are not expert in the subject of the panel's assignment but have a reputation for expertise in evaluating evidence, sound clinical judgment, and impartiality.
Financial conflicts of interest influence organizations as well as individuals. As indicated in Chapter 7 and above, the financial relationships between the NHF and the blood products industry seriously compromised the NHF's credibility. The standards for acknowledging conflicts of interest are higher than they were 12 years ago. Public health officials and the medical professions must uphold this new standard. Failure to do so will threaten the fabric of trust that holds our society together.
REFERENCES
Douglas, M. Risk Acceptability According to the Social Sciences. Russell Sage Foundation, New York; 1985
El-Sadr, W., et al. Evaluation and Management of Early HIV Infection, Clinical Practice Guideline No. 7. AHCPR Publication No. 94-0572. Rockville, MD: Agency for Health Care Policy and Research, Public Health Service, U.S. Department of Health and Human Services; January 1994.
Fischoff, B. Treating the Public with Risk Communications: A Public Health Perspective. Science, Technology and Human Values, vol. 12; 1987.
Fischoff, B., et al. Acceptable Risk. 1981.
Institute of Medicine. Clinical Practice Guidelines: Directions for a New Program. M.J. Field and K.N. Lohr, eds. National Academy Press, 1990.
Institute of Medicine. Guidelines for Clinical Practice: From Development to Use. M.J. Field and K.N. Lohr, eds. National Academy Press, 1992.
Institute of Medicine. Research Strategies for Assessing Adverse Events Associated with Vaccines, National Academy Press, 1994.
Institute of Medicine. Vaccine Supply and Innovation, National Academy Press, 1985.
Institute of Medicine. The Artificial Heart: Prototypes, Policies and Patents. J.R. Hogness and M. VanAntwerp, eds., National Academy Press, 1991.
Jacox, A., et al. Management of Cancer Pain, Clinical Practice Guideline No. 9. AHCPR Publication No. 94-0592. Rockville, MD: Agency for Health Care Policy and Research, Public Health Service, U.S. Department of Health and Human Services; March 1994.
Mariner, Wendy K., "Legislative Report: The National Vaccine Injury Compensation Program," Health Affairs, Spring 1992 vol. II.
National Research Council. Improving Risk Communication. National Academy Press, Washington, D.C.; 1989.
National Research Council. Risk Assessment in the Federal Government: Managing the Process. National Academy Press, Washington, D.C.; 1983.
National Research Council. Science and Judgment in Risk Assessment . National Academy Press, Washington, D.C.; 1994.
Nelkin, D. Communicating Technological Risk: The Social Construction of Risk Perception. Annu. Rev. Public Health, 1989.
U.S. Preventive Services Task Force. Guide to Clinical Preventive Services. Williams and Wilkins, Baltimore, Maryland; 1989.






























