2
The U.S. Blood Supply System
INTRODUCTION
The U.S. blood supply system is comprised of many organizations with different management structures and philosophies. Table 2.1 lists each of the major organizations that function to meet the nation's blood needs. To provide the context for the Committee's analysis, this chapter provides information on blood and blood products, the organizations that collect, manufacture, and distribute them, and the professional and trade associations that represent these organizations. Because of the special role of hemophilia in the Committee's analysis, this chapter also provides background information on the National Hemophilia Foundation and related organizations. Finally, this chapter also presents information on the federal agencies responsible for blood safety, the history of blood and blood product regulations, and the regulatory authority of the FDA.
BLOOD AND BLOOD PRODUCTS
There are two different types of blood collection activities. One blood collection and supply system involves the cellular elements and plasma obtained from whole blood, and the other involves large-scale collection of the plasma portion of whole blood and the subsequent manufacture of derivatives produced from that plasma as a raw material. Before describing these two types of activities, a brief summary of the products produced from whole blood and plasma is helpful.
Table 2.1 Major Organizations Comprising the Blood Supply System and Their Functions
|
Organization |
Function |
|
Federal Agencies |
|
|
Department of Health and Human Services |
Direction and oversight |
|
Public Health Service |
Direction and oversight |
|
Food and Drug Administration |
Regulation and review |
|
Center for Biologics Evaluation and Review Blood Products Advisory Committee |
Regulation, review, and research scientific advice |
|
Centers for Disease Control and Prevention |
Surveillance, investigation, and information dissemination |
|
National Institutes of Health |
Biomedical research |
|
Blood Collection Organizations |
|
|
American Red Cross |
Blood collection and supply, research |
|
Community blood banks |
Blood collection and supply, information exchange |
|
Hospital blood banks |
Blood collection and patient care |
|
For-Profit |
|
|
Plasma fractionation industry |
Plasma collection and supply, manufacturing, research |
|
Professional and Trade Associations |
|
|
American Association of Blood Banks |
Representing blood collection and transfusion services organizations, standard setting (inspection and accreditation program), and education |
|
American Blood Resources Association |
Advocacy for plasma fractionation industry, education |
|
Organization |
Function |
|
Council of Community Blood Centers |
Representing blood collection, information exchange |
|
Nonprofit-Patient Advocacy |
|
|
National Hemophilia Foundation |
|
|
Medical and Scientific Advisory Council |
Advocacy, education, and information dissemination Medical and scientific advice |
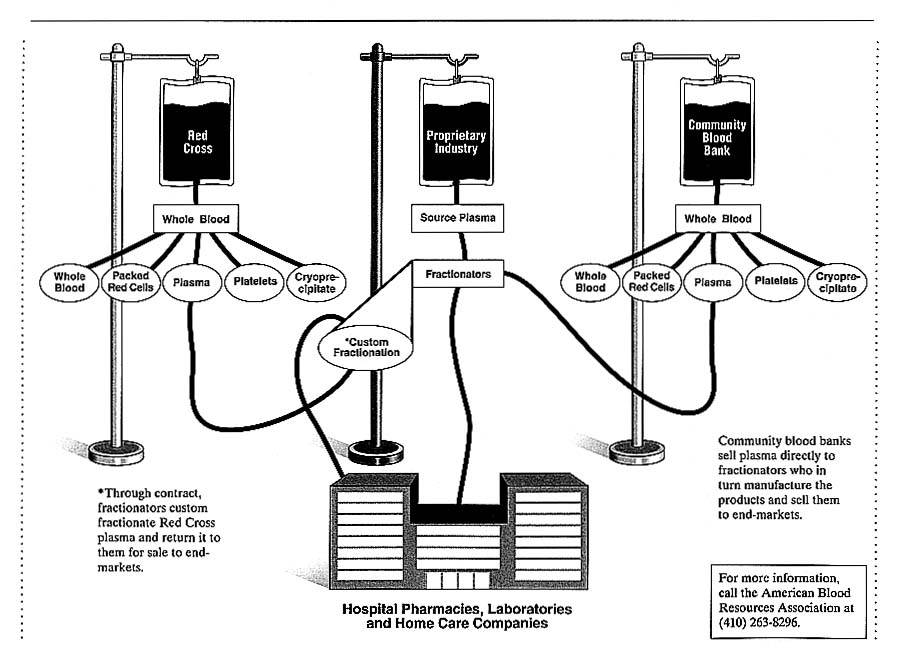
Blood is composed of plasma and several cellular elements which include red cells (erythrocytes), five kinds of white cells (leukocytes, many with important subtypes), and platelets. Either whole blood can be collected or the plasma portion of the blood can be collected with the cellular portion returning to the donor. Whole blood is collected by blood banks, which prepare the cellular products and unprocessed plasma used directly for transfusion. Plasma is collected and used as raw material to commercially produce plasma "derivatives," which are concentrated forms of selected plasma proteins (Figure 2.1).
Whole Blood and Components
Whole blood is collected by venipuncture from healthy adults into plastic bags containing a liquid anticoagulant preservative solution. About 450 milliliters of blood can be collected as often as every 56 days without harm to the donor. The whole blood is separated into components within eight hours after collection. The components are red blood cells, platelet concentrate, and fresh frozen plasma. The fresh frozen plasma can be used in one of three ways: (1) for transfusion; (2) for further processing into cryoprecipitate (i.e., fresh or frozen plasma) to be used for transfusion, and cryoprecipitate poor plasma, which serves as a source of raw material for further manufacture of plasma derivatives; or (3) as a source of raw material for subsequent manufacture of plasma derivatives as described below.
As shown in Table 2.2, among the components prepared from whole blood are red blood cells, platelets, fresh frozen plasma, and cryoprecipitate. Blood banks make many modifications of these components to obtain blood products that will be effective for specific purposes. In addition, blood banks distribute
Table 2.2 Components Produced by Blood Banks and the Medical Use of the These Components
|
Component |
Medical Use |
|
Red blood cells |
Oxygenate tissues |
|
Platelets |
Prevention or stopping of bleeding |
|
Fresh frozen plasma |
Stop bleeding |
|
Cryoprecipitate |
Stop bleeding |
|
Cryoprecipitate poor plasma |
Plasma exchange |
|
Granulocytes |
Treat infection |
|
Frozen red blood cells |
Store rare blood |
|
Leukocyte-depleted red blood cells |
Prevent reactions and certain diseases |
many of the plasma derivative products as part of their total supply program for transfusion medicine therapy, but most of these other plasma products are actually manufactured commercially by plasma fractionation companies.
Because the United States has a pluralistic system of blood collection, there is no central repository of data about the number of units of blood collected or the components produced or transfused. The American Red Cross (ARC) collects about 45 percent of the 14 million units of whole blood available for use annually in the United States. Other community blood banks collect about 42 percent, hospitals collect about 11 percent, and the remaining 2 percent is imported. In 1989, a total of 12,544,000 units of whole blood were collected by 190 blood centers and 1,685,000 units were collected by an estimated 621 hospitals (Wallace, et al. 1993).
Plasma and Derivatives
For the manufacture of derivatives, plasma can be obtained as the by-product from whole blood (plasma) or by plasmapheresis (source plasma). Plasma that is a by-product from whole blood collected by community blood banks or hospitals is sold to commercial companies in the plasma fractionation industry, who in turn manufacture the plasma derivatives and sell them in the
pharmaceutical market. See Chapter 4 for a description of the role one such product—antihemophilic factor (AHF)—in the treatment of hemophiliacs. The blood banks' sale of their plasma to the commercial plasma fractionator may, but usually does not, involve an agreement to provide some of the manufactured derivatives back to the blood bank. For example, plasma from whole blood collected by the ARC is fractionated through a contract with Baxter Healthcare, which then returns all of the derivatives produced to the ARC for sale through their blood provision system.
The amount of plasma obtained from whole blood is not adequate to meet the needs for raw material to produce plasma derivatives. Therefore, much of the plasma that will be made into derivatives is obtained by plasmapheresis. This plasma is called source plasma, which is ''the fluid portion of human blood collected by plasmapheresis and intended as the source material for further manufacturing use" [C.F.R., 1992]. Automated instruments are usually used to obtain 650–750 milliliters of plasma up to twice weekly from healthy adult donors (approximately 225 cc of plasma can be obtained from 450 ml of whole blood but most plasma is obtained directly through plasmapheresis). An individual can donate up to about 100 liters of plasma annually in the United States if the plasma protein levels and other laboratory tests and physical findings remain normal. The plasma is used as raw material for the manufacture of the derivatives shown in Table 2.3. The production of these plasma derivatives is a complex manufacturing process usually involving large batches of plasma (up to 10,000 liters) from as many 1,000–20,000, or more, donors.
The high demand for plasma products and the lengthy and often uncomfortable procedure of plasmapheresis led to the justification and legalization of compensation for plasma in the United States. Up to the early 1980s, plasma collection centers could be located in prisons and other areas where there was a high prevalence of hepatitis and other chronic infections. With the possible emergence of AIDS in the blood supply, plasma fractionators began closing their prison collection sites in December 1982, and in essence all were closed by January 1984.
Organizations and facilities need licenses for plasma collection (if shipped interstate) and the manufacture of AHF concentrate and other products from plasma.
Plasma Collection
Data regarding the plasma fractionation industry are proprietary and thus not readily available. The FDA does not routinely collect data on the nature of plasma donors, the amount of plasma each organization collects, or the number of derivative products produced. According to the American Blood Resources Association (ABRA), the U.S. plasma and plasma fractionation industry employs
over 12,000 people nationwide (Scott 1990). U.S. plasma collection facilities perform approximately 13 million plasmapheresis donor collection procedures annually. Thus, if an average of 700 ml of plasma is obtained from each donation, it could be estimated that approximately 9 million liters of plasma would be collected annually in the United States by plasma centers. Individuals who donate plasma to support the plasma fractionation industry receive between $15 and $20 per donation. According to the ABRA, donors receive compensation of more than $244 million from plasma collection facilities annually (ABRA 1994). This is in contrast to whole blood donors, who donate voluntarily and do not receive compensation. Much of the plasma obtained from whole blood collected by blood banks is also used for production. Blakestone has estimated that in 1990 approximately 12 million liters of plasma were consumed in the manufacture of plasma derivatives (Blakestone 1994).
It is estimated that plasma fractionation worldwide sales exceed $4 billion annually, with U.S. firms providing more than 60 percent of the plasma products or $2.4 billion in domestic and export sales annually (ABRA 1994). Of the $2.4 billion in domestic and export sales, $645 million is the estimated export revenue from sales of U.S. plasma products in Europe.
Plasma Processing
The collected plasma is sent from the collection site to a fractionation laboratory, which in the United States, is either owned by a pharmaceutical company or by an outside company that sells the fractionated plasma to the pharmaceutical company. Fractionation involves further separation of the plasma into proteins such as albumin, immunoglobulin, and AHF concentrates. A pool size of at least 1,000 donors is required by the FDA for the production of immunoglobulin products used in the treatment of infectious disease, because increasing the pool size concentrates the therapeutic antibody portion of plasma. Pooling was more efficient for production in the manufacturing process of AHF concentrates because clotting factor proteins are found in extremely small quantities in plasma. Pooling plasma also has the negative effect of increasing chances for contracting infectious disease (see Chapter 4).
Table 2.3 Plasma Derivative Products and Their Uses
|
Plasma Derivative |
Medical Use |
|
Albumin |
Restoration of plasma volume subsequent to shock, trauma, surgery, and burns |
|
Alpha 1 proteinase inhibitor |
Used in the treatment of emphysema caused by a genetic deficiency |
|
Anti-inhibitor coagulant complex |
Treatment of bleeding episodes in presence of Factor VIII inhibitor |
|
Anti-thrombin III |
Treatment of bleeding episodes associated with liver disease, antithrombin III deficiency, and thromboembolism |
|
Cytomegalovirus immune globulin |
Passive immunization subsequent to exposure to cytomegalovirus |
|
Factor IX complex |
Prophylaxis and treatment of hemophilia B bleeding episodes and other bleeding disorders |
|
Fibrinogen |
Treatment of hemorrhagic diathesis in hypo-, dys-, and afibrinogenemia |
|
Fibrinolysin |
Dissolution of intravascular clots |
|
Haptoglobin |
Supportive therapy in viral hepatitis and pernicious anemia |
|
Hepatitis B immune globulin |
Passive immunization subsequent to exposure to hepatitis B |
|
IgM-enriched immune globulin |
Treatment and prevention of septicemia and septic shock due to toxin liberation in the course of antibiotic treatment |
|
Immune globulin (intravenous and intramuscular) |
Treatment of agamma- and hypogamma-globulinemia; passive immunization for hepatitis A and measles |
|
Plasma Derivative |
Medical Use |
|
Plasma protein fraction |
Restoration of plasma volume subsequent to shock, trauma, surgery, and burns |
|
Rabies immune globulin |
Passive immunization subsequent to exposure to rabies |
|
Rho(D) immune globulin |
Treatment and prevention of hemolytic disease of fetus and newborn resulting from Rh incompatibility and incompatible blood transfusions |
|
Rubella immune globulin |
Passive immunization subsequent to exposure to German measles |
|
Serum-cholinsterase |
Treatment of prolonged apnea after administration of succinylcholine chloride |
|
Tetanus immune globulin |
Passive immunization subsequent to exposure to tetanus |
|
Vaccinia immune globulin |
Passive immunization subsequent to exposure to smallpox |
|
Varicella-zoster immune globulin |
Passive immunization subsequent to exposure to chicken pox |
Blood and Blood Components Distribution
Traditionally, some areas of the United States have been able to collect more blood than needed locally and have provided these extra units to other communities. The misalignment of blood use and blood collection is a longstanding phenomenon. To deal with these blood shortages, blood is "exported" from areas of oversupply and "imported" into areas of shortage—a practice called "blood resource sharing.'' The lack of an adequate local blood supply and the need to import blood causes several difficulties including complex inventory management, technical disparities, emergency donor recruitment, higher costs,
decreased independence, and higher risk-management costs (Scott 1990). Some blood centers import blood because they can obtain this blood for less than their own costs of production (Anderson 1990). For years, blood banks have participated in systems to exchange blood among themselves to alleviate shortages. Blood banks in metropolitan areas that serve large trauma, tertiary, and transplantation centers most frequently experience shortages of whole blood, components, and type-specific blood units. Although experience has demonstrated that the American public is ever-willing to donate blood in times of local disaster or national emergency, this same public has often not donated blood in sufficient supply to meet the daily needs of the local community. Less than 5 percent of the U.S. population donates blood and in certain communities the percentage is even lower. Without resource-sharing networks, many individuals would not receive the blood transfusions necessary to maintain or restore their health.
BLOOD COLLECTION ORGANIZATIONS
The United States blood collection system is heterogeneous owing to the "random development of blood centers without regard … to patient referral patterns" (Scott 1990). The American Red Cross (ARC) collects approximately half the blood in the United States. In the non-ARC covered areas, blood is collected by one or more community or hospital blood banks. In most areas of the United States, there is only one local organization that collects blood. However, in some communities, including these where the ARC operates a blood program, blood may be collected by more than one organization. When this occurs, usually several hospitals and a community blood center (ARC or non-ARC) are involved.
The adequacy of the nation's blood supply varies at different times of the year and in different parts of the United States, but, in general, the United States is almost 100 percent self-sufficient in its blood supply. Approximately 2 percent of the U.S. blood supply is imported from western Europe (Wallace, et al. 1993). Sufficiency, however, varies among geographic areas of the United States on a continual basis. The extent to which the adequacy of the blood supply is related to the public image of blood banks and the association of blood with AIDS is not clear. Public opinion surveys indicate strong support for blood banks (Gallup 1991), and despite major public education efforts by blood banks, a high (35 percent) percentage of people believe they can contract AIDS or HIV by donating blood (CDC 1991).
Community Blood Banks
Blood is collected by blood centers and hospitals. Blood centers are freestanding organizations, virtually all of which are nonprofit. These centers are governed by a board of local volunteers and are organizations whose sole function is to provide the community's blood supply. Each blood center collects blood in a reasonably contiguous area and supplies the hospitals within the blood collection area. The blood center may or may not supply the total needs of the hospitals in its area or may supply hospitals in other areas as well. The area covered by each center is determined by historical factors and did not develop according to any overall plan. Rather, local interests dictated whether, how, and what kind of community blood program developed. Not every area of the United States is necessarily covered by a blood center. There are a total of approximately 180 blood centers in United States (Scott 1990). Approximately 45 of these (25 percent) are operated by the ARC and the remainder are community blood centers as described above.
The American Red Cross Service
The ARC is the organization that collects the largest number of units of blood in the United States. The ARC Blood Service is one of many humanitarian programs operated by the ARC. The ARC is a nonprofit, congressionally chartered (but not government sponsored or operated) organization that conducts programs supported by donated funds and through cost recovery. The mission of the ARC Blood Service is to "fulfill the needs of the American people for the safest, most reliable, most cost-effective blood, plasma … services through voluntary donations." In addition, the organization attempts to be the "provider of choice for blood, plasma … services … by commitment to quality, safety, and use of the best medical, scientific, manufacturing, and business practices" (ARC 1994).
Hospital Blood Banks
Some blood is collected by blood banks that are part of hospitals. These blood banks usually collect blood only for use in that hospital and do not supply other hospitals. Very few (possibly no) hospitals collect enough blood to meet all their needs. They purchase some blood from a local or distant community blood center. Most U.S. hospitals do not collect any blood but acquire all of the blood they use from a community center. Of those that do collect blood, there are no good data available to define the proportion of their needs that they collect. This can be presumed to be quite variable.
PROFESSIONAL ASSOCIATIONS
There are three major professional associations involved with blood banking. These are the American Association of Blood Banks (AABB), the Council of Community Blood Centers (CCBC), and the American Blood Resources Association (ABRA). Other organizations such as the American Medical Association, the College of American Pathologists, the American College of Surgeons, and the American Society of Anesthesiologists, may from time to time take positions on blood-bank-related issues and maintain blood bank or transfusion medicine committees. The AABB and CCBC are the only professional organizations devoted exclusively to blood banking and transfusion medicine. The ABRA is the trade association representing the plasma fractionation industry. Each organization is described briefly in the following section.
American Association of Blood Banks
Established in 1947, the American Association of Blood Banks (AABB) is a nonprofit scientific and educational association for individuals and institutions engaged in the many facets of blood and tissue banking and transfusion and transplantation medicine. It is the only organization devoted exclusively to blood banking and blood transfusion services. Institutional members of the AABB are classified either as a community blood center, a hospital blood bank, or a hospital transfusion service. The community blood center collects blood and distributes it to several hospitals but does not transfuse blood. A hospital blood bank both collects and transfuses blood, a hospital transfusion service transfuses but does not collect blood.
Member facilities of the AABB collect virtually all of the nation's blood supply and transfuse more than 80 percent. Approximately 2,400 institutions (community, regional, and ARC blood centers; hospital blood banks; and hospital transfusion services) and 9,500 individuals are members of the AABB. The services and programs of the AABB include inspection and accreditation, standard setting, certification of reference laboratories, operation of a rare donor file, accreditation of parentage testing laboratories, group purchasing programs, certification of specialists (technologists) in blood banking, educational programs, legislative and regulatory assistance to members, participation in the National Marrow Donor Program, participation in the National Blood Foundation, which provides funds for research in transfusion medicine and blood banking, and participation in the National Blood Exchange Program, which facilitates the movement of blood among centers with surplus and those with shortages.
AABB Inspection and Accreditation Program
The AABB operates a voluntary accreditation system in which most blood collection and transfusion organizations participate. The AABB accreditation program involves a biannual inspection by AABB volunteers. The AABB Inspection and Accreditation (I&A) program was initiated in 1958. The I&A program is designed to assist directors of blood banks and transfusion services in determining that knowledge, equipment, and physical plant meet established requirements. It is also a means for detecting deficiencies in practices and provides, when needed, consultation for their correction. The I&A program provides recognition through accreditation to those institutions functioning in accordance with existing published requirements of the AABB. While increased safety in obtaining and transfusing human blood and components is the major intent and benefit of the I&A program, certain ancillary benefits such as assistance in medico-legal problems may result. Inspection and accreditation by the AABB is a prerequisite for institutional membership in the association and for full participation in the AABB National Blood Exchange Program.
Council of Community Blood Centers
The Council of Community Blood Centers (CCBC) is an association of independent (non-ARC) not-for-profit community blood centers that serves the public by assisting its members in providing excellence in blood and related health services. The association was established in 1962 by the directors of six community blood centers who recognized the need for an organization that would represent the common interests of not-for-profit community blood programs and would provide a national forum to address the unique needs in the field of blood center operations. Its policies are determined by a board of trustees comprised of one voting representative from each institutional member.
Efforts to meet the goals of safety, quality, and efficiency in blood services are accomplished through a variety of activities and services that are developed and managed by volunteers. These efforts include group purchasing of supplies, services, and liability insurance; increasing volunteer blood donation; effective sharing of blood resources; strengthening of blood center management skills and the scope of services provided to the community; training programs to assure compliance with federal regulations; assuring fair and balanced resolution of disputes between blood centers and the public they serve; influencing federal and state regulations and policies; and promoting needed research and development in the blood services area.
The CCBC also promotes information exchange between members of operational practices, new programs, policies, and ideas through surveys,
meetings of small working groups, and development of workable models. The weekly CCBC newsletter is a comprehensive chronicle of information about current government activities affecting blood centers as well as new developments in blood services and health care in general.
American Blood Resources Association
The American Blood Resources Association (ABRA) is a trade association founded in 1971 to represent the plasma collection and fractionation industry in both federal and state government relations. The ABRA's role is to educate the public at large about the commercial plasma and plasma products industry. The ABRA's mission is to promote and encourage research, to foster and monitor the promulgation of reasonable and just regulations, and to institute beneficial projects on behalf of the commercial plasma and plasma products industry. The ABRA provides facility and personnel certifications and develops industry manufacturing standards and guidelines. Its members operate under a strict code of ethics to ensure the high standards and quality. Its memberships operate over 80 percent of the U.S. commercial plasma collection facilities, and includes all of the commercial plasma product manufacturers in the United States and a majority of the manufacturers worldwide. Members manufacture and collect plasma in 42 states across the country.
HEMOPHILIA ORGANIZATIONS
The Nature of Hemophilia
Hemophilia is a rare, inherited, sex-linked disorder characterized by a deficiency in blood-clotting proteins. The estimated number of people with hemophilia in the U.S. population is approximately 15,000-16,000 (CDC, HRSA, MCHB, 1991, 1992, 1993). Hemophilia has been characterized by high mortality and a significantly lower mean age of death as compared to the general population (Chorba, et al. 1994).
There are two major types of hemophilia. The more common, hemophilia A, is characterized by a deficiency of antihemophilic Factor VIII clotting protein. The much less frequently occurring variety of hemophilia is hemophilia B, characterized by a deficiency of Factor IX clotting protein. About 85 percent of hemophilia cases are due to Factor VIII deficiency, about 14 percent to deficiencies of Factor IX. The remaining 1 percent involve the much more rare congenital clotting factors: V, VII, X, or XI (Hoffman, et al. 1994). The clinical severity of hemophilia is related to the degree to which the relevant factor is absent or deficient. The distinction of disease severity (i.e., mild,
moderate, or severe) is critical in determining treatment of the disease. Mild or moderate hemophilia is rarely complicated by episodes of spontaneous bleeding (Hoyer interview). In severe cases, which are characterized by less than 1 percent of clotting factor activity, the disorder is accompanied by spontaneous bleeding into multiple joints of the body and muscles (Chorba, et al. 1984). This can be extremely painful, can lead to severe disabling musculoskeletal disease, and can be fatal. Most of the fatality associated with hemophilia results from central nervous system bleeding. Approximately 60 percent of hemophiliacs are classified as severe (Hoffman, et al. 1994).
Chapters 4 and 7 contain more detailed information on hemophilia treatment modalities available in the 1980s.
Hemophilia Treatment Centers
On July 29, 1975, Congress passed P.L. 94-63 authorizing federal funding to establish a network of comprehensive hemophilia treatment centers [Section 606 of P.L. 94-63 amended Title XI of the Public Health Service Act]. On October 1, 1976, a total of $3 million was appropriated to fund more than 20 regional hemophilia treatment centers [Federal Register, 1976, 1977] (Smith and Levine 1984). The Hemophilia Treatment Centers became a model program for the delivery of comprehensive care services for the diagnosis and treatment of hemophilia. The centers provided education, medical, psychosocial, orthopedic, dental, and genetic counseling expertise, and the means for early application of treatment. The comprehensive care provided was aimed at preventing or reducing the complications associated with hemophilia, as well as rehabilitation of those who already had severe musculoskeletal complications (Smith and Levine 1984).
National Hemophilia Foundation
The National Hemophilia Foundation (NHF) is a nonprofit health care organization founded in 1948. Its mission is to help meet the needs of all individuals with bleeding disorders. The NHF is organized into chapters, each of which has a locally elected board of directors and officers. Each chapter's president is the chief executive officer and serves without compensation. There are 46 chapters nationwide. Chapters are self-governed and determine their own priorities, programs, and uses of funds. They have the benefit and use of the NHF's advertising, public relations materials, publications, name, and affiliation. As an affiliated member, chapters pay a monthly participation fee to the NHF. There are several hemophilia societies not affiliated with the NHF.
The NHF provides financial support for particular programs and national legislative advocacy. The NHF board of directors serves as the policymaking body of the NHF, and the current board is comprised of 22 members. The board serves to elect NHF officers, grant and terminate chapter charters, determine territorial jurisdictions for chapters, and establish and enforce uniform rules. The decision-making process of the NHF involves the four vice presidents, the president, the chairman of the board, the Medical and Scientific Advisory Council (MASAC) chair, and the executive director. The board of directors also approves all MASAC recommendations before they become "official" NHF MASAC recommendations for dissemination.
Medical and Scientific Advisory Council
One important national activity of the NHF is MASAC. In 1982, the primary mission of MASAC was to advocate for continued development and expansion of an accessible comprehensive care network, to advocate for quality treatment and care for hemophilia, to support and be involved in hemophilia research, to discuss timely issues of relevance to the hemophilia community and make recommendations concerning them, and to continue to provide technical information, educational materials, and publications. The MASAC also provided advice to the NHF board of directors concerning medical and scientific issues of relevance, and reviewed research activities. The MASAC membership included representatives from six other individual committees of NHF: research and review, nursing, mental health, social work, education, and musculoskeletal.
Membership of MASAC is generally drawn from the regional treatment centers and represents both elected and appointed members (i.e., appointed members, regionally elected members, committee liaisons, and ex-officio members). The appointed members are generally elected for their expertise in a particular area (e.g., basic research in hemophilia, etc.). The chair of MASAC is appointed by the NHF president and has a three-year term, and MASAC members serve rotational twoand four-year terms.
In 1989, a committee of medical leadership was established by the NHF to facilitate more rapid communication about major issues in the hemophilia medical and scientific community. Members include the NHF vice president for medical and scientific affairs, the MASAC chair, the medical director, associate medical directors, the chair of the AIDS task force, the president of the NHF, the chairman of the NHF board and the executive director.
ROLE OF THE U.S. PUBLIC HEALTH SERVICE
National Blood Policy of 1973
The federal government regulates blood banking, monitors the safety and efficacy of blood products, and promotes research on blood diseases (OTA 1985). In late 1972, the U.S. Department of Health, Education and Welfare reported several problems within the blood supply system, including an inadequacy in the quantity of blood supplied, an unreliability in the quality of blood owing to the high rates of transfusion-related hepatitis, an inefficiency in the system itself owing to waste in some areas and shortages in others, and excessive costs of blood and blood services. On July 10, 1973, the Assistant Secretary for Health announced the National Blood Policy, which became "the focal point around which blood banking policy has evolved over the past decade" (OTA 1985). The National Blood Policy recognized that reliance on "commercial sources of blood and blood components for transfusion therapy has contributed to a significantly disproportionate incidence of hepatitis, since such blood is often collected from sectors of society in which transmissible hepatitis is more prevalent." For this reason, the National Blood Policy encouraged efforts to establish an all-volunteer blood donation system and to eliminate commercialism in the acquisition of whole blood and whole-blood components [Federal Register 1975] (Hutt and Merrill 1991).
The National Blood Policy listed four primary goals: to provide an adequate supply of blood; to ensure a higher quality of blood; to facilitate maximum accessibility to services; and to achieve total efficiency (U.S. Senate 1979). The first actions taken to meet these goals included the adoption of an all-volunteer blood collection system (for whole blood); coordination of all costs and charges; regionalization of blood collection and distribution; and an examination of the standards of care for hemophiliacs and other special groups. The policy did not address the commercial acquisition of plasma, the preparation and marketing of plasma derivatives, and the commercial acquisition of blood for diagnostic reagents (Hagen 1982).
In 1975, the American Blood Commission (ABC) was established and funded by the National Heart, Lung, and Blood Institute and was charged with implementing the "lion's share" of the objectives set forth in the National Blood Policy (OTA 1985). The progress of the ABC was hindered by lack of funds, disagreement between the two largest blood suppliers, resistance to regionalization of blood collection and distribution, problems in obtaining data from blood banks, and a lack of knowledge of blood banking by lay members of the commission (U.S. General Accounting Office 1978). In 1985 the ABC was formally disbanded.
Public Health Service
Public health management is the responsibility of the federal government through the Public Health Service (PHS). The Public Health Service Act of July 1, 1944 [42 U.S.C. § 201], consolidated and revised substantially all existing legislation relating to the PHS. The mission of the PHS is to promote the protection and advancement of the nation's physical and mental health. The Office of the Assistant Secretary for Health in the Department of Health and Human Services plans and directs the activities of the PHS. The federal system by which public health policy decisions are made comprises the Centers for Disease Control and Prevention, the agency that conducts surveillance and reporting of disease; the National Institutes of Health, the organization that conducts research; and the Food and Drug Administration, the regulatory arm of the PHS.
Centers for Disease Control and Prevention
The Centers for Disease Control and Prevention (CDC) was established as an agency of the PHS in 1973. The CDC is charged with protecting the public health of the nation by providing leadership and direction in the prevention and control of diseases and other preventable conditions, and responding to public health emergencies. The CDC also administers national programs for the prevention and control of communicable and vector-borne diseases which includes consulting with state and local public health departments. The CDC also collects, maintains, analyzes, and disseminates national data on health status and health services.
National Institutes of Health
The National Institutes of Health (NIH) is the federal government's principal biomedical research agency. Its mission is to pursue knowledge to improve human health. To accomplish this goal, the NIH seeks to expand fundamental knowledge about the nature and behavior of living systems, to apply that knowledge to enhance the health of human lives, and to reduce the burdens resulting from disease and disability. Two of the NIH institutes have a special role in protecting blood safety.
National Institute of Allergy and Infectious Diseases
The National Institute of Allergy and Infectious Diseases conducts and supports broad-based research and research training on the causes, characteristics, prevention, control, and treatment of a wide variety of diseases believed to be attributable to infectious agents (including bacteria, viruses, and parasites), to allergies, or to other deficiencies or disorders in the responses of the body's immune mechanisms.
National Heart, Lung, and Blood Institute
In 1948 the National Heart Institute was established, and in 1969 it was reorganized as the National Heart and Lung Institute. In the 1960s, epidemiological evidence demonstrated a correlation between high rates of post-transfusion hepatitis and blood from paid donors. This, coupled with the advances in surgical techniques (especially cardiac) that increased the need for whole blood for transfusion, created a demand to increase safety measures regarding the blood supply. In addition, an increase in the use of platelets and plasma derivatives also occurred, primarily because of advances in new technologies. As a result, the National Blood Resources Program was established in 1967. The primary objective in establishing the program was to develop safe and efficient blood collection and distribution (U.S. General Accounting Office 1978).
In 1970, Congress amended the Biologics Act, which, as discussed later in this chapter, was originally enacted in 1902 to provide the framework for federal regulation of biological products for human use, to include vaccines, blood, blood components or derivatives, and allergenic products. As a result, the Blood Resources Program became the Division of Blood Diseases and Resources at the National Heart, Lung, and Blood Institute.
Food and Drug Administration
The name ''Food and Drug Administration" was established by the Agriculture Appropriation Act of 1931 [46 Stat. 392], although similar regulatory functions had been in existence under different organizational titles since January 1, 1907, when the Food and Drug Act of 1906 [21 U.S.C. §§ 1-15] became effective. The FDA's activities are directed toward protecting the health of the nation against impure and unsafe foods, drugs and cosmetics, biologics, and other potential hazards. One of the FDA's responsibilities is to administer regulation of biological products under the biological product control
provision of the Public Health Service Act and applicable provisions of the federal Food, Drug, and Cosmetics Act. The FDA's legal authority is derived from the Food, Drug, and Cosmetics Act and related laws (Hutt and Merrill 1991).
Prior to 1972, regulation of the blood supply was carried out by the NIH (Hutt and Merrill 1991). Until 1947, the control of biological products had been under the supervision of the director of the Hygienic Laboratory of NIH. In 1948 it became part of the NIH National Microbiological Institute. In 1955, the NIH was reorganized and the Division of Biological Standards (DBS) for regulating biologics was created (Hutt and Merrill 1991).
In response to a 1972 General Accounting Office report that concluded that ineffective biologics were licensed under the Biologics Act because of the failure to apply the requirements for proof of effectiveness, the Secretary of Health, Education and Welfare delegated concurrently to the FDA and the DBS the authority to administer the drug provisions of the Food, Drug, and Cosmetics Act for all biological products. On July 1, 1972, the responsibility for implementing the Biologics Act was transferred from the DBS to the FDA [37 Federal Register 12,865, 1972]. Following its assumption of responsibility for administering the Biologics Act and the formation of the Bureau of Biologics, the FDA revoked NIH's announcement to review the effectiveness of all licensed biologics. The FDA then issued its own set of detailed procedures for the review of the safety, effectiveness, and labeling of all licensed biologics. The Bureau of Biologics was given lead responsibility for overseeing blood collection, processing, testing, and marketing. It was at this point that all blood banks became federally regulated and state licensed [37 Federal Register 17,419, 1972].
In 1982, through an FDA reorganization, the Center for Drugs and the Center for Biologics merged into one unit, and the Center for Drugs and Biologics (CDB) was established. The scientific director of the CDB was responsible for integrating the scientific and research activities for biologics between the NIH and FDA. The responsibilities of the Bureau of Biologics fell under this new center and the regulation for blood products and blood banking technologies was under the purview of the Office of Biologics Research and Review. The Office of Biologics in the Division of Blood and Blood Products was responsible for approval of license applications and amendments for new blood establishments and blood products, and for approval to market blood products and related technologies (OTA 1985).
In 1988, the CDB was reorganized again and the Office of Drugs and the Office of Biologics were separated into different centers. The Center for Biologics and Review assumed oversight for all activities that previously fell under the Office of Biologics and Review. In 1993, the Center for Biologics and
Review was renamed the Center for Biologics Evaluation and Research (Fratantoni 1994).
The Center for Biologics Evaluation and Research (CBER) administers regulation of biological products under the biological product control provisions of the Public Health Service Act and applicable provisions of the Food, Drug, and Cosmetics Act. CBER plans and conducts research related to the development, manufacture, testing, and use of both new and old biological products to develop a scientific base for establishing standards designed to ensure the continued safety, purity, potency, and efficacy of biological products. It also coordinates with the Center for Drug Evaluation and Research regarding activities for biological drug products, including research, compliance, and product review and approval. CBER also plans and conducts research on the preparation, preservation, and safety of blood and blood products; the methods of testing safety, purity, potency, and efficacy of such products for therapeutic use; and the immunological problems concerned with products, testing, and use of diagnostic reagents employed in grouping and typing blood.
The CBER is the dominant focus for coordination of the Acquired Immune Deficiency Syndrome (AIDS) program, works to develop an AIDS vaccine and AIDS diagnostic tests, and conducts other AIDS-related activities. It inspects manufacturers' facilities for compliance with standards, tests products submitted for release, establishes written and physical standards, and approves licensing of manufacturers to produce biological products. In carrying out these functions, the CBER cooperates with other Public Health Service organizations, governmental and international agencies, volunteer health organizations, universities, individual scientists, nongovernmental laboratories, and manufacturers of biological products.
Blood Products Advisory Committee
The FDA makes extensive use of technical advisory committees in the support if its evaluation and regulation of drugs, biologics, and medical devices for human use. Advisory committees are utilized by the FDA to obtain independent scientific and technical advice, opinions, or recommendations on a specific matter (FDA 1994). FDA advisory committees can be established in four ways: by order of the President of the United States; by congressional statute, by the Secretary of Health and Human Services, or by the FDA commissioner. The Secretary or the FDA commissioner must approve the establishment, renewal or rechartering, or amendment of all FDA public advisory committee charters (FDA 1994). Generally, the commissioner has direct authority to charter scientific and technical advisory committees, while the Secretary issues charters for committees advising on policy issues. All public
advisory committees must be chartered, and their charters must be renewed biennially unless otherwise determined by law.
The CBER has four different standing advisory committees, one of which is the Blood Products Advisory Committee (BPAC), which provides evaluation of data related to safety, effectiveness, and labeling of blood and blood products and makes appropriate recommendations to the Secretary, the Assistant Secretary for Health, and the FDA commissioner (IOM 1992). Advisory committee nominations include candidates from relevant professional and scientific bodies, medical schools, academia, government agencies, industry and trade associations, and consumer and patient organizations. Committee members are appointed to terms not to exceed four years. Reappointment to a committee requires that one year elapse between appointments.
The general way in which an agenda is set for an FDA advisory committee involves two stages: (1) a meeting is formally scheduled and announced in the Federal Register; and (2) several days prior to the meeting, the FDA staff sends advisory committee members a detailed agenda and a list of specific questions on which their advice is sought. The FDA releases this list of questions to the public on the morning of the meeting (IOM 1992). An advisory committee meeting operates with the following separable portions: an open public hearing; an open committee discussion; a closed presentation of data; and closed committee deliberations. The BPAC's topics include investigational new drugs that meet the criteria of important diagnostic therapeutic, preventive, or other advances; novel and improved methods for product delivery; potential or apparently significant safety hazards; involvement of new biotechnology; and issues requiring additional expert review or clarification of study protocols. Product licensing agreements considered at BPAC meetings include those meeting the criteria of being a significantly new product; posing new uses for marketed products; having significant potential for risk compared to narrow therapeutic benefit; needing or being considered for postmarketing studies; presenting potential for withdrawal from market because of safety or questionable efficacy; and posing issues requiring additional expert review or clarification of study protocols.
The BPAC has 13 voting members and 2 nonvoting members. All voting members, consultants, and experts to advisory committees receive compensation for each day worked, travel, and per diem, unless waived. Industry and consumer representatives receive a salary if they have been cleared under the FDA's conflict of interest regulations as a special government employee. During the 1980s the BPAC was comprised of experts in relevant professional, scientific, and medical establishments, including academic blood banking, transfusion services, anesthesia and pharmacology, state public health departments, general medicine, biochemistry, pediatrics, laboratory medicine, infectious diseases, virology, hematology, and oncology.
BLOOD AND BLOOD PRODUCT REGULATION
Statutory Background
The history of blood and blood product regulation in the United States includes both congressional enactments (public laws) and rulemaking procedures of the FDA. The FDA regulates blood, blood components, and derivatives under two separate but overlapping statutes, one governing "biologics" and one governing "drugs." The biologics law requires that any "virus, therapeutic serum, toxin, anti-toxin, or analogous product" be prepared in a facility holding a federal license. A separate law, for food and drugs, includes drugs intended for the "cure, mitigation, or prevention of disease" and, thus, includes biologics such as blood and blood components or derivatives. Thus, blood banks and plasma product manufacturers are also subject to this drug regulatory process.
Biologics Act
In 1902, following several outbreaks of disease from contaminated vaccines, Congress enacted the Biologics Act [32 Stat. 728] which provided the framework for federal regulation of biological products for human use. The law required that biological drugs sold in interstate commerce must be licensed and produced in licensed establishments. The term biologics includes vaccines made from or with the aid of living organisms that are produced in animals or humans. Biologics also include antitoxins used to protect against diphtheria, tetanus, and whooping cough; serums for the treatment of disease; products for the treatment of allergies; and blood for transfusion and other medical purposes (Hutt and Merrill 1991).
In 1944, the Biologics Act was reenacted as part of the recodification of the Public Health Service Act [58 Stat. 682, 702, 1944], and is now codified at 42 U.S.C. § 262 (Hutt and Merrill 1991). The recodification hearings focused on the issue of possible duplicative regulatory authority of biological products under the Federal Drug and Cosmetics Act. Under the original act, the Public Health Service (PHS) licensed and controlled the manufacturing of virus serums, toxins, and other biologics. At the hearings, while PHS control of biologics was viewed as effective, the wording of the new act was seen to be suggestive of duplicative administrative control of the PHS and the FDA. In the event that some product dangerous to human life inadvertently entered the market, the FDA would have power of seizure [Section 351 of the PHS Act, referred to as the Biologics Act] (Hutt and Merrill 1991). Prior to 1970, the Biologics Act did not specifically include blood products. In 1970, Congress amended the Biologics Act
"specifically to include vaccines, blood, blood components or derivatives, and allergenic products [84 Stat. 1297, 1308]" (Hutt and Merrill 1991).
Public Health Service Act
In 1974, the FDA promulgated regulations governing good manufacturing practices in the collection, processing, and storage of human blood components [39 Federal Register 18,614, 1974; 40 Federal Register 53,532, 1975]. By combining the jurisdictional and regulatory provisions of the Biologics Act and the Food, Drug, and Cosmetic Act, the FDA brought all blood and blood products produced and used in the United States under uniform federal requirements (Hutt and Merrill 1991).
Blood Shield Laws
During the 1950s and 1960s, blood shield laws were adopted by 47 different jurisdictions. The blood shield laws were developed to exempt blood and blood products from strict liability or implied warranty claims on the basis that blood and blood products provide a service, not a sale. Accordingly (as stated in the California Health and Safety Code 1606),
the procurement, processing, distribution, or use of whole blood, plasma, blood products, and any blood derivatives for the purpose of infusing the same, or any of them, into the human body shall be construed to be, and is declared to be, for all purposes whatsoever, the rendition of a service by each and every person, firm, or corporation participating therein, and shall not be construed to be, and is declared not to be, a sale of such whole blood, plasma, blood products, or blood derivatives, for any purpose or purposes whatsoever (Westfall 1986).
Only four jurisdictions (New Jersey, District of Columbia, Rhode Island, and Vermont) did not adopt statutes protecting hospitals or blood donor services from strict liability or breach of implied warranty (Lipton 1986). Even in these jurisdictions, however, the likelihood that a court would hold a hospital or blood donor service liable under either breach or implied warranty or strict liability theories was considered remote (Lipton 1986).
In 1976 blood banks received exemption from liability under protection of blood shield law as providing a service and not a product. The court ruled that there was a rational basis for blood bank's exemption from liability, based on weighing the need for an available blood supply for surgery and other medical procedures against the "relatively minor risk of hepatitis which the blood
recipient must take" (Westfall 1986). In addition, the court found that exemption of the blood bank from liability was constitutional because protection of blood banks was related to the state's purpose of encouraging the general blood supply.
In 1977, the courts extended this protection to blood product manufacturers on the same grounds: the distribution of blood products was a service and not a sale. In a wrongful death suit concerning a hemophiliac who had died from hepatitis after using a blood product [Cutter v. Fogo 1977], the court reasoned that because the blood product was unavoidably unsafe, and because the risk of hepatitis could not be eliminated despite every attempt to screen donors (i.e., through both biological tests and avoidance of high-risk donors), the blood product manufacturers were protected from strict product liability since the blood product had been instrumental in helping many hemophiliacs (Westfall 1986).
Federal Licensure of Blood Collection Organizations
Federal licensure is thought to ensure that the facility in which the biologic is produced will ensure its purity and quality. In addition to licensing the facility or establishment, this law requires that each biologic product itself be licensed by the government. Thus, to produce a licensed biologic, an organization must have an establishment license describing the facility in which the product is produced and a product license describing the specific product being produced. Over the years, this law has been specifically amended to include the terms blood and blood component or derivative to make it clear that blood and blood products are subject to the biologics regulation.
Establishment Licensure and Registration
Presently, there are 188 FDA-licensed organizations at 790 locations for collection and interstate shipment of blood and blood components. In addition, a total of 2,900 locations are registered to collect blood but not for interstate shipment. If an organization wishes to ship the components across state lines or engage in commerce by selling the products to other organizations, the organization must obtain an FDA license for this purpose. Even if an organization does not wish to produce blood components for interstate shipment, the FDA law requires that all organizations involved in "collection, preparation, processing, or compatibility testing … of any blood product" register with the FDA (McCullough 1995). This registration allows the organization to collect blood and prepare blood components for its own use. Thus, for practical purposes, most hospitals that collect blood or prepare blood components for their
own use are registered but not licensed since they do not ship blood in interstate commerce. Most blood centers are licensed since they supply multiple hospitals, some of which may be in other states. In addition, blood centers may wish to participate in blood resource sharing with blood centers in other states and thus need to be licensed for interstate shipment of blood.
Product Licensure
Along with the establishment license, the organization must file a product license application for each product it plans to produce in the facility.
For whole blood and components, the product application involves basic information about the manufacturer (organization), establishment, product, standard operating procedures, blood donor screening tests, frequency of donation, donor medical history, presence of a physician, phlebotomy supplies, venipuncture technique, collection technique, allowable storage period, storage conditions, disposal of contaminated units, supplies and reagents, label control processes, procedures for reissue of blood, and a brief summary of experience testing 500 samples.
For the manufacture of plasma derivatives, the product license application involves the manufacturer's (organization's) name; the establishment name; procedures for determining donor suitability including medical history, examination by physician, laboratory testing, methods of preparing the venipuncture site, and collecting the plasma; methods to prevent circulatory embolism and to assure return of red cells to the proper donor; minimum intervals between donation and maximum frequency of donation; techniques for immunizing donors; laboratory tests of collected plasma; techniques of preparing source plasma and storing it; methods to ensure proper storage conditions and identification of units; label control systems; and shipping conditions and procedures.
Blood banks and plasma derivative manufacturers must submit a report annually to the FDA indicating which products are collected, tested, prepared, and distributed.
Other Required Licensure
Blood banks are subject to several other requirements or licensure systems in addition to those of the FDA. Because blood banks carry out testing on human material that is in interstate commerce, and because they provide services to Medicare and Medicaid patients, they must comply with the Clinical Laboratories Improvement Act of 1988. Several states also require that blood
banks have a license to operate or provide blood in that state. These licenses usually involve a specific application and inspection.
REGULATORY AUTHORITY OF THE FDA
Since 1972, the FDA has been the principal regulatory agency with respect to blood and blood products. Its statutory regulatory authority is extensive under the federal Food, Drug, and Cosmetics Act and the Public Health Service Act [codified as 42 U.S.C. § 262].
Compliance with Regulations
The FDA depends on the regulated industry for some amount of self-regulation. However, the FDA's enforcement cannot be by self-regulation, and the FDA's General Counsel determines if a violation of legislative mandates constitutes grounds for legal action (Hutt and Merrill 1991) (See Chapter 6, which focuses on FDA's regulation of blood and blood products during the period 1982–1986 when HIV contaminated the blood supply and before the development of a test to detect antibody to HIV, for more information).
A formal compliance program for the plasma fractionation industry was established in 1977. The responsibility for annual inspections was transferred from the Bureau of Biologics to the FDA field investigation office (OTA 1985). In addition, there was no ban on commercial collection of plasma at this time because the voluntary donor system could not meet the demand for plasma. To reduce the risks of transmission of hepatitis, source identification (as to whether the donor was paid or volunteer) was required as a federal regulation imposed by the FDA in 1978 for both whole blood and its components. This requirement, however, did not apply to source plasma or derivatives (OTA 1985).
In March 1980 a memorandum of understanding was established between FDA and the Health Care Financing Agency (HCFA) for coordination of the inspection of blood banks and transfusion services. The FDA exempted all transfusion services and clinical laboratories that are regulated by HCFA under Medicare [45 Federal Register 64,601; September 30, 1980]. HCFA adopted the FDA's blood regulation to assure uniform and efficient regulation of these facilities.
Recall Policy
The FDA's recall authority lies within the Public Health Service Act under the Biologics section [21 C.F.R. Part 7]. The FDA can issue a mandatory injunction to place the blood bank back into compliance with the regulations (Dubinsky, Falter, Foegel interviews). The FDA's Regulatory Procedures Manual requires CBER's technical staff to prepare a health hazard evaluation of a product before a recall action is initiated (FDA 1988). (A less formal discussion of recall appears in Chapter 6 and focuses on FDA's regulation of blood and blood products during the period 1982-1985 when HIV contaminated the blood supply and before the antiviral HIV test was developed.)
A recall is a method for removing or correcting marketed products that violate the laws administered by the FDA. The recall methods provide efficient and timely protection to the consumer, especially when a product has been widely marketed. Voluntary recalls may be undertaken at any time on the initiative of manufacturers to carry out their responsibility to protect the public health. The recall process is usually a voluntary action taken by a firm to remove a product from the market and may be taken as a result of FDA findings during inspections, reports from consumers, or scientific data indicating a risk (OTA 1985). If the firm decides against market withdrawal, the FDA can seize the product.
A market withdrawal is when a firm voluntarily removes a distributed product which involves a minor violation for which the FDA would not initiate legal action or which involves no violation. Requested recalls are initiated in response to a formal request from the FDA (FDA 1988). It is FDA policy that a recalling firm has the responsibility to determine whether the recall is progressing satisfactorily through the use of effectiveness checks. Because each recall is unique and requires its own strategy, the FDA reviews and/or recommends the firm's recall strategy and will develop its own strategy based on the agency's hazard evaluation and other factors, such as type or use of the product. The recall strategy is separate from, and not tied to, the class of recall selected (FDA 1988). Recall classification is a numerical designation assigned by the FDA to a product recall to indicate the relative degree of health hazard presented by the product being recalled. There are three classes of recall:
-
Class I is defined as situations in which there is a strong likelihood that the use of, or exposure to, a violative product will cause serious, adverse health consequences, or death.
-
Class II is defined as situations in which the use of, or exposure to, a violative product may cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote.
-
Class III is defined as situations in which the use of, or exposure to, a violative product is not likely to cause adverse health consequences.
Once the recall has been classified, FDA determines the depth of the recall, which depends upon the product's degree of hazard and the extent of distribution. The recall strategy will specify the level to which the recall should extend as follows [see 21 C.F.R. § 7.45]:
-
consumer or user level, which may vary with the product, including any intermediate wholesale or retail level;
-
retail level, including any intermediate wholesale level; or
-
wholesale level.
The FDA issues a warning to alert the public that a product is being recalled and presents a serious hazard to health. This is usually reserved for urgent situations where other means for preventing the use of the recalled product may appear inadequate [21 C.F.R. § 7.45]. The FDA also surveys and monitors recall actions for all biologics by following up to make sure that the recall message (i.e., a letter to the manufacturer) was received and acted upon.
The FDA can implement stronger enforcement actions if the manufacturer is not acting in accordance with the recall. However, there must be scientific and medical evidence to justify stronger enforcement actions such as a court injunction or product seizure. FDA staff must present evidence to the FDA General Counsel and the Department of Justice on the necessity of such an action (Dubinsky, Falter, Foegel interviews).
SUMMARY
The nation's blood and plasma are collected by two distinct systems that are based on different donor sources and produce different products. The blood segment of the collection system is primarily not for profit, the plasma segment is primarily for profit. The federal government regulates blood banking, monitors the safety and efficacy of blood products, and promotes research on blood diseases. Both systems are regulated by the FDA in a similar manner, although the specific requirements differ because of differences between blood and plasma products.
Since the period 1982–1986, it appears that the number of units of whole blood collected in the United States has stabilized or slightly decreased. It also appears that the substantial increase in the collection of autologous blood that occurred during recent years is slowing. There is a slight decrease in the number of community blood centers and an increase in the average number of units collected, implying that the decrease in the number of centers may be due to
mergers. Presently, members of the American Association of Blood Banks account for almost all blood collected in the United States. The number of AABB institutional members who collect blood has increased and those that transfuse blood has decreased. Because this could reflect the changing membership of the AABB, it is not proper to extrapolate these observations to changes in the blood collection or transfusion community. Membership in the Council of Community Blood Centers has increased substantially during the past decade.
It is not possible to provide accurate estimates of the amount of plasma or derivatives produced because this is proprietary information. There has been an increase in the kinds of plasma derivative products during the past decade. There has also been an increase in the number of plasma derivative manufacturers during the past decade. Although several companies that produced plasma derivatives in the early 1980s no longer do so, other companies have begun the production of plasma derivatives.
REFERENCES
American Blood Resources Association. Materials submitted to the Institute of Medicine; 1994.
Anderson, K. The Economics of Importing vs Collecting. In Adequacy of the Blood Supply, Council of Community Blood Centers conference proceedings; Clearwater, Florida. February 18, 1990.
Blakestone, M.S. Fractionation. Plasmapheresis, vol. 5, 1994.
Centers for Disease Control. Morbidity and Mortality Weekly Report , July 3, 1981.
Centers for Disease Control. Morbidity and Mortality Weekly Report , July 16, 1982.
Centers for Disease Control. Morbidity and Mortality Weekly Report , March 20, 1987.
Centers for Disease Control. Morbidity and Mortality Weekly Report , December 18, 1987.
Centers for Disease Control. HIV/AIDS Knowledge and Awareness of Testing and Treatment—Behavioral Risk Factor Surveillance . Morbidity and Mortality Weekly Report, vol. 40:704, 1991.
CDC, HRSA, and MCHB. Minimal Data Set for Risk Reduction; 1991, 1992, 1993.
Chorba, et al. Changes in Longevity and Causes of Death among Persons with Hemophilia A. American Journal of Hematology, vol. 45, 1994.
Food and Drug Administration. Policy and Guidance Handbook for Advisory Committees, 1994.
Food and Drug Administration. Regulatory Procedures Manual, May 16, 1988.
Fratantoni, J., Food and Drug Administration, Division of Hematology. Presentation to Committee, May 16, 1994.
Gallup Organization. Attitudes of U.S. Adults Towards AIDS and the Safety of America's Blood Supply. Princeton, New Jersey, 1991.
Hagen, Piet J. Blood: Gift or Merchandise? New York: Alan R. Liss, Inc., 1982.
Hoffman, et al. (editors). Hematology: Basic Principles and Practice (second edition). New York: Churchill Livingsone, 1994.
Hutt, Peter, and Merrill, Richard. Food and Drug Law: Cases and Materials . Westbury, New York: The Foundation Press, Inc., 1991.
Institute of Medicine, Committee to Study HIV Transmission Through Blood Products. Transcript of Public Meeting, September 12, 1994.
Institute of Medicine. Food and Drug Administration Advisory Committees . Washington, D.C.: National Academy Press, 1992.
Lipton, K. Blood Donor Services and Liability Issues Relating to Acquired Immune Deficiency Syndrome. Journal of Legal Medicine, vol. 7(2), 1986.
McCullough, Jeffrey. The United States Blood Collection System, 1995.
Office of Technology Assessment. Blood Policy and Technology Washington, D.C: U.S. GPO, OTA-H-260; January 1985.
Scott, E.P. Why My Blood Center Imports. In Adequacy of the Blood Supply, Council of Community Blood Centers conference proceedings, Clearwater, Florida, February 18, 1990.
Smith, Peter, and Levine, Peter. The Benefits of Comprehensive Care of Hemophilia: A Five-Year Study of Outcomes. American Journal of Public Health, vol. 74(June), 1984.
U.S. General Accounting Office [report to Congress]. Problems in Carrying Out the National Blood Policy. Washington D.C.: General Accounting Office, March 7, 1978.
U.S. Senate, Committee on Labor and Human Resources, Subcommittee on Health and Scientific Research . Hearing: Oversight on Implementation of National Blood Policy, June 7, 1979.
Wallace, E.L., et al. Collection and Transfusion of Blood and Blood Components in the United States. Transfusion, vol. 33, 1993.
Westfall, Pamela. Hepatitis, AIDS and the Blood Products Exemption from Strict Products Liability in California: A Reassessment. Hastings Law Journal, vol. 37(6), (1986).