Appendix D
Modeling of Coating Degradation
The availability of accurate models for predicting the life of coatings will become essential as future engines become increasingly reliant on coatings to protect the components from the higher firing temperatures required to improve performance. As discussed in chapter 4, coating life at high temperature is determined by many degradation mechanisms, primarily oxidation, hot corrosion, and fatigue.
CURRENT STATUS
Models for life prediction based on high-temperature oxidation and hot corrosion of coatings are not well developed and are largely empirical or semi-empirical. Existing approaches test coatings in furnaces or burner rigs to determine life and to use experience factors to relate the life in the furnace or burner rig to the life in the engine. These approaches are unsatisfactory but are used in the absence of better methods and in the presence of a large, even if questionable, database of test results and correlations. Problems with these approaches include the following:
-
The approach does not enable the life of a coating in a new engine design to be accurately determined but only to be estimated.
-
Burner-rig tests typically exhibit a large amount of scatter and are often useful only for comparison with other laboratory reference materials.
-
Furnace testing does not simulate the high-velocity gases and high heat fluxes to which coatings are exposed and which can be important to their behavior.
-
Hot-corrosion life depends on the deposition of the corrodants on the surface. The rate of this deposition is not given by the burner rig or other methods used to test hot-corrosion resistance.
-
The actual environments, including the erosive species, to which the coatings are exposed vary widely, especially for industrial engines. These conditions are difficult, if not impossible, to simulate in the laboratory and are not always known.
Although not satisfactory, determination of high-temperature oxidation life by these methods can be done for existing engines after sufficient experience has been accumulated on the engine. However, hot-corrosion life requires a determination of the deposition of the contaminants on the surface, which depends on the environment and the flow profiles through the engine.
Modeling of high-temperature oxidation life has been developed by Probst and Lowell (1988), based on isothermal oxidation and thermal cycling.
where ξ is the scale thickness, W0X is the weight of the oxide scale, Δm/A is the mass gain per unit area, kp is the parabolic rate constant, and t is time. kp is related to temperature by an Arrhenius rate law:
where kpo is a material constant, Q is the activation energy, R is the universal gas constant, and T is the absolute temperature in K or °R. kpo is a function of oxygen partial pressure, but it is not a strong function. The model tracks the formation of the oxide by a parabolic growth law (although other growth kinetics can be used), and the spallation of the oxide due to thermal cycling.
where Wspall is the weight per unit area of the oxide spalled, Q0 is an experimentally derived material constant, and Woxide is the weight per unit area of the oxide prior to spalling. (Only a portion of the oxide may spall and some of the oxide may remain adherent to the coating.) The spallation equations are empirical and are based on careful experimental measurement of the weight of the spalled oxide. Barrett and Lowell (1975) have also developed a mathematical expression to fit cyclic oxidation data. Both of these approaches rely heavily on empirical methods. The model for thermal cycling is attractive but is based on experimental calibrations of the amount of oxide spalled instead of a more fundamental, predictive model. Furthermore, spallation data for only one
chromia-forming system and one alumina-forming system are available (at least in the public domain). The committee is not aware of any actual uses of these models.
Wright et al. (1991) have developed models for erosion modified oxidation, based on an erosion rate assumed to increase with the thickness of the oxide.
where ξ is the scale thickness as a function of time, t is the time between erosion events, a is an oxidation rate term, c is the Pilling-Bedworth ratio,1 ξ1 is the scale thickness after the last erosion event (ξ l = 0 at t = 0), and k is a term describing the oxidation kinetics (k = 0 for linear kinetics and k = 1 for parabolic kinetics). A numerical solution method is used to determine the degradation of the coating.
Chang et al. (1990) have studied the interaction of erosion and oxidation and have defined the various regimes of erosion and oxidation. These last two models were developed for coal gasification and fluidized bed applications. Bernstein (unpublished work) has combined the oxidation, spallation, and erosion models for industrial gas turbines and has added erosion rate terms. An example of the approach to modeling based on correlation with engine service experience is given by Strangman (1990). For high-temperature oxidation, Strangman computes the oxidation rate as the product of an engine experience factor, a burner-rig calibration constant, and a term for the temperature dependence of oxidation. For hot corrosion, Strangman uses a lengthy expression as follows:
Hot-Corrosion Rate = Salt Deposition Factor · Salt Corrosivity Factor Salt Deposition Factor = {Salt Vapor Deposition + (Component Experience · Particulate Salt Deposition) + Fuel Salt Deposition ·
Sulfate Formation + Fuel Vanadium Deposition + Salt RetentionEvaporation}
Salt Corrosivity Factor = {(Engine Component Experience · Burner-Rig Calibration Factor · Acidic Temperature Factor · Acidic Sulfur Factor) + (Engine Component Experience · Burner-Rig Calibration Factor · Basic Temperature Factor · Basic Sulfur Factor) + (Engine Component Experience . Burner-Rig Calibration Factor · Vanadate Sulfur Factor) } · Fraction Salt Molten.
The total environmental attack is the sum of the hot-corrosion rate and the oxidation rate. These equations are solved by means of a computer program that has additional calculations for many of the terms shown above.
Nesbitt (1984, 1989) has developed a diffusion-based approach to determine the oxidation life of coatings and bulk NiAl. For bulk materials, the model computes the flux of aluminum to the surface and the removal of aluminum by oxidation. When the aluminum flux becomes less than the aluminum consumption by formation of Al203, NiAl204 becomes the stable oxide. Since this spinel is not protective, the bulk metal will be attacked at this point. For coatings, the flux of aluminum in the coating to the surface is computed and the interdiffusion of the coating with the base metal is also computed. This interdiffusion reduces the aluminum concentration (actually aluminum activity) in the coating, shortening the life of the coating.
Fracture mechanics approaches to modeling coating spallation have been and are continuing to be developed (Evans, 1994). They usually predict a dependence on the square of the temperature change, ΔT
However, the utility of these models to predict coating degradation, as well as their agreement with experimental data, remain to be determined. Continuing work on modeling coating degradation by fracture mechanics may or may not prove useful. Until a better understanding of the physical mechanisms of coating degradation and the loss of the protective oxide is obtained, it is not possible to say if this is a fracture problem or not. It must be remembered that the oxide is in a dynamic flux, in which its composition, morphology, and thickness are changing as a function of time, and its properties depend on the location within the oxide scale.
The committee did not seek, nor obtain, details of proprietary models used by engine manufacturers. However, the models used by the manufacturers, as exemplified by Strangman's model, usually are empirical in nature.
FUTURE DIRECTIONS
Thermal Barrier Coating Modeling
TBC modeling is important for life prediction, understanding TBC behavior, and design of new coatings. Life prediction is the primary modeling issue that is needed to obtain the maximum benefit of TBCs apart from process modeling, which is covered elsewhere in this report. In spite of the use of TBCs for over 30 years, there has been relatively little activity in modeling their durability and reliability. In the past 10 to 15 years, there have been several large studies sponsored by NASA. Modeling is also necessary for more efficient design of new TBCs and for examination of the complex TBC failure mechanisms. This subsection discusses previous and current models of the life and durability of TBCs and directions for future development.
It should be noted that most of the modeling has been for high-temperature oxidation combined with thermal cycling. Hot corrosion of TBCs is not well understood, and there has
been only a limited effort in modeling the hot-corrosion life of TBCs.
Early TBC studies combined modeling and experimental work to define some of the important factors in TBC durability (Sevcik and Stoner, 1978; Cassenti et al., 1981), which are:
-
the effect of residual stress generated during processing on coating spallation
-
the correlation of in-plane compressive stresses on ceramic layer spallation
-
the importance of maximum operating temperatures on TBC life
An oxidation-based failure model was proposed by Miller (1984) assuming that the strains imposed on the ceramic layer were caused by thermal-cycle effects and that time at temperature effects acted to increase the effective thermal-cycle strains caused by oxidation.
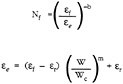
where Nf is the number of cycles to failure, ef is the failure strain in one cycle with no oxidation, er is the thermal expansion mismatch strain, W is the weight gain by oxidation, and Wc is the weight gain that should cause failure in one cycle. b and m are material constants determined from the experimental data. Miller's model correlated the cyclic life of a specific coating system tested at 1100°C for different thermal cycle times.
In the 1980s, significant advances in TBC modeling capability occurred for applications of specific TBCs. DeMasi et al. (1989) used a model similar to Miller's for plasma-sprayed TBCs, except that the thermal-cycle strains were included using a finite-element analysis that incorporated a Walker constitutive model for inelastic deformation of the ceramic during thermal cycling, and the strain to failure in one cycle, e f, changes with oxidation.
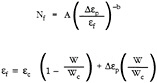
where Dep is the inelastic strain range, ec is ef at W = 0, and the other terms are as before. This same model was used for life predictions of EB-PVD (electron-beam physical vapor deposition) coatings (Manning-Meier et al., 1991), except that the EB-PVD zirconia ceramic layer was assumed elastic, the bondcoat was assumed to have inelastic behavior, and the thermal strain of the alumina scale was included. Therefore, the important cyclic strain for the EB-PVD ceramic was between the alumina layer and the substrate. Both models showed reasonable correlation to the experimental results.
Hillery et al. (1988) focused on the edges of TBCs as failure initiation sites for some TBC applications. Edge failures were driven by shear stresses in addition to normal stresses and oxidation. Oxidation was included through changes in the time-dependent material behavior and the stress state. This model was also found to give reasonable agreement with experiment.
Strangman et al. (1987) used a linear damage rule (damage from thermal cycling, oxidation, and hot corrosion) to reduce the strain-to-failure of the ceramic (instead of increasing the effective strain per cycle). This model reduces to a MansonCoffin relation. Again, the agreement to the experimentally measured lives was reasonable.
For each case the intent was to develop a model that would predict life for a given coating system rather than to determine the active degradation mechanisms. To do this, each model incorporated information known about the active degradation mechanisms for the specific coating and application domain, which was for aircraft engines. In addition, the models were heavily dependent on calibration to experimental data to predict the correct absolute lifetimes.
The vast majority of TBC modeling, including those models cited above, has been for current aircraft applications, which have a much different operational profile than land-based or marine gas turbines. Thus, for nonaircraft applications, it may be expected that the relative contributions and importance of different mechanisms to TBC failure would be different. These mechanisms include oxidation of the bondcoat, top-coat creep, sintering of the top coat, and the mechanical behavior of the alumina scale. These mechanisms have not yet been adequately characterized experimentally or theoretically.
A few models address long-term oxidation behavior and loss of alumina-forming capability (''wear out") of overlay coatings (Nesbitt and Heckel, 1984) and bondcoats for TBCs (Lee and Sisson, 1994). In these models, the life of the TBC is determined by the life of the bondcoat. While these models are not appropriate for aircraft turbines, they may address one aspect of failure for land-based and marine-based turbines.
Comprehensive models that can predict TBC life from first principles are necessary to be able to account for the wide range of conditions to which TBCs are exposed. Before a model can be developed to predict TBC life from first principles, a large number of factors must first be investigated. This
is because many of the potential contributors to TBC failure have not been adequately characterized because of the extreme difficulty in experimentally isolating these factors. Understanding of TBC behavior has been enhanced by computer modeling.
Computer modeling has been used to investigate TBC behavior by isolating and investigating issues not easily amenable to experimental study. Chang et al. (1987) and Phucharoen (1990) developed a finite-element model that examined the effect of the rough interface between the bondcoat and ceramic layer, the mechanical effects of oxidation on the stresses in ceramic layers, and the effect of bondcoat properties on TBC life. The most important of these issues are the effect of interface geometry and oxidation on TBC stress levels. Another recent computer model examining failure mechanisms, instead of life prediction, used the observed bondcoat creep response (Brindley and Whittenberger, 1993) to show that bondcoat creep can result in substantial increases in the ceramic layer delamination stresses (Brindley, 1995), which should result in decreased TBC cyclic lives. Ferguson et al. (1994) included bondcoat and top-coat creep along with a rough interface to provide a clearer picture of the role of these factors increasing the stresses generated in a TBC during thermal cycling. Apart from failure mechanisms, theoretical modeling may also shed light on heat transfer through TBCs, which in turn may guide design of more-insulating coatings.
The models discussed above have been used to guide TBC development and design. A more "design capable" model for TBCs is necessary to facilitate more rapid development and implementation of TBCs. Such a model would need to incorporate operating conditions, life prediction from first principles, and thermal modeling from first principles. It will also be necessary to establish a materials property database to support the model.
High-Temperature Oxidation
The current approach to modeling high-temperature oxidation is manageable for the original equipment manufacturer (OEM), although not desirable. It is based on laboratory testing and correlation with field experience, which has been developed over a number of years. One of the principal difficulties is the ability to accurately predict the surface temperature of the blading. Although not a coating issue, the difficulty of predicting surface metal temperature is a barrier that limits the ability of any coating degradation model to properly determine the life of the coating.
There is no current approach to predicting the degradation of the coating that the operator of the engine can use. The operator relies on OEM guidelines, which are based on design considerations and accumulated field experience. The engine operators need models that allow them to predict the coating life for their operation. This is particularly true for industrial engines that experience a wide range of conditions. Any models developed for the operator will also assist the OEM.
The current approaches to modeling do not provide the necessary insight that allow the development of more-oxidation-resistant coatings. Studies of a fundamental nature, as opposed to an engineering evaluation, are needed to determine how coatings are exposed and how they perform in the field, particularly for industrial gas turbines. Engine exposure can be fundamentally different than laboratory testing, and the nature of the protection by the coating will depend on the local exposure environment of the coating. Once these environments are determined and the coating response in the engine is established, laboratory studies simulating this behavior can be conducted to understand and predict the kinetics of the degradation of the coating. These studies can lead to the development of improved coatings and coating degradation models.
Hot Corrosion
A great deal is understood about the mechanisms and chemistry of hot corrosion, as discussed elsewhere in this report. The prediction of hot-corrosion attack of coatings in the field cannot be reliably performed, however. This inability is due in part to the difficulty in predicting the generation and deposition of corrodants and knowing the chemistry of the deposits and the surface metal temperatures.
As for high-temperature oxidation, the current approaches to modeling the life of a coating also do not provide the necessary insight that allow the development of more-corrosion-resistant coatings. (The mechanistic and chemical studies of hot corrosion do provide this insight, however.) Studies of a fundamental nature, as opposed to an engineering evaluation, are needed to determine the exposure environment of coatings and how they perform in the field, particularly for industrial gas turbines. As stated above, engine exposure can be fundamentally different from laboratory testing, and the nature of the protection of the coating will depend on the local exposure environment of the coating. Once these environments are determined and the coating response in the engine is established, laboratory studies simulating this behavior can be conducted to understand and predict the kinetics of the coating degradation. These studies can lead to the development of improved coatings and coating degradation models.
Modeling of hot corrosion is currently not as important as for high-temperature oxidation for industrial gas turbines because of the use of clean natural gas. Hot corrosion is still of importance to aircraft and marine engines, however. It will also be of importance to industrial engines if coal gasification or biomass fuels are used. Life prediction based on hot-corrosion attack is particularly difficult because the onset of attack may be caused by a local flaw in the protective oxide
that permits contact of the fused salt film with the coating. Thus the phenomenon does not obey some steady-state predictable kinetics.
Life-Prediction Models
One important aspect of the immature status of life-prediction models deserves emphasis. The overall goal for life-prediction models is to enable the component designer or user of the engine to make successful decisions regarding the selection of the superalloy, coating, and coating thickness distribution. Design decisions regarding the superalloy and coating must be made early in the engine design process (i.e., when the airfoil is still on paper). The type of information that should be available to the designer when selecting the coating and superalloy to be used is engine design parameters (e.g., gas temperature, velocity, and pressure; substrate and coating temperatures; fuel/air ratio; inlet filtration efficiency; stress; strain), anticipated application usage (e.g., fuel, location, altitude, and times at duty-cycle power points), available coating and superalloy properties, and prior engine experience knowledge bases. To make successful decisions regarding the selection of the superalloy, coating, and coating thickness distribution, the component designer or user of the engine needs mechanistic life-prediction methods that are expressed in terms that the designer and user of the engine can recognize, control, and, in some cases, adjust. For instance, life-prediction methods are needed that will enable designers to avoid thermomechanical fatigue cracking of coated superalloy components. Environmentally enhanced thermomechanical fatigue is a special case of low-cycle fatigue that is not adequately predicted with isothermal low-cycle fatigue data and models.
RECOMMENDATIONS
The understanding of the degradation and failure mechanisms of high-temperature coatings in the field need to be improved, particularly with respect to the effects of engine operation and environment on the coating performance (e.g., thermal cycling).
Qualitative and quantitative models need to be developed that predict coating life based on mechanisms observed in operational engines. These models need to be applied to optimize coating development and to estimate more accurately the remaining coating life for in-service engines. However, property measurements using reliable test methods are required before modeling.
Methods to determine the deposition rate of corrodants on in-service hot-structure components need to be developed that can be monitored by the engine operators.
REFERENCES
Barrett, C.A., and C.E. Lowell. 1975. Oxidation of Metals 9:307-355.
Brindley, W.J. 1995. Properties of plasma sprayed bond coats. Pp. 189-202 in the Proceedings of the Workshop on Thermal Barrier Coating. NASA-CP-3312. Cleveland, Ohio: National Aeronautics and Space Administration Lewis Research Center.
Brindley, W.J., and J.D. Whittenberger. 1993. Stress relaxation of low pressure plasma-sprayed NiCrAlY alloys. Materials Science and Engineering A 163(1):33-41.
Cassenti, B.N., A.M. Brickley, and G.C. Sinko. 1981. Thermal and stress analysis of thermal barrier coatings. In AIAA/SAE/ASME 17th Joint Propulsion Conference, Colorado Springs, July 27-29. New York: American Institute of Aeronautics and Astronautics, Inc.
Chang, G.C., W. Phucharoen, R.A. Miller. 1987. Finite element thermal stress solutions for thermal barrier coatings. Surface Coating Technology 32:307-325.
Chang, S.L., F.S. Pettit, and N. Birks. 1990. Interaction between erosion and high-temperature corrosion of metals: the erosion-affected oxidation regime. Oxidation of Metals 34(1/2):23.
Evans, H.E. 1994. Modeling oxide spallation. Materials at High Temperatures 12(2-3):219-227.
DeMasi, J.T., K.D. Sheffler, and M. Ortiz. 1989. Thermal Barrier Coating Life Prediction Model Development-Phase I. Final Report. NASA-CR-182230. Washington, D.C.: National Aeronautics and Space Administration.
Ferguson, B.L., G.J. Petrus, and M. Ordillas. 1994. A Software Tool to Design Thermal Barrier Coatings. Final Report. NASA Contract NAS3-2728. Washington, D.C.: National Aeronautics and Space Administration.
Hillery, R.V., B.H. Pilsner, R.L. McKnight, T.S. Cook, and M.S. Hartle. 1988. Thermal Barrier Coating Life Prediction Model Development. Final Report. NASA-CR180807. Washington, D.C.: National Aeronautics and Space Administration.
Lee, E.Y., and R.D. Sisson. 1994. The effect of bond coat oxidation on the failure of thermal barrier coatings: thermal spray industrial applications. Pp. 55-59 in Proceedings of the 7th National Thermal Spray Conference, Boston, Mass., June 20-24, C.C. Berndt and S. Sampath, eds. Materials Park, Ohio: ASM International.
Manning-Meier, S., D.M. Nissley, K.D. Sheffler, and T.A. Cruse. 1991. Thermal Barrier Coating Life Prediction Model Development. ASME Paper 91-GT-40. New York: American Society of Mechanical Engineers.
Miller, R.A. 1984. Oxidation based model for thermal barrier coating life. Journal of the American Ceramic Society 67(8):517.
Nesbitt, J.A. 1989. Predicting minimum Al concentrations for protective scale formation on Ni-base alloys. Journal of the Electrochemical Society 136(5): 1511-1527.
Nesbitt, J.A., and R.W. Heckel. 1984. Modeling of degradation and failure of Ni-Cr-Al overlay coatings. Thin Solid Films 119:281-290.
Phucharoen, W. 1990. Unpublished Ph.D. dissertation, Cleveland State University.
Probst, H.B., and C.E. Lowell. 1988. Computer simulation of cyclic oxidation. Journal of Metals 40(10):18.
Sevcik, W.R., and B.L. Stoner. 1978. An Analytical Study of Thermal Barrier Coated First Stage Blades in a JT9D Engine. NASA-CR-13560. Washington, D.C.: National Aeronautics and Space Administration.
Strangman, T.E. 1990. Turbine coating life prediction model. In 1990 Proceedings of the Workshop on Coatings for Advanced Engines, August. Washington, D.C.: U.S. Department of Energy.
Strangman, T.E., A. Liu, and J. Neumann. 1987. Thermal Barrier Coating Life-Prediction Model Development. Final Report. NASA-CR-179648. Washington, D.C.: National Aeronautics and Space Administration.
Wright, I.G., V.K. Sethis, and V. Nagarajan. 1991. An approach to describing the simultaneous erosion and high-temperature oxidation of alloys. Journal of Engineering for Gas Turbines and Power 113(0ctober):616.






