3
Decontamination Processes
Introduction
The major technology options for the D&D of the GDPs are outlined in this chapter. In addition to a brief description of the processes, comments are offered on the advantages and disadvantages of the technologies, with particular attention to their effectiveness, safety, and potential for cost reduction. The D&D experience at the BNFL Capenhurst GDP in the United Kingdom is also reviewed.
The normal sequence of decontamination operations is as follows:
- characterization or measurement of the contaminants present (radioactive and nonradioactive);
- removal of large uranium deposits;
- equipment disassembly and decontamination of surfaces;
- cleanup and demolition of buildings (assuming "greenfield"1 scenario); and
- waste management (distribution of waste products to disposal or recycling).
The sequence of processes and options is illustrated schematically in Figure 3-1. D&D operations are conducted under strict regulations in hazardous environments and require extensive safety and health protection equipment, as well as criticality controls.
Characterizations of both radioactive and hazardous materials must be carried out before, during, and after decontamination. The techniques and instruments required for characterization of the GDPs are known and have been widely used (see Appendix E).
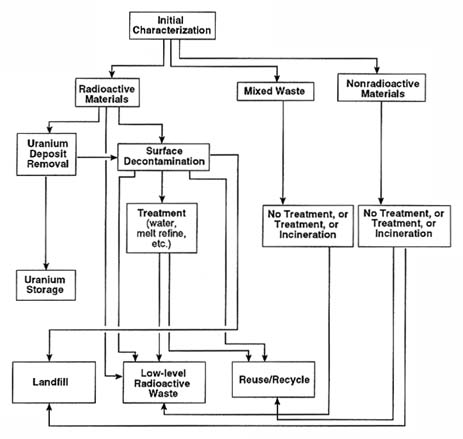
FIGURE 3-1 Simplified decontamination flow diagram.
The removal of bulk deposits of enriched uranium compounds from the process equipment is conducted first, both to reduce the possibility of nuclear criticality and to resolve security concerns regarding special nuclear materials. Two major technologies for deposit removal have been demonstrated: hot gaseous decontamination, normally performed while the process train is in operating condition; and mechanical removal, normally performed on nonoperating units after disassembly.
Following bulk uranium deposit removal, internal surfaces of the process equipment are additionally cleaned. Candidate technologies include further gaseous decontamination, chemical removal using aqueous solutions, high-pressure water jet decontamination, and dry mechanical removal technologies (abrasive or carbon dioxide [CO2] particle blasting). The last three processes require disassembling the process equipment and supporting systems.
Where building surfaces or external process equipment surfaces are contaminated, they can be cleaned by washing and wiping procedures, if contamination is light, or by various mechanical procedures, such as scabbling,2 if surfaces are deeply contaminated, or abrasive blasting, if they are contaminated by tightly adherent coatings (e.g., paint).
The technologies for performing the above decontamination operations are well known and have been demonstrated to be effective, for example, by the British D&D at Capenhurst and by the cascade improvement and cascade upgrade programs (CIP/CUP) of the U.S. GDPs. (Gaseous decontamination and aqueous washing were used during CIP/CUP, but there was no attempt to decontaminate to free-release levels; see "Capenhurst Technologies" and "CIP/CUP Technologies" later in this chapter.) Hazardous materials, such as PCBs, CFCs, asbestos, and lead paint, are known to be present in the GDPs and can also be handled with proven decontamination technologies.
The waste streams generated from the decontamination processes must be purified to release levels, recycled, or disposed of in burial sites. The waste management technologies are well established for the most part. As the cost of waste burial is increasing, or uncertain at best, a general guideline is to minimize the volume of any waste stream that is created by decontamination.
In the remainder of this chapter, the key features of technologies for each stage of the decontamination process are considered, together with opportunities for improvement. The discussion of cross-cutting areas, namely, characterization and robotics, follows the sections on deposit removal, decontamination, and waste management. The committee considered the potential of automation and robotics technology to reduce operating costs and improve safety conditions. Given the extensive technical literature on decontamination (see, for example, DOE, 1994b; DOE, 1995; DOE and IAEA, 1994), the committee felt that detailed descriptions of candidate technologies were inappropriate in the context of the present report.
The committee cannot assess the cost-benefit relationships of all of the various decontamination technologies as not only was comparative cost data lacking, but many uncertainties exist; for example, the degree and extent of contamination, the site end-states, waste release criteria, disposal sites, and disposal costs. New technologies continue to be proposed and the more promising should be investigated briefly; the committee, however, feels that it is important to initiate the D&D process without more years of delay. As mentioned above, the Capenhurst experience indicates that D&D can be performed in a cost-effective manner with existing techniques.
Nuclear Criticality
The presence of radioactive materials, specifically uranium, renders the D&D of the GDPs more complex and expensive than the demolition of large industrial plants without radioactive contamination. One distinguishing feature of the GDPs is the potential for nuclear criticality. Preventing criticality can be expensive, requiring intensive monitoring and small-scale processing under strictly controlled conditions. Thus, cost-effective D&D requires a clear understanding of where criticality will be a major concern, where it will be a relatively minor concern and easily handled, and where it is of no concern. At the GDPs, the risk of criticality during D&D is related to the sizes of deposits of uranium compounds in the process equipment, 235U enrichment levels, and the potential for the presence of a moderator (water) (see Appendix G).
The criticality hazard at the GDPs varies by site and among the buildings at each site; it can be ranked using historical characterization data, information on enrichment levels at each stage, and determinations of deposit size and location. The major concern with criticality during D&D is likely to be at the Oak Ridge GDP, particularly in the K-25 and K-27 buildings, where 235U enrichment levels are highest. It has been noted that, for these buildings, the potential for a criticality incident is greater than any potential for health physics or worker safety problems associated with D&D operations (MMES, 1992). Some deposits reported at Oak Ridge GDP (Table 2-6), if they occurred near the top of the cascade, could present a criticality hazard during D&D, even in the absence of a moderator. Under these circumstances, preliminary removal of deposits—as in the ongoing K-25 Site Deposit Removal Program (see below)—is imperative. In contrast, at 235U enrichment levels of less than 1 percent, it is essentially impossible to achieve criticality even with the most unfavorable geometry in the presence of water. Hence, there are many hundreds of stages at all three plants where criticality is not an issue during D&D.
Approaches to criticality prevention are addressed as appropriate in the following discussion of decontamination processes. Cost issues associated with criticality avoidance during D&D are considered in Chapter 6.
Uranium Deposit Removal
The first decontamination activity to be performed on GDP equipment is removal of any large uranium deposits. These deposits are the result of accidental occurrences, usually the leakage of moist air into the equipment, causing the formation of crusts of solid uranium compounds. The bulk of the crusts can be removed by gaseous treatment with ClF3 or by mechanical means. The application of gaseous deposit removal techniques differs between operating plants, such as Portsmouth and Paducah, and nonoperating (or static) plants, such as Oak Ridge, because the operating plants can heat the ClF3. The uranium deposits in the Oak Ridge plant have been characterized (Table 2-6) and must be removed to assure criticality safety and to conform to requirements for the safeguarding of special nuclear material.
The main features of both gaseous and mechanical treatments for deposit removal are summarized in the following sections. Ongoing or planned deposit removal activities at the Oak Ridge and Portsmouth GDPs are addressed. The primary objective of the K-25 Site Deposit Removal Program is to bring the Oak Ridge GDP site into compliance with DOE Order 5480.24 by removing, safely packaging, and relocating quantities of enriched uranium contamination deposited in piping and equipment (DOE, 1994a).3 DOE plans to remove highly enriched uranium deposits that are greater than 500 g of 235U and in a potentially unsafe geometry by 1999 (DOE, 1994a). Subsequent phases of this program plan for further removal of enriched uranium deposits. The use of both mechanical and gaseous methods for deposit removal is planned. Cleanup of the high-enrichment section at the Portsmouth GDP includes deposit removal (MMES, 1994). Work began in November 1991 and should be completed by the end
of fiscal year 1995. Nondestructive assay measurements are made to estimate uranium compound deposit sizes, which are then reduced to below "safe mass" using in situ gaseous treatment. If equipment inoperability precludes in situ treatment, such as at the Oak Ridge plant, equipment disassembly and mechanical deposit removal are necessary.
The survey by MMES (MMES, 1994) for the Oak Ridge GDP shows that the 235U content in most converter stages amounts to less than 100 g per stage, which would not present a criticality concern during cleanup of individual converters; it will be a concern for deposits at the high-enrichment ends of the cascade. (Data on criticality limits for 235U compounds in solid form and in aqueous solution are provided in Appendix G.) Eliminating the criticality concerns for individual converters would reduce subsequent decontamination costs (Lacy, 1994). The extent to which additional deposit removal efforts will be necessary in the high-enrichment sections at Oak Ridge and Portsmouth subsequent to the deposit removal programs must await the results of these programs.
The Ebasco cost estimate (DOE, 1991) proposed uranium deposit removal by gaseous decontamination in three ways:
- hot in situ removal in an operating plant prior to final shutdown (suitable for Portsmouth or Paducah);
- in situ deposit removal in physically isolated sections using a portable gaseous decontamination unit at relatively low temperatures (suitable for Oak Ridge); and
- removal of the process equipment and piping followed by treatment in a specially designed hot gaseous decontamination cell in a high-assay decontamination facility or a low-assay decontamination facility.
Gaseous Removal of Deposits
Gaseous removal of deposits can be carried out at an elevated temperature in operating cascades by introducing ClF3 to the closed system. The ClF3 fluorinates the solid uranium compounds present (primarily uranyl fluoride, UO2F2, and various other uranium fluorides) and removes them as UF6 (uranium hexafluoride) gas.
The principal advantage of the gaseous decontamination process is that, for the operational Portsmouth and Paducah facilities, it could be applied at elevated temperature during an organized, planned shutdown. 4 Reported experience at the Capenhurst GDP in the United Kingdom, where approximately 80 percent of the uranium deposits were removed by treatment with gaseous ClF3 prior to shutdown, suggests that much of the deposit removal can be conducted in situ (Clements and Cross, 1994). In contrast to mechanical deposit removal (see below), worker exposure to radioactivity in this process is minimized because it is totally contained in gas-tight equipment. In addition, criticality concerns are reduced because no
hydrogen-containing materials, such as water, are used; the concentration of the UF6 gas is low; and aggregation of uranium deposits is unlikely. ClF3 treatment is an expensive operation.5 However, removal of uranium deposits using an elevated temperature gaseous treatment has the potential to reduce costs substantially during subsequent decontamination of the cascade equipment as a result of reduced security controls, worker protection requirements, and contamination containment needs (Bundy and Munday, 1991). This advantage would apply primarily to the high-enrichment sections of the cascades.
The major disadvantages of gaseous decontamination are the danger in handling the toxic and highly reactive ClF3, the difficulty of applying the technique at ambient temperature to partially dismantled process equipment as in the Oak Ridge plant, and uncertainty about the amount of uranium that will be removed.6 In most cases the objective of past treatments was not decontamination but rather the improvement of gas flow impeded by the deposition of solids inside the process equipment or the removal of radioactive surface deposits to an extent that would allow operators to perform tasks requiring direct contact.
At Oak Ridge there is no in situ equipment (compressors) for heating isolated cascades, and the reactions are so slow at ambient temperatures that a cleaning cycle for one cell would likely require many months. However, a long-term, low-temperature gaseous process for deposit removal has been proposed that could be applied in situ to isolated portions of nonoperating plants as well as to operating systems (Bundy et al., 1994).7 It has been suggested that this process might be capable of reducing uranium contamination to free-release levels with a sufficiently long treatment time (Bundy, 1994). Two demonstrations of the long-term, low-temperature treatment have been proposed. Work has commenced at the Oak Ridge GDP on a mobile ClF3 demonstration unit to treat small units of the cascade equipment, such as converters and pipe sections. Heating of the unit may be tried to accelerate the removal process. A demonstration of the long-term, low-temperature process for deposit removal and subsequent decontamination is planned for an entire cell that was used for highly enriched uranium production at Portsmouth. The demonstration at the Oak Ridge GDP should be informative in choosing a bulk uranium removal process for this plant. The results from both demonstrations
should be useful in determining the degree of decontamination that can be achieved through treatment with ClF3, although a decision to use this technology would also depend on cost.
The porous nature of the nickel barriers—and their correspondingly large surface areas—impose special decontamination requirements. The evidence presented to the committee indicates that gaseous treatment with ClF3 will remove the bulk UO2F2 deposits but is unlikely to remove all of the adsorbed uranium on the barriers (Bundy, 1994). Further decontamination using aqueous or other techniques will probably be necessary. Research and development is currently in progress to determine whether an ion exchange process can be developed in which specific ions are introduced to stimulate the extensive removal of uranium and 99Tc (technetium) retained by the barrier material during gas phase treatment.8
Mechanical Removal
Uranium deposits can be removed by mechanical means, which requires the disassembly and dismantling of the process equipment, such as converters and compressors. The internal surfaces are then scraped, wire-brushed, or abrasive-blasted to remove the uranium crusts, a labor-intensive process requiring extensive health and security precautions. A glove box in a special deposit removal room has been used at Oak Ridge to demonstrate the feasibility of removing deposits mechanically from certain components, such as pipe sections and compressor parts, where criticality is an issue. Critically safe vacuum systems are used to collect and package the removed deposits in critically safe containers.
Aqueous Spray Removal
During CIP/CUP at the U.S. GDPs between 1974 and 1981, gaseous deposit removal using ClF3 was followed by aqueous solution spray booth treatment of disassembled process equipment for further uranium removal (Faulkner, 1995). Pieces of equipment were run through spray booths (analogous to a car wash), where they were washed using aqueous solutions, such as 5 percent nitric acid, and then rinsed with water. Cleaned pieces were surveyed for remaining contamination and sent through the spray booth system a second time if necessary. The objective was not to clean to free-release standards but to remove transferable contamination so that the equipment could be reassembled for use without the risk of spreading contaminants.
The diffusion barriers were removed from the converters and also run through the spray booths, but removal of uranium was difficult. The barriers were subsequently cut up using high-pressure water jets or mechanical saws. The nickel was separated from other material and the nickel pieces were packaged in 30-gallon drums. The drummed nickel pieces were eventually shipped to Paducah and the nickel barriers melted into ingots.
In the spray booths, criticality was avoided by keeping the bulk wash solutions in piping and troughs that had critically safe geometries. For example, pipe diameters were limited to 5 inches. The spray booths at each GDP were designed to provide criticality safety for the levels
of enrichment at the plant. However, only low-enriched material was of concern during CIP/CUP activities.
Again, deposit removal can be accomplished in several ways: using gaseous ClF3, mechanical removal, or an aqueous spray. The choice of an approach depends on the cost effectiveness of each of these processes for any particular case, and in some cases, on criticality considerations. It is likely that some combination will be optimal. For example, criticality is not a problem for low-enrichment cells, and simple dismantling, cutting up, and aqueous spray removal could be used. At Portsmouth, ClF3 was used effectively at the time of shutdown for the high-enrichment cells. The reported high cost of ClF3 treatment ($250,000 to $500,000/cell; Meehan, 1995) suggests that mechanical and aqueous spray need to be considered, though better estimates are needed for the cost of ClF3 treatment during an orderly shutdown of an operating plant. For higher enrichment cells at the Oak Ridge GDP, the difficulties of applying ClF3 favor mechanical removal or possibly aqueous spray removal. The spray booth technique is not suitable for all deposit removal. Criticality concerns can limit the use of aqueous spray methods, depending on the geometry of the materials, mass of the deposit, and 235U enrichment level. The washing process can result in criticality before the wash solutions enter the critically safe piping system of the spray booth. Under such circumstances, addition of a moderator (water) should be avoided, and the deposits would most likely be removed by dry techniques.
Decontamination Of Cascade Equipment
The use of aqueous decontamination techniques for the GDP cascade equipment is addressed immediately below; further details of the aqueous process used at the Capenhurst GDP are given later in the chapter. Despite some suggestions that gaseous treatment with ClF 3 might be used for decontamination of cascade equipment to free-release levels, available data from maintenance programs and CIP/CUP activities indicate that some uranium deposits are slow to react and will be difficult to remove with ClF3.9 In the committee's opinion, cleanup of the cascade equipment to free-release levels will require aqueous decontamination methods and cannot be reliably achieved solely by gaseous ClF3 treatment.
Disassembly
Large process equipment components (such as converters, compressors, motors, valves, and coolers) need to be disassembled and decontaminated. Subassemblies are removed (cut out) from major components and carried to a central location for further dismantling and decontamination. The repetitive nature of the operations may favor the use of automation and robotics.
Aqueous Decontamination
The removal of uranium deposits by aqueous solutions is a well-established procedure and was used at the Capenhurst GDP in the United Kingdom to decontaminate most of the equipment. The principal contaminant, UO2F2, is readily soluble in water. The behavior of lower valence state uranium fluorides and oxyfluorides is less well characterized (see, for example, Ritter and Barber, 1991), but these species are successfully removed by aqueous solutions of acids in the presence of air or other oxidizing agents. Since cleanup of the wash solutions is an important contributor to the cost of aqueous treatments, care is needed in the selection of the solutions to minimize the waste disposal problem.
Two different physical arrangements have been used in aqueous removal of uranium from gaseous diffusion equipment. At Capenhurst, cut-up pieces of equipment were dipped in a series of washing tanks. During CIP/CUP activities at the U.S. GDPs in the 1970s, pieces of equipment were run through aqueous solution spray booths, as described. An attractive arrangement for D&D might be to use both of these physical arrangements (Faulkner, 1995). Following removal of residual large deposits of uranium by mechanical means (e.g., at K-25), the cut pieces of equipment could be run through spray booths to remove most of the uranium, and then through a series of dip tanks for final decontamination. It is likely that many pieces—perhaps the majority—would be sufficiently clean initially to bypass the spray booths and go directly to the dip tanks. The spray booth treatment could still make unnecessary much of the labor-intensive mechanical removal, while giving good uranium recovery in a criticality-free arrangement.
Metal surfaces may be contaminated with 99Tc, as well as uranium, and with very minor quantities of other radionuclides (see Chapter 2). While these contaminants are not expected to be a problem in most of the cleanup, there may be occasions when modified wash solutions may sometimes be needed to remove these contaminants to acceptable levels. 99Tc was particularly difficult to remove during the Capenhurst D&D.
When uranium is mixed with water, the danger of criticality is greatly increased because the presence of the light element hydrogen slows or moderates neutrons and increases their chances of fissioning 235 U nuclei (see Appendix G). Criticality is affected by the mass of uranium present, the degree of 235U enrichment, the presence of moderators, and the nature of material surrounding the fissioning material that can reflect neutrons back toward the 235U. The geometry of the system can either enhance or limit the loss of neutrons from the system, and some materials, such as boron, cadmium, and gadolinium, can absorb neutrons and prevent them from fissioning 235U. In an aqueous process for decontamination, criticality prevention can be attained by several means:
- limiting the mass of 235U through continuous monitoring of its concentration, as was done at Capenhurst;
- using geometrically safe equipment, as was done in U.S. facilities during upgrading of GDP equipment; or
- inserting of neutron absorbers, or "poisons," such as boric acid. The preferred form is solid, but soluble poisons may also be used. However, soluble poisons may inadvertently precipitate from solution.
Measures to avoid criticality conditions engender additional costs. Attention must be paid to the double-contingency principle, which requires that no single mishap, regardless of its probability of occurrence, can lead to criticality. This principle requires that two unlikely, independent, and concurrent changes in process conditions occur before criticality is possible.
The aqueous decontamination process produces radioactive wastes that must be managed safely. The uranium can be removed and recovered from the wash solutions by evaporation, precipitation, ion exchange, or solvent extraction. The bulk of the water can be reused. Solid wastes containing uranium and 99Tc compounds will be produced. Organic acids in the decontamination wash solutions can be reused, biodegraded, or chemically destroyed.
For the D&D of Capenhurst, the British had a volumetric contamination standard for the free-release of cleaned material. Although analogous volumetric standards do not currently exist in the United States, it seems probable that decontamination from uranium can be successfully accomplished at the U.S. GDPs by an aqueous process. The specific aqueous treatment to be used merits some development work to determine the best alternative for design of the washing equipment and the choice of cleaning solutions. Replacement of citric acid (used at Capenhurst) by oxalic acid has been suggested (Anderson, 1994). Work at Battelle Columbus Laboratories indicates formic acid may be a desirable alternative.10 These two proposals, and possibly others, merit consideration.
As noted above, the nickel barrier contains uranium deposits within its porous structure. The removal of 99Tc, which may be present in some of the barriers, must also be addressed. Information on the efficacy of aqueous treatment for decontamination of the barriers is ambiguous. Experience in decontamination processes, notably during the CUP, provides some basis for optimism that aqueous methods can be used successfully for barrier decontamination. However, the chemical nature of the uranium complexes and the physical characteristics of the barrier material suggest that aqueous treatment with the usual decontamination agents may not be adequate.
Three possible alternative methods have been proposed for decontamination of the barriers; namely, aqueous electrolytic dissolution and redeposition, conversion to nickel carbonyl (Mond process), and melt refining. Melt refining is not expected to remove the 99Tc. There is evidence that electrochemical techniques can be used to remove 99Tc and reduce the radioactivity level to meet unconditional clearance levels of 0.3 Bq/g (8 pCi/g) recommended by the International Atomic Energy Agency (see Table E-7). This electrochemical process is estimated to cost $2/lb of nickel (Carder, 1994). Because of the value of the nickel, the committee believes that a limited effort is justified in seeking a cost-efficient process for decontamination of the barrier material.
Decontamination Of Support Systems And Buildings
Radioactively Contaminated Support Systems
The large-scale equipment of the cascades is supported by a great deal of piping, ductwork, electrical equipment, instrumentation tubing, and so forth, which is contaminated with small amounts of uranium. Easily cleaned pieces could be treated by aqueous wash, but much of this equipment, for example, small-diameter piping, is not amenable to such cleaning. In some cases, it may be worthwhile to decontaminate through melt refining. In other cases, the best disposition option may be compaction and low-level radioactive waste disposal. In practice, the extent of decontamination will depend on both waste disposal costs and recycle standards.
Radioactively Contaminated Interior Building Surfaces
Mechanical decontamination techniques for uranium contamination of parts of floors, walls, and structural components of the buildings, including washing, blasting, grinding, scabbling, scarifying, drilling, electropolishing, and ultrasonic cleaning, appear to be the most promising for these applications.11 In some cases, simple wiping with a rag may suffice. In other cases, removal of the surface to an appreciable depth may be necessary, as for example for contaminated concrete floors. Robotic equipment may be effective for much of the work, as discussed below. The large volume of material resulting from such mechanical decontamination processes will require disposal as low-level radioactive waste.
Removal of Nonradioactive Hazardous Material
As noted in Chapter 2, significant quantities of nonradioactive hazardous materials are present at the GDP facilities, including PCBs, CFCs, asbestos, and lead-based paints.12 There are also smaller quantities of other hazardous materials present, such as lubricating oils and greases, mercury in electrical switches, and chromium-contaminated wood in the cooling towers.
Proven technologies are available for removal of all of the contaminants identified at the GDP sites. In most cases, off-the-shelf, commercial technologies, such as incineration, can be used if there are no radioactive residues mixed with the hazardous materials. If radioactive contamination is present, then these methods may have to be modified to handle the materials before, or as a part of, final disposal. An exception occurs at Oak Ridge, where the TSCA incinerator handles mixed waste. Melt refining might be used to treat metal ducts contaminated with both radioactivity and PCBs. The committee believes that robotic devices may be useful in activities such as dismantling contaminated ductwork, stripping asbestos from piping, and stripping paint from structural steel to significantly reduce decontamination costs.
Waste Management
Some general guidelines govern waste management. The creation of secondary wastes from treatment should be minimized because such wastes usually must be treated and disposed of. For example, if floors are washed with large quantities of water, that wash water must be cleaned before it can be released. High disposal costs suggest that serious consideration be given to reduction of waste volumes. For example, a waste compactor should be included as part of the low-assay decontamination facility as assumed in the Ebasco cost estimate. Waste materials are either cleaned sufficiently for free-release, recycled, or buried. The disposition of the waste is governed by the level of radioactivity compared to release standards, the demand for and acceptance of recycled materials, and the cost of burial.
All decontamination processes yield some form of waste that must be managed safely and economically. These waste streams vary considerably, ranging from fragments of solids removed mechanically from converters to water from washing down walls. Accordingly, waste management must deal with handling solids, liquids, and gases containing various quantities of hazardous and radioactive materials. Established technologies exist for waste management (DOE, 1994b). A more detailed discussion of waste management issues is given in Appendix I.
The only materials at the GDPs with sufficient value for recycling are probably some of the metals (steel, stainless steel, nickel, copper, aluminum, and small quantities of mercury) and possibly the CFC refrigerants. Much of the structural steel is uncontaminated or very lightly contaminated and would require only minimal cleaning before release. As noted above, CFC refrigerants from the Oak Ridge GDP have been put to use at the other GDPs. It is likely that the current refrigerants used at the Portsmouth and Paducah plants will be replaced before the plants close; remaining refrigerants might be sold, converted to other useful compounds, or destroyed.
Melt refining, or liquid melt-slag technology, is suitable for compacting and purifying contaminated metal process equipment and accessory equipment after initial deposit removal or after subsequent surface decontamination (Worcester et al., 1993). Metals that can be treated by this process to remove uranium include mild steel, stainless steel, nickel, and copper. Thermodynamic considerations indicate that uranium cannot be removed from aluminum by melt refining. A melter unit currently operating at the Scientific Ecology Group facility at Oak Ridge has been used to treat iron and steel. Melt refining is a technically attractive option for recycling steel because the uranium can largely be removed in the slag, with the residual uranium being on the order of 1 ppm (Cavendish, 1978). Any 99Tc present that was not removed in a previous aqueous decontamination step, however, will not be removed to any great extent in the slag and will likely require special treatment. Melting substantially reduces the volume of material that must be disposed of, so disposal costs should be reduced accordingly. An additional benefit of melt refining is that organic surface contaminants, such as PCBs, are destroyed in the process.
The recycled metal may be released for unrestricted use if release standards are met or for restricted use in DOE facilities. In the latter case, it could be used for shielding blocks or waste canisters, thereby avoiding both the cost of procuring virgin metal and the cost of disposal. A dedicated facility located at a nuclear site—such as the Scientific Ecology Group
facility at Oak Ridge—is necessary to melt, cast, roll, and fabricate the lightly contaminated material. While metal waste canisters would be buried, some safeguard would be needed to ensure that other contaminated steel (e.g., shielding blocks) was not inadvertently released to the commercial market.
To determine the cost effectiveness of recycling the metals from the GDPs, additional data are required on the cost of recycling and the capability of melt refining (or other techniques) to remove radioactive contaminants, as well as the cost of disposal. However, without clearly defined release standards (see Chapter 5), the cost effectiveness of recycling technologies cannot be determined with confidence. The availability of uniform release standards in the near future would greatly expedite recycling tradeoff studies.
The cost of waste disposal at low-level radioactive or toxic waste sites depends on the volume to be stored. Consequently, chemically or thermally treating asbestos to increase its density and create a non-toxic waste form might be attractive. The economic payoff might arise from the savings in long-term storage and the avoidance of double bagging normally required for asbestos. A cost study of such an option would be worthwhile.
Characterization
Characterization of hazardous substances to determine their identities, forms, amounts, and locations will be needed before, during, and after the D&D operation. An initial survey will identify contaminated areas and estimate the magnitude of the cleanup effort needed at that location, where existing data are insufficient for these purposes. Monitoring will be performed during D&D for worker protection and criticality prevention and to determine the effectiveness of cleanup. Following D&D, characterization will again be needed to ensure compliance with regulatory limits before releasing the site, wastes, or reusable materials and equipment. The following discussion addresses the characterization approach and technologies for radionuclides and for hazardous nonradioactive substances.
Radionuclide Measurement Techniques
Survey techniques and detection instruments for radionuclides have been widely used at the three GDPs for monitoring routine operations, repairs, and plant upgrading. The suitability of available procedures and instruments and of systems under development for characterization associated with D&D has been evaluated (DOE, 1993b, 1994b; Appendix E). Existing characterization techniques should be adequate to ensure compliance with limits prior to the release of GDP sites, wastes, or reusable materials, unless the new concentration limits now under development as decommissioning limits or guidelines are significantly lower than current values. Appendix E discusses recent developments in radiation monitoring that may be applicable to D&D. Opportunities for the use of robotic systems to characterize both radionuclides and hazardous nonradioactive materials are discussed below.
In general, a carefully planned combination of field and laboratory measurements is needed to provide reliable information. In most instances, the radionuclides other than uranium cannot be determined by in situ monitoring, but require laboratory methods since they are
difficult to detect in the presence of uranium or are contained in enclosed spaces.13 Special studies have identified locations where these other radionuclides are concentrated. In particular, the general locations of 99Tc at the Oak Ridge and Paducah plants—and probably also at the Portsmouth plant—are reasonably well known.
The initial survey to locate uranium accumulation within the cascade system can be performed by gamma-ray or neutron measurements with detectors placed on external surfaces.14 In the structures that house the cascades, an extensive initial survey for contamination external to the cascades can be avoided for several reasons: monitoring will be performed in the next phase of decontamination operations for worker protection, low-levels of gamma radiation near cascade units will be overshadowed by radiation from uranium and decay products within the units, and contamination on surfaces may be increased by the cascade dismantling process.
Information on radionuclide levels of areas and structures beyond the actual cascade facilities that are known to be contaminated should be available in reports of routine monitoring and incident responses. A site-wide survey will be necessary for this purpose only if the available data are not sensitive or complete enough or if they are so difficult to convert to a consistent database that a new survey would be more cost effective. An initial survey of areas and structures believed to be uncontaminated is desirable to confirm that these locations are available for storing decontaminated materials and ready for dismantling and other activities leading to release of the site.
Hazardous Nonradioactive Materials
The methods for characterizing the hazardous materials present at the GDPs are well established (DOE, 1994b). No development work is necessary. The challenge will be to identify the number of samples required for adequate coverage and yet keep the characterization costs to a minimum. The use of available records and inventories should help minimize the amount of physical sampling. The repetitive nature of the process equipment and building structure can also be used to advantage to minimize the physical and chemical characterization program for hazardous materials.
Automation And Robotics
The enormous physical size of the GDPs and the many modules of repetitive, standardized equipment may provide opportunities for automation of processes and data management. However, the degree to which robotics and automation can be used cost effectively in the D&D of the GDPs is currently uncertain. Successful implementation of automated and
|
13 |
The non-uranium isotopes, 239Pu, 237Np, and 99Tc cannot be monitored in the presence of uranium. The higher level of radiation from uranium masks the presence of the other isotopes that are present in very low concentrations. In addition, uranium is monitored using neutron interrogation devices. However, 99Tc, for example, is a soft beta emitter that is difficult to monitor on exposed surfaces and undetectable when inside piping or equipment. |
|
14 |
A very extensive nondestructive assay survey for 235U deposits in the process equipment has already been conducted at the Oak Ridge site (see Chapter 2). |
robotic systems will require carefully planned and well-defined processes and techniques, based on experience with manual operations, to take advantage of the "learning curve." A focused, application-driven robotics development program in DOE's Office of Technology Development (EM-50) is addressing many of the areas discussed by the committee (see Chapter 6 on cost reduction and Appendix F for more detailed discussion of automated and robotic systems for D&D, including related DOE programs).
Various automated and robotic devices are commercially available for use in labor-intensive decontamination and disassembly processes. Automation and robotic technologies offer the possibility of cost reduction and improved safety for characterization, disassembly, decontamination, and material handling operations. For some tasks, hostile environments may justify the use of simple remotely controlled (teleoperated) devices for specialized work that is not labor intensive. For large numbers of repetitive tasks in structured environments, development of specialized automation (robotic and telerobotic devices) may be justified. These developments do not require fundamental research or new technologies but rather the adaptation of proven systems to specific D&D applications. Many of these opportunities and examples of such systems have been identified and discussed (DOE, 1993a, 1994b). In most cases, demonstrations are necessary to analyze the benefits, to develop the most effective operational techniques, and to train existing operators in the new systems and techniques. Logic diagrams to assess technology have been developed for environmental restoration/waste management problems at the Oak Ridge GDP site (DOE, 1993b) and have identified numerous opportunities for robotics during the D&D (Bundy et al., 1993).
Characterization will be a continuing activity during D&D operations as has been noted. Characterization consists of two operations: sampling and analysis. Manually, these are labor-intensive, repetitive operations—often requiring personnel protection and possibly large numbers of sampling points to obtain statistically reliable results. Since robots are consistent, repetitive, and patient under these conditions, their use can result in better quality data. The Mobile Autonomous Characterization System, a mobile robot, is under development at Oak Ridge National Laboratory, and evaluation tests are planned for the Oak Ridge GDP (see Appendix F; Richardson, 1994).
Characterization of less accessible areas, such as walls and ceilings, the cluttered areas around piping and process equipment, underground piping, and the internal surfaces of tanks, is not as easy for robots and will require some development work and demonstrations. However, there could be a large payback for developing of these systems. Commercial teleoperated pipe crawlers have been developed to work inside pipes and ducts. These systems may be appropriate for the GDPs.
A centralized computer database to integrate and coordinate the total information system, including both planning and operational processes, is desirable for the complex environment of the GDPs. Although real-time processing of data is desirable, a large amount of off-line processing and analysis may also be required. Automated analyses of samples collected during characterization are potentially attractive given the large volumes of data that must be handled. Such analyses are very labor intensive and time consuming.
In a highly automated system, most equipment might be dismantled and decontaminated at central facilities rather than in situ. Robotic systems for disassembly, dismantling, and transportation to a central decontamination facility are feasible and are currently under development (Thompson and Dockstader, 1994). Large systems could be dismantled remotely, eliminating the need for many of the operational personnel to wear protective clothing, and transported to a contained central disassembly/decontamination facility for further dismantling, cut-up, and aqueous decontamination.
Miles of contaminated piping and ductwork must be removed. Robotic systems are being developed for these specialized operations (see Appendix F). Removal and compaction of asbestos from pipes and ducts is a viable application. Technology demonstrations and analysis for an asbestos removal system are currently underway (The Robotics Institute, 1995). In practice, automated methods may be limited to long straight sections of pipe, which are the majority of ducts and piping at the GDPs.
The mobile robotic systems under development for characterization tasks might be re-equipped with the necessary tooling and re-employed for the decontamination of floors, walls, and ceiling surfaces. Remote and/or autonomous operations for mechanical tools, such as concrete scabblers, torches, cutting tools and scrapers, and water jets and other blasting operations, have been demonstrated and rated as a high priority need in an assessment of decontamination needs (Bundy et al., 1993). Most of this equipment is commercially available and requires only minimal adaptation to existing automated and robotic systems.
Capenhurst Technologies
The Capenhurst GDP was operated by BNFL in the United Kingdom from 1956 to 1982. The plant was very similar in design to the U.S. GDPs, although significantly smaller in production capacity and physical size.15 D&D of the facility commenced in 1982 and is scheduled for completion in late 1995 (Baxter and Bradbury, 1991; BNFL plc, 1990; Clements, 1992; Clements and Cross, 1994; Cross, 1995; Spencer, 1988). The most significant task remaining is the cleanup of approximately 4,000 tons of metals (1.5 percent of the materials from the plant) contaminated with uranium, 99Tc and neptunium (237Np) that could not be treated cost effectively by the aqueous decontamination process. The costs of D&D for Capenhurst are presented and analyzed in Chapter 4, and other details are presented in Appendix H. The present discussion provides a brief overview of the decontamination processes used at Capenhurst, many of which are applicable to the U.S. GDPs.
With regard to decontamination processes, the most significant differences between Capenhurst and the U.S. GDPs are as follows:
- At Capenhurst, the process piping and converters were mostly made of aluminum, although many were nickel-plated steel; at the U.S. GDPs these components are made of nickel-plated steel.
- The feeding of reactor-recycled UF6 containing 99Tc to the cascades was more prevalent at Capenhurst than at the U.S. GDPs.16 Therefore, there should be a smaller concentration of 99Tc in the material to be removed at the U.S. plants.
- In the United Kingdom, the Radioactive Substances Act (Substances Exemption Order, 1986) provides the framework for unrestricted release of materials for recycling.17 Comparable volumetric release criteria do not currently exist in the United States.
- At Capenhurst, the cascade equipment was located on the first floor of the process building, whereas at the U.S. plants the cascade equipment is located on the second floor.
Removal of Uranium from the Capenhurst Enrichment Cascades
At the Capenhurst plant, gaseous ClF3 (chlorine trifluoride) was circulated through the cascade equipment prior to shutdown and dismantling to convert residual uranium deposits to volatile fluorides prior to opening up the cascade system. This ClF3 treatment removed an estimated 80 percent of the UO2F2 deposits, substantially reducing the probability of a criticality accident during subsequent decontamination operations. Following gaseous treatment, further cleanup and pretreatment operations were carried out on the static plant to remove any significant remaining pockets of contamination and permit safe and cost-effective intrusions into the plant during the dismantling campaigns. Cleanup techniques included vacuuming, ridding, and machining. At the time the plant was shut down, radiological and criticality data were gathered for use in D&D planning and execution.
Decontamination of Equipment from the Capenhurst Cascades
The initial phase of disassembly involved the cut-out, removal, and storage of compressors, coolers, valves, large-diameter pipe, and large process stage units.18 Specialized workshops were built for component stripping and dry cleaning of equipment from the high-enriched section of the plant. Protection of personnel was achieved by effective ventilation and extensive alpha-in-air monitoring throughout the facility. Strict criticality prevention systems were applied at each stage of the dismantling.
Large components, such as converter shells, piping, and compressors, were reduced in size and weight to meet the physical size limitation requirements of the aqueous decontamination plant and the melter. Both hot and cold cutting methods were developed and used. Cold cutting was preferred over hot cutting for aluminum components because cold cutting does not generate fumes or airborne aluminum oxide fines, thereby reducing the need for costly heating, ventilation, and air conditioning systems. Robotic plasma-arc cutting was used for size reduction of large aluminum converter shells, and remotely controlled oxyacetylene methods were used for cutting steel converter shells and other steel components.
A wet decontamination process was used to reduce uranic contamination down to free-release levels (Anderson and Faulkner, 1989). While chemical treatment for the removal of uranium and its daughter products is a well-established process, 99Tc is difficult to remove effectively. A means of removing 99Tc had to be developed before effective disposal routes could be determined. Following extensive laboratory and pilot plant investigation, a full-scale decontamination plant was built in 1989. The flowsheet was based on achieving plant discharges having a negligible impact on the environment and on satisfying the United Kingdom's statutory regulations for recycling scrap metals to the open market.
The decontamination was achieved by dipping the metal pieces in a series of 10 tanks alternately containing wash and water-rinse solutions. The first tank contained citric acid, the third and seventh sulfuric acid, and the fifth and ninth disodium citrate as the main decontaminant. Most of the uranium and 237Np were removed in the first tank while the 99Tc came off in the third, fifth, seventh and ninth tanks.
Separate processing systems were used to clean up the spent citric acid, sulfuric acid, and disodium citrate decontamination liquors. Details of these systems are proprietary, although the following information on BNFL plans in 1989 for its decontamination process is reported by Anderson and Faulkner (1989). Uranium, along with 237Np, was recovered from the citric acid solution by evaporation. The spent sulfuric acid solutions were treated with lime to precipitate calcium fluoride, calcium sulfate, aluminum hydroxide, ferric hydroxide, and some of the 99Tc, presumably as 99Tc dioxide. The remaining solution was then passed through an anion exchanger to remove more of the 99Tc.
The spent solution from the fifth and ninth tanks containing traces of metal ions, pertechnate ion, and disodium citrate plus hydrogen peroxide, was neutralized to pH 6, filtered, and passed through a bed of activated carbon to decompose the hydrogen peroxide. The liquid was then passed through an ion exchange column containing an iminodiacetate resin that removed both the pertechnate ion and the various metal ions present.
The waste streams arising from the process were spent ion exchange resins and cleaned process liquors. The total volume of spent ion exchange resins arising from the aqueous decontamination process was about 100 yd3 for the whole plant. The liquors were neutralized, filtered, and run through ion exchange columns to remove heavy metals and radioactive species prior to release to the environment via heavy dilution with other plant wastewater streams to stay within allowable discharge concentrations.
Strict controls for criticality prevention were maintained, with detectors placed at key points in the decontamination facility. The activity of each individual piece was monitored after decontamination to ensure it met the applicable release criteria.
Following disassembly of the converters, the barriers were removed and stored in a secured area. BNFL staff report that, following a number of tests and trials, they now have a satisfactory method of recovery and recycle of the nickel from the barriers.19
Decontamination of Supporting Systems and Building Surfaces
Following process equipment removal, the remaining cell enclosures were demolished. Hazardous materials, such as asbestos, PCBs, lubricants, and laboratory chemicals, were removed and disposed of using conventional technologies such as land burial (asbestos) and incineration (PCBs). The building shell was removed from about one-half of the total structure. The floors were scabbled and removed, returning that portion of the structure to greenfield site status. A number of ancillary buildings and structures were also demolished, including 11 large natural draft cooling towers, their pump houses, and an electrical substation. Including the floor slab, this operation produced 46,000 metric tons of clean concrete rubble for off-site disposal. In practice, many items, such as structural steel and concrete, required only minimal decontamination.
Waste Management
Metallic materials recovered from the plant were categorized based on their potential for sale to the commercial market:
- clean scrap;
- contaminated scrap that could be economically decontaminated to de minimis level; and
- contaminated scrap that could not be economically decontaminated to de minimis level.
Clean scrap, such as cell cubicle structures, base plates, and some motors, was sold directly to the metals market. Scrap that was economical to decontaminate to de minimis levels was size-reduced, decontaminated and/or melted to homogenize the contamination, and sold. This class of metal included most of the steel, copper, and aluminum components. Scrap that was uneconomical to recover, consisting primarily of small-bore pipe, instruments, and swarf (metallic particles and abrasive fragments resulting from cutting or grinding), was dispatched to the low-level radioactive waste site at Drigg. Approximately 99 percent of the materials removed from the Capenhurst plant (excluding the barrier material) were recycled to the commercial markets, including bulk concrete as well as metals.
A melter was used to handle metallic components that were otherwise difficult or impossible to decontaminate cost effectively by chemical means. The main purpose of the melter was to homogenize the radioactivity in 4,500 metric tons of steel, nickel, aluminum, and other metals to provide for cost-effective monitoring.20 The melter was also used to reduce waste volume.
Characterization
The initial characterization to identify and quantify residual radioactive contaminants was performed following gaseous decontamination. Nonintrusive gamma-ray spectroscopy and neutron activation were used to characterize 237Np and 235U deposits. Counters and scintillation monitors were used to identify 99Tc and 237Np deposits. The characterization provided data on the magnitude and location of uranic alpha and soft beta 99Tc radionuclides throughout the plant.
General Considerations
Criticality prevention during aqueous decontamination was achieved by three principal methods: removing as much uranic contamination as possible during the ClF3 pretreatment and mechanical decontamination stages, designing the decontamination facility to minimize the likelihood of criticality incidents, and using batch-metering techniques to control the movement of spent citric acid solutions and their concentrations of 235U.
Research and development on cost-effective techniques for D&D formed a significant part of the Capenhurst D&D effort, constituting about 20 percent of the total project cost.21 Given the repetitive nature of GDP process equipment and building structures, the percentage of total D&D project cost spent on research and development should be much smaller for larger plants. The development of metal melting and wet chemistry decontamination processes for transuranic and fission products on metals permitted minimization of waste from the Capenhurst D&D and allowed extensive materials recycling to commercial markets. High land burial costs (greater than $2,000/yd3 [$74/ft3]) were a major driver in developing decontamination and recycling technologies in this case.22
A key principle underlying much of the Capenhurst development work was to look outside the nuclear industry for off-the-shelf equipment that would meet the D&D program needs—possibly with some modification. As noted above, robotic techniques were used during disassembly of the cascades; off-the-shelf systems were used for plasma-arc cutting to minimize costs.
Regarding health and safety, static personnel air samples and film badges were used throughout the project. Whole-body monitoring was performed twice yearly for every D&D worker. No special dispensation or relaxation of exposure limits was given for this work. Very low-levels of exposure were experienced by the work force; the mean total dose for 1993 was 0.03 mSv (3 mrem).
CIP/CUP Technologies
In response to increasing demand for low-enriched uranium for civilian nuclear power plants, the efficiency and capacity of the three U.S. GDPs were increased in the 1970s and early 1980s under the CIP/CUP. CIP increased the separative efficiency of the GDPs by installation of more efficient gaseous diffusion barriers and larger equipment and by improving the flow of the UF6 gas. CUP substantially increased the production capacity of the plants (DOE, 1993c). Some of the technologies used during CIP/CUP activities are relevant to D&D of the U.S. GDPs. However, the requirements for maintenance, upgrading, and improvement intended to enhance equipment use impose different constraints on technology applications than D&D, during which most equipment is cut up and destroyed.
One goal of the CIP/CUP activities was to reuse as much equipment as possible (Snyder, 1994). Well-known wet decontamination methods using citric acid, nitric acid, and ammonium carbonate were used to clean the process equipment for reuse. During aqueous decontamination, a tradeoff was necessary between maximizing uranium removal and recovery and avoiding damage to the nickel plating on equipment destined for reuse. Thus, although CIP/CUP made extensive use of aqueous methods for cleanup, and related data are available, decontamination to free-release standards was not demonstrated.
Scrap metal—notably aluminum and nickel-plated steel—was generated during CIP/CUP activities. The aluminum was melted and either reused or stored. The nickel-plated steel is still stored at the sites. The discarded barriers were melted and stored.
Conclusions And Recommendations
General
Conclusions
- Decontamination and decommissioning of nuclear facilities is not a new activity; it has been demonstrated successfully worldwide for many years. A substantial arsenal of safe, cost-effective technologies has been developed, including those used at the Capenhurst gaseous diffusion plant in the United Kingdom.
- Opportunities exist to optimize and reduce the cost of some decontamination technologies for application to the U.S. GDPs. However, no large research and development effort is needed to identify new decontamination technologies. Large cost reductions by developing breakthrough technologies are not anticipated.
Recommendations
- The committee recommends that a limited number of highly focused technology demonstration programs be funded to evaluate the effectiveness and system costs for specific decontamination processes (see below).
- Given the historical trend of increasing future costs of waste disposal, particular consideration should be given to processes that create a minimum volume of waste for disposal or that decontaminate sufficiently for the resultant material to be recycled or buried as nonradioactive waste.
Uranium Deposit Removal
Conclusions
- The large deposits of uranium can be removed by three methods: gaseous ClF3 treatment, mechanical scraping, or in some cases, the aqueous spray wash approach.
- At Capenhurst, 80 percent of the uranium was removed by hot ClF 3 treatment at the time of shutdown, For CIP/CUP, hot ClF3 treatment followed by spray booth wash was used to remove visible deposits.
- At Paducah and Portsmouth the opportunity will exist to remove major uranium deposits by hot ClF3 treatment at the time of shutdown. At Oak Ridge, cold treatment is being tried.
Recommendation
The deposit removal treatments to be used at each site should be carefully considered to select the most cost-effective processes. At Oak Ridge, mechanical removal or spray booth treatment appear to be the most attractive methods for most of the deposits. At Portsmouth and Paducah, ClF3 treatment at shutdown followed by spray booth treatment for removal of visible uranium deposits could be used, although spray booth treatment alone may suffice. Low-enrichment and high-enrichment cells may receive different treatments.
Decontamination of Cascade Equipment
Conclusions
- There is no obvious impediment to achieving the necessary level of decontamination of the cascade equipment (excluding the diffusion barriers) with an aqueous process. It seems likely that uranium can be decontaminated successfully using the Capenhurst treatment or an analogous liquid process.
- Aqueous washing of enriched uranium (about 2 percent enrichment and above) from metal parts will require double-contingency criticality controls; approaches include restriction
- on amounts of material and solution handled, its geometry (e.g., use of thin layers), and possible use of neutron poisons.
- A special decontamination problem exists for the diffusion barriers. They contain a large amount of valuable nickel, with internal deposits of uranium and 99Tc.
- The criticality problem is greatly reduced for material with low enrichment levels. For this material, the cleanup system can be simplified and its costs reduced.
Recommendations
- A focused demonstration program should be conducted to determine the choice of cleaning solutions and the best design for washing equipment. In choosing a set of wash solutions for aqueous decontamination, particular attention should be given to minimizing the volume of waste liquids generated; the experience at Capenhurst should be taken into account.
- Focused research and development should be conducted to establish the most economic procedure(s) for decontaminating the nickel diffusion barriers to acceptably low-levels.
Decontamination of Support Systems and Buildings
Conclusion
There is no need to pursue extensive research and development programs on new building decontamination technologies because many existing technologies have been demonstrated to work.
Waste Management and Recycling
Conclusions
- Existing technologies are generally adequate for waste management. There is no need for major programs to develop new technology, and large expenditures for research and development are not warranted.
- The decision of whether materials should be decontaminated and recycled, disposed of as nonradioactive waste, or disposed of as low-level radioactive waste will be based upon social values, relative costs, and applicable standards and laws.
Recommendations
- Radioactive wastes should be partitioned into forms for permanent disposal and forms that can be released or considered to be nonradioactive wastes.
- Priority should be given to thorough cleanup of surfaces to meet existing release standards.
- Serious consideration should be given to the recycling of metals, either for sale in commercial markets or for restricted use within the DOE complex.
Characterization
Conclusions
- Characterization of radioactive and hazardous substances is needed before, during, and after the D&D program: initially, to delineate areas for cleanup; during cleanup, to protect workers, control the spread of pollutants, and monitor progress; and finally, to ensure compliance with limits. Various characterization techniques and instruments will be needed due to the complexity of the sites.
- Instruments for monitoring uranium levels at gaseous diffusion facilities have been used and improved over 30 years for routine operations, repairs, and plant upgrading. Existing techniques can be directly applied to characterization for D&D if the radiological limits for cleanup are not much lower than past detection requirements. Laboratory techniques exist for more sensitive uranium measurement but are considerably more expensive to apply than field monitoring. In most cases, characterization of 99Tc at the GDPs will require laboratory (radiochemical) measurements.
- The cost of characterization, particularly for equipment and building surfaces, could be reduced by replacing manual surveys by extensive use of robotics.
Recommendation
The mobile robotic floor characterization system under development by Oak Ridge National Laboratory should be evaluated at the Oak Ridge GDP.
Robotics
Conclusions
- The repetitive layouts of GDP equipment and large, easily accessible floor surface areas offer many opportunities for improved D&D operations using robotics.
- D&D tasks conducted manually are labor intensive, inefficient, and time consuming and could benefit from the use of robotics. Further, D&D operations are conducted in environments that, under current and anticipated future regulations, often require extensive safety and health protection programs for implementation. The use of robotics could eliminate some safety risks and costs of corresponding worker protection measures.
- The DOE EM-50 robotics program has applications-driven developments under way to address primary D&D problems. These demonstrations are necessary for systems analysis, evaluation, and training of operators.
Recommendations
- The use of robotics should be considered as a possible way to reduce costs, improve safety, and enhance data quality in D&D. Emphasis should be placed on the use of commercial robotic systems to minimize development costs.
- Funding should be provided in a timely fashion to support the EM-50 demonstrations of robotic D&D equipment.
References
Anderson, R. W. 1994. Decontamination and Decommissioning by Wet Chemical Methods. Presented to Technology Panel of Committee on D&D of Uranium Enrichment Facilities, National Academy of Sciences, Washington, D.C., June 16, 1994.
Anderson, R.W., and R.L. Faulkner. 1989. British Nuclear Fuels plc Process for Decontamination and Decommissioning of Capenhurst Gaseous Diffusion Plant and Information from the International Conference on Decommissioning of Major Radioactive Facilities. K/QT-254. Washington, D.C.: Oak Ridge Gaseous Diffusion Plant for U.S. Department of Energy (DOE).
Baxter, S.G., and P. Bradbury. 1991. Decommissioning of the Gaseous Diffusion Plant at BNFL Capenhurst. Fairfax, Virginia: British Nuclear Fuels plc.
British Nuclear Fuels plc. 1990. Capenhurst Diffusion Plant Decommissioning. Fairfax, Virginia: British Nuclear Fuels plc, Enrichment Division.
Bundy, R.D. 1994. Overview of the Oak Ridge GDP Technology Logic Diagram . Presented to the Technology Panel of the Committee on Decontamination and Decommissioning of the Uranium Enrichment Facilities, the Beckman Center of the National Academy of Sciences, Irvine, California, May 9, 1994.
Bundy, R.D., E.B. Munday, D.W. Simmons, and D.W. Neiswander. 1994. Gas phase decontamination of GDP process equipment. Presented at International Symposium on D&D, April 25–29, 1994, Knoxville, Tennessee. Oak Ridge, Tennessee: U.S. Department of Energy.
Bundy, R.D., C.E. Benson, J.R. Devore, H.H. Haselton, E.B. Munday, and J.H. Wilson. 1993. Decontamination of facilities at K-25 and ORNL [Oak Ridge National Laboratory] sites: An assessment of needs, technologies, and development needs. Presented at the I&EC [Industrial and Engineering Chemistry] Special Symposium, American Chemical Society, held September 27–29, 1993 in Atlanta, Georgia.
Bundy, R.D., and E.B. Munday. 1991. Investigation of gas-phase decontamination of internally radioactive contaminated gaseous diffusion process equipment and piping. Presented at the 84th Annual Meeting and Exhibition of the Air and Waste Management Association, held June 16–21, 1991, in Vancouver, British Columbia.
Carder, W. 1994. commercial Recycling. Presented to Technology Panel of Committee on Decontamination and Decommissioning of the Uranium Enrichment Facilities, Radisson Hotel, Columbus, Ohio, August 23, 1994.
Cavendish, J.H. 1978. Treatment of metallic wastes by smelting. IAEA–SM–234/14. Pp. 611 in Proceeding of International Symposium on the Decommissioning of Nuclear Facilities,
held November 13–17, 1978 in Vienna, Austria. Vienna Austria: International Atomic Energy Agency.
Clements, D.W. 1992. Decommissioning Britain's gaseous diffusion plant—with special reference to volume reduction techniques . Pp. 179–188 in Nuclear DECOM 92: Decommissioning of Radioactive Facilities International Conference. London, United Kingdom: Mechanical Engineering Publications Ltd.
Clements, D., and J.R. Cross. 1994. The D&D of the Capenhurst Facility. Presented to the Committee on Decontamination and Decommissioning of the Uranium Enrichment Facilities, National Academy of Sciences, Washington, D.C., March 28, 1994.
Cohen and Associates. 1994. Analysis of the Potential Recycling of Department of Energy Radioactive Scrap Metal. McLean, Virginia: S. Cohen and Associates, Inc. for U.S. Environmental Protection Agency.
Crawford, J. 1994. Highly Enriched Uranium Suspension. Presented to the Committee on Decontamination and Decommissioning of the Uranium Enrichment Facilities, Portsmouth Uranium Enrichment Facility, Piketon, Ohio, August 22, 1994.
Cross, J. 1995. The decommissioning of the Capenhurst gaseous diffusion plant. Nuclear News, 38(2):42–44.
DOE. 1991. Environmental Restoration of the Gaseous Diffusion Plants. Technical Summary Document, vol. 1. Washington, D.C.: Ebasco Environmental and Main-1893 for the DOE. October.
DOE. 1993a. Decontamination and Decommissioning Technology Assessment. DOE/OR-1051. Washington, D.C.: DOE.
DOE. 1993b. Oak Ridge K-25 Site Technology Logic Diagram, vol. 1, Technology Evaluation. K-2073. Oak Ridge, Tennessee: Oak Ridge K-25 Site for Martin Marietta Energy Systems For DOE's Office of Technology Development. February 26.
DOE. 1993c. Lease Agreement Between the U.S. Department of Energy and the United States Enrichment Corporation. Washington, D.C.: DOE. July 1.
DOE. 1994a. U.S. Department of Energy Oak Ridge Operations Office Site Integrated Program Plan for the Implementation of Defense Nuclear Facilities Safety Board Recommendation 94–1 for K–25 Site Deposit Removal Project and Oak Ridge National Laboratory Molten Salt Reactor Experiment Remediation Project, Oak Ridge, Tennessee, vol. 1, Remediation Strategy. Part B, HEU (Highly Enriched Uranium) Deposit Removal from K-25 Building. DOE/OR/01–1333&V1, Draft.
DOE. 1994b. Decommissioning Handbook. DOE/EM–0142P. Washington, D.C.: DOE's Office of Environmental Restoration.
DOE. 1995. Decontamination and Decommissioning Focus Area, Technology Summary. DOE/EM-0253. Washington, D.C.: DOE's Office of Environmental Management Technology Development. June.
DOE and IAEA (International Atomic Energy Agency). 1994. Proceedings of the 1994 International Symposium on D&D held April 25–29, 1994 in Knoxville, Tennessee. Oak Ridge, Tennessee: DOE.
Faulkner, R. 1995. Application of Capenhurst Experience to the U.S. Gaseous Diffusion Plants. Presented to Committee on Decontamination and Decommissioning of the Uranium Enrichment Facilities, National Academy of Sciences, Washington, D.C., May 8, 1995.
Lacy, N. 1994. Gaseous Diffusion Plants Decontamination and Decommissioning Assessment Review Papers, Predecisional Draft. Washington, D.C.: Booz-Allen/Burns & Roe for DOE. January 14.
Meehan, R. 1995. Personal communication (memorandum) from Richard Meehan, DOE, to Judson Lilly, DOE, July 7, 1995, forwarded to James Zucchetto, NRC.
MMES (Martin Marietta Energy Systems). 1992. K-25/K-27 Buildings Historical Characterization. K/D-6052. Oak Ridge, Tennessee: MMES.
MMEs. 1994. Highly Enriched Uranium Program Management Plan. ERWM-HEU-100. Piketon, Ohio: MMES, Inc. for DOE.
Netzer, D. 1994. LT-Squared Project: Long-Term, Low-Temperature Gas Phase Decontamination. Presented to Committee on Decontamination and Decommissioning of Uranium Enrichment Facilities, Portsmouth Enrichment Facility, Piketon, Ohio, August 22, 1994.
Richardson, B. 1994. Mobile Automated Characterization System—MACS. Presented to Technology Panel of Committee on Decontamination and Decommissioning of Uranium Enrichment Facilities, National Academy of Sciences, Washington, D.C., June 15, 1994.
Ritter, R.L., and E.J. Barber. 1991. Aspects of uranium chemistry pertaining to UF6 cylinder handling. Pp. 3–7 in Proceedings, 2nd International Conference on Uranium Hexafluoride Handling, held October 29–31, 1990 in Oak Ridge, Tennessee.
The Robotics Institute. 1995. BOA: Asbestos Pipe-Insulation Removal Robot System. Topical Report, Phase 1. Pittsburgh, Pennsylvania: The Robotics Institute, Carnegie Mellon University for DOE.
Snyder, R. 1994. Portsmouth Cascade Improvement Program and Cascade Upgrading Program. Presented to Committee on Decontamination and Decommissioning of Uranium Enrichment Facilities, Portsmouth Uranium Enrichment Facility, Piketon, Ohio, August 22, 1994.
Spencer, A.N. 1988. Decommissioning and Decontamination of Enrichment Facilities. Presented at the 15th Annual Meeting of the World Nuclear Fuel Market, held October 16–19, 1988 in Seville, Spain.
Thompson, B., and T. Dockstader. 1994. Rosie: A Mobile Workstation for Decontamination and Dismantlement Operations: Design Description. 9316-REPT-003.2. Pittsburgh, Pennsylvania: Redzone Robotics, Inc.
Worcester, S.A., L.G. Twidwell, D.J. Paolini, and T.A. Weldar. 1993. Decontamination of Metals by Melt Refining/Slugging, An Annotated Bibliography. WINCO-1138. Idaho Falls, Idaho: Idaho National Engineering Laboratory. July.
| This page in the original is blank. |






























