3
Cellular and Molecular Effects
In this chapter, the published literature on the exposure of cultured cell systems to power-frequency electric and magnetic fields is discussed. The committee's conclusions, based on a review of the data, are presented first, followed by a discussion of the utility and limitations of in vitro studies and an examination of three categories of in vitro effects. Those effects are genotoxicity and carcinogenicity, changes in intracellular calcium concentrations, and changes in gene expression with emphasis on signal-transduction pathways.
SUMMARY AND CONCLUSIONS
From its review of the data on cellular and molecular effects from exposure to power-frequency electric and magnetic fields, the committee concludes the following:
-
Magnetic-field exposures of 50-60 Hz delivered at field strengths similar to those measured for residential exposure (0.01 to 1.0 µT) do not any significant in vitro effects that have been replicated in independent studies.
Although a few studies have reported positive effects at those field strengths, most of the studies reported negative results. Those few studies that had positive results provided no evidence that superior methods or cell systems were used that would give them precedence over the greater number that had negative results. All the studies, as with any others using cultured cell systems, are subject to experimental artifacts that can affect the results. However, the number and quality of studies with negative results are impressive, and in the absence of
-
compelling findings in animals or human beings, the committee finds no justification for any conclusion other than that magnetic fields with field strengths from 0.01 to 1.0 µT have no significant effects in cultured cell systems.
-
Magnetic-field exposures of approximately 100 µT (1 G) have been observed in independently replicated studies to produce effects on ornithine decarboxylase (ODC) activity, one of the membrane-mediated signal-transduction pathways, and numerous peer-reviewed reports have been done on the effects of magnetic fields on other components of the signal-transduction pathways. However, a mechanism through which these magnetic fields produce such biologic effects is unknown.
Positive results in the field-strength range of 50 to 500 µT (0.5 to 5.0 G) have been observed and reproduced for only the signal-transduction effect on ODC activity. For other effects—genotoxicity, intracellular calcium concentrations, and general patterns of gene expression—no convincing and reproducible results have been observed.
-
Magnetic-field strengths greater than 500 µT (5 G) have induced changes in intracellular calcium concentrations and general patterns of gene expression as well as in several components of signal transduction. However, no reproducible genotoxicity is observed at any field strength.
Again, effects of the sort seen here are typical of many experimental manipulations and do not, per se, indicate a health hazard. For the positive results that have been observed, as in the bone-healing studies, the effects cannot be extrapolated to lower field strengths; therefore, it is not known whether the effects observed at higher field strengths are induced by mechanisms distinctly different from those that might cause effects at residential and occupational field strengths.
The committee's overall conclusion based on analysis of in vitro experimentation is that magnetic-field exposures at 50-60 Hz have been shown to induce changes in cultured cells only at field strengths that exceed residential exposure levels by factors of 1,000 to 100,000.
UTILITY AND LIMITATIONS OF IN VITRO STUDIES
In vitro studies are useful for documenting responses of selected cell systems to chemical and physical agents. Interpreting probable responses of cells in culture in terms of potential or putative target-cell response in the body is problematic, however, and requires similar exposures and appropriate surrogates for target-cell populations in vivo. For example, a number of extracellular signals induce a common set of early-response genes. The early response is not, however, sufficient to determine the biologic outcome. The initial response of cells to the phorbol ester 12-O-tetradecanoylphorbol 13-acetate (TPA) is activation of protein kinase C (PKC) and induction of the early-response genes fos, myc, and jun. Phorbol esters were originally identified as promoters of papilloma development
in mice. However, TPA has also been reported to inhibit the growth and suppress the tumorigenicity of human HT29 colon cancer cells that overexpress the ß-1 isoform of PKC. Thus, phorbol ester can either stimulate or inhibit cell proliferation, depending on cell type and signal-transduction pathway (e.g., see Huang et al. 1995).
The choice of an appropriate surrogate is an absolutely crucial consideration to extrapolate from in vitro to in vivo response. If the goal in using cultured cells is to document cellular response to a general phenomenon (e.g., induction of DNA damage), then almost any cell system can be considered appropriate, provided the agent under consideration reaches the DNA in its active form. As the induced phenomenon becomes more specific, it will be observable in fewer cell systems. For instance, in vitro transformation systems used to measure induced transition from a nontumorigenic phenotype to a malignancy are specialized systems, because the genotype and phenotype of these cells can only represent limited phases in multiphase carcinogenic processes, which vary between different human somatic cells. When experiments are initiated to determine whether such agents as power-frequency electric or magnetic fields produce patterns of response in multiple-cell systems that are similar to those produced by carcinogens, neurotoxins, or developmental toxins, the results can be regarded with confidence when those systems exhibit responses similar to those produced by documented toxins and when the responses are consistent with the mechanism that is hypothesized to underlie the hazard.
GENOTOXICITY AND CARCINOGENIC POTENTIAL OF POWER-FREQUENCY ELECTRIC AND MAGNETIC FIELDS
Substantial numbers of experiments have been performed in which cultured mammalian cells or prokaryotic cells were placed in an electric or magnetic field, a putative exposure delivered, and the cell response examined for a variety of end points. Scientists conducting those experiments interpreted their results as both negative and positive mimicries of results from known carcinogens tested in the same biologic systems. The goal of this discussion is to compare the results and determine with relative confidence whether the data, taken as a whole, present a compelling argument for or against the probability that power-frequency electric and magnetic fields are a possible carcinogen. To accomplish that goal, data obtained from exposures to electric or magnetic fields must be compared with those from exposure to known carcinogens. Such a comparison must be done in the framework of a detailed knowledge of the carcinogenic process.
Using the analytic power of molecular biologic techniques, human cancer cells can be described in great detail at the molecular level. The essential biology of cancer cells is now most often described as an accumulation of multiple genetic changes that in combination lead to loss of proliferative control, loss of responsivity to differentiation signals, and loss of controlled interaction with
other tissues. The in vivo processes leading to the cancer state are less well understood. Those processes must generally be inferred from changes observed in the characteristics of cells at different stages of carcinogenesis, from the molecular action of agents that induce transition from one stage of carcinogenesis to the next, and from characteristics of cells in humans who are genetically susceptible to cancer. In vitro systems have been used effectively to describe the molecular sequelae of different agents demonstrated to induce cancer in animals and humans. In special cultured cell systems, some aspects of carcinogenesis can be mimicked, and assays for potency of in vitro transformation can be measured quantitatively. However, it must be realized that in vitro assay systems are artificial representations of target cells in vivo, and confidence in extrapolating from in vitro results to determine the relative potency for inducing cancer in vivo is limited. Using a battery of tests produces a profile of results for particular agents, and that profile can be considered a descriptor of carcinogens or types of carcinogens. Descriptors have been most widely used and accepted as indicators of carcinogenic potency for agents that are directly genotoxic. Directly genotoxic agents are generally considered to be those that can interact directly with DNA, inducing chemically stable changes in that molecule. Those changes must be repaired or restituted in some way to prevent heritable changes in DNA sequence or function; some such changes are observed in the cancer cell. Thus, biologic systems that manifest changes in DNA sequence, such as mutations or chromosomal aberrations, are useful in identifying potentially genotoxic agents, most being potential carcinogens if acting on susceptible target cells in vivo.
EXPERIMENTAL STUDIES OF IN VITRO EFFECTS
Data on the biologic effects observed in cultured mammalian cells or selected prokaryotic cells exposed to electric and magnetic fields are divided in this section into two categories: heritable changes and transient changes. Those two categories are relevant for the three categories of hazards under evaluation: carcinogenic, neurobehavioral, and reproductive effects. Of these three, the cancer phenotype is not only most clearly associated with heritable changes in the genotype of the target cells but is also clonal in origin. Neurobehavioral effects, except those induced during ontogeny, represent multicell alterations in the nervous system. Although heritable susceptibility to neurologic disease has been documented, environmental agents that induce such disease are invariably active through alteration in neurometabolism or through direct toxic effects on cell populations of the central or peripheral nervous systems. Developmental toxins need to act only for a short period during gestation, and therefore developmental effects are the most susceptible to transient changes from exposure to electric or magnetic fields. Cultured cells are limited as direct surrogate systems for the three effects of concern, but can act as a surrogate of general effects, such as mutation, growth retardation, or some changes in gene expression. If the biologic changes induced
by electric and magnetic fields are highly selective and specific, cultured cells are more limited as descriptors of induced effects.
Heritable Changes in Cells Exposed In Vitro to Electric and Magnetic Fields
Cultured cells have been widely used to detect genotoxicity of different environmental agents. Generally, end points can be divided into two categories: those that measure the induction of heritable genetic changes directly, such as mutation and certain heritable chromosomal aberrations, and those that are accepted as indicative of heritable changes, such as induction of DNA damage, DNA repair, nonheritable chromosomal aberrations, and sister chromatid exchanges (SCE).
Genotoxic effects of electric and magnetic fields have been reviewed recently by McCann et al. (1993) and by Murphy et al. (1993). According to McCann et al., there is no convincing evidence that power-frequency fields induce direct genotoxic effects and that positive effects observed for high static electric fields might result from corona, arc, or spark. They express concern that some unconfirmed results suggest that magnetic fields can act to enhance the effects of other carcinogens. According to Murphy et al., the preponderance of the data suggest that no genotoxic effects are induced by power-frequency electric or magnetic fields, but the presence of some positive data and the lack of breadth of the studies suggest the need for further study.
Table A3-1, provided in Appendix A, contains a list of published studies in which measurements were made of genotoxic responses of cultured cell systems exposed to electric and magnetic fields at 50-60 Hz. A number of additional references are included in the table of studies in which cells were exposed to static fields, high-frequency electromagnetic fields, or fields applied intermittently as modulated or unmodulated pulses. Many of those studies were discussed and analyzed by McCann and co-workers (1993) in their review of genotoxic studies of these fields. The studies also illustrate the type of data that supplements the literature strictly limited to electric-and magnetic-field exposures at 50-60 Hz. Table A3-1 lists 29 articles that report effects of exposure to electric and magnetic fields at frequencies of 2-100 Hz, with most using 50-60 Hz. In 24 of the studies, cells were exposed to fields at 50-60 Hz that are sinusoidal or approximately such. Only two of the reports, by the same laboratory (d'Ambrosia et al. 1985, 1988-1989), report positive induction of chromosomal aberrations in bovine lymphocytes; the other 22 studies, including those that are similar to the d'Ambrosia studies, are negative. From those studies, the committee concludes that genotoxic effects were not reproducible for exposures to electric and magnetic fields at 50-60 Hz delivered in a form that approximates sinusoidal exposure. Significant genotoxic effects have been reported for exposure conditions in which magnetic fields of greater than 30 µT are delivered by intermittent exposure
(Nordenson et al. 1994) or in which fields of 1 mT are delivered by pulsed exposure (Khalil and Qassem 1991). Those exposure levels are orders of magnitude larger than those encountered in everyday life. Rosenthal and Obe (1989) reported that magnetic fields induced excess SCE in human lymphocytes exposed to 50-Hz sinusoidal magnetic fields and N-nitroso-N-methylurea (NMU) or trenimon.1 However, the authors question whether their results reflect a true genotoxic effect. Other authors reported no increase in genotoxic effects when cells were exposed to magnetic fields at 1 mT and ionizing radiation at 5 grays (Gy) (Frazier et al. 1990) or to electric fields and ultraviolet light at 254 nanometer (nm) (Whitson et al. 1986). Rosenthal and Obe (1989) also produced negative results when they used diethylbenzene (DEB) rather than NMU or trenimon.
Balcer-Kubiczek and Harrison (1991) reported an interaction in producing transformed foci in C3H/10T1/2 cells between pulse-modulated microwaves of 2.45 GHz and TPA or with this combination following or preceding X-rays (0.5, 1.0, or 1.5 Gy). No effect was induced by electromagnetic fields alone. Although these data are difficult to interpret in terms of effects from exposure to fields at 50-60 Hz, they and data showing copromotion between pulse-modulated high-frequency electromagnetic fields and tumor promoters in some animal systems, as will be discussed subsequently, might warrant further study.
Although some examples of positive effects exist, the great majority of effects are negative, and for those published studies that describe positive effects, similar studies describe negative effects. There seems no imperative that would give precedence to any set of positive data. Further, a major characteristic of genotoxic agents is their induction of positive effects across a range of cell types, at least when the agent reaches DNA in its active form. Thus, the data must be interpreted as strong evidence that electric and magnetic fields are not genotoxic as defined experimentally. The positive result reported when power-frequency electric or magnetic fields were combined with certain genotoxic and nongenotoxic carcinogens is an extremely interesting observation, but one that is also extremely difficult to interpret in terms of its implications, if any, for potential carcinogenesis in human populations. Cultured cells stressed with nonphysiologic concentrations of agents might not be good models for human somatic cells in populations exposed to electric and magnetic fields.
The committee concludes from these studies that positive data either are (1) specific to particular laboratories (i.e., other laboratories using similar systems and similar exposure conditions have negative, rather than positive, results) or are (2) specific to particular biologic systems (i.e., positive results are peculiar to biologic factors in the systems used in particular laboratories). Those factors that determine the differences observed are extremely difficult to identify in retrospect.
The general conclusion from these studies is that power-frequency electric and magnetic fields are not directly a genotoxic agent; if they were, a wider
range of positive responses would have been observed. No consistent pattern is found across biologic systems or exposure conditions.
Transient Changes in Cells Exposed In Vitro to Electric and Magnetic Fields
The data showing that magnetic fields can induce transient changes in cell expression are significant. Those data fit into three categories: changes in the signal-transduction pathway including changes in concentrations of ODC, changes in gene expression, and changes in intracellular calcium levels.
Signal Transduction
Signal refers to molecular systems, both at the cell membrane and inside the cell, in which signals from the environment and from other cells are received, and which regulate intracellular processes, such as metabolic activities, gene expression, differentiation, and cell proliferation in response to the signals received. Signal-transduction processes present an interesting possible mechanism for electric and magnetic fields to influence cell function. In particular, membrane signal-transduction processes have been an area of intense focus. One reason for the interest is that the cell membrane presents a substantial barrier to electric fields, especially in the range of field strengths and frequencies present in the ambient environment. Attenuation of electric fields by the plasma membrane of mammalian cells has been estimated at 103-105 between the external plasma membrane surface and the interior of the cell (Polk 1992b). For all intents and purposes, no significant penetration of information-containing electric signal across the cell membrane can be postulated for the 60-Hz ambient fields encountered in ordinary household exposures (Polk 1992a,b). Because membrane-mediated signal transduction by hormones and other signaling agents involves the transmission of signals across the plasma membrane without requiring that the signal itself penetrate the membrane, low-frequency electric or magnetic fields have been postulated to act on intracellular processes by influencing only the initial extracellular steps of signal transduction (Adey 1992a). A number of studies have been interpreted by the investigators to indicate that weak electric or magnetic fields can produce changes in membrane signal-transduction pathways. Numerous reviews of low-frequency, low-energy electric-and magnetic-field interactions with biologic systems, including cells, animals, and humans, have been conducted (Adey 1992a,b; Cardossi et al. 1992; Cleary 1993; Liburdy 1992a; Luben 1991, 1993; Tenforde 1991, 1992; Walleczek 1992).
Although many types of signals can be found in biologic systems, the mechanisms for transmitting the information contained in those signals across the cell membrane are relatively few. In all known signal-transduction systems, a signal interacts with a cellular protein (a receptor or voltage-sensitive ion channel) and
triggers conformational changes in the protein that result in other signals or modifications of cellular metabolism. Signaling agents with limited ability to cross the cell membrane (e.g., peptide hormones, neurotransmitters, and growth factors) interact with receptor proteins that span the cell membrane. These ligand-activated receptors have an extracellular domain that is exposed to the medium surrounding the cell, and signaling agents interact with this extracellular domain. Interaction of the signal with the extracellular portion of the receptor produces conformational changes which are propagated across the membrane to the intracellular portions of the receptor molecule. Interaction of the intracellular portion of the receptor with other intracellular (effector) molecules causes changes in the activities of cellular pathways. In addition to receptors for soluble molecules (ligands), such as neurotransmitters, transduction mechanisms also exist for non-chemical signals, such as mechanical deformation, temperature, and light.
There are three well-understood methods by which signals associated with a membrane protein conformational changes are propagated across the cell membrane: (1) opening and closing of ion channels and resultant current flow; (2) changes in an intrinsic enzymatic activity of the receptor; and (3) changes in affinities of the receptor for intracellular proteins, which might have enzyme activity or be enzyme regulators. Because membrane proteins can be difficult to study, additional mechanisms undoubtedly remain to be discovered. In contrast to the relatively few signal-transduction mechanisms known, the variety of biologic responses in the cells being regulated is almost infinite. In nearly all cases, the mechanism of signal transduction distal to the receptor involves intracellular pathways being influenced either by changes in ionic composition of the cytosol (e.g., changes in intracellular ion concentrations, such as calcium or sodium) or by changes in phosphorylation of intracellular proteins (e.g., changes in enzymes, enzyme regulators, or transcriptional regulatory factors). The cellular responses to signals can be either short-term, with little or no persistence of the effect after removal of the signal, or long-term, involving persistent changes in the function of cells, such as increased or decreased proliferation, changes in gene expression or differentiation, and in some cases, apoptosis (programmed cell death). Short-term changes are generally mediated by modification of cytosolic or membrane enzyme activities of the cell; long-term changes invariably involve alteration of nuclear functions, such as transcription, cell division, cell-cycle regulation, or cytosolic and membrane effects.
A distinction should be made between the direct (biophysical) interactions of electric and magnetic fields with atoms or molecules in cells and the indirect (more general biochemical) effects, which are produced as a result of the direct interactions. All the data reported in this section deal with biologic or biochemical measurements of indirect effects on cells or tissues, although in many cases the stated purpose of the experiments was to infer the nature of postulated direct mechanisms. Several potential direct interaction mechanisms are described elsewhere in this report and will not be analyzed in detail here.
In general terms, two possible scenarios have been addressed experimentally: (1) the interaction of electric fields (either ambient or induced in the medium adjacent to the cell by oscillating magnetic fields) with ions or charged molecules at the surface of the membrane; and (2) the interaction of magnetic fields with atoms, ions, or molecules in the membrane or within the cytosol or nucleus of the cell. Either of these possible interaction mechanisms is postulated to modulate a step (or steps) in some signal-transduction event, leading to further changes in the function of the cell. The focus of some experiments was on the observable effects of exposure to electric and magnetic fields alone (i.e., attempt to show the existence of receptors for such fields). The focus of other work was on possible interactions between electric or magnetic fields and the existing signal-transduction systems for other ambient signals, such as hormones or neurotransmitters (i.e., to look for electric-or magnetic-field effects on receptors for other agents).
A number of laboratories have examined the effects of electric and magnetic fields on bone and connective tissue cells, including studies of signal transduction as well as other regulatory and differentiation processes. Those studies are summarized in Chapter 4 of this report in the section on bone healing and will not be repeated here. It is important to emphasize, however, that most of the studies on bone and connective tissue have used field strengths much higher than those encountered in either residential exposures or most occupational exposures. The lowest magnetic-field strength that has been shown to have reproducible effects on connective-tissue cells is approximately 100 µT (1 G), and most of the studies relating to clinical effectiveness of magnetic fields have been at strengths of 500-2,000 µT (5-20 G) (Brighton and McCluskey 1986).
Other examples have been presented of interactions between magnetic fields and already-recognized signal-transduction pathways. For example, Walleczek and Liburdy (1990) observed that 60-Hz magnetic fields caused increased 45Ca influx during concanavalin-A (Con-A)-induced signal transduction in lymphocytes. A 60-min exposure of rat thymic lymphocytes to a 22-mT (220-G) magnetic field (induced electric field = 1.0 mV/cm) at 37°C was performed in the presence or absence of Con-A. Nonactivated cells (no mitogen) were unresponsive to the magnetic field; 45Ca influx was not altered. When Con-A was present, the magnetic field led to an increase in 45Ca influx of 50-200%. In these studies, as in those of Luben's group on bone cells (Luben et al. 1982; Luben 1991, 1994), the effects of magnetic-field exposure were prevalent mainly at low concentrations of the signaling molecule, suggesting that power-frequency magnetic-field exposures could cause changes in the affinity of the receptor for the ligand or in the effectiveness of the transduction process at low field strengths, but could not produce a change in the maximal responsiveness of the cells to the signal. Liburdy et al. (1993a) also recently reported that cell-surface antibody binding to human lymphocytes could be altered by a 60-Hz 200-G magnetic field. T lymphocytes were reported to exhibit an approximate doubling of anti-CD3 antibody released
from cells compared with that released from the sham-treated cells (Liburdy 1992b). Again, the changes induced by power-frequency magnetic fields were most significant at low concentrations of antibody—a result consistent with a change in receptor affinity but not in the total number of receptors expressed on the cells. Those results and the anti-CD3 antibody results mentioned above suggest that the binding of ligands to their receptors would be fruitful to investigate in other cell systems. It should also be pointed out that none of the studies described have been independently replicated, and this type of replication is a pressing research need.
Blank's group has reported effects of very-low-frequency electric fields on the Na, K-ATPase ion pump in membranes (Blank 1992; Blank and Soo 1992). Electric fields at 30-300 Hz were applied for 15 min to membrane preparations at current densities of 0.05-50 mA/cm2 (0.001-1 mV/cm); the response was complex, with either increases or decreases in enzyme activity, depending on the level of Na and K ions in the medium. Field inhibition of ATPase activity occurred when the enzyme was in a medium containing optimal concentrations of activating cations, and field stimulation occurred when the enzyme activity was reduced by using ouabain or by lowering temperature. Blank estimated the threshold for effects at electric-field strengths of approximately 5 µV/cm (5 × 10-4 V/m) across the membrane, and this threshold was associated with a current density of 8 mA/cm2. This threshold value, although low by comparison with ambient electric fields in air near power lines, is much higher than those believed to be induced by environmental exposures to electric fields. The results can be interpreted in terms of the electric field inducing changes in the binding of substrate ions (Na+ and K+) to the ion pump at high and low concentrations of the ligands, similarly to the studies of Liburdy and Luben described above. Blank's work has not been replicated by other investigators, but neither have failures to replicate been reported.
ODC activity is modulated by membrane-mediated signaling events, and its activation is associated with the activity of mitogens and tumor-promoting agents of various types during carcinogenesis. Byus et al. (1987) reported that three cell lines—human lymphoma cells (CEM), mouse myeloma cells (P3), and rat hepatoma (Reuber H35) cells—exhibited increases of 50-300% in ODC activity when exposed to sinusoidal 60-Hz electric fields at 10 mV/cm. Increases in ODC were detected as low as 0.1 mV/cm in Reuber H35 cells. For comparison, phorbol ester at doses associated with tumor promotion produced activation of ODC levels by more than 1000%. The investigators interpreted these results as indicative of an electric-field effect on the cell membrane, resulting in a signal-transduction effect on ODC activation by mechanisms not directly investigated in these or subsequent studies. These findings have been used as basis for a hypothesis that electric fields might act as a copromoter with tumor-promoting agents, producing more activation of ODC and more growth promotion of carcinogen-induced cells in the presence of low electric-field strengths than in the absence of electric
fields. Several in vivo studies have been initiated to test this hypothesis directly; they are discussed in Chapter 4 of this report. Litovitz et al. (1991) also reported enhancement of ODC activity in mouse L929 cells by 60-Hz magnetic-field exposure for 8 hr at strengths of 1, 10, or 100 µT. Maximal enhancement of approximately 100% above controls was produced by 4 hr of exposure to a magnetic field at 10 µT. The studies with ODC have the distinction among signal-transduction investigations of having been independently replicated by at least two laboratories in addition to the original findings of Byus et al. (1987), although the conditions and signaling agents were slightly different in the various studies. Little effort was made in these studies to isolate the specific changes in membrane receptor mechanisms that were bringing about the observed changes in ODC activity.
The enzyme PKC is believed to be the receptor for tumor-promoting phorbol esters (Kikkawa et al. 1989), and several current research projects are investigating the possible effects of magnetic fields on PKC. Luben's group reported that exposure to 60-Hz magnetic fields at 100 µT (1 G) produced transient activation, followed by down-regulation, of PKC activity in mouse bone cells (presented as a non-peer-reviewed preliminary report by Luben 1994). Those data suggest that modification of the cellular response to tumor promoters might be an effect of the magnetic field at these relatively high field strengths. Uckun et al. (1995) also reported that PKC activity increased in human pre-B leukemia cells exposed to 60-Hz 100-µT magnetic fields; they showed that the activation of PKC was dependent on the activation of lyn kinase, a src family tyrosine protein kinase, which is known to be involved in the proliferation of leukemia cell clones. Like most other work on the cellular effects of low-frequency electric and magnetic fields, these studies have not been replicated by independent laboratories, and the positive findings are at field levels well above the constant background fields in households. However, these lines of evidence if confirmed could provide possible mechanistic links between magnetic-field exposures at 100 µT and changes in pathways known to regulate cell proliferation, differentiation, and tumorigenesis. For example, one possible mechanism to explain the apparent copromotion of tumor growth by magnetic fields and TPA in vivo (see Chapter 4) would be alteration by magnetic-field exposure of the sensitivity of PKC-dependent pathways to TPA treatment.
A potential correlation between cancer-cell growth and magnetic-field exposure was described by Liburdy et al. (1993b). In these studies, human estrogen-responsive breast cancer cells (MCF-7 cell line) were used. These cells grow rapidly in the presence of normal concentrations of female sex hormones, but their growth rate is decreased by a hormone produced by the pineal gland, melatonin. Other studies have reported that melatonin synthesis is altered by exposure of whole animals to extremely-low-frequency (ELF) EMF (Wilson et al. 1990a), and it has been proposed that disruptions of the normal daily cycles of melatonin synthesis are a risk factor for human breast cancer (Stevens 1987a,b).
Liburdy et al. (1993b) confirmed that melatonin at normal physiologic concentrations could decrease the growth rate of MCF-7 cells. However, application of a 1.2-µT (12-mG) sinusoidal magnetic field at 60 Hz prevented the oncostatic action of melatonin on the breast cancer cells. A lower field of 0.2 µT (2 mG) did not have any significant effect, suggesting that a threshold might exist between 0.2 and 2 µT. If these studies were replicated by other laboratories, they would be an exception to the observation that magnetic-field strengths near those encountered in households do not produce significant effects on cells in tissue culture.
In summary, the body of work on signal transduction suggests that power-frequency electric and magnetic fields, with magnetic fields at 100 µT (1 G) and above or electric fields at 10 k V/m and above, are likely to have some effect on a number of signal-transduction-related pathways in mammalian cells. These effects have been used clinically and might be of additional use in future treatment of disease. However, the evidence for such effects at field strengths resembling those in households (0.1 µT or 10 V/m) is essentially absent. There has been little demonstration of receptors for electric or magnetic fields in cells outside of the known pathways for signal transduction by signals that normally affect cells. Most of the studies, even those that appear to be carefully done and reliable, have not been independently replicated and thus cannot be considered conclusive. Little of the work can be interpreted in terms of possible direct (biophysical) mechanisms of interaction between cells and electric-and magnetic-field exposure. Research needs in this area include replication, more precise mechanistic studies, and studies designed to show the presence or absence of effects of exposure to environmental levels of magnetic fields.
Gene Expression and Protein Synthesis
Considerable attention has been focused on the possibility that low-strength electric and magnetic fields might produce changes in the transcription of genes, the processing or lifetime of mRNAs, or the synthesis of specific proteins by cells. Although most studies have shown that low-strength, low-frequency fields were unlikely to change the structure or function of DNA, the possibility that transcription-related events are influenced by exposure to electric or magnetic fields was raised by findings reported by Goodman and Shirley-Henderson (1991). Their results showed an increase in transcript activity for selected chromosome loci of salivary-gland cells of Drosophila and Sciara after brief exposures (<60 min) to sinusoidal fields (60-72 Hz, 0.5-1 mT, 0.3-5 × 10-4 V/m). Individual chromosomes in exposed cells were reported to possess loci with 10-1,000-fold increases in transcription over those in control cells. In general, the chromosome loci in exposed cells exhibiting field-induced changes in transcription were those loci that were active in the control cells; a change in amount of activation but not pattern of activation was the main effect postulated for exposure to electric and magnetic fields. These original studies were carried out using bone-healing
appliances that produced complex pulsed magnetic fields at field strengths up to 2 mT, four orders of magnitude above normal environmental exposures. In later studies, the fields used were sinusoidal 60-Hz fields, but the field strengths required for consistent observations remained as high as 100 µT. The original observations in the chromosome studies of Goodman and Shirley-Henderson (1991) have not been replicated, but neither have failures to replicate these studies been reported.
In subsequent studies, increases in mRNA transcripts were induced in the human promyelocytic cell line HL-60 by sinusoidal 60-Hz magnetic fields at 0.57-570 µT, 0.011-11 × 10-4 V/m (Goodman et al., 1992) and by 60-Hz sinusoidal electric fields at 0.3 × 10-4 V/m (Blank et al. 1993). Dot blots of mRNA probed with radiolabeled transcripts for c-myc, $-actin, histone H2B, and several other markers were reported to have significant increases in transcription over those observed in control cells. The c-myc proto-oncogene is a member of the family of immediate, or early-response, genes, which respond to a wide variety of mitogenic or differentiation signals; changes in activity of cmyc are potentially related to the development of tumorigenic characteristics in cells. Thus, the results from these studies were considered to be of great potential interest.
Phillips et al. (1992) also reported that 1-G 60-Hz magnetic-field exposure of a T-lymphoblastoid cell line, CEM-CM3, increased c-myc mRNA. Nuclear run-off assays, rather than the less discriminatory dot-blot technique used by Goodman et al. (1992), were used in the Phillips study to assess alterations in specific gene transcription rates for myc, jun, fos and PKC. In general, the results of Phillips et al. (1992) revealed small (30-80%), transient changes in transcription rates; the direction of the changes (increases or decreases in the activity) was dependent on the time of exposure and cell density. It is difficult to relate these results to the consistent apparent increases in total mRNA abundance reported by Goodman et al. (1992). In subsequent studies, Goodman and co-workers (Lin et al. 1994) analyzed transcription of c-myc by northern blots as well as dot blots and reported not only that the findings based on dot blots were confirmed, but also that selected regions of the upstream promoter region of the c-myc gene were required for the increased transcription induced by electric and magnetic fields, a finding that the investigators interpret as indicating that the activity of some transcriptional regulatory factor, or factors, might be altered by such exposures. This hypothesis would be consistent with a possible signal-transduction effect caused by the magnetic-field exposure (Goodman and Shirley-Henderson 1991, Goodman et al. 1992, Phillips et al. 1992). Consistent with this hypothesis, Liburdy et al. (1993c) correlated alterations in calcium influx and in c-myc mRNA gene induction in experiments that measured both parameters in the same exposed cell population—i.e., thymocytes exposed to a 220-G 60-Hz magnetic field. In the presence of the magnetic field, a 1.5-fold increase in calcium ions was observed for Con-A-treated cells; in the same cell population, the presence of the magnetic field resulted in an approximate 2-fold increase in c-myc mRNA
as measured by quantitative microdensitometry of northern blots. The field strengths used by Liburdy and co-workers were much higher than those used by Goodman and co-workers and were several orders of magnitude above those reported for residential exposures.
Studies by Czerska et al. (1992) also indicated that human lymphocytes (Daudi cell line) showed increased myc expression when exposed to field strengths similar to those used by Goodman and co-workers; in Czerska et al. (1992), the internal control ß-actin was found to be unaltered (although in the Goodman et al. studies, ß-actin appeared to be stimulated at the same field strengths as myc and other markers). None of the experiments reported in this section appears to have been carried out using blinded experimental protocols, and there has been considerable criticism of the lack of precision of some methods and the lack of consistent internal or external controls in many of the gene-expression experiments, particularly those in the Goodman et al. studies.
In response to those criticisms, at least two groups set out to perform rigorous replication studies of the findings reported by Goodman and co-workers. As reported in two current publications (Saffer and Thurston 1995; Lacy-Hulbert et al. 1995), as well as in several abstracts presented at meetings, these groups used the original equipment, exposures, and cellular end points (including experiments done in the laboratories of Goodman and Shirley-Henderson) and a more-sophisticated exposure apparatus, along with more-specific and sensitive detection techniques, to examine the claims of increased gene transcription induced by power-frequency magnetic fields. In Saffer and Thurston's (1995) study, the original exposure conditions and an extended range of exposures were used (5.7-100 µT); a number of refinements were also introduced to eliminate possible evaluator bias (double blinding) and nonuniformity of exposures (double-wrapped, double-blinded exposure coils) and to improve experimental techniques (loading techniques for RNA and internal and external standards) and detection methods (northern blots, differential display, and ribonuclease protection assays). In addition, a number of different strains of HL-60 cells (including the strain provided by Goodman and Shirley-Henderson used for the original findings) as well as Daudi cells were used. The net result of these studies was that no significant effect of magnetic-field exposure could be detected in any of the genes examined under any of the exposure conditions used. The positive control TPA was used to demonstrate that small effects on transcription could be observed under the conditions used, and a number of internal controls were used to verify the minimum levels of changes in expression that could be detected. Changes in the range of 10% could have been detected reliably, but none was found. This finding is in contrast to the increases of 30-280% reported by Goodman and co-workers in their studies. In addition to in-depth studies with c-myc and other genes used by Goodman and co-workers, Saffer and Thurston (1995) carried out differential-display polymerase-chain-reaction (PCR) analysis on HL-60 cells exposed to magnetic fields to determine whether any gene transcripts in the cell could be
shown to be affected by the exposure. The results indicate that (1) neither the exposures reported by Goodman and co-workers nor extended exposures (both in time and field strength) could be verified as producing any transcriptional effect on any of the genes reported to be modulated; and (2) no gene transcript modulation could be produced in HL-60 cells under any of the conditions tested using differential-display PCR. The authors analyzed several possible differences in technique that could have led to erroneous findings in the original studies, including insufficient controls for loading of RNA, lack of internal and external controls, and poor discrimination ability of the assay techniques. The original experiments cannot be replicated precisely because of those imprecisions; therefore, no complete explanation can be given for the complete lack of magnetic-field effects in the more-recent, more-careful experiments in contrast to the original findings.
Lacy-Hulbert et al. (1995) incorporated many of the same refinements of exposure and measurement techniques in their study as in the Saffer and Thurston (1995) study. Also in the Lacy-Hulbert et al. study, a complete lack of demonstrable effects of magnetic-field exposure on transcription of c-myc and ß-actin was found. Extensive attempts were made to force a positive result by improving the reliability of the exposure system, the assay system, and the measurement of mRNA transcripts on northern blots. The failure of these two groups to replicate the reported gene-expression effects despite elaborate precautions (including, at least in the early stages, the cooperation of Goodman and Shirley-Henderson) is difficult to analyze in any way other than to conclude that the original results are in error.
In summary, the ultimate resolution of the controversy involving the magnetic-field-induced gene expression in mammalian systems remains to be determined; however, for the purposes of this report, it can be concluded that no effects of electric-and magnetic-field exposure on gene expression have been convincingly replicated by independent laboratories. It should also be pointed out that even the originally reported results were done mainly under conditions of 2 to 4 orders of magnitude higher field strengths than those encountered in residential households. Evidence for electric-and magnetic-field effects on gene expression at residential field strengths is completely lacking.
Calcium Changes
Calcium is an important and ubiquitous inorganic ion that serves as a messenger in numerous biochemical events (Rasmussen and Barrett 1984). For example, it is involved in muscle contraction, bone formation, cell attachment, hormone release, synaptic transmission, maintaining membrane potentials, function of ion channels, and cellular regulation. It also serves as a second messenger in neural function in which the concentration of calcium inside the cell regulates a series of enzymatic events caused by kinases. Thus, any exogenous agent that affects
the flow of calcium ions either into or out of the cell could potentially have a major impact on biologic function.
Given the importance of calcium in numerous biologic processes, it is understandable that hundreds of measurements have been made and dozens of hypotheses have been set forth involving the interaction between power-frequency electric and magnetic fields and calcium. Results appearing in peer-reviewed journals (studies reviewed by experts before acceptance) from 1990 to October 15, 1994, are summarized in Table A3-2. Earlier work has been reviewed in numerous reports (e.g., Brighton and McCluskey 1986; Nair et al. 1989; EPA 1990; Goodman and Shirley-Henderson 1991; Chernoff et al. 1992; ORAU 1992) and will not be discussed here, with the exception of some of the early research on calcium efflux from chick brains.
Calcium Efflux from Chick Brains A large body of pre-1992 literature on the effects of electric and magnetic fields on calcium efflux from chick brains originally led to the concept of a complex set of ''frequency windows," "power-density windows," "temperature windows," and a dependence on the local geomagnetic field. Although that concept is currently discounted because the effects were largely attributable to temperature or pH fluctuations that occurred during tissue analysis, the work is reviewed here to place into context the concept of windows and the concept that calcium is modulated by low-frequency electric and magnetic fields. The work consists primarily of a set of peer-reviewed papers by Blackman and colleagues (Blackman et al. 1979, 1980a,b, 1982, 1985a,b 1988a, 1989, 1991; Joines and Blackman 1980; Joines et al. 1981).
The experiment, performed on thousands of chick brains, is outlined in Figure 3-1. The experiment was conducted as follows: the forebrains of four chicks (Gallus domesticus) between 1 and 7 days of age were severed along the midline and labeled by immersing them in a test tube containing Ringer's solution to which radioactive 45Ca2+ was added. The brains were incubated in these tubes for 30 min at 37°C. After incubation, the brains were thoroughly rinsed with nonradioactive solution and each half was placed in fresh solution; one half of the brain served as a control for the treated half. The control brains were incubated at 37°C for 20 min; the treated brains were either exposed to EMF of calibrated frequency and intensity, or they were sham exposed. After 20 min, 0.2 milliliter (mL) of the liquid from each control tube was added to scintillation fluid and its radioactivity counted. Typical counting times were 10 min, and typical counts were 1,800 counts per minute. This number for the control was called V c. Samples of liquid from the tubes containing treated brains were counted in an identical fashion; this number was Vt. The results from a number of runs were pooled to produce ratios of Vt:Vc for the sham-exposed and the exposed samples.
Typical data for 16-Hz exposures are shown in Figure 3-2 (Blackman et al. 1982). The statistically significant data, marked with a single asterisk (*) (p < 0.05) and double asterisk (**) (p < 0.01), show peaks at field strengths at
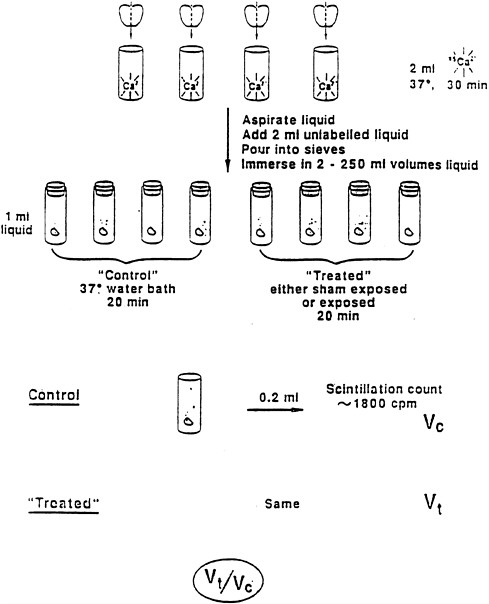
FIGURE 3-1 Experimental protocol for studies of the effects of EMF on calcium efflux in chick brain.
about 6 V/m and at about 45 V/m, respectively. Data, such as those shown in Figure 3-2, were used to compile the map of frequency and power-density windows shown in Figure 3-3 (Blackman et al. 1985a).
If accepted at face value, those and other data in the Blackman papers suggest that more is not necessarily worse when exposure to low-frequency EMF is concerned. The data exhibit sharp resonant-like responses in both frequency and
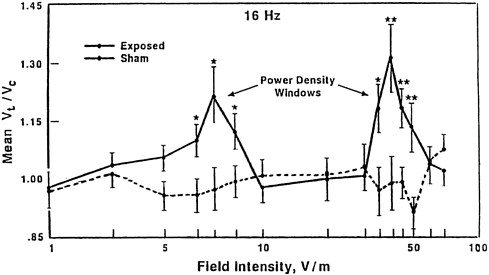
FIGURE 3-2 The ratio of Ca2+ ions in the culture media of chick-brain cultures for electric-field exposures and controls shown as a function of the electric-field strength for exposure at 16 Hz. SOURCE: Blackman et al. 1982. Reprinted with permission; copyright 1982, Radiation Research, Academic Press, New York.
field strength, and the responses are further complicated by the orientation between the oscillating ELF magnetic field and earth's dc magnetic field. It should be noted that although Blackman and co-workers observed an increase in calcium efflux, similar experiments performed earlier by Bawin and Adey (1976) showed a decrease. The disagreement between the results of Blackman et al. and those of Bawin and Adey was originally attributed to the smaller size of the ac magnetic-field component used by Bawin and Adey than that used by Blackman et al. More recently, the existence of temperature windows was proposed as one of the variables necessary to explain the differences in results between the two laboratories (Blackman et al. 1991). Either an increase or a decrease or a null result is possible depending on the temperature of the tissue samples before and during exposure.
The chick-brain preparation used in these experiments was not a particularly robust one. The solution was only weakly buffered and the large brain samples, once excised, were not maintained in a stable living state. Consequently, small variations in temperature (perhaps caused by variations in the temperature of the medium used to wash the tissue (Lee et al. 1987)) or insufficient removal of metabolic wastes by rinsing could produce large swings in pH, affecting the results. Blasiak et al. (1990) were unable to replicate specific Blackman experiments using the Blackman chick-brain preparation, and Albert et al. (1987) was unable to obtain the Blackman results using a more stable preparation with small
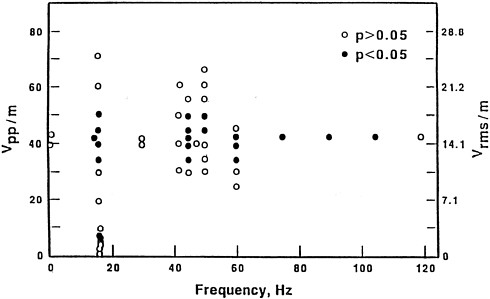
FIGURE 3-3 The ratio of Ca2+ ions in the culture media of chick-brain cultures for electric-field exposures and controls shown as a function of the electric-field strength and frequency. The electric field is given in volts per meter peak-to-peak (Vpp/m) (left) and volts per meter root-mean-square (Vrms/m) (right). SOURCE: Blackman et al. 1985 a. Reprinted with permission; copyright 1985, Bioelectromagnetics, Wiley-Liss, a subsidiary of John Wiley & Sons, New York.
tissue slices, adequate media to support the high metabolic rate of nervous tissue cells, and culture under an atmosphere of 5% carbon dioxide to 95% oxygen.
Newer Methods to Visualize Cytosolic Calcium Changes in Individual Cells During the past decade, significant advances occurred in the study of cell calcium metabolism. The advances are in two main areas: one is the development of intracellular calcium probes, coupled with ultra-sensitive imaging technology, and the second is the development of calcium-selective microelectrodes (Borle 1990; McLeod 1992). These methods have allowed the direct measurement of calcium and, in some cases, direct spatial localization within the cell of intracellular calcium concentrations (Loew 1992).
Comment on Ion Cyclotron Resonance Model Proposed to Explain How a Weak Magnetic Field Could Affect Calcium at the Membrane The ion cyclotron resonance (ICR) mechanism has been the subject of study as a possible source of interaction of low-frequency EMF with biologic systems for more than 20 years (Liboff et al. 1990). Most efforts to explain biologic effects, however, have met with little success. As an example of these studies, Lednev (1991) used the ICR model to explain how a weak magnetic field could affect calcium ions.
Calcium ions (Ca2+) either occur inside a hydration shell or are bound to a protein, producing a charged spatial oscillator. In the model, either of those states resonantly absorb energy from the appropriate alternating fields and are spiraled into or out of the cell through the protein channels in the membrane. Although a charged ion of the mass of calcium has a resonance at nearly 60 Hz in the earth's static magnetic field, such a resonance can only be observed if the state decays through an electromagnetic transition. Lednev's calculations neglected the effects of collision damping of the states (Adair 1992) and have been shown to violate the laws of physics (Halle 1988). Therefore, whether or not 60-Hz EMF cause changes in calcium concentrations, the ICR model, as currently set forth, is not a viable mechanism for biologic systems.
Even though the ICR mechanism is not viable, a number of experiments have been performed at charge-to-mass ratios (q:m) corresponding to the calculated ICR frequency for calcium (e.g., see Parkinson and Hanks 1989a; Walleczek and Budinger 1992; Yost and Liburdy 1992). One such set of resonant conditions occurs at a frequency of 16 Hz and a dc magnetic field (B) of 23.4 µT (234 mG) according to the relationship frequency (Hz = 1/2t(q/m)Bdc).
The results are conflicting. Parkinson and Hanks (1989a) see no effect on changes in cytosolic calcium concentrations (sweeping through both resonant and nonresonant EMF conditions) for BALB/c3T3, L929, V-79, and ROS cells, but other investigators (Liburdy 1992b; Walleczek and Budinger 1992; Yost and Liburdy 1992) see changes when the low-frequency magnetic-field challenge is combined with a mitogen.
Cytosolic Calcium Oscillators It is well established that the intracellular calcium concentration can display an oscillatory behavior in response to an external stimulus (Fewtrell 1993; Meyer and Stryer 1991, and references cited therein). The period of these oscillations is typically between 1 sec and several minutes. Recently, a model based on nonlinear dynamics and on the theory of self-sustained (limit-cycle) oscillators was developed that shows how a small modulation of the signal pathway at an early stage can lead to large changes in calcium metabolism of the cell (Eichwald and Kaiser 1993). These assertions have not yet been tested by experiment.
Relationship Between Very-Low-Frequency Electric and Magnetic Fields and Bone-Healing Protocols for Osteogenesis The efficacy of exposures to electric and magnetic fields in bone healing has been observed (Falugi et al. 1987; Bassett 1990), at least when applied directly to the bone. Typical bone-healing protocols have involved pulsed 20-µsec magnetic fields. The magnetic-field strength used varies from 0 to 2,000 µT (Falugi et al. 1987). One of the mechanisms attributed to the effect of magnetic fields is that the ions in the medium between the bone ends are moved back and forth by the ac field. Ions trapped by the bone matrix become bound to the matrix, thus reducing the concentration of free ions in the
medium in the vicinity of the bone. A concentration gradient is set up, and new ions diffuse to the bone, becoming available for bone repair (Parkinson and Hanks 1989b).
More recently, McLeod and Rubin (1992) demonstrated that exposure to pulsed electric fields prevented osteopenia in turkey wings, stimulating an overall 10% increase in the bone area. They used 15-, 75-, and 150-Hz sinusoidal electric fields, with an estimated peak electric field in the tissue of no more than 0.01 mV/cm. The osteogenic influence was greatest at 15 Hz stimulation.
Summary An enormous body of work seeking a relationship between exposure to electric and magnetic fields and changes in calcium concentrations has been accumulated over the past two decades. Much of it shows some sort of positive association, albeit often requiring frequency windows, temperature windows, or power-density windows for an explanation. Some of the work, particularly the early work, is flawed by unstable preparations and uncontrolled thermal and exposure conditions. Many of the effects are difficult to observe or are only borderline significant and require pooling of data to obtain statistical significance. Many of the experiments have not been replicated adequately by others, perhaps because the exact experimental protocols have not been followed; in other cases, independent investigators were unable to replicate experiments.
Of the recent experiments summarized in Table A3-2, only three meet the exacting requirements of replication by independent laboratories, publication in peer-reviewed journals, and explicit identification of exposure strengths. Those experiments were on thymic lymphocytes in which Con-A stimulated cells showed an increase in calcium transport resulting from exposure to pulsed magnetic fields having flux densities about 10,000 times larger than those found in the average human environment (Liburdy 1992b; Walleczek and Budinger 1992; Yost and Liburdy 1993).





















