4
Animal and Tissue Effects
SUMMARY AND CONCLUSIONS
The published literature regarding the exposure of animals and tissues to power-frequency electric and magnetic fields is discussed in this chapter. The committee focused on three areas of principal interest: carcinogenesis, reproduction and development, and neurobehavioral and neuroendocrine responses. On the basis of an evaluation of peer-reviewed literature, the committee has made the following conclusions:
-
There is no convincing evidence that exposure to power-frequency electric or magnetic fields causes cancer in animals.
A limited number of laboratory studies have been conducted to determine if any relationship between exposure to electric and magnetic fields and cancer exists. To date, no reports have been published showing demonstrable effects of electric-or magnetic-field exposures on the incidence of various types of cancer. However, some recent, as yet unreplicated laboratory evidence suggests a positive relationship between magnetic-field exposures at field strengths of approximately 100 µT (1 G) and the incidence of breast cancer in animals treated with carcinogens.
-
There is no convincing evidence of adverse effects from exposure to power-frequency electric and magnetic fields on reproduction or development in animals.
Reproduction and development in animals, particularly mammals, have not been shown to be affected by exposure to very-low-frequency electric or magnetic fields.
-
There is convincing evidence in animals of neurobehavioral responses to strong 60-Hz electric fields; however, adverse neurobehavioral effects of such fields have not been shown.
Laboratory evidence clearly shows that animals can detect and respond behaviorally to electric fields. Evidence of behavioral responses in animals to ac magnetic fields is much more tenuous. In either case, general adverse behavioral effects have not been shown.
-
There is evidence of neuroendocrine changes associated with 60-Hz magnetic-field exposure in animals; however, alterations in neuroendocrine functions have not been shown to cause adverse health effects.
The majority of studies that investigated magnetic-field effects on pineal-gland function suggest that these fields might inhibit night-time pineal and blood melatonin concentrations; in those studies, the effective field strengths varied from 10 µT (0.1 G) to 5.2 mT (52 G). The data supporting an effect of sinusoidal electric fields on melatonin production are not compelling. Other than the observed changes in pineal function, an effect from magnetic-field exposure on other neuroendocrine or endocrine functions has not been clearly shown in the few studies reported.
Despite the observed reduction in pineal and blood melatonin concentrations in animals as a consequence of magnetic-field exposure, no evidence to date shows that melatonin concentrations in humans are affected similarly. In animals in which melatonin changes were seen, no adverse health effects have been found to be associated with electric-or magnetic-field-related depression in melatonin.
-
There is evidence that pulsed magnetic-field exposures greater than about 0.5 mT (5 G) are associated with bone-healing responses in animals.
Replicable effects have been clearly shown in the bone-healing response of animals exposed to electric and magnetic fields at sufficiently high field strengths.
CRITERIA FOR CONSIDERATION OF LITERATURE
Consistent with the review guidelines established by the committee, only peer-reviewed literature is considered in this report unless otherwise noted. Results are reported only if they are exposure related and are statistically significant according to the authors' criteria unless otherwise noted. Greatest weight is given to studies that were confirmed in some manner in the peer-reviewed literature and that were blinded studies.
USE OF ANIMAL STUDIES IN EVALUATING RISK
Data from animal studies are an important component of estimating risk from nearly all agents. A gradient in the degree of an association between exposure to a toxic agent and the effects that agent can produce is called the dose-response relationship. The dose-response relationship forms the basis for the science of
toxicology and health physics and allows scientists to predict toxic or adverse health effects. The dose-response relationship is expected because interactions between organisms and chemicals and energy deposition occur according to the basic laws of physics and chemistry and therefore are predictable.
Dose-response relationships are of two types: one describes the response of an individual to different doses of an agent, and one describes the distribution of responses of a population of individuals to different doses. When toxic or adverse effects are considered, individual dose-response relationships are characterized by a dose-related increase in the magnitude of the response. Interpretation of individual dose-response relationships can be confused by the multiple sites of action of most toxic agents. Each site has its own dose-response relationship. Population dose-response relationships consist of a specific end point and the dose required to produce that end point for each individual in the population.
Three assumptions are made when considering dose-response relationships:
-
The response is due to the agent administered. Although this assumption seems trivial in laboratory studies, it is not so apparent in epidemiologic studies. For example, epidemiologic studies might find an association between a response (disease) and one or more variables. Use of the term "dose-response" relationship in this context is always suspect until the variable is shown to be a representative factor of the putative causative agent.
-
The response is related to the measurement of the dose. The most accurate way to determine dose-response curves is to measure the dose actually reaching the site at which an effect is detected within a cell. However, measuring the dose at the site of action generally is prohibitively expensive and has been done in only a few cases. Some measurement of exposure is nearly always substituted for a true measurement of dose.
-
A quantifiable method of measuring and a precise means of expressing toxicity are available. Early in an investigation of the toxicity of an agent, the best end point for effects might not be apparent, but as more is known about the manifestations of toxicity, the dose-response relationship should become more quantifiable.
These assumptions hold true for all types of toxic agents, presumably including extremely-low-frequency electric and magnetic fields, if such fields are found to exhibit toxicity.
Types of Animal Studies Used in Descriptive Toxicology
Two main principles underlie all descriptive animal studies of toxicity (as reviewed by Klaassen and Eaton 1991). The first is that the effects produced by an agent in laboratory animals are applicable to humans. The second is that exposure of laboratory animals to toxic agents in high doses is a valid method of discovering possible hazards in humans. Toxicity tests are not designed to
demonstrate that a chemical is safe but rather to characterize the toxic effects that can be produced.
The toxicologic studies that are generally used to predict adverse effects in humans are
-
Acute lethality
-
Skin and eye irritations
-
Sensitization
-
Repeated dose (sometimes referred to as "subacute" toxicity)
-
Subchronic toxicity
-
Chronic toxicity
-
Mutagenicity
-
Developmental and reproductive toxicity
-
Other tests, including those for immunotoxicity and toxicokinetics (absorption, distribution, biotransformation, and excretion).
The most pressing issues with regard to residential electric-and magnetic-field exposure focus on carcinogenicity and possible adverse developmental and reproductive effects. Therefore, this discussion focuses on acute lethality, repeated dose, subchronic and chronic toxicity, mutagenicity, and developmental and reproductive toxicity tests, because these tests are used most often to address carcinogenicity and adverse developmental and reproductive effects.
Acute Lethality
The initial starting point for nearly all toxicologic studies is a determination of acute toxicity. The LD50 (the median lethal dose) and other acute toxic effects are determined for one or more routes of administration in one or more species and, in most currently used test regimes, are conducted over a 14-day period. Acute toxicity tests (1) provide a quantitative estimate of acute toxicity for comparison among substances; (2) identify target organs and other clinical signs of acute toxicity; (3) establish the reversibility of toxic responses; and (4) give guidance on dosages for other studies. The information obtained in acute toxicity studies forms the basis of the dosing regimes used in repeated-dose studies.
In animal studies on the effects of exposure to electric and magnetic fields, acute toxicity studies involve effects from high-strength current flows. The physical effects and behavioral changes present in animals receiving perceptible electric shocks do not seem appropriate for the exposure conditions under which most people are exposed to electric and magnetic fields. Moreover, human data on electrocutions are sufficient to make animal testing unnecessary.
Repeated-Dose Studies
Repeated-dose studies are performed to obtain information on adverse effects after repeated administration and as an aid to establish the dosages for subchronic
toxicity studies. In most currently used test regimes, repeated-dose studies are performed after 14 days of exposure. Biologic effects reported in short-term studies using electric and magnetic fields are reviewed in this report. However, results from short-term studies often are not reproducible and are of questionable value in evaluating possible adverse health effects.
Subchronic Toxicity Studies
The principal goals of the subchronic toxicity study are to establish a no-observable-effect level (NOEL) and to further identify and characterize the organs affected by the test agent after repeated administration. Subchronic toxicity studies more precisely define the dose-response relationship of a test agent and provide the data needed to predict the appropriate dosages for chronic toxicity studies. Subchronic exposures can last for different periods of time, but in currently used test regimes, 90 days is the most common exposure duration. No subchronic toxicity studies using electric and magnetic fields have been conducted that meet the criteria necessary for defining subchronic toxicity. This deficiency is primarily due to the lack of repeatable toxic effects and the lack of a definition of dose-response relationships required from repeated-dose studies to establish dosages for a successful subchronic toxicity study.
Chronic Toxicity Studies
Dosage selection is critical to the successful completion of chronic toxicity studies. If dosages are too high, not enough animals will be alive at the end of a study to allow sufficient definition of the dose-response relationship to be useful for predicting adverse effects. If dosages are too low, not enough effects will be present to allow sufficient definition of the dose-response relationship to be useful for predicting adverse effects. Chronic exposure studies last longer than 90 days. Because humans are exposed to various types of electric and magnetic fields over their entire lifetime, exposures in chronic studies using rodents are most appropriately of 2 years duration. As is the case for subchronic toxicity studies, no chronic toxicity studies using power-frequency electric or magnetic fields have been conducted that meet the criteria necessary for defining subchronic adverse effects. The acute through subchronic dose-response relationships necessary for successful completion of chronic toxicity studies are not available.
Developmental and Reproductive Studies
Four types of animal tests are used to examine the potential of an agent to adversely affect reproduction and development—short-term, segment I, segment II, and segment III tests.
Short-term tests use whole embryos in culture, organ cultures, and cell lines.
They are not used for assessing risk directly, but they can contribute greatly to the design of developmental and reproductive studies by providing an understanding of the mechanisms by which an agent adversely affects development and reproduction.
Segment I tests are designed to address general fertility and reproductive performance. Segment I studies typically begin at an appropriate time before mating and last throughout gestation, lactation, and the first 3 weeks of life.
The potential for an agent to cause birth defects (teratogenicity) is tested in segment II studies. Segment III tests address the potential for agents to cause toxicity after birth and often include multigenerational studies.
To conduct reproductive and developmental studies properly, concentrations must be known that do not result in overt adverse effects in males and females; overt toxicity is widely known to have severe effects on reproduction and development in males and females. Thus, in the absence of good dose-response information from acute toxicity, repeated-dose, and subchronic toxicity studies, informative reproductive and developmental toxicity studies are nearly impossible to conduct.
In studies involving electric and magnetic fields, the lack of repeatable reproductive and developmental effects and the lack of a definition of reproductive and developmental dose-response relationships are not surprising given similar negative results in studies of toxicity as discussed above.
Cocarcinogenicity and Copromotion Studies of Electric and Magnetic Fields
Carcinogenesis is a multistep, multipathway process, and carcinogens probably have different potencies for each of the different steps. Experimentally, it has been difficult to identify specific steps and determine which are necessary and sufficient to cause frank malignancy. Certain systems have been developed that provide evidence of malignant transformation in vitro or malignant tumors in vivo when subjected to combinations of agents. A possible observation in these systems is the determination of whether the potency of two agents can be enhanced when they are delivered together or in a specific sequence. The term ''initiator" is used for agents that are most potent when delivered first, and the term "promoter" is used for agents that are effective when delivered after initiators. Magnetic fields have been evaluated in those systems in vitro and in vivo; the data show negative and positive results. Each system is sensitive to the effect of different initiators and promoters; thus, negative data in one system do not necessarily contradict positive data in other systems. Positive results have not been replicated, but some of the data show a dose-response relationship for exposure to magnetic fields and to the interacting carcinogen. Thus, although the pattern of interaction of electric and magnetic fields with known carcinogens is not consistent, the possibility that magnetic fields in combination with some carcinogens produce transformation in these systems cannot be excluded at this
time. However, these few systems cannot predict hazard to human populations living in realistic environments. The doses of carcinogens and promoters used in combination with test agents, such as electric and magnetic fields, are invariably large and represent nonphysiologic exposure. The extent to which the highly treated cells in these test systems are representative of actual potential target cells in the soma of exposed individuals is tenuous. In experimental systems in which combinations of agents are used to produce an end point, extrapolation to lower concentrations that represent actual exposure concentrations in human populations is difficult. Thus, although data in these systems are useful for the study of mechanisms and identification of possible interactions, they offer little information on the potency of lower exposure concentrations of agents in the human environment.
The data base that has been developed for initiation and promotion test systems is significant. These systems have shown positive results (i.e., enhanced carcinogenicity) for tests of copromotion and cocarcinogenicity with known and potent carcinogens, but positive results have also been observed when using other agents that are not considered potent carcinogens. For instance, acetic acid, beta-carotene, citrus oil, vitamin E, indomethacin, and putrescine have all yielded positive results in studies of copromotion or cocarcinogenicity using these in vivo test systems under certain conditions. Thus, the positive results in such tests are questionable until detailed studies have identified the underlying mechanism and the probable interaction of doses at environmental concentrations. Nevertheless, electric and magnetic fields, principally magnetic fields, have been shown to interact with carcinogens in some of these systems both in vitro and in vivo, and that fact raises some concern and deserves further attention. The committee provides suggestions for further study in this area in Chapter 7.
CARCINOGENIC AND MUTAGENIC EFFECTS
Because of epidemiologic reports of positive correlations between estimated exposures to power-frequency electromagnetic fields and cancer (see Chapter 5), considerable research interest has been generated concerning a possible connection between magnetic fields and cancer. To date, few laboratory animal studies have been published that bear directly on this question; however, an increasing number of investigations are being conducted. Studies that have been reported in the peer-reviewed literature examining the issue of magnetic-field exposure and cancer are discussed in the following pages and summarized in Appendix A, Table A4-1.
Several approaches and animal models can be used in laboratory cancer studies. The selection of a specific model depends largely on the hypothesis chosen to evaluate a particular underlying mechanism. For example, if an agent, such as an electric or magnetic field, is tested for its potential to be a complete carcinogen (an agent that by its application alone causes cancer to develop), 1.5
to 2 years of exposure of mice or rats to the agent is necessary. During that time, exposure to other possibly confounding agents must be kept to a minimum. In this regimen, the animals are observed during the major portion of their lifetimes, and the number, type, and time of development of tumors are the critical end points. This type of study should include several dosage groups and requires a relatively large number of animals, particularly if the natural incidence of a tumor type is low. Studies evaluating complete carcinogenicity are quite expensive due to the length of time and the number of animals involved.
Carcinogenesis is considered to be a multistep process; therefore, another approach is to assume the agent of interest acts either as an initiator or a promoter in which a two-phase protocol is required for testing. Initiation is defined as a genotoxic event in which the carcinogen interacts with the organism to affect the DNA directly. Promotion is operationally defined as an experimental protocol in which the promoting agent is applied subsequent to initiation and generally over a protracted time. Promotion is associated with a number of subcellular events that are generally nongenotoxic and is responsible for the conversion of initiated cells to cancerous cells. To evaluate electric and magnetic fields as an initiator, one high-dose exposure would be given followed by repeated exposures to a model promoter (e.g., 12-O-tetradecanoylphorbol-13-acetate, TPA) over a long-term period. If electric or magnetic fields were to be investigated for possible promotional effects, the animals would be exposed to a known initiator (e.g., 7,12-dimethylbenz[a]anthracene, DMBA) and subsequently exposed to electric or magnetic fields over a long-term period (e.g., months). The initiation and promotion approaches have the advantages of using fewer animals, less time, and less cost. However, a given model is usually limited to an evaluation of a specific type of cancer and might provide only general information on possible biologic mechanisms of the agent of interest and cancer development. Initiation and promotion studies use initiating or promoting agents, such as DMBA and TPA, respectively, at exposure concentrations that far exceed any possible comparable exposure concentrations in humans. Interpretation of such studies is for identification of possible toxic mechanisms, not for direct extrapolation to human risk.
Complete Carcinogen Studies
Few life-long animal studies examining power-frequency electric or magnetic fields as a complete carcinogen have been completed, although several are under way in the United States, Italy, Japan, and Canada. Several studies designed to evaluate magnetic fields as a promoter of cancer contained control groups that were exposed to magnetic fields without being exposed to a chemical initiator. These studies include a mammary tumor-promotion study in rats (Beniashvili et al. 1991), a lymphoma study in mice (Svedenstal and Holmberg 1993), and a mouse skin-tumor-promotion study (Rannug et al. 1993a). A major deficiency
of using such studies to evaluate complete carcinogenicity is the small size of groups involved. The Beniashvili et al. (1991) study found an increase in mammary gland tumors in rats exposed to magnetic fields at 20 µT for 3 hr per day as compared with unexposed animals. The other two studies (Rannug et al. 1993a; Svedenstal and Holmberg 1993) reported no increase in tumors with long-term exposure to magnetic fields at strengths of 500, 50, and 15 µT.
Tumor-Initiation Studies
No tumor-initiation studies of exposures to power-frequency electric or magnetic fields have been reported in the literature. Very little motivation exists for such studies because the energies involved are too weak to break chemical bonds. Furthermore, in vitro studies have not provided evidence that DNA molecules can be damaged directly by exposure to 50- or 60-Hz electric or magnetic fields.
Tumor-Promotion Studies
Despite the obvious need for promotion studies because of the suggested association between indirect measurements of exposure to electric and magnetic fields and cancer observed in epidemiologic investigations, few animal experiments have been completed. Skin-tumor promotion, after initiation with DMBA, was examined in mice exposed continuously to a 60-Hz magnetic field at 2 mT, 6 hr per day, 5 days per week, for up to 21-23 weeks (McLean et al. 1991). None of the exposed or sham-exposed mice developed papillomas. When magnetic-field exposure was combined with application of TPA, a slightly earlier development of tumors was observed in the field-exposed animals (Stuchly et al. 1992).
Rannug and co-workers (1993a,b,c) conducted skin-tumor and liver-foci studies in Sweden. In the 2-year skin-tumor-promotion study, mice were initiated with DMBA, then exposed to 50-Hz magnetic fields at either 0.5 mT or 50 µT for 19-21 hr per day. No evidence of a field-exposure effect was observed either in the development of systemic or skin tumors or in skin hyperplasia. In the liver-foci study, rats were exposed to similar magnetic-field strengths over a 12-week period. The exposed animals showed no differences in foci development from the sham-exposed rats. In animals exposed to chemical promoter (phenobarbital) and the magnetic field, foci formation was slightly inhibited when compared with initiated-only animals.
In a series of four experiments, rats were exposed for 91 days to 50-Hz magnetic fields at 30 mT (Mevissen et al. 1993). Initiation was accomplished with repeated oral doses of DMBA, and mammary tumors developed subsequently. In one experiment, the number of tumors per tumor-bearing animal was increased in animals exposed to the magnetic field. In a repeat of that experiment, however, no difference between exposed and sham-exposed animals was observed. This study is handicapped by the small number of animals in each group.
Before the Mevissen et al. (1993) study, a group in Georgia examined mammary carcinogenesis in magnetic-field exposed animals that were initiated with N-nitroso-N-methylurea (NMU) (Beniashvili et al. 1991). In the groups of animals exposed to a 60-Hz magnetic field at 20 µT for 3 hr per day for the lifetime of the animals, the incidence of NMU-induced mammary tumors increased over that in sham-exposed animals or in animals exposed for only 0.5 hr per day.
An additional mammary carcinogenesis study was performed in which DMBA was used to initiate mammary tumors in rats. Löscher and co-workers (1993) reported a significant increase in mammary-tumor induction in the rats exposed to a magnetic field. All rats received four weekly doses of 5-mg DMBA beginning at 52 days of age. After DMBA administration, 99 rats were exposed to 50-Hz magnetic fields at a flux density of 0.1 mT for 24 hr per day for 3 months. Another 99 rats were sham exposed. After 3 months of exposure, mammary-tumor incidence was about 50% higher in the exposed group (51 tumors) than in the sham-exposed group (34 tumors). The difference was statistically significant ( p < 0.05). The tumors were also larger in the exposed group (p = 0.0134), but a difference was not found in the number of tumors per tumor-bearing rat. Note that this exposure is about 1,000 times that of the usual residential field strengths.
REPRODUCTIVE AND DEVELOPMENTAL EFFECTS
This section deals with in vitro and in vivo reproductive and developmental biologic effects of electric and magnetic fields at frequencies of 50 or 60 Hz in exposures that are relevant to those associated with power transmission and use. It is divided into considerations of effects of electric fields and magnetic fields. This division is somewhat artificial because all time-varying electric fields have an associated magnetic field; however, at these low frequencies, the fields can be considered independently to a high degree of accuracy. Nonmammalian and mammalian studies are also considered separately. The studies are summarized in Appendix A, Table A4-2.
Nonmammalian Studies of 50- or 60-Hz Electric Fields
Fish
Embryonic effects of concurrent exposure to power-frequency electric and magnetic fields have been studied in Medaka fish by Cameron et al. (1985). Two-to four-cell-stage embryos were exposed for 48 hr either to 60-Hz electric fields that produced a current density of 300 mA/m2, to a magnetic field of 100 µT (1.0 G) root mean square (rms), or to combined fields. No significant developmental delays were reported immediately after exposure. Delays averaging 18 hr were detected 36-73 hr after removal from the magnetic field and
the combined field exposure. Developmental delays did not result in abnormal development or decreases in survival through hatching.
Chicken
The chicken embryo has been used to study potential effects of electric fields. Blackman et al. (1988a,b) studied brain tissue from embryos in chicken eggs exposed to 50- or 60-Hz fields at 10 V/m rms. The associated magnetic field was less than 70 nT (1 nT = 10-9 T) rms. Brain tissue was removed 1.5 days after hatching. The tissue was placed in a physiologic salt solution containing radioactive calcium and then placed in the same solution with no radioactive calcium and exposed to 50- or 60-Hz fields at 15.9 V/m rms and 73 nT rms for 20 min. The calcium efflux from the brain tissue of chicks exposed as embryos to 60-Hz fields was affected (see the description and analysis of these experiments in Chapter 3). The same phenomenon was not observed with embryos exposed to 50-Hz fields. Three replicates of the Blackman study by other laboratories have not produced consistent results.
Mammalian Studies of 50- or 60-Hz Electric Fields
Mice
Male and female mice were exposed to either horizontal or vertical electric fields in two studies by Marino et al. (1976, 1980). In the first study, mice were exposed to electric fields at 10 and 15 kV/m that led to effects attributed by the authors to microshocks. The second study involved three generations of mice. Although the postnatal-weight gains were similar in exposed and unexposed mice, a higher mortality was observed in the exposed mice. This is the only report of that phenomenon, and the results have not been supported by data from studies conducted at other laboratories.
Unlike the work of Marino and co-workers, Fam (1980) was unable to identify an exposure-related change in mortality of the progeny of mice exposed to 60-Hz electric fields at 240 kV/m. In this study, mice were exposed throughout gestation, the offspring were bred, and their litters were monitored for growth, blood histologic and biochemical changes, and histologic changes of major organs. In agreement with the results of Marino et al. (1980) except those on mortality, no changes were observed in growth or in any of the other measurements as a result of exposure.
Kowalczuk and Saunders (1990) were unable to detect any exposure-related dominant lethal mutations in male mice exposed to 50-Hz electric fields at 20 kV/m. Males were exposed for 2 weeks before breeding, and no exposure-related effects in offspring were detected in in utero death, litter size, or viability of offspring. Females were not exposed.
Zusman et al. (1990) found in vitro effects of electric fields in embryos of rats and mice, but they were unable to detect effects in fetuses exposed in vivo. Field frequencies used on mice in vitro and in vivo were 1, 20, 50, 70, or 100 Hz at 0.6 V/m with a pulse duration of 10 msec. Preimplantation mouse embryos were exposed and monitored through the blastocyst stage; 10.5-day-cultured rat embryos were exposed to the same fields. Cultured rat embryos showed abnormal limb development, and mouse embryos showed retarded development at some frequencies. When rats were exposed to the same fields and the offspring were examined at term, malformations did not increase. This study is greatly weakened because no indication is given that evaluators were blinded to the exposure group, and no correction is given for the use of multiple t-test.
Rats
Andrienko (1977) reported a study in rats exposed to 50-Hz electric fields at 5 kV/m in which they claimed to find exposure-related effects on several reproductive and developmental end points. These results are not considered further in this report because of the lack of details on experimental and statistical design furnished in the text.
Free et al. (1981) were unable to detect exposure-related effects of 60-Hz electric fields on neonatal rats. They exposed rats to 64-kV/m electric fields for 7 weeks beginning at 20 days of age and measured a spectrum of hormones that are part of the reproductive cycle (testosterone, follicle-stimulating hormone, luteinizing hormone, corticosterone, prolactin, thyroid-stimulating hormone, reduced glutathione, and thyroxin).
Burack et al. (1984) reported some effects in prenatally exposed rats; the effects appeared to be related to general stress rather than specific effects from electric fields. In this study, 17 pregnant female rats were exposed to 60-Hz electric fields at 80 kV/m on days 14-21 of gestation. Twelve pregnant females served as controls. After birth, litters were examined for viability, body weight (representing growth and maturation), and developmental landmarks (ear flap separation, eye opening, anogenital distance, age of testes descent, age of vaginal opening, and male sexual response in testosterone-treated gonadectomized animals). No exposure-related effects were detected except that exposed males displayed reduced copulatory behavior when compared with controls. As discussed in a review by Chernoff et al. (1992), the behavioral changes reported in that study match those expected from general stress.
According to Chernoff et al. (1992), Margonato and Viola (1982) were unable to detect any exposure-related effects in the offspring of male rats exposed to 50-Hz electric fields at 100 kV/m. Male rats were exposed for 30 min per day or 8 hr per day for up to 48 days. No exposure-related changes were detected in fertility, sperm viability, or morphology of exposed males or in the number of implantations, percent live per litter, or incidence of malformation in offspring.
Seto et al. (1984) was unable to find any exposure-related effects in the fetuses of rats exposed to 60-Hz electric fields at 80 kV/m for 21 hr per day for four generations. No significant effects were detected in fertility measures or postnatal growth for the first three generations, and no intrauterine effects on frequency of placental resporptions, fetal deaths, or fetal malformations were seen in the fourth generation.
Sikov et al. (1984) were unable to detect any exposure-related effects on the perinatal development of rats exposed to 60-Hz electric fields at 65 kV/m (effective field strength). Measurements included reproductive performance of adults, prenatal development of offspring, and perinatal effects of exposure through 25 days of age.
In a study designed as a follow-up to experiments done on swine (Sikov et al. 1987), Rommereim et al. (1987) exposed female rats to 60-Hz electric fields at 65 kV/m for 19-20 hr per day, beginning at 3 months of age and continuing through two breedings. Female offspring of the first breeding were exposed under the same exposure regimen of Sikov et al., bred at 3 months of age from selected animals, and killed for teratologic examination. Teratologic examinations of fetuses followed the first and second breedings. The study was conducted in two full replicates. No exposure-related effects were both replicated and statistically significant.
In the study by Rommereim et al. (1987), several effects were reported that were not replicated or consistent. In the second pregnancy of the first generation of animals, the percentage of litters with placental resorptions among exposed litters decreased significantly. Prenatal mortality decreased in litters of exposed female offspring. Neither effect was repeated during the replicate study. Significant divergent sex ratio occurred in the second pregnancy of the first generation of females, but that effect was not replicated. An increase in the degree of ossification of the skull was detected in exposed litters of the first pregnancy of the first generation, but that effect was not seen in the subsequent generation or the replicate. In the exposed group of the second pregnancy of the first generation, the incidence of reduced ossification (formation of bone) of the sternebra (primordial sternum of the embryo) was increased. The incidence of litters with reduced ossification of the phalanges was decreased in exposed litters of the second generation of females. If p < 0.12 is accepted as significant, incidence of malformations of all types (minor and major combined) was found in exposed animals in the second breeding of the first generation, but that effect was not detected in the first breedings of any experiment or in the second breeding of the replicate study. Growth and viability did not differ between exposed and control groups. The authors suggested that the pattern of significant results and the failure to replicate effects might have been due to the presence of a 65-kV/m electric-field threshold for the developing rat.
A second set of studies was conducted by Rommereim et al. (1989) to test the threshold hypothesis described above. Male and female rats were exposed
to 60-Hz electric fields at 112 or 150 kV/m. Exposures were for 19 hr per day beginning 1 month before and continuing through breeding, completion of gestation, and rearing of offspring through weaning. No differences were detected in breeding success, pregnancy rate, litter size, or postnatal growth and development.
In a third follow-up study (Rommereim et al. 1990) to the swine study conducted by Sikov et al. (1987), rats were exposed to 60-Hz electric fields at 10, 65, or 130 kV/m. Exposed males and females were bred and allowed to litter; the offspring were also exposed during breeding and subsequent pregnancies. Teratologic evaluation of over 7,000 fetuses was done at the termination of the study. No effects of exposure at any of the three field strengths were detected in reproductive outcomes, including course of pregnancy, pup weight at birth, and postnatal growth and development. No exposure-related increase in malformations was detected. The lack of reproductive effects reported in this study was even further strengthened by field-strength-related increases in chromodacryorrhea (a stress response consisting of release from the eye of a porphyrin-based material secreted by a gland behind the eye) in dams and their offspring. That effect indicated that the field strengths used were capable of producing biologic effects.
As mentioned in the previous section on mice, Zusman et al. (1990) found in vitro effects of electric fields in embryos of rats and mice, but they were unable to detect effects in fetuses exposed in vivo.
Swine
Sikov et al. (1987) reported an extensive study in which miniature swine were exposed to 60-Hz electric fields at 30 kV/m for 20 hr per day, 7 days per week for 4-18 months. Taken as a whole, this study did not show consistent exposure-related effects. Effects in some biologic results that were statistically significant were inconsistent throughout the study, and in follow-up studies in rats (Rommereim et al. 1987, 1989, 1990), no exposure-related effects were detected.
In the first generation of the study by Sikov et al. (1987), sows were exposed to electric fields for 4 months before breeding; pregnant animals were either killed before term for examination of fetuses or allowed to farrow. The mean number of live fetuses per litter increased, fetal deaths decreased, and fetal malformations decreased in offspring of exposed sows killed before term. Exposed sows that were allowed to farrow were bred again and killed before term for examination of fetuses. No differences occurred in fertility, fetal weight, or perinatal mortality in offspring from exposed animals. Exposed fetuses were smaller (including fetal mass, crown-rump length, maximal skull width, and intraorbital distance), and no exposure-related change was observed in malformation rate. Offspring from the first group of sows allowed to farrow were exposed to electric fields for 18 months and then bred. This second group of animals
were allowed to farrow, subsequently bred again, and killed before term for examination of fetuses. The increase in the number of malformations in the fetuses was significant. Offspring from the second group allowed to farrow were killed after 10 months of exposure. No significant adverse effects were detected in those animals. These studies were complicated by a disease outbreak during the course of the second breeding of the first generation of sows. The presence of the disease and the associated exposure to electric fields make interpretation of the increase in the number of malformations detected in those animals very difficult.
Cattle
Algers and Hultgren (1987) exposed pregnant cattle to 50-Hz electric fields at 4 kV/m and magnetic fields at 2.0 µT (20 mG) by keeping the animals beneath 400-kV power lines. The animals were exposed continuously for 4 months. No changes were detected in fertility, estrous cycle, progesterone levels, intensity of estrous, viability of offspring, or incidence of malformations.
Nonmammalian Studies of Time-Varying Magnetic Fields
Chicken
Numerous studies have been done in which chicken eggs were exposed to magnetic fields. For a comprehensive review of these studies, the reader is referred to Chernoff et al. (1992). Because of the plethora of conflicting results reported in the literature and the lack of replication of studies by different laboratories, a multilaboratory definitive study was conducted; nearly identical protocols in six laboratories in six locations were involved (Berman et al. 1990).
Berman et al. (1990) exposed eggs to 100-Hz pulsed magnetic fields at 1-µT amplitude, 0.5-msec duration, and 2-msec rise and fall time. Exposure occurred for the first 48 hr of incubation. Embryos were then examined for fertility, developmental stage, and morphology. Two of six laboratories detected a decrease in the percentage of normal embryos as a function of the number of fertile eggs and live embryos. That effect was significant when the results of all laboratories were pooled. Interestingly, the results of this study reflect the range of previous studies in that the strongest effect was apparently the laboratory site. Unfortunately, because the strongest effect is more related to the laboratory site than to the fields used, the significance of the results of this study is difficult to determine, and the results do not give confidence that the study shows a true biologic effect of magnetic-field exposure.
As a follow-up to the study of Berman et al. (1990), Martin (1992) exposed eggs to 60-Hz peak-to-peak fields at 3 µT for the first 48 hr of incubation. In three subsets, the embryos were evaluated immediately after incubation; in one,
the embryos were allowed to develop for another 72 hr. Despite earlier reports of positive effects by this laboratory, no exposure-related effects were detected.
Cox et al. (1993) attempted to confirm earlier studies by closely replicating appropriate exposure conditions. They exposed chicken embryos to 50-Hz fields at 10 µT with a superimposed field at 17 µT for 52 hr and allowed the embryos to develop for an additional 68 hr. No exposure-related increases in abnormal development were detected.
Mammalian Studies of Time-Varying Magnetic Fields
Mice
McRobbie and Foster (1985) exposed mice to pulsed magnetic fields ranging from 3.5 to 12 µT with pulse periods ranging from 0.33 to 0.56 msec. No exposure-related effects were detected. This study did not follow scientifically accepted test guidelines, and the data are of little value in evaluating biologic effects of magnetic fields.
Wiley et al. (1992) were unable to detect any exposure-related effects in mice exposed to fields designed to be relevant to the magnetic fields generated by video-display terminals. Mice were exposed to a magnetic field with amplitude varying in a saw-tooth shape with a repetition rate of 20 kHz and field strengths of 3.6, 17, or 200 µT from day 1 to day 19 of pregnancy. This study was unusual in that large numbers of animals were used (185 controls and three groups of 186 pregnant females). Dams were killed on day 18 of pregnancy and evaluated for implantations; litters were evaluated for fetal deaths, placental resorptions, body weights, and gross external, visceral, and skeletal malformations.
Frolen et al. (1993) exposed pregnant mice to pulsed magnetic fields (saw-toothed, linear rise time of 45 msec and a 5-msec decay time, 15-µT peak field strength, and a frequency of 20 Hz). No change in the rate of exposure-related malformations occurred. Exposures were begun on day 1 of gestation in two experiments and days 2, 5, and 7 of gestation in three additional experiments, respectively. All exposures were continued until day 19 of gestation. The number of implantations, placental resorptions, living and dead fetuses, and malformations and the length and weight of live fetuses were recorded. An increased rate of placental resorption was detected in exposed mice in all experiments except the one in which fetuses were exposed on day 7 of gestation. None of the increases in placental resorption rates was reflected in reductions in litter size. Body mass and length of exposed fetuses were reduced in the experiment in which fetuses were exposed on day 7 of gestation. The lack of correlation between increases in rates of resorptions and litter size makes it unlikely that the detected increase is of biologic significance.
Rats
Persinger et al. (1978) exposed pregnant rats to 0.5-Hz rotating fields at either 5, 100, or 1,000 µT from day 19 of gestation to 3 days after birth. Effects appeared to be unrelated to exposure.
Stuchly et al. (1988) exposed rats to 18-kHz saw-toothed waveform (44-µsec rise time, 12-µsec fall time) magnetic fields at 5.7-, 23-, or 66-µT peak-to-peak strengths. The study appeared to have largely negative results. Rats were exposed for 2 weeks before mating and throughout gestation for 7 hr per day. No exposure-related differences were detected in maternal measurements, fetal weight, or fetal malformations. A significant decrease in the incidence of bipartite or semipartite thoracic centra (primordial ossification points within the thoracic vertebra) was detected in the 2.9- and 33-µT exposure groups. A significant increase in the incidence of minor skeletal anomalies was detected in fetuses (but not litters) in the 33-µT exposure group. Because minor skeletal changes in the absence of terata (abnormalities in the developing or newborn fetus) are not likely to indicate serious adverse effects, the significance of the skeletal changes observed in this study with regard to biologic effects is difficult to assess, but the results indicate no abnormal effects on development.
McGivern et al. (1990) exposed rats to a 15-Hz pulsed magnetic field of 0.3-msec duration, 330-msec rise time, and peak strength of 800 µT. Pregnant animals were exposed for two 15-min periods on days 15 to 20 of gestation. At birth, no exposure-related effects on offspring were detected for viability, average weight, or anogenital distance. At 120 days after birth, no exposure-related effects were detected in circulating concentrations of testosterone, luteinizing hormone, and follicle-stimulating hormone. Increases in accessory-sex-organ weights and reductions in scent-marking behavior were detected in offspring of exposed dams. The committee is not aware of any attempts to replicate these results by any other study or laboratory.
Huuskonen et al. (1993) exposed mated female rats to 50-Hz (sine wave, peak-to-peak) magnetic fields at 35.6 µT or 20-kHz saw-toothed magnetic fields at 15.0 µT. No increases in malformation rates or placental resorptions were detected in the study. The mean number of implantations and living fetuses per litter was increased in rats exposed to the 50-Hz field. The increase was most likely an artifact due to the high number of resorptions in the control group. The incidence of fetuses with minor skeletal anomalies increased in both exposure groups similar to that reported by Stuchly et al. (1988). Nevertheless, such skeletal anomalies are common in teratologic studies and generally are not considered by most teratologists as indicating abnormal development.
Summary of Reproductive and Developmental Effects
The peer-reviewed literature appears to offer very little evidence of adverse effects on animals from power-frequency electric and magnetic fields. Some in
vitro biologic effects might occur, but evidence of in vivo effects from either electric or magnetic fields has very little support at strengths below those perceived (see following section) by animals. Experiments have also failed to support any mechanism for in vivo effects on reproduction or development.
NEUROBEHAVIORAL EFFECTS
A survey of the literature on the neurobehavioral effects of extremely-low-frequency electric-and magnetic-field exposure revealed that this literature has been reviewed many times. For the purposes of assessment, neurobehavioral effects considered are behavioral, anatomic, and physiologic alterations and chemical changes that may be taken as correlates of behavioral effects. Only those reports published in peer-reviewed journals and with methods adequately described to allow for replication were included in the final evaluation; those reports are summarized in Tables A4-3 through A4-6. Some reports fulfilled these requirements, but others used inappropriate controls or inadequate exposure apparatus. All studies that met the committee's basic requirements are included in the tables; however, only those reports that were repeatable and reliable are discussed herein.
This section is divided into discussions of studies using electric fields and those using magnetic fields or combined electric and magnetic fields. Simple and complex responses are also discussed separately. Simple responses include detection threshold levels (behavioral or physiologic responses) and general activity levels. Complex responses include aversion, avoidance, social behavior, learning, and analgesia.
Electric Fields
Over the past 15 years, several studies using a variety of subjects proved that mammals can detect 60-Hz electric fields as a sensory stimulus. An example that established detection and also determined the approximate threshold level was published by Sagan et al. (1987). Two operant behavioral techniques were used to estimate the minimal field strength necessary for rats to detect the electric field. The investigators found that not only did the rats respond in a way that indicated they detected the fields but also that the rats' performance was correlated accurately with the magnitude of the field. The two behavioral protocols yielded average threshold estimates of 13.3 and 7.9 kV/m rms, which were similar to the thresholds produced by other investigators (between 4 and 10 kV/m) using different behavioral protocols. Although the results clearly showed that rats can detect electric fields, these investigations did not determine the positive or negative effects of electric fields on behavior. Stern and Laties (1989) tested whether 60-Hz electric fields at 90 or 100 kV/m were perceived as an aversive stimulus to rats. In this study, the rats were given the opportunity to turn off the electric
fields that they were exposed to chronically, and when the rats turned off the electric fields, they were given the opportunity to turn it on again. None of the rats performed differently in the presence of electric fields or in control conditions where electric fields were never present. As a control for the protocol, illumination from an incandescent light was used instead of electric fields. The incandescent light served as an aversive stimulus, and the rats turned the light off at a rate dependent on the intensity of the light. Results from these studies showed that 60-Hz electric fields at 100 kV/m are not a detectably aversive stimulus to rats. However, the mechanism through which the electric field acts is not known.
In one study, investigators attempted to determine whether the electric field could be exerting its effect through stimulation of the hair follicles or the skin rather than through a direct action on neuronal membranes (Weigel et al. 1987). Using the exposed surface of an anesthetized cat's paw, 60-Hz electric fields at up to 600 kV/m were applied while simultaneously recording from the sensory dorsal root fibers, which transduce afferent impulses that originate from various receptors in the exposed paw. The results clearly showed that electric fields can elicit activation of the cutaneous mechanoreceptors with persistent duration lasting up to 90 min in some cases without fatigue. The mechanism for that response could be through the vibration of the hair follicles or through displacement of the skin by the force of the field that stimulates the receptors. Those two external mechanisms are separate from the possibility of a direct interaction of the induced currents produced in the skin with the neuronal membranes that stimulate the receptor to fire. By shaving the hair off the paw and applying mineral oil to the paw, a significant reduction in firing rate to stimulation was recorded, suggesting that the major part, but not necessarily all, of the mechanism for electric-field detection is through vibrations of the skin and hair.
Magnetic Fields
Although signal detection methods have provided evidence of the ability of mammals to detect electric fields, such evidence is not available for magnetic fields except at very high field strengths (i.e., magnetic excitation of endogenous phosphenes). A comprehensive series of studies examined the effects of chronic exposure of nonhuman primates to 60-Hz electric and magnetic fields on general health and behavioral performance, chemistry, and neurophysiology. In the first study, Wolpaw et al. (1989) exposed pigtail macaque primates to electric and magnetic fields at 3 kV/m and 10 µT, 10 kV/m and 30 µT, and 30 kV/m and 90 µT, respectively, for three 21-day periods; 21-day sham exposures preceded and followed the experimental period. General health examinations, including weight, blood chemistry, blood-cell counts, performance on a simple motor task, and postmortem examinations, were conducted on the animals. No detectable effects of electric and magnetic fields were discernible between sham exposures and experimental periods.
A companion paper (Seegal et al., 1989) reported the effects of twice-weekly-evoked potentials during the daily 6-hr field-off period. No effects of field exposure were detected on the auditory-, visual-, or somatosensory-evoked potentials of the early or mid-latency components of the response. A significant decrease in the amplitude of the late components of the somatosensory-evoked potentials was detected during two high-strength field exposures. The discussion of the results suggested that these changes might be due to opiate antagonistic effects of exposure to electric and magnetic fields. The metabolites serotonin and dopamine were changed in the monkeys exposed to electric and magnetic fields. Substantial data relates the endogenous serotonin system with analgesia; however, the mechanism is not clear through which electric and magnetic fields have an influence on serotonin and its effect on somatosensory-evoked potentials.
In a related study in rats, Ossenkopp and Cain (1988) showed that 1-hr exposures to 60-Hz magnetic fields at 100 µT (1 G) resulted in a shorter duration of fully developed seizures. These investigators also linked their results to the substantial evidence that magnetic fields inhibit the nocturnal analgesic effects of morphine in a field-strength-dependent manner (Ossenkopp and Kavaliers 1987). The mechanism of this effect is not known; however, studies using calcium-channel agonists and antagonists administered with morphine demonstrate that calcium channel antagonists inhibit and agonists enhance the analgesic effects of morphine in the presence of magnetic fields. The authors of those studies proposed that the effect of magnetic fields on analgesia is mediated through the calcium channels and cited the in vitro results of magnetic fields on calcium channels as evidence. However, direct evidence for the mechanisms of action remains undetermined.
Several studies examined the effects of magnetic fields on learning and performance in simple and complex behavioral tasks. Examples from even the best studies show mixed results. Hong et al. (1988) exposed infant rats to a static magnetic field at 0.5 T for 14 postnatal days. After a 1-month rest period, exposed and sham-exposed rats were trained to reverse a position habit in an enclosed T-maze four times. Although exposed and unexposed male and female rats differed, no differences were detected for total errors committed over the four reversal problems.
In contrast to this static-field report, Salzinger et al. (1990) reported results from rats exposed perinatally to 60-Hz electric fields at 30 kV/m and 100-µT magnetic fields for 22 days in utero and for 20 hr per day during the first 8 days postpartum. As adult rats, they were trained to emit responses for food on a random-interval schedule. When the rats were tested as adults, the exposed rats consistently responded at lower rates than the sham-exposed rats. In addition, the decrease in response was not eliminated by extinction procedures or by an additional month of testing. These results do not necessarily imply a deleterious effect of perinatal exposure to magnetic fields, but they do appear to indicate an effect was produced.
In support of an effect, Thomas et al. (1986) and Liboff et al. (1989) reported a temporary loss of stable baseline performance on a component of the multiple fixed-ratio-differential low-rate schedule dealing with differential reinforcement of low rates of responding. This loss followed a 30-min exposure to a combination of a static magnetic field at 26.1 µT and a 60-Hz linearly polarized magnetic field at 0.139 µT. Once again a decrease in performance accuracy on this task does not imply a deleterious effect of magnetic-field exposure and might be more in line with a detection or perception of the field.
To assess the potential aversion quality of 60-Hz magnetic fields, Lovely et al. (1992) tested the preference or aversion to 60-Hz magnetic fields at 3.03 mT in a shuttle box. In two sequential studies using the appropriate control and sham conditions, animals did not prefer or avoid the exposed chamber. The authors discussed their results in relation to significant responses observed with large 60-Hz electric fields. They suggested that the lack of aversion in these magnetic-field experiments indicates that aversive behavior produced by electric fields might be associated with body-surface interactions rather than internal-body currents resulting from electric-field exposure.
Summary of Neurobehavioral Effects
Mammals clearly can detect 60-Hz electric fields at relatively modest field strengths (above a few kilovolts per meter). However, the effect of electric fields, even at field strengths an order of magnitude higher, is not perceived as aversive. Further, the action of the field appears to be mediated primarily through the stimulation of the receptors and the skin through hair movement or vibration rather than through the direct interaction with neuronal membranes.
Even though little evidence exists showing that 60-Hz magnetic fields can be detected by animals, at the highest field strengths where rats appear to detect such fields (3 mT at 60 Hz), they do not produce an avoidance behavior. In addition, no general adverse health effects are detectable for field exposures, as measured behaviorally, chemically, or pathologically. However, repeated studies have reported behavioral, chemical, and electrophysiologic effects of long-term and short-term exposure to 60-Hz magnetic fields. These effects include a decrease in stable baseline performance on multiple-operant schedules dealing with reinforced behavior, on the one hand, and a suppression or decrease in induced-seizure duration, on the other hand. Both of those effects could be linked hypothetically by reports that 60-Hz magnetic fields inhibit endogenous opiate activity. A decrease in opiate activity could decrease the reinforcing properties of stimuli and exogenous opiates are known to enhance seizures. Thus, a decrease in endogenous opiates might inhibit seizures.
The underlying biologic mechanisms that mediate these effects are not known, but the results present interesting biologic questions that might or might not be construed as being health related. No link has been made between the
effects observed in animals and those observed in studies of the cellular effects of exposure to electric and magnetic fields. Those studies are discussed in Chapter 3 of this report.
IN VIVO NEUROCHEMICAL AND NEUROENDOCRINE EFFECTS
Neurochemical Effects
A variety of chemical transmitters in the brain mediate interactions between neurons. These chemical agents are released from the terminals of one neuron near the limiting membrane of another neuron, where they typically interact with specific receptors on the postsynaptic cell. The synthesis of these neurotransmitters and their release into the synaptic cleft between cells is an important aspect of cell-to-cell communication with the brain.
Relatively few studies have examined changes in brain neurotransmitter metabolism as a consequence of exposure to electric and magnetic fields. According to Vasquez et al. (1988), the chronic exposure of rats to a 60-Hz electric field at 39 kV/m changes the metabolism of brain monoamines in rats as reflected by alterations in the circadian rhythms of these chemicals. Hypothalamic and striatal norepinephrine, serotonin, dopamine, and 5-hydroxyindole acetic acid, as well as the dopamine metabolite dihydroxyphenyl acetic acid were measured following the exposure of rats to an electric field for 20 hr per day for 30 days; the measurements were made at six time points (three during the night and three during the day) throughout a 24-hr period. In the hypothalamus, the rhythms of norepinephrine, dopamine, and 5-hydroxyindole acetic acid in exposed rats differed from those in sham-exposed controls. The differences were in terms of the phasing of the rhythms rather than in their amplitude. In the striatum, only the dihydroxyphenyl acetic acid rhythm was changed by the electric-field exposure. Changes such as those, particularly in the hypothalamus, could be related to the hormonal alterations of the neuroendocrine axis that have been reported. On the other hand, static measurements, such as those reported by Vasquez and colleagues (1988), are not informative in terms of the synthesis or release of the specific chemicals in question. In addition, the neural concentrations and rhythms of the chemicals measured are highly labile (Morgan et al. 1973; Kempf et al. 1982); thus, the importance of the reported changes in terms of the physiology of the organism remains unknown.
The work of Seegal et al. (1989) also indicates that brain monoamine metabolism changes as a consequence of electric-field exposure. They observed that when macaque monkeys were exposed to a 3- to 30-kV/m electric field for 20 days, homovanillic acid, a dopamine metabolite, and 5-hydroxyindole acetic acid, a serotonin metabolite, were depressed in cerebrospinal fluid. Concentrations of these metabolites in the cerebrospinal fluid are generally reflective of brain neurotransmitter metabolism.
Amino-acid neurotransmitter concentrations have also been measured in the striatum of rats exposed to a 50-Hz electric field that ranged in strength from 20 to 180 kV/m; the exposures continued for either 14 or 58 days, and sham-exposed rats were used as controls (Vasquez et al. 1988). The neurotransmitters measured were taurine, glycine, aspartate, glutamate, gamma aminobutyric acid, and alanine. Following exposure of the rats to electric fields for 14 days, a generalized increase in striatal concentrations of all the neurotransmitters was observed. On the other hand, after 56 days of exposure, the concentrations of these neurotransmitters in the striatum were depressed. The authors pointed out that the changes were minor, and although the differences between the exposed rats and the controls were statistically significant, the mean values for the neurotransmitter concentrations were within normal limits of variation.
No data are available on the potential effects of sinusoidal magnetic fields on neurotransmitter metabolism in the central nervous system.
Melatonin Effects
Melatonin is a ubiquitously acting hormone possibly produced in all animals, including humans. In mammals, a major site of production is the pineal gland. The pineal gland is an end-organ of the visual system and is innervated by postganglionic neurons whose activity is determined by light perception at the retinas (see Figure 4-1).
Melatonin is an aminoindole and the product of the metabolism of tryptophan. Tryptophan is taken from the circulation into the pinealocytes, the hormone producing cells of the pineal gland where it is converted to serotonin (Figure 4-1). Serotonin is metabolized to melatonin in a two-step process; initially it is N-acetylated by the enzyme N-acetyltransferase to N-acetylserotonin. This product is then O-methylated by the enzyme hydroxyindole-O-methyltransferase, and melatonin (N-acetyl-5-methoxytryptamine) is formed (Reiter 1991). Melatonin production is higher at nighttime (in darkness) than daytime. After it is produced, melatonin is quickly released into the systemic circulation, causing concentrations of melatonin to be higher during the night than the day. Once in the circulation, melatonin readily enters cells to exert its effects because it is highly lipophilic.
The synthesis of melatonin is controlled by exposure to electromagnetic radiation of wavelengths in the visible regions (Reiter 1985). Similarly to visible light, certain ultraviolet wavelengths and infrared wavelengths also alter pineal melatonin production (Brainard et al. 1993; Reiter 1993a). No retinal photoreceptors are known to be activated by either the ultraviolet or the infrared wavelengths used in these studies. This fact implies that wavelengths of electromagnetic radiation outside the visible range change the ability of the pineal gland to produce melatonin; furthermore, the result is achieved by something other than the classical photoreceptor mechanisms at the level of the retinas. This finding might be germane to the subsequent discussion only in the sense that the electric and
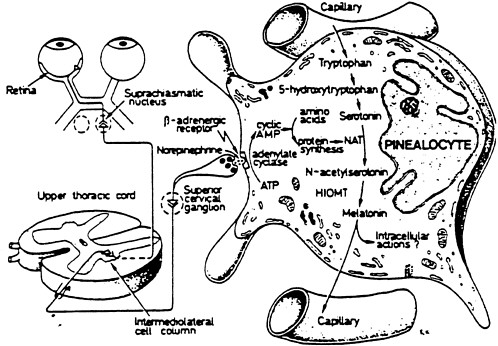
FIGURE 4-1 Illustration of the association of the retina with the pineal gland, in this case represented by a single pinealocyte. The pineal hormone melatonin is synthesized from the amino acid tryptophan; serotonin is an intermediate. The conversion of serotonin to melatonin requires two enzymes—N-acetyltransferase (NAT) and hydroxyindole-O-methyltransferase (HIOMT). The NAT-serotonin reaction is the rate-limiting step in melatonin production. SOURCE: Modified from Reiter 1991.
magnetic fields (extremely low frequencies and long wavelengths) that reportedly alter pineal melatonin synthesis are well out of the visible range. It should be emphasized, however, that the extremely low frequencies associated with power transmission differ by a factor of 1012 relative to visible and infrared radiation. Thus, the mechanisms of physical interactions of these different wavelengths might vary radically.
Effects of Electric Fields on Animals
The peer-reviewed reports that investigated the effects of sinusoidal electric fields on pineal serotonin metabolism and melatonin production and secretion are summarized in Table A4-7. Over a decade ago, Wilson and colleagues (1981) described a marked suppression of pineal melatonin production in rats exposed for 4 weeks to a 60-Hz electric field at 39 kV for 20 hr per day for 30 days. According to the experimental protocol, the authors took numerous precautions to ensure the health of the rats. After a 2-week period of isolation from other
animals, the rats were randomly divided into groups that were subsequently either exposed or sham exposed to the electric field. They were 56 days of age at the onset of the study.
The exposure apparatus was state of the art and is described in detail in a separate report (Hilton and Phillips 1980). Although the rats were subjected to an unperturbed field strength of 65 kV/m, the effective field strength was calculated to be about 35% lower because of mutual shielding, namely, 39 kV/m (Kaune 1981a). The control rats were placed in an identical exposure facility, but the coils were not energized; thus, they were true sham controls. The report does not indicate whether the investigators knew which of the two coil systems was energized during the experiment.
At the conclusion of the study, the animals were killed during either daytime (at 0800 hr or 1400 hr) or nighttime (at 2200 hr or 0200 hr, under dim red light). Pineal glands were collected and assayed for their contents of two pineal constituents, melatonin and 5-methoxytryptophol, using a gas chromatography-mass spectroscopy method developed by one of the authors of the report (Wilson et al. 1977). Additionally, pineal N-acetyltransferase (NAT) activity, which limits the rate of melatonin production, was measured by a standard radioenzymatic assay (Deguchi and Axelrod 1972).
Wilson et al. (1981) performed two similar experiments. In the first experiment, a reduced concentration of melatonin was observed 6 hr after onset of darkness in rats that had been exposed to 60-Hz electric fields for 1 month; however, a change in the 5-methoxytryptophol concentration after exposure was not significant. In the second experiment using comparable exposure conditions, pineal melatonin concentrations and NAT activity were estimated. Melatonin was found to be significantly depressed, but NAT activity remained unchanged as a consequence of exposure. The dichotomous response of the activity of NAT and the pineal content of melatonin is somewhat unusual inasmuch as the N-acetylation of serotonin is widely accepted as the rate-limiting step in melatonin synthesis (Reiter 1991). The changes observed were theorized to be a possible consequence of the reduction in the firing rate of the sympathetic neurons that terminate in the pineal gland, because earlier work (Jaffe et al. 1980) found that 60-Hz electric-field exposure reduced the frequency of action potentials in the superior cervical ganglia.
Wilson et al. (1981) gave the impression that effective electric-field strengths of 39 kV/m reduced the synthesis of melatonin in the pineal gland of rats. The results were seemingly compelling in terms of the magnitude of the inhibition; however, the field strength (39 kV/m) was high. In an erratum published 2 years later, Wilson et al. (1983) reported that the animals were actually exposed to field strengths of 1.7 to 1.9 kV/m, the difference being due to a malfunction of a transformer at the time the study was performed. In the 1-page erratum, the authors reported that the experimental results were duplicated in a study using an effective field strength of 65 kV/m, but no actual data are shown. The implication
of those findings, and one espoused by the authors, is that a wide range of field strengths (1.7-65 kV/m) reduced nocturnal pineal melatonin production in rats.
In 1986, Wilson and colleagues confirmed their original findings in studies in which rats were exposed to a 39-kV/m electric field for either 1, 2, 3, or 4 weeks (Wilson et al. 1986). After 3 and 4 weeks, the drops in nocturnal pineal melatonin concentrations were significant compared with those in sham-exposed controls. Likewise, NAT values were depressed at night in the pineal glands of rats exposed to the fields for either 3 or 4 weeks (see Table A4-9). In the same study, Wilson et al. (1986) found that withdrawal of the fields after 4 weeks caused a quick return (within 3 days) to the day-night melatonin rhythm.
As part of a larger study related to the potential consequences of electric-field exposure on fetal development, Reiter et al. (1988) exposed pregnant female rats in utero and the dams and newborns for 23 days after birth to field strengths of 10, 65, or 130 kV/m. At 23 days of age, pineal glands were collected from the young rats during the day or the night for pineal melatonin measurements. The assays for melatonin were performed blind in an independent laboratory that had not conducted electric-and magnetic-field research; a radioimmunoassay was used for measuring melatonin (Rollag and Niswender 1976; Champney et al. 1984). In the Reiter et al. (1988) study, each of the field strengths used (10, 65, and 130 kV/m) caused a slight but significant reduction in nocturnal pineal melatonin concentrations at one time point only. (Melatonin was measured at three different nighttime points.) No dose-response relationship was apparent. The nocturnal rise in pineal melatonin concentrations was also calculated to be delayed by 1.4 hr in each of the exposed groups of rats. This study used the same exposure facility as Wilson et al. (1981, 1986), but the experiments differed in that the initial studies used adult rats and the later study used fetuses and newborns. The melatonin assay procedures were different also (mass spectrometry versus radioimmunoassay).
In a study specially designed to test the effects of electric-field exposure on the melatonin synthetic activity of the pineal gland in rats, Grota and colleagues (1994) used a protocol similar to that used by Wilson and colleagues (1981, 1986). The exposure facility was state of the art, and the project was carefully supervised by a group of scientific advisors. The end points included pineal and blood melatonin concentrations as well as the activities of the two enzymes, NAT and hydroxyindole-O-methyltransferase (HIOMT) (Figure 4-1), required to convert serotonin to melatonin. Adult Sprague-Dawley rats (56 days of age at exposure onset) were exposed to a 65-kV/m 60-Hz electric field for 30 days. The control animals were sham exposed, and the pineal assays were conducted blind in an independent laboratory. After an exposure regimen of 30 days, neither daytime or nighttime pineal NAT activity, pineal HIOMT activity, nor pineal melatonin concentrations differed between the exposed and the sham-exposed animals. Serum melatonin concentrations were reported to be significantly
(p < 0.05) lower in exposed versus sham-exposed controls at one time point. In view of the failure of either pineal NAT activity, pineal HIOMT activity, or pineal melatonin concentrations to change, Grota et al. (1994) expressed concern that the measured depression in serum melatonin might have been due to chance rather than being specifically related to the field exposures. In addition, the statistical methods used in this study did not take into account the multiple hypotheses that were being tested simultaneously; such a consideration would render the differences between sham-exposed and exposed statistically insignificant.
In general, although the early studies on the suppression of melatonin synthesis by electric-field exposure were somewhat convincing, recent studies have failed to confirm a marked effect of such fields on the ability of the pineal gland to convert serotonin to melatonin. The current evidence is not convincing that electric-field exposure significantly impairs the melatonin-producing ability of the pineal gland.
Effects of Magnetic Fields on Animals
Interest in the potential neuroendocrine consequences of sinusoidal magnetic-field exposure has increased in recent years, and several approaches have been used to either directly or indirectly assess the effects of such fields on the physiologic integrity of the pineal gland.
Four studies have reported morphologic changes in the pineal gland following exposure of rats to 50-Hz magnetic fields (Table A4-8). The first study in a series investigated the ultrastructural appearance of the pineal gland of rats after their exposure to a very high magnetic-field strength of 0.7 T for 20 min daily for 2 weeks (Milin et al. 1988). Although the authors reported changes that implied significant alterations in the secretion of peptides by the pineal gland, the interpretation of the findings is greatly confounded by the restraints placed on the animals (a severe stress for rats) during the exposures. Also, no proof has been given that the pineal gland secretes any peptides. Two other studies also used high field strengths (5.2 mT), and the outcomes were inconsistent (Gimenez-Gonzalez et al. 1991; Martinez-Soriano et al. 1992). A fourth study by Matsushima et al (1993) was well controlled (exposed versus sham-exposed animals) and used a 50-Hz circularly polarized magnetic field at 5 mT, which had been claimed to influence pineal melatonin synthesis (Kato et al. 1993). Although some changes in pinealocyte size occurred in the exposed animals, they varied according to location in the gland and according to the time of year the study was conducted. How or whether these changes are significant in terms of pineal-gland function remains unknown.
An extensive number of earlier reports illustrated the suppressive effects of pulsed or perturbed static magnetic fields on pineal melatonin production (Olcese and Reuss 1986; Wilson et al. 1989; Villa et al. 1991; Reiter and Richardson
1992; Reiter 1992, 1993b,c). However, recent reports focused on the effects of sinusoidal magnetic fields on the melatonin-producing ability of the pineal gland (Table A4-9). The first report of a change in circulating melatonin concentrations associated with exposure of rats to sinusoidal magnetic fields (Martinez-Soriano et al. 1992) did not provide a complete description of the methods used; thus, their observations are of questionable significance. As in later publications by other workers, however, they did observe a reduction in blood melatonin concentrations 15 days after an intermittent exposure of the animals to a 5.2-mT (52-G) sinusoidal magnetic field.
Three subsequent studies were carried out in Japan by Kato et al. (1993, 1994a,b) who used exposed and properly sham-exposed rats. The first of these reports is comprehensive, and the methods are well described (Kato et al. 1993). In that study, rats were exposed to a 50-Hz circularly polarized magnetic-field strength of 0 (control), 0.02, 0.1, 1, 50, or 250 µT continuously for 42 days. In repetitive studies, Kato and colleagues showed that pineal and blood melatonin concentrations during daytime and nighttime were reduced by the exposures used. The main point of this report is that magnetic-field strengths of 1 µT and higher reproducibly reduced both pineal and blood melatonin concentrations at night. The degree of reduction was typically on the order of 25-30% and had p values of 0.05 to 0.01. The only perplexing and seemingly contradictory finding in the Kato et al. (1993) report is the modest rise in daytime pineal melatonin concentrations in one study in which rats were exposed to field strengths of either 1, 5, or 50 µT. Among all the reports in which pineal melatonin synthesis was studied in relation to either static or sinusoidal magnetic-field exposure, the Kato et al. (1993) result is the only hint that such fields might do something other than suppress the melatonin-producing ability of the pineal gland. In every other report, using a wide variety of exposure conditions, field exposures of all types have been reported only to suppress melatonin production (when a change in melatonin concentration was observed) (Olcese and Reuss 1986; Wilson et al. 1989; Villa et al. 1991; Reiter and Richardson 1992; Reiter 1992, 1993b,c).
In confirming the efficacy of rotating magnetic fields in suppressing pineal melatonin production, Kato et al. (1994a) made similar observations using pigmented (Long-Evans) rats and the same exposure conditions used in their original report. A comparison of the responsiveness of albino and pigmented animals to 1-µT sinusoidal magnetic fields was made because of an earlier publication in which it was claimed that the ability of static magnetic-field exposure to suppress melatonin synthesis is related to cutaneous pigmentation (Olcese and Reuss 1986).
In a series of three experiments, Kato et al. (1994b) claimed that the exposure of rats to either a horizontally or vertically oriented (as opposed to a rotating vector) 50-Hz magnetic field at 1 mT for 6 weeks was not associated with a significant alteration of either daytime or nighttime blood or pineal melatonin concentrations when compared with the controls sham exposed at about 0.02 mT. The authors speculate that the complexity of multidimensional magnetic
fields, which are a consequence of the circularly polarized fields (as opposed to the one-dimensional horizontal or vertical fields), might be operative, possibly by the induction of eddy currents, in suppressing the conversion of serotonin to melatonin in the pineal gland. These provocative findings, however, are near the limit of plausibility and must be replicated before the results can be accepted.
Until this point, the tacit assumption has been made that, for either electric-or magnetic-field exposures to suppress nocturnal melatonin production, at least part of the exposure must occur during the night when the synthesis of melatonin is increased. The studies of Yellon (1994) indicate otherwise. In successive studies performed during various seasons over several years, Yellon claimed that brief daytime exposures to unusual magnetic-field environments alter the underlying biologic clock mechanisms of the organism, thereby changing nocturnal melatonin synthesis by the pineal gland. In these studies, male and female Djungarian hamsters were exposed to a 60-Hz horizontal magnetic field at 100 µT (1 G) for 15 min beginning (at 18:00) 2 hr before onset of darkness (at 20:00). Sham-exposed hamsters served as controls. Immediately before darkness onset and during the night at 0.5 to 2-hr intervals, blood samples and pineal glands were collected from experimental and control animals. In two of three studies, the brief daytime exposure to magnetic fields significantly delayed and reduced the nighttime rise in melatonin; in the final experiment, no such observation was made. The differences are difficult to explain, but the author believes they might be related to the heterogeneity of the experimental animals. The animals were from a breeding colony maintained by the researcher, and, although all were adults, they ranged widely in age and included males as well as females, which were in different phases of their estrous cycles. Whether any of those factors confounded the outcome of the Yellon studies remains unknown. The findings point out, however, that daytime exposure to certain magnetic fields might affect the ability of the pineal gland to either synthesize properly or release melatonin on the subsequent night.
The papers summarized above represent all those published that satisfy the committee's criteria for inclusion in this report.
Effects of Combined Electric and Magnetic Fields on Animals
Lee et al. (1993) used animals in a natural setting to examine the effects of combined 60-Hz electric and magnetic fields on the circadian melatonin cycle (Table A4-10). In these complete and carefully supervised experiments, female lambs were maintained under a 500-kV high-voltage transmission line (mean electric field at 6 kV/m, mean magnetic field at 4 µT) for 10 months. Control animals were caged 229 m from the transmission line (mean electric field at < 10 V/m and mean magnetic field at < 0.02 µT). Blood samples were collected over 48-hr intervals during the 10-month period, and blood melatonin concentrations were estimated with a radioimmunoassay. Through the 10-month period,
the transmission-line-exposed and control animals (10 each) exhibited essentially indistinguishable 24-hr melatonin rhythms. The cycles were compared during different seasons and under different environmental conditions and temperatures.
Combined electric and magnetic fields were also used by Rogers et al. (1995) who examined the effects of such exposures on blood melatonin concentrations in baboons. When two baboons were subjected to a 60-Hz field at strengths of 30 kV/m and 100 µT (1.0 G) that was intermittent, irregularly scheduled, and switched rapidly on and off for 10 days, low nighttime concentrations (equivalent to those measured during the day) of melatonin were reported. The animals served as their own controls and were sham exposed several weeks before exposure to electric and magnetic fields and were found to have a normal nighttime increase in blood melatonin. One strength of the study is that the animals served as their own controls; that strength, however, also led to a major shortcoming—the control and experimental periods obviously were not simultaneous.
Effects of Electric and Magnetic Fields on Humans
Although the melatonin rhythm has been frequently studied in humans and found to be similar to that in other mammals, only on a couple of occasions has circulating melatonin been examined after the exposure of individuals to experimental electric-and magnetic-field environments (Table A4-11). In two reports, adult males were exposed to the complex electromagnetic environment used for magnetic resonance imaging (MRI) for up to 60 min. In neither male was a significant change in blood melatonin concentrations measured (Prato et al. 1988-1989; Schiffman et al. 1994). The subjects served as their own controls and were sham exposed on different nights. Although no significant alterations in blood melatonin concentrations were noted, interpretation of the data is confounded by the observation of Schiffman et al. (1994) that even bright-light exposure had relatively little effect on circulating melatonin concentrations. A single report claims that the exposure of rats to MRI fields also is without effect on pineal serotonin metabolism (LaPorte et al. 1990).
In one report of men and women using electric blankets at night, melatonin was indirectly assessed by examining the urinary excretion of 6-hydroxy melatonin sulfate (Wilson et al. 1990a). In about 25% of the individuals, some change occurred in the concentrations of the urinary metabolite when the blanket was used and when its use was discontinued. Whether these reported changes are at all related to the electric-or magnetic-field features of the blankets remains unknown. A claim has been made that humans vary in their sensitivity to electric and magnetic fields (Rea et al. 1991). That claim, albeit of potential interest and importance, requires confirmation under conditions in which the experimenter is assured that the subjects did not receive other clues that alerted them to the onset and the cutoff of the field exposures.
Neuroendocrine Effects
Compared with the amount of information related to the effects of electric and magnetic fields on pineal morphology and physiology, the data on the impact of such fields on the hypothalamus-pituitary target-organ axis are sparse. The potential activation of the pituitary-adrenal system in animals exposed to electric and magnetic fields is of particular interest. The goal of these studies was to determine whether exposures to such fields constitute a stress to the animals. The typical stress hormones are pituitary adrenocorticotropin (ACTH), adrenocortical steroids (e.g., corticosterone and cortisol), and catecholamines (e.g., norepinephrine and epinephrine), which are released during stress into the blood from a variety of sites but particularly from the adrenal medulla.
Earlier studies by Dumanskii et al. (1977) and Marino et al. (1977) concluded that electric-field exposure induces a mild stress response in animals, although the evidence presented is generally unconvincing. Unfortunately, on the basis of seemingly meager data, Marino and Becker (1977) concluded that such field exposures might have implications for human health.
In a series of 10 studies, Marino et al. (1977) exposed young rats to a 60-Hz electric field at 15 kV/m for 10 months. At the conclusion of the exposure interval, 6 of the 10 experiments found a modest suppression of blood adrenocorticoid concentrations. The suppressions, which average about 30%, were in some cases statistically significant and in others not statistically verified. In the other four studies, blood adrenosteroid concentrations did not differ in exposed and nonexposed animals. Despite the fact that stressors of any type increase, rather than decrease, the secretion of steroids from the adrenal medulla, Marino et al. (1977) concluded from their findings that electric-field exposure constitutes a stress.
In a more carefully conducted study with mice, Hackman and Graves (1981) determined that, if electric-field exposure is a stress to animals, it is a very weak one. These authors exposed groups of mice to a 60-Hz electric field at a field strength of either 25 or 50 kV/m. The onset of the fields was associated with a transient and low-amplitude rise in circulating corticosterone concentrations; that short-term peak lasted less than 15 min. Thereafter (up to 20 min), basal (unstressed) concentrations of corticosterone were measured in the exposed mice. That effect contrasts with that in control mice that were exposed to sound stress for the same duration; in the controls, circulating corticosterone concentrations were high and were maintained throughout the 120-min experiment.
The findings of Hackman and Graves (1981) are essentially consistent with those of another report that appeared at about the same time (Free et al. 1981). Free et al. exposed adult male rats to a 60-Hz electric field at 64 kV/m for 120 days, after which circulating corticosterone concentrations were measured. The outcome of the study was inconsistent in that corticosterone values in all groups of animals were highly variable. When young rats were exposed to a 60-Hz
electric field at 8 kV/m from 20 to 56 days of age, the blood corticosterone rhythm seemed to be slightly shifted.
In the seemingly carefully controlled study on the potential stress from electric-field exposure, Quinlan et al. (1985) concluded that such exposures did not evoke an activation of the pituitary-adrenocortical axis. They based their conclusion on the observation that the acute exposure of adult Long-Evans rats to a 60-Hz electric field at 100 kV/m for either 1 or 3 hr did not change blood or adrenocortical concentrations of corticosterone compared with those measured in sham-exposed controls. A particular strength of the Quinlan et al. (1985) study was the exposure facility used. The details of the facility have been published (Stern et al. 1983), and the field measurements were made by an individual from the National Bureau of Standards. Additionally, the authors took numerous precautions to ensure that the animals were isolated from known stressors.
Although Quinlan et al. (1985) reported no change in adrenocortical function as a consequence of electric-field exposure, growth hormone (GH) concentrations were raised in the blood after intermittent (16-sec on/off) electric-field exposure for 3 hr. The release of GH from the pituitary has been interpreted by some investigators to be a response to stress. Other adenohypophyseal hormones (thyrotropin and prolactin) were not changed by either continuous or intermittent electric-field exposure.
The final study on the neuroendocrine physiology of animals exposed to electric fields was published by Portet and Cabanes (1988). Following the exposure of rats to a 50-Hz, 50-kV/m electric field for 8 hr daily for 4 weeks, they observed no differences in blood corticosterone, ACTH, thyrotropin, triiodothyronine, and thyroxine concentrations or in adrenocortical corticosterone concentrations; comparisons were made with sham-exposed controls. At the conclusion of the study, the adrenal-gland weights were compared between exposed and sham-exposed rats, and the mean weights did not differ significantly; likewise, all the glands were histologically similar.
As another potential index of stress, Leung et al. (1990) quantified the degree of chromodacryorrhea around the eyes of rats exposed to a 60-Hz electric field with strengths of either 65 or 130 kV/m from day 10 of gestation until adulthood. This brown discoloration of the fur around the eyes is a consequence of secretory products from the intraorbital harderian glands and its presence, in excess, might be an indication of stress to the animals (Harkness and Ridgway 1980, Sakai 1981, Leung et al. 1990). Compared with sham-exposed control rats, both the 65- and 130-kV/m fields significantly increased the degree of chromodacryorrhea. No pituitary-adrenal function measurements were included to verify whether the field-exposed rats were actually stressed.
In general, the reports on the effects of electric fields on pituitary-adrenal function in rats suggest that this system is not significantly changed during field exposure. Likewise, no other pituitary hormones have been found to be substantially modified by electric-field exposures. Despite the fact that the emphasis
of much research has shifted to the potential biologic consequences of magnetic-field exposures, no data are available on any pituitary or target-organ hormones in animals exposed to magnetic fields under controlled conditions. However, Lee et al. (1993) reported no change in circulating progesterone in sheep maintained under a 500-kV transmission line.
Consistency and Plausibility of Results
Surprisingly few studies have examined the potential neurochemical changes associated with the exposure of animals to very-low-frequency sinusoidal electric or magnetic fields. In fact, despite the current widespread interest in the biologic consequences of time-varying magnetic fields in this area of neurobiology (exclusive of those on the pineal gland), there is a dearth of information in the peer-reviewed literature. Because it is usually intuitively assumed that the brain or its appendages would be likely to detect changes in the electric-field environment, this dearth of information is remarkable. Among many potentially important molecular biologic assessments, studies of gene expression in the central nervous system of electric-and magnetic-field-exposed animals would seem appropriate.
The effects of exposure to sinusoidal electric and magnetic fields on pineal synthesis and secretion of melatonin have generated a great deal of interest. Electric-field-induced reduction of melatonin was initially reported to be profound; however, recent studies were unable to confirm those findings. A reasonable explanation has not been presented for the apparent disappearance of the effect that was so apparent in the early studies.
Because of the failure to confirm the findings on electric-field exposures and because of the shift in emphasis of much of the work to magnetic fields, studies of melatonin suppression by changes in the magnetic field are increasing steadily. Although the publications are beyond the scope of this report, a substantial number of published peer-reviewed reports showed that exposure of mammals to perturbations of pulsed static magnetic fields reduces the nocturnal production and secretion of melatonin (Reiter 1993b). Likewise, recent studies on sinusoidal magnetic-field exposure showed that these fields also inhibit the ability of the pineal gland to transform serotonin into melatonin. Collectively, the changes in melatonin concentrations as a consequence of such exposures are shown to be highly consistent as follows: (1) When a change in melatonin concentrations has been reported, the change has always been a suppression (never a stimulation); if melatonin production were unrelated to field exposure (i.e., just a random occurrence), the field-exposed animals would have higher nighttime melatonin concentrations than controls on some occasions; such a change has never been seen. (2) When other measurements of melatonin synthesis are estimated, they change in predictable ways consistent with the reduction in melatonin production. (3) The suppression of melatonin due to exposure to sinusoidal electric and
magnetic fields has been reported in three mammals, the rat (two strains), the Djungarian hamster, and the baboon.
Another reason for the interest in the reported melatonin changes is their potential relationship to the higher incidence of cancer reported in some epidemiologic studies. Two biologically plausible, although unproved, mechanisms theoretically describe a link between reduced melatonin concentrations and cancer initiation, promotion, and progression (Figure 4-2). According to Stevens (1987a,b), the increased secretion of prolactin and gonadal steroids, which are natural consequences of melatonin suppression (Reiter 1980), could lead to excessive proliferation of stem cells in the endocrine-system organs, thereby increasing the likelihood of tumor growth in these organs (e.g., breast and prostate tissue). Another theory relies on the observations related to the intracellular action of melatonin. In recent studies, melatonin was shown to be a potent hydroxyl radical scavenger (Tan et al. 1993a) and to prevent carcinogen-induced damage to nuclear DNA (Tan et al. 1993b, 1994). Thus, reduction in melatonin due to any cause might increase the likelihood of DNA damage and cancer initiation. Considering the observations that magnetic-field exposure might induce or prolong the half-life of free radicals (Grundeler et al. 1992; Nossol et al. 1993; Harkins and Grissom 1994), which are known to be scavenged by melatonin (Tan et al. 1993a), additional justification for an association between reduced melatonin production and cancer initiation is suggested. Furthermore, melatonin has been shown in a variety of test systems to reduce the growth of already initiated cancer cells (Blask 1993), so its reduction might also promote tumor growth.
Despite the suggested associations between exposure to electric and magnetic fields, reduction in melatonin, and development of cancer, no direct experimental evidence links the field-induced reductions in melatonin to increased cancer risk. Thus, even though the explanations are biologically plausible and some experimental evidence supports the connection (Löscher et al. 1993), studies investigating the potential link need to be performed.
Other than the melatonin-production effects, other neuroendocrine and hormonal effects of electric-or magnetic-field exposures seem to be minimal, although only a few studies have examined these interactions. The field exposures used to date seem not to constitute a significant stress to the animals.
BONE HEALING AND STIMULATED CELL GROWTH
Evidence showing that exposure to electric and magnetic fields influences both the normal functions and the healing processes in bone is considerable. Bone turnover and fracture healing have been reported to respond to electric and magnetic fields under a variety of circumstances. Bone-fracture healing, in particular, represents the most thoroughly documented example of effects of low-frequency, relatively low-strength electromagnetic energy on human tissues. The effects of exposure to electric and magnetic fields on bone tissue have been studied
FIGURE 4-2 The two theories that have been proposed to explain the potential association of reduced melatonin production with the alleged increase in cancer after exposure to electric and magnetic fields. On the left, reduced melatonin concentrations lead to an increased secretion of prolactin and gonadal steroids. That increase causes proliferation of cell division in breast or prostate tissue and stimulates growth of initiated cancer cells. On the right, melatonin suppression reduced the total antioxidative potential of the organism, thereby increasing the likelihood of damage by a carcinogen to the DNA of any cell. DNA damage can increase the risk of cancer particularly if electric- and magnetic-field exposure also increases the half-life of production of free radicals.
in vivo in experimental animals and in humans, and the molecular mechanisms of the effects have been studied in in vitro systems. The evidence shows that electric and magnetic fields affect signal-transduction processes in bone cells, principally osteoblasts. The effects of magnetic-field exposure on bone have been observed almost entirely at field strengths of 0.1-15 mT (1-150 G) for magnetic fields and 1-100 mA/cm2 for current density (which is proportional to the electric field); those strengths are orders of magnitude higher than the baseline values associated with household exposures. However, the field strengths reported to produce significant effects on bone overlap field strengths that can occur during intermittent exposures to household appliances or occupational electric equipment (Wilson et al. 1994). Little evidence can be found for effects of magnetic or electric fields on bone at magnetic-field strengths below 100 µT (1 G) or at current densities below 1 mA/cm2.
Regulation and Cell Biology of Bone
Bone turnover is controlled by a number of hormones, principally parathyroid hormone (PTH) and 1,25-dihydroxyvitamin D3. Those hormones act in bone largely by regulating activities of osteoblasts (cells that synthesize and calcify bone matrix) and osteoclasts (cells that resorb bone mineral and matrix); both hormones also maintain the balance of calcium and phosphate ions in the kidney and intenstine. PTH and 1,25-dihydroxyvitamin D3 cause increased resorption and decreased formation of bone when their concentrations are acutely raised (Auerbach et al. 1985), but both agents also promote bone formation at lower concentrations or over longer periods (Tam et al. 1982). Both agents probably exert their long-term actions directly on the cellular differentiation of osteoblasts and osteoclasts (Raisz 1977). Other hormones and cytokines also carry out specialized functions or play pathologic roles in bone metabolism (e.g., calcitonin, transforming growth factor ß (TGF-ß), interleukin 1, and PTH-like peptide) (Manolagas and Jilka 1995). The central role of the osteoblast in regulation of bone metabolism is emphasized by findings that the osteoblast is probably the primary target cell for PTH (Rodan and Martin 1981), which passes the hormonal regulatory message to other cell types by paracrine mechanisms.
Bone fracture is invariably accompanied by trauma and hemorrhage. Subsequent to a fracture, a specialized remodeling structure called callus forms around the fracture site. Extensive proliferation, differentiation, and tissue turnover involving both osteoblasts and osteoclasts take place over the ensuing healing period, leading eventually to resorption of the callus tissue and strengthening of the new bone bridging the fracture (Bassett 1989). In some cases, callus formation and subsequent remodeling fail for various reasons, such as necrosis, failure to vascularize, or infection. These failures to heal might be persistent or even permanent in some cases. Resistance to healing (nonunion fractures) is the primary condition for which therapeutic electric and magnetic fields have been applied most often.
Other uses of electric and magnetic fields have been to promote bridging of congenital gaps (pseudoarthroses) in bone and to enhance the density of bone in cases of osteoporosis (Polk 1993).
Endogenous electromagnetic Properties of Bone
It is important to recognize that significant electric fields are a normal property of bone in living organisms. The modern era of research on this topic was initiated by a report showing that bone exhibited piezoelectric properties (Fukuda and Yasuda 1957). That finding was confirmed and extended to hydrated bone tissue by numerous investigators (Bassett and Becker 1962; Friedenberg and Brighton 1966; Cochran et al. 1968). Repetitive pulses of current density in the range of 10-100 mA/cm2, with electric fields of 20-200 mV/cm, are generated in bone during normal movement because of mechanical stresses on the bone (Bassett and Becker 1962; Pienkowski and Pollack 1983). Electric fields in normal bone are from two primary sources: (1) piezoelectric responses of the calcified bone matrix to mechanical loading (Anderson and Eriksson 1970), and (2) streaming potentials due to dynamic charge separation between the essentially static charges in the collagen fibers and the ionic charges in the surrounding mobile fluids, which stream during loading and relaxation of bone (Borgens 1984). The mineral component of bone (hydroxyapatite) apparently contributes to the piezoelectric process mainly as an insulator that limits dispersion of charges produced by compaction of collagen fibers (Pollack 1984). Another postulated source of endogenous electric fields in bone is the electric processes of living bone cells, which contribute significantly to the higher current densities detected in living bone as opposed to dead bone (Friedenberg et al. 1973; Bassett 1989).
It is noteworthy in the context of the overall mission of this report that no report has been made of the magnetic component of the endogenous fields produced by bone. Moreover, in studies of the effects of various electric-and magnetic-field exposures on osteogenesis, Rubin et al. (1989) concluded that regardless of the magnetic characteristics of the applied field, the only factors relevant to biologic function in bone are those associated with the induced electric field. That conclusion has been supported by many studies and is generally accepted by most investigators in the area of research on electric-and magnetic-field effects on bone tissue (Brighton and McCluskey 1986; Bassett 1989; Polk 1993).
For over a century, bone growth, remodeling, and turnover in normal organisms have been hypothesized to be subject to the influence of endogenously generated electric fields, and application of externally generated electric fields has been hypothesized to be therapeutically useful in treatment of fractures or defects in osteogenesis (reviewed in Brighton and McCluskey 1986). Fracture of bone dramatically enhances the generation of charges and the flow of current in the area around the fracture site, especially during the first few minutes or
hours after injury, and the magnitude of the currents surrounding the fracture is directly related to the healing of the fracture in subsequent processes (Friedenberg and Brighton 1966; Borgens 1984). Both piezoelectric and cellular electric processes are believed to contribute to fracture currents.
Locally generated electric phenomena in normal bone remodeling and fracture healing are believed by many researchers to be involved in a process in which areas of bone that accumulate negative charge are subject to increased deposition of bone matrix, and areas of positive charge are subject to increased resorption of existing bone matrix (Dealler 1981). That hypothesis is based on observations that, during chronic flexure of living bone, the areas of bone undergoing compression are the sites of increased bone formation (''Wolff's law"; Wolff 1892) and increased negative charge (Fukuda and Yasuda 1957; Bassett and Becker 1962), and the areas undergoing tension are the sites of increased bone resorption and increased positive charge. Moreover, numerous experimental and clinical studies (e.g., Brighton et al. 1979) have confirmed that placing dc electrodes in bone produces increased bone formation in the immediate area of the negative electrode and increased bone resorption in the area of the positive electrode. A range of current densities, roughly 10-100 mA/cm2, has been reported to be optimal for observation of these effects; no effect has been observed at lower current densities, and cell death occurs at higher current densities (Friedenberg et al. 1970, Brighton and McCluskey 1986).
Clinical Stimulation of Bone Healing with Electric and Magnetic Fields
In 1964, Bassett and colleagues reported stimulation of bone growth in vivo with the use of implanted electrodes in unfractured dog bone (Bassett et al. 1964). That report led to a number of studies using various apparatus and having widely varying results (summarized in Hassler et al. 1977; Brighton and McCluskey 1986; Polk 1993). The first clearly documented successful studies of fracture healing using implanted electrodes were those reported by Friedenberg et al. (1971a,b), in which fibular fractures in rabbits were observed to heal much faster than those in sham-treated controls. Friedenberg et al. (1971a) applied 10 mA of dc field with the negative electrode implanted directly in the fracture site. In a single human case report, a nonunion fracture was healed by the application of a similar apparatus (Friedenberg et al. 1971b). Subsequent case reports and large clinical studies have convincingly documented that nonunion fractures and congenital bone defects (pseudoarthroses and failed arthrodeses) can be healed by means of implanted dc electrodes (Brighton et al. 1979).
Pulsed fields have been used more widely than dc fields for clinical bone-healing devices, at least partly because devices producing pulsed fields can be made noninvasive. During early studies of the electromagnetic properties of bone, it was found that specific time-varying current pulses could be detected in bone undergoing stresses similar to those involved in locomotion (Bassett and Becker
1962). Evidence also suggested that pulsed current delivered by implanted electrodes would decrease the amount of tissue damage due to electrolysis at the electrode surface (Levy and Rubin 1972). Bassett et al. (1974a) set out to influence osteogenesis by reproducing those pulses of current with noninvasive means as a way of avoiding the complications encountered with invasively implanted electrodes. Early attempts were made to produce pulsing currents in bone by placing the subject between electrostatically charged plates whose charge was altered rapidly (Bassett et al. 1974a). Subsequently, the Bassett group developed the strategy of using Helmholz induction to produce intratissue current flows by means of copper-wire induction coils placed noninvasively adjacent to the tissue (Bassett et al. 1974b). Electric pulses of 10-30 V applied to the induction coils were found to produce coupled pulses of about 1 mV in adjacent tissue at current densities estimated to be about 10 mA/cm2 in tissue. Although the amplitude and general waveform of the pulses produced by this device resembled those found in living bone, the time scale was abbreviated considerably (microseconds as opposed to fractional seconds in normal locomotion) for electronic design reasons (Bassett et al. 1977). In an initial series of clinical studies with this type of device, a pulsed waveform with a single 300-µsec positive voltage pulse was used and repeated 72 times per second. At least 70% of resistant nonunion fractures and pseudoarthroses were healed by treatment with that device (Bassett et al. 1977). Subsequently, larger clinical studies reported success rates for pulsed electric-field treatment of over 80% (Bassett 1989).
Further developments in the induced pulsed electric signal involved use of a burst of about 20 200-µsec pulses, repeated 15 times per second, with a slightly improved success rate in fracture treatment (Bassett et al. 1982). The older single-pulse signal is apparently more effective in the treatment of osteonecrosis and disuse osteoporosis (Martin and Gutman 1978; Bassett 1983). A variety of other externally induced electric-and magnetic-field exposure have been used in animal and human studies (McClanahan and Phillips 1983). Success has varied. The device designed by Bassett's group and manufactured by Electro-Biology (Fair-field, N.J.) is approved by the U.S. Food and Drug Administration for clinical treatment of resistant fractures and pseudoarthroses. Side effects have been reported to be minimal. No evidence of increases in cancer or other diseases has been found despite the high field strengths used in comparison with environmental field strengths (Compere 1982; Bassett 1989). A small number of other clinical devices are also approved for use in stimulating bone healing. Most of these are based on either pulsed inductive fields or implanted dc electrodes. Some devices also use sine-wave extremely-low-frequency fields at a variety of frequencies (Polk 1993).
Despite the strong evidence that healing of nonunion fractures and pseudoarthroses is accelerated by electric and magnetic fields, there is no convincing evidence that the treatments have any influence on the healing of uncomplicated fractures. Uncomplicated fractures have not been widely treated with electric or
magnetic-field procedures, however, for the simple reason that healing begins almost immediately in normal patients. Bassett (1982, 1983) suggests that once the final repair phases of the healing process have been triggered, whether by normal events or by exposure to electric and magnetic fields, treatment with the fields might only marginally accelerate the remaining events of fracture healing. On the other hand, such fields have been used to improve incorporation of bone grafts, to facilitate spinal fusions, and to improve certain types of osteoporosis (Friedenberg and Brighton 1981; Bassett 1983).
Potential Mechanisms of Electric-and Magnetic-Field Effects on Bone
The mechanistic bases have not been clearly established for the effects of either dc or pulsed electric fields on bone healing. Most evidence suggests that changes in osteoblast activities are the major functions responsible for bone responses to electric-and magnetic-field exposure (Watson and Downes 1979; Dealler 1981; Friedenberg and Brighton 1981; Bassett 1983). Although agreement is not explicit, different mechanisms might exist for the effects on osteoblasts by dc fields and by pulsed electric and magnetic fields (Polk 1993). Direct-current fields morphologically appear to stimulate osteogenesis mainly by stimulating the proliferation and differentiation of preosteogenic cells in the fibrocartilage matrix that fills the fracture gap in nonunion fractures (Friedenberg et al. 1974). Those cells then form new bone as if they had gone through an uninterrupted differentiation induced by the fracture process itself. Brighton and Friedenberg (1974) suggested that lowered oxygen tension in the area of the cathode might play an important role in triggering differentiation. Other possibilities are local changes in ionic concentrations or pH (Jahn 1968), stimulation of local nerves or blood vessels (Becker 1974), or direct membrane effects on cells by dc (Cone 1971). Bassett (1983), on the other hand, stressed the effects of pulsed electric and magnetic fields on functions of already differentiated bone cells rather than on precursors, suggesting that pulsed fields are less effective on osteogenesis as a proliferative process per se than it is on stimulating the function of existing bone cells at or near the fracture site. Bassett (1982, 1983) classified the demonstrated tissue effects of pulsed electric-and magnetic-field exposure as (1) a major and primary effect of reducing bone destruction, possibly by decreasing the sensitivity of bone cells to parathyroid hormone, (2) increased vascularization of the fracture site, (3) increased rates of bone formation by osteoblasts, and (4) for some pulsed EMF signals, decreased intracellular calcium concentrations in chondrocytes, a decrease that promotes replacement of chondrocytes by osteoblasts.
Effects of Electric and Magnetic Fields on Signal Transduction in Bone
Although the effects of exposure to electric and magnetic fields at the tissue level have been clarified somewhat by research over the past three decades, the
primary biochemical and biophysical effects at the molecular or ionic level remain obscure. One clear likelihood for the effects of such fields on bone is that the plasma membrane of target cells is likely to be the major site of action, regardless of subsequent cellular mechanisms. The current and voltage involved in these effects are much lower than those that might be required to overcome the resistance of the plasma membrane and induce intracellular effects directly (Adey 1983). Several laboratories showed that exposure to electric and magnetic fields produces modifications in the activities of the plasma membrane of skeletal tissue cells. For example, Luben and colleagues (Luben et al. 1982; Cain et al. 1987; Cain and Luben 1987) demonstrated that exposure of bone and bone cells in vitro to pulsed electric and magnetic fields causes a membrane-mediated desensitization of the osteoblast to parathyroid hormone. Colacicco and Pilla (1983) examined calcium-transport and sodium-transport processes, factors that are likely to be related to osteoblast function, in chick tibia exposed to pulsed electric and magnetic fields. Fitton-Jackson and Bassett (1980) demonstrated positive effects of pulsed magnetic fields on chondrogenesis and osteogenesis. Rodan and colleagues examined the effects of mechanical and electric stimulation on the activity of adenylate cyclase in skeletal tissues (Norton et al. 1977; Rodan et al. 1978). A number of other membrane effects of pulsed electric and magnetic fields were reported in a variety of systems (Schmukler and Pilla 1982; Adey 1983; Borgens 1984). McLeod and colleagues used a number of in vivo and in vitro systems to study the biophysical and cellular biologic properties of bone exposed to electric and magnetic fields. They showed that electric fields induced by devices promoting bone healing are the most likely operative influence on bone-cell function (Rubin et al. 1989) and that those induced fields can prevent bone loss associated with immobility (disuse osteoporosis). Studies with different frequencies of electric fields showed that bone cells are dependent on frequency in responding to electric fields (McLeod and Rubin 1990); the most effective frequencies are in the range of 10 to 30 Hz, closely matching the frequencies most often observed in living animal bones. Field strengths were calculated to be approximately 300 mV/cm at the most effective frequencies. Further studies with isolated osteoblast-like cells (McLeod et al. 1991) suggested that 20-Hz and 60-Hz electric fields at 1-10 mV/cm could cause transient increases in cytosolic calcium-ion concentrations, a finding that corresponds with that of Ozawa et al. (1989), who used a different osteoblast-like cell line to show that calcium ions were involved in activation of DNA synthesis by pulsed electric-field exposure.
McLeod and colleagues investigated bone-cell proliferation as a function of exposure to 30-Hz electric fields at varying plating densities of cells. The cells treated with electric fields responded at medium densities by exhibiting a lowered rate of proliferation coupled with an increased alkaline phosphatase content (McLeod et al. 1993), suggesting that the field promoted differentiation toward a more active matrix-forming osteoblastic phenotype rather than a proliferative
stem-cell phenotype. Cell densities above and below the responsive densities showed no effects of the field, suggesting that some cooperative effect might be operating between cells. Such cooperative effects could include cell communication through gap junctions or more complex electric phenomena, such as the "cell-array" model of dielectric impedance proposed by Pilla (1993). A related approach was taken by Fitzsimmons et al. (1989, 1992), who suggested that the effects of exposure to low-frequency, low-strength electric fields on proliferation and differentiation of bone cells in vitro are related to the generation of growth factors or their receptors, especially insulin-like growth factor II. Field-induced changes in the differentiation state of cartilage cells in vitro were shown by Hiraki et al. (1987), whose findings indicated an increased expression of osteoblastic phenotypes in cultured rabbit chondrocytes exposed to a clinically effective bone-healing device. Changes in cell-proliferation rates were also studied by Ozawa et al. (1989), who showed that pulsed electric fields increased DNA synthesis in rapidly growing bone cells but not in bone cells that had already reached a contact-inhibited more-differentiated status. The uptake of calcium ions was correlated with those changes in DNA synthesis, suggesting a membrane-mediated mechanism. These studies indicate that exposure to electromagnetic fields induce changes in the differentiation state of treated osteogenic cultures such that increased matrix synthesis, increased calcification activities, and altered sensitivity to growth factors and systemic regulatory hormones combine to substantially increase formation and decrease resorption of bone. These findings are consistent with the in vivo observations of events during bone healing (Bassett et al. 1982). The basic mechanisms by which such changes in differentiation are brought about have not been thoroughly elucidated, although most researchers suggest that the primary locus of the effects might be at the cell membrane, where responses to most of the hormones and growth factors that regulate bone metabolism are localized (Luben 1991; Pilla 1993). A number of in vitro studies showed that pulsed electric and magnetic fields used in the most widespread clinical fracture-treatment devices (Bassett et al. 1977) produce activation of mouse osteoblasts by means of a strong inhibition of parathyroid hormone (PTH) responsiveness in the cells (Luben et al. 1982), leading to increases in synthesis of collagen (Rosen and Luben 1983), decreases in bone resorption, and accelerated differentiation of osteoblasts from stem cells (Cain and Luben 1987). The effects of electric-and magnetic-field exposure on PTH responses were found to be consistent with decreased coupling of receptors to adenylate cyclase via the stimulatory G protein (Cain et al. 1987). Release of interleukin growth factor II or other growth factors, as suggested by Fitzsimmons and colleagues (1992), could be important in the proliferative responses of less differentiated cells, and the release of transforming growth factor b might be a factor in inducing differentiation (Manolagas and Jilka 1995). However, the above chain of events, although plausible, has not been investigated in detail in any single laboratory or experimental system.
A Hypothetical Scenario for Electric-and Magnetic-Field Effects on Bone
Based on observations of the biochemical effects of exposure to electric and magnetic fields on bone in vitro, a hypothetical model was developed to find ways to induce healing of bone in vivo. One key observation is that osteoblasts exposed to pulsed electric and magnetic fields for as little as 10 min exhibit a persistent desensitization to the effects of PTH on adenylate cyclase (Luben et al. 1982; Cain et al. 1987). Studies using biochemical probes of G-protein coupling (Cain et al. 1987) indicate that the ability of bound hormone-receptor complex to activate G-protein alpha subunits is impaired by treatment of the osteoblast with pulsed fields. The desensitization of the PTH receptor results in an increased rate of synthesis of collagen by the osteoblast (Luben et al. 1982; Rosen and Luben 1983) and a decreased rate of bone resorption by ostecolasts (Cain and Luben 1987). Both effects would tend to increase the amount of bone in a localized area exposed to pulsed fields in vivo, and those effects are in fact observed in healing bone under clinical circumstances (Bassett et al. 1982).
There are some potential clues to the possible molecular mechanism of PTH receptor desensitization by electric and magnetic fields. Desensitization of other G-protein linked receptors is known to be associated with changes in the configuration of the transmembrane domains adjacent to intracellular phosphorylation sites, leading to phosphorylation of the receptor by intracellular enzymes (Sibley et al. 1988). These findings suggest that electric-and magnetic-field treatment might change the conditions at the cell-membrane surface in some as yet unknown way that changes the configuration of key residues of the PTH receptor, leading to desensitization of the receptor and a shift in the balance of osteoblast activities toward increased bone formation. In this regard, PKC is known to be involved in regulation of PTH-receptor desensitization (Ikeda et al. 1991), and the PTH receptor is known to contain phosphorylation sites for PKC but not other known protein kinases (Abou-Samra et al. 1992). Recent studies suggest that a key site of action of electric and magnetic fields might be the PKC enzyme (Uckun et al. 1995), but that finding has not been replicated independently. It should also be noted that studies of magnetic-field effects on ornithine decarboxylase (ODC) (which are well replicated) have all used protocols in which ODC is stimulated by the PKC-dependent phorbol ester signal pathway. Possible increases in cytosolic calcium (Ozawa et al. 1989; McLeod et al. 1991) might also participate in the desensitization of the PTH receptor by PKC.
DISCUSSION
Numerous studies in the laboratory have been initiated to determine the nature of the physical mechanisms involved in electric-and magnetic-field-induced effects and the extent of possible health hazards to living organisms in
an environment containing such fields. Biologic responses to exposure to electric and magnetic fields have been shown in many laboratories, and often they appear to be associated with the nervous system. In addition, unconfirmed or controversial data have been reported on observed effects that might be due to field exposure (e.g., changes in brain chemistry and morphology and alterations in reproduction and development). It is not yet known whether confirmed or putative effects are due to a direct interaction of the field with tissue or to an indirect interaction (e.g., a physiologic response due to detection or sensory stimulation by the field).
Whether a biologic effect from exposure to electric or magnetic fields constitutes a health hazard has yet to be answered. Experiments have not confirmed pathologic effects, even after prolonged exposures to high-strength magnetic (10 mT) and high-strength electric (100 kV/m) fields. In the very few tumor-promotion studies that have been reported, results seem to be mixed; most studies show no association between exposure to electric and magnetic fields and increased tumor development.
Although the data are not strong or entirely consistent, some experimental results using animal cancer models suggest a possible association of exposure to electric and magnetic fields and adverse health outcomes. The strongest laboratory evidence for an association between magnetic-field exposure and cancer development is in promotion of mammary carcinogenesis initiated by chemical carcinogens; however, the results have not been consistent. In these experiments, tumors must be initiated with a chemical carcinogen for the magnetic-field exposure to have its apparent effect. Some data also support the possibility that magnetic fields can act as a copromoter; magnetic fields alone, however, have not been shown to be effective in promoting cancer development.












































