4
Contraceptive Technology and the State of the Science: New Horizons
Beyond those contraceptive technologies that are likely to be developed within the next decade (Chapter 3), several lines of scientific research offer the promise of new and innovative approaches to contraception in the longer term. This chapter looks at these approaches within three broad categories: (1) female methods, (2) male methods, and (3) immunocontraception, areas that offer promise for both males and females. Together, these sections constitute a summary of the longer, fully referenced authored papers that were specifically commissioned for this study and appear in this report as Appendixes A, B, C, and D. Each section of this chapter is also linked to a table which summarizes the mechanisms that this committee considers important candidates for contraceptive research and the development of new methods.
A Strategy for Basic Research on Contraception
Identification of Targets Through Molecular Biology
In all organisms a small number of germ cells are set aside from somatic cells in early embryogenesis. Usually they remain in an undifferentiated quiescent state while somatic cells are dividing and forming recognizable tissues and organs. Germ cells begin to divide rather late in fetal life after they have settled in the germinal ridge.
This gonial stage is followed by cessation of mitotic cell division, differentiation, maturation, and meiosis. Every step of gamete formation is unique. In addition there are support organs that are partly or entirely devoted to conception
(testis, ovary, epididymis, prostate, fallopian tubes, uterus, sperm duct, penis, vagina) and subsequently to the protection and nourishment of the embryo (uterus). There cannot be another bodily function that has so much unique and essential paraphernalia devoted to it. It is this fact that provides the opportunities for selectively interfering with reproduction without affecting any other biological function. Drosophila geneticists estimate that about 100 genes—or 1 percent of the genome—can cause male and female sterility, respectively.
While humans may dedicate less of the genome to conception than do fruit flies, the number of tissues, organs, hormones, and the like devoted to conception suggests that there must be many genes with no other function as well. Look, for example, at the number of sterile humans who are otherwise perfectly healthy. It is this fact that provides abundant opportunities for selectively interfering with reproduction—in both males and females—without affecting any other biological function.
The basic knowledge of reproduction that is needed to conduct rational research on contraception can be summarized by two questions. First, what are the gene products that are expressed specifically in the various cells, tissues, and organs involved in reproduction? Second, which of these products is required for conception?
If we were trying to devise contraceptives for Drosophila, there would be little doubt how to proceed. Both questions would be asked simultaneously by carrying out saturation mutagenesis and isolating all sterile males and females. Saturation mutagenesis is the mutation of all genes within a species. This is accomplished by mutating each gene in one individual until all the genes have been mutated in a separate individual of Drosophila. Then a genetic screen would be used to select for the mutated individuals that are sterile to determine which genes are needed for reproduction. Each of the mutant genes would be cloned and sequenced so that some idea of their function could be predicted from similarities to genes known in the published data base. We would then have in hand a list of many genes that affect conception. We would select for further study only those genes that, when mutated, do not affect any other biological process but conception. Genes that meet these two criteria are candidates for contraceptive agents. Stated another way, the products of these genes are candidates for interference.
Next we would determine in what tissue and at what time each candidate gene is expressed. From its sequence we could predict whether the gene's product is a secreted extracellular product, a protein bound to cell membranes, a transcription factor, a component of the extracellular matrix, a growth factor, or perhaps a key hormone in the feedback loop that is required for reproduction. Having gathered this information, the basic research phase of Drosophila contraceptive development would be concluded, because we would have found our targets for contraception. We would have identified a large number of genes and their products that deal specifically with conception. Furthermore, we would
know enough about what many of their products do to consider the rational design of contraceptive agents and to select a few that seem promising.
To what extent can we apply this strategy to human contraception? What tools are available to accomplish in humans (or the closest model organism) the same results? The only higher vertebrate that currently can be mutated to saturation is the zebrafish. There are powerful genetic tools available in the mouse such as transgenesis; the introduction of a gene; "knockouts," that is, the inactivation of an existing gene; and even a large number of mutants that have been identified and maintained over the years. Nonetheless, saturation mutagenesis in the mouse is not now feasible.
However, an important truth that has emerged through decades of research is that gene sequences and functions have been remarkably preserved throughout evolution. This makes it logical to assume that genes affecting conception that are identified in a zebrafish screen could be shown to play a similar role in the mouse by selectively "knocking out" the homologous mouse gene and observing the results. If the gene turns out to play a similar role in mouse reproduction and also has a human homologue with an expression pattern resembling that of the mouse and the zebrafish—then a potential human contraceptive target has also been identified. It is by no means far-fetched to assume that Drosophila genes that are involved in conception may have homologues with the same function in humans.
A purely molecular biological method for identifying gene products that are specific for conception would use the principle of subtractive hybridization or the differential display of DNA copies of the messenger RNAs in a particular cell or tissue. Suppose, for example, that we wish to identify genes that are expressed solely in the epididymis. We would collect mRNA from epididymis tissue as well as from many other tissues and organs and then compare the populations of mRNA in order to identify genes that are expressed exclusively in the epididymis. Several genome companies are now sequencing all mRNAs (cDNAs) in many different tissues and then finding differences by computer search. Alternatively, it might be desirable to identify just those epididymis-specific genes that are regulated by androgen. Then the mRNA from control and hormone-stimulated epididymides would be compared for differentially expressed genes. One can imagine a number of important collections of tissue-specific or hormone-induced genes from which one could then choose possible targets for interfering with conception. These include cell-specific proteins of eggs, sperm, prostate, epididymis, and uterus. We would want to identify the battery of genes that is regulated by androgen, estrogen, progesterone, gonadotropin-releasing hormone (GnRH, sometimes referred to as LHRH), follicle-stimulating hormone (FSH), and luteinizing hormone (LH) in their respective target reproductive tissues.
Once we have identified genes that are specific for the target tissues and have cloned and sequenced their full-length cDNA, it is time to assay the gene for function because this method which, unlike a genetic screen, does not reveal
whether the gene is required for conception. We only know that a given gene is tissue-specific and/or regulated by a hormone. The gene sequence is critical because it gives the best clue to the gene's function. The gene can then be knocked out in the mouse and antibodies made against the gene's product to see if they neutralize its activity. There are a number of methods to test whether the gene really is essential for conception but, in each case, the best functional assay can be chosen only when the gene's product has been identified.
In summary, the fact that successful conception relies on many different, highly specific biological events and organs presents a rich array of targets for contraceptive research. What modern methods provide are new and powerful ways of identifying the molecules required for conception. In this field the basic research need not be far in advance of the practical applications.
This proposed strategy will provide an efficient means of identifying new and relevant targets. Some of these targets will be more amenable to attack than others, either because of accessibility for delivery of inhibitory agents or because of the length of the window of biological need for normal function. Thus, selection among such targets will have to be made using criteria related to successful product development and not solely scientific merit.
Identification of Targets Through Research in Reproductive Biology
In addition to any new targets identified by the strategy outlined above, there are also already available for exploitation various exciting new targets that have been identified by ongoing basic research in reproductive biology and other related fields. Figure 4-1 displays the areas of the male and female reproductive structures where today's science points to potential targets for potentially significant advances in making more contraceptive choices available to more individuals.
In both men and women, the hypothalymus secretes the gonatropin-releasing hormone (GnRH) which, in turn, stimulates the pituitary to release luteinizing hormone (LH) and follicle-stimulating hormone (FSH), both of which are needed for steroid hormone production as well as sperm and egg development. Since the primary structure of GnRH was identified two decades ago, researchers have been designing, synthesizing, and testing agents that may be able to block the hormone's action. Some of these show promise for the development of new contraceptive products, some of which could conceivably appear by the end of the next decade.
In addition to the neuron itself, there are targets throughout the GnRH neuronal network that might be activated or inhibited, providing a wealth of possible contraceptive agents. In fact, several peptidic agonists and antagonists of GnRH have already been identified. Each of these two classes has a different mode of action: While antagonists work by blocking a receptor, agonists operate by stimulating it so much that it is ultimately inhibited.
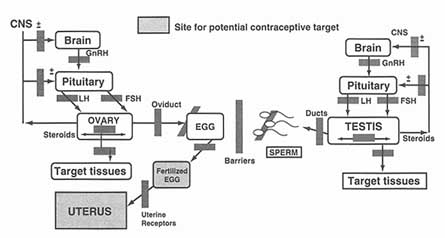
Figure 4-1
Potential targets for development of new contraceptive technologies. Source: Prepared for this report by Neena Schwartz.
The GnRH agonists available today have proved useful in the treatment of certain conditions, including prostate cancer, endometriosis, polycystic ovarian disease, and the induction of ovulation for in vitro fertilization. So far, however, they have not been potent enough to be used as contraceptives. The best hope in this area lies in the development of nonpeptide GnRH antagonists which, because they are orally active, make better drug candidates than do peptides. While all GnRH antagonists reported to date are, in fact, peptides, new approaches that have permitted development of a nonpeptidic growth hormone analogue makes the possibility of a nonpeptidic GnRH analogue more likely.
Several GnRH agonists have also been developed. Current clinical applications of these compounds include the treatment of precocious puberty and restoration of fertility in GnRH-deficient men and women, the treatment of prostate cancer in men, and the treatment of uterine fibroids, endometriosis, and polycystic ovarian disease in women. Higher doses of the agonists could be used to suppress spermatogenesis in males. While the reversibility and absence of toxicity of these analogues have been established, there are some potential problems. The most important is the need to replace testosterone, whose production is also stopped by blocking GnRH, in order to maintain libido. Also promising is current research that focuses on suppressing the production or action of FSH and LH, attractive targets because of the important role these hormones play in gametogenesis in both sexes. In males, researchers believe that FSH analogues will be able to selectively block spermatogenesis without affecting testosterone secretion. In females, the degree to which FSH affects estrogen production remains unclear. If an FSH inhibitor did decrease estrogen, the hormone would have to be
replaced. Another approach would be to target agents such as inhibin and activin, growth factors which also affect FSH secretion.
Several industrial and academic programs are working to develop FSH agonists or antagonists and are taking advantage of the new availability of cloned human FSH receptors. They are also profiting from the new techniques of combinatorial chemistry, a chemistry-based technology platform that generates large arrays of screenable compounds for rapid drug discovery that are, in effect, libraries of small organic molecules, peptides, or oligonucleotides. Nonsteroidal agents in general offer good opportunities for innovative companies, particularly those specializing in design of small molecules, drug delivery systems for proteins or large molecules, and in combinatorial chemistry.
Approaches to New Contraceptives for Females*
Recent advances in molecular and cellular biology have provided a better understanding of female reproductive processes—including oogenesis, follicular maturation, ovulation, fertilization, and implantation—than ever before. This knowledge will have major impact on the development of new contraceptives for women. The challenge now is to select from the many potential targets those that can be manipulated to achieve contraception with minimal or no impact on other organ systems; the more specific and more limited the systemic effects of a contraceptive are, the less likely they may be to produce the sorts of side effects that are troublesome in greater or lesser degree to many women. Table 4-1 summarizes those potential target areas the study committee identified as being particularly promising.
The cascade of events involving GnRH, LH, and FSH described above and depicted in Figure 4-1 is what regulates successful follicular development and ovulation. Recently, the GnRH receptors were cloned, an advance that will permit a wider range of possibilities for intervening in these processes. One possibility would be to suppress the receptor, either through agonists or antagonists, which would certainly halt follicular maturation and ovulation. However, this approach would also require steroid replacement to avoid unwanted effects elsewhere in the body. Another possibility would be to modify the intermittent or pulse-like rhythm of GnRH secretion in such a way as to exert a disproportionate effect on follicular development relative to steroid synthesis.
Another option would be to target FSH from the start. It is now considered likely that the growth factor activin plays a key role in maintaining FSH expression. Two activin inhibitors, inhibin and follistatin, have now been identified and shown to suppress both FSH production and follicular development following
systemic administration in animals. This interference was specific to activin's role in the reproductive system (DePaolo et al. 1991; Vale et al. 1994, 1990).
Once FSH reaches the ovary, there are still many ways to interfere with follicular maturation. A unique organ, the ovary contains hundreds of thousands of follicles—the sacs containing developing ova—that die naturally during a female's lifetime. Of the 400,000 follicles found in human females at puberty, for example, only about 400 will ever make it to ovulation, a process of attrition that is depicted in Figure 4-1 (see Appendix A). A better understanding of this process could lead to new contraceptive approaches.
Most ovarian cell death is caused by apoptosis, or programmed cell death. Researchers have now identified several substances in the ovary that affect apoptosis by acting either as follicular survival factors or as mediators of cell death. These substances include gonadotropins, steroid hormones, interleukins, and cytokines such as IGF- 1. Researchers have also have identified a number of transcription factors—factors controlling gene expression—that regulate apoptosis (Artini et al. 1994; Erickson and Danforth 1995; Hsueh et al. 1994).
Meiotic cell division, which allows for the combination of maternal and paternal DNA when two gametes meet, occurs only in male and female gonads. The process therefore provides a good target for contraception. One particularly promising point of intervention occurs when the ovary's primary oocytes are released from prophase I and allowed to progress to metaphase II (Grigorescu et al. 1994), an event regulated by a factor called maturation promoting factor (MPF). Further study could provide clues to new drugs that might interfere with follicular maturation (Dorce 1990).
Greater research into and understanding of the molecular and cellular events involved in oocyte maturation have provided some new and some unexpected targets. Among the unexpected are a class of ''orphan receptors," some of which may have endogenous ligands yet to be discovered, while others appear not to be ligand-dependent and rather respond to other metabolic influences or synthetic molecules. Many of these "orphans" are nuclear receptors active in gametogenesis and further study may provide important new reproductive leads (Becker-Andre et al. 1994; Chen et al. 1994; Heyman et al. 1992; Ikeda et al. 1994; Lala et al. 1992; Luo et al. 1994; O'Malley 1991; Shen et al. 1994).
Specific Targets
Contraceptive Targets Between Oocyte Development and Ovulation
The final stage of follicular development, follicular rupture, presents yet another promising contraceptive target. One factor essential to the process, the PGS-2 gene, could provide an ideal way to inhibit this one specific event while otherwise leaving the reproductive system alone (Morris and Richards 1993, 1995).
TABLE 4-1 New Horizons for Contraceptive Research and the Development of New Female Methods
|
Mechanism |
Description |
|
I. Mechanisms underlying the pulsatile release of GnRH (gonadotropin releasing hormone) and the differential regulation of FSH and LH synthesis and secretion. |
|
|
GnRH |
|
|
Long-acting agonist analogues |
Initial stimulation and eventual desensitization of the receptor and attenuation of receptor signal transduction. These agents inhibit fertility but also reduce steroid production and induce postmenopausal symptoms, and they therefore would require steroid replacement therapy. |
|
Receptor antagonists |
Immediate suppression of gonadotropin secretion, although higher doses are required than of the agonists. Potential for further optimization using high throughput GnRH receptor assays. |
|
Antagonists of SF-1 |
SF-1 is a transcription factor that controls the development of the gonadotrope. Antagonists to it could suppress the pituitary-gonadal axis; however, unless it were highly selective for oogenesis/ovulation, replacement therapy would still be required. |
|
FSH (follicle-stimulating hormone) Activin inhibitors Inhibin Follistatin |
Activin is probably the key tropic factor maintaining expression of FSH. Inhibin provides a negative feedback signal that shuts off FSH secretion; follistatin serves to limit all effects of activin. Small molecules could interfere with these functions. Inhibin suppresses only a subset of activin effects, so that the drugs could be relatively specific to the suppression of reproduction. |
II. Specific molecular events associated with maintenance of oocytes in prophase I and release of this block by the ovulatory stimulus only in mature follicles; determination of the suitability of these targets for pharmaceutical intervention. |
|
|
Meiosis |
One potential point of intervention is the regulation of progression of primary oocytes from prophase I to metaphase II. Synthetic analogues may be efficient as agonists, as well as antagonists, for pharmacologic manipulation of the onset of meiosis. MPF has been identified as an intracellular factor that regulates this transition. |
|
MPF (maturation promoting factor) |
|
|
OMI (oocyte maturation inhibitor) |
|
|
GVB (germinal vesicle breakdown) |
|
|
Mechanism |
Description |
|
III. Apoptosis research, to include the developing follicle as a target. Apoptosis research in diverse areas, including this one, could be mutually informative. An important research objective is better understanding of the mechanism by which the dominant follicle progresses while other developing follicles undergo atresia. |
|
|
FSH blockers |
Blocking the continuing maturation of preovulatory follicles or causing their premature demise using apoptotic factors is one potential contraceptive target. Gonadotropins, estrogens, growth hormone, growth factors, a cytokine, and nitric oxide act to ensure preovulatory follicle survival, while androgens, interleukin-6, and gonadal GnRH-like peptides are apoptotic factors. FSH, a gonadotropin, acts as a survival factor preventing the demise of early antral follicles, one of which is selected for final maturation and ovulation. Blocking FSH using an antagonist or a neutralizing protein would act in an ovary-specific manner, preventing ovulation. This method would therefore require physiologic replacement therapy. |
|
Deglycosylated FSH of FSH receptors |
|
|
Extracellular fragment antagonists |
|
IV. Examination of molecular and cellular aspects of follicular development and definition of the key players, their specific targets, and identification of endogenous and synthetic ligands should become major research objectives. |
|
|
Melatonin |
A hormone intimately involved in reproduction, its transcriptional effects are manifest through the orphan receptor RXR. A combination of melatonin and a synthetic progestin has been tested as a novel type of oral contraceptive preparation (see Chapter 3). |
|
Orphan receptors |
These receptors can be regulated by a synthetic pharmaceutical in a manner that impacts on relevant biological processes without knowing whether or not a given receptor has an endogenous ligand. |
|
GCNF (germ cell nuclear factor) |
A nuclear hormone restricted in expression to developing gametes and detectable in all stages of oocyte development. |
|
SF-1 (steroidogenic factor 1) |
May also play a key regulatory role in gametogenesis, in addition to being a positive transcriptional regulator of continued on next page |
|
Mechanism |
Description |
|
V. Elucidation of the factors that control follicular rupture: Inhibition of this process would be an ideal way to prevent fertilization and simulate a normal nonconception cycle with unaltered steroid patterns and levels and cycle length. |
|
|
PGS-2 (COX II) |
The LH surge induces the expression of the prostaglandin synthase 2 gene (PGS-2) that codes for an enzyme whose activity is essential for follicular rupture. If this enzyme were selectively inhibited, ovulation would be eliminated without the blocking of luteinization and the synthesis of steroid hormones. |
VI. A reexamination of established targets, for example, the steroid hormone receptors, would seem merited, given the recent observation that tissue-selective compounds can be developed to control specific subsets of genes that are regulated by the natural hormone. |
|
|
Estradiol and tamoxifen |
Both are ligands for the estrogen receptor but induce distinct conformational changes within the receptor with distinct biological consequences in vivo. |
VII. Expeditious examination of combinations of antiprogestins and other hormonal or antihormonal drugs should be undertaken, toward a method of emergency or once-a-month contraception. A more effective combination could be developed in a relatively short time frame. |
|
|
Oxytocic agents |
Drugs that stimulate uterine contractility alone or in combination with drugs that accelerate tubal transport and cause expulsion of the embryo from the uterus. |
|
Delivery |
Vaginal, instead of oral, administration of hormonal formulations based on single or combined steroids may increase efficacy and reduce gastrointestinal side effects of emergency contraception. |
|
Mifepristone |
An antiprogestin that can delay ovulation owing to temporary arrest of the growth of the dominant follicle, can offset the positive feedback of estrogen on the discharge of gonadotropin from the pituitary gland, and can disrupt the required secretory changes of the endometrium. This, in turn, could prevent either fertilization or implantation, depending on the stage of the menstrual cycle at which it is taken. Taken in combination with estrogen, it may be more effective and have fewer side effects. In addition, given in the time frame between ovulation and implantation, mifepristone prevents fertility. |
|
Mechanism |
Description |
|
Epostane and Azastene |
These enzyme inhibitors act to prevent progesterone synthesis. Epostane has been shown to terminate pregnancy in about 80 percent of women up to the eighth week of pregnancy; however, nausea was a common side effect. This approach may affect synthesis of adrenal steroids and is therefore problematic. |
|
Combinations |
Combinations of progesterone synthesis inhibitors and progesterone receptor blockers (mifepristone) might be more effective than either alone. A combination of an anti-estrogen and an antiprogestin might also be effective, given the objective of putting endometrial development out of synchrony with or making it hostile to the embryo. |
|
VIII. Studies should be carried out in nonhuman primates to develop the concept, and test the safety, of immunization against progesterone as a simple and easily reversible contraceptive method. |
|
|
Progesterone antibodies |
Active immunization against progesterone would result in exposure of the endometrium to unopposed estrogen. However, through administration of a synthetic progestin that does not cross-react with the antibody, during the last quarter of the cycle withdrawal bleeding would occur. |
|
IX. The most promising of the adhesion molecules should be studied to determine how essential they are for initial blastocyst attachment to the endometrial epithelium. |
|
|
Integrins Collagen, laminin, fibronectin, vitronectin integrin α4ß1 |
Changes in the expression of several integrins may define the putative period of uterine receptivity for blastocyst attachment. Blockade or disruption of this expression could provide a specific means of preventing implantation. |
|
High-molecular-weight (MW) glycoprotein |
A high MW glycoprotein involving N-acetyl-galactosamine and other determinants and secreted from the endometrial glands during the period of uterine sensitivity for implantation in humans, it is believed to be involved in the initial adhesion phase of implantation. |
|
Muc-1 (episialin) |
A member of the family of mucin glycoproteins and found in the endometrial epithelial cells of mouse uterus. Ways of decreasing it prematurely or delaying its down-regulation, necessary for implantation, could be the basis for a contraceptive approach. |
|
Mechanism |
Description |
|
HB-EGF (heparin-binding epidermal growth factor) |
May be important for establishing uterine receptivity for implantation and causing stromal cell proliferation. Its appearance can be blocked by antiprogestins and thus may be a good contraceptive target. |
|
Trophinin and tastin |
Although the significance of these factors in implantation remains to be determined, they may also be useful new leads for a contraceptive acting to prevent blastocyst attachment. |
|
X. Increased attention to research in growth factors and cytokines, particularly those shown to regulate endometrial functions and implantation. |
|
|
IL-1 (interleukin-1) |
Treatment of mice with IL-1R (receptor) antagonist during the preimplantation period prevented pregnancy. The importance of IL-1 remains to be seen, however, as attempts to repeat early trials have been unsuccessful. |
|
CSF-1 (colony-stimulating factor) |
May also be a critical factor for implantation. Breeding of homozygous mice lacking CSF-1 resulted in implantation failure. |
|
TNF-a (tumor necrosis factor a) |
Synthesis of this cytokine is induced through the gene regulatory action of CSF-1. TNF-a inhibits decidualization of human endometrial stromal cells in vivo; thus, the balance between CSF-1 and TNF-a may be critical for normal progression of the implantation process. |
|
LIF (leukemia inhibitory factor) |
If it can be shown that LIF is essential for implantation in species other than the mouse, then means to disrupt uterine LIF function for a short period should be sought. LIF secretion from human endometrial cell cultures peaks around the time of implantation in a conception cycle. LIF may provide an important lead in the development of specific antiimplantation agents. The most specific approach would appear to be through interference with binding of the specific LIFR a subunit to the LIF receptor complex. |
|
IGF-I (insulin-like growth factor) |
IGF-1, its receptor, and the IGF-binding proteins 1-4 are all localized to the endometrial epithelial cells and highest at the early- to mid-secretory phase of the cycle. These changes may be modulated by the embryo and are essential for implantation. |
|
FGFs (fibroblast growth factors) |
Involved in angiogenesis, disruption of the gene for FGF-4 in mice causes severe inhibition of the growth of the blastocyst inner cell mass and failure of pregnancy just after implantation. |
|
Mechanism |
Description |
|
XI. Development of nonpeptide GnRH antagonists would have utility both before and after fertilization. |
|
|
|
Nonpeptide antagonists are likely to be simpler and cheaper to produce and may be able to block the action of trophoblast GnRH in stimulating hCG secretion, which is necessary to support pregnancy. Other factors may also be involved in regulation of early hCG production and their identification and use to inhibit early hCG production could cause early pregnancy failure without disrupting menstrual cyclicity. |
In the current enthusiasm about new ovarian targets, researchers should not forget about new ways of looking at old targets, notably steroid hormone receptors. Such a reexamination is justified by the fact that it is now possible to develop tissue-selective compounds that affect a specific subset of genes regulated by these hormones. Particularly promising for the contraceptive field would be discovery of the targets of progesterone and estrogen receptors in the ovary. Such results could possibly lead to the development of tissue-selective modulators of oocyte maturation (Allan et al. 1992; McDonnell et al. 1995; Tzukerman et al. 1994).
Postovulation Contraceptive Targets
After ovulation, an ovum enters the oviduct (or fallopian tube), where it may or may not be fertilized by a spermatozoon. If it is, the fertilized egg progresses through the oviduct for another 72 hours, where it undergoes more mitotic cycles over the next 3-4 days, eventually forming a blastocyst (Croxatto 1995). The blastocyst attaches to the uterine wall, then undergoes several more steps before finally completing implantation. Thus, the consensus of many national and international medical and legal entities is that pregnancy does not begin until the completion of implantation of the fertilized ovum in the woman's uterus1 (Cook 1989). It is reasonable to believe, therefore, that agents that prevent that implantation would be more widely accepted than those that cause early pregnancy failure. From the perspective of current science, such agents are entering the realm of possibility with the discovery of new anti-implantation agents.
In many animals, administering hormones that cause embryos to be retained in the oviduct also blocks fertility. In humans, however, such interference can lead to life-threatening intratubal (ectopic) pregnancy and thus cannot be considered a contraceptive option. Another possibility may be speeding up the time it
takes a zygote to pass through the oviduct. While animal studies had indicated that premature passage through the oviduct would impede pregnancy (Adams 1980; Ortiz et al. 1991), this has not been the case in humans. Any contraceptive based on accelerating oviductal transport would also have to cause expulsion of the embryo by stimulating uterine contractility.
Potential anti-implantation targets fall into one of three categories: hormones, cell-adhesion molecules, and cytokines/growth factors. While results of experiments testing the importance of estrogen to implantation in animal models (primates) have been mixed, an absence of progesterone has inevitably produced pregnancy failure. There are three ways to stop the action of progesterone: preventing its synthesis using enzyme inhibitors, intercepting the hormone in circulation with specific antibodies, and blocking its action at the receptor level using an antiprogestin. All these methods have their drawbacks, and it has been suggested that combining the first and third may offer the most promise. All these processes offer opportunities for interference, yet even if effective combinations were to be designed, they would still have the disadvantage of being relatively nonspecific, an attribute that runs counter to the thrust of this report. As indicated in the preceding section, targets for contraceptives must be highly specific. (For research results on such methods, see Birgerson et al. 1987; Crooij et al. 1988; Gemzell-Danielsson et al. 1993; Ghosh and Sengupta 1993; Ghosh et al. 1994; Greene et al. 1992; Harper 1972; Selinger et al. 1987; Swahn et al. 1990.)
One of the first events in the implantation process is the blastocyst's attachment to the uterine epithelial surface, an event carried out by cell-adhesion molecules. Researchers have studied one family of such molecules, called integrins, in the human endometrium and concluded that blocking these molecules could provide a specific way to prevent implantation (Ilesanmi et al. 1993; Lessey 1994; Lessey et al. 1992 and 1994; Schultz and Armant 1995). A number of other cell-adhesion molecules are under investigation for their role in implantation (see, for example, Fukuda et al. 1995; Jentoft 1990; Kliman et al. 1995; Strous and Dekker 1992; Zhang et al. 1994 a and b). A potential problem with all these agents is that, unless they have a long biological half-life, it will be hard to determine the optimum time in the cycle to administer them.
It is only recently that the third category of biological agents—endogenous proteins called cytokines and/or growth factors—has been found to play a key role in implantation. Researchers have studied several of these, including interleukin-1 (De et al. 1993; Frank et al. 1995; Tabibzadeh et al. 1990; Tackacs et al. 1988; Simón et al. 1993a, 1993b, 1994), colony-stimulating factor1 (Pollard et al. 1991), and tumor necrosis factor a (Hunt et al. 1993; Inoue et al. 1994; Tartakovsky and Ben-Yair 1991). Perhaps the most promising work to date has been on leukemia inhibitory factor (LIF) (see, for example, Bhatt et al. 1991; Chen et al. 1995; Hilton et al. 1988; Stewart et al. 1992; Willson et al. 1992;
Yamamoto-Yamaguchi et al. 1986; Yang et al. 1994; Yang et al. 1995a, 1995b; Zhong et al. 1994).
In experiments where the gene for LIF was knocked out in mice, the animals, while fertile and otherwise healthy, could not maintain pregnancy because of implantation failure. While LIF's role in humans has not yet been established, there is preliminary evidence that, in rabbits, this cytokine is involved in implantation. In addition, LIF levels rise in human endometrial cells during the early and mid-luteal phase. If further research supports these results, LIF or its specific receptor subunit may make an excellent contraceptive target, although several concerns remain. These include significant structural similarities with other cytokines (suggesting a possible lack of specificity), uncertainty regarding the timing and frequency of administering the agent, and the impact on pregnancy should a LIF blocker be given too late.
Already developed for possible use before fertilization, agonists and antagonists of GnRH may also interfere with the hCG (human chorionic gonadotropin) secretion needed for luteal support and maintaining early pregnancy. While some animal studies have shown promise for agents causing luteolysis (premature demise of the corpus luteum, which interferes with progesterone secretion and thus maintenance of pregnancy), work with primates has so far produced no good leads. One alternative way to achieve the same effect would be by blocking progesterone synthesis by the corpus luteum. In rhesus monkeys, such functional luteolysis has been demonstrated following administration of either azastene or epostane during the luteal phase of the cycle (Schane et al. 1978; Snyder and Schane 1985); in pregnant monkeys, pregnancy was terminated (Schane et al. 1978).
In Chapter 3, dedicated to contraceptive technologies that have at least reached phase I in the research and development trajectory, we discussed the very small number of postcoital contraceptive options that are available on the current international market. We included in that group RU 486, the only member of the newly discovered2 group of compounds called "antiprogestins" to have become available for human use. As their categorical name suggests, these compounds are antagonists of progesterone, a steroid hormone which originates in the corpus luteum and without which pregnancy cannot be initiated or maintained (Van Look 1994). Finally, we also noted the prospects these compounds offer for intervening at various points in the female reproductive cycle, to prevent pregnancy before ovulation by inhibiting initiation of the ovulatory processes and, as well, to prevent pregnancy after ovulation up until the time of expected menses. Contraceptives designed to have effect during the postovulatory portion of the cycle are known as once-a-month contraceptives or menses-inducers and, as noted at various points in this report, are highly ranked on the women's agenda. The many processes taking place between ovulation and menses-or between ovulation and implantation if fertilization has occurred-offer abundant possibilities for once-a-month treatments (see Figure 4-2).
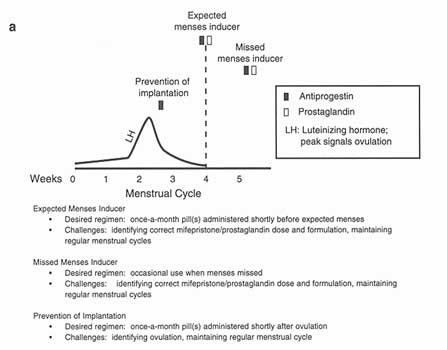
Figure 4-2
Possible regimens for using antiprogestins as menses inducers or as once a-month pills.
Sources: a—Adapted from World Health Organization. Challenges in Reproductive Health Research: Biennial Report, 1992-1993. Geneva: World Health Organization, 1994. b—Reprinted from Institute of Medicine. Clinical Applications of Mifepristone (RU 486) and Other Antiprogestins: Assessing the Science and Recommending a Research Base. MS Donaldson, L Dorflinger, SS Brown, and LZ Benet, eds. Washington, DC: National Academy Press, 1994 (Figure B12.5, p. 272).
Menses-inducers can be divided into two categories: those that are used regularly just before the onset of expected menstruation (expected menses inducers, or EMIs) and those used only when the menstrual period does not occur (missed menses inducers, or MMIs). There are crucial differences between the two in terms of legal status, frequency and ease of use, and acceptability. In general, EMIs are more acceptable in more settings than are MMIs, though the acceptability of EMIs often depends on their mechanisms of action: Agents that prevent ovulation are far more acceptable than those which interfere with implantation or terminate early pregnancies. EMIs are obviously taken more frequently (and, perhaps, unnecessarily) than MMIs, which would be taken at most three or four times a year. Finally, owing to natural, month-to-month variability in menstrual cycling, EMIs are more difficult to use; at the same time, for some women
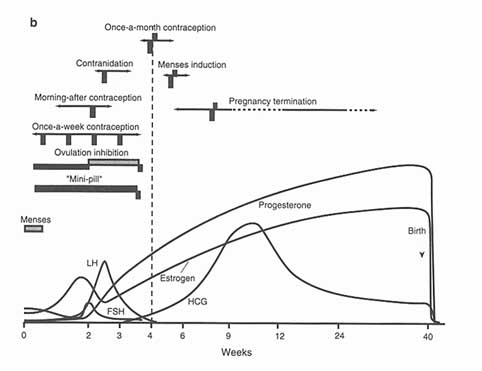
their inability to predict their next menstrual period precisely is a challenge for the use of MMIs (Van Look 1994).
Expected menses inducers work by ensuring the monthly sloughing of the endometrium at the time of expected menses, regardless of whether or not an embryo has implanted. Advance in developing such agents has been constrained by lack of understanding of the mechanisms that start and stop endometrial bleeding and by the political and religious unacceptability of the EMI approach in certain cultures and countries. Nevertheless, as indicated in Chapter 3, work with mifepristone as a possible component of an EMI has begun to produce further understanding. While the failure rate in initial studies was high (17 to 19 percent of women remained pregnant), more studies are under way combining mifepristone with the prostaglandin misoprostol. These results should be available in 1996 (WHO/HRP 1995). A related—and important—issue is the long-term safety of antiprogestin treatment. If these compounds are to be used over extended periods of time as oral contraceptives, answering the safety question will be as critical as establishing efficacy. Researchers should also consider whether novel delivery systems—such as transdermals or implants—would offer any special advantages for these compounds.
Approaches to New Contraceptives for Males*
While the biology of the male reproductive system places certain limitations on contraceptive options for men, several recently discovered—and unique—cellular and molecular events within the system have opened up new possibilities (see Table 4-2). While there appears to be little or no research on the characteristics of what men would want in a contraceptive, objectively identified criteria for male contraceptives might not be unlike those objectively identified for female contraceptives: safe, effective, reversible, ''user-controlled," and quickly effective. One criterion that appears frequently in the literature is that a contraceptive for men should have no impact on libido, a criterion that rarely if ever appears in the literature in connection with contraceptives for women.
Specific Targets
Long-loop Feedback System
Through a process that closely parallels oogenesis in the female, spermatogenesis is controlled from the brain with the production of gonadotropin-releasing hormone (GnRH) by the hypothalamus. GnRH in turn stimulates the anterior pituitary to secrete luteinizing hormone (LH) and follicle-stimulating hormone (FSH), both of which are required for normal sperm development in the testis and acquisition of fertilizing capacity.
Several studies have focused on the so-called long-loop feedback system as a contraceptive target (Wang et al. 1994) (see Table 4-2). The earliest, which tested the efficacy of androgen injections to disrupt the system (Handelsman et al. 1992), successfully achieved reversible azoospermia after a period of months. While researchers have attempted a similar strategy with GnRH agonists and antagonists, they have had to replace androgens to maintain the subjects' libido (Bagatell et al. 1993).
Targeting FSH alone should avoid this problem (Zirkin et al. 1994). Particularly promising is the hormone's ß subunit, which, unlike its a subunit, is specific to FSH and thought to be responsible for receptor specificity. New research techniques have revealed many of the molecular details surrounding FSH's interaction with its receptor. This new understanding suggests several specific tactics for FSH interference.
Sperm Development in the Testis
Once spermatogenesis is initiated in the testis by reproductive hormones,
|
* |
Please refer to Appendix B for the full text of the authored and fully referenced paper on which this section is based. |
there remain many potential ways to interfere before the process is complete. One attractive target is meiosis—which halves the number of chromosomes in a gamete's nucleus so it can combine with another gamete at fertilization—a process that occurs only in male and female gonads. A particularly promising possibility would rely on c-mos, a unique germ cell protein known to be involved in meiotic arrest in females. While in males, unlike females, meiosis is ordinarily a continuous process (and c-mos is expressed too late to have an impact), getting the protein expressed early may be able to stop meiosis and therefore sperm development.
Another possibility would be to genetically manipulate sperm cells before they are fully mature. Feasible strategies for cont
raceptive attack using genetherapy techniques include the disruption of normally functioning transcription factors, proteins that bind to DNA with the effect of switching genes on and off. While the field is still a new one, researchers have already identified several transcription factors that are specific to germ cells (Chen et al. 1994). Inappropriate expression of any of these factors (either absence when they are needed or presence when they are not) is likely to disrupt sperm differentiation.
As an alternative to transcription factors, classical genetics techniques may offer options for interfering with sperm development. Many of the most important processes in meiosis and gamete production are conserved throughout all animal phyla, and we may be able to use what we know about other species. Genetic studies of the well-known Drosophila, for example, show that mutations in any of 400 of the female's 4,000 genes cause sterility. Similar studies in the male Drosophila could identify a similar set of potential targets.
Postmeiotic cells, or spermatids, may provide the best contraceptive target in the testis. Several key events occur nowhere in the body but in these cells. One is the formation of the acrosome, a structure at the head of the sperm containing enzymes that will allow it to penetrate the egg membrane. Another is the formation of the flagellum, or tail, which allows the sperm to swim. As researchers learn more about the cellular and molecular processes underlying these unique events, specifically disrupting them to achieve contraception should become a very real possibility.
A final category of potential targets in the developing sperm arise from the plasma membranes on these cells' surfaces. Like the sperm cell itself, components of these membranes are highly compartmentalized, and at least some of them are thought to play a key functional role. Researchers have identified several sperm proteins thought to be important in fertilization and have begun to map the location of these on the sperm membrane. Because the absence of a particular sperm protein in a given region of the membrane would likely lead to dysfunctional sperm, this line of research has opened up some potential new contraceptive options (Eddy and O'Brien 1994).
TABLE 4-2 New Horizons for Contraceptive Research and the Development of New Male Methods
|
Target |
Mechanism |
|
I. Long-loop feedback mechanisms |
Injection of androgens accomplished reversible azoospermia, as did injection of GnRH agonists and antagonists combined with androgen replacement. |
|
II. Inhibition of FSH secretion and/or action |
It is generally agreed that FSH is required for spermatogenesis along with testosterone; therefore, a method targeting FSH or its receptor could disrupt spermatogenesis without affecting steroid hormones. The ß subunit of FSH is unique and apparently responsible for receptor specificity, as well; FSH binds only to a tetrameric form of FSH receptor and the interaction between the two is complicated. Possible approaches include: |
|
FSH (follicle-stimulating hormone) |
* Reducing or eliminating the secretion of FSH. This would, however, require development of improved stable androgens, GnRH analogues, or activin antagonists, and their appropriate delivery systems. * Targeting FSH-ß for immunologic destruction or neutralization through antibody binding in circulation. Although this method would afford great selectivity, it could potentially destroy the anterior pituitary, the site of FSH synthesis by cell-mediated immunity. A potential solution is the use of a B-cell epitope as a target, if one exists. |
|
FSH receptor |
* Use of anti-FSH receptor antibodies or mimetopes. This method could potentially backfire were the anti-FSH agent itself to act as a receptor activator. * Prevention of proper intracellular assembly of the tetrameric form of the FSH receptor. It would be necessary to determine how to prevent such assembly; the field of protein trafficking offers some hope in this area. |
|
III. Control of Meiosis |
Meiosis in males is a continuous event, whereas in females, it is halted at two points: the end of prophase I and during metaphase II. The responsible mechanisms for these pauses could possibly be used to arrest meiosis in the male. The arrest at prophase I is dependent on cAMP-dependent protein kinase A, which has great importance throughout the human system and is, therefore, not a likely candidate. However, |
|
c-mos |
arrest of metaphase II appears to involve c-mos, which is a unique germ cell protein. In males, its DNA is transcribed during meiosis but not translated until spermiogenesis begins. Could this translation begin earlier, during meiosis, it could potentially be a very powerful approach to fertility regulation. |
|
Target |
Mechanism |
|
IV. Genetic manipulation of sperm |
In standard gene therapy, a stem cell is targeted and new genes are inserted into these cells to overcome the effects of damaged genetic material; used contraceptively, this method could be used to insert a defect for genetic material responsible for sperm-surface components specific to spermatogonia. A certain complexity arises when considering the introduction of the new genetic material, however. Since the primary spermatocytes are protected by the blood-testis barrier, a twostage delivery system using the Sertoli cells as an intermediary would be necessary. Potential inserts would include: * Agents that disturb the functioning of transcription factors. * Replacement of nuclear histones with protamines could be targeted, since this process is specific to developing sperm cells. * Other genes responsible for fertility or infertility, using Drosophila as a model. Genes that have been conserved throughout the phyla may be identified in Drosophila and located in humans. |
|
V. Inhibition of acrosome and tail formation |
These processes are unique to the developing sperm and are thought to occur independently from hormonal regulation. * The acrosome is a product of the Golgi apparatus and first appears morphologically in spermatids. It is essential for fertility. Further exploration into its origins and development is necessary to isolate potential contraceptive targets. * Centriole attachment and tail formation occur only in spermatids, although centrioles have the potential to germinate flagella or cilia in all cells. Isolation of the causes and mechanisms of this differential development could provide unique contraceptive options. |
|
VI. Alteration of spermsurface proteins |
During spermatogenesis, sperm acquire a highly polarized morphology with a quadripartite structure in which lipids and proteins are organized into highly regionalized domains which first appear during spermiogenesis. While some fertilizationrelated proteins are restricted to certain membrane domains, others are uniformly distributed, such as fertilin and PH-20. * Since germ cells invest heavily in elaborating and maintaining the organization of proteins within and on the sperm |
|
Target |
Mechanism |
|
|
membrane, it is likely that interference with this system in any way could lead to the development of dysfunctional sperm. * Disruption of the timing of protein expression could also disorder the intricate organization of the sperm membrane. This could include fertilin, but also any other fertilizationrelated protein, or even, potentially, nonfertilization-related proteins, since the entire cascade of expression seems to be so delicately balanced. |
|
VII. Interruption of epididymal function |
The one- to two-week period that sperm spend traveling through the epididymis is essential to ultimate fertility function. The functional changes that occur—vigorous motility and ability to interact with the zona pellucida and egg membrane—are androgen dependent; however, the epididymis itself does not appear to synthesize hormones, nor do its functions appear to be linked to other biological functions. In addition, sperm are fully genetically formed by the time they reach the epididymis. For these reasons, targeting the epididymis seems potentially powerful with little adverse side effects. * Several proteins are secreted into epididymal fluid and are subsequently taken up onto the sperm surface. If any of these are necessary for sperm maturation, they could be beneficial targets. The creation of knockout mice may provide a way to pursue this possibility. * Similar to the use of organ-specific anti-estrogens in females, it may be possible to create epididymis-specific antiandrogens that could block the necessary action of androgens in epididymal sperm development. * Immunoneutralize epididymal-specific proteins. |
|
VIII. Regulation of the inhibition of immune reactions by sperm |
In many species, sperm are endowed with proteins delivered by the seminal vesicle that are immunosuppressive and make the sperm essentially immunologically silent to the female's immune system. If this event occurs in humans and could be blocked, it could offer a contraceptive target. |
|
IX. Induction of premature acrosome reaction |
Delivery of a ZP3 mimic to the sperm at some point prior to ejaculation could induce the acrosome reaction prematurely and thus make the sperm ineffective at penetrating the egg's outermost layers. However, in nature, the acrosome reaction typically occurs after spermhead capacitation, so that this |
|
Target |
Mechanism |
|
|
technique may not be effective. In addition, the quantity of ZP3 mimic would have to be quite large in order to affect the multitude of sperm cells in, or emerging from, the epididymis. However, a substance produced by a well-known mutation in the T-locus in mouse sperm, which results in the uneven success of t-bearing sperm versus T-bearing sperm, owing to the ability of the t-bearing sperm to cause a premature acrosome reaction in the T-bearing sperm, may offer a clue to the utilization of this potential contraceptive method. |
|
X. Inhibition of gamete interaction |
PH-20, fertilin, and ZRK (a ZP3 receptor), among other sperm-specific proteins required for gamete interaction, could be targeted for suppression or blockade via epididymal manipulation, "conditional knockout," or immunosuppression, ultimately resulting in the inability of the sperm to "communicate" and thereby fertilize the ovum. |
Sperm Maturation in the Epididymis
When sperm leave the testis, they must undergo more morphologic and biochemical changes before they are capable of fertilization. These final developments take place in the epididymis, which also serves as a sperm storage reservoir. Because this organ is where sperm specifically acquire their ability to fertilize eggs, it makes a promising male contraceptive target. Two advantages of contraception aimed at the epididymis are that this organ does not synthesize hormones and sperm enter it genetically fully formed; contraception aimed here is therefore less likely to produce adverse hormonal or genetic side effects than those targeting the testis.
During maturation, much of the sperm surface appears to be remodeled. These changes are mediated by products secreted by the epithelium of the epididymis. Although the precise components of this epididymal fluid have not been fully identified, it is known that its complex composition changes dramatically along the length of the tubule.
There are several possible approaches to interfering with sperm maturation. One would be to modify the expression of epididymal proteins. Researchers have identified several proteins that are secreted into epididymal fluid and subsequently adsorbed onto the sperm surface in humans and other mammals. If it is determined that any of these proteins are essential to sperm maturation, blocking their expression would constitute an excellent approach. Only recently have techniques that make this approach feasible become available (Gu et al. 1994). One way this could be accomplished would be to specifically knock out the gene
coding for the protein, a strategy that has been used successfully in mice for nonreproductive proteins.
Another way to block sperm maturation would be to administer epididymis-specific anti-androgens. Androgen is essential for the normal functioning of this organ; without it, the size of the epididymis decreases dramatically and sperm fail to mature. Recent progress in administering organ-specific antiestrogens makes this approach seem feasible today. If it works, the strategy could become one of the best options for reversible male contraception.
Finally, once epididymis-specific proteins critical to sperm maturation are identified, researchers should be able to target and neutralize these proteins using immunocontraceptive methods; a more detailed discussion of immunocontraception follows below and in Appendix B, which this section summarizes. Antibodies produced by such a method should have greater access to epididymal cells than testicular cells because of the lack of a blood-testis barrier.
Gamete Interaction
A final category of strategies to achieve male infertility would be to interfere with a sperm cell's ability to interact properly with the egg. A possible approach within this category—as yet untested—would be to block immunosuppressive proteins that attach to the sperm surface in many species (Hamilton 1993). While researchers do not yet know if similar events take place in humans, interfering with whatever mechanisms allow sperm to evade the female immune system would offer interesting contraceptive possibilities.
One of the most important determinants of successful fertilization after a sperm and egg meet is the proper timing of the acrosome reaction, the release of enzymes for the sperm's acrosome that allow it to penetrate the egg's protective coat, or zona pellucida. The early release of these enzymes, when sperm and egg are still some distance apart, results in infertility.
Recently, researchers have identified some of the sperm proteins that are important in triggering the acrosome reaction. One of these, called zona receptor kinase (ZRK), may be a receptor for the egg protein ZP3. If scientists could develop a ZP3 mimic and deliver it to the male reproductive tract, such an agent might be able to prematurely induce the acrosome reaction, resulting in infertility. If the agent could be delivered to the epididymis, it would have a long period of time to have effect.
There are two potential problems with this approach, however. One is that ZRK binds, in nature, to ZP3 after full sperm capacitation within the female reproductive tract—a poorly understood process that involves changes in the sperm cell membrane—and introducing a ZP3 mimic before this stage may not be sufficient to induce the acrosome reaction. Second, because sperm are present at such high concentrations in the epididymis, any agent that acts there would
also have to be present at a high concentration. On a more positive note, researchers have long known that a mutation on the T-locus of mouse sperm provokes a premature acrosome reaction in that species (Brown et al. 1989). Further study of this phenomenon may provide clues to achieving the same effect in humans.
Beyond the acrosome reaction, there are several other events that must take place at the proper time in order for egg and sperm to fuse successfully. These include sperm penetration of the egg's cumulus layer, primary binding to the zona pellucida (both of which take place before the acrosome reaction), secondary binding to the zona pellucida, penetration through the zona pellucida, and fusion of the sperm and egg plasma membranes (which occurs after the acrosome reaction). Researchers have recently identified several sperm proteins, including ZRK, PH-20, and fertilin, that are involved in the successful completion of all these events. Because all these steps are essential for fertilization, blocking any of them through application of new technologies offers powerful contraceptive potential.
Approaches to Immunocontraception for Males and Females*
The immune system, which under normal circumstances does not interfere with normal function of reproductive tissues, can be directed to respond to some proteins found in the reproductive system and interrupt reproductive processes. Determination of the best molecules to use for this purpose and definition of the effective dose and best manner of delivery of these proteins is an active and promising area of contraceptive research.
Unlike vaccines against most diseases, an immunocontraceptive would have to be reversible. However, it would not necessarily be given by injection; in fact, oral and vaginal preparations have shown as much promise to date as injectables. Yet while research on contraceptive immunogens has produced a number of promising results, as of this writing no such product, for humans or animals, has yet appeared on the market.
A reversible form of immunocontraception would offer several advantages over existing contraceptive methods: low cost, ease of use, infrequent administration for a long period of effectiveness, confidentiality and, above all, specificity. For an undetermined number of women and men, some or all of these attributes would be distinct advantages. For others, an immunocontraceptive might be seen as unacceptably systemic, despite certifiable specificity, and there also might be concerns about the uncertainties and individual variations in the lag time to activation and in reinstatement of fertility.
|
* |
Please refer to Appendix C for the full text of the authored and fully referenced paper on which this section is based. |
The pathway to developing an immunocontraceptive is similar to that followed in traditional vaccine development: (1) discovery and characterization of appropriate immunogens (the proteins against which the body will mount an immune response); (2) developing methods for reliably reproducing those immunogens; (3) producing and purifying the proteins in the lab; (4) formulating doses; (5) testing on small animals and primates for immunogenicity, safety, and efficacy; (6) evaluating mechanisms of action; (7) conducting human trials for immunogenicity, safety, and efficacy; and (8) developing diagnostics to monitor responses of recipients.
Of these eight steps, the first is perhaps the most complicated. It is also the stage where many research efforts on contraceptive immunogens stand today. Virtually all reproductive processes—including gamete production, gamete transport, gamete interaction, blastocyst transport, and implantation-—can be accessible to immune intervention. After targeting one process, selecting the most promising immunogens from among all possibilities can be a grueling task.
Specific Immunogens
Several criteria can help with this selection process (Griffin 1990). To make a good candidate, an immunogen should be essential to the reproductive process, act only on desired tissues, be accessible to the immune system, stimulate a sufficient number of neutralizing antibodies, be present in transient or low concentrations, and be amenable to synthesis in large quantities in the laboratory. Researchers must also have homologous animal models on which to test the candidate immunogen.
Immunogens studied for their contraceptive potential to date fall into one of four categories: (1) immunogens of the early conceptus, (2) reproductive hormones, (3) egg-surface antigens, and (4) sperm-surface antigens (see Table 4-3). Of these, those that act to prevent pregnancy before an egg is fertilized (the last three categories) have the greatest potential for acceptability. While both the male and female systems offer abundant potential for immunocontraception, research efforts to date have focused largely, though not exclusively, on methods of immunizing women against fertility or early pregnancy.
Immunogens of the Early Conceptus
Some of the most advanced immunocontraceptive products are based on human chorionic gonadotrophin (hCG), a hormone released by the early embryo that is involved in several events crucial to maintaining early pregnancy, including maintenance of the corpus luteum, progesterone production and, perhaps, implantation. To avoid undesirable side effects, researchers have focused on the hormone's beta (ß) chain (either the entire chain or portions of it) rather than on
its alpha chain (a), which is shared by several other hormones (Alexander 1992; Kharat et al. 1990).
The hCG-based immunogens have come from both natural and synthetic sources. Natural hCG formulations (derived from pregnancy urine) are under intensive study at India's National Institute of Immunology (Talwar et al. 1981; Talwar et al. 1990). In phase II clinical trials, researchers there have demonstrated an adequate immune response in 80 percent of the women tested, a response that lasted for about a year (Talwar et al. 1992 and 1994). While this formulation appears safe so far, there is a lag time of about three months between its administration and contraceptive effect, leaving women unprotected during this period. Sponsored by the World Health Organization, research on synthetic hCG vaccine formulations have progressed to phase I clinical trials, and phase II trials are now planned. Human female subjects produced significant levels of antibodies to the synthetic hCG (Jones et al. 1988; Jones 1990; Stevens et al. 1981; Stevens et al. 1990).
Egg-surface Antigens
Researchers thus far have identified three proteins on the zona pellucida, or outer membrane of ovulated eggs. Called ZP1, ZP2, and ZP3, formulations of these proteins have been tested as contraceptive immunogens in mice, dogs, rabbits, and monkeys. In all four species, fertility was significantly inhibited (Mahi-Brown et al. 1988; Millar et al. 1989; Skinner et al. 1984; Sacco et al. 1990).
However, a serious drawback of all these formulations has been a severe autoimmune reaction leading to premature ovarian failure. While such an unacceptable outcome has considerably dampened enthusiasm for a zona pellucida-based immunogen, some research groups are trying to get around the problem by identifying epitopes of the egg proteins that would induce antibody production without inducing a T-cell response (East et al. 1985; Rhim et al. 1992; Taguchi and Nishizuka 1980; Tung et al. 1987).
Sperm-surface Antigens
Sperm antigens offer at least two theoretical advantages over egg antigens. First, as proteins found only in sperm, they would have no chance of causing autoimmune reactions in women. Second, it may be possible to use the same antigen as a contraceptive immunogen for both men and women.
A woman's immune system could attack and incapacitate sperm at several points as they progress through the reproductive tract, including the vagina, cervix, uterus, and oviduct. Researchers have thus far identified and begun to study a number of different sperm antigens (see Table 5-6). Among the most advanced studies are on PH-20 (Phelps and Myles 1987; Phelps et al. 1988;
TABLE 4-3 Identified Candidate Immunogens for Contraceptive Formulations
|
Immunogen |
Development and Specifications |
|
|
I. Reproductive Hormones as Antigens |
||
|
GnRH |
Rise in anti-GnRH antibodies would reduce the levels of GnRH, LH, and FSH; steroid production would decline; and spermatogenesis would cease. The decline in androgens would result in a negative effect on libido and secondary sexual characteristics, and so this method would require supplementation with testosterone. The effects of GnRH immunogens in rats appear to be reversible. Because the immunogen causes shrinkage of male sex accessory glands, it offers a possible treatment for prostatic carcinoma or benign prostatic hyperplasia. |
|
|
GnRH-DT |
Potential prolongation of the anovulatory period occurring during postpartum amenorrhea induced by lactation. A phase I human immunogenicity trial is under way in two centers in India in postpartum women. |
|
|
FSH |
Sheep FSH |
Causes acute oligospermia, with the sperm that are produced being reduced in numbers, immature, and of poor quality, rather than complete azoospermia. This immunogen would require no exogenous androgen supplementation, has shown no toxicity in rats and monkeys, and is reversible. One of the more promising reversible male contraceptive options, the formula is likely to be based on recombinant proteins or synthetic peptides based on human or primate FSH, rather than the sheep hormone formulas currently in use. |
|
II. Egg-surface Antigens |
||
|
ZP1, -2, and -3 |
ZP3 has been shown to be an effective immunocontraceptive in rabbits and marmosets. However, ovarian pathology occurs and might lead to premature ovarian failure in humans, or even induce menopause. Tests in mice with a synthetic peptide corresponding to amino acids 328-342 of ZP3 have been shown to induce autoimmune oophoritis. Further research could produce a zona-based immunogen that could inhibit sperm-zona interaction but not cause ovarian disease (B cell epitopes rather than those which elicit T cell responses). |
|
|
Immunogen |
Development and Specifications |
|
|
III. Sperm-surface Antigens |
||
|
PH-20 |
Immunization in female guinea pigs against this integral membrane protein of both the plasma and inner acrosomal membranes of guinea pig sperm has resulted in 100% contraceptive efficacy in early trials. PH-20 is likely a zona pellucida binding protein. Primate testing of a PH-20 immunogen derived from cDNA cloning is likely in the coming year. |
|
|
LDH-C4 (LDH-X) |
This protein is found in the mature sperm associated with the midpiece cytoplasm and, according to some reports, on the plasma membrane. Three baboon trials (of native mouse LDHC4 or synthetic peptides from human LDH-C4) have been conducted and shown contraceptive efficacy of 75-80%. Deleterious side effects have not been noted and the contraceptive effect is reversed within one year after the last immunization. This antigen is a likely candidate for inclusion in a multideterminant formulation. |
|
|
RSA-1 |
Hspa18 |
RSA-1 fulfills several criteria of a zona binding protein. Hspa18, the human homologue, has been localized on both the human sperm head and tail. Trials with recombinant forms of this antigen from the human (hSp17) and mouse (mSp17) have demonstrated a significant effect on reducing fertility. |
|
hSp17 |
||
|
mSp17 |
||
|
Tcte-1 |
sp56 |
Egg-binding protein that interacts with ZP3. Tcte-1 and sp56 have the same molecular mass and sp56 was previously identified as an egg binding protein. Sufficient antibodies produced in the oviduct might block sperm binding or induce lysis. |
|
SP-10 |
Human sperm protein associated with the acrosomal matrix and membranes of mature sperm. Sufficient titers of anti-SP-10 antibody must be generated in the oviductal fluids to agglutinate or lyse the relatively few acrosome-reacted sperm, thus inhibiting sperm/egg interaction. It is not clear yet if this is possible. |
|
|
Acrosin |
Antigen restricted in the mature sperm to the acrosmal membrane. |
|
|
Others |
99 other sperm or sperm proteins have been assigned immunocontraceptive candidacy, based on sperm surface labeling. |
|
|
Immunogen |
Development and Specifications |
|
|
IV. Immunogens of the Early Conceptus |
||
|
hCG |
hCG-tt (tetanus toxoid carrier) |
Immunogen would induce formation of antibodies that inactivate the biological activity of hCG and thereby interrupt corpus luteum maintenance, endometrial receptivity, and thereby disrupt implantation. |
|
hCGß subunit vaccines |
Beta-subunit-based vaccines offer the possibility of necessary specificity. In trials, menstrual regularity is maintained and ovulation is undisturbed. |
|
|
HSD |
ßLH-DT (heterospecies dimer-ovine LH a subunit and human hCG ß subunit) |
In trials with HSD, protective levels are only reached in 80 percent of women and 3 months is required for protection to be conferred. Potential delivery methods such as slow release biodegradable microspheres or administration of recombinant antigen through vaccinia viruses may increase levels of protection. This approach is important conceptually: it shows that if sufficient antibodies to a contraceptive antigen are reached, infertility results. |
|
Synthetic hCG Peptide |
Synthetic oligonucleotide corresponding to the amino acid sequence 109-145 of the C terminus of ß hCG. Conjugated to diphtheria toxoid. Similar mode of action as above, producing antibody to hCG. |
|
Primakoff et al. 1985 and 1988), LDH-C4 (Goldberg 1975 and 1977; Hogrefe et al. 1987 and 1989; Lee et al. 1982; Wheat and Goldberg 1983 and 1985), RSA-1 (Lea et al. 1993; O'Rand et al. 1993a, 1993b; O'Rand and Widgren 1994; Yamasaki et al. 1995), and SP-10 (Anderson et al. 1987; Herr et al. 1990a, 1990b; Kurth et al. 1991). Each of these antigens acts at a different point or in a different manner on those sperm-egg interactions. PH-20, for example, functions at two stages: first, helping sperm penetrate the cumulus cells that surround the egg and, second, binding to the zona pellucida. In guinea pig studies, PH-20 immunizations have induced reversible infertility in both males and females. Recently, researchers sequenced PH-20 and found that the protein is the same in guinea pigs, mice, monkeys, and humans. They have now cloned and sequenced the monkey protein and are beginning studies to test its efficacy as a contraceptive in primates.
Ideally, a sperm immunogen would induce an immune reaction against all
sperm-surface domains: the tail, midpiece, head, and perhaps even the inner-acrosomal membrane, which forms a major part of the head's anterior surface after the acrosome reaction. While inner-acrosomal antigens alone would offer the advantage of a highly specific, precisely timed target, they would also have to stimulate antibodies at a high enough level to affect the antigens within a narrow window of time. For that reason, focusing on surface antigens may make more sense at present. Because no single antigen may provoke a strong enough immune response, researchers working on different proteins may ultimately combine their efforts to produce one formulation containing several sperm immunogens.
Multideterminant Immunogens
In research less advanced than in the case of hCG, the reproductive hormone gonadotrophin-releasing hormone (GnRH) has shown promise as the base of contraceptive immunogens for both men and women. In research with male rats, these formulations resulted in a significant suppression of spermatogenesis that proved to be reversible. One drawback in the male is that it also affects libido and secondary sexual characteristics and would therefore have to be supplemented with testosterone (Ladd et al. 1988 and 1989). In females, these formulations have been tested in primates, with mixed results (Talwar et al. 1993), and phase I human trials are currently under way in India.
More promising as a male contraceptive are immunogens based on the hormone FSH. In trials with male monkeys, FSH immunizations caused significant oligospermia (70 percent reduction in sperm count), which resulted in virtually zero fertility (Moudgal et al. 1988a, 1988b, 1992; Moudgal and Aravindan 1993). This effect was reversible, and toxicity studies in both monkeys and rats have shown no complications. A clear advantage of the FSH immunogen is that, unlike GnRH, it does not require androgen supplements to maintain libido and secondary sexual characteristics.
In immunocontraceptive research to date, the best efficacy results have been at the level of 80 percent (for the hCG-based immunogen) and 75 percent (for the LDH-C4-based formulation). Because both these percentages are well below the 95 percent efficacy of today's oral contraceptives, no formulation so far has provided a significant improvement over current contraceptive methodologies.
These results have led many researchers to believe that the ultimate contraceptive immunogen may turn out to be a combination of different immunogens, possibly hCG and one of the sperm proteins. The chances of developing such a multideterminant formulation could be enhanced by changes in current FDA requirements to test individually for safety and efficacy each component of any new drug containing several different compounds.
Several recent areas of research should contribute significantly to the development of an effective immunogen. In the area of antigen delivery, for example,
researchers have inserted the sperm antigen SP-10 into live, attenuated salmonella bacteria. When administered through the gut to female mice, this novel formulation induced an effective gamete-specific immune response (Curtiss 1990; Srinivasan et al. 1995).
Another interesting possibility comes from recent research on the direct injection of DNA rather than of an antigen. While not yet attempted with DNA encoding reproductive molecules, this work on so-called ''naked DNA immunization" has spawned a paradigm shift in the broader field of vaccine research that may yet have a major impact on the field of immunocontraception (Wolff et al. 1990).
Mucosal Immunity*
In females, the vaginal and cervical mucosa are the first line of defense against invasion by foreign molecules. Given proper reinforcements, this natural barrier could be used to block the successful entry of both microbial pathogens and sperm. The mucosal membranes of the body include the respiratory tract, conjunctiva (inner surface of the eyelids), intestinal tract, mammary glands, and the urogenital tracts of the male and female. The surface area of this complex system is nearly 400 square meters and, within it, nearly 80 percent of all immunoglobulin-producing plasma cells reside in the intestinal mucosa, making this organ the largest reservoir of immune cells in the body. Because stimulation of the mucosal immune system at one site could elicit an immune response at a distant site, researchers believe that oral administration of immunogens could lead to effective localized immunity in the urogenital tract (Haneberg et al. 1994; Lehner et al. 1992 and 1993; Ogra and Karzon 1969; Ogra and Ogra 1973). The most promising achievement would be a vaccine that produces both a local as well as a systemic immune response that would in turn supplement the mucosal immunity.
An example of where early work is going on at this interface is Reprogen's attempt to define the molecular mechanisms involved in fertility and the mucosal immune function of the urogenital tract, toward development of interventions that are both immunocontraceptive and anti-infective. One foundation concept is that, in about five percent of infertile couples, at least one member of the couple produces an antibody that binds to the male's sperm cells and blocks activity; antibodies are found in both the cervical and male urogenital mucosa. Thus the developmental effort is toward formulating sperm head and tail proteins that can induce both a strong IgA secretory and a systemic antibody response, for use in contraception. The other piece of the foundation is identification of antigens on
|
* |
Please refer to Appendix D for the full text of the authored and fully referenced paper on which this section is based. |
gonorrhea and chlamydia organisms that will generate both IgA and serum antibodies to block bacterial attachment and infection (Robbins-Roth 1994).
Three general requirements exist for successful oral immunization: (1) a formulation that achieves survival in the mucosal environment, (2) an adequate delivery of antigen to inductive sites, and (3) stimulation of an appropriate protective immune response. Researchers face several challenges to developing an oral immunization (e.g., Challacombe and Tomasi 1980; Mowat 1987). These include low absolute absorption of antigen via oral administration (meaning that a large initial dose is required) and the possibility that the systemic immune system may not respond to oral immunization. Nevertheless, because orally administered immunogens could have fewer adverse side effects than those delivered systemically, research in this area is considered important. Isolation of appropriate immunogens is a critical step in the overall process but, as with vaginal formulations, the delivery system can play a key role in the effectiveness of a mucosal vaccine, just as it does in the case of vaginal formulations. Mucosally active adjuvants, or "helpers," administered with immunogen, increase the immune response to the immunogen and decrease the quantity needed (Grun and Maurer 1989). The candidate most likely to succeed will be some combination of adjuvants or a "hybrid" mucosal immunogen.
The site of immunization may also play an important role in efficacy. Vaginal immunization has a relatively low success rate when compared with injection into the pelvic presacral space, via the intraperitoneal route, or via rectal administration. The vaginal epithelium provides a significant barrier to antigen uptake. Relatively little is known about the male urogenital mucosal system, but it would appear that rectal immunization could be effective in inducing certain mucosal immune reactions in the male as well.
Because the lower female urogenital tract already has a strong defense system in place, it makes sense to take advantage of this natural characteristic to block the entry of both sperm and pathogens. Primary oral immunization followed by repeated local boosting (vaginal or rectal) may be particularly effective. By combining the body's general natural defense mechanisms with the specificity possible through immunology, such an approach offers promise for development of a highly effective, user controlled, and reversible contraceptive as well as an anti-STD agent.
Concluding Comment
This chapter was prepared as a summary of the individually authored technical papers that constitute the appendixes to this report, to which the reader is referred. It is intended as a bridge among what the committee believes to be the pressing public health and individual needs for new contraceptive technologies; the market that will or will not respond to those needs; and the regulatory, legal,
and sociopolitical climate that will or will not make that response fall into some zone of reasonable possibility.
References
Adams CE. Retention and development of eggs transferred to the uterus at various times after ovulation in the rabbit. Journal of Reproduction and Fertility 60:309-315, 1980.
Alexander NJ. Contraceptive Vaccine Development. IN Vaccine Research and Developments. W Koff, H Six, eds. New York: Marcel Dekker. 1992.
Allan GF, X Leng, SY Tsai, et al. Hormone and antihormone induce distinct conformational changes which are central to steroid receptor activation. Journal of Biological Chemistry 267:19513-19520, 1992.
Anderson DJ, PM Johnson, WR Jones, et al. Monoclonal antibodies to human trophoblast and sperm antigens: Report of two WHO-sponsored workshops, 30 June 1986, Toronto, Canada. Journal of Reproductive Immunology 10:231-257, 1987.
Artini PG, C Battaglia, G D'Ambrogio, et al. Relationship between human oocyte maturity, fertilization and follicular fluid growth factors. Human Reproduction 9:902-906, 1994.
Bagatell CJ, AM Matsumoto, RB Christensen et al. Comparison of a gonadotropin-releasing hormone antagonist plus testosterone (T) versus T alone as potential male contraceptive regimens. Journal of Clinical Endocrinology and Metabolism 77(2):427-432, 1993.
Becker-Andre M, I Wisenberg, N Schaeren-Wiemers, et al. Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor super family. Journal of Biological Chemistry 269:28531-28534, 1994.
Bhatt H, LJ Brunet, CA Stewart. Uterine expression of leukemia inhibitory factor coincides with the onset of blastocyst implantation . Proceedings of the National Academy of Sciences, USA 88:11408-11412. 1991.
Birgerson L, A Lund, V Odlind, et al. Termination of early human pregnancy with epostane. Contraception 35:11-120, 1987.
Brown J, JA Cebra-Thomas, JD Bleil, et al. A premature acrosome reaction is programmed by mouse t haplotypes during sperm differentiation and could play a role in transmission ratio distortion. Development 106:769-773, 1989.
Challacombe SJ, TB Tomasi. Systemic tolerance and secretory immunity after oral immunization. Journal of Experimental Medicine 152:1459-1472, 1980.
Chen DB, R Hilsenrath, ZM Yang, et al. Leukemia inhibitory factor in human endometrium during the menstrual cycle: Cellular origin and action on production of glandular epithelial cell prostaglandin in vitro. Human Reproduction 10:911-918, 1995.
Chen F, AJ Cooney, Y Wang, et al. Cloning of a novel orphan receptor (GCNF) expressed during germ cell development. Molecular Endocrinology 8:1434-1444, 1994.
Cook RJ. Antiprogestin drugs: Medical and legal issues. Family Planning Perspectives 21(6):267-272, 1989.
Crooij MJ, CC deNooyer, BR Rao, et al. Termination of early pregnancy by the 3ß hydroxysteroid dehydrogenase inhibitor epostane. New England Journal of Medicine 319:813-817, 1988.
Croxatto HB. Gamete transport. IN Reproductive Endocrinology, Surgery, and Technology. E Adashi, JA Rock, Z Rosenwaks, eds. Philadelphia: Lippincott-Raven. 1995.
Curtiss R III. Attenuated Salmonella strains as live vectors for the expression of foreign antigens. IN New Generation Vaccines, GC Woodrow, MM Levine, eds. New York: Marcel Dekker. 1990.
De M, TR Sandford, GW Wood. Expression of interleukin 1, interleukin 6 and tumor necrosis factor a in mouse uterus during the peri-implantation period of pregnancy. Journal of Reproduction and Fertility 97:83-89, 1993.
DePaolo LV, M Shimonaka, RH Schwall, et al. In vivo comparison of the follicle-stimulating hormone-suppressing activity of follistatin and inhibin in ovariectomized rats. Endocrinology 128:668-674, 1991.
Dorce M. Control of M-Phase by maturation-promoting factor. Journal of Reproduction anf Fertility 97:83-89, 1993.
East IJ, BJ Gulyas, J Dean. Monoclonal antibodies to the murine zona pellucida protein with sperm receptor activity: Effects on fertilization and early development. Developmental Biology 109:268-273, 1985.
Eddy EM, DA O'Brien. The Spermatozoon. IN The Physiology of Reproduction, 2nd ed., Vol. 1. E Knobil, JD Neill, eds. New York:Raven Press. 1994.
Erickson GF, DR Danforth. Ovarian control of follicle development. American Journal of Obstetrics and Gynecology 172:736-747, 1995.
Frank GR, AK Brar, H Jikihara, et al. Interleukin-1ß and the endometrium: An inhibitor of stromal cell differentiation and possible autoregulator of decidualization in humans. Biology of Reproduction 52:184-191, 1995.
Fukuda MN, T Sato, J Nakayama, et al. Trophinin and tastin, a novel cell adhesion molecule complex with potential involvement in embryo implantation . Genes and Development 9:1199-1210, 1995.
Gemzell-Danielsson K, M-L Swahn, et al. Early luteal phase treatment with mifepristone (RU 486) for fertility regulation. Human Reproduction 8:870-873, 1993.
Ghosh D, P De, J Sengupta. Luteal phase estrogen is not essential for implantation and maintenance of pregnancy from surrogate embryo transfer in the rhesus monkey. Human Reproduction 9:629-637, 1994.
Ghosh D, J Sengupta. Anti-nidatory effect of a single, early postovulatory administration of mifepristone (RU 486) in the rhesus monkey. Human Reproduction 8:552-558, 1993.
Goldberg E. Isozymes in testes and spermatozoa. IN Isozymes: Current Topics in Biological and Medical Research. MC Rattazzi, JG Scandalios, GS Whitt, eds. New York: Alan R. Liss. 1977.
Goldberg E. Lactate dehydrogenase-X (crystalline) from mouse testes. IN Methods in Enzymology: Carbohydrate metabolism, Part B, Vol. XLI. New York:Academic Press. 1975.
Greene KE, LM Kettel, SS Yen. Interruption of endometrial maturation without hormonal changes by an antiprogesterone during the first half of the luteal phase of the menstrual cycle: A contraceptive potential . Fertility and Sterility 58:338-343, 1992.
Griffin D. Strategy of vaccine development. IN Gamete Interaction: Prospects for Immunocontraception. NJ Alexander, PD Griffin, NM Spieler, GMH Waites, eds. New York: Wiley-Liss. 1990.
Grigorescu F, MT Baccara, et al. Insulin and IGF-1 signaling in oocyte maturation. Hormone Research 42:55-61, 1994.
Grun JL, PH Maurer. T helper cell subsets elicited in mice utilizing two different adjuvant vehicles: The role of endogenous interleukin I in proliferative responses. Cellular Immunology 121:134-145, 1989.
Gu H, JD Marth, PC Orban, et al. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science 265:103-106, 1994.
Hamilton DW. Local immunity and sperm processing in the male reproductive tract. IN Local Immunity in Reproductive Tract Tissues. PD Griffin, PM Johnson, eds. New York:Oxford University Press. 1993.
Handelsman DJ, AJ Conway, LM Boylan. Suppression of human spermatogenesis by testosterone implants. Journal of Clinical Endocrinology and Metabolism 75(5):1326-1332, 1992.
Haneberg B, D Kendall, HM Amerongen, et al. Induction of specific immunoglobulin A in small intestine, colon-rectum, and vagina measured with a new method for collection of secretions from local mucosal surfaces. Infectious Immunology 62:15-23, 1994.
Harper MJK. Agents with antifertility effects during preimplantation stages of pregnancy. IN Biology of Mammalian Reproduction. KS Moghissi, ESE Hafez, eds. Springfield, IL: C.C. Thomas. 1972.
Herr JC, CJ Flickinger, M Homyk, et al. Biochemical and morphological characterization of the intra-acrosomal antigen SP-10 from human sperm. Biology of Reproduction 42:181-193, 1990a.
Herr JC, RM Wright, E John, et al. Identification of human acrosomal antigen SP-10 in primates and pigs. Biology of Reproduction 42:377-382. 1990b.
Herrmann W, R Wyss, A Riondel, et al. Effet d'un stéroide anti-progestérone chez la femme: Interruption du cycle menstruel et de la grossesse au début. Comptes Rendu de l'Académie des Sciences 294:933-938, 1982.
Heyman RA, DJ Mangelsdorf, JA Dyck, et al. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell 68:397-406, 1992.
Hilton DJ, NA Nicola, D Metcalf. Specific binding of murine leukemia inhibitory factor to normal and leukemic monocytic cells. Proceedings of the National Academy of Sciences, USA 85:5971-5975, 1988.
Hogrefe HH, JP Griffith, MG Rossmann, et al. Characterization of antigenic sites on the refined 3-A structure of mouse testicular lactate dehydrogenase-C4. Journal of Biological Chemistry 262:13155-13162, 1987.
Hogrefe HH, PTP Kaumaya, E Goldberg. Immunogenicity of synthetic peptides corresponding to flexible and antibody-accessible segments of mouse lactate dehydrogenase (LDH)-C4. Journal of Biological Chemistry 264:10513-10519, 1989.
Hsueh AJ, H Billig, A Tsafriri. Ovarian follicle atresia: Hormonally controlled apoptotic process. Endocrine Reviews 15:707-724, 1994.
Hunt JS, HL Chen, et al. Normal distribution of tumor necrosis factor-a messenger ribonucleic acid and protein in the uteri, placentas, and embryos of osteopetrotic (op/op) mice lacking colonstimulating factor-1 . Biology of Reproduction 49:441-452, 1993.
Ikeda Y, WH Shen, et al. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Molecular Endocrinology 8:654-662, 1994.
Ilesanmi AO, DA Hawkins, BA Lessey. Immunohistochemical markers of uterine receptivity in the human endometrium. Microscopic Research Technique 25:208-222, 1993.
Inoue T, H Kanzaki, M Iwai, et al. Tumor necrosis factor a inhibits in vitro decidualization of human endometrial stromal cells. Human Reproduction 9:2411-2417, 1994.
Jentoft N. Why are proteins O-glycosylated? Trends in Biochemical Science 15:291-294, 1990.
Jones WR. Lessons from an anti-human chorionic gonadotropin contraceptive vaccine trial. IN Gamete Interaction: Prospects for Immunocontraception. NJ Alexander, PD Griffin, JM Spieler, GMH Waites, eds. New York: Wiley Liss. 1990.
Jones WR, J Bradley, SJ Judd, et al. Phase I clinical trials of a World Health Organization birth control vaccine. Lancet 1:1295, 1988.
Kharat E, NS Nair, K Dhall, et al. Analysis of menstrual records of women immunized with antihCG vaccines inducing antibodies partially cross-reactive with hLH. Contraception 41:293-299, 1990.
Kliman HJ, RF Feinberg, LB Schwartz, et al. A mucin-like glycoprotein identified by MAG (mouse ascites golgi) antibodies: Menstrual cycle-dependent localization in human endometrium. American Journal of Pathology 146:166-181, 1995.
Kurth BE, K Klotz, CJ Flickinger, et al. Localization of sperm antigen SP-10 during the six stages of the cycle of the seminiferous epithelium. Biology of Reproduction 44:814-821, 1991.
Ladd A, G Prabhu, YY Tsong, T Probst, et al. Active immunization against gonadotrophin-releasing hormone combined with androgen supplementation is a promising antifertility vaccine for males. American Journal of Reproductive Immunology 17:121, 1988.
Ladd A, YY Tsong, G Prabhu, et al. Effects of long-term immunization against LHRH and androgen treatment on gonadal function. Journal of Reproductive Immunology 15:85-101, 1989.
Lala DS, DR Rice, KL Parker. Steroidogenic factor 1, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor 1. Molecular Endocrinology 6:1248-1258, 1992.
Lea I, RT Richardson, EE Widgren, et al. Cloning and sequencing of human Sp17, a sperm zona binding protein. Molecular Biology of the Cell 4:248a, 1993.
Lee CY, JH Huan, E Goldberg. Lactate dehydrogenase from the mouse. IN Carbohydrate Metabolism: Part D, Methods in Enzymology. WA Wood, ed. New York: Academic Press. 1982.
Lehner T, LA Bergmeier, C Panagiotidi et al. Induction of mucosal and systemic immunity to a recombinant simian immunodeficiency viral protein. Science 258:1365-1369, 1992.
Lehner T, R Brookes, C Panagiotidi et al. T- and B-cell functions and epitope expression in nonhuman primates immunized with simian immunodeficiency virus antigen by the rectal route. Proceedings of the National Academy of Sciences, USA 90:8638-8642, 1993.
Lessey BA. The use of integrins for the assessment of uterine receptivity. Fertility and Sterility 61:812-814, 1994.
Lessey BA, AJ Castelbaum, CA Buck, et al. Further characterization of endometrial integrins during the menstrual cycle and in pregnancy. Fertility and Sterility 62:497-506, 1994.
Lessey BA, L Damjanovich, C Coutifaris, et al. Integrin adhesion molecules in the human endometrium: Correlation with the normal and abnormal menstrual cycle. Journal of Clinical Investigation 90:188-195, 1992.
Luo X, Y Ikeda, KL Parker. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481-490, 1994.
Mahi-Brown CA, R Yanagimachi, ML Nelson, et al. Ovarian histopathology of bitches immunized with porcine zonae pellucidae. American Journal of Reproductive Immunology and Microbiology 18:94-103, 1988.
McDonnell DP, DL Clemm, T Herman, et al. Analysis of estrogen receptor function in vitro reveals three distinct classes of antiestrogens. Molecular Endocrinology 9:659-669, 1995.
Millar SE, SM Chamow, AW Baur, et al. Vaccination with a synthetic zona pellucida peptide produces long-term contraception in female mice. Science 246:935-938, 1989.
Morris JK, JS Richards. Luteinizing hormone induces prostaglandin endoperoxide synthase-2 and luteinization in vitro by A-kinase and C-kinase pathways. Endocrinology 136:1549-1558, 1995.
Morris JK, JS Richards. Hormone induction of luteinization and prostaglandin endoperoxide synthase-2 involves multiple cellular signaling pathways. Endocrinology 133:770-779, 1993.
Moudgal NR, GR Aravindan. Induction of infertility in the male by blocking follicle-stimulating hormone action. IN Immunology of Reproduction. R Naz, ed. Boca Raton, FL: CRC Press. 1993.
Moudgal NR, GS Murthy, N Ravindranath, et al. Contraception through regulation of endogenous FSH secretion: Prospects for the male. IN Human Reproduction and Contraception. S Takagi, GI Zatuchni, eds. Tokyo: Professional Postgraduate Services. 1988a.
Moudgal NR, GS Murthy, N Ravindranath, et al. Development of a contraceptive vaccine for use by the human male: Results of a feasibility study carried out in adult male bonnet monkeys (Macaca radiata) . IN Contraceptive Research for Today and the Nineties. GP Talwar, ed. New York: Springer Verlag. 1988b.
Moudgal NR, N Ravindranath, GS Murthy, et al. Long-term contraceptive efficacy of vaccine of ovine follicle-stimulating hormone in male bonnet monkeys (Macaca radiata). Journal of Reproduction and Fertility 96:91-102, 1992.
Mowat AM. The regulation of immune responses to dietary protein antigens. Immunology Today 8:93-98, 1987.
Ogra PL, DT Karzon. Distribution of poliovirus antibody in serum, nasopharynx and alimentary tract following segmental immunization of lower alimentary tract with poliovirus. Journal of Immunology 102:1423-1427, 1969.
Ogra PL, SS Ogra. Local antibody response to polio vaccine in the human genital tract. Journal of Immunology 110:1307-1311, 1973.
O'Malley BW. Steroid hormone receptors as transactivators of gene expression. Breast Cancer Research and Treatment 18:67-71, 1991.
O'Rand MG, J Beavers, EE Widgren, et al. Inhibition of fertility in female mice by immunization with a B-cell epitope, the synthetic sperm peptide, PIOG. Journal of Reproductive Immunology 25:89-102, 1993a.
O'Rand MG, RT Richardson, N Yamasaki. Zona pellucida binding of mammalian spermatozoa. Journal of Reproduction, Fertility, and Development 39(Suppl.):43-44, 1993b.
O'Rand MG, EE Widgren. Identification of sperm antigen targets for immunocontraception: B-cell epitope analysis of Sp17. Reproduction, Fertility, and Development 6:289-296, 1994.
Ortiz ME, G Bastias, O Darrigrande, H Croxatto. Importance of uterine expulsion of embryos in the interceptive mechanism of postcoital oestradiol in rats. Reproduction, Fertility, and Development 3:333-337, 1991.
Phelps BM, P Primakoff, DE Koppel, et al. Restricted lateral diffusion of PH-20, a pi-anchored sperm membrane protein. Science 240:1780-1782, 1988.
Phelps B, DG Myles. The guinea pig sperm plasma membrane protein, PH-20, reaches the surface via two transport pathways and becomes localized to a domain after an initial uniform distribution. Developmental Biology 123:63-72, 1987.
Pollard JW, JS Hunt, W Wiktor-Jedrzejczak, et al. A pregnancy defect in the osteopetrotic (op/op) mouse demonstrates the requirement for CSF-1 in female fertility. Developmental Biology 148:273-283, 1991.
Primakoff P, H Hyatt, DG Myles. A role for the migrating sperm surface antigen PH-20 in guinea pig sperm binding to the egg zona pellucida. Journal of Cell Biology 101:2239-2244, 1985.
Primakoff P, W Lathrop, L Woodman, et al. Fully effective contraception in female guinea pigs immunized with the sperm protein PH-20. Nature 335:543-546, 1988.
Rhim SH, SE Millar, F Robey, et al. Autoimmune disease of the ovary induced by a ZP3 peptide from the mouse zona pellucida. Journal of Clinical Investigation 89:28-35, 1992.
Robbins-Roth C, ed. Highlights of the Annual National Conference on Biotechnology Ventures: Reprogen, Inc—On the Trail of Mechanisms. Bioventure View 9(11), December 1994.
Sacco AG, EC Yurewicz, MG Subramanian, et al. Analysis of the porcine zona pellucida Mr=55,000 antigen for contraceptive use. IN Reproductive Immunology 1989, L Mettler, D Billington, eds. Amsterdam: Elsevier. 1990.
Schane HP, JE Creange, AJ Anzalone, et al. Interceptive activity of azastene in rhesus monkeys. Fertility and Sterility 30:343-347, 1978.
Schultz JF, DR Armant. ß1 and ß3 -class integrins mediate fibronectin binding activity at the surface of developing mouse peri-implantation blastocysts. Journal of Biological Chemistry 270:11522-11531, 1995.
Selinger M, IZ Mackenzie, MD Gillmer, et al. Progesterone inhibition in mid-trimester termination of pregnancy: Physiological and clinical effects. British Journal of Obstetrics and Gynaecology 94:1218-1222, 1987.
Shen WH, CC Moore, Y Ikeda, et al. Nuclear receptor steroididogenic factor 1 regulates the Mullerian inhibiting substance gene: A link to the sex determination cascade. Cell 77:651-661, 1994.
Simón C, A Frances, GN Piquette, et al. Embryonic implantation in mice is blocked by interleukin1 receptor antagonist. Endocrinology 134:521-528, 1994.
Simón C, GN Piquette, A Frances, et al. Interleukin-l type I receptor messenger ribonucleic acid (mRNA) expression in human endometrium throughout the menstrual cycle. Fertility and Sterility 59:791-796, 1993a.
Simón C, GN Piquette, A Frances, et al. Localization of interleukin-1 type I receptor and interleukin-1ß in human endometrium throughout the menstrual cycle. Journal of Clinical Endocrinology and Metabolism 77:549-555, 1993b.
Skinner SM, T Mills, HJ Kirchick, et al. Immunization with zona pellucida proteins results in abnormal ovarian follicular differentiation and inhibition of gonadotropin-induced steroid secretion. Endocrinology 115:2418-2432, 1984.
Snyder BW, HP Schane. Inhibition of luteal phase progesterone levels in the rhesus monkey by epostane. Contraception 31:479-486, 1985.
Srinivasan J, S Tinge, R Wright, et al. Oral immunization with attenuated Salmonella expressing human sperm antigen induces antibodies in serum and the reproductive tract. Biology of Reproduction 53:462-471, 1995.
Stevens VC, B Cinader, JE Powell, et al. Preparation and formulation of an hCG antifertility vaccine: Selection of an adjuvant and vehicle. American Journal of Reproductive Immunology 6:315-321, 1981.
Stevens VC, JE Powell, M Rickey, et al. Studies of various delivery systems for a human chorionic gonadotropin vaccine. IN Gamete Interaction: Prospects for Immunocontraception . NJ Alexander, PD Griffin, JM Spieler, GMH Waites, eds. New York: Wiley-Liss. 1990.
Stewart CC, P Kasper, LJ Brunet, et al. Blastocyst implantation depends on maternal expression of leukemia inhibitory factor. Nature 369:76-79, 1992.
Strous GJ, J Dekker. Mucin-type glycoproteins. Critical Reviews in Biochemical and Molecular Biology 27:57-92, 1992.
Swahn ML, M Bygdeman, S Cekan, et al. The effect of RU 486 administered during the early luteal phase on bleeding pattern, hormonal parameters and endometrium. Human Reproduction 5:402-408, 1990.
Tabibzadeh S, KL Kaffka, PG Satyaswaroop, et al. Interleukin-l (IL-1) regulation of human endometrial function: Presence of IL-1 receptor correlates with IL- -stimulated prostaglandin E2 production. Journal of Clinical Endocrinology and Metabolism 70:1000-1006, 1990.
Tackacs L, EJ Kavacs, MR Smith, et al. Detection of IL-la and IL-1ß gene expression by in situ hybridization: Tissue localization of IL-i mRNA in the normal C57BL/6 mouse. Journal of Immunology 141:3081-3094, 1988.
Taguchi O, Y Nishizuka. Autoimmune oophoritis in thymectomized mice: T cell requirement in adoptive transfer. Clinical Experiments in Immunology 42:324-331, 1980.
Talwar GP, SK Gupta, AK Tandon. Immunologic interruption of pregnancy. IN Reproductive Immunology. N Gleicher, ed. New York: Alan R. Liss. 1981.
Talwar GP, OM Singh, R Pal, et al. A vaccine that prevents pregnancy in women. Proceedings of the National Academy of Sciences, USA 91:8532-8536, 1994.
Talwar GP, O Singh, R Pal, et al. Vaccines against LHRH and HCG. IN Immunology of Reproduction. R Naz, ed. Boca Raton, FL: CRC Press. 1993.
Talwar GP, O Singh, R Pal, et al. Anti-hCG vaccines are in clinical trials. Scandinavian Journal of Immunology 36(Suppl. 11): 123, 1992.
Talwar GP, O Singh, R Pal, et al. Experiences of the anti-hCG vaccine of relevance to development of other birth control vaccines . IN Gamete Interaction: Prospects for Immunocontraception. NJ Alexander, PD Griffin, JM Spieler, GMH Waites, eds. New York: Wiley-Liss. 1990.
Tartakovsky B, E Ben-Yair. Cytokines modulate preimplantation development and pregnancy. Developmental Biology 146:345-352, 1991.
Tung KSK, S Smith, C Teuscher, et al. Murine autoimmune oophoritis, epididymoorchitis, and gastritis induced by day 3 thymectomy. American Journal of Pathology 126:293-302, 1987.
Tzukerman MT, A Esty, D Santioso-Mere, et al. Human estrogen receptor transcriptional capacity is determined by both cellular and promoter context and mediated by two functionally distinct intramolecular regions. Molecular Endocrinology 8:21-30, 1994.
Vale W, L Bilezikjian, C Rivier. Reproductive and other roles of inhibins and activins. IN The Physiology of Reproduction. E Knobil, JD Neill, eds. New York: Raven Press. 1994.
Vale W, A Hsueh, C Rivier. The inhibin/activin family of hormones and growth factors. IN Handbook of Experimental Pharmacology, Vol. 95/11: Peptide Growth Factors and Their Receptors, II. New York: Springer-Verlag. 1990.
Van Look PFA. Presentation on menses-inducers at an Institute of Medicine Workshop on Contraceptive Research and Development and the Frontiers of Contemporary Science, Washington, DC, Institute of Medicine, 9-10 December 1994.
Wang C, R Swerdloff, GMH Waites. Male contraception: 1993 and beyond. IN Contraceptive Research and Development 1984 to 1994. PFA Van Look, G Pérez-Palacios, eds. New York: Oxford University Press. 1994.
Wheat TE, E Goldberg. Antigenic domains of the sperm-specific lactate dehydrogenase C4 isozyme. Molecular Immunology 22:643-649, 1985.
Wheat TE, E Goldberg. Sperm-specific lactate dehydrogenase C4: Antigenic structure and immunosuppression of fertility. IN Isozymes: Current Topics in Biological and Medical Research, Vol. 7. MC Retezzi, JG Scandalios, GS Whitt, eds. New York: Alan R. Liss. 1983.
World Health Organization, Special Programme of Research, Development and Research Training in Human Reproduction, Task Force on postovulatory Methods of Fertility Regulation. Menstrual regulation by mifepristone plus prostaglandin: Results from a multicentre trial. Human Reproduction 10:308-314, 1995.
Willson TA, D Metcalf, NM Gough. Cross-species comparison of the sequence of the leukemia inhibitory factor gene and its protein. European Journal of Biochemistry 204:21-30, 1992.
Wolff JA, RW Malone, P Williams, et al. Direct gene transfer into mouse muscle in vivo. Science 247:1465-1468, 1990.
Yamamoto-Yamaguchi Y, M Tomida, M Hozumi. Specific binding of a factor inducing differentiation to mouse myeloid leukemic M1 cells. Experimental Cell Research 164:97-102, 1986.
Yamasaki N, RT Richardson, MG O'Rand. Expression of the rabbit sperm protein Sp17 in COS cells and interaction of recombinant Sp17 with the rabbit zona pellucida. Molecular Reproduction and Development 40:48-55, 1995.
Yang Z-M, S-P Le, D-B Chen, MJK Harper. Temporal and spatial expression of leukemia inhibitory factor in rabbit uterus during early pregnancy. Molecular Reproduction and Development 38:148-152, 1994.
Yang Z-M, S-P Le, D-B Chen, et al. Expression patterns of leukemia inhibitory factor receptor (LIFR) and the gp 130 receptor component in rabbit uterus during early pregnancy. Journal of Reproduction and Fertility 103:249-255, 1995a.
Yang Z-M, S-P Le, D-B Chen, et al. Leukemia inhibitory factor (LIF), LIF receptor and gp 130 in the mouse uterus during early pregnancy. Molecular Reproduction and Development 42:407-414, 1995b.
Zhang Z, C Funk, SR Glasser, et al. Progesterone regulation of heparin-binding epidermal growth factor-like factor gene expression during sensitization and decidualization in the rat uterus: Effects of the antiprogestin ZK 98.299. Endocrinology 135:1256-1263, 1994a.
Zhang Z, C Funk, D Roy, et al. Heparin-binding epidermal growth factor-like growth factor is differentially regulated by progesterone and estradiol in rat uterine epithelial and stromal cells. Endocrinology 134:1089-1094, 1994b.
Zhong Z, Z Wen, JE Darnell Jr. STAT3: A STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 264:95-98, 1994.
Zirkin BR, C Awoniyi, MD Griswold, et al. Is FSH required for adult spermatogenesis? Journal of Andrology 15:273-276. 1994.
Notes
-
2.
The first laboratory and clinical data on RU 486 were presented by Herrman et al. in 1982.









































