5
The Market for New Contraceptives: Translating Unmet Need into Market Demand
Chapter 2 documents the existence of a considerable unmet need for contraception in both the industrial and developing economies of the world. The argument is made that there is a compelling public health1 case for broadening the portfolio of contraception options, for women and men everywhere. Chapters 3 and 4—most particularly the latter—offer rich evidence for new paths in contemporary science that could expand those options and answer specific and highly critical needs for which there are now no adequate or appropriate solutions.
Yet, as irrefutable as the need may be and as promising the science, response from pharmaceutical firms in the United States and western Europe will be conditioned by the difficulties of translating need and promise into a profitable market. The high costs and risks of committing to the development of any medical technology are such that no firm will undertake commercialization without at least a strong belief in the existence of a substantial market of consumers able and willing to pay, in other words, the existence of market demand. In the case of new contraceptives, that belief is qualified by factors whose effects are economic and whose causes are several and complex. This is a major dilemma.
The present chapter explores this dilemma from several perspectives. The first is a qualitative look at present market demand as expressed in overall patterns of contraceptive use, worldwide and in the United States.
The second focus is on specific areas of contraceptive need that seem most readily translatable into market demand, that is, ''niches" that are either empty or quite inadequately filled. The indicators of these niches include the various limitations in the current array of contraceptives as expressed in the side effects experienced by users, failure and discontinuation rates reflecting side effects and
other constraints to adoption and continued use, and sterilization as a contraceptive option that is not always appropriate. This focus also encompasses the implications of the sexually transmitted diseases for contraceptive technology and their relevance for the contraceptive market.
The third perspective has to do with consumer preferences, with particular attention to the content and character of the "woman-centered agenda" and the issues it raises.
The fourth perspective is quantitative: a look at today's market for contraceptives in terms of numbers of actual and potential users and dollar values; lessons to be learned from the world vaccine market, with which the contraceptives market is in some ways analogous; and subsidized procurement as a market factor.
The chapter closes with a discussion of the cost-effectiveness of contraception and what that might mean as an incentive to investment in contraceptive R&D and the intimate and necessary relationship of that investment with the market for contraceptive technologies.
Current Contraceptive Use
Contraceptive Use Worldwide
Contraceptive prevalence2 among women currently married or in union (a group designated by the abbreviation MWRA, or "married women of reproductive age") increased worldwide from 30 percent during 1960-1965 to 57 percent in 1990. The increase was much more dramatic in the developing countries, where prevalence rose from 9 percent to 53 percent in that same period (UN 1994, cited in WHO/HRP 1995). The increase was especially dramatic in eastern Asia and Latin America, slightly less so in other parts of Asia and in North Africa, least of all in Sub-Saharan Africa. The range is wide: Contraceptive use prevalence in Africa is currently estimated at 17 percent, quite a difference from Latin America, for example, where prevalence is almost 65 percent (see Table 5-1 and Table 5-2 for data for developing countries).
There is also great variability within regions. While overall prevalence in the Arab States and Europe averages 44 percent, the range is from almost zero in some Persian Gulf countries to 68 percent in Turkey. And, in Asia, where the overall prevalence is 62.5 percent, the range is from 10 percent in Afghanistan to a use prevalence of 80 percent or more in China. Variability in contraceptive use prevalence among the industrial countries is much narrower (Guttmacher Institute 1995a; WHO/HRP 1995).
The overwhelming majority—90 percent-—of women using contraception in the developing countries are using modern methods. Globally, the most used method is female sterilization (tubectomy or tubal ligation). Thirty percent of all contracepting couples worldwide relied on female sterilization as of 1990; in the
TABLE 5-1 Basic Data, Total Population, and Contraceptive Use, World and Developing Countries, 1994 and Projected for 2005 (in thousands)
|
|
1994 |
2005 |
|
|
World |
|
|
Total population |
5,646,200 |
6,665,120 |
|
Total female population |
2,803,790 |
3,307,980 |
|
Women of reproductive age (aged 15-49) |
1,415,810 |
1,681,040 |
|
Women of reproductive age, married or in union (MWRA)a |
976,728 |
1,145,490 |
|
Contraceptive users among all women |
595,103 |
755,817 |
|
|
Developing Countries |
|
|
Total population |
4,418,180 |
5,364,550 |
|
Total female population |
2,172,230 |
2,642,990 |
|
Women of reproductive age (aged 15-49) |
1,106,570 |
1,368,950 |
|
Women of reproductive age, married or in union (MWRA)a |
784,897 |
953,815 |
|
Contraceptive users among all women |
457,759 |
625,521 |
|
Contraceptive users among married/in union women |
445,692 |
602,417 |
|
a MWRA = married women of reproductive age, defined as "married or living with a man," vis-à-vis "now widowed, divorced, or no longer living together." Source: United Nations Population Fund. Contraceptive Use and Commodity Costs in Developing Countries, 1994-2005. New York, 1995. Data for total population are from the United Nations 1992 estimates and projections. User data are derived from sample surveys carried out in 69 developing countries in the 1980s and 1990s; these countries contained 90 percent of the population and 94 percent of contraceptive users of all developing countries in 1990. Contraceptive prevalence for the period of analysis was projected using a demographic approach that takes the level of contraceptive prevalence as estimated from the latest national survey and then projects increases in contraceptive prevalence as a function of estimated changes in total fertility rates. The basis is the United Nations medium population projection. These rates are then applied to the number of MWRA and unmarried women who use contraception; the fact that there are now data from 34 countries for this second population group makes its inclusion in global calculations of contraceptive prevalence possible for the first time. |
||
TABLE 5-2 Number of Contraceptive Users, by Method, World and Developing Countries, 1994 and Projected for 2005 (in thousands)
|
|
|
Sterilization |
|
|
|
|
|
|
Total |
|
|
|
All |
Female |
Male |
Pill |
Injectable |
IUD |
Condom |
Other |
Users |
|
|
|
|
|
|
World |
|
|
|
|
|
|
|
1994 |
233,597 |
183,323 |
50,274 |
92,060 |
11,879 |
127,156 |
51,451 |
78,960 |
595,103 |
|
|
2005 |
290,599 |
232,514 |
58,085 |
120,097 |
9,375 |
152,325 |
58,965 |
4,456 |
755,817 |
|
|
|
|
|
|
Developing Countries |
|
|
|
|
||
|
1994 |
200,149 |
161,107 |
39,042 |
51,352 |
10,461 |
112,115 |
24,778 |
46,837 |
445,692 |
|
|
2005 |
258,847 |
210,031 |
48,816 |
76,603 |
17,058 |
137,079 |
35,702 |
77,128 |
602,417 |
|
|
Source: United Nations Population Fund. Contraceptive Use and Commodity Costs in Developing Countries, 1994-2005. New York, 1995. Data are derived from sample surveys carried out in 69 developing countries in the 1980s and 1990s; these countries contained 90 percent of the population and 94 percent of contraceptive users of all developing countries in 1990. Contraceptive prevalence for the period of analysis was projected using a demographic approach that takes the level of contraceptive prevalence as estimated from the latest national survey and then projects increases in contraceptive prevalence as a function of estimated changes in total fertility rates. The basis is the United Nations medium population projection. These rates are subsequently applied both to the number of MWRA (married women of reproductive age) and to the number of unmarried women who use contraception; the fact that there are now data from 34 countries for this second population group makes its inclusion in global calculations of contraceptive prevalence possible for the first time. |
||||||||||
developing countries, that figure was 38 percent (UNFPA 1994; WHO/HRP 1995). As for reversible methods, the IUD is the second most used method in the developing countries, primarily because of its extensive use in the People's Republic of China; it ranks fourth in the industrial economies, a ranking that might be higher were it not for the unavailability of the IUD in most of eastern Europe and its very limited use in the United States. The pill ranks third in the developing countries and is the most prevalent method in the industrial countries. The condom fails to even approach the levels of utilization in the developing countries that it has achieved elsewhere. In fact, all coitus-related methods (condoms, vaginal methods, withdrawal) are far less likely to be used in most developing countries than in the industrial countries. Because Norplant is so new and available in very few developing countries, use prevalence data are not included in the tables below. As of 1993, there were an estimated 1.5 million users of that method in developing countries (of whom 1.3 million were in Indonesia), with an admittedly arbitrary estimate of 6.8 million by 2005 (UNFPA 1994). It is important to remember that any ranking of method utilization reflects only what people do, not necessarily what they prefer; in much of the developing world, the full "mix" of methods that would permit individuals to truly express preference by choosing among real options is not generally available (WHO/HRP 1995) (see Table 5-3 and Table 5-4).
Contraceptive Use in the United States
In 1988, over two-thirds of women of reproductive age in the United States were at risk of unintended pregnancy, that is, they were sexually active and did not want to become pregnant but would be physically able to become pregnant if they or their partner used no contraceptive method (Forrest 1994b). Of those 39 million women, 35 million (9 in 10) were using a contraceptive and 4 million were not (Forrest 1994b).
Key problems in the use of reversible contraception in the United States and elsewhere are the high rates of discontinuation of use by 12 months after initiation and the number of unintended pregnancies among women who state that they or their partners were regularly using a method of contraception.
There is also evidence of unrealistic expectations regarding contraceptive use. This, in very small part, is due to side effects unidentified in premarketing clinical trials. In addition, known side effects are not taken into account appropriately in prescribing practice or the product information materials are so fully detailed that they are not read or fully understood (Carpenter 1989; Forrest 1994a). Further, the fact that contraceptives are used by theoretically healthy individuals who are not seeking prevention or cure, as those are medically understood, conditions the extent to which users are willing to make trade-offs, even when the costs of a potential pregnancy may be very high. The combination of all these factors with the unfettered litigiousness that characterizes the contemporary
American scene results in a distinctive and difficult environment in the United States.
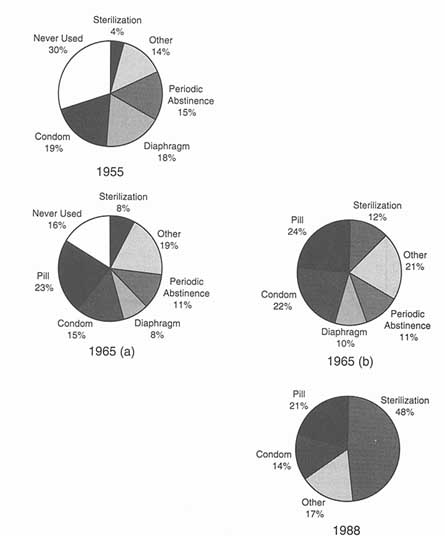
Figure 5-1 presents the proportionate use of each principal contraceptive method in the United States in 1955, 1965, and 1988. Figure 5-2 presents a more detailed picture of shifts in that trajectory between 1960 and 1988. Figure 5-2 makes clear what is sometimes forgotten, that is, that the picture in 1955 in the United States was far from being a blank slate: Among currently married white women aged 18-39 in that year, 70 percent had used contraception at some point and 34 percent had used a method before their first pregnancy. Nonetheless, what was available for use was quite limited, in variety and efficacy, and either coitus-dependent (condom, diaphragm, douche, withdrawal, spermicides) or linked to the timing of coitus (periodic abstinence); only 4 percent of U.S. women had been sterilized for contraceptive purposes.
The broad pattern changes since the availability of oral contraceptives beginning in 1960 have been:
- Increase in total contraceptive use and pill use between 1955 and 1965 and decreased use of the diaphragm, condom, and periodic abstinence.
- Steep increase in interest in coitus-independent methods and in method efficacy.
- Increased reliance on female-controlled methods, especially in the 1960s.
- Steady growth in resort to contraceptive sterilization since the mid- 1960s.
- Increased IUD use from the early 1960s till the early 1970s, then a sharp decrease to a stable but low plateau in the late 1980s.
- Decline in condom use in the early 1960s and 1970s, then increase in the 1980s.
- Rapid adoption of new methods—pill, Today sponge, injections, Norplant—as each appeared, with diminished utilization as side effects were experienced.
In addition to these larger patterns, there have been smaller patterns in contraceptive method use that have been dictated by differences and changes in the circumstances of women's lives. The result is a profile of how various female subpopulations tend to adopt or reject certain methods over time (see Table 5-5).
Specific Needs and Market Opportunities: The Limitations of Available Contraceptives
Side Effects
Like any medical intervention, all contraceptive methods have side effects. Some of those can be life threatening when a method is prescribed inappropriately for women for whom it is medically contraindicated or when an infection
TABLE 5-3 Contraceptive Use Among Married/in Union Women, by Method, and Region, 1994 (in thousands)
|
|
Africa |
Arab States and Europe |
Asia and Pacific |
|||
|
Method |
No. |
% |
No. |
% |
No. |
% |
|
Sterilization |
1,625 |
11.8 |
1,057 |
5.3 |
179,318 |
49.2 |
|
Female |
1,541 |
11.2 |
1,030 |
5.2 |
140,905 |
38.7 |
|
Male |
84 |
0.6 |
27 |
0.1 |
38,413 |
10.5 |
|
Pill |
3,507 |
25.5 |
6,081 |
30.7 |
28,579 |
7.8 |
|
Injectable |
1,748 |
12.7 |
146 |
0.7 |
7,559 |
2.1 |
|
IUD |
1,145 |
8.3 |
5,461 |
27.6 |
100,205 |
27.5 |
|
Condom |
512 |
3.7 |
1,225 |
6.2 |
21,076 |
5.8 |
|
Othera |
5,240 |
38.0 |
5,818 |
29.4 |
27,545 |
7.6 |
|
Otherc |
— |
— |
— |
— |
— |
— |
|
Total |
13,777 |
100.0 |
19,791 |
100.0 |
364,282 |
100.0 |
|
Note:—= no data available. a Data for Africa designate as "Other" vaginal methods, Norplant, and traditional methods. In the U.S. National Survey of Family Growth, "Other" included jellies and creams, suppositories and inserts, the Today sponge, douche, diaphragm, foam, periodic abstinence, and withdrawal. b No implants were available in the United States at the time these data were gathered. c 'This category includes data on users as follows: diaphragm, 2 million/5.7 percent; periodic abstinence, 0.8 million/2.3 percent; withdrawal, 0.8 million/2.2 percent; spermicides, 0.6 million/1.8 percent; sponge, 0.4 million/l. percent. Source: For all data except for the United States, United Nations Population Fund. Contraceptive Use and Commodity Costs in Developing Countries, 1994-2005 (Technical Report No. 18). New York, 1994. For the U.S. data, National Center for Health Statistics, 1988 National Survey of Family Growth, cited in Alan Guttmacher Institute, Facts in Brief: Contraceptive Use. New York, March 1993. |
||||||
results from an associated surgical intervention (Carpenter 1989; Hatcher et al. 1994). Nonetheless, while not negligible, the mortality attributable to contraceptive use is very small. For the most part, women's concerns about the contraceptive technologies that are currently available have to do with side effects that are distressing or annoying in themselves or that lead women to conclude that something bad may be going on in their bodies. These side effects include nausea, headaches, and weight gain due to the pill; increased bleeding, dysmenorrhea, and expulsion associated with the IUD; menstrual changes from implants and injectables; and the irreversibility of sterilization. These will vary among individuals according to severity, cultural meaning, and the extent to which they impinge on the ability to live life. Other health-related considerations have to do
|
|
Latin America and Caribbean |
Total Developing Countries |
USA (1988) |
|||
|
Method |
No. |
% |
No. |
% |
No. |
% |
|
Sterilization |
18,148 |
37.9 |
200,149 |
44.9 |
13,686 |
39.2 |
|
Female |
17,631 |
36.9 |
161,107 |
36.1 |
9,617 |
27.5 |
|
Male |
517 |
1.1 |
39,042 |
8.8 |
4,069 |
11.7 |
|
Pill |
13,183 |
27.6 |
51,352 |
11.5 |
10,734 |
30.7 |
|
Injectable |
1,008 |
2.1 |
10,461 |
2.3 |
—b |
— |
|
IUD |
5,303 |
11.1 |
112,115 |
25.2 |
703 |
2.0 |
|
Condom |
1,965 |
4.1 |
24,778 |
5.6 |
5,093 |
14.6 |
|
Othera |
8,233 |
17.2 |
46,837 |
10.5 |
76b |
0.6a |
|
Otherc |
— |
— |
— |
— |
4,620 |
13.1 |
|
Total |
47,840 |
100.0 |
445,692 |
100.0 |
34,912 |
100.0 |
|
Note:—= no data available a Data for Africa designate as "Other" vaginal methods, Norplant, and traditional methods. In the U.S. National Survey of Family Growth, "Other" included jellies and creams, suppositories and inserts, the Today sponge, douche, diaphragm, foam, periodic abstinence, and withdrawal. b No implants were available in the United States at the time these data were gathered. c 'This category includes data on users as follows: diaphragm, 2 million/5.7 percent; periodic abstinence, 0.8 million/2.3 percent; withdrawal, 0.8 million/2.2 percent; spermicides, 0.6 million/1.8 percent; sponge, 0.4 million/l. percent. Source: For all data except for the United States, United Nations Population Fund. Contraceptive Use and Commodity Costs in Developing Countries, 1994-2005 (Technical Report No. 18). New York, 1994. For the U.S. data, National Center for Health Statistics, 1988 National Survey of Family Growth, cited in Alan Guttmacher Institute, Facts in Brief: Contraceptive Use. New York, March 1993. |
||||||
with method qualities that produce difficulty, such as manipulation of the genitals; associated physical exams; fear of surgery, loss of potency, or diminution of libido; and random myths (Bongaarts and Bruce 1995). Table 5-6 presents the risks and side effects of currently available contraceptive methods; it also presents their noncontraceptive benefits.
Developing Countries
An extensive review of published and unpublished studies of contraceptive utilization in the developing world indicates that one in every five women with an unmet need for contraception is not using a modern contraceptive method, owing
TABLE 5-4 Contraceptive Usage, Method Rankings, Selected Regions and Countries
|
Total Developing World (1994) |
Africa |
Arab States and Europe |
Asian and Pacific |
Latin America and Caribbean |
More Developed Regionsa |
USA (1988) |
USA (1995) |
|
Tubectomy |
Other |
Pill |
Tubectomy |
Tubectomy |
Pill |
Pill |
Pill |
|
IUD |
Pill |
Other |
IUD |
Pill |
Condom |
Tubectomy |
Tubectomy |
|
Pill |
Injectable |
IUD |
Vasectomy |
Other |
Tubectomy |
Condom |
Condom |
|
Otherb |
Tubectomy |
Condom |
Pill |
IUD |
IUD |
Vasectomy |
Periodic abstinence |
|
Vasectomy |
IUD |
Tubectomy |
Otherc |
Condom |
|
Otherc |
Injectabled |
|
Condom |
Condom |
Injectable |
Condom |
Injectable |
|
Diaphragm |
Diaphragm |
|
Injectable |
Vasectomy |
Vasectomy |
Injectable |
Vasectomy |
|
IUD |
IUD |
|
Note: Tubectomy = tubal litigation. a Northern America, Japan, Europe, Australia/New Zealand, former USSR. b "Other" here includes vaginal methods, Norplant, and traditional methods. c "Other" includes vaginal methods, periodic abstinence, and withdrawal. d Depo-Provera. Sources: United Nations Population Fund. Contraceptive Use and Community Costs in Developing Countries 1991-2005. Technical Report No. 18. New York, 1994. Ortho Pharmaceutical Corporation. 1995, 1993, and 1991 Annual Birth Control Studies. Raritan, NJ, 1995. Shah IH. The advance of the contraceptive revolution. Health Statistics Quarterly 47(1):9-15. 1994. |
|||||||
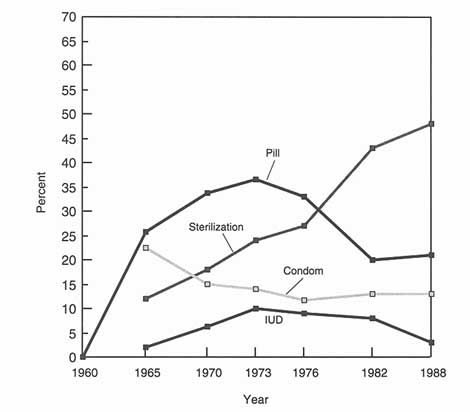
Figure 5-2
Trajectory of change in method use, United States, 1960-1988.
Source: Adapted from JD Forrest. Contraceptive Use in the United States: Past, present, and future. Advances in Population 2:29-48, 1994.
to poor or absent access to services, perception of side effects, or health concerns associated with modern contraceptives (WHO/HRP 1995). Health concerns, sometimes deriving from lack of clear understanding about the method and its side effects—are by far the most important single reason for nonadoption and, in most countries, are more frequently reported than all other concerns combined. Access in many rural settings is also a major problem. The principal foci of concern are the pill, the IUD, and sterilization. Among women with health concerns, contraceptive prevalence is reduced by an average of 86 percent for the IUD, 71 percent for the pill, and 52 percent for sterilization (Bongaarts and Bruce 1995).
This does not mean that other factors do not matter, only that they may be somehow qualified or cannot be documented as quantitatively significant. For
TABLE 5-5 Contraceptive User Characteristics, U.S. Women, 1988 and 1995, by Method
|
Method |
User Characteristics (1988) |
User Characteristics (1995) |
|
Tubal ligation |
Women 30-44; increased use among formerly married, black and Hispanic women, less educated and lowestincome women |
More married women between 35 and 50 with 2 or more children and higher incomes |
|
Vasectomy |
Currently married women, white women relying on partner |
No change identified. |
|
Pill |
Women under 25, unmarried women, women who intend to have children; increased use among better educated women, among whites, and among those with higher incomes; declines only among teenagers |
Increasing use of low-dose pills lesser extent, by women over 40 by women in their 30s and, to |
|
Condom |
Somewhat increased use, sharp increase among teenagers, unmarried and never-married women |
Increasing use by women over 40 |
|
Diaphragm |
White, college educated, and never-married women who intend to have children; slight decline overall, sharp decline among unmarried women and women under age 30 |
Use now highest between ages 25 and 40 (mainly between ages 30 and 35), women with college and postgraduate degrees; longer use |
|
IUD |
Women who intend to have no more children, previously married women, Hispanic women, those with less education; overall decline, especially among women 25-34, formerly married and less educated, sharpest decline among Hispanic women. |
More women in 30s and 40s with at least one child, in married/ mutually monogamous relationship |
|
Sources: For 1988, Alan Guttmacher Institute. Facts in Brief: Contraceptive Use. New York: The Alan Guttmacher Institute, March 1993. For 1995, Ortho Pharmaceutical Corporation, Executive Summary: 1995 Ortho Annual Birth Control Study. Raritan, NJ, 1995. |
||
TABLE 5-6 Risks, Side Effects, and Noncontraceptive Benefits of Contraceptive Methods
|
Method |
Noncontraceptive Benefits |
Risks |
Side Effects |
|
Implantable contraceptives |
May protect against acute PID, ovarian and endometrial cancers; lactation undisturbed; may decrease menstrual blood loss and pain; suppression of pain associated with ovulation |
Infection at implant site; difficult removal; not protective against viral STDs, including HIV/AIDS |
Tenderness at implant site, menstrual cycle disturbance (amenorrhea becomes less common over time), weight gain, breast tenderness, headaches, ovarian enlargement, dizziness, nausea, acne, dermatitis, hair loss |
|
Injectable contraceptives |
May protect against PID, ovarian cancer, and endometrial cancers; decreased menstrual blood loss and risk of anemia; decreased menstrual pain; suppression of pain associated with ovulation; decreased frequency of seizures |
Decreased bone density; not protective against viral STDs, including HIV/AIDS |
Menstrual cycle disturbance (amenorrhea becomes more common over time), weight gain, breast tenderness, depression, delay in return of fertility, decreased HDL cholesterol levels, headaches |
|
IUDs |
None known; progestin-releasing IUDs may decrease menstrual blood loss and pain |
Slight increase in risk of PID in first 20 days after insertion; perforation of the uterus, anemia; not protective against viral STDs, including HIV/AIDS |
Menstrual cramping, spotting, increased bleeding |
|
Oral contraceptives |
Protects against acute infection of the fallopian tubes (PID), ovarian and endometrial cancers, benign breast masses, ovarian cysts; decreased ectopic pregnancy; decreased menstrual blood loss and risk of anemia; decreased menstrual pain, suppression of pain associated with ovulation |
Estrogen-associated: slight increase in blood clot complications, stroke, liver tumors, hypertension, heart attacks, cervical erosion or ectopia, cervical chlamydia Progestin-associated: diabetesrelated changes, hypertension, heart attacks |
Estrogen-associated: nausea, headaches, fluid retention, weight gain, increased breast size, breast tenderness, stimulation of breast tumors, watery vaginal discharge, rise in cholesterol concentration in gallbladder bile, uterine fibroids |
|
Method |
Noncontraceptive Benefits |
Risks |
Side Effects |
|
Oral contraceptives |
|
Associated with increased cervical chlamydia; not protective against viral STDs, including HIV/AIDS |
Progestin-associated: weight gain, depression, fatigue, headaches, decreased libido, acne, increased breast size, breast tenderness, increased LDL cholesterol level, decreased HDL cholesterol level, chronic itch |
|
Male condoms |
Protects against bacterial and viral STDs, including HIV/AIDS; delays premature ejaculation; erection enhancement; prevention of sperm allergy |
None known |
Decreased sensation during intercourse, allergy to latex, possible interference with erection, loss of spontaneity |
|
Female condoms |
Protects against STDs, including HIV/AIDS, including on the vulva |
None known |
Decreased sensation during intercourse, allergy to polyurethane; aesthetically unappealing and awkward to use for some |
|
Barrier methods (diaphragm, cervical cap, sponge) |
Protects against bacterial STDs, prevention against HIV/AIDS not proven; diaphragm protects against cervical infection and neoplasia |
Vaginal trauma, toxic shock syndrome (rare), cervical erosion |
Vaginal and urinary tract infection (anaerobic overgrowth); vaginal discharge if not removed appropriately; allergy to spermicide, rubber, or latex; pelvic pressure, bladder or rectal pain; penile pain |
|
Method |
Noncontraceptive Benefits |
Risks |
Side Effects |
|
Spermicides |
Spermicides with nonoxynol-9 protect against gonorrhea and chlamydia: prevention against viral STDs, including HIV/AIDS, undetermined |
None proven; however, tissue irritation may enhance susceptibility to HIV infection |
Tissue irritation, yeast vaginitis, allergy to spermicidal agents Note: STDs = sexually transmitted diseases; PID = pelvic inflammatory disease. |
|
Sources: Institute of Medicine. The Best Intentions: Unintended Pregnancy and the Well-Being of Children and Families. S Brown, L Eisenberg, eds. Washington, DC: National Academy Press. 1995. Ehrhardt AA, JN Wasserheit. Age, gender, and sexual risk behaviors for sexually transmitted diseases in the United States. IN Research Issues in Human Behavior and Sexually Transmitted Diseases in the AIDS Era. JN Wasserheit, SO Aral, KK Holmes, PJ Hitchcock, eds. Washington, DC: American Society for Microbiology. 1991. Hatcher RA, J Trussell, F Stewart, et al. Contraceptive Technology, 16th Revised Edition. New York: Irvington Publishers, 1994. |
|||
example, even in areas where high fertility norms persist, birth spacing is considered highly desirable; the quintessential case is Sub-Saharan Africa. Another example is the weight of male disapproval of family planning. Evidence from Demographic and Health Surveys (DHS) and studies of fertility decision making suggests that this may either be changing in some settings or may be overstated. Women often assume the existence of male opposition simply because they are afraid to raise the topic with their partners, a reflection of very fundamental issues of power and control (Bongaarts and Bruce 1995; WHO/HRP 1995).
The United States
Research in the United States consistently reports significant lack of the kind of information among adults and adolescents that would allow them to perhaps more fully appraise the relative risks and benefits of contraception. As in the developing world, that limitation affects method adoption and continuation (Forrest 1994b; Institute of Medicine 1995).
The example of oral contraceptives (OCs), on the market for more than 35 years and used by 10 million American women, is informative. A 1993 poll commissioned by the Association for Obstetrics and Gynecology (ACOG) found that 54 percent of their sample of American women believed that there were ''substantial risks" (mainly cancer) associated with oral contraceptives (Gallup Organization 1994). Relatively few women knew the several noncontraceptive benefits of the pill and 42 percent believed there to be no health benefits from pill use other than pregnancy prevention. Only 6 percent were aware that the pill is actually protective against cancer; in fact, in the 1993 survey and an earlier ACOG survey in 1985, the same proportions of women—one-third—cited cancer as the chief risk of using oral contraceptives (Gallup Organization 1994). In both the 1985 and 1993 surveys, better than two-thirds of the sample incorrectly believed that OC use was more risky or as risky as childbirth, even though the opposite is true (Gallup Organization 1994).
A telephone poll of 1,000 American women, conducted for the Kaiser Family Foundation in 1995, found that only one-quarter of women of reproductive age are confident that oral contraceptives are "very safe" for the user; others expressed a spectrum of concern, with 43 percent considering them "somewhat safe," 18 percent "somewhat unsafe," and 11 percent "very unsafe." Six out of 10 of these women cited worries about potential health risks, while many others expressed concern that the pill does not protect against sexually transmitted diseases or that it is ineffective in preventing pregnancy. One-third incorrectly thought that oral contraceptives increased the risk of ovarian cancer and 40 percent said there was no effect; only 16 percent correctly said that the pill actually reduces that particular risk (Russell 1996).
Part of the problem is the likelihood of inadequate provider-client commu-
nication. For instance, a random-sample, telephone survey by the Kaiser Family Foundation and Louis Harris & Associates in October-November 1994 found that most American women with the potential of experiencing an unplanned pregnancy are uninformed or misinformed about the "morning-after" pill, an emergency contraceptive option currently available off label in the United States that can prevent a potential pregnancy up to 72 hours after unprotected sex (Kaiser/Harris 1995).3 A separate survey found that while 78 percent of U.S. obstetrician-gynecologists in the United States are very familiar with emergency contraceptive pills (ECPs), another 22 percent are somewhat familiar with them, and 72 percent of the entire sample consider the method both safe and effective, most have prescribed it only for a handful of their patients within the last year (Kaiser/Fact Finders 1994). Thus, only one-third of women polled indicated that they knew that anything could be done after unprotected sex to prevent pregnancy and half of those who do know are misinformed about proper timing. The difficulty seems to be that, in general, clinicians make their female patients aware of ECPs in response to an emergency situation rather than during routine contraceptive counseling; when women do call in, they may well encounter receptionists who do not know about emergency contraception (Kaiser/Harris 1995).
Many clinicians also retain negative perceptions of the IUD dating back to the Dalkon Shield disaster, even though new configurations and formulations make IUDs excellent options for many women (Westoff et al. 1992). Adolescents are especially compromised by their low level of information about contraceptives, tied up as it is in their substantial ignorance about sexuality, fertility, and sexual health. This, in turn, impinges negatively on health-seeking and family planning behavior, though lack of information is far from the only factor (Zabin et al. 1991). Nevertheless, the disposition of some U.S. media to overstate modest risks and understate the major health and social benefits of contraception does little to enhance thoughtful decision making about contraceptive use. The adverse and unbalanced media coverage of Norplant motivated women who were experiencing no problems to seek removal and, in undetermined degree, fueled a litigation "explosion" noted in the media themselves (Economist 1995; Herman 1994; Kolata 1995).
At the same time, there are unresolved uncertainties that make the use of certain methods inappropriate for some women and, in some cases, for rather large groups of women. The IUD for women with an active, recent, or recurrent pelvic infection or for women at high risk for a sexually transmitted disease is inappropriate. The jury is still out in connection with a slight increase in breast cancer risk among younger users of oral contraceptives, even though lifetime increased risk is close to zero. Nor are oral contraceptives or Norplant advisable for women with significant cardiovascular risk profiles, particularly in women over 35 (Hatcher et al. 1994). In a discussion later in this report of "informed choice," the point is made that prescribing any contraceptive method to women for whom it is contraindicated is patently a great disservice to them; it is also a
great disservice to the reputation of the technologies themselves. However, overall, modern hormonal and intrauterine contraceptives are extremely safe in comparison to the risks of pregnancy, a fact that is poorly understood.
Contraceptive Failure and Discontinuation
Contraceptive effectiveness, failure, continuation, and discontinuation are intimately linked since they are, in important respects, functions of one another. The likelihood that a contraceptive will fail to protect the user depends primarily on two factors. The first is the inherent efficacy of the method itself when used properly (perfect use); this includes technical attributes that make a method easy or difficult to use. The second factor has to do with the characteristics of the user: how often the method is used correctly and consistently, frequency of intercourse, and age (Hatcher et al. 1994).
An example: Although combination oral contraceptives have a perfect-use pregnancy rate of 0.1 percent during the first year of use, the typical-use pregnancy rate is closer to 3 percent (Hatcher et al. 1994) because, as with many medications, compliance with daily pill use is difficult for many women. A far more extreme example is the ovulation method of periodic abstinence, with first-year probabilities of failure of 3 percent during perfect use but as high as 86 percent during imperfect use (Trussell and Grummer-Strawn 1990).
In thinking about the need for new contraceptives, it is important to remember that the majority of today's reversible methods are hard to use perfectly all the time. How consistently the method is used correctly reflects both the user's skill and determination—or lack of them—as well as the inherent complexity and limitations of the methods themselves (Institute of Medicine 1995). It is also important to remember that the components of "determination" reflect some sort of internal balancing of benefits and burdens (Bulatao and Lee 1983), some personal calculus of choice (Zabin 1994), that is, in most individuals in varying measure, a labile blend of immediate and more distant circumstances and pressures, personality, attitudes, feelings, beliefs, motivation, and ambivalence that may well defy the individual's own explanatory powers (Maynard 1994). The complexity of this subject is reflected in a rich literature covering at least two decades of attempts at understanding; it is also reflected in the incompleteness of that literature, which leaves major age groups and populations almost unexamined (Institute of Medicine 1995). Nonetheless, whatever the determinants, the costs of contraceptive failure are the high rates of abortion and unintended and unwanted pregnancy among women using some reversible methods, particularly those that are coitus-related.
Developing Countries
Analysis of DHS data from 11 developing countries4 found high discon-
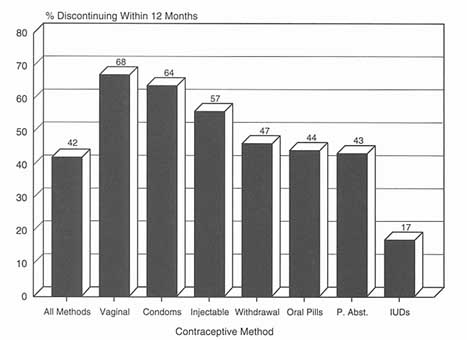
Figure 5-3
Cumulative percentage discontinuing a contraceptive method by 12 months, average for 11 developing countries. P. Abst. = periodic abstinence.
Source: WHO. Perspectives on Methods of Fertility Regulation: Setting a Research Agenda (Background Paper). Geneva: UNDP/UNFPA/WHO/World Bank/HRP. 1995.
tinuation rates for all reversible contraceptive methods over the five-year period prior to survey. Rates reflected either dissatisfaction with the method itself or with the service providing it. In the countries studied, average use of any method other than the IUD was under 15 months. Forty-two percent of all contraceptive use was terminated by the twelfth month after adoption, 11 percent because of contraceptive failure (unintended pregnancy), and 11 percent for health concerns and side effects. Overall, 68 percent of users of vaginal methods 5 and 64 percent of condom users discontinued their use by the end of the first year (see Figure 5-3). Discontinuation rates varied by country and method, from a low of 25 percent in Indonesia to a high of 65 percent in the Dominican Republic (a country where 70 percent of users are, in fact, sterilized). Failure rates for condoms and periodic abstinence were similar to typical-use rates estimated for the United States (Trussell and Kost 1987), and failure associated with the IUD was even lower in the 11 developing countries than in the United States; failure rates for the pill, vaginal methods, and withdrawal were significantly higher, however. Not surprisingly, method failures, particularly the failure of traditional methods, resulted
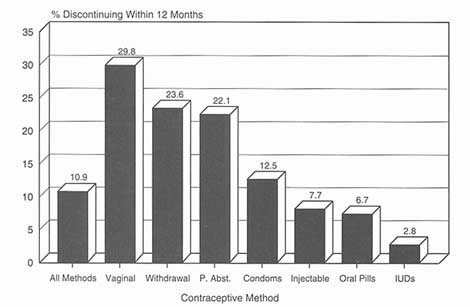
Figure 5-4
Cumulative percentage discontinuing by 12 months due to contraceptive failure, average for 11 developing countries. P. Abst. = periodic abstinence.
Source: WHO. Perspectives on Methods of Fertility Regulation: Setting a Research Agenda (Background Paper). Geneva: UNDP/UNFPA/WHO/World Bank/HRP. 1995.
in high rates of unintended pregnancy, often leading to induced abortions (WHO/ HRP 1995). This is, nonetheless, somewhat misleading: These are method-specific termination rates and many women may, in fact, change to another method, so that the more important figures have to do with continuation to some method.
As for causes of discontinuance, 1 out of 5 users of vaginal methods, periodic abstinence, and withdrawal discontinued the method before the end of the first year because of contraceptive failure; the comparable figure for the pill and injectable was 1 in 14 users (see Figure 5-4). Health concerns were the reason for discontinuance for about 1 in 5 users of the pill and injectable, but were of little consequence for users of vaginal methods, periodic abstinence, and withdrawal (see Figure 5-5). Figure 5-6 summarizes these two factors and their contribution to method discontinuance.
In the first month after discontinuation, of the 58 percent of women who discontinued contraceptive use in the four Latin American countries 6 in the sample, 16 percent became pregnant or wanted to do so, 15 percent changed to another modern method, 8 percent changed to a traditional method, and 19 percent abandoned contraception altogether even though they did not want to be-
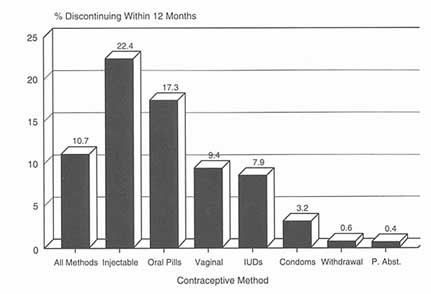
Figure 5-5
Cumulative percentage discontinuing by 12 months due to side effects and health concerns, average for 11 developing countries. P. Abst. = periodic abstinence.
Source: WHO. Perspectives on Methods of Fertility Regulation: Setting a Research Agenda (Background Paper). Geneva: UNDP/UNFPA/WHO/World Bank/HRP. 1995.
come pregnant. The IUD had the lowest discontinuation rate (17 percent) and was judged favorably with regard to both method failure and health concerns compared to other modern reversible methods. In general, there is much variability in contraceptive behavior following method discontinuation, by method and by country. In fact, there is so much variability in terms of preferences relating to future contraceptive use that it is impossible to identify a single method or set of methods as the globally preferred choice of most women. In most countries, while oral contraceptives are the preferred "next method," there is little basis for certainty that any supposedly preferred method would actually be chosen when the decision to adopt it materialized (WHO/HRP 1995).
In sum, perceived or real side effects and health concerns are major factors in the decision to use contraception; for choosing a specific method; for switching; and for abandoning use of modern methods, especially hormonal methods, entirely (WHO/HRP 1995). The totality of these findings is highly relevant to appraising the need for new contraceptive methods. The analysis by the World Health Organization's Human Reproduction Programme (WHO/HRP) concludes:
It is obvious that a substantial number of women will need to switch methods before finding one that suits them, or will need to discontinue use in order to have a pregnancy that they have only been delaying, or will need to switch methods as they make the life course transition from delaying a birth to pre-

Figure 5-6
Summary scattergram of contraceptive methods by percent discontinuing their use within 12 months because of method failure or health concerns.
Source: WHO. Perspectives on Methods of Fertility Regulation: Setting a Research Agenda (Background Paper). Geneva: UNDP/UNFPA/WHO/World Bank/HRP. 1995.
venting a birth. However, they also discontinue or switch because the current state of the available contraceptive methods, especially of hormonal methods, is far from satisfactory. Side-effects and health concerns, [perceived or real], remain major factors in discontinuing the use of hormonal methods, while barrier methods and especially vaginal and traditional methods often lead to accidental pregnancy. For these different reasons, hormonal methods and traditional methods have discontinuation rates that are high and broadly similar. Both the expansion in method mix and the improvement in contraceptive technology appear necessary to increase the satisfaction with the method use and to reduce the incidence of unintended pregnancies because of method failure or abandonment of use by the dissatisfied users (WHO 1995:45).
The United States
Table 5-7 presents failure rates and continuation rates associated with all contraceptive methods under both perfect and typical use for the U.S. population
(Hatcher et al. 1994). As common sense would predict, just as in the 11 developing countries discussed above, method failure is associated with low-efficacy reversible methods, while there are real and imagined health concerns concerning high-efficacy reversible methods, especially those that are least susceptible to user error: implants, IUDs, and injectables. There is also variability within some method categories. For instance, today's oral contraceptives contain much lower doses of estrogen and therefore produce significantly fewer side effects and implications; they also are less forgiving of human error. Failure is also significant in cumulative terms: Current U.S. estimates indicate that the typical woman in the United States will experience one contraceptive failure for every 2.25 live births (Trussell and Vaughn 1989).
As in almost everything else having to do with contraception, there is variation in cause and consequence from subpopulation to subpopulation. Formerly married (separated, divorced, widowed) women have the highest rates of failure in the use of reversible contraceptive methods; married women have the lowest. Women whose incomes are 200 percent or less of the poverty level are twice as likely as higher-income women to have a contraceptive failure, a major contributor to the high concentration of unintended pregnancy in these same groups (Forrest 1994b; Institute of Medicine 1995).
Error rates in pill taking are high and the costs of those error rates are also high. Of the 3.5 million annual unintended pregnancies in the United States, over 1 million are related to OC use, misuse, or discontinuation, with 61 percent of these occurring in women who discontinue OCs. Of that group, 67 percent did not immediately substitute other contraceptives and 33 percent adopted less reliable methods. This is a particularly important consideration for the approximately 3.7 million U.S. women who initiate OC use each year since that group commonly experiences side effects and has a high discontinuation rate. It is also an important consideration in terms of cost: The costs incurred owing to unintended pregnancies in women who discontinue OCs are close to $2.6 billion annually (Rosenberg et al. 1995).7 European women do better yet, even there, 20 percent do not maintain their regimens (Waugh 1994). There was also more failure associated with the less reliable methods; the failure of periodic abstinence rose from 16 to 25 percent during the 1980s and there were slight increases in failure rates for the condom and diaphragm as well (Institute of Medicine 1995).
Another, partially related, source of concern is the high contraceptive failure rate among females under age 20, more than one-quarter of whom experience contraceptive failure during the first 12 months of use, with the lowest-income teens having the highest failure rates. Teens have very poor success with periodic abstinence: 52 percent of low-income teens experience failure, as do 28 percent of higher-income teens (Moore et al. 1995). Younger teens appear to have a
TABLE 5-7 First-year Contraceptive Failures and Continuation Rates, United States
|
|
% of U.S. Women Experiencing Accidental Pregnancy Within the First Year of Use |
|
|
|
Method |
Typical Use |
Perfect Use |
% of U.S. Women Continuing Use at One Year |
|
Chance |
85 |
85 |
|
|
Spermicides |
21 |
6 |
43 |
|
Periodic abstinence |
20 |
|
67 |
|
Calendar method |
|
9 |
|
|
Ovulation method |
|
3 |
|
|
Symptothermal method |
|
2 |
|
|
Postovulation method |
|
1 |
|
|
Withdrawal |
19 |
4 |
|
|
Cap |
|
|
|
|
Parous women |
36 |
26 |
45 |
|
Nulliparous women |
18 |
9 |
58 |
|
Diaphragm |
18 |
6 |
58 |
|
Condom |
|
|
|
|
Female (Reality®) |
21 |
5 |
56 |
|
Male |
12 |
3 |
63 |
|
Pill |
3 |
|
72 |
|
Progestin only |
|
0.5 |
|
|
Combined |
|
0. 1 |
|
|
IUD |
|
|
|
|
Progesterone T |
2.0 |
1.5 |
81 |
|
Copper T 380A |
0.8 |
0.6 |
78 |
|
LNg 20 |
0.1 |
0.1 |
81 |
|
Depo-Provera |
0.3 |
0.3 |
70 |
|
Norplant (6 capsules) |
0.09 |
0.09 |
85 |
|
Female sterilization |
0.4 |
0.4 |
100 |
|
Male sterilization |
0.15 |
0.10 |
100 |
|
Source: Hatcher RA, J Trussell, F Stewart et al. Contraceptive Technology (16th Revised Edition). New York: Irvington Publishers. 1994. |
|||
particularly hard time taking pills properly, though older teens do almost as well as older women in contraceptive use (Guttmacher Institute 1994).
Overall continuation rates in the United States are highest for the high-efficacy, provider-controlled methods (implant, IUD, injectable), followed by the pill. Periodic abstinence and the male condom follow at some distance, with the poorest continuation rates for the low-efficacy, coitus-dependent vaginal methods (diaphragm, spermicides, cervical cap, sponge). There are a number of resemblances between these continuation rates and those in the developing countries analyzed above: When continuation rates are ranked, patterns of use of the IUD, periodic abstinence, pill, condom, and vaginal methods are similar. The major and quite striking difference is continuation with injectables, in this case Depo-Provera (depo-medroxyprogesterone acetate, or DMPA). While the continuation rate for DMPA in the United States is very high, it is low in some developing countries, overwhelmingly because of menstrual-related side effects. The IUD is also interesting in this connection. IUD use rates in the United States, while stable since 1988, are still very low; even prior to the negative sequelae and media coverage of the Dalkon Shield experience, IUD prevalence never surpassed 8 percent, well below the prevalence of the pill or tubal sterilization (Ortho 1991). Nonetheless, while IUD use in the United States is far below that in the developing countries, U.S. women continue to report high satisfaction with the method (Ortho Surveys 1991-1995), just like women in the developing countries.
If there is any global "rule" that can be derived from these reasonably definitive analyses of contraceptive use in developing countries and in the United States, it is that, overall, methods that perform better on the efficacy scale measure poorly on the health concerns scale, and vice versa, at the same time that both safety and efficacy are, in most circumstances, equally valued (WHO/HRP 1995). This rule is consistently expressed despite variation in patterns of contraceptive use, nonuse, and discontinuation; despite the remarkable safety of hormonal contraceptives; and despite such subjective criteria as preferences regarding ease of use, mode and duration of action, and willingness to tolerate the shortcomings inevitably associated with any contraceptive method. On its face, this would seem to be a profound contradiction and what might be called the "contraceptive technology predicament." However, its more subtle meaning may be that, for some women, the more mediated methods (pills and implants, for example) simply arouse greater generic concern than do less mediated ones (barrier methods, for example), even though the latter may be recognized as generally less effective. From this perspective, safety commands first priority, while efficacy may occupy a position further down the scale where "effective enough" is located. The tension between such valuations and the tradeoffs for unobtrusiveness, security, and separation from coitus that are associated with long-acting, provider-dependent methods, is substantial.
Sterilization as a Contraceptive Option
About one in three contraceptive users in the developing world has looked to sterilization as a way of terminating childbearing. There is anecdotal material that reflects user lack of knowledge about the risks and consequences of sterilization and a certain amount of prospective anxiety. While there is evidence of some regret, particularly among women sterilized at younger ages (e.g., in their 20s), there is also satisfaction among many women who have had a tubal sterilization, satisfaction primarily related to the permanence of the method and the problems associated with reversible methods. The method is rightly seen as highly effective and not difficult to ''use"; its safety, as measured in side effects, is defined as "medium," comparable to the IUD, safer than the pill and injectables, but less safe than the low-efficacy vaginal methods and periodic abstinence (WHO/HRP 1995). In considering the primary problems reported for the major contraceptive methods in the Demographic and Health Surveys, 19 percent of the problems reported for sterilization had to do with health concerns, compared to 42 percent for the pill and 35 percent for the IUD (Bongaarts and Bruce 1995).
It is reasonable to view sterilization rates, particularly among younger women and men, as an indicator that other, reversible alternatives are unavailable or unsatisfactory. Nevertheless, as noted earlier, it is only possible to reach this conclusion where options actually exist. In many countries, sterilization is a popular choice; in others, it reflects the fact that there are no other options or that the options that do exist are unappealing because they are difficult to use, because they are unreliable at a time when having no more children has transcendent importance, because users do not like the side effects or because users are simply weary of dealing with them. In countries where a range of options is available (e.g., Colombia, Costa Rica, Iran, Malaysia, Singapore, South Africa, Thailand, and Tunisia in the developing world, and Austria, Canada, and the United States in the developed world), sterilization does reflect choice. In some countries, for example, China and India, sterilization is a preferred method in national or local government programs and rates reflect that emphasis.
Despite an apparently wide range of reversible contraceptive options in the United States, sterilization is nonetheless the most common form of contraception for all racial and income groups (Ingrassia et al. 1995). One reason is that the range of choice is actually narrower than it seems. The typical woman in the United States choosing sterilization is about 30 years old and has two to three children, with 10 to 15 years of fecund life left during which she could become pregnant but does not wish to. When several IUDs were withdrawn from the U.S. market during 1985-1986, leaving only the Progestasert IUD, which had a small group of users, the remaining options for women who could not or would not use the pill formulations then available but wanted a coitus-independent, unobtrusive, and very effective method, were female or male sterilization (Mosher 1990). As noted above, although two IUDs are now available in the United States,8 the
method has a distance to go before it even regains its mid-1970s market share of around 9.5 percent.9
Finally, the promise of Norplant as a most effective alternative (FDA 1995) for women uncertain about terminating fecundity has been checked by adverse press coverage and litigation largely deriving from problems related to implant removal seen only in a small percentage of women. The fact that sterilization is often covered by public or private health insurance is a further incentive for its utilization, especially since insurance coverage for reversible methods is scanty and uneven (Kaeser and Richards 1994).
The core issue in sterilization is its suitability to the stage in the life span when it is-or is not--clear that more children are wanted. Some understandings that are relevant to sterilization appropriateness are emerging from the Collaborative Review of Sterilization, a prospective multicenter study in the United States which has followed 10,000 women undergoing tubal sterilization, each for a minimum of five years, some as long as 10-14 years. Early findings from that study indicate that failure and regret rates are higher than previously thought: 6.9 percent of women sterilized had reported regret during at least one follow-up period and 6.2 percent indicated that they had sought reanastomosis (sterilization reversal) or talked with a health professional about reversal (Wilcox et al. 1990 and 1991). Age at time of sterilization had the most pronounced effect on regret: Women under age 30 were two to three times more likely to report regret than were women aged 30-35, irrespective of parity, marital status, or education. Participants with a history of abortion and those undergoing sterilization concurrent with cesarean section were also at greater risk of regret, as were women receiving public economic assistance (Wilcox et al. 1991).10
Prevalence of Sexually Transmitted Disease , Including HIV/AIDS
There already seems to be a rather loud demand signal in the market "asking" for an industry response to the mounting risk of sexually transmitted disease (STD): Use of dual contraceptive methods, a reasonable indicator of such demand, is on the rise, at least in the United States. In 1979, just 3 percent of respondents reported dual method use; by 1988, respondents in another study reported 16 percent dual use (Plech et al. 1993; Zelnick and Kantner 1980). In the 1988 National Survey of Family Growth, women were asked which methods they used to protect themselves or their partners from infection. Responses to the question revealed that 4.24 million women reported use of the condom as an STD prophylaxis, but did not report use of the condom as a contraceptive. Altogether 10.4 million women of a total of 57.9 women aged 15-44 reported use of condoms for prevention of pregnancy or STDs or both. By the late 1980s, women seeking safer sex were buying 30 to 40 percent of all condoms in the United States (Rinzler 1987).
Since then, tracking of dual use through the annual Ortho Birth Control
Surveys has recorded a steady increase in such use. In 1991, 23 percent of all women and almost 31 percent of unmarried women in the Ortho sample reported altered practices related to STD concerns; in 1994, 46 percent of all surveyed women reported using condoms in addition to their primary contraceptive; by 1995, that figure was 52 percent (Ortho Birth Control Studies 1991, 1993, 1995). Half of those using dual methods reported doing so for STD protection. This would suggest that the other half may have been double-protecting itself against conception; if so, this may reflect perceptions of limited method efficacy and a strong commitment to not becoming pregnant. Whatever the objective, it seems clear that women in the United States are vigorously interested in methods that will protect them against sexually transmitted disease and that there is enough dual use to indicate that many are also vigorously interested in simultaneously protecting themselves from conception. There are a few clues that this is not easy to do: In the couple of instances in the United States where it has been examined, contraceptive decision making and utilization around dual method use appear to be complex and difficult (Kost and Forrest 1992; Landry and Camelo 1994). At the same time, the importance of preventing the sexually transmitted diseases, particularly the viral infections, cannot be overstated, if for no other reason than the fact that once an individual is infected with a viral STD, he or she will henceforth always be infected.
The public health aspects of the resurgence of STDs are addressed in Chapter 2. The purpose of this section is to more precisely quantify that resurgence. A very recent worldwide study by the World Health Organization's Global Programme on AIDS, done in collaboration with the Rockefeller Foundation, discovered the following sobering facts.
Sexually Transmitted Disease Worldwide
At least 333 million new cases of curable sexually transmitted diseases were predicted to occur in the world in 1995. This includes 12 million new cases of syphilis, 62 million new cases of gonorrhea, 89 million new cases of chlamydial infection, and 170 million new cases of trichomoniasis (WHO/GPA 1995). In 1990, using a modified Delphi technique, the WHO estimated that, in that year, there were over 250 million new cases of all sexually transmitted diseases (WHO/ GPA 1995). Thus, this new estimate of 333 million new cases of just four of the STDs—chancroid and the major viral STDs (e.g., herpes, human papillomavirus, and hepatitis B) were not included because of data deficiencies—is a huge increase. Table 5-8 summarizes the prevalence and incidence data for all four diseases for all of the world's regions.
Results of several large studies of human papillomavirus (HPV) and its relation to cervical cancer are also worrisome. It is clear that most, if not all, of the 500,000 cases of cervical cancer worldwide each year are caused by HPV. A 22-country study of HPV prevalence in cervical cancer patients found an overall
TABLE 5-8 Estimated New Cases of the Curable Sexually Transmitted Diseases, All Regions, 1995
|
Region |
Syphilis |
Gonorrhea |
Chlamydiaa |
Trichomoniasis |
Total by Region |
[Chancroid]b |
|
North America |
140,000 |
1.8 million |
4 million |
8 million |
14 million |
|
|
Western Europe |
200,000 |
1.2 million |
5 million |
10 million |
16 million |
|
|
Australasia |
10,000 |
0.13 million |
300,000 |
1 million |
1 million |
|
|
Latin America and the Caribbean |
1.3 million |
7.1 million |
10 million |
18 million |
36 million |
|
|
Sub-Saharan Africa |
3.5 million |
16 million |
15 million |
30 million |
65 million |
|
|
Northern Africa and the Middle East |
620,000 |
1.5 million |
2.9 million |
4.6 million |
10 million |
|
|
Eastern Europe and Central Asia |
100,000 |
2.3 million |
5 million |
10 million |
18 million |
|
|
East Asia and Pacific |
330,000 |
3 million |
6.2 million |
13 million |
23 million |
|
|
South and Southeast Asia |
5.8 million |
29 million |
40 million |
75 million |
150 million |
|
|
Global Totals |
12 million |
62 million |
89 million |
170 million |
333 million |
[7 million] |
|
a This refers to chlamydia trachomatis in adults. b No estimates could be made for chancroid using the same methodology developed for the other four diseases, since understanding of the epidemiology and natural history of the disease is poor and there is yet no good diagnostic for estimating prevalence and duration of infection. The estimate given here for chancroid is based on the ratio of syphilis to chancroid in the previous WHO (Delphi) estimates for those two diseases and the 1995 estimate for syphilis. Source: World Health Organization. An Overview of Selected Curable Sexually Transmitted Diseases (WHO/GPA/STD/95.1). Geneva: WHO/Global Programme on AIDS, August 1995. |
||||||
rate of 93 percent; it also found that just 4 of the 70 different types of HPV cause 80 percent of all cervical cancer cases, as identified in over 1,000 cervical cancer tumor specimens through use of new DNA techniques (American Health Consultants 1995; Bosch et al. 1995).
Sexually Transmitted Disease in the United States
For the four diseases analyzed by the WHO, the U.S. incidence of 14 million new cases in 1995, with an estimated prevalence of 52 million for 1995, ranks fifth among the nine WHO regions. U.S. prevalence rates are the same as those in Australasia but higher than rates in Western Europe, North Africa, the Middle East, and South and Southeast Asia. The U.S. incidence figure is also considerably higher than the estimate of 12 million new cases annually for all STDs in the United States that is commonly used (CDC 1990, cited in Rosenberg et al. 1992). The 1988 data indicated that three-quarters of all STDs in the United States were accounted for by three diseases: chlamydia (4 million cases), trichomoniasis (3 million), and gonorrhea (1.8 million).
Rates of infection in the United States vary by age and ethnicity. Among women attending family planning clinics from 1989 to 1993, chlamydia infection rates were 4.5 percent (whites), 5.5 percent (Hispanics), and 8.5 percent (African-Americans) (WHO/GPA 1995). Most worrisome is the fact that, in the United States, STD incidence increased rapidly during the 1960s and 1970s and has stayed at those high levels (Cates 1991). At present, 86 percent of all STDs occur among individuals 15 to 29 years old (NARHP 1995), two-thirds among individuals under age 25 (CDC 1992). The Maternal and Child Health Bureau reports that about 3 million U.S. adolescents contract an STD annually. Studies by the Alan Guttmacher Institute have found 15 percent of active teenage women to be infected with HPV; 15- to 19-year-olds also have a 1-in-8 chance of developing pelvic inflammatory disease (PID) and sexually active 10- to 14-year-olds are 7 times more likely to have a PID than 20- to 24-year-olds (Guttmacher Institute 1994). Nearly 200,000 cases of gonorrhea were reported among teens in 1989; visits by teenage women to fee-for-service practices for genital herpes infections grew from 15,000 in 1966 to 125,000 in 1989; and the number of visits for genital warts caused by HPV grew from 50,000 in 1966 to 300,000 in 1989, and perhaps three times as many women had asymptomatic cervical HPV infection (Hatcher et al. 1994).
Consumer Perspectives
Consumer Decision Making and Preferences
The market for any product is formed at the intersection of price, product availability, and consumer decisions. In a producer's ideal market, consumers
"vote" for products by making at least an initial commitment through purchase and, best of all, by continued use; in other words, they are predictable and loyal consumers. In a less than ideal market, consumers may be so uninformed about their options, the range of their options may be so limited, and consumer motivations may be so intricate that it is hard to interpret the meaning of initial votes or to anticipate loyalty.
By these definitions, the market for contraceptives is not ideal. Contraceptive decision making is influenced by a welter of motivational factors: method safety11 and effectiveness12; specific personal considerations; and availability, accessibility, and cost. Safety and effectiveness appear to be consistently important (WHO/HRP 1995) although, depending on the situation, there may be willingness to trade off efficacy against other method qualities, especially since side effects are tolerated differently by different women. For instance, in a setting where abortion is legal and safe, a woman' s main concern may not be contraception, but she may be quite concerned about sexual disease transmission. A young woman who was recently divorced but who might want more children in a second marriage may be willing to put up with contraceptive side effects, for instance, changes in menstrual patterns, to avoid the sterilization she does not yet want to have.
In other words, the dominance assigned to each factor in contraceptive decision making will vary by age, relationship status, family size, culture, and circumstance; by perceptions and knowledge about individual methods; by ambivalence about childbearing; by interest in spontaneity and ease of use; and by issues of power and control. The notion of risk cuts across all these variables: risks to health, method failure, getting "caught" using a method when secrecy is desired, investment in a costly method that might not work out, resort to sterilization when one might have a change of heart. All this is projected against the complicated and changing nature of women's reproductive lives, their ability to exercise control over those lives, and by sometimes considerable forces in the larger political and social environment.
There is also the matter of access. In some developing countries and in the former socialist economies, contraceptive options are severely constrained and the information individuals receive is insufficient to making well-founded choices among those options that are available (Bongaarts and Bruce 1995; Bruce and Jain 1995). The latter is also true in the United States, where provider-client exchanges concerning reproductive choice are sometimes glaringly inadequate and contraceptive users may get much of their information from the media, often incorrectly (Institute of Medicine 1995; Moore et al. 1995; Tanfer 1995).
To come to conclusions about what is needed in the marketplace for contraceptive technology, we now depend on method utilization surveys which theoretically express how consumers vote by using, continuing, or discontinuing a given method. However, the meaning of those votes may be obscured both by
whether they reflected real choice among real options and by who paid for the purchase. Thus, even though we are getting a better grasp on the specific attributes desired in contraceptive technologies by different populations in different circumstances, there is a true need for sophisticated market research in a range of settings (Snow 1994).
Perhaps the best evidence that consumers have long wanted something from the contraceptives market beyond its traditional offerings is the alacrity with which they have seized upon most new methods as they have come along. This has occurred despite the difficulty of "imagining" the new technology and seemingly without reference to anything but whether or not it might "work" (Forrest 1994a). Women are eagerly in search of something that is possibly better, only to be disappointed when they discover that no contraceptive is perfect for everybody; that there are still side effects for some users; that unexpected side effects or complications or inappropriate prescribing and patient management have contributed to litigable cases that muddy the picture of the method's utility; or that manufacturers have not adequately represented what remains unknown about their product.
The WHO/HRP review observes that users differ so markedly from one another in their criteria for selection of a contraceptive method that even people choosing a more or less similar product may be dissimilar in the relative importance they attach to the specific attributes of the product chosen. For instance, the IUD is chosen by women in India, Turkey, and the Republic of Korea primarily for its perceived effectiveness, but for its ease of use by postpartum women in the Philippines. Indian women chose the pill mainly for its ease of use, Korean women for its effectiveness. Indian women also chose the injectable DMPA for its ease of use, while Korean, Philippine, and Turkish women chose it for its convenient duration of action. For some, convenience and desire for spontaneity may determine method choice; others may care deeply about reversibility, discretion, duration of protection, no need for resupply visits, and so forth.
The review concludes that it is difficult—and probably inappropriate—to make large statements about product attributes that supposedly drive all consumer preferences; that there is really no consensus about some set of attributes that are invariable and intrinsic; and that, with the exception of safety and efficacy, all other contraceptive product attributes will vary in their meaning and priority for users according to situation (Snow 1994). In fact, some individuals in some circumstances may be willing to cede some efficacy for other, more valued or situationally appropriate attributes. This suggests that the point made in recent papers (e.g., Correa 1991; Germain 1993) that there has been, overall, undue R&D emphasis on effectiveness at the expense of safety or acceptability may also be situational. The current intent of the U.S. Food and Drug Administration to require efficacy trials of existing over-the-counter barrier methods is an example of where efficacy may, in fact, be an inappropriate emphasis. At the same time, any newly developed barrier method designed to protect against either concep-
tion or infection or both would be less attractive were its efficacy not assured at some reasonably safe level. The fact remains that the methods that have so far dominated the market for contraceptives have been those that do offer high efficacy (Ortho 1991, 1993, 1995; Snow 1994; WHO/HRP 1995). It is also true that it is the issue of safety, or the lack thereof, that is at the core of product liability.
All this raises further questions in connection with the ongoing debate about the relative merits of user control compared to provider control; reversibility compared to effectiveness; coitus-independence compared to coitus-dependence; and the need for concealment of contraceptive use compared to the desirability of partner involvement (Forrest 1994a). Some women are quite willing to trade off "having the doctor do it" for what they consider certainty and peace of mind. Some women not in a formalized relationship and whose sexual activity is sporadic are not necessarily opposed to coitus-dependence. Some women have partners with whom they can share contraceptive decision making; others do not. In sum, there appears to be a persistent divergence of opinion about the qualities that are wanted in contraceptives, who wants them, and what they mean to different groups and individuals 13 and it is this divergence that argues for the largest possible range of contraceptive options.
The "Woman-centered Agenda"
Over the past few years, importantly in connection with the International Conference on Population and Development held in Cairo in 1994, some ideas about what is missing in the contraceptives market have become clearer. There have been precise statements about specific technologies that are wanted which are so eminently desirable that they should be construed by industry as virtual instructions. These have become a core element of the "Contraception 21" Initiative launched by the Rockefeller Foundation and have come to be known as the "woman-centered agenda." As explained in Chapter 1, that agenda awards priority to the following contraceptive technologies:
- vaginal methods that protect against sexually transmitted reproductive tract infections, both in conjunction with contraception and independent from it;
- menses-inducers; and
- more methods for men.
Since current technologies are limited, and since a plausible case can be made that there is market demand for such products, these categories constitute market niches that would seem to merit new industrial investment.
Immunologic Contraception
Although it is not part of the Contraception 21 agenda, many researchers see immunologic contraception14—or what have most often been called "contraceptive vaccines"—as a potentially important new option for the next century, and several public research groups and some private companies are supporting research in that area. This research has evoked much controversy, and the Women's Global Network for Reproductive Rights (WGNRR), an advocacy group based in Amsterdam, coordinated a campaign which, by November 1994, had over 400 signatory groups from 38 countries. The WGNRR cites several concerns: (1) that these contraceptives would have a higher abuse potential than existing methods; (2) that, as presently designed, they would pose unique potential health risks to the autoimmune system; (3) that they would have cumbersome features interfering with efficacy,15 and (4) that they would offer no real advantage over existing methods. More specific analysis lists the following potential disadvantages: (1) need for additional protection during the immunologic lag period and for ancillary testing to ascertain immune levels; (2) probable allergic responses to carrier proteins; (3) possible autoimmune-mediated pathologies and crossreactive immunity to other hormones; (4) difficulty in "switching off" immune response; (5) unlikely prospect of immediate surcease from side effects; and (6) unknown consequences to woman and fetus in the event of unknown pregnancy at the time of immunization and post-immunization pregnancy (Schrater 1995). These groups advocate that research funds be focused on more user-controlled methods, not more long-acting, provider-dependent immunologic methods that provide no protection from STDs and have the potential for coercive applications.
Not all women's health advocates agree (Ravindran and Berer 1994; IDRC 1994; Schrater 1994; Snow 1994), suggesting that immunologic contraception could possibly provide safe, effective, and acceptable methods. Researchers argue that such methods could be inexpensive and convenient for women; could be designed for regimens of different duration; might be made reversible; could offer a long-acting option for men as well; and provide a high degree of confidentiality for women who required it. They note that, because the immunization principle is now widely accepted in the large majority of cultures, contraceptive immunization could be incorporated into ongoing programs for communicable diseases (J Herr 1996, personal communication). Finally, the observation is made that the frontier area of mucosal immunity might eventually offer simultaneous protection against unintended pregnancy and infection (see Appendix D).
Attempts to encourage dialogue and understanding between advocates and researchers are ongoing, as part of a process that began with a meeting between representatives of women's health groups and scientists in Geneva in February 1991. Jointly organized by the WHO Special Programme of Research, Development and Research Training in Human Reproduction (WHO/HRP) and the International Women's Health Coalition, it bore the title "Creating Common Ground:
Women's Perspectives on the Selection and Introduction of Fertility Regulation Technologies." Since then there have been six meetings to continue that dialogue, including regional meetings (Manila 1992, Nairobi 1993, Yaoundé 1994), focused topics such as anti-fertility vaccine research (Geneva 1992), the ethics of contraceptive development (Geneva 1994), and participation in regional/national research needs assessments (Yaoundé 1994). The intention is to sustain and broaden the dialogue (WHO/HRP 1995).
The analysis of the contemporary science that was a major element in this committee's work suggests to some members of the committee that there are enough avenues being opened up by immunologic research to merit continuance. Even were no female "vaccine" to result from that research, worthy advances might have been made toward methods for men and toward vaginal methods which might be protective against conception and sexually transmitted infection. Regular review of the status of progress in these areas, as well as in the broader field of relevant immunologic research, could nourish the dialogue that has been initiated between concerned women's groups and scientific researchers, and perhaps shift investment out of areas that appear to be less productive in terms of the needs of women and their potential for the market.
The Dimensions of the Market for Contraceptives
Potential demand in the market for contraceptives is composed of those who have already "voted" by using contraception and by some portion of those who are not using them. The fact that the market consists of users and nonusers implies that two kinds of market dynamics are of consequence. One is the overall size of the market and any increase or decrease in the total number of contraceptive users. The other has to do with shifts in what methods are being used or adopted. It is of interest to all producers of contraceptives that the market keep growing; it is of interest to individual producers that consumers remain loyal either to their product or, if they switch methods, to the firm.
Numbers of Contraceptive Users
The contraceptive market can be defined in terms of the number of actual and potential contraceptive purchasers or by the associated dollar value of the purchases themselves (PATH 1994b). In terms of numbers of actual and potential purchasers, the world's contraceptive user population has been growing steadily and presently stands at 57 percent of all married women of reproductive age (MWRA),16 or 595 million theoretically possible purchasers. In addition, it is estimated that an additional 220 million women state that they do not wish to be pregnant but are not using any method. Of these, 446 million are in the developing countries. Applying a medium projection, the United Nations Population Fund (UNFPA) estimates that, by 2005, there will be 755 million MWRA
worldwide using contraceptives. In the developing world, there will be 602 million MWRA using contraceptives; if unmarried women are added, that total becomes 625 million contraceptive users in the developing economies by the year 2005 (UNFPA 1994).
The Dollar Value of the Market for Contraceptives
All things being equal, a much larger population could mean a much larger potential market (even taking into account the fact that actual markets are always smaller than potential markets). That is not now the case for contraceptives. While the public sector has traditionally defined markets in terms of volume of doses, manufacturers evaluate markets in terms of revenues and profits (Mercer 1994).
As of 1992, the total world contraceptives market was somewhere around $2.5 billion annually. Oral contraceptives (OCs) are by far the biggest component of that market, accounting for around $2.0 billion in sales worldwide in 1992, up from $1.5 billion in 1988 (see Chapter 6, Table 6-8). This is a total percentage increase for the entire period of close to 40 percent, or around 10 percent annually. The United States accounted for around half of the market in 1992 with estimated sales of close to $1 billion. The next largest market is Europe ($738 million), followed at some distance by the Latin American market, where Brazil, Argentina, and Mexico accounted for $108 million in sales of oral contraceptives in 1992. The market consisting of Africa, Asia, and Australia accounted for $68 million in OC sales and undefined ''others" for another $72 million (Frost and Sullivan 1993) (Table 5-9). These figures include large-volume procurements tendered in the United States by UNFPA for programmatic use in developing countries.
Nonetheless, the oral contraceptive market is perceived as essentially saturated (Reprogen 1995). There are now at least 36 different formulations of oral contraceptives sold in the United States alone; these are primarily combination products, with about a dozen progestin-only pills (Hatcher et al. 1994). To these formulations must be added products manufactured in Europe and in some developing economies. Oral contraceptives accounted for 84 percent of U.S. sales by U.S. manufacturers in 1989; that proportion is projected to drop to 80 percent by 1999 (Table 5-10). The expectation was that there would be displacement of some of the OC market by condoms, diaphragms, and Norplant. However, the sharp decline in Norplant use which began in 1993 and the role of injectables were not accounted for in those projections, so that percentages can be expected to shift, although at this point unpredictably.
Of the remaining market balance, condom sales account for about half and are growing, apparently in response to heightened concern about sexually transmitted diseases, including HIV. Sales in Europe in 1993 were close to $493
TABLE 5-9 Worldwide Oral Contraceptive Markets, by Region and By Country, 1992 Sales (in millions of U.S. dollars)
|
|
By Country |
|
By Region |
|
|
|
1992 Sales |
|
1992 Sales |
|
|
Region and Country |
(US$) |
% Total |
(US$) |
% Total |
|
North America |
|
|
$1,104 |
52.8 |
|
United States |
$995 |
47.6 |
|
|
|
Canada |
109 |
5.2 |
|
|
|
Europe |
|
|
738 |
35.3 |
|
Germany |
227 |
10.8 |
|
|
|
France |
125 |
6.0 |
|
|
|
United Kingdom |
93 |
4.4 |
|
|
|
Italy |
72 |
3.4 |
|
|
|
Belgium |
35 |
1.7 |
|
|
|
Spain |
21 |
1.0 |
|
|
|
Latin America |
|
|
108 |
5.2 |
|
Brazil |
40 |
1.9 |
|
|
|
Argentina |
26 |
1.3 |
|
|
|
Mexico |
17 |
0.8 |
|
|
|
Africa, Asia, and Australia |
|
|
68 |
3.3 |
|
South Africa |
6 |
0.3 |
|
|
|
Australia |
2 |
1.1 |
|
|
|
Others |
301 |
14.4 |
72 |
3.4 |
|
|
— |
— |
— |
— |
|
|
$2,090 |
100.0 |
$2,090 |
100.0 |
|
Source: Frost and Sullivan. U.S. Market Intelligence Report: U.S. Contraceptive and Fertility Product Markets (Report #5021-54). New York, October 1993. |
||||
million, with a projection to $617 million by 1998 (see Table 5-1 1). In the United States, 1993 condom sales were $147 million, with a projection for 1998 of $193 million. The U.S. market currently produces over 100 different condoms (Hatcher et al. 1994), but the market is dominated by seven firms, of which just three are the primary producers of all condoms sold in the United States.
The rest of the market in 1992 consisted of spermicides, Norplant, IUDs, and diaphragms, with that descending order of importance in the U.S. market but a somewhat different order in Europe and the developing economies, where the IUD is better accepted and Norplant does not yet have widespread presence.
Between 1989 and 1994, sales by U.S contraceptives manufacturers grew at an average of 4 percent a year, with sales of oral contraceptives growing at the
slowest pace (2.64 percent annually) and condoms and IUDs growing the fastest, at 6.9 and 5.5 percent, respectively (Frost and Sullivan 1993). Sales of Norplant, introduced onto the U.S. market in February 1991, went from zero to $35 million in the method's first year on the market. In its best year, 1992, Norplant accounted for $141 million of Wyeth's $7.9 billion in worldwide sales (Kolata 1995), with a compound annual growth rate (CAGR) of 13.4 percent as of 1993 (Frost and Sullivan 1993). By 1994 that figure was down to $51 million and, as of 1995, adverse publicity and negative word-of-mouth have driven sales down from 800 a day to about 60 (Kolata 1995).
These market patterns suggest the following. First, it appears that, together, the realities and perceptions of side effects and lack of appreciation of the benefits of reversible hormonal contraceptives will continue to constrain their more widespread and effective use, since it is unlikely that further modifications of these methods will significantly reduce the current array of side effects. Much industry research over the past years has focused on improving today's hormonal contraceptives, yet it is hard to foresee any major future value added in connection with these methods, at least one substantial enough to achieve a quantum leap in utilization.
Second, the market has demonstrated that it will respond to at least some public health needs when the consumer population signals a demand for such a response. The case in point is the effect on the market of concerns about sexually transmitted disease, as different kinds of populations became aware of that public health need and the possibility of dual method use. While condoms are not costly items, the increase in their sales volume has generated respectable growth in market share.
Third, all things being equal, a new product that patently responds to unmet needs can quickly command a sizable market share, even with much of its potential volume unrealized. The predicament in which Norplant has landed does not nullify the extent to which it struck a responsive chord in the marketplace. The popularity of the IUD in developing countries and the initial success with Norplant in those countries where it has been introduced suggest that many women do, in fact, have lively interest in very effective, long-lasting methods that can resolve contraceptive needs, delay sterilization, and protect fecundity. And, although attitudes toward Depo-Provera are a mix of enthusiasm and reserve, it too has been well received in a number of developing countries, where there are 15 million users; it continues to have high initial adoption rates in the United States, particularly among younger women seeking an alternative to the pill (Ortho 1995). Again, women send a signal to the market that they want other options.
The Market for Contraceptives in the Developing Economies
The emphasis in the preceding analysis on the U.S. and European markets corresponds to the fact that the dollar value of the contraceptive market in the
TABLE 5-10 U.S. Contraceptive Sales, U.S. Manufacturers, 1989 and Projected to 1999 (in millions of U.S. dollars and product percentage of total sales)
developing economies is substantially less than that of the contraceptive market in the industrial economies, even though the latter represents a much smaller number of consumers. As of 1992, total annual contraceptive sales in developing nations represented less than 16 percent of the global contraceptive market. In contrast, the industrial economies, with less than one-third17 of all the world's users of contraceptives, generated approximately 84 percent of global contraceptive revenues (PATH 1994a) (see Figures 5-7a and b).
The market for reversible contraceptives is affected by the extent to which sterilization is selected as a contraceptive option, which becomes meaningful for the market when it is translated into absolute numbers. As of 1994, 200 million individuals in the developing countries had opted for sterilization; at current adoption rates of about 11 million women and 3 million men annually, the cumulative total of individuals who have opted for sterilization projected for 2005 will
|
|
IUDs |
Spermicides |
Norplant |
Total Sales |
Total CAGEa |
|||
|
Year |
U.S.$ |
% |
U.S.$ |
% |
U.S.$ |
% |
U.S.$ |
(%) |
|
1989 |
19.4 |
1.7 |
43.4 |
3.8 |
0.0 |
0.0 |
1,143.4 |
— |
|
1990 |
20.2 |
1.7 |
44.9 |
3.8 |
0.0 |
0.0 |
1,180.6 |
3.3 |
|
1991 |
21.3 |
1.7 |
46.4 |
3.7 |
35.1 |
2.8 |
1,253.4 |
6.2 |
|
1992 |
23.3 |
1.8 |
47.9 |
3.7 |
40.1 |
3.1 |
1,293.8 |
3.2 |
|
1993 |
24.0 |
1.8 |
48.0 |
3.6 |
45.3 |
3.4 |
1,065.6 |
3.1 |
|
1994 |
24.7 |
1.8 |
49.4 |
3.6 |
50.8 |
3.7 |
1,373.4 |
3.0 |
|
1995 |
26.8 |
1.9 |
50.9 |
3.6 |
57.9 |
4.1 |
1,412.7 |
2.9 |
|
1996 |
27.6 |
1.9 |
52.3 |
3.6 |
65.4 |
4.5 |
1,452.4 |
2.8 |
|
1997 |
29.8 |
2.0 |
52.2 |
3.5 |
74.6 |
5.0 |
1,491.8 |
2.7 |
|
1998 |
30.6 |
2.0 |
53.6 |
3.5 |
85.8 |
5.6 |
1,531.9 |
2.7 |
|
1999 |
33.0 |
2.1 |
55.0 |
3.5 |
97.4 |
6.2 |
1,571.6 |
2.6 |
|
Source: Frost and Sullivan. U.S. Market Intelligence Report: U.S. Contraceptive and Fertility Product Markets (Report #5021-54). New York, October 1993. |
||||||||
be 262 million. Sterilization now accounts for almost half of all contraceptive use in Asia and 38 percent in Latin America (UNFPA 1994).
The weight of this phenomenon has already been felt in some markets. A survey of developing countries in the 1980s found that, first, the role of the private sector diminished as use of government-provided sterilization grew and, second, that this decrease occurred at the expense of private sector sales of reversible methods (Cross et al. 1991). The other aspect of sterilization that is relevant to the market is that it is, with the IUD, the least expensive method of contraception per couple-year of protection (UNFPA 1994). The initial cost of sterilization is high—it is the second most expensive contraceptive commodity after the pill. However, it is a one-time cost and so becomes very cost-effective in terms of couple-years of protection, although any large up-front cost is very important in the decision-making processes of individual users. A country like
TABLE 5-11 Condom Retail Sales in Europe, 1993 and Projected for 1998 (in millions of U.S. dollars)
|
|
1993 |
1998 |
CAGRa |
|
Italy |
193 |
213 |
2.0 |
|
Germany |
112 |
136 |
4.0 |
|
Spain |
74 |
99 |
6.0 |
|
United Kingdom |
67 |
94 |
7.0 |
|
France |
46 |
74 |
10.0 |
|
Total |
493 |
617 |
4.6 |
|
a Compound annual growth rate. Source: Frost and Sullivan. U.S. Market Intelligence Report: U.S. Contraceptive and Fertility Product Markets (Report #5021-54). New York, October 1993. |
|||
India, with a large population and a growing economy which, all things being equal, might generate private sector purchases of reversible contraceptives in considerable numbers, has a population policy that emphasizes sterilization and thus displaces potential purchases of other, reversible options. That policy is undergirded by important cultural reasons for choosing sterilization, such as lower expectations that there will be remarriage and second families, and gender roles.
There is a virtual pandemic at present of efforts at health care reform worldwide, with heavy emphasis on privatization of health care, cost containment, and cost recovery. In settings where the trend is toward privatization, it is not inconceivable that pricing structures might be adjusted in an upward direction; at least that could occur in the upper tiers of such structures where purchases are made by those not using public health services. In settings where the public sector continues to dominate contraceptive procurement, greater incentives to the market would have to come from increased volumes of users which, in turn, would have to be motivated by government policies.
Another contributor to the disproportionate values of the two markets is the markedly higher prices that can be charged in the industrial economies. In the developing economies, private sector prices may be lower and, more critically, national public sectors or overseas development assistance agencies subsidize large contraceptive procurements for low-cost or free distribution. These distributions are made primarily through governments, which presently supply about 86 percent of all modern methods used in developing countries-95 percent of the clinical methods of sterilization and IUDs, 57 percent of pills, and 47 percent of condoms. This largely describes the picture in Asia, where the large majority of the population and contraceptive users live.
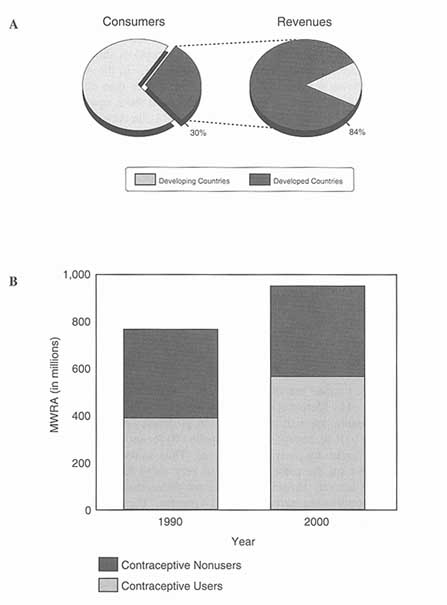
Figure 5-7
A: The contraceptive market in industrial and developing economies. B: The structure of the market in developing countries, 1994 and 2005. Source: Program for Appropriate Technology in Health (PATH). Enhancing the private sector's role in contraceptive research and development. IN Contraceptive Research and Development 1984-1994: The Road from Mexico City to Cairo and Beyond. PFA Van Look, G PérezPalacios, eds. Delhi: Oxford University Press. 1994.
At the same time, public sector dominance in the developing countries is not monolithic. In Sub-Saharan Africa, though the public sector may increasingly be the source of supply for modern methods of contraception, almost two-thirds of condom users are now supplied by the private sector. In the Arab States and Europe, the private sector supplies more contraceptives than do governments, except for sterilizations; the IUD and injectables are about equally divided between government and private sources. Finally, in Latin America, governments supply more sterilizations and IUDS than does the private sector in the ratio of 60/40, but the private sector supplies the bulk of each of the other methods (UNFPA 1994).
Lessons from the Vaccine Field
In 1993, the United Nations Children's Fund (UNICEF) and WHO commissioned Mercer Management Consulting to do a study of the commercial aspects of global vaccine supply, as a contribution to more effective implementation of the Children's Vaccine Initiative (CVI) and the Expanded Programme on Immunization (EPI).18 Even though the vaccine and contraceptives market are not fully analogous, there are enough similarities so that some of the conclusions of the Mercer analysis may be illuminating.
Similarities and Differences Between Vaccines and Contraceptives
The world vaccine market, until recently valued at around $2 billion, has been reestimated by Mercer at close to $3 billion. Of this total, the basic pediatric vaccines account for approximately $1 billion, proprietary products (Haemophilus influenza type b [Hib], hepatitis A, and hepatitis B) for another $1 billion, and the adult vaccine market for most of the balance. Thus, in dollar value, the vaccine market, at least for the present, is not so very much larger than the current contraceptives market. However, as indicated above, the annual growth rate of the global contraceptives market is around 4 percent. The annual growth rate for the number of doses of pediatric vaccines for the EPI between 1982 and 1992 was 7 percent, fueled almost entirely by developing country demand.
After years of stagnating or diminishing revenues, the world vaccine market is now growing rapidly, spurred by a new generation of proprietary products such as Hib and the hepatitis vaccines. These products have revitalized the vaccine industry and motivated manufacturers not only to continue producing vaccine but even to invest to strengthen their competitive position. A series of agreements and acquisitions has provided manufacturers with broader product portfolios, increased geographic access, and stronger R&D capacity (Mercer Management Consulting 1994).
The reinvigoration of the global vaccines market over the past decade is attributable in considerable measure to advances in the field of immunology and
the consequent launch of a few proprietary products that are of worldwide attractiveness. There are some analogies between the two markets. Both vaccines and contraceptives are what might be called "social products" that have a somewhat paradoxical value: The social returns to the development, availability, and widespread use of such products exceed the private returns, that is, what people are willing to pay for that social value. In the case of vaccines, the returns to private developers appear to be high enough (at least in industrial-economy markets) that they will invest in new products. The public sector is also willing to invest; for instance, the U.S. National Institutes of Health provides significant subsidies to vaccine development.
The cardinal difference between vaccines and contraceptives is that vaccine consumption has a commanding externality, or spillover benefit, in the form of "herd immunity," which is the protection that immunization affords nonimmune individuals by reducing the number of infected individuals in a given community below the critical level needed to sustain transmission. Within such a community, the likelihood of a susceptible individual coming into contact with someone who has a specific disease is thus diminished. This is why immunization against the major childhood diseases is compulsory and subsidized in all industrial economies and, increasingly, in many developing economies.
Contraception, at least as typically regarded, does not have an externality that is so clearly compelling. There is painfully ample justification for thinking about unwanted pregnancies as burdens on the health and well-being of societies, families, and individuals, and even as major factors in various social pathologies. It is possible to talk about "epidemics" of unintended pregnancy and unsafe abortion and to make demographic arguments about high fertility as a contributor to planetary "illness." There is also much wider societal recognition of the misery and poor prospects of the unwanted child. Yet, these analogies and arguments have not achieved the same appeal in the public mind that is attached to childhood immunization. That said, the Children's Vaccine Initiative is still searching for strategies that will engage the private sector more systematically in ways that can assure continued R&D investment and a stable level of supply.
Nonetheless, the social returns to contraception are sizable enough so that it has historically received some subsidy in the United States, as well as internationally. New analysis (Trussell et al. 1995b) now permits us to consider contraception from the standpoint of cost-effectiveness, a perspective that offers yet another rationale for investment. However, the savings that can be gotten from contraception generate benefits to industry only in the most indirect way. Thus, cost-effectiveness arguments for industrial investment are not yet persuasive, even though, as we shall see in Chapter 6, the pharmaceutical industry is, more and more, driven by considerations of cost-effectiveness.
In sum, in the case of vaccines, the science is newly productive and its results benefit a larger proportion of those able to pay a satisfactory price; subsidy is available for those who cannot pay and, at least in the United States, appears to be
politically safe. In addition, the ill health that is prevented by immunization consists of diseases that are recognized as real and direct threats to children; while the concept of pregnancy as an illness is not unknown in many contexts, the concept of pregnancy as a disease is not appealing and simply does not have the same notional status as immunization. Furthermore, childhood immunizations do not have anything to do with sexuality, about which the U.S. population entertains a large and complex ambivalence, nor is it connected to the complex and profound personal and societal issues that surround pregnancy. Finally, although there have been adverse effects from vaccines (Institute of Medicine 1992), these are now well known and can be anticipated in connection with the development of new products; also, the National Vaccine Compensation Act has set parameters around the unpredictability of litigation, so that new "blockbuster" vaccine products have not met disaster in the marketplace. Yet, in spite of these differences, there are enough commonalities between vaccines and contraceptives so that the experience with vaccines offers ideas in areas pertinent to enhancing the environment for contraceptive research and development. One of these areas is subsidy.
The Role of Subsidy and High-Volume Procurement
One of the main charges of the Mercer vaccine study was to examine the role of subsidized procurements in the vaccine market. A standard question in this connection is whether such procurements are positive incentives in the marketplace or, on the contrary, act as disincentives. The Mercer analysis concluded that, in the case of vaccines, high-volume public sector procurement does "move the market," that is, it influences manufacturers' behavior and thus can help or hinder the achievement of programmatic goals. The fact that UNICEF purchases roughly 40 percent of developing-country vaccines from 10-12 core suppliers, with the balance satisfied through direct procurement and local production, has been crucial to expanding demand for vaccine doses over the past eight years. UNICEF procurement is based on a strong tiered pricing system in which other customers, including industrial-country governments, pay a price for a given product that covers all production and overhead costs, provides research and development funds for new vaccines, and generates a reasonable return. Mercer finds this pricing structure to be a positive incentive to the market and beneficial to all parties, but notes that such positive effect requires continual reinforcement, in the form of improved country forecasting; more precise targeting of supply to countries most in need; sustained public sector knowledge about the economic and technical issues faced by suppliers; ongoing, collaborative public/private sector evaluation of procurement strategy; and evaluation of manufacturers not only on price but on supply security, R&D capacity, and access to new products (Mercer Management Consulting 1994).
Subsidized procurement is also a component of international family planning
policy. The United Nations Population Fund (UNFPA) has managed such independent, centralized procurement for contraceptives since 1983 and the pace of utilization has more than tripled over the last few years. Donors are increasingly using UNFPA procurement services because the Fund has developed the ability to manage competitive bidding for bulk purchases from manufacturers and suppliers in over 20 countries worldwide and can negotiate low prices while ensuring product quality. In 1988-1989, 47 and 59 countries, respectively, asked UNFPA to procure contraceptives using UNFPA or external resources;19 currently, the Fund serves approximately 120 countries altogether. In 1994, UNFPA spent $104 million on procurement, of which $82 million was spent on contraceptives and a little over $1 million on raw materials (for example, hormonal steroids) for the manufacture of contraceptives. The Fund's reading of the current situation is that because contraceptive prevalence rates are increasing in most countries, because the absolute number of couples of reproductive age keeps growing, and because population groups such as single persons and adolescents are increasingly in need of contraceptive services, the commodity volumes required by individual countries can only rise. This expansion, together with the customary unpredictability in the number and timing of requests for UNFPA procurement assistance, have made it clear that much greater resource allocations for contraceptive commodities and a more efficient procurement system are needed if emergency funding gaps and stockouts are not to surge in size and frequency (UNFPA 1995).
As a consequence, a proposal was prepared at the request of the UNDP and UNFPA Executive Board for the establishment and management of a Global Contraceptive Procurement Program that will create a ''revolving fund" for procurement of contraceptive commodities essential to reproductive health programs—including family planning and sexual health programs—in developing countries with economies in transition. Several multilateral (including the World Bank and the European Union) and bilateral agencies are expected to contribute to the fund, to be managed as a trust, for purchase of buffer stocks of commonly requested contraceptive commodities from established manufacturers. The program will procure contraceptives by pharmaceutical composition or generic description within pre-agreed (i.e., WHO-approved) specifications at the lowest available international price and the revolving fund principle will ensure that the buffer stock will be maintained at some predetermined level to compensate for volatility. Replenishment of the fund will be accomplished primarily by recovering funds from UNFPA country program allocations; an additional fee or premium will be put back into the program to increase the amount of available funds, offset overhead and such contingency costs as exchange rate fluctuations, as well as to help ensure that requests made to the program were truly of an emergency nature and not regular requirements. Support will also be provided through various mechanisms for improving country-level logistics and procurement capabilities.20
A major consequence of the Mercer study that is relevant to contraceptives is that UNICEF has changed its policies for donating vaccine so that, rather than giving vaccine to any nation that requests it, UNICEF now targets donations to the poorest countries. The hypothesis is that this reconstituted UNICEF customer list will force many more countries to begin buying vaccine; this may, in turn, provide a greater incentive for U.S. manufacturers to participate more fully in the developing-economy vaccine market (Institute of Medicine 1995b).
While a demographic argument for increased access to family planning options is insufficient, it is useful as another perspective on the potential market for contraceptives, including new contraceptives. UNFPA has defined the unmet need for contraceptive commodities on the basis of projected population figures from 1994 to the year 2005. The agency has calculated the numbers of contraceptive users and new acceptors that will be required to limit growth to a "medium" population projection of 950 million more people worldwide by that year. The conclusion is that, to not surpass that projection, there would have to be a modest increase—about one-half of a percentage point a year—in contraceptive prevalence in developing countries, an increase from the current rate of 57 percent to 63 percent by 2005. This small increase, combined with the large absolute growth expected in the number of married/in union women, would produce an increase of 157 million users over the period. While this sounds modest, 86 percent of that increase in users would have to come from regions with low current prevalence, primarily Sub-Saharan Africa, where most governments are severely constrained in what they can spend in the health sector and are greatly dependent on external assistance (World Bank 1993).
To achieve the level of contraceptive use needed to accomplish this demographic goal, large amounts of contraceptive commodities will be required between 1994 and 2005:196 million sterilizations, 436 million IUD insertions, 898 million injections, over 12 billion cycles of pills, and 70.3 billion condoms (55.7 billion for family planning and 14.6 billion for STD/HIV prevention), signifying a cumulative total cost of $8.1 billion for the period 1993-2005. If another $587 million is added for the projected 6.8 million Norplant users, the grand total would be almost $9 billion over the 12-year period, an average annual cost of somewhere around $650 million (UNFPA 1994), considerably more than the current market share for these regions.
UNFPA has estimated that in 1994 governments, including multilateral and bilateral donors, would provide 75 percent of all modern contraceptive methods, albeit with widely varying proportions in the commodities supplied, a total cost to governments of $398 million. Donor contributions represent approximately $100 million (in 1992, USAID spent $39.9 million on contraceptive commodities and UNFPA, using its own resources, spent $17.1 million plus $33.9 million on behalf of others, a total of $90.0 million). UNFPA goes on to conclude that developing countries would provide 56.5 percent, or $298.4 million, of the costs of contraceptive commodities in 1994, much of which is accounted for by China
and India. Thus, the private sector accounts for about 25 percent of the total, or $130 million, a large part of which is sales. Private Sector and pharmacy participation in the market is widely variable from region to region, ranging from highs of 62 and 33 percent in Latin America and the Caribbean, respectively, to lows of 7 and 0.3 percent in Asia and the Pacific, respectively (see Table 5-12).
One unknown variable in determining whether subsidy is either an incentive or disincentive to the market is the size of the subsidy and the extent to which it can be depended upon to continue. At present, direct donor subsidies account for about 25 percent of public sector participation in the provision of contraceptive commodities to developing economies. This is smaller than the UNICEF participation in the vaccine market but it is not inconsequential. The U.S. portion of direct external donor assistance in 1993 was 16.3 percent of total donor funding for contraceptive commodities and there are currently questions about whether that level will be sustained. Funding for contraceptive purchase and distribution is just part of the total funding picture for the field as a whole and is addressed as such in Chapter 6.
The Cost-Effectiveness of Contraception
As both a public and a private good, contraceptive technologies have a double identity. Both public and private decisions are made to acquire those technologies, and each decision-making process employs different criteria. However, one criterion that is shared between public sector and private sector purchasers of contraceptive technologies is value for money, that is, the potential for maximum gain for expenditure.
An important source of guidance to achieving value for money in health-related spending is estimation of the cost-effectiveness of different health interventions and medical procedures (World Bank 1993). Because the application of cost-effectiveness analysis to health is difficult, relatively little has been done in a way that permits resource allocation across a broad range of options (World Bank 1993). This is especially true for developing countries where most health services have traditionally been provided by public sectors whose agendas have been ruled by forces other than cost-effectiveness. Where such analysis has been undertaken, the emphasis has been on the curative side of the health care equation, since costing out the effectiveness of prevention, with its long-term payoffs and multiple externalities, is particularly difficult.
Until recently, little cost-effectiveness analysis had been done in connection with family planning, despite the fact that failure to avert unintended pregnancies is patently very costly to the society (Lee and Stewart 1995). This may be due to the fact that contraception is a preventive intervention with payoffs too longterm, obvious, or various to quantify, or to a perception that cost-effectiveness is unimportant relative to the successful achievement of demographic objectives. Another possibility is that contraceptive commodities are a relatively small pro-
TABLE 5-12 Estimated Sources of Supply of Modern Methods of Contraception in Developing Countries, by Method and by Region, 1994 (in %)
|
Source |
Modern |
Sterilization |
Pill |
Injectable |
IUD |
Condom |
|
Total |
|
|
|
|
|
|
|
Government |
86.3 |
95.0 |
56.7 |
66.8 |
94.4 |
47.1 |
|
Private |
13.7 |
5.0 |
43.3 |
33.2 |
5.6 |
52.9 |
|
Pharmacy |
4.1 |
0.0 |
32.7 |
5.8 |
0.2 |
40.7 |
|
NGO |
0.6 |
0.5 |
0.8 |
0.5 |
0.5 |
0.4 |
|
Other |
9.1 |
4.5 |
9.8 |
26.9 |
5.0 |
11.8 |
|
Sub-Saharan Africa |
|
|
|
|
|
|
|
Government |
65.0 |
53.2 |
67.4 |
81.3 |
62.9 |
35.5 |
|
Private |
35.0 |
46.8 |
32.6 |
18.7 |
37.1 |
64.5 |
|
Pharmacy |
4.3 |
0.0 |
7.1 |
0.3 |
0.0 |
18.6 |
|
NGO |
3.2 |
0.6 |
3.4 |
2.0 |
5.5 |
3.4 |
|
Other |
27.4 |
46.3 |
22.1 |
16.4 |
31.6 |
42.5 |
|
Arab States and Europe |
|
|
|
|
|
|
|
Government |
42.5 |
82.3 |
32.6 |
47.9 |
49.8 |
22.2 |
|
Private |
57.5 |
17.7 |
67.4 |
52.1 |
50.2 |
77.8 |
|
Pharmacy |
31.6 |
0.0 |
62.3 |
6.8 |
2.7 |
72.9 |
|
NGO |
0.7 |
0.0 |
0.9 |
2.7 |
0.9 |
0.1 |
|
Other |
25.2 |
17.7 |
4.2 |
42.5 |
46.6 |
4.8 |
portion of the costs of a family planning delivery system and, in many cases, are donated or bulk-procured at concessional prices, so that cost-effectiveness considerations seem irrelevant. For countries that construct their family planning programs around one or two methods, there is little reason for such analysis.
Now, however, as health care costs have risen everywhere and the need for sectoral reform looms large, the issue is forced. No system component is immune to the urge for savings. Family planning must compete with other interventions, curative and preventive, for its share of public sector allocations and managed care packages and for its position in the portfolios of institutions providing external assistance to developing economies. In this competition, family planning will be found to have real competitive advantage: First, it is highly cost-effective and, second, a good proportion of the ensuing savings can be realized within a 12- to 18-month time horizon (Forrest and Singh 1990; Stewart 1995). And because, in the developing world, there is accumulating pressure for a greater array of contraceptive methods, most of which cannot be locally procured, there are foreign exchange implications which may require more complex and precise calculations than has been the case.
Under the rubrics of family planning and contraception, there are two major considerations. One is the cost-effectiveness of family planning qua family planning, as a set of services that subsumes the provision of contraceptive technologies. The other is the cost-effectiveness of each contraceptive method relative to others. Each consideration plays a different role in decisions about technology utilization and the mix of methods that will be offered, and each also plays a role in the structure of the market and industry's perception of that market's value.
The Cost-Effectiveness of Contraception in the United States
Table 5-13 presents a summary of costs of the major conditions against which contraception is in some way protective, the costs per relevant intervention, and estimated savings. It includes sexually transmitted diseases, since those are transmissible through the same process as conception, and since a method that could provide simultaneous protection against both conception and infection is very high on the list of women's priorities for new technologies. The data are limited to the United States, since data elsewhere do not seem to exist at comparable levels of detail.
Figures 5-8a and b summarize the conclusions from a recent, most meticulous analysis of the economic value of contraception from the perspectives of a private payer and a publicly funded program. The study compared 15 methods in terms of their direct medical costs (the direct costs of using each method, the likelihood and costs of potential side effects, and the likelihood and cost of pregnancies due to contraceptive failure). The main outcome measures included 1- and 5-year costs and the number of pregnancies avoided compared with use of
no contraceptive method. Thus, the baseline was the cost of using no contraception, which resulted in 4.25 pregnancies per sexually active woman, an outcome that costs private insurers or patients themselves $14,663 (including the weighted costs of prenatal and delivery services, abortions, miscarriages, and ectopic pregnancies) and costs public sector payers $6,490. The copper-T IUD proved most cost-effective and, because of its high efficacy, associated savings are correspondingly high: $14,122 for private payers and $6,269 for public payers. For those who want no more children, sterilization is also highly cost-effective but saves less since its (one-time) cost is more; implants and injectables are also very cost-effective but, again, take time to be amortized. The use of emergency contraceptive pills (ECP) following unanticipated, coerced, or unprotected intercourse, or after a method failure, also results in significant cost savings. These figures are, of course, sensitive to changes in commodity prices. For example, in 1991, one study discovered average increases in the prices charged for the most-used oral contraceptives of around 42 percent (Daley and Gold 1993), which have especially powerful impact on constrained state-level health budgets.
However, the methods that are most cost-effective in terms of preventing pregnancy do not reduce the risk of sexually transmitted infection, although oral contraceptives, implants, and injectables do reduce risk for pelvic inflammatory disease. Unfortunately, the contraceptive methods that are also risk-reducing are less effective as contraceptives, so that double method use becomes necessary; this in turn reduces cost-effectiveness. Methods that are efficaciously STD-protective and contraceptive could be highly cost-effective, since STD prevalence and corresponding costs continue to rise (Donovan 1993; Hellinger 1993).
The bottom-line message from these analyses is simple. As the authors point out, "regardless of payment mechanism or contraceptive method, contraception saves money" (Trussell et al. 1995b). Even the crudest summation of putative savings is compelling: total estimated annual savings from averting unintended pregnancies ($1.8 billion for associated medical costs alone); teenage pregnancy ($10 billion, a figure which duplicates some of the medical costs of "unintended pregnancies" but introduces some welfare costs); and at least part of the costs of chlamydial infection, gonorrhea, and herpes (over $5 billion). These are not small dollars.
Contraceptive Cost-Effectiveness in the Developing World
Cost-effectiveness studies in the developing world have focused primarily on the costs of "excess fertility" to the family or society, defined from several perspectives: from a health perspective (births to women too young, too old, of too high or too closely spaced parity); from a demographic perspective (fertility that pushes population growth rates above 2 percent); or from a household perspective (what women or couples view as excess fertility). From the demographic perspective, family planning is viewed as particularly "cost-effective"
TABLE 5-13 The Cost-Effectiveness of Family Planning and Contraception, United States
|
Costs |
Intervention |
Savings |
|||
|
Event/Condition |
Dollar Costs |
Intervention |
Dollar Costs |
X Averted |
Dollar Savings |
|
Unprotected sex (85 pregnancies per 100 typical women); estimated at 5,726,412 events/ yr. - mutual intercourse w/o contraception, method failures, and rapes |
$3,795, typical managed care setting; $1,680 in publicly funded program |
Publicly funded contraceptive services |
$412 million (1987) |
3.1 million UIP/yr.a (1.3 million births) (1.4 million abortions) (0.4 million miscarriages) |
$1.8 billion/yr., immediate and short-term; $4.40 per public dollarb |
|
|
|
|
20,000 fewer LBWs 106,900 fewer births w/ no or late prenatal care |
|
|
|
|
|
Emergency contraception (I)c |
Managed care: $59 for ECP, $392 for copper-T IUD; public sector setting; $35 for ECP, $172 copper-T IUD |
53/100 women treated with ECPs; 71/100 women treated w/ postcoital insertion of Copper-T IUD: $123 in |
ECP: $142 in managed care setting, $54 in public sector setting: managed care setting, $53 for copper-T IUD in public sector setting (plus cost savings from 10 yrs. of high-efficacy contraception) |
|
|
|
Emergency contraception (II)c |
$71 for ECP in a managed care setting, $49 in public sector setting |
As above |
Per yr.: $90 for users of cervical cap, $101 male condom, $115 diaphragm, $136 spermicides, $140 |
|
Costs |
Intervention |
Savings |
|||
|
Event/Condition |
Dollar Costs |
Intervention |
Dollar Costs |
X Averted |
Dollar Savings |
|
|
|
|
|
|
withdrawal, $156 periodic abstinence, female condom. Variable by frequency and % of births calculated as unwanted vs. mistimed |
|
Teen pregnancy |
$25 billion/yr.d ($18,133 by child's 20th birthday for family begun by teenage mother in 1990) |
|
|
All births to teenage mothers |
$10 billion (40% of calculated expenditures) |
|
Abortion |
|
Government pays for abortions for poor women |
|
670,000 abortions (1982-1988) |
$4.00 per public dollar (state funds)e $340-$415 million net savings over 2 yrs. for nation as a whole |
|
STDs: Chlamydiaf herpes |
Over $5 billion/yr. |
|
|
|
|
|
Gonorrhea Chlamydiaf |
$2.18 billion/yr. (1990 projected) |
|
|
|
|
|
Ectopic pregnancy |
$1.1 billion/yr. |
|
|
|
|
|
HIV/AIDS |
$64 billion (1991) |
|
|
|
|
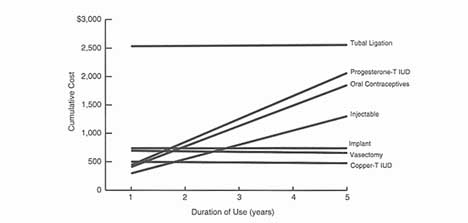
Figure 5-8a
Cumulative costs associated with selected contraceptive methods in the managed payment model.
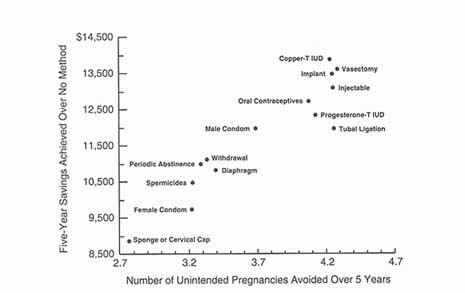
Figure 5-8b
Cost savings and pregnancies avoided over 5 years for contraceptive methods compared with no method, managed payment model. Source (Figures 5-8a and 5-8b): J Trussell, JA Leveque, JD Koenig et al. The economic value of contraception: A comparison of 15 methods. American Journal of Public Health 85(4):494-503, April 1995.
where fertility and mortality rates are high; savings are expected to be realized through reductions in maternal mortality and child deaths. More comprehensively, and as a very general rule, the higher the rates of fertility and mortality, the lower the costs per infant and child death averted, the higher the savings to government, and the greater the theoretical cost-effectiveness of family planning. As in industrialized countries, savings to government may include cost per pregnancy per mother, cost of care for a child in the first year of life, the benefits of preventing incomplete abortions and their subsequent treatment, and, more broadly, sectoral savings in education, health care, housing, infrastructure, and social services. These societal savings are also weighed against the costs of achieving fertility reduction, and these costs depend on the motivation of women to control their fertility (Birdsall et al. 1987; Cochrane and Sai 1993; Fauveau 1991; Figa-Talamanca et al. 1986; World Bank 1993) or, in more recent parlance, their reproductive intentions.
The costs of unrealized reproductive intentions, as expressed in unintended and unwanted pregnancy, may be very high indeed at the household level and even more complex to quantify than either demographic or societal costs. There is a sadly expanding body of testimony to those costs and, in many ways, they are alike in many developed and developing countries, particularly in urbanizing environments. These consequences and their costs can be severe and lasting: abortion, insufficient participation in prenatal care, greater tendency to take behavioral risks during pregnancy, low birth weight, infant mortality, poor child health and development, maternal deaths and reproductive complications, postpartum depression, domestic violence, and economic hardship for others in the family (Institute of Medicine 1995a). Virtually all studies focus on portions of these consequences and subsets of intervening variables, since any systematic, aggregated analysis of such a vast array is probably impossible.
What has not been done in developing-country contexts is the sort of method cost-effectiveness analysis described in the preceding section. Since family planning programs will remain part of the content of public health services and, in some cases, of managed care, such analysis may be timely. This dearth of analysis may be on its way to being repaired: The contraceptive cost-effectiveness model discussed above is being considered for application in Chile (personal communication, S. Díaz 1995).
The Costs of Sexually Transmitted Reproductive Tract Infections
As discussed earlier in this report, the burden of the sexually transmitted diseases in the developing world is enormous, as are its social and economic consequences. The U.S. Public Health Service has estimated the societal costs of the sexually transmitted diseases in the United States to be in excess of $3.5 billion annually (Public Health Service 1991; see also Washington et al. 1986, 1987). The quantification of those consequences remained largely uncharted
terrain until the publication of the recent work by Trussell et al. (1995a) which, in addition to calculating the savings from currently available contraceptive methods, calculates the potential impact of each method on the incidence and cost of sexually transmitted diseases and the resulting total costs or savings for the method. 21 The rank ordering of contraceptives by total costs changed only slightly when the costs of sexually transmitted diseases were included. When those costs are factored in, savings for each contraceptive method compared with ''no method" increased slightly for the barrier methods (male and female condoms, diaphragm, cervical cap, sponge, and spermicides), ranging from $283 to $183 saved per year, and actually decreased for IUDs. However, these calculations may be to some extent artifactual: First, the FDA allows only latex and plastic male condoms and the polyurethane female condom to be marketed as prophylactics against STDs and, second, the relatively small impact of STD costs on total savings from use of contraceptive methods really derives from the low incidence of sexually transmitted diseases when all women of reproductive age are considered as a group. Were the same analysis to be focused on those age cohorts among whom incidence of STDs is highest, that is, just the younger cohorts, the savings impacts would be much greater (Trussell et al. 1995a).
So far, only one published study (Over and Piot 1993) has attempted that quantification for the developing world, even though there are fragments of evidence that the economic costs associated with the sexually transmitted diseases and their complications and sequelae are substantial. The impression is that the direct costs of treating the consequences of these diseases are much greater in developing countries because of higher barriers to care and patterns of antibiotic resistance. However, the only quantification of this impression is based on proxy data such as hospital admission records from gynecology wards. These suggest that the opportunity costs of these complications are, indeed, considerable. The treatment of pelvic inflammatory disease in Sub-Saharan Africa accounts for anywhere from 17 to 44 percent of admissions to gynecology wards, and in Nigeria ectopic pregnancies alone account for 15 percent (Meheus 1992; Piot and Rowley 1992). There is also evidence of high STD-associated indirect costs, that is, the value of the labor lost from morbidity, debility, and premature mortality, as well as the value of any labor diverted from other productive uses to care for the ill (Piot and Rowley 1992). Two sets of calculations—one estimating the average annual number of discounted healthy (or productive) days of life lost by women with STDs in an urban area in Africa, the other estimating the incidence of STD complications in infants born to infected women—indicate that STDs are major contributors to adverse pregnancy outcomes and that preventing STDs produces considerable health gains.
Except for AIDS, there are few data on the effectiveness and costs of the currently available interventions against STDs in the developing world, on the benefits of averting a single case of STD, or on the cases averted because of arrested transmission. Use of condoms to prevent HIV transmission, at a cost of
$30 per Disability-adjusted Life Year (Cochrane and Sai 1993), ranks among the 10 most cost-effective interventions to improve adult health. Nonetheless, the cost of providing condoms in urban Africa alone surpasses present government per capita expenditures on all health interventions (Piot and Rowley 1992). UNFPA projects requirements for condoms for STD/AIDS prevention in developing countries between 1993 and 2005 at 14,635 million, at a total cost of about $406.5 million, around $31.3 million annually. This figure is based on use in high-risk, transient encounters, that is, outside marriage or stable union; thus, the costs of condoms for use with a regular partner would be additional (UNFPA 1994).
Paying for Contraception
The savings that can be realized from contraception are of such magnitude that it is hard to understand why they appear to be so unappreciated by virtually all providers of health insurance coverage. The probability is that there are two general health care markets that will grow dramatically: managed care for the employed and Medicaid managed care, with the latter becoming more and more a for-profit, competitive enterprise (Winslow 1995). As of July 1994, about 65 percent of all private payers, close to 115 million people, were enrolled in some form of managed care plan, an increase of about 10 percent from the preceding year, a rate that is expected to persist (Bailit 1995). As managed care plans "industrialize," expand their dominance, and consolidate in different ways, they can be expected to be increasingly capable of driving other components of the health market, including the market for pharmaceuticals. Thus, the extent to which they reimburse contraception as one strategic element in the preventive and cost-savings components of their portfolios could become more of an incentive and, perhaps, a stabilizing force in the marketplace, both of which can serve as stimuli to innovation.
At least one of the dimensions of that shift would not seem to have been predictable. In April 1995, the Wall Street Journal reported the new interest of health maintenance organizations (HMOs) in the poor. Once shunned by HMOs, those eligible for Medicaid are now seen as a major source of enrollment growth—and of profits. The logic is that by providing Medicaid patients with their own primary-care doctors, HMOs believe they can curb the use of high-cost emergency rooms for routine care, thereby reining in costs while vastly improving health care for the poor. Since family planning services are a logical part of primary care and apparently much desired by many plan participants, and since it is not hard to grasp the cost-effectiveness of contraceptives, it would not be implausible for managed care plans to incorporate those components into their service delivery packages. It may be that some states at least will seek to mandate such inclusion. As of August 1995, a landmark bill had been passed by the
California State Assembly and was pending in the State Senate, calling for all health insurance plans to include most contraceptives.
If this is to be the case, it would represent a major change in current patterns. Not surprisingly, given a tradition of covering surgical procedures but not prevention, laparoscopic tubal ligation is routinely covered by 86 percent of large-group plans, preferred provider organizations (PPOs), and HMOs, and 90 percent of point of service (POS) networks. Coverage of vasectomy is roughly the same. This may partly explain some of the high rates of surgical sterilization in the United States and the low rates of IUD use (Lee and Stewart 1995), yet another case in which availability shapes demand, rather than vice versa. Two-thirds or more of all plan types—including 66 percent of large-group fee-for-service plans, 67 percent of PPOs, 83 percent of POS networks, and 70 percent of HMOs—routinely cover induced abortions employing dilation and curettage-suction aspiration. However, almost one-fourth of all coverage of abortion by large-group plans is restricted in some way, for instance, by requiring the provider to certify the concurrent presence of a specific medical indication (Guttmacher Institute 1995c).
However, coverage of reversible contraception is, indeed, "unequal and uneven" (Guttmacher Institute 1995c). Half of typical fee-for-service plans written for large groups or PPOs cover no reversible contraception whatsoever. Less than 20 percent of large-group indemnity plans or PPOs, and less than 40 percent of POS networks and HMOs, routinely cover all five of the most effective reversible methods (IUD and Norplant insertion, Depo-Provera injection, and oral contraception) in their typical plans. What is particularly surprising is that 66 percent of large-group plans do not cover oral contraceptives, the most used reversible method in the United States, even though 97 percent of those same plans typically cover prescription drugs. Similarly, even though 92 percent of those plans cover medical devices generally, only 24 percent cover Norplant, 18 percent cover IUDS, and 15 percent cover diaphragms. Coverage of oral contraceptives is much higher in POS networks and HMOs. Only 7 percent of HMOs provide no contraceptive coverage at all, and 39 percent cover all five methods (Guttmacher Institute 1995c).
From a cost-effectiveness standpoint, these patterns are not logical. An increase of just 15 percent in new oral contraceptive users would produce enough savings in the costs of pregnancy care to cover oral contraceptives for all users in a given health insurance plan. Another instance: A 4 percent increase in copper-T IUD use continued over five years would pay for all IUD users in the plan; an 18 percent increase in one-year IUD users would produce the same result (Lee and Stewart 1995). In an environment that will see dramatic growth in managed care, expanded coverage that would offer a full array of all available contraceptive methods would produce savings to plans and to the society at large, as well as a potentially guaranteed market for both new and existing contraceptives. While these shifts are preeminently a U.S. phenomenon, they are not exclusively so, and
their power to move a market that is so responsive to U.S. demand is highly relevant to the wider market that includes the developing countries.
Public Sector Coverage of Contraception in the United States
Unlike private insurers and health maintenance organizations, all 50 states and the District of Columbia are required by law to provide reimbursement for contraceptive services, and one in three women who made a family planning visit in 1988 (the last year for which comprehensive data are available) reported going to a publicly funded family planning clinic (Guttmacher Institute 1995c).
The proportions of public funding for family planning through different channels have changed a great deal in recent years. The overall pattern has been that, since the late 1980s, Medicaid has assumed the role of lead public funder for contraceptive services, as provision of contraceptive services through other mechanisms, notably Title X, has declined (see Figure 5-9). As of 1990, Medicaid accounted for 58 percent of all federal family planning expenditures, at a level of approximately $270 million; by 1992, that amount was $319 million and amounted to 50 percent of all public funding (Guttmacher Institute 1995c).
Another overall pattern has been that, when inflation is taken into account, total public funding for contraceptive services fell by 27 percent between 1980 and 1992, with Title X funding falling by 72 percent over that same period, with a corresponding increase in unintended pregnancy. At the same time, the costs of providing those services, including costs of contraceptive commodities, have risen; for example, the average price for oral contraceptives to publicly funded family planning clinics rose 42 percent in just one year, between 1991 and 1992 (Daley and Gold 1993).
The third pattern of interest is that of the general distribution of payment source for family planning visits. A striking 41 percent of all women who received family planning services paid for their most recent visit out of their own pockets, 25 percent were completely covered by insurance, 17 percent used insurance with a copayment or deductible, and 7 percent of visits were covered by Medicaid (Kaeser and Richards 1994). The fact that women pay so much out of their own pockets for these services can be viewed in two ways: One is that they value the services and the commodities enough to pay for them; the other is that systems that could cover at least some of the costs of contraceptive services do not, for one reason or another, do so. Both possibilities can coexist and both have market implications. The first is the expression of market demand; the second is that there is a large institutional purchasing capacity that remains unused. This leaves unaddressed the economics of over-the-counter contraceptive purchases, a question that has been raised in the many discussions about the wisdom of making oral contraceptives available without prescription. The decision for the time being, at least in the United States, is that OCs will remain a prescription product, so that the economic issues are moot for now. Still, it is worth noting that such
Figure 5-9
Public expenditure for contraceptive services, United States. Source: Alan Guttmacher Institute. Uneven and Unequal: Insurance Coverage and Reproductive Health Services. New York and Washington: The Alan Guttmacher Institute. 1995.
purchases would not be covered by third-party payers but by individuals, who would assume the responsibility for purchase and for a greater share of physical risk.
Concluding Comment
At the beginning of this chapter, we used the term "dilemma," one of whose meanings is "a difficult problem . . . seemingly incapable of a satisfactory conclusion" (Merriam-Webster's New International Dictionary 1986). The analysis reflected in this chapter persuades us that the problem of translating unmet need for new contraceptive options into market demand, though difficult, is not insoluble. While the existing array of contraceptive options represents a major contribution of science and industry to human well-being, it still fails to meet the needs of significant numbers of individuals in significant populations. Even if the general need is not seen as constituting attractive market demand (defined as need plus willingness and ability to pay), substantial components of that overall need do respond to such a definition. The epidemic of sexually transmitted infections; large gaps in an array of "menses-inducers" tailored to the wide range of women's practical, physiologic, and ideologic concerns; the paucity of methods for male participation in contraception; and the persistent importance of
reducing method side effects; all are plausible indicators of commercially appreciable market demand.
A quantitative case can also be made. The numbers of contraceptive users has grown and continues to grow. While sterilization rates qualify those numbers, sterilization rates also represent an indeterminate number of consumers, particularly younger consumers, who might prefer a reversible method. Because availability shapes demand and because a full range of contraceptive options is often inaccessible, there is a potentially large population, importantly consisting of method-discontinuers and method-switchers, for new products. Availability of a good method mix has an independently positive effect on contraceptive useprevalence, as well as on reduction in crude birth rates, and might be seen in itself as a market-driver. Finally, while it is true that inability to pay conditions profit margins in many instances, the cost-effectiveness of contraception is clear enough so that it should motivate expanded coverage where third-party payment is a factor, and subsidy for bulk purchases where that is required, again improving the level of demand and the size of the market for contraceptives, in the United States and abroad.
References
Alan Guttmacher Institute. Hopes and Realities: Closing the Gap Between Women's Aspirations and Their Reproductive Experiences. New York: The Alan Guttmacher Institute. 1995a.
Alan Guttmacher Institute. Issues in Brief: The U.S. Family Planning Program Faces Challenges and Change. New York and Washington: The Alan Guttmacher Institute. 1995b.
Alan Guttmacher Institute. Uneven and Unequal: Insurance Coverage and Reproductive Health Services. New York: The Alan Guttmacher Institute. 1995c.
Alan Guttmacher Institute. Sex and America's Teenagers. New York: The Alan Guttmacher Institute. 1994.
American Health Consultants. Contraceptive Technology Update 16(9):105-120, 1995.
Ashford LS. New perspectives on population: Lessons from Cairo. Population Bulletin 5(1):22, 1995.
Bailit HL. Market strategies and the growth of managed care. IN Academic Health Centers in the Managed Care Environment. D Korn, CH McLaughlin, M Osterweis, eds. Washington, DC: Association of Academic Health Centers. 1995.
Birdsall N, SH Cochrane, J van der Gaag. The cost of children. IN Economics of Education: Research and Studies. George Psacharopoulos, ed. New York: Pergamon Press. 1987.
Bongaarts J, J Bruce. The causes of unmet need for contraception and the social content of services. Studies in Family Planning 26(2): 57-75, 1995.
Bosch FX, MM Manos, N Muñóz, et al. Prevalence of human papillomavirus in cervical cancer: A worldwide perspective. Journal of the National Cancer Institute 87:796-802, 1995.
Bruce J, A Jain. A new family planning ethos. IN The Progress of Nations. New York: UNICEF. 1995.
Bulatao RA, RD Lee. An overview of fertility determinants in developing countries. IN Determinants of Fertility in Developing Countries, Vol. 2, RA Bulatao, RD Lee, eds. New York: Academic Press. 1983.
Carpenter PF. Innovation in patient information: The importance of facilitating informed choice. Infectious and Medical Disease Letters for Obstetrics and Gynecology XI(3), 1989.
Cates W. Teenagers and sexual risk taking: The best of times and the worst of times. Journal of Adolescent Health 12(2):84-94, 1991.
Centers for Disease Control and Prevention (CDC). Division of STD/HIV Prevention Annual Report, 1991. Atlanta: CDC, 1992.
Cochrane S, F Sai. Excess fertility. IN Disease Control Priorities in Developing Countries. DT Jamison, WH Mosley, AR Measham, JL Bobadilla, eds. New York: Oxford University Press. 1993.
Correa S. Population and Reproductive Rights Component: Platform Document, Preliminary Ideas. Paper prepared for the International Conference on Population and Development [Cairo 1994]. Development Alternative with Women for a New Era. February 1993.
Cross HE et al. Contraceptive Source and the For-Profit Private Sector in Third World Family Planning. Paper presented at the Annual Meeting of the Population Association of America, Washington, DC, March 21, 1991.
Daley D, RB Gold. Public funding of contraceptive, sterilization and abortion services, fiscal year 1992. Family Planning Perspectives 25(6):244-251, 1993.
Donovan P. Testing Positive: Sexually Transmitted Diseases and the Public Health Response. New York: The Alan Guttmacher Institute. 1993.
[The] Economist. On the needless hounding of a safe contraceptive. pp. 113-114, 2 September 1995.
Fauveau V. Matlab Maternity Care Program. Review paper prepared for the World Bank Department of Population and Human Resources. Washington, DC. 1991.
Figa-Talamanca I, TA Sinnathuray, K Yusof, et al. Illegal abortion: An attempt to assess its cost to the health services and its incidence in the community. International Journal of Health Services 16:375-389, 1986.
Food and Drug Administration. FDA Talk Paper: Norplant Update. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service. 17 August 1995.
Forrest JD. Contraceptive use in the United States: Past, present and future. Advances in Population 2:29-48, 1994a.
Forrest JD. Epidemiology of unintended pregnancy and contraceptive use . American Journal of Obstetrics and Gynecology 170(5, Part 2):1485-1489, 1994b.
Forrest JD, S Singh. Public sector savings resulting from expenditures for contraceptive services. Family Planning Perspectives 22(6), 1990.
Frost and Sullivan. U.S. Market Intelligence Report: U.S. Contraceptive and Fertility Product Markets (Report #5021-54). New York: Frost and Sullivan. October 1993.
Gallup Organization. Women's Attitudes Towards Contraceptives and Other Forms of Birth Control (Poll conducted for the American College of Obsterics and Gynecology). Princeton, NJ. January 1994.
Germain A. Are we speaking the same language? Women's health advocates and scientists talk about contraceptive technology. IN Four Essays on Birth Control Needs and Risks. R DixonMueller, A Germain, eds. New York: International Women's Health Coalition. 1993.
Guttmacher Institute. See Alan Guttmacher Institute.
Hatcher RA, J Trussell, F Stewart et al. Contraceptive Technology (16th Revised Edition). New York: Irvington Publishers. 1994.
Hellinger FJ. The lifetime cost of treating a person with HIV. Journal of the American Medical Association 270:474-478, 1993.
Herman R. Whatever happened to the contraceptive revolution? Washington Post , Health Section, 13 December 1994.
Hira SK, AB Spruyt, PJ Feldblum, et al. Spermicide acceptability among patients at a sexually transmitted disease clinic in Zambia. American Journal of Public Health 85(8):1098-1103, 1995.
Ingrassia M, K Springen, D Rosenberg. Still fumbling in the dark-—Contraception: With all the condoms, pills and foams, why are so many women getting sterilized? Newsweek, 13 March 1995.
Institute of Medicine (IOM). The Children's Vaccine Initiative: Achieving the Vision. VS Mitchell, NM Philipose, JP Sanford, eds. Washington, DC: National Academy Press. 1993.
IOM. The Best Intentions: Unintended Pregnancy and the Well-Being of Children and Families, S Brown, L Eisenberg, eds. Washington, DC: National Academy Press. 1995a.
IOM. The Children's Vaccine Initiative: Continuing Activities—A Summary of Two Workshops Held September 12-13 and October 25-26, 1994. GW Pearson, ed. Washington, DC: National Academy Press, 1995b.
International Development Research Centre (IDRC). Position Paper on IDRC Support for Development of Immunological Contraceptives. Ottawa, Canada, June 1995.
Kaeser L, CL Richards. Barriers to Access to Reproductive Health Services . Paper submitted to the Institute of Medicine Committee on the Role of Planned Childbearing in the Health and WellBeing of Children, Women, and Families, Washington, DC. 1994.
Kaiser/Fact Finders. Survey on Obstetricians/Gynecologists' Attitudes and Practices Related to Contraception and Family Planning. Menlo Park, CA: The Henry J. Kaiser Family Foundation. 1994.
Kaiser/Harris. National Survey Results on Public Knowledge and Attitudes on Contraception and Unplanned Pregnancy. Menlo Park, CA: The Henry J. Kaiser Family Foundation. 1995.
Kolata G. Will the lawyers kill off Norplant? New York Times, section 3, p. 1. 28 May 1995.
Kost K, JD Forrest. American women's sexual behavior and exposure to risk of sexually transmitted diseases. Family Planning Perspectives 24:244-254, 1992.
Landry DJ, TM Camelo. Young unmarried men and women discuss men's role in contraceptive practice. Family Planning Perspectives 26:222-227, 1994.
Lee PR, FH Stewart. Editorial: Failing to prevent unintended pregnancy is costly. American Journal of Public Health 85(4):479-480, 1995.
Maynard R. The Effectiveness of Interventions on Repeat Pregnancy and Childbearing. Paper prepared for the Institute of Medicine Committee on Unintended Pregnancy. Washington, DC: Institute of Medicine. 1994.
Meheus A. Women's Health: Importance of reproductive tract infections, pelvic inflammatory disease and cervical cancer. IN Reproductive Tract Infections: Global Impact and Priorities for Women's Reproductive Health, A Germain, KK Holmes, P Piot, J Wasserheit, eds. New York: Plenum. 1992.
Mercer Management Consulting. Summary of UNICEF Study: A Commercial Perspective on Vaccine Supply. New York: Mercer Management Consulting. 1994.
Moore KA, BC Miller, D Glei, DR Morrison. Adolescent Sex, Contraception, and Childbearing: A Review of Recent Research. Washington, DC: Child Trends, Inc. June 1995.
Mosher WD, WF Pratt. Contraceptive use in the United States, 1973-88. Advance Data from Vital and Health Statistics of the National Center for Health Statistics 182:20, 1990.
Mosher WD. Contraceptive practice in the United States, 1982-1988. Family Planning Perspectives 22(5):198-205, 1990.
National Adolescent Reproductive Health Partnership. NARHP Update. Washington, DC: Association of Reproductive Health Professionals. 1995.
Ortho Pharmaceutical Corporation. Executive Summary: 1995 Ortho Annual Birth Control Study. Raritan, NJ. 1995.
Ortho Pharmaceutical Corporation. Highlights, 1993 Ortho Annual Birth Control Study. Raritan, NJ. 1993.
Ortho Pharmaceutical Corporation. Report on 1991 Ortho Annual Birth Control Study. Raritan, NJ. 1991.
Over M, P Piot. HIV infection and sexually transmitted diseases. IN Disease Control Priorities in Developing Countries, DT Jamison, WH Mosley, AR Measham, JL Bobadilla, eds. New York: Oxford University Press. 1993.
Piot P, J Rowley. Economic impact of reproductive tract infections and resources for their control. IN Reproductive Tract Infections: Global Impact and Priorities for Women's Reproductive Health. A Germain, KK Holmes, et al., eds. New York: Plenum Press. 1992.
Pleck JH, FL Sonenstein, L Ku. Changes in adolescent males' use of and attitudes toward condoms, 1988-1991. Family Planning Perspectives 25:106-110, 117, 1993.
Program for Appropriate Technology in Health (PATH). Contraceptive research and development update. Outlook (Special Issue) 13(20), 1995.
PATH. Enhancing the private sector's role in contraceptive research and development. IN Contraceptive Research and Development 1984-1994: The Road from Mexico City to Cairo and Beyond. PFA Van Look and G Pérez-Palacios, eds. Delhi: Oxford University Press. 1994a.
PATH. Market-Related Issues Affecting the Participation of the Private Sector in Contraceptive Development: A Final Report to the Rockefeller Foundation, 30 November 1994. Seattle, WA: 1994b.
Public Health Service, Department of Health and Human Services. Healthy People 2000: National Health Promotion and Disease Prevention Objectives. Washington, DC: U. S. Government Printing Office. 1991.
Ravindran TKS, M Berer. Contraceptive safety and effectiveness: Re-evaluating women's needs and professional criteria. Reproductive Health Matters 3:6-11, 1994.
Reprogen. Confidential Business Plan. Irvine, CA 1995.
Rinzler CA. The return of the condom. America's Health 6(6):97-107, 1987.
Rosenberg MJ, MS Waugh, S Long. Unintended pregnancies and use, misuse, and discontinuation of oral contraceptives. Journal of Reproductive Medicine 40(5):355-360, 1995.
Russell C. The pill is popular but not well understood: New survey shows many women overestimate the risks, underestimate the benefits. Washington Post, Health section, pg. 9, 6 February 1996.
Schrater AF. Immunization to regulate fertility: Biological and cultural frameworks. Social Science and Medicine 41(5):657-671, 1995.
Schrater AF. The pros and cons: Guarded optimism. Reproductive Health Matters 4, November 1994.
Snow R. Each to her own: Investigating women's response to contraception. IN Power and Decision: The Social Control of Reproduction. G Sen, R Snow, eds. Cambridge, MA: Harvard School of Public Health. 1994.
Stewart FH. Integrating essential public health services and managed care: Family planning and reproductive health as a case study. Western Journal of Medicine 163(Suppl.):75-77, 1995.
Tanfer K. Unpublished data on Norplant knowledge, use, and intentions, presented at National Institute of Child Health and Human Development conference on long-acting contraceptives, Bethesda, MD, September 1995.
Trussell J, L Grummer-Strawn. Contraceptive failure of the ovulation method of periodic abstinence. Family Planning Perspectives 22(2):65-75, 1990.
Trussell J, K Kost. Contraceptive failure in the United States: A critical review of the literature. Studies in Family Planning 18(5):237-283, 1987.
Trussell J, B Vaughan. Aggregate and lifetime contraceptive failure in the United States. Family Planning Perspectives 21(5):224-226, 1989.
Trussell J, J Koenig, C Ellertson, F Stewart. Emergency Contraception: A Cost-Effective Approach to Preventing Unintended Pregnancy. Unpublished manuscript. Princeton, NJ: Office of Population Research. November 1995.
Trussell J, JA Leveque, JDD Koenig, et al. Documenting the economic value of contraception: A comparison of 15 methods. Technical addendum to: The economic value of contraception: A comparison of 15 methods. American Journal of Public Health 85(4):494-503, 1995a.
Trussell J, JA Leveque, JDD Koenig, et al. The economic value of contraception: A comparison of 15 methods. American Journal of Public Health 85(4):494-503, 1995b.
United Nations Development Programme (UNDP), United Nations Population Fund (UNFPA), World Health Organization (WHO), and World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP). Perspectives on Methods of Fertility Regulation: Setting a Research Agenda. Background Paper. Geneva: WHO/HRP. 1995.
UNFPA. Global Contraceptive Commodity Programme: Report of the Executive Director (DP/ 1996/3) . Prepared for the First Regular Session of the UNDP and UNFPA Executive Board, 15-19 January 1996. New York: United Nations Population Fund. 1995.
UNFPA. Contraceptive Use and Commodity Costs in Developing Countries 1994-2005. Technical Report No. 18. New York: UNFPA. 1994.
Washington AE, RE Johnson, LL Sanders. Chlamydia trachomatis infections in the United States: What are they costing us? Journal of the American Medical Association 257(15):2070-2072, 1987.
Washington AE, PS Arno, MA Brooks. The economic cost of pelvic inflammatory disease. Journal of the American Medical Association 255:1732-1735, 1986.
Waugh MS. Report from European and U.S. surveys: OC compliance poorer among American women. Contraceptive Technology Update 15(12): 157-172, 1994.
Westoff CL, F Marks, A Rosenfield. Physician factors limiting IUD use in the US. Paper presented at a conference on A New Look at IUDs-Advancing Contraceptive Choices, New York, 2728 March 1992.
Wilcox LS, SY Chu, ED Eaker, et al. Risk factors for regret after tubal sterilization: 5 years of follow-up in a prospective study. Fertility and Sterility 55(5):927-933, 1991.
Wilcox LS, SY Chu, HB Peterson. Characteristics of women who considered or obtained tubal reanastomosis: Results from a prospective study of tubal sterilization. Obstetrics and Gynecology 75(4):661-665, 1990.
Winslow R. Medical upheaval: Welfare recipients are a hot commodity in managed care now. Wall Street Journal, 12 April 1995.
World Bank. World Development Report 1993: Investing in Health. New York: Oxford University Press. 1993.
World Health Organization (WHO). An Overview of Selected Curable Sexually Transmitted Diseases. (WHO/GPA/STD/95.1). Geneva: WHO/Global Programme on AIDS. 1995.
WHO. Perspectives on Methods of Fertility Regulations: Setting a Research Agenda (Background Paper). Geneva: UNDP/UNFPA/WHO/HRP. 1995.
Zabin LS. Addressing adolescent sexual behavior and childbearing: Self-esteem or social change. Women's Health Issues 4:93-97, 1994.
Zabin LS, HA Stark, MR Emerson. Reasons for delay in contraceptive clinic utilization: Adolescent clinic and nonclinic populations compared. Journal of Adolescent Health 12:225-232, 1991.
Zelnick M, JF Kantner. Sexual activity, contraceptive use and pregnancy among metropolitan-area teenagers: 1971-1979. Family Planning Perspectives 12:230-231, 233-237, 1980.
Notes
-
ogy, and the Public's Health. New York: Springer-Verlag, 1991, p. 1). The ability to determine the number and spacing of births and the prevention of unwanted births is understood as central to the protection of the health of women, children, families, and communities.
-
2.
The contraceptive prevalence rate is defined in terms of the percentage of currently married (or in union) women aged 15-49 using a contraceptive method at the time of survey (WHO/HRP 1995).
-
5.
Diaphragm, jelly, douche, and foam tablets.
-
9.
The acquisition in the summer of 1995 by Ortho of Gynopharma and its IUD and spermicide lines could be a noteworthy contributor to restoring the method to a greater share of the market.
-
15.
For example, a woman would be unable to determine whether or not her antibody levels were
-
providing a contraceptive effect without some sort of home diagnostic capability, for instance, a urine dipstick test.
-
16.
MWRA, or "married women of reproductive age."
-
17.
New population estimates suggest that, as of 1994, this figure is 25 percent.
-
18.
The EPI vaccines target seven diseases: diphtheria, pertussis, tetanus, tuberculosis, polio, measles, and hepatitis B.