6
The Translators: Sectoral Roles in Contraceptive Research and Development
The Role of Industry in Contraceptive Research and Development
The Pharmaceutical Industry
Stage I: The First Contraceptive Revolution and the Primacy of Industry (1951-1972)
Since the 1950s, the involvement of the pharmaceutical industry in contraceptive research and development has passed through three stages, and it is now in a fourth stage whose future pace and direction are unpredictable. The character of that involvement has shifted in each phase in response to factors in the external environment that are peculiar to the field of contraceptive research and development and, perhaps, to the entire area of women's health. Although this chapter emphasizes the history and contemporary dynamics of the U.S. pharmaceutical industry, it incorporates information about firms outside the United States that are involved in some aspect of contraceptive research and development and raises issues deriving from the ongoing processes of industrial globalization.
Stage I, "the contraceptive revolution," can be said to have begun in 1951 more or less officially, when Carl Djerassi at Syntex filed a patent for norethindrone; it ended in the early 1970s. During this period, most contraceptive research and development was sponsored and carried out by large pharmaceutical companies, especially U.S. firms. The field was "pushed" by advances in steroid chemistry and the emergence of an array of complex plastics, neither of which
had had human contraceptive uses as their original objectives. The developers of these first-phase contraceptives were well aware of cultural sensitivities about contraception and the consequent possibilities of corporate risk, but the perceived technological potential and market demand were overriding. A number of firms became engaged in the development and production of the new oral contraceptives (OCs). In the United States, those were Syntex, Searle, Upjohn, Wyeth, Merck Sharpe and Dohme, and the Syntex-Ortho partnership. Other U.S. firms were engaged in the development and production of the first modern intrauterine devices (IUDs): Ortho, Schmidt, American Caduceus Industries, Searle, and A.H. Robins. In Europe, the first firms to be involved in contraceptive research and development were Schering AG, Ciba, and Organon; British Drug House entered with two OC compounds in the early 1960s, and firms in Canada, Switzerland, and France with improved intrauterine devices in the early 1970s. Upjohn and Schering AG also presented the injectable contraceptives, Depo-Provera and Noristerat, for regulatory approval in this time period.
All this unfolded in a climate of general enthusiasm for the postwar "pharmaceutical revolution," and receptivity to effective, reversible, coitus-independent fertility regulation was rapid and enthusiastic. Regulatory requirements were fairly lenient, particularly for the IUD, and clinical studies were less sophisticated than they are today; thus, R&D time was shorter and effective patent life was longer. The taking of a daily pill over a goodly proportion of the reproductive life promised a large market, and the smaller size of the market for the longacting IUD seemed not to be a problem (Gelijns and Pannenborg 1993). Only one firm abandoned the field during this time: Parke-Davis, whose management was worried about negative consumer reaction and conflict with company values.
Stage II: The Rise of the Public and Nonprofit Private Sectors and a Worldwide Orientation (1973-1987)
The second stage of contraceptive research and development began early in the 1970s, its onset marked by negative reports in the medical and lay press on OC side effects. Senate hearings in 1970 received some sensational press coverage and OC use declined noticeably. At roughly the same time, there were reports of side effects of the IUD, primarily the Dalkon Shield, and Robins took the device off the market in 1974.
The period also saw more stringent regulatory requirements, resulting in the extension of R&D time to between 10 and 17 years and, as a consequence, much greater R&D costs and reduction in effective patent life. The eruption of litigation against Robins spilled over onto other IUDs, as well as to oral contraceptives, and public perceptions of the pill and IUD became quite negative. Although oral contraceptives accounted for just under 4 percent of the prescription drug market as the 1980s began, there were more liability suits associated with that method each year of the new decade than for any other drug product (Djerassi
1989). As for the IUD, even though the copper-releasing and medicated devices were major improvements in safety, liability insurance had become essentially unavailable in the United States; even firms with FDA-approved IUDs left the market—and contraceptive research and development—altogether. Research indicating that IUD risks had been overestimated, together with improvements, motivated numbers of European and some developing-country women to return to the method. However, in the United States, only Alza's Progestasert was able to stay on the market and the IUD became virtually a nonoption for American women, never to regain its market share (Gelijns and Pannenborg 1993; NRC/ IOM 1990). Contraceptive research and development became what it continues to be today: highly politicized, with consumer advocates and some women's groups arguing that developers and policy makers have been generically heedless of the needs and safety of women, and opponents of fertility control arguing against any contraceptive research and development whatsoever.
There was little mistaking the growing reluctance of U.S. industry to invest in contraceptive innovation; the barriers were everywhere. As something of a substitution effect, growing interest in family planning in developing countries drew the U.S. Agency for International Development (USAID) into the field, accompanied by funding that motivated research activity in universities and nonprofit organizations, especially those with strong international networks. The Center for Population Research at the National Institute of Child Health and Human Development (NICHD), motivated by U.S. domestic concerns, also became a major source of funding for contraceptive research during this stage. In 1972, the World Health Organization's Special Programme of Research, Development, and Research Training in Human Reproduction was established. Among the nonprofit entities that were either created or that became more active during this stage were the Population Council's Biomedical Research Center, Family Health International (FHI), and the Program for Appropriate Technology in Health (PATH). There were also over two dozen university-based research programs, some of which also took on roles as intermediary funders of research at other universities, for example the Institute for International Studies in Natural Family Planning (IISNFP) at Georgetown University, the Program for Applied Research in Fertility Regulation (PARFR) at Northwestern University and its successor, the Contraceptive Research and Development (CONRAD) program at Eastern Virginia Medical School.
Wyeth, Schering AG, and Organon, recognizing the long-term profit potential of developing-country markets, set up manufacturing facilities in over 20 developing countries, including Bangladesh, Egypt, India, and Indonesia (PATH 1994). Second-generation lower-dose and multiphasic oral contraceptives, minipills, and more selective progestins reduced side effects and revived the reputation of oral contraceptives; when the secondary health benefits of the method also began to be appreciated, the structure of demand shifted back in their favor. And, owing to the sophistication, greater safety, and lower relative cost of endoscopic
techniques, sterilization became by 1982 the most commonly used method in the United States. In the 1988 National Survey of Family Growth (NSFG), male and female sterilization together exceeded OC use and, in the 1990 telephone resurvey, sterilization by itself was the most commonly used method (Peterson 1990), even though, as a one-time permanent method with a limited pricing range and much subsidy, it did not offer industry a growing and profitable market niche. However, the profits that ensued from the other therapeutic and diagnostic uses of endoscopy were considerable so that the technology itself, overall, proved quite lucrative (Gelijns and Pannenborg 1993).
Yet the net result of the dynamics of Stage II was that, by the end of the 1980s, women in the United States had one effective reversible method, the pill, and one effective permanent method, sterilization.
Stage III: The Exodus of U.S. Industry and the Entry of Smaller Firms (1987-Present)
Beginning in the late 1970s, all but two of the U.S. pharmaceutical companies that had engaged in contraceptive research and development over the previous two decades had, to all intents and purposes, ceased any significant involvement in that field: Syntex, Searle, Upjohn, Mead Johnson, Parke-Davis, Merck Sharp and Dohme, Eli Lilly, and Wyeth all withdrew, although some remained involved in production. Of the nine large U.S. pharmaceutical firms that had entered contraceptive research and development in Stage I, all but two-Ortho, a subsidiary of Johnson and Johnson, and Wyeth-Ayerst-had exited by the end of Stage III, although Syntex, Searle, Upjohn, and Parke-Davis continued to produce and distribute the products their firms had developed. By the end of the 1980s, innovation in contraceptive research and development in the pharmaceutical industry resided largely in Europe, in the hands of Organon, Schering AG, and Roussel-Uclaf, a Hoechst subsidiary (Gelijns and Pannenborg 1993).
Another significant Stage III phenomenon was the entry into contraceptive research, development, production, and distribution by smaller companies. In Europe, this category has included firms like Gedeon Richter (Hungary), Alphatron (The Netherlands), Bioself (Switzerland), Cilag AG (Germany), Leiras (Finland), and Theramex (France) (see Table 6-9). In the United States a new pattern evolved, one of collaborative effort among public and private organizations: funding agencies, basic research facilities, university-based scientists, clinical trials organizations, nonprofit organizations, and smaller pharmaceutical companies, some of which were outside the U.S. (see Bronnenkant 1994). These organizational arrangements were unlike the standard model in the two preceding decades, that is, the single, large, integrated pharmaceutical company with, at most, one industry or nonprofit partner. In those years, basic research had been as likely to come out of industry as it was to emerge from the academic research community. In contrast, the multisectoral arrangements of the 1980s were flex-
ible, opportunistic, varied, and complex, and the industry component was just one of several and as likely to be a smaller firm as a larger one. Almost all of these collaborations were focused on modifications of and new delivery systems for existing or improved compounds, namely:
- VLI (purchased by Whitehall Laboratories), National Institute of Child Health and Human Development (NICHD): vaginal sponge (Today) 1
- Medisorb (formerly Stolle Research and Development Corporation), Ortho, World Health Organization (WHO), Contraceptive Research and Development Program (CONRAD), Family Health International (FHI): injectable microspheres
- Finishing Enterprises, Population Council, World Health Organization, Rockefeller Foundation, Ford Foundation: Copper T intrauterine device
- Wyeth-Ayerst, Leiras, Population Council, FHI, Program in Appropriate Technology in Health (PATH), individual clinical researchers worldwide: Norplant implants
- Ortho, Salk Institute, Medisorb (Stolle): LHRH analogues
- Alphatron, Vastech Medical Products, Population Council: nonsurgical vasectomy devices
- Upjohn, Dow Corning, Population Council, Battelle Institute, London International, Roussel UK: hormone-releasing vaginal rings
- London International, National Institute of Child Health and Human Development, Family Health International: polyurethane male condom (e.g., Avanti)
- Tactyl Technologies/SmartPractice, Contraceptive Research and Development (CONRAD) program: nonlatex (Tactylon) male condom
- Wisconsin Pharmacal, CONRAD, FHI, Reddy Health Care: female condom (Reality)
- YAMA, CONRAD, FHI: Lea's Shield.
Stage IV: The 1990s and the Biopharmaceutical Industry
The global pharmaceutical industry of the 1990s is composed of a great array of corporations devoted to the discovery, development, and commercialization of new pharmaceuticals. Over the years since the introduction of oral contraceptives, the industry has evolved and changed in response to a number of factors that are highly relevant to further advances in contraceptive research and development. Those factors include increases in the regulation of pharmaceuticals; a changing economic environment that has driven industry restructuring and stressed a global view of pharmaceutical markets; and—importantly—dramatic growth in the scientific tools and understanding of biology available to assist in the development of new pharmaceuticals. This understanding and these tools have spawned a whole new subset of the industry that is called the biotechnology
sector and, in fact, suggest that the industry can be thought of as the "biopharmaceutical industry." The emergence of the biotechnology sector, together with what some analysts have referred to as a "structural revolution," are producing an ever-wider range of companies that differ greatly in size and organization; in the variety of their product development focus; and in the extent to which they either address the entire drug development process from basic research through to the marketing and sale of approved products or, instead, focus on one or more individual steps within that process.
The Demography of the Biopharmaceutical Industry
Numerous parameters can be used to define the companies that comprise the biopharmaceutical industry. One common metric is market capitalization, or the value that the market places on a company. Market capitalization is defined as the number of shares outstanding multiplied by the price per share. Clearly, since market capitalization varies with price per share, this metric is most easily determined for public companies whose shares trade on public securities exchanges. Figure 6-1 shows the market capitalization distribution of 232 pharmaceutical/ biotechnology companies that are traded on U.S. exchanges. These companies range in market value from over $5 billion (e.g., Merck, Glaxo-Wellcome) to under $25 million (many small, public biotechnology companies). Absent from this chart is information on over 1,200 private small biopharmaceutical companies in the United States and Europe which tend to have market capitalizations of less than $100 million and most of which do not yet have any product revenues.
Figure 6-2 shows the annual net sales distributions of 244 pharmaceutical/ biotechnology companies that trade on U.S. public exchanges. The bulk of revenues produced by these companies is generated by a minority of large corporations. To emphasize this point, the accounting firm of Ernst and Young has calculated the "Merck/Biotech Index," an assessment of the entire developing biotechnology industry as compared with Merck's ethical pharmaceutical business. According to the latest Ernst and Young survey in 1995, Merck had reported $15 billion in revenues, compared to $12.7 billion in revenues for the entire biotechnology industry for the 12-month period ending June 1995. During the same period, while Merck invested $1.2 billion in research and development and had a workforce of 47,500, the biotech industry invested $7.7 billion in research and development and had a work force of 108,000. In other words, R&D investment, while intense throughout the biopharmaceutical industry, is most heavily concentrated in the emerging company subsector. Industry estimates are that, despite the large investment of public funds in disease-specific areas, 92 percent of all drugs approved between 1981 and 1990 trace their origin to private sector R&D programs. The growing importance of the R&D effort within young entrepreneurial companies is emphasized by statistics that show that even in R&D-intensive large companies such as Merck, 50 percent of all
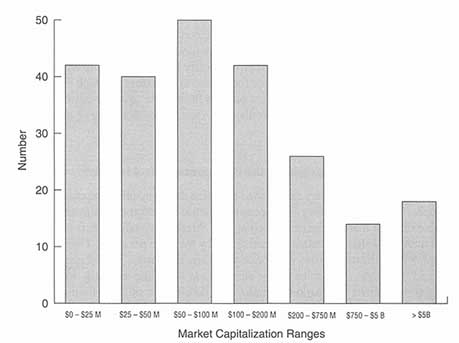
Figure 6-1
Market capitalization distributions of public pharmaceutical/biotechnology companies. B = billion; M = million. Source: Disclosure Annual Industrial Database, 1995.
products presently in clinical trials were licensed from small companies and, to a lesser extent, universities.
Contemporary Industry Dynamics
There is little disagreement that the years since the late 1980s have brought profound transformations in the marketplace for human health products. The prospect of health reform accelerated a reorganization of the health care marketplace that was, in some respects, already under way. The precipitating factor was the relentless run-up in health care expenditures in the United States and the sense of urgency about the need to contain costs and shift from fee-for-service arrangements to managed care systems. The competitiveness and profitability of such systems depend on their cost-effectiveness. As a result, fixed-fee and capitation schedules, protocols, and guidelines have been implemented or recommended. In the same vein, there is growing interest in formularies and a consequent burgeoning of pharmacy benefit management organizations (PBMs) as
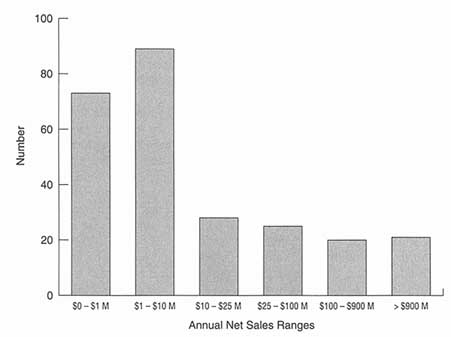
Figure 6-2
Annual net sales distributions of public pharmaceutical/biotechnology companies. M = million. Source: Disclosure Annual Industrial Database, 1995.
ways to control the costs of pharmaceuticals. Health care purchasers in the 1980s, who were primarily physicians, tended to be generally cost-insensitive. Pharmaceutical profitability in the 1980s had exceeded all other industry sectors and drug companies had been able to grow almost entirely owing to price increases. It is now clear from different challenges to industry pricing that this strategy is no longer viable (Easton 1993; Pollard 1993).
The pharmaceutical industry has been adapting to these realities by essentially reconfiguring itself. Some firms are moving toward domination of a small number of specialty areas, some toward vertical expansion throughout the health value chain. Some are increasing their size in the belief that greater critical mass will drive success, others are aiming at being total disease-management companies in which drugs are just a part of total health care solutions.
The specter of shrinking profit margins has also motivated companies to view market share—that is, the percentage of sales within a particular market segment—as the most important determinant of near- and long-term success. The sense is that market share leaders will be the most cost-efficient players, whose cost-efficiency will yield them savings for reinvestment in research or for the sourcing of higher-value products (Easton 1993). Companies are attempting
to retain market share for the drugs now coming off patent by converting them into over-the-counter (OTC) drugs; by building relationships with pharmacy benefit management organizations; by limiting price increases; or by responding to competition from generic drugs, either by building their own generic businesses, licensing out generic conversions of their drugs, or acquiring generic companies outright.
Some industry analysts argue that there will be an inevitable decline in the number of new drugs, for two reasons. One is that the science is more complicated; the other is that providers will increasingly depend on drug formularies to control costs. Furthermore, there is evidence that patients may have a much more powerful voice in determining managed care buying practices. Selection, addition, and substitution of formulary products will be based on product price and the amount of therapeutic or ''user value" added, so that product improvements that are merely incremental are likely to be far less important than in the past. Yet, while "me too" and "incremental" products will be less attractive in the future, the opportunity for cost-effective "breakthrough" products for unmet medical needs remains. As a consequence, pharmaceutical companies will not just need to be more cost-efficient inventors, developers, and marketers to make more products pay off for shareholders; they will also have to be disciplined in focusing their research investment on a smaller, more select group of therapeutic categories. Speed to market, always important, will become more so. Established, experienced pharmaceutical companies will have an advantage because they have the best scientific understanding of what is needed in the field and the greatest resources to buy the innovative research required to develop the truly novel drugs that can break into formularies. Thus, despite pressure to cut prices and reduce profit margins, innovation and heavy R&D investment will continue to fuel the industry, with high rewards for novelty and being early to market with products that lower the cost of health care (Burrill and Lee 1993; Pollard 1993). For the large pharmaceutical companies ("big pharma") and for biotechnology firms, "pharmacoeconomics," or the linking of quality-of-life and outcome measures with efficacy data in the design and conduct of clinical trials, will be essential; it will be crucial in the development of products for "difficult audiences."
The need of the large pharmaceutical industry to purchase technology in some form is highly significant for the biotech industry and there is a sense that the redefinition of the pharmaceutical industry offers biotechnology a much wider set of opportunities to prove its value. As noted above, because biotechnology firms essentially invest all of their assets in research and development, they are far from losing their identity as the incubators of much of the progress in human health care (Lee and Burrill 1995). Table 6-1 depicts different dimensions of the way the pharmaceutical industry is being reshaped, its likely future directions, and the way innovative products might be expected to fit into that picture.
The Biotechnology Industry
Industry Structure
Most analysts trace the emergence of the "biotech industry" to the late 1970s/ early 1980s, with the foundation of a series of companies (Cetus, Genentech, Amgen, Biogen) that were started to exploit the commercial potential of recombinant DNA (rDNA) and monoclonal antibody (MAb) techniques. The former involves transferring genetic information from one organism to another, splicing and "gluing" it onto a vector molecule, and replicating or cloning it for a variety of purposes. MAb technology takes advantage of antigens on the surfaces of invading agents to trigger immune-system recognition and response in the form of antibodies, proteins that attach themselves only to the foreign antigen and nothing else, signaling subsequent processes that then destroy the invader. It is the specificity of MAbs, as well as the fact that they can be enlisted as transport mechanisms, that makes them so valuable in the development of diagnostics, therapies, and vaccines.
The techniques, once seen as arcane, have become commonplace. Yet the tradition of novel technology deployed to define and address problems in human health care, agriculture, and animal health remains the hallmark of biotech companies. Thus, new technology stories are often the hallmark of new companies, so that gene therapy, xenotransplantation, genomics, and the like have been fostered first in biotech companies and then been transferred to "big pharma" via partnership, acquisition, or adoption.
From its birth, the biotechnology industry has confronted a set of factors or "hurdles" whose confluence is seen as unique: massive breakthroughs in science and technology (but quite upstream in the R&D process); enormous capital needs with a long horizon to payback; financial markets and investor expectations that turn alternatively hot and cold; a regulatory hand that is seen as heavy; uncertainty about intellectual property rights; and a cost crisis in its primary market, which is health care (Burrill and Lee 1993). Financial analysts are unable to describe public biotech companies to their investment clients using standard financial parameters because the majority of these companies are essentially R&D operations without products, revenues, and earnings. The financial community has therefore adopted a categorization of companies that describes where they are in the product development and company development process. A jargon has emerged that divides companies into first-tier, second-tier, and third-tier. The first-tier companies have products and product revenues and can be judged using standard financial criteria (e.g., Chiron, Amgen, Genzyme). Second-tier companies have products that are new or close to the market and have established the business infrastructure needed to be a self-supporting entity (e.g., Cephalon, Matrix Pharmaceuticals). The third tier has the biggest population and generally consists of companies that went public in the last three years, with
TABLE 6-1 Trends, Worldwide Pharmaceutical Industry, 1970s and Beyond
|
Early 1970s-Mid-1980s |
Mid- 1980s-Mid- 1990s |
Mid- 1990s and Beyond |
|
Consolidation |
Further Consolidation |
|
|
Many small companies |
Fewer, larger corporations |
Few, large corporations Small boutiques |
|
Domestic focus Technology driven |
Global focus Technology dependent Sales/service/distribution driven Competitive pricing Lower profitability Increased government regulation |
Global reach Technology dependent Sales/service/distribution driven Competitive pricing Potential to increase profits Increased government regulation Managed care |
|
Managed Care and the Pharmaceutical Industry |
|
|
|
Past |
|
Future |
|
Approvable products (safety/efficacy) |
|
Marketable products (pharmacoeconomic outcomes) |
|
Cost-based pricing |
|
Positioning on value |
|
Mega sales force |
|
Multiple distribution techniques |
|
Customer service |
|
Customer alliances * Disease management * Outcomes studies * Education * Customized phase III and IV clinical trials |
|
Science/sales driven |
|
Customer/market driven |
|
Early 1970s-Mid-1980s |
Mid-1980s-Mid-1990s |
Mid-1990s and Beyond |
|
Evolution of Successful Products |
||
|
Innovative products |
Innovative products |
Innovative products supported by global sales, service, and market infrastructure |
|
|
Competitive products supported by strong infrastructure |
|
|
Competitive niche products |
Competitive niche products |
Competitive niche products |
|
Sources: Burrill GS, KB Lee Jr. Biotech 94: Long-Term Value, Short-Term Hurdles (The Industry Annual Report). San Francisco, CA: Ernst and Young US, 1993. Noonan KD. Trends in the in vitro diagnostics industry in the late 1990s. Clinica, Special Supplement, April 1994(1). |
||
products still in preclinical animal testing or Phase I human clinical trials, with profits not expected till the end of the decade. Responses by several hundred industry CEOs to annual surveys by Ernst and Young continue to indicate that the largest, most mature, "top-tier" companies are mainly concerned about the complex regulatory environment, with some cyclical concerns about availability of capital. Mid-tier and small (lower-tier) companies worry most about inaccessibility and cost of capital. Unpredictability in the patent environment is significant for all biotechs, as it is for the large companies. The synergy between all these factors over the years has made the biotechnology subsector persistently volatile and its financing platform has generally been rocky.
Industry Strategies
As a result, the industry response has had to be one of very great ingenuity and flexibility, qualities that have been expressed in a plethora of strategic adaptations whose main objective was to somehow access costly resources to complete product development. A few firms tried to become fully integrated pharmaceutical companies, others to become vertically integrated firms in a niche area. A large number of firms sought to establish enough value to justify placing an initial public offering as a way of accessing capital; others worked to develop a blockbuster product as a route to acquisition by a larger company; and still others emphasized products that would require partnering with large companies in order to reach the market.
Virtual Integration Gradually, however, these strategies have been supplanted by other approaches that reflect adjustments to the realities of the changing marketplace, funding difficulties, and developmental disappointments. By 1994, the Ernst and Young annual industry analysis noted a paradigm shift to "virtual integration" and a proliferation of very pragmatic, selective, flexible relationships between biotechs and between biotechs and pharmaceutical companies (see Figure 6-3). These have included, but were hardly limited to, product swaps; development or acquisition of generic product lines; licensing in technologies, particularly late-stage technologies; partial acquisition of units or products; various combinations of different resources; and all manner of strategic partnerships (Lee and Burrill 1995).
Outsourcing Companies are also outsourcing, with high cost-effectiveness, to organizations that provide very narrowly defined services. A prime example is the "contract research organization" (CRO), an entity of possible practical interest in thinking about new strategies for revitalizing contraceptive research and development. CROs are basically third parties that provide research services on a contractual basis, focusing primarily on designing, conducting, and analyzing human clinical trials. Many CROs can also provide preclinical animal testing at
|
FUNCTION |
OWN Resources |
POTENTIAL PARTNERS |
|
Postmarketing sales support |
Statisticians, record keeping |
Specialty firms |
|
Distribution |
Warehousing, shipping Distribution |
Drug wholesalers Generic companies Direct to buyers (e.g., HMOs, government, insurance companies) |
|
Sales |
Detailing |
Customers Big pharmaceutical companies |
|
Marketing |
International and domestic coverage |
* By market/indication * By geography Speciality marketers |
|
Manufacturing |
Manufacturing plant |
Contract manufacturers Specialty-focused biotech intermediaries Big pharmaceutical companies' excess capacity |
|
Clinical/regulatory development |
Clinical expertise |
Clinical research organizations Big pharmaceutical companies Other biotech companies Specialty consultants/ contractors |
|
Development research |
R&D spending Academia |
R&D institutions Big pharmaceutical companies Government Other Biotech companies |
Figure 6-3
Virtual integration. Source: Burrill GS, KB Lee Jr. Biotech 94: Long-Term Value, Short-Term Hurdles (The Industry Annual Report). San Francisco, CA: Ernest and Young US. 1993.
the front end of the development cycle and regulatory consulting services at the back end. While CROs have existed for more than two decades, a confluence of factors seems to be driving a resurgence. These factors include pricing pressure from organized buyers; a relative paucity of investment capital; staff cutbacks; lack of expertise in most biotechs in clinical development and in regulatory affairs; perceived increases in regulatory burdens; and buyers' demands for cost-efficiency data which add a whole new layer of complexity to clinical development strategies. (Examples of such companies are Quintiles Transnational, IBAH, and Clintrials.) The role of CROs is becoming more prominent as a feature in company strategies, particularly for those that can respond to the demands of drug companies for global capabilities so that products can be tested and filed
simultaneously for approval worldwide. At one extreme, Abbott Pharmaceuticals contracts 80 percent of all its clinical development to CROs, other companies as little as 10-20 percent; industry trends are thought to be moving toward 50 percent. The volumes in dollar terms are not small: As of 1994, pharmaceutical and biotechnology companies were spending in aggregate roughly $26 billion annually. Of this amount, about two-thirds, or $17 billion, was spent on the kinds of services that CROs provide (Kreger 1994).
Alliances It is not surprising, then, that there has been steady growth in strategic alliances involving the biotech industry from the start of 1993 to mid-1995 (see Table 6-2). If the mid-1995 transaction rate is sustained, over the past three years there will have been close to 500 strategic alliances involving biotech companies, over 300 of those with large pharmaceutical companies and 150 with other biotechs. The number of mergers and biotech acquisitions is considerably less, a total of 123 projected for the same period (see Table 6-3). Table 6-3 shows the numbers of strategic alliances along with the numbers of financings, mergers and acquisitions, and downsizings as ways in which industry accesses capital, makes clear the relative importance of such alliances in overall industry strategy.
What may be surprising is that the biotech industry is not shrinking overall (see Table 6-4), despite the prevalent view among industry analysts who feel that there are too many biotechs and that some rationalization and compression will be necessary. The total number of companies, including those that have gone public, grew steadily over the same three-year period (1993-1995) and the industry's work force grew as well, even though an estimated 68 firms were seen as downsizing by the end of 1995 (see Table 6-4).
Globalization Another effect of the changes in the larger environment for "big pharma" and the biotech industry is that the industry has become increasingly
TABLE 6-2 Strategic Alliances in the Biopharmaceutical Industry, 1993 to Mid- 1995
|
|
Number of Transactions |
|
|
|
|
1993 |
1994 |
Year to Date 6/30/95 |
|
Large pharmaceutical company alliances with biotech company |
69 |
117 |
73 |
|
Biotech-and-biotech alliances |
43 |
52 |
26 |
|
Total |
112 |
169 |
99 |
|
Source: Vector Fund Management. BioWorld Financial Watch. Deerfield, IL 1995. |
|||
TABLE 6-3 How Biotechs Access Capital
|
|
Number of Transactions |
|
|
|
|
1993 |
1994 |
Year to Date, 6/30/95 |
|
Financings |
190 |
174 |
105 |
|
Mergers and acquisitions |
21 |
48 |
27 |
|
Downsizing |
6 |
26 |
18 |
|
Strategic alliances |
112 |
169 |
99 |
|
Source: Data provided by Vector Fund Management, Deerfield, IL 1995. |
|||
global. Products are being developed with the intent that they will be made available to patients in many different countries. Consequently, many companies have operations in those countries; those that do not must develop extensive contractual relationships to allow their products to reach global markets. The implications of a global biopharmaceutical marketplace are profound. From the conception of a clinical program through development and launch, decisions must be made with an eye to entering markets governed by divergent rules. The globalization of business, coupled with the need to be more cost-efficient, is behind the movement to "harmonize" criteria for drug development and approval and has led to the creation of new companies that can expeditiously facilitate new drug development across borders; international CROs are an excellent example of this adaptation.
Furthermore, the biotech industry is growing in Europe, which as of 1994 had 386 biotech companies (Lee and Burrill 1995). In consequence, many European pharmaceutical and biotech companies are creating alliances with U.S. firms, providing capital as well as manufacturing and marketing support; and regulatory filings for foreign investment have increased dramatically. In addition, Europe's often less stringent regulatory requirements offer the opportunity for U.S. companies to initiate clinical trials more quickly and possibly gain market access sooner; other operational and tax advantages also add to the general offshore allure. Finally, the biotech industry has globalized to include countries in Latin America, Eastern Europe, China, India, and the Pacific Rim, which recognize the promise of biotechnology, offer new markets, and serve as sources of innovation. Corporate partnerships and sale or licensing of product rights are the strategies favored for penetrating the European and Japanese markets, with strategic partnerships also receiving significant emphasis in the United States. Going it alone in either terrain is difficult: International expansion inevitably requires additional infu-
TABLE 6-4 Biotechnology Industry Highlights ($ billions), 1992-1995
|
|
Public Companies (Merck Index)a |
Biotech Industry Total |
||||
|
|
This Year |
Last Year |
%Change |
This Year |
Last Year |
%Change |
1995 |
|
|
|
|
|
|
|
Sales/revenues |
$15 |
$10.5 |
47 |
$12.7 |
11.3 |
12 |
|
R&D expense |
$1.2 |
$1.2 |
0 |
$7.7 |
$7.1 |
6.4 |
|
Net income (loss) |
$3.0 |
$2.2 |
26 |
$(4.6) |
($4.2) |
10 |
|
Market capitalization |
$73.0 |
$3.7 |
97 |
$52.0 |
$41.0 |
0.7 |
|
Employees |
47,500 |
47,100 |
1 |
108,000 |
103,000 |
2.3 |
|
1994 |
|
|
|
|
|
|
|
Sales |
$5.2 |
$4.3 |
20 |
$7.7 |
$7.0 |
10 |
|
Revenues |
$7.1 |
$6.0 |
17 |
$11.2 |
$10.0 |
12 |
|
R&D expense |
$3.8 |
$3.0 |
27 |
$7.0 |
$5.7 |
23 |
|
Net loss |
$2.1 |
$1.5 |
40 |
$4.1 |
$3.6 |
14 |
|
Market capitalization |
$36.0 |
$39.0 |
(8) |
$41.0 |
$45.0 |
(9) |
|
No. of companies |
265 |
235 |
13 |
1,311 |
1,272 |
3 |
|
Employees |
53,000 |
48,000 |
10 |
103,000 |
97,000 |
6 |
|
1993 |
|
|
|
|
|
|
|
Sales |
$4.4 |
$3.3 |
35 |
$7.0 |
$6.0 |
17 |
|
Revenues |
$6.1 |
$4.4 |
38 |
$10.0 |
$8.3 |
20 |
|
R&D expense |
$2.9 |
$2.4 |
24 |
$5.7 |
$5.0 |
14 |
|
Net loss |
$1.4 |
$1.4 |
0 |
$3.6 |
$3.4 |
6 |
|
Stockholders' equity |
$10.5 |
$8.1 |
30 |
$15.9 |
$48.0 |
(6) |
|
No. of companies |
235 |
225 |
4 |
1,272 |
1,231 |
3 |
|
Employees |
48,000 |
37,000 |
30 |
97,000 |
79,000 |
23 |
|
1992 |
|
|
|
|
|
|
|
Sales |
$3.4 |
$2.6 |
31 |
$5.9 |
$4.4 |
3.5 |
|
Revenues |
$4.5 |
$4.5 |
29 |
$8.1 |
$6.3 |
28 |
|
R&D expense |
$2.3 |
$1.5 |
54 |
$4.9 |
$3.4 |
42 |
|
Net loss |
$1.4 |
$0.9 |
60 |
$3.4 |
$2.6 |
32 |
|
Stockholders'equity |
$8.2 |
$4.5 |
83 |
$13.6 |
$10.7 |
27 |
|
No. of companies |
225 |
194 |
16 |
1,231 |
1,107 |
115 |
|
Employees |
37,000 |
33,000 |
12 |
79,000 |
70,000 |
13 |
|
a The MERCK Biotech Index is a measure devised by Ernst and Young, which assesses the entire developing biotech industry as compared with Merck's ethical pharmaceutical business. Sources: Ernst and Young Biotech Industry Annual Reports: Biotech 93, 94, 95, 96. |
||||||
sions of money, talent, and local know-how concerning regulatory approvals, product pricing and reimbursement, and marketing.
The theory has been advanced that an increasingly global market might be helpful in alleviating some of the constraints to contraceptive research and development that prevail in the United States, in terms of offering options for collaborative relationships (Rockefeller Foundation 1995a). Prominent among the arguments are that the regulatory environment might be less stringent elsewhere, that the pressures of liability would be less severe, that the political and ideologic environment might be less complex, and that clinical trials would be less costly. These are reasonable arguments to advance but each of them requires a complex and thoughtful examination, beyond what this study committee found feasible within its own constraints of time and resources. Another major—and persistent—constraint is the very real limitation of access to information, most importantly about prospective industrial relationships, information that is almost always well guarded. A brief retrospective glance at some partnership experiences, such as the efforts of some small U.S. firms to partner with European firms, Roussel-Uclaf s experience with RU 486, and the some of the experience with offshore production, have been too complex and the determining variables too diverse to permit easy, categorical conclusions. As just one example, the mutual perceptions of the United States and the European countries of the stringency of each other's regulatory processes appear to be quite discordant and efforts at harmonization move slowly.
The Risk-Benefit Assessment: Decisions by Private Firms to Invest
The General Process
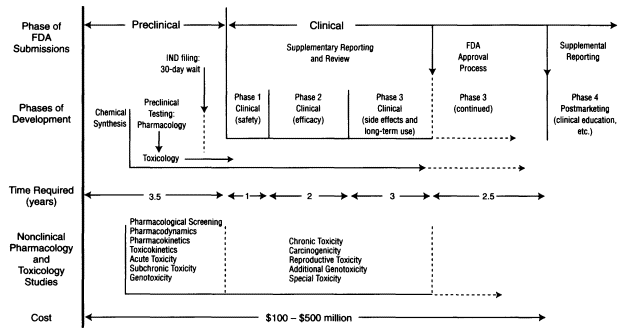
The theme of financing has pervaded the present discussion because it lies at the heart of the complicated web of interdependence that is being woven among biotechnology and large pharmaceutical companies, in the United States and overseas. And, at the heart of the financing issue is the fact that the process of developing new pharmaceutical products is complex, long, and costly (see Figure 6-4). Estimates of the time and costs involved in developing new therapeutic ethical pharmaceutical products and taking them to market vary widely. A major source of variance is the product category itself; new chemical entities (NCEs) typically take more time and cost more money (OTA 1993). A 1989 industry survey estimated that 83 percent of total U.S. R&D dollars in that year were spent in the earliest stage of the process, that is, in advancing scientific knowledge and developing new products, as opposed to improving and/or modifying existing products though distinctions between these two categories of endeavor can be fuzzy (OTA 1993). This period of conceptual work and initial synthesis is also inherently risky; it may be that, out of every 10,000 new chemicals synthesized in the laboratory, FDA approval may be sought for only 1 (NRC/IOM 1990). And,
of 20 entities submitted to FDA for approval, only two may be approved and just one may actually be introduced as a new product (Harper 1983; NRC/IOM 1990; OTA 1993).
In 1993, the Office of Technology Assessment (OTA) reanalyzed earlier seminal studies of the costs of pharmaceutical R&D and calculated an average cost of developing a new drug at ''no more than" $237 million (OTA 1993), with development time from identification of an NCE to market taking from 10 to 12 years. According to the Pharmaceutical Research and Manufacturers Association (PhRMA), the range can be from $100 million to $500 million and take a minimum of five to ten years from the time a decision is made to take a product into clinical development to the time it appears on the market. While this wide range in cost estimates may be related to product-specific needs, such as duration and number of patients in a series of clinical trials, they may also have to do with amortization of a large company's overhead vis-à-vis that of a "lean and mean" entrepreneurial biotechnology company. Still, it is simplistic to assume that product development will always be less expensive in biotech companies.
The Specific Case of Contraceptive Research and Development
One of the most important influences on the development of new contraceptives is the outcome of decisions by U.S. and European producers of pharmaceuticals and medical devices. Although governments and nonprofit organizations have made significant contributions to the funding of basic research and to the development of many contraceptive technologies, most of the currently available products were commercialized by U.S. and Western European firms.
A promising "reservoir" of basic biomedical research may be necessary, but it is by no means sufficient, to result in the introduction of new contraceptive technologies. Since basic scientific advances may have a number of possible pharmaceutical or medical device applications, choices about developing one or another of those will depend on assessment of a range of technologic, regulatory, and market factors. In addition, bringing a new contraceptive product to market takes years because of the long development cycle, numerous clinical trials, and complex regulatory approvals that are needed. Thus, today's dearth of new contraceptive products reflects decisions by pharmaceutical firms not to pursue research or development of new contraceptive products that were made 10-20 years ago.
It is tempting to seek single-factor explanations for what has or has not happened in the field of contraceptive research and development. The committee has struggled to do that and been confounded by the idiosyncracies of each "decision experience," since those respond to producer/product/provider/consumer characteristics, varying among themselves and according to time and circumstance. At the most fundamental level, these decisions are not controlled by any single factor but are the outcome of a comparison of the costs and returns
(adjusted for risk, which is unusually significant in this product area) from investment in contraceptives, relative to alternative development programs and projects. In other words, a decline in development activities in contraceptives could have nothing to do with the economic or political conditions surrounding contraceptives but instead reflect significantly improved opportunities in some other product area. In fact, the situation with respect to contraceptives appears to reflect the operation of both forces—opportunities in other areas have improved, even as the risk-adjusted returns associated with new contraceptive products may have declined.
The decisions of firms, especially those previously active in contraceptive development, not to pursue development of new products, or to exit this product area altogether, also can negatively affect the stock of expertise needed to develop new products. Firms in the pharmaceuticals industry also typically specialize to some extent in specific types of products or therapies, which reflects the fact that they have accumulated considerable scientific, technical, and market related knowledge that is specific to these areas and may not be relevant elsewhere. This type of knowledge often is not easily transferred between firms and cannot be purchased or otherwise acquired by a new firm without a long period of investment and learning. Because a number of firms formerly active in contraceptive technologies have exited this product area, much of this expertise has been lost and would be entrants into the contraceptives field (e.g., biotechnology firms) cannot easily acquire it from incumbent firms or from other sources. Reversing the effects of firm exit on this stock of "know-how" thus may take considerable time.
The factors that must be considered when industry assesses a new biopharmaceutical program are:
- market
- management/human resources
- technology assessment
- competition
- regulatory requirements
- intellectual property position
- economics
- —cost to develop (time, money, people)
- —competitive position at launch
- —projected profitability
- —opportunity cost vis-à-vis other programs
- strategic reasons
- —synergy with other efforts
- other factors
- —e.g., political risk.
Simplifying the problem considerably, and abstracting from the issue of
know-how, an individual firm's decision to pursue development of a new contraceptive technology (which may yield a family of products) is governed primarily by the expected returns to the large investment needed to commercialize this technology, i.e., the profits, relative to the costs of development (one way in which know-how enters this decision is in the confidence and reliability of a firm's judgments about costs and returns). There seems to be little evidence suggesting that the costs of developing a new contraceptive product per se are significantly higher than those involved in developing other pharmaceutical or medical products intended to be used for chronic administration: All of these products require lengthy development programs and large investments in clinical trials and regulatory approvals.
The differences between contraceptives and other pharmaceutical products appear to be more prominent, however, in the area of expected returns and the adjustments imposed by decision makers to adjust these returns for the risks associated with contraceptive products. Market success, especially for contraceptives, requires that a new product offer significant advantages in terms of factors like safety and convenience, as well as being reasonably competitive on price with existing products. Because existing contraceptive products (e.g., IUDs or oral contraceptives) are relatively inexpensive, they impose a "ceiling" on the feasible price in mass markets for new alternatives, and therefore depress projected returns from contraceptives relative to other pharmaceuticals or medical devices, whose delivery is more frequently covered by third-party reimbursement.
A rational decision maker will also adjust expected returns for risk, and this adjustment is likely to prove disadvantageous to contraceptives relative to other products. Riskier products demand a higher expected return in order to justify the investment in their development, and contraceptives appear to exhibit relatively high risks from two sources. The first is liability litigation, which is an unusually serious threat in contraceptives, just as it has been in vaccines (this factor is a serious risk mainly in the United States, but litigation risk appears to be increasing in several Western European markets as well). Like vaccines, contraceptives typically are administered to a huge market of individuals with normal health histories. As a result, the possibilities of side effects or unusual reactions, which may affect a very small fraction of the population, will yield a steady stream of claims. Moreover, many of these claims will be filed by healthy, often relatively young individuals and therefore may result in high damage awards. Thus, litigation risk in contraceptives appears to be unusually high relative to other pharmaceutical products.
The committee had entertained the idea of doing some comparative analysis of the costs of litigation for contraceptives compared to vaccines as a somewhat analogous category of products. However, considerable efforts to obtain hard data proved fruitless, since information about such costs is tightly held. The only information of this sort that appears in the public domain concerns the amounts of
awards from cases that do go to court, a small tip of a large iceberg. Of the 2,063 suits filed against Searle over the past 20 years in connection with its intrauterine device, just 24 went to trial; approximately 800 were settled out of court for undisclosed amounts (Steyer 1995). It has been customary to think of litigation as a U.S. issue; however, 346 of the complaints against Searle were "foreign," primarily in Australia and the United Kingdom.
One must add to the financial risks associated with litigation the financial and other risks associated with the strong political opposition to many forms of contraceptive technology in the United States. These risks mean that the projected "hurdle rate" of return that a firm will require to undertake a contraceptive development project will be higher than the hurdle rate associated with other projects. When one combines this requirement for a higher hurdle rate of return with the low prices of the alternatives currently available, contraceptive development projects are likely to appear less attractive than alternative applications. Obviously, for firms with considerable expertise in this product field, this gap between the hurdle rate for contraceptives and other products will be lower, and this gap will be affected by many other influences as well. Nevertheless, these factors appear to depress the projected returns for commercial contraceptive development projects relative to those in other areas in which scientific advances may offer equally enticing product development possibilities.
If, as many analysts suggest, the biotech sector is the pharmaceutical industry's "R&D department," then one surrogate of the perceived commercial attractiveness of new products in women's health might be the number of biotech firms that report to be working in this area. A 1995 survey by Goldman Sachs of the product programs in 158 public biotech companies found that out of over 450 products undergoing clinical testing, only 5 described as "for women's health" had reached that stage (Goldman Sachs 1995). Still, in 1994 PhRMA reported that, among the large number of new drugs in development, there were "143 biotechnology medicines [and] 301 medicines for women" in pharmaceutical company pipelines. In terms of numbers, medicines for women were second only to the "327 medicines for older Americans" and well ahead of the 103 medicines for AIDS and AIDS-related diseases (PhRMA 1994). There seems to be growing attention to the area of women's health in the pharmaceutical industry: Ortho, Parke-Davis, Searle, and Wyeth-Ayerst all have what are considered more or less formally as ''women's health programs." At the same time, only Ortho and Wyeth-Ayerst include contraceptives as part of that picture.
In conclusion, decisions of private firms to reduce their development efforts in contraceptives reflect the operation of a range of factors. No single factor dominates all others in the large majority of individual cases, and no single factor is inevitably the cause across all cases. Contraceptives are competing for scarce technical talent and funds with alternative product development programs, and the commercial attractiveness of other product development projects may shift for any one of a great variety of reasons. Moreover, because of the size and the
duration of the private-firm investments necessary to bring a new contraceptive technology to market, one might see a decline in commercial development activity in the face of continued public support for the basic science underpinning contraceptive technologies. But when declines in public research support are combined with factors tending to depress the relative return on investment from contraceptive development projects, development activities are likely to be curtailed even more severely.
Current Industry Involvement in Contraceptive Research and Development
As of 1993, there were 57 manufacturers or "vendors" of contraceptive and fertility products in the United States (see Table 6-5).2 Of those, 23 were manufacturers or vendors of contraceptive products. The committee identified over twice that number in the worldwide market (see Table 6-6).
One might expect contraceptives and fertility products to be areas of industrial interest with some affinity. In fact, there is surprisingly little crossover; firms active in both areas are usually subsidiaries or divisions of multinational pharmaceutical companies which have enough power and financial resources to develop products in several markets. Just five firms work in both product lines: Organon, Ortho, Syntex, Whitehall, and Wyeth-Ayerst. Ortho is a Johnson and Johnson subsidiary, Wyeth-Ayerst and Whitehall are part of American Home Products Corporation, and Organon is a subsidiary of Akzo NV. Only one, Ortho, which markets oral contraceptives, diaphragms, and spermicidal preparations is present in more than two product lines (Frost and Sullivan 1993). With its acquisition in summer of 1995 of GynoPharma, which dominated the U.S. market for IUDs and also had a line of spermicides, Ortho further increased its number of product lines and now has more product lines than any company worldwide.
The industrial profiles of the two product areas—contraception and fertility—are quite different. The fertility industry is less dominated by giants than is the contraceptives industry and is scattered among numerous, smaller competitors that tend to focus fairly narrowly, sometimes developing great expertise in a niche market and thereby becoming the dominant firm in that segment. The fertility products subgroup of the market is even more finely grained, counting many smaller participants, for example, those that develop and/or produce immunoassays and nonisotopic hormone tests and are attempting entry (Frost and Sullivan 1993).
The overall industrial picture in contraceptives is far more concentrated; in fact, it is described by at least one prominent industry analyst (Frost and Sullivan 1993) as pretty much an oligopoly, dominated by a few large and strong competitors who account for anywhere from 60 to 90 percent of total manufacturer revenues in this area. Table 6-7 shows revenues by product type and company
TABLE 6-5 Firms Manufacturing and/or Distributing Contraceptives, United States, 1993
|
Firm |
OCa |
Condom |
Diaphragm |
IUD |
Spermicide |
Implantb |
|
Aladan |
|
X |
|
|
|
|
|
Alza |
|
|
|
|
|
|
|
Ansell |
|
X |
|
X |
|
|
|
Berlex |
X |
|
|
|
|
|
|
Carter-Wallace |
|
X |
|
|
|
|
|
Finishing Enterprises |
|
|
|
X |
|
|
|
GynoPharmac |
X |
|
X |
X |
|
|
|
Mead Johnson |
X |
|
|
|
|
|
|
Milex |
|
|
X |
|
X |
|
|
National Sanitary |
|
X |
|
|
X |
|
|
Okamoto |
|
X |
|
|
|
|
|
Organon |
X |
|
|
|
|
|
|
Ortho |
X |
|
X |
|
X |
|
|
Parke-Davis |
X |
|
|
|
|
|
|
Rugby Labs |
X |
|
|
|
|
|
|
Schering Plough |
|
|
|
|
X |
|
|
Schiaparelli-Searle |
X |
|
|
|
|
|
|
Schmid |
|
X |
|
|
X |
|
|
Searle |
X |
|
|
|
|
|
|
Syntex |
X |
|
|
|
|
|
|
Thompson Medical |
|
|
|
X |
|
|
|
Warner-Chilcott |
X |
|
|
|
|
|
|
Whitehall |
|
|
|
|
X |
|
|
Wyeth-Ayerst |
X |
|
|
|
|
X |
|
a Oral contraceptives. b Norplant. c GynoPharma was sold in the summer of 1995 to Ortho Pharmaceuticals. Source: Frost and Sullivan. U.S. Contraceptives and Fertility Product Markets. New York, 1993. |
||||||
TABLE 6-6 Firms Manufacturing and/or Distributing Contraceptives Worldwide, 1993 and 1994
|
Company |
Product Manufactured or Distributed |
|
Aladan |
Condoms |
|
Alza |
IUDs (Progestasert) |
|
Ansell |
Condoms (including one with nonoxynol-9) |
|
Berlex |
Oral contraceptives (Tri-Levlen, Levlen) |
|
Boehringer Ingelheim |
Oral contraceptives |
|
Bristol-Myers Squibb |
Oral contraceptives |
|
Carter-Wallace |
Condoms (including one spermicidally lubricated) |
|
CCC (Canada)* |
IUDs |
|
Cervical Cap Ltd. |
Cervical cap (Prentif/manufactured by Lamberts/Dalston England) |
|
Chartex (United Kingdom) |
Female condom (Femidom) |
|
Cilag (UK)* |
Oral contraceptives |
|
Dongkuk Trading (Korea)* |
Condoms |
|
Finishing Enterprises* |
IUD |
|
Gedeon Richter (Hungary) |
Emergency postcoital contraceptive (Postinor) |
|
Gruenenthal |
Oral contraceptives |
|
GynoPharmaa |
IUD (CuT380A [Paragard]) (distributor) Oral contraceptive (Norcept) Diaphragms (distributor for Schmid) |
|
Hyosung (Korea)* |
Condoms |
|
Jenapharm (Germany) |
Oral contraceptives |
|
Kinsho Mataichi (Japan)* |
Spermicides |
|
Leiras Oy Pharmaceuticals |
Progestin-releasing IUD (Mirena) |
|
(Finland)* |
Norplant (manufacture) |
|
Lexis |
Oral contraceptives (NEE) |
|
Company |
Product Manufactured or Distributed |
|
London Rubber |
Condoms Diaphragms |
|
Magnafarma |
Oral contraceptives |
|
Mayer |
Condoms |
|
Mead Johnson |
Oral contraceptives (Ovcon) |
|
Medimpex* (Hungary and USA) |
Oral contraceptives/raw materials |
|
Menarini |
Oral contraceptives |
|
Milex Products, Inc. |
Diaphragms (Omniflex, Wide-seal) Jellies and creams (Shur Seal Jel has nonoxynol-9) |
|
National Sanitary |
Condoms |
|
Okamoto, USA |
Condoms |
|
Organon (Akzo) (Netherlands)* |
Oral contraceptives (Marvelon, Desogen, Jenest) IUD (Multiload) (manufacturing subsidiary, Bangladesh) |
|
Ortho Pharmaceutical* (Johnson & Johnson) |
Oral contraceptives (Loestrin; Ortho-Cept, Ortho-Cyclen, Ortho Tri-Cyclen, Ortho-Novum, Modicon) Diaphragms (Allflex, Ortho Diaphragm) Spermicides* (Gynol) IUDs |
|
Parke-Davis (Warner-Lambert) |
Oral contraceptives |
|
Polifarma |
Oral contraceptives |
|
Reddy Health Care |
Condoms |
|
RFSU of Sweden |
Condoms |
|
Roberts |
Oral contraceptives |
|
Rugby Labs |
Oral contraceptives (Genora) |
|
Safetex |
Condoms |
|
Company |
Product Manufactured or Distributed |
|
Schering AG (Germany)* |
Oral contraceptives* Injectables* |
|
Schering Plough (USA) |
Spermicides |
|
Schmid |
Condoms (including spermicidal condoms) Spermicides Diaphragms (distributed by GynoPharma) |
|
Searle (Monsanto) |
Oral contraceptives (Demulen) |
|
Seohung (Korea)* |
Condoms |
|
Syntex |
Oral contraceptives (Tri-Norinyl, Devcon, Norinyl, Brevicon) |
|
Thompson Medical |
Spermicides |
|
Upjohn* (Upjohn Belgium)* |
Injectable (Depo-Provera/DMPA) |
|
Warner-Chilcott |
Oral contraceptives (Nelova) |
|
Whitehall |
Sponge (Today)b Spermicides |
|
Wisconsin Pharmacal |
Female condom (Reality) |
|
Wyeth-Ayerst (American Home Products) |
Oral contraceptives (Lo-Ovral, Nordette, Triphasil) (joint venture, Egypt, production) Norplant (marketing, distribution) |
|
Wyeth-Pharma (Germany)* |
Oral contraceptives |
|
Wyeth (France)* |
Oral contraceptives |
|
Note: An asterisk indicates that the firm supplies to UNFPA procurement. Where the firm is listed with more than one product line and is a UNFPA source, the product supplied is also marked with an asterisk. a GynoPharma was sold to Ortho Pharmaceuticals in summer of 1995. b Whitehall decided to discontinue the Today sponge because of the costs of bringing the plant up to U.S. Food and Drug Administration specifications. Sources: Frost and Sullivan. U.S. Contraceptive and Fertility Product Markets. New York, 1993. United Nations Population Fund (UNFPA). 1993 Procurement Statistics. New York, 1993. |
|
for 1990 and 1992. Of the 11 U.S. firms competing in the U.S. oral contraceptives market in 1992, Ortho and Wyeth-Ayerst controlled 70 percent of total revenues, followed by Berlex (a Schering subsidiary), Syntex, Parke-Davis, and Mead Johnson (a Bristol-Myers Squibb subsidiary), with roughly comparable shares running around 4 to 6 percent each. The remainder was shared among the five other competitors. There is no expectation that the concentration of this subsector of the industry will change, largely because of the extent of the investment needed to develop new oral contraceptives and labeling requirements that are perceived as benefiting larger companies. Ortho, Wyeth-Ayerst, Organon, and Berlex (Schering AG) are likely to continue their market dominance with the newer progestin-based formulations as well.
The rest of the industry is similarly oligopolistic. Even though there are over 100 different brands of condoms on the market (Hatcher et al. 1994), Carter Wallace dominates with 60 percent of total revenues. There are just three competitors with diaphragm product lines and, again, Ortho got over half of 1992 revenues; Milex's share was around 40 percent. Ortho also gets half the revenues from the U.S. spermicidal preparations market, where there are six other competitors, one of which is Whitehall, like Ortho, part of American Home Products. Wyeth-Ayerst has the monopoly in the United States for the levonorgestrel contraceptive implant Norplant. Finally, the IUD line is very close to monopoly; until its sale to Ortho in summer 1995, GynoPharma was the market leader, with 94 percent of revenues. The IUD market is not likely to burgeon in terms of new entries since, as a very-long-term method that has been on the market for a long time, it generates a very modest margin of profit.
The international picture is also one of concentration. In the case of oral contraceptives, which account for the overwhelming bulk of all contraceptive revenues worldwide (over 80 percent in 1992), American Home Products and Johnson and Johnson still dominate but share the top of the worldwide OC market with the large European firms Schering AG and Organon, which increased their percentage share of sales between 1988 and 1992 (see Table 6-8). The four firms together accounted for 81 percent of all oral contraceptive sales worldwide in 1992, or $1.7 billion out of total worldwide sales of $2.1 billion. Four other large integrated firms—Searle (a Monsanto subsidiary), Syntex, Parke-Davis (a Warner-Lambert subsidiary), and Bristol-Myers Squibb—accounted for another $272 million, or 13 percent of sales. For all eight firms, oral contraceptive revenues were close to or well above the magic "$50 million-dollar market" figure; for seven other firms and miscellaneous "others," which shared the $133 million balance, that figure was much smaller and seems not to have constituted a major product line. There are, nevertheless, small firms which have evidenced commitment to engagement in new areas of contraceptive technology, notably Leiras of Finland, Gedeon Richter of Hungary, and Silesia of Brazil.
As noted at the outset of this chapter, the number of large pharmaceutical firms remaining in the research and development component of the contracep-
TABLE 6-7 Contraceptive Revenues (in millions of dollars), United States, 1990 and 1992, by Product Type and Company
|
Product |
1990 |
1992 |
|
Oral Contraceptives |
|
|
|
Ortho |
376.0 |
385.4 |
|
Wyeth-Ayerst |
306.7 |
343.7 |
|
Syntex |
69.3 |
62.5 |
|
Berlex |
59.4 |
62.5 |
|
GD Searle |
49.5 |
52.1 |
|
Parke-Davis |
49.5 |
62.5 |
|
Mead Johnson |
49.5 |
41.7 |
|
Others |
29.7 |
31.2 |
|
Subtotal |
989.6 |
1,041.6 |
|
Condoms |
|
|
|
Carter Wallace |
70.0 |
81.7 |
|
Schmid |
34.4 |
36.0 |
|
Ansell |
14.7 |
16.6 |
|
Others |
3.7 |
4.2 |
|
Subtotal |
122.8 |
138.5 |
|
Spermicides |
|
|
|
Ortho |
21.1 |
26.3 |
|
Whitehall |
9.9 |
8.6 |
|
Schmidt |
7.2 |
7.7 |
|
Others |
6.7 |
5.3 |
|
Subtotal |
44.9 |
47.9 |
|
Norplant |
0.0 |
40.1 |
|
IUDs |
|
|
|
GynoPharmaa |
15.5 |
21.2 |
|
Alza |
4.6 |
2.1 |
|
Subtotal |
20.1 |
23.3 |
|
Diaphragms |
|
|
|
Ortho |
2.1 |
2.0 |
|
Milex |
1.2 |
1.7 |
|
GynoPharmaa |
0.2 |
0.2 |
|
Subtotal |
3.5 |
3.9 |
|
Total (All product types) |
1,180.9 |
1,295.3 |
|
a GynoPharma and its IUD and diaphragm lines were sold in summer 1995 to Ortho Pharmaceuticals. Source: Frost and Sullivan. U.S. Contraceptive and Fertility Product Markets. New York, 1993. |
||
TABLE 6-8 Worldwide Oral Contraceptive Market, Sales 1988 and 1992 (in millions of U.S. dollars), by Company
|
|
1988 |
1992 |
||
|
Company |
Sales |
% Total |
Sales |
% Total |
|
Wyeth (American Home Products) |
452 |
30.2 |
610 |
29.2 |
|
Ortho (Johnson & Johnson) |
392 |
26.2 |
458 |
21.9 |
|
Schering AG |
244 |
16.3 |
372 |
17.8 |
|
Organon (Akzo) |
129 |
8.6 |
245 |
11.7 |
|
Searle (Monsanto) |
70 |
4.7 |
86 |
4.1 |
|
Syntex |
90 |
6.0 |
71 |
3.4 |
|
Parke-Davis (Warner-Lambert) |
42 |
2.8 |
69 |
3.3 |
|
Bristol-Myers Squibb |
30 |
2.0 |
46 |
2.2 |
|
Boehringer Ingelheim |
9 |
0.6 |
19 |
0.9 |
|
Menarini |
3 |
0.2 |
13 |
0.6 |
|
Polifarma |
0 |
0.0 |
13 |
0.6 |
|
Gruenenthal |
6 |
0.4 |
13 |
0.6 |
|
Rugby Labs |
4 |
0.3 |
10 |
0.5 |
|
Magnafarma BV |
0 |
0.0 |
8 |
0.4 |
|
Upjohn |
3 |
0.2 |
6 |
0.3 |
|
Others |
22 |
1.5 |
52 |
2.5 |
|
Total |
1,496 |
98.5 |
2,090 |
97.5 |
|
Source: Syntex data (I.M.S. data provided by Vector Fund Management, Deerfield, IL, 1995). |
||||
tives industry is very small: Ortho in the United States and Organon and Schering in Europe are the primary participants. Wyeth-Ayerst is engaged principally in R&D activities limited to modifications of implant technologies, and Merck, which has not been involved in contraceptives for decades, is sponsoring some research in immunologic contraception under a limited agreement with the University of Connecticut Medical Center.
The current picture of industry participation in contraceptive research and development is largely a continuance of the pattern of collaborative effort established in Stage III. These are typically with the public sector (primarily NIH/ NICHD [National Institute of Child Health and Development] and WHO/HRP [the World Health Organization's Human Reproduction Programme]); with a nonprofit entity which receives substantial infusions of public funds, for instance, the CONRAD program and Family Health International (FHI); or with a university partner. Partnerships between large and small firms are very few; at least, given information that is in the public domain, the big pharmaceutical firms remaining in contraceptive R&D appear to have no more than one or two such relationships (see Table 6-9). Furthermore, most of the products that are the
TABLE 6-9 Recent Industrial Involvement in Contraceptive Research and Developmenta
|
For-profit Companies |
Product |
Not-for-profit Partner(s) |
Other For-profit Partner(s) |
|
Advances in Health Technology |
mifepristone |
|
|
|
Allendale Labs |
Nonoxynol-9 film with benzalkonium chloride film |
CONRAD |
|
|
Alphatron (previously Ovabloc Europe) (Netherlands) |
Vas occlusion with silicone plug |
WHO/HRP AVSC |
|
|
AM Resource |
Bactericidal gel |
NIH: NICHD |
|
|
Apex Medical Technologies |
Nonlatex condoms |
NIH: NICHD |
London International Group |
|
Apothecus |
Vaginal film |
WHO/HRP NIH: NIAID FHI |
|
|
Aphton |
hCG immunocontraceptive |
WHO/HRP |
|
|
Applied Medical Research, Ltd. |
Estrogen-free minipill (B-Oval) containing melatonin and norethindrone (a progesterone) |
Dutch government |
|
|
Baxter |
Nonlatex condoms |
|
|
|
Bioself Distribution (Switzerland) |
Basal body temperature thermometer |
|
|
|
Biosyn |
Vaginal microbicides (spermicide C31 G: protection from conception, STDs) |
NIH: NICHD CONRAD University of Pennsylvania |
|
|
Biotech Australia |
Work with inhibin as component of hormonal contraception for both men and women |
|
|
|
For-profit Companies |
Product |
Not-for-profit Partner(s) |
Other For-profit Partner(s) |
|
Biotechnology General (formerly Gynex) |
Contraceptives, sublingual formulations |
|
|
|
Biotek |
Long-acting spermicide suppository; new nonoxynol-9 formulations |
NIH: NICHD Population Council CONRAD |
|
|
BKB Pharmaceuticals |
Emergency contraceptive (CDB 2914) |
NIH: NICHD RTI |
|
|
Cabot Medical |
Silicone rubber ring (Fallope) |
|
|
|
Cilag AG (Germany) |
Combined oral contraceptives |
|
|
|
Columbia Labs |
Sustained release formulations of spermicides, natural progesterone |
NIH: NICHD NIH: NIAID WHO |
|
|
Conceptus |
Non-surgical fallopian tube sterilization |
AVSC |
|
|
Curatek |
Vaginal gel (bactericidal) |
|
|
|
Cygnus Therapeutics |
Contraceptive patch 7-day contraceptive |
FHI |
Johnson & Johnson |
|
Endocon |
Biodegradable implant (Annuelle/NET) |
CONRAD FHI |
Wyeth-Ayerst |
|
Female Health Company (formerly Wisconsin Pharmacal) |
Female condom (Reality) |
CONRAD FHI |
|
|
Femcap Inc. |
Cervical cap |
CONRAD FHI |
|
|
Gynetics |
Combined oral contraceptives |
|
|
|
Integra |
Spermicide with polymer barrier |
CONRAD |
|
|
For-profit Companies |
Product |
Not-for-profit Partner(s) |
Other For-profit Partner(s) |
|
Jenapharm (Germany) |
Antiprogestins other than mifepristone |
RTI |
|
|
Leiras Pharmaceuticals (Finland) |
Progestin-releasing IUD (Levonova); levonorgestrel-IUD (Mirena); implants (Norplant) |
WHO/HRP Population Council |
|
|
Lidak |
n-Docosanol |
|
|
|
London International Group (UK) |
Nonlatex condoms |
NIH: NICHD |
|
|
Magainin |
Spermicide/microbicide |
NIH: NICHD (have CRADA) |
|
|
Medisorb (formerly Stolle Research) |
New injectable formulations using biodegradables, microspheres |
CONRAD FHI |
Ortho |
|
Merck |
Immunocontraception |
NIH: NICHD University of Connecticut Medical Center (3-yr. agreement with principal investigator) |
|
|
Novovax |
Novel delivery systems |
NIH: NICHD |
|
|
Organon (Akzo) (Netherlands) |
Antiprogestins other than mifepristone, very potent and selective (Org 317-10, Org 33628); fixed-shape IUDs (CUSafe 300, Mark II, MLCu-375, Ombrelle-250); vaginal rings; combined oral contraceptives; zona pellucida (ZP) vaccine; 1-rod implant |
University of Edinburgh Unit (licensing arrange-Reproductive Biology ment) |
|
|
Ortho (Johnson & Johnson) |
Male method (steroid hormones); spermicides; combined oral contraceptives |
|
Medisorb (see above) |
|
For-profit Companies |
Product |
Not-for-profit Partner(s) |
Other For-profit Partner(s) |
|
Reprogen |
Definition of molecular mechanisms in fertility/ mucosal immune function of urogenital tract; vaccines against STDs/ conception; endometriosis |
|
Oxford Bioscience Partners |
|
ReProtect |
Buffer gel, monoclonal antibodies |
NIH: NICHD, NIAID, FHI |
Ultrafem |
|
Roussel Labs (UK) |
Levonorgestrel-releasing vaginal ring (Femring) (licensing agreement for Phase II clinical trials, manufacture, distribution) |
NIH: NICHD, NIAID, WHO/HRP |
|
|
Schering AG (Germany) |
Antiprogestins other than mifepristone; injectable (Mesigyna); male method, steroid hormones; contraceptive (ZP) vaccine; combined oral contraceptive |
WHO/HRP Government of Indonesia |
|
|
Silesia (Chile) |
Progesterone vaginal rings |
CONRAD Population Council ICMER |
|
|
Tactyl Technologies |
Nonlatex (Tactylon) condoms |
NIH: NICHD CONRAD |
|
|
Theramex (France) |
Minipill (NOMAC, Lutenyl), primarily for breastfeeding women; Uniplant |
South-to-South |
|
|
Ultrafem |
Virucide (Buffer Gel); feminine cups (GelCup, TherapyCup) |
|
ReProtect |
|
Upjohn |
Injectable (Cyclofem) |
WHO/HRP PATH (Concept Foundation) |
|
focus of these partnerships have to do with new delivery systems or improvement of methods to enhance protectiveness against sexually transmitted infections. There is relatively little activity in radically new areas of research endeavor and truly pioneering work seems to be relatively unsupported through partnerships. Two European firms, Organon and Schering, have made considerable advances in the area of antiprogestins but have decided not to proceed, given the political dimensions of the release of RU-486, developed by Roussel-Uclaf. As for other frontier areas, Merck's involvement in immunocontraception is, for the present at least, limited. Of particular interest is the picture of some small start-up firms, for example, Applied Medical Research, Aphton, ContraVac, and Reprogen, all of which are struggling to find partnerships and support for entry with new technologies that are at varying stages of research and development.
Current Industry Involvement in Women's Reproductive Health
It may be illuminating to compare this picture with a recent picture of what is going on in the biopharmaceutical industry in connection with the larger field of women's reproductive health, excluding contraceptives, and anti-infectives for sexually transmitted diseases (see Table 6-10 and Table 6-11). In the area of women's reproductive health, emphasis is heavily on hormonal formulations, largely in connection with menopausal symptoms; work in anti-infectives consists of some work in vaccines, the balance in antiviral drugs. What is interesting about this picture is the nature of the partnerships through which a given research problem is being tackled and the ways in which these seem to differ from patterns in contraceptive research and development. The profile of relationships in both women's reproductive health and anti-infectives is one of partnerships between large pharmaceutical companies and small biotechnology companies and between biotechs. In the case of anti-infectives, the presence of venture capital seems more prominent, perhaps because larger firms that enter into partnership with small biotechs characteristically can muster up their own funds for such purposes. The committee was not able to identify any current investment in contraceptive research and development from the venture capital community.
This accords with what this committee was able to discern through its dialogues with pharmaceutical and venture capital firms in the course of the study period: That is, that the amount of partnering that is going on at present involves the large pharmaceutical industry in a relatively small way, displays no partnering between biotechnology or smaller pharmaceutical firms, and seems to enjoy very little presence of venture capital. That said, the committee readily admits that much of what goes on in the pharmaceutical industry in very new areas of research and development is-understandably-handled as highly privileged information.
Roles of the Public and Nonprofit Sectors in Contraceptive Research and Development
The public and nonprofit sectors have always played a role in contraceptive research and development, historically with their participation growing as industry has withdrawn. Table 6-12 describes the activities and functions of each of the principal participants. In general, their role can be summarized as comprising agenda-setting, motivating, and funding, the latter in research, development, production, and distribution, as well as in product evaluation and training support for investigators.
Funding for contraceptive research and development comes either from the public sector (i.e., governments or parastatal institutions established for the purpose of providing external development assistance) or from private sources (not for-profit entities such as philanthropic organizations, individual philanthropists, nongovernmental organizations/NGOs, and the for-profit pharmaceutical industry).
Data on contraceptive R&D funding are difficult to gather and complex to analyze. The entities that provide or channel such funding all maintain their accounts differently and categorize expenditures in idiosyncratic ways, primarily because their program mandates or the larger mandates of their parent organizations require it. Furthermore, as institutional priorities shift for whatever reason, portfolio emphases shift accordingly and changes in allocations from one year to the next may be large. Nomenclature makes a difference, too: The accounting for ''family planning" will not be the same as the accounting for "reproductive health," and "contraceptive research and development" will be subsumed under each of these rubrics in distinctive fashion. Also, the frontiers between basic research and everything else are sometimes hard to define; categorization of research in particular areas or further along the trajectory of development of individual methods may also be defined variously (Atkinson et al. 1985; NRC/ IOM 1990).
This elusive funding picture is further complicated by variability in the ways funds circulate. A 1993 study of the structure of international research in reproductive health by the Rockefeller Foundation noted three levels in that structure: (1) the funding of research, (2) the conduct of research, and (3) provision of technical support, all of which overlap. Beyond those entities that are the primary sources of funding, there are intermediary entities that serve to channel funds from those primary sources to the actors in research all over the world and may be themselves engaged in the conduct of research. Both intermediaries and primary funders may also provide technical support to one another, which further muddles analysis. In general, however, funds generally move from government funding agencies and private foundations to university research centers, nonprofit research organizations, and small research firms (NRC/IOM 1990).
Private industry constitutes a special case, conducting some of its own in-
TABLE 6-10 U.S. Biopharmaceutical Industry Activities and Relationships in Research and Development in Women's Reproductive Health, Excluding Contraception
|
Company |
Product |
Indication |
Partner |
|
American Home Products |
Estradiol 7-day |
Menopausal symptoms |
|
|
Ares-Serono Group |
Follicular stimulating hormone, recombinant (Gonal-F) |
Infertility |
|
|
Atrix Laboratories |
Luteinizing hormone releasing hormone, Atrigel delivery |
Prostate cancer and endometriosis |
Roche/Syntex |
|
Cell Genesys |
Follicle-stimulating hormone (FSH) produced by gene activation |
Infertility |
Akzo Nobel |
|
ContraVac Diagnostics |
Antibody test to quantitate/ characterize human sperm to measure male fertility; immunocontraception |
Infertility (male) |
Binax |
|
Cygnus Therapeutics |
Estrogen/progestin 7-day |
Menopausal symptoms |
American Home Products |
|
Cygnus Therapeutics |
Estrogen/progestin 3.5-day |
Menopausal symptoms |
American Home Products |
|
Cygnus Therapeutics |
Ethinyl estradiol 7-day |
Menopausal symptoms |
Warner-Lambert |
|
Cygnus Therapeutics |
Estradiol 7-day |
Menopausal symptoms |
Warner-Lambert, Sanofi |
|
Company |
Product |
Indication |
Partner |
|
Cygnus Therapeutics |
Estradiol 7-day |
Menopausal symptoms |
Johnson & Johnson |
|
Ligand |
Tissue-selective estrogen or progesterone agonists |
Gynecological disease, cardiovascular disease |
American Home Products |
|
Ligand |
Progesterone agonists replacement therapy |
Breast cancer, hormone |
American Home Products (option) |
|
Noven Pharmaceuticals |
Estrogen and progestogen, transdermal |
Menopausal symptoms and osteoporosis |
Rhône-Poulenc Rorer |
|
Noven Pharmaceuticals |
Estrogen (2nd generation transdermal) |
Menopausal symptoms and osteoporosis |
Ciba-Geigy, Rhône-Poulenc Rorer |
|
Noven Pharmaceuticals |
Progestogen transdermal |
Menopausal symptoms and osteoporosis |
Rhône-Poulenc Rorer |
|
Pharmos |
Estradiol-CDS |
Post-menopausal |
|
|
TheraTech |
Estradiol, transdermal |
Menopausal symptoms |
|
|
TheraTech |
Estradiol/progestin, transdermal |
Menopausal symptoms |
|
|
TheraTech |
Female hormone replacement therapy, transdermal |
Oopherectomized women |
Solvey |
|
Source: Goldman Sachs. U.S. Research: Biotechnology Products (2nd edition). New York, June 1995. |
|||
TABLE 6-11 Pharmaceutical Firms Currently Involved in Research and Development Relevant to Women's Reproductive Health, Excluding Contraception: Anti-infectives against Sexually Transmitted Diseases
|
Company |
Indication/Product |
Partner |
|
3M Pharmaceuticals |
Genital warts, antiviral (imiquimod) |
n.d. |
|
Aviron |
Herpes vaccine |
Venture-backed: Accel Advent Agingworth IVP |
|
Biocine |
Genital herpes vaccine (given postinfection as immunotherapy) |
Joint venture: Chiron Ciba-Geigy |
|
Biogen |
Hepatitis B and C, antiviral (beta interferon) |
n.d. |
|
Burroughs Wellcome |
Genital herpes, antiviral (Valtrex [valacyclovir]) |
n.d. |
|
Glaxo |
Hepatitis B, antiviral (lamivudine) |
n.d. |
|
Lidak |
Genital herpes, labialis herpes, antiviral (Lidakol [n-docosanol]) |
n.d. |
|
Hoffman-La Roche |
Uncomplicated gonorrhea, antibiotic (Megalone [fleroxacin]) |
n.d. |
|
Janssen Pharmaceutica |
Candidiasis, antifungal (Sporanox [itra-conazole]) |
n.d. |
|
North American Vaccine |
Bacterial diseases |
Affiliated with Biochem Pharma |
|
Oclassen Pharmaceuticals |
Genital warts, antiviral (Condylox Gel [podo-filox]) |
n.d. |
|
Pfizer |
Vaginal candidiasis, antifungal (Diflucan [(fluconazole]) |
n.d. |
house research and funding external research at various points along the R&D pipeline (Rockefeller Foundation 1995b). An unknown (and apparently unknowable) amount of funding also flows from large pharmaceutical firms to universities and nonprofit research organizations and, what seems to be much less, to small R&D firms. Because industry must be attentive to the bottom line in ways that are somewhat different from the bottom-line concerns of nonprofit entities, its accounting for R&D expenditures is also distinctive. Most importantly in terms of getting a grasp on industry investment in contraceptive research and development, information about industry internal allocations is treated as proprietary and there are not the same pressures to disclose such information as those typically experienced by the public sector. In sum, there is a wide margin of error and ignorance in terms of tracking funding trends in contraceptive research and development and making comparisons within and between sectors.
TABLE 6-12 Participants Active in Contraceptive Research and Development, Public and Private Sectors, 1995a
|
U.S. Government Agencies |
|
|
Contraceptive Development Branch and Contraceptive and Reproductive Evaluation Branch, Center for Population Research, National Institute of Child Health and Human Development, National Institutes of Health (CDB/CPR, CAREB/CPR, NICHD/NIH) |
Established in 1968 as part of the National Institute of Child Health and Human Development of the U.S. National Institutes of Health, the Center for Population Research began its institutional life by supporting fundamental mission-oriented research; it expanded into CR&D in 1971. It does no research itself but manages research through contracts to universities, industry, and nonprofit institutions. The CPR is the world's principal funder of fundamental nondirected reproductive research and a major funder of research into the long-term safety and efficacy of marketed methods. The CDB currently supports research on male contraception (androgen replacement, peptide contraceptives, nonhormonal drugs, barrier methods) and female contraception (hormonal drugs, chemical barriers, immunocontraception). The CDB has established three Cooperative Contraceptive Centers, funded under congressional mandate, two of which focus on immune contraception, the other on vaginal methods. It also supports a biological testing facility, synthetic chemical and peptide facilities, and a primate testing facility, and has initiated a multicenter Clinical Trials Network to enable faster testing of promising products. It issues Requests for Proposals (RFPs) and for Applications (RFAs) and is developing its ability to enter more easily and quickly into Cooperative Research and Development Agreements (CRADAs) and Small Business Innovation Research (SBIR) grants. NICHD has also opened up a "Discretionary Funding Zone" as an alternative to RFAs that will channel support into High Priority Research Areas (HPRA), explicitly contemplated as a mechanism for accelerating contraceptive research and development. |
|
U.S. Agency for International Development (USAID) |
Of all U.S. agencies, only USAID and NICHD/NIH (q.v.) support contraceptive research and development, although the Department of Agriculture, the Department of Energy, and the National Science Foundation provide modest funding for reproductive biology. USAID conducts no research of its own but provides virtually all of FHI's and CONRAD's support through cooperative agreements. It is also the second largest bilateral funder of the WHO's Special Programme of Research, Development, and Research Training in Human Reproduction (WHO/HRP) and provides much of the funding for the Program for Appropriate Technology in Health (PATH) and for the work of the Population Council's International Center for Contraceptive Research (ICCR). USAID's major product accomplishments include development and/or evaluation of tubal bands and clips for female sterilization; evaluation and introduction of low-dose combined oral contraceptives and progestin-only pills; supporting the research that led to development of |
|
|
the contraceptive vaginal sponge; evaluation and FDA approval of the Reality female condom; evaluation, introduction, and extension of lifetime effectiveness of the Copper-T-380A intrauterine device; evaluation and introduction of Norplant and Norplant II implants; and delineation and evaluation of the Lactational Amenorrhea Method (LAM) for child spacing. At present, USAID cooperating agencies (CAs) are involved with preclinical and clinical testing of two woman-controlled barrier methods, Lea's Shield and Femcap; testing of nonlatex male condoms; preclinical testing of compounds and formulations for spermicidal and virucidal activity; and clinical testing of vaginal contraceptive film preparations. |
|
John E. Fogarty International Center National Institutes of Health |
In October 1995, the Center announced the funding of initial awards under an International Training and Research in Population and Health Program jointly sponsored by Fogarty and NICHD. Seven awards have been made to U.S. universities to support programs for scientists and health professionals from developing countries concerned with population issues, including research and training in areas related to reproductive biology. These grants will be funded at a level of $1 million/year for 5 years. |
|
U.S. Nonprofit Organizations |
|
|
|
Nonspecialized Research Institutions |
|
Research Triangle Institute (RTI) Salk Institute |
Biodegradable polymers; delivery of contraceptive drugs, antiprogestins, male contraceptives, spermicides. Peptide antagonists of LHRH (GnRH) as ovulation inhibitors. |
|
Specialized Contraceptive Research Institutions |
|
|
Contraceptive Research and Development (CONRAD) program, Eastern Virginia Medical School |
Established in 1986 under a cooperative agreement with USAID, CONRAD's focus is on the improvement of existing contraceptives and development of new methods that are safe, effective, acceptable, and suitable for use in the United States and developing countries. CONRAD's mandate is to move contraceptive leads from the laboratory through phase I and II clinical trials. To this end, it supports timely and high-quality extramural subprojects conducted by collaborating investigators at universities, research institutes, and private companies worldwide. Current priority is assigned to methods that are controlled by the individual female user, are suitable for lactating women, are long-acting, interfere in the maturation/transportation/ fertilization processes, and decrease transmission of HIV and other STDs, as well as to generic products for use by public sector family planning programs at less cost than trade-name products. From January 1987 to November 1995, CONRAD managed approximately 175 extramural subprojects related to research, development, and testing of new contraceptive methods. |
|
Family Health International (FHI) |
FHI was founded in 1971 with funds from USAID, which continues its support. It has expanded its original mandate, which was to test and improve available contraceptive methods, to include development of new methods, the study of the impact of contraceptive use on the health of developing-country populations, and the strengthening of the research capacity of developing countries. As of 1995, around 15 percent of its overall budget was devoted to contraceptive development of new contraceptive methods, primarily in the clinical trials phase. Most recently, FHI conducted trials for the Reality female condom and Lea's Shield and has developed modified condoms for men. |
|
Population Council |
The Population Council's International Center for Contraceptive Research (ICCR) was established in 1971 to pursue scientific leads deemed ready for dosage formulation and clinical trials. It is the only R&D group that conducts extensive product formulation work in its own laboratories, under the aegis of its Center for Biomedical Research. Its present focus is on development and improvement of technology for unserved or underserved groups: methods under the user's control; methods for men, nursing and postmenopausal women, and teenagers; methods that do not alter women's menstrual cycles; compounds protective against STDs, including HIV; and methods of medical pregnancy termination. Under active investigation are contraceptive rings, levonorgestrel-releasing IUDs, improved implants and transdermal delivery systems for |
|
|
women, microbicides, medical abortifacients, a two-implant system and immunocontraception for men, and "no-scalpel" vasectomy. The Council receives grants from foundations (Ford, Rockefeller, and Mellon), international organizations, and federal agencies (including NICHD and USAID), and also uses funds from its endowment to support some contraceptive research. |
|
Program in Appropriate Technology in Health (PATH) |
Founded in 1976, PATH's work in contraceptive R&D focuses on the production end of the R&D trajectory. The organization has worked with colleagues in 39 developing countries (including Bangladesh, Brazil, Egypt, India, Indonesia, Mexico, People's Republic of China, Philippines, Thailand, Turkey, Vietnam, and Zimbabwe) to conduct feasibility studies and provide technical assistance in technology transfer and good manufacturing practices in connection with the production of condoms, injectables, Copper-T IUDs, oral contraceptives, and spermicidal products. PATH also collaborated with the Population Council and other groups in introduction of the Copper-T-380A IUD and Norplant. Its support comes from a number of public- and private sector sources, including USAID, foundations, UNFPA, World Bank, and WHO. |
|
Program for the Topical Prevention of Conception and Disease (TOPCAD) |
Established in October 1993, TOPCAD's mandate is to develop and evaluate new woman-controlled vaginal methods of preventing conception and/or STDs; foster international collaboration and training to test novel methods and train new investigators; and study consumer acceptance and use of vaginal topicals and the optimal delivery of newly developed methods. Funding comes from the Rockefeller Foundation, the CONRAD Program, and the Andrew W. Mellon Foundation; its headquarters are at Rush-PresbyterianSt. Luke's Medical Center, Chicago. |
|
Consortium for Industrial Collaboration in Contraceptive Research (CICCR) |
The CICCR was initiated in 1995 by the Rockefeller Foundation and the Andrew W. Mellon Foundation at the CONRAD Program, to foster research on new contraceptives, with emphasis on barrier methods, a range of monthly regimens for women, and methods for males. The CICCR will award grants for contraceptive R&D to not-for-profit research institutions working in collaboration with for-profit industrial partners, in the expectation that sharing cost and risk in the early stages of research will provide the momentum needed to drive the field. |
|
Universities |
Research and training in reproductive technology is ongoing at over two dozen U.S. universities. |
|
International Agencies |
|
|
World Health Organization Special Programme of Research, Development, and Research Training in Human Reproduction |
Established by the WHO in 1971-1972, the "HRP" mobilizes and coordinates a worldwide effort to develop appropriate technologies and generate information in areas of reproductive health of high priority to developing countries. It works to strengthen human and material resources for research, to enable developing countries to address their own research needs and participate in global research efforts, and it (WHO/HRP) supports collaborative research centers in 56 countries. Its present portfolio is organized around seven methods, including postovulatory, long-acting, and male methods, and immunocontraception. HRP is funded by voluntary contributions from member states in developed and developing regions and is cosponsored by the United Nations Development Programme (UNDP), United Nations Population Fund (UNFPA), and the World Bank. HRP's income for the 1994-1995 biennium came from a total of 23 funders worldwide, including 18 bilateral agencies, 3 multilateral organizations, and 2 foundations (Rockefeller and Ford). The United Kingdom is the largest contributor ($7.8 million for the 1994-1995 biennium), followed by the UNDP ($7.0 million), the United States ($5.5 million), the World Bank ($3.75 million), Norway ($2.38 million), Sweden ($2.0 million), Denmark ($1.9 million), and The Netherlands and Australia ($1.0 million each). The HRP's recently revised mission goals are to increase reproductive choices for women, expand male responsibility in reproductive health, respond to needs of developing countries, and coordinate and expand global research. |
|
South to South Cooperation in Health |
South to South is a network of contraceptive researchers from 14 countries, headquartered in Brazil. Research includes development of single-rod implants; a new progestin, ST 1435; menses inducers and emergency contraception, gossypol and Praneem polyherbal preparations; vaginal contraceptives; and immunocontraception. |
|
United Nations Population Fund (UNFPA) World Bank |
UNFPA provides support for contraceptive development activities, notably its support to WHO/HRP, as well as a major procurement activity for developing countries. Support for WHO/HRP. |
|
Government Agencies (worldwide) |
|
|
International Development Research Centre (IDRC) (Canada) |
Support for contraceptive development activities. |
|
Indian Council of Medical Research (ICMR) |
Supported by the government of India at a level of $3.0 million in 1992. |
|
Medical Research Council (MRC) (United Kingdom) |
The British government provides support to its Medical Research Council (MRC) for work on fertility, contraception, and abortion ($5.8 million in 1992), as well as for basic research in molecular genetics ($17.7 million), fetal development and child health ($16 million), and infections and immunity (HIV) ($47 million). |
|
Overseas Development Assistance (ODA)(United Kingdom) Institute National de la Santé et de Recherche Médicale (INSERM/France) |
The British government is also the leading bilateral supporter of the WHO/HRP and its contraceptive R&D activities. Biomedical research; instrumental in development of RU 486. |
|
National Research Institute for Family Planning (China) |
The government of the People's Republic of China (GOPRC) has collaborated with the United Nations in the buildup of research capacities in human reproduction and family planning since early 1979 when agreement was reached for joint activities with the WHO/HRP. Between 1979 and 1994, UNFPA provided $13 million and WHO/HRP some $15 million to research institutes in Beijing, Chengdu, Guangzhou, Shanghai, and Tianjin for institutional strengthening and collaborative research activities. The GOPRC invested at least twice those amounts toward the same objectives. The Chinese National Research Programme for Family Planning has focused its priorities on development of new types and improvement of currently available IUDs, long-acting steroid contraceptives (once-a-month oral contraceptives, implants, vaginal rings, monthly injectables), male methods of fertility regulation (gossypol, long-acting testosterone esters), no-scalpel and reversible methods of sterilization, immunocontraception (antisperm antigen, ZP antibodies), medical abortion (herbal approaches, RU 486), and basic and applied research on reproductive physiology (implantation mechanism, testicular and epididymal function, ovarian function and regulation, IUD- and steroid-induced bleeding). There are 33 contraceptive manufacturers in China, producing over 40 varieties of oral contraceptives, injectables, spermicides, IUDs, and condoms. |
|
Nonprofit Private Sector |
|
|
Foundationsb |
|
|
Rockefeller Foundation |
The Rockefeller Foundation has been providing support for population research since 1963, when it declared population as a primary area of interest. In 1991, the Foundation began a collaboration with the WHO/HRP to support an initiative in Technical Cooperation Among Developing Countries (TCDC) to foster cooperation in research and research training in biomedical, social sciences, and public health research in reproductive health between two or more institutions in developing countries. In its 1995 Annual Report, the Foundation includes ''Mobilization for Unmet Demand" among its nine core strategies, with a commitment of $14.75 million (the second largest allocation) toward mobilizing during the next decade "the resources to ensure availability of high-quality reproductive health and family planning services to all women in the developing world." |
|
Andrew W. Mellon Foundation |
The Andrew W. Mellon Foundation's interest in the population field dates from its beginning in 1969, when the Avalon and Old Dominion Foundations, which had a history of grants in the population field, were amalgamated to form the Andrew W. Mellon Foundation. Beginning in 1977, the Foundation has been providing grants to U.S. centers of reproductive biology, whose main purpose has been to provide support for postdoctoral fellows and junior faculty members working in areas relevant to contraceptive development. In 1995, Mellon joined Rockefeller in supporting the CICCR, described above. |
|
Burroughs Wellcome Fund |
Burroughs Wellcome has entered this arena with a 1995 funding level of $800,000 dedicated entirely to the training of researchers. |
|
Howard Hughes Medical Institute (HHMI) |
While HHMI does not support research in contraceptive research and development specifically, it does support fellowships in fundamental science of high relevance. As of 1994, the Institute was supporting 8 fellowships in the area of molecular and cell biology and genetics with particular relevance for contraceptive research and development. |
Funding Patterns Over Time: 1970-1990
Between 1973 and 1987, the pattern in federal funding was one of apparent overall increase. During that period, support for basic research in reproductive biology rose in current dollars from $30 million to $135 million, for contraceptive development from $7 million to $36 million. In constant dollars, the increases were 64 percent and 78 percent, respectively. However, that apparently positive trend conceals smaller, more significant, and less positive patterns that can be seen as predicting trends in the 1990s (NRC/IOM 1990).
First, foundation support for contraceptive research and development dropped dramatically in relative terms, from 22 percent of all federal and foundation funding for research in reproductive biology in FY1973 to around 3 percent by FY 1987. Support for contraceptive development from the Ford, Rockefeller, and Mellon Foundations, and the Population Council, consistently much smaller than support for basic research, fell from about 18 percent to 10 percent in those same years (NRC/IOM 1990).
Second, while U.S. federal support for population research from FY1970 to FY 1990 rose from $34.3 million to $231.2 million in current dollars, the trend in constant dollars was fairly flat. After 1982, the swings were less extreme than they had been in the 1970s: Increases and decreases in constant-dollar funding levels remained more or less in the low single digits except for a one-time 12 percent increase in 1987 to almost $74 million in U.S. federal funds for population research. However, each of the subsequent three years brought a decline in constant dollars, with each of the three successive years bringing a constant-dollar decline in federal support for population research. Federal support for population research totaled $232.2 million in 1990 current dollars, $6.4 million less than in 1989, a 3 percent drop; the drop from the 1989 constant-dollar level of $69.0 million to $63.5 million in 1990 was an 8 percent decrease. At the same time, the median cost of all population research projects rose by 4 percent; the median costs for contraceptive development and contraceptive evaluation projects exceeded that overall average considerably (USDHHS 1990).
Third, federal support for reproductive processes research fell from $141.6 million in 1989 to $134.5 million in 1990, the first time since 1977 that funds for this area declined. Contraceptive development also received less funding, dropping from the $26.9 million of 1989 to $25.6 million. Support for contraceptive evaluation decreased to $4.8 million in 1990, a far cry from its peak of $15.1 million in FY1980.
Fourth, as in the case of the foundations, federal funding for contraceptive development continues to be substantially less than the amount devoted to basic research. Since 1981, over 50 percent of all federal funding for population research has gone to basic research in reproductive biology, with studies of reproductive endocrinology dominating heavily. In 1990, 58 percent of all federal funding for population research went to reproductive processes, compared to
11 percent for contraceptive development and 2 percent for contraceptive evaluation.
Fifth, federal funding for research on reproductive processes has been heavily dominated by reproductive endocrinology, with 30 percent of all projects and 23 percent of the funds. Demography ranked second in funding, cell and molecular biology third. In terms of numbers of projects, cellular and molecular biology was second, demography third. The gaps between the disciplines in terms of funding were large: The dollar amount for reproductive endocrinology was twice that of demography and almost three times that of cell and molecular biology, which received only 15 percent of federal funds for research on reproductive processes. And, of all federal funding for population research, 22.5 percent was for research on the female reproductive system, 12.5 percent on the male reproductive system (USDHHS 1990).
Sixth, trends in worldwide funding, after peaking in 1972, fell sharply in 1975 (Lincoln and Kaeser 1987) and stayed relatively constant between 1977 and 1983. In the rest of the decade, a percentage increase of 138 percent in USAID support for contraceptive research and development and, subsequently, the onset of UNDP (United Nations Development Programme), UNFPA (United Nations Population Fund), and World Bank contributions to the World Health Organization's Human Reproduction Programme constituted the funding bulwark for the period.
Table 6-13 presents a compilation of funding trends in the public and private sectors between 1990 and 1995. The pattern was erratic over the five-year period but, overall, was one of modest increase in current dollars, but either flat or declining in constant dollars. The picture as this committee completes its work is mixed. At present, only four private foundations worldwide provide funding explicitly for contraceptive research and development: The Burroughs Wellcome Fund (U.S.A.), the Andrew W. Mellon Foundation, the Rockefeller Foundation, and the Wellcome Trust (United Kingdom). In 1995, all of these entities made some increase in the volume of funding in some way supportive of contraceptive research and development. The Burroughs Wellcome Fund, concerned about assuring maintenance of and fostering increase in the cadre of scientists for the field, made a first-year investment of $800,000 toward the training of scientists. The Rockefeller Foundation committed $14.75 million toward mobilizing during the next decade "the resources to ensure availability of high-quality reproductive health and family planning services to all women in the developing world" and, with Andrew W. Mellon, provided startup funds for the Consortium for Industrial Collaboration in Contraceptive Research (CICCR). The Wellcome Trust made a public commitment to invest £50 million in the area of population, including contraceptive research and development, primarily in the developing world.
Yet, as the Rockefeller Foundation notes in its related mission statement (Fathalla 1994),3 foundation funds are small relative to the very large amounts required for the development of a new contraceptive method (see Figure 6-4), so
TABLE 6-13 Funding for Contraceptive Research and Development, Selected Institutions, Selected Years (in millions of U.S. dollars)
|
|
Annual Average |
|
|
|
|
|
|
|
|
1980-1983a |
1990 |
1991 |
1992 |
1993 |
1994 |
1995 |
|
NIH/NICHD/CPR/CDB |
8.4 |
11.5 |
13.5 |
15.25 |
17.0 |
15.2 |
18.7 |
|
USAID |
8.0 |
6.7b |
9.9 |
9.1 |
9.8 |
9.0 |
11.3 |
|
|
|
10.9 |
20.5 |
16.9 |
22.4 |
19.0 |
21.1 |
|
WHO/HRP |
6.7 |
6.7c |
6.7 |
5.3 |
5.3 |
4.5b |
4.5 |
|
|
|
18.0 |
18.0 |
16.4 |
16.4 |
15.1 |
15.1 |
|
Andrew W. Mellon Foundation |
|
|
|
|
|
|
|
|
Reproductive biology |
n.d. |
1.4 |
1.7 |
1.3 |
1.9 |
1.5 |
1.5 |
|
Contraceptive development |
n.d. |
0.7 |
0.7 |
0.9 |
1.1 |
1.1 |
1.5 |
|
Total |
n.d. |
2.2 |
2.5 |
2.25 |
3.0 |
2.6 |
3.0 |
|
Rockefeller Foundation |
n.d. |
5.0 |
2.6 |
5.6 |
4.3 |
4.7 |
5.4 |
|
Notes: USAID = United States Agency for International Development; NIH/NICHD/CDC/CPR = National Institutes of Health, National Institute for Child Health and Human Development, Center for Population Research, Contraceptive Development Branch; WHO/HRP = UNDP/UNFPA/WHO/World Bank Special Programme of Research Development and Research Training in Human Reproduction. Information in this table was obtained from telephone conversations or written exchanges with the entities listed. Because of the complexity of funding flows and passthroughs, which can lead to misleading doublecounting, the table includes only the largest U.S. funders of contraceptive research and development, with the exception of the listing for the World Health Organization's Special Programme for Research, Development, and Research Training in Human Reproduction (WHO/HRP), whose funding represents support from 18 bilateral and 3 multilateral agencies and 2 foundations) (see Table 6-12 for detail). Owing to the proprietary nature of this type of information, the table does not include figures on pharmaceutical industry investment in contraceptive R&D. Older estimates of such investment (see Atkinson et al. 1985) and estimates calculated by the Program for Appropriate Technology in Health (PATH) in 1994, have ranged around $25 million a year, a figure said by some industry representatives to be considerably understated. a Source: LE Atkinson, R Lincoln, JD Forrest. Worldwide trends in funding for contraceptive research and evaluation. Family Planning Perspectives 17(5):196-207, 1985. b The first row of figures presents the approximate values for contraceptive development research through FDA approval; the second row is that portion of USAID support for population that goes to biomedical research, which constitutes about 50 percent of all population research. c The first row of figures refers to the line item for Technology Development and Assessment, which includes funding for long-acting agents, postovulatory methods, vaccines, male methods, natural methods, IUD research, and infertility, under the heading "Research and Development." The second row presents total expenditures on research and development and institutional strengthening for research, which also includes Technology Introduction and Transfer (some of which might be considered contraceptive R&D), Epidemiological Research, Social Science Research, and Regional Resources for Research. Sources: WHO/ HRP. Financial Reports (1990-1991, 1992-1993, 1994-1995). Geneva. 1996. |
that their value—and, in this sense, that value is substantial—is as a catalytic agent. There is other, related foundation investment: The Pew Charitable Trusts, Ford Foundation, John D. and Catherine T. MacArthur Foundation, the Hewlett Foundation, and the David and Lucile Packard Foundation all allocate some funding to population and reproductive health, but their funding streams are oriented toward policy, social scientific research, and family planning services.
A very large concern as this committee completes its work is the resurgence of political, judicial, and legislative controversy over family planning that is expressed in some measure in the November 1994 election of a new, more conservative U.S. Congress; ongoing attempts to reverse the Supreme Court's decision on abortion through adoption of a constitutional amendment (a prospect dormant since 1983); and the conceptual blurring of the lines of demarcation between contraception and abortion, not unrelated to the modes of action of certain types of fertility control (Rosoff 1995). All this, together with the prevailing lack of enthusiasm for U.S. foreign assistance and a climate of budgetary austerity unlikely to dissipate in the foreseeable future, are imposing a substantial chill on what has been a substantial funding flow-$550 million for all USAID population assistance program activity in FY 1995, cut by 35 percent for FY 1996. While this year's budget of the National Institutes of Health, of which the National Institute of Child Health and Human Development is a component, has not been affected by the austerities imposed on other government agencies, the future is always a question.
Concluding Comment
Some of the tables in this chapter, though accurate, are misleading. The reader might gather the impression that much is happening in contraceptive research and development and ask why more is wanted.
There are two responses. The first is that, for the most part, current activity emphasizes enhancement of existing methods. This sort of "me-too" development can be important and is characteristically part of the development life of all technologies, few of which arrive on the human scene with no room for improvement. Yet, these modifications will still leave significant needs in significant populations unaddressed, needs that will require pharmaceutical pioneering.
Second, pioneering activity in contraceptive research and development will, in turn, require investment in basic research and in the early, highest-risk stages of the development process. Thus, funding to prime such activity, to share risk with academic researchers and corporate developers and, possibly, to leverage other infusions of funds, becomes key. The rationale is that, once a product completes phase II clinical trials, the chance of its getting into the marketplace is 50 percent, a notably brighter outlook for a corporation looking to develop new contraceptives (Harper 1996).
An increasingly frequent pattern of work in the contemporary pharmaceuti-
cal industry is a sequence of "virtual partnerships" and contract research arrangements at different junctures in the research and development processes. As noted above, this is not an unfamiliar pattern in contraceptive R&D; in fact, the willingness of small companies to risk engagement, and the willingness of the public and non-profit sectors to venture along with them, have been pivotal in advances in the field. It is now possible that the power and speed of combinatorial chemistry and high-speed computer modelling of compounds may make it more plausible for these sorts of collaborations to occur earlier in the research sequence. The partial nature of such early commitments might be attractive to biotechnology companies and might also mean that large pharmaceutical companies, wary of involvement in full product launch, would be willing to engage in different kinds of collaboration "closer to the bench."
The committee recognizes the difficulties international agencies face in obtaining additional funding for contraceptive research and development at a time of fiscal austerity. Still, the committee's scrutiny of funding for contraceptive research and development over the past 15 years indicates that it has, at best, remained flat in constant dollars and actually may have diminished. This means that cuts of any size have major implications. Targeted financial support from the public sector, notably by USAID and NICHD, has been critical in drawing U.S. university and not-for-profit institutional researchers into the field of reproductive biology and in catalyzing and sustaining the creative research partnerships that produced the advances in contraceptive research and development over the past two decades. The public sector also has particular strength and expertise in supporting the later stages of contraceptive research and development, for example, the clinical trials necessary to obtain the data required by the drug regulatory process. Any function that can expeditiously catalyze these sorts of ad hoc mechanisms across and within sectors could provide considerable value added. The reverse is also true; that is, any function impeding the healthy growth of such mechanisms is value lost.
References
Atkinson LE, R Lincoln, JD Forrest. Worldwide trends in funding for contraceptive research and evaluation. Family Planning Perspectives 17(5): 196-207, 1985.
Bronnenkant L. Panel discussion on vaccine development to meet U.S. and international needs. AIDS Research and Human Retroviruses 10:Supplement 2, 1994.
Burrill GS, KB Lee Jr. Biotech 94: Long-Term Value, Short-Term Hurdles (Industry Annual Report). San Francisco: Ernst and Young. 1993.
Burrill GS, KB Lee Jr. Biotech 93: Accelerating Commercialization (Industry Annual Report). San Francisco: Ernst and Young. 1992.
Djerassi C. The bitter pill. Science 245:354-361, 1989.
Easton R. Reform re-slices the market share pie. In Vivo: The Business and Medicine Report 11(7), 1993.
Fathalla MF. Mobilization of resources for a second contraceptive technology revolution. IN Contraceptive Research and Development 1984-1994: The Road from Mexico City to Cairo and Beyond. PFA Van Look, G Pérez-Palacios, eds. Delhi: Oxford University Press. 1994.
Frost and Sullivan. U.S. Contraceptives and Fertility Product Markets. New York, 1993.
Gelijns AC, CO Pannenborg. The development of contraceptive technology: Case studies of incentives and disincentives to innovation. International Journal of Technology Assessment in Health Care 9(2):210-22, 1993.
Goldman Sachs. U.S. Research: Biotechnology Products (2nd ed.). New York. June 1995.
Harper MJK. Funding For a New Partnership Between the Pharmaceutical Industry and Not-for profit Research Institutions. Arlington, VA: Consortium for Industrial Collaboration in Contraceptive Research (CICCR). 1996.
Harper MJK. Birth Control Technologies: Prospects by the Year 2000. Austin: University of Texas Press. 1983.
Hatcher RA, J Trussell F Stewart, et al. Contraceptive Technology: 16th Revised Edition. New York: Irvington Publishers. 1994.
Kreger JC. Industry Overview: Contract Research Organizations (CROs)—A Clinically-Proven Route to Profits. Deerfield, IL. November 1994.
Lee KB Jr, GS Burrill. Biotech 96: Pursuing Sustainability: The Tenth Annual Industry Report. Palo Alto, CA: Ernst and Young LLP. 1995.
Lee KB Jr, GS Burrill. Biotech 95: Reform, Restructure, Renewal (9 th Annual Industry Report). Palo Alto, CA: Ernst and Young LLP. 1994.
Lincoln R, L Kaeser. Whatever happened to the contraceptive revolution? Family Planning Perspectives 13(4):141-145, 1987.
National Research Council and the Institute of Medicine (NRC/IOM). Developing New Contraceptives: Obstacles and Opportunities. L Mastroianni Jr, PJ Donaldson, TT Kane, eds. Washington, DC: National Academy Press. 1990.
Office of Technology Assessment (OTA). Pharmaceutical R&D: Costs, Risks and Rewards. Washington, DC: Congress of the United States. 1993.
Peterson LS. Contraceptive Use in the United States: 1982-90—Advance Data from Vital and Health Statistics of the Centers for Disease Control and Prevention/National Center for Health Statistics No. 260. Hyattsville, MD: National Center for Health Statistics. 1995.
Pharmaceutical Research and Manufacturers of America (PhRMA). IN Development: New Medicines for Infectious Diseases. Washington, DC: 1994.
Pollard MR. Pharmaceutical innovation in the United States: Factors affecting future performance. International Journal of Technology Assessment in Health Care 9(2): 167-173, 1993.
Program for Appropriate Technology in Health (PATH). Market-related Issues Affecting the Participation of the Private Sector in Contraceptive Development: A Final Report to the Rockefeller Foundation, 30 November 1994. Seattle, WA: 1994.
Rockefeller Foundation. Public/Private Sector Collaboration in Contraceptive Research and Development: A Call for a New Partnership. Report from a Bellagio Conference, 10-14 April 1995. New York: Rockefeller Foundation. 1995a.
Rockefeller Foundation. International Research in Reproductive Health: A Guide to Agencies/ Organizations. Paper prepared by World Health Organization Special Programme for Research, Development and Research Training in Human Reproduction. New York: The Rockefeller Foundation. 1995b.
Rosoff JI. The political storms over family planning: Supplement to chapters 1 and 7. IN The Best Intentions: Unintended Pregnancy and the Well-Being of Children and Families. S Brown, L Eisenberg, eds. Washington, DC: National Academy Press. 1995.
Steyer R. Searle nearing end of lawsuits over Copper 7 contraceptive. St. Louis Post-Dispatch P.1E, 15 October 1995.
U.S. Department of Health and Human Services (USDHHS). Inventory and Analysis of Federal Population Research, Fiscal Year 1990. Bethesda, MD: Office of Science Policy and Analysis and Center for Population Research, National Institute of Child Health and Human Development, for the Interagency Committee on Population Research. 1990.
Notes
-
2.
The committee is grateful to the industry research firm Frost and Sullivan for giving the Institute of Medicine permission to use copyrighted information presented in this section.
7
Issues of Law, Regulation, Information, and the Environment for Contraceptive Research and Development
The 1990 report on contraceptive development by the National Research Council and Institute of Medicine (NRC/IOM 1990) paid detailed attention to the magnitude of regulatory factors and product liability as obstacles to new advances in the field. The study committee did not find the U.S. Food and Drug Administration (FDA) and its regulatory authority over contraceptives to be major obstacles, owing to important legislative and procedural changes that had occurred in the decade of the 1980s. In fact, the committee voiced support for the FDA's rigorous review and approval processes and described its own limited recommendations as ways to make the evaluation of product safety more meaningful and more specific to different user populations.
In contrast, the study committee concluded that the impact of product liability litigation, particularly on the cost and availability of liability insurance, was a very large contributor to the climate of disincentives for the development of contraceptive products. The weightiest aspects of litigation were its unpredictability and the fact that evidence of compliance with FDA regulations was granted no special status in liability lawsuits in most states. Thus, the committee recommended that the U.S. Congress enact a product liability statute that would, first, establish uniform standards for lawsuits involving contraceptives and, second, give manufacturers of an FDA-reviewed contraceptive product a defense based on FDA's acceptance of that product.
This chapter is an update of the work of that 1990 committee, meant to respond to the following questions: What of note has happened since 1990 in the areas of regulation and product liability related to contraceptive research and development; has the weight of those two variables on the field increased or
diminished; and what does that suggest, if anything, in the way of recommendations for action?
The first section of this chapter examines changes in the regulatory climate since 1990; the second section examines changes in the legal climate since that year, in each instance from the perspective of how those affect—or might affect—contraceptive research and development. The chapter then turns to an area where law, culture, politics, scientific research, medicine, and the thoughts and needs of contraceptive users intersect: the area of information. The chapter closes with a scan of those aspects of the environment that are most critical in generating the controversy that so often attends the development and use of modern contraceptive technologies.
The emphasis in this chapter is primarily on the United States. Even though we know that regulatory structures and legal frameworks differ significantly in many other countries in ways that could be illuminating, resources limited what the committee could deal with adequately. It may also be, at least at present, that breakthroughs in contraceptive research and development are not likely to occur without full-fledged U.S. participation. Finally, regulatory, legal, and political and legal decisions that are made in the United States continue to produce repercussions for other countries, particularly developing countries; examples include the controversy around Depo-Provera1 and the persistent controversy in the United States around the abortion issue.
This is not to say that no other nation is present on the international contraceptive research and development stage. As perhaps the most prominent examples, Organon of The Netherlands and Schering AG of Germany are leading industrial players; until recently, French scientists at INSERM (Institute National de la Santé et de Recherche Médicale) were actively involved in the development of antiprogestins; India and China both have major research endeavors, and China is also a major producer of contraceptives; and The Wellcome Trust of the United Kingdom has recently committed £50 million to priorities in population and contraceptive research and development.
There are also informative similarities between the United States and other countries. Many European countries and the United Kingdom have experienced some of the same controversy around contraceptives for postcoital use that has been experienced in the United States in connection with RU 486 (Hughes 1995). Still, no nation is as litigious as the United States, although this may be changing (Steyer 1995), and few have a health system as driven by the market (Hutton et al. 1994). Each of these characteristics affect contraceptive research and development and the availability of contraceptive services.
Regulatory Influences on Contraceptive Research and Development
Regulatory Developments Specifically Pertaining to Contraceptives
Changes in Requirements for Safety Data
Contraceptive products are subject to the same basic statutory and regulatory approval requirements as are other medical products in the corresponding regulatory category (i.e., drugs, biologics, or devices). Historically, however, the FDA has imposed special requirements on contraceptive products, particularly in the area of safety data, requirements which have had the practical result of delaying or obstructing the approval of such products. Among the most onerous was the long-standing requirement that new contraceptive drugs undergo long-term toxicology testing in several animal species, including seven-year studies in dogs and up to 10 years of study in monkeys. FDA's rationale for this unusually extensive testing was that contraceptives are intended for regular long-term use by millions of healthy women, most of whom have alternative contraceptive options, so that greater than usual rigor in ensuring long-term safety was necessary (NRC/IOM 1990).
This approach—though not lacking in logic—was, in its original form, somewhat exaggerated. Pregnancy, after all, poses its own set of health risks for many women, including serious complications for some women if the pregnancy is carried to term; these risks are likely to be far more sizable in developing countries where women's poor health, low economic status, and uneven access to good-quality care may be major factors. Thus, the risk-benefit calculus of approving a new contraceptive may not be quite as heavily weighted toward the reduction of product-associated risks as FDA's original model had it. At the same time, the calculation of risk in relation to both contraception and pregnancy is terribly complex; much debated; and highly various by age, culture, and socioeconomic environment. For instance, a young woman in the United States, even in a difficult socioeconomic environment, is unlikely to factor the possibility of maternal mortality into her ''personal calculus of choice" about using contraceptives or getting pregnant (Zabin 1994), while a very young woman in a very remote village or urban slum in the developing world may do so as a matter of course. Similarly, older women in what is probably the majority of countries will construct their "burden-benefit" calculations with a keen awareness of the relationship between late childbearing and maternal mortality (Bulatao and Lee 1983; Scheper-Hughes 1995).
FDA's thinking on long-term toxicology studies has evolved over the years in the direction of greater flexibility. As noted in the 1990 NRC/IOM report, by 1987, the 10-year monkey study requirement had been eliminated and the seven-year dog (beagle) study requirement reduced to three-years. The time and cost
burden imposed by this latter test, however, was still substantial. In addition, the test was scientifically controversial because of the innately high risk of breast tumors in beagles, which, in turn, could increase the likelihood of "false positive" results.
The injectable contraceptive Depo-Provera (DMPA or depo-medroxyprogesterone acetate) became something of a test case for the three-year beagle toxicology requirement. The Upjohn Company first sought FDA approval of this product in 1967. After a long and rather tortured regulatory history (NRC/IOM 1990), the FDA rejected the application, largely on the ground that the drug had produced a significant increase in benign and malignant mammary tumors in beagles. Subsequently, however, the results of large-scale epidemiologic studies of actual users of Depo-Provera in developing countries, conducted by the World Health Organization and others, became available and showed that DMPA had at most a weak association with an increased risk of breast cancer, and that such risk, if any, was of the same order of magnitude as that posed by oral contraceptives (Jordan 1992).
As a consequence, the FDA concluded that not only did "women using Depo-Provera run no greater risk for breast cancer (or any other type of cancer) than women taking other approved steroidal contraceptives," but that "at least in the case of [Depo-Provera], the results in the beagle did not accurately predict the effects in humans."2 The agency accordingly eliminated the beagle test from the required toxicology testing for steroidal contraceptives (Jordan 1992) and approved Depo-Provera, on the ground that the "major safety issue of the possible relation between breast cancer and the use of this drug for contraception has been adequately addressed" (Corfman 1992).
With the elimination of the beagle testing requirement, the preclinical requirements for steroidal contraceptives have now been brought generally into line with those that apply to other categories of drugs, although their specifics still differ in certain respects (Jordan 1992). This represents a substantial step forward in reducing regulatory barriers to the development of contraceptive products.
Requirements for Contraceptive Methods Protecting Against Sexually Transmitted Diseases (STDs)
In other areas, however, the FDA continues to exhibit a restrictive approach to contraceptives that tends to delay product availability, sometimes in perplexing ways. On the one hand, the agency has responded to the AIDS emergency with greater flexibility and speed (Fox 1995; Washington Post 1995) and appeared to be disposed to extend that flexibility to those contraceptive methods that also offered protection against STDs. Nevertheless, the female condom, Reality, took 6.5 years to be approved. The producer, Wisconsin Pharmacal, attempted to gather clinical data justifying dual claims of protection against
pregnancy and STDs, but was frustrated in that endeavor by the FDA and the product was ultimately approved only for contraception (J Trussell, personal communication, 1995). The matter of approval of vaginal contraceptives, discussed below, is another puzzling case in point.
Prevention of sexually transmitted infections is an unquestionably critical public health objective, and barrier contraception is on a very short list of ways that objective can be accomplished. As a public health agency, FDA clearly has the right, and the responsibility, to pay attention to this problem, including efforts to foster development of safe and effective barrier contraceptives that can also block STD infection (FDA 1990). But the other, and original, intended use of female barrier devices—to prevent pregnancy—is also an important public health concern. In this respect, FDA's approach to the basic issues of safety and efficacy for contraceptive products at times seems at odds with practical realities. Contraceptives are used by a wide variety of people, in a wide variety of settings, with a wide variety of motivations. Many of those users, particularly women at risk of an unwanted pregnancy, would be happy to have an inexpensive, safe, and convenient contraceptive product available when needed, even were the efficacy of such a product to be less than perfect, provided they are well informed about failure rates.
Again, the female condom serves as an example. In addition to the apparent confusion about STD prevention, the FDA also seemed to apply unrealistically high standards of efficacy to the product in terms of pregnancy prevention. Although the contraceptive efficacy of the female condom was shown to be the same as that for the sponge, cervical cap, and diaphragm (Trussell et al. 1994), the FDA insisted on comparing it with the male condom, despite the fact that a clinical trial of the male condom that conforms to modern standards of research design, execution, and analysis does not exist. Thus, while Reality's effectiveness in this regard was perhaps not as high as that of some alternative contraceptive products, it clearly offered much better protection than nothing. For a woman not using an alternative method, and whose partner was himself unwilling to use a condom, the female condom could well mean the difference between less-than-perfect (but still significant) protection and no protection at all. The question was raised: Why did not FDA simply require disclosure in the labeling of relative rates of efficacy and let women decide for themselves?
Other Issues Around Vaginal Contraceptives
Interestingly, FDA itself expressed an approach somewhat along these lines 15 years ago, in its comments on the report of the Advisory Panel on Over-the Counter Vaginal Contraceptive Products. There the agency said: "FDA is . . . aware that there is a strong consumer interest in knowing the actual percentage effectiveness of each OTC vaginal contraceptive product. . . . FDA concurs with the Panel that the most valuable labeling is that which expresses effectiveness in
terms of the percent of women for whom the product is effective under described conditions of use" (FDA 1980). This statement reflects what might be called a consumer-oriented approach to contraceptive products. The premise is: Give the consumer clear and reliable information about how contraceptive products actually perform (assuming, of course, a reproducible minimum level of safety and efficacy), and let her make her own decision about which product to use under which circumstances, the hypothesis being that greater variety of method choice will increase the likelihood that women will have authentic access to contraception when and as they need it.
However, unlike the changes in long-term toxicology testing, some of FDA's recent actions in the area of contraceptives seem to have been moving in the direction of less flexibility. Of particular note is the issuance by the agency in February 1995 of a notice of proposed rulemaking on vaginal contraceptive products that would require manufacturers of over-the-counter (OTC) vaginal contraceptive products to conduct expensive and time-consuming clinical trials and obtain approved new drug applications (NDAs) in order to continue to market their products (FDA 1995b). This proposal was based on the premise that the effectiveness of this category of products is dependent upon their final formulation. Therefore, FDA's argument goes, the typical OTC review approach of categorizing particular active ingredients as generally recognized as safe and effective, and allowing manufacturers to market those active ingredients in formulations of their choice as long as the inactive ingredients used are safe and suitable, would not work for OTC vaginal contraceptives.
The agency has attempted to mitigate the impact of the regulation by streamlining the data requirements for NDAs for currently marketed products (e.g., waiving the requirement for preclinical data for such applications) and by assuring manufacturers that they would have sufficient time to complete studies and submit data to FDA before the proposal took final effect. However, many OTC products are manufactured or marketed by smaller companies that will not have the resources or expertise to conduct full-scale clinical studies and put together an NDA. As the National Women's Health Network and the Boston Women's Health Book Collective observed in their written comments in mid-1995: "We believe that the proposed rule, which requires every manufacturer of spermicides designed to be used alone to conduct controlled contraceptive effectiveness trials, is over-burdensome, may lead to the withdrawal of some products, and will ultimately leave women with fewer methods of contraception available over-the counter. . . . Our organizations do not object to the requirement that manufacturers conduct clinical studies of vaginal irritation" (National Women's Health Network 1995). The comments add that the contraceptive effectiveness of vaginal spermicides is known well enough to permit women to make reasonably well-informed choices. Were they, on the contrary, new products, both organizations would support both controlled trials of contraceptive effectiveness and vaginal irritation and studies of STD prevention.
While there is disagreement about whether enough is, in fact, known about the contraceptive efficacy of spermicides to allow reasonably well-informed choices, enthusiasm about lengthy and costly controlled effectiveness trials is hard to find. One possible approach might be development of less time-consuming research designs that would build on existing survey, clinical data, and/or current trials with microbicides or, perhaps, a comparative controlled contraceptive trial coordinated by a third party, with each manufacturer contributing a share of the cost (National Women's Health Network 1995). Another approach would take on the issue at a different level and require that the quality and quantity of clinical data on a particular product be reflected more explicitly in the product's labeling.
General Regulatory Developments
The drug development process, as a product of both science and society, evolves over time. In the early 1990s, several factors influenced that evolution: changing attitudes toward risks and benefits, the Prescription Drug User Fee Act of 1992, cooperation among international regulatory authorities, integration of computers into the drug review process, and a shift from the "honor system" to the "trust but verify" system of regulatory surveillance and enforcement (Mathieu 1994).
Changes in Regulation of Combination Products
Thus, recent years have brought a number of broader changes in how FDA reviews products generally; current internal and external pressures for further regulatory reform may produce even greater alterations (Lasagna 1995a). Several of the changes that have already occurred have significant implications for the agency's regulation of contraceptive products. Establishment in 1991 of a specific policy on FDA review of those products that combine elements of two or more of the three major product categories (drugs, biologics, and devices) has cleared away much of the procedural confusion that previously hampered the review of such products. Required by the Safe Medical Devices Act of 1990, FDA's rules on the regulation of these combination products assign jurisdiction within the agency on the basis of a product's "primary mode of action," and establish procedures for determining which component of the agency will handle the review of combination products (FDA 1991a). As part of this initiative, intercenter agreements were concluded between the drugs, biologics, and device centers specifying in more detail how particular products would be handled.
For contraceptive products that combine, for example, a device component with a drug component, the combination product policy reduces the chances of review delays caused by jurisdictional confusion within FDA. The intercenter agreement between the drug center and the devices center lists, for instance, a
"condom, diaphragm, or cervical cap with contraceptive or antimicrobial agent" as an example of a combination product that is to be regulated by the device center under the statutory provisions pertaining to devices, on the grounds that such products have the "primary intended purpose of fulfilling a device function," although they also "have a drug component that is present to augment the safety and/or efficacy of the device" (FDA 1991 a).
Further, the advent in 1992 of user fees and corresponding deadlines for FDA review of new drugs and biologics subject to user fee requirements, combined with FDA's overall efforts to manage the review process more efficiently, offer the prospect of faster review times generally for new contraceptive drugs and biologics. Average review times for new drugs seem to have dropped substantially since user fee requirements came into play, according to analysis carried out under the aegis of the White House task force on reinventing government (RIGO), although the time prior to acceptance of a new filing has increased (Clinton and Gore 1995). Another, more recent analysis, prepared by the U.S. General Accounting Office in response to a bipartisan congressional request, also found that there had been a considerable reduction in approval times for new drugs during 1987-1992 (Barnett 1995) and that the FDA is actually faster in approving drugs than its European counterparts (Schwartz 1995). The FDA's recently issued Annual Report to Congress showed that, in FY1994, the agency approved 93 percent of all drugs within a year of the companies' applications, surpassing the 55 percent benchmark mandated by the Prescription Drug User Fee Act (Associated Press 1995). The numbers may be debated by congressional and industry critics, yet there appears to be no industrial enthusiasm for diluting the advantages that the FDA approval stamp currently gives to products that have it, including some degree of legal protection and consumer confidence in their safety (Washington Post 1995).
Prospective Regulatory Changes
A number of changes being contemplated or implemented as part of current FDA reform initiatives could be of specific benefit to the development of contraceptive products. For instance, the reduction or elimination of requirements for FDA review of postapproval changes in manufacturing facilities or processes for drugs and biologics (BIO 1995; Clinton and Gore 1995), a concept that has already been implemented to some degree for biologics (FDA 1995b), is intended to reduce the regulatory burden on manufacturers of other products affected by those requirements, a category which includes contraceptives. The same is true for the proposed elimination of the costly and often irrelevant requirement for inclusion of an environmental assessment in most new drug applications (NDAs).3 The biotechnology industry will also benefit from the recent issuance of proposed new rules that effectively allows FDA to treat well-characterized biotechnology products (e.g., monoclonal antibodies) like other drugs. The expectation is that
this particular piece of reform will save biotechnology companies hundreds of millions of dollars and hasten passage of new biotechnology products through regulatory approval processes (Schwartz 1995).
Inclusion of Women in Clinical Trials
In July 1993, the FDA released a new Guideline for the Study and Evaluation of Gender Differences in the Clinical Evaluation of Drugs, which modifies and revises the section of its 1977 guidelines that recommended exclusion of women from Phase I and early Phase II of drug development. The new guidelines provide for the inclusion of women in those phases and state that the broad principles for that inclusion will also be applied to FDA approval processes for biological products and medical devices (IOM 1994a).
The exclusion of women from trials of contraceptives, products developed explicitly for women, is patently not an issue. What is an issue is the potential for interactions of new contraceptives with other drugs for which data on adverse effects and contraindications in women are lacking because women were excluded from the original trials. The importance of the pharmacokinetics of drugs is addressed in the 1988 FDA Guidelines for the Format and Content of the Clinical and Statistical Sections of New Drug Applications, which requires that consideration during drug development be given to effects on pharmacokinetics of estrogen replacement therapy and systemic contraceptives (IOM 1994a).
As women of reproductive age come to be increasingly included in the early phases of drug trials as a consequence of these new guidelines, there will be another issue, that is, the possibility of their becoming pregnant while they are participants in trials. This means that informed consent protocols must include information about contraception and the alternatives of voluntarily withdrawing from the study and terminating a pregnancy should conception take place (IOM 1994b). If the participant opts for contraception, this raises the issue of pharmacokinetics referred to above. Snow and Hall (1994) point to an apparent regional variability in pharmacologic response to contraceptives, specifically steroids. They also point to the limited and highly speculative state of the science in this regard, a situation that derives from study designs in the early phases of human trials. The WHO-recommended approach is that pharmacokinetic studies of contraceptive steroids be conducted separately from studies of the efficacy and side effects of those same steroids, in order to control for confounding with the research design. This precludes investigation of the relationships between pharmacokinetic variability among women and their experience of contraceptive side effects (Snow and Hall 1994).
Legal Influences on Contraceptive Research and Development
Manufacturers' Concerns
Beyond the extensive regulatory requirements a potential contraceptive developer must contemplate when making product development decisions, there is the legal environment to consider. A pharmaceutical company generally expects to have some liability expenditure after marketing almost any product and will factor that anticipated expenditure into development calculations; in fact, companies may set aside a "kitty" for that purpose (IOM 1995a). As extensive as the regulatory process may be and as exhaustive in its consideration of possible unwanted effects on different categories of users, no clinical trial, no matter how well designed, can anticipate every single contingency when a product is marketed to what may be millions of consumers. Human physiology and individual medical and personal histories are simply too diverse and unpredictable, as are other contextual variables that cannot be incorporated into clinical trials. The law requires manufacturers to warn not only about known risks but about foreseeable risks that should have been known had the manufacturer applied "reasonable, developed human skills and foresight." While risks designated as "unexpected and unknown" will not trigger strict liability, sellers still are deemed to be experts and are imputed to have all "knowledge of the product's risks based on reliable and obtainable information" (Flannery and Greenberg 1994). Thus, development decision-making incorporates the assumption that a product could cause harm to some of its future users, some estimation of the magnitude of those problems, and prediction of the likelihood that a liability suit will result. Woven into these imponderables are concerns about the following:
- causing injury, whether from known or unknown side effects;
- loss of public confidence because of adverse publicity from lawsuits, whether lost or won;
- the costs ensuing from any legal action, even when the plaintiffs case is weak and liability is uncertain, including such indirect costs as work disruption, and the direct costs of litigation and settlement;
- delayed liability exposure from side effects that may not manifest for 5, 10, or 20 years;
- the potential for mass litigation, that is, class action lawsuits, particularly those that ensue from active recruitment of plaintiffs, some injured by the product, many not, and all of whom are "classed" together;
- government investigations by the FDA or by U.S. or state's attorneys after a lawsuit (Flannery 1995).
There are other issues. First, with most pharmaceuticals, a U.S. firm is likely
to be marketing its product on a national scale, so that unpredictability almost inevitably derives from the variability among the individual product liability rules of as many as all 50 states, the District of Columbia, and two territories (Foote 1988; IOM 1990).
Second, there is the uncertainty introduced by having highly technical and complex scientific issues evaluated by a judge and jury untrained in the sciences (IOM 1994b).4 This relative unfamiliarity may amplify the weight of personal feelings and opinions in final rulings. An ancillary consideration is that there seems to be a tendency for juries to be more sympathetic to injured plaintiffs than to large corporations.
Third, there is the manufacturer's inability to rely on FDA approval as protection against liability. As rigorous as FDA approval processes may be, most courts have held that obtaining FDA approval of a drug5 does not provide a manufacturer with an absolute shield from state tort liability, although evidence of compliance with FDA warning regulations may be introduced as evidence of the adequacy of such warnings (Flannery and Greenberg 1994). This is addressed later in this chapter in the context of a government standards defense.
Fourth is the cost and unavailability of comprehensive general liability insurance, which includes product liability. This grew to be a big issue for contraceptive manufacturers and entities engaged in research in the 1980s. The reasons were both general—the size and number of liability awards soared in those years—and specific to contraceptives because they are used by so many women over long periods of time and because their risks may be latent for years and include risks to offspring (NRC/IOM 1990). Bigger corporations can self-insure or find other ways to secure the necessary insurance, but smaller companies and independent and nonprofit organizations do not have comparable avenues; for them, insurance is a more sizable impediment.
Finally there is the political climate. All aspects of liability pertain in a climate that is, for contraceptives, especially volatile and ideologically fraught because they are tethered to profound beliefs about abortion, the mechanisms of contraceptives, and conservative perspectives about family planning as a general matter. All this, in turn, tends to inflate the liability issue in virtually every dimension.
Product Liability Rules
Tort law encompasses civil wrongs where one person's conduct causes injury to another in violation of a duty imposed by law (Foote 1988). In the context of product liability, tort law encompasses both negligence, which is based on fault, and strict liability, which is based on no-fault principles (Prosser and Keeton 1984).
The objectives of product liability rules are to compensate individuals injured by unreasonably dangerous products, to deter the marketing of dangerous
or defective products, and to resolve disputes between those injured and manufacturers (Smith 1987). Since tort law is the province of the states, these rules generally are created through common law, not statutory law; that is, they are rules made for the most part by judges, not laws enacted by Congress or state legislatures. There is no uniform federal product liability law that would preempt those state rules (IOM 1994a; NRC/IOM 1990).
Liability pertains at two major junctures in the project development trajectory from laboratory to market. The first is during research, that is, during clinical studies6 when a product is applied to or ingested by a living human being. The second is the liability of a manufacturer (and possibly a provider) after the finished pharmaceutical product is launched on the market and public consumption begins. While legal action in these areas is in some respects based on the same theories, there are significant differences between them.
There are five legal theories under which a product liability case can fall: (1) express warranty, (2) implied warranty (both generally superseded by commercial codes in each state), (3) fraudulent misrepresentation (not commonly applied), (4) negligence, and (5) strict liability (see Table 7-1). It is the last two areas that are pertinent to both product liability and research liability.
An additional theory underlies research liability: the theory of battery, which is defined as unlawful and intentional bodily contact directed at another person without that person's consent. In research liability cases, battery has to do with use of an individual as a research subject without his or her knowledge or consent and there may be a punitive element in damage awards (IOM 1994a). Battery does not apply in product liability cases.
Negligence
Negligence in Product Liability Negligence is deviation from acceptable standards of conduct or standards of care. It entails breach of a legal duty owed by the defendant to the plaintiff and injury consequent to that breach. To recover damages for negligence, causation must be proved. An area of dissensus in negligence theory is whether a defendant is liable for injuries to offspring that occur as a result of any injury to the woman before the child's conception. In most negligence cases, only compensatory damages are awarded, except for cases of gross negligence, wherein some states allow a punitive element. Third parties may also recover money damages for injury to a research participant caused by negligence (Reisman 1992).
In product liability, a manufacturer can breach the "duty of care" in three ways: (1) by adopting a design for the product that causes it to be unreasonably dangerous; (2) making mistakes or omissions in the manufacturing process that result in a properly designed product becoming unreasonably dangerous; (3) or by failing to provide adequate warnings about the product's hazards and instructions concerning its use. In most jurisdictions, the manufacturer must warn only
TABLE 7-1 Five Theories of Liability
|
Theory |
Explanation |
Usage |
|
Express Warranty |
A written or oral affirmation of fact or promise made by the seller of the product to the buyer about the condition, efficacy, or safety of the product. |
(1) Whether the seller actually made a statement of fact to the buyer about the productexpressions of opinions are not supportive; (2) the meaning or interpretation of said statements; (3) whether the statement was true or false; and (4) whether the product caused the plaintiff harm. |
|
Implied Warranty |
Representation by the seller that is implied in a contract for the sale of the product that the product is "merchantable"—"fit for the ordinary purposes for which such goods are used." |
The seller must be a ''merchant" and, in most states, manufacturers have been held to be so. Because sales are contracts, this presents a broad avenue for recovery against sellers of defective goods, but recovery is generally governed by the relevant provisions of the Uniform Commercial Code as adopted by each state. |
|
Fraudulent Misrepresentation |
Similar to warranty theory but the plaintiff must also prove fraud and deceit. |
Plaintiffs must prove (1) that the defendant made a false representation about the product, (2) that the defendant knew the representation was false, (3) that the defendant intended to induce the plaintiff to act or refrain from acting on the basis of the misrepresentation, (4) that the plaintiff justifiably relied on the representation, and (5) that the plaintiff was injured thereby. [Product liability cases are not usually based on this theory owing to the common requirement of "clear and convincing" evidence.] |
|
Theory |
Explanation |
Usage |
|
Negligence |
Plaintiffs must show that: the defendant-manufacturer owed the plaintiff a "duty of care"; the defendant breached this duty of care; the plaintiff was injured; and the defendant's lack of care was the proximate cause of the injury. A manufacturer owes a duty of care to avoid an unreasonable risk of harm to the user of a product. |
A manufacturer can be said to have breached this duty of care in three broad respects: (1) by adopting a design for the product that causes it to be unreasonably dangerous; (2) by making mistakes or omissions in the manufacturing process that result in a properly designed product becoming unreasonably dangerous; (3) or by failing to provide adequate warnings about the product's hazards and instructions concerning its use. As a general matter, only the medical provider-the "learned intermediary"-not the patient, must be directly warned. |
|
Strict Liability |
Relatively recent; plaintiff may recover without demonstrating negligence. |
Plaintiff must demonstrate (1) design defect, including defective testing; (2) manufacturing defect; or (3) failure to warn. |
|
Source: National Research Council and Institute of Medicine. Developing New Contraceptives: Obstacles and Opportunities. L Mastroianni, PJ Donaldson, TT Kane, eds. Washington, DC: National Academy Press. 1990. |
||
the medical profession, not the patient—the "learned intermediary" rule—and the adequacy of the warning is frequently in contention (Harper et al. 1986).
Negligence in the Context of Research. In research liability, legal actions for injury based on a negligence theory often involve the doctrine of "informed consent." There is a difference between the nature of consent needed to avoid a legal action for battery, which is a form of assent to a bodily intervention that is sometimes termed "simple consent," and what is needed to avoid an action for negligence, which requires "informed consent.'' The latter is defined as consent based on the disclosure of all facts, including the risks and benefits of the proposed intervention, as well as alternatives and their risks and benefits, that are necessary to form the basis of willing, uncoerced, intelligent consent by the
patient to the procedure (Jonsen et al. 1982; Reisman 1992; Wadlington 1984). Legal action will be based on whether the information given to the participant before securing consent sufficiently warned of potential risks and whether the degree of disclosure was reasonable given the circumstances. Decisions in this area depend heavily on definitions of "adequacy" (Wadlington 1984). The statute of limitations that limits the number of years during which a legal action can be initiated is usually longer for a negligence action (IOM 1994a).
Strict Liability
Strict liability is fairly new in tort law, but this less-than-definitive theory leaves much room for interpretation. While a plaintiff must demonstrate more than simply some injury consequent to use, under this theory, use by a manufacturer of all possible care in design and manufacture does not absolve it from liability. Under strict liability, then, a person injured by a product can recover damages without having to show that the manufacturer was negligent.
The Restatement of Torts (Second), a compendium of the views of leading legal scholars, recommends that the principle of strict liability not apply to products that present generic risks, termed "unavoidably unsafe" products, and cites vaccines and drugs as examples (Foote 1988). A manufacturer may be exempted from this general rule under the comment k exemption of the Restatement, Section 402A, which states that a drug is not "unreasonably dangerous" and the manufacturer is not subject to strict liability, as long as the drug is "properly prepared and marketed and a proper warning is given" (American Law Institute 1977). Comment k specifically refers to experimental drugs, "as to which, because of lack of time and opportunity for sufficient medical experience, there can be no assurance of safety" (Flannery and Greenberg 1994). However, though a manufacturer may keep a product from being considered "unreasonably dangerous'' by giving appropriate warnings, the adequacy of these warnings is very often the legal issue brought into contention. Furthermore, while some state courts have consistently upheld this exception to strict liability, others have not (Foote 1988).
Manufacturers can generally satisfy their duty to warn for prescription and investigational drugs by informing physicians and investigators of any risks of harm. It then becomes the responsibility of the physician—the "learned intermediary"—to prescribe drugs only for appropriate indications and for monitoring their use (IOM 1994a). For prescription contraceptives, however, courts have reached differing conclusions as to whether manufacturers have a duty to warn consumers directly (Flannery and Greenberg 1994).
Liability for Injuries to Offspring
The greatest concern about liability is the possibility of injury to offspring
when women of childbearing potential are included in clinical drug trials. Both researchers and manufacturers must not only be concerned with the health and well-being of the woman (or man) using its product—in our context a contraceptive product—but also with the future health of a child who might be born to that contraceptive user. These concerns reflect the experience with diethylstilbestrol (DES) and thalidomide and the ensuing realization that, as complex as proof of causation might be, the harm that might be alleged to result from in utero exposure to a drug is great. Other considerations are the greater length of the statute of limitations for cases of injury to children and the fact that a parent cannot waive a child's rights to sue (IOM 1994a). In fact, claims on behalf of a child of a contraceptive user can be filed against a manufacturer as many as 20 years after the child's birth, even when the birth itself may have occurred several years after the initial purchase of the product; this is because children's claims are typically tolled, which means that the statute of limitations does not begin to run during their minority (Clayton 1994). A highly specific "anticipatory release" signed by the parent may relieve the manufacturer of liability but, since this could generate future conflict between parent and child, it is typically disallowed (Clayton 1994).
Potential plaintiffs in a legal action for injuries to offspring include the living child and the parents. The most important questions are whether the child was born alive and what the parents would have done had they known about the risks: Would they have avoided the drug or put off procreation (Clayton 1994)? In the case of research, when the plaintiff is the child, the success of a legal action depends in part on the ability of the parents to show that they would not have participated in the research had they known about the risks (IOM 1994a). A child may bring an action for wrongful life when the parents claim that they would not have had the child if they had been informed of the risks; such actions have rarely been recognized by the courts. Parents who have a live but injured child might bring a wrongful birth action if they show that they would have avoided childbearing had they known of the risks (Clayton 1994). If the child is stillborn as a result of a research injury, some states allow the parents to bring an action for wrongful death. In some of those states, a wrongful death action for a stillborn child is allowed only if the child was deemed to have been a viable fetus at the time the injury occurred; in other states, a wrongful death action is allowed regardless of the fetus's initial viability.
Current Proposals for Tort Reform
If a manufacturer loses a suit, there are three kinds of damages that can be awarded to a claimant:
- Compensatory damages, which encompass economic damages for whatever economic injury may have occurred, for example, medical expenses, loss of income, or estimates of future judicial expenses; as well as
-
- Noneconomic damages, awarded to compensate for emotional distress, pain, and suffering; and
- Punitive damages7 intended to punish and deter wrongdoing.
A punitive dimension in liability awards, once relatively rare, has become increasingly present in many tort judgments for damages (Flannery 1995; Peterson 1987). In response to that fact and to the growing numbers of cases and individuals entering into product liability litigation—and, of compelling interest, explosion in the dollar magnitudes involved—pressure for tort reform has mounted. Recognition of the need for a set of standards governing product liability on a federal level is, nevertheless, not new. Since at least the 101st Congress, members of both houses have made attempts to address the issue and some state legislatures have, for the first time, enacted legislation that, in some circumstances, limits damages awards against defendant manufacturers.8
The National Vaccine Injury Compensation Program (NVICP)
Beyond efforts at the state level, very specific federal reform legislation (H.R. 5184, 99th Cong. 2d sess., 1986) established the National Vaccine Injury Compensation Program (NVICP), essentially no-fault insurance against possible injuries from the seven pediatric vaccines children were mandated to receive in order to attend school in the United States. Beginning in the mid-1970s, a steady increase in numbers of vaccine-related lawsuits against manufacturers, as well as in the sizes of awards to plaintiffs, led to withdrawal of many companies from vaccine manufacturing and marketing (IOM 1985 and 1993). The NVICP was an attempt to compensate families of children adversely affected by government-mandated vaccines and to shore up the vaccine industry by eliminating liability risk through imposition of a vaccine excise tax. The trust fund into which the taxes were paid had a balance of about $620 million at the beginning of 1993, when the tax was lifted. In 1993, an IOM committee concluded that impact of the program was not yet clear (IOM 1993). Despite an apparent decrease in vaccine-related lawsuits and an increase in vaccine-related R&D, no company that had dropped out of vaccine manufacturing in the United States in the 1970s and 1980s had returned as of mid-1993. Nonetheless, foreign companies, many of which had traditionally shied away from the U.S. vaccine market, were readying themselves to enter it, either by applying for FDA licenses for their products or by entering into alliances with other companies and entities holding U.S. product licenses (IOM 1993). A very recent analysis of the effect of the NVICP indicates that industry now, 10 years later, views the program as "working" (Day 1996).
The program is regarded by some analysts as an inappropriate model for replication in other areas of health because of its loose handling of causation, among other matters (Wadlington, personal communication, 1995). However, it is not the only model: Analysis in the mid-1980s found that Denmark, Germany,
France, Japan, Switzerland, and the United Kingdom all had compensation programs for injuries whose reasonable cause was a vaccine mandated or recommended by law (IOM 1985). Replication in the area of contraception would encounter at least one contextual obstacle, that is, the difference in public perceptions of vaccines and contraceptives (Djerassi 1989). Vaccines are generally perceived as a public good that is also protective of the individual; the pediatric vaccines are seen as particularly protective of children who are in themselves generally perceived as a good thing. There is little or no perception of contraceptives as addressing a present or putative public health problem; thus, unlike vaccines, contraceptives are not seen as a public good.
Current Legislation
One outcome of this mounting concern is that two pieces of legislation, H.R.956 and S.565, passed the House and the Senate of the U.S. Congress in March and May 1995, respectively, and await reconciliation in conference. A third bill (H.R. 10), included in the Republican Party's "Contract with America," contains many of the same legislative elements. The three bills are compared in Table 7-2.
It is perilous to take up the topic of legislation that is still in the making, since much of its content may change and, of course, there may be none.9 After all, product liability legislation has been vigorously debated since it was first proposed in the early 1980s. Nonetheless, there are generic components whose inclusion in any final legislation could contribute significantly to enhancing the environment for improving existing contraceptives and developing new ones, in ways that will better respond to current national and international public health needs and demands. The most pertinent are the following:
A Government Standards Defense. This aspect of reform would standardize what some states have already adopted, that is, exclusion of the punitive element in awards for damages, assuming that there had been FDA review and approvals at appropriate junctures. The defense would not be available if the manufacturer withheld relevant information from the FDA (NRC/IOM 1990), nor would it prevent plaintiffs from receiving compensatory damages.
Punitive Limits. Another aspect of reform would be the imposition of limits on the size of punitive elements in awards and/or how those are awarded.
Limits on the Liability of Biomaterials Suppliers. Another element in tort reform would be limits on the liability of biomaterials suppliers.
Others. Other reform elements include caps on compensatory damages; modifications of statutes of repose and statutes of limitations; and issues related to pleadings found to be frivolous, including "loser-pays" provisions.
TABLE 7-2 Comparison of Pending Tort Reform Legislation, November 1995, United States
|
Area of Reform |
H.R.956 |
S.565 |
H.R. 10 |
|
Punitive Damages |
Impose a $250,000 cap on punitive damages, or limit them to three times any economic losses. The bill would apply to any civil litigation, not just product disputes. |
Cap at $250,000 or three times economic losses, which can include lost wages or medical expenses, whichever is greater. The bill would apply only to product liability cases. |
Same cap would apply only to product liability actions and would require a specific showing of intentional malice for such awards. |
|
Regulatory Approval Defense (Government Standards Defense) |
Bar punitive damages in cases in which a medical device or drug had won approval from the FDA before the product was sold. |
Provide no special exemptions from punitive damages against manufacturers of products that had won approval from the FDA. |
Same as H.R.956. |
|
Time Limits |
Retain current law |
Allow a plaintiff to bring a lawsuit up to two years after discovering both the cause of an injury and the injury itself. Under existing statutes of limitations, the clock begins to run as soon as a product causes an injury, even if the victim cannot detect it or cannot discern its source. |
Same as H.R.956. |
|
Area of Reform |
H.R.956 |
S.565 |
H.R.10 |
|
Frivolous Lawsuits |
Expose plaintiffs found to have filed a frivolous lawsuit to the potential burden of having to pay the other side's legal fees and other sanctions. |
Retain current law. |
Same as H.R.956. |
|
Statute of Repose |
Limit the time for filing product liability cases for most products to 15 years after delivery. |
Limit the time for filing a product liability suit to 20 years after a product is delivered. This limit would apply to durable goods such as machinery used in the work place, but not to toxic materials, nor to commercial trains or airplanes. States would be allowed to keep in place any law providing a shorter limit. The measure would retain the right of plaintiffs to file suits when the manufacturer issued a specific warranty guaranteeing the product beyond the 20-year term limit. |
No time limit provision. |
|
Source: HJ Hyde. Products Liability Fairness Act of 1995; Biomaterials Access Assurance Act of 1995 (H.R. 956) [Common Sense Products Liability and Legal Reform Act of 1995.] Legi-Slate Report for the 104th Congress, Quick Bill, 7 August 1995. |
|||
Government Standards Defense
The 1990 NRC/IOM report on contraceptive development recommended that the U.S. Congress enact a federal product liability statute that would give contraceptive manufacturers credit for approval of contraceptive drugs and devices (and their labeling) by the FDA. The gist of such an "FDA Defense"—sometimes termed "Government Standards Defense" or "Regulatory Defense"—is as follows: If it is established that an injury-causing aspect of a contraceptive drug or device was in compliance with all applicable requirements of U.S. federal food and drug law at the time that drug or device was made or sold, then a manufacturer or seller of a contraceptive drug or device would not be liable under any of the relevant legal theories (misrepresentation, warranty, negligence, or strict liability) for any injury related to design, nor liable for failure to provide adequate warning or instruction regarding any danger associated with its use, nor liable if the FDA had not asserted that the contraceptive drug or device was not in compliance.
However, the defense would not be available if a claimant were able to establish that the manufacturer should have made design modifications or given different or additional warnings or instructions; if the manufacturer or seller knew or should have known of studies showing increased risk of harm from the contraceptive drug or device that indicated increased likelihood of serious injury; or if the claimant established that the FDA was not informed of dangers regarding the contraceptive drug or device that were known to the manufacturer or seller, and that the injury to the claimant or persons sharing the claimant's medical characteristics was attributable to such dangers (NRC/IOM 1990). The arguments for the FDA defense are that punitive elements of damage awards are meant to punish egregious behavior, not to punish companies that have complied with the strict rules of the regulatory system and could not have predicted the side effects alleged to have caused the injury.
Counterarguments are that the proposed legislation would protect irresponsible corporations at the expense of people victimized by faulty products (Mayer 1995; Schrage 1995). In general, manufacturers of all types of products have sought to limit their liability exposure and consumer groups have generally opposed these efforts. The pivotal issue appears to be that proposals for some kind of government standards defense have generally overlooked the widely held public belief that people are entitled to compensation for harm, particularly harm caused by negligence (Foote 1988). Other countries (e.g., New Zealand, Canada) have addressed comparable concerns through comprehensive accident compensation plans, of which the National Vaccine Injuries Compensation Program (NVICP) in the United States is an example. An indicator of the tensions over the concept of a government standards defense is the fact that, although such a provision appears in the bill passed by the House of Representatives, it is not included in the Senate-passed bill owing to fears that opposition to the provision
would kill the possibility of passage. Nonetheless, it is very important to note that the argument for some kind of government standards defense does not do away with liability. FDA compliance is, indeed, a shield, but noncompliance makes that shield useless.
Arguments raised against a government standards defense often mention the histories of diethylstilbestrol (DES)10 and the Dalkon Shield as illustrative of the risks to consumers inherent in such a defense. In these cases, however, the primary lesson may have less to do with the fact of corporate irresponsibility than it does with the perils of nonregulation. In the case of DES, it was not until 1962, with the amendments to the 1938 Food, Drug, and Cosmetic Act, that the FDA's jurisdiction was expanded and its character shifted from being a premarket notification system to being a premarket approval system (Merrill 1994). The Dalkon Shield came on the market in the late 1960s, when FDA approval was not yet required for devices. The device proved to carry large risks to the user and approval would not be likely today.
The Case of the Dalkon Shield The costs of the Dalkon Shield were high in several respects. After paying $250 million to settle approximately 4,400 suits, and after juries in 11 cases had awarded $24.8 million in punitive damages against it, the manufacturer, AH Robins, declared bankruptcy and was ordered to set up a trust fund to compensate those injured by its product (NRC/IOM 1990). As of October 1995, the Dalkon Shield trust had paid $1.42 billion to 185,000 women in the United States and 110 other countries, with another $800 million to be distributed by the end of the year and $654 million and 6,000 claimants remaining (Steyer 1995).
Another cost was that other manufacturers, notably G.D. Searle, a Monsanto Company subsidiary, whose Copper-7 and Tatum-T IUDs had been tested before receiving FDA approval and were never found defective, pulled those products off the market in 1986. Nonetheless, the company got some of the backwash from the general legal turbulence and has had to defend against 2,063 suits. At the end of 1995, over 20 years after Searle first started selling its intrauterine devices, 40 suits and 346 foreign complaints remained. Information from the company indicates that, unlike Robins, the costs of litigation did not have significant impact on the company or its parent. Of the total suits filed, 24 went to trial, of which Searle won 19 and lost five. Of the five losses, four cost a total of $689,300. The fifth, Kociemba v. Searle, considered somewhat aberrant, was an award of $8.75 million by a jury critical of Searle's testing and marketing of the IUD; of that total, $1 million was for emotional distress, $750,000 for pain and disability, and $7 million for punitive damages, the only award in any of the Searle trials that had a punitive element (Steyer 1995).
Searle was not alone in bearing costs from the Dalkon Shield experience. The costs to women were also high, surely to those who were injured and, to an
unknowable degree, to those women who lost contraceptive options they would have had if the safe IUDs had not disappeared from the market.
Limits on Punitive Elements in Awards of Damages
Another area of proposed reform targets the excesses of punitive elements in damage awards. There appears to be no disposition toward total forgiveness of punitive damages; the objective is to standardize a national cap on punitive amounts. In general, it is proposed that there be an absolute cap of around $250,000 or some multiple or sum of compensatory damages. The argument is that this reform would allow manufacturers to estimate the potential costs of suits resulting from a new product and remove the uncertainty created by the disparate tort rules among the states. Some of the proposed reforms also include an "escape hatch," which provides the judge hearing the case the discretion to disallow the limits if the defendant's behavior is found to have been flagrantly negligent, in which instance the potential size of the punitive award will once again become essentially limitless. Punitive elements in awards would be predicated on clear and convincing proof of conscious, flagrant indifference to safety; compensatory damages would not be affected. Another option is the filing of criminal proceedings against those individuals responsible for the corporate behavior resulting in injury; this would punish the guilty rather than those who have no direct control over management decisions, that is, shareholders, employees, or the public. Yet another possible variation would be articulation of a set of explicit standards for behavior warranting punitive damage awards that would provide clearer guidance for judges and juries; those who propose this variation note that punitive damages thereby become more predictable in some respects and more precisely targeted on the kinds of behavior that most merit strong deterrence (Garber 1993).
The fundamental argument in favor of limits is that the states' patchwork of laws and unpredictable, arbitrary, and excessive punitive damage awards raise corporate costs; discourage research, development, and innovation; hurt the competitive position of U.S. companies in an increasingly international marketplace; and expose companies to risks that are disproportionate (Mayer 1995; Spayd 1995). One specific consumer perspective in support of limits is that threats of liability may motivate companies to take off the market drugs that are of identified toxicity but still needed by some subpopulation(s) willing to trade off the risks, because the costs of not having the medication available are so high (Mayer 1995).
Arguments against limits are that, first of all, they would encourage the marketing of unsafe products that could be more profitable, since predictability would permit industry to price them with greater assurance. Secondly, limits would dilute the primary deterrent value of punitive elements, that is, the unpredictability and arbitrariness that industry finds so unsettling. A more ge-
neric argument is that, as a general matter, egregious corporate conduct should be harshly punished without limits.
Limited Liability of Biomaterials Suppliers
Currently, suppliers of raw materials are held liable for damage awards even though they have no direct role in the use of their material as a biomaterial. In March 1993, as a result of many and costly claims related to its silicone breast implants,11 Dow Corning stopped supplying silicone for permanent medical implants and reproductive, contraceptive, obstetric, and cosmetic applications. When DuPont was caught up in suits against Vitek, which made temporomandibular jaw (TMJ) implants using DuPont's Teflon as a component, the company discontinued supply of materials including Teflon, Dacron, and Delrin to the medical implant industry, and it appears that the supply of polyethylene, used for the bearing surface of all total joint replacements, will be exhausted by the end of the year as well (Greenberger 1995). Though the precipitating factors, as well as the corporate responsibility, are different in each case, the net effect is the same: the departure of these and other companies from this industrial area and a loss of materials badly needed for medical purposes. Particularly affected are small producers of finished implants who cannot guarantee the stringent indemnity insurance and other requirements for testing and consumer education that raw materials suppliers are now demanding (Baker 1995). Manufacturers of contraceptive devices that use silicone-based or analogous biomaterials, for example cervical caps and implants, will soon need to find other sources or re-engineer their products. It may be that offshore producers may be a source but, so far, non U.S. producers seem reluctant to enter the U.S. market because of liability and insurance concerns.
The critical distinguishing factors in determining bulk supplier liability are whether the component was defective when it left the seller's control, whether the seller adequately warned of known or knowable risks associated with the buyer's intended use of the component, and whether the component was transformed into an entirely new and different product (Baker 1995). The Biomaterials Access Assurance Act of 1995 being considered in the U.S. Congress would expressly preempt all tort claims brought against biomaterials suppliers in state or federal courts. As currently written, the Act specifies that a biomaterials supplier is liable for harm to the consumer of the finished product only if the product supplied did not meet contract specifications; it also specifies that the supplier may not also be the device manufacturer. The buyer must also be producing implants approved by, and registered with, the FDA, pursuant to the Federal Food, Drug, and Cosmetic Act, an oblique reflection of the relevance of government standards to defense against liability. Whether this sort of statutory protection will entice bulk suppliers back into the medical device market cannot be predicted. The inevitable restructuring of this market will be driven by two forces: Large multi-
national corporations, with their deep pockets, will leave the field to smaller producers of biomaterials; at the same time, the probability that indemnification agreements will become standard in all supply contracts to the medical implant industry may drive out the smaller producers of medical implants who cannot afford to honor those agreements, some of whom would then be absorbed by larger companies (Baker 1995; Hyde 1995).
Liability Protection During Research
Another proposal, under internal consideration at the National Institutes of Health, contemplates a statutory product liability exemption for institutions, investigators, and manufacturers from claims for non-negligent injury occurring in PHS-funded research involving (1) human subjects and (2) use of an Investigational New Drug or Device (IND or IDE) approved by the FDA for testing (NIH 1995).
At present, with a few exceptions for small subpopulations, the only recourse for a human subject injured in a clinical trial is through the courts. Correspondingly, research institutions, investigators, and manufacturers who are not federal employees but are involved in PHS-funded research can be sued for any injury that may befall a subject of the research. This is so even though the drug or device and its use in the research has been made as safe as the current state of scientific knowledge is able to make it. In other words, it has been cleared by the FDA for human testing, the research protocol has passed scientific peer review and human subjects protection review by an Institutional Review Board (IRB); and the subject has given informed consent acknowledging the possibility of unknown risks at this stage of investigation. To protect themselves, manufacturers and institutions must purchase product liability insurance, often prohibitively expensive or simply unavailable. This constrains research and the marketing of new drugs and devices and has already affected development of new means of fertility regulation and the testing of new vaccines against infectious diseases. The constraint is especially severe for small businesses who, for the same reason, have been unable to proceed with clinical testing of devices developed in the Small Business Innovation Research (SBIR) program (NIH 1995).
The NIH is concerned, nonetheless, that a statutory exemption from product liability suits would be prejudicial for the rare injured subject who would, then, have no recourse whatsoever. This would be contrary to Guideline 13 of the International Ethical Guidelines for Biomedical Research Involving Human Subjects, which states that:
Research subjects who suffer physical injury as a result of their participation are entitled to such financial or other assistance as would compensate them equitably for any temporary or permanent impairment or disability. In the case of death, their dependents are entitled to material compensation. The rights to
compensation may not be waived (Council for International Organizations of Medical Sciences [CIOMS] 1993).
Thus NIH recommends a companion legislative provision that would broaden the definition of ''federal employee" for purposes of eligibility for benefits and compensation distributed under the Federal Employees' Compensation Act (FECA), an action for which there is precedent (NIH 1995). A similar proposal was offered in the form of a recommendation by the IOM committee that reviewed the Fialuridine (FIAU) Clinical Trials (IOM 1995a). The recommendation was that there be a system of no-fault compensation for research injury by government, sponsors, or some combination of both. In effect, this is another application of the Government Standards Defense, but during the research period rather than the postmarketing period. The NIH proposition would not require a waiver of rights to compensation, but rather would simply remove the risk of potentially excessive punitive awards from manufacturers or researcher(s) involved in clinical trials funded by the NIH.
Legal Cases Related to Contraceptive Development
The 1990 NRC/IOM contraceptive technology report, in its summary of trends in litigation involving contraceptives, noted that, while every single contraceptive method then available had been the subject of product liability litigation, the history of each was diversely patterned. Litigation around the intrauterine devices has been characterized by extremes—massive liability for one product, but only limited recovery by claimants in cases involving other IUDs. In the case of oral contraceptives, litigation has been characterized by its long-running nature, an almost exclusive focus on warnings given by manufacturers about side effects, and only uneven success with defense premised on the learned intermediary rule. Most relevant to consideration of a government standards defense is that most courts hearing cases concerning oral contraceptives have held that compliance with FDA regulations does not, of itself, necessarily constitute adequate warning; FDA compliance is only considered along with all other evidence of whether, in the circumstances, the warning was adequate (MacDonald v. Ortho Pharmaceutical; McEwen v. Ortho Pharmaceutical). Finally, three other cases involving a diaphragm, a spermicide, and a condom were so variable in adherence to the rules of evidence that they must be considered anecdotal, if not aberrant (NRC/IOM 1990).
The Case of Norplant
The most substantial contemporary case of litigation involving a contraceptive is the current experience of Wyeth-Ayerst, the distributor of the silicone-coated levonorgestrel-containing implant, Norplant. With 2.3 million users else-
where in the world, a cumulative five-year failure rate of only 3.7 percent,12 virtually no reports of major problems, and 27,000 physicians trained by WyethAyerst in insertion techniques, Norplant looked like a safe, effective, long-term yet easily reversible approach to family planning (White 1995). Indeed, when it was introduced into the United States in February 1991, sales moved briskly and soon 1 million U.S. women had adopted the method. Norplant's sales in its first full year—its best—amounted to $141 million and ran at about 800 units a day (Kolata 1995).
However, American Home Products Corporation or Wyeth-Ayerst have now been served with over 500 lawsuits alleging injury as a result of the insertion, use, or removal of the Norplant System, with approximately 12,800 plaintiffs named in those suits. From 85-90 percent of the plaintiffs are located in Texas, where one lawyer alone accounts for over 5,300 plaintiffs. One-third of the suits are in state courts, principally in Texas, Illinois, and Indiana. Two-thirds of the suits were pending in federal courts and were consolidated for pretrial purposes in Beaumont, Texas, as master class action complaint MDL-1038, 13 on counts of negligence; product liability; various permutations of fraud, misrepresentation, and breaches of warranties; and allegations of scarring and side effects (U.S. District Court, Beaumont, Texas 1995). As of late 1995, court actions in Beaumont, Chicago, and San Francisco, were occupied with matters of class certification. The first state case likely to come to trial is Ulrich v. Wyeth-Ayerst , set for Superior Court in San Francisco in early 1996. The original allegation was infection at the implant site, but plaintiffs' counsel have recently recast the case as one involving an unspecified autoimmune disorder caused by Norplant's Silastic tubing.
In contemplating the ramifications of litigation, it is important to note that many other parties have been sued along with American Home Products and Wyeth-Ayerst. These include: physicians or health care providers (including Planned Parenthood), who were involved in either the insertion or removal of the implant, or treatment of plaintiffs; Leiras Oy, the manufacturer of Norplant or its parent company, Huhtamaki Oy;14 Dow Corning, which supplied the Silastic tubing, or other Dow entities; Schering AG, the German company which supplies the bulk levonorgestrel; and the Population Council, which developed Norplant.
The impetus for the Norplant litigation appears to have come from attorneys for a group of breast implant plaintiffs who filed "cookie-cutter" complaints that were then picked up by other plaintiffs' lawyers. The allegations of injury fall roughly into three categories:
- Removal difficulty claims, including those related to capsule displacement, lengthy removals, or improper insertion;
- Levonorgestrel-related claims, including acne, headaches, depression, fatigue, mood swings, weight gain, weight loss, excessive bleeding, ovarian cysts,
- increased intracranial hypertension, premature birth, birth defects, and other allegedly hormonal-related injuries; and
- Silastic-related claims, including autoimmune problems, fear of silicone, and other injuries allegedly related to the hardened silicone elastomer tubing which contains the levonorgestrel.
The rates of removal difficulties are within the expected rates stated in the Norplant labeling, as are the reported adverse events attributed to the levonorgestrel component, which do not differ substantially from those alleged against oral contraceptives over the last 20 years. As for silicone issues, there is no reputable scientific opinion linking the Silastic tubing used in Norplant with any autoimmune or related problems in women; the silicone elastomer used as the delivery system for Norplant has been used for over 30 years in a wide variety of medical products, including catheters, shunts, pacemaker leads, joint replacements, and tubal litigation bands.
In late summer 1995, the FDA publicly reaffirmed its support for Norplant in written testimony before the Subcommittee on Human Resources and Intergovernmental Relations of the U.S. House of Representatives Committee on Government Reform and Oversight:
One specific area where biological effects [of silicone] have been assessed is with the contraceptive implant, Norplant. This product is a piece of closed tubing of silicone elastomer filled with crystals of drug that delivers the drug over a five-year period. The biological safety of the tubing has been studied in laboratory and animal toxicity tests. The silicone materials caused the expected local reactions, but tests to detect immunologic reactions were negative. In addition, reported cases of autoimmune or potentially immune-related disorders among women using Norplant are consistent with the expected rate in this population (Kessler 1995).
In a subsequent Talk Paper, the agency announced that it had recently approved a new patient acknowledgement form that has been incorporated into the product's labeling, which allows patients to acknowledge receipt of information and the opportunity for thorough discussion regarding Norplant prior to insertion. The FDA was also developing information to help assure that patients are appropriately informed of the product's risks and benefits before implantation. It also wrote that its ongoing analysis of adverse reaction reports and postmarketing surveillance studies had found no basis for questioning the safety and effectiveness of Norplant when the product is used as directed in the labeling. FDA's review had already assessed the safety and effectiveness of the hormone levonorgestrel for long-term contraception and the safety of Norplant's silicone-based delivery system. The organized medical community also continues to support Norplant. Wyeth-Ayerst insists that it will stay with the product and will soon file a new drug application for Norplant II, a two-rod, three-year formulation (White 1995).
Nonetheless, the experience is seen as chilling (Economist 1995; Kolata 1995; White 1995). In addition, the method has also become a lightning rod for other sorts of controversy. Because it was perceived in some quarters as a way to control fertility in certain populations, Norplant has been used at times inappropriately, coercively, and even punitively, and a number of minority women's groups have charged that such pressures have been inequitably placed on women of color. This, of course, is not the fault of the manufacturers but it does add to the general weightiness of the atmosphere. Furthermore, the method has also been inaccurately determined to be an abortifacient, such that the State of Pennsylvania, which for the first time in recent history appropriated state funds for contraceptives, explicitly excluded Norplant, as well as Depo-Provera and IUDs from inclusion. Finally, there have been tensions over the cost of the method (U.S. Congress 1994), although those may be resolved soon in negotiations for a public sector price. Nevertheless, the fact that women worldwide continue to use the method and find it appropriate to their needs provides testimony to the value of other perspectives.
Alternative Models
Any evaluation of tort, product liability, and regulatory systems must identify the criteria against which those systems are to be evaluated. As this report goes to press, the fate of product liability reform in the United States is uncertain and regulatory reform is still in process. Yet, even if there are changes, in the United States or elsewhere, those changes and any subsequent proposals for reform need to be evaluated in terms of the way they meet broader social and economic goals since they quite naturally serve a variety of goals and interests. For example, analysts who stress the goal of deterrence as a criterion for evaluating tort law assess the pressures that can be exerted so that product manufacturers and marketers will act to minimize the possibilities of accidental risks and maximize information to potential users about known risks and their prevention or minimization (Schwartz 1994). Analysts concerned with individualized justice will stress goals of access to compensation, while those more concerned with collective or distributive justice will focus on the just allocation of risks and of resources for compensation among populations of consumers. Finally, analysts concerned with market-incentive and consumer-choice goals will balance market accommodation of improved products and consumer choice against consumer protection and the innovator's just responsibility for avoidable and unavoidable defects in their products and services (Dewees and Trebilcock 1992). Since different models of legal and regulatory reform serve different objectives, priorities, and economic or ideologic interests, and since a model that advances one goal may obstruct or frustrate another, selection of the "right" model has to wait until those goals and interests have been determined and priorities ordered. Table 7-3 summarizes four alternative models of liability by way of reference.
|
TABLE 7-3 Alternative Models for the Reform of Tort Law |
|
Fault-Based Liability Model |
|
Conventional tort law bases liability on fault, meaning failure to maintain the standard of care and performance the law expects of those whose actions can harm others. Most actions bear minimal risks of harm to others that no amount of care can reduce, and victims bear these losses alone, subject to any insurance coverage they may arrange for themselves. Fault attracts liability through individual access to a system of remedy, particularly the court system. Historic distinctions between civil compensation of individuals proportionate to the injury they have suffered and criminal punishment of wrongdoers for their wrong against the community at large are blurred when civil courts award punitive damages for egregious wrongs proven by civil plaintiffs. Punitive damages are justified by proven egregious fault, but reward the individual victim who sues first with a windfall profit disproportionate to the compensable loss actually suffered, and may deplete the resources from which others equally harmed may recover just compensation. This model serves the goal of individualized remedy, but may defeat the goal of distributive justice. |
|
Strict Liability Model |
|
This model permits recovery of compensation without a plaintiff demonstrating a defendant's negligence or other fault. Responsive to the goals of consumer protection, it fixes product manufacturers and perhaps intermediaries with liability for even the irreducible minimum of defects that products cause. The justification is that manufacturers and others seek profits from the distribution of products known to result in injury to a broadly defined population, although not necessarily to any individual user. Manufacturers and others are made insurers for injuries associated with promotion of products, and the model claims to balance individuals' interests in compensation with manufacturers', distributors', and others' interest in profit from trade. This model accepts that potential manufacturers, etc., economically unable or unwilling to trade on this basis, will be deterred from entering or remaining in a market. Conviction that a product should be on the market in the public interest will disfavor this model over that of no-fault liability. |
|
No-Fault Insurance Model |
|
This model, of which the U.S. National Vaccine Injury Act of 1986 is an example, balances the social need of a product being available to the public with the need to ensure that those injured through its use receive convenient and effective compensation. Built into the system of public supply will be a means to accumulate funds, from which those proven to have suffered injury related to use of the product will recover just compensation for their financial losses and their pain and suffering. Means to develop compensation funds include special taxes collected by government; add-on user fees that distributors return to manufacturers from which to meet claims; and, for instance, government provision of funds from general revenues. |
|
This model serves the goal of protecting individual users, innovators who serve a public interest in product development, and public service. The model serves distributive justice regarding products whose availability serves the public interest, but it denies the goal of individualized justice in that it excludes an individual victim of injury from entering the "forensic lottery" that offers the prospect of windfall riches in the form of punitive damages. An alternative is to afford victims the choice between, on the one hand, certain and prompt compensation for losses and for pain and suffering, and on the other hand, the delayed, uncertain outcome of no recovery or high reward through litigation. |
|
Reproductive Health Model |
|
This model is less a discrete model of legal reform than it is a framework or context within which choice may be made among the models outlined above. This framework might favor fault-based liability over strict liability, or the no-fault insurance model over faultbased liability, on grounds of social justice and the importance of reproductive health to the well-being of individuals and countries. The framework would provide a range of public sector incentives to encourage the private sector to respond to reproductive health needs as and when they arise. This report has identified the need for combined barrier methods, male methods, postcoital and once-a-month methods, and novel non-barrier female methods with fewer systemic effects, and has pointed to the potential of immunocontraception for providing leads to innovation in some of these priority areas. The overall principle of this model would be the development and distribution of these and other means conducive to the protection and promotion of reproductive health. Public sector incentives might include financial, tax, and other such incentives as extension of a patent period for contraceptive and STD products to public and private organizations, or incentives to develop and publicize new means to improve reproductive health. |
The Role of Information
Informed Consent , Informed Choice , and Consumer Education
This leads to a necessary but difficult intersection where we feel compelled to raise the following questions. First, are there other ways of making the liability climate more "temperate" without sacrificing the rights and recourse of consumers—in this case, the consumers of contraceptives—that principles of justice require? Second, does any of this intersect with other areas of rights, importantly the right to know? Third, without reference to law or penalty, what circumstances will produce the kind of information that is needed for individuals and families to freely and fairly determine the number and timing of the progeny they bring into the world?
Issues of Definition
Informed Consent in Tort Law "Informed consent" is the general principle of civil law describing the duty to disclose to a patient or user of some health technology the risks and benefits of a proposed intervention, as well as the optional or alternative technologies or courses of treatment that are available, so that the patient may exercise his or her judgment by balancing probable risks against probable benefits. It is this knowledge that constitutes the basis of willing, uncoerced, and intelligent consent by the patient or user to a proposed intervention (IOM 1989; Jonsen et al. 1982; Reisman 1992; Wadlington 1984).
Informed consent is a pivotal doctrine under the theory of strict liability. Under this theory, liability derives from negligence in failing to adequately disclose alternative choices and the risks of serious potential consequences, or "failure to warn." Legal action will be based on whether the information given to the participant before securing consent adequately warned of potential risks and whether the degree of disclosure was reasonable given the circumstances. Patients are seen as needing special information, since their mere compliance with what health professionals propose to do to them may not be considered in law to amount to true consent, so that liability may then arise for negligence. The doctrine is seen as protecting patient autonomy and reflects the view that, first, mature individuals have the legal right to make decisions that affect them, a concept developed by courts, originally in the United States (Cook and Dickens 1989) and, second, that physicians may not remain silent because divulgence might prompt the patient to forego needed therapy (Faden and Beauchamp 1986). While the legal individualism that leads to the doctrine of autonomy may not be favored in other cultures, even countries whose laws do not require informed consent recognize that the health professional—client relationship imposes on the professional special duties of assessment and disclosure before treatment is undertaken (Cook and Dickens 1989). Thus, decisions in this area depend heavily on definitions of adequacy (IOM 1994a; Wadlington 1984), as well on concepts of validity, understanding, and voluntariness (Gray and Osterweis 1986; IOM 1994a).
Informed Consent in Research While the term "informed consent" is used both in connection with tort law and in connection with research, it has somewhat different meanings in each context, differences that stem from the differing bases for liability in terms of which party is the defendant. A pharmaceutical company, for example, might be sued on the basis of the theory of strict liability; a researcher ordinarily would be sued only on the basis of negligence, including battery in certain cases (IOM 1994a). "Informed consent" also has a somewhat different meaning in the context of the processes of IRBs that are required for all research supported by the U.S. Public Health Service. IRBs are charged with the review of risk-benefit ratios, confidentiality protections, and procedures for selection of subjects. An IRB is also responsible for ensuring that investigators obtain the consent of participants and reviewing the protocols prepared for doing so, although they only rarely observe the process of obtaining consent itself (Robertson 1982). As women are increasingly included in clinical trials, there may be more coincidence between the universe of research and the tort system in connection with research-related injury (IOM 1994a). This possibility could also expand in the context of introductory trials, since those straddle the research and marketing phases squarely; this signals an area where work needs to be done on improved approaches to and standards for informed consent in international settings, as well as ensuring consistent and effective use of the procedure (WHO/HRP 1991).
Informed Choice The argument has been made that, as a legalistic term deriving in most respects from the practice of law, informed consent is limited as a principle of social policy. In law, informed consent has as its primary goal the legal protection of manufacturers and/or health providers; thus, another conceptual framework is needed whose primary orientation is the protection of the patient or client (Carpenter 1989).
As with informed consent, the principle of patient autonomy is pivotal in informed choice, as is the question of adequacy of information. However, the emphasis is on information for the protection of the patient or client, a desirable objective even if there were no threat of liability (Dukes and Swartz 1988). That protection is achieved by avoiding injury; injury, in turn, is avoided by fully informing potential users about all aspects of a product they are considering and encouraging them not to use that product if they find any of those aspects unacceptable. A key element is the informing process. The assumption here is that provision of correct information is insufficient in itself; presentation, intelligibility, and confirmation that the information has been understood are necessary components of insuring that individual choices are truly informed (Carpenter 1989).
The Notion of Informed Choice
The term "informed choice" has been accumulating currency in the family planning community and among women's advocacy groups (WHO/HRP 1991). Table 7-4 disaggregates the concepts of informed consent and informed choice in terms of their relevance to the stages of contraceptive research and development and adds a third term, "consumer education," as a member of the same conceptual family. All concepts clearly overlap.
Elsewhere in this report we observe that there is no "universal woman" to whom the information in the report or its recommendations are meant to apply in some inevitable, culture-blind fashion. Nonetheless, there are two principles in the women's agenda that are arguably universal: (1) the goal of enhancing women's control over their own bodies, including control over the occurrence and timing of pregnancy; and (2) equity in women's physical safety and wellbeing.
Other commonalities among women are objective circumstances that can make the legal premises of informed consent, as well as the practical premises of informed choice, virtually empty. True freedom to participate in a research trial or to use a given contraceptive depends on the client's access to alternative methods. Poor, illiterate women, especially, may often have little or no choice among either clinical services or methods (IOM 1995a). Many women worldwide lack access to the full and accurate information, sympathetic and respectful provider-client interaction, continuity of care, and availability of other basic reproductive health services that are the sine qua non for both informed consent,
TABLE 7-4 The Relative Roles of Informed Consent, Informed Choice, and Consumer Education
|
|
Informed Consent |
Informed Choice |
Consumer Education |
|
Research Clinical trials phases I-III |
X |
|
|
|
Introductory trials Research cum Marketing |
X |
X |
|
|
Clinical provision of contraceptives/ contraceptive services |
Xa |
X |
X |
|
Over-the-counter provision of contraceptives |
|
X |
X |
|
Postmarketing research |
|
X |
X |
|
a At present, informed consent sign-off sheets are used in connection with the provision of the Progestasert and Paragard IUDs and, imminently, Norplant. |
|||
where that operates in law, or for exerting informed choice (Bruce and Jain 1995; Carpenter 1989; WHO/HRP 1991). Women who are particularly needy are also likely to be particularly vulnerable to incentives in cash or kind for participating in trials. Furthermore, culture, language, and educational level condition understanding to such a degree that the users of contraception—female or male—are often neither informed, consenting, nor freely choosing research participants or consumers (IOM 1994a, WHO/HRP 1991).
Each of these potentiating situations enhances the likelihood that something will go wrong, if only because no contraceptive method is appropriate for all users and the range of their preferences, and none is free of side effects, although some are more severe for certain users than for others. Another potentiating circumstance resides in the nature of technology itself, independent of the possibility that information about some method side effects was not uncovered in premarketing clinical trials (Forrest 1994b). As the histories of oral contraceptives and intrauterine devices make clear, the first drug in a new therapeutic class is unlikely to be the optimal version. Incremental improvements after initial adoption play an important role in pharmaceutical and biological development
and take place not only in industrial R&D laboratories but in clinical practice as well. Thus, new technologies retain some degree of uncertainty long after their initial adoption (Gelijns and Rosenberg 1994).
Another key element in making informed choices is having the knowledge on which to assess relative risks. For example, as maternal mortality rates associated with pregnancy and childbirth fall in most places in the world, and as those declines are perceived as real, the risk equation shifts and women will want different kinds of information on which to base their contraceptive decisions; they will also be less inclined to accept really bothersome contraceptive side effects (Germain and Dixon-Mueller 1993). Women who must confront unsafe abortion as the only alternative to contraception will want to understand their contraceptive options on the basis of efficacy. Other women may need information that will permit them to make tradeoffs between risks of an infection that could be lethal for them or their children, vis-à-vis anti-infective and/or contraceptive side effects. The point is that the parameters for decision-making around contraceptive and anti-infective use are more complex than ever, so that the knowledge for exercising informed choice is ever more critical.
Consumer Education
Chapter 6 discusses the restructuring of health services and the biomedical commodities industry as a component of health care reform efforts worldwide. Among the most relevant of these dynamics is the proliferation of health care delivery models which fall under the rubric of "managed care." These include a shift from hospital-based health care to any model that is cost-effective, including preventive interventions, particularly those that are cost-effective in the shorter as opposed to the longer term. Concurrent with these shifts is a consumer movement which incorporates notions of the "educated patient," patient autonomy, health promotion, client-centered care, and the significance of client-provider information exchange. In the context of contraceptive use and family planning, these have become standard themes. The point is often made that at least some of the failure rates associated with contraceptive methods (e.g., barrier methods) are a function of inadequate information and support (WHO 1991) or, as in the case of emergency contraception, an almost total lack of communication from provider to client. Furthermore, there is ample evidence that women everywhere, to a varying extent, are inadequately prepared by providers for the possibility of contraceptive side effects, their implications, and appropriate response.
One implication of all this is that accuracy of information, its level of completeness, intelligibility, and overall effectiveness, always important, are becoming critical to health care in general and to reproductive health in particular. Though by far from the only element in consumer education, the packaging and labeling of contraceptives play-or could play-a much more useful role in the educational process of clinicians and consumers than they now do (Guest et al.
1993). At present, the labels and inserts in contraceptive packaging are daunting visually and technically, a fact often attributed to exigencies imposed by the FDA and to concerns about liability. At the same time, the FDA only has jurisdiction to approve package inserts as accurate statements of the safety and efficacy of their contents; they are not in the business of approving new, perhaps more effective ways of informing consumers and clinicians.
Labeling and package inserts can be dismissed as a small matter, or at least smaller than other forces that impinge on the availability and use of contraceptives, such as the roles of the media and advocacy groups, and the ideas, information and, sometimes, misinformation that flow through them. Still, a single categorical location where intelligible, accurate information about the technical aspects of individual contraceptive technologies—their risks, side effects, contraindications, benefits, and proper use—is consistently available could serve to anchor the information base. It is also the case that the media pay attention to package labels; in the case of the Reality female condom, an apparent misreading of the label published in a major trade journal, sent the manufacturer's stock plummeting (AIDS Alert 1994).
Nor is labeling a small matter in its technical complexity, regulatory considerations, and legal implications; even ''cosmetically" it will take considerable thought and work. And, even assuming that the method in question is physiologically and socially appropriate for use by a given population, there will always be some part of that population for whom newly accessible information will make no direct difference, either because it does not fit into the ways they calculate risk, or because they receive the information inadequately transmitted through an intermediary. Nor is there any easy way to calculate the costs and benefits of better labeling. Revising labeling in some generic way would inevitably impose costs, but these would seem unlikely to be crippling for large companies (IOM 1994c).15 In a clinic setting, helping individuals make informed choices takes time and effort, both at a premium for busy health care providers who may also have patient load quotas. A well-informed consumer may decide not to use a product after all, and a possible sale does not occur, yet another cost. Still, these costs must be arrayed against others: the putative harm to a user or a user's progeny, the costs of compensating that harm, the costs to the reputation of the firm, and the costs to other appropriate consumers of losing an option when a contraceptive method is withdrawn from the market.
The Environment
Whatever may come to pass in regulation, law, and improvements in information about contraception, after all is said and done, each of these is nested in individual and societal systems of belief and behavior. To an extent that is highly variable, chronologically and by local and national setting, contraception has evoked a sometimes stunning range of controversy. This situation persists, again
with great variety, despite the fact that contraception has become an accepted fact of life for millions of individuals worldwide and despite major changes in attitudes concerning women's rights to equity and choice.
At the heart of controversy reside two fundamental clusters of issues: beliefs about human sexuality, especially female sexuality, and beliefs pertaining to abortion. In many parts of the world, sometimes as a correlate of economic reversals, controversy may be expressed in a resurgence of conservative ideologies regarding women, their sexuality, and their rights, a resurgence that may originate in religious fundamentalism, ethnic nationalism, or social conservatism in reaction to concerns about excessive sexual and personal freedom (IWHC/ UWI 1994).
Sexuality
The United States is no exception. One scholar quoted in the recent IOM study of unintended pregnancy (IOM 1995b) notes that "Few if any societies exhibit a more perverse combination of permissiveness and prudishness in their treatment of sexual issues" and adds that the U.S. popular media are "filled with sexual material, while, on the other hand, there is a noted absence of equal attention to contraception, responsible personal behavior, and values in sexual expression."
Other analysts point to the deep strains of ambivalence about contraceptives among some groups in the United States. In some cases, the ambivalence is rooted in economic inequality, racial discrimination, and a history sometimes marked with coercive or punitive uses of contraception (Samuels and Smith 1992; Segal et al. 1994). In others, it derives from the asynchrony between a tradition of high demand for technologic solutions, particularly in health, and the broad strain of technologic minimalism born in the 1960s. Another dimension of this line of thought includes worries about the "hypermedicalization" of women's health and the high priority that is placed by some women's advocacy groups on the need for women to have total control over their own fertility. A more specific ambivalence springs from disappointments as limitations and, in some cases, real deficiencies of new contraceptives are revealed in use in large populations (Forrest 1994b). Finally, there is an underlay of cynicism about the profit motives of the private sector, currently fueled by attention to issues of pharmaceutical pricing and the privatization of health care.
All these areas of dissonance necessarily affect the exchange of accurate information about sexual behavior, contraception, and the avoidance of unintended pregnancy (Chen 1995). At the same time, the disjoints foster dissatisfaction with current contraceptive methods and, somewhat ironically, strong support for continued research and development of new ones (Forrest 1994b).
Abortion
The other major element of this conceptual environment has to do with beliefs about childbearing and the relative rights of parents and offspring, with definitions of the beginning of viable life ex utero, and with the ways abortion and contraception are seen to overlap. This overlap, real or not, matters greatly, whether or not abortion is defined as in itself a contraceptive method; whether it is defined as a last recourse in case of contraceptive failure; whether a specific method is defined as having abortifacient effect, even though science may provide evidence that no such effect is in play; or whether artificial contraception is seen, in itself, to be morally wrong. The resurgence of abortion as a central theme in the current U.S. political campaign, attempts to reverse Roe v. Wade, and efforts to eliminate the national family planning program are ample indication of the durable, penetrating character of these matters. For example, the State of Pennsylvania just, for the first time, appropriated state funds for contraceptives but excluded Norplant, Depo-Provera, and IUDs from being provided because they are considered abortifacients, a definition that is scientifically incorrect.
There appears to be more clarity about current methods of emergency contraception, which are well accepted by medical and legal authorities in the United States and Europe as not being abortifacients, but as methods that either prevent fertilization altogether or stop the fertilized egg from implanting in the uterine wall. In other words, methods that prevent implantation from occurring, or perhaps even completing, are appropriately regarded as contraceptives, not abortifacients (Holt 1995).16 The same clarity of perception may be less likely in connection with the antiprogestins, since these offer potential for a number of interventions across the whole menstrual cycle, before and after implantation, a matter that will require special consideration.
Revisiting the Analogy with Vaccines
Issues of regulation and liability were also central in the years of discourse around vaccines (IOM 1985 and 1993). Vaccines and contraceptives are alike in that neither is a curative drug for people who are already ill; rather, both are administered to healthy individuals to prevent a condition they may never get. As a result, adverse reactions are much more noticeable and less tolerated by the vaccine (IOM 1993), so that both product categories have been especially susceptible to "superlitigation" (Djerassi 1989). However, as discussed earlier in this chapter and in Chapter 3, the vulnerability of the pediatric vaccines to litigation has been much attenuated by the National Vaccine Injury Compensation Program (NVICP) in the United States and was already less of an issue in the European countries, many of which had instituted some kind of vaccine injury compensation program (IOM 1985).
The vaccines market, after decades of modest, birth-driven growth, entered a
phase of dramatic expansion beginning in the mid-1980s. As of 1995, global vaccines sales reached approximately $2.5 billion, a market size close to that of the global market for contraceptives (FIND/SVP 1996).17 Part of the expansion is attributed to industry's gradual appreciation of the potential of the NVICP (Day 1996) and part to a confluence of other policy decisions, importantly the establishment of pediatric vaccination as part of the health care regimen for all newborns in most developed countries and intensification of vaccination campaigns in developing countries under the guidance of the World Health Organization (FIND/SVP 1996). WHO efforts were pivotal in raising immunization coverage of the developing world's children from about 10 percent in the late 1970s to 80 percent in many countries by the late 1980s. These efforts received further impetus from the 1990 World Summit for Children, where most of the world's nations committed themselves to achieving 90 percent national coverage by the year 2000 (UNICEF 1992). Consequent to the Summit, WHO, in conjunction with other United Nations' agencies, the World Bank, and the U.S. Agency for International Development, established an International Children's Vaccine Initiative (CVI) that is working toward development of a multivalent pediatric "supervaccine" that would require just one or two doses administered orally and would be heat stable, affordable, and have a low rate of side effects (IOM 1993).
The vaccines market was further driven by development of new, genetically engineered vaccines (HiB-related meningitis, hepatitis A, and hepatitis B), as well as efforts to develop vaccines for such key needs as prevention of AIDS (FIND/SVP 1996). Analysts also attribute vaccine market growth to recognition of the emergence and resurgence of diseases, appreciation of infectious etiologies for some chronic diseases, and mounting drug resistance (IOM 1992). Greater awareness of the cost-effectiveness of vaccines is also considered a factor (FIND/ SVP 1996) and by subsidized bulk procurement for vaccine provision to developing countries that has been able to evoke positive commercial response (Mercer Management Consulting 1994). The bottom line is that there are over a dozen new pediatric vaccines in development, 18 in addition to almost two dozen diseases targeted for preventive vaccines for adults.19 To these must be added efforts at developing therapeutic vaccines, obviously including AIDS, cancer, and herpes, and work in novel delivery systems, including liposomes, microspheres, and nanoparticles.
However, vaccines are not about human sexuality nor have they confronted anywhere near the diversity of public opinion that swirls around contraception. Furthermore, vaccines are not generically blurred as technologies qua technologies, nor are issues around their delivery as culturally fraught. Vaccines are what they are: They prevent disease and their modes of action are not confused by the public with any other purpose or effect. In addition, it is understood that vaccines are to be delivered by a provider in a formal health system and, while they may be required by law, they are not perceived as coercive except by small minorities who resist vaccination for their children largely on religious grounds. In contrast,
the provider role in contraception is frequently brought into question (Forrest 1994b; Richter 1994).
Concluding Comment
Despite the imperfection of the analogy between vaccines and contraceptives, the vaccine experience does inform us that the issues around particularly controversial medical products, whatever the sources of controversy, tend to be incremental and systemic in their resolution. The vaccine experience took time—two decades of working groups charged with solving "the vaccine problem," a decade of legislative attempts to construct a passable bill, and close to a decade for industry to perceive that the legislative remedy was effective. Change came from several sources—from a surge of discovery in the science; from legislative action that modified public policy; and, in lesser measure, from the decision of a major international procurer of commodities to seek professional assistance in assessing its processes and their impact. This does not mean that the same amount of time will be needed for improvement in the contraceptive landscape, nor will the solutions be the same. The central implication is that there is not likely to be any "silver bullet" solution to the dilemmas faced by the field of contraceptive research and development, but that each piece of the dilemma will need to be considered and addressed as part of a coherent strategy.
References
AH Robins Company. 1988 Annual Report and Form 10-K. Richmond, VA: AH Robins Company. 1988.
AIDS Alert. Barriers to better condom "killing people"; regulatory, political hurdles stifle development. Atlanta, GA: American Health Consultants. 1994.
American Law Institute (ALI). Restatement (Second) of Torts §402A. 1977.
Associated Press. FDA Defends Its Drug Program. Washington, DC: 12 December 1995.
Baker FD. Effects of products liability on bulk suppliers of biomaterials. Food and Drug Law Journal 50:455-460, 1995.
Bankowski Z, J Barzelatto, AM Capron, eds. Ethics and Human Values in Family Planning: 22nd CIOMS Conference, Bangkok, Thailand, 19-24 June 1988. Geneva: WHO Special Programme of Research, Development and Research Training in Human Reproduction and Council for International Organizations of Medical Sciences. 1989.
Barnett AA. FDA criticized by Republicans despite success. Lancet 346:16-17, 1995.
Biotechnology Industry Organization (BIO). FDA Regulatory Reform: A Proposal by the Biotechnology Industry Organization. Washington, DC: BIO. 27 February 1995.
Bruce J, A Jain. A new family planning ethos. The Progress of Nations. New York: UNICEF. 1995.
Bulatao RA, RD Lee. An overview of fertility determinants in developing countries. IN Determinants of Fertility in Developing Countries, Vol. 2. RA Bulatao, RD Lee, eds. New York: Academic Press. 1983.
Carpenter PF. Informed choice as a way to reduce risks and prevent injury. IN No Fault Compensation in Medicine: Proceedings of a Joint Meeting of the Royal Society of Medicine and the
British Medical Association, 12-13 January 1989. RD Mann, J Havard, eds. London: Royal Society of Medicine. 1989.
Carpenter PF. Perspectives of industry, the physician, and government: Responsibility, risk, and informed consent. IN New Medical Devices: Invention, Development, and Use. KB Ekelman, ed. Washington, DC: National Academy Press. 1988.
Cates W Jr., KM Stone. Family planning: The responsibility to prevent both pregnancy and reproductive tract infections. IN Reproductive Tract Infections: Global Impact and Priorities for Women's Reproductive Health. A Germain, KK Holmes, P Piot, JN Wasserheit, eds. New York: Plenum Press. 1992.
Chen V. A new social norm . . . all pregnancies intended. Carnegie Quarterly Fall-Winter: 10-11, 1995.
Clayton EW. Liability exposure when offspring are injured because of their parents' participation in clinical trials. IN Women and Health Research: Ethical and Legal Issues of Including Women in Clinical Studies, Vol. 2, Workshop and Commissioned Papers, AC Mastroianni, R Faden, D Federman, eds. Washington, DC: National Academy Press. 1994.
Clinton W, A Gore. Reinventing Drug and Medical Device Regulations, 3. Washington, DC: Office of the President, Task Force on Reinventing Government. 1995.
Cook RJ. Antiprogestin drugs: Medical and legal issues. Family Planning Perspectives 21(6):267-272, 1989.
Cook RJ, BM Dickens. Ethics and human values in family planning: Legal and legislative aspects. IN Ethics and Human Values in Family Planning: 22nd CIOMS Conference, Bangkok, Thailand, 19-24 June 1988. Z Bankowski, J Barzelatto, AM Capron, eds. Geneva: WHO Special Programme of Research, Development and Research Training in Human Reproduction and Council for International Organizations of Medical Sciences. 1989.
Corfman P. Memorandum to the Record re Action on NDA 20-246, 27 October 1992. Rockville, MD: Food and Drug Administration. 1992.
Council for International Organizations of Medical Sciences (CIOMS). Guidelines for Biomedical Research Involving Human Subjects. Geneva: CIOMS. 1993.
Cunningham G, et al. Williams Obstetrics. 19th Edition, pp. 1335-1336. 1993.
Day K. Vaccine maker gets a shot in the arm. Washington Post, Business section, pp. 18-19, 11 March 1996.
Dewees D, M Trebilcock. The efficacy of the tort system and its alternatives: A review of the empirical evidence. Osgoode Hall Law Journal 30:58-138, 1992.
Dixon-Mueller R. Abortion is a method of family planning. IN Four Essays on Birth Control Needs and Risks. R Dixon-Mueller, A Germain, eds. New York: International Women's Health Coalition. 1993.
Djerassi C. The bitter pill. Science 245:345-361, 1989.
Dukes MNG, B Swartz. Responsibility for Drug-induced Injury. Amsterdam: Elsevier. 1988.
[The] Economist. On the needless hounding of a safe contraceptive method. pp. 113-114, 2 September 1995.
Faden RR, TL Beauchamp. A History and Theory of Informed Consent. New York: Oxford University Press. 1986.
FIND/SVP. Pharmaceutical Market Reports: Summary of Research Report on the World Market for Vaccines. New York: FIND/SVP. 1996.
Flannery EJ. Products Liability Issues and Tort Reform Update. Presentation to the Institute of Medicine Workshop on Private Sector Participation in Contraceptive Research and Development: Opportunities, Challenges, Strategies, 11 May 1995. Washington, DC: Covington and Burling. 1995.
Flannery EJ, SB Greenberg. Liability exposure for exclusion and inclusion of women as subjects in clinical studies. IN Women and Health Research: Ethical and Legal Issues of Including Women in Clinical Studies, Vol. 2, Workshop and Commissioned Papers, AC Mastroianni, R Faden, D Federman, eds. Washington, DC: National Academy Press. 1994.
Food and Drug Administration (FDA). Talk Paper, 17 August 1995a.
FDA. Vaginal Contraceptive Drug Products for Over-the-Counter Human Use. Federal Register, 60:68-92, 1995b.
FDA. Assignment of Agency Component for Review of Premarket Applications. Federal Register 56:58,754, 1991a.
FDA. Intercenter Agreement Between the Center for Drug Evaluation (CDER) and Research and the Center for Devices and Radiological Health (CDRH), section VII.A.2, 31 October 1991b.
FDA. Premarket Testing Guidelines for Female Barrier Contraceptive Devices Also Intended to Prevent Sexually Transmitted Diseases. 4 April 1990.
FDA. Vaginal Contraceptive Drug Products for Over-the-Counter Human Use: Establishment of a Monograph; Proposed Rulemaking. Federal Register 45:82,014, 1980.
Food Labeling and Nutrition News, Sample Issue, 4 January 1996.
Foote SB. Product liability and medical device regulation: Proposal for reform. IN New Medical Devices: Invention, Development, and Use. KB Ekelman, ed. Washington, DC: National Academy Press. 1988.
Foreman J. FDA allows Depo Provera as injectable contraceptive. Boston Globe p. 1, 30 October 1992.
Forrest JD. Contraceptive use in the United States: Past, present and future. Advances in Population, Vol. 2. London: Jessica Kingsley Publishers Ltd. 1994a.
Forrest JD. Acceptability of Contraceptives in the United States. Paper prepared for the Ad Hoc Group on Population of the International Council of Scientific Unions (ICSU). New York: The Alan Guttmacher Institute. 1994b.
Fox JL. For AIDS, the FDA may be reforming itself. Biotechnology 13:314-315, 1995.
Garber S. Products Liability and the Economics of Pharmaceuticals and Medical Devices. Santa Monica, CA: RAND Institute for Civil Justice. 1993.
Gelijns A, N Rosenberg. The dynamics of technological change in medicine. Health Affairs13(3):28-46, 1994.
Germain A, R Dixon-Mueller. Whose life is it, anyway? Assessing the relative risks of contraception and pregnancy. Women's health advocates and scientists talk about contraceptive technology. IN Four Essays on Birth Control Needs and Risks. R Dixon-Mueller, A Germain, eds. New York: International Women's Health Coalition. 1993.
Gray BH, M Osterweis. Ethical issues in a social context. IN Application of Social Science to Clinical Medicine and Health Policy. L Aiken, D Mechanic, eds. New Brunswick, NJ: Rutgers University Press. 1986.
Greenberger P. Memorandum: Press Conference on Biomaterials Availability Legislation, and Statement on Biomaterials Access Assurance Act. Washington, DC: Society for the Advancement of Women's Health Research. 1 February 1995.
Guest F, F Stewart, J Trussell, C Ellertson. Enhancing Safe and Effective Use of Oral Contraceptives. Paper prepared for meeting of FDA Fertility and Maternal Health Drugs Advisory Committee, 29 October 1993. Rockville, MD: Food and Drug Administration. 1993.
Harper F, F James, O Gray. Torts, 2nd ed., Vols. 3 and 5. Boston: Little, Brown and Company. 1986.
Holt R. Emergency Contraception: Working Paper on Pharmaceutical Company Involvement. Los Angeles, CA: Pacific Institute for Women's Health, Western Consortium for Public Health. 1995.
Hughes S. Emergency contraception-A hard pill to swallow? Scrip Magazine, September 1995:48-52.
Hunt AR, A Murray. Courting success: The Republican Contract with America sets its sights on the legal system. SmartMoney, March 69-70, 1995.
Hutton J, M Borowitz, I Oleksy, BR Luce. The pharmaceutical industry and health reform: Lessons from Europe. Health Affairs 13(3):98-111, 1994.
Hyde HJ. Products Liability Fairness Act of 1995; Biomaterials Access Assurance Act of 1995 (H.R. 956) [Common Sense Products Liability and Legal Reform Act of 1995]. Legi-Slate Report for the 104th Congress, Quick Bill, 7 August 1995.
Institute of Medicine (IOM). Review of the Fialuridine (FIAU) Clinical Trials. FJ Manning, M Swartz, eds. Washington, DC: National Academy Press. 1995a.
IOM. The Best Intentions: Unintended Pregnancy and the Well-Being of Children and Families. S Brown, L Eisenberg, eds. Washington, DC: National Academy Press. 1995b.
IOM. Women and Health Research: Ethical and Legal Issues of Including Women in Clinical Studies, Vol. 1. AC Mastroianni, R Faden, D Federman, eds. Washington, DC: National Academy Press. 1994a.
IOM. Women and Health Research: Ethical and Legal Issues of Including Women in Clinical Studies, Vol. 2, Workshop and Commissioned Papers. AC Mastroianni, R Faden, D Federman, eds. Washington, DC: National Academy Press. 1994b.
IOM. Nutrition Labeling: Issues and Directions for the 1990s. D Porter, RO Earl, eds. Washington, DC: National Academy Press. 1994c.
IOM. The Children's Vaccine Initiative: Achieving the Vision. VS Mitchell, NM Philipose, JP Sanford, eds. Washington, DC: National Academy Press. 1993.
IOM. Emerging Infections: Microbial Threats to Health in the United States. J Lederberg, RE Shope, SC Oaks Jr., eds. Washington, DC: National Academy Press. 1992.
IOM. Medical Professional Liability and the Delivery of Obstetrical Care, Vol. I. Washington, DC: National Academy Press. 1989.
IOM. Vaccine Supply and Innovation. Washington, DC: National Academy Press. 1985.
International Women's Health Coalition, and Women and Development Unit, University of the West Indies Challenging the Culture of Silence: Building Alliances to End Reproductive Tract Infections. [Report of a Conference on Reproductive Tract Infections among Women in the Third World, Barbados, March 1992]. P Antrobus, A Germain, S Nowrojee, rapporteurs. New York: International Women's Health Coalition. 1994.
Jones EF, JD Forrest, SK Henshaw, J Silverman, A Torres. Pregnancy, Contraception, and Family Planning Services in Industrialized Countries. New Haven: Yale University Press. 1989.
Jonsen AR, M Siegler, WJ Winslade. Clinical Ethics: A Practical Approach to Ethical Decisions in Clinical Medicine. New York: Macmillan. 1982.
Jordan A. FDA requirements for nonclinical testing of contraceptive steroids. Contraception 46:499-509, 1992.
Kessler DA. Testimony Before the Subcommittee on Human Resources and Intergovernmental Relations of the House Committee on Government Reform and Oversight. Washington, DC, 1 August 1995.
Kolata G. Will the lawyers kill off Norplant? New York Times, Section 3, p. 1, 28 May 1995.
Lasagna L. Impact of Funding and Policy Changes on the Food and Drug Administration. Manuscript submitted for publication. Boston: Tufts Center for the Study of Drug Development. July 1995a.
Lasagna L. What's Wrong with the FDA? Unpublished manuscript. Boston: Tufts Center for the Study of Drug Development. July 1995b.
Levine C. Women and HIV/AIDS research: The barriers to equity. Evaluation Review 14(5):447-463, 1990.
Lewin T. Fears, suits and regulations stall contraceptive advances. New York Times, Business section, pp. 1, 12, 27 December 1995.
Macklin R. Ethics and human values in family planning: Perspectives of different cultural and religious settings. IN Ethics and Human Values in Family Planning: 22nd CIOMS Conference, Bangkok, Thailand, 19-24 June 1988. Z Bankowski, J Barzelatto, AM Capron, eds. Geneva: WHO Special Programme of Research, Development and Research Training in Human Reproduction and Council for International Organizations of Medical Sciences. 1989.
Mathieu M. New Drug Development: A Regulatory Overview, 3rd ed. Waltham, MA: Parexel International Corporation. 1994.
Mayer CE. Getting personal on product liability: Two lawmakers' opposing views stem from their own painful experiences. The Washington Post, pp. D1-4, 7 March 1995.
Mercer Management Consulting. Summary of UNICEF Study: A Commercial Perspective on Vaccine Supply. New York: Mercer Management Consulting. 1994.
Merrill RA. Regulation of drugs and devices: An evolution. Health Affairs 13(3):47-69, 1994.
National Institutes of Health. Fiscal Year 1997 Legislative Proposal: Products Liability Exemption for PHS IND and IDE Research. Bethesda, MD: National Institutes of Health. 1995.
National Institutes of Health (NIH). Long-term Effects of Exposure to Diethylstilbestrol (DES), NIH Workshop, Falls Church, VA, 22-24 April 1992.
National Research Council and Institute of Medicine. Developing New Contraceptives: Obstacles and Opportunities. L Mastroianni Jr, PJ Donaldson, TT Kane, eds. Washington, DC: National Academy Press. 1990.
National Women's Health Network. Memorandum to Dockets Management Branch, Food and Drug Administration: Vaginal Contraceptive Drug Products for Over-the-Counter Human Use (Docket No. 80N-0280). Washington, DC: National Women's Health Network. 2 June 1995.
Newbille CI. The "injectable contraceptive"-Depo Provera: Cause for alarm, not celebration. Health and Fitness 1:6, 1993.
Peterson MA. Civil Juries in the 1980s. Santa Monica, CA: Rand Institute for Civil Justice. 1987.
Prichard JRS. Canadian FeralProvincial/Territorial Review on Liability and Compensation Issues in Health Care. Toronto: University of Toronto Press. 1990.
Prosser W, P Keeton. Prosser and Keeton on Torts, 5th ed. St. Paul, MN: West Publishing. 1984.
Reisman EK. Products liability: What is the current situation and will it change (and how) when more women are included in studies? Paper presented at the Women in Clinical Trials of FDA Regulated Products Workshop, Washington, DC, Food and Drug Law Institute, 5 October 1992.
Richter J. Beyond control: About antifertility vaccines, pregnancy epidemics and abuse. IN Power and Decision: The Social Control of Reproduction. G Sen, R Snow, eds. Cambridge, MA: Harvard University Press. 1994.
Robertson JA. The law of institutional review boards. UCLA Law Review 25:484-549, 1982.
Samuels SE, MD Smith, eds. Norplant and Poor Women. Menlo Park, CA: The Henry J. Kaiser Family Foundation. 1992.
Sathyamala C. Depot-medroxyprogesterone acetate and breast cancer: A critique of the WHO's multinational case control study. Medico Friend Circle Bulletin 220:1-5, 1995.
Scheper-Hughes N. Death Without Weeping: The Violence of Everyday Life in Brazil. Berkeley: University of California Press. 1995.
Schrage M. Shielding companies from suits is just industrial policy in disguise. The Washington Post, p. B3, 10 March 1995.
Schwartz GT. Reality in the economic analysis of tort law: Does tort law really deter? University of California at Los Angeles Law Review 42:377-444, 1994.
Schwartz J. FDA revises biotechnology rules: Changes are designed to consolidate and speed approval process. Washington Post, Federal Page, p. A19, 13 November 1995.
Segal SJ, GP Talwar, RG Edwards. The Agenda for Contraceptive Research. Paper prepared for the Ad Hoc Group on Population of the International Council of Scientific Unions (ICSU). New York. 1994.
Sivin I. International experience with NORPLANT® and NORPLANT®-2 contraceptives. Studies in Family Planning 19(2):81094, 1988.
Smith SD. The critics and the "crisis": A reassessment of current conceptions of tort law. Cornell Law Review 72:765-798, 1987.
Snow R, P Hall. Steroid Contraceptives and Women's Response: Regional Variability in SideEffects and Pharmacokinetics. New York: Plenum Press. 1994.
Spayd L. America, the plaintiff: In seeking perfect equity, we've made a legal lottery. The Washington Post, Outlook section, 5 March 1995.
Squires S. DES daughters and their children. The Washington Post, February 19:14. 1991.
Steyer R. Searle nearing end of lawsuits over Copper 7 contraceptive. St. Louis Post-Dispatch, p. 1E, 15 October 1995.
Swenson S. Depo-Provera: Loopholes and double standards. Hastings Center Report 17:3, 1987.
Trussell J, K Sturgen, J Strickler, R Dominik. Comparative contraceptive efficacy of the female condom and other barrier methods. Family Planning Perspectives 26(2):66-72, 1994.
United Nations Children's Fund (UNICEF). The State of the World's Children 1992. New York: Oxford University Press. 1992.
United States Congress, House of Representatives. Impact of the high cost of long-term contraceptive products on federally sponsored family planning clinics, welfare reform efforts, and women's health initiatives. Hearing before the Subcommittee on Regulation, Business Opportunities, and Technology of the Committee on Small Business, 103rd Congress, 2nd session. Washington, DC, 18 March 1994.
United States District Court for the Eastern District of Texas, Beaumont Division. Memorandum Opinion and Order Granting Plaintiffs' Motion to Remand (Case No. 1:94CV5006). Beaumont, TX, 17 March 1995.
Wadington W. Breaking the silence of doctor and patient (review of J. Katz's The Silent World of Doctor and Patient). The Yale Law Journal 93(8):1640-1651, 1984.
Washington Post. Editorial: Drug Regulation and Reform. 5 December 1995 .
White K. Contraceptive makers chilled by court challenges. Journal of Women's Health 4(3):223-224, 1995.
World Health Organization. Creating Common Ground: Women's Perspectives on the Selection and Introduction of Fertility Regulation Technologies-Report of a Meeting Between Women's Health Advocates and Scientists, 20-22 February 1991. Geneva: WHO/Special Programme of Research, Development and Research Training in Human Reproduction and the International Women's Health Coalition. 1991.
World Health Organization Collaborative Study of Neoplasia and Steroid Contraceptives. Depomedroxyprogesterone Acetate (DMPA) and Cancer: Memorandum from a WHO Meeting. Bulletin of the WHO 64(3):375-382, 1986.
Zabin LS. Addressing adolescent sexual behavior and childbearing: Self esteem or social change. Women's Health Issues 4:93-97, 1994.
Notes
-
groups—for example, the National Black Women's Health Project and Women of All Red Nations (WARN) in the United States, and several women's advocacy groups in India—expressed concern about Depo-Provera (Foreman 1992; Newbille 1993; Sathyamala 1995, Swenson 1987). Concerns have tended to fall into two categories: issues of side effects and the method's potential for coercive applications.
-
1992). The ensuing legal actions were numerous and costly, with over a thousand pending nationwide as of February 1991 (Squires 1991). Of high relevance for the area of research liability were the awards for battery made to women involved in clinical trials of DES who had not been informed that they were part of a study (IOM 1994a). The experience encouraged exclusion of pregnant and potentially pregnant women from clinical research and the writing of FDA guidelines to reinforce that practice (IOM 1994a).
-
13.
MDL = Multidistrict litigation.