C: Immunocontraceptive Approaches
John Christian Herr, Ph.D.
Department of Cell Biology and The Center for Recombinant
Gamete Contraceptive Vaccinogens, University of Virginia
Introduction
The concept of an immunocontraceptive might be stated as follows: A formulation of certain molecules is injected or taken orally by a man or woman, resulting in the production by his or her own body of circulating antibodies or immune effector cells that interrupt reproductive processes and sustain a period of infertility, without side effects. As of the summer of 1995, no immunocontraceptive had been marketed in any country, either for human or veterinary application.
This review discusses some general concepts regarding contraceptive immunization, reviews several promising immunogen candidates, identifies hurdles on the path to developing an immunocontraceptive, and cites new approaches that may offer opportunities for significant scientific advances.
Differences Between Traditional Vaccines and Immunocontraceptives
The vaccine paradigm was established nearly two centuries ago when, in 1796, Edward Jenner inoculated an 8-year-old boy with cowpox and effectively prevented smallpox. Since then, many vaccines have been developed, their purpose being to provide protection against debilitating or life-threatening microorganisms. The purpose of an antifertility immunogen is to prevent fertility or early pregnancy. Traditional immunization against micro-organisms may often be the only means available for controlling a given disease; in the case of contraception, alternative methods are already available.
Another major difference is that traditional vaccines are also based on, and directed against, foreign antigens contained in the disease-causing micro-organism; in contrast, antifertility immunization is directed against isologous antigens from the egg, sperm, or certain reproductive hormones. Since isologous antigens may not be as immunogenic as foreign antigens, development of sustained immune responses to them may be difficult, owing in part to mechanisms of immunologic tolerance.
Finally, the ideal traditional vaccine confers long-term protective immunity, usually aided by boosting throughout the lifetime of the individual as a result of exposure to the natural antigen. Because it will be generally desirable for an immunocontraceptive to be reversible, the immunity induced should be of relatively short duration, that is, measured in months or a few years, and should not be boosted naturally by exposure to the target antigen; in other words, insemination should not boost a sperm-based immunocontraceptive.
The Pathway to Development of an Immunocontraceptive
The pathway for development of an immunocontraceptive follows a series of steps not unlike those followed in traditional vaccine development. These include: (1) fundamental discovery and characterization of appropriate immunogens derived from reproductive hormones and/or from the sperm, egg, egg investments, conceptus, or accessory reproductive organs; (2) development of methods for producing the immunogens to high standards of purity through (a) genetic engineering of genes encoding specific immunogens, (b) peptide syntheses, or (c) isolation of the antigen from natural sources; (3) production and purification of immunogens under good laboratory practices (GLP); (4) formulation of immunogen doses; (5) small animal and primate testing of immunogen formulations for immunogenicity, safety, and efficacy; (6) evaluation of mechanisms of immunogen action; (7) human trials for immunogenicity, safety, and efficacy, using formulations produced under good manufacturing practices (GMP); and (8) development of diagnostics to monitor infertility status in recipients of effective immunogens.
The first step—discovery and characterization of immunocontraceptive components—is perhaps the most complicated; it is also the stage at which many research projects currently stand. This step is subdivided into several milestones: (1) definition of events or processes in reproduction accessible to immune intervention; (2) identification of molecules (immunogens) whose elimination or neutralization will have an antifertility effect; (3) biochemical characterization of the structure of the immunogen(s); and (4) determination of whether the immunogen is unique to the reproductive event or tissue targeted and is absent in all other tissues.
In considering which molecules might be best for a contraceptive immuniza-
tion, consideration should be given to events in the process of reproduction that are accessible to immune intervention. For purposes of simplification, mammalian reproduction may be divided into several key stages, each of which may offer an opportunity for intervention: (1) gamete production (spermatogenesis and oogenesis); (2) gamete shedding and transport (copulation; sperm transport in the cervix, uterus, and oviduct; ovulation and oviductal transport of the cumulus mass); (3) gamete interaction (capacitation, the acrosome reaction, penetration of the egg investments, fusion with the oolemma); (4) blastocyst transport and hatching; and (5) implantation. All of these events can theoretically be inhibited by eliciting an immune response directed against functionally and structurally important molecules that are accessible to immune effectors (antibody or cell mediated).
Many cells and cellular products are involved in the stages of mammalian reproduction enumerated above, including hypothalamic, pituitary, and gonadal hormones; gamete surface components, including proteins, glycoproteins, and glycolipids; and secretions of reproductive organs including cervical, uterine, oviductal, prostatic, and seminal vesicular secretory products. Thus, the field of potential targets would seem to be extensive. However, there are criteria that should be applied to this universe of possible candidates, in order to identify those suitable for development of immunocontraceptives for clinical use (Griffin 1990). These criteria are discussed immediately below; their specifications narrow the range of targets considerably.
Criteria for Candidate Prioritization
Relevance. An antifertility immunogen should be based on a molecule involved in and essential for the process of reproduction, so that immunological neutralization, blockage, or removal of the antigen will cause infertility.
Specificity. In order to avoid complications, it is particularly important that the molecules selected for immunocontraceptive development exhibit tissue specificity, that is, that they are secreted or expressed only in the intended target tissue. This issue should be addressed at the immunogen discovery phase rather than later during toxicological and teratological safety studies, so as to avoid unnecessary effort and expense studying unsuitable immunogens. The potential complications of immunization with contraceptive vaccines include: (1) immediate hypersensitivity and anaphylactic responses; (2) delayed hypersensitivity responses; and (3) autoimmune response and autoimmune disease owing to immune reaction with endogenous antigens of the immunized subjects (Tung 1986). Many of these concerns may be obviated by selection of non-crossreactive immunogens specific to the reproductive target tissues.
Existence of a Homologous Animal Model. The target antigen should, ideally, have an identical or closely related form and function in an animal model in which immunogenicity, safety, and efficacy studies can be carried out.
Accessibility. The target antigen must be accessible to antibody or sensitized lymphocytes in the circulation and/or lumen of the genital tract of the recipient. The target antigen must therefore be secreted by the target cells and/or appear displayed on the surface membrane or glycocalyx of the target cells. In the case of sperm, which undergo remodeling of the sperm surface after the acrosome reaction, this criterion includes the inner-acrosomal membrane, which is the limiting membrane on the anterior portion of the sperm head after the completion of the acrosome reaction. ''Coating antigens" derived from the male sex accessory glands might also be included.
Amplitude. The concentration of neutralizing antibodies induced by immunization is important. Not only must sufficient antibody be induced, but it must be induced in the right place and at the right time. The amount of antibody must be sufficient to neutralize the target antigen to achieve contraceptive efficacy as well as safety. Thus knowledge of the concentration of the target molecule is important, as is information on fluctuations in its concentration. The neutralizing concentrations of antibody must be available at the appropriate location in the circulation or lumen of the reproductive tract at the time when the antigen is accessible. Negative effects of too high an antibody response must also be avoided. Insoluble immune complex formation in the circulation in vascular sites may provoke immunopathology (immune complex diseases). Immediate and delayed hypersensitivity reactions may occur in the reproductive organs. Thus, a balance must be struck between reaching an amplitude of antibody or immune effector cells necessary to achieve contraceptive efficacy while, at the same time, avoiding inflammatory complications.
The immunogens chosen for incorporation into an immunocontraceptive must include epitopes recognized by most, if not all, recipients. In an outbred population such as the human race, variability in host responsiveness to any single epitope is likely to be large. A given immunogen may be effective in one group but ineffective in another. This implies that a formulation that includes a variety of immunogens to which all groups respond has the best likelihood of achieving high contraceptive efficacy.
Transience. Reproductive antigens that are present only transiently or in low concentrations have some advantages: (1) The risk of chronic formation of immune complexes is minimized; (2) The immune response will be called into effect only when the antigen is present; (3) Excess antigen is not likely to overwhelm available antibody.
Synthetic Capability. Capacity to synthesize the immunogens in large quantities under good laboratory and good manufacturing practices is an important practical requirement for selecting molecules for clinical testing. Use of the natural tissues as starting materials for immunocontraceptive production is possible; however, isolation processes from natural sources are much more difficult to control from the standpoint of safety than are standard synthetic or genetic engineering procedures. For example, isolating a sperm antigen from human
semen would require safety assurances that viruses such as HIV and hepatitis B were absent from the starting semen preparation. Thus, recombinant proteins and synthetic peptides offer important advantages.
The Timing of Intervention
Pre- and Postfertilization Immunocontraception
Based on assumptions regarding their hypothesized mechanism of action, immunocontraceptives based on antigens associated with the sperm, egg, or early conceptus are frequently referred to as pre- or postfertilization immunocontraceptives, although such designations are largely speculative since the mechanism of action of any immunocontraceptive is only partially understood. The basic hypotheses are, first, that an antibody to a sperm membrane component would act in the oviduct to prevent fertilization or to agglutinate or lyse sperm during passage through the female tract; in other words, immunocontraception would take place prior to fertilization, that is, a prefertilization method. In contrast, an immunocontraceptive based on antibody to chorionic gonadotrophin, which is a product of the early embryo's syncytiotrophoblast, would act within the uterus to induce early abortion and would therefore be a postfertilization intervention.
Gender Focus
The emphasis in research to date has been on methods of immunizing women, either to prevent fertilization or early pregnancy. This focus has been a function of historical developments in reproductive biology in which detailed knowledge about female reproductive biology has preceded understandings about the male reproductive system. This is partly because women are most immediately concerned with conception and its implications and partly for a range of cultural reasons.
Biologic considerations also come into play. For example, both men and women would seem to be equal candidates for immunocontraceptive approaches using formulations based on sperm antigens, since natural models of infertility due to antisperm antibodies occur in both males and females. In males, antisperm antibodies arise following vasectomy in all animal models studied and in human males (Linnet 1983). Indeed, the lowered incidence in male fertility observed following vasectomy reversal (vasovasostomy or reasnatomosis) may be due to immunologic mechanisms. Women also exhibit antisperm antibodies and, in some women with unexplained infertility, levels of these antisperm antibodies may be quite high (Mathur et al. 1981). Yet, despite the capability of both men and women to develop antisperm antibodies, development of amounts of such antibody sufficient to reduce fertility in the female reproductive tract appears much more feasible, simply by virtue of numbers. The numbers of sperm in the
oviduct at the time of fertilization may only be in the tens, possibly hundreds; the number of sperm requiring immunologic interdiction in the male tract is on the order of 108 to 109. Thus, generating contraceptive titers of antisperm antibody in the oviduct is a less challenging prospect than producing a comparable effect in the male reproductive tract.
Antigens with Contraceptive Potential
The molecules that have been considered as contraceptive immunogens fall into the following groups:
- Reproductive hormones. This group consists of two basic, somewhat overlapping subsets:
- A group which includes the hypothalamic gonadotropin-releasing hormone, GnRH; the pituitary gonadotrophins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH); and the gonadal steroids; and
- A group which includes hormones produced by the conceptus such as human chorionic gonadotrophin (hCG), a glycoprotein hormone produced by the placental syncytiotrophoblast.
Reproductive Hormones as Antigens
GnRH-based Immunogens for Both Males and Females
The decapeptide gonadotrophin-releasing hormone (GnRH) is synthesized in the hypothalamus. It regulates the synthesis of the pituitary gonadotrophins, LH and FSH, that are the key regulators of spermatogenesis and oogenesis. LH acts on Leydig cells and luteal cells to regulate steroid synthesis. FSH acts on the seminiferous epithelium to stimulate spermatogenesis at puberty in males, and on the theca and granulosa cells to stimulate follicular maturation in females. The
role of FSH during the later stages of sexual life in the male is less well understood, but it appears to be important for spermatogonial proliferation.
A GnRH formulation offers a particularly promising approach to a reversible male contraceptive. The mechanism of action of such a formulation posits the following cascade of events: a rise in circulating anti-GnRH antibodies would reduce levels of GnRH, LH, and FSH; levels would then fall, steroid production would subsequently decline, and spermatogenesis would cease (Bremner et al. 1986). Secondary effects, including decrease in testicular size, possible decline in libido, and alterations in secondary sexual characteristics, are practical drawbacks to the commercial acceptance of this formulation. Current GnRH formulations have coupled this decapeptide to various carriers, including tetanus toxoid, and have found that, in rats inoculated with GnRH-TT immunogen on five occasions over a 15-week period, there was atrophy of the testes and accessory sex organs (prostate and seminal vesicles), decreased testosterone as antibodies to GnRH increase, suppressed spermatogenesis, and a decline in libido (Ladd et al. 1989). At the same time, because the formulation shrinks male sex accessory glands, it has been viewed as a possible treatment for prostatic carcinoma or benign prostatic hyperplasia and is being tested in men with prostate cancer under Population Council sponsorship at The University of Texas at San Antonio; similar studies are being conducted through the National Institute of Immunology in Delhi, India.
Thus, to become marketable as a contraceptive for males, a GnRH formulation would require supplementation with testosterone to maintain libido and avoid alterations in secondary sexual characteristics (Ladd et al. 1988). Key to possible acceptance will be the ability to deliver correct doses of supplementary androgen to achieve those objectives. In addition, because administration of exogenous androgen is well known for decreasing LH and FSH through the pituitary feedback loop, it could also reinforce contraceptive action by depressing LH and FSH and inhibiting spermatogenesis. Development of slow-release formulations of androgen, possibly coupled to transdermal delivery, may constitute an important parallel technology that is necessary for success in developing a GnRH immunocontraceptive for men. Reversibility will also be pivotal and appears to be possible. Immunized rats supplemented with testosterone display normal sexual behavior yet still show 100 percent infertility; after cessation of immunization, antibody titers have gradually fallen, testosterone production returned, and testis and accessory gland weights have increased (Bremner et al. 1986; Ladd et al. 1989).
The GnRH approach has also been tested on female primates, whose menstrual cyclicity has been impaired in response to administration of a GnRH formulation. However, because of an accompanying decline in estrogen synthesis which could be associated with eventual osteoporosis, application of this formulation would be inappropriate for some percentage of women (Talwar et al. 1993). Use of a GnRH formulation has also been contemplated for use during the
postpartum period, the objective being to prolong the anovulatory period that occurs during the period of postpartum amenorrhea that is induced by lactation. Researchers have concluded that concerns about possible effects on infants of anti-GnRH antibodies being passively transferred through breast milk have largely been laid to rest, since there appears to be no active uptake of these antibodies by the infant gut. A phase I human immunogenicity trial of a GnRHDT (diphtheria toxoid) immunogen is currently under way in postpartum women at two centers in India.
FSH Immunogen for Males
Immunization of male monkeys with sheep FSH has led to a high degree of contraceptive efficacy (Moudgal et al. 1988b). The immunogen in this case was isolated from natural sources (sheep pituitaries) and formulated with aluminum hydroxide gel as an adjuvant. Doses of 1.0, 0.3, 0.1, and 0.1 mg were given at days 1, 20, 40, and 70, followed by a booster 100 days thereafter (Moudgal and Aravindan 1993). A 70 percent reduction in sperm count (oligospermia) was observed three months after the start of injection. Flow cytometry of testicular cells harvested on day 80 indicated that the primary spermatocyte population had been reduced by >90 percent, the spermatogonial population by 32 percent, and the round spermatid population by 53 percent; testis weight decreased by only 10 percent (Moudgal et al. 1988a, 1988b, 1992). Fertility testing in monkeys was started as early as 90 days after immunization and ten immunized males, fertile prior to immunization, were cohabitated with at least five females each on days 9 to 14 of the menstrual cycle. The efficacy of the FSH formulation was excellent: None of 52 mated females became pregnant (Moudgal et al. 1992). Furthermore, toxicity studies in rats and monkeys have shown no complications, even in monkeys immunized for over five years, and the formulation's contraceptive effect is reversible; following cessation of boosting and fall in antibody titers, sperm counts gradually returned to normal and 9 out of 10 monkeys regained fertility (Moudgal and Aravindan 1993).
Among the possible hormone-based male immunocontraceptives that have undergone trial, the FSH formulation is the only one that does not require supplementation with exogenous androgens. Although the formulation has no effect on androgen-dependent libido and should therefore be at least theoretically acceptable to the human male, and although it does not produce azoospermia (total absence of sperm), the formulation does cause acute oligospermia (reduced sperm numbers), a state that is compatible with infertility, and the sperm that are produced in immunized animals are not only reduced in numbers but are immature and of poor quality.
As indicated, the FSH formulation is currently made with hormone obtained from sheep pituitaries. Future work on this contraceptive immunogen is likely to move toward a formula based on recombinant proteins or synthetic peptides
corresponding to epitopes of human or primate FSH. As an avenue for a commercially acceptable, effective, reversible male contraceptive, the FSH formulation is one of the more promising possibilities.
Immunogens of the Early Conceptus
The human chorionic gonadotrophin (hCG) formulations are the most extensively studied, are furthest along the development pathway, and serve as a model for a postfertilization immunocontraceptive. The hCG hormone is a glycoprotein produced by the placental trophoblast. It has a molecular weight of approximately 38 kd and a carbohydrate content of 30 percent. The hCG molecule is comprised of two dissimilar, noncovalently linked subunits designated a and ß (Alexander 1992). Because the hormones FSH, LH, thyroid-stimulating hormone (TSH), and hCG have a similar a subunit, antibodies to the whole hCG molecule crossreact with these hormones (Kharat et al. 1990). As a consequence, formulations based on whole hCG are open to possible immunopathologic complications resulting from impairment of FSH, LH, or TSH functions or immune complex formation. Because the ß subunit is distinctive in this regard, a formulation based upon this subunit offers a possibility of greater specificity.
The hCG hormone responds to several criteria for a suitable contraceptive target. It is made in appreciable amounts in a physiologically active form only in pregnancy (specificity) and in trophoblastic or nontrophoblastic cancers. Its synthesis starts at the preimplantation stage of blastocyst development, being detectable on day 21 of the menstrual cycle, and increases rapidly after implantation as the mass of the trophoblast expands (accessibility) (Talwar et al. 1993).
The precise mechanism(s) by which an hCG formulation might exert antifertility effects are unknown, but there are several possibilities. First, because the hCG hormone is the stimulus for continued maintenance of the corpus luteum and progesterone production which, in turn, sustains the endometrium in a receptive state for receiving the embryo, it may play a direct role in implantation. In other words, not only does hCG act early but it is vital for pregnancy to occur. Antibodies to hCG that would be induced by an hCG formulation might inactivate the hormone's biologic activity and thereby interrupt the ßhCG-dependent events of corpus luteum maintenance and endometrial receptivity, in so doing interrupting implantation.
Another possible mechanism of action is by direct immune attack on the hCG-producing cells of the peri-implantation blastocyst (Stevens 1988). Unlike steroid contraception, which blocks ovulation and replaces natural steroids with synthetic compounds, ovulation and sex steroid synthesis should continue when hCG formulations are used.
The hCG Formulation from Natural Sources Pregnancy urine is the source of the hCG that has been used in a formulation being tested at the National Institute
of Immunology in India (Talwar et al. 1981, 1990, 1994). Since women are generally tolerant to the ß subunit of hCG, in this formulation the ß subunit has been chemically linked to a foreign carrier molecule, tetanus toxoid, to mobilize T helper cell function. Women immunized with this ßhCG-TT conjugate develop antibodies to both hCG and tetanus toxoid, so that the formulation confers protection against tetanus. When the immunogen is administered four times at two-week intervals, menstrual regularity is maintained, ovulation is undisturbed, uterine biopsies appear to remain normal, and titers are sustained for a year, after which the response declines to near zero levels between 300 and 500 days following immunization. Immune complexes formed as a result of the presence of antibody appear to be appropriately handled by the body's scavenging system. Studies in hyperimmune monkeys showed that no immune deposits were detected in the pituitaries, choroid plexus, or kidneys after repeated challenge with hCG.
At the same time, because the antibody titers achieved with this vaccine were highly variable, several modifications to the formula have been made. The first injection now contains an adjuvant, SPLPS (sodium phthalyl derivative of lipopolysaccharide), which boosts antibody response. Attempts were also made to enhance the formulation's immunogenicity by (1) making a mixture of the ß subunit of ovine LH conjugated to diphtheria toxin (ßLH-DT) and the hCG-TT, or (2) by forming a heterospecies dimer (HSD) consisting of the ß subunit of ovine LH and the ß subunit of hCG.
The HSD formulation proved to be the most immunogenic in humans, and phase II trials have been conducted with this formulation at a dose of 300 µg per injection (Talwar et al. 1992; Talwar et al. 1994). The formulation was highly effective in those women who had reached sustained serum concentrations of anti-hCG antibody of at least 50 ng/ml (Talwar et al. 1994). However, these protective levels are only reached in 80 percent of the women inoculated. Thus, 20 percent of women did not achieve contraceptive protection with the current formulation. Another drawback is the lag that occurs after a woman is first immunized but before titers reach protective levels. Women receive three injections at six-week intervals; a three-month period is required for protection to be conferred, so that there is a window at the outset of the therapy during which a woman is unprotected.
The current hCG formulation that is derived from natural materials appears safe, with no major complications observed and with ovulation continuing to occur in inoculated women. With improvements in delivering this immunogen, either through use of slow-release biodegradable microspheres or administration of live recombinant antigen through vaccine viruses (Talwar et al. 1992, 1993), it is possible the formulation might achieve levels of protection that could make it a commercially viable product. The results from human trial of this hCG formulation (Talwar et al. 1994) are of general importance to the field of immunocontraceptives since they demonstrate for the first time "proof of prin-
ciple": that is, if sufficient levels of antibody are generated to a defined reproductive antigen, a sustained period of contraception occurs and, as the antibody titers decline to preimmunization levels, fertility is restored.
A Synthetic hCG Peptide Formulation This formulation is being developed under sponsorship of the World Health Organization (Jones et al. 1988). It has already been tested in women for immunogenicity (phase I) (Jones 1990), and phase II efficacy trials in humans were under way as of 1995.
Because the C-terminal region of ß hCG is unique to this subunit and because antibodies directed to it do not crossreact with LH, considerable effort has been directed toward development of a peptide formulation based on the C terminus. A series of peptides have been synthesized representing sequences from 6 to 47 amino acids of the C terminus. Since peptides of fewer than 20 amino acids showed relatively lower immunogenicity and induced antibodies incapable of neutralizing hCG (Stevens 1988) and peptides of 35 amino acids or more in length consistently produced antibodies capable of neutralizing hCG, only peptides of this length or longer have been utilized in subsequent development. These peptides require coupling to a carrier to elicit the optimum antibody response and bovine gamma globulin, tetanus toxoid, and diphtheria toxin have all been tested as potential vehicles.
Data on actively immunized marmosets (Hearn et al. 1988) and baboons (Stevens 1976) showed an hCG peptide formulation to be capable of blocking fertility at an early stage of pregnancy, with no discernible alterations of menstrual cycling. In baboons (n = 15) receiving a control injection of diphtheria toxin alone, 14 of 20 ovulatory menstrual cycles resulted in pregnancy, for a 70 percent fertility rate. In baboons inoculated with the hCG peptide coupled to diphtheria toxin, 2 of 44 menstrual cycles resulted in pregnancy, a 4.6 percent fertility rate. Thus, this hCG formulation was 95 percent efficacious. These studies were conducted over three menstrual cycles following completion of the immunization regimen (Stevens 1979).
The hCG peptide formulation that has now progressed to a phase I human clinical trial for immunogenicity is composed of a synthetic oligopeptide corresponding to the amino acid sequence 109-145 of the C terminus of ßhCG (Jones et al. 1988). The peptide is conjugated to diphtheria toxoid to form a haptencarrier complex and a water-soluble adjuvant, muramyl dipeptide (MDP), is incorporated into the aqueous phase of the formula. Prior to injection, a saline-oil emulsion is created, with an oil phase consisting of 4 parts squalene to 1 part mannide monooleate as an emulsifying agent (Stevens et al. 1981, 1990).
To date, 30 premenopausal human female subjects, already surgically sterilized prior to the study, were inoculated with this formulation (Jones et al. 1988). Subjects were assigned to five dosage groups of six subjects each, with each subject receiving a variable dose intramuscularly, injected twice six weeks apart, with the highest dose being 1 mg peptide in 0.5 ml of vehicle. The study revealed
that significant levels of antibodies to hCG were attained in the female subjects (Jones 1990). Animal teratology studies are under way with this formulation, and a phase II efficacy study in humans was begun in Sweden. However, because of problems with both vehicle and adjuvant, as of this writing, trials were suspended. Still, this synthetic peptide immunocontraceptive bears close watching.
Egg-surface Antigens
At least three glycoproteins, ZP1, ZP2, and ZP3, have been identified in the zona pellucida of pig, human, mouse, and rabbit eggs, homologies among the zona proteins in these species having been identified (Hedrick 1993). Pig zona pellucida has been injected into mice (Millar et al. 1989), dogs (Mahi-Brown et al. 1988), rabbits (Skinner et al. 1984), and monkeys (Sacco et al. 1990), and in each species significant inhibitory effects on fertility have been observed.
For example, immunization of 50 female squirrel monkeys with 200 µg each of ZP3 (isolated from pig zona), in conjunction with Freund's adjuvant, induced significant antibody titers. The monkeys were followed for four breeding seasons. For the first two years, the group immunized with ZP3 had no pregnancies, while the controls had a 16 percent fertility rate. In the third and fourth breeding seasons, 6 percent of the immunized group became pregnant versus a 21 percent pregnancy rate in controls. The monkeys showed initial disturbances in ovarian function as measured by estradiol and progesterone assays. However, by 10-15 months postimmunization, normal hormonal function had returned as assessed by both hormone determinations and histological observation of normal folliculogenesis (Sacco et al. 1990). There have been similar contraceptive effects in marmosets immunized with porcine ZP3; however, induction of high-titer antibodies to the zona were invariably associated with the appearance of ovarian pathology and depletion of the primordial follicle pool (Paterson et al. 1992).
Severe ovarian disease (Taguchi et al. 1980a,b; Tung et al. 1987), termed autoimmune oophoritis, has been induced by injecting zona proteins in other models, particularly rabbit (Skinner et al. 1984) and mouse (Rhim et al. 1992). In rabbits, injection of pig zona pellucida caused loss of growing follicles, a failure to ovulate in response to hCG, and increased levels of FSH and LH in serum compared to control animals (Skinner et al. 1984). In mice, a synthetic peptide corresponding to amino acids 328--342 of ZP3 has been shown to induce autoimmune oophoritis (Rhim et al. 1992).
These findings suggest that a zona-based formulation might induce immunologic attack on many of the eggs in the ovary and, in effect, induce a type of premature ovarian failure, in other words, induced menopause, a finding that has somewhat diminished enthusiasm for zona-based immunocontraceptives. Still, in theory, zona antibodies could exert their potential contraceptive effects through two mechanisms: (1) inhibition of sperm-zona interaction, as demonstrated by in vitro and passive immunization experiments (East et al. 1984); and (2) induction
of autoimmune oophoritis. If antibodies could be developed that would act only to block fertilization without causing oophoritis, the path to an immunocontraceptive based on the zona pellucida would clear.
It is in this area that the research challenges lie. One possible approach is to define epitopes that induce antibodies without inducing T cell responses. Studies with peptides derived from the zona proteins may offer some experimental insights into those B cell epitopes that may be incorporated into an immunocontraceptive but not cause ovarian disease.
Sperm-surface Antigens
Background
Immunizing women with sperm to render them infertile is not a new concept. It was proposed many years ago (Baskin 1932; Rosenfield 1926) and a U.S. patent (2,103,240) was awarded to Baskin for a nonspecific spermatoxic vaccine and for the process involved in its production (Katsh 1959). Despite 12 claims made in the patent, there is no record in the literature as to whether the patented product achieved the objectives stated. Immunization with human seminal plasma proteins or diluted semen resulted in anaphylactic responses in several women, usually after the seventh to tenth injection; inoculation with sperm antigen did not (Otani et al. 1971). Similar hypersensitivity reactions to seminal plasma antigens have been recorded elsewhere (Bernstein et al. 1981).
In animal models, injection of female rabbits with sperm membrane extracts has been shown to significantly reduce fertility (Menge et al. 1979). Rabbit sperm precoated with specific monoclonal antibodies prior to insemination also have shown reduced fertility (Naz et al. 1984). Kummerfeld and Foote achieved a 98 percent suppression of fertility in female rabbits injected with ejaculated, epididymalor amylase-treated sperm (Kummerfeld and Foote 1976); similarly, virtually complete fertility suppression was obtained in female rabbits after immunization with homologous sperm (Muñoz and Menge 1978).
With crude complex sperm extracts demonstrating some contraceptive efficacy, the principal research effort has been directed subsequently toward identifying and characterizing individual sperm molecules that have similar antifertility effects. A number of antigens have been described that first appear during spermatogenesis and then persist on the mature sperm. As a class, these antigens are often referred to as "intrinsic differentiation antigens," as opposed to extrinsic or coating antigens of sperm. Such differentiation antigens often fulfill the important criterion of tissue specificity discussed above. Several promising sperm antigens are discussed below in connection with possible mechanisms of action induced by a sperm-based immunocontraceptive.
The overall purpose of studies of sperm-based molecules is to develop an immunocontraceptive that will induce antibodies in the female reproductive tract
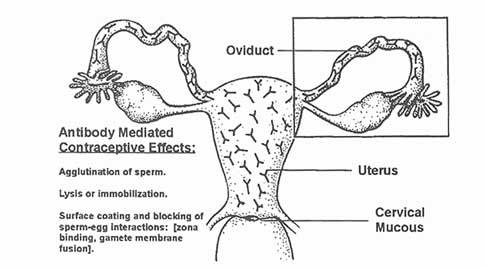
at sufficient levels to block fertilization. It is envisioned that antibodies developed by this formulation will act to agglutinate, immobilize, or coat the sperm in the oviduct, uterus, cervix, or vagina (see Figure C-1). Thus, the formulation will exert its effects before the fertilization event, as a ''prefertilization contraceptive." This contraceptive strategy is deemed to have the highest likelihood of widespread acceptance by individuals of varied religious, ethical, and political persuasions.
Multideterminant Sperm Immunocontraceptives
An ideal sperm immunocontraceptive might contain sperm-specific immunogens that would induce antibodies to all the sperm-surface domains that are accessible to antibody, that is, the sperm head, midpiece, and tail plasmalemma, as well as the inner-acrosomal membrane that forms a major part of the anterior surface of the sperm head following the acrosome reaction. This concept guides the work in this field overall and suggests that there is an opportunity for various groups, each working on individual immunogens, to eventually combine their efforts to create the "ultimate immunocontraceptive."
As sperm progress through the female reproductive tract, immune effectors in female secretions may exert contraceptive effects that act in various regions (see Figure C- 1). There are numerous possibilities. Antisperm antibodies present
in cervical mucus might agglutinate or lyse sperm in the vagina prior to sperm passage through the cervix. Sperm, coated with surface antibody, might be prevented from swimming through and penetrating cervical mucus, a condition often referred to clinically as the "shaking phenomenon" that is well described in the literature on sperm-cervical mucus interaction. Another possibility is that sperm might either be trapped or lysed by antibodies present in uterine secretions so that they are prevented from reaching the oviduct or, perhaps, antibody coating of sperm might interfere with the critical process of capacitation. Antibodies present in oviductal fluid might likewise bind to sperm antigens and prevent the key steps in fertilization that occur in the oviduct. Finally, antibodies to spermsurface domains, if present in oviductal fluids or embedded in the zona pellucida (which is permeable to immunoglobulins) might coat key sperm receptors at any of the key stages of sperm-egg interaction: penetration of the cumulus mass, zona binding, capacitation, induction of the acrosome reaction, shedding of the acrosomal ghost, penetration of the zona pellucida, binding to the oolemma, or internalization of the spermatozoon by the egg (see Figure C-2 and Figure C-3).
LDH-C4 LDH-C4 (also referred to as LDH-X in older literature) is an isoenzyme of lactate dehydrogenase, a glycolytic enzyme that is found only in male
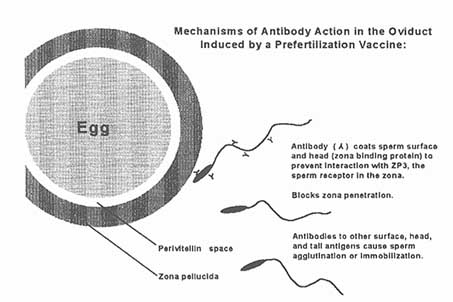
Figure C-2
Possible mechanism of antibody action to a sperm-surface antigen where antibody coats the sperm head to prevent sperm binding to the zona pellucida and blocks zona penetration.
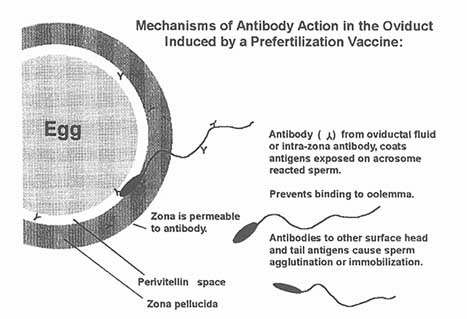
Figure C-3
Possible mechanism of antibody action to a sperm antigen which is on the acrosomal membrane. Antibody in the zona pellucida and oviduct fluid coats the sperm antigen, preventing interaction of the sperm with the oolema.
germ cells, thus fulfilling the important criterion of tissue specificity (Goldberg 1977, 1975; Wheat and Goldberg 1983). The complete amino acid sequence for human LDH-C4 has been deduced from cDNAs (Millan et al. 1987); in addition to being one of the best characterized human sperm proteins, it is the sperm antigen that has undergone the most extensive animal and primate testing (Lee et al. 1982; Hogrefe et al. 1987, 1989; Wheat and Goldberg 1985). LDH-C4 first appears with the onset of puberty during prophase of the first meiotic division of the primary spermatocyte. The protein is found within the mature sperm in association with the midpiece, where it appears localized both to the mitochondrial sheath and, to a lesser extent, to plasma membrane overlying the midpiece and principal piece. As far as we know, LDH-C4 is never expressed in female tissues and because of its stage-specific expression in primary spermatocytes, it is also sequestered from the immune system of the male by the blood-testis barrier.
In considering the mechanism of action of an LDH-C4 antigen, the following considerations are germane. Although primarily an intracellular constituent, LDH-C4 is presumably accessible to immune attack owing to its presence to some degree on the plasma membrane overlying the midpiece. Antibodies to LDH-C4 partially suppress fertility in female mice and rabbits, the antibodies cause some agglutination, and in some cases complement mediated lysis. Both
auto- and isoimmunogenic responses to LDH-C4 have been noted in mice and rabbits (Goldberg 1973; Lerum and Goldberg 1974). Oviductal fluids are a transudate of serum. Although the contraceptive mechanism is not entirely known, what seems to happen is that high titers of circulating antibody to LDHC4 result in quantities of antibody in the oviduct that are sufficient to agglutinate or lyse oviductal sperm, thus preventing fertilization. Ovulatory cycles in these animal models were not affected by immunization, nor were other deleterious side effects noted.
A formulation consisting of purified mouse LDH-C4 was injected into female baboons, which subsequently underwent fertility testing. In the experimental group, 8 of 30 matings resulted in pregnancy for a 27 percent fertility rate; 21 of 29 matings in the control group resulted in pregnancy, a 72 percent fertility rate. A second formulation based on mouse LDH-C4, consisting of a synthetic peptide (amino acids 5-16, coupled to diphtheria toxoid), has also been shown to reduce fertility in female baboons. Ten of 14 baboons in the control group became pregnant (71 percent fertility), compared to 3 of 14 baboons in the experimental group, a 21 percent fertility rate (Goldberg 1990).
More recently, a formulation based on another human LDH-C4 sequence (amino acids 9-20) was fertility-tested in female baboons. In the control group 8 of 15 animals became pregnant (53 percent), compared to 2 out of 14 animals in the experimental group (15 percent). If the pregnancy rate is scored as the number of pregnancies occurring per number of cycles of mating, the controls had 8 pregnancies in 34 cycles of mating (23 percent), whereas the experimental group had 2 pregnancies out of 45 mated cycles (4 percent). The contraceptive effect was reversed within one year after the last immunization (O'Hern et al. 1995), nor have there been detectable side effects with LDH-C 4 immunization in baboons in any of the studies to date (O'Hern et al. 1995). The thinking is that, since serum antibody levels were not directly correlated with infertility in these studies, cell-mediated immunity rather than humoral immunity might be the critical effector mechanism.
It is clear that an immunocontraceptive based upon the sperm-derived LDHC4 immunogen has a contraceptive effect in primates, although not currently of a magnitude sufficient to suggest a product that is competitive with oral contraceptives. Nevertheless, this antigen is a likely candidate for inclusion in a mixture of immunogens to form a multideterminant immunocontraceptive. Because LDHC4 has undergone primate tests and has repeatedly shown that a sperm antigen can reduce fertility, it is serving as the model for other sperm antigen development programs. A new formulation of the hC9-20 peptide from LDH-C4 has been prepared by coupling to a promiscuous T cell epitope from tetanus toxoid; in 1995, fertility studies were under way in animals (monkeys).
RSA-1 The RSA-1 differentiation antigen appears on the surface of rabbit pachytene spermatocytes and on the surface of rabbit sperm. This molecule is a
potent autoantigen, and polyclonal as well as monoclonal reagents to this molecule inhibit fertilization. RSA-1 fulfills several criteria for a zona pellucida binding protein (O'Rand et al. 1984, 1988, 1993a).
A family of RSA peptides in the 14-18 kDa range has been observed and the human homologue (Hspa 18) of the rabbit sperm autoantigen RSA has been cloned (Lea et al. 1993). The cloned molecule, termed hSp17 to distinguish it from the native molecule, is 151 amino acids in length, with a predicted mass of 17,534 daltons. The human Sp17 sequence is 76.7 percent identical to a rabbit 17-kd sperm autoantigen, termed RSA. The amino terminus hSp17 has 43 percent identity with a known protein from human testis, the cAMP-dependent, protein kinase, type II a regulatory subunit.
Human Sp 17 has been studied by Northern analysis and all data indicate that it is testis specific. A recombinant hSp17 has been generated and an antisera made to the recombinant protein (rec-hSp17). This anti-rec hSP17 recognizes a doublet of 29 kDa on Western blots of human sperm extracts. The antigen has been localized by immunofluorescence on both the human sperm head and tail. Human Sp17 binds fucoidan and human zona with saturation kinetics. Further, hSp17 is recognized by sera from vasectomized men who have antisperm antibody titers, indicating that hSpa18 is a human sperm autoantigen (Lea et al. 1993).
The mouse homologue of hSp17 has also been cloned, sequenced, and expressed. Mouse Sp17 is 74 percent identical to rabbit and 72 percent identical to human. When female mice are immunized with mSp17, they make an antisera that reacts with a 24-kDa band on Western blots. Fertility testing in mice with a peptide (plOG) derived from the rabbit autoantigen RSA reduced fertility by 80 percent in those animals that developed high titers (O'Rand et al. 1993b). Both human and mouse recombinant Sp17 have been synthesized and tested in mice, and a significant decrease in fertility has been observed (Yamasaki et al. 1995).
Tcte-1 A recently cloned mouse gene within the T complex appears to encode a sperm-specific protein known as Tcte-1 (Sarvetnick et al. 1990). The Tcte-1 gene product is a 56-kD protein that possesses certain properties of an eggbinding protein including interaction with ZP3. Antibodies to Tcte-1 bind principally to the plasma membrane overlying the head of acrosome-intact mouse sperm and compete with mouse ZP3 for binding to the sperm head. In addition, antibodies to Tcte- 1 inhibit the binding of mouse sperm to mouse eggs (L. Silver, personal communication, 1995). Since Tcte-1 is a candidate for an egg-binding protein on the sperm head, a Tcte-1 formulation could inhibit fertilization if sufficient antibodies were generated in the oviduct to block this protein or induce sperm lysis. Because the product of the Tcte-1 gene is the same molecular mass as sp56 (a protein previously identified as an egg-binding protein by photo affinity labeling using purified mouse ZP3 and the Denny-Jaffee reagent [Bleil et al. 1988]), there is considerable interest in Tcte-1 and it is considered promising for
immunocontraceptive development. Immunogenicity and fertility tests of recombinant forms of Tcte- 1 in mice could take place in the not too distant future.
PH-20 PH-20 is an integral membrane protein of 64 kDa, present on both the plasma membrane and inner-acrosomal membrane of guinea pig sperm. The protein is anchored in the plasma membrane by phosphatidylinositol and undergoes proteolytic processing during the acrosome reaction (Phelps et al. 1988). Some monoclonal antibodies to PH-20 block sperm interaction with the zona pellucida, suggesting that PH-20 is a zona pellucida binding protein (Primakoff et al. 1985).
PH-20 has provided the field of sperm-based immunocontraceptives with a compelling model for continuing the quest for a human immunocontraceptive based on sperm components (Primakoff et al. 1988). Immunization of female guinea pigs with PH-20 purified from the natural source (sperm) has provided remarkable evidence that full but reversible contraception can be achieved. Two injections of PH-20 were given over a one-month period and the animals were mated two months after the first injection. Of 25 immunized females, none became pregnant, a 100 percent contraceptive effect; in contrast, out of 36 controls, 34 became pregnant (94 percent fertility) (Primakoff et al. 1988). No further immunizations were given to the female guinea pigs and they continued to be mated. By 6 months after the first injection, 17 percent of the animals conceived; by 9-11 months, 46 percent had regained fertility (Primakoff et al. 1988). PH-20 cDNAs have been cloned for both guinea pig and human, and primate testing of the PH-20 formulation to determine its immunogenicity is likely in 1996. Because of the 100 percent contraceptive efficacy shown in the guinea pig model, the outcome of primate trials of PH-20 are awaited with high expectations.
At the same time, in view of a finding of hyaluronidase activity associated with the molecule (Gmachl et al. 1993), some caution with regard to PH-20's tissue-specificity is appropriate, since this finding raises the question as to whether PH-20 or PH-20 domains are present in other tissues that express hyaluronidase. An extensive RT-PCR study of PH-20's tissue-specificity, including bone marrow and circulating blood elements, may be warranted to confirm--or not confirm-the molecule's testis-specificity.
SP-10 In 1985 and 1986, the World Health Organization sponsored workshops to identify sperm antigens that could function as potential immunogens (Anderson et al. 1987). The human sperm protein SP-10 was identified as a potential contraceptive immunogen, both on the basis of its apparent germ cell specificity, as well as on the basis of functional assays that indicated that a monoclonal antibody (MHS-10) specific to SP-10 was able to inhibit sperm penetration in a hamster egg penetration assay (Anderson et al. 1987).
Electron microscopic immunocytochemical studies using the MHS- 10 mono-
clonal antibody have indicated that the human SP-10 protein is localized to the developing acrosome of round and elongating spermatids and remains associated with the acrosomal matrix and membranes of mature sperm (Herr et al. 1990a; Kurth et al. 1991). By immunofluorescence microscopy in intact human sperm, the SP-10 antigen is found to be distributed as a cap-shaped fluorescent pattern on the anterior sperm head. After induction of the acrosome reaction with ionophore, SP-10 remains associated with the inner-acrosomal membrane and the equatorial segment, where it displays immunofluorescent images of a faint acrosomal cap or a fluorescent equatorial bar on the acrosome-reacted sperm (Herr et al. 1990b). This finding is relevant to the hypothesized mechanism of action of an SP-10 antigen, since the equatorial segment and postacrosomal regions of the sperm are thought to be involved in the initial interaction of the sperm surface with the egg plasma membrane.
The Inner-acrosomal Membrane as a Target for Immunocontraception
While considerable attention has been given to sperm plasmalemma antigens as targets for immunocontraception, less consideration has been given to components associated with the acrosomal membranes. Human sperm must undergo the acrosome reaction in order to penetrate the zona pellucida (Singer et al. 1985) so as to fuse with the egg plasma membrane (Sathananthan and Chen 1986). After the acrosome reaction, the acrosomal contents are externalized and the inner-acrosomal membrane then becomes the limiting membrane of the anterior sperm head (Nagae et al. 1986; Yudin et al. 1988). Most sperm observed on the human zona after one minute of binding in vitro have intact acrosomes, but the numbers of acrosome-reacted sperm on the zona rapidly increase with time as the zona acts as a potent inducer of the reaction (Cross et al. 1986). The combined events of zonal binding and acrosome reaction have been referred to as "primary binding," an event presumably mediated in humans by interaction between zonal proteins and a human equivalent of the ZP3 receptor (Bleil et al. 1988). Penetration through the zona is mediated by components released from the acrosome and exposed on the inner-acrosomal membrane; the latter process is referred to as "secondary binding." After penetration of the zona, initial fusion between sperm and egg in humans, as in other eutherian animals, is thought to occur between the plasma membrane over the equatorial segment of the sperm and the egg plasma membrane (Bedford et al. 1979). The SP-10 molecule resides within the acrosomal compartment in association with the acrosomal membranes and is observed on the inner-acrosomal membrane and equatorial segment of acrosome-reacted sperm. Thus, the proposed mechanism of contraceptive action of a SP-10 based immunocontraceptive is induction of sufficient titers of anti-SP-10 within the oviductal fluids to bind (agglutinate/lyse) the relatively few acrosome-reacted sperm found there, thus inhibiting sperm/egg interaction. (Included in this concept is the notion that the zona pellucida is permeable to immunoglobulins, and
that anti-SP-10 antibody of serum origin may infiltrate the zonal matrix during follicle maturation and remain embedded in the zona during oviductal passage.) SP-10 thus fulfills the criterion of transience noted at the beginning of this paper. The molecule is exposed during a key step of the fertilization process but remains within the acrosomal compartment until the acrosome reaction is initiated.
It is important to consider acrosomal antigens as two categories that have some significant differences in terms of the criteria listed at the beginning of this paper. The first group comprises antigens that have both a plasma membrane form and an intra-acrosomal form (e.g., PH-20 [hyaluronidase]); the second group comprises antigens that are restricted in the mature sperm to the acrosomal membranes and matrix (e.g., acrosin, SP-10). Proteins that have dual localizations both on the sperm plasma membrane and within the acrosome are accessible to antibody at many stages of sperm transport within the female tract. However, proteins that are restricted to the acrosomal membranes and matrix afford a much narrower window for immunologic interdiction and contraceptive action; such antigens within the acrosomal compartment become accessible to antibody as the acrosome reaction is initiated, fusion pores form, hybrid vesicles develop, and the acrosomal membranes and matrix are exposed to the surrounding medium.
Restricted localization of acrosomal proteins to the matrix and membranes of the acrosomal compartment has both advantages and disadvantages from the perspectives of contraceptive development. As an advantage, intra-acrosomal proteins (which remain sequestered until initiation of the acrosome reaction) may afford precise staging of immunizing action to events occurring after sperm-zona binding. In theory, this targeted timing of action should offer an attractive model for a prefertilization immunocontraceptive. On the other hand, it must be appreciated that, for inhibition of fertilization to occur in vivo, antibody to intra-acrosomal antigens must be generated at sufficient levels in the oviduct and/or zona pellucida to contact these antigens at the egg surface within the narrow window of time when these antigens "decloak" and are accessible. Antibody levels must be sufficient to block one or several stages in the cascade of events involving primary and secondary binding to the zona, induction of the acrosome reaction, shedding of the acrosomal ghost, penetration of the zona pellucida, and binding to the oolemma. Sperm plasma membrane antigens should undoubtedly receive the major focus of attention in research efforts; it is still too soon to tell whether intra-acrosomal antigens will have a place as one component of a multideterminant immunocontraceptive.
Other New Approaches
This review has touched on only a few of the potential immunogens that may eventually become the immunocontraceptive of the future. New methods and experimental approaches are emerging that offer unprecedented opportunities and challenges for new investigators in the immunocontraceptive arena.
New Delivery Systems
An important application of the human intra-acrosomal protein SP-10 model has been to insert the SP-10 gene into avirulent Salmonella for use in oral administration. The advent of recombinant DNA technology has enabled foreign genes to be expressed in Salmonella, thus converting this bacterium into an efficient delivery system for the induction of immune responses to the expressed antigens. Salmonella typhimurium strains with deletions of the adenylate cyclase (cya) and cyclic AMP receptor protein (crp) genes are avirulent and immunogenic, at the same time that they retain their ability to colonize gut-associated lymphoid tissue (GALT) and internal organs (Curtiss and Kelly 1987). Since S. typhimurium naturally invades and persists in GALT (Carter and Collins 1974), oral immunization with attenuated Salmonella expressing foreign antigens stimulates antigen-specific secretory, humoral, and cellular immune responses (for a review, see Curtiss et al. 1990). A cDNA sequence encoding human SP-10 was cloned on an asd+ vector and expressed to a high level in an avirulent Delta cya, Delta crp, and Delta asd strain of S. typhimurium. Oral immunization of female BALB/c mice with this recombinant Salmonella elicited high-titer anti-SP-10 IgG antibodies in serum and IgA antibodies in vaginal secretions. Anti-SP- 10 antibody titers could be further elevated by secondary and tertiary oral administrations of the recombinant Salmonella.
These quite recent results are the first indication that a gene encoding a human sperm antigen can be delivered in an oral immunogen vector and induce a secretory immune response against sperm-specific antibodies in the reproductive tract. This discovery could lead to the development of a simple, safe, efficient, and easy to use immunocontraceptive and opens the way for development of additional vectors that induce secretory immunity in the female reproductive tract (Srinivasan et al. 1995).
Novel Antigens
Because the sperm, egg surface, early embryo, and embryonic membranes represent unique differentiated cell types, it is likely that quite a few candidate immunogens will eventually be discovered that will be both specific for the gametes and/or conceptus (specificity) and will also be presented on the cell surface (accessibility). In the case of the sperm, for example, use of lectins and monoclonal antibodies has revealed antigens that cover the entire sperm surface or show a mosaic of topographically restricted domains. Some domains overlie anatomically and functionally distinct regions of cytoplasm; some antigens arise during spermatogenesis; some are secreted by the epididymis and bind to the sperm during epididymal maturation; and some antigens coat the sperm surface at the time of ejaculation when sperm mix with the secretions of the accessory glands. There may well be similar differentiation markers on the trophectoderm,
the hatched blastocyst, or the oolemma. Thus, a rich opportunity awaits new investigators who would seek to define and characterize novel surface antigens of the gametes and early conceptus, and to manipulate their encoding genes so as to produce recombinant immunogens.
Vectorial Labeling and 2-D Electrophoresis
One direction for research characterizing immunogen candidates is to create data bases from 2-D electrophoresis and to couple this method with vectorial labeling to define surface antigens on egg, sperm, or trophoblast (Naaby-Hansen et al. 1995). Presented with the problem of defining which antigens on the sperm might be appropriate for inclusion in an immunocontraceptive, knowledge of which proteins are exposed on the sperm surface would provide a key rationale for candidacy. A two-dimensional gel protein data base for the majority of human sperm proteins has been created (Naaby-Hansen et al. 1995) and both isoelectric focusing (IEF) and nonequilibrating gel electrophoresis (NEPHGE) have been employed to separate human sperm proteins, which were then stained with silver to obtain protein spots over a range of pH four to ten. Using vectorial labeling of the sperm surface with I125 as well as surface biotinylation this data base has provided definition of a repertoire of the major proteins exposed on the human sperm surface. The Western blotting method with antibodies to known proteins was employed in conjunction with autoradiography of surface-labeled proteins to co-identify individual protein spots and thus validate the surface labeling procedure and to orient known proteins on the 2-D map. The 2-D gels were stained, the gel images were digitized, and now data from more than 50 2D gels representing sperm proteins from eight different people has been digitized. A consensus repertoire, termed the "sperm protein encyclopedia" which contains 1,293 silver-stained human sperm proteins that have been assigned coordinates, molecular weight, pI, and shape of spot. Vectorial labeling with biotin has shown 214 surface proteins. None of the controls for known intercellular proteins were labeled.
This approach to designing a sperm-surface-directed immunocontraceptive will require (1) microsequencing selected surface proteins after 2-D preparative SDS-PAGE; (2) developing oligonucleotide probes for cloning and sequencing these proteins; and (3) tissue-specificity studies.
Mixtures of Immunogens
The results noted for the hCG formulation, which currently shows an 80 percent efficacy, and the LDH-C4 formulation, which shows roughly 75 percent efficacy, highlight the fact that an immunocontraceptive product has yet to be achieved with efficacy equal to the 95 percent usually quoted for oral steroid pills. The FDA requirement that each component of a pharmaceutical consisting
of several compounds must be tested in its own right for safety and efficacy has, to date, focused attention on the testing of single-component formulations. With several immunogens now being tested as single compounds, it is likely that a future test of a multideterminant formulations, possibly consisting of several sperm antigens or several sperm antigens in conjunction with hCG, will occur in the coming years.
Gene Therapy
As noted at the outset, the traditional paradigms for immunization began with Jenner's use of cowpox to induce immunity to smallpox, the first example of injection of a closely related, less virulent strain of a virus to attenuate or inactivate disease organisms. With the advent of subunit vaccines consisting of molecules from the organism that were particularly immunogenic or were capable of inducing neutralizing antibodies, the fundamental concept then moved beyond using whole organisms. With advances in protein chemistry came the possibility of using synthetic peptides based on important epitopes or immunodominant regions and, more recently, with recombinant DNA technology, there is now the capability for selectively attenuating organisms, engineering and expressing recombinant protein subunits, and developing immunogens consisting of recombinant proteins.
Another quantum leap in the field builds on the work by Wolff et al. (1990). The research showed that skeletal muscle fibers randomly distributed in the muscle belly expressed the plasmid DNA encoding several reporter proteins, apparently taking up the DNA without a need for special delivery systems such as calcium precipitation or gene guns. DNA expression continued for several months and the plasmids are not integrated into the genome but persist as episomes. Subsequent analysis by the Liu group (Ulner et al. 1993) confirmed the Wolff paradigm and showed that antigens expressed in skeletal muscle after direct naked DNA injection are presented to the immune system: Plasmid DNA-encoding influenza A nucleoprotein, driven by either Rous sarcoma virus or cytomegalovirus promoters and injected into mouse skeletal muscle, generated both specific antibody to nucleoprotein and nucleoprotein-specific cytotoxic lymphocytes. Furthermore, this immune response also protected mice upon subsequent challenge with a heterologous strain of live influenza A virus. It has also been reported that cDNA expression vectors encoding interleukin 2 or interleukin 4, when injected into mouse skeletal muscle, cause expression of these proteins and in vivo cytokine effects (Raz et al. 1993). Further gene inoculation of human immunodeficiency virus envelope protein (pM160) into mouse muscle also has been shown to generate antibodies to HIV (Wang et al. 1993).
Thus, it would seem that the principle of naked DNA innoculation is established. The possibility is now at hand to pursue development of DNA vaccination into muscle, using genes encoding immunogens-from the sperm, egg, or
early conceptus—with potential contraceptive effects, an area potentially rich for discovery but with potential products at some distance in the future.
References
Alexander NJ. Contraceptive vaccine development. IN Vaccine Research and Developments. W Koff, H Six, eds. New York: Marcel Dekker. 1992.
Anderson DJ, PM Johnson, WR Jones, et al. Monoclonal antibodies to human trophoblast and sperm antigens: Report of two WHO-sponsored workshops, 30 June 1986, Toronto, Canada. Journal of Reproductive Immunology 10:231-257, 1987.
Baskin MJ. Temporary sterilization by the injection of human spermatozoa: A preliminary report. American Journal of Obstetrics and Gynecology 24:892, 1932.
Bedford JM, HDM Moore, LE Franklin. Significance of the equatorial segment of the acrosome of the spermatozoa in eutherian mammals. Experimental Cell Research 119:119-126, 1979.
Bernstein L, B Englander, J Gallagher, et al. Localized and systemic hypersensitivity reactions to human seminal fluid. Annals of Internal Medicine 94:459, 1981.
Bleil JD, JM Greve, PM Wassarman. Identification of a secondary sperm receptor in the mouse egg zona pellucida: Role in maintenance of binding of acrosome-reacted sperm to egg. Developmental Biology 128:376-385, 1988.
Bremner WJ, AM Matsumotoa. Endocrine control of human spermatogenesis: Possible mechanism for contraception. IN Male Contraception: Advances and Future Prospects, GI Zatuchni, A Goldsmith, JM Spieler, JJ Sciarra, eds. Philadelphia: Harper and Row. 1986.
Carter PB, FM Collins. The route of enteric infection in normal mice. Journal of Experimental Medicine 139:1189-1203, 1974.
Cross NL, P Morales, JW Overstreet, et al. Two simple methods for detecting acrosome-reacted human sperm. Gamete Research 15:213-226, 1986.
Curtiss R III. Attenuated Salmonella strains as live vectors for the expression of foreign antigens. IN New Generation Vaccines, GC Woodrow, MM Levine, eds. New York: Marcel Dekker. 1990.
Curtiss R III, SM Kelly. Salmonella tymphimurium deletion mutants lacking adenylate cyclase and cyclic amp receptor protein are avirulent and immunogenic. Infectious Immunology 55:3035-3043, 1987.
Dunbar BS, AB Dudkiewicz, DS Bundman. Proteolysis of specific porcine zona pellucida glycoproteins by boar acrosin. Biology of Reproduction 32:619-630, 1985.
East IJ, DR Mattison, J Dean. Monoclonal antibodies to the major protein of the murine zona pellucida: Effects on fertilization and early development. Developmental Biology 104:49, 1984.
Freemerman AJ, RM Wright, JC Herr. Cloning and sequencing of baboon and cynomolgus monkey intra-acrosomal protein SP-10 and comparison with human SP-10 and mouse sperm antigen 63. Molecular Reproduction and Development 34:140-148, 1993.
Gmachl M, JS Sagan, S Ketter, et al. The human sperm protein PH-20 has hyaluronidase activity. FEBS 336, 545-548, 1993.
Goldberg E. LDH-C4 as an immunocontraceptive model. IN Gamete Interaction: Prospects for Immunocontraception , NJ Alexander, PD Griffin, JM Spieler, GMH Waites, eds. New York: Wiley-Liss. 1990.
Goldberg E. Isozymes in testes and spermatozoa. IN Isozymes: Current Topics in Biological and Medical Research, Vol. 1. MC Rattazzi, JG Scandalios, GS Whitt, eds. New York: Alan R. Liss. 1977.
Goldberg, E. Lactate dehydrogenase-X (crystalline) from mouse testes. IN Methods in Enzymology, Vol. XLI: Carbohydrate Metabolism, Part B. New York: Academic Press. 1975.
Goldberg E. Infertility in female rabbits immunized with lactate dehydrogenase X. Science 181:458, 1973.
Golden WL, C von Kap-Herr, BE Kurth, et al. Refinement of the localization of the gene for human intra-acrosomal protein SP-10 to band q24 of chromosome 11 by nonisotopic in situ hybridization. Genomics 18:446-449, 1993.
Griffin PD. Strategy of vaccine development. IN Gamete Interaction: Prospects for Immunocontraception. NJ Alexander, PD Griffin, JM Spieler, GMH Waites, eds. New York: Wiley-Liss. 1990.
Hearn JP, AA Gidley-Baird, JK Hodges, et al. Embryonic signals during the preimplantation period in primates. Journal of Reproduction and Fertility 36(Suppl.):49, 1988.
Hedrick JL. The pig zona pellucida: Sperm binding ligands, antigens and sequence homologies. IN Reproductive Immunology. PM Johnson, F Dondero F, eds. New York: Raven Press. 1993.
Herr JC, CJ Flickinger, M Homyk, et al. Biochemical and morphological characterization of the intra-acrosomal antigen SP-10 from human sperm. Biology of Reproduction 42:181-193, 1990a.
Herr JC, RM Wright, E John, et al. Identification of human acrosomal antigen SP-10 in primates and pigs. Biology of Reproduction 42:377-382, 1990b.
Herr JC, K Klotz, J Shannon, RM Wright, et al. Purification and microsequencing of the intra-acrosomal protein SP-10: Evidence that SP-10 heterogeneity results from endoproteolytic processes. Biology of Reproduction 47:11-20, 1992.
Herr JC, RM Wright, CJ Flickinger, et al. Assignment of the gene for human intra-acrosomal protein SP-10 to the p12->q13 region of chromosome 11. Journal of Andrology 12(5):281-287, 1991.
Hogrefe HH, JP Griffith, MG Rossmann, et al. Characterization of antigenic sites on the refined 3A structure of mouse testicular lactate dehydrogenase-C4. Journal of Biological Chemistry 262:13155-13162, 1987.
Hogrefe HH, PTP Kaumaya, E Goldberg. Immunogenicity of synthetic peptides corresponding to flexible and antibody-accessible segments of mouse lactate dehydrogenase (LDH)-C4. Journal of Biological Chemistry 264:10513-10519, 1989.
Jones WR. Lessons from an anti-hCG contraceptive vaccine trial. IN Gamete Interaction: Prospects for Immunocontraception. NJ Alexander, PD Griffin, JM Spieler, GMH Waites, eds. New York: Wiley-Liss. 1990.
Jones WR, J Bradley J, SJ Judd, et al. Phase I clinical trial of a World Health Organization birth control vaccine. Lancet 1:1295, 1988.
Katsh S. Immunology, fertility and infertility: A historical survey. American Journal of Obstetrics and Gynecology 77:946, 1959.
Kharat E, NS Nair, K Dhall, et al. Analysis of menstrual records of women immunized with Antioch vaccines inducing antibodies partially cross-reactive with hLH. Contraception 41:293-299, 1990.
Kummerfeld HL, RH Foote. Infertility and embryonic mortality in female rabbits immunized with different sperm preparations. Biology of Reproduction 14:300-305, 1976.
Kurth BE, K Klotz, CJ Flickinger, et al. Localization of sperm antigen SP- 10 during the six stages of the cycle of the seminiferous epithelium in man. Biology of Reproduction 44:814-821, 1991.
Ladd A, G Prabhu, Y-Y Tsong, et al. Active immunization against gonadotrophin-releasing hormone combined with androgen supplementation is a promising antifertility vaccine for males. American Journal of Reproductive Immunology 17:121, 1988.
Ladd A, Y-Y Tsong, J Lok, et al. Active immunization against LHRH: I: Effects of conjugation site and dose. American Journal of Reproductive Immunology 22:56, 1990.
Ladd A, Y-Y Tsong, G Prabhu, et al. Effects of long-term immunization against gonadal function. Journal of Reproductive Immunology 15:85-101, 1989.
Lea I, RT Richardson, EE Widgren, et al. Cloning and sequencing of human Sp17, a sperm zona binding protein. Molecular Biology of the Cell 4:248a, 1993.
Lee CY, JH Huan, E Goldberg. Lactate dehydrogenase from the mouse. IN Carbohydrate Metabolism, Part D, Methods in Enzymology. WA Wood, ed. New York: Academic Press. 1982.
Lerum JE, E Goldberg. Immunological impairment of pregnancy in mice by lactate dehydrogenase X. Biology of Reproduction 11:108, 1974.
Linnet L. Clinical immunology of vasectomy and vasovasostomy. Urology 22:101, 1983.
Liu M-S, R Aebersold, C-H Fann, et al. Molecular and developmental studies of a sperm acrosome antigen recognized by HS-63 monoclonal antibody. Biology of Reproduction 46:937-948, 1992.
Madhwa Raj HG. Effects of active immunization with follicle stimulating hormone (FSH) on spermatogenesis in the adult crab-eating monkey: Evaluation for male contraception. IN Immunological Approaches to Contraception and Promotion of Fertility . G Talwar, ed. New York: Plenum Press. 1986.
Mahi-Brown CA, R Yanagimachi, ML Nelson, et al. Ovarian histopathology of bitches immunized with porcine zonae pellucidae. American Journal of Reproductive Immunology and Microbiology18:94, 1988.
Mathur S, ER Baker, HO Williamson, et al. Clinical significance of antisperm antibodies in infertile couples. Fertility and Sterility 36:486, 1981.
Menge AC, H Peegel, ML Riolo. Sperm fractions responsible for immunologic induction of pre- and postfertilization infertility in rabbits. Biology of Reproduction 20:931, 1979.
Millan JL, CE Driscoll, KM LeVan, et al. Epitopes of human testis-specific lactate dehydrogenase deduced from a cDNA sequence. Proceedings of the National Academy of Sciences, USA 84:5311-5315, 1987.
Millar SE, SM Chamow, AW Baur, et al. Vaccination with a synthetic zona pellucida peptide produces long-term contraception in female mice. Science 246:935, 1989.
Moudgal NR. A need for FSH in maintaining fertility of adult male subhuman primates. Archives of Andrology 7:117-125, 1981.
Moudgal NR, GR Aravindan. Induction of infertility in the male by blocking follicle-stimulating hormone action. IN Immunology of Reproduction. R Naz, ed. Boca Raton, FL: CRC Press. 1993.
Moudgal NR, GS Murthy, AL Rao, et al. Development of FSH as a vaccine for the male-A status report on the recent researches carried out using the bonnet monkey M. radiata. IN Proceedings of the International Symposium on Immunological Approaches to Contraception and Promotion of Fertility. GP Talwar, ed. New York: Plenum Press. 1986.
Moudgal NR, GS Murthy, N Ravindranath, et al. Contraception through regulation of endogenous FSH secretion: Prospects for the male. IN Human Reproduction and Contraception, S Takagi. GI Zatuchni, eds. Japan: Professional Postgraduate Services. 1988a.
Moudgal NR, GS Murthy, N Ravindranath, et al. Development of a contraceptive vaccine for use by the human male: Results of a feasibility study carried out in adult male bonnet monkeys (Macaca radiata). IN Contraceptive Research for Today and the Nineties. GP Talwar, ed. New York: Springer Verlag. 1988b.
Moudgal NR, N Ravindranath, GS Murthy, et al. Long-term contraceptive efficacy of vaccine of ovine follicle-stimulating hormone in male bonnet monkeys (Macaca radiata). Journal of Reproduction and Fertility 96(1):91-102, 1992.
Muñoz MG, AC Menge. Infertility in female rabbits isoimmunized with subcellular sperm fractions. Biology of Reproduction 18:669, 1978.
Naaby-Hansen S, JC Herr. A 2-D protein database for the human spermatozoa. Journal of Andrology 16 Jan.-Feb., Suppl.), 1995.
Nagae T, R Yanagimachi R, P Srivastava, et al. Acrosome reaction in human spermatozoa. Fertility and Sterility 45:701-707, 1986.
Naz RK, BB Rosenblum, AC Menge. Characterization of a membrane antigen from rabbit testes and sperm isolated by using monoclonal antibodies and effect of its antiserum on fertility. Proceedings of the National Academy of Sciences, USA 81:857, 1984.
O'Hern PA, Bambra CS, M Isahakia, et al. Reversible contraception in female baboons immunized with a synthetic epitope of sperm-specific lactate dehydrogenase. Biology of Reproduction 52:331-339, 1995.
O'Rand MG. Sperm-egg recognition and barriers to interspecies fertilization. Gamete Research 19:315-328, 1988.
O'Rand MG, J Beavers, EE Widgren, et al. Inhibition of fertility in female mice by immunization with a B-cell epitope, the synthetic sperm peptide, P10G. Journal of Reproductive Immunology 25:89-102, 1993a.
O'Rand MG, RT Richardson, N Yamasaki. Zona pellucida binding of mammalian spermatozoa. Journal of Reproductive Development 39(Suppl.):43-44, 1993b.
O'Rand MG, EE Widgren, SJ Fisher. Sperm antigens relevant to infertility. IN Perspectives in Immunoreproduction. S Mathur, CM Fredericks, eds. New York: Hemisphere Publishing. 1988.
O'Rand MG, EE Widgren, SJ Fisher. Characterization of the rabbit sperm membrane autoantigen, RSA, as a lectin-like zona binding protein. Developmental Biology 129:231, 1984.
Otani Y, I Hiroshi, S Inoue, et al. Immunization of human female with human sperm and semen. International Journal of Fertility 16:19, 1971.
Paterson M, T Koothan, K Morris, et al. Biology of Reproduction 46:523-534, 1992.
Phelps BM, P Primakoff, D Koppel, et al. Restricted lateral diffusion of PH-20, a pi-anchored sperm membrane protein. Science 240:1780, 1988.
Primakoff P, H Hyatt, D Myles. A role for the migrating sperm surface antigen Ph-20 in guinea pig sperm binding to the egg zona pellucida. Journal of Cell Biology 101:2239, 1985.
Primakoff P, W Lathrop, L Woodman, et al. Fully effective contraception in female guinea pigs immunized with the sperm protein PH-20. Nature 335:543-546, 1988.
Raz E, A Watanake, SM Baird, et al. Systemic immunologic effects of cytokine genes injected into skeletal muscle. Proceedings of the National Academy of Sciences, USA 90:4523, 1993.
Rhim SH, SE Millar, F Robey, et al. Autoimmune disease of the ovary induced by an 8 amino acid zona pellucida peptide. Journal of Clinical Investigation 89:28, 1992.
Rosenfield SS. Semen injections with serologic studies. American Journal of Obstetrics and Gynecology 12:385, 1926.
Sacco AG, EC Yurewicz, MG Subramanian, et al. Analysis of the porcine zona pellucida Mr = 55,000 antigen for contraceptive use. IN Reproductive Immunology. L Mettler, D Billington, eds. Amsterdam: Elsevier. 1990.
Sarvetnick N, JY Tsai, H Fox, et al. A mouse chromosome 17 gene encodes a testes-specific transcript with unusual properties. Immunogenetics 31:283, 1990.
Sathananthan AH, C Chen. Sperm-oocyte fusion in the human during monospermic fertilization. Gamete Research 15:177-186, 1986.
Singer SL, H Lambert, JW Overstreet, et al. The kinetics of human sperm binding to the human zona pellucida and zona-free hamster oocyte in vitro. Gamete Research 12: 29-39, 1985.
Skinner SM, T Mills, HJ Kirchick, et al. Immunization with zona pellucida proteins results in abnormal ovarian follicular differentiation and inhibition of gonadotropin-induced steroid secretion. Endocrinology 115:2418, 1984.
Srinivasan J, S Tinge, R Wright, et al. Oral immunization with attenuated Salmonella expressing human sperm antigen induces antibodies in serum and the reproductive tract. Biology of Reproduction 53:462-471, 1995.
Stevens VC. Development of hCG antifertility vaccine. IN Perspectives in Immunoreproduction. S Mathur, CM Fredericks, eds. New York: Hemisphere Publishing. 1988.
Stevens VC. Human choronic gonadotropin: Properties and potential immunological manipulation for clinical application. IN Clinics of Obstetrics and Gynecology, Vol. 6. W. Jones, ed. London: W.B. Saunders. 1979.
Stevens VC. Perspective on development of a fertility control vaccine from hormonal antigens of trophoblast. IN Development of Vaccines for Fertility Regulation: A World Health Organization Symposium. Copenhagen: Scriptor. 1976.
Stevens VC, B Cinader, JE Powell, et al. Preparation and formulation of an hCG antifertility vaccine: Selection of an adjuvant and vehicle. American Journal of Reproductive Immunology 6:315-321, 1981.
Stevens VC, JE Powell, M Rickey, et al. Studies of various delivery systems for hCG vaccine . IN Gamete Interaction: Prospects for Immunocontraception. NJ Alexander, PD Griffin, JM Spieler, GMH Waites, eds. New York: Wiley-Liss. 1990.
Taguchi O, Y Nishizuka. Autoimmune oophoritis in thymectomized mice: T cell requirement in adoptive transfer. Clinical Experiments in Immunology 42:324, 1980a.
Taguchi O, Y Nishizuka, T Sakakura, et al. Autoimmune oophoritis in thymectomized mice: Detection of circulating antibodies against oocytes. Clinical Experiments in Immunology 40:540, 1980b.
Talwar GP, SK Gupta, AK Tandon. Immunologic interruption of pregnancy. IN Reproductive Immunology. N Gleicher, ed. New York: Alan R. Liss. 1981.
Talwar GP, O Singh, R Pal, et al. A vaccine that prevents pregnancy in women. Proceedings of the National Academy of Sciences USA 91:8532-8536, 1994.
Talwar GP, O Singh, R Pal, et al. Vaccines against LHRH and HCG. IN Immunology of Reproduction. R Naz, ed. Boca Raton, FL: CRC Press. 1993.
Talwar GP, O Singh, R Pal, et al. Anti-hCG vaccines are in clinical trials. Scandinavian Journal of Immunology 36(Suppl. 11):123, 1992.
Talwar GP, O Singh, R Pal, et al. Experiences of the anti-hCG vaccine of relevance to development of other birth control vaccines. IN Gamete Interaction: Prospects for Immunocontraception. NJ Alexander, PD Griffin, JM Spieler, GMH Waites, eds. New York: Wiley-Liss. 1990.
Tung KSK. Immunopathologic consideration of antifertility vaccines. IN Male Contraception: Advances and Future Prospects. Philadelphia: Harper and Row. 1986.
Tung KSK, S Smith, C Teuscher, et al. Murine autoimmune oophoritis, epididymoorchitis, and gastritis induced by day 3 thymectomy. Immunology. American Journal of Pathology 126:293-302, 1987.
Ulner JB, JJ Donnelly, SE Parker, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259:1745, 1993.
Wang B, KE Ugen, U Svikartan, et al. Proceedings of the National Academy of Sciences, USA 90:4156, 1993.
Wheat TE, E Goldberg. Antigenic domains of the sperm-specific lactate dehydrogenase C4 isozyme. Molecular Immunology 22:643-649, 1985.
Wheat TE, E Goldberg. Sperm-specific lactate dehydrogenase C4: Antigenic structure and immunosuppression of fertility. IN Isozymes: Current Topics in Biological and Medical Research, Vol. 7. MC Retezzi, JG Scandalios, GS Whitt, eds. New York: Alan R. Liss. 1983.
Wolff JA, RW Malone, P Williams, et al. Direct gene transfer into mouse muscle in vivo. Science 247:1465, 1990.
Wright RM, E John, K Klotz, et al. Cloning and sequencing of cDNAs coding for the human intra-acrosomal antigen SP-10. Biology of Reproduction 42:693-701, 1990.
Yamasaki N, RT Richardson, MG O'Rand. Expression of the rabbit sperm protein Sp17 in COS cells and interaction of recombinant Sp17 with the rabbit zona pellucida . Molecular Reproduction and Development 40(1):48-55, 1995.
Yudin AI, W Gottlieb, S Meizel. Ultrastructural studies of the early events of the human sperm acrosome reaction as initiated by human follicular fluid. Gamete Research 20:11-24, 1988.





























