3
Epidemiology of the HIV/AIDS Epidemic
As noted in Chapter 1, the global HIV/AIDS epidemic consists of many separate, individual epidemics spread unevenly through sub-Saharan Africa, each with its own distinct characteristics that depend on geography, the specific population affected, the frequencies of risk behaviors and practices, and the temporal introduction of the virus. In addition, biological factors may influence the spread of the epidemic by increasing or decreasing susceptibility to the virus, altering the infectiousness of those with HIV, and hastening the progression of infection to disease and death. Such biological factors may include the presence of classical STDs, male circumcision, and the viral characteristics of both HIV-1 and HIV-2 and their multiple genetic strains.
In sub-Saharan Africa, many of the behavioral patterns and biological conditions that can precipitate rapid HIV transmission were present at the time HIV was introduced into selected populations. Within a relatively brief period of time, massive HIV epidemics were ignited in some areas, affecting over 11 million African adults and resulting in 3 million AIDS-related deaths to date, with many more expected in the next few years (World Health Organization, 1995a). These estimates represent over two-thirds of the worldwide total of all HIV infections and AIDS cases. By the year 2000, as many as 20 million individuals on the continent of Africa will be HIV infected, and at least 8 million people will have died of AIDS (World Health Organization, 1993). It is within the African region that HIV will clearly have its greatest impact on morbidity and mortality, in addition to profound economic, demographic, and social consequences.
This chapter gives an overview of the epidemiology of the HIV/AIDS epidemic
in sub-Saharan Africa, including its status and modes of transmission. The chapter ends with a discussion of remaining gaps in knowledge and a set of recommendations for future research.
STATUS OF THE EPIDEMIC
The origin of HIV continues to be an enigma, and the timing of the first human infection remains unknown. Attempts to determine the origins of the disease led to early speculation that AIDS originated in Africa. Not surprisingly, this speculation led many African leaders to resent the implication that Africans were to blame for AIDS. The controversy about the origin of AIDS resulted in a "backlash" and denial that HIV even existed within high-risk populations in sub-Saharan African countries and proved very unhelpful for designing effective prevention programs. Efforts to acknowledge that the problem existed and to initiate efforts to control its spread were delayed in some countries for several years. Because theories about where and when AIDS originated have become so entangled in politics, and because the epidemic is now too far advanced for the question to really matter, attempts to find definitive answers to these questions have been given a low priority. There are a few isolated reports in the literature in the 1970s and even earlier of people dying of opportunistic infections that have now become known as the trademarks of AIDS (Henig, 1993). However, AIDS was not recognized as a clinical entity until 1981. In Africa the first reports of AIDS-like syndromes and "slim" appeared in the literature between 1983 and 1985 (Van de Perre et al., 1984; Piot et al., 1984; Serwadda et al., 1985). Since the early 1980s, the prevalence of HIV infection among certain populations in Africa has increased dramatically, and it is expected to grow even more rapidly in the future. Factors in the spread of HIV are discussed in Chapters 2 and 4 with regard to the larger societal context and individual attitudes and behavior, respectively.
HIV/AIDS Statistics
As of December 1994, nearly 350,000 AIDS cases had been reported from the African region (World Health Organization, 1995a). As noted earlier, this sum represents one-third of the global number (1,025,073) of AIDS cases reported since the start of the epidemic. Allowing for under-diagnosis, incomplete reporting, and reporting delays, WHO estimates that more than 3 million cases of AIDS have occurred in Africa, comprising 70 percent of the global total of 4.5 million. In sub-Saharan Africa, 11 million adults are estimated to have been infected with HIV. This number represents nearly two-thirds of the estimated 18 million cumulative HIV infections that have occurred worldwide. More than half of these 11 million infected adults are women, and as many as 1 million African
children are estimated to have been infected as a result of mother-to-child transmission (World Health Organization, 1994).
As discussed previously, sub-Saharan Africa is geographically, demographically, socially, and culturally heterogeneous, and the extent and spread of HIV infection and AIDS have accordingly been heterogeneous in the region. Thus, it is difficult if not impossible to generalize about the AIDS epidemic within the region. Yet some overall characteristics and trends can been seen. Wherever possible, we provide specific examples, with the proviso that the quoted rates of infection are pertinent only to the specific population and geographical area for which they are cited.
There have been only a few nationally or regionally representative seroprevalence studies conducted to date in sub-Saharan Africa, and information is available predominantly on the groups with the highest risk of HIV infection. In addition, sentinel surveillance systems have been developed to monitor changes in the levels of HIV infection among specific segments of the population, including those with high-risk behaviors, such as women engaged in commercial sex activities or patients receiving care for STDs, as well as groups more representative of the general population (i.e., at lower risk), such as blood donors and women seeking prenatal care.
HIV prevalence is not uniformly distributed even among all countries of sub-Saharan Africa. As described in Chapter 1, to date the epidemic has disproportionately affected East and Southern Africa. Among certain urban populations in the worst-afflicted parts of the region, such as those in Kigali, Rwanda, and Kampala, Uganda, up to one in every three adults is infected with HIV (Rwandan HIV Seroprevalence Study Group, 1989; Ministry of Health [Uganda], 1989). Overall HIV-1 infection patterns for lower-risk urban populations in Africa are shown in Figure 3-1. High levels of infection (in excess of 10 percent) are found among these populations in many urban areas throughout East and Southern Africa and in Abidjan, Côte d'Ivoire, in West Africa (U.S. Bureau of the Census, 1994a).
Recent data suggest that HIV seroprevalence is still low in most rural as compared with urban settings (Figure 3-2). However, HIV infection appears to be increasing in rural areas as well. In Tanzania, the Bukoba district probably always had a higher HIV seroprevalence than Dar es Salaam. However, even within the Bukoba district, urban centers exhibit higher rates of infection than do rural areas: 24 percent versus 5 percent, respectively (Mhalu et al., 1987; Schmutzhard et al., 1989).
Even within a particular geographic area, some population groups are disproportionately affected by the epidemic. The highest infection rates are usually found among men and women between 20 and 40 years old; people with STDs and tuberculosis; and, as discussed in Chapter 2, certain occupational groups, such as long-distance truck drivers, military personnel, and women employed in the commercial sex and entertainment industries (including those who work in
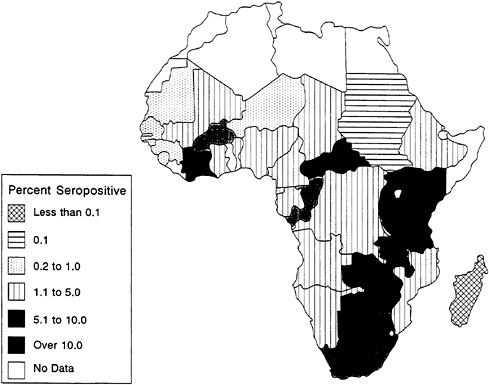
FIGURE 3-1 African HIV-1 Seroprevalence for Lower-Risk Urban Populations.
SOURCE: U.S. Bureau of the Census (1994b).
bars and hotels). HIV infection rates of well over 80 percent have been reported for commercial sex workers in East and Central Africa (Piot et al., 1987, 1988; Quinn, 1991). Figure 3-3 shows the levels of HIV seroprevalence among populations of commercial sex workers in selected countries. Indeed, the AIDS epidemic in each country can be seen as a series of epidemics among subpopulations with varying levels of risk. For example, in Nairobi, Kenya, available data clearly show HIV infection spreading first and most extensively to commercial sex workers, followed by STD clinic patients—no doubt including many clients of those commercial sex workers (Piot et al., 1987). Finally, infection can be seen to be spreading among the general population, as evidenced by the initially slow but accelerating spread among pregnant women (Figure 3-4).
In serologic surveys, pregnant women are often used as surrogates for the general population. Such surveys are convenient because in many countries, pregnant women attend government clinics to receive prenatal care and may be readily tested there. To some extent, pregnant women can be considered as being at slightly higher risk than the general population because they are demonstrably sexually active. Moreover, they are drawn from a limited age range and tend to
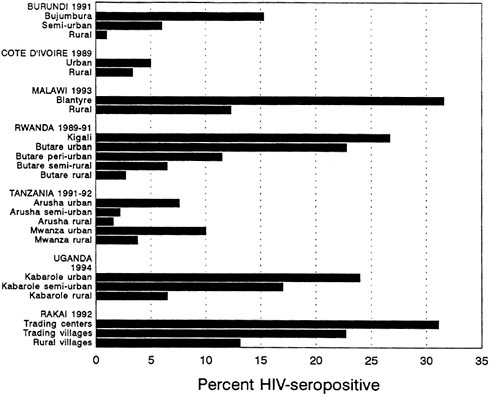
FIGURE 3-2 Urban/Rural Differentials in HIV Infection.
SOURCE: U.S. Bureau of the Census (1994a).
be younger than adult women in general, given typical patterns of age-specific fertility rates. The population of pregnant women may also be biased toward those in marital (formal or informal) unions. Nevertheless, in many countries data on pregnant women provide the most representative picture of HIV infection among the general population.
Seroprevalence data from a number of studies of pregnant women conducted since the mid-1980s demonstrate the heterogeneity mentioned above (Figure 3-4) (U.S. Bureau of the Census, 1994c). There has been a consistent and rapid increase in HIV infection levels among pregnant women in Francistown, Botswana; Blantyre, Malawi; and Kampala, Uganda. By 1992, between 25 and 35 percent of pregnant women in these cities were infected. Infection rates among pregnant women rose at a much more moderate pace in Nairobi, Kenya; Bangui, the Central African Republic; and Dar es Salaam, Tanzania. However, they still reached 15 percent by 1993. Infection levels among pregnant women in Abidjan, Côte d'Ivoire, increased rapidly to about 10 percent by 1987, appeared to have reached a plateau below 15 percent through 1991, but have increased recently above 15 percent. Meanwhile, infection levels in Kinshasa, Zaire, have
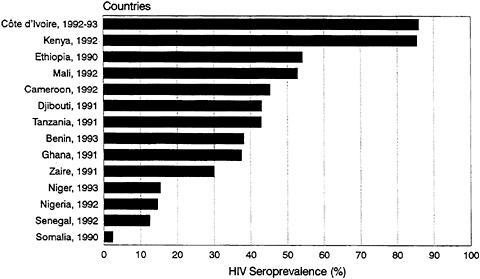
FIGURE 3-3 HIV Seroprevalence for Commercial Sex Workers in Sub-Saharan Africa: Circa 1992.
NOTE: Includes infection from HIV-1 and/or HIV-2. SOURCE: U.S. Bureau of the Census (1994a).
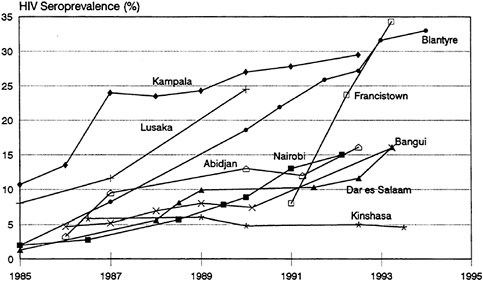
FIGURE 3-4 HIV Seroprevalence for Pregnant Women in Selected Urban Areas of Africa: 1985-1994.
NOTE: Includes infection from HIV-1 and/or HIV-2. SOURCE: U.S. Bureau of the Census (1994a).
been relatively stable at 5 to 6 percent since the mid-1980s (Piot et al., 1990; Piot and Tezzo, 1990).
Although the epidemic in Africa was first recognized in Central and East Africa, and these regions continue to have the highest infection levels, there is increasing evidence that the epidemic is spreading into West and Southern Africa. In Abidjan, Cô d'Ivoire, in West Africa, HIV-1 prevalence among adults increased from 1 percent in 1986 to more than 15 percent in 1992. In Nigeria, a country with more than 105 million inhabitants, the largest population in the region, studies indicate that the prevalence of HIV-1 and HIV-2 among prostitutes in Lagos may be rising rapidly (Dada et al., 1993; Olaleye et al., 1993; see also below).
Within the last few years, investigators have noted the introduction of HIV among high-risk populations in Nigeria. Although relatively rare during the late 1980s, HIV has been increasing since 1990 throughout Nigeria, according to several surveys. This trend is of critical importance since the population of Nigeria, estimated at over 105 million, represents more than one-sixth of the total population of sub-Saharan Africa (World Bank, 1995). In one recent study, 12.3 percent and 2.1 percent of 885 female prostitutes in Lagos State were infected with HIV-1 and HIV-2, respectively, a rise from a combined prevalence of only 1.7 percent 2 years previously (Dada et al., 1993). Women in the youngest age group, ages 12 to 19, had the highest prevalence (20 percent). In addition, prostitutes residing in the port area of Lagos, which serves as a major convergence of overland and sea routes within and outside Nigeria, had the highest prevalence of HIV-1 infection. A highway region that is traversed by the overland interstate highway also had high rates. Because Lagos is the largest cosmopolitan city in Africa, the constant migratory movement of people into and out of this major trade center provides further opportunity for HIV dissemination.
The virus may be spreading even more rapidly in Southern Africa than in West Africa. For example, in Botswana, HIV prevalence among pregnant women increased from 10 percent in 1991 to 34 percent in 1993 in Francistown, and from 6 percent in 1990 to 19 percent in 1993 in Gaborone (U.S. Bureau of the Census, 1994c). Similar disturbing data are emerging from South Africa, suggesting a three-fold increase in HIV prevalence between 1990 and 1993 among women attending prenatal clinics in most regions of the country. Aggregated data collected in prenatal clinics across South africa show a rapid increase in overall prevalence from under 1 percent in 1991 to 1.7 percent in 1992, 2.8 percent in 1993, and 6.4 percent in 1994 (U.S. Bureau of the Census, 1994a).
Thus, although HIV infection rates are high among many populations and subgroups in sub-Saharan Africa, there remains much variation in incidence and prevalence rates recorded to date, both geographically and by population subgroups. The probable causes of this heterogeneity in seroprevalence are multiple, and include behavioral, biological, and societal factors. Trying to explain the phenomenon by a single factor such as civil war, male circumcision, STDs, or
rate of partner change is simplistic. Instead, it appears that the simultaneous occurrence of several risk factors for HIV transmission determines how rapidly and to what level HIV spreads among the population and who will become infected. In the absence of facilitating factors, HIV infection could remain endemic at low levels over long periods of time until a critical prevalence of infection is reached, and the spread of HIV-1 accelerates (Nzilambi et al., 1988). This epidemiologic diversity not only reflects differences in sexual and other behaviors, but also suggests that the epidemic has not reached an equilibrium in most areas.
The epidemiological evidence suggests that HIV prevalence may be stabilizing in some large urban centers (see Figure 3-4) and potentially in some rural areas (Wawer et al., 1994b; U.S. Bureau of the Census, 1994a). However, it must be recognized that a stable prevalence can conceal a significant level of new HIV infection replacing those who die (Wawer et al., 1994b). For example, in the absence of migration, a stable adult seroprevalence of 20 percent suggests that up to 2 percent of adults become newly infected each year, replacing the approximately 10 percent of the infected who are expected to die annually in African settings.
Demographics of HIV Infection
The HIV epidemic and the demographic structure of sub-Saharan populations will have complex interactions over time. The population of sub-Saharan Africa is predominantly young, in sharp contrast with the age structure in developed countries; 45 percent of the population of the region is under the age of 15, compared with one-third or less for the other major geographic regions (Decosas and Pedneault, 1992; Quinn, 1994). Among persons aged 15 and over, those in the 15-39 age group represent over two-thirds of all sub-Saharan adults; only in Latin America do young adults so predominate, whereas in Asia and the developed regions, young adults represent at most half of all adults (United Nations, 1993). In urban areas, one finds a prominent one-sided bulge caused by the migration of young males into the cities for employment (with some rural areas reporting a proportional ''deficit" of young males who have migrated away) (Serwadda et al., 1992). For example, the prevalence of HIV infection among both urban and rural populations in Uganda is highest in the 25-to-44-year-old bracket among males and in the 15-to-34-year-old bracket among females (Figure 3-5) (Wagner et al., 1993; Serwadda et al., 1992).
A significant contributor to the elevated prevalence of infection in sub-Saharan Africa is the fact that behavioral factors associated with HIV transmission—including multiple partners and impermanent relationships—are generally more common among the young, this coupled with the high proportion of young adults found in sub-Saharan African countries (Anderson et al., 1991). Accordingly, the large number of young persons under age 15, who will soon enter their
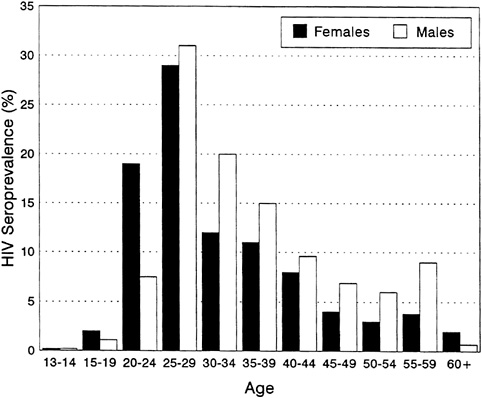
FIGURE 3-5 HIV Seroprevalence of Adult Population of Rural Rakai District, Uganda, by Age and Sex, 1992.
SOURCE: Maria Wawer (personal communication, 1995).
sexual and reproductive lives, must represent a priority group for AIDS and STD prevention.
The difference in the age distribution of peak HIV prevalence between men and women occurs in the region because, on average, sexual partnerships are formed between older men and younger women (see also Chapter 2). The distortion of the urban population profile caused by male migration initially resulted in equal numbers of infected men and women (Quinn, 1994). However, male-to-female transmission of the virus is more efficient than female-to-male transmission in the absence of other cofactors (Haverkos and Quinn, 1995), so that as the epidemic has spread into the larger rural population, the absolute number of infections has become higher among women than men (Rowley et al., 1990; Anderson et al., 1991).
HIV-2 Infection
One unique feature of the AIDS epidemic in Africa is the remarkable viral heterogeneity of HIV infection. Within HIV-1 there are now nine recognized
subtypes, labeled A through I, as well as the more recently identified subtype O. In addition to HIV-1, which is common predominantly in Central, Southern, and East Africa, a more distinct variant, labeled HIV-2, has been identified, predominantly in West African countries. Originally identified in 1986, HIV-2 was recognized among high-risk populations such as commercial sex workers in urban centers in West Africa. While having some genetic relationship to HIV-1, in evolution HIV-2 may be more closely related to a simian immunodeficiency virus (SIV) (Kanki, 1994). Although similar in terms of morphology, cell tropism, and overall genetic organization, HIV-1 and HIV-2 differ significantly in terms of nucleotide sequences, with only 42 percent homology (Clavel et al., 1986; Guyader et al., 1987). Genomic studies further demonstrate that HIV-2 has 70 percent or more homology with SIV. Genetic sequencing of HIV-2 isolates also shows a wide divergence among individual strains of HIV-2, similar to that observed with HIV-1. Thus, it is highly probable that the divergence of HIV-1 and HIV-2 occurred earlier than the beginning of the current epidemic (Myers, 1994). A common ancestor with similar properties and pathogenic potential may have existed a long time ago (Myers, 1994); the spread of HIV in Africa was most likely a result of simultaneous modifications of epidemiologic parameters in West and Central Africa, such as rapid urbanization and increased mobility, leading to infection of larger populations with HIV-1 and HIV-2 (Rowley et al., 1990; Anderson et al., 1991; Decosas et al., 1995) (see also Chapter 2).
Although HIV-2 can cause AIDS, it is increasingly clear that its pathogenic potential is lower than that of HIV-1. In cross-sectional studies, individuals infected with HIV-2 were found to have immunologic abnormalities similar to although less marked than those associated with HIV-1. In a prospective study among prostitutes in Senegal who were HIV-2 positive, no reduction in CD4 lymphocyte levels and no clinical abnormalities were found (Marlink, 1994). A less aggressive course of HIV-2 infection is also suggested by other observations in Dakar, Senegal; whereas HIV-2 is predominant among asymptomatic people, HIV-1 is more frequent among hospitalized patients with AIDS (Poulsen et al., 1993; Kanki et al., 1994; Marlink, 1994).
The routes of transmission and risk factors for HIV-1 and HIV-2 are similar. Like HIV-1, HIV-2 is transmitted primarily sexually (Kanki et al., 1994). However, the latency period for HIV-2 appears to be longer, and vertical transmission of HIV-2 from mother to infant is rare (Matheron et al., 1990; Poulsen et al., 1992; Adjorlolo-Johnson et al., 1994).
HIV-2 infection rates have risen steadily over the past two decades in countries of West Africa, including Côte d'Ivoire, Senegal, Guinea-Bissau, Burkina Faso, The Gambia, and Cape Verde (Kanki, 1991; Naucler et al., 1991; Markovitz, 1993; Poulsen et al., 1993). In several urban centers of West Africa, 15 to 64 percent of female prostitutes are infected. In Guinea-Bissau and The Gambia, HIV-2 is the prevalent infection, and HIV-1 is rare. In Côte d'Ivoire and Burkina
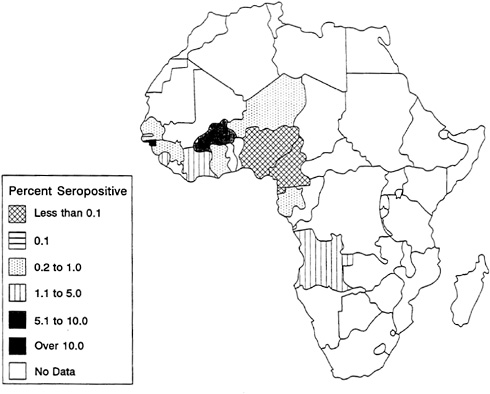
FIGURE 3-6 African HIV-2 Seroprevalence for Lower-Risk Urban Populations.
SOURCE: U.S. Bureau of the Census (1994b).
Faso, HIV-2 and HIV-1 are both present among appreciable proportions of the population.
The geographic pattern of HIV-2 shows a higher prevalence in West Africa and in other African countries with a Portuguese colonial history (Kanki, 1991). Troop movements among these former Portuguese colonies and travel facilitated by cultural ties surely contributed to the spread of HIV-2 in these select countries. Conversely, several countries bordering those with substantial HIV-2 infection have as yet shown little evidence of an HIV-2 epidemic.
The highest prevalence of HIV-2 infection among high-risk urban adults is found in Côte d'Ivoire and The Gambia, where infection rates are 37 percent and 27 percent, respectively. HIV-2 seroprevalence among low-risk urban adults is far lower (see Figure 3-6); only in Guinea-Bissau does seroprevalence exceed 10 percent (U.S. Bureau of the Census, 1994b). The highest HIV-2 infection rates are found among populations with high HIV-1 prevalence, including people with tuberculosis or STDs and female prostitutes. As noted above, in contrast to HIV-1, which shows a distinct peak at ages 25 to 40, the age-specific prevalence of
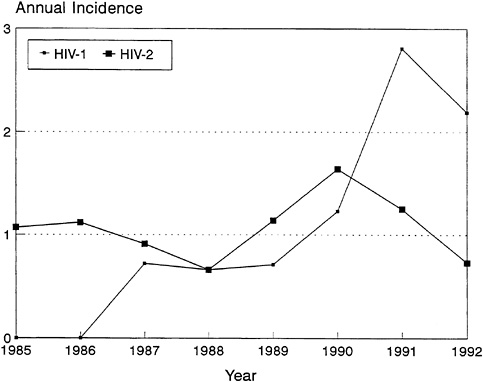
FIGURE 3-7 Incidence of HIV-1 and HIV-2 Among Female Prostitutes in Senegal.
NOTE: Incidence measured as the percentage per 100 person-years of observation.
SOURCE: Data from Kanki et al. (1994).
HIV-2 infection increases gradually with age. This increase may be the result of a lower case fatality rate or a longer latency period of HIV-2 infection.
It appears that with time, HIV-1 is increasing among these populations at a faster rate than HIV-2 (Kanki et al., 1994). Although HIV-2 appears to have been in West Africa longer than HIV-1, levels of HIV-1 infection have now surpassed those of HIV-2 in many West African countries. For example, in a recent study in Dakar, Senegal, 1,452 registered female prostitutes were followed prospectively between 1985 and 1993 (Kanki et al., 1994). Initially, HIV-2 was more common than HIV-1. In 1985, HIV-2 was present with a prevalence of 11.3 percent, whereas HIV-1 was present with a prevalence of 6.2 percent. The 1,277 women with a seronegative sample were evaluated over the next 8 years for evidence of seroconversion. While the incidence of HIV-2 remained stable, the annual incidence of HIV-1 increased substantially (Figure 3-7). There was a 1.4-fold increase in risk per year and a 12-fold increase in risk over the entire study period. This study suggests that the heterosexual spread of HIV-2 is significantly slower than that of HIV-1, which may reflect the differences in the viruses'
infectivity potential (Kanki et al., 1994). Consistent with the idea that HIV-2 may be less virulent than HIV-1 and may have been present in West Africa for many decades, seropositivity increases with age. Among prostitutes in Dakar, Senegal, almost 100 percent of those aged 50 and over are infected with HIV-2 (Marlink, 1994).
The disparity in the speed with which HIV-1 and HIV-2 are spreading is particularly striking in Abidjan, Côte d'Ivoire, where HIV-1 has become by far the predominant virus, causing a major epidemic of AIDS. In contrast with HIV-1, HIV-2 has barely increased in Abidjan during the past 6 years (Figure 3-8). This finding suggests that under identical conditions and among populations with the same behavior, HIV-2 is less readily transmissible through sexual intercourse than HIV-1. Nevertheless, studies in Côte d'Ivoire and The Gambia have found that HIV-2 infection correlates with factors associated with HIV-1 infection, such as prostitute contact, history of past STDs, and past and current genital ulcer disease. In Côte d'Ivoire and Guinea-Bissau, 40 percent of spouses of people with HIV-2 were also infected.
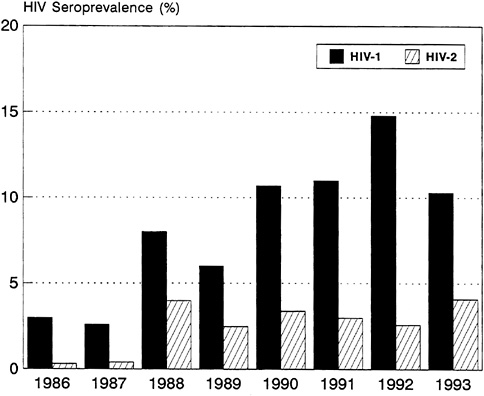
FIGURE 3-8 HIV Seroprevalence for Pregnant Women, Abidjan, Côte d'Ivoire: 1986-1993.
SOURCE: U.S. Bureau of the Census (1994a).
The clinical and epidemiologic diversity between HIV-1 and HIV-2 may also have important biological and immunologic implications. Intrinsic biological properties of these viruses include infectivity and replication capacities that contribute to different epidemic curves. In addition to the higher rates of sexual and perinatal transmission and more rapid clinic course of disease associated with HIV-1, HIV-2 infection may provide some immunologic protection from HIV-1 (Travers et al., 1995). In another recent paper on commercial sex workers in Dakar, Senegal, the HIV-1 incidence was shown to be higher for seronegative commercial sex workers than for their HIV-2 infected counterparts. The lower HIV-1 seroconversion rate among those infected with HIV-2 occurred despite the same frequency of other STDs in both groups, suggesting that differences in sexual behavior between the two groups were not responsible. Further analysis indicated that HIV-2-seropositive women with CD4 cell counts of less than 800 per cubic millimeter were likely to become infected with HIV-1 than were those women with higher CD4 counts, suggesting that with increasing immunosuppression, the protective effect of HIV-2 infection against subsequent HIV-1 may decrease (Travers et al., 1995). Further studies are needed to determine whether cross-reactive immunity can occur between different strains of HIV, and whether this information can be used in the development of more effective vaccines.
Differences in the patterns of infection are likely to be the result of different types of interrelations and contacts within West Africa, where HIV-2 predominates, and between West Africa and Central Africa, where HIV-1 predominates. In general, it seems likely that the migration of sexually active, high-risk populations played a major role in the observed spread of these sexually transmitted viruses. One example is seasonal migration of young men and women in Senegal and The Gambia. In one study of 3,230 persons residing in rural Senegal, 0.8 percent were HIV-2 seropositive, and 0.1 percent were HIV-1 seropositive. Seropositivity was directly associated with seasonal migration and a history of blood transfusion, injections, or STDs (Pison et al., 1993). In another study of 278 female prostitutes from Ziguinchor, Senegal, HIV-2 seroprevalence was associated with women from Guinea-Bissau and with increased years of sexual activity. Women from Ghana and Guinea-Bissau also constituted a significant portion of study participants (commercial sex workers) in other sites in Senegal (Kanki et al., 1992). Both of these nationalities were associated with higher HIV-2 prevalence.
These findings suggest that even in rural areas, with the exception of a few cases of transmission by blood transfusion or injection, HIV-2 is transmitted mainly sexually from migrant female prostitutes to adult men, and secondarily to their wives or regular partners upon return home. Ghanaian and Gambian prostitutes report that they migrate and work in a number of other West African countries, such as Burkina Faso, Côte d'Ivoire, and Mali, all having significant HIV-2
2 rates (Pepin et al., 1991; Pickering et al., 1992; Dada et al., 1993; Olaleye et al., 1993).
Several cases of mother-to-child transmission of HIV-2 have been reported from The Gambia. However, the risk of perinatal transmission of HIV-2 is much lower than that of HIV-1 (Adjorlolo-Johnson et al., 1994). In a community-based survey in Guinea-Bissau, all HIV-2-seropositive infants lost HIV-2 antibodies before the age of 9 months, and no evidence of mother-to-child transmission of HIV-2 was found in prospective studies in Burkina Faso or Côte d'Ivoire. Additional data from prospective studies are needed before the risk of HIV-2 transmission from mother to child can be more accurately assessed.
HIV-2 infection can also be acquired by blood transfusion. This is illustrated by studies among hospitalized patients in Guinea-Bissau, where previous blood transfusions were found to be a risk factor for HIV-2, and in Côte d'Ivoire among children with multiple transfusions, who had an increased prevalence of infection with HIV-2 (Horsburgh and Holmberg, 1988; De Cock and Brun-Vezinet, 1989).
MODES OF TRANSMISSION
The primary mode of HIV transmission is sexual, with heterosexual transmission accounting for at least 80 percent of adult HIV infections in sub-Saharan Africa (Piot et al., 1988). HIV is also acquired through perinatal and parenteral transmission.
Sexual Transmission
Risk Factors in Sexual Transmission
Behavioral risk factors for HIV transmission among heterosexuals include number of sex partners, frequency of unprotected intercourse, commercial sex, a history of or concurrent infection with an STD, lack of male circumcision, and anal intercourse; many women are at risk only because they have unprotected intercourse with a regular partner or spouse who is infected. Of these factors, the importance of STDs as a cofactor for HIV transmission among heterosexuals has been emphasized in a number of studies (Wasserheit, 1992). The increase in seroprevalence in recent years among STD clinic patients in comparison with the increase among the overall population was 2-fold in Tanzania, 3-fold in Zambia, 4-fold in Burundi, and 20-fold in rural Rwanda (Plummer et al., 1991; Laga et al., 1993). Even among commercial sex workers, the transmission of HIV is associated with the presence of STDs.
The statistical risk of HIV transmission appears to be small for any single episode of penile-vaginal intercourse, regardless of which partner is infected (Haverkos and Quinn, 1995). Studies of male-to-female transmission suggest
that the risk may be as low as 0.1 percent per episode of intercourse. Female-to-male transmission by intercourse is less efficient than male-to-female spread (Mastro et al., 1994; Haverkos and Quinn, 1995). Reasons for the greater susceptibility of women may include greater trauma to the genitalia and the vaginal epithelium in women during intercourse and longer exposure to HIV when infected ejaculate is retained in the vagina. Similar mechanisms may account for greater female susceptibility to non-ulcerative STDs, such as gonorrhea, chlamydia, and trichomoniasis (Wasserheit, 1992). Studies of HIV transmission among heterosexual couples have demonstrated a seroprevalence of 20 to 50 percent among originally discordant couples; in two African studies, annual seroconversion rates among discordant couples were on the order of 4 to 9 percent per year (Allen et al., 1992; Serwadda et al., 1995). From European data, factors that appear to increase heterosexual transmission include and-receptive intercourse, more advanced illness in the male partner, and history or presence of STDs (de Vincenzi, for the European Study Group on Heterosexual Transmission of HIV, 1994).
It is generally accepted that homosexual contact between men is a minor route of HIV transmission in sub-Saharan Africa, given that such behavior is reported only rarely in the region (see Chapter 4). Although homosexual transmission may be rare, underreporting of such behavior may be common because homosexuality is highly stigmatized in most African societies. However, when homosexual sex does occur, male-to-male transmission may play a part in sustaining the epidemic; there is little reason to think that the risk factors would not be similar to those in Western countries, where anal-receptive intercourse appears to be a primary risk factor for homosexual transmission of HIV. Similarly, other studies among male homosexual populations have demonstrated an association between HIV-1 and other STDs, such as syphilis and herpes.
HIV and STDs
A growing body of data suggests that HIV transmission cannot be considered in isolation from the classical STDs, principally syphilis, gonorrhea, chlamydia, chancroid, trichomoniasis, and herpes simplex virus type 2 (HSV-2). HIV shares modes of transmission and behavioral risk factors with these other STDs. More important, there is evidence that classical STDs may increase susceptibility to and transmission of HIV; their control may thus serve as an important element in curbing the HIV epidemic. Thus, the following sections on sexual transmission, as well as some subsequent sections on behavior and care seeking, refer to both HIV and the classical STDs.
Prevalence of STDs in Africa Although accurate determination of the prevalence of STDs in Africa is hindered by a lack of population-based data and adequate surveillance, existing information suggests that infection rates are very
high in many African settings. Serological evidence of a prevalence of syphilis ranging from 11 to 21 percent has been documented among women attending prenatal clinics in a number of sub-Saharan countries (Ratman et al., 1982; Watson, 1985; Cooper-Poole, 1986). Between 7 and 15 percent of pregnant women have been found to have Neisseria gonorrhoeae (N. gonorrhoeae) infection in similar settings (Nasah et al., 1980; Mabey et al., 1984; Laga et al., 1986; Mason et al., 1989; Welgemoed et al., 1986; Widy-Wirsky and D'Costa, 1989). In a recent study of pregnant Ugandan women, Nsubuga et al. (1994) found a prevalence of 42.5 percent for trichomoniasis, 10.4 percent for active syphilis, and 7.5 percent for chlamydia. Hemophilus ducreyi (chancroid) is a frequent etiologic agent of genital ulcers (World Health Organization Expert Committee on Venereal Diseases and Treponematoses, 1986; Mabey et al., 1987). Of 293 male STD clinic clients in Nairobi, 149 had acquired genital ulcer disease following contact with a prostitute; chancroid was clinically diagnosed in 89 percent of the cases (followed by genital herpes in 5 percent) (Cameron et al., 1989). Non-ulcerative Chlamydia trachomatis (C. trachomatis) infection is another common genital tract pathogen, isolated in over 13 percent of male and female patients with urethritis or discharge in several studies (Bowie et al., 1977; Mabey and Whittle, 1982; Ballard et al., 1986; Leclerc et al., 1988).
Data from community-based cohort studies suggest that high STD rates are not confined to self-selected, urban clinic populations. High rates of positive syphilis serology, indicative of active syphilis in over 10 percent of the adult population, have been reported from population-based studies in rural Uganda (Mulder, 1993; Hudson, 1993). Preliminary data from another predominantly rural district of Uganda indicate that up to half of the rural women in Rakai residing in small towns on secondary roads have Trichomonas vaginalis infection (Wawer et al., 1995a).
In contrast with HIV, whose prevalence has been found to vary widely within countries or districts in relation to the degree of urbanization and proximity to roads, the classical STDs may be more uniformly distributed among the general population within a given region. In Uganda, adult HIV rates ranged from 11 percent in the stratum of relatively isolated agrarian villages to over 30 percent in communities along main roads (Wawer et al., 1991). In contrast, the rates of active syphilis varied far less, from 9 percent in the most rural stratum to 13 percent in the main-road towns (Nelson K. Sewankambo, personal communication, 1995). The older infections may have become established more uniformly over time in any one particular region; given that STDs may enhance HIV transmission, the data also suggest that STDs represent an important risk factor in a very wide segment of the African urban and rural populations. Given the data described above, however, it is obvious that the rates of classical STDs may vary substantially across regions, and such underlying rates may have contributed to the unequal pace at with which HIV infection has spread in different parts of Africa.
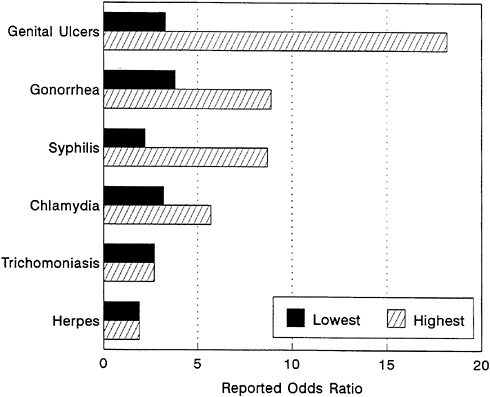
FIGURE 3-9 Relative Risk of HIV Infection by Type of STD. NOTE: Summary of risk estimates of STDs and HIV infection, drawn from prospective cross-sectional or case control studies. All reported studies included physical examinations and/or laboratory confirmation of the STD and multivariate adjustment for behavioral risk factors. Data reported are for heterosexual men or women. All risks are expressed as odds ratios and indicate the highest and lowest reported risk (odds ratio) of HIV infection in the presence of the STD, as compared with subjects without the STD. SOURCE: Wasserheit (1992).
Synergy of HIV and STDs A number of studies conducted in sub-Saharan Africa have demonstrated that both genital ulcers—mainly chancroid, syphilis, and herpes—and non-ulcerative STDs—such as gonorrhea, chlamydial infection, and trichomoniasis—are associated with increased risk of sexual transmission and acquisition of HIV (Figure 3-9).
That HIV-1 can be isolated directly from genital ulcers strongly suggests the potential for increased transmission associated with genital ulcers (Kreiss et al., 1989); the reduced epithelial integrity of the ulcers may render uninfected sexual partners more susceptible to HIV infection. In a prospective study in Kenya, Cameron et al. (1989) demonstrated a high incidence of HIV infection among men who developed genital ulcer disease after contact with a commercial sex
worker. Among the same population of seropositive female commercial sex workers, HIV was detected by culture from 4 of 36 ulcers (11 percent).
The case for non-ulcerative STDs as risk factors for HIV transmission/acquisition is less well documented. Cervical inflammation has been shown to be associated with cervical shedding of HIV DNA (Kreiss et al., 1994). In a prospective study among female prostitutes in Kinshasa, Zaire, gonorrhea, chlamydia, and trichomoniasis during the presumed period of exposure were all significantly associated with HIV seroconversion, even after controlling for sexual exposure in terms of the number of sexual contacts and frequency of condom use (Laga et al., 1993).
Based on data from this observational cohort study, Laga et al. (1994) reported lower HIV incidence among those sex workers who regularly attended clinical services for STD diagnosis and treatment as compared with workers who received STD services irregularly, after adjustment for condom use. Among prostitutes in Nairobi, Kenya, genital ulcer disease was independently associated with HIV infection (Simonsen et al., 1990). It should also be noted that although the association with HIV infection is generally stronger for ulcerative than non-ulcerative STDs, the latter are more prevalent. It is thus possible that in many settings, the increase in attributable risk to the whole community of HIV infection due to the presence of other STDs may be greater as a result of non-ulcerative STDs than genital ulcer disease (Piot and Laga, 1989; Wasserheit, 1992). In addition, as suggested above, although the high prevalence of untreated STDs found in many African settings may have contributed to the generally rapid spread of HIV in the region, underlying differences in STD rates may play a role in the unequal spread of HIV on the continent.
It has been argued that the consistency of the findings and the strength of the associations between HIV infection and STDs lend strong support to a causal association: that STDs facilitate HIV transmission. However, interpretation of the findings is difficult because of potential confounding variables. Classical STDs may be markers of sexual activity and not causal risk factors, and accurately controlling for the sexual activity of the patient and of his/her partner(s) is frequently not possible (Pepin et al., 1989; Wasserheit, 1992). Even longitudinal observational studies do not provide conclusive evidence because of potential misreporting of partner information, problems in determining the time sequence of the infections (the latter being dependent on the frequency of HIV testing and the accuracy of the information regarding the time the STD was acquired), the variable delay in HIV seroconversion following infection, and the inability to assess accurately the STD status of the partner(s) of the index case (Pepin et al., 1989; Piot and Laga, 1989; Mertens et al., 1990). It has thus been proposed that ''a more direct approach to establish a causative association would be through the conduct of randomized controlled trials, which seek to determine whether intervening against STDs reduces the transmission of HIV" (Mertens et al., 1990:63).
The attributable risk of STDs for the transmission of HIV remains to be determined, as does the impact of STD control on the spread of HIV-1.
Data from a recent randomized community-based intervention trial conducted in Mwanza, Tanzania, indicate significantly lower HIV incidence among adults residing in communities that received improved STD case management at the primary health care level, as compared with control communities (Grosskurth et al., 1995). HIV incidence was lower for both males and females, and for all age groups between 15 and 54, in the treatment communities as compared with the controls; the data further suggest that the observed differences were not due to factors such as condom use or numbers of partners. However, the study had only limited information on STD end points, and no significant reductions in STD rates were observed. The authors hypothesize that "the most plausible explanation for our results is that the STD treatment programme reduced HIV incidence by shortening the average duration of STDs …" (Grosskurth et al., 1995:535).
A number of other randomized controlled community-based trials of STD prevention and treatment are under way currently in Africa (Wawer et al., 1995b; Mulder et al., 1994a). Data from these trials should elucidate further the effects of STD reduction on HIV transmission among the general population and should add to our understanding of the role of individual STDs, and of symptomatic and asymptomatic infections, in HIV transmission in the community setting.
HIV may also have a reciprocal effect on other STDs; that is, HIV infection may alter the natural history, diagnosis, and response to therapy of other STDs (Johnson et al., 1991; Wasserheit and Holmes, 1992; Aral and Wasserheit, forthcoming). For example, although data are as yet limited, there is some evidence that the incidence of pelvic inflammatory disease may be greater among HIV-positive women with gonorrhea than among HIV-negative women with gonorrhea (Plummer et al., 1989). Increased rates of chancroid treatment failure have been reported among HIV-infected persons in Zimbabwe and Kenya (Latif, 1989; MacDonald et al., 1989). More research is needed to determine whether HIV infection results in atypical STD presentations, including more resistant lesions or more frequent recurrence (Aral and Wasserheit, forthcoming), which could presumably result in greater STD transmission.
Aral and Wasserheit (forthcoming) conclude that the multiple potential interactions between STDs and HIV, coupled with their joint modes of transmission, result in epidemics that are "inextricably intertwined, particularly in heterosexual populations. … These interrelationships mean that HIV infection and other STDs may amplify one another by establishing a mutually reinforcing spiral of infection. … Clearly, this suggests that in communities with high rates of STDs, augmented STD prevention and control efforts should be a central HIV prevention strategy."
Other Health Consequences of STDs Regardless of the STD/HIV synergy, STD control is highly desirable in itself. STDs result in substantial acute adult morbidity;
male and female infertility; maternal postpartum complications; fetal loss; and other negative reproductive outcomes, including congenital sequelae.
High incidence of pelvic inflammatory disease has been reported in several African countries (Muir and Belsey, 1980), with C. trachomatis and N. gonorrhoeae being the two most frequently recognized pathogens (Mabey et al., 1985; Frost et al., 1987). In a prospective Kenyan study, the incidence of postpartum upper genital tract infections was 20 percent, nearly one-third of these being infections caused by N. gonorrhoeae, C. trachomatis, or both (Plummer et al., 1987).
Pelvic inflammatory disease is thought to have contributed substantially to the historical "infertility belt" of Central Africa, a region that includes Cameroon, the Central African Republic, northern Zaire, the Congo, Gabon, and parts of Uganda (Arya et al., 1973; Arya and Taber, 1975; World Health Organization Scientific Group on the Epidemiology of Infertility, 1975; Frank, 1983). Based on World Fertility Survey and Demographic Health Survey data from 17 sub-Saharan African countries, Larsen (1994) estimates that at the age of 34, the proportion of sterile women ranges from between 33 and 41 percent in Cameroon to between 10 and 15 percent in Burundi, Kenya, Ondo State, Nigeria, and Togo. These data, as well as those from a WHO-sponsored multicenter collaborative investigation of infertility, are strongly suggestive of previous genital infection (World Health Organization Scientific Group on the Epidemiology of Infertility, 1975). Indeed, it has been estimated that between 50 and 80 percent of cases of female infertility in Africa may be due to reproductive tract infections; in industrialized countries, the proportion is from 10 to 35 percent (Cates et al., 1985; Wasserheit and Holmes, 1992). Brunham et al. (1991) have estimated that a 20 percent prevalence of gonorrhea among sexually active adults may produce up to a 50 percent reduction in net population growth, an estimate consistent with fertility rates observed in parts of Uganda. Male fertility is also affected by STDs: sexually transmitted organisms, in particular N. gonorrhoeae and C. trachomatis, are the most common cause of epididymitis among heterosexual men under the age of 35. Epididymitis and occlusion of the vas deferens are associated with reduced male fertility (Berger, 1990). Such conditions arise more frequently in Africa than in other regions because of inadequate treatment (Mputo et al., 1986; Arya and Taber, 1975).
STDs can also result in adult mortality, particularly among women, through a number of major complications, including ectopic pregnancy and postpartum infection. It should be noted further that in addition to the reproductive effects of STDs in adults, the cardiovascular and neurological sequelae of syphilis represent important public health concerns.
Pregnancy and birth outcomes are also affected by STDs. A Zambian study determined that 57 percent of pregnancies among women with untreated syphilis ended in abortion, stillbirth, preterm birth, or low birth weight, as compared with 10 percent among women without syphilis (Hira et al., 1990). Shulz et al. (1987)
have estimated that with a 10 percent prevalence of syphilis among pregnant women—not uncommon in Africa—5 percent or more of all pregnancies surviving past the twelfth week of gestation will have congenital syphilis or one of the other serious adverse syphilitic outcomes of the type cited above.
The incidence of many other STD-related birth sequelae is poorly documented, but data suggest that Trichomonas vaginalis and C. trachomatis can also be associated with negative pregnancy outcomes (Berman et al., 1987; Sweet et al., 1987; Cotch and Pastorek, 1991). Approximately 30 to 45 percent of infants exposed to gonorrhea in utero acquire gonococcal eye infection (gonococcal ophthalmia neonatorum or GON) in the absence of prophylaxis; GON rates of 4 to 6 percent have been documented in African centers (Laga et al., 1986; Shulz et al., 1987; De Schryver and Meheus, 1990; Hammerschlag, 1991). Bacterial vaginosis is characterized by a marked (20- to 1,000-fold) increase in vaginal Gardnerella vaginalis, Mycobacteria hominis, Ureaplasma urealyticum, and various anaerobic bacteria and reduced numbers of peroxide-producing Lactobacillus species (Hillier et al., 1992; Eschenbach, 1993; Hillier, 1993; Hillier et al., 1993). Bacterial vaginosis, particularly in early pregnancy, has been associated with chorioamnionitis and intramniotic infection; such infections may lead to premature rupture of the membranes and premature delivery (McDonald et al., 1991; Gibbs, 1993; McGregor et al., 1993; Read and Klebanoff, 1993; Riduan et al., 1993).
In another example of STD and HIV interactions, it has been hypothesized that STDs that compromise the integrity of the placental membranes and result in placental barrier defects may increase the rate of vertical (mother-to-child) HIV transmission (St. Louis et al., 1993). There is some evidence that active genital ulcer disease in the mother at the time of delivery also increases maternal-to-infant transmission (Wabwire-Mangen, 1995).
In addition to the substantial, direct effects of STDs on adult and infant health, STDs have major social and economic implications. Although male infecundity may be responsible for up to one-third of all "couple" infecundity, the woman is more likely to be identified as the responsible partner and to suffer the social consequences (de Bruyn, 1992). To avoid rejection and divorce, the woman in an infertile couple may seek an outside partner to make her pregnant, feeding a vicious cycle of STD-HIV transmission. Economically, STDs place substantial demands on the health systems of sub-Saharan Africa, representing one of the top five reasons for clinic attendance in many settings (Meheus et al., 1990). STDs have been found to account for up to 10 percent of adult outpatient visits in Zambia (Hira and Sunkutu, 1993). The substantial negative effects of congenital syphilis, gonorrhea, and chlamydia on child growth, development, and survival, described earlier, also affect the provision of health and social services.
More intensive STD control is thus critically needed, in particular since evidence from countries such as Burkina Faso and Kenya suggests that the prevalence and incidence of STDs are rising (Damiba et al., 1990; Hammerschlag,
1991; Cates and Hinman, 1991). Although some of the reported increase may be due to improved diagnosis, the trends appear to be real, presumably driven by the same social factors (mobility, urbanization, changing mores) that are fueling the HIV/AIDS epidemic.
Transmission Models of STDs and HIV The basic biological and behavioral factors that determine the transmission dynamics of STDs, including HIV, are reflected in the formula R0 =βDc (Anderson and May, 1988). R0 represents the reproductive rate of infection of a particular pathogen, i.e., the number of new infections transmitted by one infected person in the susceptible population. There are three direct determinants of R0, or the rate of spread:
β, which represents the mean probability of sexual transmission per partnership
D, the mean number of years an infected person remains infectious
c, the average rate of new sexual partner selection per year
Each of these variables can be viewed as a category that encompasses certain classes of risk factors. If R0 exceeds 1.0, the disease will grow, whereas if R0 is less than 1.0, the disease will die out since each new infection will fail to replace itself. Added complexity is introduced since the duration of the infectious period, the probability of transmission, and the rate of new partner acquisition will certainly vary among individuals.
Each STD, including HIV, has unique biological characteristics that affect its transmissibility or β. The number of infectious particles necessary to establish infection, or the infectious dose, is an example. A factor associated with HIV infectiousness is increased viremia, seen in acute primary infection or advanced clinical disease. Moreover, as indicated above, conditions that compromise the vaginal mucosal or penile epithelial barriers, such as genital ulcers or inflammatory conditions provoked by non-ulcerative STDs, may increase susceptibility to HIV infection. It has also been postulated that HIV infection, by compromising immunocompetence, may lead to longer duration of infection by classical STDs. Theoretically, cervical ectopy (a condition in which part of the cervical surface becomes covered by the delicate mucus-secreting columnar cells that normally line the cervical canal), such as that arising during pregnancy or use of some contraceptives (Moss et al., 1991; Daly et al., 1994), may increase β by increased viral shedding or susceptibility to infection. Adolescent females may be at higher risk of HIV infection because of a larger zone of cervical ectopy that is associated with puberty; the effect could be direct, or indirect through increased susceptibility to gonococcal or chlamydial cervicitis (Bulterys et al., 1994). It has also been suggested that the immunosuppression of pregnancy may have similar effects (Biggar et al., 1989). Some of the broad number of biological and behavioral
TABLE 3-1 Factors That Affect Infectiousness of or Susceptibility to HIV
|
Infectiousness |
Susceptibility |
|
Acute primary HIV infection |
Genital ulcerations |
|
Advanced clinical stage of HIV |
Other STDs |
|
Genital ulcerations (chancroid, syphilis, herpes) |
Cervical ectopy |
|
Other STDs (gonorrhea, chlamydia, trichomoniasis) |
Lack of male circumcision |
|
Cervical ectopy |
Traumatic sex |
|
Antiretroviral therapy (decreased infectiousness) |
Lack of condom use |
|
Consistent condom use (decreased infectiousness) |
Anal intercourse |
|
|
Sex during menses |
factors that may influence either the infectiousness of or susceptibility to HIV are listed in Table 3-1.
Areas with the highest HIV prevalence correspond roughly to geographic areas where most men are not circumcised (Bongaarts et al., 1989; Caldwell and Caldwell, 1993; Caldwell, 1995a, 1995b; Piot et al., 1994). Lack of circumcision in men has been associated with an increased prevalence and incidence of HIV in several studies in Nairobi (Cameron et al., 1989), although not in studies in Rwanda or Tanzania (Borgdorff et al., 1991, cited in Piot et al., 1994; see also Chapter 4). Vaginal trauma or abrasions caused by traumatic sex or vaginal application of plant extracts or desiccating products have been associated with an increased risk of HIV acquisition among women in several countries (see Chapter 4).
Since β is defined as the probability of transmission per partnership, it depends in part on sexual behaviors, such as coital frequency, anal intercourse, and dry sex. The most important measure of sexual behavior is c, the rate of sexual partner change within a population (which through its effect on STDs also affects β). Note that c is not the average annual number of new sexual partners per person, µ, but instead is σ+ σ2/µ where σ2 is the variance of the number of new sexual partners per year. It is the number of partners associated with the average new partnership, which exceeds the average number of new partners per person, just as the size of the city in which the average person resides exceeds the average size of cities. The lesson is that both mean and variance matter. Those few individuals with many new partners ensure that infections will spread more rapidly.
The structure of the sexual mixing environment—sexual interactions among individuals—has a major influence on the shape of the HIV/AIDS epidemic (Anderson and May, 1992). A high number of sexual partners within a relatively circumscribed group partially explains the very rapid rise of HIV infection among homosexual men in the early phase of the epidemic in the United States. In Africa, sexual contact with commercial sex workers, who represent a group of high-frequency transmitters, may have been partially responsible for escalating and sustaining the epidemic in urban settings. The activities of such a core group sustain the hyperendemic levels of STDs and HIV within the core; because the core is not a closed population, the epidemic is spread and sustained beyond the core. As discussed earlier, in sub-Saharan Africa, female commercial sex workers, their male clients, truck drivers, migrant workers, and the military form the core groups of heterosexual HIV transmission, resulting in the high HIV seroprevalence and seroincidence described earlier.
It should also be noted, however, that once HIV infection has achieved high levels among the general population, the role of multiple sexual partnerships and core groups in sustaining the epidemic becomes less important (Wawer et al., 1994a). Serwadda et al. (1992) report that in rural Uganda, having one sexual partner in communities with high underlying HIV prevalence was associated with as much risk of HIV infection as having multiple partners in areas of lower prevalence. Thus, where prevalence is already high, programs targeted at high-risk groups may have limited impact. In addition, concurrency—a pattern of long-term overlapping relationships—provides the basis for stable connected networks that can amplify HIV spread through populations (Morris and Kretzschmar, forthcoming; Kretzschmar and Morris, forthcoming). Such concurrent relationships are not uncommon in African settings (Pison, 1989; Parkin and Nyamwaya, 1987).
In Africa, the high prevalence of STDs, low rates of male circumcision, substantial incidence of commercial sex, heterogeneous sexual mixing, low rates of condom use, and long duration of the HIV/AIDS epidemic may explain the current high levels of heterosexual transmission of HIV as compared with the levels in other regions of the world. Nevertheless, the recent explosion of HIV infection among heterosexuals in Asia demonstrates how quickly HIV can spread within core groups, in this case commercial sex workers, their clients, and injecting drug users, and from these groups to the general population.
Underlying ecological and behavioral factors that operate through one or more of these direct determinants of the spread of the epidemic lie on a continuum. Some of the factors are more remote and cannot be readily modified, such as cultural, economic, and social history. Factors that are more proximate on the continuum, and more modifiable, include a number of behaviors influenced by recent technological and commercial product development, such as use of condoms and spermicides for family planning and STD/HIV prevention, care
seeking for STDs, and patterns of alcohol and illicit drug use (which influence sexual behaviors).
Of particular interest is the potential effect of a vaccine against HIV on the course of the epidemic. The efficacy of a vaccine can be expressed as the product of three factors (Blower and McLean, 1994): the vaccine take (the fraction of recipients in whom the vaccine induces any immunological effect), the degree of the vaccine (the reduction in susceptibility per sexual partnership among those in whom the vaccine takes), and the fraction of those ceasing sexual activity before the vaccine-induced protection wanes. Whether a vaccine could ultimately lead to eradication of HIV and if so, the extent of coverage necessary to achieve eradication depend on R0, the reproductive rate of HIV. Modeling results show that vaccines with moderate efficacy or those administered to a population with a severe HIV epidemic (as measured by R0) could not achieve eradication; for example, 100 percent of the population at risk would need to be vaccinated if R0 were 2.0 and the efficacy of the vaccine were 50 percent (Blower and McLean, 1994). These calculations assume no change in risk behavior.
Perinatal Transmission
The second major mode of HIV transmission in Africa is perinatal, which accounts for approximately 15 to 20 percent of all AIDS cases in sub-Saharan Africa, in contrast with 5 to 10 percent worldwide (Quinn et al., 1994). The large numbers of infected children in Africa are explained by the high proportion of women infected with the HIV virus and the large number of children each women bears. Serologic surveys of pregnant women in Africa find that between 6 and 30 percent are HIV-positive (U.S. Bureau of the Census, 1994c). Sub-Saharan Africa accounts for three of every four women who have been infected with HIV worldwide (World Health Organization, 1995b).
Perinatal transmission may occur in utero through transplacental infection, at the time of delivery, or through breastfeeding or other routes. The probability of mother-to-child transmission varies according to different studies: 27 percent in Kampala, Uganda; 30 percent in Kigali, Rwanda; 39 percent in Lusaka, Zambia; 39 percent in Nairobi, Kenya; 39 percent in Kinshasa, Zaire; and 42 percent in Brazzaville, Congo (Ryder et al., 1989; Hira et al., 1989; Lallemant et al., 1989; Miotti et al., 1990). In comparison, transmission rates have been lower in North America and Europe, ranging from 7 to 30 percent (Blanche et al., 1989; Rogers et al., 1989; Oxtoby, 1990; European Collaborative Study, 1991). Unfortunately, the results of various studies published to date are not strictly comparable because of differences in recruiting strategies for prospective studies and the criteria used to determine HIV infection among children. Nevertheless, risks of perinatal transmission reported in African studies appear to be generally higher than those reported in North American and European studies, probably because of large differences between the duration and intensity of breastfeeding by seropositive
women in Europe and North America compared with women in sub-Saharan Africa. To resolve the issue of lack of standardization of study protocols, a consensus meeting was held in Ghent, Belgium, in 1992. It resulted in a definition of HIV infection in children and a clinical classification of HIV-infected mothers.
Factors that affect the probability of perinatal transmission include the disease stage and immune status during pregnancy, as measured by CD4 and CD8 cell counts; the conditions of pregnancy and delivery; the particular viral strain involved; the infectious, parasitic, and nutritional environment in which the mother and child live; the presence of chorioamnionitis and funisitis; and whether the infant is breastfed (Ryder et al., 1989; St. Louis et al., 1993; Semba et al., 1994). Of these factors, the rate of transmission associated with breastfeeding is most difficult to evaluate because infants are often exposed to HIV infection during pregnancy and at birth, as well as postnatally. Although the majority of transmission occurs pre-or intrapartum, there have been several documented cases of postnatal transmission to the infant in which the mothers were infected after delivery (Van de Perre et al., 1991; Lepage et al., 1987). Transmission in these cases occurred from mother to infant during the first year of life while the infant was being breastfed. Postnatal transmission in these instances was probably facilitated because the mothers were seroconverting and therefore had a high level of viremia, and because no immunity was passively transferred to the infants transplacentally or through breast milk. The rate of postnatal transmission estimated from these studies might be higher than that estimated for asymptomatic seropositive mothers who breastfed their infants, but is still likely to be low. The majority of infants who are infected with HIV-1 acquire the infection in utero or during childbirth. When the mother is infected prenatally, the additional risk of HIV-1 transmission via breastfeeding is estimated to be 14 percent (Dunn et al., 1992); when the mother is infected postnatally, the risk of HIV-1 transmission is 29 percent (Dunn et al., 1992). As noted earlier, the risk of perinatal transmission is much higher for HIV-1 than for HIV-2.
All babies born to an HIV-infected mother carry passively acquired maternal antibodies to HIV. Those infants who are not infected will gradually lose those antibodies, which may nevertheless persist in some cases beyond a year. Since standard tests for HIV can detect only HIV antibodies and not the virus itself, they cannot be used reliably to determine which infants born to HIV-positive mothers have been infected until the maternal antibodies have been lost (Hardy, 1991). The problem, therefore, is that the HIV status of infants born to HIV-infected mothers cannot be ascertained until well after birth. It is possible that a new inexpensive HIV test will be developed that can reliably yield positive results only if the infant is HIV-positive when cord blood is tested, although a negative result would not mean conclusively that the infant was HIV-negative (Miles et al., 1993). If such a test were developed, HIV-positive mothers might be advised that an infant who tested positive could be breastfed.
There has been much discussion concerning whether breastfeeding should be discouraged in areas where HIV is very prevalent. In 1992, a special group representing WHO/UNICEF concluded that breastfeeding should be promoted in all developing countries, regardless of HIV infection rates (World Health Organization, 1992). Breastfeeding provides a mechanism for increased spacing of births, as well as better nutrition and protection against diarrheal diseases, pneumonia, and other infections. Where the primary causes of infant deaths are infectious diseases and malnutrition, the benefits of breastfeeding outweigh the risk of HIV transmission via breastfeeding, even for women known to be infected with HIV. However, in areas with low infant mortality rates from infectious diseases, women known to be infected with HIV should be advised to use a safe feeding alternative to breastfeeding; women whose HIV status is unknown should be advised to breastfeed (World Health Organization, 1992). Several studies support the WHO/UNICEF recommendations (Choto, 1990; Kennedy et al., 1990; Nicoll et al., 1990; Ryder et al., 1991; Dunn et al., 1992; Hu et al., 1992).
Mortality among HIV-infected infants is much higher in Africa than in North America or Europe. In examining mortality rates, it is necessary to distinguish between African studies, in which survival is reported globally for infants born to seropositive mothers because of difficulty in diagnosing HIV infection during the first year, and American studies, in which mortality is often reported among infected infants only (Quinn et al., 1994). In Africa, the ultimate cause of death is not easily determined because the diagnostic tools are lacking, and because infants whose clinical deterioration is rapid do not always reach the hospital before they die. In Kinshasa, Zaire, and Brazzaville, Congo, mortality at 12 months was found to be 21 and 37 percent, respectively, for children born to HIV-seropositive mothers and 4 percent for controls (Ryder and Hassig, 1988). In a study in Malawi, mortality rates were 32 percent for the first 24 months for infants born to seropositive mothers and 11 percent for controls (Taha et al., forthcoming). In rural Rakai, Uganda, infant mortality rates were 210 per 1,000 live births for children born to HIV-seropositive mothers and 111 per 1,000 for those born to HIV-seronegative mothers (Sewankambo et al., 1994).
High mortality rates among HIV-infected children are due not only to the direct effects of HIV, but also to the profound disruption of the family unit associated with the infection (Preble, 1990). In many cases, the parents themselves are incapacitated by HIV infection, becoming progressively less capable of caring for their families. Parental loss and worsening socioeconomic status affect the survival of children in the family regardless of HIV serologic status.
In countries with large numbers of HIV-seropositive women, the impact of AIDS on overall childhood survival is already being felt. In Zimbabwe, approximately half of pediatric hospital admissions were from HIV-associated illness. However, the extent of illness among hospitalized children that is due to HIV infection and AIDS is unknown because of the difficulty involved in making a diagnosis in young children. Using available HIV seroprevalence data for women
living in several African countries, Valleroy et al. (1990) estimated the percentage increase in infant-child mortality rates that is due to HIV-attributable mortality. They estimated that infant mortality rates would increase by 6 to 38 percent in Kampala and by 1 to 6 percent in Nairobi as a result of HIV infection (Valleroy et al., 1990). WHO also estimated that within the previous 10 years, nearly 500,000 infants in Africa had been born with HIV infection (Chin, 1990). By the year 2000, there will be an additional 10 million HIV-infected children in Africa. In addition, 5 to 10 million children under age 10 are expected to become orphans during the 1990s because of the death of one or both parents from AIDS. During the decade, it is estimated that infant and child mortality rates in some African countries will increase by 50 percent as a result of AIDS.
Parenteral Transmission
The third mode of HIV infection is parenteral transmission, which includes blood transfusions, injections, and scarification. This mode represents less than 10 percent of all HIV cases in sub-Saharan Africa (Piot et al., 1988).
Blood transfusion with HIV-infected blood is known to be a very efficient means of transmission, with over 90 percent of recipients becoming infected. Unfortunately, blood screening, which is universal in industrialized countries, is not widely available in many developing areas of the world. The impact of not screening is substantial. In Central Africa, HIV seroprevalence is between 2 and 18 percent among blood donors (Mhalu and Ryder, 1988). The public health impact of exposing African populations to unscreened blood units has been documented in several countries. In one survey of 2,384 health care workers in Kinshasa, 9 percent of HIV-seropositive individuals had received blood transfusions as compared with 5 percent of HIV-seronegative individuals (Mann et al., 1986c). In a study of children who were admitted to general pediatrics or measles wards and whose mothers were HIV-seronegative, 31 percent of seropositive children had received blood transfusions, as compared with only 7 percent of seronegative children (Mann et al., 1986a). In another study of older children admitted to a pediatrics ward but not diagnosed with AIDS, 60 percent of children infected with HIV had received blood transfusions, whereas only 33 percent of the HIV-seronegative children had received transfusions (Mann et al., 1986b).
HIV transmission via blood transfusion is often associated with preventable endemic tropical diseases. In pediatric populations, malaria-associated anemia is highly prevalent, and patients are often given multiple transfusions for treatment. In one study in Kinshasa, 87 percent of blood transfusions at one hospital had been given to children for malaria-induced anemia. In that study, it was estimated that as many as 561 new pediatric cases of HIV infection would occur each year in one hospital if donated blood were not screened for HIV (Greenberg et al., 1988).
With increasing awareness of the transmission of HIV through blood transfusion,
transmission through infected blood and blood products is being reduced as appropriate screening of donated blood is introduced. Other measures being taken to protect the blood supply include recruiting donors from among low-risk population groups on a voluntary and unpaid basis. Health-care workers are also being encouraged to revise their guidelines on transfusion to ensure that the procedure is carried out only when absolutely necessary, and that saline solutions are used as blood substitutes whenever possible.
Needles and other sharp instruments used by traditional African healers are an unproved but possible important mode of HIV transmission (N'Galy et al., 1988; Berkley et al., 1989). Traditional healers normally establish patient practices in a village, or have a mobile practice in which they visit a circuit of different towns and villages. Many practitioners give their patients injectable antibiotics; injection equipment used is sterilized poorly or not at all. Ironically, the practice of giving prophylactic antibiotics may facilitate HIV transmission because the patient receives an additional injection with blood-contaminated needles and syringes. Because needle use is so ubiquitous in Africa, it is difficult to determine a true causal relationship between needle use and needle transmission. Cosmetic scarification, with its custom of using communally shared cutting utensils, is another possible mode of HIV transmission, as is tattooing with unsterilized needles.
HIV transmission through the use of unsterilized paraphernalia by injecting drug users has not been documented as a major mode of HIV transmission in Africa. In contrast with the situation in developed countries, injectable drugs such as heroin or cocaine are not commonly found or used in Africa, although they are becoming more popular in certain port areas and among the more affluent population (World Health Organization, 1994). The low incidence of injectable drug use in Africa has been attributed to the expense of the drugs and associated paraphernalia.
REMAINING GAPS IN KNOWLEDGE
Limitations of Existing African Behavioral Data
With a more complex set of data, it would be possible to understand better the social and behavioral processes that underlie the HIV/AIDS epidemic. To develop more effective AIDS-and STD-prevention strategies, additional information is needed on sexual behaviors (including sexual networking), particularly their determinants (as discussed in Chapter 4), and on barriers to the effective and rapid adoption of preventive measures, including reduced numbers of partners, safe and nonpenetrative sex, condom use, and care seeking for symptoms of STDs (as discussed in Chapter 5). Specifically, data on timing of entrance into a sexual network, dominant sexual practices, and timing of permanent or transitory exit from and re-entry into sexual networks would provide a better understanding
of the social and behavioral aspects of HIV transmission. More complex data would also provide information on the conditional probability of infection and its association with particular practices, the presence of other diseases, and possible duration dependencies.
Unfortunately, social science research related to sexuality, AIDS, and STDs in Africa has most frequently been conducted in urban areas and among groups that fit the Western concept of high-risk behavior, such as commercial sex workers and the military; other groups in the general population that may also be at high risk because of elevated underlying HIV prevalence are less likely to be contacted (Udvardy, 1990). Logistical problems associated with community-based research have further resulted in a preponderance of urban clinic, hospital, and high-risk group studies, and more recently, community-based urban and rural serosurveys involving little or no behavioral research (Kaheru, 1989; Rwandan HIV Seroprevalence Study Group, 1989). The number of studies combining HIV serologic and behavioral research among representative community-based populations, particularly among rural dwellers, remains small (Konde-Lule et al., 1989; Killewo et al., 1990; Serwadda et al., 1992; Mulder et al., 1994b). The need for such research is underlined by the fact that the vast majority of Africans reside in rural areas, and may account for the bulk of the region's HIV infection.
Only recently have researchers started to collect data on actual African sexual practices and patterns. It has been noted that ''anthropologists have devoted relatively little attention to the systematic study of sexual behavior. There are many studies of marriage and divorce, of the social, economic and ritual roles of women, of changes in male-female relationships and in other institutionalized forms of gender behavior. Where sexual practices are mentioned, however, they are often of a generalized nature" (Brokensha, 1988:167-168). Similarly, data on sexual networks and the determinants of partner selection remain limited in quantity and scope (Orubuloye et al., 1990; Obbo, 1993), so that empirical findings are incomplete at best.
We still have much to learn about the acceptance of and barriers to other aspects of HIV/AIDS prevention, including condom use. As yet, there are inadequate data on care seeking for STDs and AIDS, and on the determinants of acceptance of HIV serological testing.
When considering available data, as well as data that will become available in the next decade, we must be careful not to overgeneralize findings from one African setting to another (Ntozi and Lubega, 1990). As emphasized throughout this report, Africa is culturally diverse, and neighboring groups of closely related peoples can have very different cultural expectations. Given evidence of variability in sexual beliefs and practices, the utility of overarching models of African sexuality has been questioned (Schoepf, 1990).
To summarize, available social and behavioral data have limitations from the viewpoint of supporting the development of more effective AIDS-prevention strategies or projecting future transmission. Little is known about the range of
sexual options open to individuals; the types of sexual practices conducted with different partners; and changes brought about by migration, various degrees of urbanization, and AIDS itself. The next chapter surveys the available knowledge on these issues. Chapter 7 examines means of building an indigenous capacity for HIV/AIDS-related research in Africa.
The Need to Combine Epidemiological and Social/Behavioral Research
To date, the interpretation and utility of much epidemiological and social/behavioral research have been limited by the lack of a multidisciplinary approach. Data on reported behavior change may be difficult to assess in the absence of biological validation that such change is sufficient to reduce STD/HIV acquisition. Efforts to model the demographic effects of the HIV/AIDS epidemic are hindered by a paucity of data sets that combine fertility, mortality, migration, and other sociodemographic information with HIV serology. Conversely, serological studies that fail to collect adequate behavioral data miss an important opportunity to assess the effects of factors such as sexual practices, sexual networks, and injecting drug use practices within given populations. There is also a growing realization that the design, execution, and analysis of clinical trials for HIV vaccines, STD control, antiretroviral drugs, and genital barrier methods/viricides all depend on appropriate behavioral research to guide enrollment, ensure adherence to trial protocols, and permit adequate interpretation of epidemiological results (including the very basic need to control for potential differential behavioral change among study groups).
The disjunction between epidemiological and social/behavioral research has been due in part to a perception by behavioral scientists that biological specimen collection is difficult, intrusive, and unacceptable to subjects and to caution among clinical/epidemiological researchers in posing questions about potentially intimate behaviors within the context of studies that collect substantial biological samples. However, such reluctance to implement multidisciplinary research is rapidly losing its rationale as the STD/HIV epidemic continues to intensify and as techniques for and experience in the application of combined epidemiological/social science studies improve.
On the biological front, assessment of HIV prevalence/incidence has been greatly facilitated by the development of serological collection methods that do not call for venous blood collection. Finger prick/filter paper and saliva tests are now well established as tools in HIV epidemiology (Behets et al., 1992; Belec et al., 1994; Nyambi et al., 1994; Pappaioanou et al., 1993; Frerichs et al., 1994), and HIV assessment from urine samples is under development (Cao et al., 1988; Berrios et al., 1995). Such samples can be collected by lay personnel in nonclinic settings. Urine samples can also be used to quantify gonorrhea and chlamydia prevalence (Chernesky et al., 1994; Lee et al., 1995; Smith et al., 1995), and there is positive experience with home-based collection of self-administered vaginal
swabs for trichomonas culture (Wawer et al., 1995a) and the determination of bacterial vaginosis (Nugent et al., 1991; Speigel et al., 1983). From the behavioral viewpoint, detailed data on sexual networks and practices have been collected on subjects in diverse cultural settings (Dyson, 1992; Caldwell et al., 1993; Orubuloye et al., 1994).
A few studies have integrated biological specimen and detailed behavioral information collection in community surveys and have achieved high participation rates (Mulder et al., 1994a; Wawer et al., 1995a). The Demographic and Health Surveys (DHS) project is currently planning a pilot survey that would combine biological sample collection with standard DHS sociodemographic and contraceptive information (Cynthia Stanton, personal communication, 1995). Although experience is still limited, data suggest that a combined epidemiological/social science research approach will prove acceptable in many clinic and population-based settings.
Ethical Issues in STD/HIV Research
According to guidelines for research involving human subjects developed jointly by the Council for International Organizations of Medical Sciences (CIOMS) and WHO, when research is conducted by investigators of one country on subjects of another, the ethical standards applied should be no less exacting than if the research were carried out in the initiating country (Council for International Organizations of Medical Sciences and World Health Organization, 1992). It has also been noted, however, that "the great complexity, varied presentation, and wide distribution of HIV infection challenge this stance" (Christakis, 1988:31) and that the stated purpose of the CIOMS/WHO guidelines is, in part, to anticipate such issues and suggest they can be applied to the special circumstances of developing countries (Christakis, 1988).
Ethical standards related to biological and behavioral data collection, and to intervention trials of medical and behavioral prevention modalities, include voluntary informed consent, confidentiality, randomization, avoidance of physical/psychological risk, and provision of STD/HIV counseling and preventive services (Christakis, 1988; Barry, 1988). The concept of justice is also relevant, particularly in the case of international research: the burdens of research should be justly distributed, and disadvantaged communities should be assured of reaping an equal share of potential benefits—such as access to effective vaccines that have been tested in part in developing countries (Beauchamp and Childress, 1983; Christakis, 1988; Garner et al., 1994). Each of these issues is complex, and only a few salient points can be summarized here.
Voluntary informed consent represents an ethical imperative in behavioral and medical research. In studies conducted in Africa (or other regions, for that matter), informed consent procedures must take cultural practices into consideration. Thus, community-based research may require the consent of the head of
the household prior to the enrollment of other household members, in addition to confidential individual consent. It is obvious that consent forms require careful translation into the local language (or prevalent European language or dialect, if appropriate). In addition, the legalistic language required by U.S.-based institutional committees is frequently incomprehensible and inappropriate for African contexts. Some flexibility is required to develop appropriate formulations, while safeguarding the inviolate principal of voluntary and informed participation in research activities.
A recent review of HIV/AIDS intervention research concludes that the majority of behavioral intervention studies reported in the literature were methodologically inadequate for assessing intervention effectiveness (Oakley et al., 1995). The authors consider randomized controlled trials most appropriate for evaluating the effectiveness of behavioral interventions, but note that "it is commonly argued by behavioral researchers that random allocation to experimental groups is ethically more dubious than the uncontrolled experimentation resulting from less robust designs or from the implementation of unevaluated programs." The authors conclude that the resulting methodological weaknesses have led to a situation in which there is "a troubling lack of … soundly based preventive interventions," and they call for more randomized controlled trials to provide adequate guidance for investment in HIV/AIDS behavioral interventions (Oakley et al., 1995:484). A similar call for controlled trials has been sounded by Aral and Peterman (1993). Involving subjects in inadequately controlled trials is itself ethically questionable, as it requires commitment (and potentially some inconvenience and risk) on the part of study subjects with no assurance that this commitment will result in useful data or effective programs.
Assessment of reasonable research risk must take into consideration existing services, prevention strategies, and medical care in a given setting. It is obvious that regardless of where it is conducted, research must never take advantage of a lack of alternative services to test strategies that are of dubious benefit or are associated with inappropriate risk. However, in places where diagnostic and service delivery alternatives are very limited, testing of STD/HIV prevention, diagnostic, and treatment strategies that would not be applicable in the North American or European context may be appropriate, provided such research offers distinct potential for the development of locally appropriate and sustainable approaches that would otherwise not exist. Examples include innovative STD interventions based on limited diagnostics. In any case, the involvement of host country researchers, care givers, and policy makers in ethical decision making is essential in weighing the local risks and benefits of particular research efforts.
A potential barrier to the integration of behavioral and epidemiological research has been the perception that serological testing for HIV must always include mandatory HIV counseling (i.e., that for ethical reasons and in keeping with U.S. domestic federal regulations, subjects cannot be enrolled unless they agree, a priori, to receive their HIV results). Researchers (both behavioral and
other) have thus shied away from collecting blood specimens or at best have collected unlinked, anonymous specimens, thus severely constraining the analysis and interpretation of results. In reality, the desirability of voluntary HIV counseling may be dictated by both the value of enrolling truly representative population samples in some intervention trials (and not only those persons who agree to receive their results) and host country regulations and standards. Indeed, large population-based HIV studies in countries such as Uganda and Tanzania have adopted voluntary testing strategies, with the proviso that HIV results be made readily available and that the programs provide information and motivation for subjects to receive their results. In Nairobi, Kenya, women were tested in perinatal HIV transmission studies after giving voluntary informed consent and were given an appointment one week later to collect their results. Among the 243 women who were told that they were infected, three-quarters did not report their HIV-positive status to their partner, 1 committed suicide, 7 were beaten, and 11 were replaced by another wife or expelled from their home. The investigators subsequently adopted a policy respecting women's right not to know their HIV test results (Temmerman et al., 1995).
Finally, although it is ethically important to provide an appropriate level of HIV education and prevention services to all study participants, in a randomized trial it is also important to ensure sufficient difference between treatment and control groups to allow interpretation of data. Studies that "overtreat" control-group subjects and thus do not meet this basic criterion are themselves of questionable ethical standing, as they may lead to false conclusions and subsequent ineffective programs or the dismissal of a useful approach to HIV prevention. Services provided to control-group subjects must at a minimum meet local standards of care; it is not necessarily appropriate for them to meet U.S. standards of care. To borrow an example from medical interventions, WHO recently concluded that comparing simplified intrapartum Zidovudine (AZT) regimens with untreated controls can be ethically appropriate in settings where AZT is not otherwise available, even if such a control group would no longer be appropriate in the United States (World Health Organization, forthcoming).
RECOMMENDATIONS
KEY RECOMMENDATION 1. Basic surveillance systems for monitoring the prevalence and incidence of STDs and HIV must be strengthened and expanded.
Good social science research is as dependent as public health and medical research on reliable and valid HIV/AIDS surveillance data. With the implementation of various interventions aimed at controlling HIV transmission, periodic
monitoring of STD and HIV prevalence and incidence among selected populations is essential both for assessment of the impact of these programs and for decision making on program design and implementation.
Recommendation 3-1. More emphasis must be placed on HIV incidence studies for monitoring trends in HIV infection rates.
Although seroprevalence provides important information regarding currently infected individuals in an area, measuring incidence is also critically important for estimating the rate of change in the spread of HIV infection in a given population. In particular, data on current incidence provide the most direct and immediate information regarding the potential effects of a given intervention. Together, prevalence and incidence studies can provide information regarding the current status of the epidemic in terms of numbers of infected individuals and the rate of spread within a given population on an annual basis.
Recommendation 3-2. STD and HIV prevalence and incidence data should be combined with behavioral and demographic information.
Current surveillance systems are often limited, incomplete, and inconsistent, and they rarely measure behavioral or demographic variables. Given new, noninvasive techniques for the collection and analysis of biological specimens (including blood, urine, vaginal secretions, and saliva), accurate assessment of STD and HIV prevalence and incidence can readily be combined with behavioral and demographic information.
In conjunction with periodic serosurveys, demographic information is needed to elucidate the differential spread of STD and HIV infection in rural and urban settings and variations in seroprevalence and incidence by gender, educational level, profession, income level, age, and other demographic factors. This type of information is critical for targeting prevention messages to selected groups at risk of acquiring and transmitting HIV and for projecting the effects of HIV and other STDs on a population over time.




































