15
Drug-Induced Delay of Hypothermia
André L. Vallerand1
INTRODUCTION
Humans have always been considered tropical animals because of their high capacity for heat loss and poor resistance to cold (LeBlanc, 1975). It comes as no surprise, therefore, that numerous experiments have attempted to enhance humans' tolerance to cold. Various diets, different exercise regimens, repeated exposures to cold air or cold water, as well as the administration of various hormones and pharmacological agents, have all been used (Vallerand and Jacobs, 1992a; Vallerand et al., 1989). A pharmacological approach with thermogenic agents is certainly an attractive and promising avenue, particularly from a metabolic point of view. In view of the importance and number of animal studies in this field, they will be briefly reviewed first before focusing on human studies.
ENHANCEMENT OF COLD TOLERANCE IN ANIMALS
During studies of endocrine response of animals to the cold, it was found that the administration of several hormones (for various periods of time) could markedly delay the onset of hypothermia. Such hormones include catecholamines, thyroxine, mixtures of thyroxine and cortisol, and growth hormone (for a review see LeBlanc, 1975; Sellers, 1972). Although these studies were very useful to the understanding of cold-induced thermogenesis—also known as thermoregulatory thermogenesis—it is now apparent that they have little direct application to humans. For instance, the catecholamine-induced improvement in heat production is usually directly related to brown adipose tissue, the long-term use of high doses of thyroxine is not recommended in euthyroid subjects, the long-term use of high cortisol doses increases protein breakdown and is associated with Cushing's syndrome, and finally the long-term use of growth hormone leads to insulin resistance and is associated with acromegaly (for a review, see LeBlanc, 1975; Sellers, 1972). Interestingly, in parallel to the above, a wide variety of pharmacological agents have also been shown to be effective in delaying the onset of hypothermia in animals (Vallerand, 1993).
Dinitrophenol uncouples oxidative phosphorylation, and it is therefore an extremely potent thermogenic agent (Hall et al., 1948). It is rather unfortunate that this uncoupling effect happens to be generalized to virtually all body tissues. The strong thermogenic effect of dinitrophenol is even magnified in the presence of thyroxine (Frommel and Valette, 1950), but again it is difficult to find an application to humans. Other effective compounds in the cold include vitamin C, α-amino acids, strophanthin, chlorpromazine, coramine, and cardiozol (see Vallerand and Jacobs, 1992a). Of much greater interest today are methylxanthines.
Caffeine, the most well-known methylxanthine, is an established and effective thermogenic agent at comfortable ambient temperatures (Acheson et al., 1980; LeBlanc, 1987). In the cold, caffeine has been shown to improve cold tolerance since it significantly reduced the drop in rectal core temperature (Tre) in animals (Estler et al., 1978; Gennari, 1940). Another effective xanthine is theophylline. During the last decade, L. C. H. Wang has shown on numerous occasions that the acute administration of theophylline in cold-exposed rats produces significantly warmer Tre via its enhancement of thermoregulatory thermogenesis (Wang, 1981; Wang and Anholt, 1982; Wang and Lee, 1990). Other potent agents in the cold include amphetamines, the well-known central nervous system stimulants (Gilman and Goodman, 1970) that, with or without epinephrine, have been shown to markedly increase metabolic heat production (M) and to produce significantly warmer Tre in the cold (Pick, 1948). The present animal data could therefore be interpreted as indicating that sympathomimetics and methylxanthines form two classes of pharmacological agents that are likely to be useful for humans exposed to the cold.
ENHANCEMENT OF COLD TOLERANCE IN HUMANS
As early as 1942, it was reported that the ingestion of caffeine in men exposed to a cool ambient temperature reduced the drop in mean skin temperature (![]() sk) and thus ensured a warmer
sk) and thus ensured a warmer ![]() sk (Scheurer and Hugo, 1942). Similarly, LeBlanc (1987) found that caffeine ingestion before retiring for the night in a cool environment significantly increased oxygen consumption and provided a warmer
sk (Scheurer and Hugo, 1942). Similarly, LeBlanc (1987) found that caffeine ingestion before retiring for the night in a cool environment significantly increased oxygen consumption and provided a warmer ![]() sk, but without any change in Tre. Other studies that have analyzed the effect of caffeine in the cold have confirmed that it has little influence on Tre. Contrary to LeBlanc's observations, they have also reported that it tends to exaggerate the drop in
sk, but without any change in Tre. Other studies that have analyzed the effect of caffeine in the cold have confirmed that it has little influence on Tre. Contrary to LeBlanc's observations, they have also reported that it tends to exaggerate the drop in ![]() sk in cold air (Graham et al., 1991; McNaughton et al., 1990) or that it offers no beneficial effect in cold water, in spite of an important increase in M (Doubt and Hsieh, 1991).
sk in cold air (Graham et al., 1991; McNaughton et al., 1990) or that it offers no beneficial effect in cold water, in spite of an important increase in M (Doubt and Hsieh, 1991).
Following up on his animal work (described earlier), Wang and colleagues showed that the drop in Tre in cold-exposed individuals can be greatly reduced with the prior administration of theophylline. This was demonstrated in acute cold air studies, with the subjects either at rest or performing intermittent exercise (Wang et al., 1986, 1987, 1989). It was suggested that, as in animals, the effectiveness of theophylline in enhancing human cold tolerance resided in an enhancement of energy substrate mobilization, a factor that was thought to be limiting for cold-induced thermogenesis and consequently cold tolerance (Wang, 1978, 1981; Wang and Anholt, 1982; Wang et al., 1986). Although this theory seems well supported by the animal studies, the corresponding metabolic data in humans are not as convincing, particularly since the marked improvements in the subjects' Tre, described above, were not accompanied by any significant increase in M (Wang et al., 1986, 1987, 1989). To clarify the mechanisms by which cold tolerance can be enhanced, Vallerand and coworkers decided to reinvestigate the concept linking energy substrate mobilization, thermoregulatory thermogenesis, and cold tolerance.
Energy Substrate Mobilization and Cold Tolerance
Based on the theory above, which stated that energy substrate mobilization regulates thermoregulatory thermogenesis, a commercially available recreation and sports bar (Cold Buster™) has been recently developed. It is purported to delay markedly the onset of hypothermia in humans as a result of its energy-containing substrates and theobromine content. After proper subject familiarization (i.e., prior exposure to cold and instruments) to ensure a good reproducibility between cold tests (< 5 percent variability) (Vallerand and Jacobs, 1989) and while fasting seminude (jogging shorts only), subjects were
exposed to a single-blind protocol.2 Following the ingestion of either a placebo (artificially-sweetened water disguised as experimental treatment), pure carbohydrates (Canadian Forces Survival Rations), or the above-mentioned sports bar in isocaloric amounts (340 kcal or 1,422 kJ), subjects were exposed for 3 hours on three occasions 1 week apart to cold and wind at 5°C (41°F) and 1 m/s (3.3 ft/s), respectively.
Results showed no differences across all three trials with respect to Tre, ![]() sk, M, and the heat debt (balance of heat production minus all heat losses) (Figure 15-1). Furthermore, whole body carbohydrate oxidation was increased following the ingestion of either the pure carbohydrates or the sports bar (also high in carbohydrates), exactly as expected. For both treatments however, this increase in carbohydrate oxidation took place entirely at the expense of lipid oxidation, which resulted in no change in M (Vallerand et al., 1993). Surprised
sk, M, and the heat debt (balance of heat production minus all heat losses) (Figure 15-1). Furthermore, whole body carbohydrate oxidation was increased following the ingestion of either the pure carbohydrates or the sports bar (also high in carbohydrates), exactly as expected. For both treatments however, this increase in carbohydrate oxidation took place entirely at the expense of lipid oxidation, which resulted in no change in M (Vallerand et al., 1993). Surprised
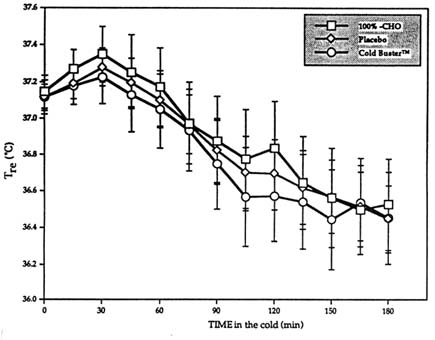
FIGURE 15-1 Influence of Cold Buster™ sports bar, pure carbohydrate (100%-CHO), or placebo on Tre profile rectal core temperature during 3-h exposure at rest to 5°C (41°F) and 1 m/s (3.3 ft/s) wind. Tre in °C (°F): 36.0°C (96.8°F), 36.2°C (97.1°F), 36.4°C (97.5°F), 36.6°C (97.9°F), 36.8°C (98.2°F), 37.0°C (98.6°F), 37.2° (99°F), 37.4°C (99.3°F), and 37.6°C (99.7°F). Results are mean ± SEM. SOURCE: Adapted from Vallerand et al. (1993).
by the fact that the beneficial effect of the sports bar on cold tolerance noted by Wang et al. (Unpublished data, University of Alberta, Edmonton, Alberta, Canada) could not be reproduced, the investigators immediately retested the theory (and the sports bar) in another study performed in different conditions with different subjects. Identical results were obtained (Figure 15-2).
The first question that arose from the conflicting results between these studies and those of Wang was related to laboratory procedures: Could methodological differences possibly explain the fact that the energy substrate mobilization theory of Wang et al. (1986, 1987, 1989) could not be confirmed? A first possible explanation was that the subjects were at rest in the cold in the sports bar study. With shivering alone, M rose to about 2.5 to 3.5 times over resting M (Vallerand et al., 1992, 1993). Wang and colleagues have suggested, based on the results of some studies (Wang et al., 1987, 1989), but not all (Wang et al., 1986), that a higher M, such as that seen during intermittent exercise, was required to fully exploit the energy-mobilizing effect of ingesting substrates and/or xanthines on thermoregulatory thermogenesis and cold tolerance. Vallerand and coworkers therefore attempted to reproduce such a protocol. However, when M was raised in the cold by about 3.4 times and by
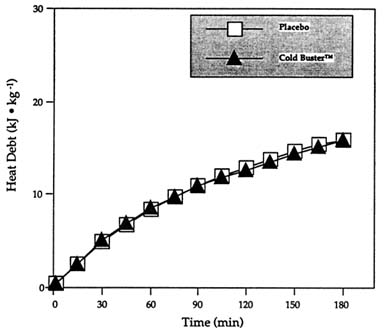
FIGURE 15-2 Heat debt (minute-by-minute balance of heat production minus all heat losses) during 3-h exposure at 10°C (50°F), < 0.4 m/s (1.3 ft/s) wind following the ingestion of Cold Buster™ sports bar or placebo. SOURCE: Adapted from Vallerand et al. (1992).
as much as 6.7 times over resting values at thermal neutrality during an intermittent rest-exercise protocol respectively (similar to Wang et al. [1987, 1989]), the ingestion of a food supplement containing substrates and theobromine had no influence on M, Tre, ![]() sk (not shown), or heat debt (Vallerand and Jacobs, 1993a) (Figures 15-1–15-3). Exactly as in the two previous trials described in Figures 15-1 and 15-2, the food supplement, which was high in carbohydrates, also increased whole body carbohydrate oxidation at the expense of lipid oxidation with no change in M (Figure 15-3).
sk (not shown), or heat debt (Vallerand and Jacobs, 1993a) (Figures 15-1–15-3). Exactly as in the two previous trials described in Figures 15-1 and 15-2, the food supplement, which was high in carbohydrates, also increased whole body carbohydrate oxidation at the expense of lipid oxidation with no change in M (Figure 15-3).
A second possible explanation for the conflicting results may have been that the dose of energy substrates was simply not optimal in the sports bar study to observe a positive influence of substrate mobilization on M in this laboratory. However, this explanation is unlikely for two precise reasons. First, previous trials, like those in this laboratory, have mainly used about 300 kcal (1,255 kJ) (Vallerand and Jacobs, 1993a; Vallerand et al., 1992, 1993; Wang et al., 1986, 1987, 1989). Second, this laboratory recently completed a study where subjects ingested as much as 710 kcal (2,970 kJ) in a high-carbohydrate supplement in an effort to maximize substrate mobilization in the cold (Vallerand and Jacobs, 1993b). As above, this treatment had no beneficial effect
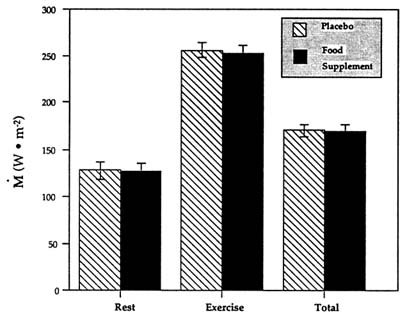
FIGURE 15-3 Average metabolic heat production (M) in the cold following the ingestion of a food supplement or a placebo while performing intermittent exercise. Data are expressed in watts per square meter of body surface area for the periods of rest, exercise, and timeweighted total. SOURCE: Adapted from Vallerand and Jacobs (1993a).
on any measured thermal physiology parameters, even though carbohydrate mobilization and oxidation were increased at the expense of lipid mobilization and oxidation. Although energy substrate utilization remains crucial and essential to fuel M in the cold (Vallerand and Jacobs, 1992b), data from this laboratory do not support the theory that energy substrate mobilization is limiting for thermoregulatory thermogenesis in humans. It is suggested that other factors are required to enhance heat production, which in turn could reduce the heat debt and thus ameliorate cold resistance. Additional experiments were carried out to explore these factors.
The Effects of β-Adrenergic Agonists and Methylxanthines on Cold Tolerance
Numerous studies have firmly established that β-adrenergic drugs—such as ephedrine—and xanthines—such as caffeine and theophylline—significantly increase resting M in humans who are exposed to comfortable ambient temperatures, either on a short- or long-term basis (Astrup et al., 1985, 1992; Dulloo and Miller, 1986; Dulloo et al., 1990). These studies also demonstrated the efficacy and safety of these antiobesity compounds. Whether mixtures of ephedrine and xanthines can enhance cold thermogenesis and cold tolerance, and by which mechanisms, was examined in two separate studies.
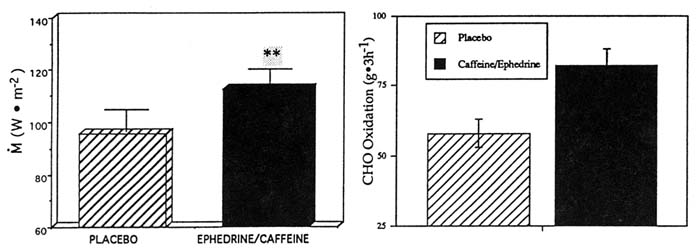
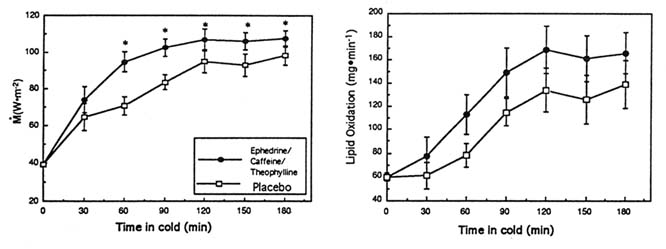
In one study, the ingestion of ephedrine and caffeine (1 mg/kg, 2.5 mg/kg, respectively; double-blind protocol) in the cold significantly increased overall M by 19 percent compared to the same subjects receiving the placebo (p < 0.05) (Figure 15-4). This increment in M was fueled almost entirely by a large increase in whole body carbohydrate oxidation (p < 0.05), with no change in lipid or protein oxidation and was associated with significantly smaller drops in (and thus warmer) Tre and ![]() sk, a lesser body heat debt, and slightly higher catecholamine levels. In another study of ephedrine and xanthines in the cold, the ingestion of ephedrine, caffeine, and theophylline (44, 60, and 100 mg, respectively) significantly increased M by 17 percent over 3 hours in the cold, in contrast to the placebo condition in the same subjects (Figure 15-5). This was achieved through a significantly greater whole body lipid oxidation and a slightly greater carbohydrate oxidation (Figure 15-5). This enhanced thermoregulatory thermogenesis was directly related to a significantly lower heat debt, slightly warmer Tre, and significantly warmer
sk, a lesser body heat debt, and slightly higher catecholamine levels. In another study of ephedrine and xanthines in the cold, the ingestion of ephedrine, caffeine, and theophylline (44, 60, and 100 mg, respectively) significantly increased M by 17 percent over 3 hours in the cold, in contrast to the placebo condition in the same subjects (Figure 15-5). This was achieved through a significantly greater whole body lipid oxidation and a slightly greater carbohydrate oxidation (Figure 15-5). This enhanced thermoregulatory thermogenesis was directly related to a significantly lower heat debt, slightly warmer Tre, and significantly warmer ![]() sk.
sk.
These results clearly demonstrate the beneficial effects of mixtures of ephedrine with one or more xanthines in the cold. The mixtures tested significantly enhanced thermoregulatory thermogenesis, produced warmer body temperatures, and reduced the heat debt (Figure 15-4 and 15-5). When compared to the results of other similar cold studies (Table 15-1), the improvements in several important thermophysiological parameters such as the
TABLE 15-1 Summary of Important Studies on the Pharmacological Approach to Increase Human Cold Resistance: Comparisons with a Placebo
changes in M, dry heat loss (R + C), heat debt (S), as well as ΔT re and ∆![]() sk appear more favorable with ephedrine and xanthines than with the other treatments. Further, this lab has recently been made aware that a TTCP (the Technical Cooperation Program) Nation (N2) has fielded a mixture of ephedrine/xanthine as a medical countermeasure against performance degradation for survival conditions (Personal communication, I. Jacobs, Defence and Civil Institute of Environmental Medicine, North York, Ontario, Canada, 1996). Table 15-1 also highlights the advantages of a heat balance analysis and the necessity of performing it more often since the heat debt appears as a more robust index of cold resistance than Tre alone.
sk appear more favorable with ephedrine and xanthines than with the other treatments. Further, this lab has recently been made aware that a TTCP (the Technical Cooperation Program) Nation (N2) has fielded a mixture of ephedrine/xanthine as a medical countermeasure against performance degradation for survival conditions (Personal communication, I. Jacobs, Defence and Civil Institute of Environmental Medicine, North York, Ontario, Canada, 1996). Table 15-1 also highlights the advantages of a heat balance analysis and the necessity of performing it more often since the heat debt appears as a more robust index of cold resistance than Tre alone.
The exact mechanism of cellular action of ephedrine and xanthines in the enhancement of thermoregulatory thermogenesis is still uncertain, but an increase in sympathomimetic effect is likely. On the one hand, it is well known that ephedrine is an adrenergic agonist that stimulates both β and α receptors and can increase plasma catecholamine levels (Dulloo et al., 1990; Hoffman and Lefkowitz, 1990). On the other hand, xanthines, such as caffeine and theophylline, act either by inhibition of phosphodiesterase activity, by antagonistic effect at the level of adenosine receptors, or by a translocation of intracellular calcium ions (Dulloo et al., 1990; Rall, 1990). By combining these actions, ephedrine-xanthines compounds could thus increase liver and skeletal muscle glycogenolysis, white adipose tissue lipolysis, and the activation of the sympathetic nervous system (Astrup et al., 1985, 1992; Dulloo and Miller, 1986; Dulloo et al., 1990; Hoffman and Lefkowitz, 1990; Rall, 1990; Vallerand et al., 1989). An increase in either whole body carbohydrate or lipid oxidation would probably be dependent on the actual dosage of ephedrine and xanthines used. However, it is not clear which particular tissue is responsible for the ephedrine and xanthine-induced increase in thermoregulatory thermogenesis. It could well take place in the skeletal muscle, which is a major site of heat production during shivering (see LeBlanc, 1975; Vallerand et al., 1989) and during the ephedrine-induced thermogenesis at comfortable ambient temperature (Astrup et al., 1985). Since ephedrine-xanthines do increase heat production without muscular contractions at comfortable ambient temperatures and since ephedrine-xanthines increase cold thermogenesis without any indication of greater shivering, it is thus possible that ephedrine-xanthines act via mechanisms unrelated to shivering, either at the level of the striated muscle or at the level of the smooth muscle in the vascular bed, as recently suggested (Colquhoun and Clark, 1991). These exciting hypotheses require further study.
AUTHOR'S CONCLUSIONS AND RECOMMENDATIONS
In summary, once the insulation provided by the microclimate and by peripheral vasoconstriction has been maximized, increasing metabolic heat production (M) represents the last line of defence against the cold. This is best illustrated in survival conditions where additional insulation from the environment may not be available. Although several techniques have been used to further enhance M and cold tolerance, a pharmacological approach has attracted a lot of attention. Indeed, recent experiments in cold-exposed humans have shown that the ingestion of a mixture of ephedrine and caffeine enhances M and reduces both the heat debt (body heat deficit) and the drop in Tre (P < 0.05). The ingestion of a slightly different thermogenic mixture, where theophylline (another methylxanthine) was added to ephedrine and caffeine, produced about the same beneficial effect (P < 0.05). Although some authors have reported that theophylline alone reduces the drop in Tre (i.e. warmer Tre),
thus enhancing cold tolerance, these improvements were found difficult to explain in view of the absence of changes in M and mean skin temperature. A theobromine-based (another xanthine) Recreation and Sports bar (Cold Buster™) is purported to greatly reduce the drop in Tre and delay the onset of hypothermia. However, these claims could not be confirmed in two different studies performed in this lab. The administration of caffeine (alone) did not alter Tre in the cold, despite an increase in M in some studies. In conclusion, it is suggested that ephedrine/xanthine mixtures represent at the moment, a safe and one of the best pharmacological means to enhance thermoregulatory thermogenesis and cold resistance. Future work is required to determine their effectiveness at deeper levels of hypothermia as well as during much longer albeit less severe ''survival-like" exposures.
ACKNOWLEDGMENTS
It is with great pleasure that the expert assistance of Ingrid Schmegner, the collaboration of Ira Jacobs, as well as the dedication of all subjects employed throughout the cold stress studies are acknowledged by the author.
REFERENCES
Acheson, K.J., B. Zhorska-Markiewicz, P. Pittet, K. Anantharaman, and E. Jéquier 1980 Caffeine and coffee: Their influence on metabolic rate and substrate utilization in normal weight and obese individuals. Am. J. Clin. Nutr. 33:989–997.
Astrup, A., J. Bulow, J. Madsen, and N.J. Christensen 1985 Contribution of BAT and skeletal muscle to thermogenesis induced by ephedrine in man. Am. J. Physiol. 248 (Endocrinol. Metab. 11):E507–E515.
Astrup, A., B. Buemann, N.J. Christensen, S. Toubro, G. Thorbek, O.J. Victor, and F. Quaade 1992 The effect of ephedrine/caffeine mixture on energy expenditure and body composition in obese women. Metabolism 41(7):686–688.
Colquhoun, E. Q., and M.G. Clark 1991 Open question: Has thermogenesis in muscle been overlooked and misinterpreted? News Physiol. Sci. 6:256–259.
Doubt, T.J., and S.S. Hsieh 1991 Additive effects of caffeine and cold water during submaximal leg exercise. Med. Sci. Sports Exerc. 23(4):435–442.
Dulloo, A.G., and D.S. Miller 1986 The thermogenic properties of ephedrine methylxanthine mixtures: Human studies. Int. J. Obes. 10:467–481.
Dulloo, A.G., J. Seydoux, and L. Girardier 1990 Dietary and pharmacological effectiveness of thermogenic stimulation in obesity treatment. Prog. Obes. Res. Pp. 135–144.
Estler, C.J., H.P.T. Ammon, and C. Herzog 1978 Swimming capacity of mice after prolonged treatment with psychostimulants. I. Effect of caffeine on swimming performance and cold stress. Psychopharmacol. 58:161–166.
Frommel, E., and F. Valette 1950 De l'influence de la thyroxine sur la temperature dinitrée du cobaye . Arch. Sci. Genève 3(1):54–56.
Gennari, G. 1940 The effect of caffeine on passive hypothermia in experimental cooling. Rendic Ist. San. Pubb. Roma 2(3):515–522.
Gilman, A.G., and L.S. Goodman 1970 Sympathomimetics. P. 187 in The Pharmacological Basis of Therapeutics, A.G. Gilman and L.S. Goodman, eds. New York: Macmillan.
Graham, T.E., P. Sathasivam, and K.W. McNaughton 1991 Influence of cold, exercise and caffeine on catecholamines and metabolism in men. J. Appl. Physiol. 70(5):2052–2058.
Hall, V.E., F.P. Attardo, and J.H. Perryman 1948 Influence of dinitrophenol on body temperature threshold for thermal polypnea. Proc. Soc. Exp. Biol. Med. 69:413–415.
Hoffman, B.B., and R.J. Lefkowitz 1990 Catecholamines and sympathomimetics. P. 187 in The Pharmacological Basis of Therapeutics, A.G. Gilman, T.W. Rall, and A.S. Nies, eds. New York: Pergamon.
LeBlanc, J. 1975 Man in the Cold. Springfield, Ill.: Charles C Thomas.
1987 Various influences on the response to sleeping in the cold. Pp. 35–43 in 27th DRG Seminar on Sleep and its Implications for the Military. NATO Report DS/A/DR(88)47. Lyon, France: North Atlantic Treaty Organization.
McNaughton, K.W., P. Sathasivam., A.L. Vallerand, and T.E. Graham 1990 Influence of caffeine on metabolic responses of men at rest in 28 and 5°C. J. Appl. Physiol. 68(5):1889–1895.
Pick, E.F. 1948 Thermogenic action of adrenalin and benzedrine on the brain. Arch. Int. Pharm. Dyn. 77(2):219–225.
Rall, T.W. 1990 Drugs used in the treatment of asthma. The methylxanthines, chromolyn sodium and other agents. P. 618 in The Pharmacological Basis of Therapeutics, A.G. Gilman, T.W. Rall, and A.S. Nies, eds. New York: Pergamon.
Scheurer, O., and W. Hugo 1942 The effects of cardiovascular agents on skin temperature. Munchen Med. Wochensch. 89:907–911.
Sellers, E.A. 1972 Hormones in the regulation of body temperatures. Pp. 57–71 in The Pharmacology of Thermoregulation, E. Schonbaum and P. Lomax, eds. Basel, Switzerland: Karger.
Vallerand, A.L. 1993 Effects of ephedrine/xanthines on thermogenesis and cold tolerance. Int. J. Obes. 17(suppl 1):S53–S56.
Vallerand, A.L., and I. Jacobs 1989 Influence of cold exposure on plasma triglyceride clearance in humans. Metabolism 39:1211–1218.
1992a Review of pharmacological approach to improve cold tolerance. Pp. 119–123 in DCIEM Report No. 92-10. North York, Ontario: Defence and Civil Institute of Environmental Medicine.
1992b Energy metabolism during cold exposure. Int. J. Sports Med. 13(suppl 1):S191–S193.
1993a Interaction of a food supplement, intermittent exercise and cold exposure on heat balance. DCIEM Report No. 93-19. North York, Ontario: Defence and Civil Institute of Environmental Medicine.
1993b High-energy food supplement, energy substrate mobilization and heat balance in cold-exposed humans. DCIEM Report No. 93-36. North York, Ontario: Defence and Civil Institute of Environmental Medicine.
Vallerand, A.L., I. Jacobs, and M.F. Kavanagh 1989 Mechanism of enhanced cold tolerance by an ephedrine-caffeine mixture in humans. J. Appl. Physiol. 67(1):438–444.
Vallerand, A.L., I.F. Schmegner, and I. Jacobs 1992 Influence of the Cold Buster™ sports bar on heat debt, mobilization and oxidation of energy substrates. DCIEM Report No. 92-60. North York, Ontario: Defence and Civil Institute of Environmental Medicine.
Vallerand, A.L., P. Tikuisis, M.B. Ducharme, and I. Jacobs 1993 Is energy substrate mobilization a limiting factor for cold thermogenesis? Eur. J. Appl. Physiol. 67:239–244.
Wang, L.C.H. 1978 Factors limiting maximum cold-induced heat production. Life Sci. 23:2089–2098.
1981 Effects of feeding on aminophylline induced supra-maximal thermogenesis. Life Sci. 29:2459—2466.
Wang, L.C.H., and E.C. Anholt 1982 Elicitation of supramaximal thermogenesis by aminophylline in the rat. J. Appl. Physiol. 53:16–20.
Wang, L.C.H., and T.F. Lee 1990 Enhancement of maximal thermogenesis by reducing endogenous adenosine activity in the rat. J. Appl. Physiol. 68:580–585.
Wang, L.C.H., S.F.P. Man, and A.N. Belcastro 1986 Improving cold tolerance in men: Effects of substrates and aminophylline. Pp. 22–26 in Homeostasis and Thermal Stress, K. Cooper, P. Lomax, E. Schonbaum, and J. Veale, eds. Basel, Switzerland: Karger.
1987 Metabolic and hormonal responses in theophylline-increased cold resistance in males. J. Appl. Physiol. 63:589–596.
Wang, L.C.H., S.F.P. Man, A.N. Belcastro, and J.C. Westly 1989 Single, optimal oral dosage of theophylline for improving cold resistance in man. Pp. 54–58 in Thermoregulation: Research and Clinical Applications, P. Lomax and E.Schonbaum, eds. Basel, Switzerland: Karger.