21
Oxidative Stress at High Altitudes and Effects of Vitamin E
I. Simon-Schnass1
INTRODUCTION
In the course of evolution, life found a way to use the high-energy potential of reducing oxygen to water within the mitochondrial respiratory chain. At the same time, however, it was not possible to prevent the formation of other reduced forms of oxygen with toxic properties (Reznick et al., 1993). Therefore, the life and development of cells within an oxygen-containing environment would not be possible without a defense system against these prooxidants. This defense system comprises enzymes as well as low-molecular weight compounds that are categorized as antioxidants (Halliwell et al., 1987).
Aerobic life is characterized by a steady state between prooxidants and antioxidants. To maintain this homeostasis a continuous regeneration of the antioxidative capacity is necessary. If this capacity is insufficient, there is an accumulation of oxidative damage. A shift to the prooxidative state is called oxidative stress (Sies, 1993). The antioxidative ''strategy" of aerobic cells is targeted at inhibiting or blocking potentially toxic oxygen species or their
derivatives at the various levels of formation, or blocking their reaction with biomolecules (Elstner, 1990). There is growing evidence that oxidative injury mediated by free radicals is an important factor in various pathologies, including adverse metabolic reactions at high altitudes. Table 21-1 summarizes the many intra- and extracellular sources of free radicals. The mitochondrial electron transport chain as an important source of free radicals is described in more detail below.
PHYSICAL EXERCISE AS A CAUSE OF OXIDATIVE STRESS
Each physical movement is associated with the turnover of substrates including oxygen and thus with energy consumption. From a simplified point of view, blood circulation is no more than an aid to substrate delivery and removal.
Circulation increases during dynamic activity; otherwise, the increased consumption of substrates and the removal of end products would not be possible. However, muscular contraction also leads to vascular compression, which in turn causes a regional and short-term reduction in circulation with limited hypoxia. This effect particularly applies to persons participating in sports activities with concentrated power development. However, when performing
TABLE 21-1 Sources of Oxidative Stress
|
1) Autoxidation |
2) Enzymatic Reactions |
|
Redox cycling |
Cytochrome P-450 |
|
Quinones |
Hemoglobin |
|
Redox dyes |
Xanthine oxidase |
|
Melanin |
Peroxidases |
|
Iron complexes |
Aldehyde oxidases |
|
3) Cellular Sources |
4) Environment |
|
Mitochondrial and microsomal electron transport chains |
UV radiation |
|
Leukocytes |
Ultrasound |
|
Macrophages |
γ rays, X rays |
|
|
Toxic chemicals, including drugs |
|
|
Metal ions |
|
SOURCE: Adapted from Elstner (1990). |
|
sports with an emphasis on overall endurance rather than on individual muscles, the transport capacities of the blood and oxygen exchange into the cell become the limiting factors. In both cases, transient oxygen deficiency can occur, despite the high oxygen turnover (Berg et al., 1987).
Classical sports physiology has concerned itself intensively with the phenomena of availability, turnover, and regeneration of substrates for obtaining energy. In addition, the effects on the structured components of the cell are of essential relevance, a fact that has as yet hardly been acknowledged. It is no coincidence that compartmentalization of the cell is a major feature of higher organisms. The cell membrane contains important switching points for transport processes as well as for reactive processes. Indeed this is the basis of cell function. Membranes participate in some form in the vast majority of metabolic processes. The main objective, even in the case of intense physical performance, is always to maintain the integrity of the membrane structures or at least to ensure that changes are reversible (Berg et al., 1987).
The major cause of membrane damage is the formation of free radicals that can arise from various processes during metabolism. In the aerobic energy supply, most of the adenosine triphosphate (ATP) is formed during endoxidation, when electrons of a substrate (e.g., pyruvate or succinate) are transformed via a so-called redox chain to oxygen. The end product formed is water (reduction of the oxygen to water), where:
It is known that free radicals can arise in the case of incomplete oxygen reduction. If less than four electrons are made available, the following activated oxygen forms are created:
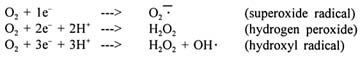
In the resting state, 250 to 300 ml of oxygen per minute are usually taken up. Under physical exertion, oxygen uptake can increase to 4,700 ml/min, or even more, depending on training conditions. Around 3 to 10 percent of the metabolized oxygen is not completely reduced to water but to these different radicals (Demopoulos et al., 1986). The increased formation of oxygen radicals is also termed oxidative stress (Demopoulos et al., 1986).
Other metabolic processes also lead to the generation of free radicals. Physically strenuous activity induces certain inflammatory-like reactions that are associated with the increased formation of radicals (e.g., leukocyte activation with phagocytosis, leukotriene synthesis). In addition, radicals from outside are taken up into the body, for example, from air pollution, ultraviolet radiation, and cigarette smoke. These sources can also be of great relevance
because the damaging reactive mechanisms of these radicals do not differ greatly from the reactions mentioned above (Packer, 1984).
One characteristic of free radicals is their high, at times extremely high, reactive capability. Activated oxygen species react very readily with other substances and thus form other radicals. Particular mention should be given to reactions with lipids. A generalized scheme of lipid peroxidation is given in Figure 21-1.
Free radicals also react with proteins, particularly those that contain functional sulfhydryl (SH) groups, which leads to inactivation and the formation of carbonyls. Destructive chain reactions, which will be mentioned later, can be set off that may lead to functional impairments ranging up to complete destruction of the cell (Packer, 1984).
In the case of extreme physical activity (the term extreme should always be considered in a relative manner and defined on an individual basis), the following factors can lead to an increased discharge of free radicals. Oxygen turnover can be increased up to 20 times that of resting consumption. Accordingly, the incomplete reduction of oxygen to water and the associated formation of free radicals can also increase. Hypoxic cells are particularly susceptible to oxidative stress, a phenomenon commonly referred to as the oxygen
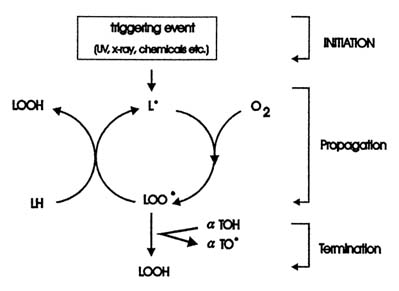
FIGURE 21-1 Scheme for lipid peroxidation chain reactions initiated by free radicals. LH, lipid molecule; L·, lipid radical; LOO·, lipid peroxyl radical; LOOH, lipid hydroperoxyl radical; α-TOH, alpha-tocopherol; α-TO·, alpha-tocopherol radical. SOURCE: Adapted from Sies (1993).
paradox (Demopoulos et al., 1986). If there is not enough oxygen available to accept electrons, they will be transferred to other low-molecular-weight molecules, which in turn induce radical chain reactions (Demopoulos et al., 1986). When the pH drops (metabolic acidosis during extreme physical exertion), superoxide anion (![]() ) can be converted into the highly toxic peroxyl (OOH·) radical.
) can be converted into the highly toxic peroxyl (OOH·) radical. ![]() is derived from the respiratory chain, whereas the required hydrogen is supplied by the lactic acid that is formed. At physiological pH, only around 1 percent of the superoxide is converted to a peroxyl radical. However, the percentage increases with increasing acidosis (Demopoulos et al., 1986; Simon-Schnass, 1993).
is derived from the respiratory chain, whereas the required hydrogen is supplied by the lactic acid that is formed. At physiological pH, only around 1 percent of the superoxide is converted to a peroxyl radical. However, the percentage increases with increasing acidosis (Demopoulos et al., 1986; Simon-Schnass, 1993).
THE DEFENSE SYSTEM
As mentioned above, the antioxidative strategy of aerobic cells is targeted at inhibiting or blocking potentially toxic oxygen species or their derivatives at the various levels of formation or blocking their reaction with biomolecules (Elstner, 1990). The most important components of this defense system against oxidative stress are:
Vitamins and other antioxidants
- vitamin E,
- vitamin C,
- β-carotene,
- flavonoids,
- ubiquinones, and
- glutathione.
Enzymes
- catalases,
- superoxide dismutases
- peroxidases, and
- transferases.
Typical detoxification enzymes are superoxide dismutase, the catalases, and various peroxidases. If the organism is confronted with radicals, as is the case in physical activity, these enzymes are reactively synthesized to an elevated degree (Simon-Schnass, 1993). Clearly, a certain time lag between the occurrence of the noxae (radicals) and the higher enzymatic level as a protective measure cannot be avoided. If the stimulus for the increased enzyme formation ceases, the enzyme drops back to its original level (Simon-Schnass, 1993). This effect suggests that enzymatic protection against oxidation can only be effectively boosted by regularly taking part in sports. From this point of view, athletes who train irregularly and/or at varying degrees of intensity
are relatively insufficiently protected against oxidative stress by enzymes (Simon-Schnass, 1993).
However, the defense system comprises various antioxidants in addition to the antioxidative enzymes. Many substances that function as antioxidants in vitro also occur physiologically (in vivo). Until now, practical relevance has only been found for vitamins E and C, β-carotene, and other carotenoids, as well as glutathione, some flavonoids, the ubiquinones, and, in certain cases, uric acid, taurine, the amino acids cysteine and histidine, and several xenobiotics (Elstner, 1990).
Vitamin E is a lipophilic radical inhibitor. Correspondingly, its action is mainly limited to the region of the lipophilic membrane. Because it is stored there in the immediate vicinity of the substances in danger of oxidation, vitamin E can very effectively circumvent the peroxidation of fatty acids, as well as the oxidation of cholesterol and proteins. This is of great importance for membrane integrity. Even during vitamin E deficiency, no other antioxidant has yet been shown to serve as a substitute to break the radical chain reaction in the lipophilic area of the cell (Elstner, 1990). This means that vitamin E cannot be replaced by any other substances at its functional site. In addition to the inhibition of lipid peroxidation in the lipophilic centers of biological membranes, vitamin E also appears to play a role in the repair of oxidized amino acids, which otherwise may contribute to the formation of lipofuscin (cross-linking of protein and other high-molecular fragments). In this way, vitamin E could also be attributed a special role in the repair of damaged amino acids in the integral membrane proteins (Elstner, 1990).
Vitamin C is known as an excellent water-soluble antioxidant with a strong reducing potential. It is capable of scavenging a wide variety of different oxidants. For example, it has been shown to scavenge effectively superoxide anions, hydrogen peroxide, the hydroxyl (OH·) radical, aqueous peroxyl radicals, as well as singlet oxygen (Stocker and Frei, 1991). As an antioxidant, vitamin C undergoes a two-electron oxidation to dehydroascorbic acid with the intermediate formation of a relatively unreactive ascorbyl radical (Bielski and Richter, 1975). Dehydroascorbic acid is unstable and hydrolyses readily to diketogulonic acid. However, it also can be reduced back to ascorbic acid by glutathione in erythrocytes and other blood cells (Hughes and Maton, 1968).
As a water-soluble substance, vitamin C is predominantly found in plasma and the aqueous phase of the cell. In particular, vitamin C deactivates the extracellular oxidants generated by activated neutrophils (Halliwell et al., 1987) but also quenches radicals diffused to the extracellular space. Moreover, vitamin C interacts with the membrane-bound vitamin E by reducing the tocopheryl radical back to tocopherol (Packer et al., 1979).
In addition to vitamin E, β-carotene, which is also lipid soluble, has been shown to be an effective chain-breaking antioxidant (Sies, 1993). The peroxyl-trapping activity of β-carotene and possibly other carotenoids is
dependent on the partial pressure of oxygen (pO2). It is less effective in the presence of air, but becomes a good peroxyl radical trap at the low pO2 that prevails in biological tissues (Burton and Ingold, 1984). At very low pO2 (4 torr), it inhibited lipid peroxidation even better than vitamin E (Vile and Winterbourn, 1988).
The antioxidative strategy of aerobic cells is aimed at reducing or blocking the potentially toxic effects of activated oxygen species that are generated by various metabolic processes and which may cause oxidative stress. The question now is whether oxidative stress occurs to a greater extent during a prolonged stay at high altitudes. In the following discussion, several possible sources of free-radical generation are discussed.
EVIDENCE FOR INCREASED OXIDATIVE STRESS AT HIGH ALTITUDES
With respect to life, the main characteristics of high altitude are the decreased availability of oxygen, with its influence on metabolism and thereby on physical and mental performance, as well as increased ultraviolet radiation, wide temperature differences, sometimes increased psychological stress, dehydration, and insufficient nutrient intake.
High-Altitude Hypoxia
Aerobic energy supply is necessary for all higher life forms. In accordance, an inadequate oxygen supply leads to unnoticed metabolic impairments that may ultimately create a direct life threat. A drop in arterial oxygen pressure below a nominal level may be due to a number of factors, including a decrease in inhaled oxygen because of the low partial pressure that occurs at high altitudes.
Acute exposure to high altitudes results in a decreased amount of oxygen available to the body and thus to a decreased arterial blood oxygen saturation. This leads to a reduction in maximal aerobic power of approximately 1 percent for every 100 m (328 ft) above 1,500 m (4,921 ft) (Buskirk, 1966). However, because the body has compensation mechanisms to counteract the resulting hypoxia, acclimatization is possible. This effect includes an increase in hematocrit and hemoglobin to improve the transport capacity for oxygen (compensatory polycythemia) and a shift in oxygen binding to hemoglobin, as well as an increase in capillary density, mitochondria, and tissue myoglobin (Eckhardt et al., 1989; Hannon et al., 1969). Moreover, muscle glycogen is saved, and mobilization and metabolism of free fatty acids are improved as shown by a decrease in blood lactate and ammonia during submaximal exercise (Young et al., 1982, 1987). These changes are remarkably similar to
those induced by endurance training and are aimed at reducing the oxygen demand of the body.
Direct Studies on Oxidative Stress at High Altitudes
The exhalation of pentane can be considered a result of lipid peroxidation (Kappus, 1985). In animal as well as in human studies it was related to vitamin E status (Bland, 1986; Dillard et al., 1978; Schwarz, 1962). Various conditions, such as an increased energy turnover and/or hypoxia, favor lipid peroxidation (Demopoulos et al., 1984; Dillard et al., 1978). Vitamin E is one of the most effective membrane-bound radical scavengers. In animals, exercise-induced lipid peroxidation was prevented by vitamin E administration (Dillard et al., 1977; Packer, 1984).
To test if there is indeed an increased oxidative stress leading to increased lipid peroxidation at high altitudes, the amount of exhaled pentane was determined by gas chromatography (Simon-Schnass and Pabst, 1988). Twelve mountaineers were supplemented with 400 mg of vitamin E per day (test group) or a placebo (control group) during an expedition to K2 (8,611 m [28,250 ft]). Baseline tests were done in Scardu, Pakistan. Supplementation began upon departure from Scardu to base camp, which was established at 5,100 m (16,732 ft) 2 weeks after departure from Scardu. Pentane was measured twice—the baseline value at Scardu and a second value after a 2-wk stay at base camp. The results are shown in Figure 21-2.
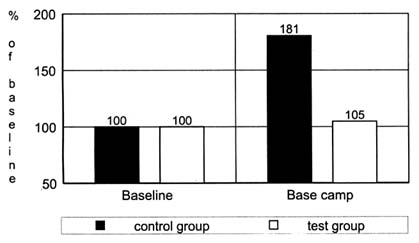
FIGURE 21-2 Pentane exhalation (percent of baseline) of 12 mountaineers during an expedition to K2 with (test group) or without (control group) a supplementation with 400 mg of vitamin E per day. SOURCE: Adapted from Simon-Schnass and Pabst (1988).
There was no significant difference between the initial pentane exhalation of the two groups, but after 4 weeks of supplementation and 2 weeks at high altitudes, the exhaled pentane increased significantly (p < 0.01) in the control group. In the treatment group, there was no noticeable change. It can be concluded from this study that high-altitude climbing incurs a considerable risk of metabolically induced cell damage. This risk can be counteracted by supplementation with antioxidants.
As mentioned above, membranes are most susceptible to oxidative stress because of their high amount of polyunsaturated fatty acids. Erythrocytes are able to change their shape due to their membrane fluidity, among other factors. The loss of this fluidity can be influenced by factors such as acidosis, hyperthermia, and immobilization (stasis), due to, for example, aggregation, membrane defects, and cell aging (Thews and Vaupel, 1982). Because the important underlying phenomenon is considered to be an oxidative change of membrane lipids and proteins, it is suggested that these changes may be triggered by free radicals (Kappus, 1985). The filterability of the red blood cells is considered a measurement of their flexibility (Schmidt-Schönbein et al., 1973). This parameter was tested during two other expeditions at high altitudes.
During the first study, 13 mountaineers were supplemented with either 400 mg of vitamin E per day (test group) or a placebo (control group) during an expedition to Annapurna (8,091 m [26,545 ft]) (Simon-Schnass and Korniszewski, 1990). Baseline tests were done in Kathmandu, Nepal. Supplementation began upon departure from Kathmandu to base camp, which was established at 4,300 m (14,108 ft) 2 weeks after departure from Kathmandu. The destination of the second expedition was the Solo Khumbu area near Mount Everest. The members were scientists who stayed for several weeks at an altitude of 5,000 m (16,404 ft) for research reasons. Ten of them took part in the study described later. Baseline tests were done after arrival at this altitude. Supplementation began immediately after the baseline tests.
For standardization reasons, erythrocyte filterability is expressed as the ratio of the turbidity of an unfiltered to a filtered sample. These data are given in Figure 21-3.
It was shown that red blood cell filterability deteriorates at high altitudes. The fact that no change in erythrocyte filterability was detected in the test group indicates that protection from oxidation was adequate there. The significant drop in filterability in the control group (p < 0.05), however, permits the conclusion that the oxidative stress led to depletion of vitamin E and/or other antioxidative substances.
To prove that the reason for this depletion was indeed the effect of an increased free radical production, the test was repeated during the above described expedition to Solo Khumbu (Simon-Schnass, 1994). Ten scientists were supplemented with either 400 mg of vitamin E per day (test group) or a
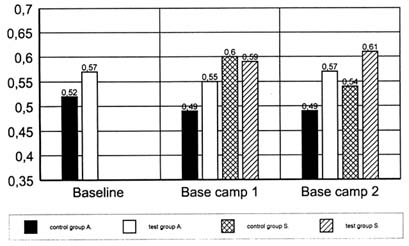
FIGURE 21-3 Filterability of red blood cells (without dimension) from subjects of two different expeditions to high altitudes. In both studies the subjects were divided into a test group and a placebo group. The test group was supplemented with 400 mg of vitamin E per day, whereas the control group received a placebo. In one study (Annapurna), there is a baseline value from a blood sample taken before the start of the expedition and two more with 2-wk difference. In the other study (Solo Khumbu), a baseline value at low altitude could not be achieved; so, there are only two samples at high altitude, one taken shortly after arrival and the other 2 weeks later. SOURCE: Annapurna, adapted from Simon-Schnass and Korniszewski (1991); Solo Khumbu, adapted from Simon-Schnass (1995).
placebo (control group). The tests were conducted in a permanently established laboratory at an altitude of 5,000 m (16,393 ft). The results are shown in Figure 21-3. Unfortunately, only data from 1 and 3 weeks after arrival at the laboratory were available because the laboratory heads would not be convinced to agree to the determination of low-altitude values as well. In this case, the first term at altitude is the baseline value. Although the values are higher in general, the trend is very similar to the first experiment. The difference between the two groups after 2 weeks at altitude is again significant (p < 0.05).
In addition during the second experiment (study at the Solo Khumbu area), the susceptibility of the erythrocytes of the same blood sample to peroxidation was tested by measuring the amount of thiobarbituric acid reactive substances (TBARS), which were determined at the Rowett Research Institute in Aberdeen, Scotland by a method established in this laboratory. In contrast to the slight decrease in TBARS formation in the test group, there was a tremendous increase in the control group. This result clearly documents increased oxidative stress at high altitudes. As shown later, the average daily intake of vitamin E of this group was 16.8 mg. Obviously this amount was
inadequate to meet the demand at high altitudes. The data are given in Figure 21-4.
There was also a negative correlation between the filterability of the erythrocytes and the amount of TBARS measured during this test (r = –0.9190 for the test group and r = –0.8218 for the control group). This result indicates that membranes that are more susceptible to oxidative stress in vitro (as determined by increased TBARS) are also more susceptible in vivo (as measured by decreased filterability) (Simon-Schnass, 1994). Given the results of the pentane measurement, these results show that there is increased oxidative stress at high altitudes and that supplementation with vitamin E, an antioxidant, can counteract its negative consequences.
Increased Ultraviolet Radiation with Increasing Altitude
The skin is exposed to radiation of a wavelength between about 300 and 3,000 nm, including infrared, visible light, and ultraviolet (UV) radiation (made up of UV-A and UV-B wavelengths). The proportion between the different kinds of radiation is not constant but is influenced by the sun's position (depending on locus, season, and time of day), altitude, ozone content of the stratosphere, and extent of air pollution (Kindl and Raab, 1993). Radiation intensity is proportional to the angle at which the sunlight has to
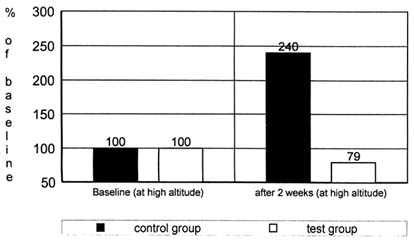
FIGURE 21-4 Thiobarbituric acid reactive substances (nm/g Hb) generated by erythrocytes of 10 subjects after arrival at high altitudes and after 2 weeks shown as percent of baseline. After taking the baseline samples, the test group was supplemented with 400 mg of vitamin E per day, whereas the control group received a placebo. SOURCE: Adapted from Simon-Schnass (1995).
pass before it reaches the ground. The shorter the wavelength, the higher the losses (Kindl and Raab, 1993). Therefore UV-B radiation is much more susceptible to scattering than UV-A radiation and visible light. This is also the reason why UV-B radiation increases dramatically in clean air and high altitudes. As shown in Table 21-2, for every 1,000 m (3,281 ft) of altitude, the UV-B intensity increases by 15 to 20 percent, whereas the UV-A intensity increases to a much lower degree (Kindl and Raab, 1993).
The basis for a photochemical reaction in the skin is the interaction between a light quantum and biological material, which means that an electron is lifted to a higher orbit. In the case of a stable electron condition, the energy is absorbed. However, there is a characteristic borderline, the so-called ionization potential. If the absorbed energy is higher than the binding energy of the electron, the electron is detached (ionized). Visible light and UV radiation have a quantum energy of 40 to 150 kcal/mol. This amount is within the order of the intramolecular binding energy, which is the reason why singlet and triplet forms as well as free radicals can be produced (Kindl and Raab, 1993). The increased UV-B radiation at high altitudes may thus be an important reason for increased oxidative stress at high altitudes.
Irradiation of the skin with UV-A and UV-B radiation can induce the formation of lipid peroxidation products. In human skin, surface lipids are peroxidized by UV-A radiation, whereas UV-B causes a remarkable decline in unsaturation of fatty acids (Fuchs and Packer, 1991). This increased lipid peroxidation could be proved by the elevated levels of TBARS in chronically sun-exposed human skin (Niwa et al., 1987). Lipid peroxides are cytotoxic and have a proinflammatory potency. Both are thought to play a role in skin inflammation induced by UV-B radiation (Ohsawa et al., 1984).
Various cellular compounds, such as carotenoids, vitamin E, vitamin C, and sulfhydryls, are effective radical scavengers. Especially carotenoids may play a role in protection against UV-radiation damage. Carotenoids have been
TABLE 21-2 Increase of Ultraviolet Radiation, UV-B and UV-A, with Increasing Altitude
|
Altitude Difference (m [ft]) |
UV-B Increase (%) |
UV-A Increase (%) |
|
1,000 (3,281) |
20 |
17 |
|
2,000 (6,562) |
35 |
27 |
|
3,000 (9,843) |
50 |
34 |
|
5,000 (16,404) |
70 |
44 |
|
SOURCE: Adapted from Kindl and Raab (1993). |
||
shown to inhibit UV-B-induced epidermal damage and tumor formation (Mathews-Roth, 1985). Vitamin E is increased in chronically sun-exposed skin, perhaps as an adaptive reaction of the body (De Simone et al., 1987). However, the vitamin E level is decreased immediately after UV-B irradiation of mouse skin (Fuchs et al., 1989). The total amount of vitamin C does not change with sun exposure to mouse skin, but there is no information about the ratio between ascorbic and dehydroascorbic acid (Fuch et al., 1989).
Oral β-carotene administration significantly increased the minimal erythema dose of solar radiation in humans (Mathews-Roth et al., 1972). This result may be caused by the photoprotective mechanism of β-carotene, a potent singlet oxygen scavenger at low partial pressure of oxygen (Mathews-Roth et al., 1972). A similar effect of vitamin E is likely (Shindo et al., 1993).
Because UV radiation increases tremendously at high altitudes (see Table 21-2), prophylactic supplementation for people who want to go to the mountains is highly recommended. Until now, no specific investigations have examined this topic.
Indirect Studies on Oxidative Stress at High Altitudes
Physical Exercise
One of the very few studies on the influence of an antioxidant (vitamin E) on physical performance at varying altitudes is that of Nagawa et al. (1968). The authors concluded that vitamin E might have an enhancing effect on endurance due to its ability to increase activity of respiratory enzymes in mitochondria of muscle cells and thereby improve the utilization of oxygen in muscle during activity, especially at high altitudes.
This hypothesis is supported by the results of a later study on the effect of vitamin E on physical performance at high altitudes (expedition to K2, described earlier) (Simon-Schnass and Pabst, 1988). As an objective parameter of performance, the anaerobic threshold of subjects was determined before departure and three times at base camp separated by intervals of 2 weeks. Anaerobic threshold is generally defined as the work load that leads to a lactic acid blood level of 4 mmol/liter. Accumulation of lactic acid in plasma was caused by performing graded exercise until exhaustion on a bicycle with 3-min load steps starting at 50 watts and with increments of 50 watts. Given the baseline performance at the anaerobic threshold as 100 percent, performance during the other three time periods is expressed in percent of baseline. These data are shown in Figure 21-5.
There was no significant difference between the baseline values of the two groups. During the experiment, the anaerobic threshold of the treatment group increased, while in the control group it initially increased to a smaller degree
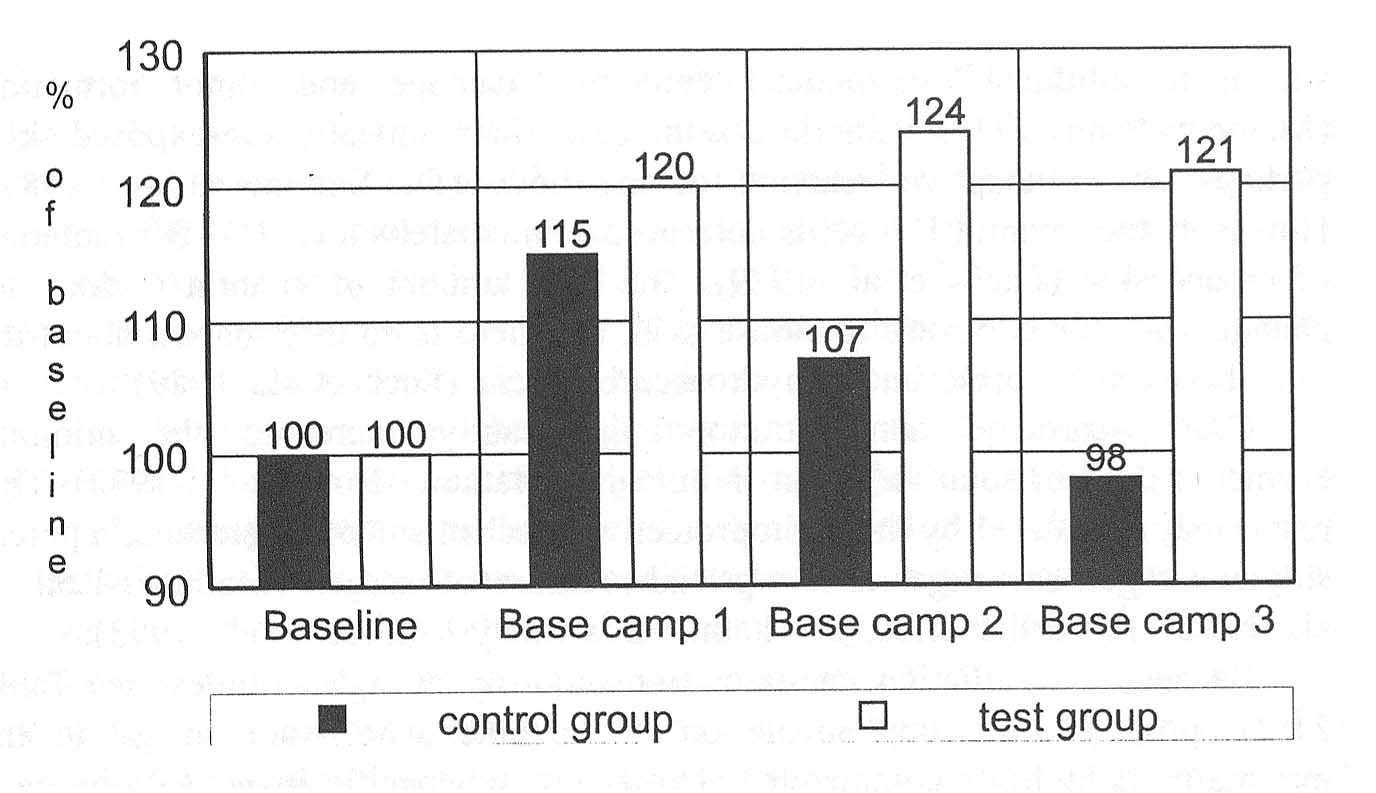
FIGURE 21-5 The anaerobic threshold given in watts as percent of mountaineers at baseline, after arrival in base camp 2 weeks later, and after 2- and 4-wk stay at base camp. Subjects were divided into a test group and a placebo group. The test group was supplemented with 400 mg of vitamin E per day, whereas the control group received a placebo. SOURCE: Adapted from Simon-Schnass and Pabst (1988).
and then decreased compared to the initial value. The difference between the changes of the anaerobic threshold of the treatment group and the control group became significant (p < 0.01) after 4 weeks, as shown in the third and fourth panels from the left in Figure 21-5.
Little research has been performed on the effect of altitude on anaerobic threshold. Results of the placebo group in the above described study, however, confirm the previous observation that a prolonged stay at high altitudes leads to reduced physical performance, reflected in a decreased anaerobic threshold.
By its stabilizing effect on various components of the respiratory chain, vitamin E contributes to aerobic energy production (Cormier, 1977; Schwarz, 1962, 1972). A local vitamin E deficiency leads to disturbances of electron transport and thus to reduced cell respiration (Carabello, 1974; Carabello et al., 1971; Fedelesova et al., 1971). This effect is especially apparent when the available oxygen is also limited, which occurs due to high demand, poor local supply, or low partial pressure of oxygen. It can be expected that the impairment of metabolism is especially pronounced under conditions of increased physical load at high altitudes. Investigations have shown that a prolonged stay at extreme altitudes leads to a loss of activity of succinate and lactate dehydrogenase (Cerretelli and di Prampero, 1985). The activity of both enzymes in skeletal muscle is also decreased by vitamin E deficiency (Bertolotty et al., 1965; Chen and Lin, 1980; Tureen and Simons, 1968). This decrease can be explained by the enzymes' content of labile SH groups, which have to be protected by the antioxidant.
The possible involvement of labile SH groups suggests that free radical reactions must be taken into consideration. Only very few studies have shown an increased risk of free radical production during exercise at high altitudes. Three of them are described in more detail here.
As shown, increased free radical production not only influences physical performance by damaging energy metabolism but also has a negative influence on membranes. This effect was demonstrated by the experiments on red blood cells and is of central importance especially at high altitudes.
Blood Flow
At high altitudes the body attempts to boost the blood's oxygen transport capacity by increasing erythropoiesis, resulting in a compensatory polycythemia. This effect has significant influence on capillary blood supply, which is mainly determined by cardiac output, vascular resistance, and the rheological properties of the blood or its constituents.
The special rheological properties of blood arise from its two-phase composition of plasma and blood cells. The viscosity of the blood depends largely on the packed cell volume, plasma viscosity, the deformability of the erythrocytes, and their tendency to aggregate (Ernst et al., 1985).
The viscosity of blood varies as a function of flow conditions. The very same blood can at one time be highly fluid and rapidly flowing and at another highly viscous with sluggish flow. In in vitro measurements, an elevated hematocrit level is discernible immediately, whereas the situation in vivo is much more complex. Because of the special two-phase composition of blood, under normal conditions a high hematocrit does not necessarily cause changes in flow characteristics in the terminal vessels because the local hematocrit in the capillaries is much lower (with a high flow rate) than in the larger blood vessels (Schmidt-Schönbein, 1982). There are limits even to this mechanism, however. Thus, it is assumed that at hematocrit levels of greater than 50 to 55 percent, the oxygen transport capacity falls again, not only at high altitudes but also in pulmonary diseases (Oelz, 1984; Winslow, 1984). Hematocrit levels of 60 percent or more are not a rare finding in high-altitude climbers. In the event of a general or localized reduction in flow velocity, however, the capillary hematocrit level approaches the venous hematocrit, and aggregation occurs. In this case, the hematocrit becomes highly important.
In the case of a high hematocrit, one of the major factors determining if local oxygen supply is improved or decreased is the flexibility of the erythrocytes, which is highly dependent on membrane integrity. As discussed above, hypoxia leads to oxidative stress. There is increased formation of free radicals, which triggers lipid peroxidation. As a result, membrane fluidity (measured by erythrocyte filterability) deteriorates. Because vitamin E is
directly incorporated into the membranes, it should counteract such processes there.
In addition to blood viscosity, the elasticity and integrity of the vascular wall play an important role in capillary blood supply. Here too, oxidative changes are discussed as a pathogenetic factor (Kappus, 1985). The release of tissue hormones, such as histamine, kinins, and prostaglandins, also plays an important part in the damage of the vascular wall (Schmidt-Schönbein and Neuman, 1985). Endothelial lesions may lead to disturbances in microcirculation. These disturbances may be compounded by activation of the coagulation system, with resultant consumption coagulopathy (see Stedman's Pocket Medical Dictionary, 1987, p. 149). This result can lead to the formation of microthrombi and, consequently, increased reactive fibrinolysis, which in turn results in an increased tendency to hemorrhages (Hiller and Reiss, 1988).
The aforementioned disturbances are frequently found during a prolonged stay at high altitudes. Here, the pathological changes are mainly in the area of the pulmonary and cerebral capillaries, but changes also occur in the retina and the mucosa (Volger, 1984).
Both the radical-binding properties of vitamin E and its involvement in the metabolism of eicosanoids (which may be related to each other) indicate that this vitamin has an effect on the phenomena described above (Chow, 1979; Leibovitz and Siegel, 1980; Simon-Schnass and Koeppe, 1983).
In the aforementioned study (expedition to Annapurna), the influence of supplementation of 400 mg of vitamin E per day on several of these rheological parameters was tested. The parameters included whole blood and plasma viscosity, white blood cells, platelets, antithrombin III, and protein C (Simon-Schnass and Korniszewski, 1990). In this chapter, only the data on white blood cells and two antiaggregational substances are discussed.
The viscosity of blood is mainly determined by the amount of blood cells, their flexibility, and the plasma viscosity. Results from this author's studies (Simon-Schnass, 1994) and the experience of mountaineers (Oelz, 1982) show that an adequate fluid intake not only maintains the hematocrit in a physiologically acceptable range, but it can also prevent hemoconcentration and a related increase in plasma viscosity. Because there was no correlation between hematocrit and plasma viscosity, it can be assumed that the volunteers in the study did not experience any appreciable dehydration. The increase in whole blood viscosity was therefore mostly the result of the increase of blood cells and their membrane rigidity.
Because of their rigidity and spherical shape, leukocytes cannot pass through the terminal vessel as easily as erythrocytes. Even under physiological conditions there may be a reduction in flow velocity or even temporary stasis in the passage of leukocytes through the capillaries (Asano et al., 1973). If the perfusion pressure falls, pronounced disturbances of microcirculation occur, mainly due to the white blood cells. Thus, there may also be, for example, occlusion of the arterioles and venules due to adhesions to the vascular walls
(Bagge et al., 1986; Lipowsky et al., 1980). After stimulation (e.g., by activation of complement, endotoxins, immune complexes, or leukotriene B4), there is a particularly substantial rise in the tendency of the leukocytes and, in particular, the granulocytes, to aggregate. This aggregation in turn leads not only to a further increased risk of occlusion, but also to increased release of intracellular proteases (Harlan et al., 1981). As shown in Figure 21-6 a marked increase in white blood cells was shown in the control group, whereas no change occurred in the supplemented group.
On the basis of data in the literature, an increased granulocyte stimulation can be assumed to occur in such a situation. The proteases then released can split not only the endothelial cells and the proteins bound to the endothelial cells, but also proteins free in the plasma (Benjamini and Leskowitz, 1988; Weis and Regiani, 1984). This assumption could explain the significant drop in protein C observed in the control group (p < 0.05) as well as the drop in antithrombin III, which did not reach the borderline level for significance (p < 0.052). The data are shown in Figure 21-7.
Another possible cause of disturbances in microcirculation seems to be a modulation of endothelial cell function in hemostasis. On the one hand, endotoxins (the presence of which was indicated by the rise of leukocytes) can
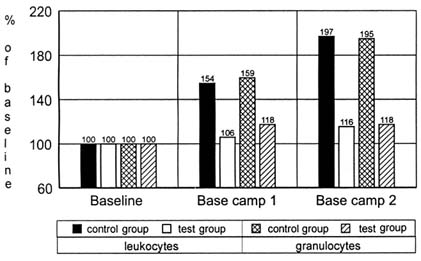
FIGURE 21-6 Leukocyte and granulocyte counts (× 109/Liter) of 13 mountaineers during an expedition to Annapurna. Subjects in the test group were supplemented with 400 mg of vitamin E per day, whereas the control group received a placebo.
SOURCE: Adapted from Simon-Schnass and Korniszewski (1991).
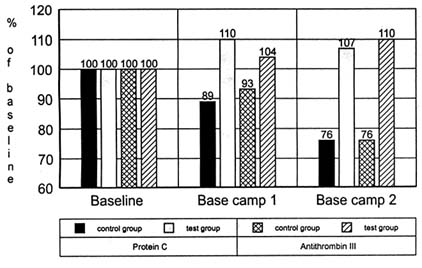
FIGURE 21-7 Activity of protein C and of antithrombin III (percent of activity compared to standard provided by the manufacturer) of 13 mountaineers during an expedition to Annapurna. Subjects in the test group were supplemented with 400 mg of vitamin E per day, whereas the control group received a placebo.
SOURCE: Adapted from Simon-Schnass and Korniszewski (1991).
reduce the concentration of available thrombomodulin so that there is only reduced protein C activation (Moore et al., 1987). Blockade of the binding sites could then result in increased protein C clearance. On the other hand, increased formation of the inflammation mediator interleukin-1 may cause similar reactions (Nawroth et al., 1986). Many of these reactions produce or are influenced by free radicals. Supplementation with antioxidants seems to stabilize both leukocytes and the endothelial cells and to protect against splitting of proteins.
All the mentioned changes in the various tested parameters point clearly to impaired blood rheology at high altitudes. In this context it should be borne in mind that mountain sickness is often attributed to general microcirculation disturbances (Olez, 1984), which explains the tendency to frostbite, retinal hemorrhage, and cerebral and pulmonary edema. Because supplementation with 400 mg of vitamin E per day was able to prevent some of these changes (Simon-Schnass and Korniszewski, 1990) and vitamin E is known to be an effective radical scavenger, free radical-related reactions are most likely. Unfortunately there are still many more questions than answers in this field of research.
Wide Temperature Differences at High Altitudes
Wide temperature differences are typical at high altitudes. There are not only considerable temperature differences between day and night but also temperature differences related to the presence of sunshine or especially wind. For example, at an altitude of more than 4,000 m [13,123 ft], it is not unusual for the afternoon temperature in the sun to be about 30°C (86°F) or more and after sunset for the temperature to drop within 15 minutes to below 0°C (32°F). Although there have been no known investigations of changes in metabolism caused by these rapid changes in air temperature, one can speculate about such a relationship.
Until now no data have been collected concerning the increased production of free radicals in hot environments. However, it has been suggested that work in hot environments could create a hypoxic condition in muscle due to the redistribution of blood from the muscle to the skin (Young, 1990). Although no studies have examined the amount of lipid peroxidation in hot environments, it is possible that the combination of hypoxia, dehydration, and other changes such as heat stress could exacerbate oxidative stress in the muscle. If this hypothesis can be confirmed, the use of antioxidants should be recommended (Clarkson, 1993).
Hypothermia may also be an important reason for damage to the blood vessels and may induce a deterioration in the rheological properties of blood. The degree of deterioration varies, but it is clearly temperature dependent. Schmidt-Schönbein and Neumann (1985) gave an excellent overview. Under hypothermic conditions, erythrocytes tend to aggregate more easily, and the aggregates are more resistant to hydrodynamic dispersion. This result is consistent with the observation that together with a membrane stiffening, the deformation of aggregated red blood cells in stasis is made worse in (Schmidt-Schönbein and Neumann, 1985). From the start it has been impossible to decide if the stiffening of the membranes is only a temperature phenomenon or the result of an increased lipid peroxidation. In any case, after stasis occurs, tissue hypoxia is most likely, and thus oxidative stress increases.
Until now, blood rheology has been understood to be dependent on many factors. Clearly some minor changes in variables may result in big effects. The prophylactic use of antioxidants at high altitudes or in the cold may be one of these variables. Certainly they will not prevent frostbite in all conditions, but it seems worthwhile to do more research on the possibility that influencing the metabolism in a way that makes blood cells and vessel intima more resistant to factors that initially disturb blood rheology may prevent harmful events in some cases.
It is well known that temperature influences hormonal status. Noradrenalin, for example, plays an important role in nonshivering thermogenesis. It was shown that incubation of mitochondria with noradrenalin caused a significant increase in hydrogen peroxide production and, thus, of oxidative stress. The
same author was able to demonstrate this same effect in animals exposed to cold temperatures (Swaroop et al., 1983).
Dehydration
Fluid demand increases at high altitudes depending on temperature and humidity of the air. Working in hot or even warm surroundings causes more or less intensive perspiring. As mentioned earlier, even at high altitudes temperatures can rise considerably. Working to a more or less intensive degree is obligatory when staying in the mountains. Thus, fluid loss through perspiration is common. Undoubtedly these losses of water as well as of electrolytes must be replaced, and in this case, an electrolyte drink is useful.
With increasing altitude, fluid losses from the lungs as a consequence of breathing the increasingly dry air tend to dominate over perspiration. There is evidence that this fluid loss can increase to 6 or more liters per day at extremely high altitudes (Olez, 1984). Because the melting of 1 liter of water from snow takes about 1 hour under these conditions, it is not surprising that high-altitude climbers generally do not meet their fluid demand and return to base camp more or less dehydrated (Personal communications with experienced climbers during the various expeditions, 1986, 1988, 1990). In this case, the fluid deficit can be compensated for by water only, but in practice it is usually by tea (Personal communications with experienced climbers during the various expeditions, 1986, 1988, 1990).
Because dehydration is one of the important risk factors for frostbite, it is necessary to test for a proper fluid balance before starting to climb. This balance can be easily measured by checking hematocrit or urine osmolarity regularly.
No known studies have examined whether dehydration is involved in increased free radical production. Clarkson (1993) mentions dehydration as a possible risk factor. Because dehydration is such a big problem at high altitudes, studies on this question are highly desirable.
CONSEQUENCES OF OXIDATIVE STRESS WITH RESPECT TO NUTRITIONAL RECOMMENDATIONS
Nutrient deficiency may be a problem at low altitudes, but it most certainly is one at high altitudes. In Table 21-3, some data are shown that were produced by calculating nutrient intake during two different expeditions. The food supply of the first (K2, 1986) was determined carefully by a nutrition professional, taking the limited food items available during an expedition into
TABLE 21-3 Average Daily Intake of Selected Nutrients from Food during Three Different Expeditions to High Altitudes, Given as Percentage of the Recommended Dietary Allowance
|
Nutrient |
K2, 1986 |
Solo Khumbu, 1990 |
Kangchenjunga, 1992 |
|
Vitamin E |
159 |
140 |
203 |
|
Vitamin C |
85 |
183 |
76 |
|
Vitamin B2 |
105 |
50 |
165 |
|
Zinc |
44 |
25 |
45 |
|
Selenium |
103 |
43 |
129 |
|
SOURCE: Adapted from Simon-Schnass (1995). |
|||
consideration. The second expedition was a scientific project in the Solo Khumbu for which the food supply was organized by experienced mountaineers. In the first case, data were obtained by weighing the food consumed by the group over the entire period and then calculating the average intake by dividing this amount per person and day at the base camp. In the second case, nutrient intake was calculated from 7-d food records. Because there was not much chance for varying the meals individually during the time period, the data can be considered representative for the entire expedition (Simon-Schnass, 1994). Data shown in Table 21-3 are limited to the intake of water and some vitamins and minerals.
From Table 21-3 it can be seen that nutrient intake may be lower than recommended intakes, and thus, an increase risk of deficiency may easily occur during high-altitude climbing. There can be no trend identified, which nutrients are the most critical ones. That depends completely on the knowledge and financial resources of the organizing team and whether fresh food items are available at base camp. Because price and weight of food as well as loss of appetite often are limiting factors, such concerns are not surprising.
No known investigations have looked at nutrient intake during trekking and altitude training of athletes. Because increased oxidative stress at high altitudes can be presumed, especially the antioxidative nutrients such as β-carotene, vitamin E, vitamin C, selenium, and zinc should be taken into consideration. The relatively high intake of vitamin C during the second expedition (Table 21-3) was due to a regular supply of fresh potatoes, cabbage, and apples. Such supplies depend largely on area and season and cannot be relied upon. In general, the food supply is limited, especially with respect to fresh food. Therefore, supplementation is advisable.
SUMMARY
Considerable evidence suggests that there is increased oxidative stress at high altitudes. The most important causes of free radical formation are the respiratory chain itself, hypoxia, and increased ultraviolet radiation. Whether high and/or low temperatures and dehydration lead to an increased formation of free radicals is not clear at the present time. Certainly there are other causes for increased oxidative stress, such as disease and/or intake of medication. However, these should be discussed separately.
Several studies indicate that oxidative stress impairs physical performance as well as blood flow. Both are of particular importance for people at high altitudes.
A nutritional survey (Simon-Schnass, 1995) showed that inadequate nutrient intake, especially of antioxidants, may not always meet the increased demand at high altitudes. Inadequate levels of antioxidants clearly impair metabolic functions at high altitudes, and the recommendations for people living at lower altitudes are insufficient for this special situation. Because the food supply is limited during a prolonged stay at high altitudes (e.g., during a trekking tour or an expedition), supplementation with antioxidants is advisable. The only known studies (Simon-Schnass, 1994; Simon-Schnass and Korniszewski, 1990; Simon-Schnass and Pabst, 1988) available have shown a beneficial influence of vitamin E on physical performance, blood flow, and some parameters, indicating an increased oxidative stress at high altitudes. However, the synergistic functions of other antioxidants, such as β-carotene and vitamin C (Halliwell et al., 1987), justify the recommendation of a supplementation.
Not all of the aforementioned factors influence all groups of people to the same degree. However, in general their importance and occurrence increases with altitude. A great deal of research still needs to be done in this field.
REFERENCES
Asano, M., P.I. Branemark, and A. Castenholz 1973 A comparative study of continuous qualitative and quantitative analysis of microcirculation in man. Adv. Microcirc. 5:1–31.
Bagge, U., A. Blinxt, and M. Braide 1986 Macromodel experiments on the effect of wall-adhering white cells on flow resistance. Clin. Hemorrheol. 6:365–372.
Benjamini, E., and S. Leskowitz 1988 Überempfindlichkeitsreaktionen. Pp. 197–212 in Immunologie, E. Benjamini and S. Leskowitz, eds. Stuttgart: Schwer Verlag.
Berg, A., I. Simon-Schnass, L. Rokitzki, and J. Keul 1987 Die bedeutung des vitamin E für den sportler. Deut. Z. Sportmed. 38:416–424.
Bertolotty, E., G. Loidodice, and G.F. Quazza 1965 Sugli eventuali rapporti tra anomalia citogenetiche ed alterazioni citochimiche. La leuco fosfatasi alcalina nel mongolosmo. Minerva Pediatr. 17:873–877.
Bland, J. 1986 Vitamin E, ein pharmakologisch wirksamer Nähr-stoff. Pp. 47–59 in Frei Radikale: Vitamin E—Therapeutische Bedeutung, V. Böhlau and J. Reimann, eds. München: Otto Hoffmanns Verlag.
Burton, G.W., and K.U. Ingold 1984 β-carotene: An unusual type of lipid antioxidant. Science 224:569–573.
Buskirk, E.R. 1966 Physiology and performance of track athletes at various altitudes in the United States and Peru . Pp. 65–72 in The Effects of Altitude on Physical Performance, R.F. Goddard, ed. Chicago: Athletic Institute.
Carabello, F.B. 1974 Role of tocopherol in the reduction of mitochondrial NAD. Can. J. Biochem. 52:679–688.
Carabello, F.B., F. Liu, O. Eames, and J. Bird 1971 Effect of vitamin E deficiency on mitochondral energy transfer. Fed. Proc. 30:639.
Cerretelli, P., and P.E. di Prampero 1985 Aerobic and anaerobic metabolism during exercise at altitude. Pp. 1–19 in High Altitude Deterioration, J. Rivolire, P. Cerretelli, J. Foray, and P. Segantini, eds. Basel: Karger.
Chen, L.H., and C.I. Lin 1980 Some enzymatic changes associated with pathological changes in rats with long-term vitamin E deficiency. Nutr. Rep. Int. 21:387–395.
Chow, C.K. 1979 Nutritional influence on cellular antioxidant defense systems. Am. J. Clin. Nutr. 32:1066–1081.
Clarkson, P.M. 1993 The effect of heat on vitamin requirements. Pp. 137–171 in Nutritional Needs in Hot Environments, Applications for Military Personnel in Field Environments, B.M. Marriott, ed. A report of the Committee on Military Nutrition Research, Food and Nutrition Board, Institute of Medicine. Washington D.C.: National Academy Press.
Cormier, M. 1977 Regulatory mechanisms of energy needs: Vitamins in energy utilization. Prog. Food Nutr. Sci. 2:347–356.
Demopoulos, H.B., J.P. Santomier, M.L. Seligman, and D.P. Pietronigro 1984 Free radical pathology: Rationale and toxicology of antioxidants and other supplements in sports medicine and exercise science. Pp. 139–189 in Sport, Health and Nutrition: The 1984 Olympic Science Congress Proceedings, vol. 2, F.I. Katch, ed. Champaign, Ill.: Human Kinetics.
De Simone, C., L. Rusciani, and A. Vernier 1987 Vitamin E and coenzyme Q10 content in epidermis and basal cell epithelioma. J. Invest. Dermatol. 89:317.
Dillard, C.J., E.E. Dumelin, and A.L. Tappel 1977 Effect of dietary vitamin E on expiration of pentane and ethane by the rat. Lipids 12:109–114.
Dillard, C.J., R. Litov, W. Savin, E.E Dumelin, and A.L. Tappel 1978 Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J. Appl. Physiol. 45:927–932.
Eckhardt, K., U. Boutellier, A. Kurtz, M. Schopen, E. Koller, and C.J. Bauer 1989 Rate of erythropoietin formation in humans in response to acute hypobaric hypoxia. Appl. Physiol. 66:1785–1788.
Elstner, E.F., ed. 1990 Der Sauerstoff. Mannheim: BI-Wissenschaftsverlag.
Ernst, E., A. Matrai, and E. Aschenbrenner 1985 Blood rheology in athletes. J. Sports Med. 25:207–210.
Fedelesova, M.P., P.V. Sulakhe, J.C. Yates, and N.S. Dhalla 1971 Biochemical basis of heart function. IV. Energy metabolism and calcium transport in hearts of vitamin E deficient rats. Can. J. Physiol. Pharmacol. 9:909–918.
Fuchs, J., and L. Packer 1991 Photooxidative stress in the skin. Pp. 561–583 in Oxidative Stress: Oxidants and Antioxidants, H. Sies, ed. London: Academic Press .
Fuchs, J., R.J. Mehlhorn, and L. Packer 1989 Free radical reduction mechanism in mouse epidermis skin homogenates. J. Invest. Dermatol. 93:633–640.
Halliwell, B., M. Wasil, and M. Grootveld 1987 Biologically significant scavenging of the myeloperoxidase-derived oxidant hypochlorous acid by ascorbic acid. FEBS Lett. 213:15–18.
Hannon, J.P., J.L. Shields, and C.W. Harris 1969 Effects of altitude acclimatization on blood composition of women. J. Appl. Physiol. 26:540–547.
Harlan, J.M., P.D. Killen, and L.A. Harken 1981 Neutrophile-mediated endothelial injury in vitro: Mechanisms of cell detachment. J. Clin. Invest. 68:1394–1403.
Hiller, E., and H. Riess 1988 Erworbene Gerinnungsstörungen. Pp. 86–109 in Hämorrhagische Diathese und Thrombose, E. Hiller and H. Ries, eds. Stuttgart: Wissenschaftliche Verlagsgesellschaft mbH.
Hughes, R.E., and S.C. Maton 1968 Passage of vitamin C across the erythrocyte membrane. Br. J. Haematol. 14:247–253.
Kappus, H. 1985 Lipid peroxidation. Mechanisms, analysis, enzymology and biological relevance. Pp. 273–310 in Oxidative Stress, H. Sies, ed. London: Academic Press.
Kindl, G., and W. Raab, eds. 1993 Licht und Haut. Frankfurt: Govi-Verlag.
Leibovitz, B.E., and B.V. Siegel 1980 Aspects of free radical reactions in biological systems: Aging. J. Gerontol. 35:45–56.
Lipowsky, H., S. Usami, and S. Chien 1980 In vivo measurements of ''apparent viscosity" and microvessel hematocrit in the mesentery of the cat. Microvasc. Res. 19:297–319.
Mathews-Roth, M.M. 1985 Carotenoid dose level and protection against UV-B induced skin tumors. Photochem. Photobiol. 42:35–38.
Mathews-Roth, M.M., M.A. Pathak, J. Parrish, T.B. Fitzpatrick, E.H. Kass, K. Toda, and W. Clemens 1972 A clinical trial of the effects of oral β-carotene on the responses of human skin to solar radiation. J. Invest. Dermatol. 59:349–353.
Moore, K.L., S.P. Andreollo, and N.L. Esmon 1987 Endotoxin enhances tissue factor and suppresses thrombomodulin expression of human vascular endothelium in vitro. J. Clin. Invest. 79:124–130.
Nagawa, T., H. Kita, J. Aoki, T. Maeshima, and K. Shiozawa 1968 The effect of vitamin E on endurance. Asian Med. 11:619–633.
Nawroth, P.P., D.A. Handley, C.T. Esmon, and D.M. Stern 1986 Interleukin 1 induces endothelial cell procoagulant while suppressing cell-surface anticoagulant activity. Proc. Natl. Acad. Sci. 83:3460–3464.
Niwa, Y., T. Kanoh, T. Sakane, H. Soh, S. Kawai, and Y. Miyachi 1987 The ratio of lipid peroxides to superoxide dismutase activity in the skin lesions of patients with severe skin diseases: An accurate prognostic indicator. J. Clin. Biochem. Nutr. 2:245–251.
Oelz, O. 1982 How to stay healthy while climbing Mount Everest. Pp. 298–300 in High Altitude Physiology and Medicine, W. Brendel and R.A. Zink, eds. New York: Springer-Verlag.
1984 Höhenhypoxie—Physiologische und medizinische Aspekte. Pp. 205–220 in Hypoxie, S. Daum, ed. München-Deisenhofen: Dustrie Verlag.
Ohsawa, K., T. Watanabe, R. Matsukawa, Y. Yoshimura, and K. Imaeda 1984 The possible role of squalene and its peroxide of the sebum in the occurrence of sunburn and protection from the damage caused by U.V. irradiation. J. Toxicol. Sci. 9:151–159.
Packer, J.E., T.F. Slater, and R.L. Wilson 1979 Direct observation of a free radical interaction between vitamin E and vitamin C. Nature 278:737–738.
Packer, L. 1984 Vitamin E, physical exercise and tissue damage in animals. Med. Biol. 62:105–109.
Reznick, A.Z., B. Rappaport, S.V. Landvik, I. Simon-Schnass, and L. Packer 1993 Vitamin E and the aging process. Pp. 435–454 in Vitamin E in Health and Disease, L. Packer and J. Fuchs, eds. New York: Marcel Dekker.
Schmidt-Schönbein, H. 1982 Blood rheology in hemoconcentration. Pp. 109–116 in High Altitude Physiology and Medicine, W. Brendel and R.A. Zink, eds. New York: Springer-Verlag.
Schmidt-Schönbein, H., and F.J. Neumann 1985 Pathophysiology of cutaneous frost injury: Disturbed microcirculation as a consequence of abnormal flow behavior of the blood. Pp. 20–38 in High Altitude Deterioration, J. Rivolire, P. Cerretelli, J. Foray, and P. Segantini, eds. New York: Karger.
Schmidt-Schönbein, H., H.J. Klose, E. Volger, and J. Weiss 1973 Hypothermia and blood flow behavior. Res. Exp. Med. 161:58–68.
Schwarz, K. 1962 Vitamin E, trace elements, and sulfhydryl groups in respiratory decline. Vit. Horm. 20:463–484.
1972 The cellular mechanisms of vitamin E action: Direct and indirect effects of alpha-tocopherol on mitochondrial respiration. Ann. N.Y. Acad. Sci. 203:42–52.
Shindo, Y., E. Witt, and L. Packer 1993 Antioxidant defense mechanisms in murine epidermis and dermis and their responses to ultraviolet light. J. Invest. Derm. 100:260–265.
Sies, H. 1993 Vitamin E in biologischen Systemen: Die Biochemie van Tocopheryl-Radikalen und ihre Rolle bei Krankheiten im Menschen. Pp. 11–20 in Vitamin E in der modernen Medizen, K. Schmidt and W. Wildmeister, eds. Lenggries/Obb. MKM Verlagsgesellschaft.
Simon-Schnass, I. 1993 Vitamin E requirements for physical activity: Vitamin E. Pp. 144–153 in World Review of Nutrition and Dietetics, A.P. Simopoulos, ed. Basel: Karger.
1994 Risk of oxidative stress during exercise at high altitude. Pp. 191–210 in Exercise and Oxygen Toxicity, C.K. Sen, L. Packer, and O. Hänninen, eds. Amsterdam: Elsevier.
1995 Benefit of supplementation with antioxidants at high altitude. Nutrition under Extreme Stress Symposium. April 2–3, ACS National Meeting, Anaheim, Calif.
Simon-Schnass, I., and H.W. Koeppe 1983 Vitamin E und arteriosklerose. Z. Allgemeinmedizin 59:1474–1476.
Simon-Schnass, I., and L. Korniszewski 1990 The influence of vitamin E on theological parameters in high altitude mountaineers. Int. J. Vit. Nutr. Res. 60:26–34.
Simon-Schnass, I., and H. Pabst 1988 Influence of vitamin E on physical performance. Int. J. Vit. Nutr. Res. 58:49–54.
Stedman's Pocket Medical Dictionary 1987 Baltimore: Williams and Wilkins.
Stocker, R., and B. Frei 1991 Oxidants and antioxidants. Pp. 213–243 in Oxidative Stress, H. Sies, ed. London: Academic Press.
Swaroop, A., M.S. Patole, R.S. Puranam, and T. Ramasarma 1983 Noradrenaline treatment of rats stimulates H2O2 generation in liver mitochondria. Biochem. J. 214:745–750.
Thews, G., E. Mutschler, and P. Vaupel, eds. 1982 Anatomie, Physiologie und Pathophysiologie des Menschen. Stuttgart: Wissenschaftliche Verlags-gesellschaft mbh.
Tureen, L., and R. Simons 1968 Enzyme changes within muscle fibres in genetic and nutritional muscular dystrophy. Proc. Exp. Biol. Med. 129:384–390.
Vile, G.F., and C.C. Winterburn 1988 Inhibition of adriamycin-promoted microsomal lipid-peroxidation by β-carotene, alpha-tocopherol and retinol at high and low oxygen partial pressures. FEBS Lett. 238:353–356.
Volger, E. 1984 Einflüsse der hypoxie auf die erythropoese, sauerstoffbindung des hämoglobins, gerinnung und rheologie des blutes. Pp. 225–240 in Hypoxie, S. Daum, ed. München-Deisenhofen: Dustrie Verlag.
Weis, S.J., and S. Regiani 1984 Neutrophils degrade subendothelial matrices in the presence of alpha-1-proteinase inhibitor. Cooperative use of lysosomal proteinases and oxygen metabolites. J. Clin. Invest. 73:1297–1303.
Winslow, R.M. 1984 High-altitude polycythemia. Pp. 163–172 in High Altitude and Man, J.B. West and S. Lahiri, eds. Bethesda, Md.: American Physiological Society.
Young, A.J. 1990 Energy substrate utilization during exercise in extreme environments. Exerc. Sports Sci. Rev. 18:65–117.
Young, A.J., W.J. Evans, A. Cymerman, K.B. Pandolf, J.J. Knapik, and J.T. Maher 1982 Sparing effect of chronic high-altitude exposure on muscle glycogen utilization. J. Appl. Physiol. 52:857–862.
Young, P.M., P.B. Rock, and C.S. Fulco 1987 Altitude acclimatization attenuates plasma ammonia accumulation during submaximal exercise. J. Appl. Physiol. 63:758–764.


























