5
Mediated Electrochemical Oxidation Silver II
Process Description
Silver II is a patented electrochemical process. It was originally developed in 1987 by AEA Technology at Dounreay, Scotland, as a means for destroying solid and liquid radioactive organic waste streams from the U.K. Fast Reactor fuel development program. AEA Technology submitted the Silver II technology to the Army for consideration as an alternative technology for agent destruction at the Aberdeen and Newport sites and will therefore be referred to as the TPC (technology proponent company) for the Silver II process in the remainder of this report.
Most of the TPC's effort to date has been dedicated to operation of a 4-kW pilot plant for destroying inactive fuel solvent composed of 10 percent tributyl phosphate in kerosene. In addition, laboratory tests conducted at Dounreay since 1987 have demonstrated destruction of 68 organic compounds encountered in industrial wastes, including HD (distilled S-mustard), VX, and GB (another unitary chemical nerve agent).
Figure 5-1 is a schematic diagram of the heart of the Silver II process as described by the TPC for destruction of VX and mustard. The core reactions take place in two separate 180-kW, electrochemical cells (model ICI FM21), which are connected in parallel through a 360-kW power supply. Each FM21 cell comprises 45 anode-cathode compartments, each 10 mm wide by 240 mm high; each electrode is separated by a Nafion1 membrane, which is permeable to cations and water but impermeable to anions (Figure 5-2). The anode-cathode chambers are connected in parallel, each pair requiring a normal operating current of 2,000 A at a nominal 2 volts DC. Thus, the 360-kW power supply unit for a standard module must provide a total of 90 kA and 180 kW to each of the two-cells that make up the module. The aggregate volume of all the anode-cathode chambers within a cell is 2.5 m3.
At the start of operation, the composition of the anolyte is approximately 8 molar in nitric acid, 0.5 molar in silver nitrate, and 0.02 to 0.03 molar in agent. The catholyte is 4 molar nitric acid.
When power is applied to the cell, Ag(I) ions are oxidized at the anode to the highly reactive Ag(II). The Ag(II) species has been shown to exist in the form of AgNO3+ ions (Po et al., 1968), which impart a brown color to the solution in the absence of organics. In the presence of organics, AgNO3+ ions oxidize water into intermediates such as hydroxyl radicals that rapidly oxidize the organic species. Simultaneously, Ag(II) is
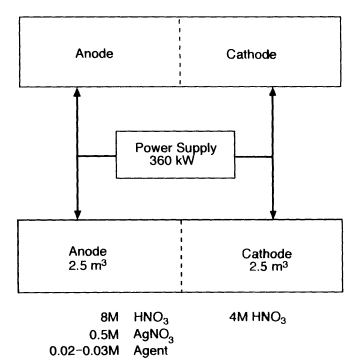
Figure 5-1
Schematic diagram of the basic cell module for mediated electrochemical oxidation.
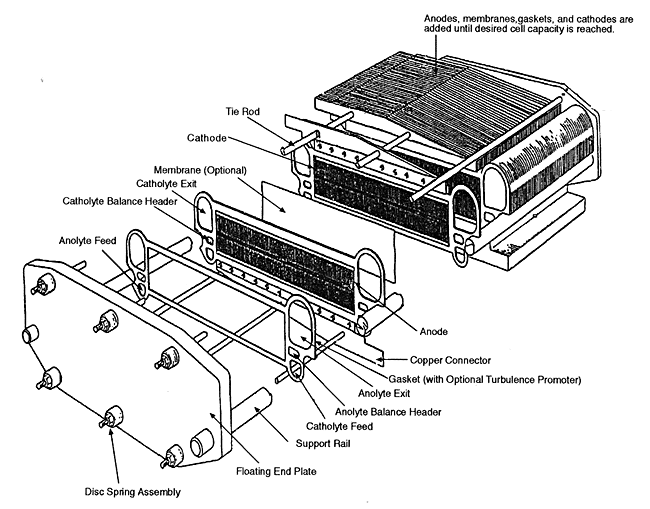
Figure 5-2
Exploded view of the FM21 electrochemical cell. Source: AEA Technology.
reduced back to Ag(I), which migrates back to the anode surface where it is reoxidized to Ag(II). Silver therefore serves as an electron transfer intermediate that is not consumed in the process. However, when chloride ions or organic chlorides are present, as in HD, Ag(I) precipitates as AgCl.
The anticipated overall anode reactions for VX and HD are as follows:
VX: C11H26SNPO2 + 31H2O = 11CO2 + H3PO4 + H2SO4 + HNO3 + 82H+ + 82e
HD: C4H8SCl2 + 12H2O = 4CO2 + H2SO4 + 2HCl + 28H+ + 28e
Some CO will form as well, by analogous reactions, but laboratory tests have shown that carbon is converted primarily to CO2. Hydrated protons (hydronium ions, H3O+) move across the membrane toward the cathode, where the primary reaction is reduction of nitric acid to nitrous acid:
HNO3 + 2H+ + 2e- = HNO2 + H2O
Nitrous acid will partially decompose to NO gas, nitric acid, and water. In the laboratory tests observed by the AltTech Panel, the gas leaving the cathode compartment had the characteristic red-brown color of NO2, which can form by oxidation of NO in the gas-phase when O2 is present.
The overall cell reactions are:
VX: C11H26SNPO2 + 40HNO3 = 11CO2 + H3PO4 + H2SO4 + 41HNO2 + 10H2O
HD: C4H8SCl2 + 14HNO3 = 4CO2 + H2SO4 + 2HCl + 14HNO2 + 2H2O
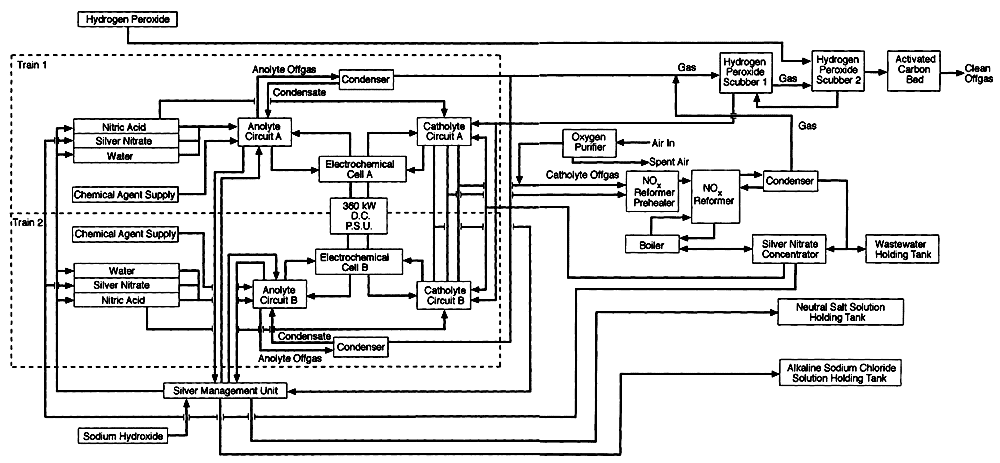
Figure 5-3
Block flow diagram of the Silver II process total system. Source: AEA Technology.
The reaction products are treated in subsequent steps outside the cell to reoxidize HNO2 to HNO3 and to neutralize the acids to their corresponding sodium salts. Therefore, the net reactions are as follows:
VX: C11H26SNPO2 + 20.5O2 + 6NaOH = 11CO2 + Na3PO4 + Na2SO4 + NaNO3 16H2O
HD: C4H8SCl2 + 7O2 + 4NaOH = 4CO2 + Na2SO4 + 2NaCl + 6H2O
The overall reactions are similar to the overall reactions for incineration of VX and HD, but they occur at low temperature (less than 90°C) and close to atmospheric pressure. In both processes, carbon is released to the gas-phase primarily as CO2. In the electrochemical process, the sulfur, phosphorus, and chlorine components of the agent appear in the final effluent as hydrated anions in aqueous solution (sodium is the principal cation). This solution can be analyzed and treated further, if necessary, prior to release. In combustion processes like the baseline incineration system, these elements yield gases (assuming oxidation is complete), which must be removed in a treatment train, but the treated process gas stream is difficult to analyze prior to release to the atmosphere.
Three additional reactions that can occur will affect the energy efficiency of the process. First, Ag(II) can react directly with water in the anode compartment to
form oxygen gas (O2). Second, the Ag(I) can migrate across the membrane to the cathode compartment. Third, cationic impurities in the agent can migrate across the membrane to the cathode compartment. Analyses of the HD stored at Aberdeen show that such impurities are likely to include iron, copper, and possibly mercury. Organic impurities in the agent will be oxidized in the anode compartment by reactions analogous to the reactions with agent.
The process reactions involving agent cannot be reversed. Therefore, once agent is destroyed, it cannot reform. However, agent destruction is likely to proceed in several steps, some of which may produce volatile organic intermediates that will enter the gas-phase and require further treatment. In laboratory tests, for example, the TPC identified varying levels of alkyl nitrates in the anolyte offgas, which was mainly CO2. Nonvolatile organic intermediates that may also form will remain in the anode compartment and will ultimately undergo complete conversion to simpler inorganic products, such as sulfate, phosphate, chloride, and CO2/CO.
In common with virtually all commercial electrochemical processes, Silver II requires continuous feed systems to both the anolyte and catholyte chambers and treatment systems for anolyte and catholyte products. Figure 5-3 is a block flow diagram of a total system, which comprises the following components:
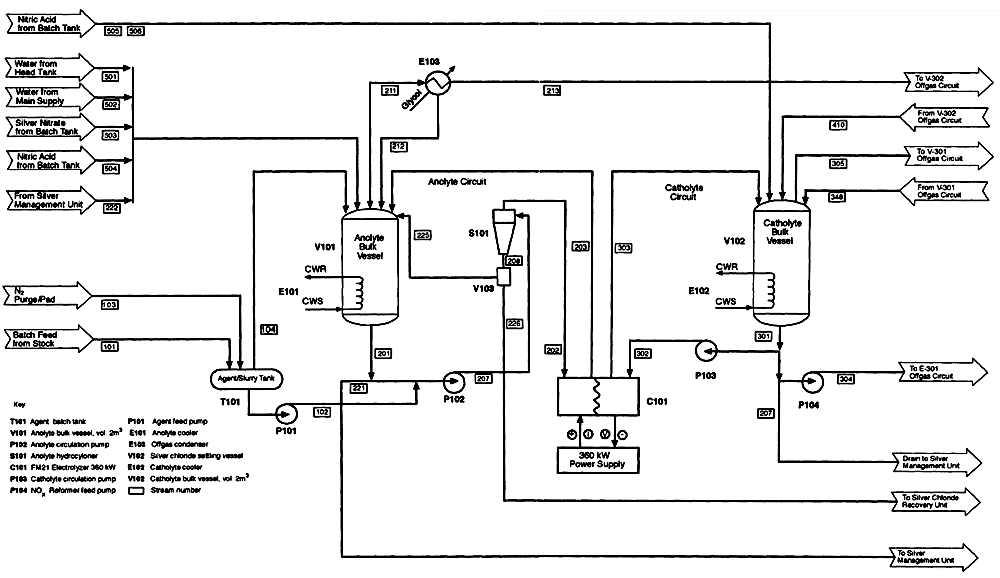
Figure 5-4
Process flow diagram for a single Silver II cell. Source: AEA Technology.
- agent receipt and supply
- anolyte feed circuit
- catholyte feed circuit
- electrochemical cell
- anolyte offgas condenser
- NOx reformer system
- catholyte silver nitrate recovery circuit
- combined offgas treatment circuit
- silver management system
- utilities infrastructure
Figure 5-4 is a process flow diagram. Each of the key system components is discussed below.
Agent Receipt and Supply. The TPC plans to use the same systems developed and tested by the Army for the baseline system.
Anolyte Feed Circuit. The anolyte feed circuit includes a 2-m3 anolyte vessel, the anolyte compartment of the electrochemical cell, a circulation pump, and connecting pipework. For HD processing, a hydrocyclone is added to remove some of the silver chloride precipitate. The anolyte vessel is fed from batch tanks of silver nitrate and nitric acid, a head tank of water, the catholyte silver nitrate recovery circuit, and an agent-slurry tank.
Catholyte Feed Circuit. The catholyte feed circuit consists of a single loop by which 4.0 molar nitric acid is pumped from a 2-m3 bulk vessel through the cathode compartment of the electrochemical cell and back to the bulk vessel. The nitric acid concentration in the bulk vessel is maintained by additions from the NOx reformer, which reclaims nitric acid from spent catholyte and NOx separated from the catholyte.
Electrochemical Cell. Anolyte and catholyte solutions circulate through the cell at flow rates up to 45 m3/h and temperatures up to 90°C.
These four components make up the basic agent destruction system. This system runs in a semibatch, or campaign, mode. Each of the FM21 electrochemical cells has an associated agent receipt and supply unit to process a ton container of agent, as well as its own anolyte and catholyte feed circuits. A campaign consists of processing a ton container of agent through this system. A campaign for the standard 360-kW module (two FM21 cells) therefore involves handling and processing two ton containers of agent simultaneously. The TPC expects each campaign to last 7 to 10 days, during which time the system will be run continuously. The 360-kW module is the basic unit of facility scale. Increased throughput, or facility scale-up, consists of adding additional 360-kW modules and the infrastructure to support them. The silver management system is operated in batch mode at the end of a campaign. It operates totally apart from the agent destruction process and does not affect the time for destroying agent (throughput rate).
Anolyte Offgas Condenser, NOx Reformer, Catholyte Silver Nitrate Recovery Circuit, and Combined Offgas Treatment Circuit. These four components, which are shown in Figure 5-5, operate continuously throughout a campaign. They constitute the auxiliary and downstream processing and recycling components of a fully functioning agent destruction system. The anolyte offgas condenser removes water vapor, nitric acid vapor, and condensable organics from the offgas. The NOx reformer reconstitutes nitric acid from the products of the cathode reaction. The catholyte silver nitrate recovery circuit captures silver that has migrated across the cell membrane from the anolyte. The offgases from the cell and the noncondensable overheads from the distillation circuits are processed through the combined offgas treatment circuit before being released to the atmosphere.
Silver Management System. The silver management system, shown in Figure 5-6, operates independently of the agent destruction system. At the end of a campaign, it is used to treat residual chemicals that have accumulated in the anolyte and catholyte circuits and to recover silver. Residuals in the anolyte circuit can include phosphate, sulfate, nitrate, and chloride anions in acid solutions. The specific anionic mix depends on whether HD or VX has been treated. The anode compartment of an FM21 cell, at 2.5 m3, is large enough to keep the phosphate from VX and the sulfate from VX or HD in solution throughout a campaign. After a campaign, the silver management system removes the phosphates and sulfates from the cell electrolytes and recovers any silver remaining in the catholyte and anolyte circuits. Not shown in Figure 5-6 is the auxiliary system that will be needed to recover silver from the solid silver chloride formed when HD is processed.
Utilities Infrastructure. The Silver II process is energy-intensive. The electrical energy required is 72,600 kW·h per metric ton of HD destroyed and 134,900 kW·h per metric ton of VX destroyed.
Scientific Principles
Ag(II) in an acidic medium is one of the most powerful oxidizing agents known (Lehmani et al., 1996). The standard reduction potential of the Ag(II)/Ag(I) couple is 1.98 volts, whereas the standard reduction potential of the O2/H2O couple is only 1.23 volts in nitric acid. Several published studies report on the use of anodically generated Ag(II) to oxidize organics in an acid solution (e.g., Lehmani et al., 1996; Farmer et al., 1992; Steele, 1990; Mentasti et al., 1984).
The basic half cell reactions for the Silver II process are as follows:
Anode: 2Ag+ → 2Ag++ + 2e Eº= -1.98 V
Cathode: HNO3 + 2H+ + 2e → HNO2 + H2O Eº = +0.94 V
The net reaction is therefore:
2Ag+ + HNO3 + 2H+ → 2Ag++ + HNO2 + H2O Eº= -1.04 V
In these equations, E°is the standard equilibrium potential at zero current flow when all reactants and products are at unit activity. In practice, the required potential is larger than the standard equilibrium
potential because of ohmic heating and other effects. The TPC uses an applied potential of 2 V.
Oxidation of Ag(I) to Ag(II) at the surface of a platinum anode is rapid, and the required overpotential is low: 120 mV at 5kA/m2. The principal Ag(II) species formed is AgNO3+, which has a dark brown color. The color disappears almost instantaneously in the presence of organics due to several complex reaction steps that result in the complete oxidation of the organics and the reduction of Ag(II) back to Ag(I). Silver is not consumed in the process but functions as a mediator between the electric power fed into the cell and the organic compounds being destroyed.
The reaction mechanisms in silver-mediated electrochemical oxidation are not well understood but are believed to involve highly reactive, short-lived species, including hydroxyl and other radicals. In a study of the electrochemical oxidation of ethylene glycol and benzene by Ag(II), several relatively long-lived reaction intermediates were identified, but with sufficient time complete oxidation was achieved as evidenced by measurement of stoichiometric quantities of CO2 in the final product. (Farmer et al., 1992)
Technology Status
The Silver II process has yet to be operated on a commercial-scale. The largest-scale pilot-tests have been conducted with 4-kW cells consisting of a single anode-cathode pair. The most extensive tests have been conducted with spent tributyl phosphate dissolved in kerosene, from the Purex process, as the feed material. These tests, which were run continuously, 24 hours per day for up to 14 days, destroyed a total of 150 liters of the feed material. The TPC has successfully completed laboratory tests on 10-g batches of agent and has constructed a pilot plant at Porton Down, United Kingdom, that is suitable for tests on 15-liter batches of agent. All of the tests prior to startup of the Porton Down plant had been conducted with only the electrochemical cell component of the agent destruction system. The Porton Down facility also includes anolyte and catholyte feed circuits, an anolyte offgas condenser, an NOx reformer system, and a modified version of the combined offgas treatment circuit, which culminates in a sodium hydroxide scrubber. The silver management system will be tested at Dounreay on the effluent generated at Porton Down.
A preliminary draft report received by the panel on May 31, 1996, summarizes the results of a test conducted by the TPC at Porton Down on 14.62 kg of "as supplied VX," which contained 12.7 kg of agent. The test consisted of a single continuous run of 6.5 days. At the end of the run, no agent was detected in the catholyte or in the process residuals. The lower detection limits for VX were 7.6 mg/m 3 in the anolyte, 9.2 mg/m3 in the catholyte, and 1.7 mg/m3 in the residuals discharged during the trial. The corresponding volumes were 0.0724 m3 of anolyte, 0.0854 m3 of catholyte, and 0.0929 m3 of process residuals. The total residual VX was therefore less than 1.5 mg out of an input of 12.7 kg of VX, corresponding to an agent destruction efficiency of greater than 99.99998 percent.
The TPC calculated that the 14.62 kg of "as supplied VX" contained 7.21 kg of organic carbon. At the end of the run, the total organic carbon remaining in the anolyte and catholyte circuits was 0.816 kg. Therefore, the destruction and removal efficiency for conversion of organic carbon to CO2 and CO was 88.7 percent. The TPC suggests that further removal might have been possible by continuing the operation of the cell after the organic feed was ended.
The TPC operated the test cell at Porton Down at currents between 600 and 1,400 A. The test was not able to operate at the design current of 2,000 A because of pressure increases in the anolyte compartment when VX was added. The TPC traced the problem to lower than expected efficiency of the NOx reformer, which resulted in the passage of more than expected unreacted O2 and NOx gas through the condenser and into the scrubber. This increased the pressure drop across the scrubber, causing an increase in pressure in the anolyte gas stream.
Operational Requirements and Considerations
Process Operations
In concept, the Silver II process as a complete system will operate as follows. Prior to the introduction of agent to the system, all other constituents are present in the anolyte and catholyte solutions, the feed circuits are operating, and all systems are at their set-point temperatures. Once flows and temperatures are stable, the current is turned on and agent is pumped into the circulating anolyte solution from the 1-m3 agent-slurry tank. The flow rate of this agent feed is about 0.01 m3/hr, which should maintain the agent concentration in the anolyte
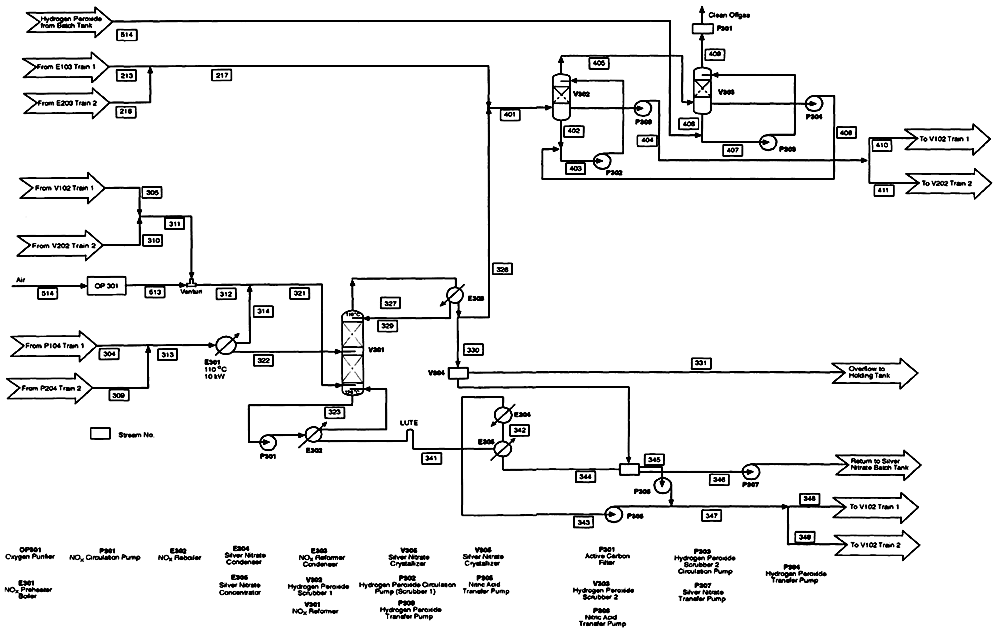
Figure 5-5
Anolyte offgas condenser, NOx reformer, silver nitrate recovery circuit, and combined offgas treatment circuit. Source: AEA Technology.
at about 5,000 ppm. To ensure good mixing of the agent with the anolyte feed, the agent is added at the inlet to the circulating pump (see Figure 5-4).
The TPC has proposed several options for transfer-
ring agent from a ton container to the agent-slurry tank. The agent transfer system that the Army has proposed for use in the neutralization process (see Chapter 7) is equally well suited to Silver II.
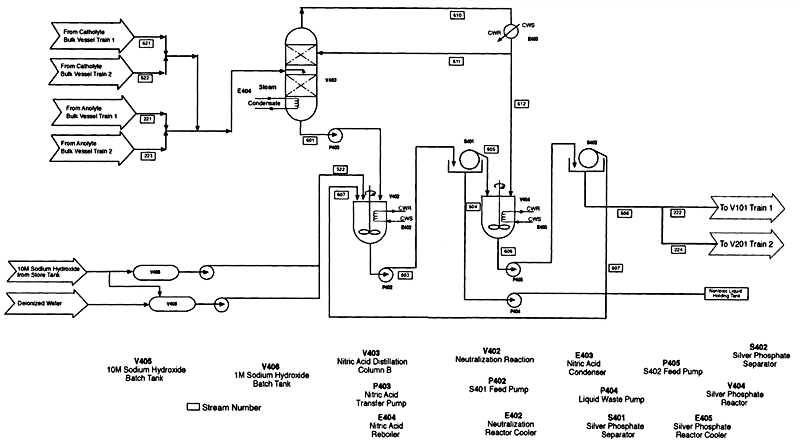
Figure 5-6
Silver management system. Source: AEA Technology
Compositional Changes during Normal Operation
Normal cell operation depletes certain constituents of both the anolyte and catholyte, so continuous addition of makeup chemicals is required. Silver nitrate must be added to the anolyte circuit; nitric acid must be added to the catholyte circuit.
The loss of silver nitrate has two causes: the transport of Ag(I) from the anode to the cathode compartment, which occurs with any organic feed material, and the precipitation of silver chloride, which happens when a feed material contains chlorine, as does HD. The TPC reports that transport of Ag(I) accounts for about 1 percent of the total charge transferred. The total theoretical charge
transfer per metric ton of agent destroyed is 17 x 109 coulombs for HD and 29.6 x 109 coulombs for VX. The anolyte circuit starts out with 2.5 m3 of solution that is 0.5 molar in silver nitrate, which represents an initial inventory of 1.25 kg-mols of silver nitrate or 134 kg of silver. During the course of an HD campaign (one ton container), 190 kg of silver will transfer from the anode to the cathode compartment; during a VX campaign, 332 kg will transfer. In both cases, therefore, the total quantity of silver transferred to the cathode compartment during a campaign exceeds the initial amount of silver in the anolyte circuit. The catholyte silver nitrate recovery circuit, which is discussed below, recovers the silver from the catholyte by crystallizing silver nitrate
from the concentrated solution and dissolving it in nitric acid for return to the anolyte circuit.
During the destruction of HD, major losses of silver from the anolyte occur from precipitation of insoluble silver chloride. By the end of the campaign, 12.58 kg-mols of silver chloride, containing 1,357 kg of silver, has precipitated. Therefore, the silver nitrate additions during an HD campaign must make up for silver losses of 1,547 kg from both Ag(I) transport and AgCl precipitation. This means that 1.5 metric tons of silver must be added to the anolyte circuit for each metric ton of HD destroyed.
The makeup silver nitrate is added to the anolyte feed circuit through a manifold in the top of the anolyte vessel and mixes into the bulk anolyte as the solution circulates. Silver concentration must be monitored during a campaign, and feedback systems must be designed to automate the addition of proper quantities of silver nitrate to the anolyte circuit.
The acidity of the anolyte solution increases substantially during a campaign. Sulfur from the feed becomes sulfuric acid; phosphorus becomes phosphoric acid; and in HD processing, chlorine precipitates with Ag(I) as AgCl, leaving nitric acid. It appears that the resulting increases in acidity will not be corrected during a campaign.
The catholyte solution loses nitric acid continuously because the nitric acid is reduced to nitrous acid as the principal cathode reaction. The nitrous acid subsequently decomposes to NOx gases. To compensate for this loss, a bleed stream from the catholyte circuit is pumped continuously to the NOx reformer system, where some of the excess water is boiled off and the nitrous acid is oxidized to nitric acid for return to the catholyte circuit. (The NOx reformer system is discussed in detail below.)
Water Management System
A water management system is needed to control the water level in both the anode and cathode compartments. The water balance is complex, involving two countervailing forces. Water flows from the anode compartment across the membrane to the cathode compartment in the form of hydrated protons (hydronium ions, H3O+) generated as a product of the anode reaction. Water flows in the opposite direction, from the cathode compartment to the anode compartment, because of the osmotic pressure maintained by the lower acidity (i.e., higher water concentration) in the cathode compartment.
The transport of hydrated protons from the anode compartment to the cathode compartment can be calculated readily from the basic electrochemistry of the cell (Appendix E). The compensating effect of osmotic diffusion must be determined empirically. In pilot plant commissioning tests observed by the panel at Porton Down, in which triethyl phosphate was the organic feed, the level of the anolyte visibly rose within a few hours of operation, while the level of the catholyte fell. Thus, under those conditions, the rate of osmotic diffusion was clearly exceeding the rate of water transport via hydrated protons. Further tests with agent as the organic feed will be required to engineer the system for proper water balance. The osmotic flow will also vary during a campaign, as the acidity of the anolyte increases.
NOx Reformer
The principal reaction at the cathode is the reduction of nitric acid to nitrous acid. A bleed stream (flow rate of 0.168 m3/h) from the bottom of each of the two catholyte bulk vessels used in a standard 360-kW module is pumped to a boiler, where the nitrous acid undergoes thermal decomposition to NO gas and nitric acid. The NO gas is mixed with 90 percent pure oxygen, heated to 110°C, and fed at a rate of 196.7 m3/h to the base of a distillation column. This column forms the heart of the NOx reformer (see Figure 5-5). The aqueous phase from the boiler, containing nitric acid and silver nitrate, is fed into the midsection of the distillation column at a rate of 0.377 m3/h. The overhead stream from the distillation column passes through a condenser. The condensate stream, a dilute solution of nitric acid, is split; one part returns to the top of the distillation column, and the rest goes to a holding tank for reuse or eventual discharge (after being neutralized to a salt such as sodium nitrate). Noncondensables enter the combined offgas treatment circuit (discussed below).
Catholyte Silver Nitrate Recovery Circuit
The bottom stream from the NOx reformer column passes to a boiler. Nitric acid and water vapor from the boiler return to the bottom of the distillation column, and
the remaining, more concentrated solution of silver nitrate in nitric acid passes to a concentrator. Approximately every 6 hours, the liquid accumulated in the concentrator is transferred to a crystallizer, where the solution is cooled. Silver nitrate crystallizes out, and the supernatant nitric acid is drained off and returned to the catholyte circuit. The silver nitrate crystals are then redissolved in the dilute nitric acid from the overhead of the NOx reformer. This solution returns to the batch tank for silver nitrate solution, to be used as makeup for the anolyte circuit.
Anolyte Offgas Condenser
Reactions in the anode compartment produce several gaseous products including CO2, O2, and possibly CO. Volatile organic products of incomplete oxidation may also form from the stepwise oxidation of agent. These gaseous reaction products form an offgas saturated with water and nitric acid vapors. The offgas is released from the anolyte vessel to a condenser chilled by a mixture of water and glycol at 0°C. The gases are cooled to 10°C, causing any nitric acid, water, chemical agent, or condensable organic products to condense and drain back to the anolyte bulk tank. The noncondensable gases enter the combined offgas treatment circuit.
Combined Offgas Treatment Circuit
The noncondensable gases from the anolyte offgas condenser and the NOx reformer are combined for further treatment (Figure 5-5). The combined gases pass through two hydrogen peroxide scrubbers that are 30 to 35 feet tall. The scrubbers reduce the concentration of NOx to less than the permitted discharge limit. The scrubbed gas passes through an activated carbon filter bed and is released to the atmosphere. The process flow diagrams do not show a condenser and reheater that will be required upstream from the carbon filter bed to remove water from the scrubbed gas. Gas from the hydrogen peroxide scrubbers will be saturated in water vapor, which, if not removed, would impair the capacity of the carbon bed to adsorb trace organics.
Silver Management System
At the end of a campaign, solutions from both the anolyte and catholyte circuits are transferred to the silver management system (Figure 5-6). The combined solutions are distilled through two columns in series (columns A and B in Figure 5-6). Still bottoms from the first column are drained to a mixing tank, where they are neutralized by sodium hydroxide added from a batch tank. These highly acidic still bottoms contain a solution of silver nitrate in nitric, sulfuric, and phosphoric acids; as nitric acid is removed by distillation, silver sulfate and silver phosphate may precipitate. The exact composition depends on which agent was treated. Addition of sodium hydroxide converts the acids to their sodium salts in solution, which becomes a process residual. Any precipitated silver salts (silver sulfate, phosphate, or oxide) are filtered out and reacidified to recover silver.
Figure 5-7 shows the adjunct to the silver management system that will be required after an HD campaign. As previously discussed, the residuals in the anolyte circuit will contain more than a metric ton of precipitated silver chloride, which must be filtered out. This filtration could be difficult because precipitated silver chloride tends to form very small particles. The supernatant acid mixture is double-distilled as described above, and silver nitrate is ultimately recovered from the still bottoms.
The precipitated silver chloride is transferred to a separate mixing vessel to which excess sodium hydroxide is added. Any sulfuric and nitric acids accompanying the silver chloride are converted to dissolved sodium salts. The silver chloride is partially converted to silver oxide (Ag2O) via a solid-state, diffusion-controlled reaction. This conversion therefore proceeds from the outside of the particle in, so that each particle has a core of silver chloride and a coating of silver oxide. The liquid, containing sodium salts, is filtered off and becomes a process residual. The precipitate is reacidified with nitric acid, which dissolves the silver oxide as silver nitrate. The silver nitrate solution is filtered off for reuse as anolyte feed. Any remaining silver chloride solids are recycled to repeat the treatment with sodium hydroxide for conversion to silver oxide. This sequence is repeated until all the silver chloride from the campaign has been converted back to silver nitrate solution in nitric acid.
The TPC has not described this post-campaign neutralization and silver recovery system in detail. It appears that neither part of the system has been tested. Actual quantities and compositions of feed and product streams were not reported to the panel. The silver management system will operate as a batch process totally separate from the agent destruction campaign. The proposed process, which appears to be scientifically
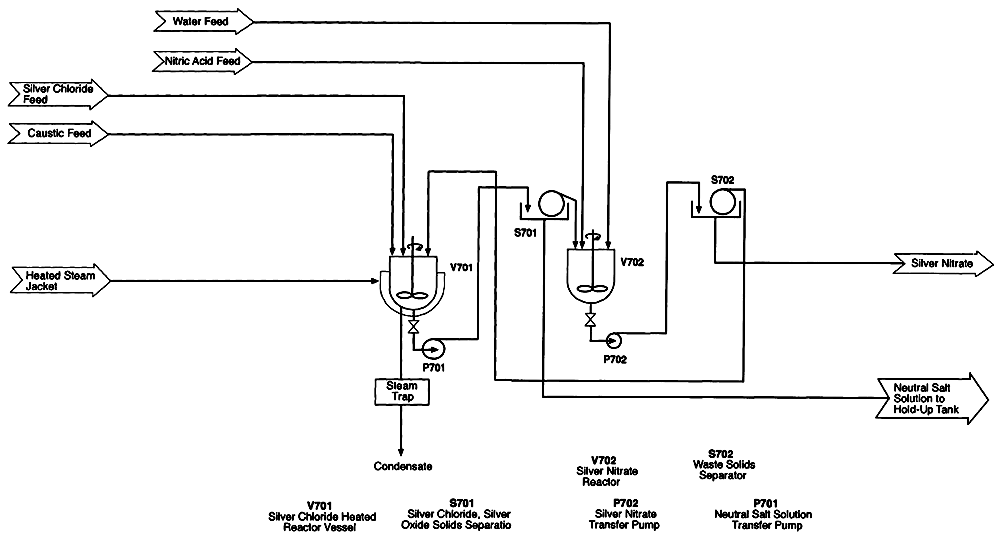
Figure 5-7
Silver chloride treatment system. Source: AEA Technology.
sound, will be tested by the TPC on the post-campaign electrolyte solutions from the pilot-tests at Porton Down.
Energy Requirements
The Silver II process consumes a great deal of electrical energy for cell operation and for auxiliary heating, refrigeration, and pumping. The theoretical energy for a 2-volt cell is about 9,400 kW·h per metric ton of HD and 16,440 kW·h per metric ton of VX. The TPC assumes a 60 percent electrochemical efficiency, which raises the energy requirements to 15,700 and 27,400 kW·h per metric ton of agent destroyed for HD and VX, respectively.
The TPC estimates the total electric power consumption for operation of a basic two-cell module and auxiliary equipment at 1.7 MW, consisting of:
|
Cell requirement |
360 kW |
|
DC power supply losses |
360 kW |
|
Refrigeration |
2 kW |
|
Steam |
622 kW |
|
Compressor for plant air |
10 kW |
|
Instrumentation and control |
10 kW |
|
Blast air coolers |
360 kW |
Based on the TPC's estimates that a single 360-kW module, operated 24 hours per day, could destroy 137.6 metric tons of mustard or 74.1 metric tons of VX in 245 days, the total electric energy consumption is 72,600 kW·h per metric ton of HD destroyed and 134,900 kW·h per metric ton of VX destroyed.
The silver management system, which requires additional electric power of 507 kW, is expected to operate for about 6 hours following completion of each
campaign. The electrical energy consumption for silver management after a two-cell (two ton containers) campaign is therefore about 3,000 kW·h.
The power requirement shown above is for one 360-kW module. The TPC's design for processing HD calls for two modules; the plan for VX calls for three modules. This scaling of facilities would provide sufficient capacity to destroy the agent inventories at Aberdeen and Newport in 6 years. It would require 3.4 MW of power for the HD facility and 5.1 MW for the VX facility. About 40 percent of this power must be transformed to 2 volts and then rectified to DC (direct current) to supply the electrochemical cells. The remainder is needed for motors and resistance-heating to produce steam. A power system of this scale will have to be carefully designed, although it is well within the state of practice. The power requirement is large enough that either facility will require its own power substation, where power will probably be drawn directly from a high voltage grid (around 13,800 volts) and transformed down to the voltages needed. There will probably be a requirement for phase correction. These requirements do not appear to pose any unusual problems for a local utility. Destruction of the agent inventories in a shorter time period would require additional modules and, of course, additional power.
All of this electrical energy input becomes heat. Additional heat is generated by the reactions (effectively the same as the heat of combustion of the agent being destroyed), which amounts to another 10 percent on top of the total electrical energy input. The heat from both sources must be removed, primarily by cooling water. The location of heat transfer equipment is shown on the various flow diagrams, and Figure 5-8 summarizes the various heating and cooling requirements. More than 1,000 square feet (93 m2) of heat exchanger surface is required for each module. The heat exchanger materials must be suitable for service in contact with concentrated nitric acid.
Startup and Shutdown
It is preferable to run the agent destruction system continuously during a campaign. Although agent oxidation can be stopped and restarted with a touch of the switch that controls current to the electrochemical cell, procedures need to be defined for shutting off electrolyte flows and downstream systems, if necessary. Before resuming cell operations after a shutdown, flows and temperatures of many process streams would have to be re-established. There is no time pressure in restarting the system because no reaction occurs until the cell current is turned on.
Emergency shutdown procedures have not been fully worked out, but if conditions do not require immediate shutdown of the cell, the sequence of steps would probably be as follows:
- Shut off agent injection.
- Shut off feedstock chemical injection.
- When the total organic content in the anolyte circuit has been reduced to a predetermined level, shut off the current to the cell.
- Shut off the circulation pumps in the anolyte and catholyte circuits.
- Continue operating all scrubber, stripping, gas stream, and ancillary circuits until the system is purged, and then shut them down.
The same procedure would be followed for planned maintenance and at the end of each campaign.
Feed Streams
Table 5-1 summarizes the data submitted by the TPC on feed stream compositions and mass requirements per metric ton of agent destroyed. Tables 5-2 and 5-3 show the overall mass balances, also supplied by the TPC, for the destruction of 2 metric tons of HD and VX, respectively, in a single module campaign. The panel assumes that the obvious discrepancies between the quantities in Table 5-1 and the mass balance quantities in Tables 5-2 and 5-3 will be resolved as the TPC continues to develop the technology toward a detailed engineering basis. (Appendix E contains the elemental balances corresponding to Tables 5-2 and 5-3.) Silver nitrate is not included as an input stream in the mass balance on the assumption that there is no significant net loss of silver. The mass balances are presented to the nearest tenth of a ton. They therefore do not address trace quantities of organics (i.e., concentrations of I percent or less) that might be present in the offgas. Nor do they include trace quantities of silver that might be present in the neutral salt solution. Material balances showing the flow of all fluids into and out of each component subsystem of the Silver II process are not available.
TABLE 5-1 Feed Stream Compositions and Quantities
|
|
|
Tons Per Ton of Agent Destroyed |
|
|
Feed Streams |
Composition |
VX |
HD |
|
Nitric acid |
69 wt% (16 M) |
4.4 |
5.0 |
|
Silver nitrate |
200 g Ag/liter (1.2 M) |
4.2 |
5.9 |
|
Water |
|
47.1 |
41.3 |
|
Hydrogen peroxide |
35 wt% |
19.0 |
10.9 |
|
Sodium hydroxide |
10 M NaOH |
0.2 |
0.1 |
|
Oxygen |
90 vol% (10% nitrogen) |
2.6 |
0.7 |
Process Effluent Streams
The thermodynamics and kinetics of the electrochemistry underlying the Silver II process, coupled with the TPC's design conditions, such as a low concentration of agent in a highly acidic anolyte, clearly indicate that, in principle, the required DRE of six 9's or higher should be technically feasible. In laboratory-scale tests on both surrogates and agents (10 g per test), no agent was detected in the residuals. However, because of the small quantities involved in these tests and the limits of detectability of the analytical methods used, the computed DREs are only four 9's (99.99 percent). As was noted above (under Technology Status), preliminary results for VX from the pilot-testing under way at Porton Down indicate a destruction efficiency of at least six 9's (actually, 99.99998 percent or almost seven 9's), with detectability again being the limiting factor. These results show that the technology can destroy agent. However, even the more sensitive analyses being run at the current Porton Down facility do not demonstrate that a full-scale cell (an FM21 cell), configured for the operating conditions of a fully functioning basic agent destruction system over the course of a campaign, will in fact achieve or exceed the required DRE. In addition, the destruction efficiency for agent does not address issues of the composition and concentration of process products in the residual streams, including trace quantities of toxic residuals or the environmental burden of residuals. (For further discussion, see Scale-Up Requirements below.)
Under normal operating conditions, the submitted design for Silver II anticipates that the following process residuals will be produced:
- End-of-pipe gaseous emissions from the combined offgas treatment circuit will be a mixture primarily of carbon dioxide, oxygen, and nitrogen.
- Aqueous effluent from the silver management system will be a solution of sodium nitrate, sodium sulfate, sodium phosphate, and sodium chloride. The exact composition will depend on the agent that was treated.
- Sodium nitrate solution is the residual from neutralization of the effluent (0.6 percent nitric acid, pH 1, 13.2 m3 per ton of agent) generated from the NOx reformer. This salt solution is likely to be combined for discharge with the aqueous effluent from the silver management system.
No residuals have been tested for toxicity, but the principal constituents are common materials that are not considered hazardous to health or the environment.
The gases are released to the atmosphere after passing through two hydrogen peroxide scrubbers in series and a filter bed of activated carbon. This treatment should reduce any organics in the offgas to nondetectable levels, but the final emissions will not be retained for analysis prior to release. The panel considers it highly improbable that any agent will escape from the anolyte to the offgas. In any case, the severe treatment
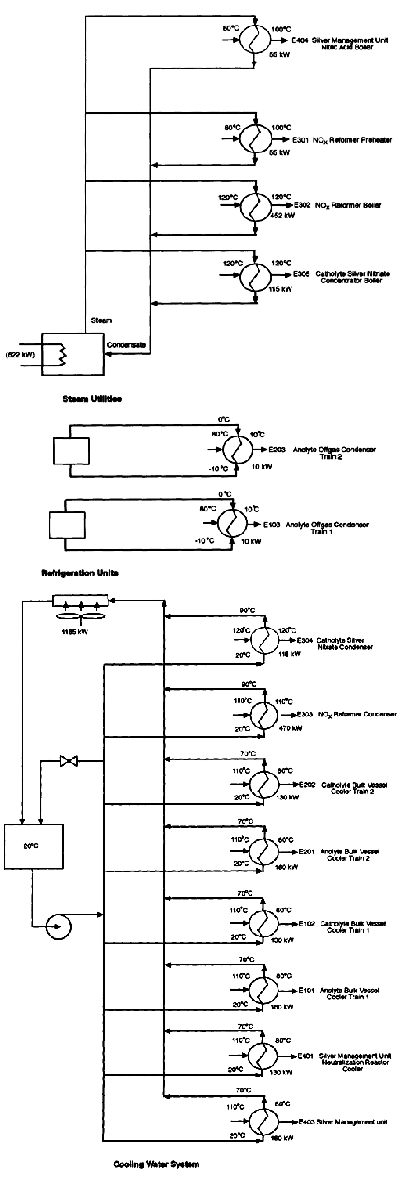
Figure 5-8
Process flow diagram for services and utilities. Source: AEA Technology.
of the offgas with hydrogen peroxide, followed by carbon filtering, will remove both agent and volatile organics from the offgas.
The TPC reports that the aqueous effluent from the silver management system is slightly acidic (pH 6). Although this effluent is primarily a solution of
sodium salts, it could contain trace quantities of silver salts as well. The TPC also reports that laboratory experiments show that the silver concentrations in the effluent will be on the order of 50 µg/m3 (about 50 parts per trillion); the panel did not receive details of these experiments. The maximum allowed concentration in
TABLE 5-2 Mass Balance for HD Destruction (all figures in metric tons)
|
Inputs |
Agent |
Nitric Acid |
Hydrogen Peroxide |
Sodium Hydroxide |
Oxygen |
Total |
|
HD (mustard) |
2.0 |
|
|
|
|
|
|
HNO3 |
|
0.4 |
|
|
|
|
|
H2O |
|
0.2 |
|
|
|
|
|
H2O2 |
|
|
1.1 |
|
|
|
|
H2O |
|
|
2.0 |
|
|
|
|
NaOH |
|
|
|
2.0 |
|
|
|
H2O |
|
|
|
0.1 |
|
|
|
O2 |
|
|
|
|
2.8 |
|
|
N2 |
|
|
|
|
0.3 |
|
|
Total Input |
2.0 |
0.6 |
3.1 |
2.1 |
3.1 |
10.9 |
|
Outputs |
|
Offgas |
Waste Acid |
|
Neutral Salt Solution |
Total |
|
CO2 |
2.2 |
|
|
|
|
|
|
O2 |
0.1 |
|
|
|
|
|
|
N2 |
0.3 |
|
|
|
|
|
|
NOx |
0.002 |
|
|
|
|
|
|
HNO3 |
|
|
0.6 |
1.8 |
|
|
|
H2O |
|
2.2 |
|
|
1.5 |
|
|
|
|
|
2.2 |
|
|
|
|
Total Output |
2.6 |
|
2.8 |
|
5.5 |
10.9 |
the United States is 50 ppb (parts per billion). The expected volume of aqueous discharge per metric ton of agent treated is 11.2 m3 when treating HD and 4.7 m3 when treating VX.
The aqueous residuals from the silver management system and the NOx reformer are retained in a holding tank for analysis. After that, disposal may be by one of three routes: (1) direct discharge to the environment in accordance with an National Pollutant Discharge Elimination System(NPDES) permit; (2) indirect discharge to a publicly owned treatment works (POTW); or (3) transport to an off-site facility for recovery of the salts. The third option will have to be preceded by evaporating the solution to dryness, if the Army does not allow transport of liquid residuals.
Ton container cleanout will follow the protocol established and tested by the Army. (This protocol is described in Chapter 7.)
TABLE 5-3 Mass Balance for VX Destruction (all figures in metric tons)
|
Inputs |
Agent |
Nitric Acid |
Hydrogen Peroxide |
Sodium Hydroxide |
Oxygen |
Total |
|
VX |
2.0 |
|
|
|
|
|
|
HNO3 |
|
0.7 |
|
|
|
|
|
H2O |
|
0.3 |
|
|
|
|
|
H2O2 |
|
|
1.9 |
|
|
|
|
H2O |
|
|
3.6 |
|
|
|
|
NaOH |
|
|
|
1.8 |
|
|
|
H2O |
|
|
|
0.1 |
|
|
|
O2 |
|
|
|
|
4.9 |
|
|
N2 |
|
|
|
|
0.5 |
|
|
Total Input |
2.0 |
1.0 |
5.5 |
1.9 |
5.4 |
15.8 |
|
Outputs |
Offgas |
|
Waste Acid |
|
Neutral Salt Solution |
Total |
|
CO2 |
3.8 |
|
|
|
|
|
|
O2 |
0.1 |
|
|
|
|
|
|
N2 |
0.5 |
|
|
|
|
|
|
NOx |
0.004 |
|
|
|
|
|
|
HNO3 |
|
|
1.1 |
|
|
|
|
H2O |
|
|
3.9 |
|
|
|
|
NaNO3 |
|
|
|
|
|
0.6 |
|
Na2SO4 |
|
|
|
|
|
1.1 |
|
Na3PO4 |
|
|
|
|
|
1.2 |
|
H2O |
|
|
|
|
|
3.6 |
|
Total Output |
4.4 |
5.0 |
6.5 |
|
|
15.9 |
Process Instrumentation and Control
The heart of the proposed system of process instrumentation and control is a computer-based system for supervisory control and data acquisition (SCADA). This system allows the operators to monitor and control facility operations from a dedicated control room or cabin. To protect cabin personnel from on-site gases, the
control cabin would have its own filtered air supply and be ventilated at positive pressure relative to the rest of the facility.
The control parameters to be monitored by a suitable SCADA software package are listed in Table 5-4, as are basic requirements and features. Key elements of this integrated system that are particularly relevant to Silver II are discussed below.
The current and voltage measurements indicate whether the cell is operating properly. They provide warning of cell malfunctions such as membrane failures.
Electrolyte flow rates must be monitored because high flow rates through the cells are necessary for good mixing. Each cell compartment is 10 mm wide by 240 mm high. Electrodes occupy about half the volume of a cell. The volumetric flow through a full cell is 45 m3/h. The TPC estimates the hydraulic radius of each electrode compartment to be 4.8 mm, giving a Reynolds number of around 4,600 (density = 1,000 kg/m3; viscosity = 1 centipoise), which is at the lower end of the turbulent range.
Gases released from the anolyte and catholyte circuits will be monitored for CO2, O2, NOx, CO, volatile organics, and chemical agent as indicators of proper cell operation. For instance, an abrupt elevation of oxygen concentration indicates that direct oxidation of water by Ag(II) has become the predominant anode reaction. The same gaseous components are monitored in the offgas before and after carbon filtration to ensure safety and to confirm proper operation of the hydrogen peroxide scrubber train.
Liquid composition must be monitored to obtain the feedback necessary for the controlled addition of key constituents in the electrolytes. For satisfactory cell operation throughout a campaign, the addition of chemical agent is controlled to maintain about 5,000 ppm in the anolyte circuit. Monitoring data are also needed to control the addition of silver nitrate to the anolyte circuit and the addition of nitric acid to the catholyte circuit. Composition monitoring also follows the progressive buildup of sulfate or phosphate in the anolyte and indicates whether agent and organic intermediates are being oxidized.
Monitoring temperatures and pressures is important for confirming proper operation of the cooling system, particularly because of the large heat-transfer requirements for sustained operation of the Silver II process. (See preceding discussion of electrical energy and heat of reaction as sources of heat to be removed.)
During an HD campaign, another important parameter to monitor is the amount and location of precipitated silver chloride. By the end of a campaign a large amount of silver chloride will have precipitated in the anolyte circuit. The hydrocyclone in this circuit is intended to deposit most of the precipitate in a collection vessel (shown in Figure 5-4). The efficiency of the hydrocyclone is critical to proper functioning of the anolyte circuit. Some sampling at various points in this circuit will be needed to determine the solids content, with particular attention to the anolyte flowing into the electrochemical cell and the possible retention of precipitate in the cell.
All the parameters listed in Table 5-4 must be monitored without human intervention and the results fed into the SCADA system for control of operations. Analogous monitoring and control systems are used for industrial processes but will have to be adapted specifically for the Silver II process.
One of the commercially available SCADA-type software packages that operate on a personal computer and are used in the chemical industry may prove suitable for use in Silver II. Higher-integrity packages based on the UNIX operating system are also available. The SCADA system that the TPC is testing at Porton Down uses Paragon TNT software with Allen Bradley controls. The system was not yet fully operational at the time of the panel's visit. In any case, final SCADA system selection and integration will not be part of the piloting program under way at Porton Down. These actions are being deferred to an early stage of detailed design for a full-scale operating facility.
For agent monitoring, which will be required throughout the plant, the standard equipment approved by the U.S. Army will be used. All agent sensors must interface with the SCADA system to ensure automatic alarm and response capability.
Process Stability, Reliability, and Robustness
Stability
The Silver II process as presented in the submitted designs is composed of two systems that operate independently of one another. One is the agent destruction system, which is composed of the electrochemical cell and its supporting circuits; the offgas treatment circuits; and all supporting unit operations, processes, and plumbing. The other is the silver management system, which operates separately at the end of a campaign. Separation of the two systems contributes to stability and ease of operation.
TABLE 5-4 Elements of a Supervisory Control and Data System for Silver II
|
Control Parameters to be Monitored |
|
DC current and voltage, particularly to the cells |
|
Electrolyte flow rates |
|
Gas flow rates |
|
Gas composition (volume percent O2, CO2, CO, NOx, volatile organics, and chemical agent vapor) |
|
Liquid composition (pH; dissolved silver, sulfate, phosphate, total organic carbon, and chemical agent; suspended silver as AgCl) |
|
Temperature |
|
Pressure |
|
Additional Required Software Features |
|
Validation of operation inputs |
|
Interlocks to prevent inappropriate operator commands |
|
Mimic diagrams of plant subsystems |
|
Alarms that are triggered from process or facility sensors and that can initiate plant responses |
|
Software control and display of data from subsystems |
|
Operator control of plant actuators and processes based on graphical interface display of piping and instrumentation diagrams |
|
Automatic data logging |
|
Trend display of logged data |
|
Plant data (i.e., SCADA system data) accessible from remote sites |
|
Automatic report generation |
|
Multiple SCADA displays around the plant |
|
Automatic responses to fault conditions |
|
Detection of rate of change alarms |
The agent destruction system operates in a semibatch mode. Catastrophic failure from uncontrolled reactions is highly unlikely because of the nature of the process and the conditions under which the various modules operate. Agent is fed slowly to the anolyte to maintain a constant, low concentration and therefore will not accumulate in the anolyte circuit. The agent feed rate is controlled by monitoring the CO2 concentration in the anolyte offgas. If the CO2 level drops below a set-point determined by the agent feed rate (i.e., by the carbon feed to the process), a fault condition exists and the agent feed will shutdown automatically.
For a runaway condition to occur, the cell reactions must release enough heat to raise the electrolyte temperature from the normal 90°C at which it is controlled to 105°C, the boiling point of nitric acid. For this to happen, three independent trip or interlock systems must malfunction: the cooling circuit controls, the anolyte high temperature trip, and the agent addition inhibition interlock. Simultaneous failure of these three control systems is highly improbable. Minor process fluctuations under normal operating conditions might vary the temperature between 87°C and 93°C.
During the course of a campaign, some process conditions will change substantially, particularly in the anolyte circuit, but the rate of change is slow under normal operating conditions. Therefore, the response time for most control instrumentation is not very
demanding. For example, as stated previously, the total outflow of silver from the anolyte circuit during an HD campaign of 5 days at 24 hours per day is about 1,550 kg. The required makeup is therefore 12.9 kg per hour, which is less than 10 percent of the initial 134 kg inventory of silver in the anolyte compartment. The required silver makeup in a VX campaign is about 2.8 kg per hour, which is 2 percent of the initial silver inventory. Silver makeup is in the form of 1.2 molar silver nitrate, which contains 12.84 kg/ m3 of silver.
None of the processes in the system modules is particularly sensitive to small excursions in composition or temperature. However, compositions of some constituents will change substantially during the course of a campaign, and a test program is needed to verify that the planned control systems are adequate to ensure stable operation over the full range of operating compositions.
Both the agent destruction and silver management systems operate at low temperatures and close to atmospheric pressure, which substantially reduces the requirements for sensitivity and response time of control systems, compared with high temperature systems. Even though the system can tolerate small temperature excursions and a runaway reaction is unlikely, there are large heat loads produced in a system with relatively small volumes. Therefore, temperature control in each of the modules and in the system as a whole must be tested and validated.
A large loading of silver chloride precipitate during an HD campaign can cause many problems, including malfunction of the electrochemical cells, inadequate heat transfer in the heat exchangers, and pump malfunctions. The pilot demonstration is critical not only to determining the effectiveness of the hydrocyclone in removing the very fine precipitate expected but also to assessing the effect of suspended particles on cell operation. The pilot plant at Porton Down is testing only a single anode-cathode pair. In a full-scale cell, if one compartment should become plugged, the flow will increase through the remaining anode compartments and further precipitation will occur in the plugged compartment. Plugging would lower cell efficiency and, in the plugged anode compartments, increase the alternative reaction of Ag(II) with water to produce O2. The TPC has identified the further potential consequences of plugging as overheating and failure of the Nafion membranes in the blocked compartment. To reduce the risk of solids settling in the anode compartments, the TPC has designed the system for turbulent flow. In addition, the temperature of the anode compartment will be monitored to detect overheating in time to exercise process controls, if plugging does occur.
Reliability
With respect to the reliability of equipment, the electrochemical cell to be used in Silver II is identical in design to commercial cells that have been used reliably for decades to manufacture chlorine gas and caustic (NaOH) by electrolysis of brine (NaCl solution). However, the two applications are totally different from a process perspective. Cells that produce chlorine and caustic operate in a pH-neutral to alkaline environment. The Silver II process requires a highly acidic environment. Furthermore, the anode and cathode reactions in the two processes are completely different.
Laboratory and pilot-tests conducted by the TPC for reprocessing radioactive waste and for destroying many other organic materials have demonstrated that the general Silver II cell technology and conceptual framework are sound. There have been no commercial applications to date.
The other components of the agent destruction system are standard unit processes and operations to be conducted with readily available, off-the-shelf equipment. Tests conducted as of May 1996 have not included these other components. The key components are included in the scheduled pilot-testing at Porton Down, but the facility itself and the planned tests will not provide an end-to-end proof of design sufficient for scaling to full operation. A higher level of pilot-testing will be required to verify materials of construction (the Porton Down plant is constructed largely of glass), operational reliability for the full-scale FM21 cell under varying conditions, and integration of all system components that must operate simultaneously and in concert for the duration of a campaign.
In addition to the reliability of equipment and the reliability of the basic processes, there are several additional aspects of reliability relevant to an assessment of the Silver II process. With respect to reliability of agent detoxification, the agent is hydrolyzed, and therefore detoxified, upon contact with nitric acid in the anolyte circuit. The agent feed to the anolyte circuit is maintained at a level low enough that this hydrolysis occurs immediately.
With respect to reliability as backup operability, the standard 360-kW module for the basic agent destruction system consists of two identical, separately fed 180-kW
cells. If one cell fails, it can be removed for cleaning and replacement, while the other continues to operate.
The design includes a standby generator to provide electrical backup in the event of a power failure. This backup power must be adequate to continue operating scrubbers and pumps in the event of an emergency shutdown.
With respect to reliability against unplanned downtime, an individual 180-kW cell can be removed to a remote area for repair or maintenance while a replacement module in good working order is substituted and processing continues. Thus, the modular design of the system reduces the risk of unplanned downtime.
Robustness
In the panel's judgment, the Silver II system is capable of operating satisfactorily over a wide and varying range of temperature, pressure, energy input, and feed composition. Anionic and cationic impurities in the agent could reduce cell current efficiency but would not otherwise interfere with the basic process operations.
With a well-designed SCADA system, upsets in feed, in key reaction conditions (temperature, pressure, agent concentration, and reactant concentrations), or in energy input or heat removal should be readily detectable in time to take appropriate corrective action. However, repeated upsets, although not a major threat to human health or the environment, would be highly undesirable from an operational standpoint. Current test data are insufficient to estimate the probable frequency of events that could lead to upsets.
Materials of Construction
Systems and Materials
In the design submitted for Silver II, the core agent destruction process is carried out in aqueous concentrated nitric acid at close to atmospheric pressure and at temperatures below 90°C. Temperatures at points in the secondary circuits where nitric acid solutions are distilled will reach the boiling point of the still bottoms. (The boiling point of concentrated nitric acid is 105°C; additional salts in the still bottoms may further elevate the boiling point.) The NOx reformer heats the NO gas stream to 110°C.
The technology design, including the selection of materials of construction, is based mainly on the TPC's experience with nitric acid for reprocessing radioactive wastes. The materials selected, which are well known to be compatible with concentrated nitric acid, include titanium, low-carbon stainless steels, platinum, zirconium, and polytetrafluoroethylene (used for Nafion 324 cell membranes and for gaskets).
In the submitted design, anodes are made of platinum or platinized titanium. Cathodes are made of low-carbon stainless steel. The piping and vessels in the anolyte feed circuit are made from titanium to ensure integrity. Boilers are constructed from zirconium because their conditions of operation were judged to be too close to a corrosion band for titanium.
Although the materials and design are conventional for applications involving concentrated nitric acid, the panel believes the following issues require further consideration:
- The primary metals of construction (stainless steel, titanium, and zirconium) all sustain stress corrosion cracking in nitric acid solutions at various concentrations and potentials. The possibility of stress corrosion cracking must be carefully investigated, particularly given the presence of a high concentration of dissolved silver.
- The possibility of intergranular corrosion should be addressed because nitric acid is highly oxidizing, and the chemistry of oxidation at grain boundaries is not well defined for any of the metals being considered for Silver II. Of particular concern are changes in chemical potentials at grain boundaries, as a result of adsorption.
- Plugging of the anode compartments, particularly in HD campaigns, may significantly affect reliability. The conditions under which plugging occurs are not known at present. Also, a simple and reliable technique for replacing an FM21 cell when the system is on line is highly desirable. A means of detecting plugging and conditions that could lead to a short circuit or hot spots should be pilot-tested and incorporated into the final design.
- The electrochemical oxidation of agent in nitric acid will produce species containing carbon, sulfur, and phosphorus (VX only) in the anolyte. This environment is substantially different from the environments in previous industrial experience with nitric acid baths. In addition, the concentrations of species containing sulfur and phosphorus
- increase throughout the duration of a campaign. The effects on corrosion resulting from this wide and cyclical variation in electrolyte composition should be examined.
- The pilot plant at Porton Down is constructed of glass and therefore will not test the construction materials to be used in a full-scale installation.
- The plant will be designed for a 20-year lifetime, but membranes will have to be replaced every two years at a minimum and possibly more frequently when processing HD.
Environmental Conditions and Chemistry
The principal issues for the internal environment of materials of construction derive from exposure of materials to concentrated nitric acid and have been addressed above. The SCADA system will be able to detect changes in temperature from a loss of circulation or cooling in time for appropriate actions to be taken. Local hot spots at a Nafion membrane, caused by plugging or some other loss of electrolyte circulation, may damage the membrane. Methods of monitoring for hot spots and plugging in the cell are necessary. Although the system operates at close to atmospheric pressure, the equipment is designed to withstand internal pressures of up to 4 atmospheres.
Startup and Shutdown
The procedures for startup and shutdown are described under Process Operations. Neither normal nor emergency procedures will cause significant thermal stress on the materials of construction.
Failure Definition
The TPC assembled a multidisciplinary team for two days in September 1995 to conduct a first phase hazard and operability study for the design of a Silver II facility for chemical agent destruction. The team assessed the consequences of the hazard challenges listed in Table 5-5 to each of the key system components individually and to the facility as a whole, including the interfaces between components.
For each challenge and each component, the team identified causes, consequences, and safeguards. The
TABLE 5-5 Hazard and Operability Challenges
|
Fire |
Human error |
|
Explosion/implosion |
Corrosion |
|
Maintenance |
Erosion |
|
Containment |
Effluents |
|
Contamination |
Missiles |
|
Toxicity |
Terrorism and sabotage |
|
Loss of services |
Other external events |
|
Extreme weather |
Industrial hazards |
team then recommended additional safety measures or additional information required to assess whether further controls were needed. Fifty recommendations were made. Most of the cases leading to an accidental release to the atmosphere were generated for the challenges of missiles, terrorism and sabotage, and other external events (seismic events, aircraft crashes, or fire affecting the agent receipt and supply system). The fact that atmospheric releases were identified for these external challenges does not reveal any particular vulnerability of the Silver II technology or the TPC's design because these challenges were not specific to the agent destruction process at the facility. Other consequences worth noting were release of nitric acid as the result of corrosion or maintenance problems, in-plant fires, and releases of agent inside the secondary containment. The majority of consequences from these internal events affected the operability of the plant but not the safety of the public.
The TPC assembled a team to review this initial hazard and operability study for two days in May 1996. Taking into account the likelihood and severity of potential failures, the team identified only one possible occurrence of concern: the possibility that chemical contamination of the electrical system might degrade cable insulation or seals, leading to potential failures.
Operations and Maintenance
See Process Operations above for the operational details of each system component. This section
describes operational experience of the TPC relevant to operating an agent destruction facility and maintenance planning for such a facility.
Operational Experience
Operational experience with the Silver II process has been limited to the electrochemical cell. However, the pilot-testing under way at Porton Down will combine the electrochemical cell with the auxiliary fluid systems (anolyte and catholyte feed circuits, anolyte offgas condenser, NOx reformer, and a modified version of the combined offgas treatment circuit). This pilot system will include all the key components of the agent destruction system except the agent feed and supply and the catholyte silver nitrate recovery circuit.
The TPC has conducted 12 laboratory tests to demonstrate the destruction of organophosphorous and mustard agents, including three nerve agents (GA, GB, and VX) and three mustard agents (HD, HT, and THD). The tests were performed with an FM01 electrochemical cell, which is a 1/35th scale model of the FM21 cell that would be used in full-scale operations. Figure 5-9 is a schematic flow diagram of the test rig for the FM01 cell.
In each test, 10 g of agent was injected into the anolyte vessel of the test rig. The anolyte vessel contained a 0.5 molar silver nitrate solution in 8 molar nitric acid. The catholyte vessel contained 4-molar nitric acid. Anolyte temperature was maintained at 50°C. Tests lasted for up to six hours. In all cases, final agent concentration was below detectable limits for the analytical methods used, but the limits of detectability were not specified. The anolyte offgas, which was measured throughout each experiment, contained varying levels of nitrous oxide and volatile alkyl nitrates.
The preliminary results from the Porton Down pilot-testing of VX are discussed in the section above on Technology Status. Longer duration tests of a Silver II cell on a scale similar to the scale of the Porton Down facility have been undertaken with mixtures of tributyl phosphate and kerosene. In these tests, an FM01 cell was operated continuously, 24 hours per day, for up to 14 days.
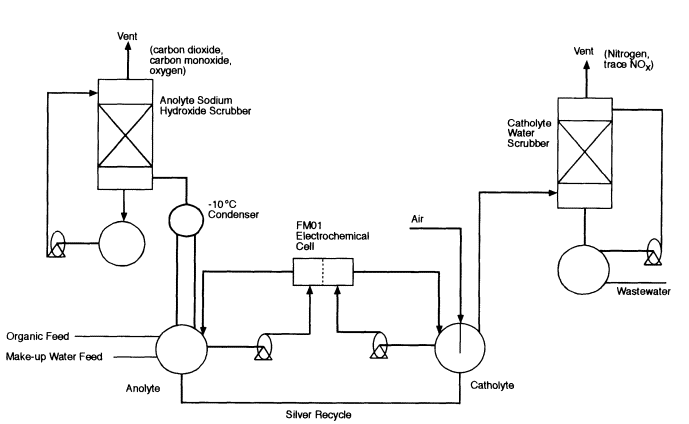
Figure 5-9
Schematic flow diagram of the FM01 test rig. Source: AEA Technology.
Maintenance
No maintenance schedule has been established at this stage of technology development for Silver II. Because the plant would operate under highly corrosive conditions with a hazardous working fluid (nitric acid), continuous inspection and maintenance must be a priority.
The electrolysis cell is the same as the cells used for chlorine production. Membrane cells have revolutionized that industry and have a good record for durability. The TPC states that a normal maintenance schedule for replacing membranes in chlorine production is 27 months. The maintenance required during agent destruction will have to be developed; process conditions for Silver II are quite different from the conditions for chlorine production.
Scale-Up Requirements
Plant scale-up in the submitted design is based on adding 360-kW modules (two 180-kW FM21 cells per module) to the facility. However, neither that module nor its 180-kW cell unit has been piloted for Silver II, and the FM21 cell represents a large scale-up from the 4-kW pilot-test at Porton Down.
The TPC has stated that scale-up from the Porton Down pilot plant to a 180-kW cell with 45 electrode pairs and 45 parallel flow paths for circulating fluid will not be a problem because the FM21 cell has been used successfully in industry. However, the reagents and reaction chemistry for Silver II are very different from those in industrial production of chlorine and caustic from brine.
A technical issue of concern to the panel is the precipitation of silver chloride in HD campaigns. The TPC expects the hydrocyclone to be highly effective in removing silver chloride, with a solids concentration in the underflow of about 0.9 percent by volume. The TPC states that blockage of the hydrocyclone discharge line is unlikely below 30 volume percent solids. The TPC also states, based on information from the vendor of the FM21 cell, that heavy solids loading will not adversely affect cell operation. However, in chlorine production the brine is treated with soda ash (crude Na2CO3) or caustic (NaOH) to precipitate out oxides and hydroxides of calcium, iron, and magnesium prior to electrolysis, because precipitation within the cell has been found to foul the membrane. Therefore, the effect of the anticipated loading of silver chloride solids on cell operation in the Silver II process clearly must be pilot-tested.
The NOx reforming process to regenerate nitric acid is conventional; it is very similar to the process used commercially to treat offgases from the manufacture of nitric acid. Nonetheless, inefficiency of the NOx reformer in the first pilot-test at Porton Down indicates that the design must be improved and more tests must be done. The hydrogen peroxide scrubbing is also conventional, although not commonly used at the scale proposed. The silver management system is not conventional but appears to be based on sound chemistry.
There are certainly significant heat transfer requirements, although none seems unconventional. As an example, in the silver management system, which operates independently from the agent destruction system, a concentrated acid solution (well over 8 molar) of a mixture of nitric, sulfuric, and phosphoric acids, plus silver nitrate, silver chloride, and various impurities, is neutralized with sodium hydroxide. This reaction has a high heat release and is prone to spattering, but the operation is well within the current state of practice.
Process Safety
Plant Safety and Health Risks
Based on the first-level hazard and operability study performed by the TPC and on the panel's preliminary, qualitative evaluation, the possibility of a catastrophic accident with a cause internal to the Silver II technology is extremely low. However, anode and cathode reactions are carried out in concentrated nitric acid, which has been described as the common chemical most frequently involved in reactive incidents because of its exceptional ability to function as an effective oxidant even when fairly dilute or at ambient pressure (Bretherick, 1985). Many reported incidents have involved closed or nearly closed vessels that have failed from internal gas pressure created either by oxidation of organic compounds to CO2 or auto-decomposition of nitric acid to NOx fumes and oxygen.
Such incidents are unlikely in the Silver II process because the system is essentially open and the concentration of organics in contact with nitric acid is low. As was already noted, three independent controls or interlocks would have to fail simultaneously for a sufficiently high concentration of agent and derived
organics to build up in the nitric acid and create potentially explosive conditions.
Community Safety, Health, and Environmental Risks
The planned containment system will reduce the risk of a release of either agent or other hazardous chemicals to negligible levels during normal operations. Abnormal events that might threaten the health or safety of the community or the surrounding environment are unlikely because the system is operated at low temperature and atmospheric pressure, the chemical reactions are slow and easily controllable, and the agent is processed at low total amounts at any one time.
Schedule
The panel anticipates that pilot-testing of a 360-kW module at Newport will require 12 months for design, 12 months for construction and commissioning tests, and an additional 12 months for agent testing. Installation of additional modules and associated infrastructure will require 12 months; commissioning tests, 6 months; and agent processing, 36 months. Pilot-testing at Aberdeen is to take longer because of the added complication of silver chloride precipitation.
The duration of operation to complete destruction of agent at the Aberdeen or Newport sites depends on the number of basic modules installed for simultaneous operation. If full-scale operations start on January 1, 2001, and agent destruction must be completed by December 31, 2004, then the facility for destruction of VX at Newport will require five 360-kW modules with a total footprint of 33 m by 61 m. Under the same schedule requirements, the facility for HD at Aberdeen will require three 360-kW modules with a footprint of 33 m by 37 m. The footprint is only for the operating plant and does not include agent handling buildings, administrative offices, workshops, electrical substation, and tank farms. Agent destruction could be completed in a shorter time by adding modules. As noted in the section on Utility Requirements, the electrical power requirement correlates with the number of modules.






























